Vaccine
A technology of vaccines and vaccine products, applied in the field of vaccines, to achieve the effects of maintaining biological activity, enhancing specific CTL killing effect, and mild preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] (1) For the preparation of splenocytes, see the ELSPOT procedure.
[0071] (2) Preparation of stimulating cells
[0072] ① Preparation of splenocytes from normal syngeneic mice, the method is the same as (1). Adjust the cell concentration to 5×10 with 1640 complete medium 6 cell / ml.
[0073] ② Add mitomycin C (MMC) to a final concentration of 25 μg / ml, at 37°C, 5% CO 2 Incubate for 2 hours.
[0074] ③ Add 30ml of serum-free 1640 medium, centrifuge at 400g for 10 minutes, and wash 3 times to remove residual mitomycin C.
[0075] ④Use 1640 complete medium containing 20U / ml rIL-2 to adjust the cell concentration to 5×10 6 cell / ml, add HBsAg MHC class I peptide S 28-39 , the final concentration was 20 μg / ml.
[0076] ⑤ at 5% CO 2 After incubating at 37°C for 4 hours in the incubator, the cell concentration was adjusted to 1×10 6 cell / ml.
[0077] (3) Preparation of effector cells
[0078] ① The splenocytes and stimulator cells of the immunized mice were mixed at ...
Embodiment 1
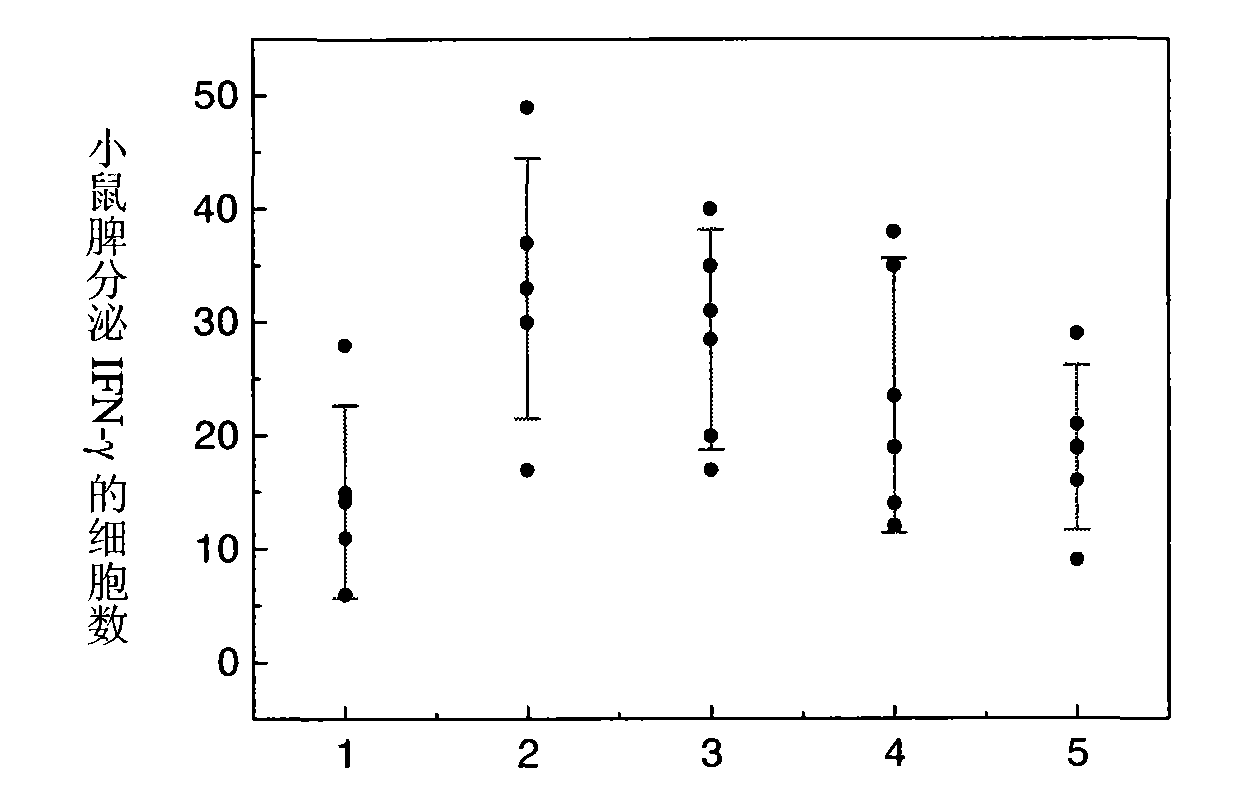
[0095]Add 10 mg of PLGA blank microspheres (average particle size of 1.5 μm, molecular weight of 10 kDa) to 1 ml of Hansen antigens with concentrations of 10 μg / ml, 40 μg / ml, 100 μg / ml, 200 μg / ml, and 400 μg / ml, The pH of the antigen solution is 5.5, and the concentration of NaCl in the solution is adjusted to be 0.9% w / v, and the oscillating adsorption is carried out at room temperature for 12 hours to obtain the vaccine product. ml, 400 μg / ml antigen was diluted to 40 μg / ml, each batch of microsphere antigen compound preparation and simple Hansen antigen were used to immunize mice with 4 μg antigen dosage. After 7 days, use the ELISPOT method to detect the secretion of IFN-γ in mouse spleen MNC, see figure 1 , the ordinate represents the number of cells secreting IFN-γ per 4×105 splenic MNCs stimulated by HBsAg MHC class I polypeptide S28-39, that is, the number of spot-forming cells (SFC); Effects on IFN-γ secretion of mice after 7 days, 1-10 μg / ml; 2-40 μg / ml; 3-100 μg / ml...
Embodiment 2
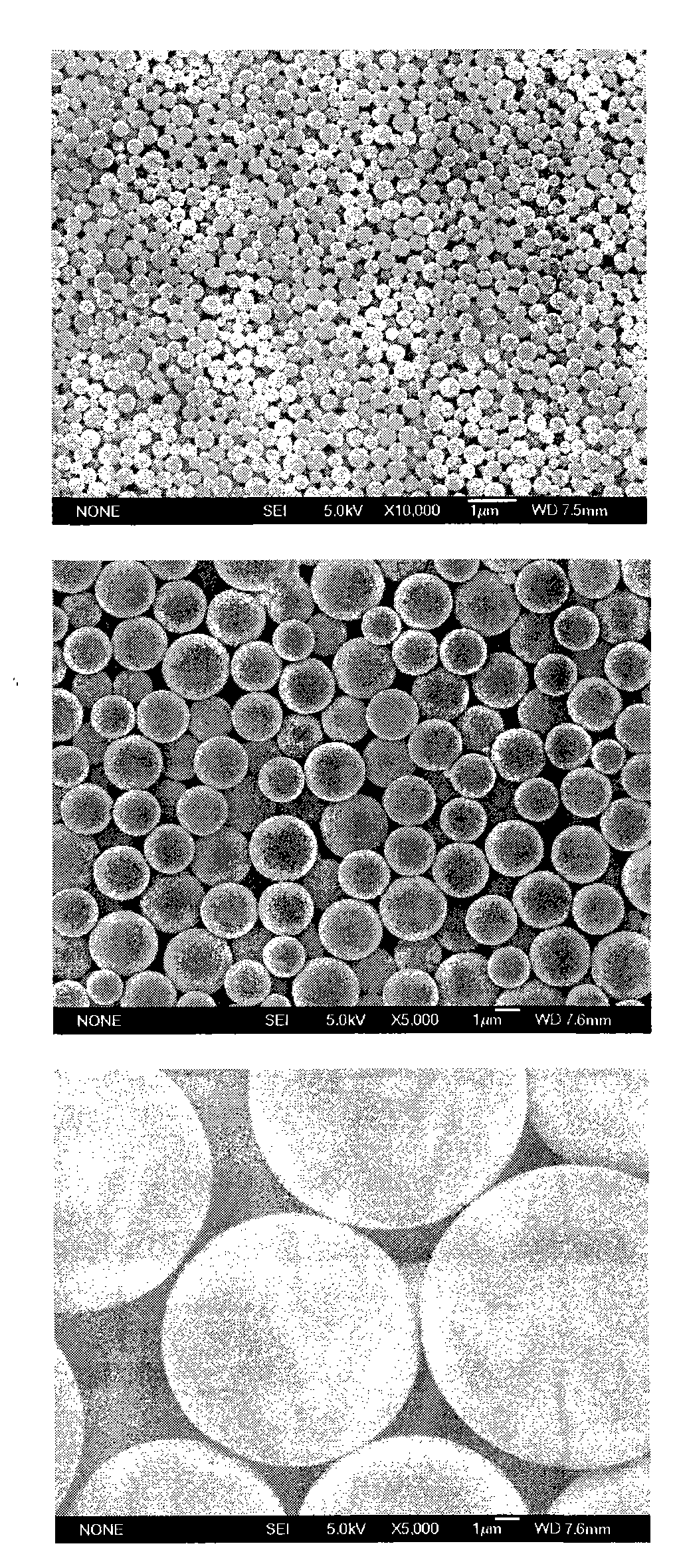
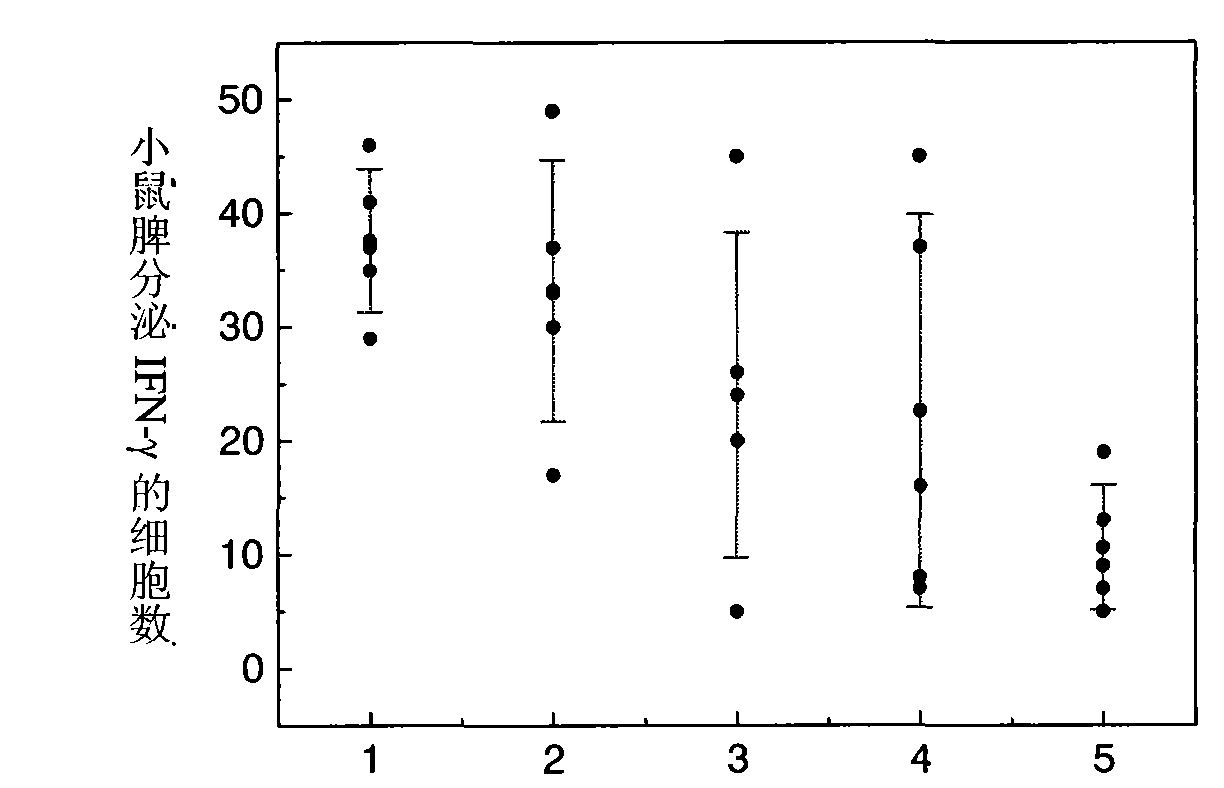
[0097] Three different particle sizes of PLA (molecular weight 15kDa) blank microspheres were used as vaccine adjuvants, with average particle sizes of 0.35 μm, 1.9 μm and 12.1 μm, respectively. figure 2 . Add 10 mg of the above three microspheres to 1 ml of Hansen antigen with a concentration of 45 μg / ml, adjust the pH of the antigen solution to 5.9, adjust the concentration of NaCl in the solution to 1.5% w / v, and oscillate for adsorption at 25°C 5h, each batch of microsphere antigen compound preparations were used to immunize mice with 4 μg of antigen, and 7 days later, the ELISPOT method was used to detect the secretion of IFN-γ in mouse spleen MNC, see image 3 . The ordinate represents the number of cells secreting IFN-γ per 4×105 splenic MNCs stimulated by HBsAg MHC class I polypeptide S28-39, that is, the number of spot-forming cells (SFC); The effect of IFN-γ secretion after 7 days, among which 1-0.35 μm microspheres; 2-1.9 μm microspheres; 3-12.1 μm microspheres; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com