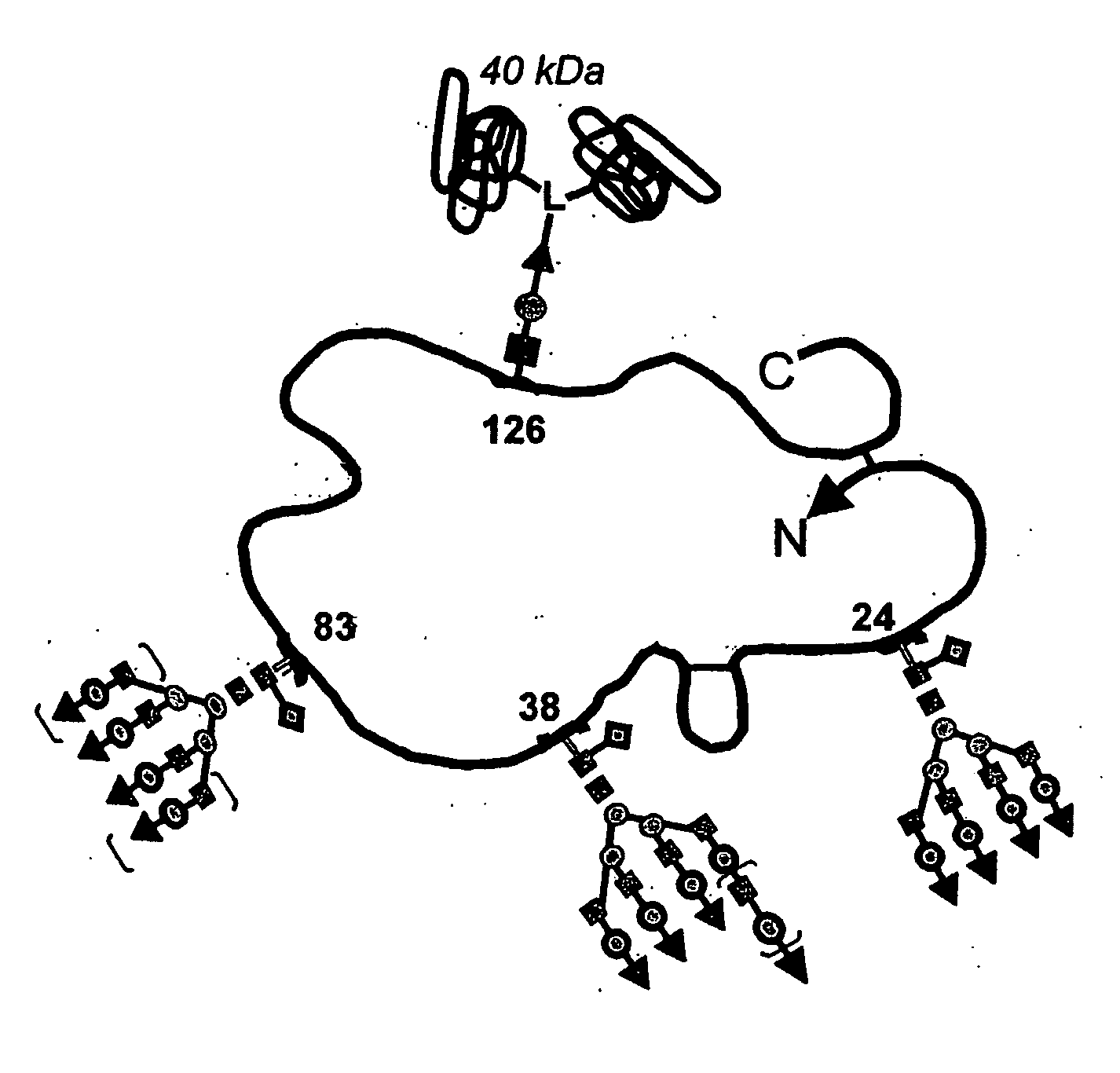
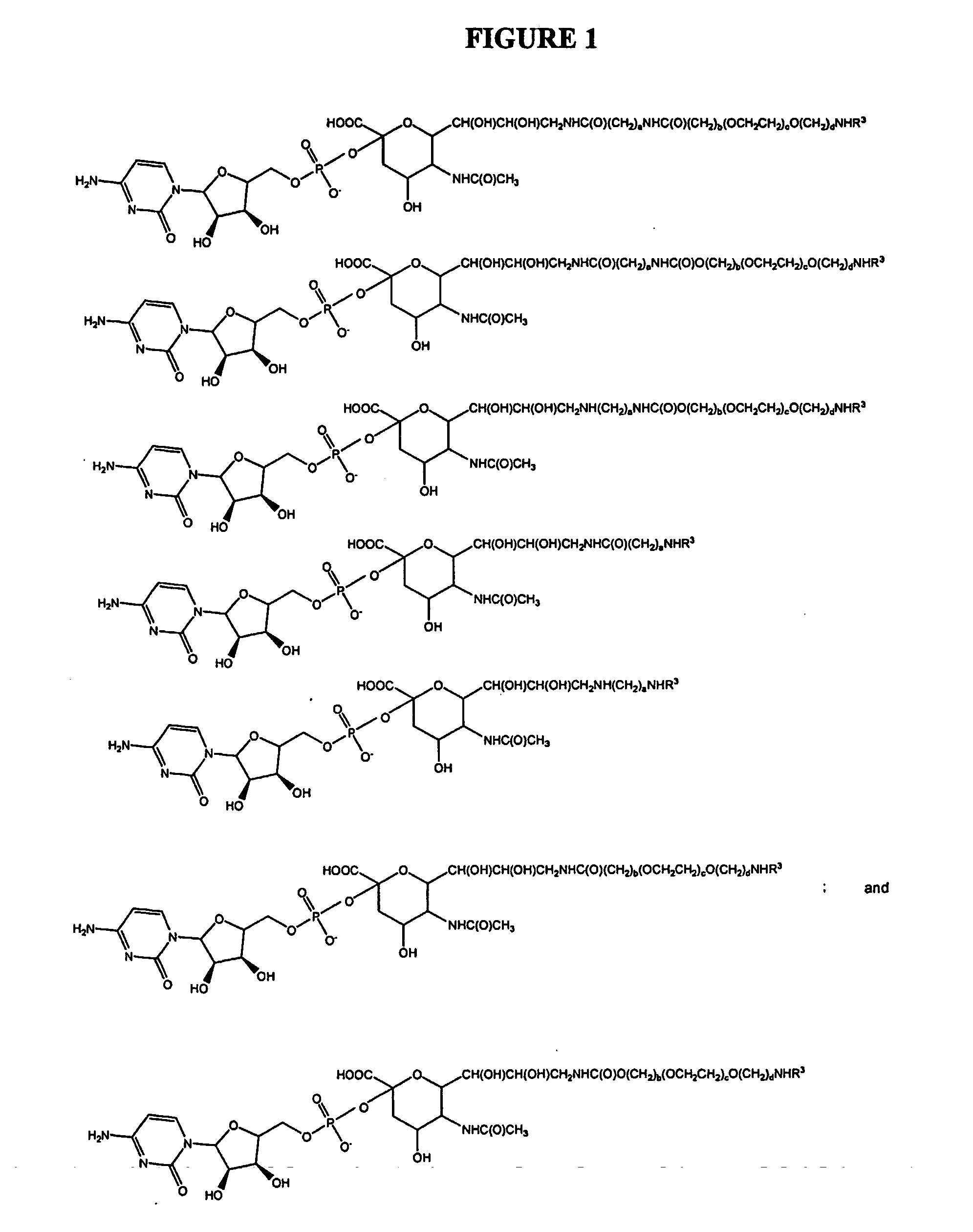
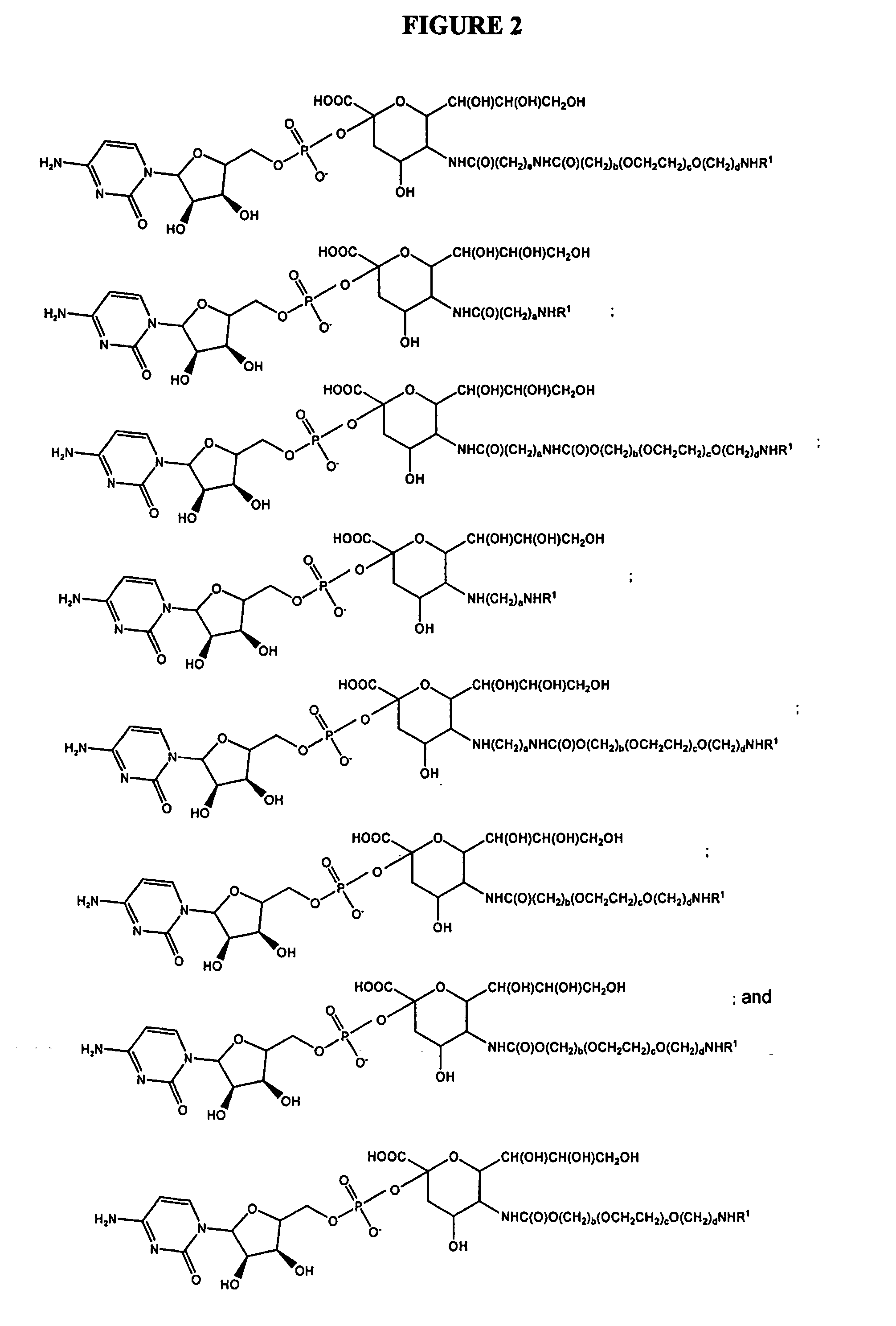
Glycopegylated Erythropoietin
a technology of erythropoietin and erythropoietin, which is applied in the field of glycopegylated erythropoietin, can solve the problems of microheterogeneity, protein glycosylation, and numerous problems, and achieve the effect of improving pharmacokinetic properties and cost-effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of UDP-GalNAc-6′-CHO
[0371] UDP-GalNAc (200 mg, 0.30 mmoles) was dissolved in a 1 mM CuSO4 solution (20 mL) and a 25 mM NaH2PO4 solution (pH 6.0; 20 mL). Galactose oxidase (240 U; 240 μL) and catalase (13000 U; 130 μL) were then added, the reaction system equipped with a balloon filled with oxygen and stirred at room temperature for seven days. The reaction mixture was then filtered (spin cartridge; MWCO 5K) and the filtrate (˜40 mL) was stored at 4° C. until required. TLC (silica; EtOH / water (7 / 2); Rf=0.77; visualized with anisaldehyde stain).
example 2
Preparation of UDP-GalNAc-6′-NH2):
[0372] Ammonium acetate (15 mg, 0.194 mmoles) and NaBH3CN (1M THF solution; 0.17 mL, 0.17 mmoles) were added to the UDP-GalNAc-6′-CHO solution from above (2 mL or ˜20 mg) at 0° C. and allowed to warm to room temperature overnight. The reaction was filtered through a G-10 column with water and the product collected. The appropriate fractions were freeze-dried and stored frozen. TLC (silica; ethanol / water (7 / 2); Rf=0.72; visualized with ninhydrin reagent).
example 3
Preparation of UDP-GalNAc-6-NHCO(CH2)2—O-PEG-OMe (1 KDa).
[0373] The galactosaminyl-1-phosphate-2-NHCO(CH2)2—O-PEG-OMe (1 KDa) (58 mg, 0.045 mmoles) was dissolved in DMF (6 mL) and pyridine (1.2 mL). UMP-morpholidate (60 mg, 0.15 mmoles) was then added and the resulting mixture stirred at 70° C. for 48 h. The solvent was removed by bubbling nitrogen through the reaction mixture and the residue purified by reversed phase chromatography (C-18 silica, step gradient between 10 to 80%, methanol / water). The desired fractions were collected and dried at reduced pressure to yield 50 mg (70%) of a white solid. TLC (silica, propanol / H2O / NH4OH, (30 / 20 / 2), Rf=0.54). MS (MALDI): Observed, 1485, 1529, 1618, 1706.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| effective radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com