Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
74 results about "Allogeneic graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allogeneic graft allograft. autodermic graft (autoepidermic graft) a skin graft taken from the patient's own body. autologous graft (autoplastic graft) a graft taken from another area of the patient's own body; called also autograft.
Cortical bone-based composite implants
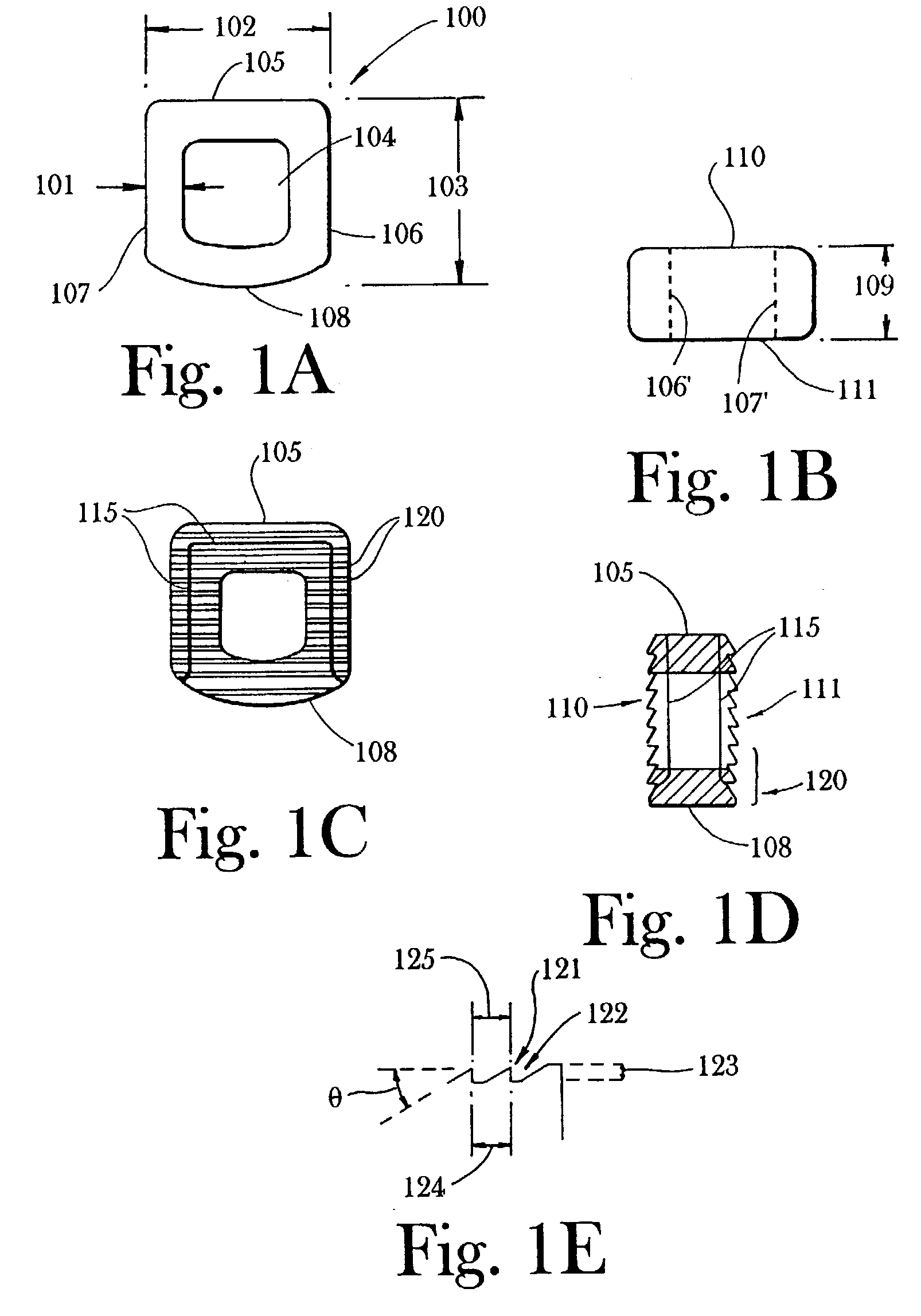
An implant composed substantially of cortical bone is provided for use in cervical Smith-Robinson vertebral fusion procedures. The implant is derived from allograft or autograft cortical bone sources, is machined to form a symmetrically or asymmetrically shaped (e.g. a substantially "D"-shaped) implant having a canal running therethrough according to methods of this invention, and inserted into the space between adjacent cervical vertebrae to provide support and induce fusion of the adjacent vertebrae. Osteogenic, osteoinductive or osteoconductive materials may be packed into the canal of the implant to expedite vertebral fusion and to allow autologous bony ingrowth.
Owner:REGENERATION TECH
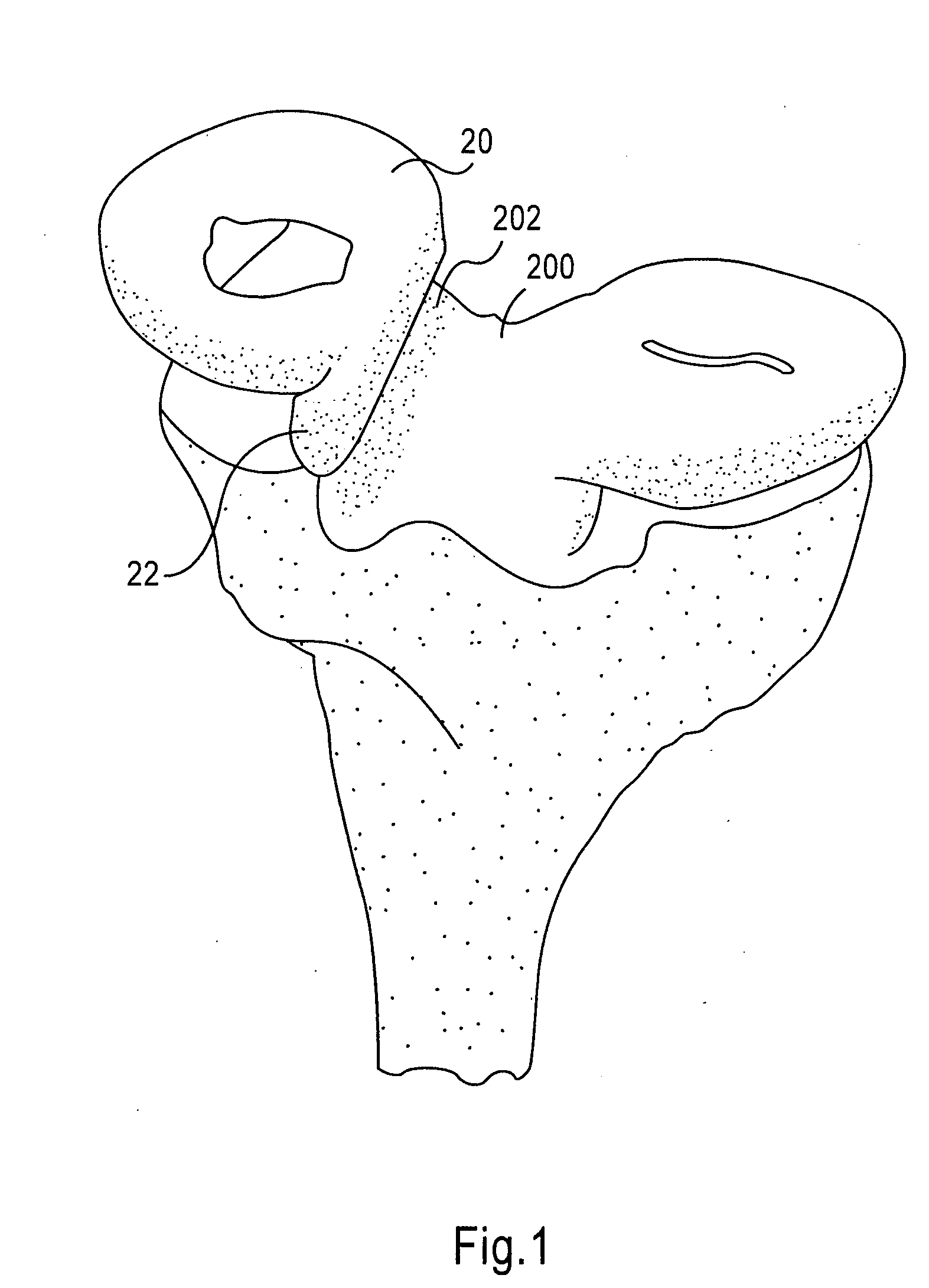
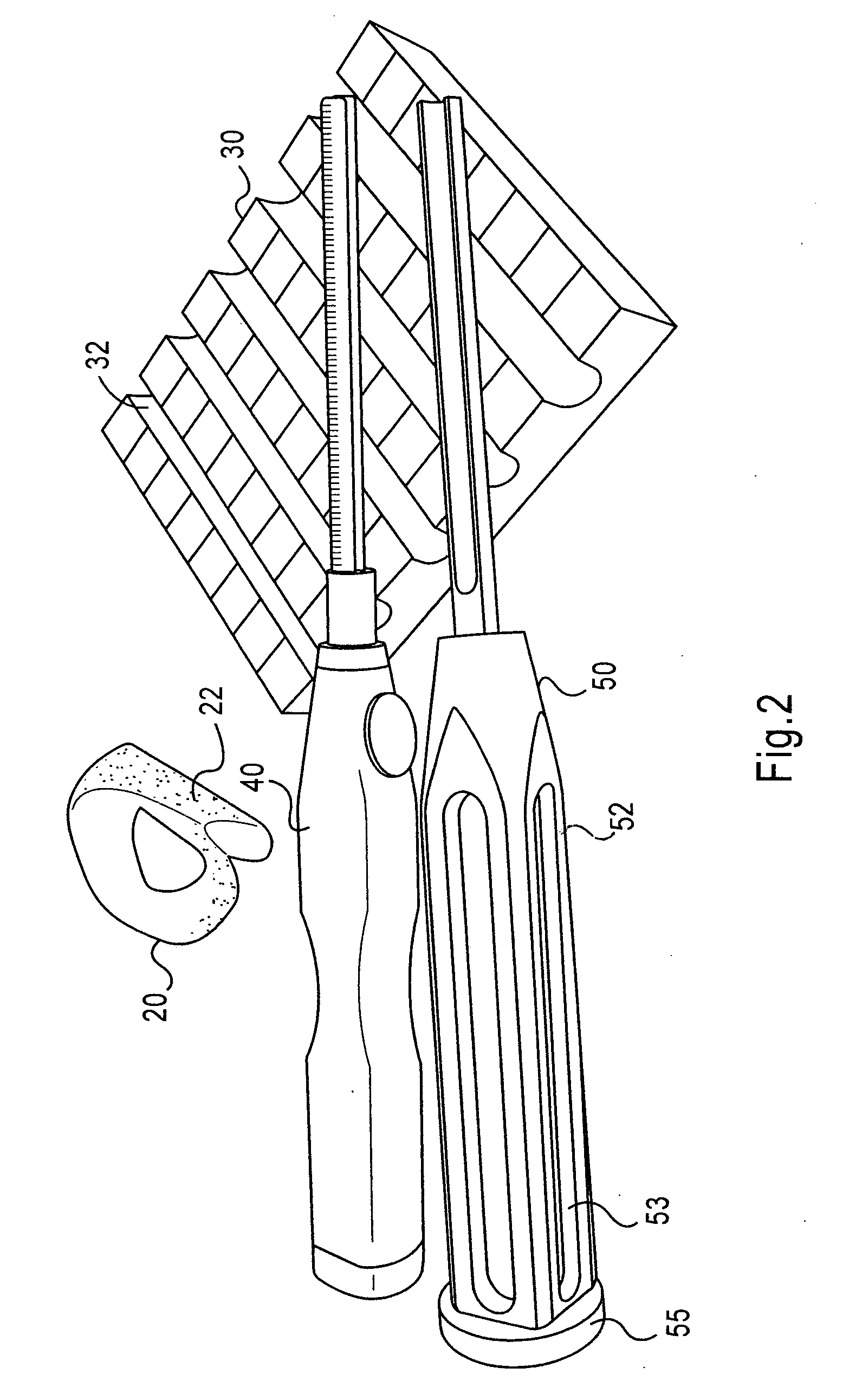
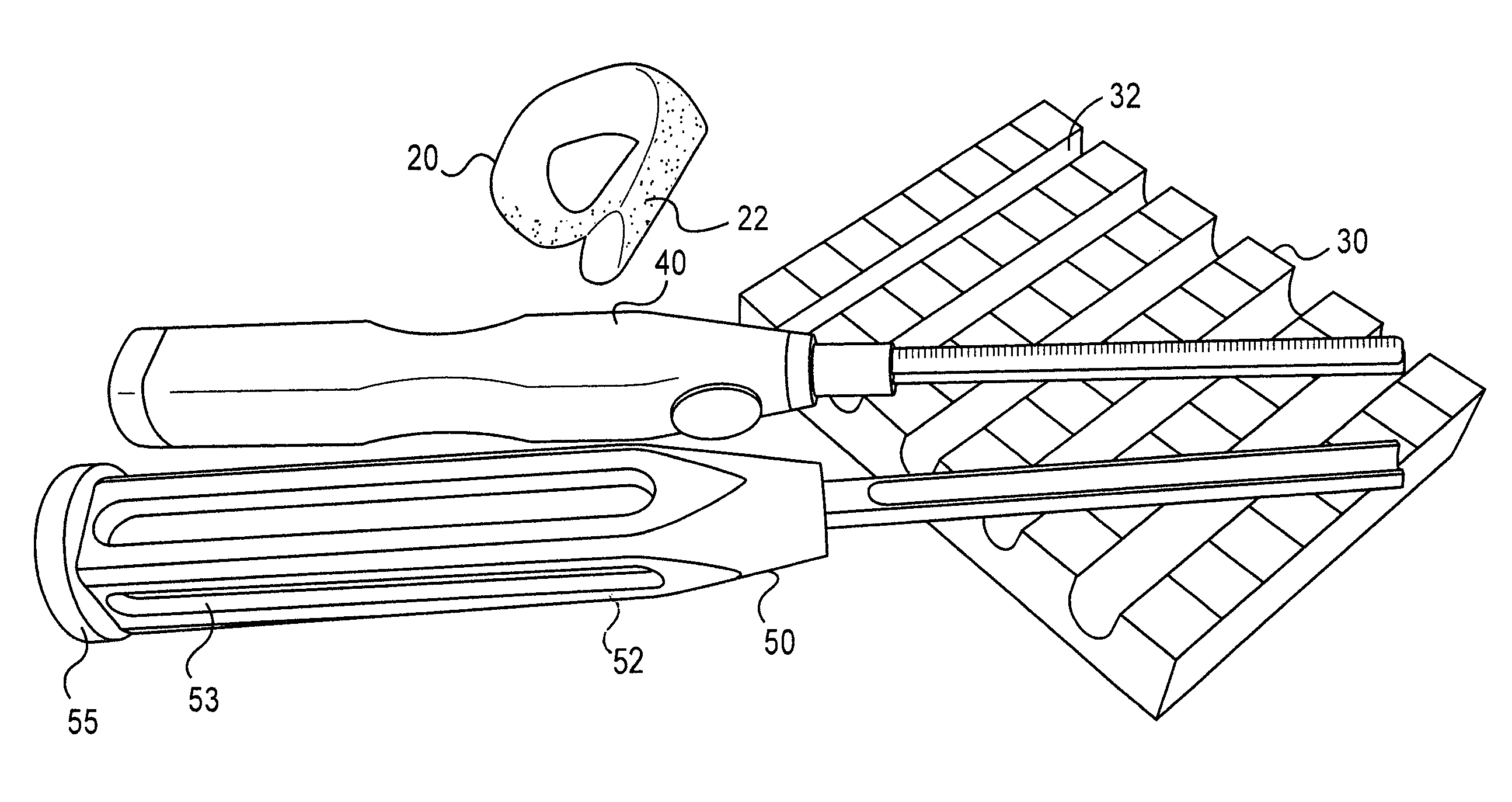
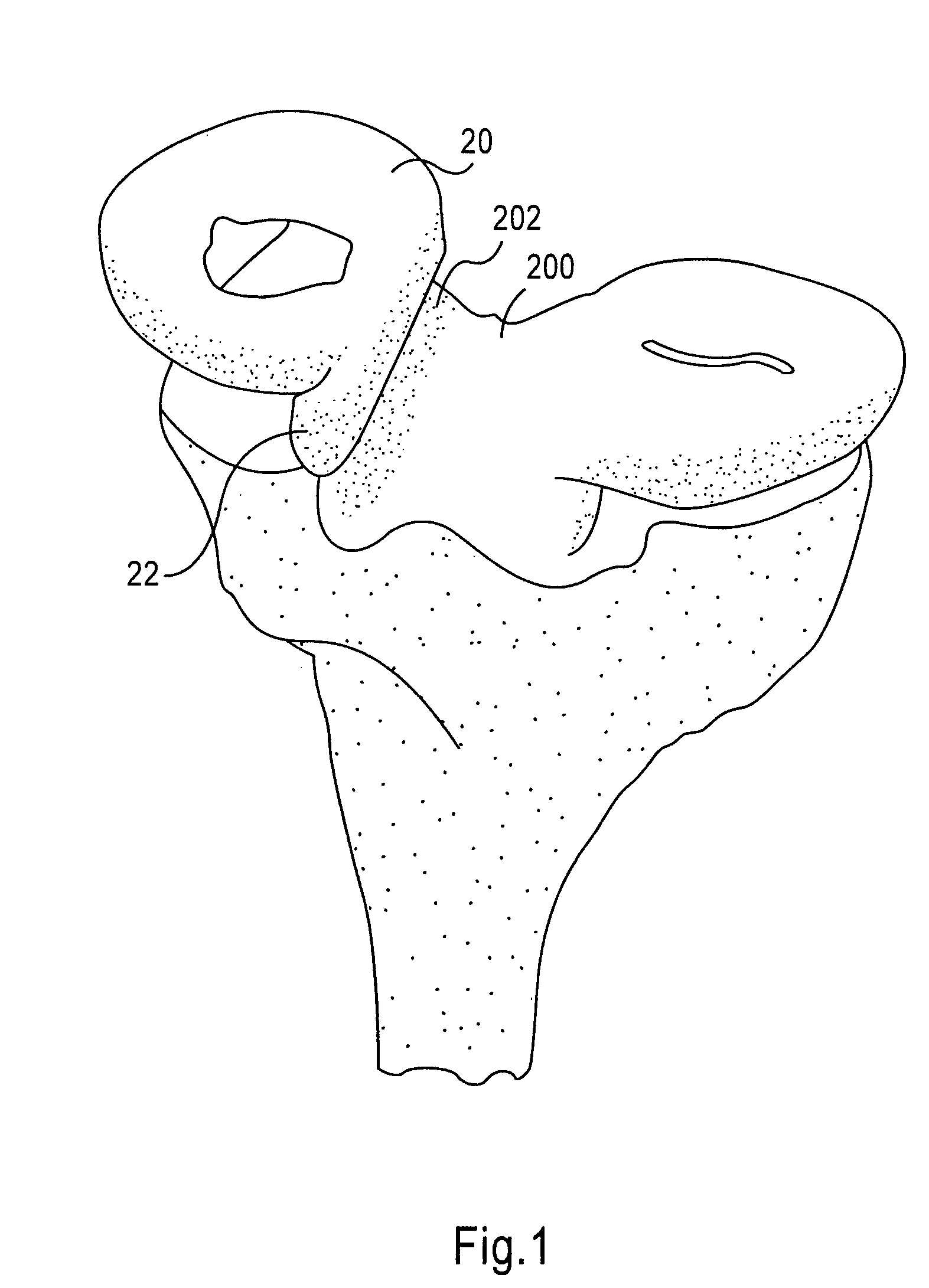
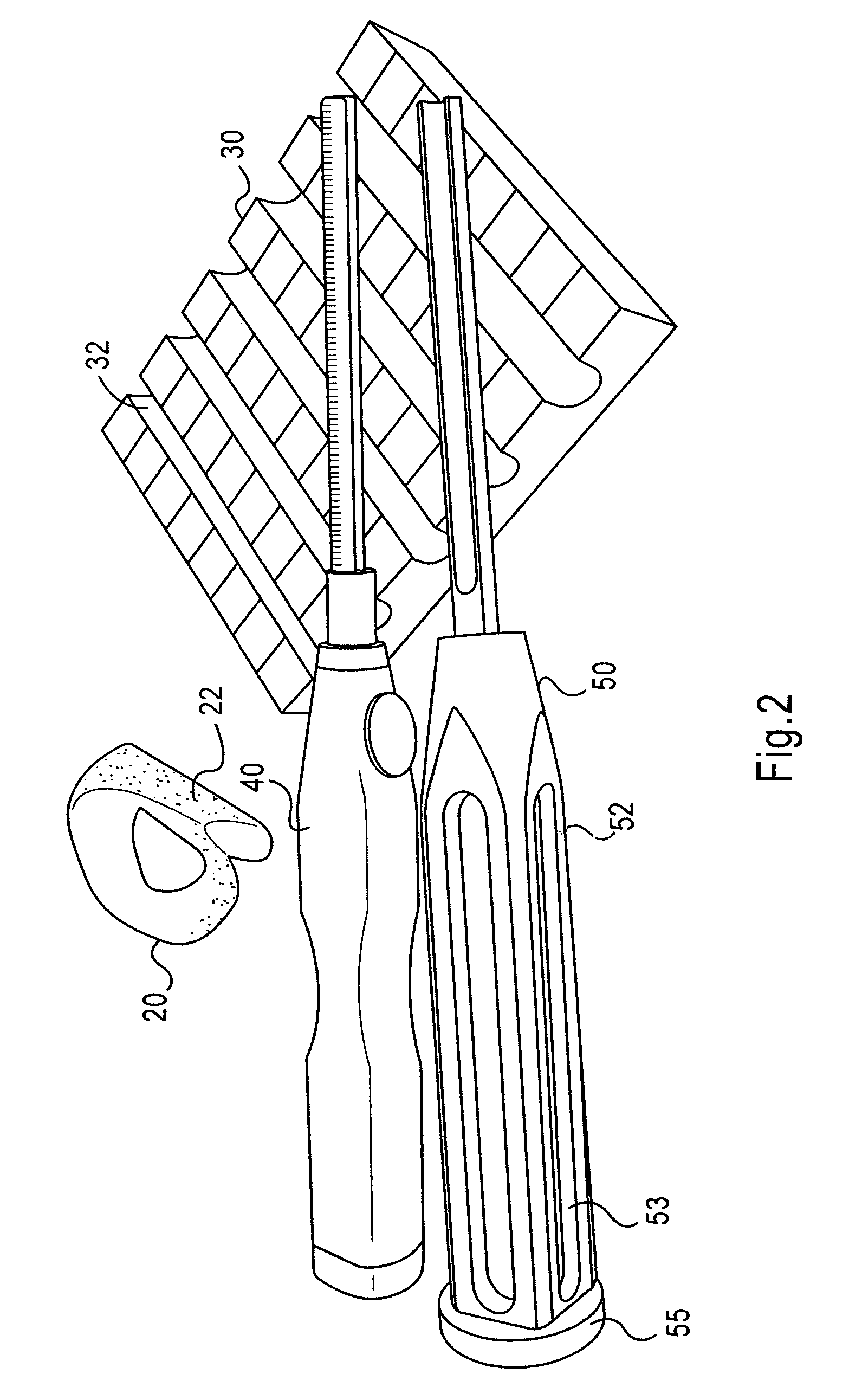
Instrumentation and method for repair of meniscus tissue
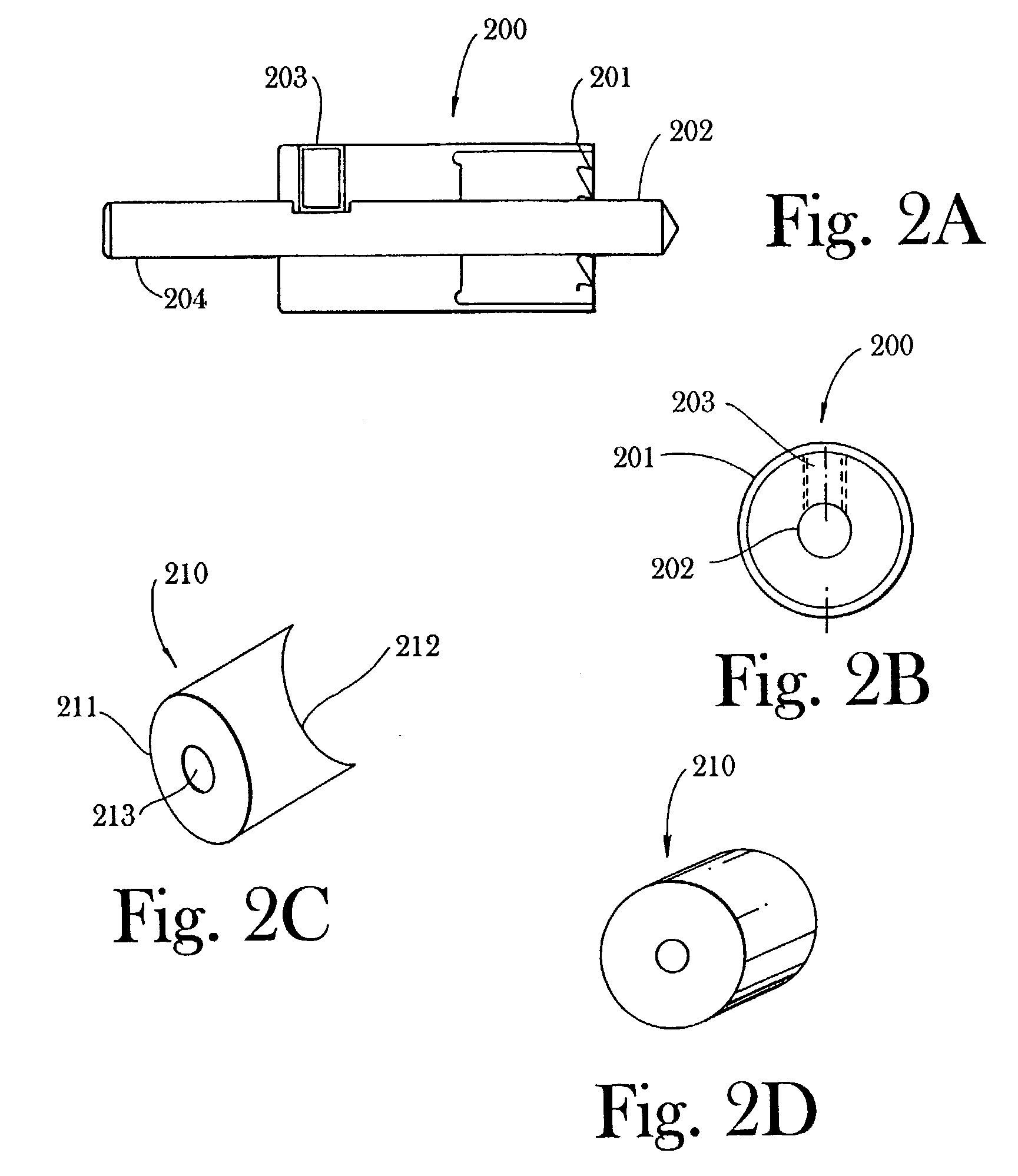
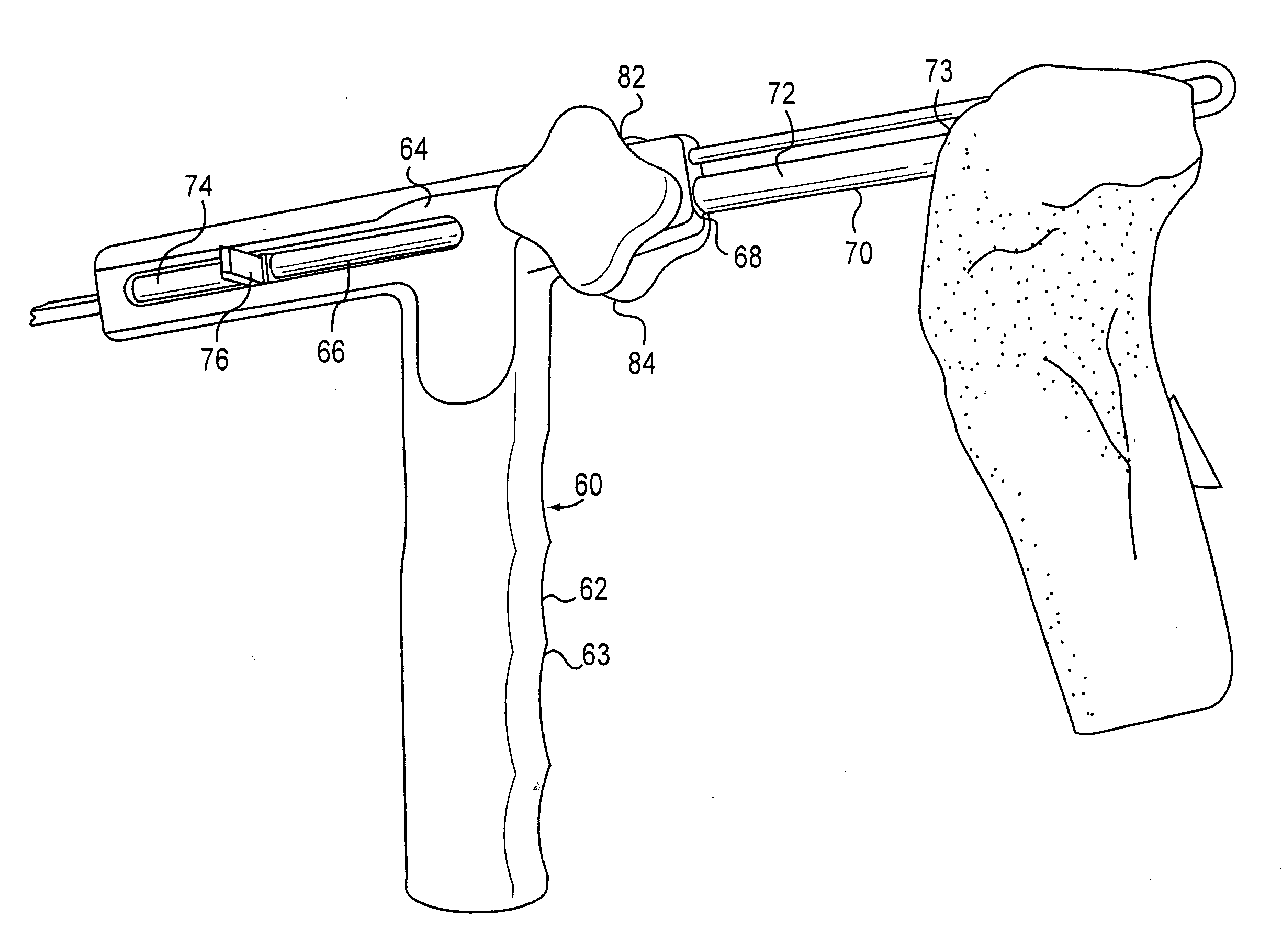
The invention is directed toward a method and instrumentation to replace a damaged human knee joint meniscus with an allograft meniscus. The implant has its bone base cut to a desired width in a workstation. The finished base is measured in the sizing groove of the sizing block for width and length. The tibia is then drilled with drill to the appropriate depth and length and groove is formed in the tibia with a tissue chisel so that the width is the same as the width of the bone base. The bone base is press fit into the tibia groove and may be secured with a bone screw.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
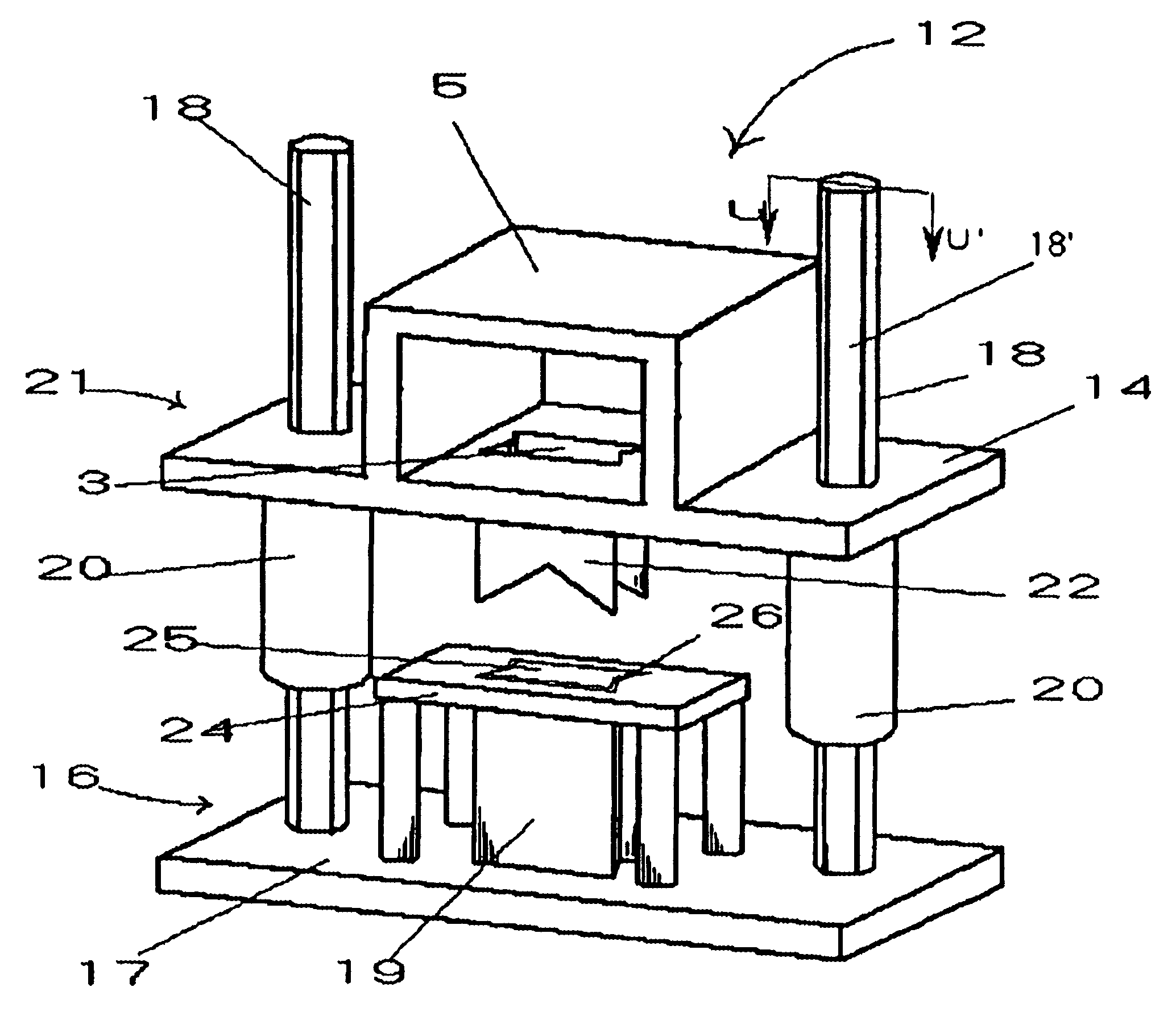
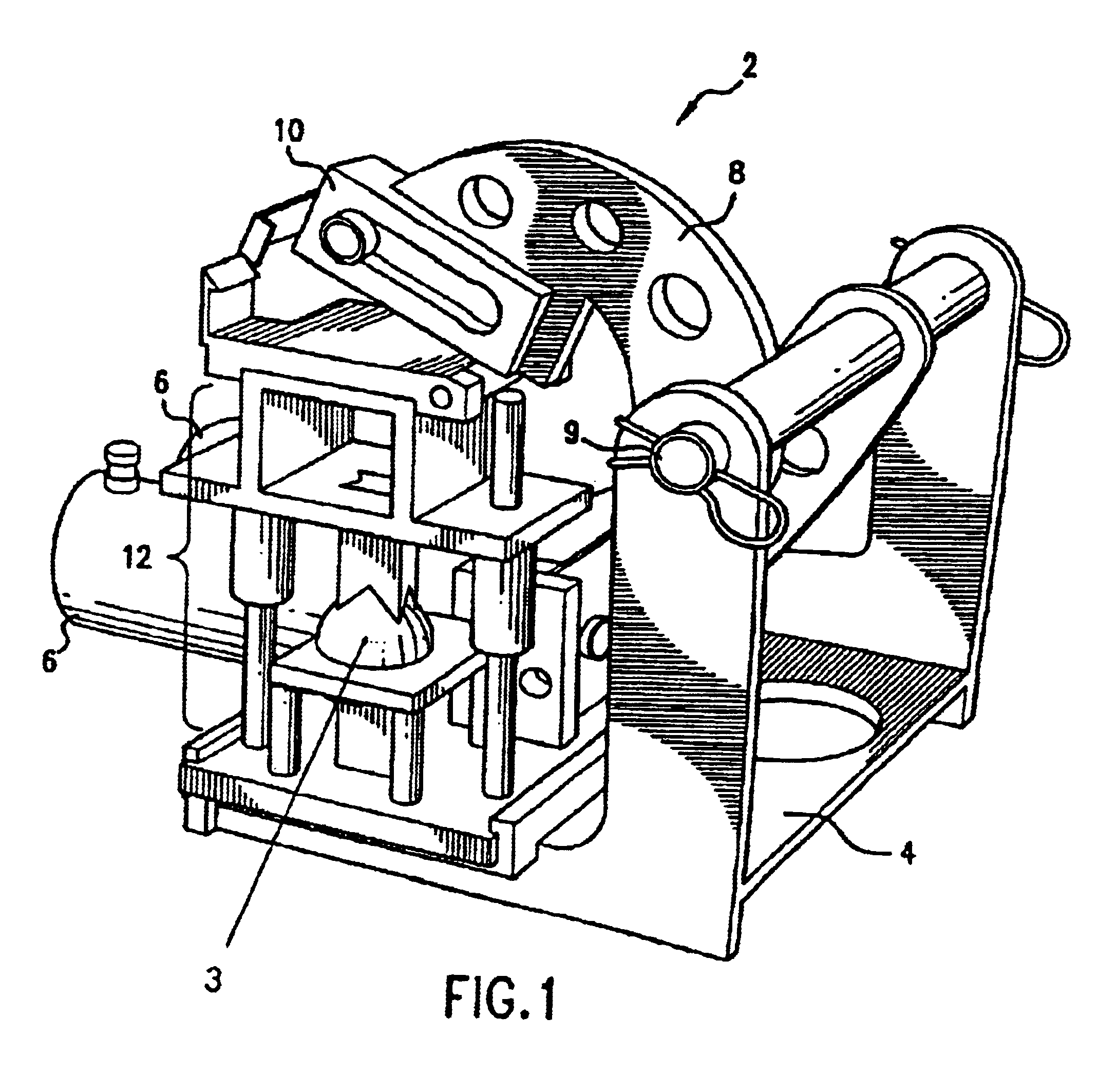
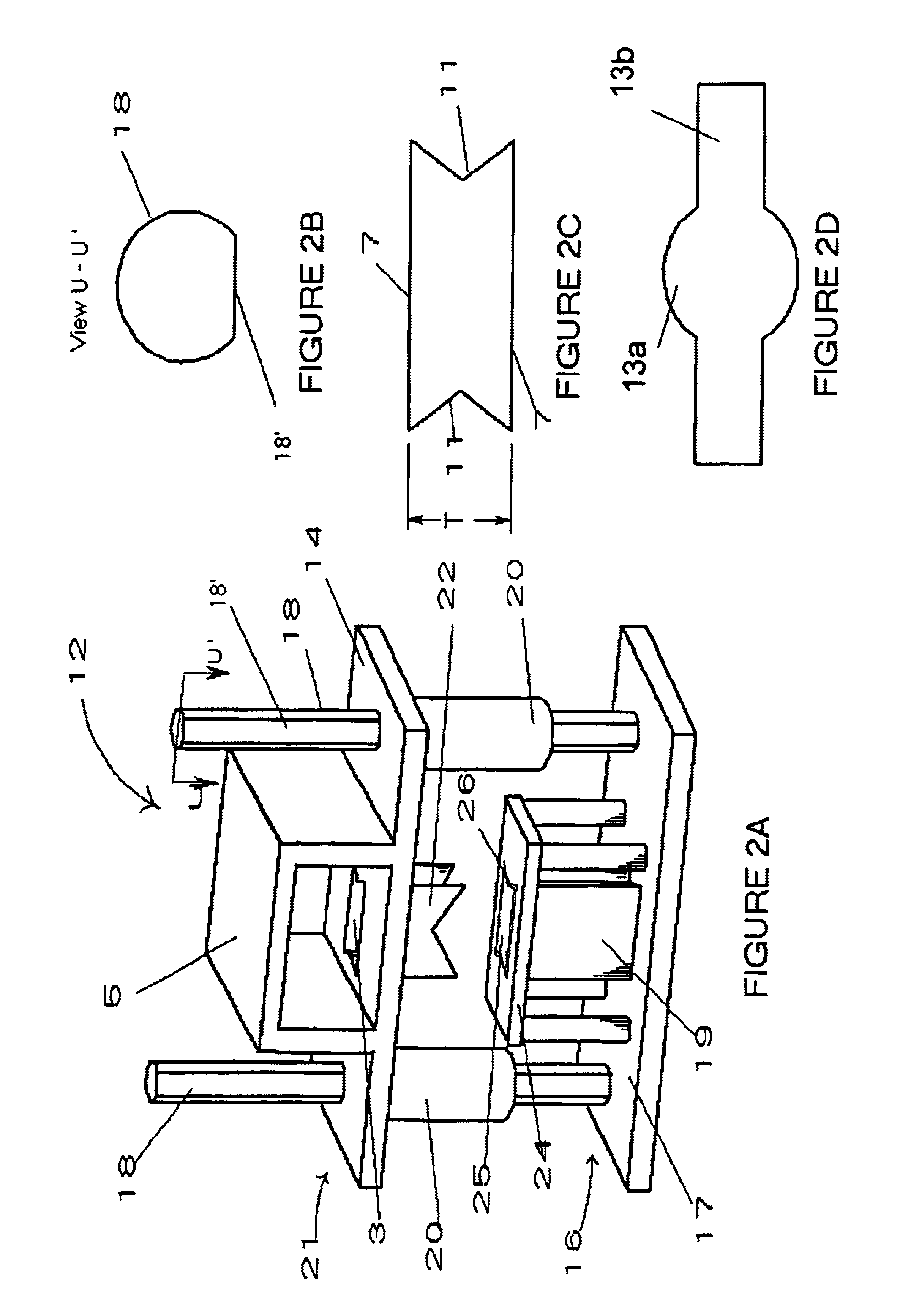
Allograft implant cutting machine
The present invention is an apparatus for cutting allograft bone implants from donor bone. Die sets of standard sizes are installed in a press. Donor bone is then placed in position between the upper and the lower parts of a matched cutting set comprising a hollow cylindrical cutting blade, a mandrel, and a table which establishes a gap around the mandrel which guides the blade. After the bone to be shaped has been properly placed, a shaped cutting blade is driven through the donor bone, producing a precisely shaped allograft implant. The invention provides for rapid change out of blades, rapid cleaning, and easy maintenance.
Owner:ALPHATEC SPINE INC
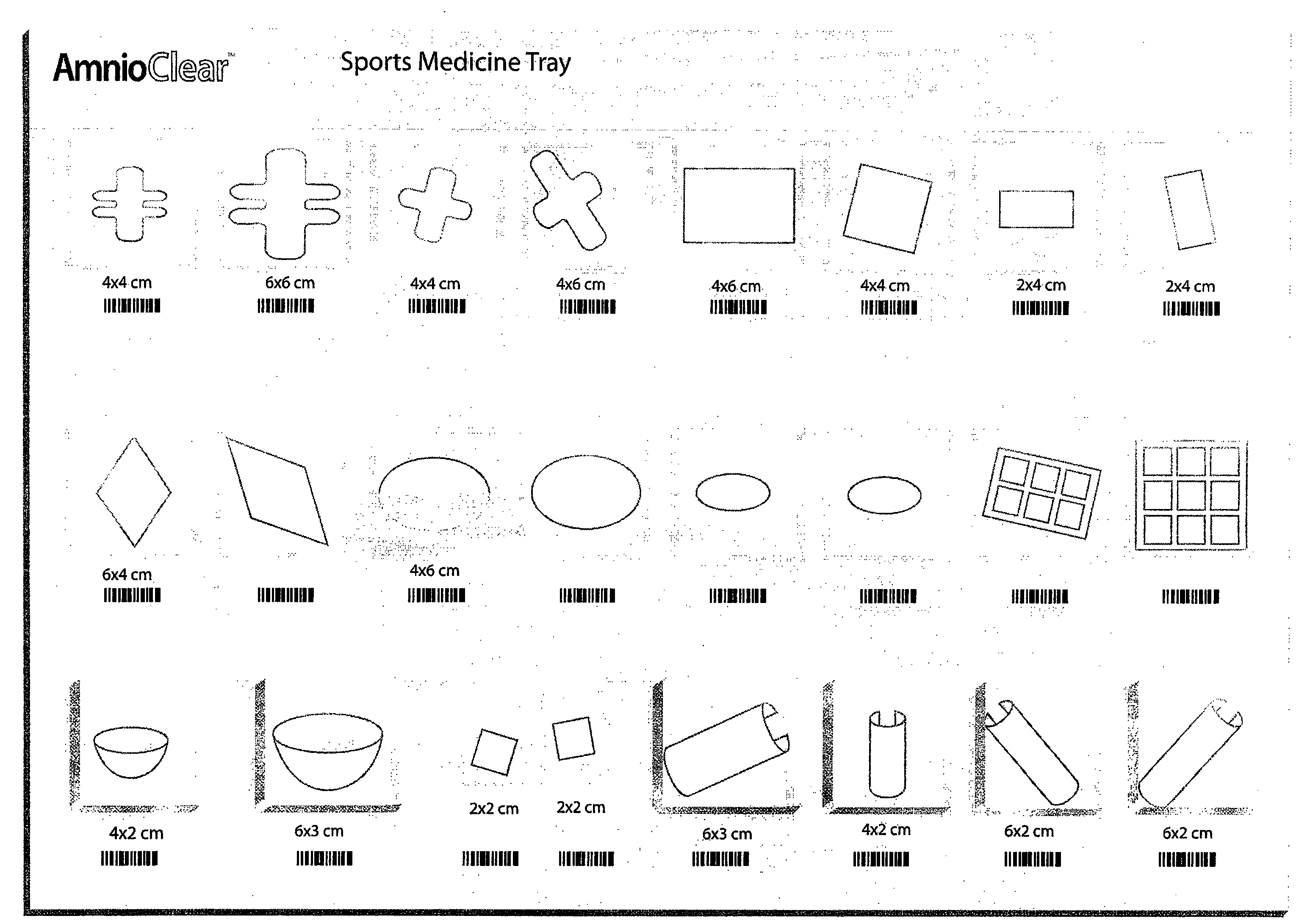
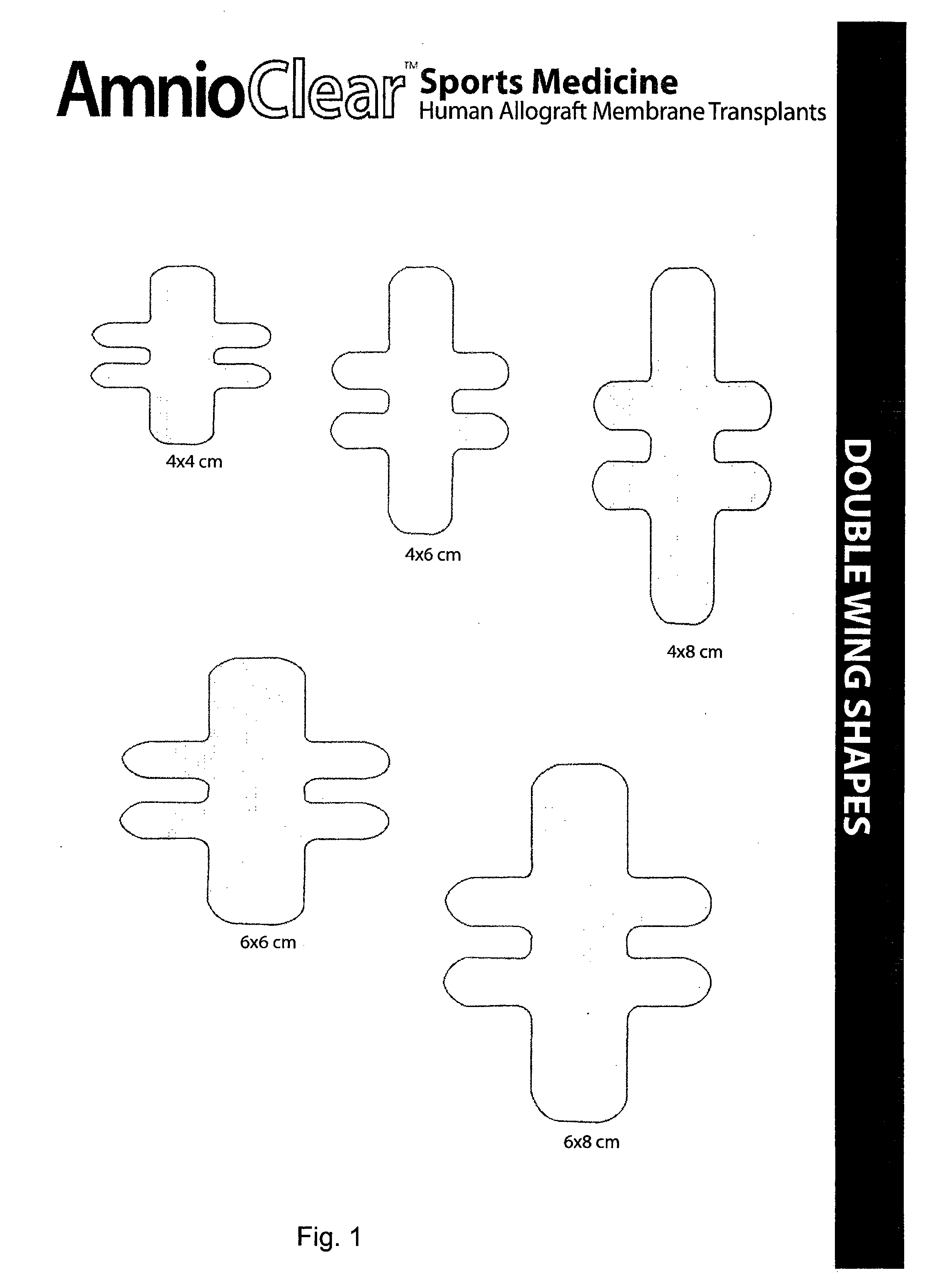
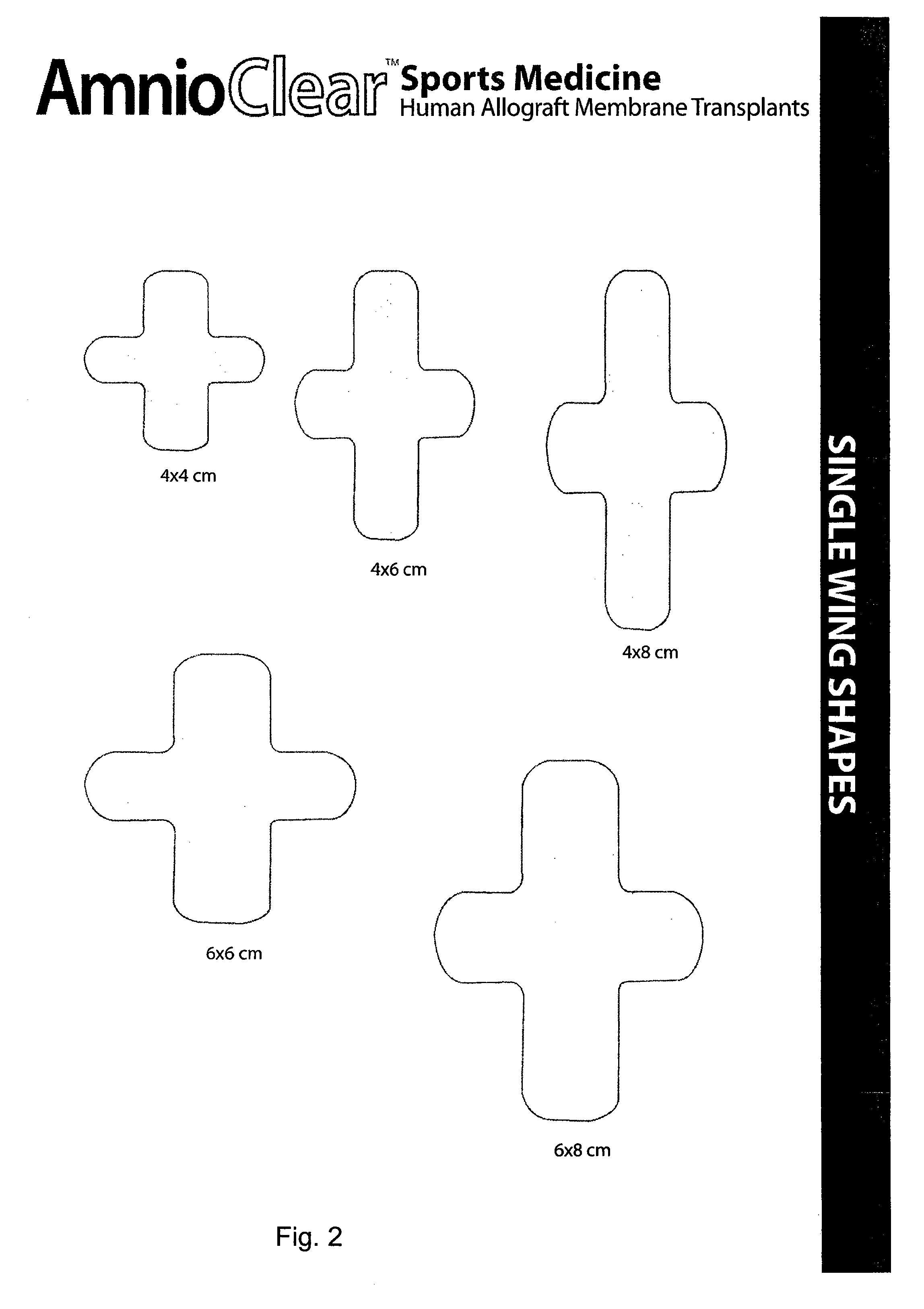
Amnion and chorion constructs and uses thereof in sport injury surgeries
Improved methods for sport injury surgeries are described. The improvement includes covering a damaged site of fascia with at least one of an amniotic fluid and a construct for use in surgical repair of the sport injury prior to wound closing. The construct contains an allograft comprising at least one layer of human amnion and chorion tissues and the construct has a size and shape suitable for covering the damaged site of fascia. The method improves fascial membrane repair, reduces complications and recovery time of sport injury surgeries.
Owner:LIVENTA BIOSCI
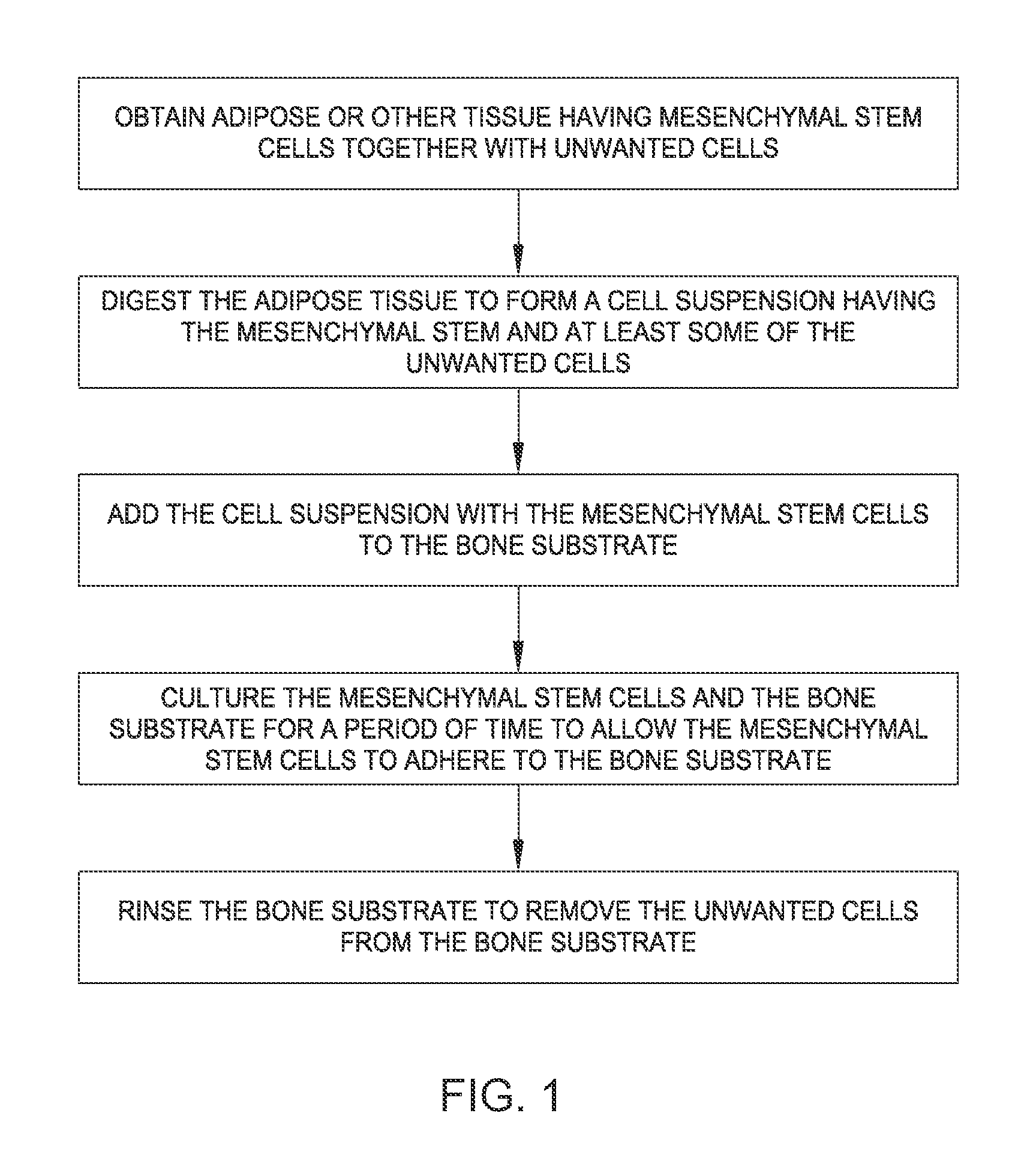
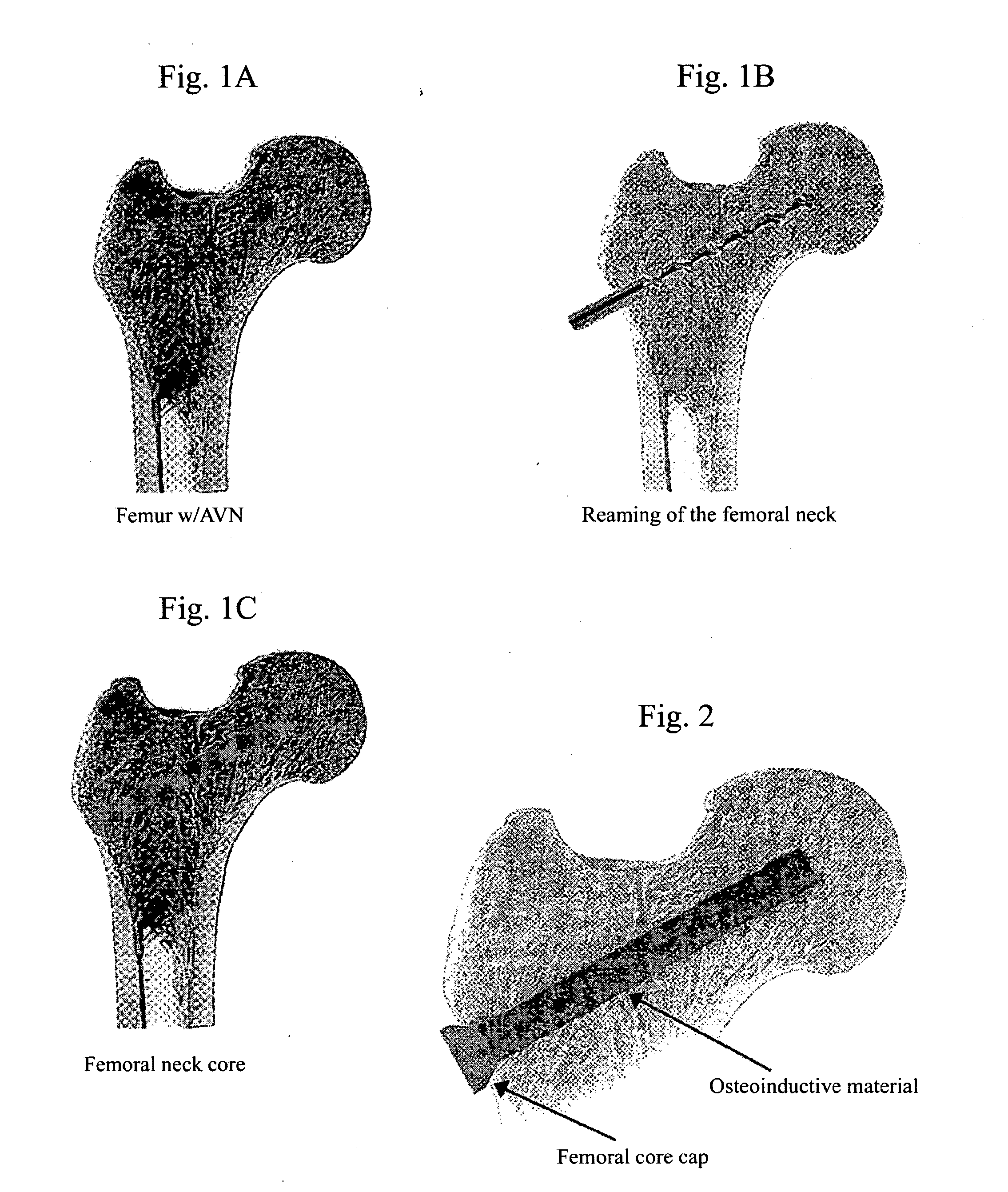
Process to treat avascular necrosis (AVN) with osteoinductive materials
InactiveUS20060057184A1Improved core decompression procedureEffectively prevent and treat avascular necrosisPeptide/protein ingredientsBone implantAllogeneic graftAnatomy
A method of treating avascular necrosis (“AVN”) comprising administering one or more osteoinductive formulations to the site of AVN disease progression. The method involves the combination of a core decompression technique, followed by the introduction of one or more osteoinductive formulations into the decompression core, and concluding with capping of the lateral aspect of the decompression core with a femoral core cap. The osteoinductive formulations of the invention comprise one or more osteoinductive agents and suitable carrier molecules. The femoral core cap retains the osteoinductive formulation within the decompression core, thereby preventing leakage of the osteoinductive formulation from the decompression core. The method of the invention optionally comprises introduction of autograft or allograft with the osteoinductive formulations of the invention. The method of the invention further optionally comprises incorporation of sustained release compositions to provide extended periods of osteogenesis.
Owner:WARSAW ORTHOPEDIC INC
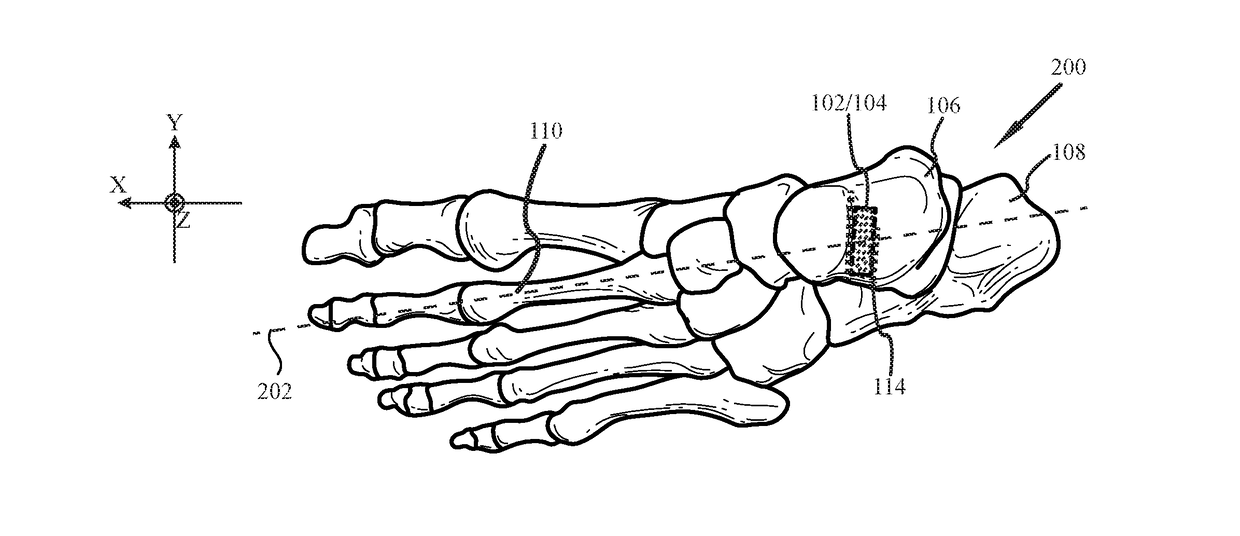
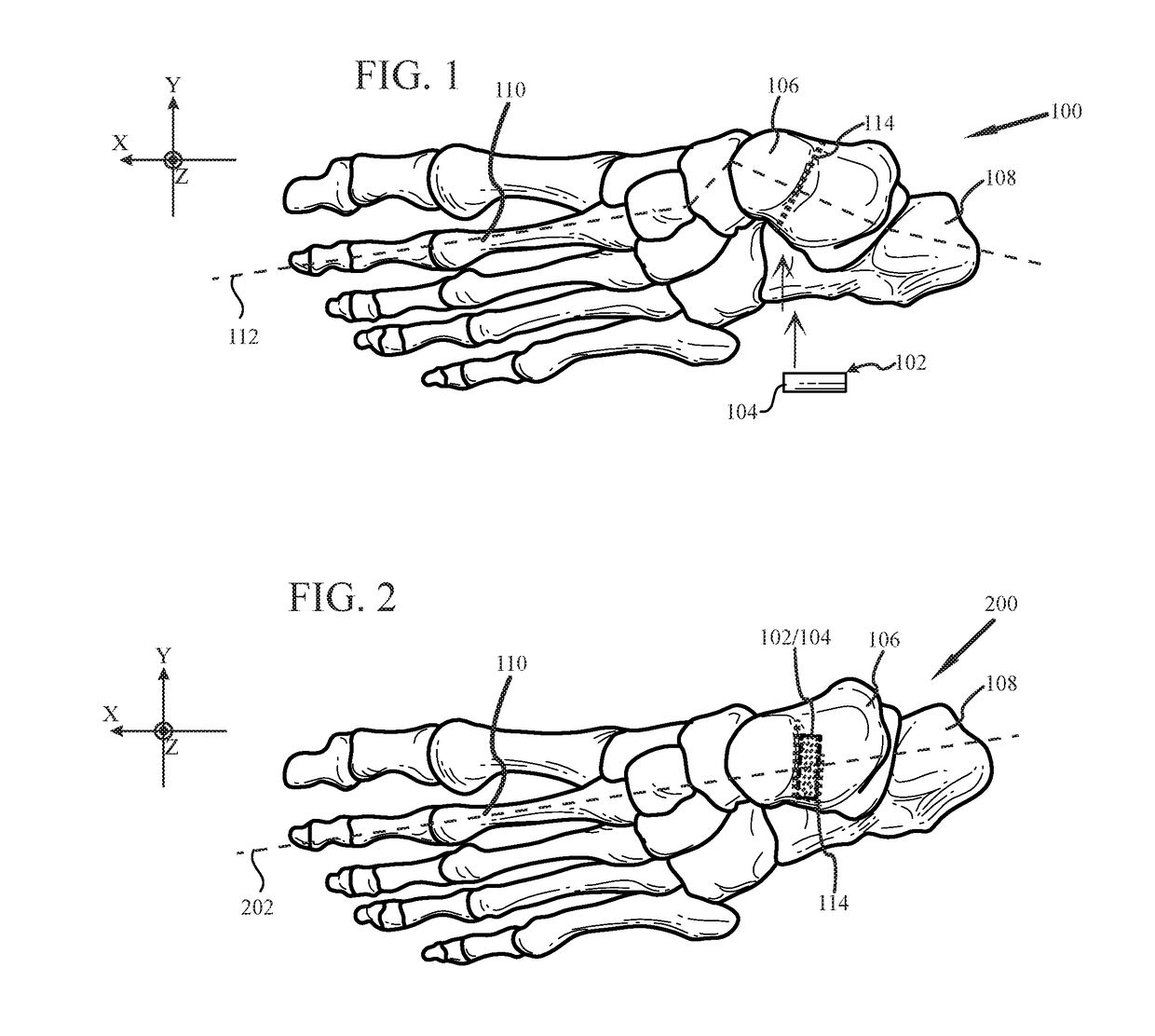
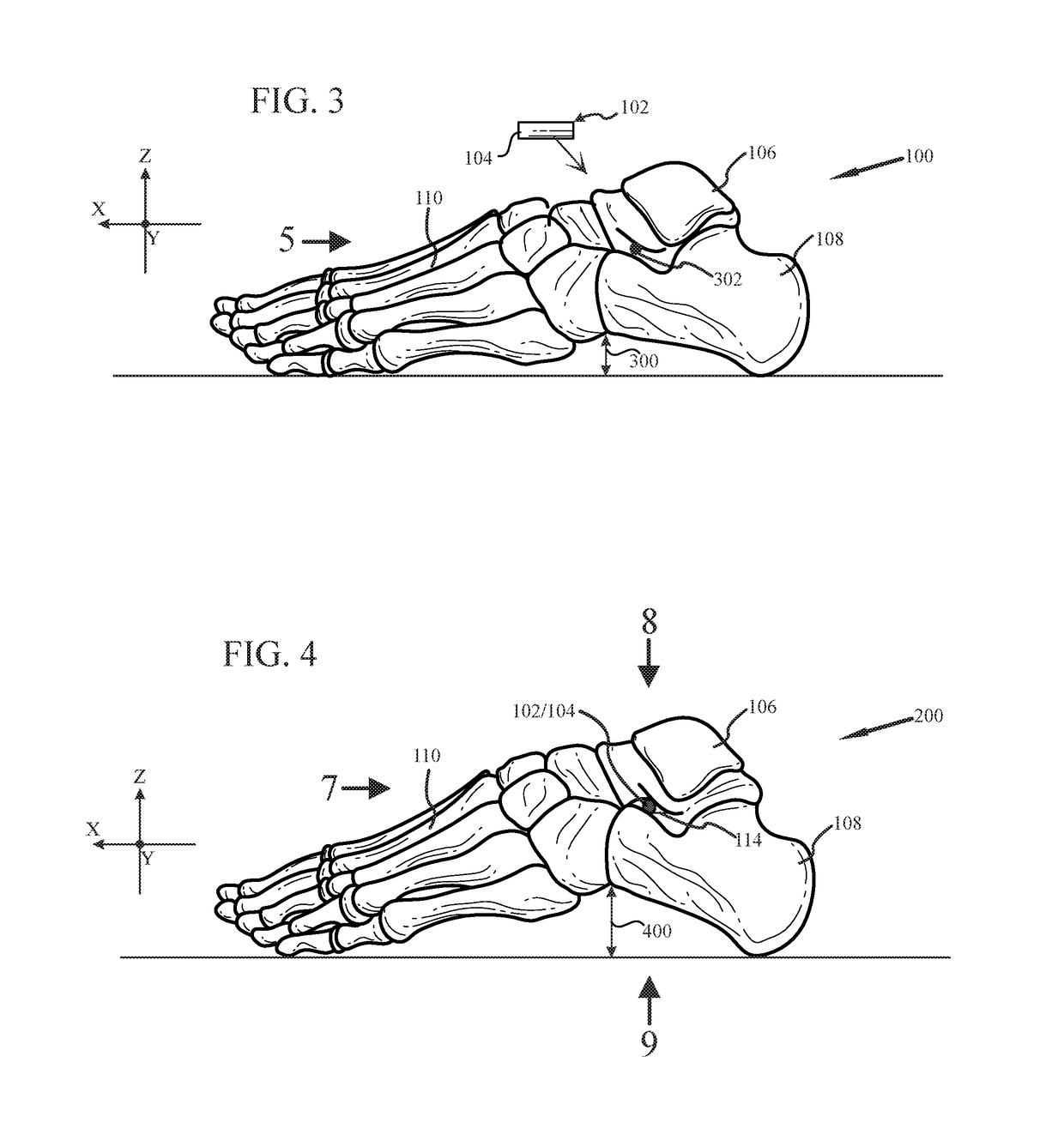
System and method for non-binding allograft subtalar joint implant
Provided is a system and method for providing a non-binding allograft subtalar joint implant for surgical implant into a person's foot proximate to the ankle. This system for repair includes at least one sterile non-binding allograft subtablar joint implant provided as a pre-formed allograft rod plug “ARP” having a diameter about equal to an average width of a canal between a person's talus and calcaneus bones, the ARP being resiliently compressible and flexible. When snuggly disposed between the person's talus and calcaneus bones, the ARP compresses during normal use of the person's foot and maintains the canal in an anatomically correct alignment and reduces a tendency for abnormal motion between the person's talus and calcaneus bones. An associated method of use is also provided.
Owner:ARTHROSURFACE
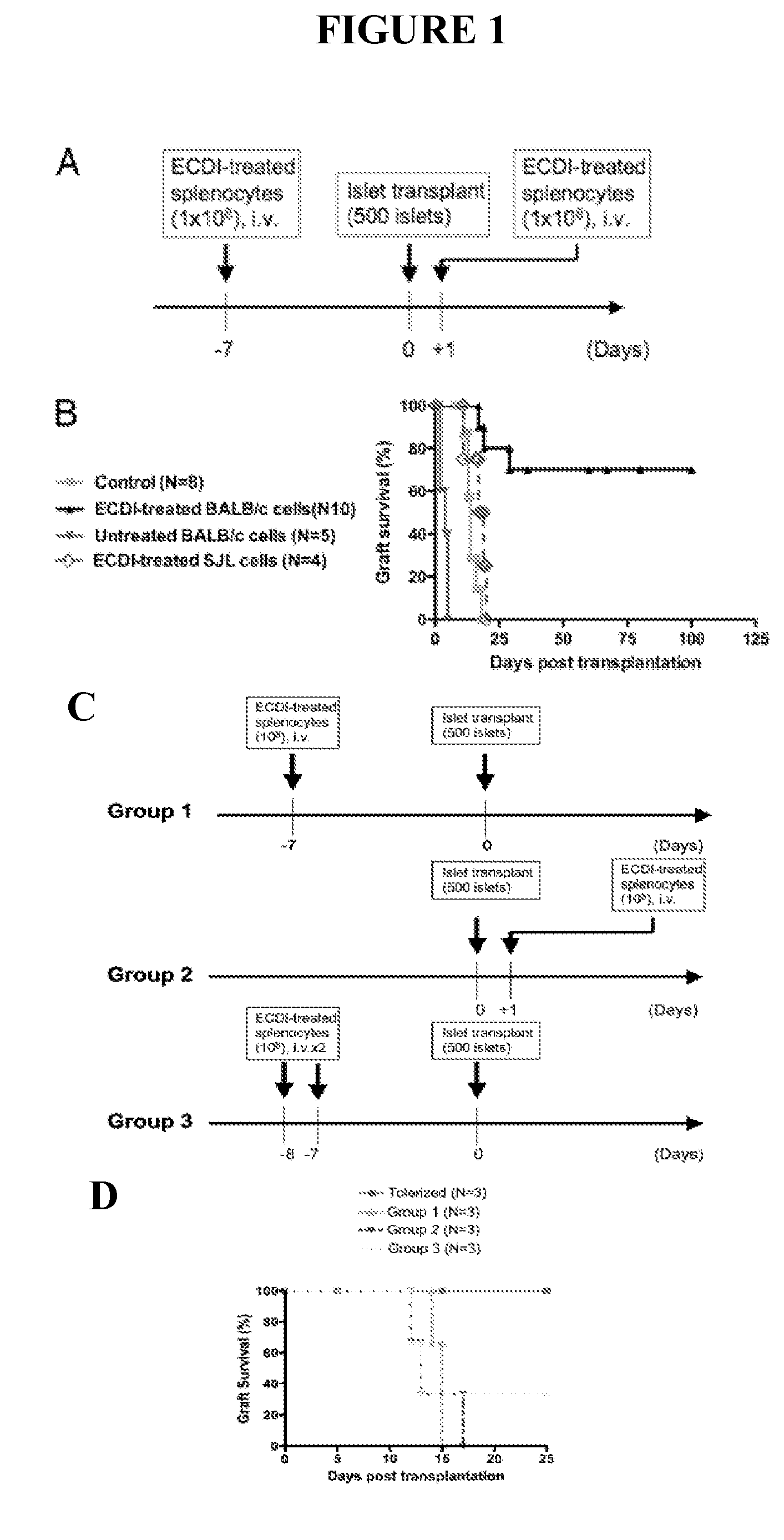
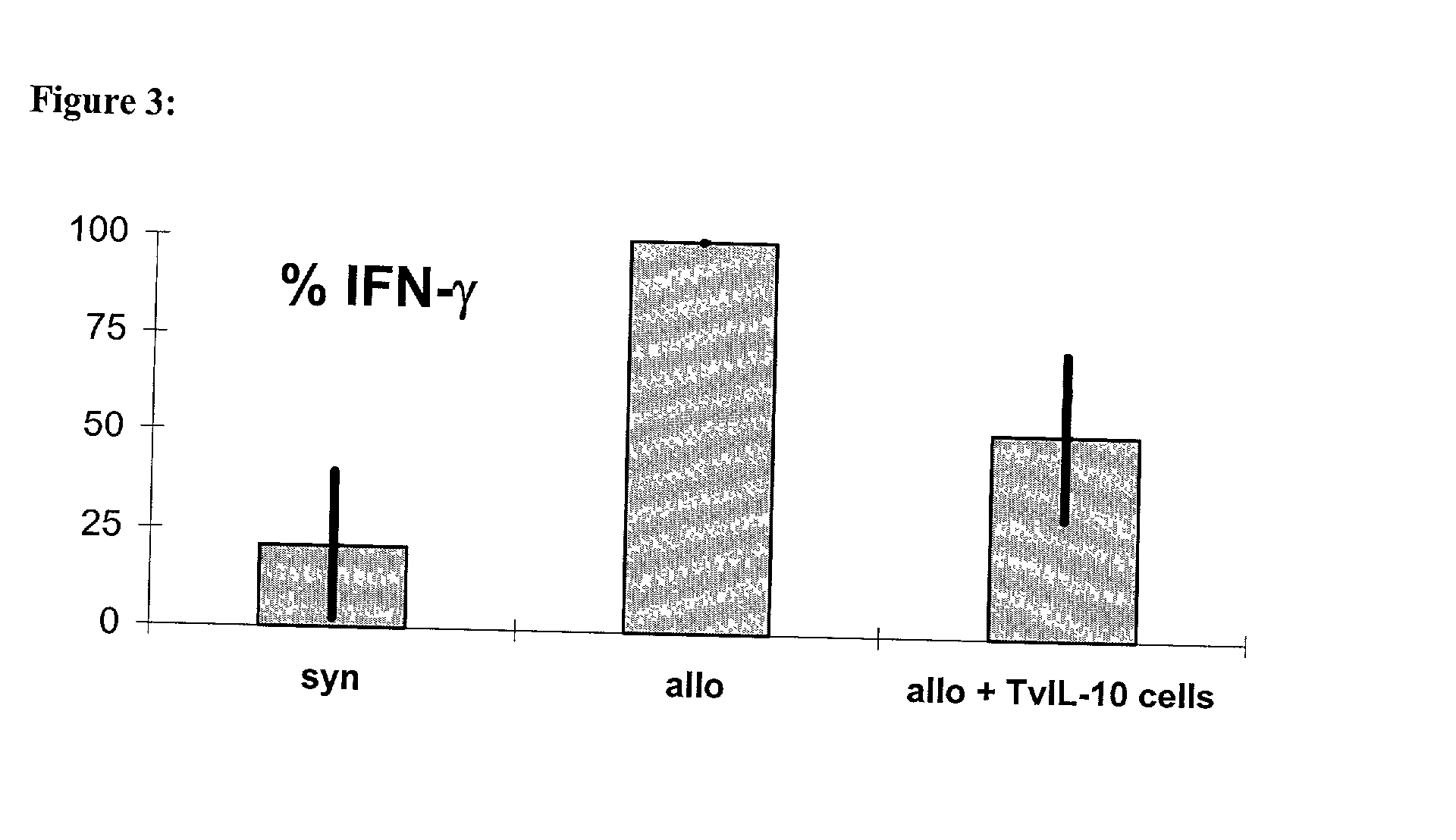
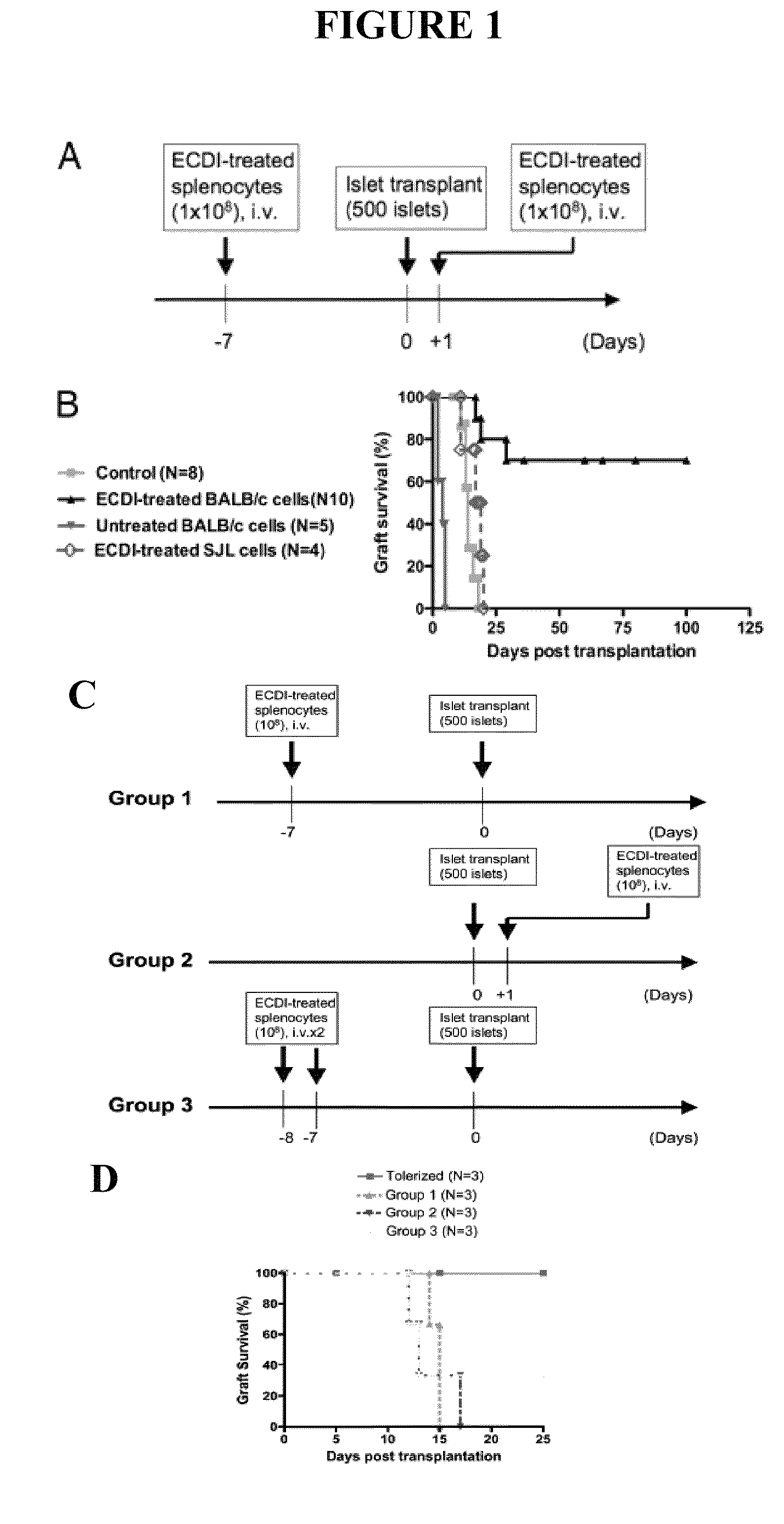
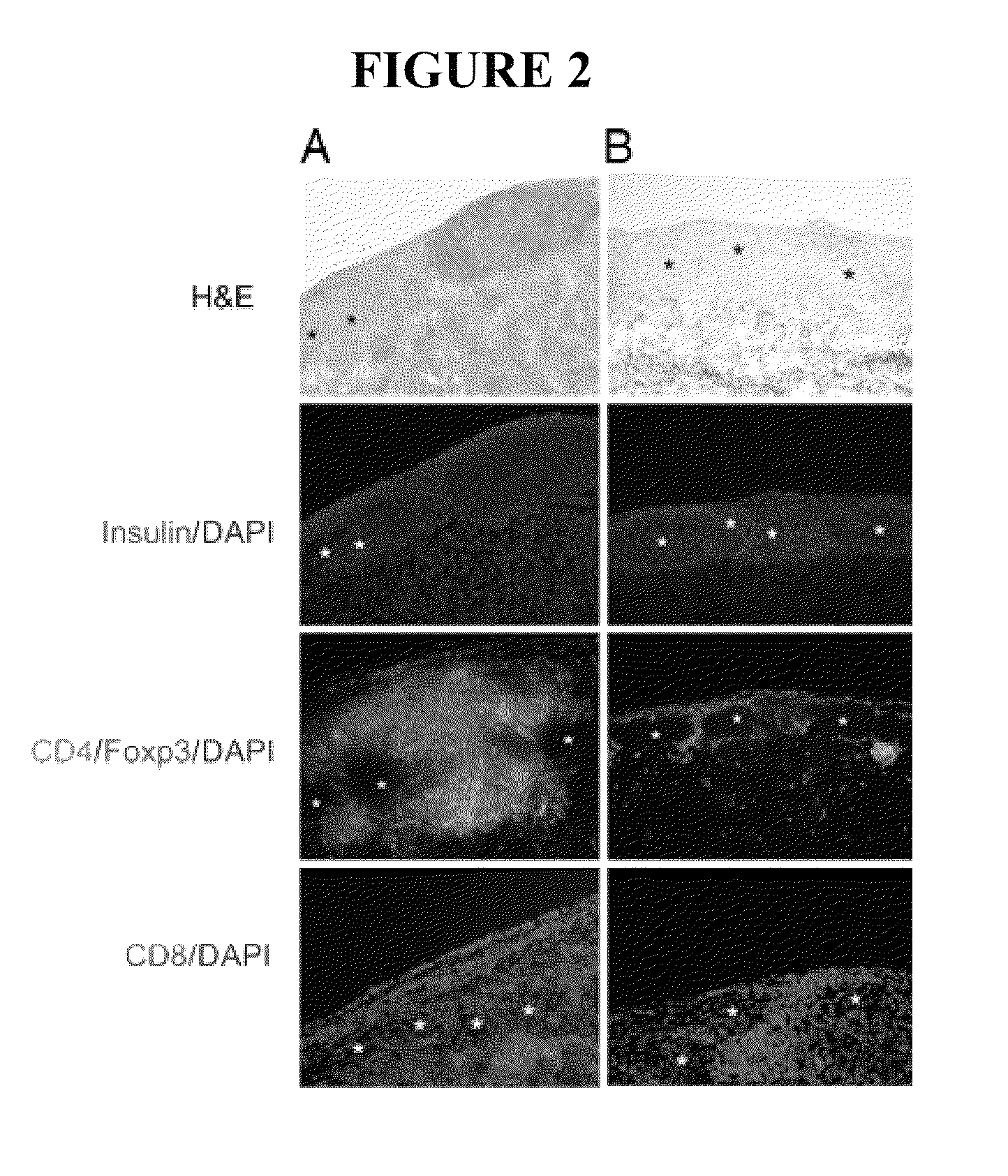
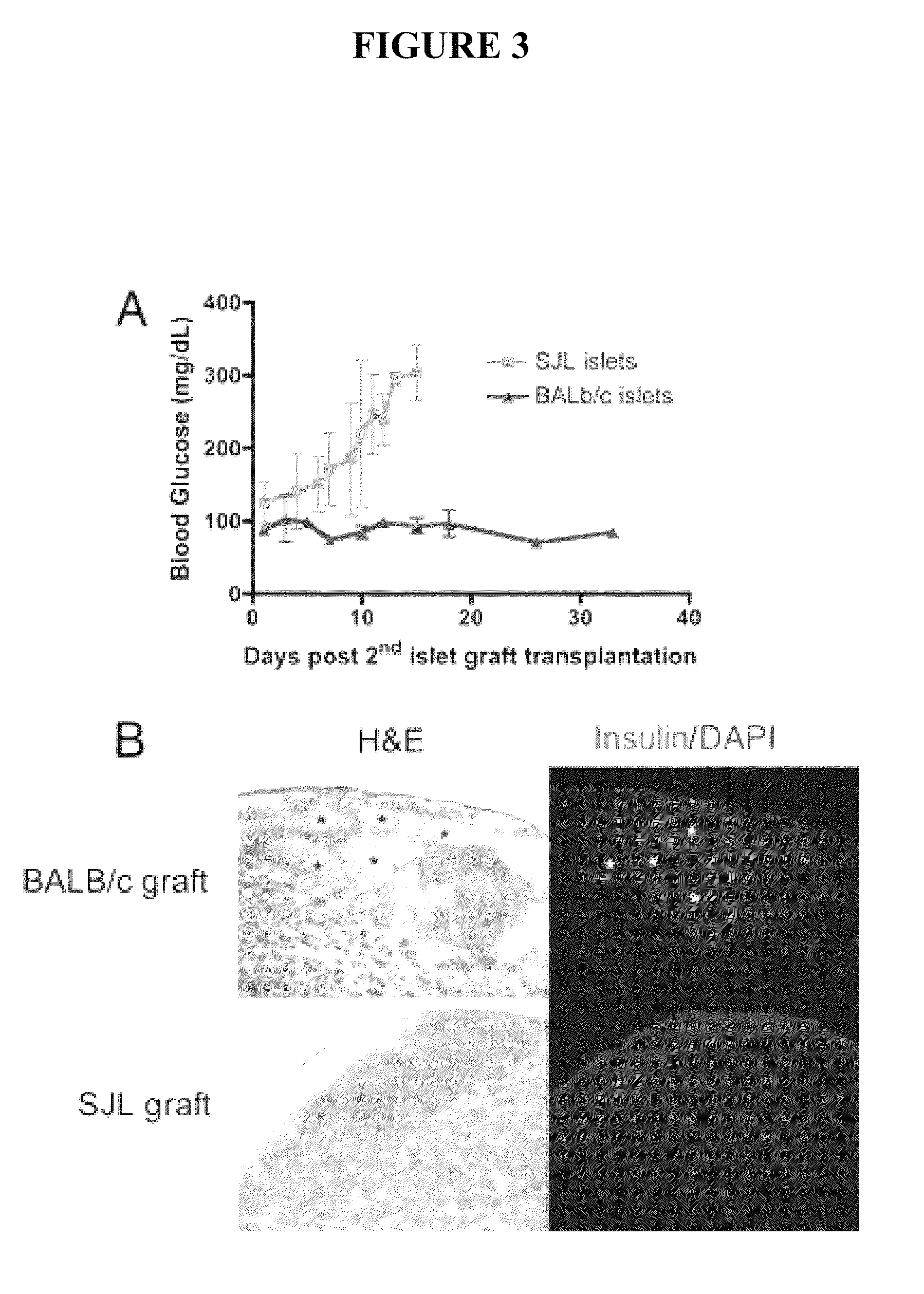
Use of ecdi-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
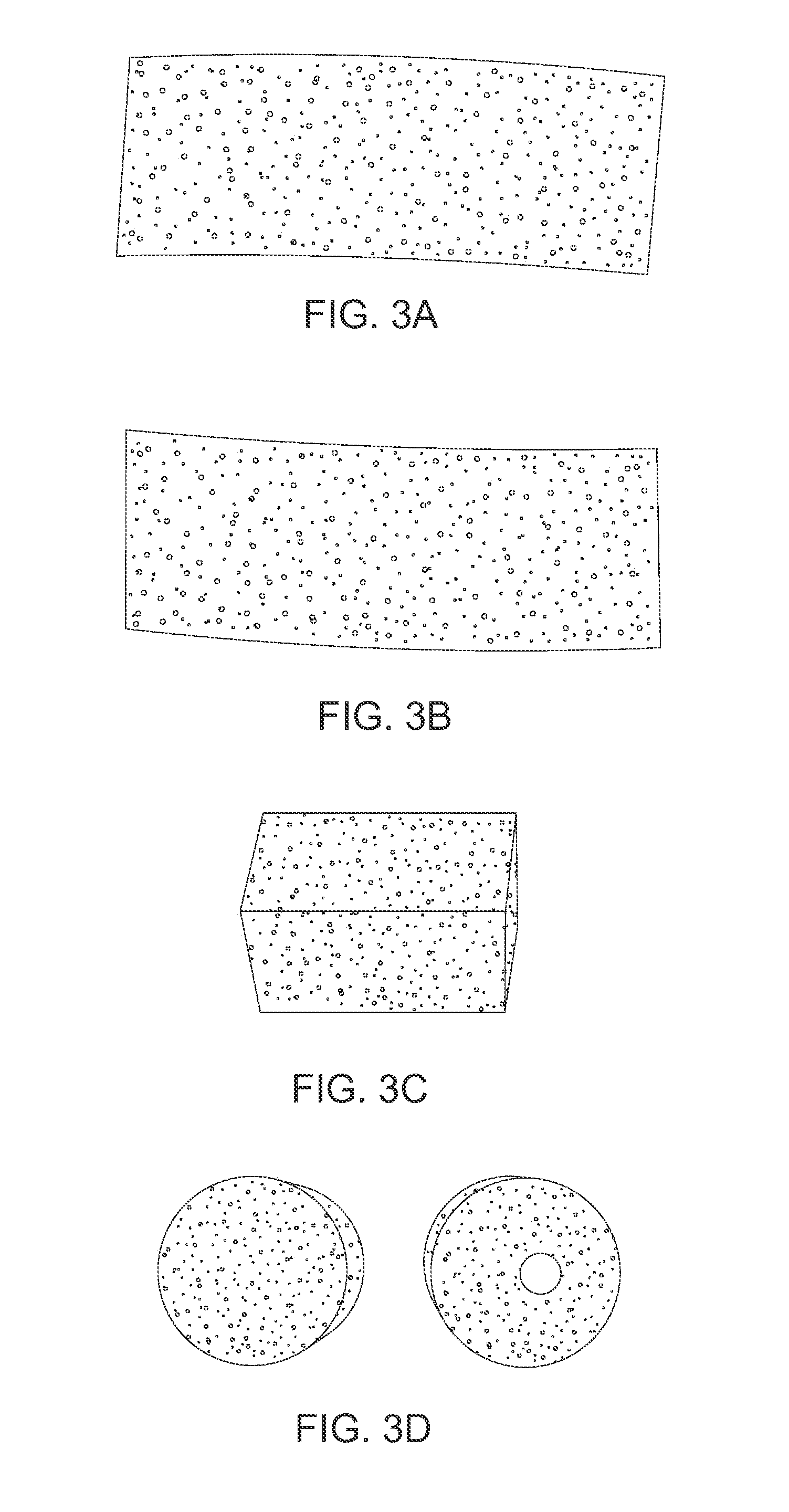
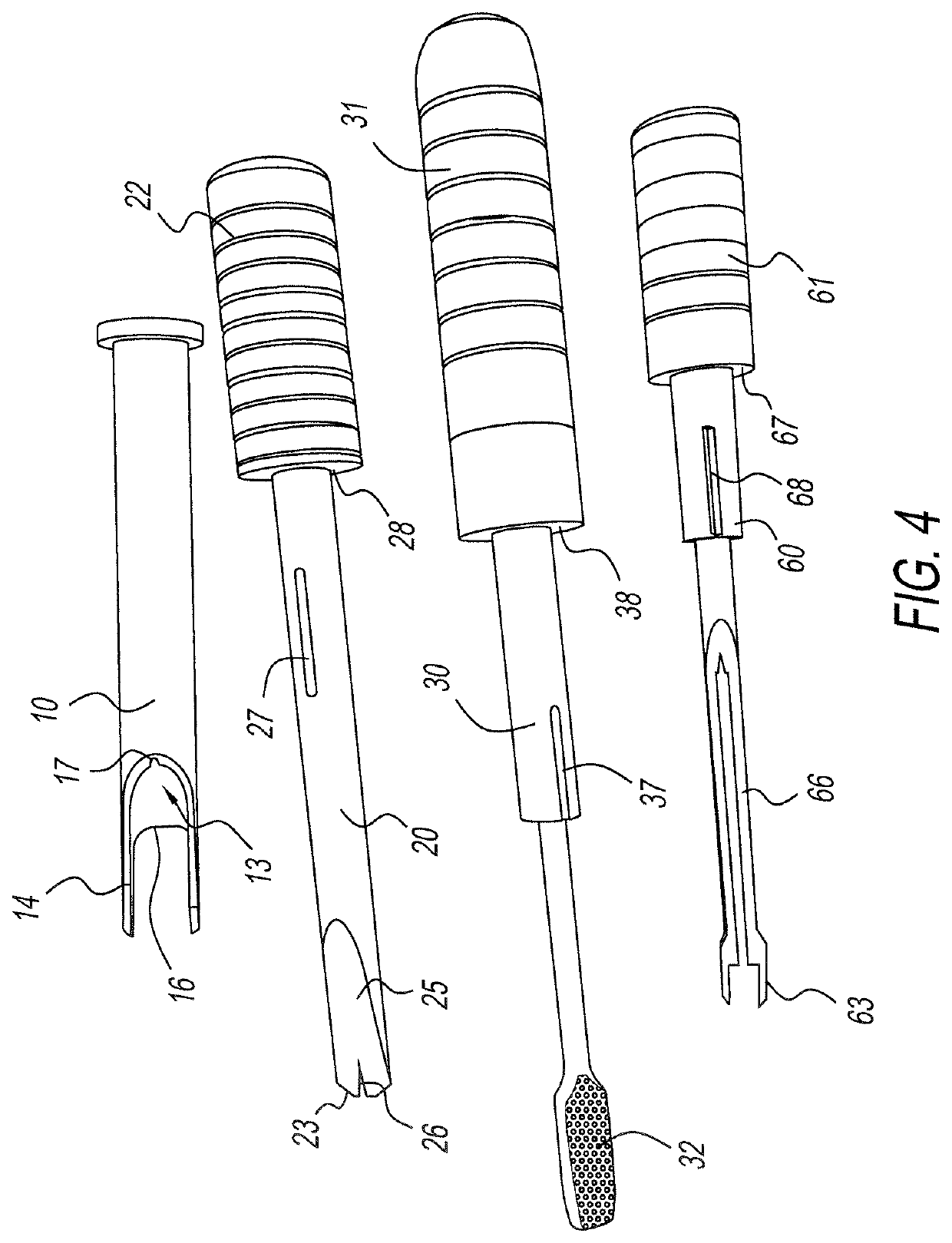
Multiple wafer cortical bone and cancellous bone allograft with cortical pins
InactiveUS20110137417A1Reduce the possibilityRelieve pressureBone implantSpinal implantsHuman bodyAllogeneic graft
A bone allograft and cortical pin for inserting into a surgically altered site of a human. The cortical pin is cylindrically shaped and has a thick middle diameter surrounded by two smaller end portions. The allograft has two end cortical wafers with small canals to receive the small ends of the cortical pin. At least one cancellous wafer is disposed adjacently between the end cortical bone wafers. A plurality of cancellous and / or cortical wafers may be inserted between the end cortical wafers to form the allograft. The wafers inserted between the end cortical wafers have larger canals to receive the thick diameter of the cortical pin. The size of the allograft may be adjusted as desired by either adding or removing inner wafers, or adjusting the size of the inner wafers of the allograft. The allograft and cortical pin are inserted parallel to the plane of insertion into the human.
Owner:TRANSPLANT TECH OF TEXAS
Amnion and chorion constructs and uses thereof in minimally invasive surgeries
A construct for use in a minimally invasive surgery is described. The construct contains an allograft having at least one layer of human amnion and chorion tissues, and is adapted for insertion into a small incision or a cannula employed in the minimally invasive surgery for access to the surgical site. The allograft has a shape appropriate for covering the surgical site. Methods of preparing the construct and using it in a minimally invasive surgery are also described. The products and methods improve the performance of the minimally invasive surgery, e.g., by reducing adhesions, scar formation while also reducing inflammation and risk of post-operative infection.
Owner:LIVENTA BIOSCI
Preparation and storage of stable, biologically active materials
InactiveUS20100068245A1Improve temperature stabilityLow costBiocidePeptide/protein ingredientsActive agentCytokine
A method for the preparation of biologically active materials is presented. The invention involves taking a base material such as allografts, xenografts, polymers, metals, and ceramics and combining it with a biologically active agent, such as proteins, cytokines, growth factors, and enzymes after which it is irradiated with ionizing radiation to sterilize and stabilize the material. The resulting biologically active material may then be stored at ambient temperature while maintaining its biological activity and the structural integrity of the base material. The invention is particularly useful for eliciting desired biological responses in human and animal medicine, and in certain industrial applications.
Owner:PROMETHEAN LIFESCIENCES INC
Vascular graft sterilization and decellularization
ActiveUS20070260109A1Efficient removalOptimal biological functionalityTissue regenerationBlood vesselsBlood Vessel GraftingHeterografts
The present technology is related to the field of sterilization and decellularization of allografts or xenografts, specifically to processes that achieve effective removal of the cells contained within a vascular tissue matrix and an effective reduction in potential harmful organisms to create grafts suitable for human implantation. In some embodiments, vascular grafts, vascular tissue and / or blood vessels are contacted with cleaning solution under conditions suitable conditions to reduce immune reaction in patients. More specifically, the present technology is directed to sterilization and decellularization of vascular grafts.
Owner:RTI BIOLOGICS INC
Immunogenic peptides and their use in transplantation
ActiveUS20110110964A1Preventing allograft rejectionPeptide/protein ingredientsAntibody mimetics/scaffoldsRedoxAllograft rejection
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C—(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
Immunogenic peptides and their use in transplantation
ActiveUS9248171B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsOxidation-Reduction AgentRedox
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C-(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
Genetically modified T-cells, method for producing them and use thereof
InactiveUS20020119571A1Prevent rejectionBiocideGenetic material ingredientsT cellDrainage lymph nodes
T cells of a graft recipient are stimulated in-vitro by cells of a graft donor or by cells which express dominant MHC-molecules, while they are transduced with immuno-modulatory genes using gene transfer. Following the gene transfer the transduced T cells start to express immuno-modulatory genes. The gene transfer can be accomplished using retroviruses, other viral vector systems or liposomes. Due to the chosen set-up of the experiment, which leads to the generation and expansion of allospecific T cells, the T cells migrate, after the in-vivo application, specifically into the allogeneic graft as well as into the draining lymph nodes and are then able to express the immuno-modulatory genes there. Rejection of allogeneic grafts (cells, tissues, organs) can be successfully prevented, tolerance towards allogeneic grafts can be induced and maintained.
Owner:UNIVSKLINIKUM CHARITE MEDIZINISCHE FAKULTAET DER HUMBOLDT UNIV ZU BERLIN
Use of ECDI-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
Method of using amnion allograft in heart valve repair surgery
InactiveUS20130211504A1Speed up the repair processSuitable shapeVenous valvesBlood vesselsHeart valve repairAnesthesia
Improved methods for heart valve repair surgery are described. The methods utilize an allograft comprising a layer of amnion to improve the performance and reduce complications of heart valve repair surgery and the allograft has a pre-made size and shape suitable for the application.
Owner:LIVENTA BIOSCI
Method of using amnion allograft in heart transplant surgery
InactiveUS20130209524A1Suitable shapeReduce sizeBiocidePharmaceutical delivery mechanismHeart transplantationImproved method
Owner:LIVENTA BIOSCI
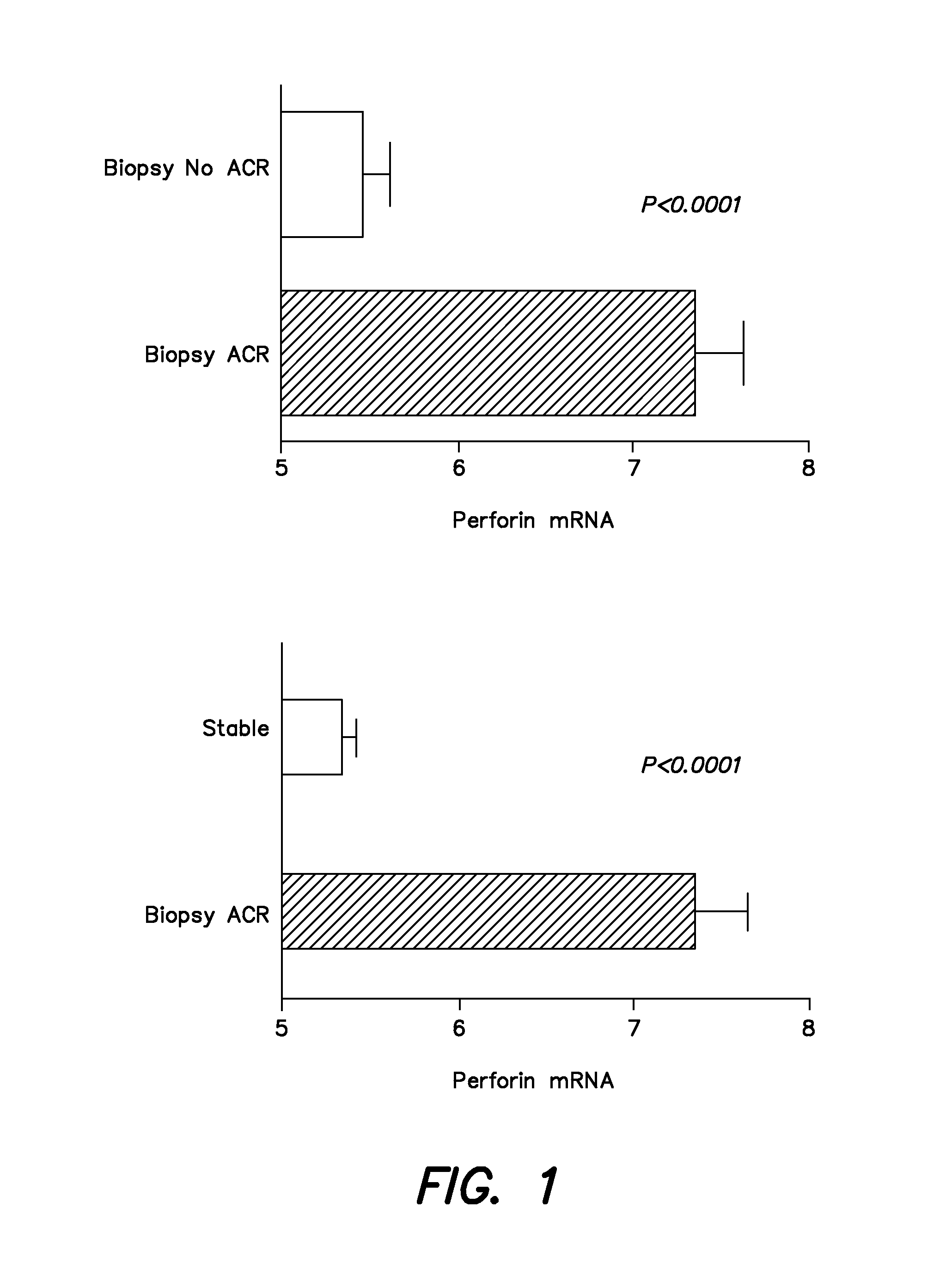
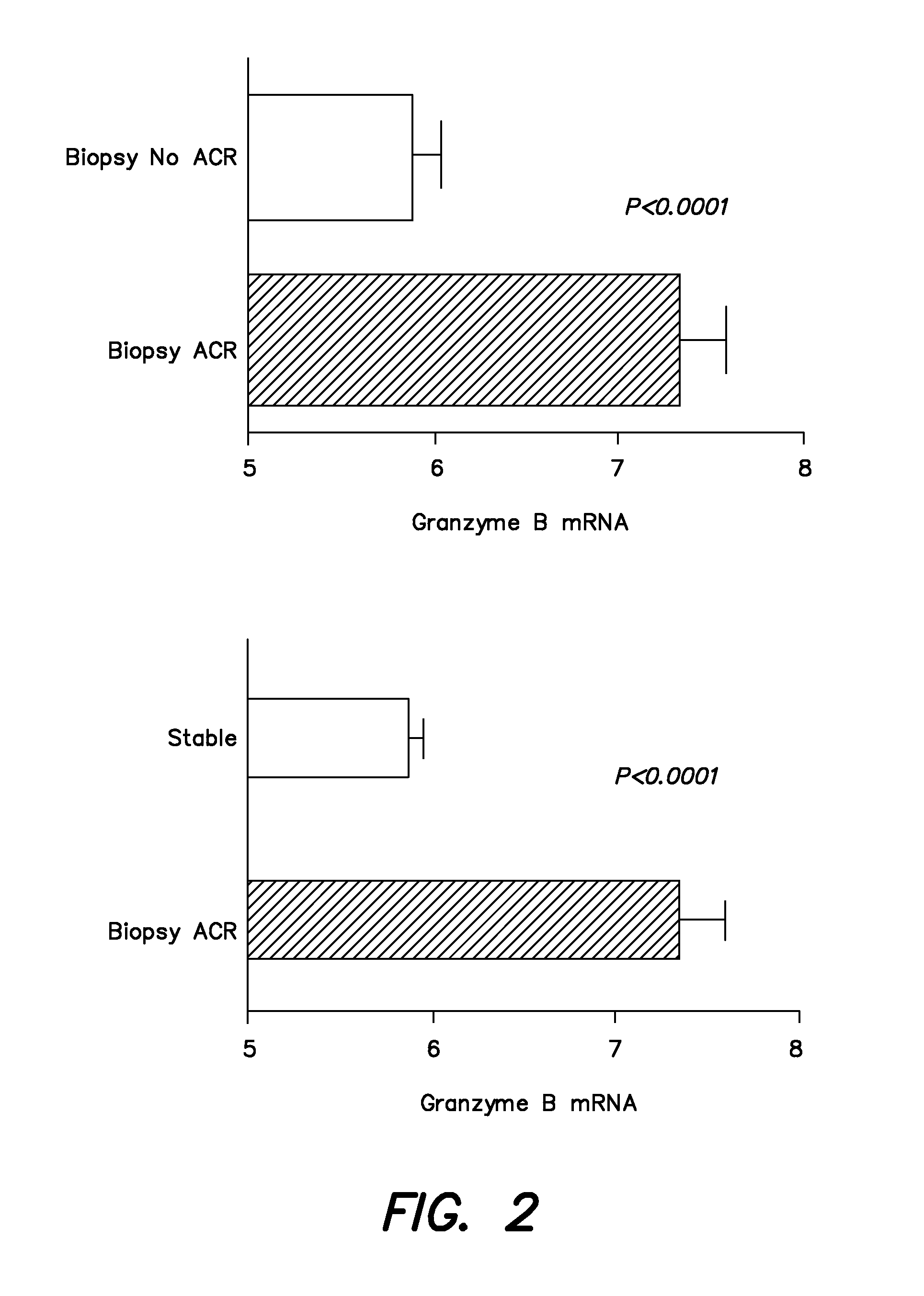
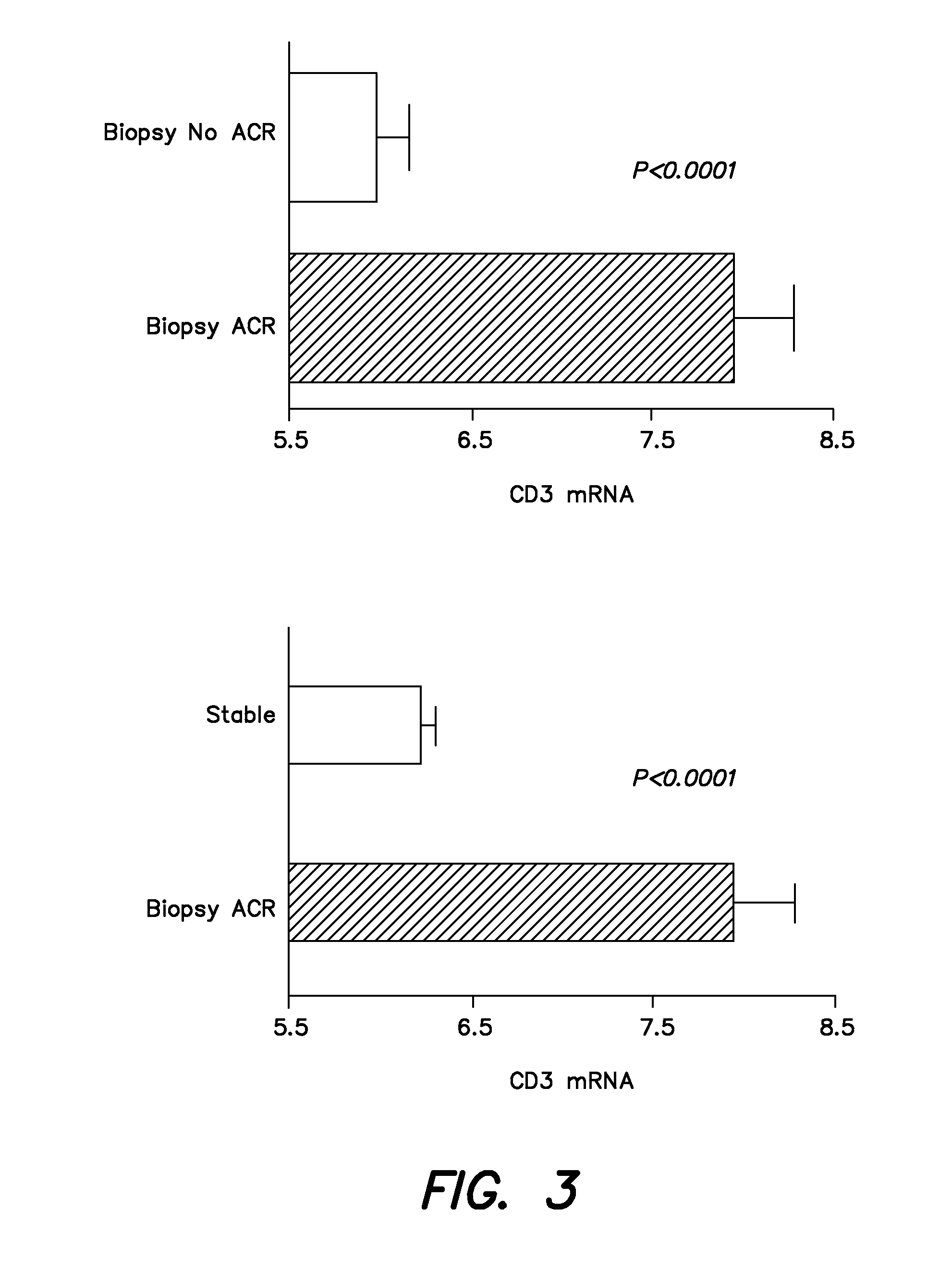
Methods and compositions to predict and detect acute rejection
ActiveUS20130012860A1Other blood circulation devicesMicrobiological testing/measurementTransplant rejectionTransplanted Organs
In some embodiments, a method to detect acute rejection in allograft from is described. In some embodiments, a method to anticipate an episode of acute rejection in allografts is also described. In some embodiments, a kit for detecting or predicting acute transplant rejection of a transplanted organ is described.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
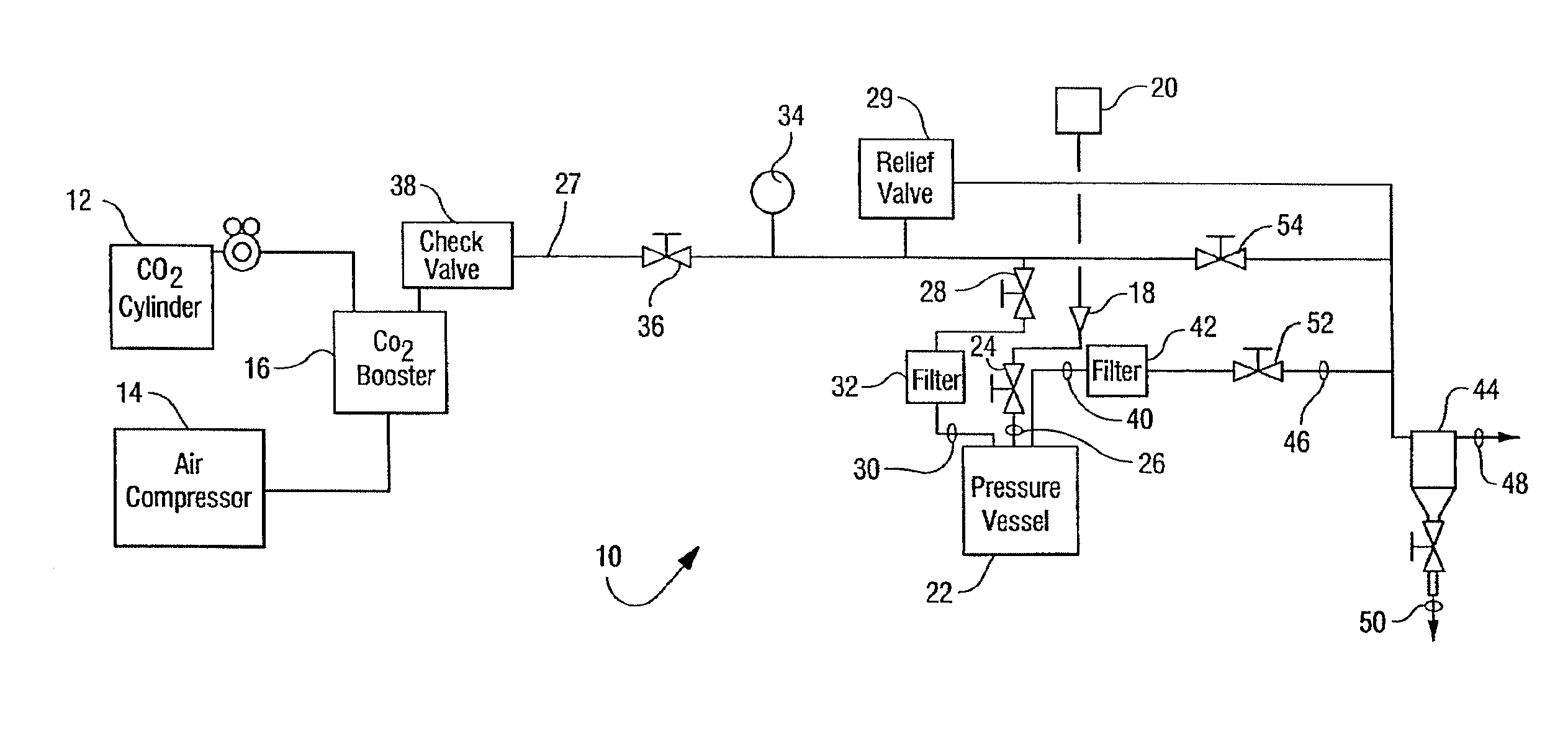
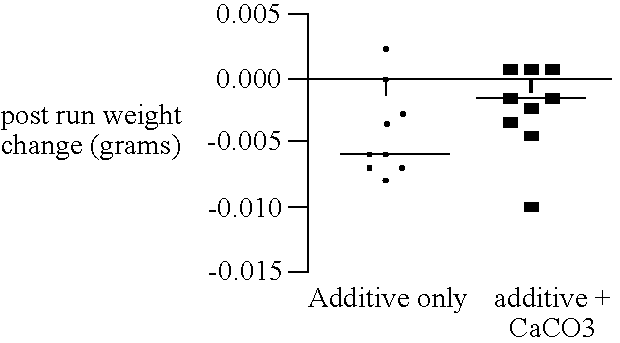
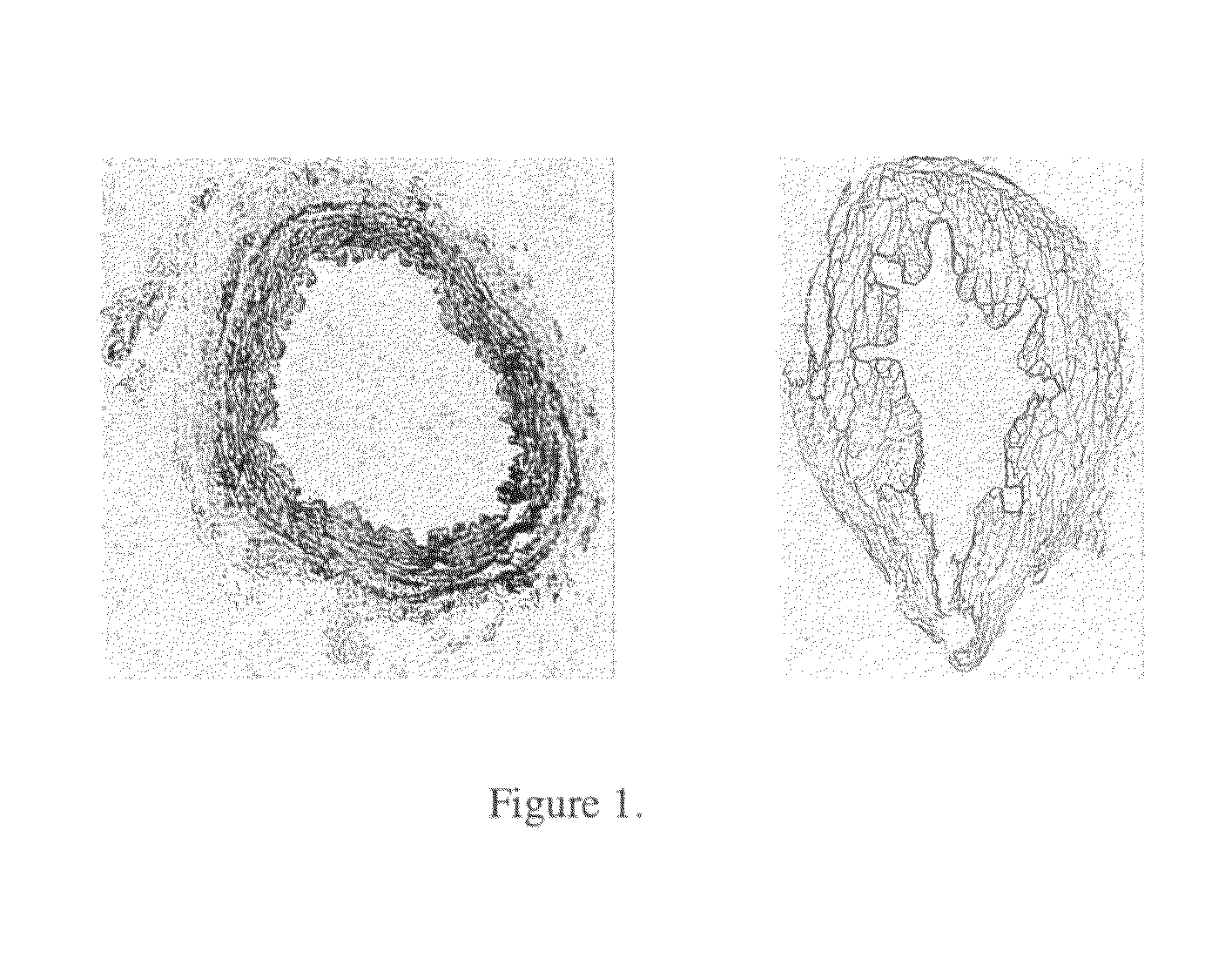
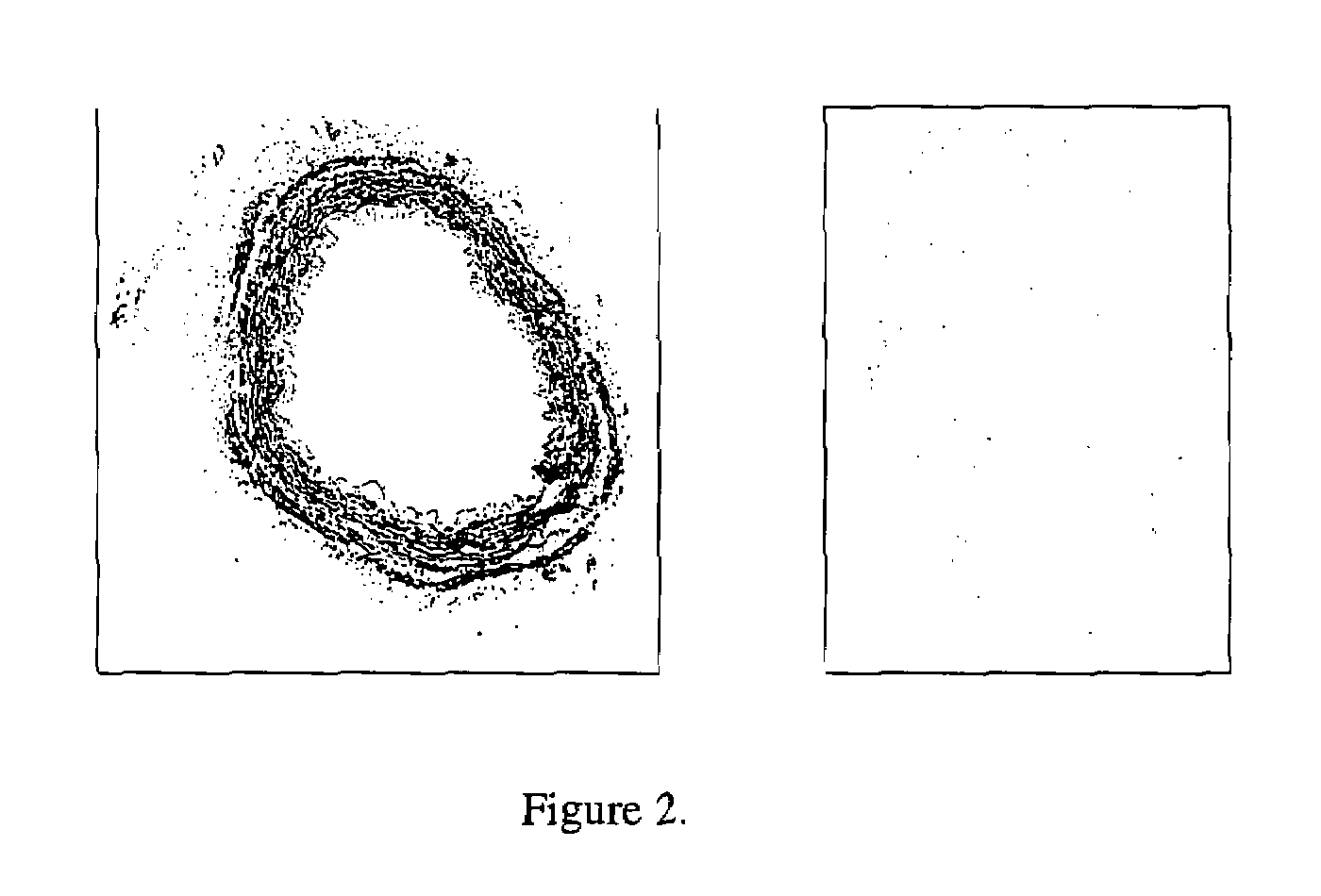
Combined use of an alkaline earth metal compound and a sterilizing agent to maintain osteoinduction properties of a demineralized bone matrix
A method is disclosed that produces allografts from matrices typically containing demineralized bone matrix (DBM) powder, demineralized bone matrix gel, demineralized bone matrix paste, bone cement, cancellous bone, or cortical bone and mixtures thereof. The matrices are sterilized utilizing supercritical CO2 in the presence of a sterilizing additive and an entrainer such as an alkaline earth metal compound, preferably CaCO3. The resultant allograft materials have a reduced rate of rejection when used in allograft procedures including, bone, cartilage, tendon, and ligament grafting procedures.
Owner:NOVASTERILIS
Vascular graft sterilization and decellularization
ActiveUS7658706B2Effective sterilization and decellularizationEffective reduction in potential harmful organismsTubular organ implantsTissue regenerationVascular tissueBlood Vessel Tissue
The present technology is related to the field of sterilization and decellularization of allografts or xenografts, specifically to processes that achieve effective removal of the cells contained within a vascular tissue matrix and an effective reduction in potential harmful organisms to create grafts suitable for human implantation. In some embodiments, vascular grafts, vascular tissue and / or blood vessels are contacted with cleaning solution under conditions suitable conditions to reduce immune reaction in patients. More specifically, the present technology is directed to sterilization and decellularization of vascular grafts.
Owner:RTI BIOLOGICS INC
Methods and compositions for allogeneic transplantation
InactiveUS7544355B2Improve efficacyImprove securityBiocideBone implantAllogeneic graftAllogeneic transplantation
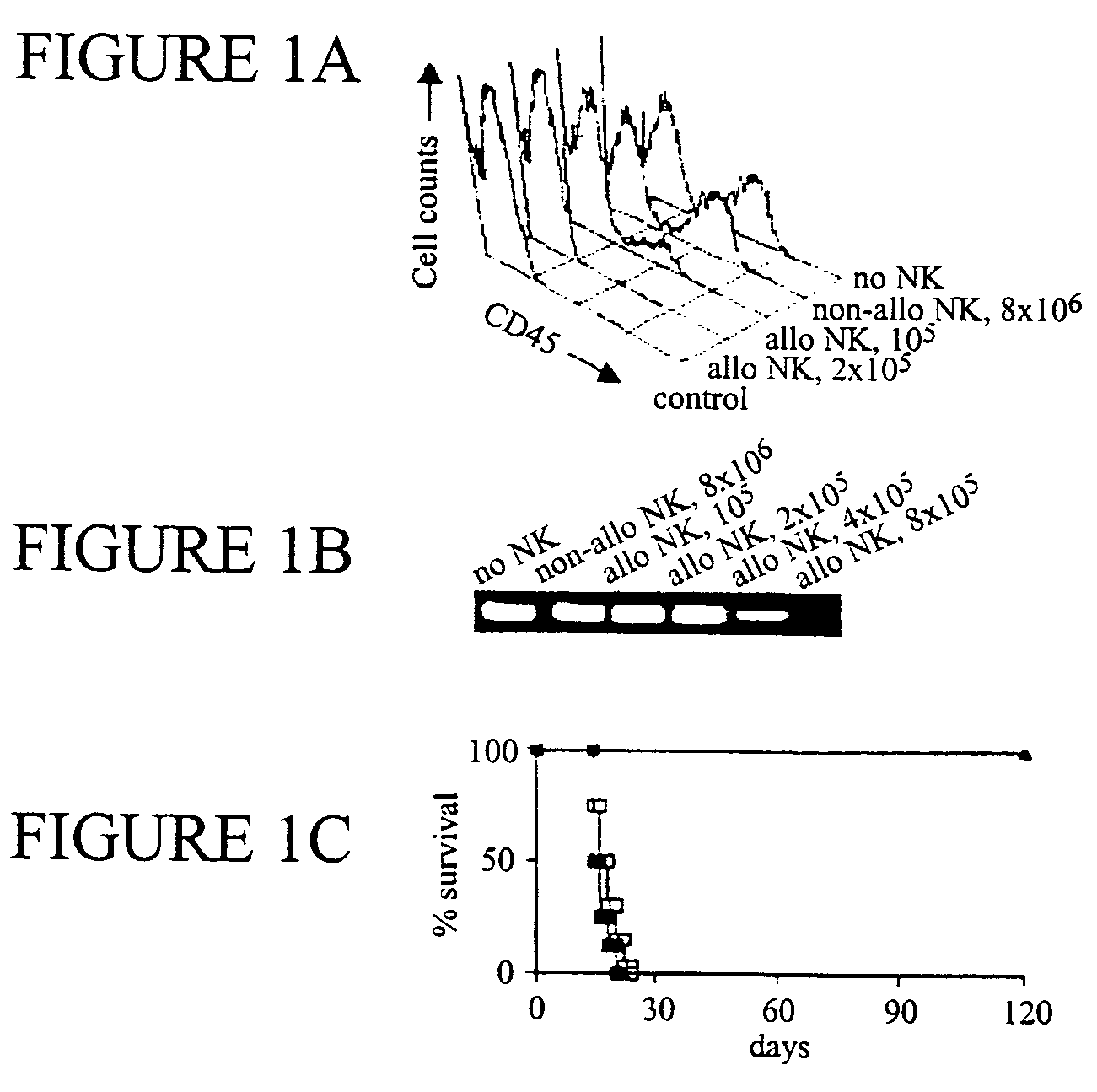
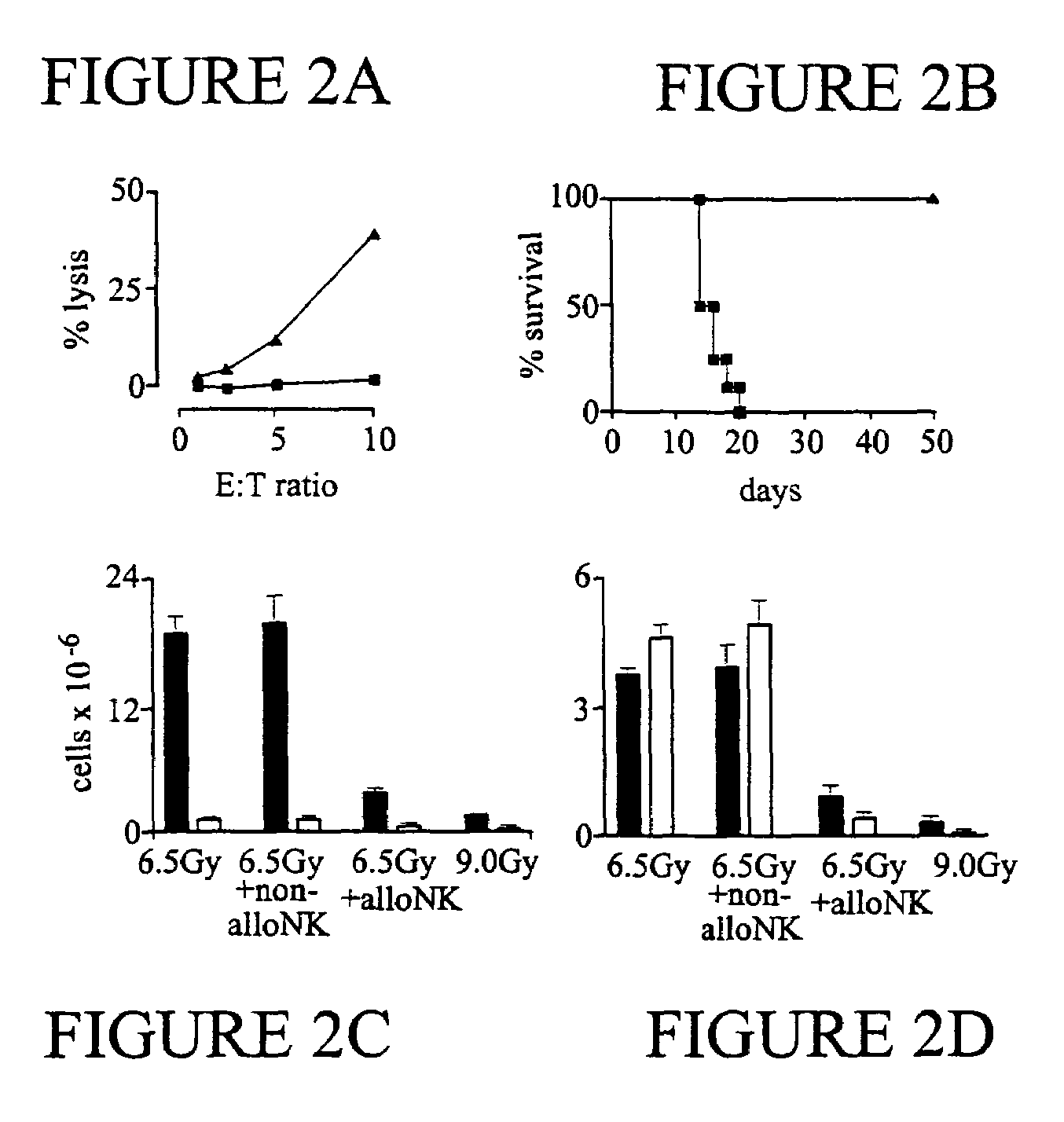
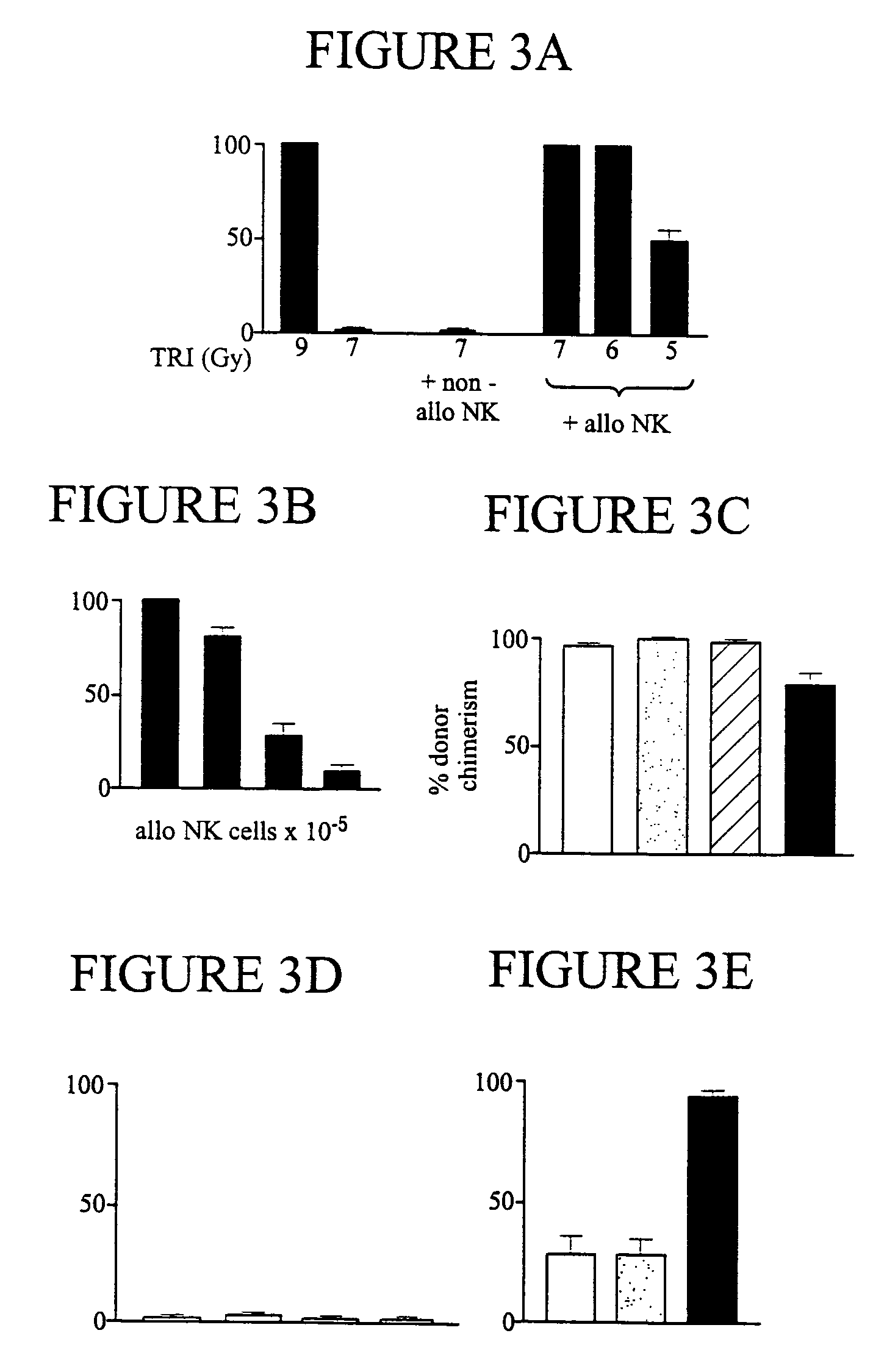
The present invention relates, generally, to methods and compositions for allogeneic transplantation. More particularly, the invention relates to the use of alloreactive natural killer cells in order to enhance the efficacy and / or safety of allogeneic grafts in human subjects. The invention allows to increase the engraftment of an allogeneic grafts, even in myelo-reductive conditioning, to protect against GVHD and / or to eradicate malignant cells. The invention is particularly suited in allogeneic hematopoietic transplantations, particularly bone marrow transplantations.
Owner:UNIV DEGLI STUDI DI PERUGIA
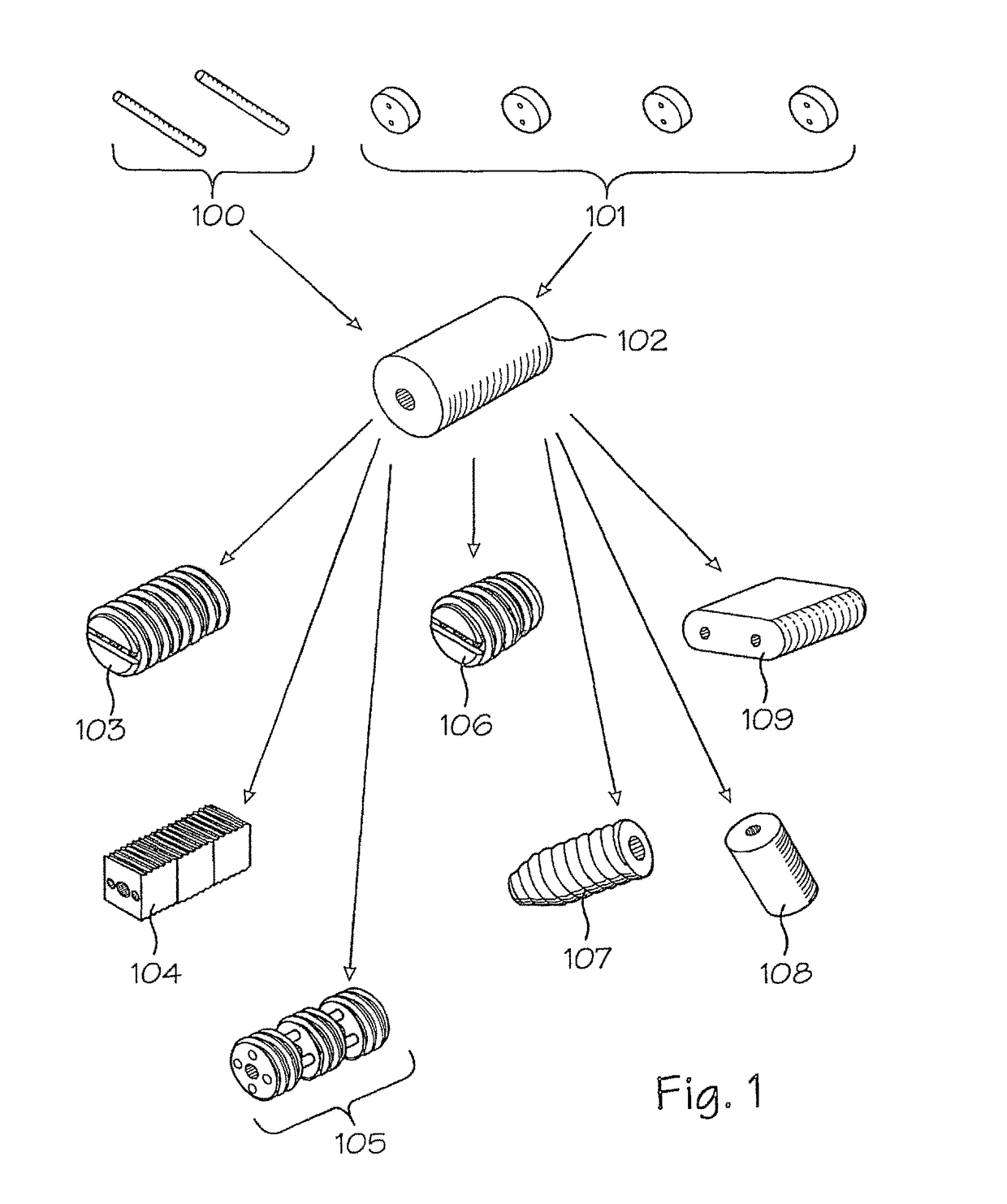
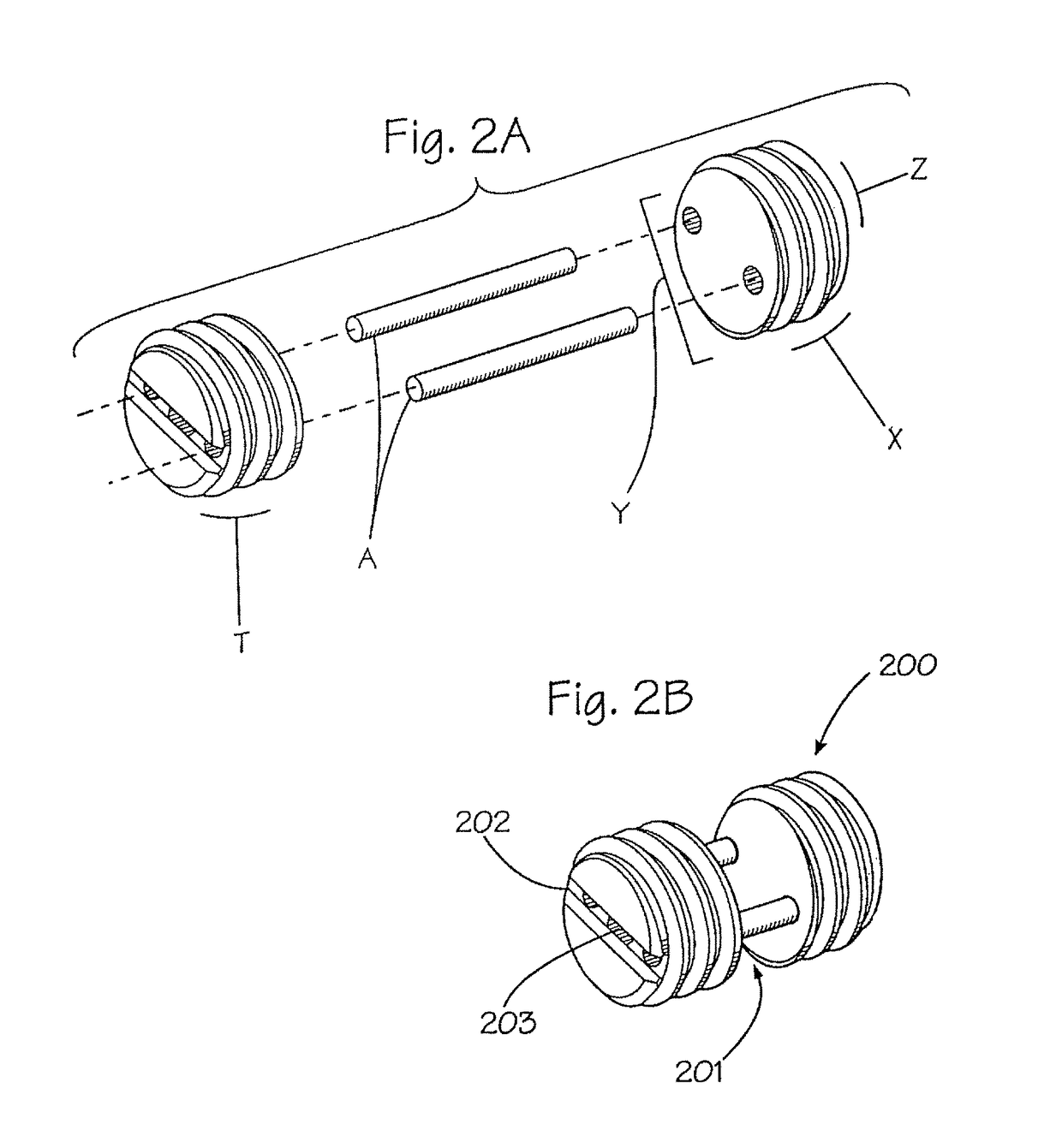
Assembled implant
This invention is directed to an assembled implant comprising two or more portions of bone that are held together in appropriate juxtaposition with one or more biocompatible pins to form a graft unit. Preferably, the pins are cortical bone pins. Typically, the cortical pins are press-fitted into appropriately sized holes in the bone portions to achieve an interference fit. The bone portions are allograft or xenograft.
Owner:RTI BIOLOGICS INC
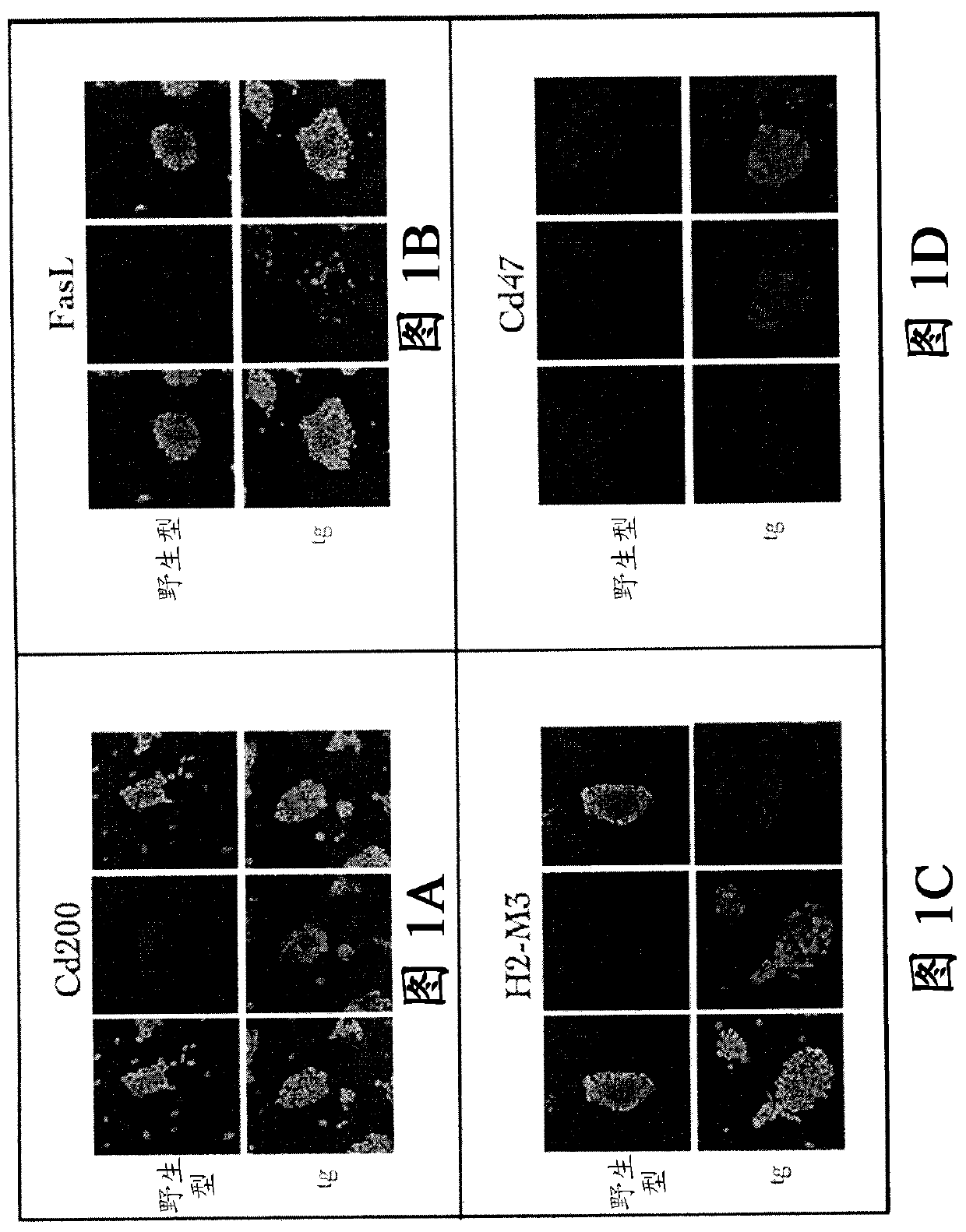
Allograft tolerance without the need for systemic immune suppression
A cell genetically modified to comprise at least one mechanism for providing a local immunosuppression at a transplant site when transplanted in an allogeneic host, and methods for making and using the same are provided. The cell comprises a set of transgenes, each transgene encoding a gene product that is cytoplasmic, membrane bound, or local acting, and whose function is one or more of: to mitigate antigen presenting cell activation and function; to mitigate graft attacking leukocyte activity or cytolytic function; to mitigate macrophage cytolytic function and phagocytosis of allograft cells; to induce apoptosis in graft attacking leukocytes; to mitigate local inflammatory proteins; and to protect against leukocyte-mediated apoptosis. The transgenes comprise two or more of the followinggenes: PD-L 1, HLAG or H2-M3, Cd47, Cd200, FASLG or Fasl, Ccl21 or Ccl21b, Mfge8, and Serpin B9 or Spi6.
Owner:SINAI HEALTH SYST
Generation of antigen specific T suppressor cells for treatment of rejection
InactiveUS20050250161A1Preventing autoimmune diseaseReduce riskGenetically modified cellsMicrobiological testing/measurementDiseaseHeterografts
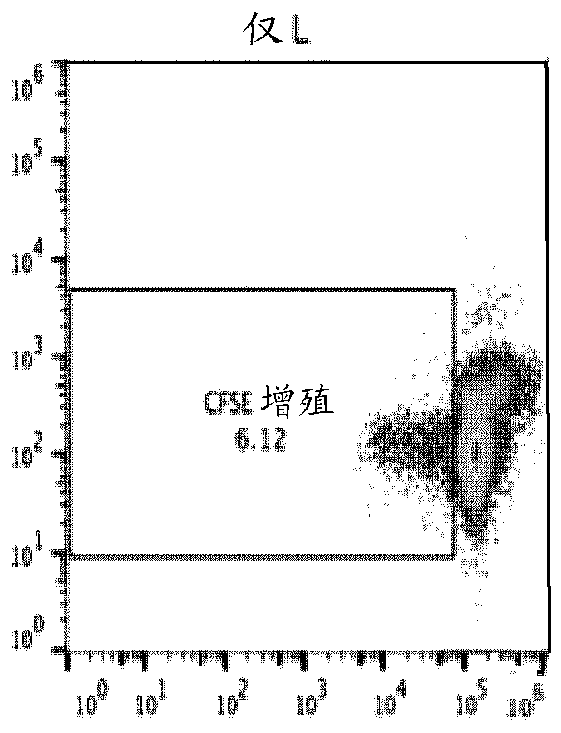
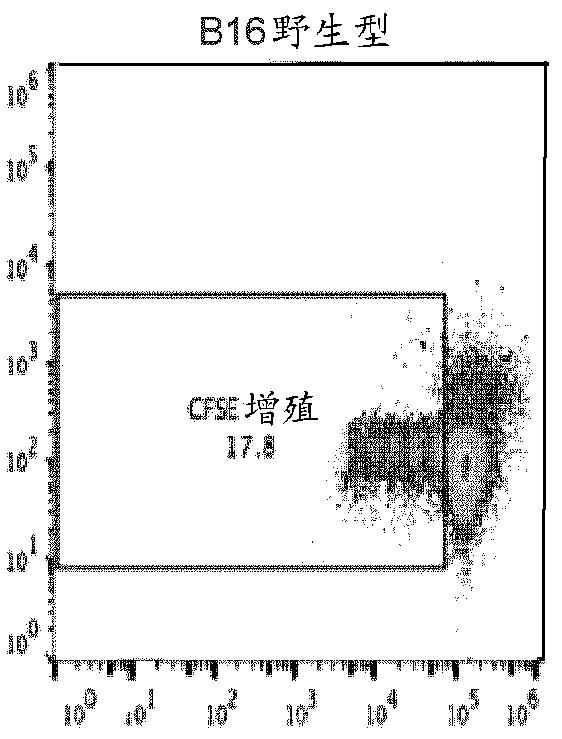
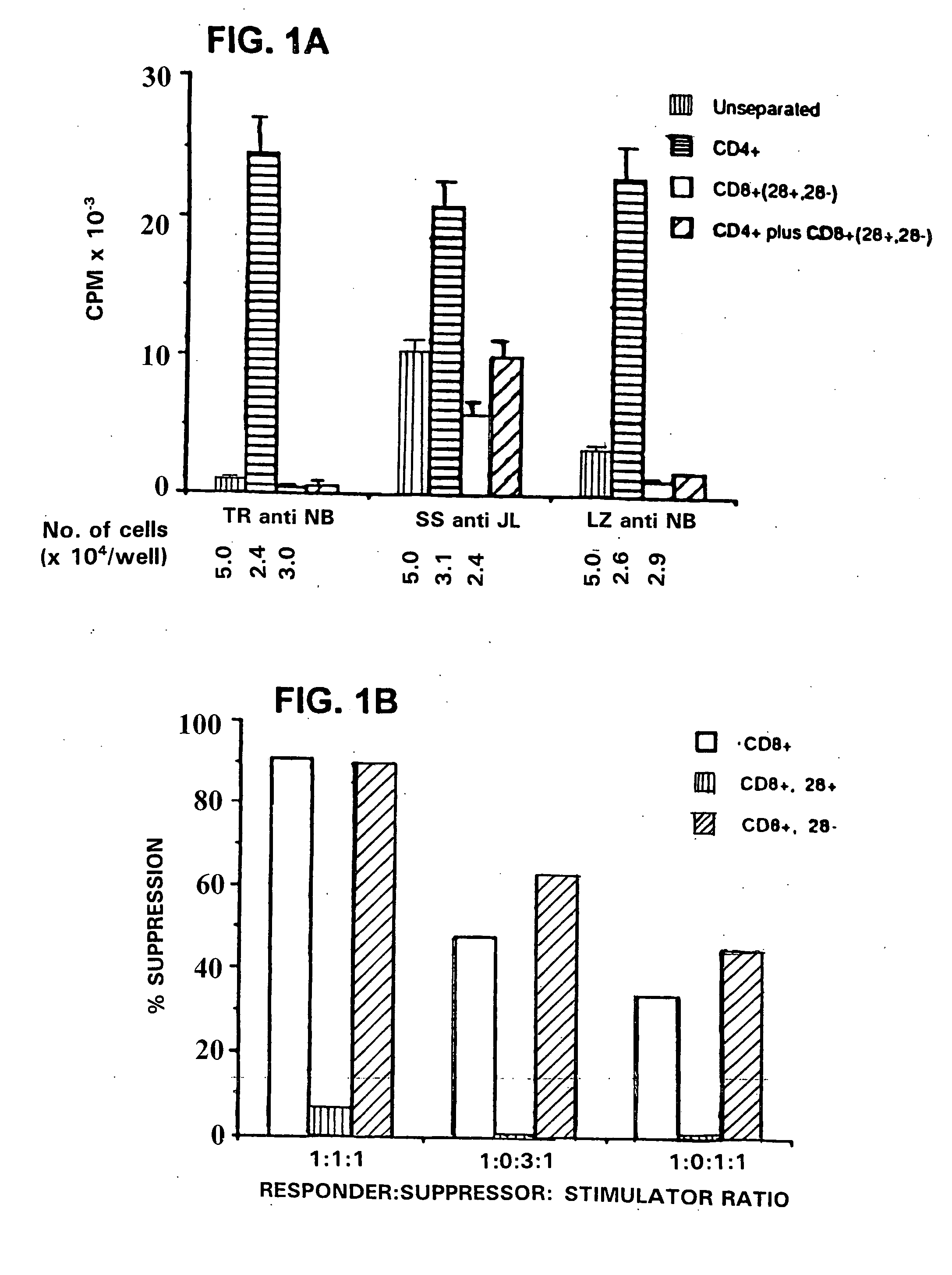
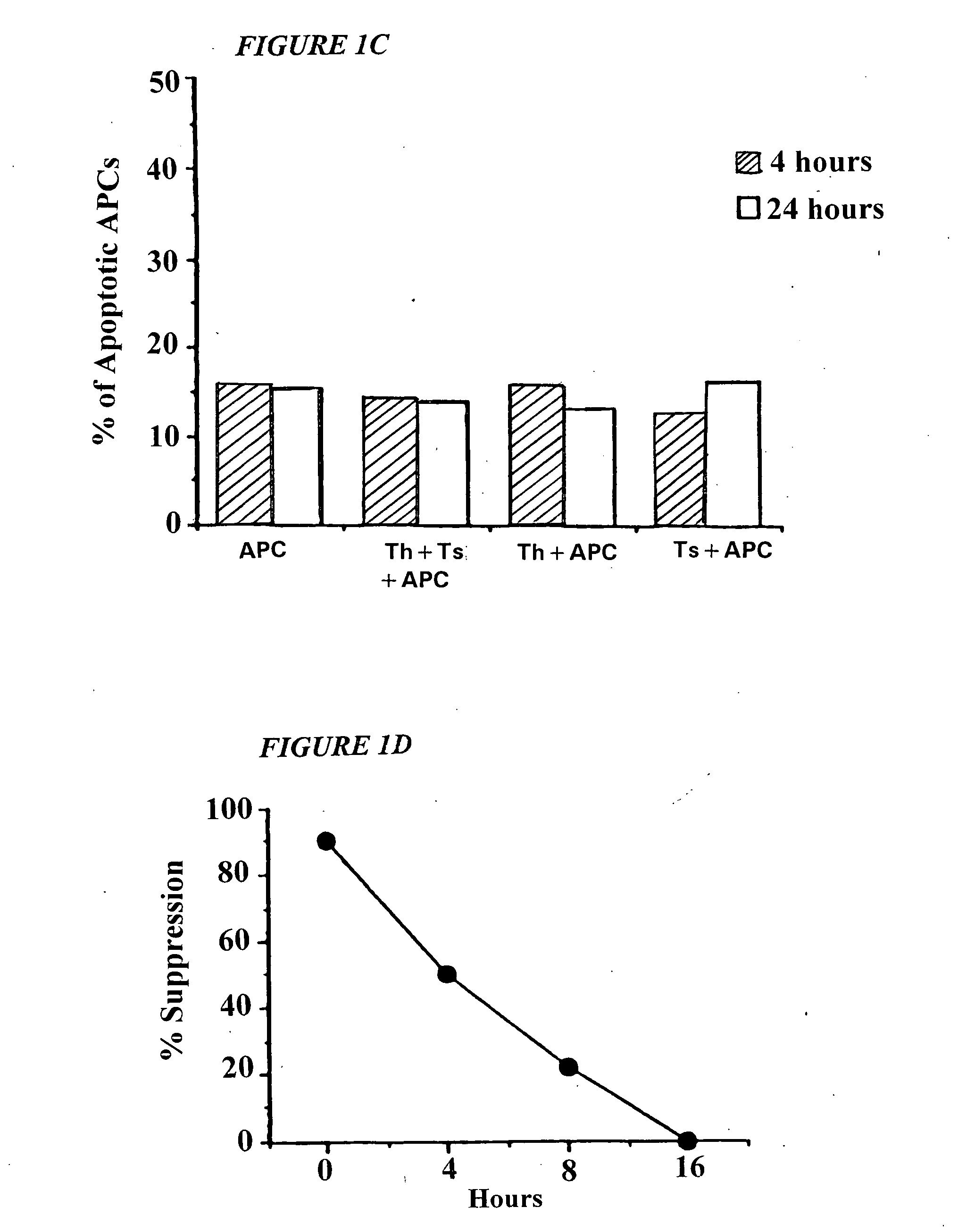
This invention provides a method of generating antigen specific allospecific human-suppressor CD8+CD28− T cells. This invention also provides a method of generating xenospecific human suppressor CD8+CD28− T cells. This invention further provides a method of generating allopeptide antigen specific human suppressor CD8+CD28− T cells. Methods of treatment for, reduction of risk of rejection of allografts and xenografts and autoimmune diseases using the human suppressor CD8+CD28− T cells so produced are also provides, as are methods of preventing rejection and autoimmune diseases, and vaccines comprising the produced suppressor T cells. Methods of diagnosis to determine whether a level of immuno-suppressant therapy requires a reduction are provided.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Application of TIGIT-ECD recombinant protein in resisting allogeneic immunological rejection
InactiveCN108948182ARegulation PolarizationHelps induce toleranceCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTIGITMacrophage polarization
The invention provides an application of a TIGIT-ECD recombinant protein in resisting allogeneic immunological rejection. The amino acid sequence of the recombinant protein TIGIT-ECD is shown as SEQ ID NO:1. The nucleotide sequence coding the recombinant protein TIGIT-ECD is shown as SEQ ID NO:2. The recombinant protein is capable of effectively regulating macrophage polarization by activating a TIGIT-CD155 signal path, and contributes to inducing allogeneic graft tolerance. Therefore, the recombinant protein TIGIT-ECD can serve as a graft rejective reaction inhibitor, and can also be appliedto preparing drugs for preventing and treating the graft rejective reaction.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Agonistic Anti-tumor necrosis factor receptor 2 antibodies
InactiveUS20190135929A1Improve the immunityImprove solubilityPeptide/protein ingredientsBiological material analysisImmunologic disordersRegulatory T cell
The invention provides agonistic TNFR2 antibodies and antigen-binding fragments thereof and encompasses the use of these antibodies as therapeutics to promote the proliferation of regulatory T cells (T-reg) for the treatment of immunological diseases. Antibodies of the invention can be used to potentiate the T-reg-mediated deactivation of self- and allergen-reactive T- and B-eases. Antibodies and can thus be used to treat a wide variety of indications, including autoimmune diseases, allergic reactions, asthma, graft-versus-host disease, and allograft rejection, among others.
Owner:THE GENERAL HOSPITAL CORP
Instrumentation and method for repair of meniscus tissue
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
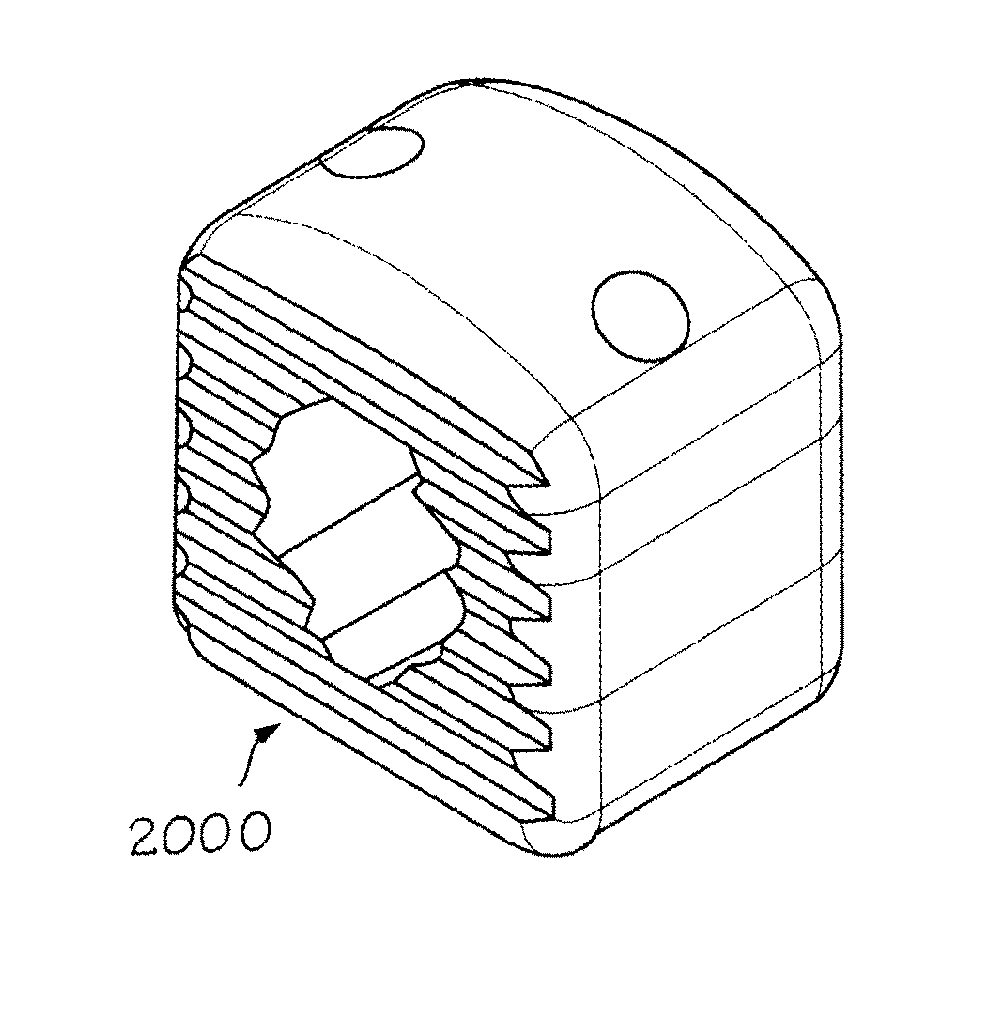
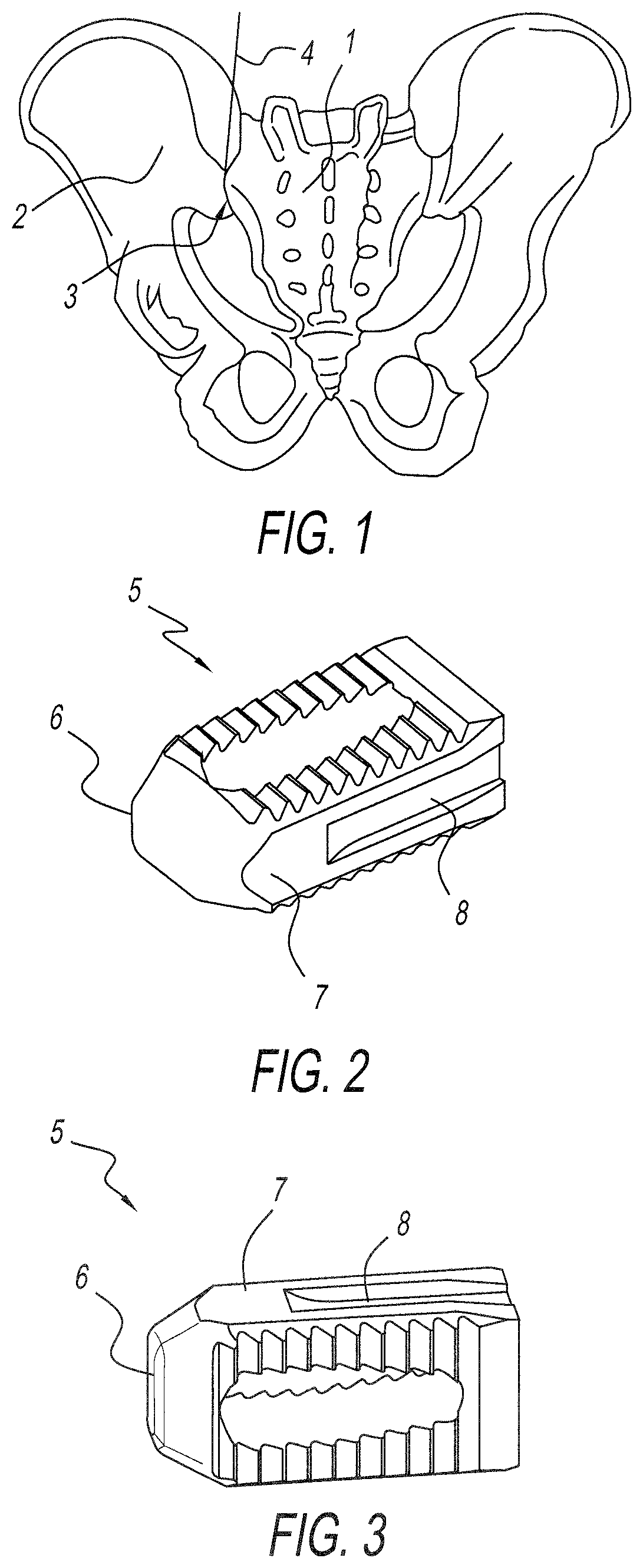
Allograft implant for fusing a sacroiliac joint
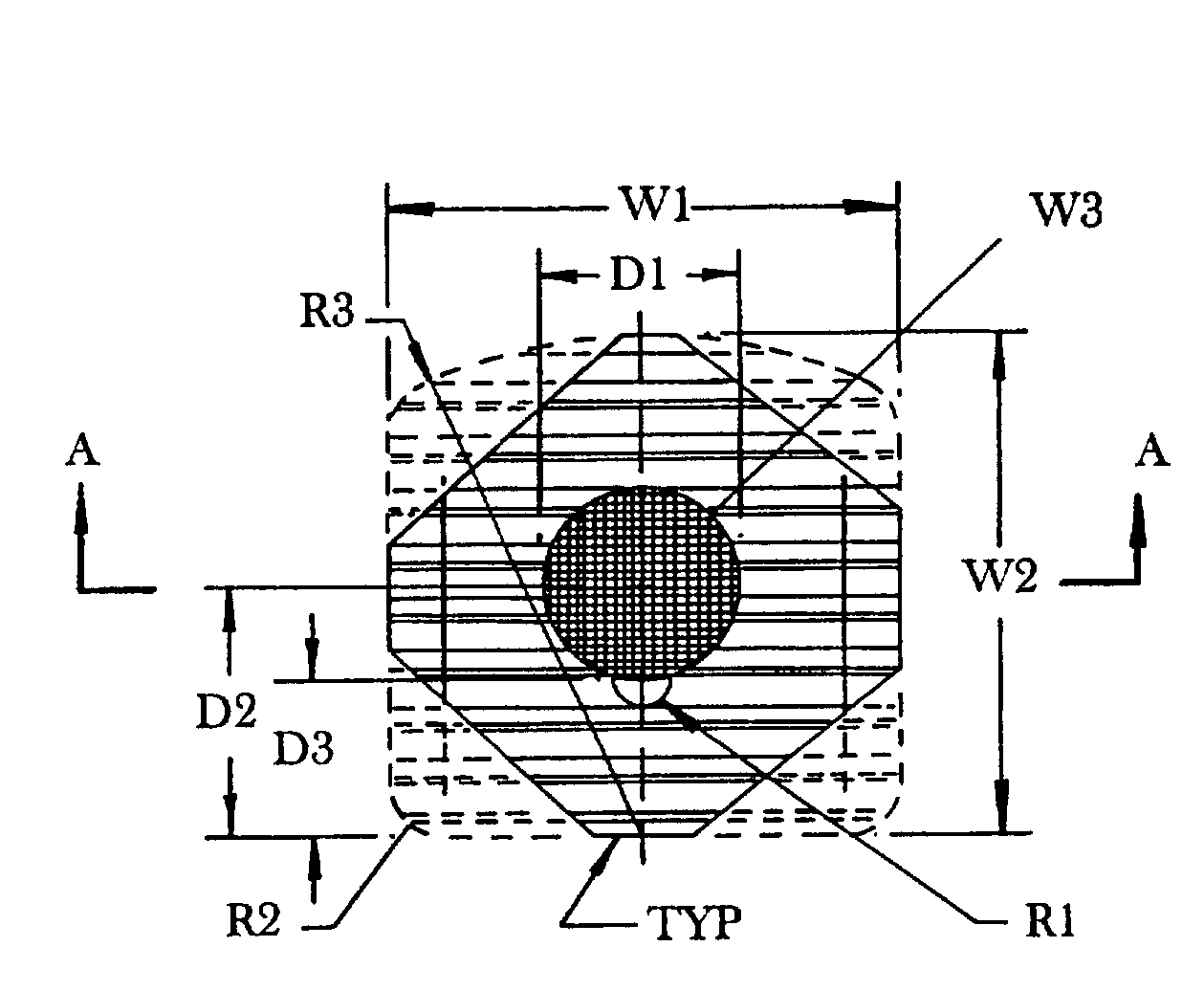
An allograft implant for fusing a sacroiliac joint (“SI Joint”), the allograft implant comprising a body having two opposing faces. Each face has one or more anti-migration features, and each face is configured for abutting contact with a sacrum or an ilium of the SI Joint. A graft window is disposed between the two opposing faces, the graft window providing passage through the body between the two opposing faces. The graft window has a cross-sectional area that is about 35% to about 40% of a first cross-sectional area of the body, and about 60% of an area of each opposing face that makes contact with the sacrum or the ilium of the SI Joint.
Method of using amnion allograft in congenital heart disease surgery
Improved methods, compositions and kits for congenital heart disease surgeries are described. The methods utilize amniotic fluid and / or an allograft comprising a layer of amnion to improve the performance and reduce complications of the surgeries and the allograft has a pre-made size and shape suitable for the application.
Owner:LIVENTA BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com