Small compounds that correct protein misfolding and uses thereof
A protein and compound technology, applied in the field of synthesis, can solve problems such as damage to proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: Analysis of protein folding using cell lines expressing P23H opsin
[0135] P23H mutant and wild-type opsins were expressed in tetracycline-induced stable HEK293 cell lines in the presence of 11-cis-retinal and various inhibitors, respectively. At 48 hours, the aforementioned folded protein was purified by immunoaffinity and quantified by UV-visible spectroscopic analysis. The total amount of opsin was analyzed at 280nm. The amount of rhodopsin in a biochemically functional conformation was analyzed at 500 nm. Immunofluorescence microscopy was also used to determine the cellular location of the protein.
Embodiment 2
[0136] Example 2: Inhibition of the proteasome improves reversion to correctly folded P23H
[0137] MG132, a reversible proteasome inhibitor, was added to the culture medium of the HEK293 cell line described in Example 1 at the time of induction. Such as Figure 1A As shown, proteasome inhibition resulted in more than 200-250% recovery of rhodopsin. In contrast, the yield of wild rhodopsin was only increased by 35-40% ( Figure 1B ).
Embodiment 3
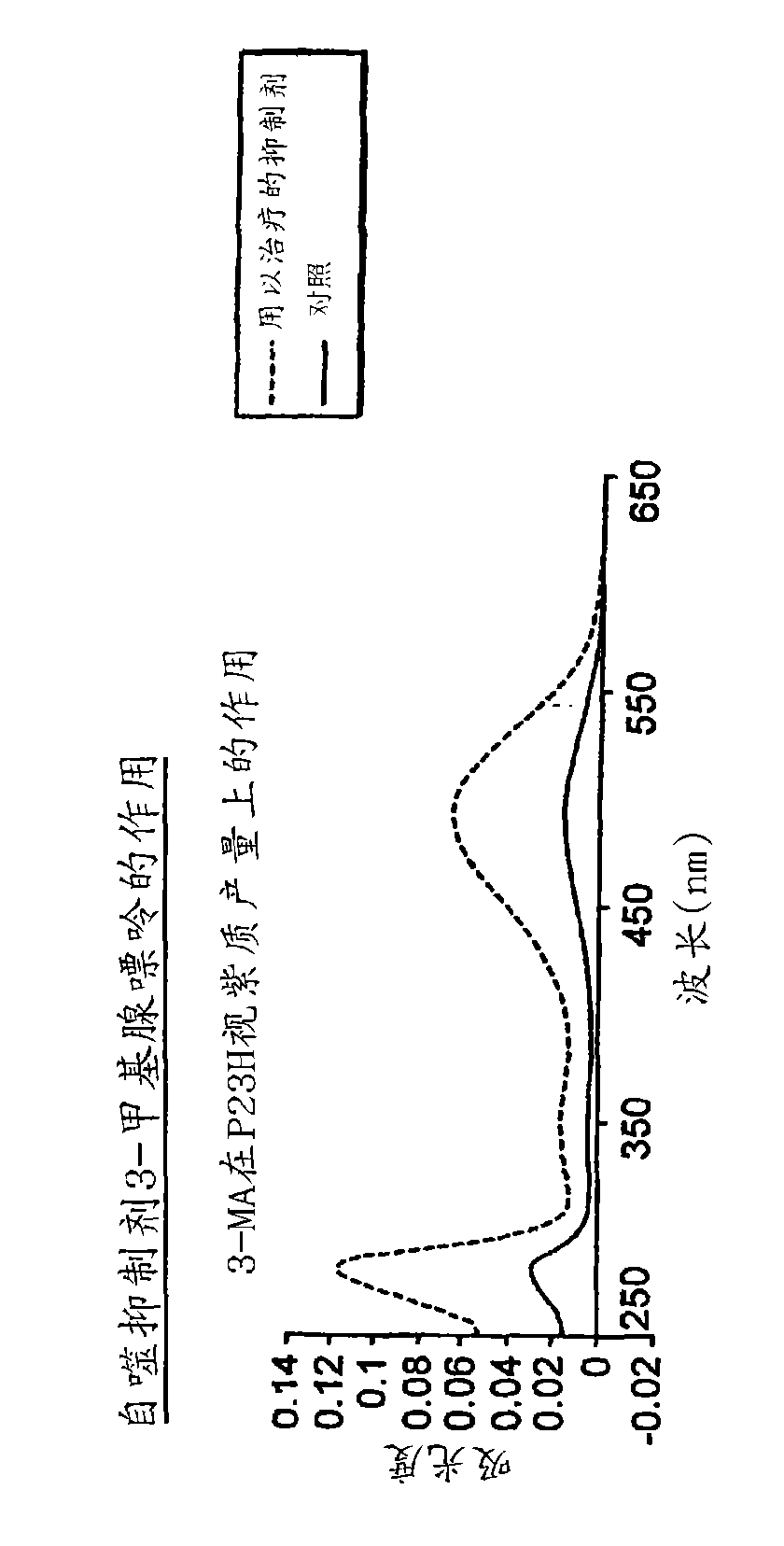
[0138] Example 3: Inhibition of autophagy improves reversion to correctly folded P23H
[0139] By adding 3-methyladenine to the medium during induction, the autophagy of HEK293 cells in Example 1 was inhibited. This resulted in a 350-400% increase in P23H rhodopsin recovery compared to only 50-60% recovery of wild-type rhodopsin ( Figure 2A and 2B ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com