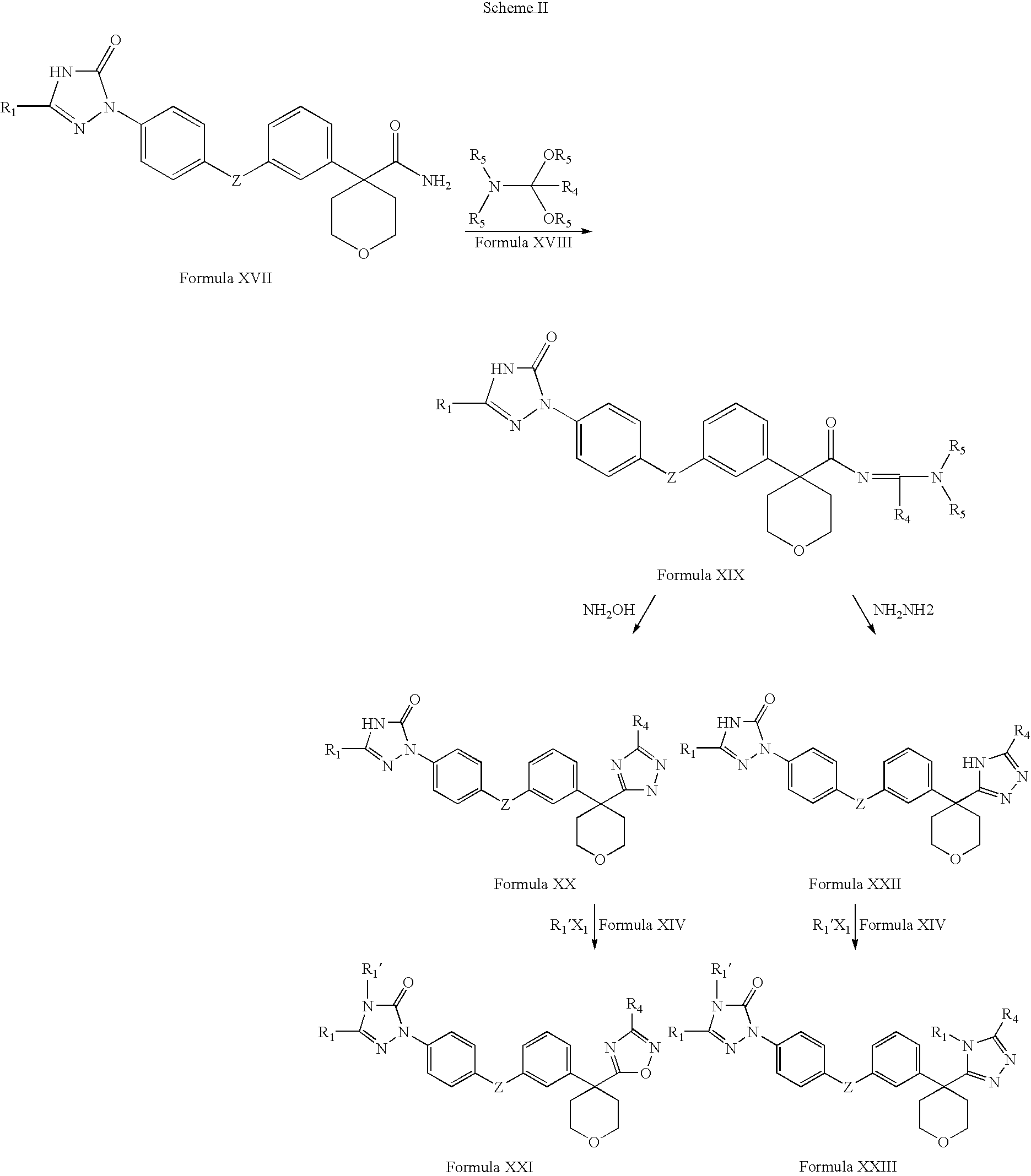Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
86 results about "Allograft rejection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allograft rejection. Also found in: Dictionary, Thesaurus, Encyclopedia. the rejection of tissue transplanted between two genetically different individuals of the same species. The rejection is caused by T lymphocytes responding to the foreign major histocompatibility complex of the graft.
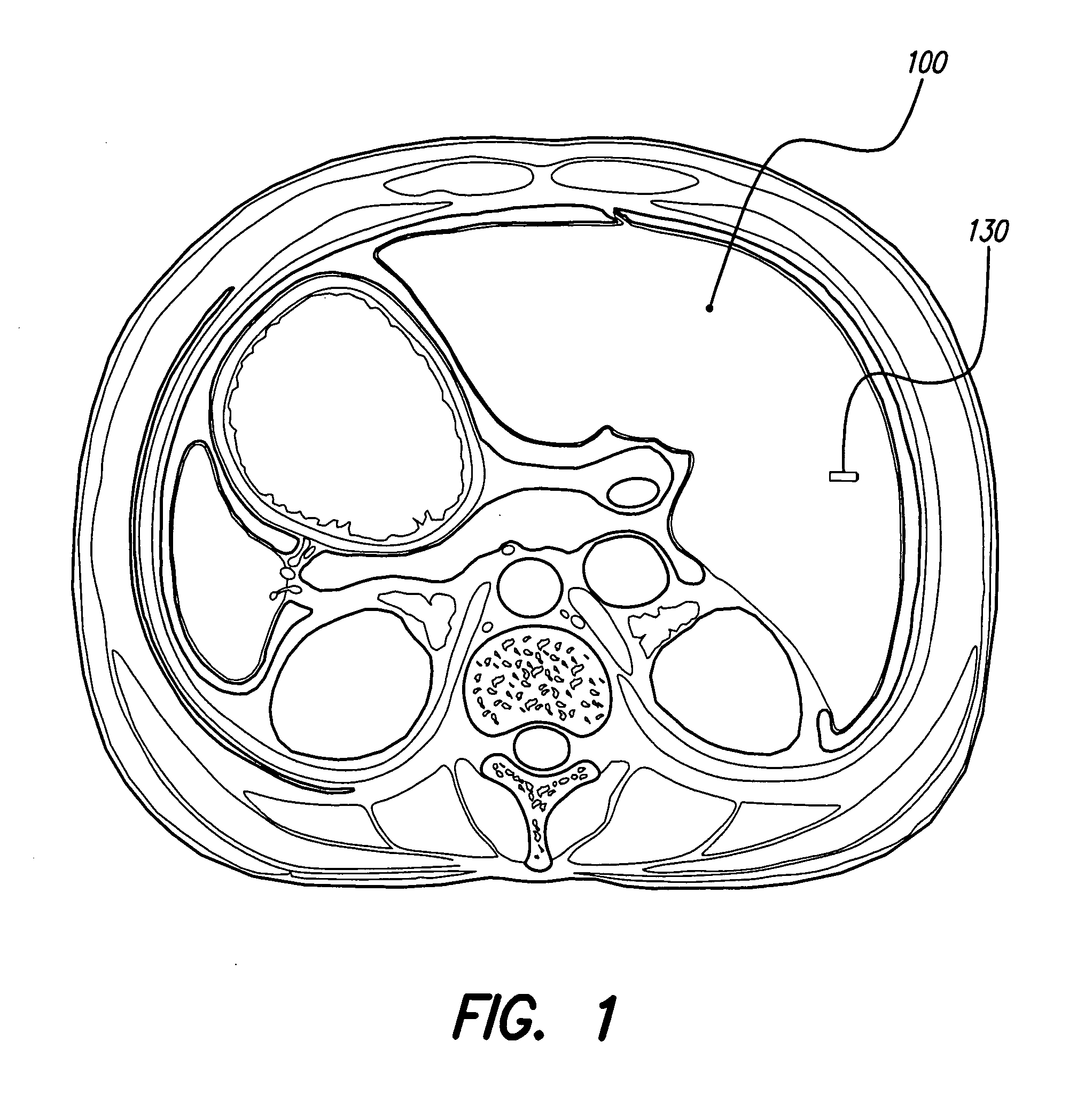
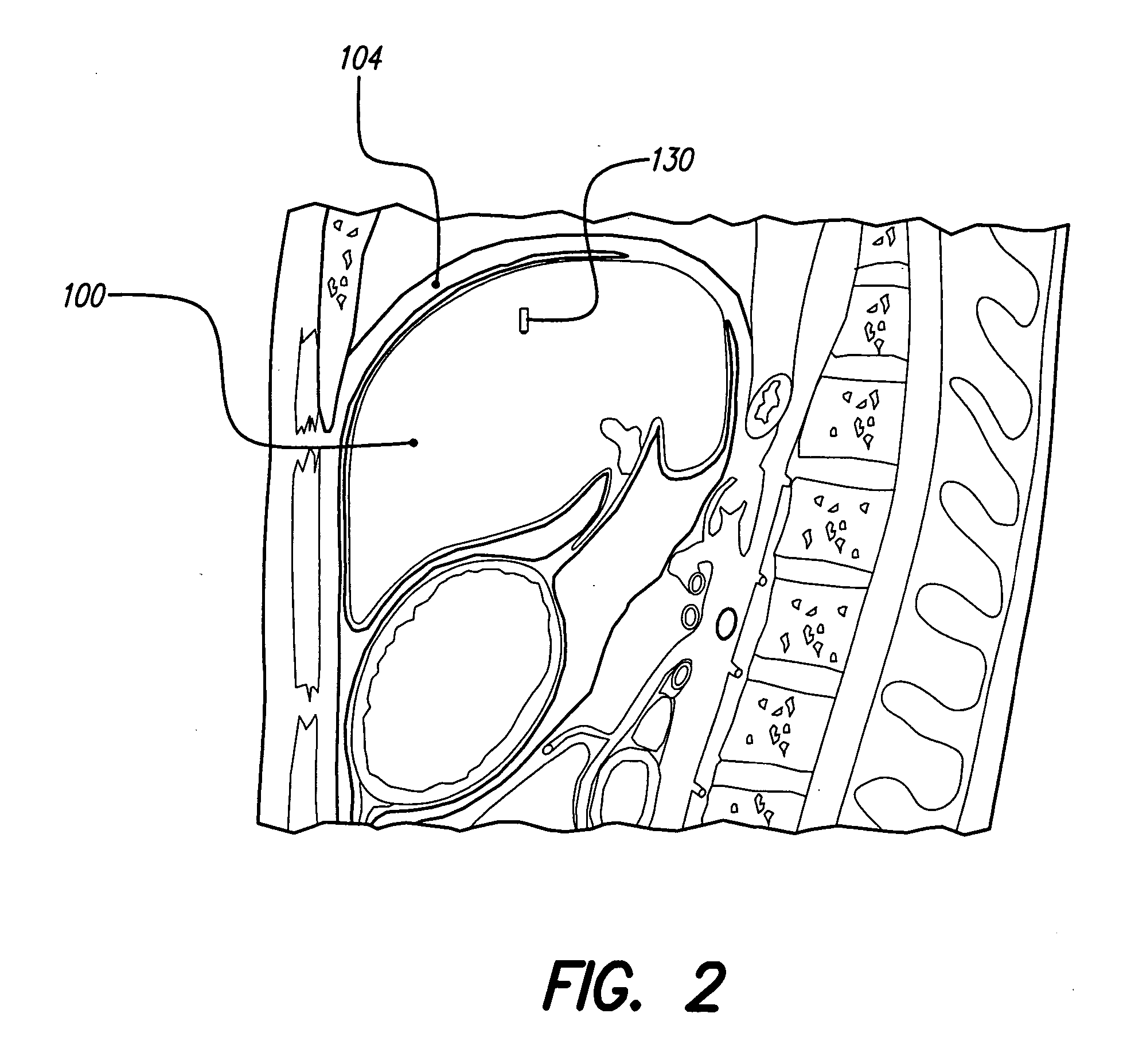
Monitoring, preventing, and treating rejection of transplanted organs
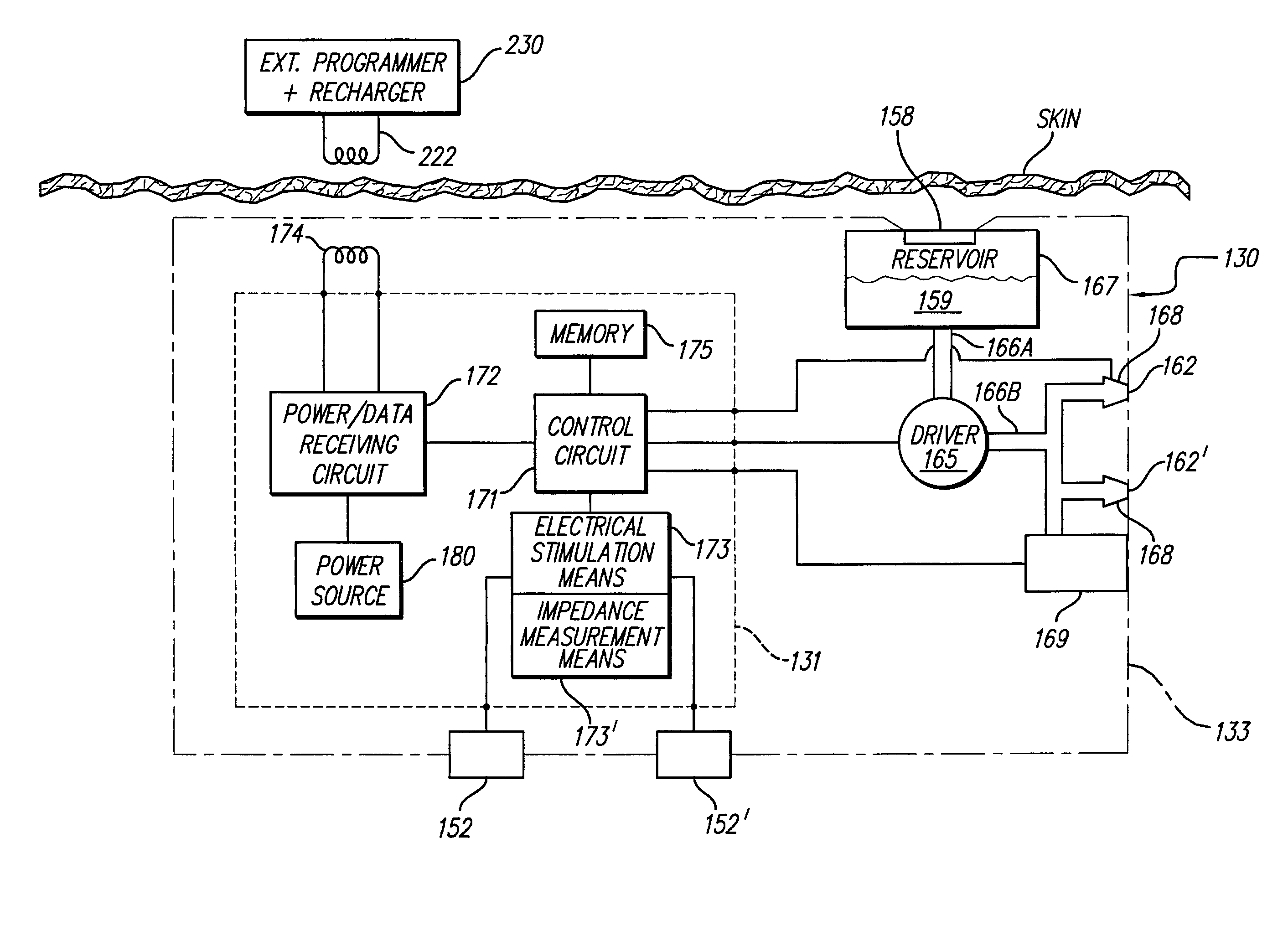
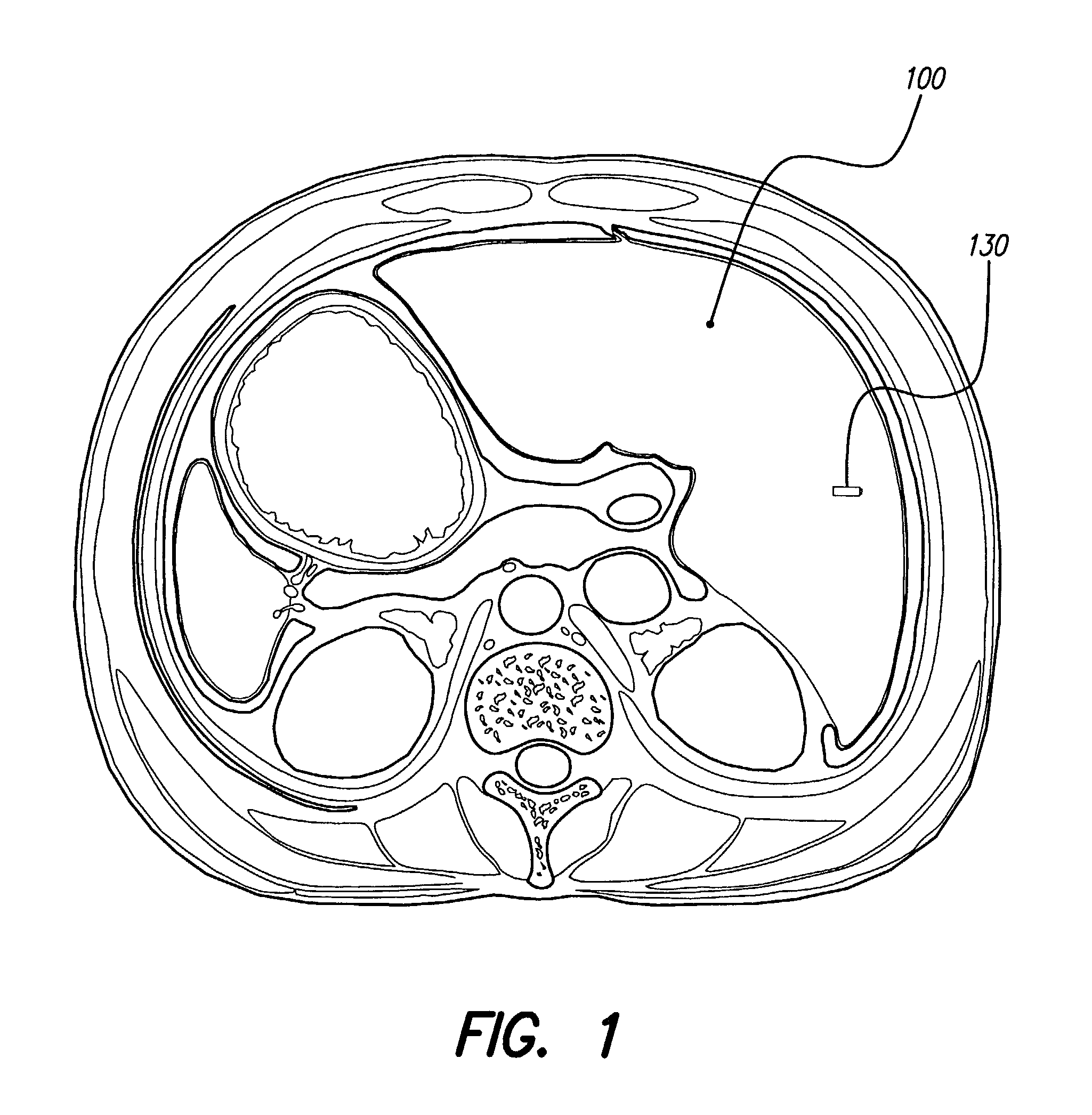
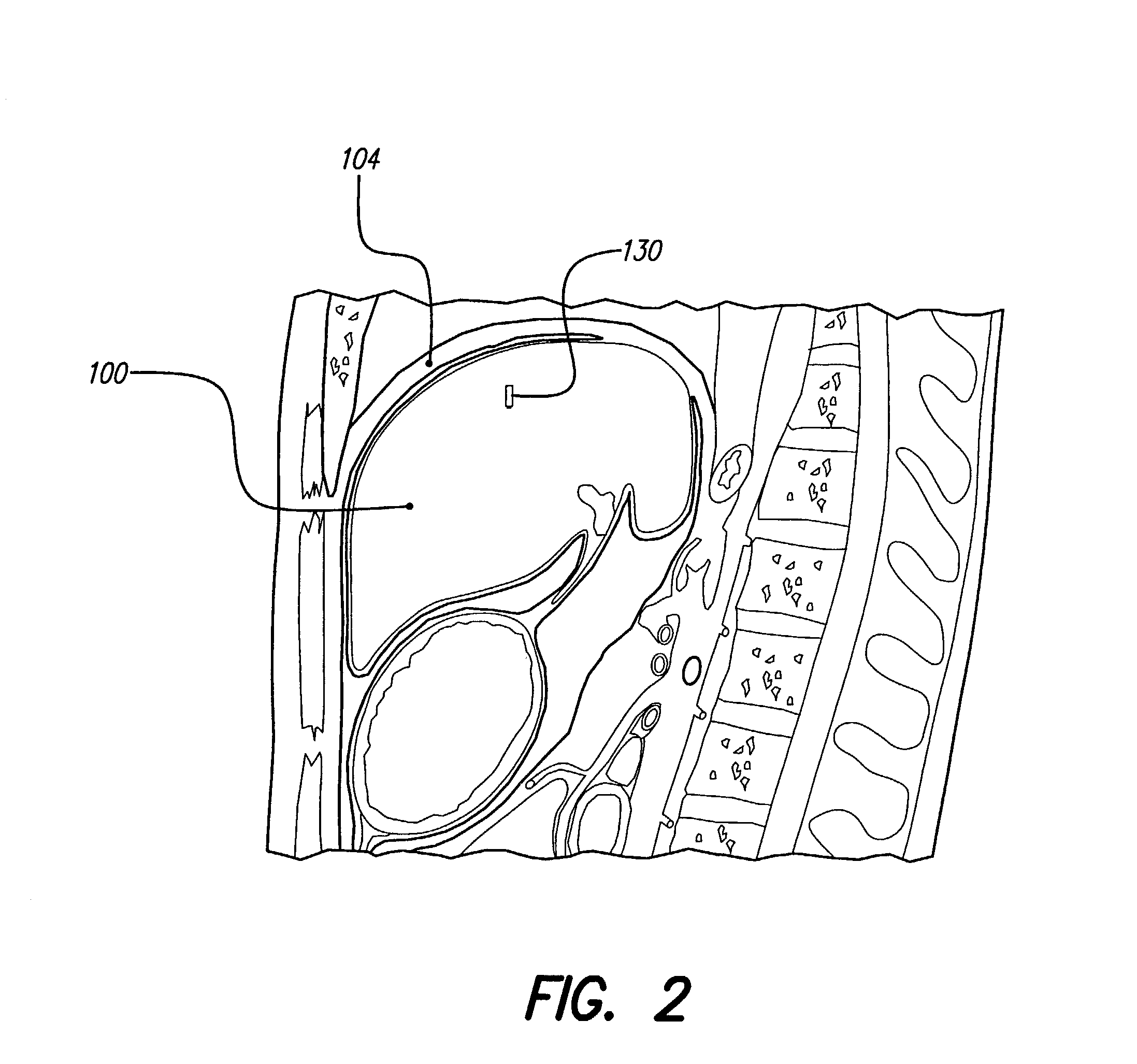
An implantable system control unit (SCU) includes means for measuring tissue impedance or other condition to determine allograft health, in order to predict or detect allograft rejection. The SCU also includes at least two electrodes coupled to means for delivering electrical stimulation to a patient within whom the device is implanted, and may also include a reservoir for holding one or more drugs and a driver means for delivering the drug(s) to the patient. In certain embodiments, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters. Alternatively, this sensory “SCU” sounds an alarm, communicates an alarm to an external device, and / or is responsive to queries regarding sensed information, such as tissue impedance.
Owner:BOSTON SCI NEUROMODULATION CORP
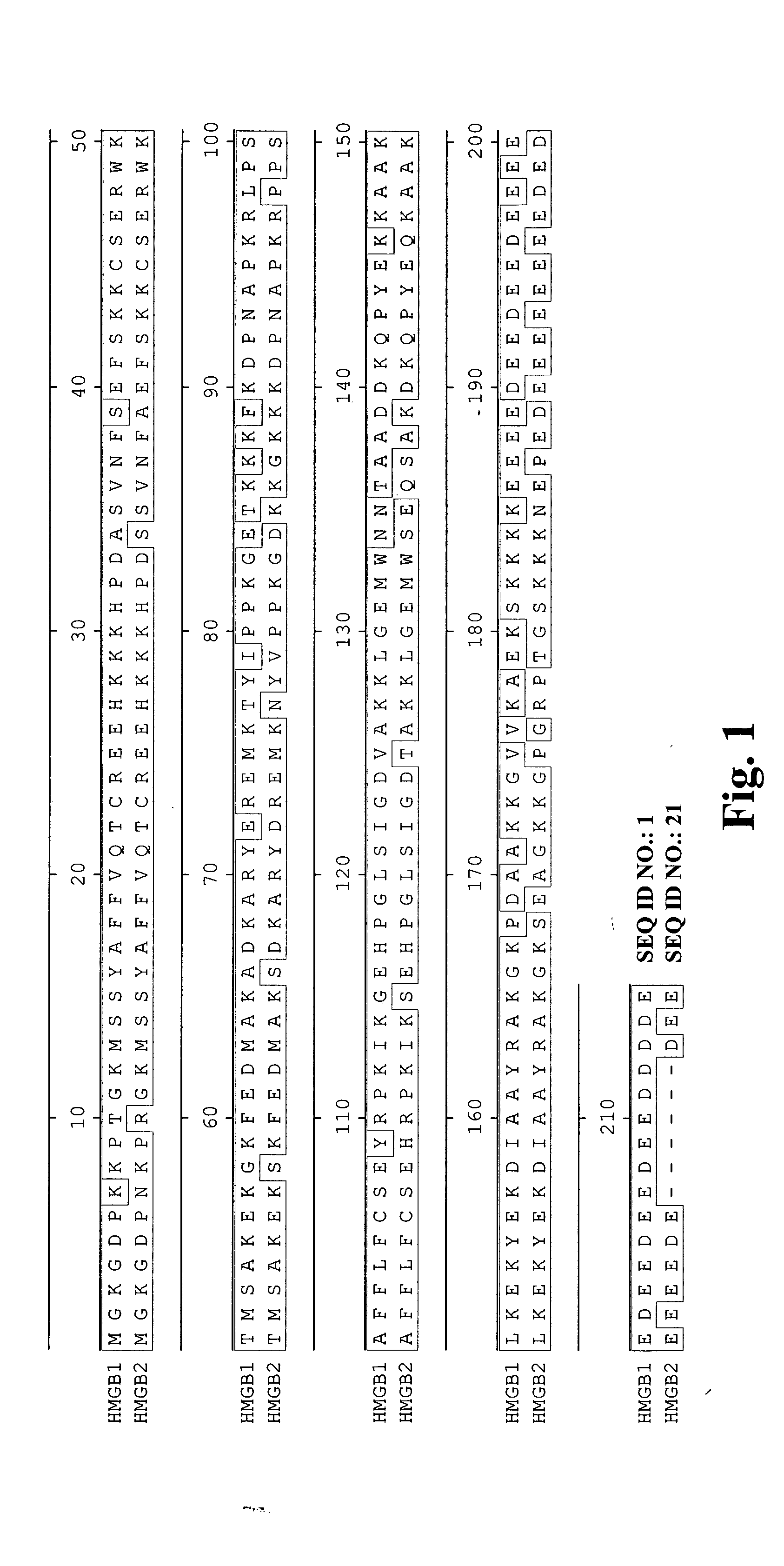
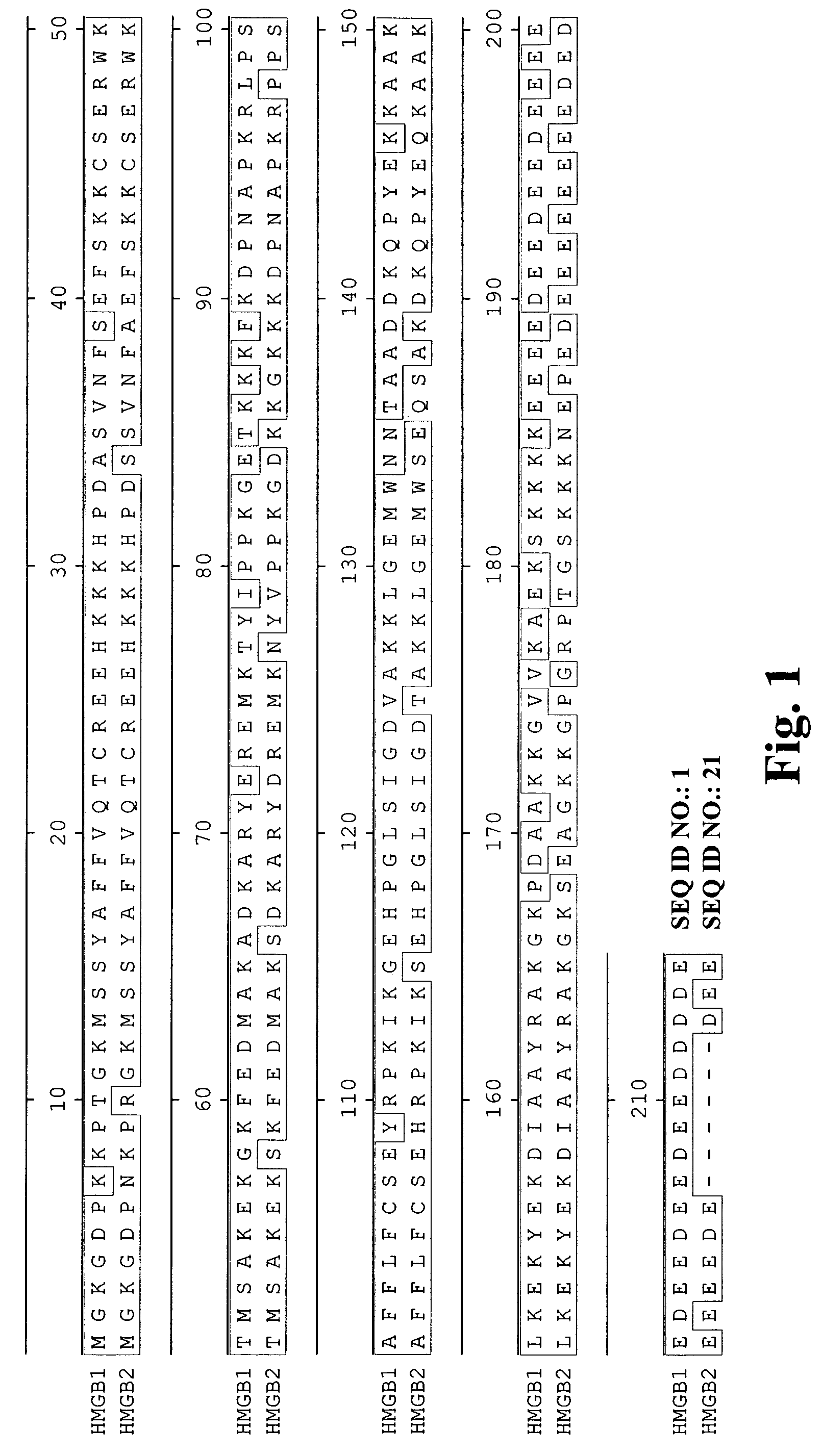
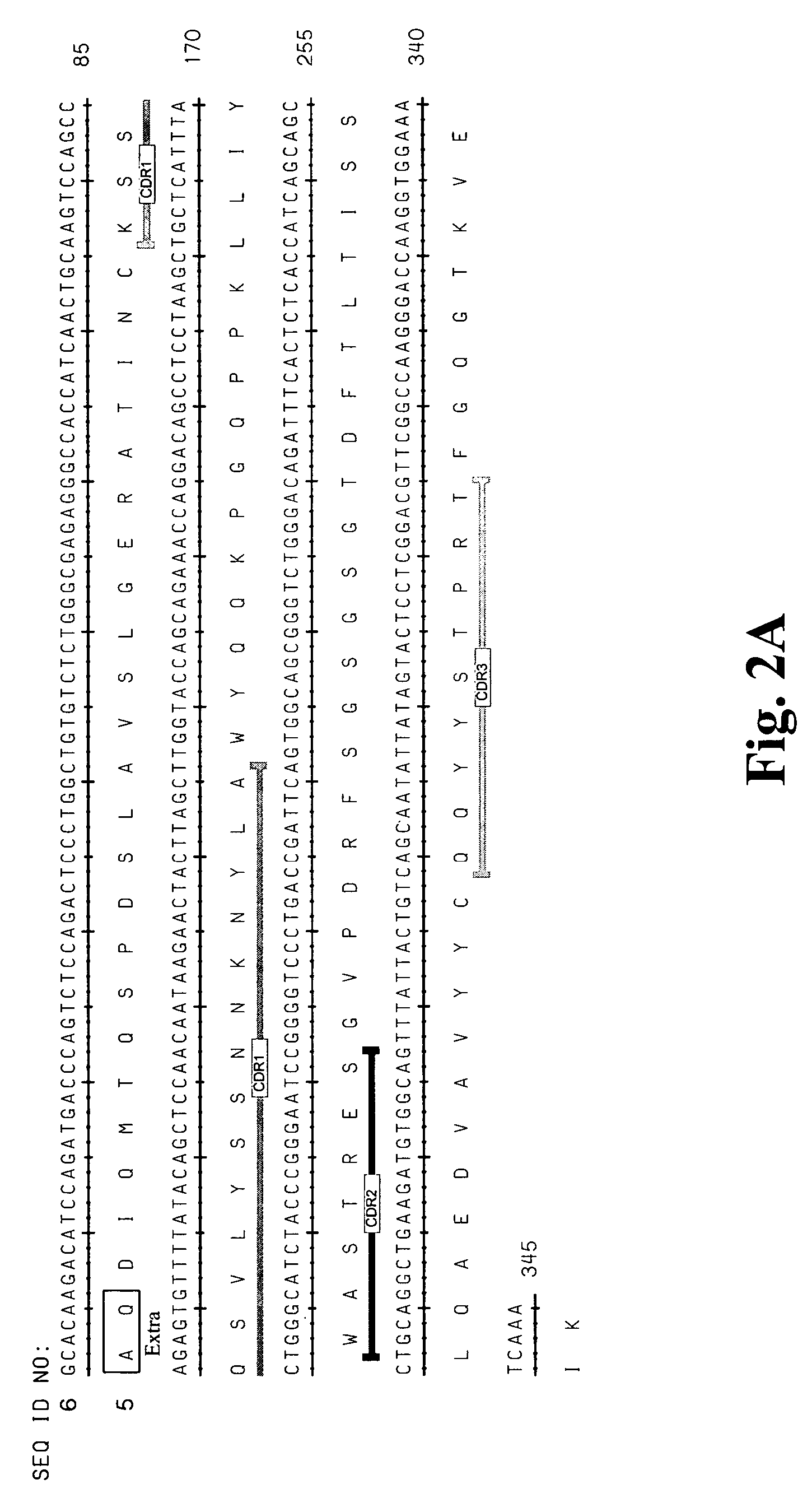
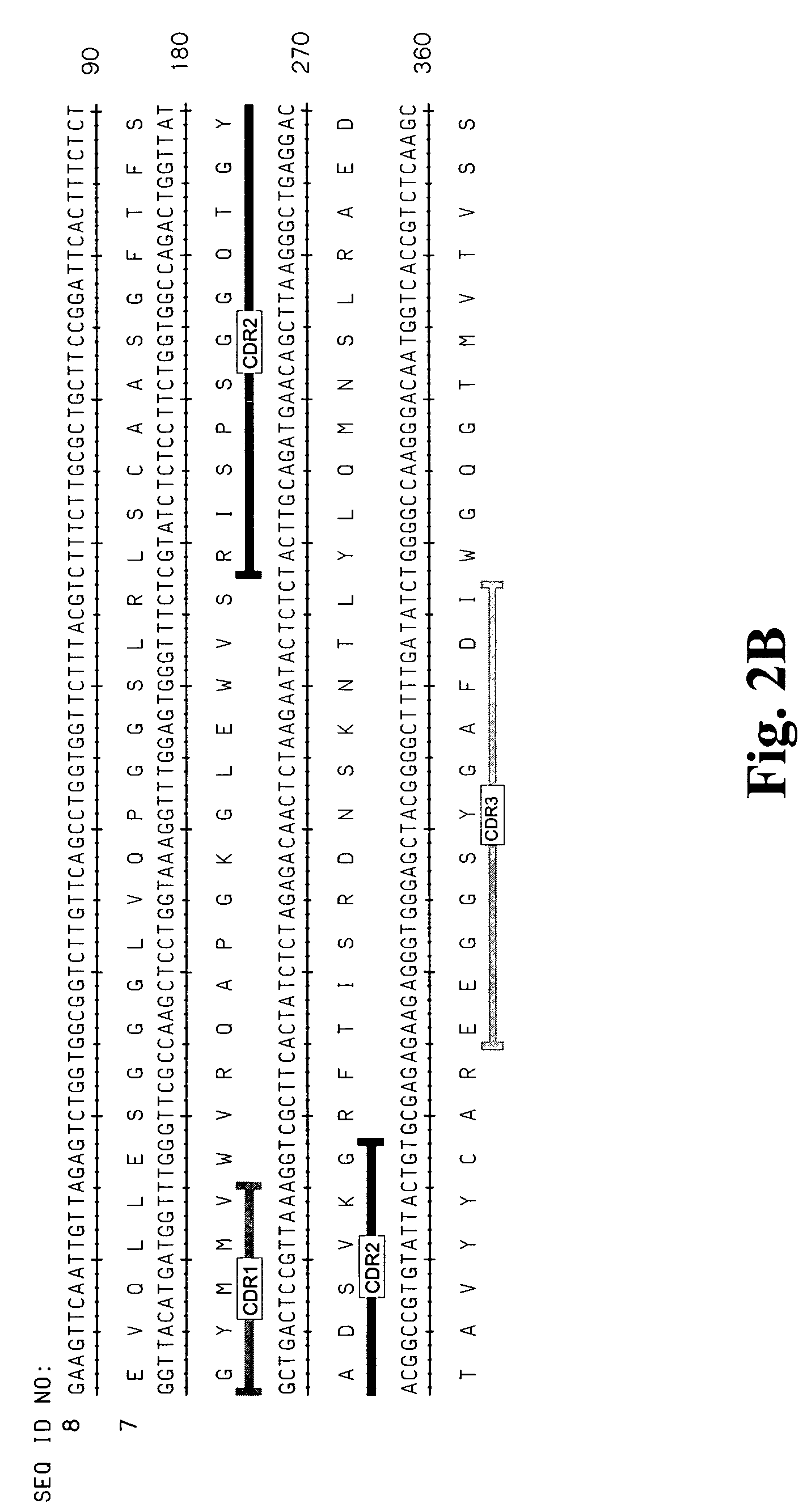
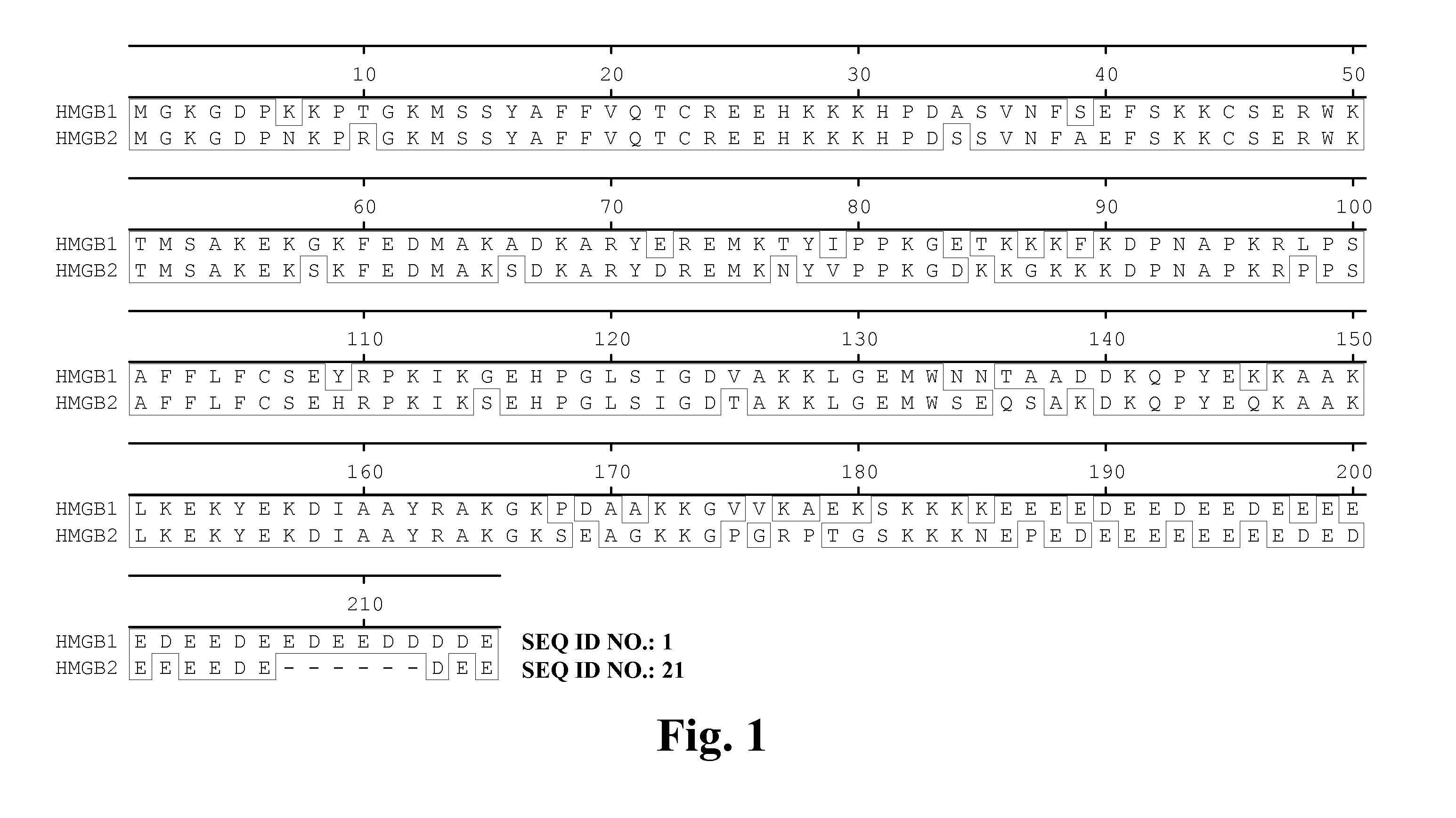
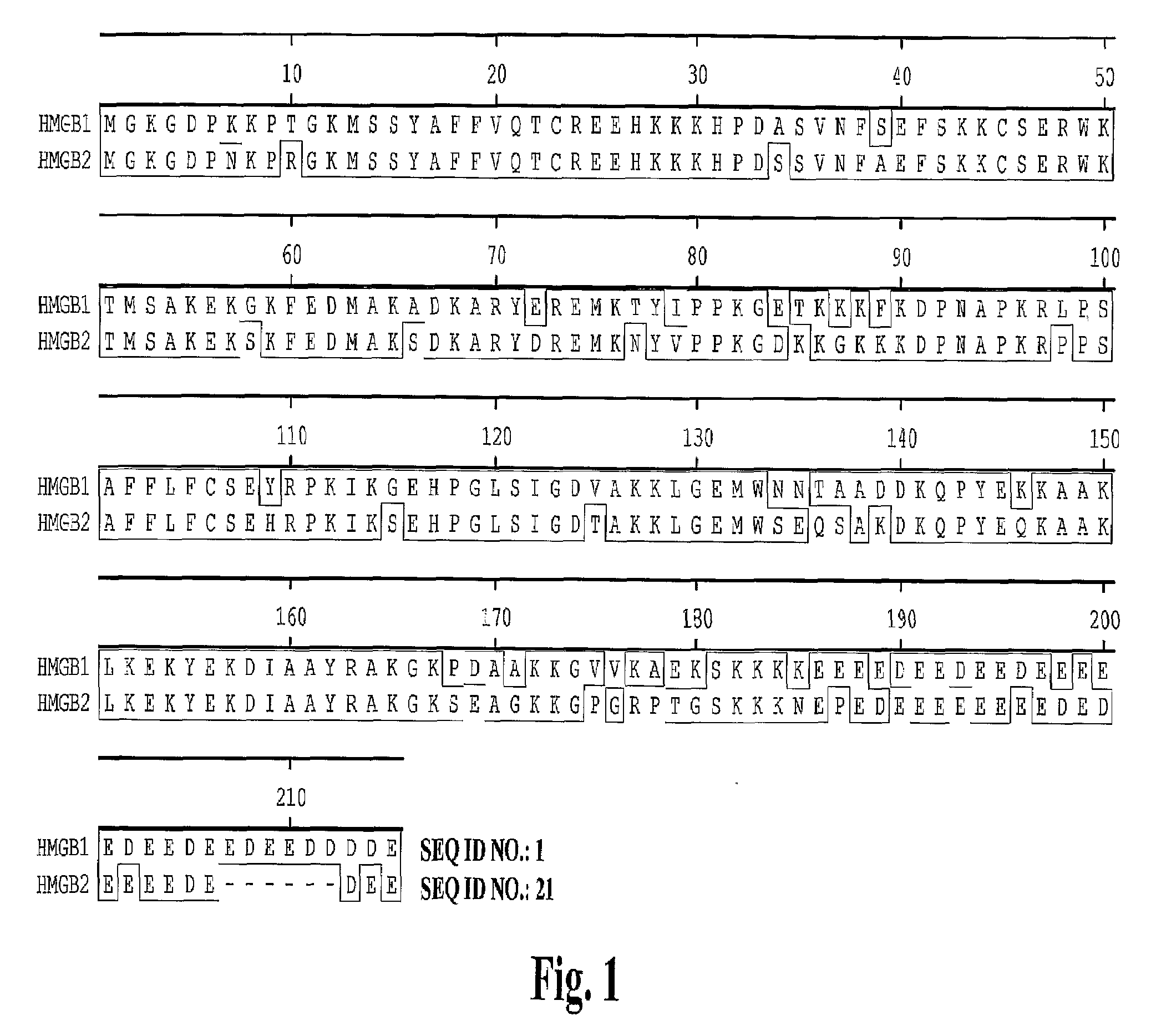
High affinity antibodies against HMGB1 and methods of use thereof
InactiveUS20060099207A1Reduce bone loss and/or cartilage damageAntibacterial agentsAntibody mimetics/scaffoldsReperfusion injuryAllograft rejection
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn's disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:MEDIMMUNE LLC
Methods and compositions for inducing oral tolerance in mammals
The present invention relates to methods and pharmaceutical formulations for orally delivering an antigen to induce tolerance. The antigen is combined with derivatized amino acids or salts thereof. The induction of oral tolerance may be applied clinically for the prevention or treatment of auto-immune diseases and clinical allergic hypersensitivities, and for the prevention of allograft rejection.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Oligonucleotide compositions and methods for treating disease including inflammatory conditions
InactiveUS20050153919A1Reduced activityReduce expressionAntibacterial agentsSenses disorderPhosphodiesteraseAllograft rejection
The invention relates to therapeutic antisense oligonucleotides directed against genes coding for phosphodiesterase (PDEs) and the use of these in combination. These antisense oligonucleotides may be used as analytical tools and / or as therapeutic agents in the treatment of disease associated with reduced cellular cAMP in a patient, such as inflammatory diseases of the respiratory tract including, for example, asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome, bronchitis, chronic bronchitis, silicosis, pulmonary fibrosis, lung allograft rejection, allergic rhinitis and chronic sinusitis as well as other conditions in which an increase in cyclic AMP or a decrease in PDE levels is beneficial.
Owner:TOPIGEN PHARMA
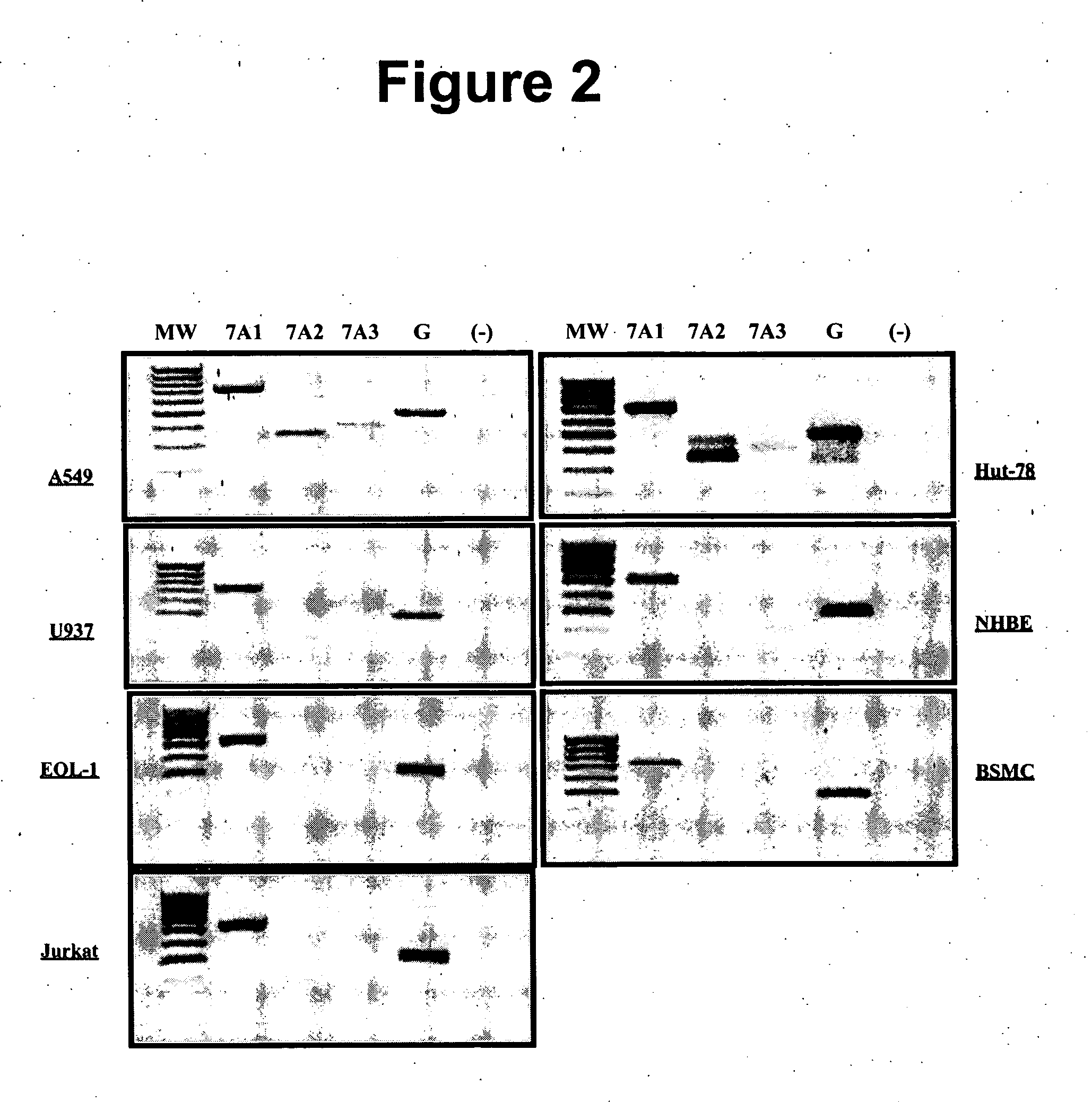
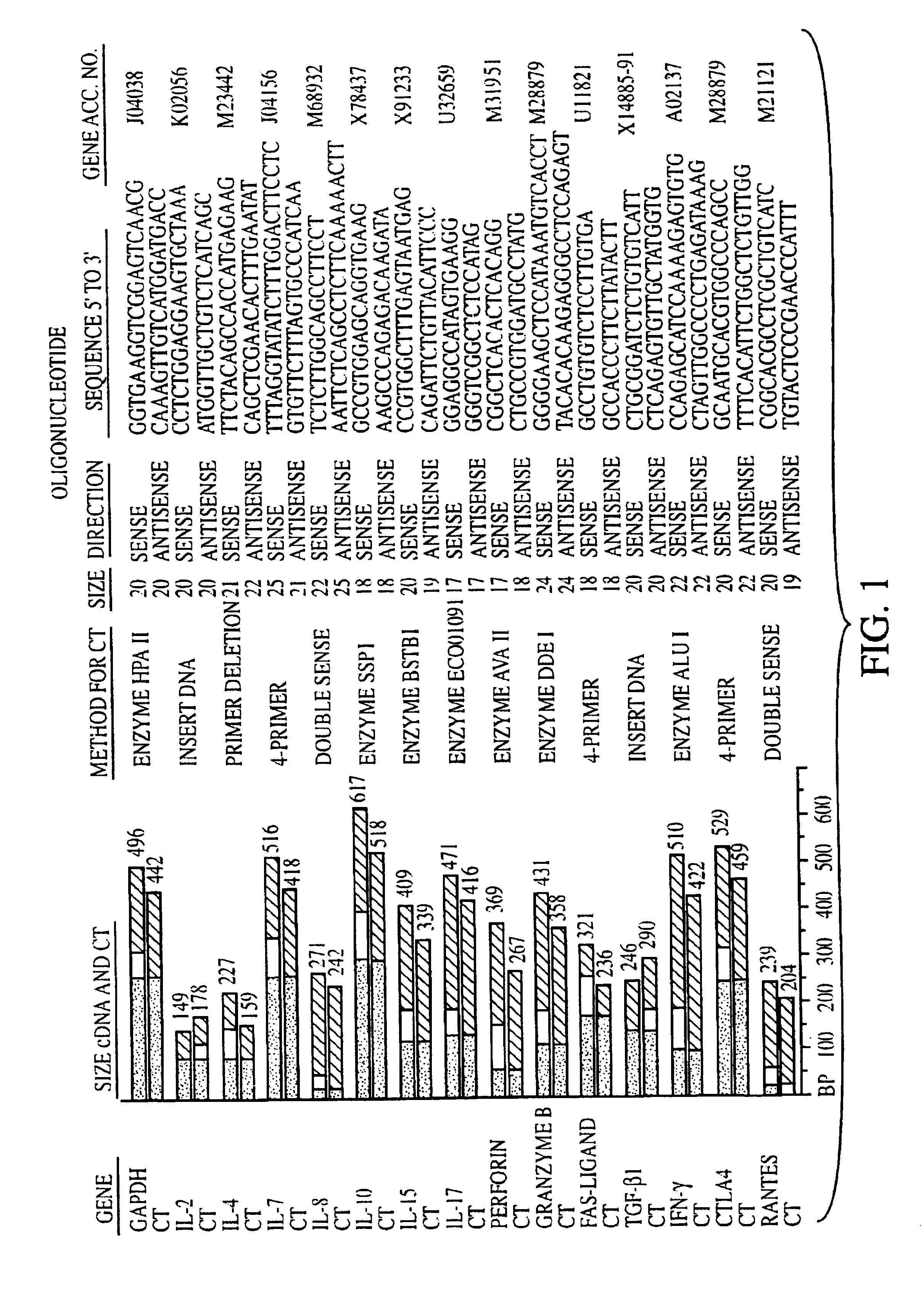
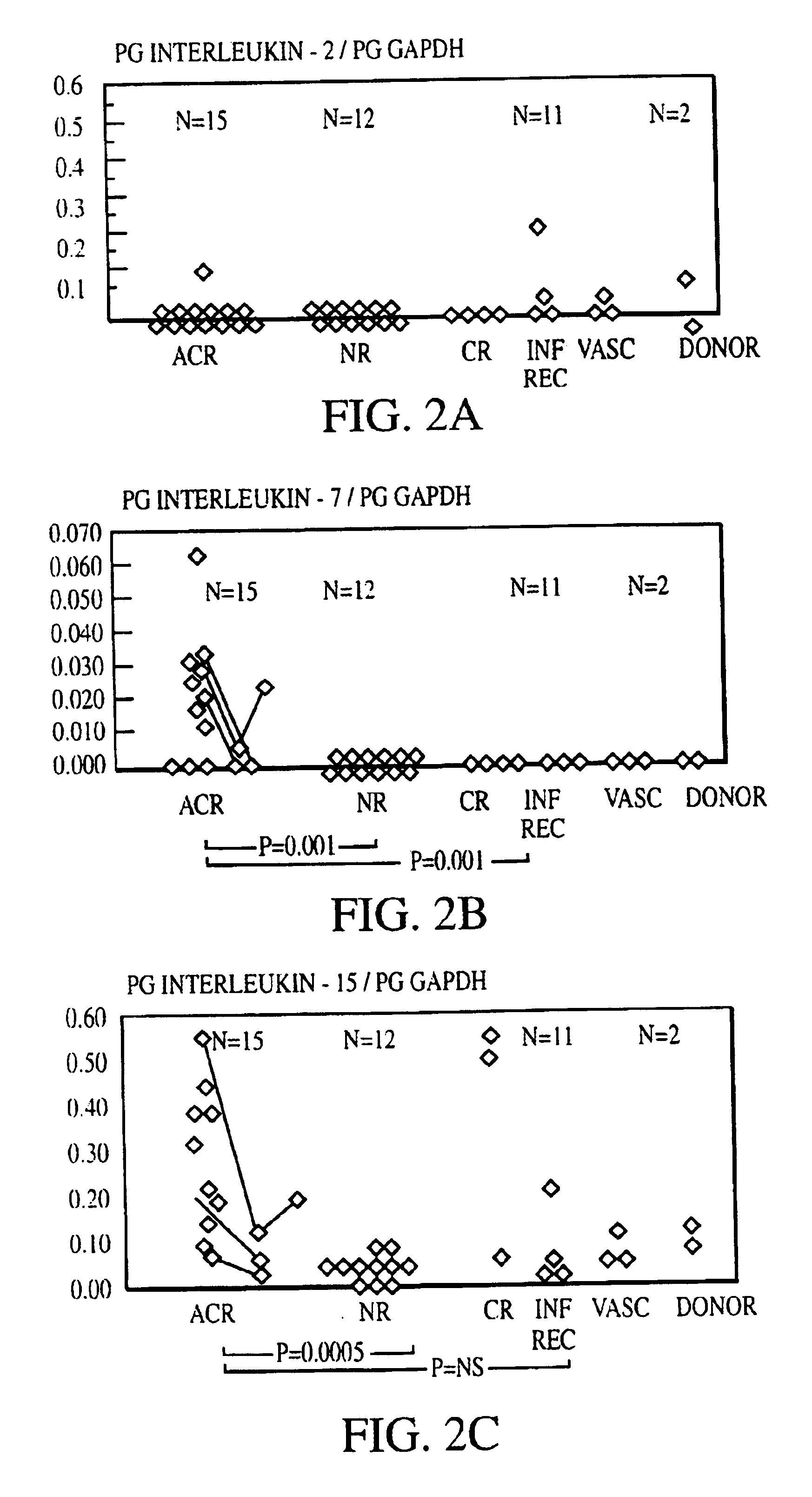
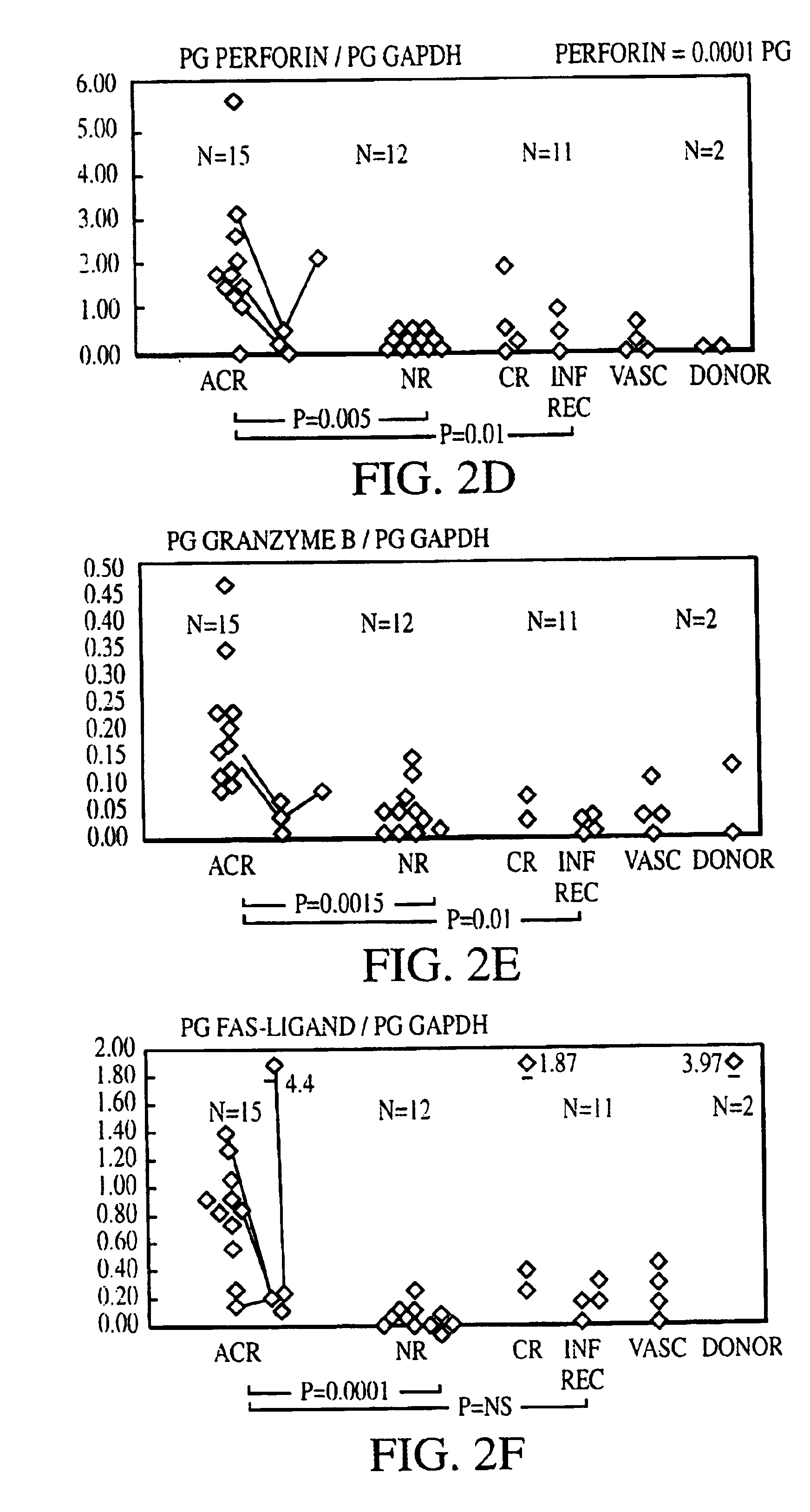
Measurement of protective genes in allograft rejection
InactiveUS6900015B2Quantitative precisionAccurately allograft rejectionMicrobiological testing/measurementDisease diagnosisCytotoxicityAllograft rejection
The invention relates to methods of evaluating transplant rejection in a host comprising determining a heightened magnitude of gene expression of genes in rejection-associated gene clusters. The disclosed gene clusters include genes that are substantially co-expressed with cytotoxic lymphocyte pro-apoptotic genes, cytoprotective genes and several other cytokine and immune cell genes.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Short peptides useful for treatment of ischemia/reperfusion injury and other tissue damage conditions associated with nitric oxide and its reactive species
ActiveUS20080182797A1Avoid tissue damageLevel of protectionNervous disorderTetrapeptide ingredientsPregnancyAllograft rejection
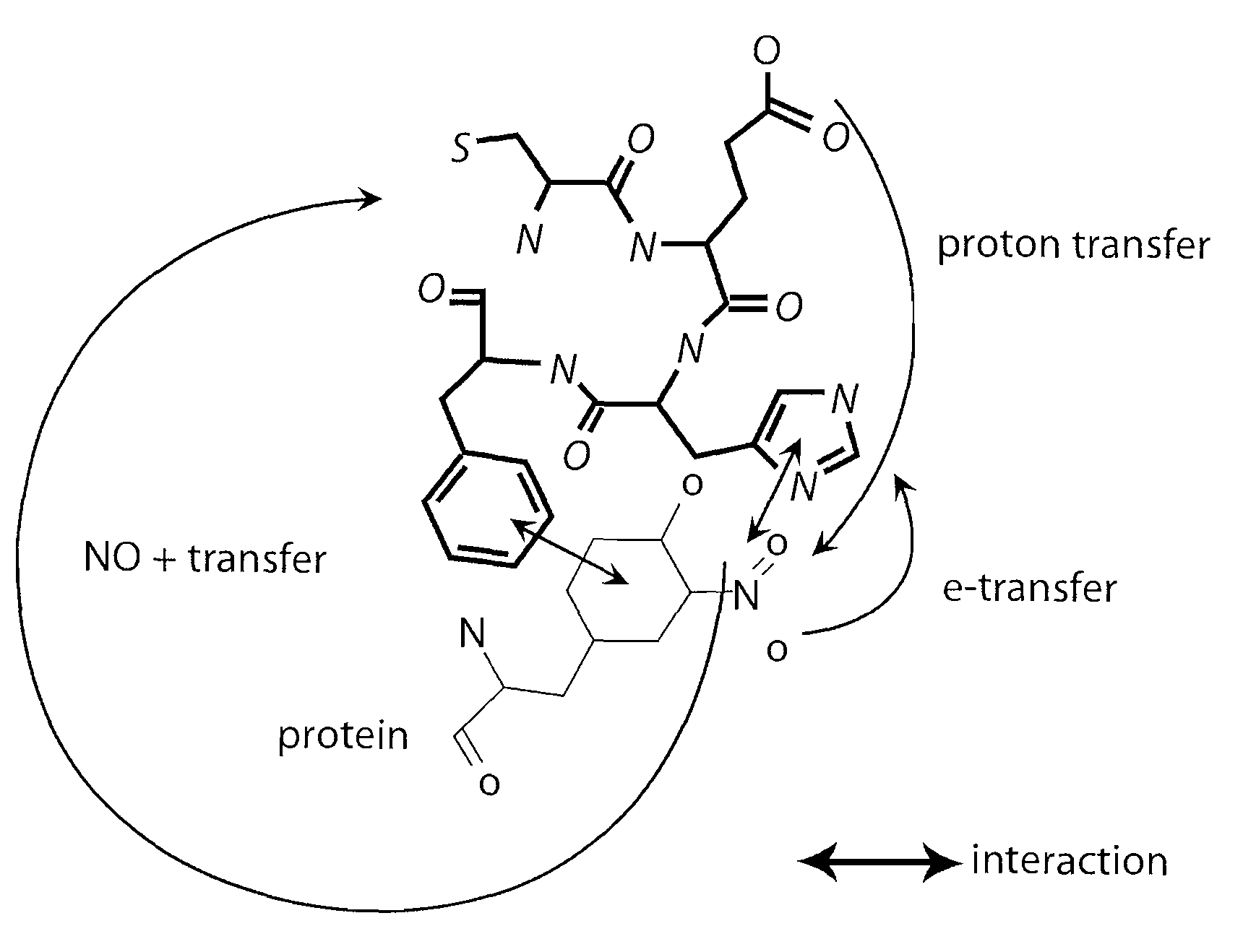
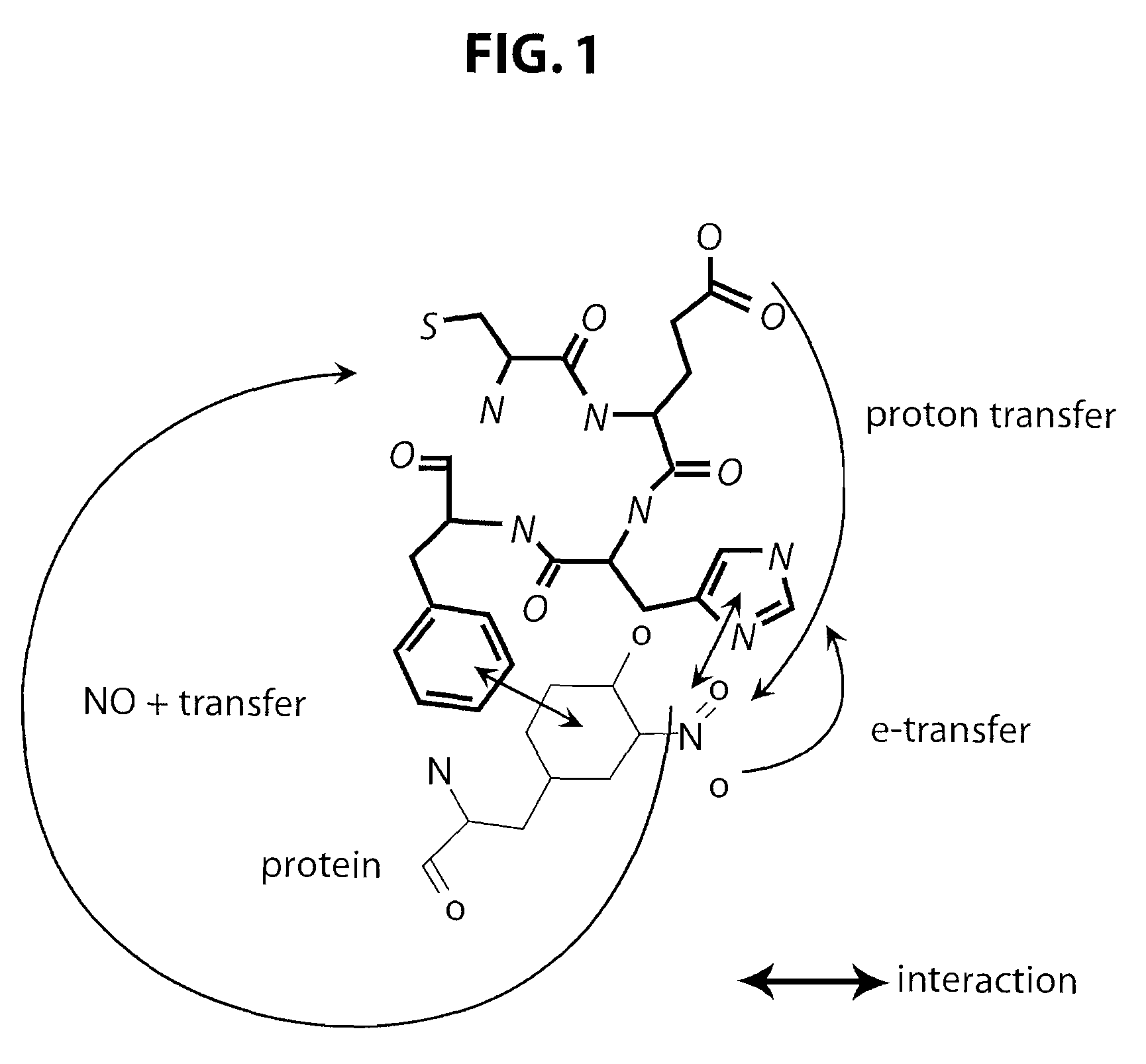
This invention discloses isolated short peptides comprising the amino acid sequence Cys-Glu-Phe-His (CEFH) and analogs thereof as well as compositions comprising CEFH peptides and analogs thereof. The CEFH peptides disclosed herein are effective in mediating the denitration of 3-nitrotyrosines (3-NT) in cellular proteins thereby preventing tissue damage associated with excess nitric oxide (NO) and its reactive species. The CEFH peptides disclosed herein are useful in the treatment of ischemia / reperfusion (I / R) injury of various tissues (e.g., I / R injury of heart muscle associated with heart attack or cardiac surgery, I / R injury of brain tissue associated with stroke, I / R injury of liver tissue, skeletal muscles, etc.), septic shock, anaphylactic shock, neurodegenerative diseases (e.g., Alzheimer's and Parkinson's diseases), neuronal injury, atherosclerosis, diabetes, multiple sclerosis, autoimmune uveitis, pulmonary fibrosis, oobliterative bronchiolitis, bronchopulmonary dysplasia (BPD), amyotrophic lateral sclerosis (ALS), sepsis, inflammatory bowel disease, arthritis, allograft rejection, autoimmune myocarditis, myocardial inflammation, pulmonary granulomatous inflammation, influenza- or HSV-induced pneumonia, chronic cerebral vasospasm, allergic encephalomyelitis, central nervous system (CNS) inflammation, Heliobacterium pylori gastritis, necrotizing entrerocolitis, celliac disease, peritonitis, early prosthesis failure, inclusion body myositis, preeclamptic pregnancies, skin lesions with anaphylactoid purpura, nephrosclerosis, ileitis, leishmaniasis, cancer, and related disorders.
Owner:NEW YORK UNIVERSITY
Alteration of immune response using pan DR-binding peptides
InactiveUS7202351B1Avoid immune responsePeptide-nucleic acidsSugar derivativesAutoimmune responsesBinding site
The present invention provides compositions and methods of inhibiting or inducing activation of T cells in a patient. The methods comprise administering a therapeutically effective dose of pharmaceutical compositions comprising a pharmaceutically acceptable carrier and peptides of between about 4 and about 20 residues, that bind antigen binding sites on MHC molecules encoded by substantially all alleles of a DR locus. These peptides are referred to as pan DR binding peptides. The pan DR binding peptides can be used to inhibit immune responses associated with immunopathologies, such as autoimmunity, allograft rejection and allergic responses. The peptides can also be used in combination with CTL peptides to enhance a CTL response.
Owner:EPIMMUNE
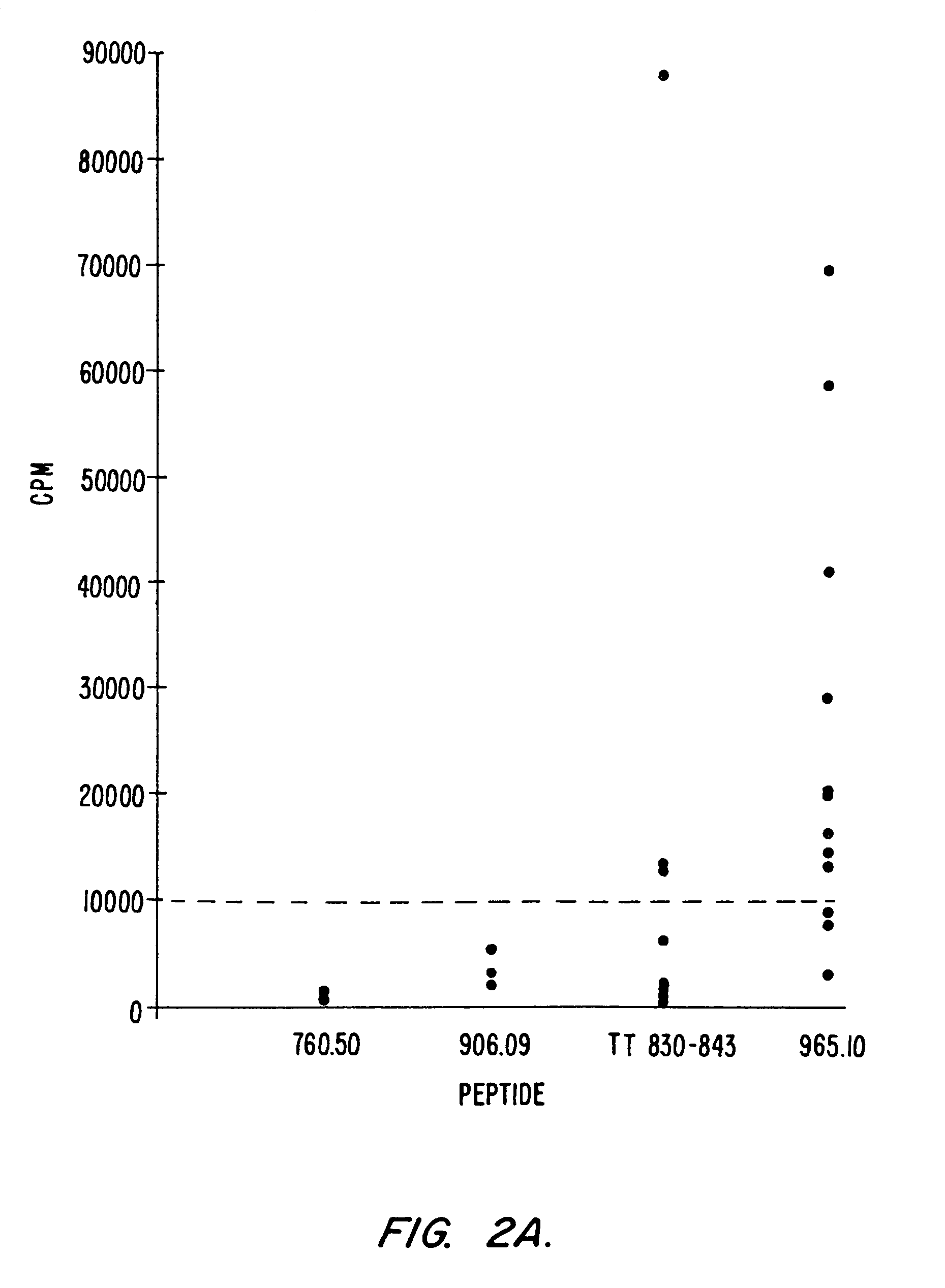
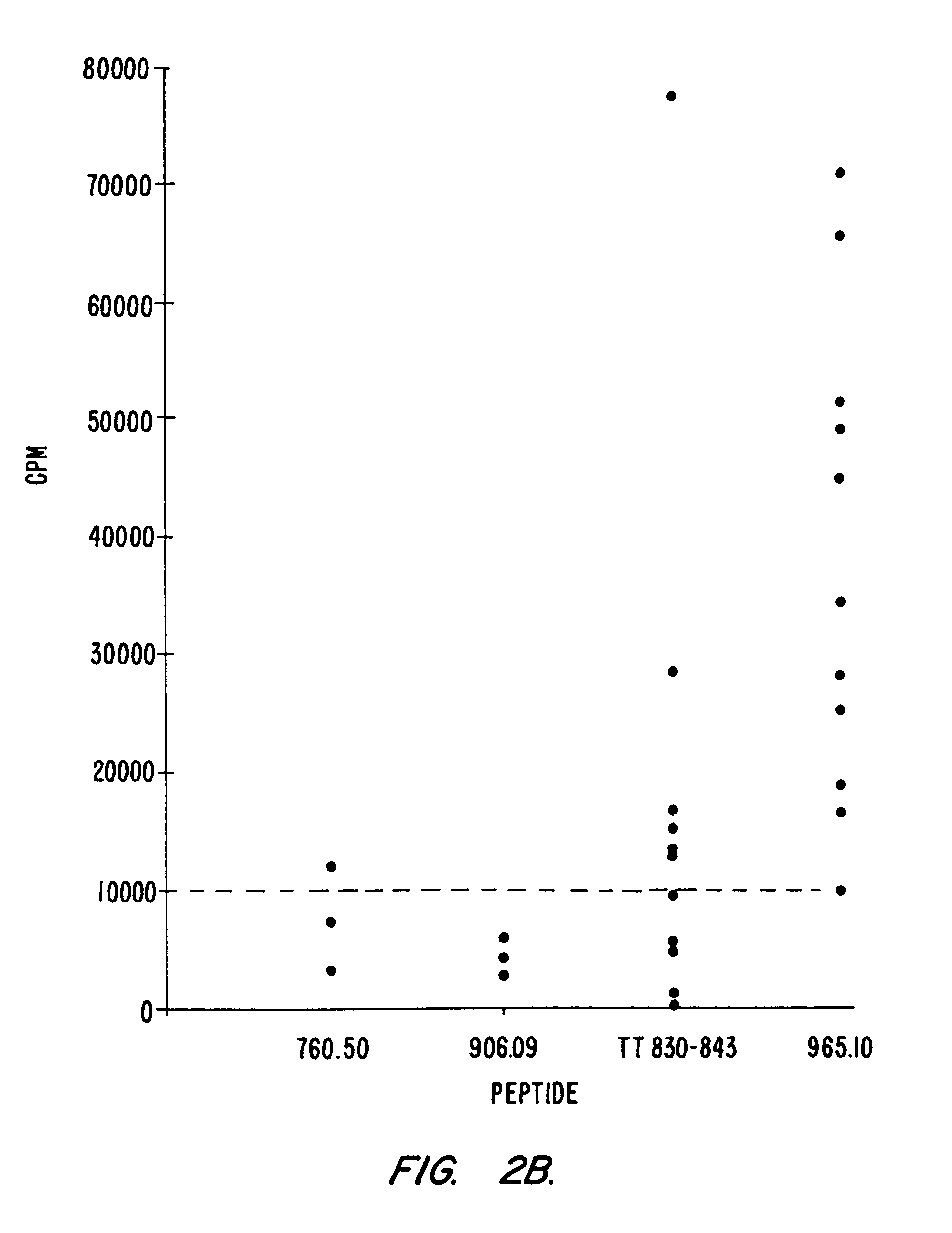
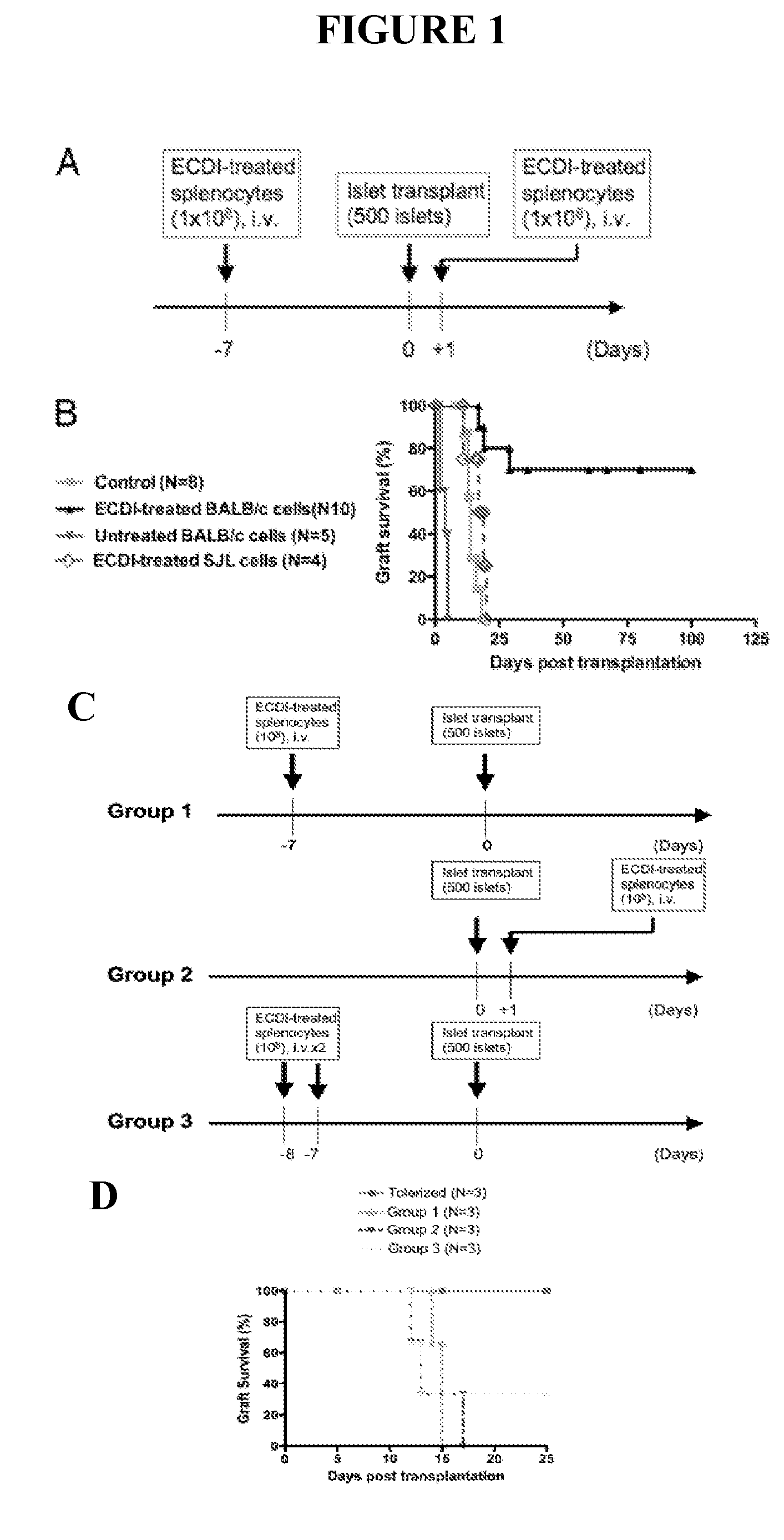
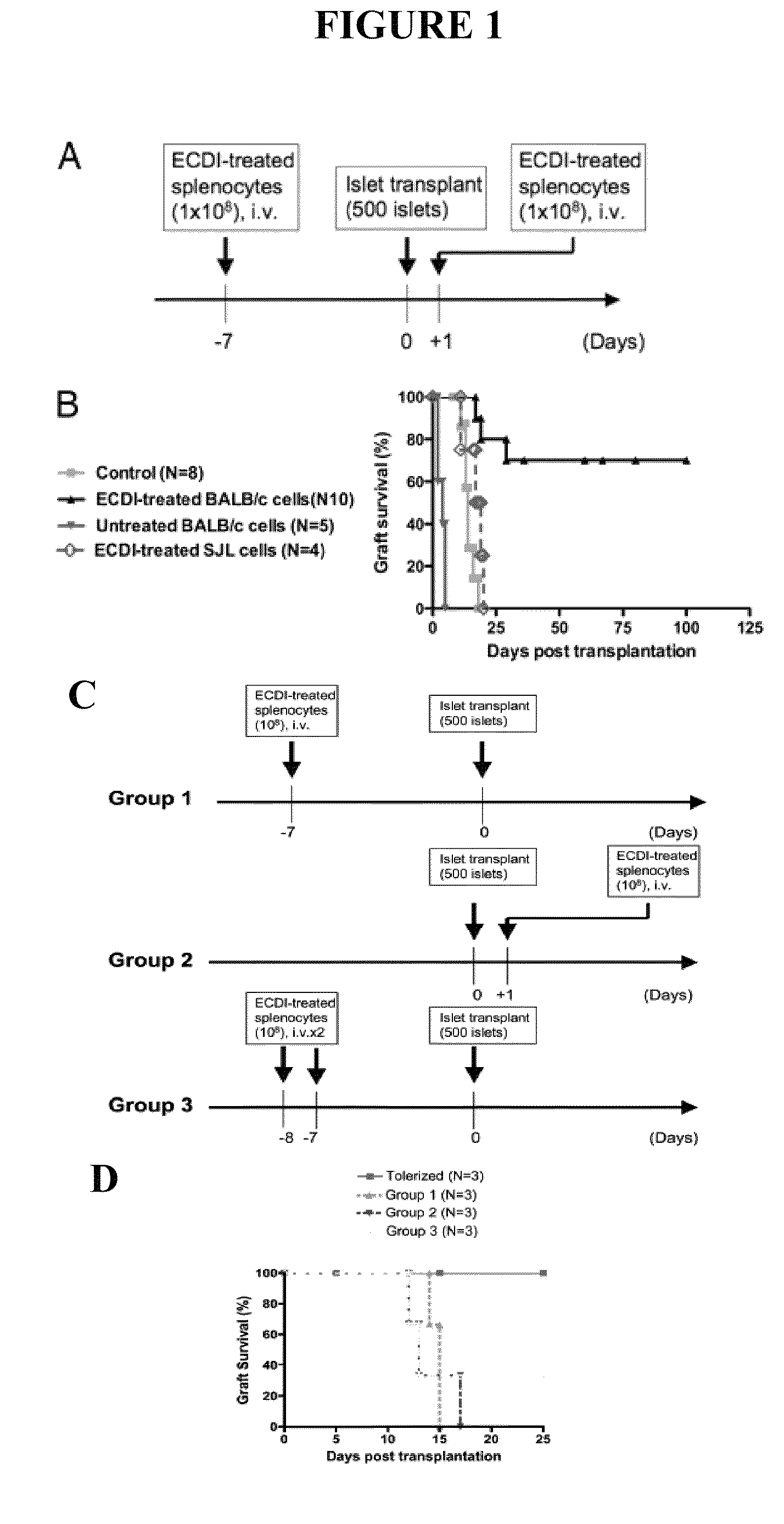
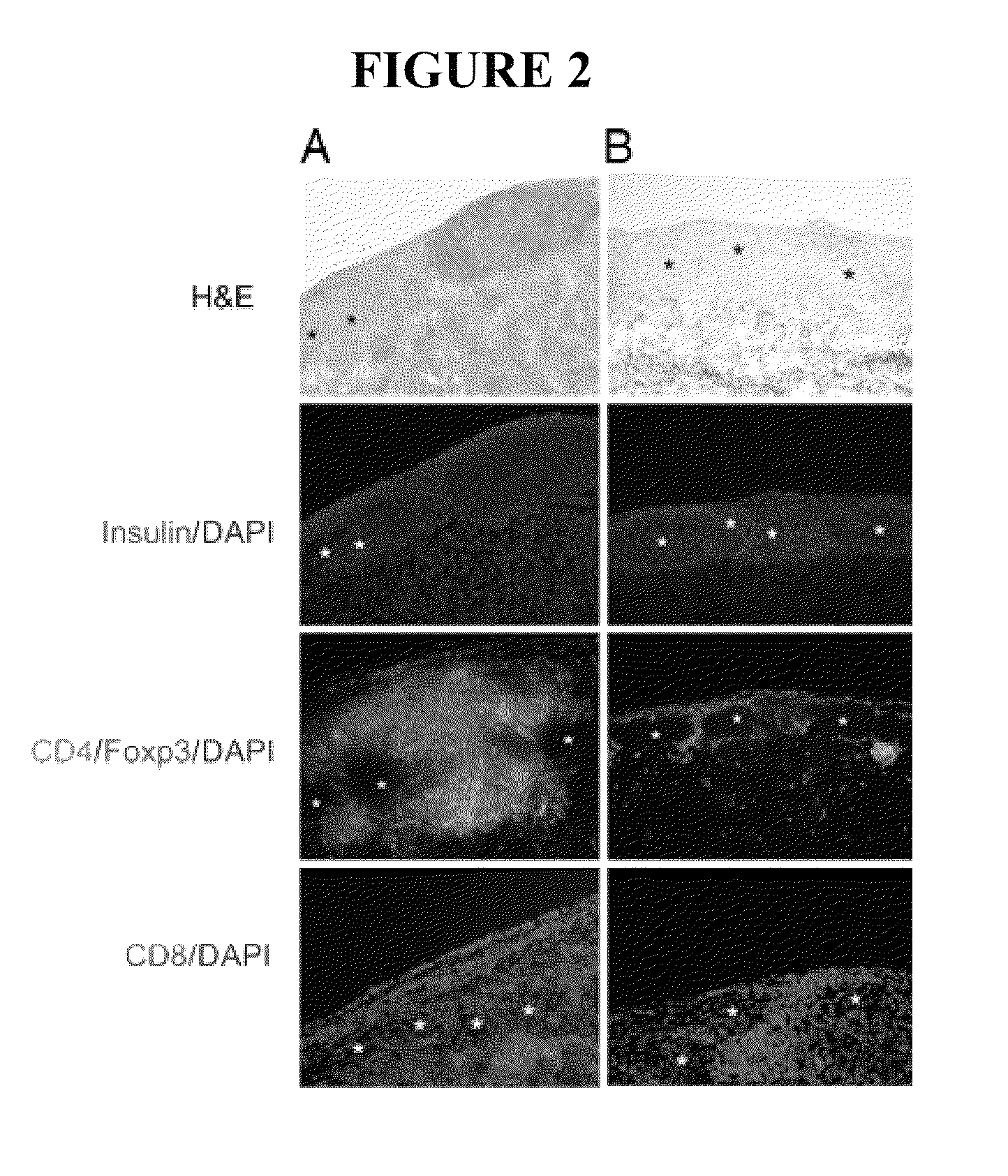
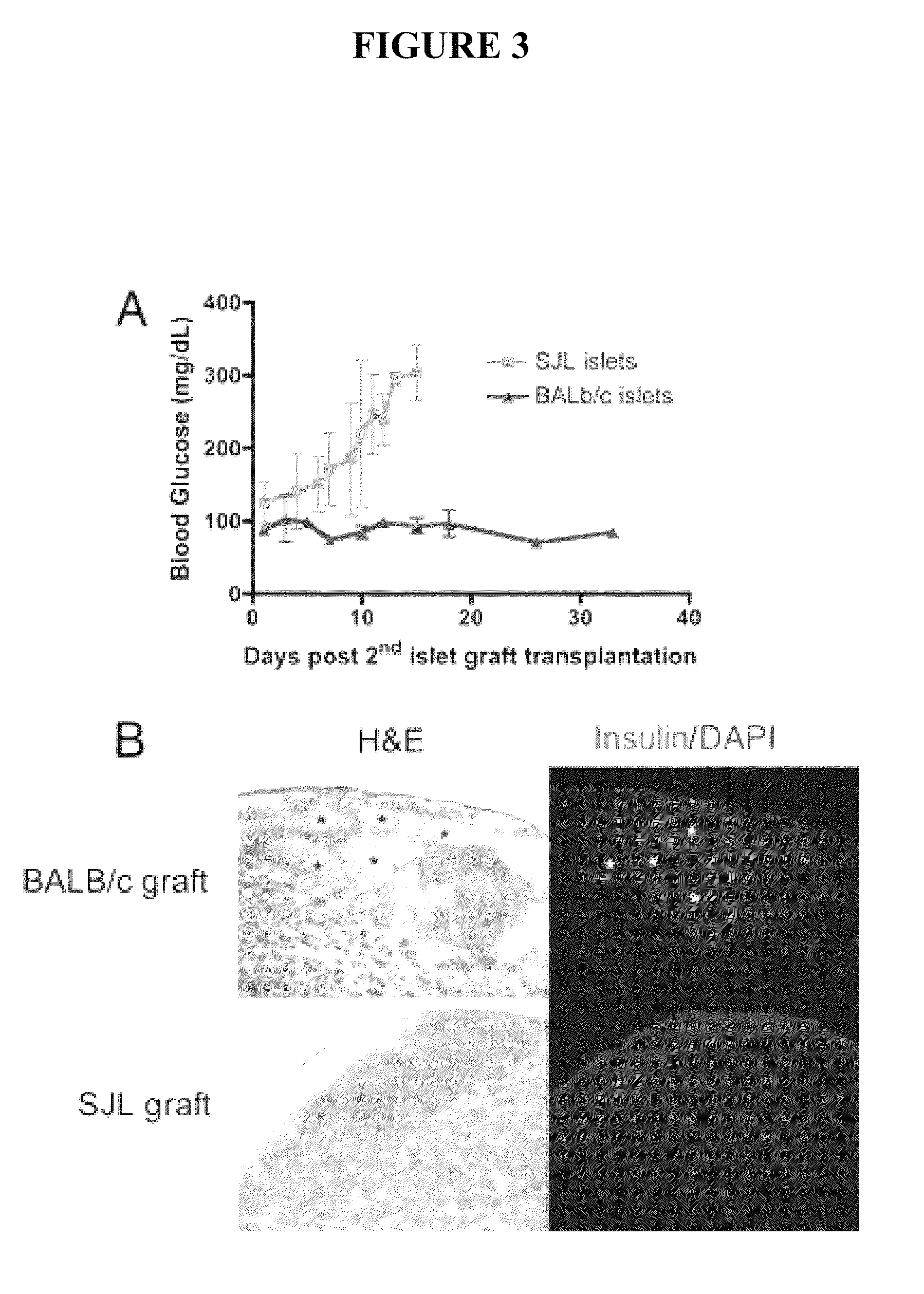
Use of ecdi-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
Monitoring, preventing, and treating rejection of transplanted organs
InactiveUS20060036286A1Good effectReduced responseElectrotherapyPressure infusionElectricityClosed loop
An implantable system control unit (SCU) includes means for measuring tissue impedance or other condition to determine allograft health, in order to predict or detect allograft rejection. The SCU also includes at least two electrodes coupled to means for delivering electrical stimulation to a patient within whom the device is implanted, and may also include a reservoir for holding one or more drugs and a driver means for delivering the drug(s) to the patient. In certain embodiments, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters. Alternatively, this sensory “SCU” sounds an alarm, communicates an alarm to an external device, and / or is responsive to queries regarding sensed information, such as tissue impedance.
Owner:BOSTON SCI NEUROMODULATION CORP
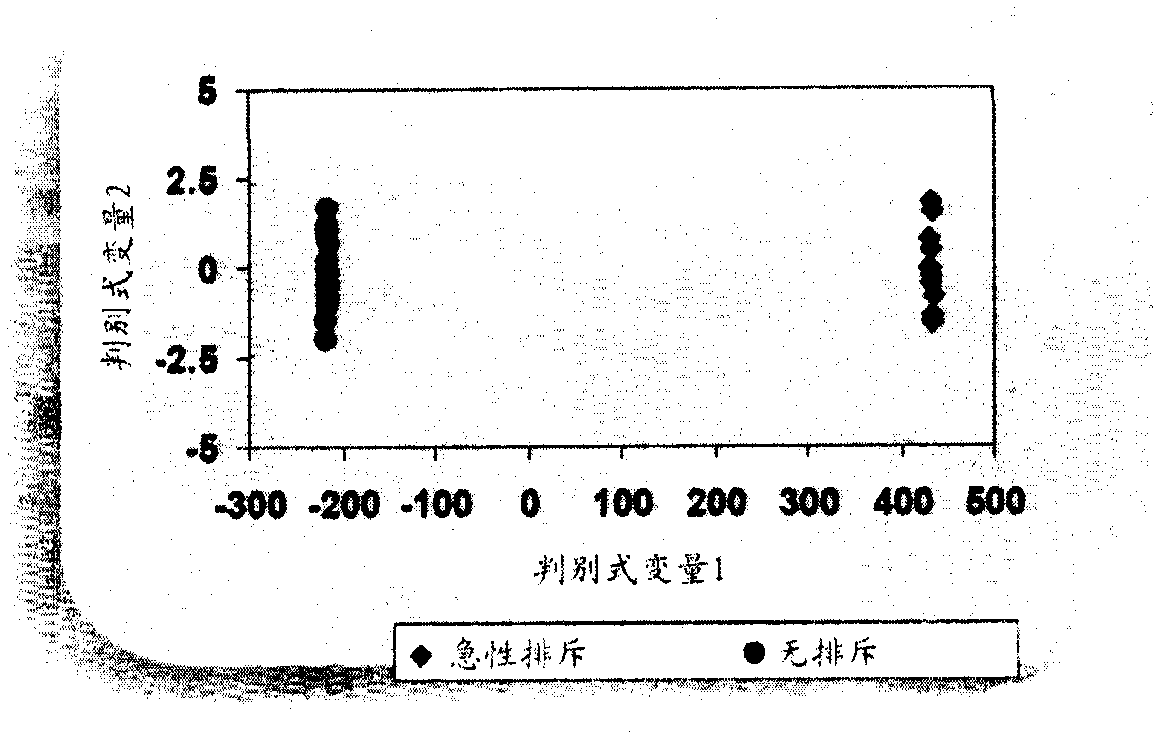
Methods of Diagnosing Rejection of a Kidney Allograft Using Genomic or Proteomic Expression Profiling
InactiveUS20110189680A1Reduce errorsLess detectableMicrobiological testing/measurementIsotope separationAllograft rejectionGenome
A method of determining the acute allograft rejection status of a subject, the method comprising the steps of: determining the nucleic acid expression profile of one or more than one nucleic acid markers, or one or more than one proteomic markers in a biological sample from the subject; comparing the expression profile of the one or more than one nucleic acid markers to a control profile; and determining whether the expression level of the one or more than one nucleic acid markers is increased relative to the control profile, wherein the increase of the one or more than one nucleic acid markers is indicative of the acute rejection status of the subject.
Owner:THE UNIV OF BRITISH COLUMBIA
Compositions and methods for modulation of suppressor t cell activation
InactiveUS20100061984A1Relieve symptomsIncreased acetylation levelsCompounds screening/testingAntibacterial agentsAntigenImmunologic disorders
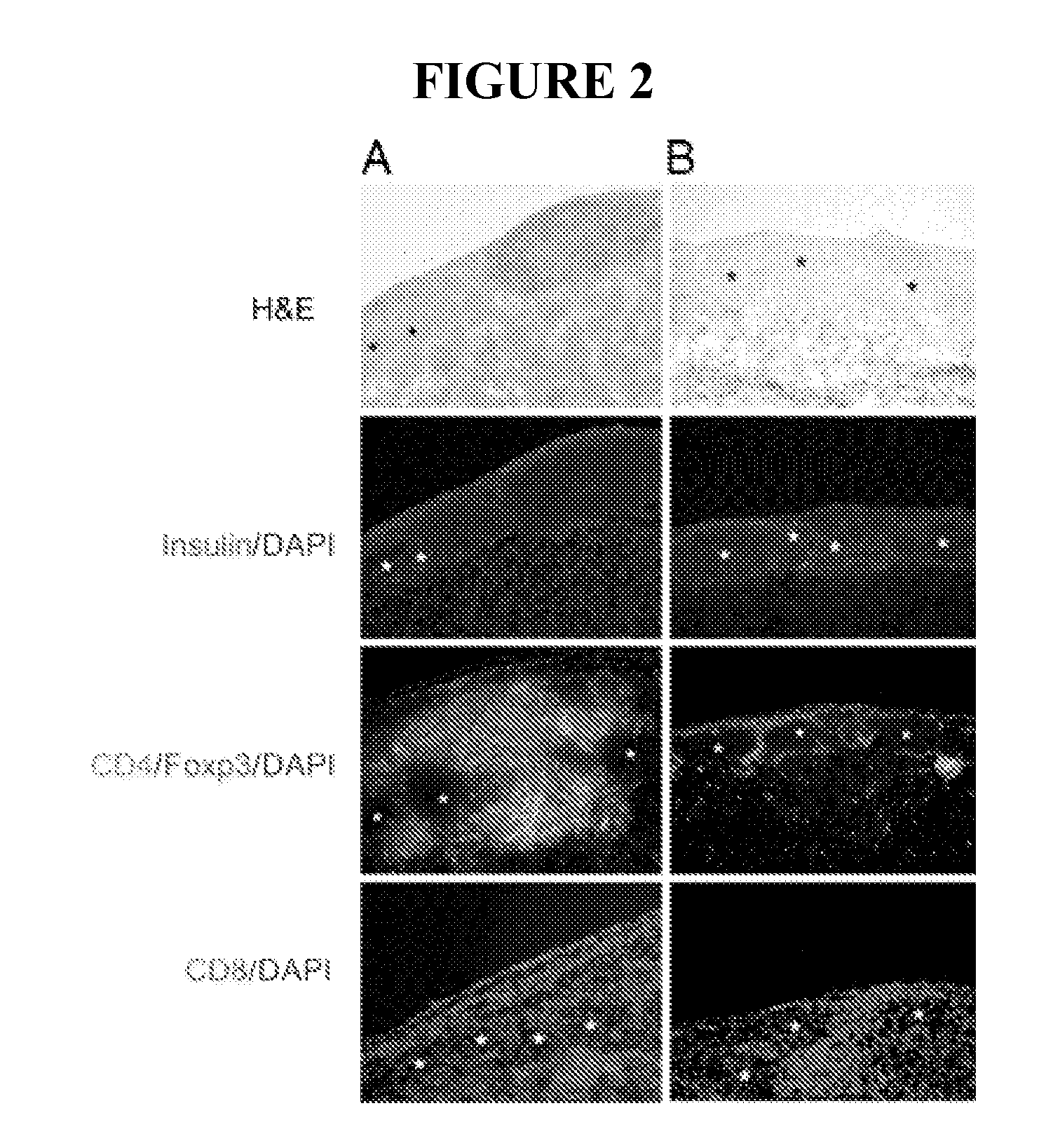
Methods of treating autoimmune disorders, coronary artery disease, allergy symptoms, allograft rejection sepsis / toxic shock are disclosed. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3 in combination with a T suppressor stimulus and / or an antigen. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3. Some methods comprise administering soluble GITR or antibodies that bind to GITR ligand. Methods of treating cancer, infectious diseases, and immune deficiency are also disclosed as are vaccination methods. The methods comprise administering one or more regulatory compositions to inactivate the T suppressor cells by reducing the acetylation level and / or protein level of FOXP3. Improved vaccines and vaccination methods are disclosed. Methods of identifying compounds that are useful to modulate acetylation level and / or protein level of FOXP3 and treat diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Methods for the treatment and prevention of inflammatory diseases
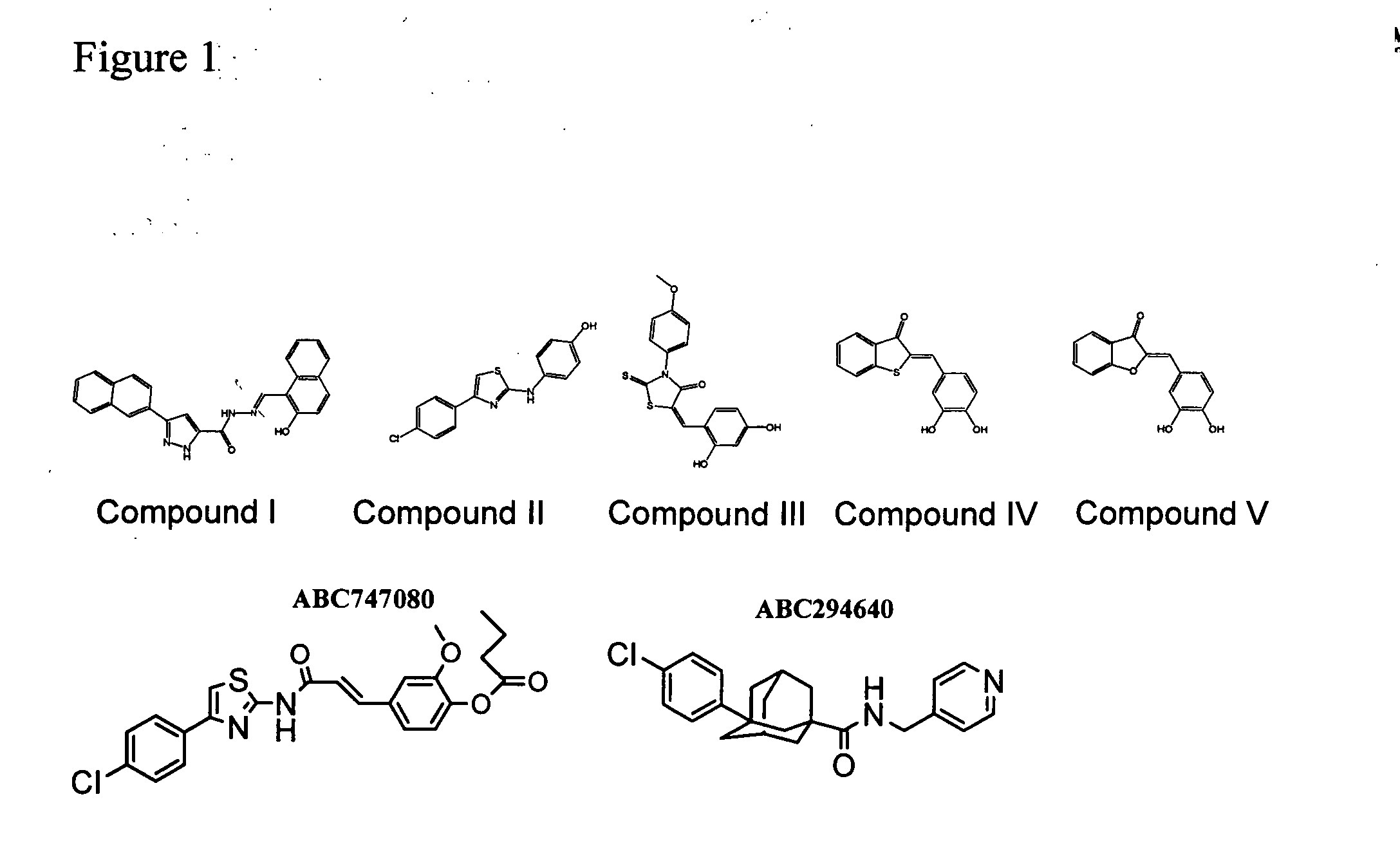
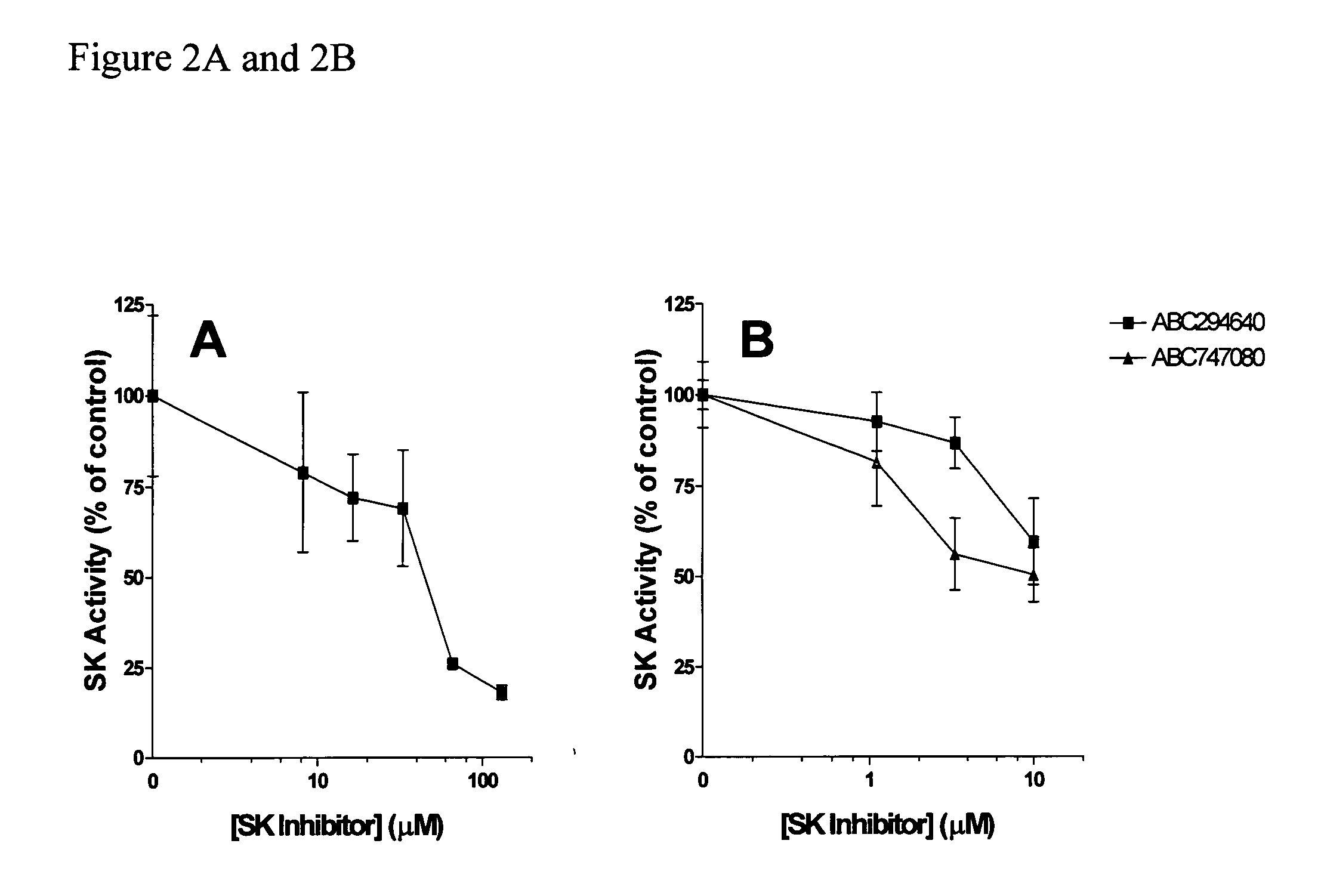
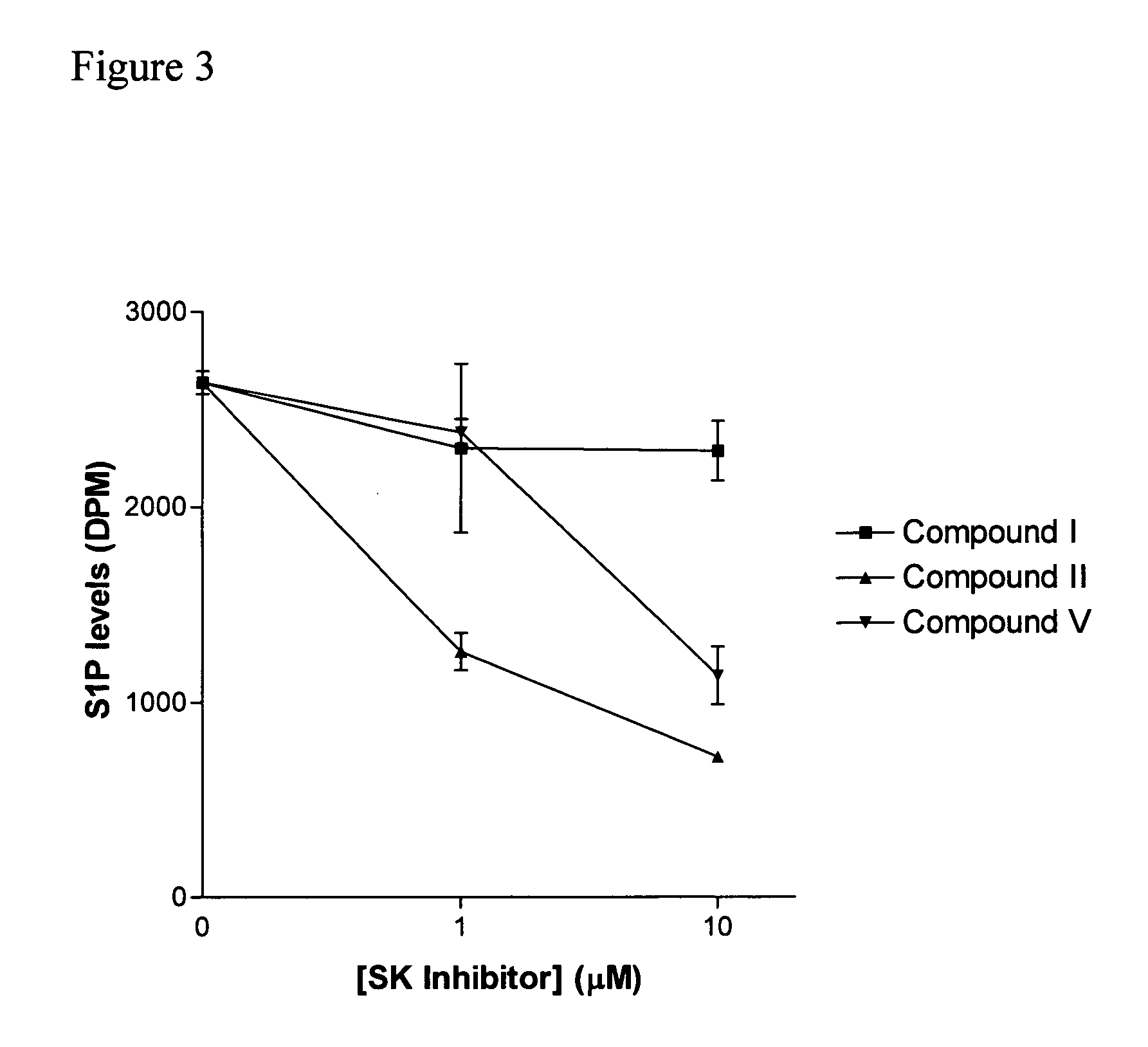
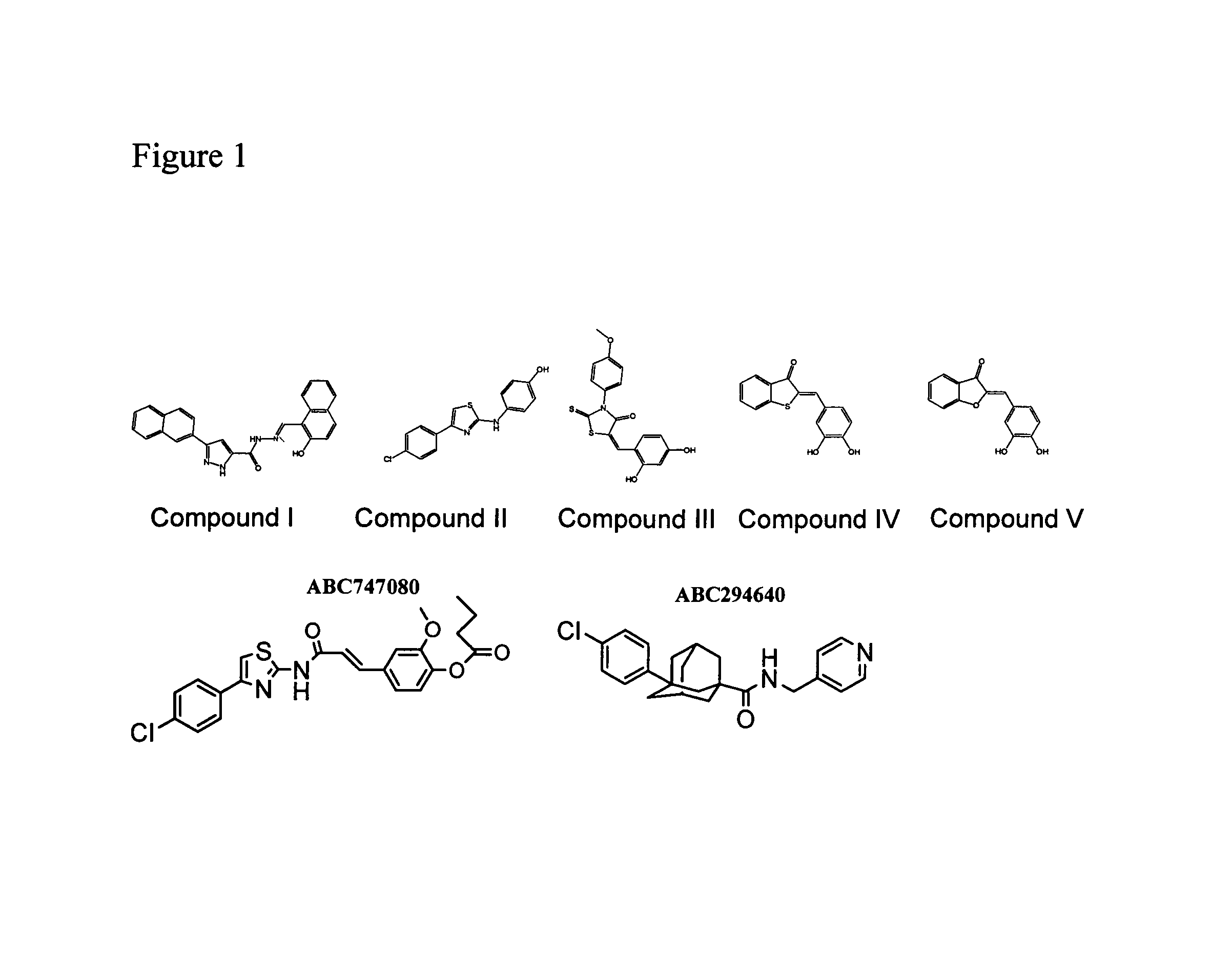
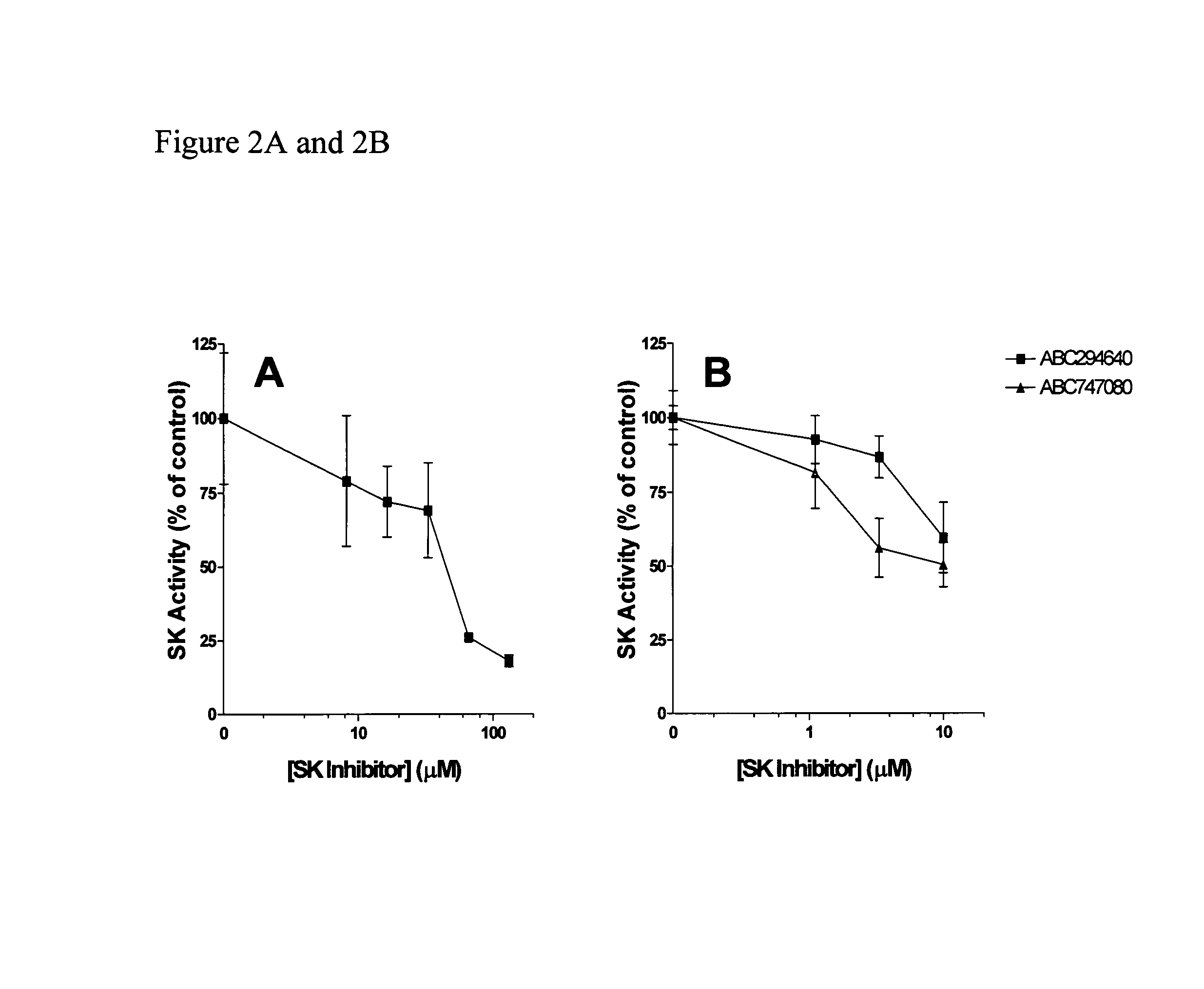
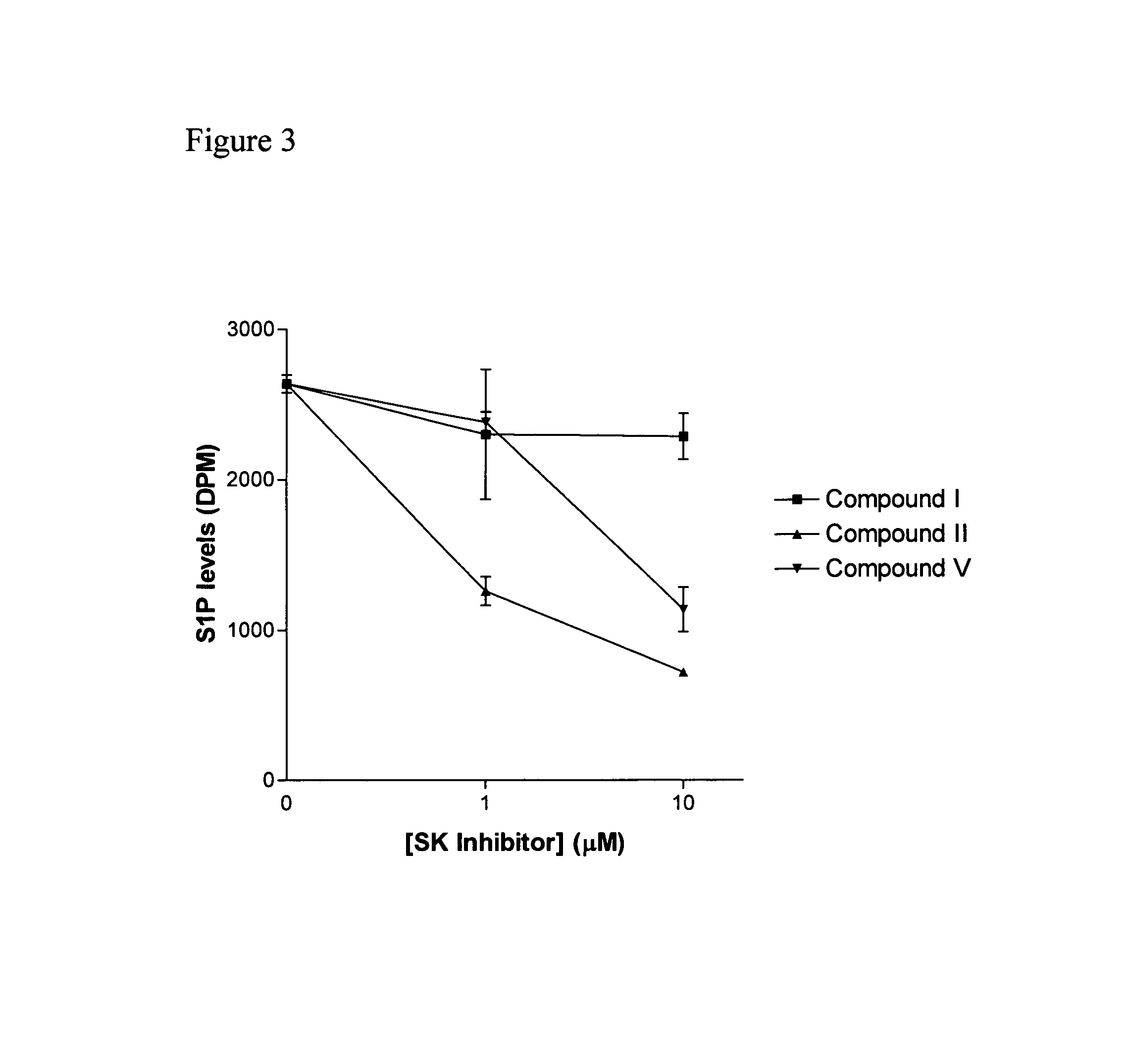
The invention includes processes mainly for the treatment of a inflammatory diseases, such as inflammatory bowel disease, arthritis, atherosclerosis, asthma, allergy, inflammatory kidney disease, circulatory shock, multiple sclerosis, chronic obstructive pulmonary disease, skin inflammation, periodontal disease, psoriasis and T cell-mediated diseases of immunity, including allergic encephalomyelitis, allergic neuritis, transplant allograft rejection, graft versus host disease, myocarditis, thyroiditis, nephritis, systemic lupus erthematosus, and insulin-dependent diabetes mellitus. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:APOGEE BIOTECHNOLOGY CORP
Adenine based inhibitors of adenylyl cyclase, pharmaceutical compositions, and method of use thereof
InactiveUS6887880B2Inhibit and prevent fibroproliferative vasculopathyBiocideOrganic chemistryAdenylyl cyclasePercent Diameter Stenosis
The present invention relates to derivatives and analogues of adenine of the formula: wherein L, A, Y and Z are those defined herein. Compounds of the present invention are useful in inhibiting adenylyl cyclase activity. The present invention also relates to a method of preventing and inhibiting a patient's fibroproliferative vasculopathy following vascular injury or a vascular surgical operation which includes administering to the patient, an effective amount of a compound according to the invention subsequent to a vascular injury, or subsequent to a vascular surgical operation, for one to two weeks after the injury or surgical operation, effective to treat or prevent a patient's fibroproliferative vasculopathy such as chronic allograft rejection or vascular restenosis following vascular trauma. The present invention also relates to a method for measuring the inhibition of adenylyl cyclase activity and a method for treating congestive heart failure.
Owner:MILLENNIUM PHARMA INC
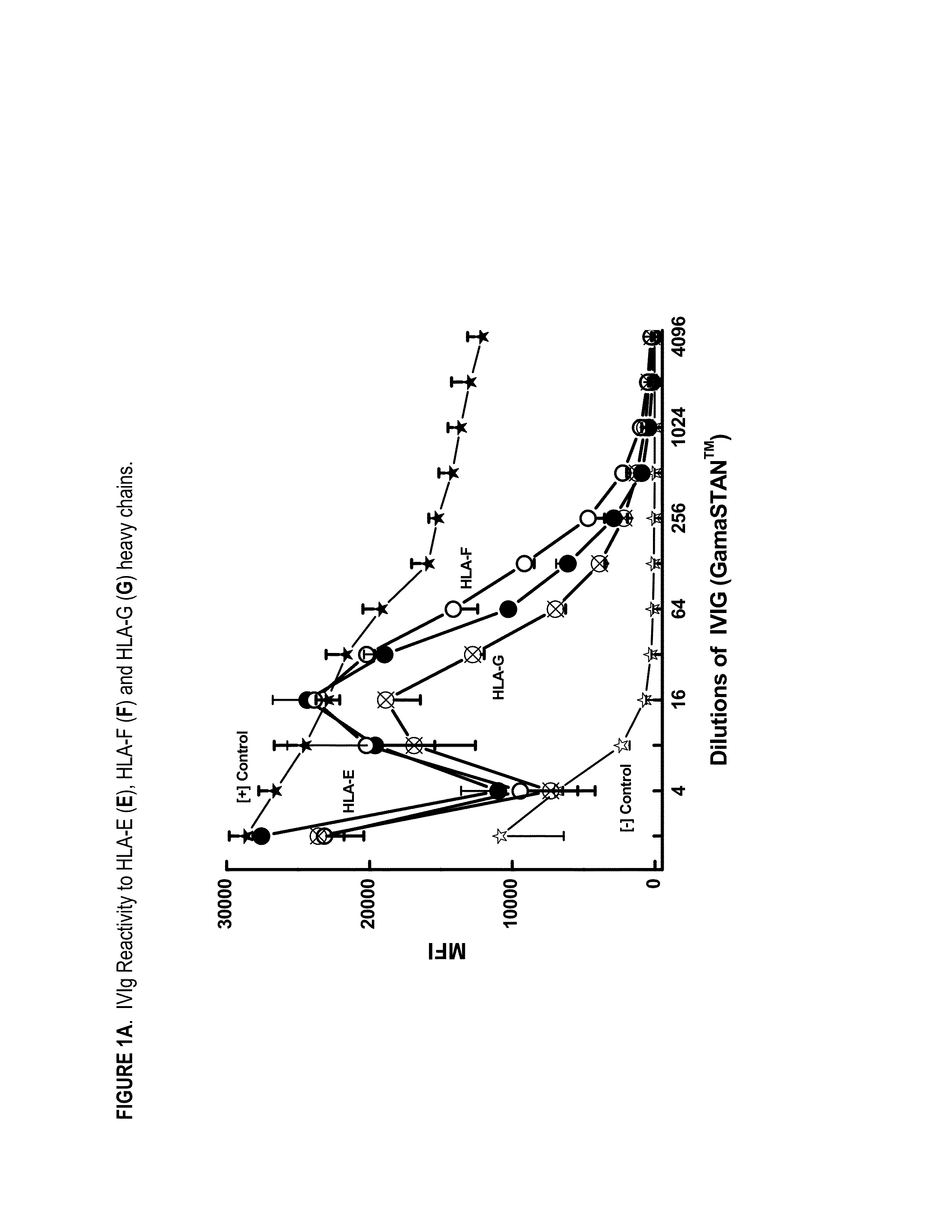
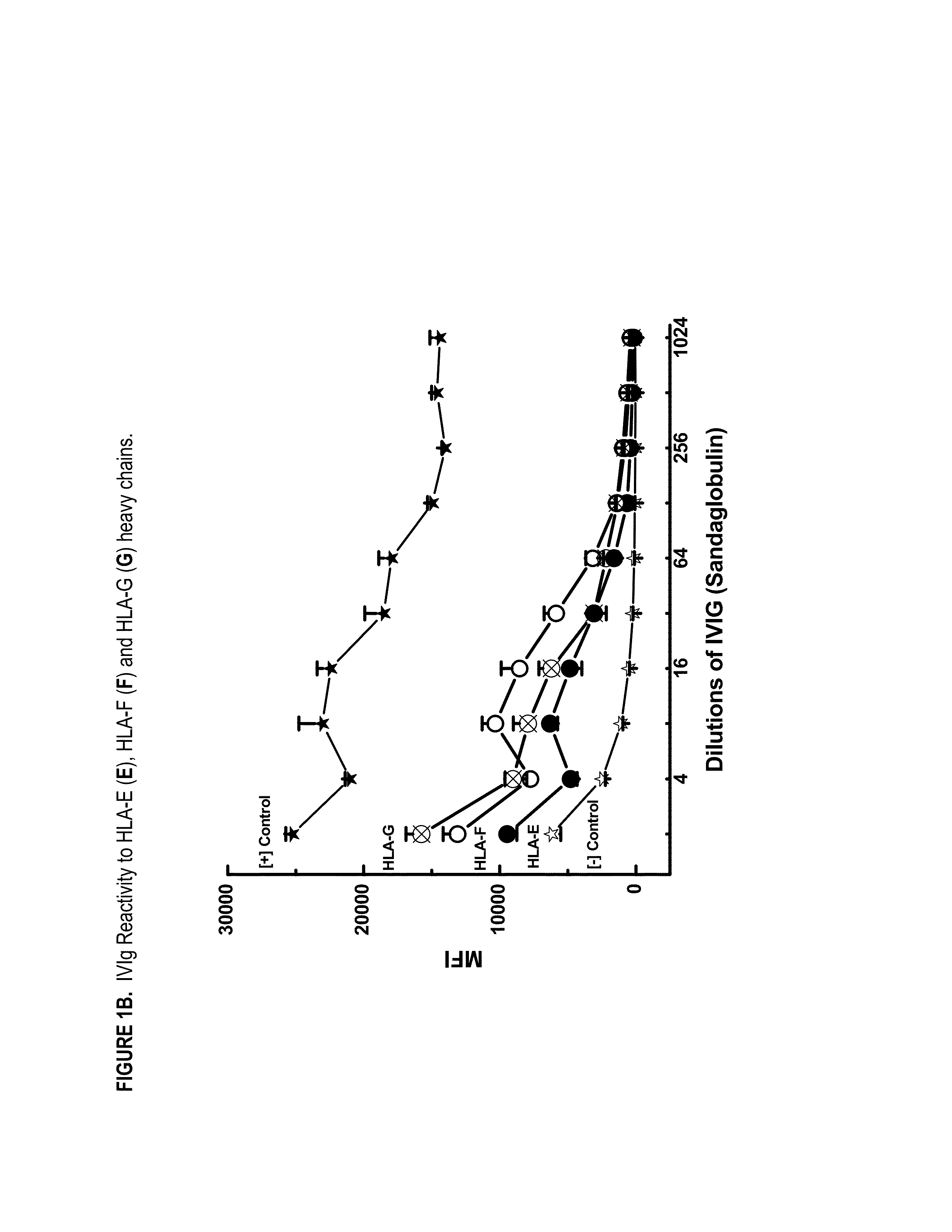
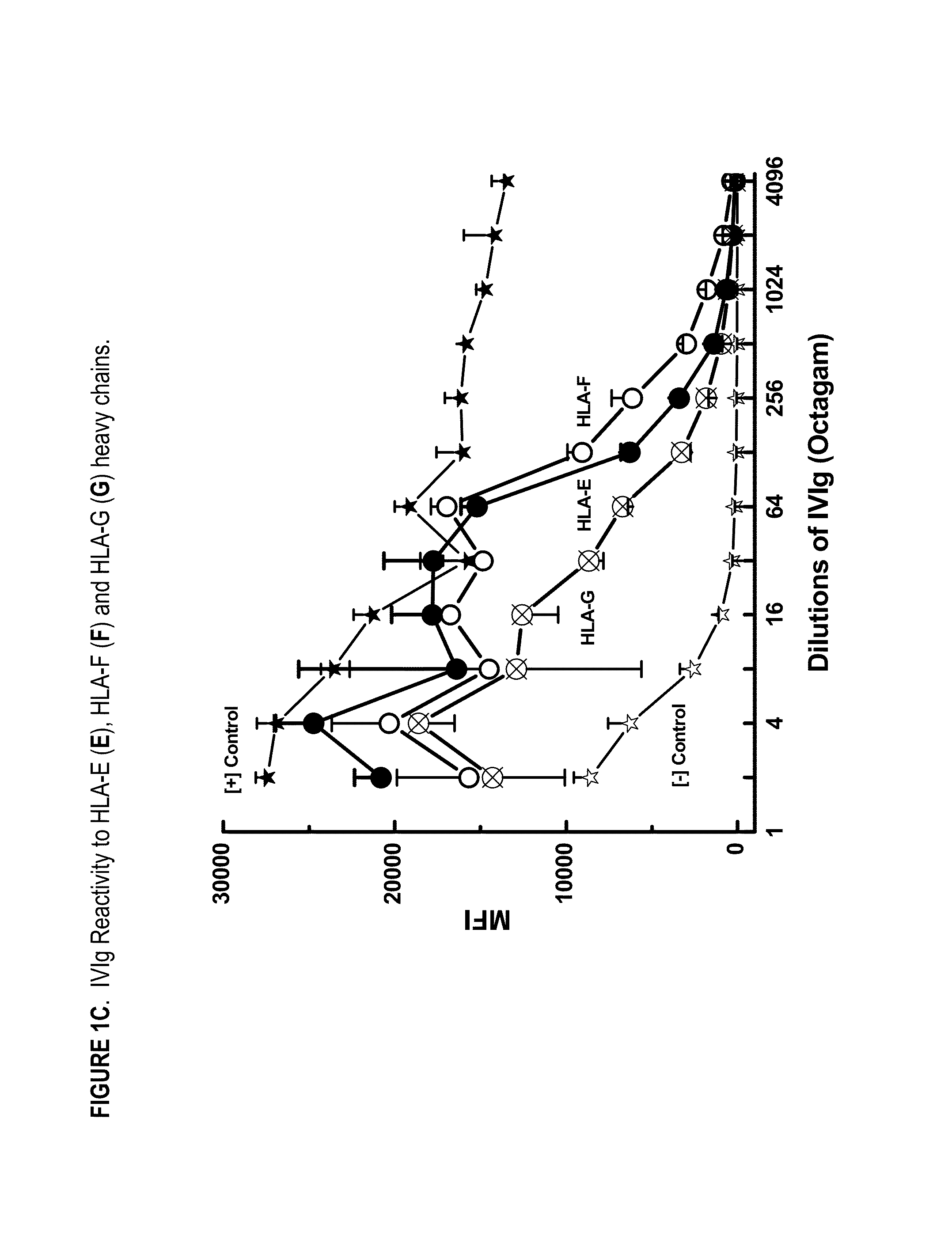
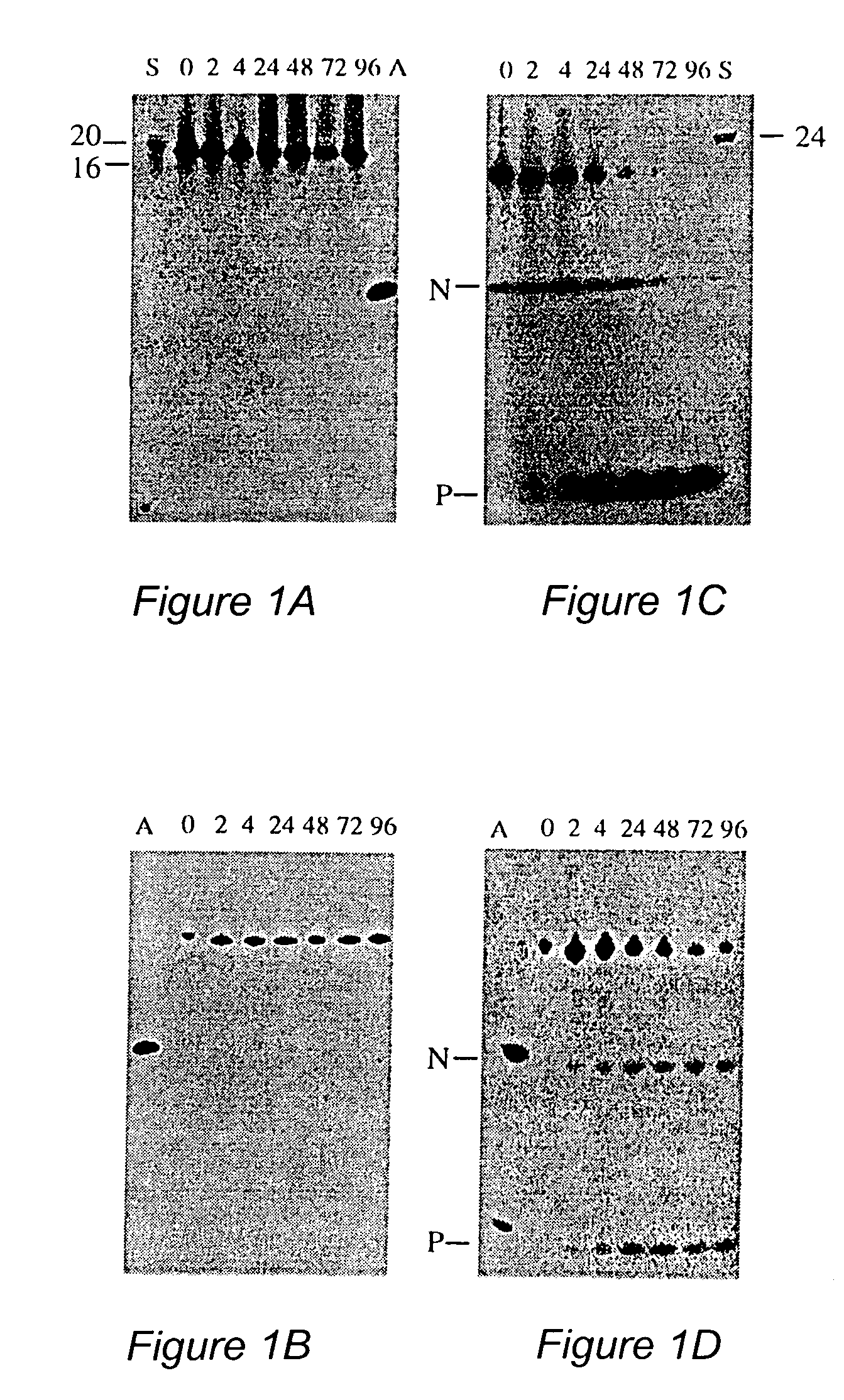
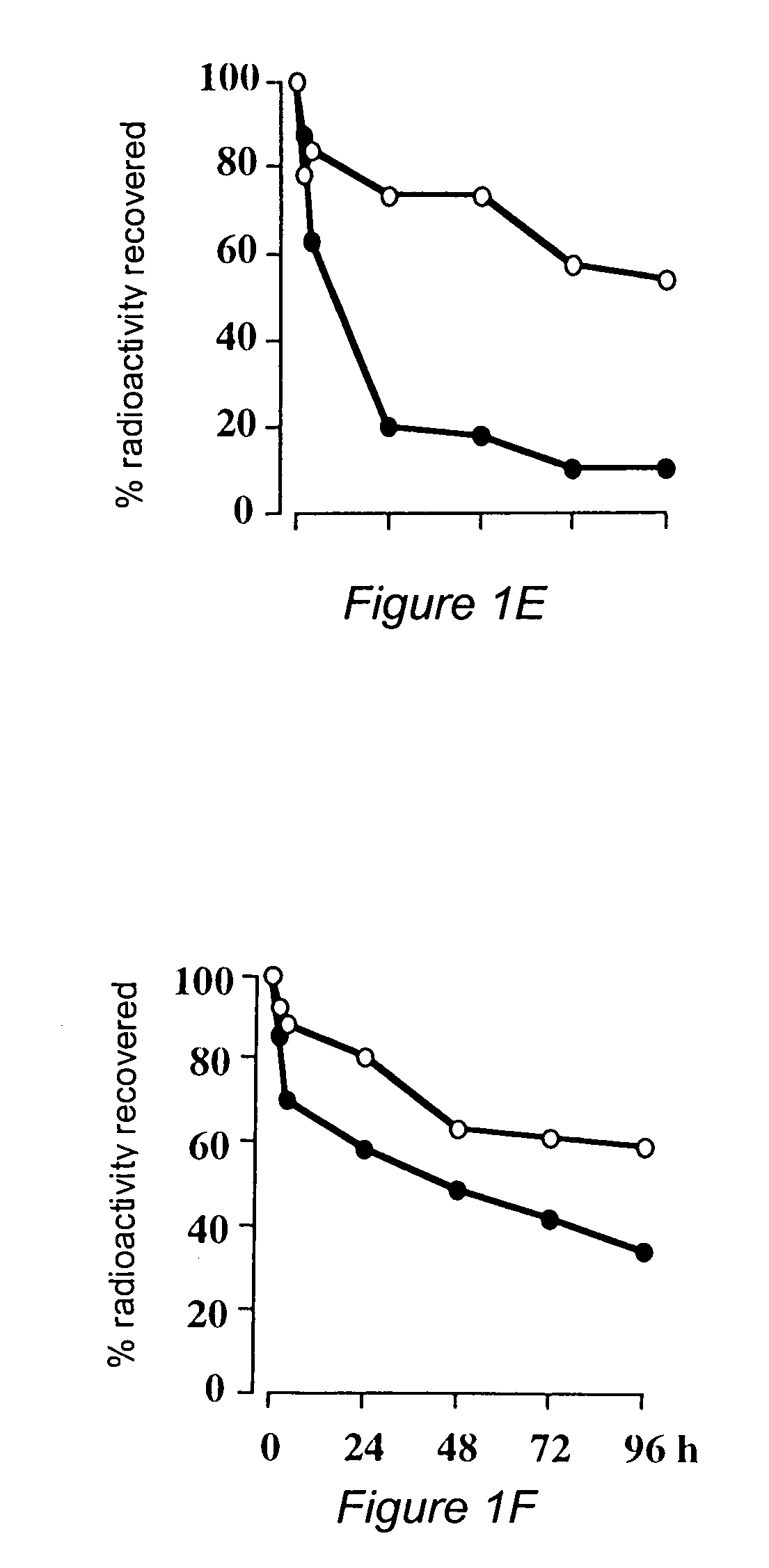
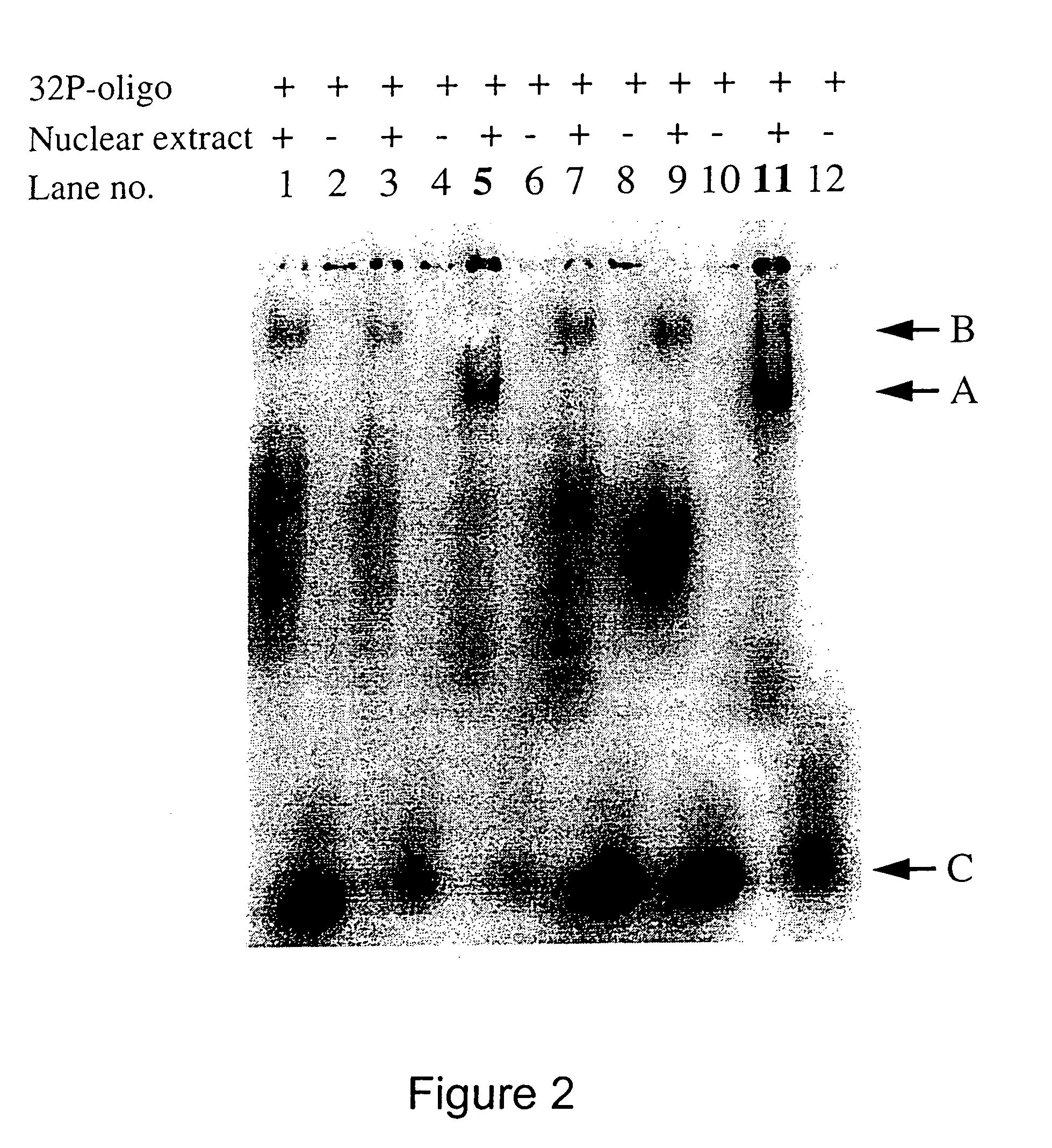
ANTI-HLA CLASS-Ib ANTIBODIES MIMIC IMMUNOREACTIVITY AND IMMUNOMODULATORY FUNCTIONS OF INTRAVENOUS IMMUNOGLOBULIN (IVIg) USEFUL AS THERAPEUTIC IVIg MIMETICS AND METHODS OF THEIR USE
InactiveUS20130177574A1Market price thereof has been risingMinimizing IVIg related side effectAntipyreticAnalgesicsDiseaseAntigen
Provided herein are compositions comprising anti-HLA-Ib antibodies as IVIg mimetics and methods for using the same for the prevention, treatment, therapy and / or amelioration of inflammation induced diseases and allograft rejection. In certain embodiments, the anti-HLA-Ib antibodies (monoclonal antibodies or mixed monoclonal antibodies, recombinant or chimeric or humanized or human antibodies) strongly mimic IVIg in immunoreactivity to HLA class Ia (HLA-A, HLA-B and HLA-Cw) and Ib antigens (HLA-E, HLA-F and HLA-G). In certain embodiments, the anti-HLA-Ib antibodies (monoclonal or mixed monoclonal antibodies; recombinant, chimeric, humanized or human antibodies) strongly mimic IVIg in immunomodulatory or immunosuppressive activities. While anti-HLA-Ib mAbs can be used to restore anti-tumor activities of CD8+ T cells and Natural killer cells by passive therapy in cancer patients, methods are also provided herein to induce production of polyclonal anti-HLA-Ib antibodies in cancer patients for restoring anti-tumor activities of CD8+ T cells and NK cells, by active specific immunotherapy.
Owner:RAVINDRANATH MEPUR DR
G-rich oligo aptamers and methods of modulating an immune response
InactiveUS6994959B1Modulate T cell responseInhibit transcriptionOrganic active ingredientsNervous disorderInsulin dependentAllograft rejection
Aptamer oligonucleotides specifically bind to the DNA binding site of proteins such as Sp1 and Sp1-related proteins which regulate the genes which encode costimulatory molecules such as CD28 and cytokines such as IL-2 and GMCSF. The oligonucleotides compete with the DNA-binding sites of regulatory proteins which specifically regulate molecules to modulate T-cell activation. This serves to modulate gene expression by preventing transcription of the gene. Aptamers are administered to provide therapies for diseases which involve aberrant T-cell activation such as psoriasis, Type I (insulin-dependent) diabetes mellitus, multiple sclerosis, autoimmune uveitis, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease (Crohn's and ulcerative colitis), and septic shock and to regulate normal T-cell activation such as in allograft rejection.
Owner:VALEANT RES & DEV
High affinity antibodies against HMGB1 and methods of use thereof
InactiveUS7585504B2Reduce bone loss and/or cartilage damageAntibacterial agentsAntibody mimetics/scaffoldsReperfusion injuryAllograft rejection
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn's disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:MEDIMMUNE LLC
Methods of diagnosing rejection of a kidney allograft using genomic or proteomic expression profiling
A method of determining the acute allograft rejection status of a subject, the method comprising the steps of: determining the nucleic acid expression profile of one or more than one nucleic acid markers, or one or more than one proteomic markers in a biological sample from the subject; comparing the expression profile of the one or more than one nucleic acid markers to a control profile; and determining whether the expression level of the one or more than one nucleic acid markers is increased relative to the control profile, wherein the increase of the one or more than one nucleic acid markers is indicative of the acute rejection status of the subject.
Owner:THE UNIV OF BRITISH COLUMBIA
Immunogenic peptides and their use in transplantation
ActiveUS9248171B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsOxidation-Reduction AgentRedox
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C-(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
Immunogenic peptides and their use in transplantation
ActiveUS20110110964A1Preventing allograft rejectionPeptide/protein ingredientsAntibody mimetics/scaffoldsRedoxAllograft rejection
The present invention relates to the use of immunogenic peptides comprising a T-cell epitope derived from an allograft antigen and a redox motif such as C—(X)2-[CST] or [CST]-(X)2-C in the prevention and / or treatment of allograft rejection and in the manufacture of medicaments therefore.
Owner:IMCYSE
Use of ECDI-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
High Affinity Antibodies Against HMGB1 and Methods Of Use Thereof
InactiveUS20100061987A1Reduce bone loss and/or cartilage damageAntibacterial agentsAntipyreticAntiendomysial antibodiesAllograft rejection
Owner:MEDIMMUNE LLC
Oral tolerance induction by collagen to prevent allograft rejection
InactiveUS20030078208A1Peptide/protein ingredientsSnake antigen ingredientsAllograft rejectionOral tolerance
The present invention relates to the use of collagen and MHC-like compounds to down regulate immune responses. Methods for administration of such compounds that induce immune tolerance are described. The invention is important in the context of allograft rejection, transplantation, graft rejection, pleural disease and immunotolerance.
Owner:INDIANA UNIV RES & TECH CORP
High affinity antibodies against hmgb1 and methods of use thereof
InactiveUS20090169546A1Useful in treatmentReduce hyperostosisAntipyreticMicrobiological testing/measurementReperfusion injurySpinal cord
Compositions and methods are disclosed for inhibiting the release of a proinflammatory cytokine from a vertebrate cell, and for inhibiting an inflammatory cytokine cascade in a patient. The compositions comprise, for example, high affinity antibodies that specifically bind HMG1 and antigenic fragments thereof. The high affinity antibodies of the present invention and pharmaceutical compositions comprising the same are useful for many purposes, for example, as therapeutics against a wide range of inflammatory diseases and disorders such as sepsis, rheumatoid arthritis, peritonitis, Crohn s disease, reperfusion injury, septicemia, endotoxic shock, cystic fibrosis, endocarditis, psoriasis, psoriatic arthritis, arthritis, anaphylactic shock, organ ischemia, reperfusion injury, and allograft rejection. In addition, the high affinity antibodies of the present inventions are useful as diagnostic antibodies.
Owner:CORNERSTONE THERAPEUTICS +1
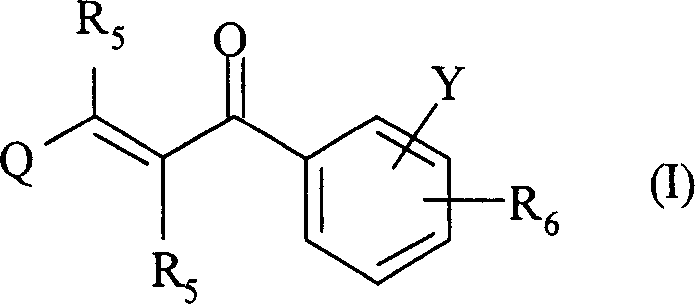
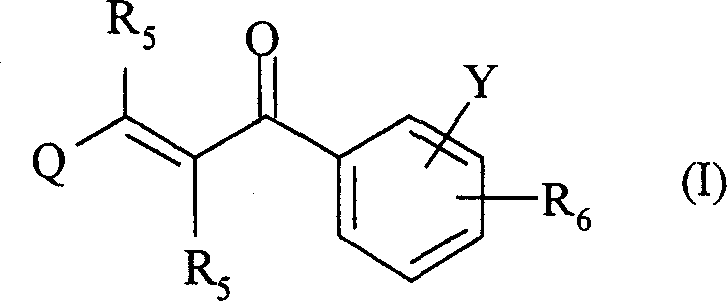
2-propylene-1-ones as hsp70 inducer
The present invention relates to novel compounds of 2-propene-1-one series, of general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, pharmaceutically acceptable salts, pharmaceutically acceptable solvates and pharmaceutically acceptable compositions containing them, wherein R5, R6, Q and Y are as defined in the specification. The present invention also relates to a process for preparing such compounds, compositions containing such compounds, and use of such compound and composition in medicine. The compounds of the general formula (I) induce HSP-70 and are useful for the treatment of diseases accompanying pathological stress in a living mammalian organism, including a human being, such as stroke, myocardial infarction, inflammatory disorder, hepatotoxicity, sepsis, diseases of viral origin, allograft rejection, tumourous diseases, gastric mucosal damage, brain haemorrhage, endothelial dysfunctions, diabetic complications, neuro-degenerative diseases, post-traumatic neuronal damage, acute renal failure, glaucoma and aging related skin degeneration.
Owner:TORRENT PHARMA LTD
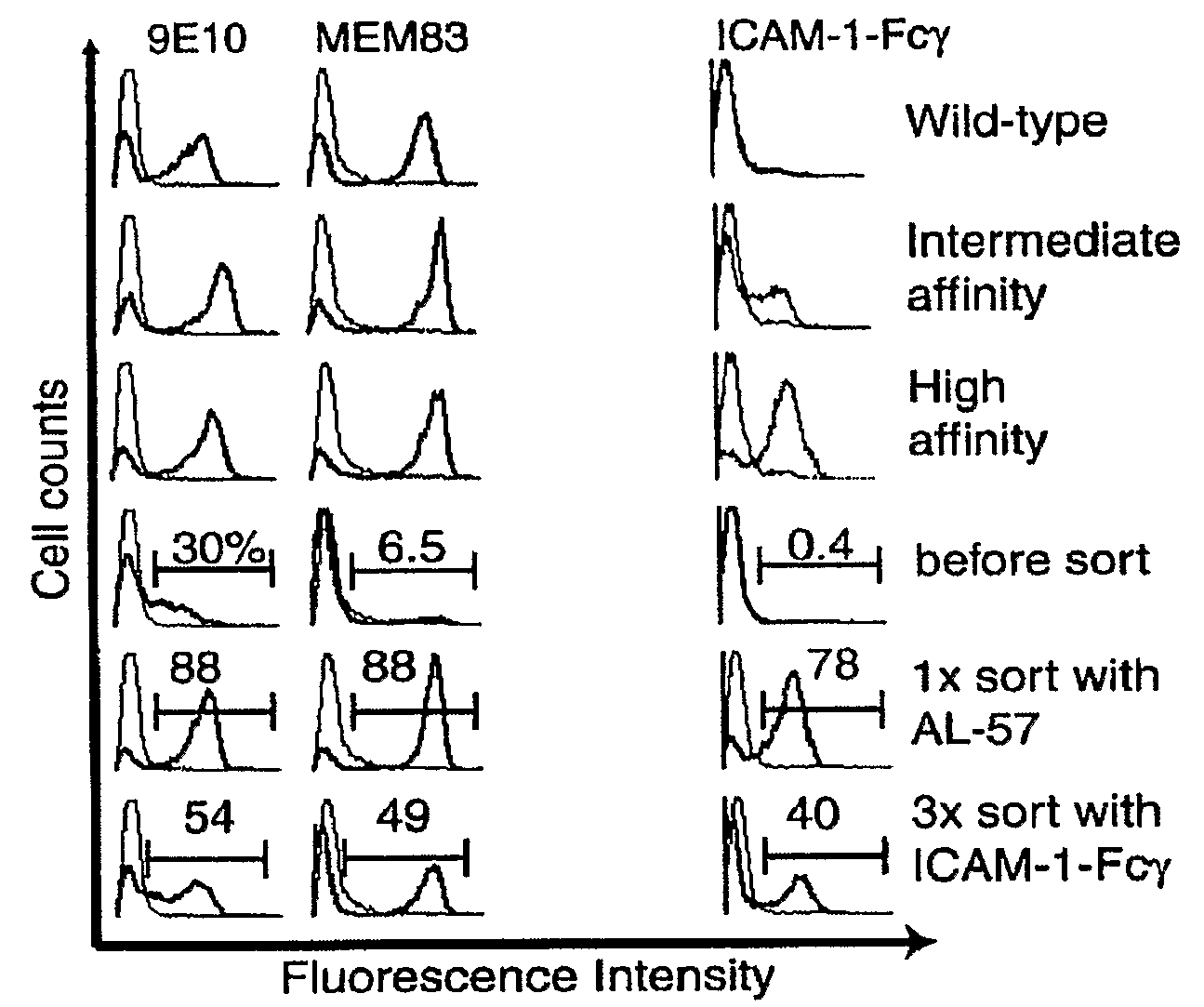
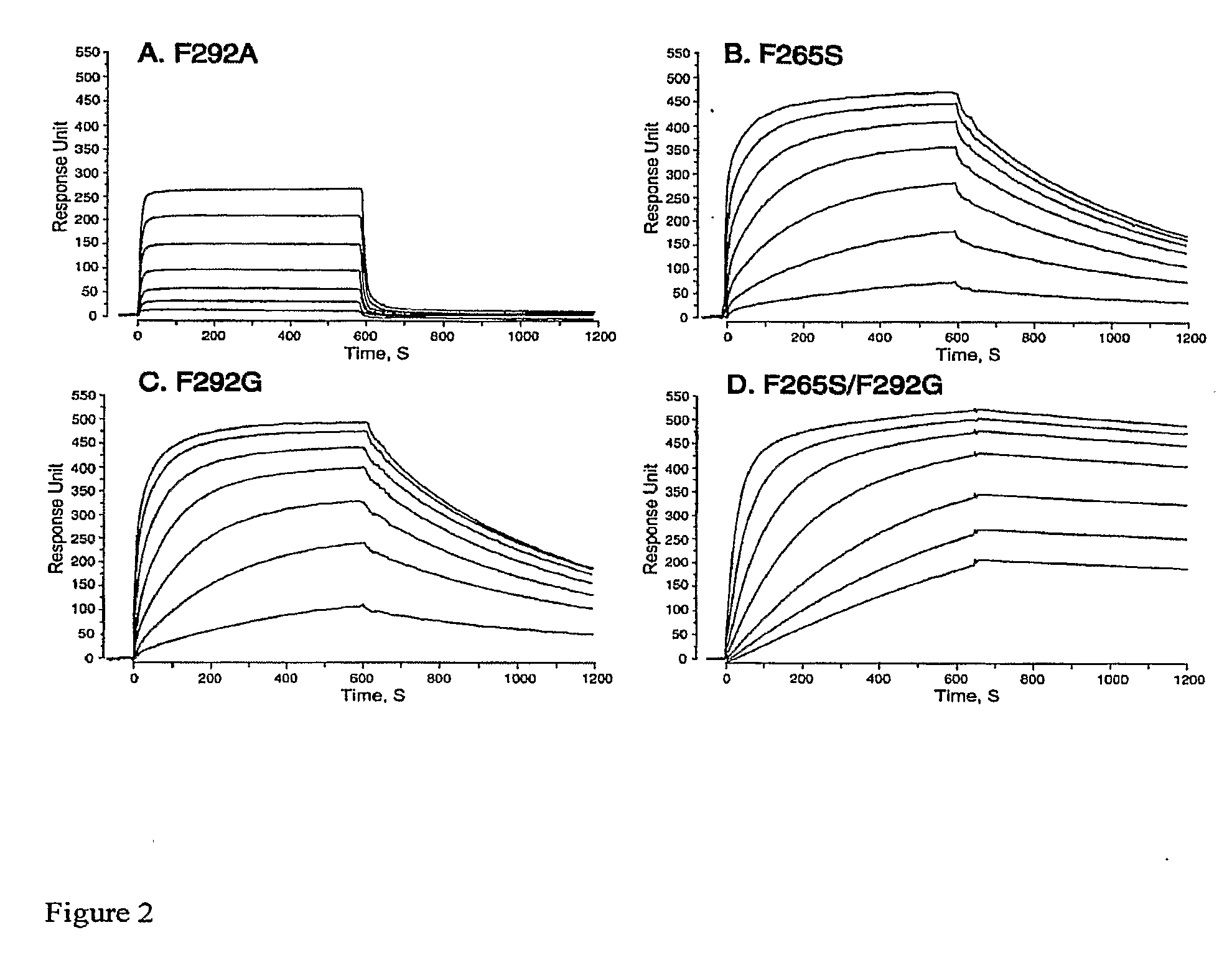
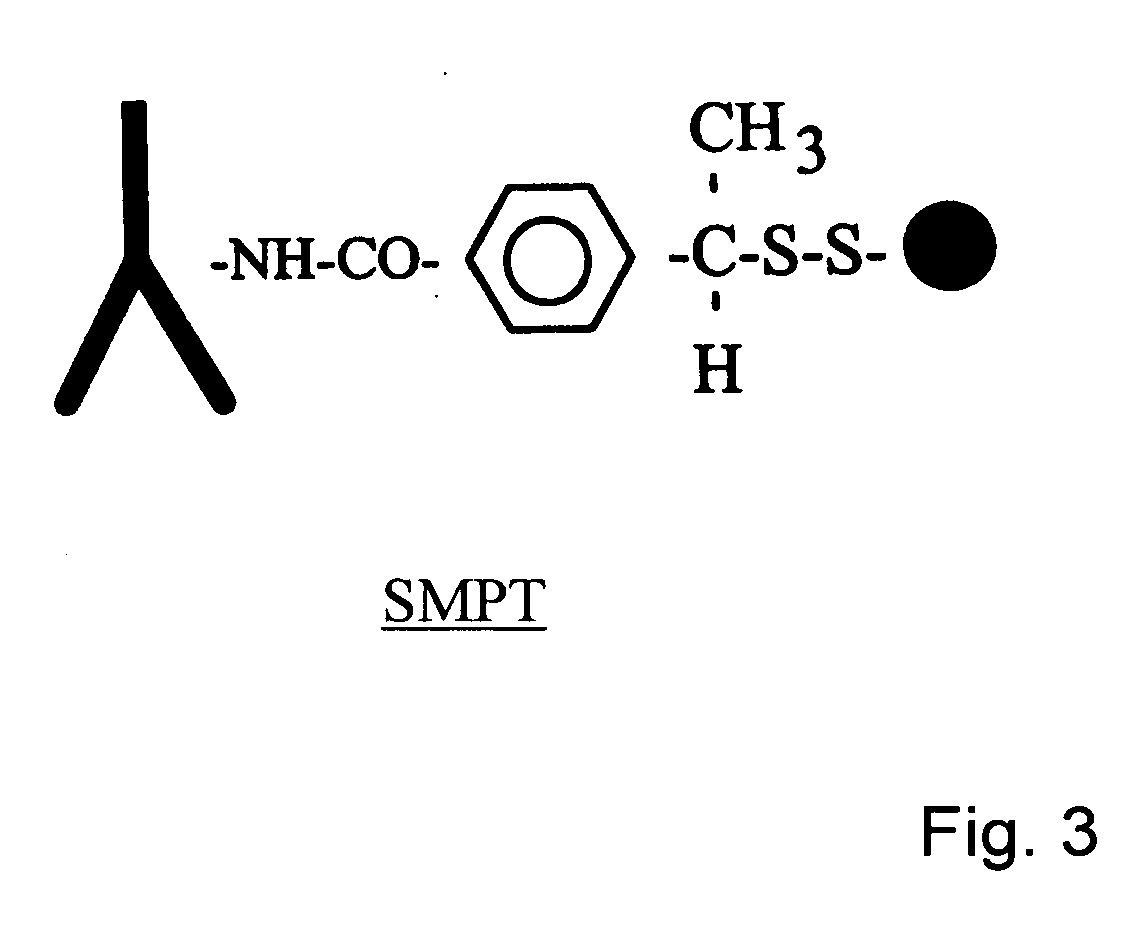
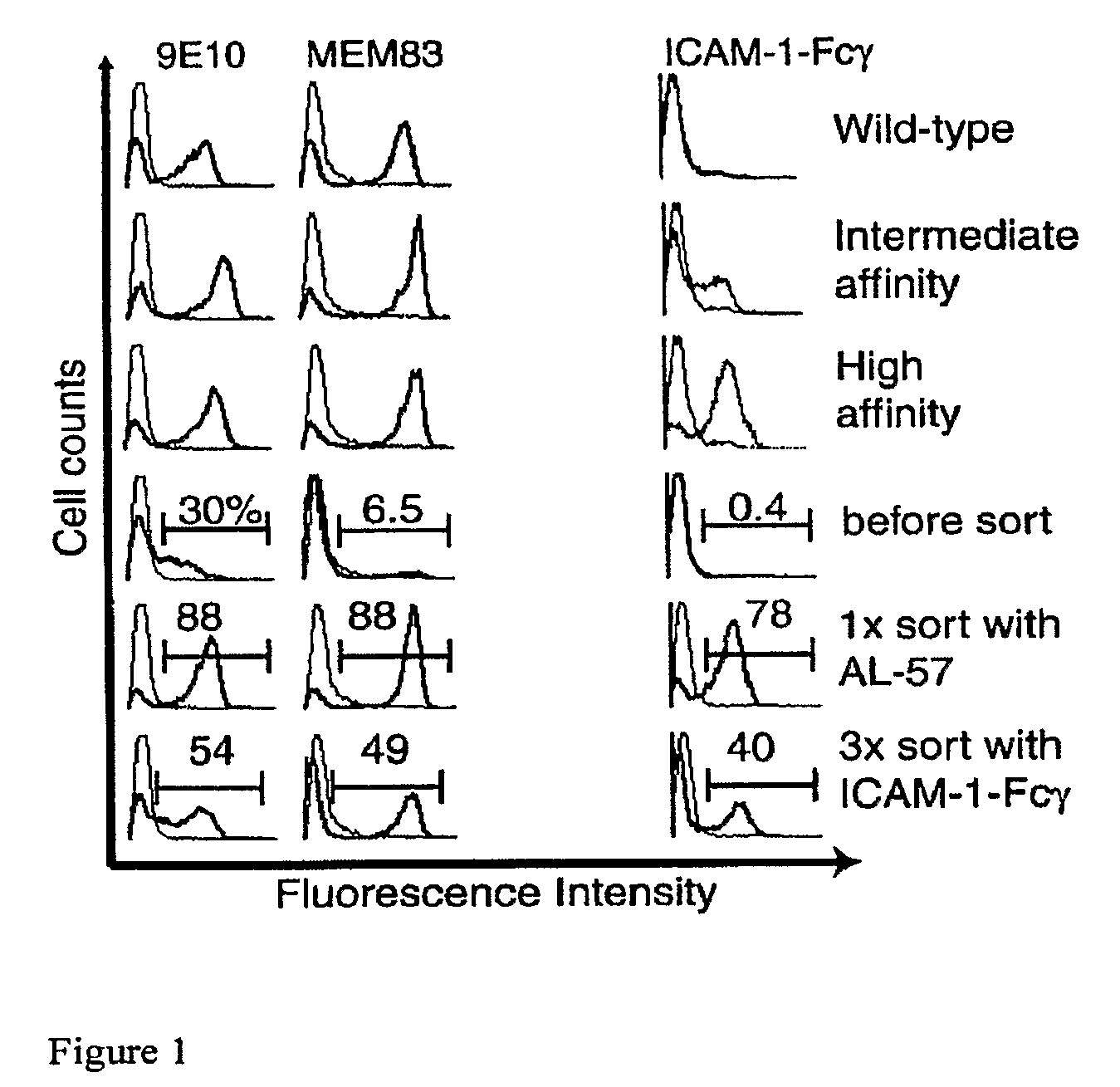
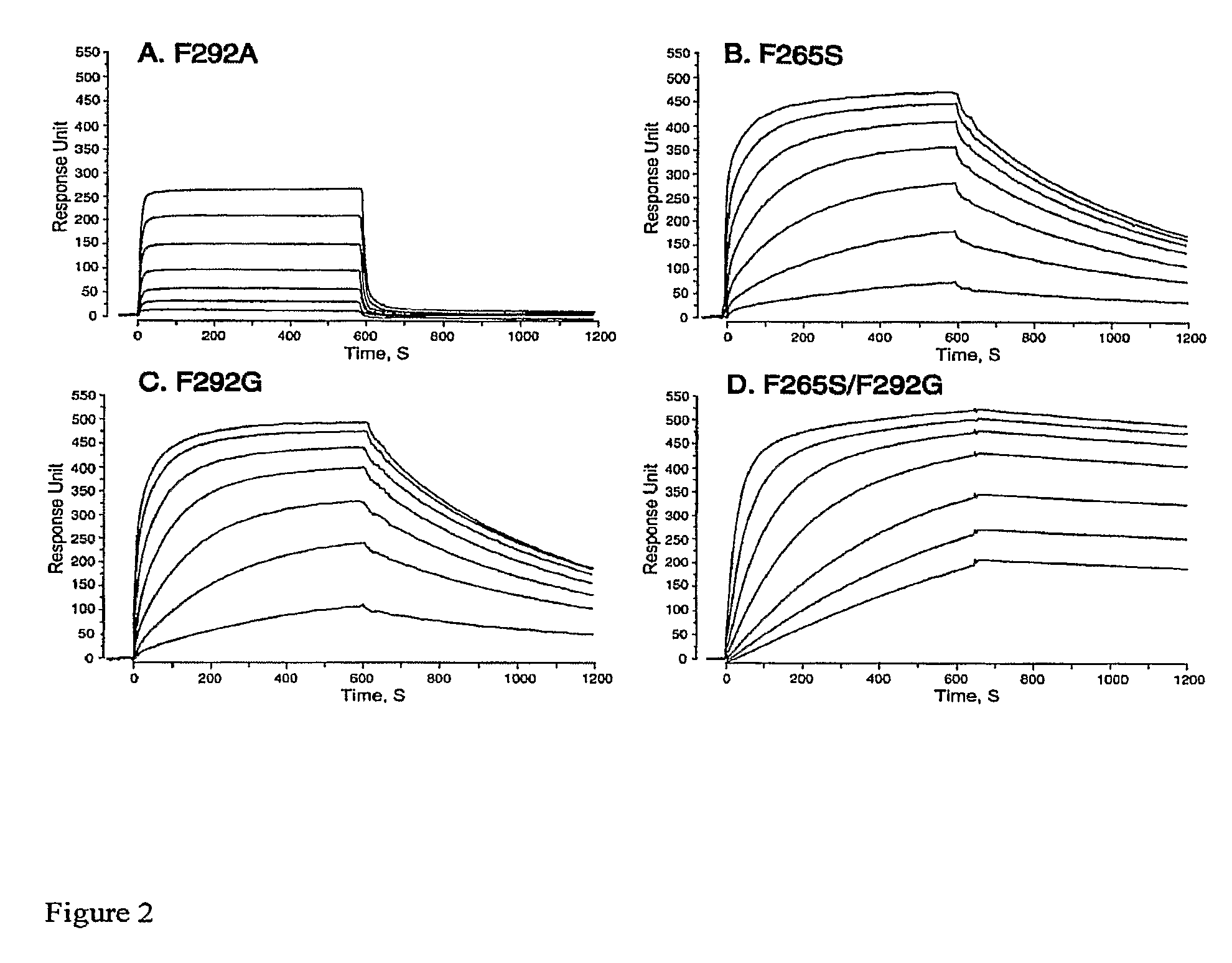
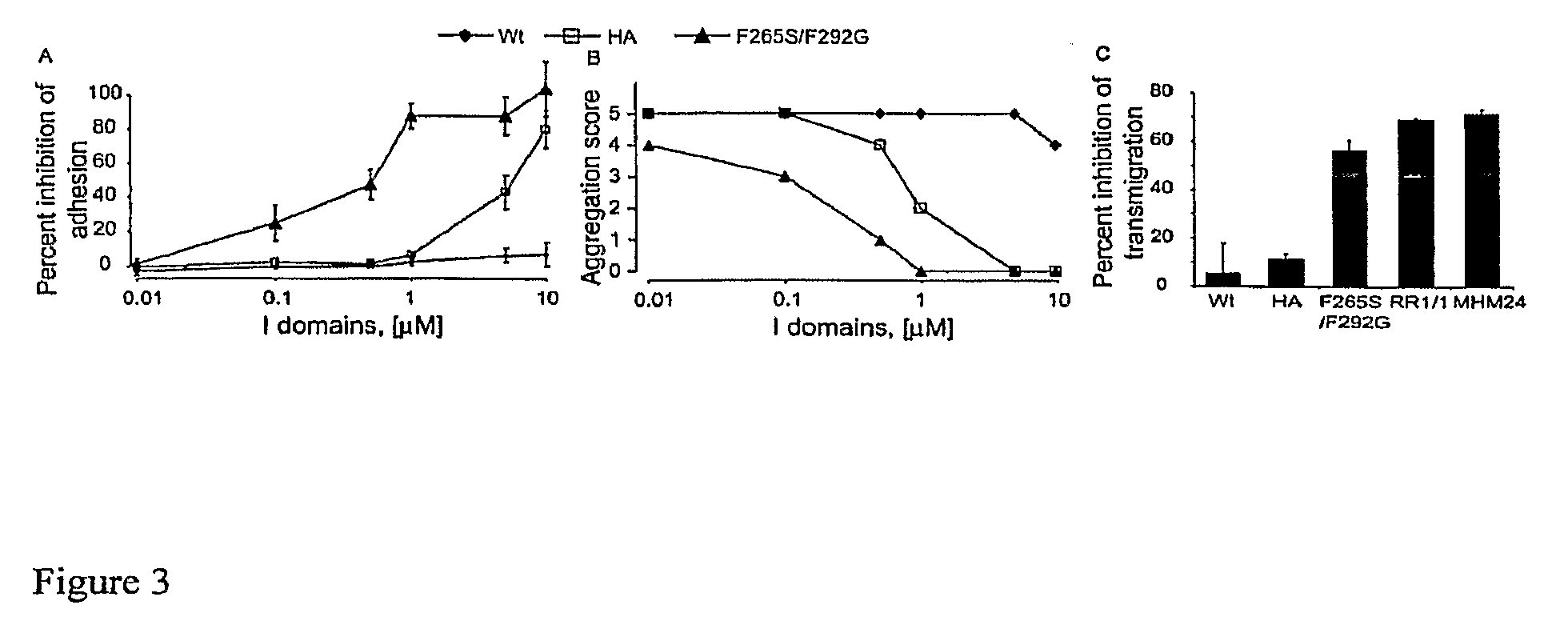
Integrin Alpha L I Domain Mutants with Increased Binding Affinity
The present invention provides an isolated polypeptide capable of binding to aCAM-1, comprising the integrin (XL I domain or biologically active portion thereof, wherein one or more residues is substituted, wherein the substituted polypeptide binds ICAM-I at a higher affinity than wild type integrin CCL protein. The invention provides a method for inhibiting ICAM-I and a pharmaceutical composition comprising an integrin (XL I domain polypeptide or biologically active portion of the polypeptides. The invention also provides a method of treating or preventing an LFA-I mediated ICAM-1 associated disease such as inflammation, artherosclerosis, allograft rejection, diabetes, T-cell mediated sensitization reaction, psoriasis, HIV infection, or rheumatoid arthritis.
Owner:IMMUNE DISEASE INST INC
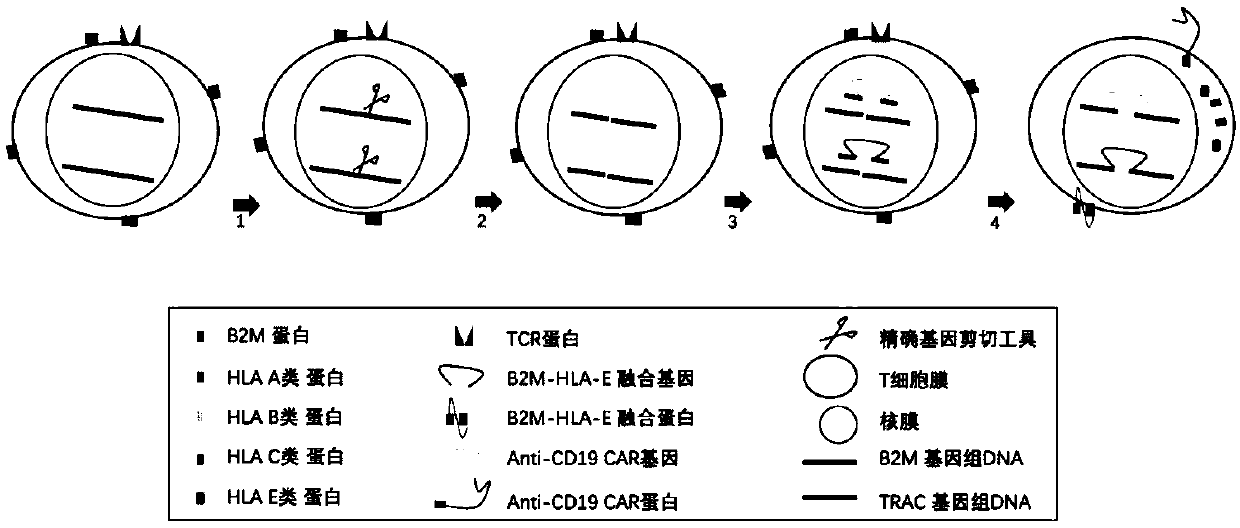
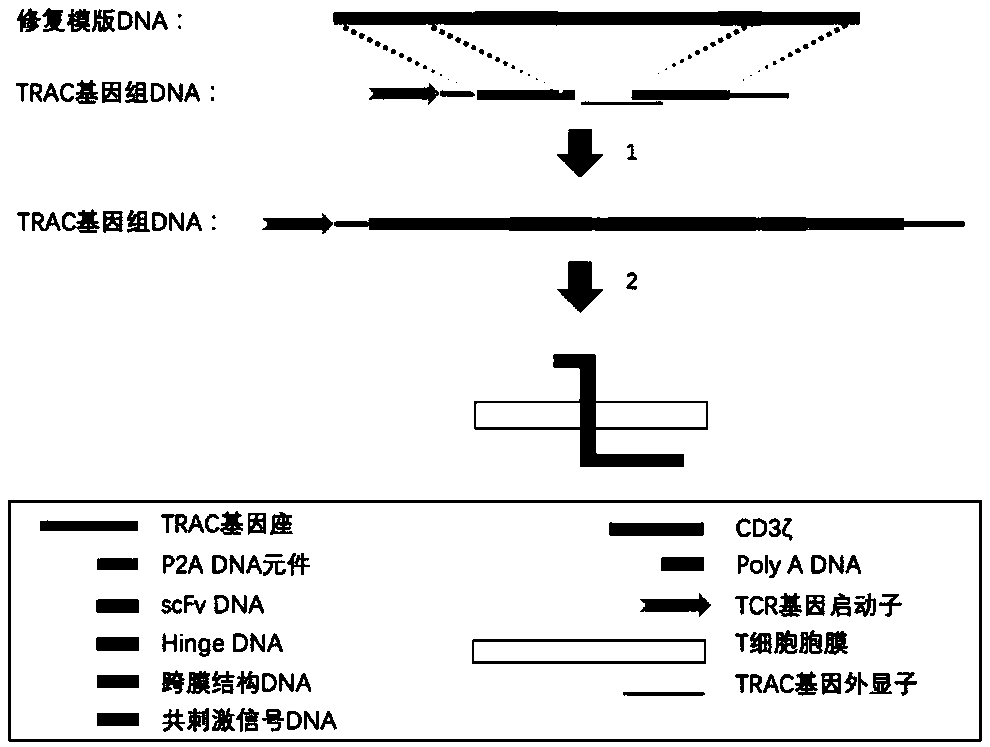
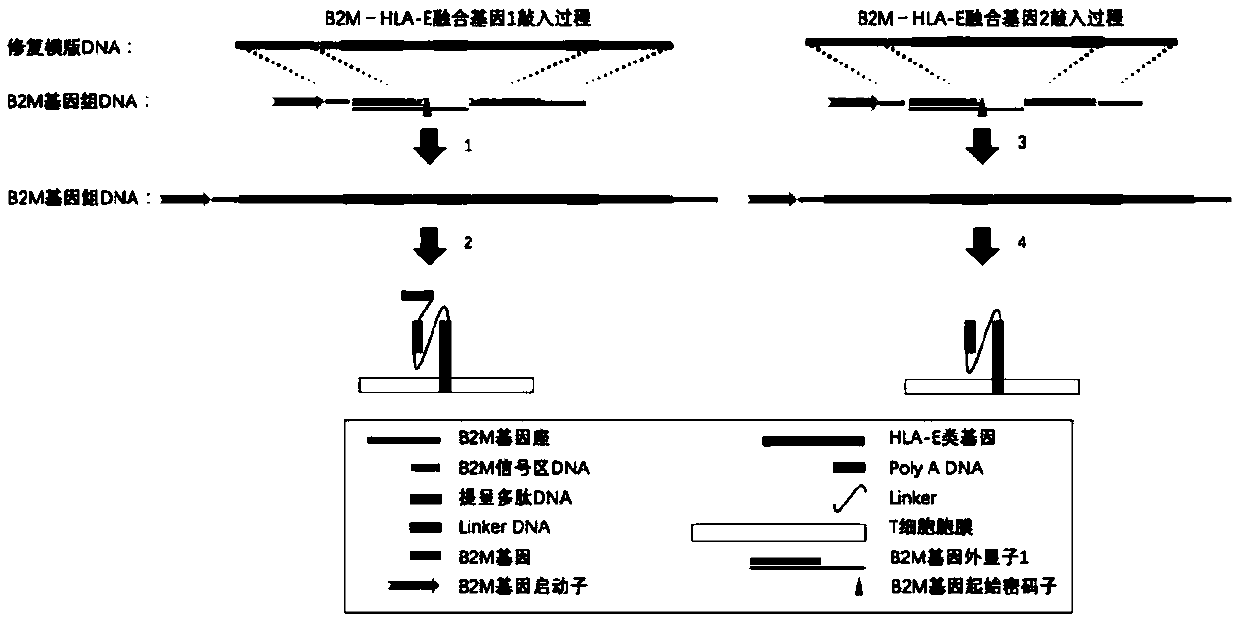
Preparation and Application of Universal Targeting CD19 Antigen Chimeric Receptor T Cells
ActiveCN110616189AVigorousReduce rejectionAntibody mimetics/scaffoldsMammal material medical ingredientsAllograft rejectionCardiac allograft
The invention relates to a preparation and an application of universal targeted CD19 antigen chimeric receptor T cells. Concretely, the invention relates to a method for preparing targeted CD19 antigen chimeric receptor T cells. The method comprises delivering a gene editing substance to the T cell to shear TRAC and B2M genes; and cloning the targeted CD19 CAR and B2M-HLA-E fusion gene into a repair template vector to simultaneously introduce targeted CD19 CAR and B2M-HLA-E fusion gene homologous recombination repair templates into T cells. The universal targeted CD19 antigen chimeric receptorT cell has the advantages of small allograft rejection reaction, high yield, high safety, immediate availability, wide application range and the like.
Owner:西安桑尼赛尔生物医药有限公司
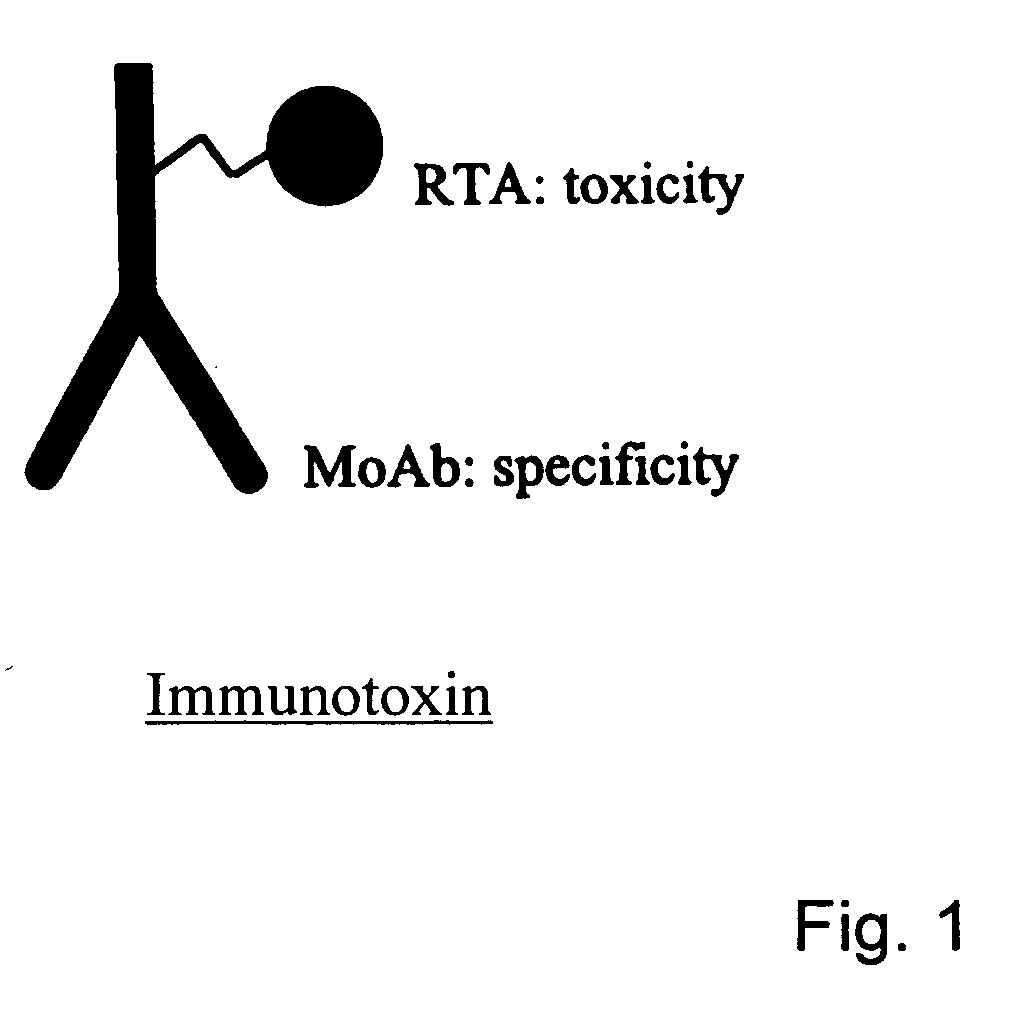
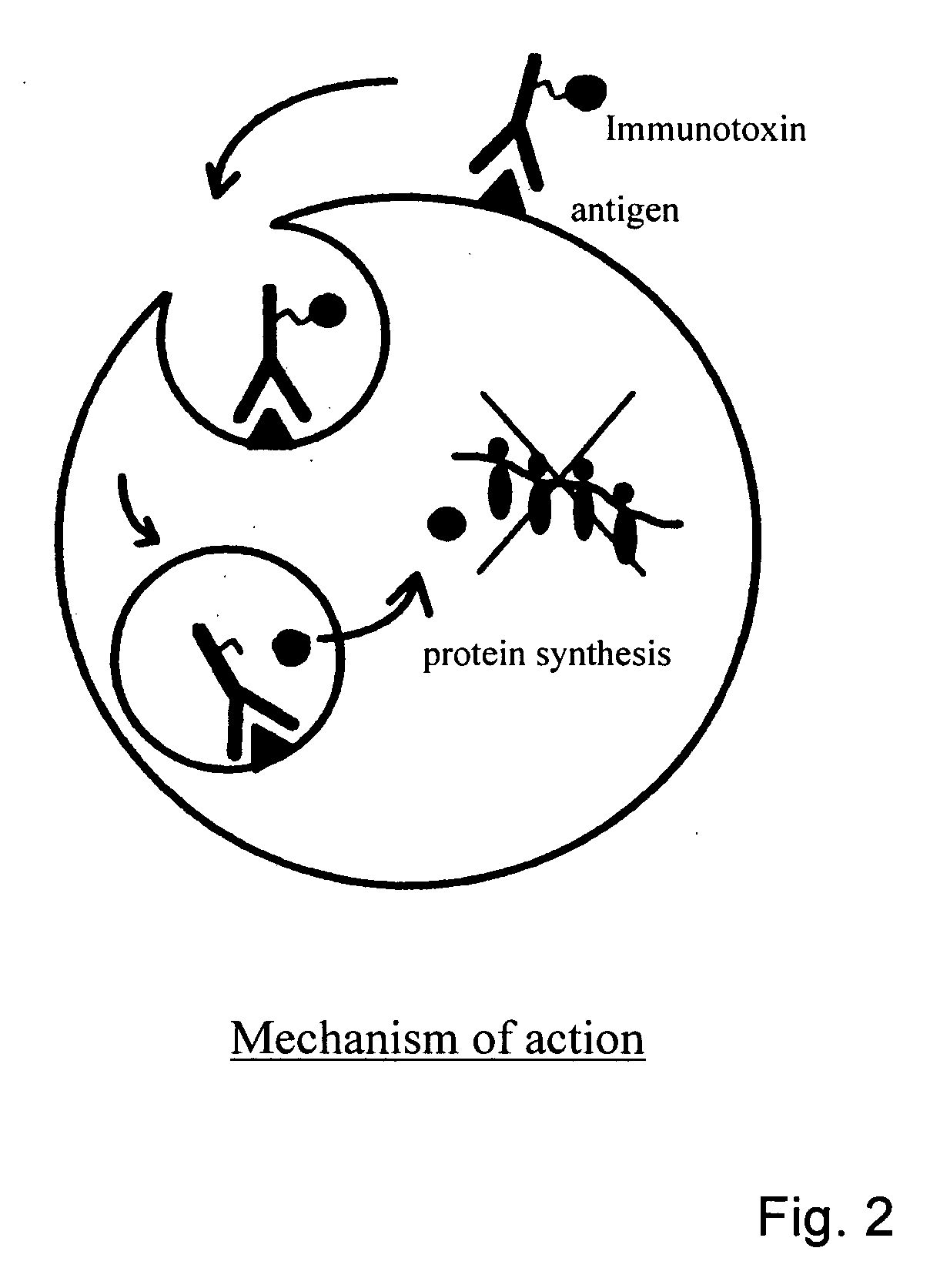
Methods and means for the treatment of immune-related diseases
The present invention provides novel means and methods for treating unwanted side effects in transplantations, such as GVHD and allograft rejection. The invention provides immunotoxins comprising an antibody and a toxic substance, whereby cocktails of such conjugates are directed to different targets associated with one population of cells, wherein one target is chosen from CD3 or CD7. The preferred combination is a cocktail directed against both CD3 and CD7.
Owner:HENOGEN BV
Integrin alpha L I domain mutants with increased binding affinity
The present invention provides an isolated polypeptide capable of binding to aCAM-1, comprising the integrin (XL I domain or biologically active portion thereof, wherein one or more residues is substituted, wherein the substituted polypeptide binds ICAM-I at a higher affinity than wild type integrin CCL protein. The invention provides a method for inhibiting ICAM-I and a pharmaceutical composition comprising an integrin (XL I domain polypeptide or biologically active portion of the polypeptides. The invention also provides a method of treating or preventing an LFA-I mediated ICAM-1 associated disease such as inflammation, artherosclerosis, allograft rejection, diabetes, T-cell mediated sensitization reaction, psoriasis, HIV infection, or rheumatoid arthritis.
Owner:IMMUNE DISEASE INST INC
5-lipoxygenase inhibitors
The present invention relates to 5-lipoxygenase inhibitors. Compounds disclosed herein can be useful in the treatment of bronchial asthma, chronic obstructive pulmonary disorder, arthritis, type I diabetes, multiple sclerosis, allograft rejection, psoriasis, inflammatory bowel disease, ulcerative colitis, acne, atherosclerosis, cancer, pruritis, urticaria, atopic dermatitis, allergic rhinitis, other inflammatory and autoimmune diseases. Processes for the preparation of disclosed compounds, pharmaceutical compositions containing the disclosed compounds and their use as 5-lipoxygenase inhibitors are also provided.
Owner:RANBAXY LAB LTD
Methods for the treatment and prevention of inflammatory diseases
The invention includes processes mainly for the treatment of a inflammatory diseases, such as inflammatory bowel disease, arthritis, atherosclerosis, asthma, allergy, inflammatory kidney disease, circulatory shock, multiple sclerosis, chronic obstructive pulmonary disease, skin inflammation, periodontal disease, psoriasis and T cell-mediated diseases of immunity, including allergic encephalomyelitis, allergic neuritis, transplant allograft rejection, graft versus host disease, myocarditis, thyroiditis, nephritis, systemic lupus erthematosus, and insulin-dependent diabetes mellitus. The processes involve treating a patient with a pharmaceutical composition containing an active ingredient that inhibits the activity of sphingosine kinase.
Owner:APOGEE BIOTECHNOLOGY CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com