LNA oligonucleotides and the treatment of cancer
a technology of oligonucleotides and cancer, applied in the field of pharmaceutical compositions, can solve the problems of increased cancer recurrence, poor prognosis, resistance to therapy,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Monomer Synthesis
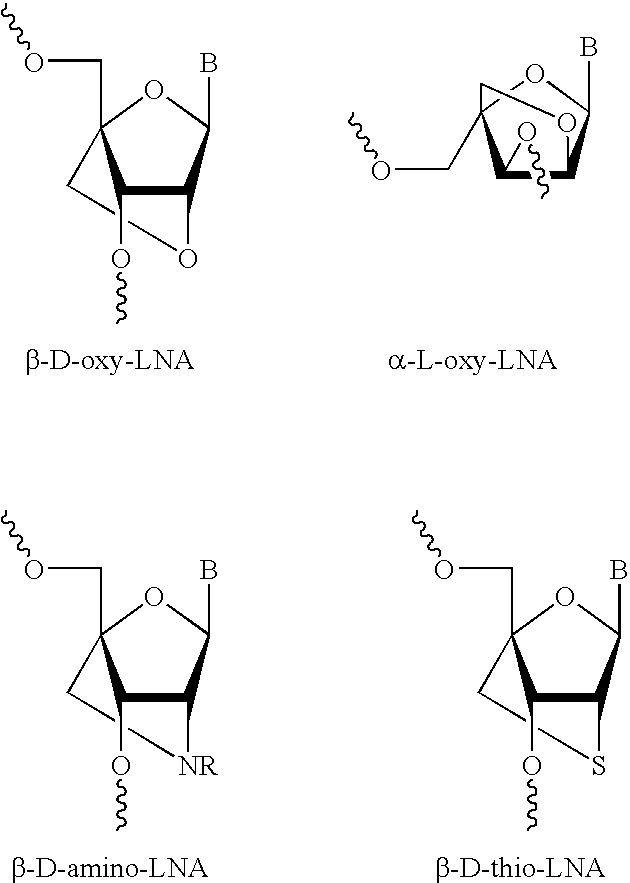
[0191] The LNA monomer building blocks and derivatives thereof were prepared following published procedures and references cited therein, see:
[0192] WO 03 / 095467 A1
[0193] D. S. Pedersen, C. Rosenbohm, T. Koch (2002) Preparation of LNA Phosphoramidites, Synthesis 6, 802-808.
[0194] M. D. Sørensen, L. Kværnø, T. Bryld, A. E. Håkansson, B. Verbeure, G. Gaubert, P. Herdewijn, 3. Wengel (2002) α-L-ribo-configured Locked Nucleic Acid (α-l-LNA): Synthesis and Properties, J. Am. Chem. Soc., 124, 2164-2176.
[0195] S. K. Singh, R. Kumar, J. Wengel (1998) Synthesis of Novel Bicyclo[2.2.1] Ribonucleosides: 2′-Amino- and 2′-Thio-LNA Monomeric Nucleosides, J. Org. Chem. 1998, 63, 6078-6079.
[0196] C. Rosenbohm, S. M. Christensen, M. D. Sørensen, D. S. Pedersen, L. E. Larsen, J. Wengel, T. Koch (2003) Synthesis of 2′-amino-LNA: a new strategy, Org. Biomol. Chem. 1, 655-663. D. S. Pedersen, T. Koch (2003) Analogues of LNA (Locked Nucleic Acid). Synthesis of the 2′-Thio-LNA Thymi...
example 2
Oligonucleotide Synthesis
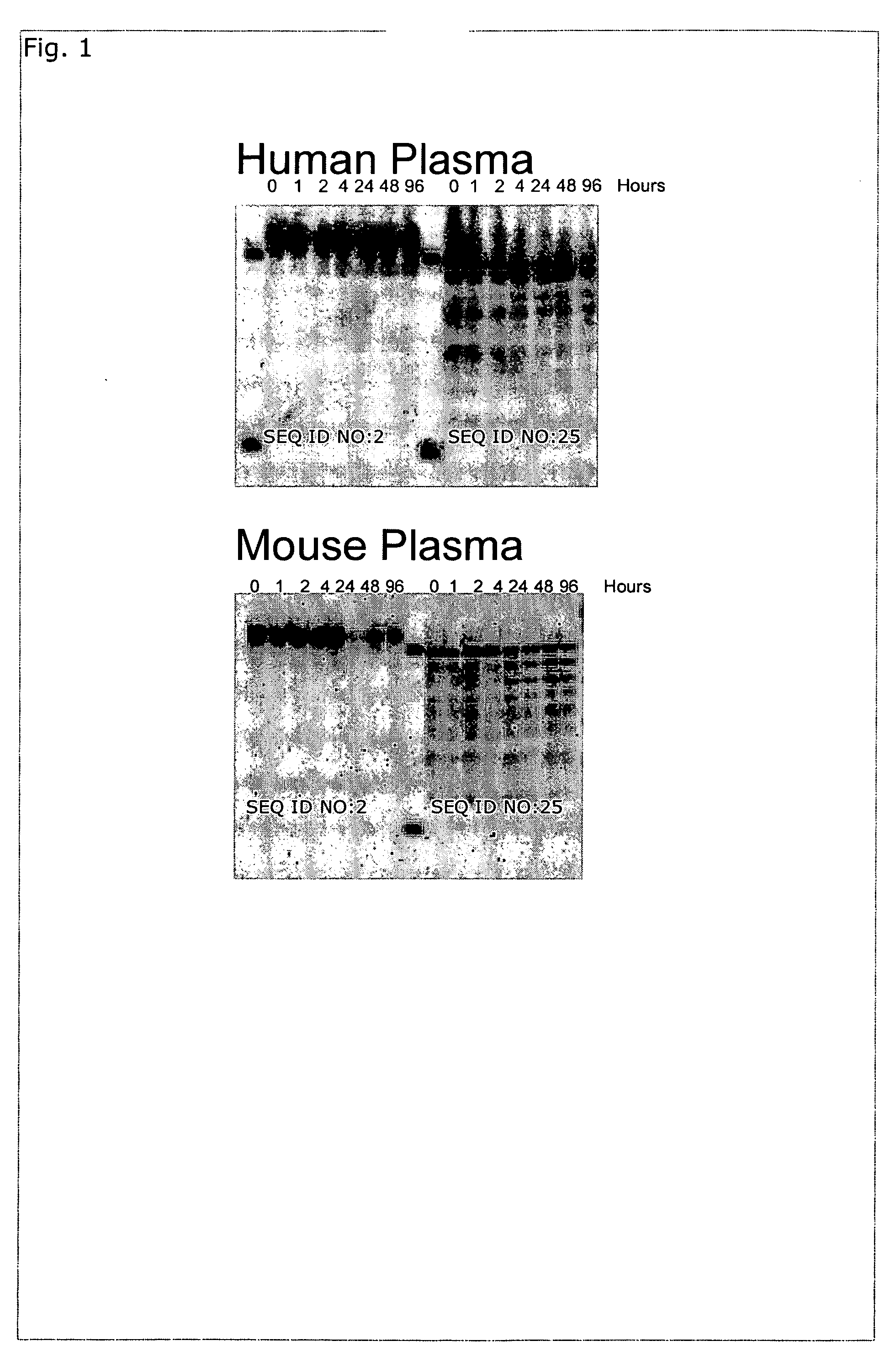
[0197] Oligonucleotides were synthesized using the phosphoramidite approach on an Expedite 8900 / MOSS synthesizer (Multiple Oligonucleotide Synthesis System) at 1 μmol or 15 μmol scale. For larger scale synthesis an Äkta Oligo Pilot was used. At the end of the synthesis (DMT-on), the oligonucleotides were cleaved from the solid support using aqueous ammonia for 1-2 hours at room temperature, and further deprotected for 4 hours at 65° C. The oligonucleotides were purified by reverse phase HPLC (RP-HPLC). After the removal of the DMT-group, the oligonucleotides were characterized by AE-HPLC, RP-HPLC, and CGE and the molecular mass was further confirmed by ESI-MS. See below for more details.
[0198] Preparation of the LNA-Solid Support:
[0199] Preparation of the LNA Succinyl Hemiester
[0200] 5′-O-DMT-3′-hydroxy-LNA monomer (500 mg), succinic anhydride (1.2 eq.) and DMAP (1.2 eq.) were dissolved in DCM (35 mL). The reaction was stirred at room temperature overnig...
example 3
Design of the LNA Oligonucleotide
[0220]
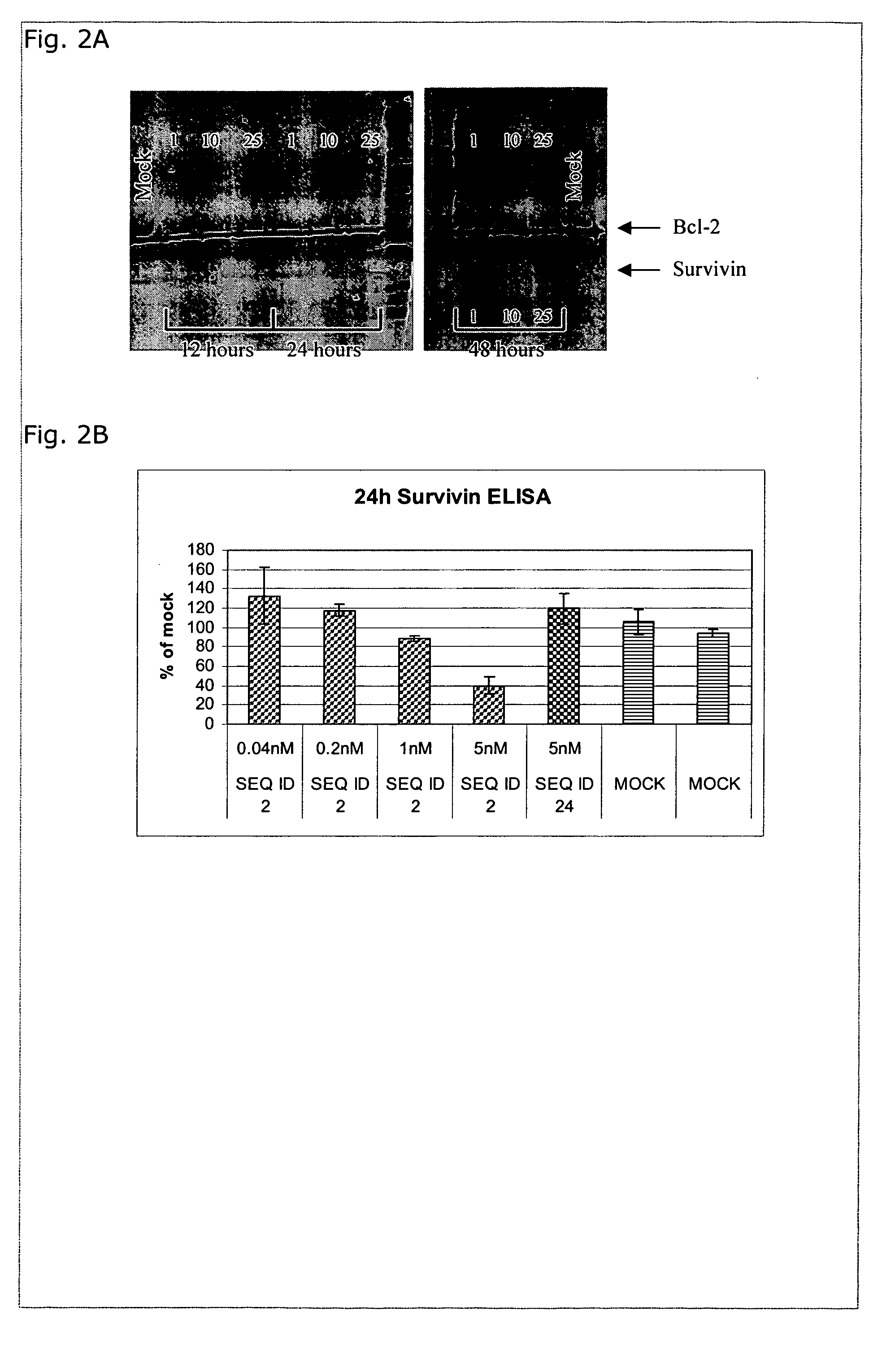
TABLE 2LNA OligonucleotidesSEQIDNO.Sequence and designdesigns15′-MeCxTxMeCxAsastscscsastsgsgsMeCxAxGxc-3′25′-MeCsTsMeCsAsastscscsastsgsgsMeCsAsGsc-3′4-8-3-1Reducing the amount of phosphorothioate links35′-MeCTMeCAsastscscsastsgsgsMeCAGsc-3′4-8-3-145′-MeCTMeCAsastscscsastsgsgsMeCAGc-3′4-8-3-1Reducing the amount of LNA monomers units55′-MeCsTsMeCsAsastscscsastsgsgsMeCsAsgsc-3′4-8-2-265′-csTsMeCsAsastscscsastsgsgsMeCsAsGsc-3′1-3-8-3-1Reducing the amount of phosphorothioatelinks and LNA monomers units75′-MeCTMeCAsastscscsastsgsgsMeCAgsc-3′4-8-2-285′-csTMeCAsastscscsastsgsgsMeCAGsc-3′1-3-8-3-195′-MeCTMeCAsastscscsastsgsgsMeCAgc-3′4-8-2-2105′-cTMeCAsastscscsastsgsgsMeCAGc-3′1-3-8-3-1LNA oligonucleotides containing a-L-LNA monomer units115′-MeCasTasMeCasAasastscscsastsgsgsMeCasAasGasc-3′4-8-3-1125′-MeCsTsMeCsAasastscscsastsgsgsMeCasAsGsc-3′4-8-3-1135′-MeCaTaMeCaAasastscscsastsgsgsMeCaAaGasc-3′4-8-3-1145′-MeCaTaMeCaAasastscscsastsgsgsMeCaAaGac-3′4-8-3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionic strength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com