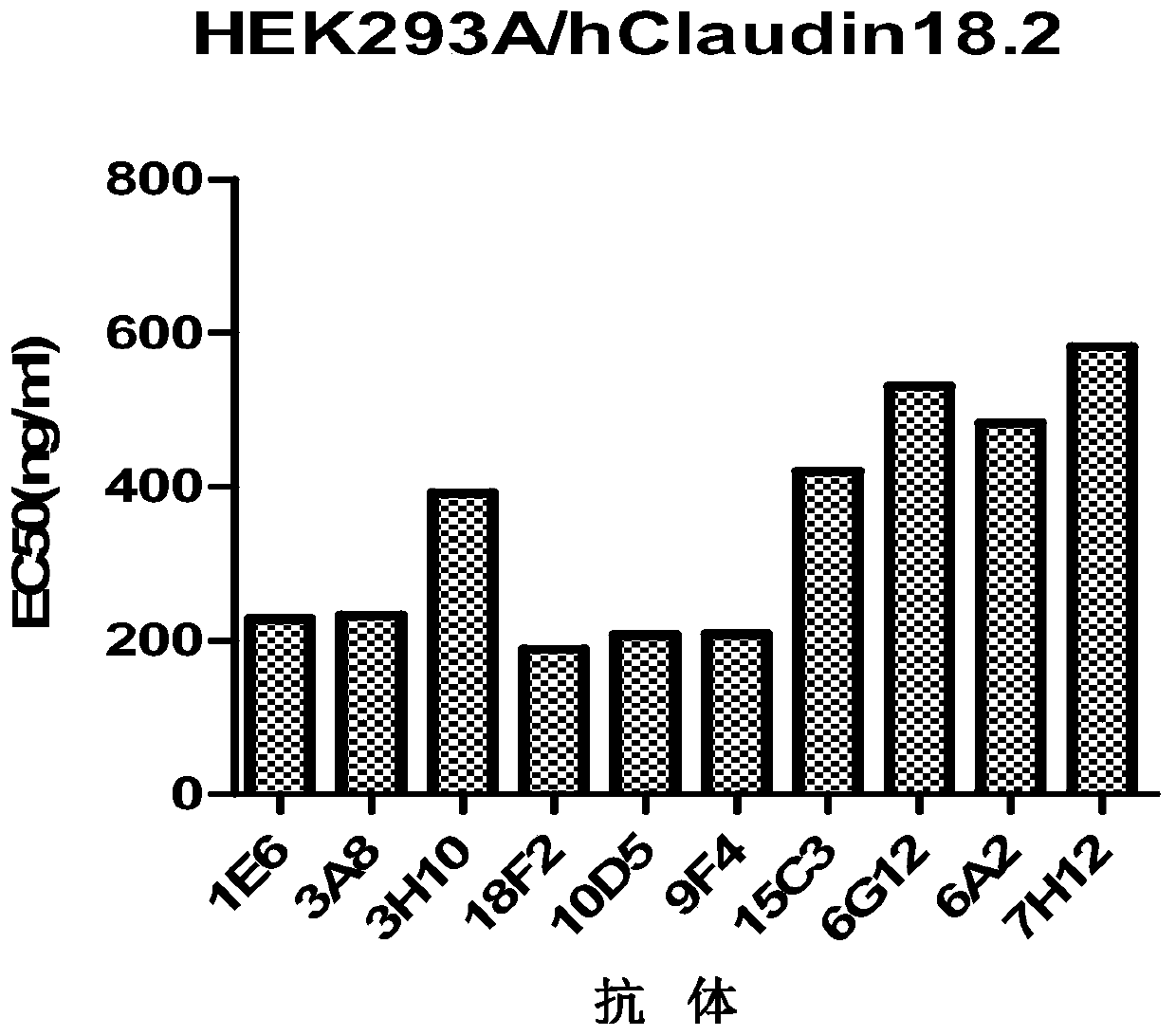
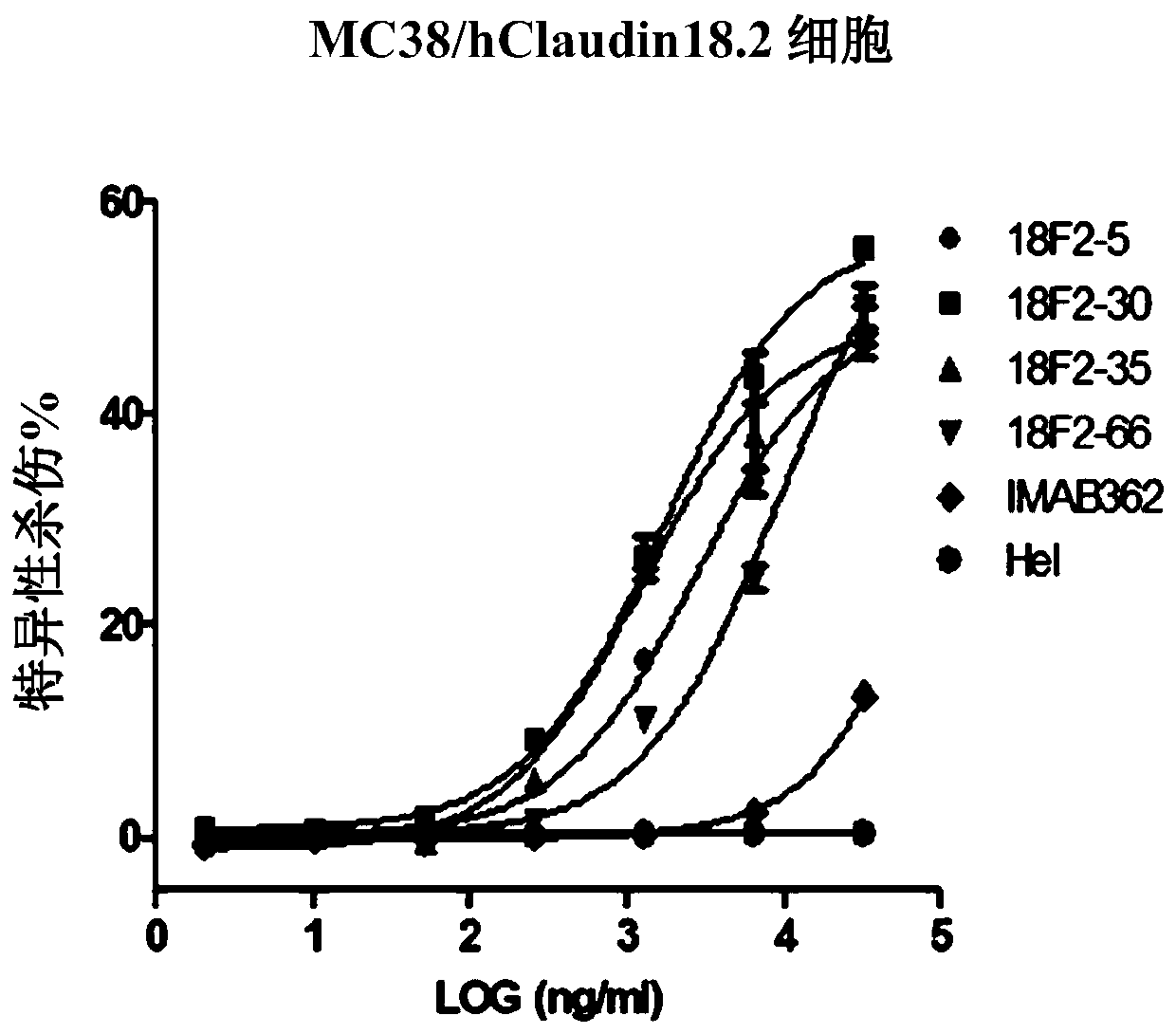
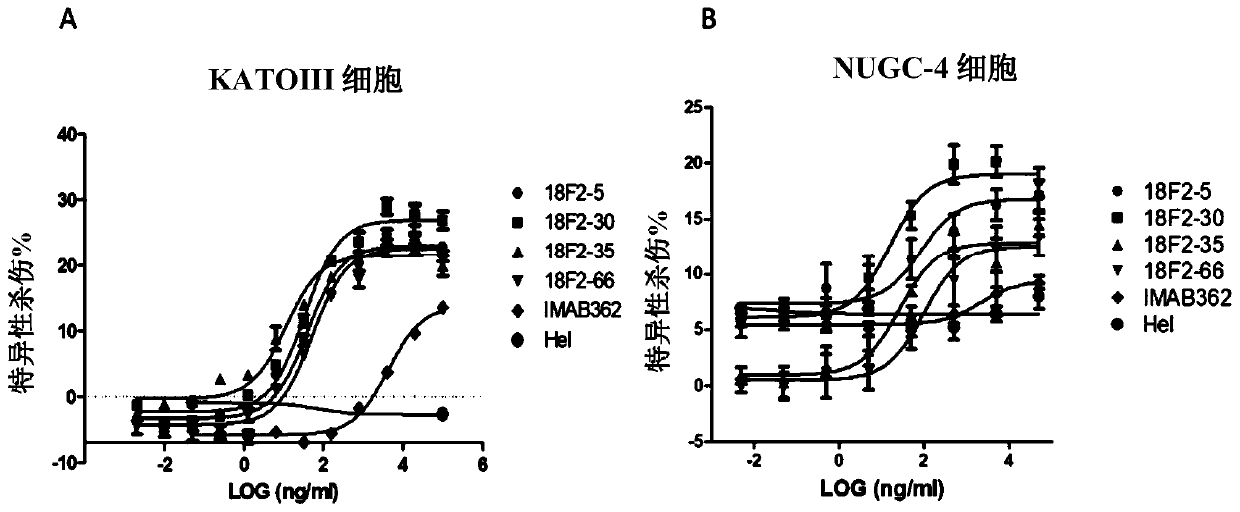
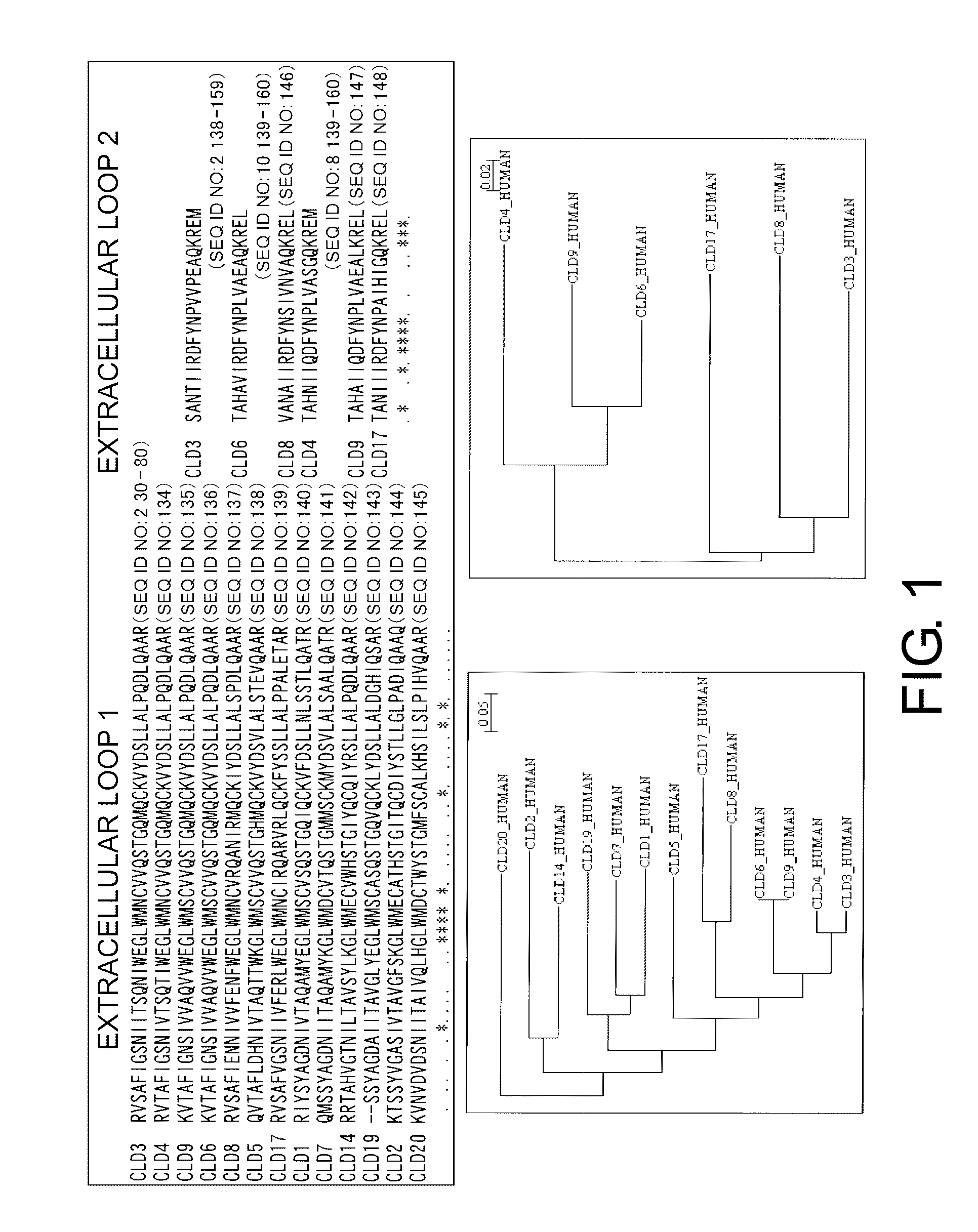
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
144 results about "Claudin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
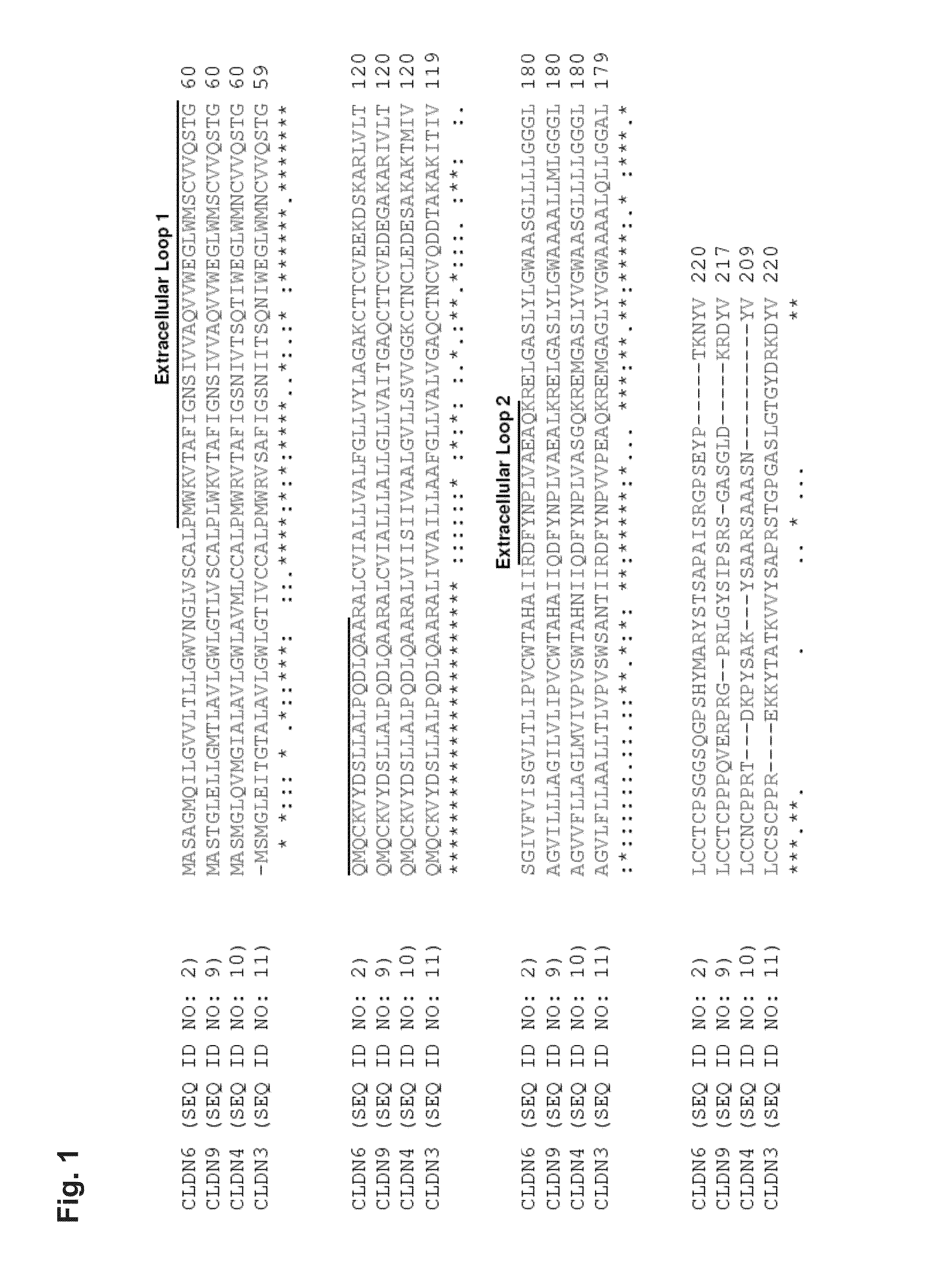
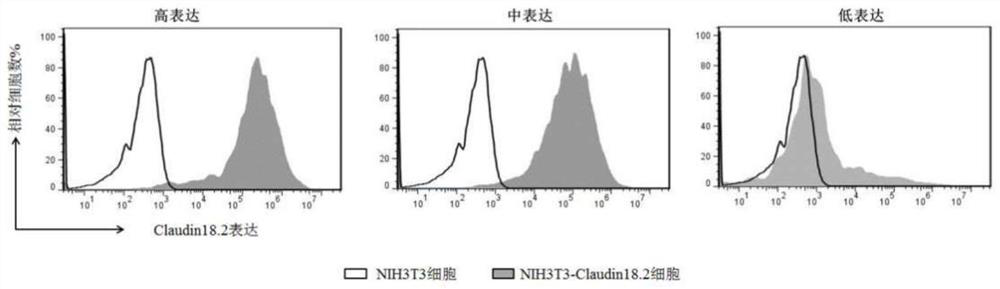
Claudins are a family of proteins which, along with occludin, are the most important components of the tight junctions (zonulae occludentes). Tight junctions establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.
Isolated monoclonal antibodies that specifically bind to human Claudin 18.2
ActiveCN109762067AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonal antibody 14G2AAntigen receptors
Owner:BEIJING MABWORKS BIOTECH
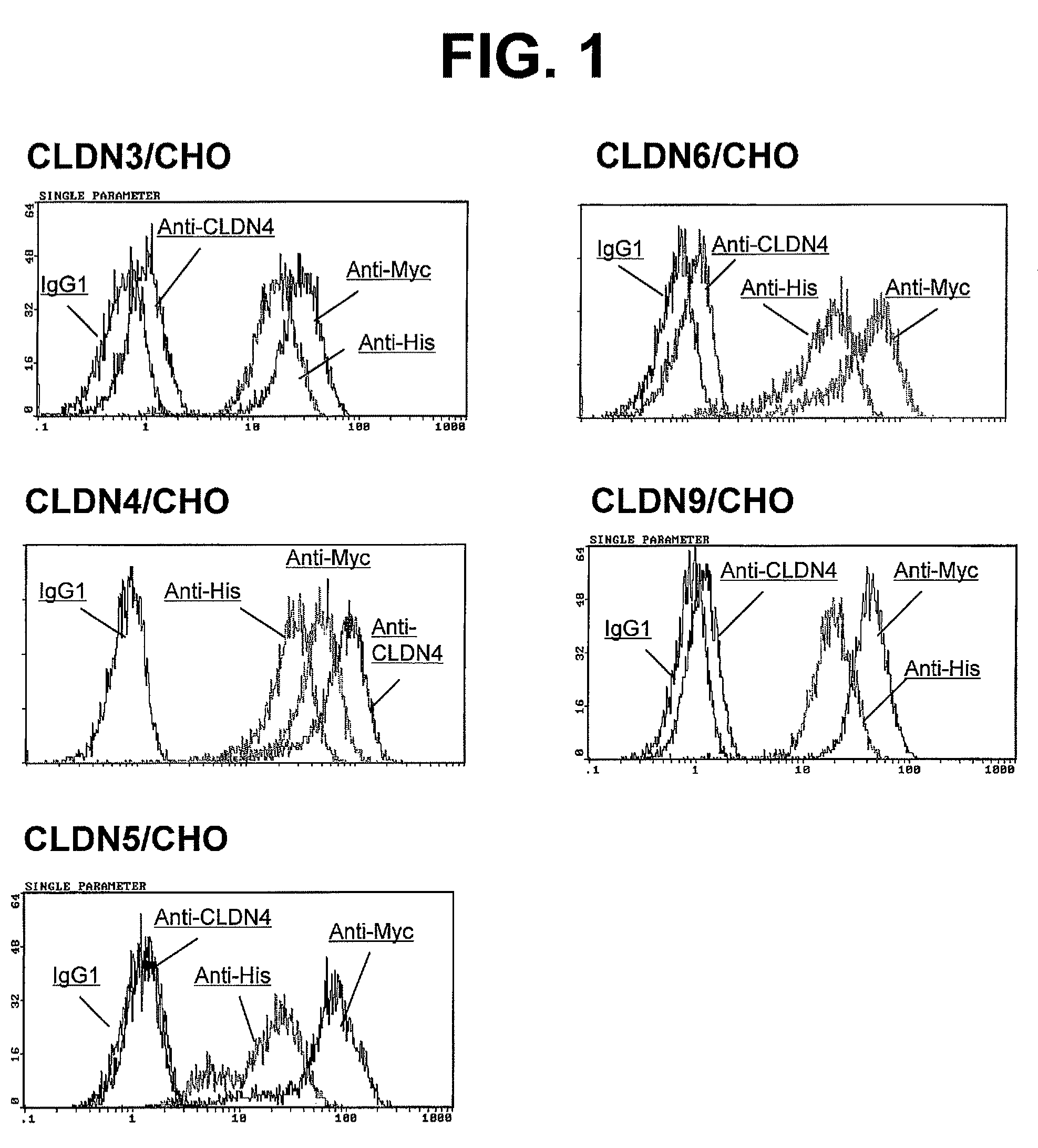
Anti-Claudin 3 Monoclonal Antibody and Treatment and Diagnosis of Cancer Using the Same
InactiveUS20100111852A1Inhibit cell proliferationIn-vivo radioactive preparationsBiological material analysisProstate cancerCytotoxicity
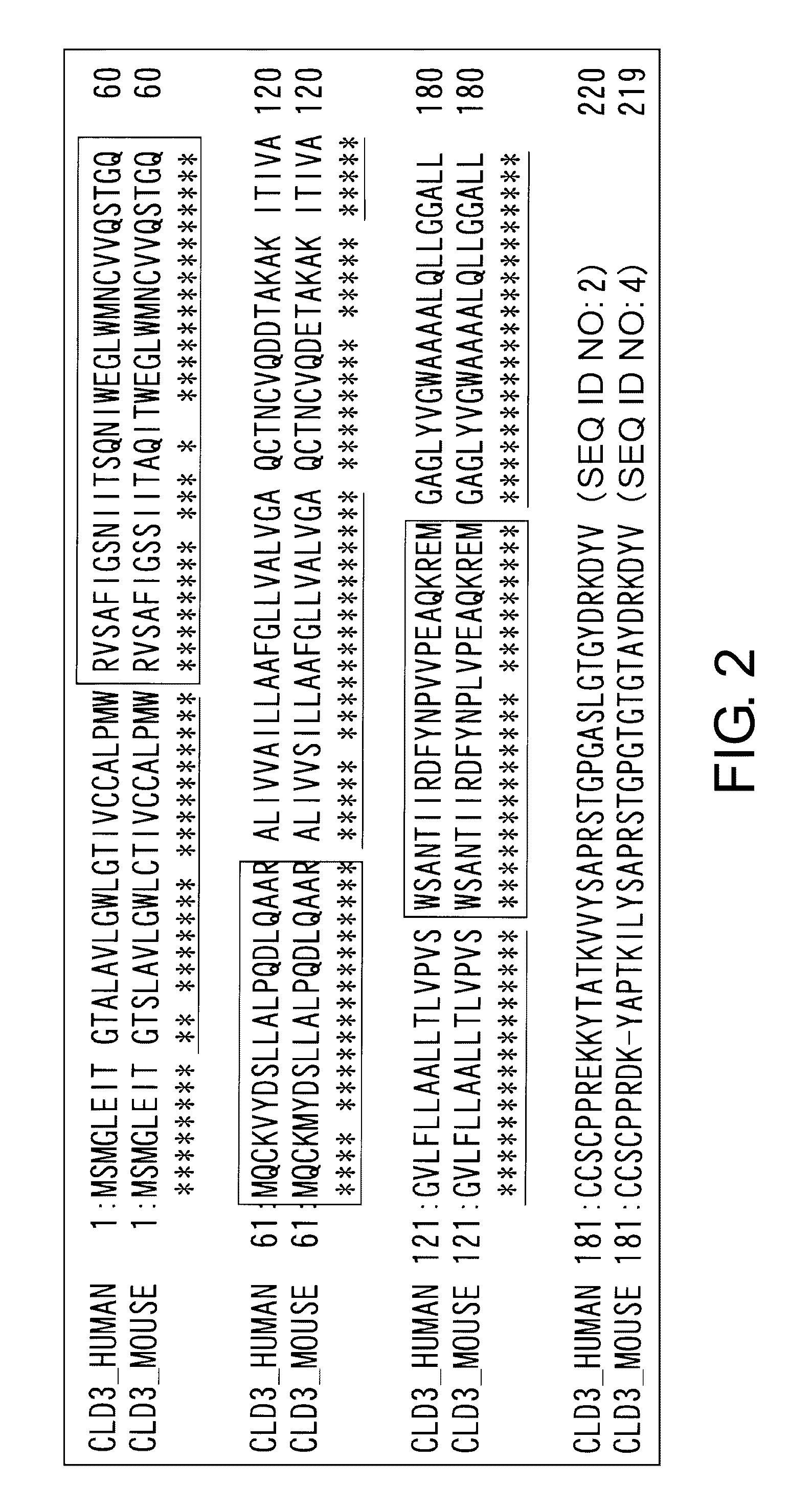
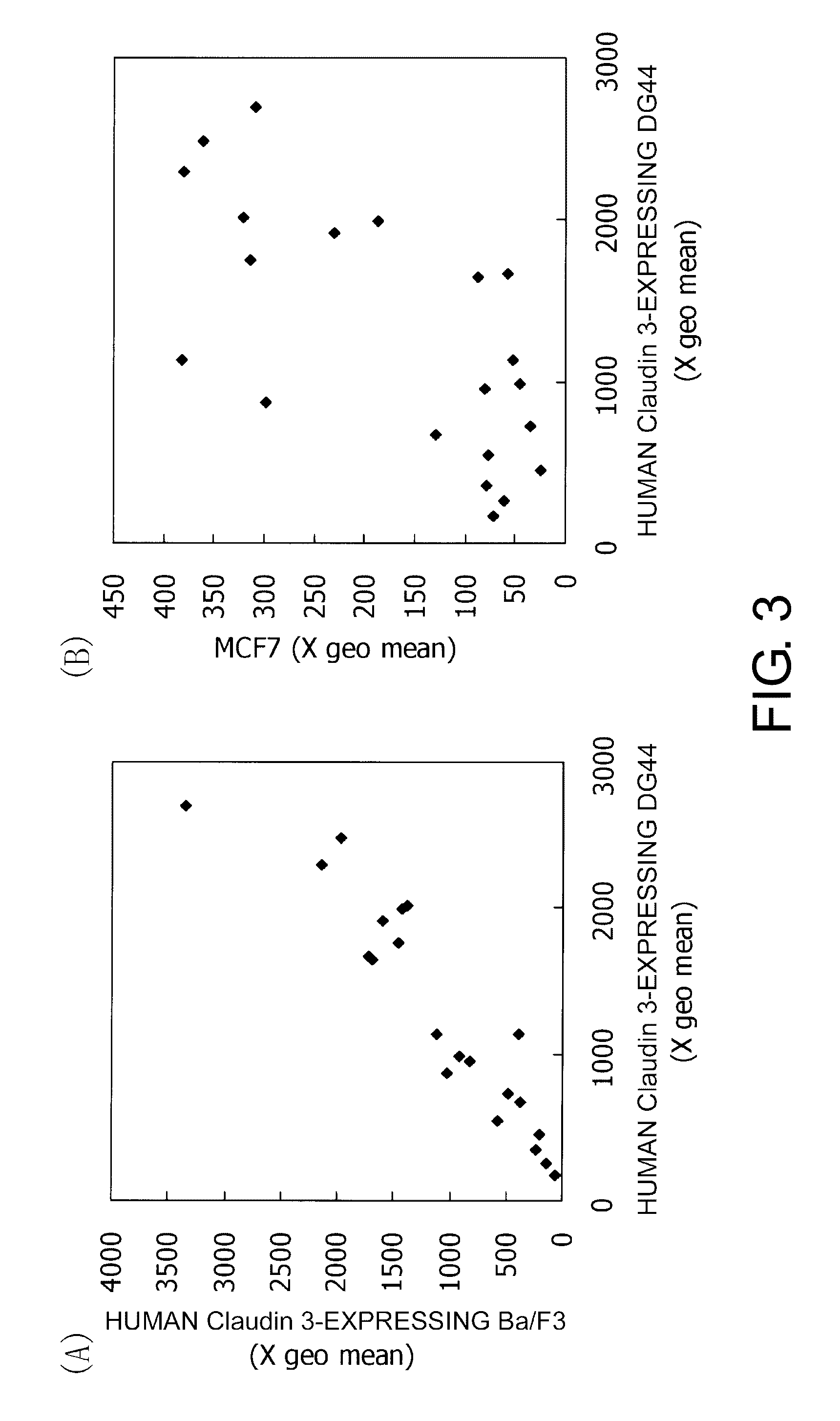
Monoclonal antibodies that bind specifically to Claudin 3 expressed on cell surface are provided. The antibodies of the present invention are useful for diagnosis of cancers that have enhanced expression of Claudin 3, such as ovarian cancer, prostate cancer, breast cancer, uterine cancer, liver cancer, lung cancer, pancreatic cancer, stomach cancer, bladder cancer, and colon cancer. The present invention provides monoclonal antibodies showing cytotoxic effects against cells of these cancers. Methods for inducing cell injury in Claudin 3-expressing cells and methods for suppressing proliferation of Claudin 3-expressing cells by contacting Claudin 3-expressing cells with a Claudin 3-binding antibody are disclosed. The present application also discloses methods for diagnosis or treatment of cancers.
Owner:CHUGAI PHARMA CO LTD
Gene expression profiling in primary ovarian serous papillary tumors and normal ovarian epithelium
InactiveUS20060078941A1Highligthing the divergence of gene expressionMicrobiological testing/measurementBiological testingKallikrein-10Gene family
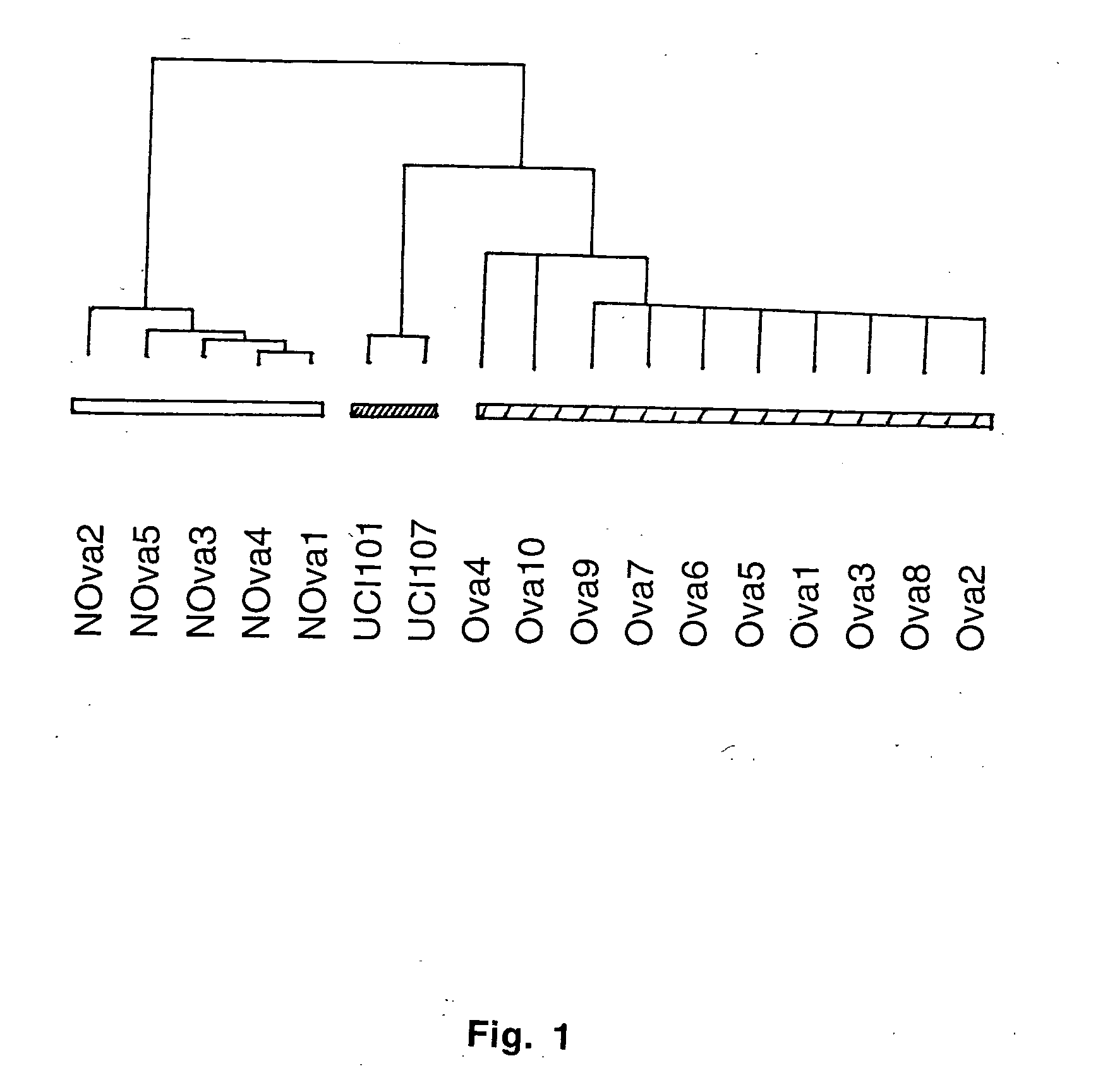
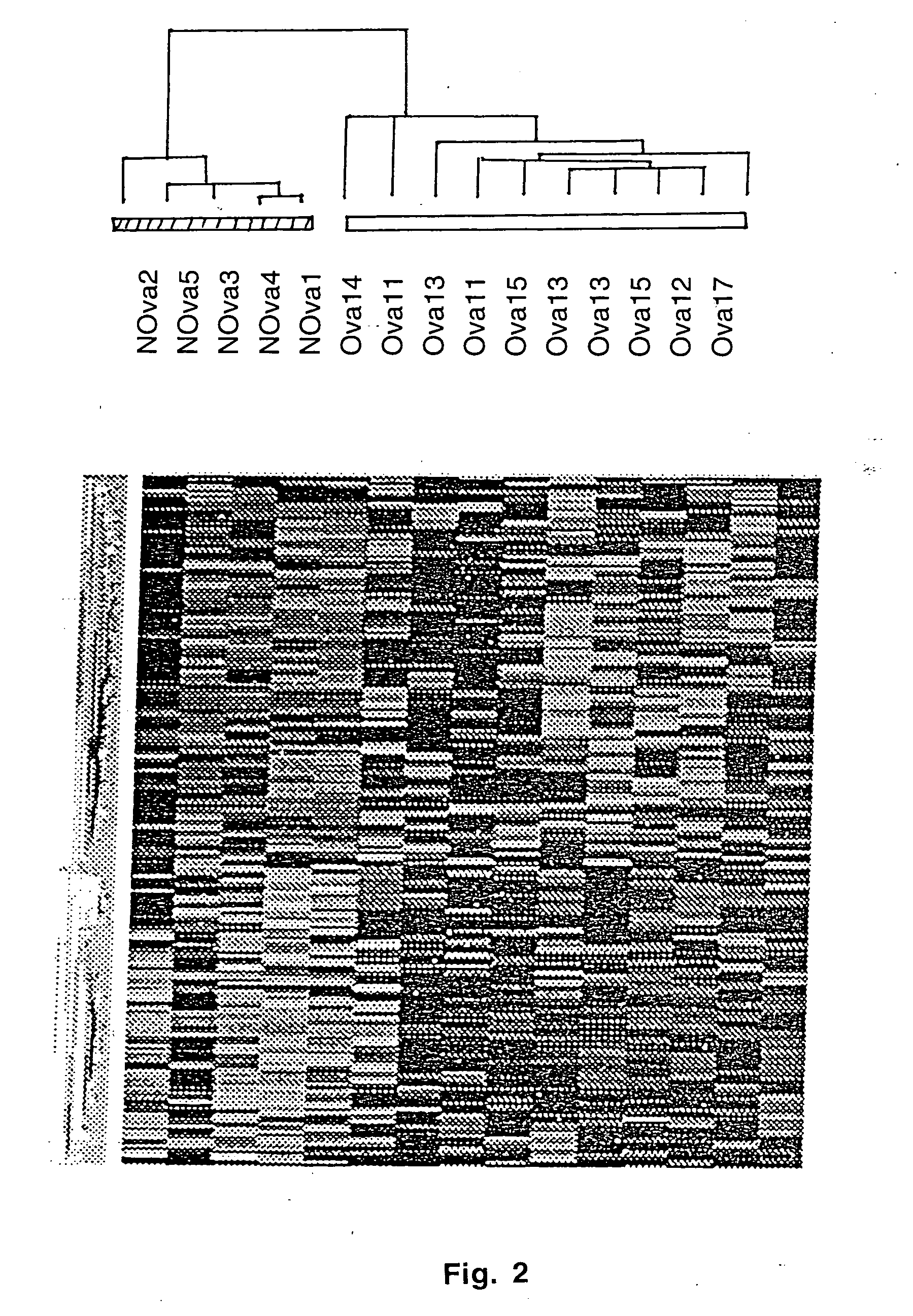
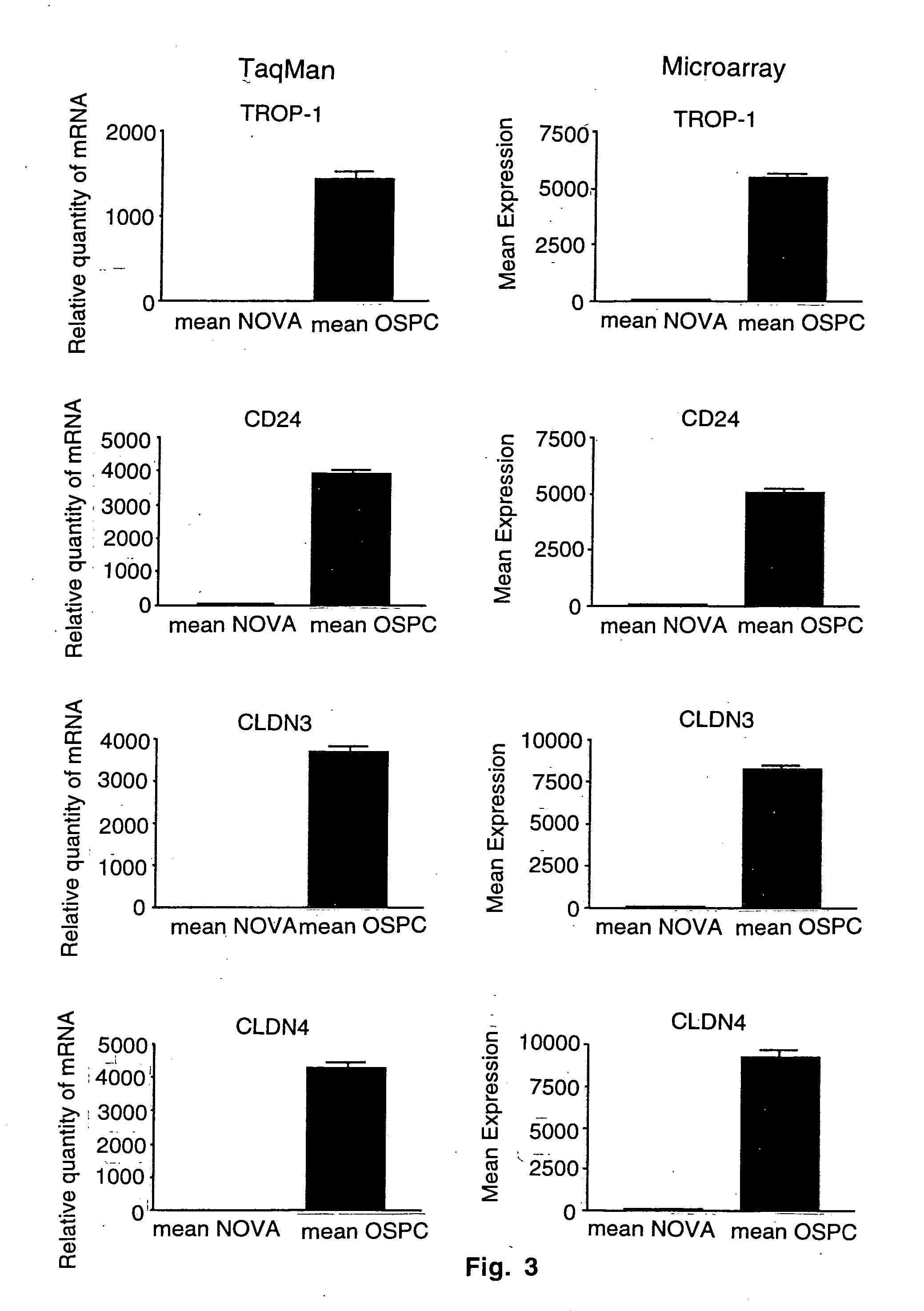
Gene expression profiling and hierarchial clustering analysis readily distinguish normal ovarian epithelial cells from primary ovarian serous papillary carcinomas. Laminin, tumor-associated calcium signal transducer 1 and 2 (TROP-1 / Ep-CAM; TROP-2), claudin 3, claudin 4, ladinin 1, S100A2, SERPIN2 (PAI-2), CD24, lipocalin 2, osteopontin, kallikrein 6 (protease M), kallikrein 10, matriptase and stratifin were found among the most highly overexpressed genes in ovarian serous papillary carcinomas, whereas transforming growth factor beta receptor III, platelet-derived growth factor receptor alpha, SEMACAP3, ras homolog gene family, member I (ARHI), thrombospondin 2 and disabled-2 / differentially expressed in ovarian carcinoma 2 (Dab2 / DOC2) were significantly down-regulated. Therapeutic strategy targeting TROP-1 / Ep-CAM by monoclonal chimeric / humanized antibodies may be beneficial in patients harboring chemotherapy-resistant ovarian serous papillary carcinomas. Claudin-3 and claudin-4 being receptors for Clostridium Perfringens enterotoxin, this toxin may be used as a novel therapeutic agent to treat ovarian serous papillary tumors.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Noveltreatment for neurological disorders
InactiveUS20100172919A1Lower protein levelLower Level RequirementsAntibacterial agentsBiocideAmyloid betaBiomarker (petroleum)
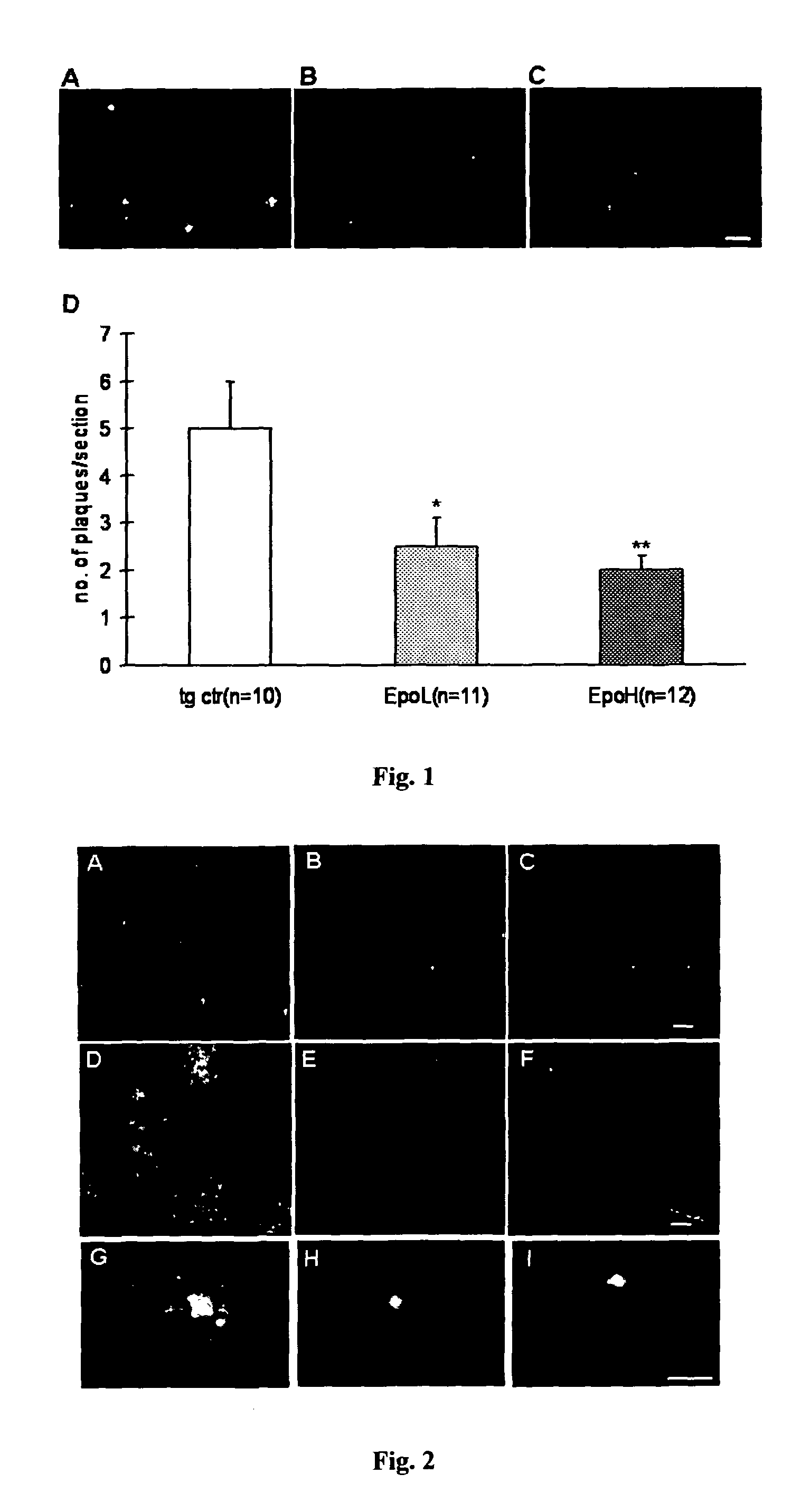
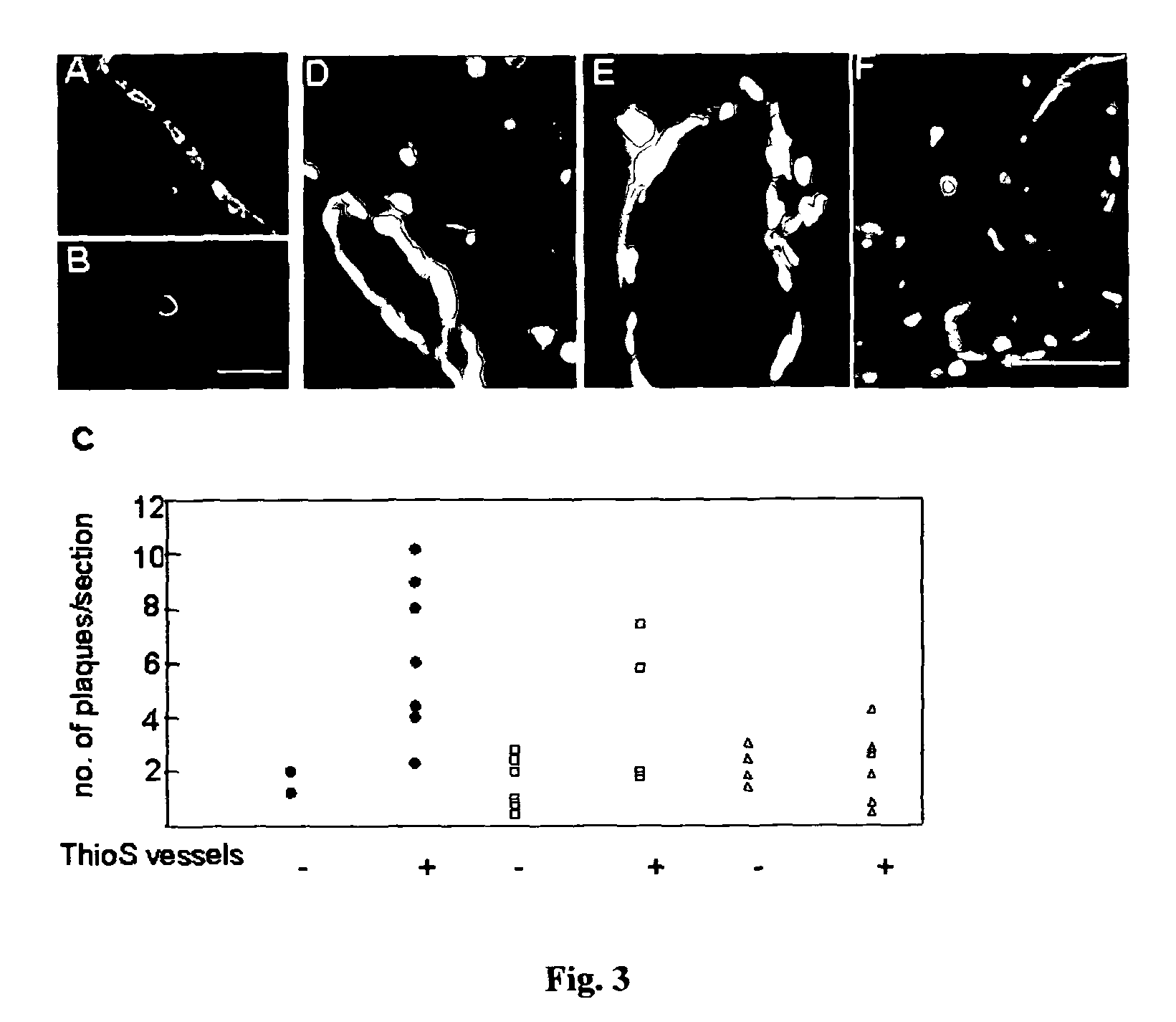
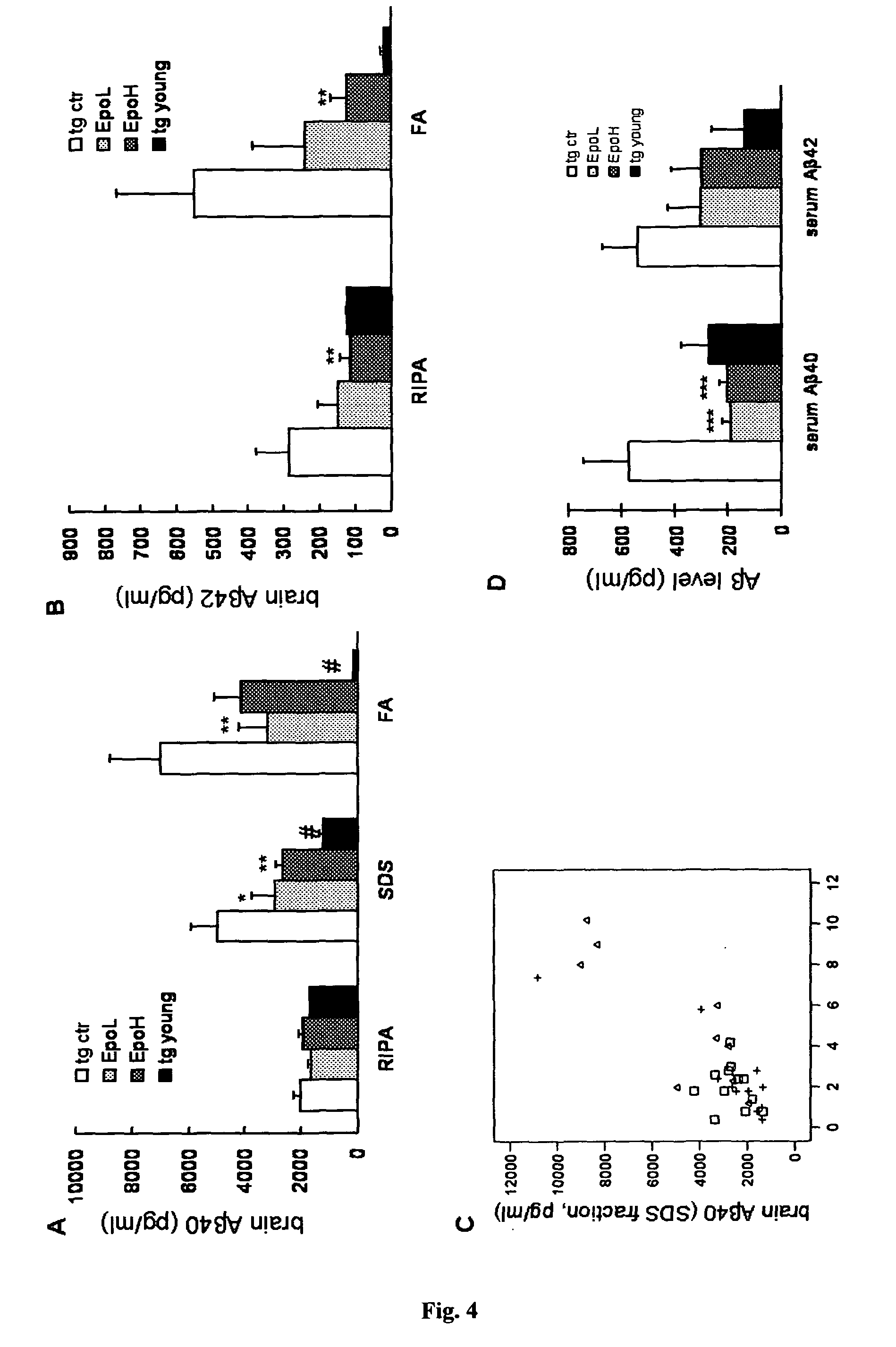
Provided are novel drugs and methods in the treatment as well as diagnosis of neurological disorders such as Alzheimer's disease and amyloid-beta pathology / amyloidosis. More specifically, the use of erythropoietin and analogs thereof for the treatment of Aβ peptide related brain impairments is described. Furthermore, the use of claudin-5 and variants thereof as biomarker for Alzheimer's disease and for the progression of Alzheimer's disease, respectively, is provided.
Owner:UNIV ZURICH
Cancer-specific promoters
InactiveUS20090192101A1Improve overall utilizationShrink tumorOrganic active ingredientsTumor rejection antigen precursorsNucleotideGene product
The present invention regards cancer-specific control sequences that direct expression of a polynucleotide encoding a therapeutic gene product for treatment of the cancer. Specifically, the invention encompasses breast cancer-specific and ovarian cancer-specific control sequences. Two breast cancer-specific sequences utilize specific regions of fatty acid synthase and claudin 4 promoters, particularly in combination with a two-step transcription amplification sequence and / or a post-transcriptional control sequence. Two ovarian cancer-specific sequences utilize specific regions of hTERT and survivin promoters, particularly in combination with a two-step transcription amplification sequence and / or a post-transcriptional control sequence. In more particular embodiments, these polynucleotides are administered in combination with liposomes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Anti-human claudin 18.2 monoclonal antibody and application thereof
The invention relates to the field of biomedicine, in particular to an anti-human claudin 18.2 monoclonal antibody and application thereof. The antibody has three heavy chain complementary determiningregions (heavy chain CDR1, heavy chain CDR2, heavy chain CDR3) and three light chain complementary determining regions (light chain CDR1, light chain CDR2, light chain CDR3), and has good specificity, high affinity and stability.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
Combination therapy involving antibodies against claudin 18.2 for treatment of cancer
InactiveUS20150132253A1Effectively preventingEffectively treatingHeavy metal active ingredientsOrganic active ingredientsDiseaseCombined Modality Therapy
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:ASTELLAS PHARMA INC +1
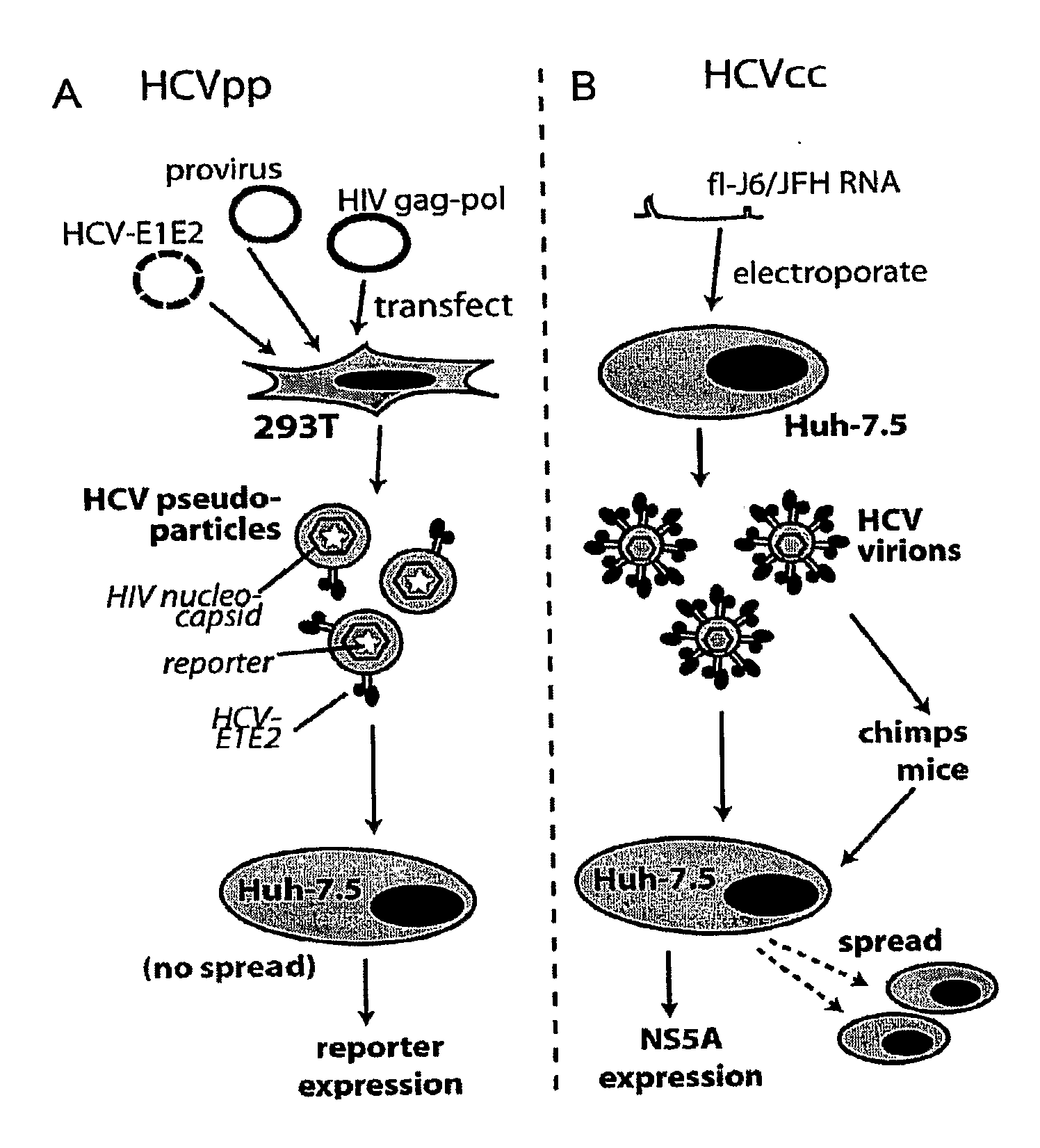
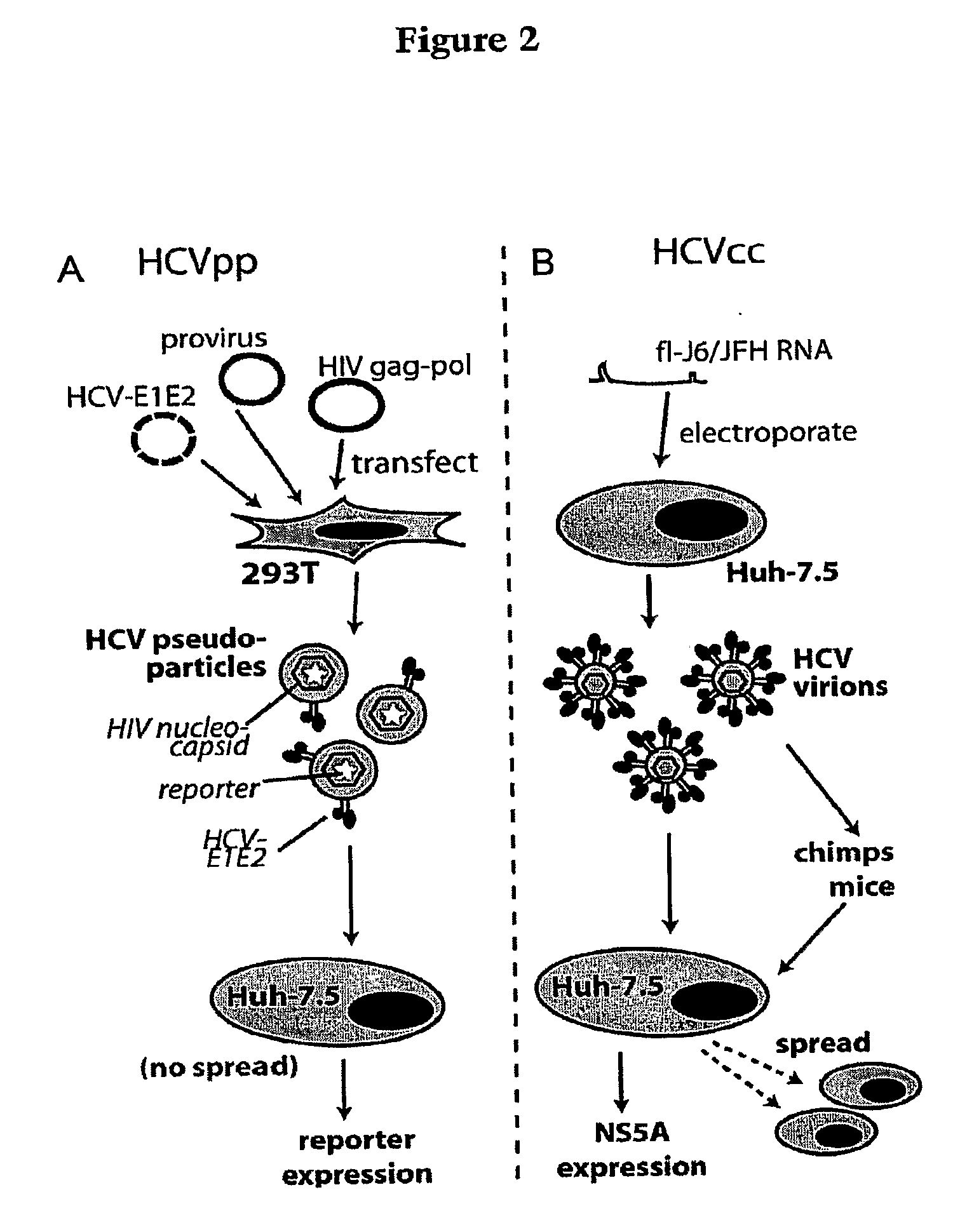
Hcv coreceptor and methods of use thereof
ActiveUS20090098123A1Prevents and mitigates interactionReduce retentionPeptide/protein ingredientsMicrobiological testing/measurementCo-receptorProtein function
The invention relates to the discovery that the Claudin-1 protein functions as a co-receptor for entry of HCV into cells. Methods of inhibiting, preventing or mitigating HCV infections by inhibiting HCV interactions with Claudin-1 are provided. Methods of identifying agents or compounds that interfere with HCV interactions with Claudin-1 are also provided. Finally, useful kits, cell culture compositions, agents, and compounds related to the inhibition of HCV interactions with Claudin-1 are also disclosed.
Owner:THE ROCKEFELLER UNIV
Method for Opening Tight Junctions
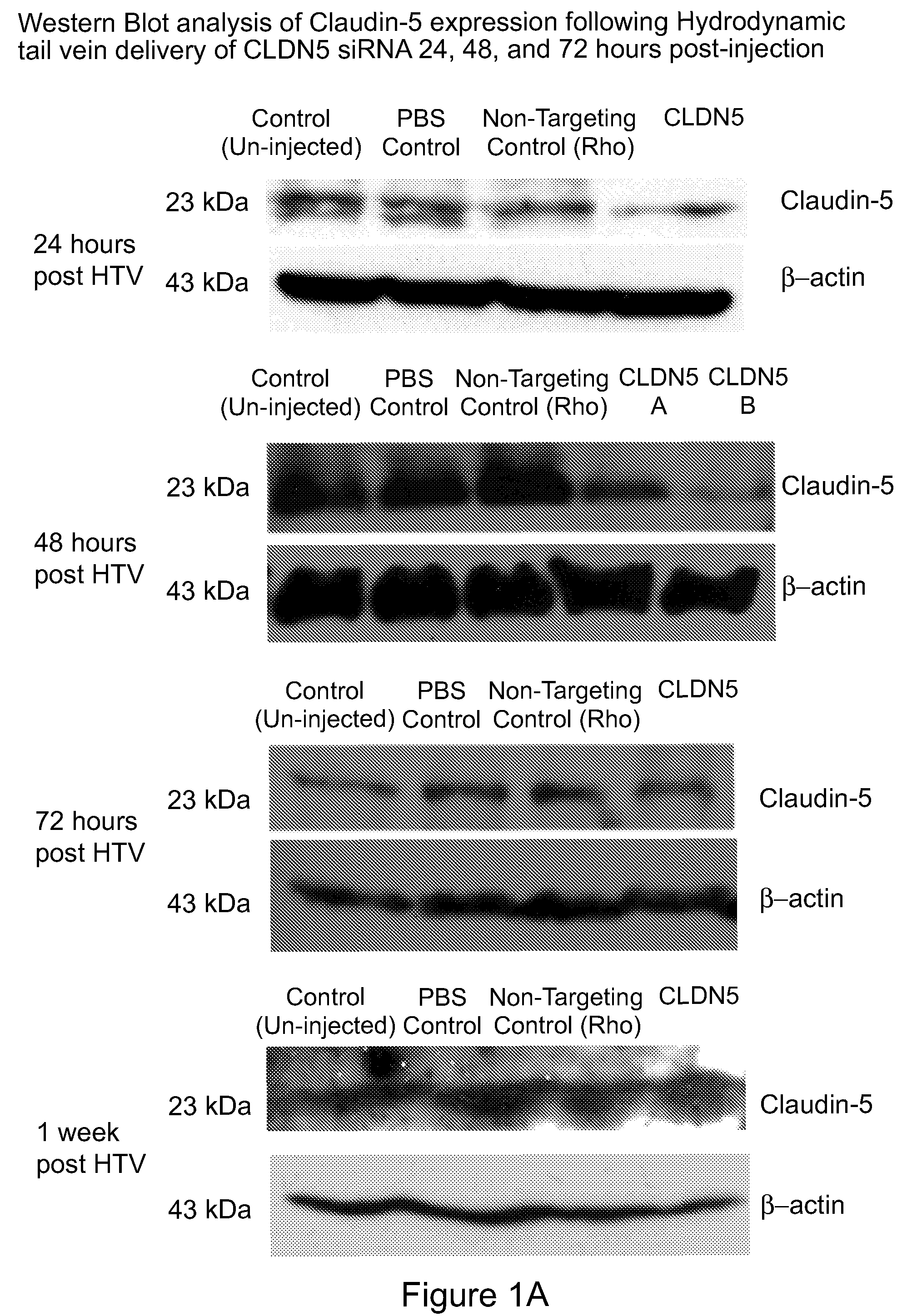
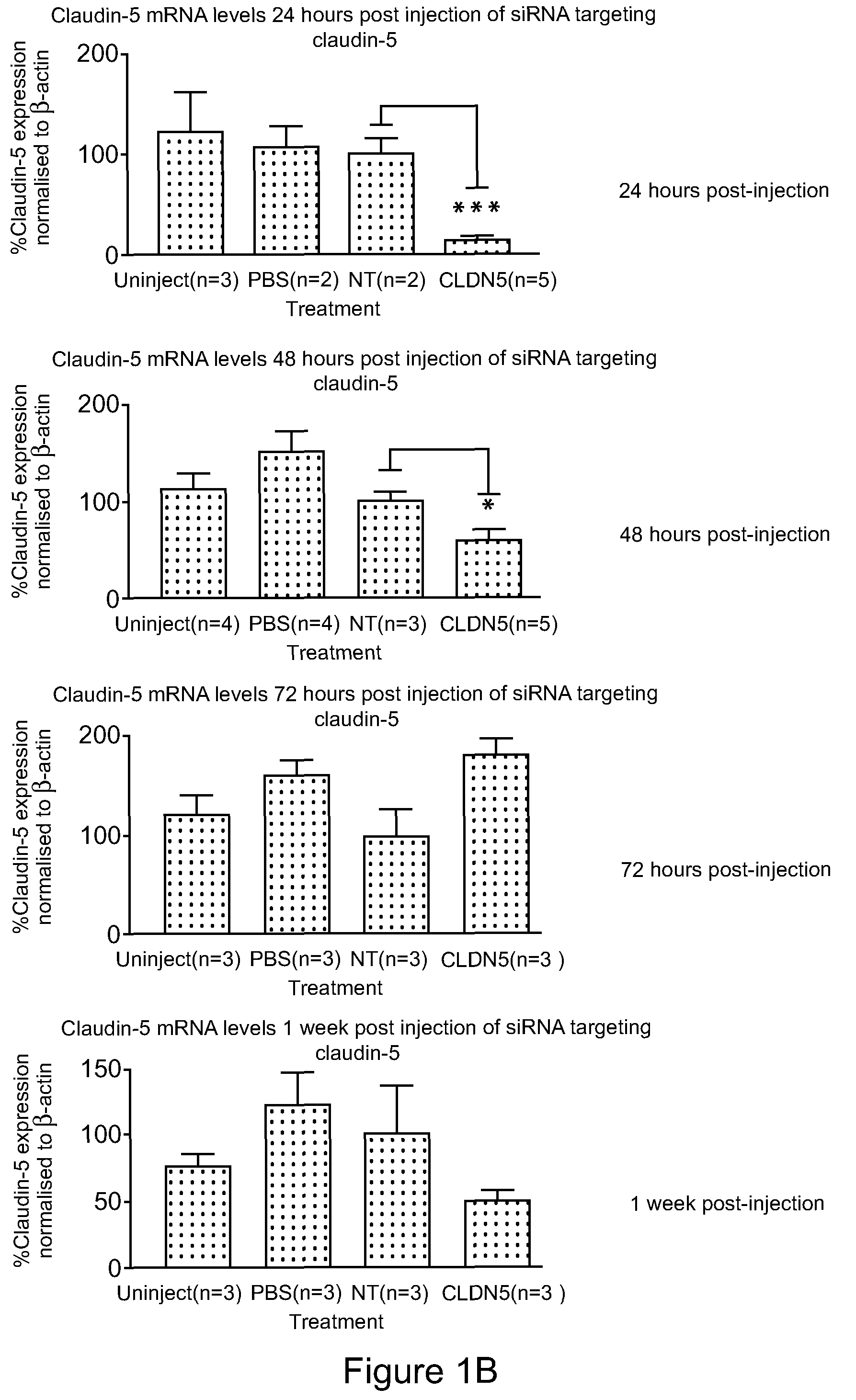
The present invention is directed to a method and use of RNA interference (RNAi) for the transient, reversible and controlled opening of the tight junctions of the blood brain barrier and / or the blood retinal barrier. This method may be used in the treatment of many diseases and disorders which require the opening of the blood brain barrier and / or blood retinal barrier. Such methods generally involve the use of an RNAi-inducing agent, such as siRNA, miRNA, shRNA or an RNAi-inducing vector whose presence within a cell results in production of an siRNA or shRNA, targeting tight junction proteins to open the blood brain barrier and / or blood retinal barrier.
Owner:TRINITY COLLEGE DUBLIN
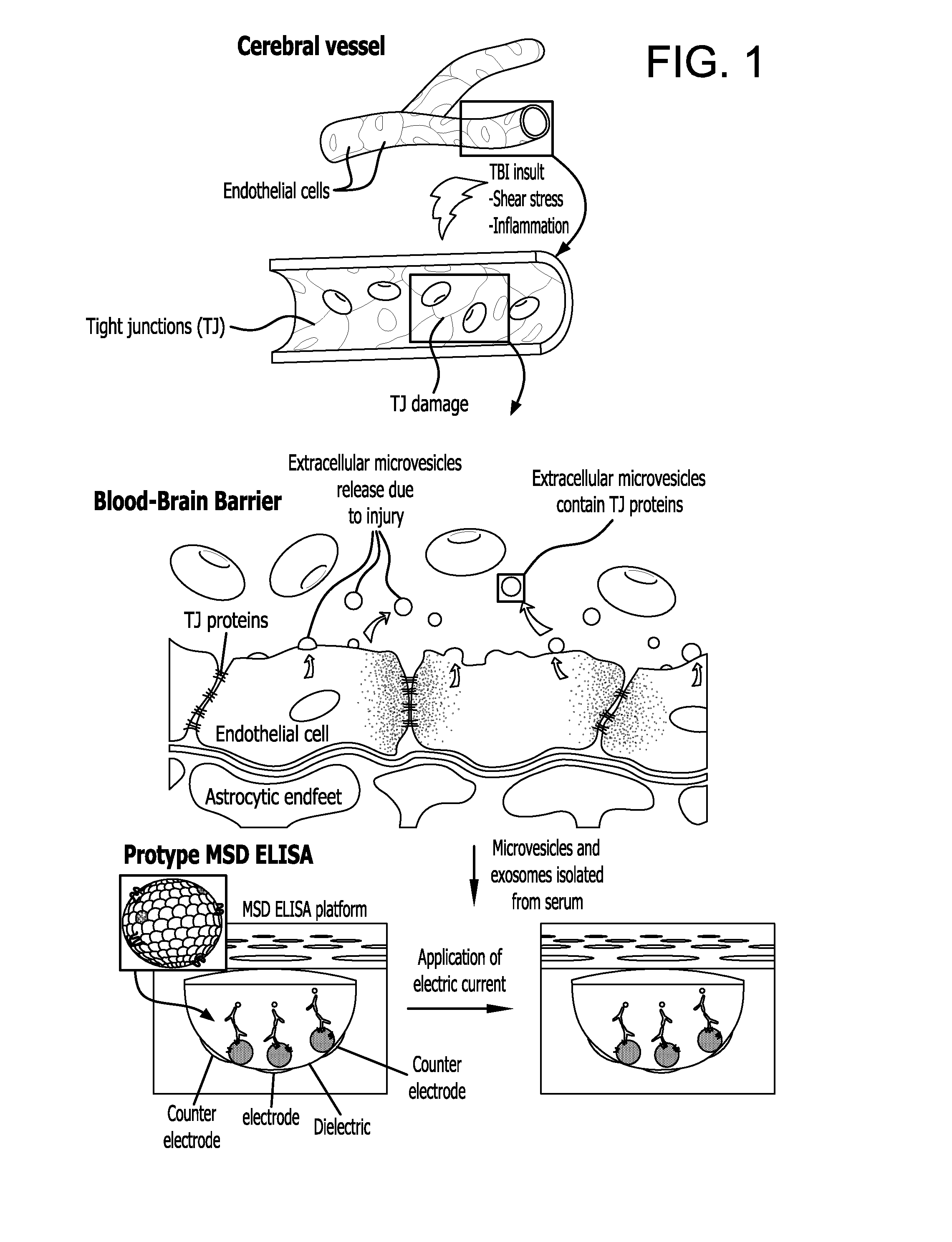
Method for detecting injury to the brain
Provided is a method for detecting injury to the brain comprising: a) determining the level of a tight junction (TJ) protein in exosomes isolated from a test sample from a subject, wherein the TJ protein is occludin, claudin-3, claudin-5, claudin-12, ZO-1, ZO-2, ZO-3, JAM-A, JAM-B or JAM-C, or any combination thereof; b) comparing the level of the TJ protein in the test sample to the level of the TJ protein in a control sample, wherein an elevated level of the TJ protein in the test sample relative to the level of the TJ protein in the control sample indicates that the subject has an injury to the brain.
Owner:TEMPLE UNIVERSITY
Combination therapy involving antibodies against claudin 18.2 for treatment of cancer
ActiveUS20150157711A1High expressionStabilizing and increasing expressionHeavy metal active ingredientsPeptide/protein ingredientsDiseaseCombined Modality Therapy
The present invention provides a combination therapy for effectively treating and / or preventing diseases associated with cells expressing CLDN18.2, including cancer diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder and metastases thereof.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
Claudin-18.2-specific immunoreceptors and T cell epitopes
ActiveCN107960056APolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsSpecific immunity
Owner:BIONTECH CELL & GENE THERAPIES +1
Claudin 18.2-targeting CAR molecule, immune cell modified by Claudin 18.2-targeting CAR molecule and application of Claudin 18.2-targeting CAR molecule
PendingCN111848809AHigh activityHigh affinityImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsTransmembrane domainIntracellular domain
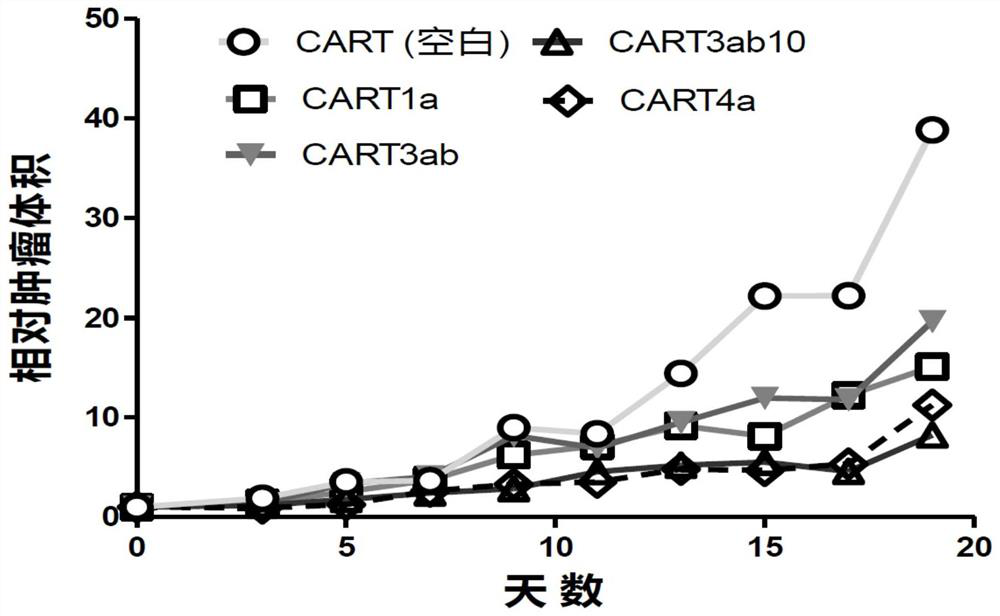
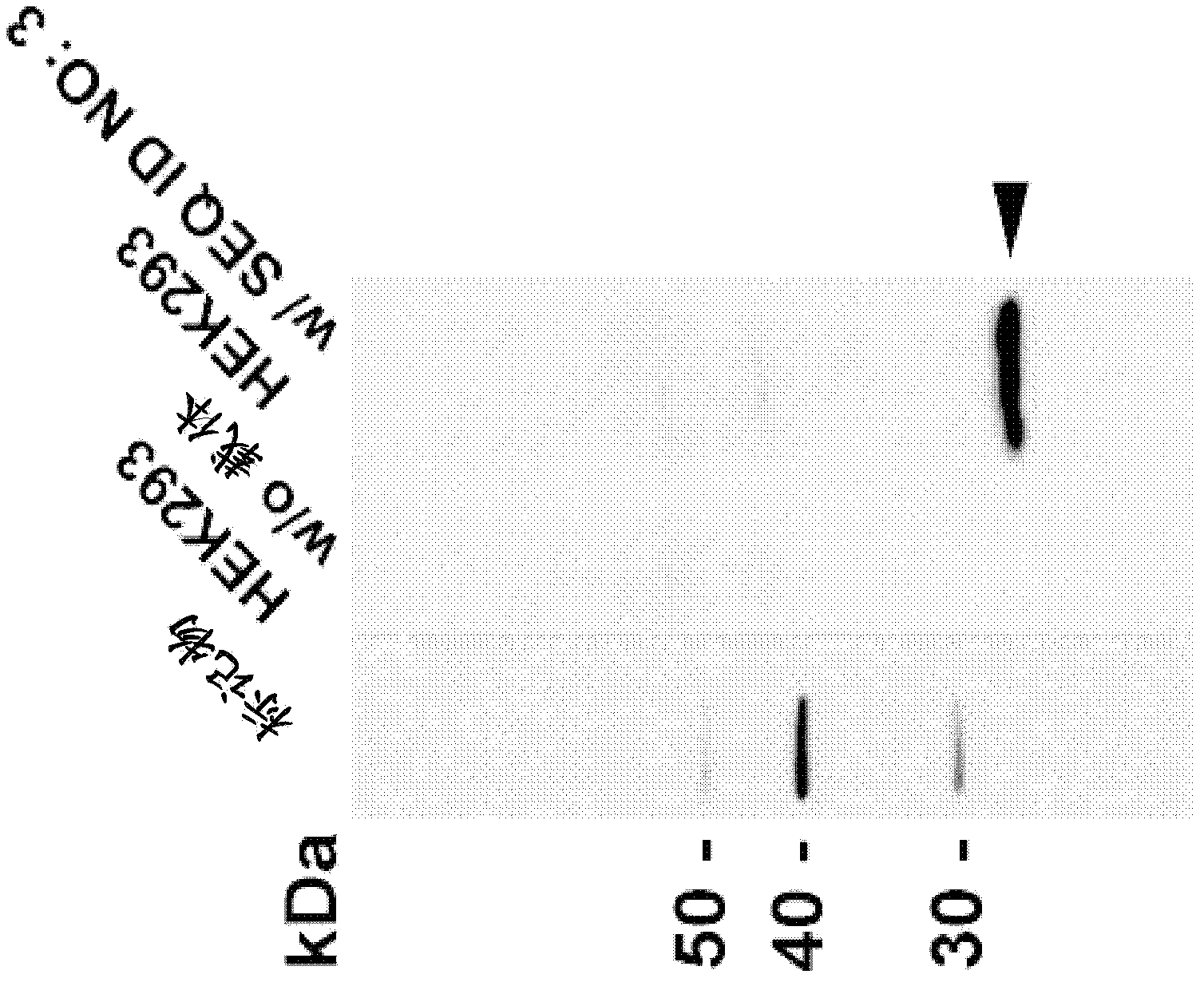
The present invention discloses a nucleic acid construct. The nucleic acid construct is characterized in that the nucleic acid construct has a structure as shown in the formula car-[(IRES)-f]<m>, wherein the IRES is a sequence of an internal ribosome entry site, f encodes a functional protein F, m is 0 or a non-0 natural number, car encodes a CAR, the CAR comprises (a) an extracellular binding domain that specifically recognizes CLDN18.2, (b) a hinge domain, (c) a transmembrane domain, (d) a costimulating intracellular domain, and (e) a signal transduction domain, the extracellular binding domain comprises scFv, and the amino acid sequences and functions of the extracellular binding structural domain are described in the specification. The CAR molecule provided by the invention has excellent safety and effectiveness and shows a very good tumor inhibition effect.
Owner:L&L BIOPHARMA CO LTD
Monoclonal antibodies against claudin-18 for treatment of cancer
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing CLD18, including tumor-related diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder.
Owner:ASTELLAS PHARMA INC +1
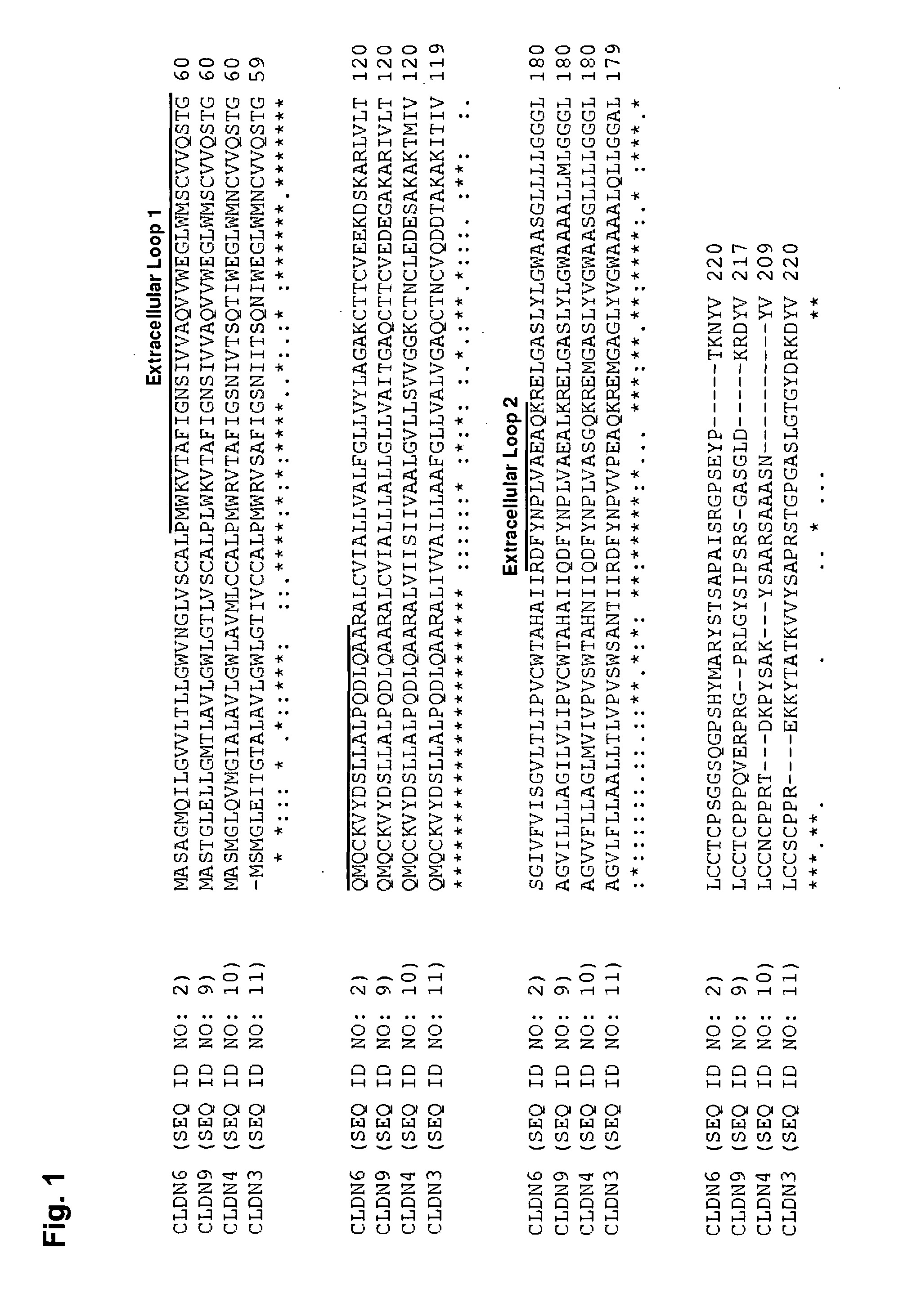
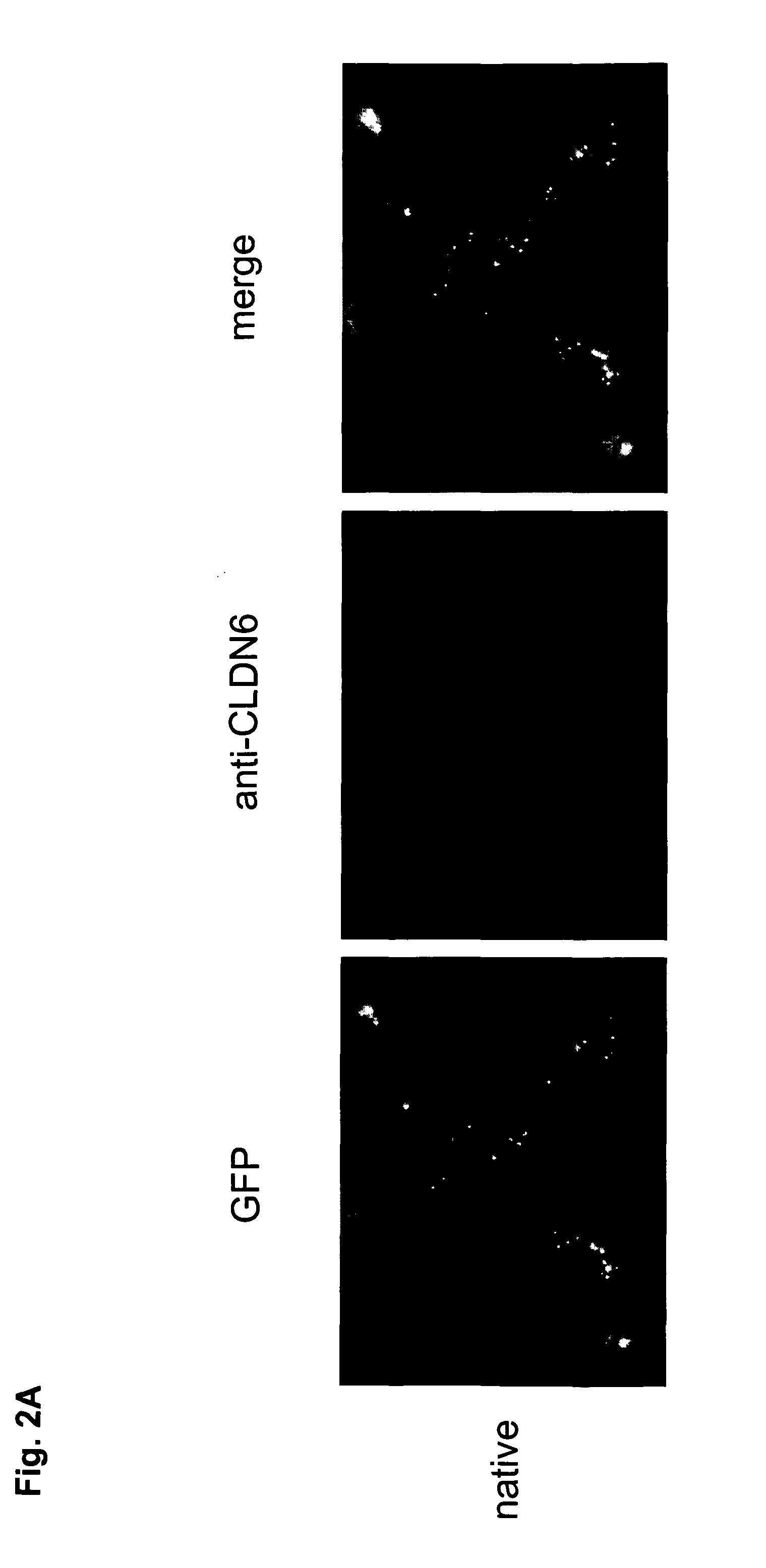
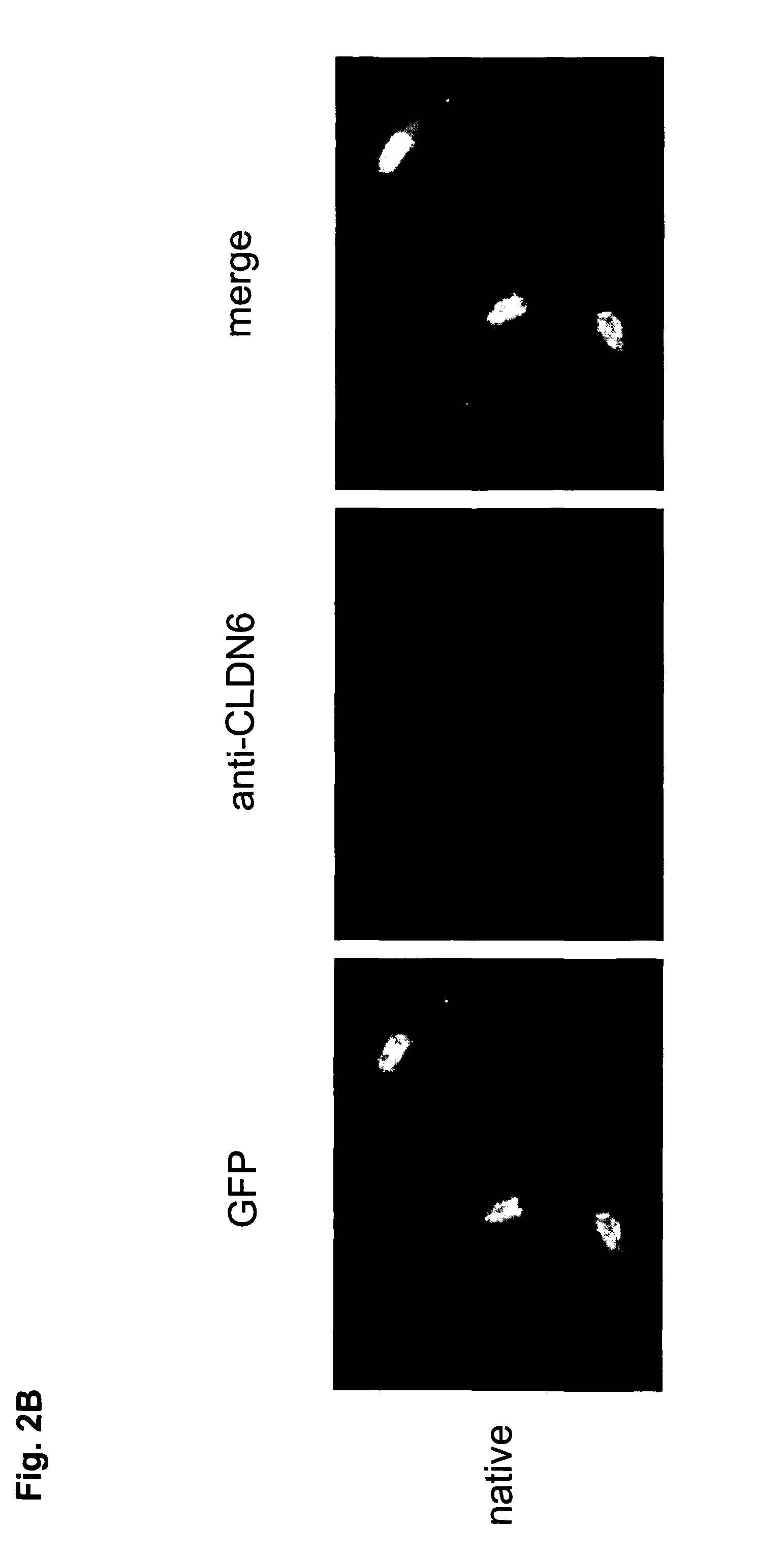
Antibodies specific for claudin 6 (CLDN6)
ActiveUS20120308478A1Minimize adverse effectsHigh levelPeptide/protein ingredientsGenetic material ingredientsDiseaseMelanoma
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing Claudin-6 (CLDN6), including tumor-related diseases such as ovarian cancer, lung cancer, gastric cancer, breast cancer, hepatic cancer, pancreatic cancer, skin cancer, malignant melanoma, head and neck cancer, sarcoma, bile duct cancer, cancer of the urinary bladder, kidney cancer, colon cancer, placental choriocarcinoma, cervical cancer, testicular cancer, and uterine cancer.
Owner:GANYMED PHARMA AG +1
Monoclonal antibodies against claudin-18 for treatment of cancer
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing CLD18, including tumor-related diseases such as gastric cancer, esophageal cancer, pancreatic cancer, lung cancer, ovarian cancer, colon cancer, hepatic cancer, head-neck cancer, and cancer of the gallbladder.
Owner:ASTELLAS PHARMA INC +1
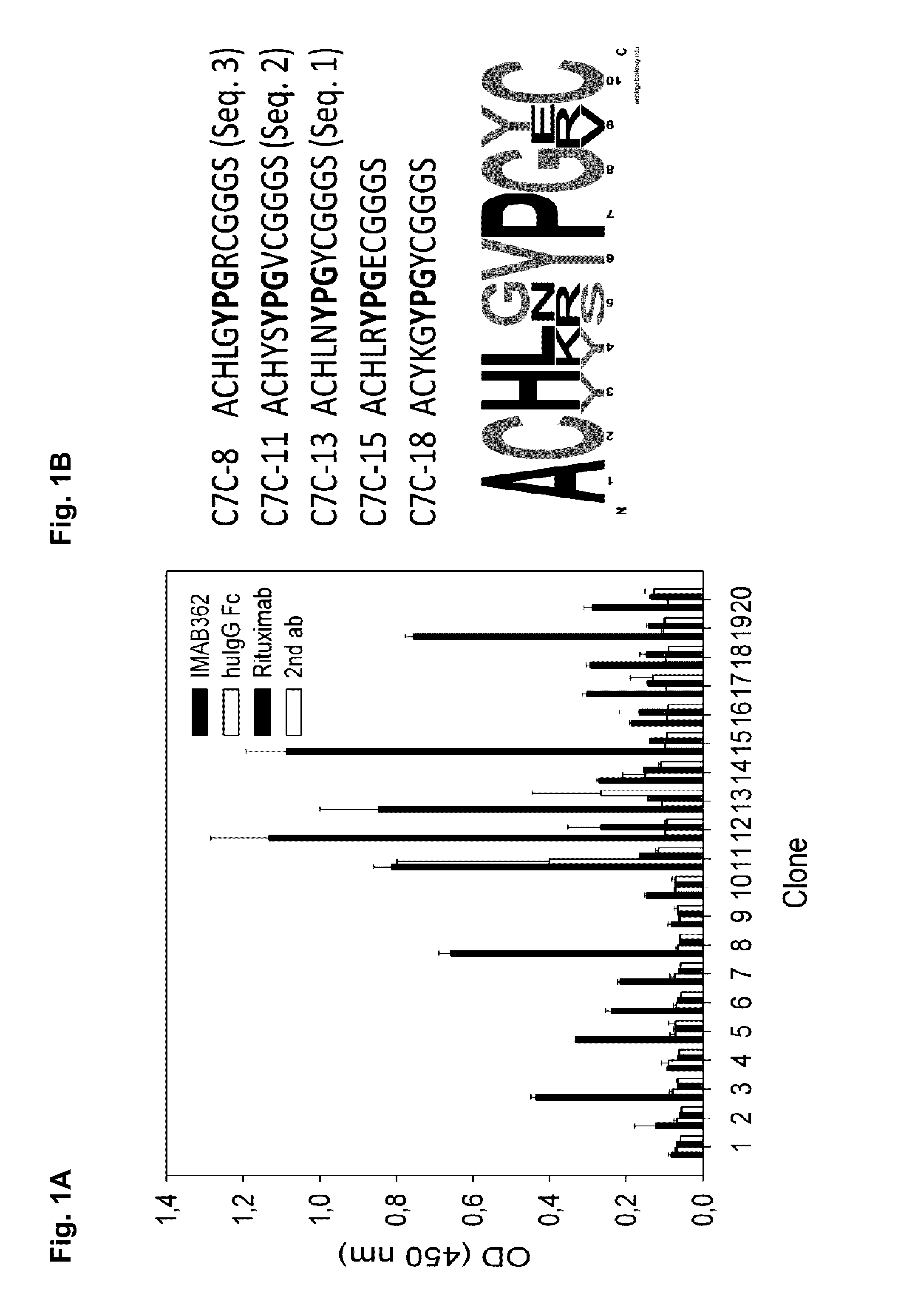
Peptide mimotopes of claudin 18.2 and uses thereof
ActiveUS20160347815A1Optimization of binding propertyCompound screeningCell receptors/surface-antigens/surface-determinantsAntigenBinding domain
The present invention provides molecules that mimic antigenic determinants of the integral transmembrane protein claudin 18.2 (CLDN18.2). These molecules compete with CLDN18.2 for binding to a CLDN18.2 binding domain, e.g. a CLDN18.2 binding domain of an antibody, and are capable of detecting antibodies against CLDN18.2. The mimotopes of the invention may be used to generate or inhibit immune responses in animals and preferably humans. Furthermore, they can be used for purposes of detecting agents comprising a CLDN18.2 binding domain in biological samples as well as for purifying agents comprising a CLDN18.2 binding domain.
Owner:JPT PEPTIDE TECH +1
Antibodies binding human Claudin 18.2 and uses thereof
ActiveUS10421817B1High activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsChimeric antigen receptorImmunoconjugate
An isolated monoclonal antibody that specifically binds human Claudin 18.2. A nucleic acid molecule encoding the antibody, an expression vector, a host cell and a method for expressing the antibody are also provided. The present invention further provides an immunoconjugate, a bispecific molecule, a chimeric antigen receptor, an oncolytic virus and a pharmaceutical composition comprising the antibody, as well as a diagnostic or treatment method using an anti-Claudin 18.2 antibody of the invention.
Owner:BEIJING MABWORKS BIOTECH
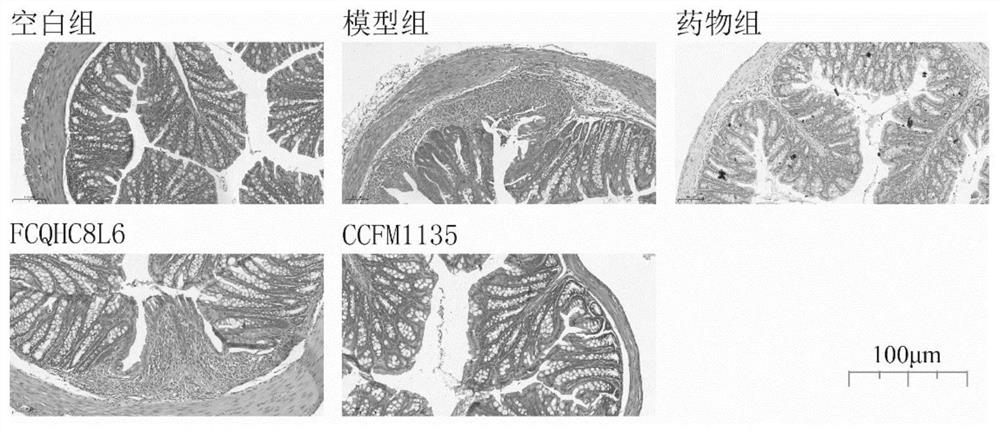
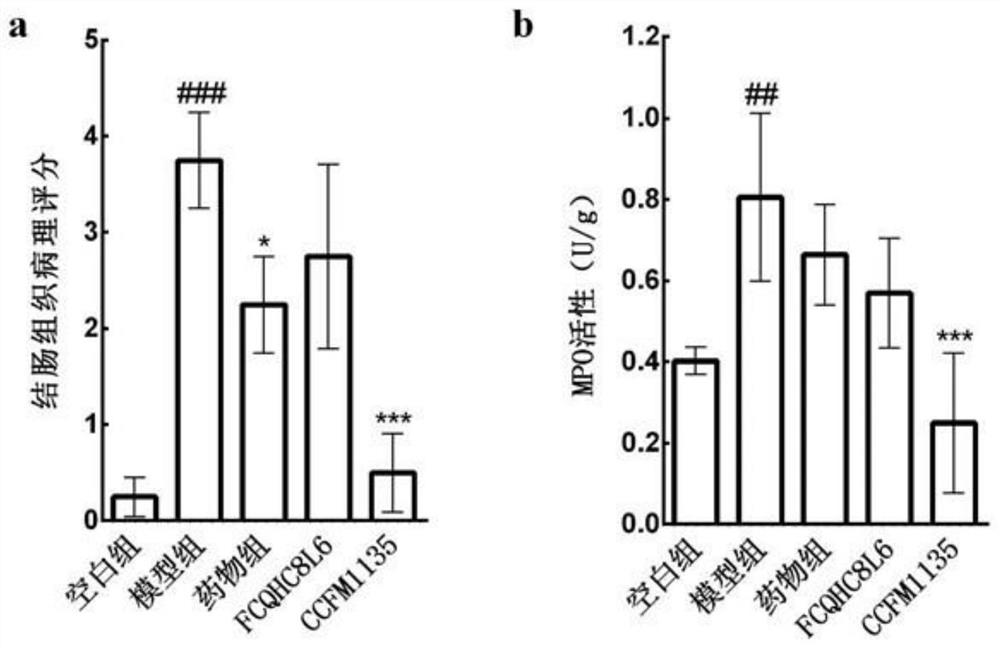
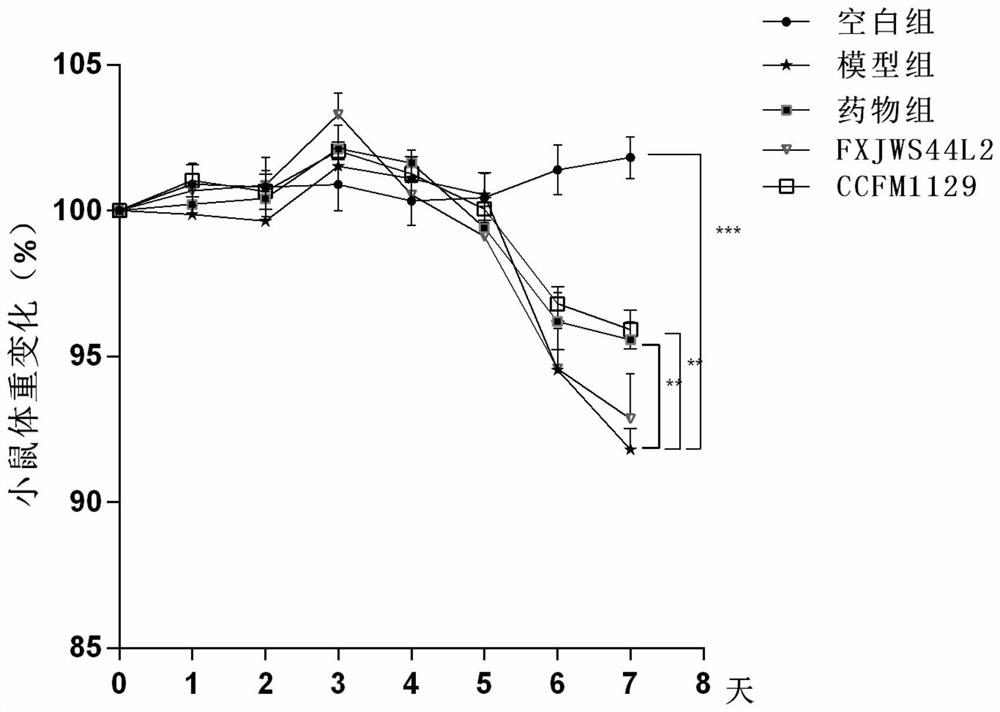
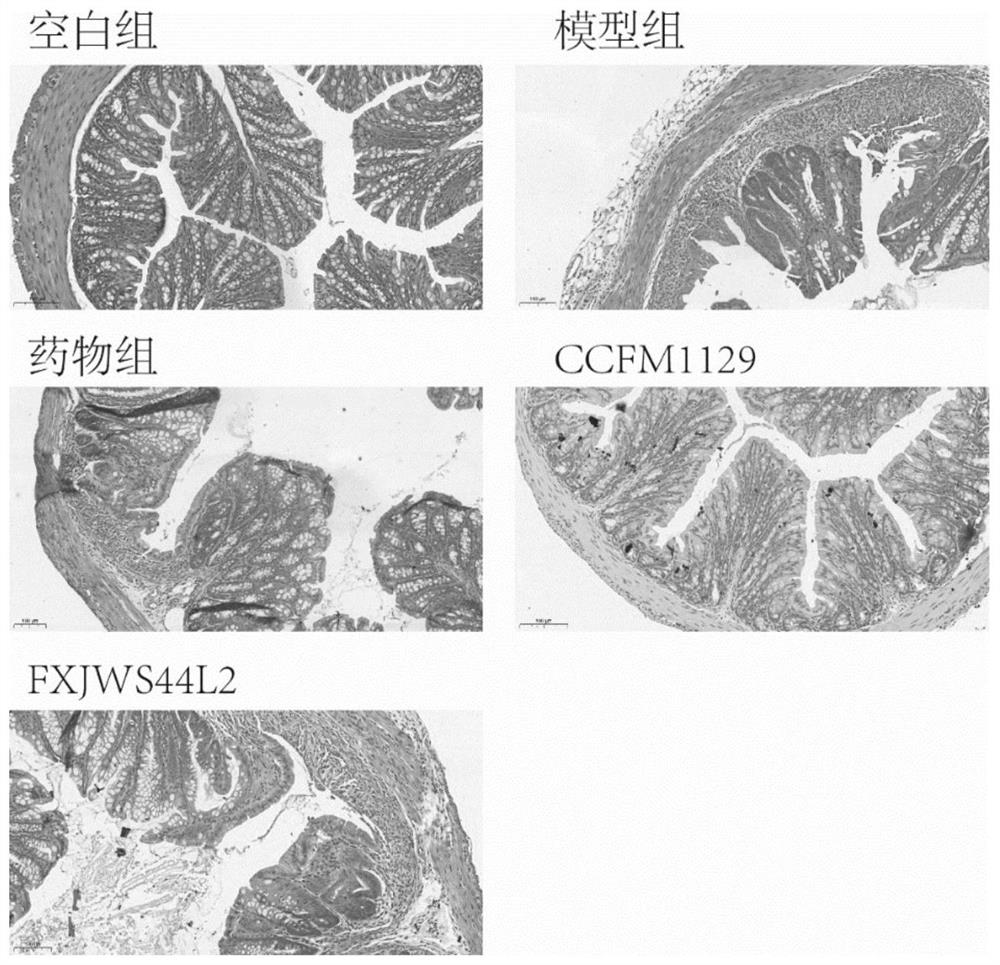
Application of lactobacillus reuteri in preventing and relieving ulcerative colitis
ActiveCN112646744AImprove toleranceReduce abundanceMilk preparationBacteriaUlcerative colitisDisease activity
The invention discloses application of lactobacillus reuteri in preventing and relieving ulcerative colitis, and belongs to the technical field of functional microorganisms. Lactobacillus reuteri CCFM1135 can tolerate the gastrointestinal environment of a human body, significantly reduce the disease activity index during the ulcerative colitis, improve colon mucosal injury, reduce MPO activity, reduce the content of proinflammatory factors TNF-alpha, IL-6 and IFN-gamma in colon, up-regulate the transcription level of colon tight junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, up-regulate the transcription levels of colon antibacterial peptides Reg3g and Reg3b, improve the diversity of intestinal flora, and reduced the relative abundance of acinetobacter in excrement.
Owner:JIANGNAN UNIV
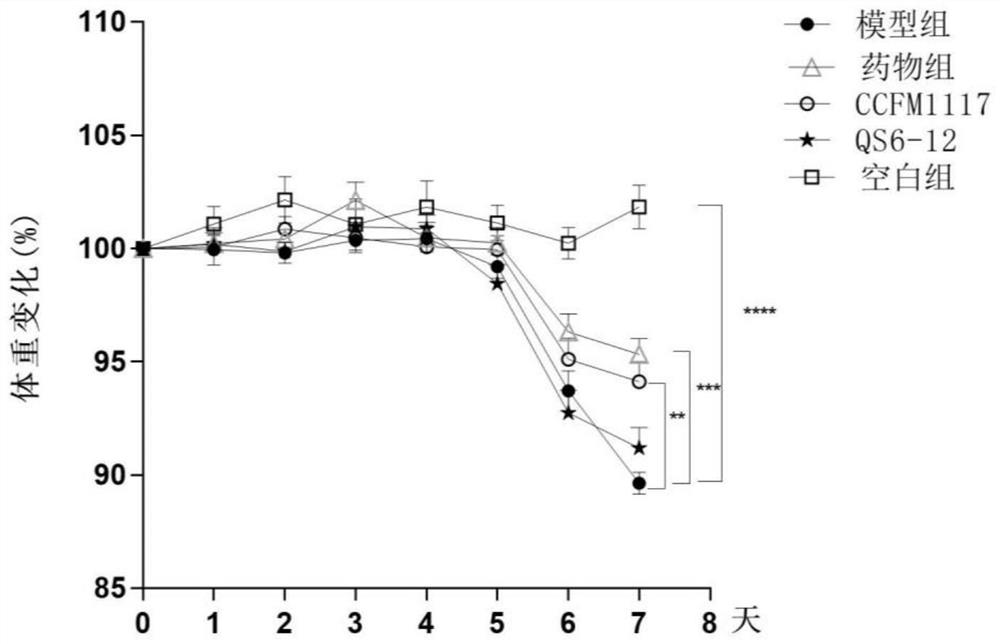
Lactobacillus plantarum for relieving ulcerative colitis and application thereof
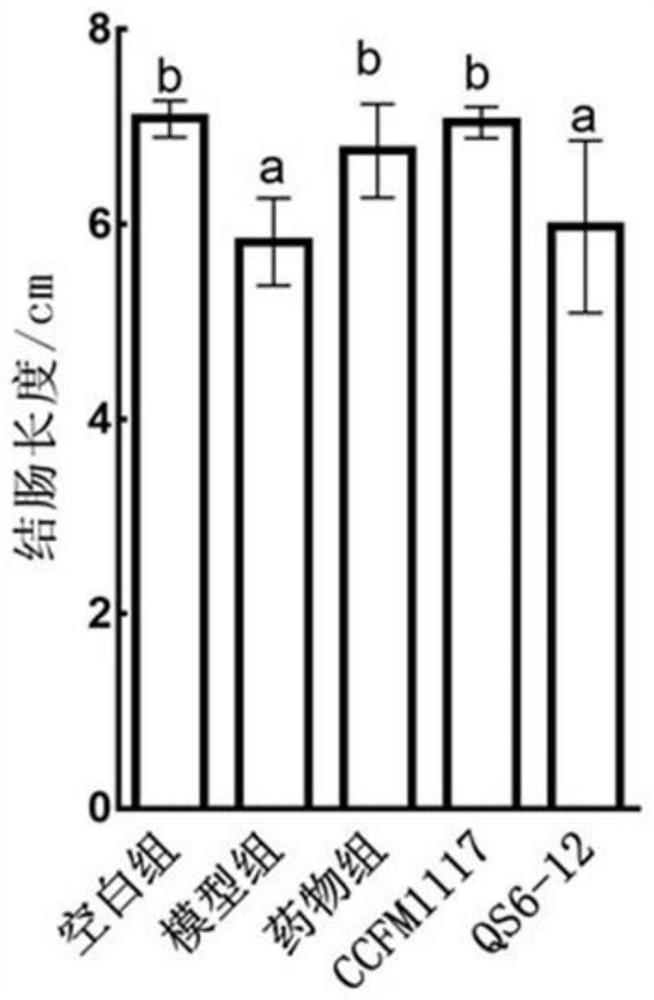
The invention discloses a lactobacillus plantarum for relieving ulcerative colitis and application thereof, and belongs to the technical field of functional microorganisms. The lactobacillus plantarum CCFM1117 can tolerate the gastrointestinal environment of a human body, reduce weight loss in the ulcerative colitis disease period, improve fecal characters and hemafecia conditions, relieve length shortening of the colon, improve colonic mucosal injury, reduce the content of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IFN-gamma in the colon, up-regulate the gene transcription level of colon tight junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, increase the content of short-chain fatty acid, increase the abundance of short-chain fatty acid producing bacteria Coprococcus and butyric acid producing bacteria Faecalibacterium, and improve the diversity of intestinal flora.
Owner:JIANGNAN UNIV
Bifidobacterium longum subsp.infantis for relieving colitis and application thereof
PendingCN114574390ANo side effectsLower disease activity indexBacteriaDigestive systemBiotechnologyMyeloperoxidase level
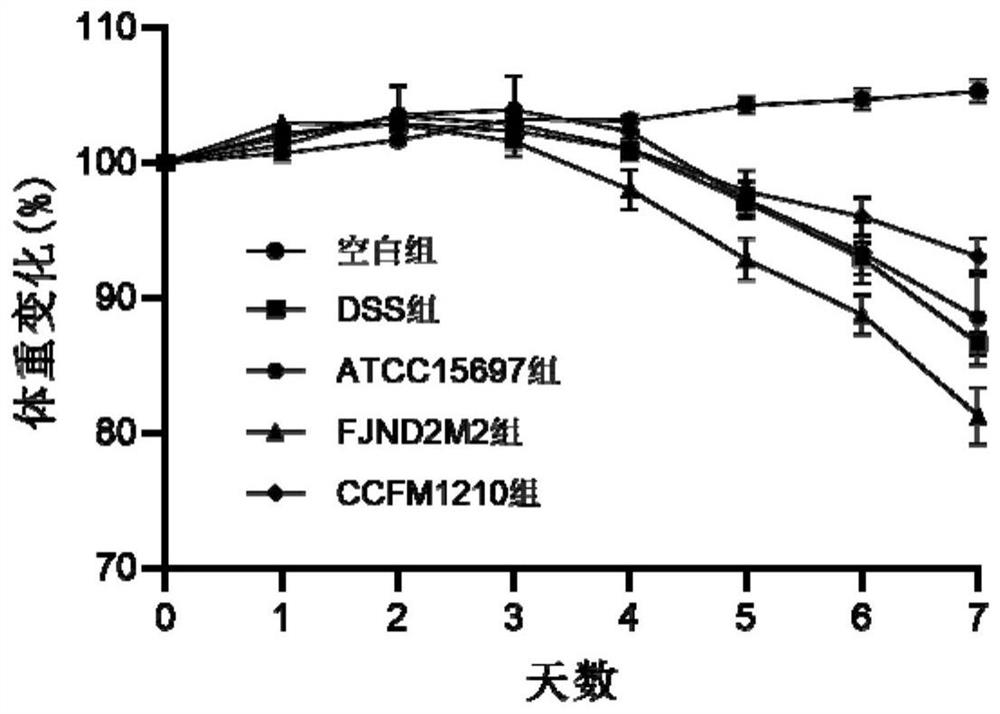
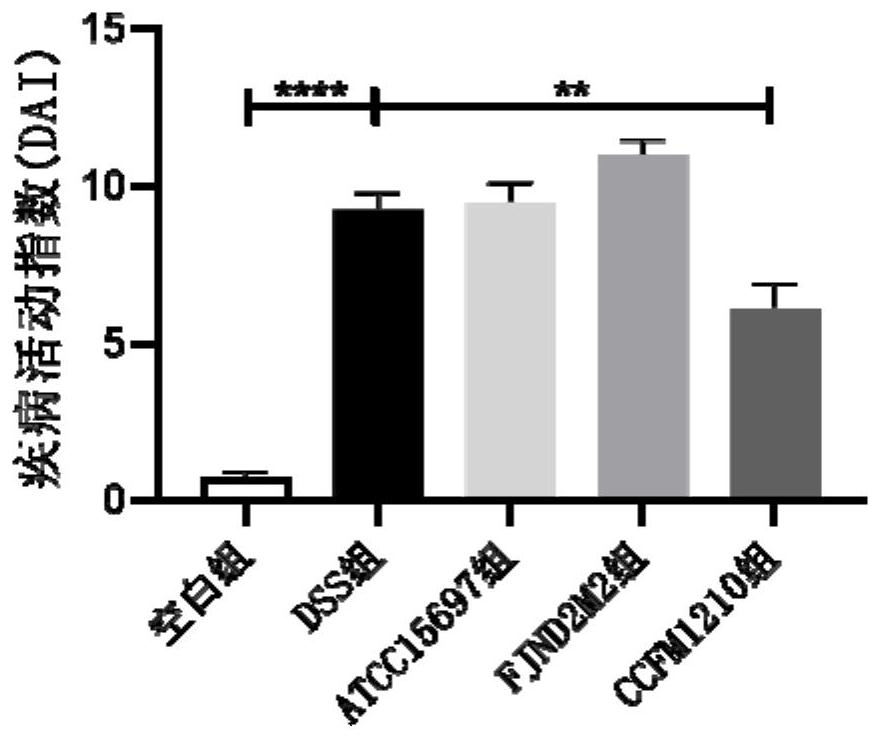
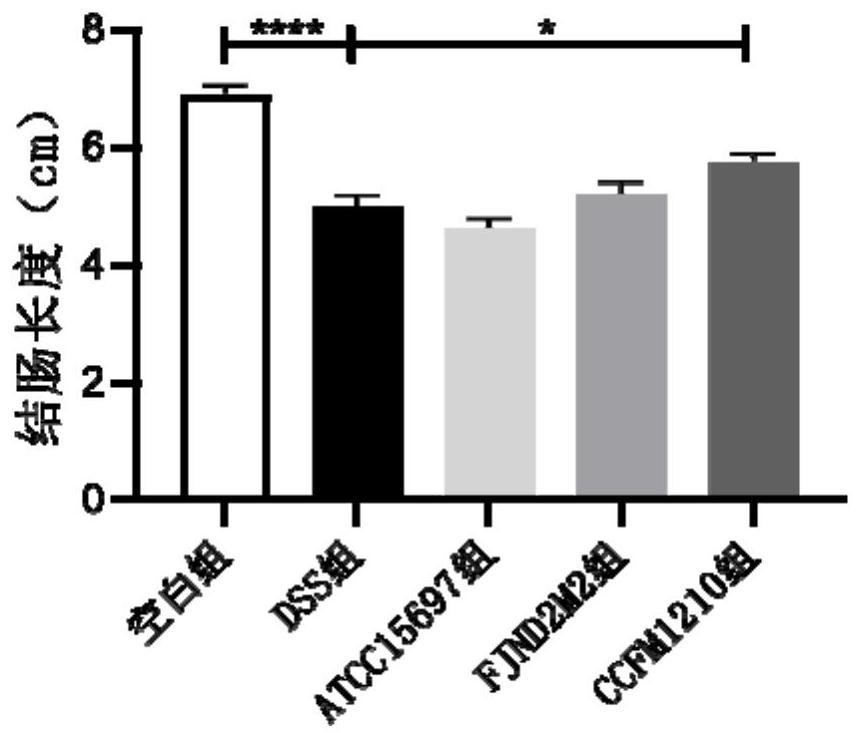
The invention discloses bifidobacterium longum subsp.infantis for relieving colitis and application, and belongs to the technical field of biology. The bifidobacterium longum subsp. Infantis CCFM1210 provided by the invention has the immunoregulation capability, and can improve the intestinal barrier and relieve colitis. Animal experiments prove that through intervention of the bifidobacterium longum subsp.infantis CCFM1210, tissue damage of a mouse can be remarkably relieved, the myeloperoxidase level can be remarkably reduced, the content of tight junction protein in colon tissue can be remarkably increased, the levels of proinflammatory cytokines IL-1beta and IL-6 can be remarkably reduced, and the level of an anti-inflammatory cytokine IL-10 can be remarkably increased; the concentration of butyric acid is obviously increased, the diversity of intestinal flora is obviously improved, and the disorder of the flora is relieved. Compared with a traditional medicine for treating colitis, a medicine or a probiotic preparation prepared from the bifidobacterium longum subsp. Infantis CCFM1210 disclosed by the invention has certain advantages and has a wide market prospect.
Owner:JIANGNAN UNIV
Antibodies specific for claudin 6 (CLDN6)
ActiveUS20160222125A1Improve survivalMinimize adverse effectsPeptide/protein ingredientsGenetic material ingredientsDiseaseMelanoma
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing Claudin-6 (CLDN6), including tumor-related diseases such as ovarian cancer, lung cancer, gastric cancer, breast cancer, hepatic cancer, pancreatic cancer, skin cancer, malignant melanoma, head and neck cancer, sarcoma, bile duct cancer, cancer of the urinary bladder, kidney cancer, colon cancer, placental choriocarcinoma, cervical cancer, testicular cancer, and uterine cancer.
Owner:JOHANNES GUTENBERG UNIV +1
Application of lactobacillus rhamnosus to preventing and relieving ulcerative colitis
ActiveCN112625964AEasily damagedIncrease richnessMilk preparationBacteriaLactobacillus rhamnosusUlcerative colitis
The invention discloses an application of lactobacillus rhamnosus to preventing and relieving ulcerative colitis, and belongs to the technical field of functional microorganisms. The lactobacillus rhamnosus can tolerate the gastrointestinal environment of a human body, significantly reduce weight loss during the period of ulcerative colitis, improve fecal traits and hematochezia, improve colon mucosal injury, reduce MPO activity, and reduce the content of proinflammatory factors TNF-alpha, IL-1beta, IL-6 and IFN-gamma in colon. The transcription levels of colon close junction related proteins Claudin-3, ZO-1, ZO-2 and Occludin, antibacterial peptides Reg3g and Reg3b and mucoprotein MUC2 are up-regulated, the abundance of short-chain fatty acid producing bacteria Coprococcus and Faecalibacterium and the content of short-chain fatty acid are improved, the abundance of beneficial bacteria Lactobacillus in intestinal tracts is increased, the abundance of the conditional pathogenic bacteria Acinetobacter is reduced, and the abundance and diversity of intestinal flora are increased.
Owner:JIANGNAN UNIV
Antibody combined with tight junction protein-18.2 and application of antibody
The present disclosure relates to an antibody or an antigen-binding portion thereof combined with a tight junction protein-18. 2 and application of the antibody or the antigen-binding portion thereof.The antibody can be combined with the tight junction protein-18. 2 with high affinity, and can be used for detecting the tight junction protein-18. 2, and diagnosing, treating or preventing diseasesrelated to cells of the tight junction protein-18. 2.
Owner:CHENGDU CONMED BIOSCI CO LTD +2
Monoclonal anti-claudin 1 antibodies for the inhibition of hepatitis C virus infection
ActiveUS8518408B2Preventing HCV infectionLess susceptible to developmentAnimal cellsPeptide/protein ingredientsExtracellular StructureHybridoma cell
The present invention provides monoclonal antibodies that specifically bind to the extracellular domain of human Claudin-1 on the cell surface, thereby inhibiting HCV entry into susceptible cells and preventing HCV infection of these cells; and hybridoma cell lines which produce such monoclonal antibodies. Also provided are reagents that comprise such antibodies, and pharmaceutical compositions comprising such antibodies. Methods of treating or preventing HCV infection by administration of an inventive monoclonal antibody, or a pharmaceutical composition thereof are also described.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
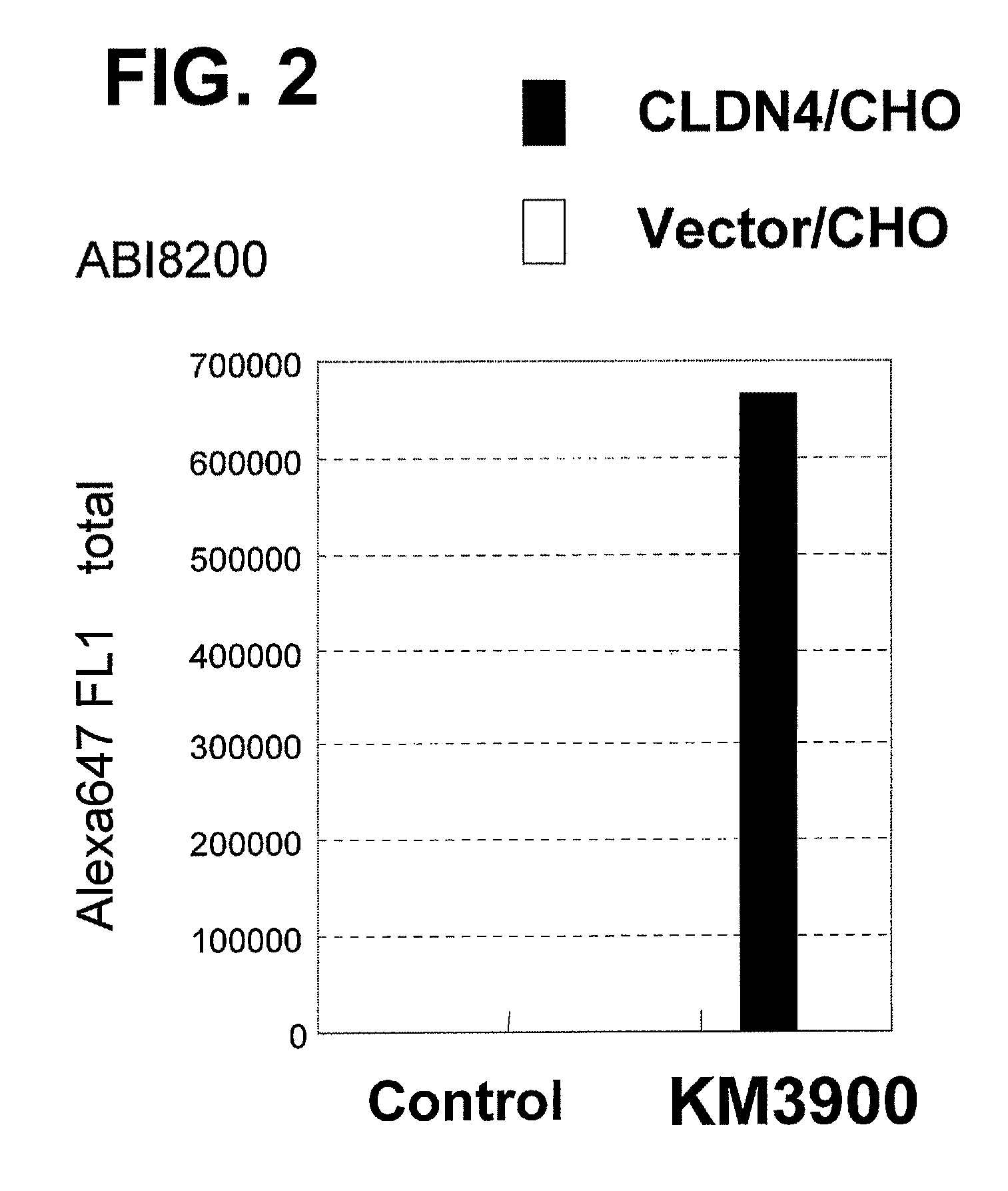
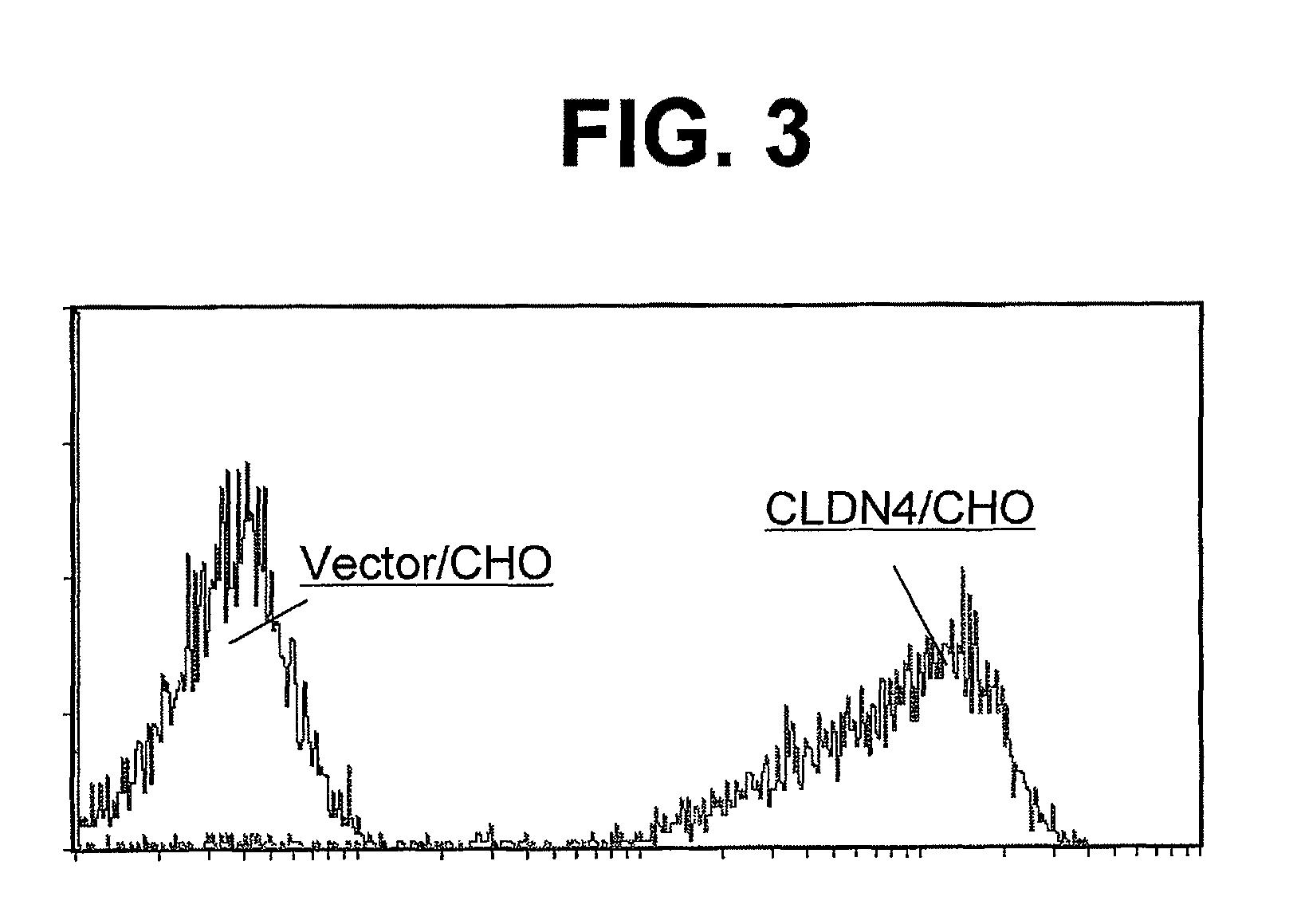
Anti-claudin-4 antibody
An object of the present invention is to provide a monoclonal antibody which is useful as a diagnostic agent or a therapeutic agent for a disease relating to a polypeptide encoded by Claudin-4 (hereinafter referred to as “CLDN4”) gene or a polypeptide encoded by a Claudin-3 (hereinafter referred to as “CLDN3”) gene, or a method for using the same. Accordingly, the present invention provides a monoclonal antibody or an antibody fragment thereof, which specifically recognizes three-dimensional structure of an extracellular region of CLDN4 and binds to the extracellular region; a monoclonal antibody or an antibody fragment thereof, which specifically recognizes three-dimensional structure of both of extracellular regions of CLDN3 and CLDN4 and binds to the extracellular regions; a hybridoma which produces the antibody; a DNA which encodes the antibody; a vector which comprises the DNA; a transformant obtained by transforming the vector; a process for producing an antibody or an antibody fragment thereof using the hybridoma or the transformant; and a diagnostic agent or a therapeutic agent for a disease relating to a polypeptide encoded by CLDN4 gene and / or CLDN3 gene using the antibody or the antibody fragment.
Owner:KYOWA HAKKO KIRIN CO LTD
Monoclonal Anti-Claudin 1 Antibodies for the Inhibition of Hepatitis C Virus Infection
ActiveUS20110236347A1Neutralize HCV infectivityLess susceptible to developmentAnimal cellsPeptide/protein ingredientsExtracellular StructureHybridoma cell
The present invention provides monoclonal antibodies that specifically bind to the extracellular domain of human Claudin-1 on the cell surface, thereby inhibiting HCV entry into susceptible cells and preventing HCV infection of these cells; and hybridoma cell lines which produce such monoclonal antibodies. Also provided are reagents that comprise such antibodies, and pharmaceutical compositions comprising such antibodies. Methods of treating or preventing HCV infection by administration of an inventive monoclonal antibody, or a pharmaceutical composition thereof are also described.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Bifidobacterium capable of relieving colitis and application thereof
The invention discloses a rapid screening method and application of bifidobacterium capable of relieving colitis and belongs to the technical field of biology. The bifidobacterium pseudocatenulatum is preserved in China Center for Type Culture Collection (CCTCC), Wuhan, on July 5, 2021, and the preservation number is CCTCC M2021820. The bifidobacterium pseudocatenulatum can be used for significantly improving weight loss, colon length and disease activity index of mice during DSS-inducing colitis, enhancing intestinal barrier integrity, and inhibiting tissue damage, myeloperoxidase (MPO) activity, PGE2 level and proinflammatory factors. Compared with mice of a model group, the bifidobacterium pseudocatenulatum KLDS N2 has the advantages that the expression of proinflammatory cytokine mRNA of a DSS stimulated mouse is obviously down-regulated, and the expression of mRNA of mucoprotein and tight junction protein is up-regulated.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Claudin-18.2-specific immunoreceptors and t cell epitopes
ActiveUS20180282389A1Minimize adverse effectsMinimizing chancePolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsSpecific immunity
Owner:BIONTECH CELL & GENE THERAPIES +1
Norrin regulation of junction proteins and the use thereof to treat epithelial or endothelial membrane leakage induced edema
A method of tightening inter-cellular junctions in endothelial or epithelial cells includes exposing the endothelial or epithelial cells cells to norrin. Upon sufficient contact time, for norrin to selectively up- regulate gene expression of Cadherin or claudin-5 in the endothelial or epithelial cells, the inter-cellular junctions are tightened.
Owner:RETINAL SOLUTIONS LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com