Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Pathway signaling" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding (or signal sensing) in a receptor give rise to a signaling cascade, which is a chain of biochemical events along a signaling pathway.
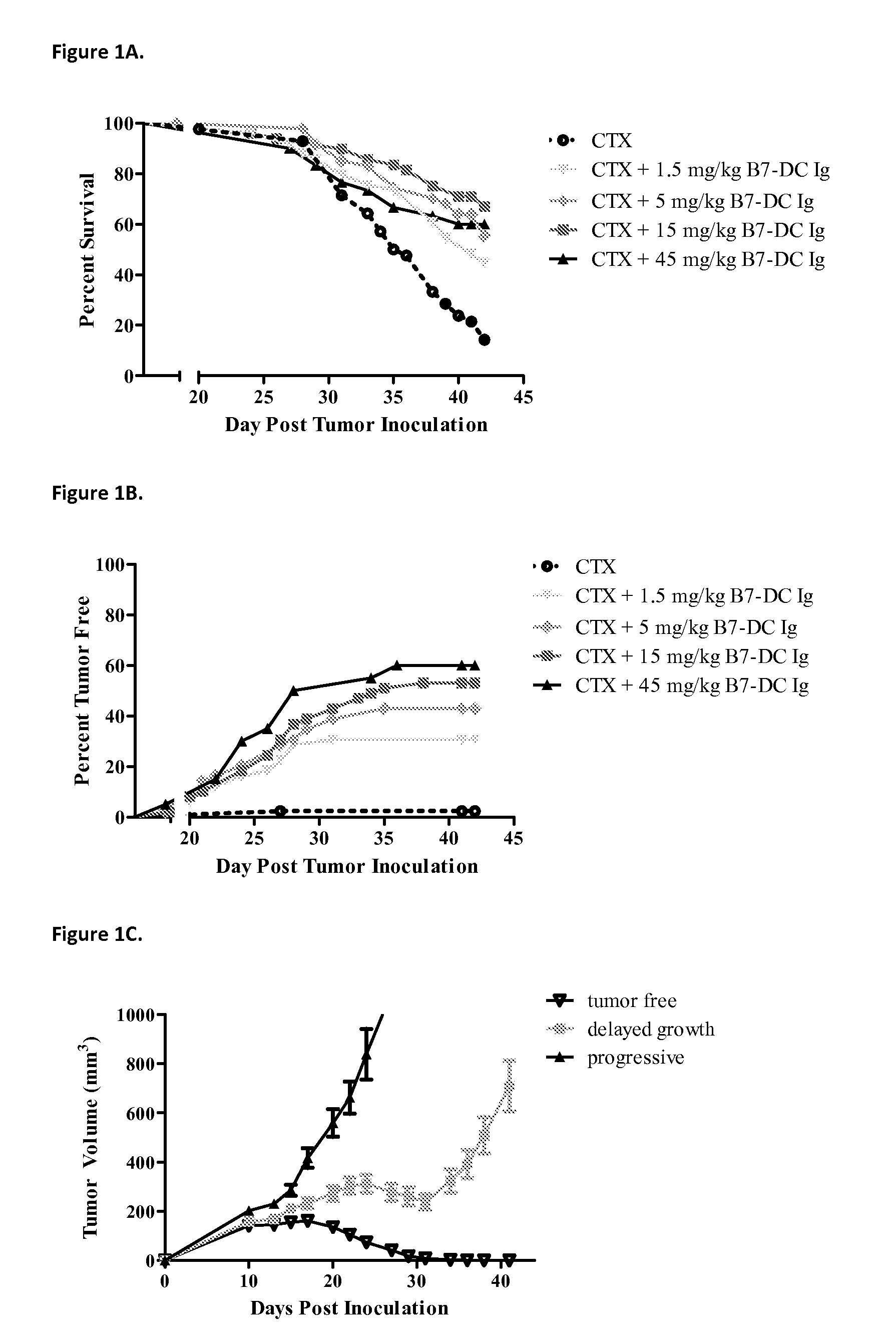
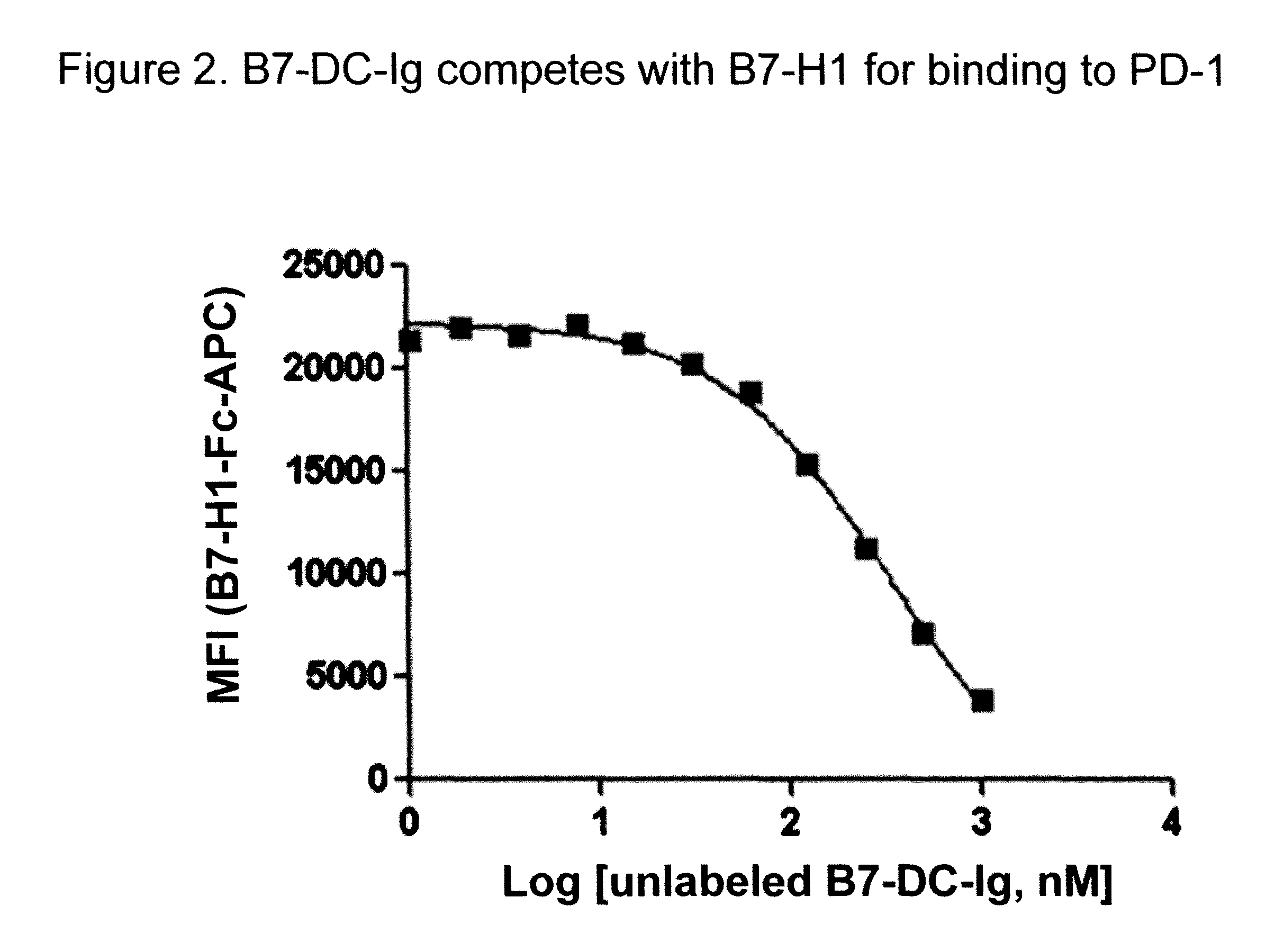
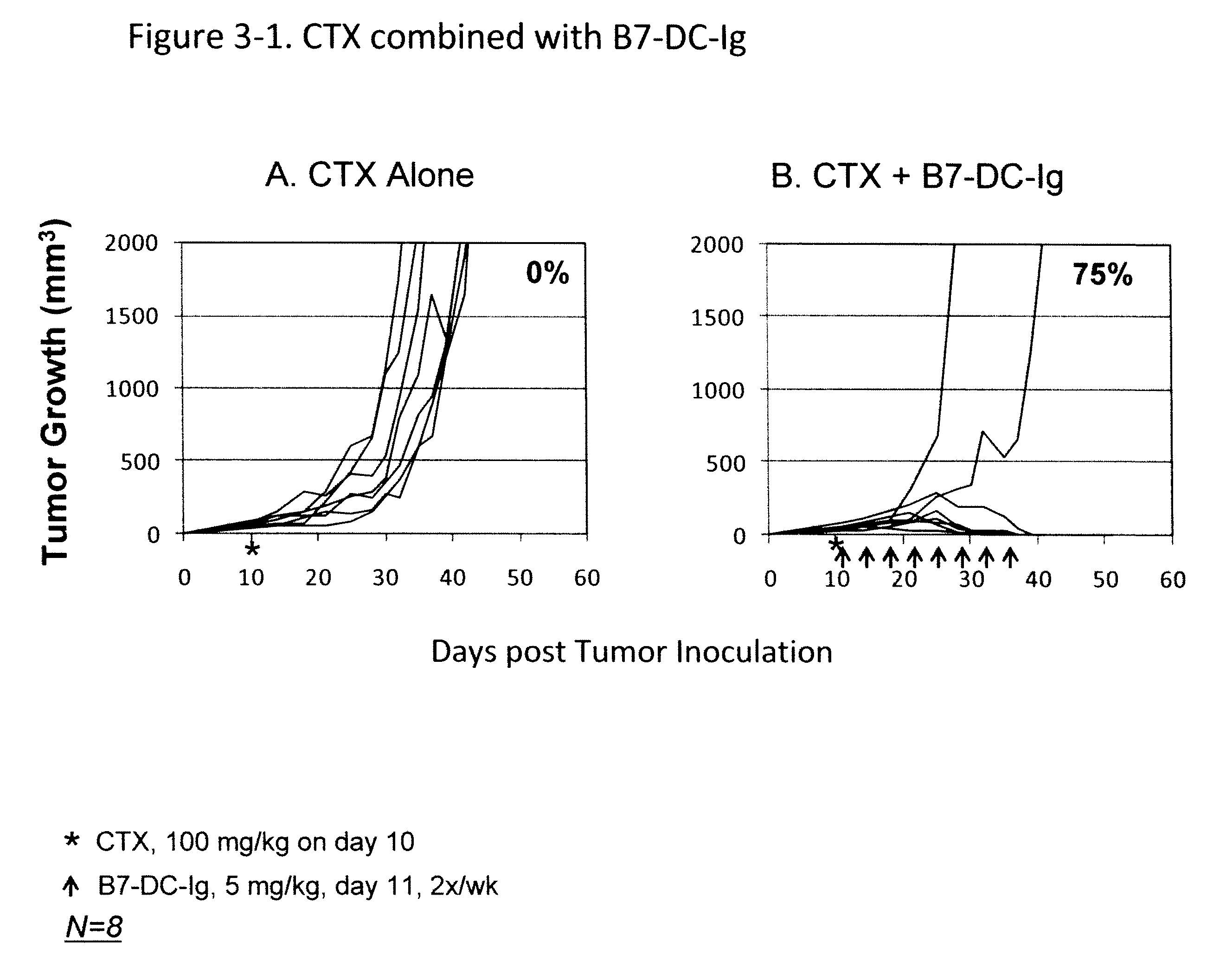
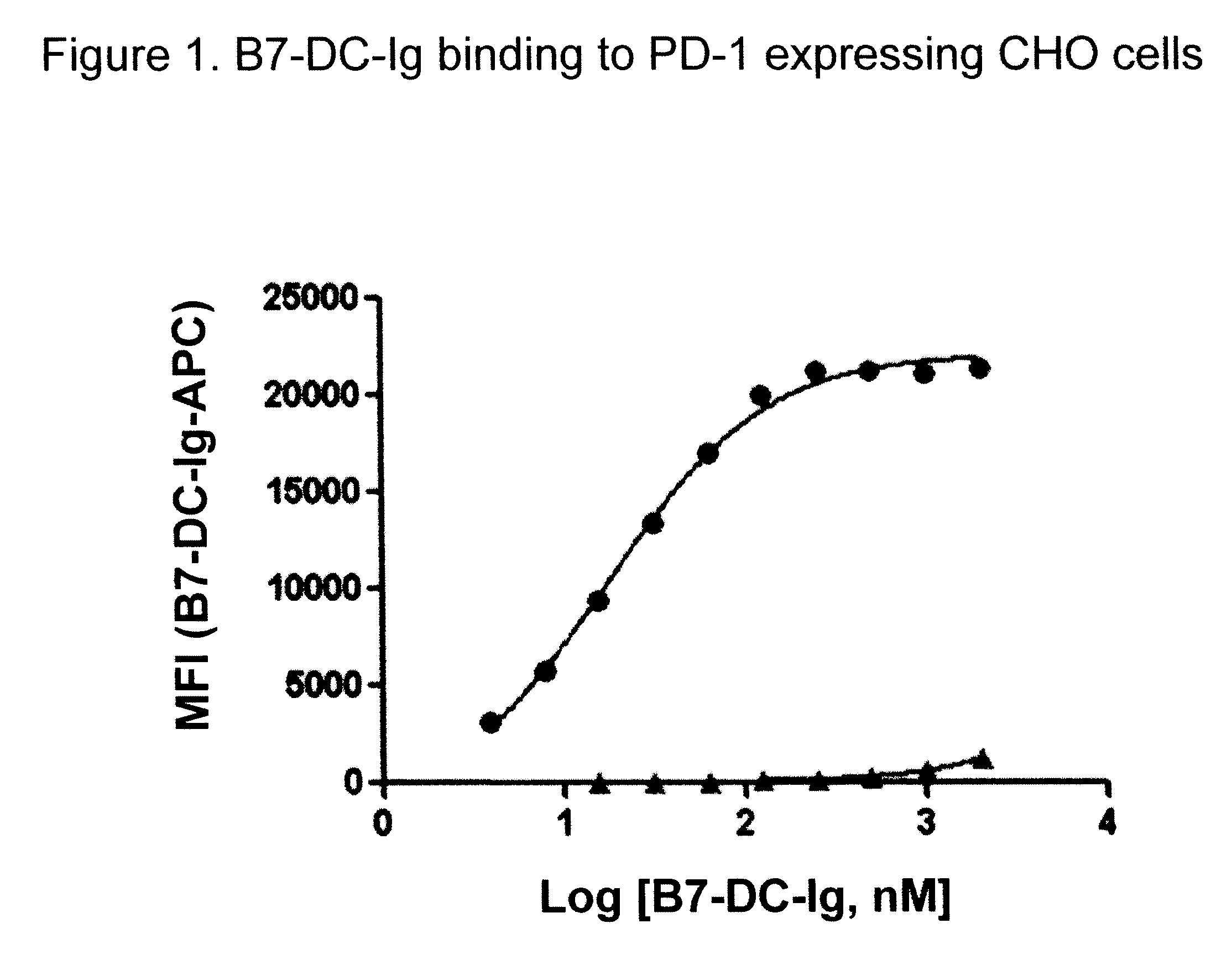
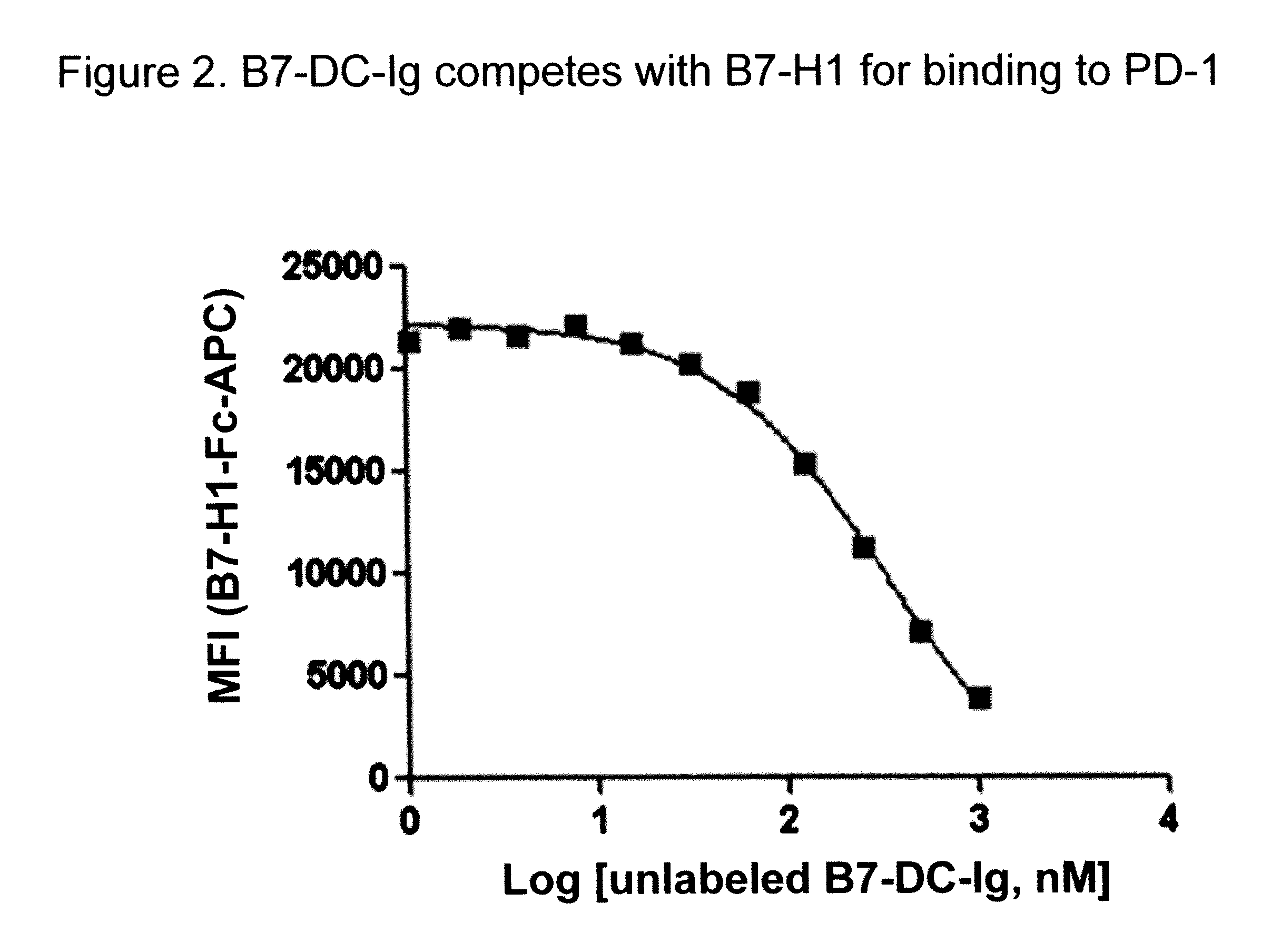
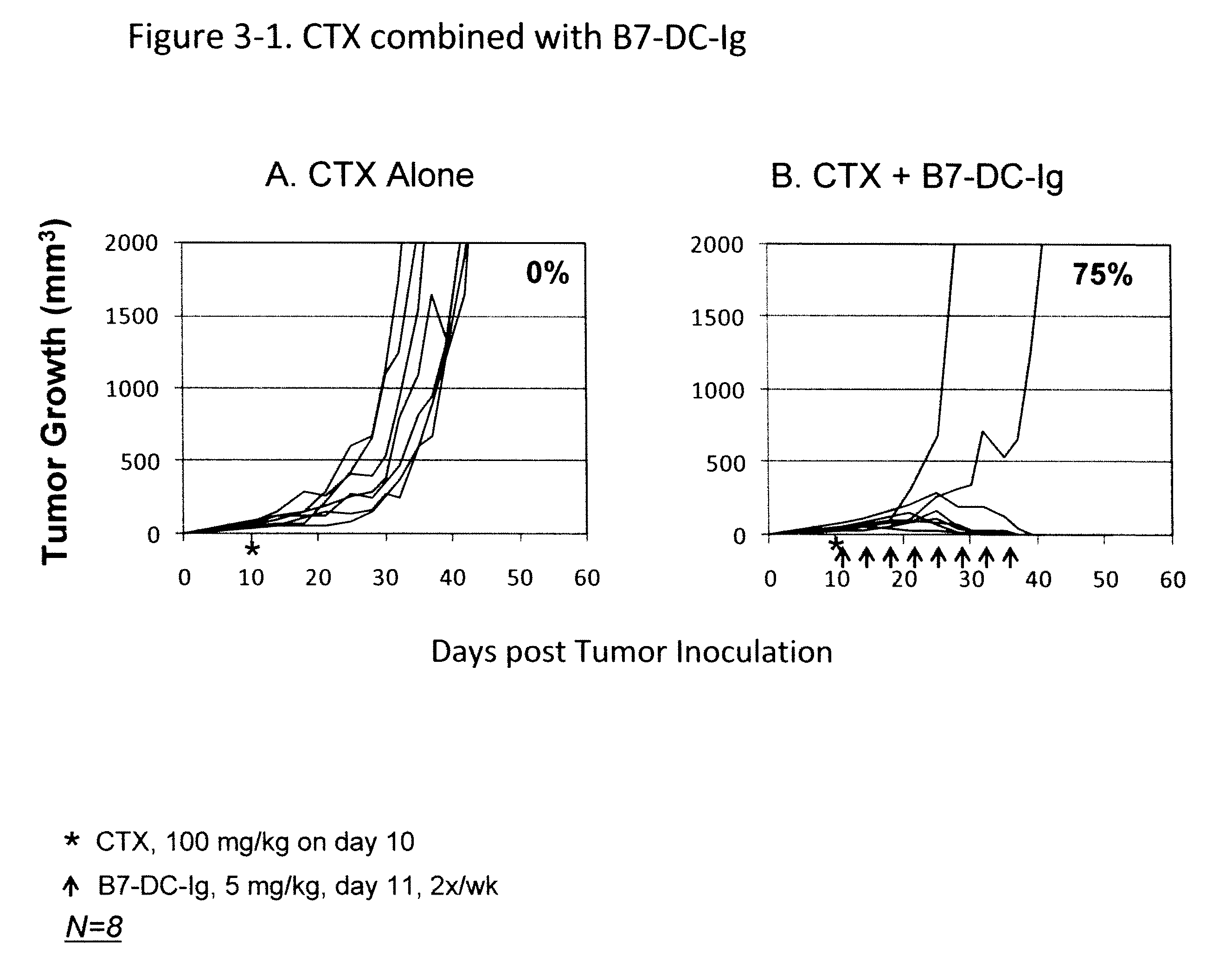
Compositions of PD-1 antagonists and methods of use
InactiveUS8114845B2Improve responseInhibitory signal transductionAntibacterial agentsOrganic active ingredientsCompound (substance)T cell
Owner:MEDIMMUNE LLC
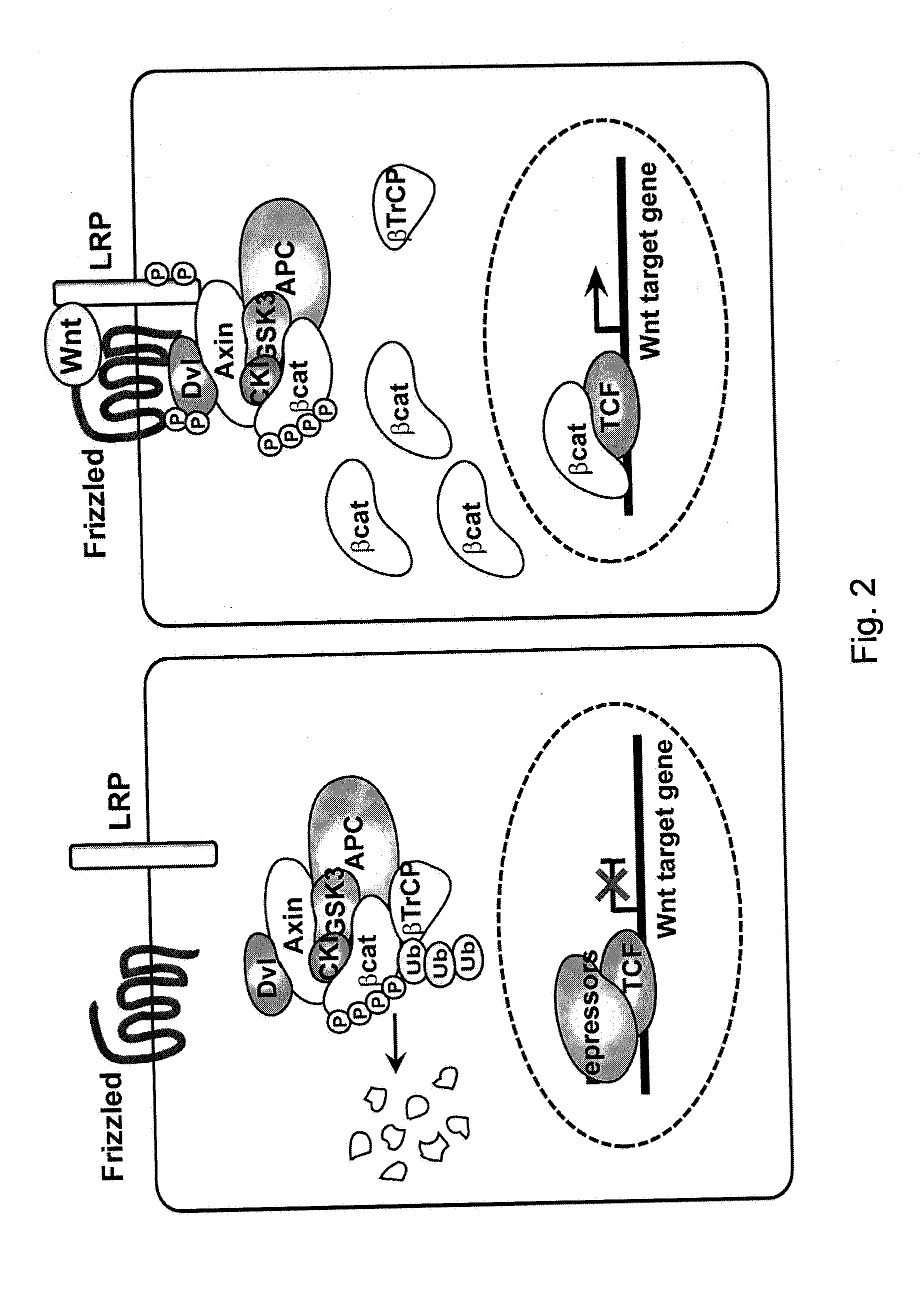
Indazoles as wnt/b-catenin signaling pathway inhibitors and therapeutic uses thereof
Described herein are methods of treating a disorder or disease in which aberrant Wnt signaling is implicated, with a variety of compounds, including Wnt inhibitor compounds. More particularly, it concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases due to mutations in Wnt signaling components.
Owner:SAMUMED LLC
Indazole inhibitors of the wnt signal pathway and therapeutic uses thereof
Indazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases due to mutations in Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Indazole inhibitors of the WNT signal pathway and therapeutic uses thereof
Indazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases due to mutations in Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
INDAZOLE-3-CARBOXAMIDES AND THEIR USE AS WNT/Beta-CATENIN SIGNALING PATHWAY INHIBITORS
Indazole-3-carboxamide compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole-3-carboxamide compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases and neurological conditions / disorders / diseases due to mutations or dysregulation of the Wnt pathway and / or of one or more of Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Combinations of signal transduction inhibitors
InactiveUS20050222163A1Promote growthPromote proliferationOrganic active ingredientsOrganic chemistryAnticarcinogenCell Cycle Inhibition
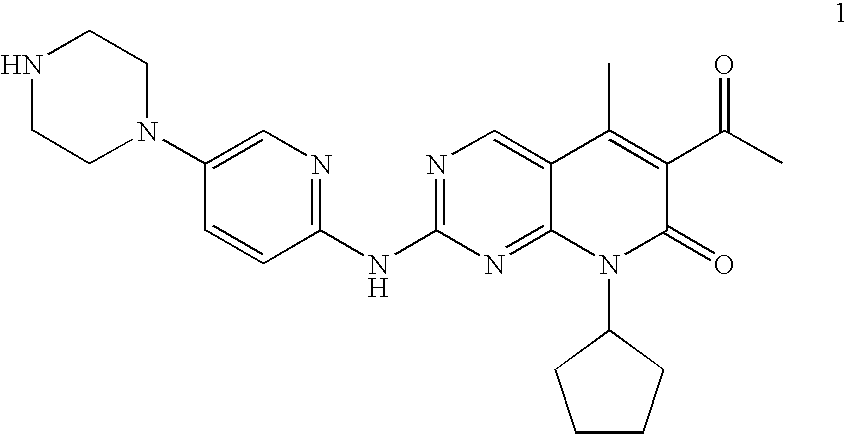
The present invention relates to methods for treating cancer comprising utilizing a combination of signal transduction inhibitors. More specifically, the present invention relates to combinations of so called cell cycle inhibitors with mitogen stimulated kinase signal transduction inhibitors, more specifically combinations of CDK inhibitors with mitogen stimulated kinase signal transduction inhibitors, more preferably MEK inhibitors. Other embodiments of the invention relate to additional combinations of the aforesaid combinations with standard anti-cancer agents such as cytotoxic agents, palliatives and antiangiogenics. Most specifically this invention relates to combinations of 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8H-pyrido[2,3-d]pyrimidin-7-one including salt forms, which is a selective cyclin-dependent kinase 4 (CDK4) inhibitor, in combination with one or more MEK inhibitors, most preferably N-[(R)-2,3-dihydroxy-propoxy]-3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-benzamide. The aforementioned combinations are useful for treating inflammation and cell proliferative diseases such as cancer and restenosis.
Owner:PFIZER INC
Indazole inhibitors of the Wnt signal pathway and therapeutic uses thereof
Indazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease, lung disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases and neurological conditions / disorders / diseases due to mutations or dysregulation of the Wnt pathway and / or of one or more of Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Compositions of pd-1 antagonists and methods of use
InactiveUS20100055102A1Reduces inhibitory signal transductionEnhanced T cell responseAntibacterial agentsOrganic active ingredientsT cellInfective disorder
Methods of treating cancer and infectious diseases utilizing a treatment regimen comprising administering a compound that reduces inhibitory signal transduction in T cells, in combination with a potentiating agent, such as cyclophosphamide, to produce potent T cell mediated responses, are described. Compositions comprising the PD-1 antagonists and potentiating agents useful in the methods of the invention are also disclosed.
Owner:MEDIMMUNE LLC
Use of inhibitors of the EGFR-mediated signal transduction for the treatment of benign prostatic hyperplasia (BPH)/prostatic hypertrophy
The present invention relates to the use of specific EGF-receptor antagonists for preparing a pharmaceutical composition for the prevention and / or treatment of benign prostatic hyperplasia and / or prostatic hypertrophy, a method for the treatment or prevention of benign prostatic hyperplasia / prostatic hypertrophy comprising administering an EGF-receptor antagonist of groups (A), (B) or (C), described herein optionally in combination with known compounds for the treatment of benign prostatic hyperplasia / prostatic hypertrophy, as well as associated pharmaceutical compositions.
Owner:BOEHRINGER INGELHEIM PHARM KG
3-(pyrolyllactone)-2-indolinone compounds as kinase inhibitors
The invention relates to certain indolinone compounds, their method of synthesis, and a combinatorial library consisting of the indolinone compounds of the invention. The invention also relates to methods of modulating the function of protein kinases using indolinone compounds of the invention and methods of treating diseases by modulating the function of protein kinases and related signal transduction pathways.
Owner:SUGEN INC
Indazole-3-carboxamides and their use as wnt/beta-catenin signaling pathway inhibitors
Indazole-3-carboxamide compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole-3-carboxamide compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases and neurological conditions / disorders / diseases due to mutations or dysregulation of the Wnt pathway and / or of one or more of Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
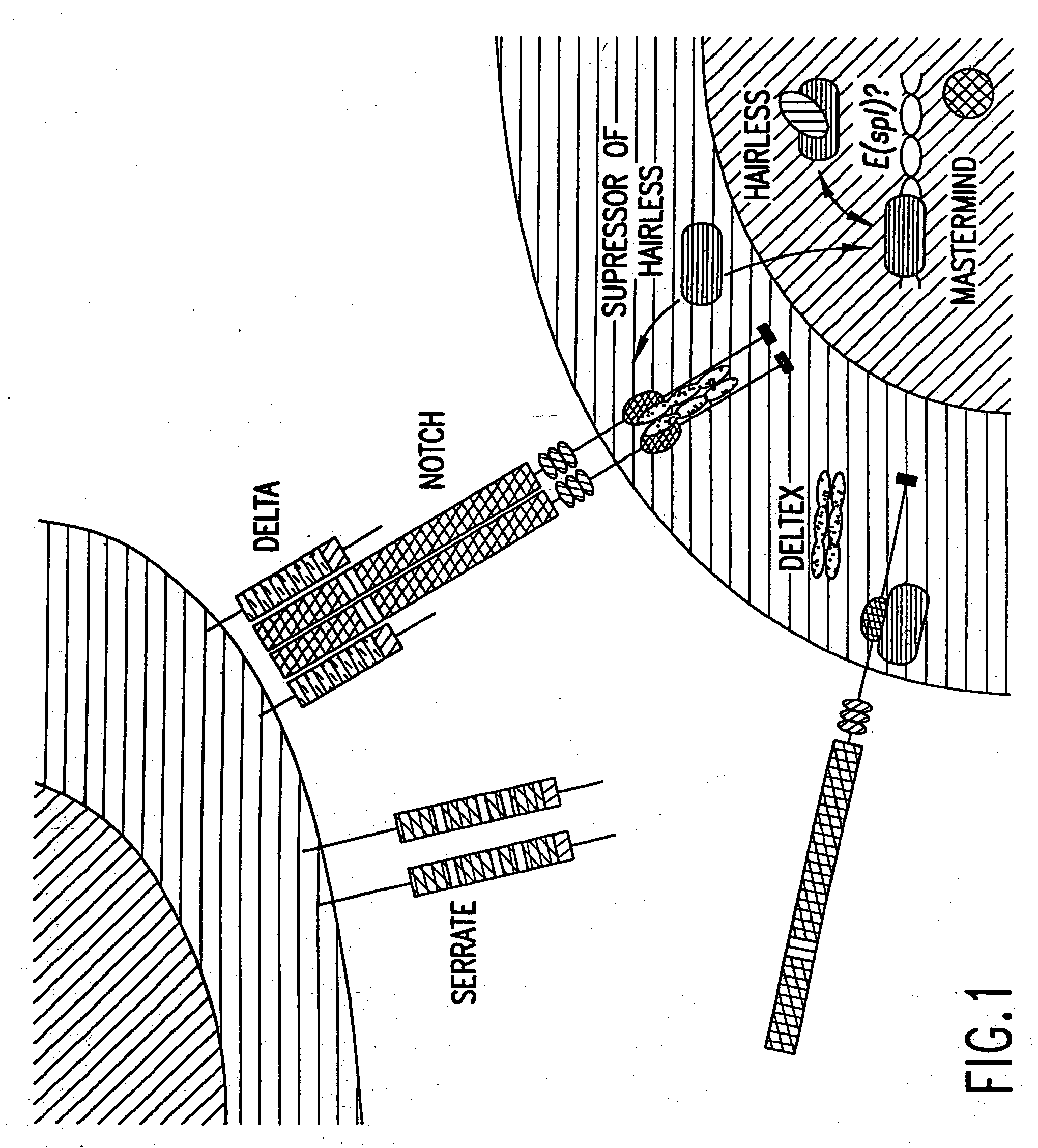
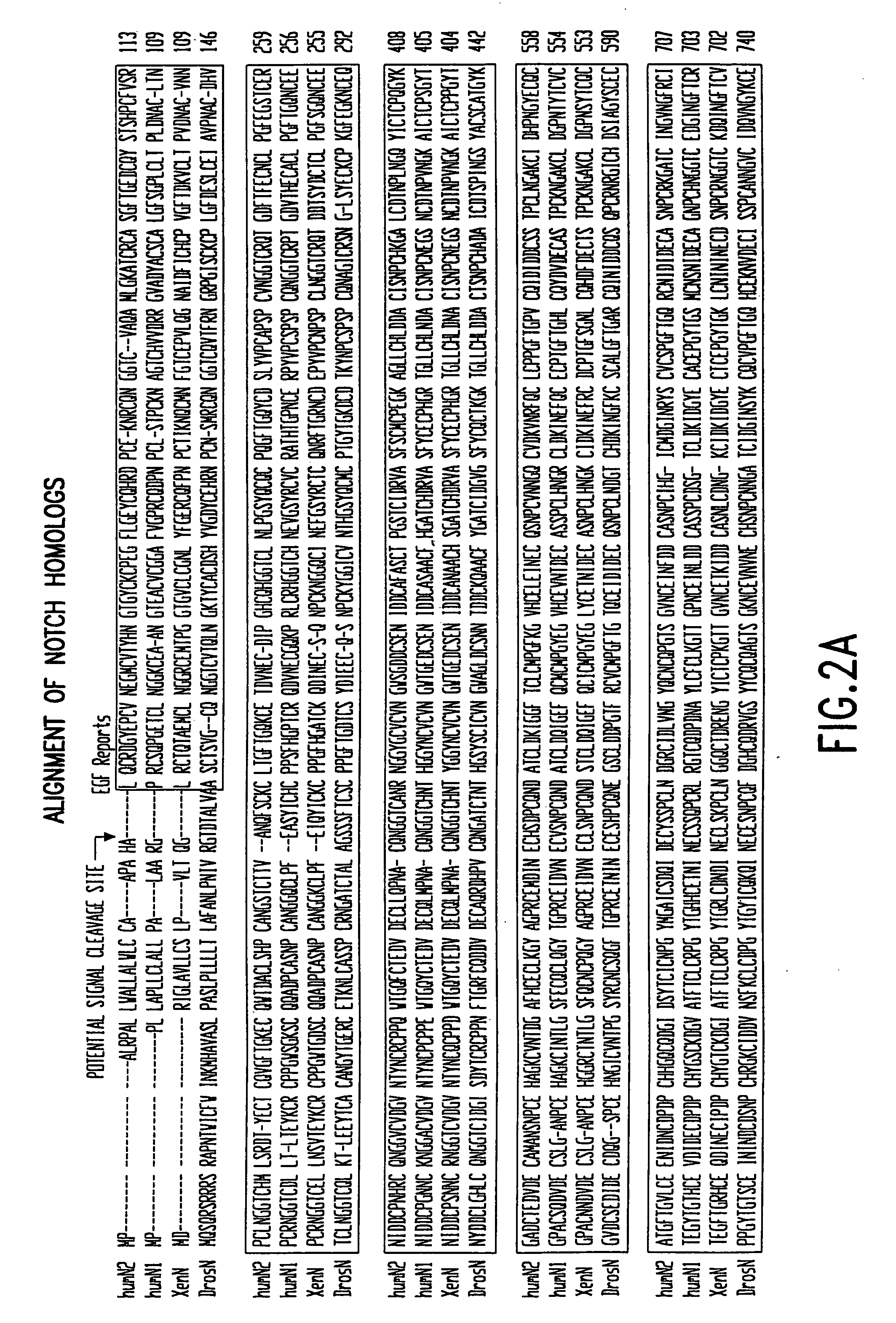
Activated forms of notch and methods based thereon
Owner:YALE UNIV
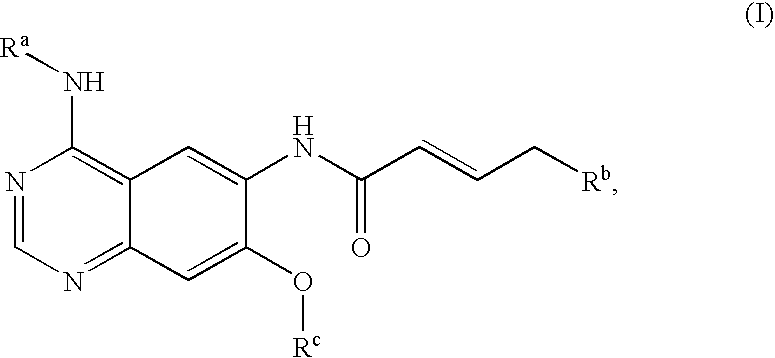
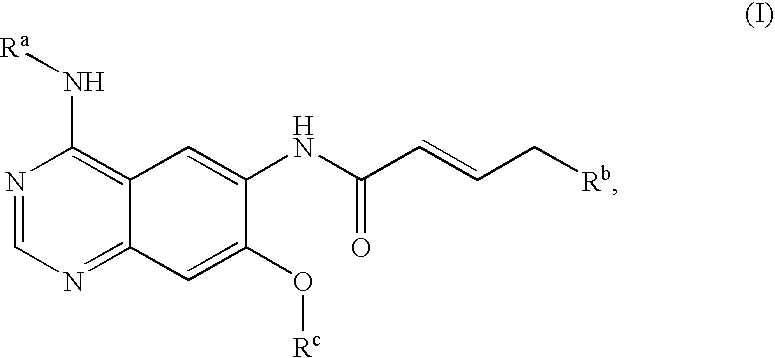
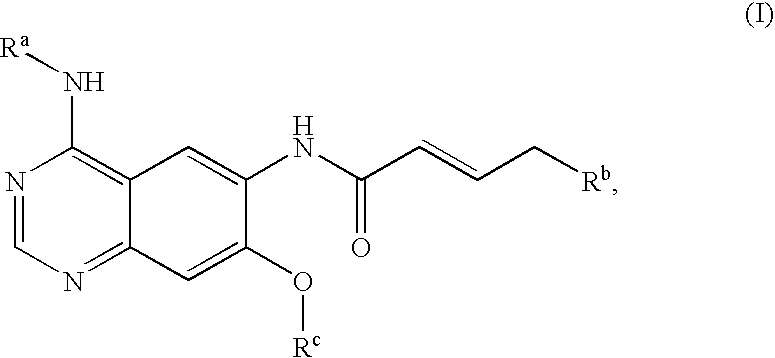
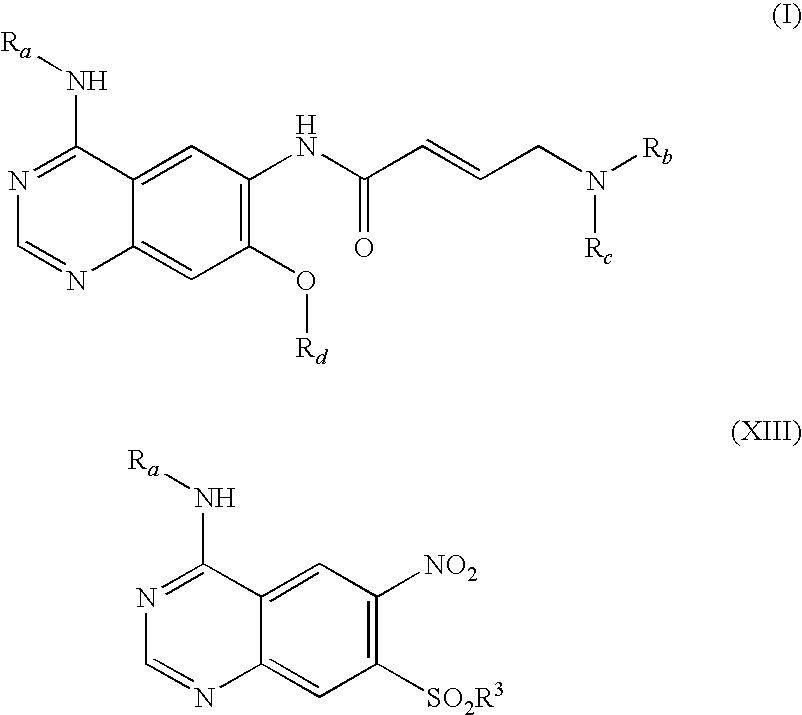
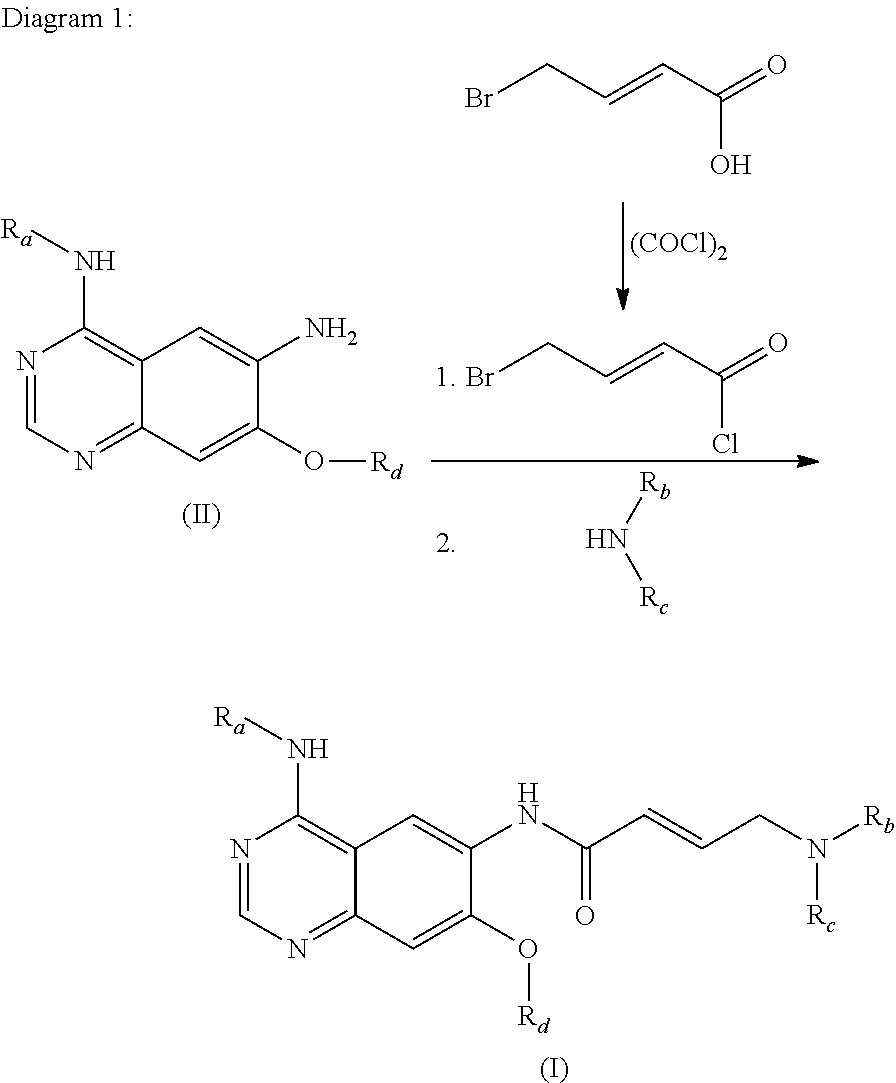
Process for preparing aminocrotonylamino-substituted quinazoline derivatives
The invention relates to an improved process for preparing aminocrotonylamino-substituted quinazoline derivatives of general formula (I) wherein the groups Ra, Rb, Rc and Rd have the meanings given in the claims, as well as sulphonyl derivatives of formula (XIII) and the use thereof as synthesis components for preparing quinazolines of formula (I). The quinazoline derivatives of formula (I) are inhibitors of signal transduction mediated by tyrosinekinases and by the Epidermal Growth Factor-Receptor (EGF-R) and are therefore particularly suitable for the treatment of tumoral diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Bicyclic heterocycles, pharmaceutical compositions containing these compounds, their use and processes for the preparation thereof
The present invention relates to bicyclic heterocycles of general formula wherein Ra, Rb, Rc, A, B, C, D, E and X are defined as in claim 1, the tautomers, the stereoisomers, the mixtures and the salts thereof, particularly the physiologically acceptable salts thereof with inorganic or organic acids which have valuable pharmacological properties, particularly an inhibitory effect on signal transduction mediated by tyrosine kinases, the use thereof for treating diseases, particularly tumoral diseases, as well as benign prostatic hyperplasia (BPH), diseases of the lungs and respiratory tract, and the preparation thereof.
Owner:BOEHRINGER INGELHEIM PHARMA KG
Indazole inhibitors of the wnt signal pathway and therapeutic uses thereof
Owner:BIOSPLICE THERAPEUTICS INC
Indazole inhibitors of the wnt signal pathway and therapeutic uses thereof
Indazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases due to mutations in Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Identification and use of molecules implicated in pain
The invention relates to the use of: (a) an isolated gene sequence that is down-regulated in the spinal cord in response to streptozocin-induced diabetes; (b) an isolated gene sequence comprising a nucleic acid sequence of Tables I to X. (c) an isolated gene sequence having at least 80% sequence identity with a nucleic acid sequence of Tables I to X; (d) an isolated nucleic acid sequence that is hybridizable to any of the gene sequences according to (a), (b) or (c) under stringent hybridisation conditions; (e) a recombinant vector comprising a gene sequence or nucleic acid sequence according to any one of (a) to (d); (f) a host cell containing the vector according to (e); (g) a non-human animal having in its genome an introduced gene sequence or nucleic acid sequence or a removed or down-regulated gene sequence or nucleic acid sequence according to any one of (a) to (d); (h) an isolated polypeptide comprising an amino acid sequence at least 90% identical to an amino acid sequence encoded by a nucleotide sequence according to any one of (a) to (d), or a polypeptide variant thereof with sequential amino acid deletions from the C terminus and / or the N-terminus; or (i) an isolated polypeptide encoded by a nucleotide sequence according to any one of (a) to (d); or (k) an isolated antibody that binds specifically to a polypeptide according to (h) or (i); in the screening of compounds for the treatment of pain, or for the diagnosis of pain. The invention also relates to the use of naturally occurring compounds such as peptide ligands of the expression products of the above gene sequences and their associated signal transduction pathways for use in the treatment of pain.
Owner:BROOKSBANK ROBERT ALAN +3
Identification and use of molecules implicated in pain
The invention relates to the use of: (a) an isolated gene sequence that is down-regulated in the spinal cord of a mammal in response to mechanistically distinct first and second models of neuropathic or central sensitization pain; (b) an isolated gene sequence comprising a nucleic acid sequence of any of Tables I to VI; (c) an isolated gene sequence having at least 80% sequence identity with a nucleic acid sequence of any of Tables I to VI; (d) an isolated nucleic acid sequence that is hybridizable to any of the gene sequences according to (a), (b) or (c) under stringent hybridisation conditions; (e) a recombinant vector comprising a gene sequence or nucleic acid sequence according to any one of (a) to (d); (f) a host cell containing the vector according to (e); (g) a non-human animal having in its genome an introduced gene sequence or nucleic acid sequence or a removed or down-regulated gene sequence or nucleic acid sequence according to any one of (a) to (d); (h) an isolated polypeptide comprising an amino acid sequence at least 90% identical to an amino acid sequence encoded by a nucleotide sequence according to any one of (a) to (d), or a variant polypeptide thereof with sequential amino acid deletions from the C terminus and / or the N-terminus; (i) an isolated polypeptide encoded by a nucleotide sequence according to any one of (a) to (d); or (j) an isolated antibody that binds specifically to a polypeptide according to (h) or (i); in the screening of compounds for the treatment of pain, or for the diagnosis of pain. The invention also relates to the use of naturally occurring compounds such as peptide ligands of the expression products of the above gene sequences and their associated signal transduction pathways for use in the treatment of pain.
Owner:BROOKSBANK ROBERT ALAN +3
Identification and use of molecules implicated in pain
The invention relates to the use of: (a) an isolated gene sequence that is up regulated in the spinal cord in response to streptozocin-induced diabetes; (b) an isolated gene sequence comprising a nucleic acid sequence of Tables I to X. (c) an isolated gene sequence having at least 80% sequence identity with a nucleic acid sequence of Tables I to X; (d) an isolated nucleic acid sequence that is hybridizable to any of the gene sequences according to (a), (b) or (c) under stringent hybridisation conditions; (e) a recombinant vector comprising a gene sequence or nucleic acid sequence according to any one of (a) to (d); (f) a host cell containing the vector according to (e); (g) a non-human animal having in its genome an introduced gene sequence or nucleic acid sequence or a removed or down-regulated gene sequence or nucleic acid sequence according to any one of (a) to (d); (h) an isolated polypeptide comprising an amino acid sequence at least 90% identical to an amino acid sequence encoded by a nucleotide sequence according to any one of (a) to (d), or a polypeptide variant thereof with sequential amino acid deletions from the C terminus and / or the N-terminus; or (i) an isolated polypeptide encoded by a nucleotide sequence according to any one of (a) to (d); or (k) an isolated antibody that binds specifically to a polypeptide according to (h) or (i); in the screening of compounds for the treatment of pain, or for the diagnosis of pain. The invention also relates to the use of naturally occurring compounds such as peptide ligands of the expression products of the above gene sequences and their associated signal transduction pathways for use in the treatment of pain.
Owner:BROOKSBANK ROBERT ALAN +3
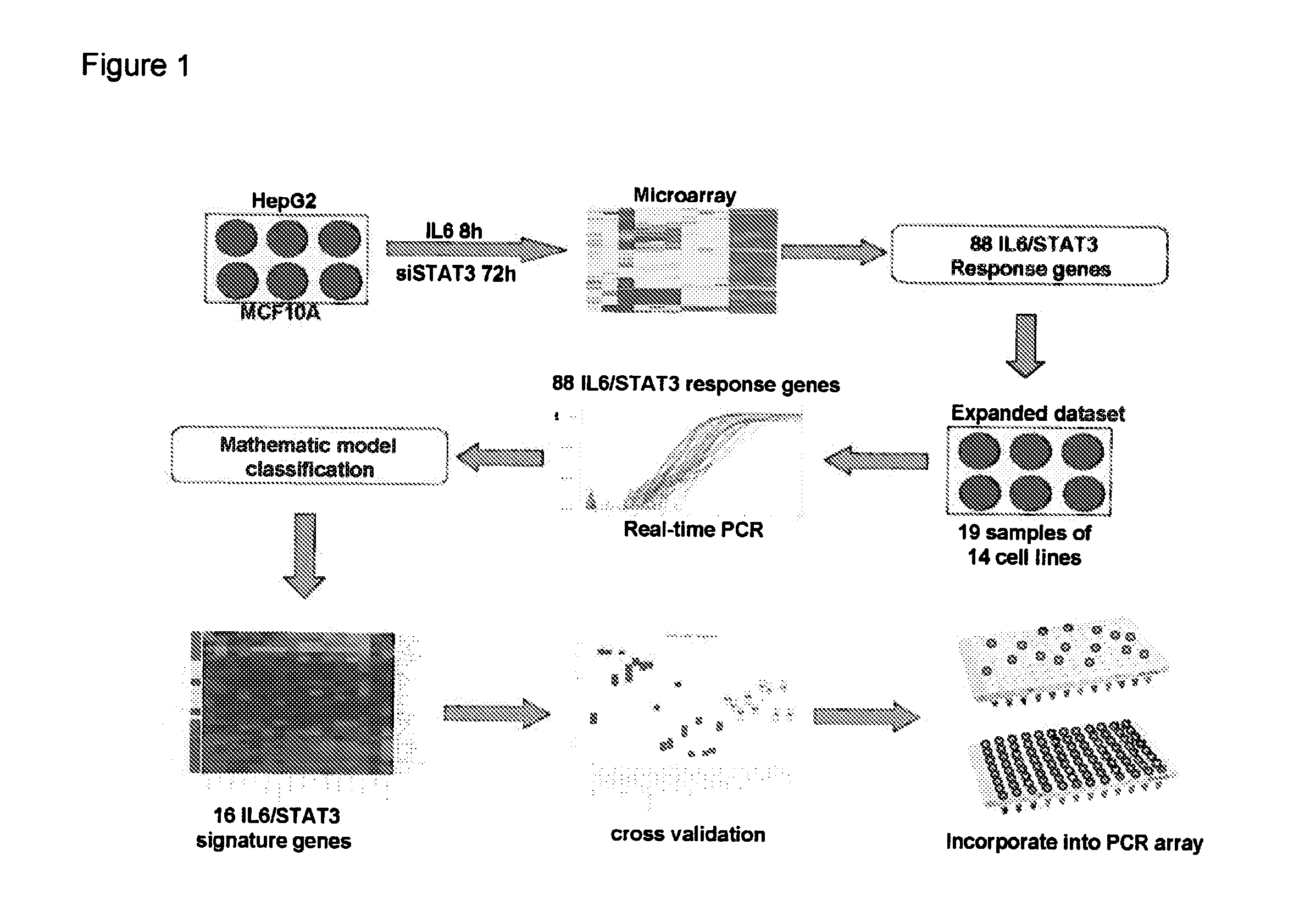
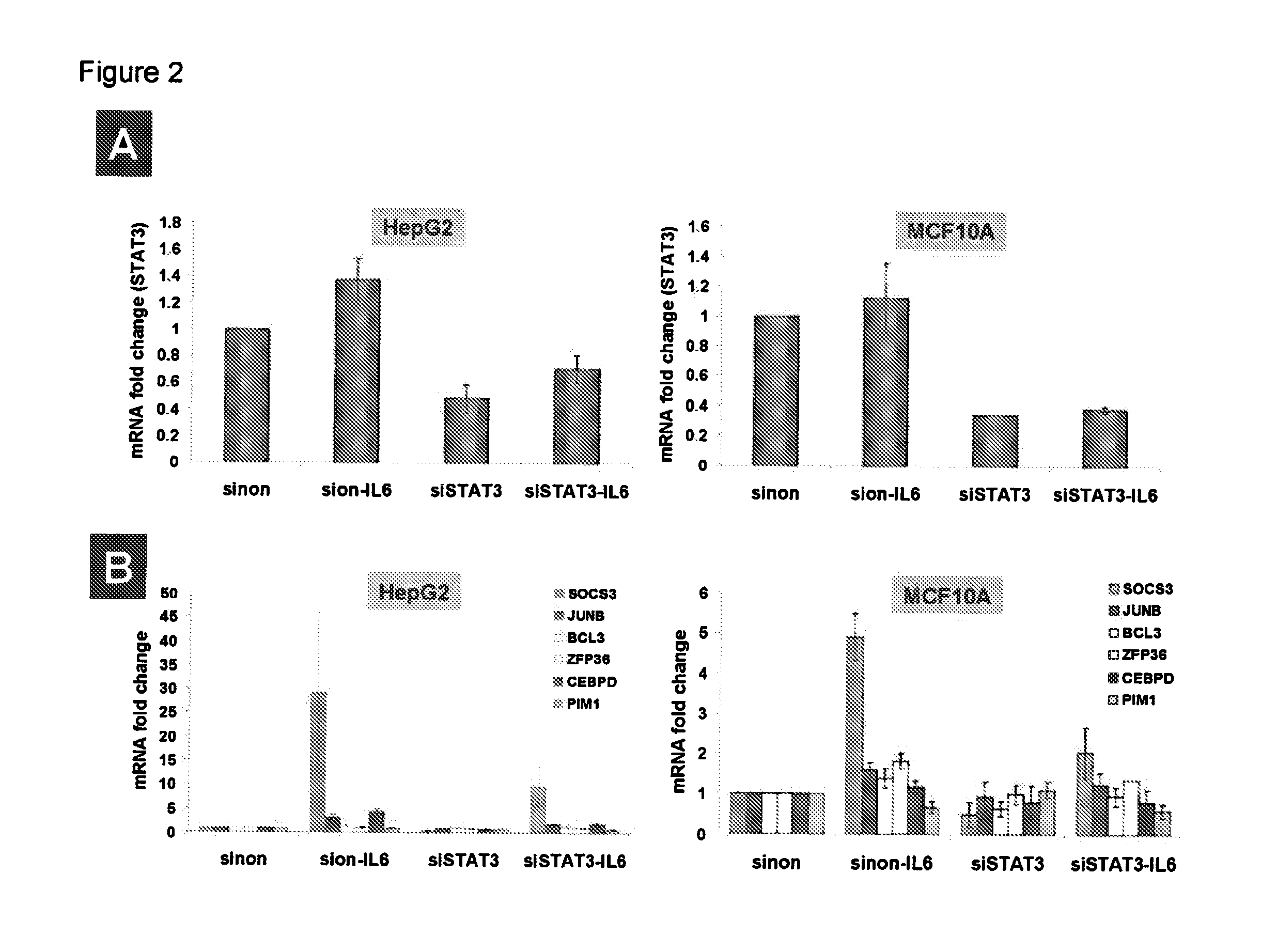
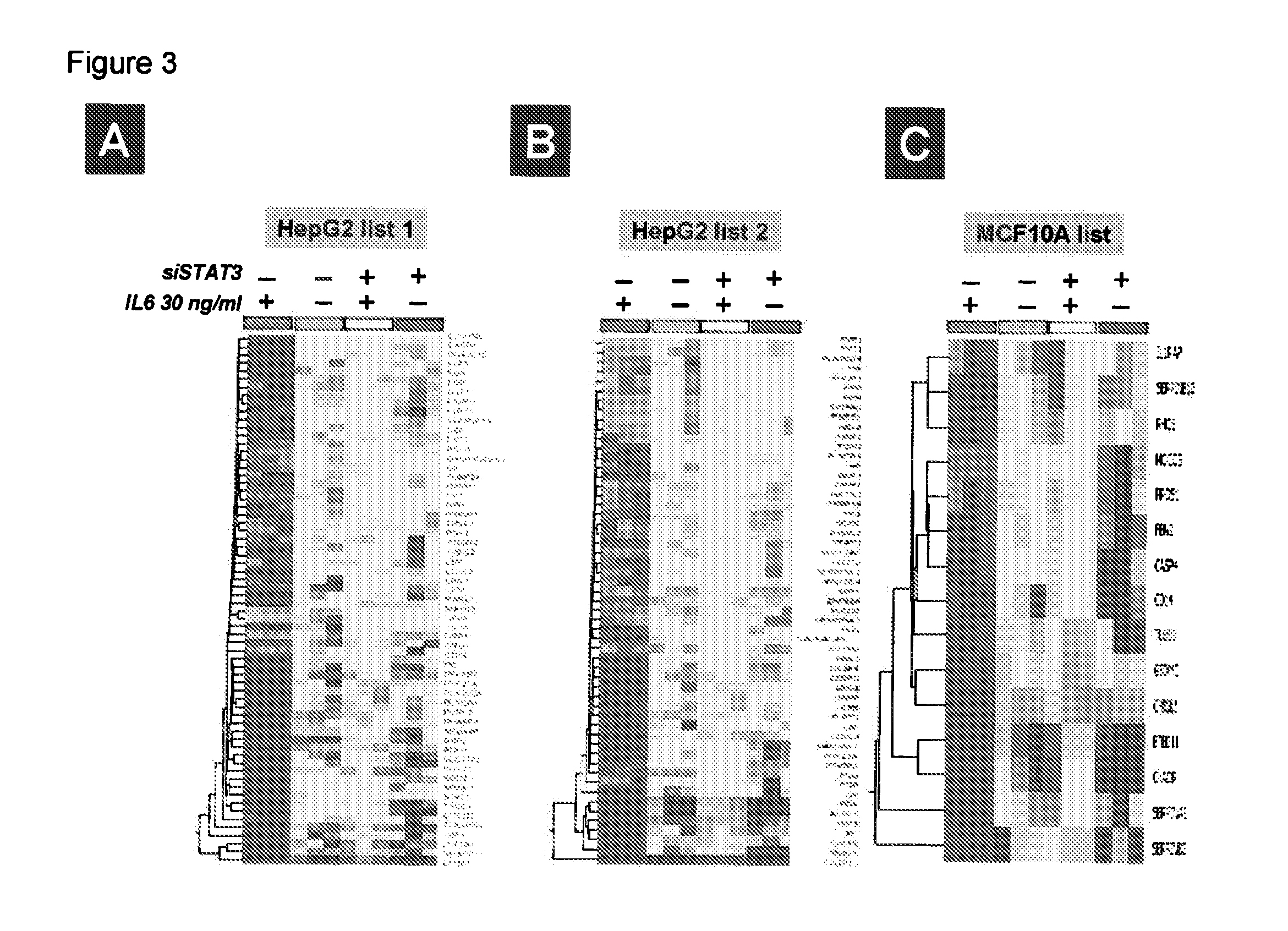
Gene expression signature for il-6/stat3 signaling pathway and use thereof
ActiveUS20150232926A1Reliable measurementMicrobiological testing/measurementDNA/RNA fragmentationRandom forestStat3 Signaling Pathway
The present invention relates to a set of biomarkers, microarrays that provide for detection thereof, an expression signature comprising 16 genes or a subset thereof, and the use thereof in determining the regulation status of IL-6 / STAT3 signaling pathway in a cell sample or subject, as well as compositions for the detection thereof. The regulation status of IL-6 / STAT3 signaling pathway in a cell sample or subject may be assayed based on the level of expression of one or more of these genes. The methods and compositions provided herein may be used to evaluate IL-6 / STAT3 pathway regulation status in a sample; classify a cell sample as having a deregulated or regulated IL-6 / STAT3 signaling pathway; determine whether an agent modulates the IL-6 / STAT3 signaling pathway; predict the response of a subject to an agent that modulates the IL-6 / STAT3 signaling pathway; assign treatment to a subject; and / or evaluate the pharmacodynamic effects of therapies designed to regulate IL-6 / STAT3 pathway signaling. Expression of the biomarkers is preferably determined by RT-PCR using SYBR Green methods, and the expression data analyzed and compared to a control sample by use of the random forest method.
Owner:QIAGEN SCIENCES LLC +1
Method for Determining Responsiveness to Chk1 Inhibitors
InactiveUS20080145358A1Robust dose dependent increase in phospho-H2AXOrganic active ingredientsPeptide/protein ingredientsA-DNACancer research
The invention is directed at a method for predicting a patient's responsiveness to a CHK1 inhibitor The method includes providing a sample from a test patient who is being treated with a DNA damaging agent; and determining for the presence of an activated CHK1 signal transduction pathway molecule, wherein the presence of an activated CHK1 signal transduction pathway molecule is indicative that a CHK1 inhibitor should be administered to the patient.
Owner:ASTRAZENECA AB
Application of micro ribose nucleic acid (RNA) (miR)-574-5p
ActiveCN102416182AGenetic material ingredientsMicrobiological testing/measurementLymphatic SpreadBeta-catenin
The invention discloses application of micro ribose nucleic acid (RNA) (miR)-574-5p, relates to miR of biomedical materials, and provides application of the miR-574-5p to preparation of a medicine for treating colorectal cancer and a diagnostic reagent for colorectal cancer. The miR-574-5p regulates target genes Qki (quaking) of the miR-574-5p to influence signal transduction of beta-catenin / Wnt and p27, promote proliferation of colorectal cancer cells and inhibit differentiation of the colorectal cancer cells, and promote migration and invasion of the colorectal cancer cells. In the colorectal cancer cells, the expression of the miR-574-5p is extremely high, so that the expression of the miR-574-5p and downstream QKI, beta-catenin and p27 can be detected so as to diagnose tumor, and evaluate the tumor metastasis and prognosis; and in the colorectal cancer cells in which the expression of the miR-574-5p is extremely high, the expression of the miR-574-5p can be inhibited so as to inhibit the occurrence and development of colorectal cancer.
Owner:厦门祥安利康生物科技有限公司
Indazole inhibitors of the wnt signal pathway and therapeutic uses thereof
Indazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an indazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, Alzheimer's disease, lung disease and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases and neurological conditions / disorders / diseases due to mutations or dysregulation of the Wnt pathway and / or of one or more of Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Combination Therapy for Treatment of Cancer
InactiveUS20150132301A1Eliminate side effectsImprove therapeutic indexPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCombined Modality Therapy
The present invention provides methods comprising combination therapy for treating cancer. In particular, the present invention provides Wnt pathway inhibitors in combination with MAPK pathway inhibitors for the treatment of cancer and other diseases. In some embodiments, the MAPK pathway signaling activation is due to a mutation in a MAPK pathway component. In some embodiments, the MAPK pathway signaling component is Ras, Raf, MEK, or ERK.
Owner:ONCOMED PHARMA
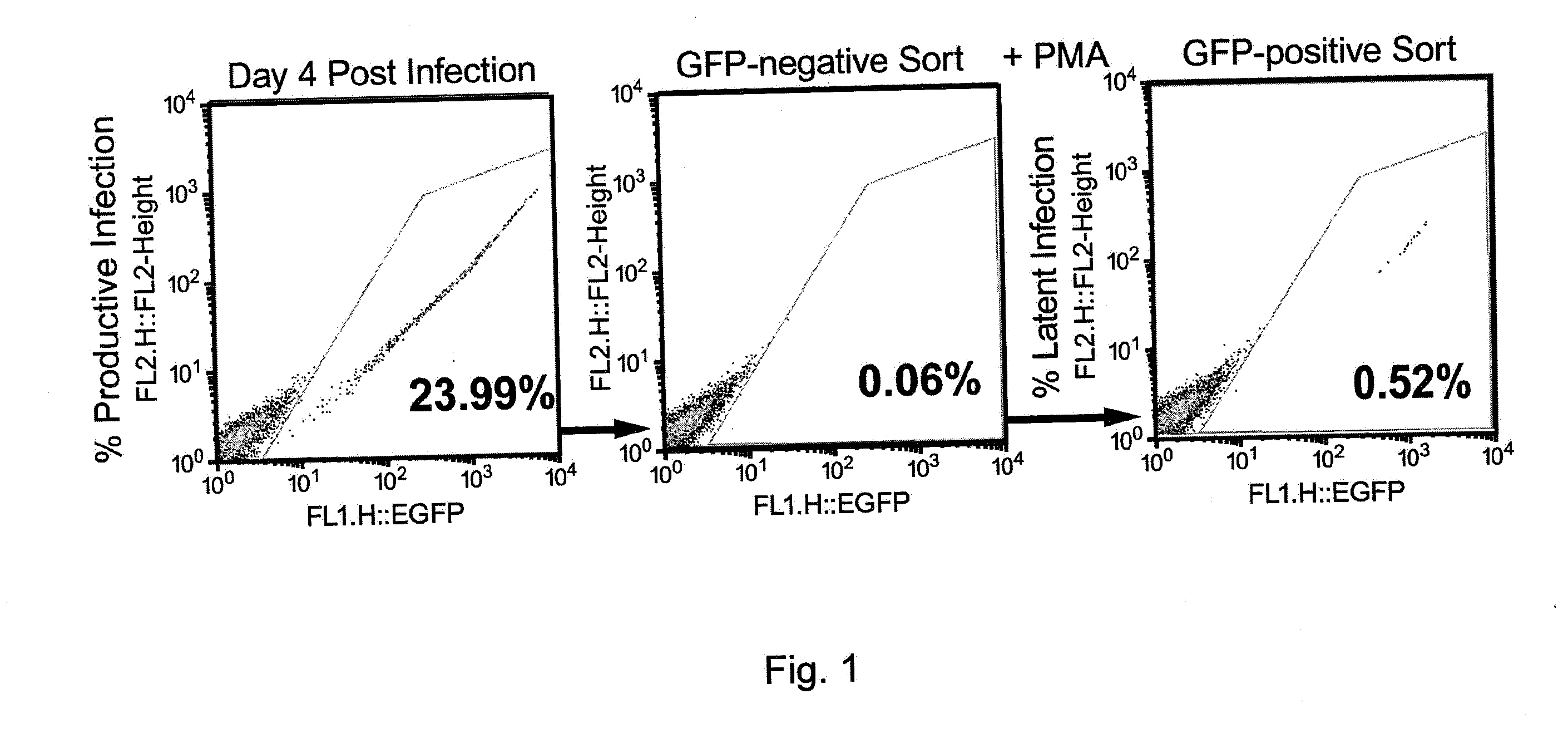
Methods for activating retrovirus in latent infected cells, and compounds for use therein
InactiveUS20150099012A1Increase transcriptionEnhanced signalBiocidePeptide/protein ingredientsInfected cellLong terminal repeat
The present invention relates to a method for increasing retrovirus transcription in an infected eukaryotic cell, comprising increasing Wnt pathway signaling in said cell such that transcription of said retrovirus is increased. Also, the present invention relates to a method of treating a subject infected with a retrovirus, said method comprising administering to said subject an activator of the Wnt pathway in an amount that increased Wnt pathway signaling in resting memory CD4+ T cells of said subject such that the retroviral long terminal repeat (LTR) in said cells is activated or de-repressed.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Gene expression signature for wnt/b-catenin signaling pathway and use thereof
InactiveUS20120252689A1Nucleotide librariesMicrobiological testing/measurementBeta-cateninΒ catenin signaling
The present invention relates to a novel set of 16 biomarkers, microarrays that provide for the detection thereof, an expression signature comprising 16 genes or a subset thereof, and the use thereof in determining the regulation status of Wnt / β-catenin signaling pathway. The regulation status of Wnt / β-catenin signaling pathway may be assayed based on the level of expression of one or more of these genes. The expression of these biomarkers may be used to evaluate Wnt / β-catenin pathway deregulation status; classify a cell sample as having a deregulated or regulated Wnt / β-catenin signaling pathway; determine whether an agent modulates the Wnt / β-catenin signaling pathway; predict response of a subject to an agent that modulates the Wnt / β-catenin signaling pathway; assign treatment to a subject; or evaluate the pharmacodynamic effects of therapies designed to regulate Wnt / β-catenin pathway signaling.
Owner:SABIOSCIENCES CORP
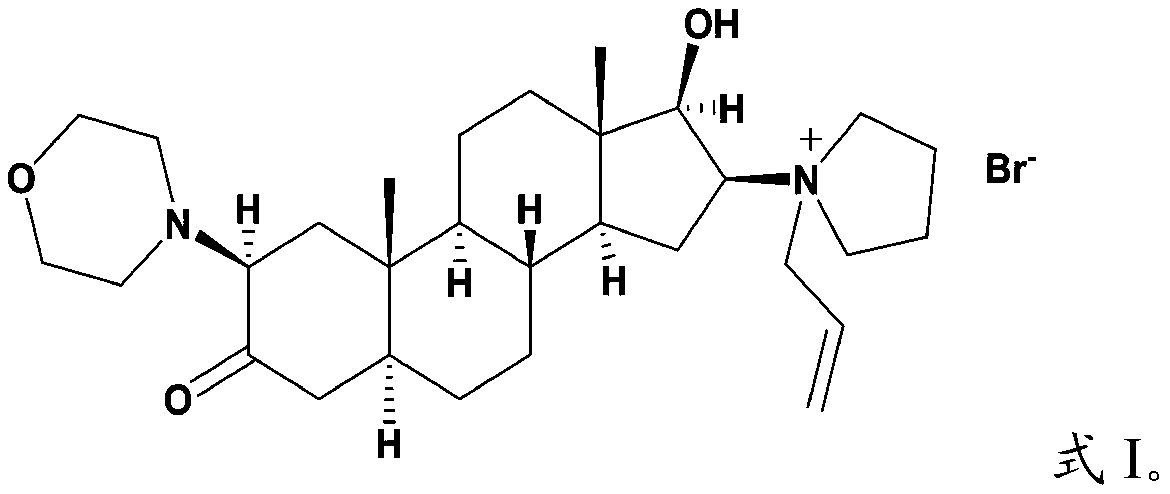
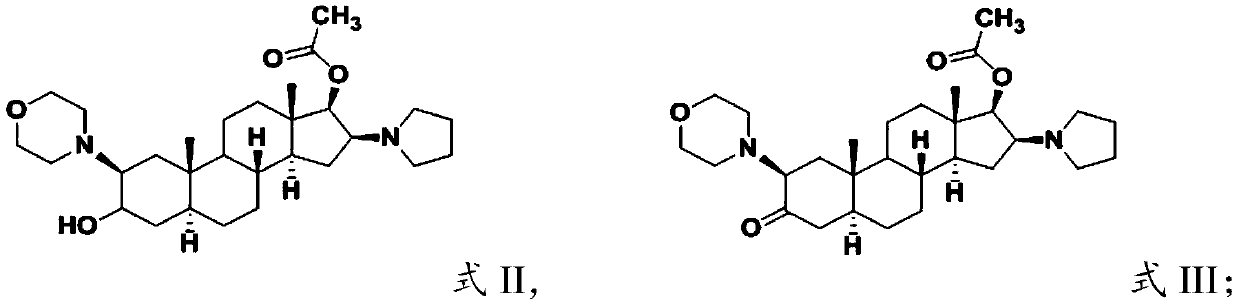
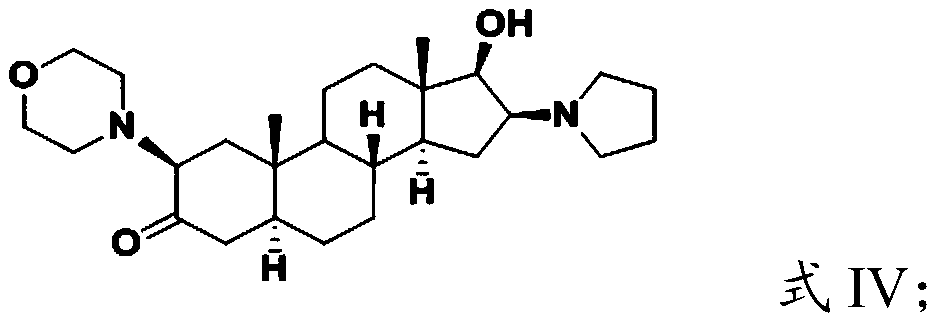
Androstane derivative, preparation method and applications thereof
ActiveCN110240624AEnhanced inhibitory effectOrganic active ingredientsSteroidsHedgehog signaling pathwayIc50 values
The invention provides an androstane derivative, a preparation method and applications thereof, and belongs to the technical field of medicine. According to the present invention, the androstane derivative has good inhibitory effect on Hedgehog signaling pathway so as to provide an effective treatment drug for patients with chronic myelogenous leukemia; the results of the embodiments show that the androstane derivative has the Hedgehog pathway signaling inhibition average IC50 value measured by using mouse [s12] and human [MZ24] Gli reporter cell lines of 0.020 [mu]m while the average IC50 value of the existing FDA-approved Hedgehog pathway inhibitor is 0.022 [mu]M, such that the androstane derivative has Hedgehog signaling pathway inhibition effect, and further achieve the effect comparable to Vismodegib.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Indazole compounds and pharmaceutical compositions for inhibiting protein kinases, and methods for their use
InactiveUS20040192735A1Reducing and inhibiting abnormal cell growthDecreasing endothelial cell migrationBiocideSenses disorderDiabetic retinopathyProtein kinase domain
Indazole compounds that modulate and / or inhibit the ophthalmic diseases and the activity of certain protein kinases are described. These compounds and pharmaceutical compositions containing them are capable of mediating tyrosine kinase signal transduction and thereby modulate and / or inhibit unwanted cell proliferation. The invention is also directed to the therapeutic or prophylactic use of pharmaceutical compositions containing such compounds, and to methods of treating ophthalmic diseases and cancer and other disease states associated with unwanted angiogenesis and / or cellular proliferation, such as diabetic retinopathy, neovascular glaucoma, rheumatoid arthritis, and psoriasis, by administering effective amounts of such compounds.
Owner:AGOMRON PHARMA +1
Method for inhibiting signal transduction, signal transduction inhibitor to be used therein and use thereof
ActiveUS8173365B2Avoid developmentPrevention and treatment of cancerElectrolysis componentsPeptide/protein ingredientsSignalling pathwaysHuman cell
Owner:THE UNIV OF TOKYO +2
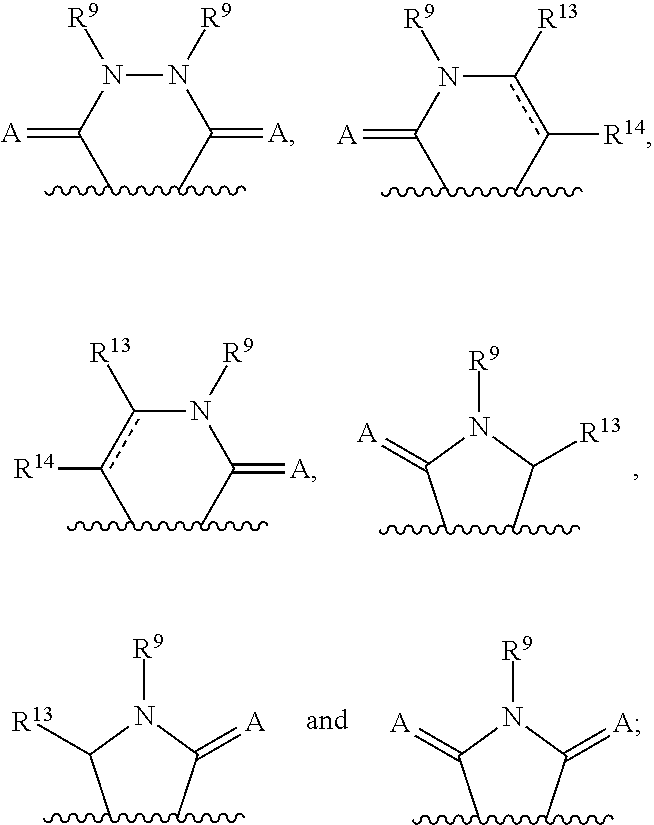
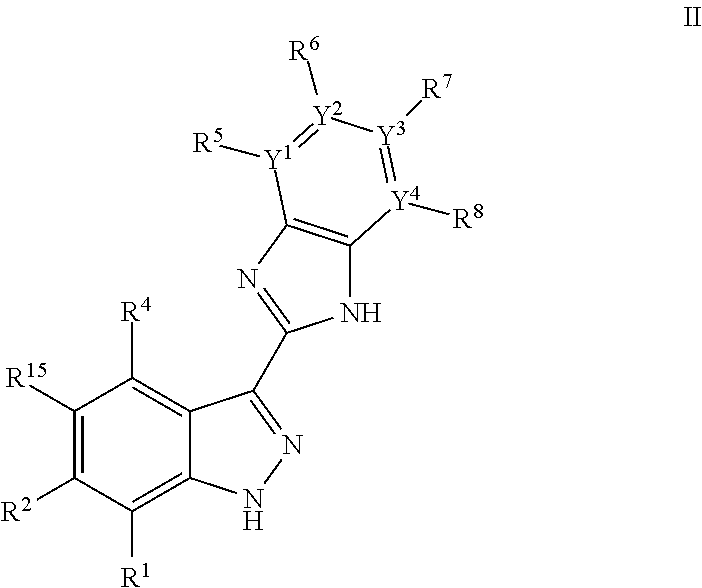
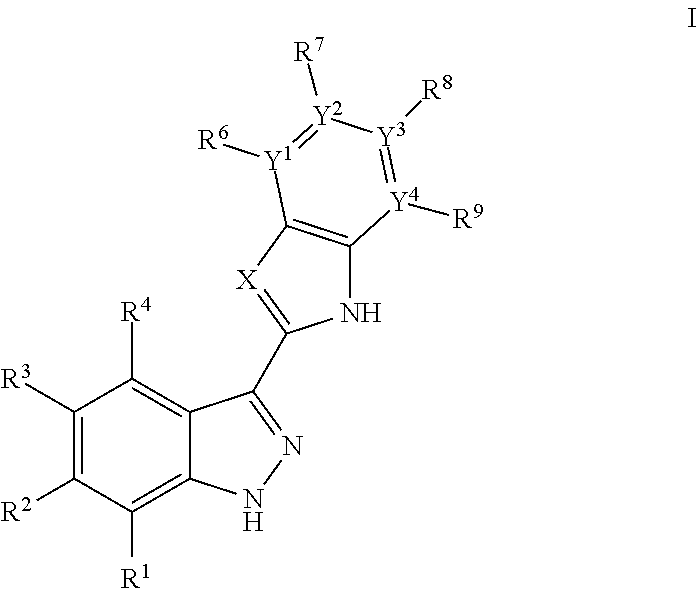
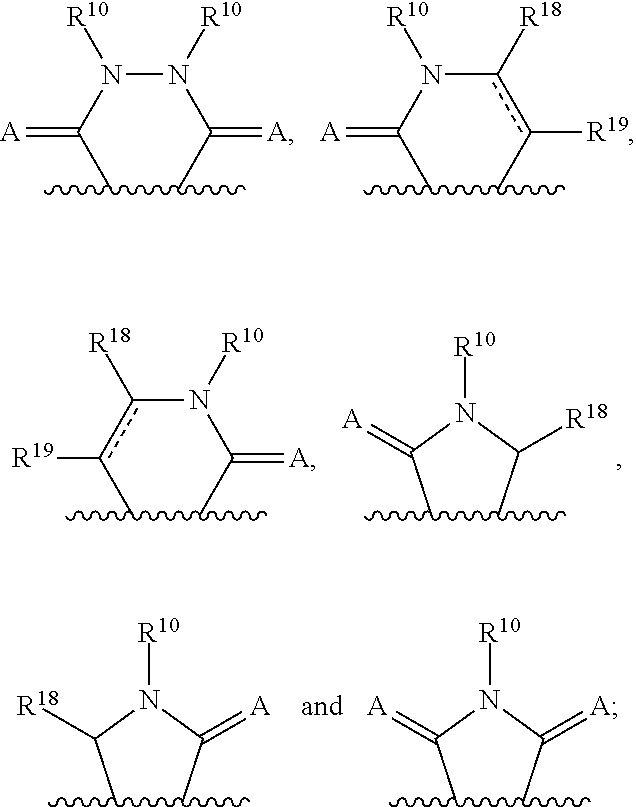
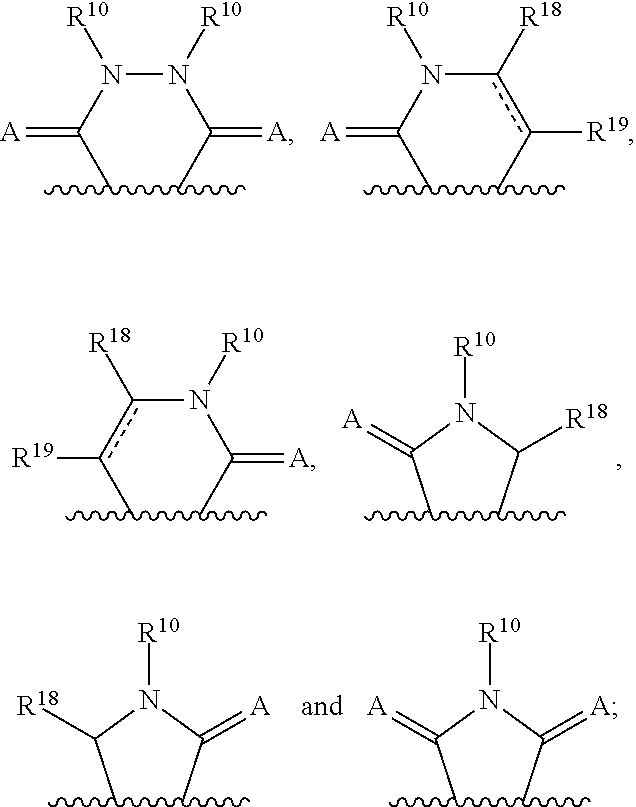
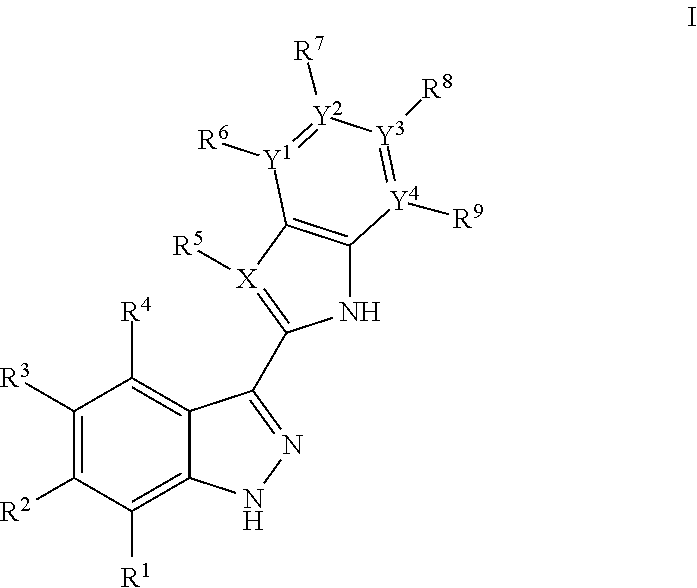
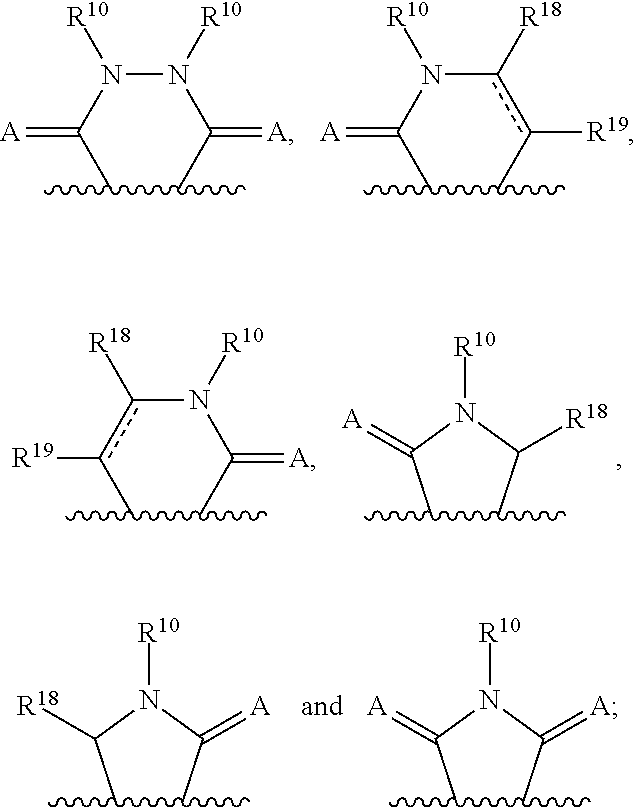
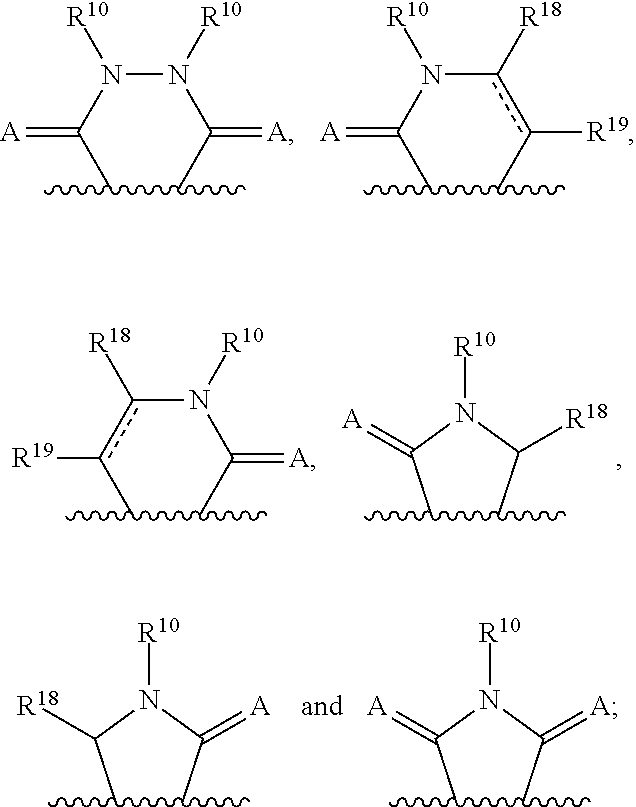
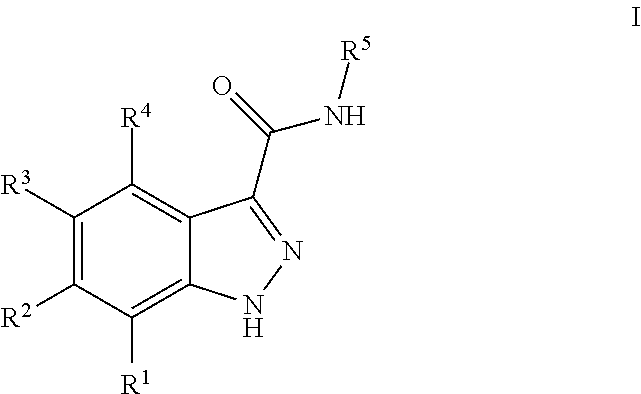
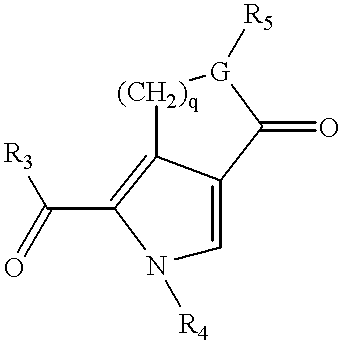
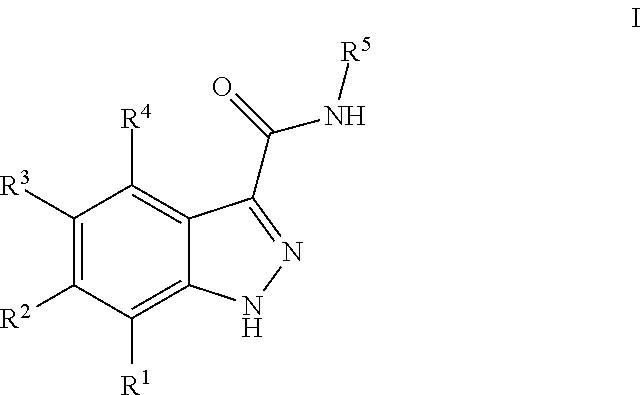
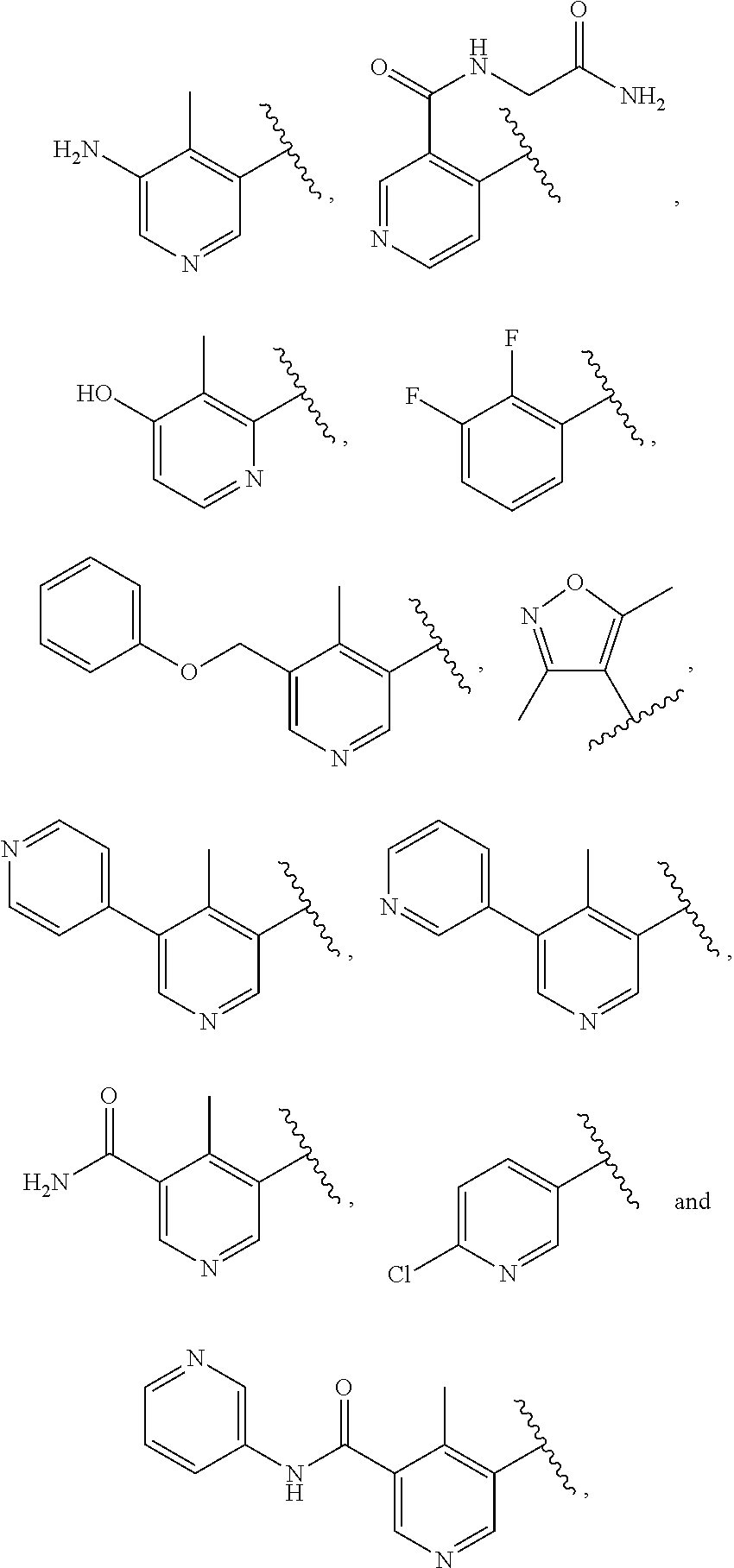
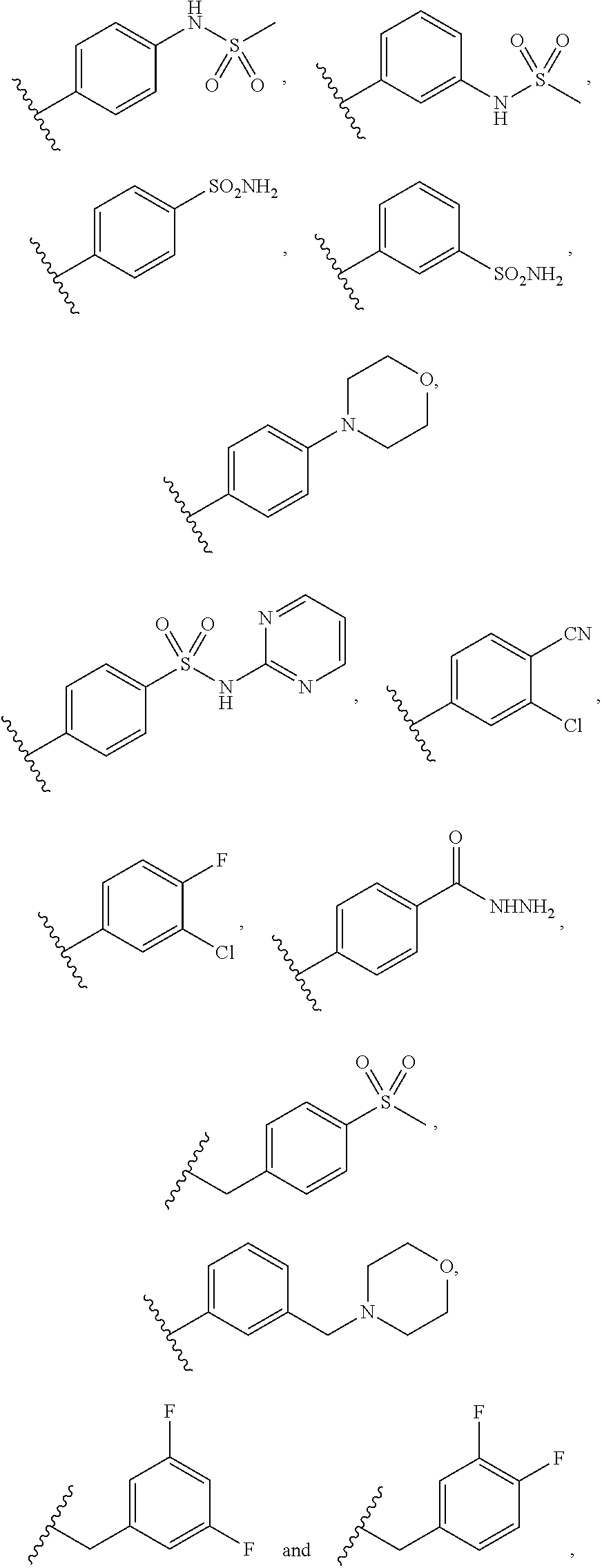
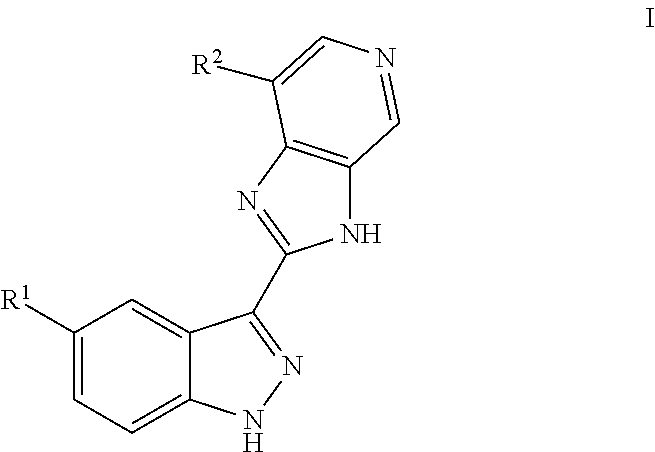
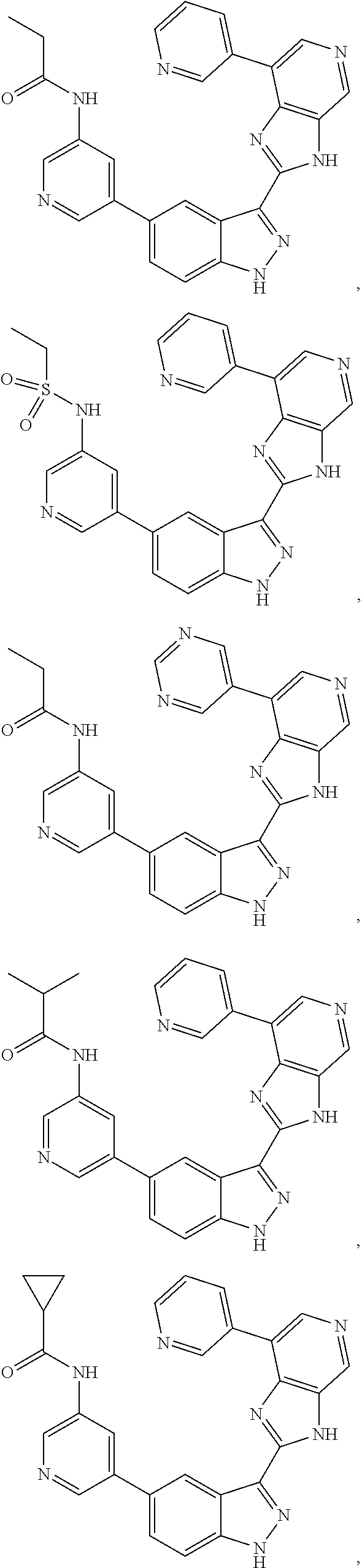
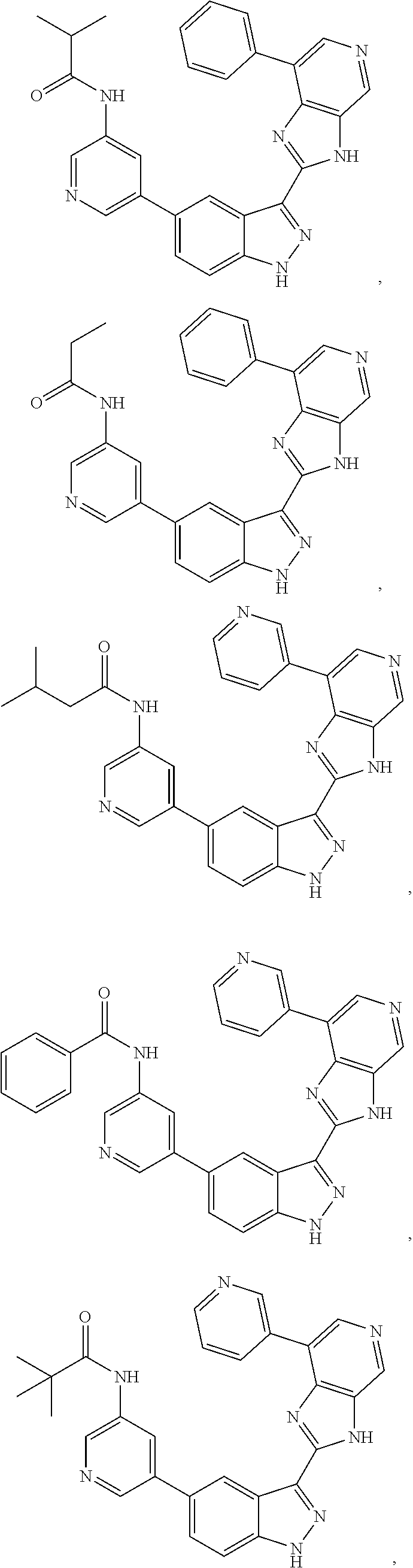
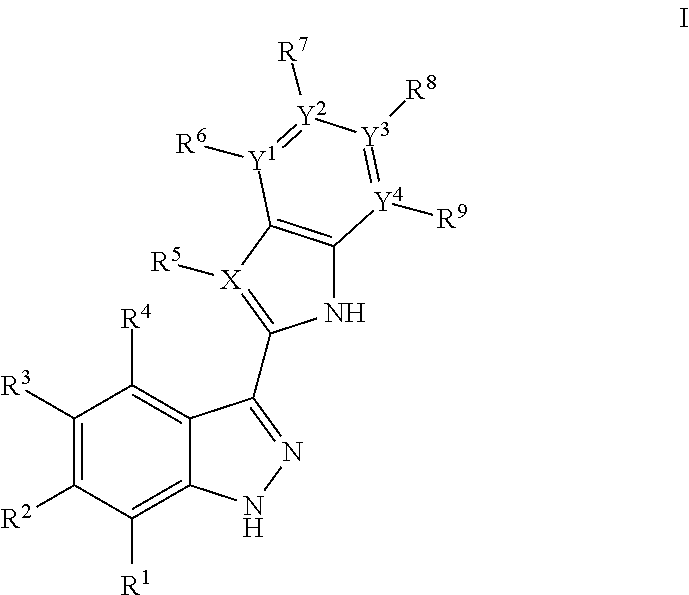
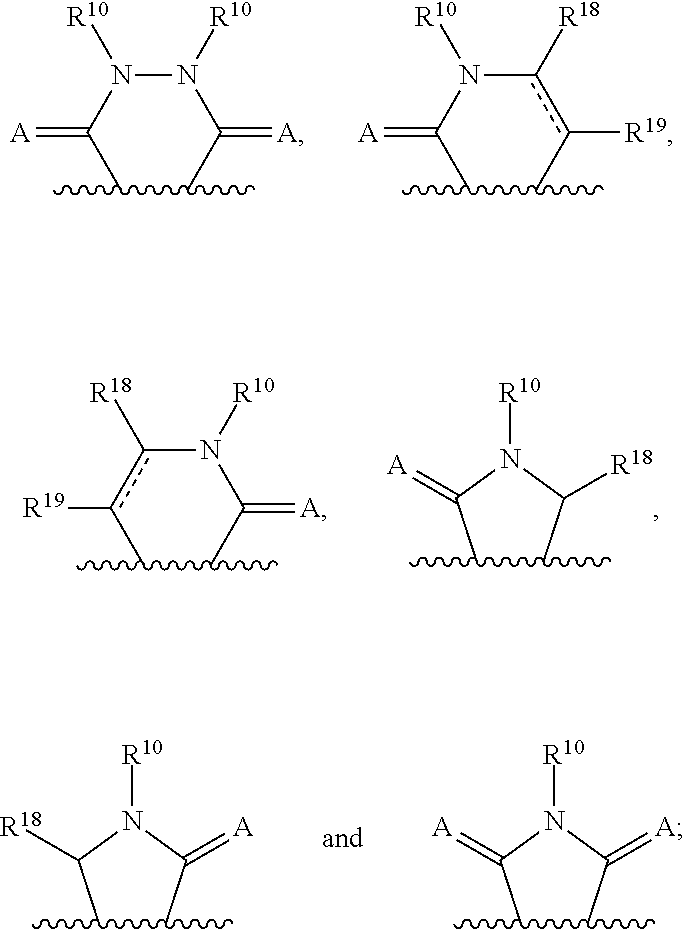
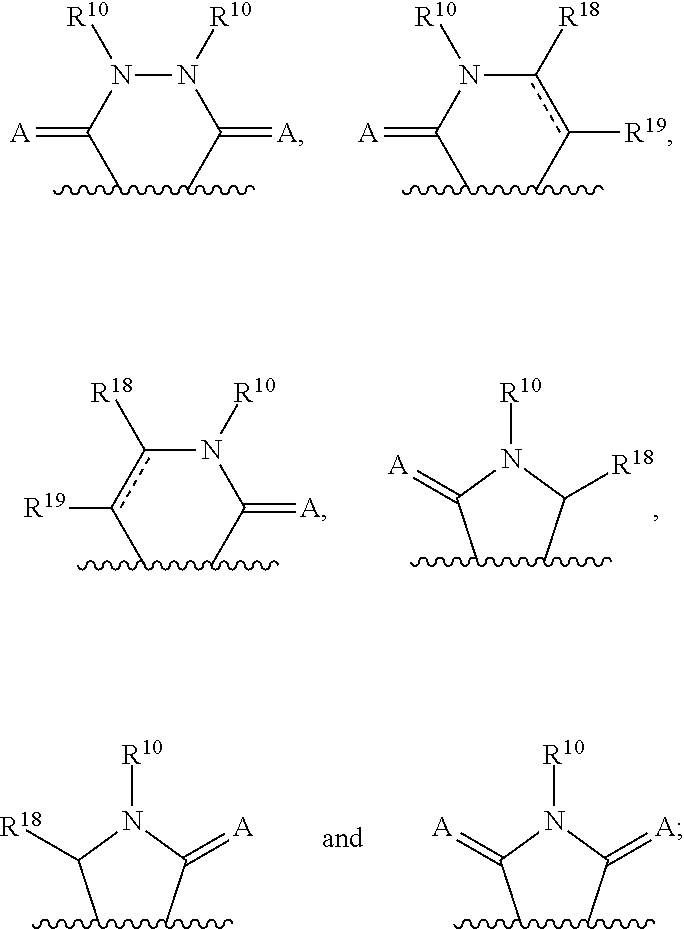
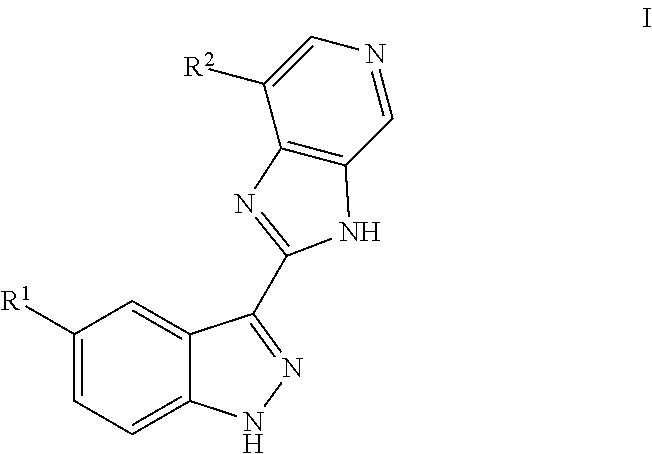
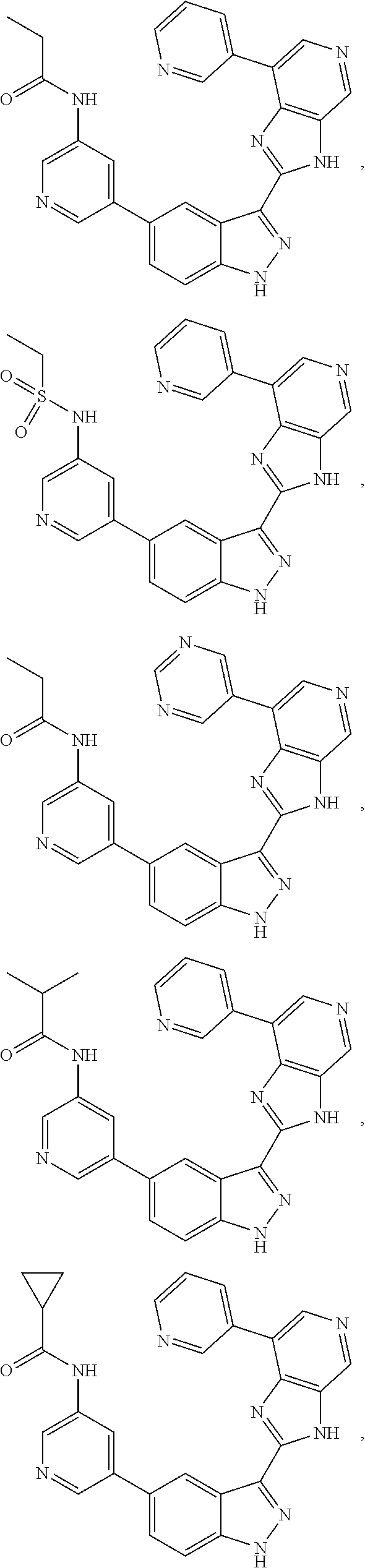
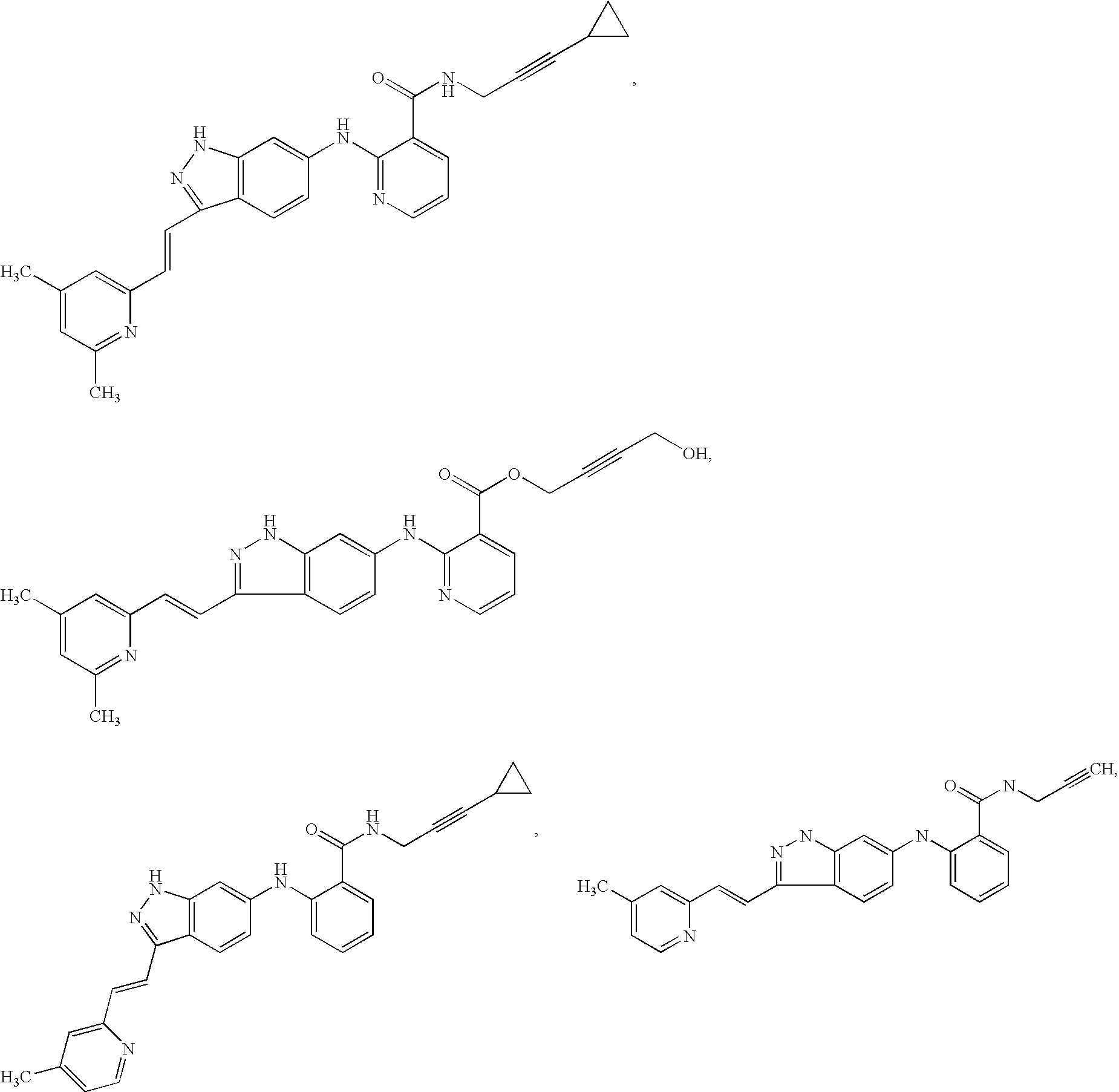
3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof
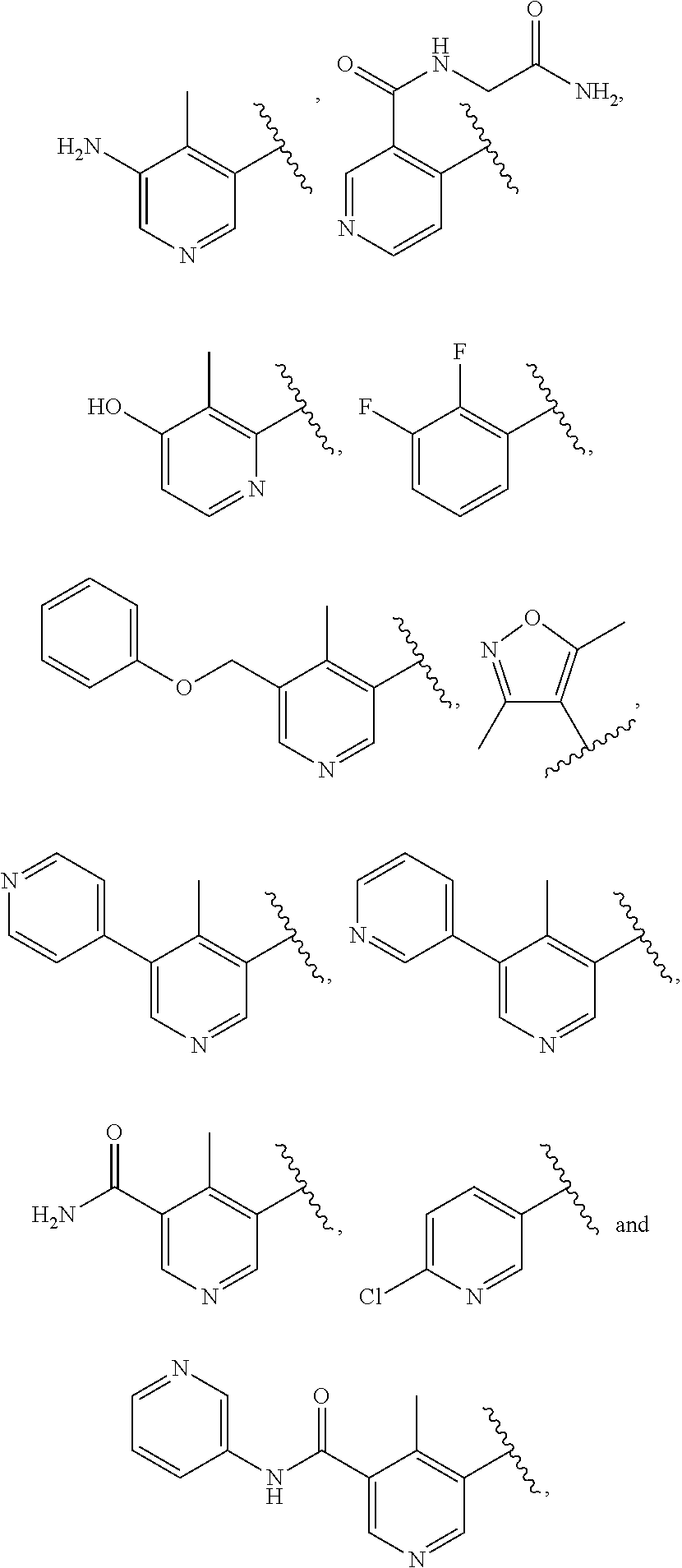
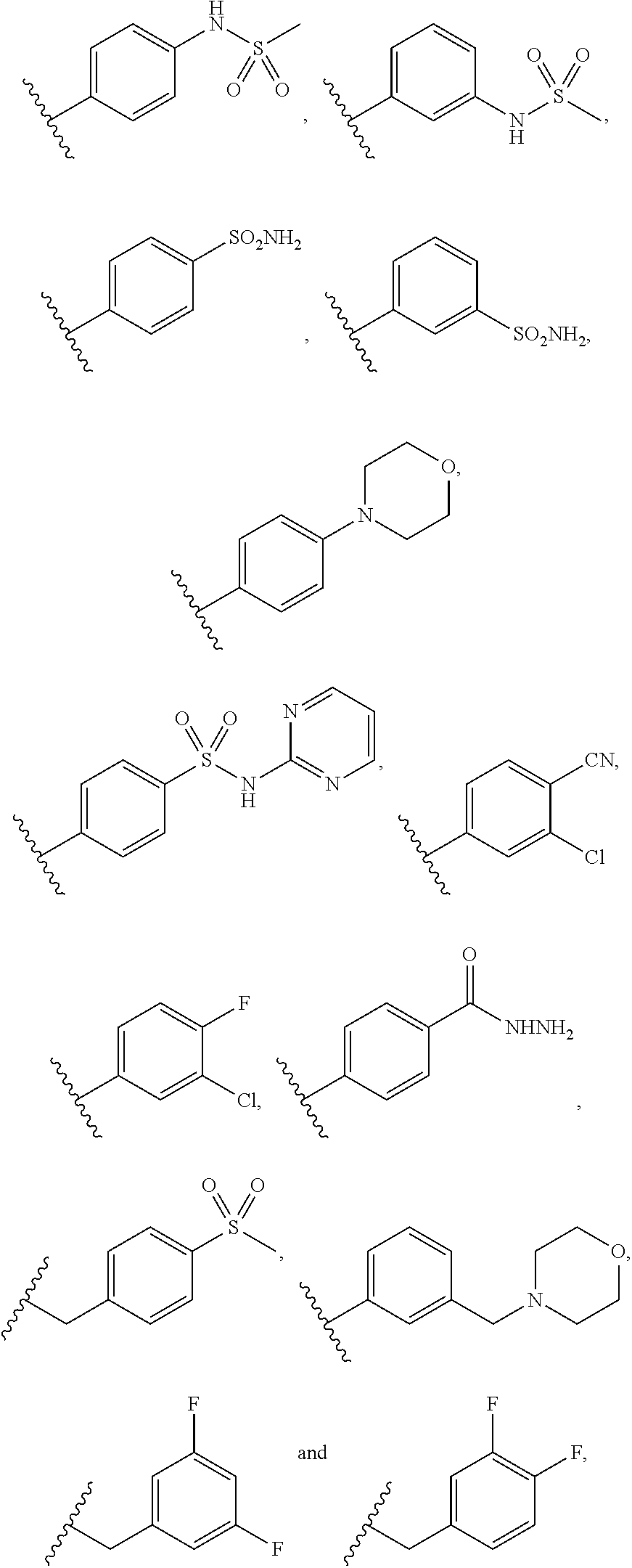
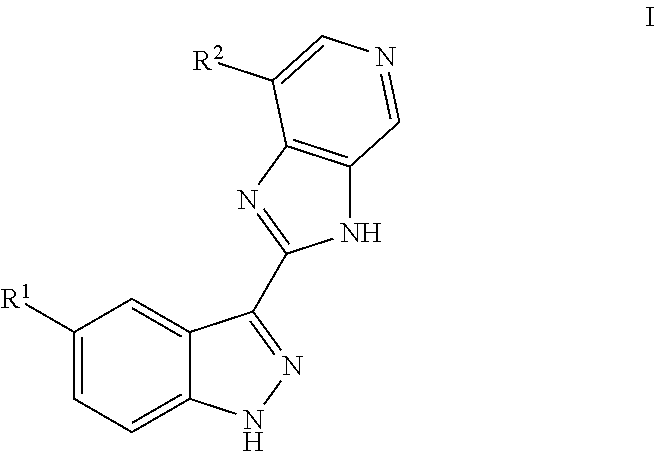
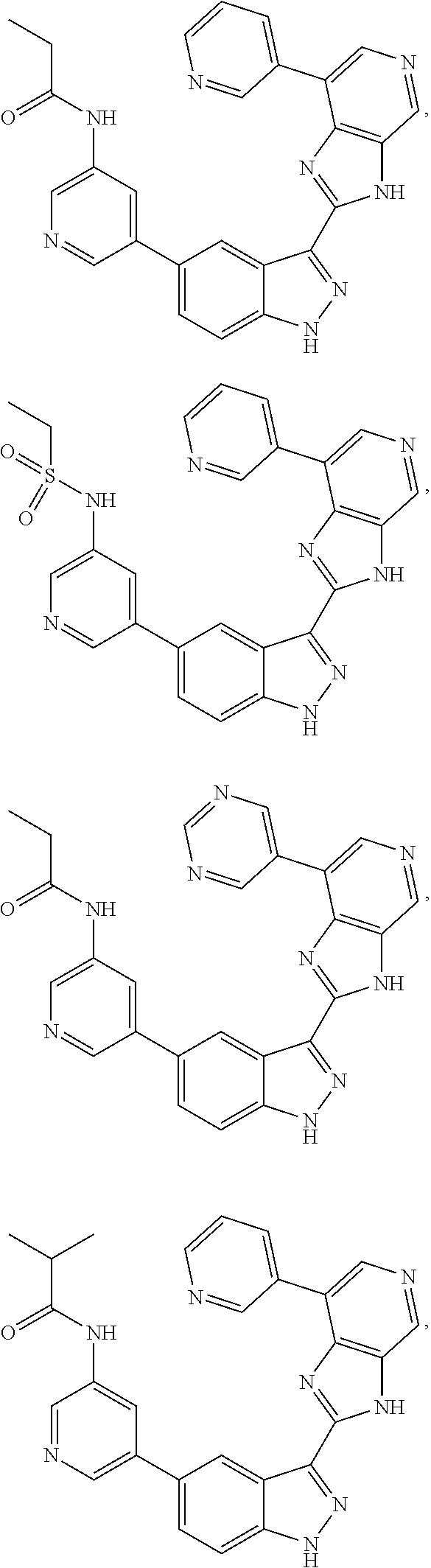
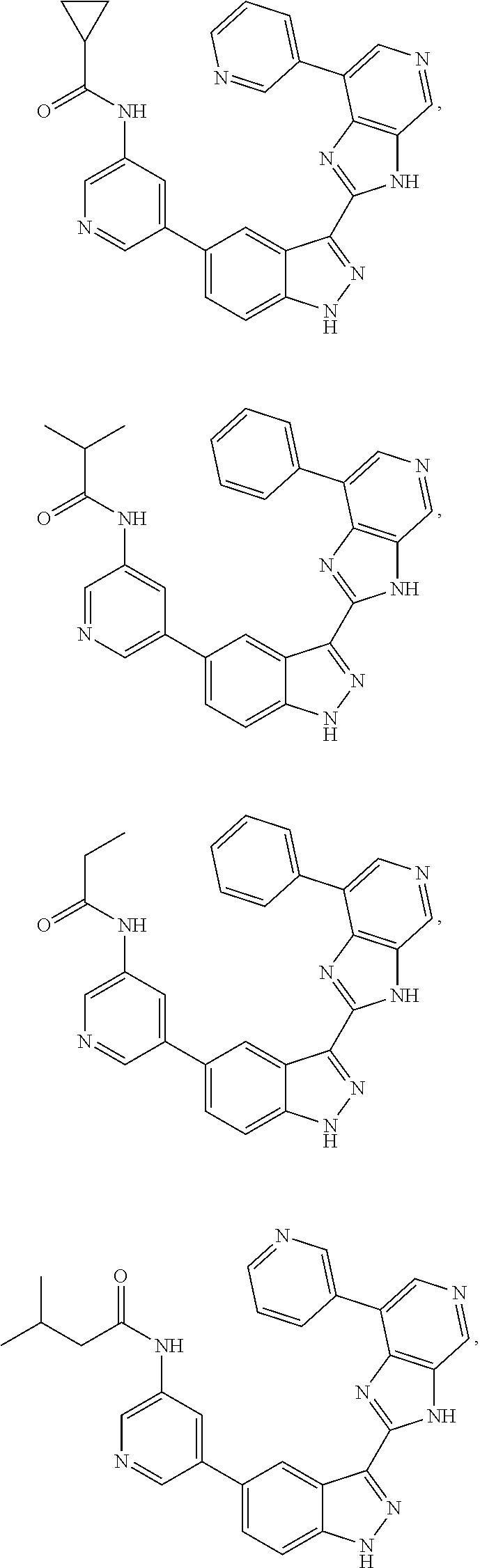
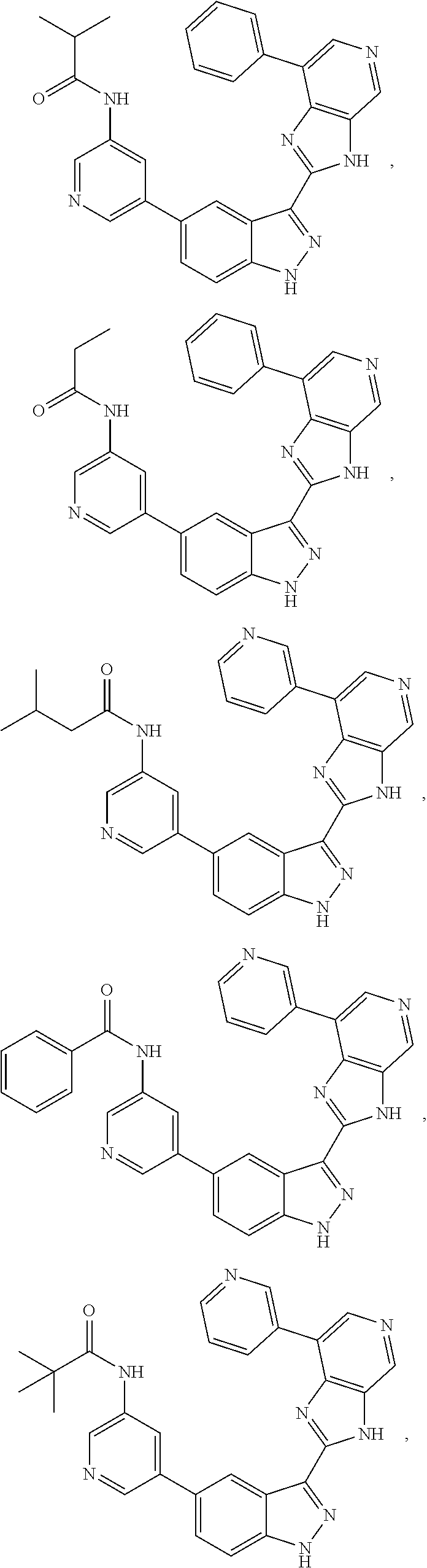
ActiveUS9475825B2Reduce and inhibit angiogenesisInhibit and reduce proliferationOrganic active ingredientsOrganic chemistryBiological activationCartilage Diseases
Azaindazole compounds for treating various diseases and pathologies are disclosed. More particularly, the present invention concerns the use of an azaindazole compound or analogs thereof, in the treatment of disorders characterized by the activation of Wnt pathway signaling (e.g., cancer, abnormal cellular proliferation, angiogenesis, fibrotic disorders, bone or cartilage diseases, and osteoarthritis), the modulation of cellular events mediated by Wnt pathway signaling, as well as genetic diseases and neurological conditions / disorders / diseases due to mutations or dysregulation of the Wnt pathway and / or of one or more of Wnt signaling components. Also provided are methods for treating Wnt-related disease states.
Owner:BIOSPLICE THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












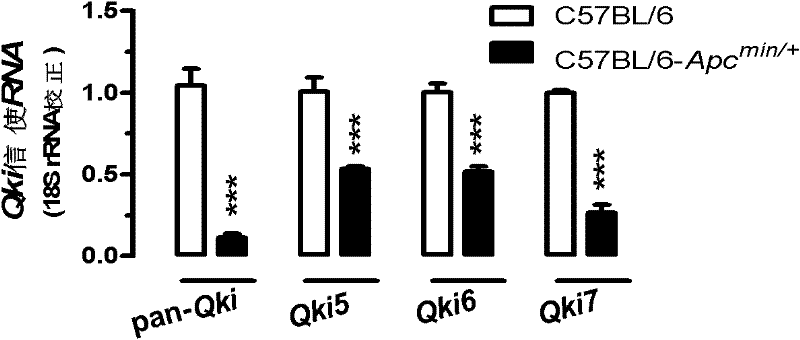











![3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof 3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe8836ec-5cab-45c0-989d-a576cad8b67a/US09475825-20161025-C00001.PNG)
![3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof 3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe8836ec-5cab-45c0-989d-a576cad8b67a/US09475825-20161025-C00002.PNG)
![3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof 3-(3H-imidazo[4,5-C]pyridin-2-yl)-1H-pyrazolo[3,4-C]pyridine and therapeutic uses thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe8836ec-5cab-45c0-989d-a576cad8b67a/US09475825-20161025-C00003.PNG)