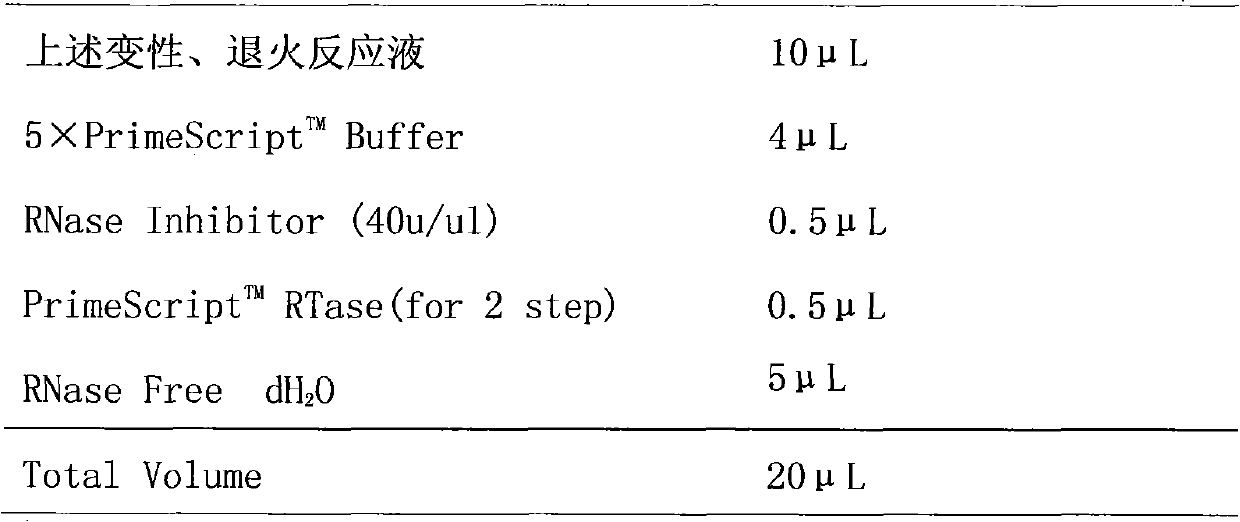
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Local immunity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Local immunity. a state of protection against disease in a particular organ, tissue, or anatomical site, mediated by localized antibodies or lymphoid cells.
New effectors of dipeptidyl peptidase iv for topical use
InactiveUS20030092630A2Reduced activityOrganic active ingredientsPeptide/protein ingredientsDiseaseSide chain
Abstract of Disclosure The invention relates to compounds for topically influencing the activity of dipeptidyl peptidase of the general formula wherein A is an amino acid having at least one functional group in the side chain;B is a chemical compound covalently bound to a functional group of the side chain of A, chosen from the group consisting of (a) oligopeptides having a chain length of up to 20 amino acids, (b) homopolymers of glycine consisting of up to 6 glycine monomers, and (c) polyethylene glycols having molar masses of up to 20 000 g / mol; and C is a group amide-bonded to A chosen from the group consisting of thiazolidine, pyrrolidine, cyanopyrrolidine, hydroxyproline, dehydroproline or piperidine. The invention further relates to the use of said compounds for targeted intervention in local immunological processes (chemotaxis, inflammatory processes, autoimmune diseases), as well as effective and targeted treatment of pathophysiological and physiological processes related thereto (psoriasis, periodontitis, arthritis, allergies, inflammation), inter alia.
Owner:VIVORYON THERAPEUTICS NV
Biological rack material for articular cartilage and its prepn process
The present invention relates to one kind of biological rack material for articular cartilage and its preparation process. The porous rack material for articular cartilage is prepared through mixing natural cartilage matrix component with the cell, antigen and type I and type X collagen eliminated and type II gollagen, and serial physical and chemical treatments with sodium chloride crystal grain as pore forming agent. The rack material has the components the same as those of natural cartilage, high biomechanical strength, high porosity, proper degradation speed, use convenience and no influence on the body's and local immunity. The present invention may be used in the clinical repair of articular cartilage defect and in vivo and in vitro construction of tissue engineering cartilage.
Owner:孔清泉
Use of pigmented retinal epithelial cells for creation of an immune privilege site
InactiveUS20060147437A1Eliminate the effects ofUseful in treatmentBiocideOrganic active ingredientsLocal immunityTherapeutic protein
The present invention relates to a novel in vivo method for creation of a localized immunosuppressive environment in tissue. The method involves the transplanting of pigmented retinal epithelial cells into a mammal thereby producing a localized immunosuppressive environment. The transplanted pigmented retinal epithelial cells may also be used to produce therapeutic proteins or other biologically active molecules that may be useful in treatment of diseases.
Owner:TITAN PHARMA
Non-specific complex polysaccharide immunity milk and preparation method thereof
InactiveCN101336661AExpand the connotationExtended epitaxyMilk preparationFood preparationBiotechnologyDisease
This invention discloses a nonspecific compound polysaccharides immune milk,which is formulated from the following raw materials, by weight,0.3 to 60 parts of fungi polysaccharides, 0.9 to 15 parts of plant polysaccharides, 35 to 1000 parts of functional oligosaccharides, 8000 to 10000 parts of skim cow milk or skim sheep milk. This invention extends the connotation and the extension of immune milk, solves some problems of immune milk produced by local immunity and / or systemic immunity, such as high cost, high operational difficulty, long period and anaphylaxis, has the obvious function of immune regulation, promotes the body resistance to diseases, has good health effect to the people who is weak and liable to have cold. The nonspecific compound polysaccharides immune milk of this invention has a steady character, no risk of anaphylaxis, simple processing technology,is easy to produce on a large scale and has great developmental prospect.
Owner:NORTHWEST A & F UNIV
Allograft tolerance without the need for systemic immune suppression
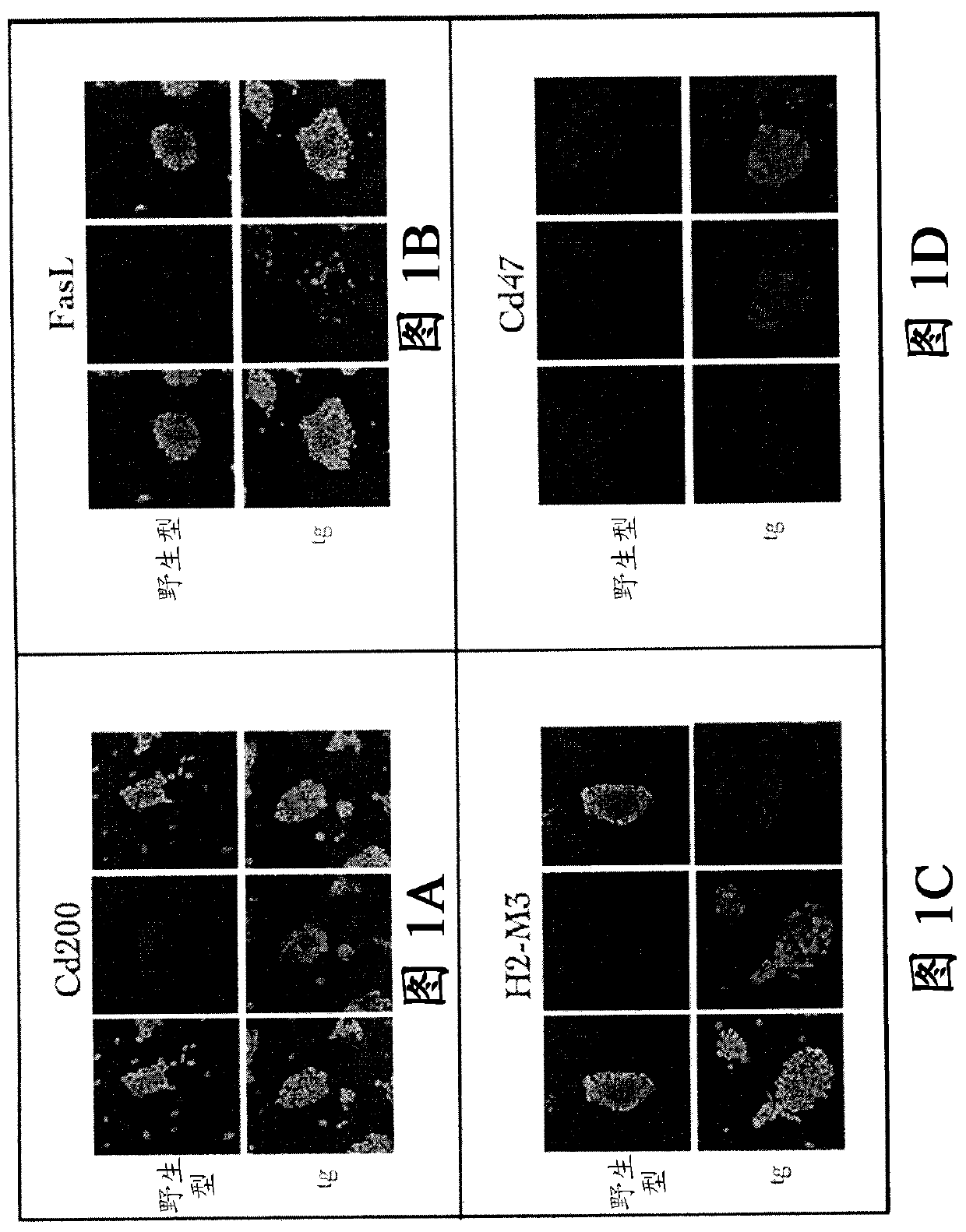
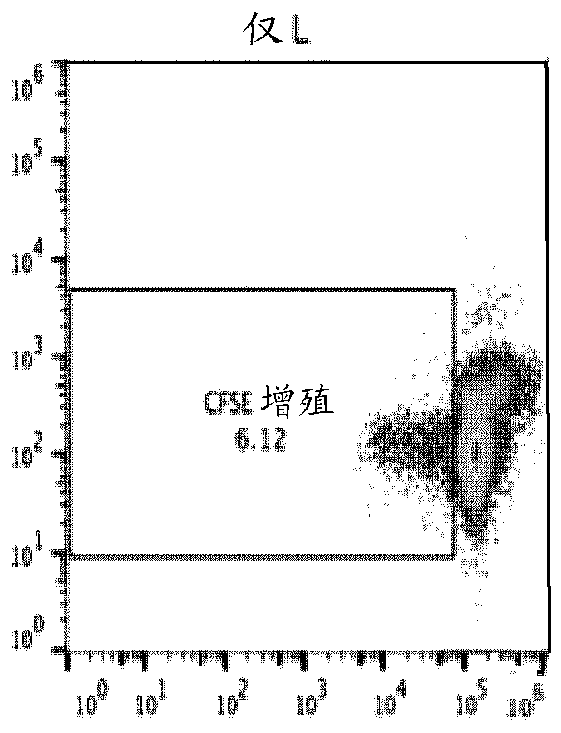
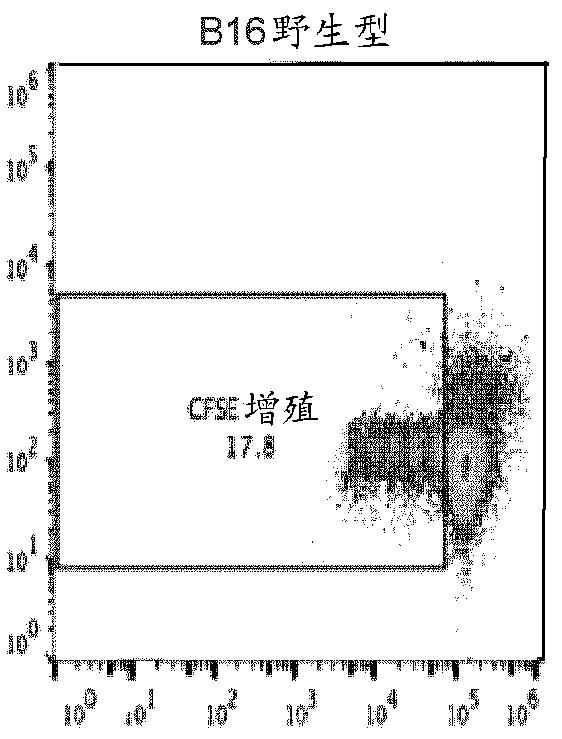
A cell genetically modified to comprise at least one mechanism for providing a local immunosuppression at a transplant site when transplanted in an allogeneic host, and methods for making and using the same are provided. The cell comprises a set of transgenes, each transgene encoding a gene product that is cytoplasmic, membrane bound, or local acting, and whose function is one or more of: to mitigate antigen presenting cell activation and function; to mitigate graft attacking leukocyte activity or cytolytic function; to mitigate macrophage cytolytic function and phagocytosis of allograft cells; to induce apoptosis in graft attacking leukocytes; to mitigate local inflammatory proteins; and to protect against leukocyte-mediated apoptosis. The transgenes comprise two or more of the followinggenes: PD-L 1, HLAG or H2-M3, Cd47, Cd200, FASLG or Fasl, Ccl21 or Ccl21b, Mfge8, and Serpin B9 or Spi6.
Owner:SINAI HEALTH SYST
Preparation method of porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
ActiveCN102988971AAvoid stressAvoid absorptionAntiviralsAntibody medical ingredientsStaphylococcus lactisLocal immunity
The invention discloses a preparation method of a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine. The method comprises the following steps of: 1) synthesizing an M gene; 2) constructing an expression vector; 3) performing protein induction expression; 4) carrying out recombinant strain preservation experiment; and 5) preparing the vaccine. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:北京信得威特科技有限公司
Nasal cavity immunity composite adjuvant for avian influenza inactivation antigen
InactiveCN101085348ALow costImprove immunityAntiviralsAntibody medical ingredientsNasal cavityLocal immunity
The invention relates to compound intranasai immunization adjuvant used for deactivated avian influenza antigen, belongs to biotechnical field, and is special for the application of deactivated avian influenza antigen. The compound adjuvant is mixed or combined by CpG DNA and sodium cholate according to weight ratio of 5:2 according to dosage of immunological adjuvant of 0.1mg / kg. through combining with deactivated avian influenza antigen and intranasai immunization, the inventive compound intranasai immunization adjuvant used for deactivated avian influenza antigen can effectively local immunity of mucous membrane and humoral immunity of whole body, avoid immunization schedule through intramuscular injection, reduce stress reaction of chickens, provide guarantee for improving chicken quality, and provide an ideal Immunization approach for prevention of avian influenza.
Owner:NANJING AGRICULTURAL UNIVERSITY
Antigen-drug vehicle enabling transmucosal and transdermal administration, and method of inducing mucosal immunity and mucosal vaccine and DDS using the same
ActiveUS8268321B2Excellent infection defense effectReinforces and promotes preventive and therapeutic effect of drugPeptide/protein ingredientsViral antigen ingredientsSecretory IgA antibodyLocal immunity
Owner:UNIVERSITY OF TOKUSHIMA
Conjugate of hapten and amino-containing carrier and preparation method and usage thereof
InactiveCN101856497AImprove local immune microenvironmentCarcinomaAntipyreticAnalgesicsLocal immunityIn vivo
The invention belongs to the field of drug synthesis, and relates to a combined conjugate of a hapten and an amino-containing carrier, and a preparation method as well as the usage thereof. In the invention, the combined conjugate of the hapton and the amino-containing carrier is obtained via Sanger's reaction. A signal can be amplified during micro determination by the immunity of the hapten and the function of the carrier which are combined with the monoclonal antibody for the hapten, and the sensitivity of determination is obviously improved. Amino-containing dinitrobenzol structure is taken as a war head with a killing function when inducing cellular immunity in vivo, is combined with the carrier with a targeting function, has peculiar discrimination on cancer and chronic infection, locally induces multiple immunity activating factors at targeting lesion parts, improves local immunity micro environment, and treats cancer or infection by strengthening immunization. The conjugate can be used for micro determination of immunization and the preparation of drug for preventing and curing tumor.
Owner:复旦大学附属华东医院
Nasal cavity spraying inactivated influenza virus vaccine and its prepn process
InactiveCN1810291AImprove effectivenessExtended stayAerosol deliveryAntiviralsNasal cavityLocal immunity
The nasal cavity spraying inactivated influenza virus vaccine contains the following components: cell immunizing factor, natural polysaccharide and influenza virus antigen. The effective synergistic reaction of IL-2 and natural polysaccharide raises the permeating of the influenza virus antigen in nasal cavity, avoids the degradation of enzyme on the antigen and raises the effectiveness of the medicine obviously. It is revealed that the vaccine composing scheme may be well applied in inoculating macro biomolecule to nasal cavity to produce excellent protection effect while stimulating body to generate local immunity reaction and systemic immunity reaction.
Owner:侯云德
Oral cavity anaerobium bacteriostatic and preparation
InactiveCN101234183ACool tasteSweet tasteAntibacterial agentsDigestive systemBacteroidesLocal immunity
The invention relates to a bacteriostatic agent for oral anaerobe and a preparation method thereof. The weight proportion of each raw material in every 100 weight shares is as follows: 1-50 shares of natural bamboo extract, 0.1-5 shares of edible alkali, 0.1-0.01 shares of edible natural spice, 0.1-0.01 shares of edible nonnutritive sweetener and the rest shares of water. The steps of the preparation method are as follows: adding the natural bamboo extract into the water according to the proportion and mixing well, wherein the natural bamboo extract is acid; adding a 7-10 percent edible alkali solution into the natural bamboo extract solution slowly, and stopping adding when the pH value of the solution is 5.0-6.0; then adding the edible nonnutritive sweetener and the natural spice and filtrating all the materials with a bacterial resisting pellicle filter after complete dissolving. The bacteriostatic agent of the invention has the beneficial effects of clear, cool and sweet taste, no sugar the oral bacteria can make use of, no stimulation to the oral mucosa, promoting the inhibiting and killing effect of the natural bamboo extract on the oral anaerobe, enhancing the local immunity in the surrounding of lesion tissues and facilitating the repair function of the ulcer part on the oral mucosa.
Owner:叶敏 +2
Local medicament for treating senile vaginitis by replacing estrogen
InactiveCN101766638AImprove immunityNormal secretion is not disruptedHydroxy compound active ingredientsAntiinfectivesLocal immunityTherapeutic effect
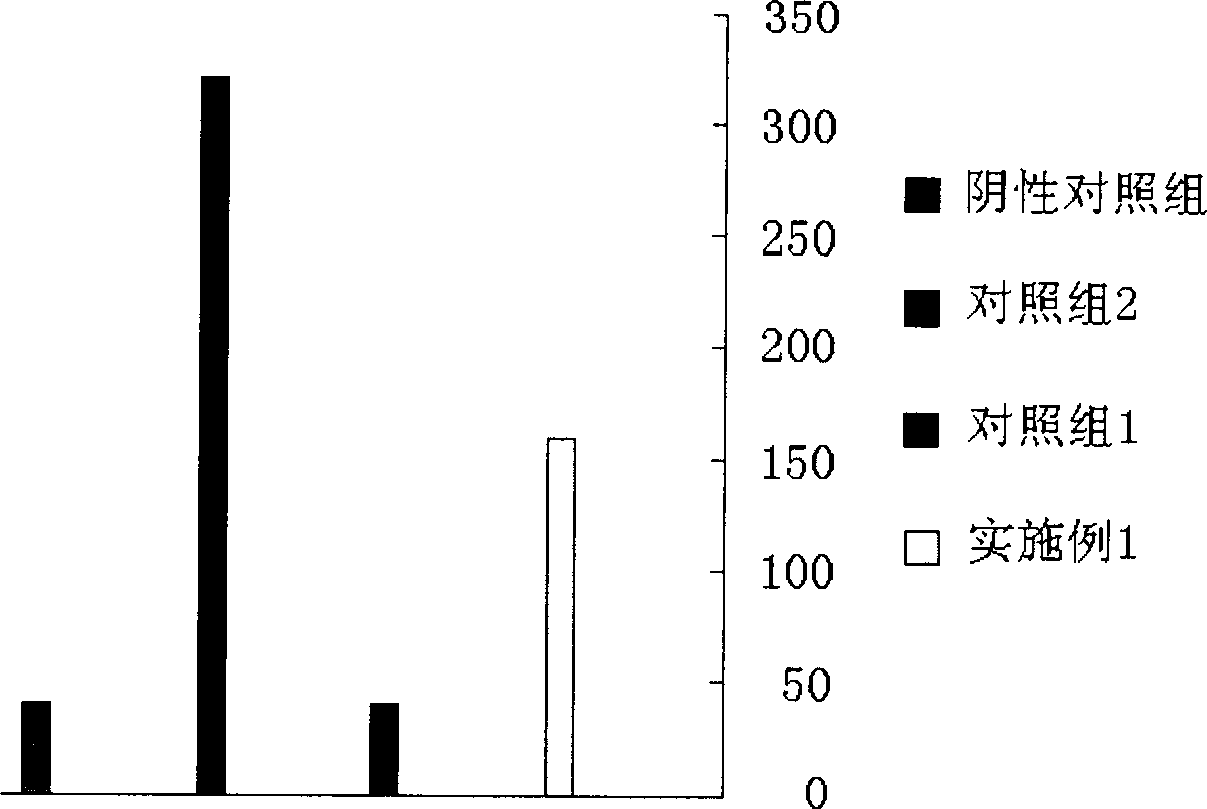
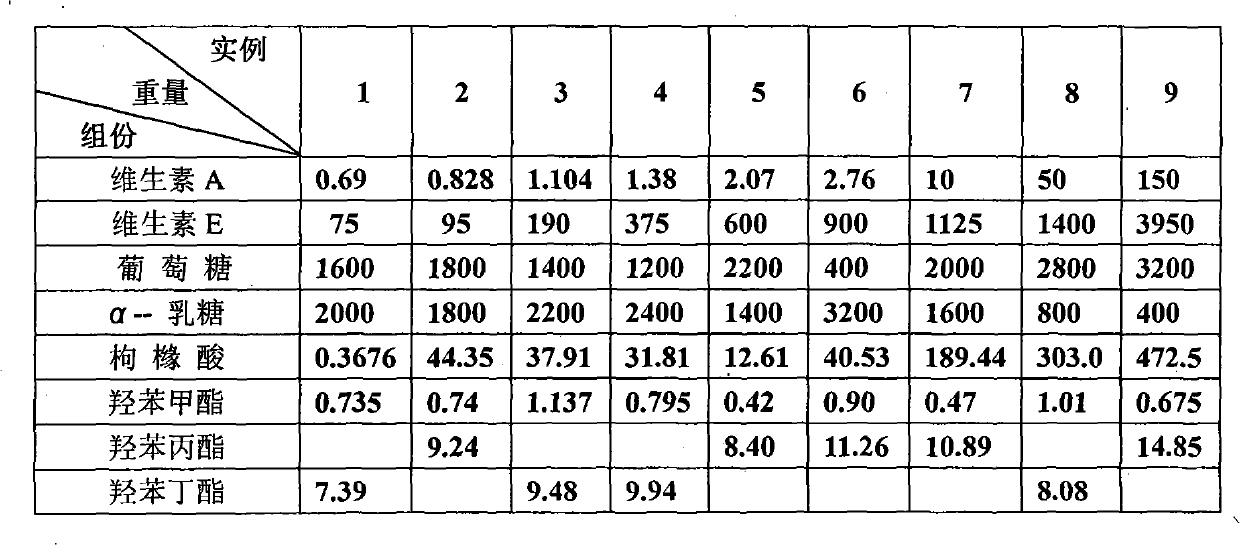
The invention relates to a local medicament for treating senile vaginitis by replacing estrogen. The medicament consists of vitamin A, vitamin E, glucose, alpha-lactose, citric acid and butylparaben bacteriostat. The invention adopts the most common and familiar medicaments, utilizes the unique treatment effect of each medicament and synergism after combination, and provides the medicament for treating the senile vaginitis by replacing the estrogen for people; and the medicament is safe, has special effect, can rapidly change diseased environment of the senile vaginitis, promotes the propagation of lactic acid bacillus, further reduces pH value, inhibits and kills pathogenic bacteria, replenishes nutrition, regulates the moistening degree, strengthens local immunity function, improves local microcirculation, promotes metabolism, and recovers injured mucosal of vagina to a normal state.
Owner:邹月芝
Nasal drops and preparation method thereof
InactiveCN104398579AGood curative effectGood effectHydroxy compound active ingredientsPharmaceutical delivery mechanismBacterial virusDisease
The invention relates to the technical field of rhinitis medication, which discloses nasal drops and a preparation method thereof. The preparation method comprises the following steps: 1)respectively weighing raw materials according to weight part; 2)immersing lithospermum, xanthium, dahurian angelica root and magnolia flower by sesame oil; 3)decocting; and 4)adding borneol, mint cream and liquid paraffin, continuously decocting, depositing, separating, and filtering to obtain a filtrate which is the nasal drops. The nasal drops can effectively inhibit growth of bacterial virus, promote tissue restoration of nasal cavity and increase local immunization state; the nasal drops is prepared by traditional Chinese medicinal materials, have the characteristics of no side effect, excellent curative effect, good effect and high cure rate, are suitable for various rhinopathy patients, have obvious alleviation effect for acute cold and nasal obstruction, rhinorrhea and dizziness, can shorten the course of disease, and obviously improve the life quality.
Owner:郝杰
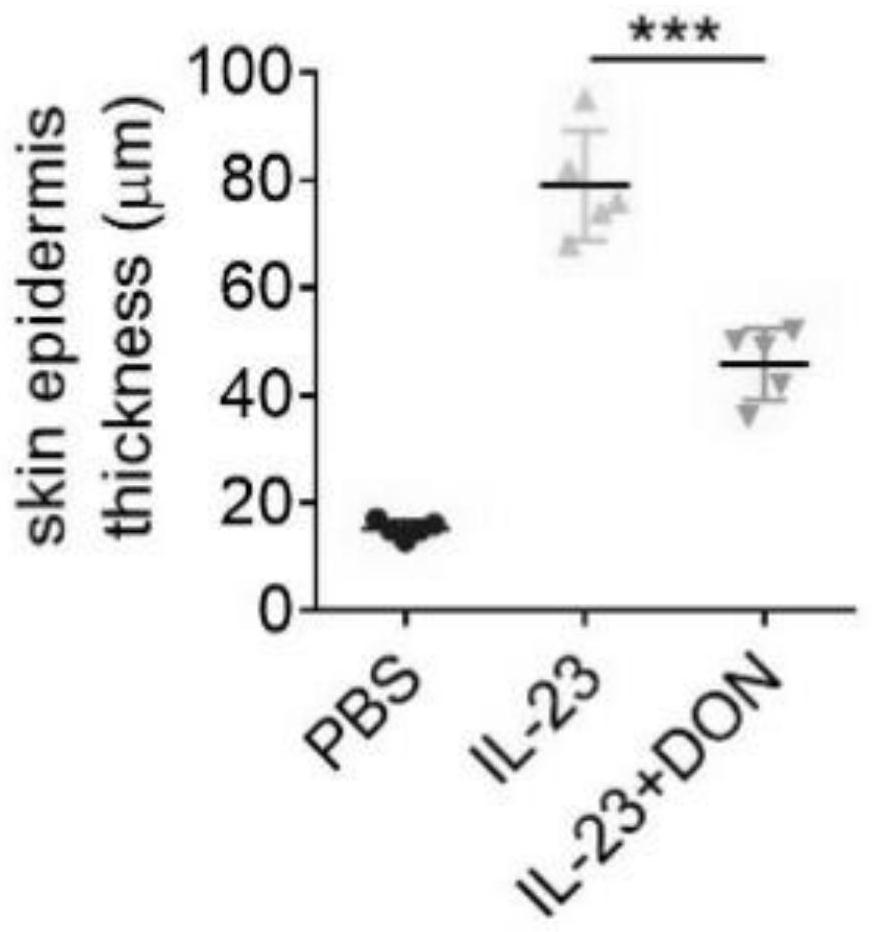
Application of 6-diazo-5-oxo-L-leucine in preparation of drugs for preventing and treating psoriasis
InactiveCN112807297ASuppress inflammatory immune responseRelieve symptomsOrganic active ingredientsAntipyreticLocal immunityPharmaceutical drug
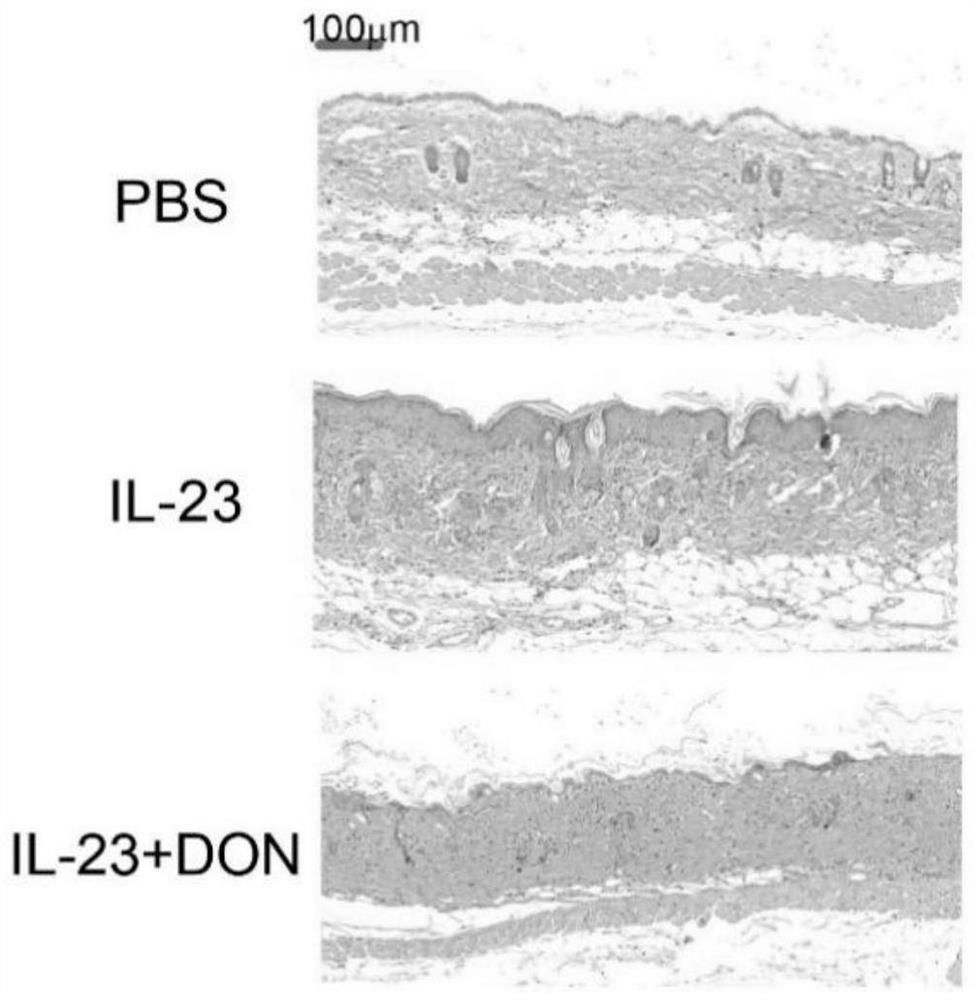
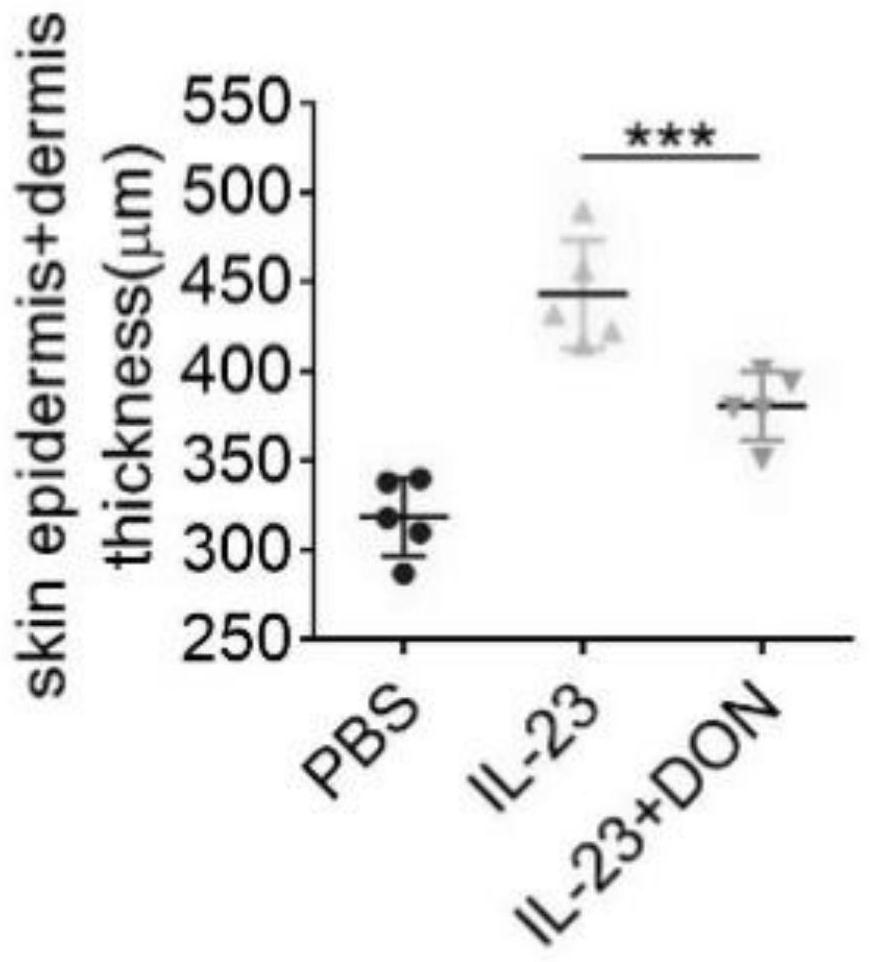
The invention discloses application of 6-diazo-5-oxo-L-leucine in preparation of drugs for preventing and treating psoriasis, and relates to the technical field of medicine. Experimental comparisons discover that the skin thickening at the psoriasis lesion after DON metabolic intervention is obviously reduced, the infiltration of immune cells is reduced, and DON can effectively inhibit inflammatory local immune response; at the same time, DON alleviates the spleen swelling phenomenon of psoriasis, and also effectively inhibits the inflammatory immune response of psoriasis; and by detecting the expression of proinflammatory factors at the skin inflammation site of psoriasis, DON also inhibits the expression of such genes, and DON effectively alleviates the symptoms of psoriasis.
Owner:深圳市福田区风湿病专科医院
Methods and materials for immunomodulation of tissue grafts
PendingUS20200139012A1Organic active ingredientsPharmaceutical non-active ingredientsLocal immunitySpinal cord
Embodiments described herein relate to restorative solutions for segmental peripheral nerve (PN) defects using allografted PNs for stimulating PN repair. More specifically, embodiments described herein provide for localized immunosuppression (LIS) surrounding PN allografts as an alternative to systemically suppressing a patient's entire immune system. Methods described herein provide for injection of ISV agents into a nerve graft prior to implantation of the nerve graft into a recipient. Methods described herein also provide for injection of ISV agents with a polymerizable carrier into a nerve graft, polymerization of the carrier within the graft, and implantation of the nerve graft into a recipient. Certain embodiments provide for reinnervation of central nervous system (CNS) axons in a spinal cord utilizing a peripheral nerve graft.
Owner:UNIVERSITY OF WYOMING
Duck-origin coronavirus N protein gene, clone method and application thereof
InactiveCN101948851AWide spectrum of infectionDegree of large variationGenetic material ingredientsAntiviralsLocal immunitySerotype
The invention relates to a N protein gene of duck-origin coronavirus ZZ2004, and a clone method and application thereof. The N protein gene sequence has the overall length of 1230 bp, and codes N proteins of 409 amino acid residues. The virus sequence with higher homology is found through random PCR, a pair of specific primers is designed by using the sequence as the template and the corresponding fragments are amplified, and then the fragments are respectively connected with a pMD18-T carrier to clone the N protein gene of the virus. Due to that the N gene is an important immune gene and has important effect in the aspects of cellular immunity, humoral immunity and local immunity. The research of the N gene of the invention favors the discovery of the IBV heteromorphosis regularity, the distinguishing of the different serotypes and the further development of novel genetic engineering vaccine, and plays important role in the effective prevention of the IBV.
Owner:HENAN INST OF SCI & TECH
Riemerella anatipestifer bacterial ghost vaccine adopting chitosan oligosaccharide as adjuvant
InactiveCN104971346AImprove the level ofAvoid the stress of immunizationAntibacterial agentsBacterial antigen ingredientsPenicillinAdjuvant
The purpose of the present invention is to provide a riemerella anatipestifer bacterial ghost vaccine loaded with polyinosinic acid-polycytidylic acid having interferon inducing activity and added with chitosan oligosaccharide as an adjuvant. According to the technical scheme, a temperature control expression vector is constructed and is electrotransformed into riemerella anatipestifer protoplast, positive clones are screened and then are subjected to bacterial amplification culture at a temperature of 28 DEG C, lysis gene expression is induced at a temperature of 42 DEG C, polylysine is added to continuously act when the OD600 is no longer be reduced so as to completely lyze the live bacteria, centrifugation is performed to separate the bacteria, the dried bacteria and a 6 mg / mL Poly I:C solution according to an equal ratio so as to make the Poly I:C be embedded into the bacterial ghosts, a 2% chitosan oligosaccharide aqueous solution is added, stirring is performed to form a uniform mixture, sub-packaging is performed into penicillin bottles, and freeze-drying is performed so as to obtain the novel riemerella anatipestifer bacterial ghost vaccine. According to the present invention, the prepared riemerella anatipestifer bacterial ghost vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, the humoral immunity can be stimulated, and the protection effect on the human body is not different from the injection immunity.
Owner:QINGDAO AGRI UNIV
Application of Nocardia rubra cell wall skeleton as neutrophil modulator
The invention relates to application of a Nocardia rubra cell wall skeleton as a neutrophil modulator. The present application provides a neutrophil modulator, which comprises a component derived from the cell wall of Nocardia rubra, and which can modulate the chemotactic function, phagocytosis function, and bactericidal function (reactive oxygen species (ROS) generation, degranulation formation) of neutrophil. The neutrophil regulator disclosed by the invention can also improve the local immune microenvironment of a chronic infection wound.
Owner:LIAONING GREATEST BIO-PHARM CO LTD
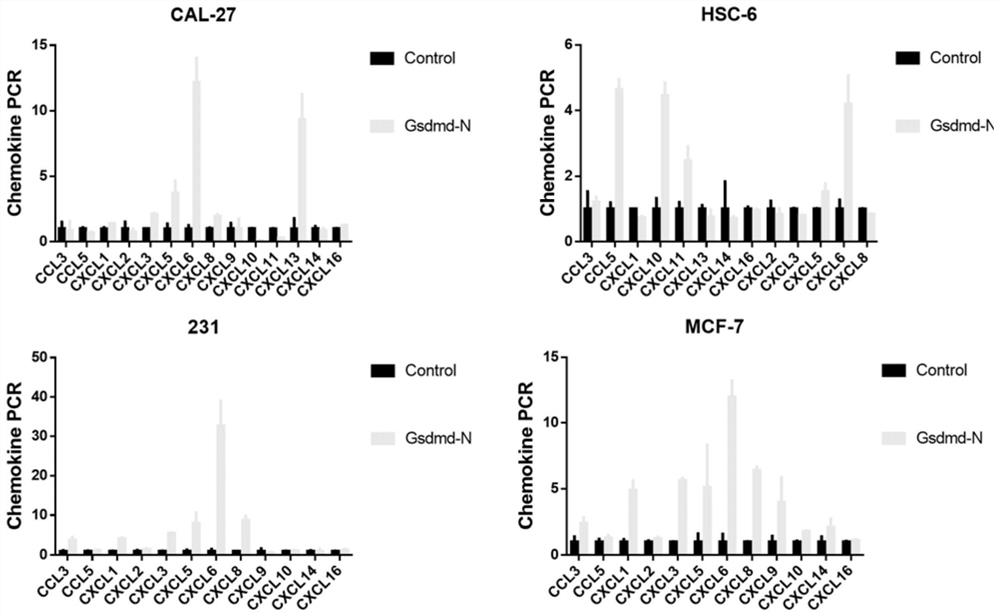
Nanometer material based on loaded GSDMD protein N-terminal peptide fragment and application thereof
PendingCN114657210AEasy to operateReduce manufacturing costPeptide/protein ingredientsNanomedicineLocal immunityBiochemistry
The invention relates to the field of biological medicine, in particular to a nanometer material based on a loaded GSDMD protein N-terminal peptide fragment and application of the nanometer material. The invention provides a plasmid loaded with a GSDMD-N terminal nucleotide sequence. The GSDMD-N terminal nucleotide sequence is as shown in SEQ ID NO: 2. The invention also provides the GSDMD-N-PDPA nano material, the core of the GSDMD-N-PDPA nano material is the GSDMD-N plasmid, and the entrapment material of the GSDMD-N-PDPA nano material is the nano carrier PDPA. The GSDMD-N-PDPA nano material has the effects of killing tumors and enhancing the local immune function, the preparation cost is low, the core of the nano material is the GSDMD-N plasmid, the plasmid amplification operation is simple and convenient after preparation, and the preparation cost is low; the entrapment material is a nanometer material PDPA, so that the preparation cost is not high.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Medicament for treating vaginitis and capable of preventing lactobacillus in vagina from being killed
ActiveCN105727021APlay the role of vaginal self-cleaningImprove local immunityAntibacterial agentsAntimycoticsLocal immunityEcological environment
The invention discloses a medicament for treating vaginitis and capable of preventing lactobacillus in a vagina from being killed. The pH value of the medicament is 2.5 to 6.5, and the medicament is prepared by mixing bamboo vinegar or / and wood vinegar with a matrix and water, wherein in percentage by weight, the bamboo vinegar or / and wood vinegar accounts for 2 to 50 percent of the weight of the medicament, and the matrix accounts for 0.1 to 35 percent of the weight of the medicament; the water accounts for the balance percentage of the weight of the medicament. An experiment shows that the medicament disclosed by the invention has a specific killing or suppressing effect on harmful pathogenic bacteria, but cannot kill or suppress probiotics and lactobacillus; the shortcoming that vaginitis is mainly treated with relevant medicaments such as antibiotic is overcome; the ecological environment of the vagina is well maintained, the vagina self-cleaning effect of the lactobacillus is developed, and local immunity of the vagina is enhanced; the medicament is expected to be an optimal medicament for treating the vaginitis and has a good clinical application prospect.
Owner:朱有名
Medicine for repairing intestinal mucosa and improving mucosa immune function and preparation method of medicine
PendingCN113813373AReduce congestionIncrease heightPeptide/protein ingredientsDigestive systemLocal immunitySpecific immunity
The invention provides a pharmaceutical composition for repairing an intestinal mucosa and improving a mucosa immune function for poultry. The pharmaceutical composition is prepared from the following components in parts by weight: 5 to 10 parts of arteannuic acid, 8 to 12 parts of quinine hydrochloride, 4 to 10 parts of a solanum lyratum extract, 6 to 10 parts of a bidens pilosa extract, 3 to 8 parts of lysozyme, 8 to 15 parts of citric acid, 10 to 20 parts of chitosan and the balance of a carrier. According to the pharmaceutical composition provided by the invention, through protecting a cecum gland structure of an infected chick, the development of intestinal tissues is promoted, and the integrity of the intestinal mucosa is protected, so that absorption and digestion functions of intestinal tracts are recovered, and the weight gain rate of chicks is increased; and the proliferation of pathogenic bacteria in the intestinal tracts is reduced through a specific immune way, the local immune function of intestinal mucosa is enhanced, and the healing and recovery of intestinal mucosa injury are promoted. The pharmaceutical composition provided by the invention has a good prevention and treatment effect on intestinal mucosa injury and hemorrhagic diarrhea caused by mixed infection of chicken coccidiosis and secondary colibacillosis.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Preparation method of gynecological gel
InactiveCN113134066ANon-traumaticNo stimulationAntibacterial agentsAntimycoticsLocal immunityAngelica Sinensis Root
The invention discloses a preparation method of gynecological gel. The gel is prepared from the following traditional Chinese medicines: ginseng, cortex phellodendri, catechu, radix salviae miltiorrhizae, fructus cnidii, radix paeoniae alba, herba lycopi, radix scutellariae, fructus kochiae, radix stemonae, aloe, borneol, saffron crocus, cananga odorata, geranium, radix sophorae flavescentis, folium artemisiae argyi, dragon's blood, angelica sinensis, frankincense, propolis, radix angelicae, herba epimedii and the like. The gynecological gel is prepared by setting a proper ratio of thetraditional Chinese medicines; and further, the extracting solution of the traditional Chinese medicines is used as a main effective component, so that the traditional Chinese medicine composition is noninvasive, non-irritant, hormone-free, free of any toxic and side effects and free of a microbial tolerance phenomenon. The active ingredients of traditional Chinese medicines are used for inhibiting the activity of viruses and bacteria, quickly nourishing and repairing germ cells and improving the self-secretion function and local immunity, so that the survival of viruses and bacteria is intervened, and finally, the treatment on vaginitis, cervicitis and cervical erosion is realized. The gynecological gel disclosed by the invention has a bacteriostatic effect on staphylococcus aureus, candida albicans and escherichia coli of the female pudendum.
Owner:诸暨市博美生物科技有限公司
Riemerella anatipestifer pre-bacterial ghost vaccine preparation method
ActiveCN104975038AIntact surface structureAntigenicity is not affectedAntibacterial agentsBacterial antigen ingredientsLocal immunityFreeze-drying
The present invention relates to a method for conversion preparation of a riemerella anatipestifer pre-bacterial ghost vaccine by using phage PhiX174 E gene and staphylococcal nuclease A gene, and an application method. According to the technical scheme, the phage PhiX174 E gene and the staphylococcal nuclease A gene are coupled to form the series coupling gene through the flexible coupling arm (Gly4Ser), a temperature control expression vector pBV-E-SN is further constructed, the pBV-E-SN is transformed into riemerella anatipestifer protoplast through electric shock conversion, the positive clones are screened and are subjected to bacterial amplification culture at a temperature of 28 DEG C, the bacteria are separated, a protection agent is added, and sub-packaging and freeze-drying are performed. According to the present invention, the prepared live bacterial vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, and the humoral immunity can be stimulated.
Owner:QINGDAO AGRI UNIV
Localized immunosuppression of allografts for peripheral nerve repair
ActiveUS10064938B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAllogeneic graftRegulatory T cell
Embodiments described herein relate to restorative solutions for segmental peripheral nerve (PN) defects using allografted PNs for stimulating PN repair. More specifically, embodiments described herein provide for localized immunosuppression (LIS) surrounding PN allografts as an alternative to systemically suppressing a patient's entire immune system. Methods include localized release of immunosuppressive (ISV) agents are contemplated in one embodiment. Methods also include localized application of immunosuppressive (ISV) regulatory T-cells (Tregs) in other embodiments. Hydrogel carrier materials for delivery of ISV agents and are also described herein.
Owner:UNIVERSITY OF WYOMING
Localized immunosuppression of allografts for peripheral nerve repair
Embodiments described herein relate to restorative solutions for segmental peripheral nerve (PN) defects using allografted PNs for stimulating PN repair. More specifically, embodiments described herein provide for localized immunosuppression (LIS) surrounding PN allografts as an alternative to systemically suppressing a patient's entire immune system. Methods include localized release of immunosuppressive (ISV) agents are contemplated in one embodiment. Methods also include localized application of immunosuppressive (ISV) regulatory T-cells (Tregs) and / or mesenchymal stomal cells in other embodiments. Hydrogel carrier materials are also described herein.
Owner:UNIVERSITY OF WYOMING
Porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
InactiveCN102989010AAvoid stressAvoid absorptionGenetic material ingredientsAntiviralsLocal immunityInfectious Disorder
The invention discloses a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine which is collected in China Center for Type Culture Collection (CCTCC) and the collection number thereof is lactococcus lactis MG1363 / pMG36e-M CCTCC M 2012354. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:SHANDONG SINDER TECH
Parenteral Nutrition Formulation
ActiveUS20210378998A1Improve Gut HealthMaintaining or amelioratingAntipyreticAnalgesicsPediatric patientLocal immunity
The present disclosure relates to parenteral nutrition formulations, including ready-to-use parenteral nutrition formulations which are reconstituted from multi-chamber containers and amino acid formulations. More particularly, the present disclosure is directed to formulations comprising butyrate derivatives, specifically arginine butyrate, for use with adult or pediatric patients. The disclosure further provides for methods of reducing or preventing systemic and local inflammation of patients receiving parenteral nutrition, and methods of maintaining or ameliorating their systemic immunity and local immunity, as well as the patients' gut barrier functions.
Owner:BAXTER INT INC +1
External capsule preparation for preventing and treating vaginitis
ActiveCN109464655AInhibit and eliminate growth functionImprove immunityAntibacterial agentsPeptide/protein ingredientsLocal immunityBULK ACTIVE INGREDIENT
The invention discloses an external capsule preparation for preventing and treating vaginitis. The preparation comprises a capsule A and a capsule B, wherein the capsule A is prepared from lactobacillus plantarum ST-III as the main active ingredient, and the capsule B is prepared from main active ingredients of transdermal EGF and transdermal bFGF. In the external capsule preparation, the viable bacteria number of lactobacillus plantarum ST-III is not smaller than 0.25*106 CFU / particle, the transdermal EGF is not less than 1000 ng / particle, and the transdermal bFGF is not less than 200 ng / particle. The external capsule preparation has the functions of enhancing local immunity and repairing vaginal mucosa barrier, and is worthy of wide application in clinical treatment.
Owner:FUJIAN LONGSHENG BIOLOGICAL TECH CO LTD
Biomarkers of immune response in mucosal lesions and their use with therapeutic vaccination
InactiveUS20150177241A1Effective amountViral antigen ingredientsDisease diagnosisClinical trialT lymphocyte
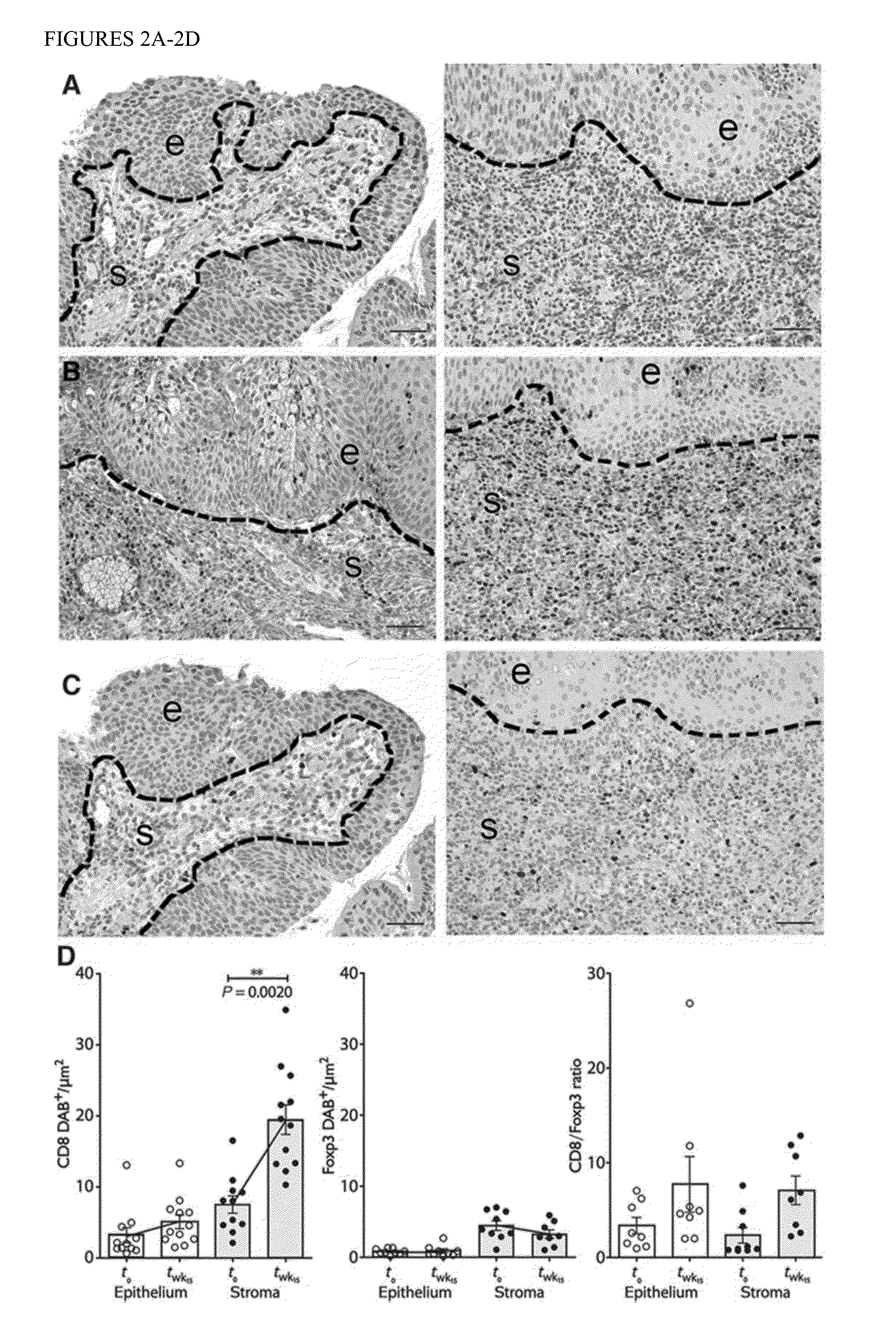
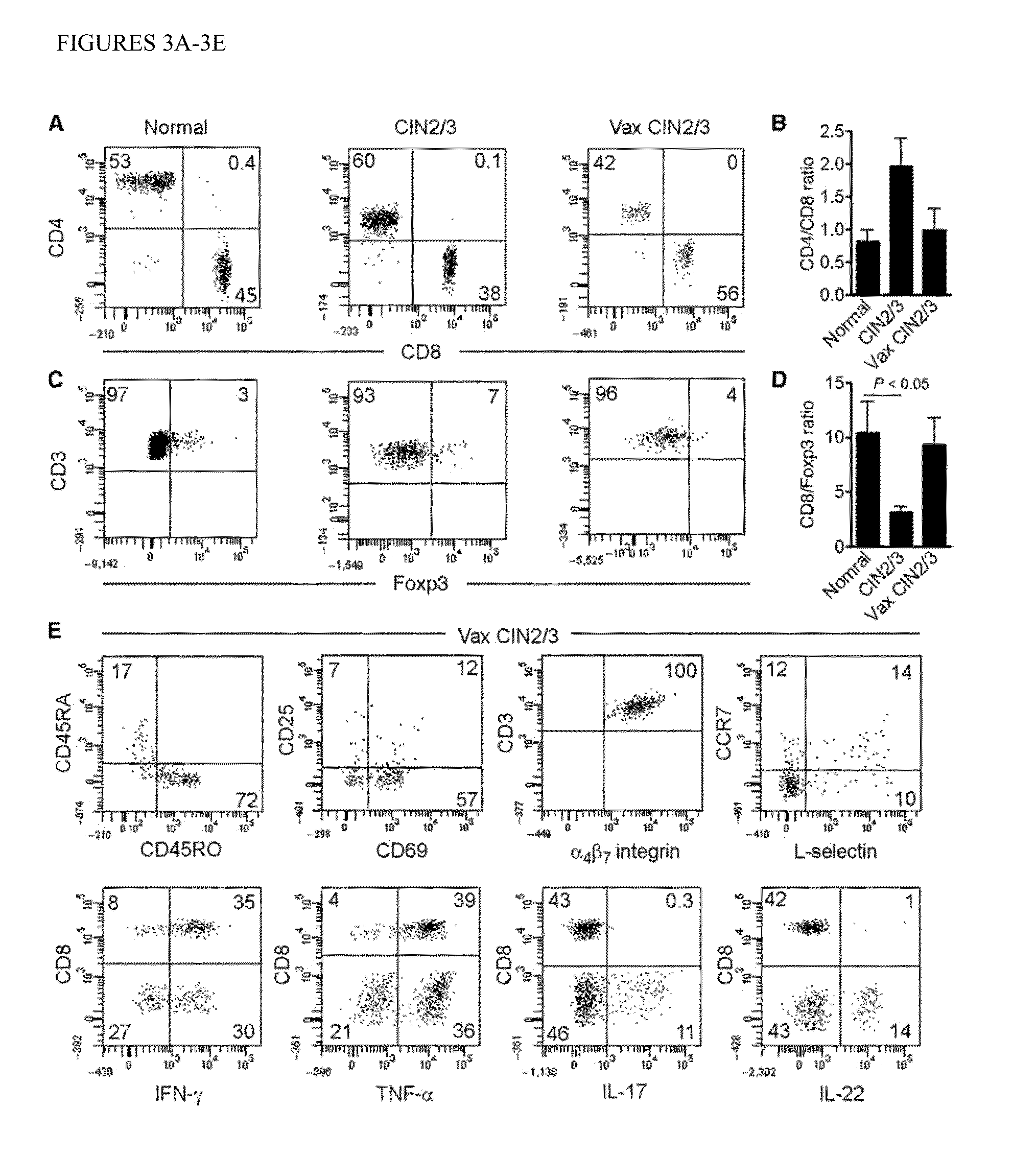
The present invention provides evidence of post-vaccination immunologic changes in target HPV induced cervical intraepithelial neoplasias that suggest the induction of clinically relevant tissue-localized immune responses, despite modest detectable responses in circulating T lymphocytes. The inventive methods allow for identification of immunological responses to HPV immunotherapies, and means for monitoring responses during clinical trials and treatment regimens.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Preparation method and application of immune gel for regulating and controlling tumor microenvironment
PendingCN113425671AHas catalase activityAchieve fixOrganic active ingredientsHeavy metal active ingredientsGel preparationLocal immunity
The invention relates to a preparation method of immune gel for regulating and controlling a tumor microenvironment. The preparation method comprises the following steps of: (1) synthesizing NO-doped Prussian blue nanoparticles; (2) carrying out amination modification on the Prussian blue nanoparticles; (3) modifying the surfaces of the Prussian blue nanoparticles with Anti-SIRP alpha; (4) loading a CSF-1R small-molecule inhibitor BLZ945 into holes of the Prussian blue particles; (5) preparing gelling preparation and thrombin solutions; and (6) uniformly mixing the BLZ945-loaded Prussian blue nanoparticles obtained in the step (4) with the gelling preparation solution, and adding the thrombin solution to obtain the immune gel under the stimulation of thrombin. According to the invention, the preparation method is simple, materials are easy to obtain, the effect is excellent, prussian blue serves as a carrier, so that efficient loading of the medicine can be implemented; the prepared immune gel is used for treating cancer postoperative recurrence, not only continuous controlled release of local immunotherapy nanoparticles is guaranteed, but also postoperative wound repair and healing are implemented, and remarkable social and economic benefits are achieved.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com