Method for producing swine pseudorabies live vaccine by using passage cell source, and product thereof
A technology for subculture cells and pseudorabies, applied in biochemical equipment and methods, medical preparations containing active ingredients, microorganisms, etc., can solve the lack of freezing and cold storage facilities in grassroots epidemic prevention departments, long-term vaccine storage and long-distance transportation restrictions, epidemic diseases epidemic and other problems, to achieve good immune protection, improve vaccine production and quality, and high immune efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Preparation of heat-resistant protective agent
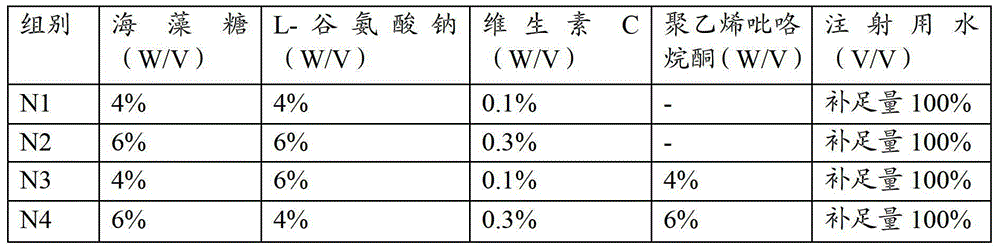
[0048] The composition and component content of the heat-resistant protective agent are shown in Table 1.
[0049] Table 1 Composition and content of heat-resistant protective agent
[0050]
[0051] For each heat-resistant protectant, 1 L of water for injection was heated to a temperature of 60° C. under agitation, and then the compound was slowly added to hot water while maintaining agitation via a magnetic bar to facilitate its dissolution. Agitation was maintained for about 20 minutes after the last addition, resulting in a homogeneous solution.
[0052] Then the solution was cooled to room temperature, and after cooling, the heat-resistant protective agent was sterilized by filtration through a filter membrane with a pore size of 0.22 μm, and stored at room temperature.
Embodiment 2
[0053] Example 2 Production process of porcine pseudorabies live vaccine produced by passage cells
[0054] The production process of producing porcine pseudorabies live vaccine with passaged cells, the concrete steps are as follows:
[0055] (1) Select passage cells as cells for seedling production.
[0056] (2) Subculture and culture of seedling cells: the above-mentioned subcultured cells were digested and subcultured with trypsin cell dispersion, and the cells containing 90% to 97% by volume of MEM culture medium and 3% to 10% by volume of fetal bovine serum were used for passage. The growth medium (pH adjusted to 7.0-8.0) was further cultured at 36°C-38°C to form a good monolayer, which was used for continued passage or virus inoculation.
[0057] (3) Propagation of cytotoxic species: Inoculate the well-grown monolayer of the above passaged cells with the attenuated strain of porcine pseudorabies virus, and use MEM culture medium containing 95% to 99% by volume and fetal...
Embodiment 3
[0060] Example 3 Preparation of virus liquid
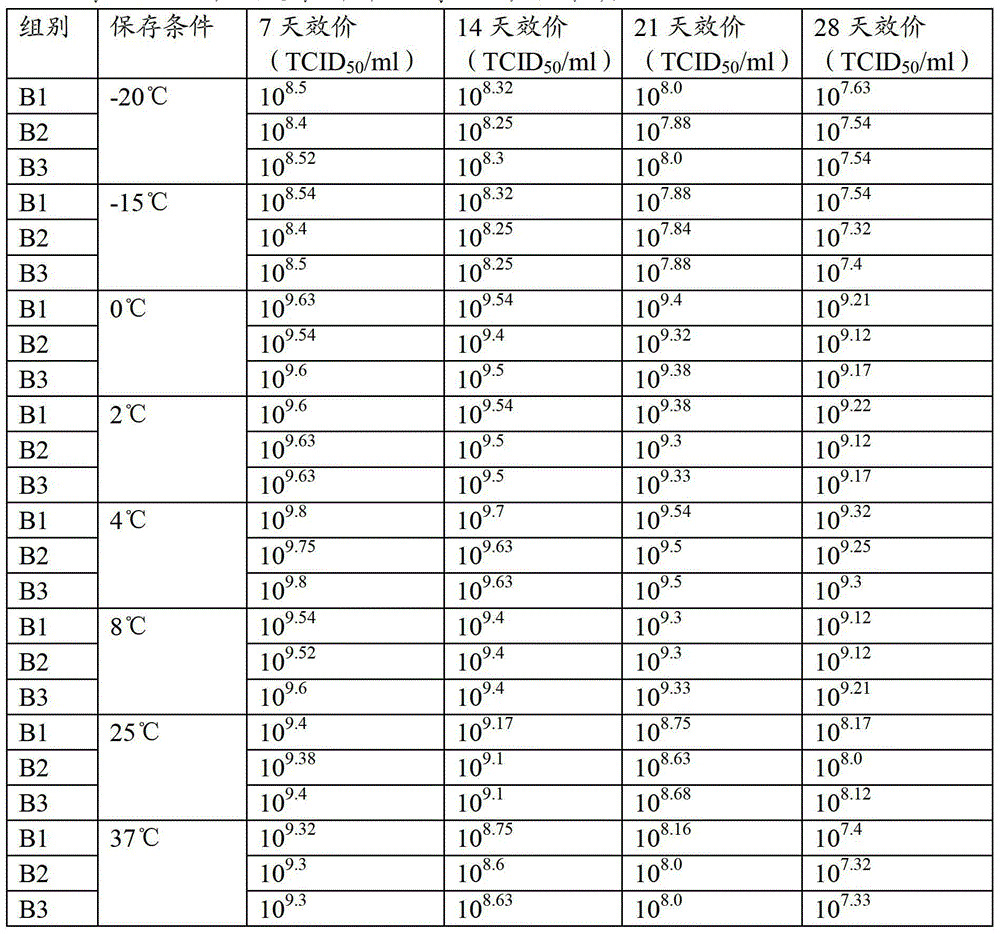
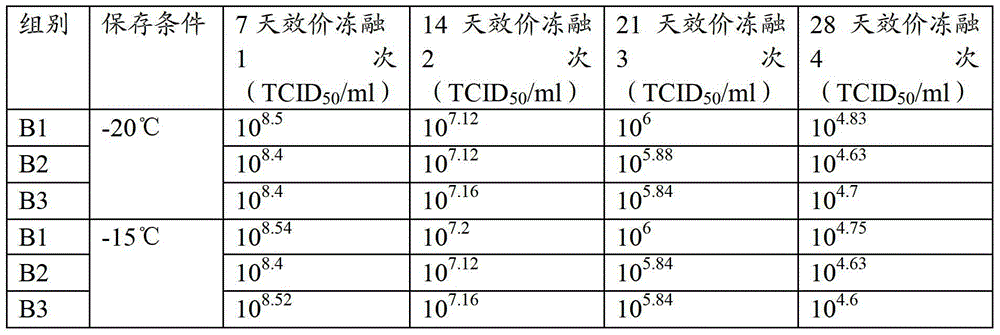
[0061] Select porcine testis (ST) passage cells, pig kidney (PK15) passage cells and pig kidney (IBRS-2) passage cells, the attenuated strain of porcine pseudorabies virus is selected from porcine pseudorabies virus strain SA215, and the production process according to Example 2 is respectively Porcine pseudorabies virus liquid B1, B2 and B3 were prepared, and the titer was measured, and the virus content per 1ml was respectively 10 9.8 TCID 50 、10 9.75 TCID 50 、10 9.8 TCID 50 , stored above 0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com