Death receptor-5 agitated polyvalent antibody and application thereof in preparation of anti-tumor medicines
A technology of death receptors and multivalent antibodies, which is applied in the direction of anti-tumor drugs, anti-animal/human immunoglobulins, antibodies, etc., to achieve the effect of reducing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Preparation of DR5 single-chain antibody
[0028] Routine immunization was performed by emulsifying 100 μl (100 μg) of DR5 antigen (PeproTech) with an equal volume of complete Freund's adjuvant. Spleen of mice was taken, total RNA was extracted, and cDNA was synthesized by reverse transcription. According to the protocol of "Recombinant Antibodies" (Shen Beifen, Science Press), cDNA was used as a template to amplify a complete set of VH and VL genes, and the amplified heavy and light chain products were subjected to overlap extension PCR to amplify ScFv fragments. The ScFv fragment and the carrier PAK100 were digested with SfiI, ligated with T4 ligase, and electrotransformed into E. coli XL1-Blue competent cells to prepare a phage antibody library. Coat 96-well plates with DR5 antigen (100 μg / ml in the first round, 30 μg / ml in the second round, 10 μg / ml in the third round, 1 μg / ml in the fourth round, and 0.5 μg / ml in the fifth round), After blocking with 1%...
Embodiment 2
[0079] Example 2 Construction of Tetravalent Antibody Expression Vector
[0080] Using the DR5 single-chain antibody gene as a template and L1 and H2 as primers, ScFv was amplified by PCR. The reaction conditions were: 95°C for 3 minutes, 95°C for 30 sec, 60°C for 40 sec, and 72°C for 30 sec, 30 cycles. Using P53a and P53b as primers, the P53 fragment was amplified by PCR, and the reaction conditions were: 95°C for 30 sec, 60°C for 40 sec, 72°C for 30 sec, 30 cycles. Using the above product as a template and L1 and P532 as primers, overlap extension PCR was carried out. The reaction conditions were: 95°C for 3 min, 95°C for 30 sec, 60°C for 40 sec, 72°C for 30 sec, 30 cycles. Take the recovered product VL+VH2+P53 of the target gene, and use the restriction endonucleases XhoI and Hind III as a double-digestion system for fragments. Take the empty vector plasmid pcDNA3.1, and cut it with restriction endonucleases XhoI and HindIII. The target gene was recovered by gel cutting, ...
Embodiment 3
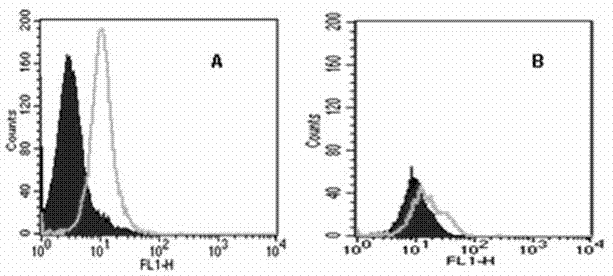
[0086] Example 3 Screening of agonistic tetravalent antibodies
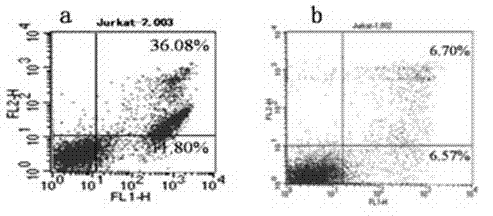
[0087] The tetravalent antibody plasmids of each clone were transiently transfected into COS7 cells, and the supernatant was harvested after 48 hours, and the expression of the tetravalent antibody was confirmed by westenblot (results in image 3 ). Collect and prepare Jurkat cell suspension, add to 24-well culture plate, the final concentration of cells is 1×10 5Each well was cultured at 37°C and 5% CO2 for 12 hrs, and 50 μl of COS7 cell culture supernatant was added; negative control wells used RPMI1640 culture medium. at 37°C and 5% CO 2 Incubate for 20 h under certain conditions, and collect all the cells in each well into corresponding centrifuge tubes. After washing the cells with PBS, suspend the cells in 100 μl of AnnExin V binding solution in each well, add 5 μl of AnnExin V-FITC solution and 5 μl of PI solution, mix well, incubate at room temperature in the dark for 20 min, and use binding Make up ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com