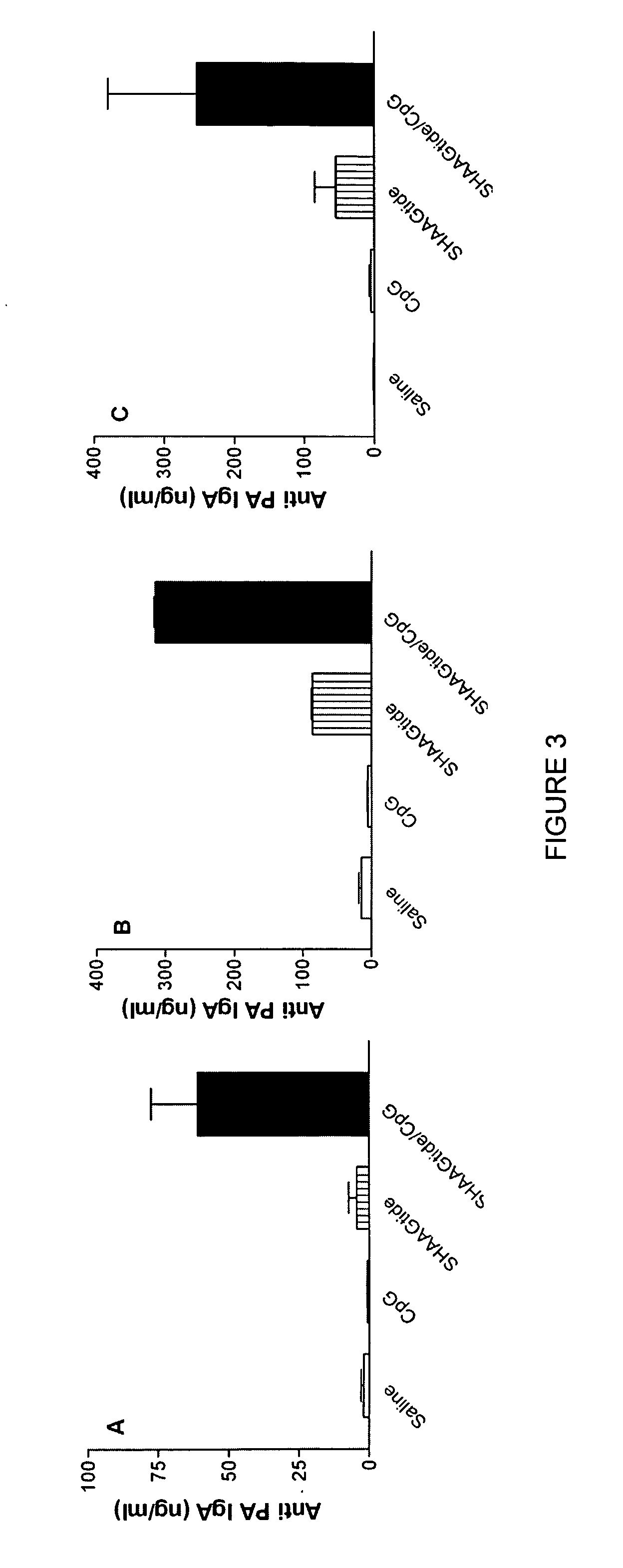
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
216 results about "Mucosal immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mucosal immunity is formed by mucosa-associated lymphoid tissue, which functions independently of the systemic immune system, and which has its own innate and adaptive components.
Immunogenic compositions and vaccines comprising carrier bacteria that secrete antigens
InactiveUS20040101531A1Snake antigen ingredientsDepsipeptidesMucosal Immune ResponsesAntigen delivery
Disclosed are vaccines and immunogenic compositions which use live attenuated pathogenic bacteria, such as Salmonella, to deliver ectopic antigens to the mucosal immune system of vertebrates. The attenuated pathogenic bacteria are engineered to secrete the antigen into the periplasmic space of the bacteria or into the environment surrounding the bacteria. The vertebrate mounts a Th2-mediated immune response toward the secreted antigen.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Novel Vaccine Containing Adjuvant Capable Of Inducing Mucosal Immunity
InactiveUS20070219149A1Easy vaccinationEffectiveSsRNA viruses negative-senseAntibacterial agentsAntigenAdjuvant
The present invention provides an adjuvant that possesses a greater adjuvant potential than that of a conventional adjuvant, and that is capable of producing a protective reaction across different strains. This problem has been solved by the finding that a double-stranded RNA (for example, Poly(I:C)) unexpectedly exhibits the above capability when used in combination with a subunit antigen. Accordingly, the present invention provides a vaccine for mucosal administration containing A) a double-stranded RNA and B) a subunit antigen or inactivated antigen of a pathogen.
Owner:THE RES FOUND FOR MICROBIAL DISEASES OFOSAKA UNIV +2
Lactobacillus fermentum and application thereof
InactiveCN102226157ADisease resistantGrowth-promotingAntibacterial agentsBacteriaLactobacillus fermentumFeed additive
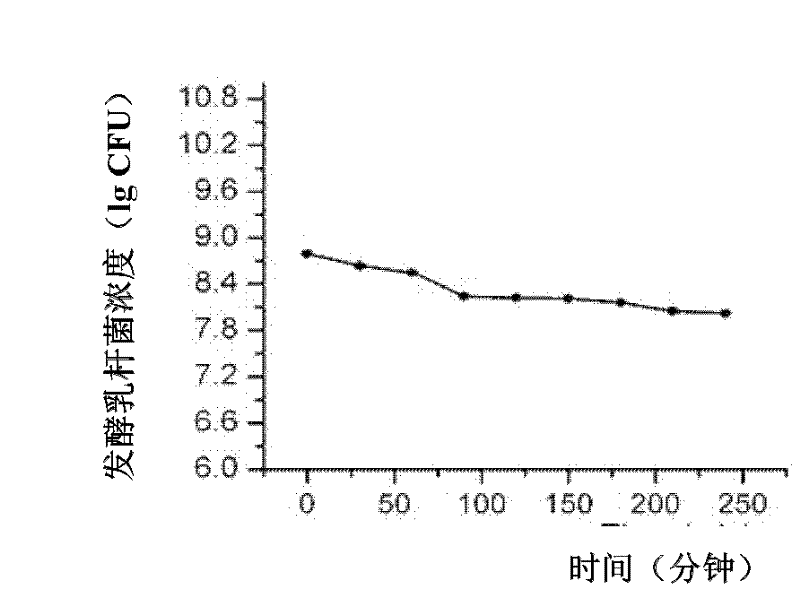
The invention discloses a lactobacillus fermentum and an application thereof. The lactobacillus fermentum HAFI5007 is preserved in the China General Microbiological Culture Collection Center on March 11, 2011 and the registration number of the lactobacillus fermentum HAFI5007 is CGMCC No.4648. The lactobacillus fermentum is mainly utilized as an additive of animal feeds, can improve a nutrient digestibility, a microbial composition in a gastrointestinal tract, functions of intestinal mucous protein expression and intestinal mucosa immunization and an antioxidant defense system of a pig, and reduce oxidative damages produced by an oxidative stress. Therefore, the lactobacillus fermentum can improve a production performance of pigs.
Owner:北京龙科方舟生物工程技术有限公司
Potent mucosal immune response induced by modified immunomodulatory oligonucleotides
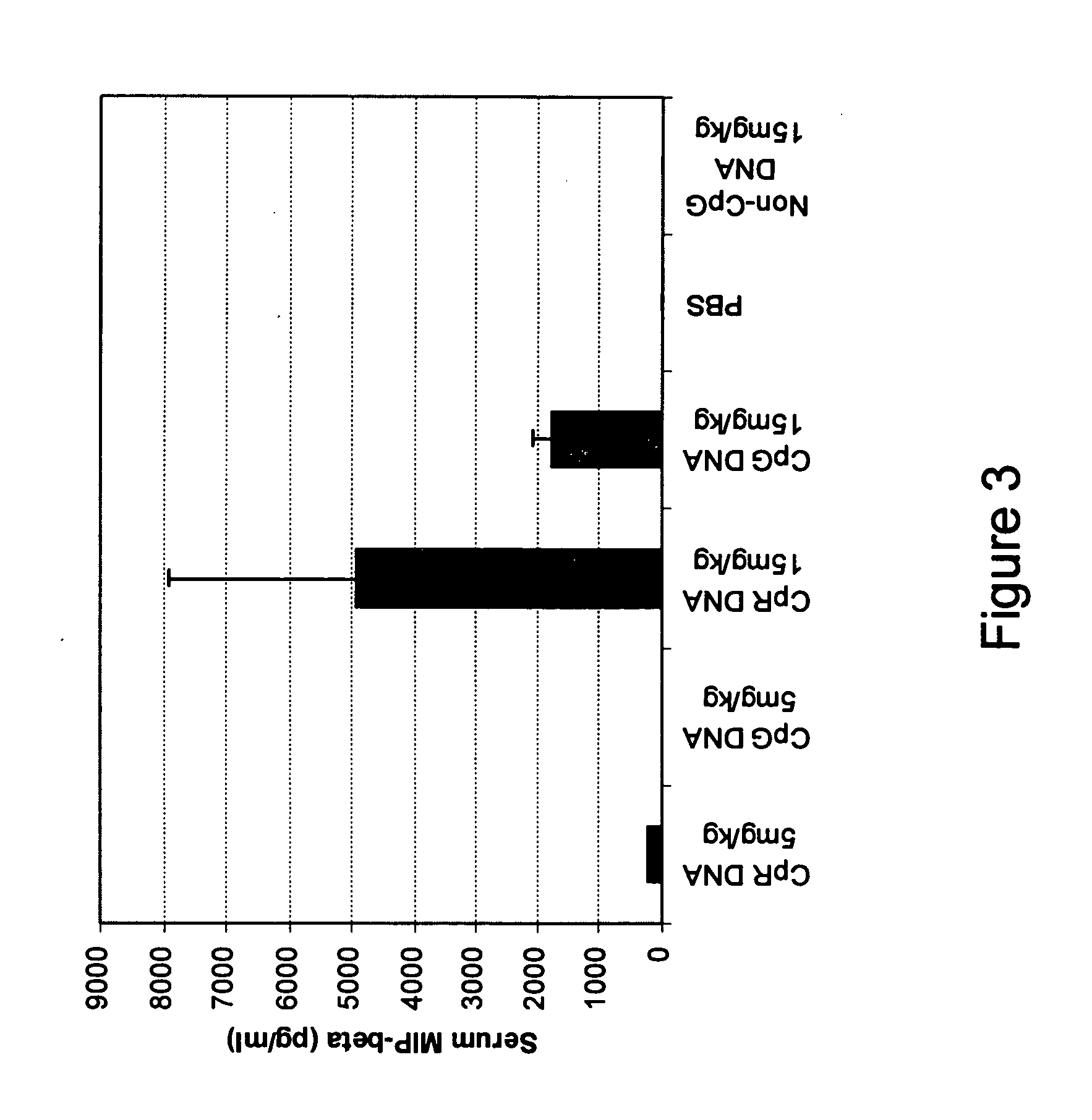
InactiveUS20050222072A1Increases generationConducive to survivalAntibacterial agentsAntipyreticOligonucleotideMucosal immunity
Owner:IDERA PHARMA INC
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
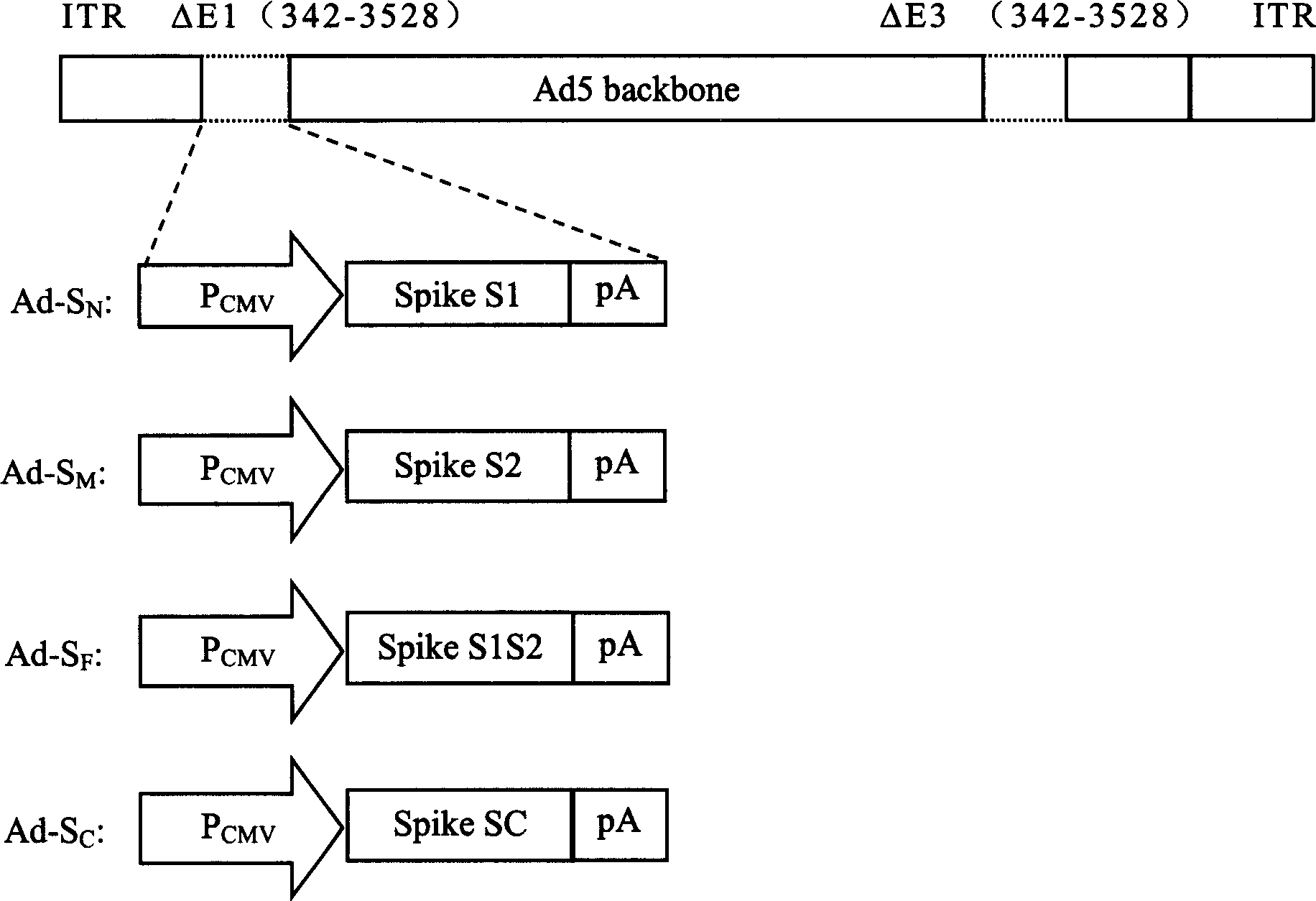
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
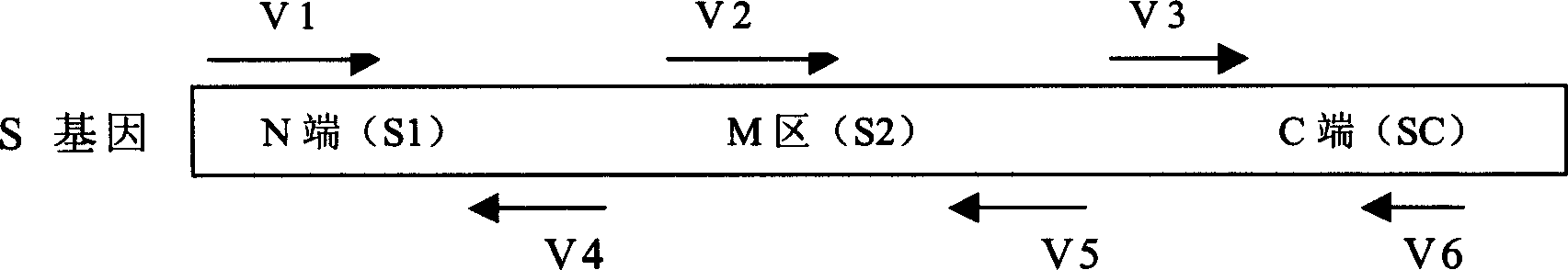
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
Recombinant helicobacter pylori protein vaccine and preparation method thereof
InactiveCN107298716ARetain the infrastructureRetain activityAntibacterial agentsBacterial antigen ingredientsAdjuvantVaccine antigen
The invention discloses a recombinant helicobacter pylori protein vaccine and a preparation method thereof. The active ingredient recombinant fusion protein of the vaccine consists of recombinant LTAl-Ureal protein and LTB protein, the amino acid sequence of the recombinant LTAl-Ureal protein is shown as Seq ID No.1, the amino acid sequence of the LTB protein is shown as Seq ID No.2. Epitope-containing gene segments of Hp urease A subunit is inserted in an LTA subunit-encoded gene, a toxic part-containing segment is replaced to prepare a recombinant plasmid so as to express and obtain recombinant fusion protein polymer as a vaccine antigen, the fusion antigen and LTB protein pentamer are combined to form a hexamer structure, so that not only can the structure basis and activity of an LT mucosa adjuvant be remained, but also the toxicity can be removed, the immune response of organism muscosa can be effectively induced through immunity of mucosa path, to generate specific IgA antibody. The recombinant helicobacter pylori protein vaccine provides a vaccine manner for preventing and treating infection of helicobacter pylori.
Owner:成都亿妙生物科技有限公司
Induction of mucosal immunity by vaccination via the skin route
InactiveUS20040109874A1SsRNA viruses negative-senseBacterial antigen ingredientsVaccinationImmunity response
Methods for generating an immune response at a mucosal surface are described. Compositions suitable for use in the methods for generating an immune response at a mucosal surface are also described. In addition, methods for treating or preventing a disease caused by the entry of a pathogen into the body of a subject via a mucosal surface are provided.
Owner:POWDER JECT VACCINES INC
Active lactic acid bacteria capsules with treatment and prevention effect on vaginitis
InactiveCN102727531ABroaden your optionsEasy to rebalanceBacteria material medical ingredientsSexual disorderImmunity responseIntestino-intestinal
The invention relates to active lactic acid bacteria capsules with a treatment and prevention effect on vaginitis. The lactic acid bacteria capsules capable of simultaneously adjusting dysbacteriosis of intestinal tracts and vagina and emphasizing treatment and prevention of the vaginitis are developed for achieving comprehensive effects. According to the technical scheme, excipient and lactic acid bacteria mixed bacteria powder are wrapped in the active lactic acid bacteria capsules with the treatment and prevention effect on vaginitis, and the lactic acid bacteria mixed bacteria powder comprises lactobacillus crispatus powder and other lactic acid bacteria subspecies powder with an effect of adjusting the intestinal flora. The active lactic acid bacteria capsules can be used for treating the vaginitis by adopting oral administration and intra-vaginal use; the oral lactobacillus can effectively improve the immunity of mucosa by stimulating the immune response of the intestinal mucosa, adjust the overall function state of a human body and well treat the vaginitis; and a large quantity of lactobacillus proliferated in the intestinal tract can inhibit growth of intestinal bacteria and reduce the risk of intestinal bacteria infection in vagina.
Owner:郑州金森生物科技工程有限公司
Microneedle array vaccine adjuvant transmission system built by using lipid modifying carrier
InactiveCN104083759AImprove stabilityEnhance internal and external stabilityImmunological disordersAntibody medical ingredientsExcipientTransmission system
The invention discloses a microneedle array which contains lipid modifying carrier and is used for a vaccine adjuvant transmission system. The microneedle array comprises a substrate and a plurality of microneedles fixed on the substrate, wherein the substrate is composed of saccharides, povidone, cellulose, or starch auxiliary materials; each microneedle comprises lipid A modifying carrier, the auxiliary materials (excipients) and vaccine ingredients; the lipid A modifying carrier can be lipidosome, lipid, micro-capsule or nanoparticle, and the like; the vaccine ingredients mainly refer to causative agent antigen proteins. Compared with the existing vaccine preparations, the lipid A-modified lipidosome microneedle array vaccine adjuvant transmission system can contain different vaccine ingredients to form vaccines aiming to different pathogens and therefore has a wide application scope. Biodegradable materials are selected and therefore the safety is high. The microneedle array vaccine adjuvant transmission system is a solid preparation which is high in stability and convenient to inoculate. Inoculation can be completed by a user self through oral mucosa, the vaccine can be prevented from running away along with saliva, and a body can be induced to establish mucosal immunity.
Owner:ANHUI MEDICAL UNIV
Female Reproductive Tract and Anal Prophylaxes
InactiveUS20110104121A1Reduce and eliminate effectBiocidePeptide/protein ingredientsBacteroidesGlucocorticoid
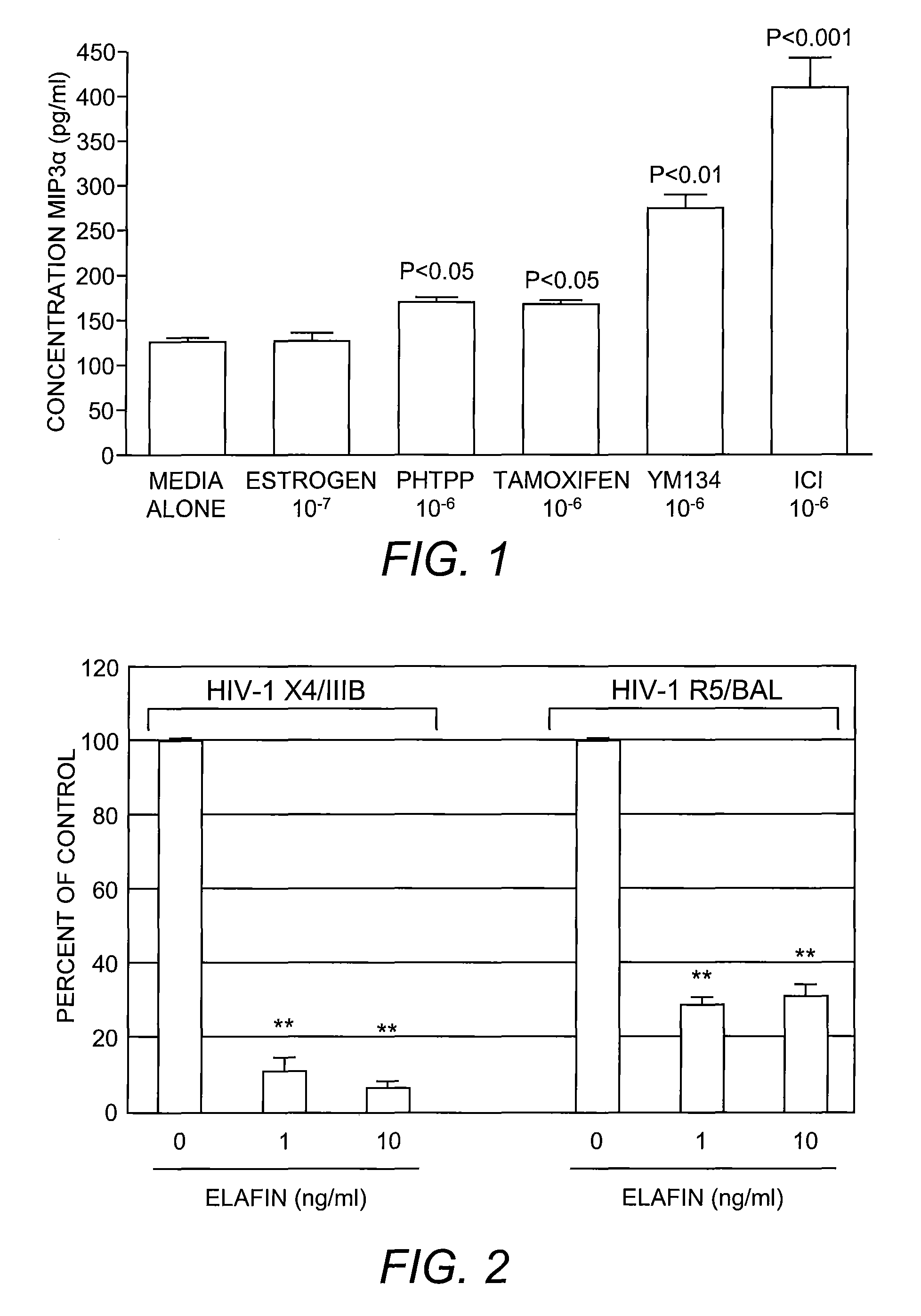
The present invention provides methods for boosting mucosal immunity in the female reproductive tract of pre- and post-menopausal women using a TGF-beta inhibitor, a Selective Estrogen Receptor Modulator, and / or a recombinant commensal bacterium that expresses endogenous microbicides into the intestinal tract or reproductive tract of a subject. It also provides methods for boosting innate and adaptive immunity by providing a glucocorticoid. Methods for preventing sexually transmitted infections including HIV infection are also provided.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application
InactiveCN102399806AReduce colonizationPromote accumulationBacteriaGenetic material ingredientsBacillus megateriumCell wall
The invention discloses a porcine epidemic diarrhea S1 protein fusion gene, recombinant bacillus megaterium strain and application. An antigen fusion gene shown as SEQ ID No. 1. is obtained by connecting an antigen locus of porcine epidemic diarrhea virus (PEDV) S glycoprotein and a cell wall anchoring sequence. The invention further constructs a secretion expression vector containing the antigen fusion gene, and secretion expression vector is converted into the bacillus megaterium to express recombinant protein on the cell wall or surface of the bacillus megaterium. Immunoblotting experiments indicate that the expressed recombinant protein can react with PEDV immune serum and has the same antigenicity as PEDV natural antigens. Immunofluorescent tests of live bacteria which are subjected to induced expression indicate that the expressed recombinant protein is positioned to the surfaces of the bacteria. Experiment results indicate that the recombinant protein can be prepared into safe and effective mucous immune live vaccines for preventing and treating porcine epidemic diarrhea.
Owner:WUHAN HUAYANG ANIMAL PHARMA
Hepatitis b virus surface antigen as a mucosal immunostimulator and the resulting formulations
InactiveUS20050025780A1Enhance immune responseViral antigen ingredientsReverse transcribing DNA virusesHeterologous AntigensAdjuvant
The invention relates to a mucosal surface antigen which is used to promote and increase in the immune response against co-administered antigens in the formulations out line in the invention. Said novel formulations are obtained from the dual use of the surface antigen as an immunostimulatory agent and, at the same time, as a vaccine antigen. In this way it is possible to obtain multiple formulations of the hepatitis B surface antigen and heterologous antigens, with immunogenicity levels similar to those obtained following parenteral administration and with a reduction in components that can dispense with the use of nasal adjuvants, thereby converting same antigens into elements that can promote an increase in the response to the other co-administered antigens. Said novel use of the hepatitis B virus surface antigen and the resulting antigen formulations can be used in the pharmaceutical industry as therapeutic and preventive vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
Mucosal immunogenic substances comprising a polyinosinic acid - polycytidilic acid based adjuvant
InactiveUS20070166239A1Enhance immune responseImprove responseSsRNA viruses negative-senseOrganic active ingredientsMucosal Immune ResponsesAdjuvant
The present invention provides a polynucleotide adjuvant composition and methods of use in eliciting an immune response, in particular a mucosal immune response. The present invention also provides an immunogenic composition comprising the polynucleotide adjuvant composition together with other immunogenic compositions such as an antigen (e.g., as in a vaccine). The present invention further contemplates methods of use of such adjuvant compositions, particularly in eliciting an immune response, in particular a mucosal immune response to an antigenic compound.
Owner:YISHENG BIOPHARMA SINGAPORE
Pharmaceutical Proteins, Human Therapeutics, Human Serum Albumin Insulin, Native Cholera Toxin B Subunit on Transgenic Plastids
InactiveUS20110179530A1Eliminate needLarge biomassBryophytesMicroorganismsOral tolerizationInsulin-like growth factor
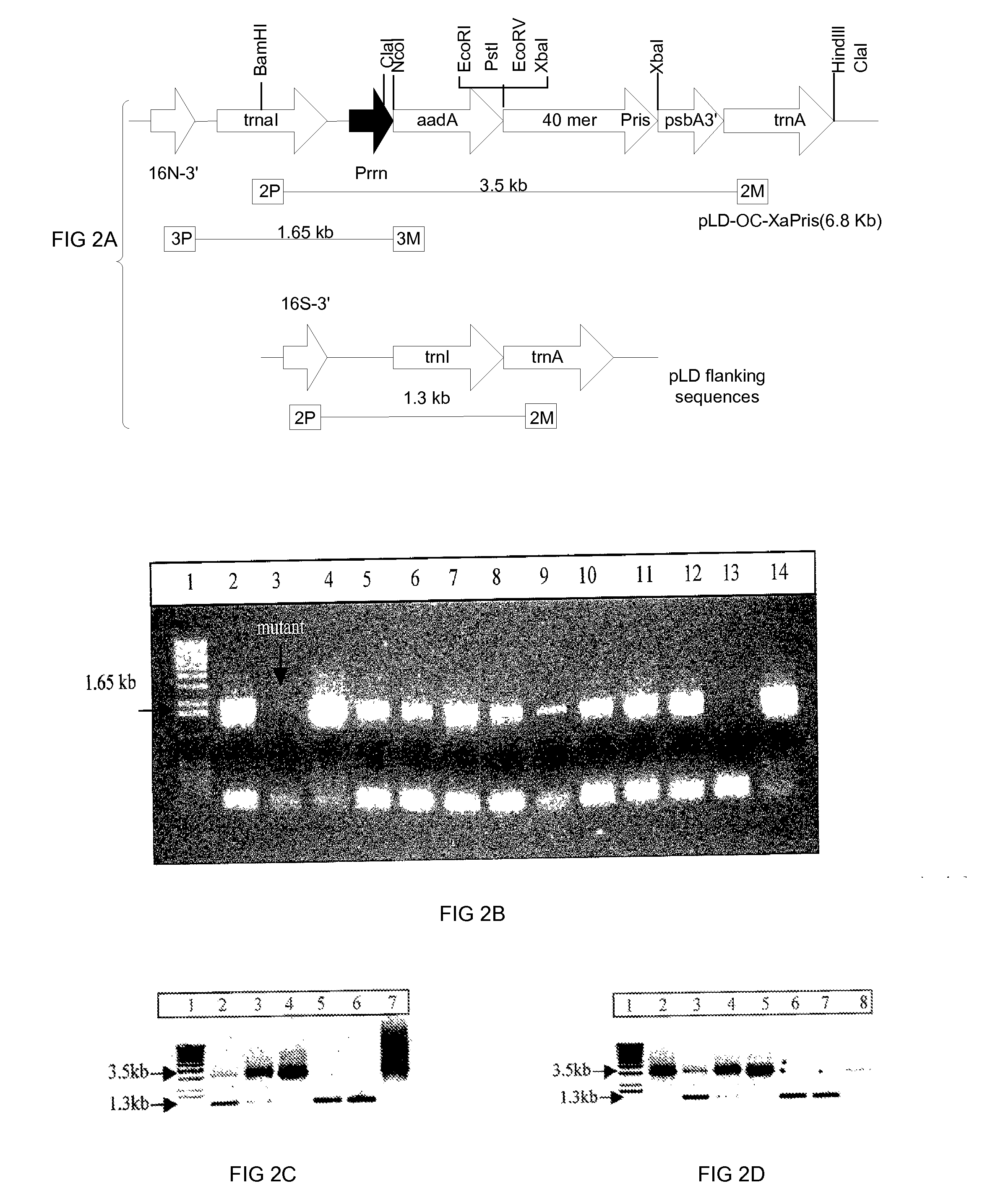
This invention relates in part to synthesizing high value pharmaceutical proteins in transgenic plants by chloroplast expression for pharmaceutical protein production. We use poly(GVGVP), for example, as a fusion protein to enable hyper-expression of insulin and to accomplish rapid one step purification of fusion peptides utilizing the inverse temperature transition properties of this polymer. We also use insulin-CTB fusion protein in chloroplasts of nicotine free edible tobacco (LAMD 605) for oral delivery. This invention includes expression of native cholera toxin B subunit gene as oligomers in transgenic tobacco chloroplasts which may be utilized in connection with large-scale production of purified CTB, as well as an edible vaccine if expressed in an edible plant, as a transmucosal carrier of peptides to which it is fused to enhance mucosal immunity, and / or to induce oral tolerance of the products of these peptides. The present invention also relates in part to recombinant DNA vectors for enhanced expression of human serum albumin, insulin-like growth factor I, and interferon-α 2 and 5, via chloroplast genomes.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Novel mucosal vaccination approach for herpes simplex virus type-2
InactiveUS20120027841A1High potencyPrevent relapseOrganic active ingredientsViral antigen ingredientsHeterologousLiposome
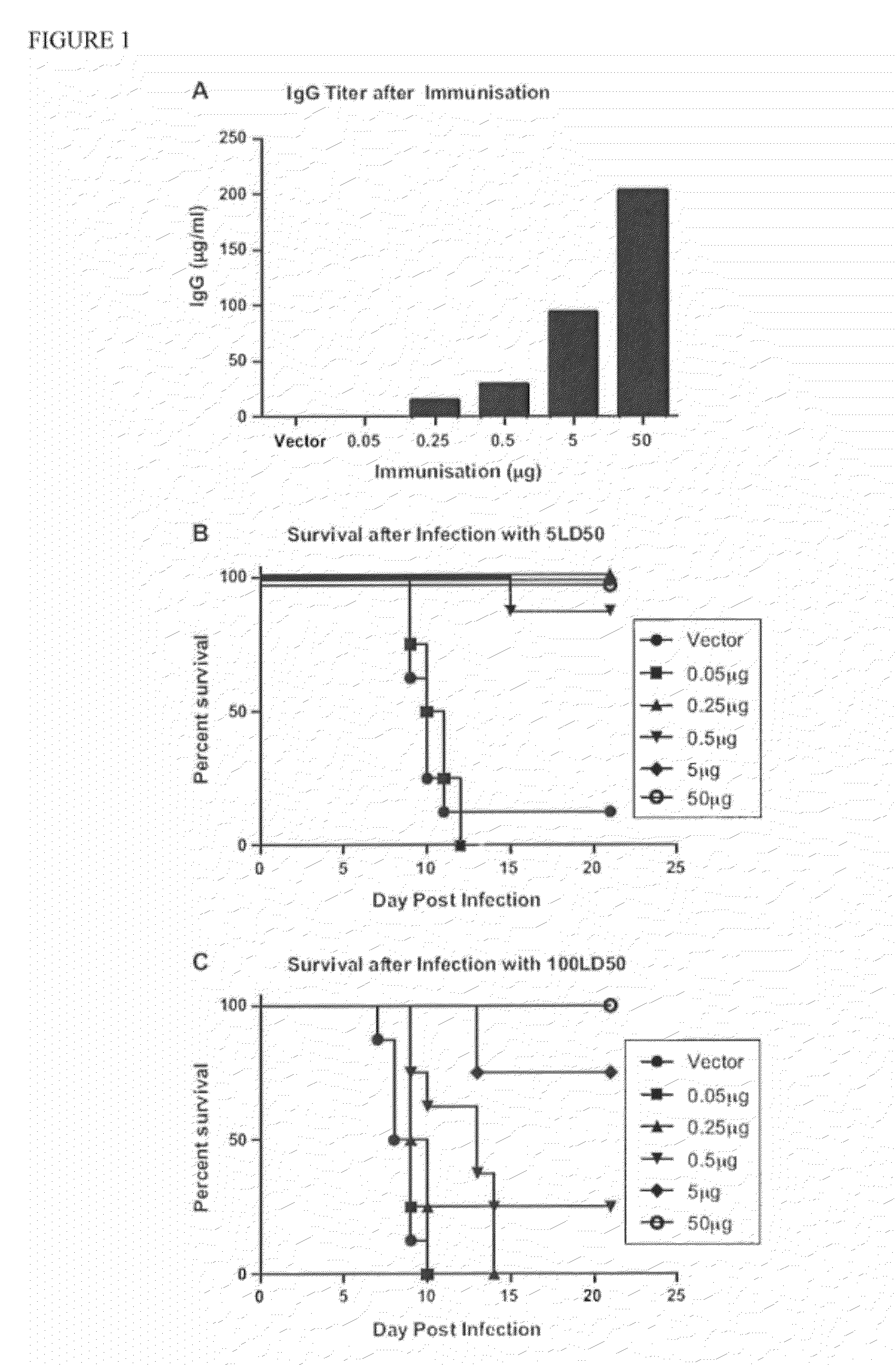
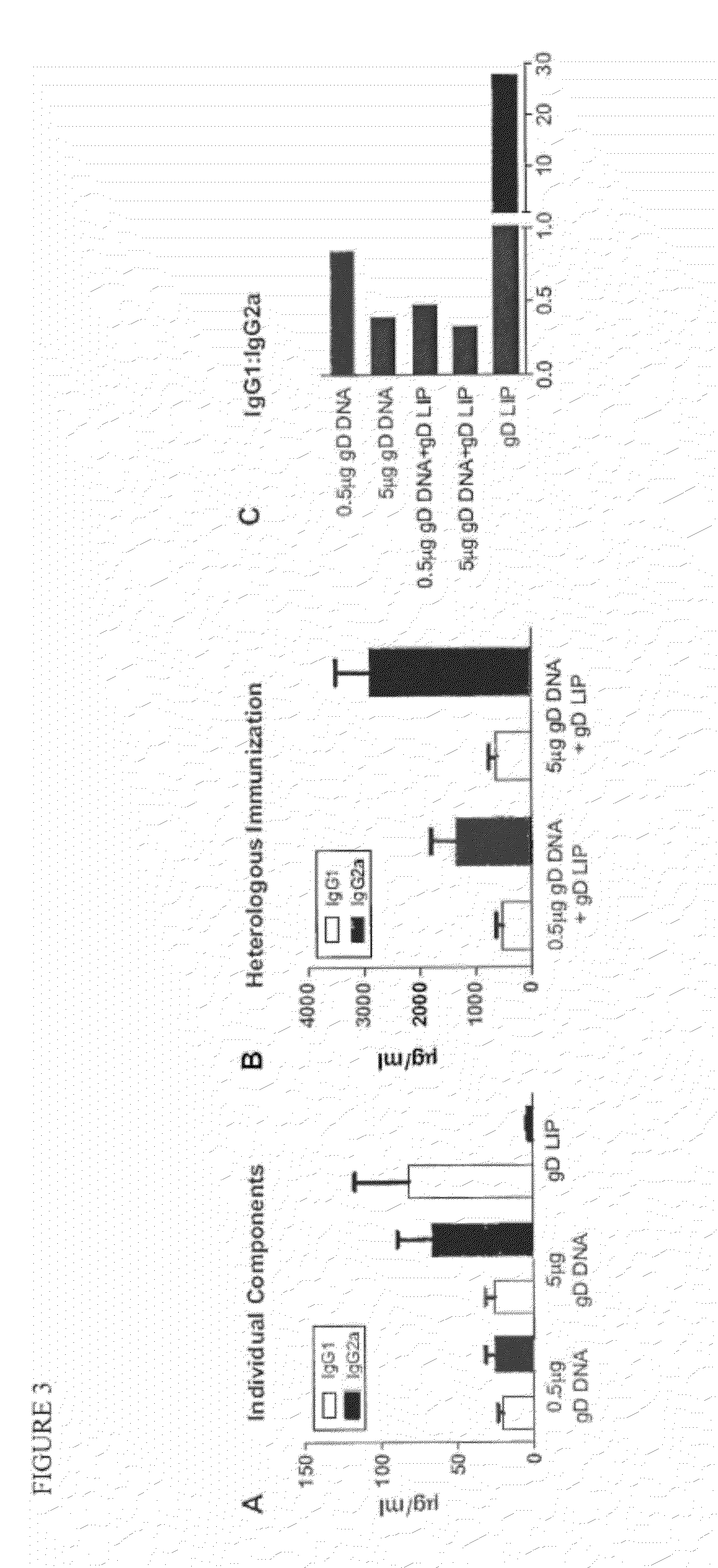
The invention provides methods and kits for immunizing animals (e.g. mammals) against viral antigens, including herpes-simplex virus type 2. The protective immune response elicited by the methods and kits of the invention is characterized by robust humoral, cellular, and mucosal immunity. In particular, the invention provides a heterologous immunization method comprising a priming DNA vaccine encoding an antigen and a boosting protein vaccine, in which the protein form of the antigen is encapsulated in liposomes. Methods of preventing primary acute, latent and recurrent viral infections, such as that caused by HSV-2 virus, and methods of providing passive protective immunity against a viral pathogen such as HSV-2 virus to a mammal are also disclosed.
Owner:MUCOSAL VACCINE TECH LLC
Immunological adjuvant with immunity vegulating agent for treating and preventing diabetic from insulin-dependent
InactiveCN1831012AReduce immune damageDoes not induce atherosclerosisPeptide/protein ingredientsMetabolism disorderInsulin dependent diabetesInsulin dependent
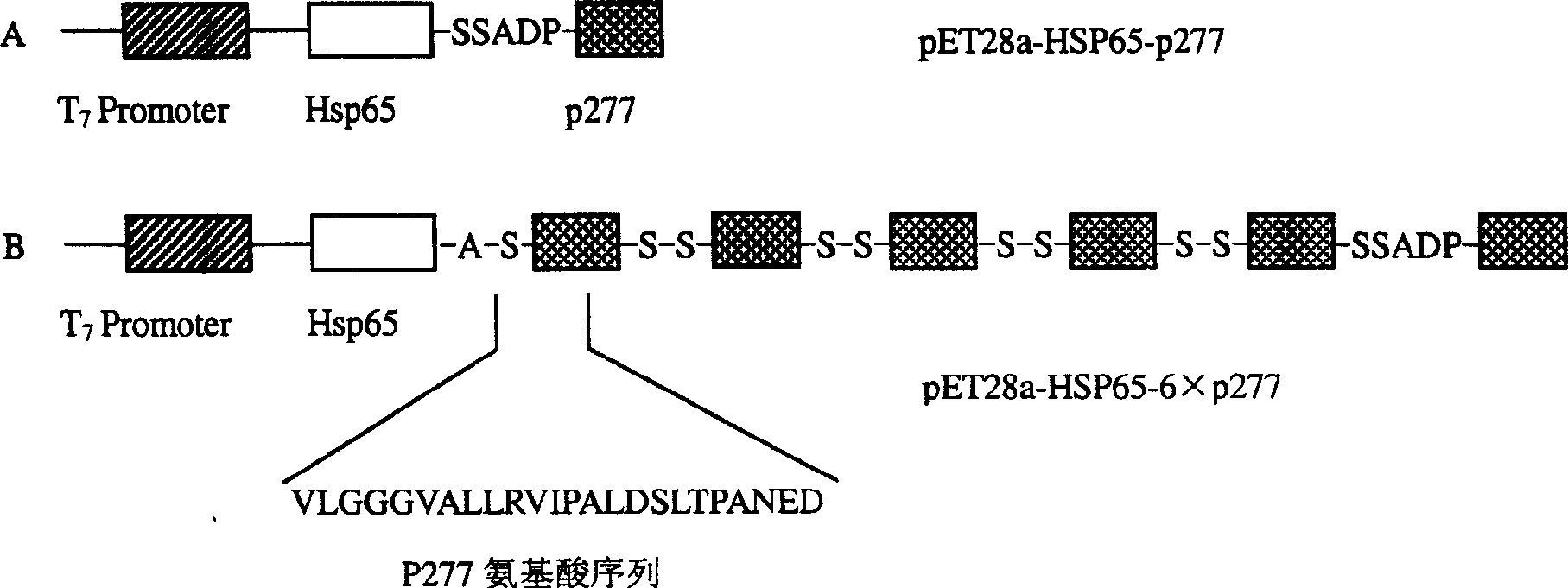
The invention provides the immunomodulator with the free adjuvant having the function of preventing and curing the insulin-dependent diabetes mellitus. Aiming at the weak immunogenicity, the antigen epitope polypeptide gene (a length of antigen epitope p277 rooted from human HSP60) is associated repeatedly six times and inserted repeatedly into the backward position of the heat shock protein HSP65 gene rooted from the mycobacterium bovis, the fusing expression between the repeat series connecting antigen polyeptide and the HSP65 is realized. The produced fusing albumen did not need the adjuvant; the antigen polyeptide don't need be coupled with the carrier chemistry to the immunity. The fusing albumen can inspire the organism to produce the high titer aiming at p277 by means of the mucous membrane immunity, so the nosogeny of NOD chmice diabetic are depressed highly. The invention can prevent the 1 type and 1.5 type diabete characterized in depending on the insulin.
Owner:CHINA PHARM UNIV
Antigen-Drug Vehicle Enabling Switch From Selective Production of IgA Antibody to Production of Both of IgA and IgG Antibodies and Transnasal/Mucosal Vaccine Using the Same
ActiveUS20090130131A1Easy to solveReinforces and promotes prophylactic/therapeutic effectSsRNA viruses negative-senseViral antigen ingredientsMucosal vaccineBody fluid
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid; allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain as well as construction method and application thereof
PendingCN112011521AImprove growth characteristicsHas immune effectSsRNA viruses negative-senseSsRNA viruses positive-senseCoronavirus vaccinationNucleotide
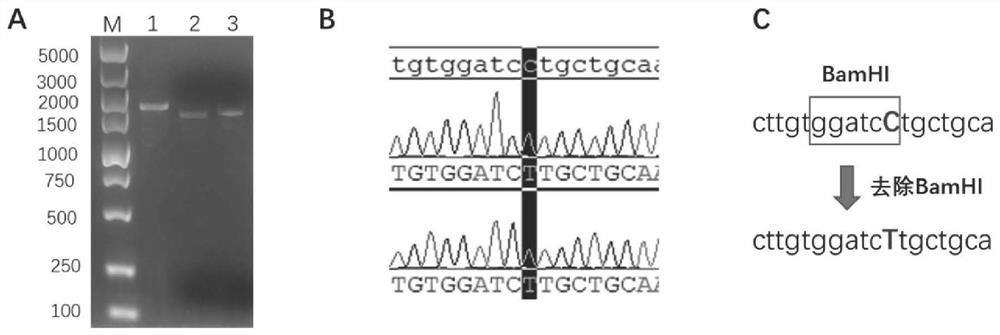
The invention relates to a recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain as well as a construction method and application thereof, and belongs to the technicalfield of genetic engineering vaccines. According to the vaccine candidate strain disclosed by the invention, a Newcastle disease virus LaSota strain is used as a carrier, and a mutated novel coronavirus S gene (C3756T, BamHI site is removed) is inserted between a P gene and an M gene of the Newcastle disease virus LaSota strain; and the nucleotide sequence of the mutated novel coronavirus S gene is shown as SEQ ID NO. 1. The recombinant Newcastle disease virus vector novel coronavirus vaccine candidate strain provided by the invention is safe, effective and low in production cost, can stimulate an organism to generate mucosal immunity through nasal cavity inoculation, and has important application value and outstanding public health safety significance.
Owner:ZHEJIAN DIFFERENCE BIOLOGICAL TECH CO LTD
Preparation method of recombinant antimicrobial peptide extracted from small intestine of pig and application thereof
ActiveCN103417574AHigh yieldLess quantityAntibacterial agentsPeptide/protein ingredientsUltrafiltrationAntimicrobial peptides
The invention discloses a preparation method of a recombinant antimicrobial peptide extracted from a small intestine of a pig and an application thereof. The preparation method comprises the following steps: mashing, homogenizing and repeatedly freezing and thawing the small intestine tissue of the pig with a meat grinder and a colloid mill, and preliminarily crushing cells; then performing the water boiling heat treatment, and extracting with acetic acid solution to obtain a large amount of antimicrobial peptides; centrifuging to enable the quantity of other proteins to be reduced greatly, filtering the supernate obtained through centrifuging by a tangential flow micro-filtration membrane and a tangential flow ultrafiltration membrane sequentially, adjusting the pH value, filtering the supernate by a tangential flow nanofiltration membrane, concentrating and dialyzing to desalt, and disinfecting after adjusting the osmotic pressure to obtain the recombinant antimicrobial peptide extracted from the small intestine of the pig. According to the invention, the application of the recombinant antimicrobial peptide adopts effects of the antimicrobial peptide on aspects of mucosal immunity, immunopotentiators, novel antibiotics and the like, and the mucosal immunity character of the antimicrobial peptide can play an important role in the prevention and control of transmissible gastroenteristis, epidemic diarrhea and mycoplasma gallisepticum; the antimicrobial peptide replaces traditional antibiotics, and is remarkable in effect.
Owner:派生特(福州)生物科技有限公司 +1
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and vaccine of APP OMVs
ActiveCN105420161AExperimental evaluation of immunostimulatory effectsAssessing immunostimulatory effectsAntibacterial agentsBacterial antigen ingredientsIMMUNE STIMULANTSFiltration
The invention relates to a preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and a vaccine of the APP OMVs, and belongs to the technical field of biology. The preparation method comprises the steps of culturing APP shope strains in vitro by using a culture medium which is in iron ion limit, obtaining acellular cultural supernatant after carrying out centrifugation and 0.22-[mu]m filtration treatment, and preparing the OMVs released by germs through ultracentrifugation, wherein the observation through a transmission electron microscope shows that the diameter of most OMVs is 50 to 100 nm, the OMVs are used as subunit vaccines to carry out secondary intranasal immunization on a mouse, the weight of the OMV immune mouse is increased for a long time, and the visual forms of lungs have no obvious difference from a PBS (Phosphate Buffer Saline) immune group. Meanwhile, an experiment shows that the OMVs are efficient immune stimulants, not only can high-level IgG (Immunoglobulin G) be stimulated to be generated by mouse sera, but also high-level IgA (Immunoglobulin A) can be generated in mouse lungs, mucosal immunity of the mouse lungs is effectively stimulated, and a better application prospect of using the OMVs as the subunit vaccines is expressed.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
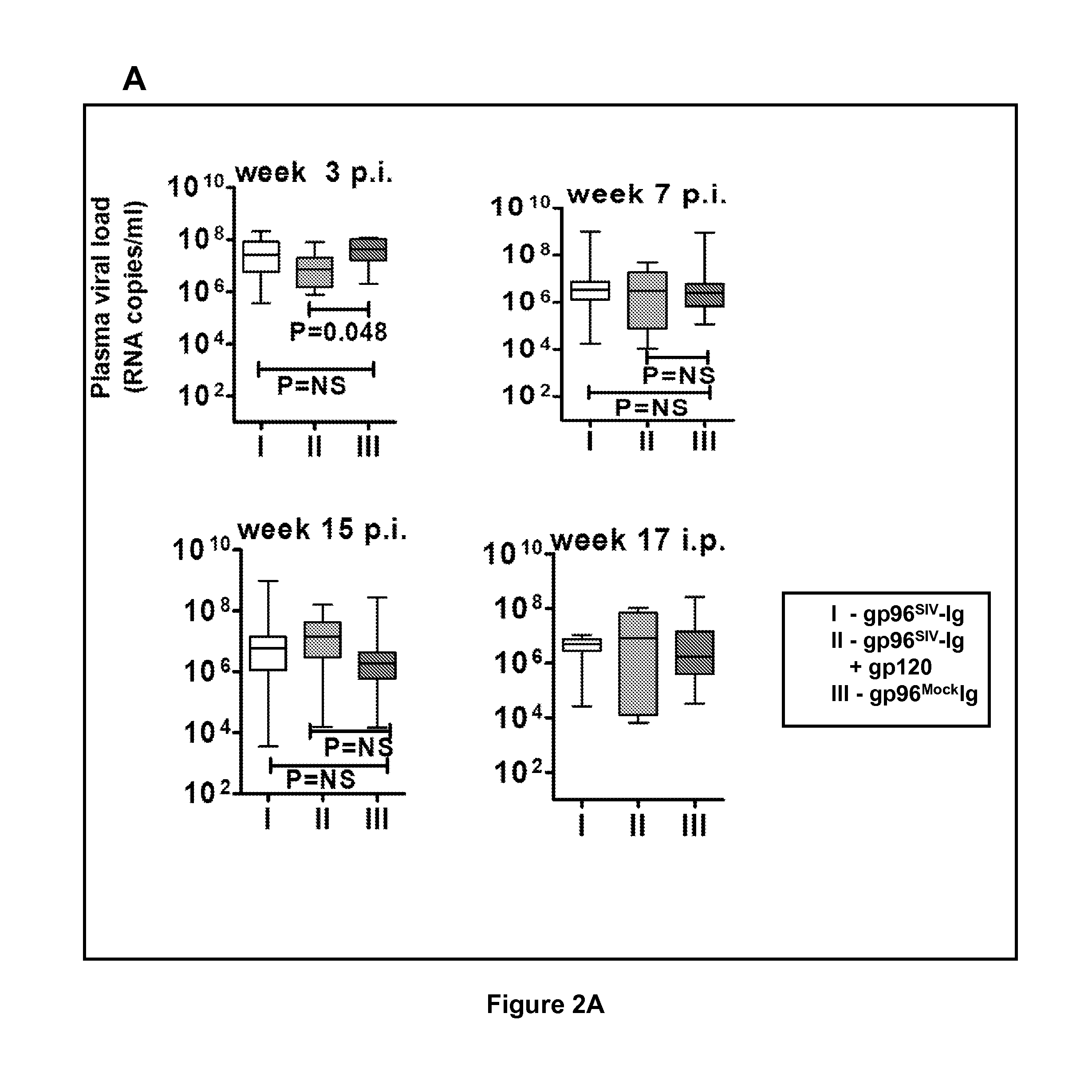
Combined cell based gp96-ig-siv/hiv, recombinant gp120 protein vaccination for protection from siv/hiv
InactiveUS20140286991A1Viral antigen ingredientsMammal material medical ingredientsAntigenVaccination
Compositions are provided comprising heat shock protein, immunoglobulins and retroviral antigens to induce systemic and mucosal immunity to infection from retroviruses such as Human Immunodeficiency Virus (HIV). Methods of treatment provided comprise administration of the compositions, which boost the immune systems response to the retroviral antigens or immunogens.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
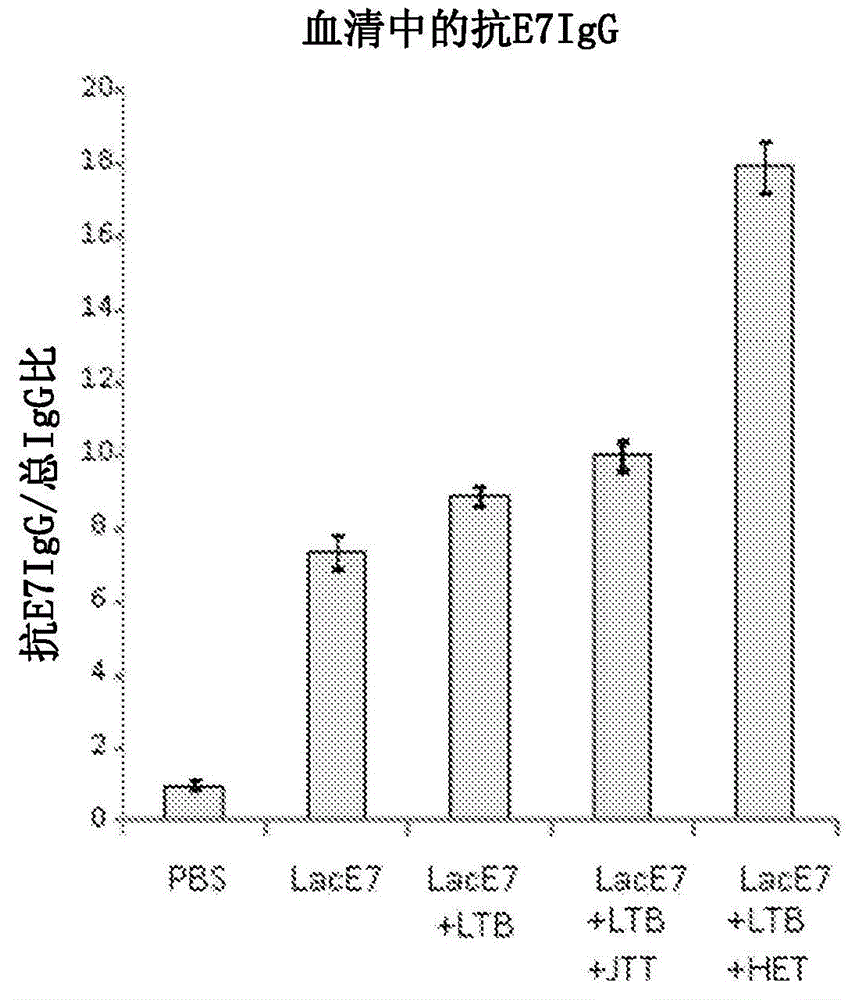
Mucosal immunity-stimulating agent, and oral pharmaceutical composition for treating hpv infection
A mucosal immunity-stimulating agent comprises a Kampo medicine having a revitalizing activity and a mucosal adjuvant. An oral pharmaceutical composition for treating HPV infection comprises at least E7 polypeptide of HPV and a Kampo medicine having a revitalizing activity.
Owner:THE UNIV OF TOKYO
Infant formula milk powder capable of promoting development of intestinal mucosal immunity function of newborn
InactiveCN105746713AMeet normal growth and developmentPromote growth and developmentMilk preparationLactoseVitamin
The invention relates to infant formula milk powder capable of promoting the development of the intestinal mucosal immunity function of a newborn and belongs to the technical field of infant formula milk powder production.The infant formula milk powder contains necessary substances such as protein, lactose, lipids, vitamins and minerals, and each 100g of the infant formula milk powder also contains 200-300mg of lysozyme and 3-5g of lactoferrin.The infant formula milk powder has the advantages that the infant formula milk powder uses breast milk as the reference, and the lysozyme and the lactoferrin are specially added according to the physiological and nutritional features of the newborn of 0-28 days; experiments prove that the intestinal structure, immunocompetent cell number and bacterial flora distribution conditions of the mice eating the infant formula milk powder are evidently better than those of a control group, and accordingly the infant formula milk powder can evidently improve the intestinal mucosal immunity function of the newborn.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Recombinant Newcastle disease virus vector containing novel coronavirus double-antigen target sequence combination and corresponding vaccine strain and vaccine
PendingCN113881704AImproving immunogenicitySmall toxicitySsRNA viruses negative-senseSsRNA viruses positive-senseReceptorSpecific immunity
Owner:ZHEJIAN DIFFERENCE BIOLOGICAL TECH CO LTD
Mucosal vaccine enabling switching from production of IgA antibody to production of both of IgA and IgG antibodies
ActiveUS8211442B2Easy to solveReinforces and promotes effectSsRNA viruses negative-senseViral antigen ingredientsBody fluidMucosal vaccine
In the aim of practical utilization of a safe and effective transnasal / inactivated / mucosal vaccine and establishment of a technology for imparting capacity of producing both of IgA and IgG antibodies to a conventional inactivated vaccine, toxoid, allergen, or the like, a means for prevention and treatment of allergy, and the like, it is intended to provide an antigen-drug vehicle (AD vehicle) enabling transnasal, transmucosal, and transdermal administrations, an inactivated vaccine simultaneously inducing a mucosal immunity and humoral immunity by using the AD vehicle, a production method of the inactivated vaccine, an AD vehicle enabling a switch from induction of selective production of IgA antibody to induction of both of IgA and IgG antibodies, and a transnasal vaccine, a mucosal vaccine, a therapeutic / prophylactic agent for allergy, and the like using the AD vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Antigen-drug vehicle enabling transmucosal and transdermal administration, and method of inducing mucosal immunity and mucosal vaccine and dds using the same
ActiveUS20070141073A1Excellent infection defense effectReduce sensitizationPeptide/protein ingredientsViral antigen ingredientsMucosal immunityAntigen specific
Disclosed are an antigen-drug vehicle (AD vehicle) exerting a function of inducing the preferential and selective production of an antigen-specific secretory IgA antibody, local immunity or mucosal immunity; a method of inducing mucosal immunity, a mucosal vaccine and a preventive or a remedy for allergy using the above AD vehicle; and a transmucosal or transdermal DDS using the above vehicle.
Owner:UNIVERSITY OF TOKUSHIMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com