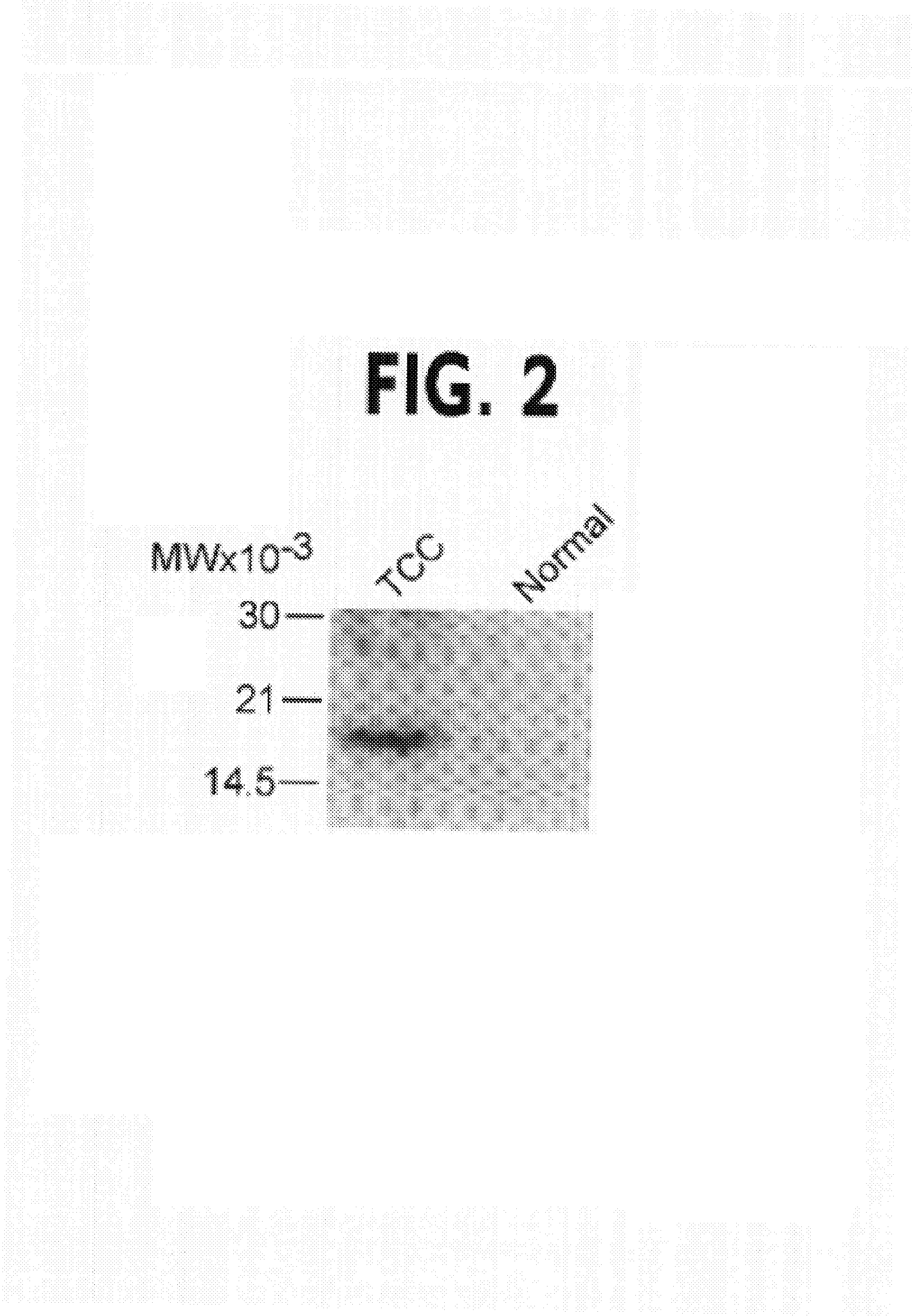
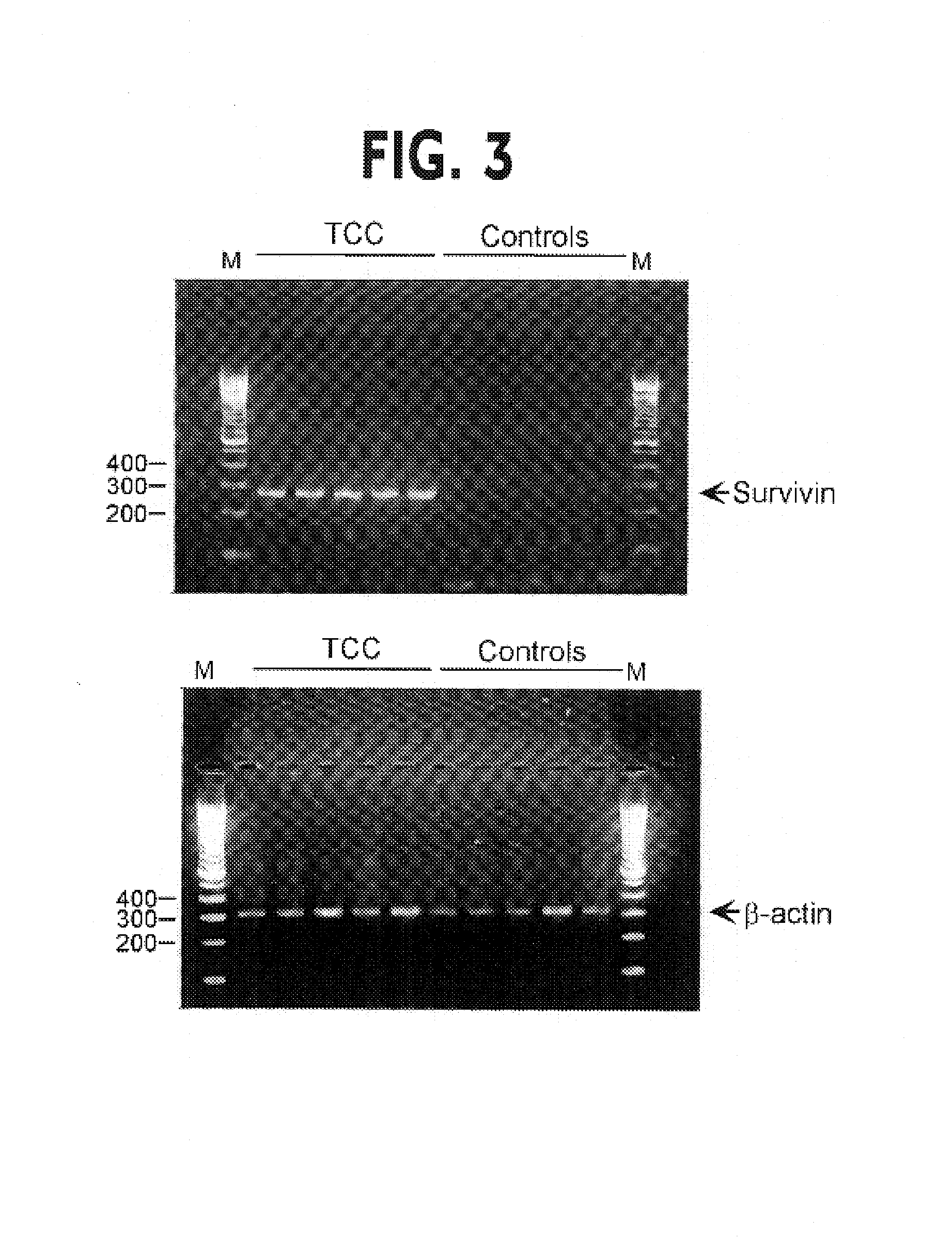
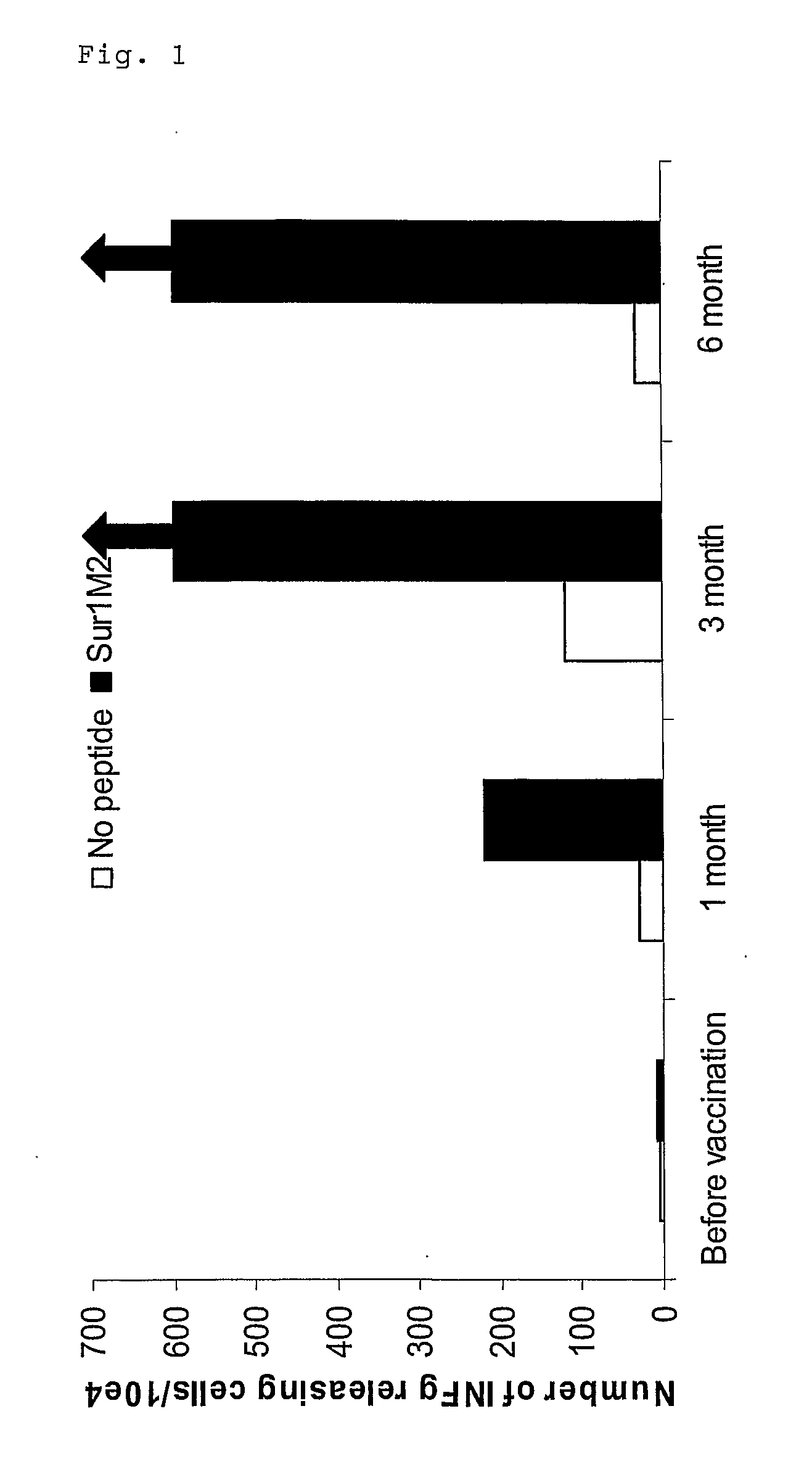
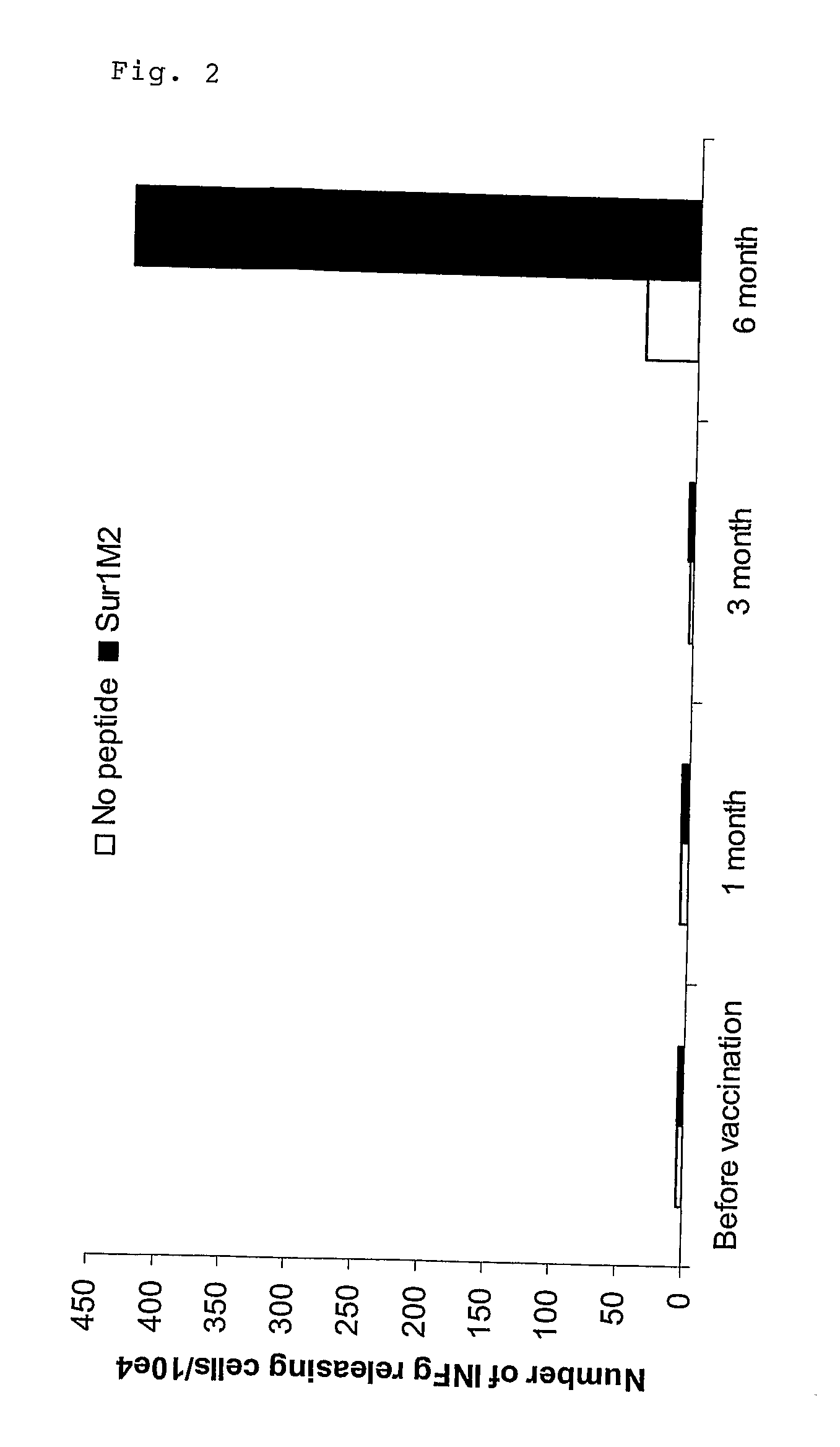
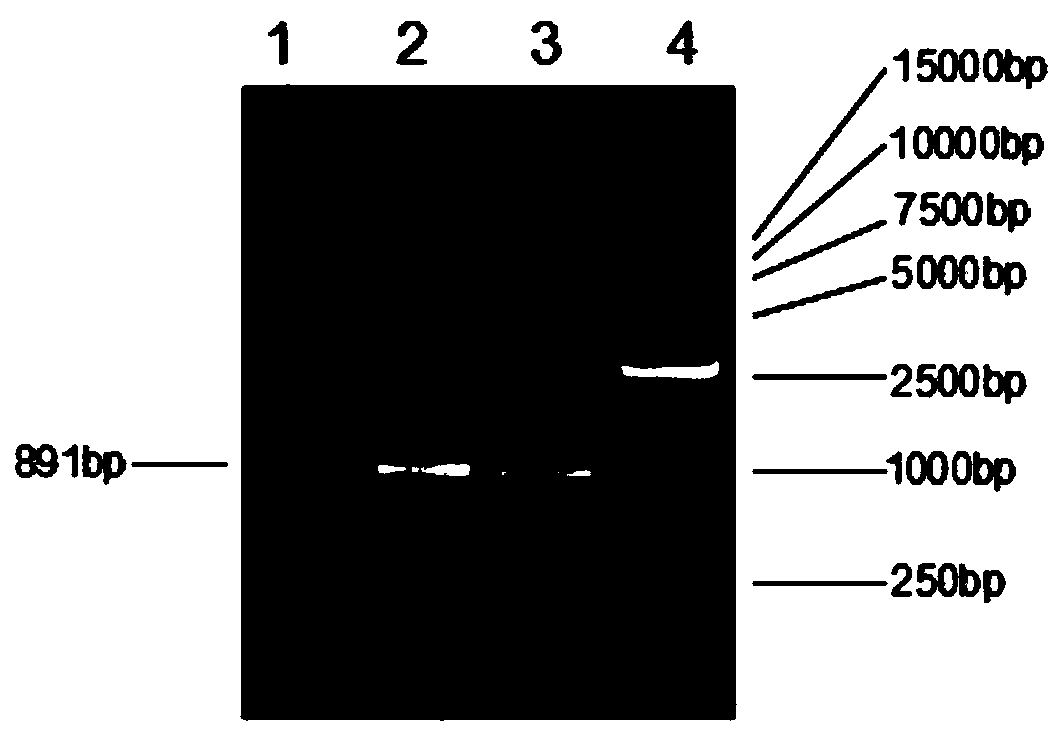
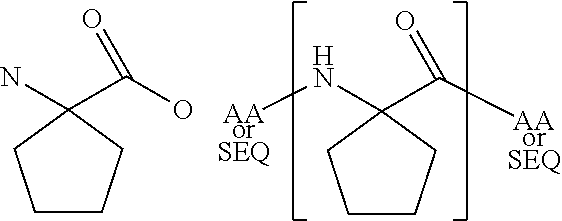
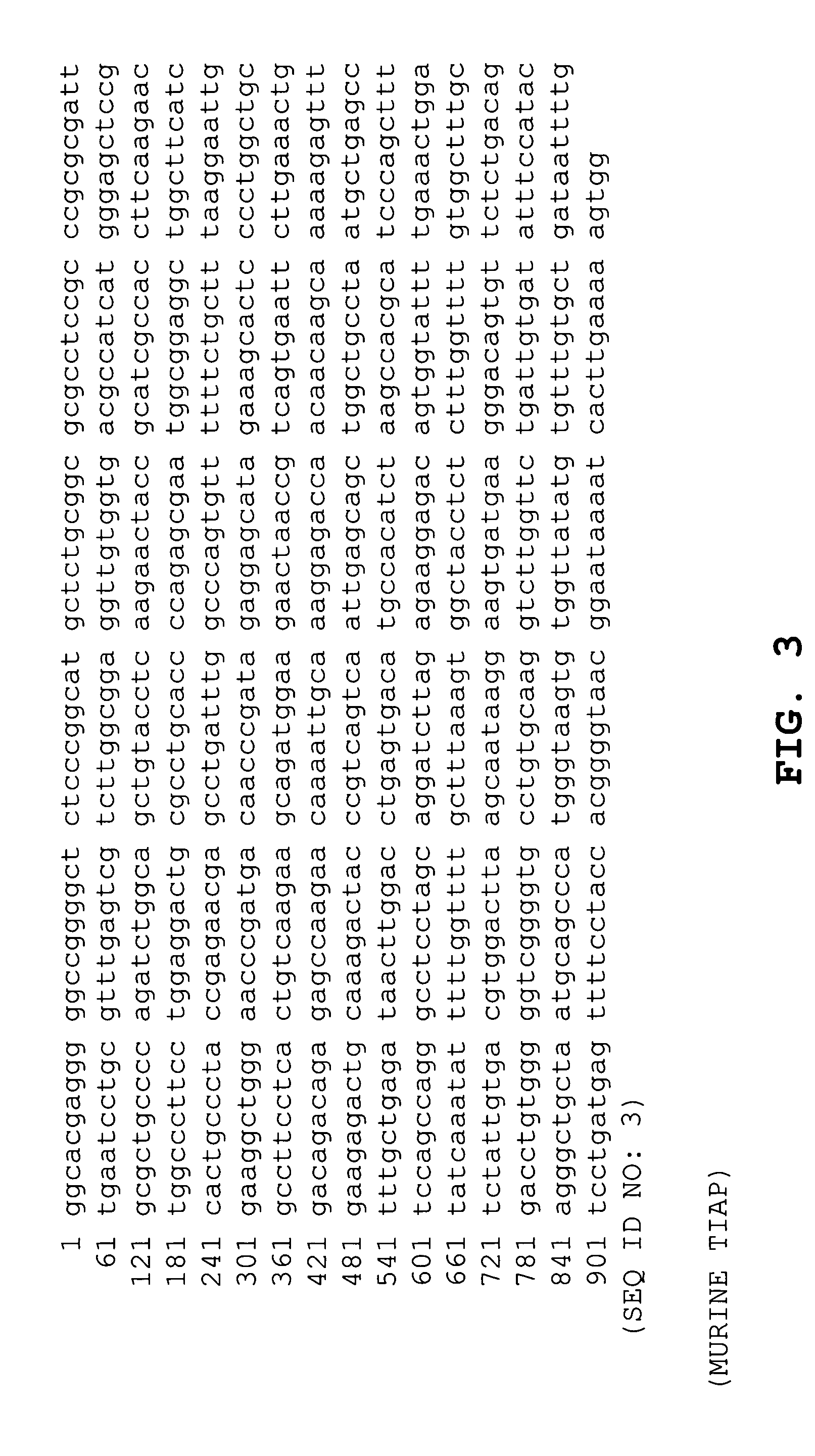
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
231 results about "Survivin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5, is a protein that, in humans, is encoded by the BIRC5 gene. Survivin is a member of the inhibitor of apoptosis (IAP) family. The survivin protein functions to inhibit caspase activation, thereby leading to negative regulation of apoptosis or programmed cell death. This has been shown by disruption of survivin induction pathways leading to increase in apoptosis and decrease in tumour growth. The survivin protein is expressed highly in most human tumours and fetal tissue, but is completely absent in terminally differentiated cells. These data suggest survivin might provide a new target for cancer therapy that would discriminate between transformed and normal cells. Survivin expression is also highly regulated by the cell cycle and is only expressed in the G2-M phase. It is known that Survivin localizes to the mitotic spindle by interaction with tubulin during mitosis and may play a contributing role in regulating mitosis. The molecular mechanisms of survivin regulation are still not well understood, but regulation of survivin seems to be linked to the p53 protein. It also is a direct target gene of the Wnt pathway and is upregulated by beta-catenin.
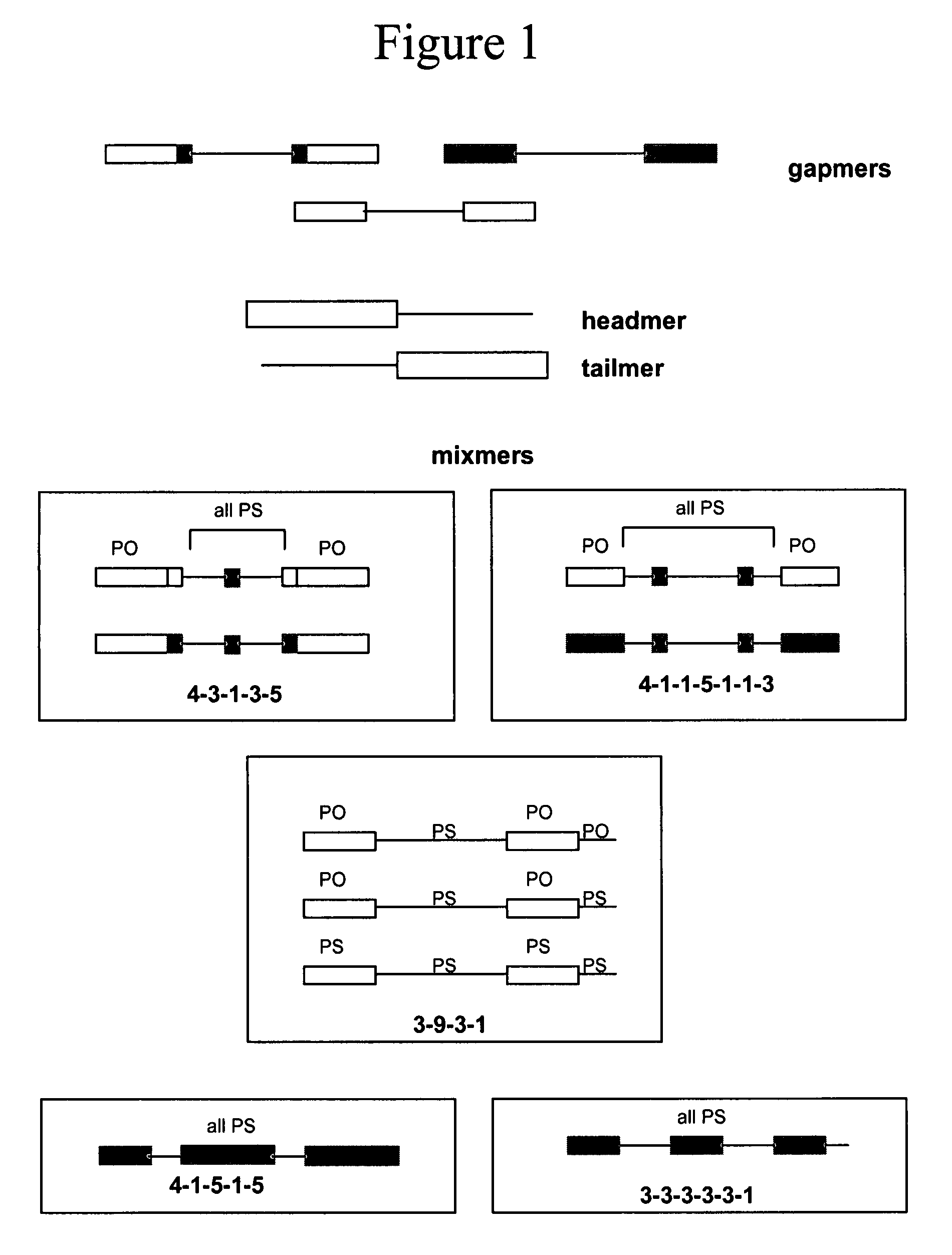
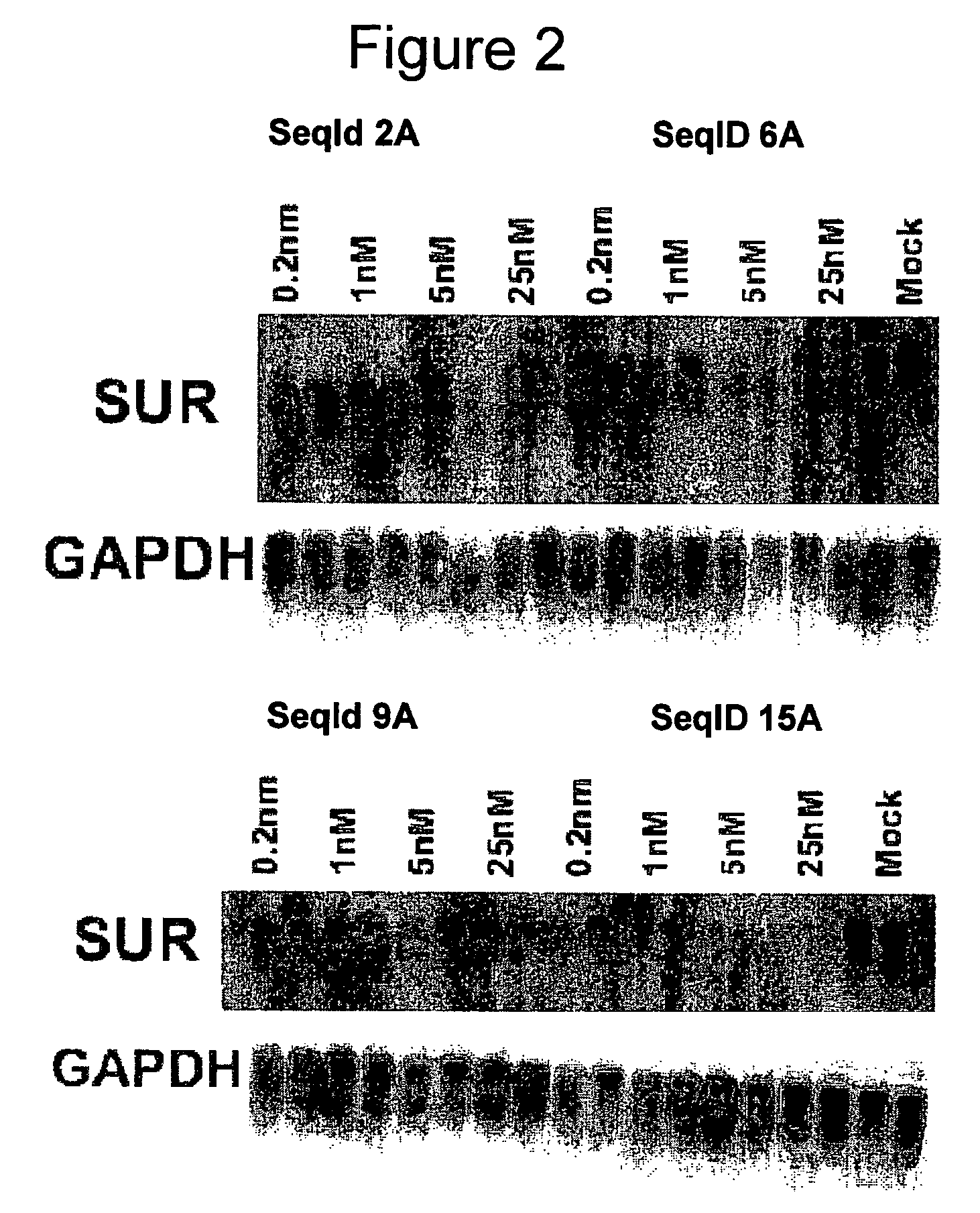
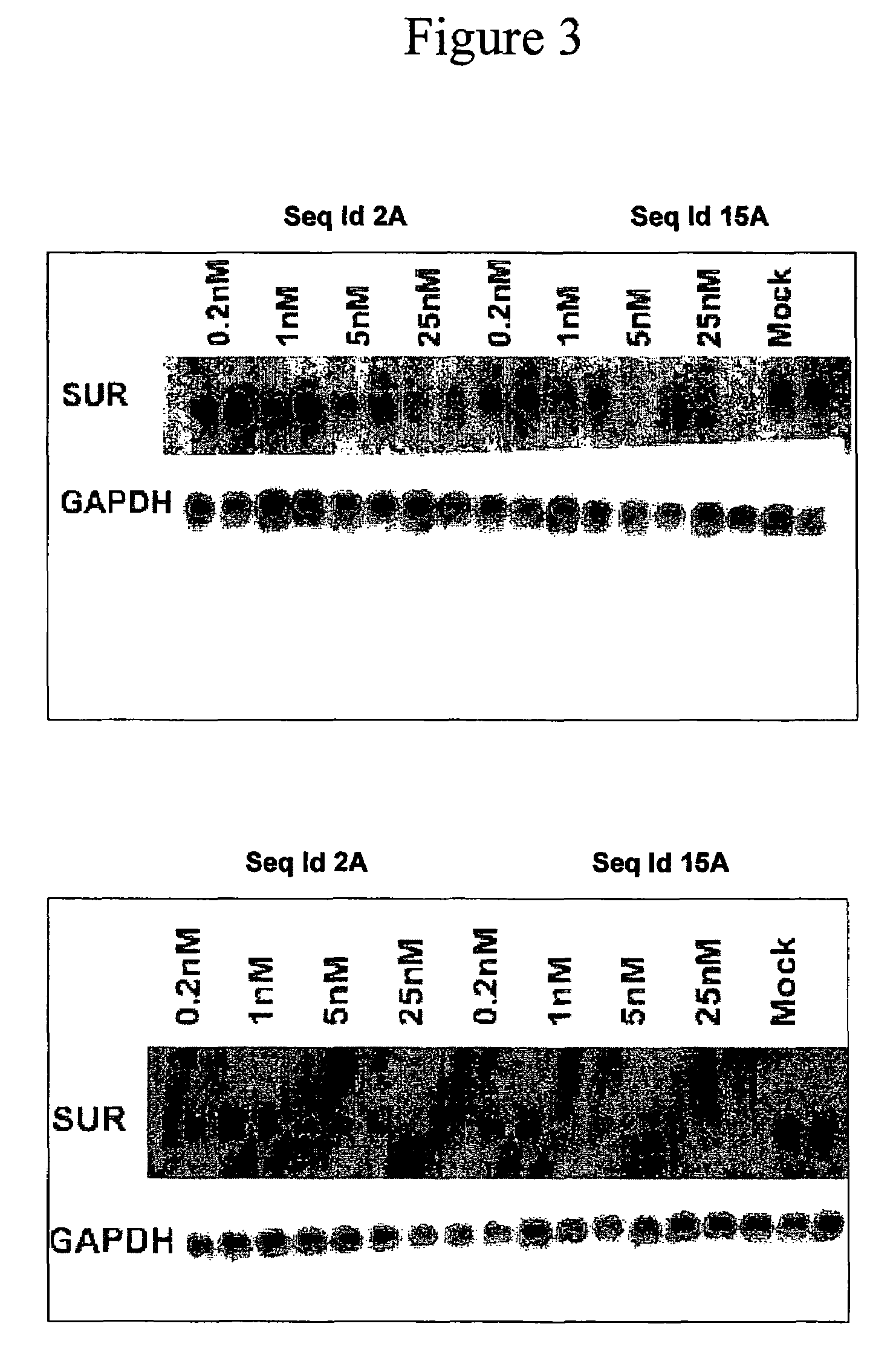
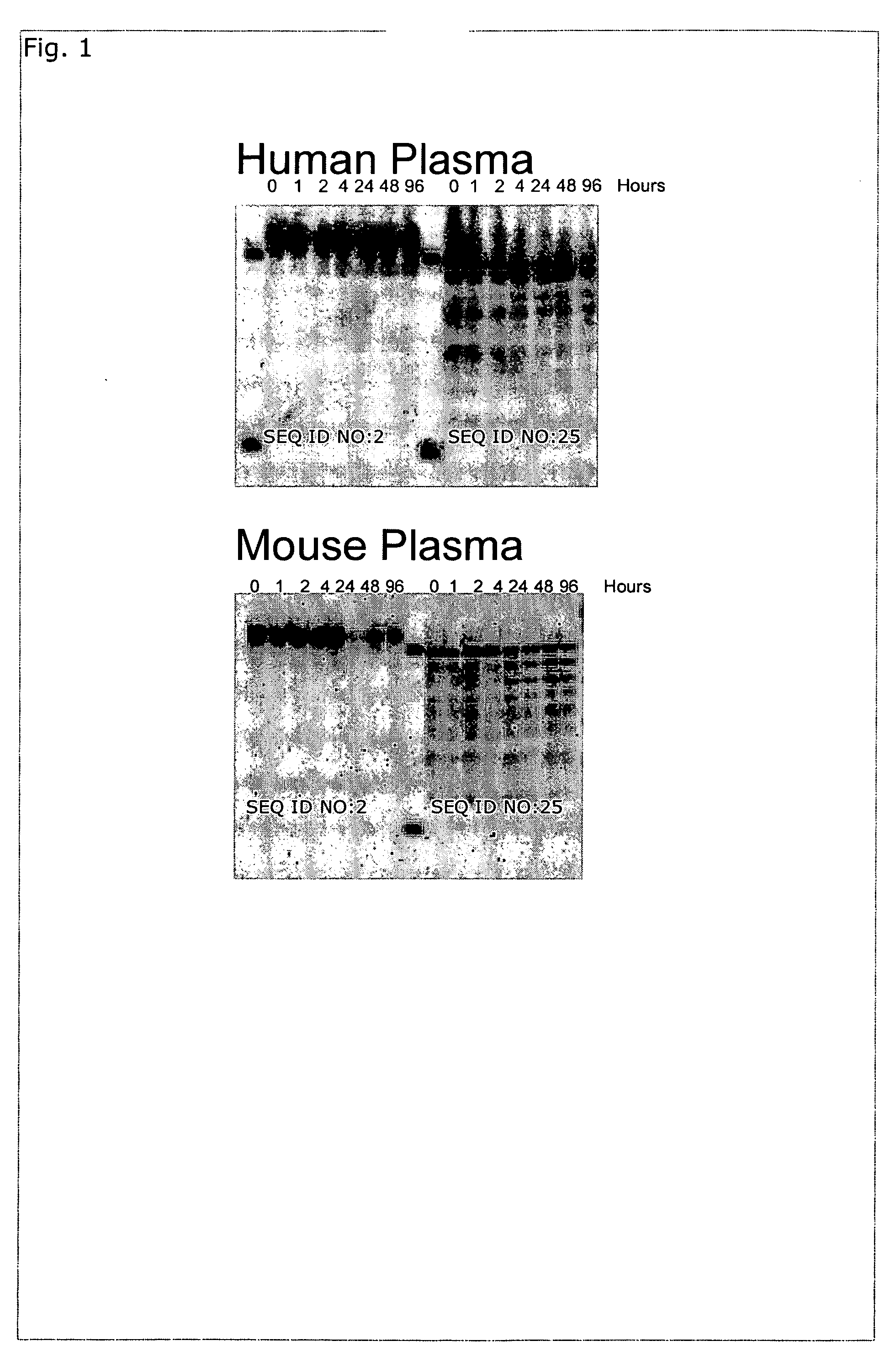
Oligomeric compounds for the modulation of survivin expression
Oligonucleotides directed against the survivin gene are provided for modulating the expression of survivin. The compositions comprise oligonucleotides, particularly antisense oligonucleotides, targeted to nucleic acids encoding the survivin. Methods of using these compounds for modulation of survivin expression and for the treatment of diseases associated with either overexpression of survivin, expression of mutated survivin or both are provided. Examples of diseases are cancer such as lung, breast, colon, prostate, pancreas, lung, liver, thyroid, kidney, brain, testes, stomach, intestine, bowel, spinal cord, sinuses, bladder, urinary tract or ovaries cancers. The oligonucleotides may be composed of deoxyribonucleosides or a nucleic acid analogue such as for example locked nucleic acid or a combination thereof.
Owner:ENZON PHARM INC
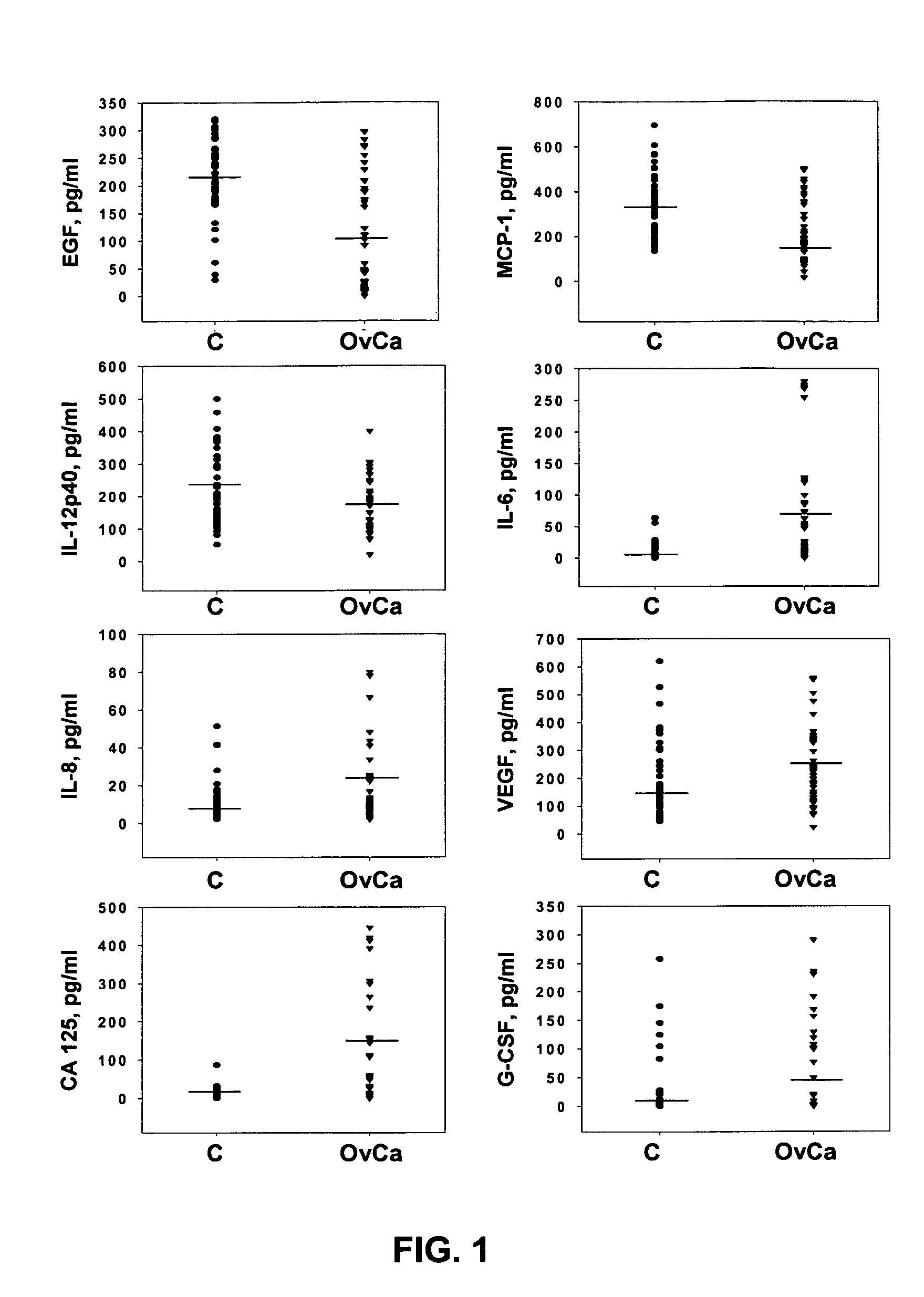
Multifactorial assay for cancer detection
InactiveUS20050069963A1Rapid and early detectionPeptide/protein ingredientsMicrobiological testing/measurementAnti her2Anti-MUC-1
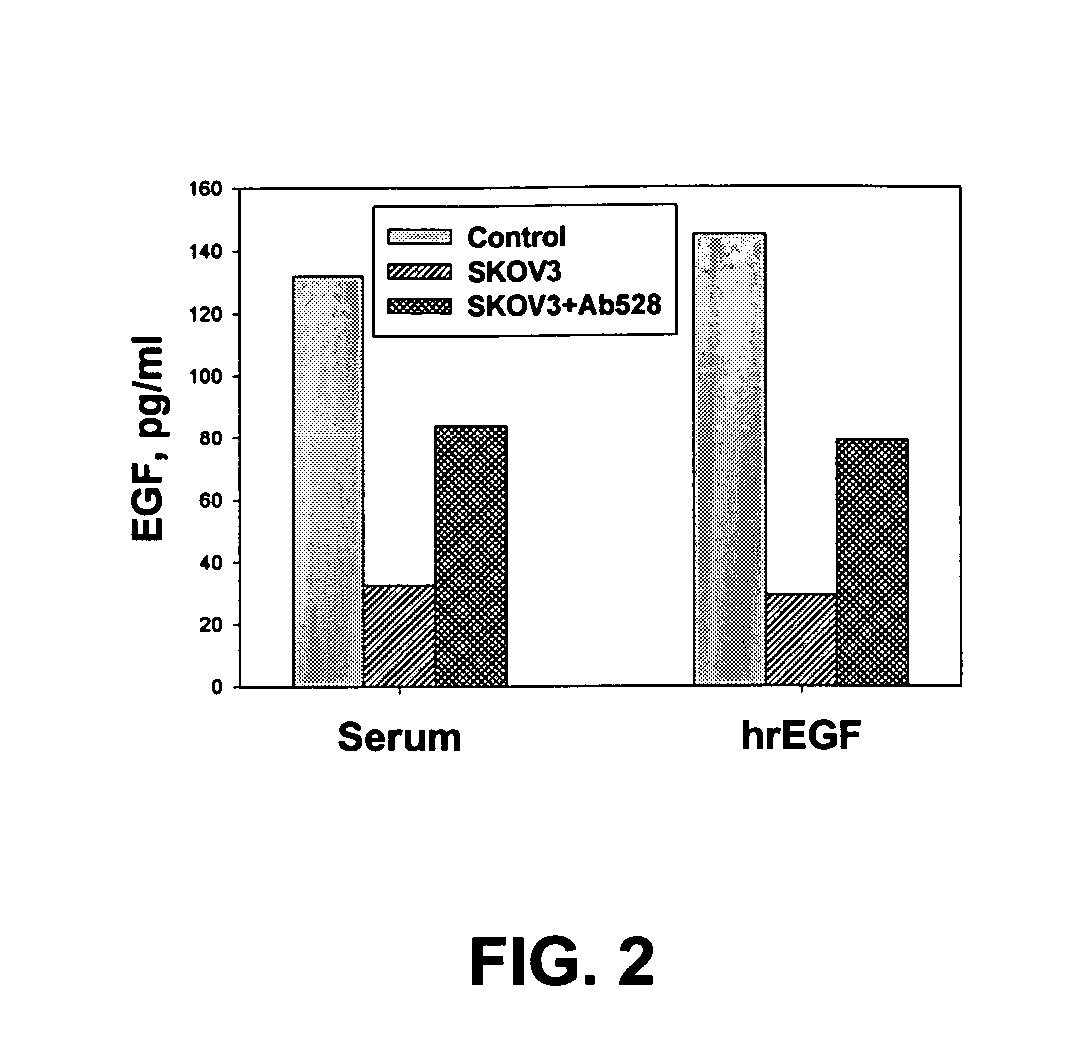
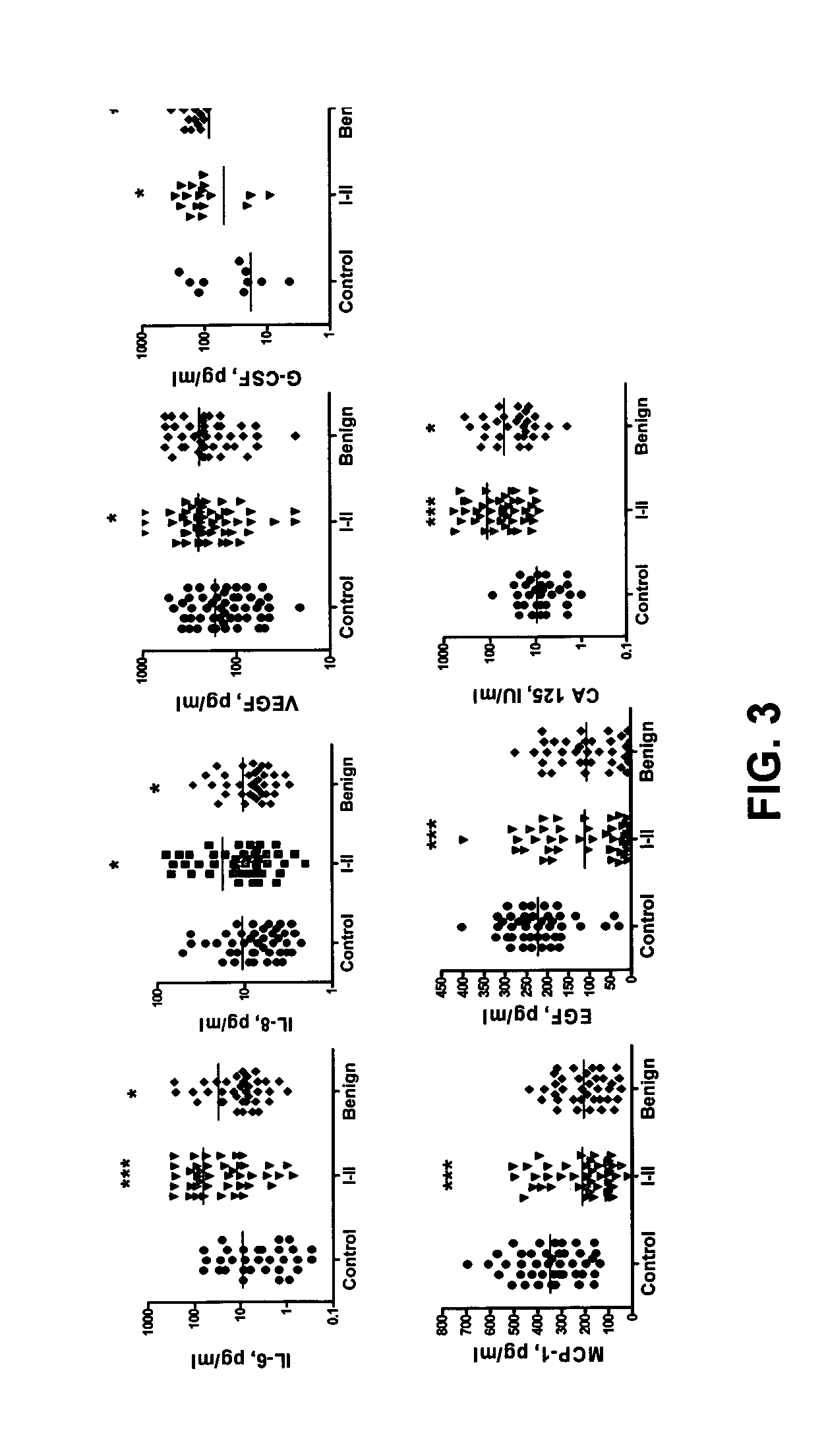
Provided are methods for the rapid detection of ovarian cancer. The methods employ a multiplex immunoassay to detect levels of two or more of the markers EGF, G-CSF, IL-6, IL-8, CA-125, VEGF, MCP-1, anti-IL6, anti-IL8, anti CA-125, anti-c-myc, anti-p53, anti-CEA, anti-CA 15-3, anti-MUC-1, anti-survivin, anti-bHCG, anti-osteopontin, anti-PDGF, anti-Her2 / neu, anti-Akt1, anti-cytokeratin 19, cytokeratin 19, EGFR, CEA, kallikrein-8, M-CSF, FasL, ErbB2 and Her2 / neu in a sample of the patient's blood, where the presence of abnormal levels of two or more of the markers indicates the presence of ovarian cancer in the patient. An array also is provided to quantitate levels of these markers in a patient's blood. Also provided is a method of predicting onset of clinical ovarian cancer comprising determining the change in concentration over time of two or more of anti-Her2 / neu, anti-MUC-1, anti-c-myc, anti-p53, anti-CA-125, anti-CEA, anti-CA 72-4, anti-PDGFRα, IFNγ, IL-6, IL-10, TNFα, MIP-1α, MIP-1β, EGFR and Her2 / neu in a patient's blood.
Owner:UNIVERSITY OF PITTSBURGH
Survivin, a protein that inhibits cellular apoptosis and its modulation
The present invention provides the amino acid of a protein that inhibits cellular apoptosis, herein termed the Survivin protein and nucleic acid molecules that encode Survivin. Based on this disclosure, the present invention provides isolated Survivin protein, isolated Survivin encoding nucleic acid molecules, methods of isolating other members of the Survivin family of proteins, methods for identifying agents that block Survivin mediated inhibition of cellular apoptosis, methods of using agents that block Survivin mediated inhibition or Survivin expression to modulate biological and pathological processes, and methods of assaying Survivin activity.
Owner:YALE UNIV
Lna oligonucleotides and the treatment of cancer
Owner:ENZON PHARM INC
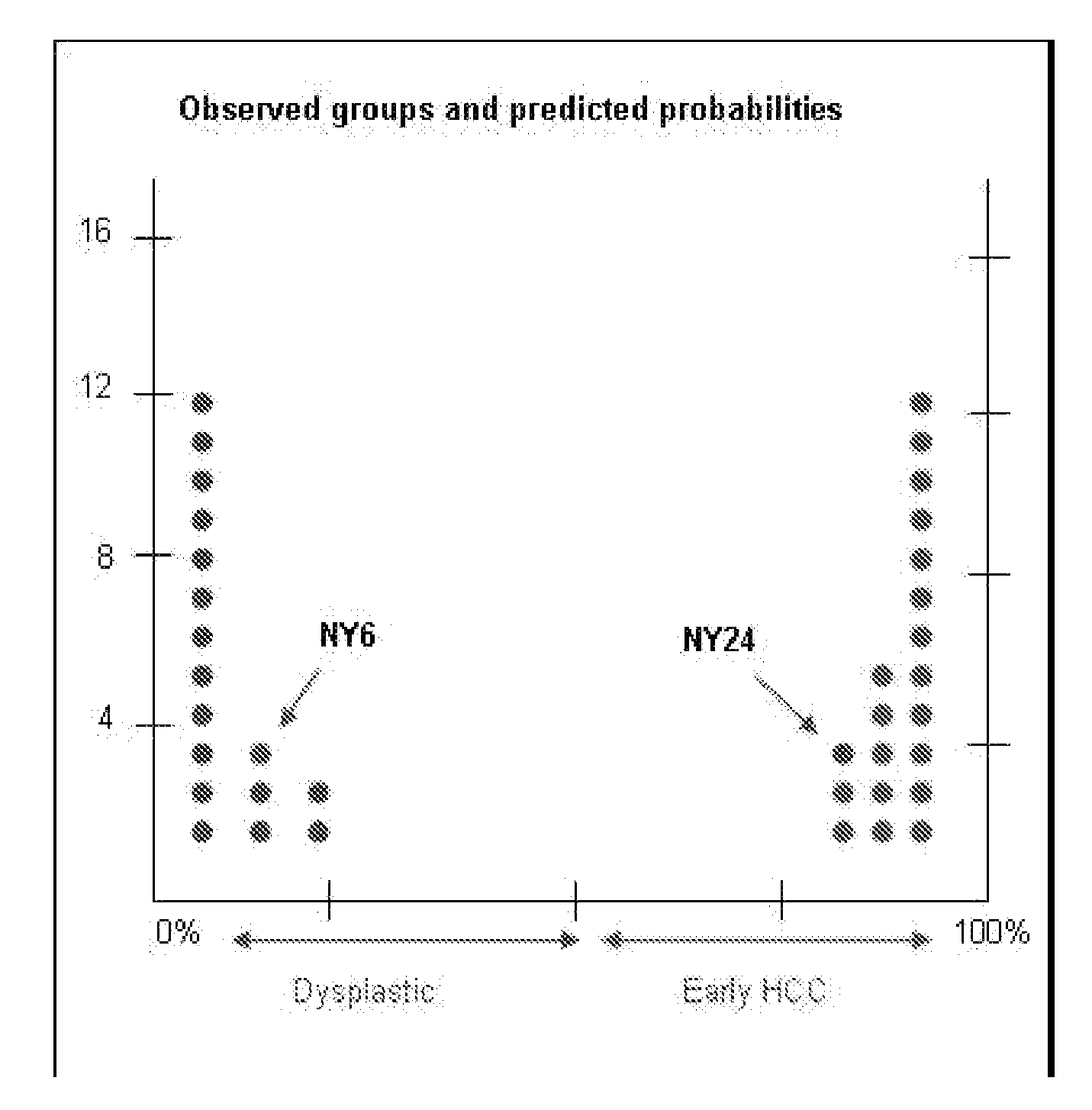
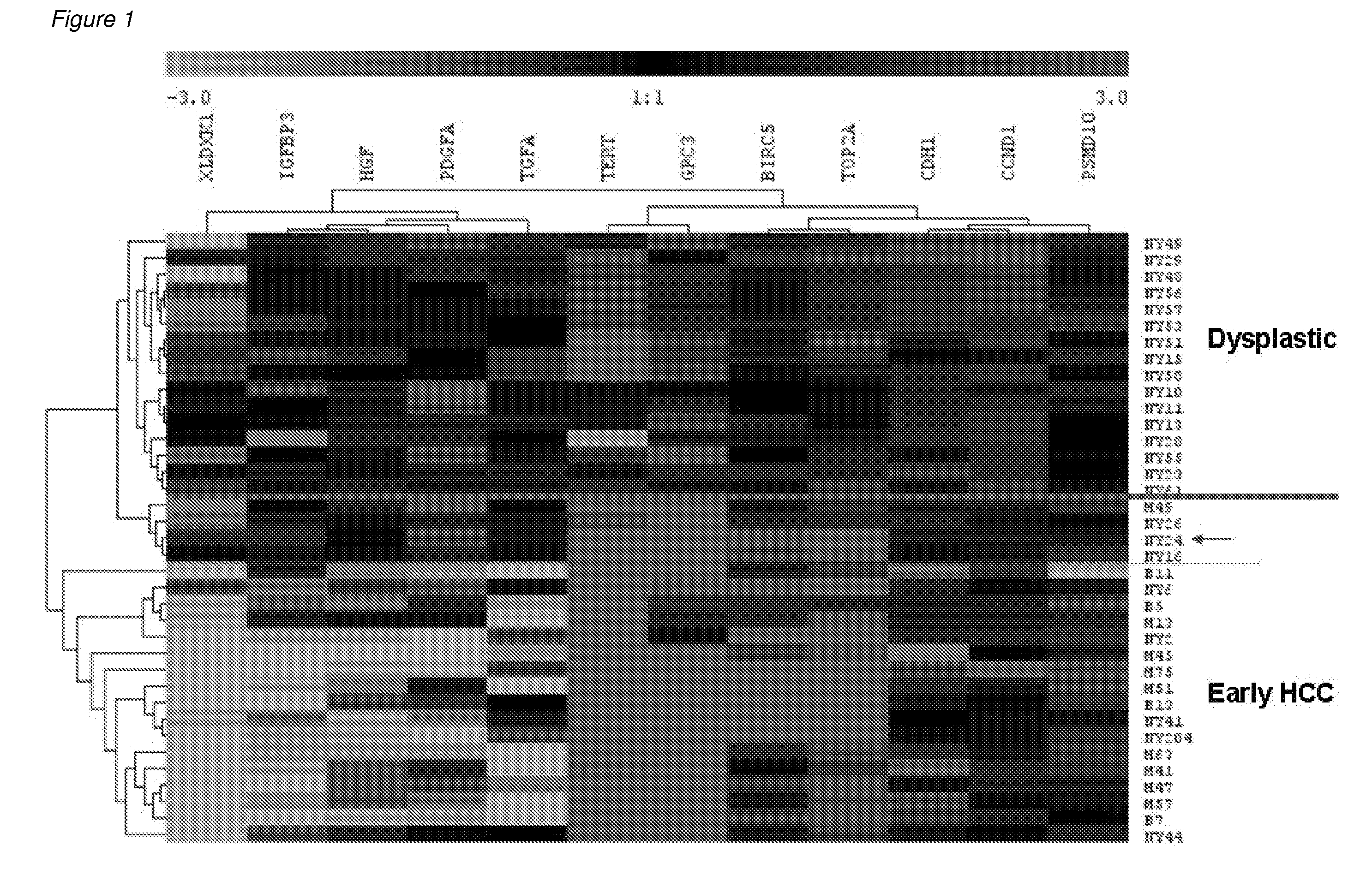
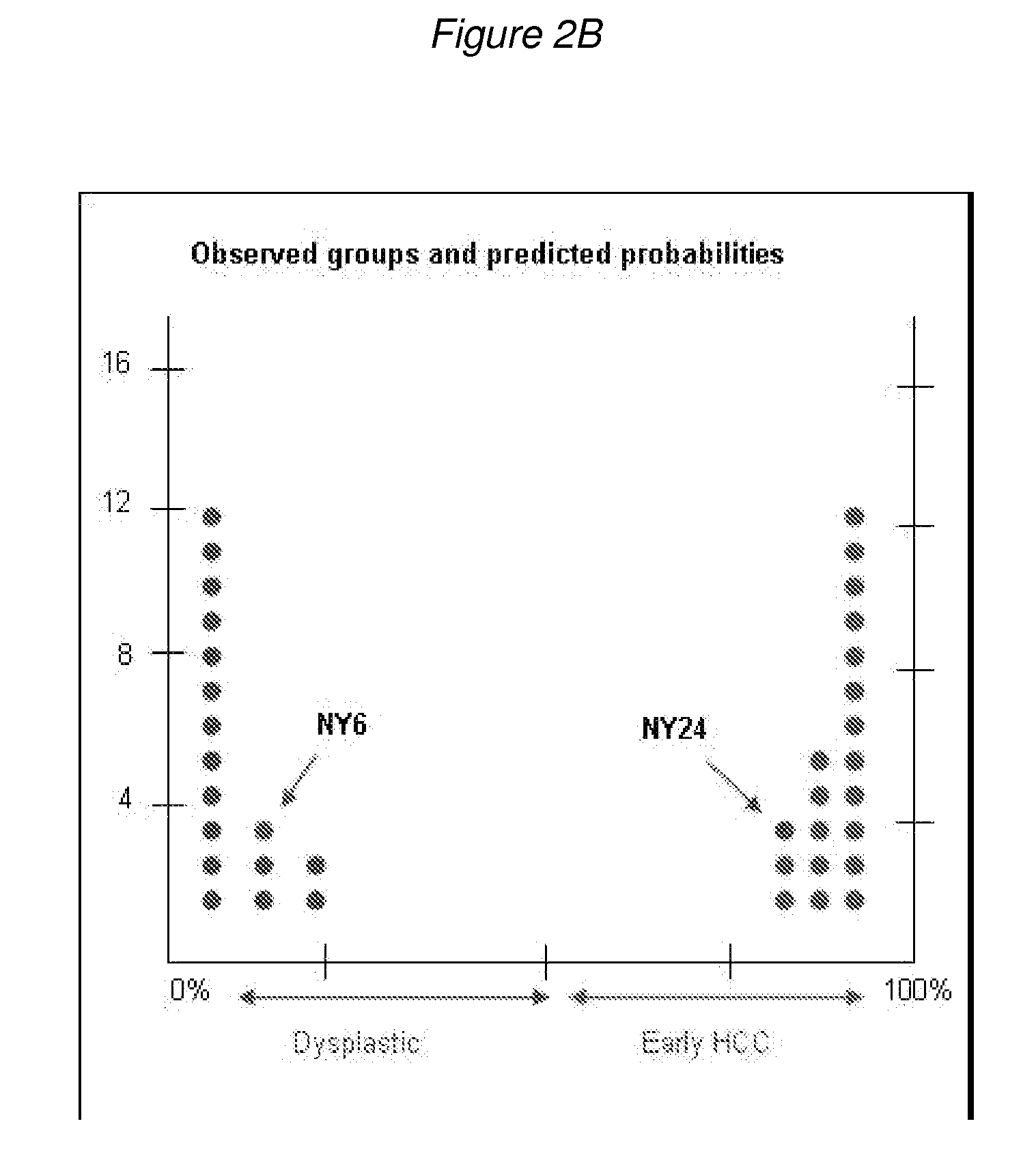
Methods and compositions for the diagnosis for early hepatocellular carcinoma
ActiveUS20080038736A1Microbiological testing/measurementBiological testingCyclin D1Early Hepatocellular Carcinoma
Methods and compositions are provide to allow discrimination of dysplastic nodules from early HCC nodules. More specifically, it has been determined that TERT, GPC3, gankyrin, survivin, TOP2A, LYVE1, Ecadherin, IGFBP3, PDGFRA, TGFA, cyclin D1 and HGF are differentially expressed in HCC as compared to normal liver cells and liver cells that have dysplastic, non-cancerous nodules.
Owner:MT SINAI SCHOOL OF MEDICINE
Antisense modulation of survivin expression
Antisense compounds, compositions and methods are provided for modulating the expression of Survivin. The compositions comprise antisense compounds, particularly antisense oligonucleotides, targeted to nucleic acids encoding Survivin. Methods of using these compounds for modulation of Survivin expression and for treatment of diseases associated with expression of Survivin are provided.
Owner:IONIS PHARMA INC
Enhancing Class I Antigen Presentation With Synthetic Sequences
InactiveUS20080206270A1Enhance antigen presentationInduces antitumor and antiviral CTLTumor rejection antigen precursorsCell receptors/surface-antigens/surface-determinantsCancer cellT lymphocyte
The invention relates generally to the treatment and prevention of human cancer and viral diseases. More specifically, this invention relates to development of a new generation of vaccines that rely on eliciting cellular immune responses, specifically induction of cytotoxic T lymphocytes (CTL), against cancer cells and virus-infected cells via administration of a vaccine comprising a fusion peptide or a modified peptide. Such a fusion peptide is composed of an insertion signal sequence and a peptide derived from a tumor antigen or a viral antigen, which improves antigen presentation and induces CTL with higher efficiency against cancer cells and virus-infected cells. An exemplary antigen utilized in the invention is HER2 / neu. The peptides peptide vaccines of the invention are derived from the antigens PRAME, OFA / iLRP, STEAP and SURVIVIN.
Owner:RGT UNIV OF CALIFORNIA
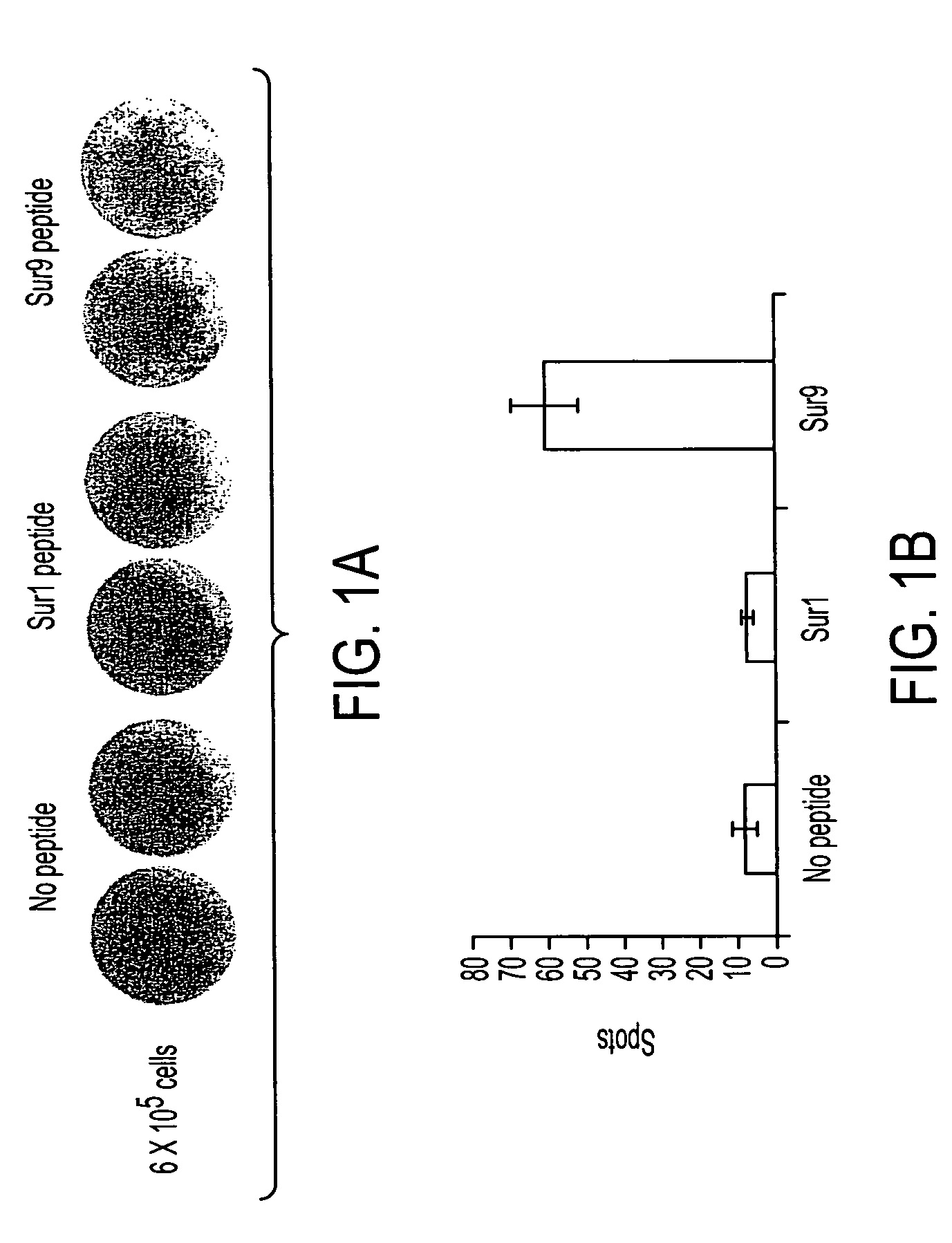
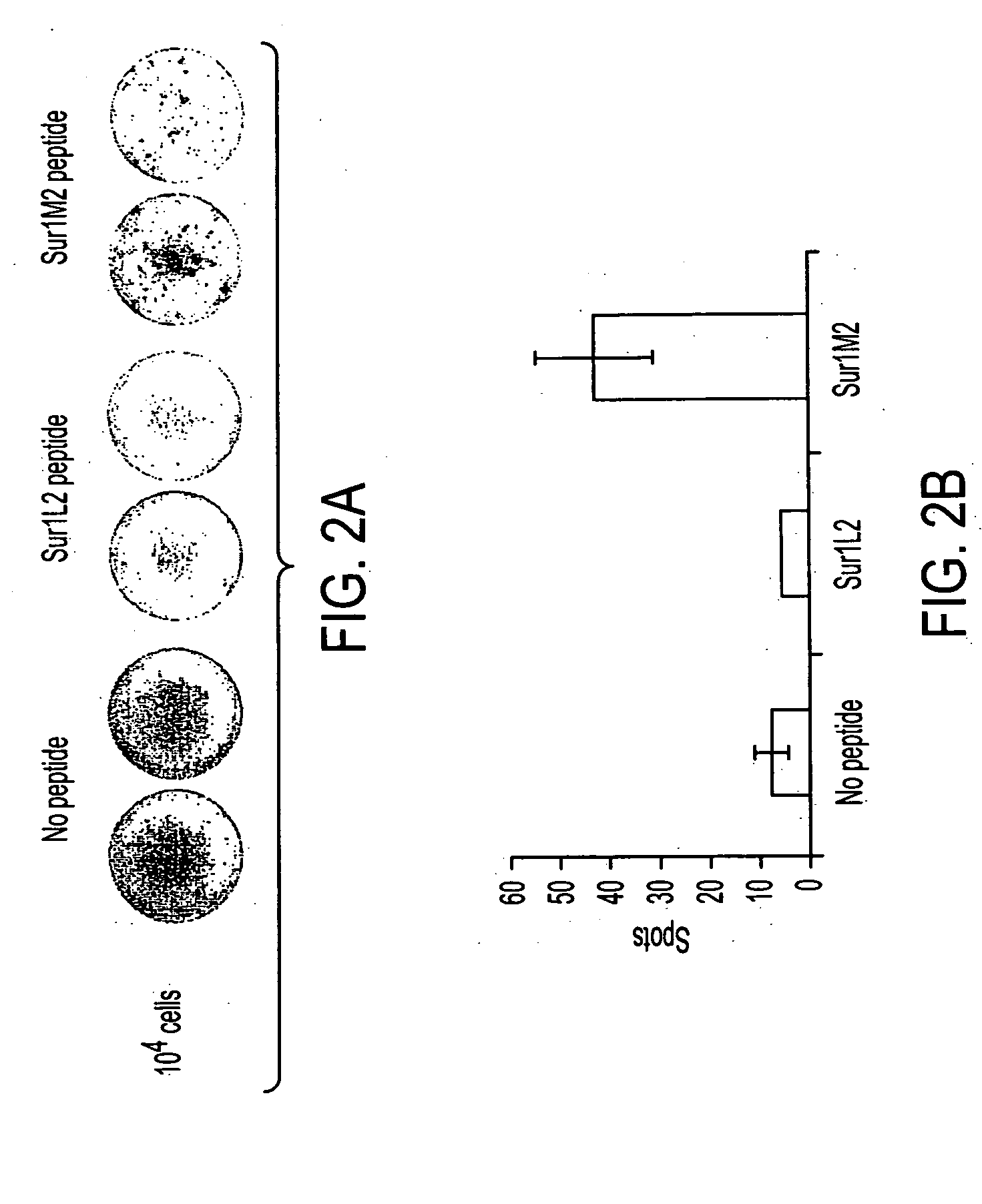
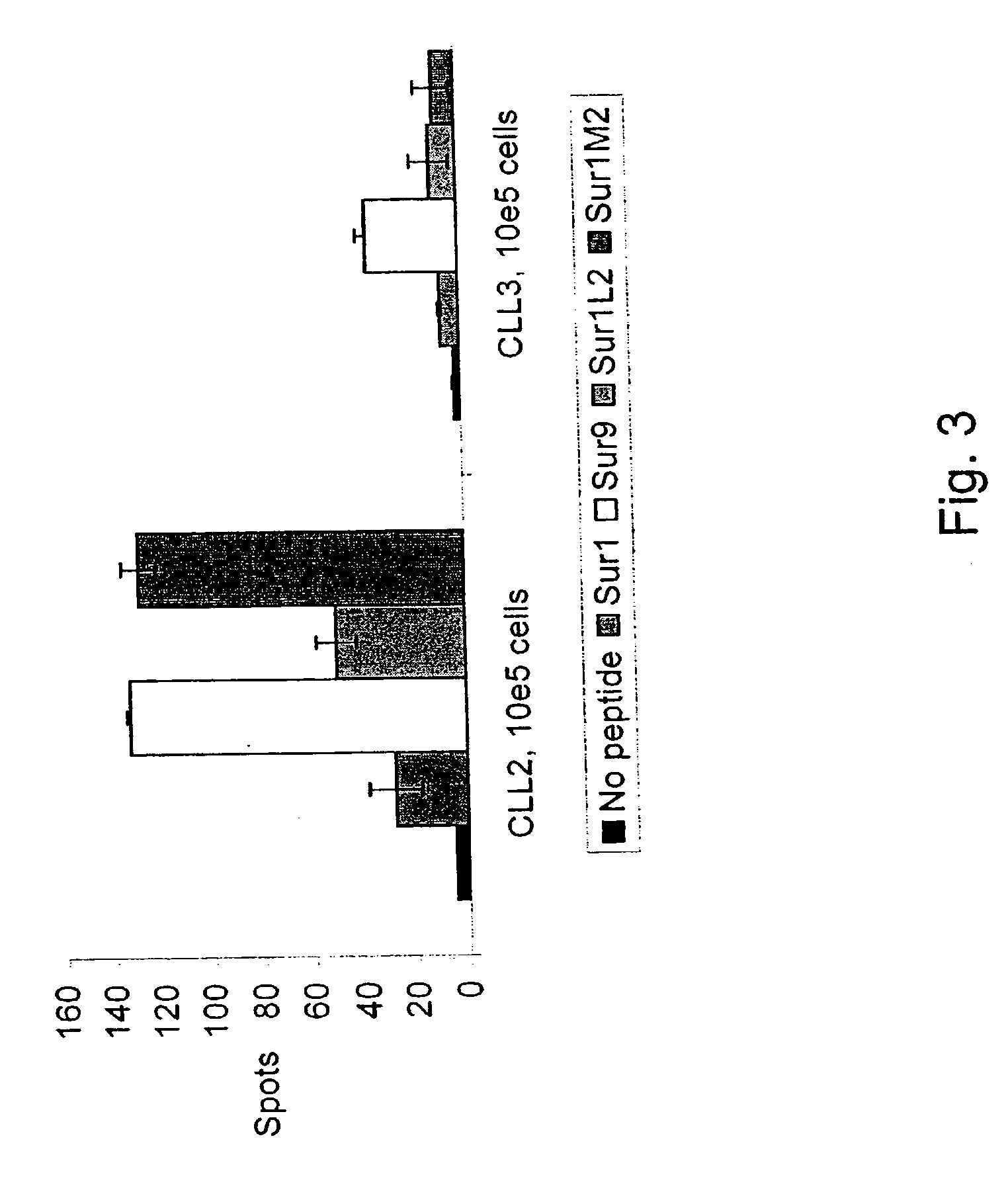
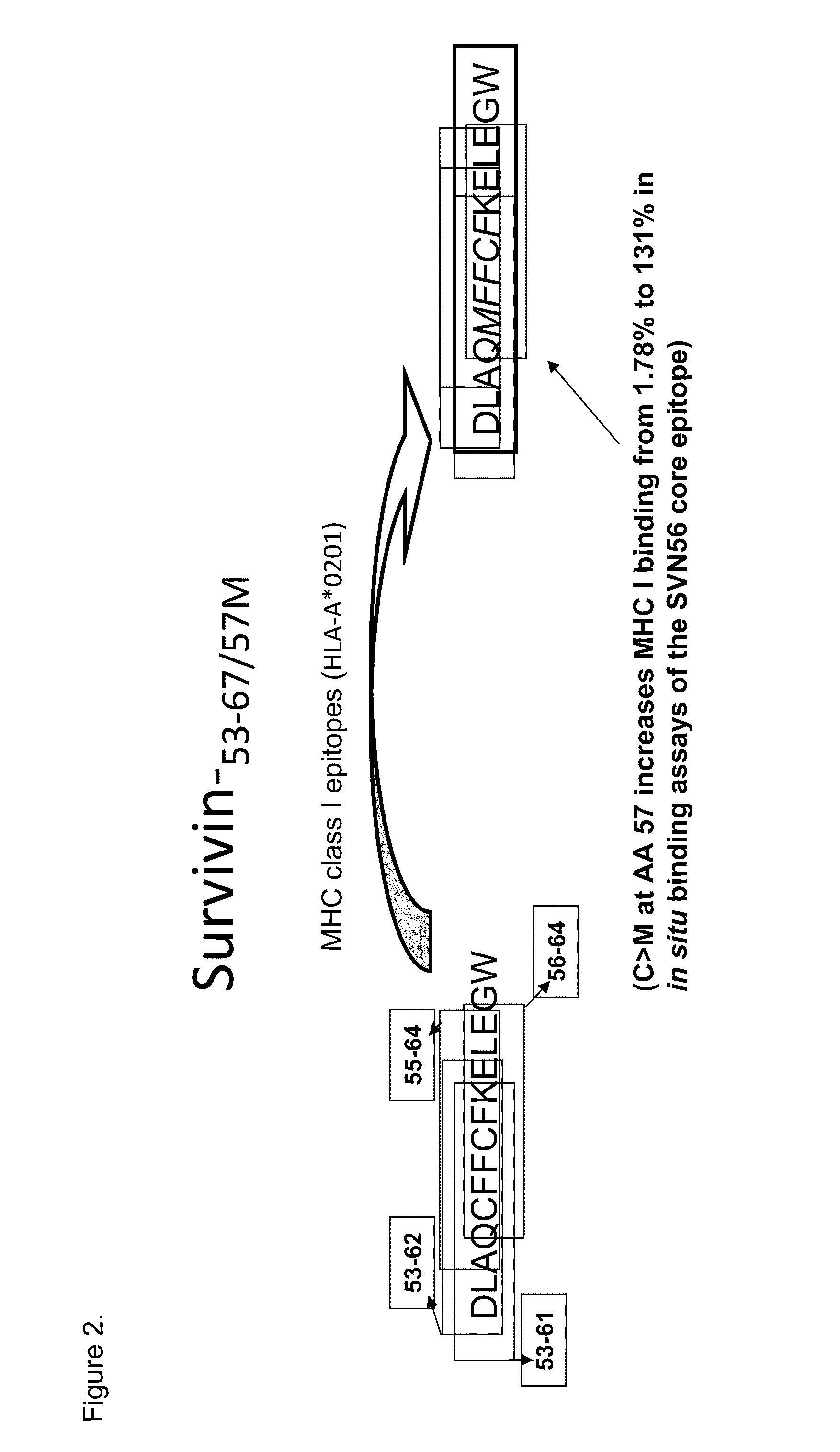
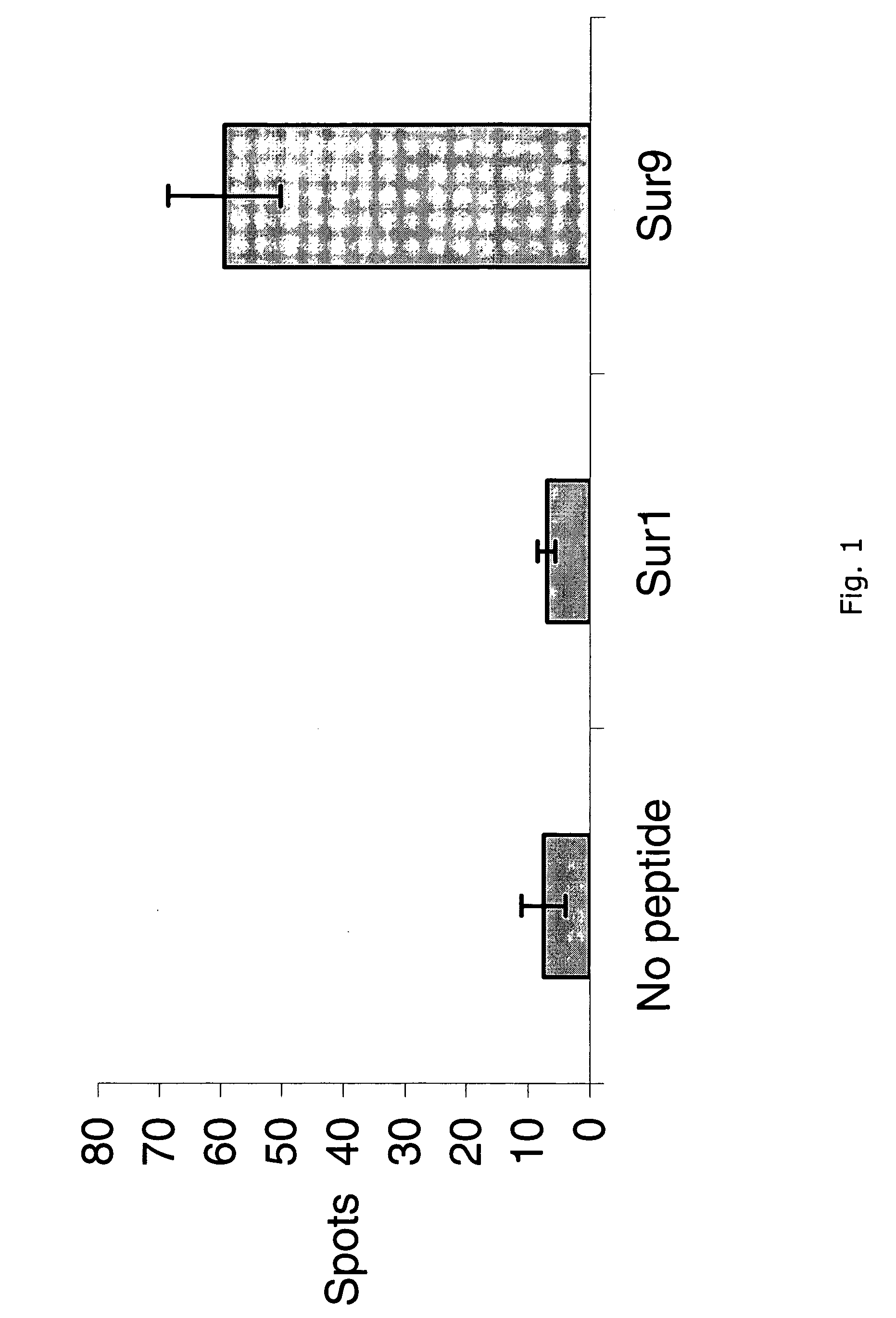
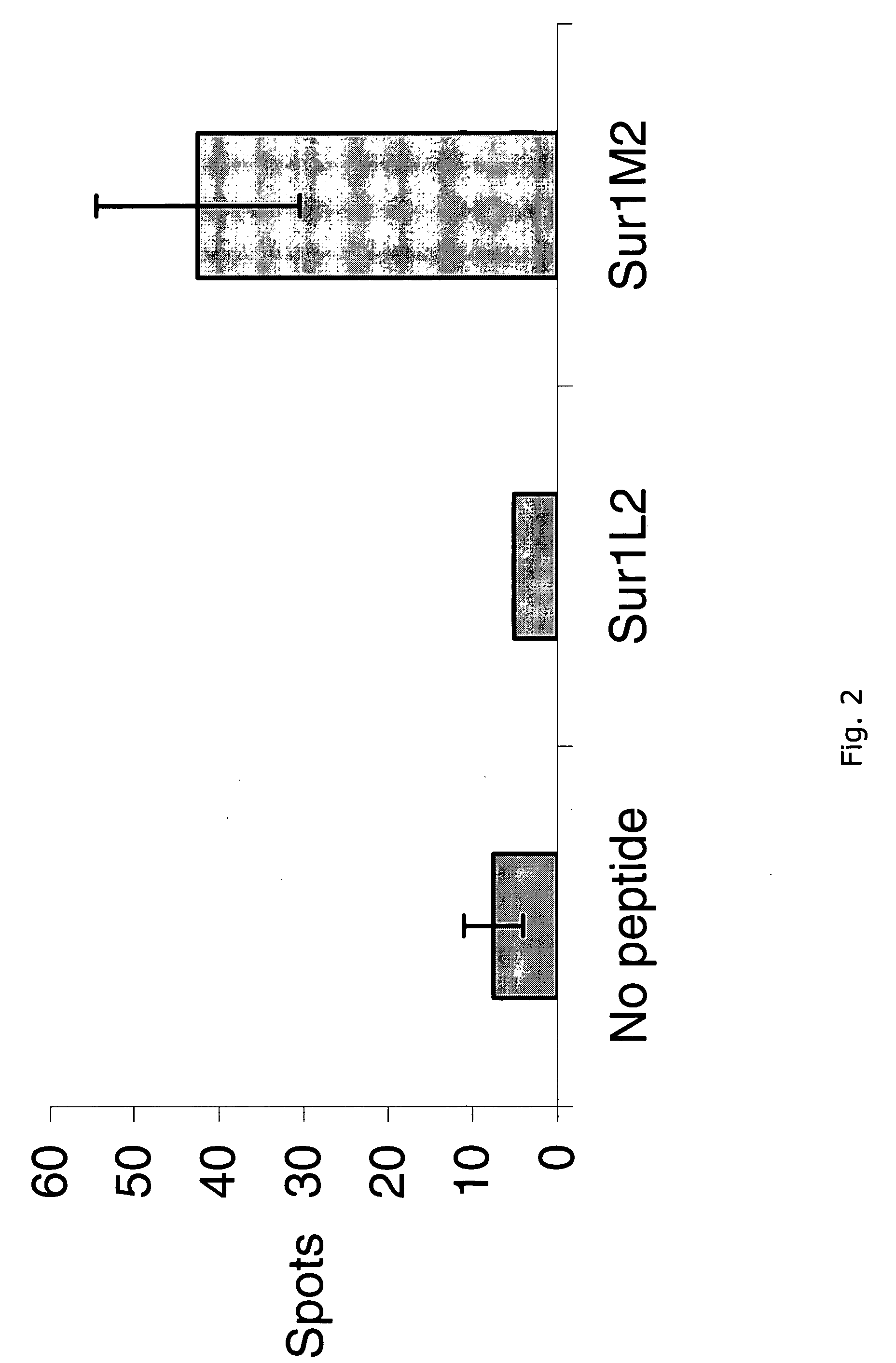
Survivin-derived peptides and use thereof
InactiveUS20040210035A1Easy to filterWeak affinityPeptide/protein ingredientsDepsipeptidesMHC class IWilms' tumor
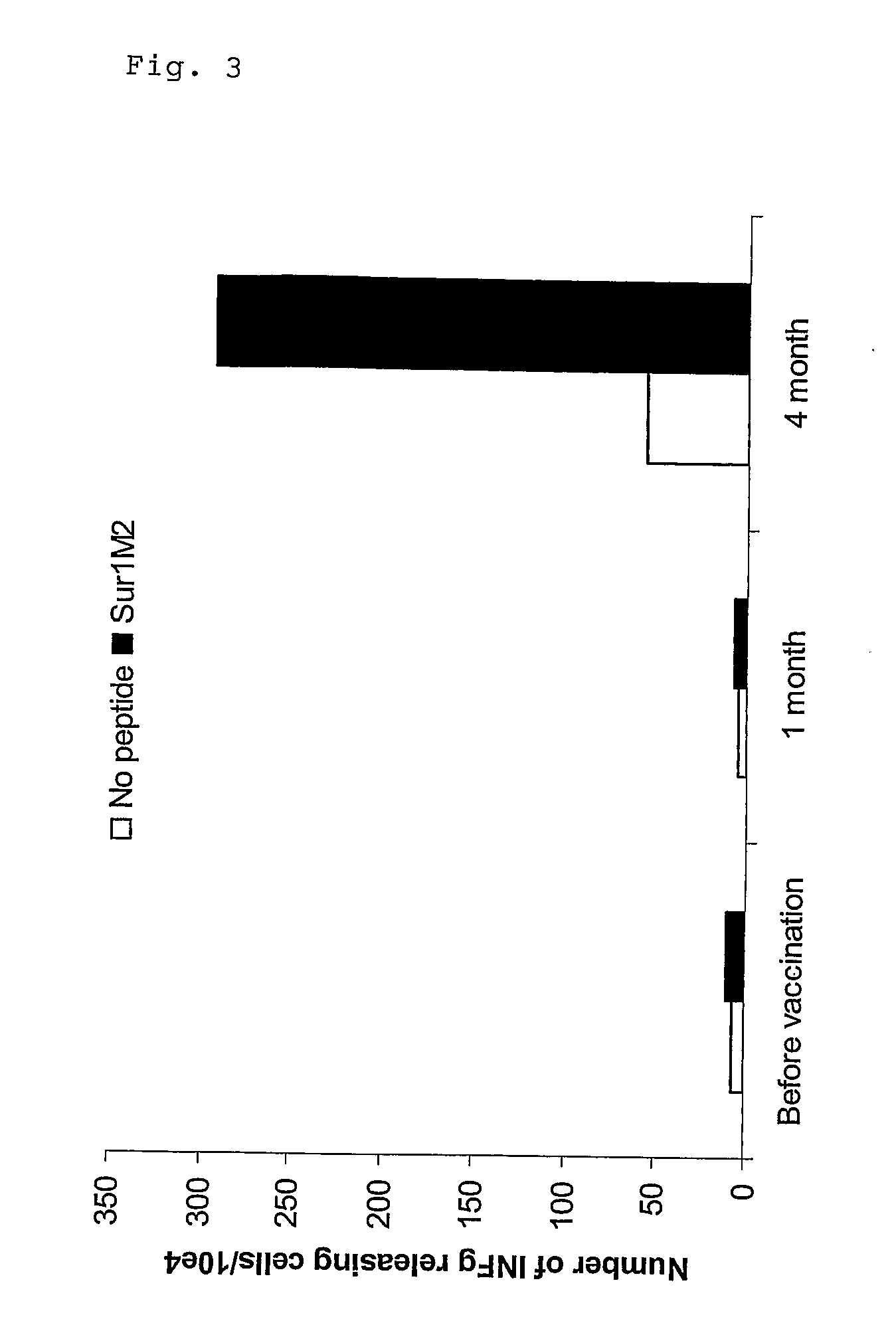
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
LNA oligonucleotides and the treatment of cancer
InactiveUS20060154888A1Organic active ingredientsPeptide/protein ingredientsCancer cell linesApoptosis induction
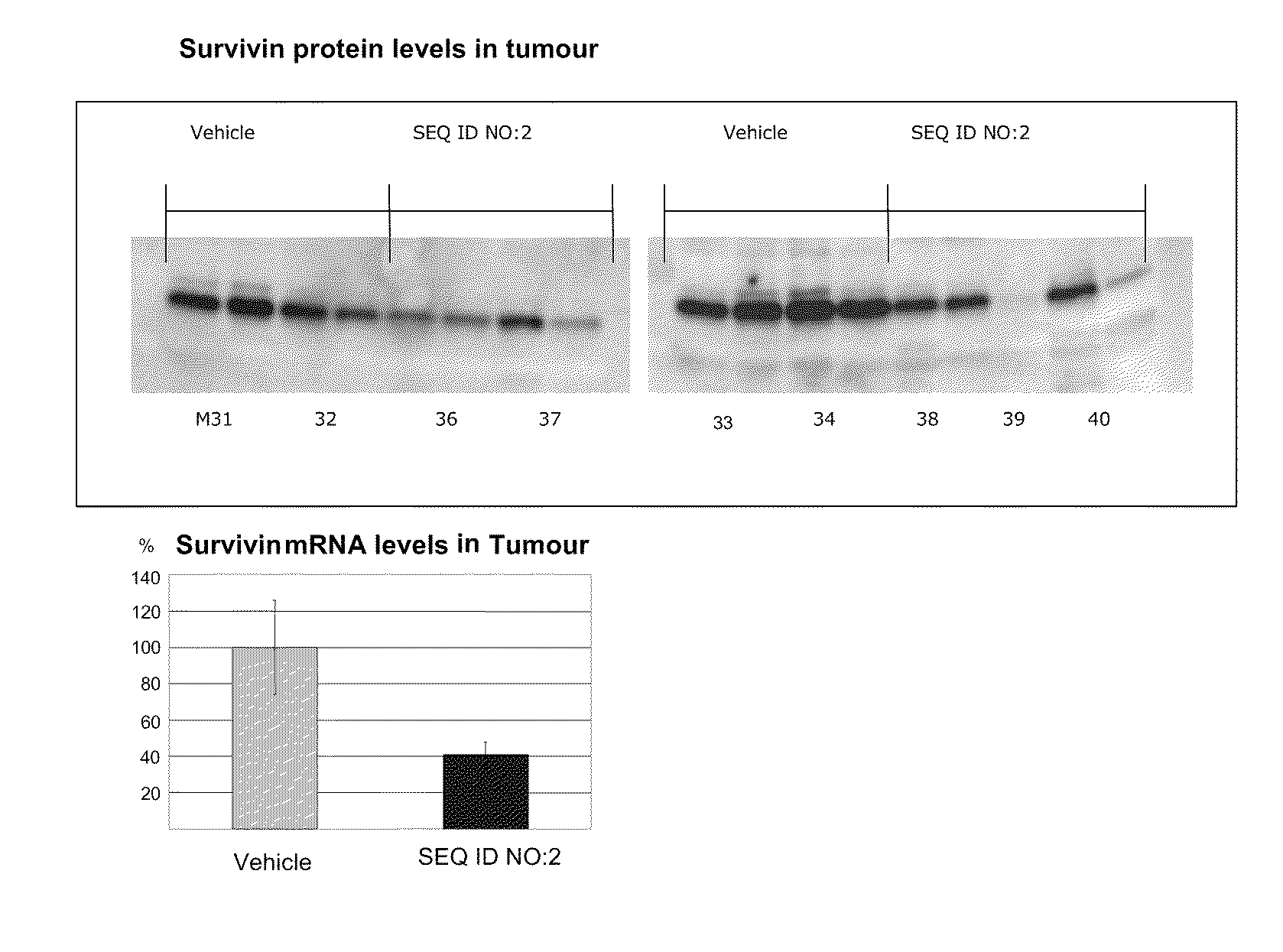
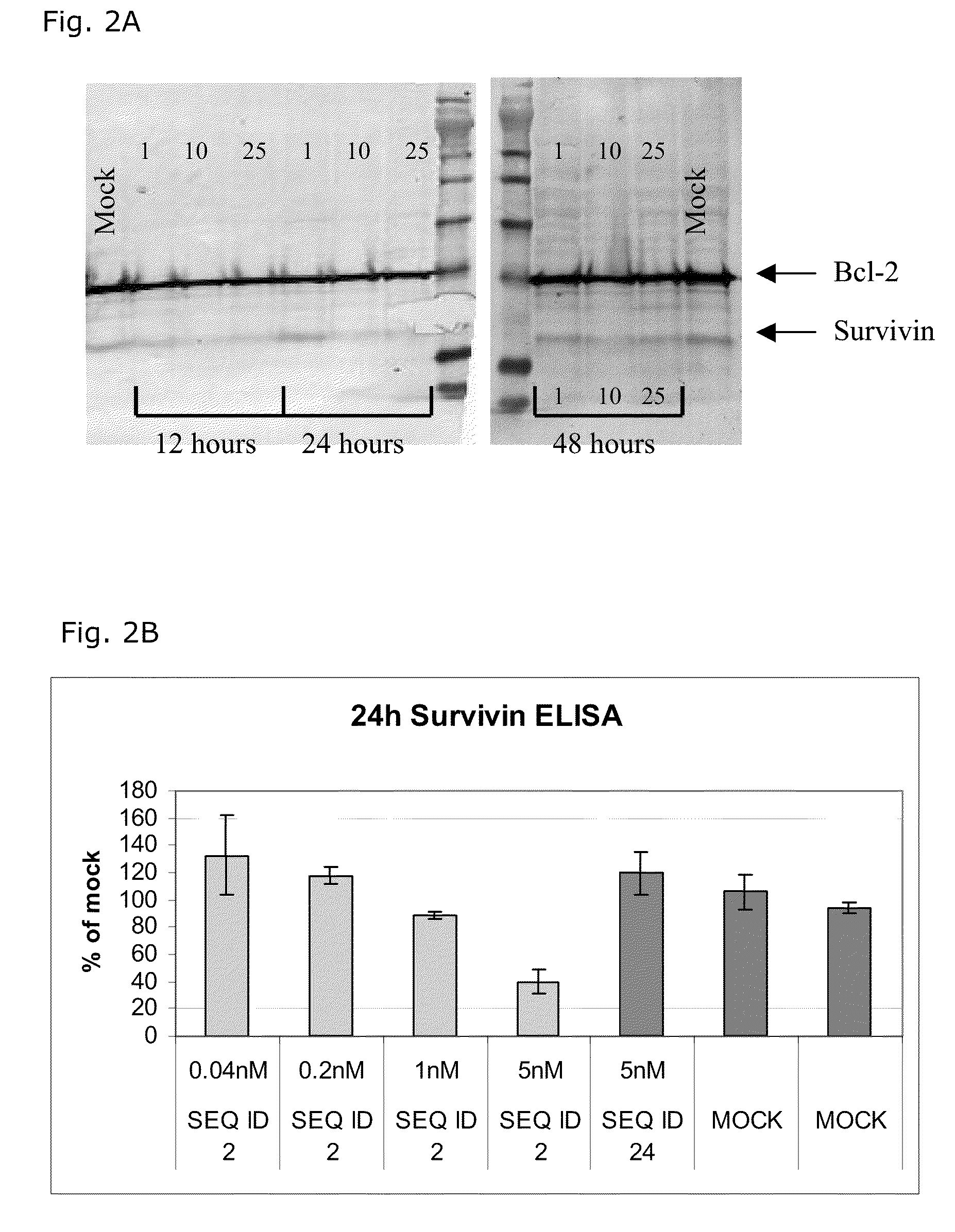
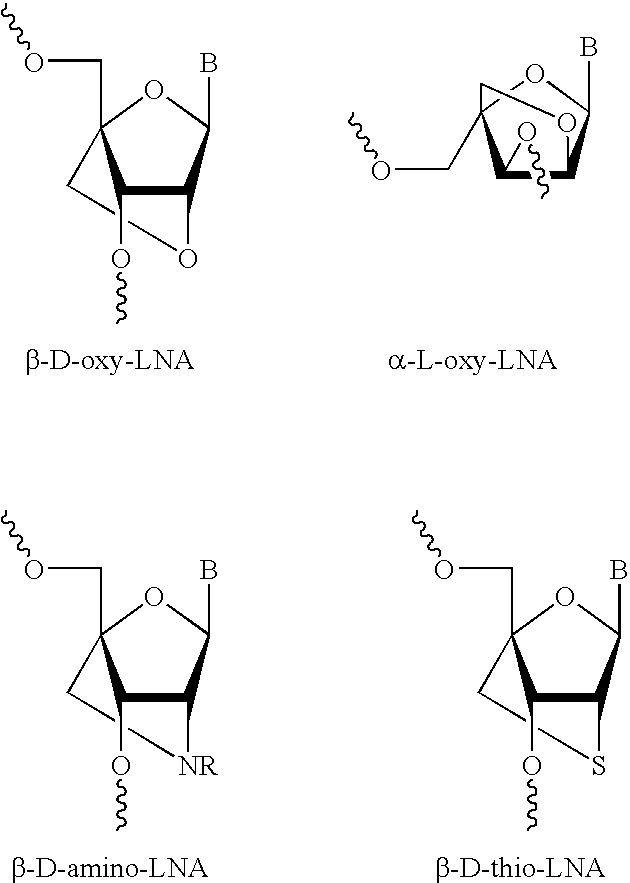
The present disclosure concerns LNA oligonucleotides having a (sub)sequence of the general formula 5′-(MeCx)(Tx)MeCxAsAstscscsastsgsgsMeCxAx(Gx)(c)-3′, and preferably of the general formula 5′-MeCxTxMeCxAsastscscsastsgsgsMeCxAxGxc-3′, wherein capital letters designate an LNA nucleotide analogue selected from β-D-oxy-LNA, β-D-thio-LNA, β-D-amino-LNA and α-L-oxy-LNA, small letters designate a deoxynucleotide, and underline designates either an LNA nucleotide analogue as defined above or a deoxynucleotide. Such LNA oligonucleotides exhibit surprisingly good properties with respect to inhibition of the expression of Survivin by means of an anti-sense mechanism, and thereby lead to reduction or inhibition of tumour development in vivo. The LNA oligonucleotides are superior to other LNA oligonucletides targeting Surviving mRNA measured by functional read outs such as apoptosis induction and proliferation inhibition, and is potent in down-regulating Survivin mRNA and protein in transfected cancer cell lines, and induce apoptosis in combination with Taxol superior compared to other LNA oligonucleotides.
Owner:ENZON PHARM INC
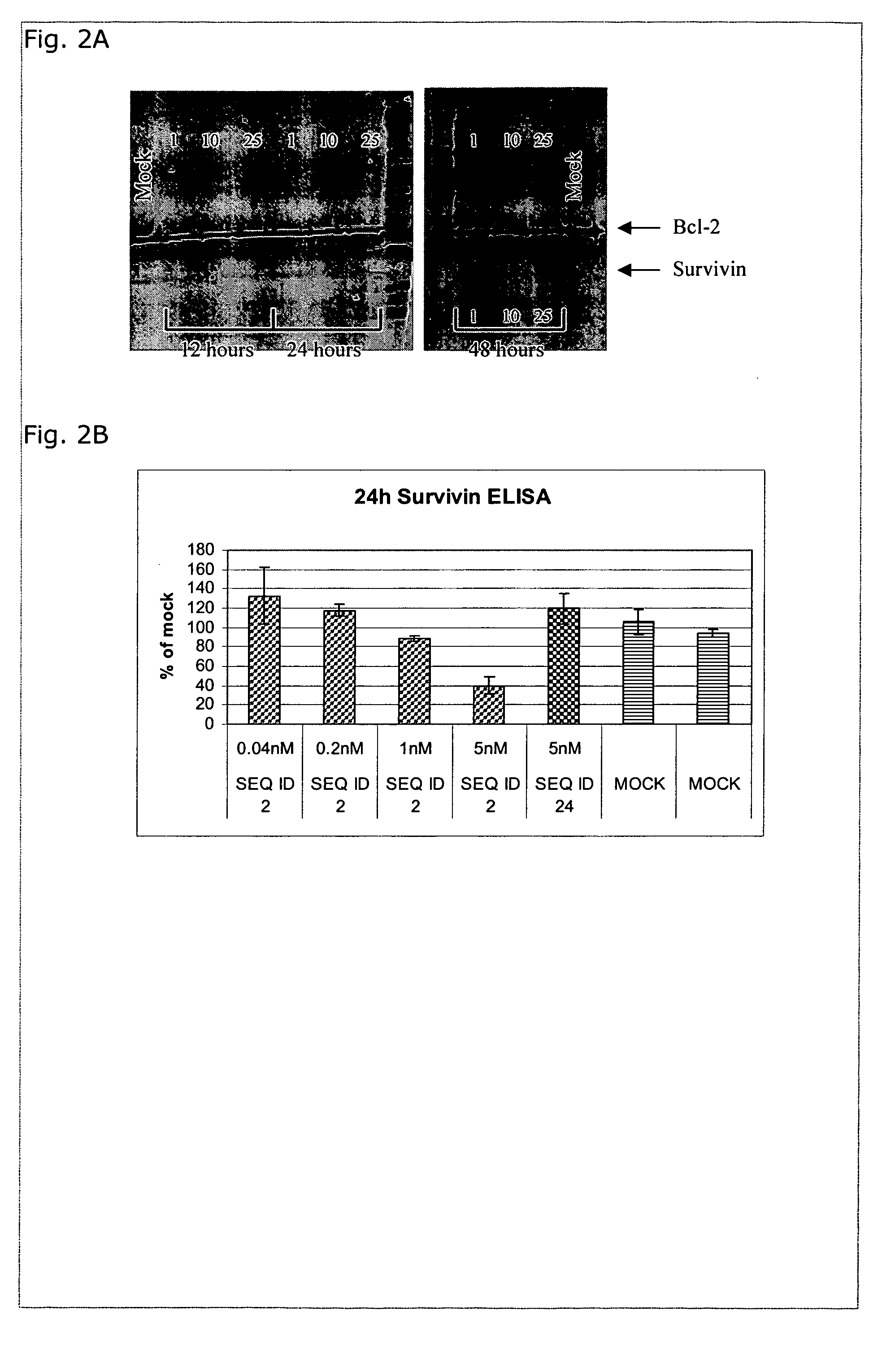
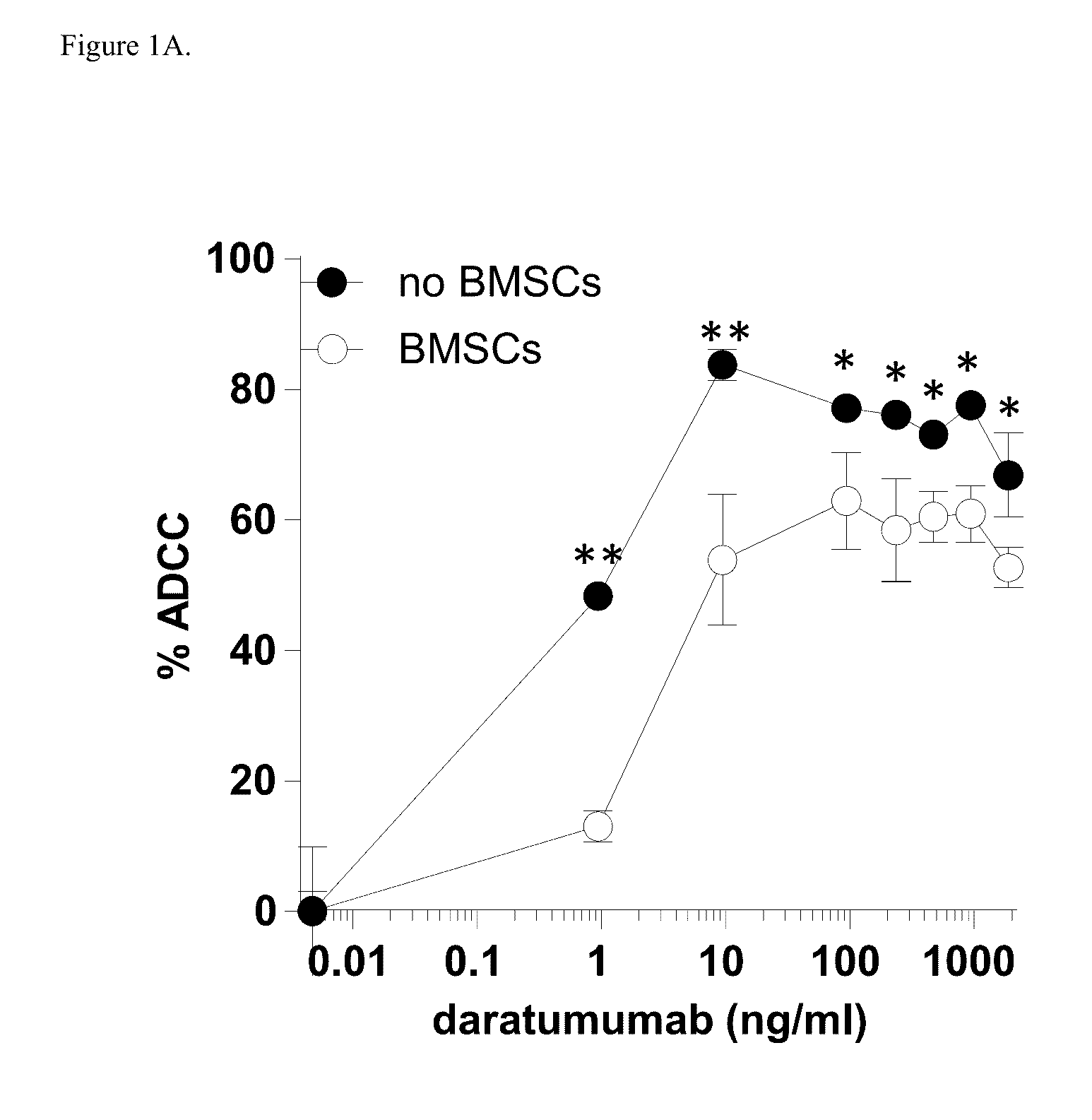
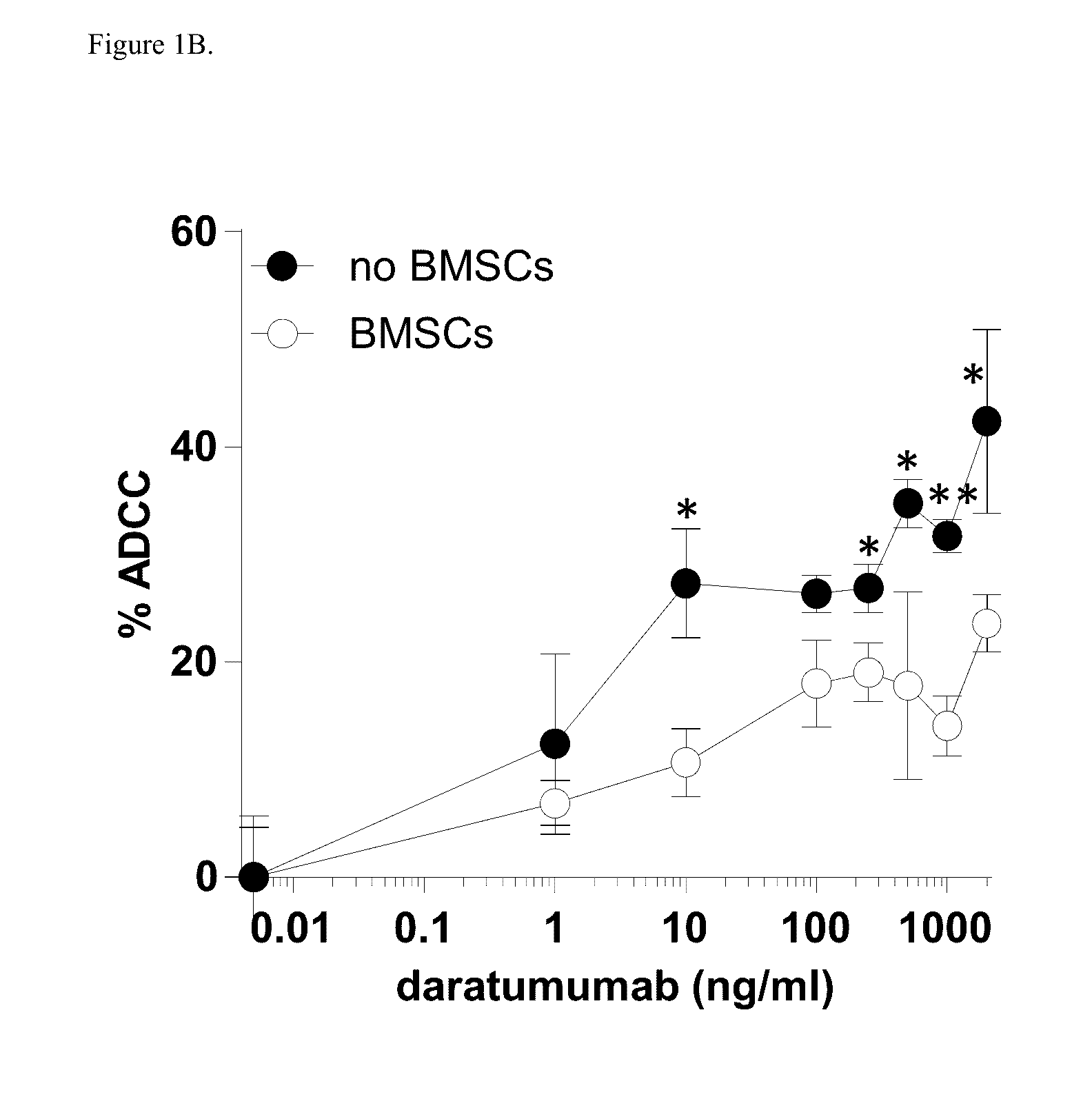
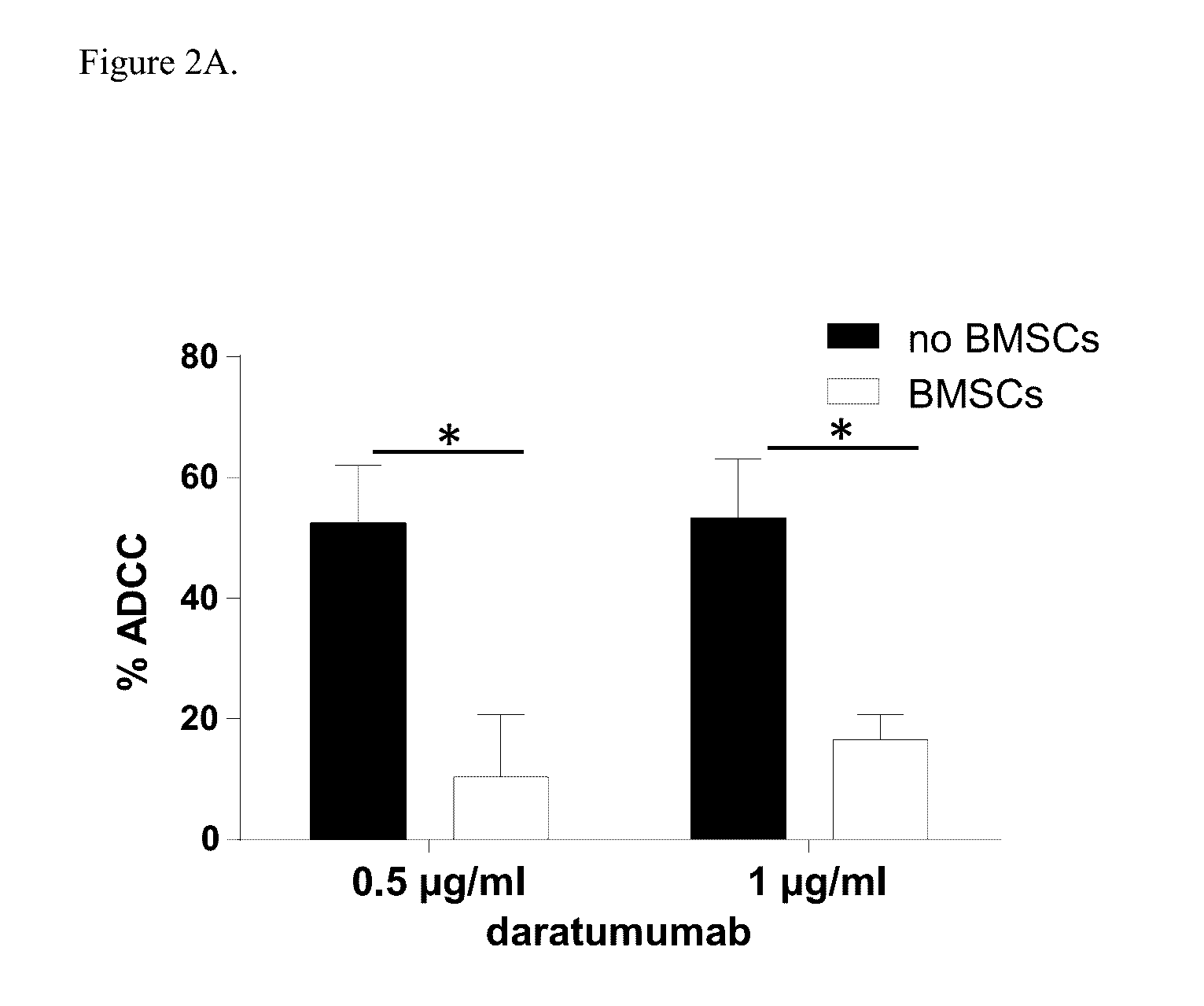
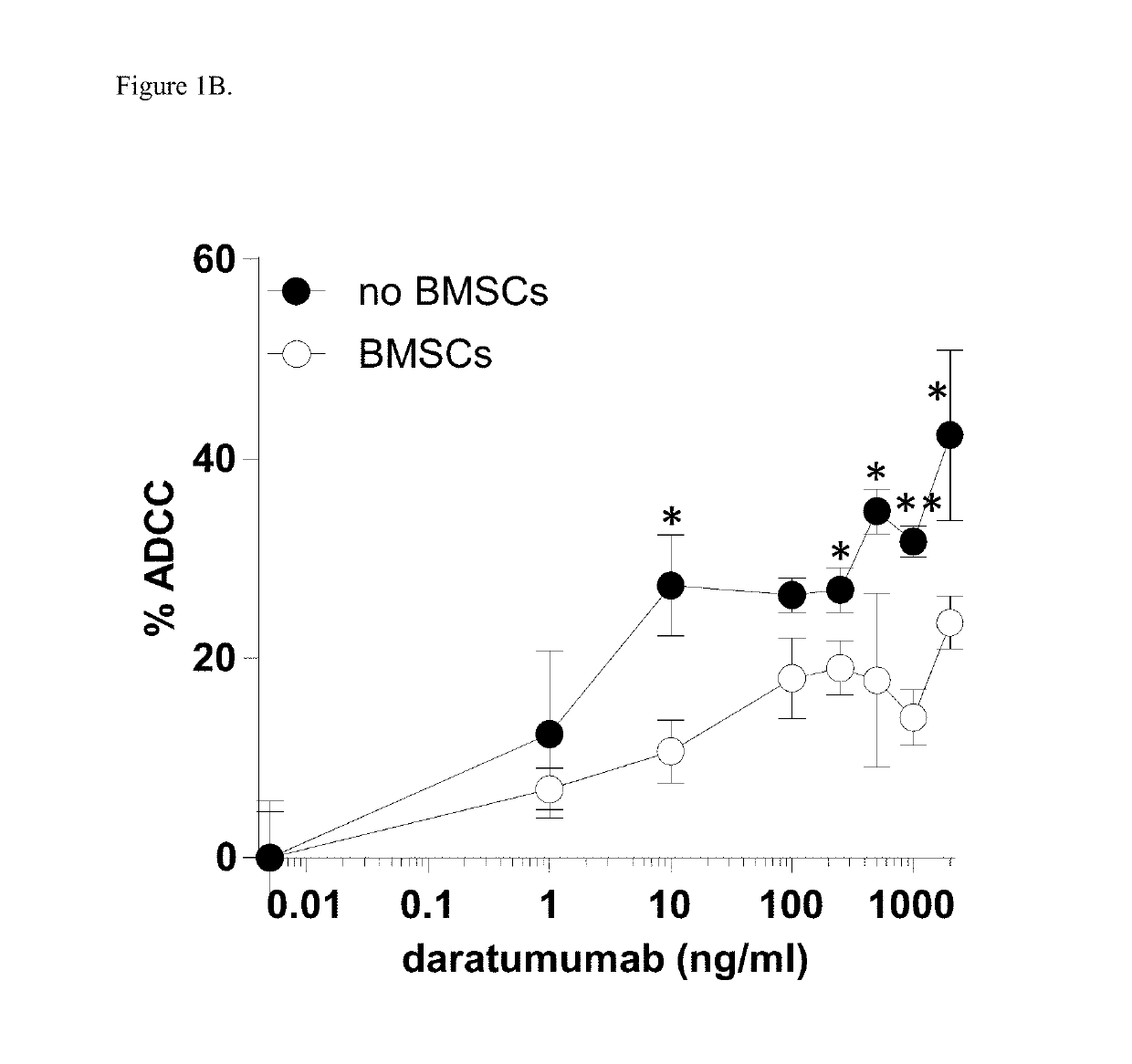
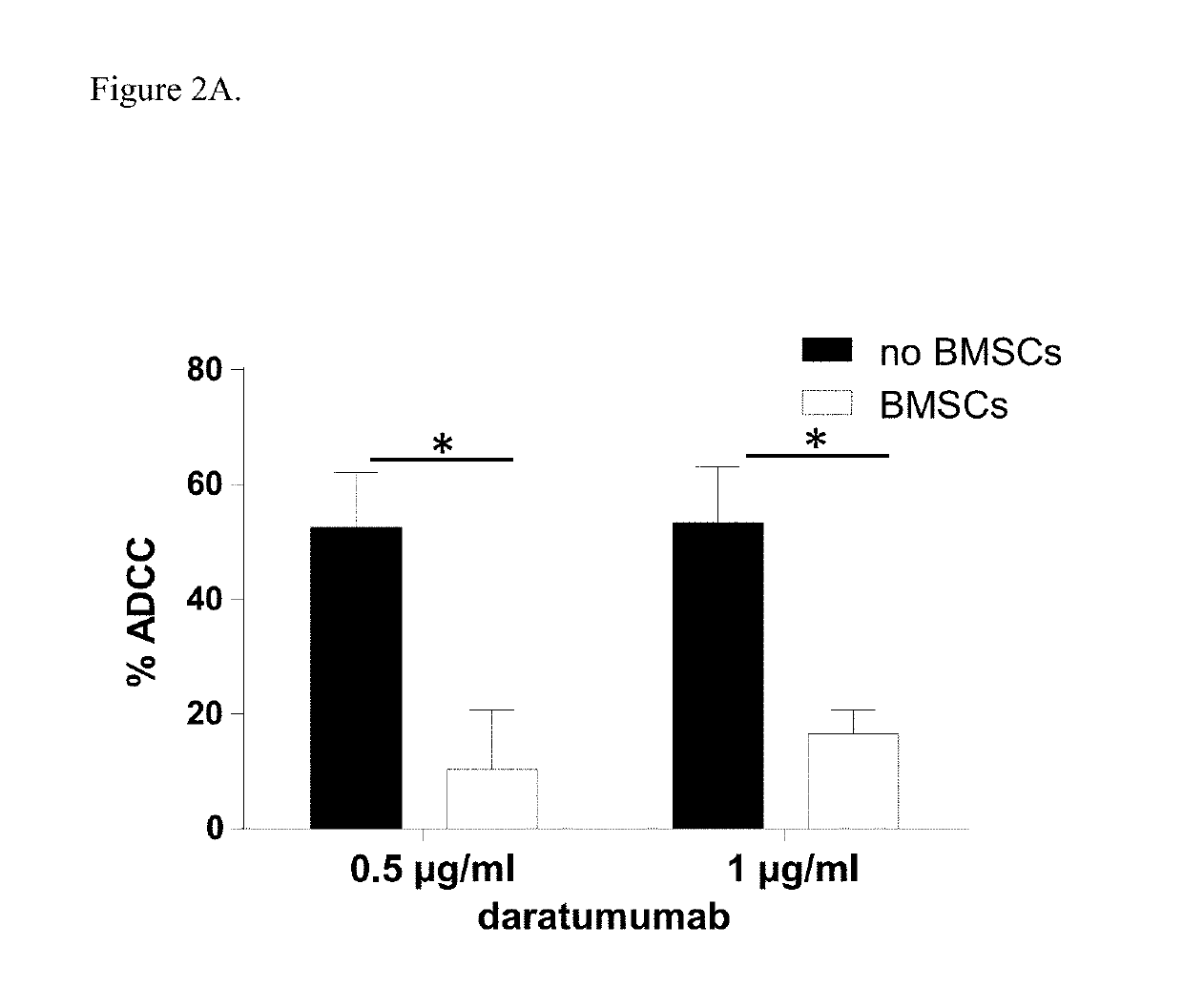
Combination therapies for heme malignancies with Anti-CD38 antibodies and survivin inhibitors
ActiveUS20160367663A1Ensure adequate treatmentOrganic active ingredientsPeptide/protein ingredientsSerum igeHeme
Owner:JANSSEN BIOTECH INC
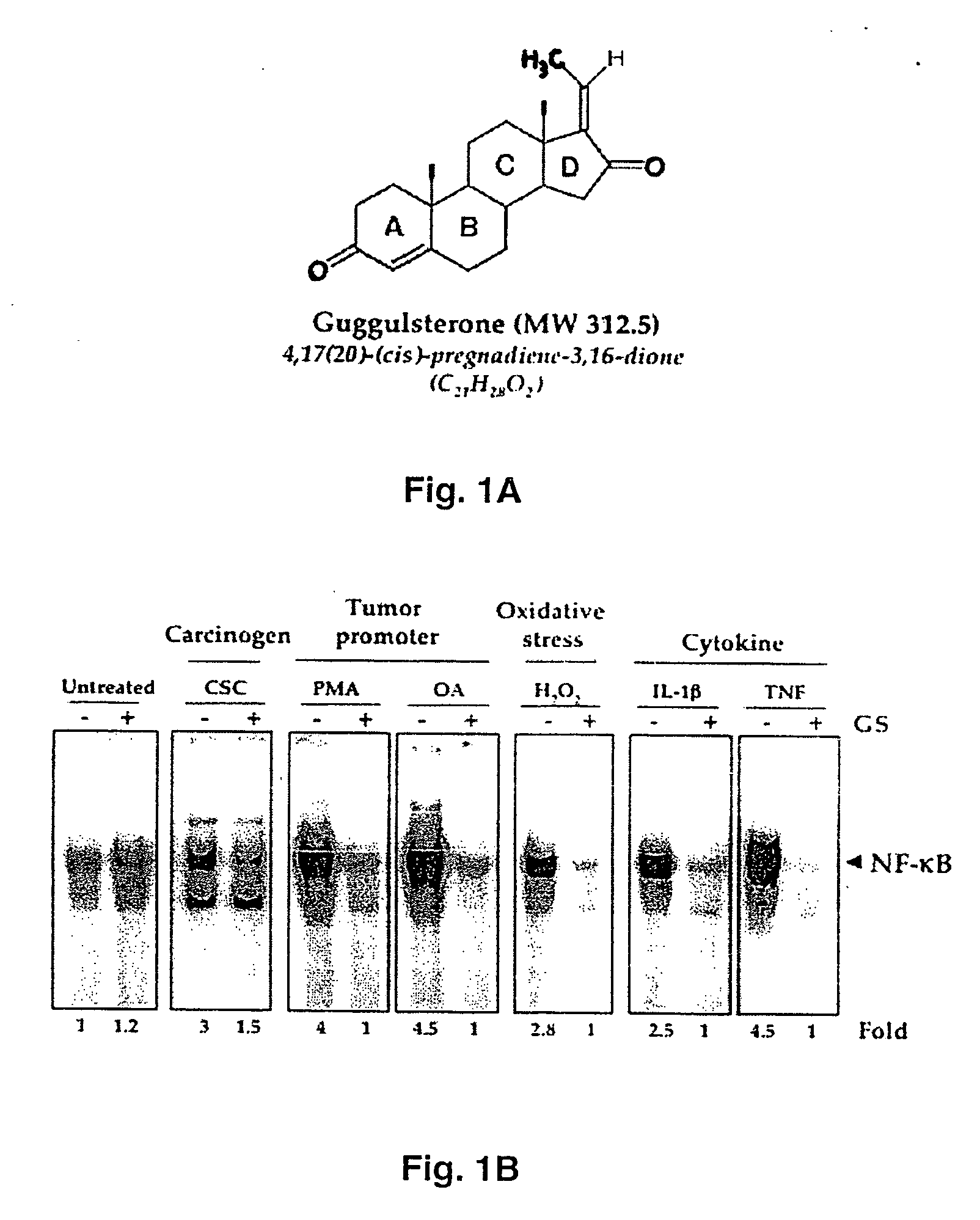
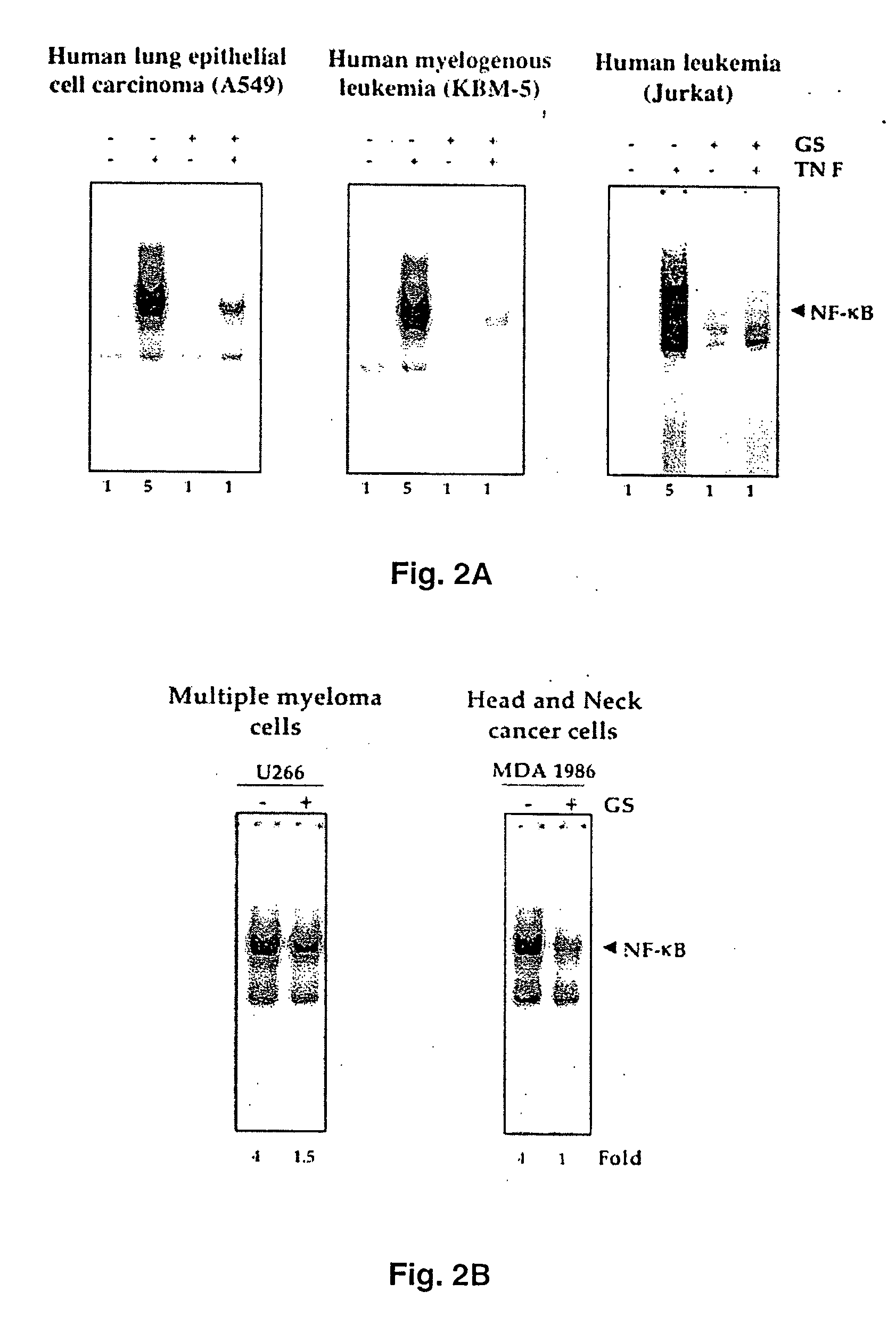
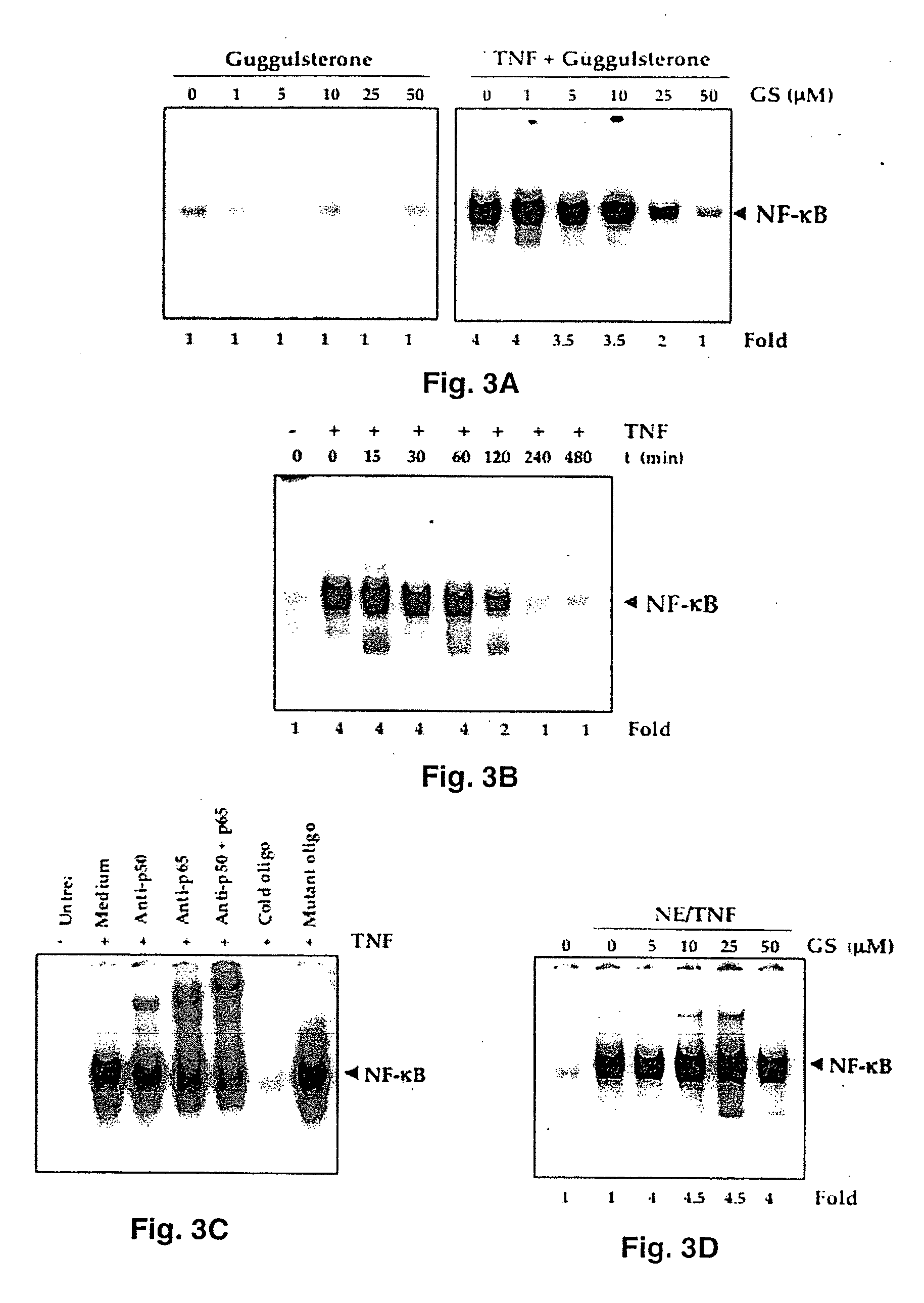
Guggulsterone: an inhibitor of nuclear factor - kappaB and IkappaBalpha kinase activation and uses thereof
InactiveUS20060019907A1Good effectHigh activityBiocideOrganic active ingredientsLymphatic SpreadCyclin D1
The present invention provides an inhibitor of NF-κB, guggulsterone and its analogs. Guggulsterone suppresses NF-κB activation induced by TNF, phorbol ester, okadaic acid, cigarette smoke, H2O2 and IL-1β, as well as constitutive NF-κB activation expressed in most tumor cells. One mechanism by which guggulsterone inhibits activation of NF-κB is through suppression of IκBα phosphorylation and IκBα degradation. NF-κB-dependent gene transcription is modulated by guggulsterone and its analogs. In particular, induction by TNF, TNFR1, TRADD, TRAF2, NIK and IKK, is modulated by guggulsterone and its analogs. In addition, guggulsterone decreased the expression of genes involved in anti-apoptosis (IAP1, XIAP, Bfl-1 / A1, bcl-2, cFLIP, survivin), proliferation (cyclin D1, c-myc) and metastasis (MMP-9, COX2 and VEGF).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Novel and powerful mhc-class ii peptides derived from survivin
ActiveUS20100029571A1Tumor rejection antigen precursorsPeptide/protein ingredientsHuman tumorAdditive ingredient
The present invention relates to peptides, nucleic acids, and cells for use in the immunotherapy of cancer. The present invention furthermore relates to survivin-derived tumor-associated cytotoxic T cell (CTL) peptide epitopes, alone or in combination with other tumor-associated peptides that serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses. The present invention specifically relates to three novel peptide sequences and variants thereof derived from HLA class I and class II molecules of human tumor cells that can be used in vaccine compositions for eliciting anti-tumor immune responses.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
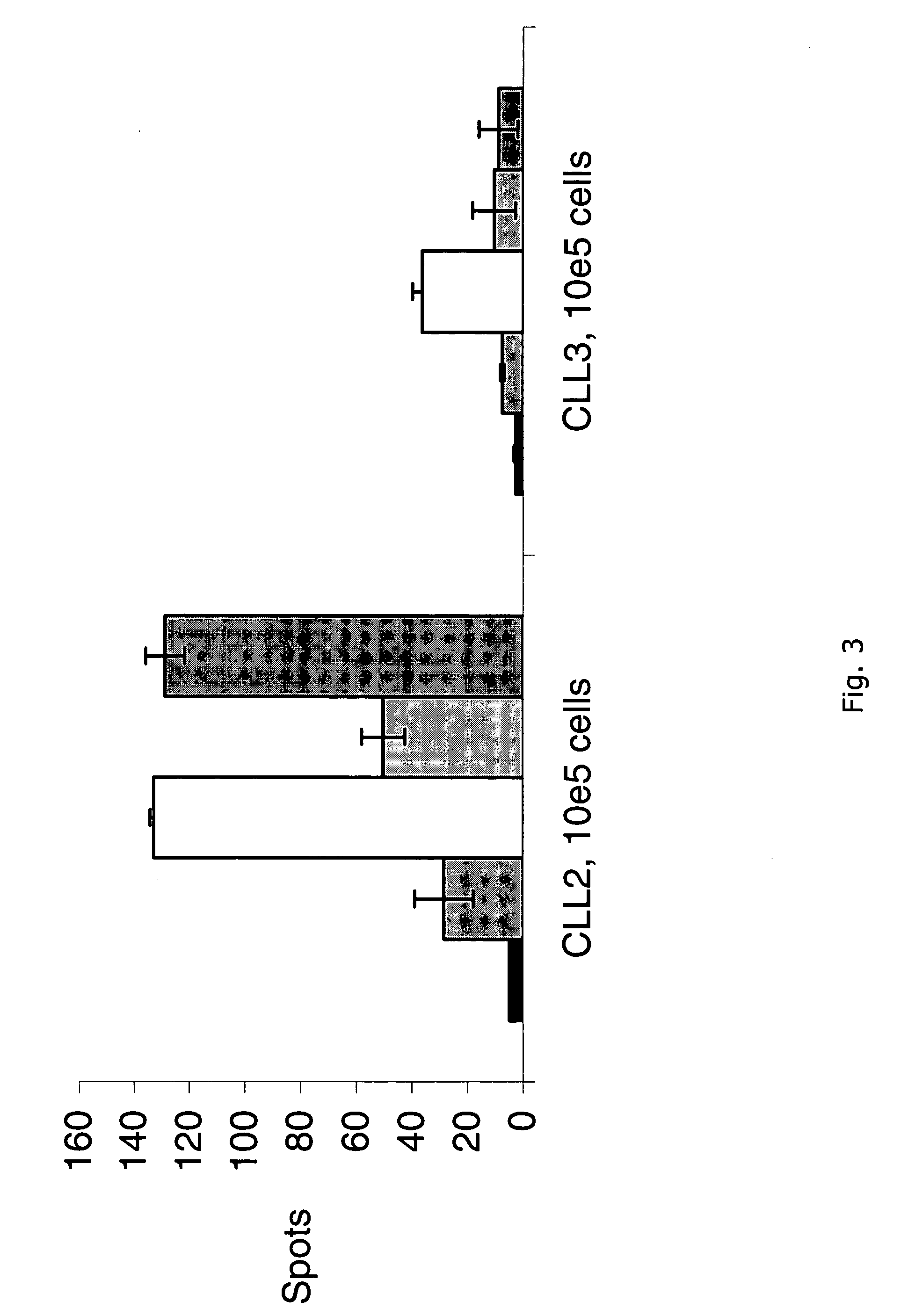
Method for effectively culturing tumor infiltrating lymphocytes (TILs)
InactiveCN102174469AShorter hospital stayReduce the burden of hospitalizationBlood/immune system cellsAntineoplastic agentsClinical efficacyApoptosis Regulatory Proteins
The invention discloses a method for effectively culturing tumor infiltrating lymphocytes (TILs). The method comprises the following steps: extracting mononuclear cells from the pectoral ascite or tumor tissue of a patient, washing, inoculating into a culture bottle coated with recombinant human fibrin and cluster-of-differentiation-3 antibody, culturing for 24 hours, adding interleukin II, then using a serum-free culture medium containing the interleukin II and the supernatant pectoral ascite to enlarge and subculture every two or three days, and adding apoptosis-regulated protein survivin and mucoprotein-1 on the twelveth or thirteenth day to activate the killing activity; and harvesting cells on the fourteenth day. By adopting the method, the culture time, which is only 14 days, is greatly shortened, so that the hospital stays of the patient can be greatly shortened and the hospital burden of the patient can be reduced. Meanwhile, the problems that the cell proliferation speed is low and the proliferation ratio is low can be solved, thus the number of the cultured cells can meet the clinical requirement. The cultured TILs has strong cell specificity so as to enhance the clinical curative effect.
Owner:宋鑫 +1
Cancer-specific promoters
InactiveUS20090192101A1Improve overall utilizationShrink tumorOrganic active ingredientsTumor rejection antigen precursorsNucleotideGene product
The present invention regards cancer-specific control sequences that direct expression of a polynucleotide encoding a therapeutic gene product for treatment of the cancer. Specifically, the invention encompasses breast cancer-specific and ovarian cancer-specific control sequences. Two breast cancer-specific sequences utilize specific regions of fatty acid synthase and claudin 4 promoters, particularly in combination with a two-step transcription amplification sequence and / or a post-transcriptional control sequence. Two ovarian cancer-specific sequences utilize specific regions of hTERT and survivin promoters, particularly in combination with a two-step transcription amplification sequence and / or a post-transcriptional control sequence. In more particular embodiments, these polynucleotides are administered in combination with liposomes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Survivin peptides as cancer vaccines
ActiveUS7943138B2Improve survivalEnhance immune responseBiocidePeptide/protein ingredientsCancer cellCancer research
Provided are compositions and methods for treating survivin expressing cancers. The compositions contain peptide survivin peptide mimics with improved MHC-I binding characteristics. The method involves administering a survivin peptide mimic with improved MHC-I binding characteristics to an individual to effect inhibition of the growth of survivin expressing cancer cells in the individual.
Owner:HEALTH RES INC
Method for treatment of tumors using nordihydroguaiaretic acid derivatives
InactiveUS8318815B2Inhibiting survivin productionImprove the level ofBiocideOrganic chemistryCancer researchSurvivin
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Survivin Peptides As Cancer Vaccines
ActiveUS20090041732A1Improved human cell mediated immune responseImprove survivalBiocidePeptide/protein ingredientsCancer cellCancer research
Provided are compositions and methods for treating survivin expressing cancers. The compositions contain peptide survivin peptide mimics with improved MHC-I binding characteristics. The method involves administering a survivin peptide mimic with improved MHC-I binding characteristics to an individual to effect inhibition of the growth of survivin expressing cancer cells in the individual.
Owner:HEALTH RES INC
Survivin-derived peptides and use thereof
ActiveUS20040176573A1Easy to filterIncrease the immuogenicity of survivin-derived peptidesPeptide/protein ingredientsApoptosis related proteinsMHC class IWilms' tumor
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
Detection of survivin in the biological fluids of cancer patients
InactiveUS7097966B2Peptide/protein ingredientsMaterial analysis by observing effect on chemical indicatorBody fluidDiagnosis cancer
The present invention includes a method for diagnosing cancer comprising detecting the presence of survivin in the biological fluid of a patient. The present invention also provides kits comprising one or more agents that detect survivin polypeptide or survivin nucleic acid and a container for collecting biological fluid for testing.
Owner:YALE UNIV
Survivin peptide vaccine
InactiveUS20110091489A1Evaluate usefulnessStrong responsePeptide/protein ingredientsCancer antigen ingredientsDiseaseCurative treatment
The present invention relates to a therapeutic vaccine comprising one or more survivin polypeptide fragments. The vaccine can be used for prophylactic, ameliorating and / or curative treatment of e.g. cancer diseases. The invention further relates to methods of combination treatment.
Owner:SURVAC
Compositions comprising survivin sirna and methods of use thereof
InactiveUS20110053862A1Reduce severityReduce the severity of the diseaseOrganic active ingredientsSugar derivativesSurvivinNucleic acid molecule
The present invention provides siRNA nucleic acid molecules that inhibit survivin expression. Methods of using the nucleic acid molecules are also provided.
Owner:INTRADIGM CORP
Combination therapies for heme malignancies with anti-CD38 antibodies and survivin inhibitors
ActiveUS10668149B2Ensure adequate treatmentOrganic active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesHeme
Owner:JANSSEN BIOTECH INC
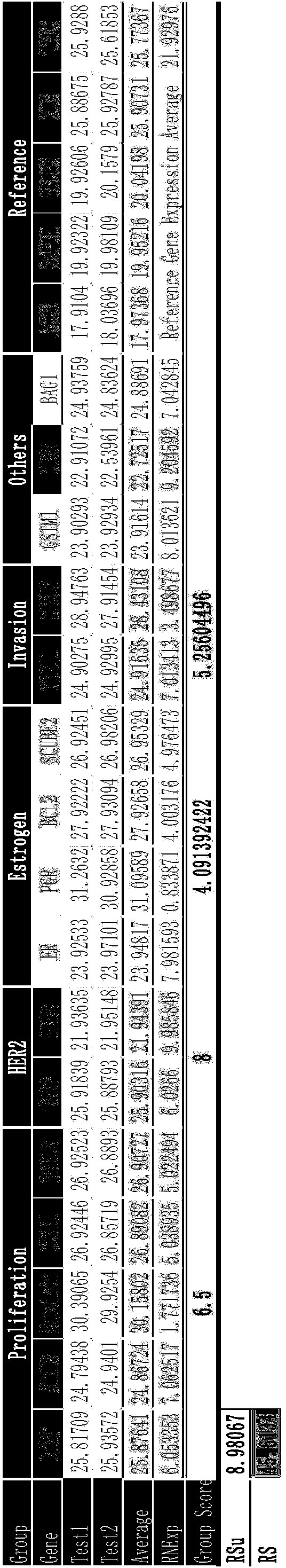
Kit for jointly detecting breast cancer 21 genes and preparation method of kit
InactiveCN104004844AEasy to operateShorten detection experiment timeMicrobiological testing/measurementSingle sampleBAG1
The invention discloses a kit for jointly detecting breast cancer 21 genes. A PCR (polymerase chain reaction) amplification primer consists of 21 primer pairs, and can be used for jointly detecting 21 gene-related expression quantities: Ki67, STK15, Survivin, CCNB1, MYBL2, MMP11, CTSL2, GRB7, HER2, GSTM1, CD68, BAG1, ER, PGR, BCL2, SCUBE2, ACTB, GAPDH, PRLPO, GUS, and TFRC. Output fragments of the 21 pairs of primers related in the invention are smaller than 100bp, so that PCR efficiency after reverse transcription of mRNA (messenger ribonucleic acid) extracted by a paraffin specimen is greatly improved, all primers of 21 genes are premixed, and time and labor for designing and synthesizing 21 pairs of genes by an experimenter are saved; the corresponding primers of the 21 genes are not needed to be added for premixing in mother liquor, and therefore, errors of reaction holes are greatly reduced. The kit disclosed by the invention is simple to operate, and capable of detecting on a quantitative PCR instrument by only adding a template, so that detection experimental time is greatly shortened. The kit disclosed by the invention is applicable to a single sample and multiple samplers, so that results can be quickly obtained, and therefore, scientific research and clinical detection requirements can be satisfied very well.
Owner:杭州美中疾病基因研究院有限公司
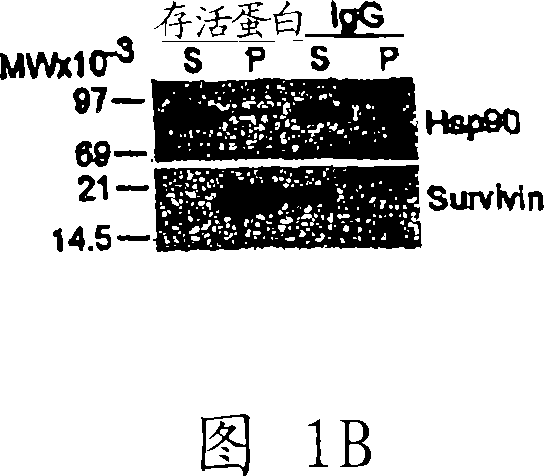
Compounds that inhibit hsp90 protein-protein interactions with iap proteins
InactiveCN101065138APeptide/protein ingredientsApoptosis related proteinsIAP ProteinHSP90 Heat-Shock Proteins
Owner:UNIV OF MASSACHUSETTS
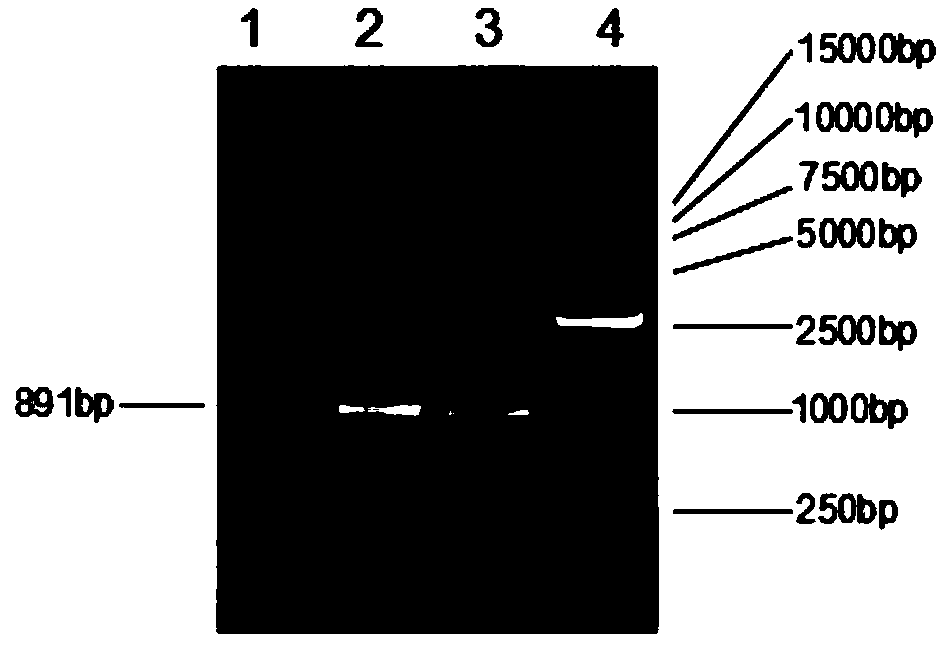
Survivin-directed RNA interference-compositions and methods
InactiveUS20060276423A1Poor prognosis of survivalHigh sensitivityBiocideOrganic active ingredientsDiseaseDNA
The present invention is directed to compositions and methods for inhibiting the expression of survivin in cells expressing survivin. The invention is also directed to methods of treating conditions associated with elevated survivin, such as hyperproliferative disorders. More particularly, the invention is directed to inhibition of survivin expression using short interfering RNAs (si-RNAs) or through administration of DNA sequences yielding the expression of short hairpin RNAs (sh-RNAs).
Owner:ALTURA RACHEL +2
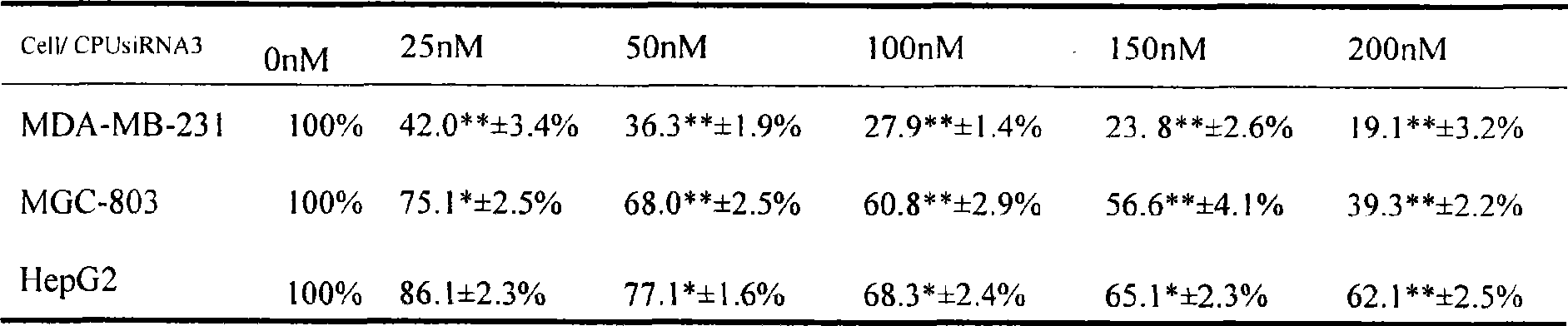
Use of high efficiency siRNA for preparing tumor migration inhibition drug
InactiveCN101445534AInhibit migrationInhibited DiffusionOrganic active ingredientsSugar derivativesAbnormal tissue growthType IV collagen
The invention belongs to the biological medicine field, in particular relating to the use of a high efficiency small interfering RNA for preparing a tumor migration inhibition drug. The high efficiency small interfering RNA can silence the expression of survivin, change the adhesive force of breast cancer, gastric cancer and liver cancer cells against IV collagen, and inhibit the migrating ability of the breast cancer, gastric cancer and liver cancer cells at two-and three-dimensional levels. The small interfering RNA can also prepare the drug for inhibiting the tumor migration.
Owner:YICHUN UNIVERSITY
Recombinant oncolytic gene-adenovirus targeting cancer and construction method and application of recombinant oncolytic gene-adenovirus
PendingCN110628728AGood treatment effectLow toxicityPeptide/protein ingredientsPeptidesCancer cellTherapeutic effect
The invention discloses a recombinant oncolytic gene-adenovirus targeting cancer, and the recombinant oncolytic gene-adenovirus is Ad-sp-E1A-delta E1B-VGLL4. The recombinant oncolytic gene-adenovirusAd-sp-E1A-delta E1B-VGLL4 is driven by a survivin promoter to drive a virus E1A gene, an E1B gene region is knocked out off the virus genome at the same time, and a VGLL4 expression frame fragment isinserted on the basis. The invention also discloses a construction method and application in preparing drugs for treating cancer of the recombinant oncolytic gene-adenovirus Ad-sp-E1A-delta E1B-VGLL4.The recombinant oncolytic gene-adenovirus Ad-sp-E1A-delta E1B-VGLL4 of the invention specifically replicates in cancer cells with the aid of an adenovirus vector, and achieves good therapeutic effects in anticancer tests.
Owner:SHANGHAI YUANSONG BIOTECHNOLOGY CO LTD
MHC-I restricted epitopes containing non-natural amino acid residues
ActiveUS9109007B2Slow off-rateMaintain good propertiesCompound screeningTumor rejection antigen precursorsMHC class IEpitope
The invention provides for the synthesis of more effective generators of a T-cell immune response by providing peptide derivatives that are MHC class I-restricted antigens. The peptide derivatives of the present invention are prepared or derived from a parent peptide of 8 to 11 amino acid residues in length, wherein the peptide derivative contains a non-natural amino acid substituted in place of a naturally-occurring amino acid residue at one or more primary anchor positions in the parent peptide or at position 6, position 7, or the C-terminus. The exemplary polypeptides are derived from the survivin, hTERT, CYP1B1 and MART-1 antigens.
Owner:PURDUE PHARMA LP
DNA vaccines against tumor growth and methods of use thereof
A DNA vaccine suitable for eliciting an immune response against cancer cells comprises a DNA construct operably encoding a cancer-associated Inhibitor of Apoptosis-family protein and an immunoactive gene product, such as a cytokine or a ligand for a natural killer cell surface receptor, in a pharmaceutically acceptable carrier. A preferred cytokine is CCL21. Preferred ligands for a natural killer cell surface receptor include human MICA, human MICB, human ULBP1, human ULBP2, and human ULBP3. The cancer-associated Inhibitor of Apoptosis (IAP)-family protein is preferably a survivin protein or livin protein. Method of inhibiting tumor growth by administering the vaccine of the invention to a mammal is also described.
Owner:THE SCRIPPS RES INST
Methods for selectively modulating survivin apoptosis pathways
The present invention, based on the discovery of a new biological phenomena, provides methods and compositions for use in identifying agents that modulate the phosphorylation of survivin, the interaction between survivin and p34cdc2-cyclin B1 kinase complex, and the interaction between survivin and caspase-9. Related methods and compositions can be used to modulate survivin regulated apoptosis.
Owner:YALE UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com