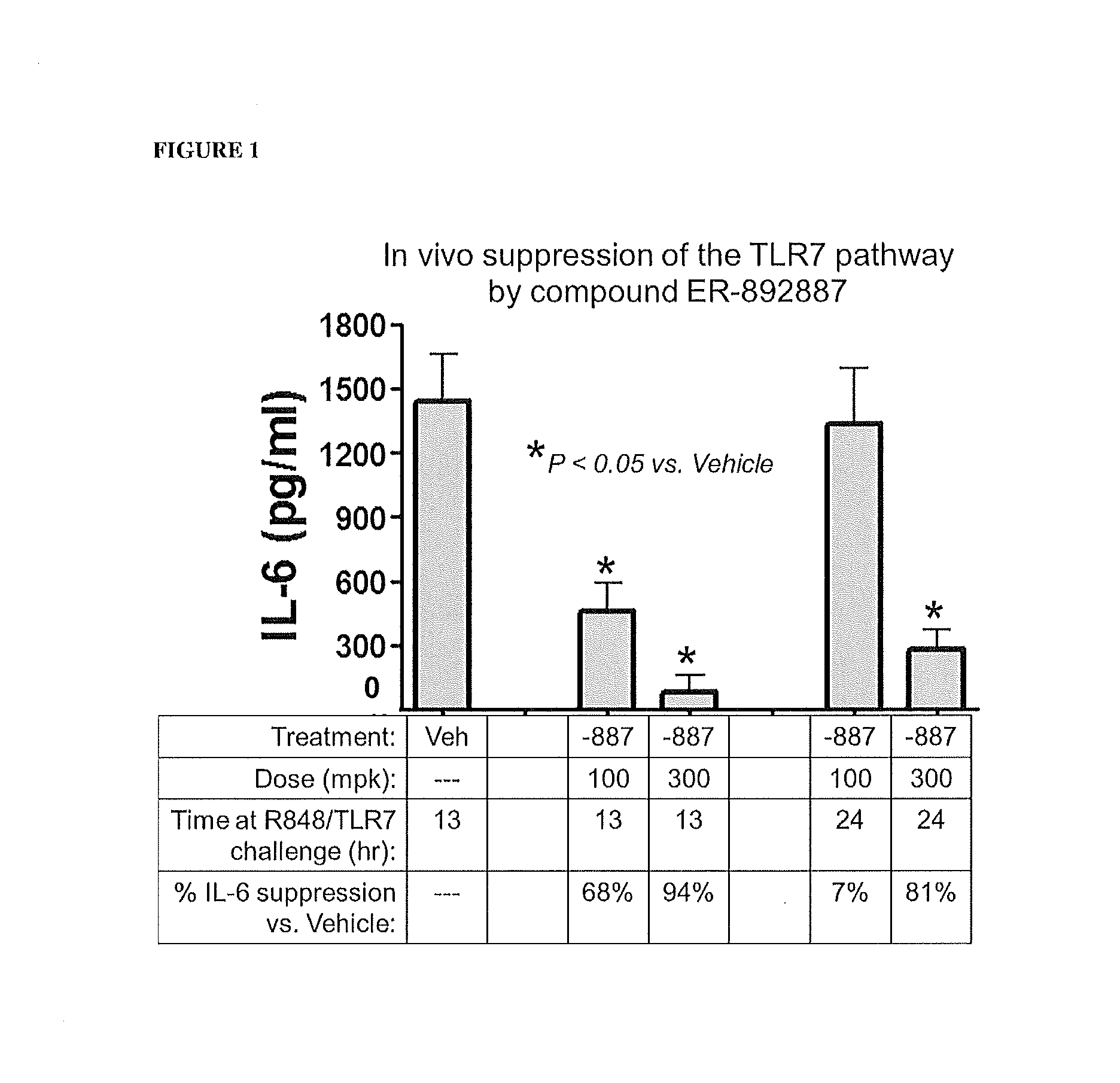
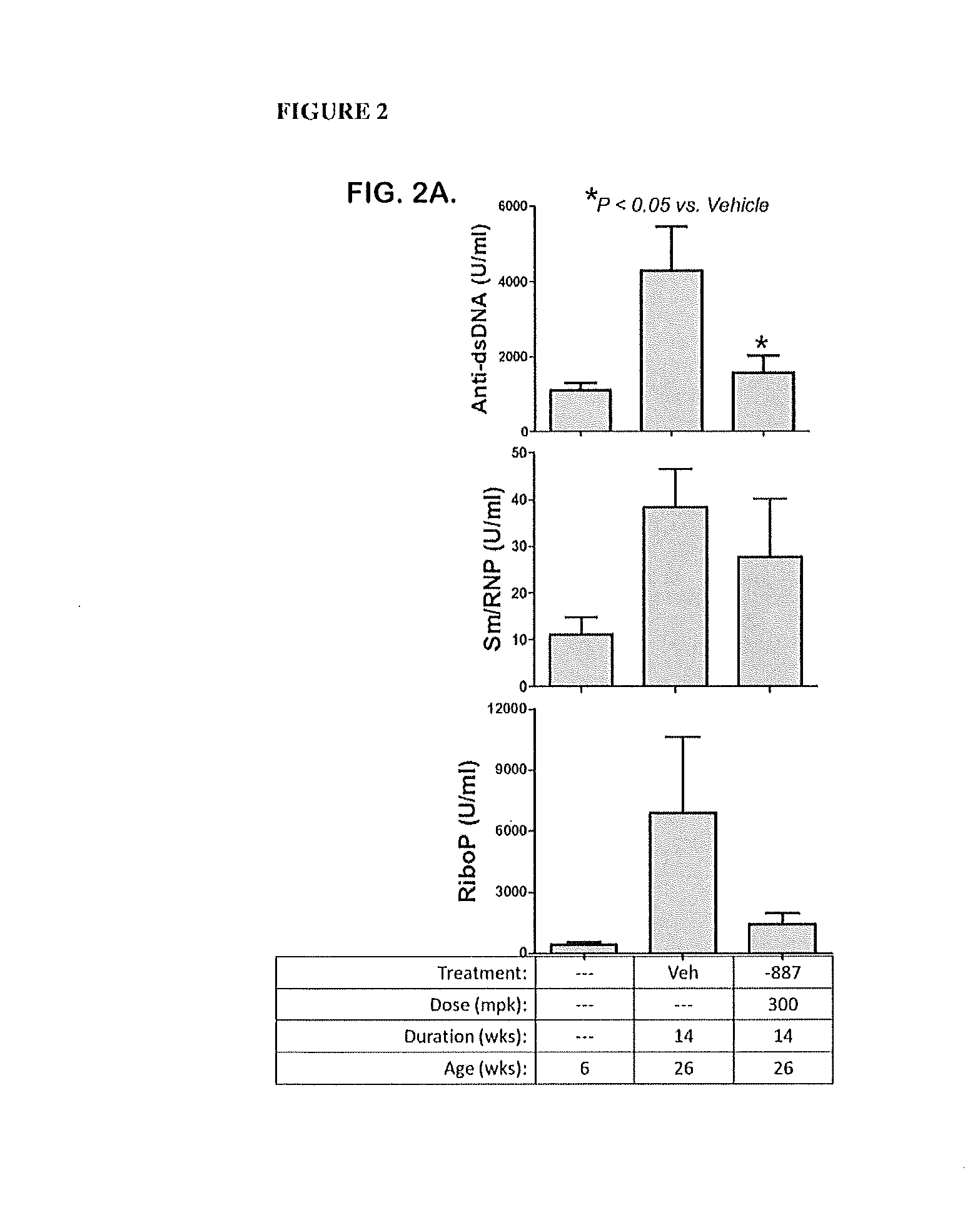
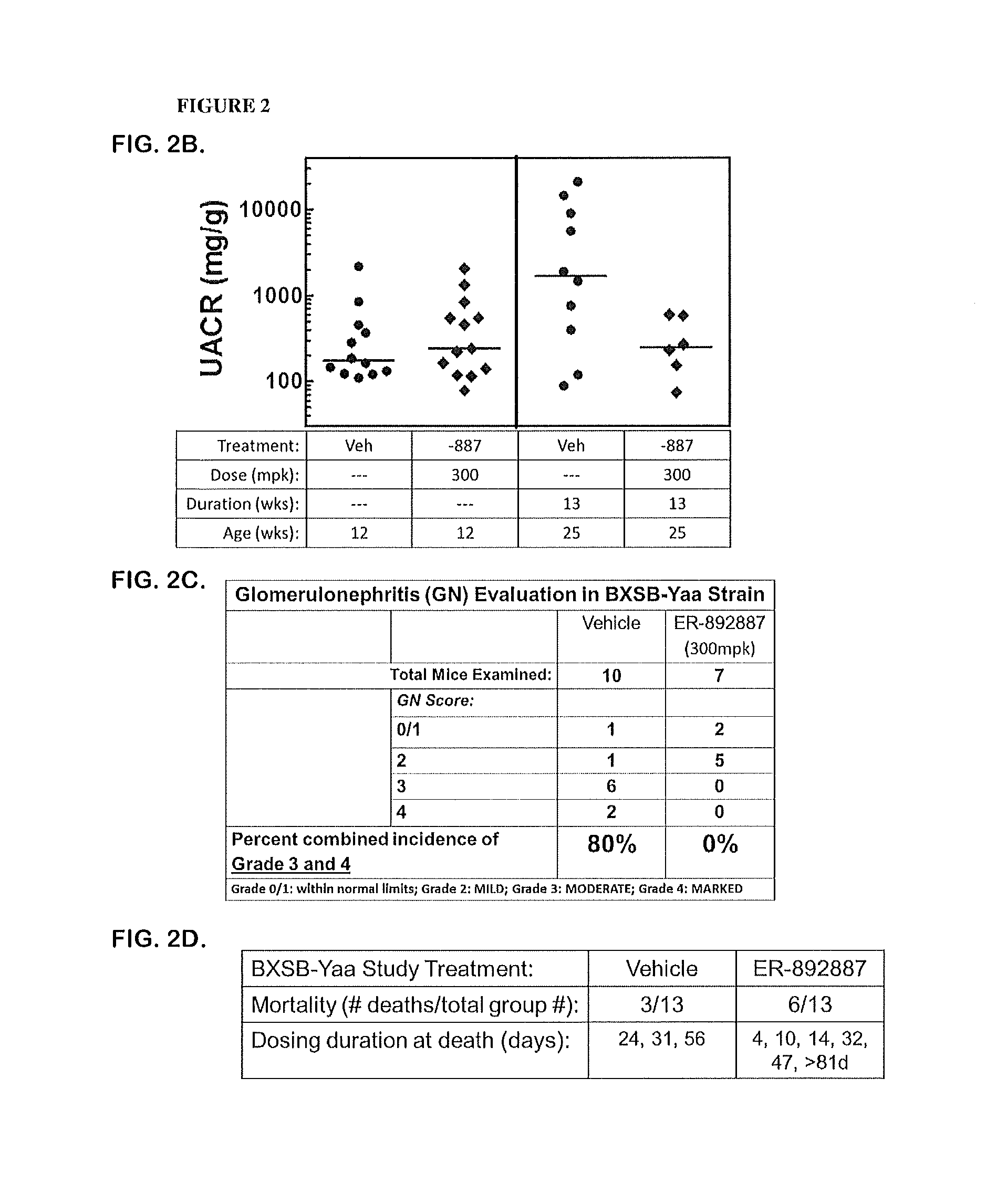
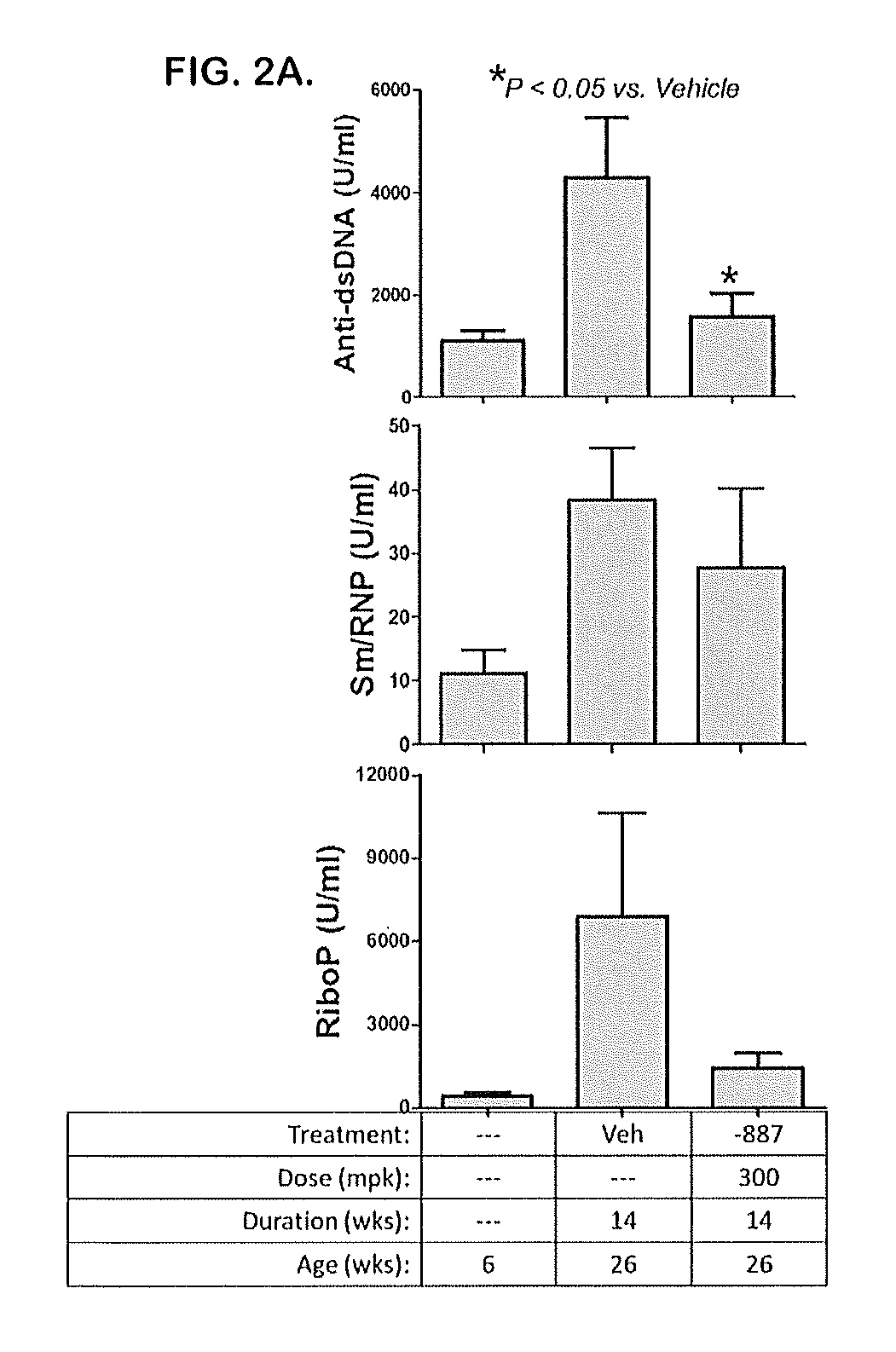
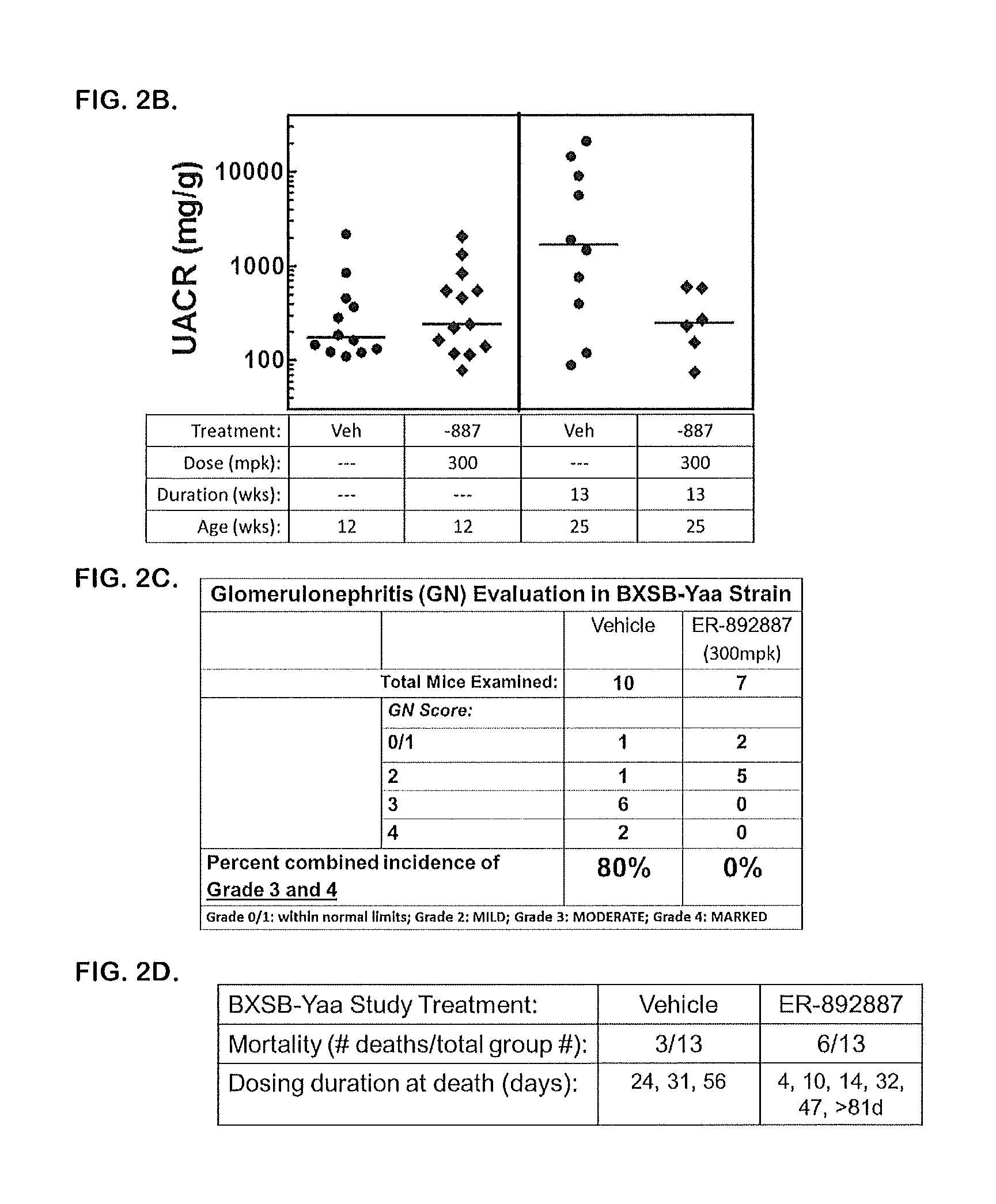
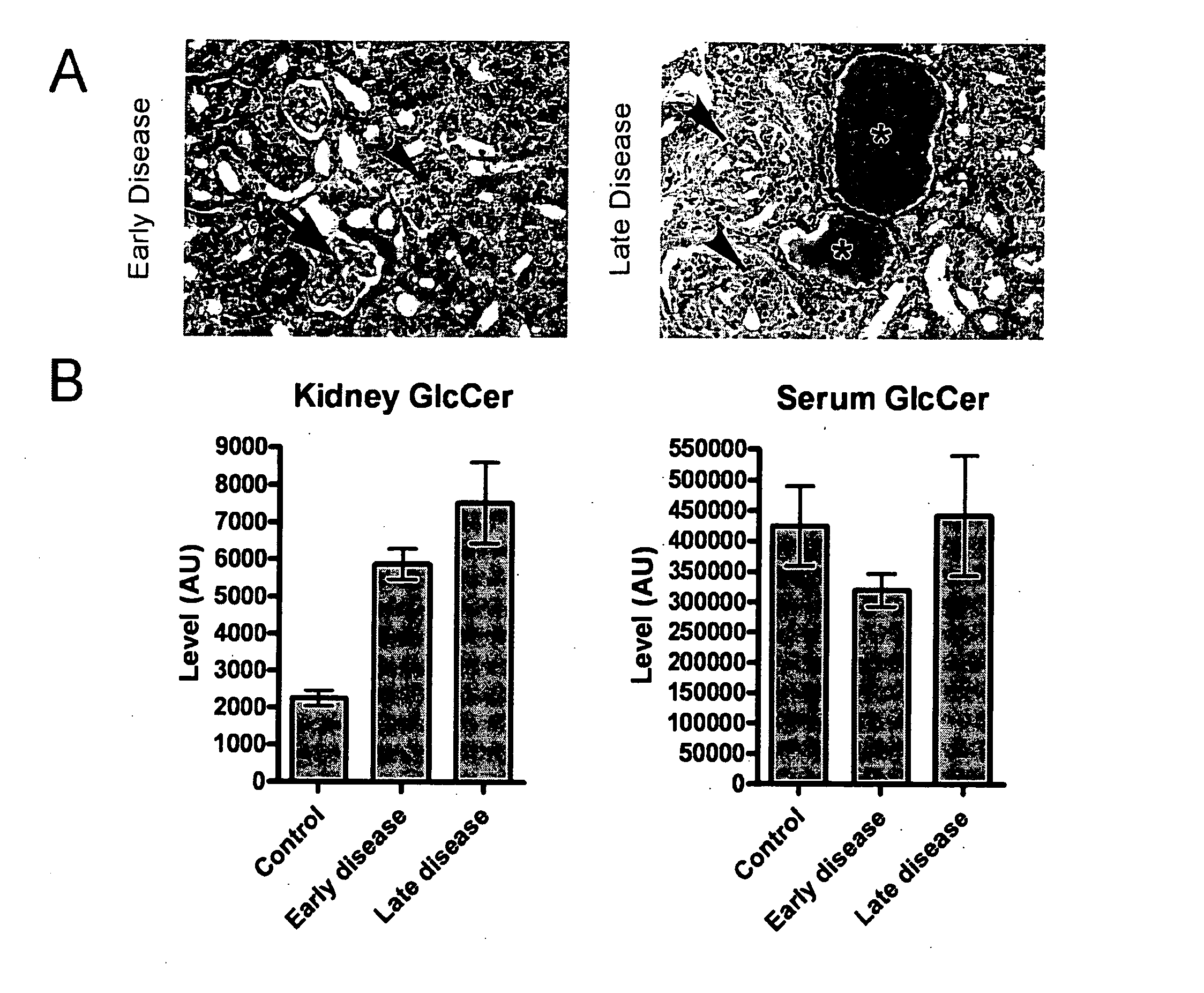
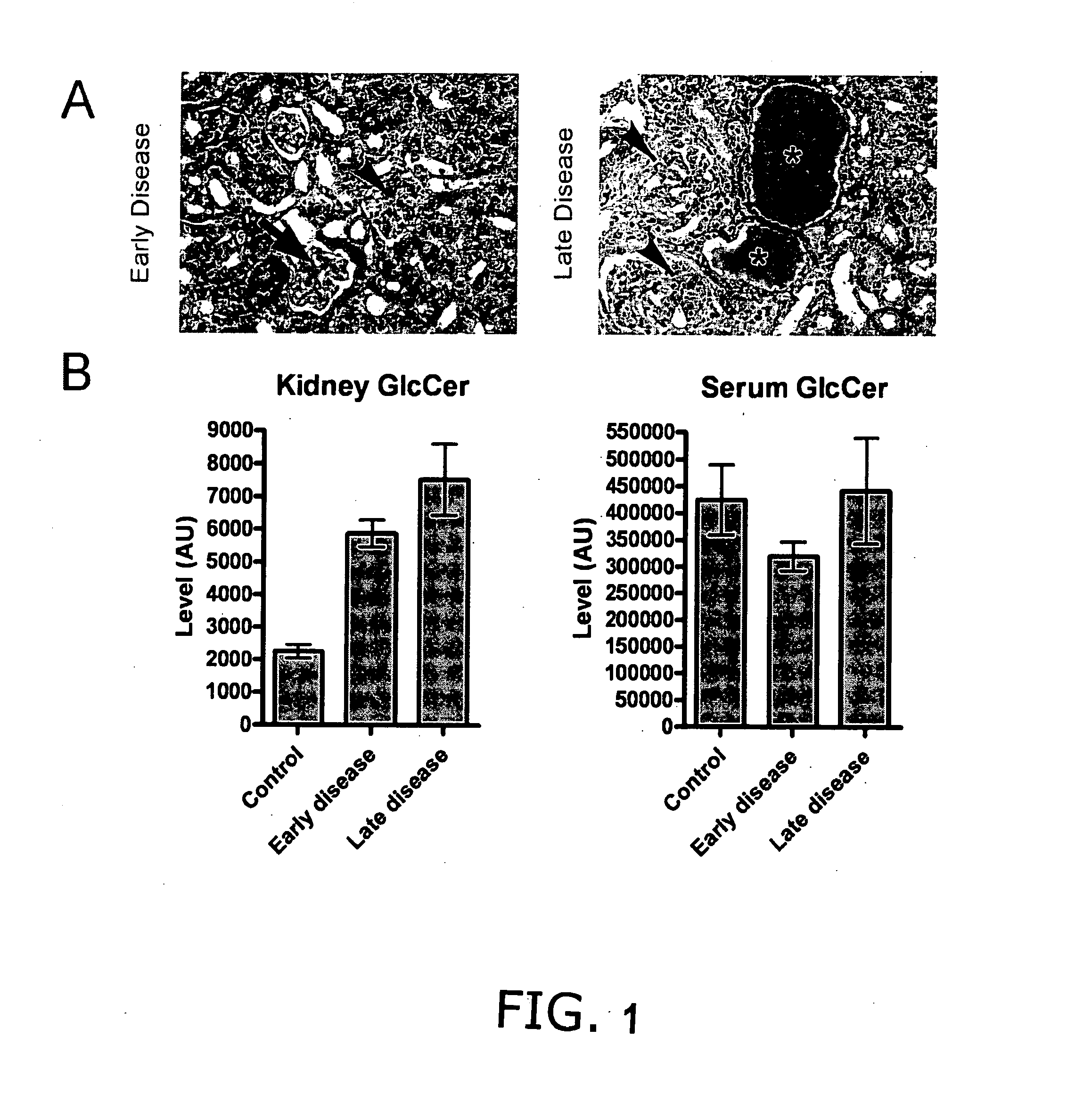
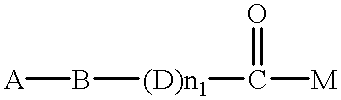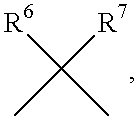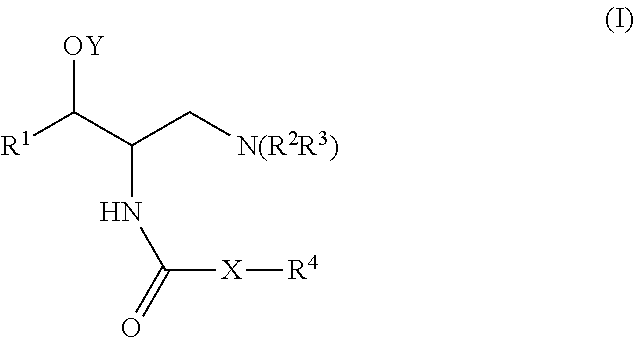Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
737 results about "Nephritis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inflammation of the kidneys that may involve the glomeruli, tubules or interstitial tissue surrounding them.
Pharmaceutical composition for treatment of diseases caused by IL-6 production
Pharmaceutical compositions for prevention or treatment of diseases caused by interleukin-6 production, comprising an antibody to interleukin-6 receptor (IL-6R antibody). As the IL-6R antibody, an antibody of animals other than the human such as mice, rats, etc., a chimeric antibody between these and a human antibody, a reshaped human antibody, etc. may be used. The pharmaceutical compositions are useful for prevention or treatment of diseases caused by interleukin-6 production such as plasmacytosis, anti-IgGl-emia, anemia, nephritis, etc.
Owner:KISHIMOTO TADAMITSU +2
Il-6 production inhibitors
InactiveUS20050119305A1Easy to prepareLow toxicityAntibacterial agentsBiocideAutoimmune conditionHydroxamic acid
An IL-6 production inhibitor which comprises a hydroxamic acid derivative of formula (I) (wherein all the symbols have the same meanings as defined in the specification), an equivalent thereof, a non-toxic salt thereof or a prodrug thereof as an active ingredient. Because of having an IL-6 production inhibitory activity, the compound of formula (I) may be useful for the prevention and / or treatment of various inflammatory diseases, sepsis, multiple myeloma, plasma cell leukemia, osteoporosis, cachexia, psoriasis, nephritis, renal cell carcinoma, Kaposi's sarcoma, rheumatoid arthritis, hypergammaglobulinemia (gammophathy), Castleman's disease, intra-atrial myxoma, diabetes, autoimmune disease, hepatitis, colitis, graft-versus-host disease, infectious diseases, endometriosis and solid cancer.
Owner:ONO PHARMA CO LTD
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
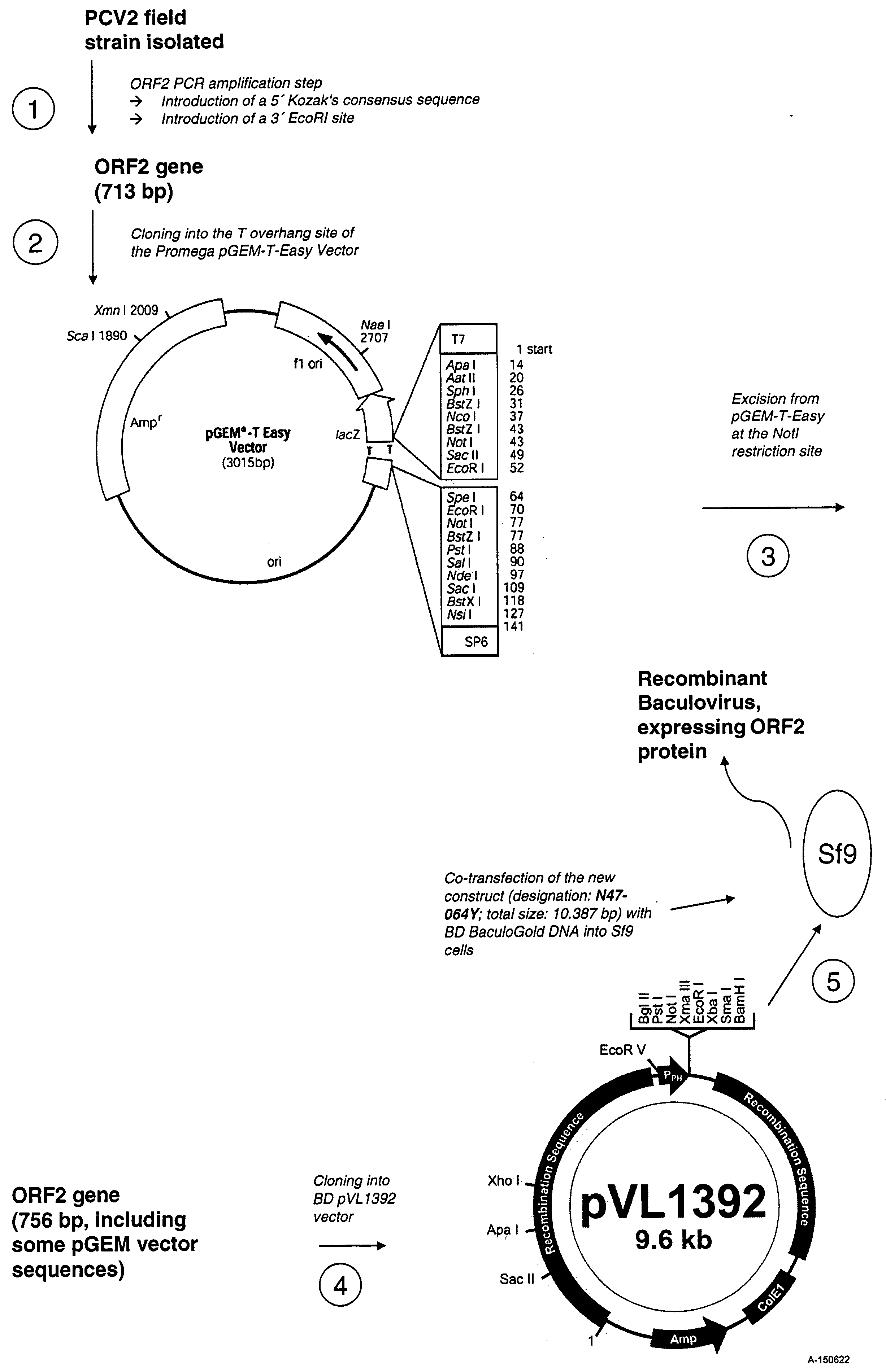
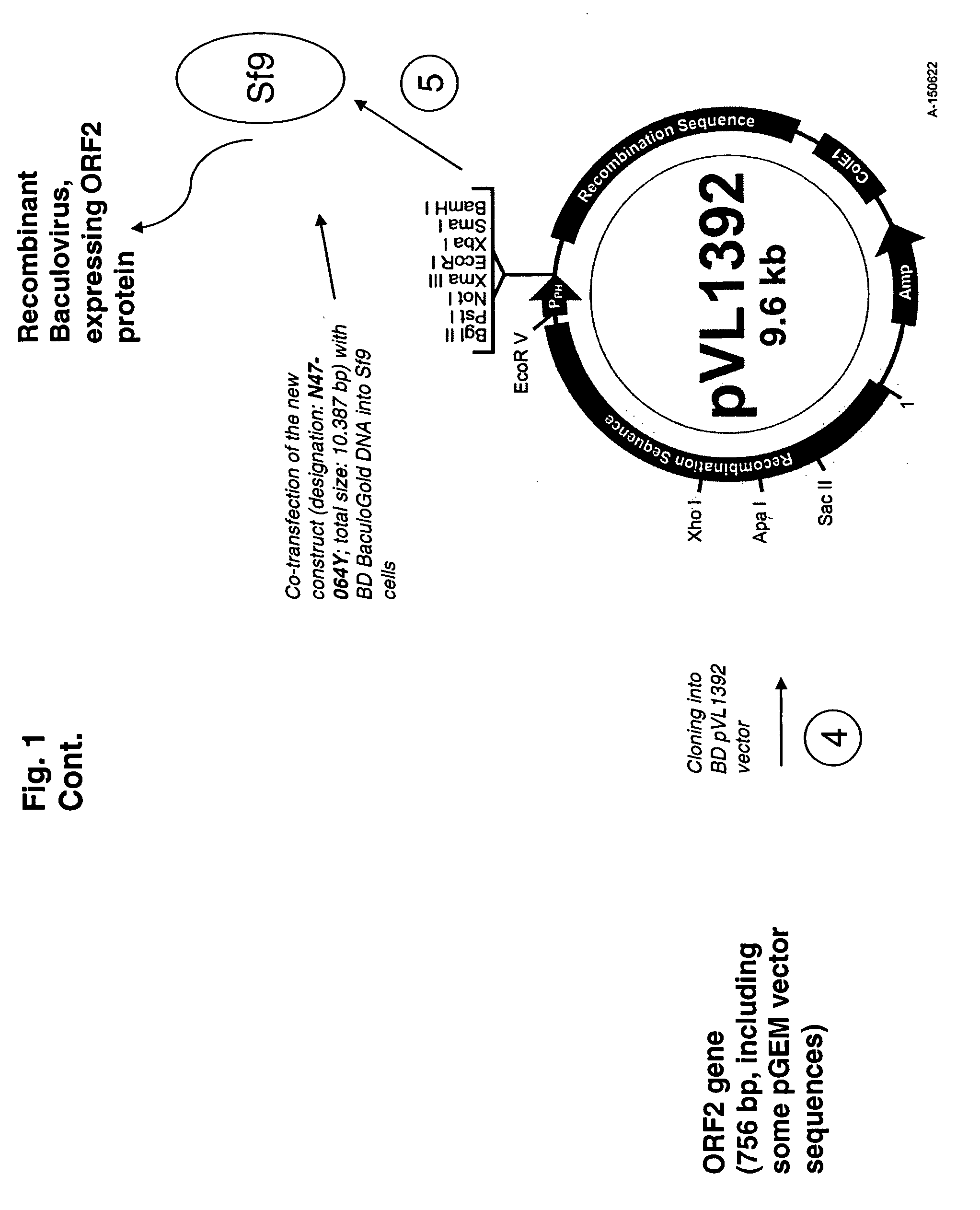
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
InactiveUS20080181910A1Reduces circovirus loadImprove the level ofViral antigen ingredientsDigestive systemClinical manifestationPorcine circovirus
The present invention relates to the use of an immunogenic composition that comprises a porcine circovirus type 2 (PCV2) antigen for treatment of several clinical manifestations (diseases). Preferably, the clinical manifestations are associated with a PCV2 infection. Preferably, they include lymphadenopathy, lymphoid depletion and / or multinucleated / giant histiocytes. Moreover, the clinical symptoms include lymphadenopathy in combination with one or a multiple of the following symptoms in pigs: (1) interstitial pneumonia with interlobular edema, (2) cutaneous pallor or icterus, (3) mottled atrophic livers, (4) gastric ulcers, (5) nephritis and (6) reproductive disorders, e.g. abortion, stillbirths, mummies, etc. Furthermore the clinical symptoms include Pia like lesions, normally known to be associated with Lawsonia intracellularis infections.
Owner:BOEHRINGER INGELHEM VETMADICA INC
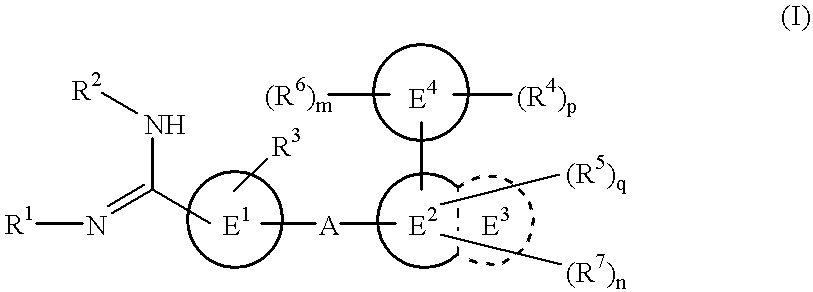
Amidino derivatives and drugs containing the same as the active ingredient
InactiveUS6358960B1BiocideGroup 5/15 element organic compoundsExtracorporeal circulationDisseminated coagulopathy
The novel amidino derivatives of the formula (I):wherein all the symbols are as in specification defined;have an inhibitory activity of a blood coagulation factor VIIa and are useful for treatment and / or prevention of several angiopathy caused by enhancing a coagulation activity, such as disseminated intravascular coagulation, coronary thrombosis, cerebral infarction, cerebral embolism, transient ischemic attack, cerebrovascular disorders, pulmonary vascular diseases, deep venous thrombosis, peripheral arterial obstruction, thrombosis after artificial vascular transplantation and artificial valve transplantation, post-operative thrombosis, reobstruction and restenosis after coronary artery bypass operation, reobstruction and restenosis after PTCA or PTCR, thrombosis by extracorporeal circulation and procoagulative diseases such as glomerlonephriitis.
Owner:ONO PHARMA CO LTD
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
ActiveUS20080261887A1Reduce severityReduce morbidityPeptide/protein ingredientsAntiviralsDiseaseClinical manifestation
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Retinoic acid agonists as preventive and therapeutic agents for nephritis
The present invention provides a therapeutic or prophylactic agent as a substitute for conventional steroids or immunosuppressive agents to treat or prevent systemic erythematosus, glomerulonephritis, lupus nephritis, idiopathic thrombocytopenic purpura or autoimmune anemia. The agent comprises a retinoic acid receptor agonist, specifically a retinoic acid receptor subtype alpha (RARalpha) agonist, including for example:(1) carboxylic acid compounds having condensed rings represented by the following formula: (wherein the rings L and M are condensed, are the same as or different from each other, and represent an aromatic hydrocarbon which may have a substituent group or a heterocycle which may have a substituent group; the rings A and B are independent of each other and represent an aromatic hydrocarbon ring or heterocycle which may have a substituent group; and D represents a carboxyl group which may have a protective group),(2) 4-{[(3,5-bistrimethylsilylphenyl)carbonyl]amino}benzoic acid, 4-{2-[5-(3-methoxymethyl-5,6,7,8-tetrahydro-5,5,8,8-tetramethylnaphthalene-2-yl)pyrrolyl]}benzoic acid, etc.
Owner:EISIA R&D MANAGEMENT CO LTD
Human TIMP-1 antibodies
Human antibodies that bind to TIMP-1 can be used as reagents to diagnose and treat disorders in which TIMP-1 is elevated, such as liver fibrosis, alcoholic liver disease, cardiac fibrosis, acute coronary syndrome, lupus nephritis, glomerulosclerotic renal disease, benign prostate hypertrophy, colon cancer, lung cancer, and idiopathic pulmonary fibrosis.
Owner:BAYER CORPORATION
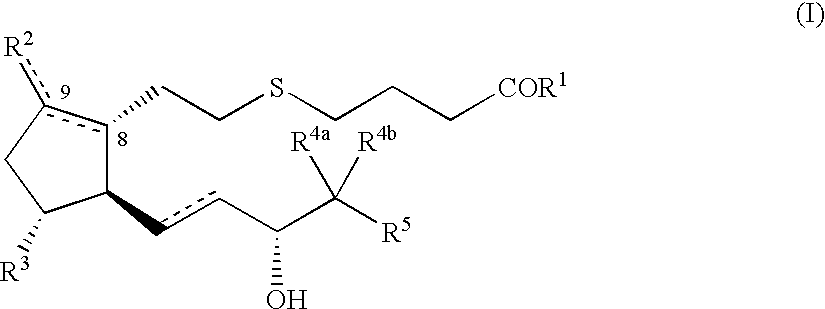
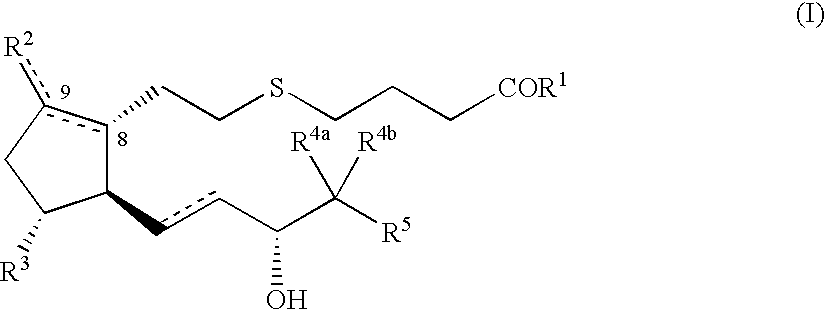
5-thia-omega-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient
The present invention relates to 5-thia-omega-substituted phenylprostaglandin E derivatives of the formula (I)(wherein, all the symbols are as defined in the specification), process for producing them and pharmaceutical compositions comprising them as active ingredient.The compounds of the formula (I) can bind to PGE2 receptors (especially, subtype EP4) strongly, so they are expected to be useful for prevention and / or treatment of immunological diseases (autoimmune diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Sjoegren's syndrome, chronic rheumarthrosis and systemic lupus erythematosus etc., and rejection after organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, lung failure, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardiac ischemia, systemic inflammatory response syndrome, ambustion pain, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, and shock etc. Further, it is thought that EP4 subtype receptor relates to sleeping disorder and blood platelet aggregation, so the compounds of the present invention are expected to be useful for the prevention and / or treatment of such diseases.
Owner:ONO PHARMA CO LTD
Selectively substituted quinoline compounds
ActiveUS20150105370A1Change is minimalHigh expressionBiocideMicrobiological testing/measurementToll-like receptorQuinoline
Embodiments of the disclosure relate to selectively substituted quinoline compounds that act as antagonists or inhibitors for Toll-like receptors 7 and / or 8, and their use in pharmaceutical compositions effective for treatment of systemic lupus erythematosus (SLE) and lupus nephritis.
Owner:EISIA R&D MANAGEMENT CO LTD
TH2-specific gene
InactiveUS6190909B1Increase the number of cellsEffective in number of cellOrganic active ingredientsFungiContact dermatitisTransgene
The present invention relates to the discovery, identification and characterization of nucleic acids that encode a novel protein differentially expressed within the TH2 cell subpopulation (hereinafter referred to as STIF). The invention encompasses STIF nucleotides, host cell expression systems, STIF proteins, fusion proteins, polypeptides and peptides, antibodies to the STIF protein, transgenic animals that express a STIF transgene, or recombinant knock-out animals that do not express the STIF protein, and compounds that modulate STIF gene expression or STIF activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or used to treat STIF based disorders, such as proliferative disorders and T-lymphocyte-related disorders including, but not limited to, chronic inflammatory diseases and disorders, such as Crohn's disease, reactive arthritis, including Lyme disease, insulin-dependent diabetes, organ-specific autoimmunity, including multiple sclerosis, Hashimoto's thyroiditis and Grave's disease, contact dermatitis, psoriasis, graft rejection, graft versus host disease, sarcoidosis, atopic conditions, such as asthma and allergy, including allergic rhinitis, gastrointestinal allergies, including food allergies, eosinophilia, conjunctivitis, glomerular nephritis, certain pathogen susceptibilities such as helminthic (e.g., leishmaniasis) and certain viral infections, including HIV, and bacterial infections, including tuberculosis and lepromatous leprosy.
Owner:MILLENNIUM PHARMA INC
Heterocyclic compounds and their use as angiotensin antagonists
InactiveUS6100252AHigh yieldPotent angiotensin II receptor antagonistic activityBiocideNervous disorderDiseaseHeart disease
Compounds shown by the above formula or salt thereof show a strong angiotensin II antagonistic activity and hypotensive action and CNS activity, and are useful as therapeutic agents of circulatory diseases such as hypertensive diseases and heart diseases (e.g. hypercardia, heart failure, cardiac infarction), strokes, cerebral apoplexy, nephritis, atherosclerosis, Alzheimer's disease, senile dementia, etc.
Owner:TAKEDA PHARMACEUTICALS CO LTD
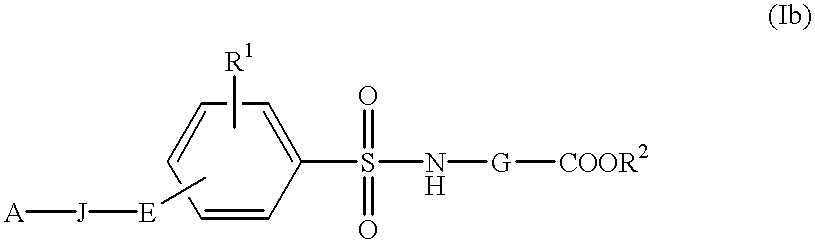
Sulfonylamino acid derivatives
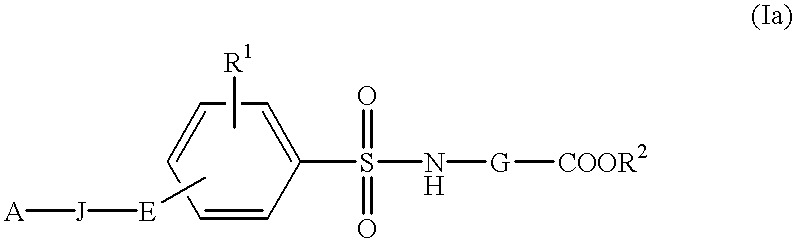
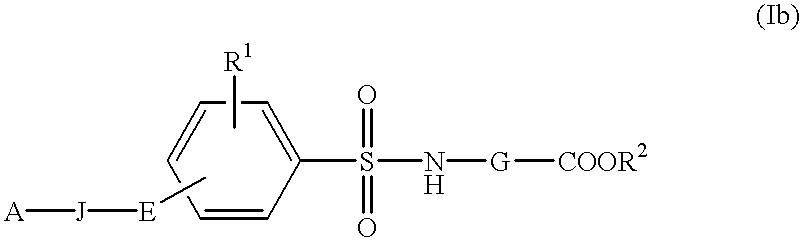
InactiveUS6177466B1Easy to useLow toxicityBiocideOrganic chemistryAutoimmune conditionWhite blood cell
The present invention is related to:(i) matrix metalloproteinase inhibitors containing sulfonylamino acid derivatives of the formula (Ia):wherein R1 is hydrogen, C1-4 alkyl; R is hydrogen, C1-8 alkyl etc.; E is -CONR3-, in which R3 is hydrogen, C1-4 alkyl etc., -NR3CO-, -CO-O-, -O-CO- etc.; A is hydrogen, C1-8 alkyl, C3-7 cycloalkyl, or Ar; J is bond, C2-4 alkylene etc.; G is -(CH2)m- in which m is 2, 3 or 4, orin which R6 and R7 is hydrogen, C1-8 alkyl etc.; and non-toxic salts thereof,(ii) novel sulfonylamino acid derivatives of the formula (Ib):wherein all the symbols are the same meaning as (i); and non-toxic salts thereof, and(iii) process for the preparation of the compound of the formula (Ib). The compounds of the formula (Ia) are useful for prevention and / or treatment of diseases induced by overexpression and excess activity of MMP. The diseases such as above, for example, are rheumatoid, arthrosteitis, unusual bone resorption, osteoporosis, periodontitis, interstitial nephritis, arteriosclerosis, pulmonary emphysema, cirrhosis, cornea injury, metastasis of, invasion of or growth of tumor cell, autoimmune disease (Crohn's disease, Sjogren's syndrome etc.), disease caused by vascular emigration or infiltration of leukocytes, arterialization.
Owner:ONO PHARMA CO LTD
Tetrahydropyrazolopyrimidine compounds
ActiveUS20130324547A1High expressionLower Level RequirementsBiocideOrganic chemistrySystemic lupus erythematosusNephritis
Embodiments of the disclosure relate to tetrahydropyrazolopyrimidine compounds that act as antagonists or inhibitors for Toll-like receptors 7 and / or 8, and their use in pharmaceutical compositions effective for treatment of systemic lupus erythematosus (SLE) and lupus nephritis
Owner:EISIA R&D MANAGEMENT CO LTD
Tetrahydropyrazolopyrimidine compounds
ActiveUS9126999B2High expressionLower Level RequirementsBiocideOrganic chemistryMedicineToll-like receptor
Owner:EISIA R&D MANAGEMENT CO LTD
Glucosylceramide Synthase Inhibition For The Treatment Of Collapsing Glomerulopathy And Other Glomerular Disease
A method of treating a glomerular disease selected from the group consisting of mesangial proliferative glomerulonephritis, collapsing glomerulopathy, proliferative lupus nephritis, crescentic glomerulonephritis and membranous nephropathy in a subject comprises administering to the subject an effective amount of a glucosylceramide synthase inhibitor.
Owner:GENZYME CORP
Antagonists of MCP-1 function and methods of use thereof
InactiveUS20050054668A1Useful in treatmentBiocideOrganic chemistryAutoimmune conditionAutoimmune disease
Compounds which are antagonists of MCP-1 function and are useful in the prevention or treatment of chronic or acute inflammatory or autoimmune diseases, especially those associated with aberrant lymphocyte or monocyte accumulation such as arthritis, asthma, atherosclerosis, diabetic nephropathy, inflammatory bowel disease, Crohn's disease, multiple sclerosis, nephrtitis, pancreatitis, pulmonary fibrosis, psoriasis, restenosis, and transplant rejection; pharmaceutical compositions comprising these compounds; and the use of these compounds and compositions in the prevention or treatment of such diseases.
Owner:TELIK INC +1
Selectively substituted quinoline compounds
ActiveUS9428495B2High expressionLower Level RequirementsOrganic active ingredientsOrganic chemistry methodsQuinolineSystemic lupus erythematosus
Embodiments of the disclosure relate to selectively substituted quinoline compounds that act as antagonists or inhibitors for Toll-like receptors 7 and / or 8, and their use in pharmaceutical compositions effective for treatment of systemic lupus erythematosus (SLE) and lupus nephritis.
Owner:EISIA R&D MANAGEMENT CO LTD
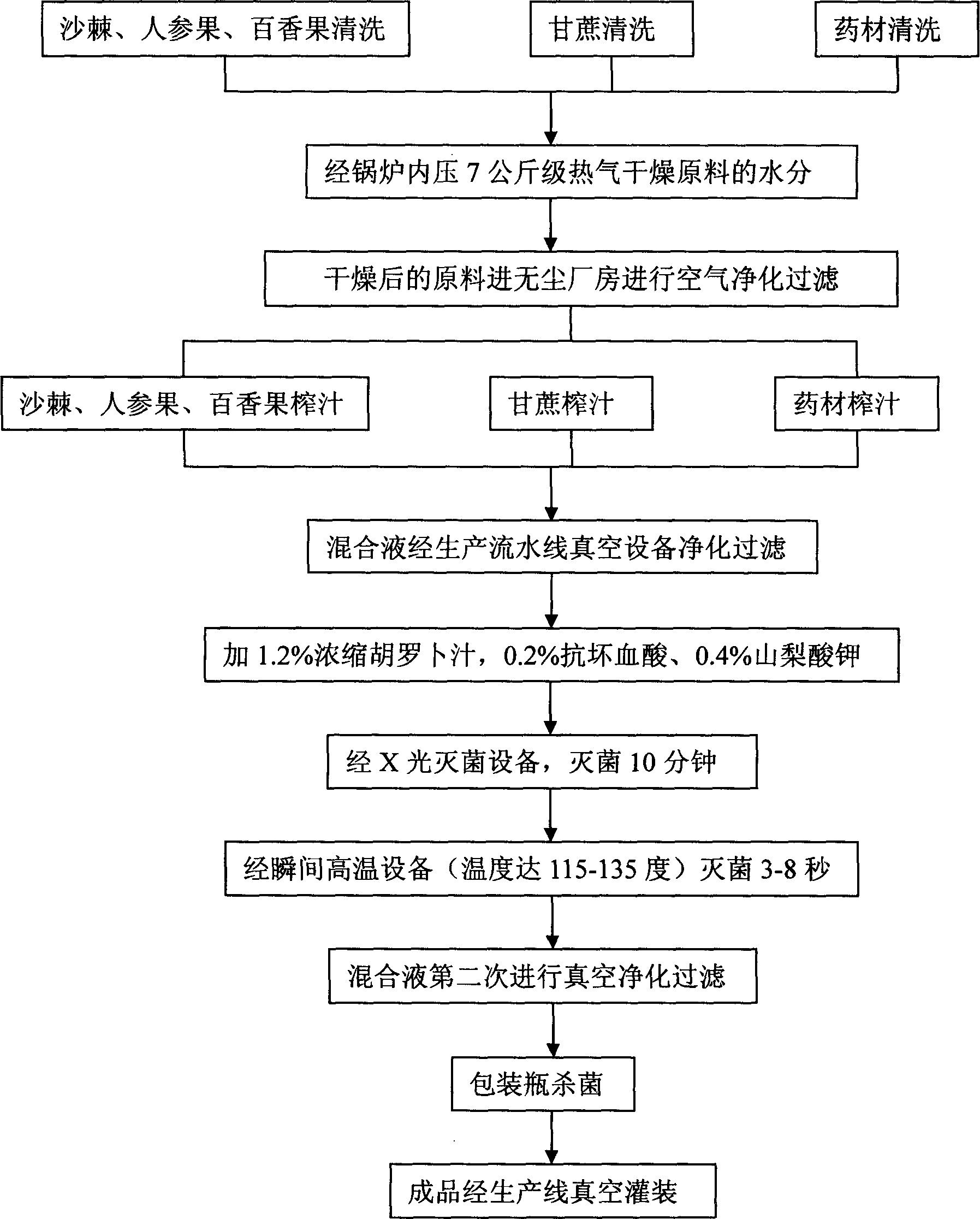
Toxin expelling and beautifying tea and preparation method thereof
ActiveCN101878833AImprove immunityEnhanced stomach ailmentsPre-extraction tea treatmentTea extractionPolygonum fagopyrumRed yeast rice
The invention discloses toxin expelling and beautifying tea and a preparation method thereof. A formula comprises the following components in percentage by mass: 20 to 70 percent of tartary buckwheat, 10 to 30 percent of honey powder, 5 to 40 percent of oligosaccharide and 10 to 65 percent of auxiliary material, wherein the auxiliary material is plant or plant extract; the plant is one or mixtureof Pu'er tea, red yeast rice, cassia seed, rhubarb, white paeony root, officinal magnolia bark, lotus leaf, senna leaf and aloe; and the plant extract is extract of the plants. The tartary buckwheat serving as a main component is combined with other medicament-food homological plants in the formula, so the tea can clear the toxin in vivo, plays a role in conditioning the daily life aiming at the crowds with high blood fat and long-term constipation, and has the effects of expelling toxin and reducing fat, enhancing human immunity, treating stomach disease, removing dampness and releasing toxin, treating nephritis and the like. The tea has comprehensive regulating effect on sub-health condition caused by current ubiquitous factors such as environmental pollution, working pressure, poor dietary habit and the like.
Owner:IDEALITY TECH GRP
Method of treating disease with sulfonylamino acid derivatives
InactiveUS6403644B1Easy to useLow toxicityBiocideOrganic active ingredientsAutoimmune conditionWhite blood cell
The present invention is related to:(i) matrix metalloproteinase inhibitors containing sulfonylamino acid derivatives of the formula (Ia); wherein R1 is hydrogen, C1-4 alkyl; R2 is hydrogen, C1-8 alkyl etc.; E is -CONR3-, in which R3 is hydrogen, C1-4 alkyl etc., -NR3CO-, -CO-O-, -O-CO- etc.; A is hydrogen, C1-8 alkyl, C3-7 cycloalkyl, or Ar; J is bond, C2-4 alkylene etc.; G is -(CH2)m-, in which m is 2, 3 or 4, orin which R6 and R7 is hydrogen, C1-8 alkyl etc.; and non-toxic salts thereof,(ii) sulfonylamino acid derivatives of the formula (Ib):wherein all the symbols are the same meaning as (i); and non-toxic salts thereof, and(iii) process for the preparation of the compound of the formula (Ib).The compounds of the formula (Ia) are useful for prevention and / or treatment of diseases induced by overexpression and excess activity of MMP. The diseases such as above, for example, are rheumatoid, arthrosteitis, unusual bone resorption, osteoporosis, periodontitis, interstitial nephritis, arteriosclerosis, pulmonary emphysema, cirrhosis, cornea injury, metastasis of, invasion of or growth of tumor cell, autoimmune disease (Crohn's disease, Sjogren's syndrome etc.), disease caused by vascular emigration or infiltration of leukocytes, arterialization.
Owner:ONO PHARMA CO LTD
Humanized anti-VLA4 immunoglobulins
InactiveUS7435802B2Peptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadAIDS dementia
The invention provides methods of treatment using humanized immunoglobulins that specifically bind to alpha-4 integrin. The methods are useful for treatment of asthma, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, transplant rejection, graft versus host disease, tumor metastasis, nephritis, atopic dermatitis, psoriasis, myocardial ischemia, and acute leukocyte mediated lung injury.
Owner:BIOGEN MA INC
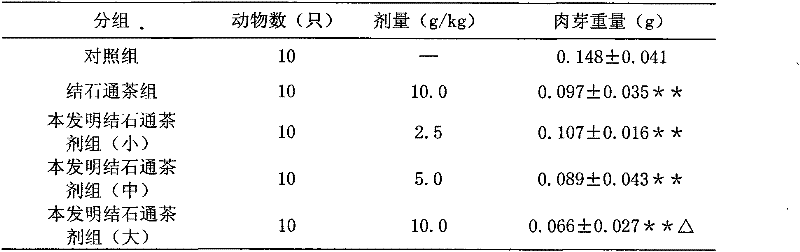
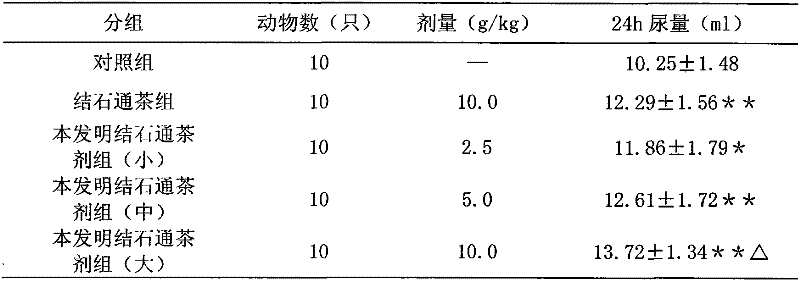
A kind of calculus tea composition and preparation method thereof
ActiveCN102266501ANormal frequency of urinationGood curative effectUrinary disorderPlant ingredientsUrinary catheterUpper urinary tract infection
The invention relates to a Jieshitong tea composition and a preparation method thereof. The Jieshitong tea composition is prepared from eight Chinese medicines of snowbell-leaf tickclover herb, corn stigma, abrus herb, tuckahoe, pyrrosia leaf, lalang grass rhizome, plantain herb and lygodium. The Jieshitong tea composition has the effects of inducing diuresis and diminishing inflammation, treating stranguria and relieving pain and stopping bleeding and eliminating stone, and is mainly used for urinary tract infection, cystitis, nephritis edema, lithangiuria, hematuresis, turbid dribbling and causalgia in urinary catheter. Clinical pharmacodynamic tests prove that the Jieshitong tea composition is obvious in an effect and high in bioavailability and does not have toxic or side effect.
Owner:王疆宏
Traditional Chinese medicine for treating acute renal glomerulus nephritis
InactiveCN101129862ACompatibility is simpleLow costUrinary disorderPlant ingredientsDiseaseRenal glomerulus
Disclosed is a Chinese medicament for treating acute glomerular nephritis wherein the recipe includes astragalus root 20g, root of red rooted saliva 15g, cogongrass rhizome 40g, rhizome smilacis glabrae 100g, motherwort 20g, malt 30g, arctium fruit 10g, bletilla tuber 12g, kudzuvine root 8g, cordate houttuynia 10g, plantain seeds, polyporus umbellatus each 20g, alkanna tinctoria, eclipta, notoginseng each 30g, Achyranthes bidentatae 25g, sweet basil, ledebouriella root each 30g. The Chinese medicament has simplified recipe, easy preparing process and low price.
Owner:尹克山
Health care beverage of sugar cane juice
InactiveCN1957771APrevent nephritisPrevent kidney failureFood preparationPlant ingredientsMedicinal herbsGLYCYRRHIZA EXTRACT
A health-care sugar cane juice beverage for preventing nephritis, renal failure and uremia, and treating cough and bronchitis is prepared from sugar can juice and 16 Chinese-medicinal materials including wolfberry fruit, honeysuckle flower, liquorice root, scutellaria root, etc.
Owner:曹喜元
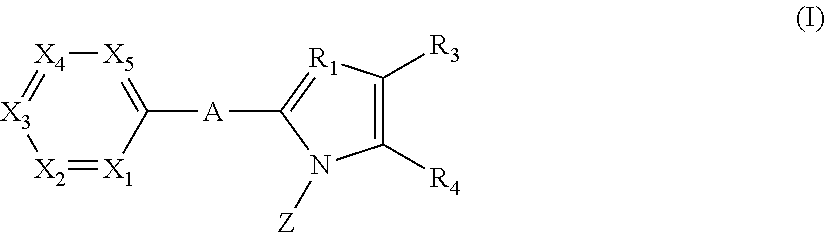
Pyridine derivative
Provided is a pyridine derivative represented by formula (I), a prodrug thereof, a pharmaceutically acceptable salt of the pyridine derivative or the prodrug, or a solvate of the pyridine derivative, the prodrug or the pharmaceutically acceptable salt, which is useful for treatment or prophylaxis of diseases associated with URAT1 such as gout, hyperuricemia, hypertension, kidney diseases such as interstitial nephritis, diabetes, arteriosclerosis and Lesch-Nyhan syndrome.
Owner:TEIJIN LTD
Humanized anti-VLA 4 immunoglobulins and uses thereof
InactiveUS20060110394A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthLymphatic Spread
The invention provides methods of treatment using humanized immunoglobulins that specifically bind to alpha-4 integrin. The methods are useful for treatment of asthma, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, transplant rejection, graft versus host disease, tumor metastasis, nephritis, atopic dermatitis, psoriasis, myocardial ischemia, and acute leukocyte mediated lung injury.
Owner:BIOGEN MA INC
Sulfonyl urea derivatives and their use in control of interleukin-1 activity
A compound of formula (I) wherein R<1> and R<2> are as defined in the description, R<2> being an aromatic group, useful in the treatment and condition selected from the group consisting of the group meningitis and salpingitis, septic shock, disseminated intravascular coagulation, and / or adult respiratory distress syndrome, acute or chronic inflammation, arthritis, cholangitis, colitis, encephalitis, endocarditis, glomerulonephritis, hepatitis, myocarditis, pancreatitis, pericarditis, reperfusion injury, vasculitis, acute and delayed hypersensitivity, graft rejection, and graft-versus-host disease, auto-immune diseases including Type 1 diabetes mellitus and multiple sclerosis, periodonate diseases, interstitial pulmonary fibrosis, cirrhosis, systemic sclerosis, keloid formation tumors which produce IL-1 as an autocrine growth factor, cachexia, Alzeimer's disease, percussion injury, depression, atherosclerosis, osteoporosis in a mammal, including a human.
Owner:PFIZER INC
Sugar-free traditional Chinese medicine granules for treating lithangiuria and its prepn. method
InactiveCN1947781AAvoid lostAvoid the disadvantage of hygroscopicityUrinary disorderGranular deliverySucroseUrethra
A Chinese medicine in the form of non-sugar particles for treating infection in urinary system, cystitis, nephritis, urethra calculus, hematuria, etc is prepared from 8 Chinese-medicinal materials including lysimachia, tuckahoe, pyrrosia leaf, imperate rhizome, etc. Its preparing process is also disclosed.
Owner:王玫
2-aryl-propionic acid and medicine composition containing same
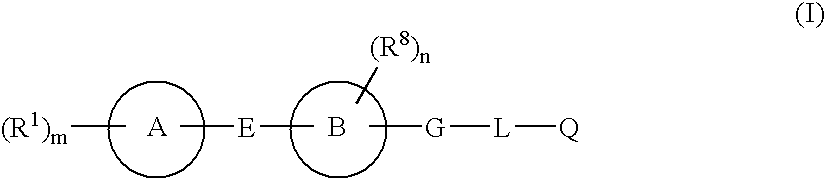
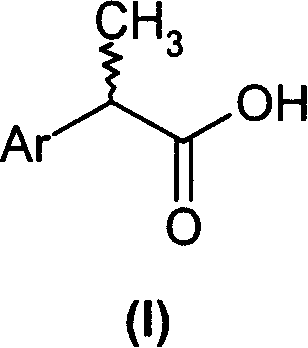
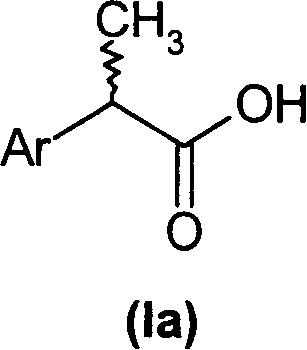
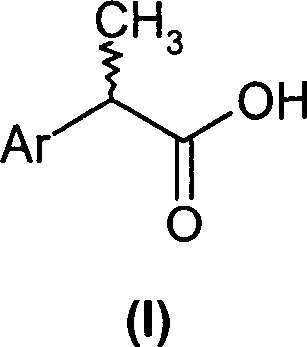
(R,S)-2-Aryl-propionic acids are useful as inhibitors of interleukin-8 induced human polymorphonucleated neutrophils (PMN) chemotaxis. (R,S)-2-Aryl-propionic acids of formula (I), their single (R) and (S) enantiomers or salts are useful as inhibitors of interleukin-8 (IL-8) induced human polymorphonucleated neutrophils (PMN) chemotaxis. [Image] Ar : phenyl ring (substituted by T in meta position, T1 in para position or T2 in ortho position); T : linear or branched 1-5C alkyl, 2-5C alkenyl, or 2-5C alkynyl (optionally substituted by 1-5C alkoxycarbonyl, optionally substituted phenyl, linear or branched 1-5C hydroxyalkyl or arylhydroxymethyl); T1benzoyloxy, benzoylamino, benzenesulfonyloxy, benzenesulfonylamino, benzenesulfonylmethyl (all optionally substituted), 1-5C acyloxy, 1-5C acylamino, 1-5C sulfonyloxy, 1-5C alkanesulfonylamino, 1-5C alkanesulfonylmethyl, 3-6C cycloalkyl, 2-furyl, 3-tetrahydrofuryl, 2- thiophenyl, 2-tetrahydrothiophenyl or ((1-8C)-alkanoyl, -cycloalkanoyl or -arylalkanoyl)-1-5C-alkylamino (preferably acetyl-N-methyl-amino or pivaloyl-N-ethyl-amino); and T2arylmethyl, aryloxy or acylamino (all optionally substituted by 1-4C alkyl, 1-4C-alkoxy, chlorine, fluorine or trifluoromethyl). The meta linear or branched 1-5C alkyl together with a substituent in ortho or para position and the benzene ring forms optionally saturated or optionally substituted bicyclo aryls. INDEPENDENT CLAIM are included for the following: (1) preparation of (I); and (2) use of (I) in the preparation of a medicament for the treatment of e.g. psoriasis, ulcerative colitis, melanoma and chronic obstructive pulmonary disease (COPD). - ACTIVITY : Antipsoriatic; Antiulcer; Respiratory-Gen.; Antirheumatic; Antiarthritic; Nephrotropic; Vasotropic; Antiinflammatory; Gastrointestinal-Gen.; Cytostatic. - MECHANISM OF ACTION : Interleukin-8 (IL-8) inhibitor; GROalpha inhibitor; CXCR2 agonist / antagonist. The ability of (R,S) 2-[(3'-isopropyl)phenyl]propionic acid (A) to inhibit IL-8 induced chemotaxis of human monocytes was tested as described in Van Damme J. et al. (Eur. J. Immunol., 19, 2367, 1989). (A) showed inhibition (%) of 51+-12.
Owner:DOMPE FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com