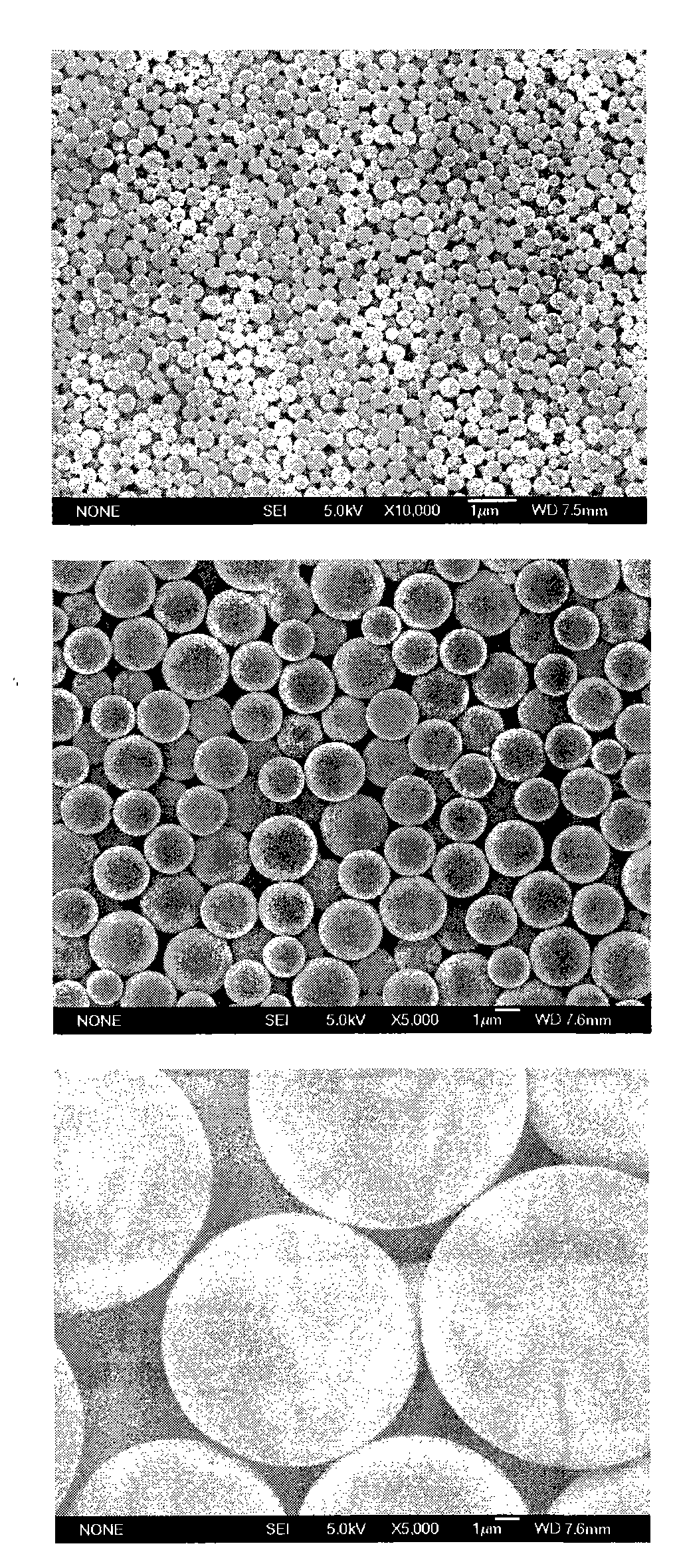
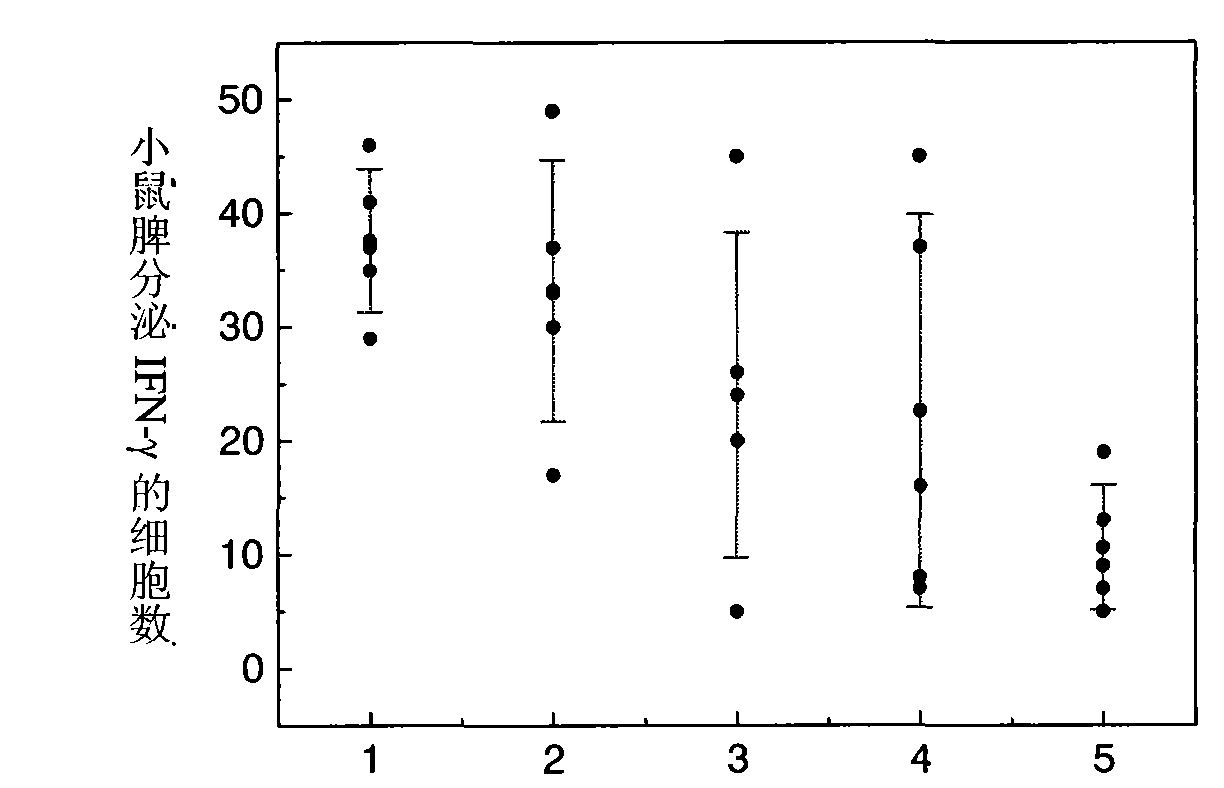
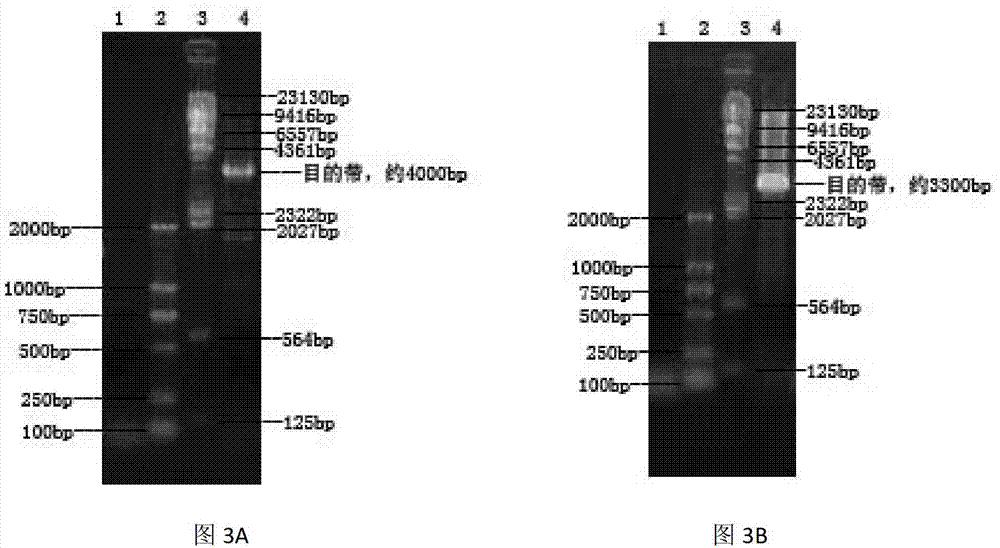
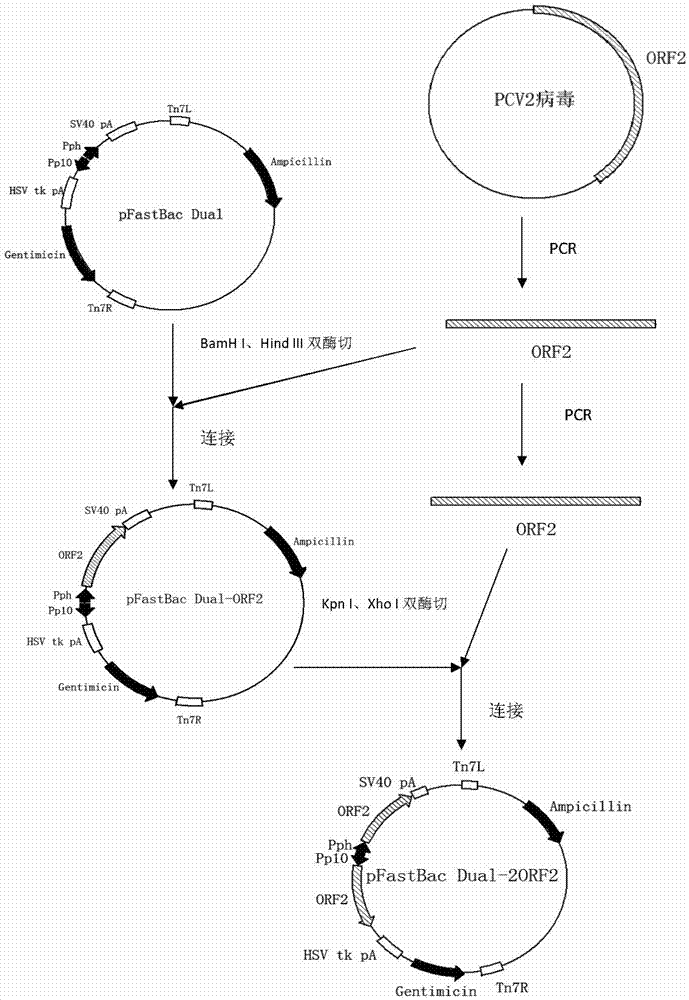
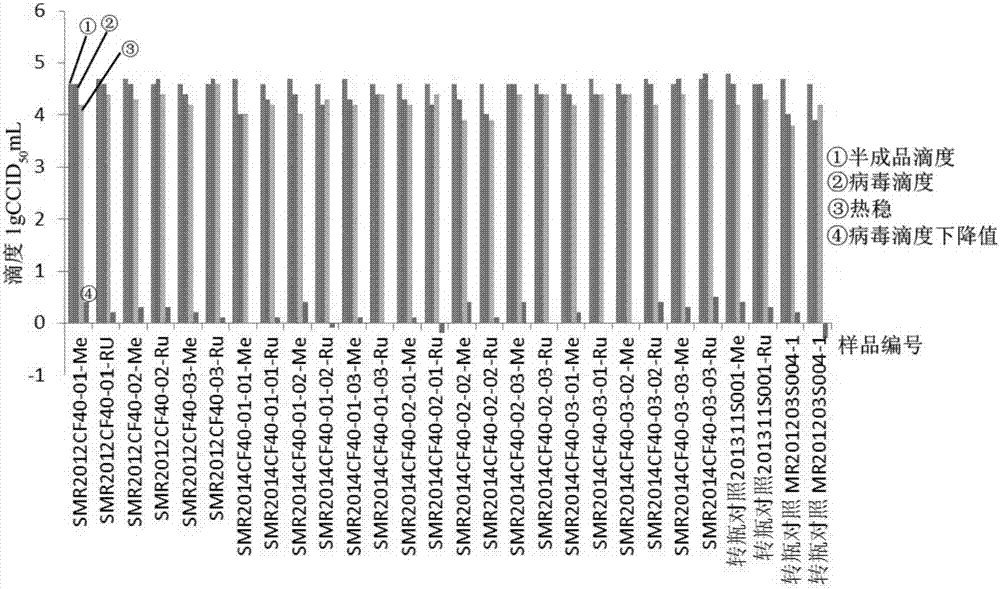
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
147 results about "Vaccine product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lawsonia intracellularis of European origin and vaccines, diagnostic agents and methods of use thereof
The present invention relates to Lawsonia intracellularis vaccines and methods for protecting against and diagnosing L. intracellularis infection. The products and processes of the invention are attainable, in part, as the result of an improved method for cultivating large scale supplies of L. intracellularis, including both a novel isolate of L. intracellularis of European origin and a method of preparing a lyophilized product containing the attenuated European isolate as vaccine product.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Lawsonia intracellularis of European origin and vaccines, diagnostic agents and methods of use thereof
The present invention relates to Lawsonia intracellularis vaccines and methods for protecting against and diagnosing L. intracellularis infection. The products and processes of the invention are attainable, in part, as the result of an improved method for cultivating large scale supplies of L. intracellularis, including both a novel isolate of L. intracellularis of European origin and a method of preparing a lyophilized product containing the attenuated European isolate as vaccine product.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Preparation method and product of H9N2 subtype avian influenza inactivated vaccine
ActiveCN101816785AHigh titerSimple production methodAntiviralsAntibody medical ingredientsVirus multiplicationVaccine Production
The invention relates to a preparation method and a product of an H9N2 subtype avian influenza inactivated vaccine. The technical points of the invention mainly relate to the screening, the determination and the domestication of a virus-adapted cell line, the primary amplification cultivation and the continuous cultivation of a virus-adapted cell, the preparation of virus fluid by virus-inoculated culture and the preparation of final inactivated vaccine products. Firstly, the invention avoids the virus propagating method using a large amount of chick embryos in the avian influenza production at present, thereby avoiding the problem of biological potential safety hazards, and overcoming the problem that the mass production of vaccines is enslaved to the supply of the chick embryos; secondly, the invention provides a safe, continuous and closed cell culture virus production method, is used for the preparation of the H9N2 subtype avian influenza inactivated vaccine, enables the use of the cell culture method, and can simultaneously produce high-titer viruses to meet the requirements for the immunological production; and finally, the vaccine production method of the invention is simple and fast, thereby realizing the fast vaccine supply at the epidemic situation.
Owner:扬州优邦生物药品有限公司
Vaccine
InactiveCN101601860ASimple preparation processGentle preparationDigestive systemAntiviralsBody fluidCytokine
The invention discloses a vaccine for prevention and treatment, wherein the antigen is in two forms with one absorbed on a biodegradable polymer particle and the other unabsorbed (free antigen). The average grain diameter of the particles is 0.1-20um; the antigen has specific structure and antiviral effect, preferably hepatitis B surface antigen and vaccines used for human being. The vaccine is prepared by the following methods: mixing polymer particles with antigen solution to obtain suspension under the absorption of the polymer particles and antigen solution, that is vaccine product; and adding cryoprotector in the obtained suspension-like vaccine to obtain lyophilized powder of the vaccine. The vaccine can induce immune response rapidly in the engine body, induces the engine body to generate high-level humor immune and cell immune, and eliminates or controls virus by secretoryantibody or cytokine. Besides, the vaccine has simple preparation process and mild preparation process.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as preparation method and applications thereof
ActiveCN103033622AImprove expression efficiencyImproving immunogenicityMicroorganism based processesGenetic engineeringSorbentMonoclonal antibody
The invention relates to a PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as a preparation method and applications thereof, wherein the detection kit comprises an elisa plate of a polyclonal antibody of peridium anti-PCV2-Cap (nucleocapsid) protein, seal liquids, sample diluent, an antigen standard product, a second antibody of a monoclonal antibody of HRP marked anti-PCV2-Cap protein, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen standard product is purified reconstructed PCV2-Cap protein. The specificity of the kit provided by the invention achieves 100%, and the sensitivity is as high as 4ng / ml, and the kit can be used for swinery PCV2 antigen detection and PCV2 vaccine product quantitative detection.
Owner:WUHAN CHOPPER BIOLOGY
EV-71 virus seed, inactivated vaccine for human and method of producing the same
ActiveCN101402944AEffective immunogenicityGood growth and replication abilityInactivation/attenuationAntiviralsAntigenVaccine Immunogenicity
The invention discloses an EV-71 virus species, a human inactivated vaccine and a preparation method thereof. The classification name of the virus species is intestinal virus 71 with a storage number of CGMCC No.2701. The prepared human EV-71 inactivated vaccine comprises the following components: 100 Mu g / ml of EV-71 alive purified antigen, 0.8-1mg / ml of aluminum hydroxide, and 0.05-0.1mg / ml of merthiolate. The experiments show that the vaccine products have good immunogenicity and safety.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Cell-derived viral vaccines with low levels of residual cell DNA
ActiveUS20090304729A1Enhance immune responseLess recognizableSsRNA viruses negative-senseViral antigen ingredientsViral VaccineA-DNA
The present invention relates to vaccine products for the treatment or prevention of viral infections. Further provided are methods of reducing contaminants associated with the preparation of cell culture vaccines. Residual functional cell culture DNA is degraded by treatment with a DNA alkylating agent, such as β-propiolactone (BPL), thereby providing a vaccine comprising immunogenic proteins derived from a virus propagated on cell culture, substantially free of residual functional cell culture DNA.
Owner:SEQIRUS UK LTD
Recombinant nerve putrescence virus protein vaccine and its coding sequence and uses
The invention relates to a recombinant nerve putrescence virus protein vaccine, the nucleic acid sequence coding the recombinant protein, and the use of the recombinant protein in preparing fish use vaccine product for nerve putrescence viruses resistance. Wherein the cDNA of the capsid nucleocapsid for the grouper nerve putrescence viruses is utilized to obtain the polypeptide recombinant proteins through DNA recombination process and pronucleus expression method.
Owner:SUN YAT SEN UNIV
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
A kind of mite allergen freeze-dried vaccine and preparation method thereof
ActiveCN102258780AAvoid inconvenienceAvoid instabilityAllergen ingredientsAntiparasitic agentsSide effectAdjuvant
The invention provides a novel lyophilized mite allergen vaccine and a preparation method thereof. The vaccine is prepared by mixing and lyophilizing mite allergen activated protein, a lyophilization protective agent and phosphate buffer solution with pH value of 7.0-8.5 based on the weight ratio of 1:(100-1000):(70-140). Compared with the traditional water preparation, glycerol preparation and preparation containing an aluminum hydroxide adjuvant of the mite allergen vaccine, the lyophilized mite allergen vaccine overcomes use inconvenience of the glycerol preparation, instability of the water preparation and side effects resulting from the preparation containing the aluminum hydroxide adjuvant, thus providing an efficient vaccine product with good stability and good safety for patients allergic to dust mites.
Owner:北京新华联协和药业有限责任公司
Method for producing pseudorabies attenuated vaccine by using bioreactor and pseudorabies attenuated vaccine product
ActiveCN101695572AImprove immune efficiencyIncrease growth densityMicroorganism based processesAntiviralsVaccine ProductionAntibiotic Y
The invention provides a method for producing a pseudorabies attenuated vaccine by using a bioreactor and a pseudorabies attenuated vaccine product. After being sterilized, the bioreactor and a micro carrier are inoculated with cells for producing the vaccine, and a cell growth medium is added for culture. A maintenance medium containing attenuated strains of pseudorabies viruses are inoculated into the bioreactor to continue culturing the cells. 2 to 3 days after virus inoculation, cell culture virus liquid is obtained and added with a stabilizer and antibiotics, and the cell culture virus liquid is refrigerated and dried under vacuum to obtain the pseudorabies attenuated vaccine. In the method, the cell density and virus concentration are improved greatly, the titer of the vaccine is improved, the side reactions, labor intensity and product cost are reduced, the monitoring performance of vaccine production is improved and uniform and stable product quality is guaranteed. The pseudorabies attenuated vaccine produced by the method has high safety, immune efficacy and good immune and protective effect against the attack by the virulent pseudorabies viruses.
Owner:广东永顺生物制药股份有限公司
Chicken new castle disease-infectious bronchitis trivalent combined vaccine and preparation method thereof
InactiveCN103599533AImprove the chance of preventionMeet the needs of preventionPowder deliveryViral antigen ingredientsDiseaseAntigen
The invention discloses a chicken new castle disease-infectious bronchitis trivalent combined vaccine and a preparation method thereof. The trivalent combined vaccine comprises antigens and a freeze-drying protective agent. The antigens comprise a chicken kidney-type infectious bronchitis virus K136 strain having an accession number of CCTCC-V201320, a chicken breath-type infectious bronchitis virus H120 strain, and a chicken new castle disease virus La Sota strain. A mode of inoculating the K136 strain and the La Sota strain in a homeomorphism manner and inoculating the H120 strain individually is adopted for preparing the antigens. Based on the mode, after culture products are mixed according to a ratio to prepare the antigens, a product of the vaccine is obtained by adding the freeze-drying protective agent. The trivalent combined vaccine can prevent the chicken new castle disease and the chicken infectious bronchitis simultaneously, thus solving effectively a problem that vaccines at present can only provide a part of protection or cannot provide protection for the kidney-type IB, and achieving an objective that one injection can be used for preventing two diseases.
Owner:TIANJIN RINGPU BIO TECH
Expression vector of Cap protein of porcine circovirus (PCV) 2 as well as construction method and application thereof
InactiveCN105087624AReduce manufacturing costSimple production processViral antigen ingredientsVirus peptidesEscherichia coliCircovirus
The invention discloses an expression vector of a Cap protein of a porcine circovirus (PCV) 2 as well as a construction method and application thereof. The expression vector is obtained by cloning an optimized codon obtained after optimization of a gene ORF2 of the PCV 2 in a pET-28a vector. The expression vector and the construction method have the beneficial effects that the prokaryotic expression vector capable of efficiently expressing the main immunogenic Cap protein of the PCV 2 can be constructed and the Cap protein of the PCV 2 is efficiently expressed by utilizing escherichia coli, thus obviously reducing the antigen production cost, simplifying the production process and providing the cheap and fine vaccine product for the pig industry.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +2
Protein recombinant lactococcus lactis for secretory expression of core antigen COE of PEDV (Porcine Epidemic Diarrhea Virus) as well as preparation method and application of protein recombinant lactococcus lactis
ActiveCN104120142AStrong immune responseGood industry prospectsBacteriaMicroorganism based processesStaphylococcus lactisTGE VACCINE
The invention belongs to the field of animal biomedicine engineering, and in particular discloses protein recombinant lactococcus lactis for secretory expression of a core antigen COE of a PEDV (Porcine Epidemic Diarrhea Virus) as well as a preparation method and an application of the protein recombinant lactococcus lactis. The method comprises the following steps: connecting a signal peptide and other sequences onto a gene of the core antigen COE of the PEDV via an overlap extension PCR method by designing multiple primers, connecting the gene to a lactococcus lactis expression vector pNZ8048 and introducing the vector with the gene into a cell of the lactococcus lactis NZ9000 in an electrotransformation manner so as to obtain recombinant bacteria; and inducing the recombinant bacteria with nisin so as to obtain an expression, and directly taking all induced cultures as oral vaccines capable of stimulating mice and inducing strong cellular immune responses. Thus, the protein recombinant lactococcus lactis can serve as a novel oral vaccine product with a good industrial prospect and has a positive effect for reducing harms of the PEDV to pig industry, thereby playing a great practical significance for promoting the healthy development of the pig industry.
Owner:SOUTH CHINA AGRI UNIV
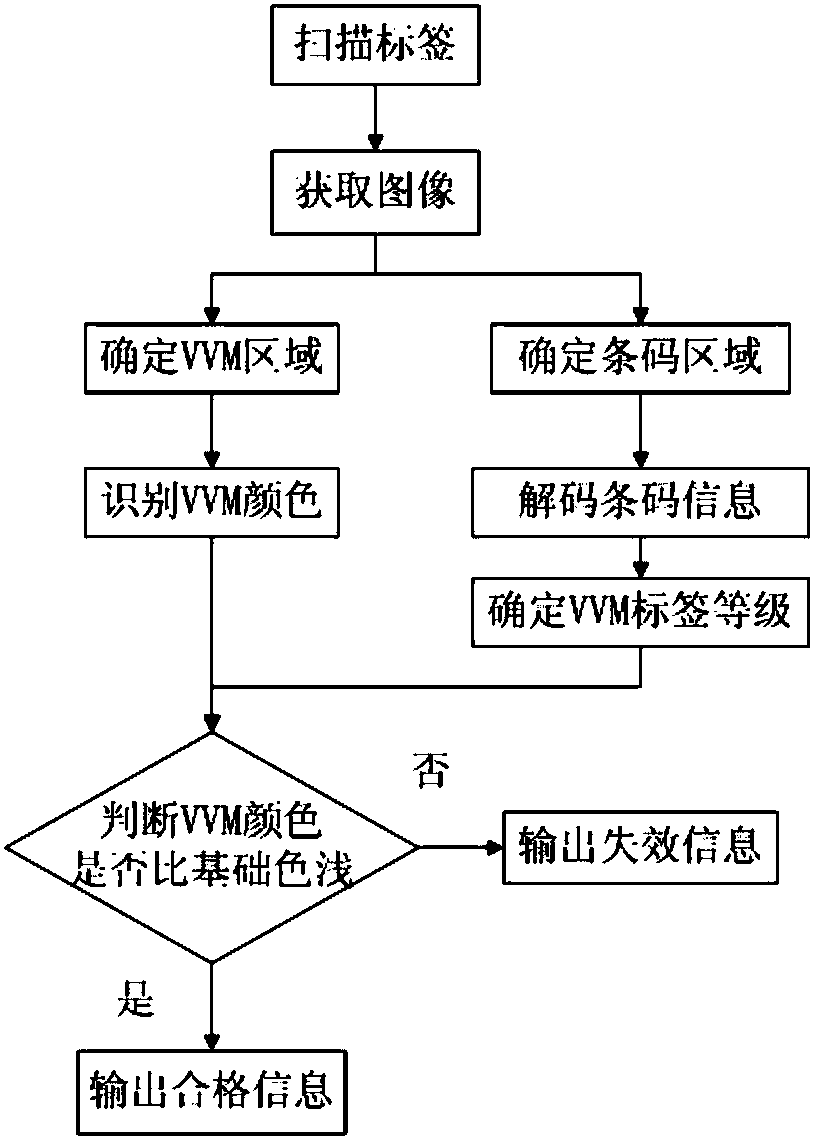
Thermochromic material bar code tag and reading method thereof
InactiveCN108256607ARealize the combinationSpeed up entryMemory record carrier reading problemsRecord carriers used with machinesCold chainVaccine vial monitor
The present invention provides a thermochromic material bar code tag and a reading method thereof. The thermochromic material bar code tag is a tag configured to mark vaccine product information and storage state, and comprises a thermochromic area and an area comprising product information, and the thermochromic area is formed by VVM (Vaccine Vial Monitor) thermal tags. A reading device is employed to read the thermochromic material bar code tag to achieve one-step reading of the product production information and the storage state information, accelerate the input process of vaccine cold chain transportation and cold chain information, improve the input accuracy and avoid the problem that workers are fatigable and low in efficiency in a batch reading mode.
Owner:FUJIAN NEWLAND AUTO ID TECH CO LTD
After treatment method for attenuated strain polio inactivated vaccine
ActiveCN1616095AMeet production needsStable production processViral antigen ingredientsInactivation/attenuationAntigenVaccine Immunogenicity
The present invention is after-treatment process of deactivated vaccine of attenuated polio strain. The large scale cultured virus liquid is three stage filtered in filtering columns of 06-1.0 micron, 0.45-0.6 micron and 0.22-0.45 micron pore size separately, clarified, ultrafiltered and concentrated, chromatographic column purified and deactivated to obtain semi-finished vaccine product with high purity, good immunogenicity, high safety and stable quality for the requirement of producing the attenuated polio strain. The semi-finished vaccine product can meet the requirement of product and may be used to produce Aabin IPV vaccine with stable quality, high safety and excellent immunogenicity.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
F-gene type attenuated live mumps vaccine and preparation method and application thereof
InactiveCN102018956AGood humoral immunityExcellent neutralization potencyInactivation/attenuationAntiviralsBiotechnologyGenotype
The invention discloses an F-gene type attenuated live mumps vaccine and a preparation method and application thereof, relating to an attenuated live mumps vaccine which is prepared in such a way that a separated F-gene type mumps attenuated live seed is inoculated to the human diploid cells (KMB17 strain) and is subjected to subsequent processes and the freezing-drying technology. The vaccine product has good immunogenicity and safety. The invention provides a cryoprotectant which does not contain glutin, thereby ensuring that the attenuated live mumps vaccine has good appearance, low moisture content and good stability.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Method for proliferating avian influenza viruses in bioreactor with cell carrier
InactiveCN102127524AUniform product qualityStable virulenceMicroorganism based processesViruses/bacteriophagesAutomatic controlVaccine Production
The invention discloses a method for replicating and proliferating avian influenza viruses in the passage cells which are absorbed and grow in the microcarrier of a bioreactor. The invention is based on the method which uses cells as carrier to proliferate viruses in the bioreactor. The obtained avian influenza viruses and the avian influenza virus vaccine product have uniform quality and stable toxicity. The automatic control of vaccine production can be realized, the problem of large-scale production and application can be solved, the production matrix of the influenza vaccine can be completely changed, the technology that chick embryo is adopted to culture viruses can be changed, the problems caused by chick embryo such as adventitious agent pollution and viral pollution and the interferences caused by heterologous proteins such as chick embryo can be reduced, the purity, safety and immune effect of vaccine can be increased and the method can play an important role in the prevention of avian influenza.
Owner:深圳市南海潮生物技术有限公司
CoxA16 virus strain and human CoxA16 inactivated vaccine
ActiveCN102839159AEffective immunogenicityGood growth and replication abilityMicroorganism based processesAntiviralsNucleotideVaccine virus
The invention provides a CoxA16 virus strain, named CoxA16 (GX-20K-D virus strain) with CGMCCNo.6350 and including three bases, namely an original seed base, a master seed base and a working seed base; in addition, the CoxA16 virus strain has a nucleotide sequence shown as SEQNo.1. The invention has the following obvious beneficial effects: 1, a virus strain is provided for preparing a human CoxA16 inactivated vaccine and has effective immunogenicity and good growth and replication capacity on cells; 2, a production and preparation method of the human CoxA16 inactivated vaccine which is prepared from KMB17 human diploid cell matrix is provided, and the vaccine product has excellent immunogenicity and safety; and a suspended absorption technical method is provided so as to ensure the effective infection of the CoxA16 inactivated vaccine virus strain on KMB17 cell and excellent growth of the CoxA16 inactivated vaccine virus strain on the matrix.
Owner:JIANGSU CONVAC BIO TECH CO LTD
Cell-factory-based measles virus stock solution and measles-series attenuated live vaccine preparation
InactiveCN107418936AAchieve trainingSmall batch-to-batch varianceSsRNA viruses negative-senseSsRNA viruses positive-senseCell factoryCulture fluid
The invention provides a cell-factory-based measles virus stock solution and a measles-series attenuated live vaccine preparation. A preparation method of the measles virus stock solution includes selecting SPF chick-embryo cells, and adding cell culture fluid to prepare cell suspension liquid, and adding the cell suspension liquid into a cell factory; inoculating the cell factory with working seeds of measles viruses and the cell suspension liquid according to the ratio of the working seeds to the cell suspension liquid being (0.005-0.05):1, and standing and cultivating the cell factory at the temperature of 33+ / -1 DEG C for 3-4 days, pouring out archeocyte culture fluid, and replacing with fresh cell growth liquid for continuous cultivation; during cytopathy, collecting single measles virus liquid step by step. In an equal production scale, the batch number of cell dissociation is reduced, and the high-titer measles virus liquid is obtained, so that quality uniformity of measles-series vaccine products is improved effectively, and product yield is increased.
Owner:BEIJING BIOLOGICAL PROD INST CO LTD
Method for preparation of swine pseudorabies virus vaccine and vaccine product
InactiveCN105727277AReduce investmentSimple production processAntiviralsAntibody medical ingredientsHighly pathogenicVirus strain
The present invention relates to a method for preparation of a swine pseudorabies virus vaccine, the method comprises the follwoing steps: (1) preparation of suspension passage cells; (2) multiplication culture of the suspension passage cells prepared by the step (1); and (3) inoculation of pseudorabies virus onto the suspension passage cells after the multiplication culture of the step (2), and multiplication culture of the pseudorabies virus. Compared with production methods in the prior art, production process is simplified, and cost is reduced. The swine pseudorabies virus vaccine can well avoid attacks from market popular highly pathogenic pseudorabies virus strains, and economic losses caused by pseudorabies can be reduced.
Owner:PU LIKE BIO ENG
Method for removing residual DNA in hydrophobia vaccine product by utilizing ultrasound combined with EDTA solution
ActiveCN101780276AEffective removal of contentBreak through the quality bottleneckAntiviralsAntibody medical ingredientsAntigenHybrid protein
The invention provides a method for removing residual DNA in a hydrophobia vaccine product by utilizing ultrasound combined with EDTA solution. The method solves the problems that a method for extracting hydrophobia vaccine through concentration, purification and the like can only remove dissociative DNA in a certain percentage and cannot remove host DNA combined with antigen protein and clinical adverse reactions commonly occur. The method has main points of: adding the EDTA solution into hydrophobia vaccine concentrate; performing ultrasonic treatment on the hydrophobia vaccine by using ultrasonic wave to make the host DNA broken more easily under the action of the ultrasound; and removing the host DNA through chromatography and purification. The method has the advantages that: on the premise of ensuring the valence of the vaccine, the quality of a vaccine product is improved, mass hybrid protein and the host DNA are removed so that the residual DNA content of the vaccine product is less than 100 pg / dose, and the quality problem in the hydrophobia vaccine industry at present is solved.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Vaccine product with respectively packaged freeze-dried vaccine and dilute solution thereof and method for preparing dilute solution
The invention provides a vaccine product with respectively packaged freeze-dried vaccine and dilute solution thereof and method for preparing the dilute solution, which aims to solve the problem that the patent No. 93105862.7 records a vaccine in liquid state made by the combination of polyinosinic cytidilic acid containing kanamycin and calcium ions with lyssa virus antigen, both of which are in liquid states, has a guarantee period that is strictly limited to result in the limitation of the application of the product; and the method for preparing the adjuvant of polyinosinic cytidilic acid containing kanamycin and calcium ions is not disclosed in the patent No. 93105862.7. The invention has the following essentials: (1) freeze-dried vaccine and dilute solution thereof are respectively packaged, and the dilute solution is the polyinosinic cytidilic acid containing kanamycin and calcium ions; (2) the method for preparing the dilute solution. The invention overcomes the defect that the freeze-dried vaccine can not use PIKA adjuvant, and widens the practical application range of the PIKA adjuvant.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Method for producing avian influenza vaccine by using cells cultured by spherical microcarrier bioreactor
InactiveCN102657859AHigh titerShorten the production cycleInactivation/attenuationMicroorganism based processesEmbryoBottle
The invention provides a method for producing an avian influenza vaccine by using cells cultured by a spherical microcarrier bioreactor. The method comprises the following steps of: selecting seed cells; passaging cells reproduced in a spinner bottle; harvesting the cells in the spinner bottle; inoculating the cells; culturing the inoculated cells in the bioreactor; inoculating avian influenza virus; reproducing the avian influenza virus; collecting avian influenza virus liquid; inactivating the avian influenza virus liquid; preparing an avian influenza cell vaccine; and immunizing the avian influenza cell vaccine. By the method, the defects that the avian influenza vaccine produced by the traditional process is long in production period, large in inter-batch difference, high in endotoxin content and influenced by the supply of embryo eggs, and a tablet carrier is difficult to amplify are overcome; and the prepared avian influenza vaccine product is small in inter-batch difference, free from bacterial pollution, low in endotoxin content and formaldehyde content, stable in quality and short in the production period, and the tablet carrier is easy to amplify.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
Lyophilized mite allergen vaccine and preparation method thereof
ActiveCN102258780BAvoid inconvenienceAvoid instabilityAllergen ingredientsAntiparasitic agentsSide effectAdjuvant
Owner:北京新华联协和药业有限责任公司
Method for producing swine pseudorabies live vaccine by using passage cell source, and product thereof
ActiveCN104367996AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesRoom temperatureDiluent
The present invention provides a method for producing a swine pseudorabies live vaccine by using a passage cell source, and a swine pseudorabies live vaccine product thereof. The method comprises: adopting passage cells to culture a swine pseudorabies virus attenuated strain; harvesting to obtain a cell culture virus solution; and adding a stabilizer, and carrying out freezing vacuum drying to obtain the passage cell derived swine pseudorabies live vaccine. During the use, the diluent of the prepared vaccine of the present invention can further be used. According to the present invention, the method has advantages of simple and stable production process, easy operation, high virus content, low difference between batches, controllable quality, significant vaccine yield and quality improving, and the like, the obtained product can be stored for a long time at the room temperature, especially at the temperature of 2-8 DEG C, the shelf life can be 36 months, and the diluted vaccine obtained by diluting the vaccine with the diluent of the present invention can be stored for a long time at the room temperature and can maintain the stability.
Owner:PU LIKE BIO ENG
CV-A10 virus species and inactivated vaccine thereof for human
PendingCN109536460AIncrease productivityImprove cultivation efficiencySsRNA viruses positive-senseViral antigen ingredientsAntigenAluminium hydroxide
The invention discloses a CV-A10 virus species and an inactivated vaccine thereof for human. The classification name of the species is coxsackie virus A group 10-type with the preservation number being CGMCC No.16218; and the prepared CV-A10 inactivated vaccine for human consists of 100 [mu]g / ml of CV-A10 inactivated purified antigens and 1mg / ml of aluminium hydroxide. Results indicate that the vaccine products have favorable immunogen and safety.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Purification method for split influenza virus vaccine
ActiveCN103721251AGuaranteed uniformityHigh removal rateAntiviralsAntibody medical ingredientsHemagglutininPurification methods
The invention provides a purification method for split influenza virus vaccine. An influenza virus strain is inoculated onto a chick embryo and cultured to obtain a virus solution; the virus solution is sequentially subjected to inactivation and ultrafiltration concentration to obtain an ultrafiltrate; the obtained ultrafiltrate is subjected to cane sugar density gradient centrifugation by using a KII continuous flow centrifuge to obtain an ultra centrifugal solution; the ultra centrifugal solution is subjected to ultrafiltration dialysis for cane sugar removal and molecular sieve gel chromatography to obtain a virus purification solution; the obtained virus purification solution is subjected to virus split by using a split agent TritonX-100 and sodium deoxycholate; after the split is over, the split agent is removed via ultrafiltration dialysis; impure protein is centrifugally removed from the obtained split solution; supernatant is collected, filtered and sterilized so as to obtain the purified influenza virus vaccine primary solution. The purification method is simple and convenient to operate, high in centrifugation capacity and suitable for large-scale production; by adopting a dual-split agent and a centrifugal process, the finished product is greatly improved in activity (hemagglutinin content / protein total content), and approaches the activity ratio of subunit vaccine, as a result, the high-grade split influenza virus vaccine product is obtained.
Owner:SINOVAC BIOTECH
Method for detecting content of D antigen in poliovirus type III
InactiveCN106290886AQuantitatively accurateEasy to operateBiological material analysisMonoclonal antibodyPolyclonal antibodies
The invention provides a method for detecting the content of D antigen in poliovirus type III and belongs to the technical field of antigen detection. Through matching of a poliovirus type III specific polyclonal antibody and a monoclonal antibody, the content of D antigen in poliovirus type III can be detected in a highly targeted mode without detecting C antigen. The method is easy to operate, the detection result can truly reflect the content of effective antigen in poliovirus, a basis can be well provided for follow-up poliomyelitis vaccine immunogenicity testing usage and the final concentration of a vaccine product, and the quality of poliomyelitis vaccine can be improved finally. The method has a high economic value and a high market application value.
Owner:SINOVAC BIOTECH
Heat-resistant freezing and drying protecting agent for contagious ovine ecthyma virus cell attenuated vaccine as well as preparation method and application of heat-resistant freezing and drying protecting agent
ActiveCN103893776AEasy to transportEasy to storePharmaceutical non-active ingredientsAgricultural scienceImpetigo contagiosa
The invention discloses a heat-resistant freezing and drying protecting agent for a contagious ovine ecthyma virus cell attenuated vaccine as well as a preparation method and application of the heat-resistant freezing and drying protecting agent. According to the characteristics of the contagious ovine ecthyma virus cell attenuated vaccine, the heat-resistant freezing and drying protecting agent for the contagious ovine ecthyma virus cell attenuated vaccine is prepared, wherein the heat-resistant freezing and drying protecting agent contains 30g / L-70g / L of trehalose, 10g / L-30g / L of skim milk powder, 10g / L-30g / L of polyvinylpyrrolidone, 5g / L-15g / L of gelatin, 0.5g / L-4g / L of arginine, 1g / L-5g / L of vitamin C, and the balance being of ultra-pure water. By virtue of the heat-resistant freezing and drying protecting agent disclosed by the invention, the vaccine can be stored for 24 months at 2 DEG C-8 DEG C while viral titer is not changed greatly; before and after freezing and drying a vaccine product, virus loss ratio is 0.3 titer, and the virus loss ratio is only 1.0 titer while the vaccine is stored for 30 days at 37 DEG C. According to the heat-resistant freezing and drying protecting agent disclosed by the invention, a bottleneck technology that the contagious ovine ecthyma virus cell attenuated vaccine only can be stored and transported at (-)15 DEG C is solved, so that the vaccine is more convenient to store and transport.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com