Use of penetrant for preparation of medicament for treatment of ocular disorders
A technology of penetrants and uses, applied in the field of penetrants, can solve problems such as high cost of surgical intervention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Inhibitory effect of osmotic agents on amyloid fibrillation
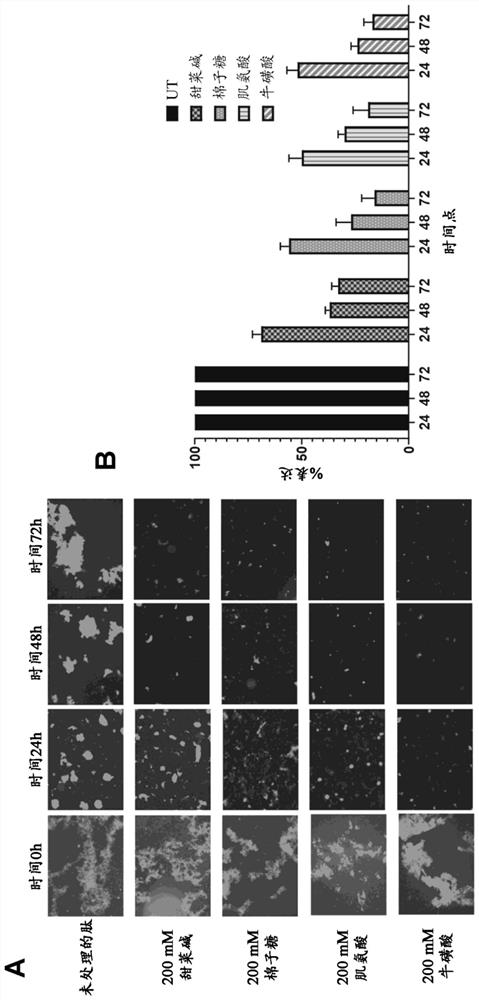
[0066] In the present study, a 23 amino acid long peptide from the fourth FAS1 domain of TGFBIp (TGFBIp611-633c.623G>R) with a rapidly forming amyloid fibril-forming substitution G623→R (EPVAEPDIMATNRVVHVITNVLQ) was used. Peptides were dissolved (0.6 mg / ml) in PBS and allowed to form amyloid fibrils in 50 ml Falcon tubes with and without the addition of osmotic agents in a shaking incubator at 37°C and 180 rpm. To study the effect of osmotic agents on amyloid fibrillation kinetics, the model peptide TGFBIp 611-633 G623R was treated with 200 mM betaine, raffinose, sarcosine and taurine (Sigma-Aldrich Inc., MO, USA), respectively, and its chemical structure is shown in figure 1 Treatment in 50 ml Falcon tubes for 24 hours (h), 48 hours and 72 hours, respectively, is indicated. TGFBIp incubated with PBS 611-633 The G623R peptide was used as a control.
[0067] Thioflavin T (ThT) assay:
[0068] P...
Embodiment 2
[0090] Example 2: Effect of osmotic agents on deaggregation of preformed amyloid fibrils
[0091] In vitro amyloid fibrillation:
[0092] TGFBIp 611-633 The G623R peptide was incubated in PBS for 24 hours to allow the formation of homogeneous amyloid fibrils. Then for TGFBIp 611-633 G623R peptide solutions were subjected to circular dichroism spectroscopy to confirm the formation of amyloid fibrils. The results showed an absorption minimum of about 218 nm and an absorption maximum of about 195 nm, which is very typical of the β-sheet rich secondary structure of amyloid fibrils. This indicates that the peptide forms amyloid fibrils within 24 hours.
[0093] Thioflavin T (ThT) assay:
[0094] Peptide solutions containing pre-formed amyloid fibrils were treated with 200 mM betaine, raffinose, sarcosine and taurine each in 50 ml Falcon tubes for 24 hours (h), 48 hours and 72 hours, respectively. A solution of preformed amyloid fibrils incubated with PBS was used as a control...
Embodiment 3
[0109] Example 3: Synergistic effect of taurine and sarcosine on amyloid fibrillation inhibition and preformed amyloid fibril deaggregation To investigate the effect of taurine and sarcosine on amyloid fibrillation inhibition The synergistic effect of the model peptide TGFBIp 611-633 G623R was treated with 100 mM taurine and 100 mM sarcosine (Sigma-Aldrich Inc., MO, USA) in 50 ml Falcon tubes for 24 hours (h), 48 hours and 72 hours, respectively, and the results were as follows Figure 9 shown. To investigate the synergistic effect of taurine and sarcosine on the deaggregation of preformed amyloid fibrils, the model peptide TGFBIp 611- 633 Pre-formed amyloid fibrils of G623R were treated with 100 mM taurine and 100 mM sarcosine (Sigma-Aldrich Inc., MO, USA) in 50 ml Falcon tubes for 24 hours (h), 48 hours and 72 hours, respectively, which The result is as Figure 10 shown.
[0110] Thioflavin T (ThT) fluorescence assay, circular dichroism (CD) assay, and ThT fluorescence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com