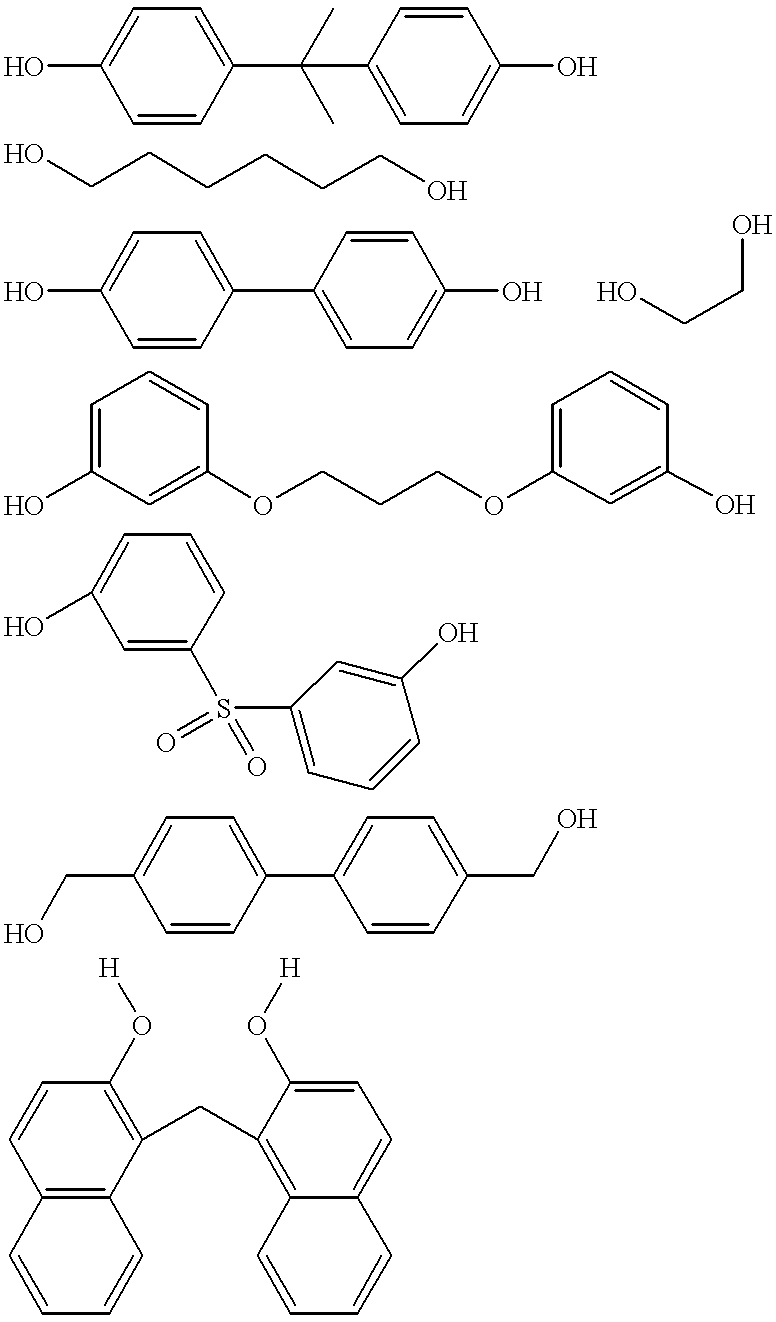
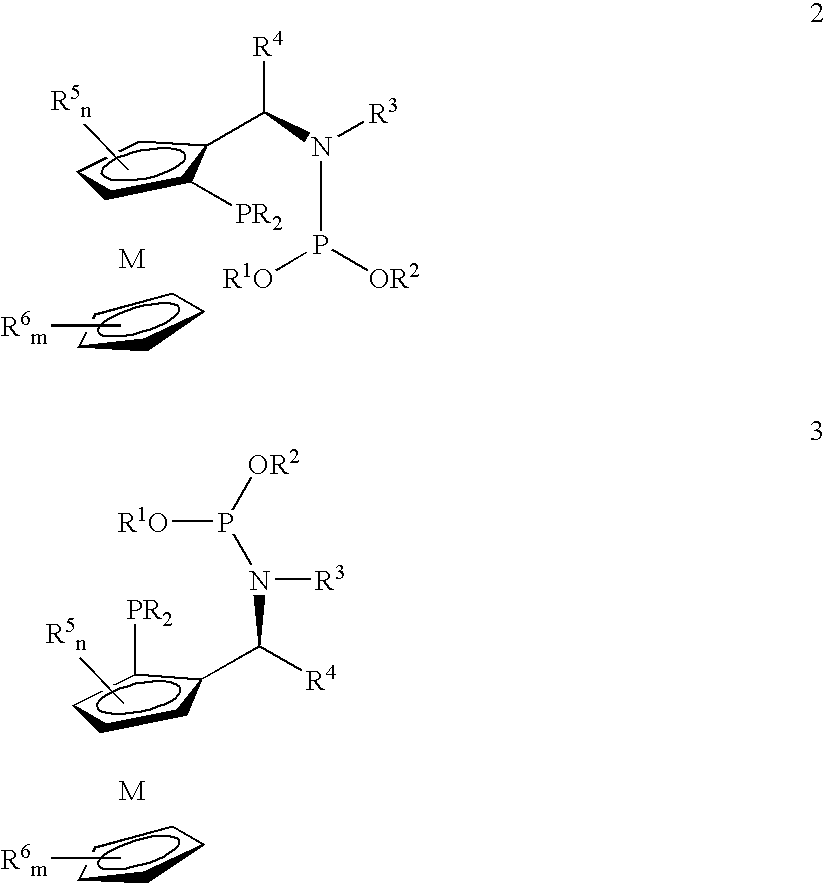
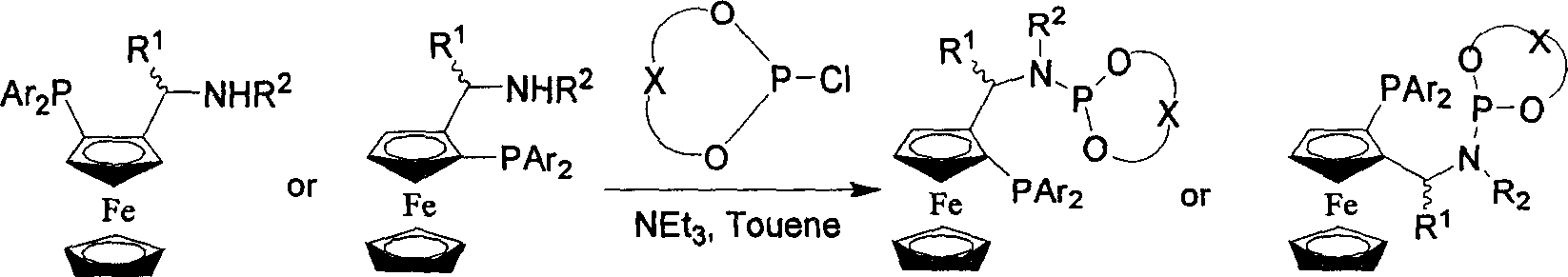
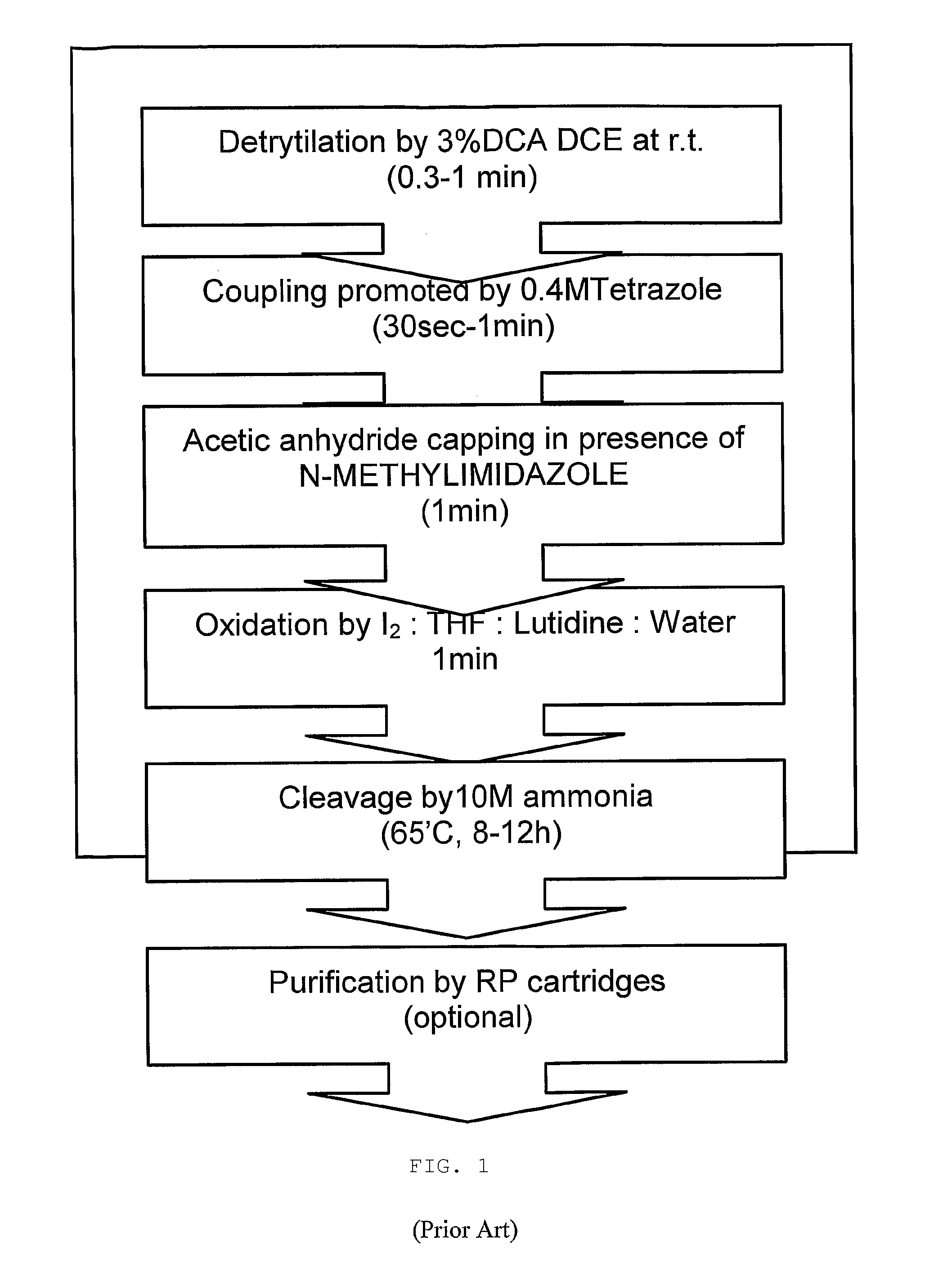
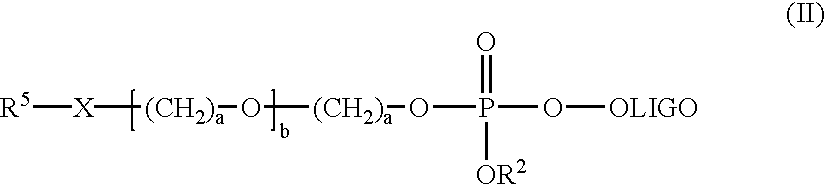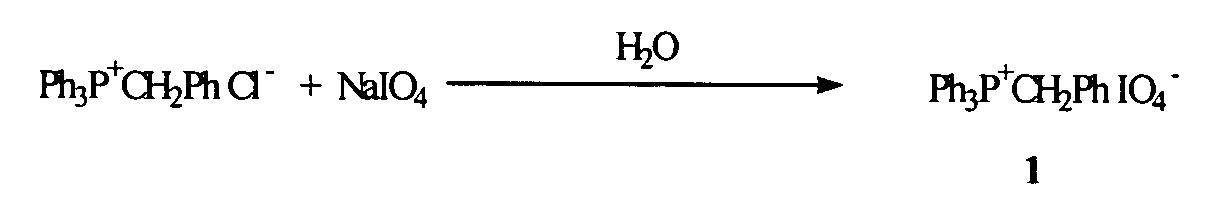Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
239 results about "Phosphoramidite" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
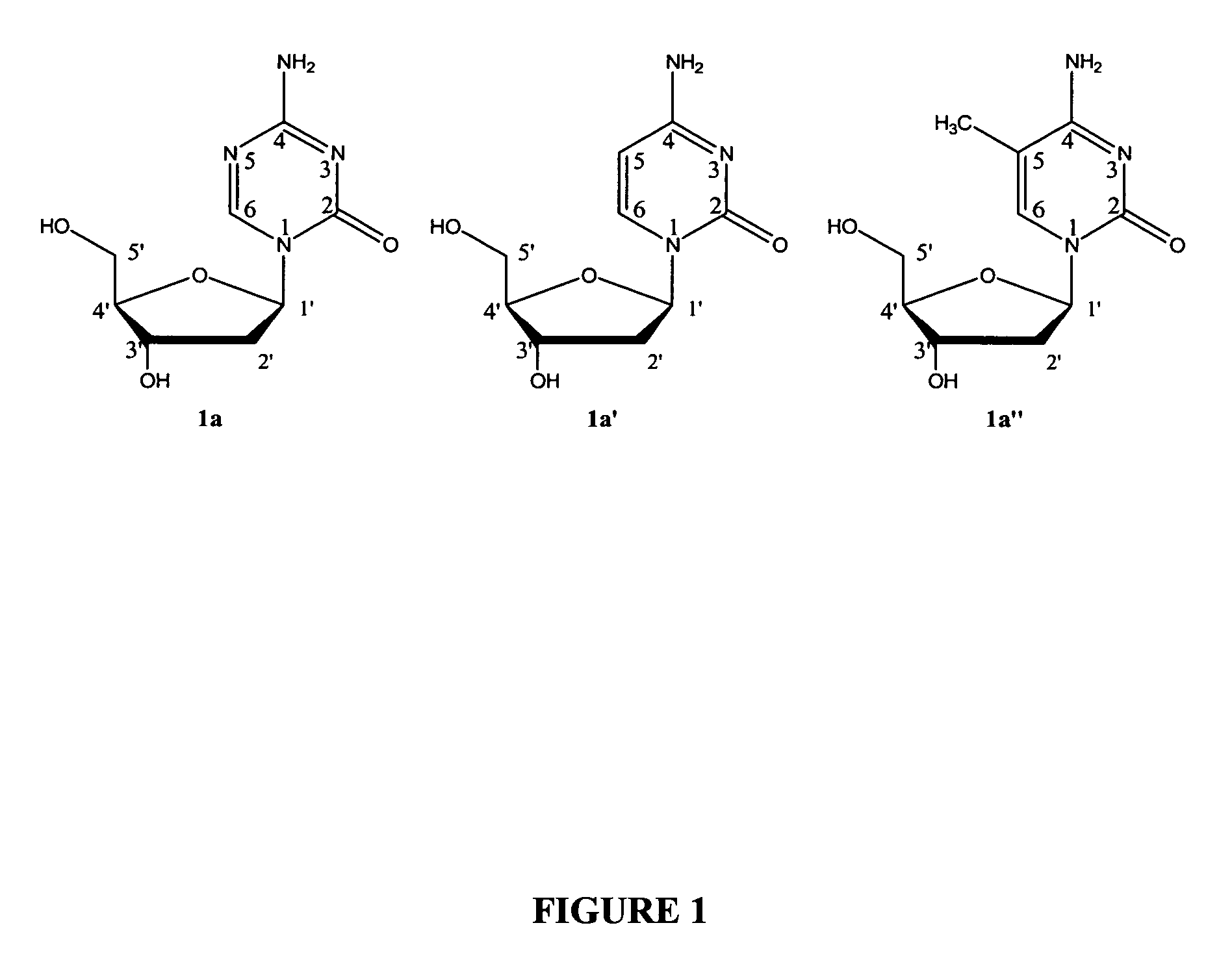
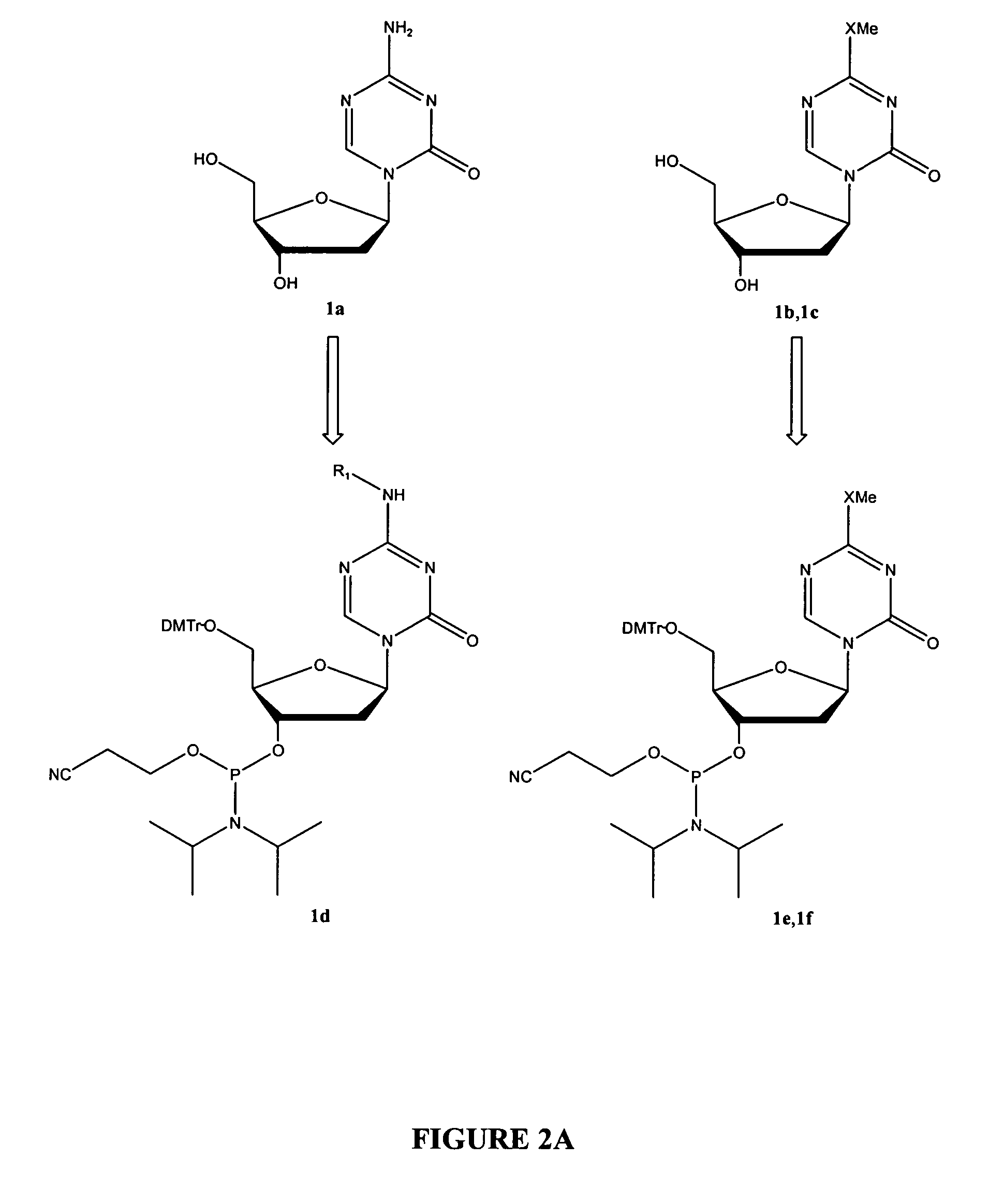
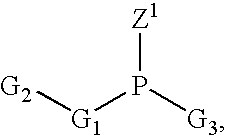
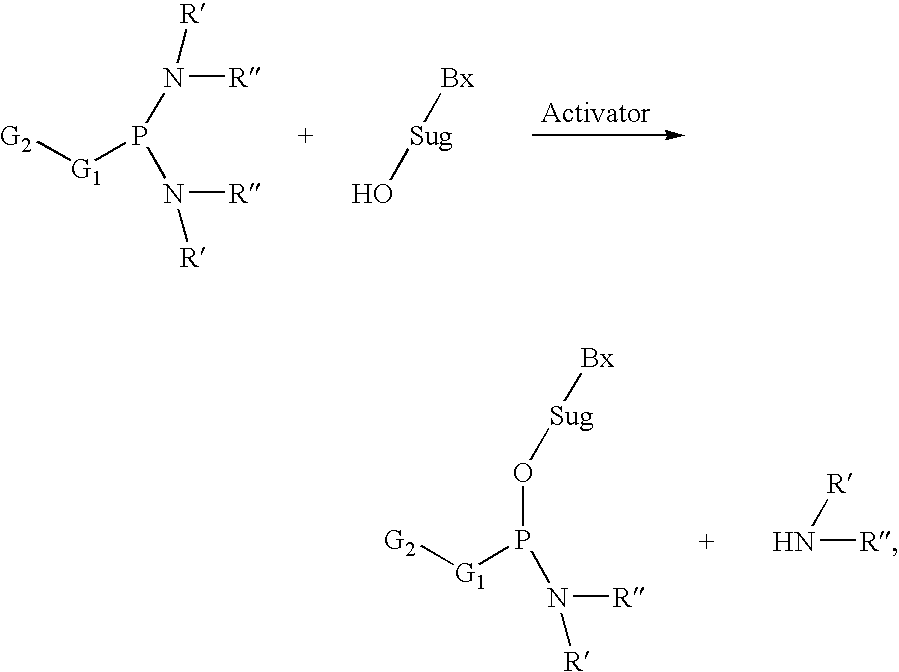
A phosphoramidite (RO)₂PNR₂ is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids e.c., triethylammonium chloride or 1H-tetrazole. In these reactions, the incoming nucleophile replaces the NR₂ moiety.
Oligonucleotide synthesis with alternative solvents
ActiveUS7276599B2Efficient synthesisSugar derivativesMicrobiological testing/measurementPhosphateOligonucleotide synthesis
The invention provides for methods of manufacturing an oligonucleotide comprising a pentavalent phosphate triester. In particular, the method comprises providing a 5′ blocked-nucleoside, deblocking the 5′ blocked-nucleoside to form a 5′ OH-nucleoside, coupling the 5′ OH-nucleoside with a phosphoramidite to form and oligonucleotide comprising a trivalent phosphite triester; and oxidizing the oligonucleotide comprising a trivalent phosphite triester to the oligonucleotide comprising a pentavalent phosphate triester. In some embodiments, the wash between any of the steps above is with at least one solvent wash comprising a toluene.
Owner:IONIS PHARMA INC
Method of down-regulating gene expression
InactiveUS6936593B1Down-regulated expressionOrganic active ingredientsBiocideCarbamateDithiophosphoric acid
Disclosed is a method of down-regulating the expression of a gene in an animal, wherein a pharmacological formulation comprising a chimeric oligonucleotide complementary to the gene is orally administered to an animal. The oligonucleotide administered has at least one phosphorothioate internucleotide linkage and at least one alkylphosphonate, phosphorodithioate, alkylphosphonothioate, phosphoramidate, phosphoramidite, phosphate ester, carbamate, carbonate, phosphate triester, acetamidate, or carboxymethyl ester internucleotide linkage.
Owner:IDERA PHARMA INC
Method for preparation of LNA phosphoramidites
The present invention relates to large scale preparation of LNA phosphoramidites using a 2-cyanoethyl-N,N,N′,N′-tetra-substituted phosphoramidite and a nucleophilic activator, e.g. 2-cyanoethyl-N,N,N′,N′-tetraisopropylphosphoramidite and 4,5-dicyanoimidazole. The method is faster and more cost efficient that previously known methods.
Owner:SANTARIS PHARMA AS
Novel methods for the synthesis and purification of oligomers
InactiveUS20130137861A1Carbamic acid derivatives preparationSugar derivativesOligomerOligonucleotide synthesis
A reagent for oligonucleotide synthesis or purification, wherein the reagent has a structure of:X—C-L-H (Formula A)wherein X is a phosphoramidite group, an H-phosphonate group, an acetal group, or an isocyanate; C is a direct bond or a cleavable adaptor represented by —Ca-Cb—; L is a hydrocarbyl chain; and H is a terminal alkyne or an activated cyclooctyne. The reagent of Formula (A) can be used in the synthesis and purification of oligonucleotides.
Owner:AGILENT TECH INC
Oligonucleotide analogues incorporating 5-aza-cytosine therein
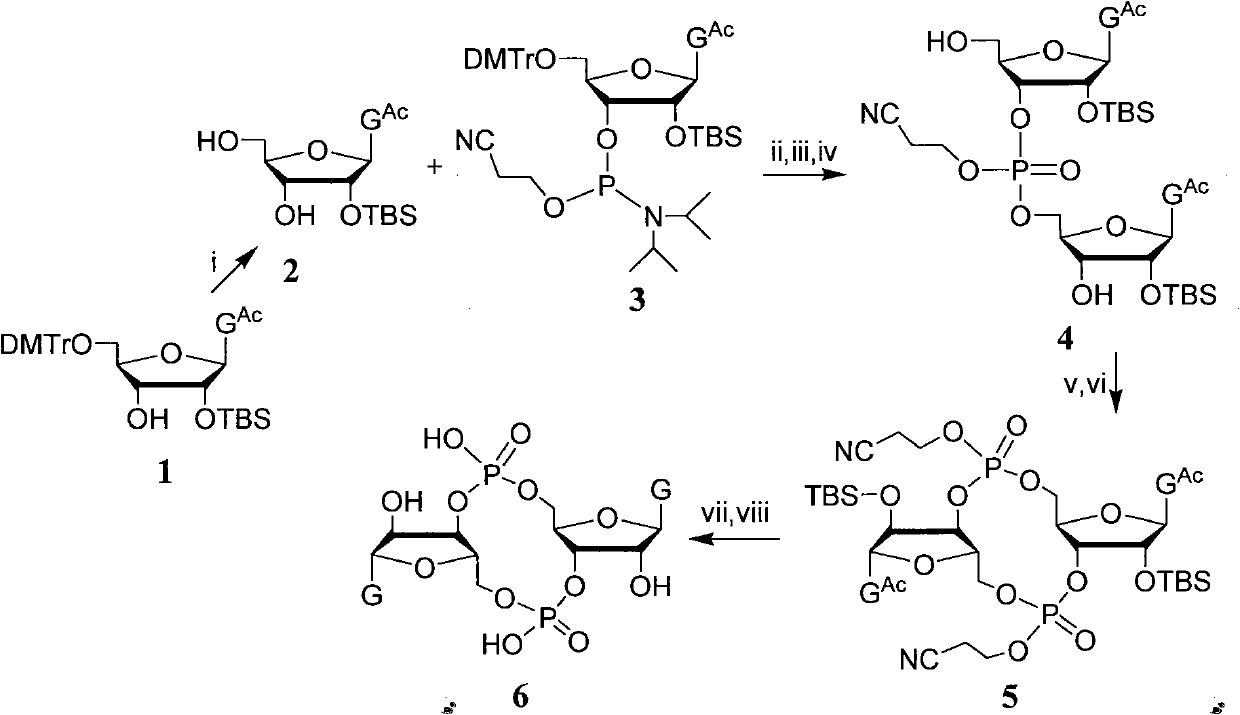
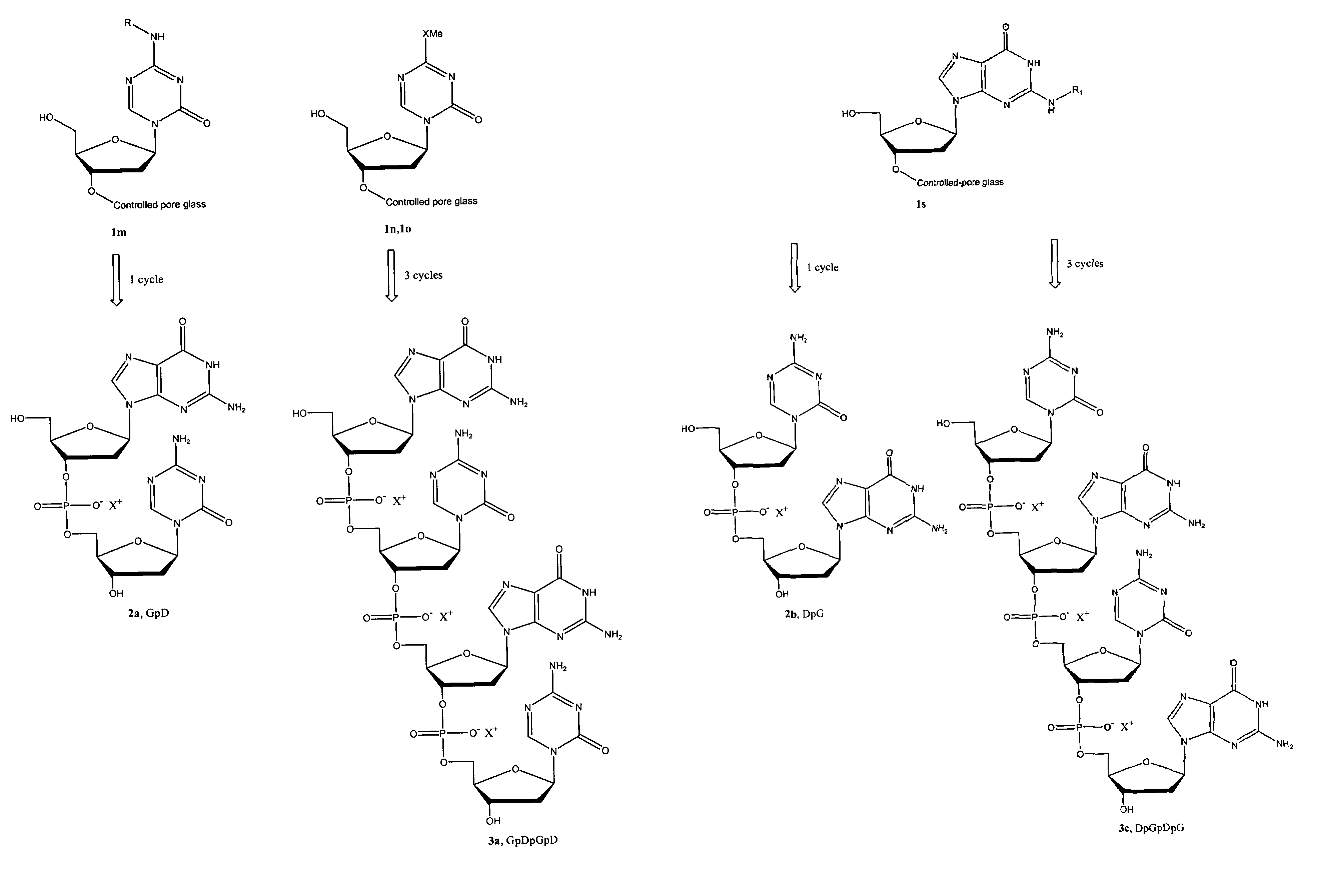
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Oligonucleotide ligation
ActiveUS20130046084A1Efficient methodImprove thermal stabilitySugar derivativesSugar derivatives preparationSynthesis methodsAlkyne
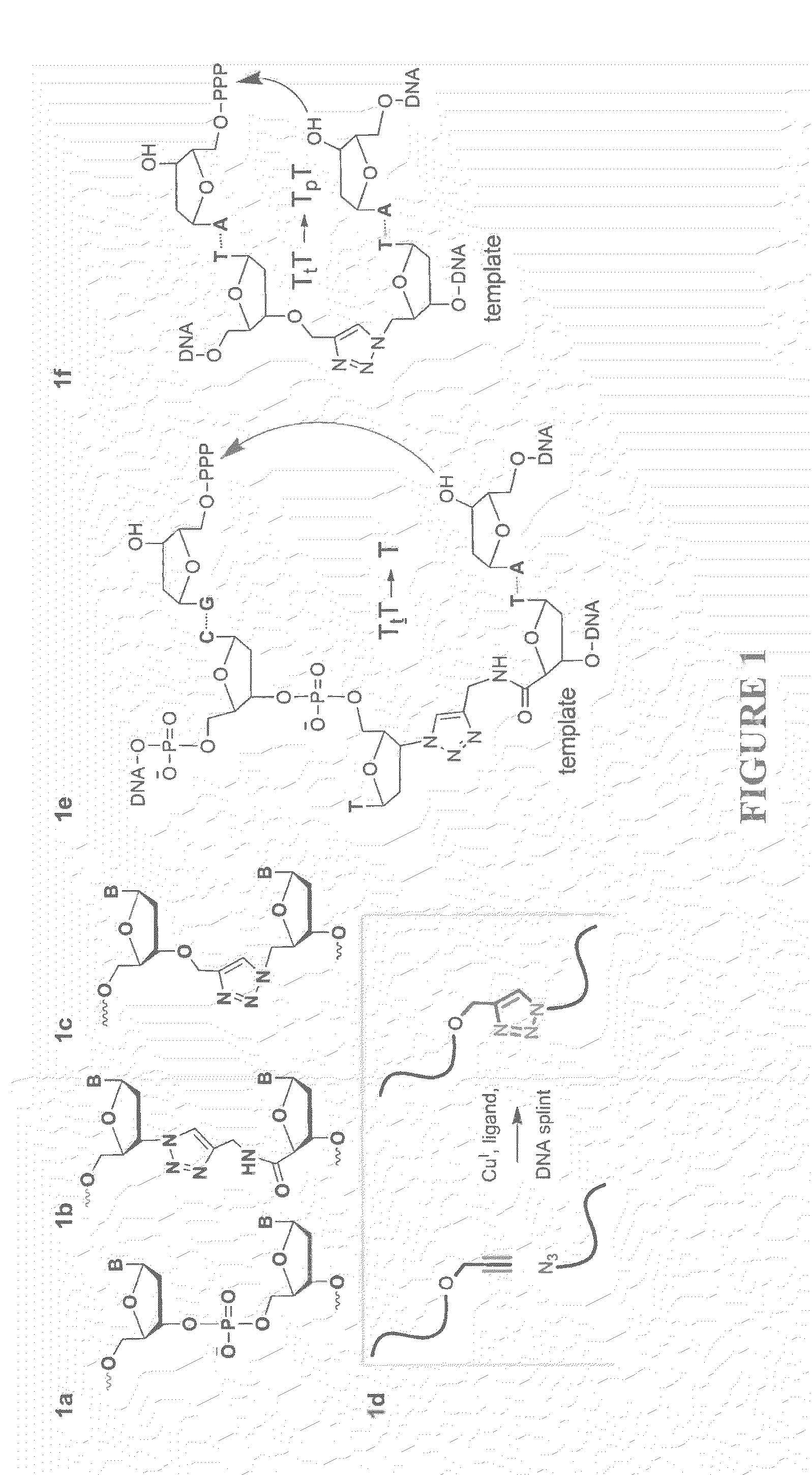
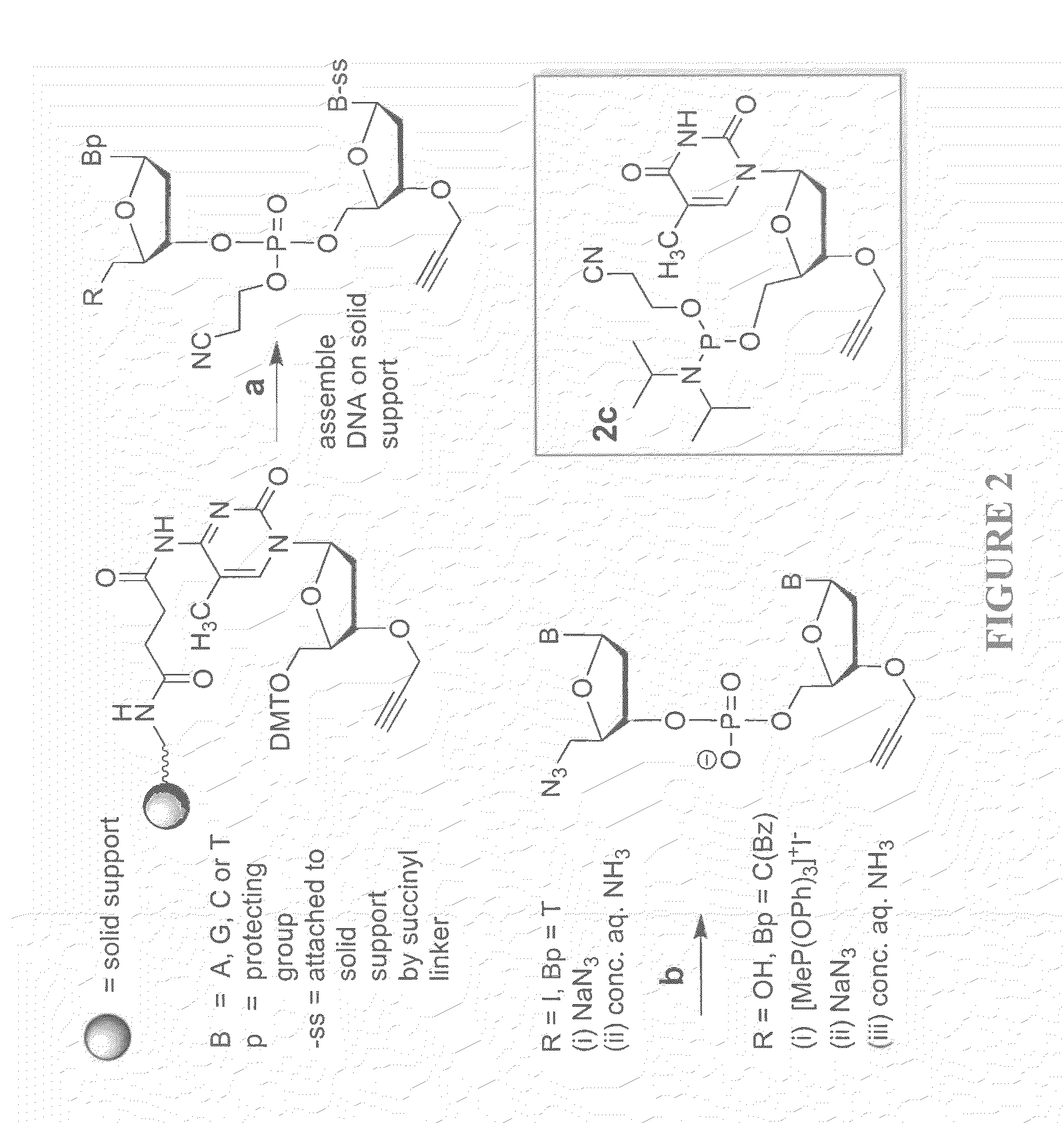
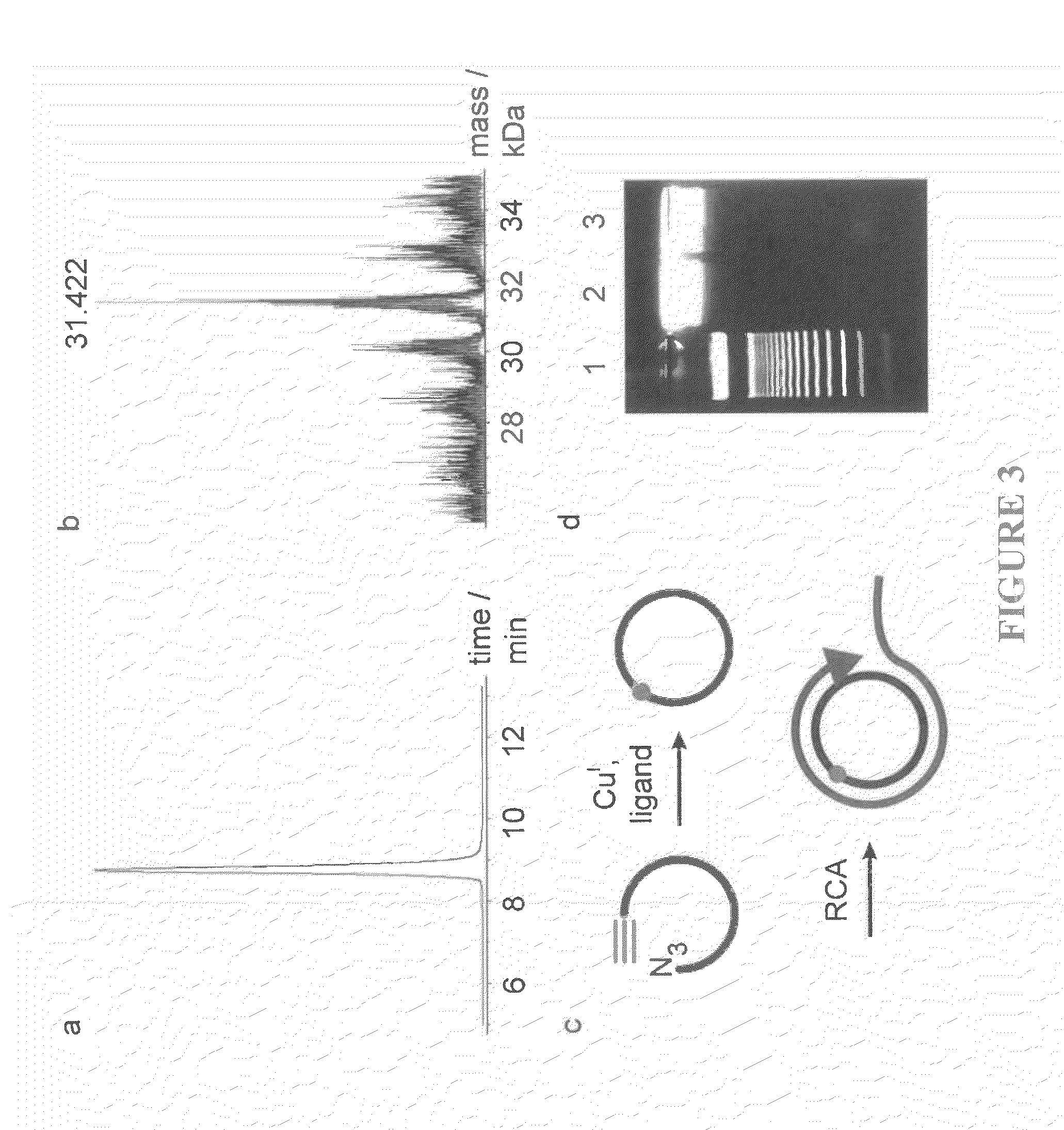
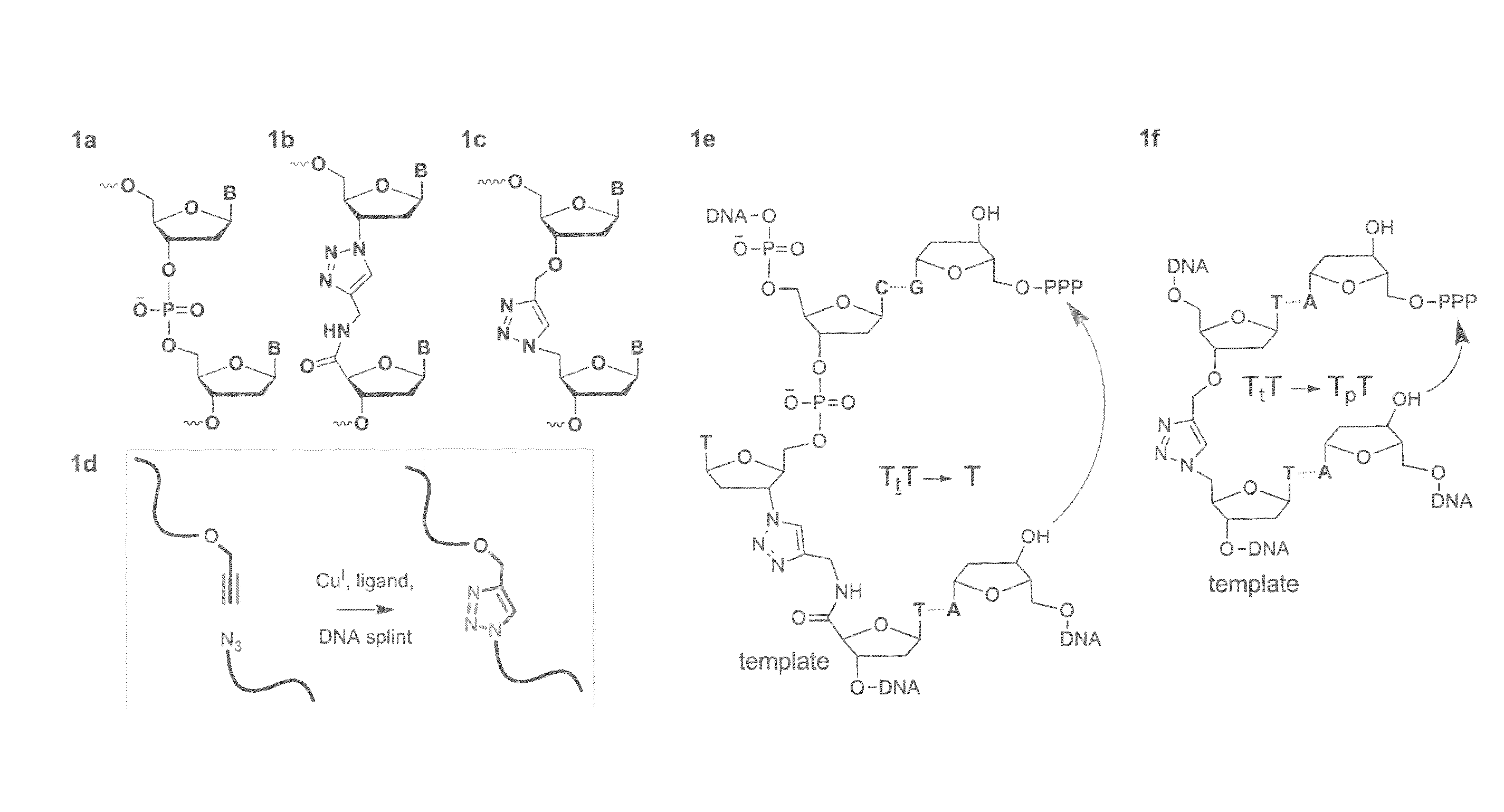
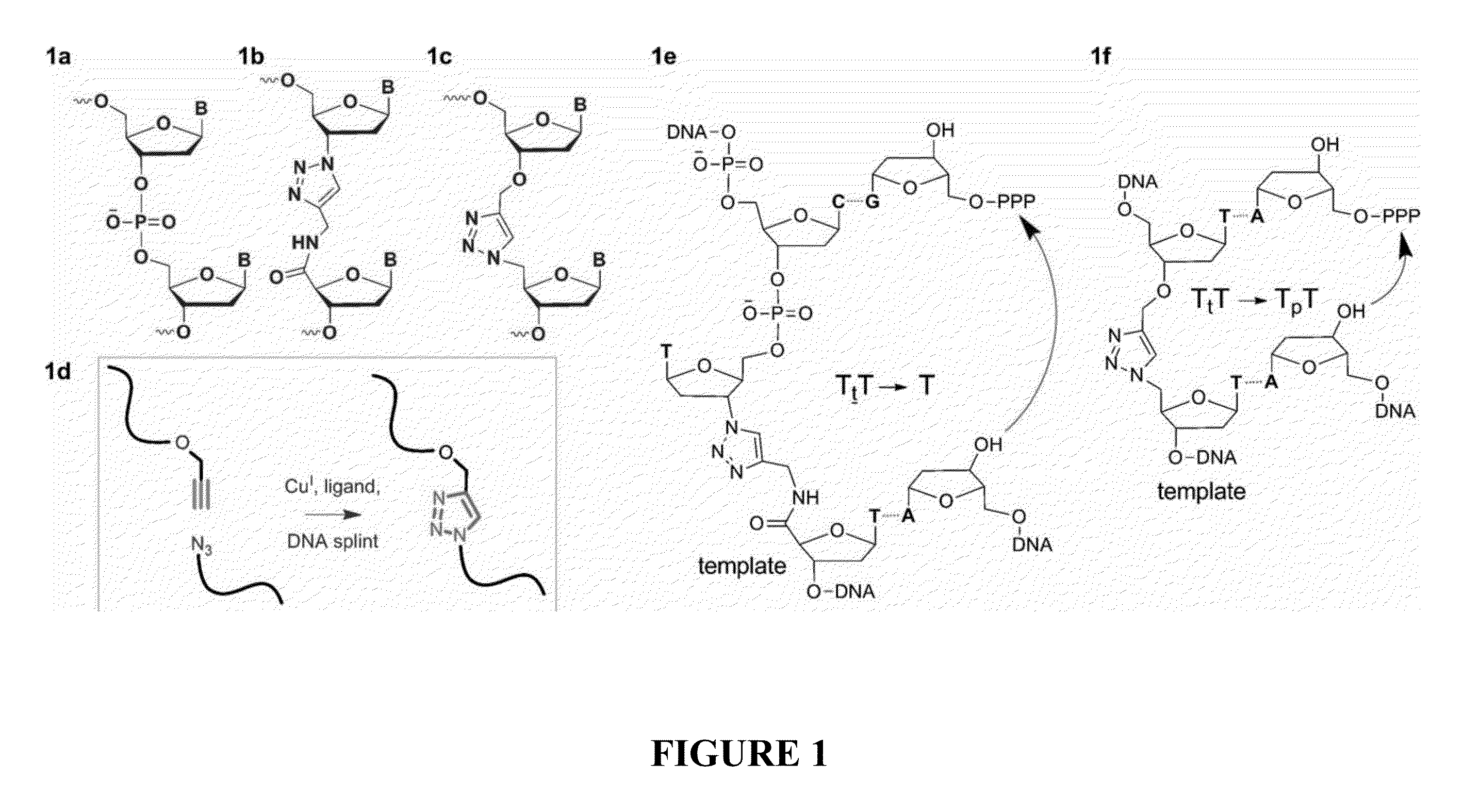
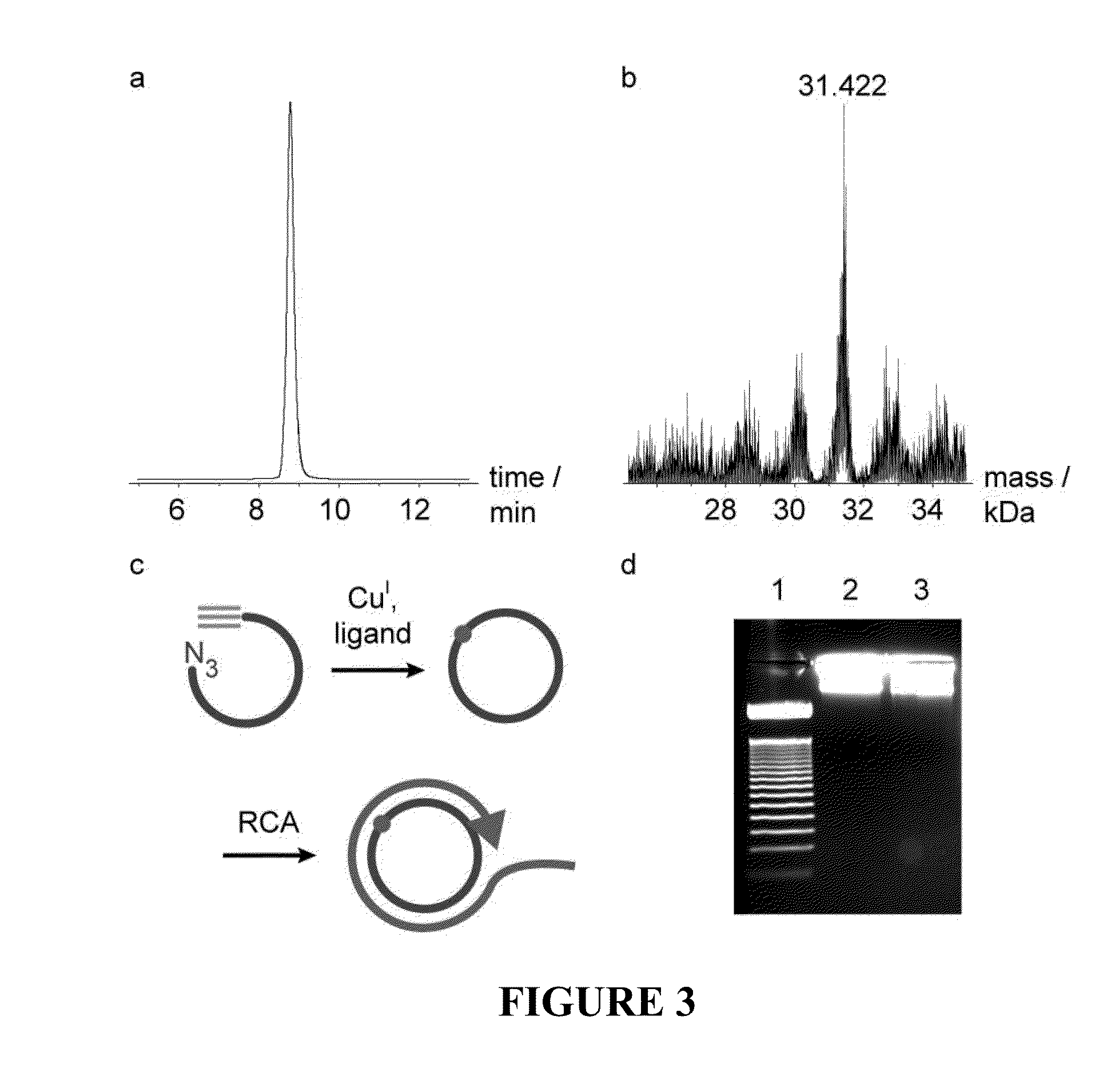
Oligonucleotide chemistry is central to the advancement of core technologies such as DNA sequencing, forensic and genetic analysis and has impacted greatly on the discipline of molecular biology. Oligonucleotides and their analogues are essential tools in these areas. They are often produced by automated solid-phase phosphoramidite synthesis but it is difficult to synthesize long DNA and RNA sequences by this method. Methods are proposed for ligating oligonucleotides together, in particular the use of an azide-alkyne coupling reaction to ligate the backbones of oligonucleotides together to form longer oligonucleotides that can be synthesized using current phosphoramidite synthesis methods.
Owner:ATDBIO LTD
Methods and compositions for the tandem synthesis of two or more oligonucleotides on the same solid support
InactiveUS20060149046A1Simple and smooth and efficientWide range of applicationsEsterified saccharide compoundsSugar derivativesOligonucleotide primersCombinatorial chemistry
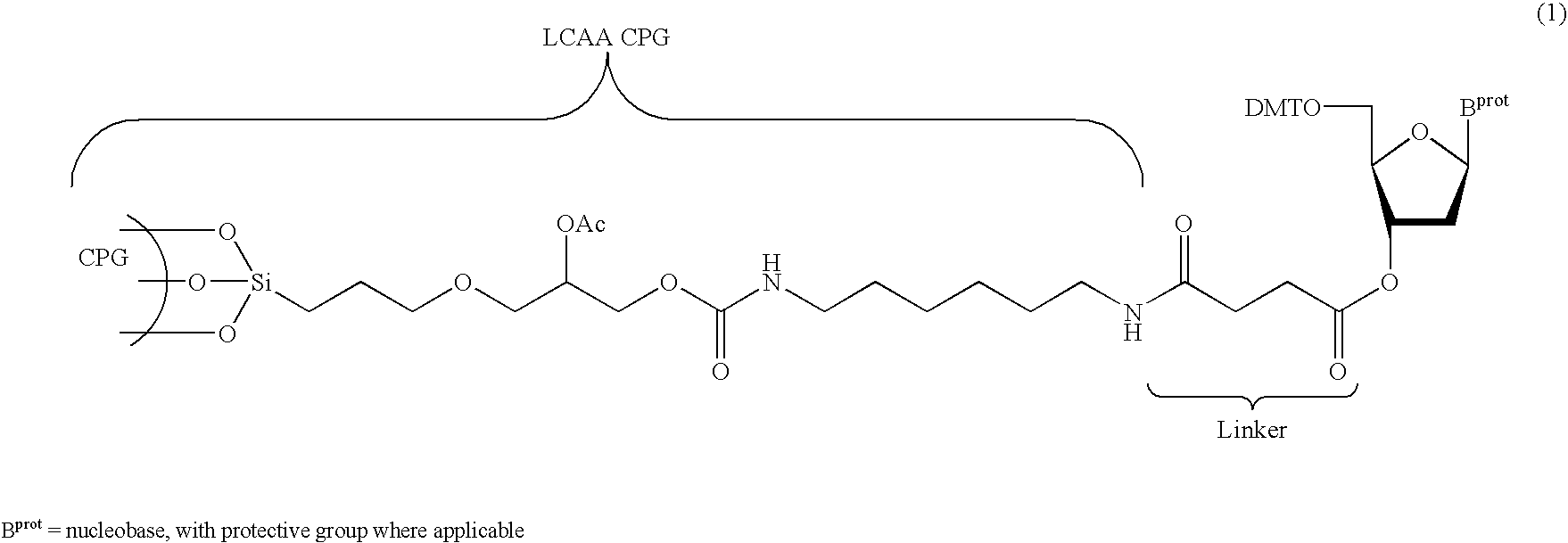
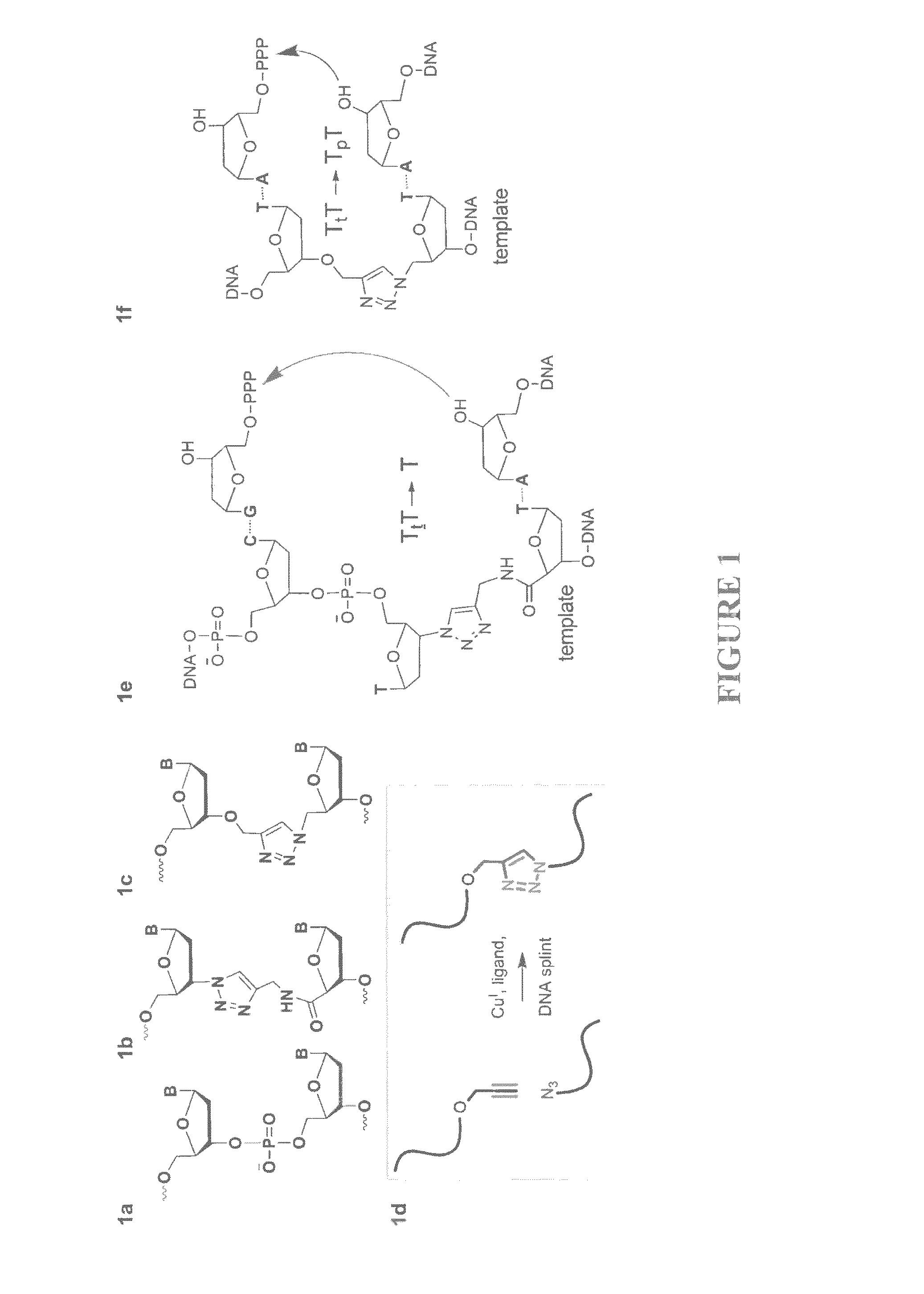
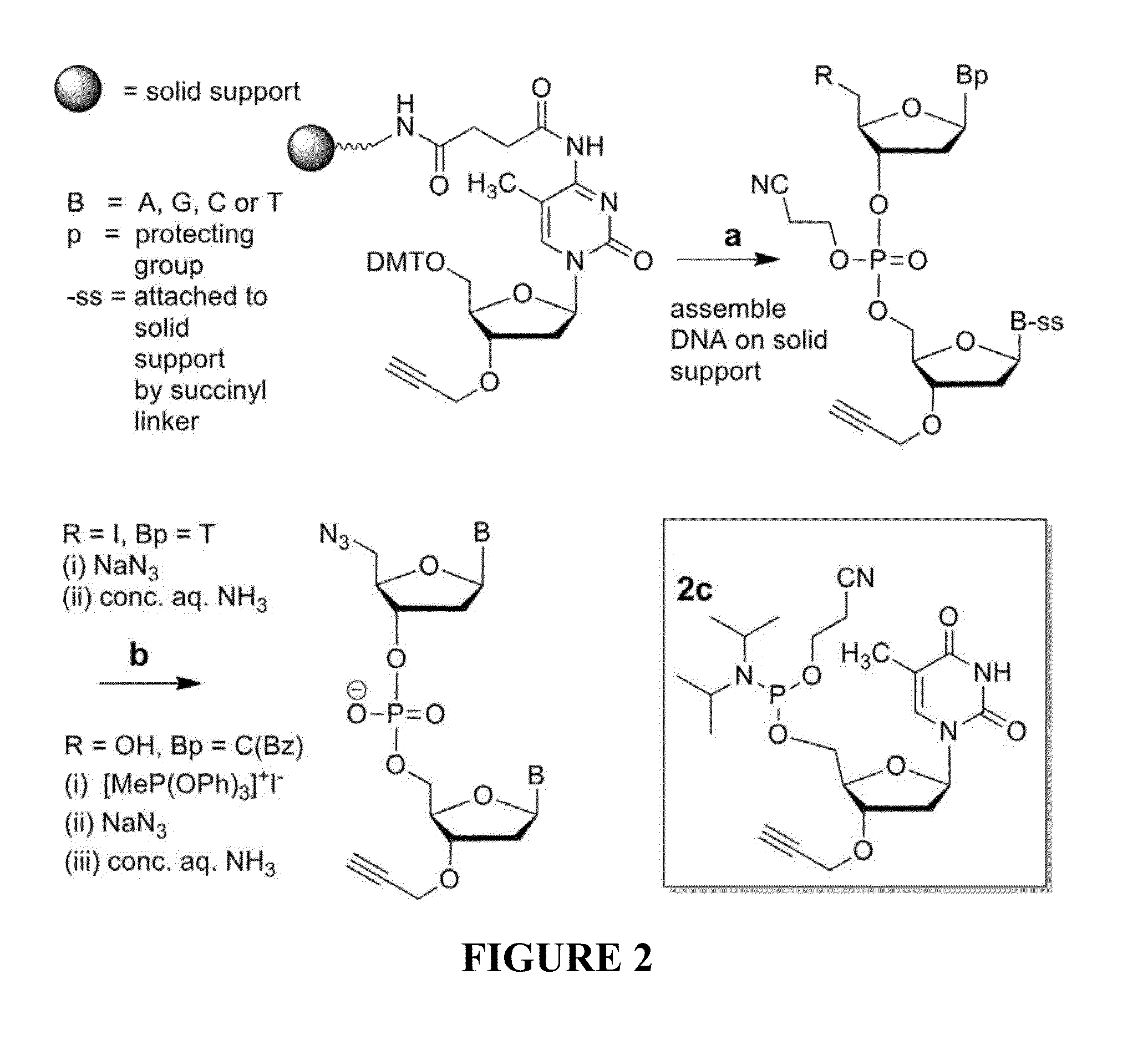
The present invention relates to novel methods and novel solid support materials for the tandem synthesis of two or more different oligonucleotides on the same solid support in one synthetic run. The methods involve novel support preparations comprised of two or more types of orthogonally protected anchor groups. Subsequent to the selective removal of the first of the respective protective groups, the first oligonucleotide is assembled on the deblocked anchor groups according to standard methods, preferably via phosphoramidite chemistry. Following the capping of said first oligonucleotide, the anchor groups blocked by the second type of protective group are selectively liberated and serve is the starting point for the assembly of a second oligonucleotide, and so forth. After completion of all of the syntheses on the solid support, the oligonucleotides are released from the solid support and deprotected at the nucleobases, using standard methods. Preparations obtained using the method of this invention, generally contain two or more different oligonucleotides. Such preparations are particularly useful in applications that require pairs of oligonucleotide primers, several probes at a time, duplexed nucleic acid fragments, or other combinations of oligonucleotides that are useful in applications such as PCR, sequencing, multiplexed genotyping, cloning and RNA interference. The invention includes procedures for the preparation of the novel solid supports of the invention.
Owner:SIGMA ALDRICH CO LLC
Design, Synthesis and Assembly of Synthetic Nucleic Acids
ActiveUS20080300842A1Enhanced couplingEasy subsequent assemblyMicrobiological testing/measurementAnalogue computers for chemical processesNucleotideOligonucleotide synthesis
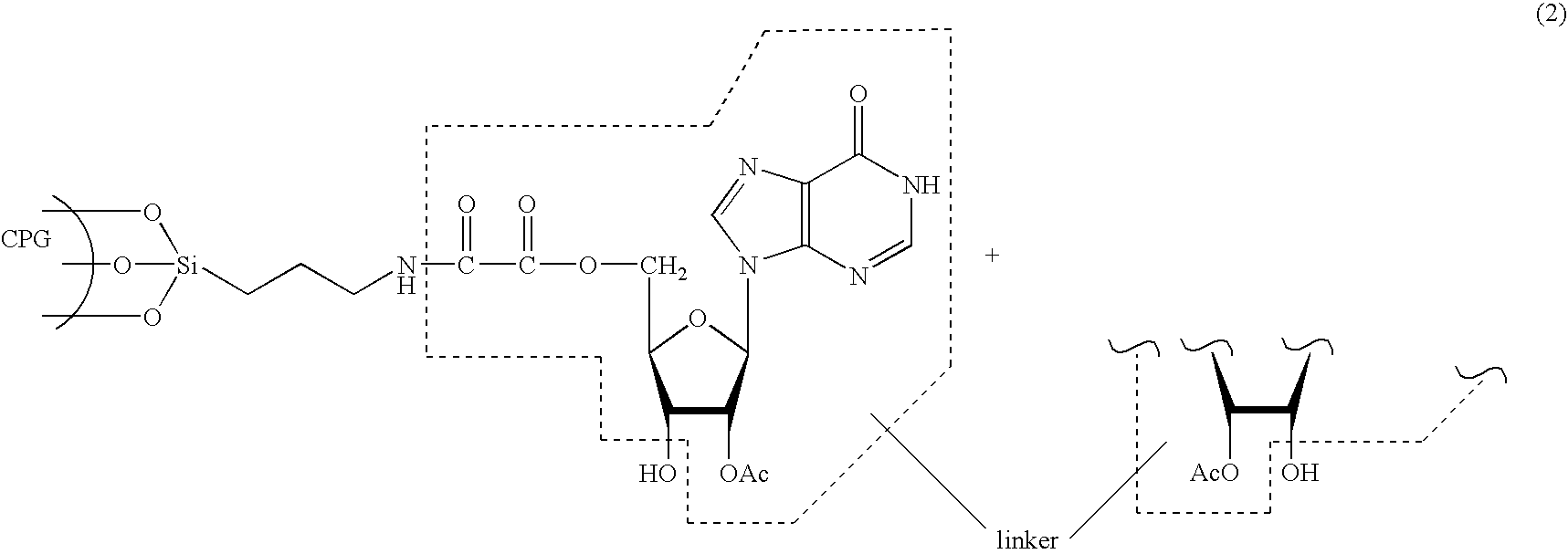
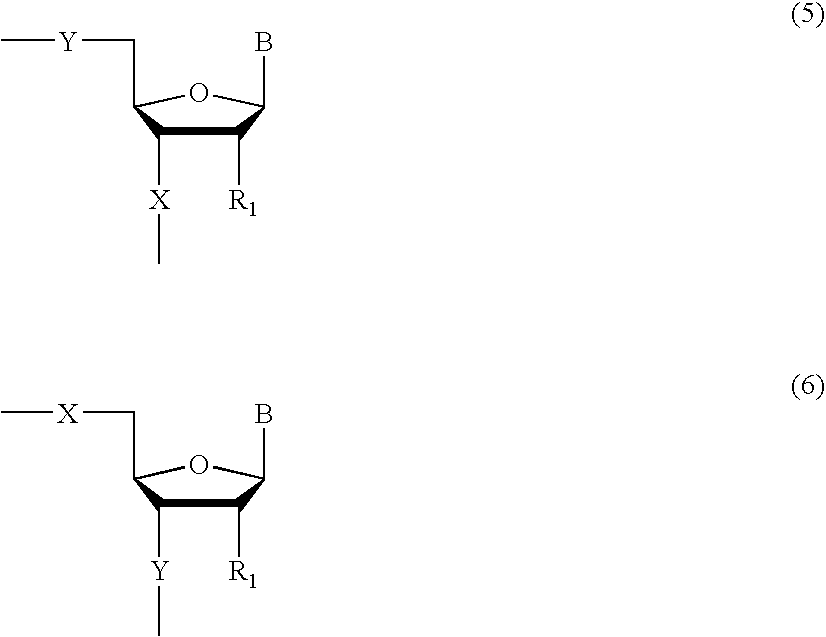
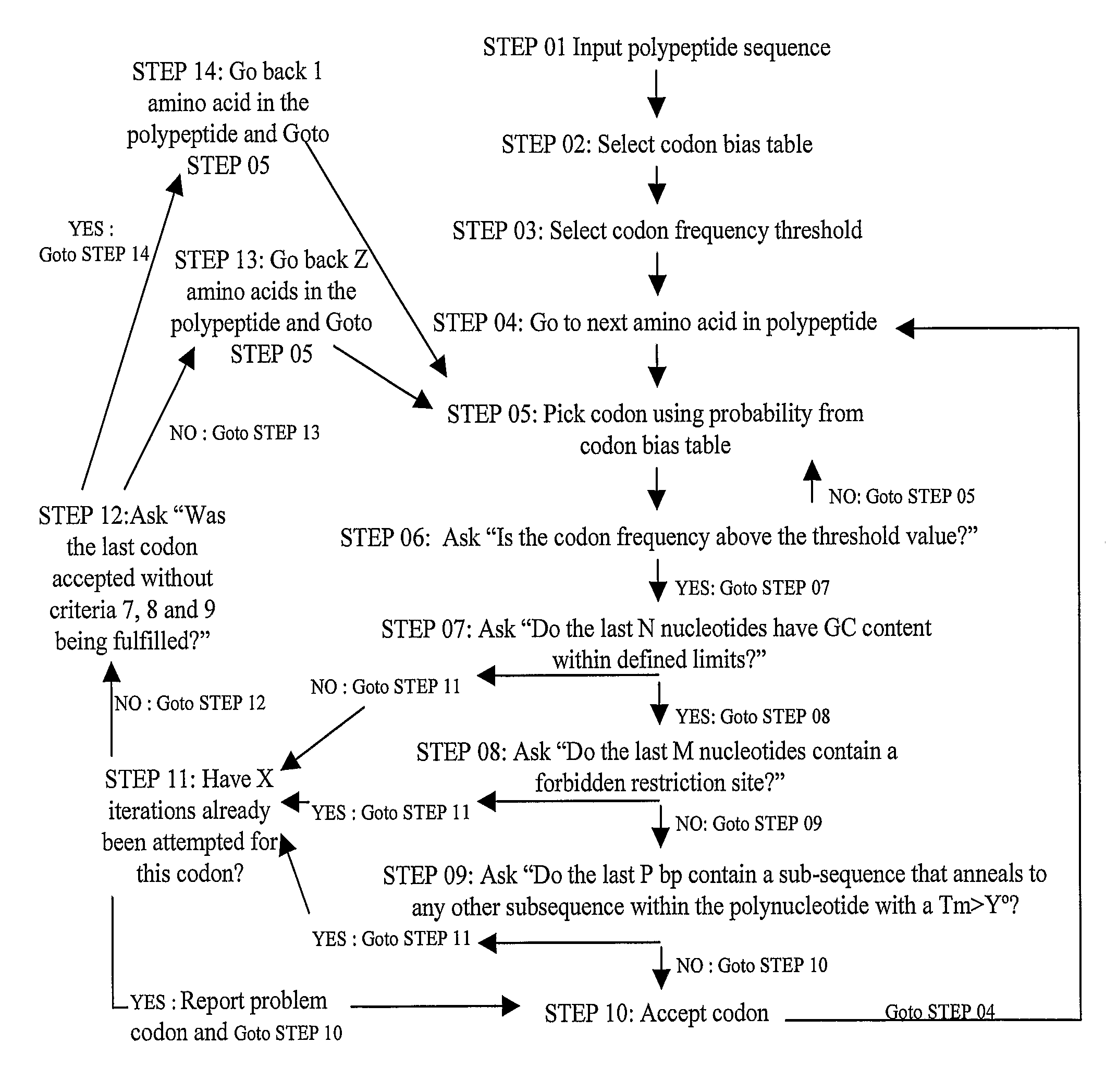
Methods of synthesizing oligonucleotides with high coupling efficiency (>99.5%) are provided. Methods for purification of synthetic oligonucleotides are also provided. Instrumentation configurations for oligonucleotide synthesis are also provided. Methods of designing and synthesizing polynucleotides are also provided. Polynucleotide design is optimized for subsequent assembly from shorter oligonucleotides. Modifications of phosphoramidite chemistry to improve the subsequent assembly of polynucleotides are provided. The design process also incorporates codon biases into polynucleotides that favor expression in defined hosts. Design and assembly methods are also provided for the efficient synthesis of sets of polynucleotide variants. Software to automate the design and assembly process is also provided.
Owner:DNA TWOPOINTO
Methods for the synthesis and purification of oligomers
InactiveUS8889851B2Carbamic acid derivatives preparationSugar derivativesPurification methodsOligomer
A reagent for oligonucleotide synthesis or purification, wherein the reagent has a structure of:X—C—L—H (Formula A)wherein X is a phosphoramidite group, an H-phosphonate group, an acetal group, or an isocyanate; C is a direct bond or a cleavable adaptor represented by —Ca—Cb—; L is a hydrocarbyl chain; and H is a terminal alkyne or an activated cyclooctyne. The reagent of Formula (A) can be used in the synthesis and purification of oligonucleotides.
Owner:AGILENT TECH INC
C-di-GMP, analogues thereof and preparation method thereof
ActiveCN102199183AInhibition formationPrevent proliferationSugar derivativesSugar derivatives preparationDrug developmentBiological membrane
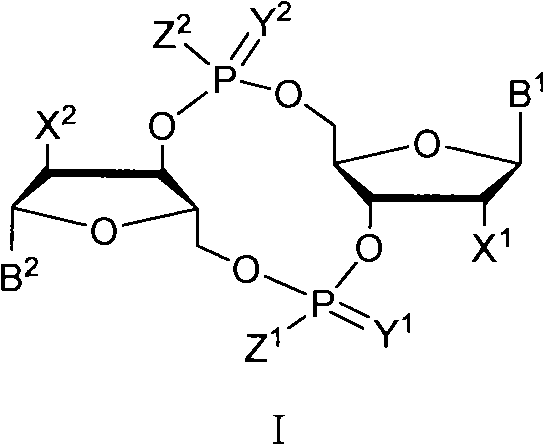
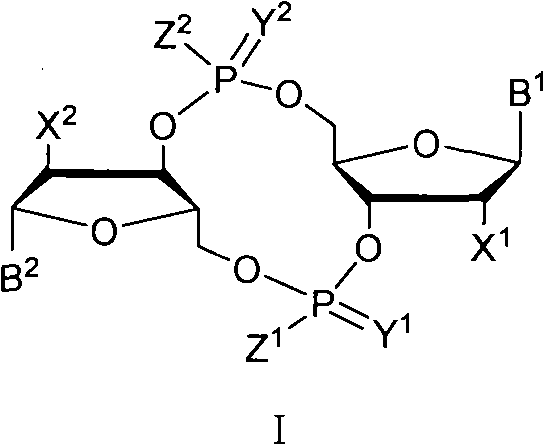
The invention discloses a c-di-GMP and analogues thereof which have structures of a general formula I. The invention also discloses a novel preparation method-a kettle phosphoramidite method which can be used for rapidly, simply and conveniently preparing c-di-GMP compounds with high yield and low cost on a large scale and at mild conditions. The c-di-GMP is prevalent in bacteria and is a novel second messenger molecular which takes part in regulating multiple physiological functions. The research shows that the c-di-GMP and the analogues thereof can inhibit the formation of bacterium biological membranes and the multiplication of eukaryotic cells; and thereof the c-di-GMP and the analogues thereof have good medicine development prospect.
Owner:PEKING UNIV
Microarray with hydrophobic barriers
InactiveUS7498176B2Material nanotechnologyBioreactor/fermenter combinationsCrystallographyProtecting group
The present invention is a microarray having a plurality of subarrays with a hydrophobic barrier that defines each subarray of the microarray, and a method for preparing such a microarray. The hydrophobic barrier is prepared using a microarray synthesis instrument, where NPPOC photoprotected and other hydrophobic group-bearing phosphoramidites are coupled to the microarray using light from a digital micromirror to direct formation of the hydrophobic barrier. The method utilizes hydrophobicity, a well-established property, of conventional phosphoramidite protecting groups for an entirely new application, the synthesis of hydrophobic barriers on microarrays.
Owner:ROCHE NIMBLEGEN
Oligonucleotide analogues incorporating 5-aza-cytosine therein
Oligonucleotide analogues are provided that incorporate 5-aza-cytosine in the oligonucleotide sequence, e.g., in the form of 5-aza-2′-deoxycytidine (decitabine) or 5-aza-cytidine. In particular, oligonucleotide analogues rich in decitabine-deoxyguanosine islets (DpG and GpD) are provided to target the CpG islets in the human genome, especially in the promoter regions of genes susceptible to aberrant hypermethylation. Such analogues can be used for modulation of DNA methylation, such as effective inhibition of methylation of cytosine at the C-5 position. Methods for synthesizing these oligonucleotide analogues and for modulating nucleic acid methylation are provided. Also provided are phosphoramidite building blocks for synthesizing the oligonucleotide analogues, methods for synthesizing, formulating and administering these compounds or compositions to treat conditions, such as cancer and hematological disorders.
Owner:SUPERGEN
Phosphoramidite Compound And Method For Producing Oligo-Rna
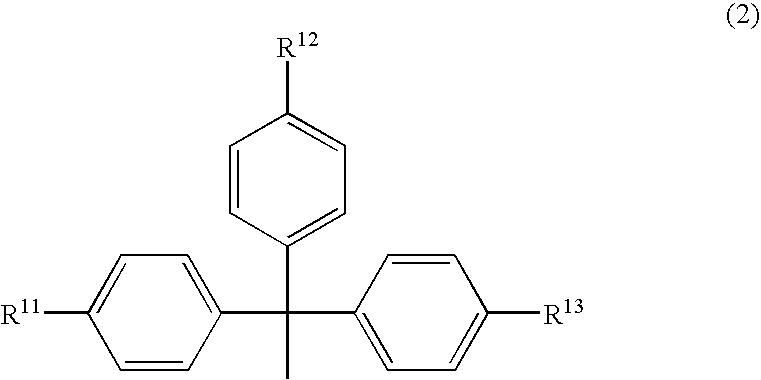
ActiveUS20070282097A1Simple and high-yield methodSimple and high-yieldSugar derivativesOrganic compound preparationNitrogen atomPhosphoramidite
An object of the present invention is to provide a useful and novel phosphoramidite compound for the synthesis of oligo-RNA. A phosphoramidite compound represented by general formula (1), wherein: BX represents a nucleobase optionally having a protecting group; and R1 is a substituent represented by general formula (2), wherein: R11, R12 and R13 are the same or different and each represents hydrogen or alkoxy; R2a and R2b are the same or different and each represents alkyl, or R2a and R2b taken together with the adjacent nitrogen atom may form a 5- to 6-membered saturated amino cyclic group, the amino cyclic group optionally having an oxygen or sulfur atom as a ring-composing member in addition to the adjacent nitrogen atom; and WG1 and WG2 are the same or different and each represents an electron-withdrawing group.
Owner:NIPPON SHINYAKU CO LTD
Oligonucleotide ligation
ActiveUS8846883B2Improve thermal stabilitySugar derivativesMicrobiological testing/measurementSynthesis methodsAlkyne
Oligonucleotide chemistry is central to the advancement of core technologies such as DNA sequencing, forensic and genetic analysis and has impacted greatly on the discipline of molecular biology. Oligonucleotides and their analogues are essential tools in these areas. They are often produced by automated solid-phase phosphoramidite synthesis but it is difficult to synthesize long DNA and RNA sequences by this method. Methods are proposed for ligating oligonucleotides together, in particular the use of an azide-alkyne coupling reaction to ligate the backbones of oligonucleotides together to form longer oligonucleotides that can be synthesized using current phosphoramidite synthesis methods.
Owner:ATDBIO LTD
Polymeric phosphite composition and hydrocyanation of unsaturated organic compounds and the isomerization of unsaturated nitriles
InactiveUS6855799B2Reduce solubilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsIsomerizationOrganic compound
A polymeric composition, a process for producing the composition, and a process for using the composition in, for example, hydrocyanation or isomerization are disclosed. The composition comprises repeat units derived from (1) a carbonyl compound, a monomer, and phosphorochloridite; (2) phosphorus trichloride, a polyhydric alcohol, and an aromatic diol; or (3) combinations of (1) and (2) in which the monomer can be a polyhydric alcohol, an amine, combinations thereof. The composition can further comprise a Group VIII metal and optionally a Lewis acid. The composition can be produced by (1) contacting a carbonyl compound with the monomer to produce an intermediate and contacting the intermediate with phosphorochloridite; (2) contacting phosphorus trichloride with a second polyhydric alcohol under a condition sufficient to produce a phosphorus-containing polymer and contacting the phosphorus-containing polymer with an aromatic diol; or (3) contacting an N,N-dialkyl dichlorophosphoramidite with a second polyhydric alcohol to produce a polymer phosphoramidite, contacting the polymer phosphoramidite with an acid such as HCl to produce the phospphorus-containing polymer, which is then contacted with an aromatic diol. The composition can be used as catalyst, for example, for converting an unsaturated organic compound to a nitrile and isomerizing a nitrile.
Owner:INVISTA NORTH AMERICA R L
Mobility-modified nucleobase polymers and methods of using same
InactiveUS6743905B2Expanded repertoireAdd additional massSugar derivativesMicrobiological testing/measurementCapillary electrophoresisPhosphate
The present invention relates generally to nucleobase polymer functionalizing reagents, to mobility-modified sequence-specific nucleobase polymers, to compositions comprising a plurality of mobility-modified sequence-specific nucleobase polymers, and to the use of such polymers and compositions in a variety of assays, such as, for example, for the detection of a plurality of selected nucleotide sequences within one or more target nucleic acids. The mobility-modifying polymers of the present invention include phosphoramidite reagents which can be joined to other mobility-modifying monomers and to sequence-specific oligonucleobase polymers via uncharged phosphate triester linkages. Addition of the mobility-modifying phosphoramidite reagents of the present invention to oligonucleobase polymers results in unexpectedly large effects the mobility of those modified oligonucleobase polymers, especially upon capillary electrophoresis in non-sieving media.
Owner:APPL BIOSYSTEMS INC
Phosphine-phosphoramidite compounds
ActiveUS6906212B1Hydrocarbon by hydrogenationGroup 8/9/10/18 element organic compoundsIsomerizationSilica hydride
Disclosed are novel phosphine-phosphoramidite compounds which may be employed in combination with a catalytically-active metal to effect a wide variety of reactions such as asymmetric hydrogenations, asymmetric reductions, asymmetric hydroborations, asymmetric olefin isomerizations, asymmetric hydrosilations, asymmetric allylations, asymmetric conjugate additions, and asymmetric organometallic additions. Also disclosed are a process for the preparation of the phosphine-phosphoramidite compounds, metal complex compounds comprising at least one of the phosphine-phosphoramidite compounds and a catalytically-active metal and hydrogenation processes utilizing the metal complex compounds.
Owner:JOHNSON MATTHEY PLC
Catalyst using phosphine-phosphoramidite ester as ligand, its preparation method and application
InactiveCN1768944AThe synthetic route is simpleHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsIridiumPt element
The invention relates to a catalyst which uses the phosphor-phosphamide as ligand, which is the phosphor-phosphamide ligand metallic complex of chiral ferrocenyl bone, wherein, the metal is rhodium, ruthenium, iridium, platinum or palladium; and the mol rate between the ligand and the metal precursor is 1.1:1-2.2:1. The ligand is compounded from chiral ferrocenyl to intermediate compound via several reaction steps, to be processed condensation with phosphite ester chloridate to attain the phosphor-phosphamide ligand with different chiral centers. Said ligand has new structure, stable property, simple compounding method, and wider application to the catalyst, which has higher catalysis activity (TON reaches 10000) and higher spatial selectivity (ee reaches more than 99%) in catalyzing asymmetry hydrogenization of itaconic acid, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Design, synthesis and assembly of synthetic nucleic acids
InactiveUS20130196864A1Peptide librariesMicrobiological testing/measurementOligonucleotide synthesisPolynucleotide
Methods of synthesizing oligonucleotides with high coupling efficiency (>99.5%) are provided. Methods for purification of synthetic oligonucleotides are also provided. Instrumentation configurations for oligonucleotide synthesis are also provided. Methods of designing and synthesizing polynucleotides are also provided. Polynucleotide design is optimized for subsequent assembly from shorter oligonucleotides. Modifications of phosphoramidite chemistry to improve the subsequent assembly of polynucleotides are provided. The design process also incorporates codon biases into polynucleotides that favor expression in defined hosts. Design and assembly methods are also provided for the efficient synthesis of sets of polynucleotide variants. Software to automate the design and assembly process is also provided.
Owner:DNA TWOPOINTO
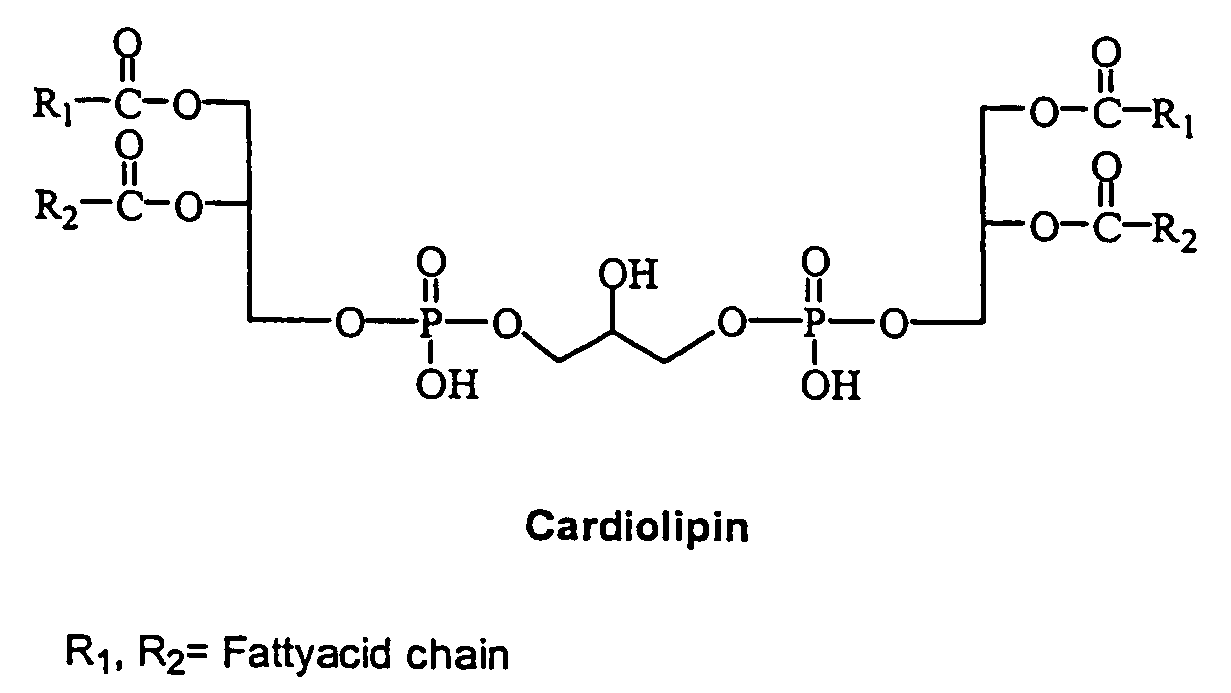
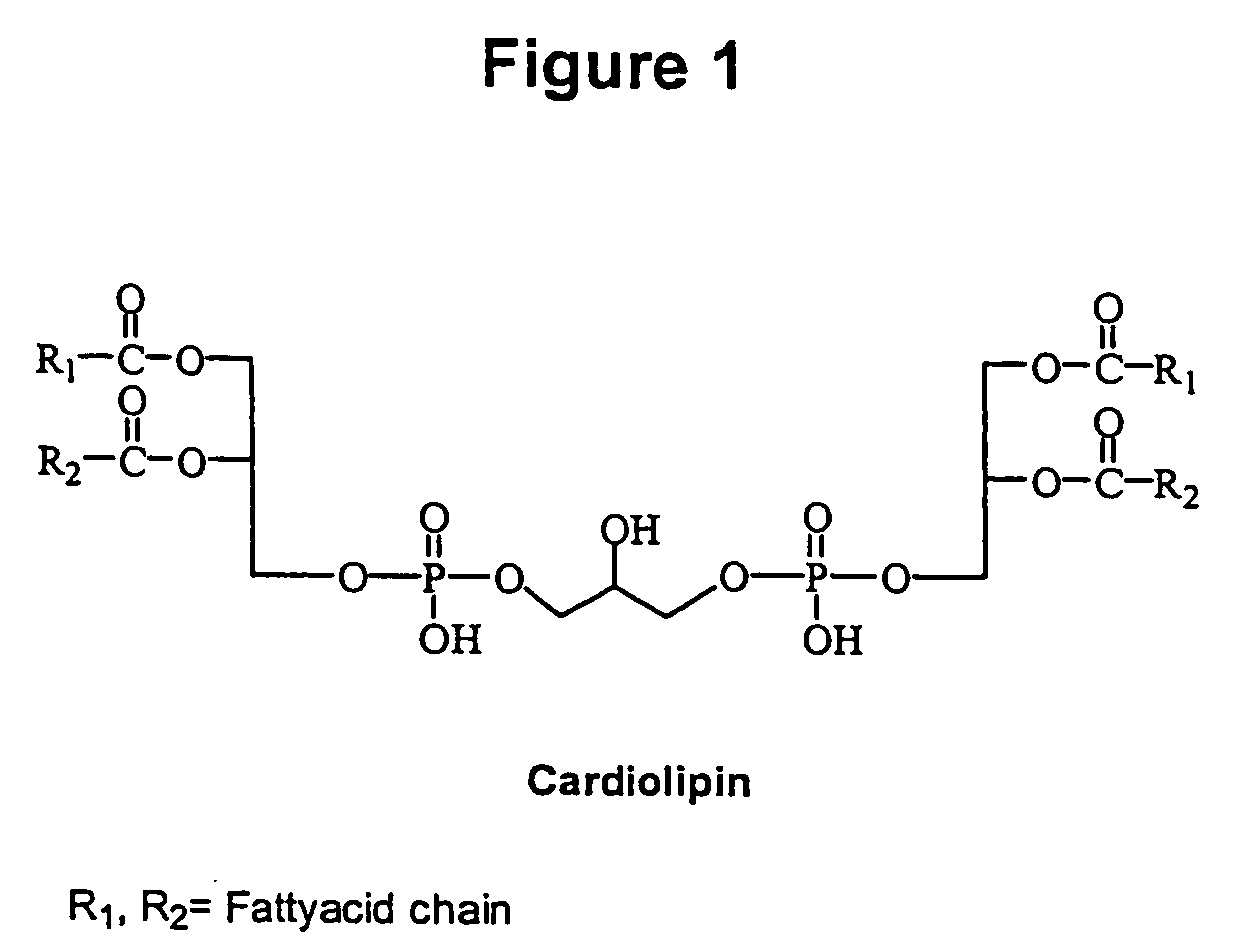
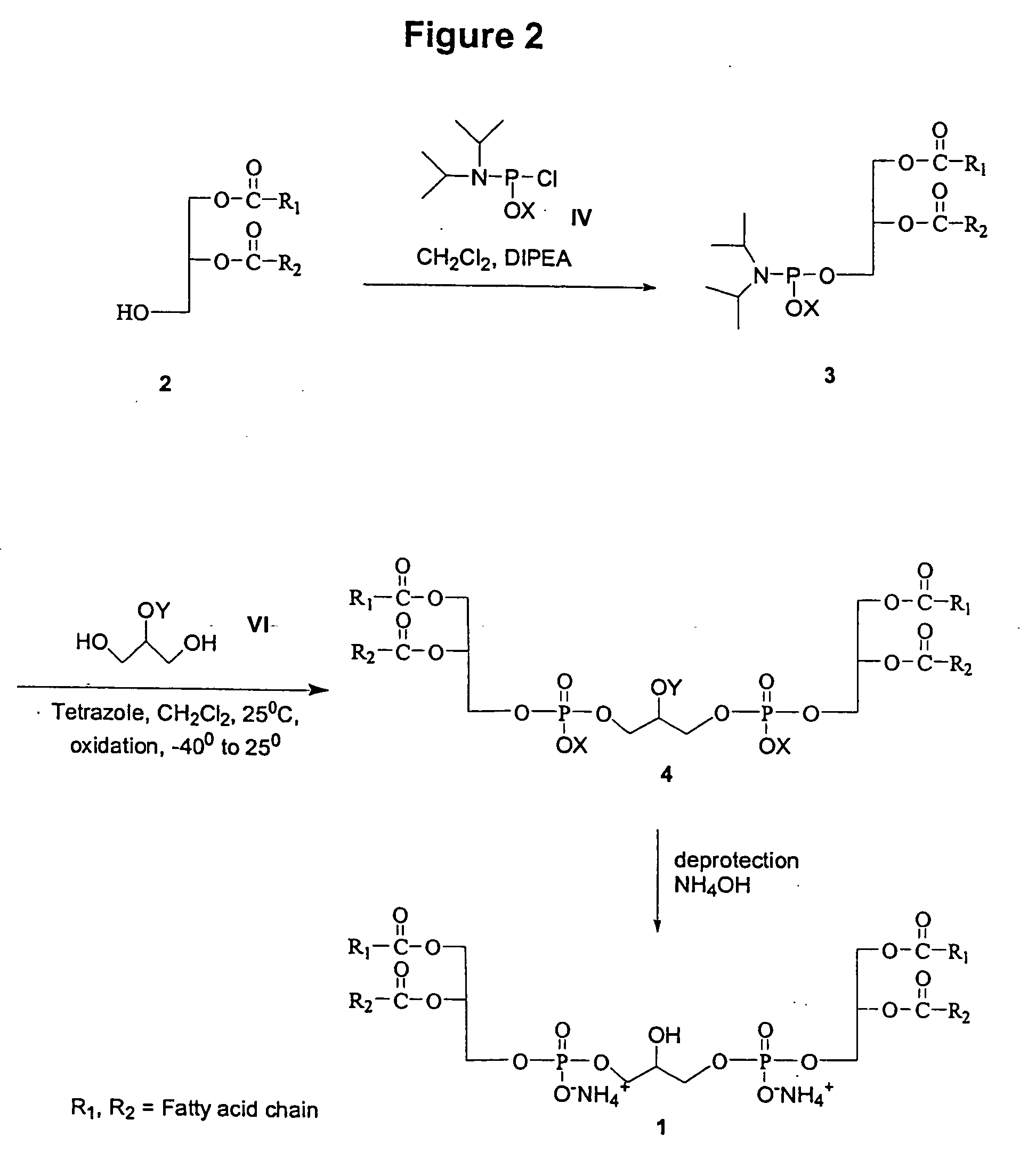
Cardiolipin molecules and methods of synthesis
The invention provides new synthetic routes for cardiolipin with different fatty acids and / or alkyl chains with varying chain length and also with or without unsaturation, particularly a short-chain cardiolipin. The methods comprise reacting a 1,2-O-sn-diacyl / 1,2-O-sn-dialkyl glycerol or a 2-O-protected glycerol, with a phosphoramidite reagent or a phosphate triester to produce a protected cardiolipin, which is deprotected to prepare the short chain cardiolipin. The reaction schemes can be used to generate new variants of cardiolipin. The cardiolipin prepared by the present methods can be incorporated into liposomes, which can also include active agents such as hydrophobic or hydrophilic drugs. Such liposomes can be used to treat diseases or in diagnostic and / or analytical assays. Liposomes can also include ligands for targeting a particular cell type or specific tissue.
Owner:NEOPHARMA INC
Processes and reagents for oligonucleotide synthesis and purification
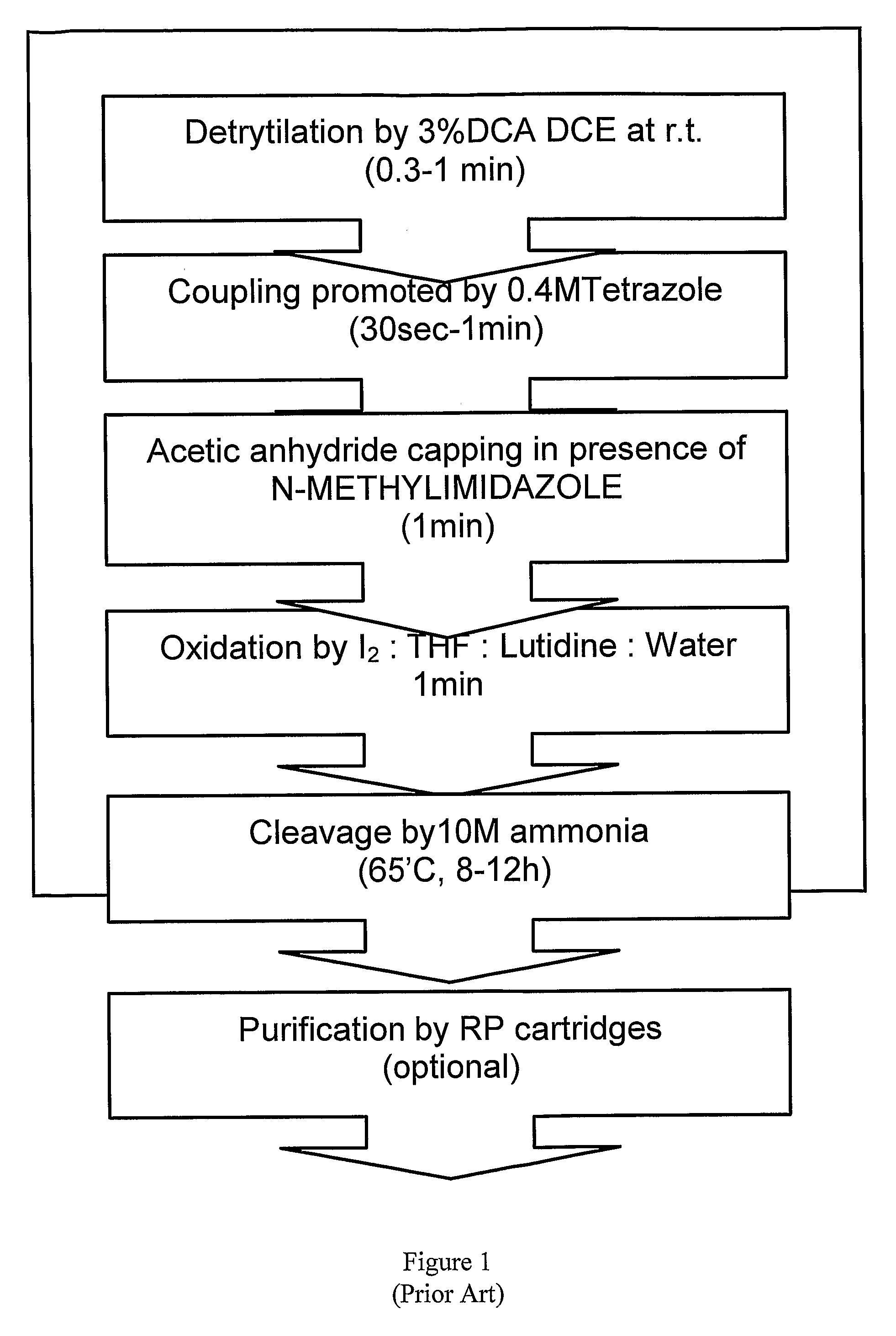
The present invention relates to processes and reagents for oligonucleotide synthesis and purification. One aspect of the present invention relates to compounds useful for activating phosphoramidites in oligonucleotide synthesis. Another aspect of the present invention relates to a method of preparing oligonucleotides via the phosphoramidite method using an activator of the invention. Another aspect of the present invention relates to sulfur-transfer agents. In a preferred embodiment, the sulfur-transfer agent is a 3-amino-1,2,4-dithiazolidine-5-one. Another aspect of the present invention relates to a method of preparing a phosphorothioate by treating a phosphite with a sulfur-transfer reagent of the invention. In a preferred embodiment, the sulfur-transfer agent is a 3-amino-1,2,4-dithiazolidine-5-one. Another aspect of the present invention relates to compounds that scavenge acrylonitrile produced during the deprotection of phosphate groups bearing ethylnitrile protecting groups. In a preferred embodiment, the acrylonitrile scavenger is a polymer-bound thiol. Another aspect of the present invention relates to agents used to oxidize a phosphite to a phosphate. In a preferred embodiment, the oxidizing agent is sodium chlorite, chloroamine, or pyridine-N-oxide. Another aspect of the present invention relates to methods of purifying an oligonucleotide by annealing a first single-stranded oligonucleotide and second single-stranded oligonucleotide to form a double-stranded oligonucleotide; and subjecting the double-stranded oligonucleotide to chromatographic purification. In a preferred embodiment, the chromatographic purification is high-performance liquid chromatography.
Owner:ALNYLAM PHARM INC
Spirocyclophophorous amine
InactiveCN1342652AHigh stereoselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhosphorous acidItaconic acid
A spirocyclic phosphorous amide is prepared though reaction of spirocyclicdiphenols on substituted phosphorous amide, and features that it has two mutamers: dextro-and levo-spirocyclicphosphorous amides. It can be used for asymmetric catalytic hydrogenating reaction with very high stereo-selectivity (more than 99% of e.e. value).
Owner:NANKAI UNIV
Oligonucleotide ligation
InactiveUS20130046083A1Inhibition formationSugar derivativesSugar derivatives preparationSynthesis methodsAlkyne
Oligonucleotide chemistry is central to the advancement of core technologies such as DNA sequencing, forensic and genetic analysis and has impacted greatly on the discipline of molecular biology. Oligonucleotides and their analogues are essential tools in these areas. They are often produced by automated solid-phase phosphoramidite synthesis but it is difficult to synthesize long DNA and RNA sequences by this method. Methods are proposed for ligating oligonucleotides together, in particular the use of an azide-alkyne coupling reaction to ligate the backbones of oligonucleotides together to form longer oligonucleotides than can be synthesized using current phosphoramidite synthesis methods.
Owner:UNIV OF SOUTHAMPTON
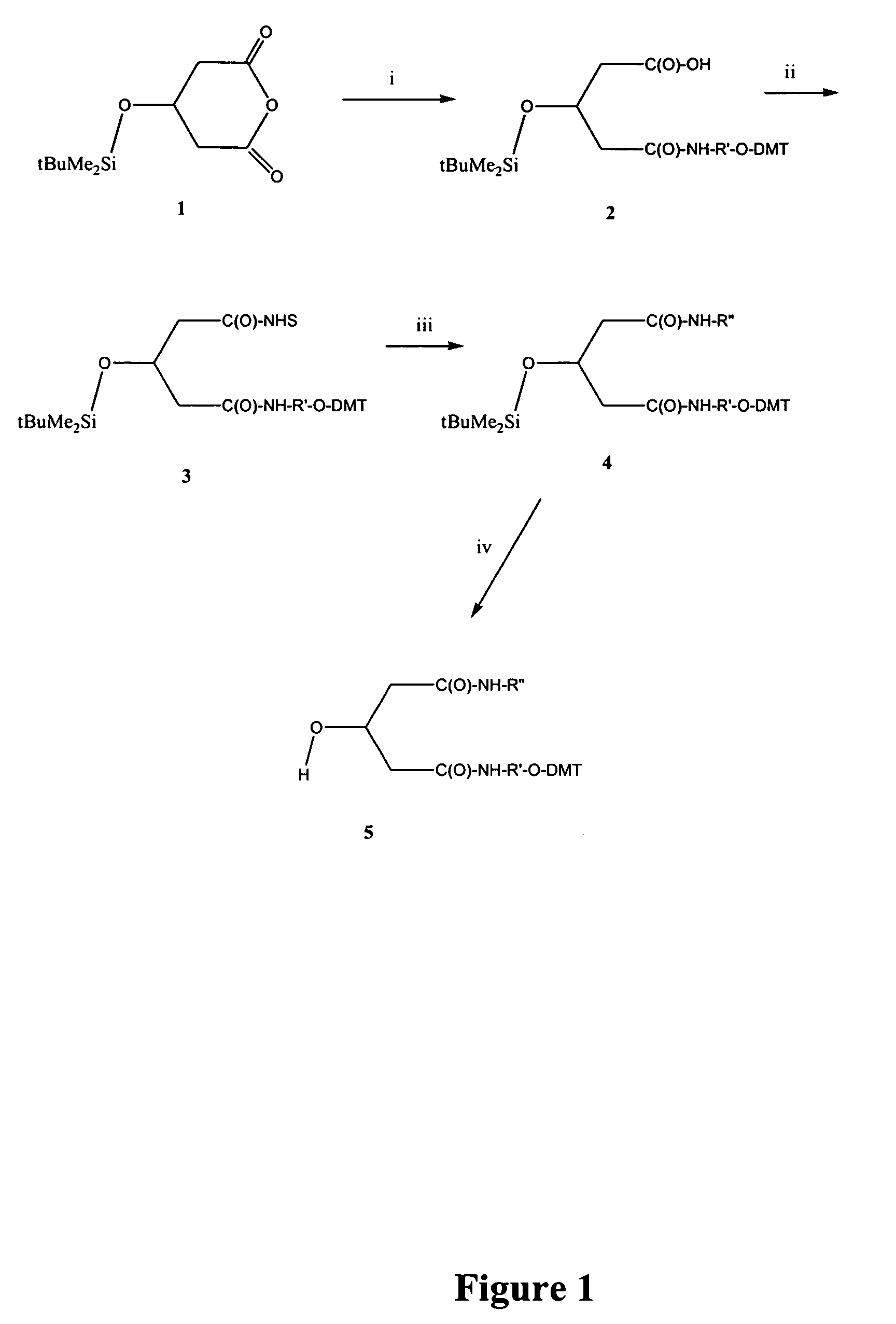
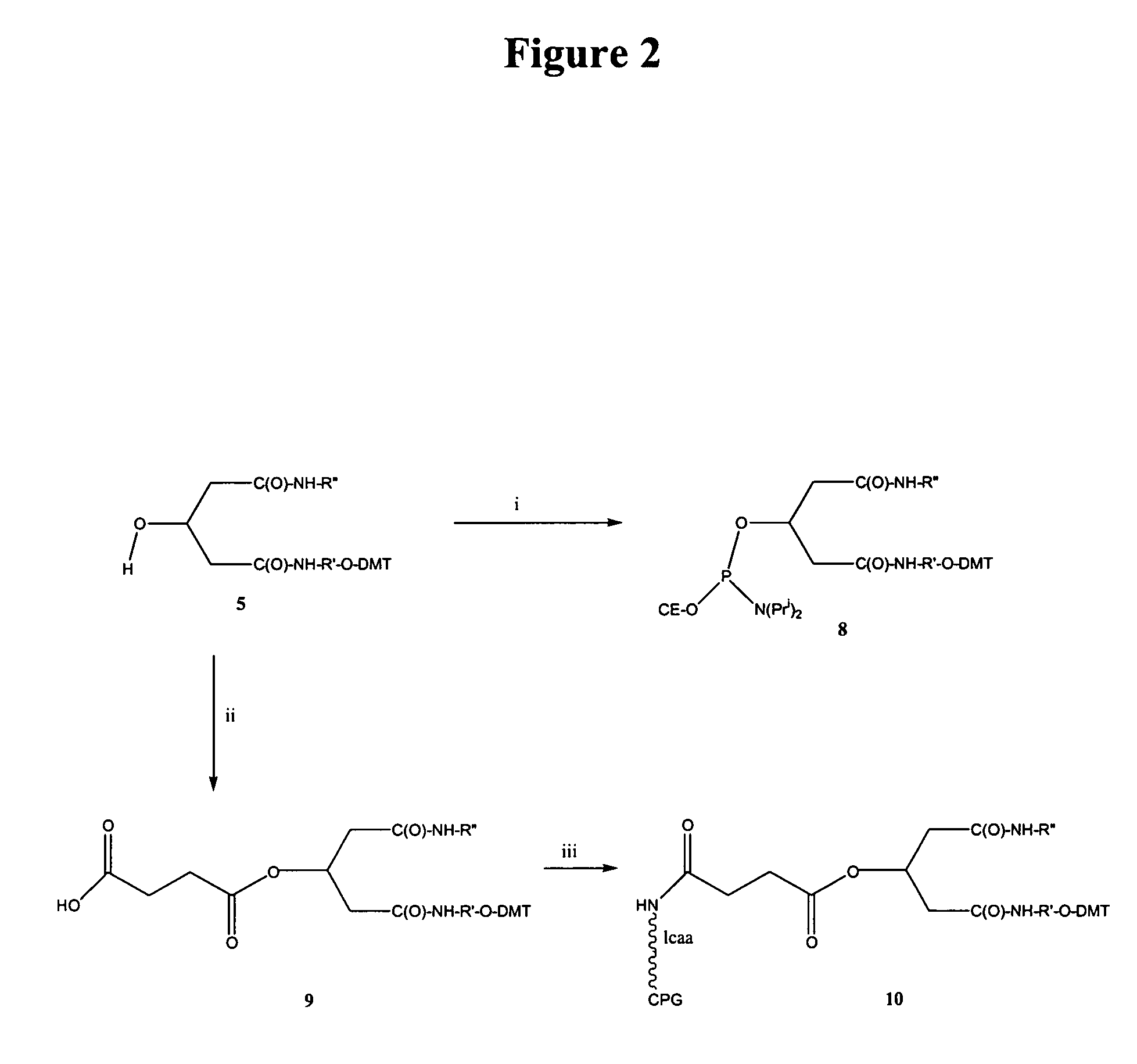
Process of purifying phosphoramidites
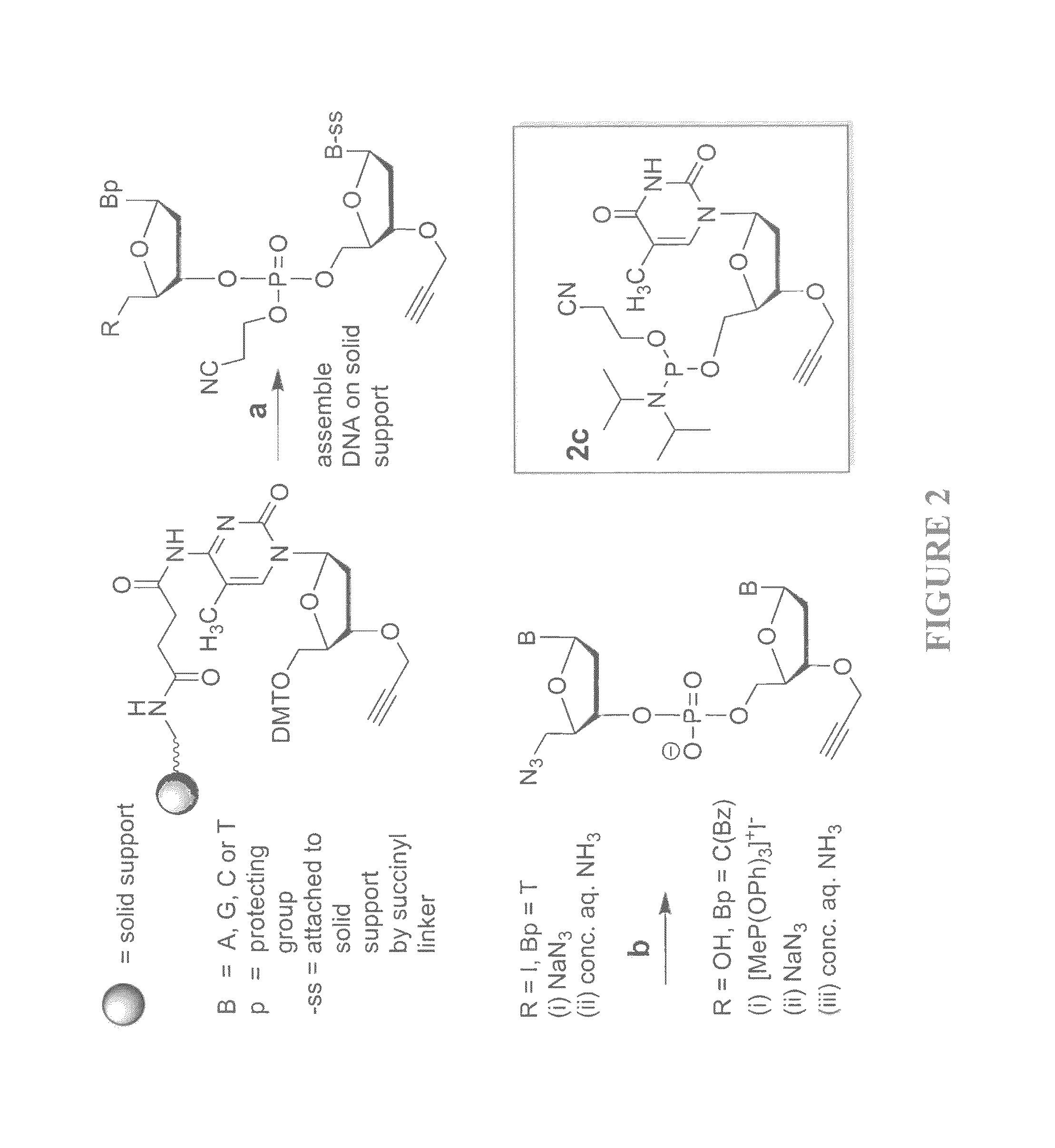
A process of purifying phosphoramidite precursors useful in inter alia synthesis of oligonucleotides comprises dissolving a crude phosphoramidite in a polar phase, adding a basic compound to the polar phase, adding a portion of water to the polar phase, contacting the polar phase with a first apolar phase to extract impurity into the apolar phase, separating the first apolar phase from the polar phase, adding a second aliquot of water to the polar phase, and contacting the polar phase with a second apolar phase, whereby the phosphoramidite partitions into the second apolar phase.
Owner:IONIS PHARMA INC
Reactive functional groups
The present invention relates to compositions and methods for the preparation of modified nucleic acids. In particular, the present invention provides novel reagents and chemistries for the generation of linkers, modified phosphoramidites, and modified solid supports.
Owner:GEN PROBE INC
Compositions for modifying nucleic acids
The present invention relates to compositions and methods for the preparation of modified nucleic acids. In particular, the present invention provides novel reagents and chemistries for the generation of linkers, modified phosphoramidites, and modified solid supports.
Owner:GEN PROBE INC
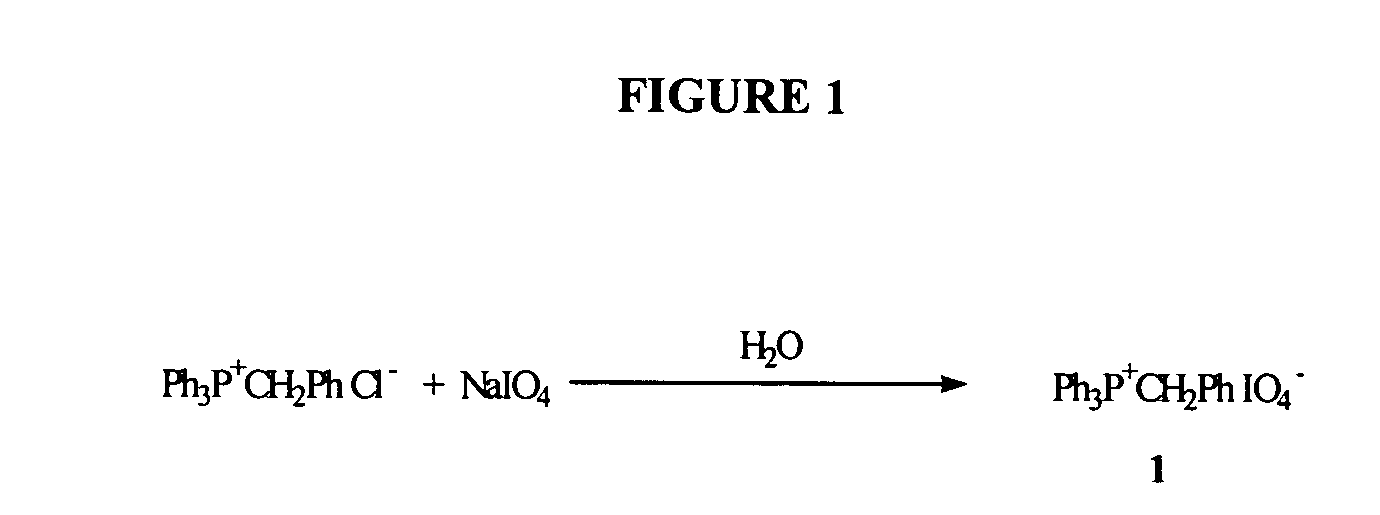
Oligonucleotide synthesis using periodate salts
The present invention relates generally to nucleic acid chemistry and to the chemical synthesis of oligonucleotides. More particularly, the invention relates to improved methods for synthesizing oligonucleotides wherein periodate salts are used (e.g., in organic solvents) as an oxidation reagent in oligonucleotide synthesis (e.g., for automated phosphoramidite synthesis of oligonucleotides). The invention finds utility in the fields of biochemistry, molecular biology, and pharmacology, and in medical diagnostic and screening technologies.
Owner:THIRD WAVE TECH
Kit and method for modifying vitro synthesized RNA
ActiveCN101550175AMild reaction conditionsHigh yieldSugar derivativesPeptide preparation methodsChemical synthesisDecomposition
The present invention discloses a kit and method for modifying vitro synthesized RNA, particular: (1) in chemical synthesis of RNA, using phosphoramidite monomer with 1, 3-di-dipole group or pro-dipole group to synthesis ribonucleic acid; (2) the after or during the synthetic process, modified ribonucleic acid and corresponding pio-dipole group or biomolecular probe of the 1, 3-di-dipole group performing 'click' reaction at the present of cuprous ion compounds catalyst, the ribonucleic acid is modified. The present invention provided kit and method for modifying RNA avoid shortcomings of traditional molecule connecting method active molecule decomposition and inactivation in RNA synthesis or doff protecting group process. The invention is provided with advantages of low cost, simple operation, mild condition, good modify impression and will not affect biological activity of any RNA.
Owner:GUANGZHOU RIBOBIO
Ribonucleoside analogs with novel hydrogen bonding patterns
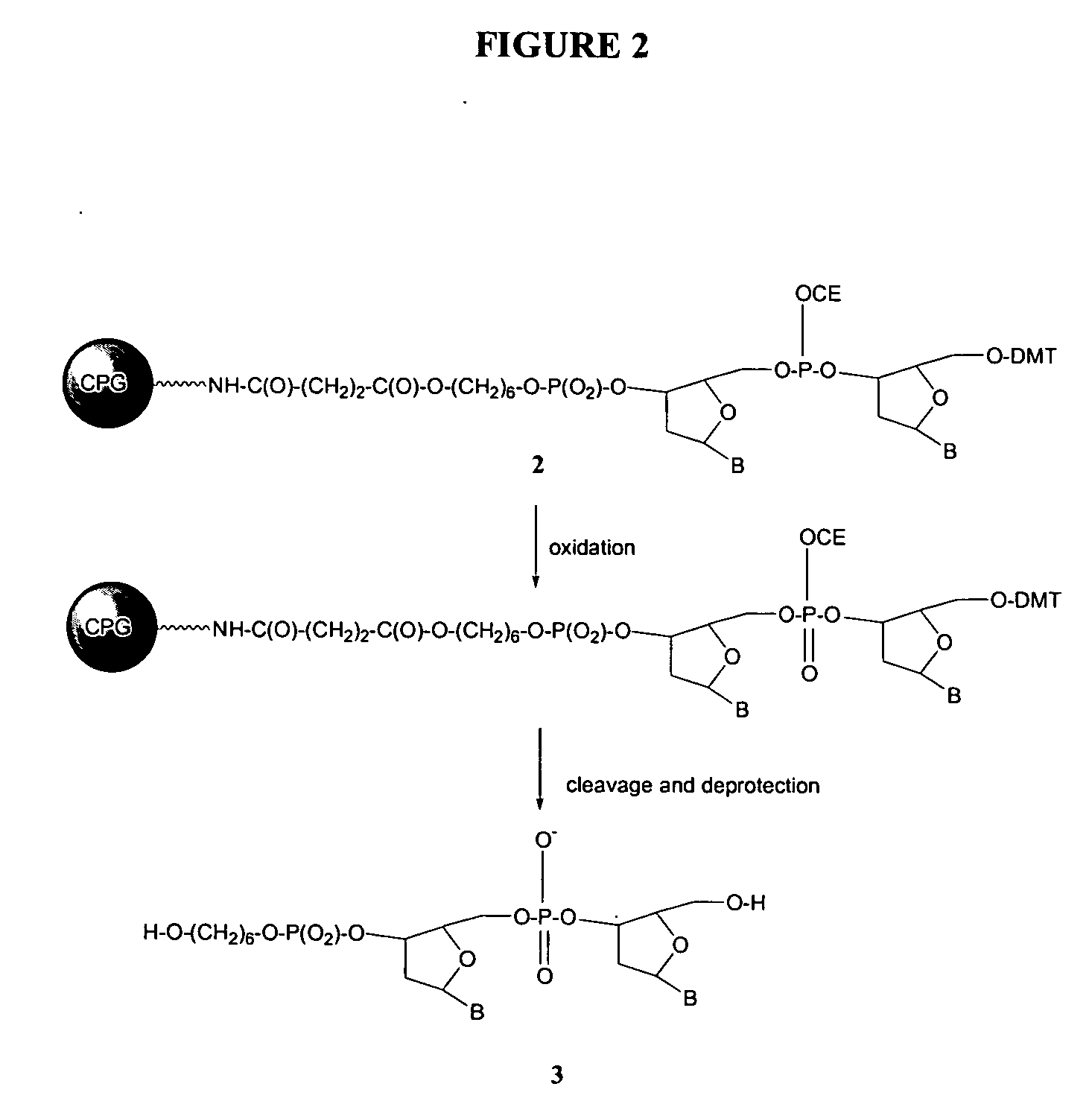
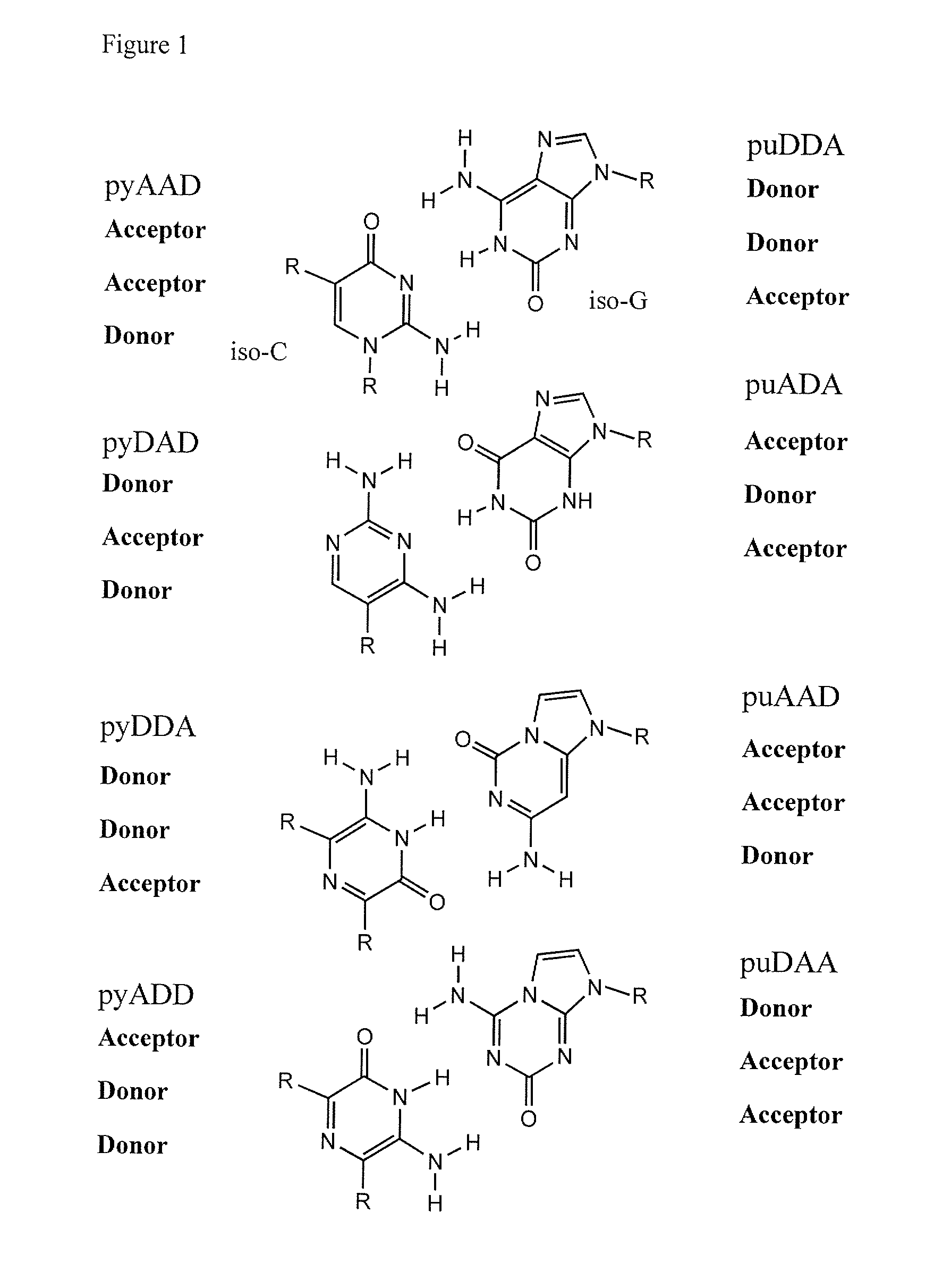
This invention relates to nucleoside, nucleotide, and oligonucleotide analogs that incorporate non-standard nucleobase analogs, defined to be those that present a pattern of hydrogen bonds to a paired nucleobase analog in a complementary strand that is different from the pattern presented by adenine, guanine, cytosine, and thymine. The invention is specifically concerned with nucleotide analogs that present the donor-donor-acceptor, hydrogen bonding patterns on pyrimidine analogs, and especially those that are analogs of ribonucleotides, including protected ribonucleotides suitable for phosphoramidite-based synthesis of RNA. The heterocycles on these nucleoside analogs are aminopyridones that have electron withdrawing groups attached to the position analogous to the 5-position of the ring in standard pyrimidines, including nitro, cyano, and carboxylic acid derivatives.
Owner:BENNER STEVEN A +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com