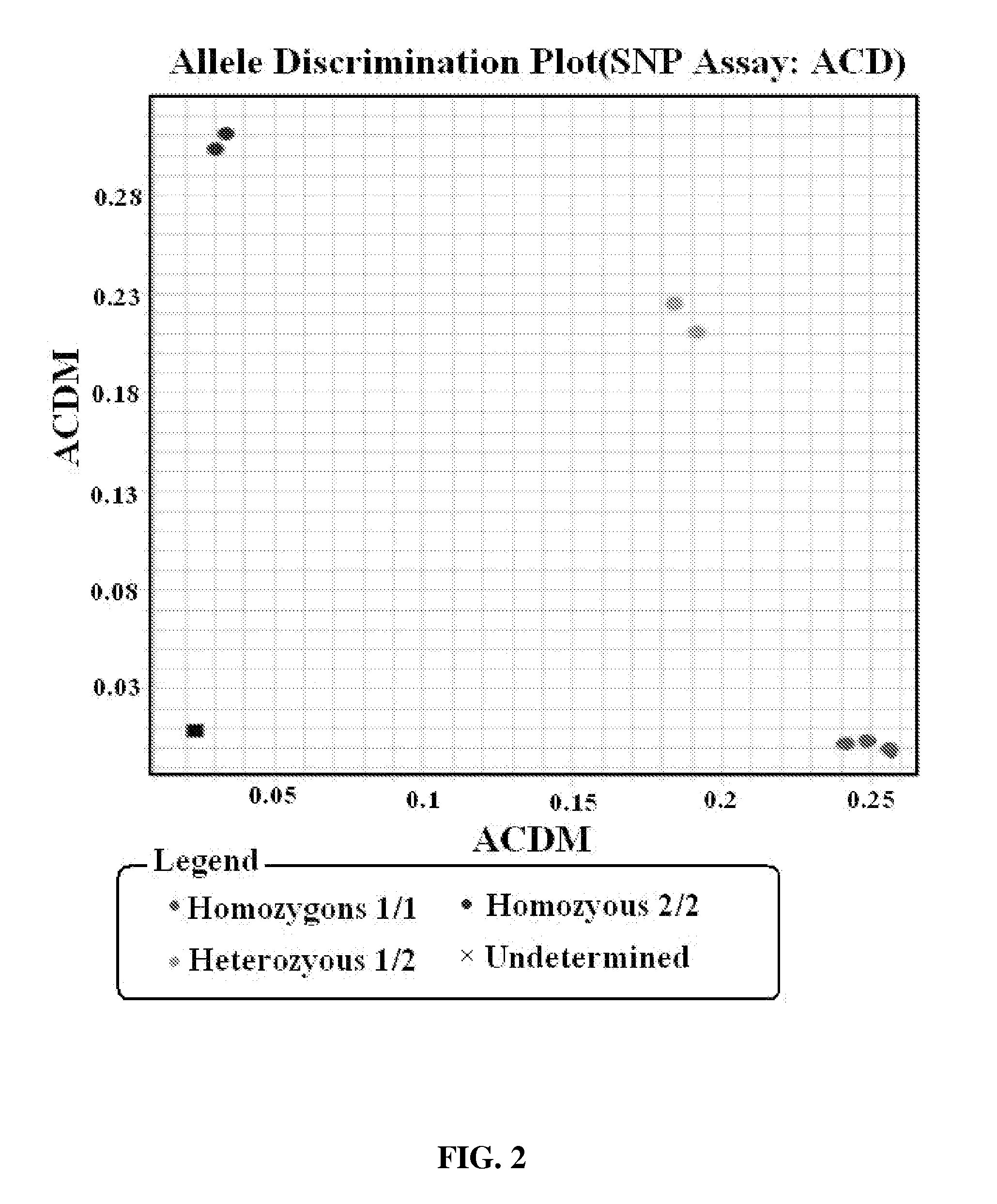
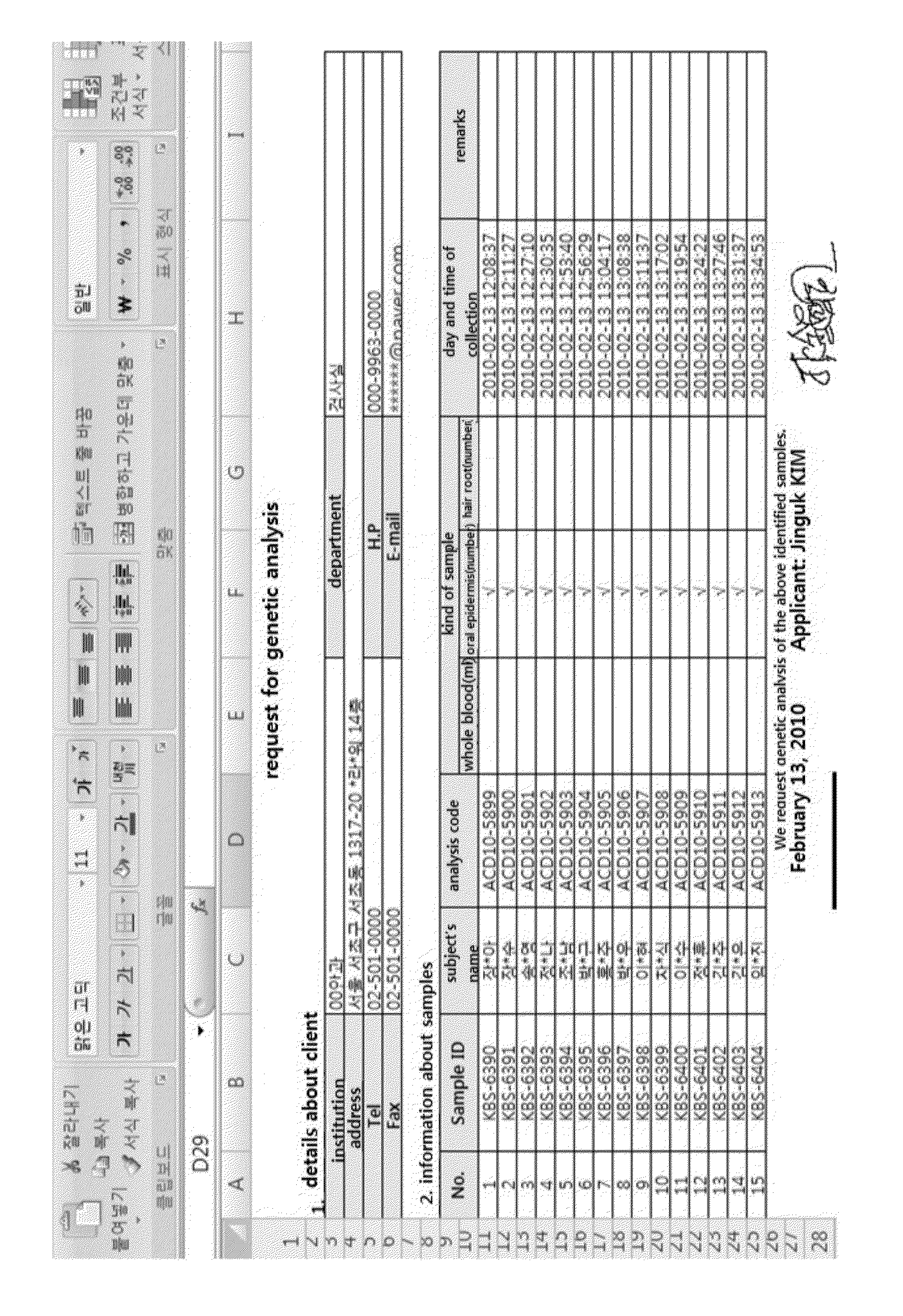
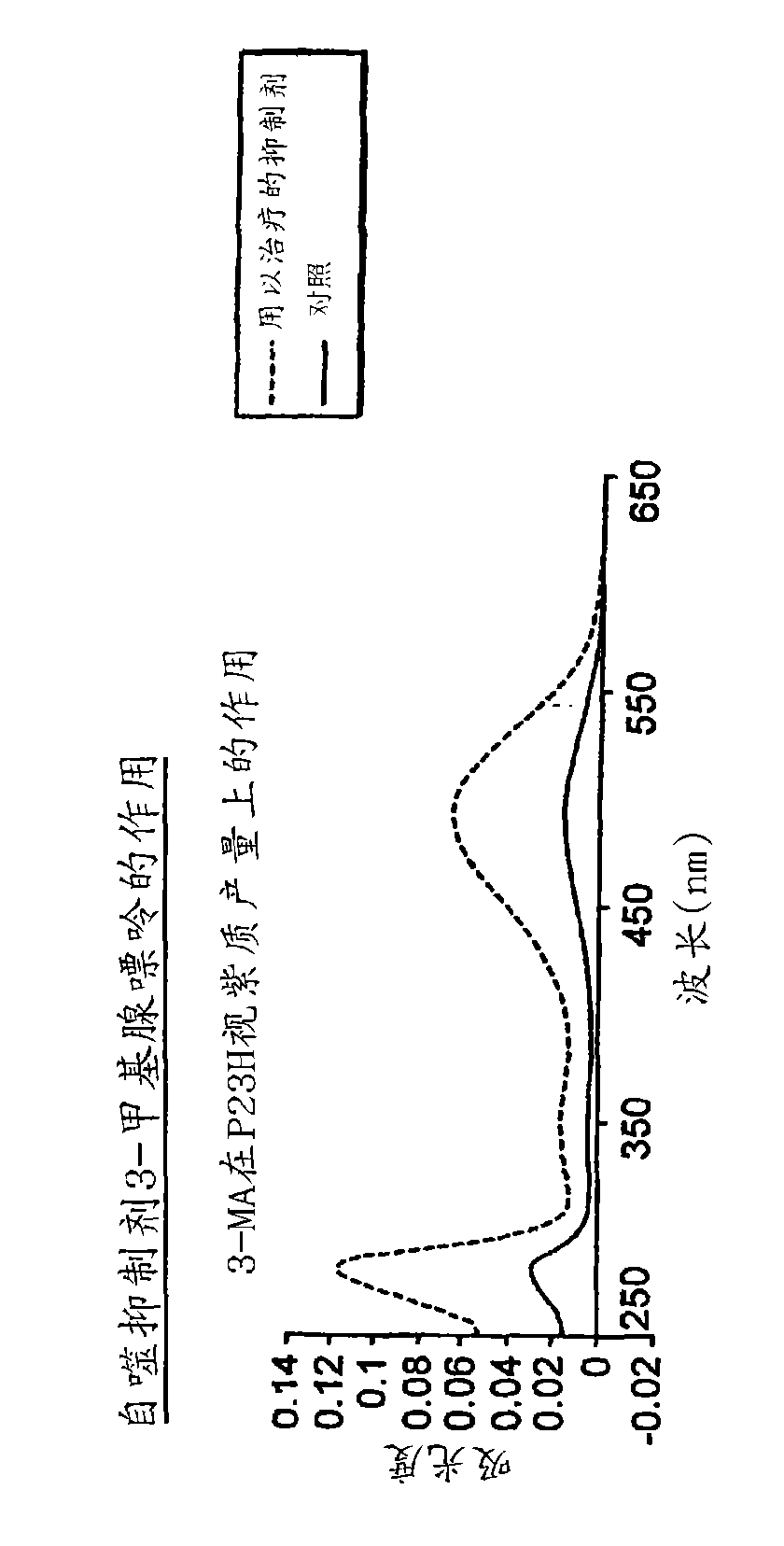
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Cornea dystrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Corneal dystrophy, Gelatinous drop-like. Corneal dystrophy is a group of rare hereditary disorders characterised by bilateral abnormal deposition of substances in the transparent front part of the eye called the cornea.
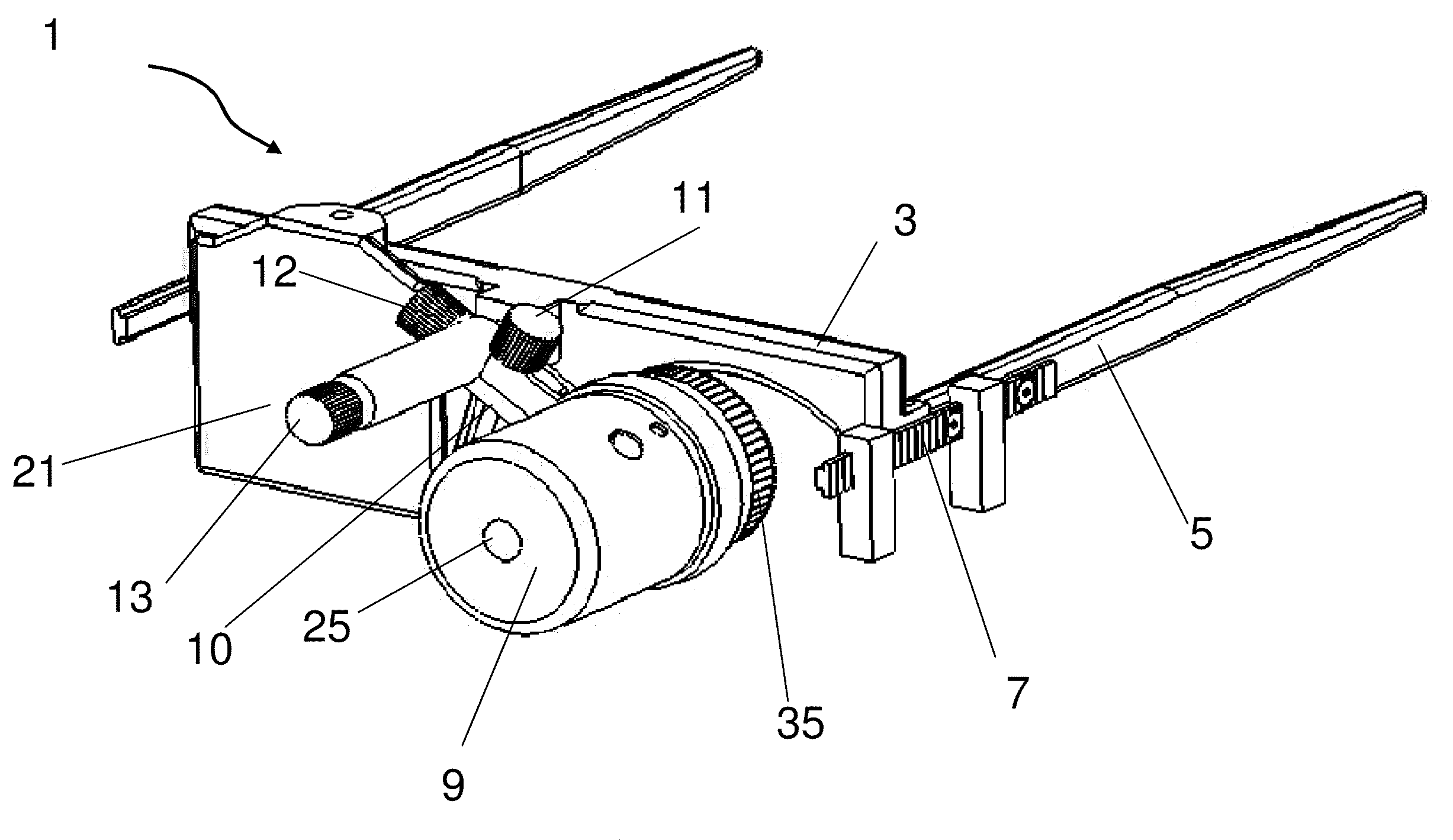
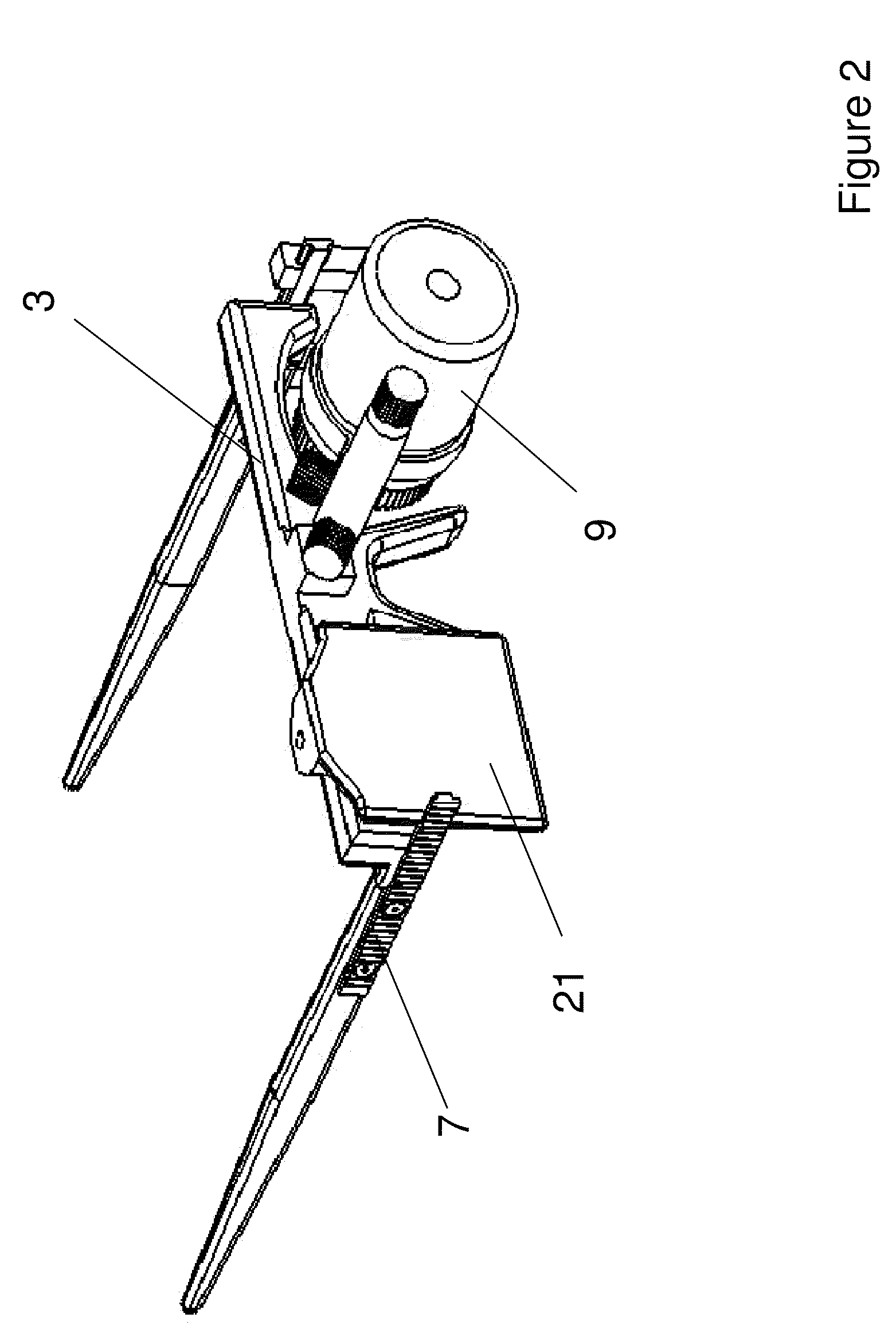
Wearable photoactivator for ocular therapeutic applications and uses thereof
The invention provides a wearable device for delivery of light of a desired wavelength and power to the cornea of a subject. The device includes a frame for attachment of a light source housing which includes a light source and a lens positioned in the housing to allow light to be directed to the eye of the subject, and the light source is operably linked to a power source. The invention provides method for the prevention and treatment of ocular disease including infection, neoplasia, and corneal dystrophies. The device of the invention can be used in conjunction with photoactive therapeutic agents.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Pharmaceutical Composition for Treating Avellino Cornea Dystrophy Comprising Blood Plasma or Serum
InactiveUS20080299212A1Efficient removalSenses disorderMammal material medical ingredientsSerum igeCornea dystrophy
The present invention relates to a pharmaceutical agent for treating Avellino corneal dystrophy, and more particularly, to a pharmaceutical composition for treating Avellino corneal dystrophy comprising pharmaceutically effective amount of blood plasma or serum as an active ingredient. The pharmaceutical composition of the present invention has an effect of improving symptoms by dissolving away hyaline granules in the cornea of a patient with severe Avellino corneal dystrophy due to LASIK surgery.
Owner:MEDIGENES CO LTD
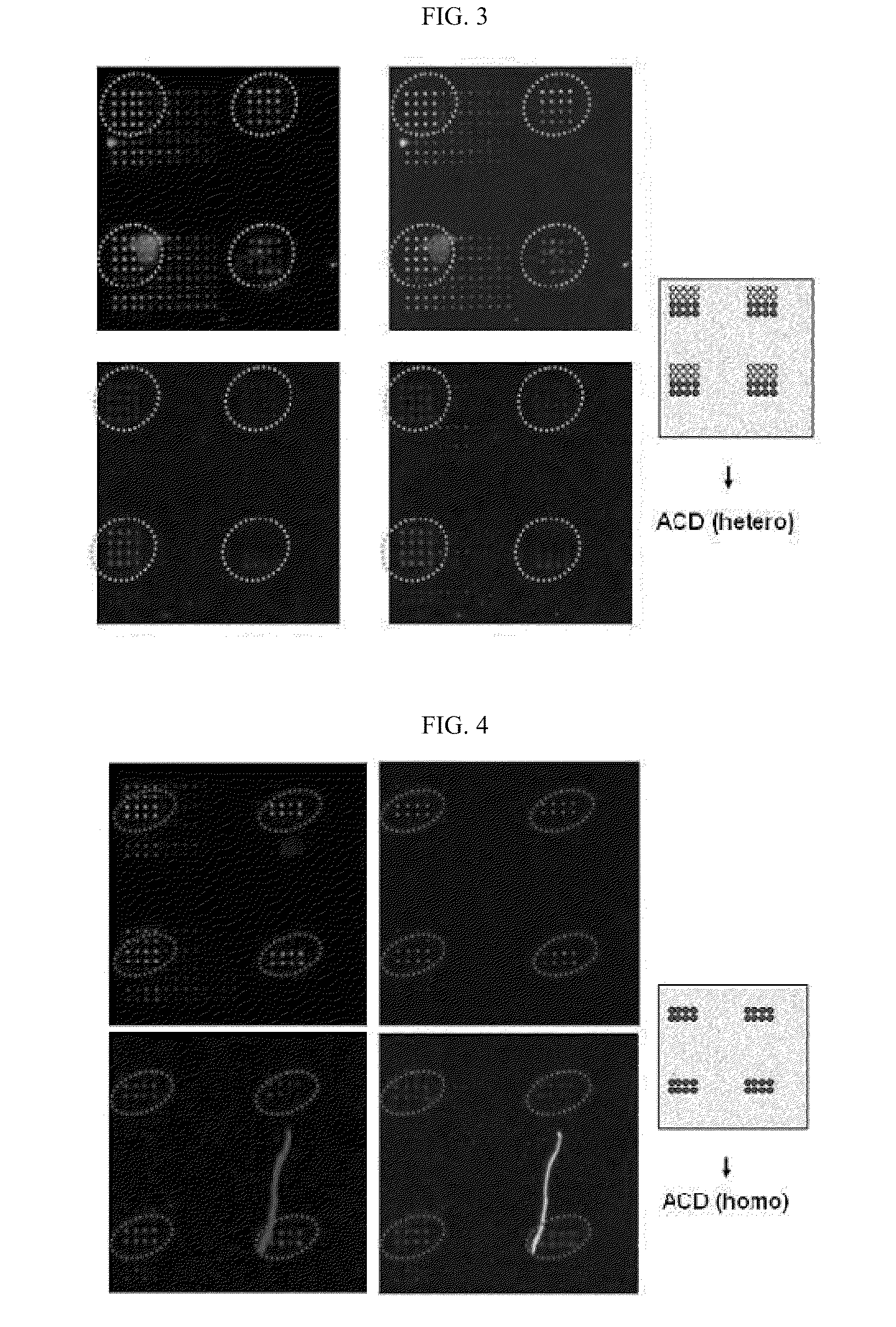
DNA chip for diagnosis of corneal dystrophy
InactiveCN101374850ASugar derivativesMicrobiological testing/measurementCornea dystrophySurgery procedure
The present invention relates to oligonucleotides for diagnosis of corneal dystrophy. More particularly, the present invention relates to oligonucleotides for detecting mutation of BIGH3 gene for diagnosis of corneal dystrophy including Avelllino corneal dystrophy, which must be precisely diagnosed before vision correction surgery, and a DNA chip for diagnosis of corneal dystrophy, which has the oligonucleotides fixed thereon. According to the present invention, conventional microscopic diagnosis of corneal dystrophy can be replaced with a precise genetic method, which prevents a patient with corneal dystrophy from losing eyesight by eyesight correction surgery after erroneous diagnosis.
Owner:MEDIGENES CO LTD
DNA chip for diagnosis of corneal dystrophy
InactiveUS20090305394A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCornea dystrophyOligonucleotide
The present invention relates to oligonucleotides for diagnosis of corneal dystrophy. More particularly, the present invention relates to oligonucleotides for detecting mutation of BIGH3 gene for diagnosis or corneal dystrophy including Avellino corneal dystrophy, which must be precisely diagnosed before vision correction surgery, and a DNA chip for diagnosis of corneal dystrophy, which has the oligonucleotides fixed thereon. According to the present invention, conventional microscopic diagnosis of corneal dystrophy can be replaced with a precise genetic method, which prevents a patient with corneal dystrophy from losing eyesight by eyesight correction surgery after erroneous diagnosis.
Owner:MEDIGENES CO LTD
Pharmaceutical Composition for Treating Avellino Cornea Dystrophy Comprising an Antibody Against Tgf-Beta
InactiveUS20080267946A1Relieve symptomsSpectales/gogglesSenses disorderAdditive ingredientCornea dystrophy
The present invention relates to a medicine for treating Avellino corneal dystrophy (ACD), and more particularly, to a pharmaceutical composition for treating Avellino corneal dystrophy containing an antibody against TGF-β as an effective ingredient. The pharmaceutical composition of the present invention has an effect of improving symptoms of a patient with severe Avellino corneal dystrophy due to TGF-β induced by exposure to intense light, such as UV etc.
Owner:MEDIGENES CO LTD
Primers for diagnosing avellino corneal dystrophy
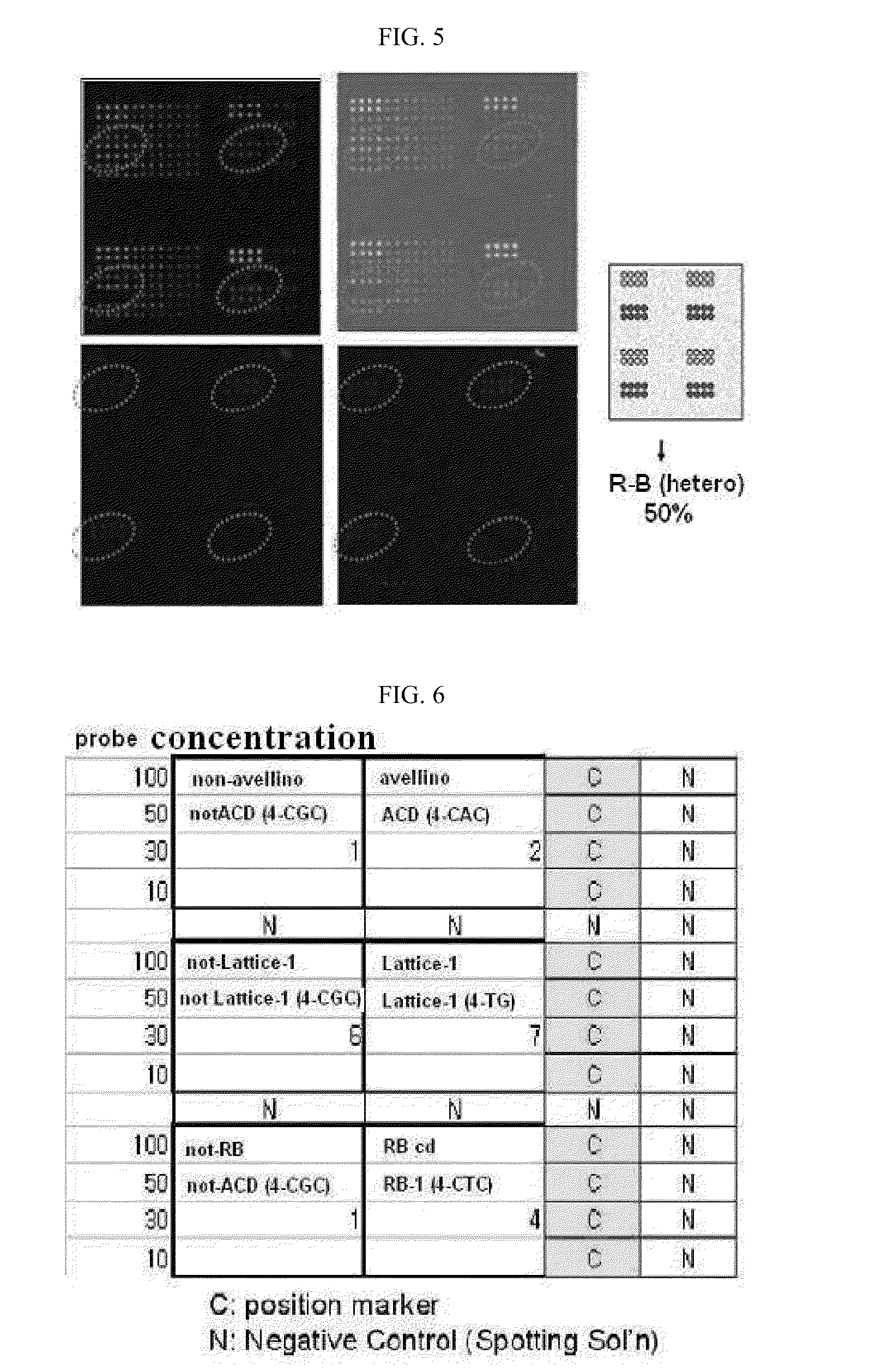
InactiveUS20120077200A1Efficiently and accurately diagnosingSugar derivativesMicrobiological testing/measurementReal-Time PCRsCornea dystrophy
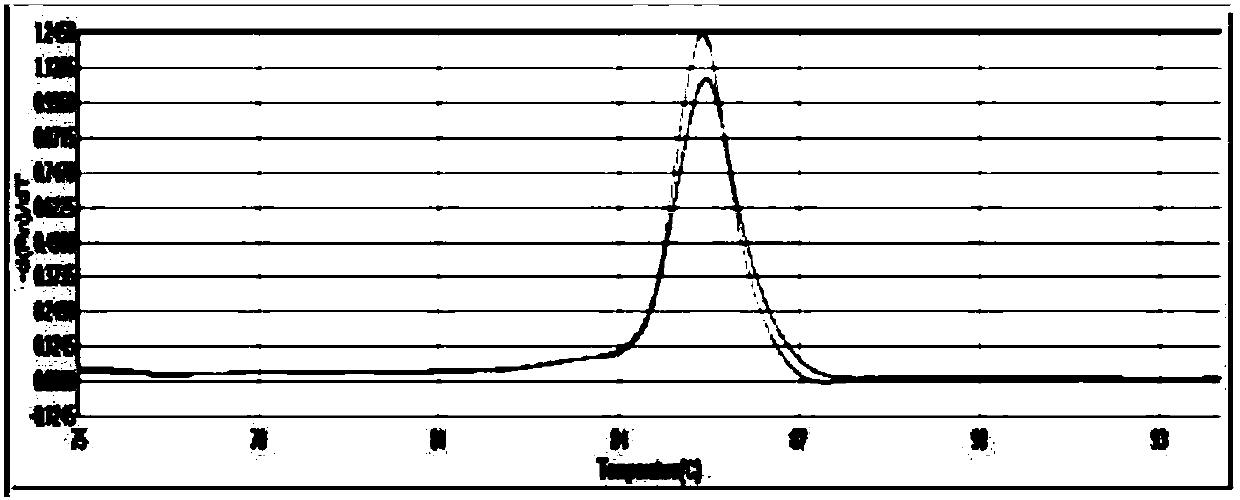
The present invention relates to a real-time PCR primer pair and probe for diagnosing Avellino corneal dystrophy, and more particularly to a real-time PCR primer pair and probe for diagnosing Avellino corneal dystrophy, which can accurately diagnose the presence or absence of a mutation in exon 4 of BIGH3 gene, which is responsible for Avellino corneal dystrophy. The use of the primer pair and probe according to the invention can diagnose Avellino corneal dystrophy in a more rapid and accurate manner than a conventional method that uses a DNA chip or PCR.
Owner:AVELLINO
System for diagnosing avellino corneal dystrophy
ActiveUS20130302811A1Bioreactor/fermenter combinationsBiological substance pretreatmentsOphthalmologyCornea dystrophy
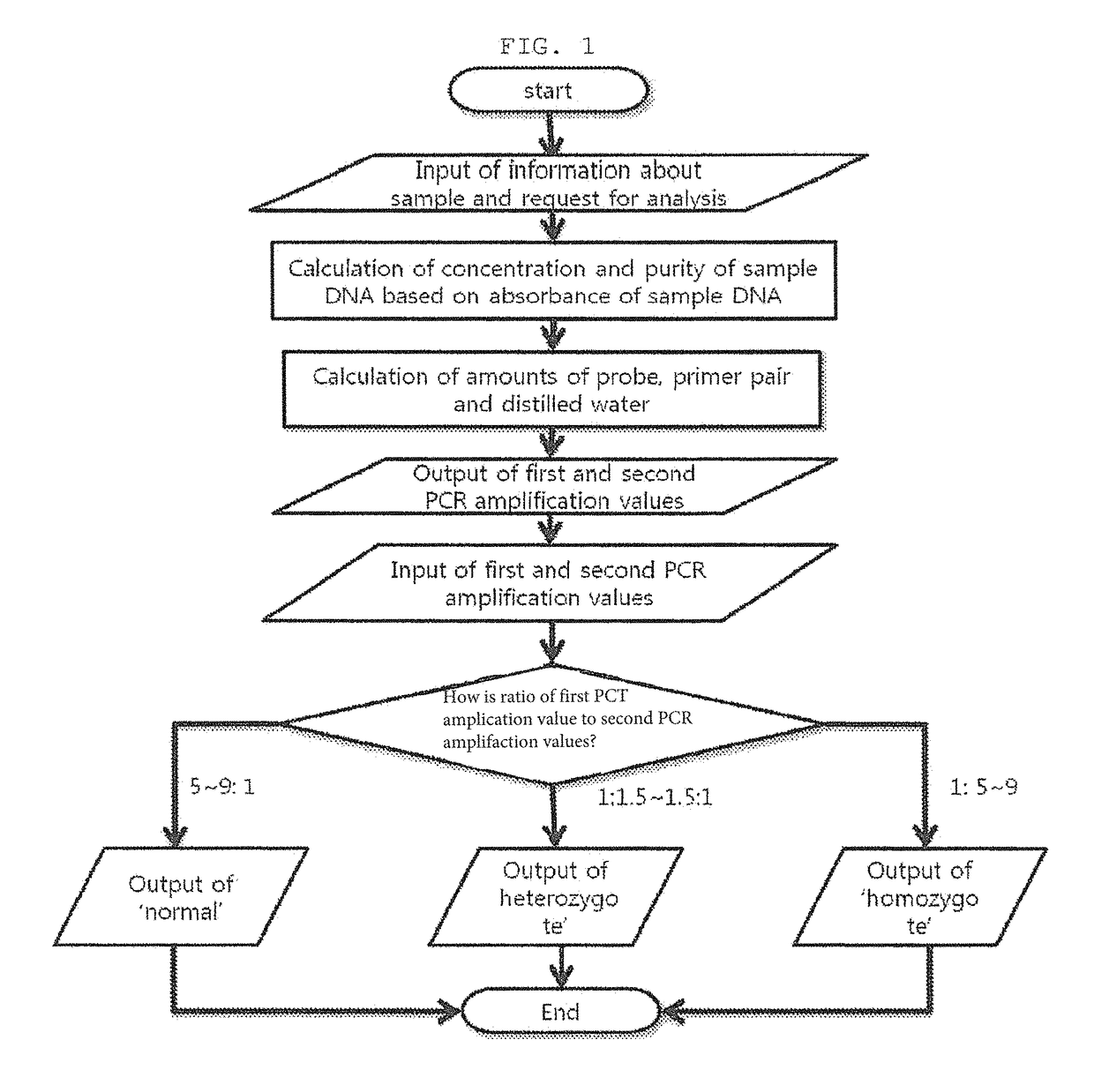
The present invention relates to a system for diagnosing Avellino corneal dystrophy, and more particularly to a system for diagnosing Avellino corneal dystrophy, in which whether a sample is normal or Avellino corneal dystrophy is determined based on the ratio of the input first PCR amplification value and the second PCR amplification value. The system makes it possible to diagnosis Avellino corneal dystrophy in a simpler and accurate manner without being influenced by the doctor's skill. Particularly, the inventive system makes the overall process systematic, and thus provides accurate diagnosis. In addition, the system can also easily administer a number of test subjects.
Owner:AVELLINO
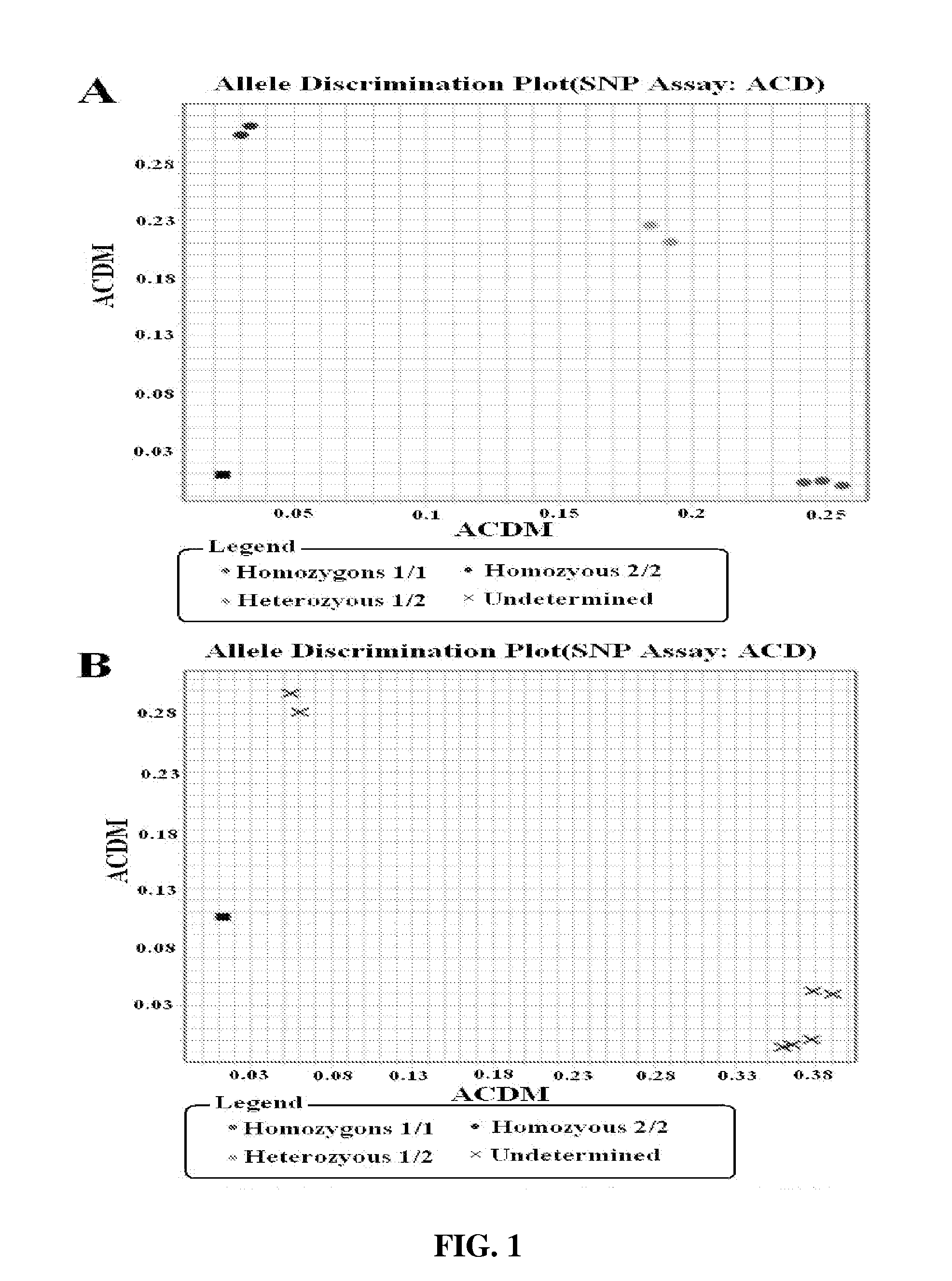
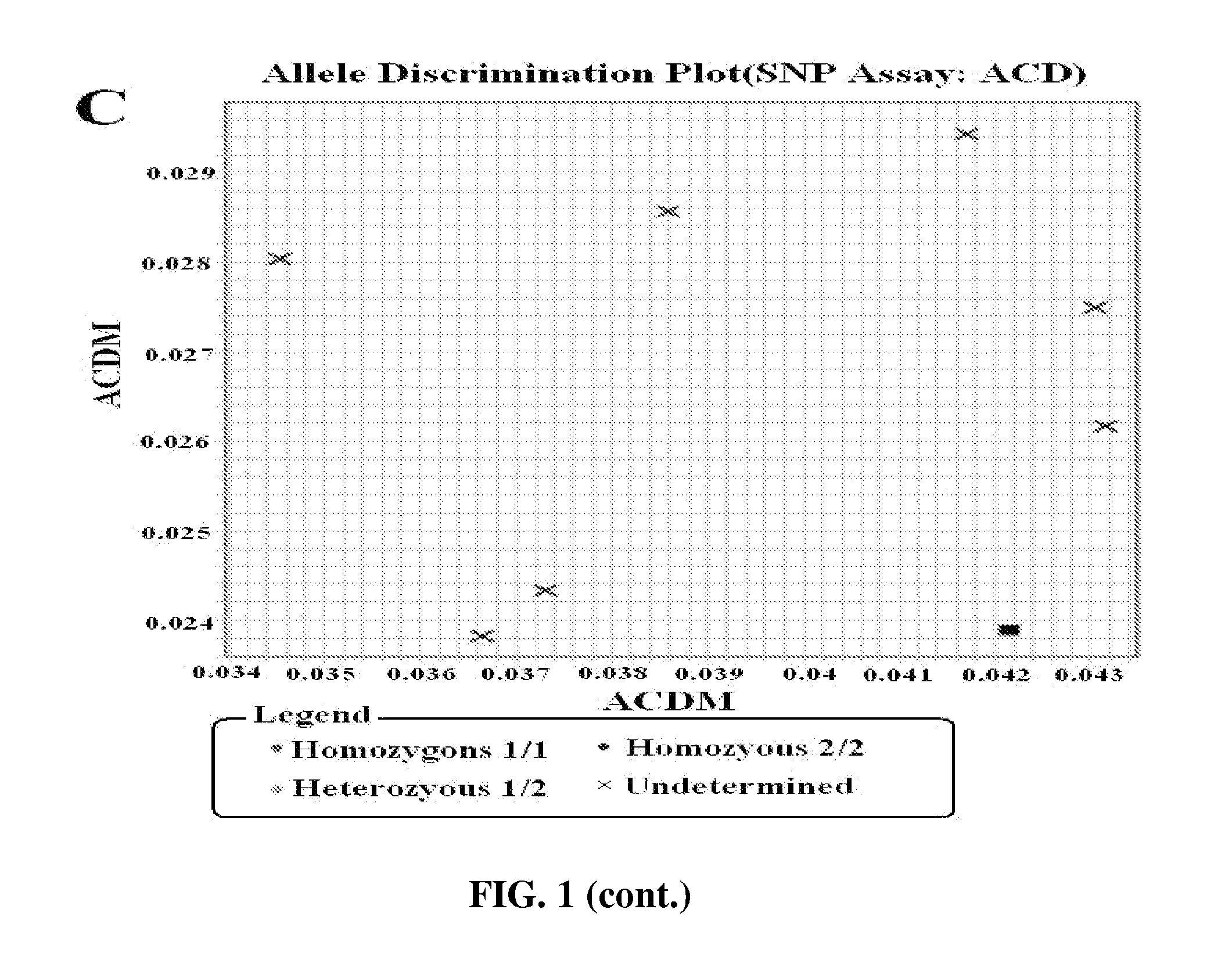
Methods for multiplex detection of alleles associated with ophthalmic conditions
Systems and methods for detecting at least two genomic alleles associated with corneal dystrophy in a sample from a human subject are disclosed in which cells (e.g., epithelial) of the subject are adhered to a tip of a substrate. The tip of the substrate is agitated in a lysis solution that lyses cells adhered to the substrate. The substrate is removed from the lysis solution upon completion of this agitation. The resulting lysis solution is incubated and then genomic DNA from the lysis solution is isolated to form a gDNA solution. From this, identity of at least two nucleotides present in the human TGFpI gene is determined using at least two oligonucleotide primer pairs and the gDNA solution. These at least two nucleotides are located at respective independent positions of the TGFpI gene corresponding to respective independent single nucleotide polymorphisms (SNPs) associated with corneal dystrophy.
Owner:AVELLINO LAB USA
Multi-spot metal-deposited nucleic acid chip with nanostructure arrays for diagnosing corneal dystrophy, and method for producing same
InactiveCN102648291AMaterial nanotechnologyMicrobiological testing/measurementHereditary Eye DiseasesSurface plasmonic resonance
The present invention relates to a multi-spot metal-deposited nucleic acid chip with nanostructure arrays for diagnosing corneal dystrophy, and more particularly, to a multi-spot metal-deposited nucleic acid chip with nanostructure arrays which uses the optical characteristics of localized surface plasmon resonance (LSPR), to a method for producing the chip, and to a multi-spot metal-deposited nucleic acid chip with nanostructure arrays for diagnosing BIGH3 gene mutations and various types of corneal dystrophies. According to the present invention, the metal-deposited nucleic acid chip with nanostructure arrays, and an analysis device including a light source, a detector, a spectrophotometer and a computer are combined to be used as a label-free biosensor based on the optical characteristics of LSPR. The multi-spot metal-deposited nucleic acid chip with nanostructure arrays for diagnosing BIGH3 gene mutations and corneal dystrophy according to the present invention diagnoses various types of corneal dystrophies, which are hereditary eye diseases, at the same time.
Owner:KOREA ADVANCED INST OF SCI & TECH +1
Small compounds that correct protein misfolding and uses thereof
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Method for detecting genotype of polymorphic sites of corneal dystrophy gene and its kit
ActiveCN108085375AIncrease the number of mismatched basesImprove low efficiencyMicrobiological testing/measurementFluorescenceWild type
The invention discloses a method for detecting a genotype of polymorphic sites of a corneal dystrophy gene and its kit. In a same reaction system, real-time fluorescence quantification PCR detection is respectively carried out on a same to-be-detected sample by an enhanced ARMS primer aiming at a gene wild type template for polymorphic sites of corneal dystrophy and an enhanced ARMS primer aimingat a mutant template; according to a Ct mutant type detected by the real-time fluorescence quantification detection by the enhanced ARMS primer aiming at the gene wild type template, a Ct wild type detected by the real-time fluorescence quantification detection by the enhanced ARMS primer aiming at the mutant template, and a difference delta-Ct value of the Ct mutant type and the Ct wild type, and the mutant genotype of the corneal dystrophy gene can be determined according to the types of the polymorphic site. The method has the advantages of simple operation and high accuracy.
Owner:北京华大通瀛科技有限公司
Multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing of corneal dystrophy and preparation method thereof producing same
ActiveUS20140243222A1Accurate diagnosisMaterial nanotechnologyNucleotide librariesHereditary Eye DiseasesNanostructure
A multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing corneal dystrophy, and more particularly to a multi-spot metal-capped nanostructure array nucleic acid chip capable of employing LSPR (localized surface plasmon resonance) optical properties, a preparation method thereof, and a multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing BIGH3 gene mutations, which can diagnose various corneal dystrophies. The metal-capped nanostructure array nucleic acid chip can be combined with analysis devices, including a light source, a detector, a spectrophotometer and a computer, to provide an LSPR optical property-based optical biosensor, and the use of the multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing BIGH3 gene mutations allows the simultaneous diagnosis of various corneal dystrophies that are genetic ocular diseases.
Owner:AVELLINO
Method for analyzing pathogenesis of cornea related diseases
InactiveCN104914277AUnderstanding Structural Pathogenic MechanismsScanning probe microscopyDiseaseOphthalmology
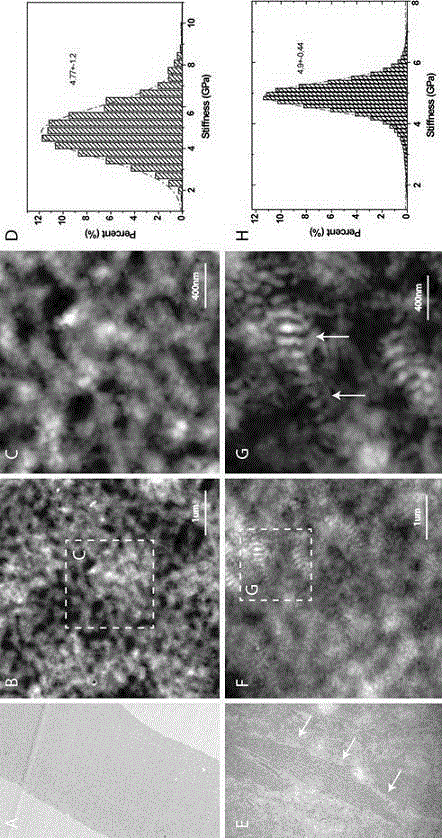
The invention relates to a method for analyzing pathogenesis of cornea related diseases, and belongs to the field of biomedical technologies. Specifically, the invention relates to a method for recognizing and analyzing the pathogenesis of the cornea related diseases at a nanometer scale by using a specific instrument. According to the invention, an auxiliary analysis method for the pathogenesis of the cornea related diseases is provided from such two aspects as morphology and related mechanical properties at the nanometer scale by using a quantitative nanomechanics atomic force microscope, and detailed elaboration is conducted by taking a Fuchs endothelial cornea dystrophy disease as an example, thereby providing the universality of the method in cornea related disease research.
Owner:JIANGSU UNIV
Oligonucleotide, virus vector, application of virus vector and RNAi medicinal preparation
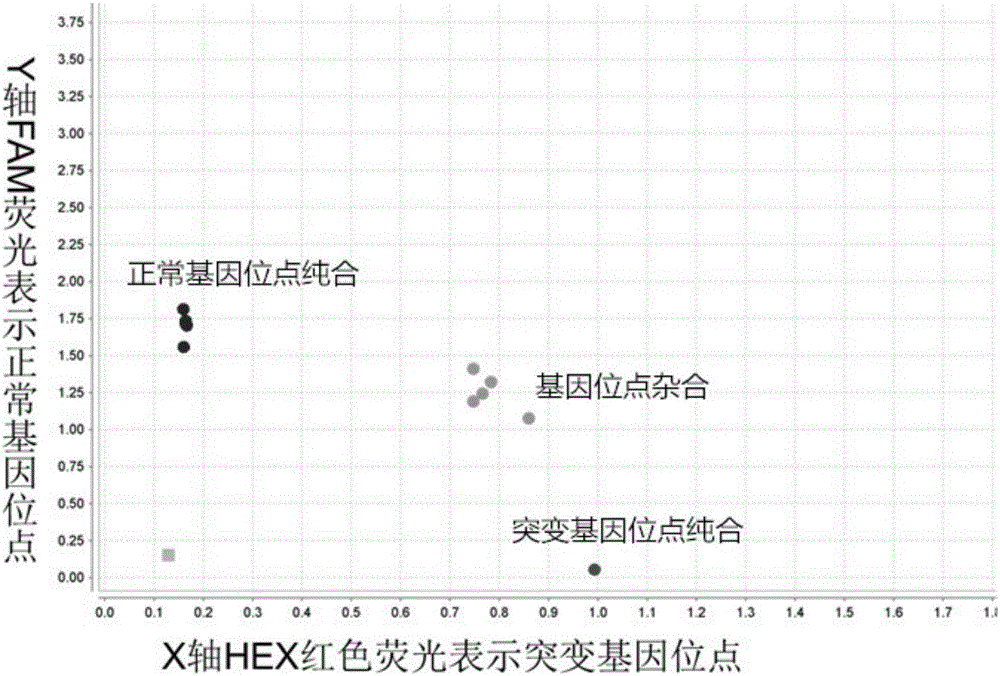
ActiveCN111926015AReduced protein content of mutant TGFBIAvoid badOrganic active ingredientsSenses disorderNucleotideTGFBI
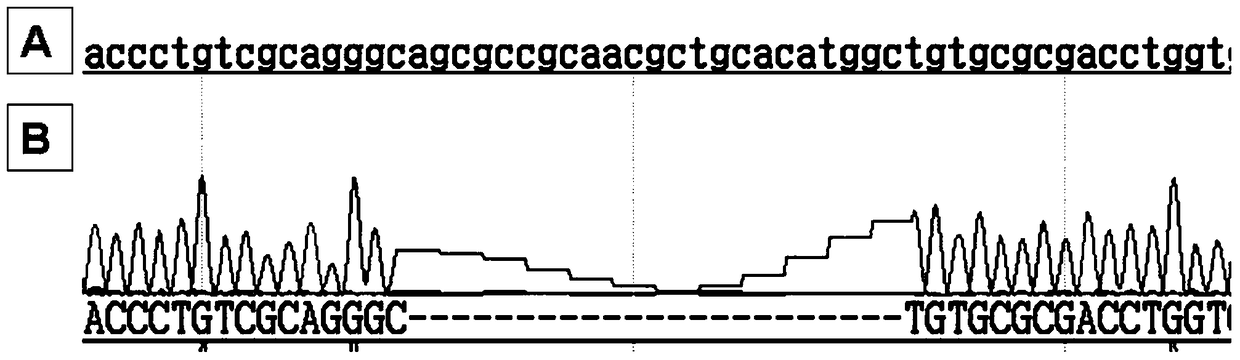
The invention relates to the field of medicine, in particular to oligonucleotides, virus vectors, application of the oligonucleotides and the virus vectors and an RNAi medicinal preparation. The oligonucleotide is one sequence from SEQ ID NO: 3 to SEQ ID NO: 21; or a nucleic acid sequence having no less than 80% consistency with one of the sequences from SEQ ID NO: 3 to SEQ ID NO: 21. The oligonucleotide, the virus vector and the RNAi pharmaceutical preparation provided by the invention can be used for effectively treating and preventing corneal dysplasia or corneal dystrophy caused by TGFBI Met619Lys mutation.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Primer probe for diagnosing corneal dystrophy caused by mutation of site 124 of human TGFbetaI gene and detection method
InactiveCN107858420AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceWild type
The invention provides a primer probe for diagnosing corneal dystrophy caused by mutation of a site 124 of a human TGFbetaI gene. The primer probe comprises a specific primer designed for the site 124of the TGFbetaI gene, a specific wild type Taqman fluorescent probe for a wild type site R124, and at least one of four specific mutation type Taqman fluorescent probes for mutation type sites C124,S124, H124 and L124, wherein each probe is connected with a different fluorescent report group, so as to collect different fluorescent signals to detect, and internal references are not added. The invention further provides a primer probe-based detection method for mutation of the site 124 of the human TGFbetaI gene. The detection method has advantages of being highly sensitive, highly specific, being not easy to contaminate, being highly accurate, and being convenient and quick, and is applicable to clinical case analysis and inspection work.
Owner:奥斯汀生命科学技术公司
System for diagnosing Avellino corneal dystrophy
ActiveUS9970051B2Bioreactor/fermenter combinationsBiological substance pretreatmentsProcess systemsCornea dystrophy
Owner:AVELLINO
Application of BIGH3 in preparation of animal model with corneal dystrophy
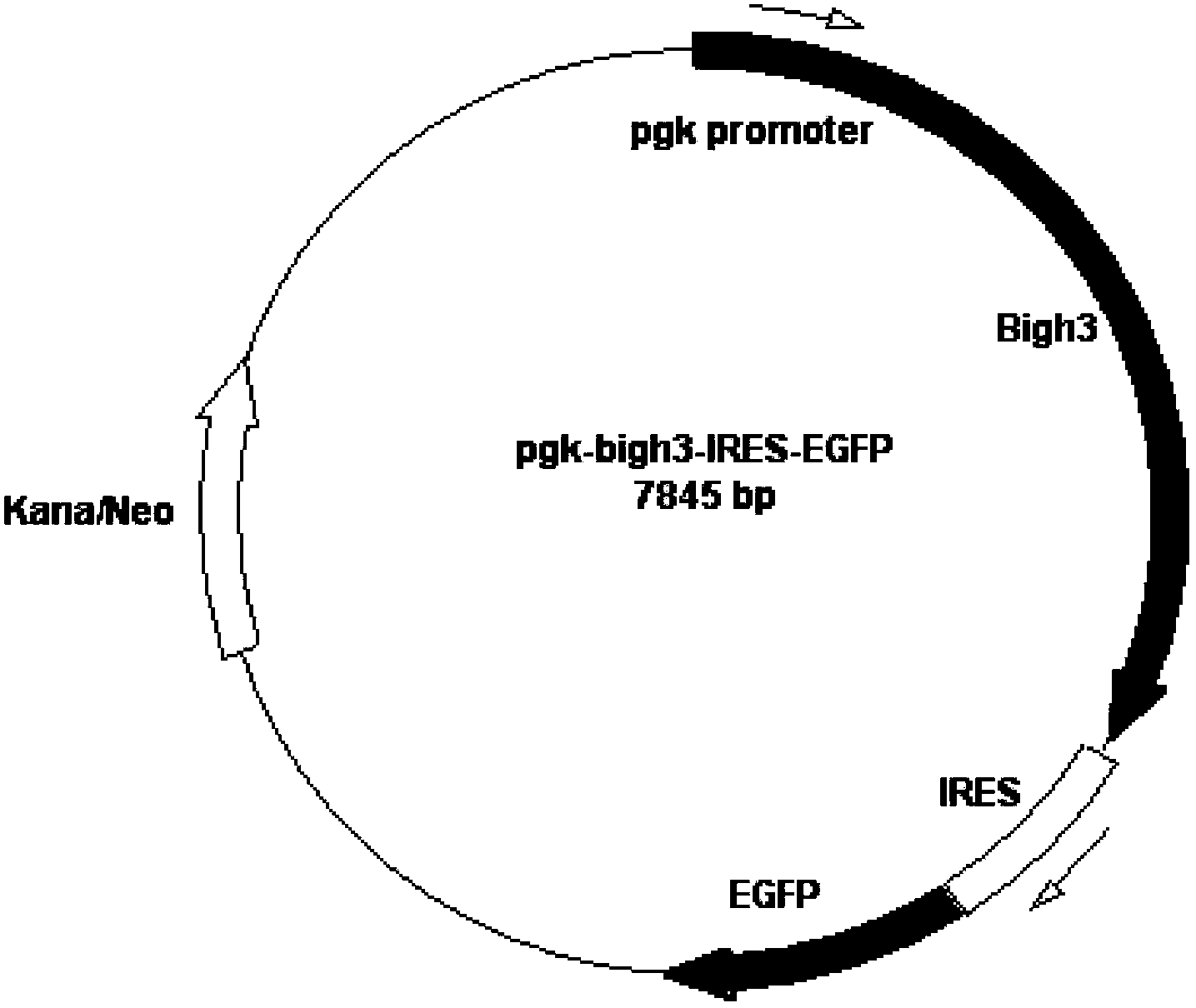
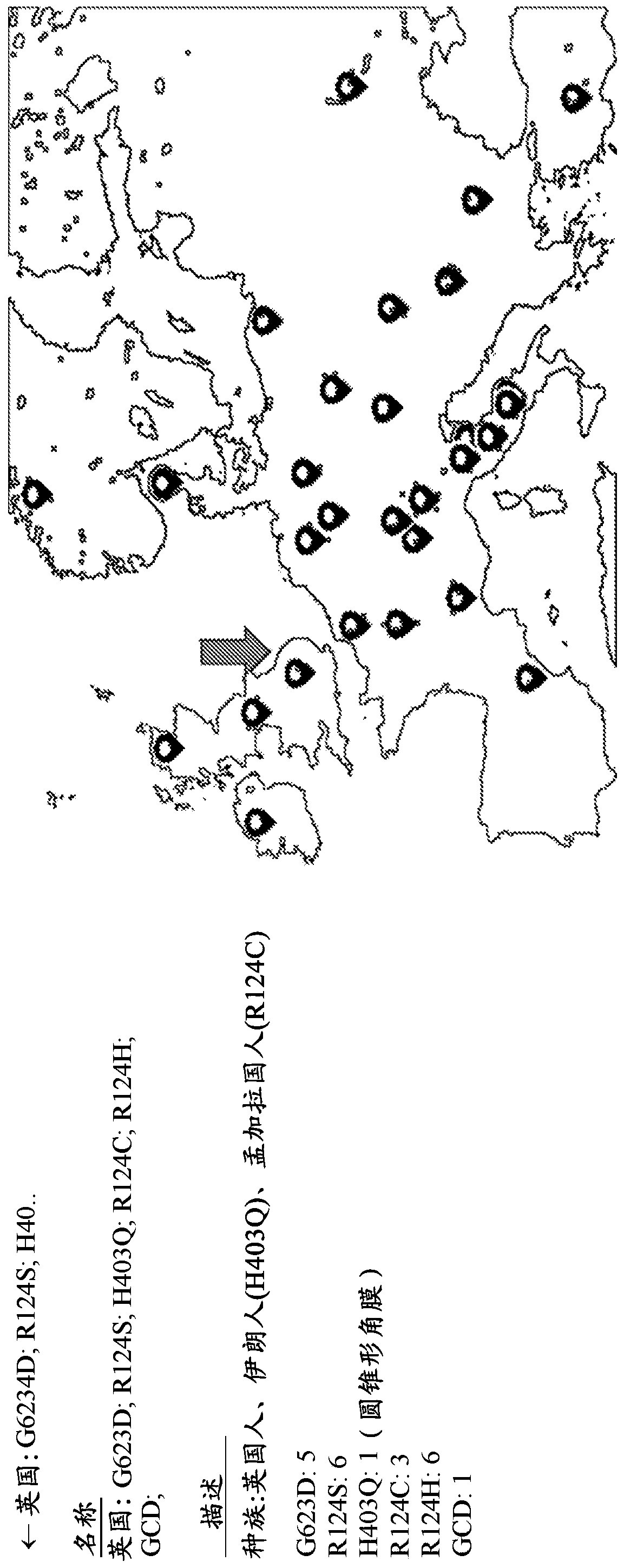
The invention relates to an application of BIGH3 in preparation of an animal model with corneal dystrophy. The corneal dystrophy model with stability in genetics and stability in phenotype is established, and the model is realized by mutating the 124th site of a Bigh3 protein of a non-human mammalian from Arg to His. By taking the transgenic animal model provided by the invention as a research model, the feasibility of preventing and treating corneal dystrophy diseases can be investigated on the gene level, and a research basis is provided for gene diagnosis and treatment of granular corneal dystrophy.
Owner:SHANGHAI TENTH PEOPLES HOSPITAL
Quick and efficient detection method of mutation sites of genes associated with corneal dystrophy
InactiveCN106480200AHigh sensitivityImprove throughputMicrobiological testing/measurementForward primerTGFBI gene
The invention discloses a quick and efficient detection method of mutation sites of genes associated with corneal dystrophy. The method comprises the steps of firstly, preparation of a mutational DNA template and design of primers, wherein TGFBI gene fragments containing four mutation sites are artificially synthesized respectively and used for detecting the detection efficiencies of the mutation sites; meanwhile, specific amplification primers aiming at normal sites and the mutation sites are designed respectively, corresponding reverse primers are designed, three primers are composited to form a primer premix liquid, and in addition, the 5' ends of two forward primers are connected with different primer detection sequences respectively and used for fluorescence detection; secondly, establishment of a PCR detection system; thirdly, result interpretation. Compared with the prior art, the technology has the advantages of being accurate and reliable in detection result, higher in flux, flexible in experimental design, convenient and efficient in operation, fast in detection speed, easy for popularization and low in cost.
Owner:神州亿昊基因技术(北京)有限公司
System for Diagnosing of Avellino Corneal Dystrophy
PendingUS20180251816A1Simpler and more skillMicrobiological testing/measurementCornea dystrophyDiagnostic system
The present invention relates to a system for diagnosing Avellino corneal dystrophy, and more particularly to a system for diagnosing Avellino corneal dystrophy, in which whether a sample is normal or Avellino corneal dystrophy is determined based on the ratio of the input first PGR amplification value and the second PGR amplification value. The system makes it possible to diagnosis Avellino corneal dystrophy in a simpler and accurate manner without being influenced by the doctor's skill. Particularly, the inventive system makes the overall process systematic, and thus provides accurate diagnosis. In addition, the system can also easily administer a number of test subjects.
Owner:AVELLINO
Human TGFBI gene mutation detection kit and detection method thereof
PendingCN109161589AStrong specificityHigh sensitivityMicrobiological testing/measurementDiseaseTGFBI gene
The invention provides a human TGFBI gene mutation detection kit and a detection method thereof. The invention provides effective and targeted protection at an early stage for carriers of a corneal abnormality mutation gene, and can delay the development of a disease course and even avoid the onset of a disease. The invention provides a primer and a probe for detecting corneal dystrophy caused bymutation of a human TGFBI gene at a 124-site and a 555-site, and the primer and the probe are capable of detecting the mutation of the TGFBI gene at the 124-site and the 555-site, high in sensitivityand strong in specificity. The invention provides the corneal abnormality mutation gene detection kit which is capable of rapidly, efficiently and accurately detecting the mutation of the human TGFBIgene at the 124-site and the 555-site. The invention further provides the detection method for detecting the mutation of the human TGFBI gene at the 124-site and the 555-site by the kit, and the detection method has the advantages of simple operation, high sensitivity, high specificity, wide application models, true and reliable detection results, easy promotion and the like.
Owner:SHUWEN BIOTECH CO LTD
Nucleotides, viral vectors and their applications and RNAi pharmaceutical preparations
ActiveCN111944813BAvoid malnutritionOrganic active ingredientsSenses disorderNucleotideCornea dystrophy
The present invention relates to the technical field of biomedicine, in particular to nucleotides, viral vectors and their applications and RNAi pharmaceutical preparations. The nucleotide is selected from one of the following nucleic acid sequences: the nucleic acid sequence is one of SEQ ID NO: 3 or SEQ ID NO: 4; at 80% of the nucleic acid sequence. The promoter-optimized RNAi medicine of the invention has the effect of treating and preventing corneal dystrophy caused by the Q455K mutation of COL8A2 gene.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Oligonucleotide, virus vector, application of oligonucleotide or virus vector, and RNAi medicinal preparation
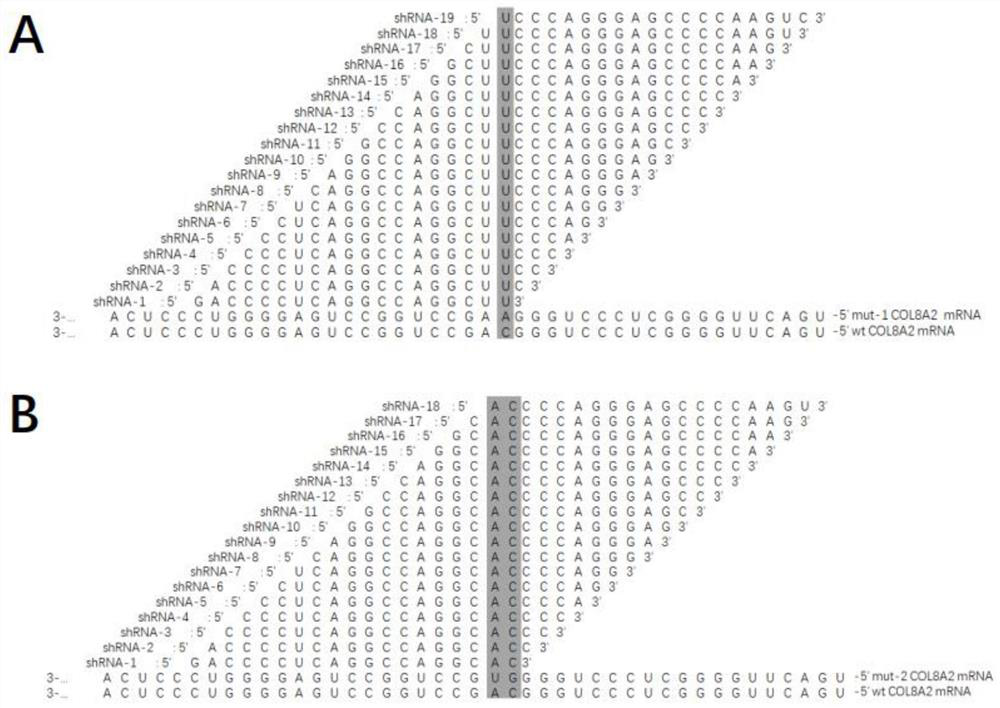
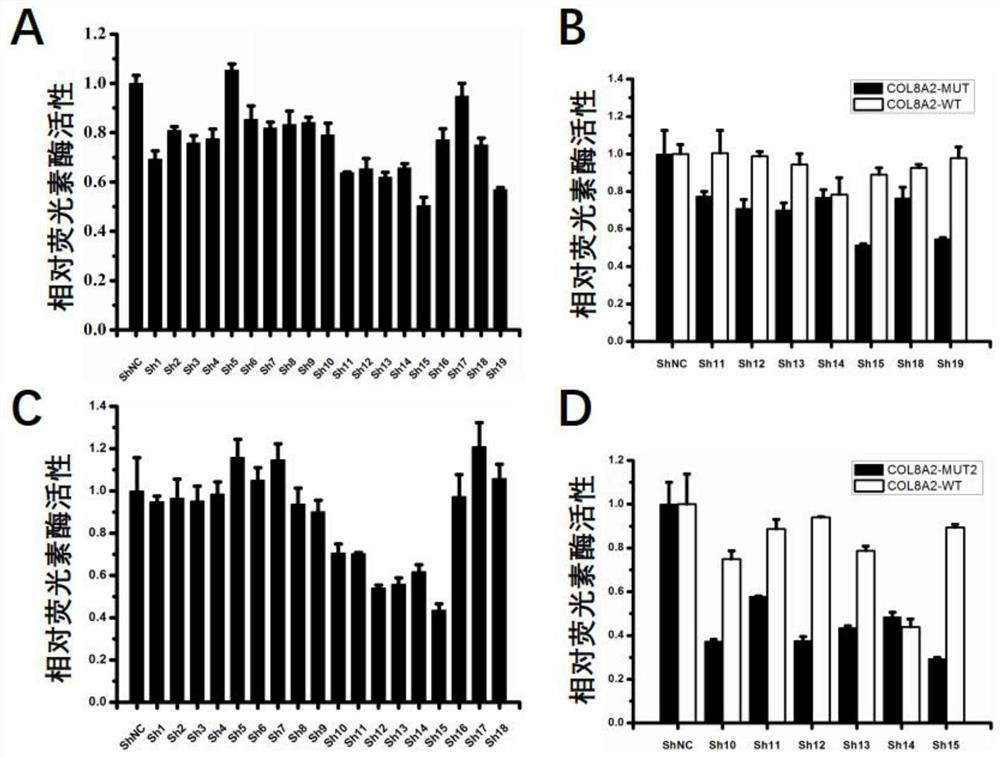
The invention relates to the field of medicine, in particular to oligonucleotide, a virus vector, application of the oligonucleotide or the virus vector, and an RNAi medicinal preparation. The oligonucleotide is one of sequences from SEQ ID NO: 4 to SEQ ID NO: 40; or a nucleic acid sequence having no less than 80% consistency with one of the sequences from SEQ ID NO: 4 to SEQ ID NO: 40. The oligonucleotide, the virus vector and the RNAi medicinal preparation provided by the invention can effectively treat and prevent corneal dystrophy caused by COL8A2 mutation.
Owner:WUHAN NEUROPHTH BIOTECHNOLOGY LTD CO
Construction method and delivery system of plaque corneal dystrophy animal model and gene therapy vector
PendingCN114774470AAchieve healingHigh eye symptomsSenses disorderPeptide/protein ingredientsOphthalmologyWild type
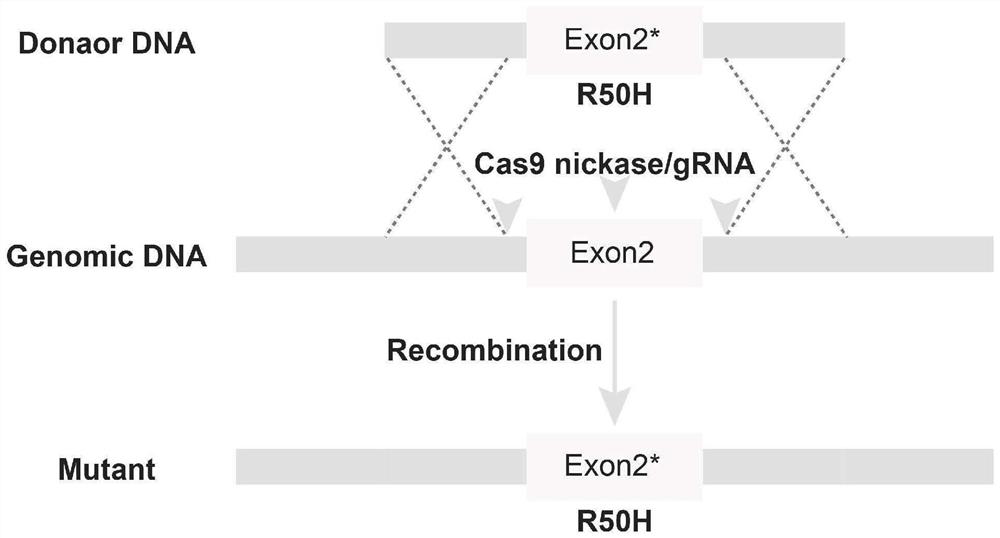
The invention provides a plaque corneal dystrophy animal model, a construction method of a gene therapy vector and a delivery system, and relates to the technical field of model construction. According to the animal model, animal Chst5 genes are knocked out from corneal endothelium in an anterior chamber injection mode or through Chst5R50H point mutation, and compared with an existing animal model, the eye symptom similarity is higher. The invention also provides a gene therapy vector which is realized by injecting the adeno-associated virus vector into the anterior chamber to carry the wild type mouse Chst5 gene. In the embodiment of the invention, the treatment of plaque corneal dystrophy can be realized by injecting the gene therapy vector into the anterior chamber of the mouse model.
Owner:EYE INST OF SHANDONG FIRST MEDICAL UNIV
An SNP locus for detection of plaque corneal dystrophy
ActiveCN105274225BQuick checkMicrobiological testing/measurementDNA/RNA fragmentationDiseaseAmniotic fluid
The invention provides an SNP (single nucleotide polymorphism) locus for detection of macular corneal dystrophy. The SNP locus is a 250th-272th basic group from an initiation codon in a CHST6 gene coding region. Due to providing of a new usage of a CHST6 gene, an effective approach of macular corneal dystrophy gene diagnosis, prenatal gene screening and genetic counseling is provided. According to application effects, the SNP locus and detection primers can be effectively applied to fast CHST6 gene mutation site test of clinical patients and chorionic villus or amniotic fluid.
Owner:SHANDONG EYE INST
Methods for multiplex detection of alleles associated with corneal dystrophy
The present disclosure provides a method for detecting corneal dystrophy in a subject, comprising a reaction mixture, the reaction mixture comprising one or more labeled probes comprising a mutant TGFBI nucleotide sequence; the reaction mixture further comprises at least one amplification primer pair for amplifying a TGFBI gene sequence from a biological sample from the subject; and detecting one,two, three, four, five or six mutations selected from the group consisting of G623D, M502V, R124S, A546D, H572R, and H626R mutations in TGFBI gene, wherein the detecting comprises detecting the one or more mutations using the labeled detection probes. Further provided is a reaction kit comprising the reaction mixture.
Owner:AVELLINO LAB USA INC
Primers for diagnosing avellino corneal dystrophy
ActiveUS20150093751A1Efficiently and accurately diagnosingMicrobiological testing/measurementDNA/RNA fragmentationCornea dystrophyExon
The present invention relates to a real-time PCR primer pair and probe for diagnosing Avellino corneal dystrophy, and more particularly to a real-time PCR primer pair and probe for diagnosing Avellino corneal dystrophy, which can accurately diagnose the presence or absence of a mutation in exon 4 of BIGH3 gene, which is responsible for Avellino corneal dystrophy. The use of the primer pair and probe according to the invention can diagnose Avellino corneal dystrophy in a more rapid and accurate manner than a conventional method that uses a DNA chip or PCR.
Owner:AVELLINO
Methods for the treatment of corneal dystrophies
Methods and compositions for the treatment of a corneal dystrophy in a subject in need thereof are provided. In one aspect, the method includes the step of obtaining a plurality of stem cells comprising a nucleic acid mutation in a corneal dystrophy target nucleic acid from the subject and manipulating the nucleic acid mutation in one or more stem cells of the plurality of stem cells to correct the nucleic acid mutation, thereby forming one or more manipulated stem cells. The manipulated stem cells are isolated and then transplanted into the subject. In some embodiments, the nucleic acid mutation is manipulated using CRISPR system.
Owner:AVELLINO LAB USA
Multi-spot metal-capped nanostructure array nucleic acid chip for diagnosis of corneal dystrophy and preparation method thereof
ActiveUS9145583B2Accurate diagnosisMaterial nanotechnologyBioreactor/fermenter combinationsGenes mutationHereditary Eye Diseases
A multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing corneal dystrophy, and more particularly to a multi-spot metal-capped nanostructure array nucleic acid chip capable of employing LSPR (localized surface plasmon resonance) optical properties, a preparation method thereof, and a multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing BIGH3 gene mutations, which can diagnose various corneal dystrophies. The metal-capped nanostructure array nucleic acid chip can be combined with analysis devices, including a light source, a detector, a spectrophotometer and a computer, to provide an LSPR optical property-based optical biosensor, and the use of the multi-spot metal-capped nanostructure array nucleic acid chip for diagnosing BIGH3 gene mutations allows the simultaneous diagnosis of various corneal dystrophies that are genetic ocular diseases.
Owner:AVELLINO
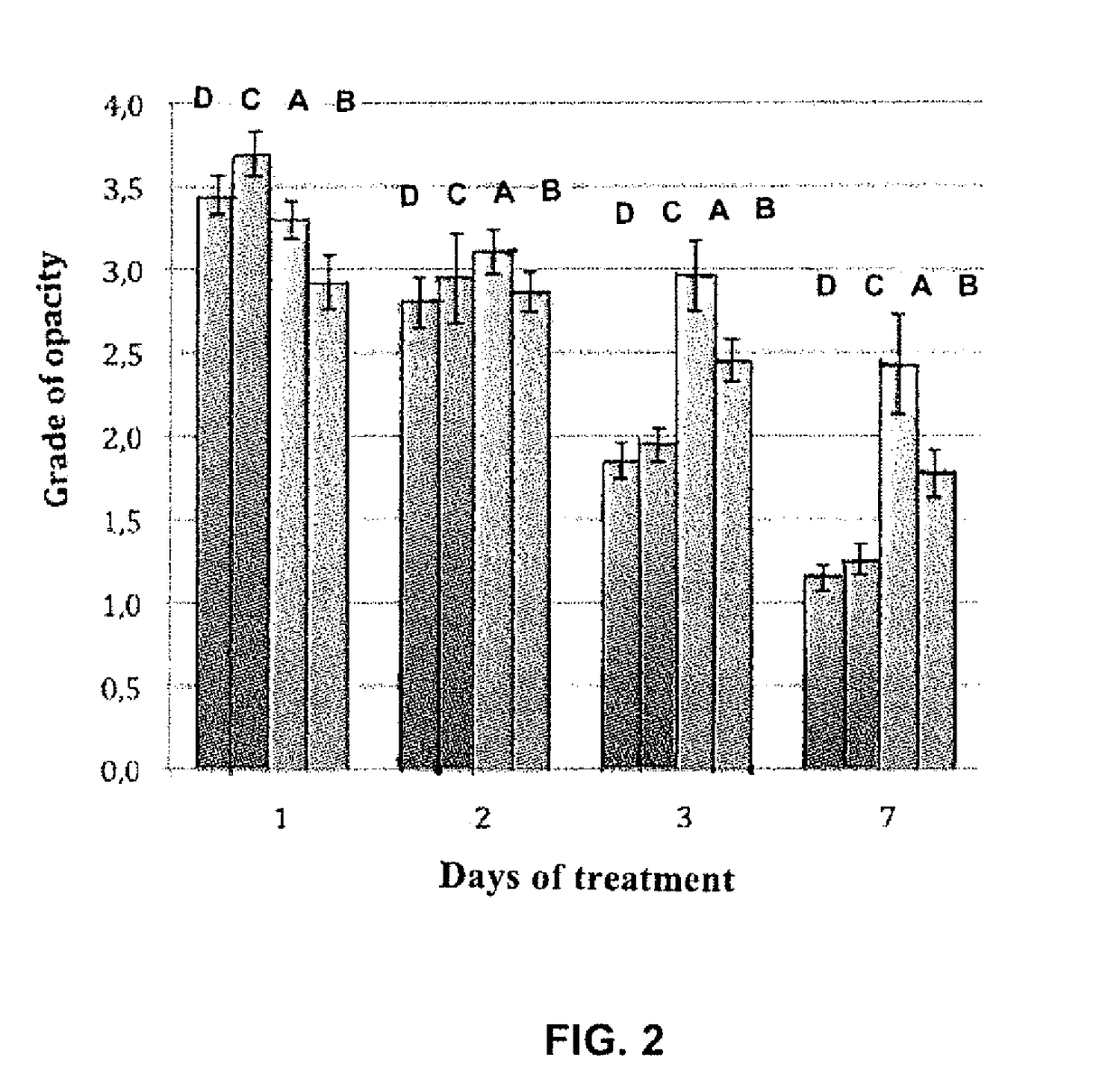
Compositions based on saffron for the prevention and/or treatment of corneal dystrophies
ActiveUS10092585B2Advantage in of in preventionAvoid treatmentSenses disorderHydroxy compound active ingredientsAntioxidantCornea dystrophy
The present invention relates to a pharmaceutical, dietary and / or food composition, comprising saffron for use in the prevention and / or treatment of corneal dystrophies. The present invention also relates to a combination comprising saffron and at least one antioxidant and to a pharmaceutical dietary and / or food composition comprising said combination for use in the prevention and / or treatment of corneal dystrophies.
Owner:HORTUS NOVUS SRL
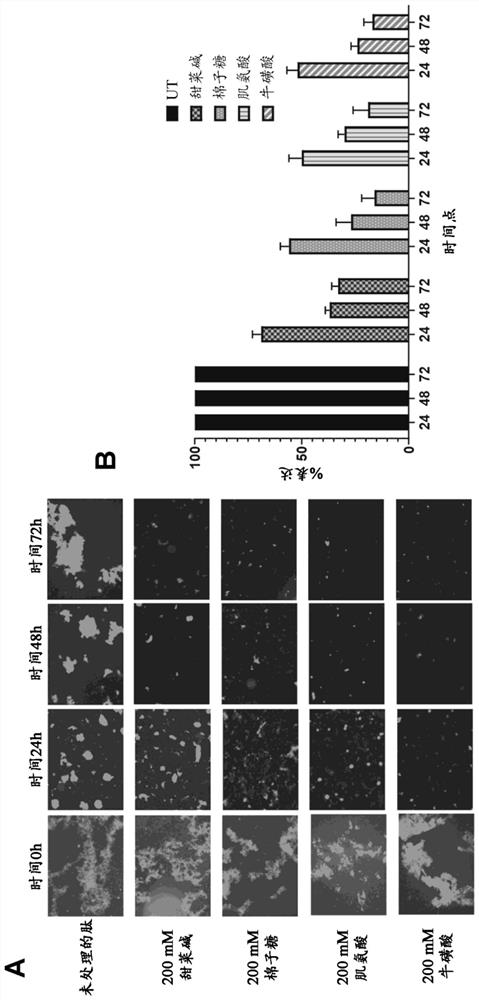
Use of penetrant for preparation of medicament for treatment of ocular disorders
The invention provides an application of a penetrant in preparation of a medicine for treating protein aggregation related disorders, and particularly provides an application of a penetrant in preparation of a medicine for treating TGFBI corneal dystrophy. The penetrant is selected from betaine, raffinose, sarcosine, taurine and / or any pharmaceutically acceptable derivative thereof.
Owner:SINGAPORE HEALTH SERVICES PTE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com