Full-humanized agonist single-chain antibody resistant to human death receptor 5 and application thereof
A death receptor and single-chain antibody technology, which is applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody, anti-animal/human immunoglobulin, etc., can solve the problem of short half-life, etc. Achieve the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
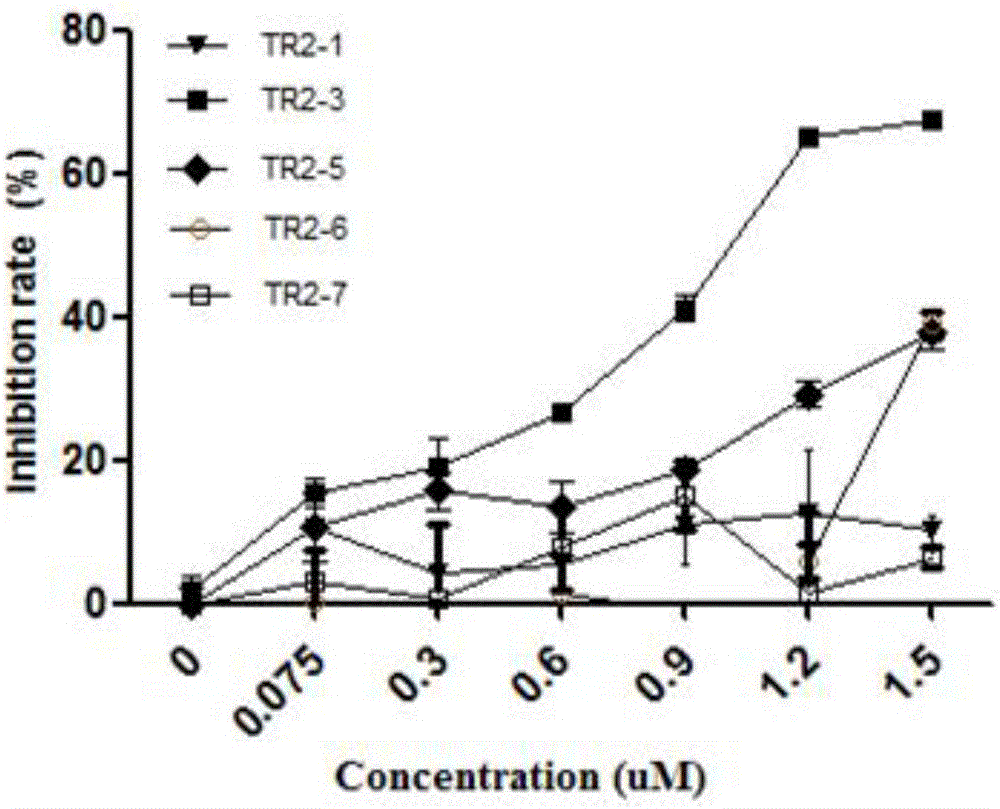
Examples
Embodiment 1
[0033] Example 1. Expression and purification of human death receptor DR5 protein
[0034] Using the cDNA of the human death receptor DR5 (product of Beijing Yiqiao Shenzhou Co., Ltd.) as a template, PCR amplifies the gene fragment of the extracellular region (1-130aa) of the death receptor DR5, clones it into the expression vector pET21b, and transforms it into Escherichia coli BL21 (DE3) for protein expression. The expression conditions are as follows: inoculate the seed solution in 2×YT-AG medium with 2% inoculum amount and culture with shaking at 37°C until OD 600nm =0.6, add IPTG with a final concentration of 0.5mM, and induce at 20°C for 20h. Centrifuge at 6000rpm at 4°C for 15min to collect the cells; resuspend the cells in PBS and sonicate (sonication for 3s, interval of 3s, 20min in total); centrifuge at 12000rpm at 4°C for 30min to collect the supernatant; pass the supernatant through a nickel ion affinity column (GE company product) and molecular exclusion chromat...
Embodiment 2
[0035] Example 2. Construction of phage human single-chain antibody library
[0036] The construction of the phage human single-chain antibody library was carried out according to the literature [Sblattero D., et al. Nat Biotechnol, 2000, 18: 74-80; Sblattero D., et al. Immunotechnology, 1998, 3: 271-278]. Using the peripheral blood of 105 healthy people and 58 peripheral blood of tumor patients as sources, the peripheral blood lymphocytes producing antibodies were separated by density gradient centrifugation, the total RNA was extracted, and cDNA (product of Treasure Biotech Co., Ltd.) was synthesized by reverse transcription. As a template, 6 heavy chain upstream primers, 4 heavy chain downstream primers, 7 lambda chain upstream primers and 3 lambda chain downstream primers were selected for PCR amplification of the variable region of the human-specific antibody [Sblattero D., et al. Nat Biotechnol, 2000, 18: 74-80; SblatteroD., et al. Immunotechnology, 1998, 3: 271-278], an...
Embodiment 3
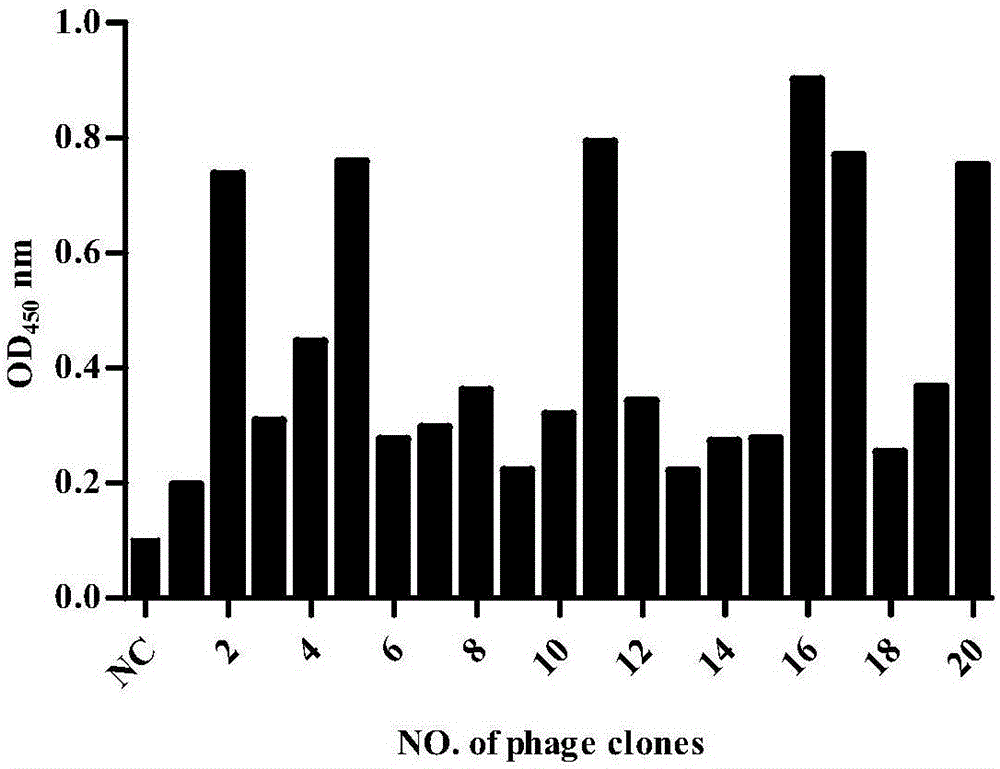
[0037] Example 3. Screening of fully human anti-human death receptor DR5 single chain antibody
[0038] Coating buffer (50mM NaHCO 3 , pH9.6) to dilute the death receptor DR5 extracellular domain protein to 20ug / ml, take 2ml and add it to the immune tube, and coat it at room temperature overnight; the next day, discard the supernatant, and wash the immune tube 3 times quickly with PBS; 2% MPBS ( PBS containing 2% skimmed milk), 37°C for blocking for 2h; discard the blocking solution, and wash the immunotube 3 times quickly with PBS; phage antibody library (~10 12 pfu) suspended in 4ml 2% MPBS and added to the immune tube, placed on a rotary shaker and rotated repeatedly for 2h, washed the immune tube 15 times with PBS containing 0.1% Tween-20, and then washed the immune tube 15 times with PBS; add 1ml 100mM triethylamine, rotate and incubate at room temperature for 10min for specific elution; 0.5ml 1M Tris-HCl (pH7.4) buffer is used to quickly neutralize the eluted phage; the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com