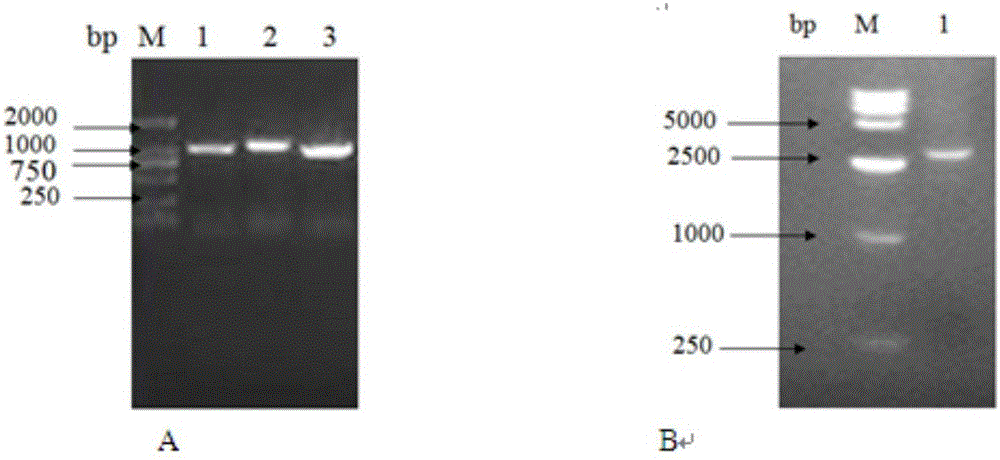
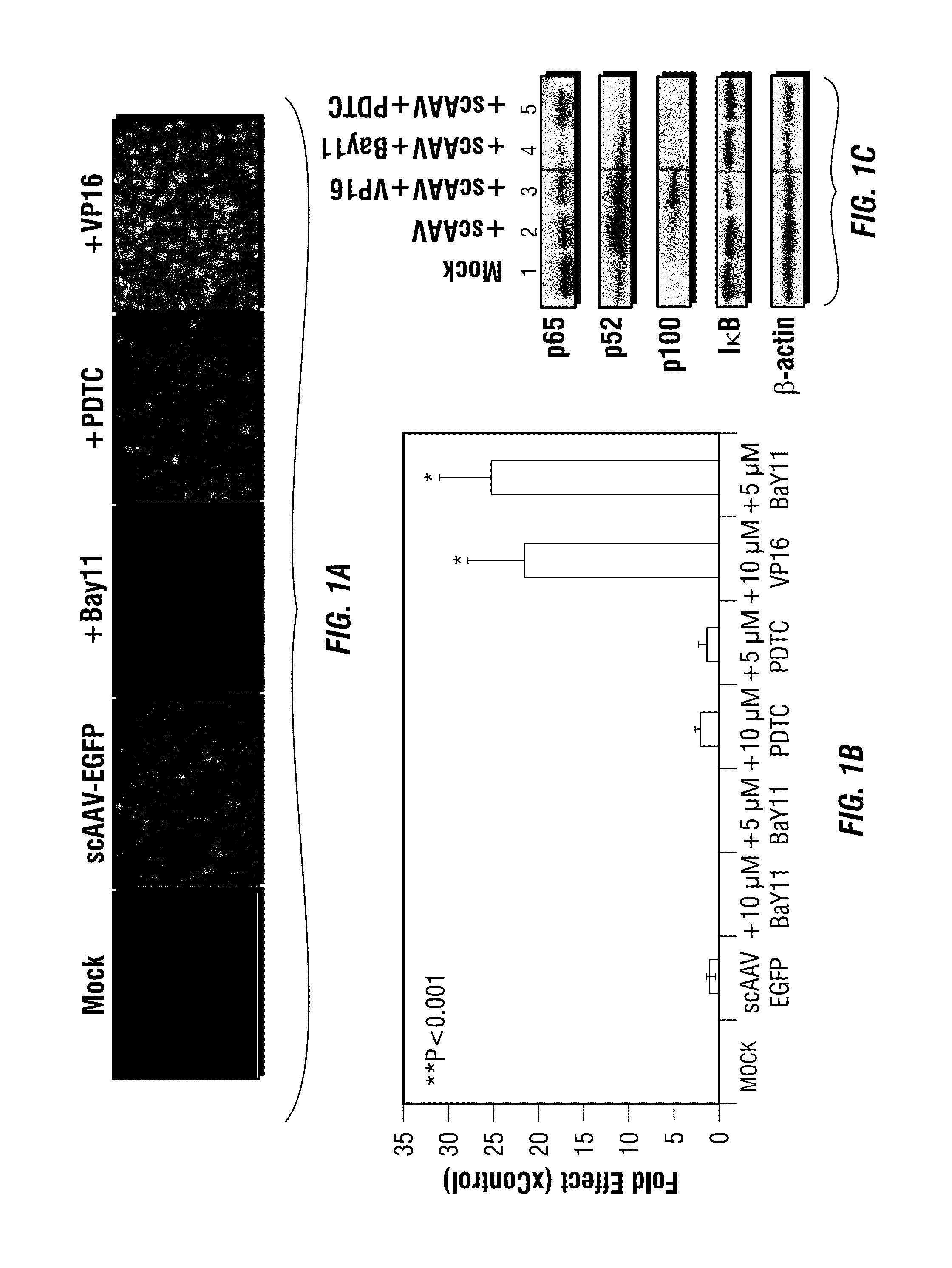
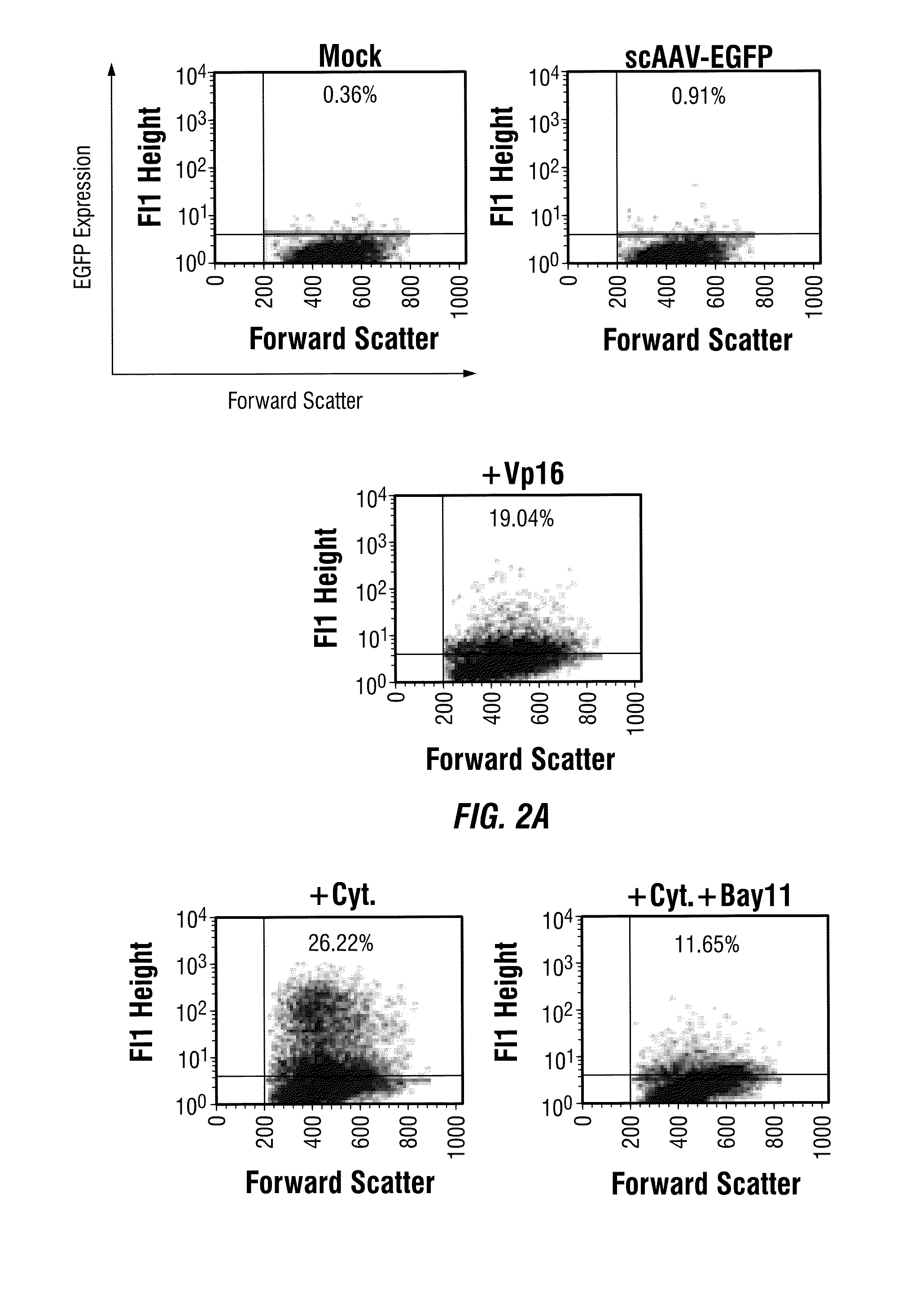
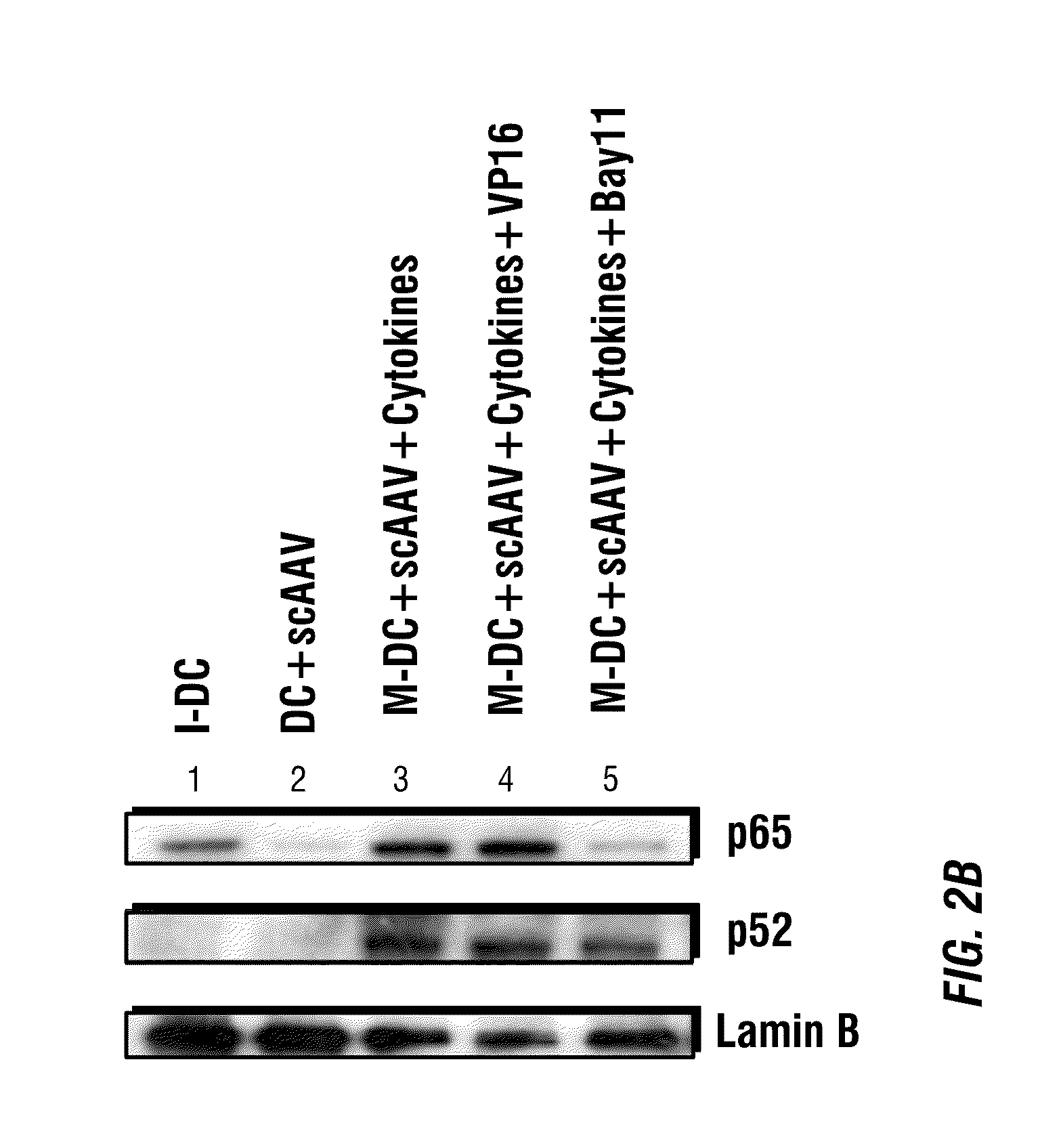
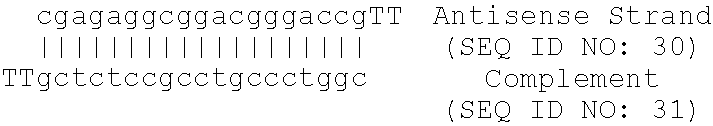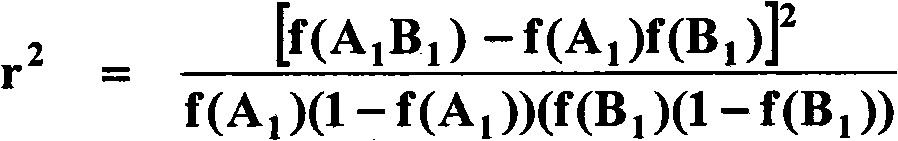Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
159 results about "Animal gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Effects of apolipoprotein B inhibition on gene expression profiles in animals
InactiveUS20060009410A1Inhibition of secretionPeptide/protein ingredientsMetabolism disorderCellular pathwaysSerum ige
Methods are provided for modulating the expression of genes involved in lipid metabolism, useful in the treatment of conditions associated with cardiovascular risk. Antisense oligonucleotides targeted to apolipoprotein B reduce the level of apolipoprotein B mRNA, lower serum cholesterol and shift liver gene expression profiles from those of an obese animal towards those of a lean animal. Further provided are methods for improving the cardiovascular risk of a subject through antisense inhibition of apolipoprotein B. Also provided are methods for employing antisense oligonucleotides targeted to apolipoprotein B to modulate a cellular pathway or metabolic process.
Owner:KASTLE THERAPEUTICS LLC
Method of down-regulating gene expression
InactiveUS6936593B1Down-regulated expressionOrganic active ingredientsBiocideCarbamateDithiophosphoric acid
Disclosed is a method of down-regulating the expression of a gene in an animal, wherein a pharmacological formulation comprising a chimeric oligonucleotide complementary to the gene is orally administered to an animal. The oligonucleotide administered has at least one phosphorothioate internucleotide linkage and at least one alkylphosphonate, phosphorodithioate, alkylphosphonothioate, phosphoramidate, phosphoramidite, phosphate ester, carbamate, carbonate, phosphate triester, acetamidate, or carboxymethyl ester internucleotide linkage.
Owner:IDERA PHARMA INC
Method for knocking off animal myostatin gene by using CRISPR-Cas9 system
InactiveCN104531705AThe identification rules are simpleEasy to operateMicroinjection basedVector-based foreign material introductionBiotechnologyTranscription initiation site
The invention provides a method for knocking off an animal myostatin gene by using a CRISPR-Cas9 system. The method comprises the following steps: firstly, acquiring a DNA sequence aiming at an sgRNA recognition area of a second myostatin exon, wherein the base sequence of the DNA sequence is as shown in SEQ ID NO.1; secondly, establishing an sgRNA expression structure of the second myostatin exon, inserting a T7 starter before an sgRNA transcriptional start site, establishing an in-vitro transcription carrier of Cas9 protein, and regulating and controlling by using the T7 starter. Cas9 mRNA and sgRNA are obtained through the in-vitro transcription carrier of Cas9 and sgRNA, and the method can be used for knocking off the animal myostatin gene.
Owner:CHINA AGRI UNIV
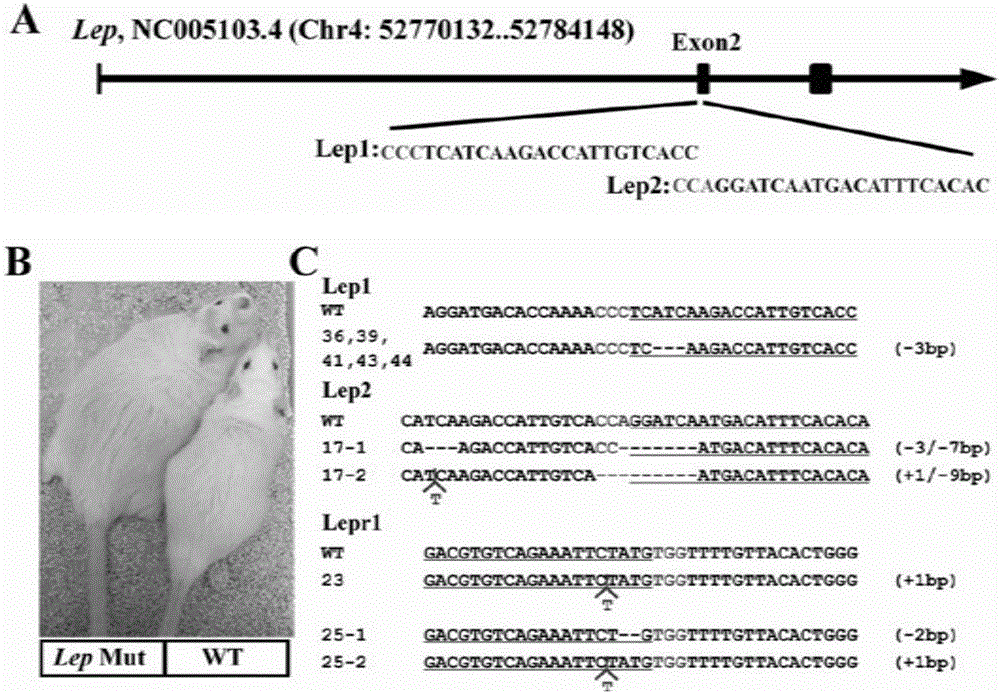
Animal model for obese rats and establishing method
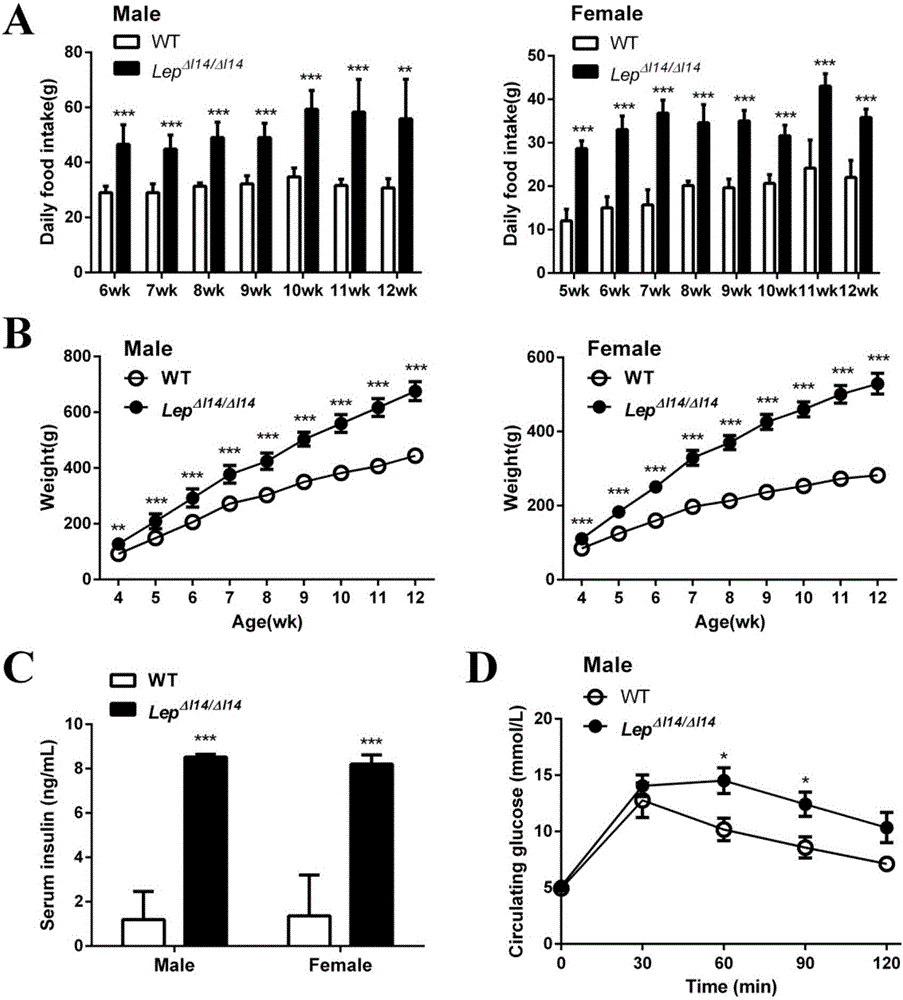
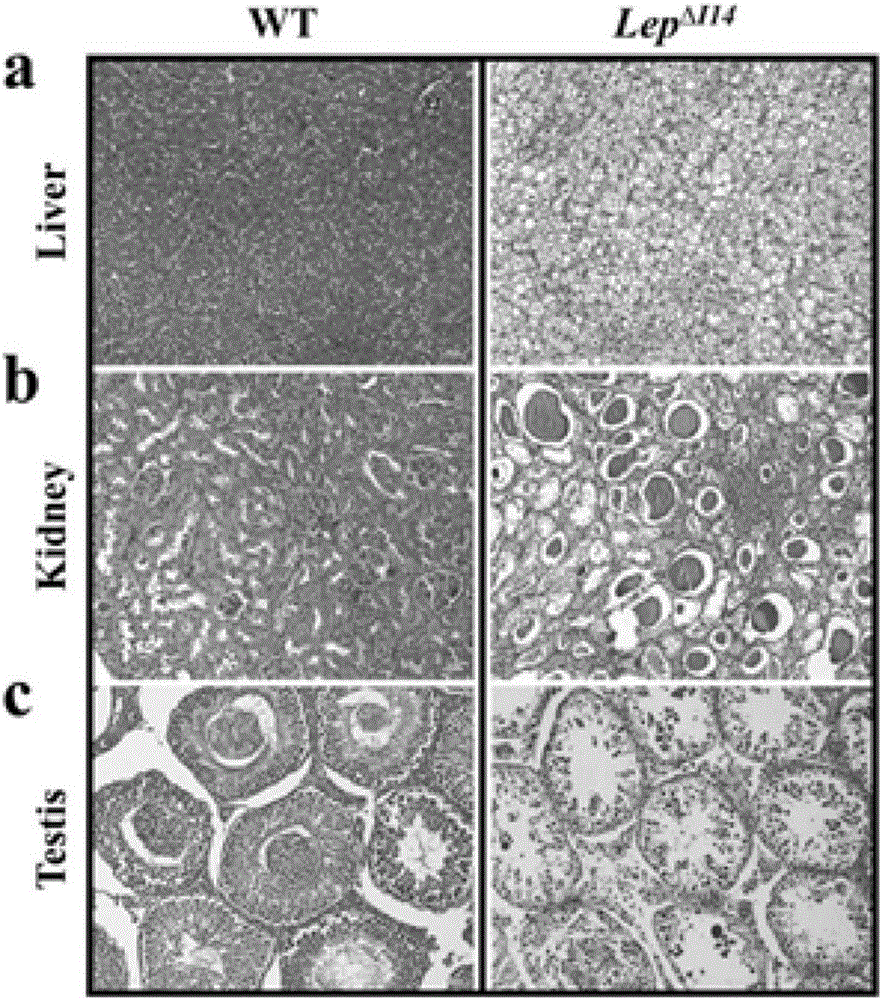
The invention relates to an animal model for obese rats and an establishing method, and belongs to the field of animal genetic engineering and genetic modification. The animal model for obese rats and the establishing method are characterized in that rats with LEP and LEPR genes knocked out are obtained through the CRISPR / Cas9 technology. The rats with LEP and LEPR genes knocked out have a typical obesity characteristic, the reliable animal model can be provided for studying the obesity disease of people, and the rats with LEP and LEPR genes knocked out are expected to become a standard experiment animal of obese rats.
Owner:SUZHOU RUIQI BIO PHARMA CO LTD
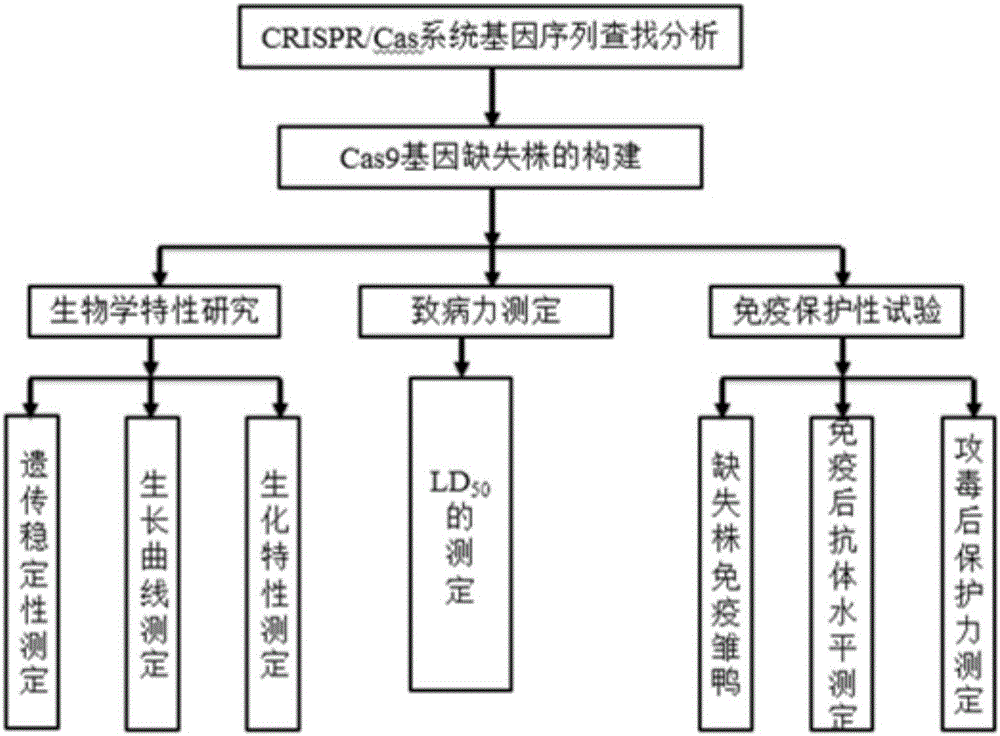
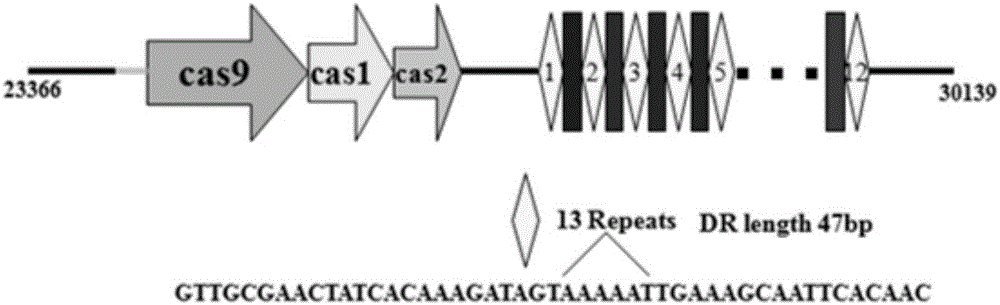
Riemerella anatipestifer mutant strain with Cas9 gene deletion and applications of riemerella anatipestifer mutant strain
InactiveCN106190903ALow toxicityReduced toxicityAntibacterial agentsBacterial antigen ingredientsCRISPRBacterial strain
The invention discloses a riemerella anatipestifer mutant strain with Cas9 gene deletion and applications of the riemerella anatipestifer mutant strain, belonging to the technical field of preparation of animal genetically engineered vaccines. The strain is preserved in the China Center for Type Culture Collection (CCTCC), and is assigned with the accession number of CCTCC NO:M 2016247. According to the strain, 2070bp of the Cas9 gene of the CRISPR-Cas system is deleted, so that the toxicity of the bacterial strain is obviously reduced, but the good immunogenicity is still reserved. Compared with the traditional inactivated vaccines, the strain with the Cas9 gene deletion has the advantages that the culture method is simple, the production cost is low, the inoculation can be carried out through the manners of nasal inhalation, mist spraying and the like, the operation is simple and rapid, the body can be stimulated to generate effective mucosal immunity, and meanwhile, the systemic immunity of the body also can be stimulated.
Owner:HUAZHONG AGRI UNIV
Effects of apolipoprotein b inhibition on gene expression profiles in animals
Methods are provided for modulating the expression of genes involved in lipid metabolism, useful in the treatment of conditions associated with cardiovascular risk. Antisense oligonucleotides targeted to apolipoprotein B reduce the level of apolipoprotein B mRNA, lower serum cholesterol and shift liver gene expression profiles from those of an obese animal towards those of a lean animal. Further provided are methods for improving the cardiovascular risk of a subject through antisense inhibition of apolipoprotein B. Also provided are methods for employing antisense oligonucleotides targeted to apolipoprotein B to modulate a cellular pathway or metabolic process.
Owner:KASTLE THERAPEUTICS LLC
Construction method and application of immunodeficient mouse animal model
InactiveCN107460196AImprove the level ofPromote basic researchCompounds screening/testingGenetically modified cellsKnockout animalWilms' tumor
The invention relates to a construction method and application of an immunodeficient mouse animal model, belonging to the field of animal gene engineering and genetic modification. Il2rg gene knockout mice can be obtained by using a CRISPR / Cas9 technology; the Il2rg gene knockout mice have an immunodeficient phenotype. The construction method can be widely applied to multiple fields such as humanized animal models as well as stem cell and tumor research.
Owner:TONGJI UNIV
CAPSID-MODIFIED rAAV VECTOR COMPOSITIONS HAVING IMPROVED TRANSDUCTION EFFICIENCIES, AND METHODS OF USE
ActiveUS20140050701A1Improve efficiencyHighly efficient transductionBiocidePeptide/protein ingredientsDrugPharmaceutical formulation
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Bovine CASTgene SNP and meat tenderness
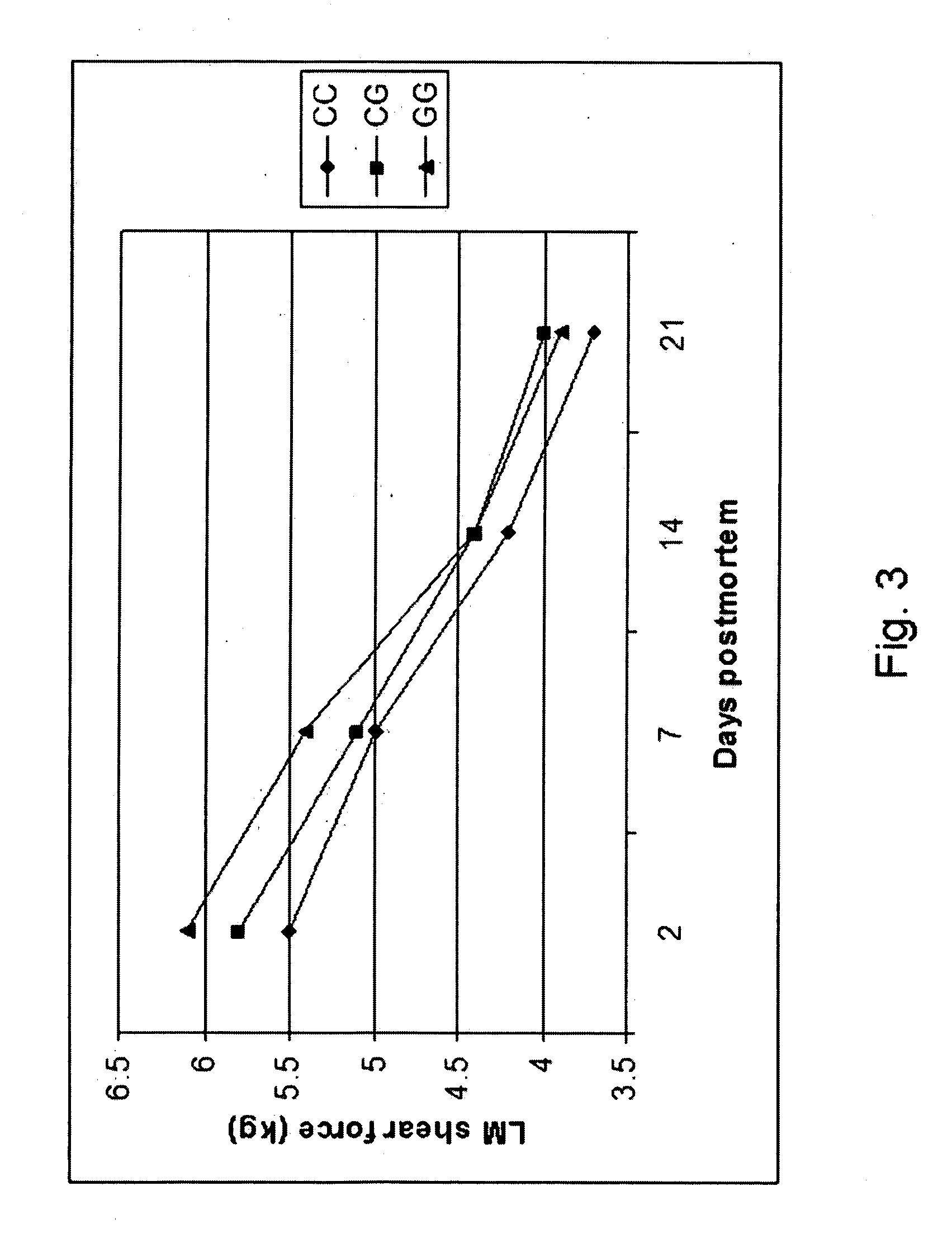
ActiveUS20060211006A1Increase productivityHigh frequencySugar derivativesMicrobiological testing/measurementNucleotideComputer-aided
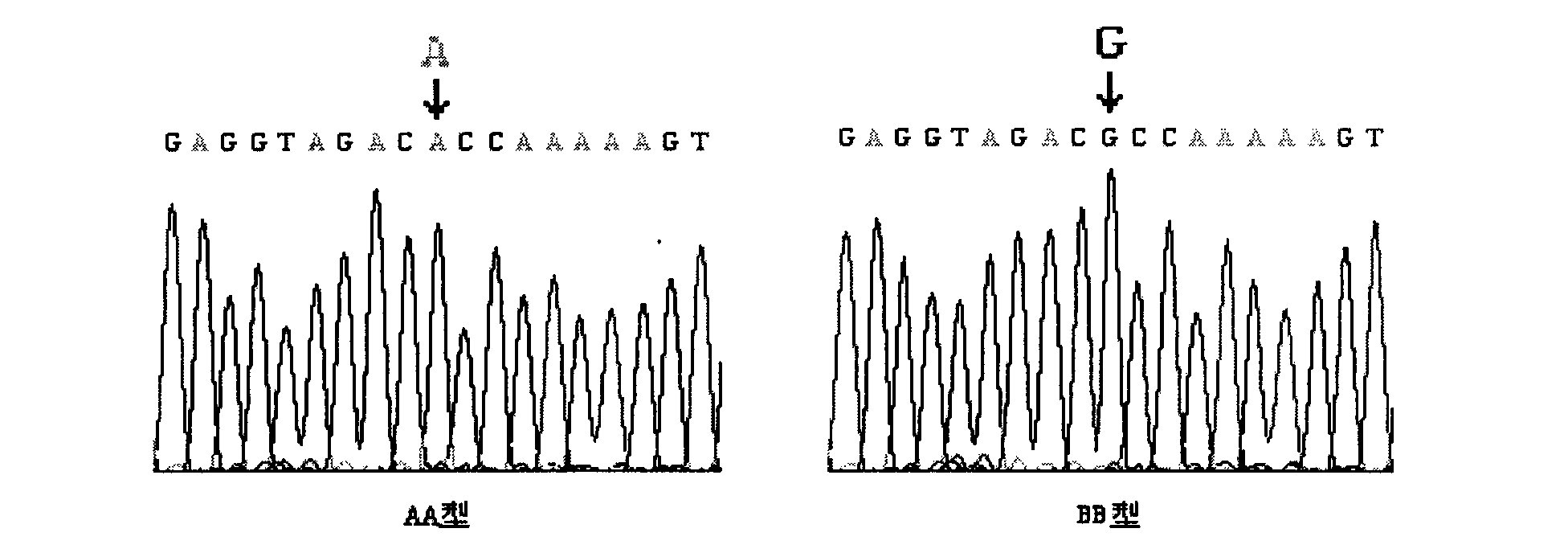
The present invention relates to the identification of a single nucleotide polymorphism (SNP) within the bovine CAST locus encoding the calpastatin protein, wherein the allelic variation of the SNP is a G / C transversion associated with post-mortem muscle tenderness. The invention further relates to oligonucleotides useful in identifying the genotype of bovines as it relates to the CAST locus polymorphic site. The invention also encompasses computer-assisted methods and systems for improving the production efficiency for livestock having marketably tender meat using multiple data, and in particular the genotype of the animals as it relates to the CAST SNP. These methods of the invention encompass obtaining a genetic sample from each animal in a herd of livestock, determining the genotype of each animal with respect to specific quality traits as defined by a panel of at least two single polynucleotide polymorphisms (SNPs), one SNP corresponding to a site between exons 5 and 6 of the bovine CAST locus, grouping animals with like genotypes, and optionally, further sub-grouping animals based on like phenotypes.
Owner:UNIVERSITY OF GUELPH
Modified pig propagation and respiratory syndrome virus ORF5 gene and use thereof
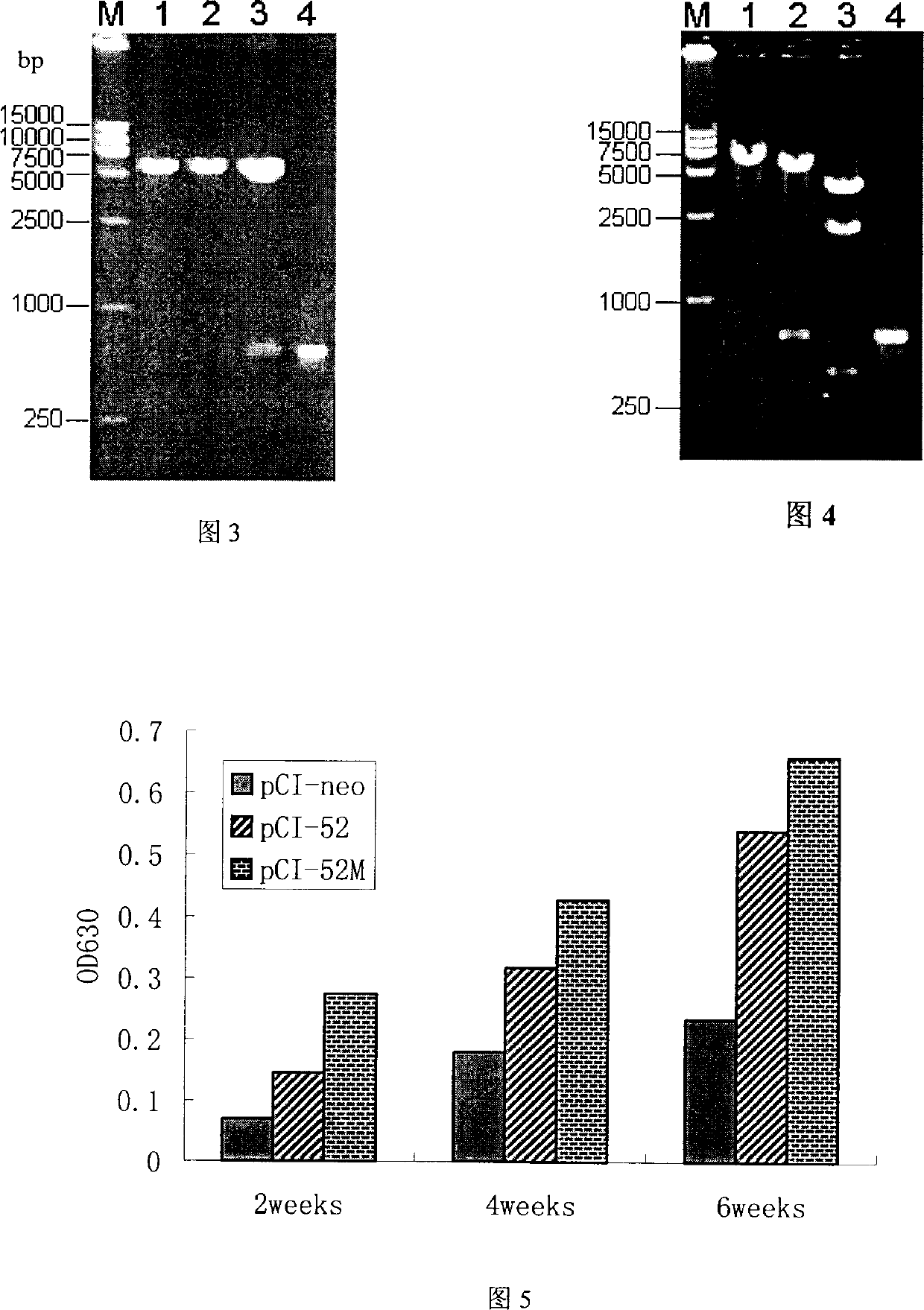
The invention belongs to the animal gene engineering technology field. It is about a gene and its application, the gene is modificatory porcine reproductive and respiratory syndrome virus ORF5. Its attribute is to insert nucleotide sequence between the neutralization and cover list of GP5, the nucleotide sequence is artificial synthesis coding accessorial T-lymph cell list. The sequence of this nucleotide is just as the sequence list of SEQ ID NO:1 and attached figure 1. This modificatory gene is included in eukaryotic expression plasmid, and the Escherichia coliDH5 / pCI-52M, which includes the plasmid, is conserved in CCTCC, and the number is CCTCC NO: M204080. This invention also presents the use of this gene in the preparation of pig bread and respiratory syndrome DNA vaccineíú
Owner:HUAZHONG AGRI UNIV
Authentication method of cattle CAPN1 gene as longisimus dorsi tenderness molecular marker and application
InactiveCN101624598AMicrobiological testing/measurementGenetic engineeringAgricultural sciencePolymorphism analysis
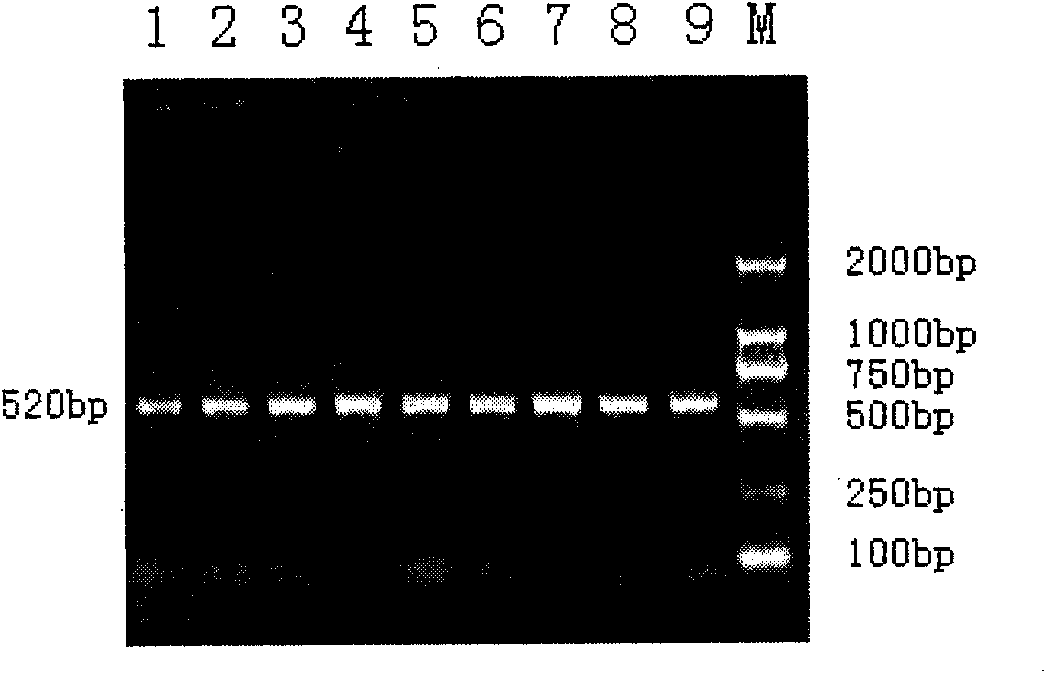
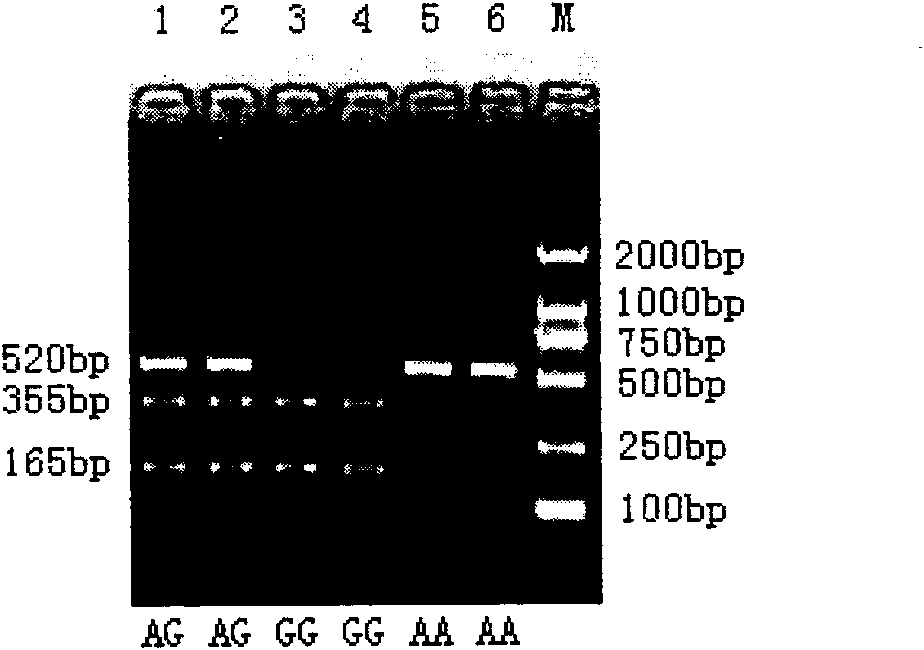
The invention belongs to the technical field of animal gene engineering, relating to clone of cattle longisimus dorsi tenderness related CAPN1 gene segment and an application thereof in cattle marker assisting selection. The obtained CAPN1 gene segment has the nucleotide sequence length of 520bp and comprises full fourteenth exons. A base mutation from A to G exists at the 166bp of the segment, which results in the generation of PsuI-RFLP restriction enzyme polymorphism. The mutation site is taken as a molecular marker, the restriction enzyme PsuI is used for detecting the mutation site, and association analysis can be carried out on the detection result and the cattle longisimus dorsi tenderness. Analysis result shows that striking difference exists among the longisimus dorsi tenderness of different genotype individuals, thus providing a theoretical basis for the seed selection and breeding of cattle. The invention is characterized by obtaining the CAPN1 gene part segment related to the cattle longisimus dorsi tenderness and providing the molecular marker and the application for the marker assisting selection of cattle by using a PCR-RFLP method to carry out polymorphism analysis on the segment.
Owner:JILIN UNIV
Method and markers for determining the genotype of horned/polled cattle
ActiveUS20070134701A1Microbiological testing/measurementBiostatisticsNucleic acid sequencingNucleic acid sequence
Provided herein are methods to discover and use single nucleotide polymorphisms (SNP) for determining the genotype of a horned / polled ruminant subject. The present invention further provides specific nucleic acid sequences, SNPs, and SNP patterns that can be used for determining the genotype of a horned / polled ruminant subject.
Owner:IGENITY
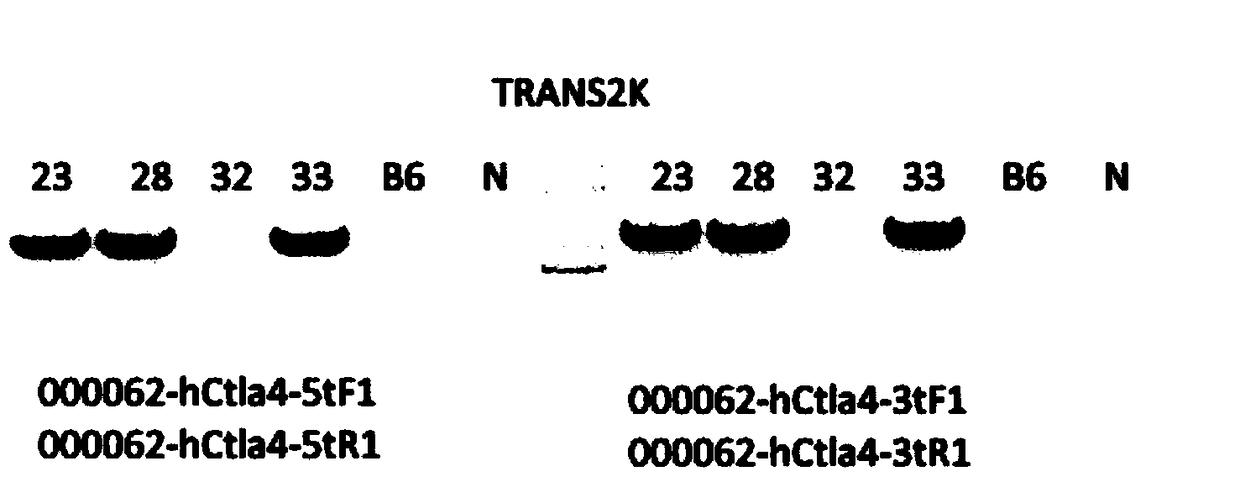
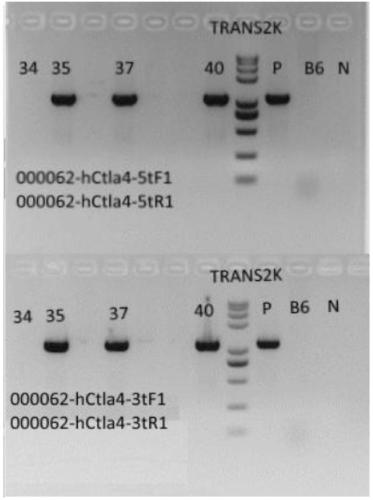
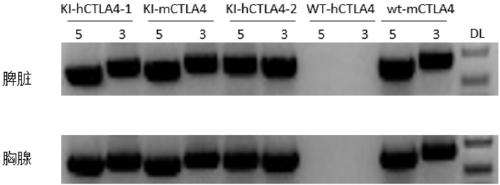
Establishment method of CTLA4 gene humanized animal model and application thereof
ActiveCN109022443AIncreased odds of homologous recombination repairIntracellular signaling is not affectedStable introduction of DNAPeptidesDrug developmentIn vivo
The invention belongs to the fields of animal gene engineering and genetic modification and particularly relates to an establishment method of a CTLA4 gene humanized animal model and an application thereof. In the invention, the extracellular domain of mouse CTLA4 gene is replaced by a human-source sequence while the intracellular domain holds a complete mouse-source sequence. The CTLA4 gene humanized animal model not only can be used for evaluating the efficacy on tumors of antibodies and has important guiding significance on clinical effect of medicine development, but also is an excellent model for security evaluation on anti-human CTLA4 antibody medicines. The establishment method also includes pre-clinical in-vivo evaluation on the toxicology of the CTLA4 antibody medicine, thus filling market gap.
Owner:GEMPHARMATECH CO LTD
Method for efficient RNA interference in mammalian cells
The present invention relates to methods of making RNAi libraries using E. coli RNAse III for inhibition of mammalian gene expression.
Owner:RGT UNIV OF CALIFORNIA
Clone and application of pig skeletal muscle specificity expression gene alpha-actin promoters
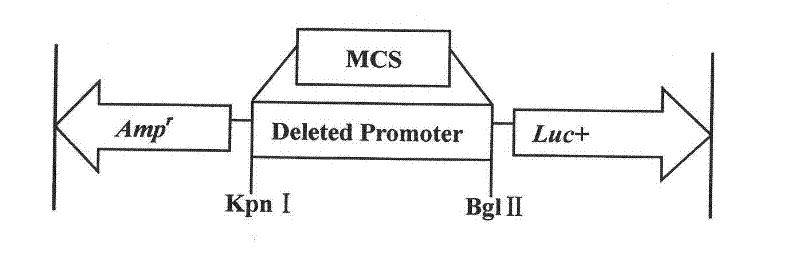
The invention belongs to the technical field of animal gene engineering and particularly relates to isolated identification and functional verification of different-length promoter regions of pig skeletal muscle specificity expression genes alpha-actin. Eight upstream different-length promoters of the skeletal muscle specificity expression genes alpha-actin (the Gene Bank accession number is 100154254) are cloned from the pig genome, and the nucleotide sequences of the promoters are respectively shown as SEQ ID No: 2 to SEQ ID No: 9. The results show that the region with the length being 249bp has independent promoting activity and muscle tissue specificity, the nucleotide sequence is shown as the SEQ ID No: 9 in a sequence table. The invention also discloses a method for obtaining eight different deletion promoter segments, a method for preparing corresponding recombinant expression vectors and an application of a dual-luciferase enzymatic activity detection system to the promoter activity analysis.
Owner:HUAZHONG AGRI UNIV
Immunodeficient mice, manufacturing method thereof and application
PendingCN106661593AEasy to observeEasy to observe growthMicroinjection basedAnimal husbandryAnimal GeneticsImmunodeficiency
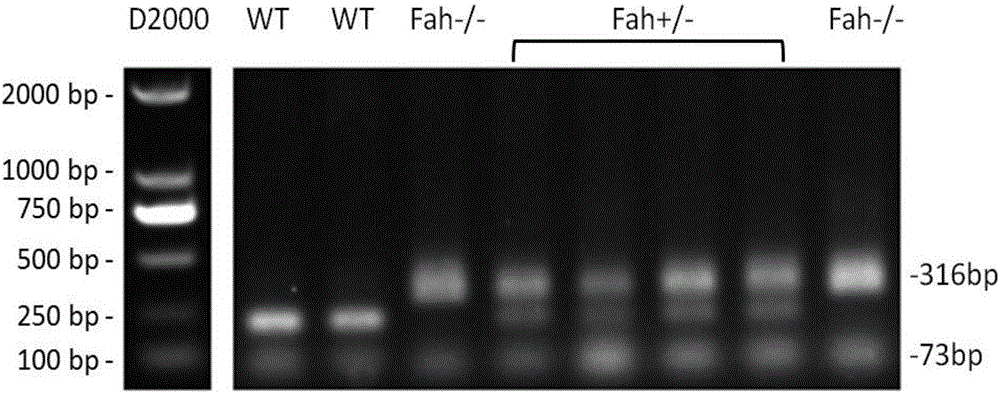
The invention belongs to technical field of animal genetic engineering, and specifically relates to immunodeficient mice, a manufacturing method thereof and applications. The manufacturing method comprises using NOD-Scid IL2rg- / - immunodeficient mice (NSI mice), and deleting Foxnl gene or Fah gene. Through deleting the Fah gene on the NSI mice, NSIF mice are obtained. The NSIF mice can be used to efficiently establish a novel humanized mouse model, used for liver physiological and pathological research. Through deleting the Foxnl gene on the NSI mice, the NSIF mice are obtained. The NSIF mice have no body hair, and immune system is further defected. The NSIF mice provide convenience for establishment, monitoring, and measurement of solid tumor.
Owner:湖南昭泰生物医药有限公司
Mycoplasma hyopneumoniae fusion gene and application
ActiveCN104293816AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
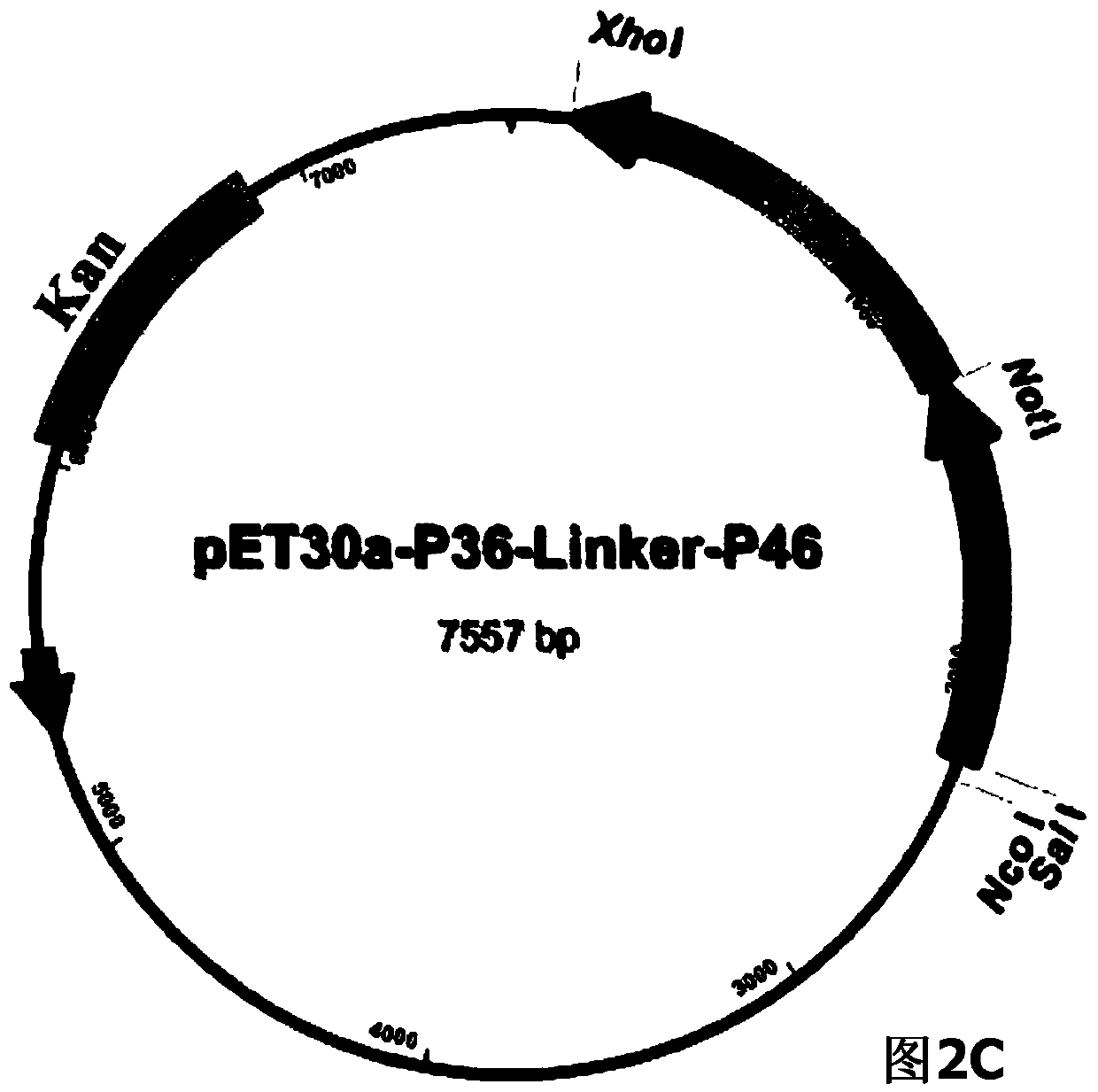
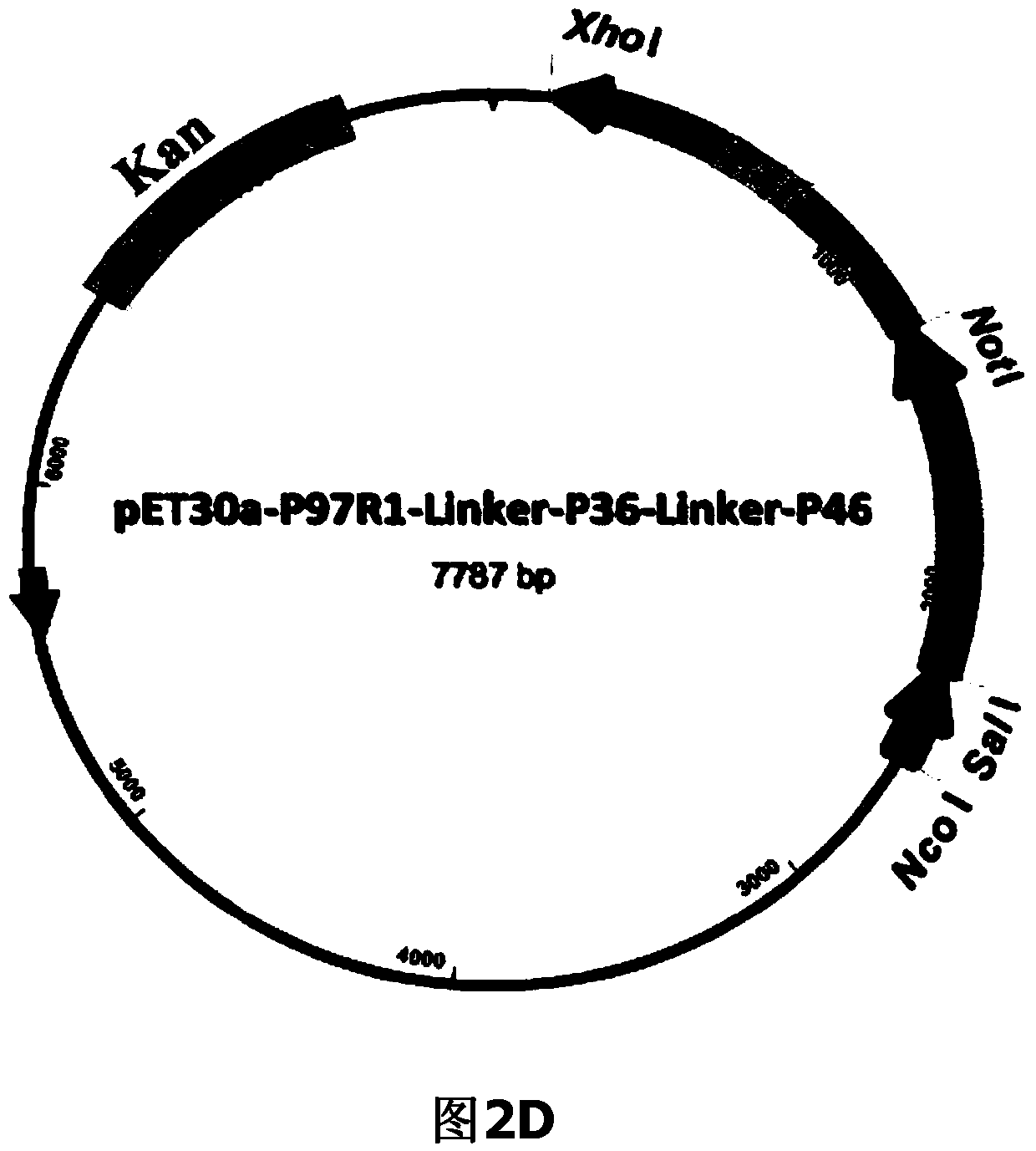
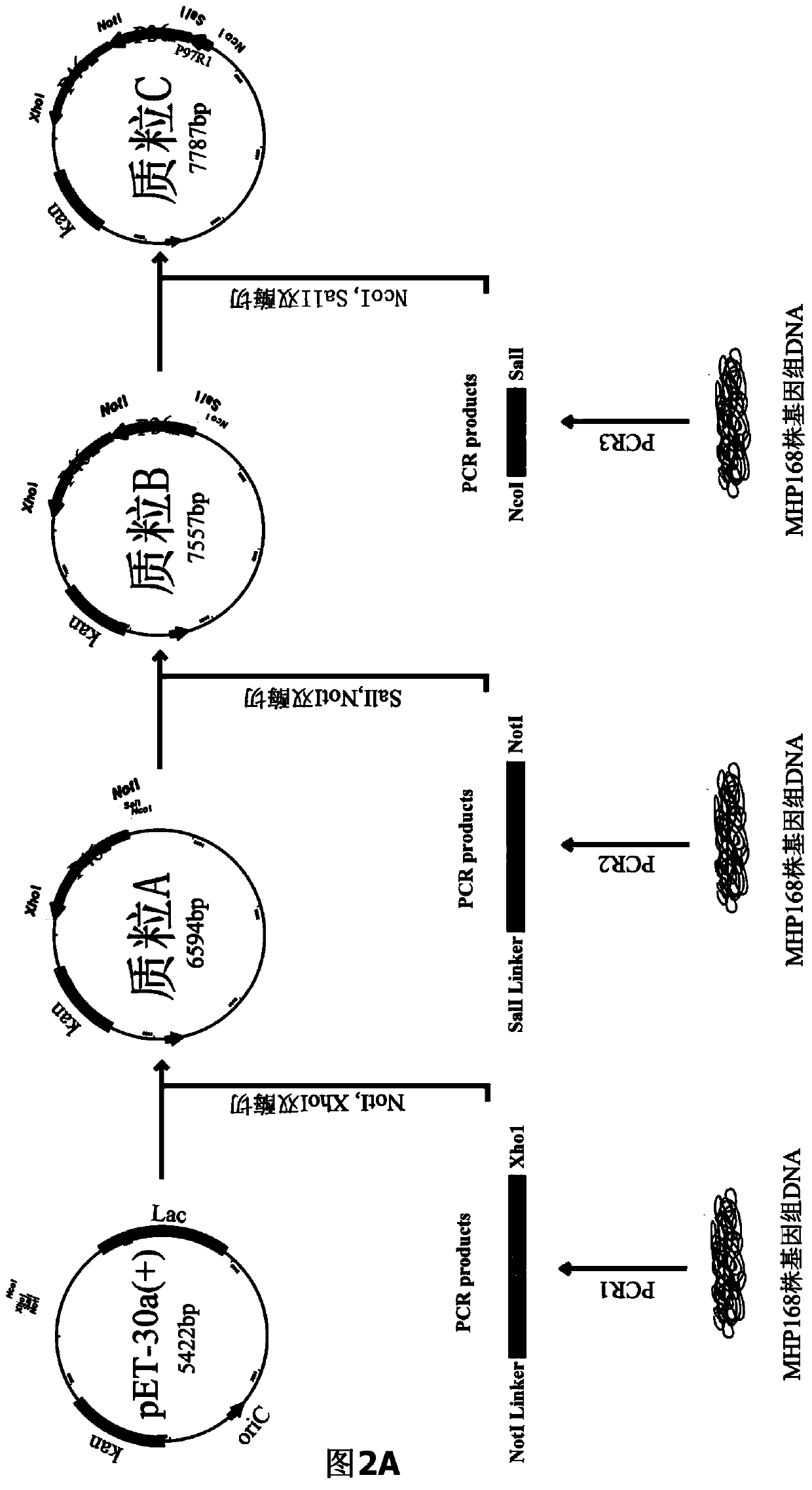
The invention belongs to the field of animal gene engineering, and in particular relates to a synthetic fusion gene for expressing mycoplasma hyopneumoniae and application. The fusion gene is characterized in that a mycoplasma hyopneumoniae P97R1 gene is connected in series with a P36 gene through a Linker, the termination codon of the P36 gene is deleted, the P46 gene with signal peptide removed is fused at the C end of the P36 gene through Linker, and then the fusion gene P97R1-P36-P46 is obtained, wherein the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The fusion gene is included in a prokaryotic expression plasmid, and escherichia coli BL21 / pET30a-P97R1-Linker-P36-Linker-P46 containing the plasmid is collected in CCTCC with the collection number CCTCC NO: M2014269. The invention further discloses immune efficacy evaluation of the fusion gene and application of the fusion gene in a novel vaccine.
Owner:HUAZHONG AGRI UNIV
Identification method taking growth hormone receptor gene as Chinese grassland red bull excellent slaughter trait molecular marker and application thereof
The invention belongs to the technical field of animal genetic engineering, and particularly relates to a growth hormone receptor gene taken as a Chinese grassland red bull excellent slaughter trait molecular marker and an application thereof. The length of a nucleotide sequence of an obtained GHR genetic segment is 489bp and the nucleotide sequence is a part of an exon. A base mutation exists at a 289-position basic group to cause the generation of restrictive fragment length polymorphism. The mutant site is taken as the molecular marker and detected by a PCR-RFLP (polymerase chain reaction-restrictive fragment length polymorphism) method using restriction enzyme BstACI, and detection results are associated with the Chinese grassland red bull slaughter traits so as to provide theoretical basis for selection breeding of Chinese grassland red bull. The invention is characterized in that partial segment of GHR gene associated with the Chinese grassland red bull excellent slaughter traits is obtained, and polymorphism analysis is conducted to the segment for providing the molecular marker for marker assisted selection of the Chinese grassland red bull.
Owner:JILIN UNIV +1
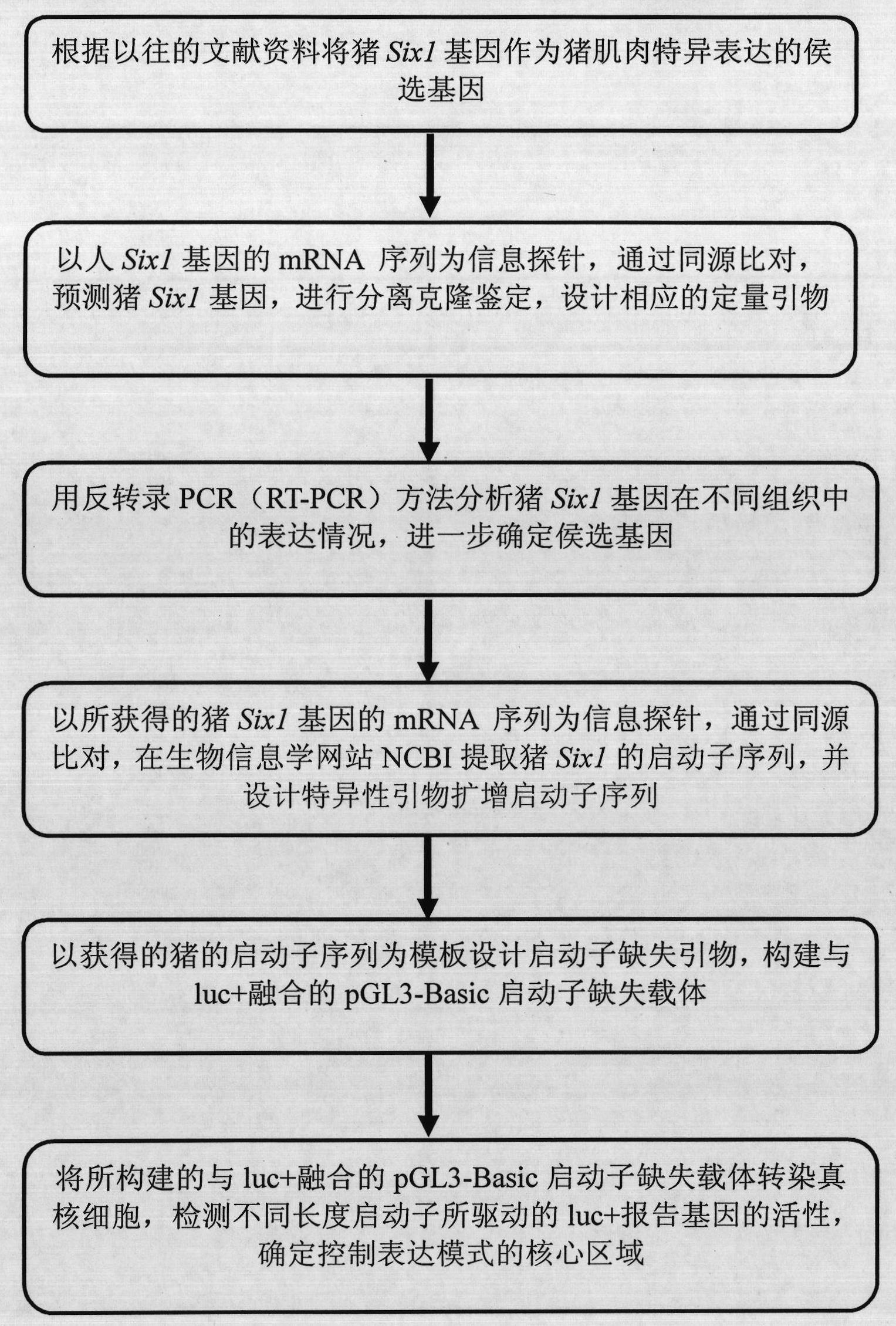
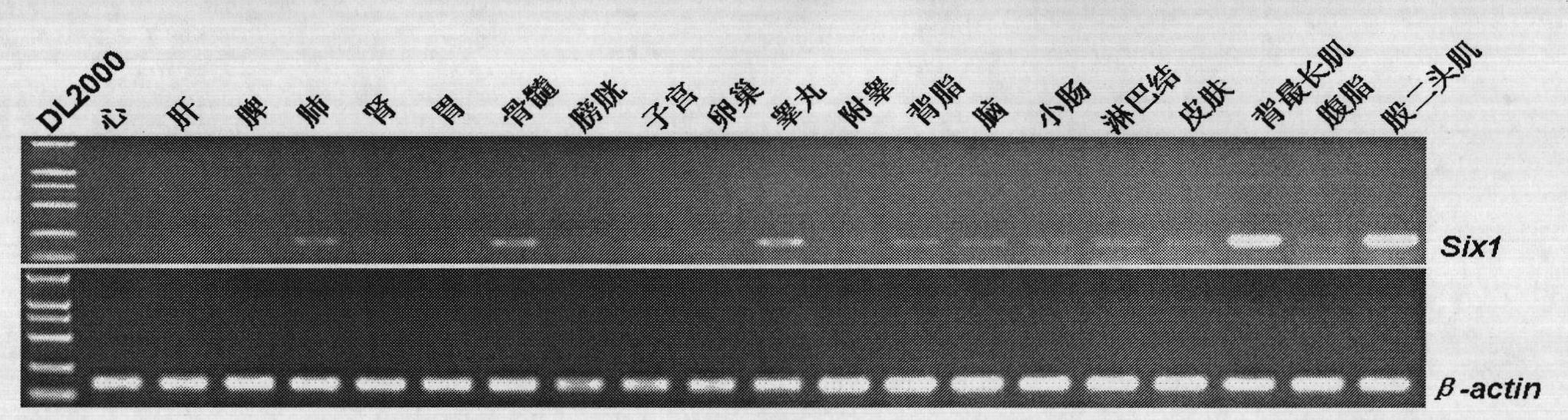
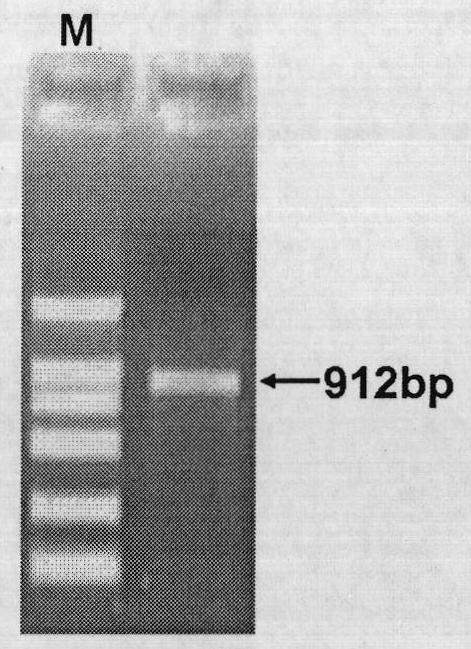
Identification of isolation cloning and core region of promoters suitable for gene expression of skeletal muscles in pigs
InactiveCN101979547AImprove reliabilityIncrease authenticityAnimal husbandryDNA/RNA fragmentationBiotechnologyPromoter activity
The invention belongs to the field of animal gene engineering. Seven promoters Six1-P, P1, P2, P3, P4, P5 and P6 with different lengths in the upstream of a specific expression gene of skeletal muscles in pigs are cloned from a pig genome. Nucleotide sequences of the promoters are shown in SEQ ID NO:1; 2; 3; 4; 5; 6; and 7. The lengths of the nucleotide sequences are respectively 1,692, 180, 530, 844, 1,143, 1,135 and 1,494bp. The activity analysis on promoter fragments with different lengths of a pig Six1 gene in three eukaryotic cells shows that the promoters of the pig Six1 gene are promoters which express the muscle specificity; except for the P1, other promoters P2, P3, P4, P5 and P6 all have the capacity of promoting gene expression; and in the promoters P2, P3, P4, P5 and P6, except for the promoter P2, other promoters all show the muscle specificity. The invention discloses the seven promoters, a method for preparing corresponding expression vectors of the seven promoters and identification of the promoter activity of the pig Six1 gene by using a dual-luciferase reporting system.
Owner:HUAZHONG AGRI UNIV
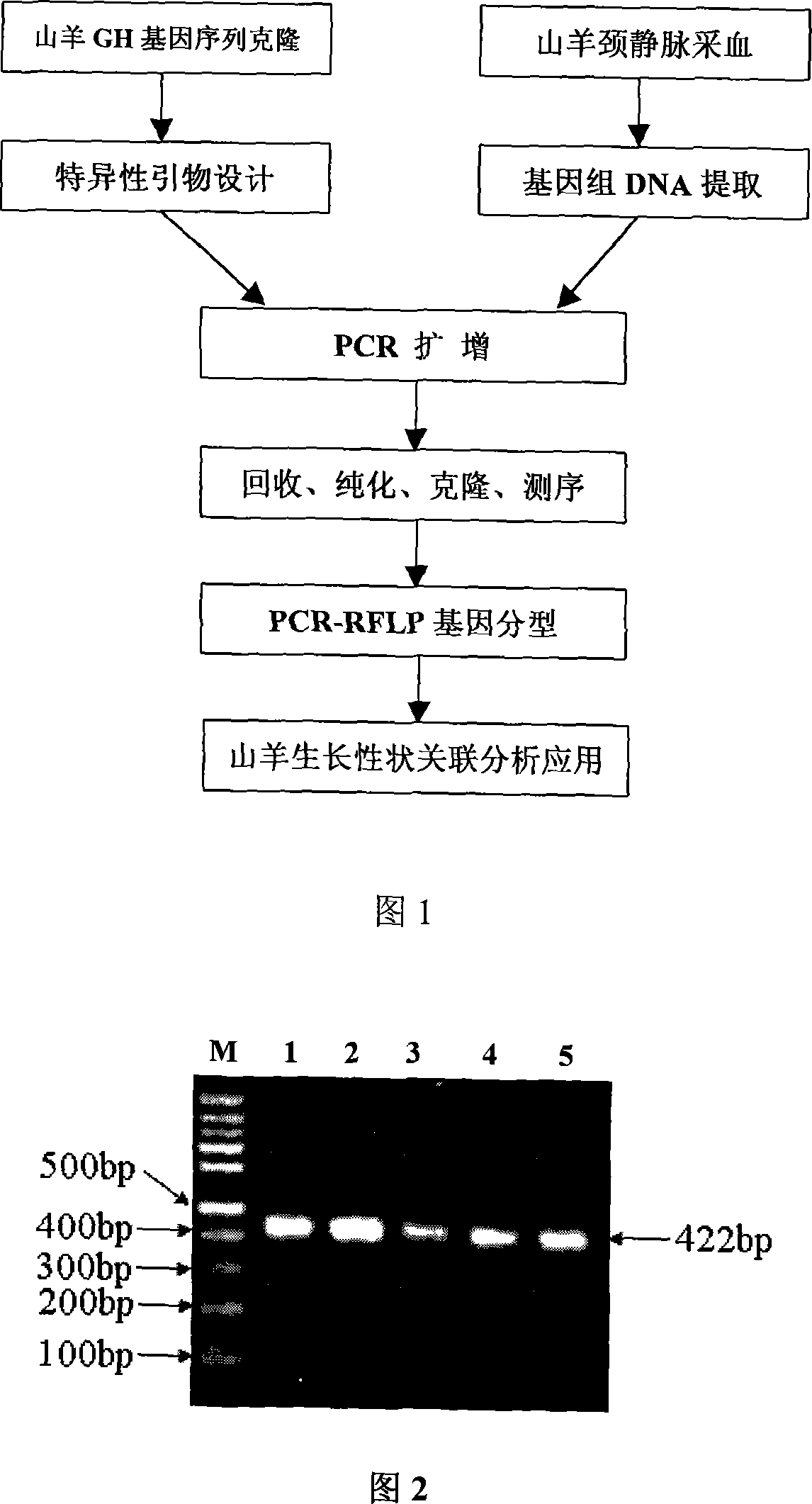
Goat growth trait GH gene clone and its application as molecule mark
The present invention belongs to the field of animal gene engineering, and is especially the cloning and polymorphism application of goat's growth hormone (GH) gene. The present invention features that two DNA sequences of goat's GH gene as shown is obtained through cloning and two base mutations affecting goat's growth characters are detected. The present invention discloses two goat's GH gene sequences and two base mutational sites affecting goat's growth characters. The present invention also discloses the preparation process and application of the said genes, and provides new molecular markers for the auxiliary selection of goat's growth characters.
Owner:HUAZHONG AGRI UNIV
TEF-1 gene of weight increment per day of pig and application in assistant selecting molecule markers of pigs
InactiveCN101092622AMicrobiological testing/measurementGenetic engineeringMarker-assisted selectionGenetics
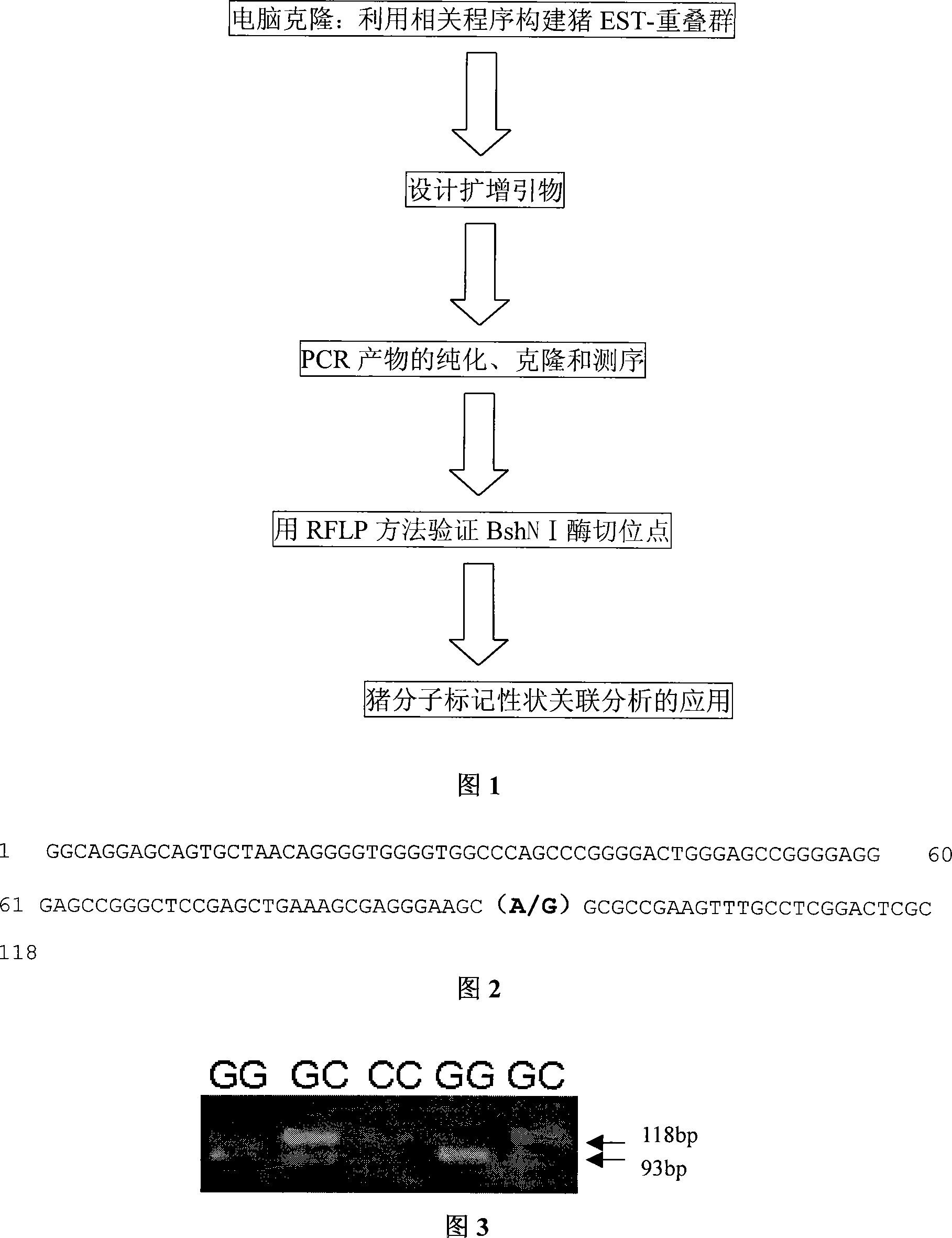
This invention relates to cloning and application of pig daily gain-related TEF-1 gene. The cDNA sequence of TEF-1 gene is shown in SEQ ID No.1. There is an A-G mutation at 93bp of SEQ ID No.1, which leads to BshN I-RFLP polymorphism. This invention also discloses a method for preparing pig daily gain-related TEF-1 gene, its primer design method, and the application of TEF-1 gene in molecular marker-assisted selection of pigs.
Owner:HUAZHONG AGRI UNIV
Application of long-chain non-coding RNA IncMGPF in regulation and control of muscle development functions of pigs
ActiveCN111154763AImprove production efficiencyShort cycleNucleic acid vectorFermentationSlow virus infectionGenetic engineering
The invention belongs to the technical field of animal genetic engineering, and particularly relates to application of long-chain non-coding RNA IncMGPF in regulation and control of muscle developmentfunctions of pigs. An over-expression vector and interfere vector of long-chain non-coding RNA IncMGPF are constructed, skeletal muscle satellite cells of the pigs are infected at a cellular level ina slow virus infection manner, biceps femoris muscles of the pigs are infected at a living level and are collected after being infected by viruses for multiple times, and the influences of IncMGPF onthe growth and development of the muscles are proved according to the individual pig, so that a technical basis is laid for the cultivation of new species of pork-type pigs with higher yield.
Owner:HUAZHONG AGRI UNIV
ZB (zebrafish) transposon system and gene transfer method mediated by same
ActiveCN105018523AEfficient transfer mediationImprove transfer efficiencyFermentationVector-based foreign material introductionCloning SiteBiology
The invention belongs to the field of animal genetic engineering, and relates to a ZB (zebrafish) transposon system of and a gene transfer method mediated by the ZB transposon system. The ZB transposon system is derived from zebra fish, and comprises two transgenic donor plasmids with terminal repetition sequences and capable of being inserted into a target gene box, and transposase auxiliary plasmids for providing activity of transposition; the transgenic donor plasmids comprise terminal repetition sequences and Msc1 insertion cloning sites at two sides of ZB transposon. The invention further discloses a gene transfer method based on the ZB transposon system, which is characterized that target genes are guided into a receptor genome through the ZB transposon system. The system and the method can be applied to multiple field of biotechnology: (1) the method can be used for effectively inserting the target gene box into a host cell genome, so that the gene transfer efficiency is improved; (2) by combining with the gene trapping technology, the researches on functions of animal genes can be effectively developed; (3) human gene therapy can be realized through mediation.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Genetic markers for horned and polled cattle and related methods
InactiveCN101883869AIncrease concentrationIncrease temperatureMicrobiological testing/measurementBiotechnologyHerd
Embodiments of the present invention provide methods for improving desirable animal traits including the horned / polled phenotype in bovine animals. Also provided are methods for determining a dairy animal's genotype with respect to multiple markers associated with polled, fitness and / or productivity traits. The invention also provides methods for selecting or allocating animals for predetermined uses such as progeny testing or nucleus herd breeding, for picking potential parent animals for breeding, and for producing improved progeny animals. Also provided are methods for identifying genetic markers associated with polled, fitness and / or productivity traits that are in allelic association with the SNPs disclosed herein.
Owner:ZOETIS SERVICE LLC
Method for improving pig meat quality
InactiveCN105039402AImprove qualityHigh in fatFermentationVector-based foreign material introductionBiotechnologyMuscle tissue
The invention belongs to the technical field of animal genetic engineering and particularly relates to a method for improving pig meat quality. The method is characterized in that a peroxisome proliferator-activated receptor (PPAR) gamma gene serves as an important candidate gene for improving the meat quality, a fibroblast cell line of a 30-day-old fetus of a large white pig is established, a SwaI linearized pN1-MCK-PPAR gamma2 expression vector is shifted to the 30-day-old fetus fibroblast cell line of the large white pig through an electrotransfection method, and a PPAR gamma transgenic pig is prepared in a method of somatic nucleus transplantation. The influence of muscle tissue overexpression PPAR gamma genes on meat traits such as intramuscular fat deposition is verified in a transgenic pig, the contradiction of simultaneously selecting meat quality and meat quantity in conventional animal breeding is overcome, and the novel method is provided for cultivating lean meat pigs with good meat quality.
Owner:HUAZHONG AGRI UNIV
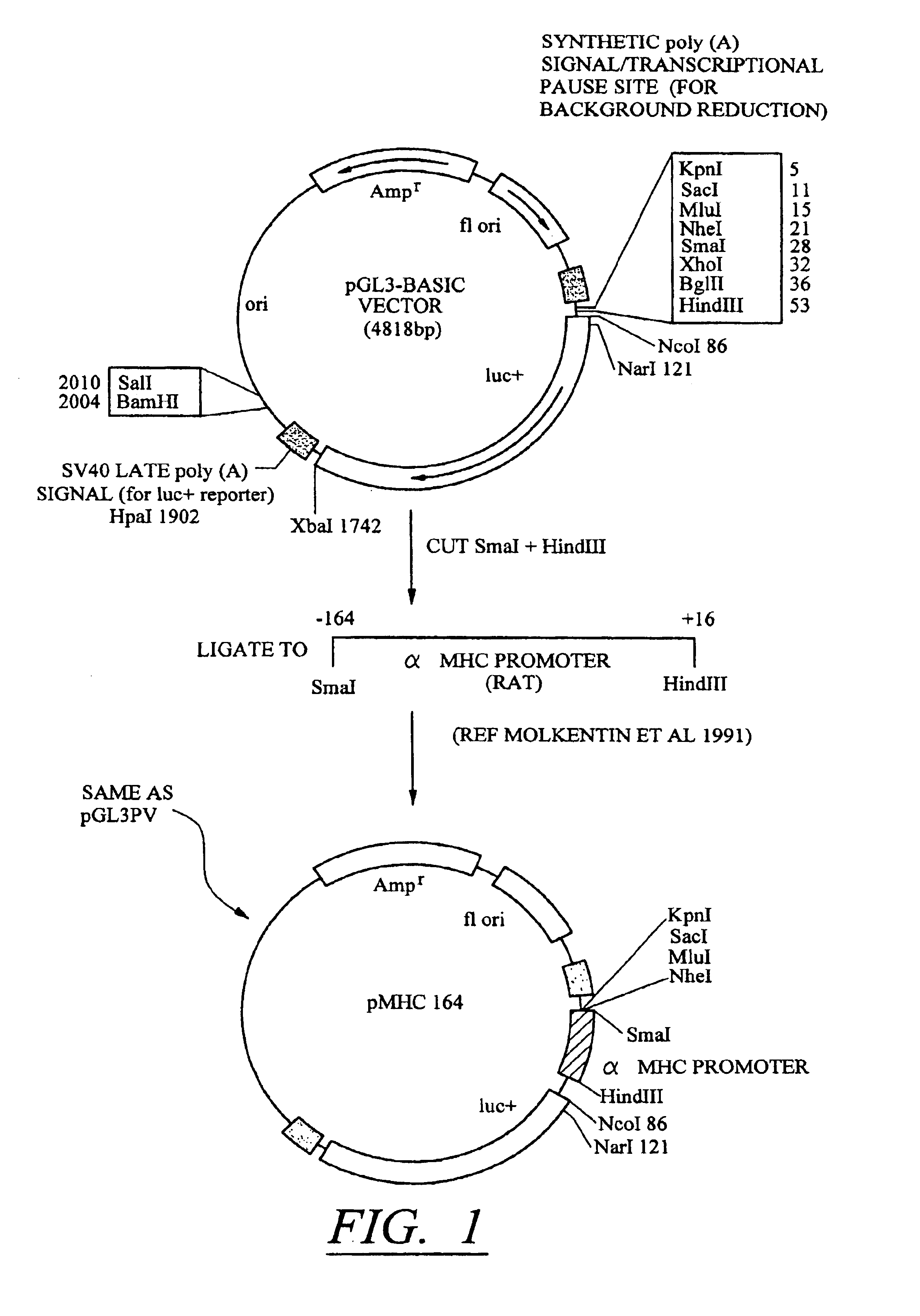
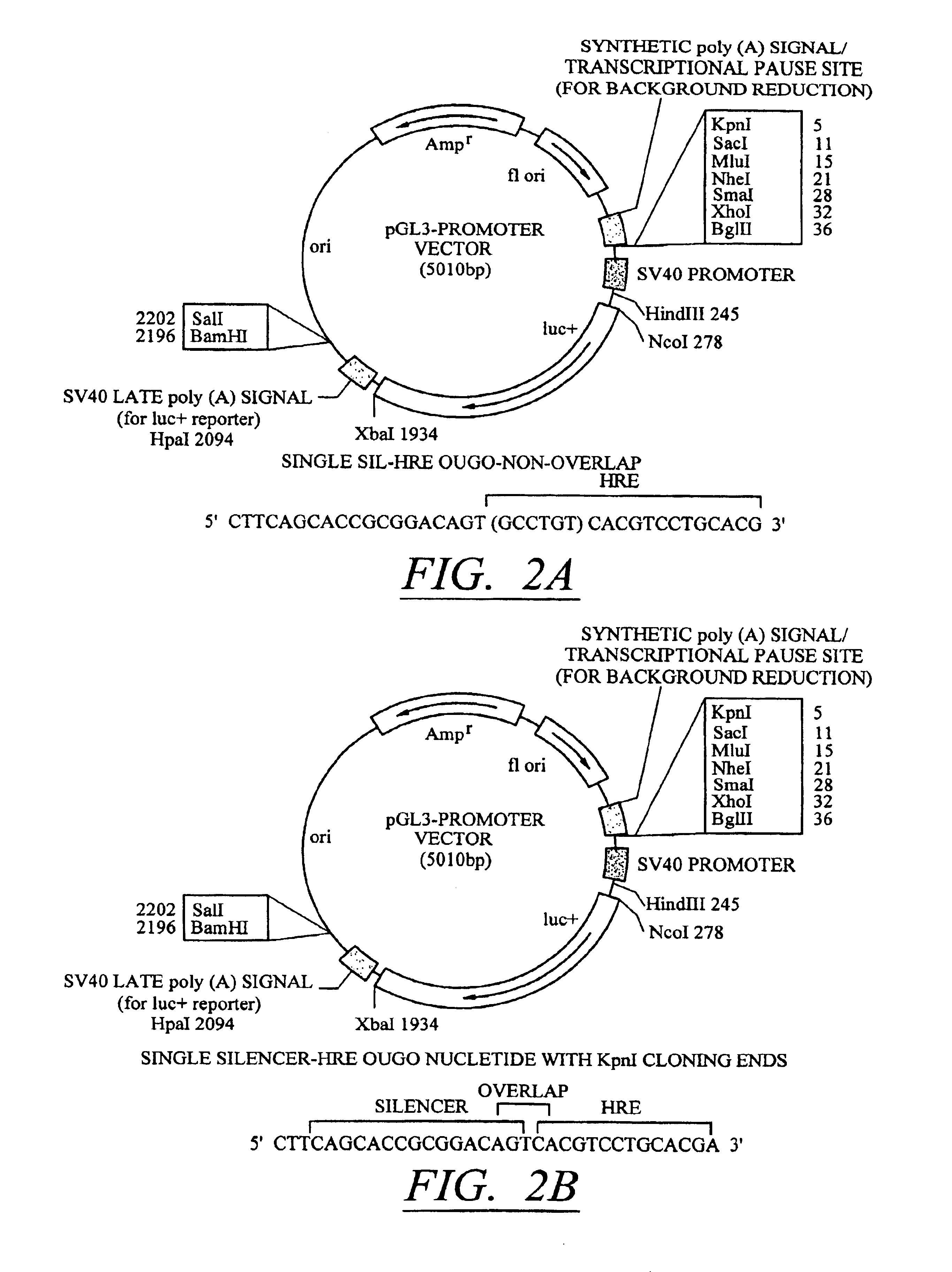
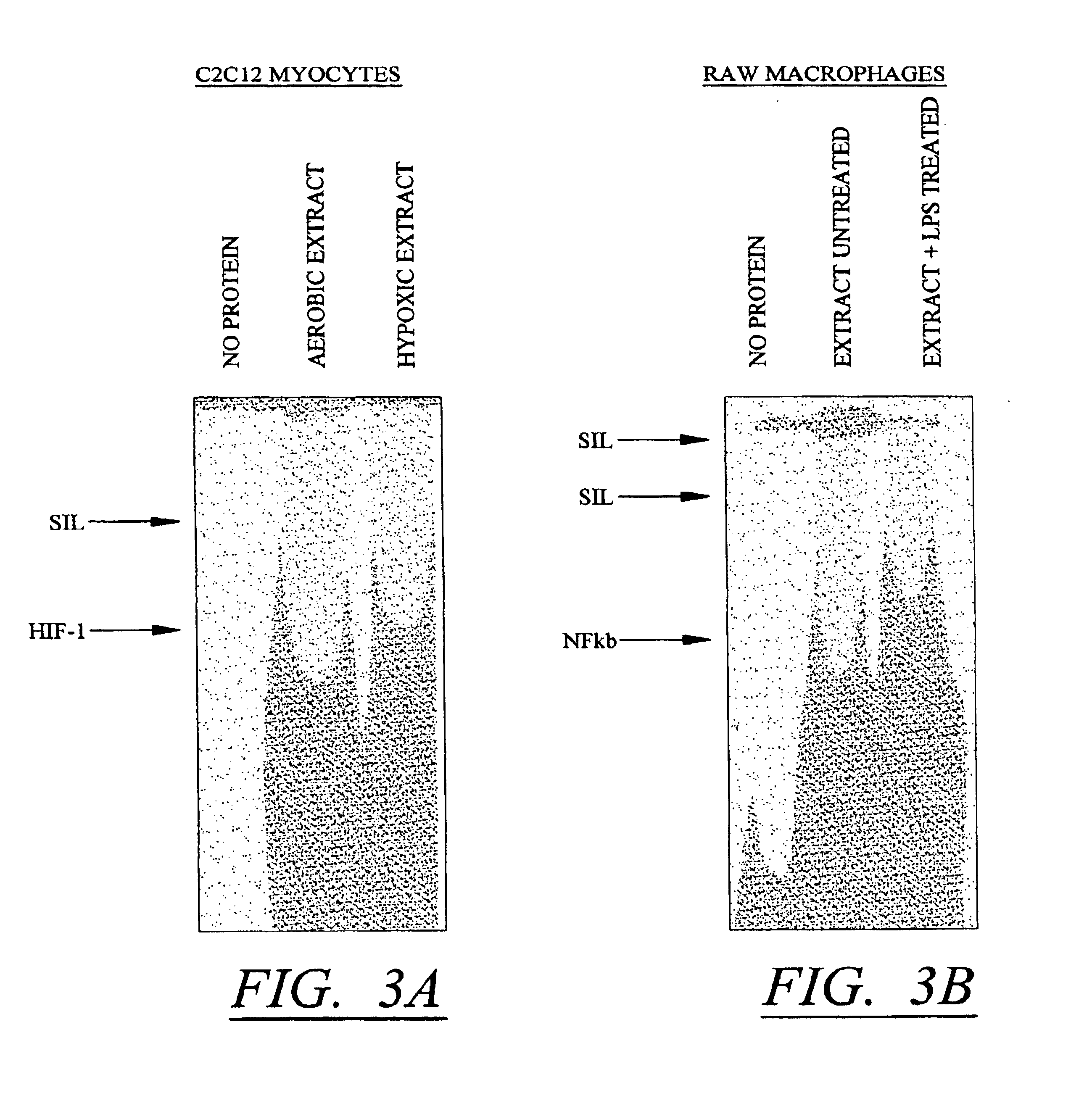
Molecular switch for regulating mammalian gene expression
Expression vectors are disclosed that are comprised of (a) one or more silencer elements and conditionally inducible elements to form silencer-inducible regions and (b) promoters in operative linkage upstream of at least one expressed region. The expression vector thereby regulates expression of at least one downstream region by conditional silencing in which an expressed DNA region of a gene is transcribed to produce RNA transcripts, which may or may not be translated to produce polypeptides. Genetically engineered mammalian cells and non-human mammals can be made using such expression vectors through transfection and transgenesis techniques. Moreover, processes of making and using the aforementioned products are disclosed (e.g., the expression vector may be used diagnostically, therapeutically, or prophylactically).
Owner:KEITH WEBSTER +1
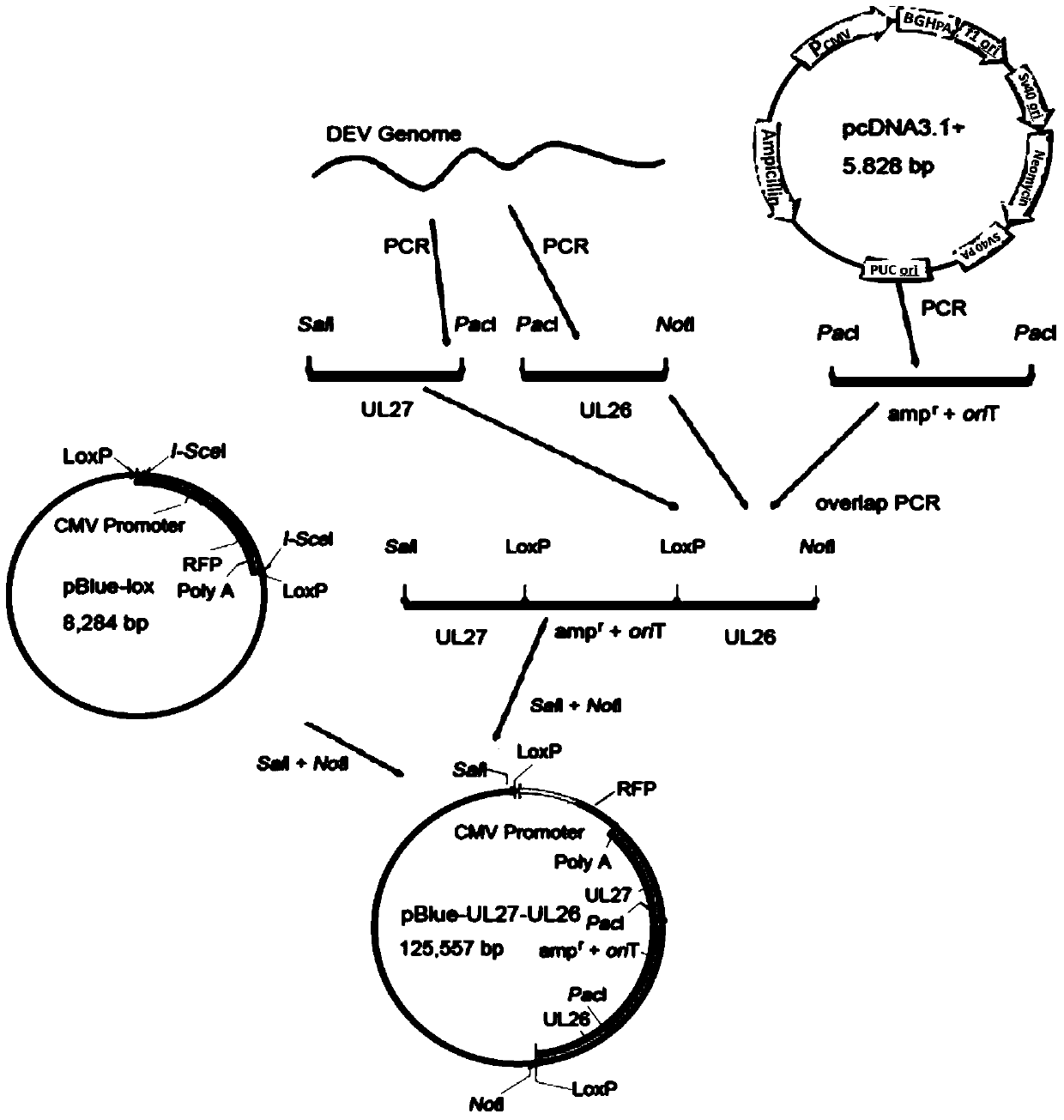
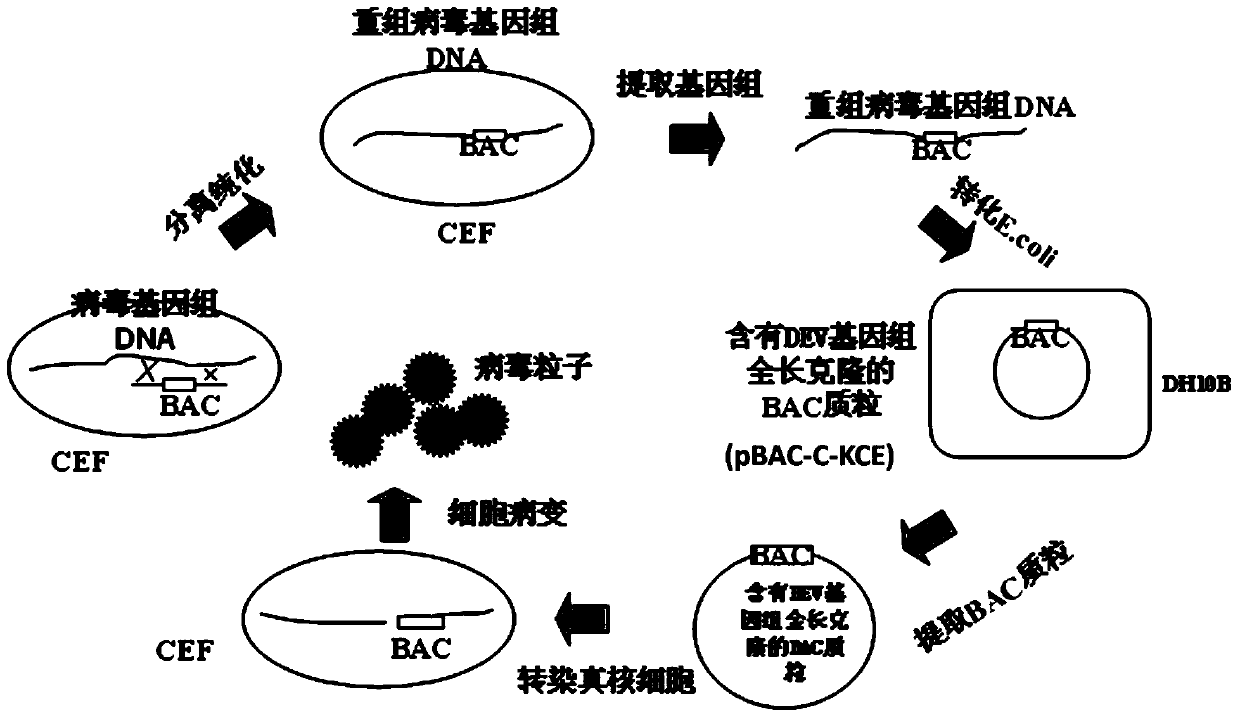
Construction method and use of duck enteritis virus bacterial artificial chromosome
InactiveCN103497967AShort cycleThere is no cross-species transferViruses/bacteriophagesVector-based foreign material introductionDuck enteritis virusEngineered genetic
The invention belongs to the technical field of animal gene engineering and relates to a construction method and a use of a duck enteritis virus bacterial artificial chromosome. Through cloning, a duck enteritis virus bacterial artificial chromosome pBAC-C-KCE plasmid is obtained, is preserved in the China center for type culture collection (CCTCC) and has an accession number of CCTCC NO: M2013377. The invention also discloses the use of the duck enteritis virus bacterial artificial chromosome pBAC-C-KCE in recombinant duck enteritis virus packaging.
Owner:HUAZHONG AGRI UNIV
Oral recombined DNA vaccine for accelerating growth of animal, and application
InactiveCN101050448AIncrease secretion levelImprove immunityBacteriaGenetic material ingredientsBiologyRecombinant DNA
This invention relates to preparation and application of orally administered recombinant DNA vaccine for accelerating animal growth, which Salmonella typhimurium CSO22 / pGMCSF-SS (CCTCC M206141) is containing fusion expression plasmid pGMCSF-SS. Salmonella typhimurium CSO22 / pGMCSF-SS is prepared by: cloning GMCSF, fusing with plasmid pcS / 2SS containing somatostatin gene to obtain fusion expression plasmid pGMCSF-SS, and transforming Salmonella typhimurium. This invention also discloses the application of Salmonella typhimurium CSO22 / pGMCSF-SS in orally administered recombinant DNA vaccine for accelerating animal growth.
Owner:HUAZHONG AGRI UNIV
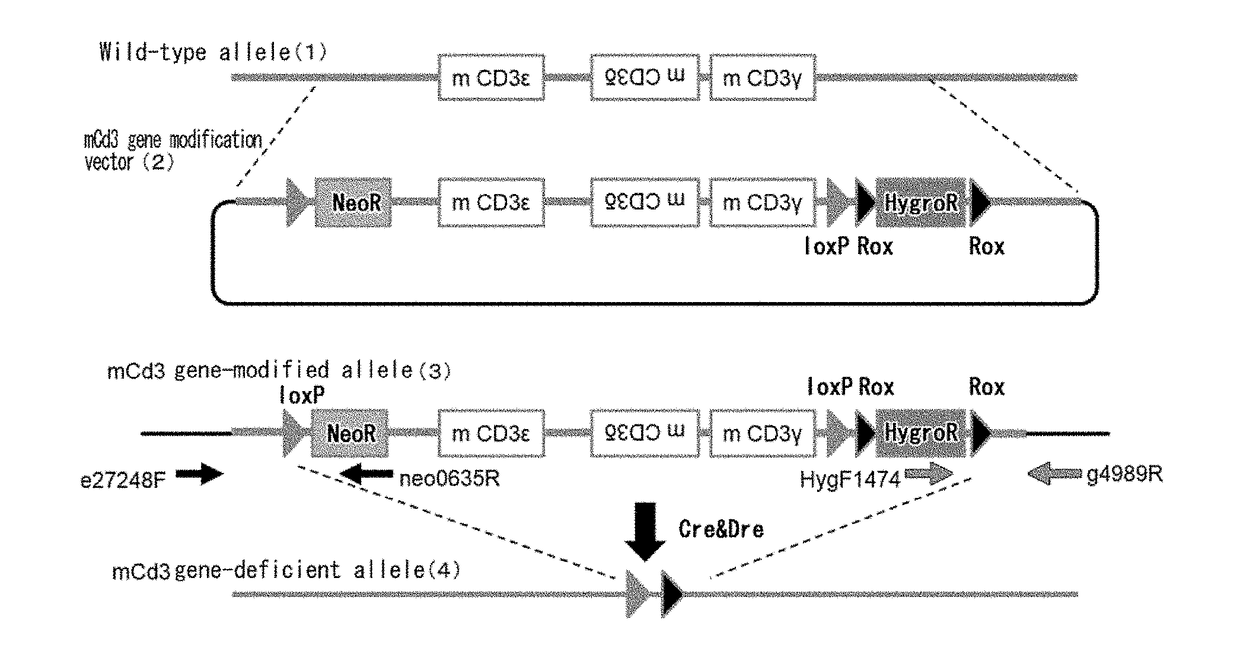
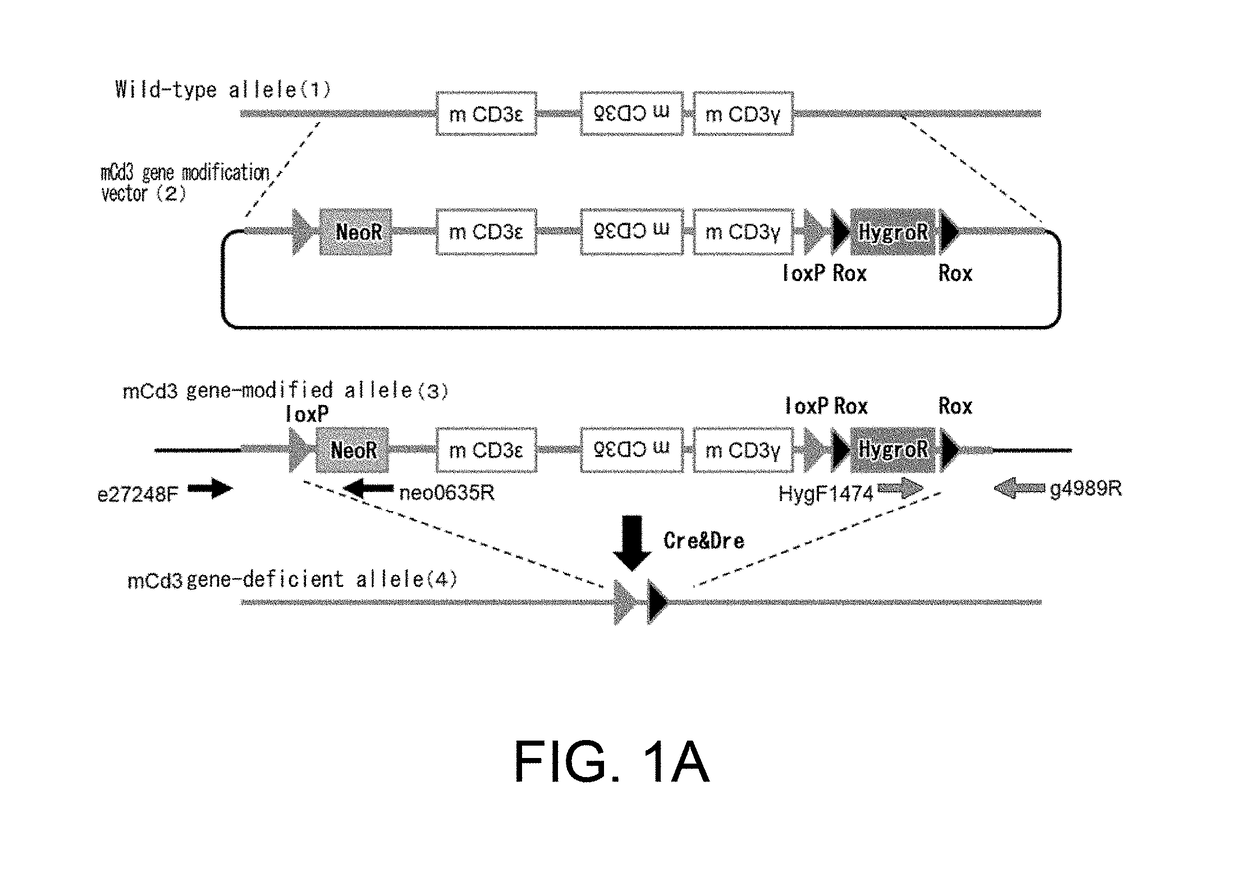
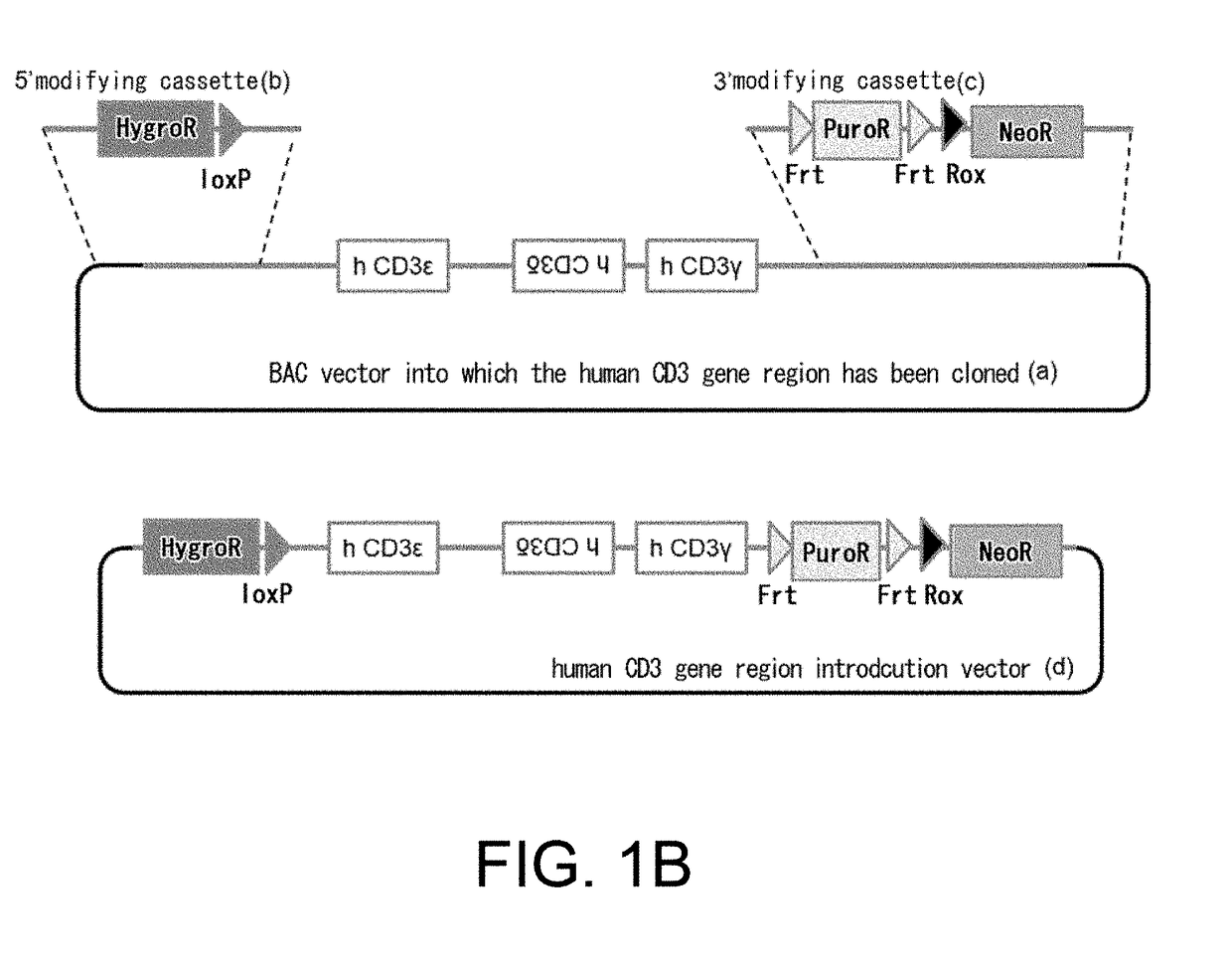
Non-human animal having human cd3 gene substituted for endogenous cd3 gene
ActiveUS20180192623A1Appropriately evaluatedImmunoglobulin superfamilyImmunoglobulinsMature T-CellT cell
The present invention provides genetically modified non-human animals which are deficient in at least one or more types of CD3 genes selected from the group consisting of endogenous CD3ε, CD3δ, and CD3γ in its genome and functionally express at least one or more types of human CD3 genes selected from the group consisting of human CD3ϵ, CD3δ, and CD3γ. In the genetically modified non-human animals of the present invention, mature T cell differentiation and production can take place, and immunocompetent cells including T cells can exert their functions. The genetically modified non-human animals of the present invention enable efficient evaluation and screening in the development of therapeutic agents and therapeutic methods that use human CD3-mediated targeted drugs.
Owner:CHUGAI PHARMA CO LTD
Clone for pork generation character related gene BTG1 of pig and application thereof in pig molecule mark auxiliary selection
InactiveCN101148668AMicrobiological testing/measurementGenetic engineeringBiotechnologyMarker-assisted selection
The present invention belongs to the field of animal gene technology, and is especially one pig gene BTG1 related to its pork producing character and its cloning and application in auxiliary selection of pig's molecular marker. The present invention clones pig gene BTG1 related to its pork producing character with the sequence of SEQ ID No. 1, which has one A179-T179 base mutation in No. 179 bp and one C280-T280 base mutation in No. 280 bp to result in Psu1-RFLP polymorphism and Bsh1236I-RFLP polymorphism separately. The present invention also discloses the preparation process of the said gene and its primer, and provides new molecular marker for auxiliary selection of pig.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com