Cell programmed death acceptor 1 antibody preparation and use thereof
A formulation and pharmaceutical preparation technology, applied in the field of PD-1 antibody preparations, can solve the problems of different structures and properties, lack of protein preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Sample Test Method
[0021] 1.1. Osmotic pressure detection
[0022] The pipette draws 20 μl of the sample and two osmotic pressure standards of 290 mOsmol / kg, and the Advanced 2020 osmometer is used to measure the osmotic pressure values of the samples and the standard.
[0023] 1.2. SEC-HPLC
[0024] Agilent 1260 high performance liquid chromatography, TOSOH TSK G3000 (300*7.8 mm) chromatographic column was used to analyze the sample, the column temperature was room temperature, the flow rate was 0.5 mL / min, and the detection wavelength was 280 nm. The composition of the mobile phase was 100 mM PB, 0.5% NaCl, pH 6.8, the test sample was diluted to about 1.0 mg / mL with the mobile phase, 50 μL was injected, and the stop time was 30 min.
[0025] 1.3. CEX-HPLC
[0026] Agilent 1260 HPLC, Thermo ProPac WCX-10 column (4 × 250 mm) was used to analyze the sample, the column temperature was 35°C, the flow rate was 1.0 mL / min, the detection wavelength was 280 n...
Embodiment 2
[0031] Example 2. Preparation of Antibodies
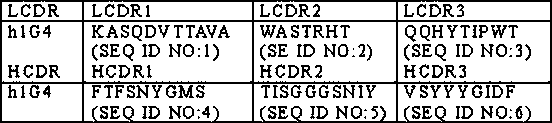
[0032]The PD-1 antibody h1G4 is derived from the h1G4 disclosed in the patent application WO2018052818, and its preparation method fully refers to WO2018052818. The amino acid sequence of the PD-1 antibody h1G4 is as follows:
[0033] Table 2 CDR sequence of h1G4
[0034]
[0035] h1G4 antibody heavy chain variable region (VH) SEQ ID NO: 7
[0036] QVQLVESGGGLVKPGGSLRLSCAASG FTFS NYGMS WIRQAPGKGLEWVS TISGGGSNIY YADSVKGRFTISRDNAKNSLYLQMNSLRAEDTAVYYC VSYYYGIDF WGQGTSVTVSS
[0037] h1G4 antibody light chain variable region (VL) SEQ ID NO: 8
[0038] DIQMTQSPSSLSASVGDRVTITC KASQDVTTAVA WYQQKPGKAPKLLIY WASTRHT GVPSRFSGSGSGTDFTLTISSLQPEDFATYYC QQHYTIPWT FGGGTKLEIK
[0039] h1G4 antibody light chain (LC) SEQ ID NO: 9
[0040] DIQMTQSPSSLSASVGDRVTITCKASQDVTTAVAWYQQKPGKAPKLLIYWASTRHTGVPSRFSGSGSGTDFLTISSLQPEDFATYYCQQHYTIPWTFGGGTKLEIKRTVAAPSVFIFPPSDEQLKSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLGSKADYEVECKQGLSS ...
Embodiment 3
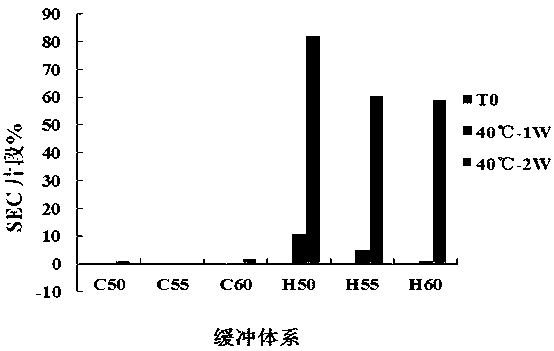
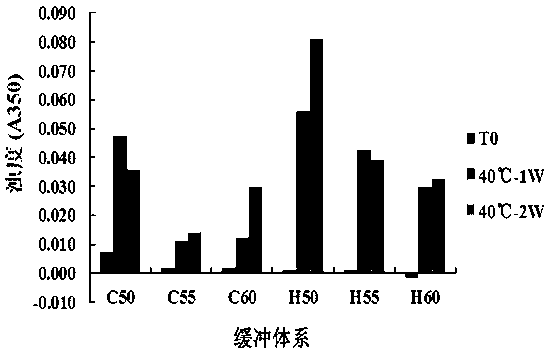
[0043] Embodiment 3. Buffer system screening research
[0044] In this study, two groups of different formulation buffer systems were selected as alternative formulations (hereinafter referred to as B1~B6), and the concentration of the target drug h1G4 antibody was 10.0 mg / mL. Among them, B1~B3 are 20 mmol / L citric acid-sodium citrate buffer system, the pH values are 5.0, 5.5 and 6.0; B4~B6 are 20 mmol / L histidine-histidine hydrochloric acid buffer system, pH The values are 5.0, 5.5 and 6.0; the above buffer systems all contain 3% mannitol, 0.02% polysorbate 80 and 0.3% sodium chloride as auxiliary materials, and the concentration percentages of auxiliary materials in this material refer to the mass volume ratio (w / v , g / L). See Table 3 for information on alternative prescriptions for screening research on the buffer system of h1G4 antibody preparations.
[0045] Table 3. Alternative buffer system compositions for h1G4 antibody preparations
[0046]
[0047] Take an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com