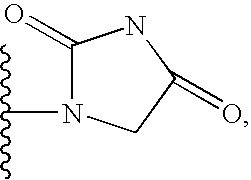
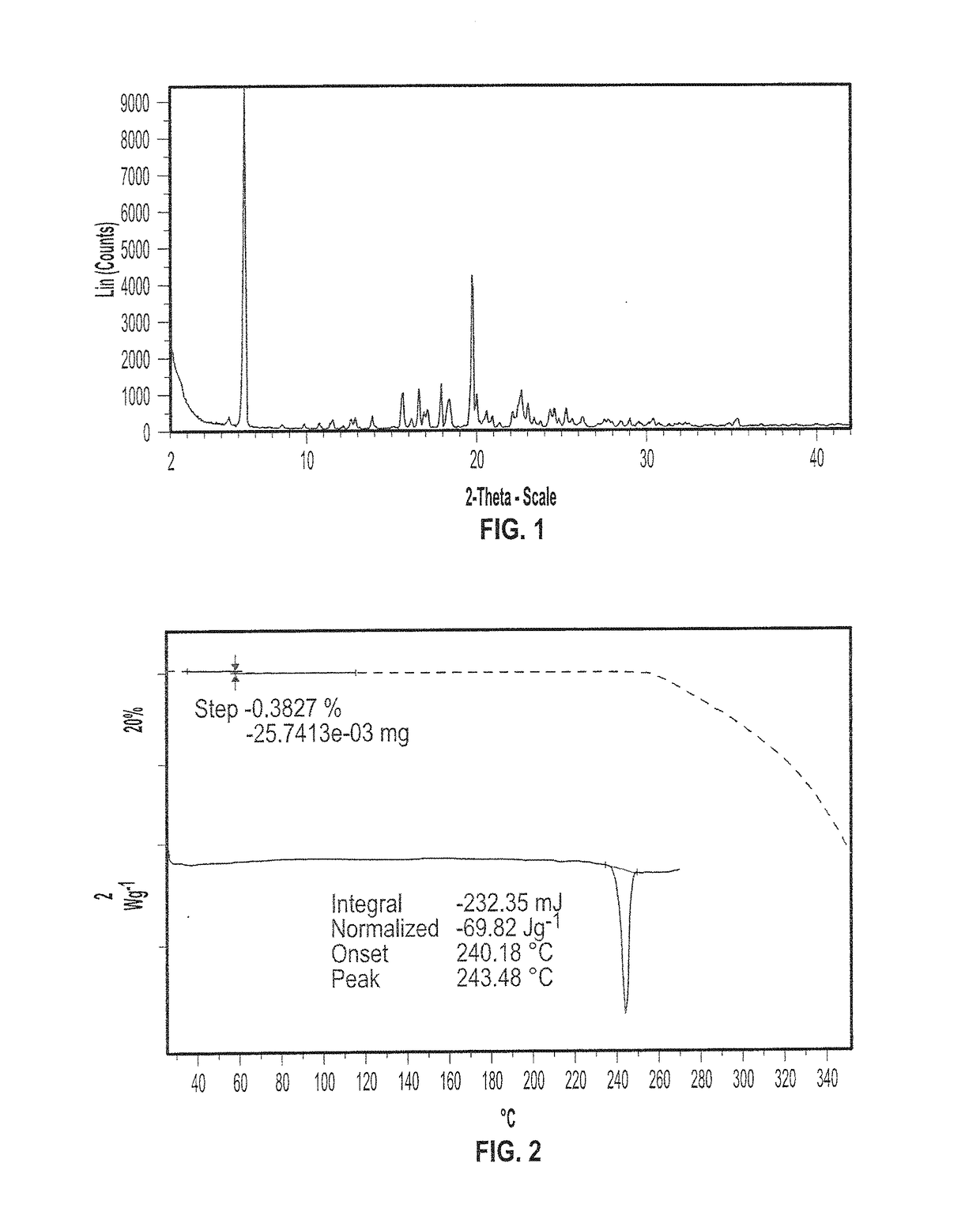
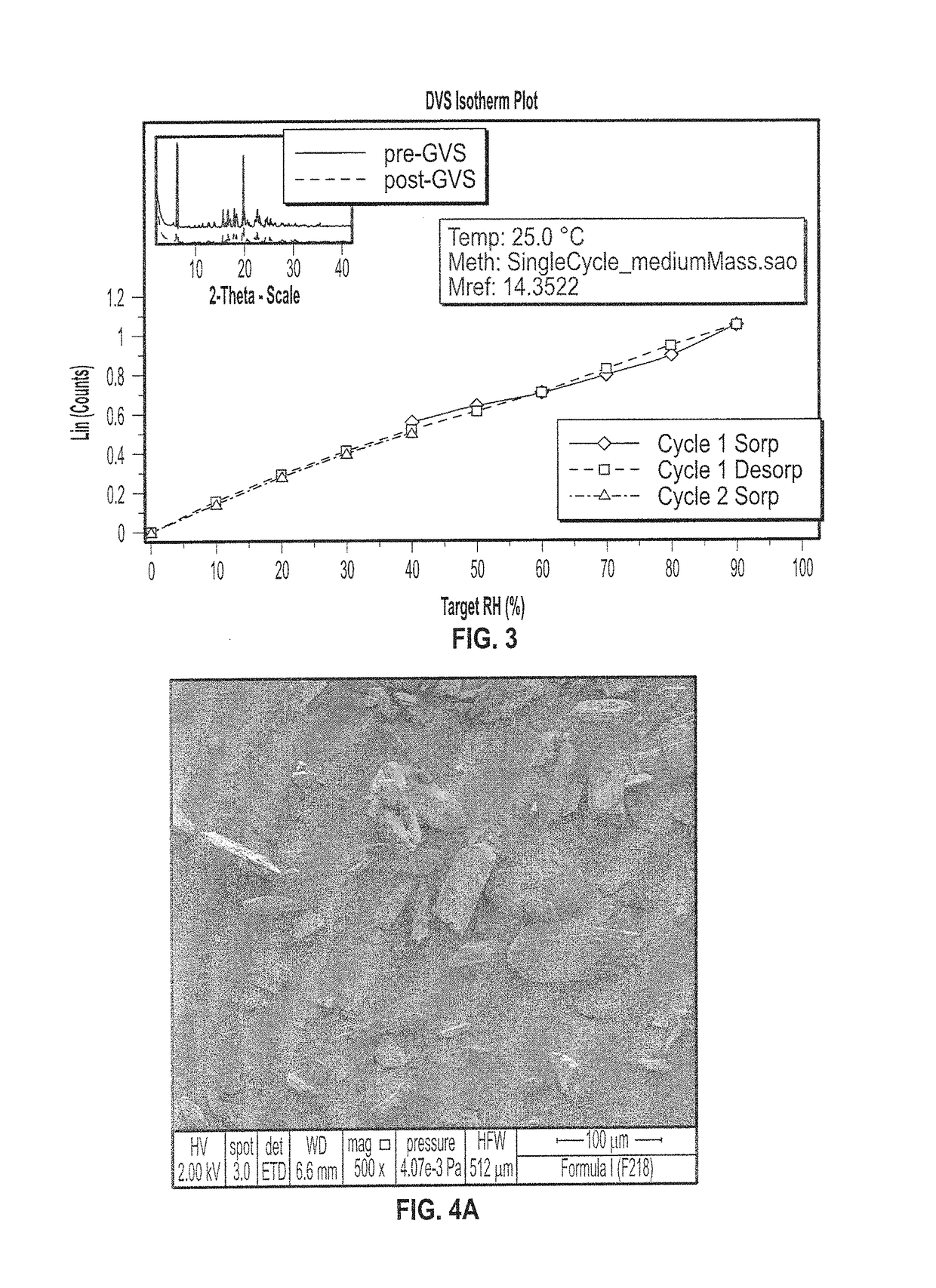
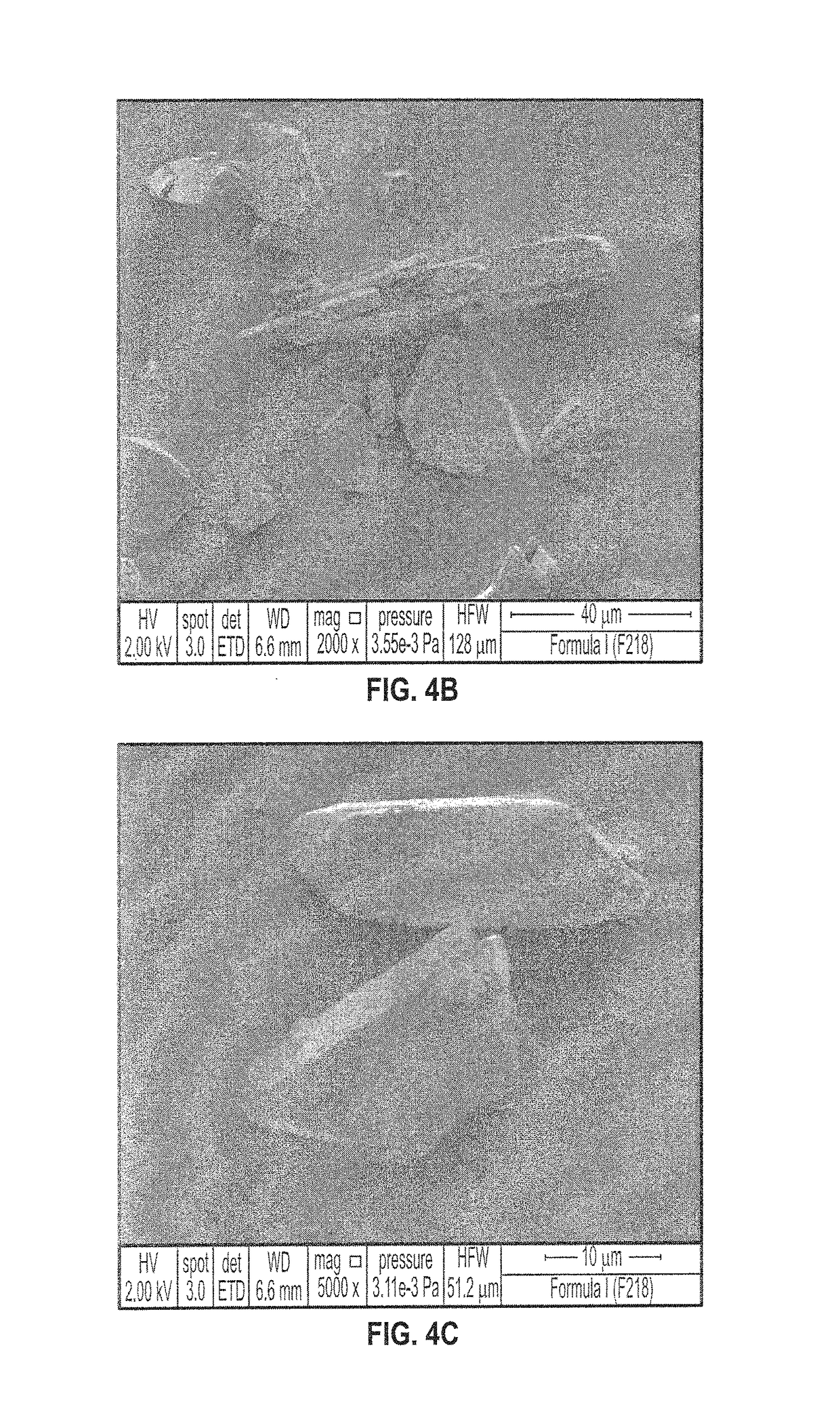
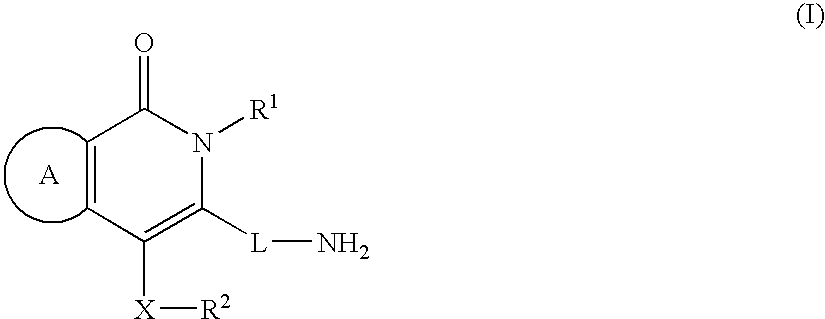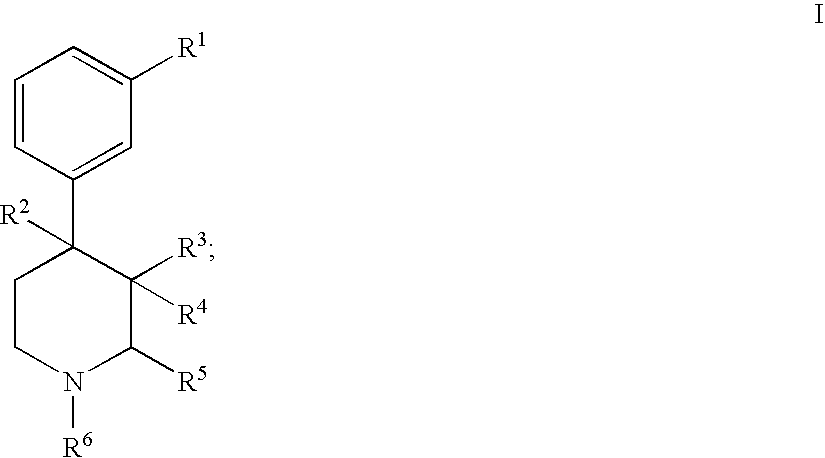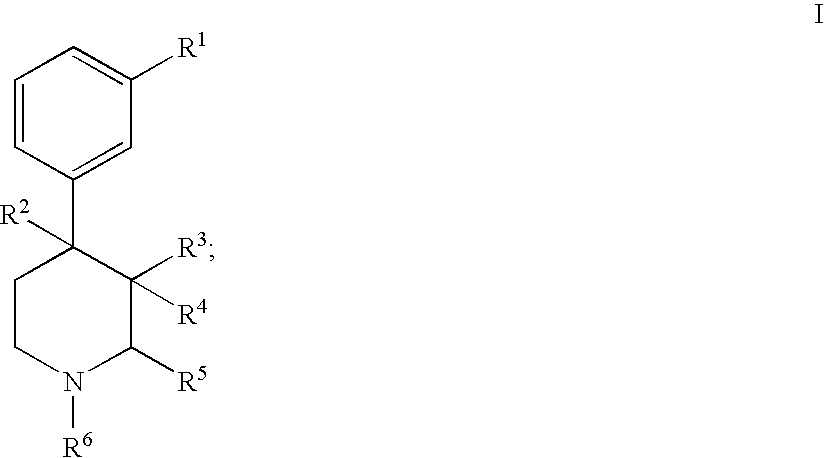Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1496 results about "Isoquinoline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
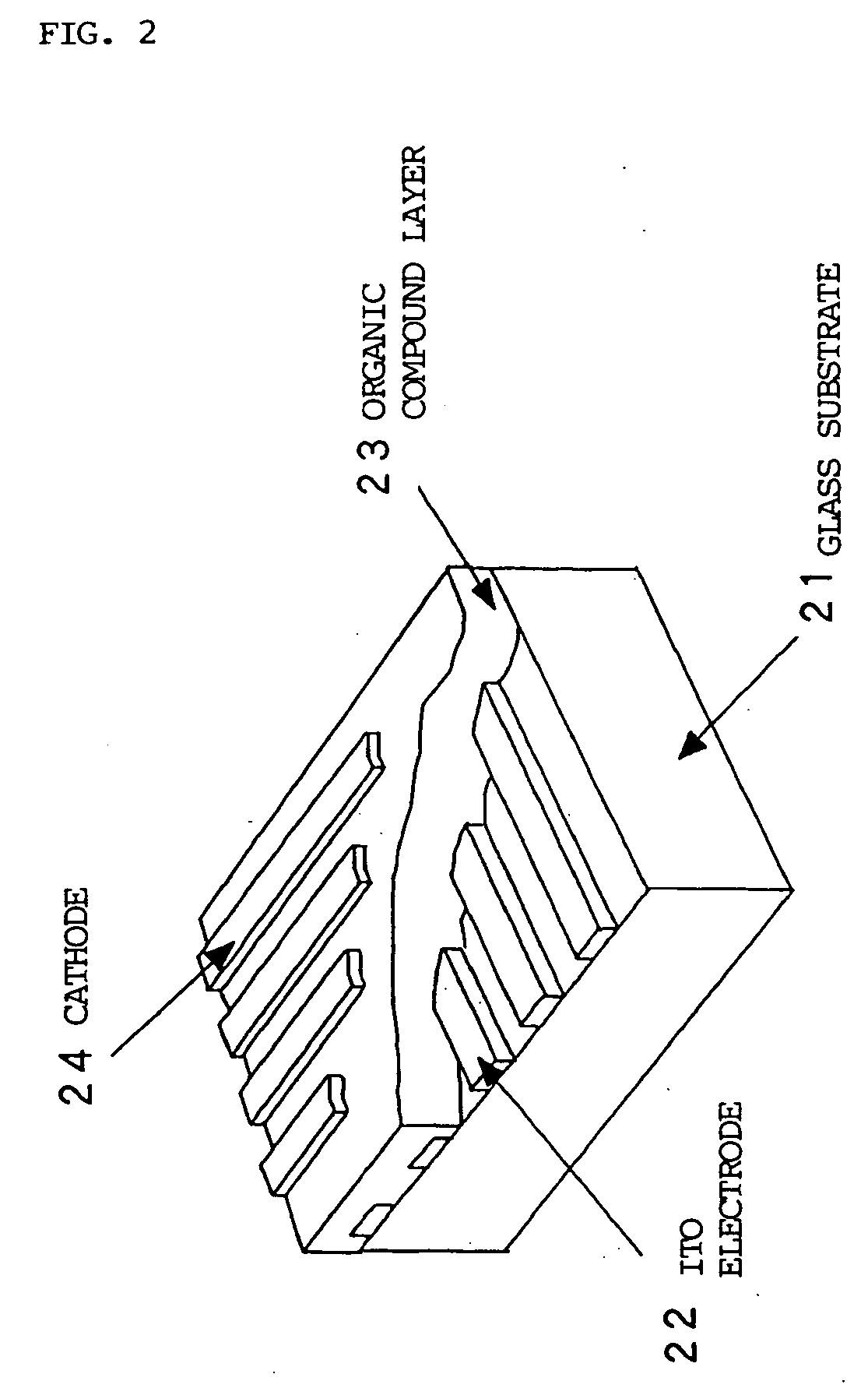
Organic electroluminescent devices
InactiveUS20050123791A1Useful white light emissionSolid-state devicesSemiconductor/solid-state device manufacturingIridiumIsoquinoline
Disclosed is an electroluminescent device comprising a cathode and anode, and therebetween, at least two light-emitting layers wherein the first layer, layer A, comprises a phosphorescent light-emitting organometallic compound comprising iridium and an isoquinoline group and a second layer, layer B, comprising a light-emitting material. Such devices provide useful white light emissions.
Owner:EASTMAN KODAK CO
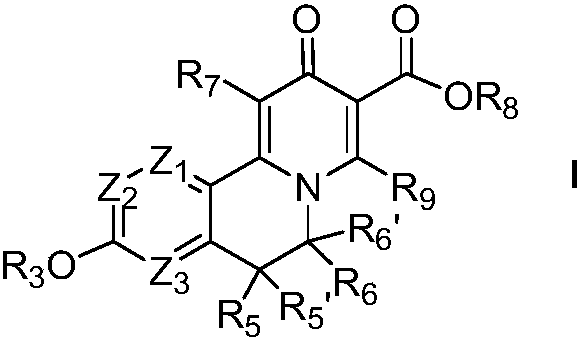
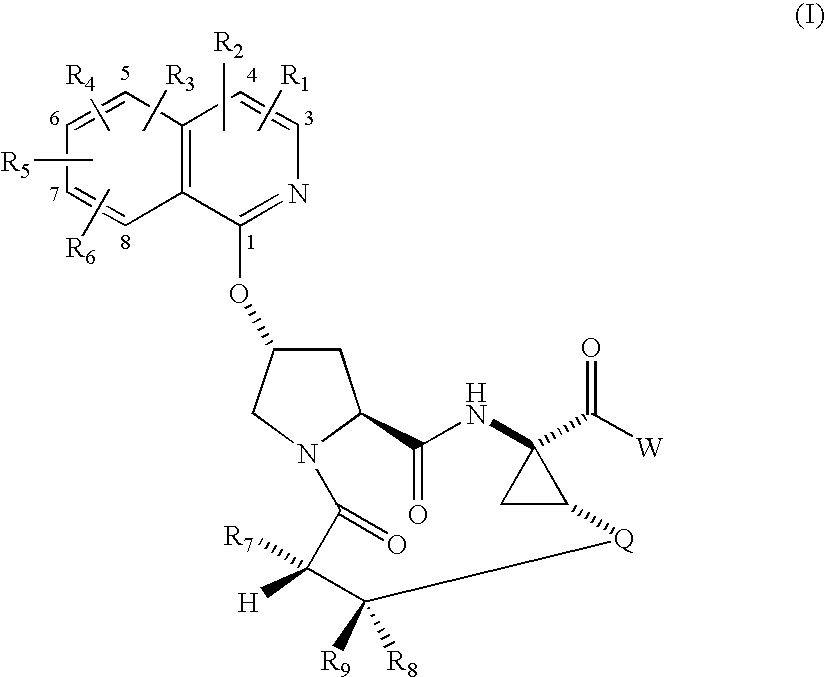
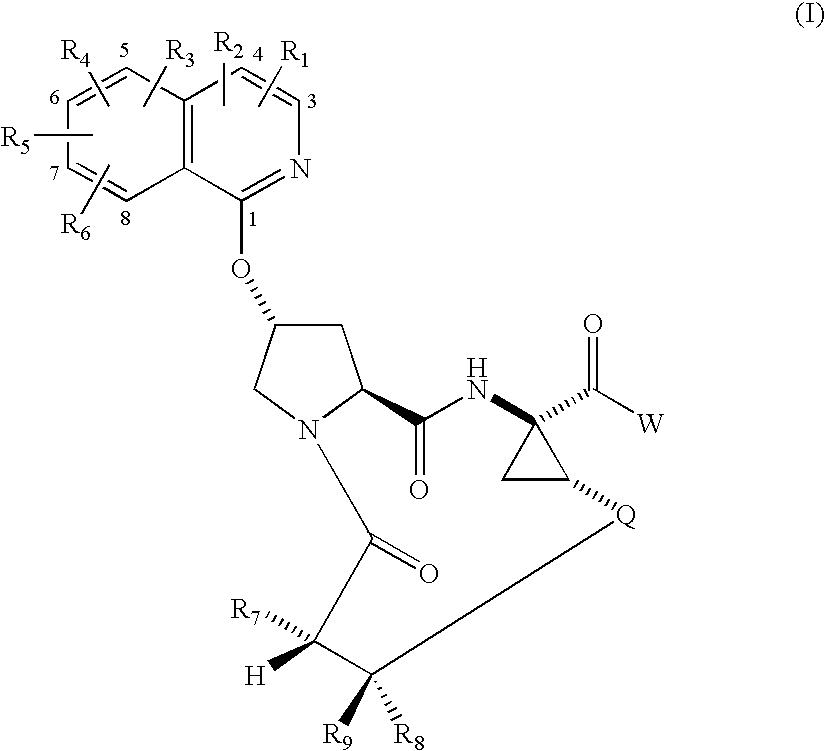
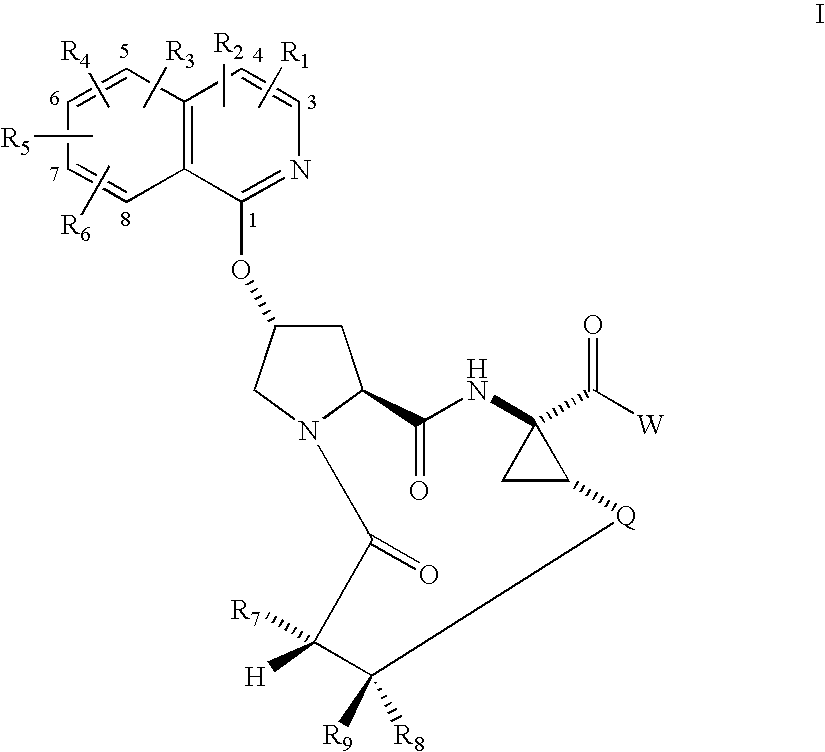
Macrocyclic isoquinoline peptide inhibitors of hepatitis C virus
InactiveUS20060257980A1Inhibit functioningEffective treatmentDigestive systemAntiviralsIsoquinolineCombinatorial chemistry
Macrocyclic isoquinoline peptides are disclosed having the general formula: A compound of formula I: wherein R1 to R9, Q and W are described in the description. Compositions comprising the compounds and methods for using the compounds to inhibit HCV are also disclosed.
Owner:BRISTOL MYERS SQUIBB CO
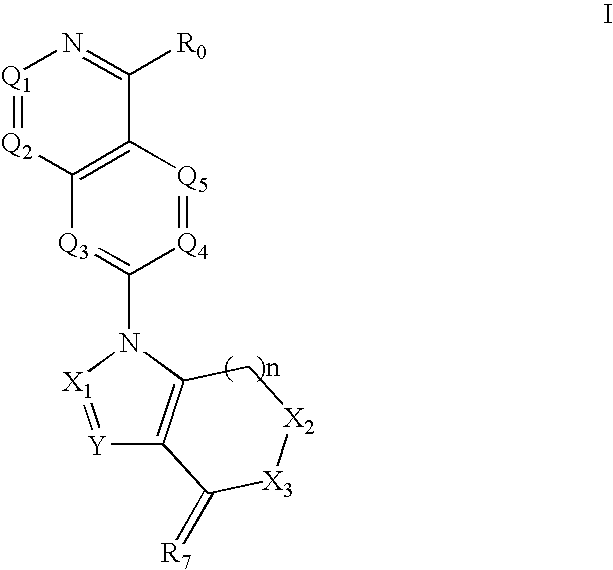
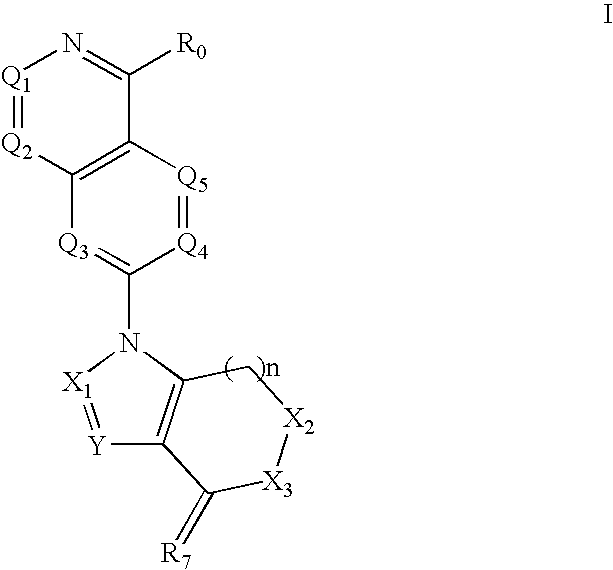
Isoquinoline, Quinazoline and Phthalazine Derivatives
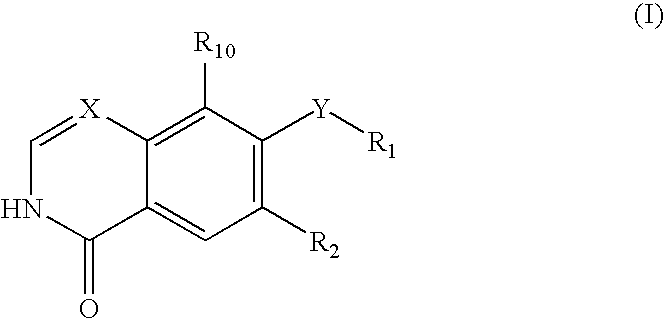
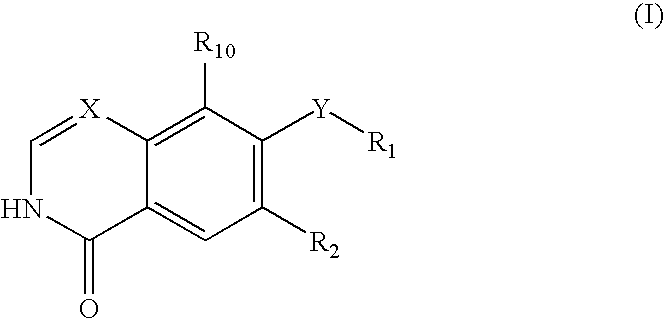
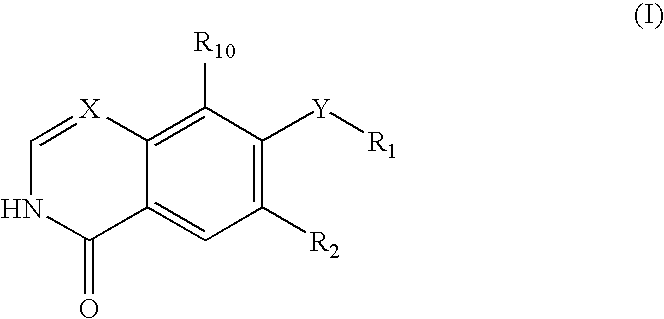
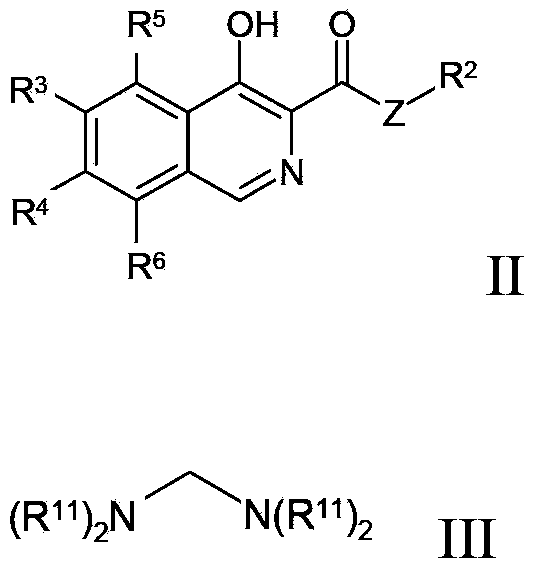
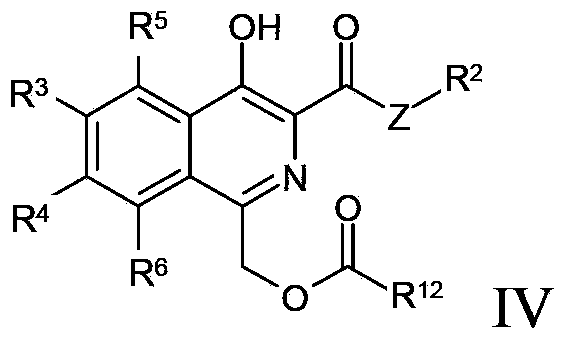
Disclosed are compounds and pharmaceutically acceptable salts of Formula I wherein R0, R5, R6, R7, n, Q1-Q5, Y, and X1-X3 are as defined herein. Compounds of Formula I are useful in the treatment of diseases and / or conditions related to cell proliferation, such as cancer, inflammation, arthritis, angiogenesis, or the like. Also disclosed are pharmaceutical compositions comprising compounds of the invention and methods of treating the aforementioned conditions using such compounds.
Owner:ESANEX
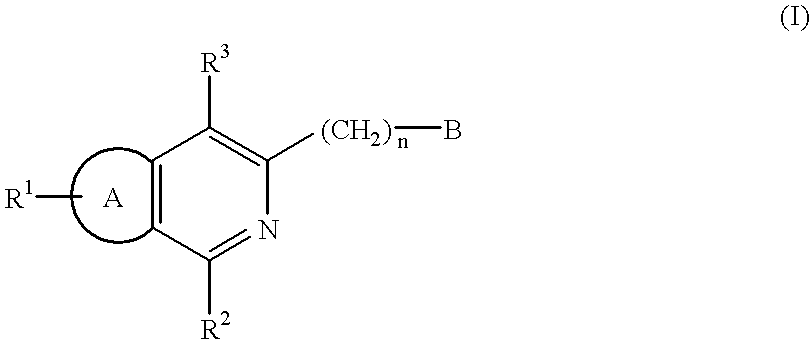
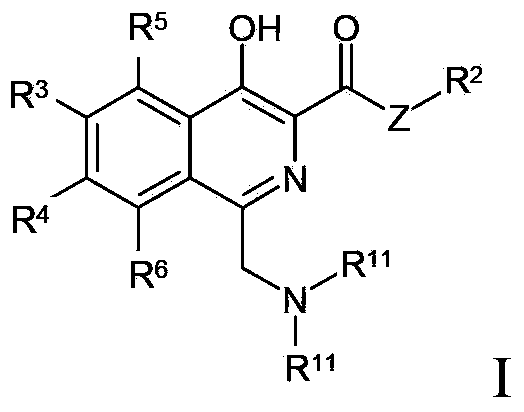
Certain 1,3-disubstituted isoquinoline derivatives
Condensed pyridine compounds represented by formula (I):wherein: R<1 >and R<3 >are, independently, hydrogen, halogen, lower alkyl, or lower alkoxy; R<2 >represents an amino substituent; ring A is a benzene ring, pyridine ring, thiophene ring, or furan ring; and B represents a substituent containing a ring structure. Also, pharmaceutically acceptable salt and hydrates thereof. These compounds are clinically useful medicaments having; serotonin antagonism, and in particular, for treating, ameliorating, or preventing spastic paralysis. They are also useful as central muscle relaxants for ameliorating myotonia.
Owner:EISIA R&D MANAGEMENT CO LTD
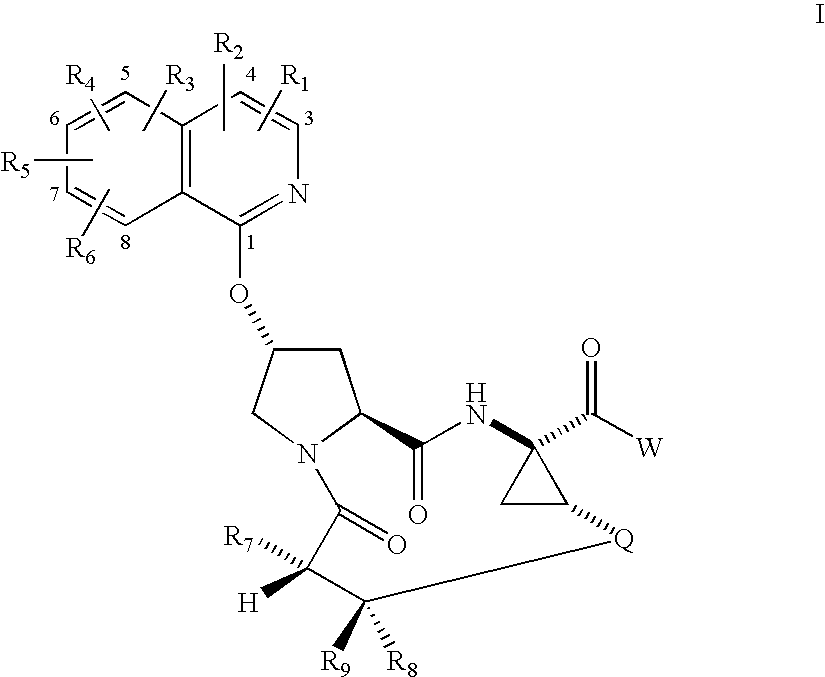
Macrocyclic isoquinoline peptide inhibitors of Hepatitis C virus
ActiveUS20050090432A1Inhibit functioningEffective treatmentBiocideAntiviralsIsoquinolineCombinatorial chemistry
Macrocyclic isoquinoline peptides are disclosed having the general formula: A compound of formula I: [0001]wherein R1 to R9, Q and W are described in the description. Compositions comprising the compounds and methods for using the compounds to inhibit HCV are also disclosed.
Owner:BRISTOL MYERS SQUIBB CO
Method for preparing isoquinoline compounds
The invention relates to a method for preparing isoquinoline compounds and intermediate compounds obtained thereby. Such compounds can be used for preparing compounds and compositions which can reduce the activity of HIF hydroxylase enzyme, thereby improving the stability and / or activity of hypoxia inducible factors (HIF).
Owner:FIBROGEN (CHINA) MEDICAL TECHNOLOGY DEVELOPMENT CO LTD
Isoquinoline compound melanocortin receptor ligands and methods of using same
The invention relates to melanocortin receptor ligands and methods of using the ligands to alter or regulate the activity of a melanocortin receptor. The invention further relates to tetrahydroisoquinoline aromatic amines that function as melanocortin receptor ligands and as agents for controlling cytokine-regulated physiologic processes and pathologies, and combinatorial libraries thereof.
Owner:LION BIOSCIENCE AG
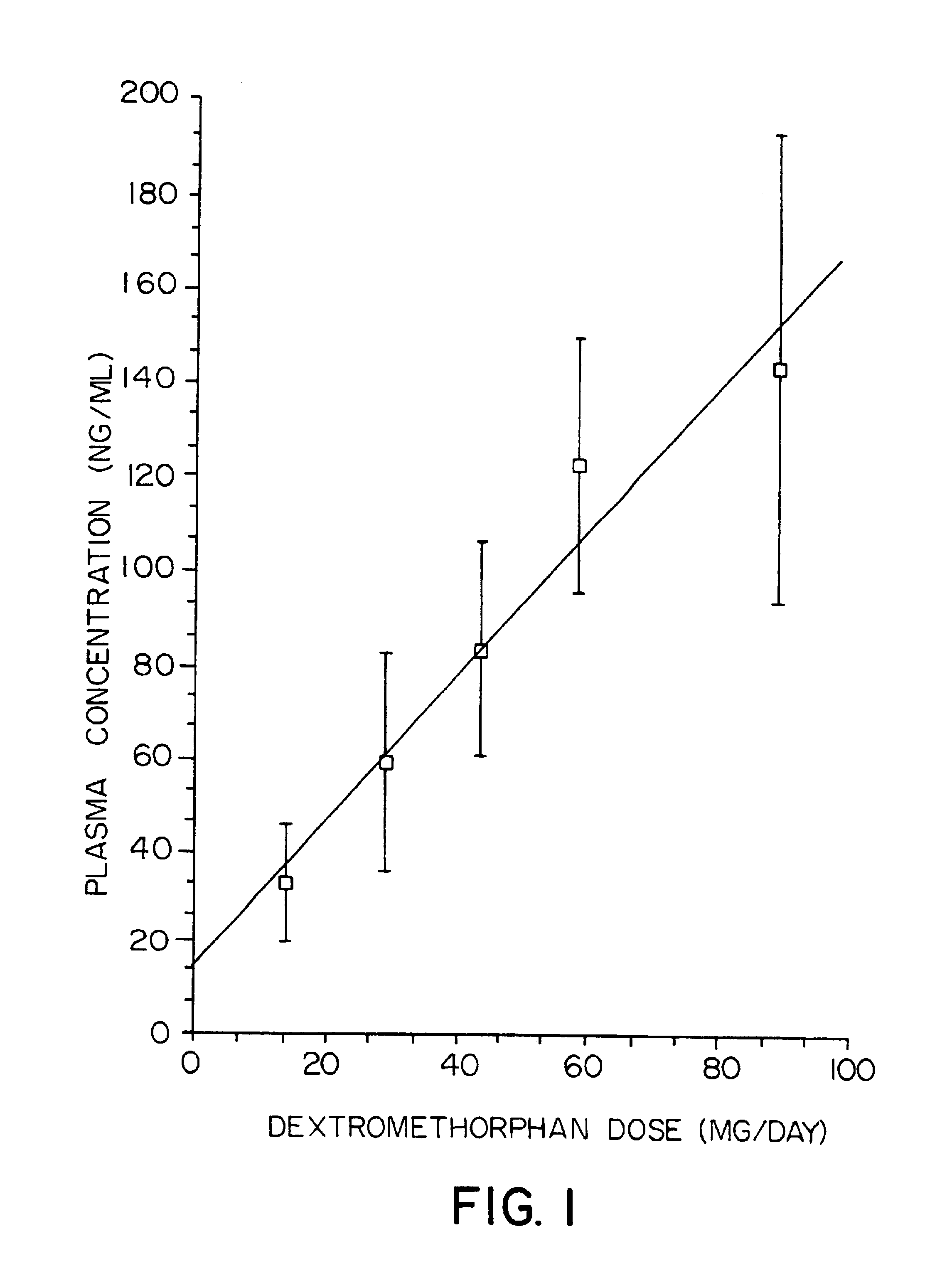
Dextromethorphan and an oxidase inhibitor for treating intractable conditions
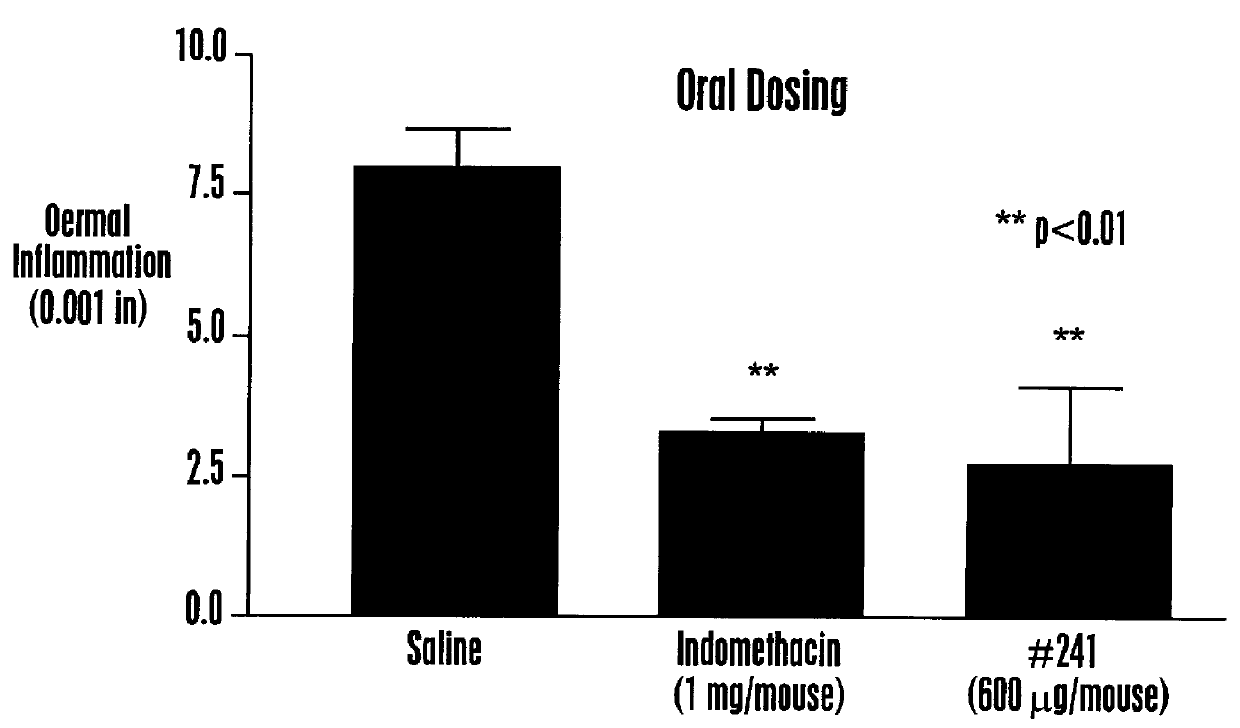
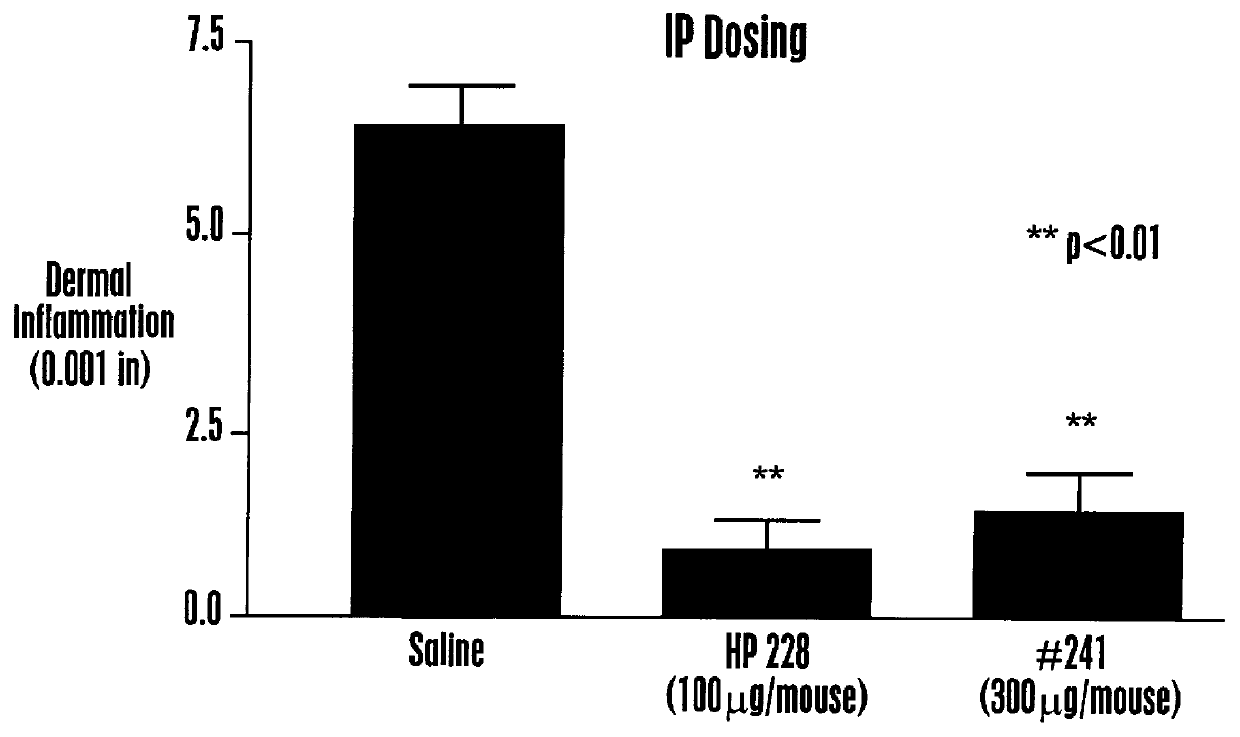
Methods are disclosed for increasing the effectiveness of dextromethorphan in treating chronic or intractable pain, for treating tinnitus and for treating sexual dysfunction comprising administering dextromethorphan in combination with a therapeutically effective dosage of a debrisoquin hydroxylase inhibitor. A preferred combination is dextromethorphan and the oxidative inhibitor quinidine.
Owner:AVANIR PHARMA
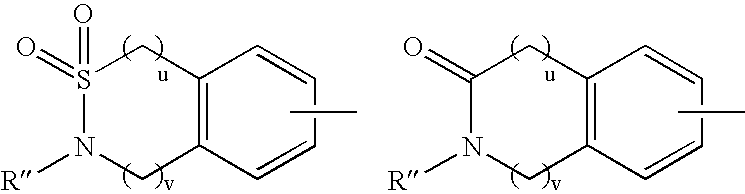
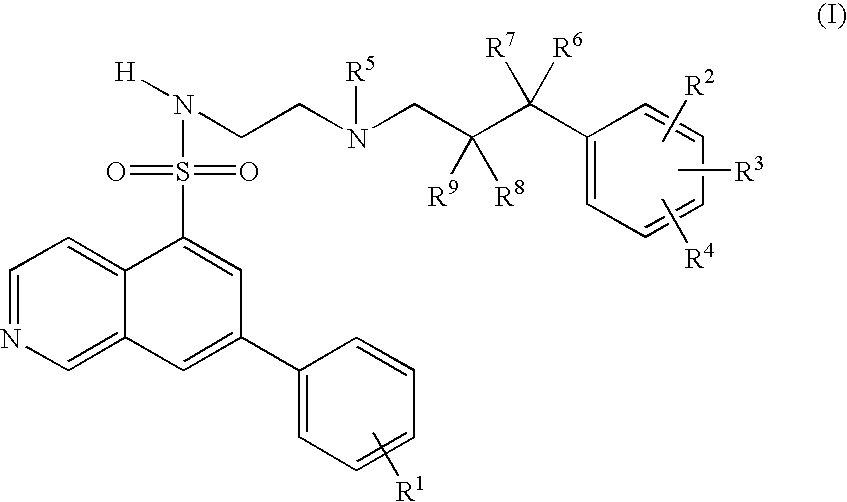
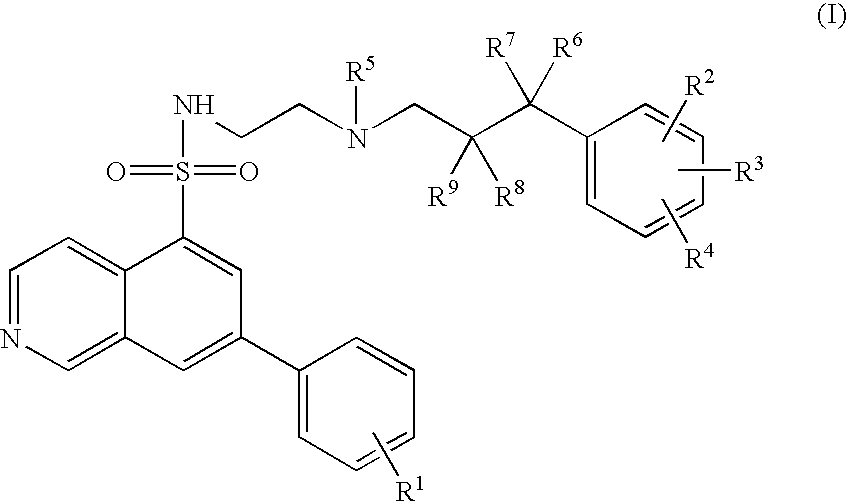
7-phenyl-isoquinoline-5-sulfonylamino derivatives as inhibitors of akt (protein kinase B)
Owner:ELI LILLY & CO
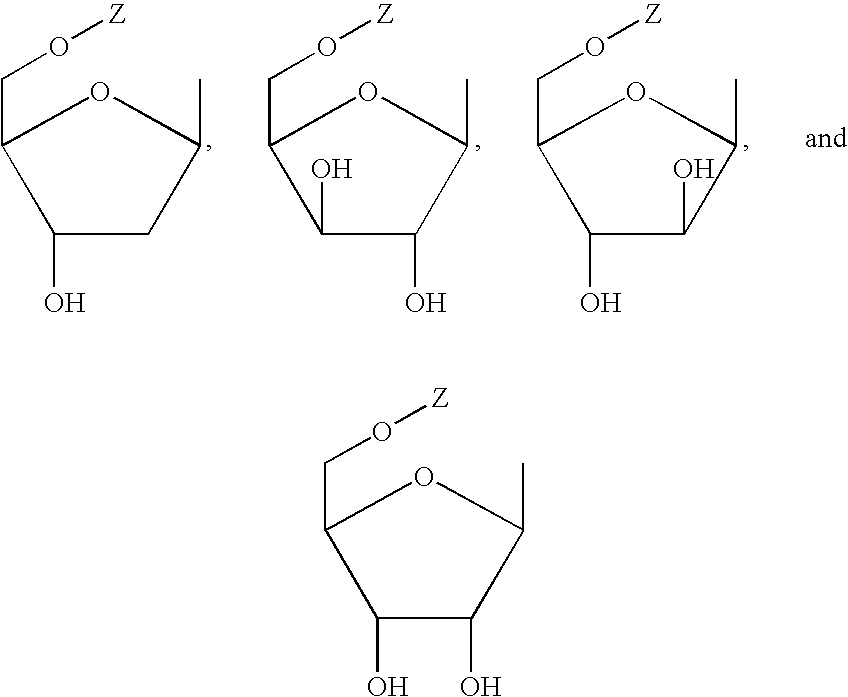
N²-quinoline or isoquinoline substituted purine derivatives
Novel compound having the following formula: wherein W represents a hydrogen, an optionally substituted C1-6 alkyl, an optionally substituted C3-6 cycloalkyl, or an optionally substituted C1-6 haloalkyl, Y represents a hydrogen, or a saccharide, Q represents a quinoline or isoquinoline. Also disclosed are a pharmaceutical compositions comprising the same, methods for treating cancer using the same, and methods for the synthesis of the same.
Owner:WU NA +1
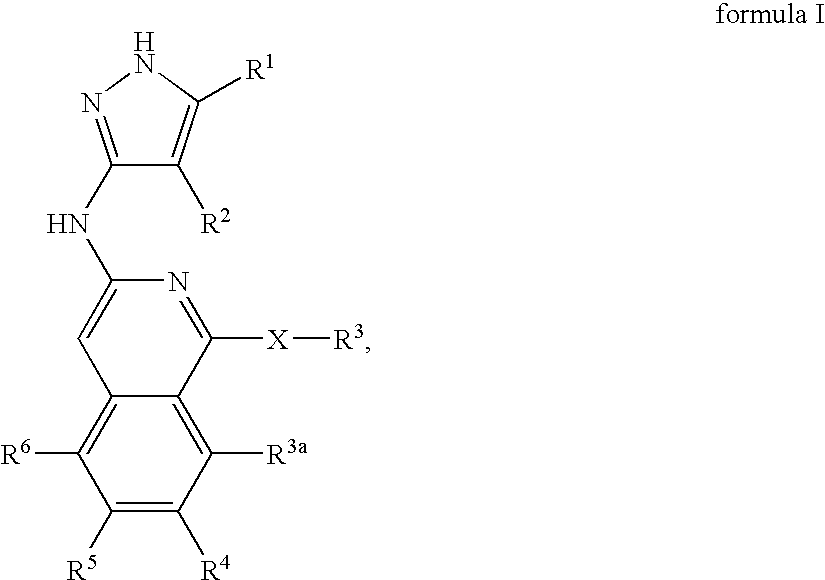
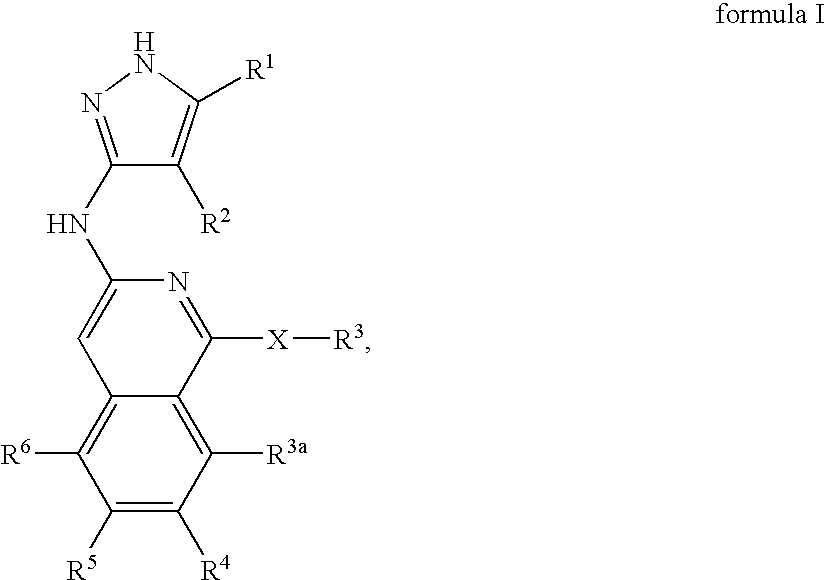
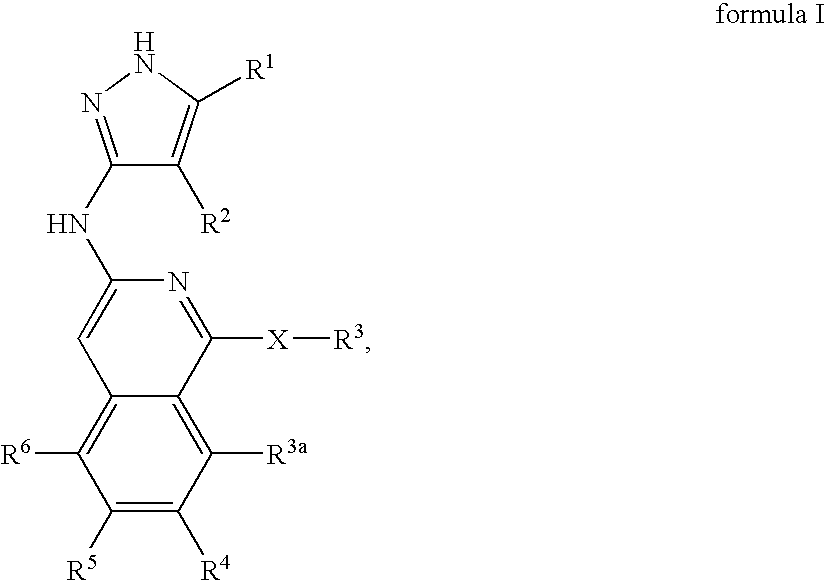
Isoquinoline aminopyrazole derivatives
The present invention relates to the compounds of formula I: their pharmaceutically acceptable salts or esters, enantiomeric forms, diastereoisomers and racemates, the preparation of such compounds, pharmaceutical compositions containing them and their manufacture, as well as the use of such compounds in the control or prevention of illnesses such as cancer.
Owner:F HOFFMANN LA ROCHE & CO AG
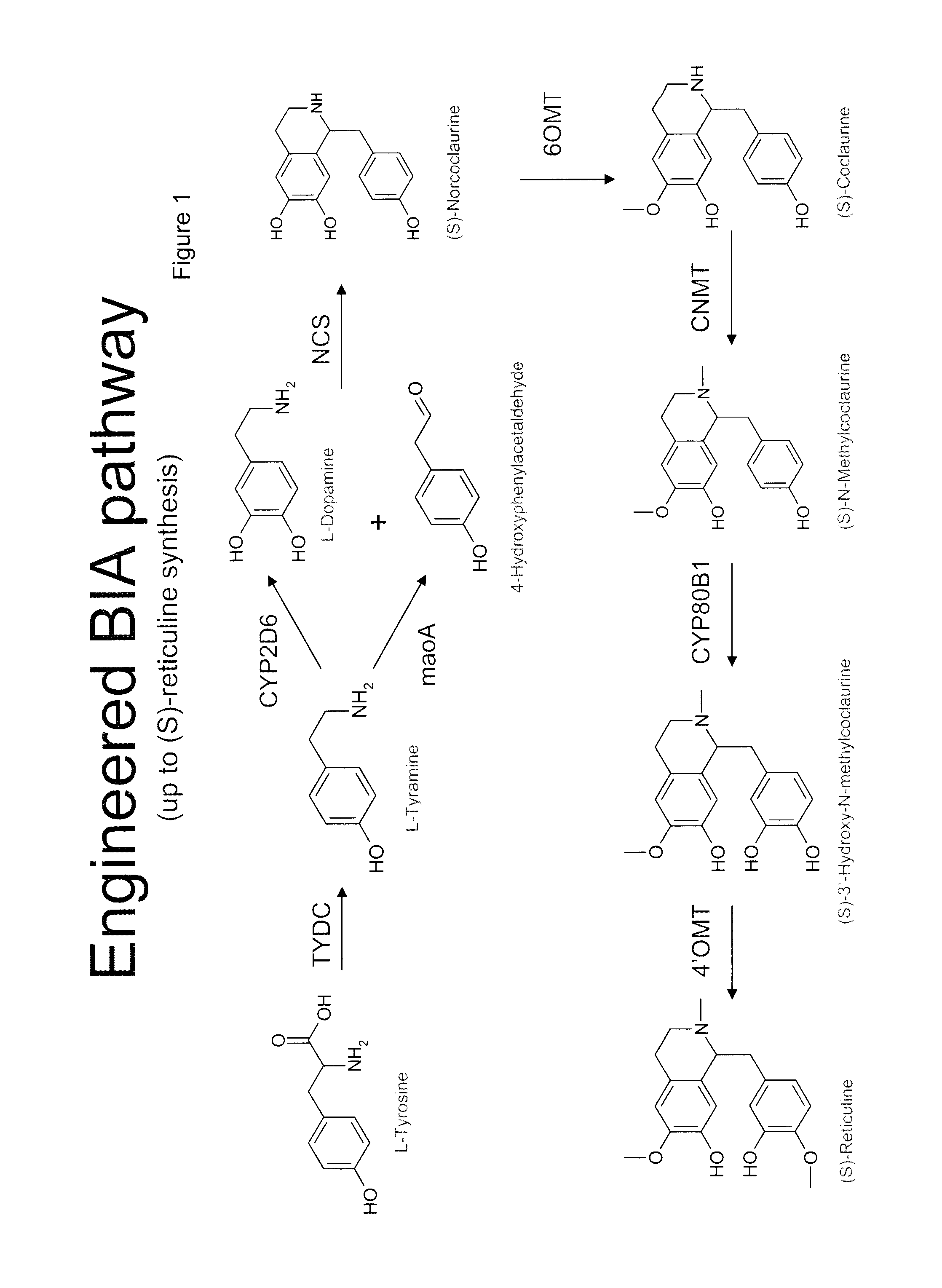
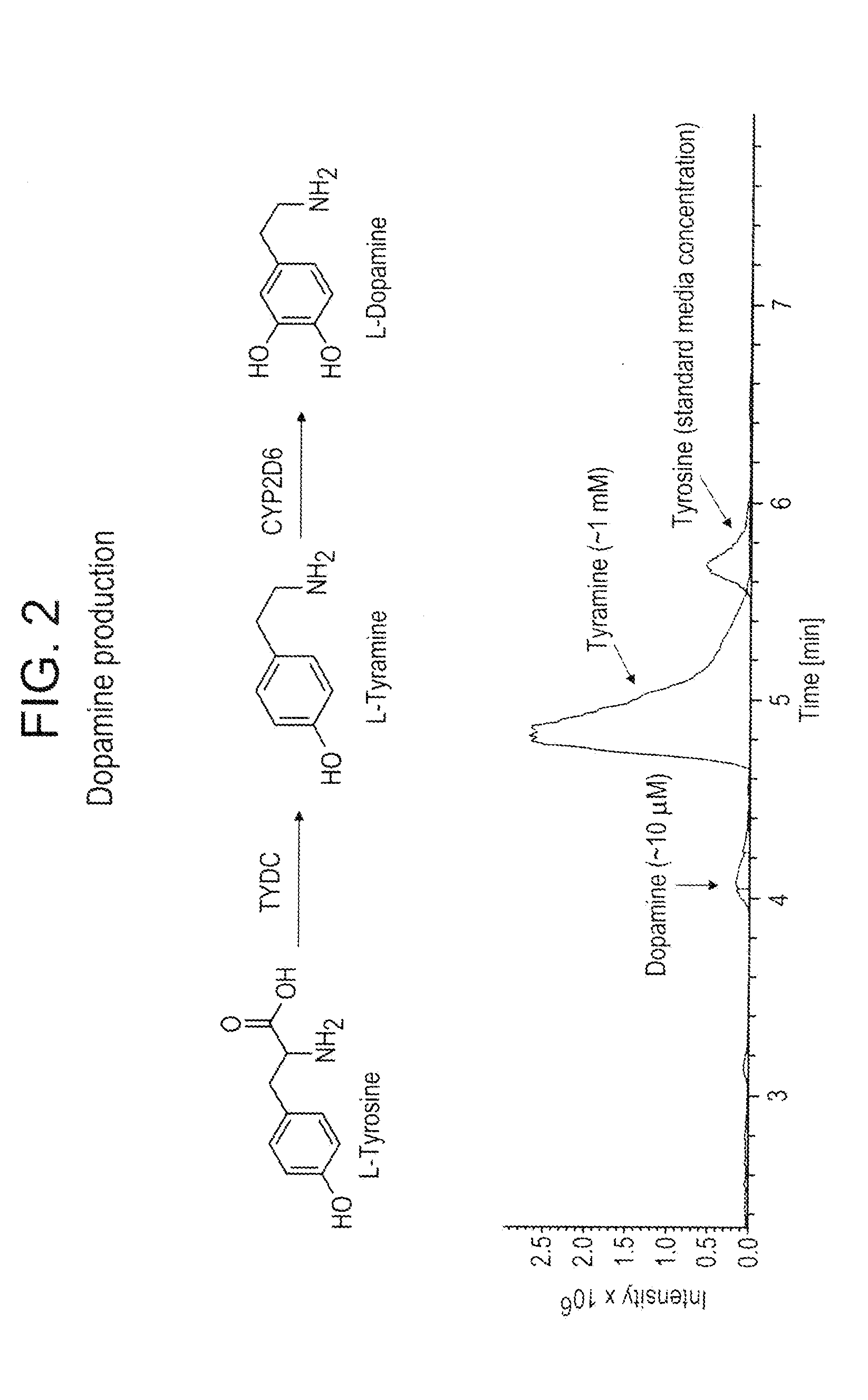
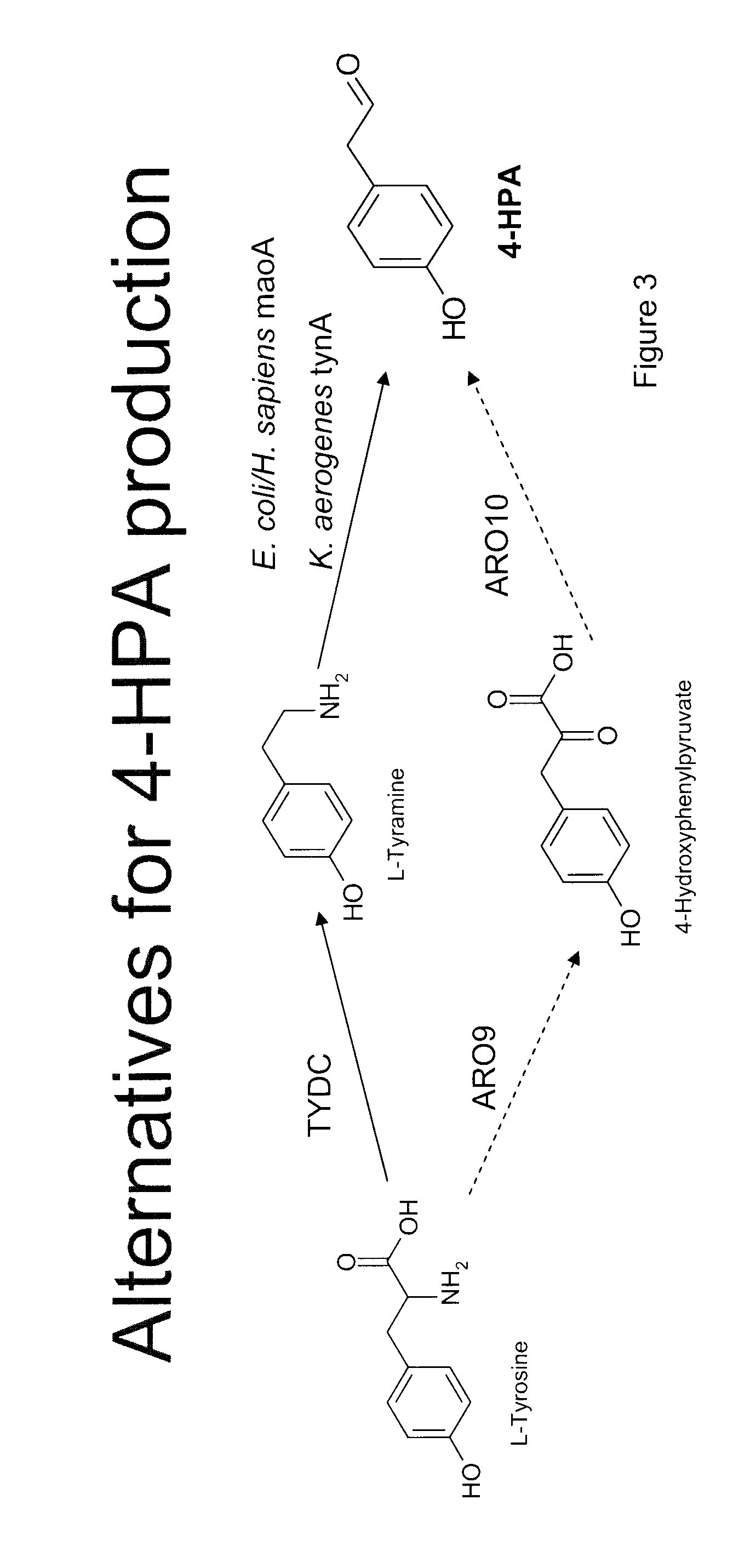
Compositions and methods for producing benzylisoquinoline alkaloids
ActiveUS20080176754A1Optimize growth ratePromote expression and activityTissue cultureLibrary member identificationMetabolic pathwayBenzylisoquinoline
The present invention relates to host cells that produce compounds that are characterized as benzylisoquinolines, as well as select precursors and intermediates thereof. The host cells comprise one, two or more heterologous coding sequences wherein each of the heterologous coding sequences encodes an enzyme involved in the metabolic pathway of a benzylisoquinoline, or its precursors or intermediates from a starting compound. The invention also relates to methods of producing the benzylisoquinoline, as well as select precursors and intermediates thereof by culturing the host cells under culture conditions that promote expression of the enzymes that produce the benzylisoquinoline or precursors or intermediates thereof.
Owner:CALIFORNIA INST OF TECH
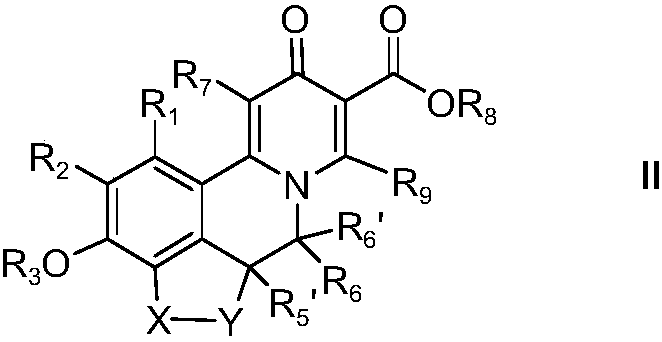
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto
Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-ol compounds are disclosed that are inhibitors of the vesicular monoamine transporter 2 (VMAT2). The compounds of this invention have the structure:wherein R1 is as defined herein, including stereoisomers and pharmaceutically acceptable salts and solvates thereof. Also disclosed are compositions containing a compound of this invention in combination with a pharmaceutically acceptable carrier, as well as methods relating to the use in a subject in need thereof.
Owner:NEUROCRINE BIOSCI INC
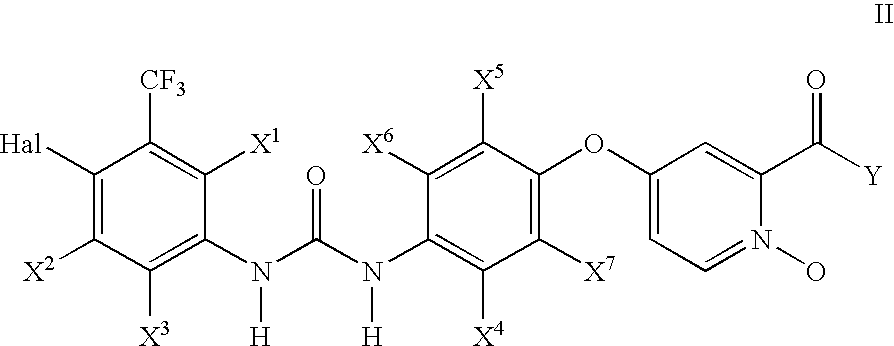
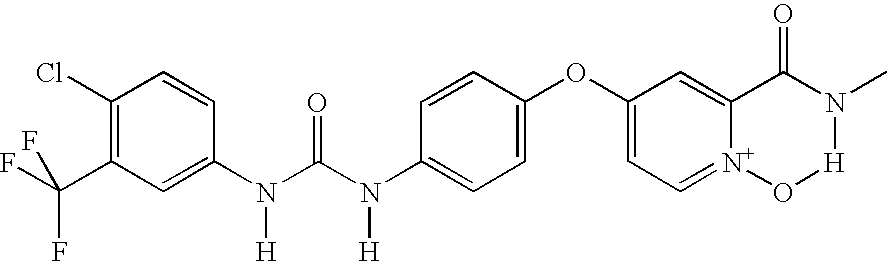
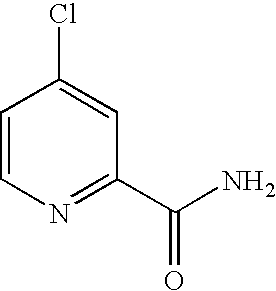
Pyridine, quinoline, and isoquinoline N-oxides as kinase inhibitors
This invention relates to urea compounds containing a pyridine, quinoline, or isoquinoline functionality which is oxidized at the nitrogen heteroatom and which are useful in the treatment of (i) raf mediated diseases, for example, cancer, (ii) p38 mediated diseases such as inflammation and osteoporosis, and (iii) VEGF mediated diseases such as angogenesis disorders.
Owner:BAYER HEALTHCARE LLC
Synthetic method and application of covalent organic framework (COF) material
ActiveCN103755588AOrganic-compounds/hydrides/coordination-complexes catalystsHydrazide preparationMetal-organic frameworkNitromethane
The invention discloses a synthetic method of a covalent organic framework (COF) material. The method comprises the following steps: after mixing 1,3,5-benzenetricarboxaldehyde with 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide uniformly in an organic solvent, reacting in the presence of a catalyst acetic acid to obtain the COF material, wherein the mole ratio of 1,3,5-benzenetricarboxaldehyde to 2,5-di(N,N-dimethyl)amino-1,4-benzdihydrazide is 1:(0.5-3). The COF material obtained by adopting the method has relatively large specific surface area and regular open framework structure with adjustable diameter, thus being beneficial for mass transfer of reactants and products in photoabsorption and catalytic processes; the material can serve as a photocatalyst and can increase the yield of the dehydrogenative coupling reaction between 2-phenyl-1,2,3,4-tetrahydroisoquinoline and nitromethane from 39% in the absence of catalysts to 89%.
Owner:LANZHOU UNIVERSITY
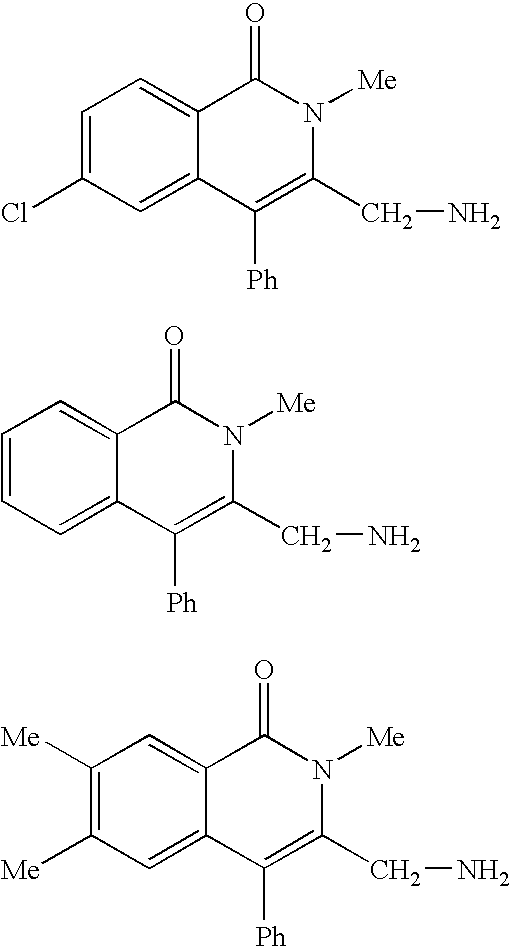
Fused heterocyclic compounds
The present invention provides a compound of the formula: wherein ring A is an optionally substituted 5 to 10-membered aromatic ring; R1 and R2 are the same or different and each is an optionally substituted hydrocarbon group or an optionally substituted heterocyclic group; X is a bond and the like; and L is a divalent hydrocarbon group, and a salt thereof, except 3-(aminomethyl)-2,6,7-trimethyl-4-phenyl-1(2H)-isoquinolinone, 3-(aminomethyl)-2-methyl-4-phenyl-1(2H)-isoquinolinone, 3-(aminomethyl)-6-chloro-2-methyl-4-phenyl-1(2H)-isoquinolinone and 3-(aminomethyl)-2-isopropyl-4-phenyl-1(2H)-isoquinolinone. The compound shows a superior peptidase-inhibitory activity and is useful as an agent for the prophylaxis or treatment of diabetes and the like.
Owner:TAKEDA PHARMA CO LTD
Isoquinoline Compounds And Methods For Treating HIV
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
Isoquinolinone Rho kinase inhibitors
Owner:AERIE PHARMA
Isoquinoline-1,3,4-trione compounds, the synthetic method and the use thereof
InactiveUS20060135557A1Inhibitory activityInhibition effectBiocideNervous disorderIsoquinolineIschemic injury
The invention relates to various substituted isoquinoline-1,3,4-trione, the synthetic method thereof and the use for treating neurodegenerative diseases, especially as the medicine for Alzheimer's disease, apoplexy and brain ischemic injuries.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
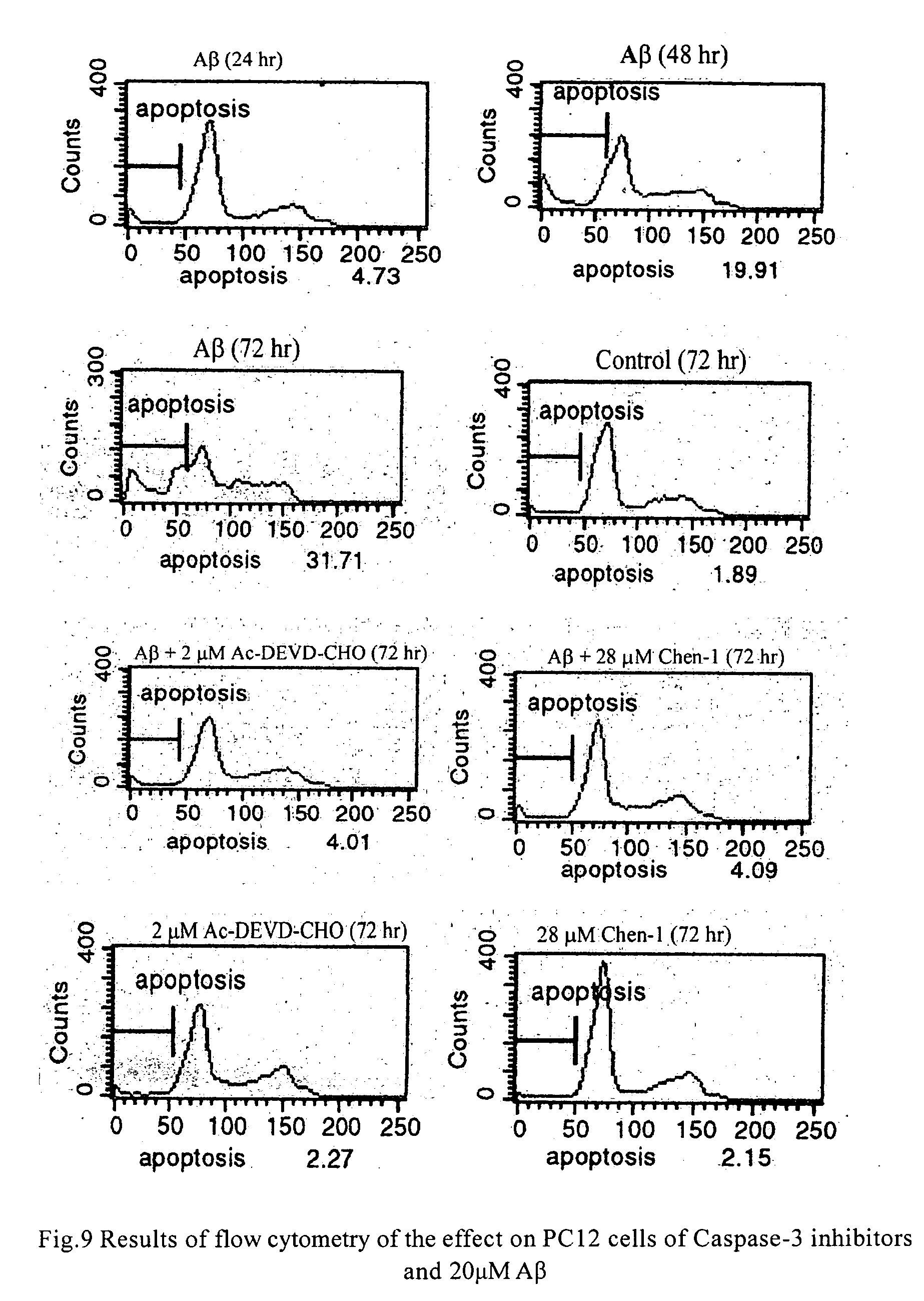
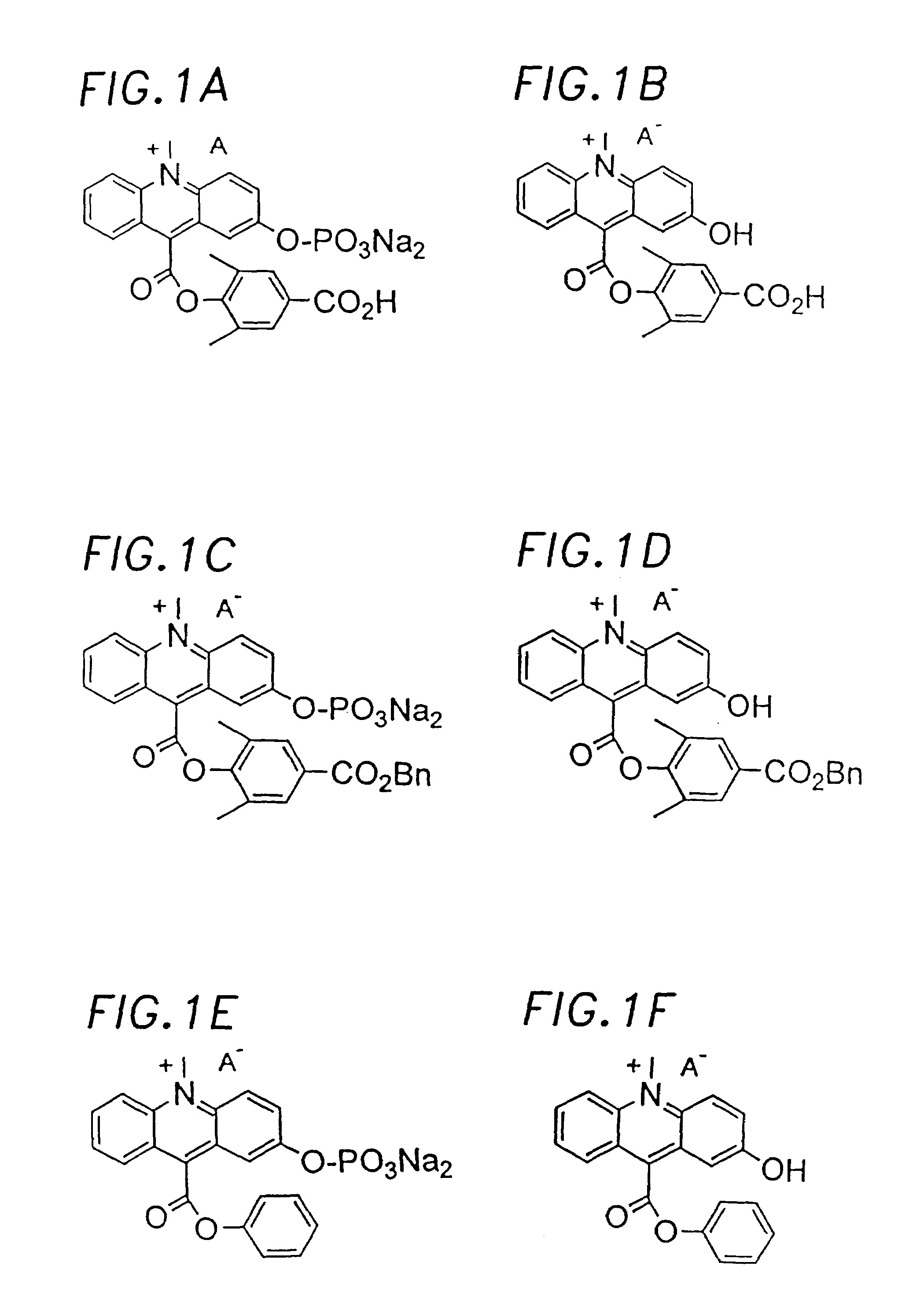
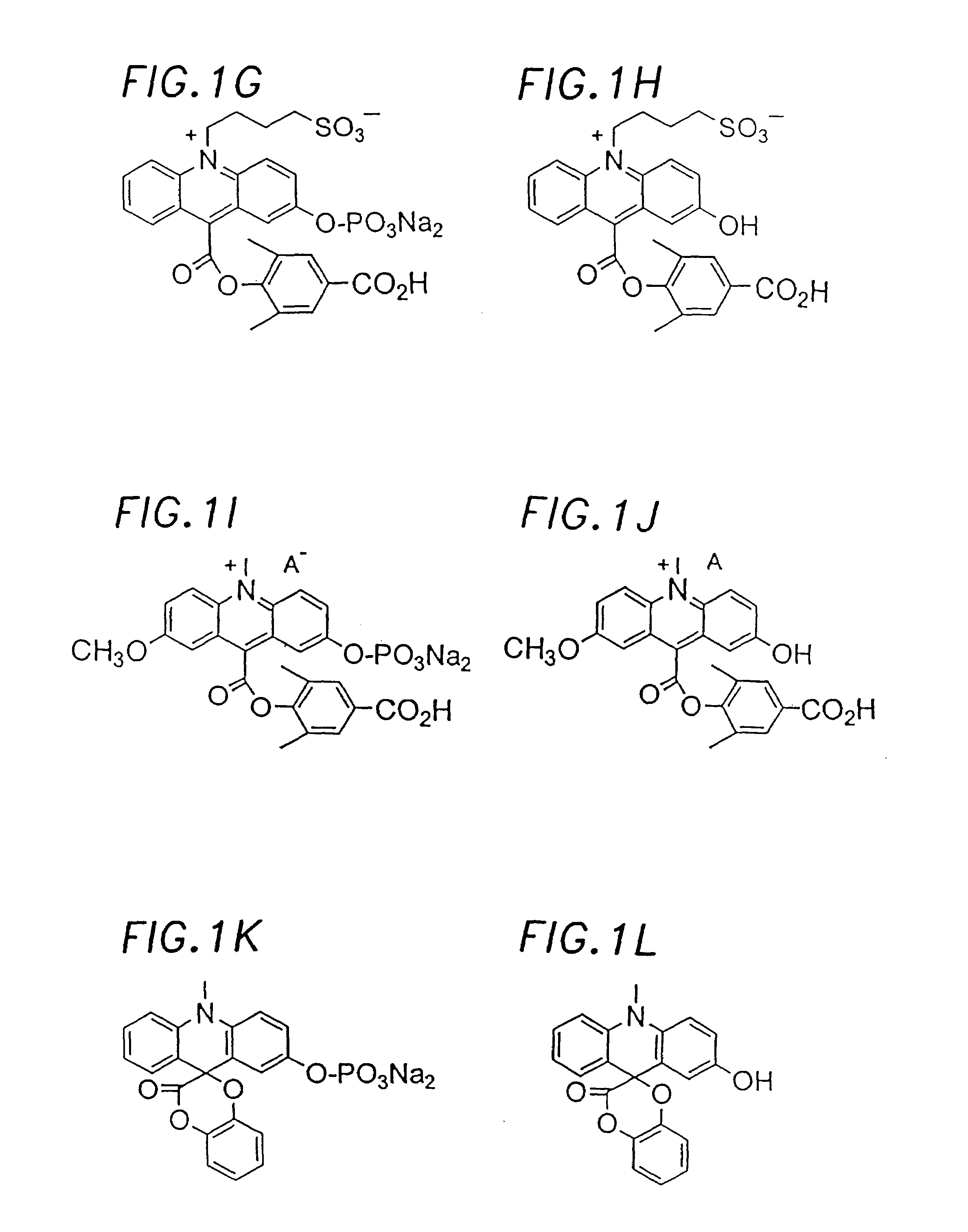
Chemiluminescent acridinium compounds and analogues thereof as substrates of hydrolytic enzymes
InactiveUS7097995B2Improve light outputIncrease productionSugar derivativesMicrobiological testing/measurementBenzeneIsoquinoline
A chemiluminescent substrate of a hydrolytic enzyme having the following general Formula I is disclosed, as follows:Lumi-M-PFormula Iwhere “Lumi” is a chemiluminescent moiety capable of producing light (a) by itself, (b) with MP attached and (c) with M attached. Examples of Lumi include chemiluminescent acridinium compounds, benzacridinium compounds, quinolinium compounds, isoquinolinium compounds, phenanthridinium compounds, and lucigenin compounds, spiroacridan compounds, luminol compounds and isoluminol compounds. M is a multivalent heteroatom having at least one lone pair of electrons selected from oxygen, nitrogen and sulfur, directly attached to the light emitting moiety of Lumi at one end and to P at the other end. P is a group that can be readily removed by hydrolytic enzymes. An enzymatic reaction utilizing the above compound is the following:where HE is a hydrolytic enzyme. Lumi-M is a chemiluminescent product having physical and / or chemical properties different from those of Lumi-M-P.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Substituted piperidine compounds and methods of their use
Certain 4-aryl-piperidine compounds, including N-substituted 9β-substituted-5-(3-substituted-phenyl)morphans and N-substituted octahydro-4a-(3-hydroxyphenyl)-10a-methyl-benzo[g]isoquinolines, pharmaceutical compositions, and methods of their use, inter alia, as opioid antagonists are disclosed.
Owner:APOLOR CORP
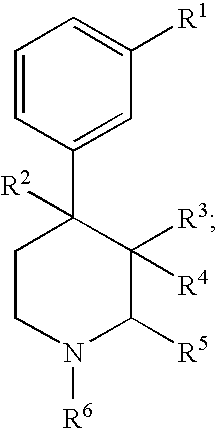
Substituted piperidine compounds and methods of their use
Certain 4-aryl-piperidine compounds, including N-substituted 9β-substituted-5-(3-substituted-phenyl)morphans and N-substituted octahydro-4a-(3-hydroxyphenyl)-10a-methyl-benzo[g]isoquinolines, pharmaceutical compositions, and methods of their use, inter alia, as opioid antagonists are disclosed.
Owner:APOLOR CORP
Valbenazine salts and polymorphs thereof
Provided herein are salts of (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester in amorphous and crystalline forms, and processes of preparation, and pharmaceutical compositions thereof. Also provided are methods of their use for treating, preventing, or ameliorating one or more symptoms of neurological disorders and diseases including hyperkinetic movement disorders or diseases.
Owner:NEUROCRINE BIOSCI INC
Isoquinolone compound and application thereof
The invention relates to an isoquinolone compound and application thereof. The invention in particular relates to an isoquinolone compound used as a HIF prolyl hydroxylase inhibitor (HIF-PHI) and a pharmaceutical composition thereof. Moreover, the present invention provides the application of the isoquinolinone compound or the pharmaceutical composition thereof in the preparation of a medicament for preventing or treating HIF-associated or HIF-mediated diseases as anemia or ischemia, local ischemia or hypoxia.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
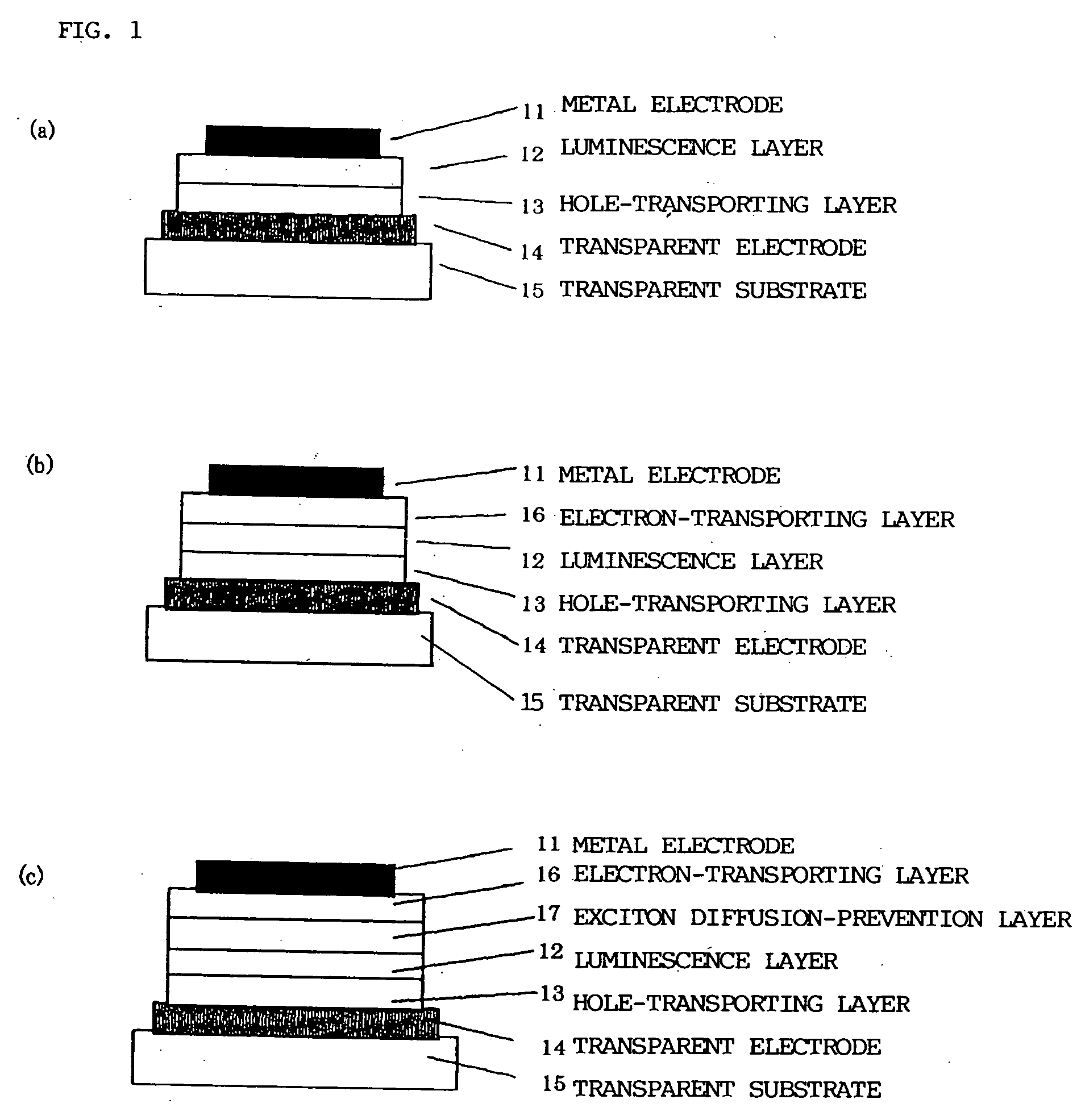
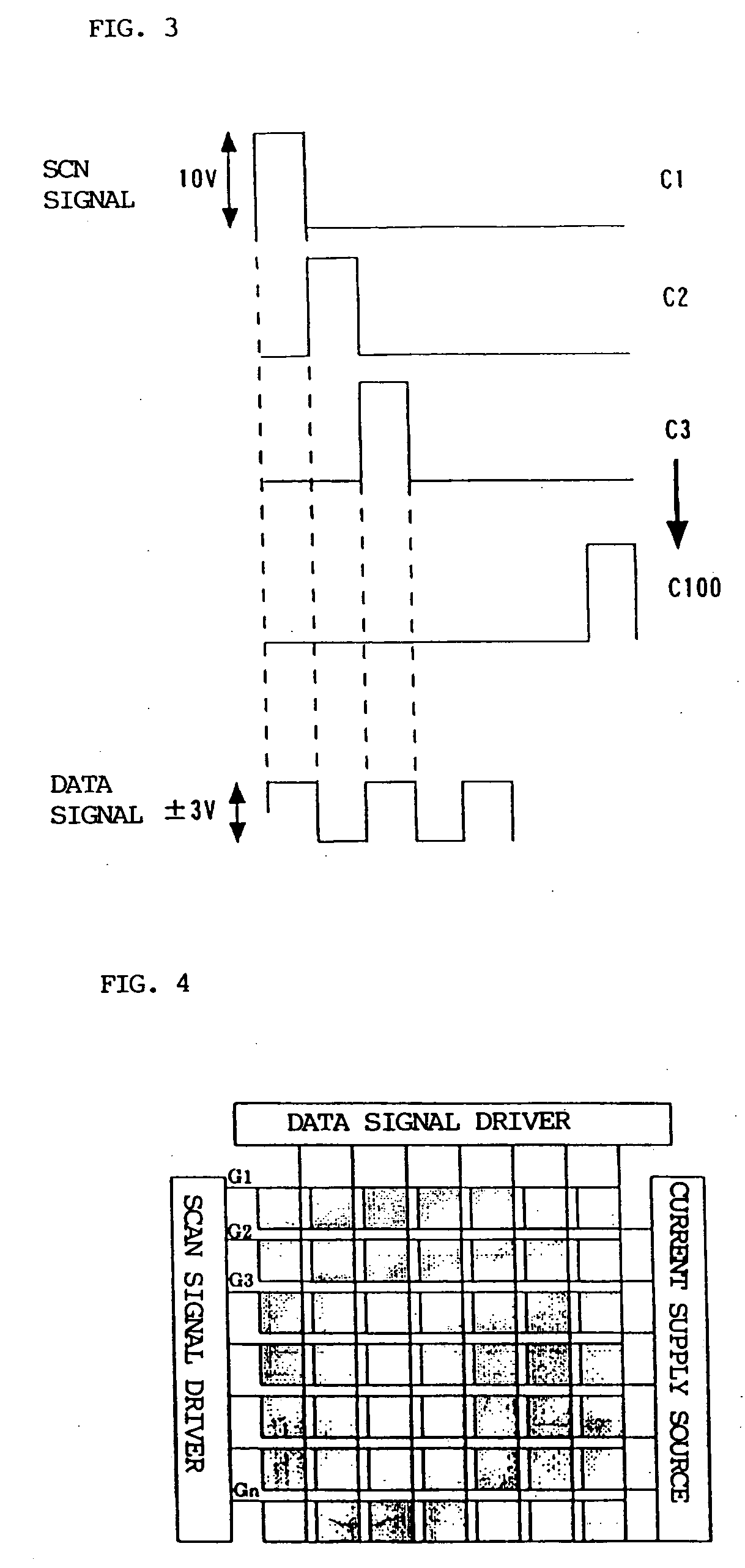
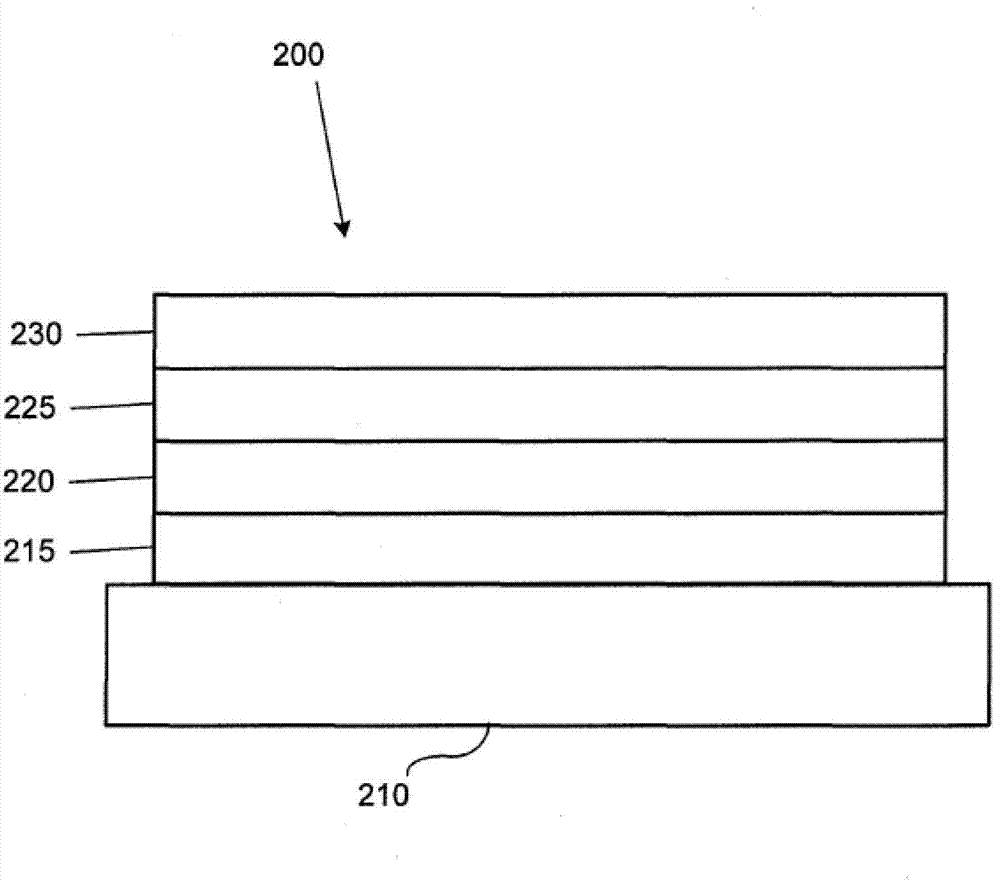
Luminescence device and display apparatus
InactiveUS20060177694A1High efficiency luminescenceExtend device lifeIndium organic compoundsDischarge tube luminescnet screensLuminescenceHigh luminance
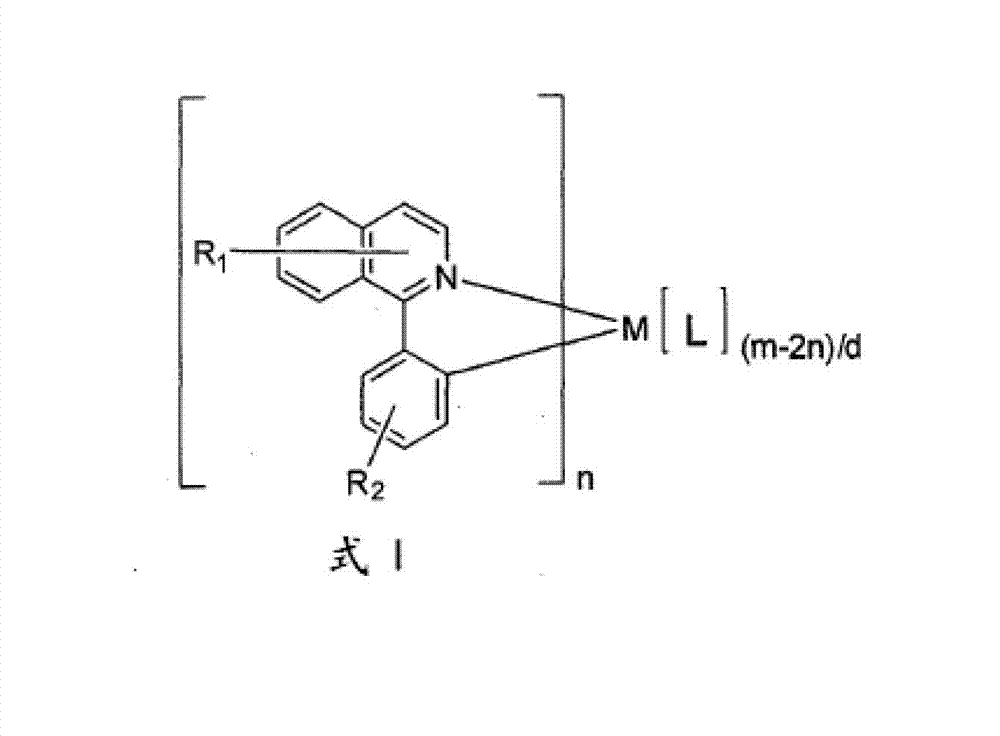
A luminescence device having a layer containing a metal coordination compound which has a partial structure. MLm of formula (2) below and is preferably entirely represented by formula (3) below: MLmL′n (3) wherein M denotes a metal atom of Ir, Pt, Rh or Pd; represent mutually different bidentate ligands; m is 1 or 2 or 3; n is 0 or 1 or 2 with the proviso that m+n=2 or 3; the partial structure MLm is represented by formula (2) below (wherein B is an isoquinolyl group bonded to the metal M with its N and including a position-1 carbon atom bonded to a cyclic group A which includes the C bonded to the metal M), and the partial structure ML′n is represented by formula (4), (5) or (6) shown below. There is provided a luminescence device capable of high-efficiency luminescence and long-term high luminance and adapted to red luminescence.
Owner:CANON KK
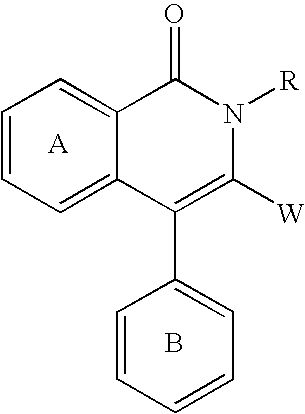
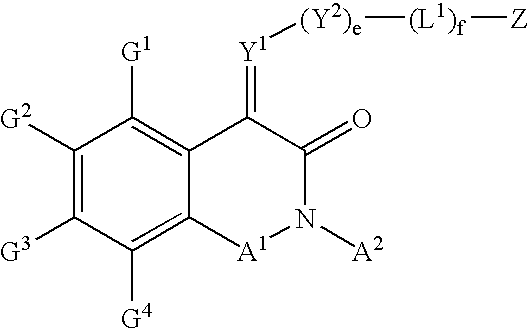
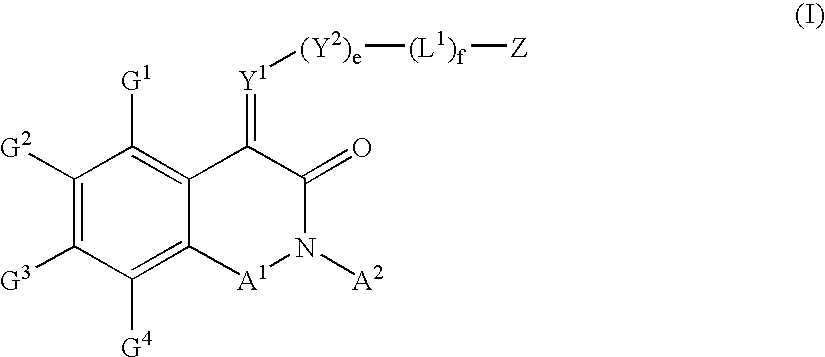
Substituted isoquinoline-1,3(2H,4H)-diones, 1-thioxo,1,4-dihydro-2H-isoquinoline-3-ones and 1,4-dihyro-3 (2H)-isoquinolones and methods of use thereof
This invention provides compounds of Formula (I), having the structurewhere G1, G2, G3, G4, A1, A2, Y1, Y2, L1, Z, e and f are defined herein, or a pharmaceutically acceptable salt thereof, which are useful for treating or preventing cancer.
Owner:WYETH LLC
Valbenazine salts and polymorphs thereof
Provided herein are salts of (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester in amorphous and crystalline forms, and processes of preparation, and pharmaceutical compositions thereof. Also provided are methods of their use for treating, preventing, or ameliorating one or more symptoms of neurological disorders and diseases including hyperkinetic movement disorders or diseases.
Owner:NEUROCRINE BIOSCIENCES INC
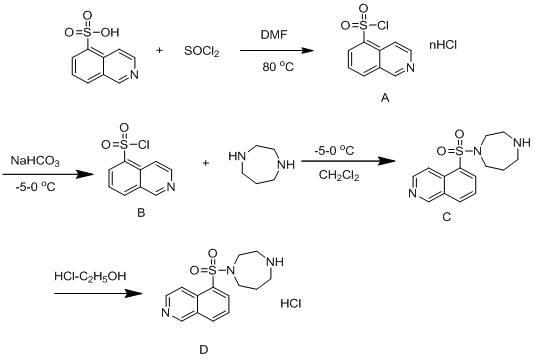
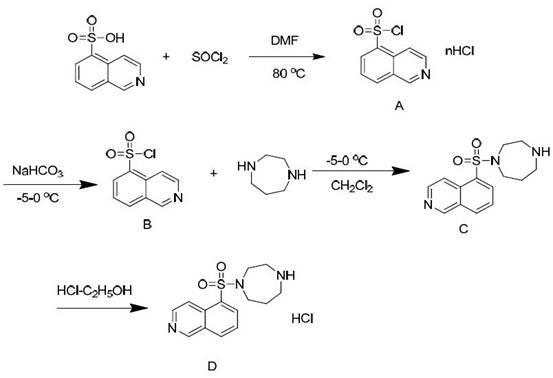
Method for synthesizing and purifying Fasudil hydrochloride
InactiveCN102020636AReduce generationSolve excessive residualOrganic chemistryBenzodiazepineSodium bicarbonate
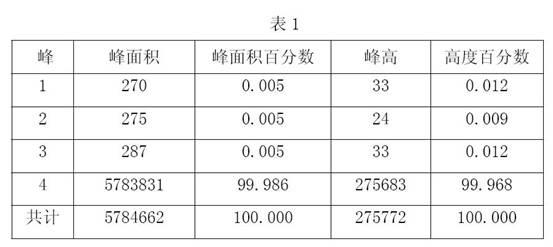
The invention relates to a method for synthesizing and purifying Fasudil hydrochloride, comprising the following steps of: adding N,N-dimethylformamide and thionyl dichloride to 5-isoquinoline sulfonic acid to obtain a 5-isoquinoline sulfonyl chloride hydrochloride; adding the 5-isoquinoline sulfonyl chloride hydrochloride, water and sodium bicarbonate to dichloroethene to obtain a 5-isoquinoline sulfonyl chloride solution; dripping the 5-isoquinoline sulfonyl chloride solution to homopiperazine at zero DEG C to obtain a hexahydro-1-(5-sulfonyl isoquinoline)-1(H)-1,4-benzodiazepine solution; removing impurities in three steps; adjusting the pH value to be 4.5-5.5 with an acidizing fluid, extracting, and discarding an organic phase dissolved with a dipolymer impurity; adjusting the pH value to be 9.5-10.5 with alkali, extracting and discarding a water phase dissolved with homopiperazine impurities and a majority of pigments; removing the residue pigment by passing through silica gel; and finally, washing, drying, filtering, then dripping a hydrochloric acid-ethanol solution at 0 DEG C, and crystallizing while stirring to prepare the Fasudil hydrochloride. The invention has the advantages that the three kinds of impurities are effectively removed through the three-step purification process, and the purity of a product reaches more than 99.9%.
Owner:JIANGSU WANBANG BIOPHARMLS +1
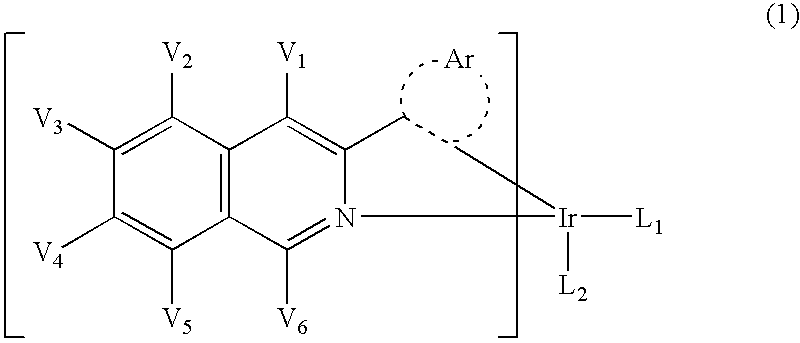
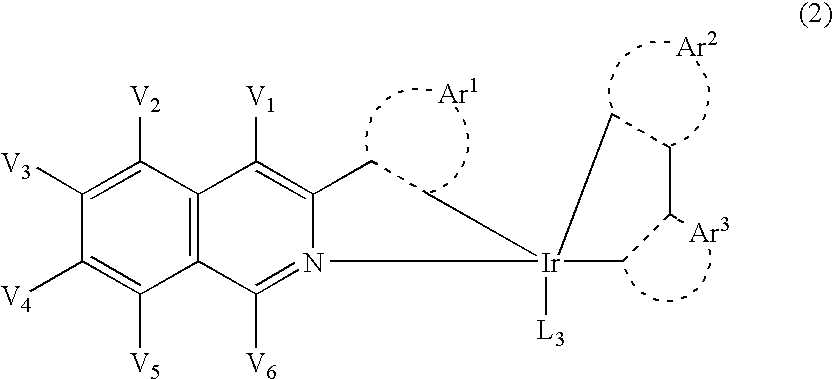
Novel organic light emitting materials
InactiveCN103159798AIndium organic compoundsElectroluminescent light sourcesIsoquinolineLuminescent material
Novel phosphorescent metal complexes containing 2-plienylisoquinoline ligands with at least two substituents on the isoquinoline ring are provided. The disclosed compounds have low sublimation temperatures that allow for ease of purification and fabrication into a variety of OLED devices.
Owner:UNIVERSAL DISPLAY
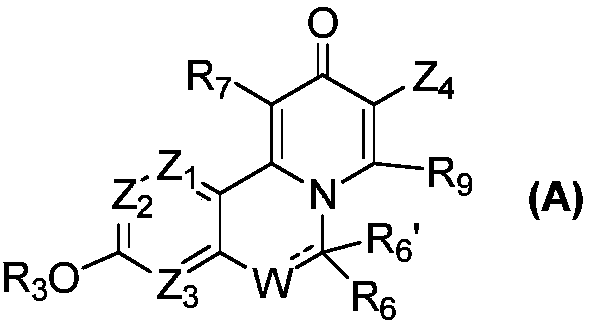
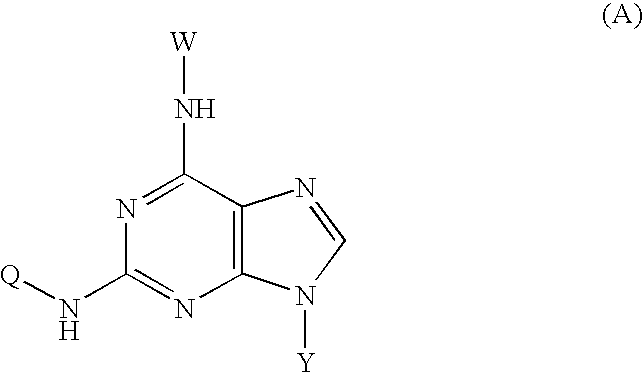
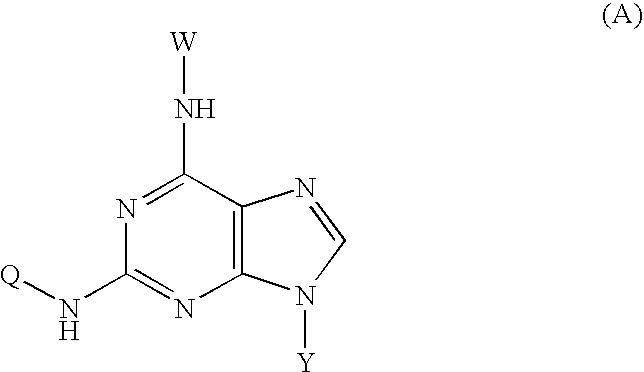
Novel isoquinoline compound and medical application thereof
InactiveCN108727378AImprove bioavailabilityLow toxicityGroup 5/15 element organic compoundsBoron compound active ingredientsAntigenViral infectious disease
The invention relates to an isoquinoline compound shown as a formula (A) or a stereoisomer, a pharmaceutically acceptable salt, a hydrate, a solvate or a crystal thereof, a medicinal composition thereof and application thereof as antiviral medicines. The isoquinoline compound inhibits hepatitis B DNA activity and hepatitis B surface antigen activity at the same time. The invention particularly relates to application thereof to preparation of medicines for treating and / or preventing hepatitis B, hepatitis B viruses (HBV) thereof and other viral infectious diseases, in particular to treatment and / or prevention of the hepatitis B and the hepatitis B viruses as HBV Surface antigen inhibitor medicines and HBV DNA production inhibitor medicines.
Owner:GINKGO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

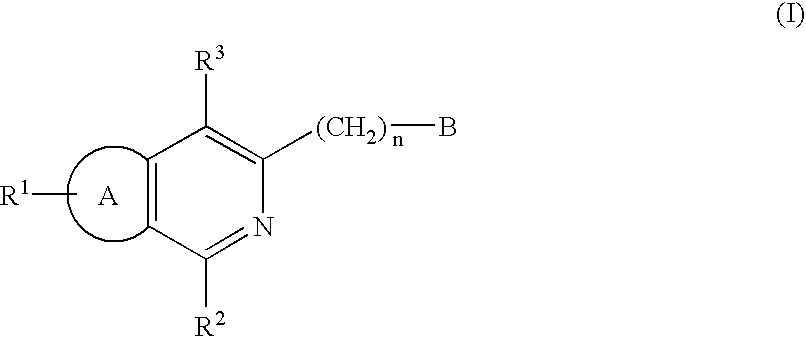
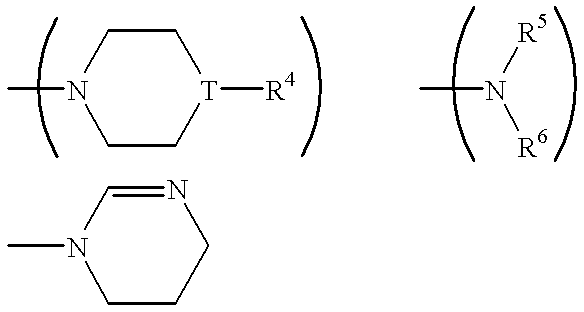
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00001.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00002.png)
![Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto Substituted 3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2-ol compounds and methods relating thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/a0bd7133-284f-4ef7-95fe-2ba89ead4c06/US20080167337A1-20080710-D00003.png)