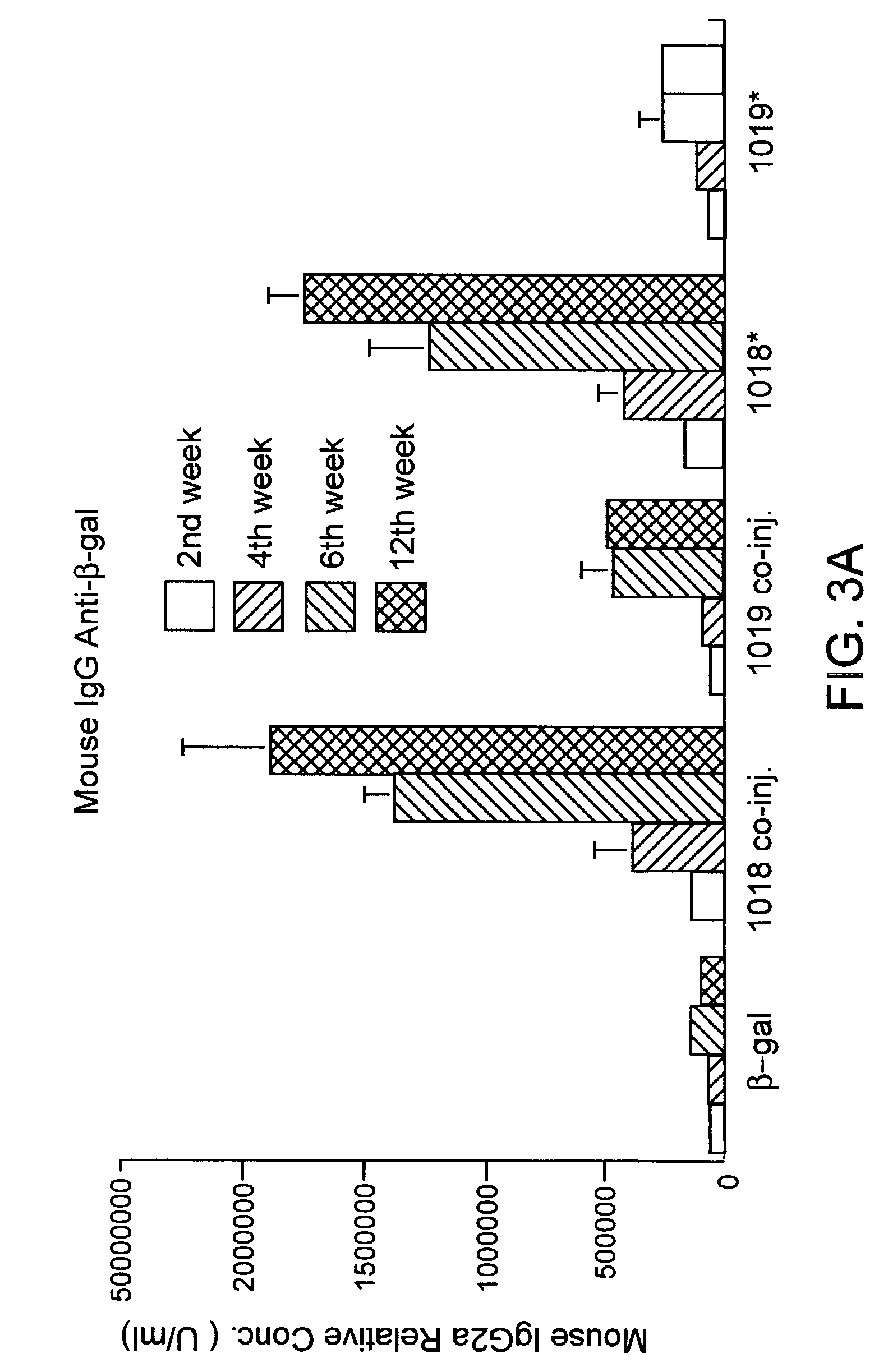
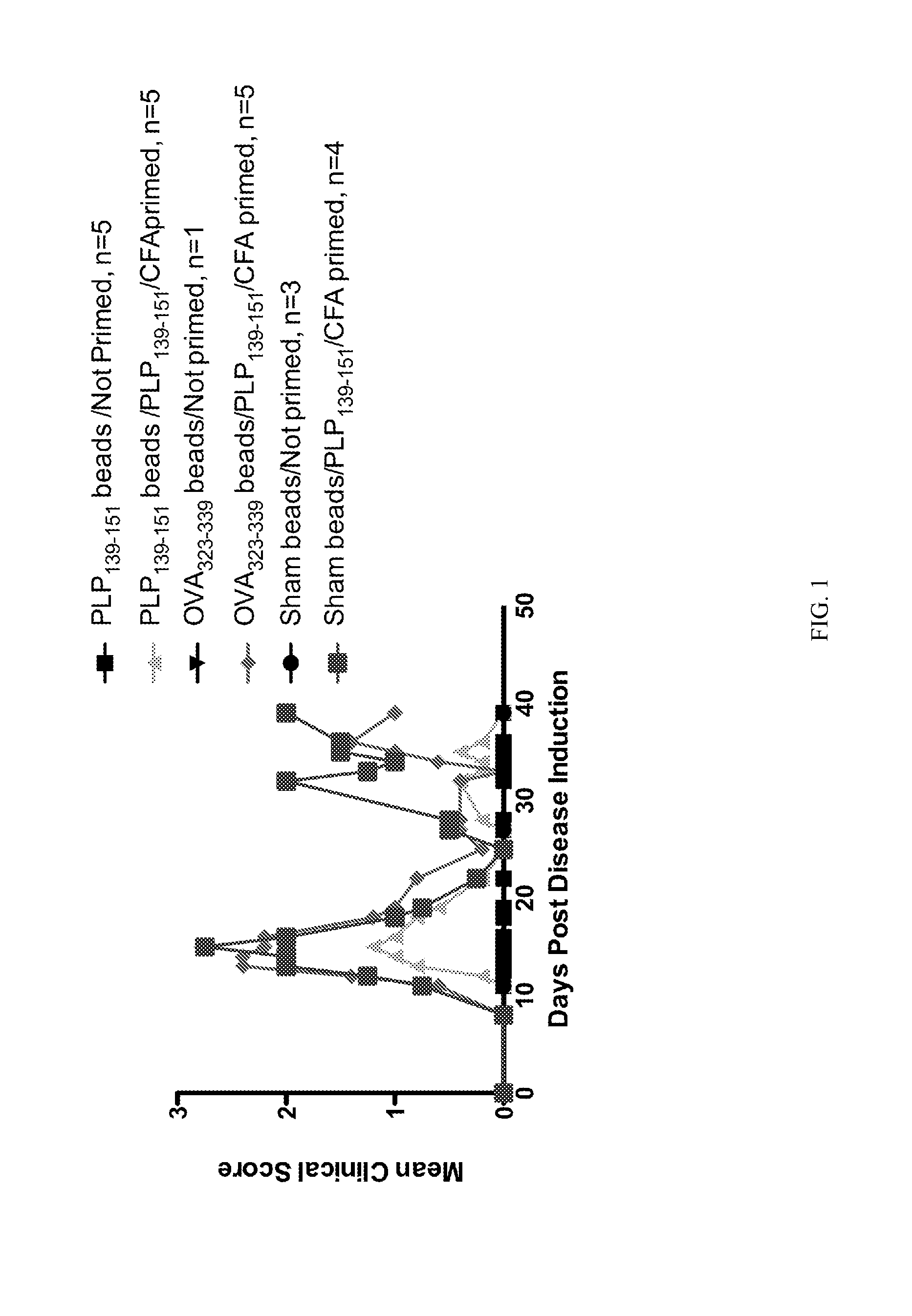
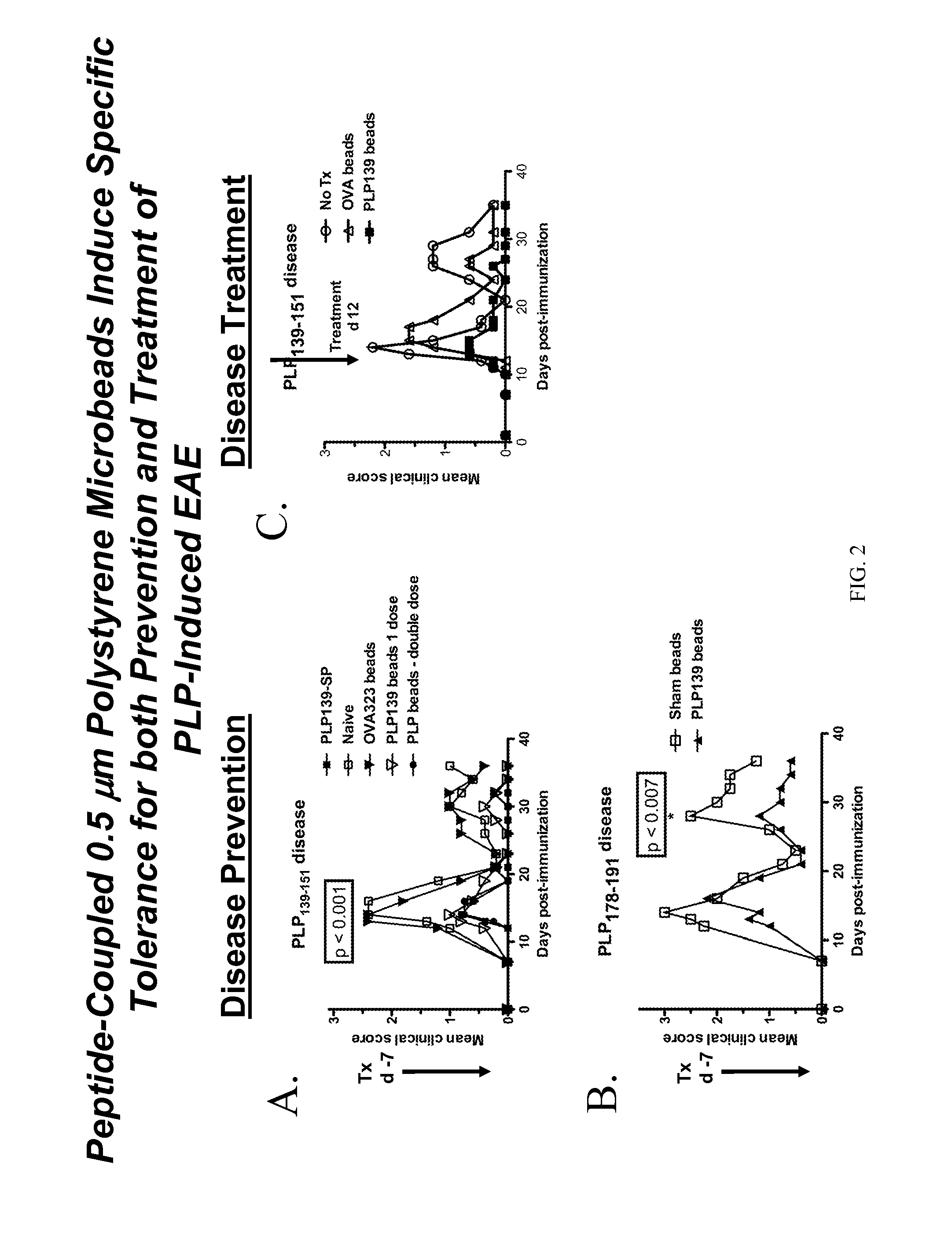
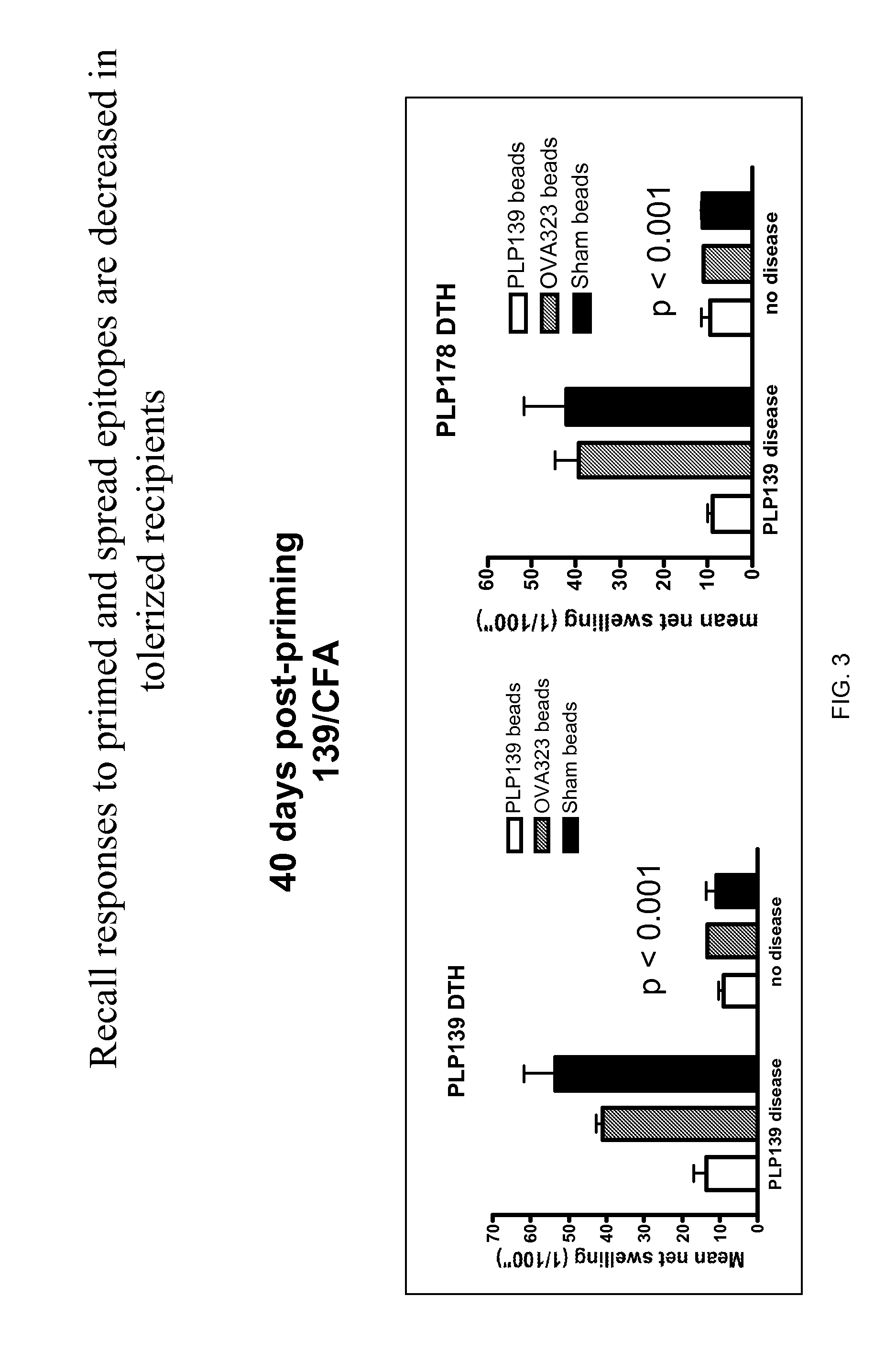
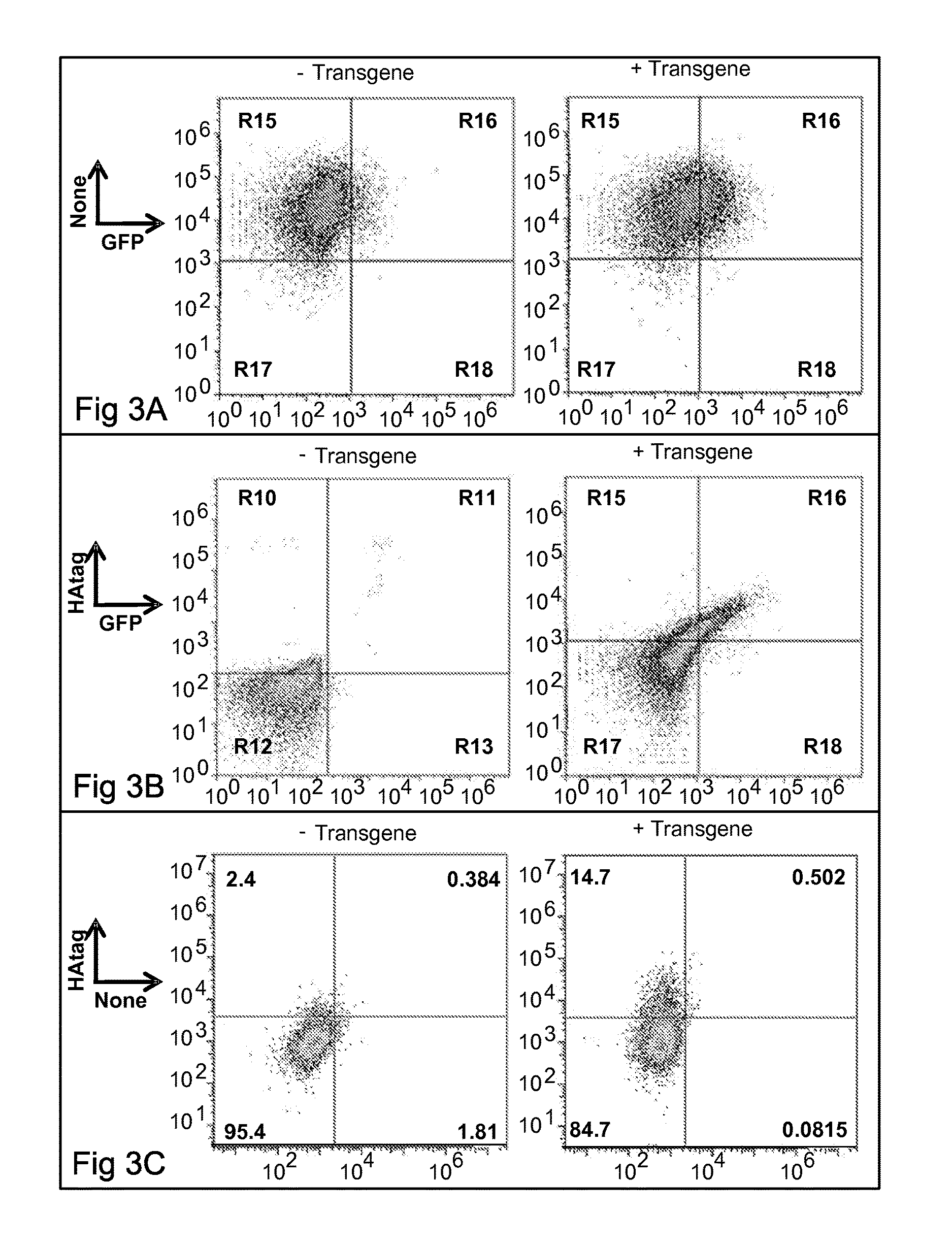
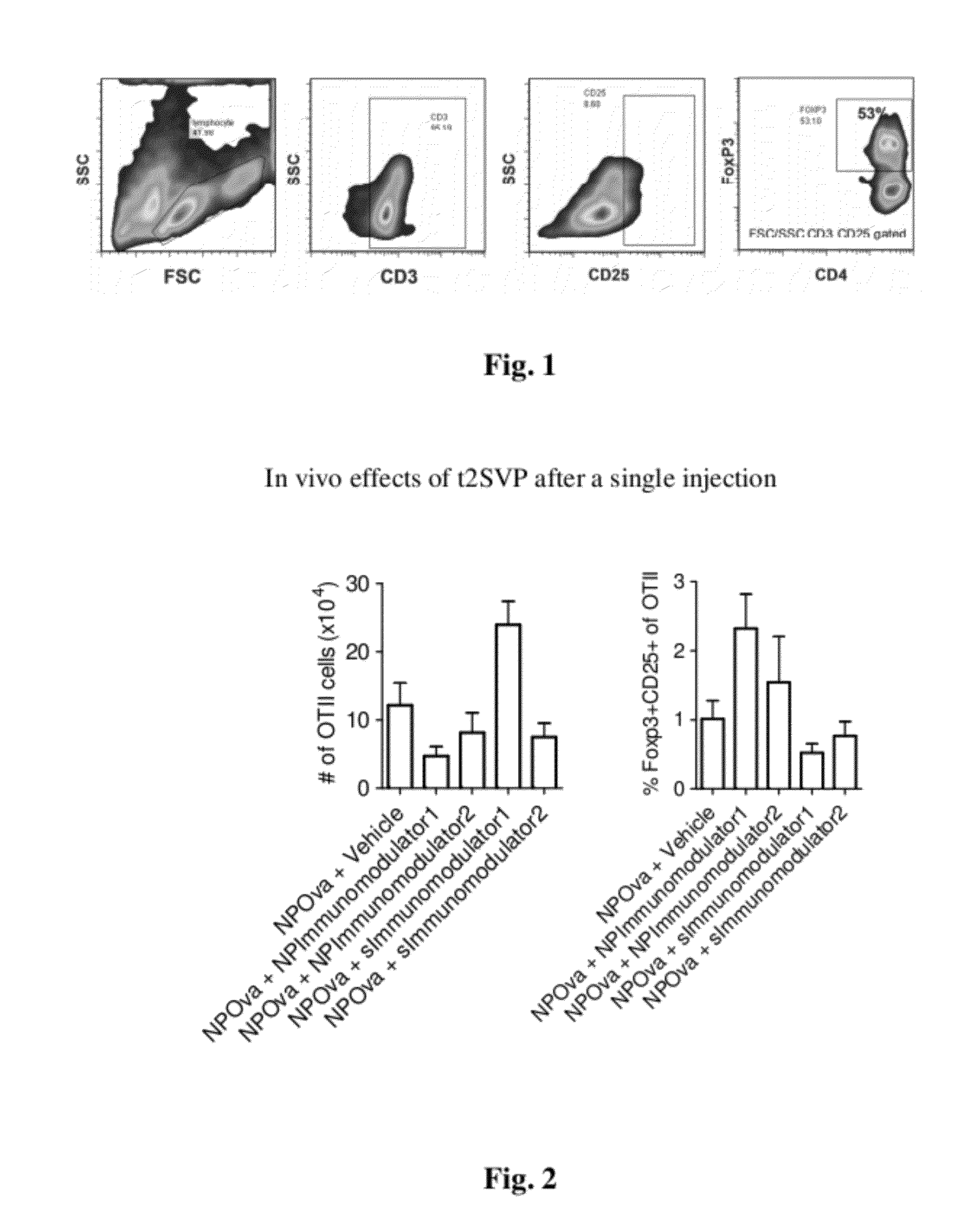
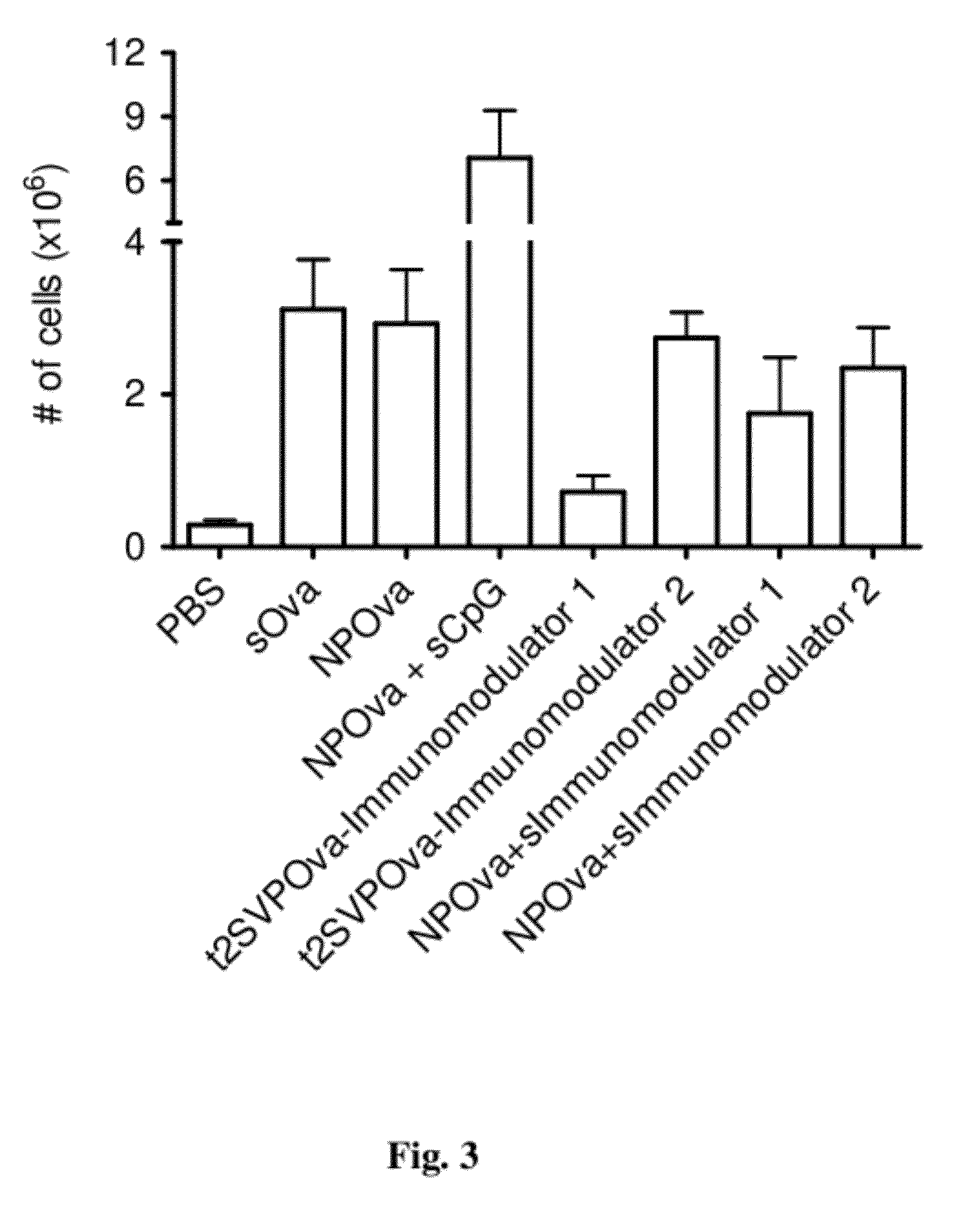
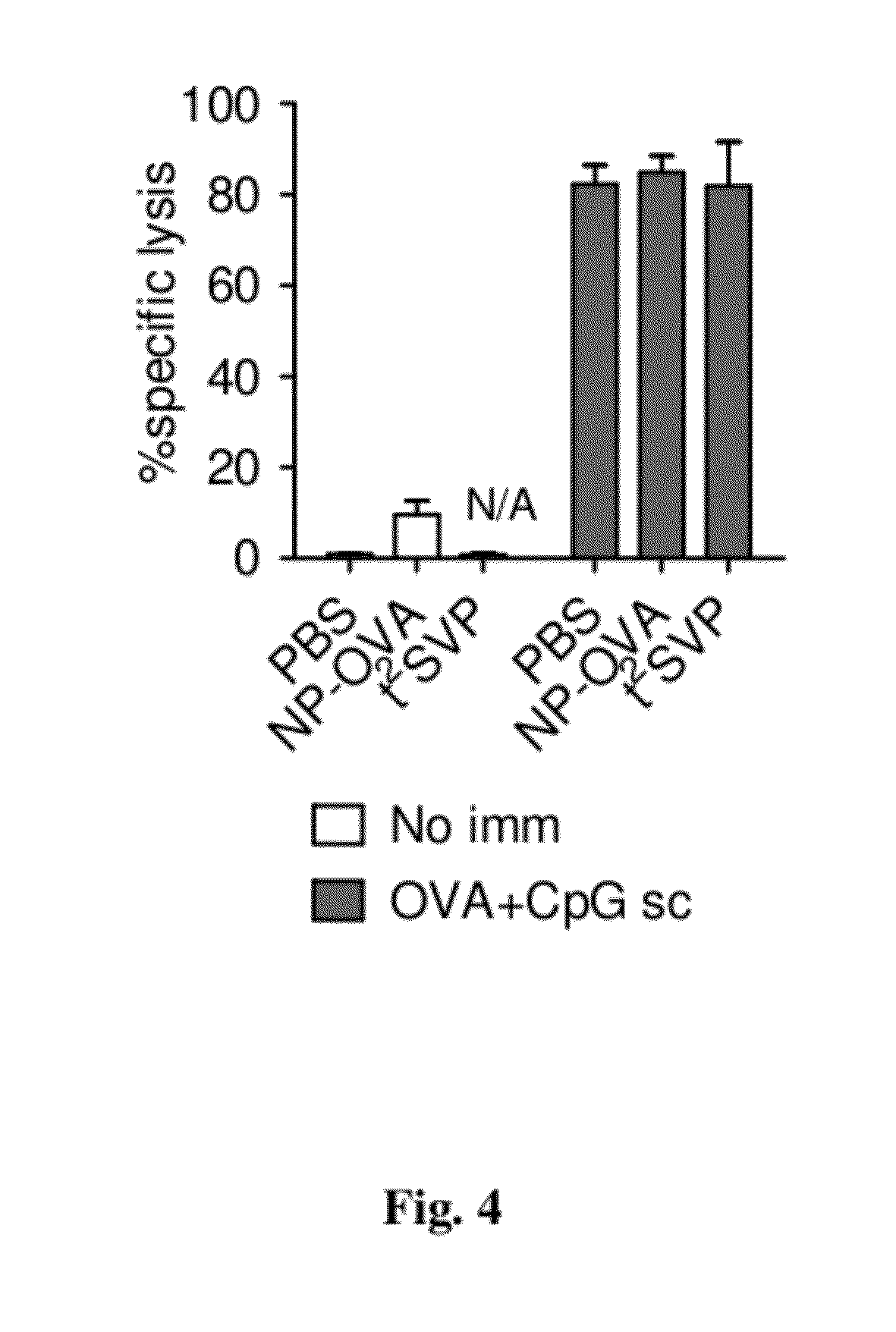
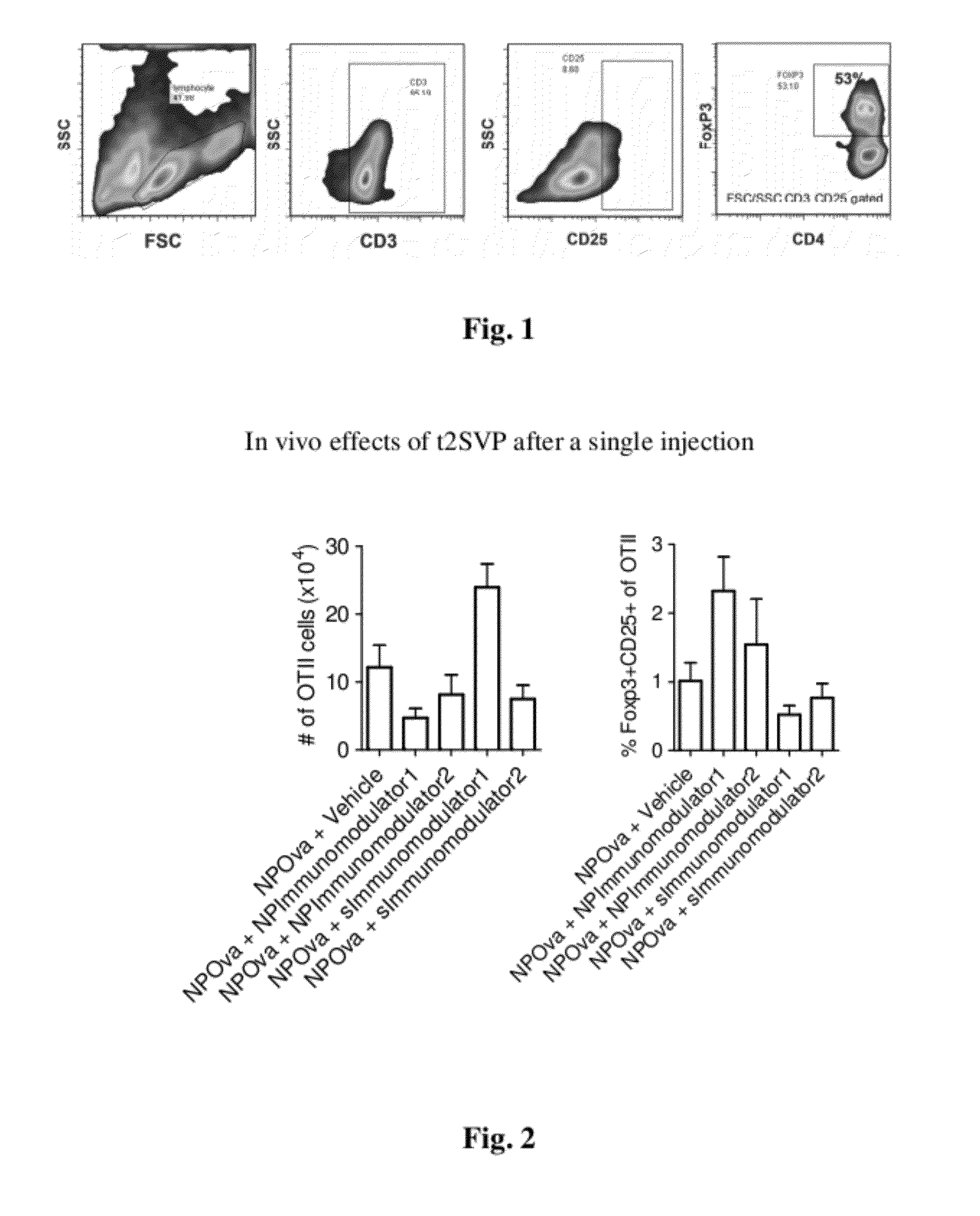
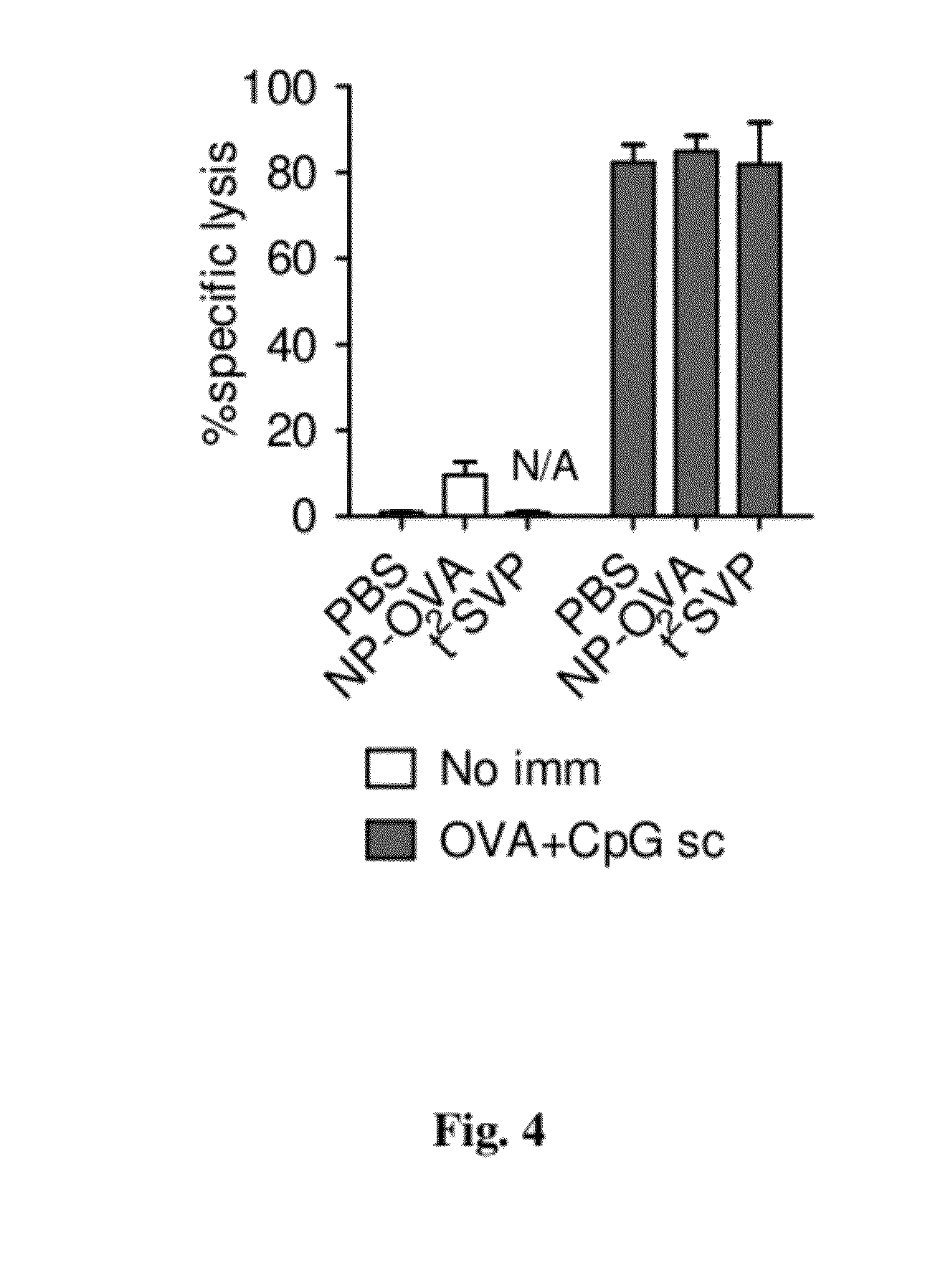
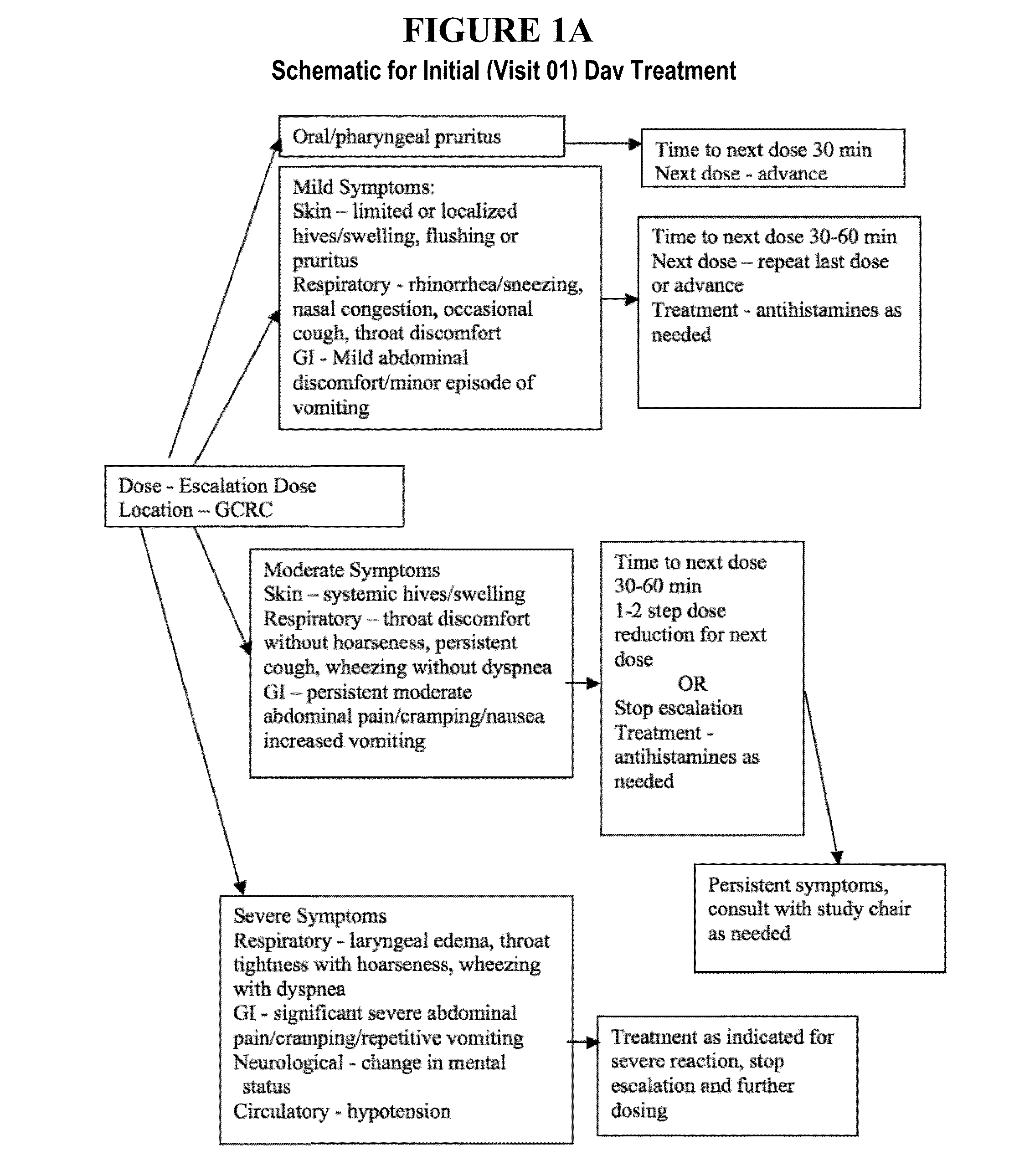
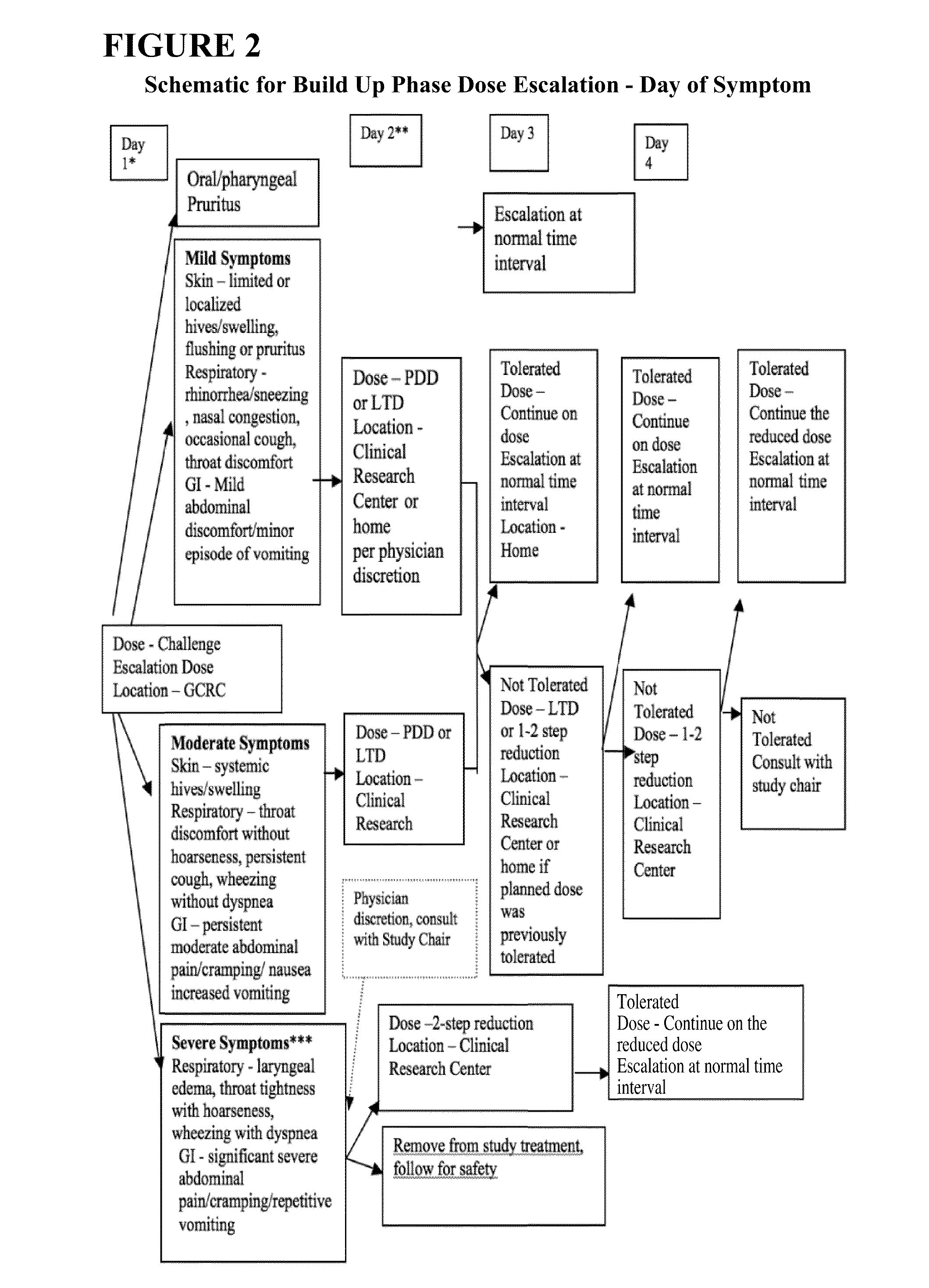
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
903results about "Allergen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
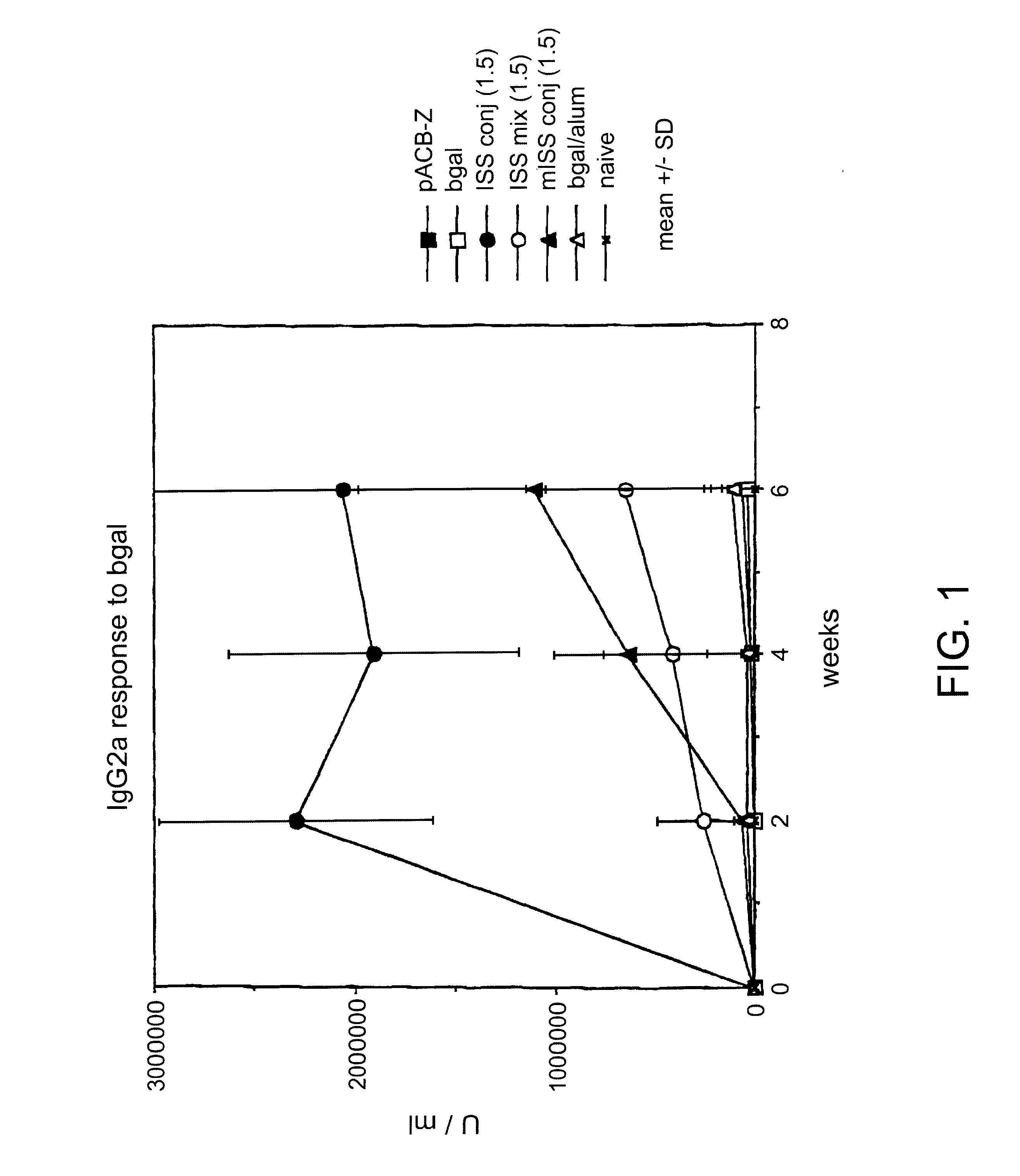
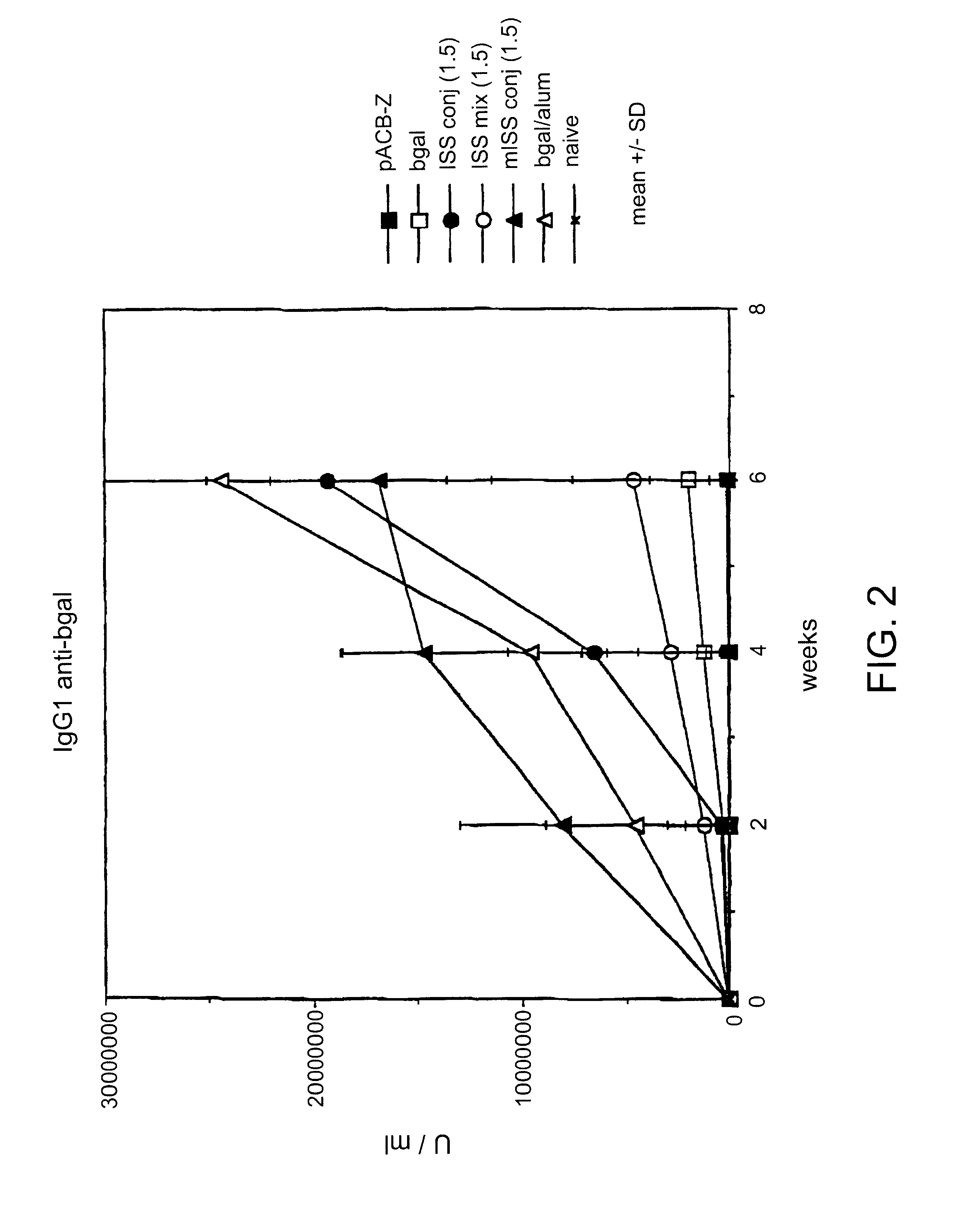
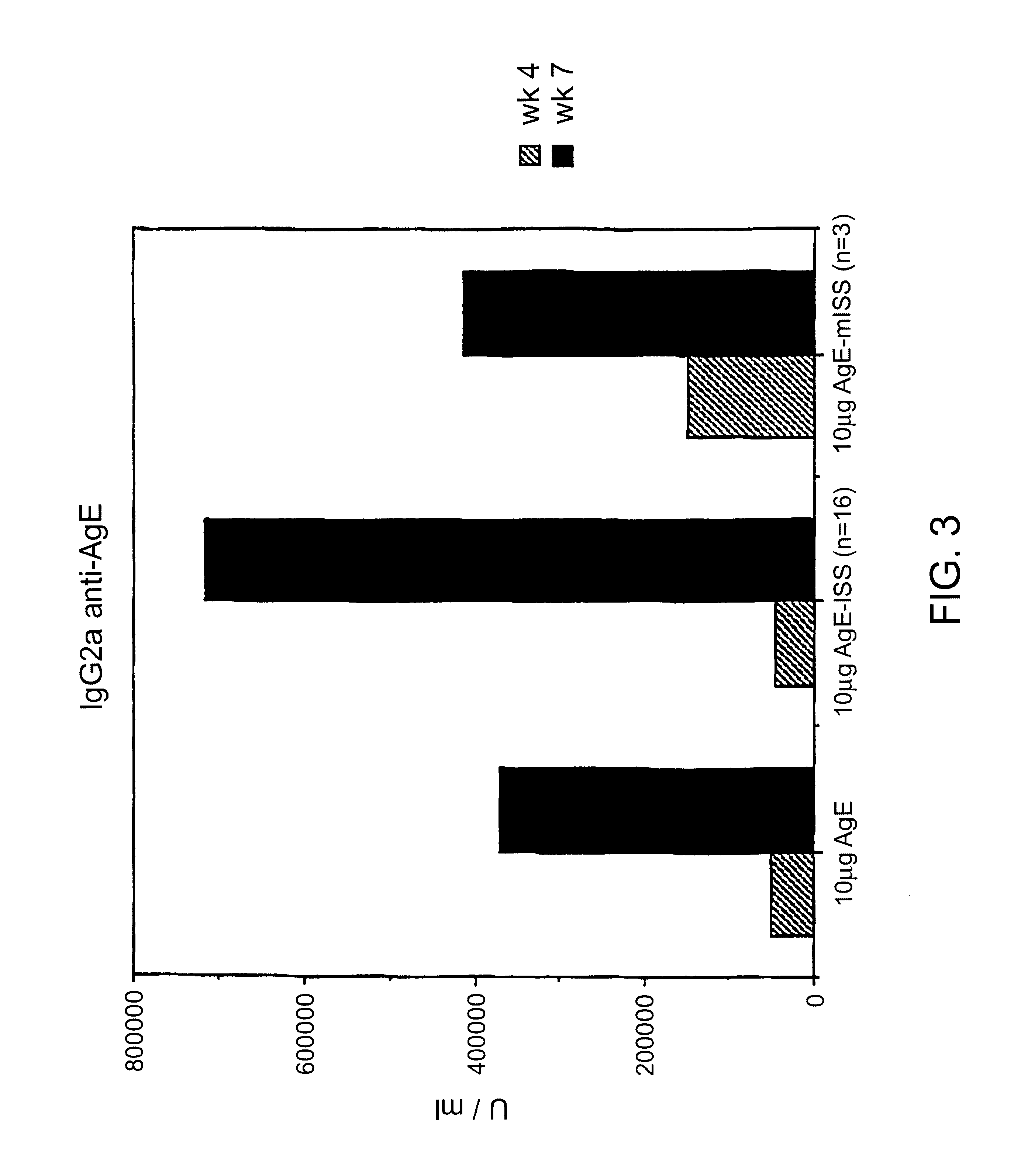
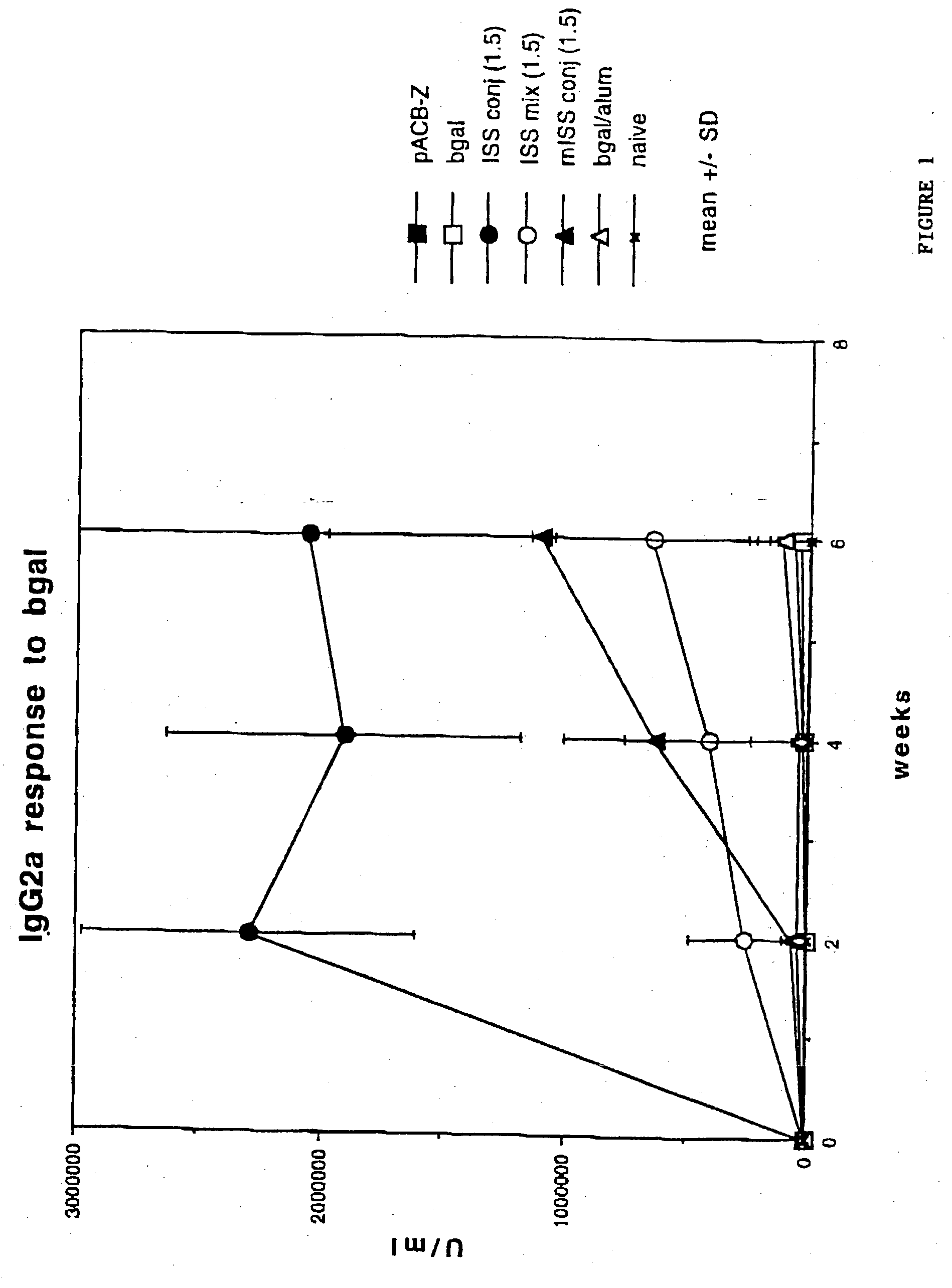
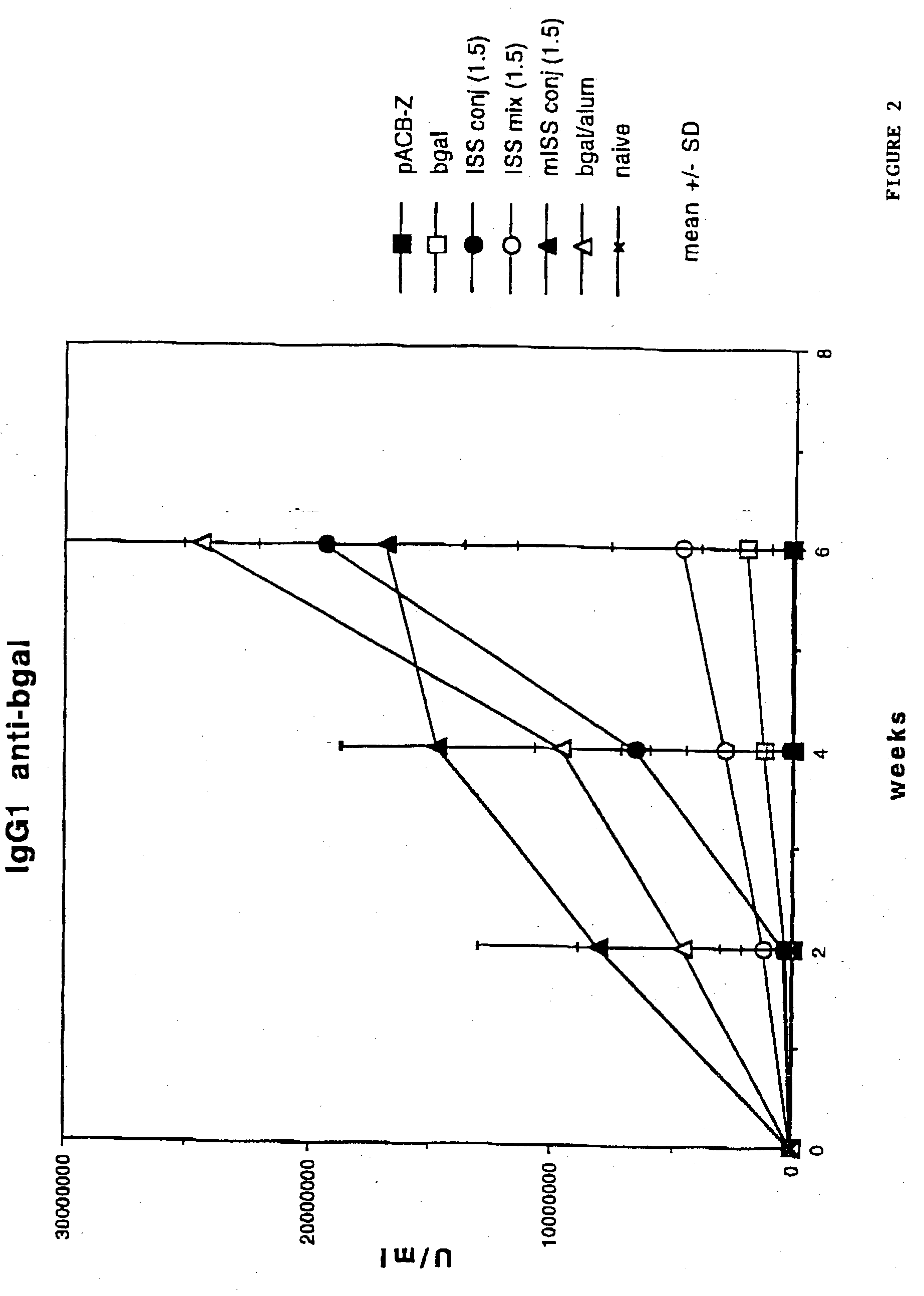
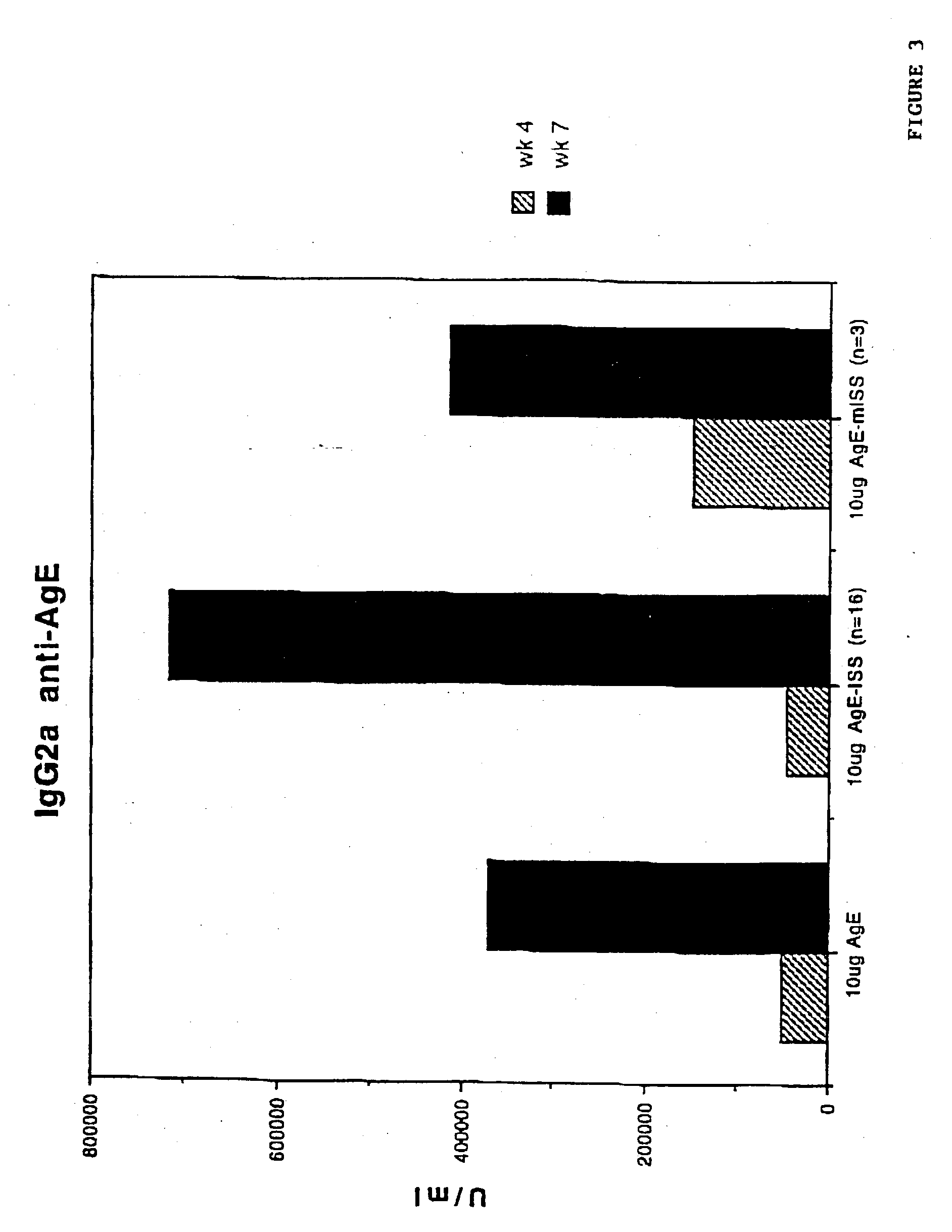
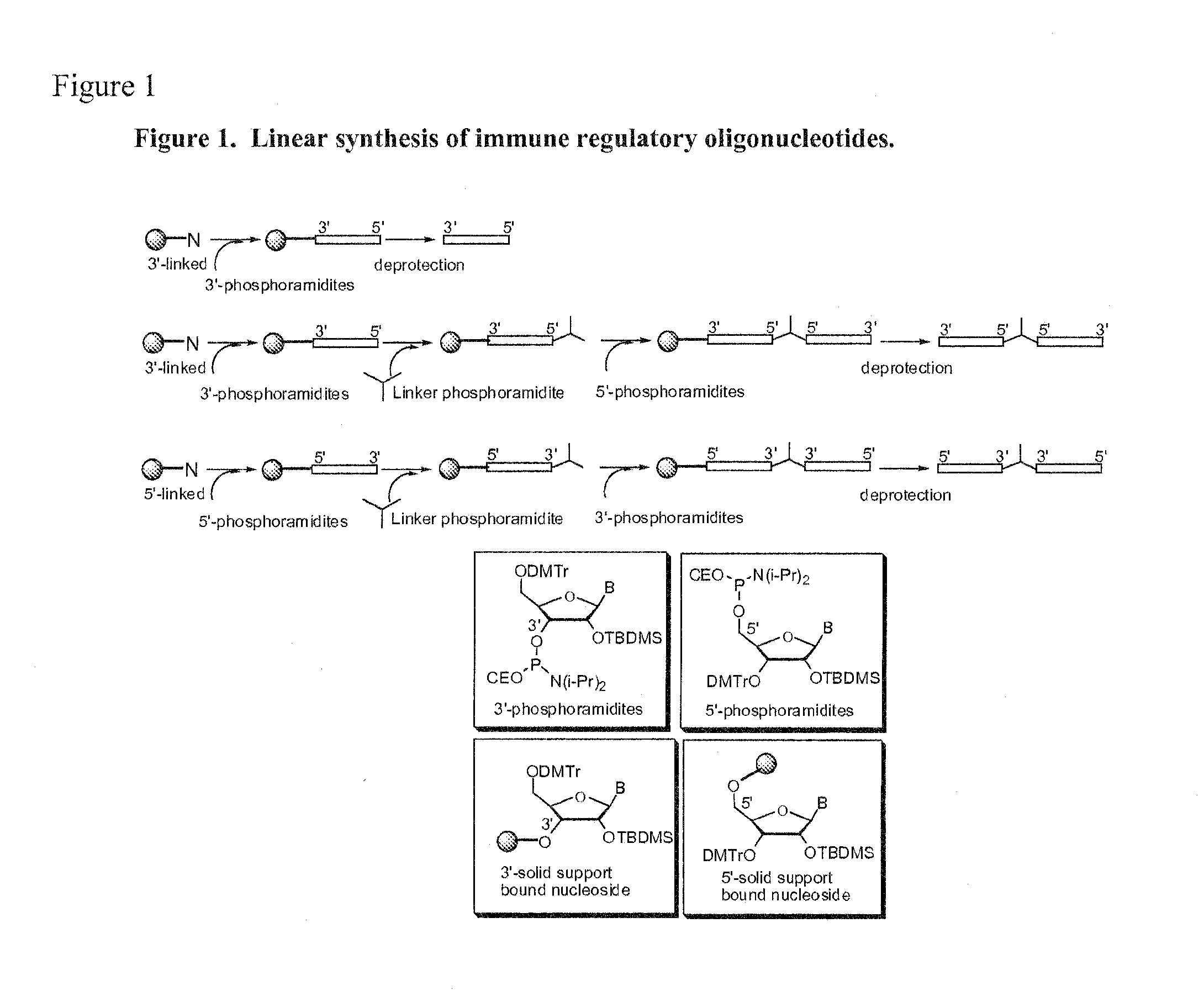
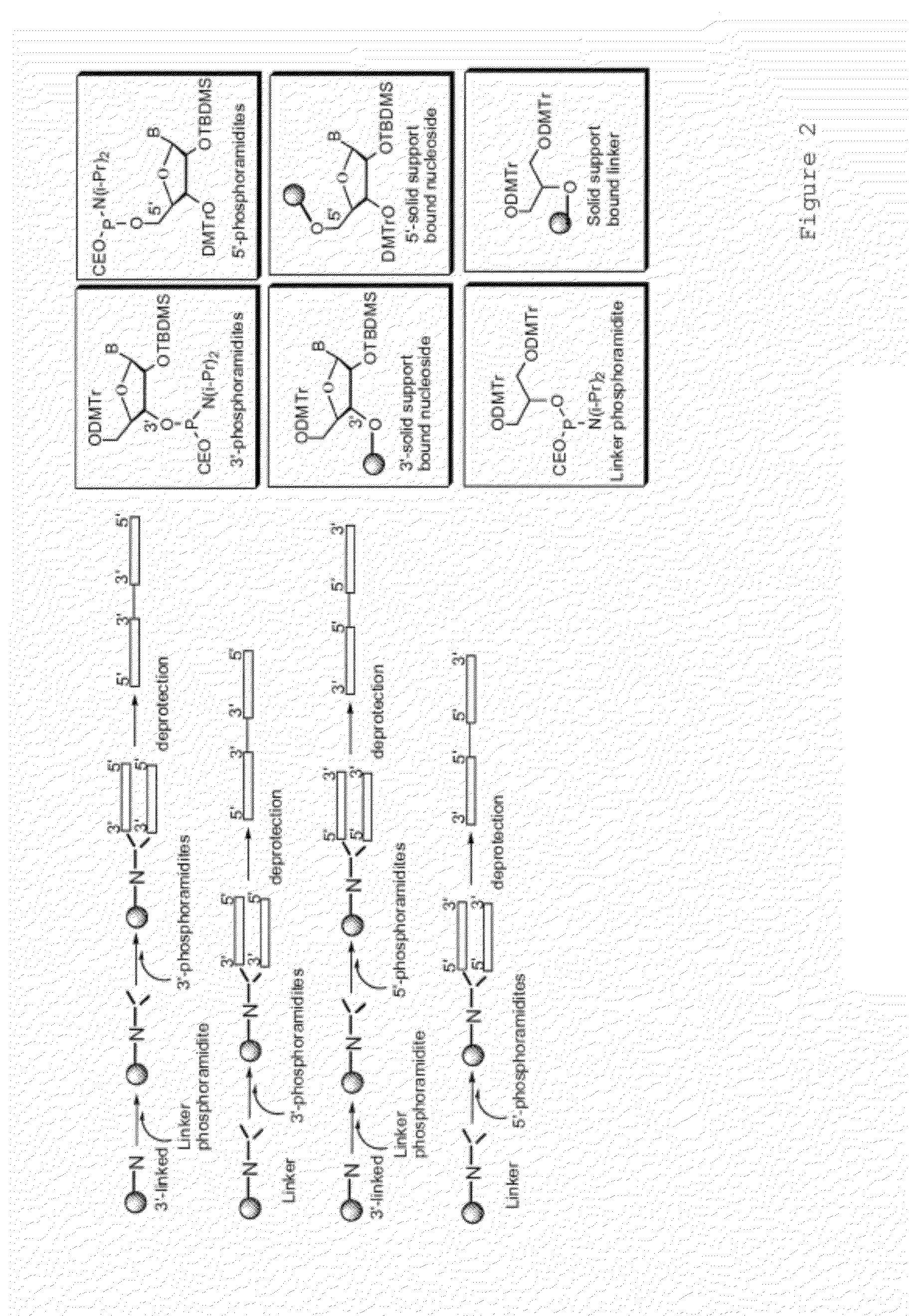
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
InactiveUS6610661B1Boost magnitudeBoost both humoral (antibody)Peptide/protein ingredientsGenetic material ingredientsAntigenAdjuvant
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
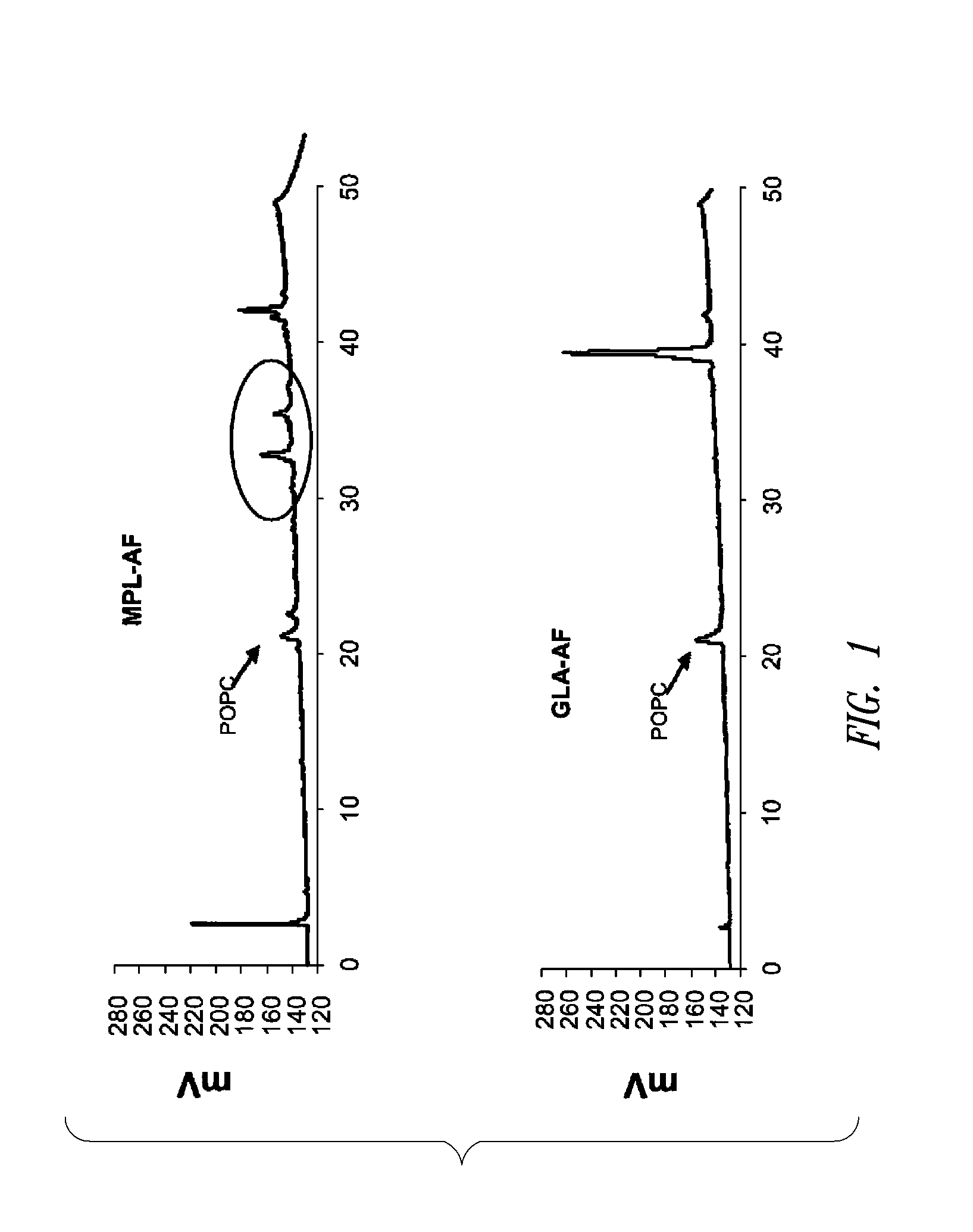
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
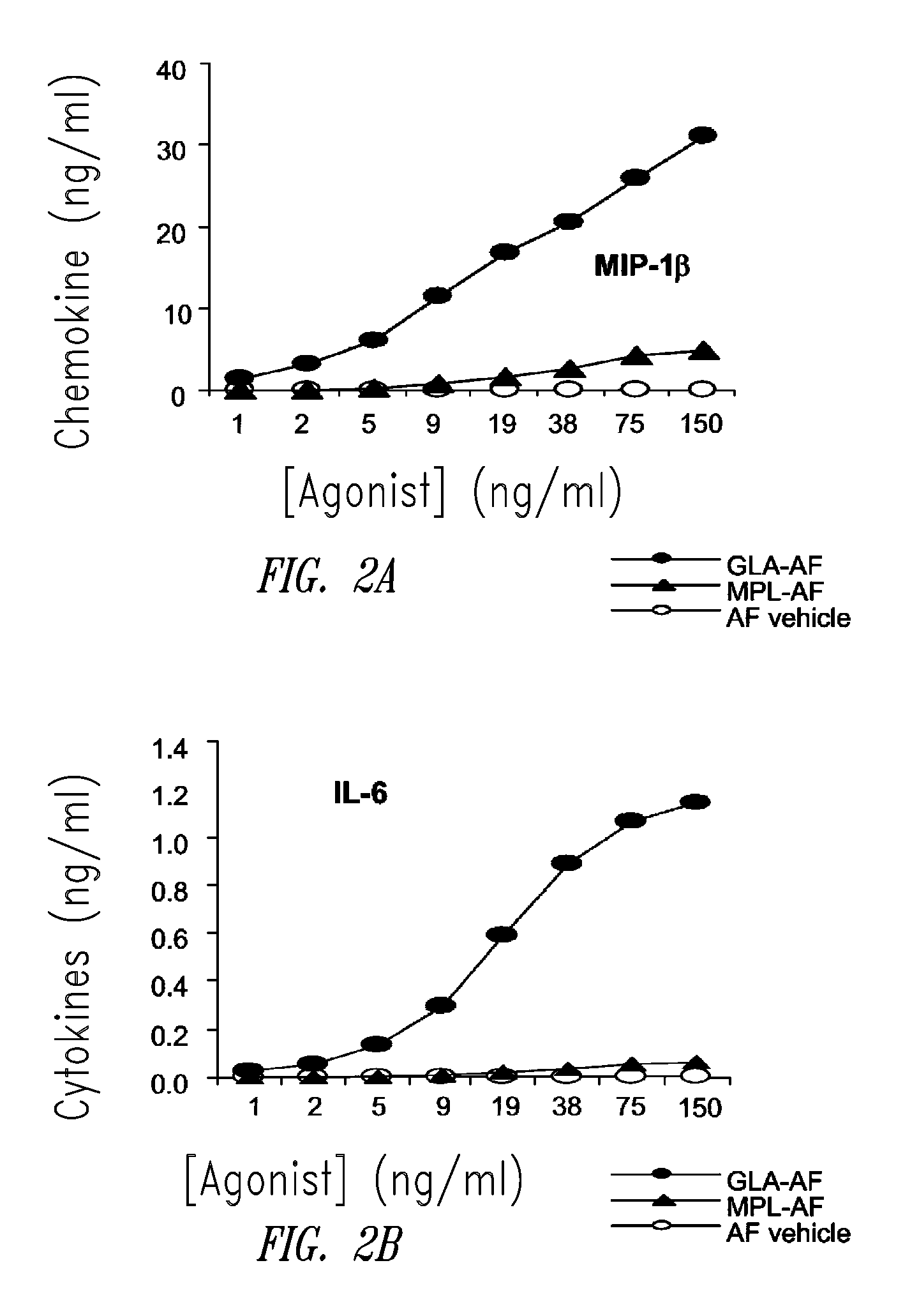
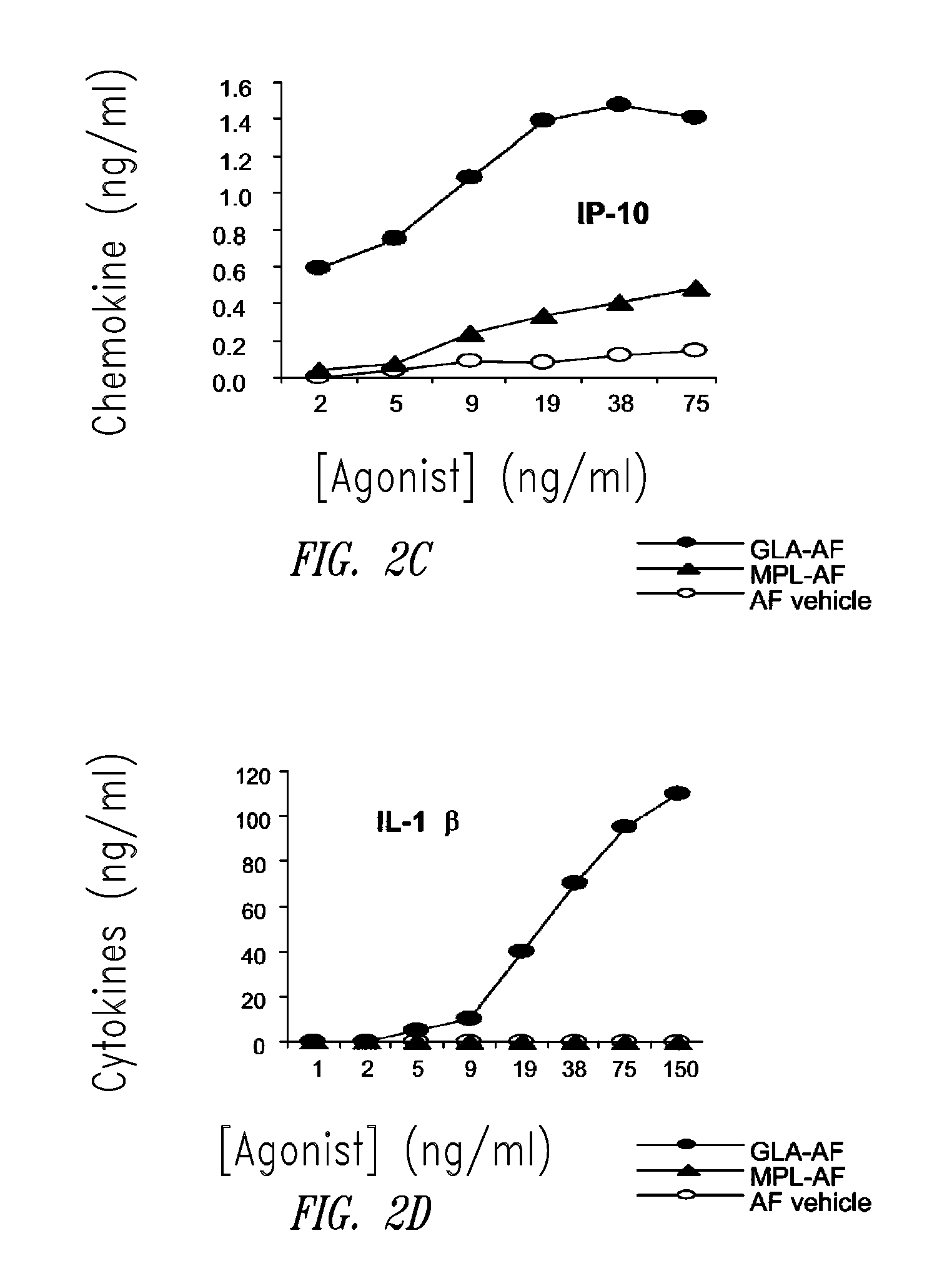
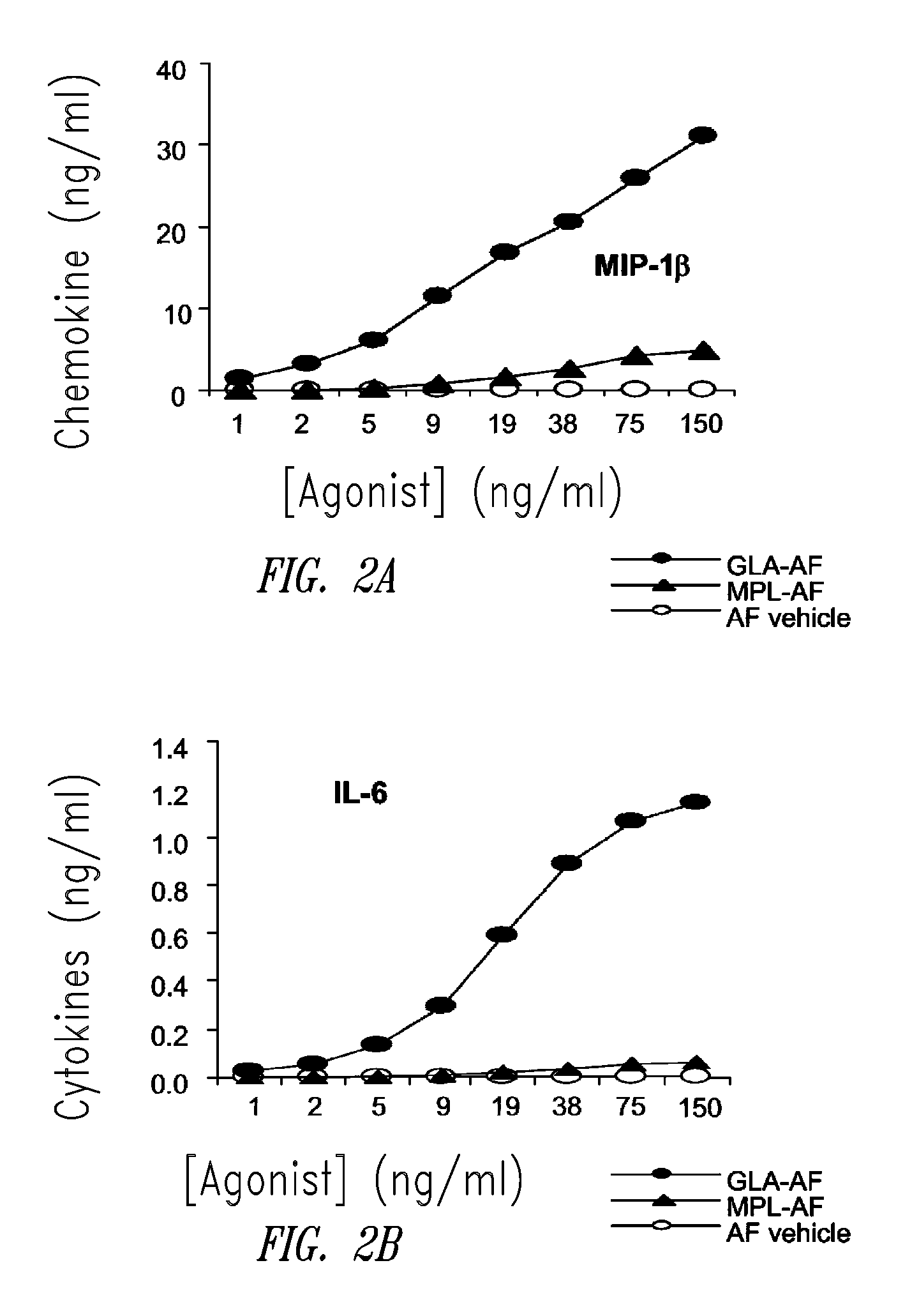
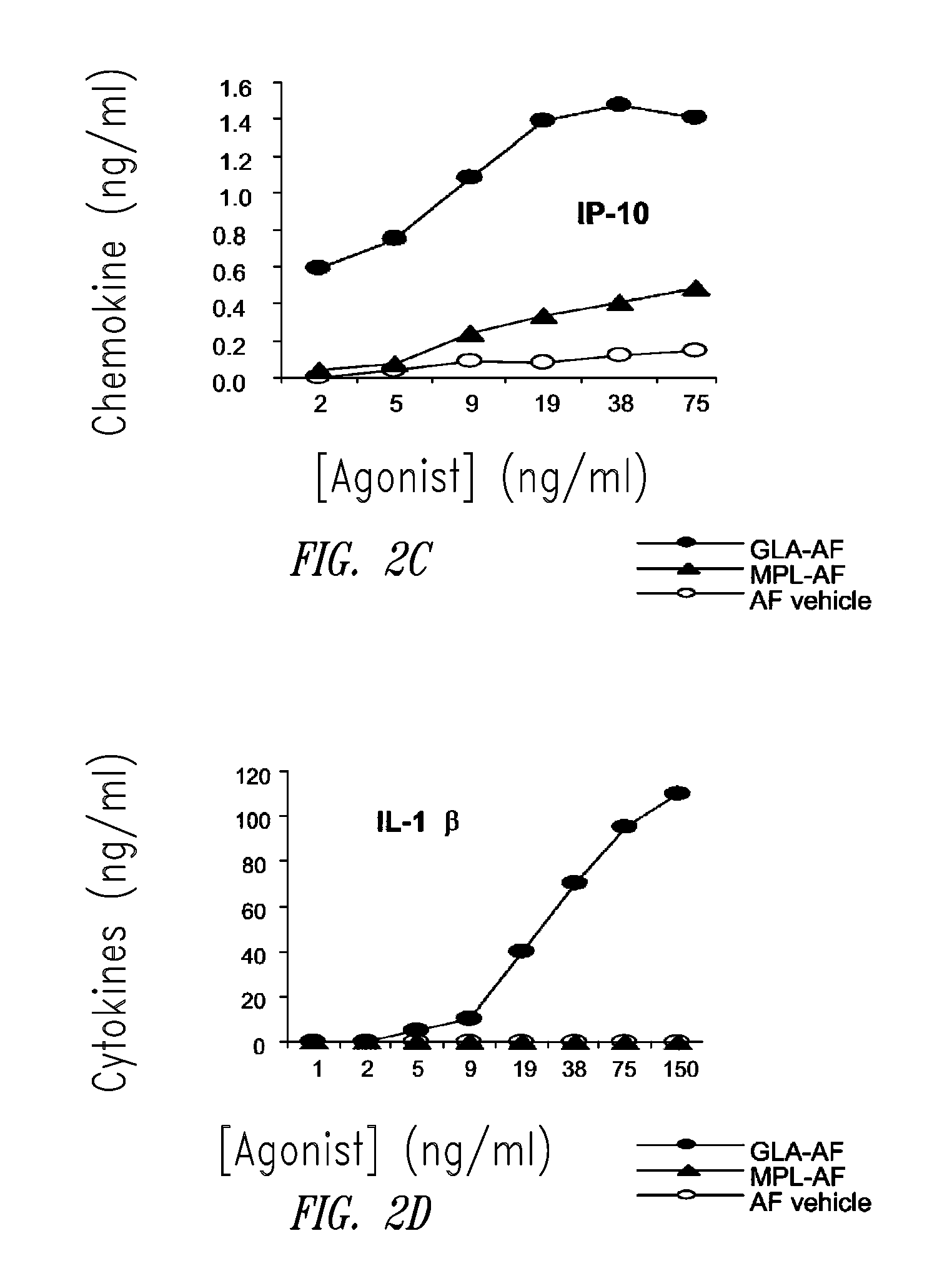
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
Compositions and methods for modulating immune responses
ActiveUS20100151000A1Lower Level RequirementsDecreases functional activityPowder deliveryAntipyreticDiseaseAutoimmune responses
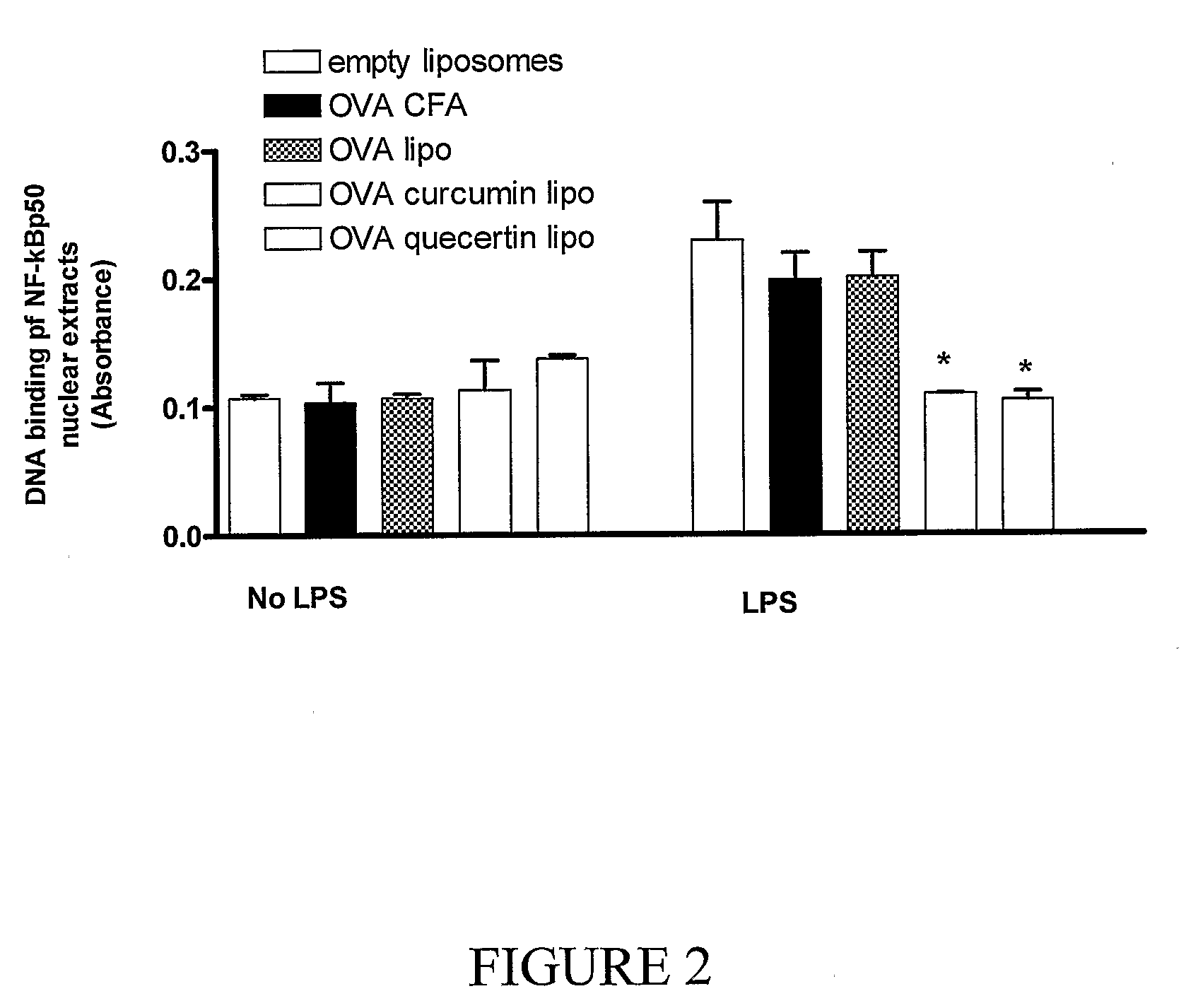
This invention discloses methods and compositions for modulating immune responses, which involve particulate delivery of agents to immune cells, wherein the agents comprise an inhibitor of the NF-κB signaling pathway and an antigen that corresponds to a target antigen. The methods and compositions of the present invention are particularly useful in the treatment or prophylaxis of an undesirable immune response associated with the target antigen, including autoimmune diseases, allergies and transplantation associated diseases.
Owner:THE UNIV OF QUEENSLAND
Immunostimulatory nucleic acid molecules for activating dendritic cells
InactiveUS20070065467A1Th responseEffective adjuvantOrganic active ingredientsSugar derivativesDendritic cellPyrimidine Nucleotides
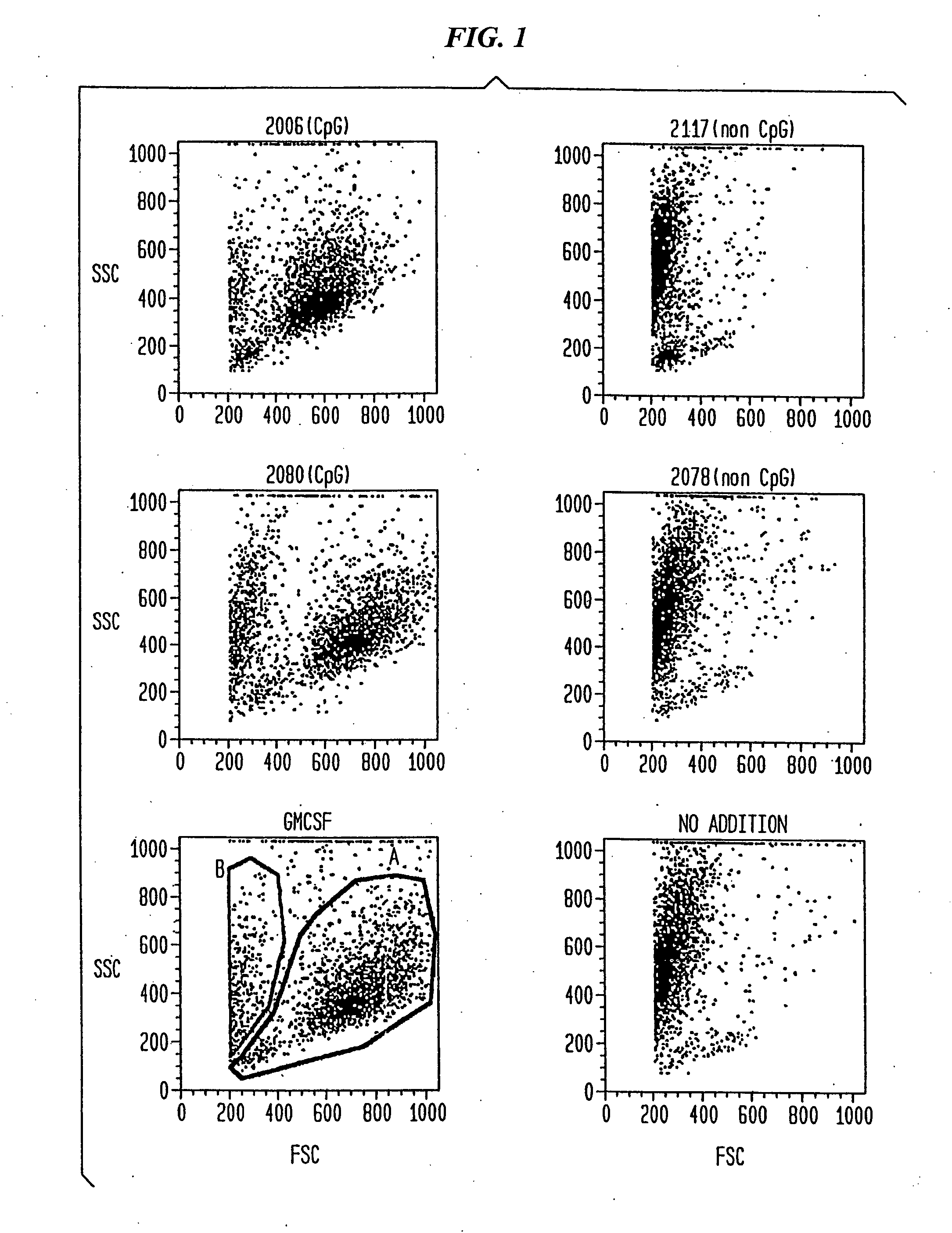
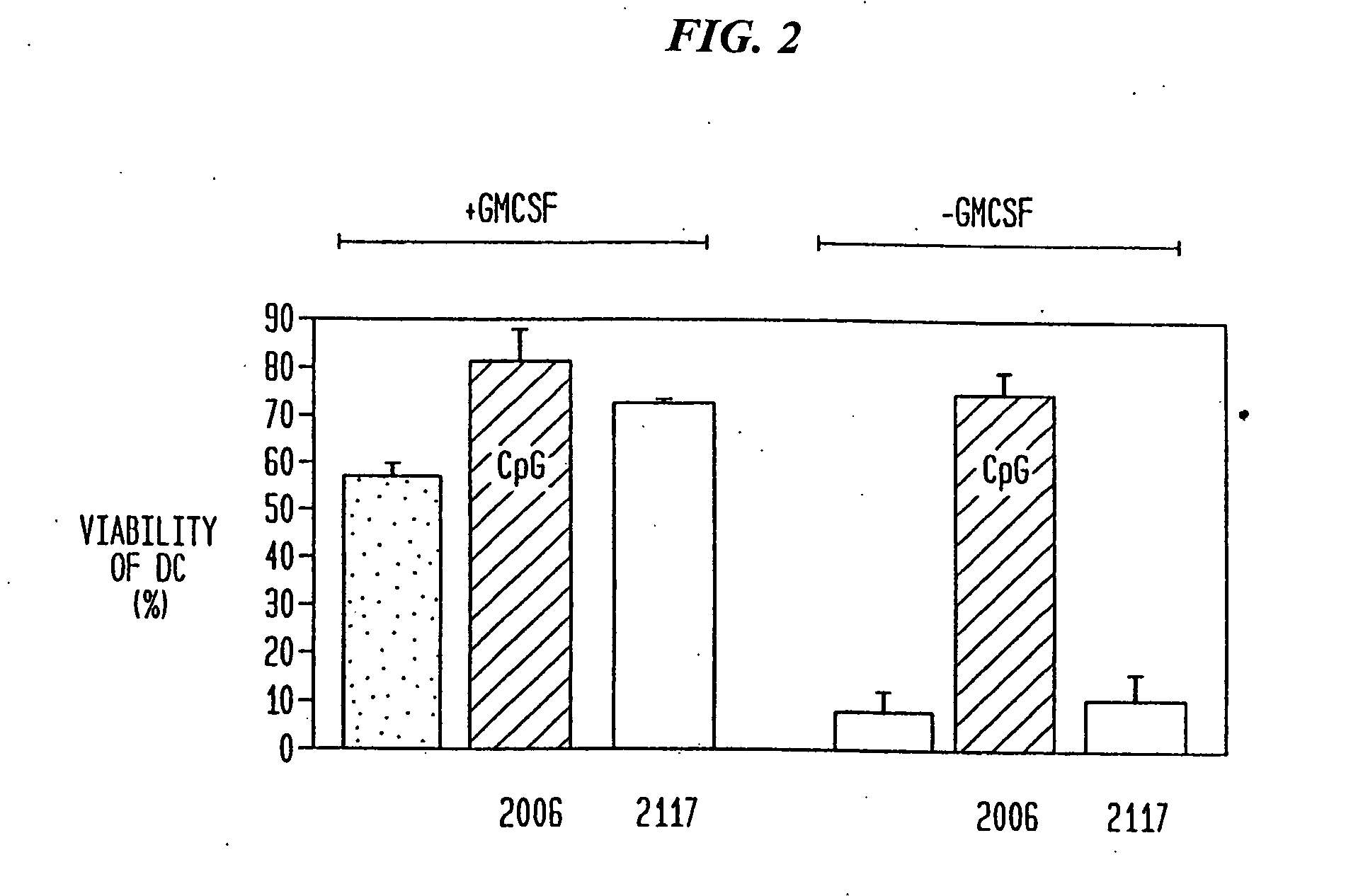
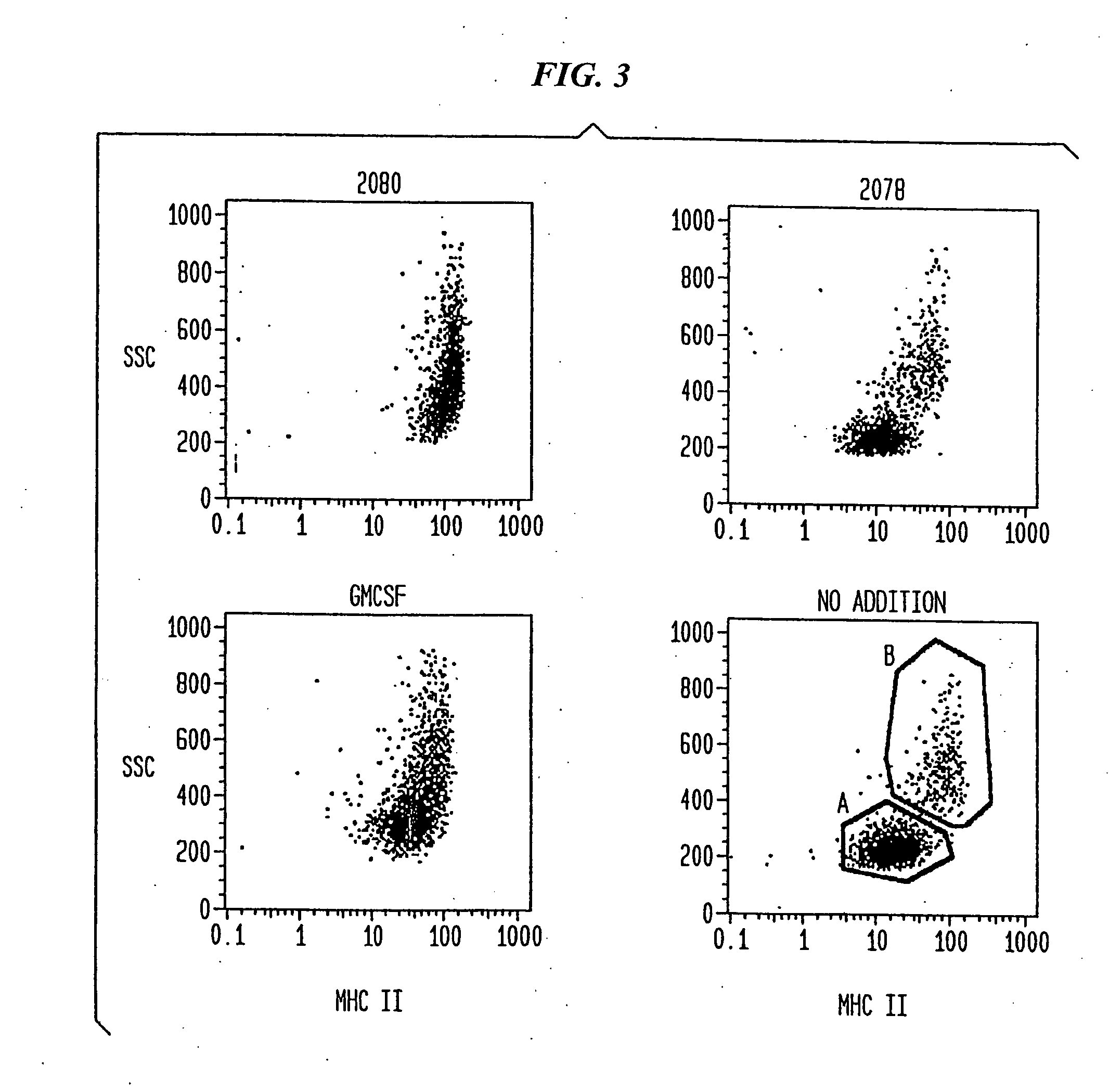
The present invention relates generally to methods and products for activating dendritic cells. In particular, the invention relates to oligonucleotides which have a specific sequence including at least one unmethylated CpG dinucleotide which are useful for activating dendritic cells. The methods are useful for in vitro, ex-vivo, and in vivo methods such as cancer immunotherapeutics, treatment of infectious disease and treatment of allergic disease.
Owner:UNIV OF IOWA RES FOUND
Immunostimulatory polynucleotide/immunomodulatory molecule conjugates
InactiveUS20040006010A1Boost magnitudeBoost both humoral (antibody)BiocideOrganic active ingredientsAntigenAdjuvant
Immunostimulatory polynucleotide-immunomodulatory molecule conjugate compositions are disclosed. These compositions include a polynucleotide that is linked to an immunomodulatory molecule, which molecule comprises an antigen and may further comprise immunomodulators such as cytokines and adjuvants. The polynucleotide portion of the conjugate includes at least one immunostimulatory oligonucleotide nucleotide sequence (ISS). Methods of modulating an immune response upon administration of the polynucleotide-immunomodulatory conjugate preparation to a vertebrate host are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
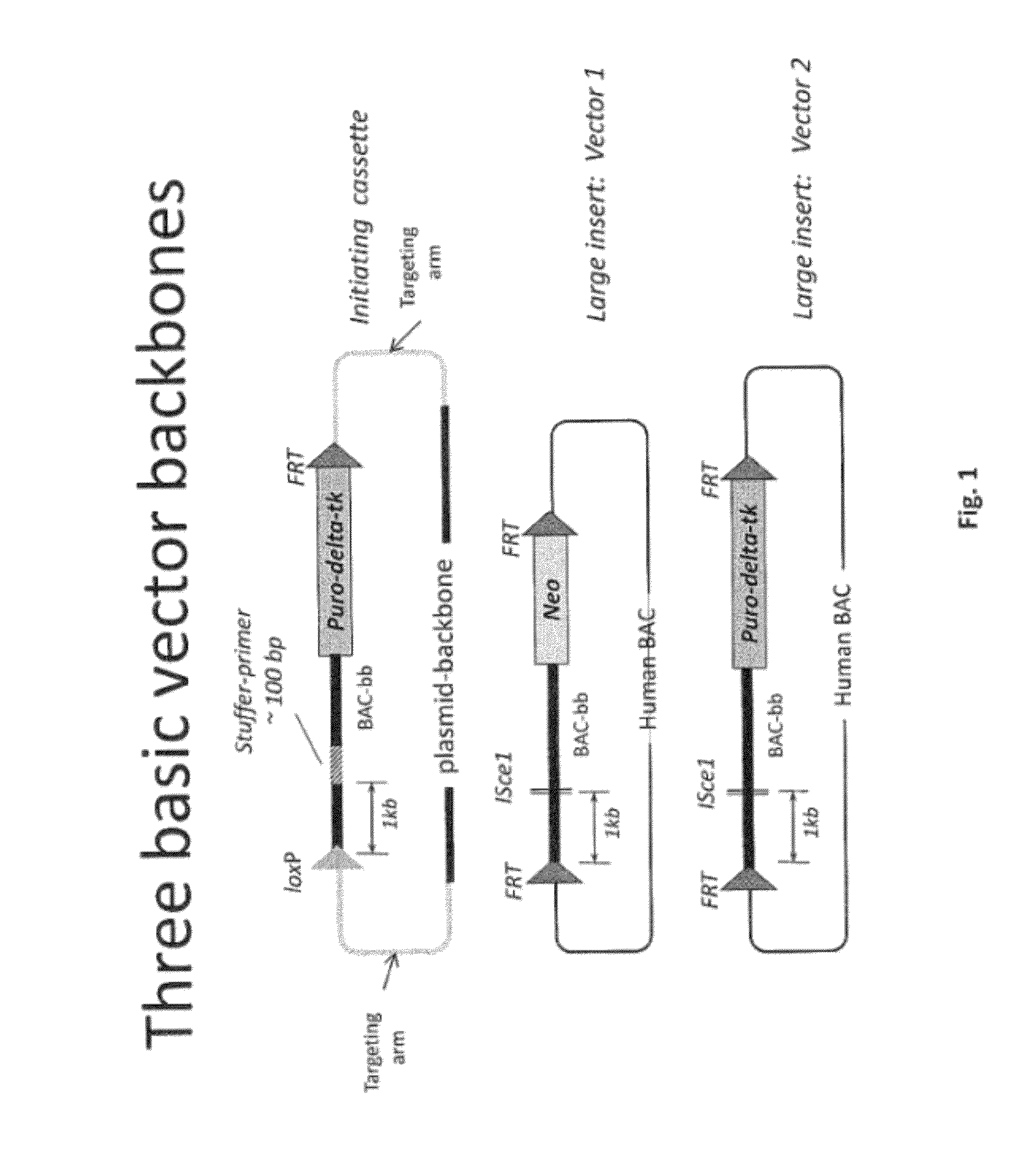
Animal models and therapeutic molecules
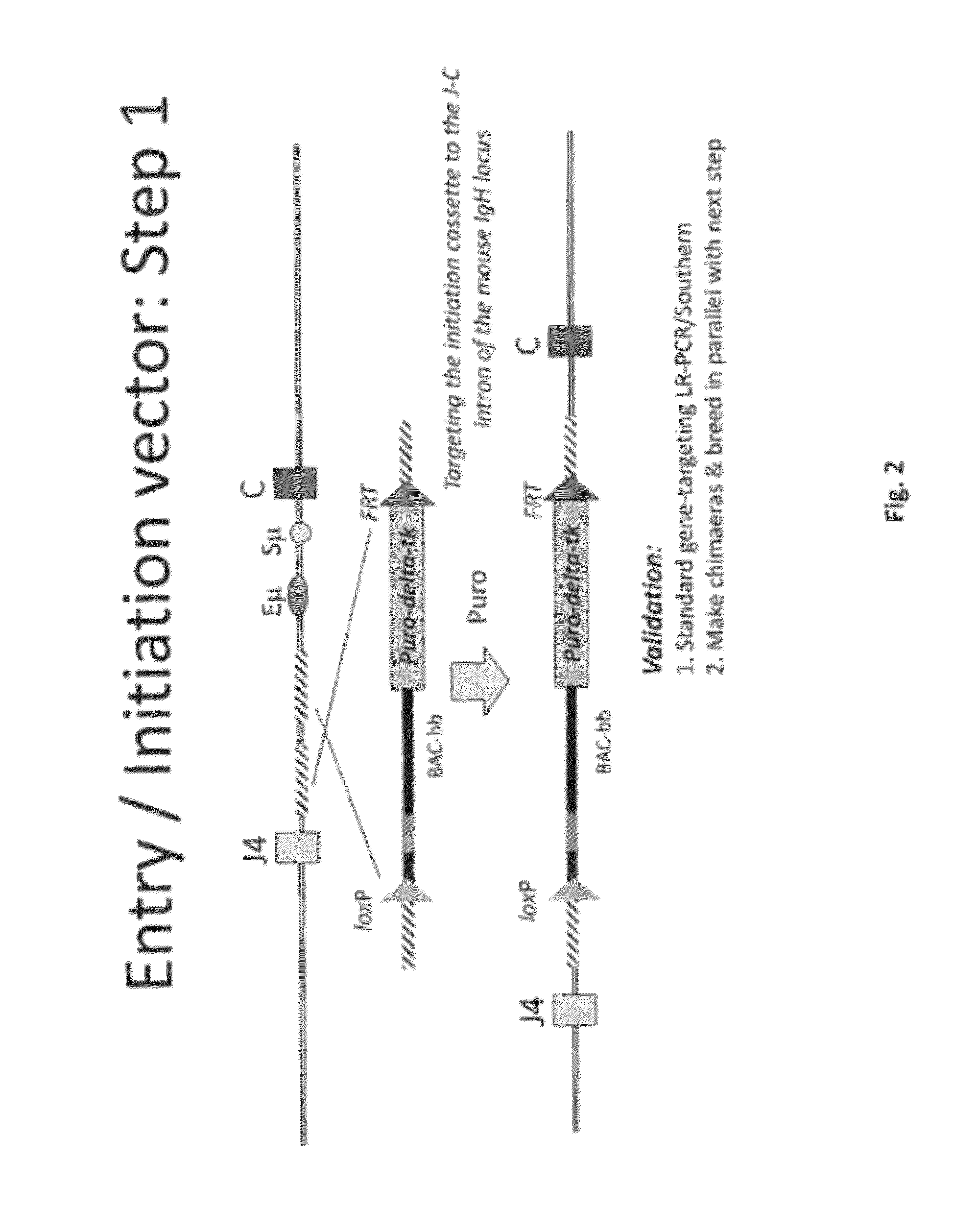
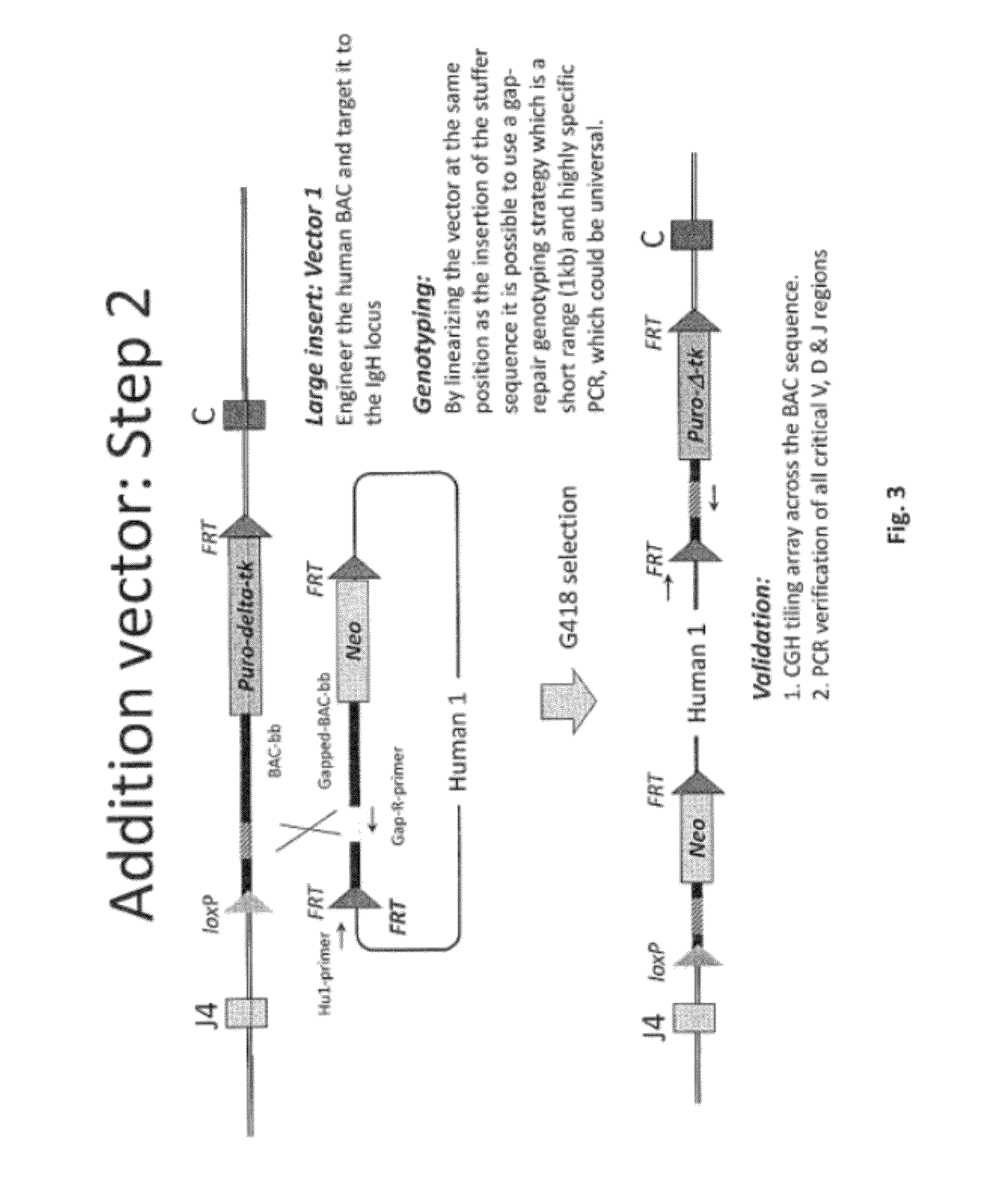
The invention discloses methods for the generation of chimaeric human—non-human antibodies and chimaeric antibody chains, antibodies and antibody chains so produced, and derivatives thereof including fully humanised antibodies; compositions comprising said antibodies, antibody chains and derivatives, as well as cells, non-human mammals and vectors, suitable for use in said methods.
Owner:KIMAB LTD
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7332174B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAntigenAdjuvant
Mutant cholera holotoxins having single or double amino acid substitutions or insertions have reduced toxicity compared to the wild-type cholera holotoxin. The mutant cholera holotoxins are useful as adjuvants in antigenic compositions to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus, or parasite, a cancer cell, a tumor cell, an allergen, or a self-molecule.
Owner:WYETH HOLDINGS CORP +1
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7285281B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAdjuvantCancer cell
Owner:UNIV OF COLORADO FOUND +1
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a Th1 phenotype
InactiveUS20030092663A1Useful in treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsBiocideAntigen stimulationTherapeutic intent
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for induction of antigen-specific tolerance
InactiveUS20120076831A1Efficiently inducing long-term immune tolerancePowder deliveryNervous disorderDiseaseTransplant rejection
The present invention utilizes carrier particles to present antigen peptides and proteins to the immune system in such a way as to induce antigen specific tolerance. The carrier particle is designed in order to trigger an immune tolerance effect. The invention is useful for treatment of immune related disorders such as autoimmune disease, transplant rejection and allergic reactions.
Owner:MILLER STEPHEN +4
Immunomodulatory compositions containing an immunostimulatory sequence linked to antigen and methods of use thereof
InactiveUS7223398B1Reduced antibody productionReduce productionGenetic material ingredientsAntiviralsNucleotideImmuno modulation
The invention provides classes of immunomodulatory compositions which comprise an average of one or more immunostimulatory sequence (ISS) containing polynucleotide conjugated, or attached, to antigen. The extent of conjugation affects immunomodulatory properties, such as extent of antigen-specific antibody formation, including Th1-associated antibody formation, and thus these various conjugate classes are useful for modulating the type and extent of immune response. The invention also includes methods of modulating an immune response using these compositions.
Owner:DYNAVAX TECH CORP
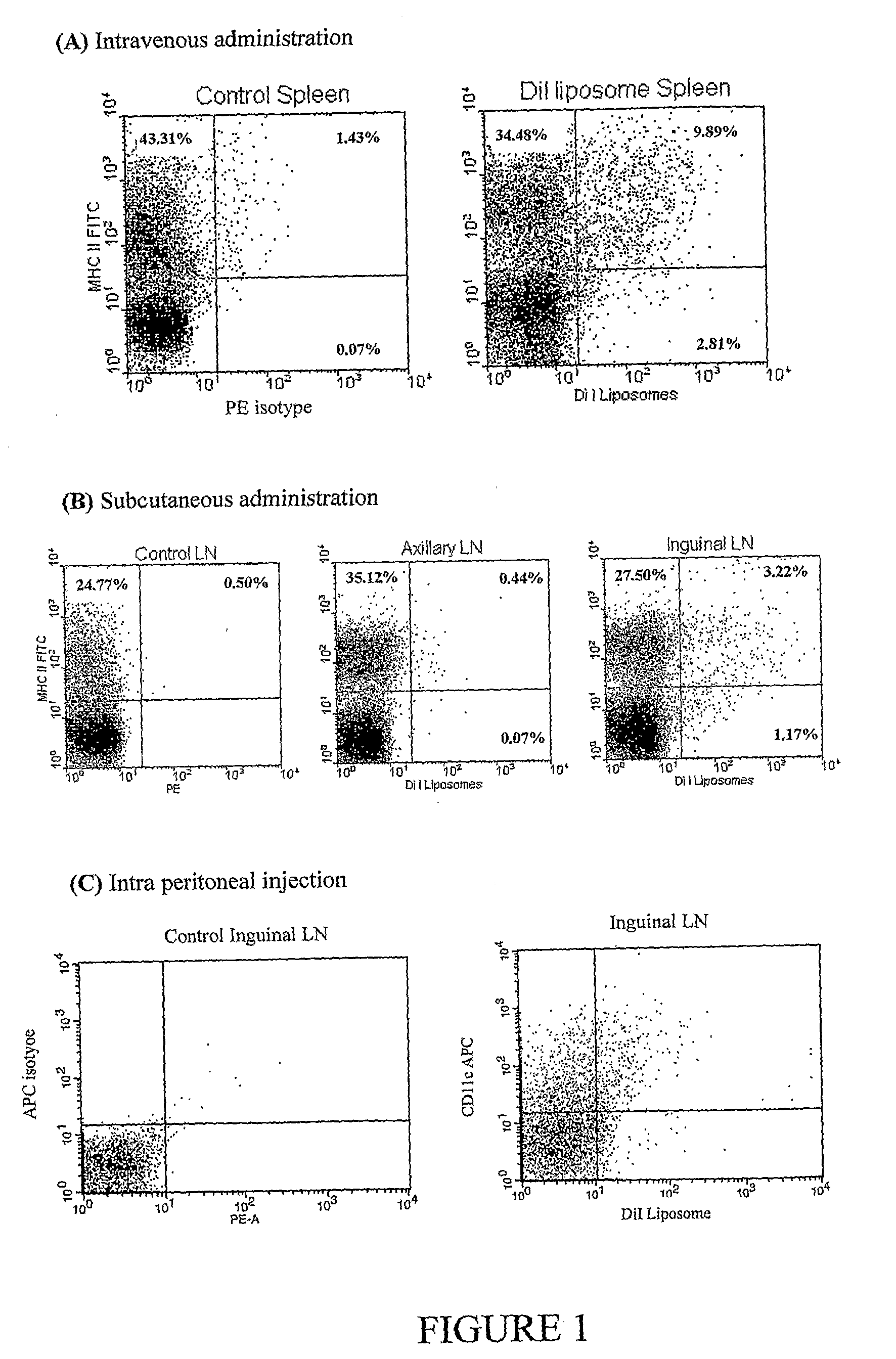
Synergistic Liposomal Adjuvants
InactiveUS20070298093A1Improve actionEnhance immune responseAntibacterial agentsAntimycoticsVascular diseaseAdjuvant
The present invention relates to liposome, mixtures or liposomes and liposomal compositions comprising at least two different adjuvants and a therapeutic agent, their production and use for the prevention and therapy of proliferative diseases, infectious diseases, vascular diseases, rheumatoid diseases, inflammatory diseases, immune diseases, in particular autoimmune diseases and allergies.
Owner:PHARMEXA
Immunomodulatory formulations and methods for use thereof
InactiveUS7183111B2Regulate immune responseMore resistant to immunomodulationOrganic active ingredientsNanotechNanocarriersImmunomodulations
The invention provides new compositions and methods for immunomodulation of individuals. Immunomodulation is accomplished by administration of immunomodulatory polynucleotide / microcarrier (IMP / MC) complexes. The IMP / MC complexes may be covalently or non-covalently bound, and feature a polynucleotide comprising at least one immunostimulatory sequence bound to a nonbiodegradable microcarrier or nanocarrier.
Owner:DYNAVAX TECH CORP
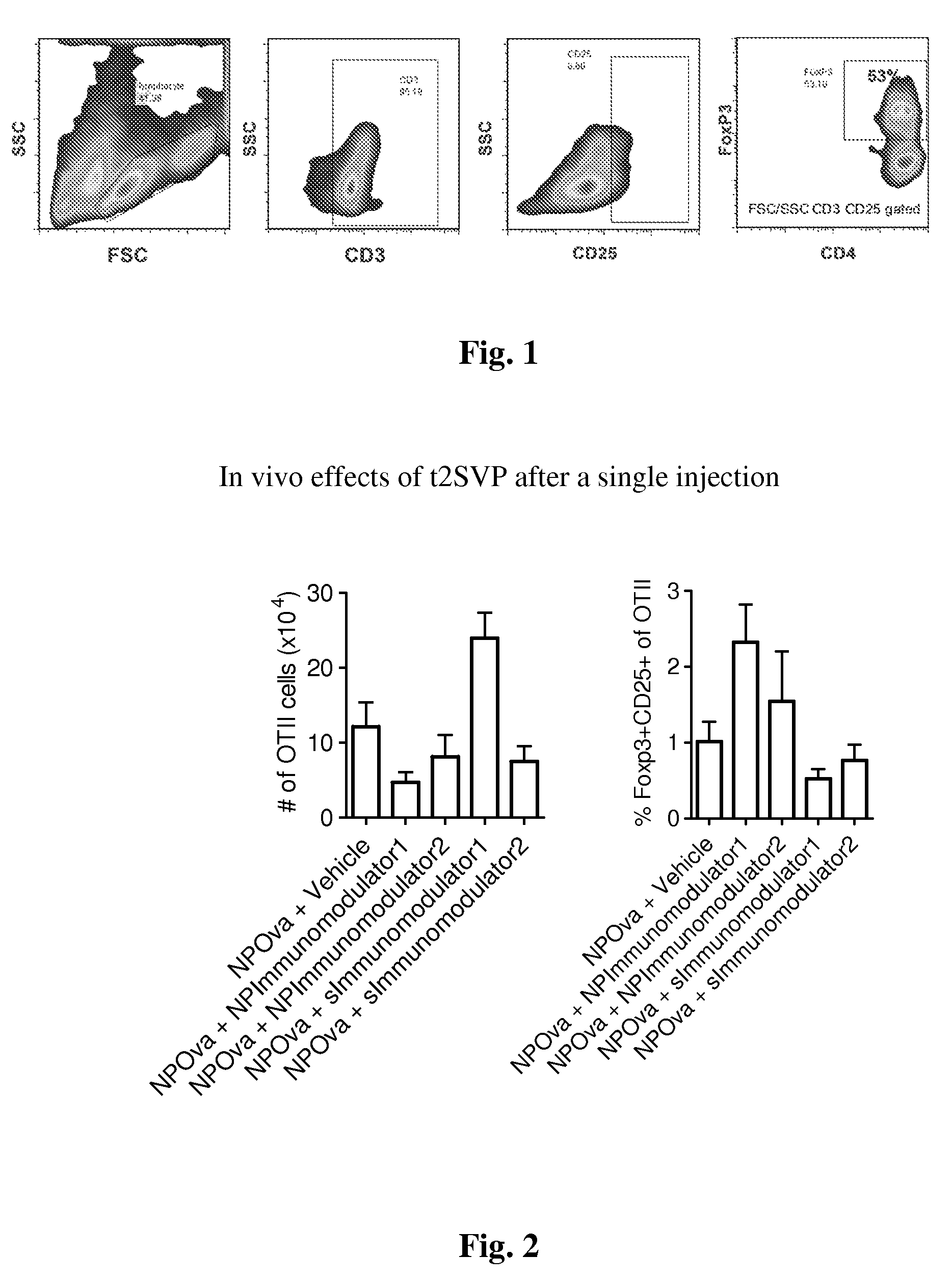
Methods and compositions for immunomodulation
ActiveUS20170020926A1Efficient managementLess side effectsNervous disorderAntipyreticImmunomodulationsExogenous antigen
Owner:RUBIUS THERAPEUTICS
Tolerogenic synthetic nanocarriers to reduce cytotoxic t lymphocyte responses
Disclosed are synthetic nanocarrier compositions, and related methods, comprising MHC Class I-restricted and / or MHC Class II-restricted epitopes associated with undesired CD8+ T cell responses and immunosuppressants that provide tolerogenic immune responses against antigens that comprise the epitopes.
Owner:SELECTA BIOSCI
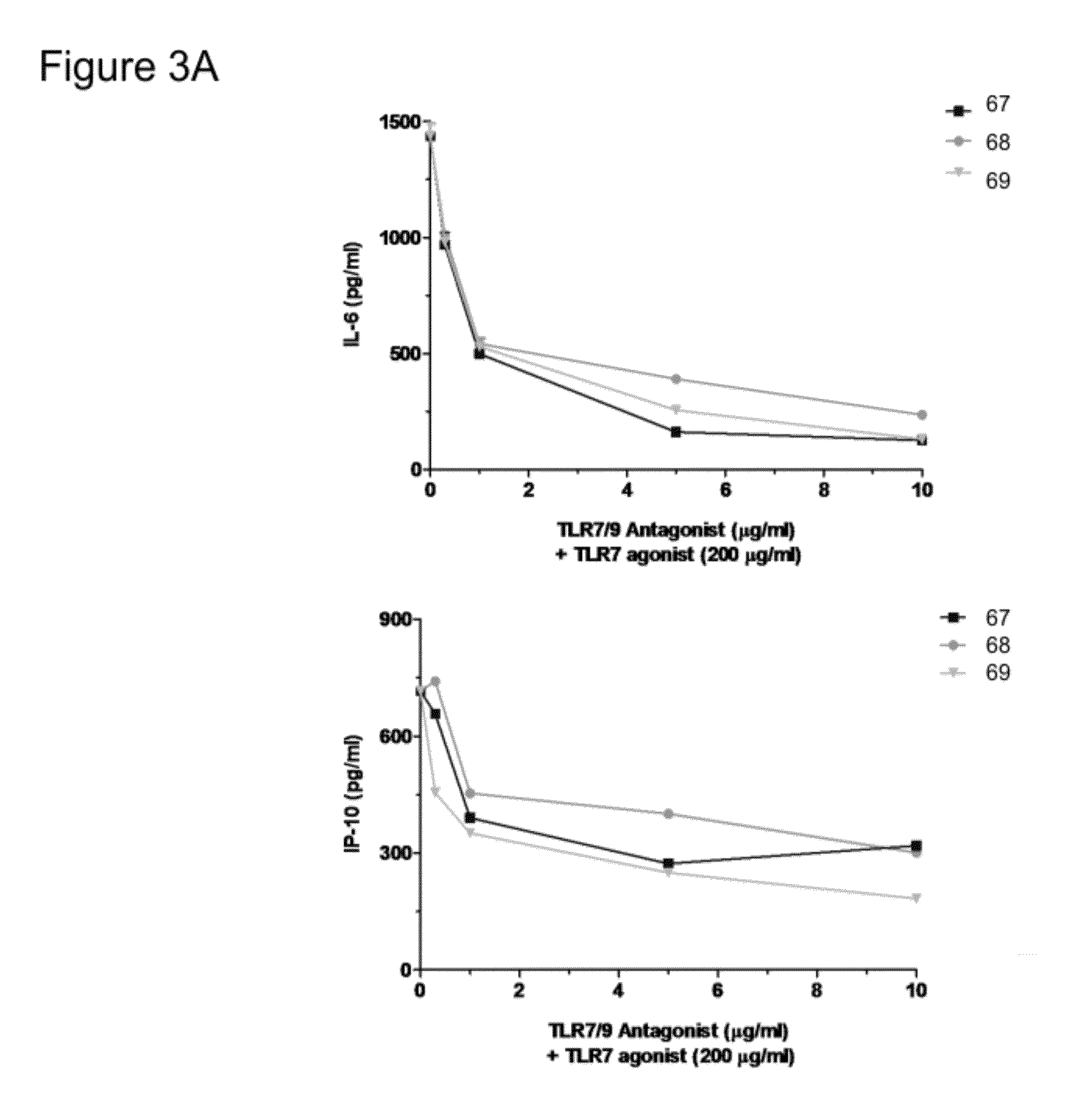
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS20120128699A1Antagonize inhibit suppress preventAntagonize, inhibit suppress or prevent the cytokine and chemokine responseNervous disorderAntipyreticTlr agonistsAutoimmune disease
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These TROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Tolerogenic synthetic nanocarriers for antigen-specific deletion of t effector cells
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering immunosuppressants and MHC Class I-restricted and / or MHC Class II-restricted epitopes that can generate tolerogenic immune responses (e.g., antigen-specific T effector cell deletion).
Owner:SELECTA BIOSCI
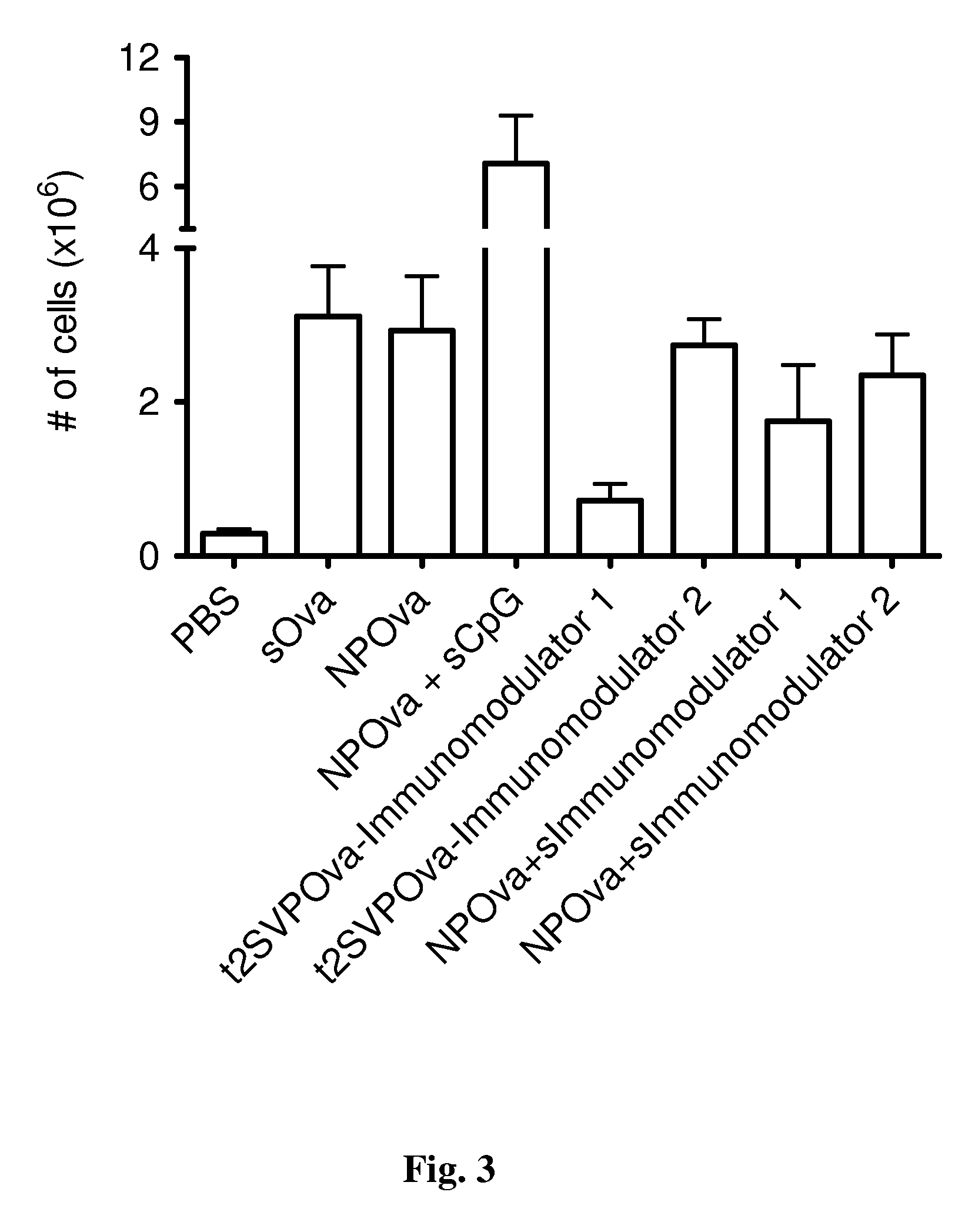
Novel proteosome-liposaccharide vaccine adjuvant
InactiveUS20030044425A1Increase secretionUniform processSsRNA viruses negative-senseBiocideImmunotherapeutic agentCytokine
An adjuvant complex composed of bacterial outer membrane protein proteosomes complexed to bacterial liposaccharide is prepared to contain the component parts under a variety of conditions. The complex can be formulated with antigenic material to form immunogenic compositions, vaccines and immunotherapeutics. An induced immune response includes protective antibodies and / or type 1 cytokines is shown for a variety of protocols.
Owner:ID BIOMEDICAL CORP LAVAL
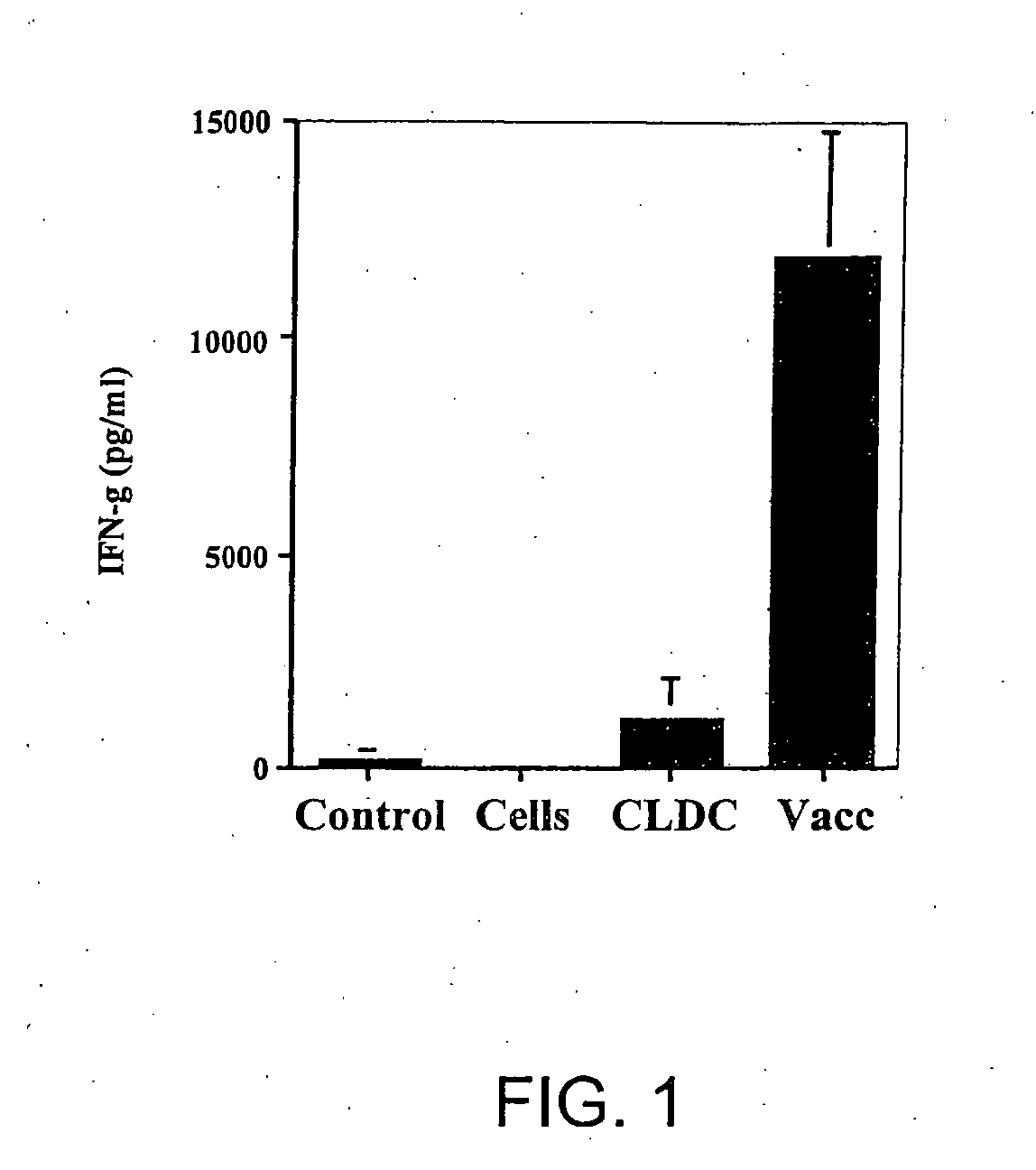
Vaccines using nucleic acid-lipid complexes
InactiveUS20060223769A1SsRNA viruses negative-sensePeptide/protein ingredientsInfectious DisorderSelf-Antigens
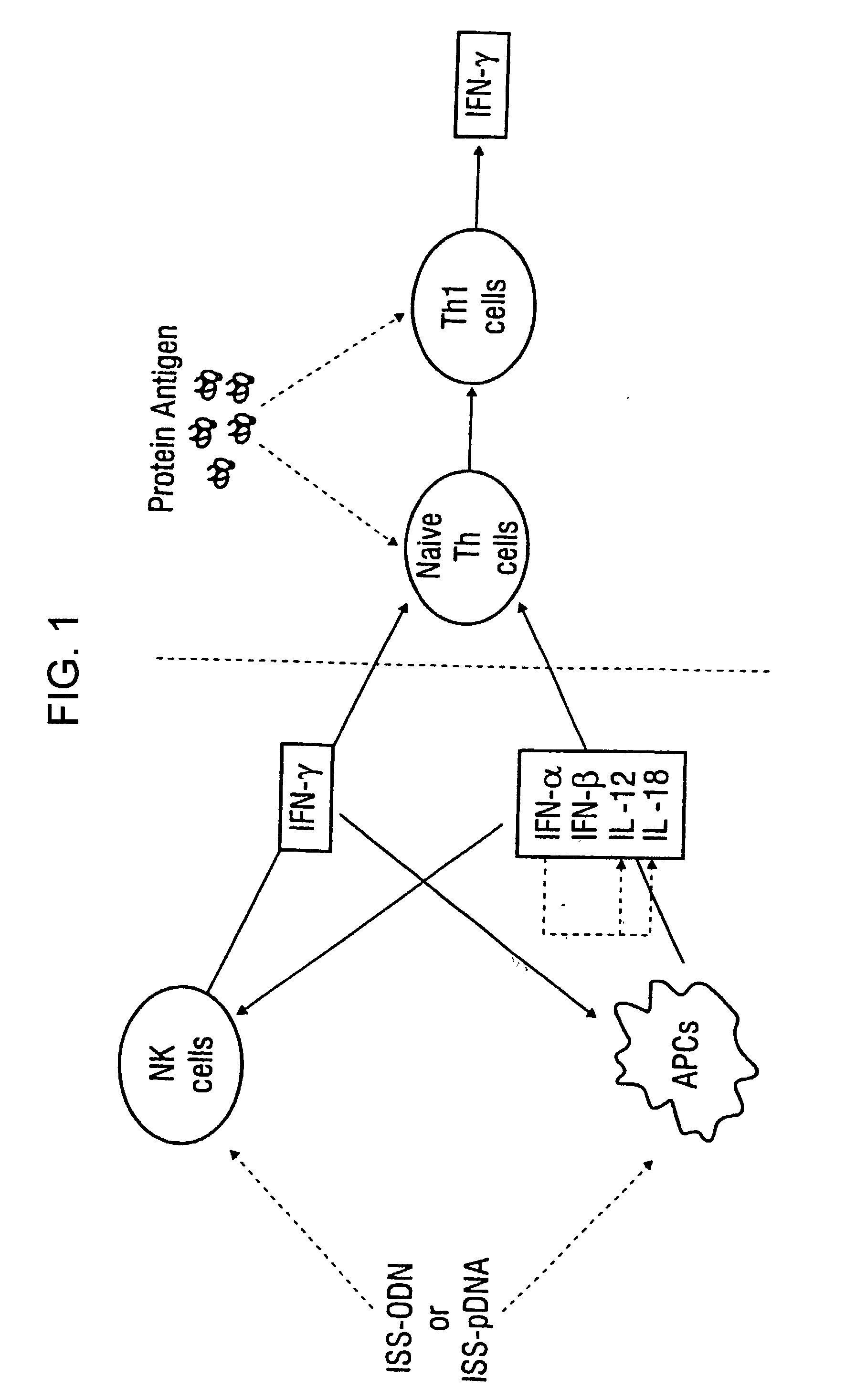
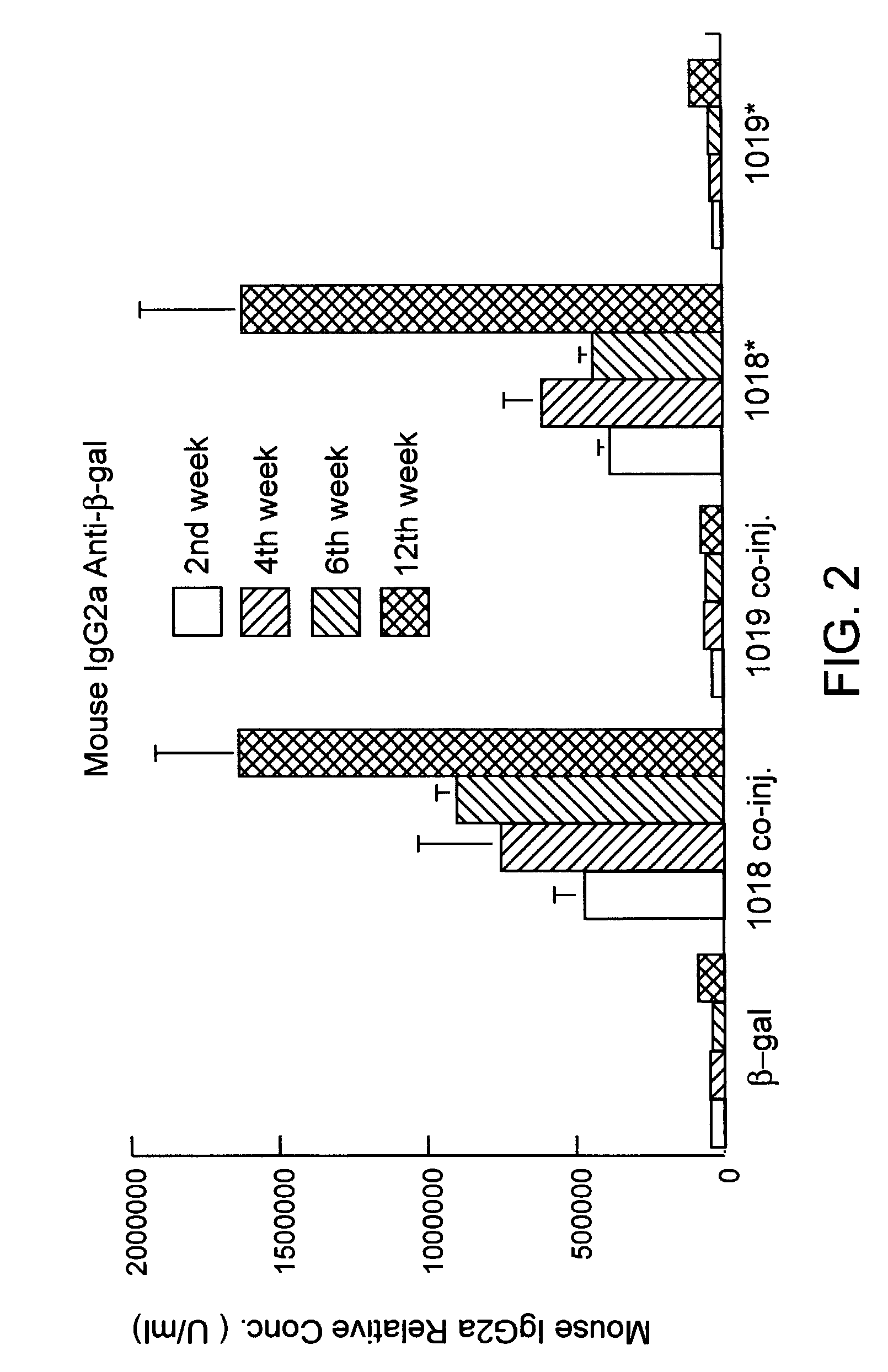
This invention relates to a vaccine and a method for immune activation which is effective for eliciting both a systemic, non-antigen specific immune response and a strong antigen-specific immune response in a mammal. The method is particularly effective for protecting a mammal from a disease including cancer, a disease associated with allergic inflammation, an infectious disease, or a condition associated with a deleterious activity of a self-antigen. Also disclosed are therapeutic compositions useful in such a method.
Owner:NAT JEWISH MEDICAL & RES CENT
Methods and compositions for inducing oral tolerance in mammals
The present invention relates to methods and pharmaceutical formulations for orally delivering an antigen to induce tolerance. The antigen is combined with derivatized amino acids or salts thereof. The induction of oral tolerance may be applied clinically for the prevention or treatment of auto-immune diseases and clinical allergic hypersensitivities, and for the prevention of allograft rejection.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Tolerogenic synthetic nanocarriers for regulating innate immune responses
InactiveUS20120276160A1Suppressing antigen-specific activationReduce in quantityOrganic active ingredientsPowder deliveryB cellAntigen specific
Disclosed are synthetic nanocarrier methods, and related compositions, comprising administering B cell and / or MHC Class II-restricted epitopes of an antigen and immunosuppressants in order to reduce antigen-specific activation of innate immune cells.
Owner:SELECTA BIOSCI
Methods of manufacture and use of calcium phosphate particles containing allergens
The present invention relates to the use of calcium phosphate particles in formulation with allergens for allergic desensitization. Particularly, the invention relates to novel calcium phosphate core particles, particularly nano- and micron-sized particles, as allergen adjuvants and in compositions for inducing allergic desensitization. Methods of making such particles and to methods of inducing a specific immune response using the particles of this invention are also provided.
Owner:CAPTIVATE PHARMACEUTICALS LLC
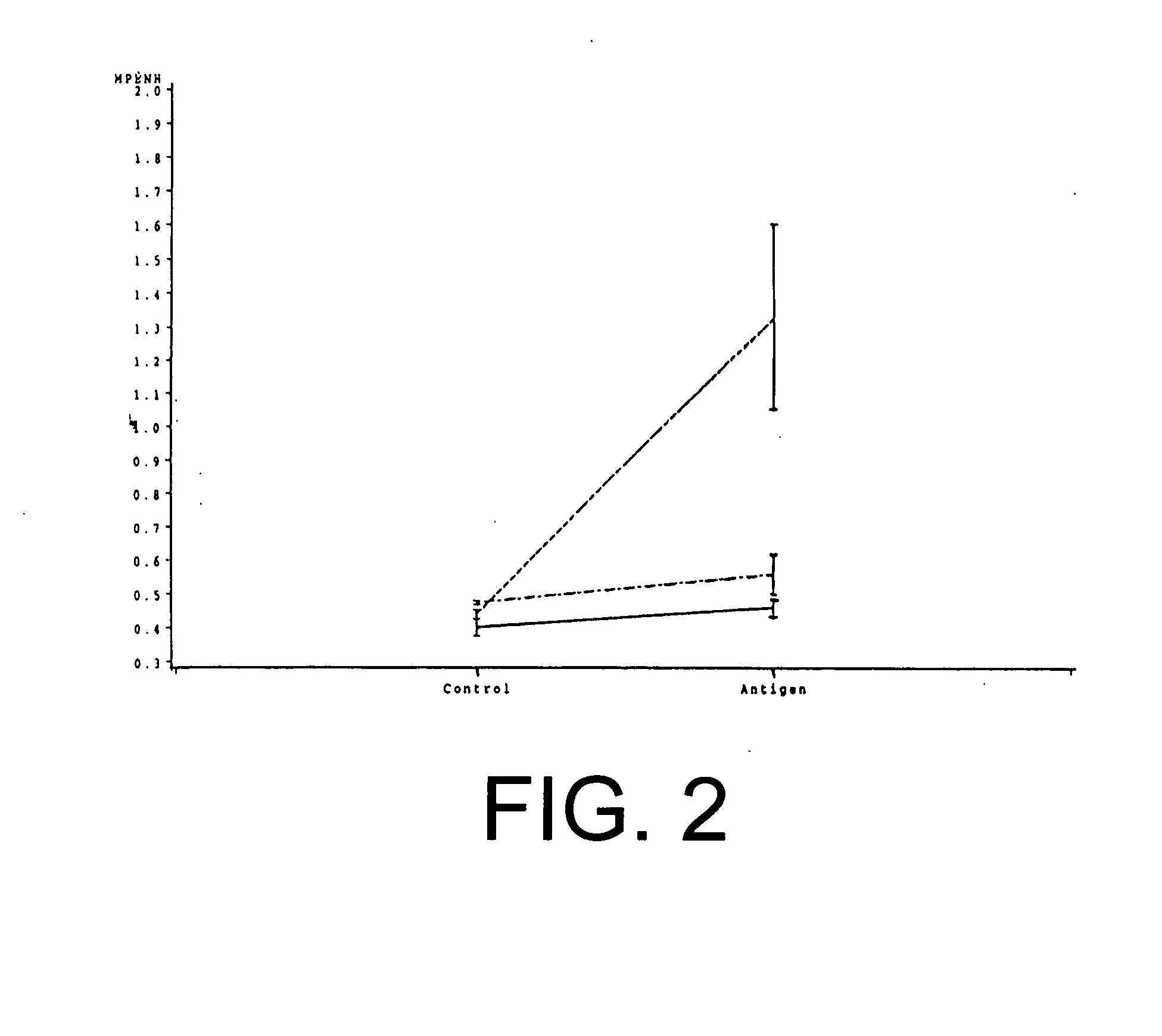
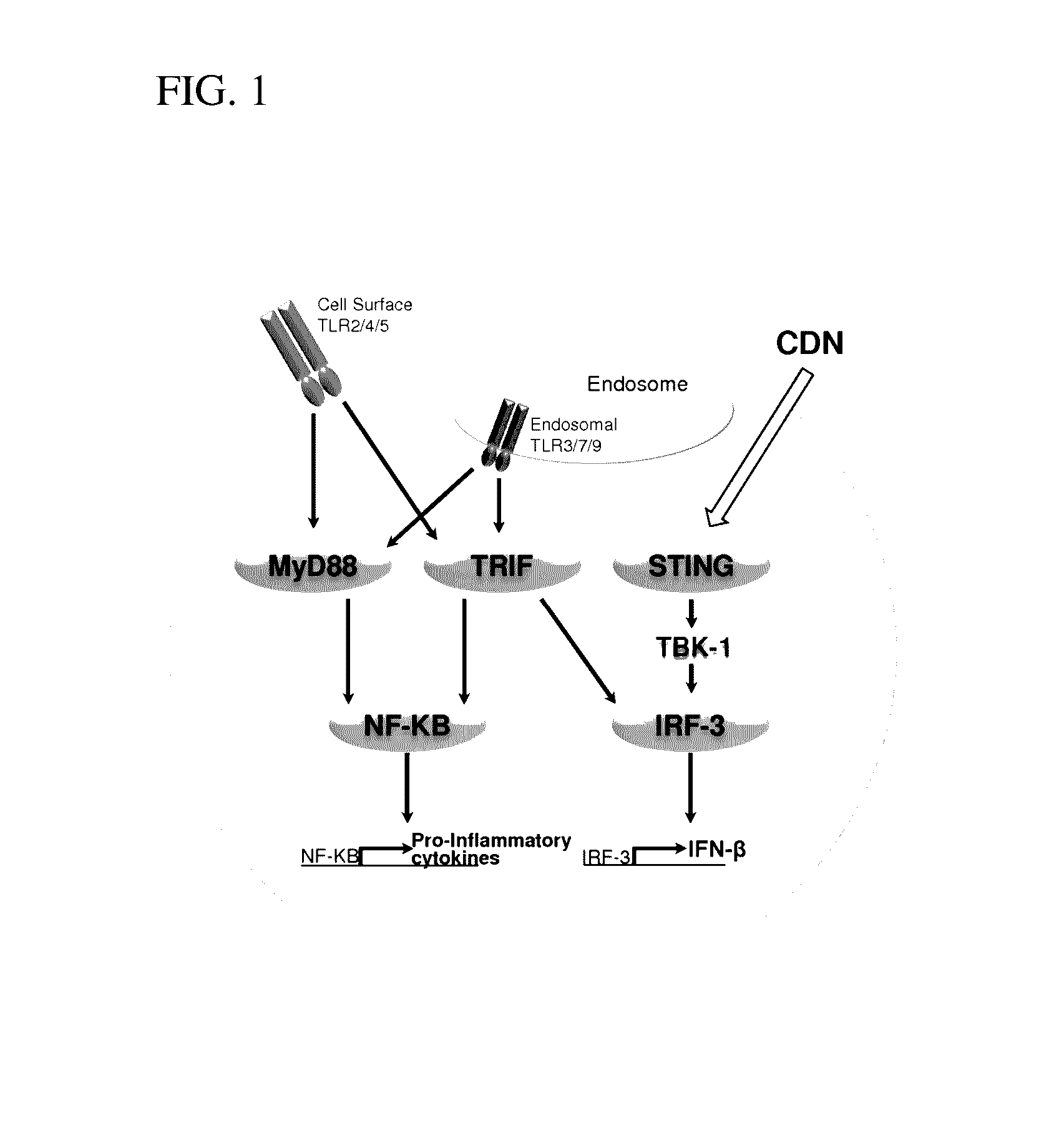
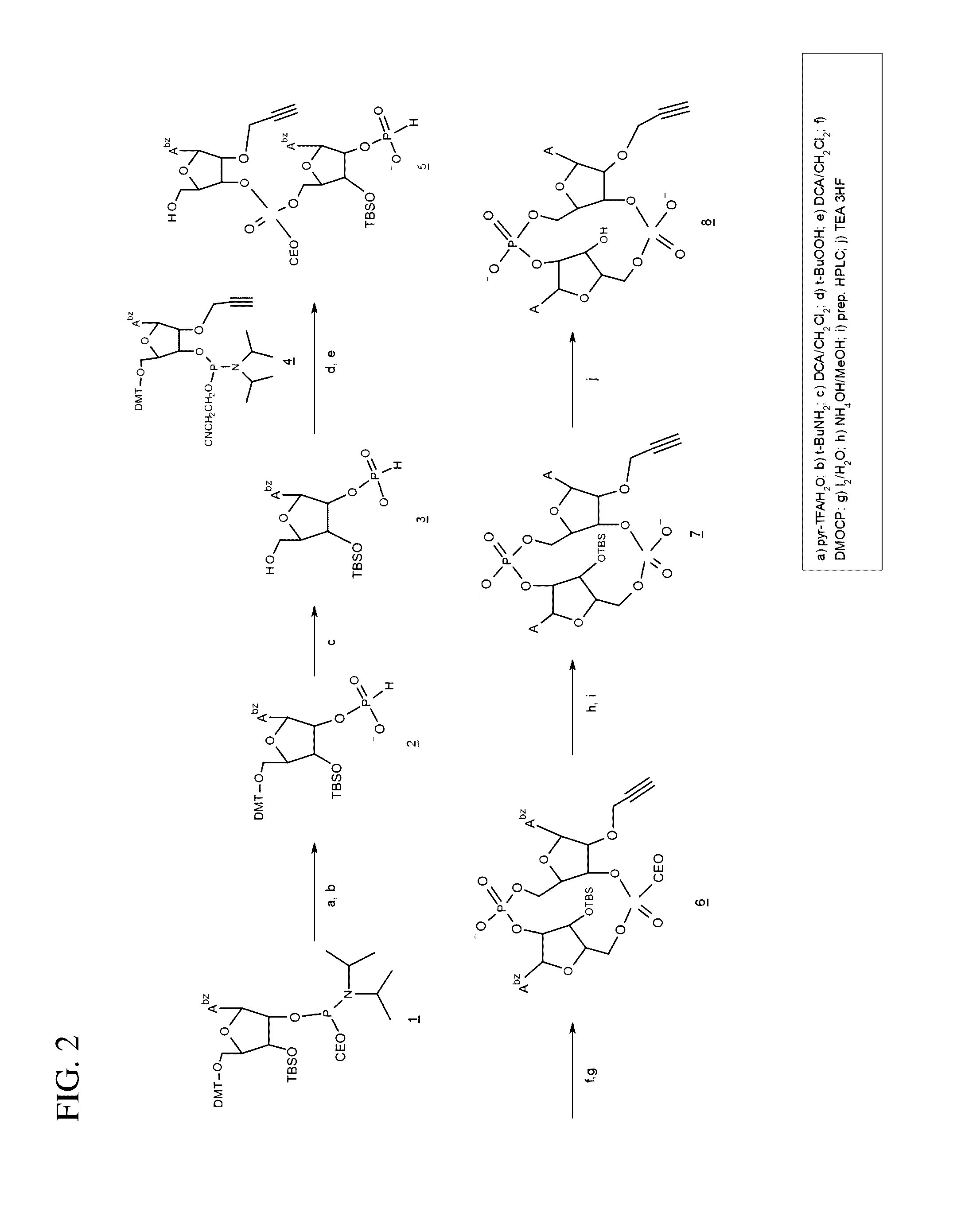
Compositions and methods for inhibiting “stimulator of interferon gene”—dependent signalling
Owner:ADURO BIOTECH
Tolerogenic synthetic nanocarrier compositions with transplantable graft antigens and methods of use
InactiveUS20120276156A1Eliminate side effectsReduce generationAntipyreticAnalgesicsNanocarriersPlant Antigens
Disclosed are synthetic nanocarrier compositions, and related methods, comprising APC presentable transplant antigens and immunosuppressants that provide tolerogenic immune responses (e.g., a reduction in CD8+ T cell proliferation and / or activity) specific to the APC presentable transplant antigens.
Owner:SELECTA BIOSCI
Peanut formulations and uses thereof
The present application relates to compositions for oral immunotherapy of peanut allergies. Further, the present application relates to methods for the preparation of the compositions for immunotherapy, and their use in immunotherapy.
Owner:SOC DES PROD NESTLE SA
Compositions and methods for modulating immune responses
This invention discloses methods and compositions for modulating immune responses, which involve particulate delivery of agents to immune cells, wherein the agents comprise an inhibitor of the NF-kappa B signaling pathway and an antigen that corresponds to a target antigen. The methods and compositions of the present invention are particularly useful in the treatment or prophylaxis of an undesirable immune response associated with the target antigen, including autoimmune diseases, allergies and transplantation associated diseases.
Owner:THE UNIV OF QUEENSLAND
Vaccine composition containing synthetic adjuvant
Owner:ACCESS TO ADVANCED HEALTH INST
Packaged virus-like particles
InactiveUS20060251623A1Improve responseEnhanced T cell responseSsRNA viruses negative-senseBiocideAbnormal tissue growthDisease
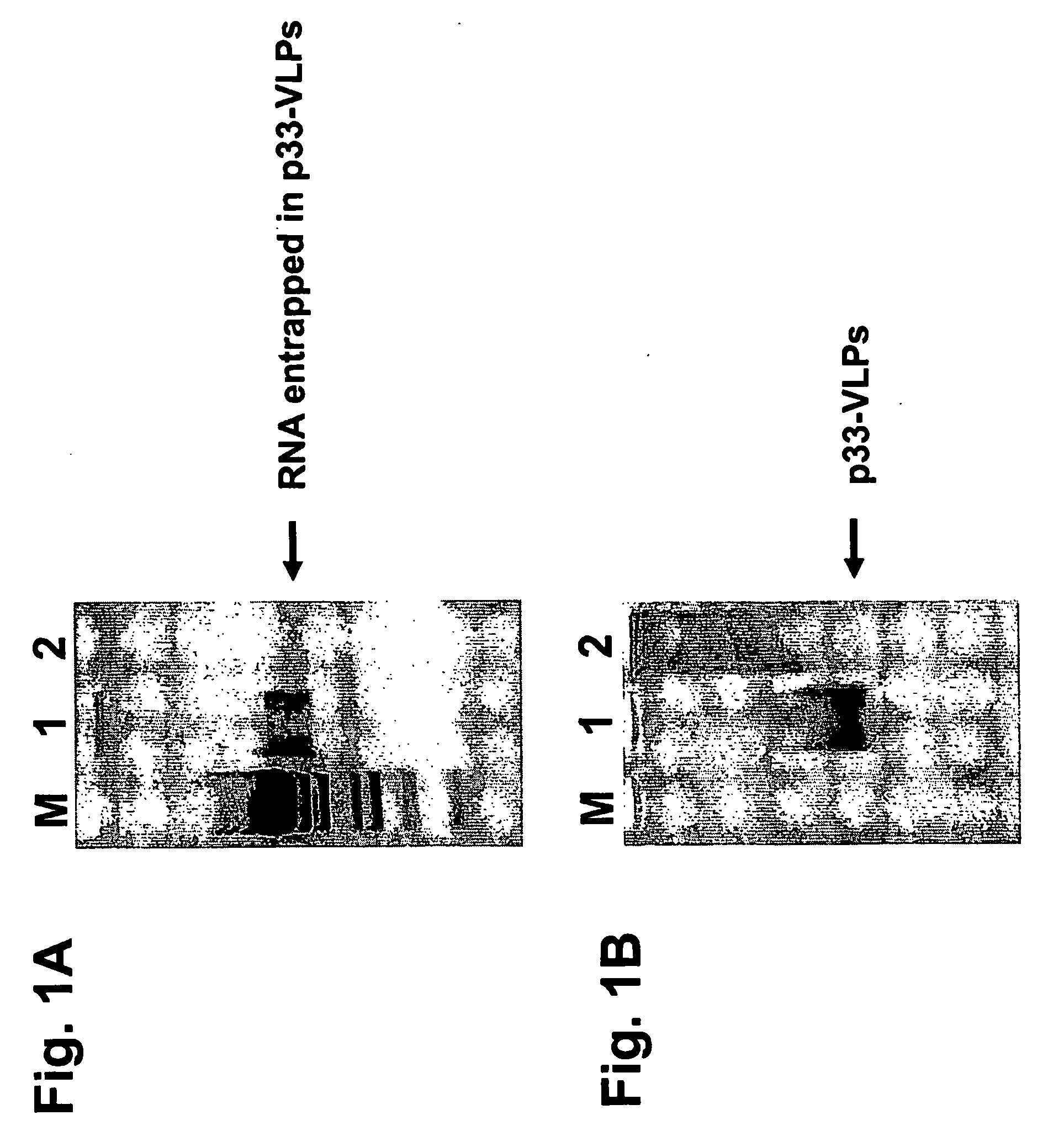
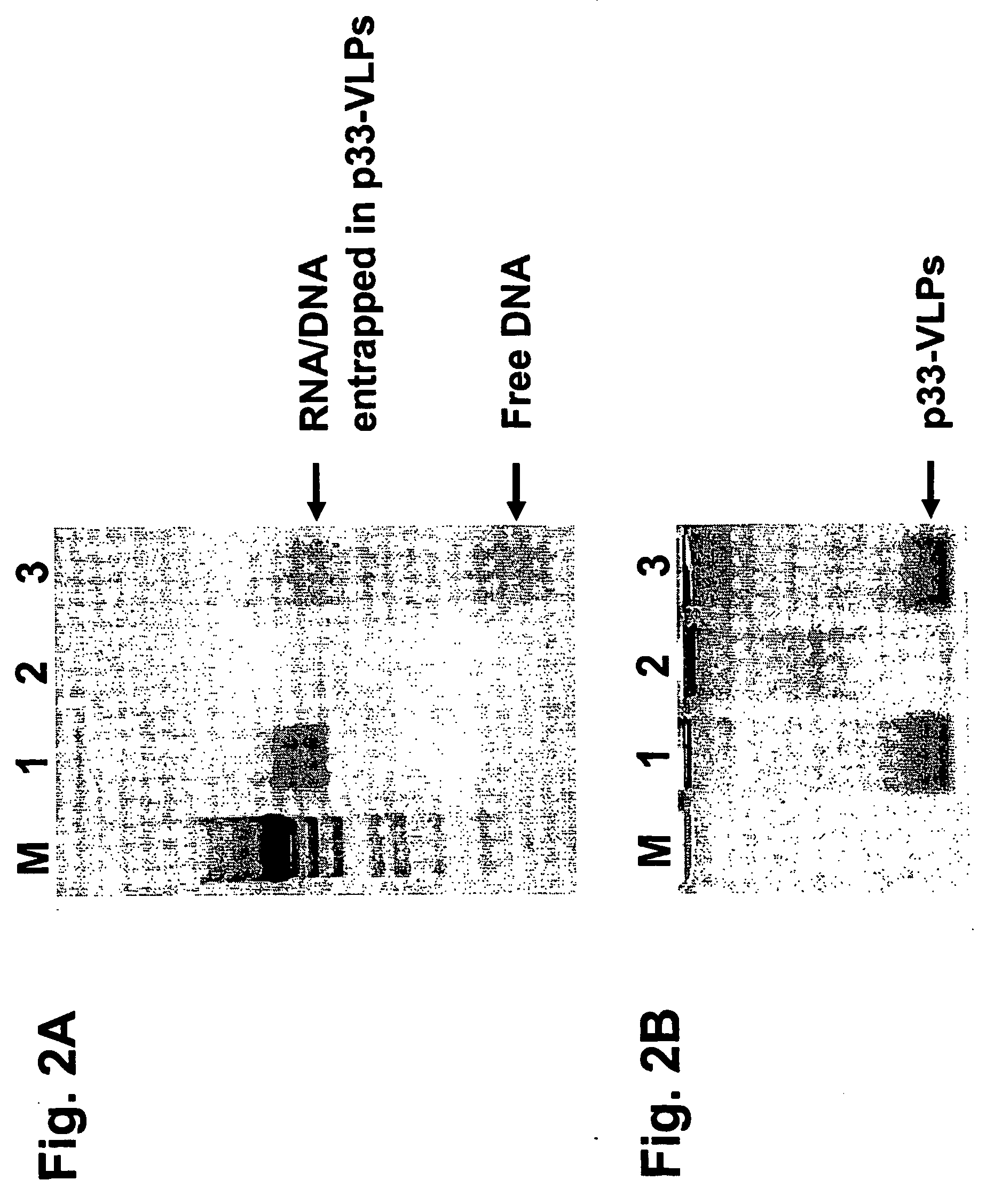
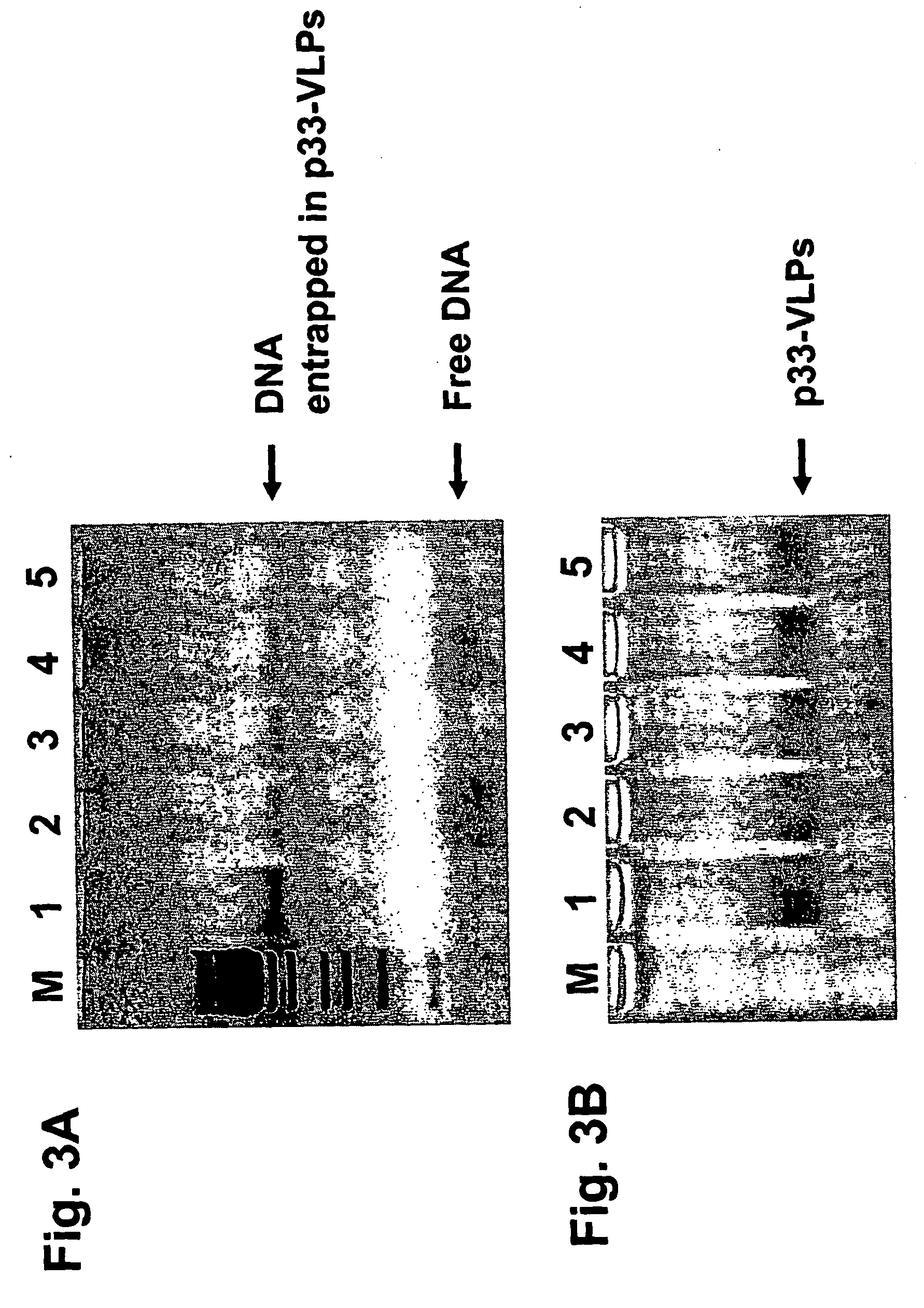
The present invention is related to the fields of vaccinology, immunology and medicine. The invention provides compositions and methods for enhancing immunological responses against antigens coupled or fused to virus-like particles (VLPs) packaged with immunostimulatory nucleic acids, preferably oligonucleotides containing at least one non-methylated CpG sequence and a toll-like receptor (TLR) ligand. The invention can be used to induce strong antibody and T cell responses particularly useful for the treatment of allergies, tumors and chronic viral diseases as well as other chronic diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Solid Vaccine Formulation
InactiveUS20090155351A1High propertyReadily re-hydratedPowder deliverySnake antigen ingredientsAntigenAlcohol sugars
The invention relates to a solid vaccine formulation adapted for mucosal administration comprising at least one antigen as active substance, wherein the formulation comprises a lyophilisate of a suspension comprising an oxygen-containing metal salt, the antigen(s) and one or more excipients selected from (i) saccharides, (ii) sugar alcohols, and (iii) amino acids or pharmaceutically acceptable salts thereof.
Owner:ALK ABELLO SA
Immunostimulatory oligonucleotides and uses thereof
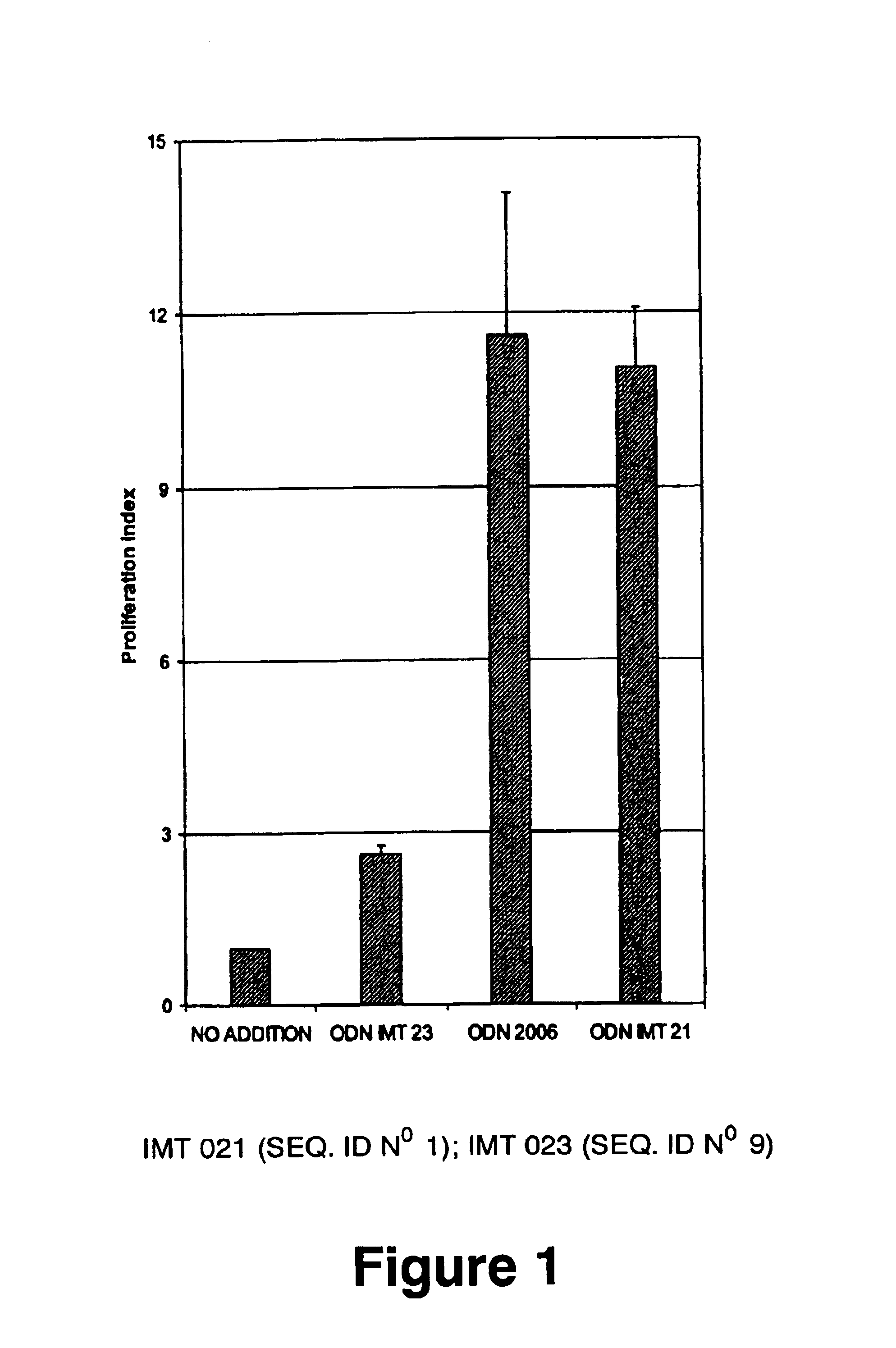
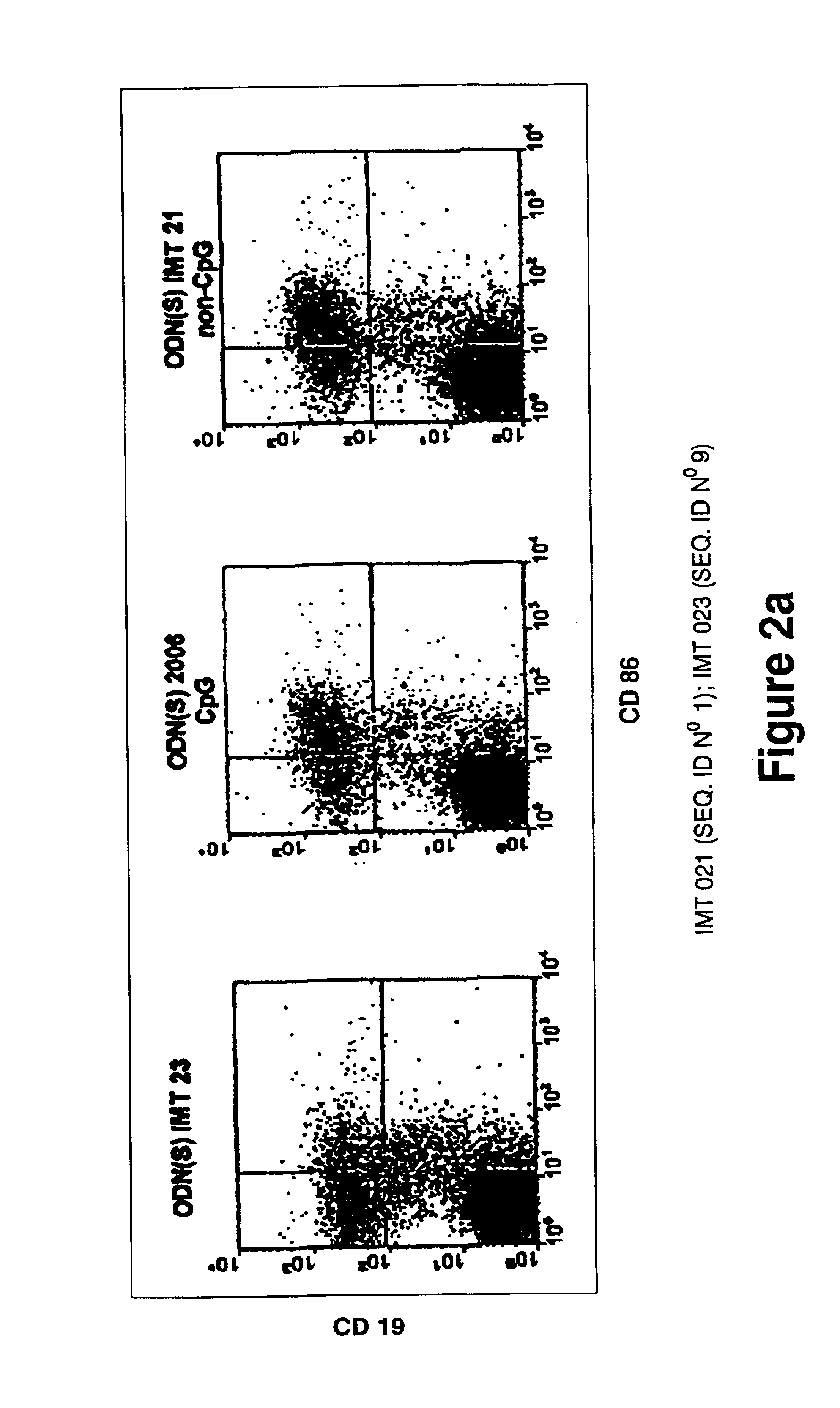
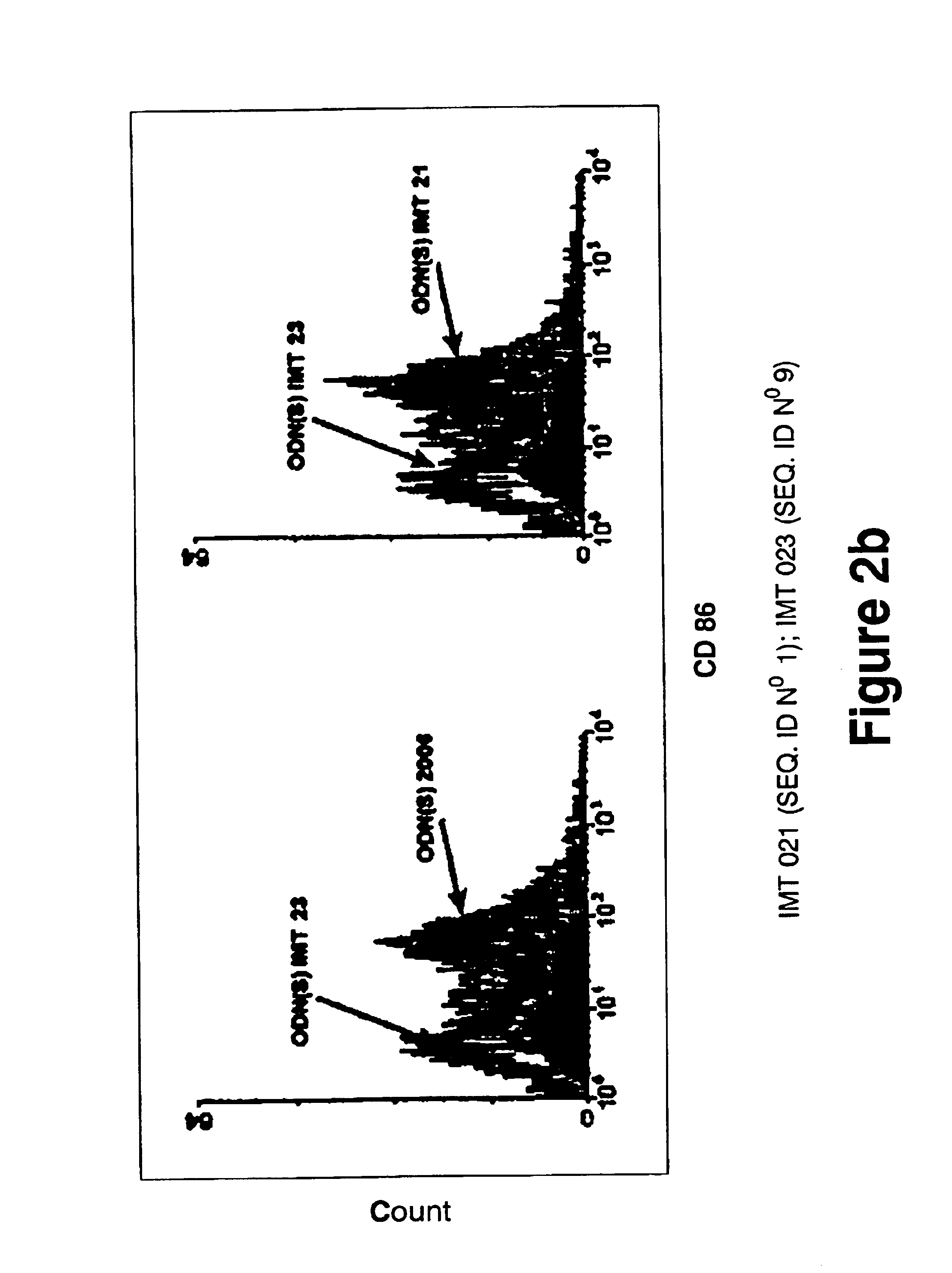
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com