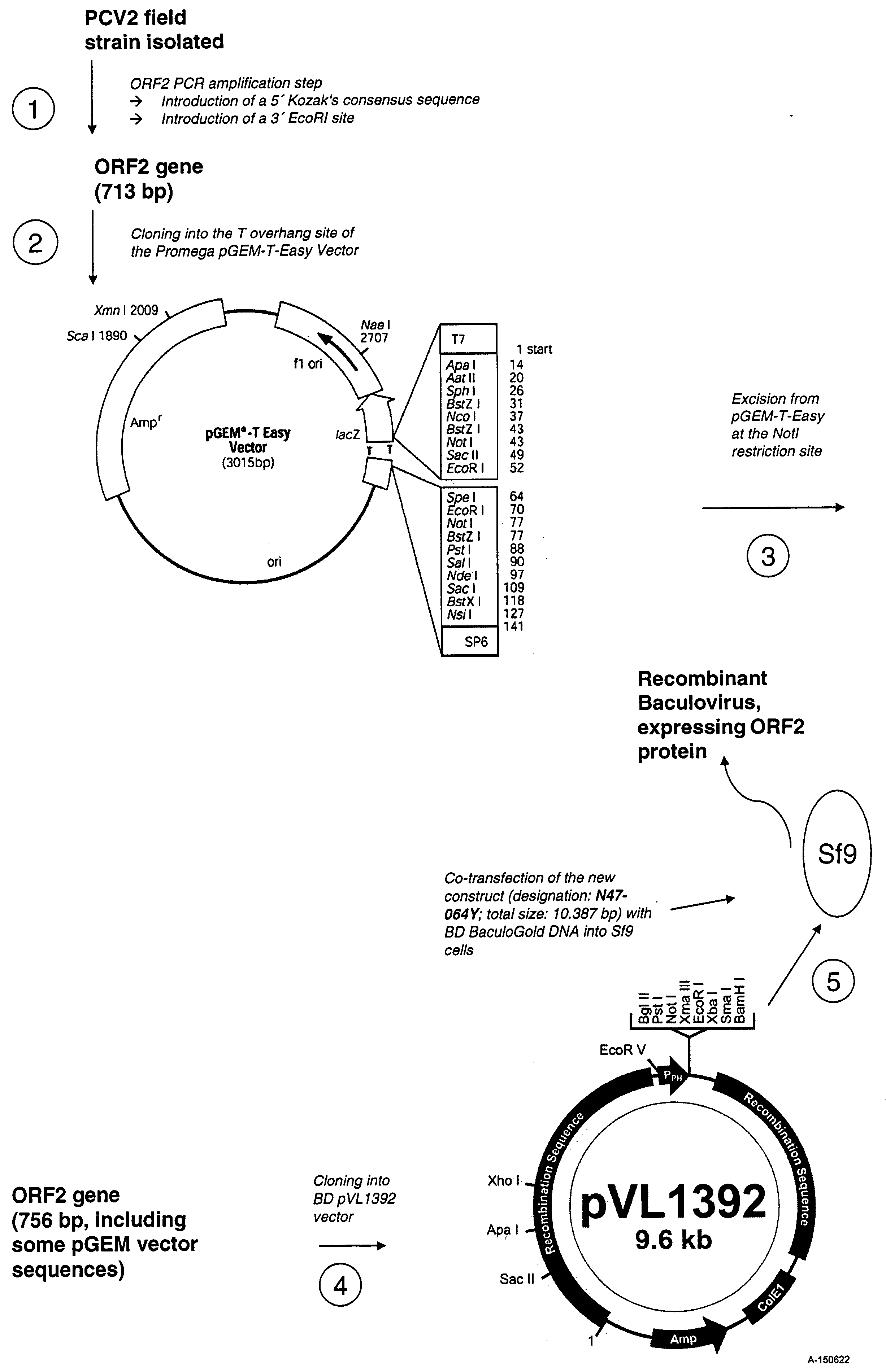
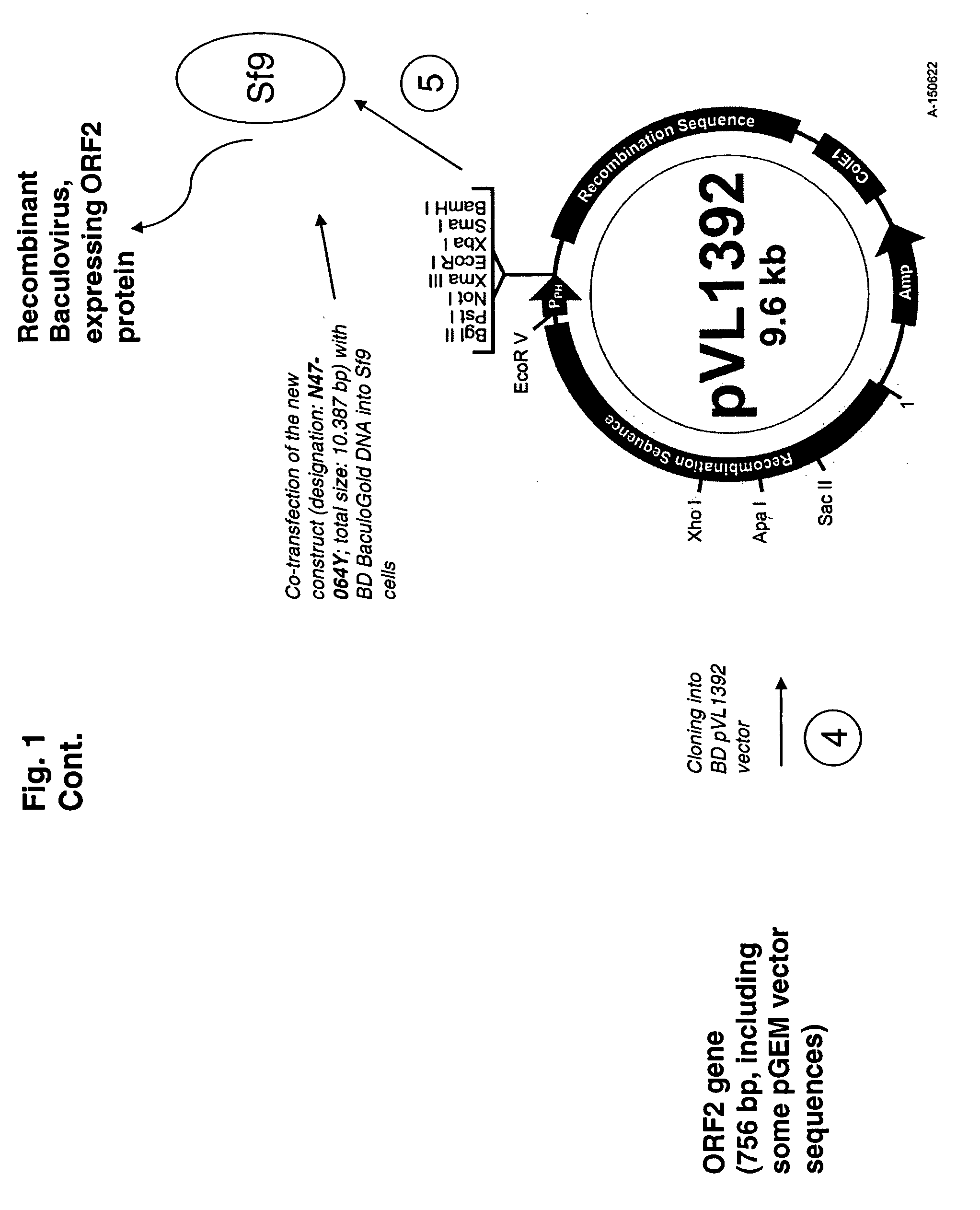
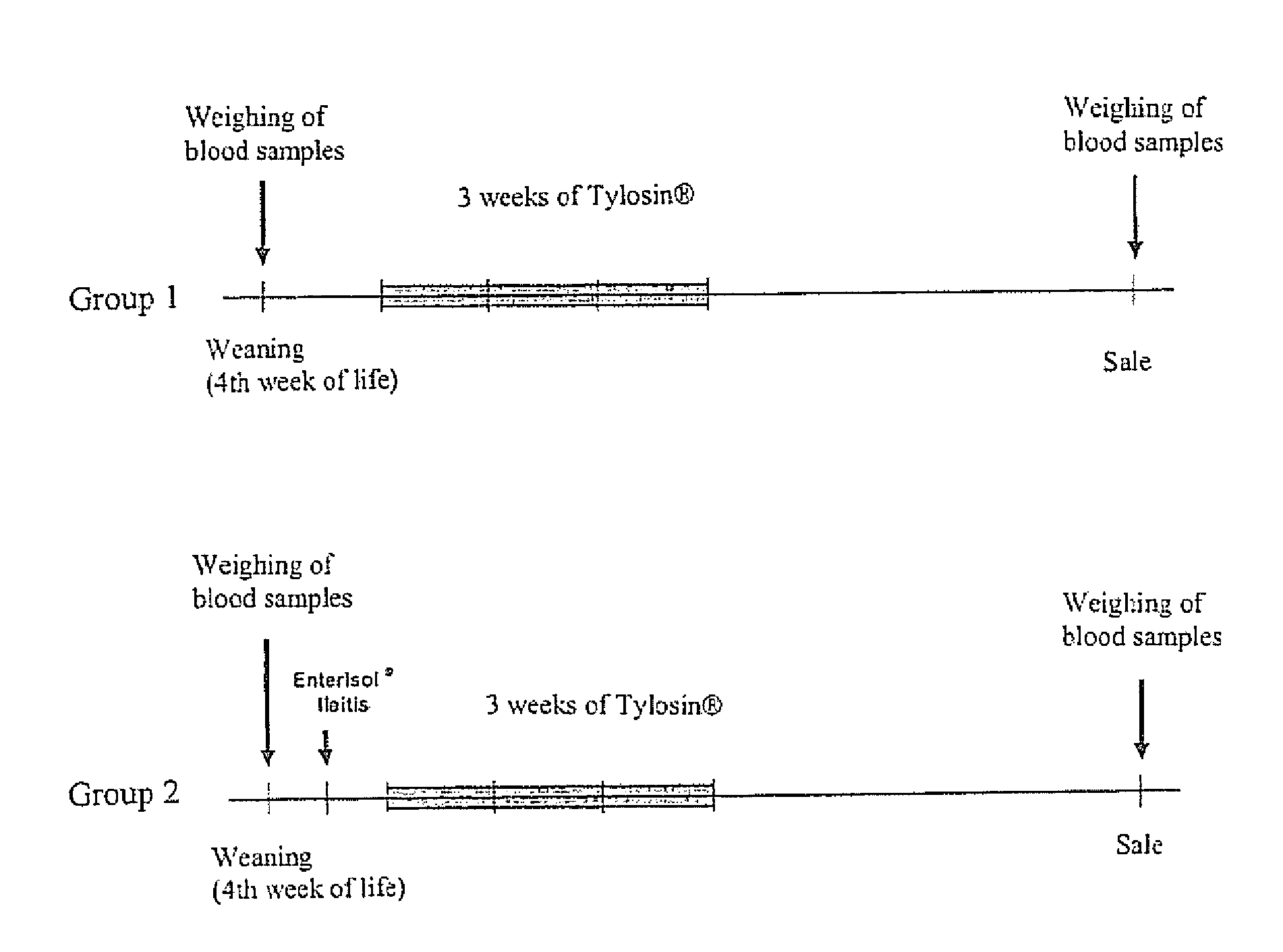
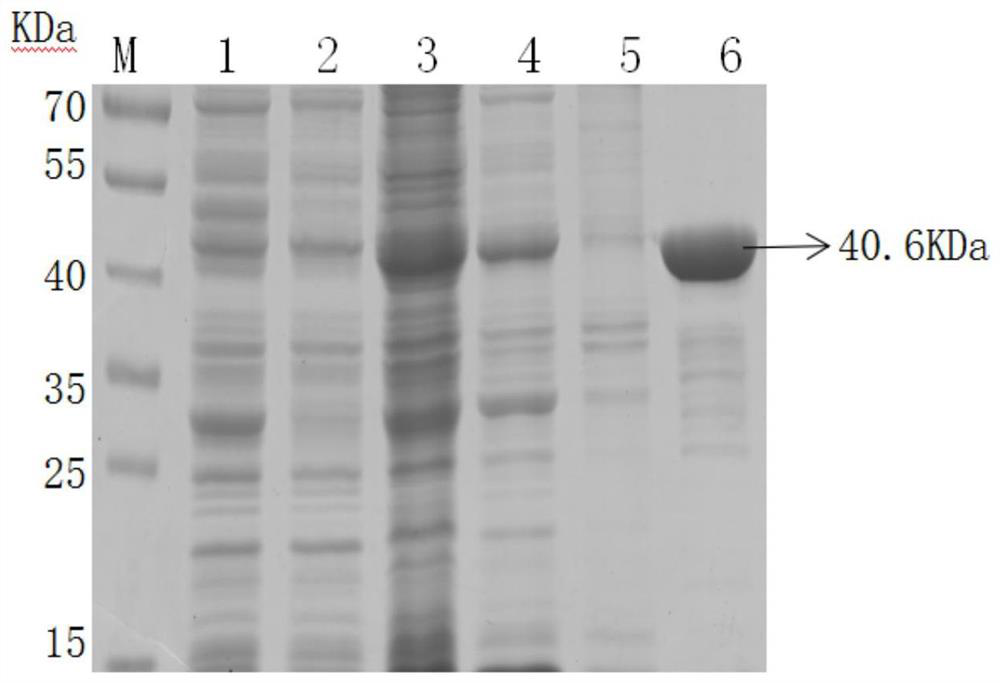
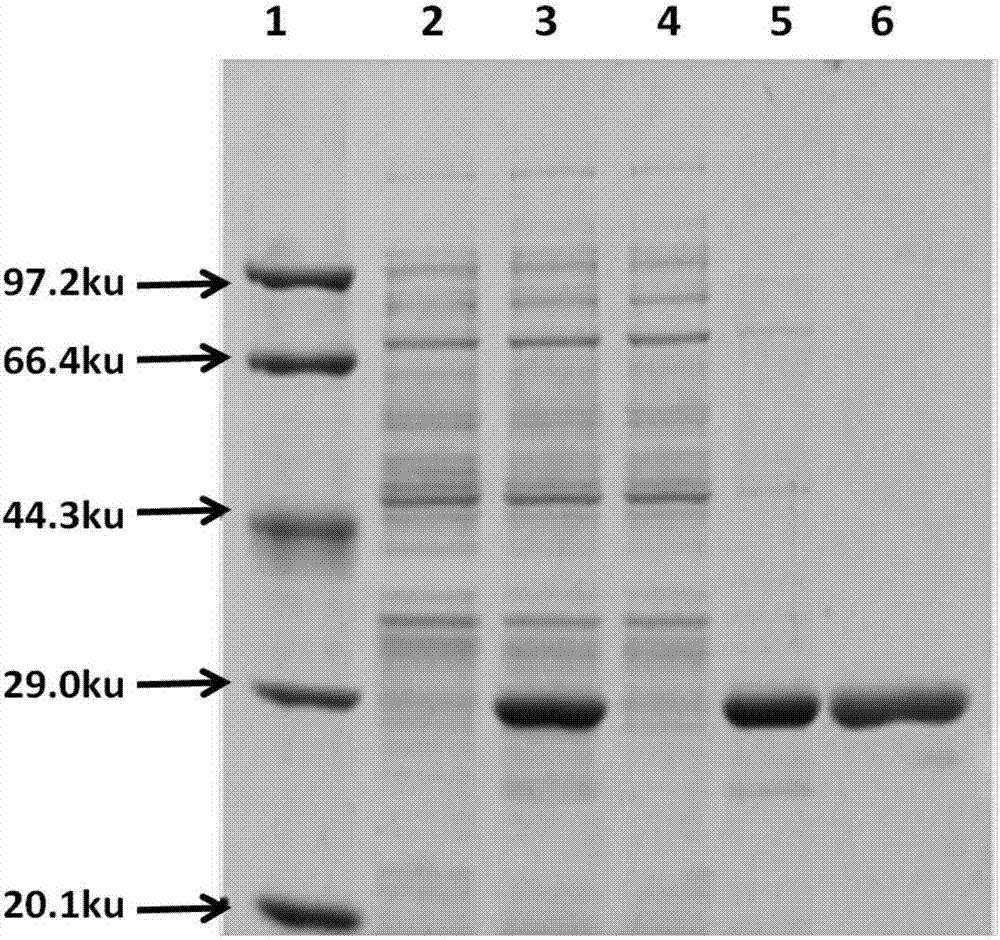
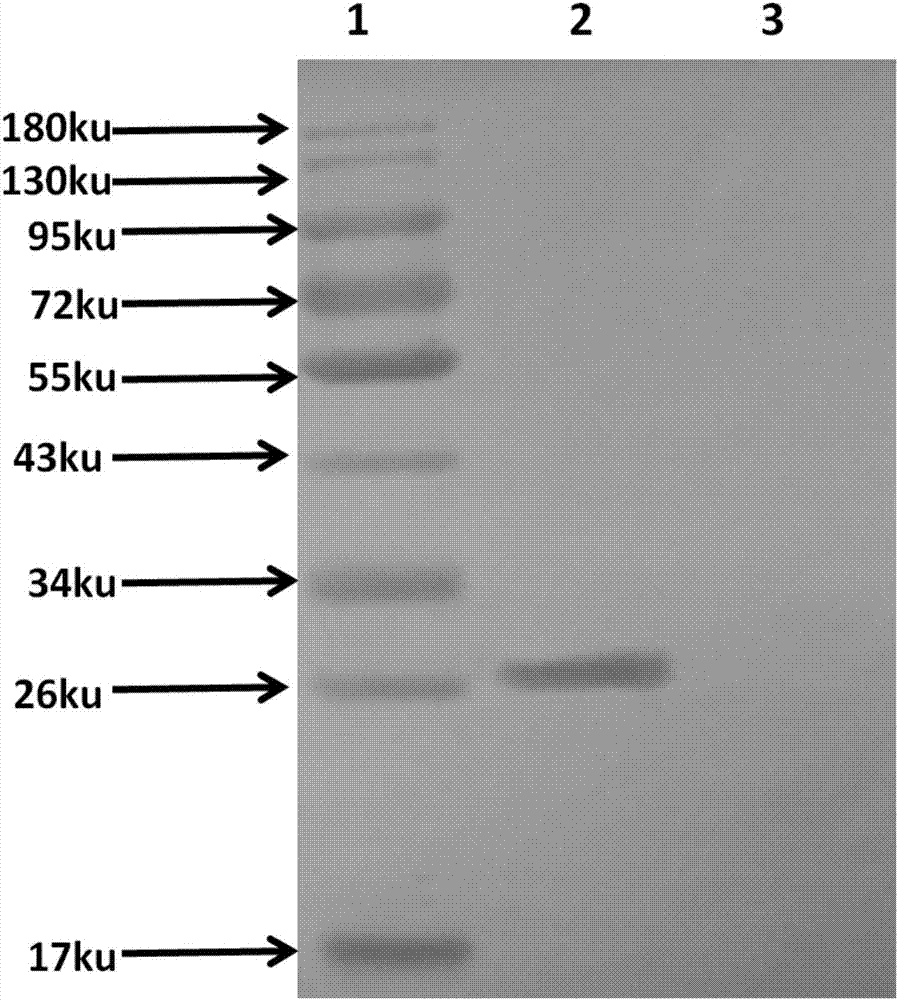
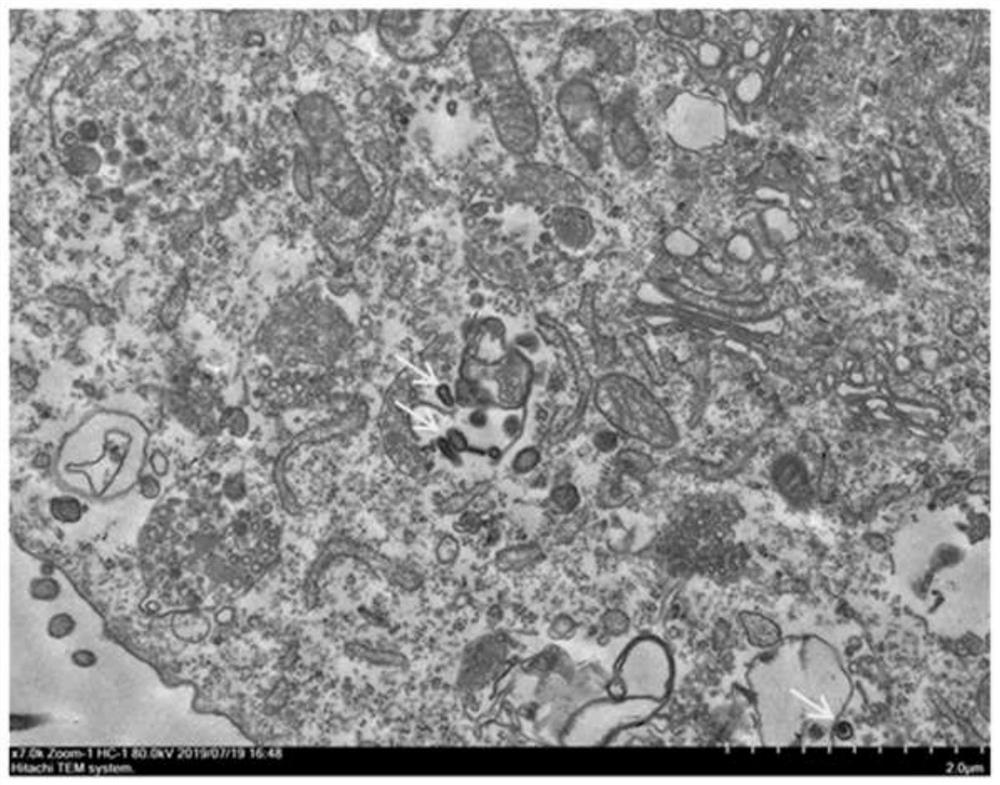
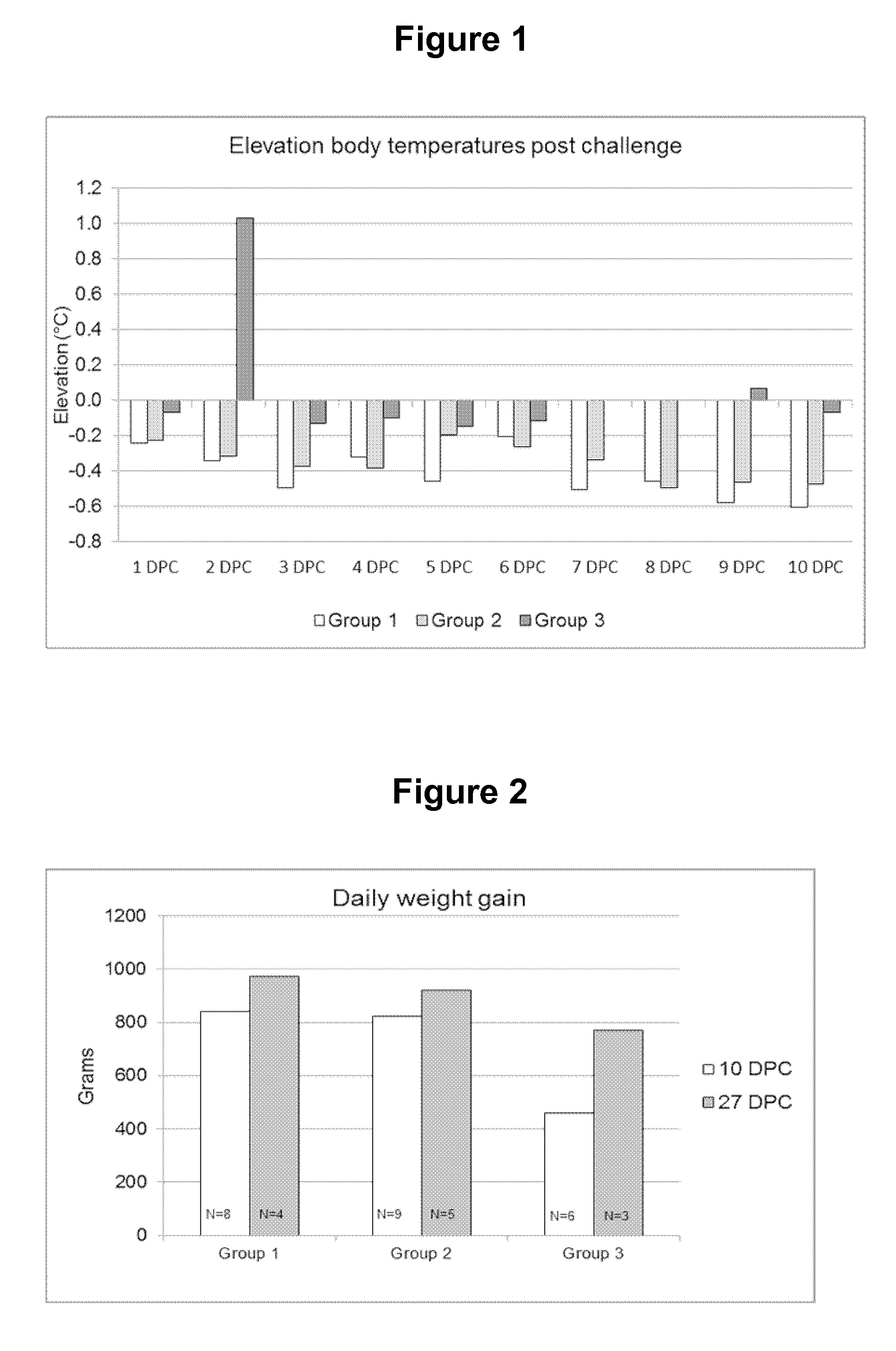
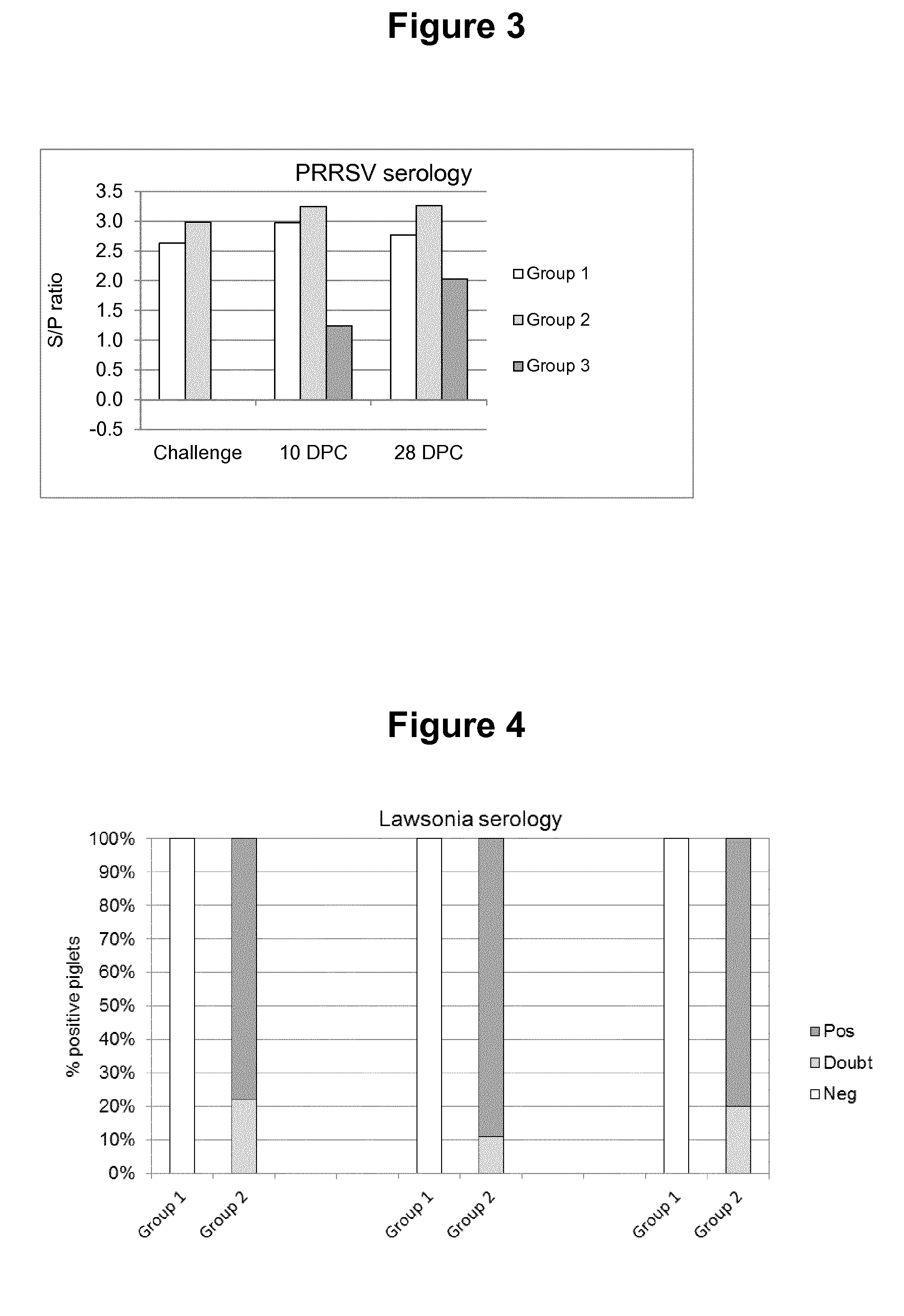
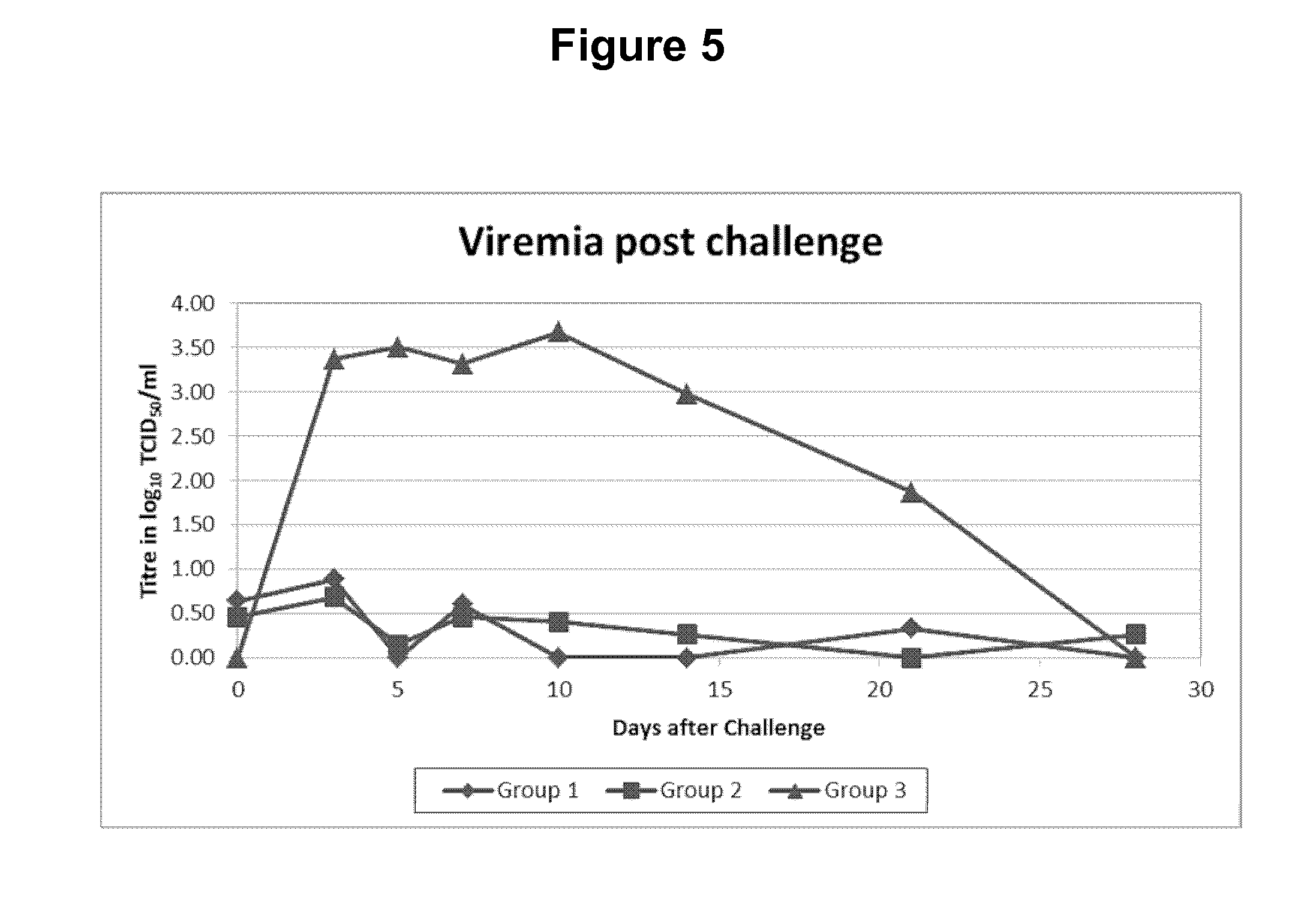
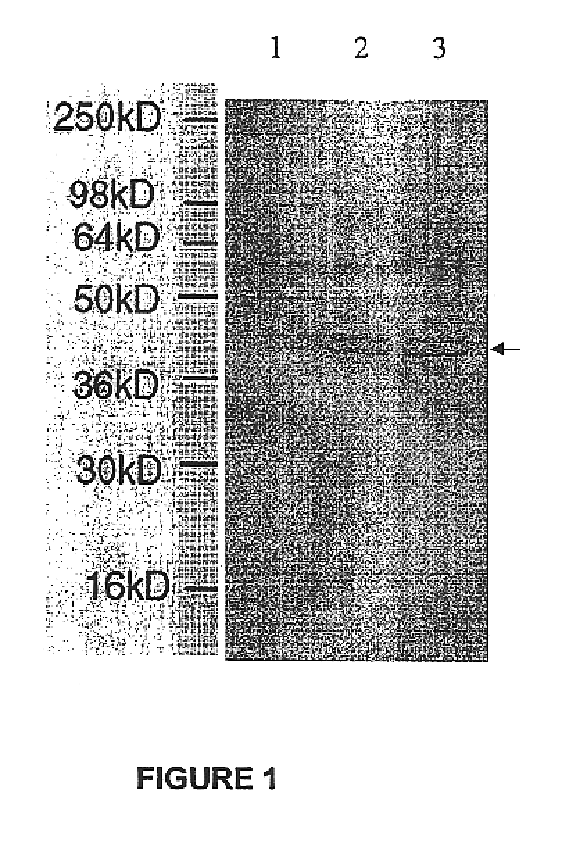
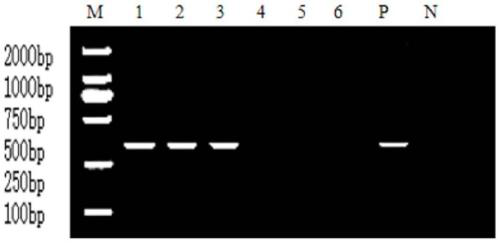
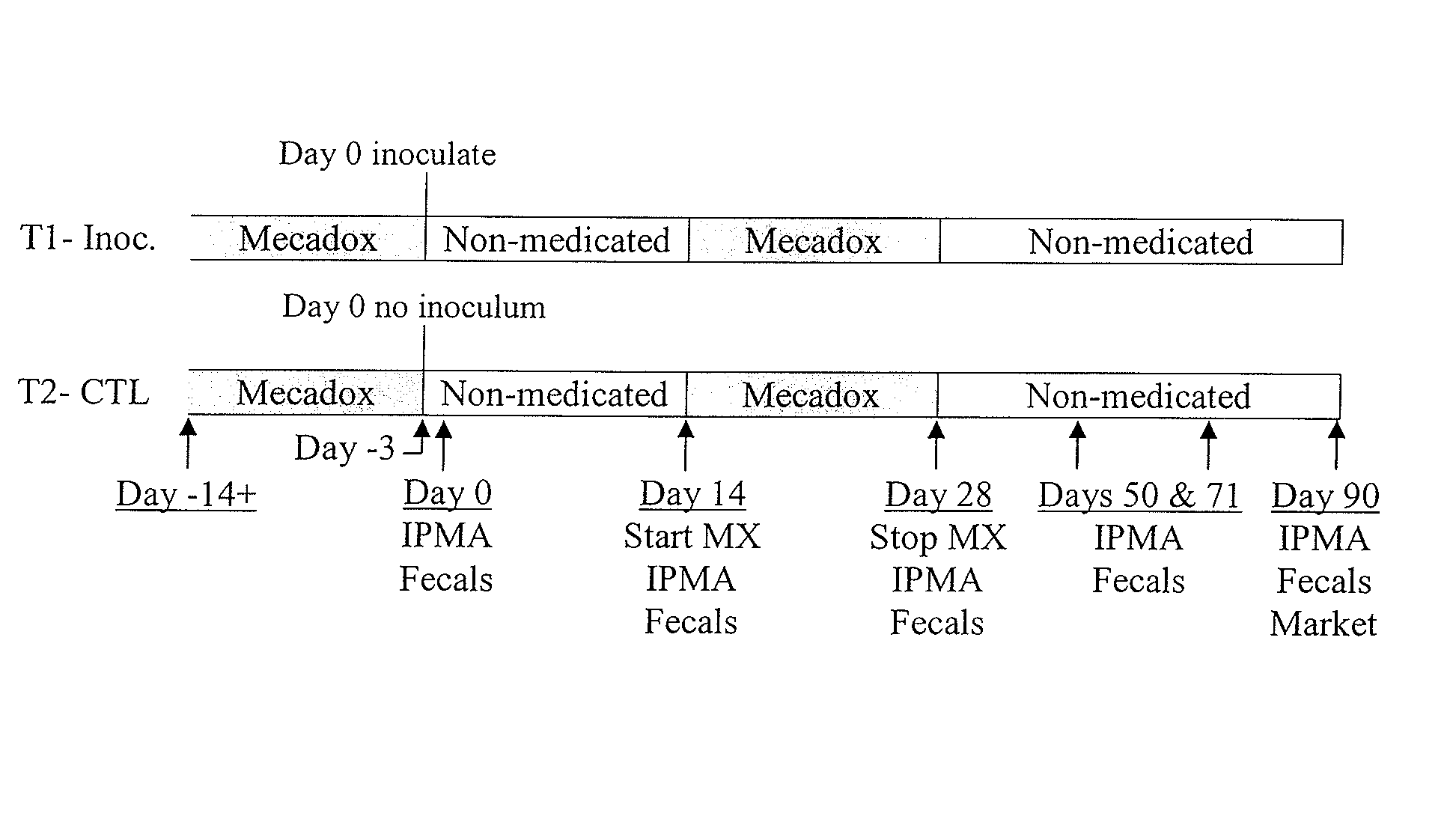
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Lawsonia intracellularis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lawsonia intracellularis is a species of bacterium. It is obligately intracellular and was isolated from intestines of pigs with proliferative enteropathy disease.
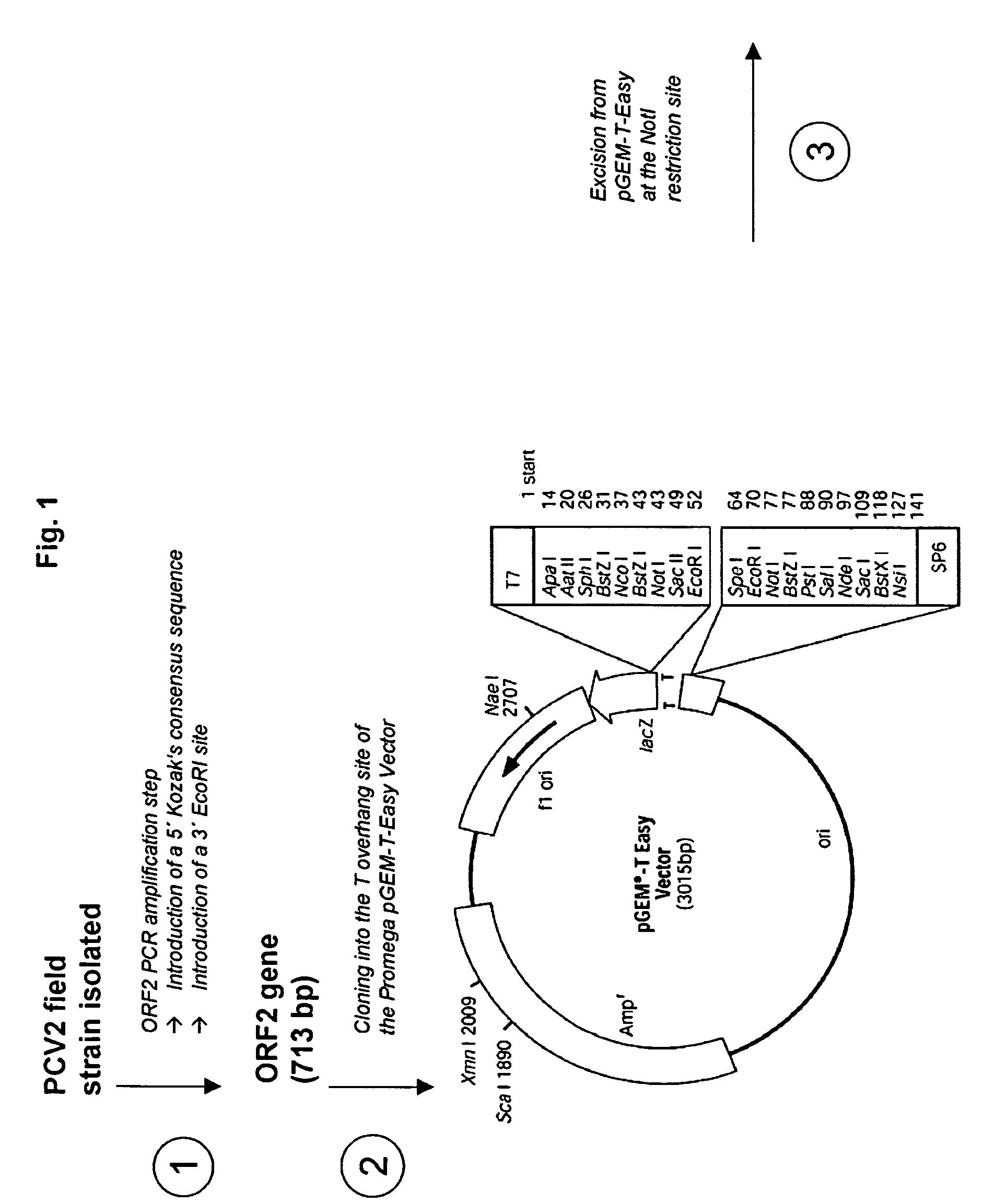
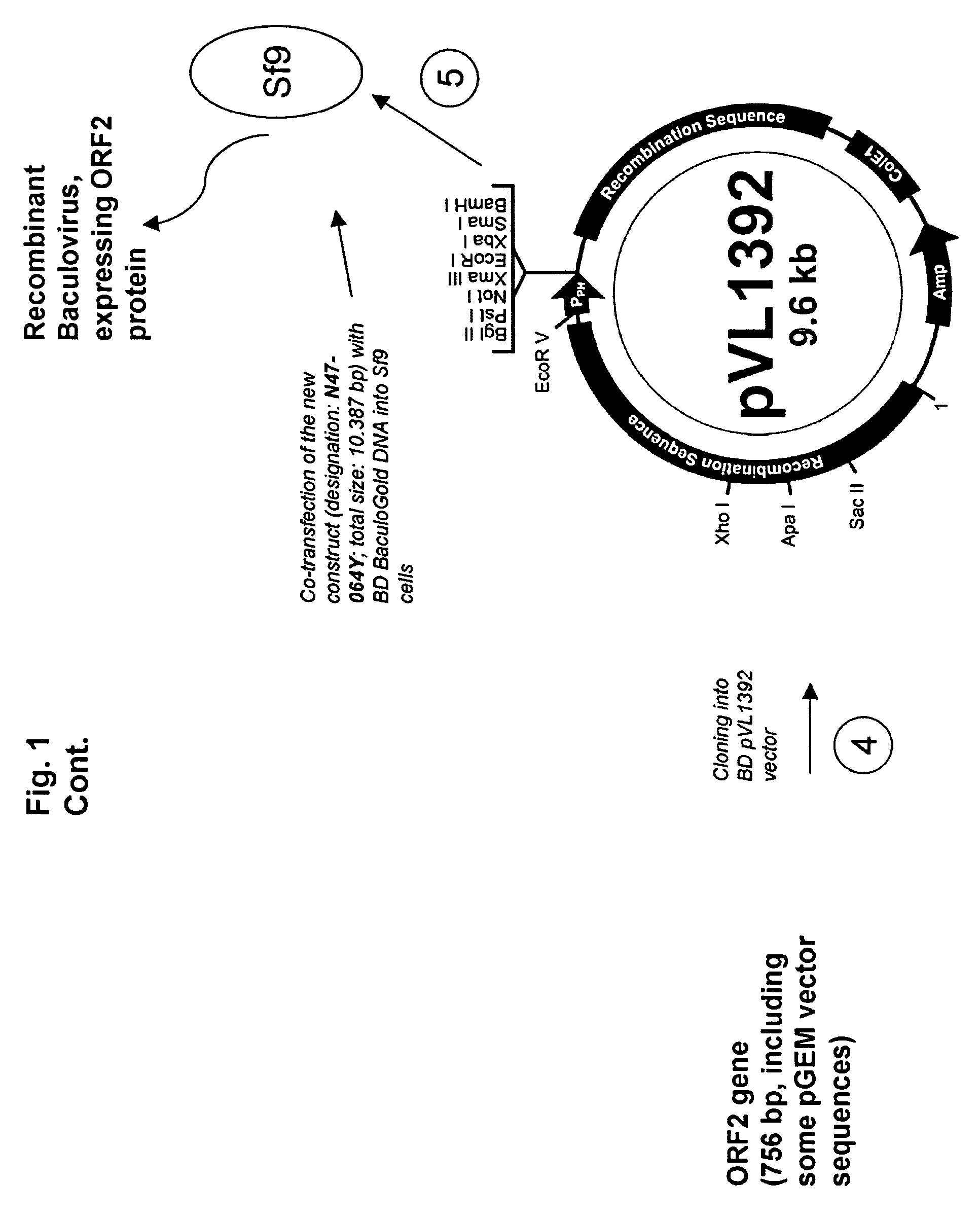
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
InactiveUS20080181910A1Reduces circovirus loadImprove the level ofViral antigen ingredientsDigestive systemClinical manifestationPorcine circovirus
The present invention relates to the use of an immunogenic composition that comprises a porcine circovirus type 2 (PCV2) antigen for treatment of several clinical manifestations (diseases). Preferably, the clinical manifestations are associated with a PCV2 infection. Preferably, they include lymphadenopathy, lymphoid depletion and / or multinucleated / giant histiocytes. Moreover, the clinical symptoms include lymphadenopathy in combination with one or a multiple of the following symptoms in pigs: (1) interstitial pneumonia with interlobular edema, (2) cutaneous pallor or icterus, (3) mottled atrophic livers, (4) gastric ulcers, (5) nephritis and (6) reproductive disorders, e.g. abortion, stillbirths, mummies, etc. Furthermore the clinical symptoms include Pia like lesions, normally known to be associated with Lawsonia intracellularis infections.
Owner:BOEHRINGER INGELHEM VETMADICA INC
Lawsonia intracellularis of European origin and vaccines, diagnostic agents and methods of use thereof
The present invention relates to Lawsonia intracellularis vaccines and methods for protecting against and diagnosing L. intracellularis infection. The products and processes of the invention are attainable, in part, as the result of an improved method for cultivating large scale supplies of L. intracellularis, including both a novel isolate of L. intracellularis of European origin and a method of preparing a lyophilized product containing the attenuated European isolate as vaccine product.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Use of a pcv2 immunogenic composition for lessening clinical symptoms in pigs
ActiveUS20080261887A1Reduce severityReduce morbidityPeptide/protein ingredientsAntiviralsDiseaseClinical manifestation
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Lawsonia intracellularis of European origin and vaccines, diagnostic agents and methods of use thereof
The present invention relates to Lawsonia intracellularis vaccines and methods for protecting against and diagnosing L. intracellularis infection. The products and processes of the invention are attainable, in part, as the result of an improved method for cultivating large scale supplies of L. intracellularis, including both a novel isolate of L. intracellularis of European origin and a method of preparing a lyophilized product containing the attenuated European isolate as vaccine product.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Lawsonia intracellularis vaccine
The present invention relates i.a. to nucleic acid sequences encoding novel Lawsonia intracellularis proteins. It furthermore relates to DNA fragments, recombinant DNA molecules and live recombinant carriers comprising these sequences. Also it relates to host cells comprising such nucleic acid sequences, DNA fragments, recombinant DNA molecules and live recombinant carriers. Moreover, the invention relates to proteins encoded by these nucleotide sequences. The invention also relates to vaccines for combating Lawsonia intracellularis infections and methods for the preparation thereof. Finally the invention relates to diagnostic tests for the detection of Lawsonia intracellularis DNA, the detection of Lawsonia intracellularis antigens and of antibodies against Lawsonia intracellularis.
Owner:INTERVET INT BV
Method of detecting Lawsonia intracellularis antibodies
ActiveUS7303891B2Animal cellsMicrobiological testing/measurementDiagnostic testLawsonia intracellularis
The present invention relates to the field of animal health and in particular to Lawsonia intracellularis. In particular, the invention relates to a method of diagnosing Lawsonia intracellularis infection and a diagnostic test kit using Lawsonia intracellularis-specific antibodies. The invention also relates to the use of the method or test kit for diagnosing Lawsonia intracellularis infections.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Therapeutic and diagnostic compositions
The present invention relates generally to therapeutic compositions for the treatment and / or prophylaxis of intestinal disease conditions in animals and birds caused or exacerbated by Lawsonia intracellularis or similar or otherwise related microorganism. The present invention also contemplates methods for the treatment and / or prophylaxis of such intestinal disease conditions and to diagnostic agents and procedures for detecting Lawsonia intracellularis or similar or otherwise related microorganism.
Owner:AGRI VICTORIA SERVICES PTY LTD +1
Lawsonia intracellularis immunological proteins
InactiveUS20080279893A1Improve the immunityReduce the severity of the diseaseAntibacterial agentsPeptide/protein ingredientsA-DNAImmunogenicity
The present invention provides nucleic acid and amino acid sequences useful as the immunogenic portion of vaccines or immunogenic compositions effective for lessening the severity of the clinical symptoms associated with Lawsonia intracellularis infection or conferring protective immunity to an animal susceptible to such infection. Preferred amino acid sequences are selected from the group consisting of 1) a polypeptide comprising a sequence selected from the group consisting of SEQ ID Nos.: 1-455, SEQ ID No 466, or the polypeptide encoded by SEQ ID No: 456, SEQ ID No: 457 or SEQ ID No: 466; 2) any polypeptide that has at least 85% sequence homology, more preferably at least about 90% sequence homology, still more preferably at least about 95% sequence homology, even more preferably at least about 97% sequence homology, still even more preferably at least about 98% sequence homology, and even more preferably at least about 99% sequence homology to the polypeptide of 1); 3) any immunogenic portion of the polypeptides of 1) and / or 2) 4) the immunogenic portion of 3), comprising at least 300, 290, 280, 270, 260, 250, 240, 230, 220, 210, 200, 190, 180, 170, 160, 150, 140, 130, 120, 110, 100, 90, 80, 70, 60, 50, 45, 40, 35, 30, 25, 20, 18, 15, 13, 10, or most preferably 9 contiguous amino acids included in the sequences of SEQ ID No: 1-455, SEQ ID No: 456, or the amino acid sequence encoded by SEQ ID No: 457 or SEQ ID No: 466; and / or 5) a polypeptide that is encoded by a DNA that codes for a peptide comprising the sequence of SEQ ID No: 1-455 or SEQ ID No: 466. Thus, the nucleic acid sequences encoding such proteins, or the proteins themselves are included in vaccine compositions, together with veterinary-acceptable carrier and administered to an animal in need thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Ileitis diagnostic assay
Improved immunoassays for the protection of antibodies against Lawsonia intracellularis are provided which permit rapid, easy detection of low concentrations of anti-Lawsonia antibodies in animal-derived specimens. The preferred assay is an ELISA assay employing an antigenic extract of L. intracellularis lipopolysaccharide.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Vaccines for proliferative ileitis and methods of making and using the same
InactiveUS20060193874A1Avoid developmentAntibacterial agentsBacterial antigen ingredientsAdjuvantVaccine Production
A proliferative ileitis vaccine comprising tissue culture grown Lawsonia intracellularis and methods of making said vaccines. Proliferative ileitis vaccines described include those containing whole L. intracellularis, extracts of L. intracellularis, protective immunogenic subunits of L. intracellularis, recombinant immunogens of L. intracellularis and naked DNA of L. intracellularis. The vaccines of this invention may be inactivated or modified live and contain adjuvants and / or stabilizers. The vaccines of this invention may be in a liquid or lyophilized form. Also disclosed are monoclonal antibodies which neutralize the growth of L. intracellularis and which may be used for diagnosing proliferative ileitis as well as for quantitating antigen during vaccine production.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Lawsonia derived gene and related OmpH polypeptides, peptides and proteins and their uses
The present invention relates generally to therapeutic compositions for the treatment and / or prophylaxis of intestinal disease conditions in animals and birds caused or exacerbated by Lawsonia intracellularis or similar or otherwise related microorganism. In particular, the present invention provides a novel gene derived from Lawsonia intracellularis which encodes an immunogenic OmpH peptide, polypeptide or protein that is particularly useful as an antigen in vaccine preparation for conferring humoral immunity against Lawsonia intracellularis and related pathogens in animal hosts. The present invention is also directed to methods for the treatment and / or prophylaxis of such intestinal disease conditions and to diagnostic agents and procedures for detecting Lawsonia intracellularis or similar or otherwise related microorganisms.
Owner:PIG RES DEV CORP +1
Vaccination of young animals against lawsonia intracellularis infections
ActiveUS20080063648A1Reduced gross pathology associatedImprove the immunityAntibacterial agentsBiocideAntigenVaccination
The present invention provides a method of vaccinating a young animal against L. intracellularis infection comprising the step of administering to said animal an effective dose of L. intracellularis antigen. It also provides a method of vaccinating an animal, preferably a young animal, having anti-L.intracellularis antibodies or is exposed to anti-L.intracellularis antibodies. In particular, those anti-L.intracellularis antibodies are maternally derived antibodies.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Vaccination of horses against lawsonia intracellularis
The present invention is broadly concerned with vaccination of horses against proliferative enteritis, preferably equine proliferative ileitis, which is caused by an obligate intracellular bacterium Lawsonia Intracellularis (L. intracellularis). Specifically, the invention provides for a method of providing immune protection against L. intracellularis by vaccinating horses, preferably foals starting from one (1) week of age. Preferably the foals are vaccinated with about 4.9 log 10 to about 6.9 log 10 of a live modified L. intracellularis bacteria per dose.
Owner:BOEHRINGER INGELHEM VETMADICA INC
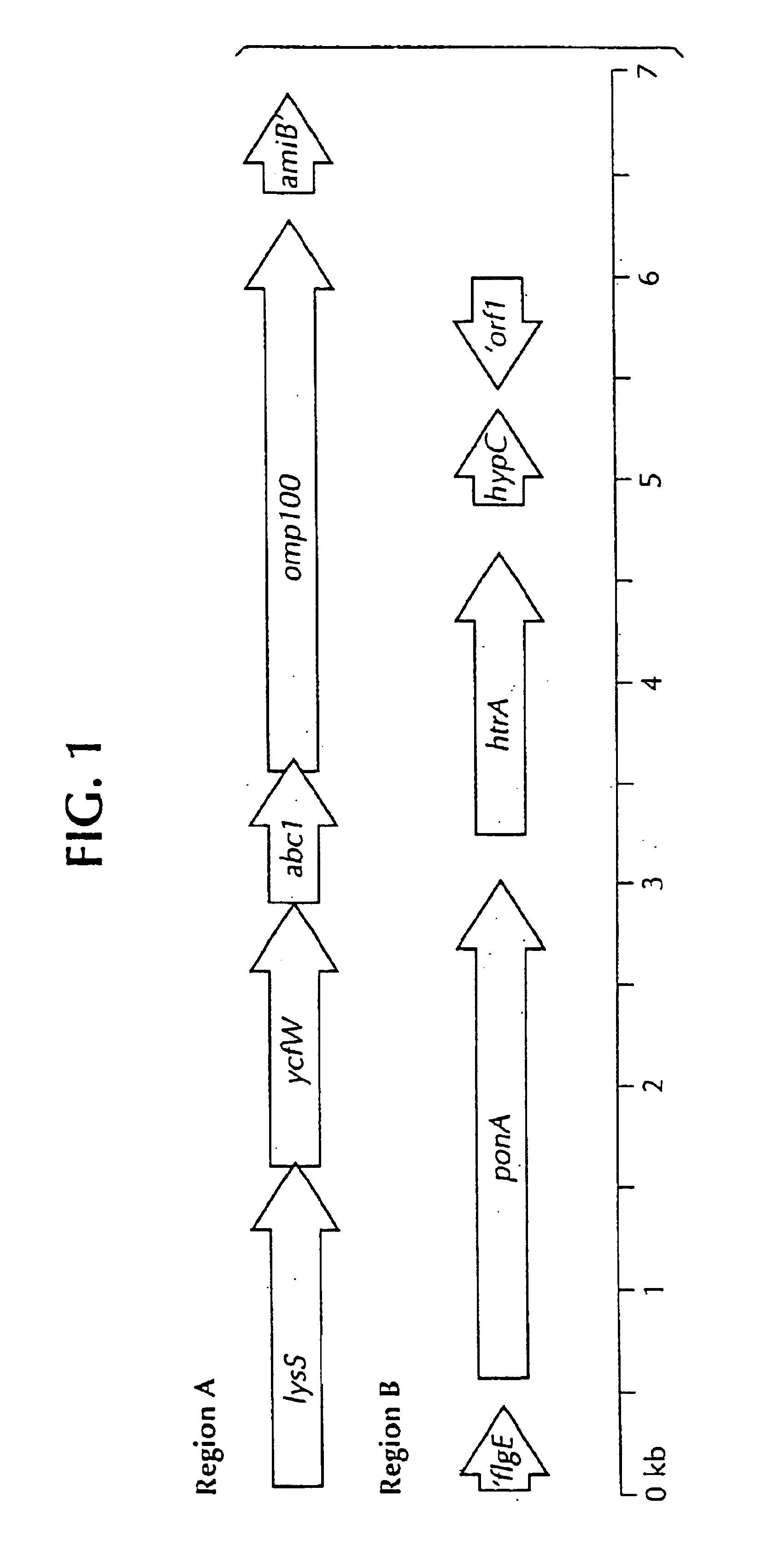
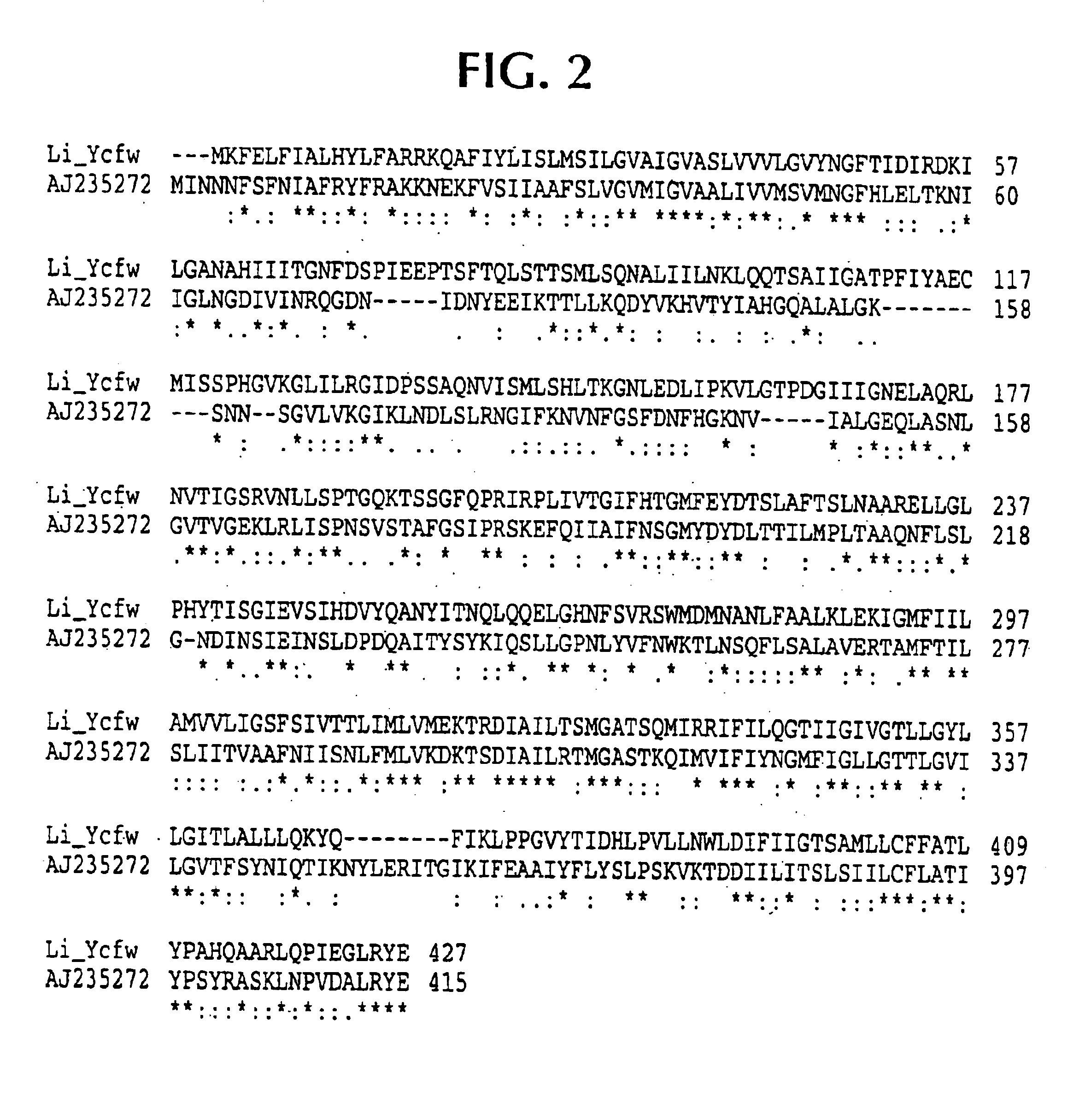
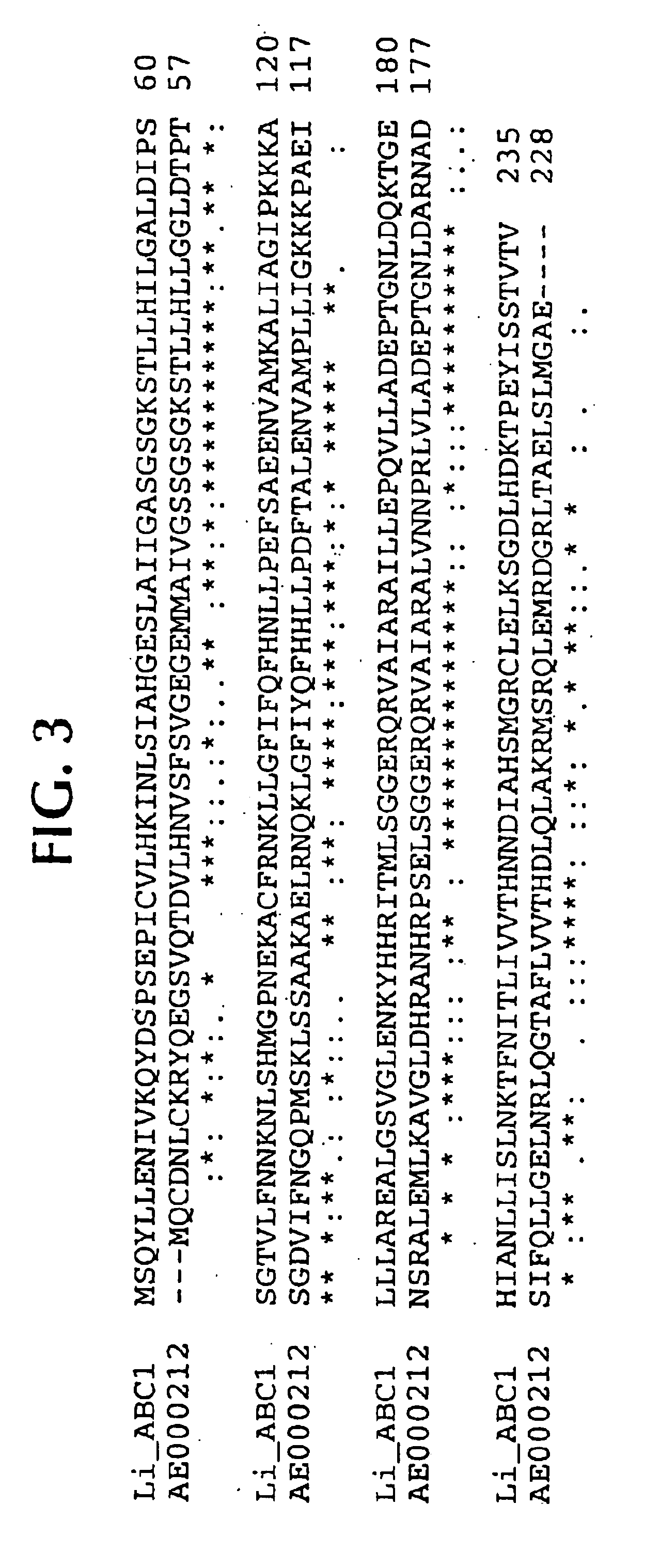
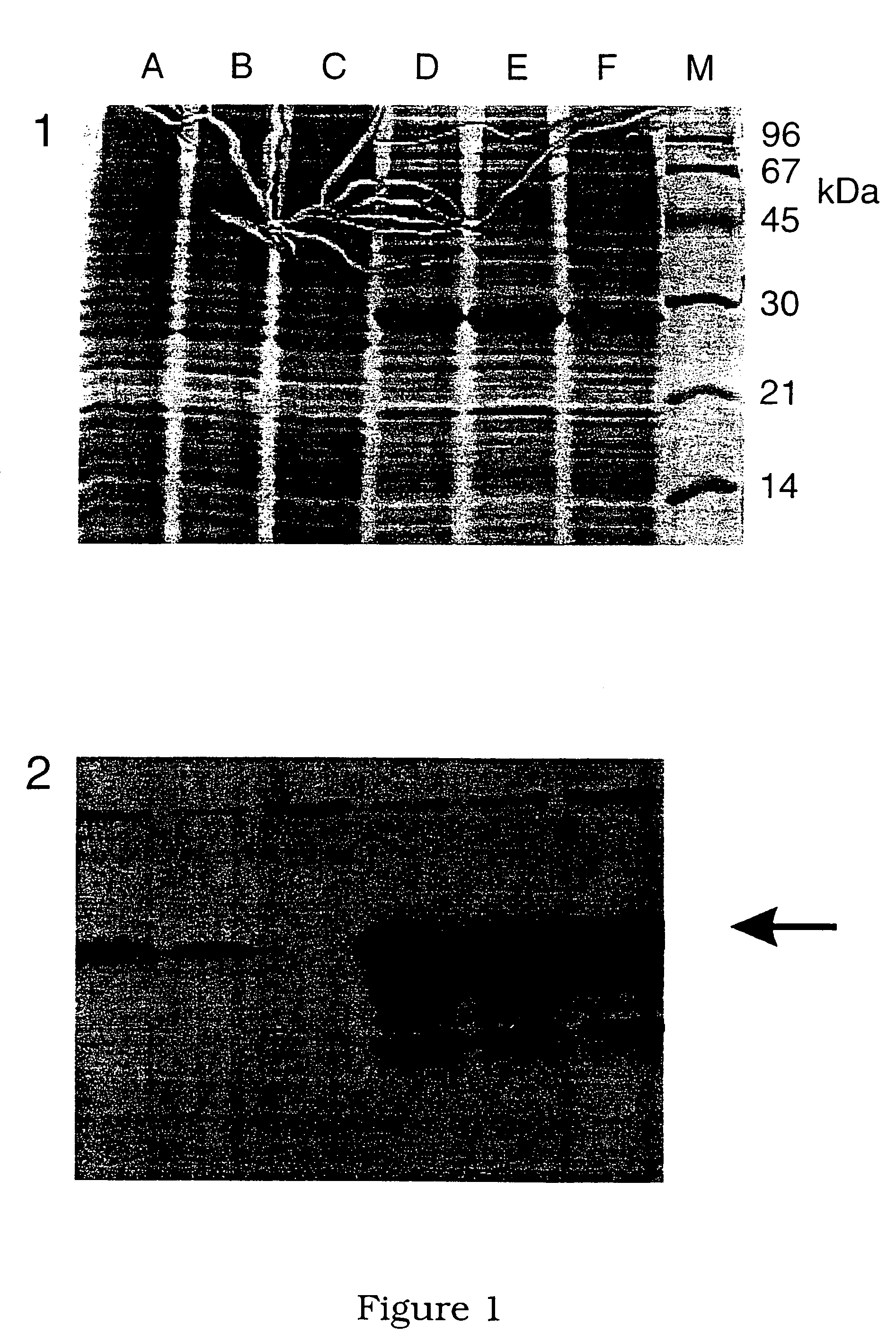
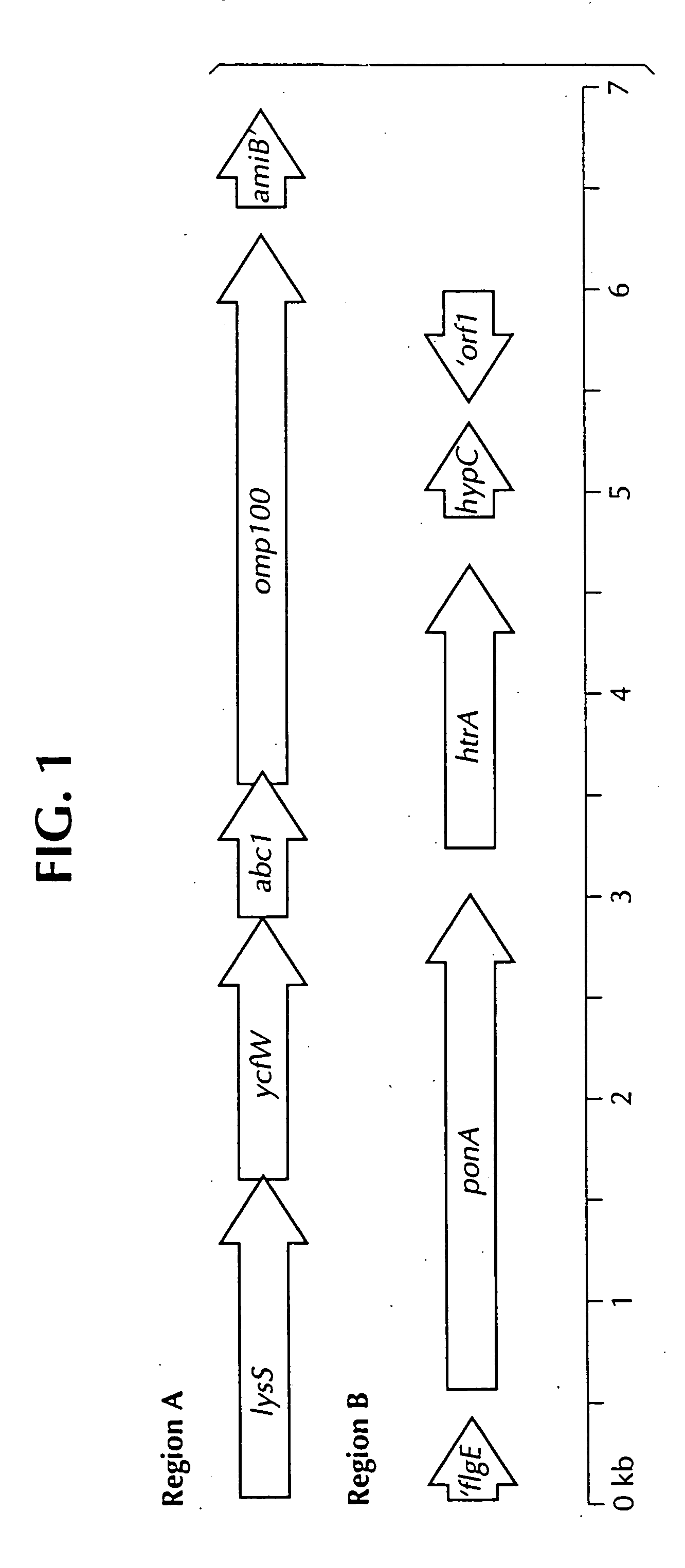
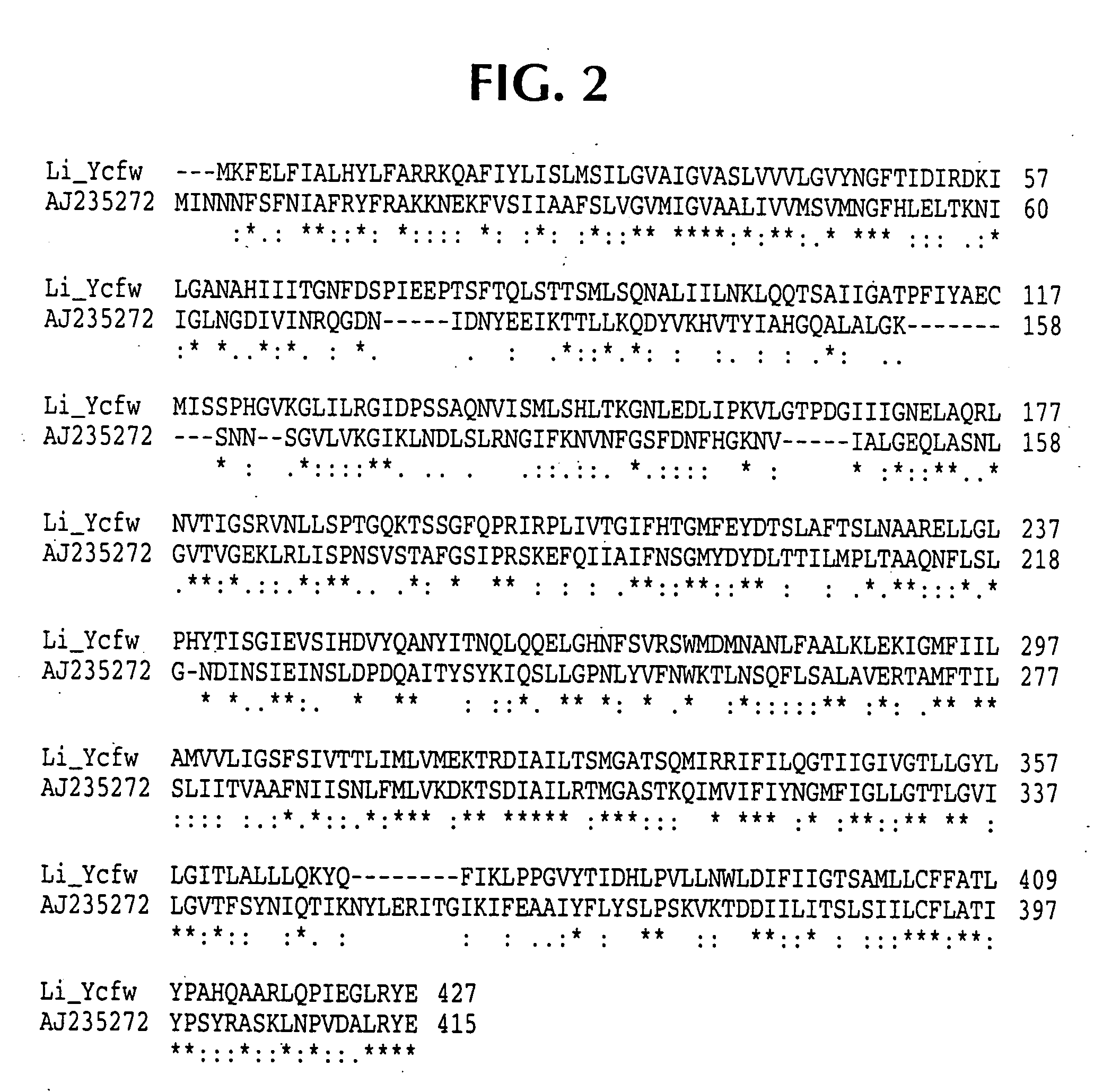
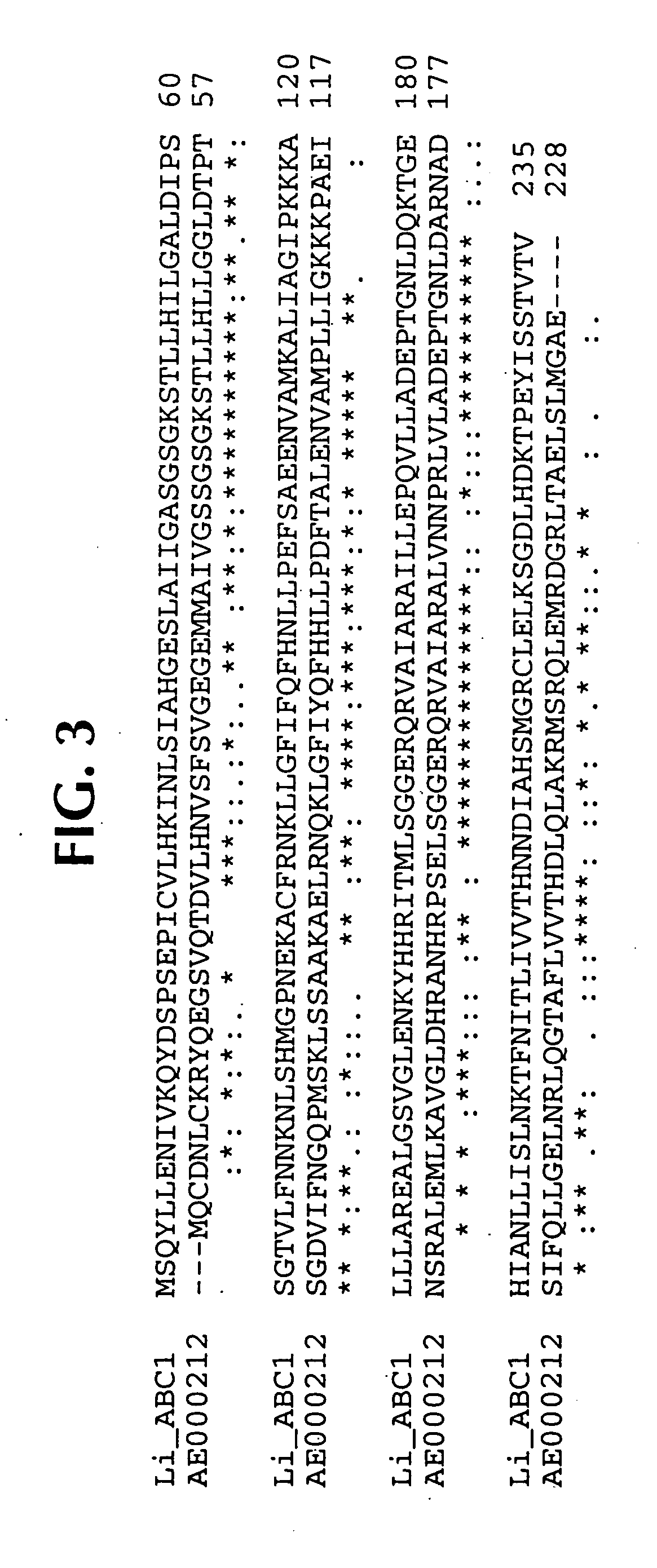
Lawsonia intracellularis proteins, and related methods and materials
Isolated polynucleotide molecules contain a nucleotide sequence that encodes a L. intracellularis HtrA, PonA, HypC, LysS, YcfW, ABC1, or Omp100 protein, a substantial portion of the sequences, or a homologous sequence. Related polypeptides, immunogenic compositions and assays are described.
Owner:ROSEY EVERETT
Method of preventing early lawsonia intracellularis infections
ActiveUS20100266637A1Reduce in quantityEffective protectionBiocideOrganic active ingredientsAntibiotic YLawsonia intracellularis
The present invention relates inter alia to the use of a combination of a vaccine against Lawsonia intracellularis and an anti-Lawsonia antibiotic for the prevention or reduction of early, preferably fulminant Lawsonia intracellularis infections. The present invention relates particularly to the use of a live Lawsonia intracellularis vaccine in conjunction with an antibiotic that is effective against Lawsonia intracellularis, for the avoidance or reduction of early Lawsonia intracellularis infections in animals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Lawsonia protein useful as a component in subunit vaccine and methods of making and using thereof
InactiveUS20090215698A1Improve the immunityReduce the severity of the diseaseAntibacterial agentsOrganic active ingredientsNucleic acid sequencingProtective immunity
The present invention provides nucleic acid and amino acid sequences useful as the immunogenic portion of vaccines or immunogenic compositions effective for lessening the severity of the clinical symptoms associated with Lawsonia intracellularis infection or conferring protective immunity to an animal susceptible to such infection. Preferred amino acid sequences include at least 9 contiguous amino acids from SEQ ID NOS 1 (IDFKAKGVWDFNNFE), 3 (IDFKAKGVWDFNNFEWQQSSFMKG), or 7 (MKLGYKISAGFAIGMIMVVLM). Thus, the nucleic acid sequences encoding such proteins, or the proteins themselves are included in vaccine compositions, together with veterinary-acceptable carrier and administered to an animal in need thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Ileitis diagnostic assay
ActiveUS20060035287A1Bacterial antigen ingredientsSugar derivativesLawsonia intracellularisElisa assay
Improved immunoassays for the protection of antibodies against Lawsonia intracellularis are provided which permit rapid, easy detection of low concentrations of anti-Lawsonia antibodies in animal-derived specimens. The preferred assay is an ELISA assay employing an antigenic extract of L. intracellularis lipopolysaccharide.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Method of preventing early Lawsonia intracellularis infections
The present invention relates inter alia to the use of a combination of a vaccine against Lawsonia intracellularis and an anti-Lawsonia antibiotic for the prevention or reduction of early, preferably fulminant Lawsonia intracellularis infections. The present invention relates particularly to the use of a live Lawsonia intracellularis vaccine in conjunction with an antibiotic that is effective against Lawsonia intracellularis, for the avoidance or reduction of early Lawsonia intracellularis infections in animals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Porcine lawsonia intracellularis IPMA antigen detection method and application thereof
PendingCN111830257AGood antigenicityImprove hydrophilic abilitySerum immunoglobulinsMaterial analysis by observing effect on chemical indicatorInfected cellStaining
The invention relates to a porcine lawsonia intracellularis immunoperoxidase monolayer assay (IPMA) antigen detection method. The rabbit anti-lawsonia intracellularis SodC protein polyclonal antibodyis prepared by taking purified recombinant SodC protein as immunogen, and the titer and the reactogenicity are detected. The lawsonia intracellularis IPMA antigen detection method is established by taking lawsonia intracellularis positive bacteria as a coating antigen and taking a prepared polyclonal antibody as a primary antibody. The method has good specificity, sensitivity and repeatability, expensive instruments such as a fluorescence inversion microscope, a microplate reader and a PCR instrument are not needed, operation is easy and convenient, judgment is more visual, the dyed microporous plate can be stored for a long time, the method is suitable for screening of a large number of samples, and a new means is provided for positioning and detection of lawsonia intracellularis in infected cells.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Enzyme-linked immunosorbent assay (ELISA) test kit for testing lawsonia intracellularis (LI) of pigs
InactiveCN107576791AAchieving high-level expressionIncrease profitMaterial analysisTrue positive rateLawsonia intracellularis
The invention discloses an enzyme-linked immunosorbent assay (ELISA) test kit for testing lawsonia intracellularis (LI) of pigs. The ELISA test kit for testing the LI of the pigs comprises a solid phase carrier and an antigen enveloped in the solid phase carrier, wherein the antigen is LsaA recombinant protein, and the amino acid sequence of the LsaA recombinant protein is as shown in SEQ ID No. 1. The kit has the characteristics of being higher in sensitivity, high in specificity, good in stability, and the like, and has the advantages of convenient use, simple operation, short detection timeand low use cost; the kit can be used for carrying out serological investigation, antibody detection, vaccine immune effect evaluation and the like on the LI infection condition of the pigs, and is suitable for large-scale popularization and use.
Owner:HENAN AGRICULTURAL UNIVERSITY
Separation and culture method for Lawsonia intracellularis
PendingCN111961627AAvoid pollutionIncrease success rateAntibacterial agentsBacterial antigen ingredientsAttenuated vaccineCryopreservation
The invention discloses a separation and culture method for Lawsonia intracellularis. The method comprises the following steps: preparation of Lawsonia intracellularis isolation; subculture of McCoy cells; bacterium inoculation and culture; generation transferring and cryopreservation of Lawsonia intracellularis; and identification on the Lawsonia intracellularis. According to the method, the McCoy cells are used as host cells and are applied to the separation and culture of the Lawsonia intracellularis, the vacancy that the Lawsonia intracellularis is not successfully separated and cultured from a body of a pig yet domestically is filled up, and a foundation is settled for research and development of attenuated vaccines of the Lawsonia intracellularis and research on a pathogenesis mechanism.
Owner:NANJING AGRICULTURAL UNIVERSITY
Swine Vaccine Against PRRS and Lawsonia Intracellularis
InactiveUS20160303219A1Less-safe and effectiveComplicate safetyAntibacterial agentsBacterial antigen ingredientsAntigenLawsonia intracellularis
The present invention pertains to a swine vaccine, in particular a vaccine comprising in combination live attenuated PRRS virus and inactivated Lawsonia intracellularis antigen, for the protection of a swine against an infection with PRRS virus and Lawsonia intracellularis bacteria. The invention also pertains to a method to protect a swine against an infection with PRRS virus and Lawsonia intracellularis bacteria using this vaccine.
Owner:INTERVET INC
Therapeutic compositions for treating infection by Lawsonia spp.
The present invention relates generally to therapeutic compositions for the treatment and / or prophylaxis of intestinal disease conditions in animals and birds caused or exacerbated by Lawsonia intracellularis or similar or otherwise related microorganism. In particular, the present invention provides a novel gene derived from Lawsonia intracellularis, which encodes an immunogenic polypeptide that is particularly useful as an antigen in a vaccine preparation for conferring humoral immunity against Lawsonia intracellularis and related pathogens in animal hosts, wherein said polypeptide is selected from the group consisting of flhB, fliR, ntrC, glnH, motA, motB, tlyC, ytfM, and ytfN polypeptides, or a homologue, analogue or derivative of any one or more of said polypeptides. The present invention is also directed to methods for the treatment and / or prophylaxis of such intestinal disease conditions and to diagnostic agents and procedures for detecting Lawsonia intracellularis or similar or otherwise related microorganisms.
Owner:PFIZER INC +2
Vaccine against lawsonia intracellularis and porcine circovirus 2
ActiveUS20160303218A1Reduce vaccine efficacyIncrease local adverse reactionAntibacterial agentsBacterial antigen ingredientsBacteroidesPorcine circovirus
The present invention pertains to a vaccine comprising in combination killed whole cell Lawsonia intracellularis bacteria and porcine circo virus 2 (PCV2) ORF2 protein for use in protecting a pig against an infection with Lawsonia intracellularis and PCV2 by an intradermal administration of the vaccine. The invention also pertains to a method to protect a swine against an infection with Lawsonia intracellularis bacteria and PCV2.
Owner:INTERVET INT BV +1
Method for establishing PK-PD synchronous model of tilmicosin to lawsonia intracellularis of pigs
InactiveCN111549156ADelay drug resistanceProtect and maintain effectivenessMicrobiological testing/measurementMicroorganism based processesBiotechnologyDosing regimen
The invention discloses a method for establishing a PK-PD synchronous model of tilmicosin to Lawsonia intracellularis of pigs. The method comprises the following steps: drawing a growth curve of Lawsonia intracellularis in IPEC-J2 cells; determining intracellular and extracellular minimum inhibitory concentrations, anti-mutation concentrations and antibacterial post-effects of tilmicosin on lawsonia intracellular and extracellular of pigs, and drawing in-vitro and semi-in-vivo intracellular sterilization curves; selecting proper PK-PD parameters according to a measured intracellular sterilization curve in a lawsonia intracellularis half body, fitting data, establishing a PK-PD synchronous model of tilmicosin to lawsonia intracellularis, and formulating a reasonable dosing scheme. Accordingto the invention, an administration scheme of tilmicosin to Lawsonia intracellularis can be formulated, stable medication data support is provided for scientific culture, and clinical medication is scientifically guided; the drug resistance of the lawsonia intracellularis to the tilmicosin is effectively relieved, the effectiveness of the tilmicosin is protected and maintained, and a guide is provided for researching the PK-PD synchronous model of the antibacterial drug to the intracellular bacteria.
Owner:HUAZHONG AGRI UNIV
Lawsonia intracellularis cultivation, anti-lawsonia intracelluaris vaccines and diagnostic agents
InactiveCN1519311AThe cultivation method is simpleCultivate large-scaleBacterial antigen ingredientsBacteriaBacteroidesInfected cell
A method for large scale cultivation and attenuation of L. intracellularis bacteria by inoculating cells with L. intracellularis bacteria to infect the cells, incubating the infected cells in a reduced oxygen concentration and maintaining the infected cells in suspension. Anti-L. intracellularis vaccines are prepared from cultures grown in suspension. Diagnostic agents are also disclosed.
Owner:NOBL LAB
Methods for cultivating lawsonia intracellularis
InactiveUS20090232848A1Accelerate cultivationAntibacterial agentsBacterial antigen ingredientsHydrogenLawsonia intracellularis
This invention relates to methods for cultivating Lawsonia intracellularis. In particular, the present invention provides improved methods for cultivating Lawsonia intracellularis by employing reducing agents other than molecular hydrogen; or alternatively, by employing a combination of one or more reducing agents with molecular hydrogen. This invention also relates to vaccines and diagnostic reagents prepared from Lawsonia intracellularis cultivated by employing the methods disclosed herein.
Owner:PFIZER INC
Vaccination for Lawsonia intracellularis
The present invention include a method of protecting an animal against Lawsonia intracellularis infections, the method including administering a dose of live virulent L. intracellularis to the animal, allowing a subclinical L. intracellularis infection to develop in the animal, and administering one or more antibiotics to the animal, wherein the one or more antibiotic is administered in a dose sufficient to abbreviate the subclinical L. intracellularis infection.
Owner:WINKELMAN NATHAN LEN
Lawsonia intracellularis cultivation, anti-lawsonia intracellularis vaccines and diagnostic agents
InactiveCN1670188AThe cultivation method is simpleCultivate large-scaleBacterial antigen ingredientsBacteriaInfected cellBacteroides
A method for large scale cultivation and attenuation of L. intracellularis bacteria by inoculating cells with L. intracellularis bacteria to infect the cells, incubating the infected cells in a reduced oxygen concentration and maintaining the infected cells in suspension. Anti-L. intracellularis vaccines are prepared from cultures grown in suspension. Diagnostic agents are also disclosed.
Owner:NOBL LAB
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com