Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Stimulator of interferon genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
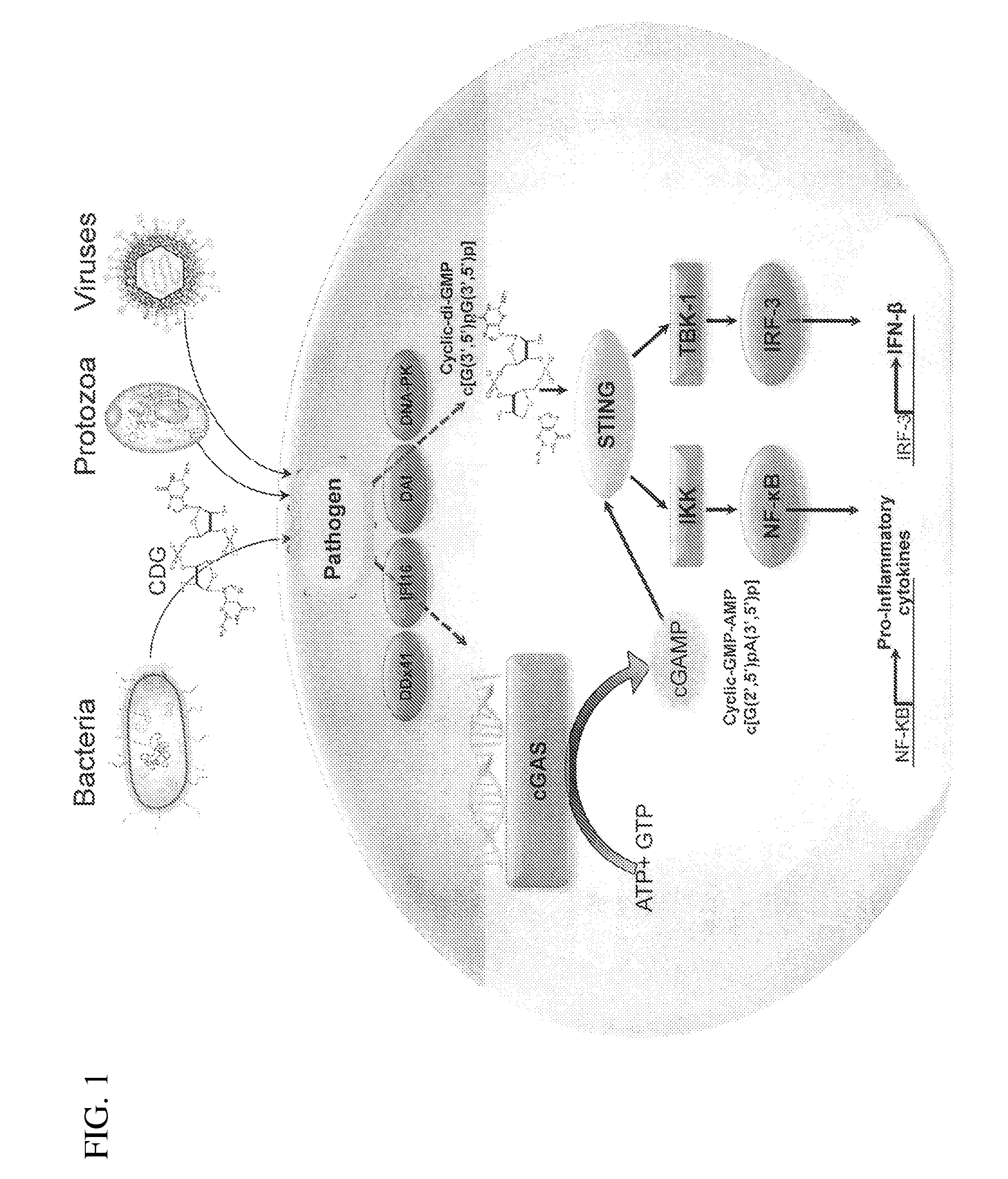
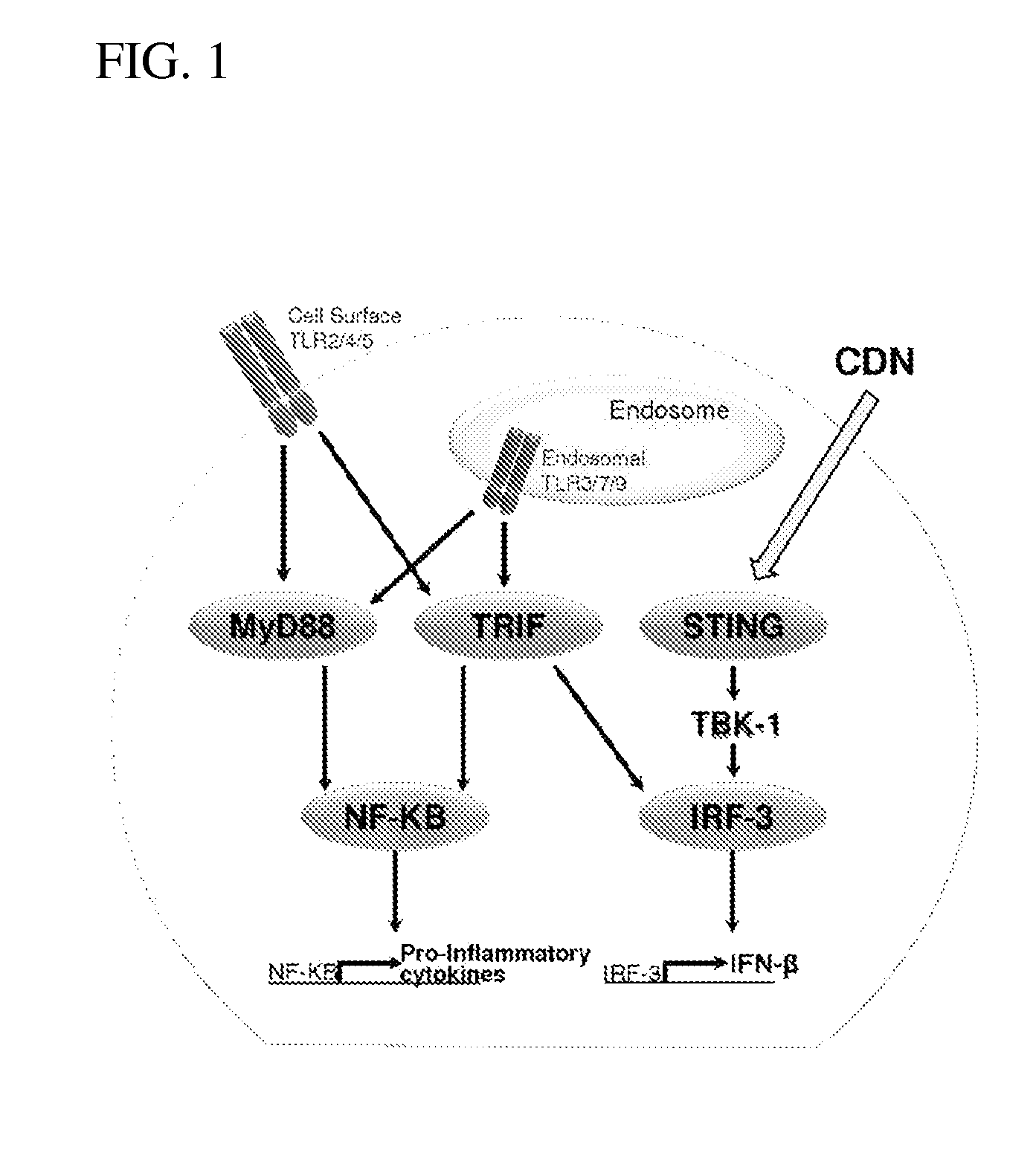
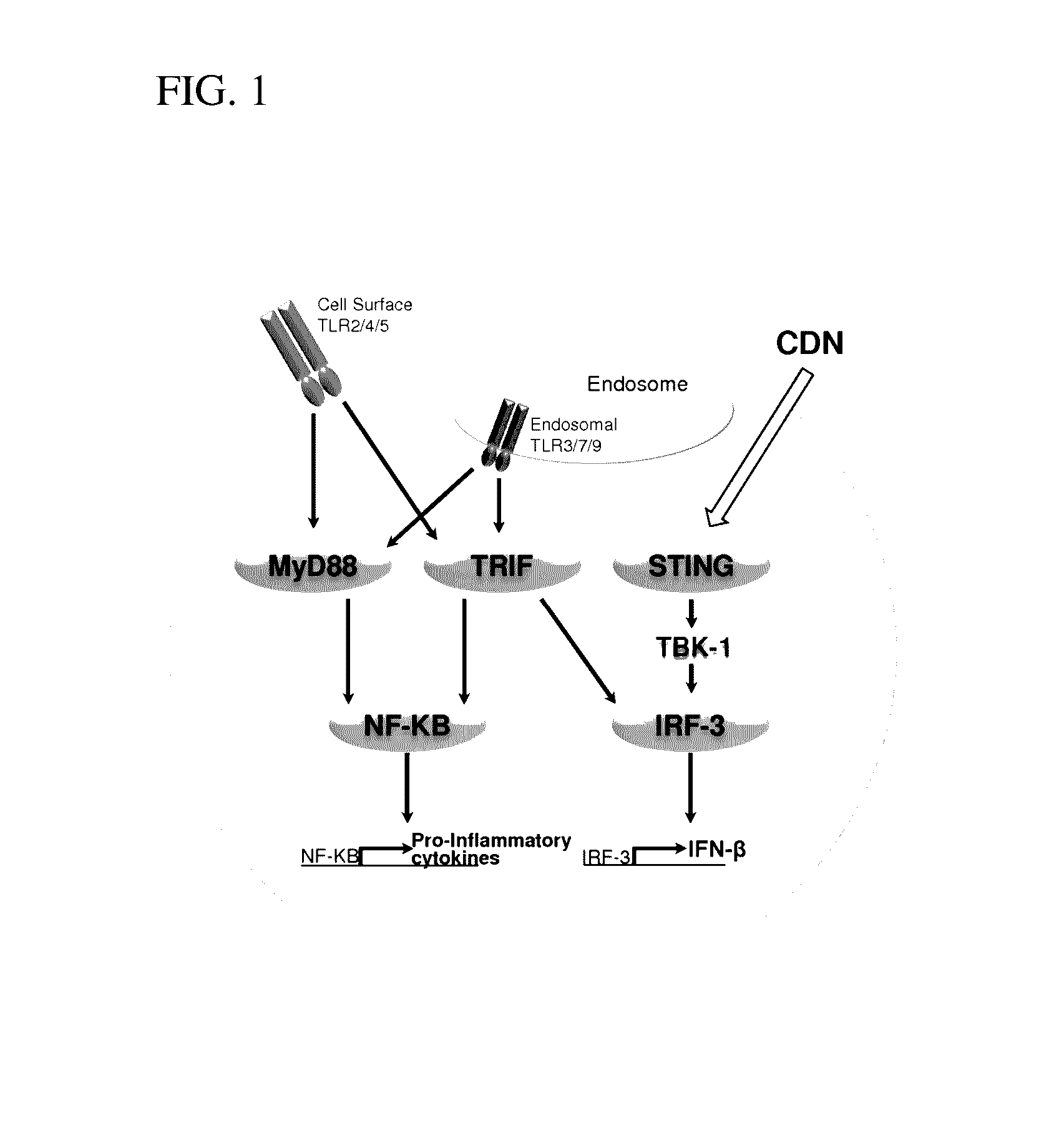
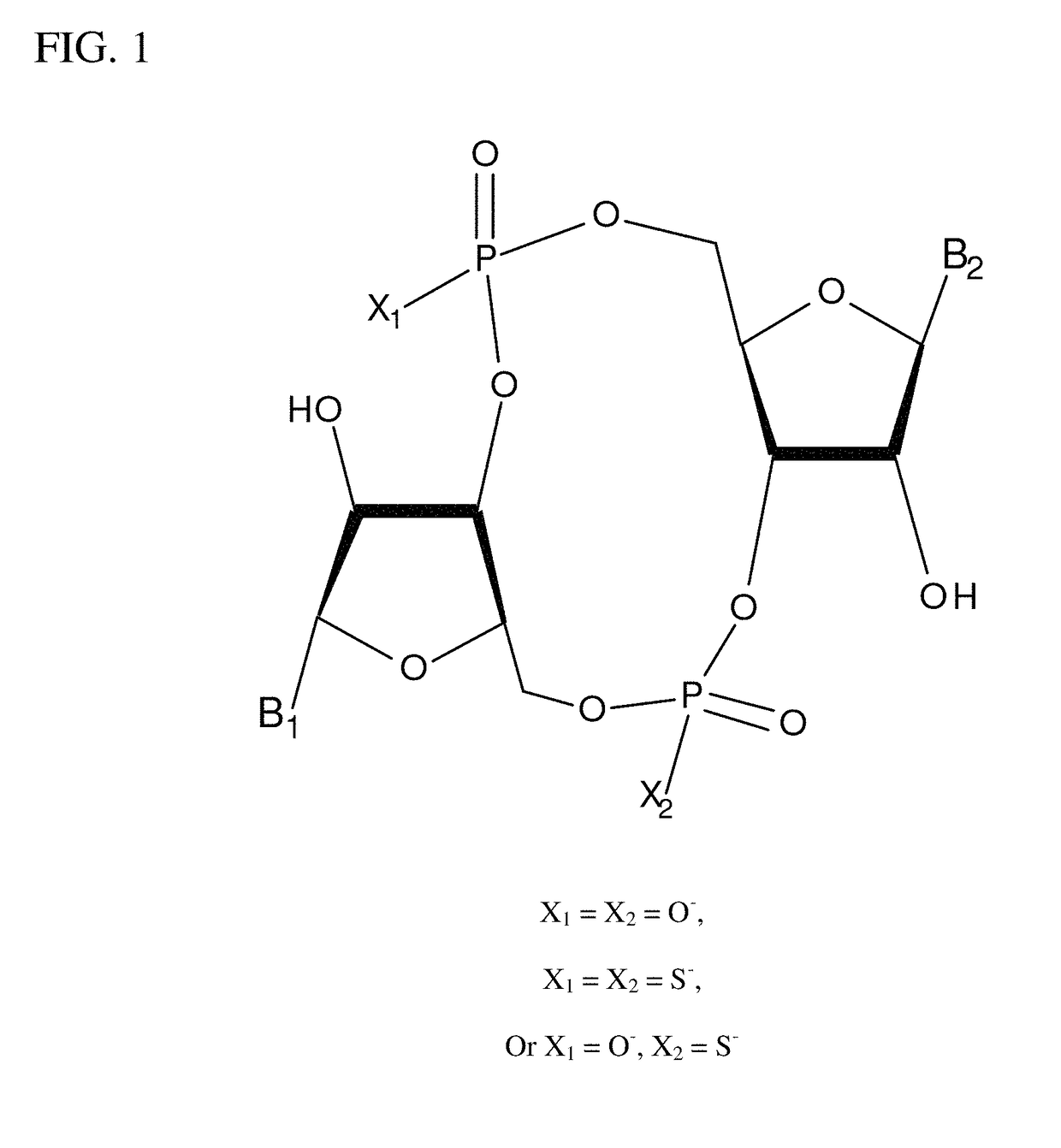
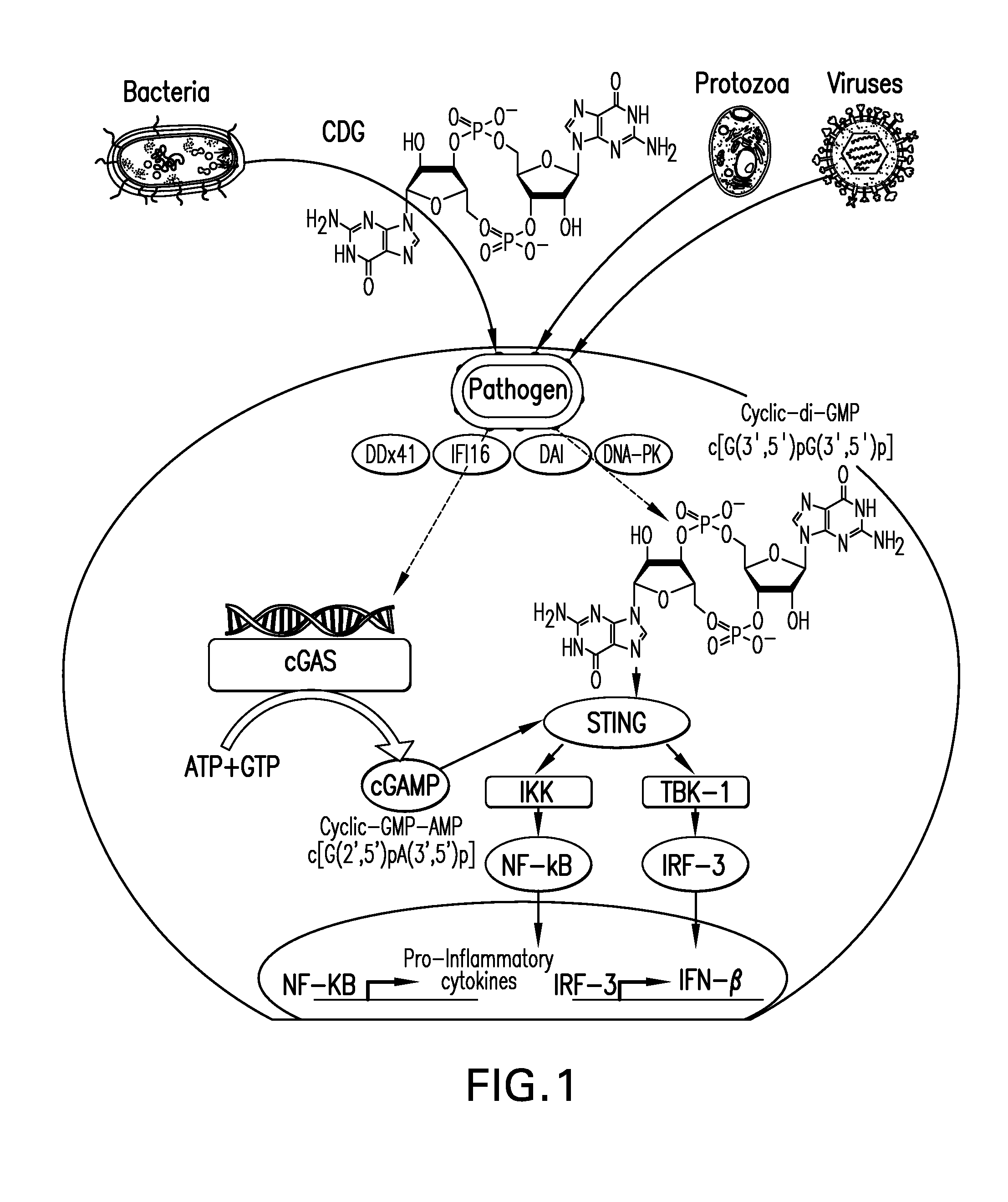
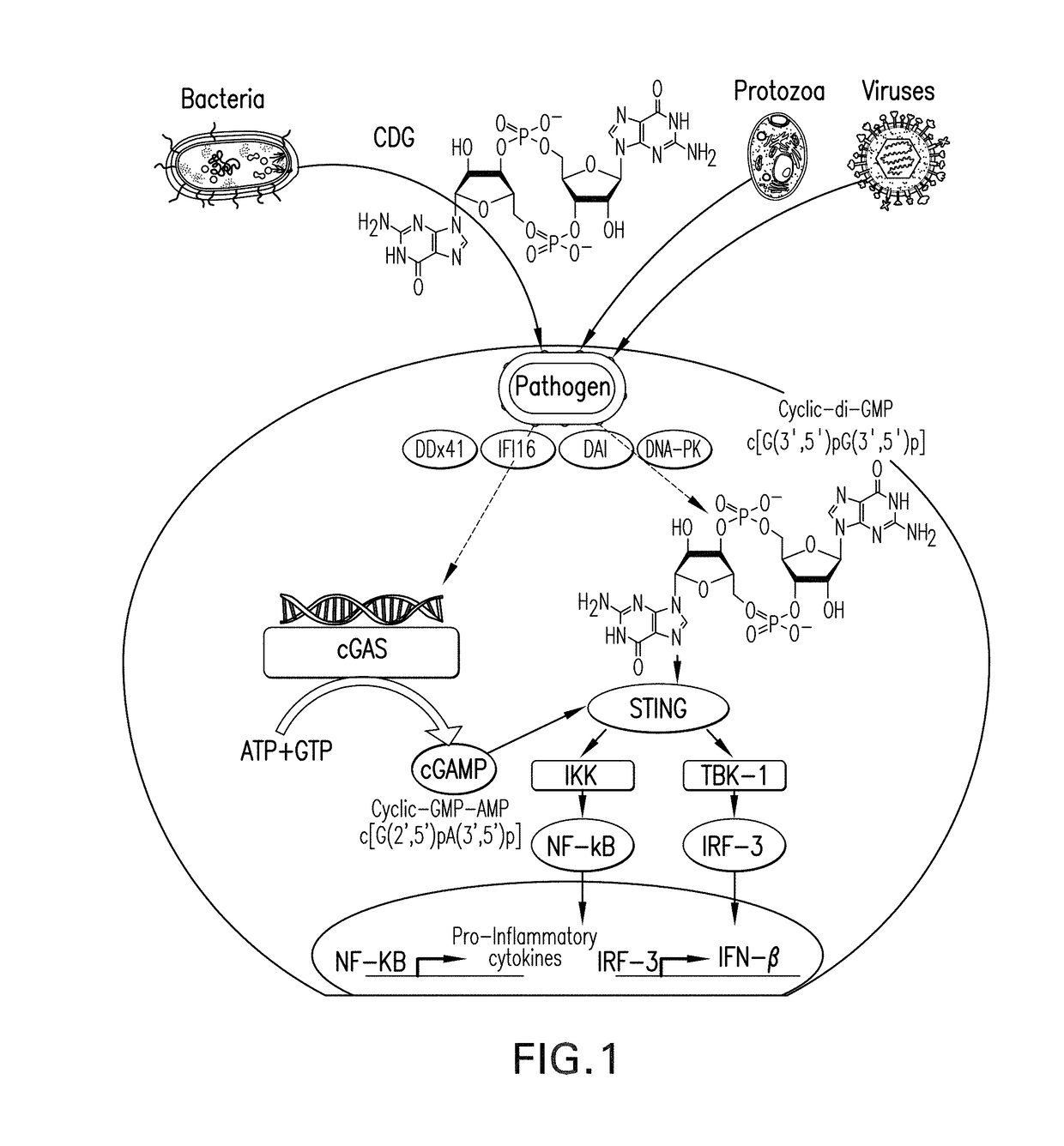
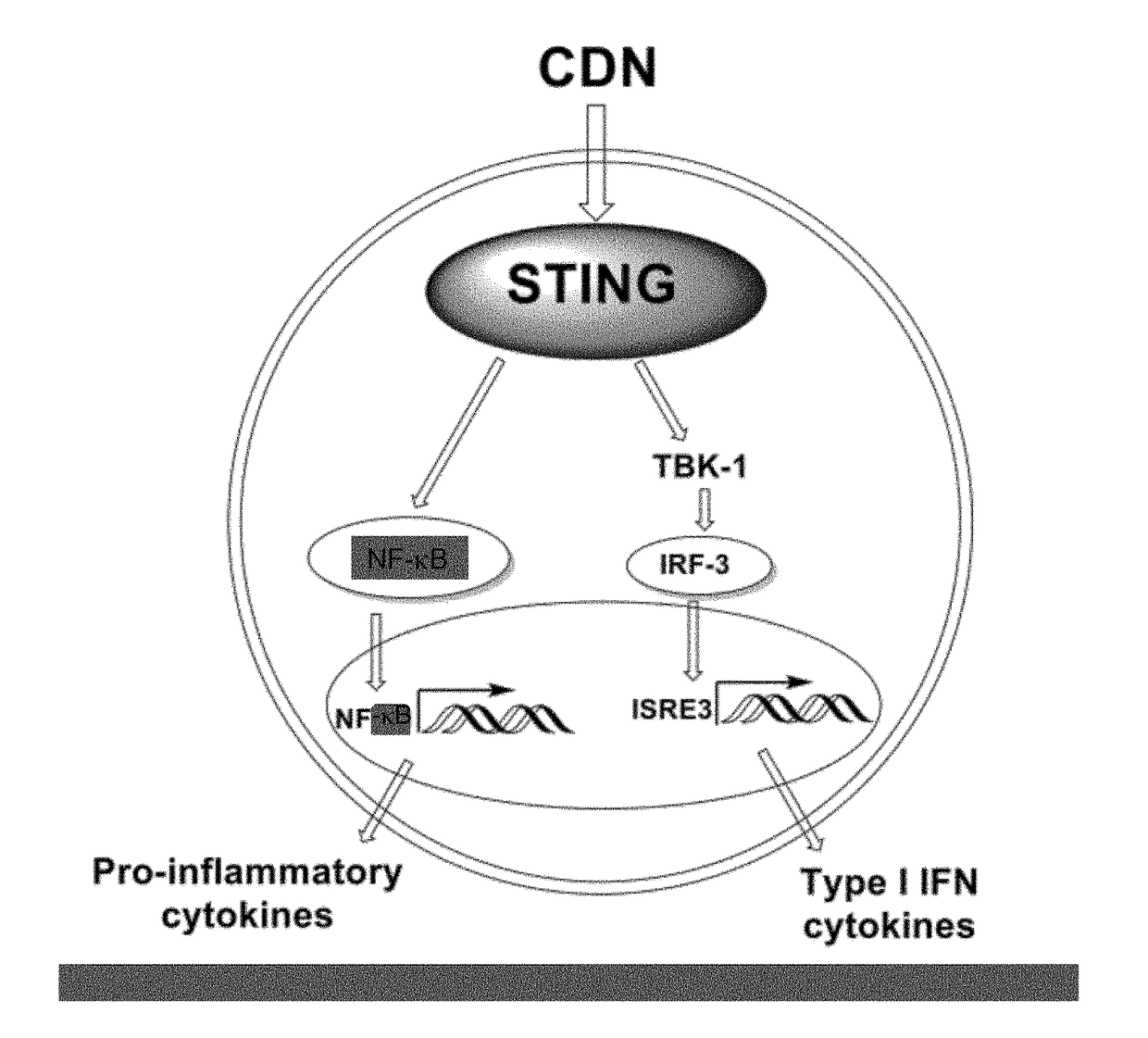
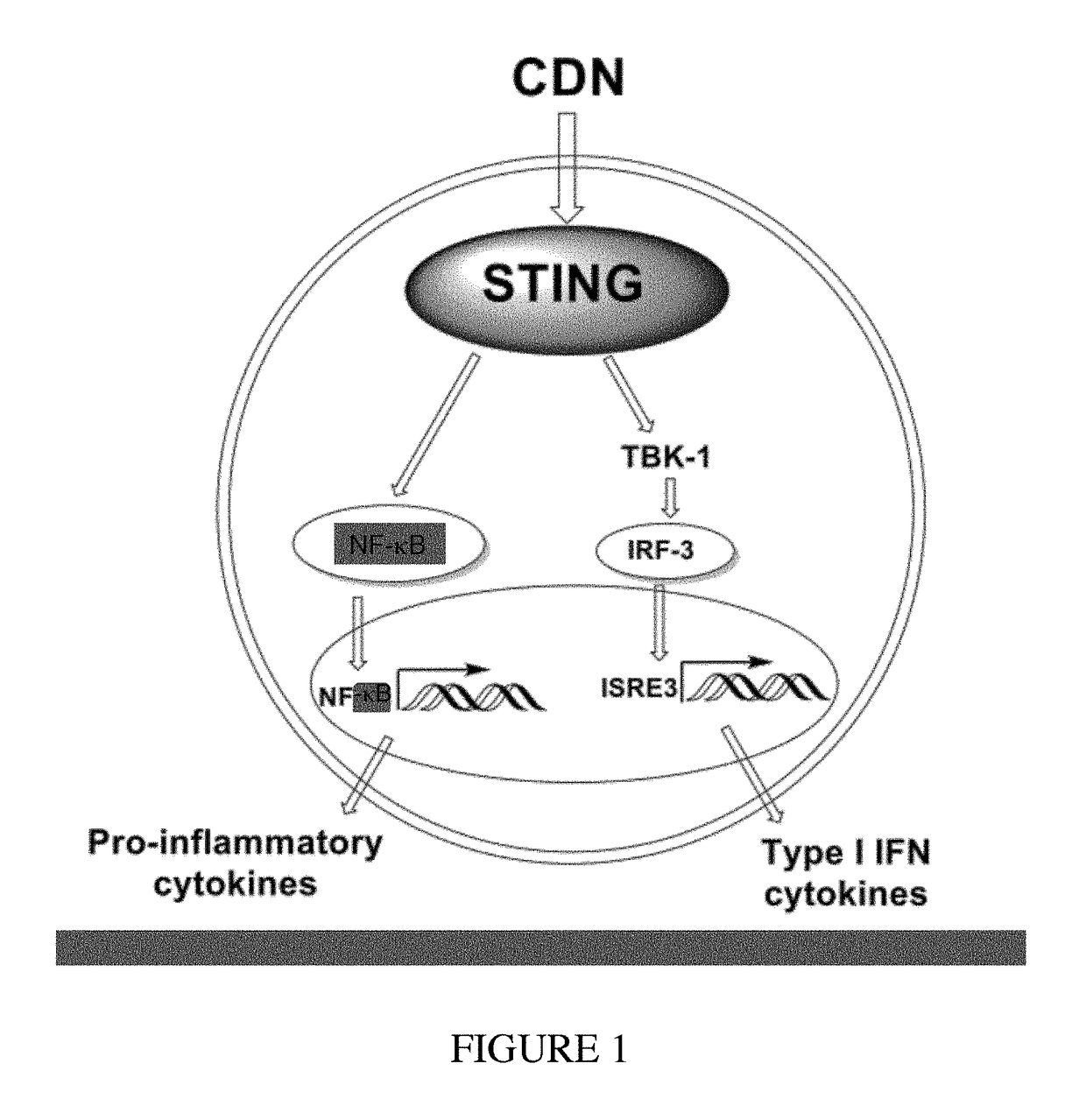
Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173) and MPYS/MITA/ERIS is a protein that in humans is encoded by the TMEM173 gene. STING plays an important role in innate immunity. STING induces type I interferon production when cells are infected with intracellular pathogens, such as viruses, mycobacteria and intracellular parasites. Type I interferon, mediated by STING, protects infected cells and nearby cells from local infection by binding to the same cell that secretes it (autocrine signaling) and nearby cells (paracrine signaling.)
Compositions and methods for activating stimulator of interferon gene-dependent signalling
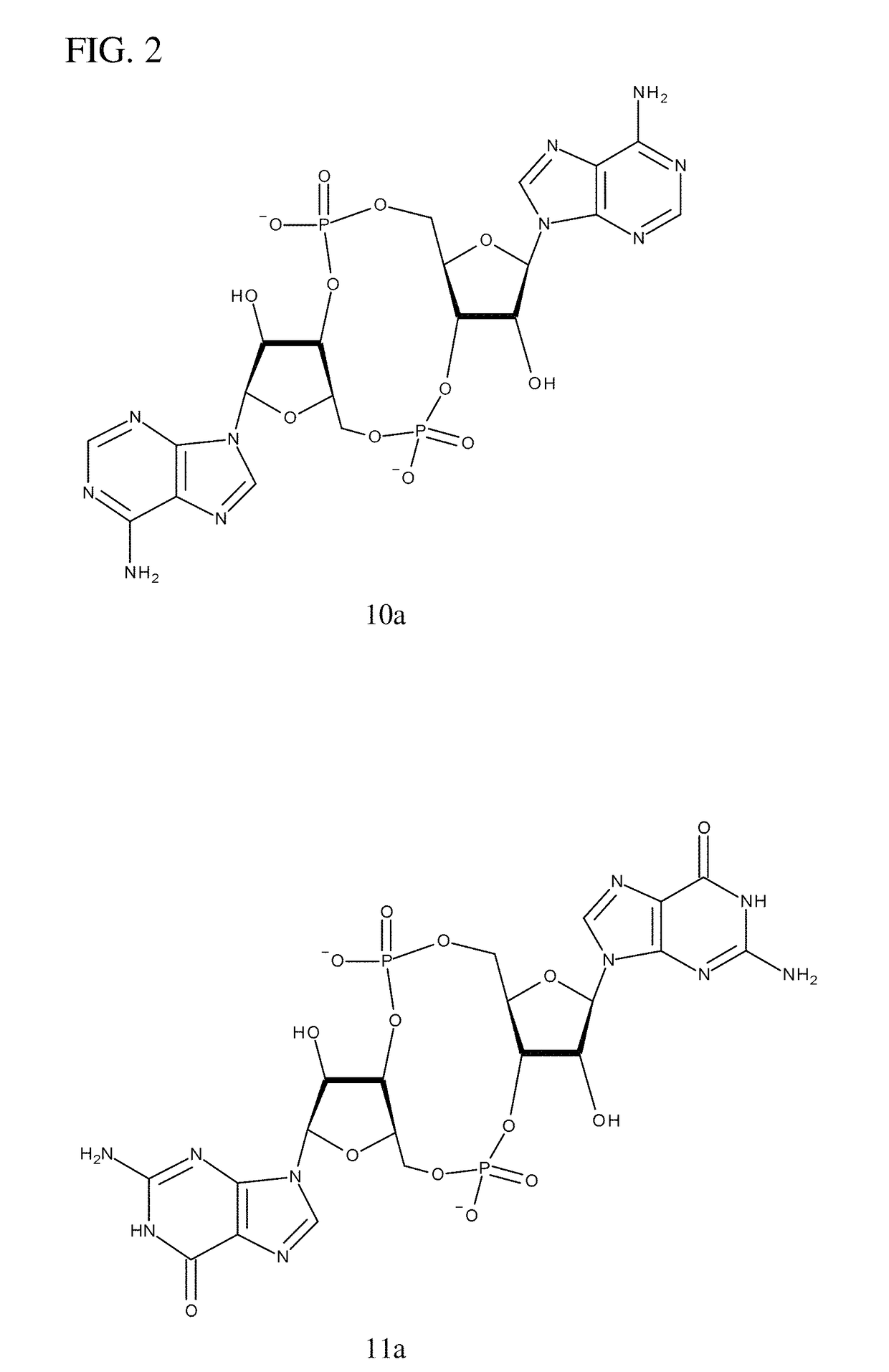
The present invention provides highly active cyclic-di-nucleotide (CDN) immune stimulators that activate DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides induce STING-dependent type I interferon production, wherein the cyclic purine dinucleotides present in the composition are substantially pure 2′,5′,2′,5′ and 2′,5′,3′,5′ CDNs, and preferably Rp,Rp stereosiomers thereof.
Owner:RGT UNIV OF CALIFORNIA +1
Compositions and methods for inhibiting "stimulator of interferon gene" -dependent signalling
ActiveUS20140341976A1Suppress overreactionOrganic active ingredientsSugar derivativesPurineStimulator of interferon genes
The present invention provides cyclic-di-nucleotide (CDN) compounds that inhibit signaling at a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides which inhibit STING-dependent TBK1 activation and the resulting production of type I interferon.
Owner:ADURO BIOTECH
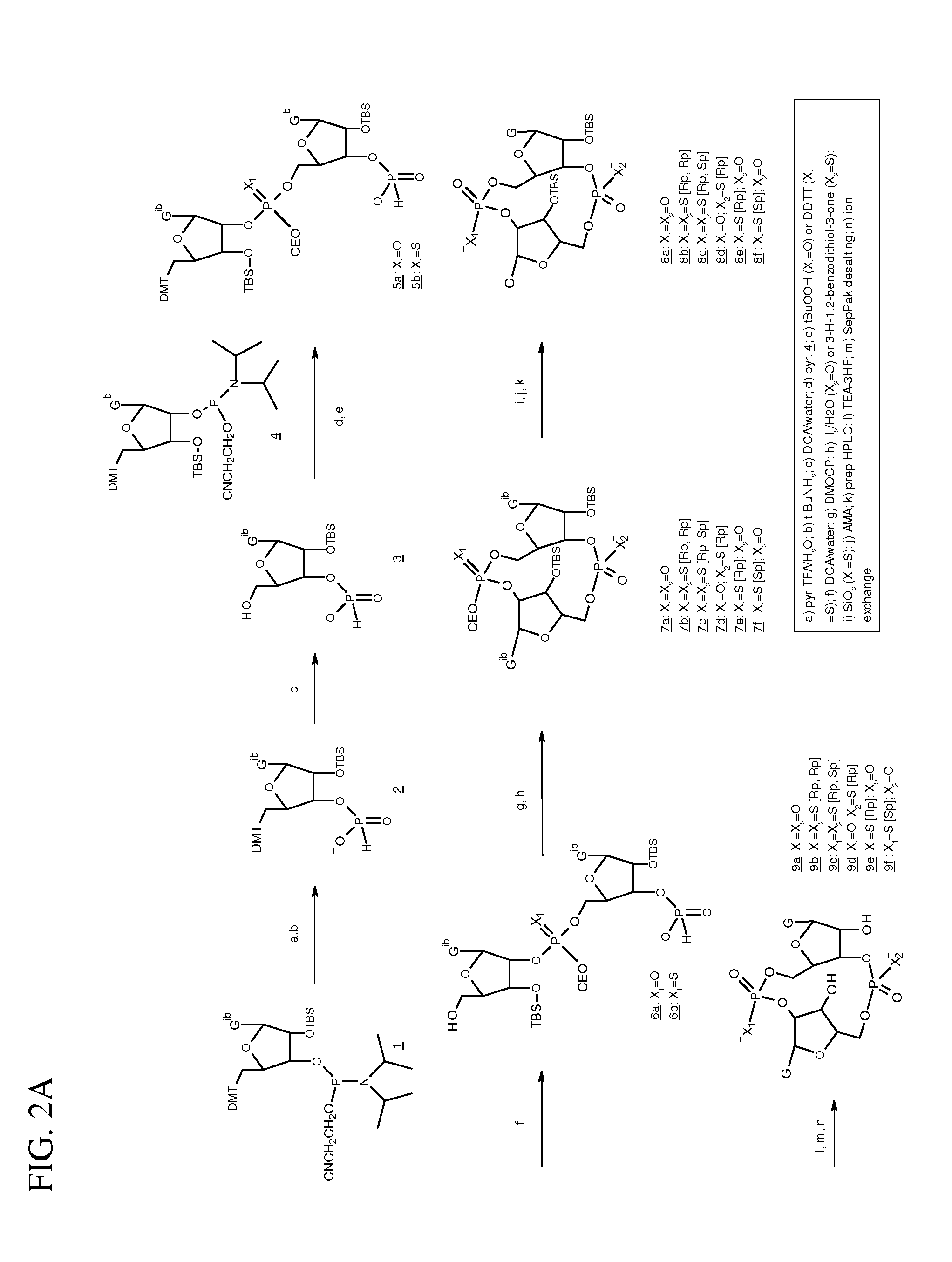
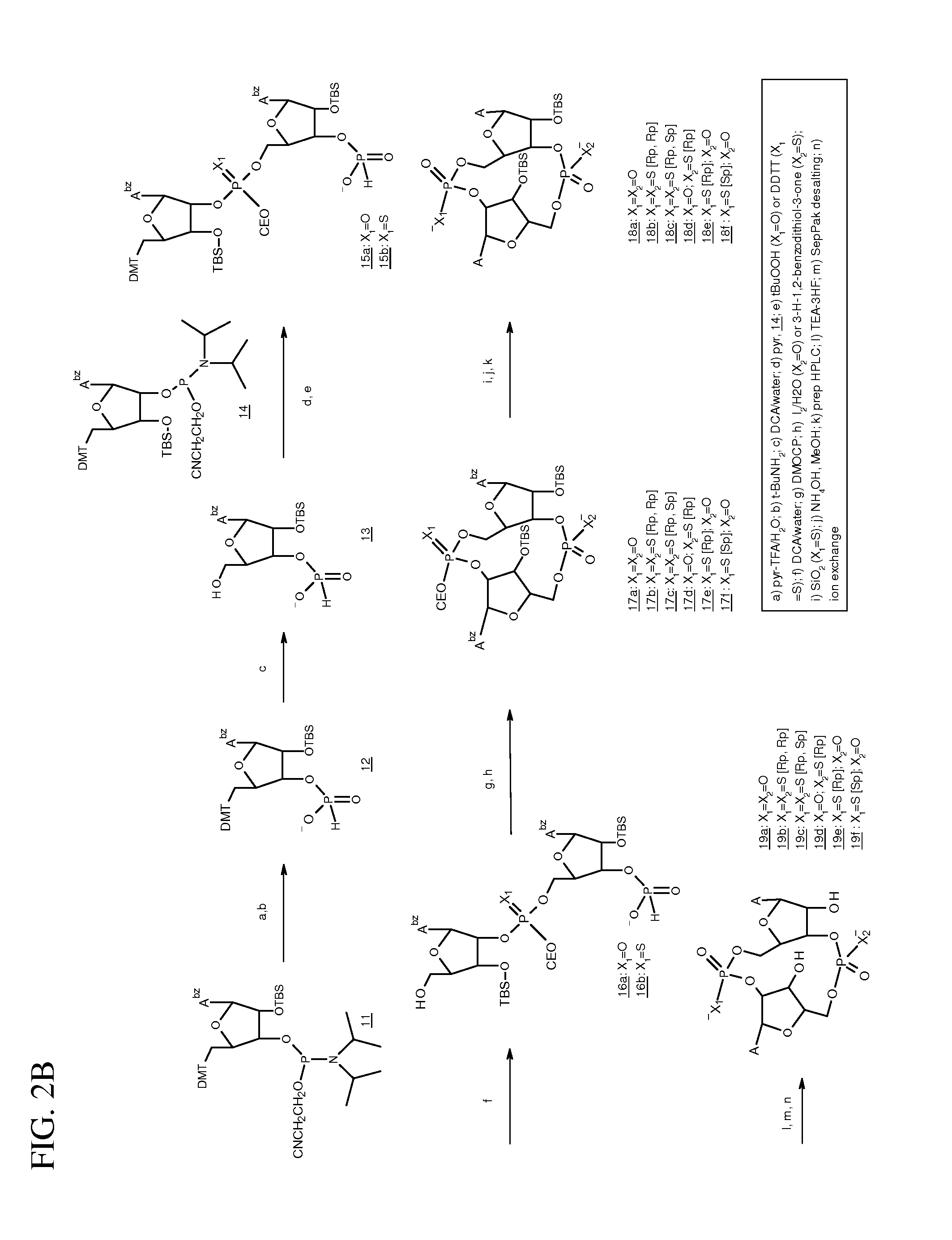
Compositions comprising cyclic purine dinucleotides having defined stereochemistries and methods for their preparation and use
ActiveUS20140205653A1Suppress overreactionOrganic active ingredientsBacterial antigen ingredientsEnantiomerPurine
It is an object of the present invention to provide novel and highly active cyclic-di-nucleotide (CDN) immune stimulators that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides that induce STING-dependent TBK1 activation, wherein the cyclic purine dinuclotides present in the composition are substantially pure Rp,Rp or Rp,Sp stereoisomers, and particularly substantially pure Rp,Rp, or RpSp CDN thiophosphate diastereomers.
Owner:CHINOOK THERAPEUTICS INC
Compositions and methods for inhibiting “stimulator of interferon gene”—dependent signalling
Owner:ADURO BIOTECH
Compositions and methods for activating stimulator of interferon gene-dependent signalling
The present invention provides highly active cyclic-di-nucleotide (CDN) immune stimulators that activate DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides induce STING-dependent type I interferon production, wherein the cyclic purine dinucleotides present in the composition are substantially pure 2′,5′,2′,5′ and 2′,5′,3′,5′ CDNs, and preferably Rp,Rp stereoisomers thereof.
Owner:RGT UNIV OF CALIFORNIA +1
Compositions comprising cyclic purine dinucleotides having defined stereochemistries and methods for their preparation and use
ActiveUS9695212B2Suppress overreactionOrganic active ingredientsBacterial antigen ingredientsEnantiomerPurine
It is an object of the present invention to provide novel and highly active cyclic-di-nucleotide (CDN) immune stimulators that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes). In particular, the CDNs of the present invention are provided in the form of a composition comprising one or more cyclic purine dinucleotides that induce STING-dependent TBK1 activation, wherein the cyclic purine dinuclotides present in the composition are substantially pure Rp,Rp or Rp,Sp stereoisomers, and particularly substantially pure Rp,Rp, or RpSp CDN thiophosphate diastereomers.
Owner:CHINOOK THERAPEUTICS INC
Compositions and methods for cancer immunotherapy
ActiveUS20150010613A1Improve propertiesHigh activityOrganic active ingredientsPeptide/protein ingredientsAntigenHuman tumor
The present invention provides a combination therapy which relies on a small molecule immune stimulator—cyclic-di-nucleotide (CDN)—that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes) formulated with allogeneic human tumor cell lines engineered to secrete high amounts of GM-CSF. This combination therapy can provide an ideal synergy of multiple tumor associated antigens, DC recruitment and proliferation, coupled with a potent DC activation stimulus.
Owner:ADURO BIOTECH +2
Compositions and methods for cancer immunotherapy
ActiveUS9770467B2Improve propertiesHigh activityPeptide/protein ingredientsViral antigen ingredientsAntigenHuman tumor
The present invention provides a combination therapy which relies on a small molecule immune stimulator—cyclic-di-nucleotide (CDN)—that activates DCs via a recently discovered cytoplasmic receptor known as STING (Stimulator of Interferon Genes) formulated with allogeneic human tumor cell lines engineered to secrete high amounts of GM-CSF. This combination therapy can provide an ideal synergy of multiple tumor associated antigens, DC recruitment and proliferation, coupled with a potent DC activation stimulus.
Owner:ADURO BIOTECH +2
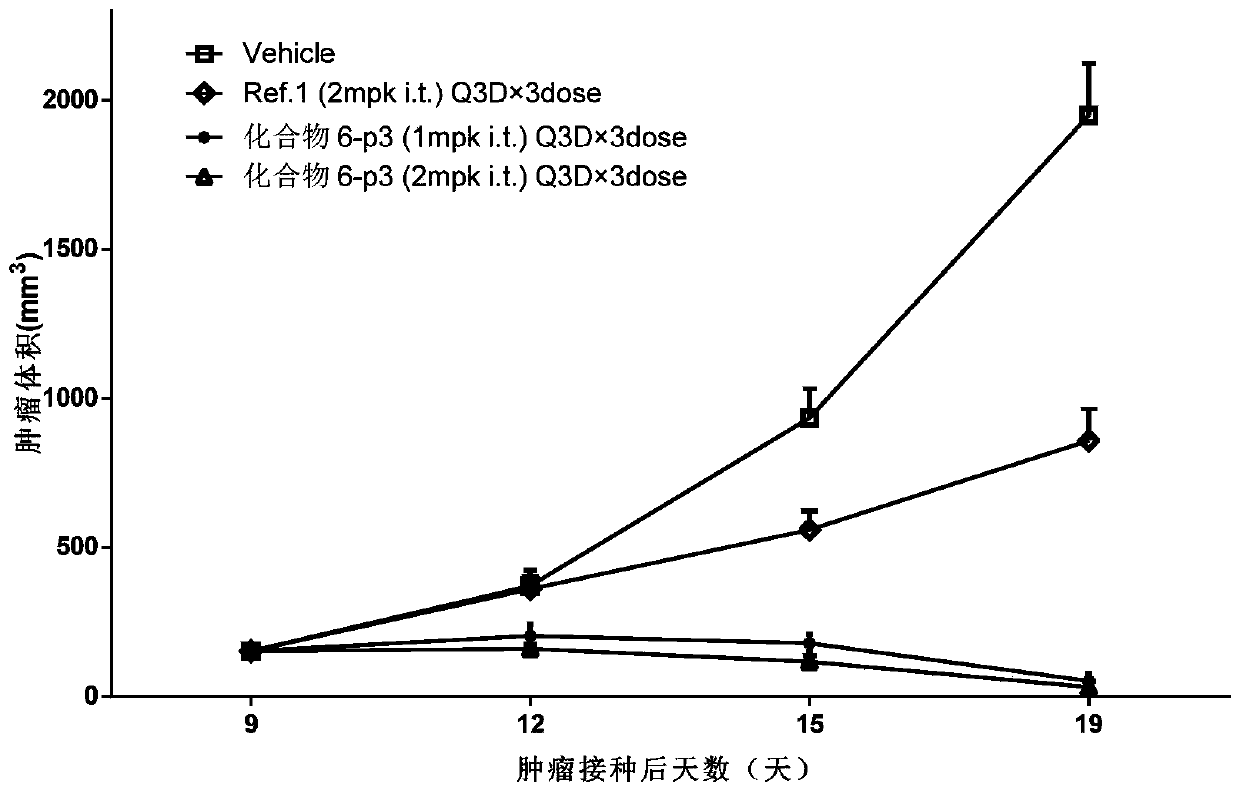
Use of sting agonist as cancer treatment
InactiveUS20160287623A1Sufficient effectOrganic active ingredientsAntineoplastic agentsStimulator of interferon genesAgonist
Methods and compositions for treating cancer by intratumorally administering a stimulator of interferon genes (STING) agonist are disclosed herein. In some embodiments, there are provided compositions and methods concerning methods for treating cancer in a subject comprising administering to the subject an effective amount of a stimulator of interferon genes (STING) agonist, wherein the STING agonist is administered intratumorally.
Owner:UNIVERSITY OF CHICAGO +1
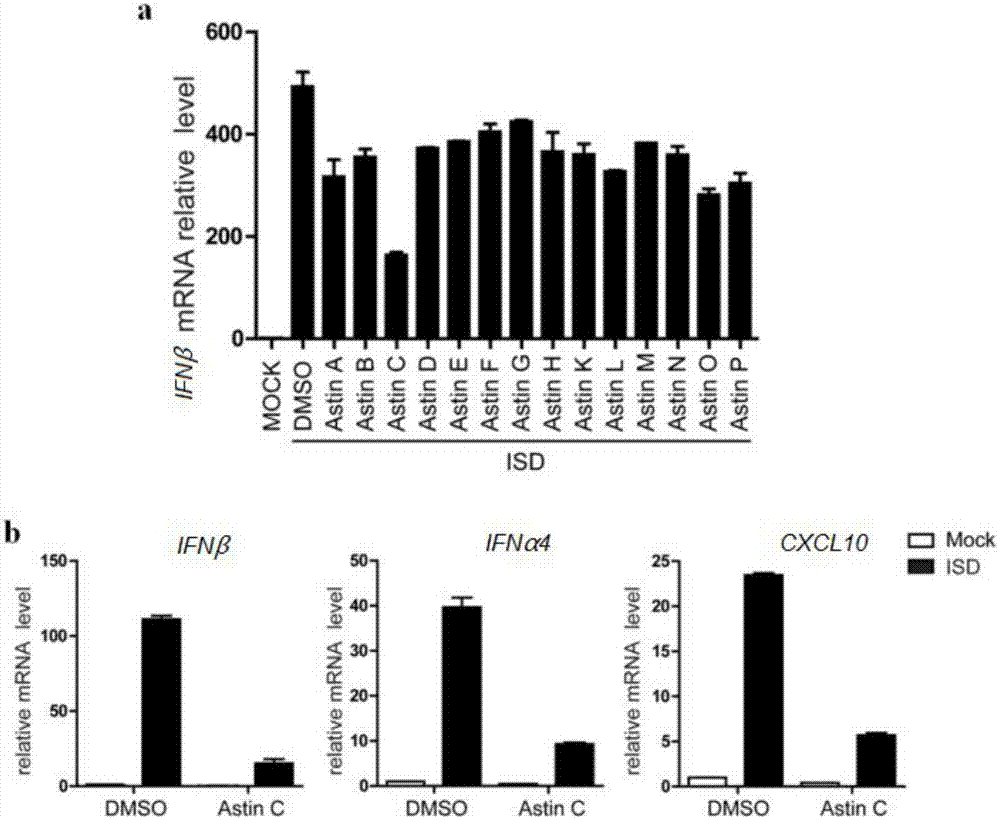
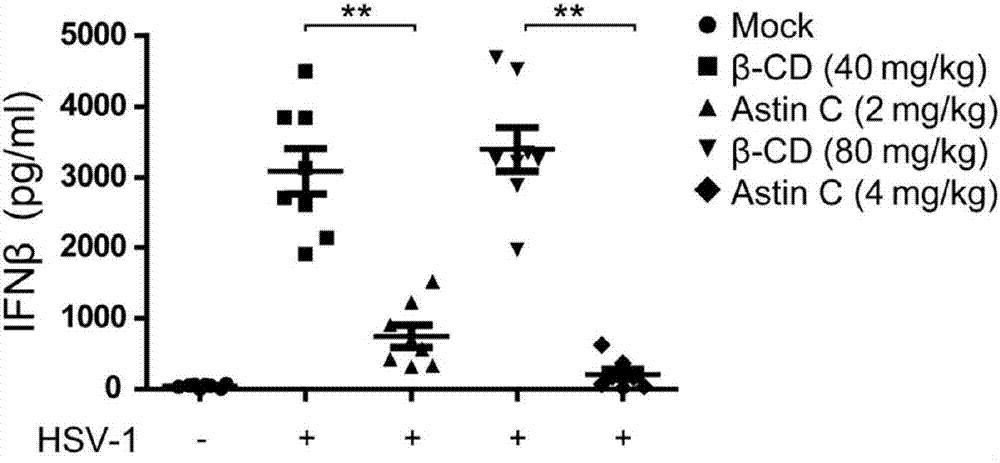
Application of Asteraceae cyclopeptides as cGAS-STING (stimulator of interferon genes) signal channel inhibitor
The invention discloses an application of Asteraceae cyclopeptides or pharmacologically admissible salts thereof as a cGAS-STING (stimulator of interferon genes) signal channel inhibitor as well as an application of the Asteraceae cyclopeptides or the pharmacologically admissible salts thereof as the cGAS-STING signal channel inhibitor to preparation of drugs for treating cGAS-STING signal channel abnormally activated diseases. The Asteraceae cyclopeptides are natural compounds, adopt diverse forms and administration ways and have extensive clinical application prospect.
Owner:CHINA PHARM UNIV
Use of sting agonist as cancer treatment
InactiveUS20180028553A1Organic active ingredientsAntineoplastic agentsAgonistStimulator of interferon genes
Owner:UNIVERSITY OF CHICAGO +1
Combined use of a chemotherapeutic agent and a cyclic dinucleotide for cancer treatment
InactiveUS20170340658A1Good treatment effectEffective treatmentOrganic active ingredientsAntineoplastic agentsCombined useAgonist
A kit of parts includes a) gemcitabine or a pharmaceutically acceptable salt thereof and b) a cyclic dinucleotide or pharmaceutically acceptable salt thereof, wherein the cyclic dinucleotide or pharmaceutically acceptable salt thereof is an agonist of the receptor known as “stimulator of interferon genes” (STING), for use in the treatment of solid pancreatic cancer.
Owner:INVIVOGEN
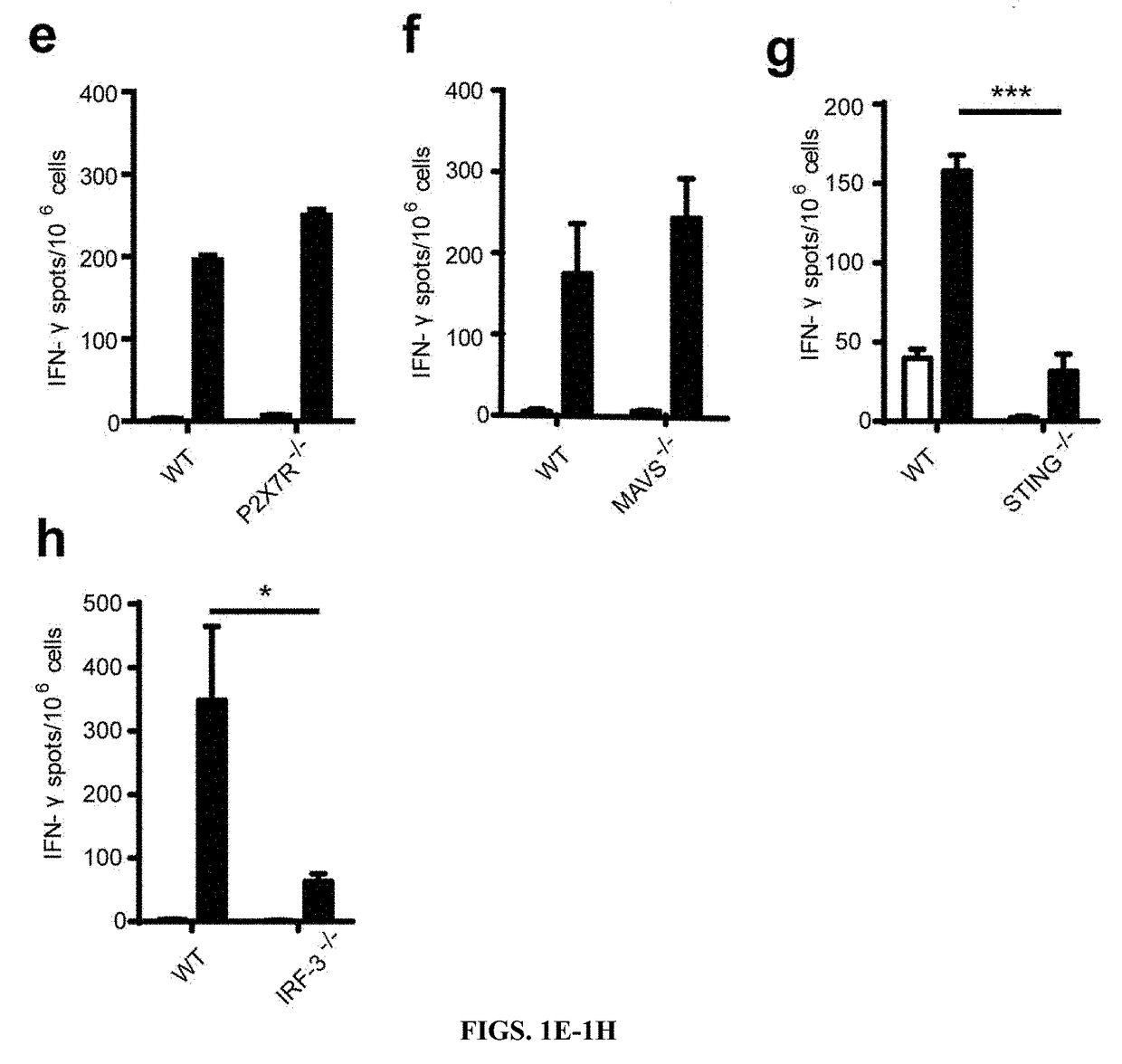
Methods for identifying inhibitors of "stimulator of interferon gene"- dependent interferon production
InactiveUS20180369268A1Suppress overreactionReduced activityOrganic active ingredientsOrganic chemistryDiseaseNucleotide
The present invention relates to the use cyclic-di-nucleotide and related scaffold molecules that measurably inhibit STING signaling, and methods for their use in identifying more potent inhibitors of STING signaling. In particular, the methods provided can be used to identify potent inhibitors of STING signaling, which are useful in the treatment of autoimmune and inflammatory diseases. Also provided are compounds having STING inhibitory activity useful in the treatment of autoimmune and inflammatory diseases.
Owner:ADURO BIOTECH
Cyclic dinucleotides containing benzimidazole, method for the production of same, and use of same to activate stimulator of interferon genes (sting)-dependent signaling pathways
ActiveUS11001605B2Good membrane permeabilityImprove biological activityOrganic active ingredientsSugar derivativesMembrane permeabilizationNucleotide
Cyclic dinucleotides are described, which in contrast to their natural congeners carry lipophilic nucleobases and have higher membrane permeability and increased biological activity.
Owner:BIOLOG LSI FORSCHUNGS & BET GMBH
Combinations of pd-1 antagonists and cyclic dinucleotide sting agonists for cancer treatment
InactiveUS20190328762A1Organic active ingredientsSugar derivativesStimulator of interferon genesAgonist
Therapeutic combinations that comprise at least one antagonist of the Programmed Death 1 receptor (PD-1) and at least one cyclic dinucleotide compound that activates the Stimulator of Interferon Genes (STING) pathway are disclosed herein. Also disclosed is the use of such therapeutic combinations for the treatment of cancers.
Owner:MERCK SHARP & DOHME CORP
Immunostimulatory bacteria engineered to colonize tumors, tumor-resident immune cells, and the tumor microenvironment
PendingCN113748124AAvoid lossImprove anti-tumor efficacyBacteriaPeptide/protein ingredientsAdenosinePurine
Provided are delivery immunostimulatory bacteria that have enhanced colonization of tumors, the tumor microenvironment and / or tumor-resident immune cells, and enhanced anti-tumor activity. The immunostimulatory bacteria are modified by deletion of genes encoding the flagella or by modification of the genes so that functional flagella are not produced, and / or are modified by deletion of pagP or modification of pagP to produce inactive PagP product. As a result, the immunostimulatory bacteria are flagellin" and / or pagP'. The immunostimulatory bacteria optionally have additional genomic modifications so that the bacteria are adenosine and / or purine auxotrophs. The bacteria optionally are one or more of asct, purl' and msbB'. The immunostimulatory bacteria, such as Salmonella species, are modified to encode proteins that induce type I interferon (IFN) expression, or that are variants thereof that have increased activity to induce type I IFN expression, or that are variants thereof that result in constitutive expression of type I IFN. The bacteria can encode a modified Stimulator of Interferon Genes (STING) protein from a non-human species, that has lower NF-kappaB signaling activity, and, optionally, higher type I IFN pathway signaling activity, compared to human STING. The bacteria preferentially infect immune cells in the tumor microenvironment, or tumor-resident immune cells, and / or induce less cell death in immune cells than in other cells. Also provided are methods of inhibiting the growth or reducing the volume of a solid tumor by administering the immunostimulatory bacteria.
Owner:阿克蒂姆治疗有限公司
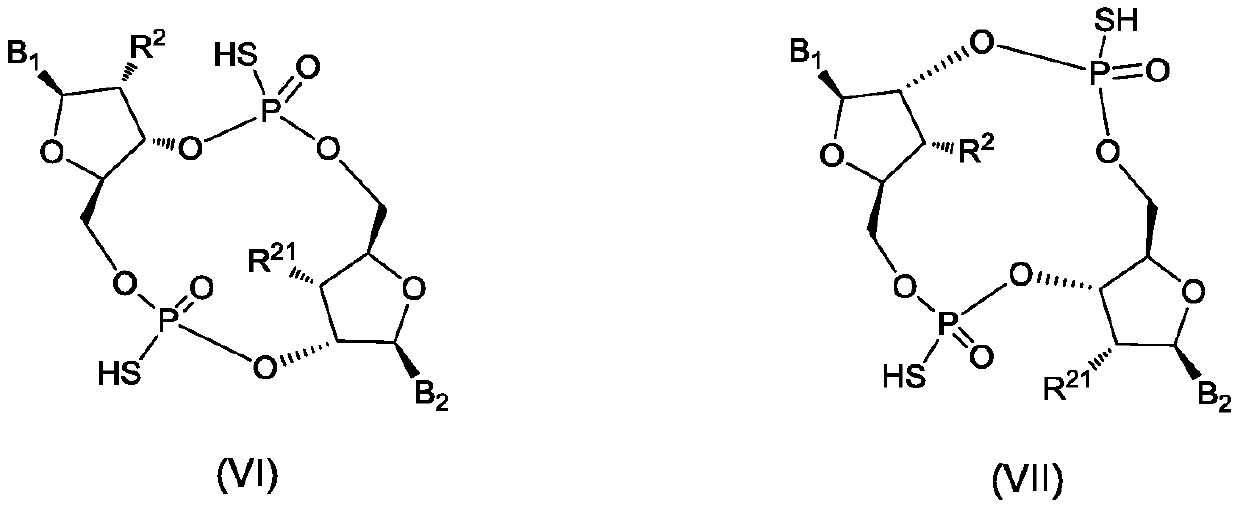
Cyclic dinucleotide analogues, pharmaceutical composition of analogues and applications of analogues and pharmaceutical composition
The invention discloses cyclic dinucleotide analogues, a pharmaceutical composition of the analogues and applications of the analogues and the pharmaceutical composition. The cyclic dinucleotide analogs (I), an isomer, prodrug, stable isotope derivative or pharmaceutically acceptable salt of the analogs have a structure shown in the specification. The cyclic dinucleotide analogs provided by the invention can be used as regulators of stimulator of interferon genes (STIG) and related signaling pathways, and can effectively treat and / or alleviate multiple types of diseases, including but not limited to malignant tumors, inflammation, autoimmune diseases and infectious diseases; and in addition, the STING regulators can also be used as vaccine adjuvants.
Owner:SHANGHAI DE NOVO PHARMA
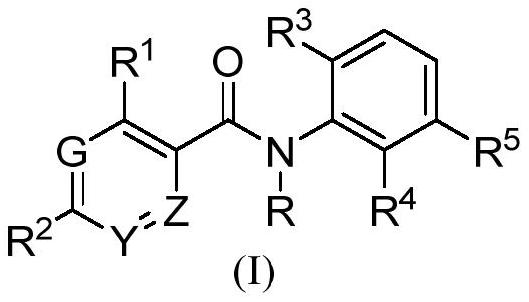
Benzamide derivatives as cgas-sting pathway agonists
Pharmaceutical compositions of the invention comprise functionalized benzamide derivatives useful as cyclic GMP-AMP synthase- Stimulator of interferon gene (cGAS-STING) pathway agonists, and useful for treating viral diseases and boost antitumor immunity.
Owner:BARUCH S BLUMBERG INST
Detection method, primer pair and kit for chicken-original STING (stimulator of interferon genes) fluorogenic quantitative PCR (polymerase chain reaction)
InactiveCN105483277AQuick checkQuantitatively accurateMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyStimulator of interferon genes
The invention discloses a pair of specificity real-time fluorogenic quantitative PCR (polymerase chain reaction) primers, namely an upstream primer S1-F and a downstream primer S1-R. Accordingly, the inventor designs and sets up a corresponding kit and a corresponding detection method. It is shown through experiments that the detection method, the primer pair and the kit have the advantages of being high in detection speed, accurate in quantification, high in specificity and sensitivity, good in repeatability and stability and free of pollution. A technological platform is provided for fast and accurately detecting the relative expression level of chicken-original STING (stimulator of interferon genes) mRNA, and a technological base is provided for further inquiring an innate immunity mechanism of chicken-related diseases.
Owner:GUANGXI UNIV
Stimulator of interferon genes (STING) ligands and uses thereof
ActiveUS20210299250A1Enhanced antigen-specific antibody productionEnhances B cell immunitySsRNA viruses negative-senseOrganic active ingredientsStimulator of interferon genesTGE VACCINE
Provided herein are Stimulator of Interferon Genes (STING) ligand for use in enhancing immune response and / or as adjuvants in vaccines. In some embodiments, STING ligand is used alone or in combination with Alum in an adjuventation system for early life immunization.
Owner:CHILDRENS MEDICAL CENT CORP
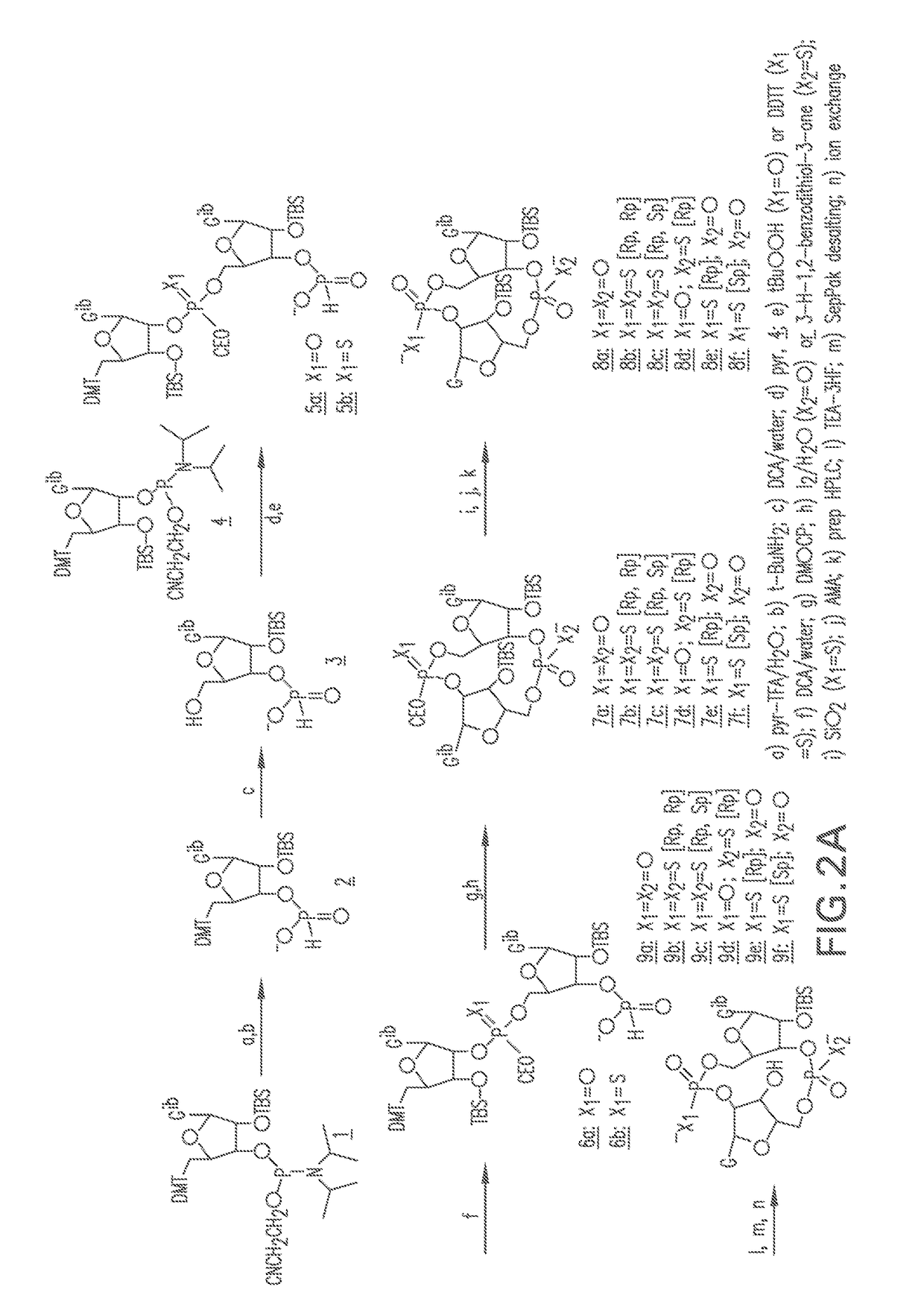
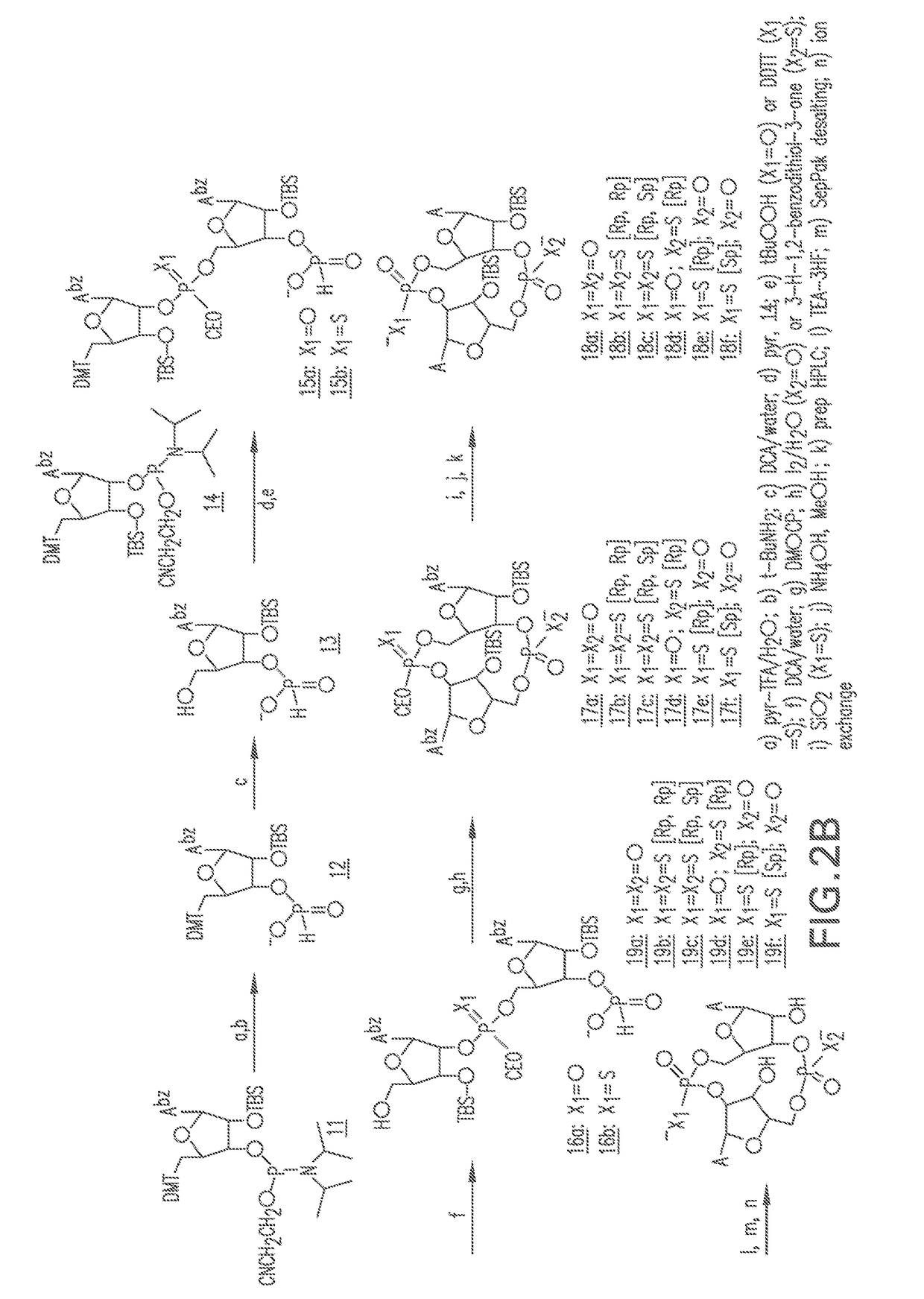
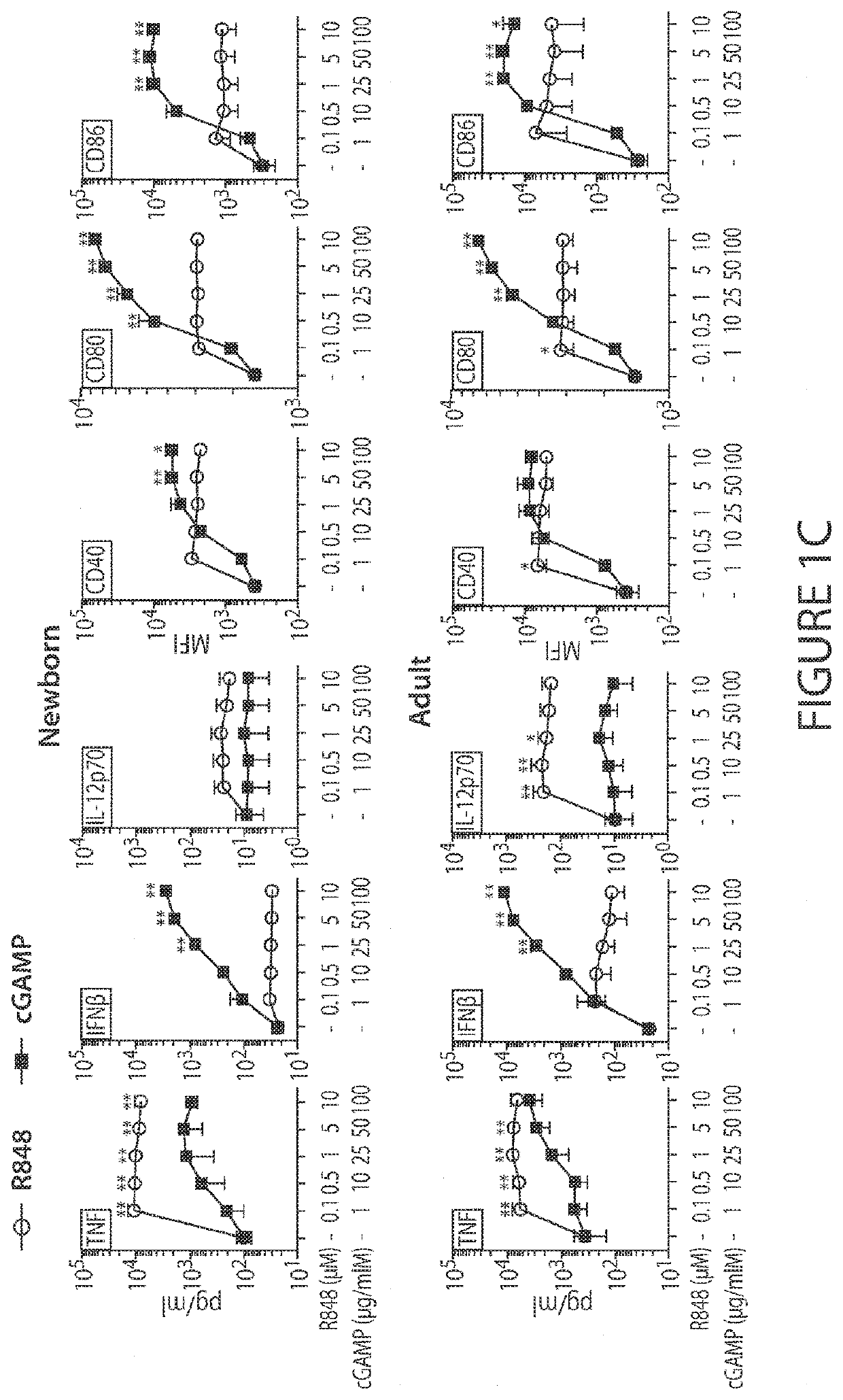
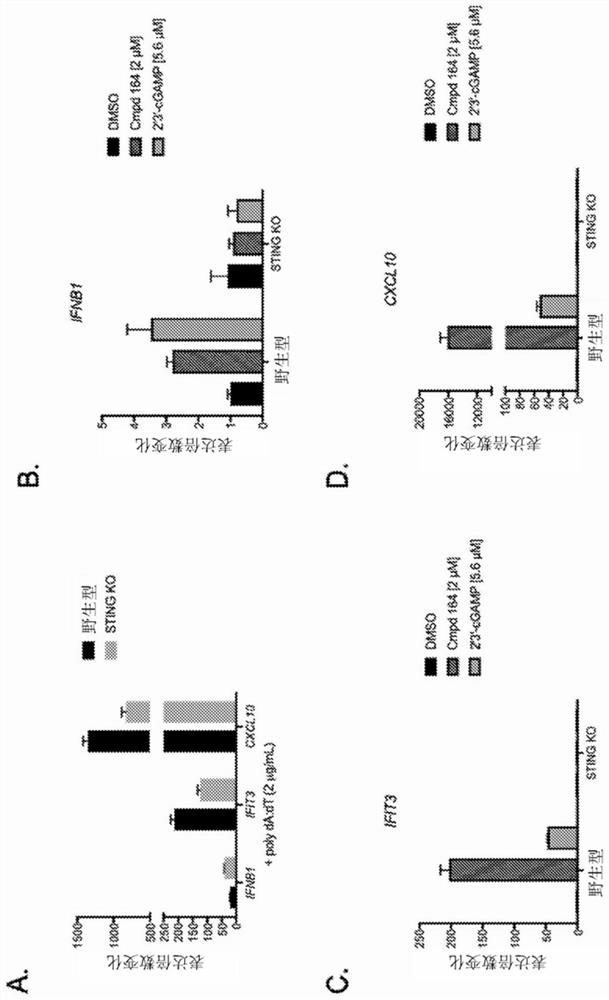
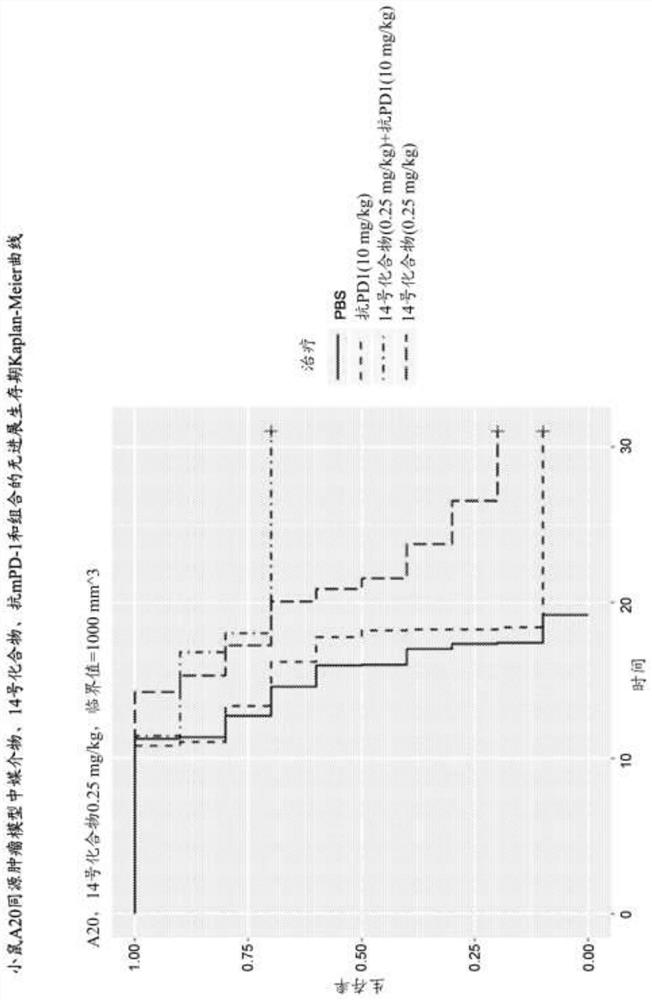
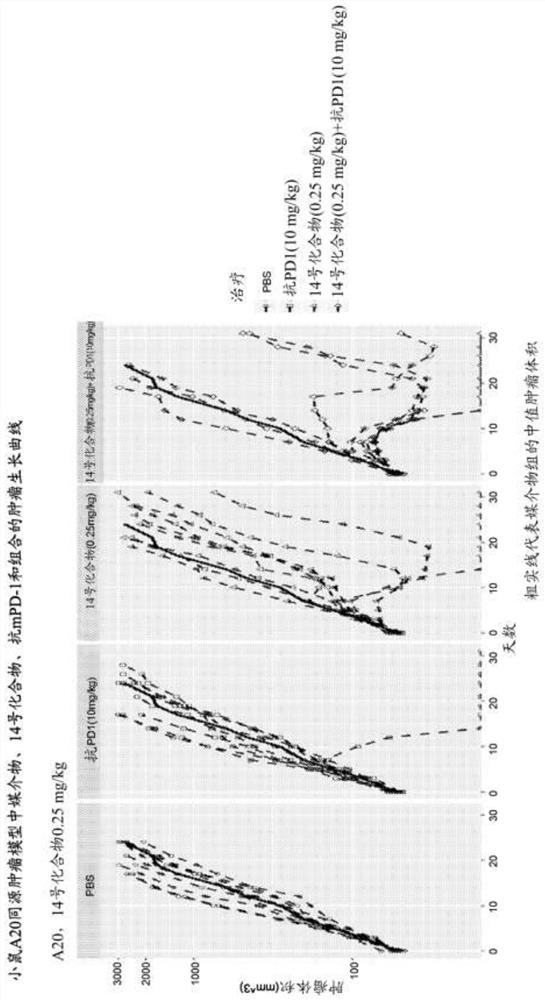
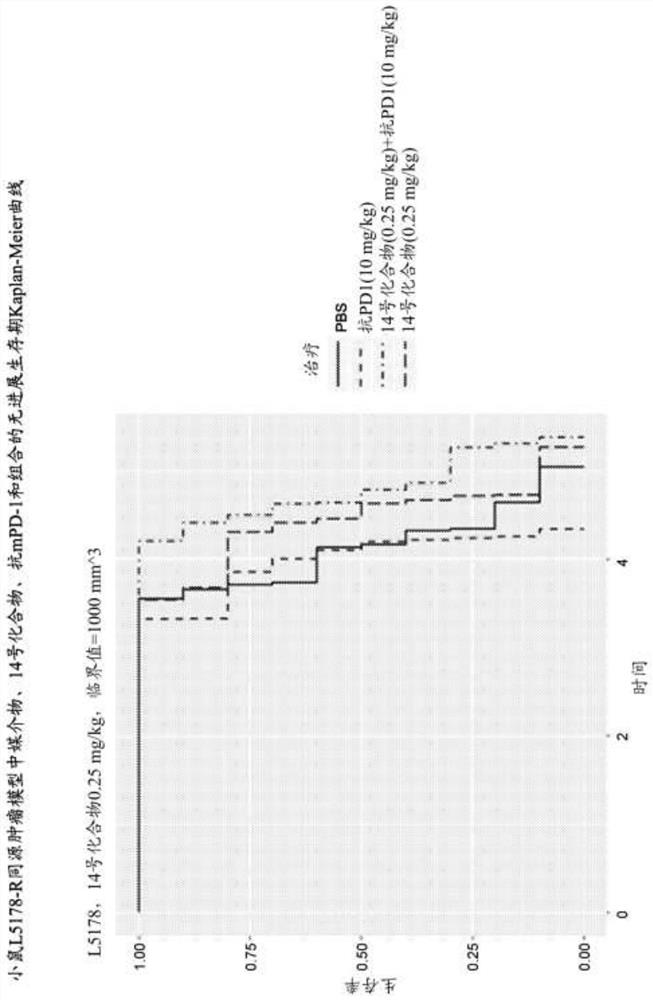
Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment
ActiveUS20210130466A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDeath ReceptorsStimulator of interferon genes
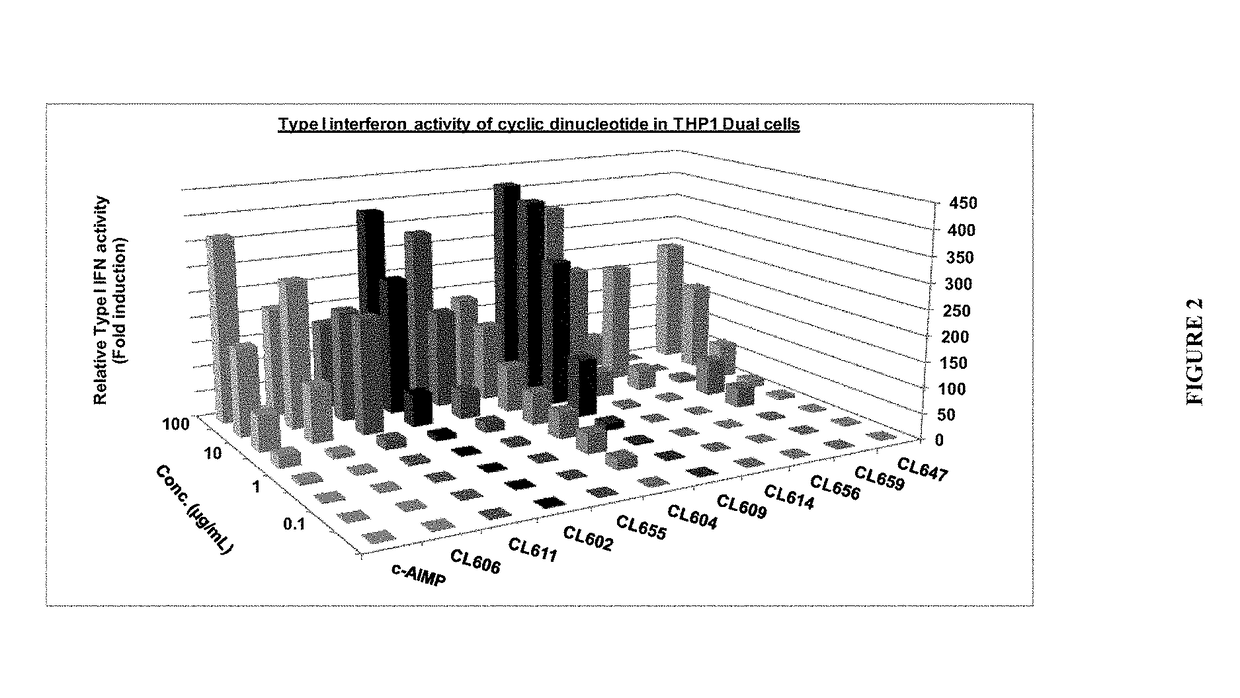
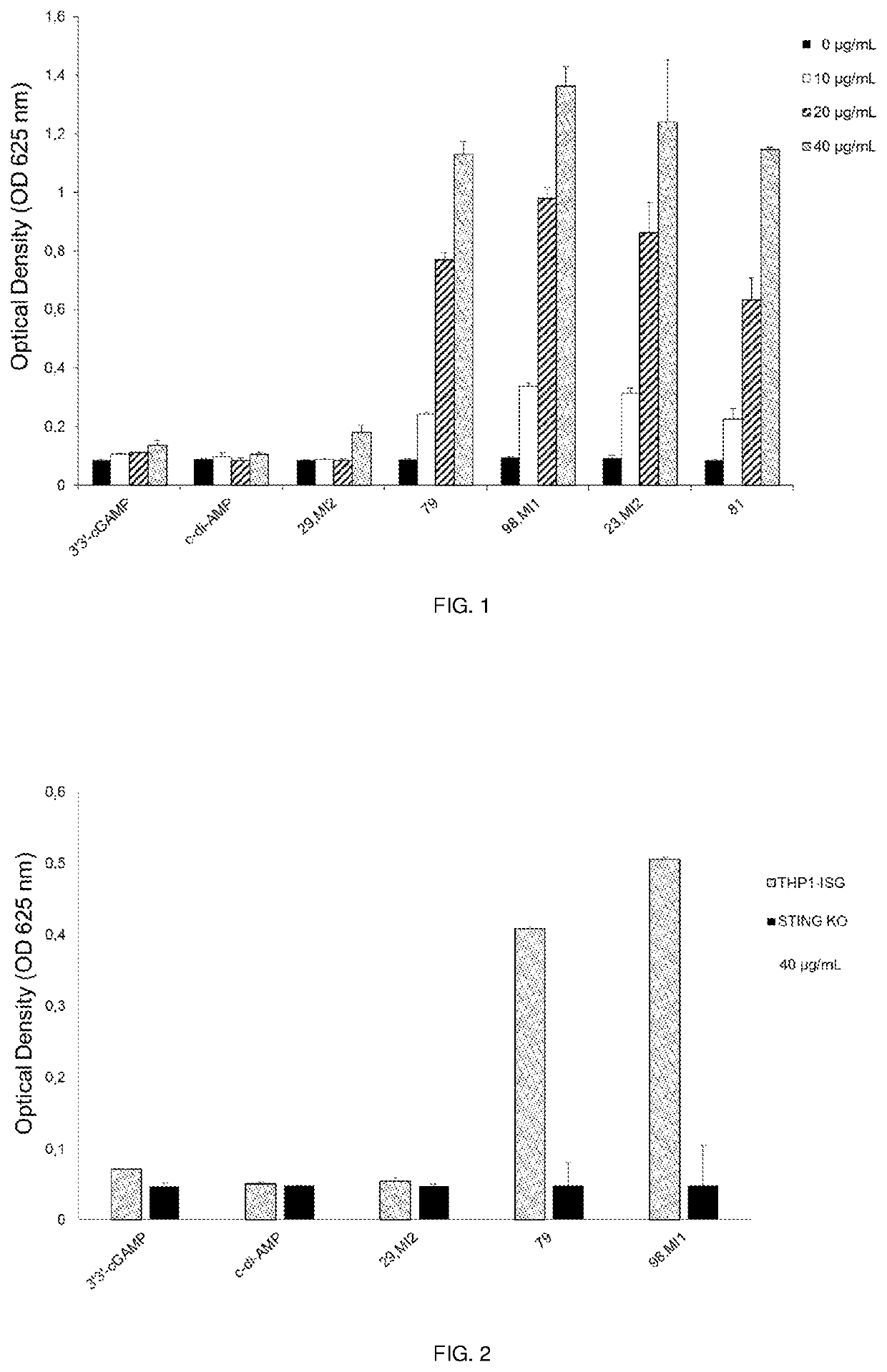
Therapeutic combinations that comprise at least one antagonist of the Programmed Death 1 receptor (PD-1) and at least one benzo[b]thiophene compound that activates the Stimulator of Interferon Genes (STING) pathway are disclosed herein. Also disclosed is the use of such therapeutic combinations for the treatment of cancers.
Owner:MERCK SHARP & DOHME LLC
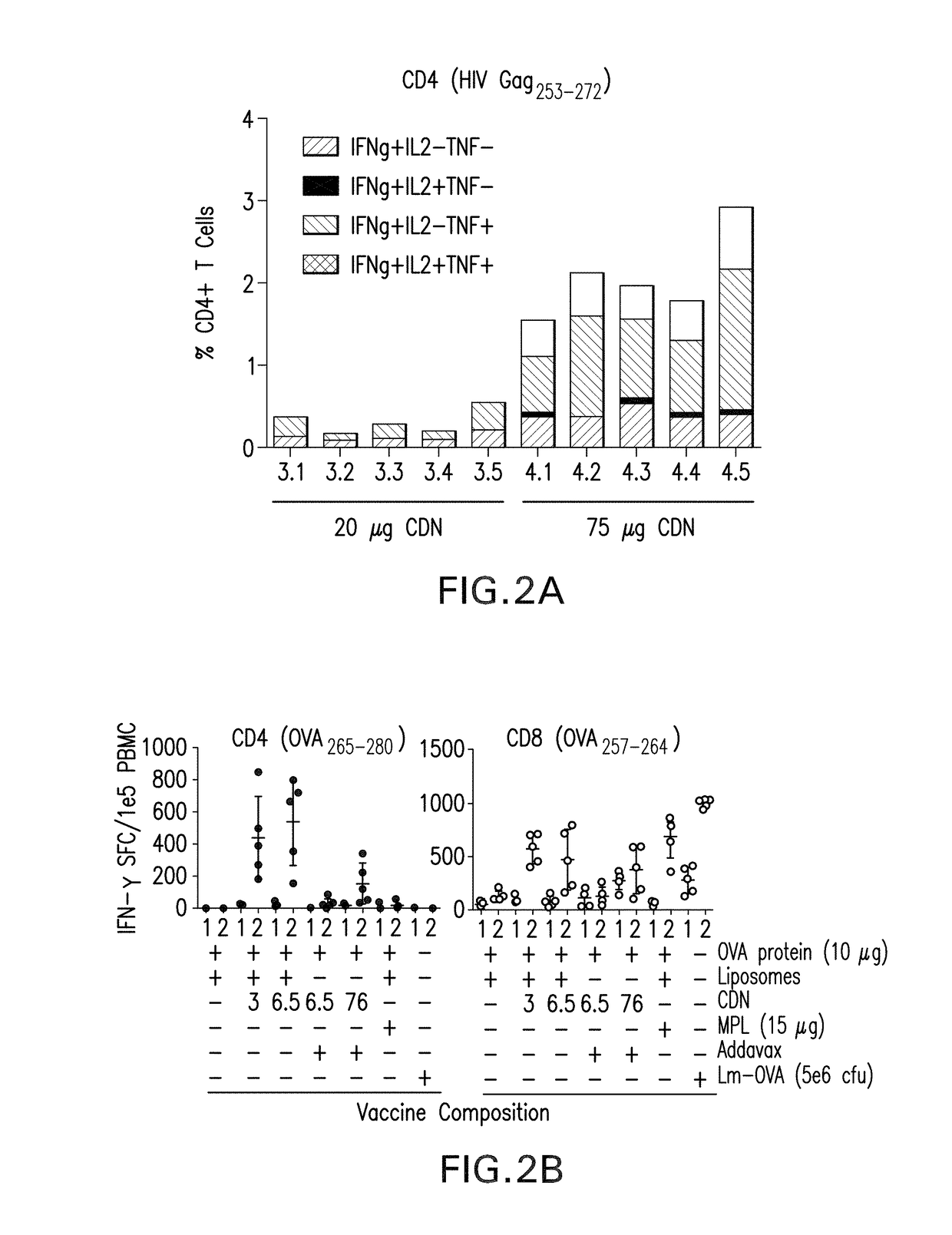
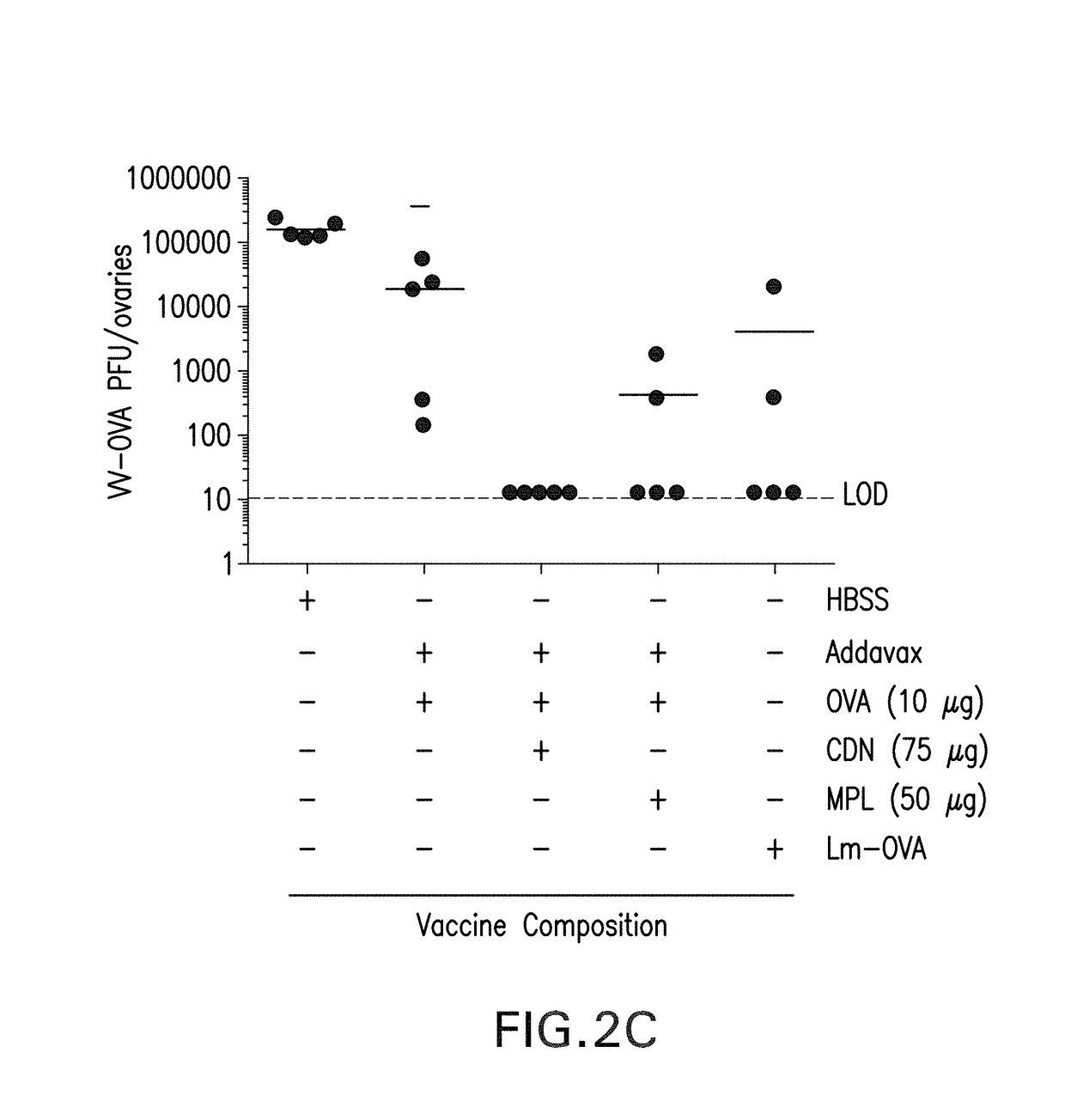
Intranasal delivery of a cyclic-di-nucleotide adjuvanted vaccine for tuberculosis
ActiveUS20200338182A1Antibacterial agentsBacterial antigen ingredientsVaccination against tuberculosisAntigen
A vaccine against Mycobacterium tuberculosis (M. tuberculosis) formulated for intranasal administration, comprises a first vaccine component comprising one or more M. tuberculosis, Mycobacterium vaccae (M. vaccae) or Mycobacteroium bovis (M. bovis) antigens, and a second vaccine component comprising a Stimulator of Interferon Genes (STING) activator.
Owner:RGT UNIV OF CALIFORNIA
Cyclic dinucleotides containing benzimidazole, method for the production of same, and use of same to activate stimulator of interferon genes (STING)-dependent signaling pathways
ActiveUS20200040028A1Good membrane permeabilityImprove biological activityOrganic active ingredientsSugar derivativesMembrane permeabilizationNucleotide
Cyclic dinucleotides are described, which in contrast to their natural congeners carry lipophilic nucleobases and have higher membrane permeability and increased biological activity.
Owner:BIOLOG LSI FORSCHUNGS & BET GMBH
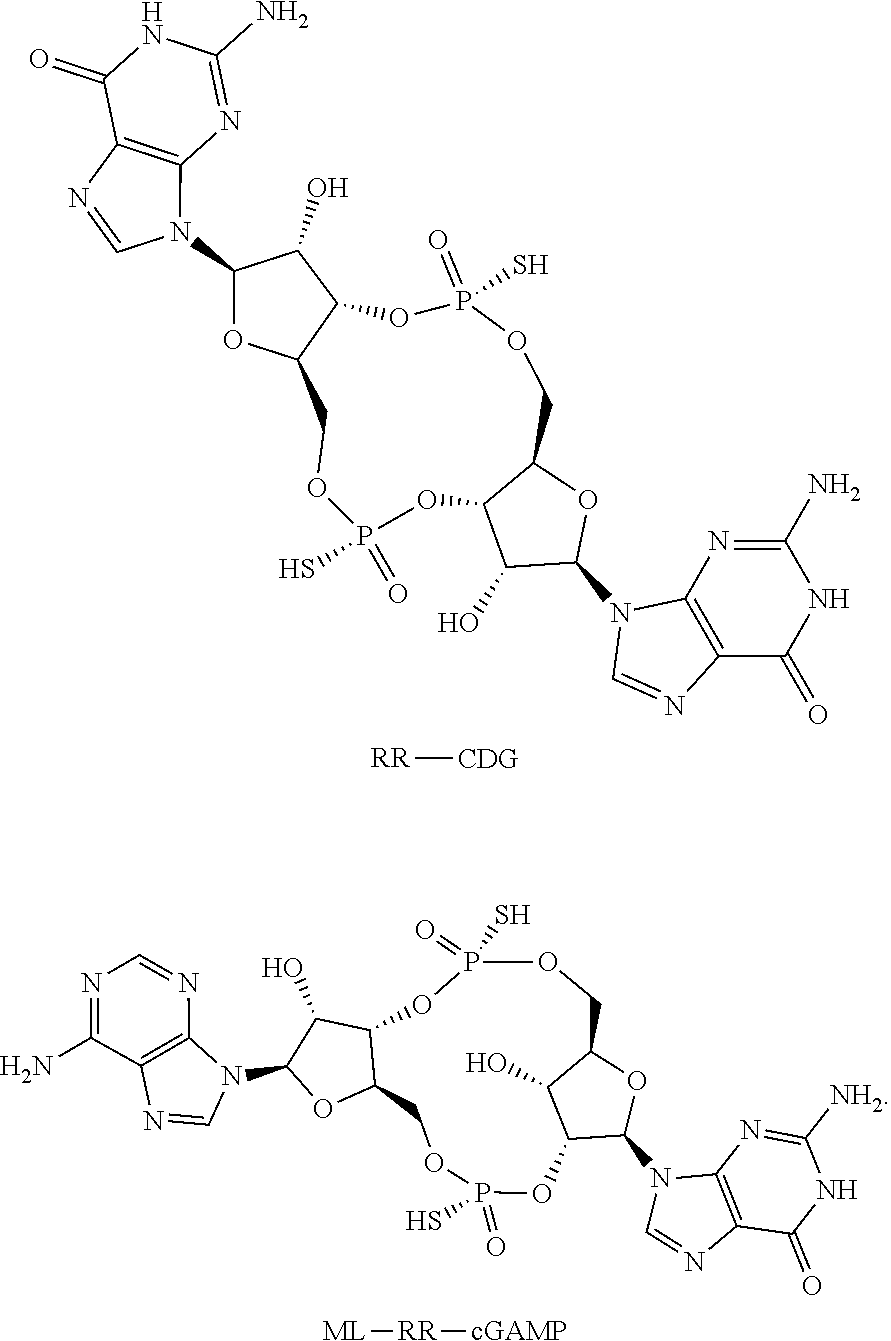
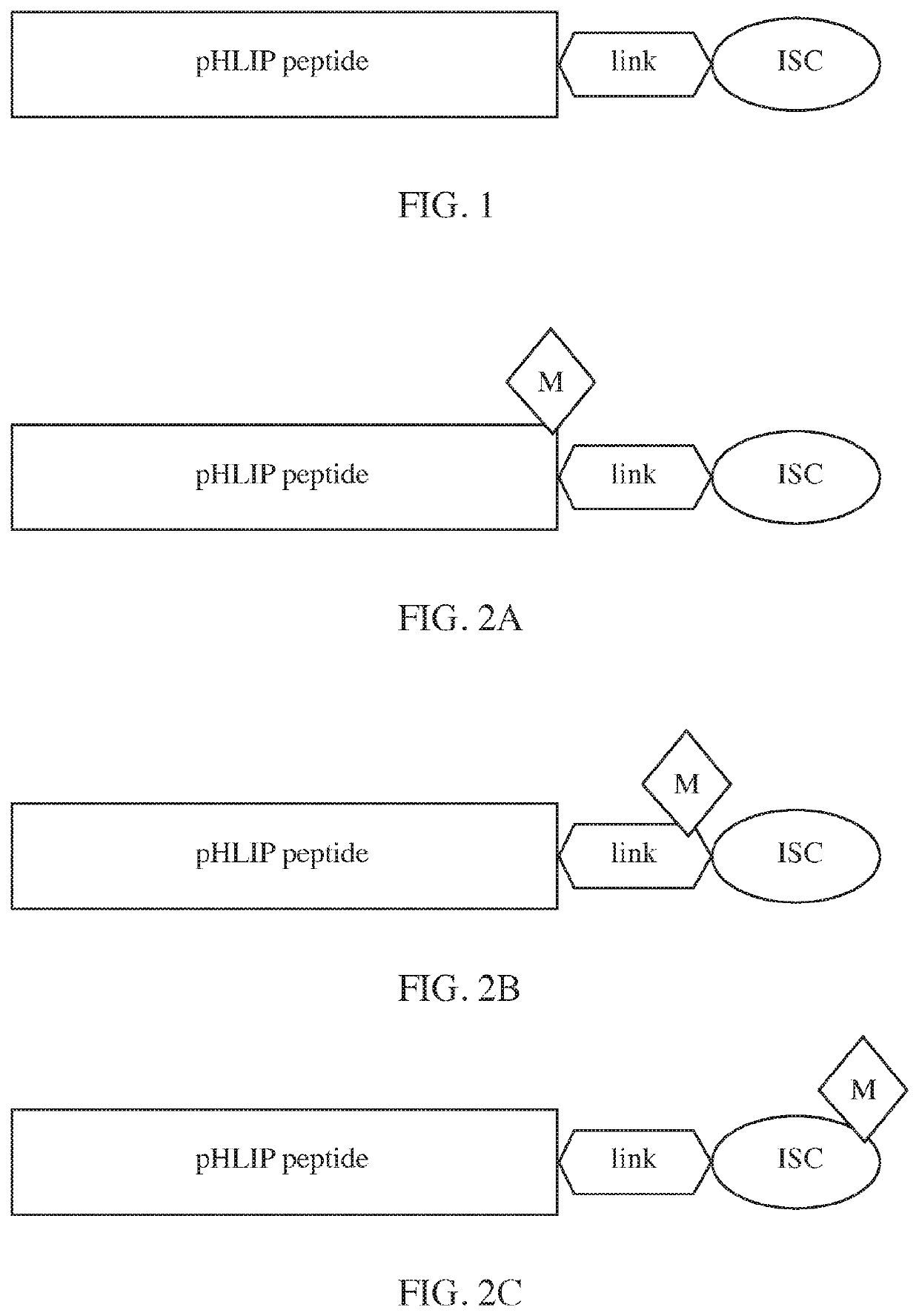
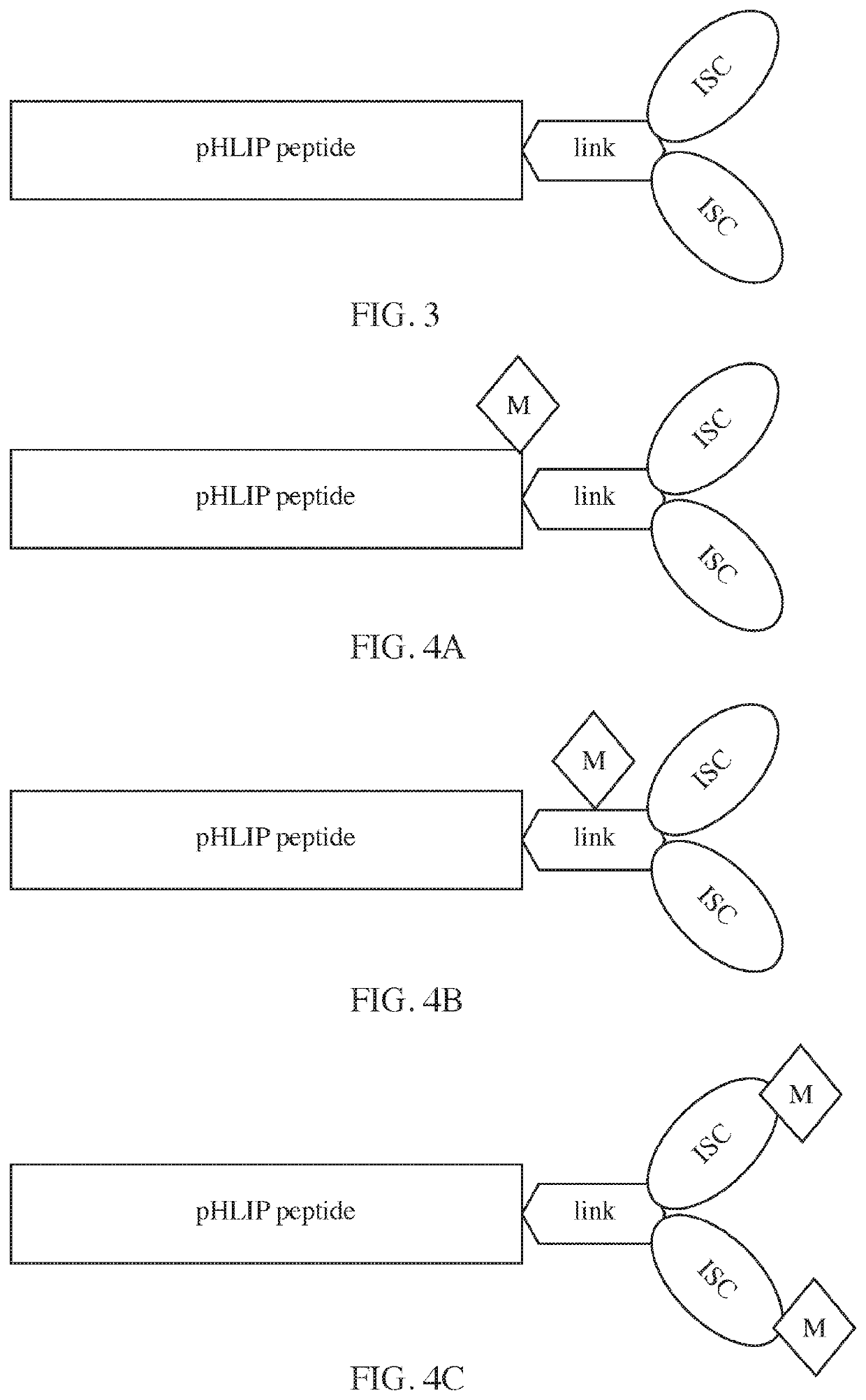
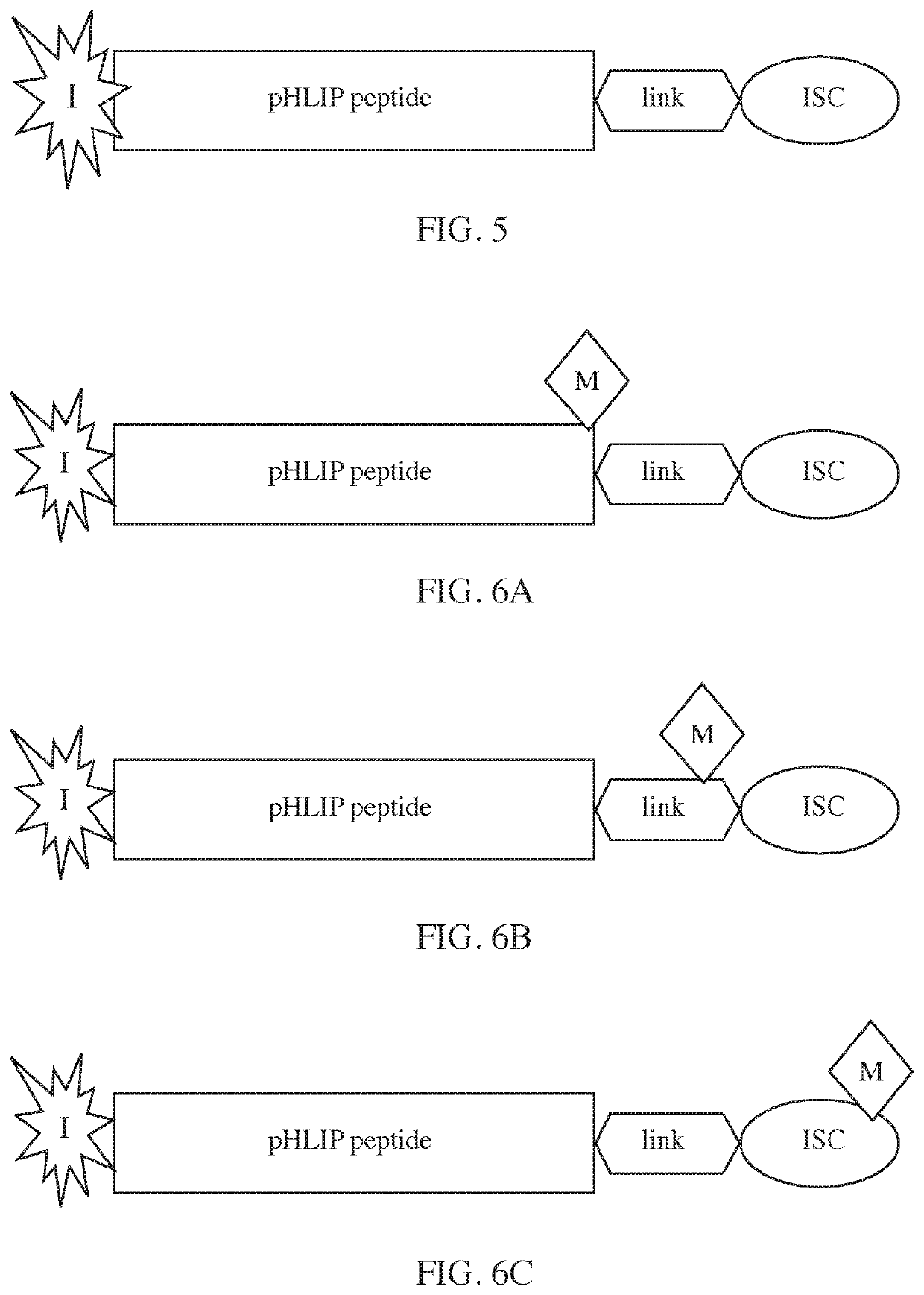
Phlip®-mediated intracellular delivery of immuno-stimulatory compounds
PendingUS20220088208A1Reduce deliveryLower pHPharmaceutical delivery mechanismImmunological disordersIntracellularNucleotide
The invention features a composition comprising an immuno-stimulatory compound and a pHLIP® peptide, e.g., an immunostimulatory compound that comprises a cyclic purine dinucleotide, which binds to a stimulator of interferon genes (STING) such as a cGAMP cyclic compound inside a cell.
Owner:YALE UNIV +1
Modulators of STING (Stimulator of Interferon Genes)
PendingUS20210087180A1Relieve symptomsOrganic active ingredientsOrganic chemistryStimulator of interferon genesPharmaceutical medicine
Owner:PFIZER INC
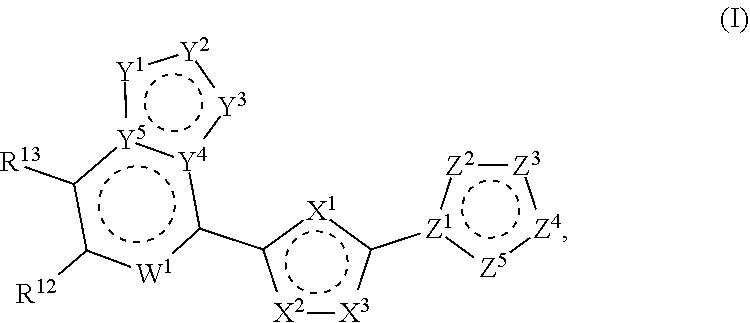
Agonists of stimulator of interferon genes sting
PendingCN111971045AOrganic active ingredientsOrganic chemistryPharmaceutical drugStimulator of interferon genes
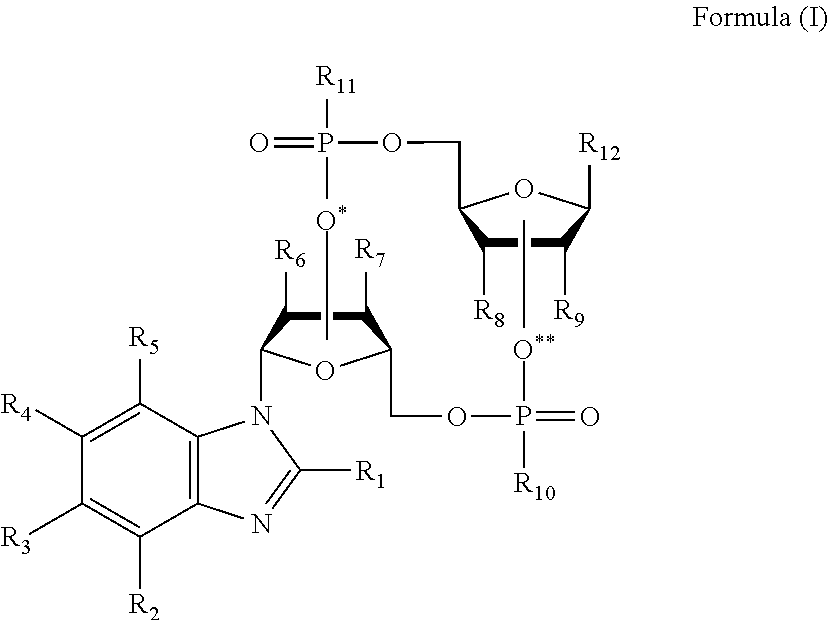
The invention provides compounds having STimulator of INterferon Genes (STING) agonistic bioactivity that can be used in the treatment of tumors in patients afflicted therewith. The compounds are of formula (I): as defined herein. Compounds for practice of a method of the invention can be delivered via oral delivery for systemic exposure, as well as delivered intratumorally. Antitumor therapy using a compound of formula (I) can further comprise administration of an effective dose of an immune-checkpoint targeting drug.
Owner:THE SCRIPPS RES INST
Polyheterocyclic modulators of STING (stimulators of interferon gene)
PendingCN114728946AOrganic active ingredientsOrganic chemistryMedicineStimulator of interferon genes
Owner:PFIZER INC
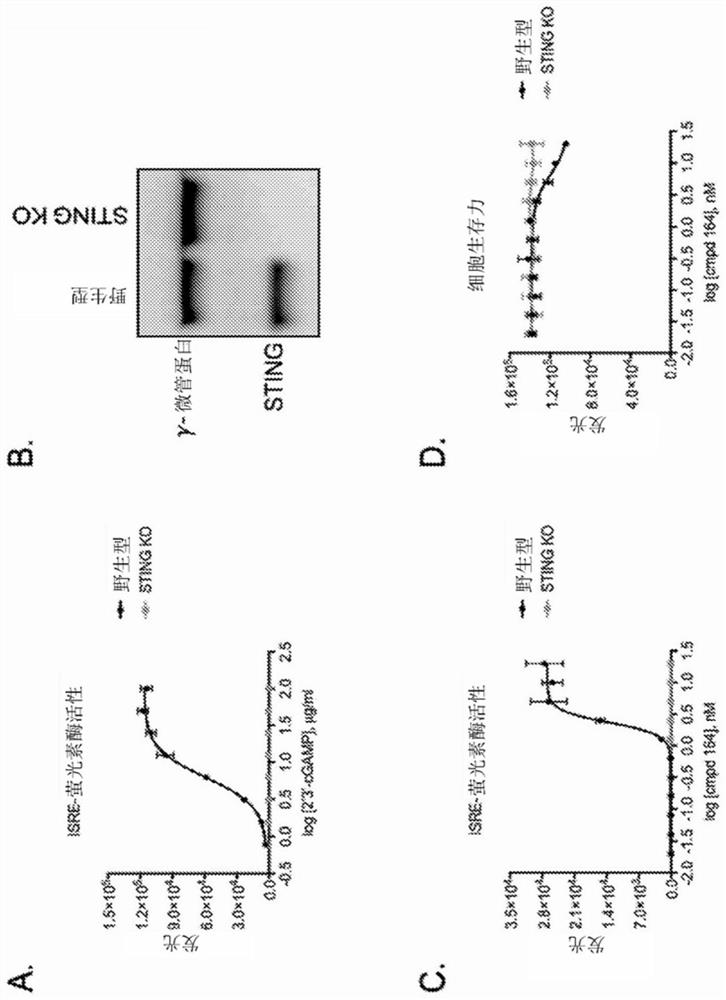
Administration of sting agonist and checkpoint inhibitors
PendingCN114080228AOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsPharmaceutical drugStimulator of interferon genes
The present disclosure provides methods, pharmaceutical compositions, and kits for treating cancer in patients in need thereof. The methods comprise administering to a patient in need a STING (stimulator of interferon genes) agonist, such as Compound No. 14 as defined in the description, or a pharmaceutically acceptable salt thereof, in combination with one or more checkpoint inhibitors. Also provided are medicaments for use in treating cancer.
Owner:TAKEDA PHARMA CO LTD
pHLIP-MEDIATED INTRACELLULAR DELIVERY OF IMMUNO-STIMULATORY COMPOUNDS
The invention features a composition comprising an immuno-stimulatory compound and a pHLIP peptide, e.g., an immunostimulatory compound that comprises a cyclic purine dinucleotide, which binds to a stimulator of interferon genes (STING) such as a cGAMP cyclic compound inside a cell.
Owner:RHODE ISLAND COUNCIL ON POSTSECONDARY EDUCATION +1
Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment
ActiveUS11312772B2Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDeath ReceptorsStimulator of interferon genes
Therapeutic combinations that comprise at least one antagonist of the Programmed Death 1 receptor (PD-1) and at least one benzo[b]thiophene compound that activates the Stimulator of Interferon Genes (STING) pathway are disclosed herein. Also disclosed is the use of such therapeutic combinations for the treatment of cancers.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com























![Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8efadd20-a259-4bb0-9e08-267bc393be3c/US20210130466A1-D00001.png)
![Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8efadd20-a259-4bb0-9e08-267bc393be3c/US20210130466A1-D00002.png)
![Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment Combinations of PD-1 Antagonists and Benzo[b]thiophene STING Agonists for Cancer Treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/8efadd20-a259-4bb0-9e08-267bc393be3c/US20210130466A1-D00003.png)











![Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6190ea79-ec27-4162-8609-7242d923fa1d/US11312772-D00001.png)
![Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6190ea79-ec27-4162-8609-7242d923fa1d/US11312772-D00002.png)
![Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment Combinations of PD-1 antagonists and benzo [b] thiophene STING agonists for cancer treatment](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/6190ea79-ec27-4162-8609-7242d923fa1d/US11312772-D00003.png)