Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1645 results about "Quinazoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
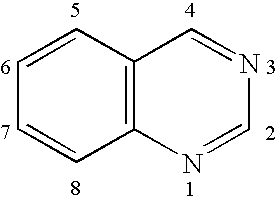
Quinazoline is an organic compound with the formula C₈H₆N₂. It is an aromatic heterocycle with a bicyclic structure consisting of two fused six-membered aromatic rings, a benzene ring and a pyrimidine ring. It is a light yellow crystalline solid that is soluble in water. Also known as 1,3-diazanaphthalene, quinazoline received its name from being an aza derivative of quinoline. Though the parent quinazoline molecule is rarely mentioned by itself in technical literature, substituted derivatives have been synthesized for medicinal purposes such as antimalarial and anticancer agents. Quinazoline is a planar molecule. It is isomeric with the other diazanaphthalenes of the benzodiazine subgroup: cinnoline, quinoxaline, and phthalazine.
Quinazoline ditosylate salt compounds
Ditosylate salts of 4-quinazolineamines are described as well as methods of using the same in the treatment of disorde4rs characterized by aberrant erbB family PTK activity.
Owner:NOVARTIS AG
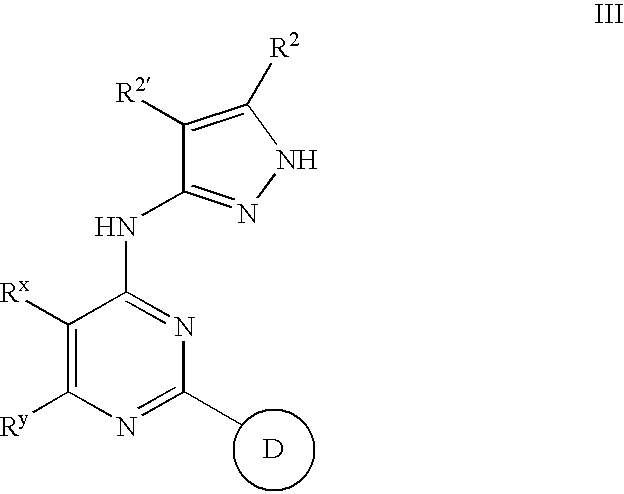
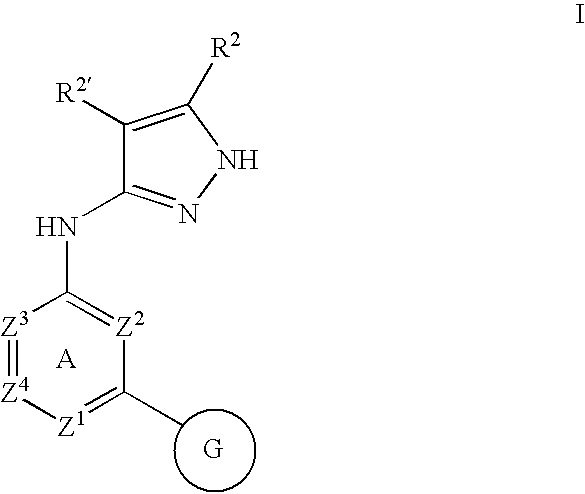
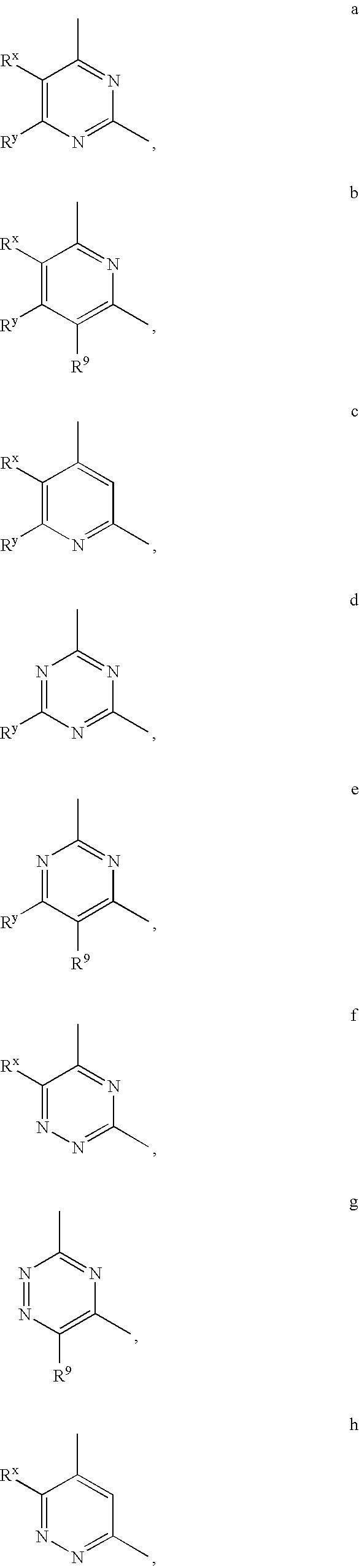
Pyrazolylamine substituted quinazoline compounds useful as protein kinase inhibitors
This invention describes novel pyrazole compounds of formula III:wherein Ring D is a 5–7 membered monocyclic ring or 8–10 membered bicyclic ring selected from aryl, heteroaryl, heterocyclyl or carbocyclyl; Rx and Ry are taken together with their intervening atoms to form a fused, unsaturated or partially unsaturated, 5–8 membered carbocyclo ring; and R2 and R2′ are as described in the specification. The compounds are useful as protein kinase inhibitors, especially as inhibitors of aurora-2 and GSK-3, for treating diseases such as cancer, diabetes and Alzheimer's disease.
Owner:VERTEX PHARMA INC
Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-II receptor antagonists
The present invention relates to urotensin II receptor antagonists, pharmaceutical compositions containing them and their use.
Owner:ENCYSIVE PHARMA INC
Quinazoline based EGFR inhibitors containing a zinc binding moiety
InactiveUS20080139590A1Enhanced and unexpected propertyHigh activityOrganic active ingredientsBiocideDiseaseZinc binding
The present invention relates to quinazoline containing zinc-binding moiety based derivatives that have enhanced and unexpected properties as inhibitors of epidermal growth factor receptor tyrosine kinase (EGFR-TK) and their use in the treatment of EGFR-TK related diseases and disorders such as cancer. The said derivatives may further act as HDAC inhibitors.
Owner:CURIS INC
Rho kinase inhibitors
Disclosed are novel substituted 2H-isoquinolin-1-one and 3H-quinazolin-4-one derivatives useful as inhibitors of Rho kinase and for treating a variety of diseases and disorders that are mediated or sustained through the activity of Rho kinase, including cardiovascular diseases, pharmaceutical compositions comprising such compounds, methods for using such compounds and processes for making such compounds.
Owner:AERIE PHARMA
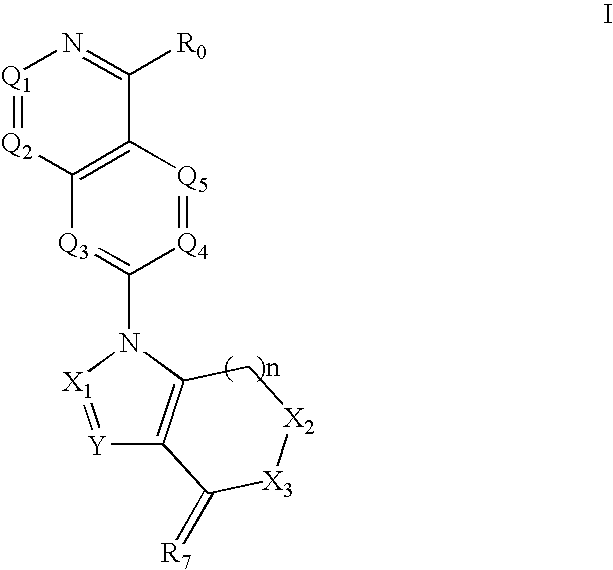
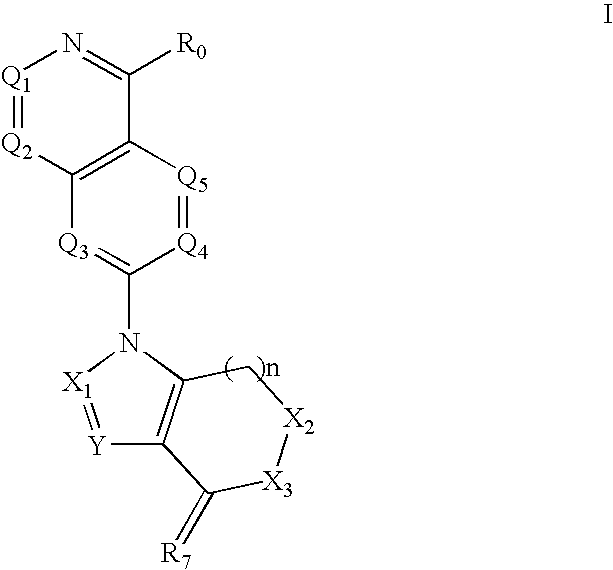
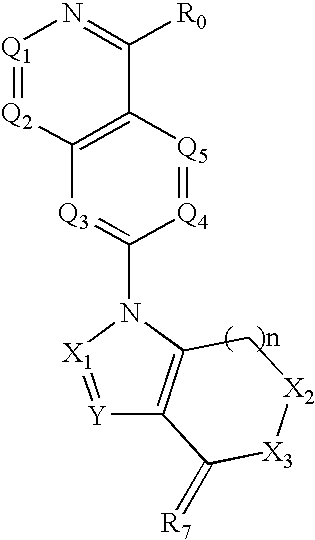
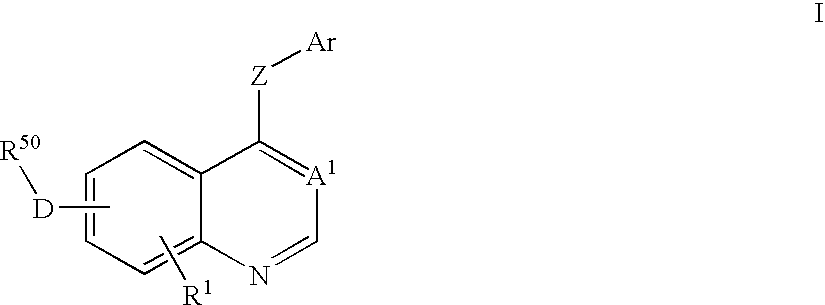
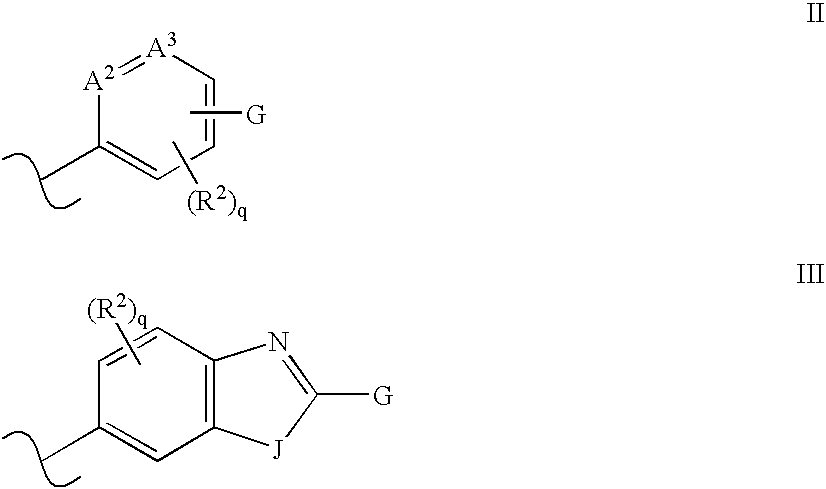
Isoquinoline, Quinazoline and Phthalazine Derivatives
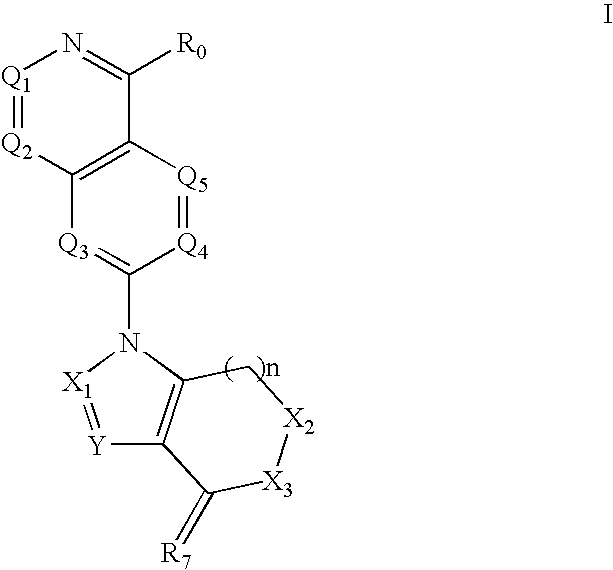
Disclosed are compounds and pharmaceutically acceptable salts of Formula I wherein R0, R5, R6, R7, n, Q1-Q5, Y, and X1-X3 are as defined herein. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer, inflammation, arthritis, angiogenesis, or the like. Also disclosed are pharmaceutical compositions comprising compounds of the invention and methods of treating the aforementioned conditions using such compounds.
Owner:ESANEX
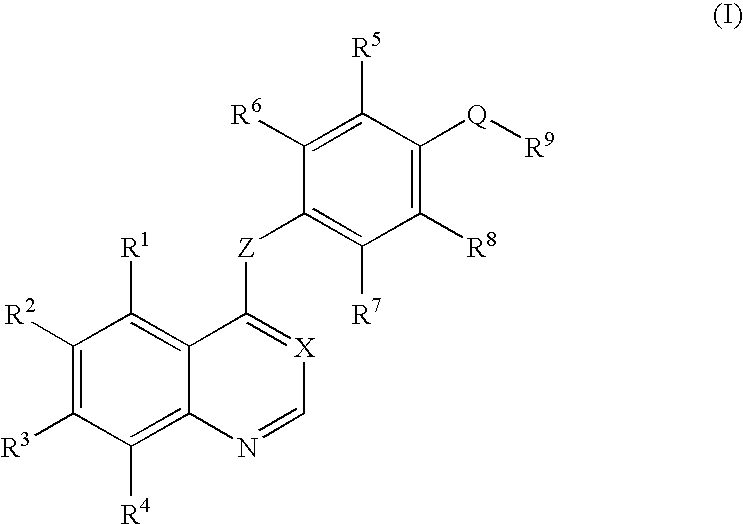
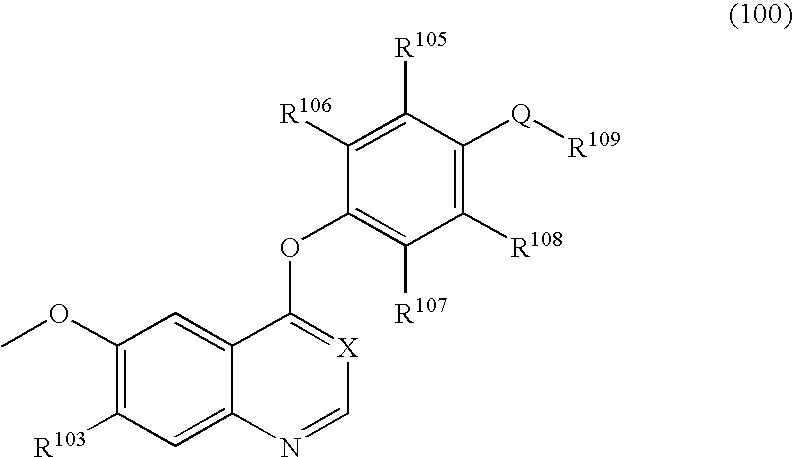
Quinazoline derivatives for the treatment of cancer
The current invention is directed toward compounds and pharmaceutically acceptable salts of Formula Iwherein Q1 is CR1, Q2 is N, Q4 and Q5 are each CR1, Q3 is CR2, X1 is N or CRc, Y is CRc, X2 and X3 are each C(R5)(R6), R7 is O. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer The current invention is also directed toward pharmaceutical compositions comprising compounds of the invention.
Owner:ESANEX
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
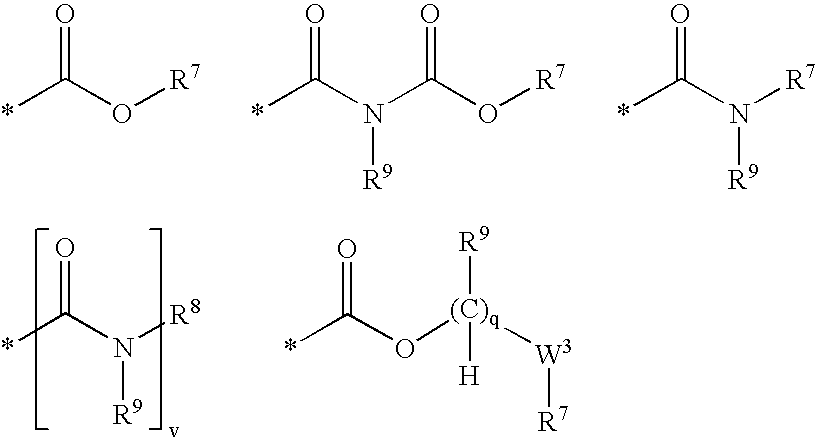
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Method for treating cancer harboring EGFR mutations
ActiveUS20090318480A1Advantageously effective in treatmentReduced responseBiocideOrganic active ingredientsAcquired resistanceActivating mutation
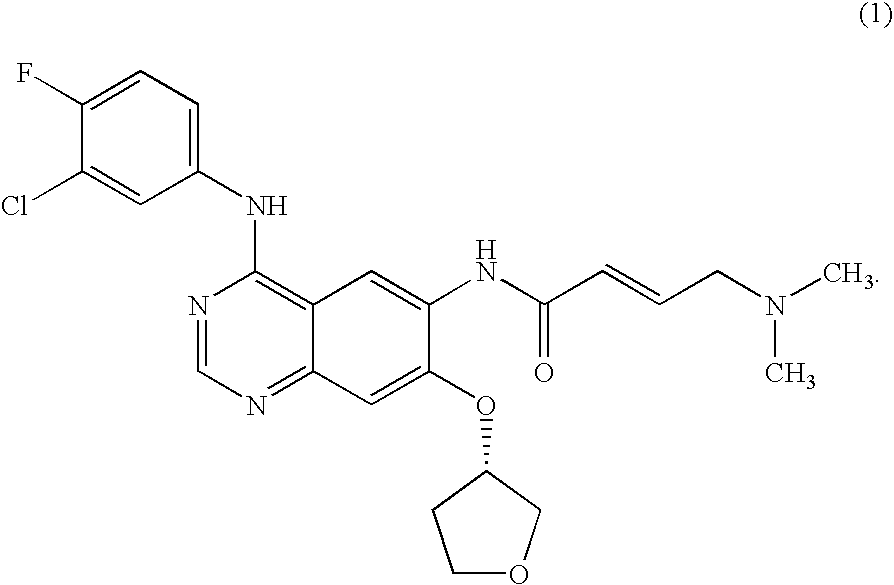
The present invention relates to a method of treatment of patients suffering from cancer and harbouring mutations of EGFR in the tumour, for instance an activating mutation of the EGFR or a mutation responsible for resistance or the emergence of acquired resistance to treatment with reversible EGFR and / or HER2 inhibitors or irreversible inhibitors such as CI-1033, EKB-569, HKI-272 or HKI-357, comprising administering an effective amount of the irreversible EGFR inhibitor BIBW2992 (1) 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino}-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, to a person in need of such treatment, optionally in combination with the administration of a further chemotherapeutic agent, in combination with radiotherapy, radio-immunotherapy and / or tumour resection by surgery, and to the use of a BIBW 2992 (1) for preparing a pharmaceutical composition for the treatment of patients suffering from cancer and harbouring mutations of EGFR in the tumour.
Owner:BOEHRINGER INGELHEIM INT GMBH
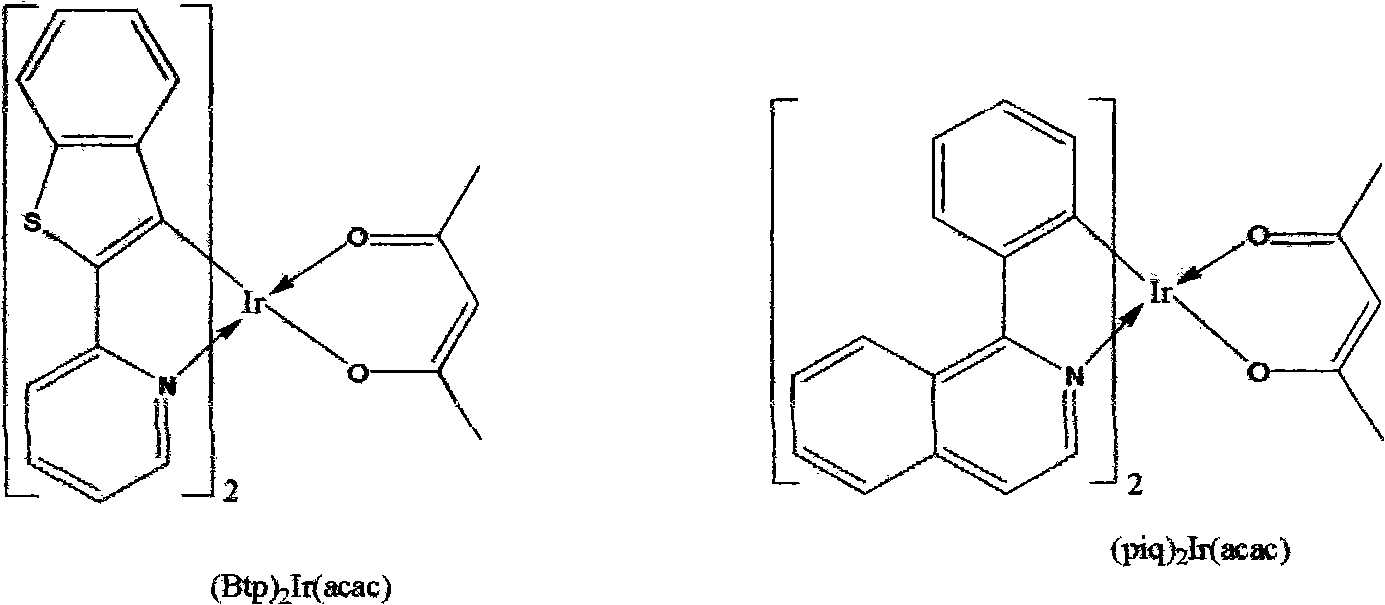
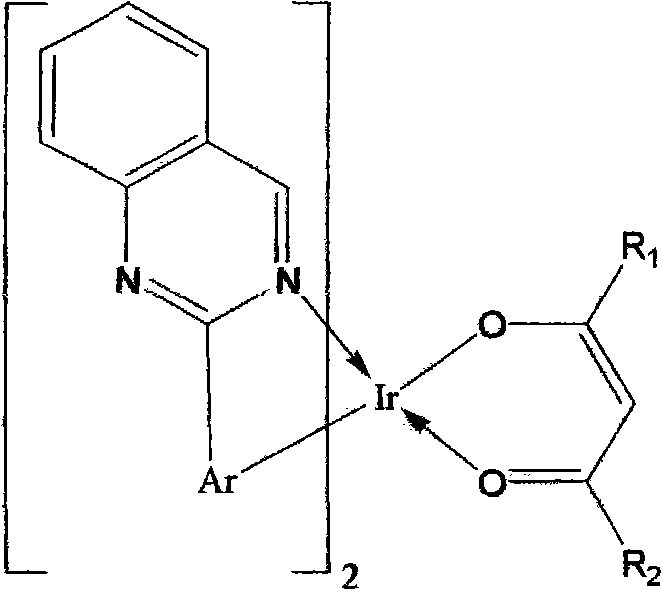
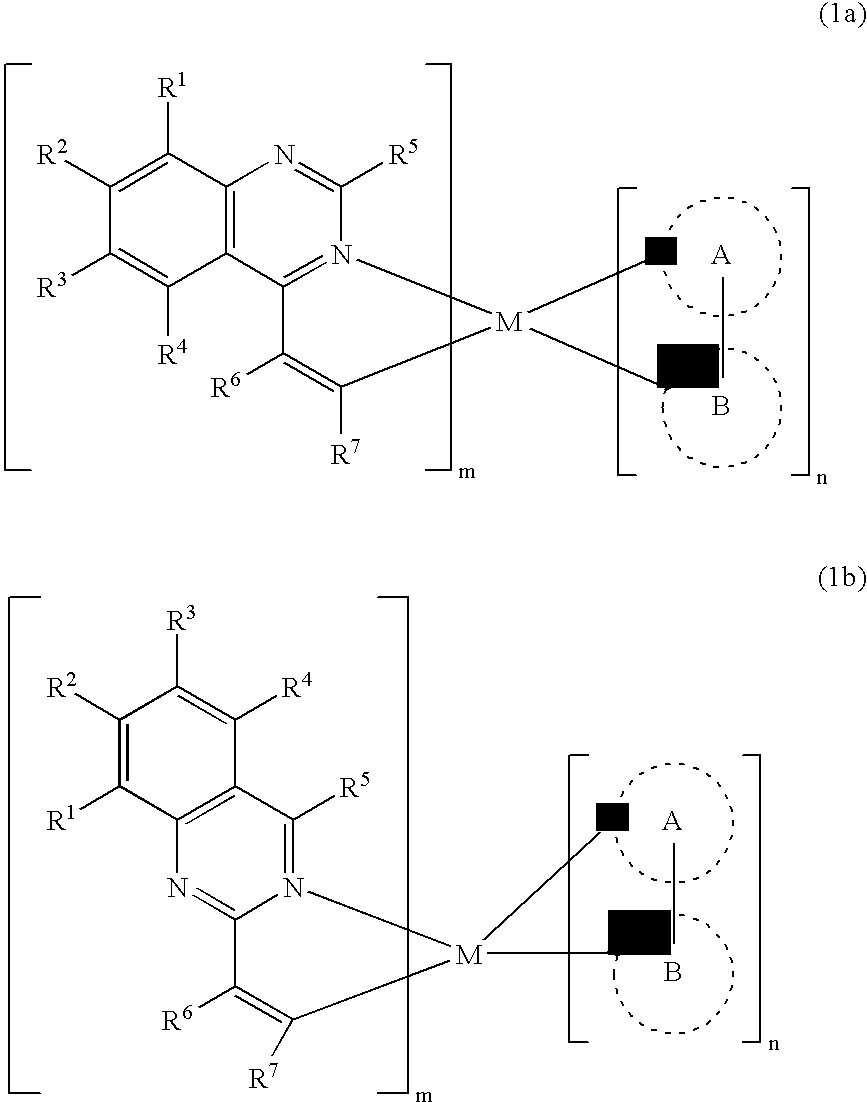
Electroluminescent device with quinazoline complex emitter
ActiveUS20070122655A1Useful emissionUseful stabilityGroup 5/15 element organic compoundsSolid-state devicesLuminophoreHost material
An OLED device comprises a cathode, an anode, and located therebetween a light-emitting layer containing a host material and a tris-CˆN-cyclometallated complex of Ir or Rh wherein at least one of the ligands comprises a substituted quinazoline moiety. The device provides useful emission and stability attributes.
Owner:GLOBAL OLED TECH
c-Met modulators and methods of use
The present invention provides compounds for modulating protein kinase enzymatic activity for modulating cellular activities such as proliferation, differentiation, programmed cell death, migration and chemoinvasion. More specifically, the invention provides quinazolines and quinolines which inhibit, regulate and / or modulate kinase receptor, particularly c-Met, KDR, c-Kit, flt-3 and flt-4, signal transduction pathways related to the changes in cellular activities as mentioned above, compositions which contain these compounds, and methods of using them to treat kinase-dependent diseases and conditions. The present invention also provides methods for making compounds as mentioned above, and compositions which contain these compounds.
Owner:EXELIXIS INC
Quinoline or quinazoline derivatives inhibiting auto-phosphorylation of fibroblast growth factor receptors
InactiveUS20050049264A1Antitumor activityGrowth inhibitionBiocideOrganic chemistryCancer cellHalogen
An objective of the present invention is to provide novel compounds which have inhibitory activity against autophosphorylation of an FGF receptor family and, when orally or intraveneously administered, can suppress the growth of cancer cells. The compounds of the present invention are represented by formula (I) or a pharmaceutically acceptable salt or solvate thereof: wherein X represents CH or N; Z represents O or S; Q represents NR10, CR11R2, carbonyl, O, S(═O)m, wherein m is 0 to 2, or urea; R1 to R3 each independently represent H, OH, halogen, nitro, amino, alkyl, alkoxy or the like in which the alkyl and alkoxy groups are optionally substituted; R4 represents H; R5 to R8 each independently represent H, halogen, alkyl, or alkoxy; and R9 represents an optionally substituted carbocyclic or heterocyclic group.
Owner:KYOWA HAKKO KIRIN CO LTD
Substituted quinazoline compounds and methods of use thereof
ActiveUS20160297774A1Organic active ingredientsGroup 5/15 element organic compoundsDiseaseChemical compound
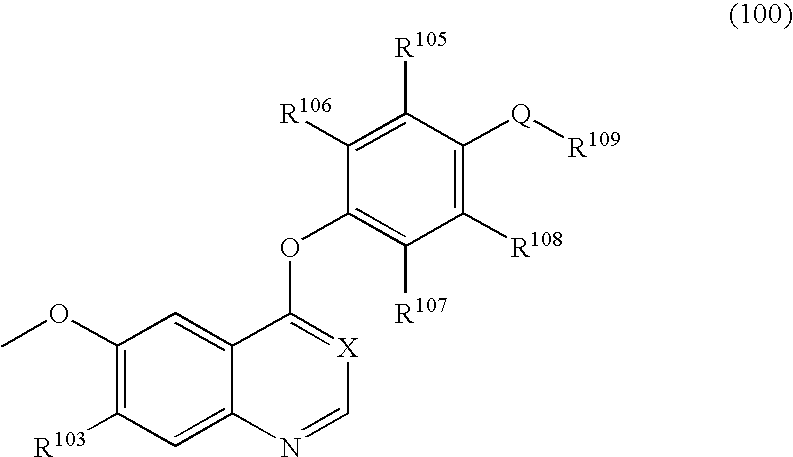
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have one of the following structures (I), (II) or (III):or a pharmaceutically acceptable salt, stereoisomer or prodrug thereof, wherein R1, R2a, R2b, R2c, R3a, R3b, R4a, R4b, R5a, R5b, R6, A, B, G1, G2, L1, L2, m1, m2, n, x, y, X and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Organic Compound, Light-Emitting Element, Display Module, Lighting Module, Light-Emitting Device, Display Device, Electronic Device, and Lighting Device
ActiveUS20160240794A1Improve heat resistanceLess crosstalkOrganic chemistrySolid-state devicesElectron injectionDisplay device
A novel organic compound that can be used as an electron-injection material or an electron-transport material of a light-emitting element is provided. An organic compound with which a display device having less crosstalk can be obtained is provided. A light-emitting device, a display device, and an electronic device each having less crosstalk are provided. An organic compound including two or three benzo[h]quinazoline rings is provided. In the organic compound, two or three benzo[h]quinazoline rings are preferably included in the substituent including an aromatic ring or a heteroaromatic ring and having 3 to 30 carbon atoms. When two or three benzo[h]quinazoline rings are included in a substituent particularly including a heteroaromatic ring and having 3 to 30 carbon atoms, a high electron-transport property can be obtained.
Owner:SEMICON ENERGY LAB CO LTD
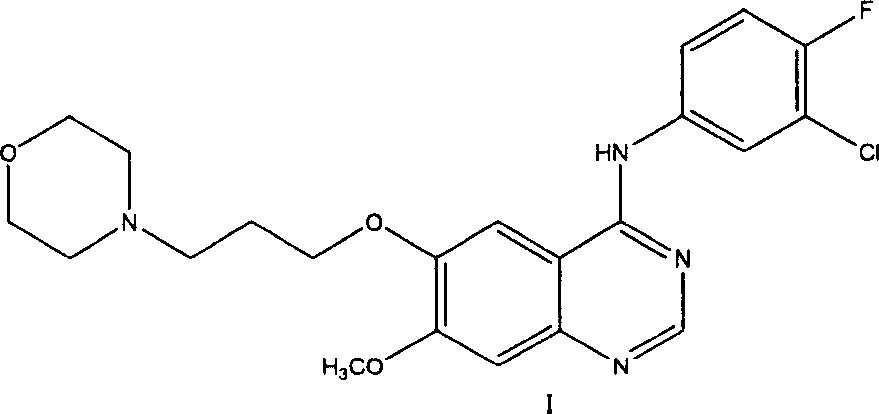
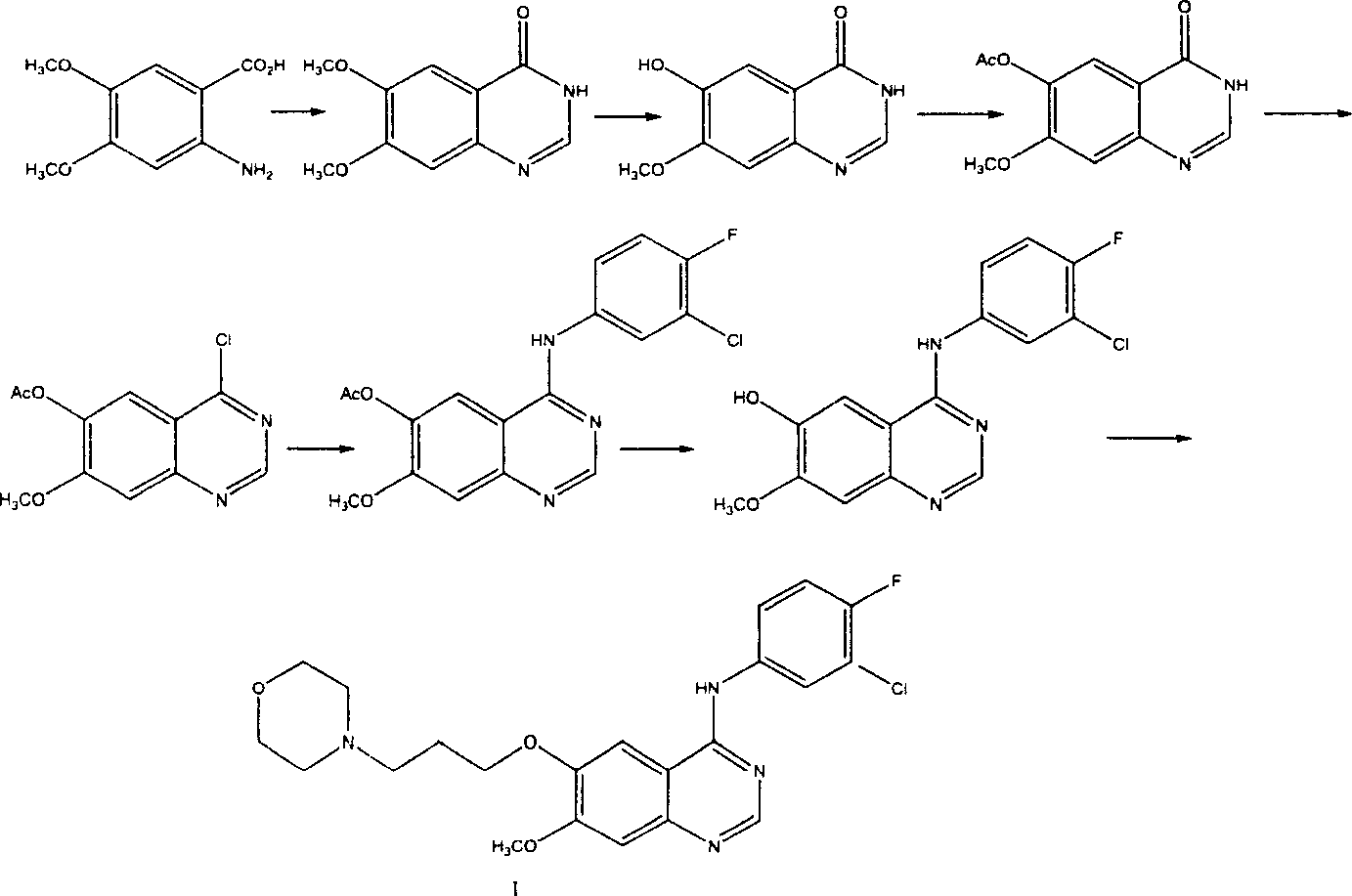
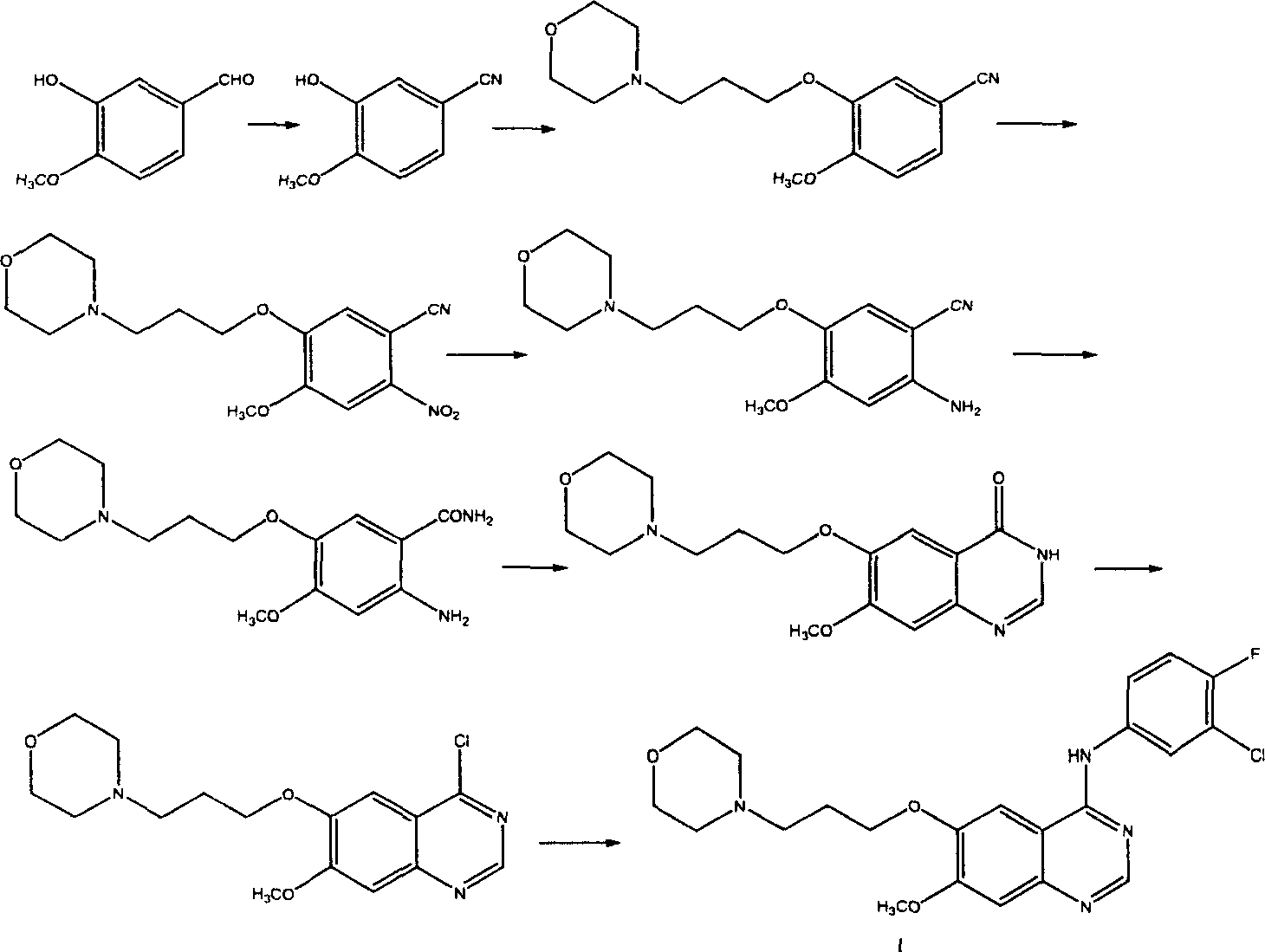
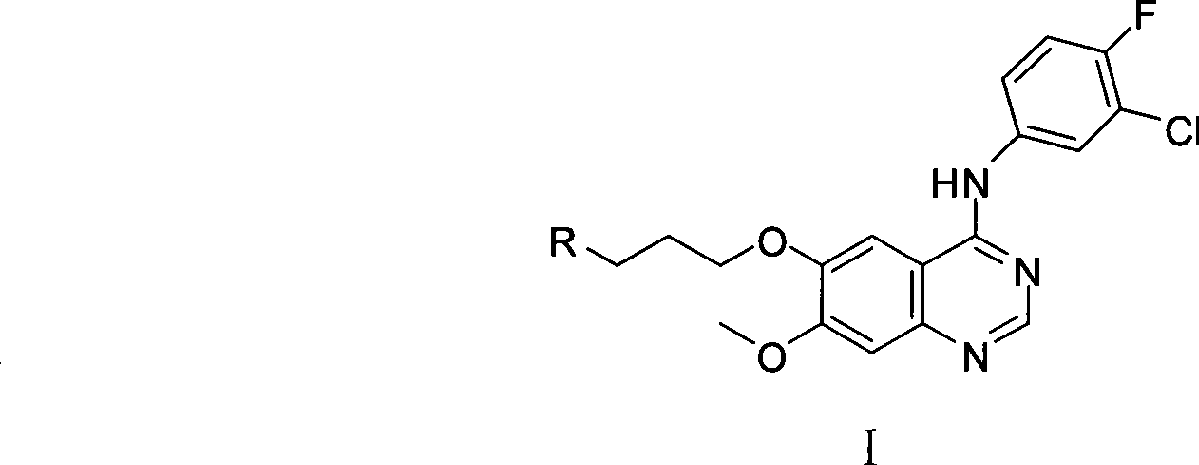
Preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline
InactiveCN1733738AReduce pollutionReduce manufacturing costOrganic chemistryAntineoplastic agentsMorpholine3-chloro-4-fluoroaniline
The invention relates to a preparation method of 4-(3-chlor-4-fluorobenzeneamidocyanogen)-7-methoxy-6-(3-morpholine oxypropyl)quinazoline, which comprises using 3,4-dimethoxybenzoic acid (II) as raw material, synthesizing 2-amido-4-methoxy-5-hydroxybenzoic acid (V), cyclizing to obtain 6-hydroxy-7-methoxy-3,4-dihydroquinazolin-4-one (VI), directly chloridizing to obtain 4-chloro-hydroxy-7-methoxy-quinazoline (VII), reacting directly with 3-chloro-4-fluoroaniline, carrying out amination to obtain 4-(3-chloro-4-fluoroanilino)-6-hydroxy-7-methoxy-quinazoline (VIII), finally reacting with morpholinyl chloropropane to obtain Geftinat (I).
Owner:江苏吴中苏药医药开发有限责任公司
Amino-quinazoline derivative with antineoplastic activity and its salts
The present invention provides an amido quinazoline derivative which has recipient singal conductance for inhibition of epidermal growth factors with anti-tumor activity. The novel compounds with a structure identical to quinazoline has quite high activity for inhibition of tumor cells, in particular to the remarkable inhibition effects on the growth of tumor cells of EGFR high expression. And the effective inhibition concentration is 5 times higher than the medicine IRESSA on the market.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Substituted quinoline and quinazoline inhibitors of quinone reductase 2
The present invention provides composition and methods of inhibiting quinone reductase 2 (QR2). The methods are useful in the treatment of malaria and autoimmune diseases. The compositions of the invention comprise quinoline and quinazoline derivatives. The invention also provides methods for inhibiting the activity of QR2 by contacting the enzyme with one or more compositions of the invention.
Owner:SERENEX INC
Quinoline or quinazoline derivatives inhibiting auto-phosphorylation of fibroblast growth factor receptors
InactiveUS7495104B2Antitumor activityGrowth inhibitionBiocideOrganic chemistryCancer cellAutophosphorylation
An objective of the present invention is to provide novel compounds which have inhibitory activity against autophosphorylation of an FGF receptor family and, when orally or intraveneously administered, can suppress the growth of cancer cells. The compounds of the present invention are represented by formula (I) or a pharmaceutically acceptable salt or solvate thereof:wherein X represents CH or N; Z represents O or S; Q represents NR10, CR11R2, carbonyl, O, S(═O)m, wherein m is 0 to 2, or urea; R1 to R3 each independently represent H, OH, halogen, nitro, amino, alkyl, alkoxy or the like in which the alkyl and alkoxy groups are optionally substituted; R4 represents H; R5 to R8 each independently represent H, halogen, alkyl, or alkoxy; and R9 represents an optionally substituted carbocyclic or heterocyclic group.
Owner:KYOWA HAKKO KIRIN CO LTD
Substituted quinazolines as inhibitors of KRAS G12C
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I):or a pharmaceutically acceptable salt, tautomer, prodrug or stereoisomer thereof, wherein R1, R2a, R3a, R3b, R4a, R4b, G1, G2, L1, L2, m1, m2, A, B, W, X, Y, Z and E are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided.
Owner:ARAXES PHARMA LLC
Pyridine, pyrimidine, quinoline, quinazoline, and naphthalene urotensin-II receptor antagonists
The present invention relates to urotensin II receptor antagonists, pharmaceutical compositions containing them and their use.
Owner:ENCYSIVE PHARMA INC
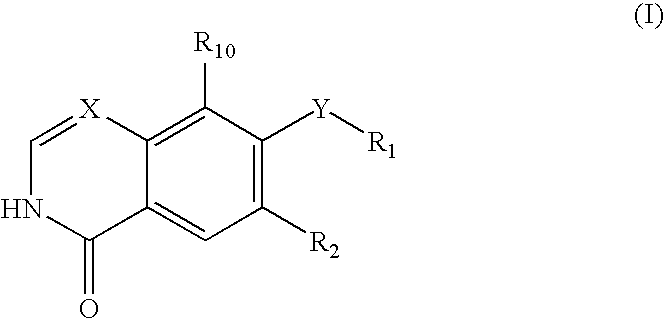
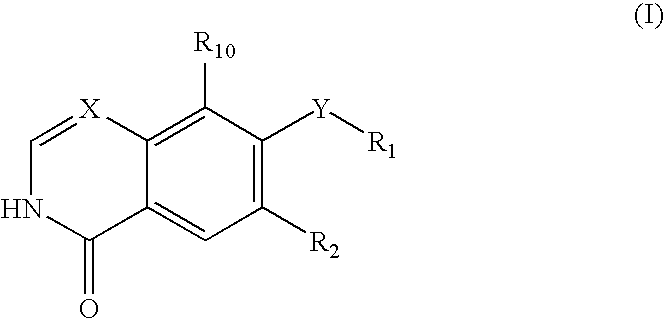
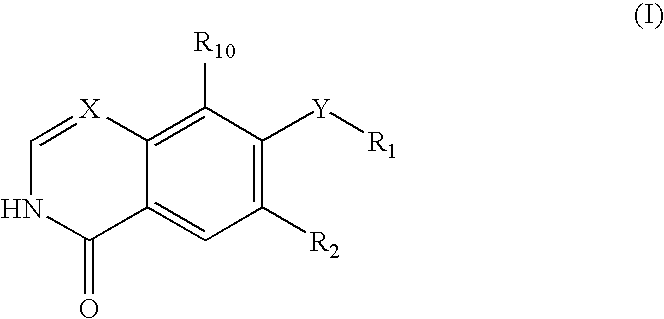
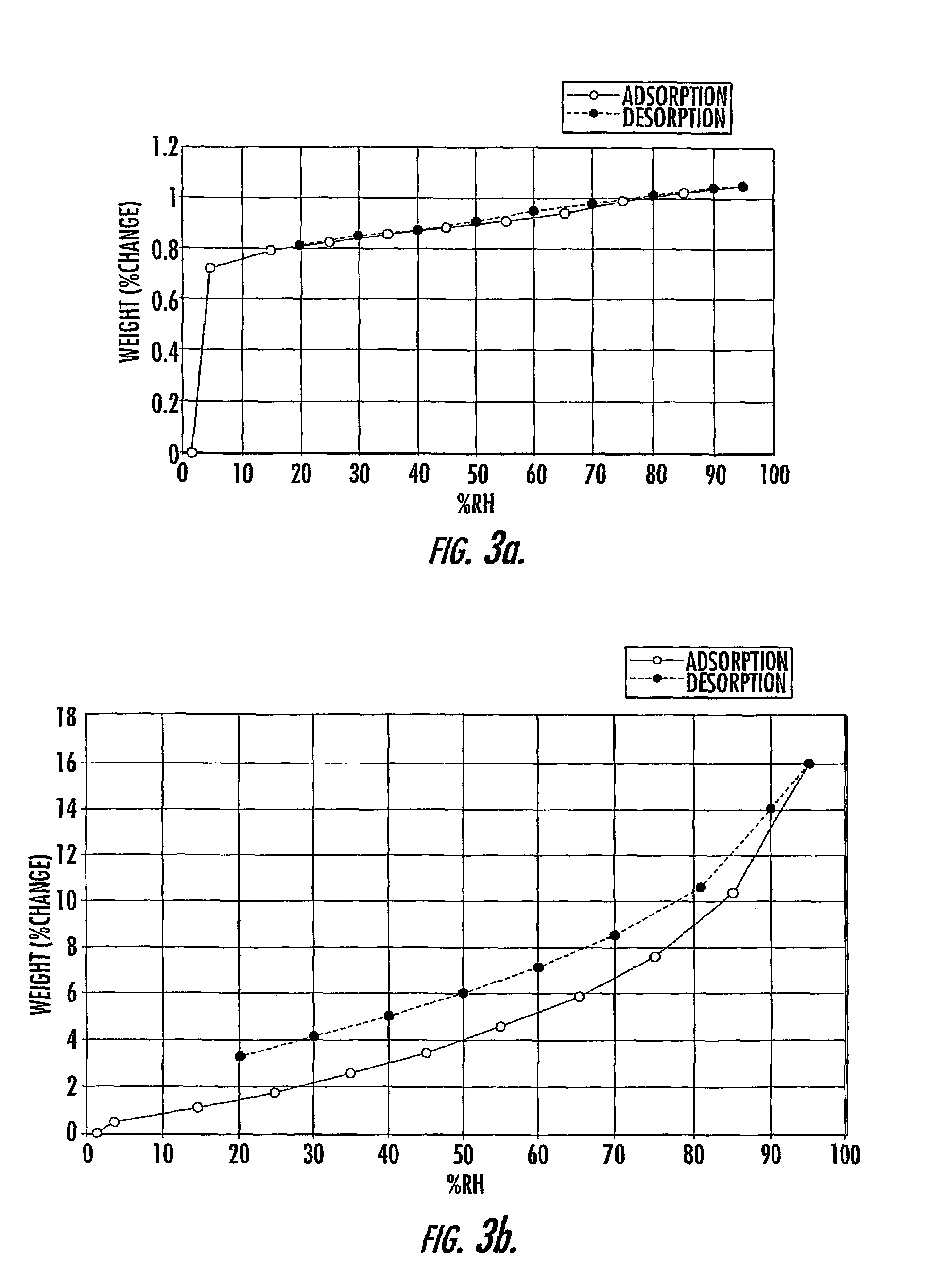
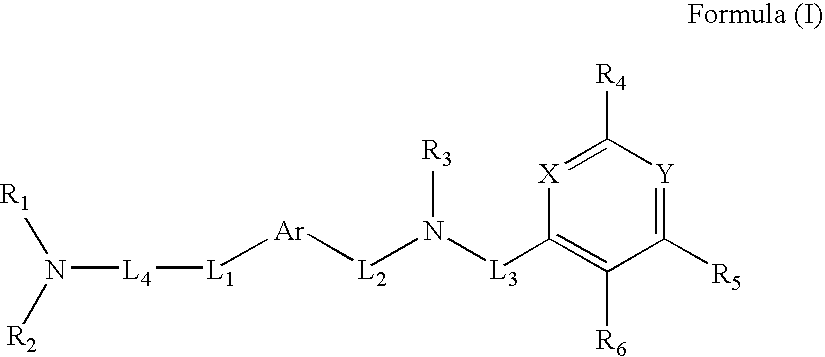
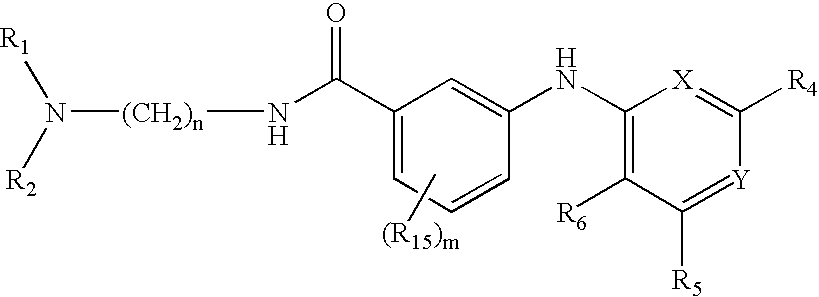
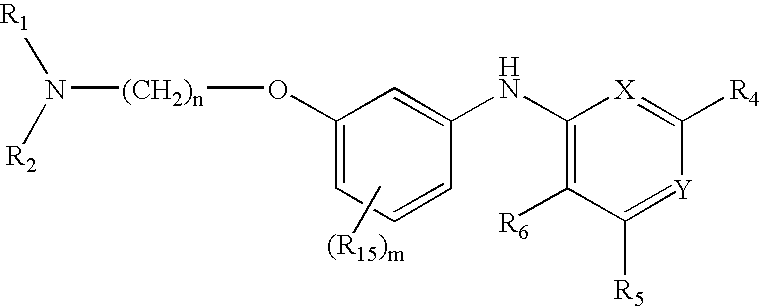
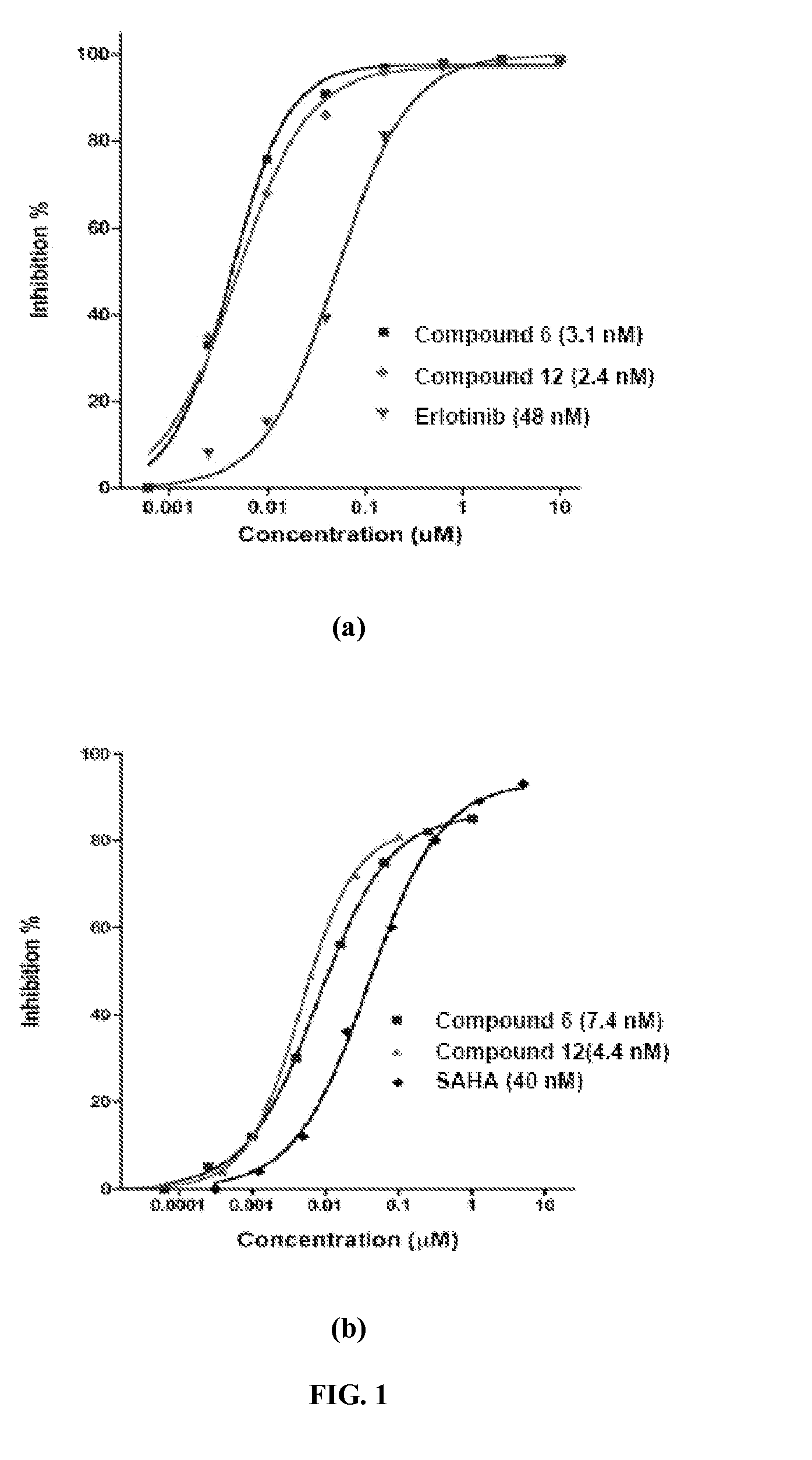
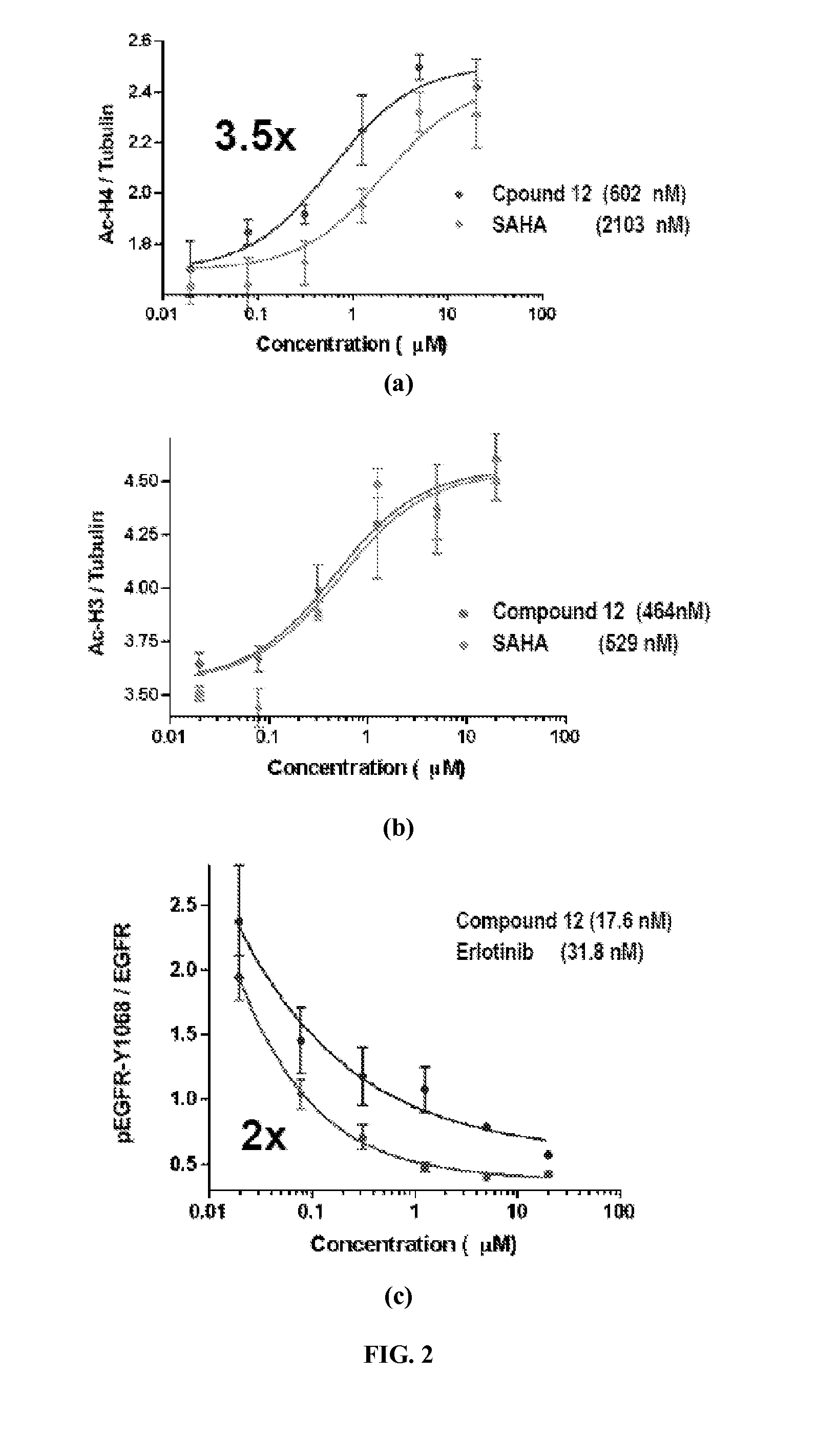
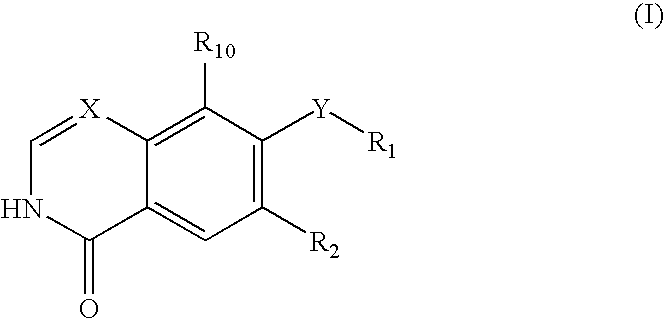
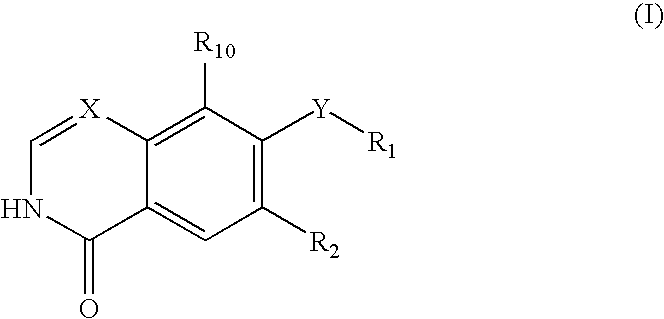
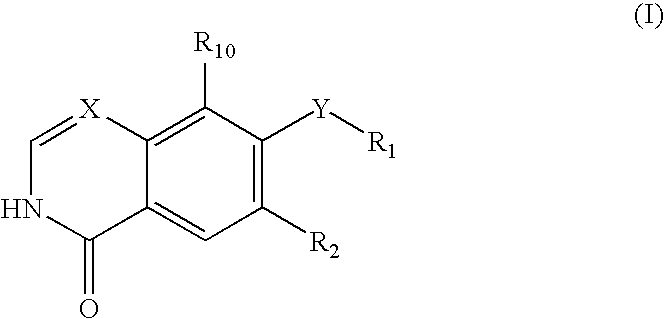
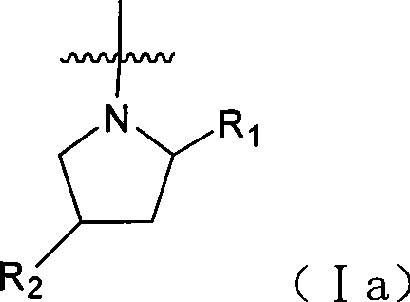
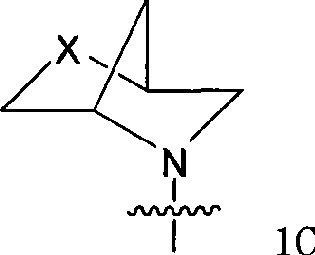
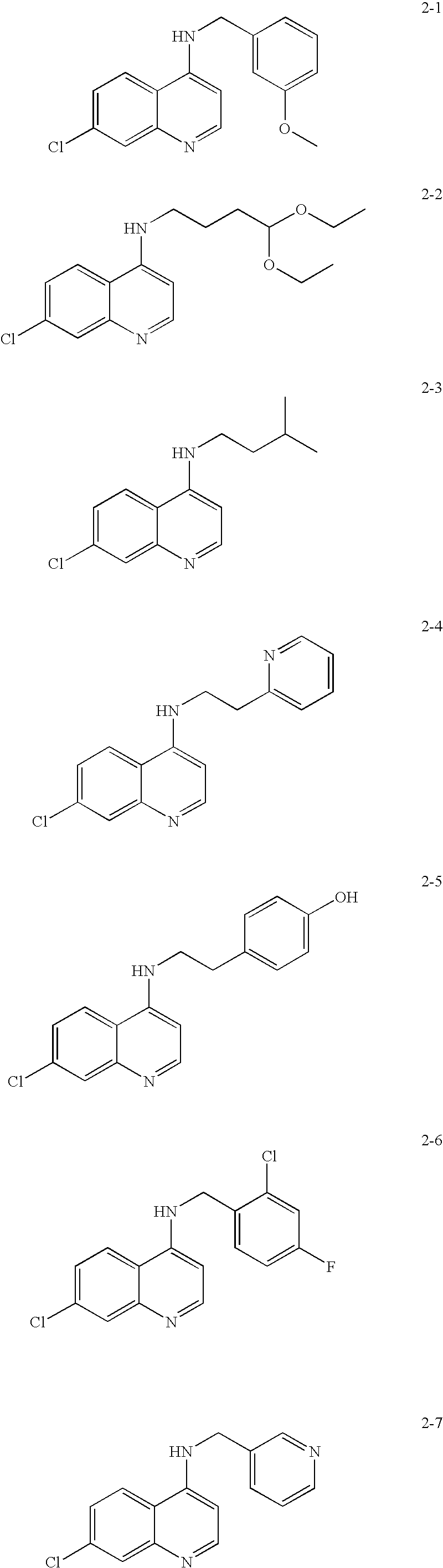
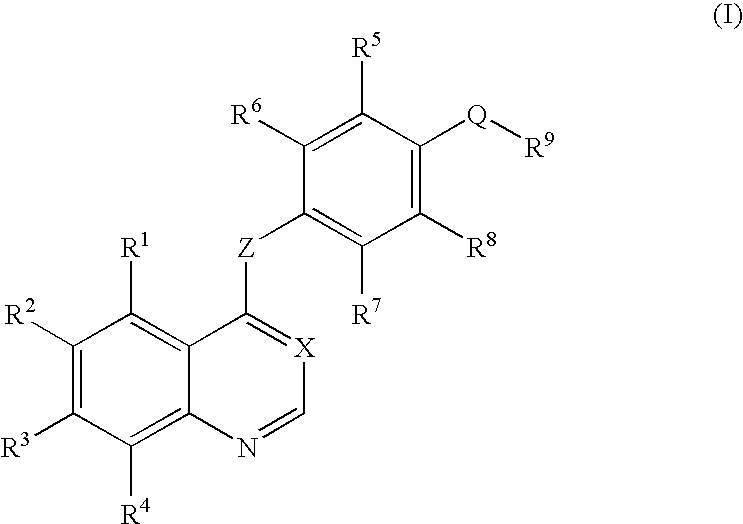
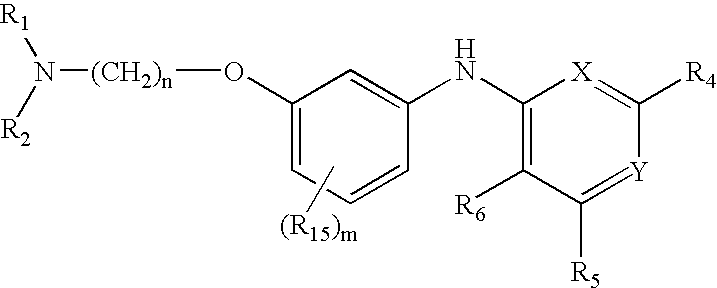
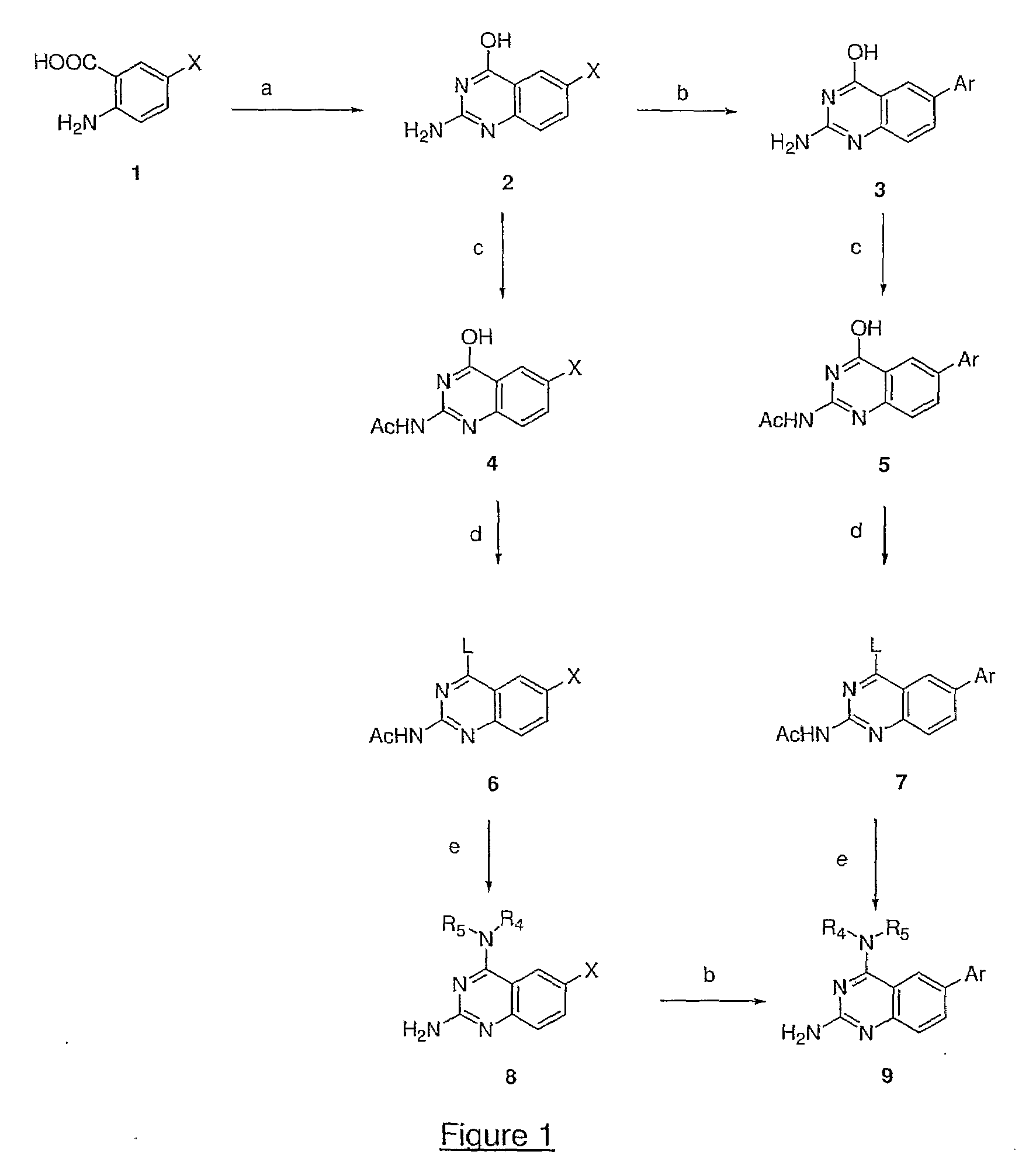
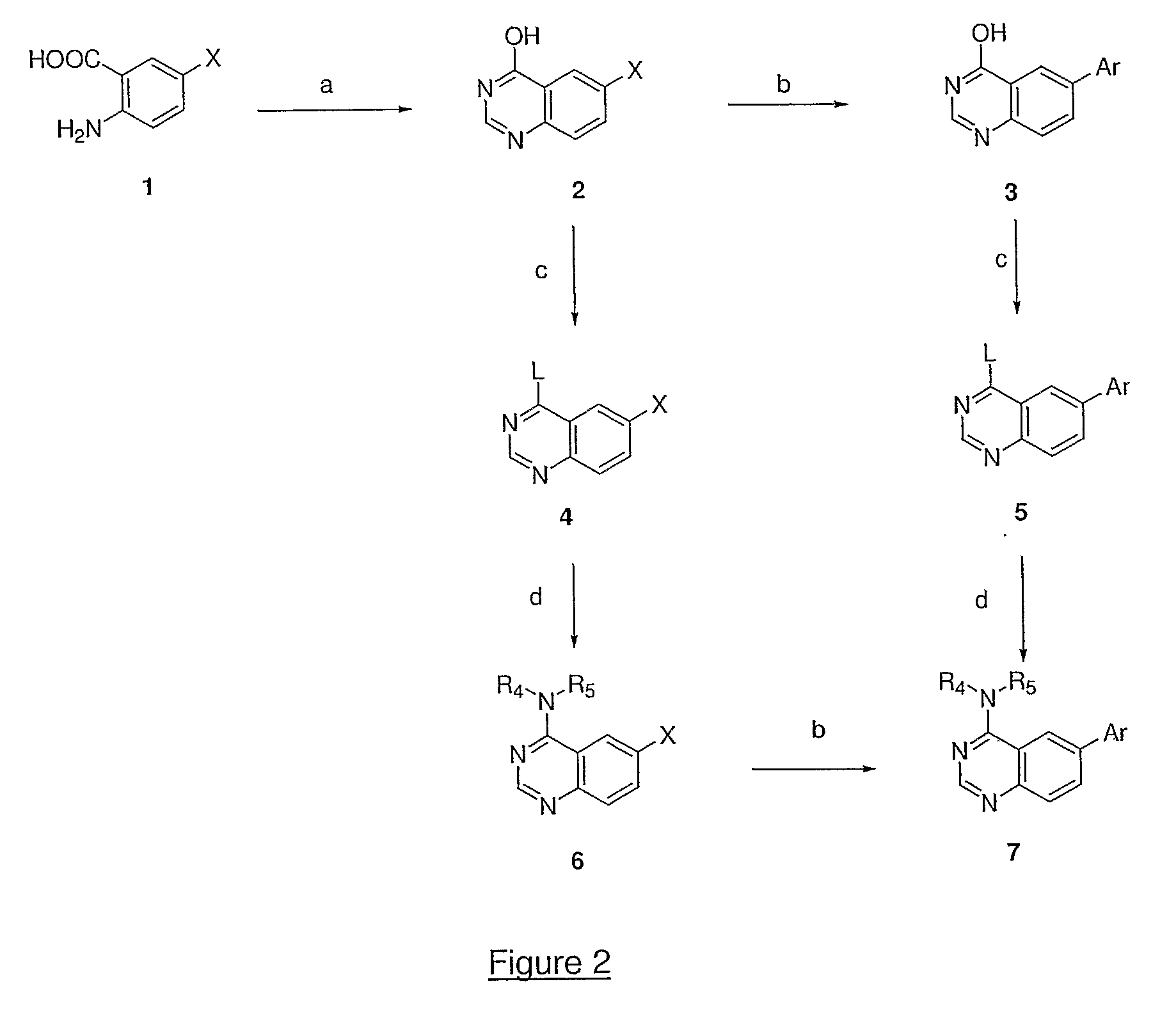
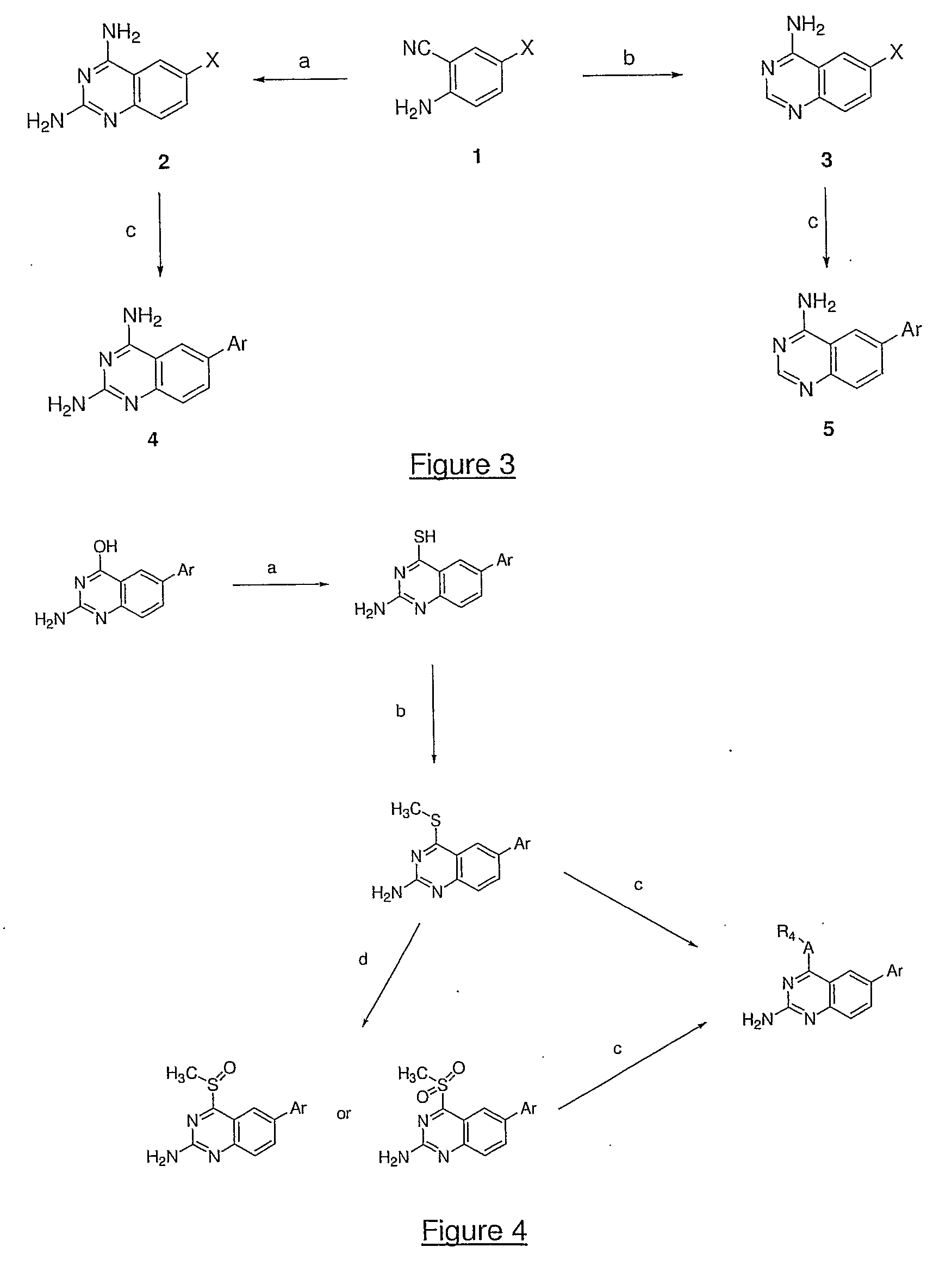
Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7
Owner:BRISTOL MYERS SQUIBB CO
4,6-di- and 2,4,6-trisubstituted quinazoline derivatives useful for treating viral infections
This invention provides quinazoline derivatives represented by the structural formula: (I); wherein: R2 is hydrogen, NR′R″, C1-7 alkyl, arylC1-7 alkyl or C3-10 cycloalkyl; R4 is amino, C1-7 alkyl, C2-7 alkenyl, C3-10 cycloalkyl, C3-10 cycloalkenyl, aryl, heterocyclic, arylalkyl, heterocyclic-substituted C1-7 alkyl or C3-10 cycloalkyl-C1-7 alkyl; R5 is hydrogen or C1-7 alkyl, or R5 and R4 together with the nitrogen atom to which they are attached form a heterocyclic ring; Y is a single bond, C1-7alkylene, C2-7 alkenylene or C2-7 alkynylene; R6 is halogen, heteroaryl or aryl; R′ and R″ are each independently hydrogen, C1-7 alkyl-carbonyl or C1-7 alkyl; provided that R4 is not phenyl substituted with morpholino when R2 is H and R5 is H, and provided that when NR4R5 is piperazinyl, said NR4R5 is either non-substituted or substituted with methyl or acetyl; a pharmaceutically acceptable addition salt, a stereoisomer, a mono- or a di-N-oxide, a solvate or a pro-drug thereof, for the treatment of viral infections.
Owner:GILEAD SCI INC
Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7
Quinazoline and pyrido[2,3-d]pyrimidine phosphodiesterase 7 (PDE 7) inhibitors of the following formula wherein R1, R2, L, Y1, Y2, Y3 and Z are as described herein, are provided which are useful in treating T-cell mediated diseases.
Owner:BRISTOL MYERS SQUIBB CO
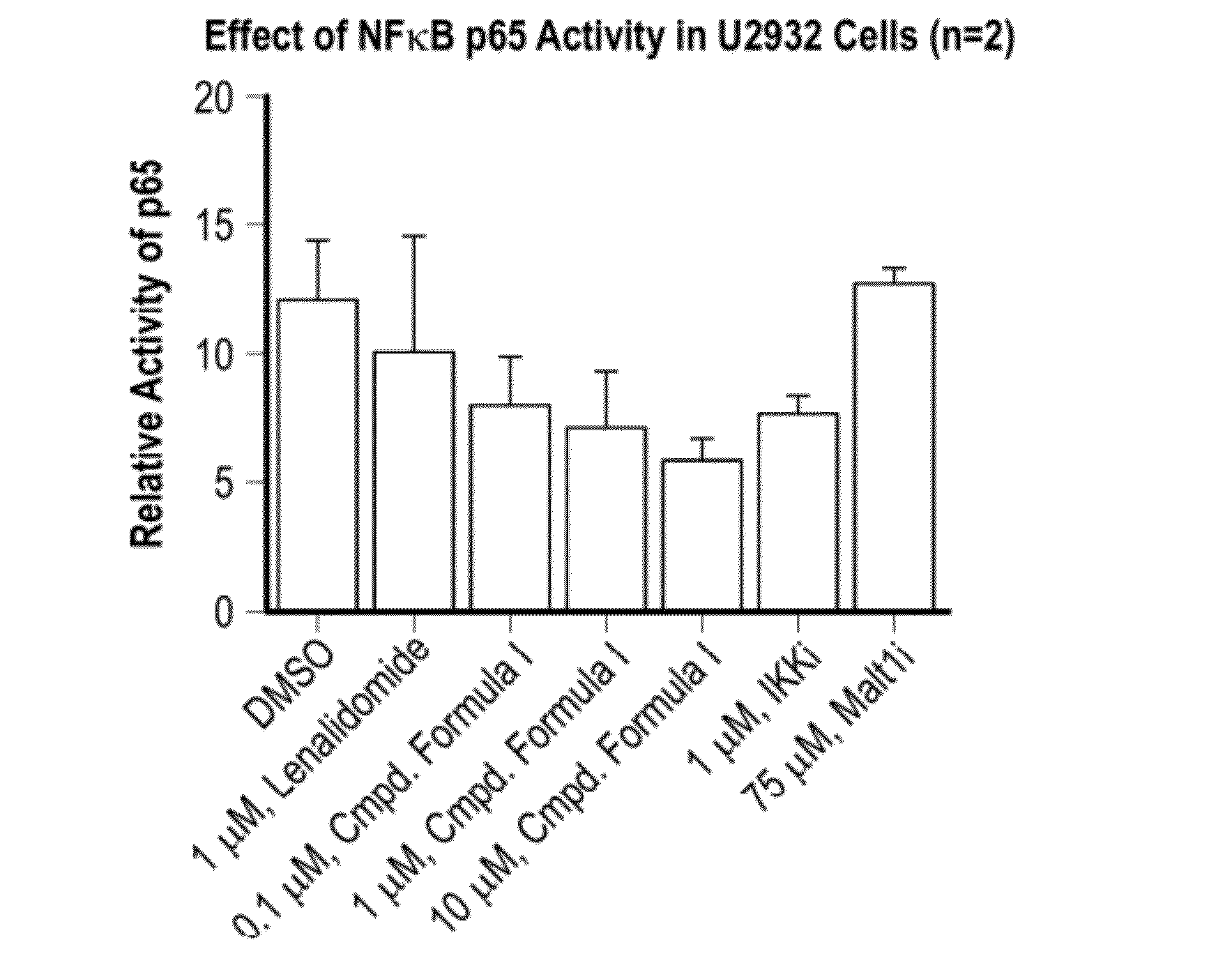
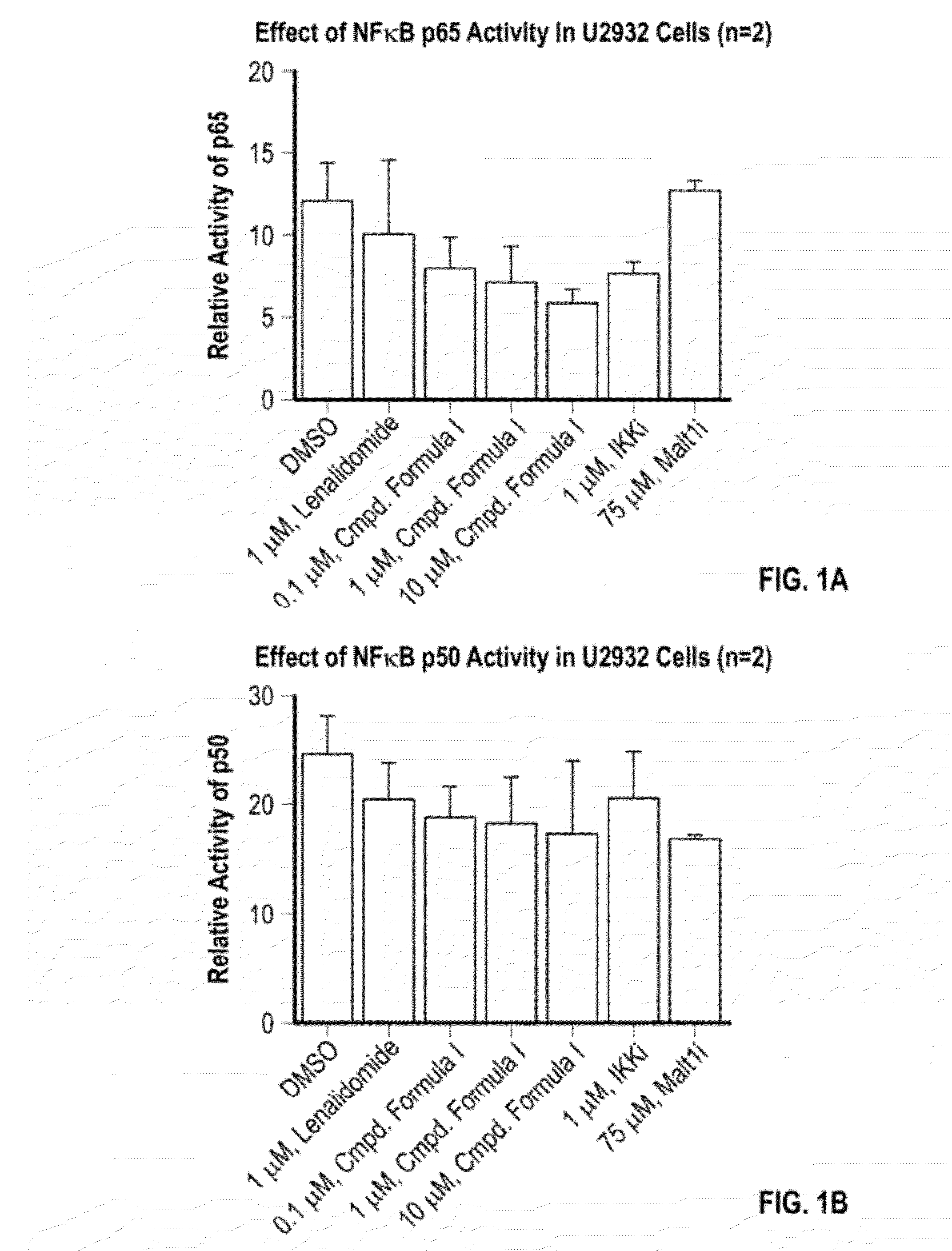
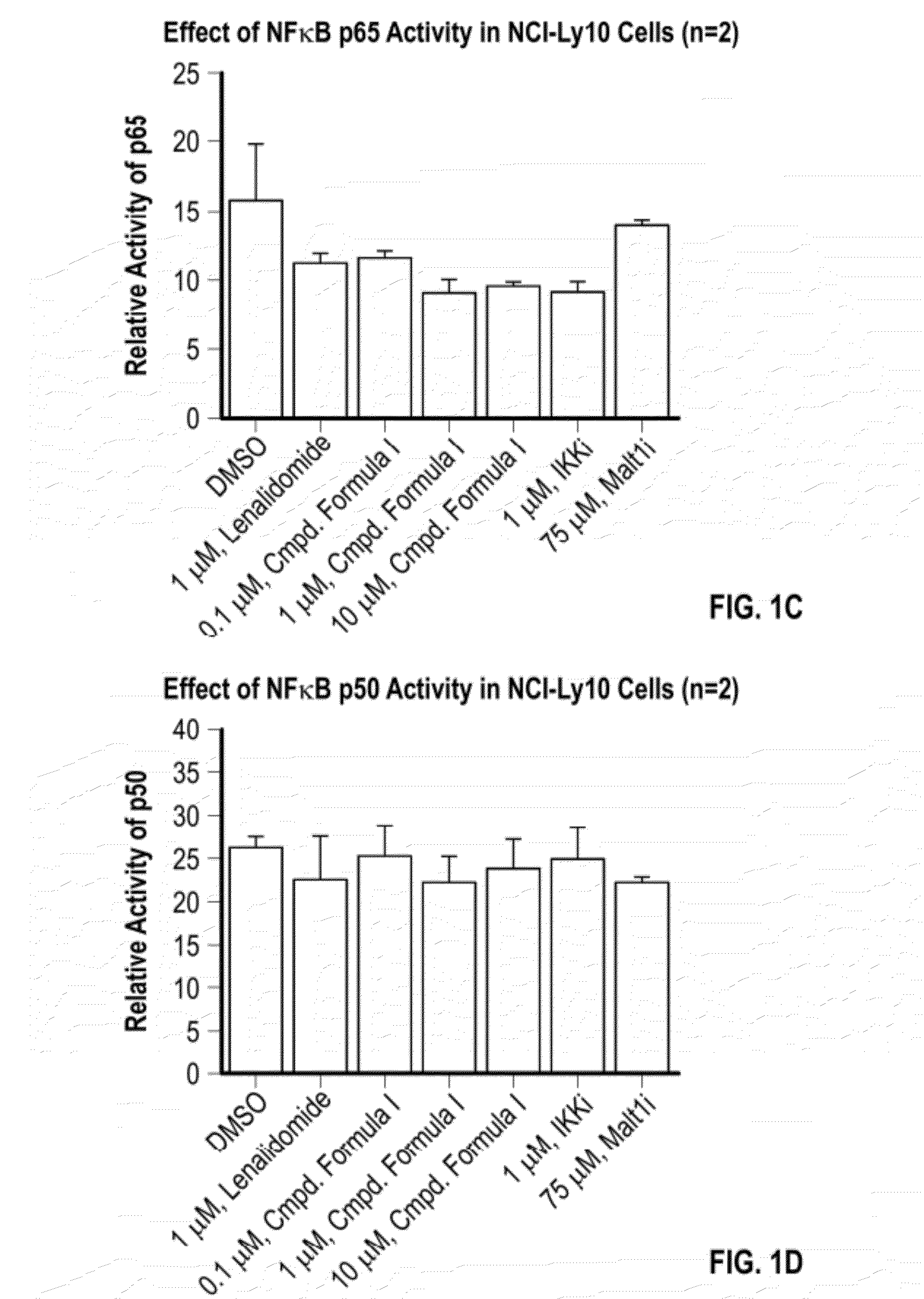
Methods of treating cancer using 3-(5-amino-2-methyl-4-oxo-4h-quinazolin-3-yl)-piperidine-2,6-dione
Owner:CELGENE CORP
4,6-di- and 2,4,6-trisubstituted quinazoline derivatives and pharmaceutical compositions useful for treating viral infections
This invention provides the treatment of viral infections with a 4,6-disubstituted or 2,4,6-trisubstituted quinazoline derivative represented by the structural formula [(I)] wherein: R2 is selected from the group consisting of hydrogen, NR′R″ and C1-7 alkyl; —A is selected from the group consisting of a bond, O, S(O)n, C1-7 alkylene, C2-7 alkenylene and C2-7 alkynylene; R4 is selected from the group consisting of C1-7 alkyl, C2-7 alkenyl, C3-10cycloalkyl, C3-10 cycloalkenyl, aryl, heterocyclic, arylalkyl, heterocyclic-substituted alkyl and cycloalkyl-alkyl; —Y is selected from the group consisting of a single bond, C1-7 alkylene, C2-7 alkenylene, and C2-7 alkynylene; n is 0, 1 or 2; and R6 is selected from the group consisting of halogen, heteroaryl and aryl; a pharmaceutically acceptable addition salt, a stereoisomer, a mono- or a di-Λ / -oxide, a solvate or a pro-drug thereof.
Owner:GILEAD SCI INC
Compounds and therapeutical use thereof
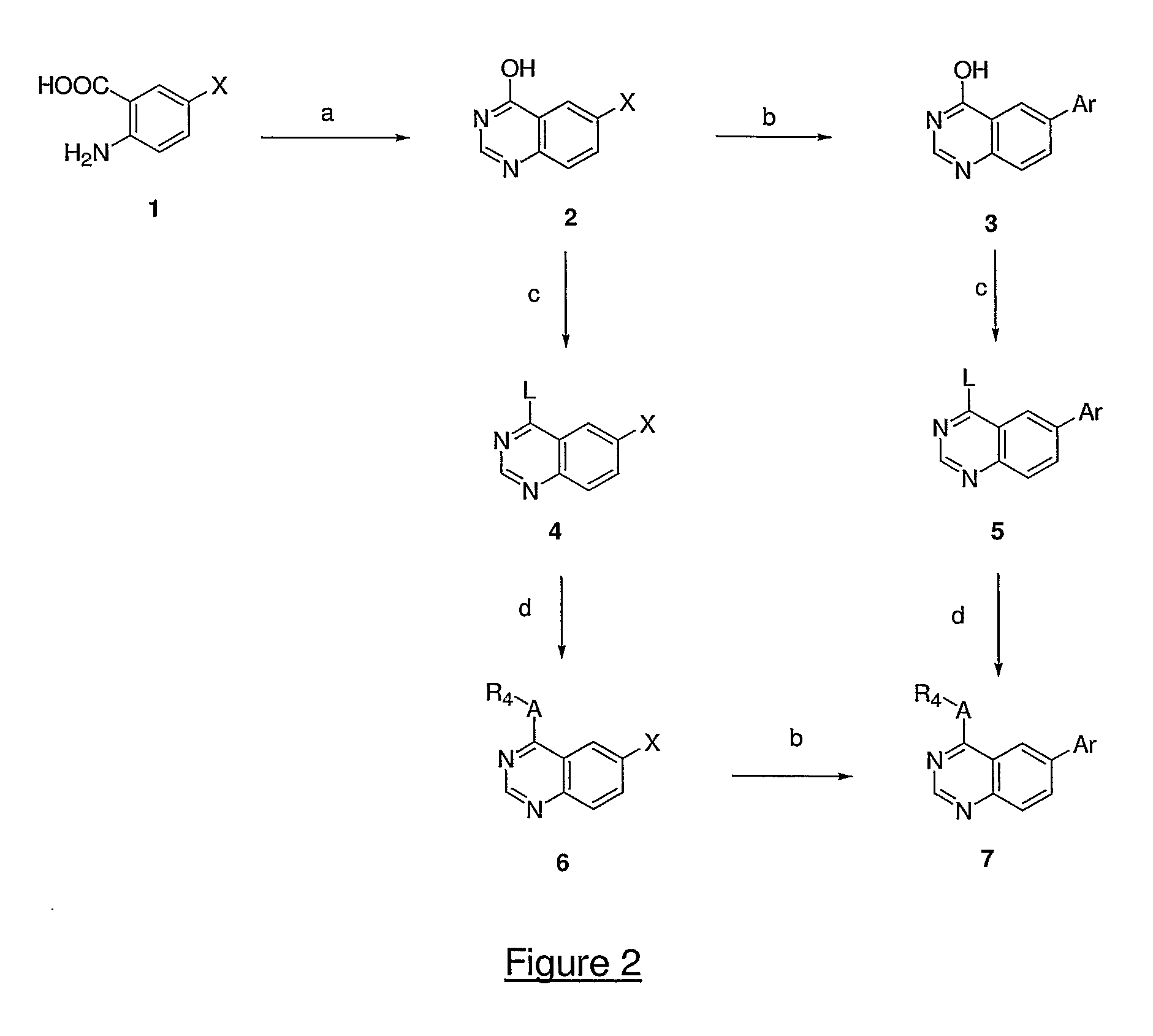
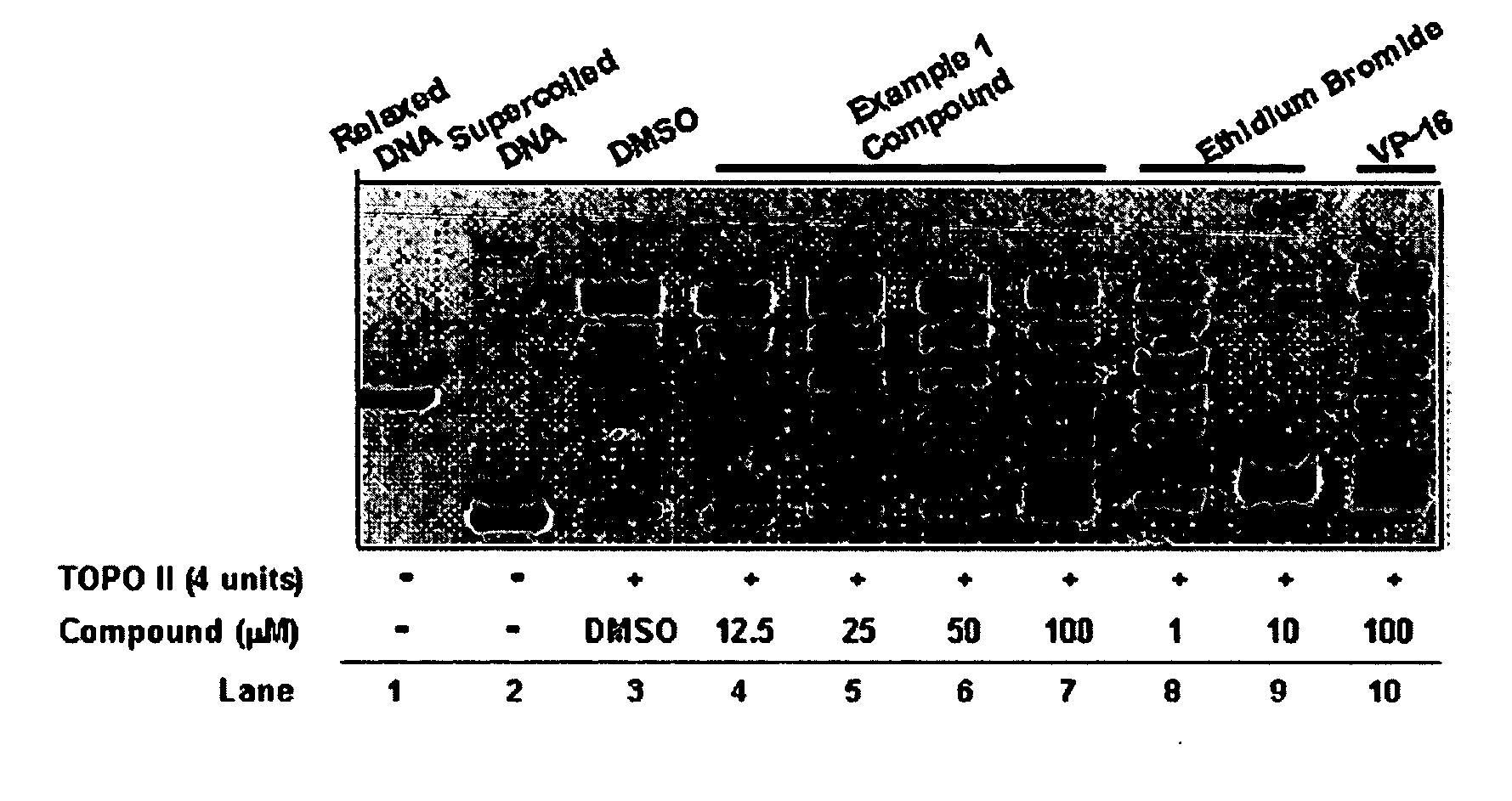
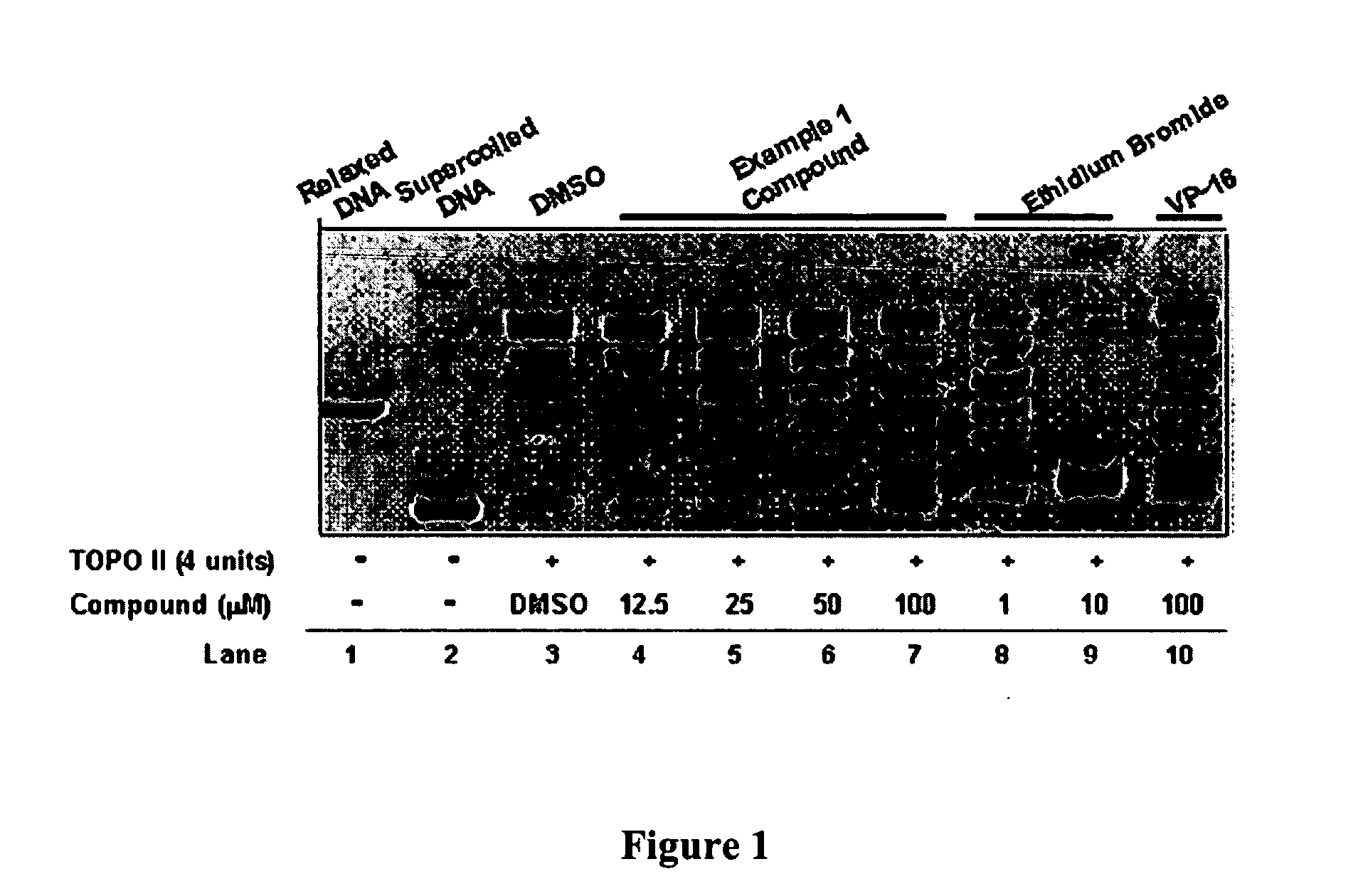
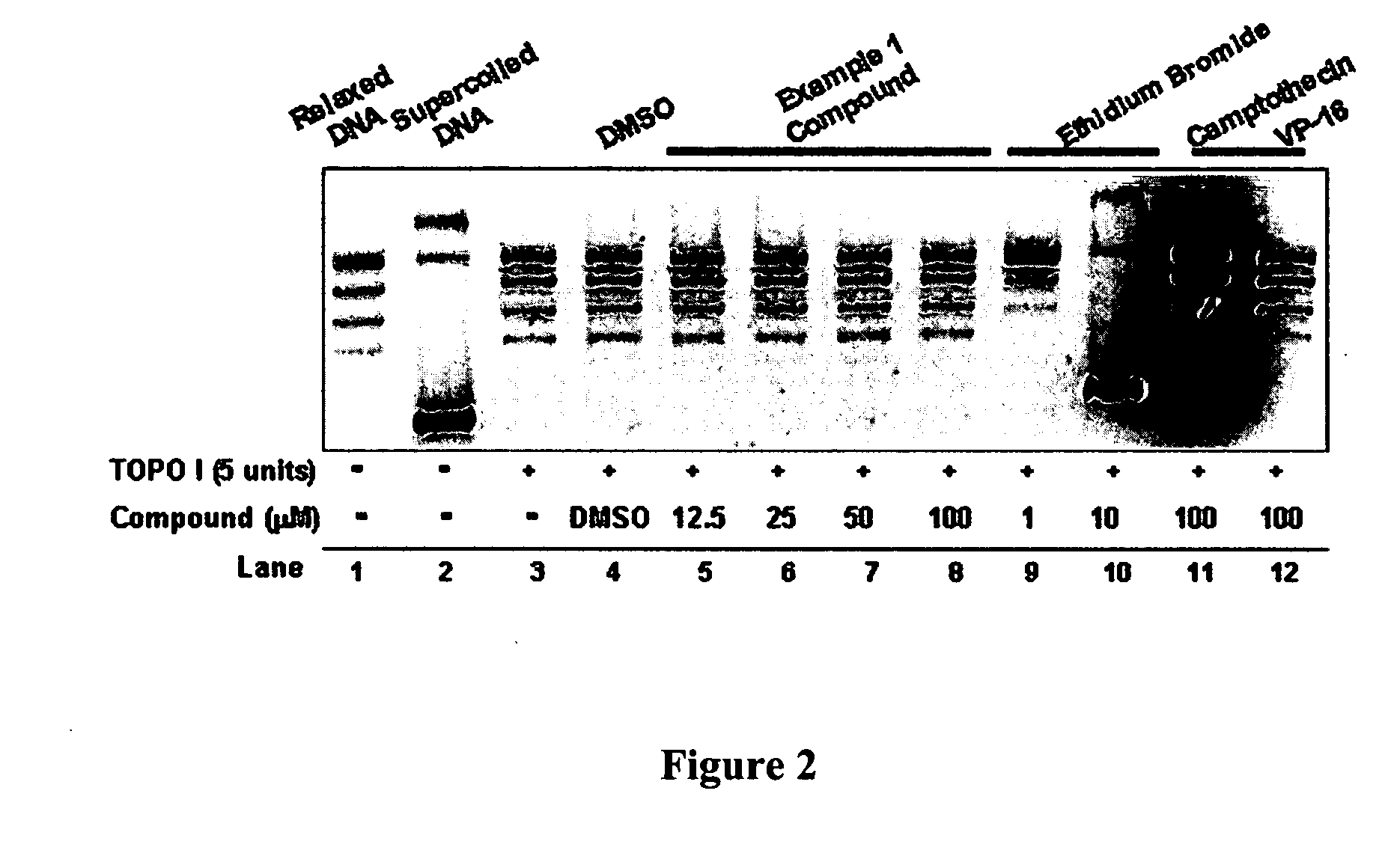
InactiveUS20050137213A1Inhibiting topoisomeraseBiocideOrganic active ingredientsApoptosisAbnormal cell
Disclosed are 4-arylamino-quinazolines and analogs thereof effective as activators of caspases and inducers of apoptosis. The compounds of this invention are useful in the treatment of a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
Owner:CYTOVIA INC +1
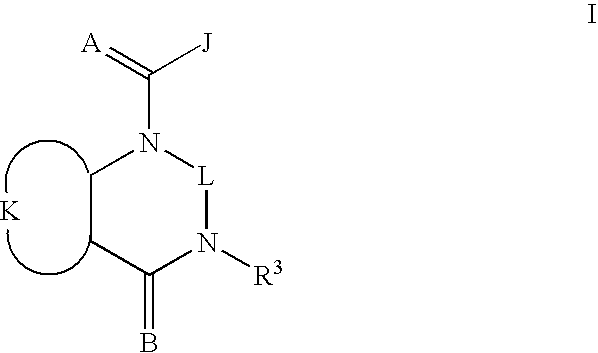
Quinazoline(di)ones for invertebrate pest control
Compounds of Formula I including all geometric and stereoisomers, N-oxides, and agriculturally suitable salts thereof, are disclosed which are useful as invertebrate pest control agents, (I) wherein A, B, J, K, L and R3 are as defined in the disclosure. Also disclosed are compositions for controlling an invertebrate pest comprising a biologically effective amount of a compound of Formula I an N-oxide thereof or a suitable salt thereof and at least one additional component selected from the group consisting of surfactants, solid diluents and liquid diluents. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a compound of Formula I, its N-oxide or a suitable salt of the compound (e.g., as a composition described herein).
Owner:EI DU PONT DE NEMOURS & CO
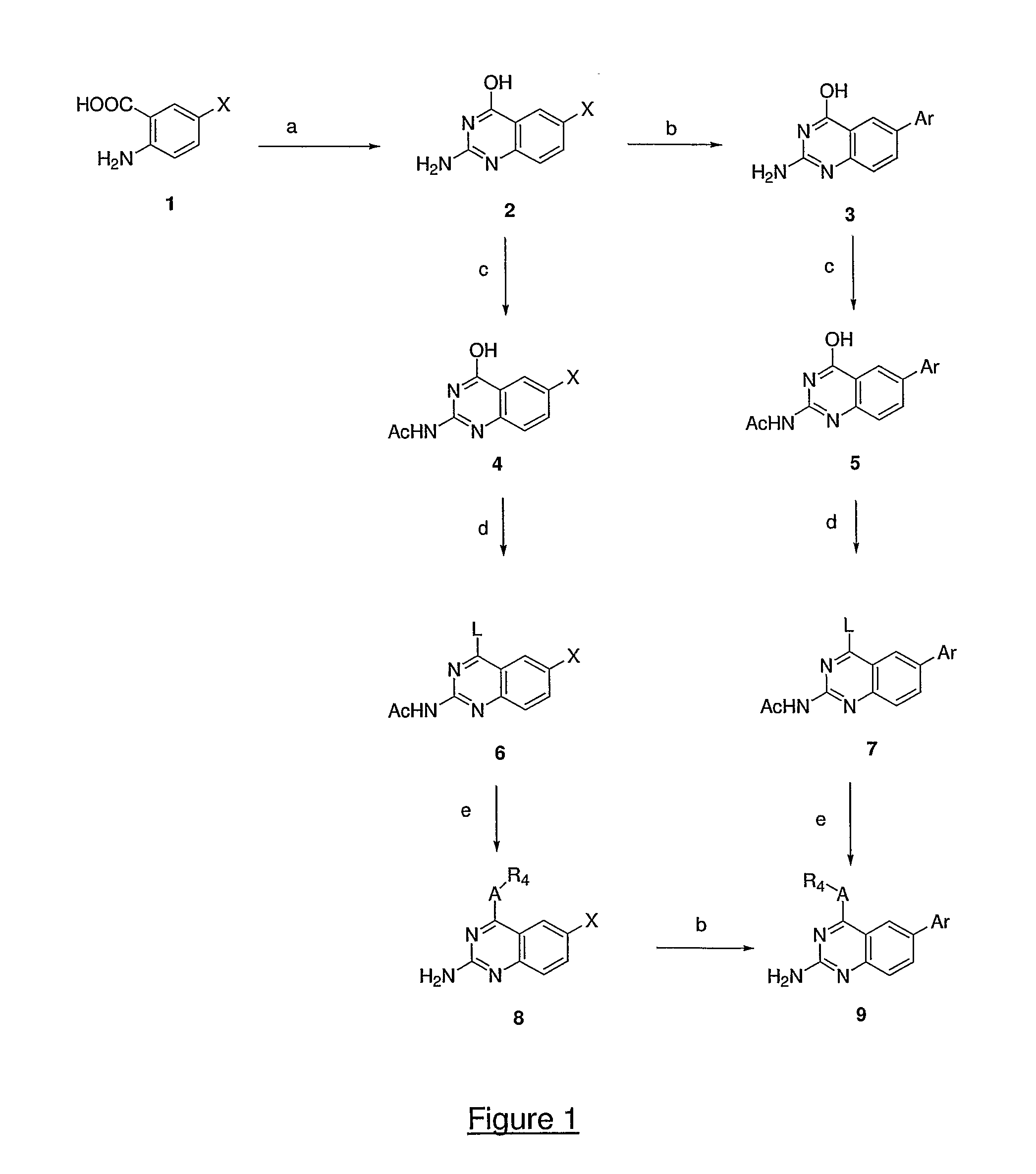
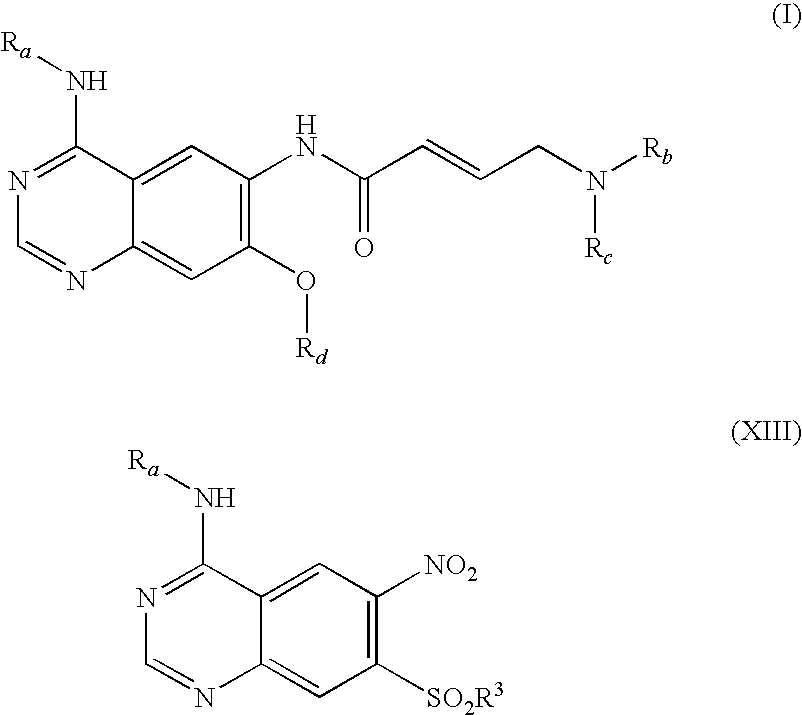
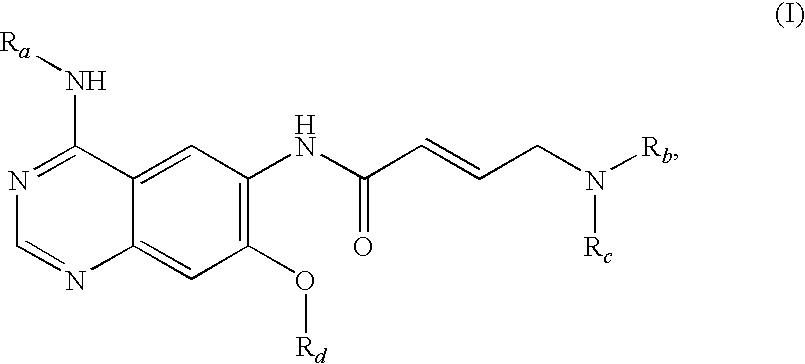
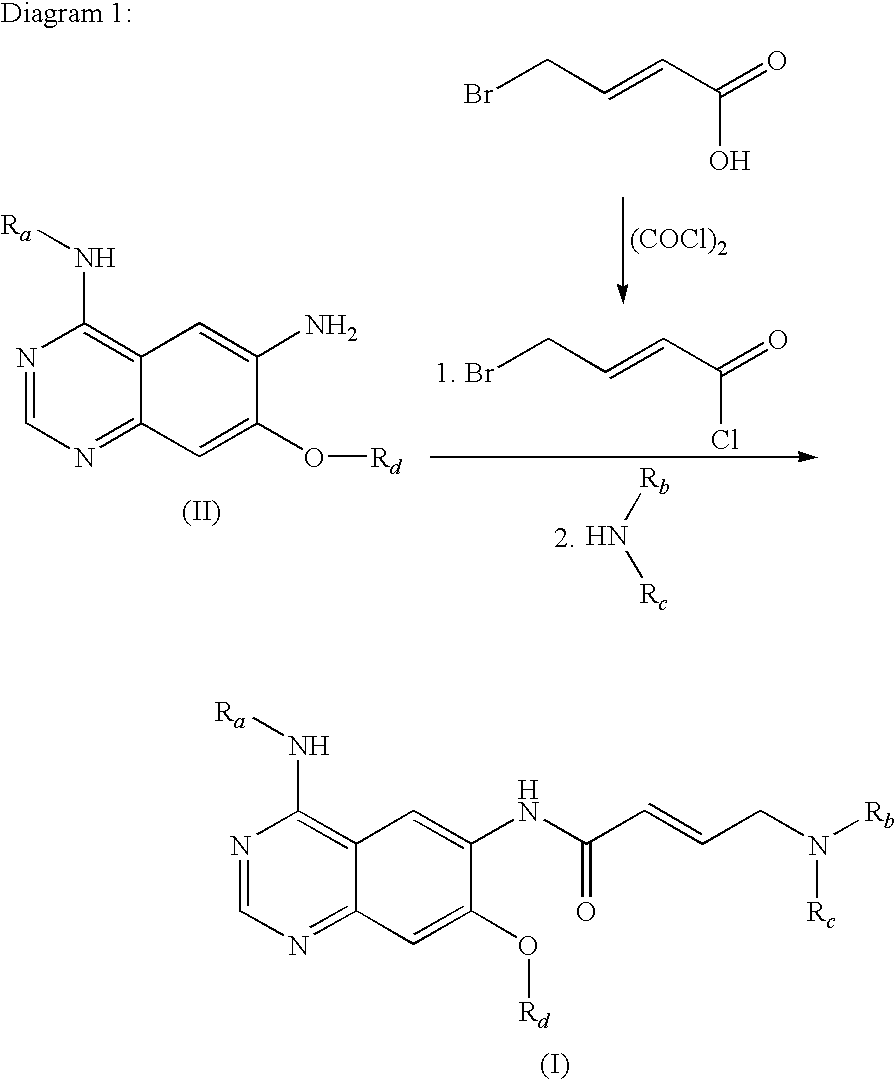
Process for preparing aminocrotonylamino-substituted quinazoline derivatives
The invention relates to an improved process for preparing aminocrotonylamino-substituted quinazoline derivatives of general formula (I) wherein the groups Ra, Rb, Rc and Rd have the meanings given in the claims, as well as sulphonyl derivatives of formula (XIII) and the use thereof as synthesis components for preparing quinazolines of formula (I). The quinazoline derivatives of formula (I) are inhibitors of signal transduction mediated by tyrosinekinases and by the Epidermal Growth Factor-Receptor (EGF-R) and are therefore particularly suitable for the treatment of tumoral diseases.
Owner:BOEHRINGER INGELHEIM INT GMBH
Isoquinolinone Rho kinase inhibitors
Owner:AERIE PHARMA
Red organic electroluminescent phosphorescent material containing aryl united quinazoline metal iridium complexes and organic electroluminescent device thereof
InactiveCN101899296AHigh color purityImprove efficiencyGroup 8/9/10/18 element organic compoundsSolid-state devicesDopantIridium
The invention discloses a red organic electroluminescent phosphorescent material containing aryl united quinazoline metal iridium complexes and an organic electroluminescent device thereof. The organic electroluminescent device is in a layered doped structure, wherein Zn(BTZ)2 is selected as a substrate material of a luminescent layer, and serial complexes Ir(III) are selected as dopants. The invention provides a basic skeleton structure, i.e. a red organic electroluminescent phosphorescent material containing the aryl united quinazoline metal iridium complexes, modifies the structure with various different groups, is combined with the structural optimization of the organic electroluminescent device using the groups as dopants and realizes red phosphorescence emission with better purity and higher efficiency as comparison with a traditional red light material. The invention has simple manufacture process and provides excellent material for full color display and illumination application.
Owner:XIAN RUILIAN NEW MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




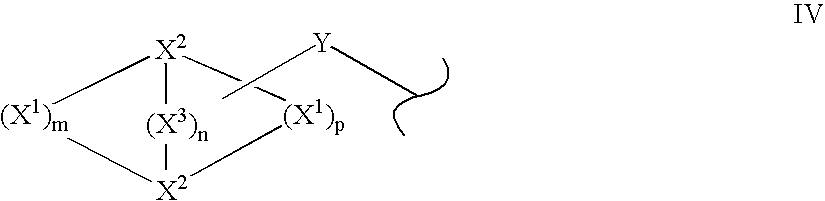







![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00001.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00002.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4ed49fc3-cda5-4b77-abdb-a8271bf83186/US20060116516A1-20060601-C00003.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00001.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00002.png)
![Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7 Quinazoline and pyrido[2,3-d]pyrimidine inhibitors of phosphodiesterase (PDE) 7](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/dae0fdd8-4850-4e47-9c9e-a14298d41e43/US07022849-20060404-C00003.png)