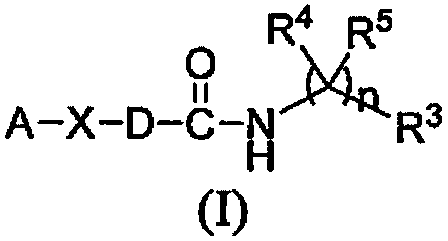
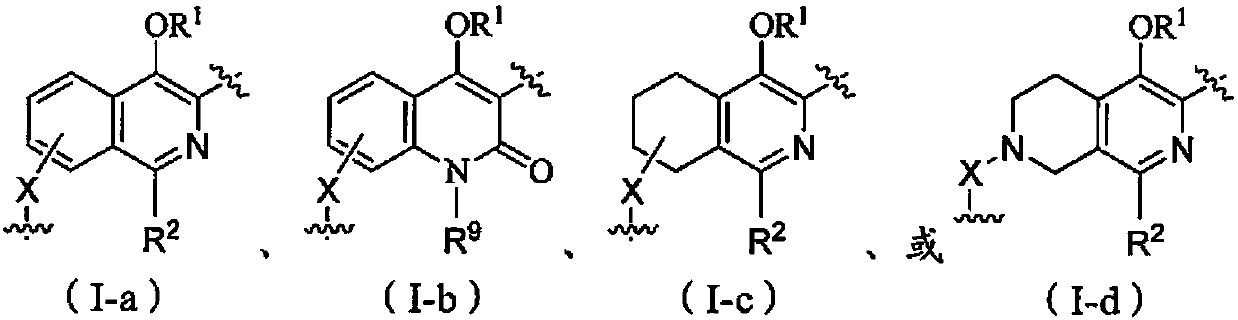
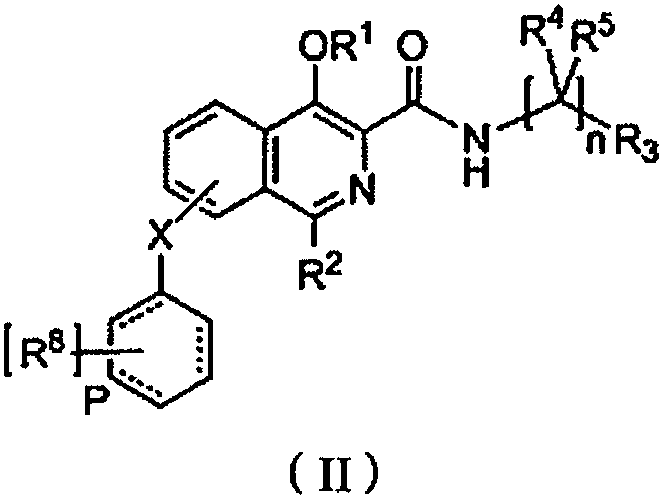
Isoquinolone compound and application thereof
A compound and selected technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems of undisclosed compound regulation, unsatisfactory therapeutic effect, and restrictions on widespread use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1: 2-(7-(4-chloro-2-fluorobenzyl)-4-hydroxyl-1-methylisoquinoline-3-carboxamide)acetic acid (Compound 1)
[0179]
[0180] The first step: methyl 4-bromo-2-(chloromethyl)benzoate (1b)
[0181] 5-Bromoisobenzofuran-1(3H)-one (10.00g, 47.2mmol), benzyltriethylammonium chloride (2.20g, 9.4mmol), boron trifluoride ether (1.20mL, 9.40mmol ), xylene (50 mL) were added into the reaction flask, and the reaction solution was replaced with nitrogen three times, and then heated to 110° C. for 30 min. Then, thionyl chloride (8.60 mL, 118.0 mmol) was slowly added dropwise into the above reaction solution, and after the drop was completed, the temperature was raised to 130°C for 6 h, then cooled to 40-50°C, and methanol (20 mL) was slowly added dropwise to quench the reaction. The solvent was evaporated from the reaction liquid under reduced pressure, and the title compound 1b (11.40 g, 93.0%) was obtained by separation on a silica gel column.
[0182] The second step: m...
Embodiment 2
[0199] Example 2: N-((2H-tetrazol-5-yl)methyl)-4-hydroxyl-1-methyl-7-phenoxyisoquinoline-3-formamide (compound 2)
[0200]
[0201] The first step: 5-phenoxyisobenzofuran-1(3H)-one (2b)
[0202] Phenol (20.4g, 0.22mol) and DMF 50mL were added to the reaction flask, stirring was started, and 5-bromoisobenzofuran-1(3H)-one (34.0g, 0.16mmol), acetylacetone (3.2 g, 0.03mol), cuprous bromide (3.6g, 0.03mol), potassium carbonate (30.8g, 0.22mol), replaced with nitrogen three times, heated to 90°C and stirred overnight, added 1000mL of purified water to the reaction solution, and suction filtered. Dissolve the filter cake with 800 mL of dichloromethane, wash the organic phase with 800 mL of 1N hydrochloric acid solution, wash with 1000 mL of purified water, dry the organic phase, and concentrate to dryness under reduced pressure to obtain a yellow solid. Title compound 2b (22.5 g, 63%).
[0203] The second step: methyl 2-(chloromethyl)-4-phenoxybenzoate (2c)
[0204] 5-phenoxyi...
Embodiment 3
[0216] Example 3: N-((2H-tetrazol-5-yl)methyl)-4-hydroxyl-1-methyl-7-phenoxyisoquinoline-3-carboxamide (compound 3)
[0217]
[0218] At room temperature, methyl 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylate (200mg, 0.65mmol), (1H-1,2,3-triazol-4-yl) Methylamine (500mg, 3.68mmol), 2mL of methanol, and 1mL of sodium methoxide in methanol were added to a sealed tube, and the reaction was sealed overnight at 110°C. After suction filtration, the filtrate was added to DCM, and a solid precipitated out. The obtained solid was purified by silica gel column chromatography to obtain the title Compound 3 (75 mg, 30%). MS m / z (ESI): 376 (M+1).
[0219] 1 H NMR (400MHz, CDCl 3 )δ8.44(d, J=9.6Hz, 1H), 7.81(s, 1H), 7.57(d, J=9.6Hz, 2H), 7.23-7.04(m, 3H), 7.12(d, J=8.0 Hz, 2H), 4.83 (s, 2H), 2.89 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com