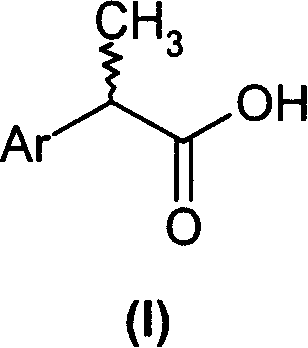
2-aryl-propionic acid and medicine composition containing same
A technology of phenylpropionic acid and aryl, which is applied in the field of 2-aryl-propionic acid and pharmaceutical compositions containing them, and can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] 2-[3′-(isopropenyl)phenyl]propanoic acid
[0169] The title acid was synthesized starting from ethyl 3'-perfluorobutanesulfonyloxy-2-phenylpropanoate (7.63 mmol) dissolved in N-ethylpyrrolidone (30 mL); in this mixture Anhydrous lithium chloride (0.94 g, 22.9 mmol), triphenylarsine (90 mg, 0.3 mmol) and dipalladium trimenzylideneacetone (0.173 g, 0.15 mmol palladium) were added. After standing at room temperature for 5 minutes, tributylisopropenyltin (2.83 g, 8.55 mmol) was added, and the solution was stirred at 90° C. for 5 hours. After the solution had cooled to room temperature, the mixture was diluted with hexane and a saturated solution of potassium fluoride was added; after filtration and separation of the two phases, the organic phase was dried over sodium sulfate and evaporated in vacuo. The residue was purified by flash chromatography to give ethyl 2-[3'-isopropenylphenyl]propionate (Ritter K., Synthesis, 735, 1993 and Mitchell T.N., Synthesis, 803, 1992).
...
Embodiment 2
[0173] 2-[3′-(α-Ethyl-propenyl)phenyl]propanoic acid
[0174] With reference to the above method, tributyl-(α-ethyl) propenyl tin synthesized by known methods (Ritter K., Synthesis, 735,1993 and Mitchell T.N., 803,1992) is a starting reagent , to synthesize the title acid.
[0175] 1 H-NMR (CDCl 3 ): δ10.0(bs, 1H, COOH); 7.28(m, 1H); 7.15(m, 1H); 7.05(m, 2H); 5.5(m, 1H); 3.75(m, 1H); 1.6(q, 2H); 1.45(d, 3H, J=7Hz); 0.85(d, 3H, J=7Hz); 0.78(t, 3H, J=7Hz).
Embodiment 3
[0177] 3-[3′-(1″-Styryl)phenyl]propionic acid
[0178] With reference to the above-mentioned method, tributyl-(α-styryl tin) synthesized by a known method (Ritter K., Synthesis, 735, 1993 and Mitchell T.N., 803, 1992) is a starting reagent to synthesize Title Sour.
[0179] 1 H-NMR (CDCl 3 ): δ 11.0 (bs, 1H, COOH); 7.38-7.13 (m, 9H); 3.95 (m, 2H); 3.81 (m, 1H); 1.72 (d, 3H, J=7Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com