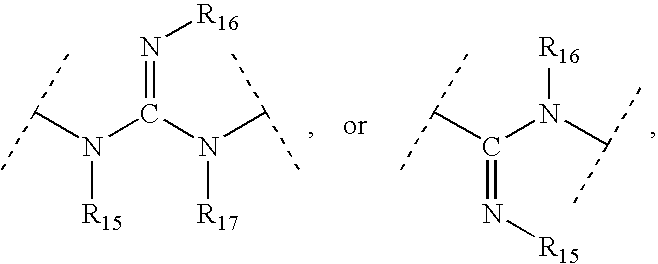
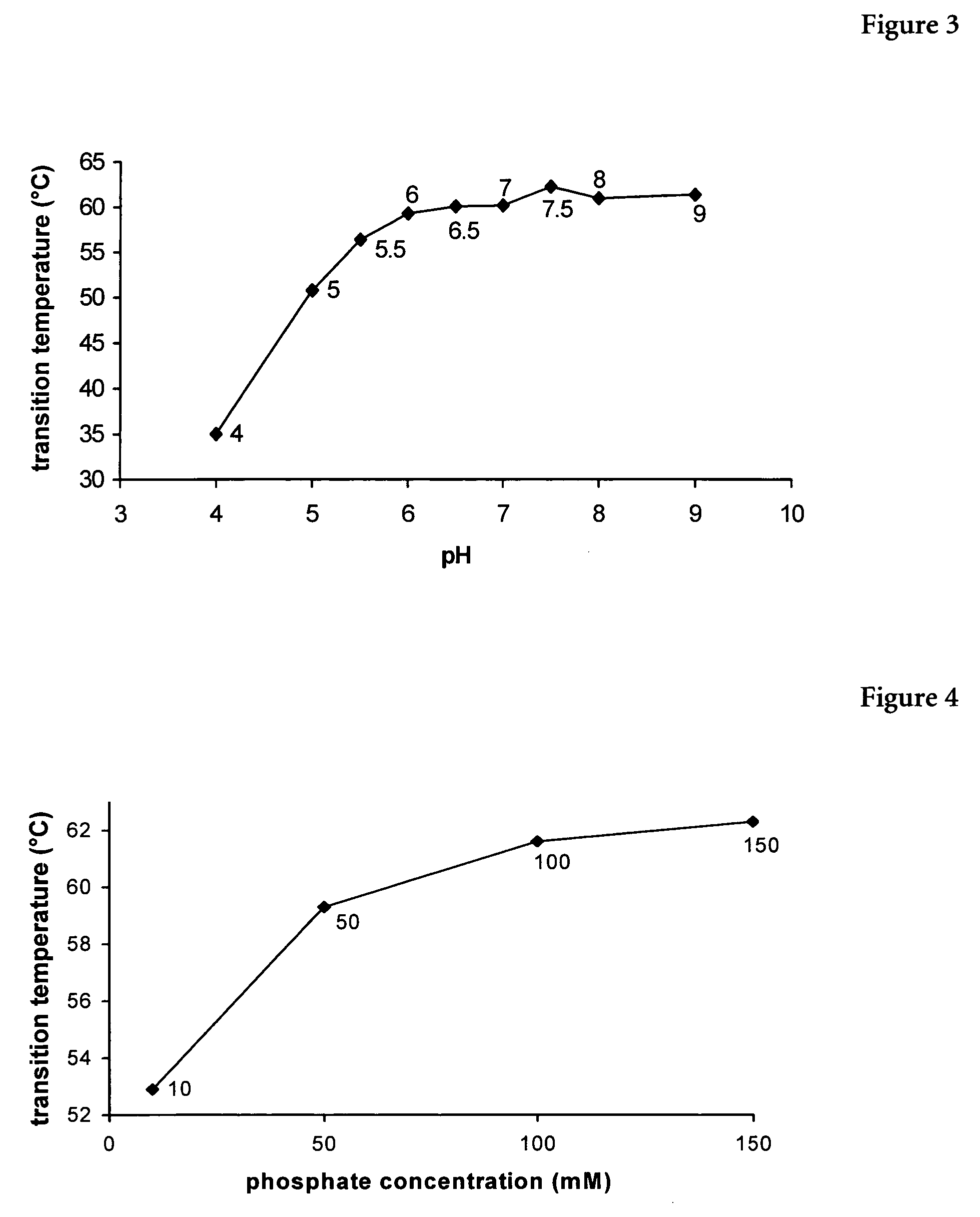
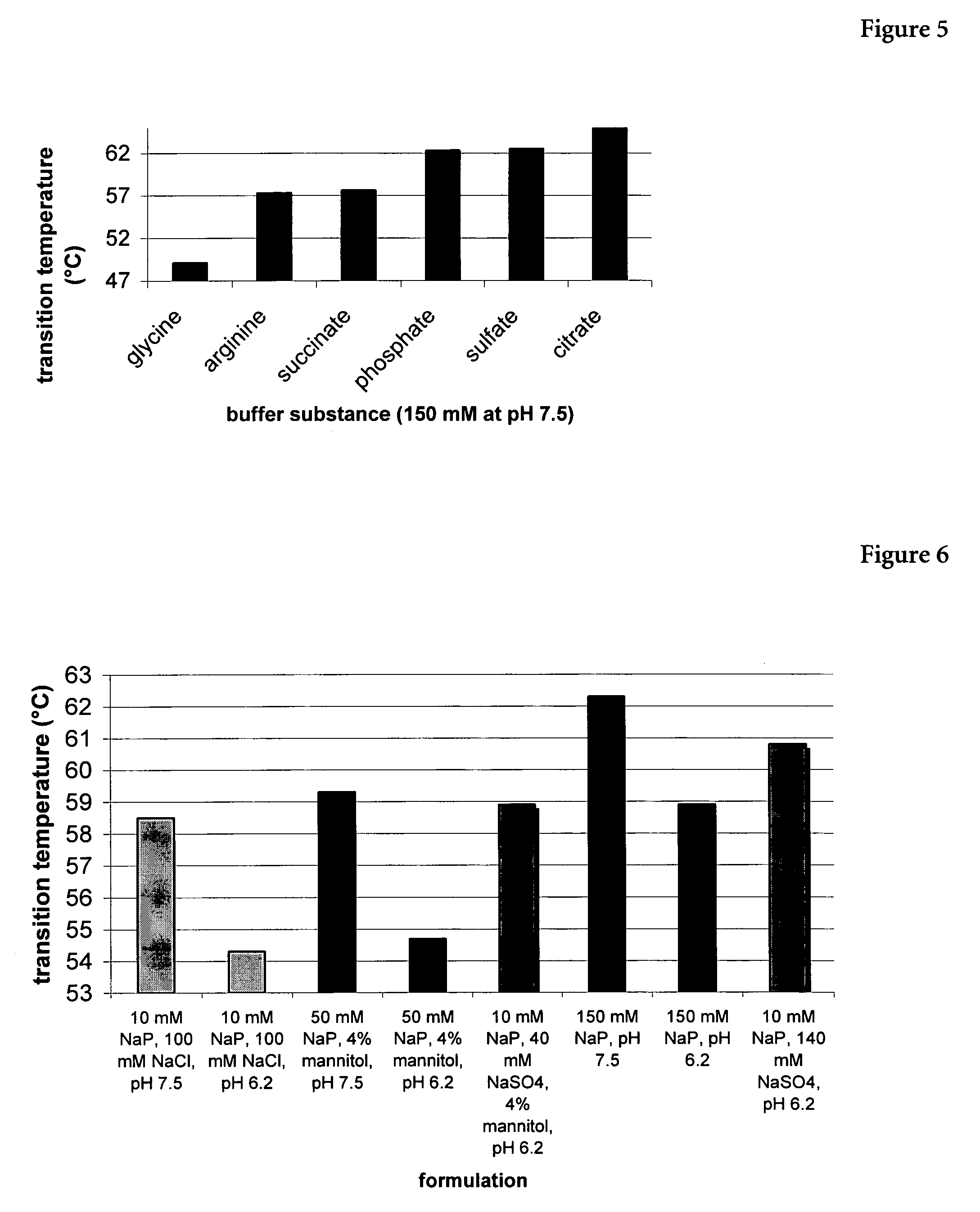
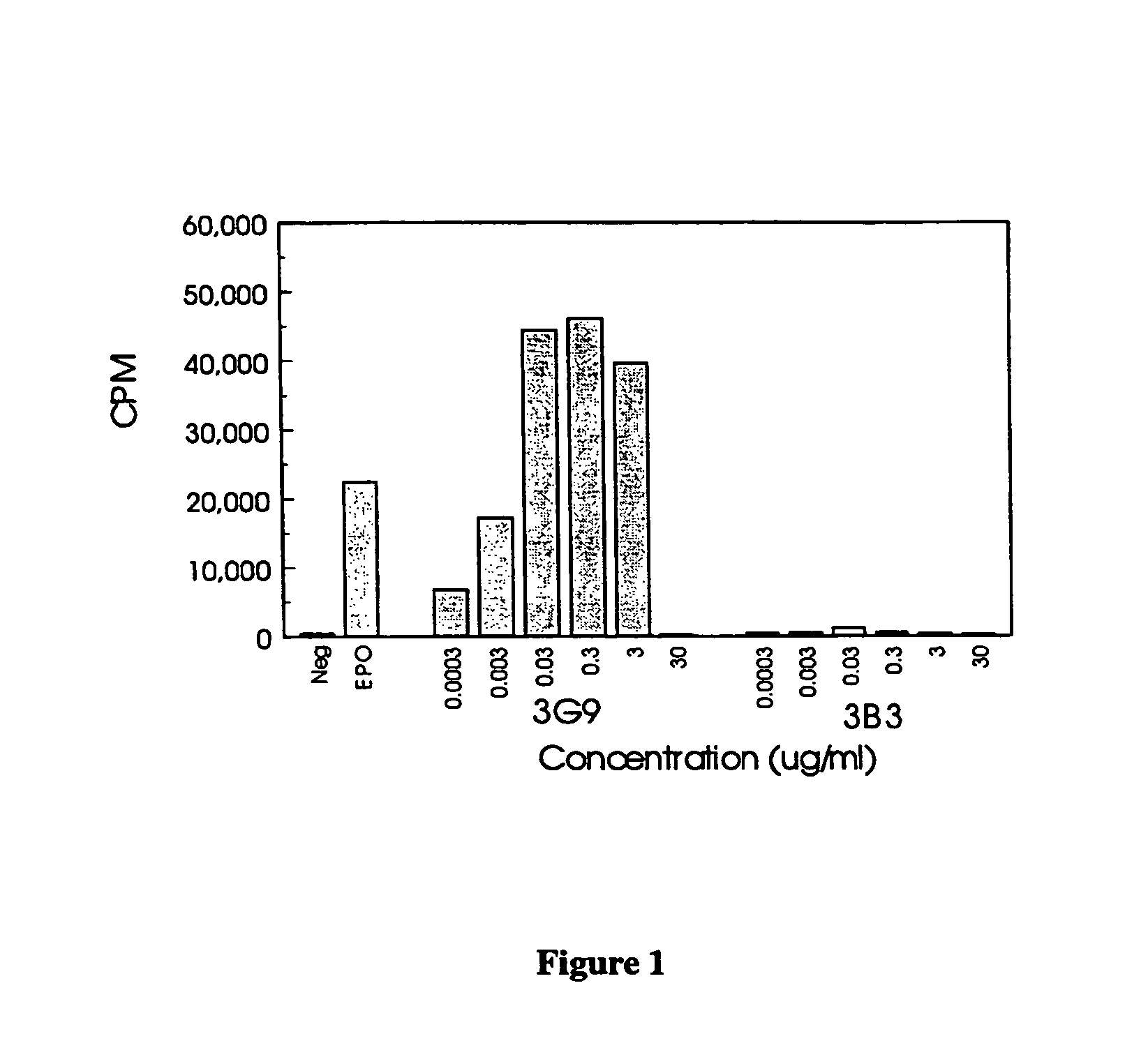
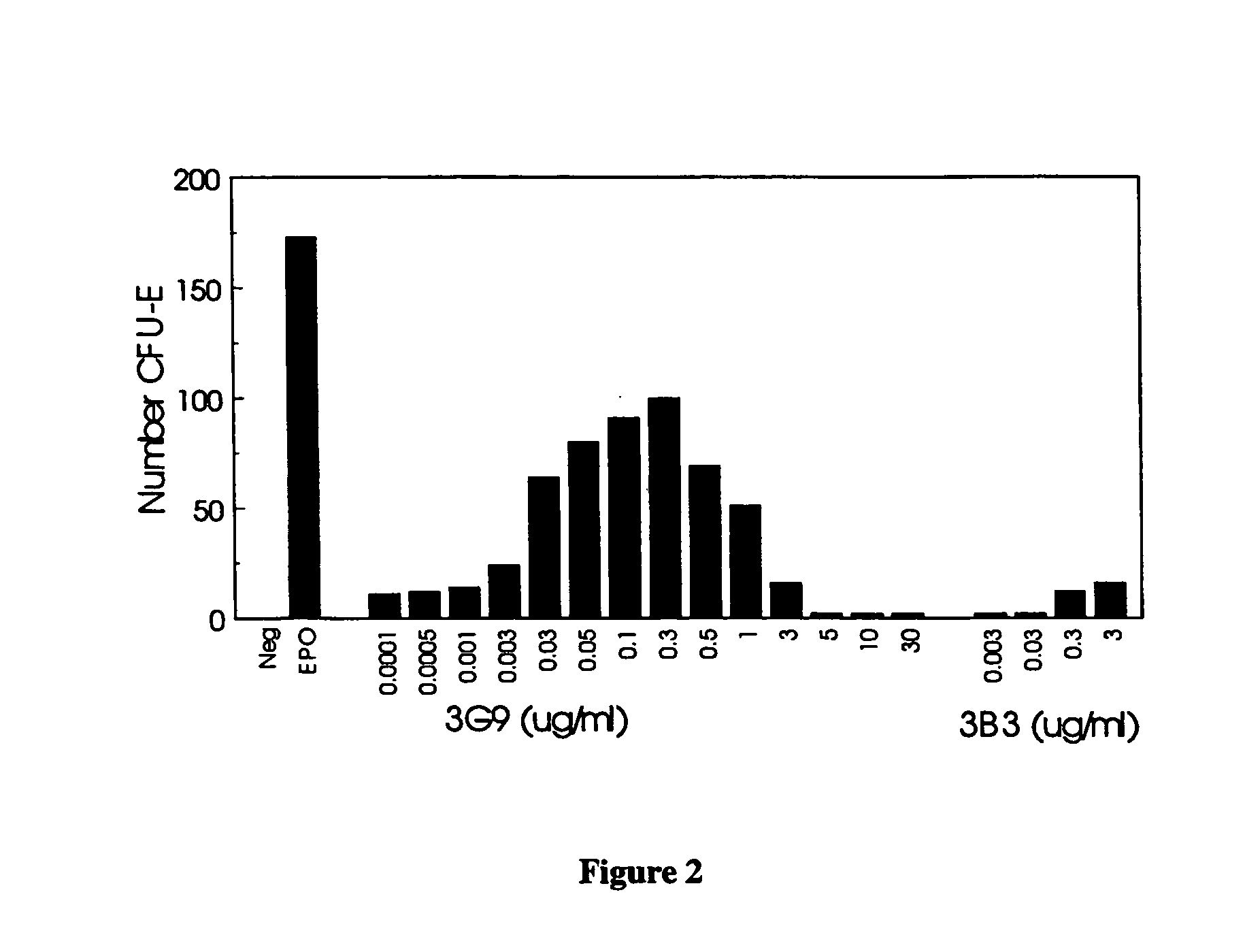
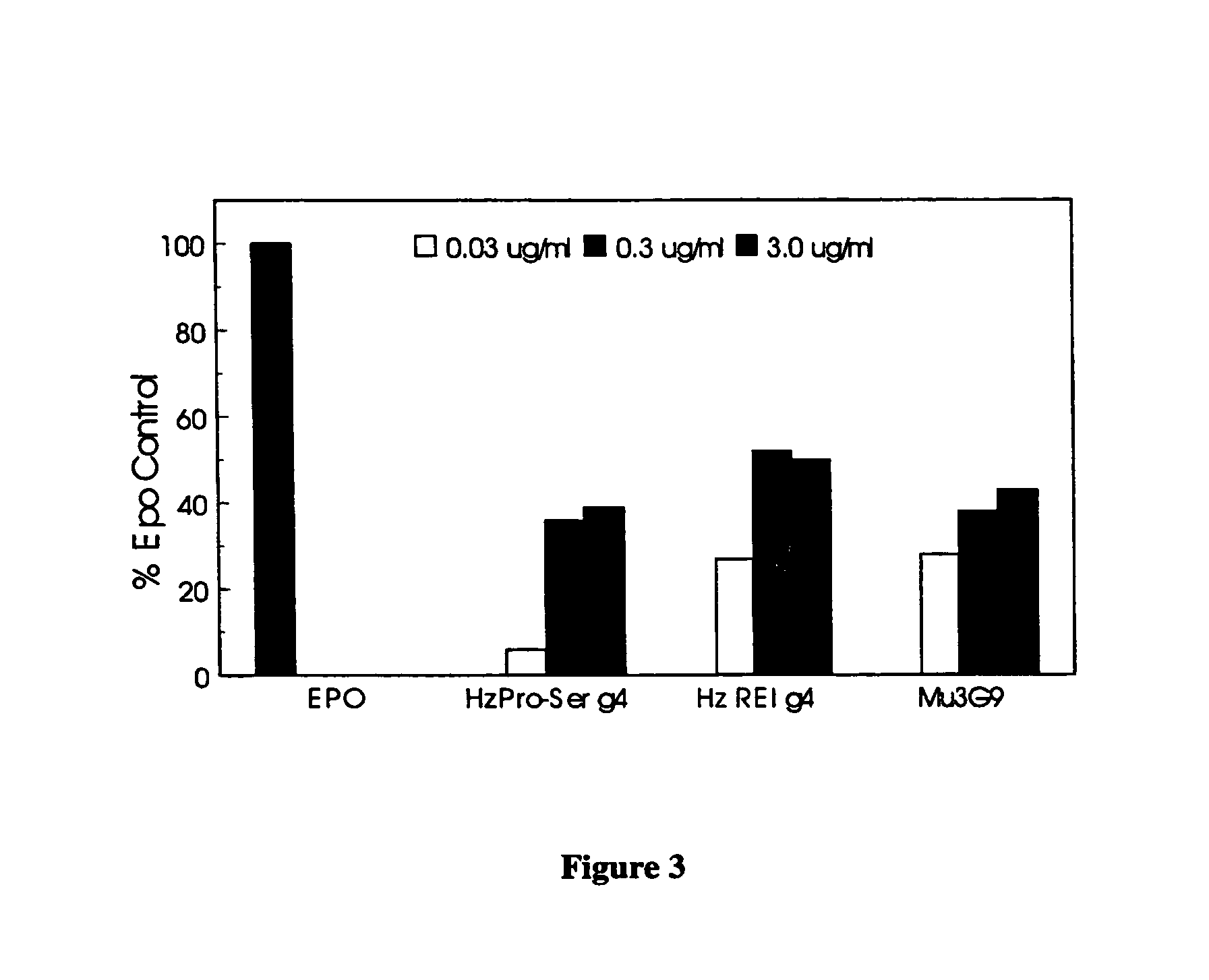
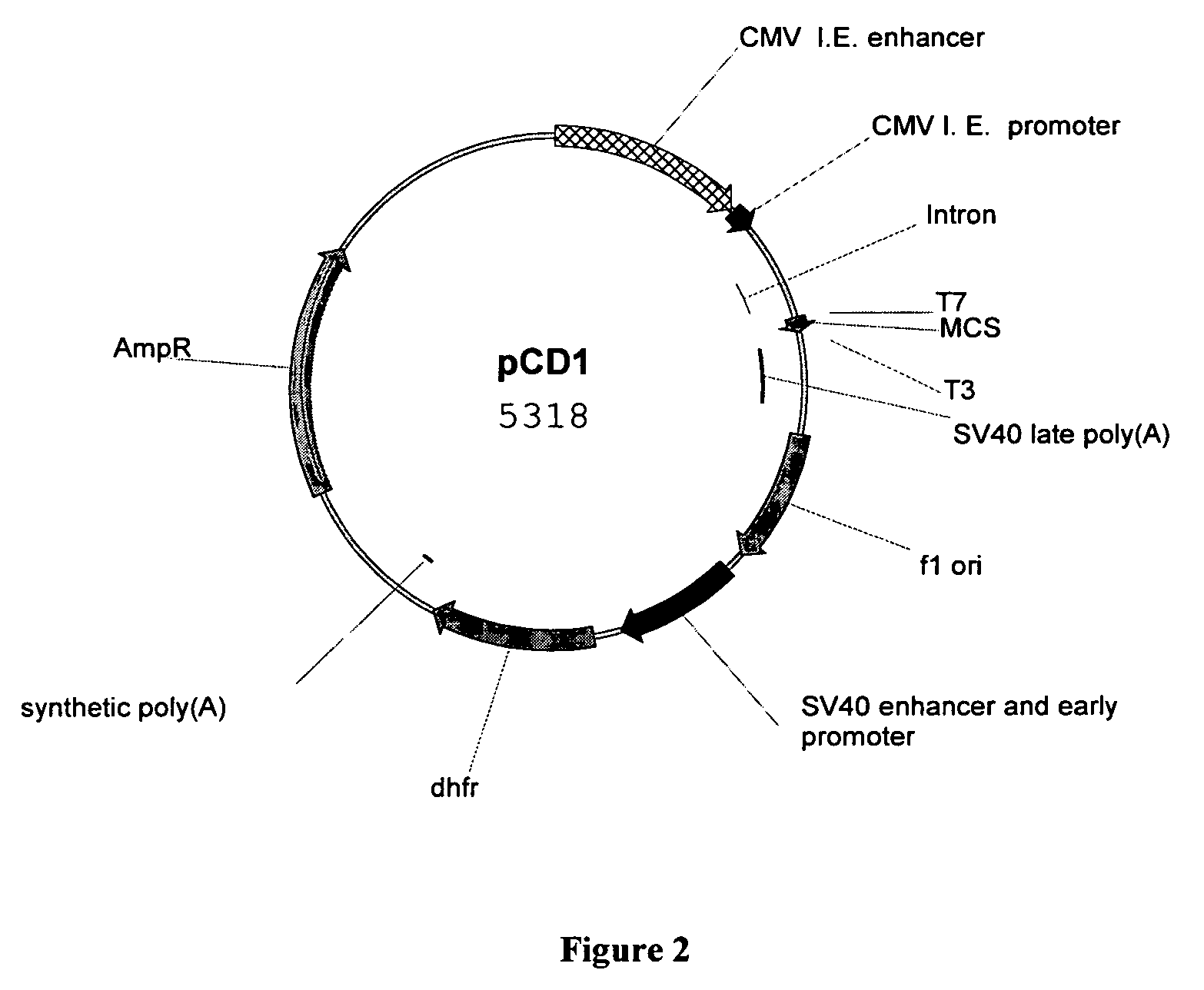
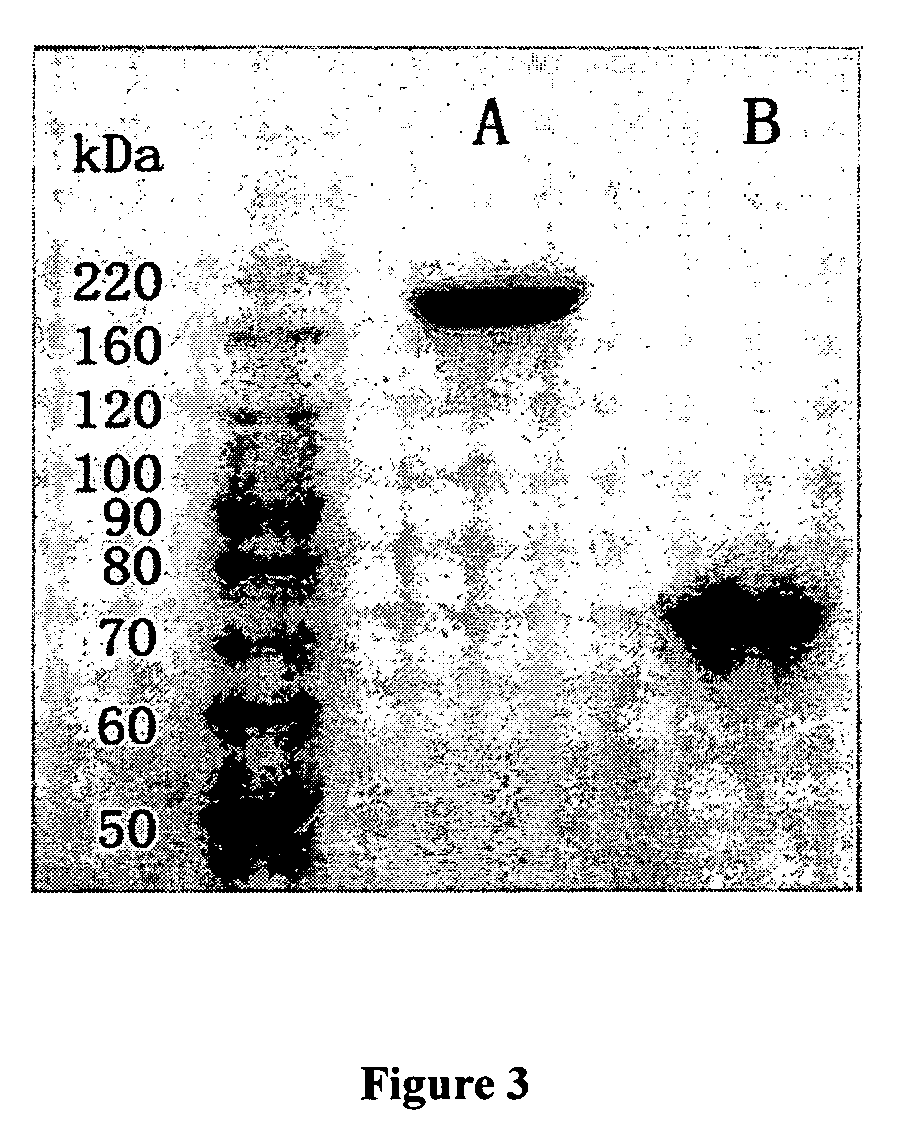
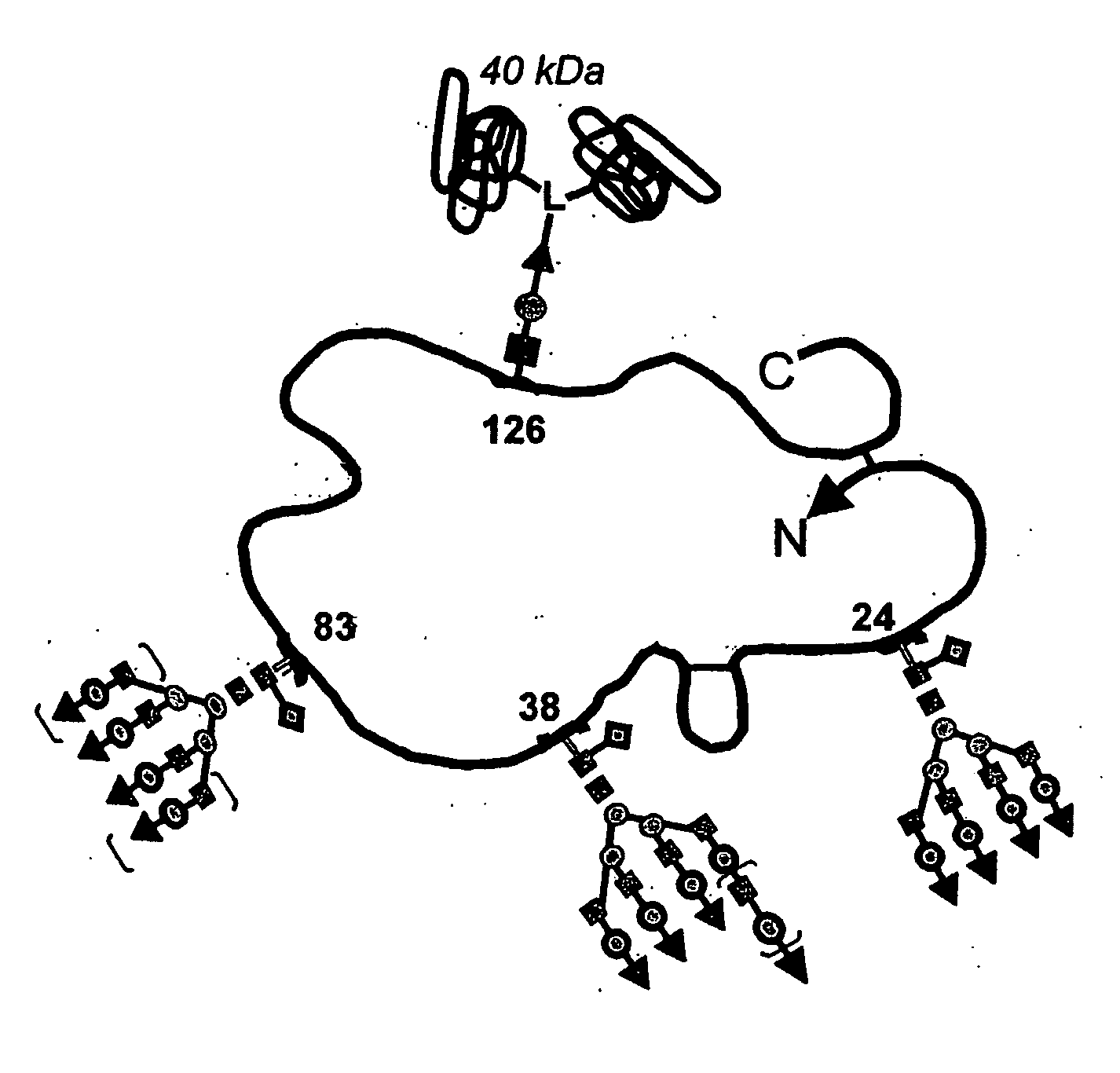
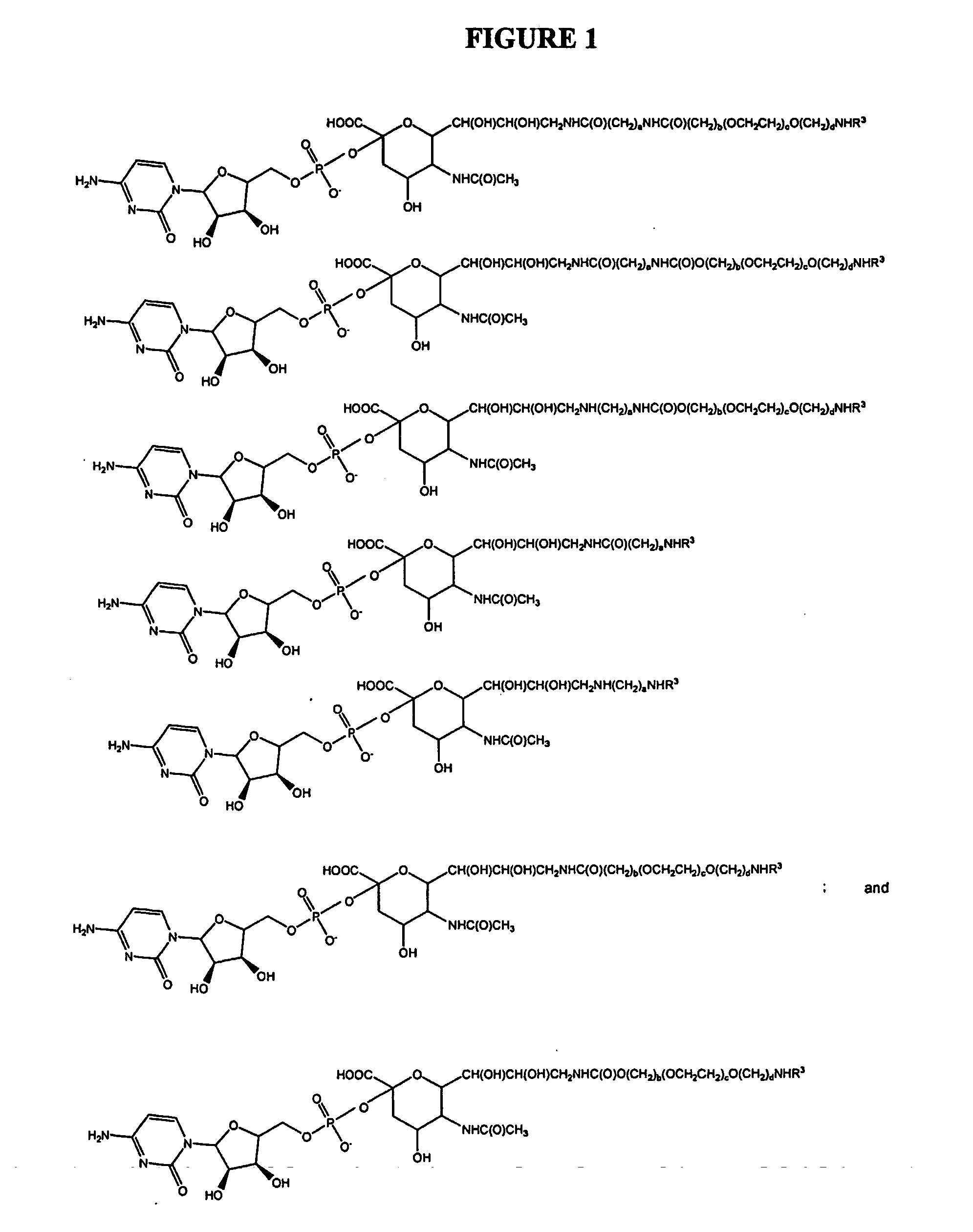
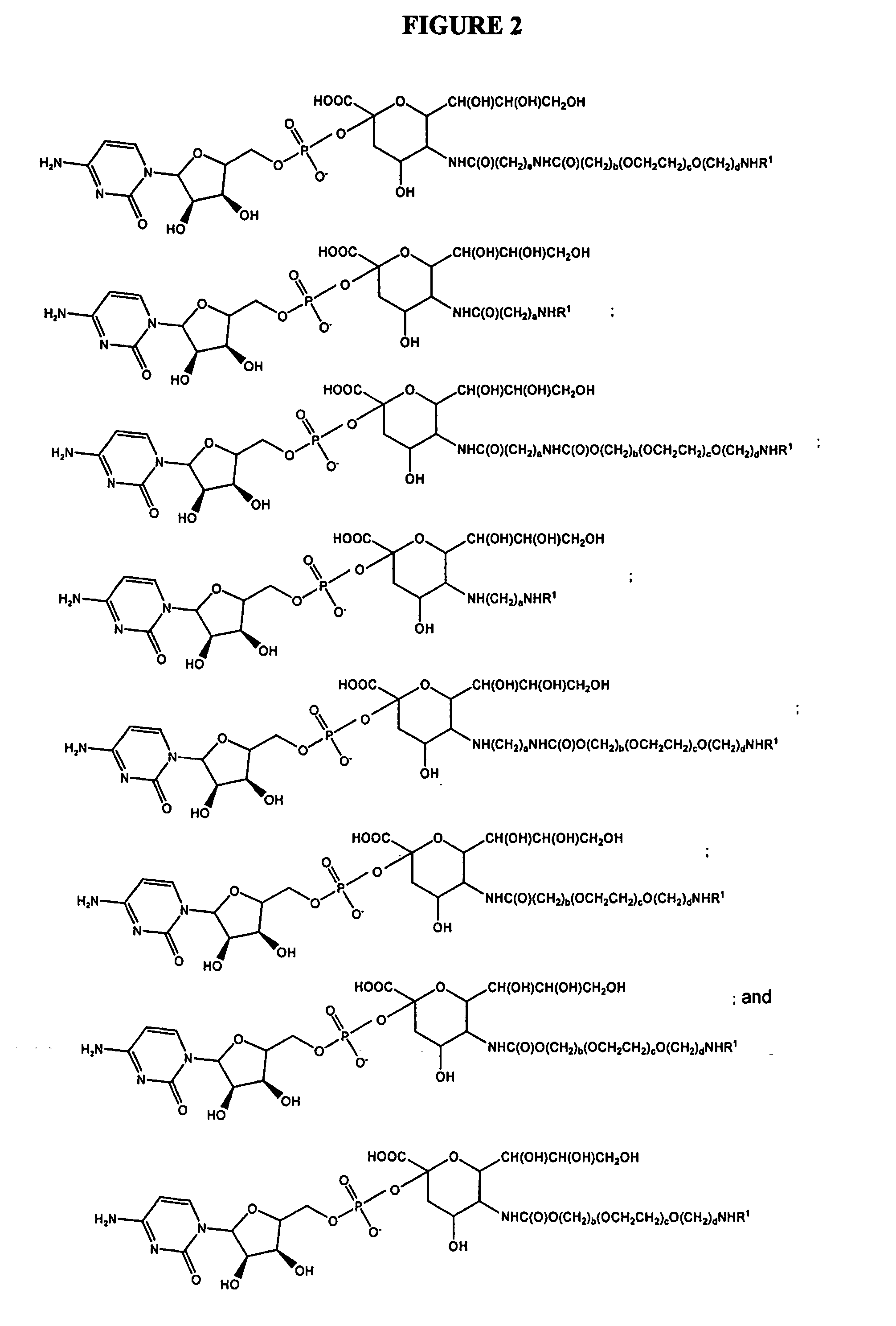
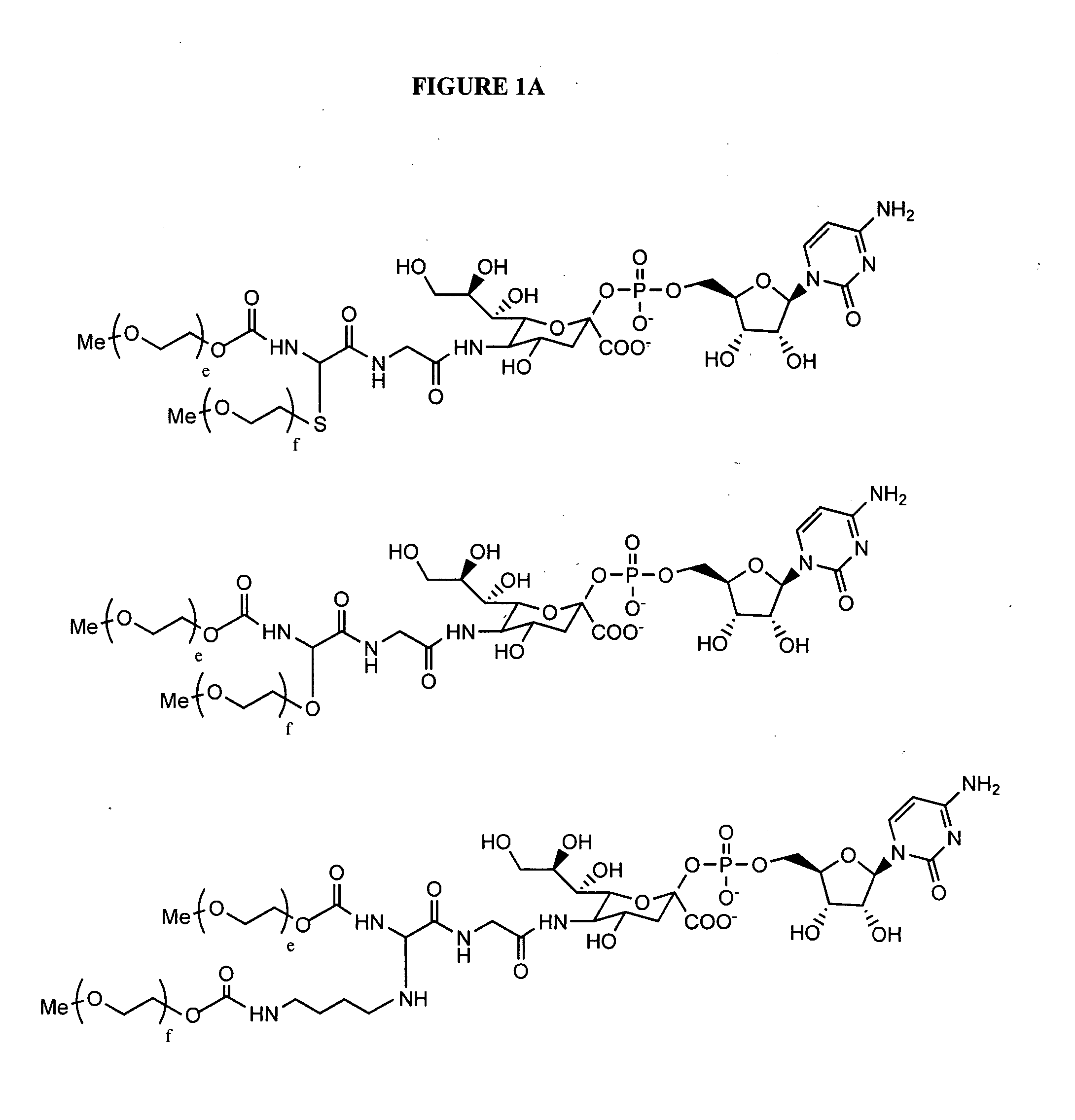
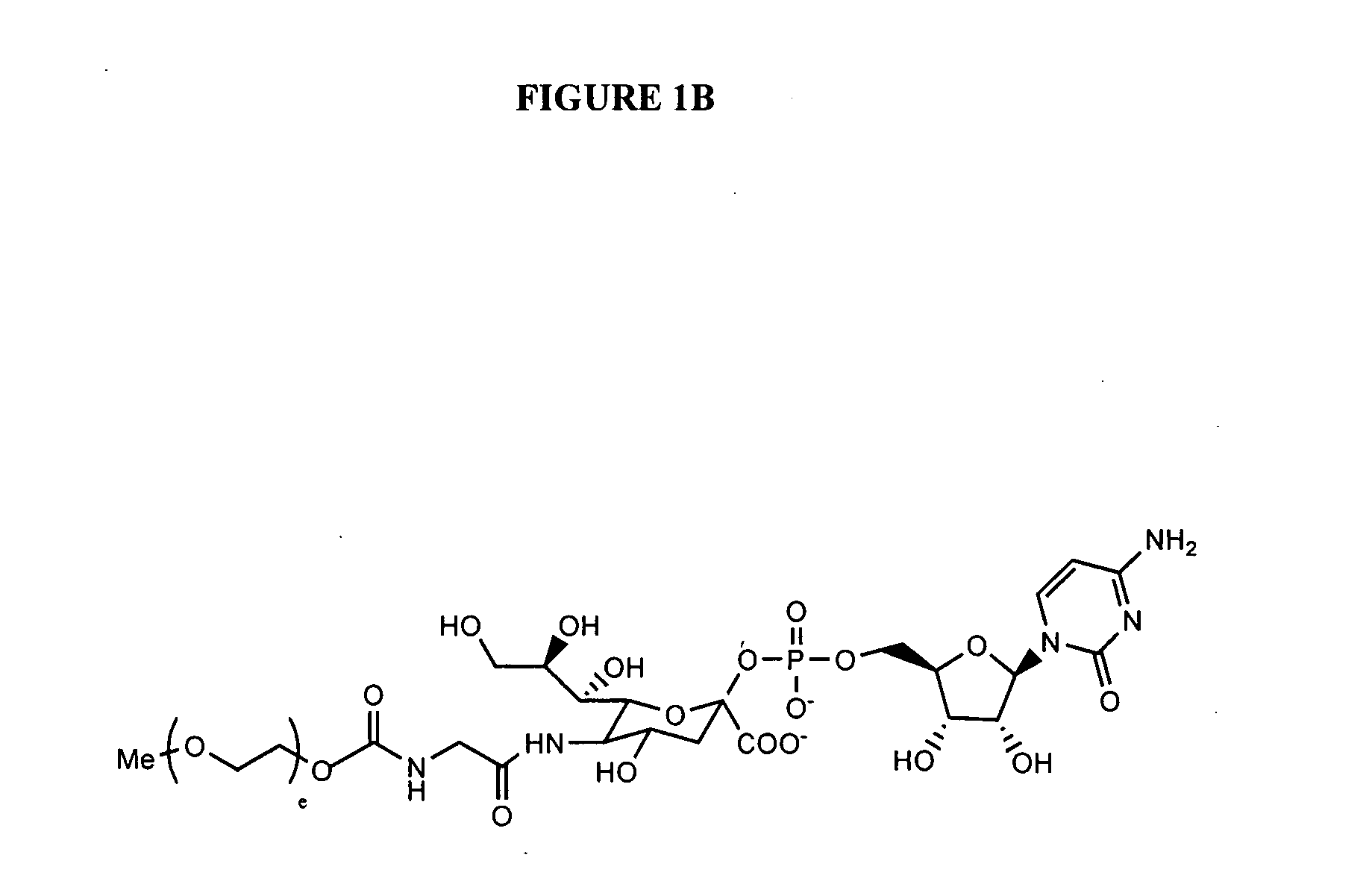
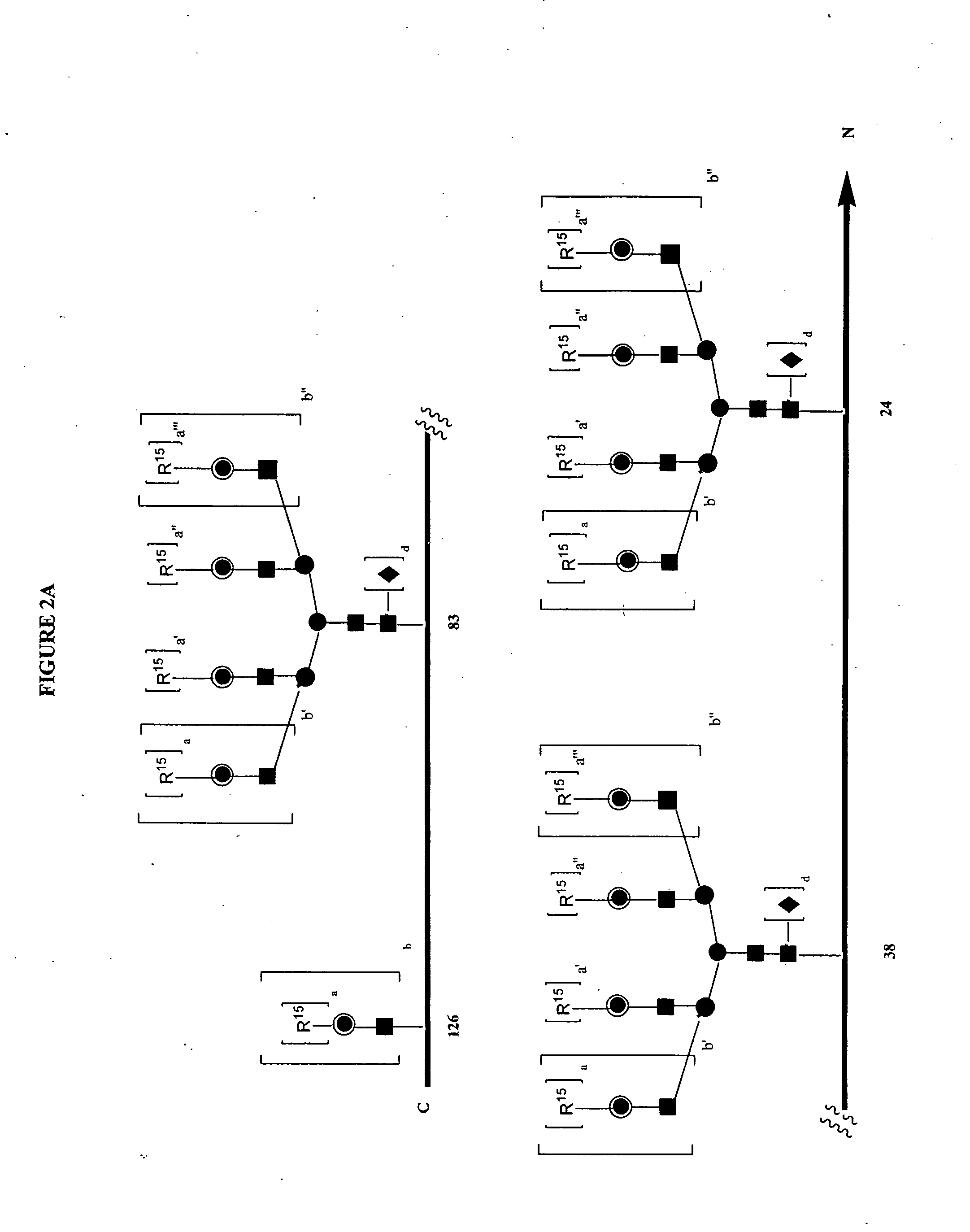
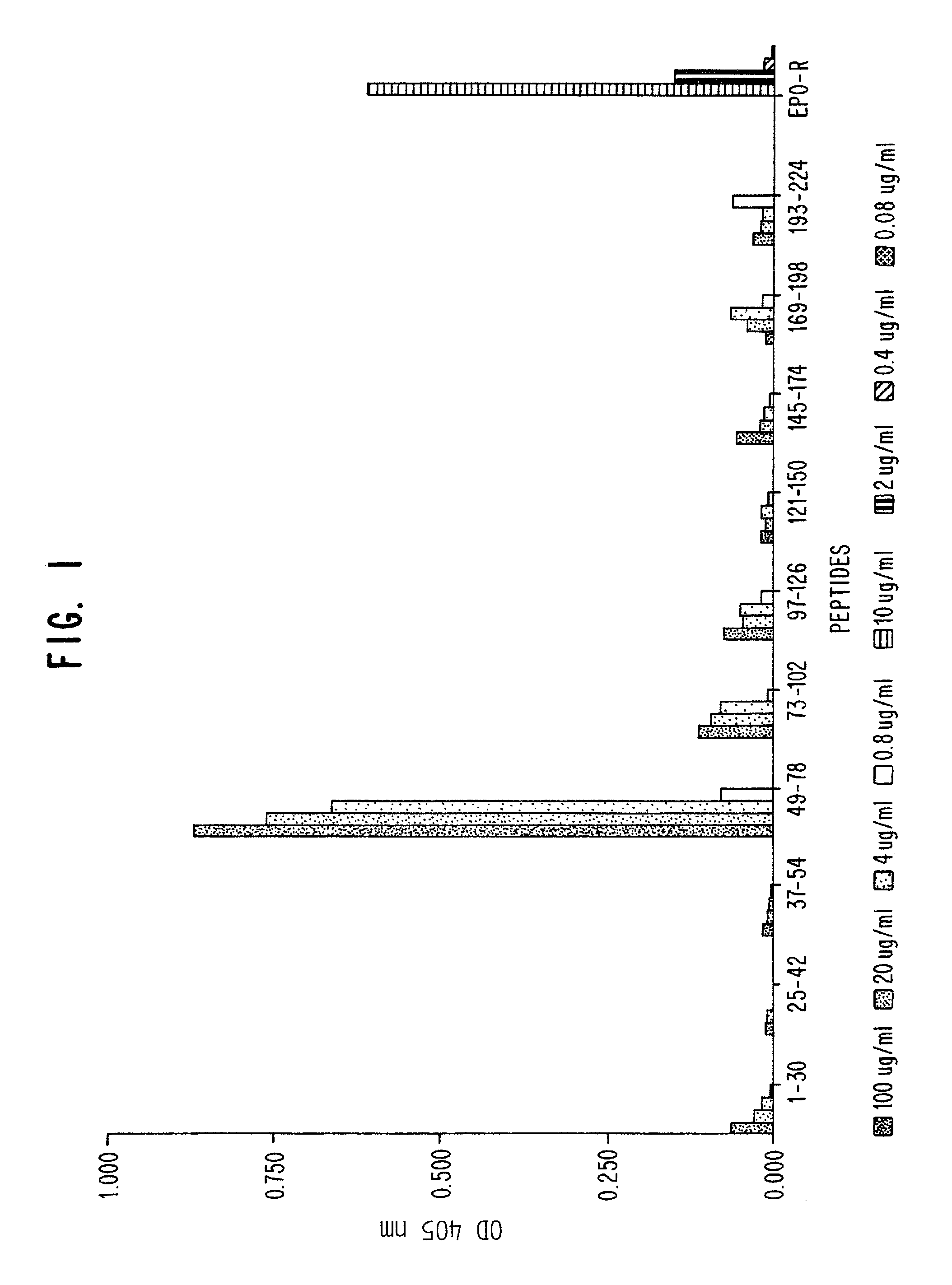
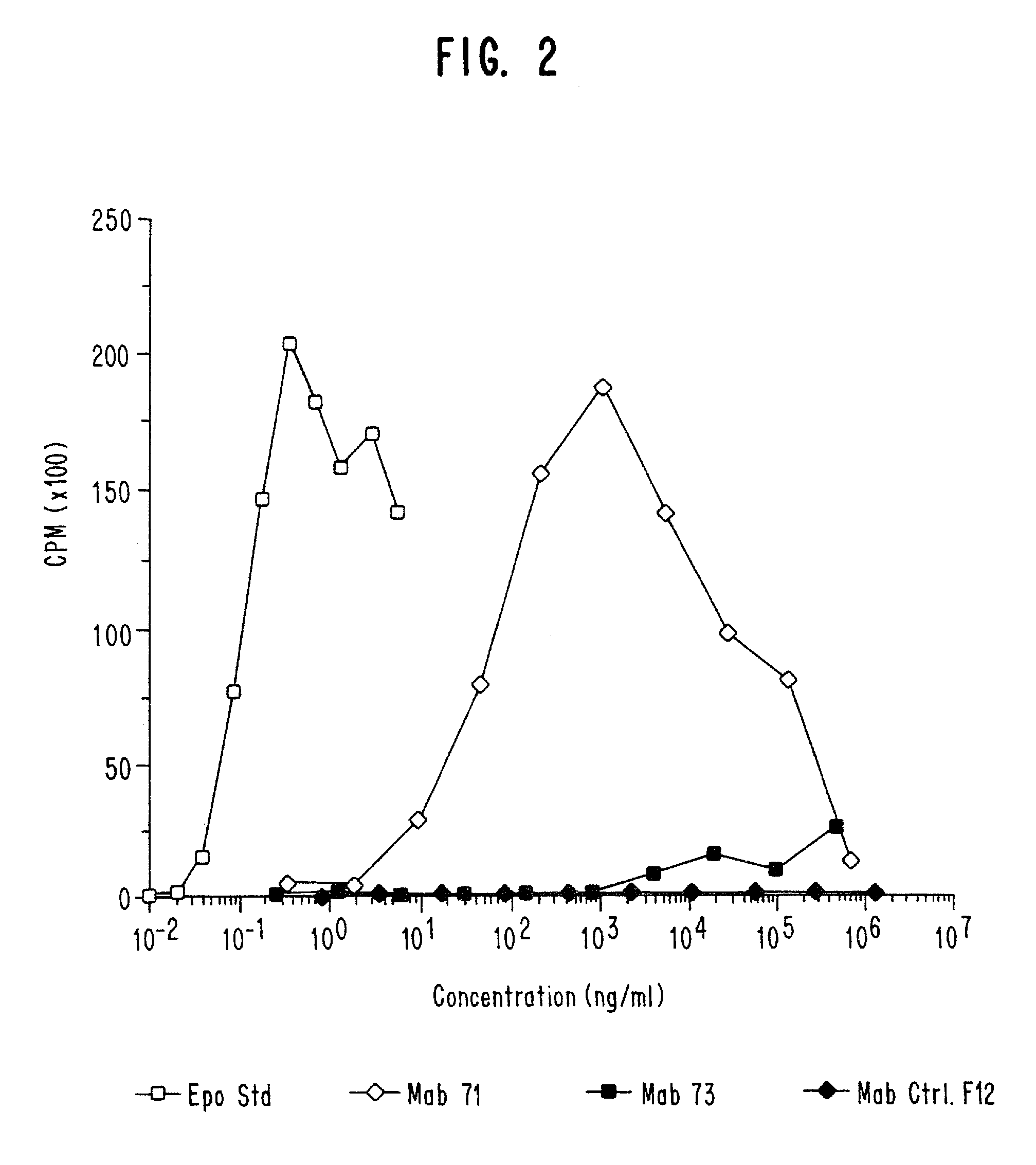
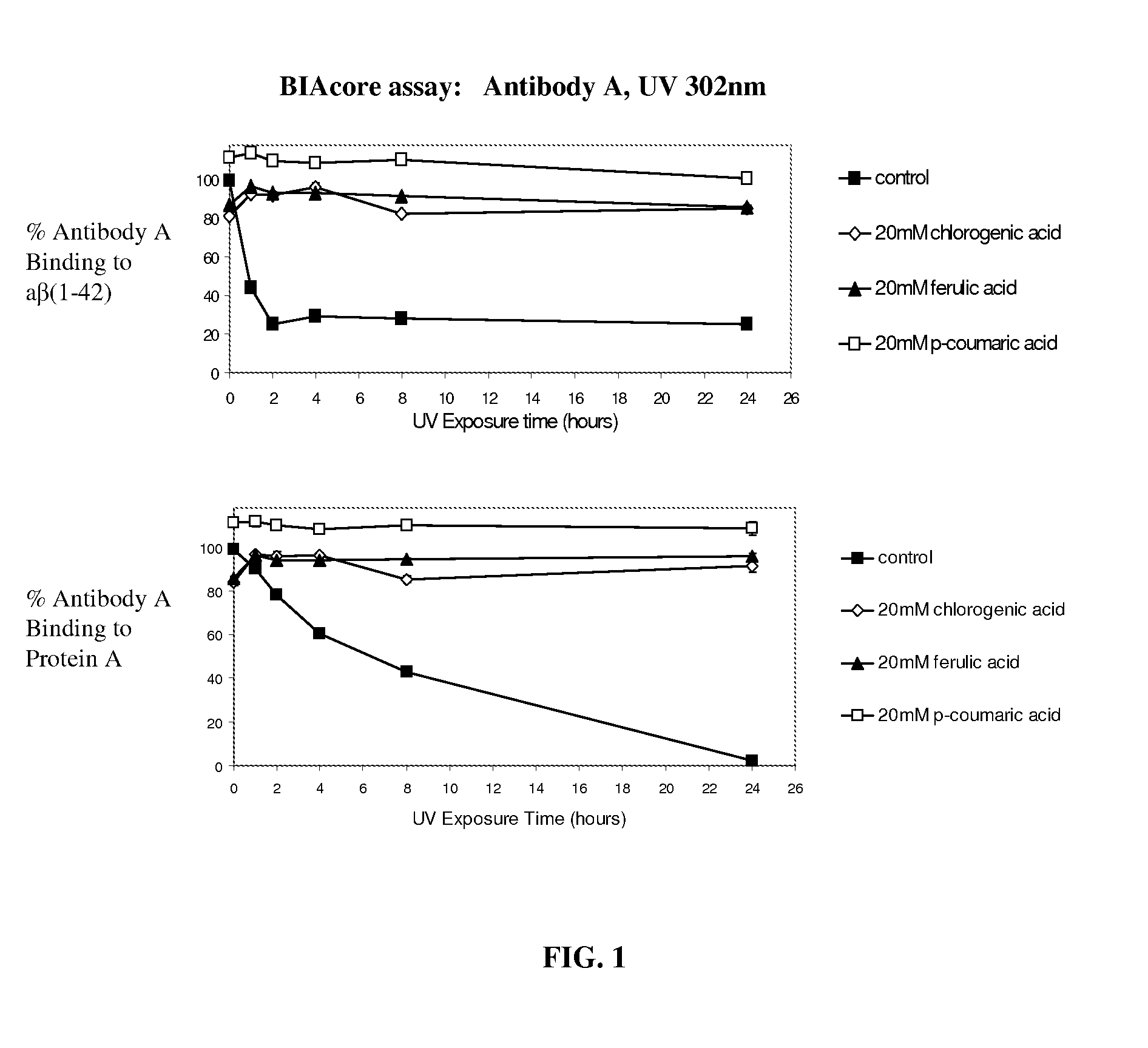
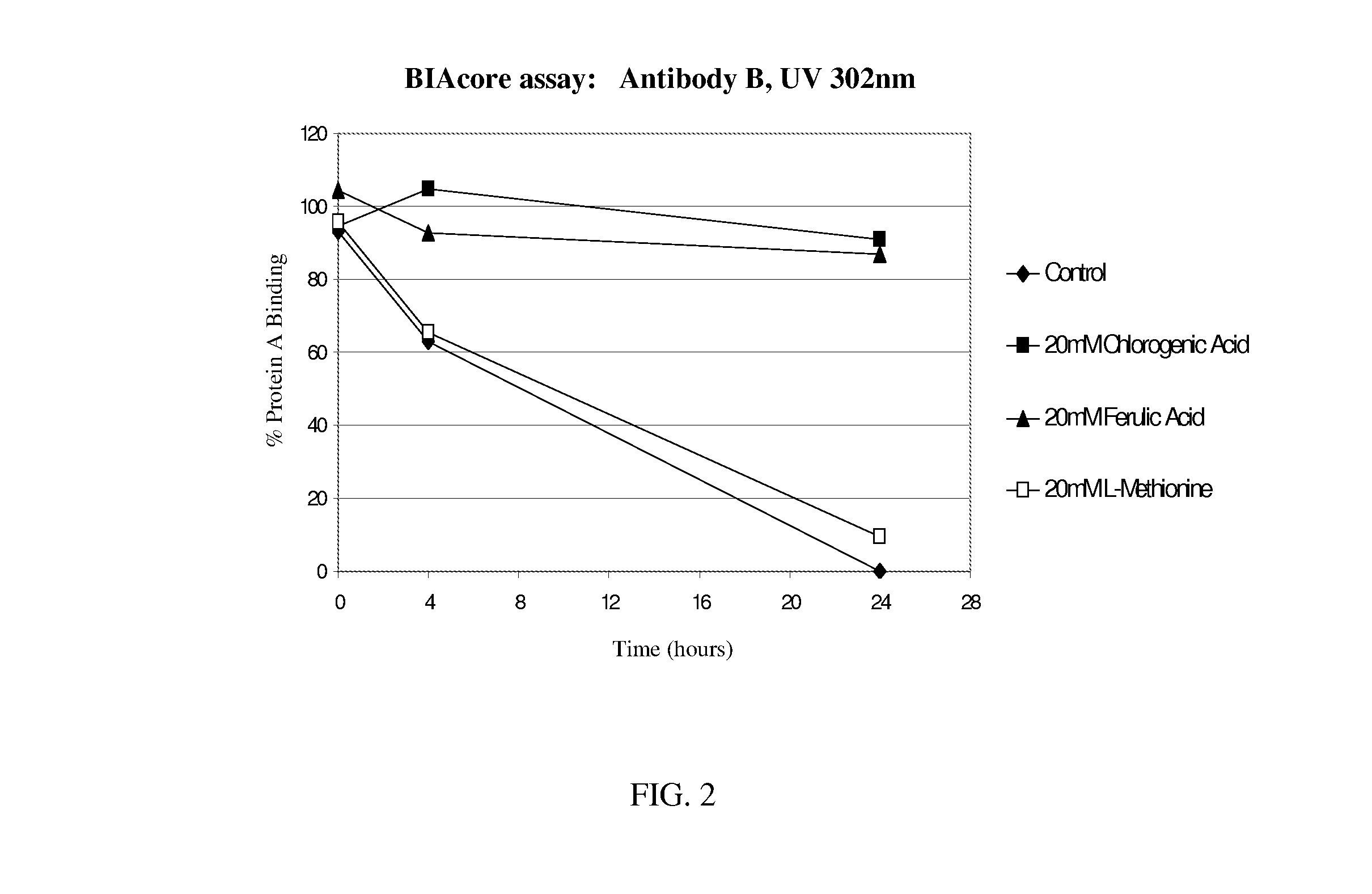
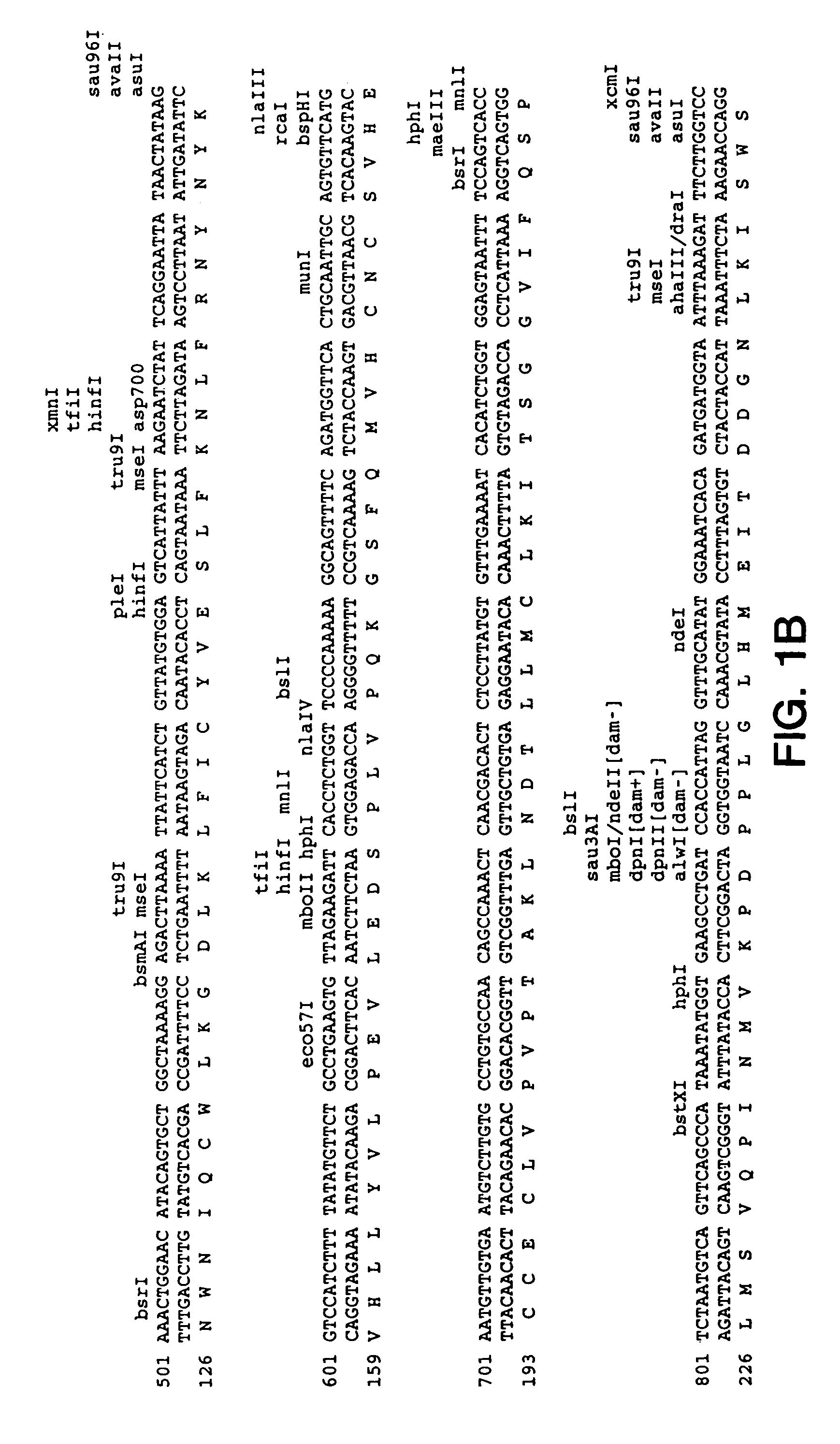
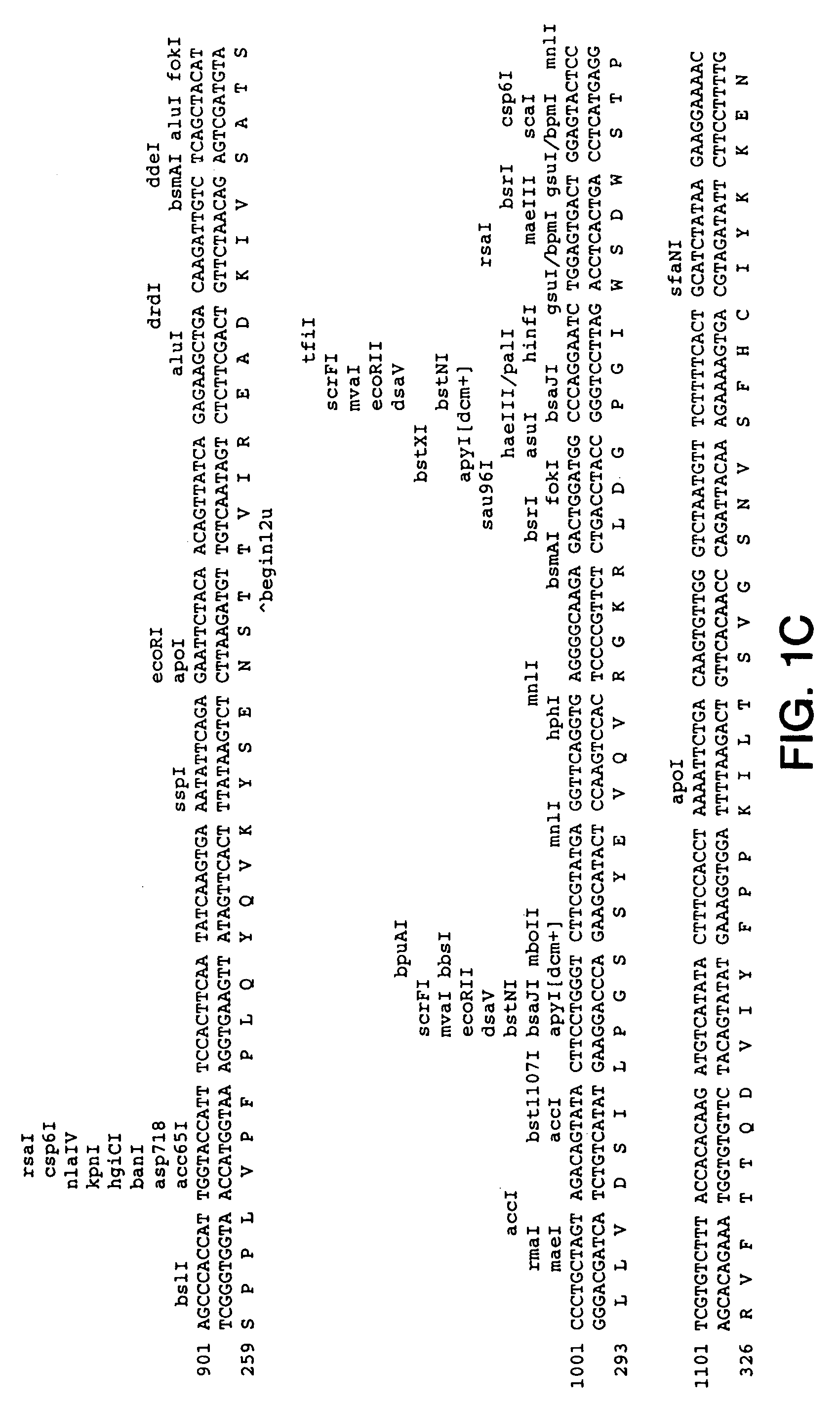
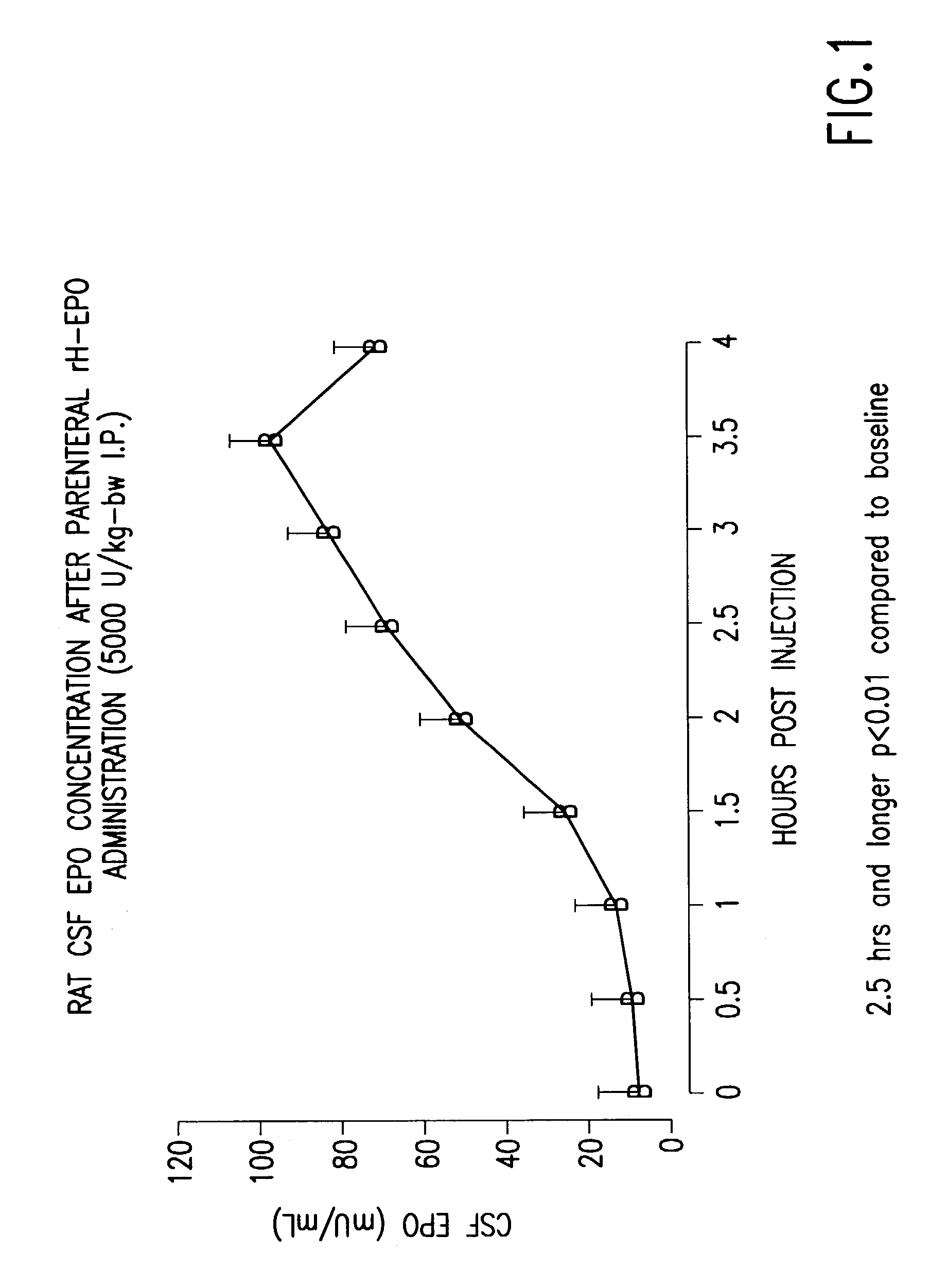
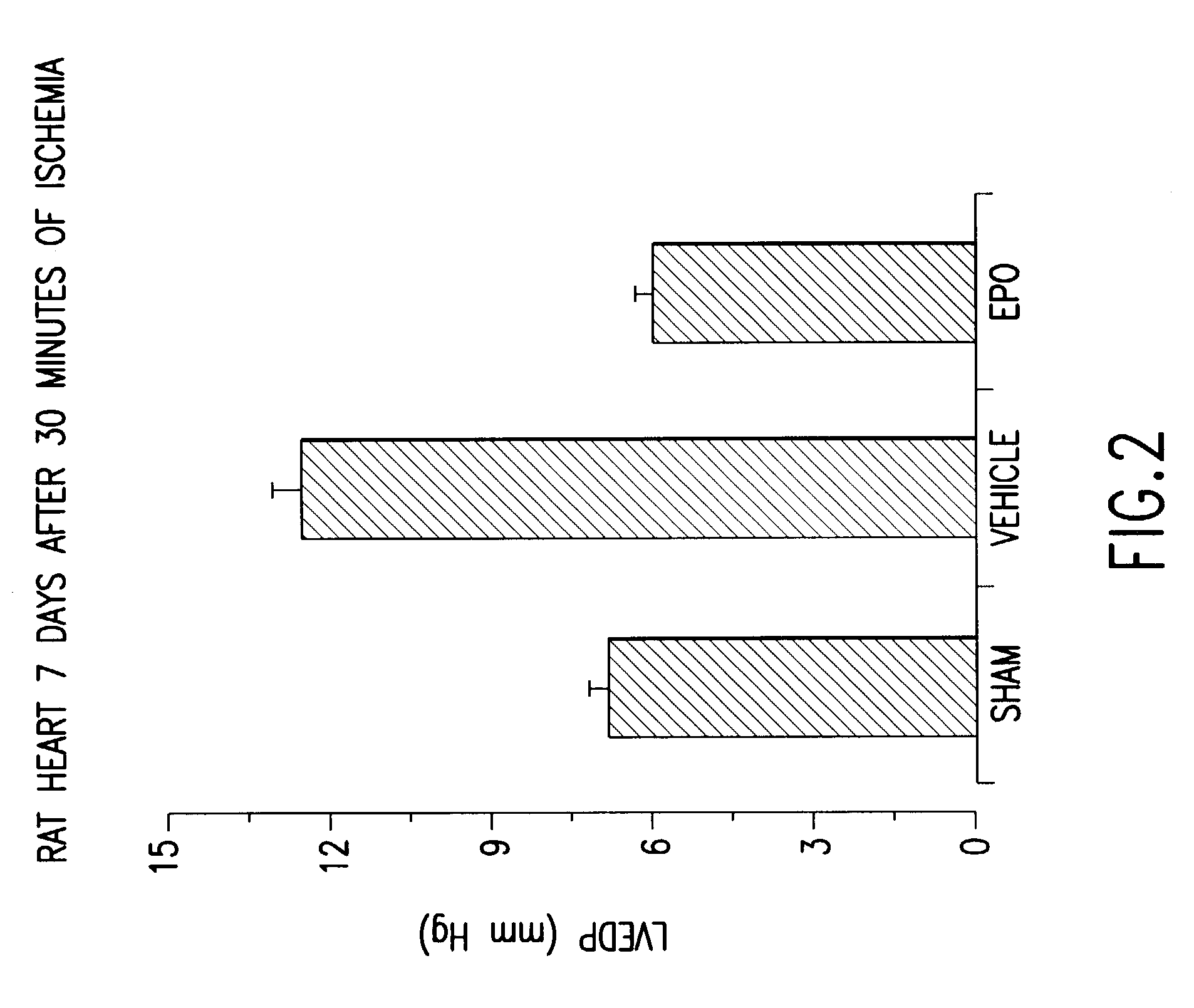
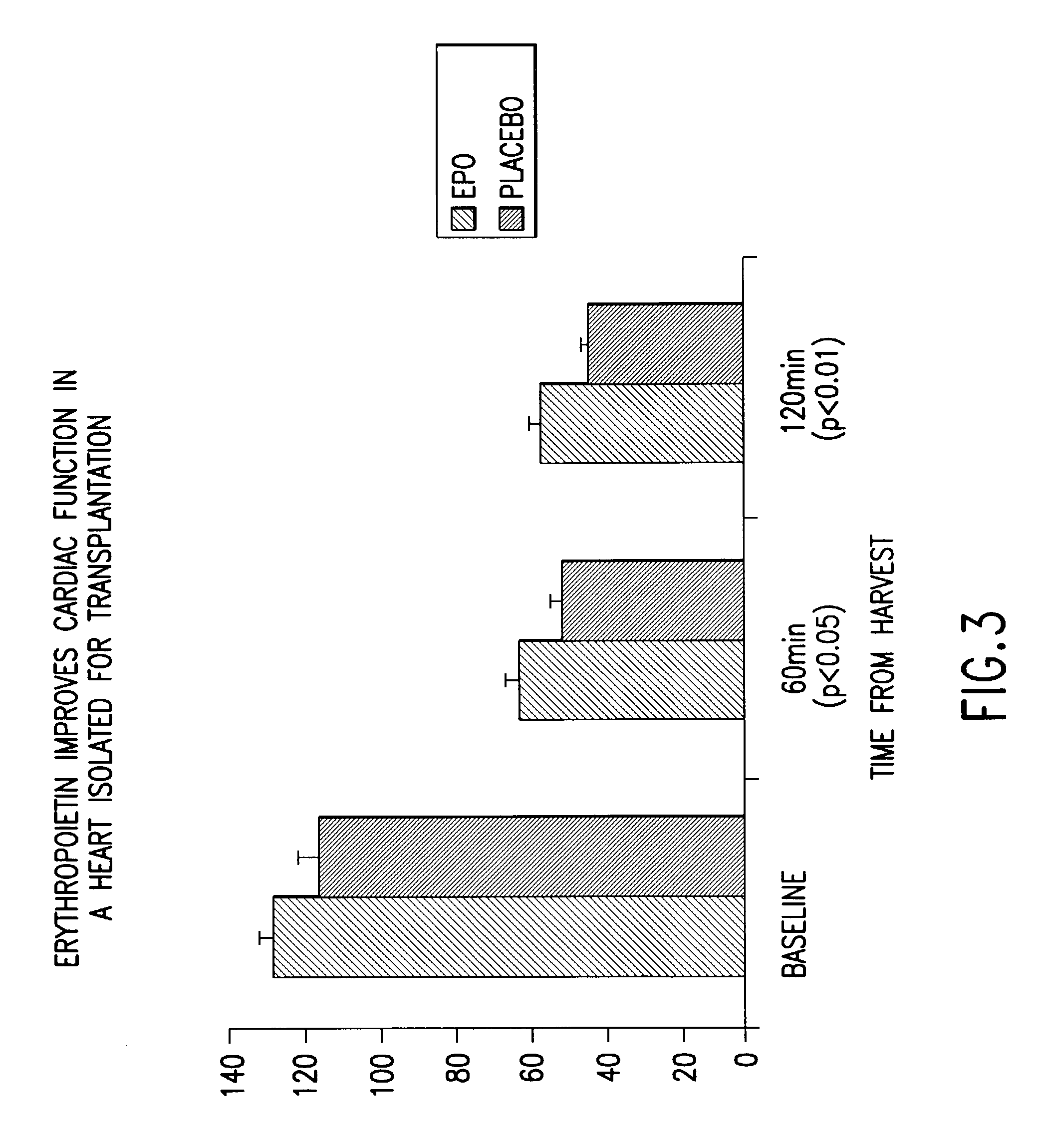
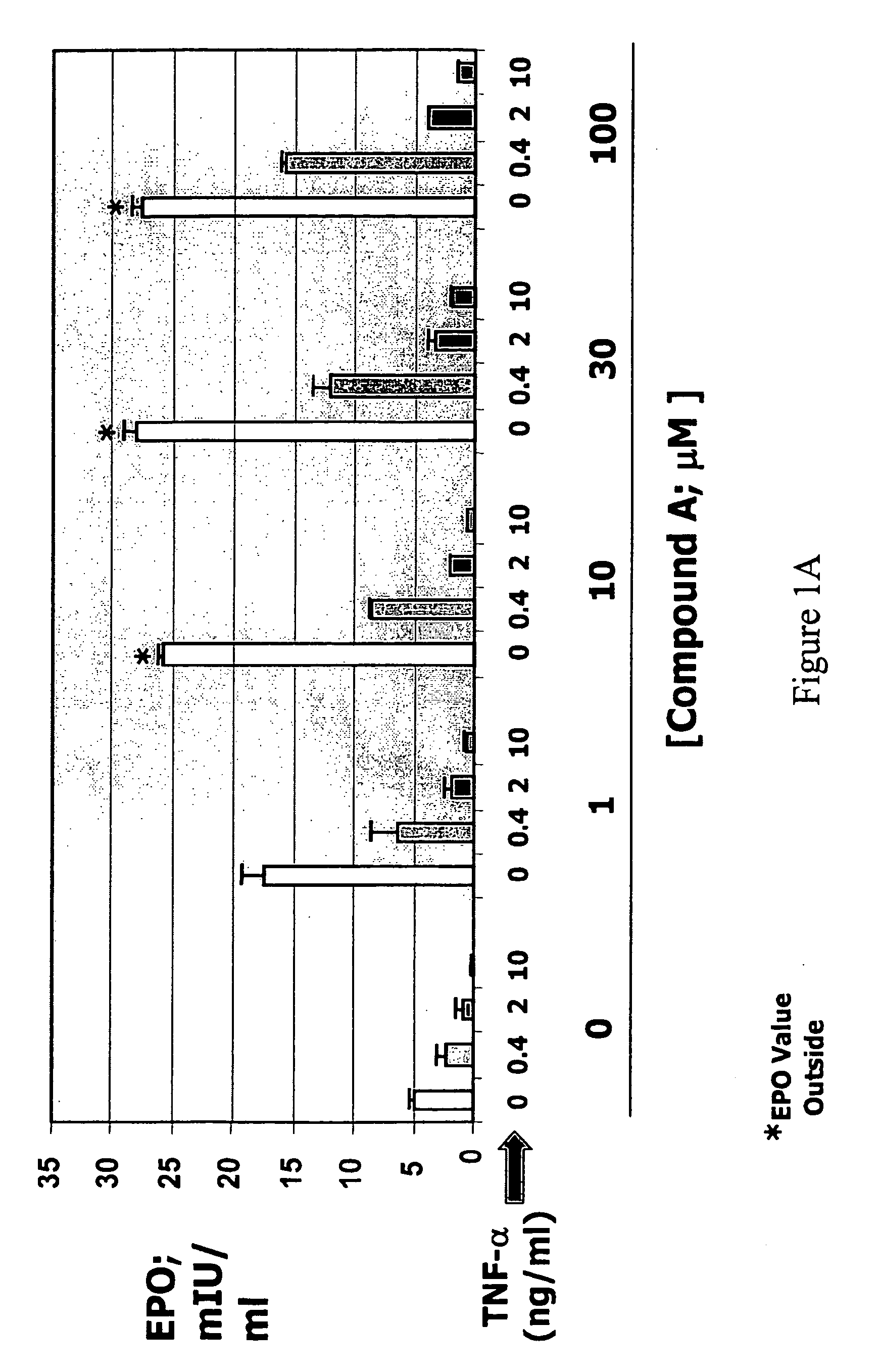
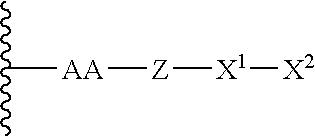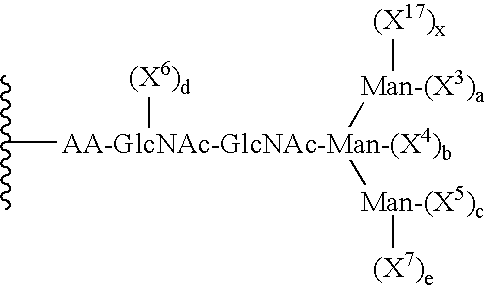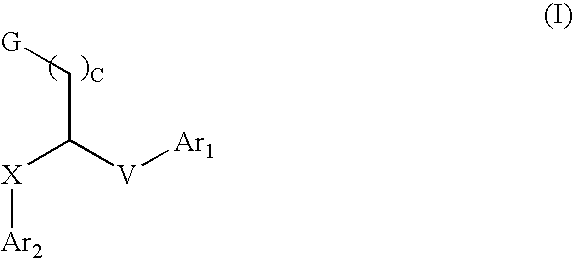Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Erythropoiesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Erythropoiesis (from Greek 'erythro' meaning "red" and 'poiesis' meaning "to make") is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.
Erythropoietin: remodeling and glycoconjugation of erythropoietin
InactiveUS20060088906A1Increase hematocrit levelImprove the level ofPeptide/protein ingredientsPeptide preparation methodsErythropoiesisErythropoietin
Owner:NEOSE TECH
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Erythropoietin receptor antibodies
InactiveUS20050244409A1Increased erythropoiesisAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsErythropoietin receptorMedicine
Erythropoietin receptor agonist and antagonist antibodies and their use in enhancing erythropoiesis are disclosed.
Owner:SMITHKLINE BECKMAN CORP
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
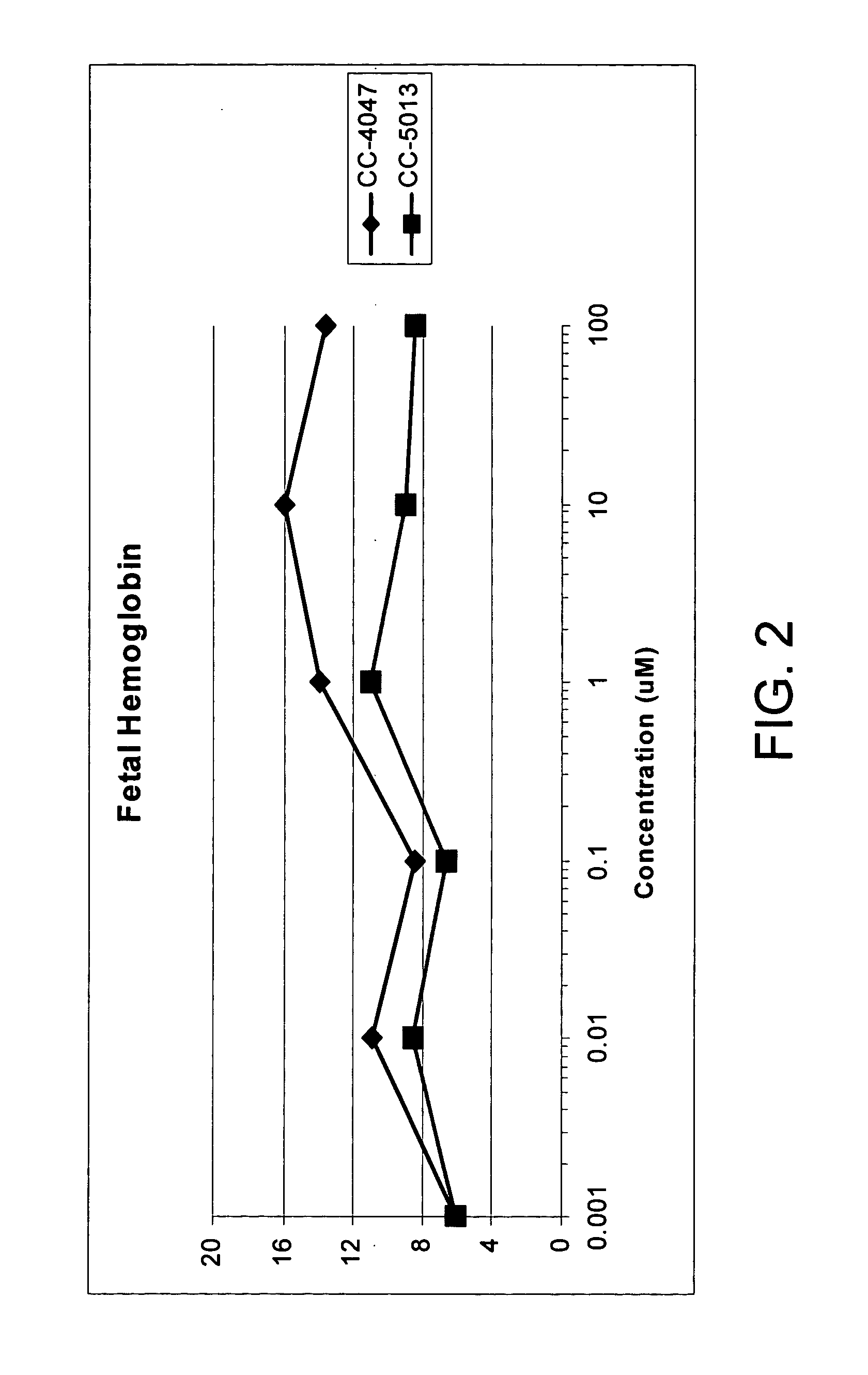
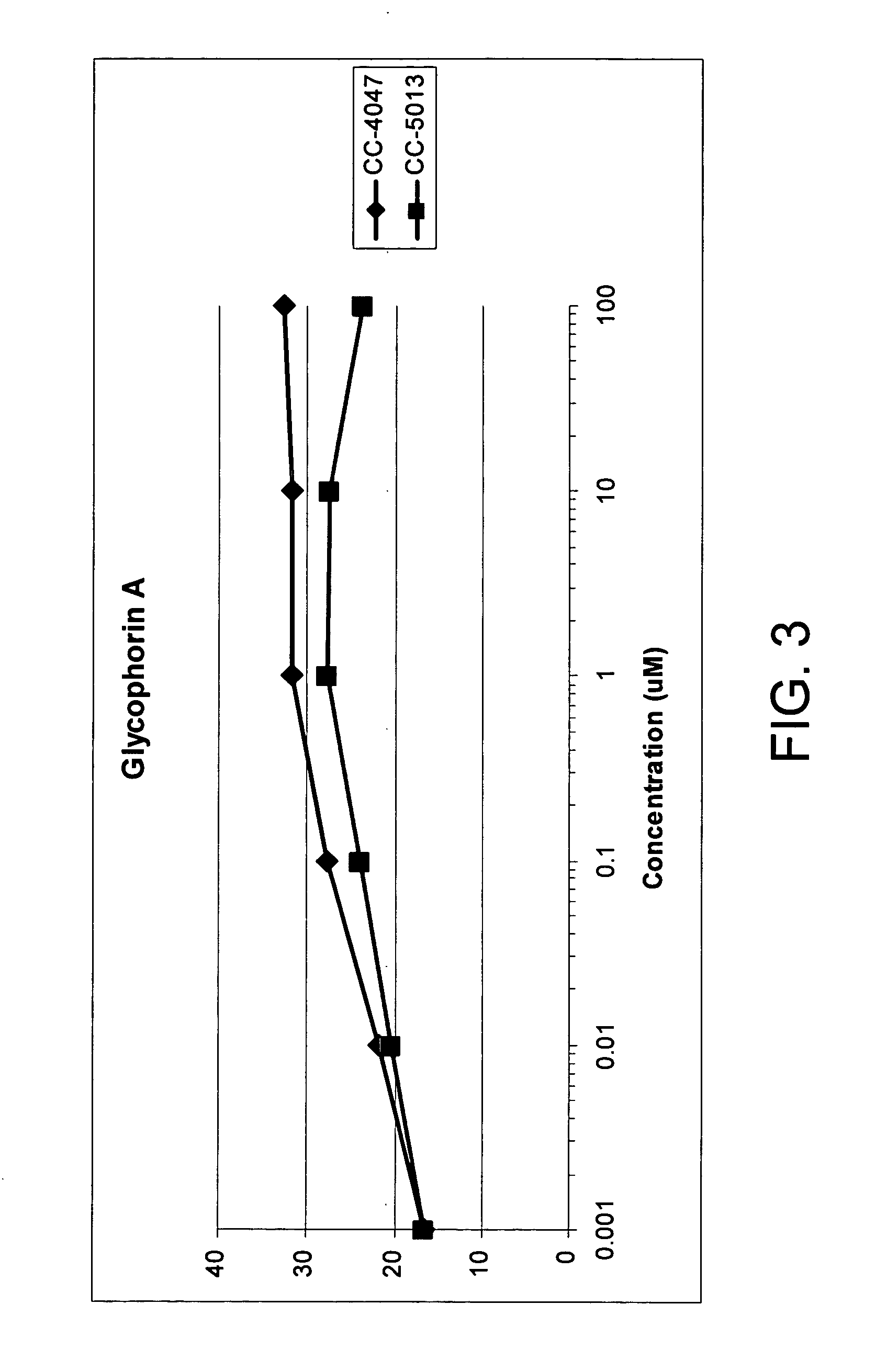
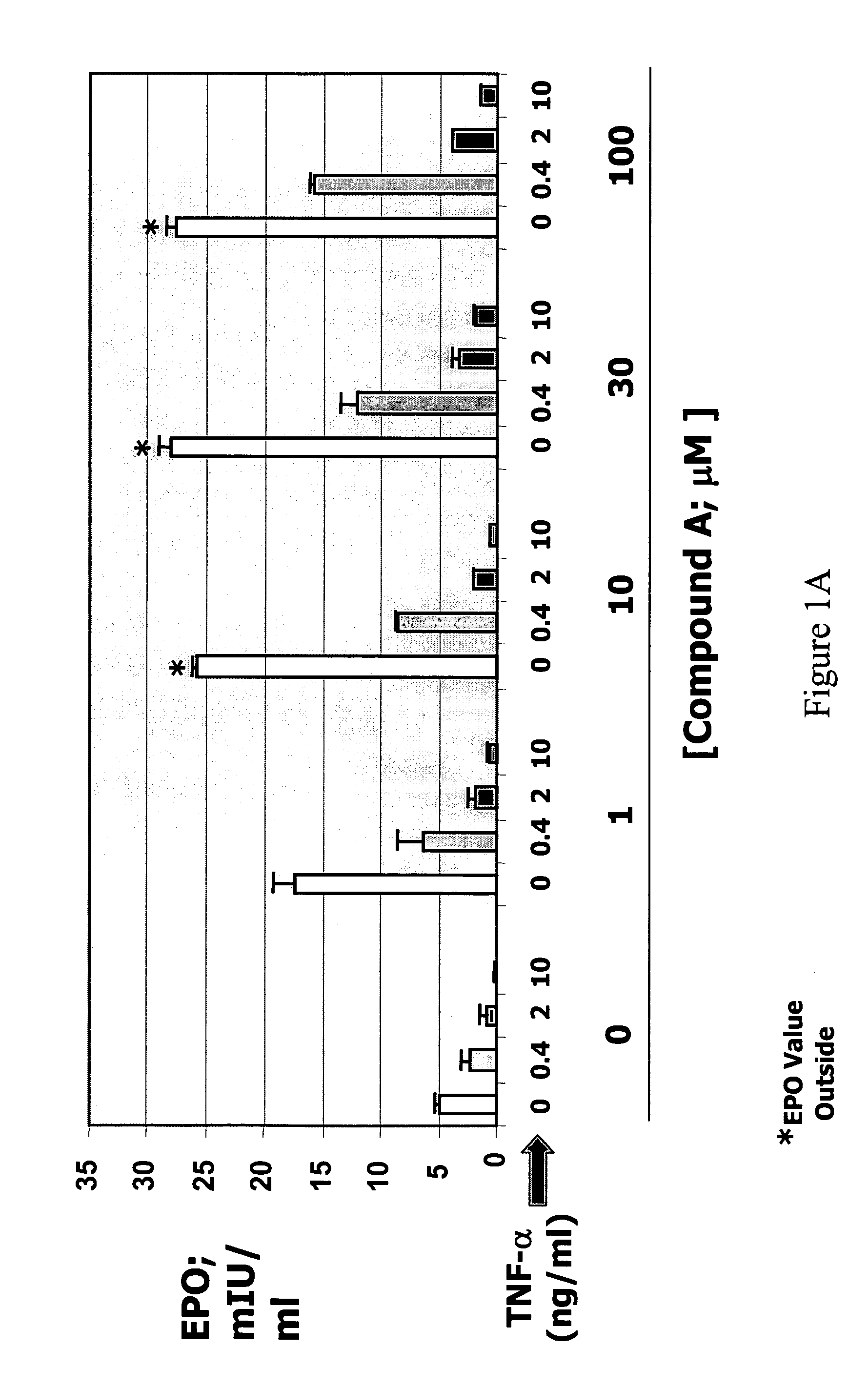
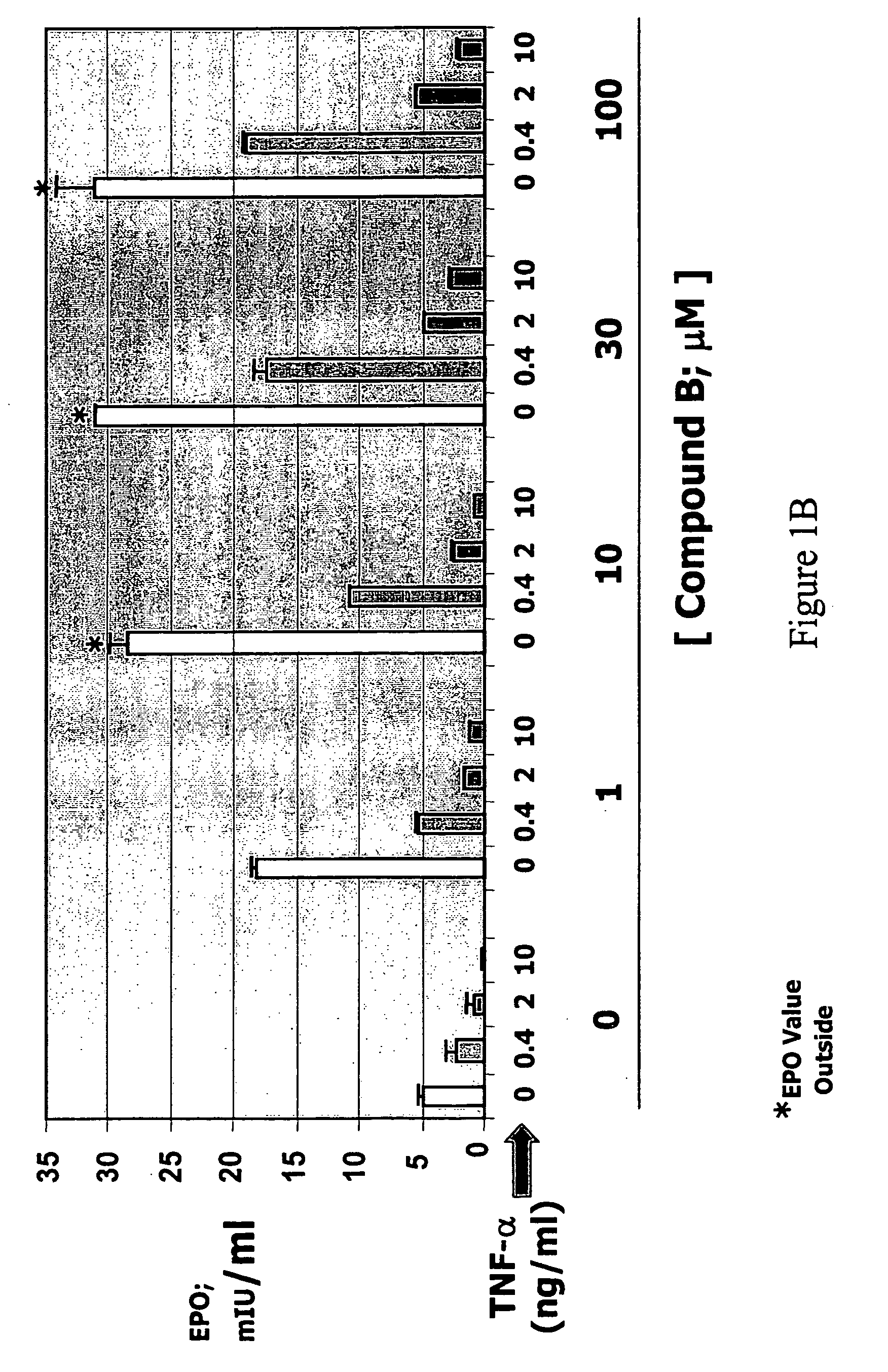
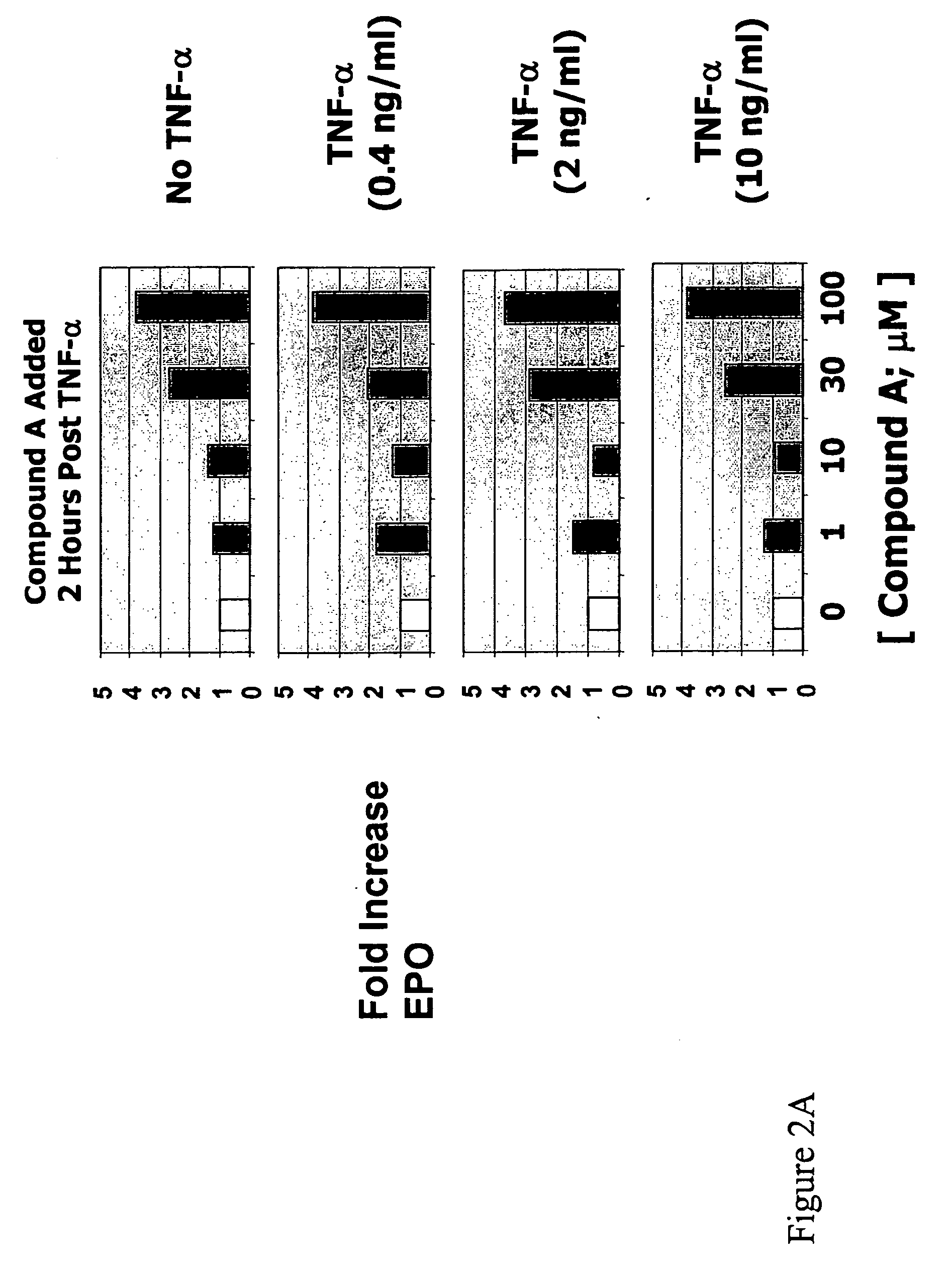
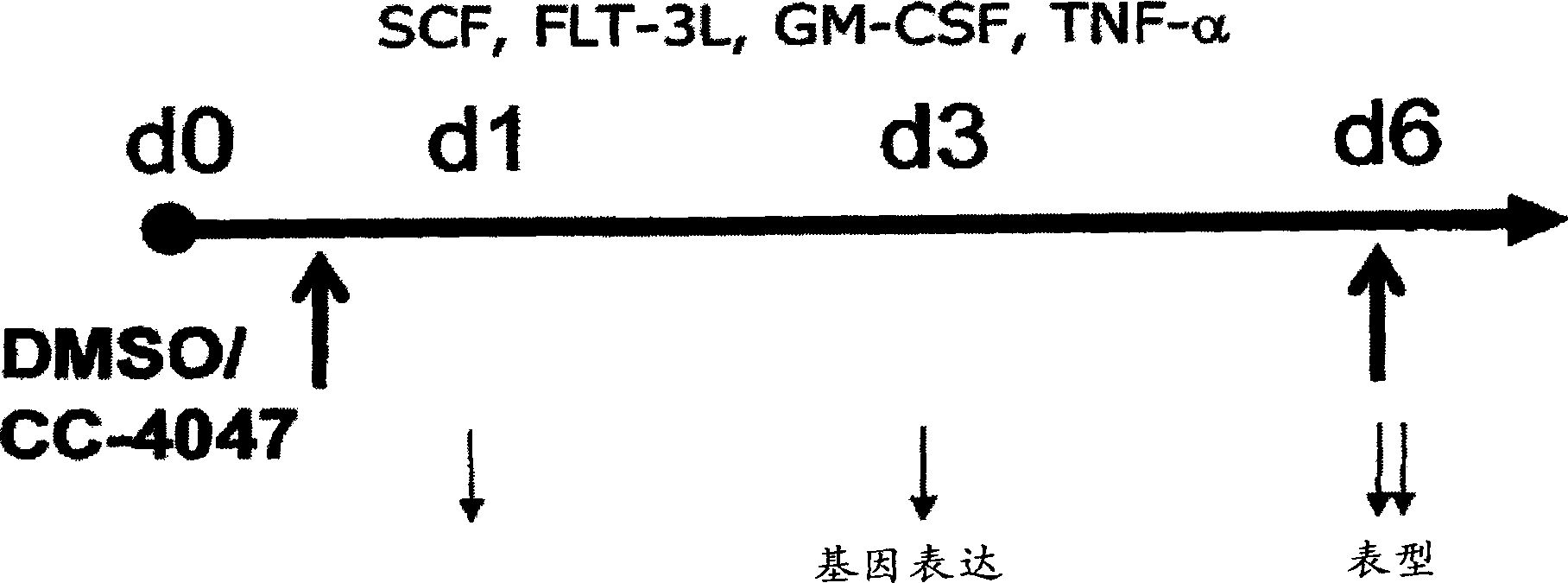
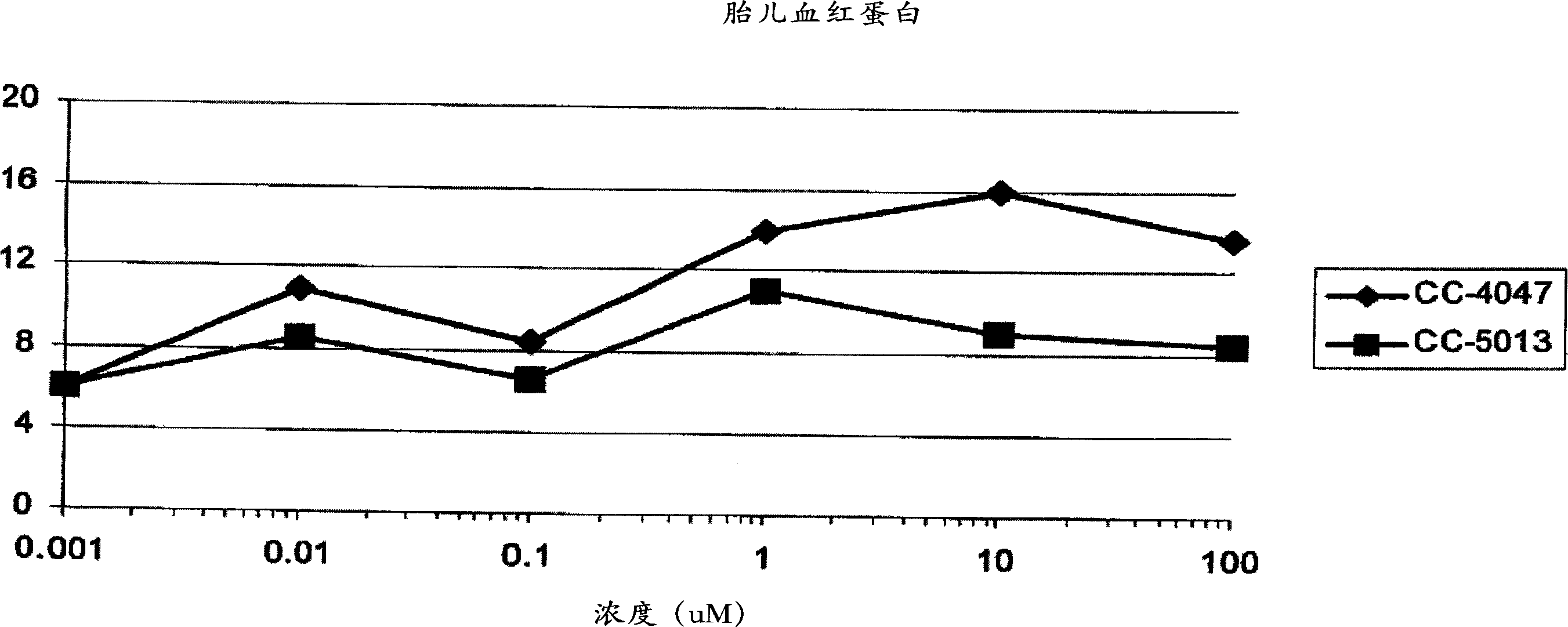
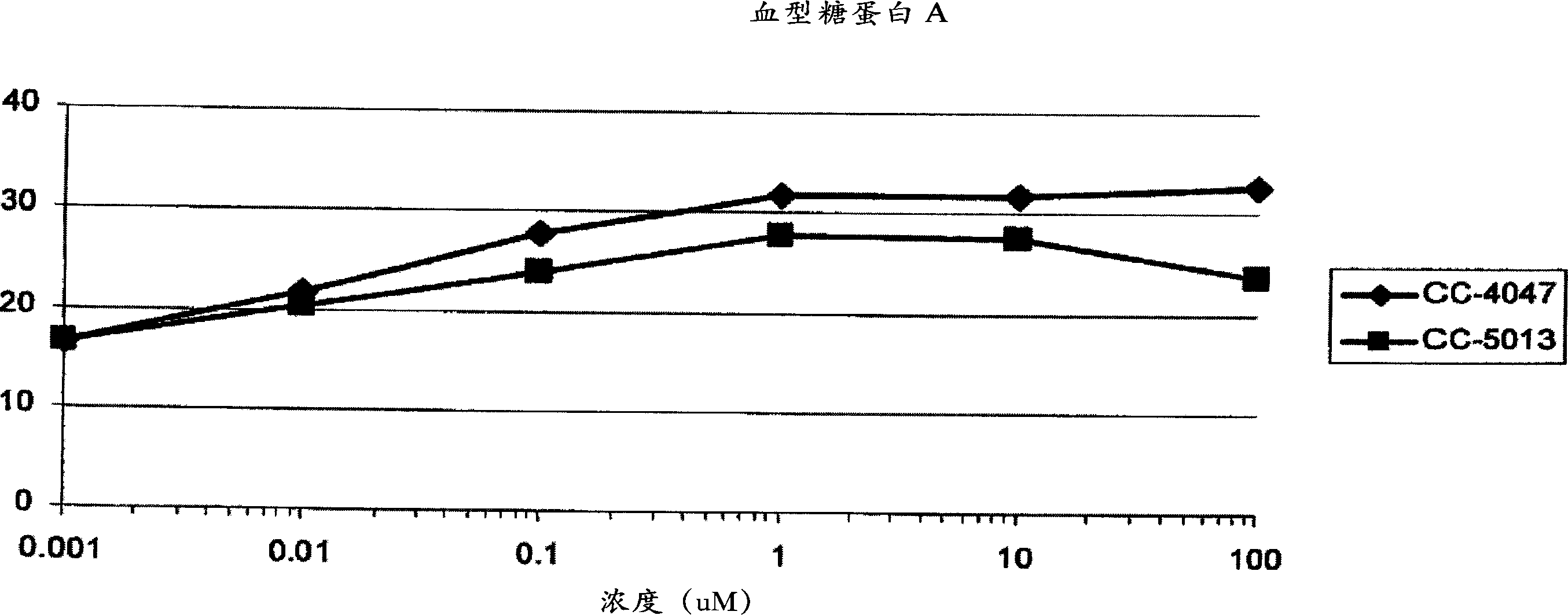
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
Enhanced erythropoiesis and iron metabolism
ActiveUS20050020487A1Increase productionReduce microcytosisBiocidePeptide/protein ingredientsDiseaseRed blood cell
The present invention relates to methods and compounds for regulating or enhancing erthropoiesis and iron metabolism, and for treating or preventing iron deficiency and anemia of chronic disease.
Owner:FIBROGEN INC
Aryl and heteroaryl compounds, compositions and methods of use
InactiveUS20050049310A1Reduce hypoxiaInduce red blood cell productionBiocideOrganic chemistryArylErythropoietin receptor
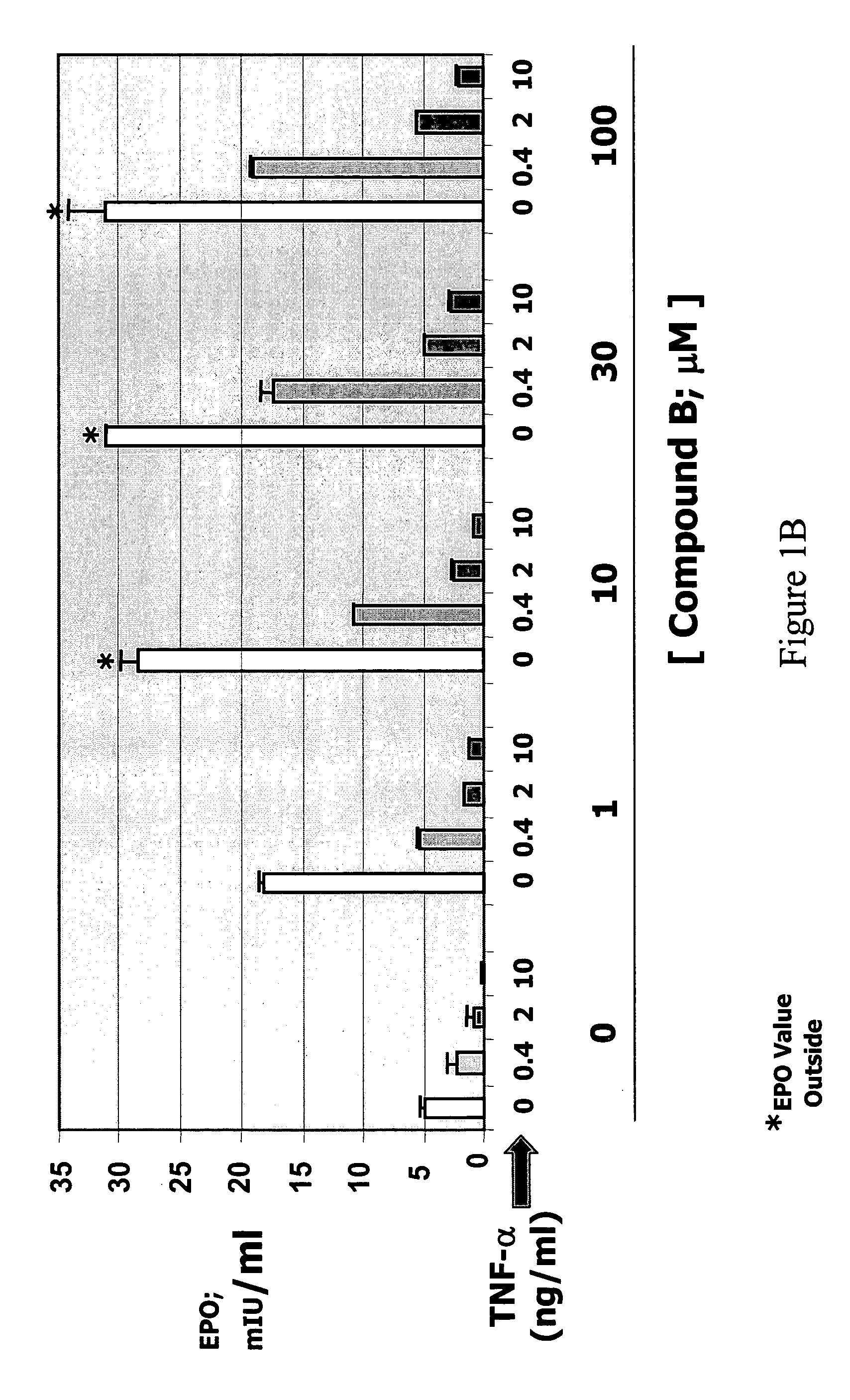
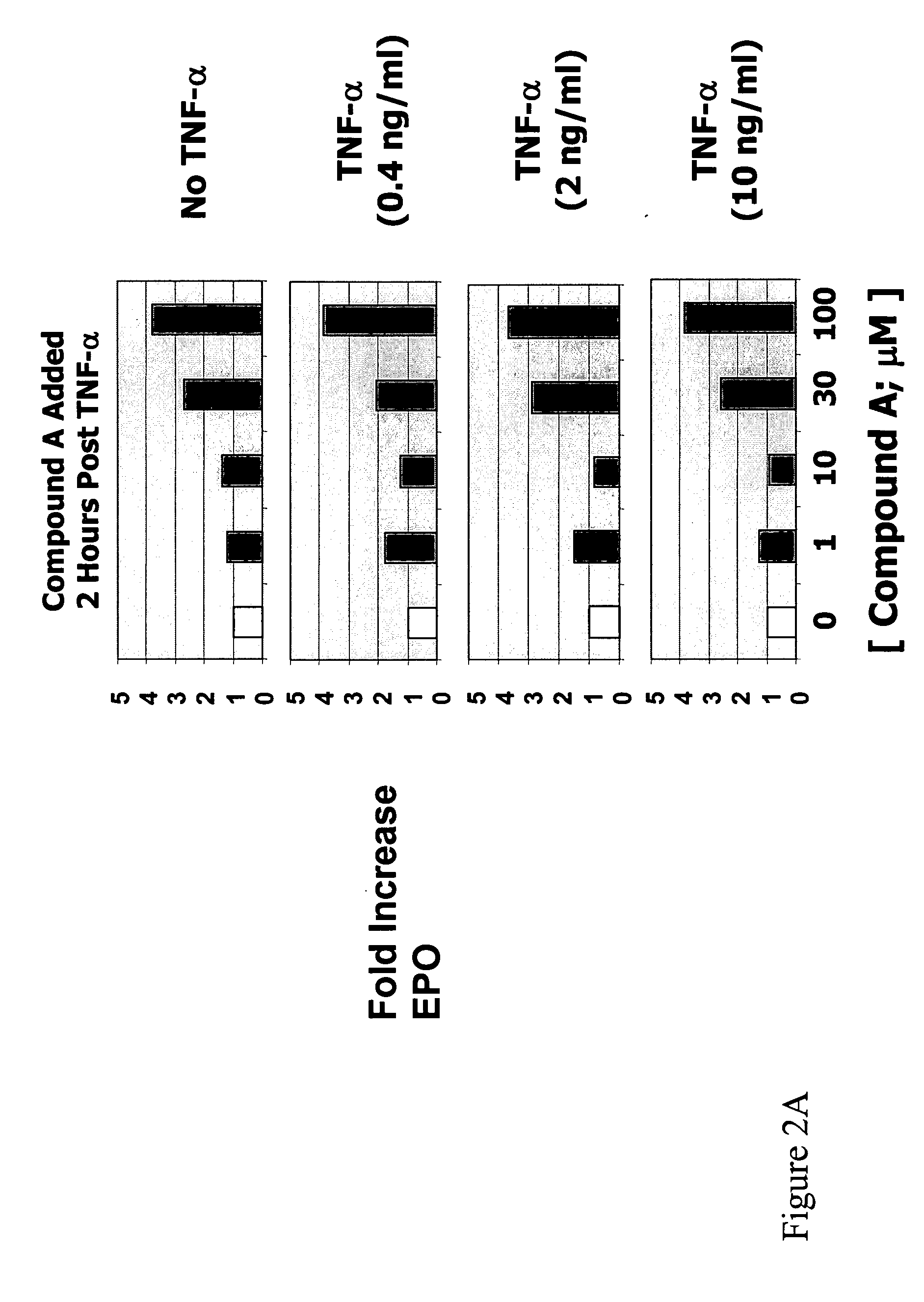
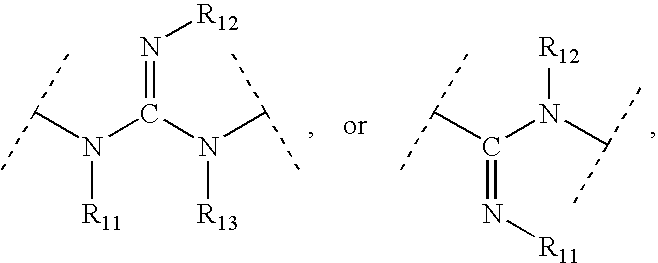
This invention provides aryl and heteroaryl compounds of Formula (I) as described herein, and methods of their preparation. Also provided are pharmaceutical compositions made with the compounds of Formula (I) and methods for making such compositions. The compounds of Formula (I) may activate an erythropoietin receptor and thus, may be useful to induce red blood cell production. The compounds of Formula (I) and compositions including compounds of Formula (I) may be useful in a variety of applications including the management, treatment and / or control of diseases caused at least in part by deficient (or inefficient) EPO production relative to hemoglobin level.
Owner:VTV THERAPEUTICS LLC
Erythropoietin composition
InactiveUS7202208B2Improve stabilityWithout loosingPeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseErythropoiesis
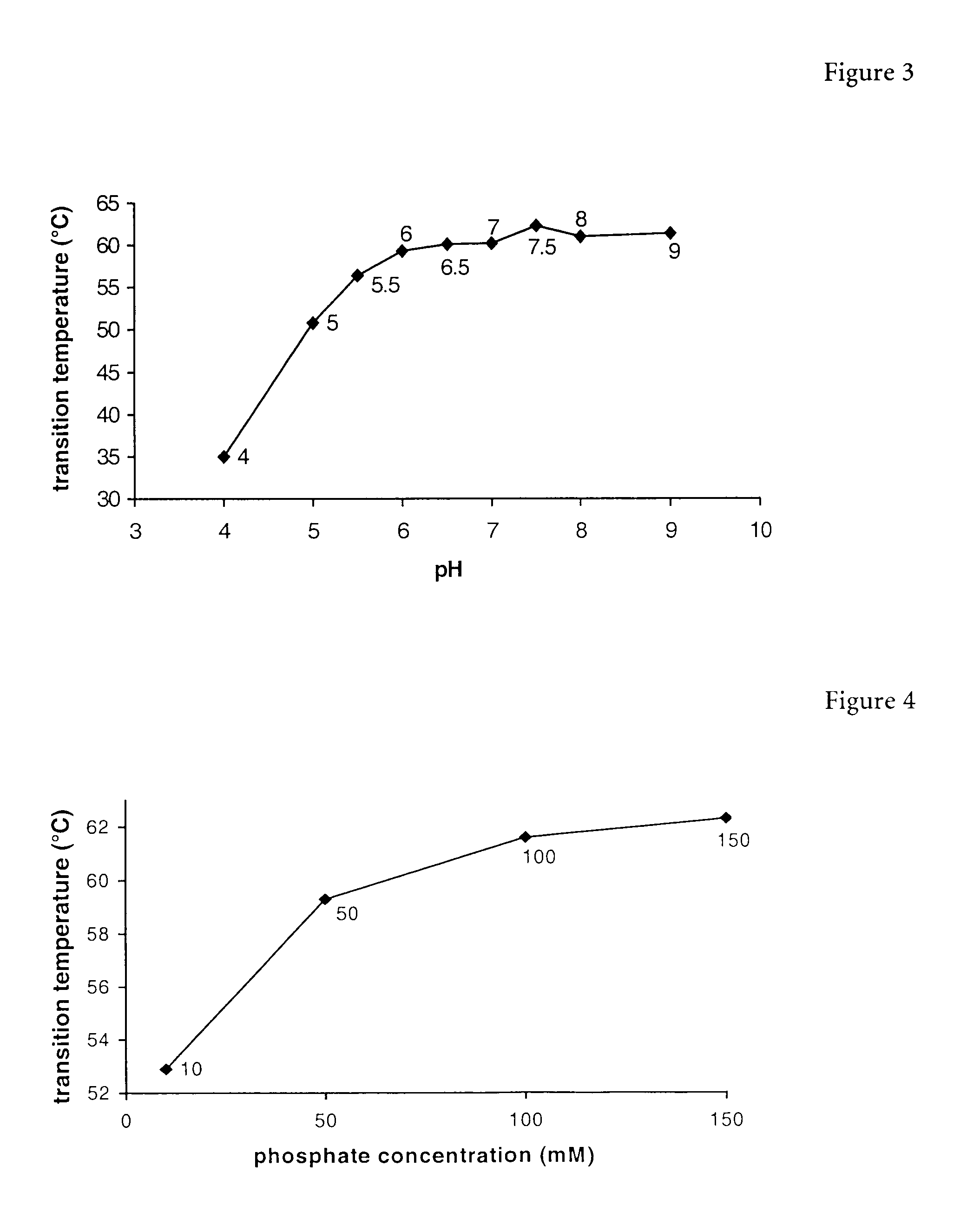
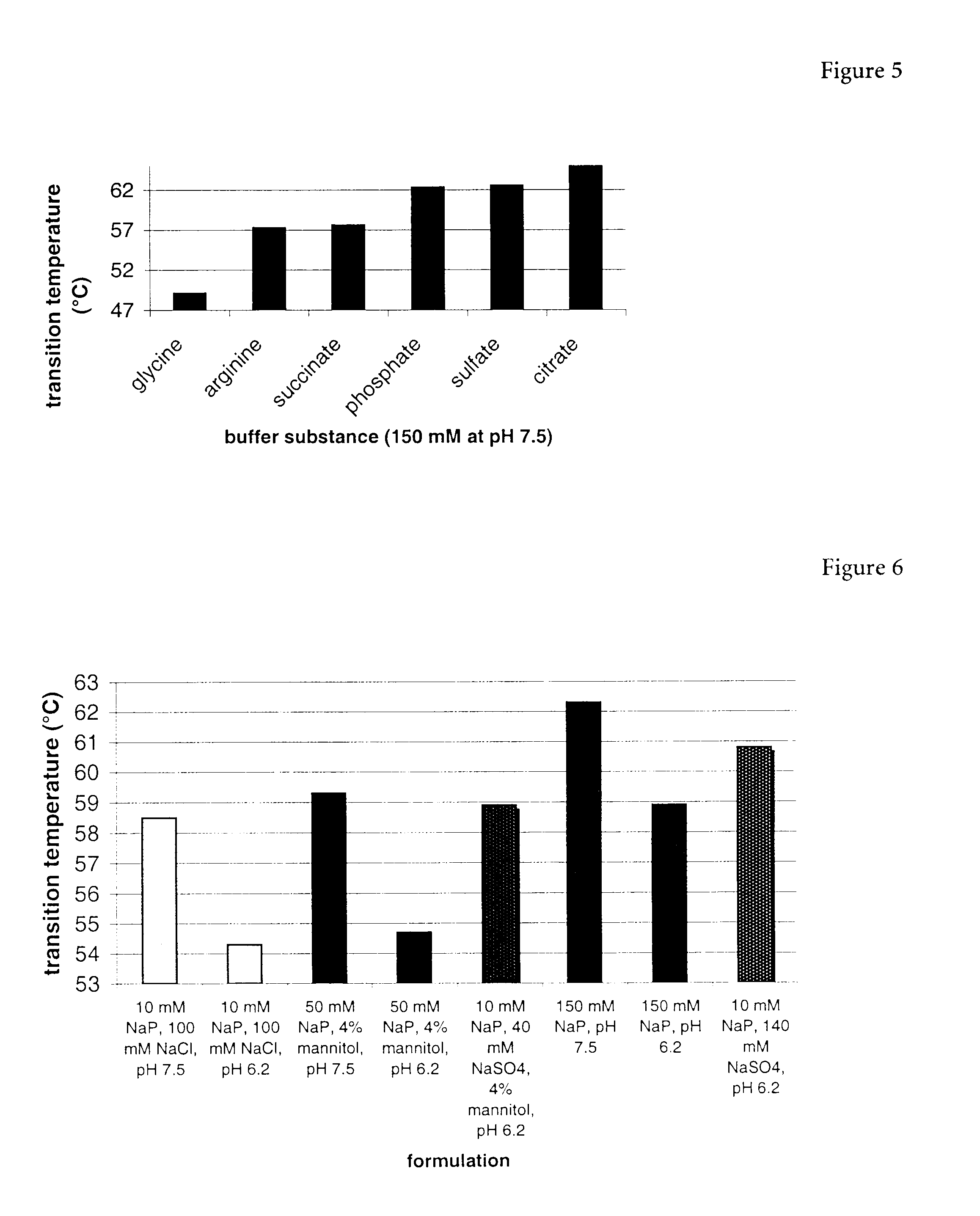
The present invention relates to a liquid pharmaceutical composition consisting essentially of an erythropoietin protein, a multiple charged inorganic anion in a pharmaceutically acceptable buffer suitable to keep the solution pH in the range from about 5.5 to about 7.0, and optionally one or more pharmaceutically acceptable excipients. This composition is especially useful for the prophylaxis and treatment of diseases related to erythropoiesis.
Owner:F HOFFMANN LA ROCHE & CO AG
Treatment of mitochondrial diseases with an erythropoietin mimetic
InactiveUS20090291092A1Stimulating erythropoiesisNervous disorderPeptide/protein ingredientsDiseaseRed blood cell
Methods of treating mitochondrial disorders that are not respiratory chain disorders using compositions comprising EPO mimetic compounds or compounds capable of increasing endogenous EPO levels or stimulating erythropoiesis are disclosed. Methods of treating Friedreich's ataxia, Leigh's syndrome, or other disorders by increasing the expression of frataxin with an EPO mimetic compound or a compound capable of increasing endogenous EPO levels or stimulating erythropoiesis are also disclosed.
Owner:EDISON PHARMA
Erythropoietin receptor antibodies
InactiveUS6998124B1Increased erythropoiesisAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsErythropoietin receptorErythropoiesis
Erythropoietin receptor agonist and antagonist antibodies and their use in enhancing erythropoiesis are disclosed.
Owner:SMITHKLINE BECKMAN CORP
Erythropoietin conjugates
InactiveUS7128913B2Easy to synthesizePeptide/protein ingredientsMammal material medical ingredientsRed blood cellBone marrow cell
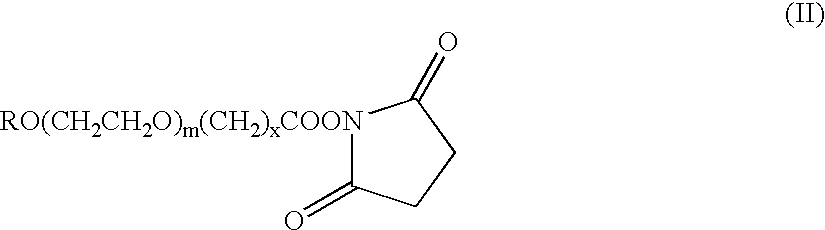
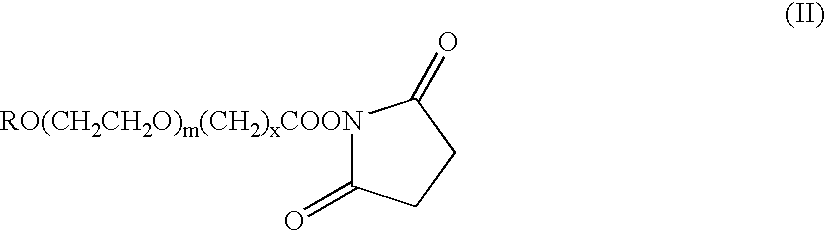
The present invention refers to conjugates of erythropoietin with poly(ethylene glycol) comprising an erythropoietin glycoprotein having an N-terminal α-amino group and having the in vivo biological activity of causing bone marrow cells to increase production of reticulocytes and red blood cells and selected from the group consisting of human erythropoietin and analogs thereof which have the sequence of human erythropoietin modified by the addition of from 1 to 6 glycosylation sites or a rearrangement of at least one glycosylation site; said glycoprotein being covalently linked to one poly(ethylene glycol) group of the formula—CO—(CH2)x—(OCH2CH2)m—ORwherein the —CO of the poly(ethylene glycol) group forms an amide bond with said N-terminal α-amino group; and wherein R is lower alkyl; x is 2 or 3; and m is from about 450 to about 1350.
Owner:F HOFFMANN LA ROCHE INC
Recombinant human EPO-Fc fusion proteins with prolonged half-life and enhanced erythropoietic activity in vivo
ActiveUS7625564B2Enhanced erythropoietic bioactivityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeRed blood cell
A recombinant fusion protein comprising a human erythropoietin peptide portion linked to an immunoglobulin peptide portion is described. The fusion protein has a prolonged half-life in vivo in comparison to naturally occurring or recombinant native human erythropoietin. In one embodiment of the invention, the protein has a half-life in vivo at least three fold higher than native human erythropoietin. The fusion protein also exhibits enhanced erythropoietic bioactivity in comparison to native human erythropoietin. In one embodiment, the fusion protein comprises the complete peptide sequence of a human erythropoietin (EPO) molecule and the peptide sequence of an Fc fragment of human immunoglobulin IgG1. The Fc fragment in the fusion protein includes the hinge region, CH2 and CH3 domains of human immunoglobulin IgG1. The EPO molecule may be linked directly to the Fc fragment to avoid extraneous peptide linkers and lessen the risk of an immunogenic response when administered in vivo. In one embodiment the hinge region is a human Fc fragment variant having a non-cysteine residue at amino acid 6. The invention also relates to nucleic acid and amino acid sequences encoding the fusion protein and transfected cell lines and methods for producing the fusion protein. The invention further includes pharmaceutical compositions comprising the fusion protein and methods of using the fusion protein and / or the pharmaceutical compositions, for example to stimulate erythropoiesis in subjects in need of therapy.
Owner:NOVAGEN HLDG CORP
Glycopegylated Erythropoietin
InactiveUS20070254834A1Improved pharmacokinetic propertiesCost effectiveOrganic active ingredientsBiocideDiseaseErythropoiesis
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Glycopegylated erythropoietin
InactiveUS20100210507A9Improved pharmacokinetic propertiesCost effectiveSaccharide peptide ingredientsPeptide preparation methodsDiseaseErythropoiesis
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Synthetic hyperglycosylated, and hyperglycosylated protease-resistant polypeptide variants, oral formulations and methods of using the same
ActiveUS20060204473A1Increased protease resistanceNervous disorderPeptide/protein ingredientsHybrid typeInterferon receptor
The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites. The present invention provides synthetic Type I interferon receptor polypeptide agonists comprising consensus or hybrid Type I interferon receptor polypeptide agonists, containing one or more native or non-native glycosylation sites, as well as erythropoietin and darbepoetin alfa, each of which are linked to a penetrating peptide that facilitates translocation of a substance across a biological barrier as well as pharmaceutical compositions, including oral formulations, of the same. The present invention further provides oral formulations of hyperglycosylated or protease-resistant, hyperglycosylated polypeptide variants, which polypeptide variants lack at least one protease cleavage site found in a parent polypeptide, and thus exhibit increased protease resistance compared to the parent polypeptide, which polypeptide variants further include (1) a carbohydrate moiety covalently linked to at least one non-native glycosylation site not found in the parent protein therapeutic or (2) a carbohydrate moiety covalently linked to at least one native glycosylation site found but not glycosylated in the parent protein therapeutic. The present invention further provides compositions, including oral pharmaceutical compositions, comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides containers, devices, and kits comprising the synthetic Type I interferon receptor polypeptide agonist, the hyperglycosylated polypeptide variant, or the hyperglycosylated, protease-resistant polypeptide variant. The present invention further provides therapeutic methods involving administering an effective amount of an oral pharmaceutical composition comprising a synthetic Type I interferon receptor polypeptide agonist, a hyperglycosylated polypeptide variant, or a hyperglycosylated, protease-resistant polypeptide variant to an individual in need thereof.
Owner:ALIOS BIOPHARMA INC +1
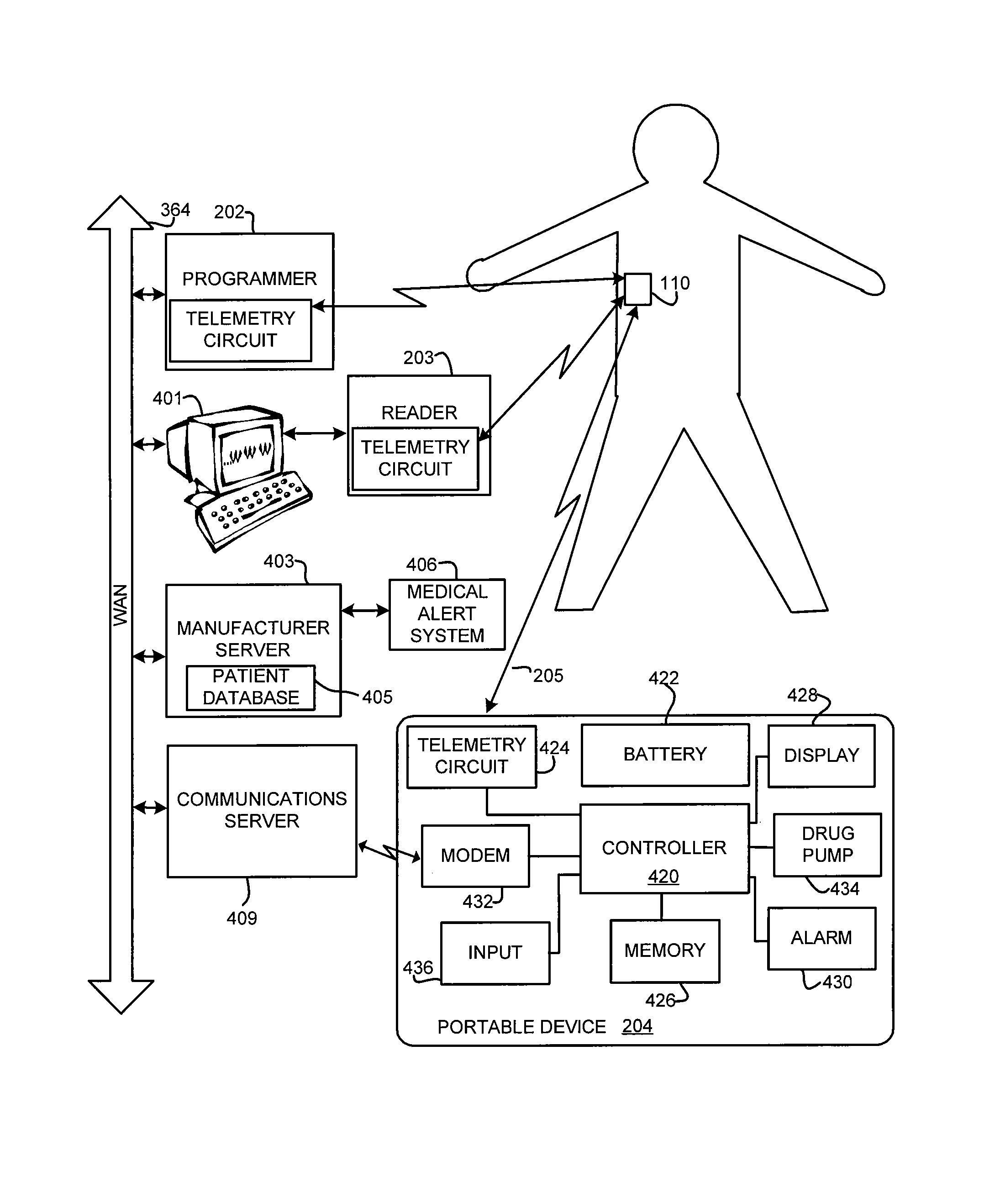
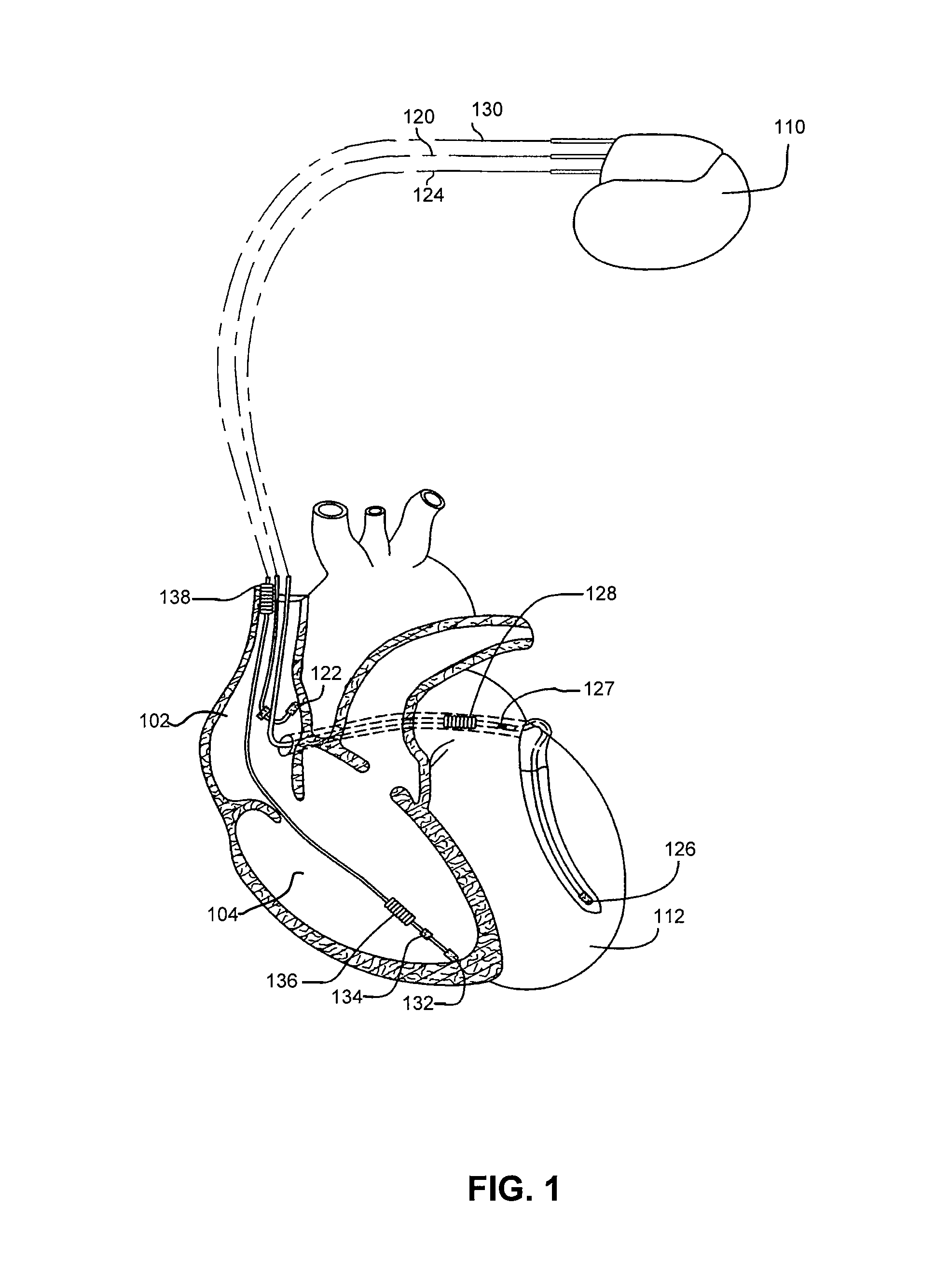
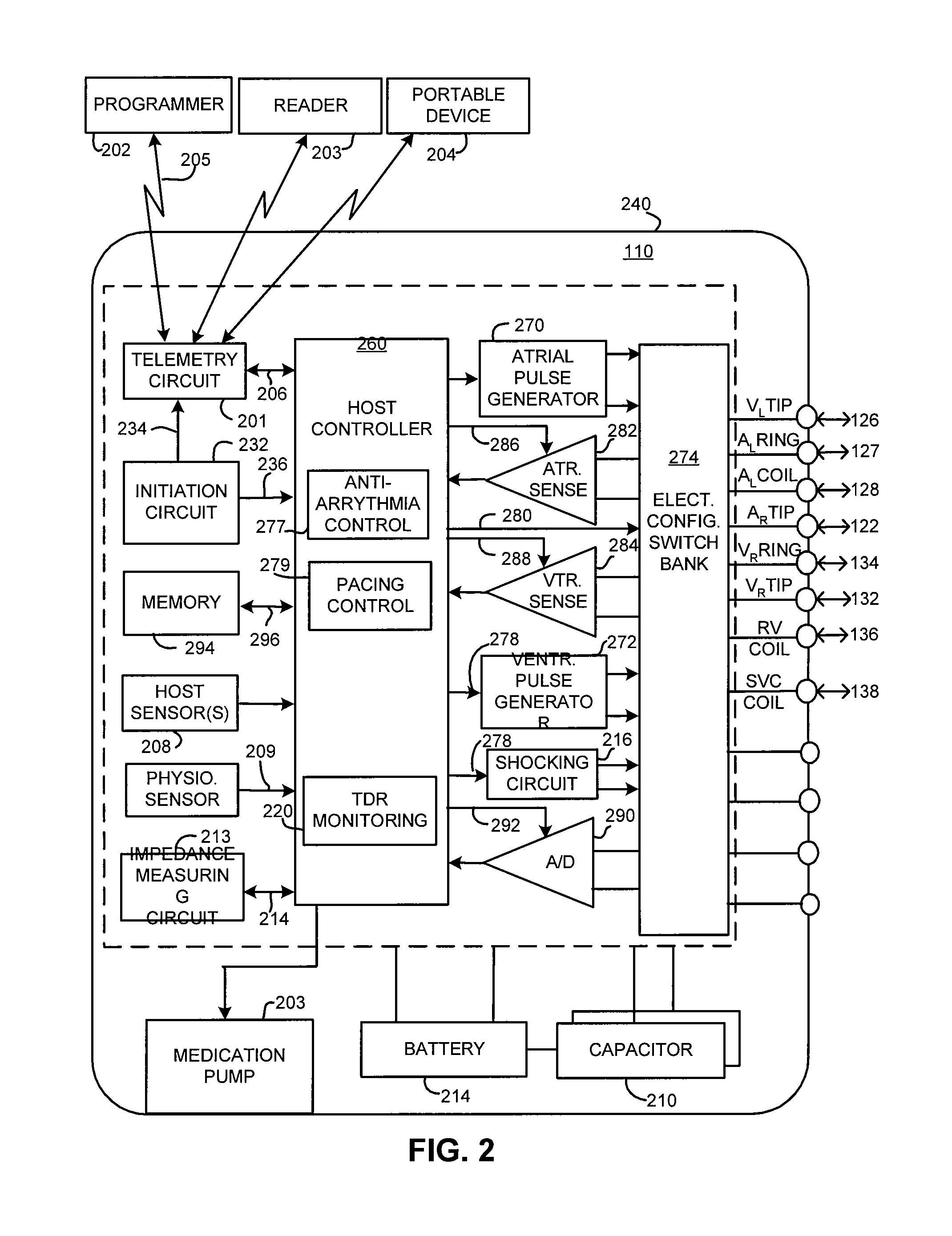
Method and apparatus for monitoring arrythmogenic effects of medications using an implantable device
ActiveUS8934963B1Reduce riskContinuous monitoringElectrotherapyElectrocardiographyT waveErythropoiesis
An implantable device and method for monitoring changes in the risk of arrhythmia induced by medications. The implantable device monitors risk of arrhythmia by analyzing an aspect of T-wave morphology to generate a metric of transmural dispersion of repolarization (“TDR”) as a proxy for the risk of arrhythmia. The implantable device generates an index of change in the risk of arrhythmia by comparing values of the metric of TDR obtained for different time periods. The implantable device generates a warning if the change in risk of arrhythmia is outside acceptable limits. The implantable device can also communicate with other devices to correlate changes in risk of arrhythmia with medications taken by the patient.
Owner:PACESETTER INC
Erythropoietin composition
InactiveUS7169754B2Increase productionImprove stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseRed blood cell
The present invention relates to a liquid pharmaceutical composition comprising an erythropoietin protein, a multiple charged inorganic anion in a pharmaceutically acceptable buffer suitable to keep the solution pH in the range from about 5.5 to about 7.0, and optionally one or more pharmaceutically acceptable excipients. This composition is especially useful for the prophylaxis and treatment of diseases related to erythropoiesis.
Owner:F HOFFMANN LA ROCHE & CO AG
Antibodies which activate an erythropoietin receptor
InactiveUS20080182976A1Peptide/protein ingredientsMicroorganism based processesErythropoietin receptorRed blood cell
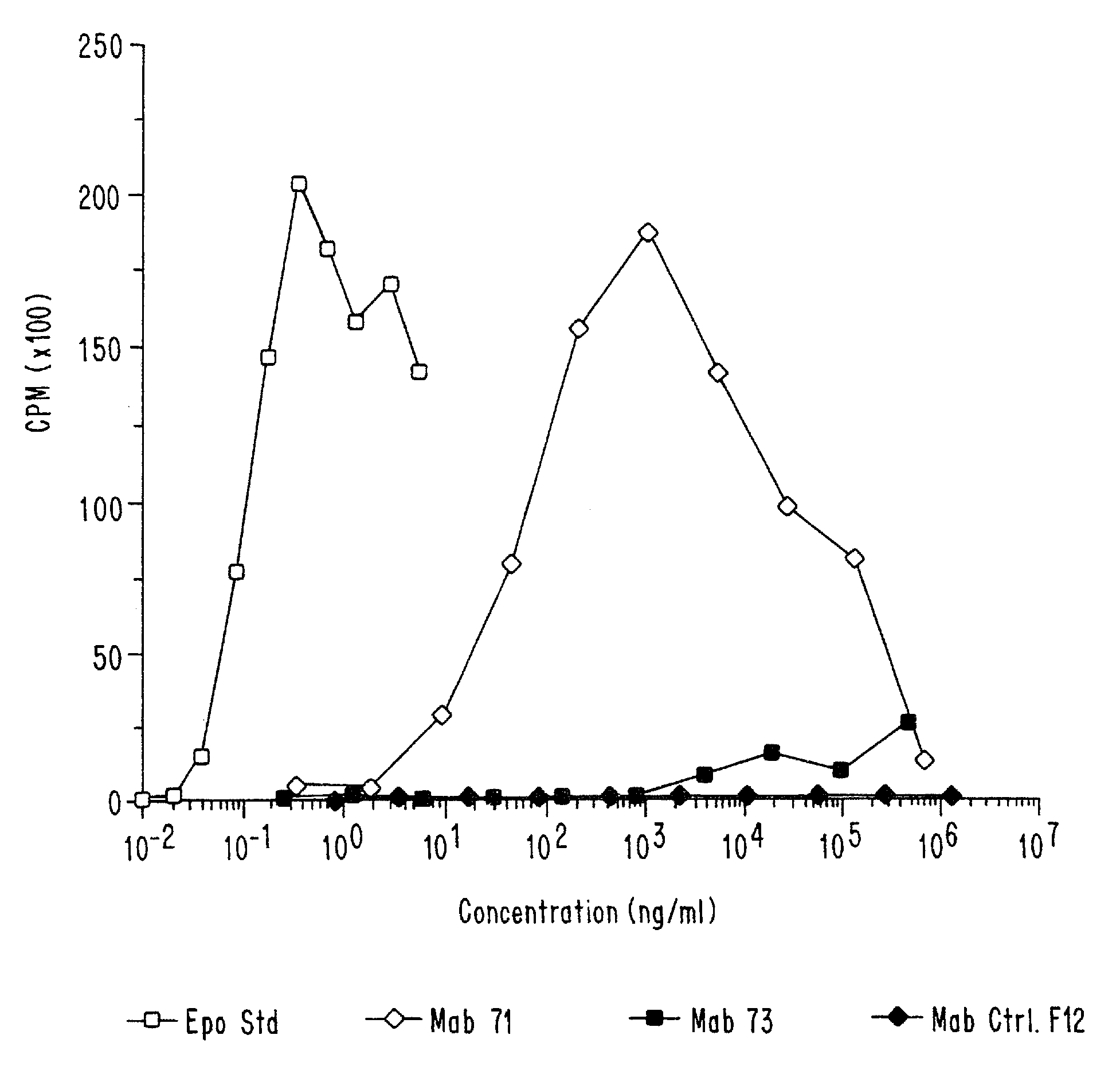
Antibodies and fragments thereof which activate an erythropoietin receptor and stimulate erythropoiesis are described. Also described are hybridoma cell lines which produce the antibodies and methods and compositions for the treatment of anemia.
Owner:AMGEN INC
Aqueous formulation of erythropoiesis stimulating protein stabilised by antioxidants for parenteral administration
ActiveUS20100297117A1Improve stabilityIncrease resistancePeptide/protein ingredientsPharmaceutical delivery mechanismParenteral nutritionAntioxidant
The present invention relates to stable aqueous protein formulations. In particular, disclosed herein are therapeutic protein formulations suitable for parenteral administration having one or more antioxidants.
Owner:AMGEN INC
Recombinant human EPO-Fc fusion proteins with prolonged half-life and enhanced erythropoietic activity in vivo
ActiveUS20070178112A1Enhanced erythropoietic bioactivityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsRed blood cellHalf-life
A recombinant fusion protein comprising a human erythropoietin peptide portion linked to an immunoglobulin peptide portion is described. The fusion protein has a prolonged half-life in vivo in comparison to naturally occurring or recombinant native human erythropoietin. In one embodiment of the invention, the protein has a half-life in vivo at least three fold higher than native human erythropoietin. The fusion protein also exhibits enhanced erythropoietic bioactivity in comparison to native human erythropoietin. In one embodiment, the fusion protein comprises the complete peptide sequence of a human erythropoietin (EPO) molecule and the peptide sequence of an Fc fragment of human immunoglobulin IgG1. The Fc fragment in the fusion protein includes the hinge region, CH2 and CH3 domains of human immunoglobulin IgG1. The EPO molecule may be linked directly to the Fc fragment to avoid extraneous peptide linkers and lessen the risk of an immunogenic response when administered in vivo. In one embodiment the hinge region is a human Fc fragment variant having a non-cysteine residue at amino acid 6. The invention also relates to nucleic acid and amino acid sequences encoding the fusion protein and transfected cell lines and methods for producing the fusion protein. The invention further includes pharmaceutical compositions comprising the fusion protein and methods of using the fusion protein and / or the pharmaceutical compositions, for example to stimulate erythropoiesis in subjects in need of therapy.
Owner:NOVAGEN HLDG CORP
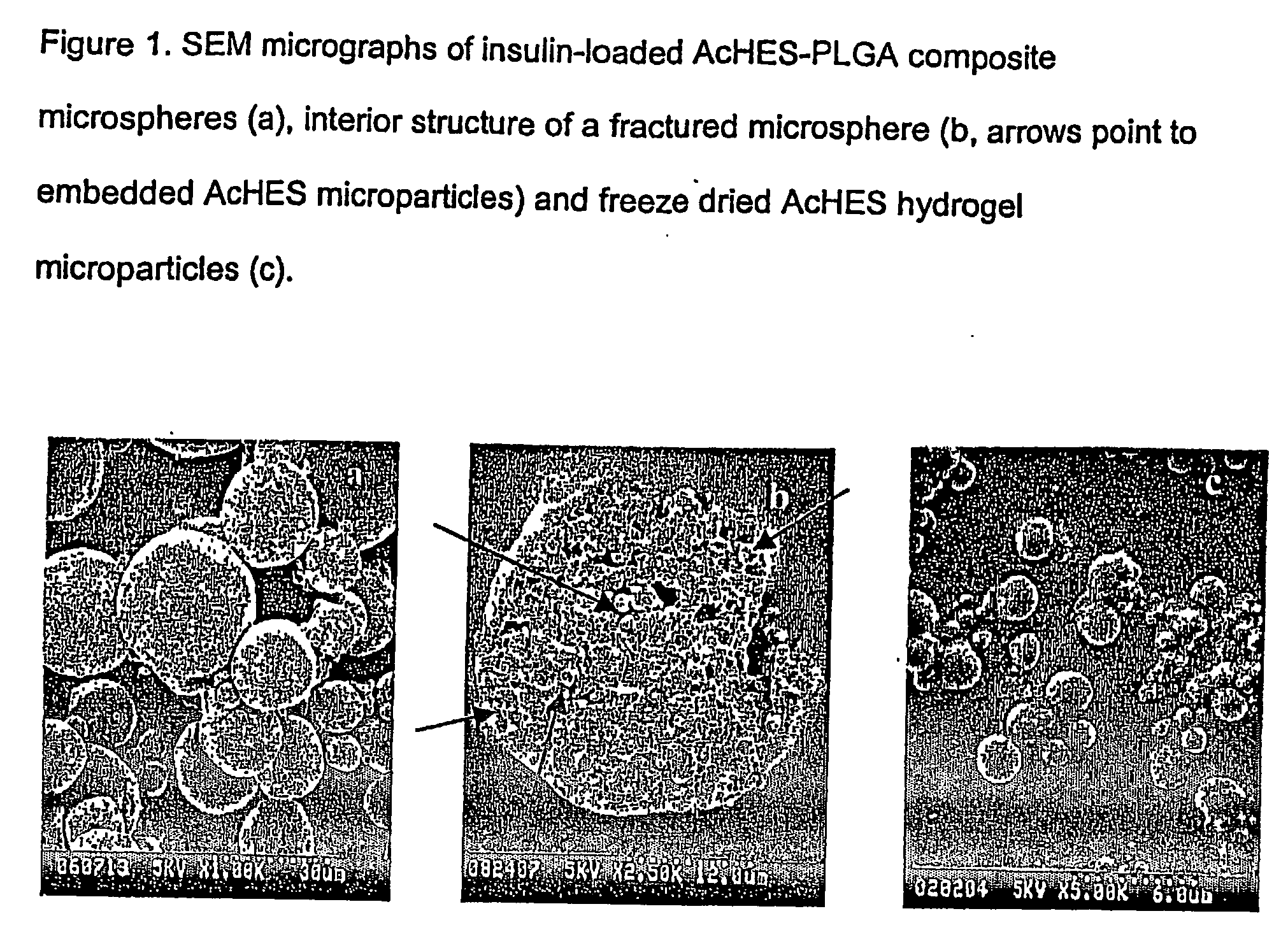
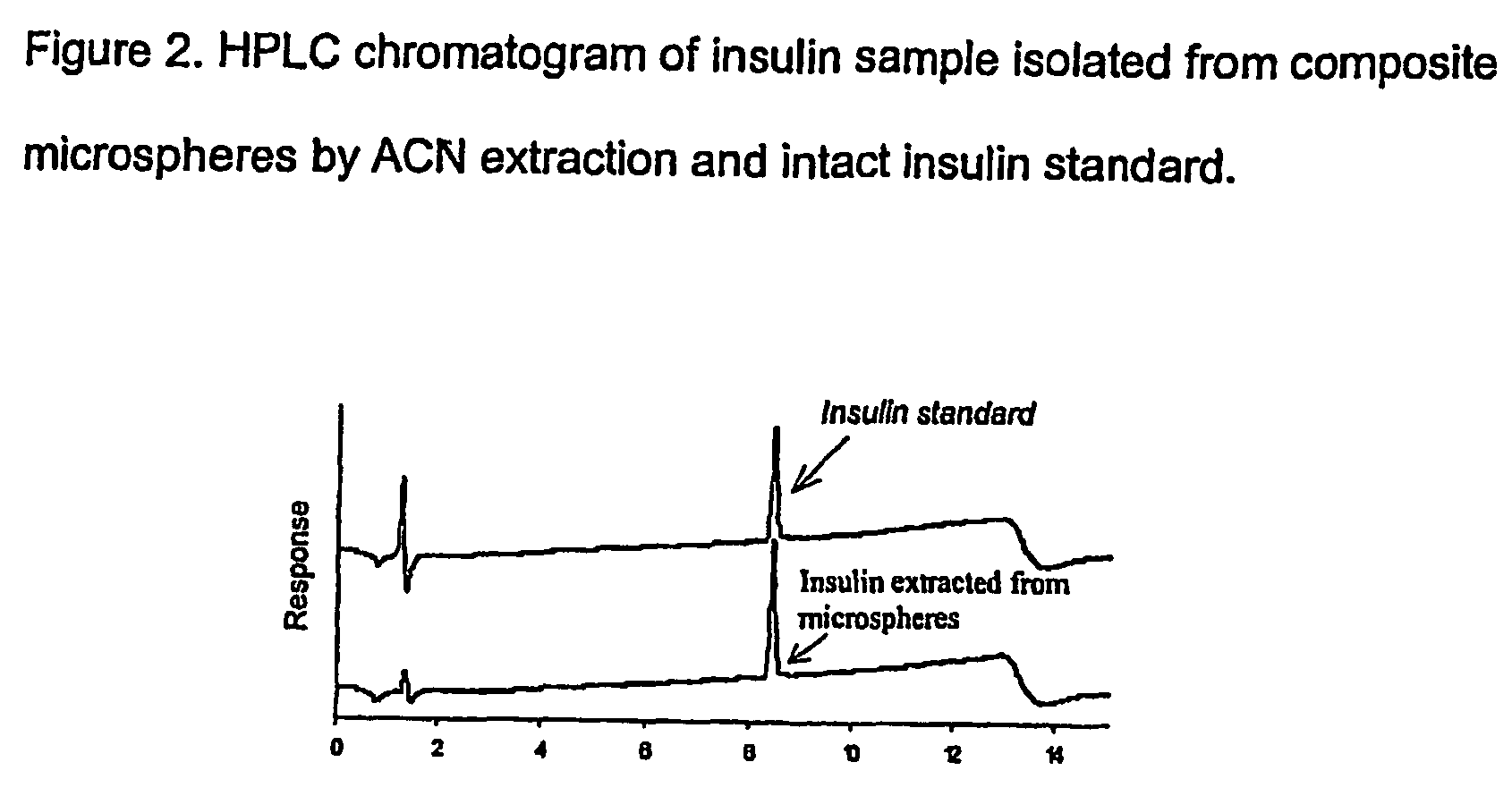
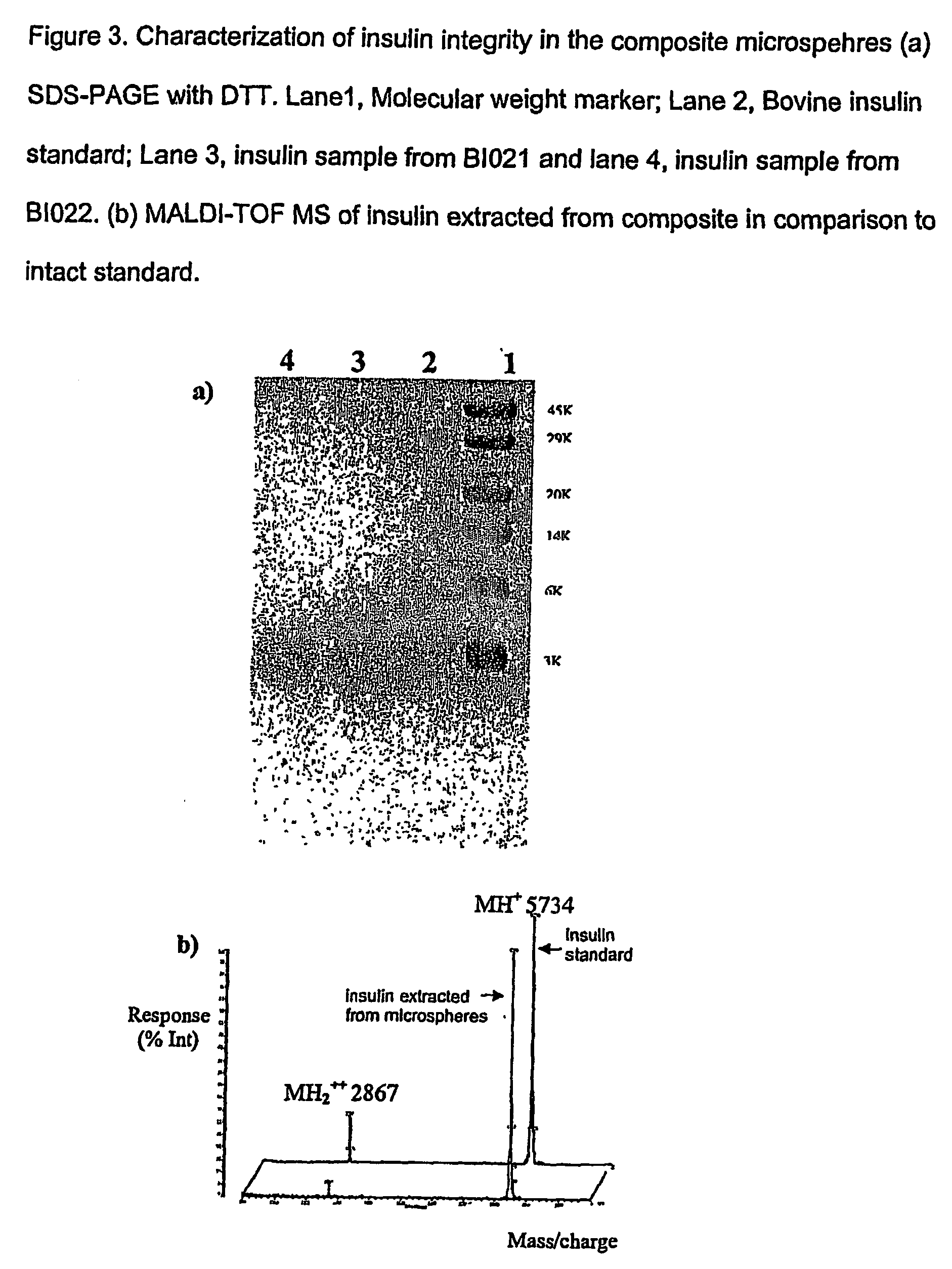
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Genetically modified non-human animals expressing human epo
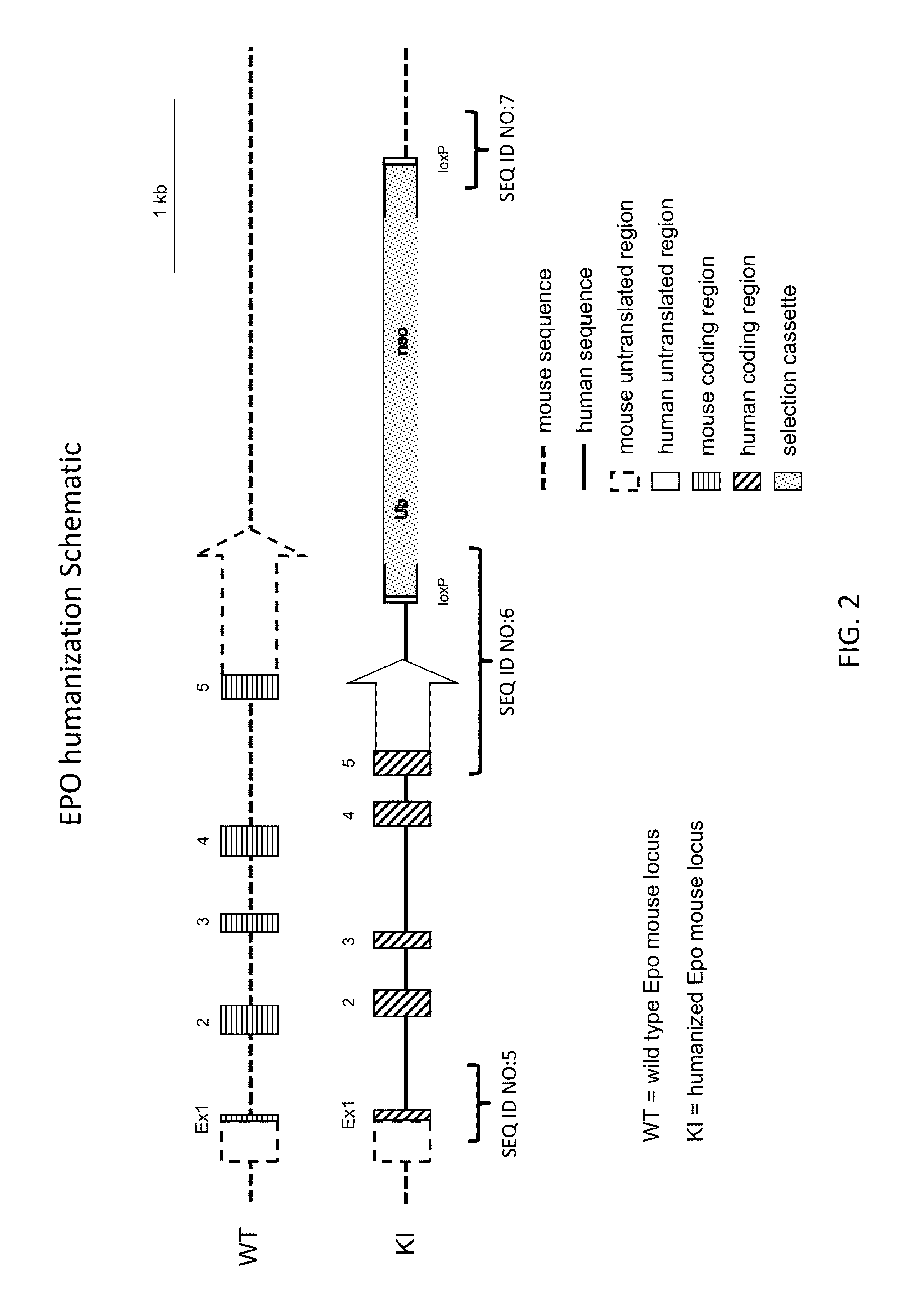
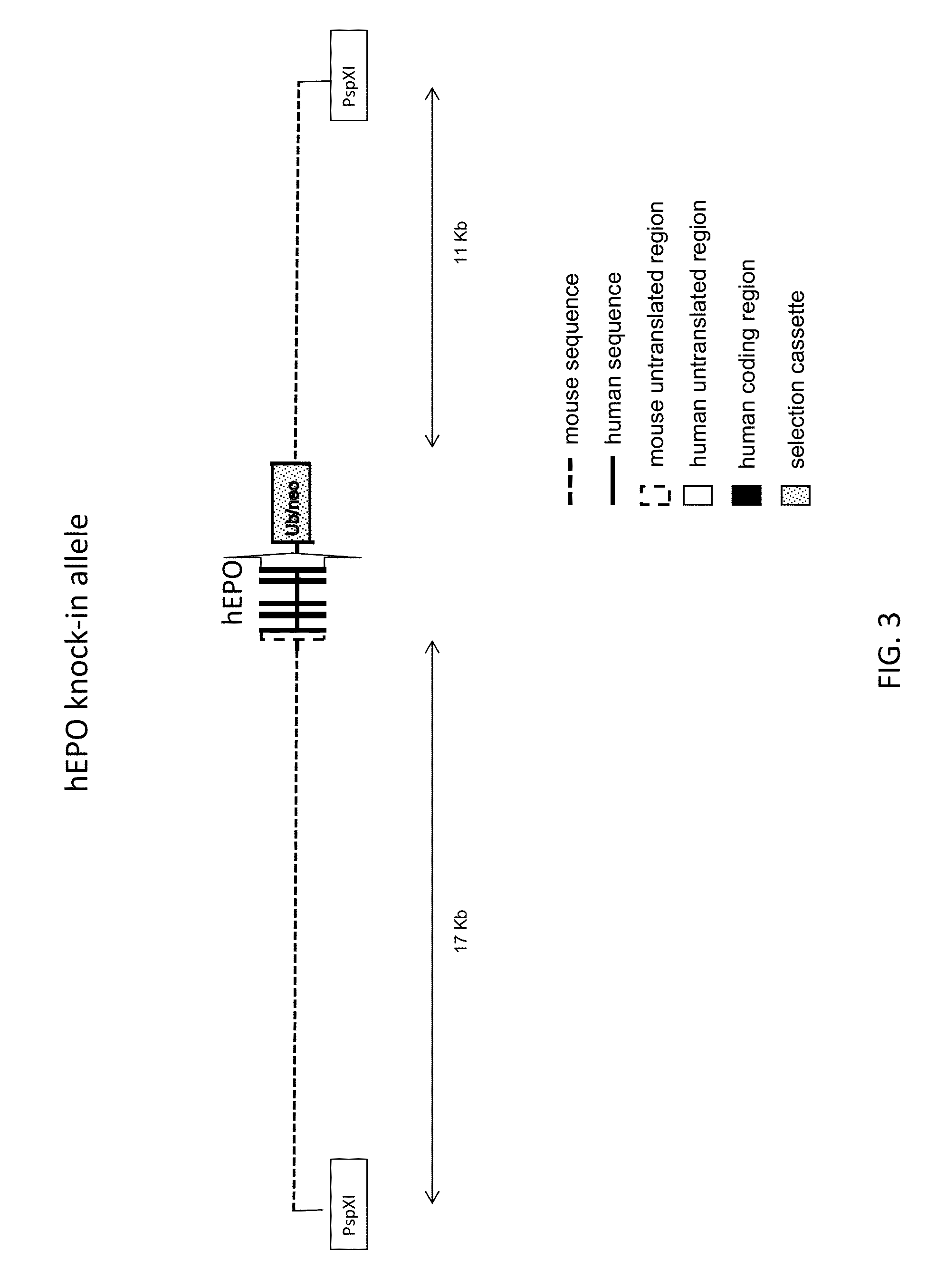
ActiveUS20150327524A1Reduce the amount requiredConducive to survivalCompounds screening/testingHydrolasesDiseaseProgenitor
Genetically modified non-human animals expressing human EPO from the animal genome are provided. Also provided are methods for making non-human animals expressing human EPO from the non-human animal genome, and methods for using non-human animals expressing human EPO from the non-human animal genome. These animals and methods find many uses in the art, including, for example, in modeling human erythropoiesis and erythrocyte function; in modeling human pathogen infection of erythrocytes; in in vivo screens for agents that modulate erythropoiesis and / or erythrocyte function, e.g. in a healthy or a diseased state; in in vivo screens for agents that are toxic to erythrocytes or erythrocyte progenitors; in in vivo screens for agents that prevent against, mitigate, or reverse the toxic effects of toxic agents on erythrocytes or erythrocyte progenitors; in in vivo screens of erythrocytes or erythrocyte progenitors from an individual to predict the responsiveness of an individual to a disease therapy.
Owner:REGENERON PHARM INC +2
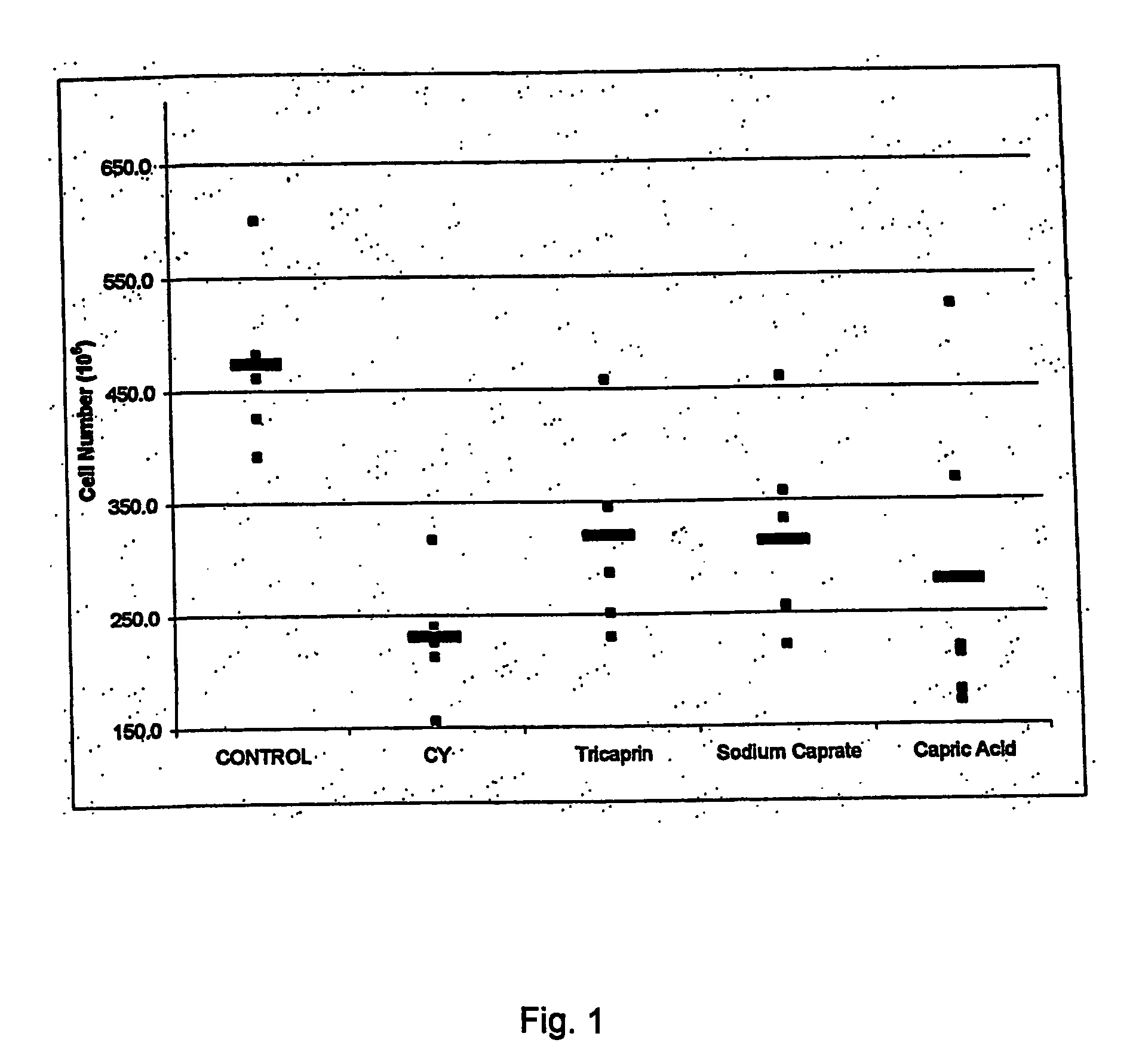
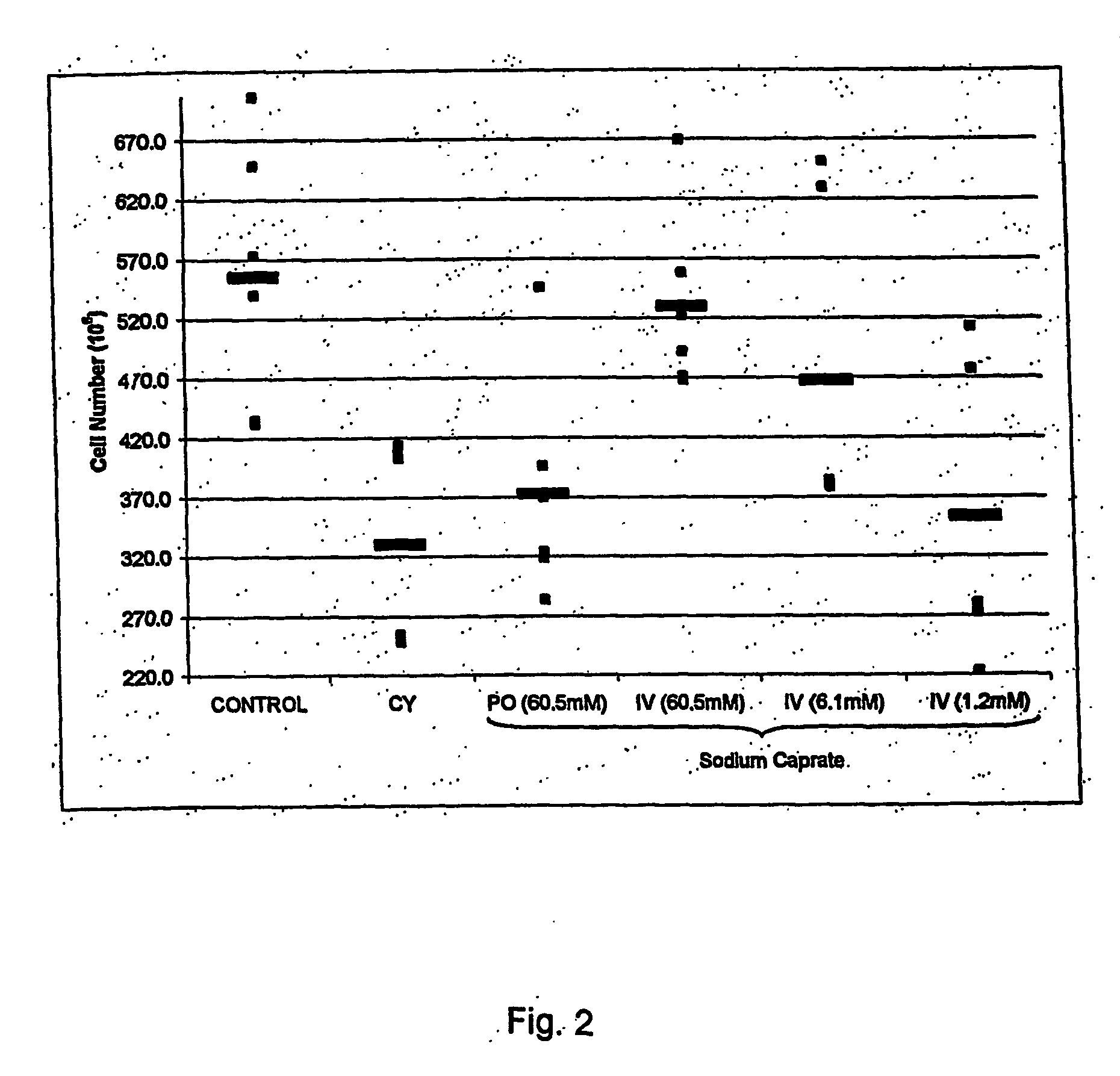
Medium-chain length fatty acids, glycerides and analogues as stimulators of erythropoiesis
ActiveUS20060128800A1Satisfies needMeet actual needsBiocidePharmaceutical non-active ingredientsRed blood cellErythropoiesis
Use of a composition comprising a compound of any of formulae I, II, Ila, III and Illa; or a combination thereof wherein each R1 is independently C7-11 alkyl; A and B are independently H or CO—R1; R2 is H or C1-4 alkyl; M is a metal monocation (k=1) or dication (k=2); Y is 0 or NH; and Z is 0, NH, CH2O or a bond; for the manufacture of a medicament for stimulating erythropoiesis. Preferably, the composition further comprises human erythroporietin.
Owner:PROMETIC PHARMA SMT LTD
Method for enhancing proliferation or differentiation of a cell using ob protein
InactiveUS7074397B1Easy to implantFacilitates mobilizationPeptide/protein ingredientsDepsipeptidesMyelopoiesisIn vivo
Uses for WSX ligands in hematopoiesis are disclosed. In particular, in vitro and in vivo methods for stimulating hematopoiesis (e.g., myelopoiesis, erythropoiesis and especially, lymphopoiesis) using a WSX ligand (e.g., anti-WSX receptor agonist antibodies or OB protein), and optionally another cytokine, are described.
Owner:GENENTECH INC
New application of sodium ferrous chlorophyll to promotion of erythropoiesis
The invention discloses new application of sodium ferrous chlorophyll to the promotion of erythropoiesis, and belongs to the field of medicines. Sodium ferrous chlorophyll has the effect of erythropoietin, and can be prepared into medicaments for treating anemia such as 'anemia of chronic disease' or 'renal anemia' which are caused by hyposecretion in erythropoietin.
Owner:武汉联合药业有限责任公司
Methods and apparatuses for predicting the effects of erythropoiesis stimulating agents, and for determining a dose to be administered
InactiveUS20130052136A1Prevent from overdosing ironAdverse overdosing of iron may be advantageously be preventedMaterial analysis by electric/magnetic meansMaterial analysis by optical meansRed blood cellErythropoiesis-stimulating agent
The present invention relates to a method for predicting the concentration or the mass of hemoglobin or an approximation thereof, respectively, in a body fluid and / or an extracorporeal sample thereof of a patient at a later, second point of time, the patient having theoretically or in reality been administered a certain dose of an erythropoiesis stimulating agent at an earlier, first point of time. It relates further to a method for determining the dose of an erythropoiesis stimulating agent to be administered to a patient, to a method for determining whether a patient is affected by circumstances leading to the loss of hemoglobin, to corresponding devices and to an erythropoiesis stimulating medicament for use in the treatment of anemia.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
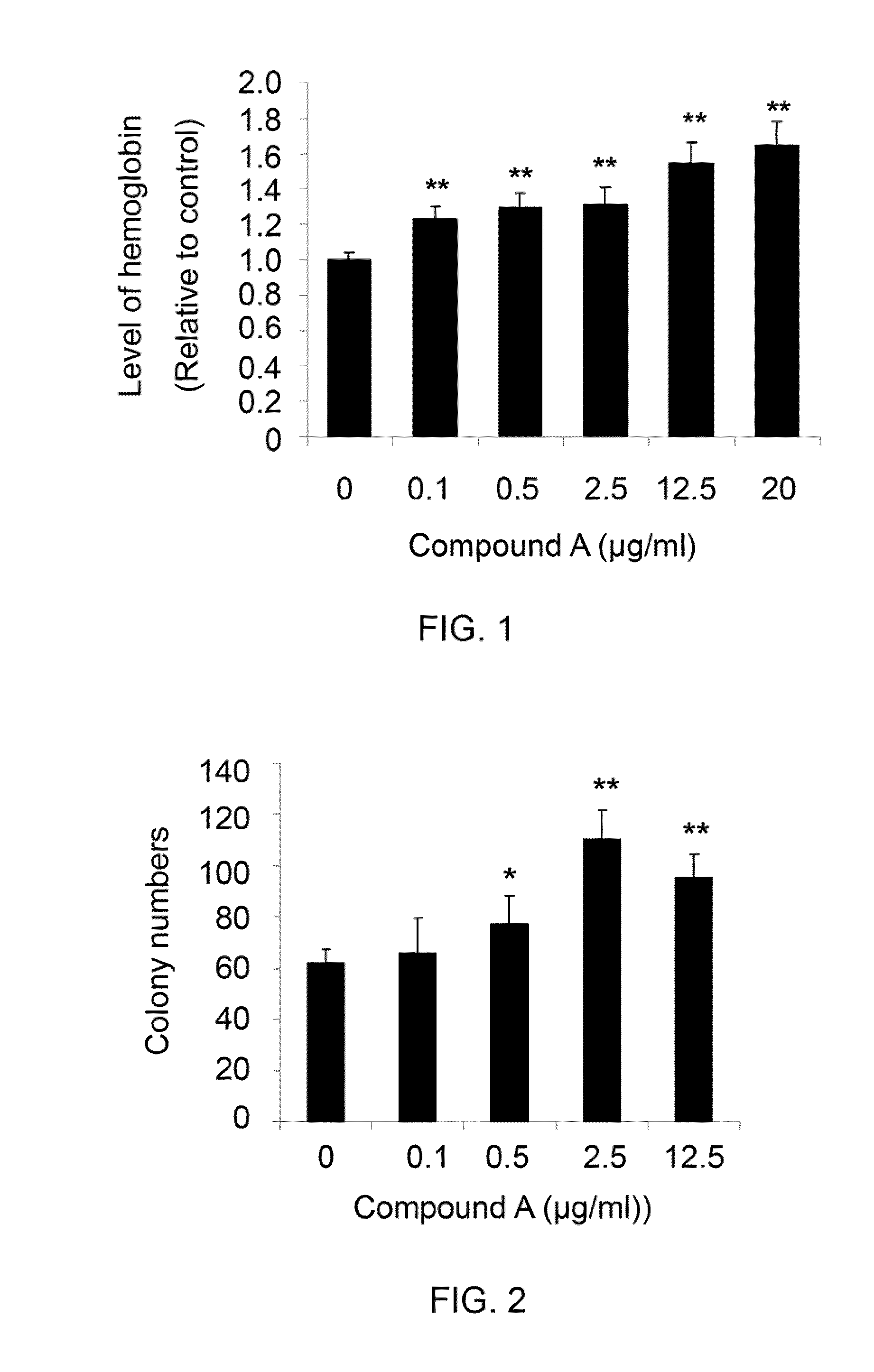
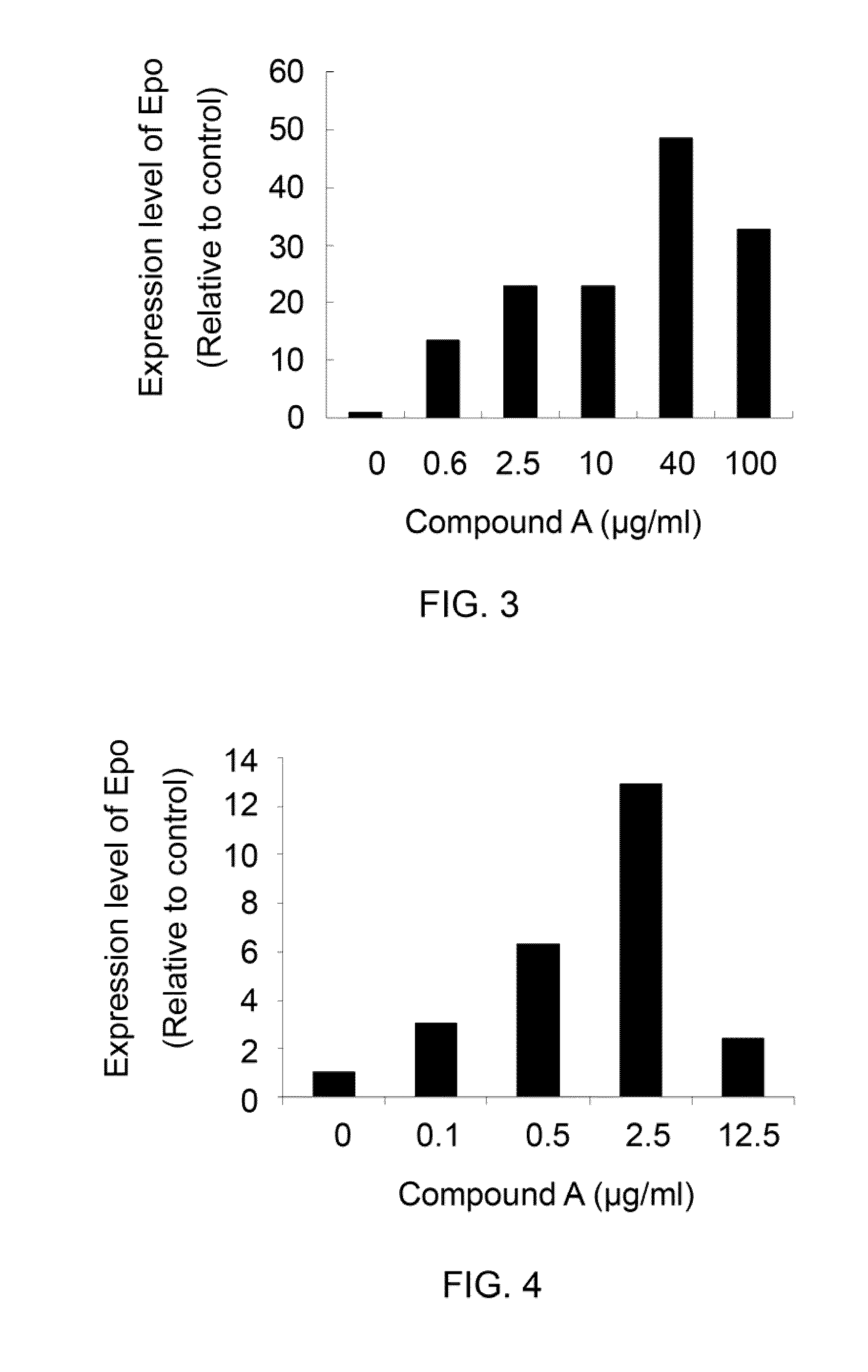
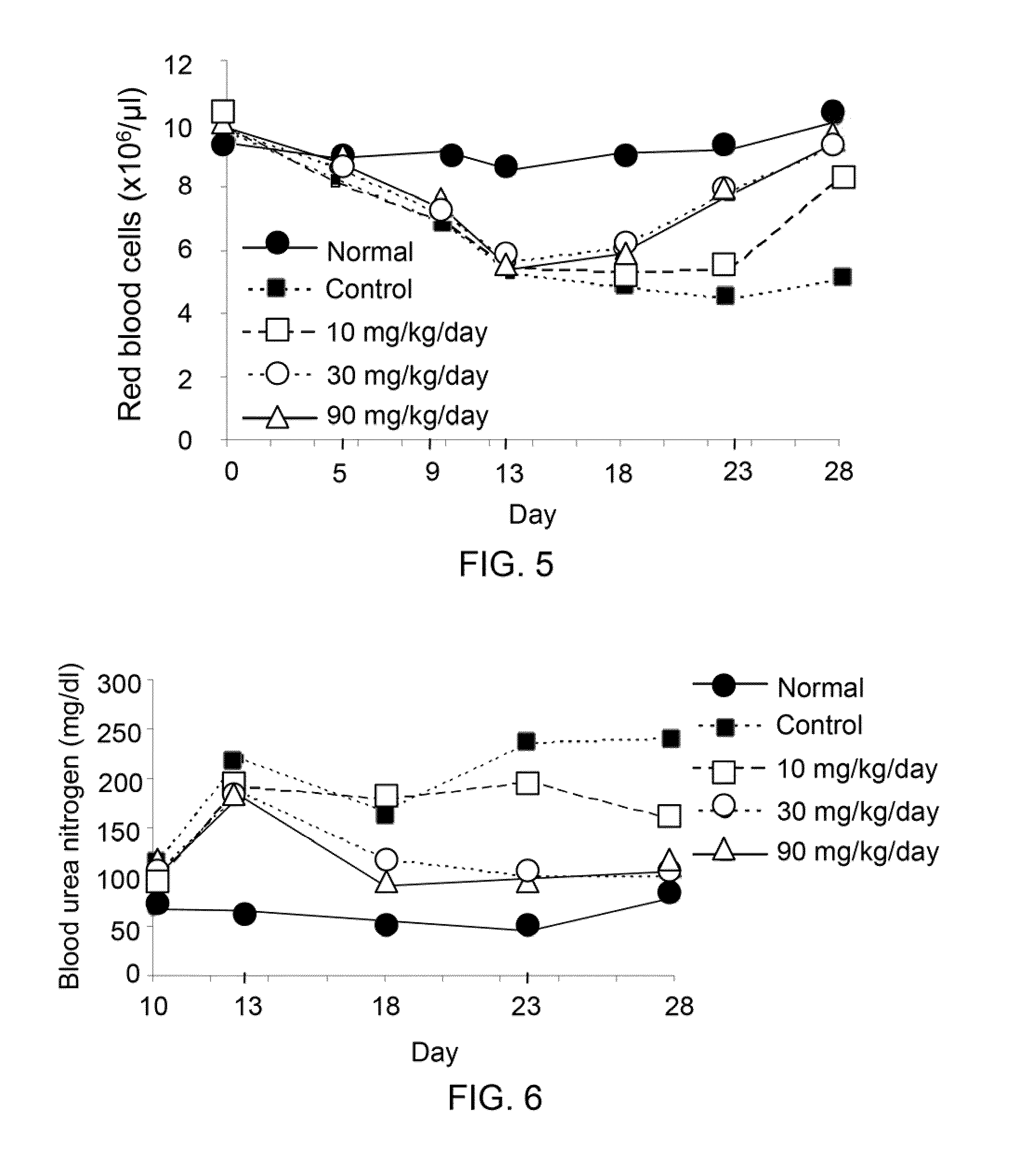
Compounds and methods for enhancing erythropoiesis
Compounds and methods for enhancing erythropoiesis. The compound contains a chemical structure of the formula (I) indicated below, in which R is a glucosyl group. In addition to having an erythropoiesis effect, the compound of the formula (1) is effective in enhancing erythropoietin formation, and increasing kidney function and expression of hepatocyte growth factor. The method includes the step of administering an effective amount of the compound of the formula (I) to a subject in need thereof and thereby results in an enhancement of erythropoiesis.
Owner:NATIONAL YANG MING UNIVERSITY
Protection, restoration, and enhancement of erythropoietin-responsive cells, tissues and organs
InactiveUS7767643B2Restoration and of dysfunctionImprove survivalBiocideSenses disorderWhole bodyMammal
Methods and compositions are provided for protecting or enhancing an erythropoietin-responsive cell, tissue, organ or body part function or viability in vivo, in situ or ex vivo in mammals, including human beings, by systemic or local administration of an erythropoietin receptor activity modulator, such as an erythropoietin or a modified erythropoietin.
Owner:THE KENNETH S WARREN INST
Enhanced erythropoiesis and iron metabolism
ActiveUS20100278941A1Increase productionTo promote metabolismBiocideHeavy metal active ingredientsDiseaseRed blood cell
The present invention relates to methods and compounds for regulating or enhancing erthropoiesis and iron metabolism, and for treating or preventing iron deficiency and anemia of chronic disease.
Owner:FIBROGEN INC
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveCN1913896AOrganic active ingredientsPeptide/protein ingredientsBeta thalassemiaSickle cell anemia
Owner:CELGENE CORP
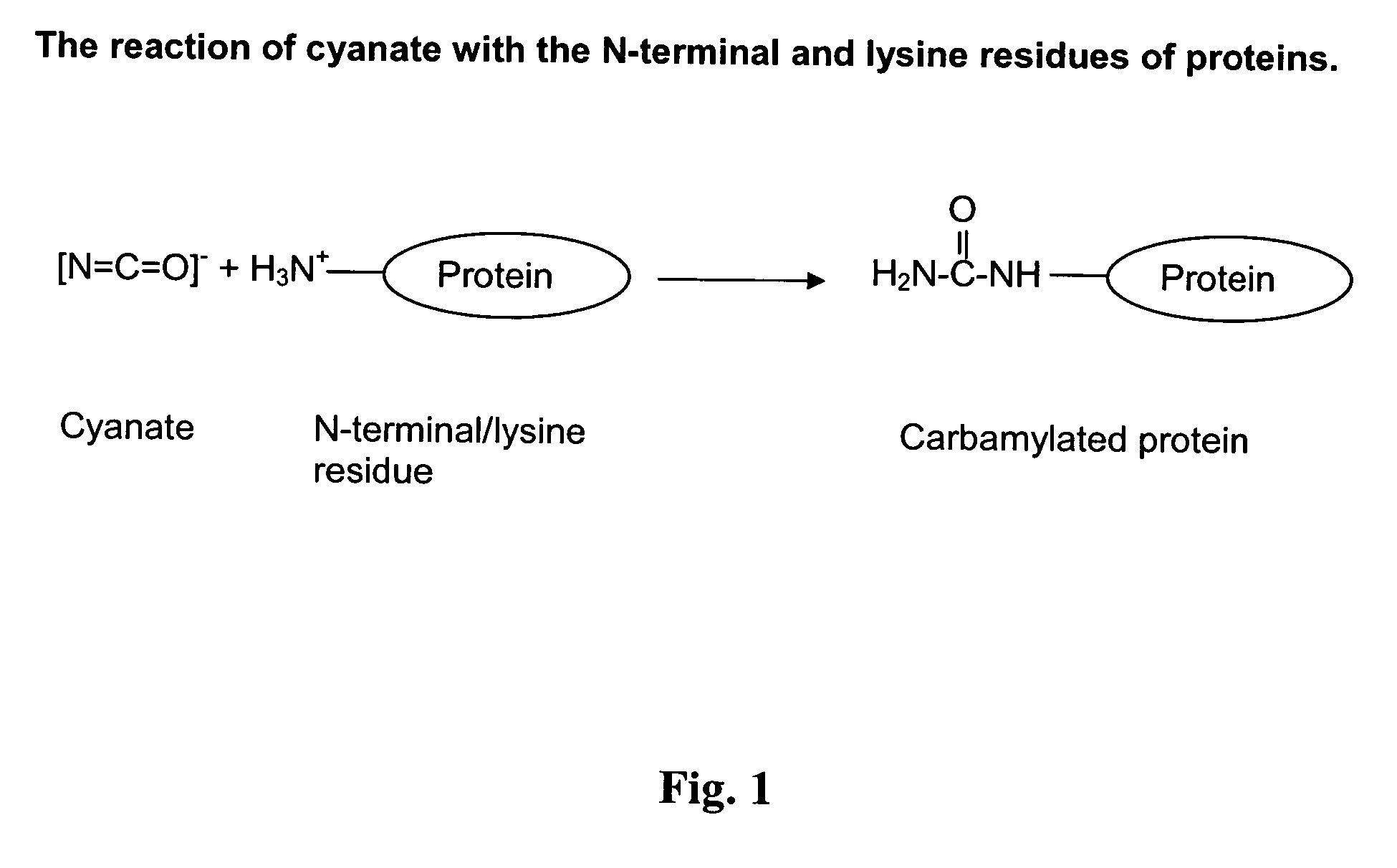
Novel carbamylated EPO and method for its production
InactiveUS20060135754A1Low formationMinimum lossPeptide/protein ingredientsDepsipeptidesMedicineCarbamylated erythropoietin
The present invention discloses a method for production of novel carbamylated erythropoietin and compositions comprising the novel carbamylated erythropoietin and pharmaceutical compositions comprising this and uses thereof.
Owner:H LUNDBECK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com