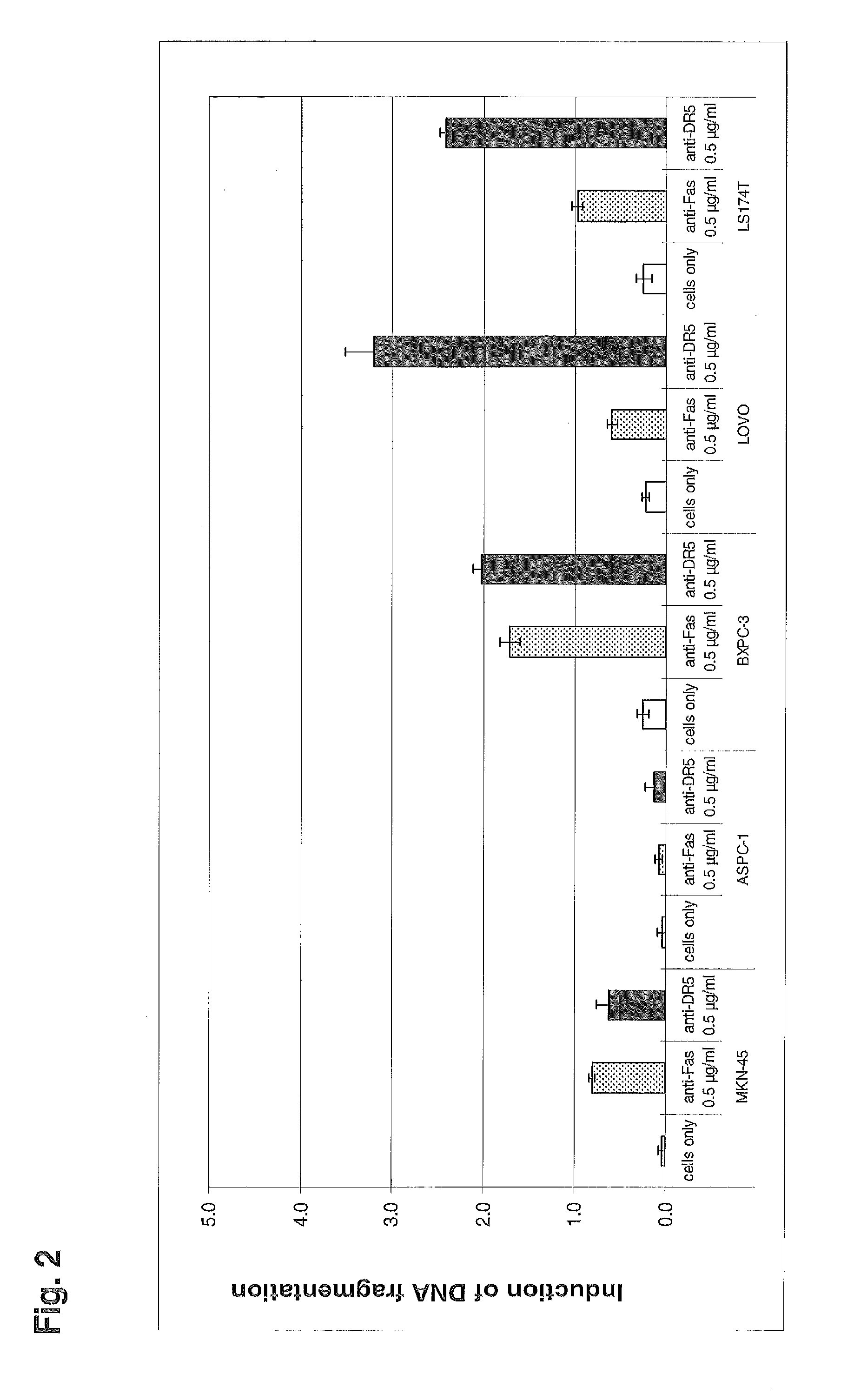
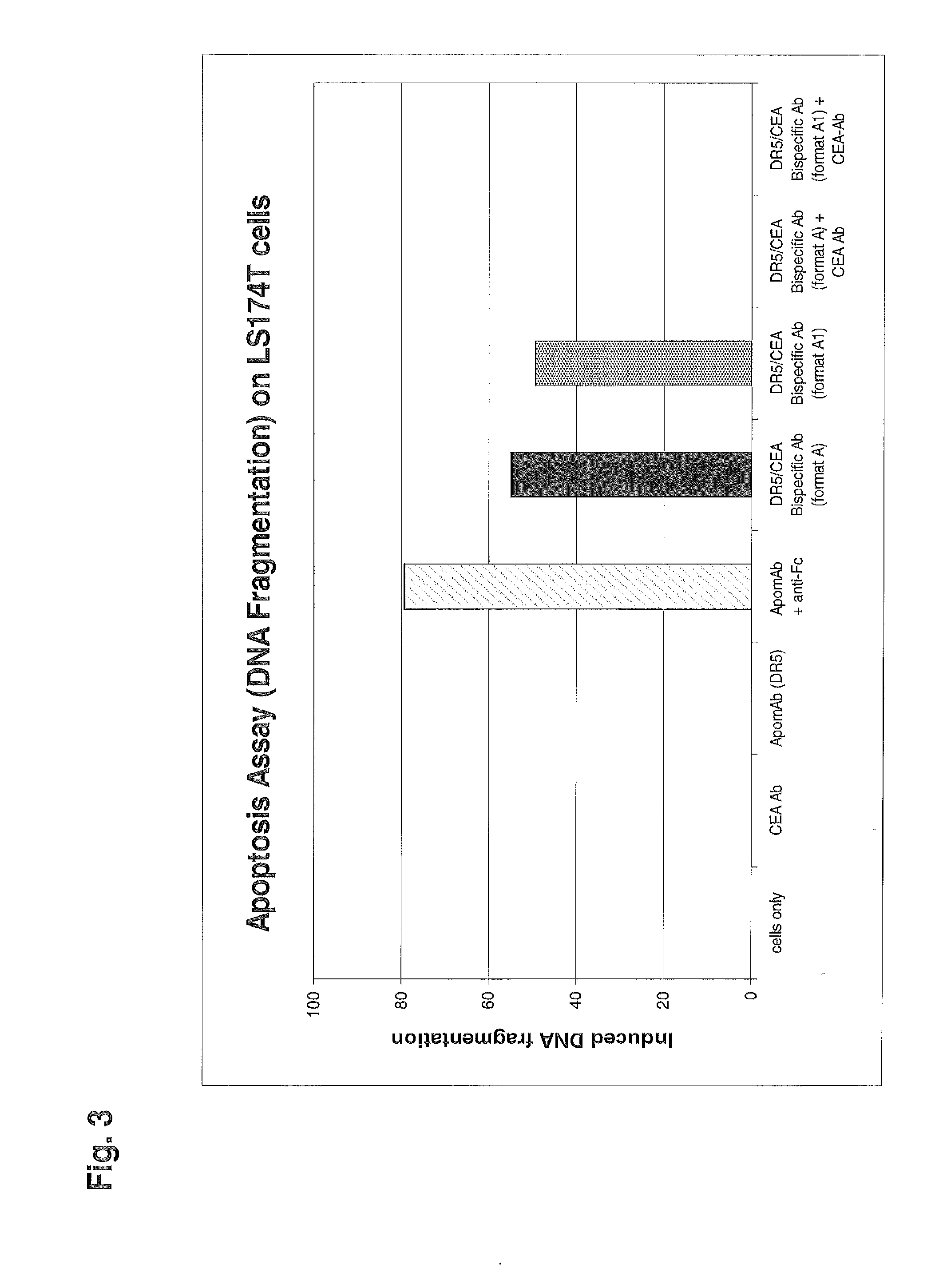
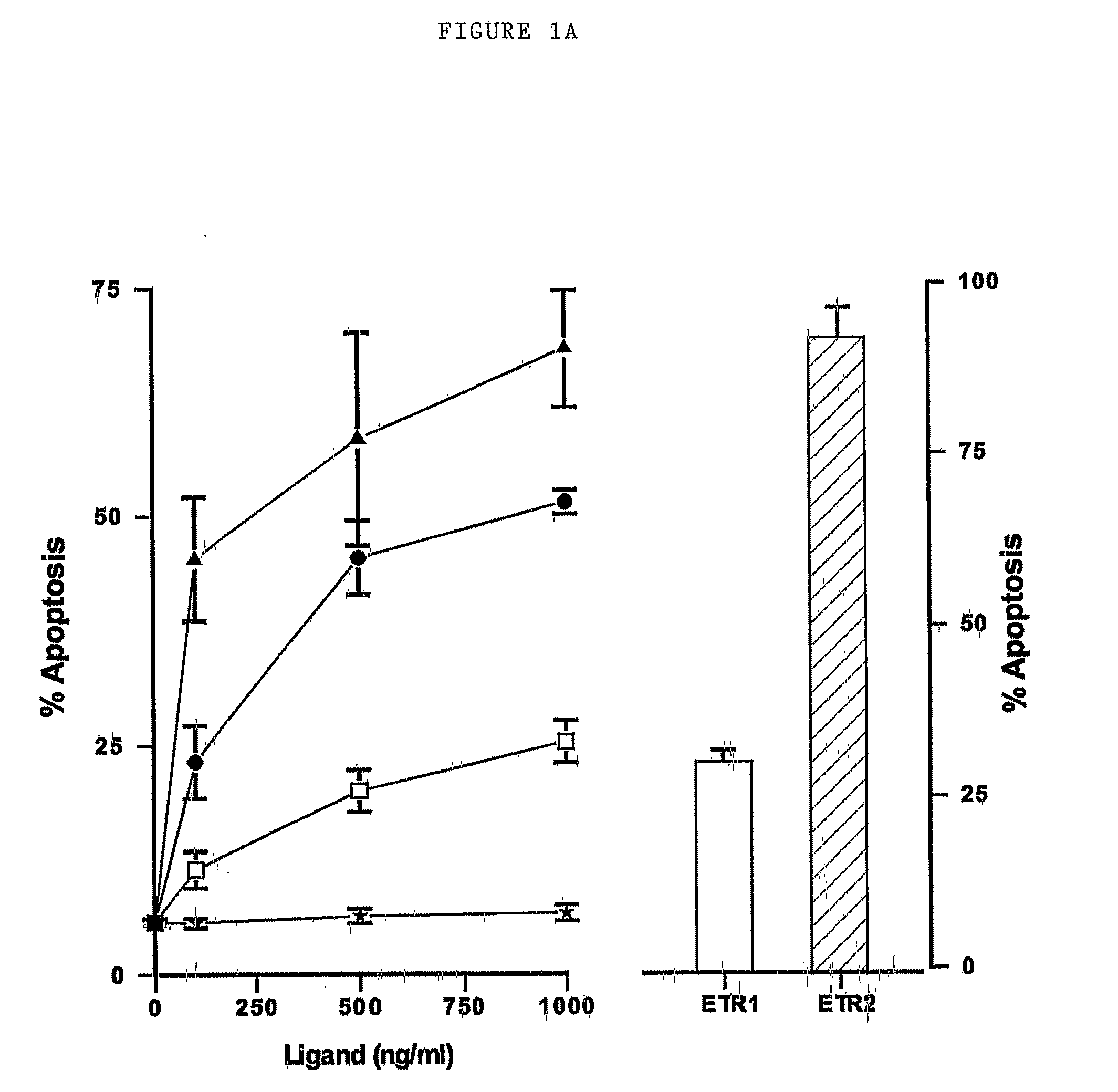
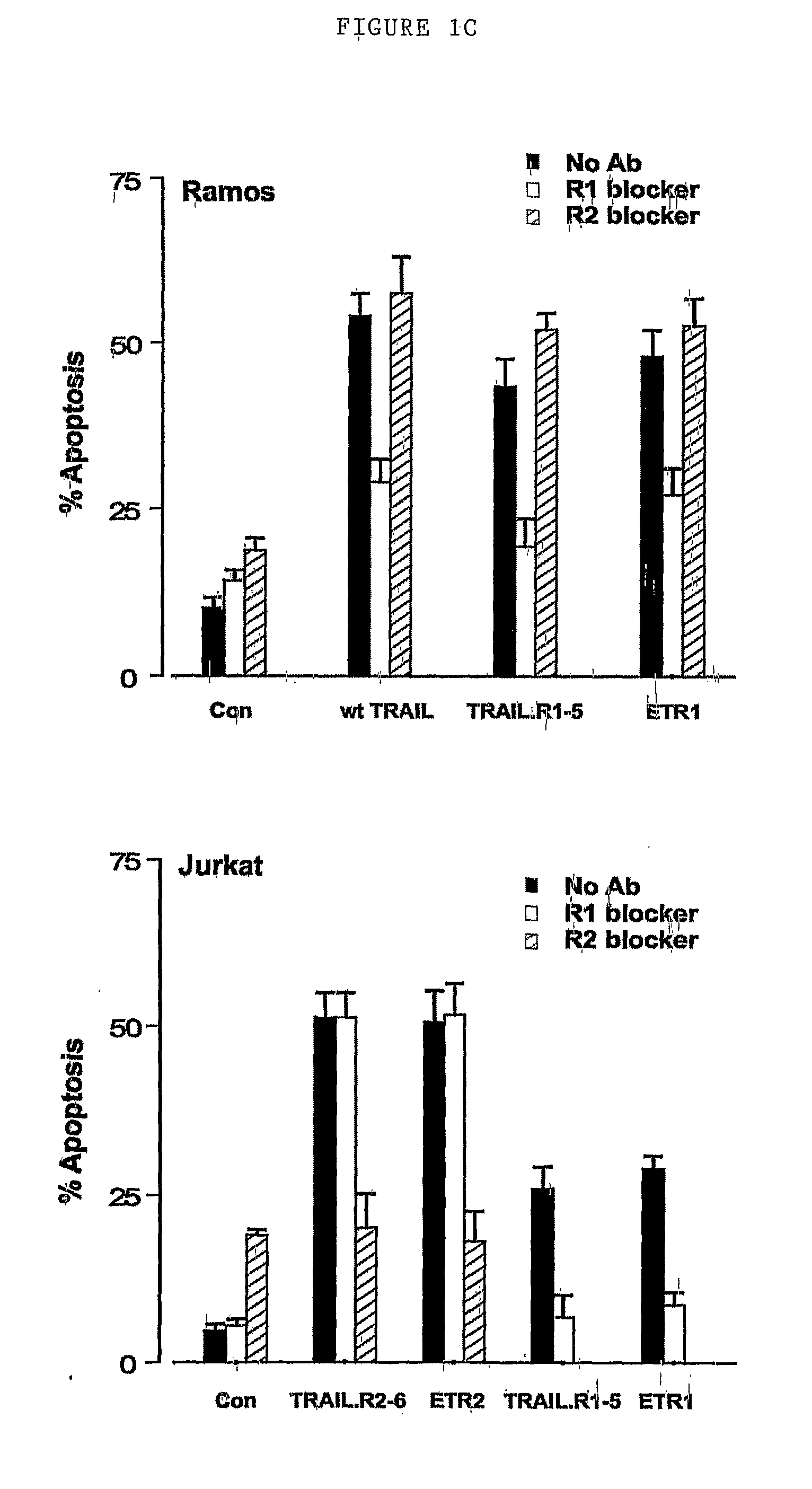
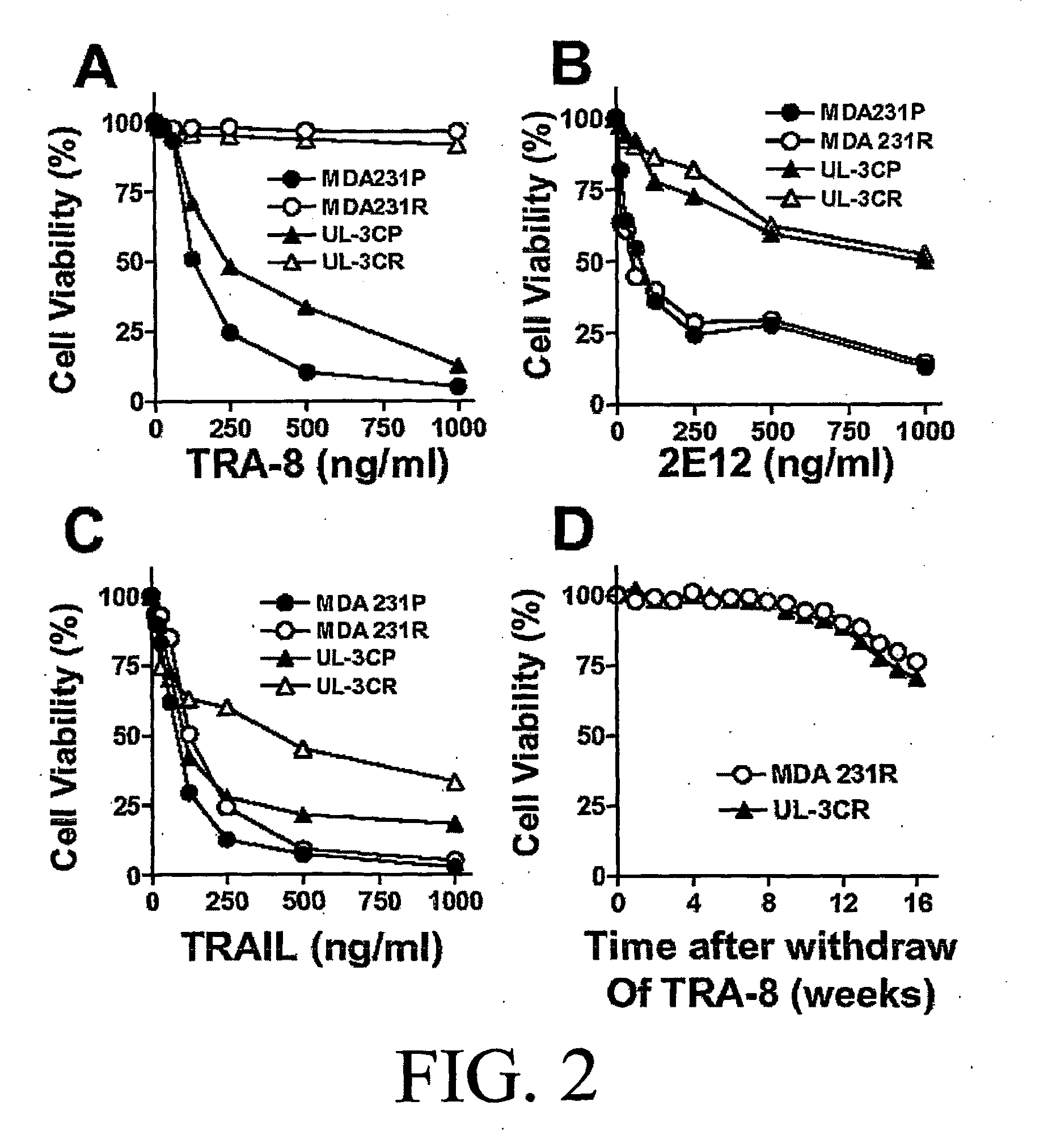
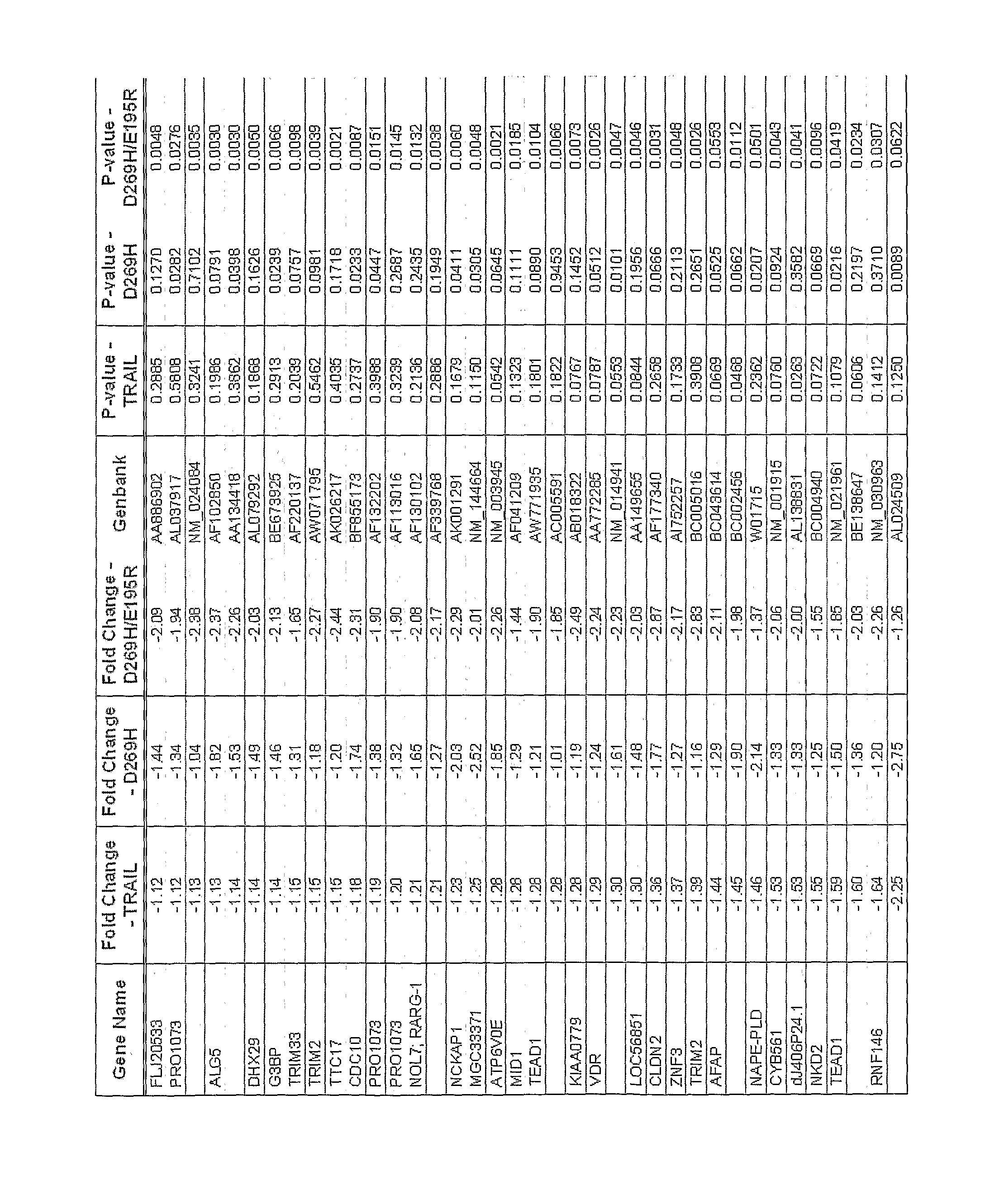
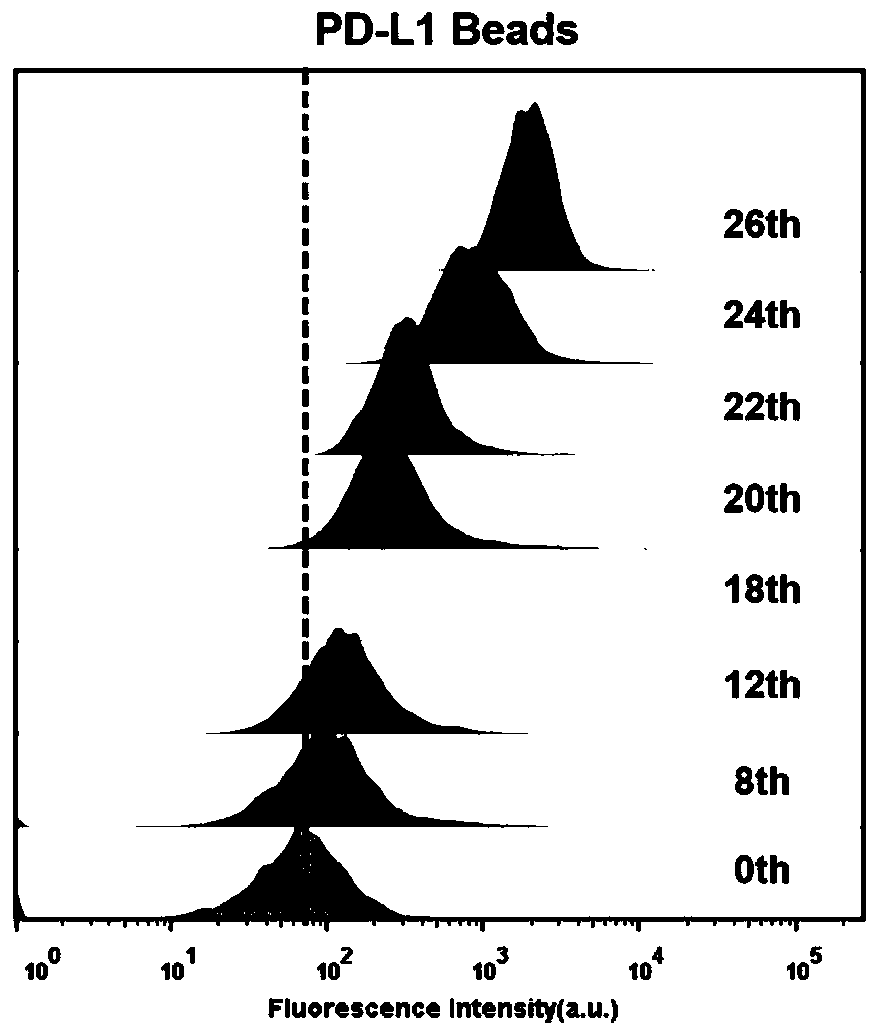
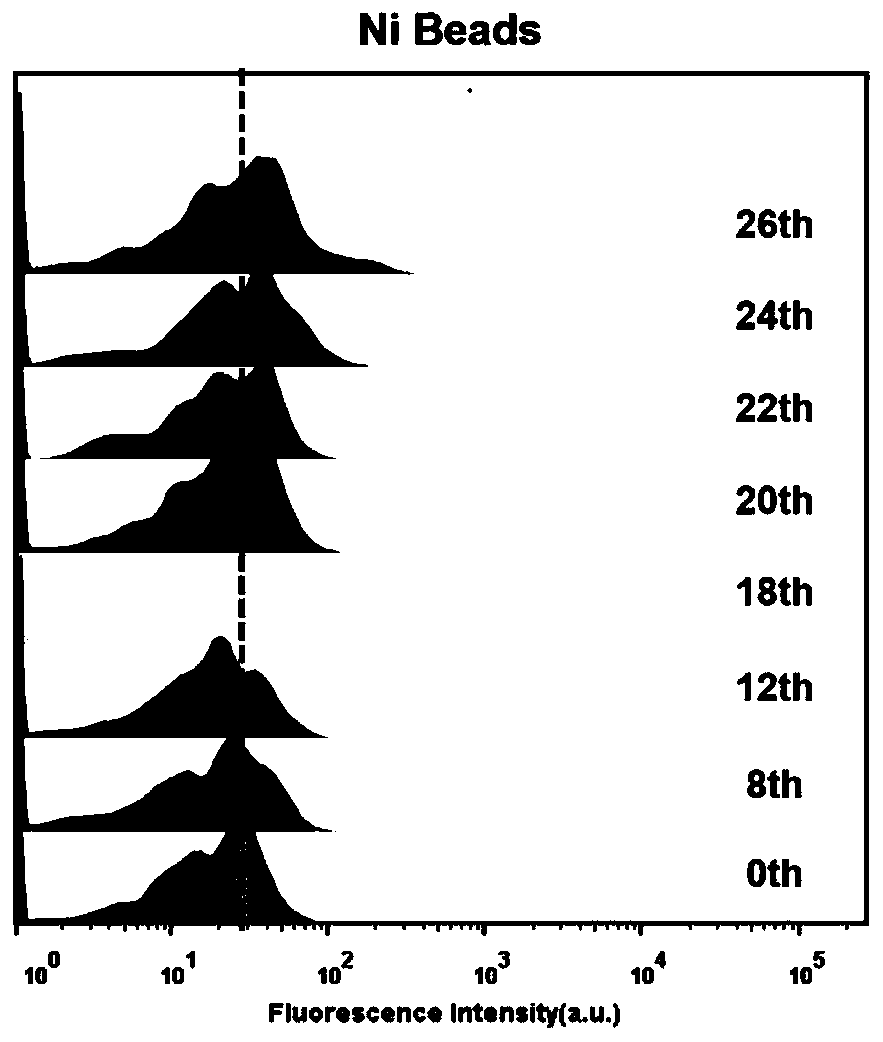
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Death Receptors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
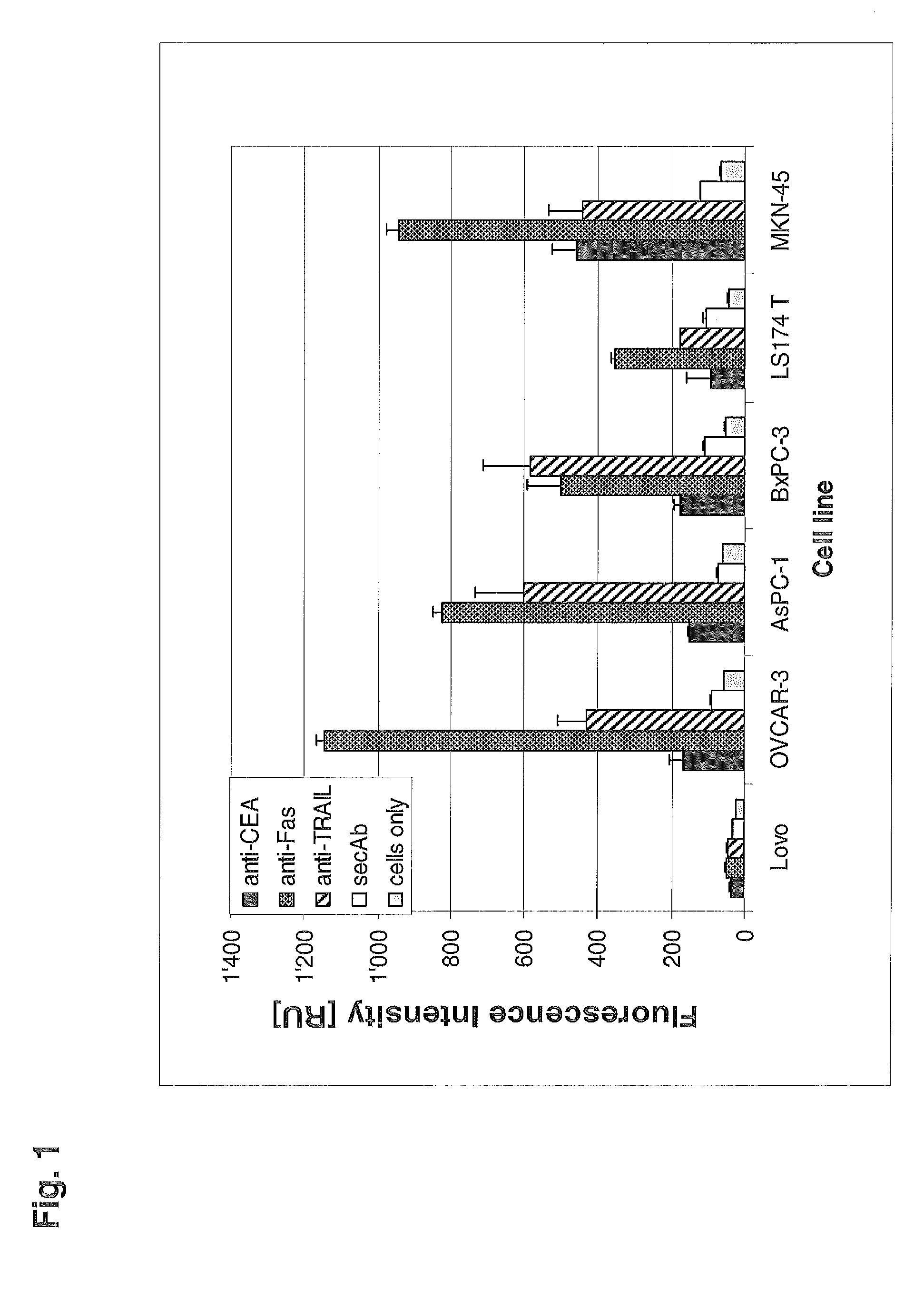
Bispecific death receptor agonistic antibodies
ActiveUS20120184718A1Skeletal disorderImmunoglobulins against cell receptors/antigens/surface-determinantsBinding siteDeath Receptors
The present invention relates to bispecific antibodies comprising a first antigen binding site specific for a death receptor and a second antigen binding site specific for a second antigen, methods for their production, pharmaceutical compositions containing said antibodies, and uses thereof.
Owner:ROCHE GLYCART AG
Receptor-specific tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) variants
InactiveUS20090325867A1Reduce and eliminate liver toxicityImprove bindingPeptide/protein ingredientsNGF/TNF-superfamilyLymphocytic leukaemiaApoptosis
The invention relates to a tumour necrosis factor- (TNF-) related apoptosis-inducing ligand (TRAIL) which is capable of selectively signalling through death receptor 4 (DR4), comprising Y at position 189. Preferably the TRAIL further comprises 19 IL and / or 199V; preferably also 201R, 213W and 215D, and / or preferably further comprises 193S. The invention also relates to uses of such TRAIL mutants which are capable of selectively signalling through DR4 in the treatment of cancer, and in the manufacture of medicaments for use in treatment of cancer. Preferably the cancer is chronic lymphocytic leukaemia, mantle cell lymphoma or non-Hodgkin's lymphoma. The invention also relates to kits comprising same.
Owner:UNIVERSITY OF LEICESTER +1
DR5—FAP bispecific death receptor agonistic antibodies
ActiveUS9481730B2Skeletal disorderImmunoglobulins against cell receptors/antigens/surface-determinantsBinding siteDeath Receptors
The present invention relates to bispecific antibodies comprising a first antigen binding site specific for a death receptor and a second antigen binding site specific for a second antigen, methods for their production, pharmaceutical compositions containing said antibodies, and uses thereof.
Owner:ROCHE GLYCART AG
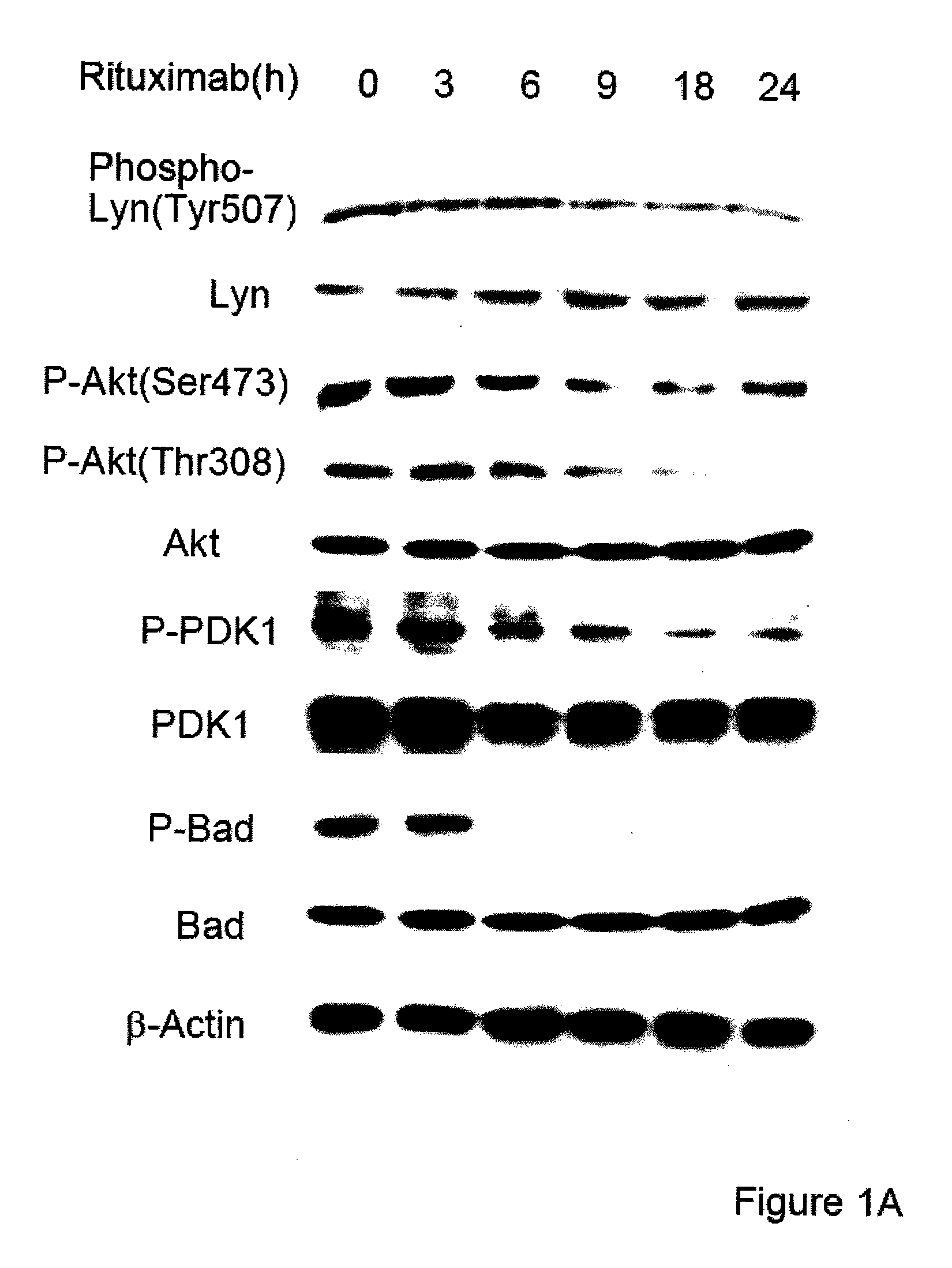
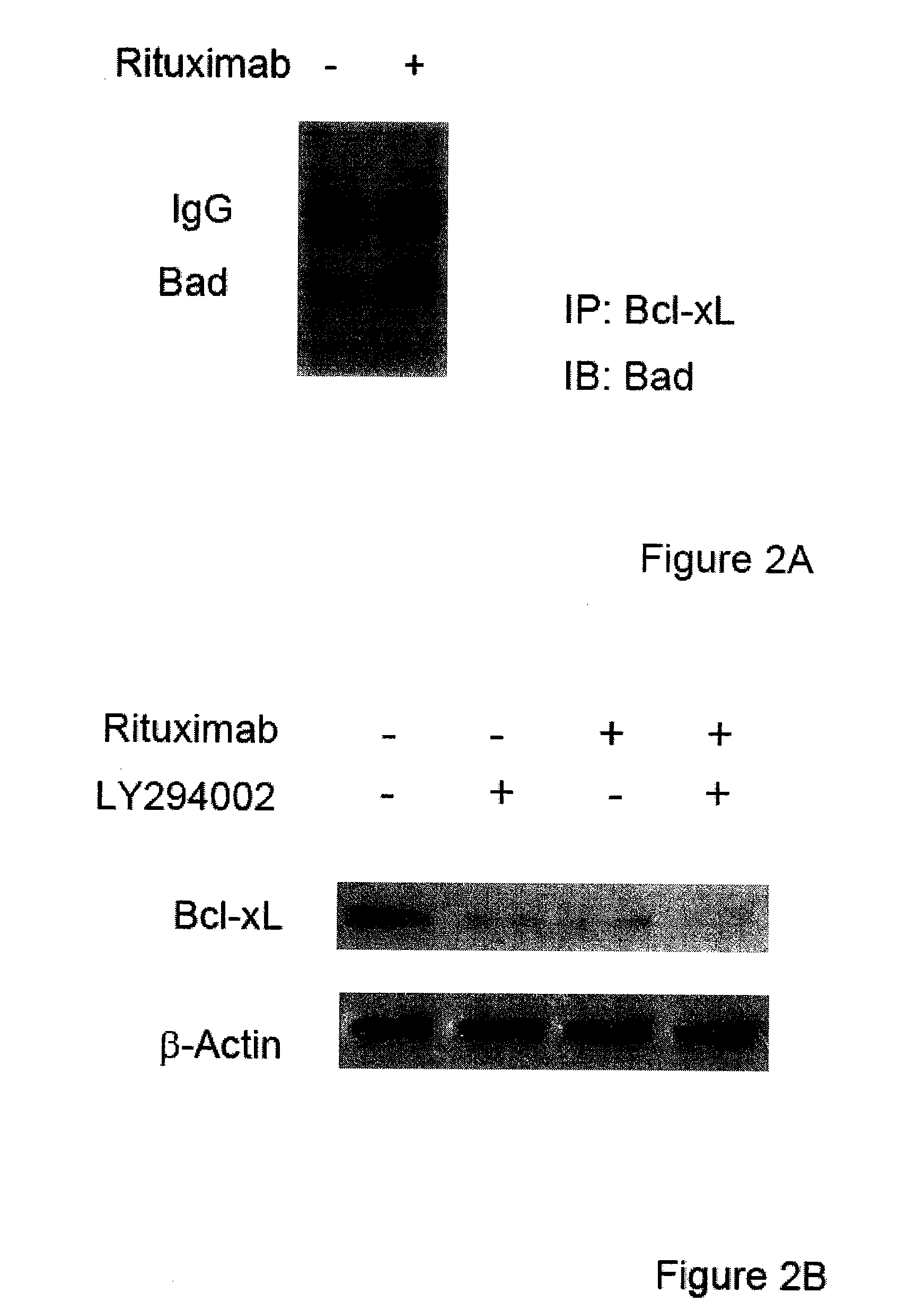
Molecular signaling pathways triggered by rituximab: prognostic, diagnostic, and therapeutic uses
InactiveUS20070172847A1RegulateRegulate the cancer cells' sensitivity to immunotherapyMicrobiological testing/measurementMaterial analysisAutoimmune conditionCancer cell
The present invention provides markers associated with activated molecular signaling pathways (example: p38 MAKP, NF-κB, ERK1 / 2, YY-1 and AKT) inhibited by rituximab in cancer cells as well as pathways activated by rituximab (such as death receptors, RKIP, PTEN) all of which are associated with the regulation of chemo and immunoresistance. The present invention provides methods of prognosis and providing a prognosis for cancer such as lymphoma, leukemia, and autoimmune disease, as well as, methods of drug discovery. These markers are also therapeutic targets for treatment of cancer resistant to conventional and experimental cancer therapeutics. Inhibition or activation of expression and / or activity of targeted gene products sensitizes resistant tumor cells to subtoxic doses of cytotoxic treatment including chemotherapy, radiation therapy, or immunotherapy and gene therapy, and the cytotoxic molecules.
Owner:RGT UNIV OF CALIFORNIA
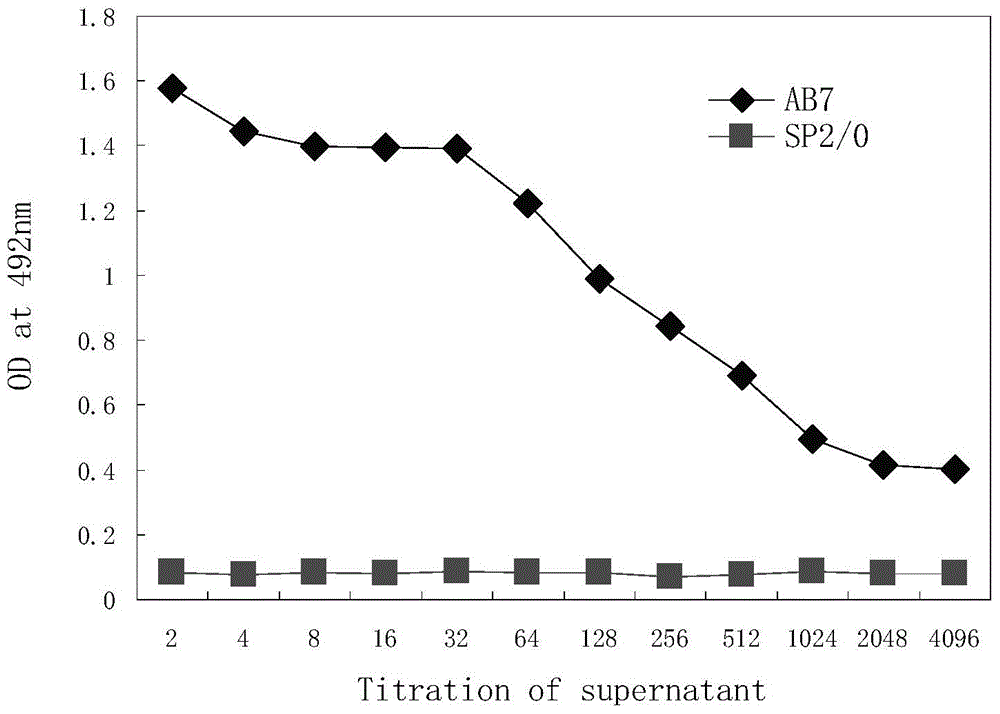
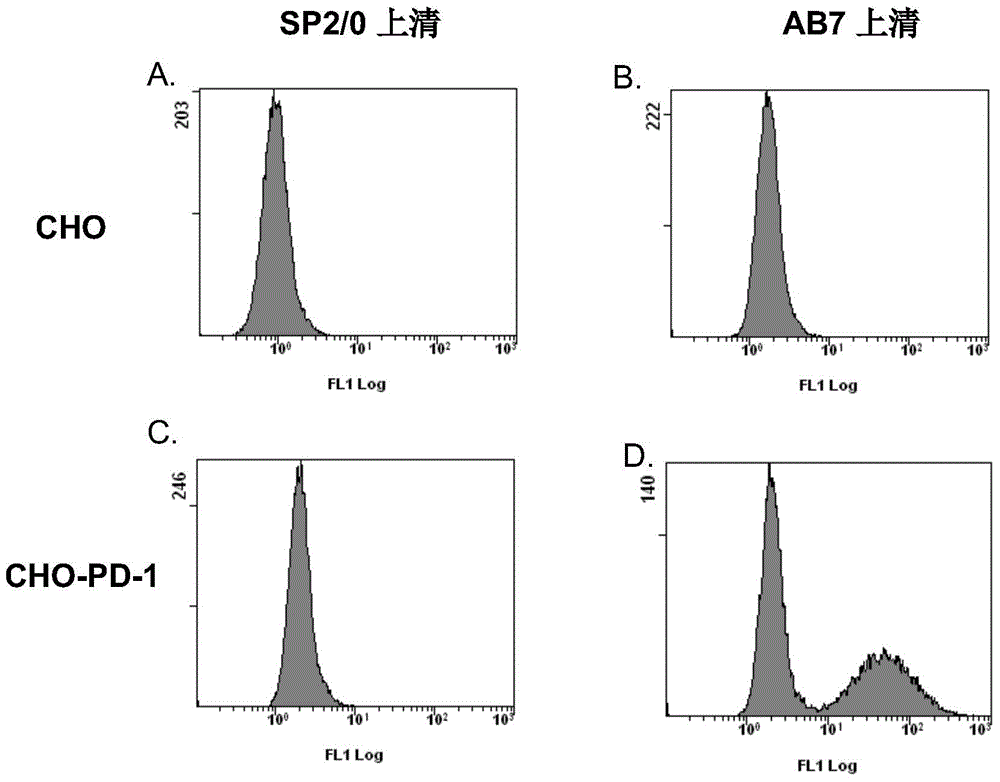
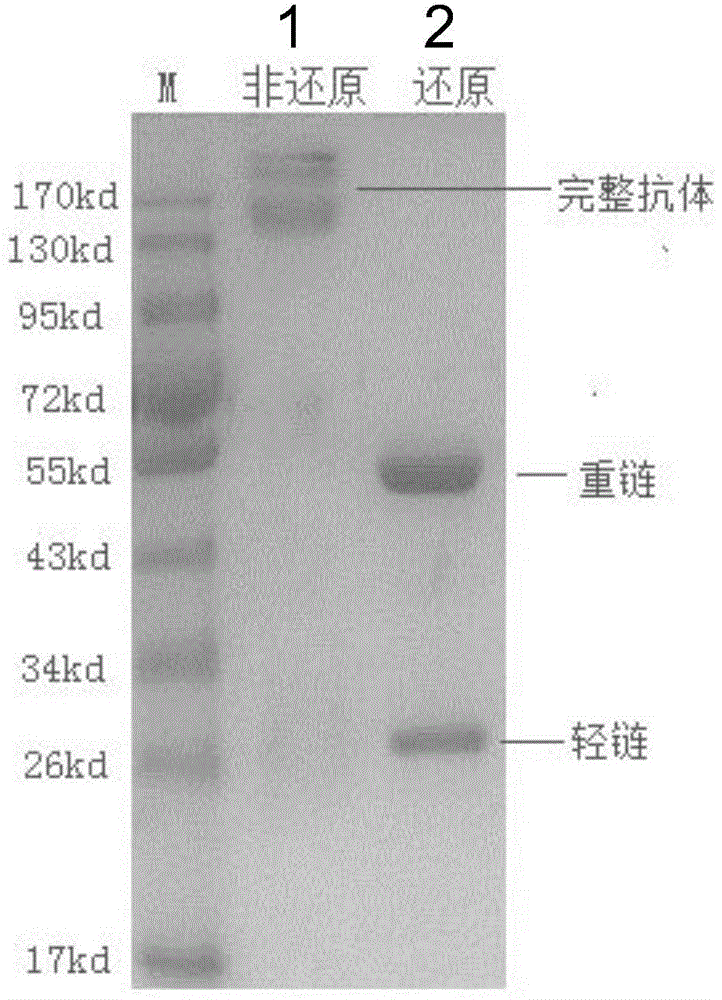
Monoclonal antibody capable of antagonizing and inhibiting bonding of programmed death-1 receptor (PD-1) and ligand thereof as well as encoding sequence and use of monoclonal antibody
ActiveCN104558177AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsProtein detectionHeavy chain
Disclosed in the present invention are a mouse monoclonal antibody for antagonizing and inhibiting the binding of a programmed death-1 (PD-1) to the ligand thereof, and the heavy chain variable region and light chain variable region amino acid sequences thereof. Also disclosed in the present invention is a DNA molecule nucleotide sequence encoding the heavy chain variable region and light chain variable region of the antibody. Also disclosed in the present invention are a method for preparing a human-mouse chimeric antibody of the antibody and derivatives thereof, and the use thereof in PD-1 protein detection.
Owner:ACROIMMUNE BIOTECH CO LTD
Epidithiodioxopiprazines and uses thereof in treating cancer
InactiveUS20120219568A1Good effectGood curative effectHeavy metal active ingredientsOrganic chemistryNatural resourceCancer cell
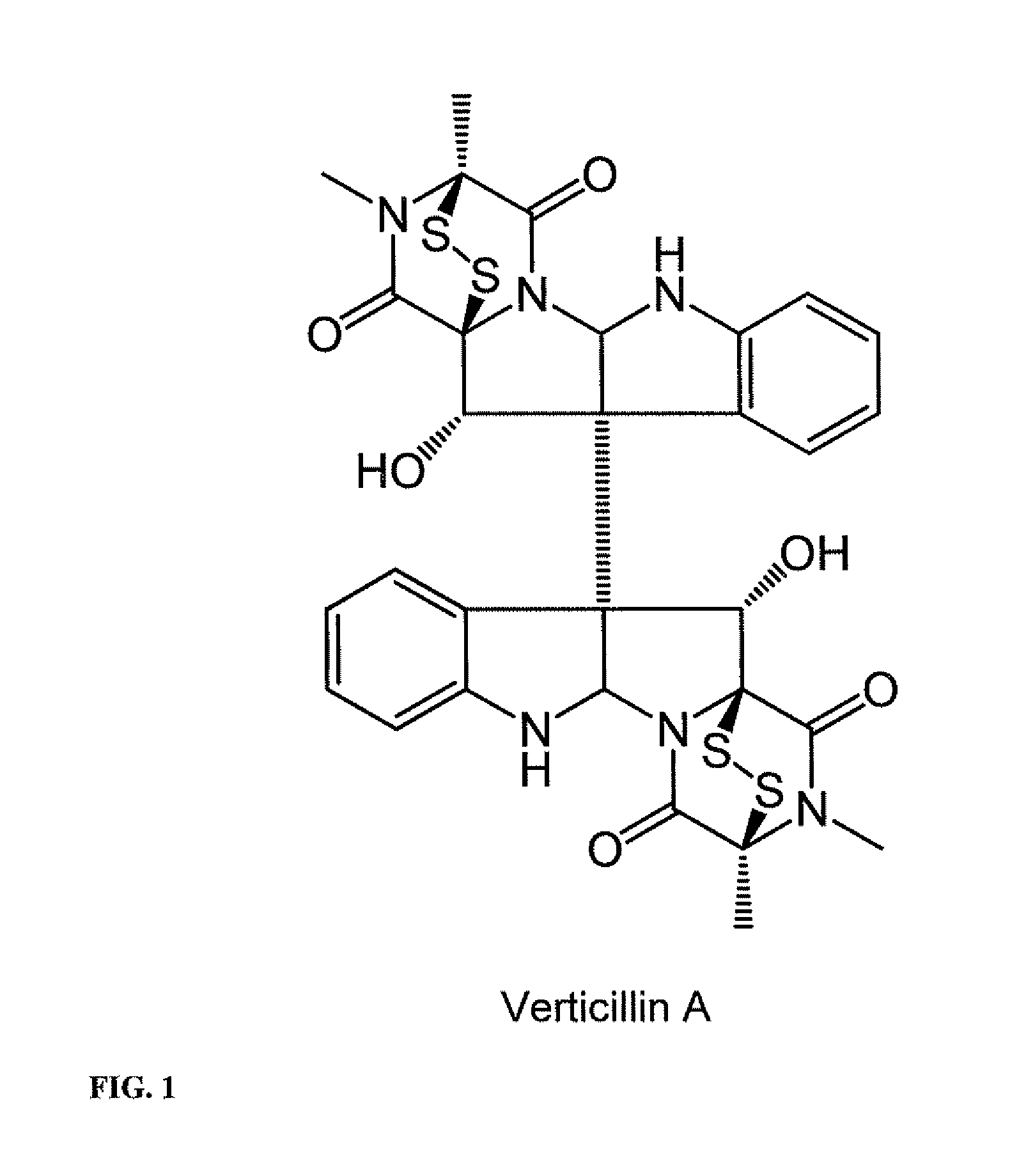
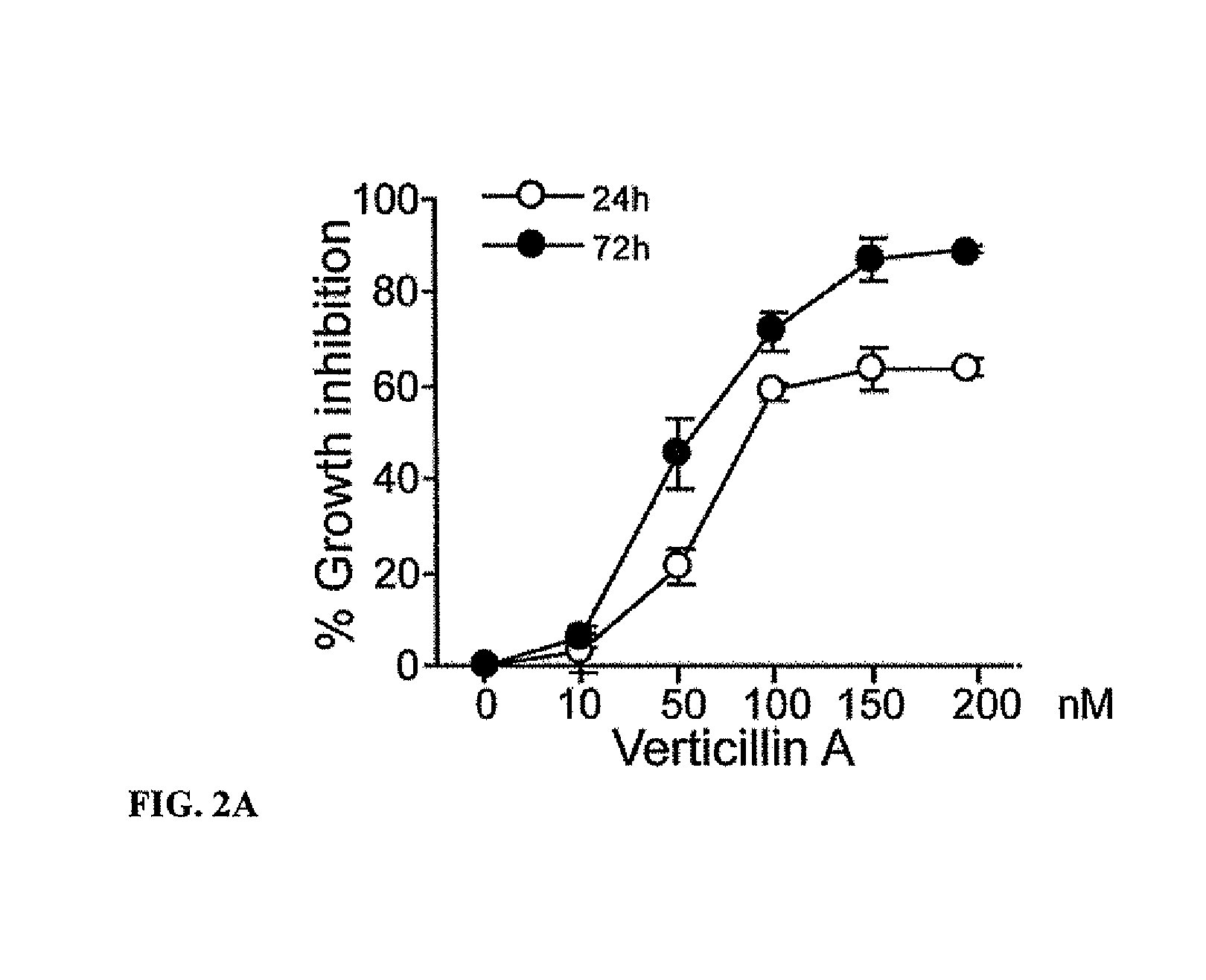
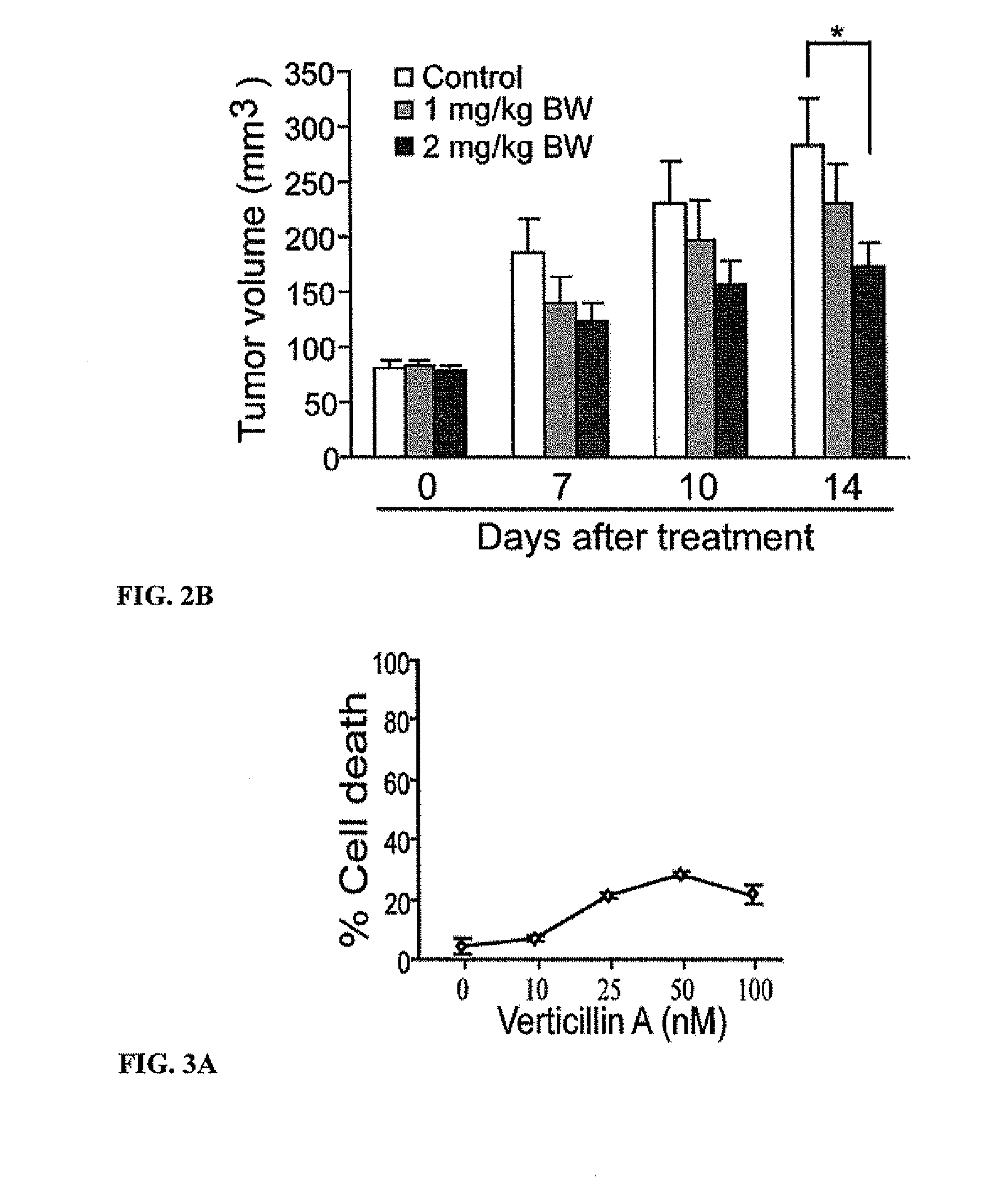
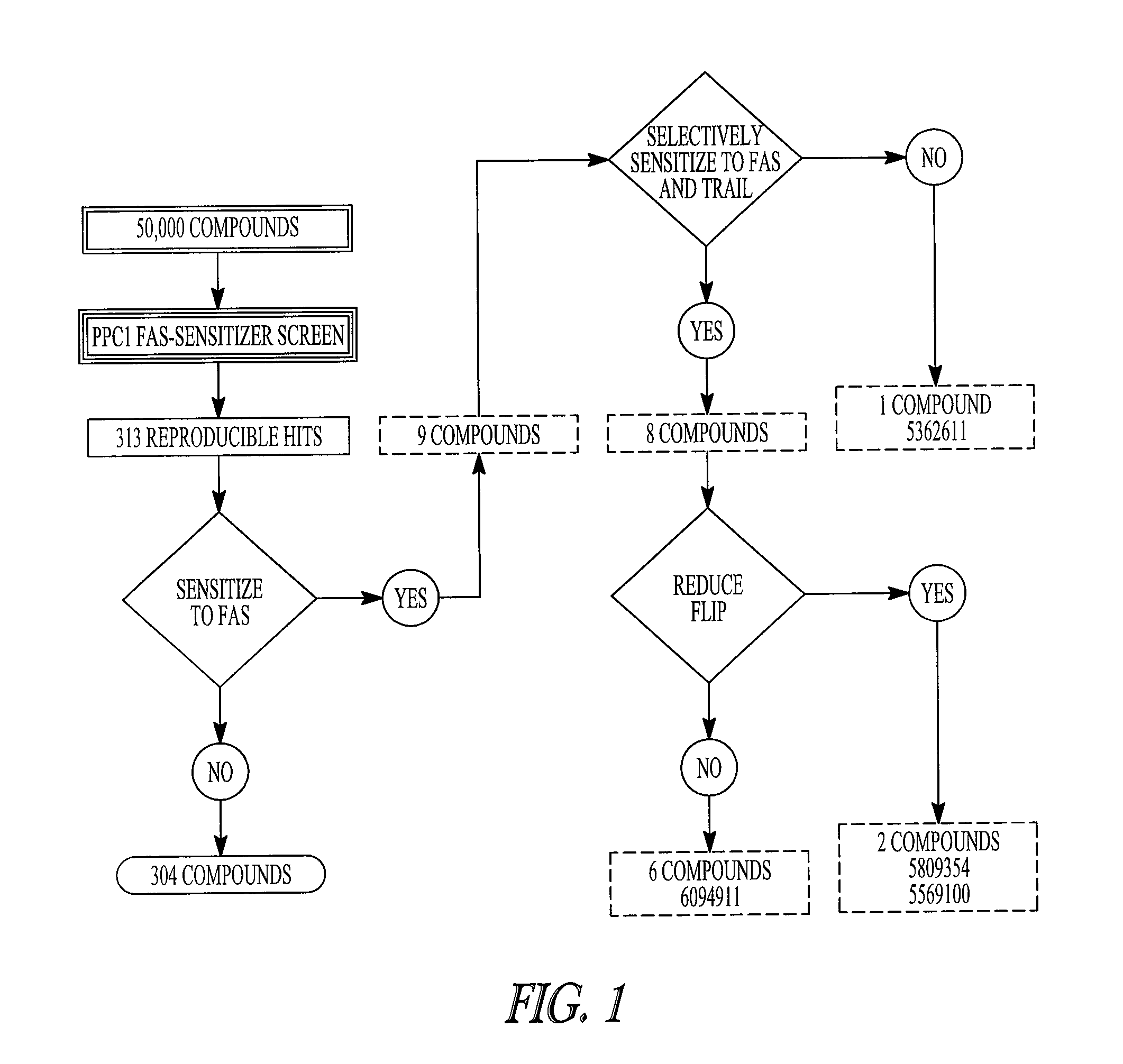
Compositions containing epidithiodioxopiprazines and methods of their use are provided. Epidithiodioxopiprazines can be isolated from natural resources or synthesized de novo. Moreover, epidithiodioxopiprazines, including Verticillin A, are shown to effectively sensitize multiple types of tumor cells to TRAIL-induced apoptosis. In addition, epidithiodioxopiprazines, including Verticillin A, are shown to effectively overcome cancer cell resistance to existing drugs (i.e. Etoposide, Cisplatin, 5-FU and Doxorubicin). Therefore, compositions and methods are provided for use in sensitizing target cancer cells to death receptor- and other anticancer drugs-induced apoptosis. Methods of treating cancer in a subject in need thereof are also provided.
Owner:ZHEJIANG UNIV +1
Death receptor sensitizing compounds and methods of use therefor
InactiveUS20080166378A1Accelerated deathIncreased apoptosisHalogenated hydrocarbon active ingredientsBiocideDeath ReceptorsReceptor for activated C kinase 1
Owner:BURNHAM INST THE
Polypeptides that Bind TRAIL-R1 and TRAIL-R2
Owner:ANAPHORE INC
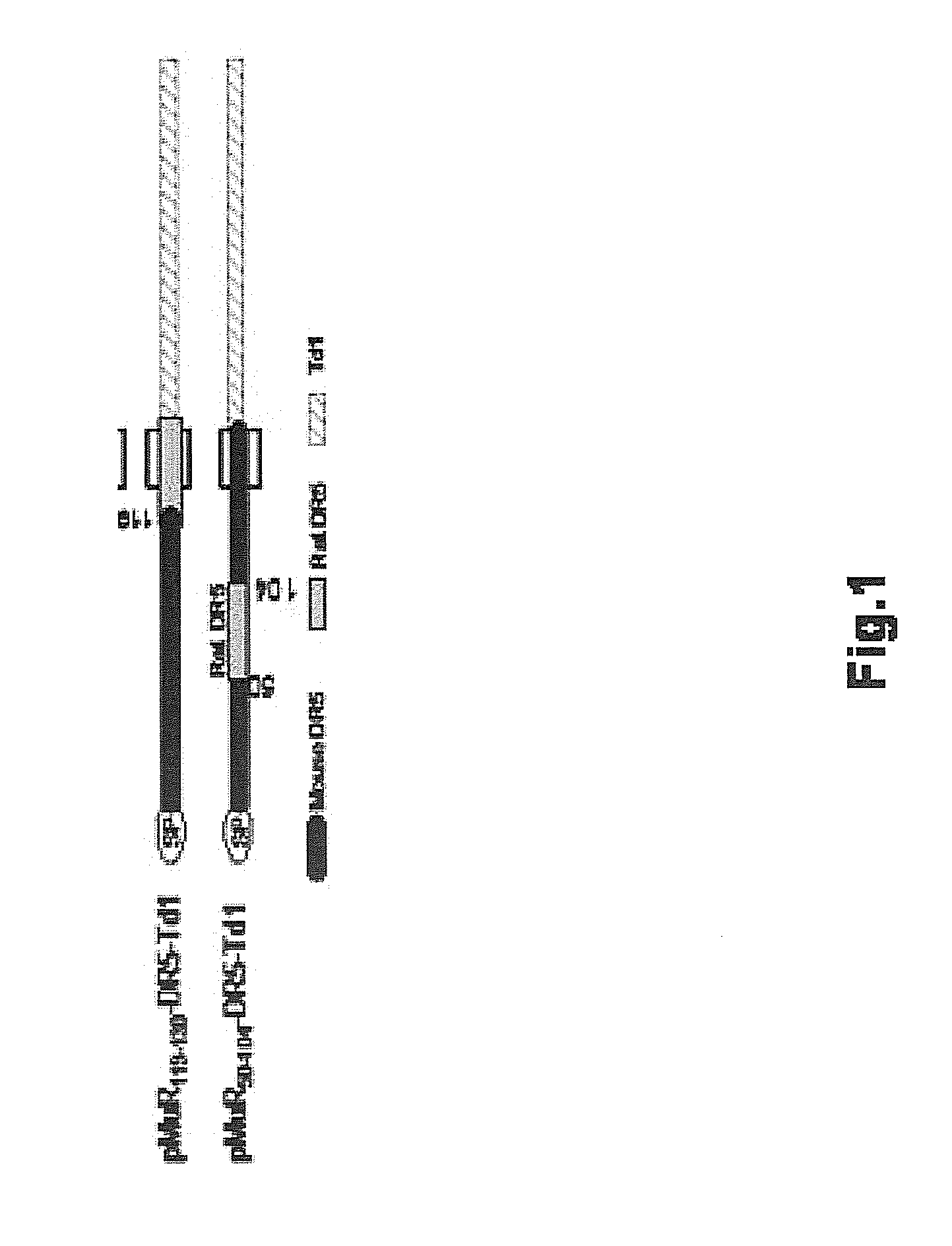
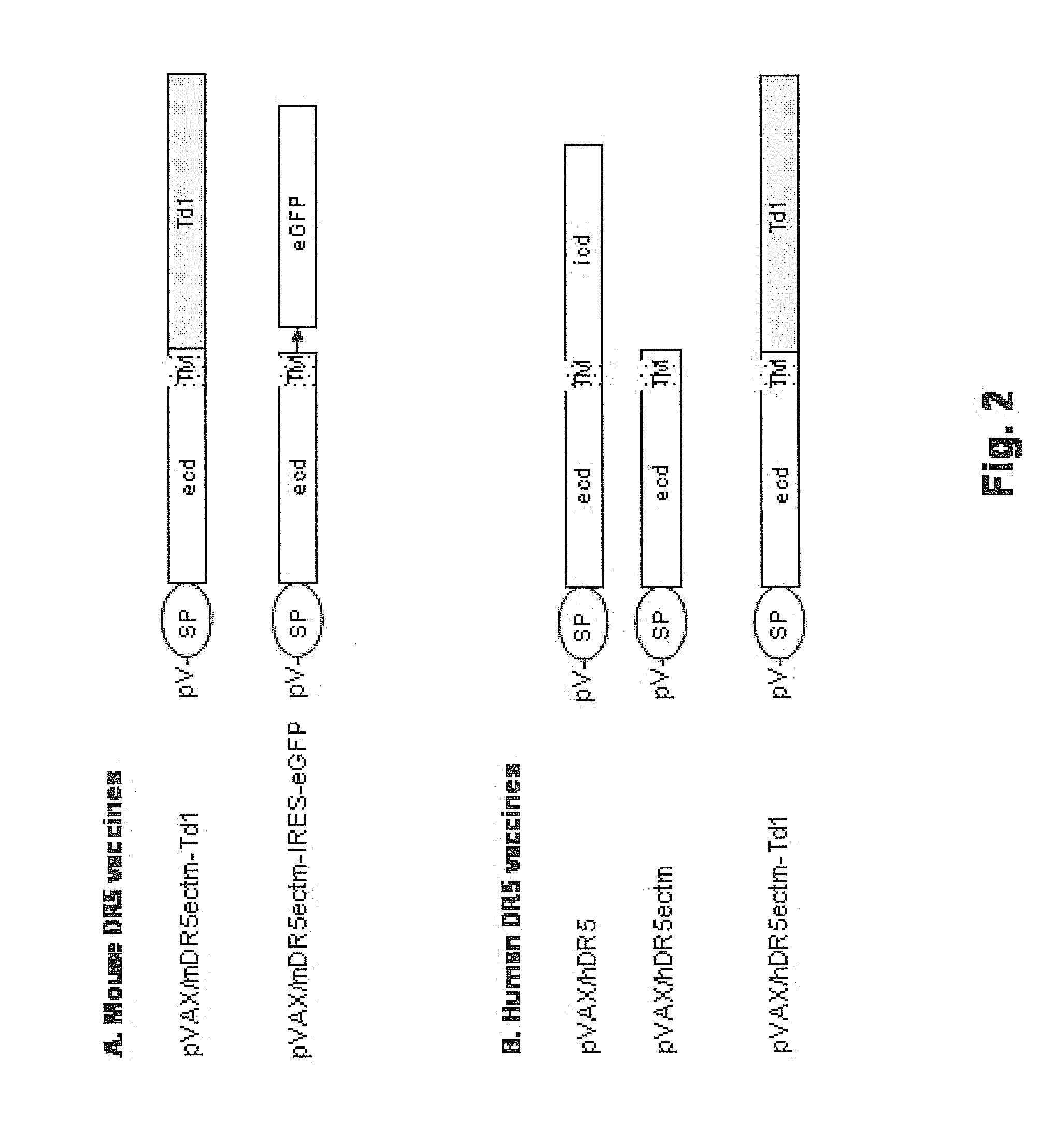
Vaccines targeting cellular death receptors
InactiveUS20140205538A1Improving immunogenicityTolerating abilityCompounds screening/testingPeptide/protein ingredientsCancer cellImmunocompetence
The invention provides therapeutics and methods to induce a mammalian host, including a human, to produce antibodies, which agonize death receptors and cause the apoptotic death of target cells within the host's body. The therapeutics are vaccine compositions, including genetic vaccines encoding death receptor antigens of the tumor necrosis factor receptor family. Also provided are means and methods for overcoming host immunological tolerance to death receptors. The vaccines are useful against cancer cells and other death receptor bearing target cells within the host, and can be used in both therapeutic and prophylactic settings. The vaccines are also useful for diagnostic testing of the immunocompetence of a host.
Owner:WAYNE STATE UNIV
Novel death receptor 5 antibody fusion protein and application thereof
ActiveCN107022557AImprove biological activityImprove stabilityOrganic active ingredientsPeptide/protein ingredientsAutoimmunityFhit gene
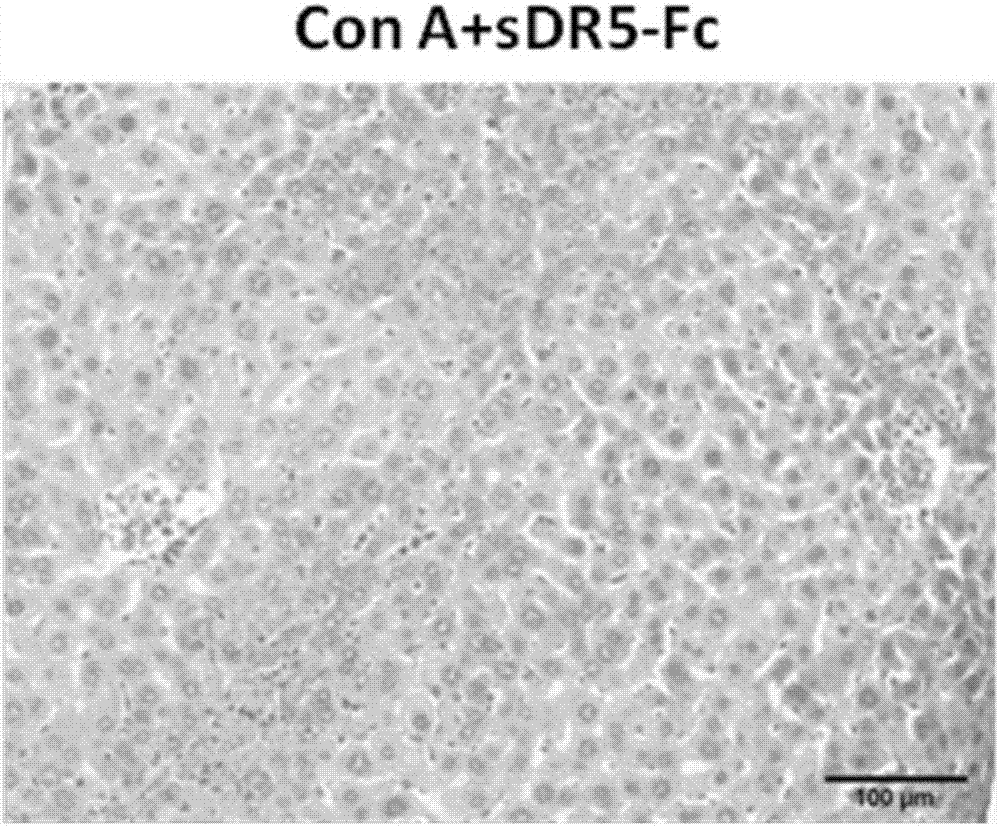
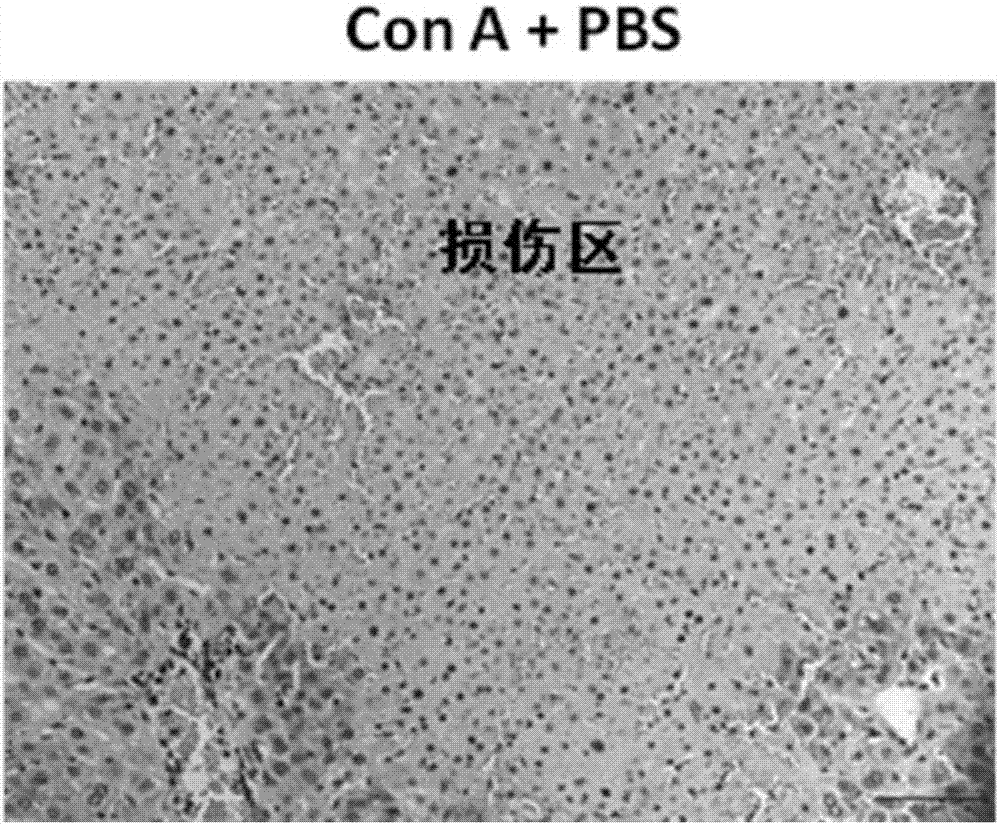
The invention relates to sDR5-Fc recombinant protein (ZJ501-5) with an amino acid sequence shown as SEQ ID NO: 2 and a gene shown as SEQ ID NO: 1 of the recombinant protein, and meanwhile, the invention discloses an application of the sDR5-Fc recombinant protein and the gene thereof in preparing medicines for treating immune liver diseases. In addition, bioactivity of the sDR5-Fc protein in vitro is 3 times higher than that of an sDR5-Fc product from R&D systems Company, the proportion of an N terminal splice variant is just about 1% and the sDR5-Fc protein is high in protein drug stability; and the sDR5-Fc protein provided by the invention, which can significantly improve a survival rate of mice having Con A induced acute autoimmune hepatitis, has a potential application value for treating autoimmune hepatitis in human being.
Owner:SHENZHEN ZHONGKE AMSHENN MEDICINE CO LTD
Double-target-spot fusion protein capable of enhancing TRAIL antitumor activity
InactiveCN103524627AImprove solubilityImprove recycling efficiencyPolypeptide with localisation/targeting motifPeptide/protein ingredientsTRAIL ProteinCell membrane
The invention belongs to the technical field of genetic engineering drugs, and discloses a double-target-spot fusion protein capable of enhancing the TRAIL antitumor activity. The cDNA sequence of the overall-length fusion gene sequence 1 is shown in SEQ ID NO1; the coding amino acid sequence of the overall-length fusion gene sequence 1 is shown in SEQ ID NO2; the coding cDNA sequence of the overall-length fusion gene sequence 2 is shown in SEQ ID NO4; and the coding amino acid sequence of the overall-length fusion gene sequence 2 is shown in SEQ ID NO5. In comparison with the prokaryotic expression carrier of separate TRAIL protein soluble fragment coding cDNA sequence, the fusion protein of the prokaryotic expression carrier has higher soluble expression, and the recycling and purifying efficiency of the fusion protein is higher; and the in-vitro biological activity analysis indicates that the fusion protein can activate death receptors on the cell membrane and inhibit expression of an XIAP gene in the cell plasma, enhance the activity of inducing tumor cell apoptosis through the double-target-spot function in apoptotic pathway and ensure stronger antitumor effect.
Owner:CHENGDU HUACHUANG BIOTECH CO LTD
Vaccines targeting cellular death receptors
ActiveUS20120189572A1Reduce and eliminate cell populationPreventing initiationCompounds screening/testingPeptide/protein ingredientsCancer cellTolerability
The invention provides therapeutics and methods to induce a mammalian host, including a human, to produce antibodies, which agonize death receptors and cause the apoptotic death of target cells within the host's body. The therapeutics are vaccine compositions, including genetic vaccines encoding death receptor antigens of the tumor necrosis factor receptor family. Also provided are means and methods for overcoming host immunological tolerance to death receptors. The vaccines are useful against cancer cells and other death receptor bearing target cells within the host, and can be used in both therapeutic and prophylactic settings. The vaccines are also useful for diagnostic testing of the immunocompetence of a host.
Owner:WAYNE STATE UNIV
Molecular signaling pathways triggered by rituximab: prognostic, diagnostic, and therapeutic uses
InactiveUS20090203050A1Regulate the cancer cells' sensitivity to immunotherapyOrganic active ingredientsMicrobiological testing/measurementAutoimmune conditionCancer cell
The present invention provides markers associated with activated molecular signaling pathways (example: p38 MAKP, NF-κB, ERK1 / 2, YY-1 and AKT) inhibited by rituximab in cancer cells as well as pathways activated by rituximab (such as death receptors, RKIP, PTEN) all of which are associated with the regulation of chemo and immunoresistance. The present invention provides methods of prognosis and providing a prognosis for cancer such as lymphoma, leukemia, and autoimmune disease, as well as, methods of drug discovery. These markers are also therapeutic targets for treatment of cancer resistant to conventional and experimental cancer therapeutics. Inhibition or activation of expression and / or activity of targeted gene products sensitizes resistant tumor cells to subtoxic doses of cytotoxic treatment including chemotherapy, radiation therapy, or immunotherapy and gene therapy, and the cytotoxic molecules.
Owner:RGT UNIV OF CALIFORNIA
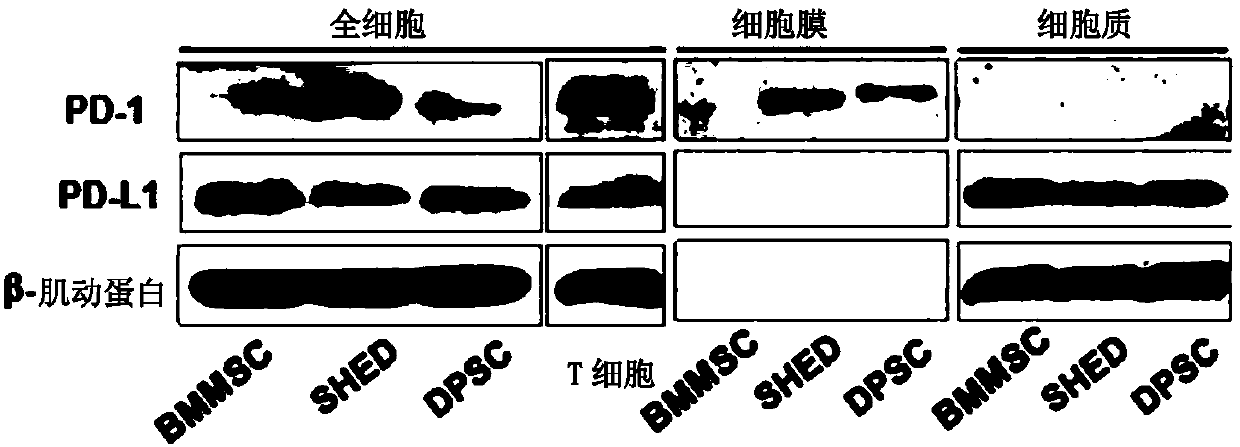
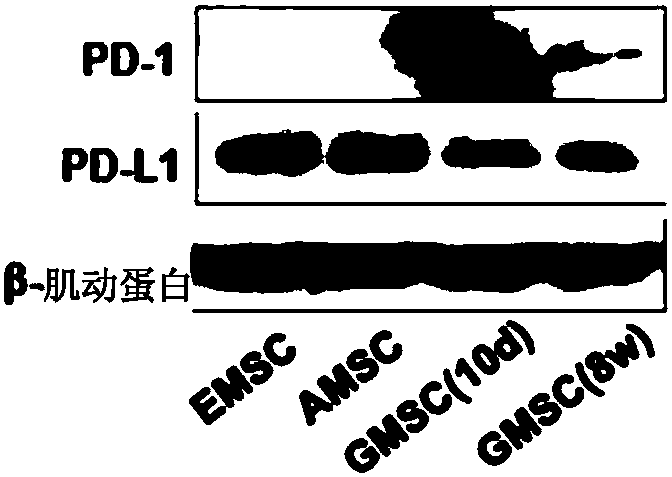
Stem cells for specifically expressing PD-1 as well as identifying and separating method and application of stem cells
The invention discloses stem cells for specifically expressing a programmed death receptor 1 (PD-1), and particularly relates to the stem cells from an organic cavity. The stem cells comprise but notlimited to dental pulp stem cells, gingival mesenchymal steam cells (GMSCs), periodontal ligament stem cells (PDLSCs), stem cells of apical papilla (SCAPs) and dental follicle stem cells (DFSCs) or any combination thereof, and preferably comprise stem cells from human exfoliated deciduous teeth (SHED) and / or dental pulp mesenchymal stem cells for permanent teeth (DPSC); the stem cells also includemesenchymal stem cells from other tissues, which do not express the PD-1 at first and express the PD-1 after being modified by CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), suchas PD-1 plus bone mesenchymal stem cells (BMMSC) modified by the CRISPR. The invention further discloses an identifying and separating method for the stem cells for specifically expressing the PD-1,a method for preparing the PD-1 plus mesenchymal stem cells modified by the CRISPR as well as application of the mesenchymal stem cells for expressing the PD-1, disclosed by the invention, in tissue regeneration, pain relief and treatment of chronic pain and a series of diseases.
Owner:北京泰盛生物科技有限公司
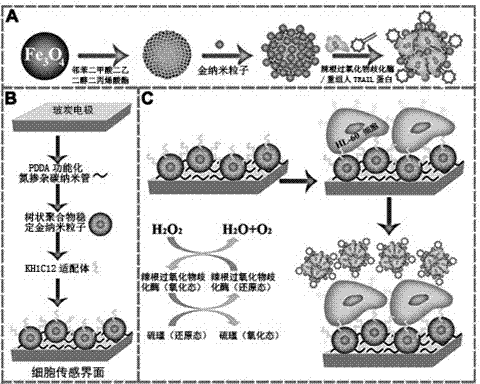
Nanometer structure electrochemical cell sensor preparation method, produced nanometer structure electrochemical cell sensor and use thereof
InactiveCN103713028AImprove hydrophilicityGood biocompatibilityMaterial electrochemical variablesAptamerNucleotide
The present invention discloses a composite nanometer structure electrochemical cell sensor, which comprises two assemblies of a multi-functional mixed nanometer probe and a nanometer structure electrode interface, wherein the multi-functional mixed nanometer probe (HRP-TRAIL-Fe3O4@Au) for electrochemical cell sensing comprises Au nanoparticle-modified magnetic Fe3O4 nanometer spheres immobilized with recombinant human TRAIL protein and horseradish peroxidase (HRP) through a co-immobilization effect, and the nanometer structure electrode interface is an electrode cell sensing interface constructed through a layer-by-layer assembling method, wherein the nanometer structure electrode interface integrates high biocompatibility Au nanoparticles (AuDSNPs) stabilized by a dendrimer, high resistivity nitrogen-doped carbon nano-tubes (CNx) and a high specificity cellular localization oligonucleotide aptamer, and has a nanometer layered structure. The composite nanometer structure electrochemical cell sensor can be provided for carrying out selective quantification detection on expression of DR4 / DR5 death receptors on leukemic cells and surfaces thereof. The present invention further discloses the preparation method.
Owner:NANJING UNIV
Death receptor-5 agitated polyvalent antibody and application thereof in preparation of anti-tumor medicines
ActiveCN102924600ANo toxicityLow immunogenicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesAntiendomysial antibodies
The invention provides an antibody in an extracellular region of a death receptor-5 of a tumor necrosis factor-related apoptosis inducing ligand. By a death receptor-5 agitated polyvalent antibody, a single-chain antibody of the death receptor-5 forms a polyvalent antibody through an oligomerization structural domain, and the amino acid sequence is shown as SEQIDNO.1. The single-chain antibody of the death receptor-5 forms a tetravalent antibody through a p53 structural domain, and the amino acid sequence is shown as SEQIDNO.2. After multimerization, the antibody can induce apoptosis of tumor cells, and is nontoxic to normal cells. The invention also provides an application of the death receptor-5 agitated polyvalent antibody in preparation of anti-tumor medicines.
Owner:HENAN UNIVERSITY
Bi-specific antibodies for medical use
InactiveUS20140314764A1High affinityImprove avidityNervous disorderAntipyreticDiseaseCell Surface Antigens
A bispecific antibody format devoid of an active Fc moiety comprising a monovalent binding site for a death receptor and at least one binding site for a cell surface antigen expressed on B-cells, for use in the treatment or prevention of B cell mediated autoimmune diseases.
Owner:JUNG GUNDRAM
Use of cyclosporin A to sensitize resistant cancer cells to death receptor ligands
InactiveUS20080206287A1High sensitivityIncrease killMicrobiological testing/measurementTissue cultureDeath receptor ligandCyclosporins
Cyclosporin A has been discovered to sensitize cancers resistant to TNF-family death receptors such as TRAIL and Fas to ligand-mediated apoptosis. Therefore, compositions that include cyclosporin A are useful in treating such cancers.
Owner:REED JOHN C +1
Full-humanized agonist single-chain antibody resistant to human death receptor 5 and application thereof
ActiveCN106397594AEnhanced inhibitory effectGood antitumor activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEscherichia coliTumor target
The invention relates to a single-chain antibody capable of specific binding and capable of activating human death receptor 5 (DR5), and belongs to a full-humanized antibody; the single-chain antibody is obtained by performing quintuple screening in a high-capacity full-humanized phage antibody library by using human death receptor DR5 extracellular protein as a target antigen; the single-chain antibody may be acquired by induced expression in Escherichia coli, nickel ion affinity chromatography and molecular-exclusion chromatographic purification; pharmacodynamic experiments prove that the single-chain antibody is efficient in suppressing the growth of DR5 positive colon cancer cell COLO205 and breast cancer cell MDA-MB-231; the single-chain antibody and other antibodies modified therefrom, including full-length, bispecific or fusion protein forms, may act as drugs for positive tumor targeting therapy of death receptor DR5; the single-chain antibody has the advantages such as low immunogenicity, high specificity and high penetrability and is an ideal targeting tumor therapeutic drug.
Owner:CHINA PHARM UNIV
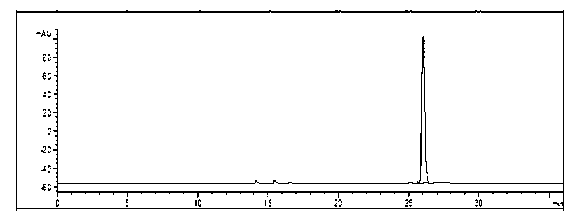
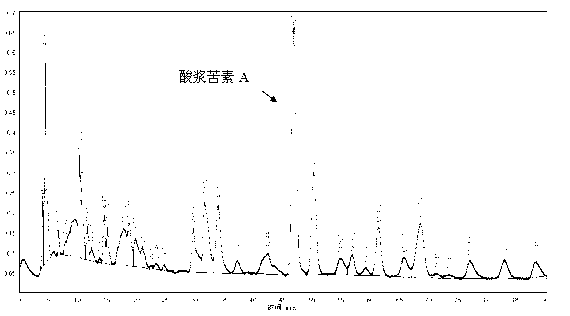
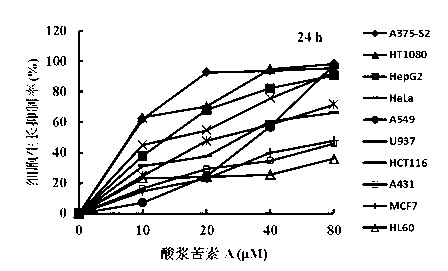
Physalin A extracting process and medical application thereof
The invention belongs to the technical field of medicines, in particular relates to a physalin A extracting process and medical application thereof, in particular relates to a novel application of physalin A in preparation of an anti-tumor dug and in particular relates to the use of physalin A in treatment of human fibrosarcoma and human malignant melanoma. After process optimization, the extracting rate of physalin A is 0.2133%. Physalin A can be used for inhibiting the growth of various tumor cells, particularly has obvious inhibiting action on the growth of the human fibrosarcoma and human malignant melanoma, but does not inhibit the activities of the human normal cells obviously. The mechanism is that the downstream caspase family-associated protein is activated by activating Fas death receptors, so that the tumor cells are induced to generate apoptosis. Meanwhile, the physalin A can be used for inducing the tumor cells to generate autophagy for achieving autophagy antagonism apoptosis in the HT1080 and the A375-S2 cells; and the p53 protein and the MAPK (Mitogen-Activated Protein Kinase)-familty p38 protein have a key regulation effect. The physalin A can be used for preparing a digestive tract dosage form or a non-digestive tract dosage form, which can be used for treating tumors including the human fibrosarcoma and human malignant melanoma.
Owner:SHENYANG PHARMA UNIVERSITY
Antibody specifically binding to dr5 and composition for preventing or treating cancers comprising the same
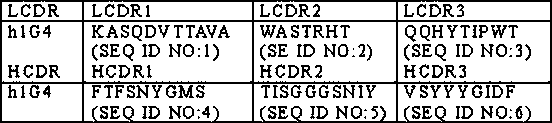
The present invention provides an antibody specifically binding to death receptor 5 (DR5), which is selected from the group consisting of: an antibody comprising a heavy chain variable region (VH) having the amino acid sequences of SEQ ID NOs: 1 to 3 at complementary determining regions (CDRs) and a light chain variable region (VL) having the amino acid sequences of SEQ ID NOs: 4 to 6 at CDRs; and an antibody comprising a (VH) having the amino acid sequences of SEQ ID NOs: 7 to 9 at CDRs and a (VL) having the amino acid sequences of SEQ ID NOs: 10 to 12 at CDRs, and a composition for preventing or treating a cancer comprising the same. The antibody of the present invention can be effectively used for the prevention or treatment of various cancers, through inducing autophagic cell death of TRAIL-sensitive cancer cells as well as TRAIL-resistant cancer cells by specific binding to DR5.
Owner:AJOU UNIV IND ACADEMIC COOP FOUND
Combinations of pd-1 antagonists and cyclic dinucleotide sting agonists for cancer treatment
InactiveUS20190328762A1Organic active ingredientsSugar derivativesStimulator of interferon genesAgonist
Therapeutic combinations that comprise at least one antagonist of the Programmed Death 1 receptor (PD-1) and at least one cyclic dinucleotide compound that activates the Stimulator of Interferon Genes (STING) pathway are disclosed herein. Also disclosed is the use of such therapeutic combinations for the treatment of cancers.
Owner:MERCK SHARP & DOHME CORP
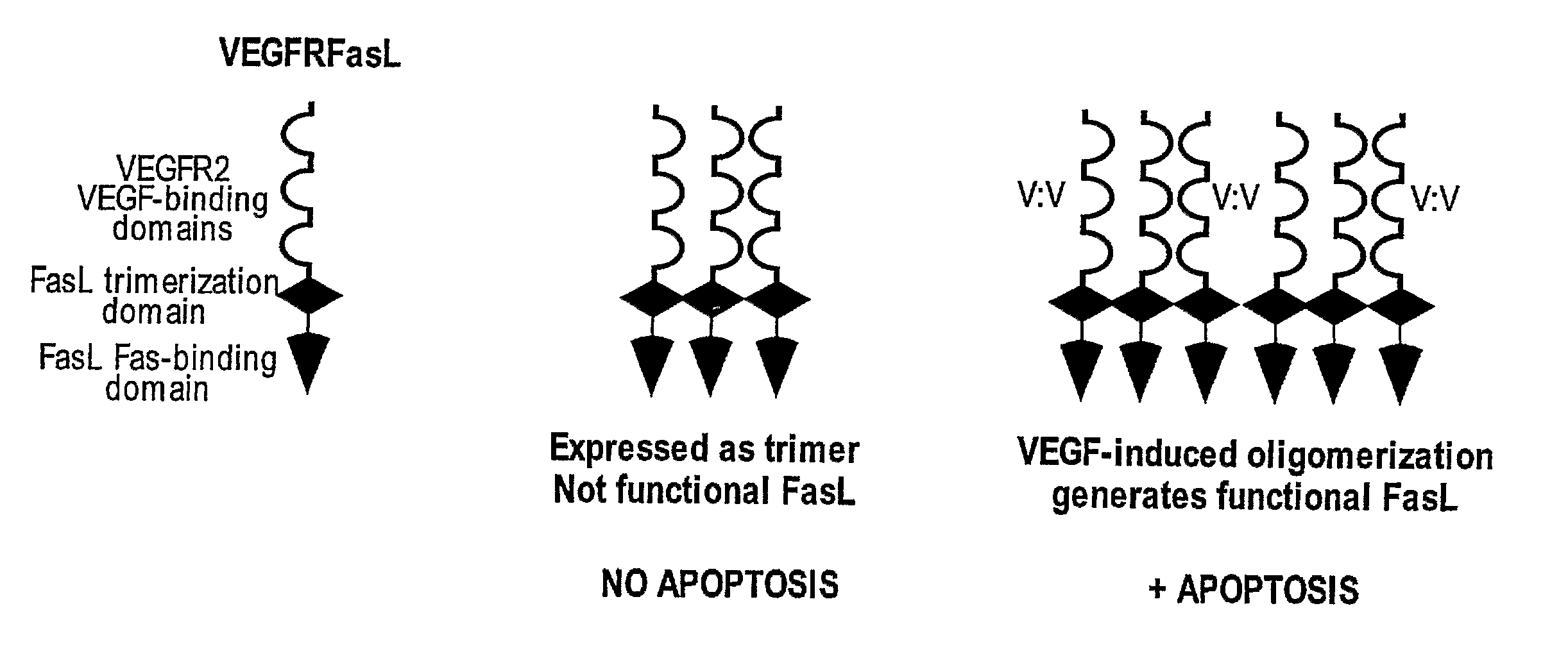
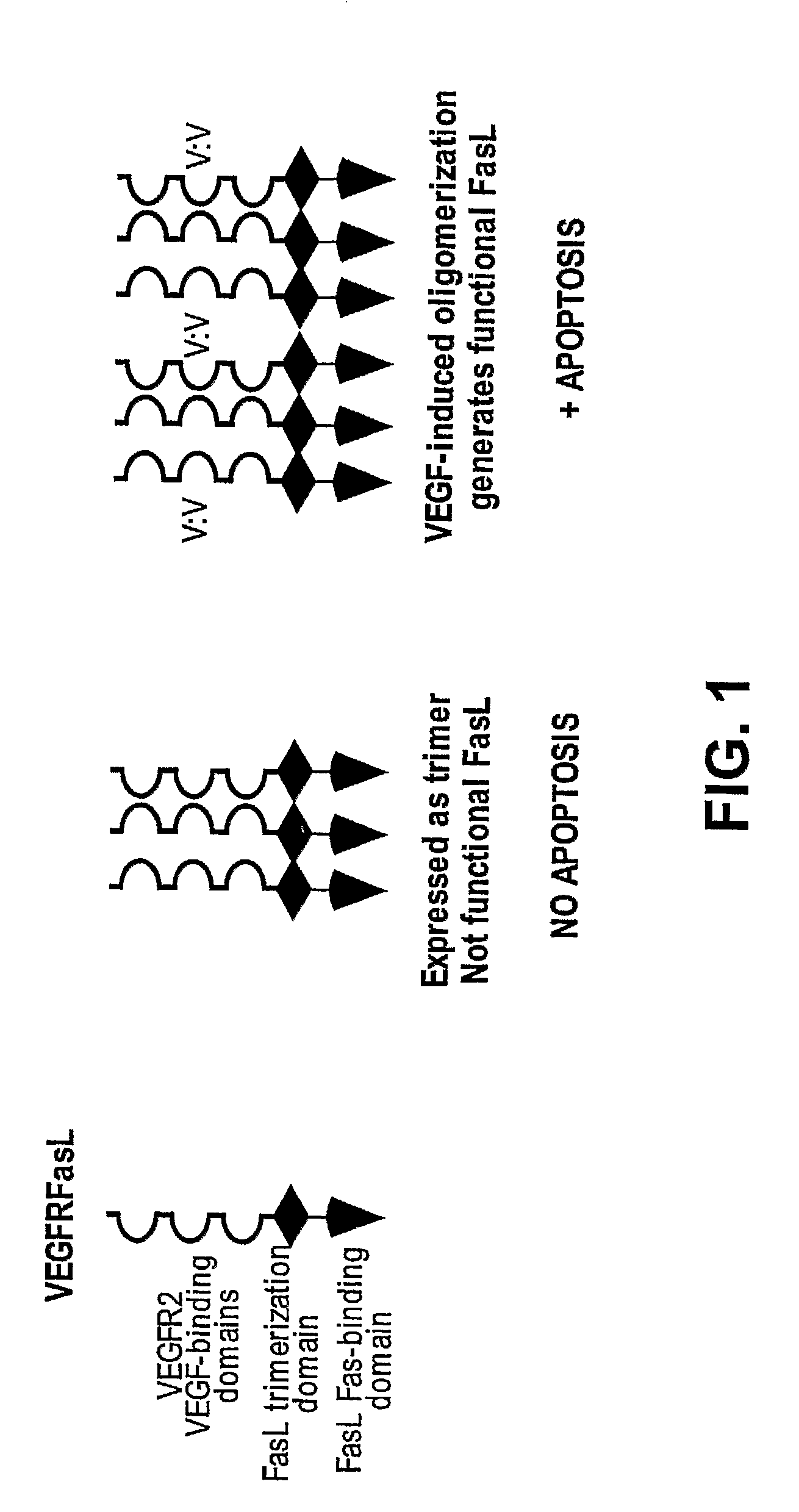
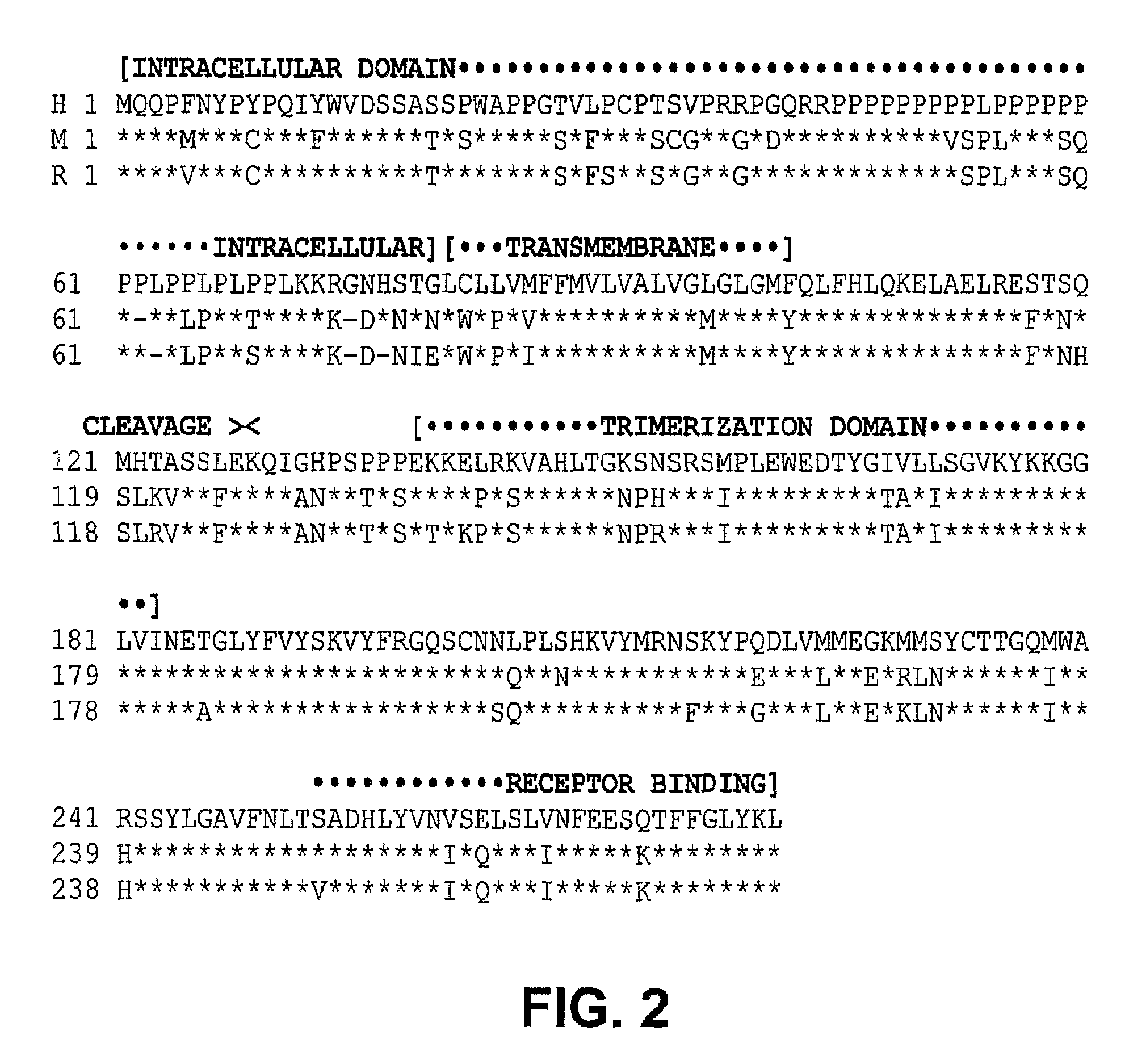
VEGF-activated ligands
The present invention provides fusion proteins comprising an extracellular domain of a VEGF receptor and a death ligand. The fusion proteins bind to VEGF and to death receptors on tumor cells thereby inhibiting VEGF activation of VEGF receptors and inducing apoptosis in the tumor cells. Fusion proteins of the present invention are useful for inducing apoptosis and cytotoxic effects in cells, treating cancer and diseases or disorders related to unregulated angiogenesis and / or vasculogenesis. Thus, this invention further provides methods for treating angiogenesis related diseases using the fusion proteins, polynucleotides encoding the fusion proteins, vectors containing the polynucleotides, pharmaceutical compositions and kits containing the fusion proteins or the polynucleotides encoding the fusion proteins.
Owner:RGT UNIV OF CALIFORNIA
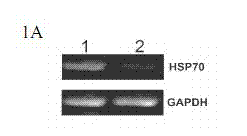
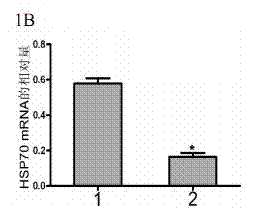
Method for regulating expression, quantity and activity of heat shock protein 70 and application thereof
ActiveCN102816792AIncreased sensitivityAdded treatment strategyPeptide/protein ingredientsGenetic material ingredientsDeath ReceptorsTranscription Factor NF-kB
The invention belongs to the field of biotechnology, and in particular relates to a method for regulating expression, quantity and activity of a heat shock protein 70 and application thereof. The method provided by the invention can also regulate tumor tissue, activity of a transcription factor NF-kB, expression and activity of a death receptor DR4 and DR5, phosphorylation and activity of JNK and c-Jun, expression and activity of a p53 protein, expression and proapoptotic activity of proapoptotic molecules Bax, Bid, t-Bid, capsase 3, capsase 8 and capsase 9, and expression and activity of c-FLIP and Bcl-2, and promote apoptosis of tumor tissues and cells. The invention also relates to application of the above method to preparation of drugs cooperatively applied with cell apoptosis induced drug.
Owner:NANJING UNIV
Methods and Compositions for Treatment of Sepsis
InactiveUS20090012025A1Reduce cell deathAntibacterial agentsOrganic active ingredientsMitochondrial pathwayDeath Receptors
Methods of treatment of sepsis are disclosed. These methods comprise administering to a subject a composition comprising at least one siRNA directed against at least one gene encoding a pro-apoptotic polypeptide. The pro-apoptotic polypeptide, in some aspects, can be other than Fas or caspase-8. In some embodiments, an siRNA can be directed against a pro-apoptotic component of the mitochondrial pathway, such as a pro-apoptotic bcl-2 protein. In some aspects, an siRNA can be directed against a BH3-only bcl-2 protein, while in other aspects, siRNAs can be directed against multi BH domain Bcl-2 family members such as bax and bak. In some embodiments, an siRNA can be directed against a death receptor pathway molecule such as FADD. In various configurations, a composition can also comprise a cationic lipid such as DOTAP, or nanoparticles comprising a cyclodextrin-containing polycation and a polymer such as a poly(ethylene glycol).
Owner:HOTCHKISS RICHARD +3
Biologic compounds directed against death receptor 5
The present invention relates to amino acid sequences that are directed against TRAIL cell surface receptor 2 (herein also “DR5”), as well as to compounds or constructs thereof, and in particular proteins and polypeptides and nucleotides that encode them (referred to herein in their entirety as “NB agents”) and fragments thereof, and pharmaceutically effective variants thereof, and their use in the diagnosis and treatment of DR5 associated diseases and disorders.
Owner:ABLYNX NV +1
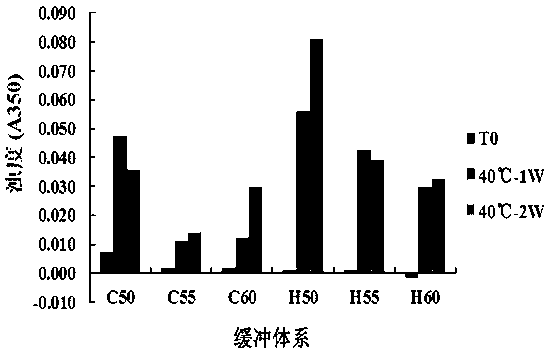
Cell programmed death acceptor 1 antibody preparation and use thereof
The invention provides a pharmaceutical preparation containing a cell programmed death acceptor 1 monoclonal antibody. The pharmaceutical preparation comprises ingredients: a PD-1 monoclonal antibody,a citric acid-sodium citrate buffer solution, a protein protectant, a surfactant and an isotonicity regulator. According to the pharmaceutical preparation, proven by a longest-4-week accelerated experiment at a temperature of 40 DEG C, major detection indexes, i.e., testing equivalent standards such as appearance, concentration, turbidity (A350), pH value, osmotic pressure and purity are all freeof obvious changes, and good stability is shown.
Owner:SHANGHAI HENLIUS BIOTECH INC +2
Agents and Methods Related to Reducing Resistance To Apoptosis-Inducing Death Receptor Agonists
InactiveUS20100135951A1Reduce resistancePeptide/protein ingredientsAntipyreticApoptosisDeath Receptors
Provided herein is a method of reversing or preventing a target cell's resistance to a death receptor agonist. Also provided are methods of screening for biomarkers resistance of and monitoring resistance to death receptor agonists. Also provided are methods of selectively inducing apoptosis in a target cell, treating a subject with cancer, autoimmune or inflammatory diseases, comprising administering compositions provided herein. Further provided are compositions comprising agents that modulate CARD containing proteins.
Owner:UAB RES FOUND
Treatment of proliferative disorders with a death receptor agonist
InactiveUS20110262455A1Improve the immunityIncrease ratingsOrganic active ingredientsPeptide/protein ingredientsMedicineDeath Receptors
A method of treating a proliferative disorder, and a pharmaceutical composition for use in such a method, comprises administering to the patient a combination of an agonist of a death receptor and an antagonist of Egr-1. The death receptor agonist and the Egr-1 antagonist may be administered sequentially, separately or in combination
Owner:NATIONAL UNIVERSITY OF IRELAND
Nucleic acid aptamer of programmed death receptor-ligand 1 (PD-L1) and application of nucleic acid aptamer
ActiveCN110004149AEasy to synthesizeMark easyBiological material analysisPharmaceutical non-active ingredientsPD-L1 inhibitorDynamic monitoring
The invention discloses a nucleic acid aptamer of a programmed death receptor-ligand 1 (PD-L1) and application of the nucleic acid aptamer, and relates to a nucleic acid. The nucleic acid aptamer withhigh specific recognition on PD-L1 and high affinity combination force on PD-L1 is provided. The nucleic acid aptamer can be screened and prepared by means of a protein SELEX method based on agarosemicrobead separation-flow cytometry analysis. The nucleic acid aptamer has the advantages that G basic groups are abundant, a stem-loop structure is provided, synthesis and marking are easy, the costis low, the stability is high, and non-immunogenicity is excellent; the nucleic acid aptamer is utilized to achieve the specific recognition of PD-L1 proteins, tumor cell lines of PD-L1 expression proteins and exudates of the PD-L1 expression proteins excreted by the tumor cell lines, the application of the nucleic acid aptamer in the field of biochemical analysis and detection is verified, the nucleic acid aptamer is expected to be used as a molecular recognition tool for detecting the expression level of PD-L1 and applied to precise prediction and dynamic monitoring of the treatment effect of PD-1 / PD-L1 inhibitors in the field of clinical medical tumor immunity research.
Owner:XIAMEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com