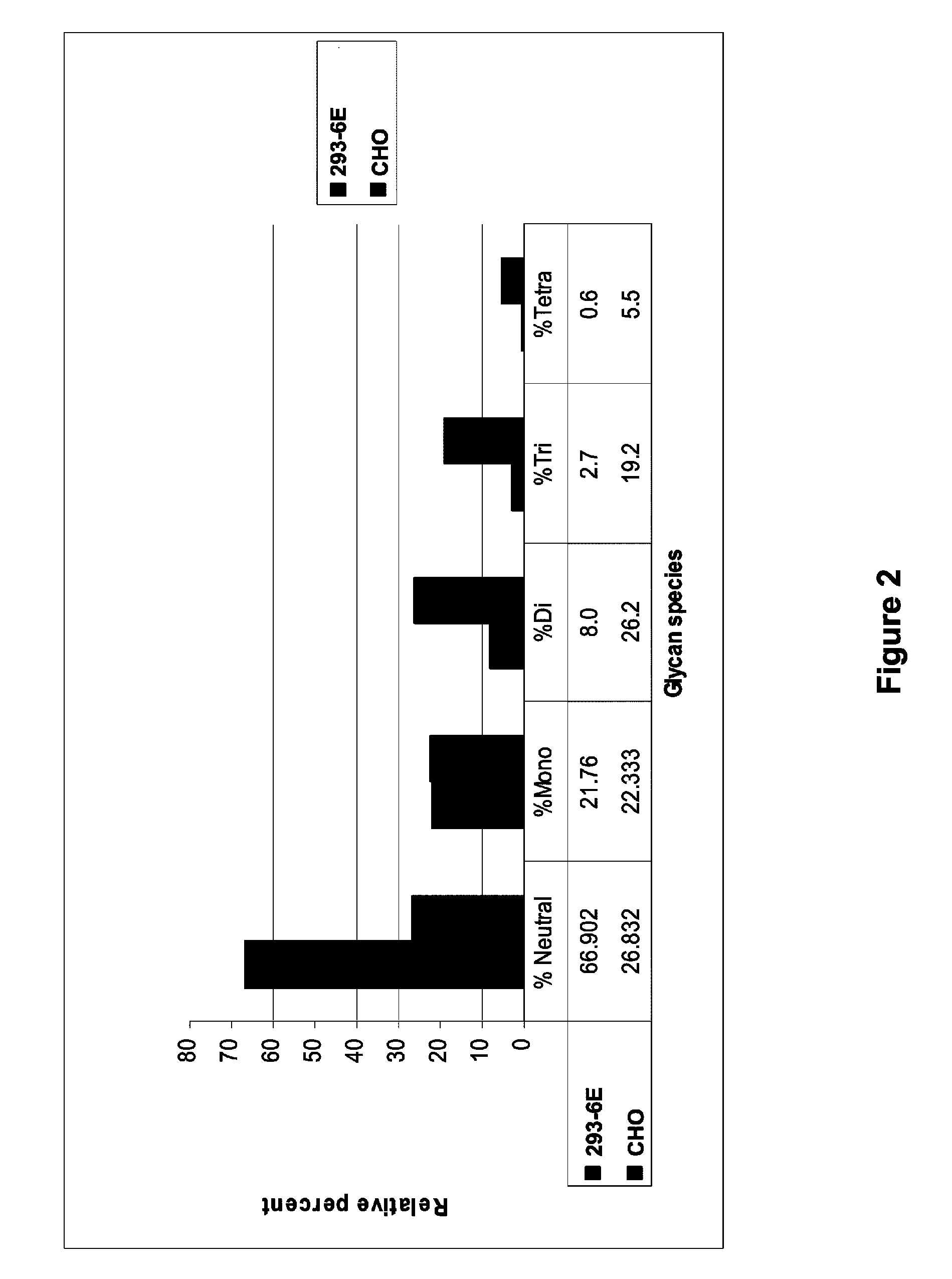
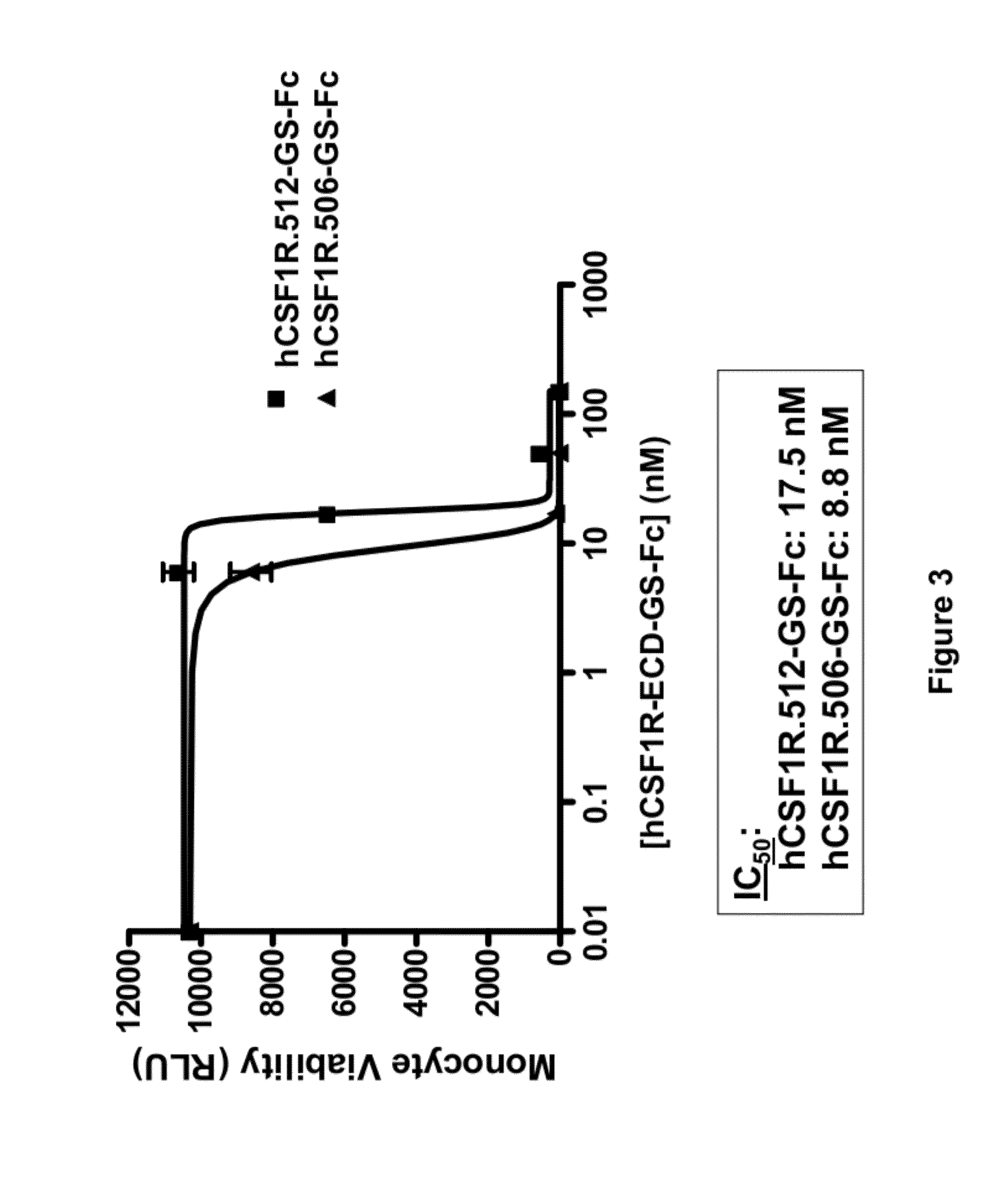
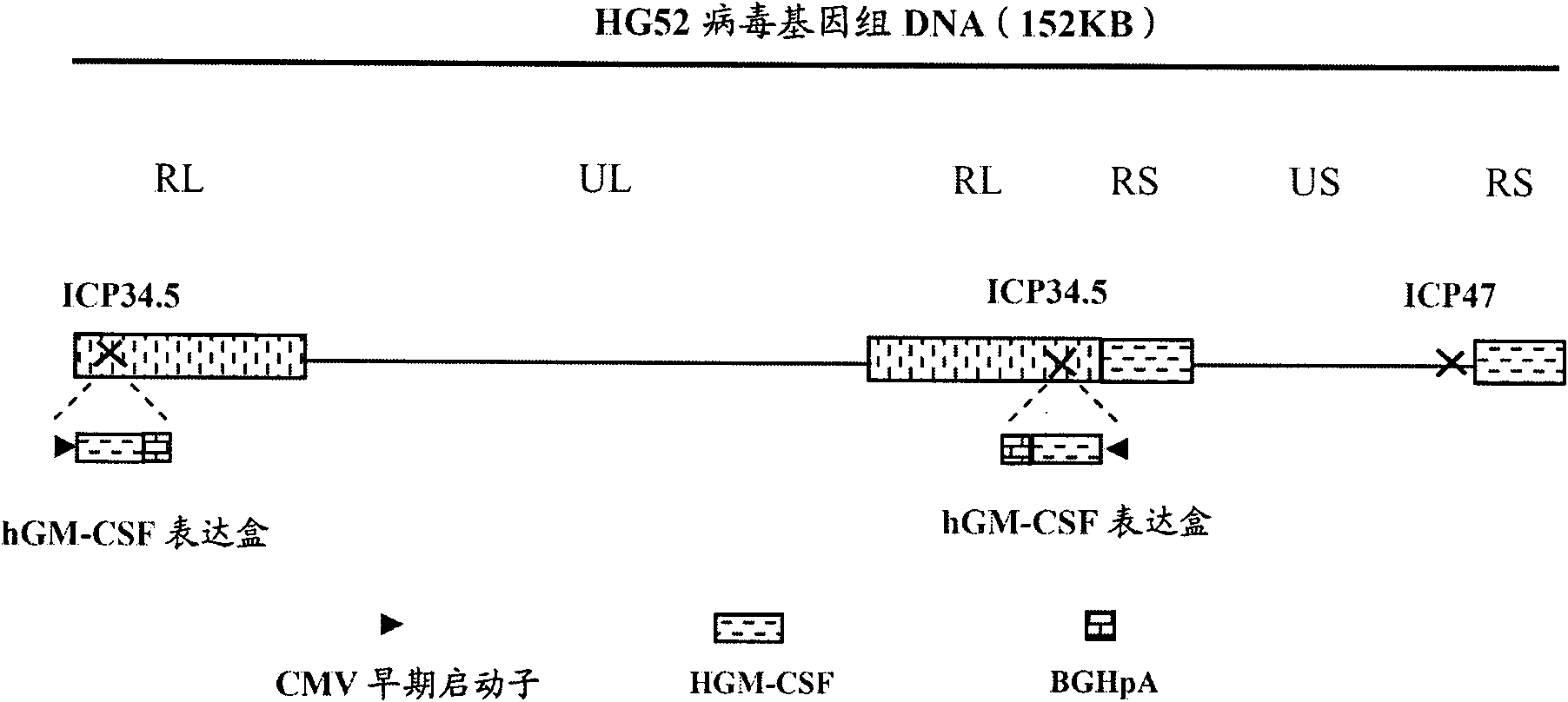
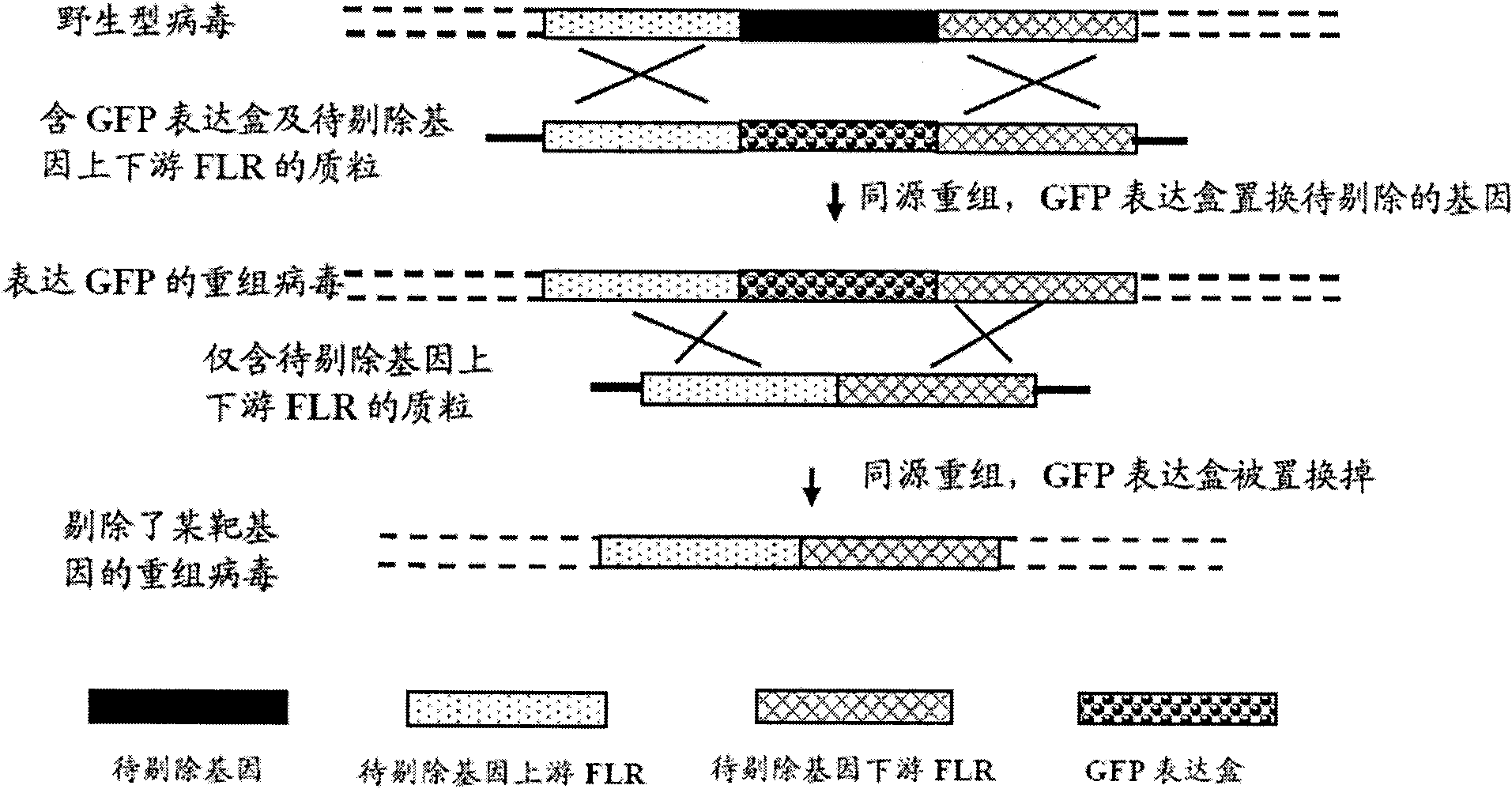
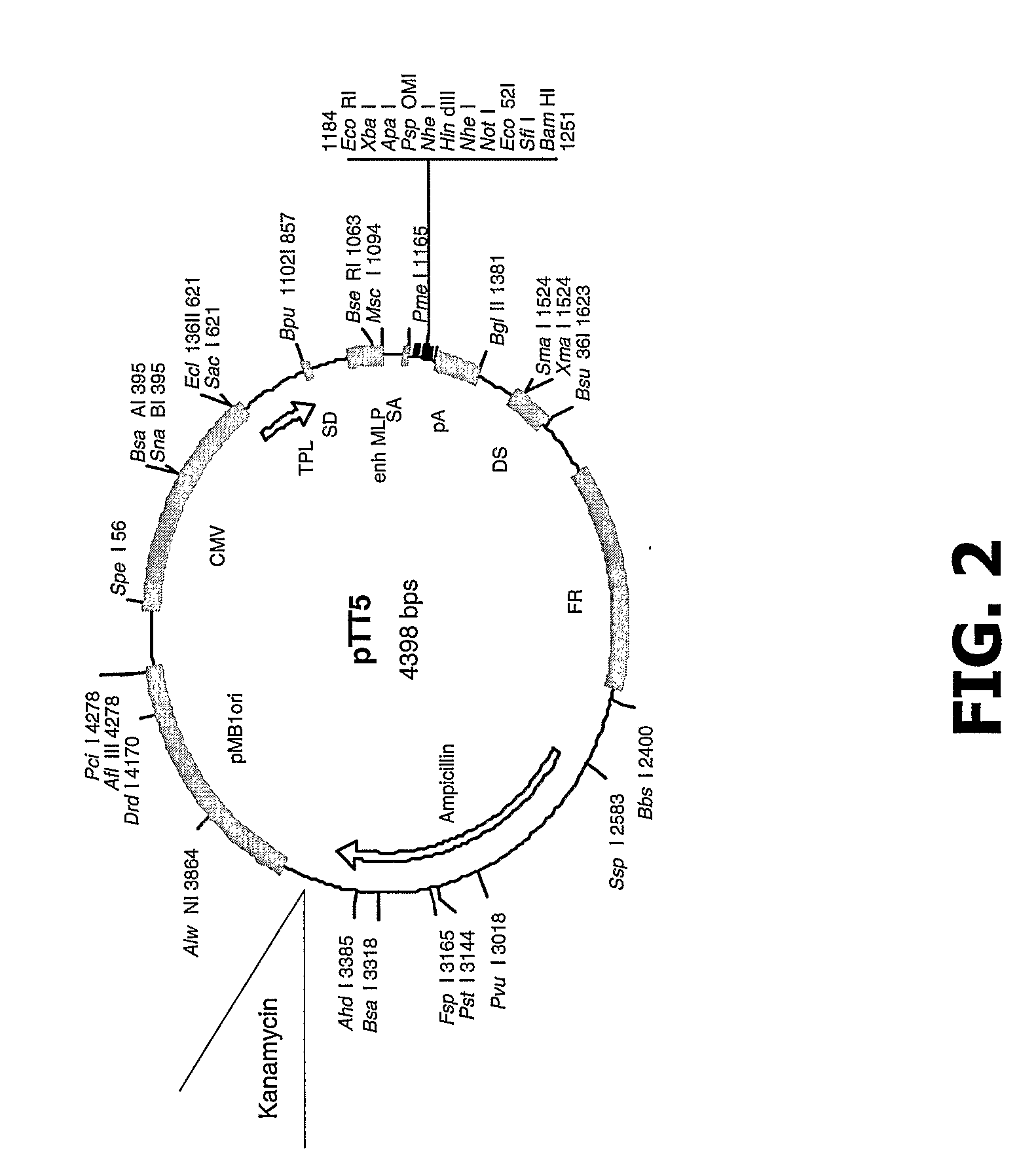
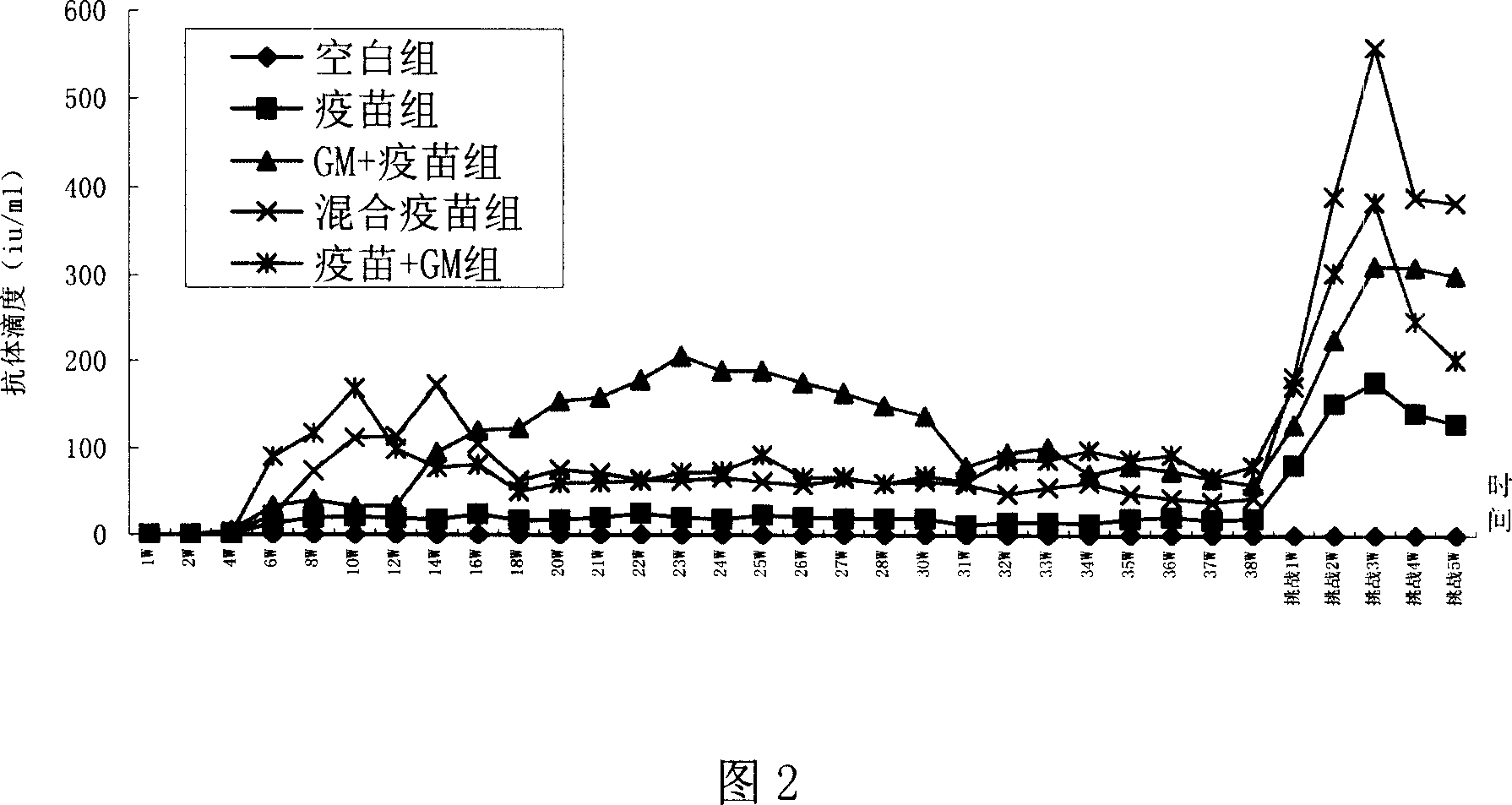
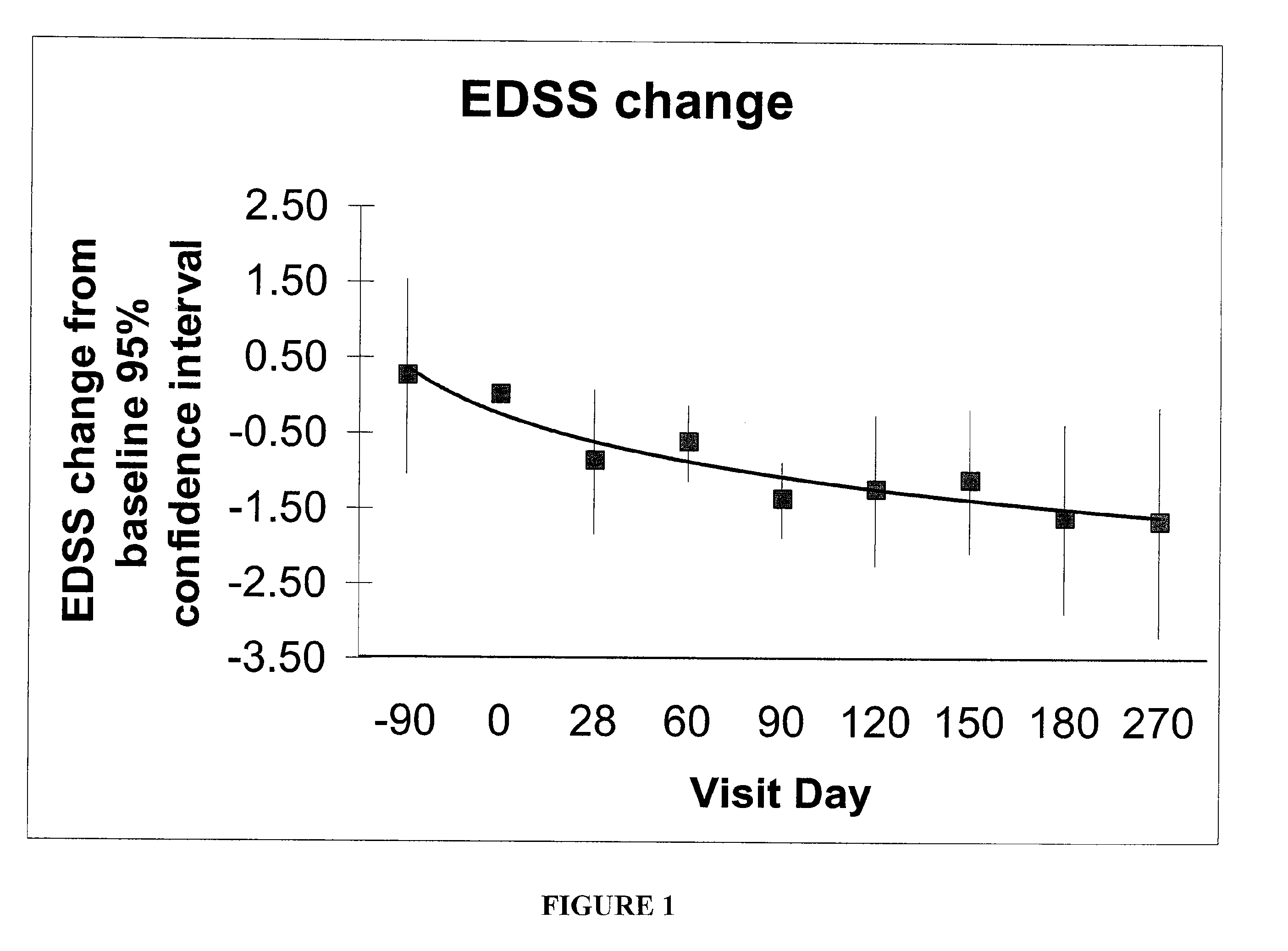
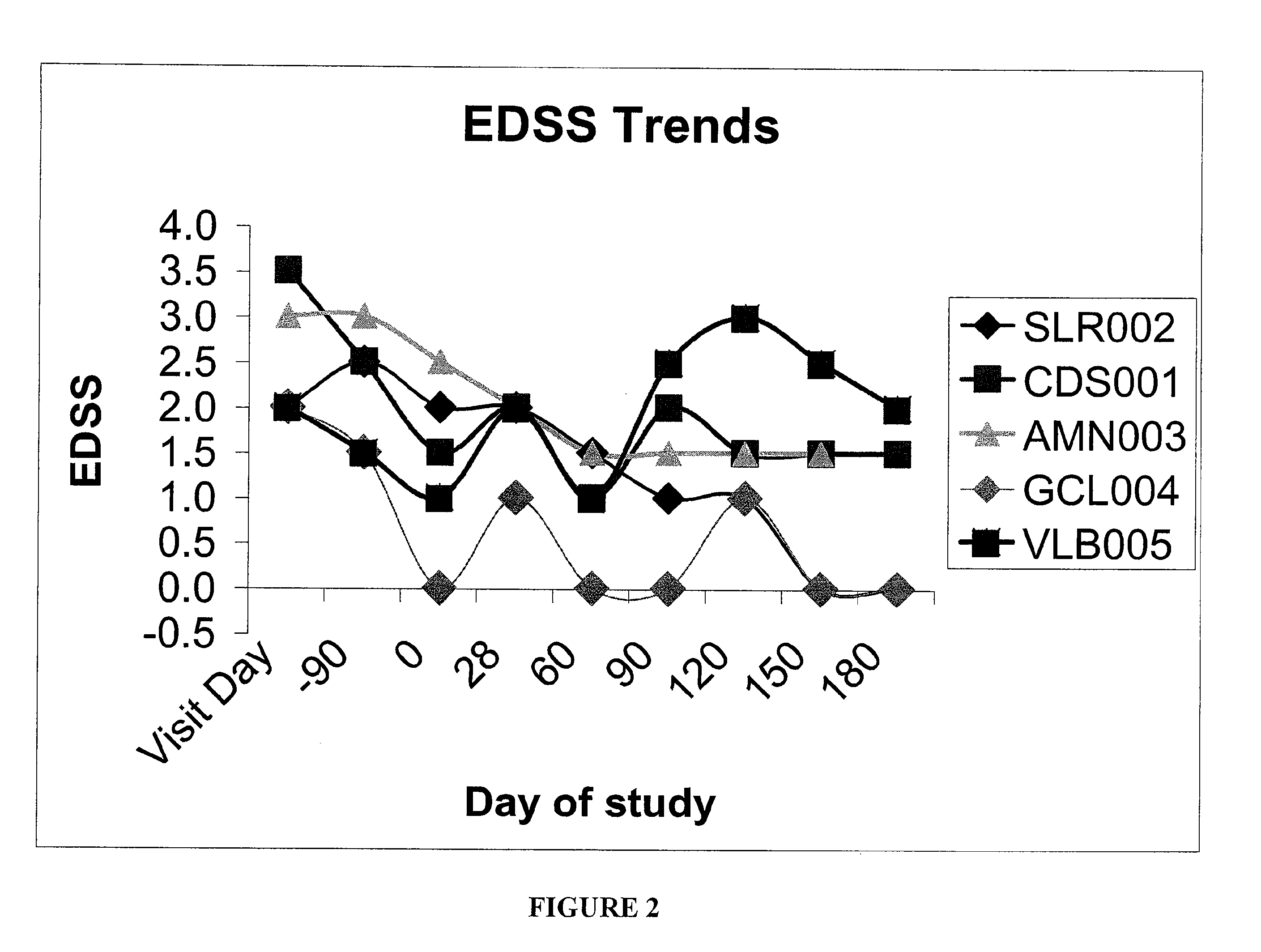
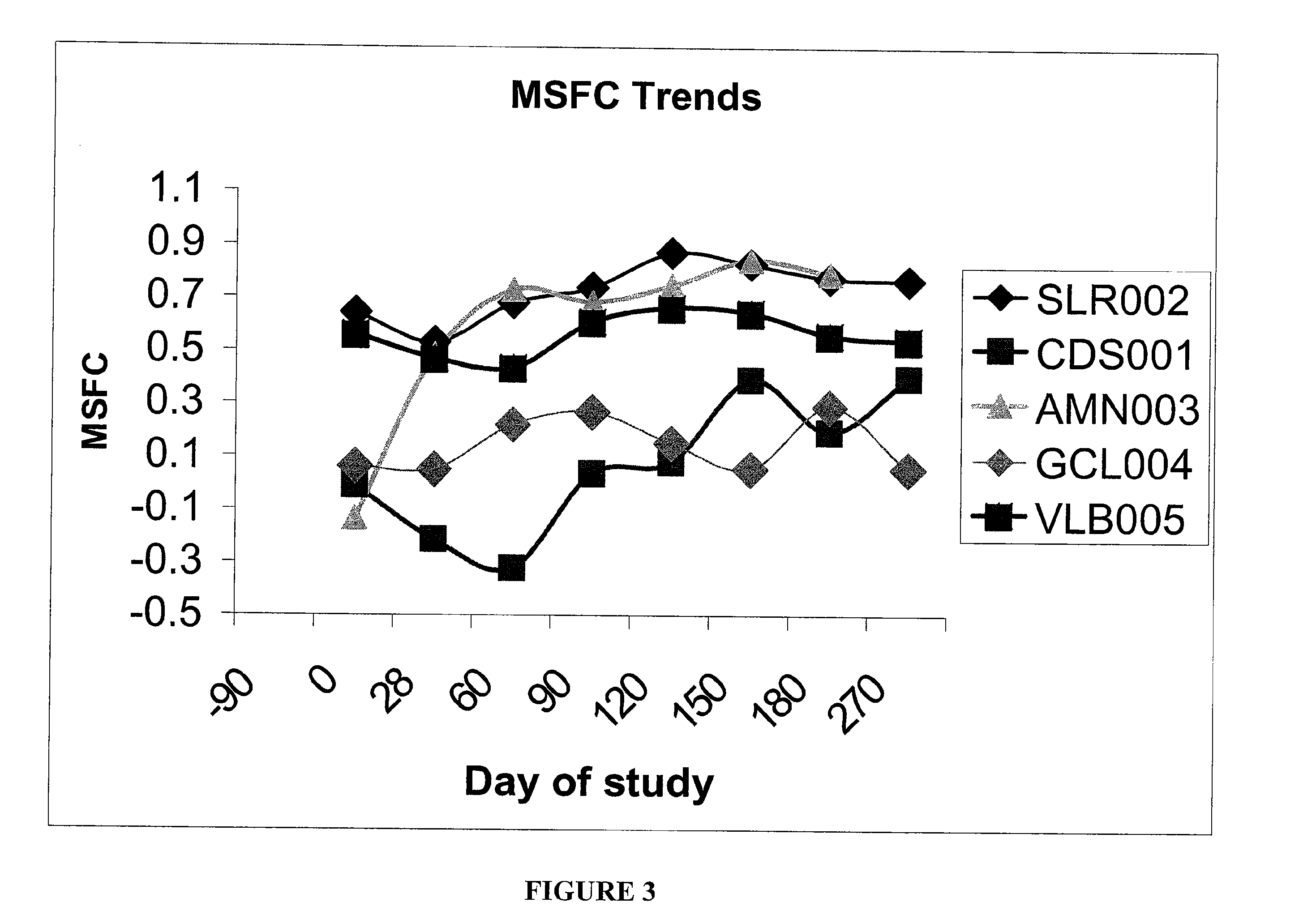
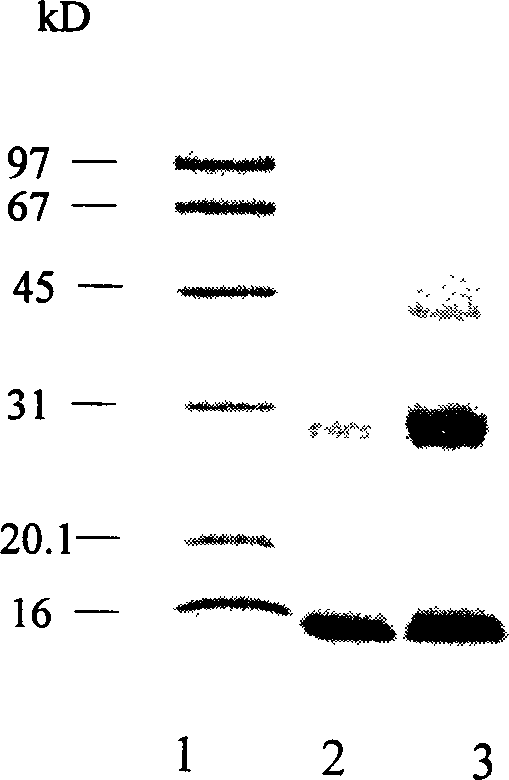
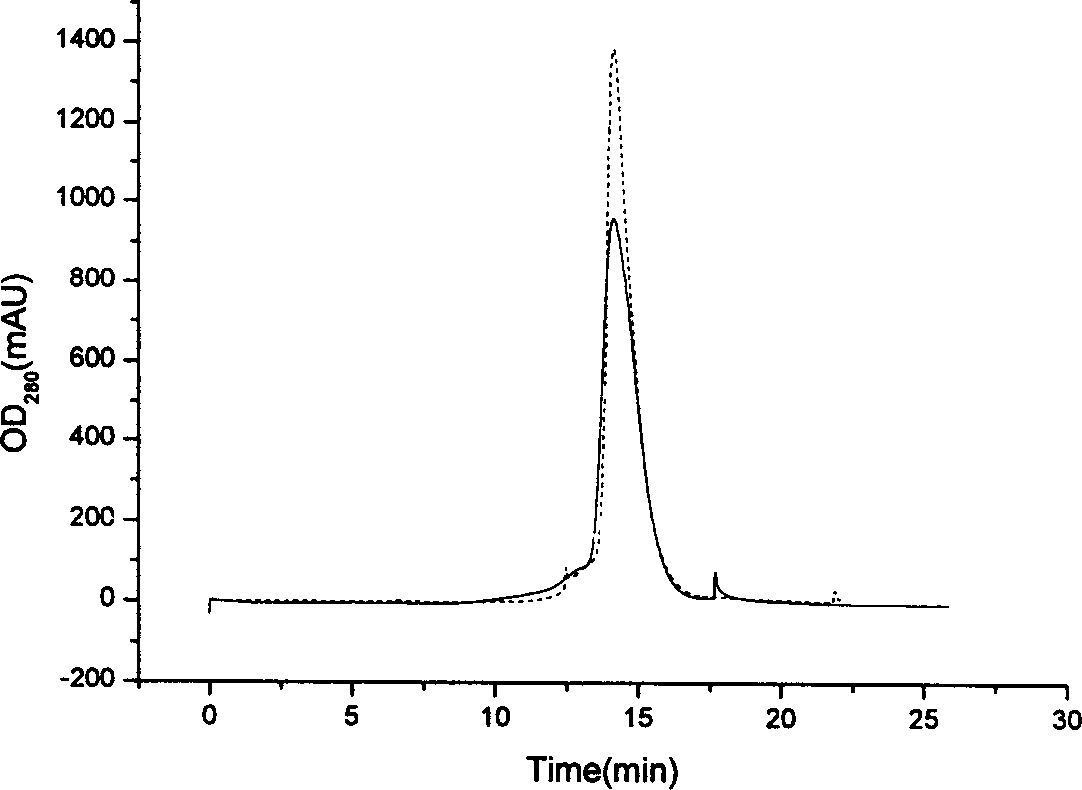
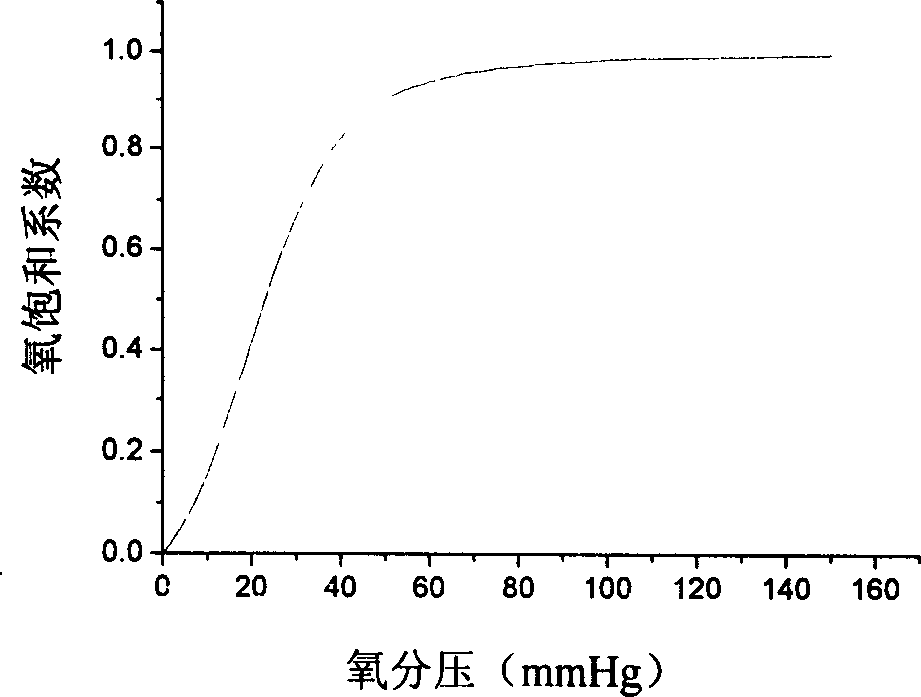
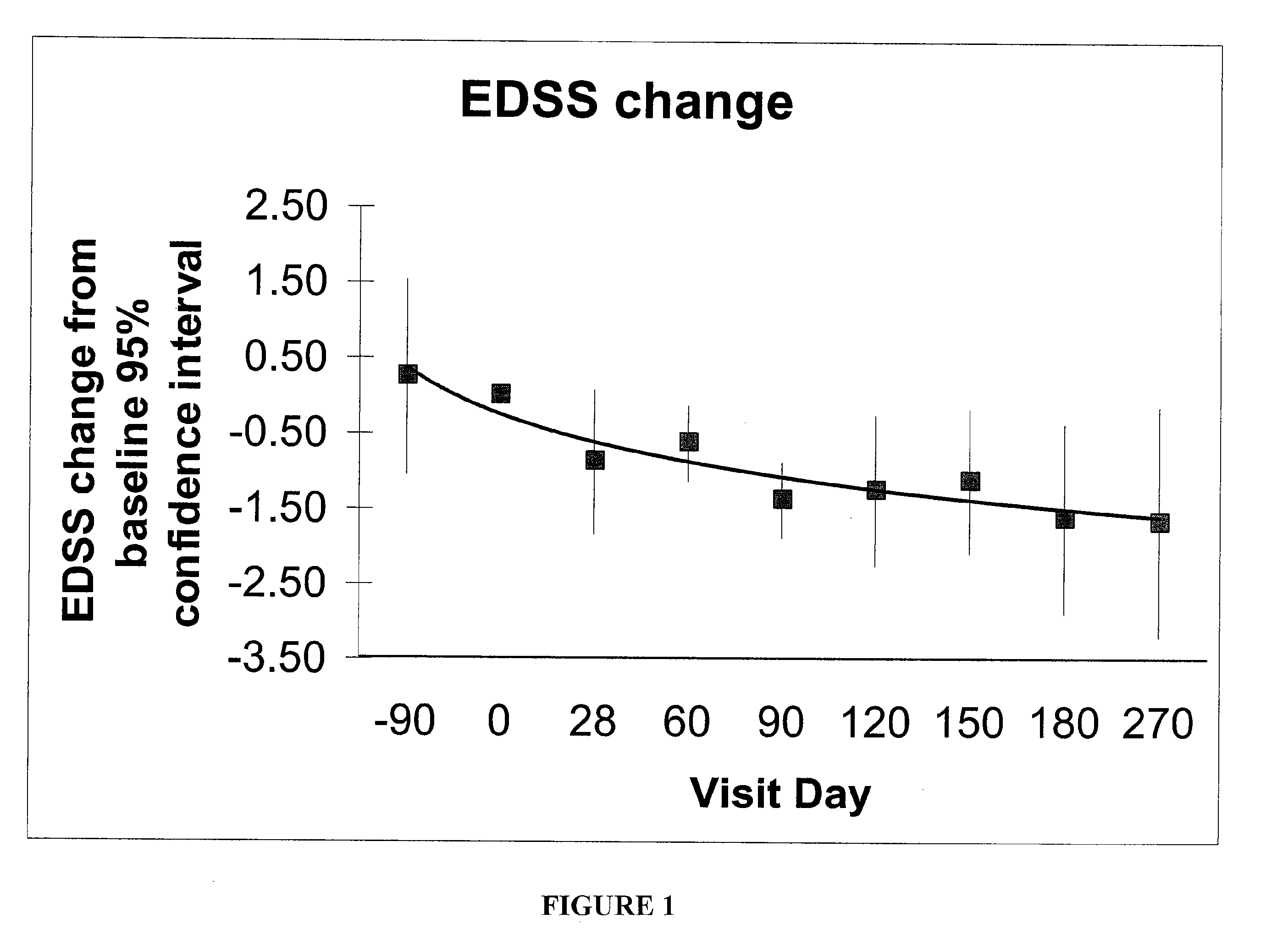
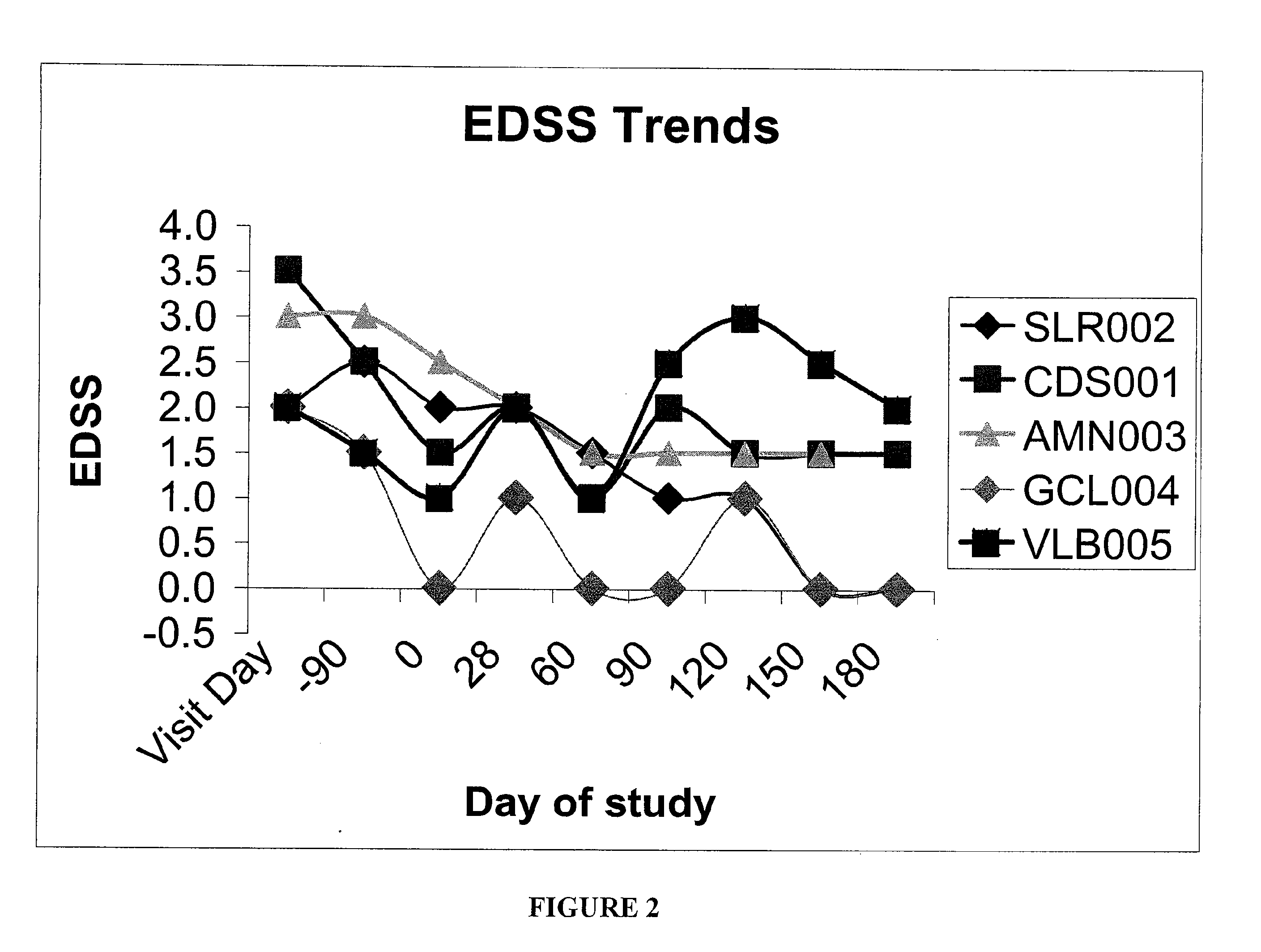
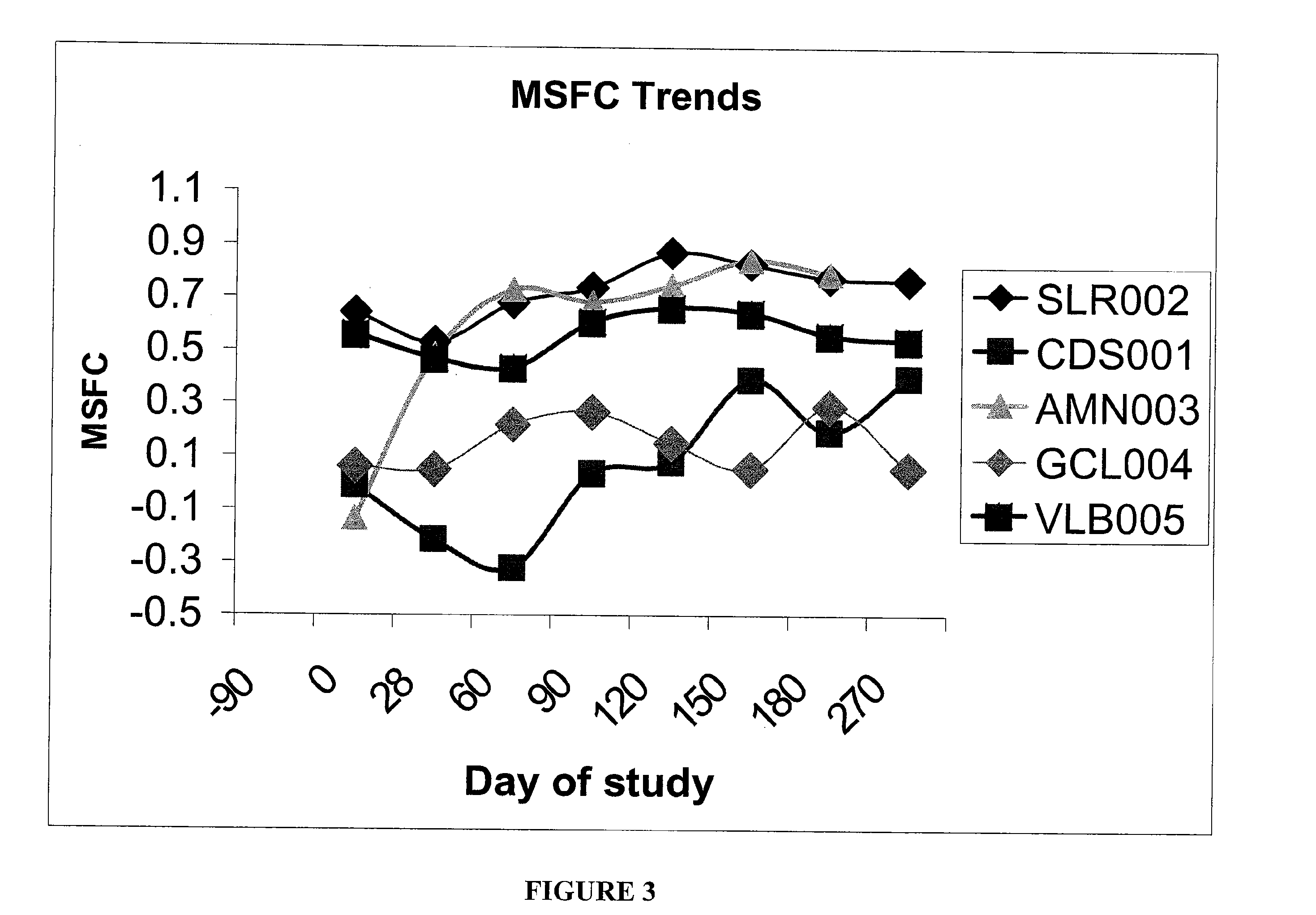
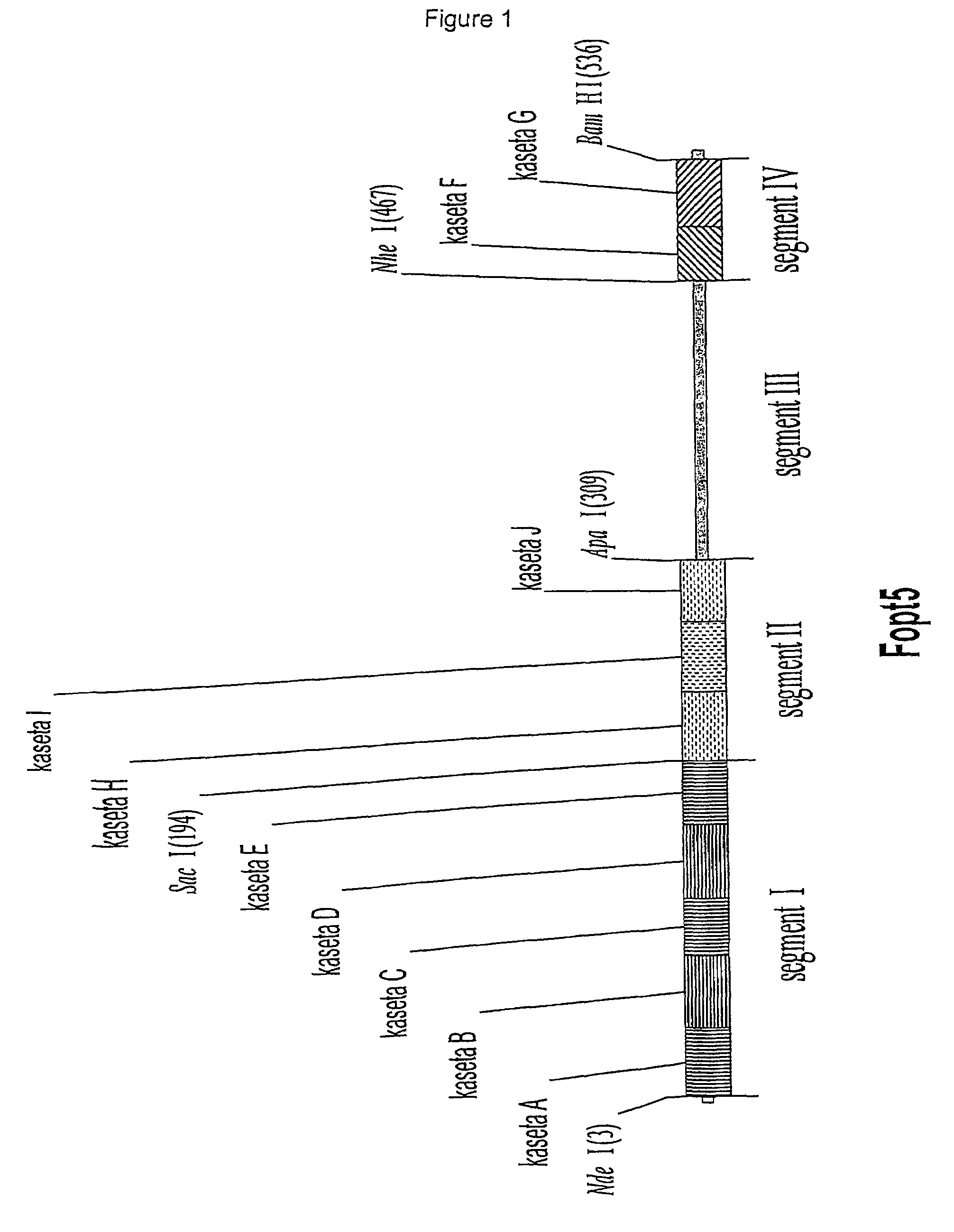
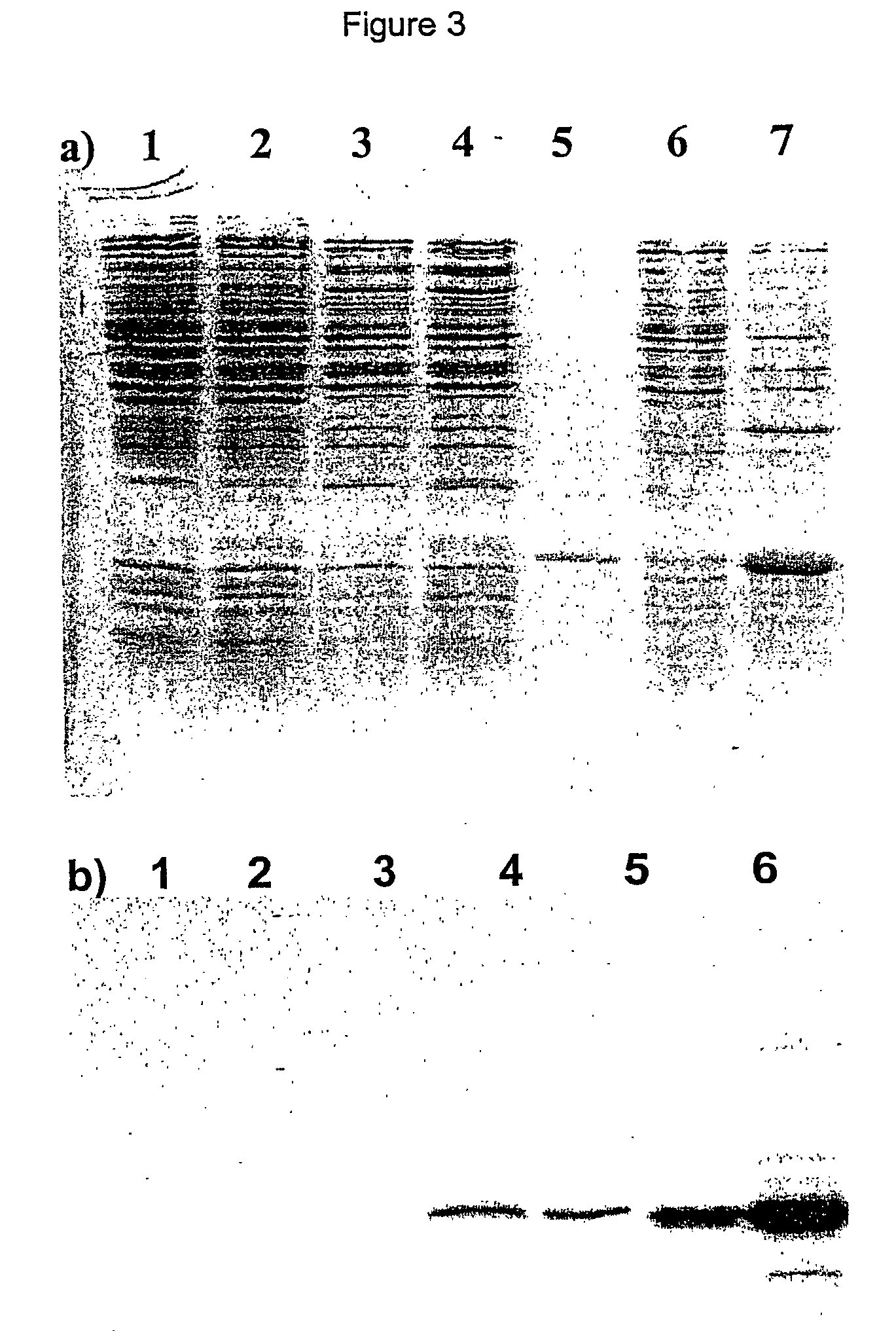
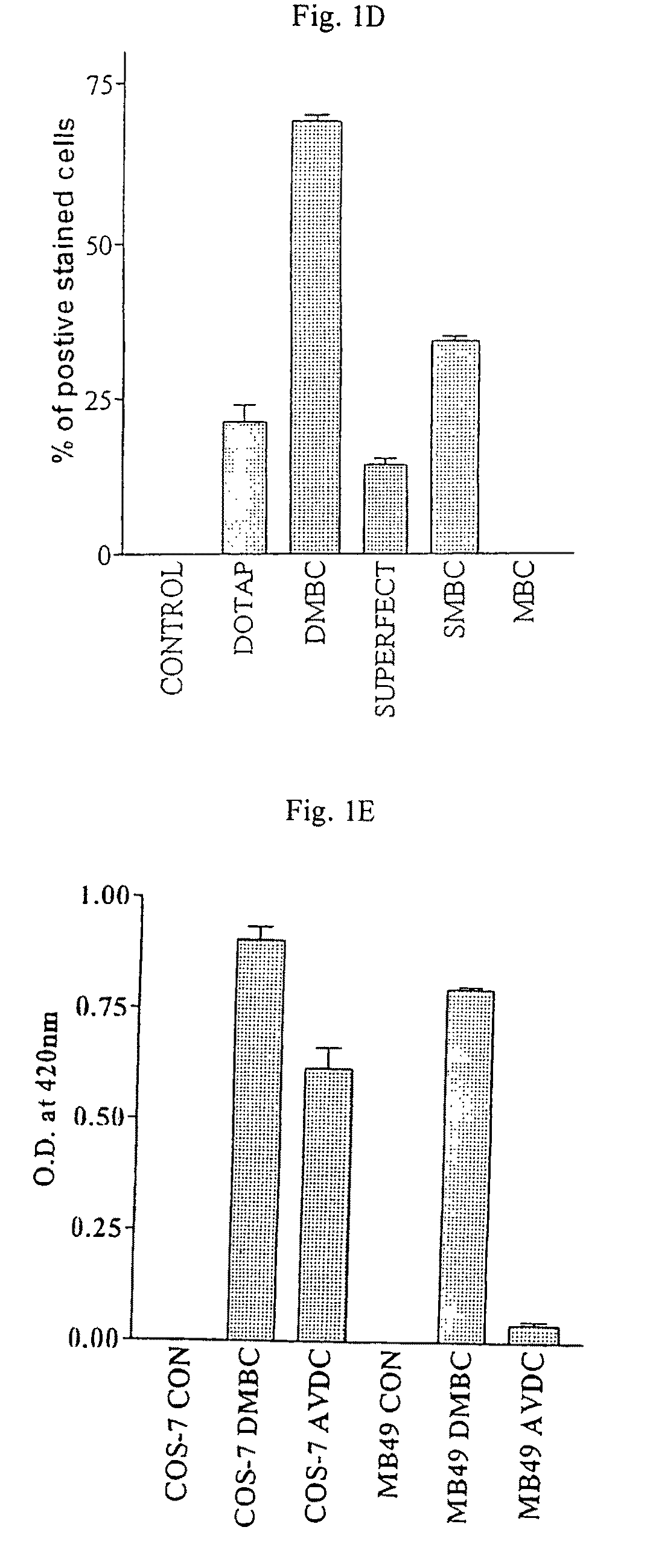
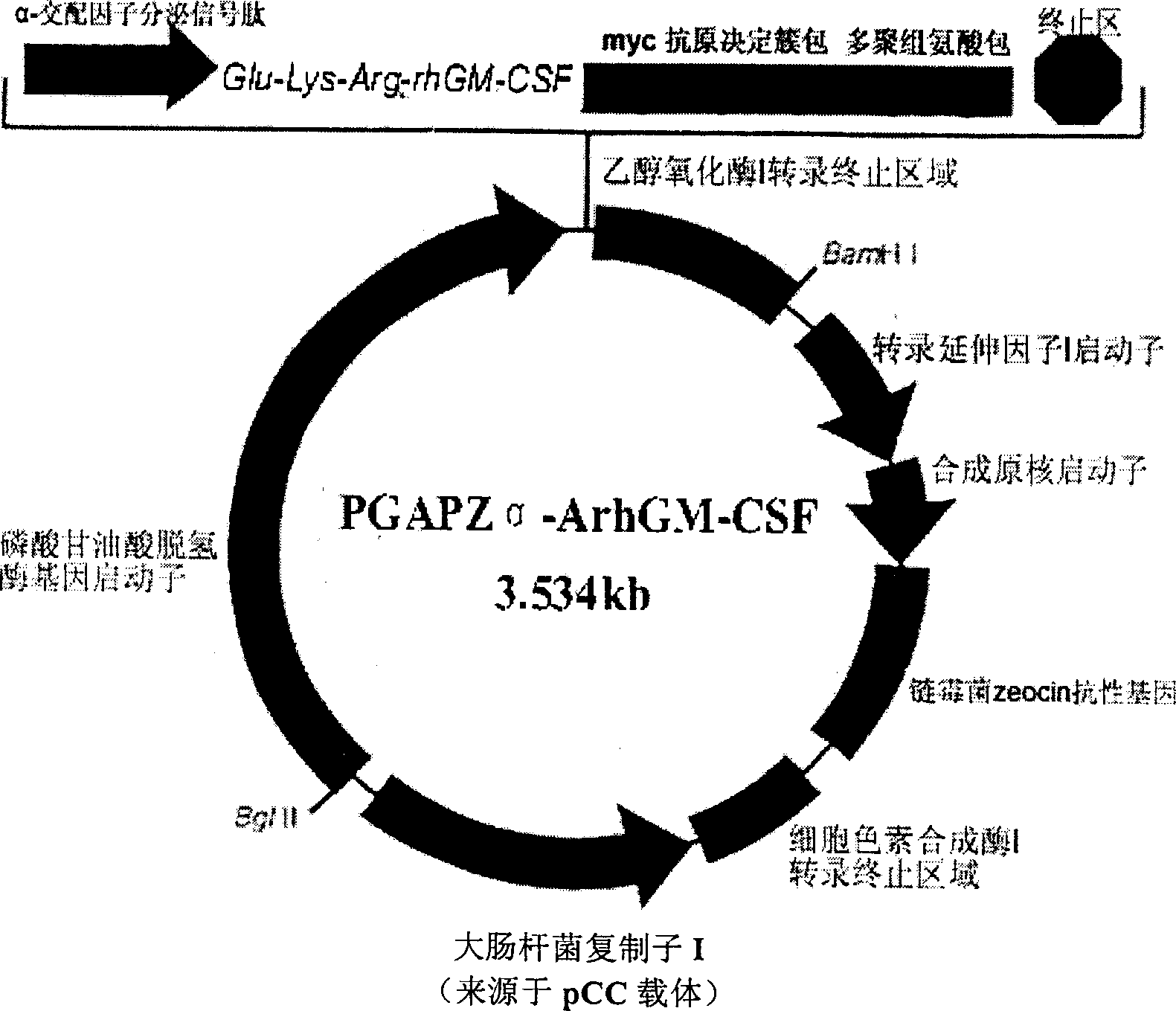
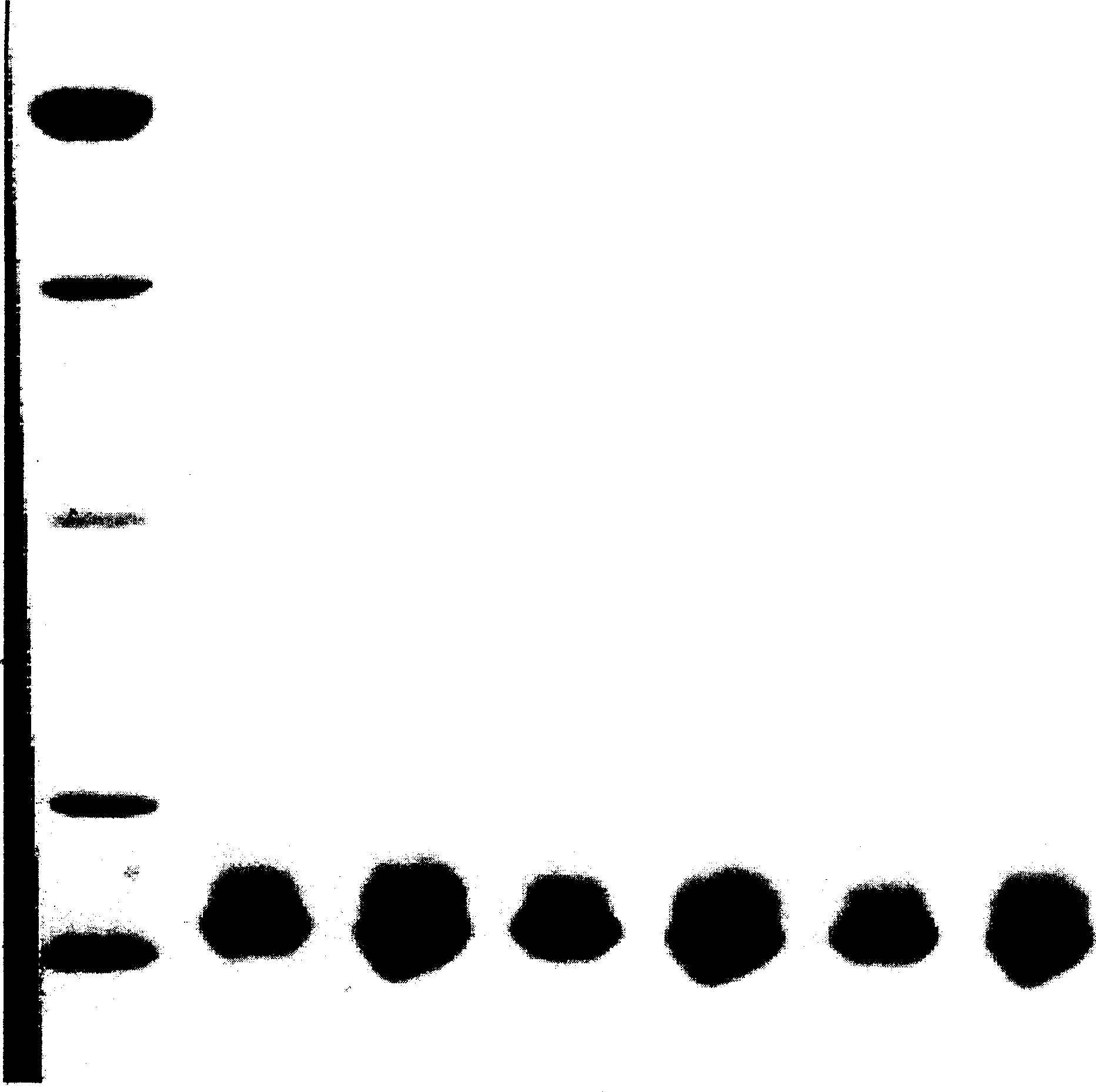
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
174 results about "Colony-stimulating factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colony-stimulating factors (CSFs) are secreted glycoproteins that bind to receptor proteins on the surfaces of hemopoietic stem cells, thereby activating intracellular signaling pathways that can cause the cells to proliferate and differentiate into a specific kind of blood cell (usually white blood cells. For red blood cell formation, see erythropoietin).
Methods of treating inflammation using antibodies to M-CSF
InactiveUS7108852B2Inhibiting and minimizing capabilityInhibiting and minimizing accumulationSnake antigen ingredientsImmunoglobulins against cytokines/lymphokines/interferonsAutoimmune responsesAutoimmune disease
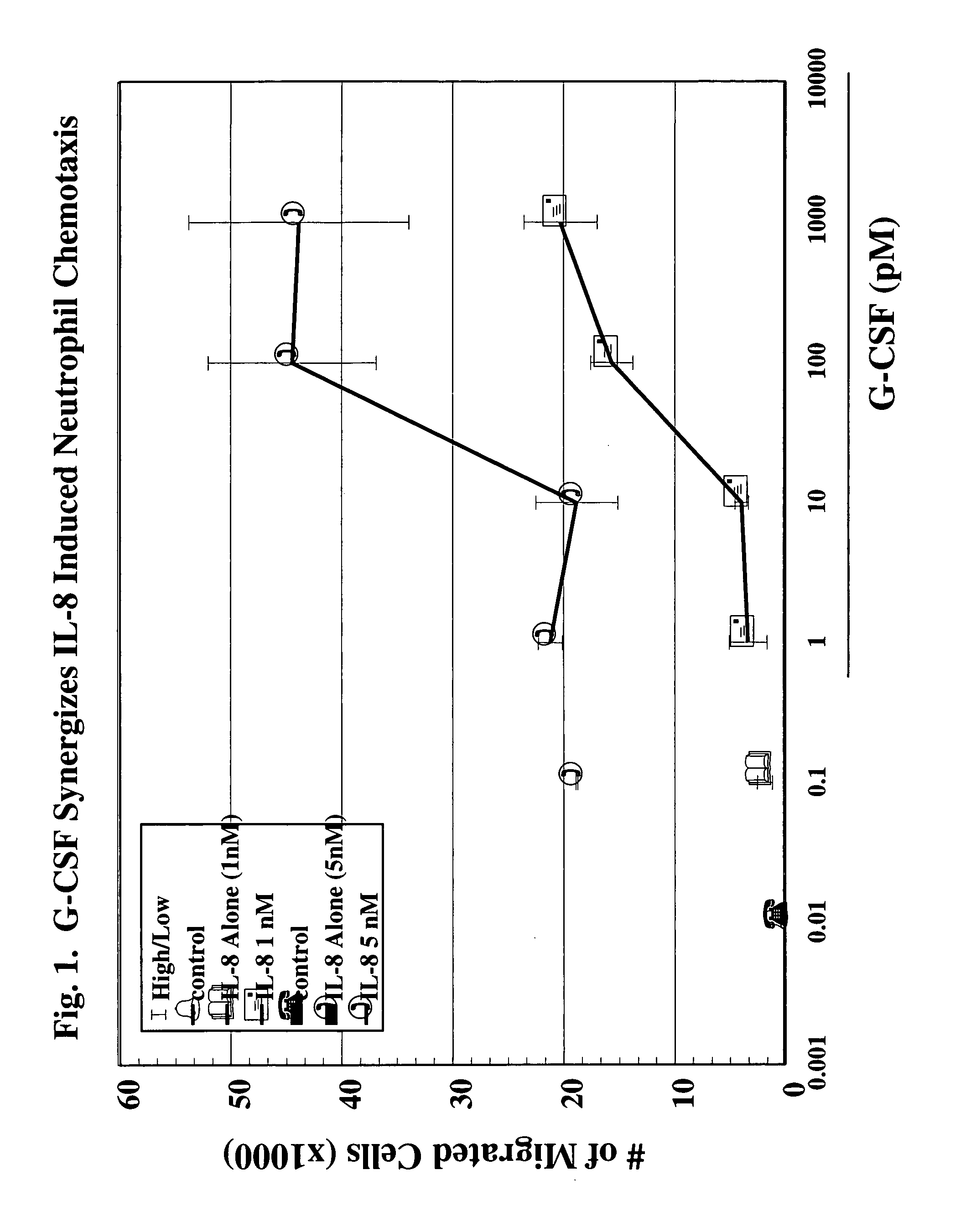
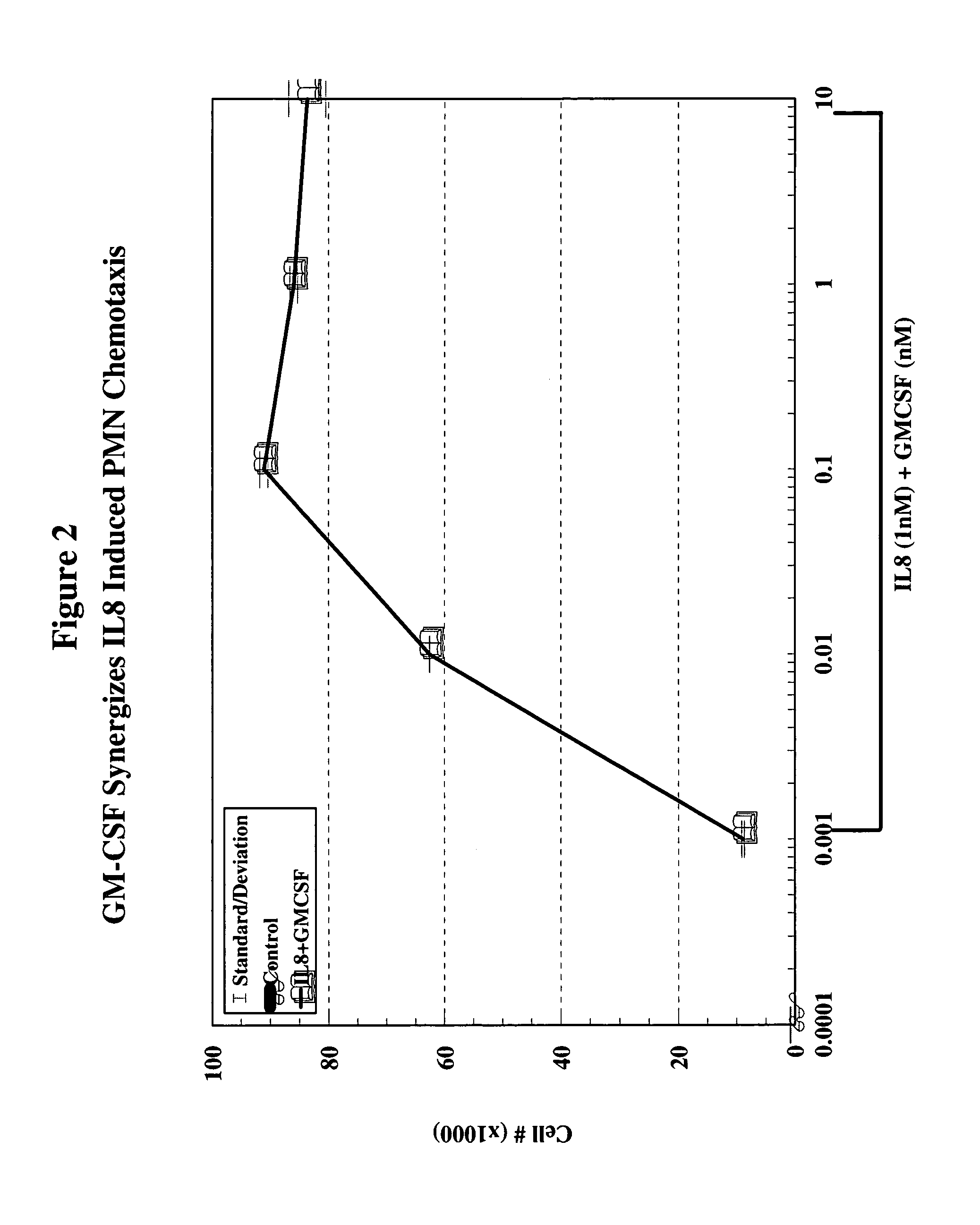
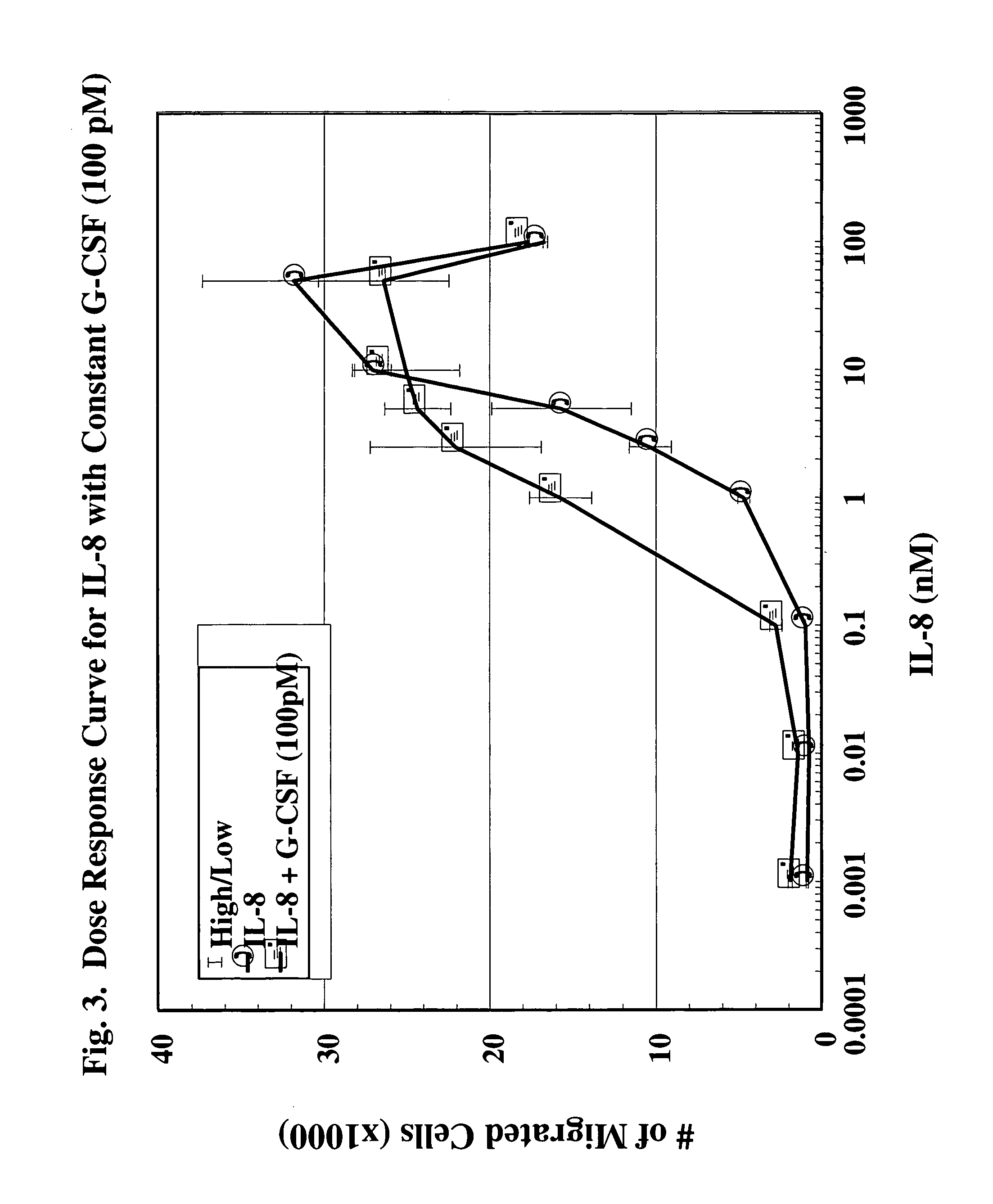
A hematopoetic factor called “colony stimulating factor” (CSF) is capable of synergizing the attracting capabilities of chemokines and of inducing the accumulation and / or activation in vitro and in vivo of key components of inflammatory responses. Various types of agents that inhibit or otherwise hinder the production, release or activity of CSF could be used therapeutically in the treatment of ischemia and other inflammatory diseases, such as autoimmune disease, and various chronic inflammatory diseases such as rheumatoid arthritis and psoriasis.
Owner:WARNER-LAMBERT CO
G-csf site-specific mono-conjugates
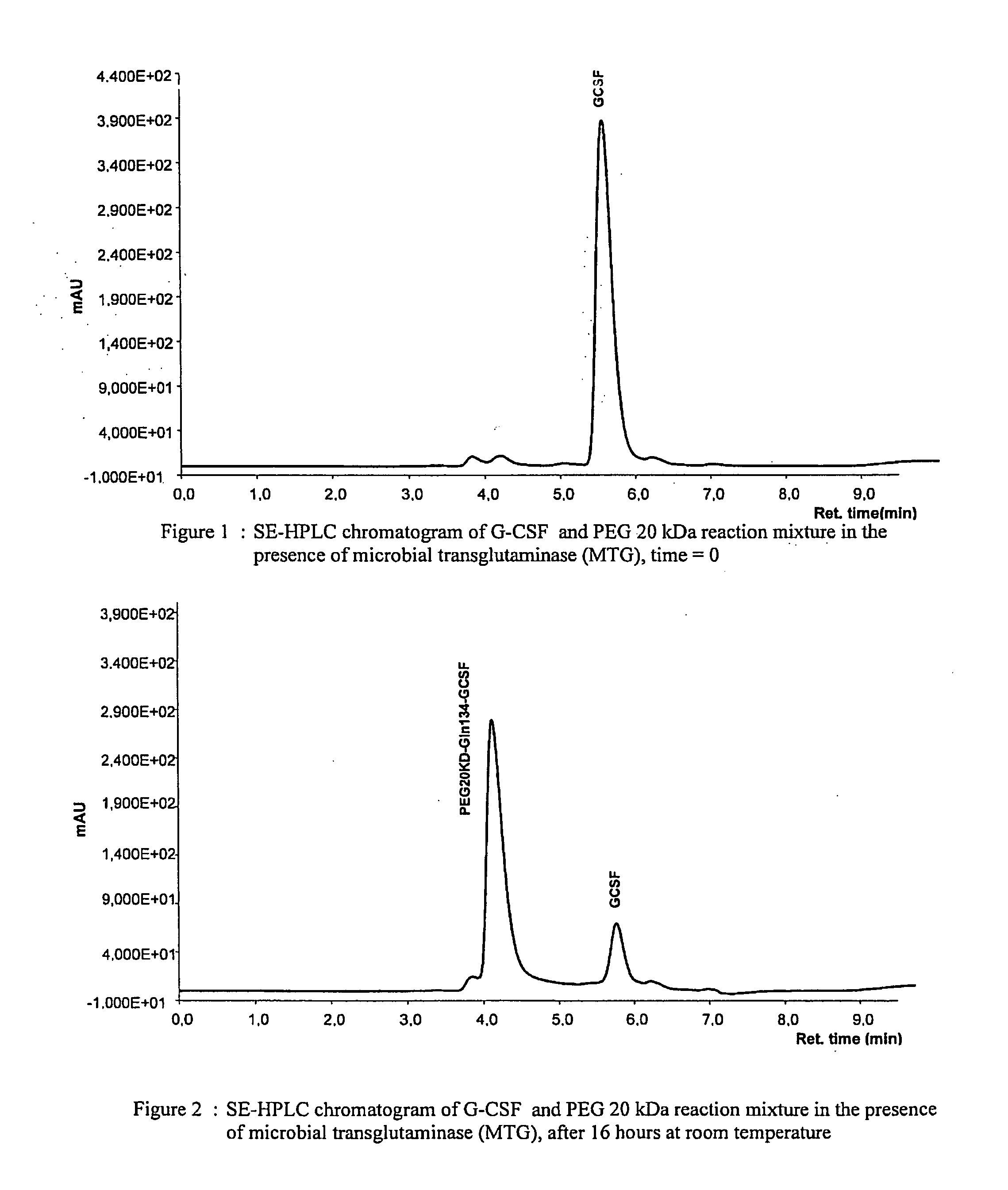
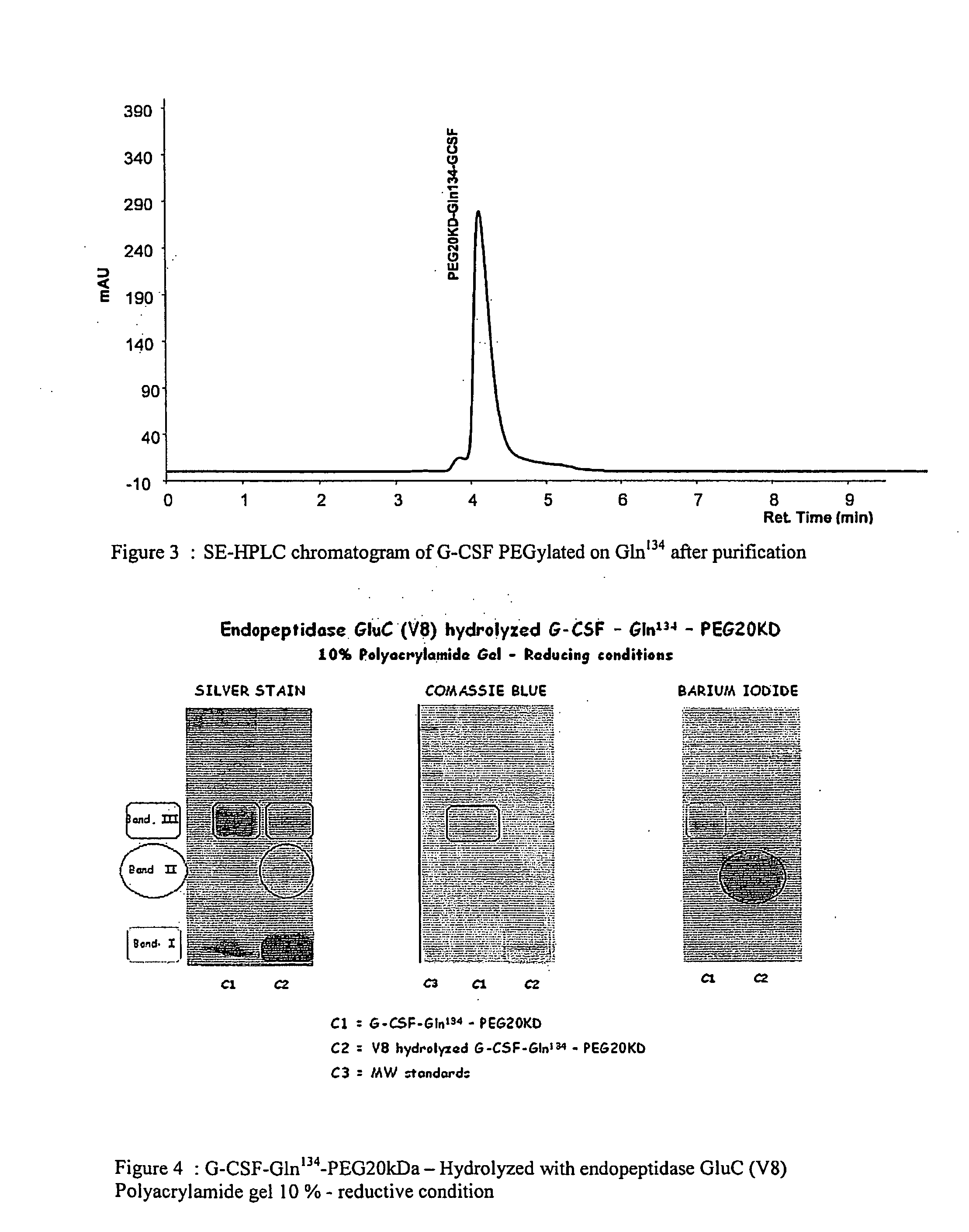
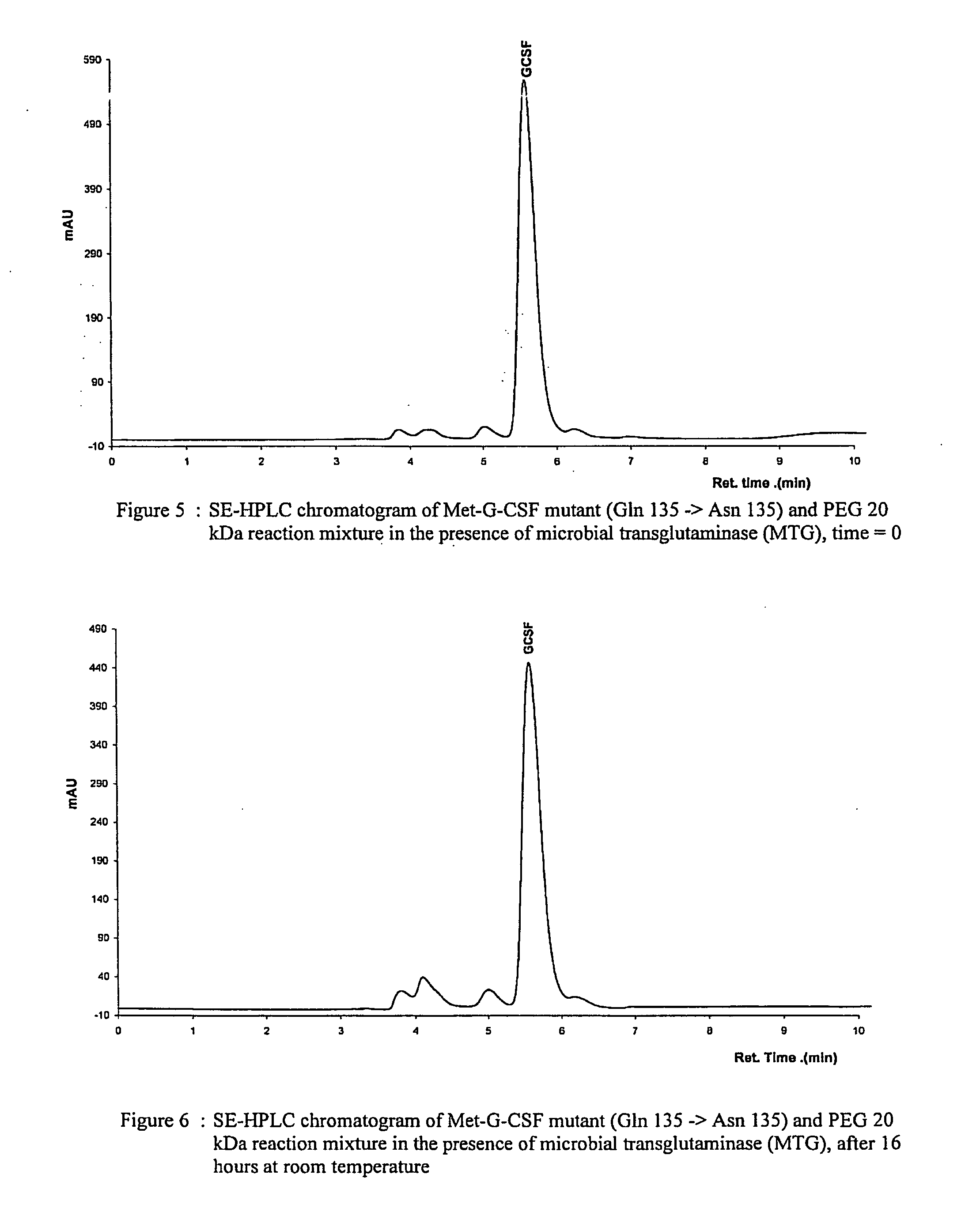
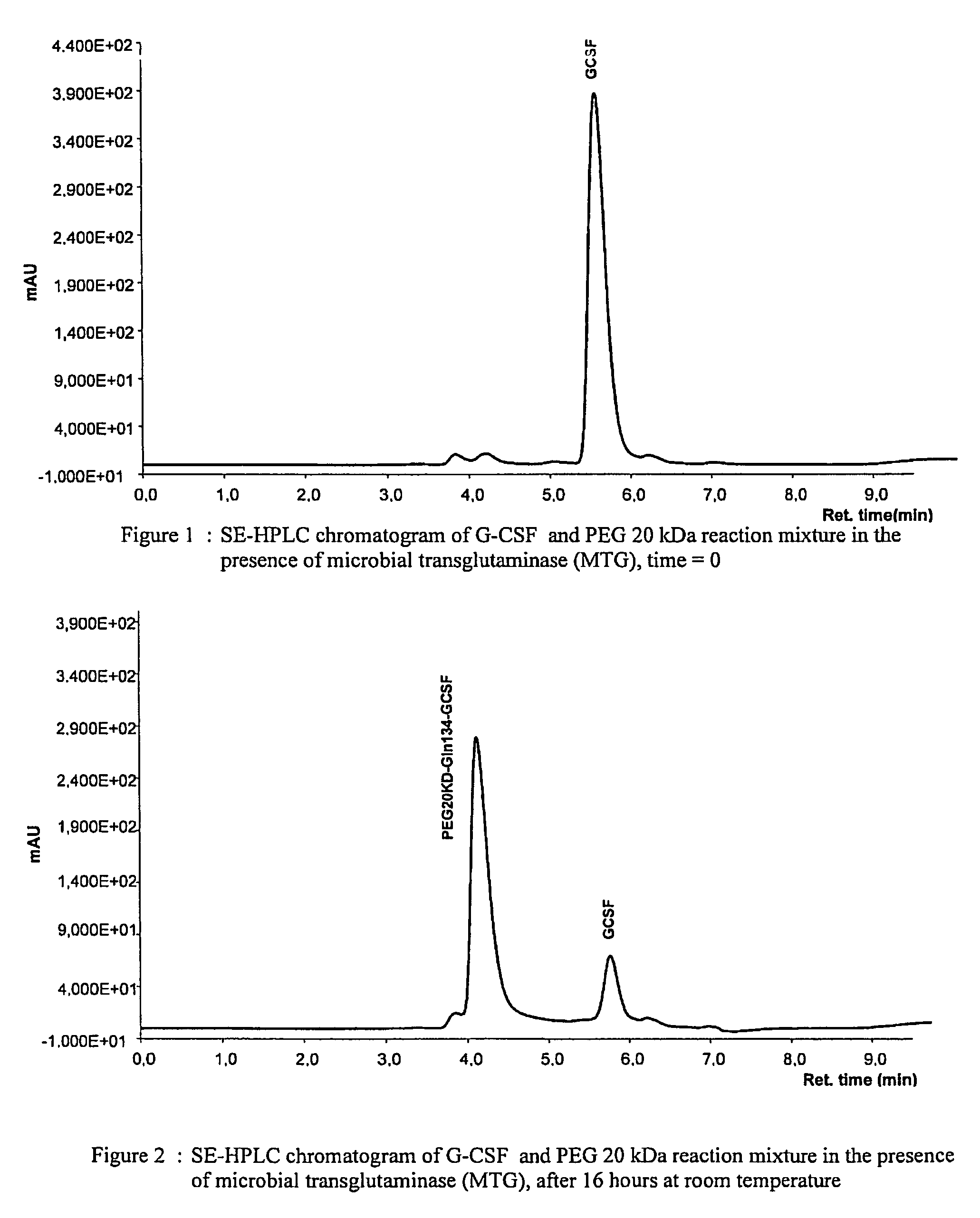
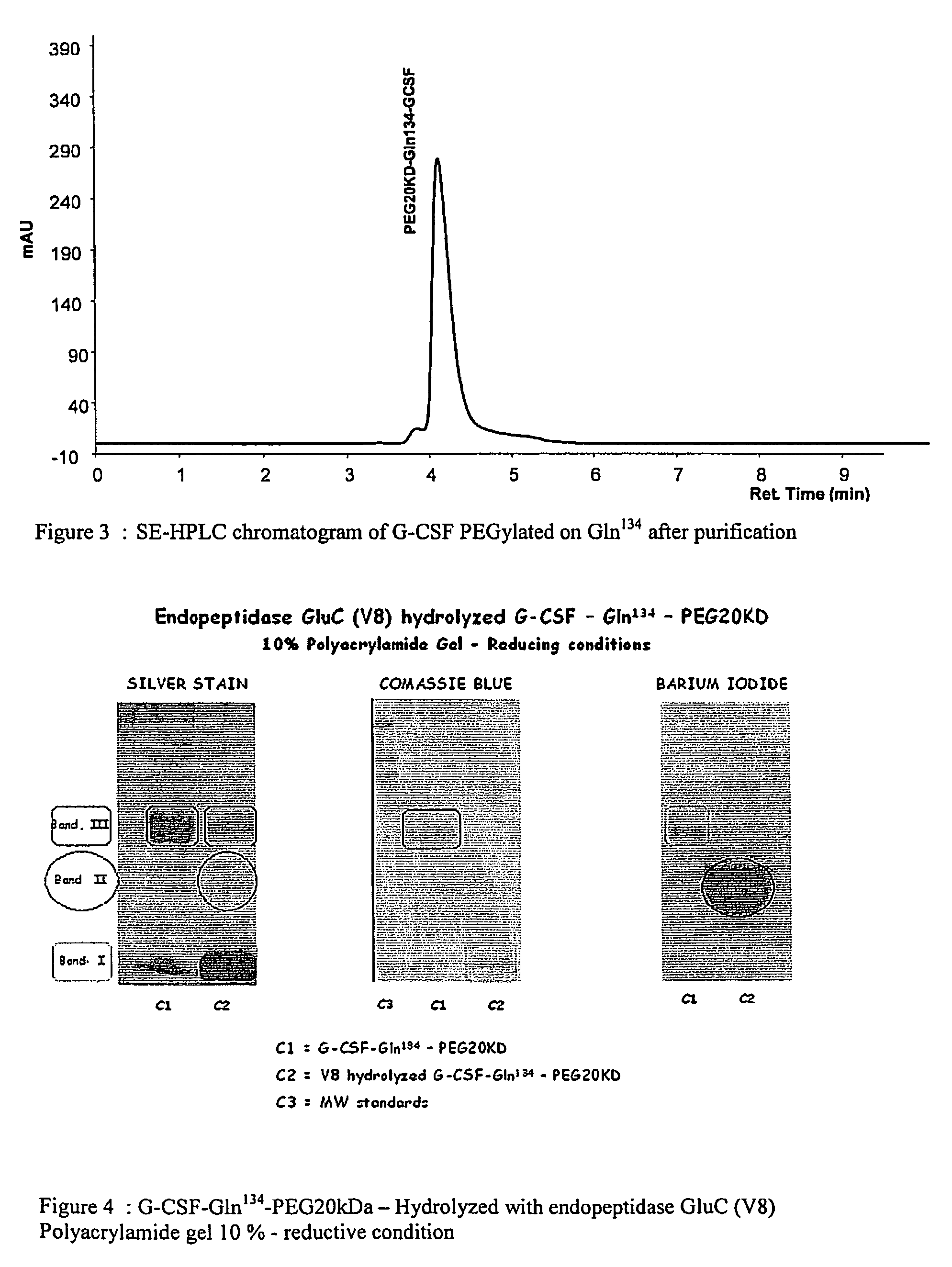
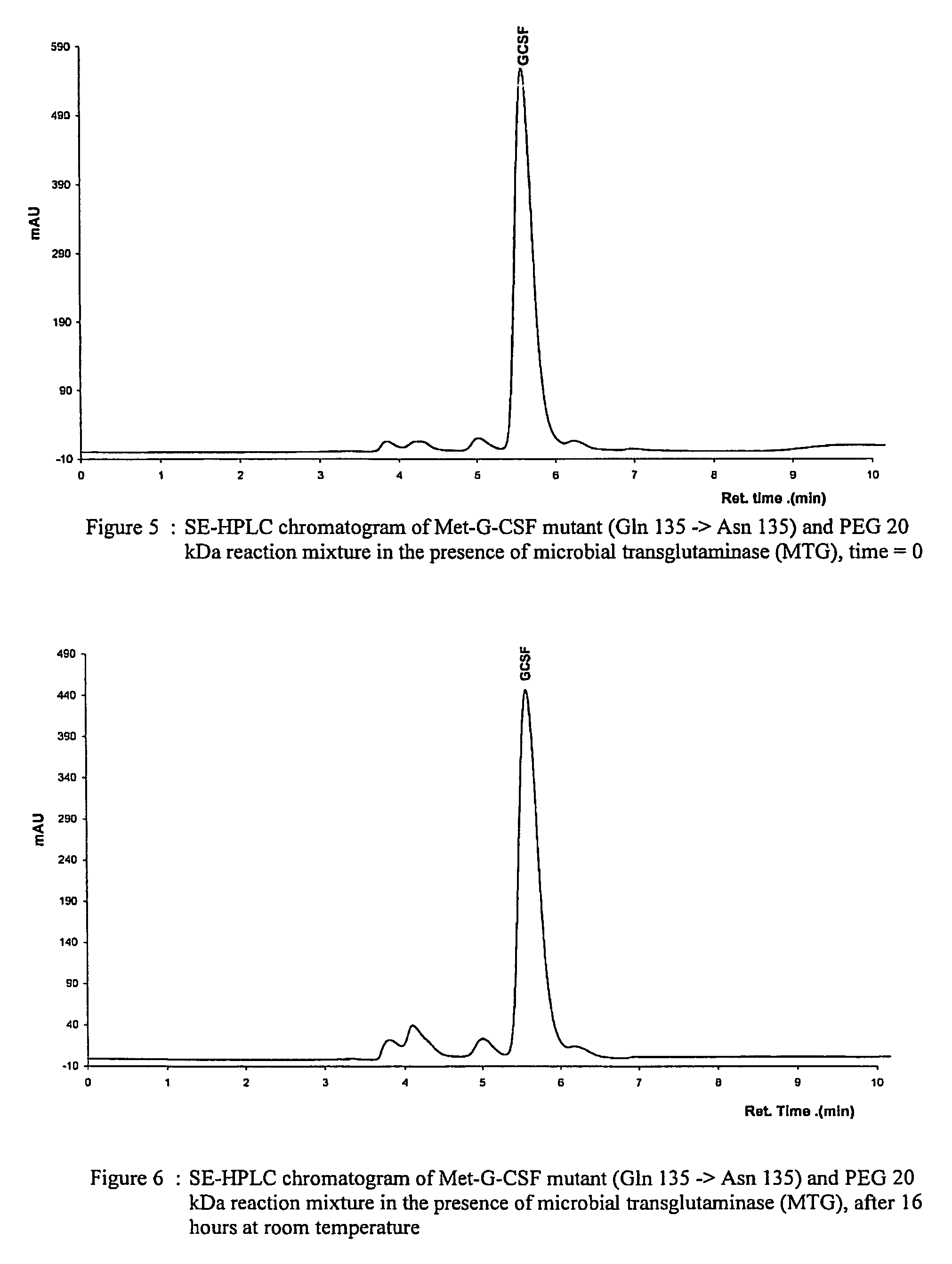
Novel site-specific mono-conjugates of Granulocyte Colony Stimulating Factor (G-CSF) are hereby described, with analogues and derivatives thereof, which stimulate proliferation and differentiation of progenitor cells to mature neutrophiles. These conjugates have been obtained using transglutaminase to covalently and site-specifically bind a hydrophilic, non-immunogenic polymer to a single glutamine residue of the human G-CSF native sequence and analogues thereof. These novel site-specific mono-conjugated derivatives are recommended for therapeutic use since they are stable in solution and exhibit significant biological activity in vitro and a longer bloodstream half-life, as compared to the non-conjugated protein, with a consequent prolonged pharmacological activity.
Owner:BIO KER
G-CSF site-specific mono-conjugates
Owner:BIO KER SRL
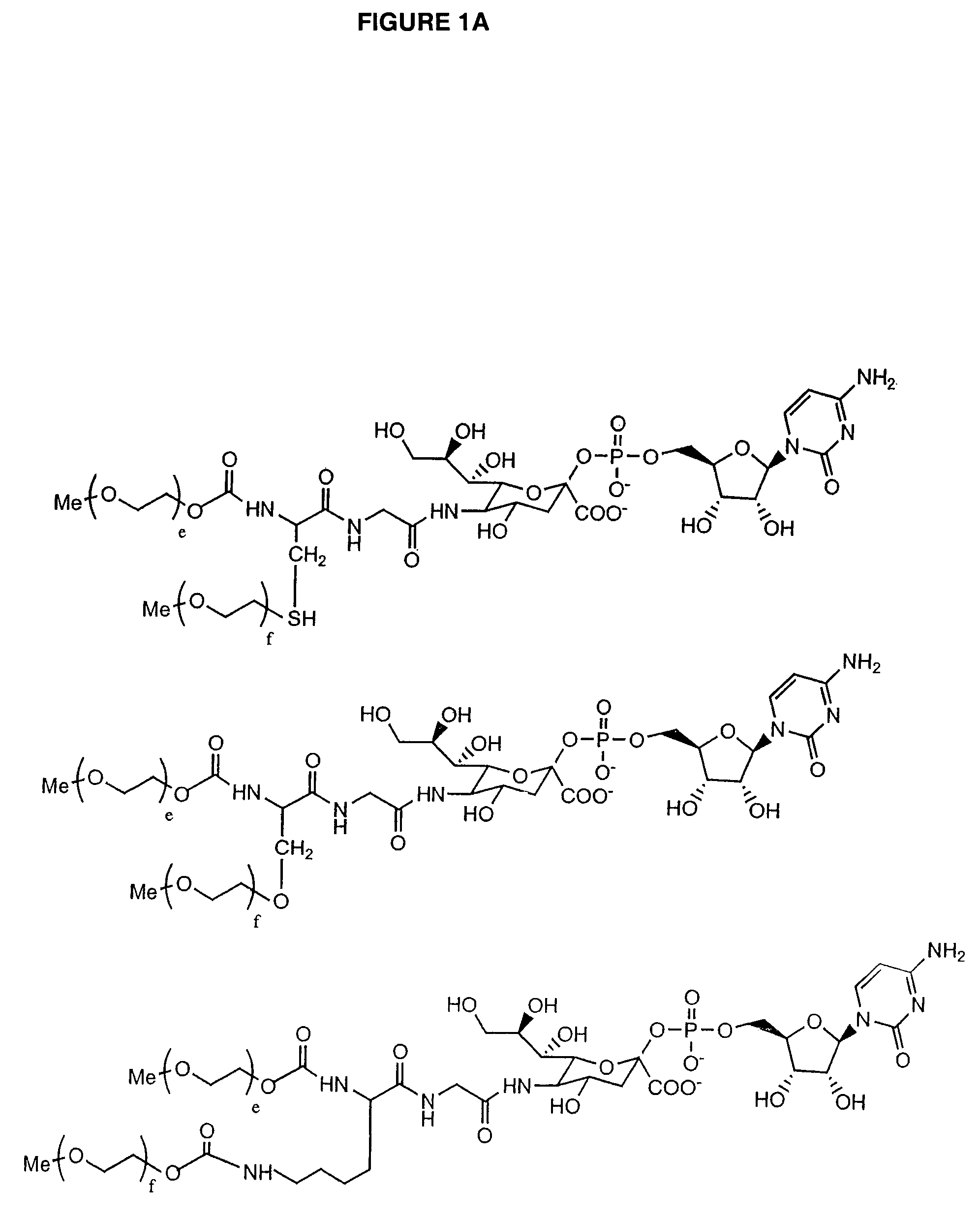
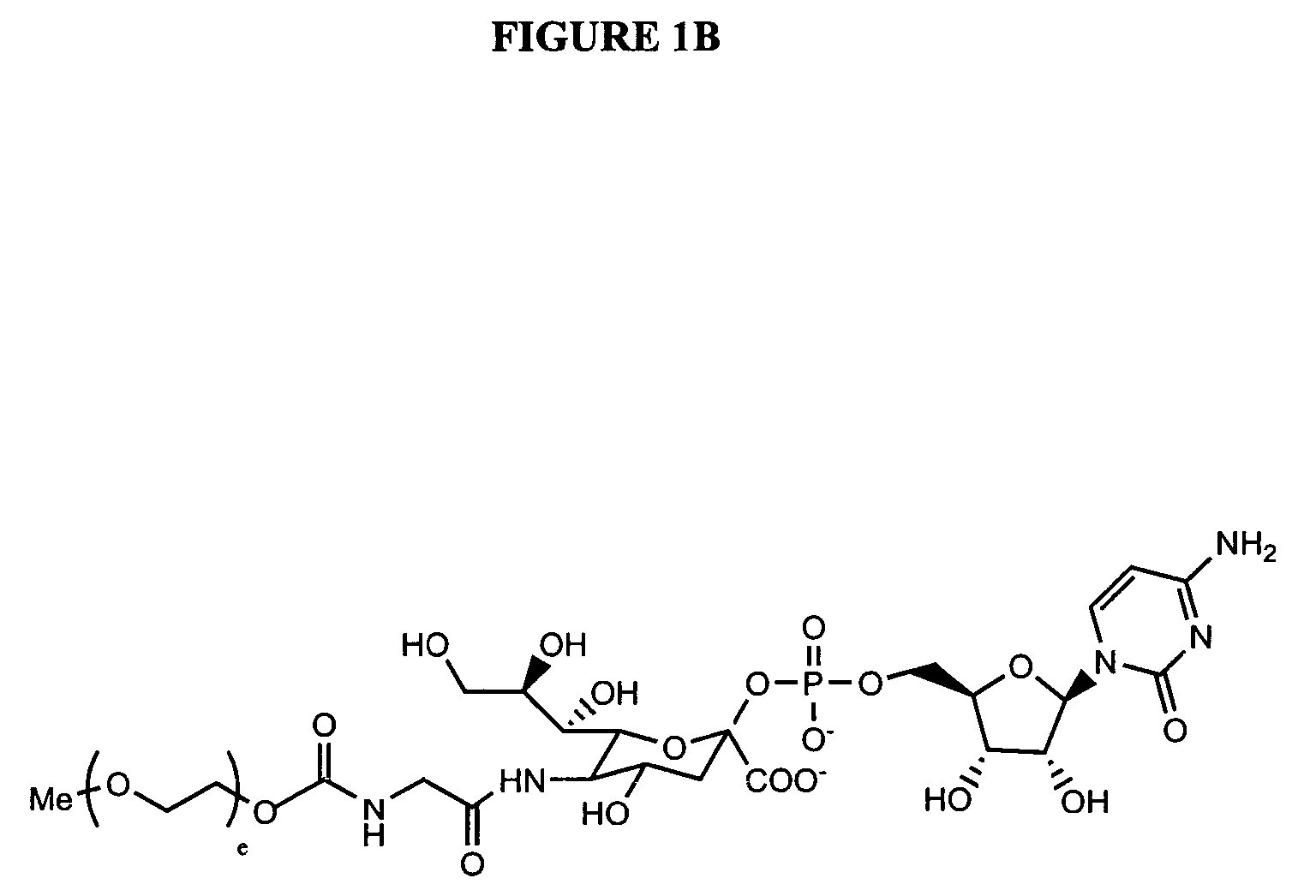
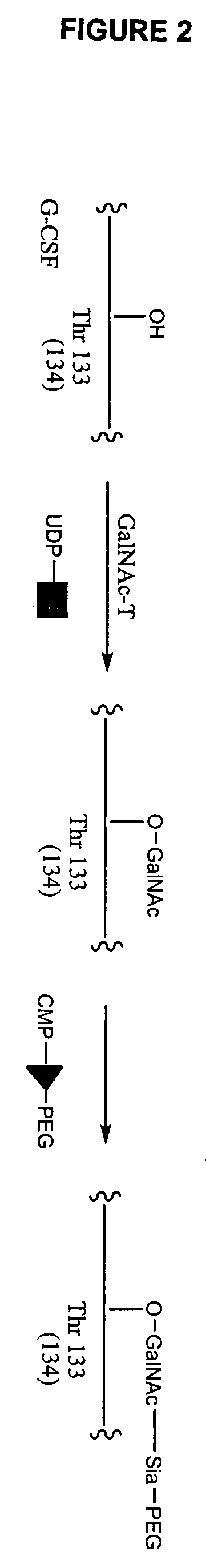
Glycopegylated Granulocyte Colony Stimulating Factor
InactiveUS20090203579A1Improved pharmacokinetic propertiesPeptide/protein ingredientsPeptide preparation methodsColony-stimulating factorG-csf therapy
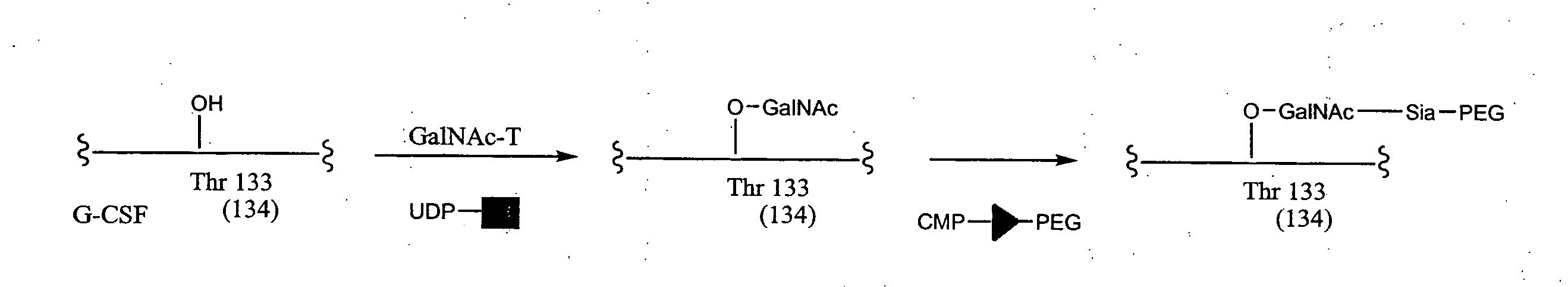
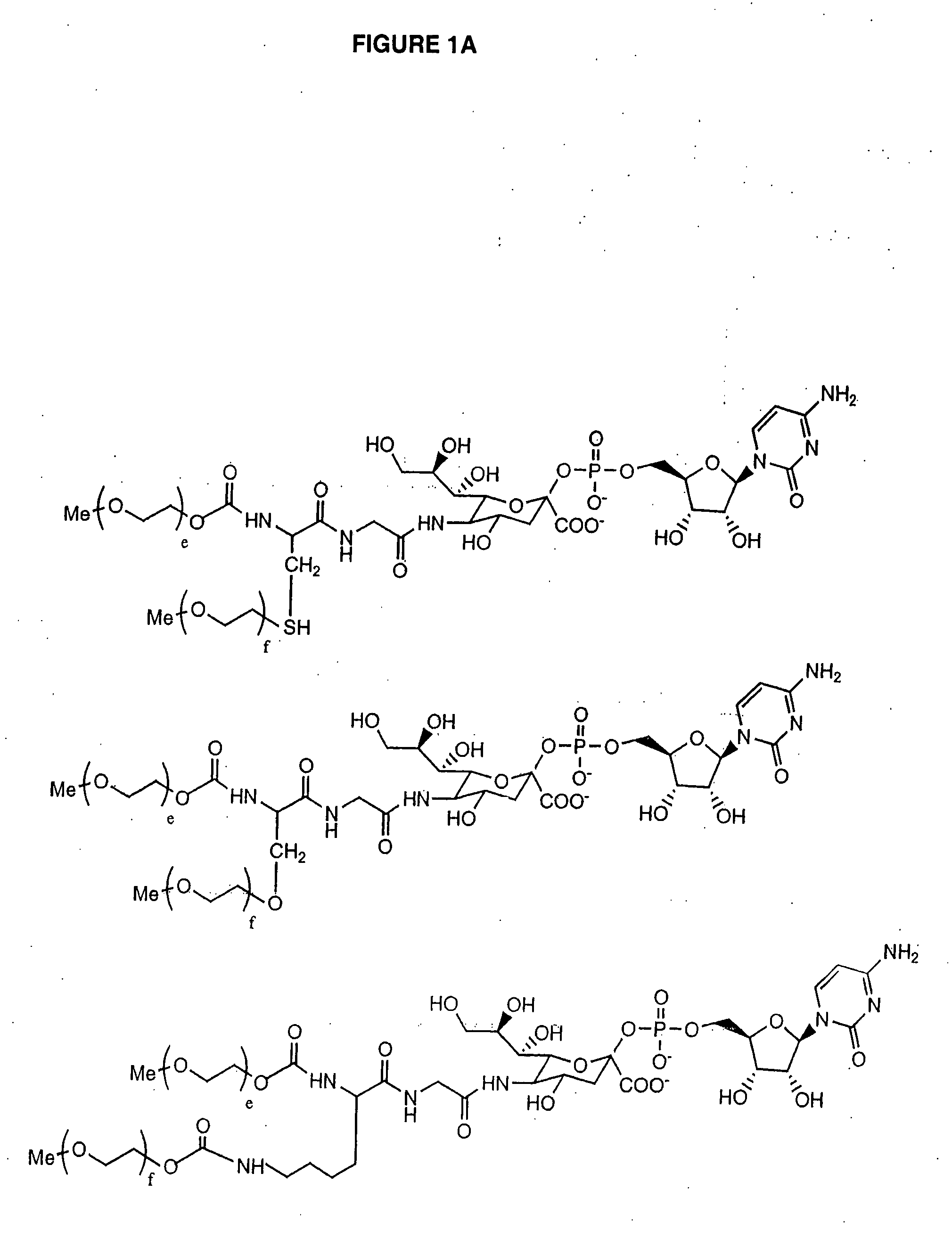
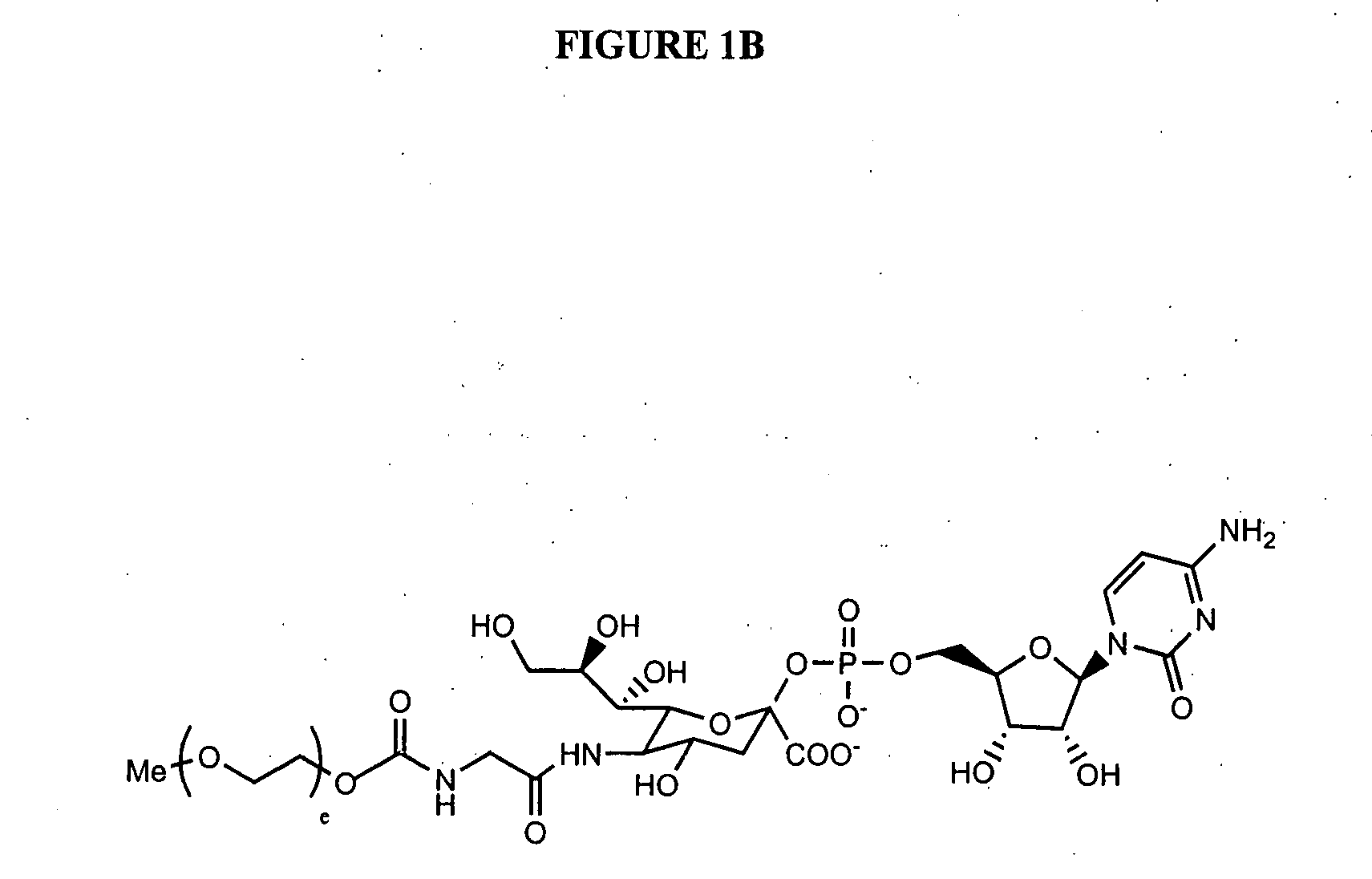
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Glycopegylated granulocyte colony stimulating factor
ActiveUS7956032B2Improved pharmacokinetic propertiesPeptide/protein ingredientsFermentationColony-stimulating factorG-csf therapy
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Degradable wound repair material and preparation method thereof
InactiveCN103830768AGood biocompatibilityGood for degradation and absorptionAbsorbent padsBandagesWhite blood cellPancreatic hormone
The invention relates to a degradable wound repair material and a preparation method thereof. The degradable wound repair material includes a matrix component and an auxiliary component, wherein the matrix component includes a protein ingredient, and the auxiliary component includes at least one of an antibacterial agent and an active factor. Specifically, the antibacterial agent is a synthetic antibacterial drug, an inorganic antibacterial agent, an organic antibacterial agent, or a natural antibacterial agent, and the active factor is at least one of the following active factors: an epidermal growth factor, an FGF vascular endothelial growth factor, a platelet-derived growth factor, a platelet activating factor, an insulin-like growth factor, a tumor necrosis factor, interleukin, colony stimulating factor-1, various bone morphogenetic proteins or transforming growth factors. By loading the antibacterial agent and active factor component on the basis of the matrix component, the degradable wound repair material provided by the invention can have biological activity on the basis of meeting degradability, can better promote wound repair, and has good anti-infection properties during use.
Owner:SHENZHEN LANDO BIOMATERIALS
Inhibition of colony stimulating factor-1 receptor signaling for the treatment of brain cancer
InactiveUS20150119267A1Compound screeningApoptosis detectionAfter treatmentColony-stimulating factor
The present invention provides a method of screening brain tumor patients for treatment with inhibitor of CSF-1R, based on differential gene expression including adrenomeduUin (ADM), arginase 1 (ARG1), clotting factor F13A1, mannose receptor C type 1 (MRC1 / CD206), and protease inhibitor SERPINB2 after treatment with the inhibitor. Based on the same differential gene expression profile, the present invention also provides a method of screening a compound to treat brain cancer.
Owner:SLOAN KETTERING INST FOR CANCER RES
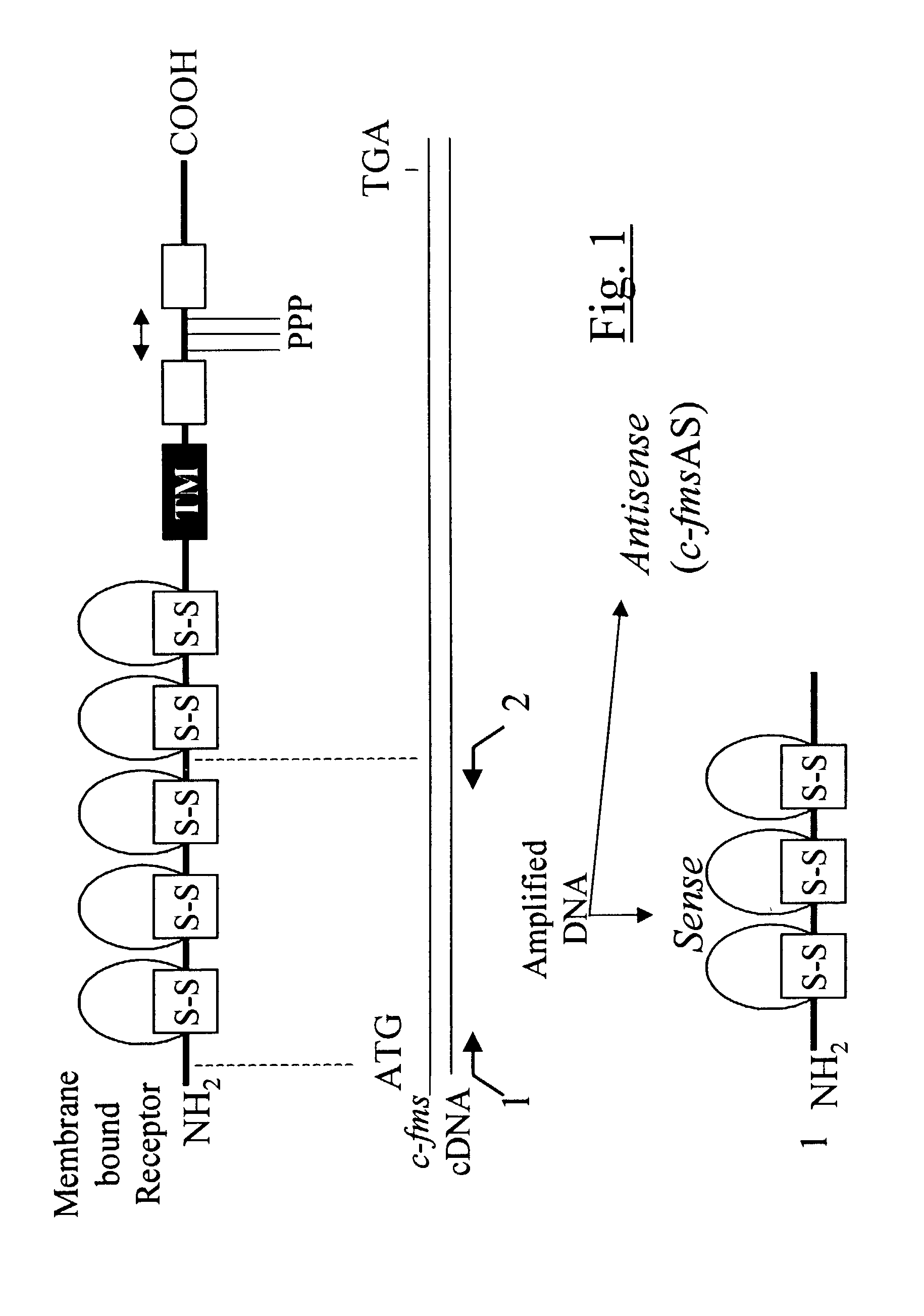
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
Method of prodcing megacaryocyle using umbilical blood CD 344+cell in vitro induction method
InactiveCN1556197AIncrease multipleImprove production efficiencyTissue cultureHuman plateletWhite blood cell
A process for generating megacaryocyte by in vitro induction to umbilical blood cell CD34+ includes in vitro amplifying the CD34+ in the culture medium containing fetal calf serum, human SCF, human TPO and / or ligand flt-3 and inducing the generation of megacaryocytes in the non-serum culture medium containing human TPO, human interleukin 3(IL-3) and / or human GM-CSF. Its advantage is high inducing efficiency.
Owner:上海伯瑞生物技术发展有限公司 +1
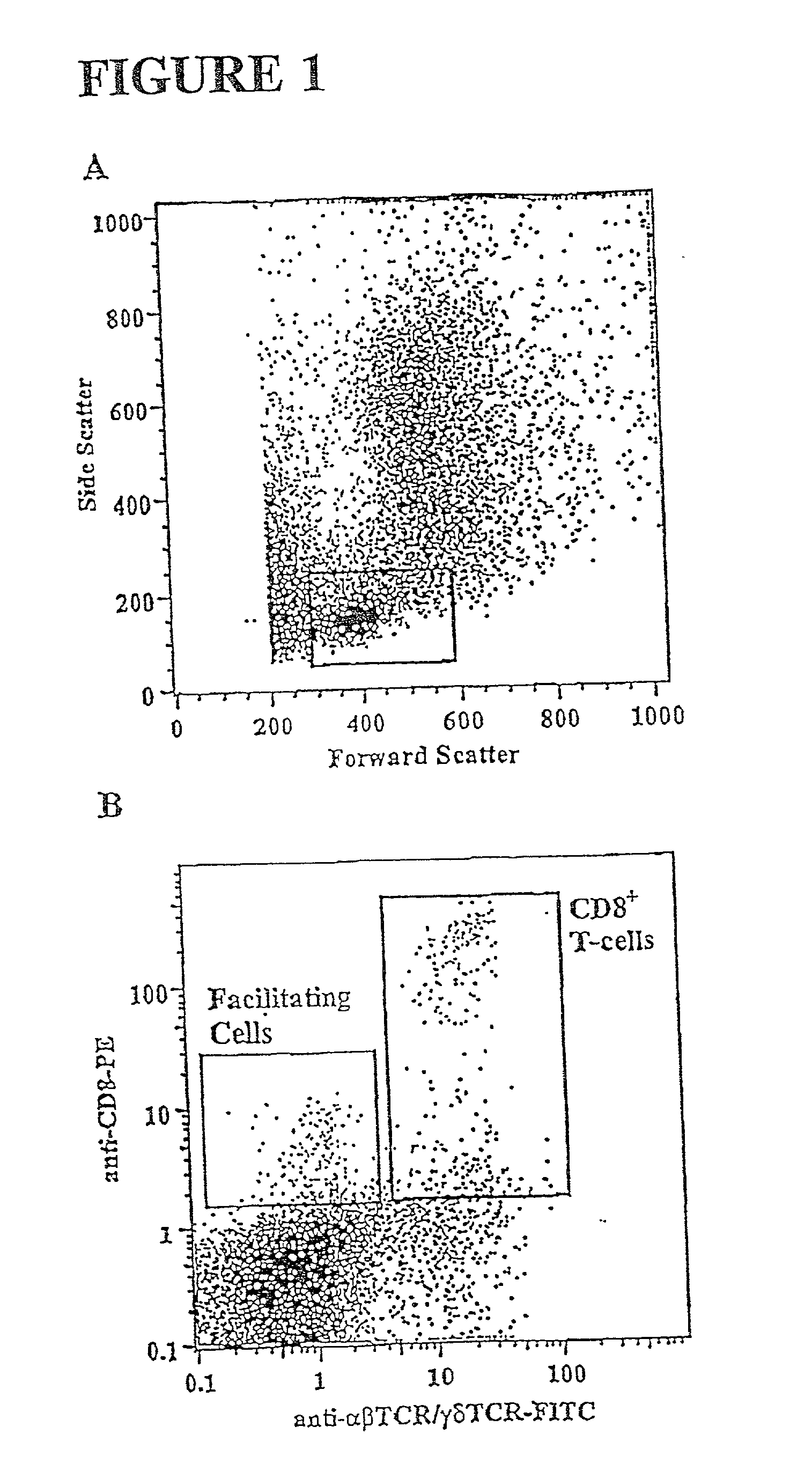
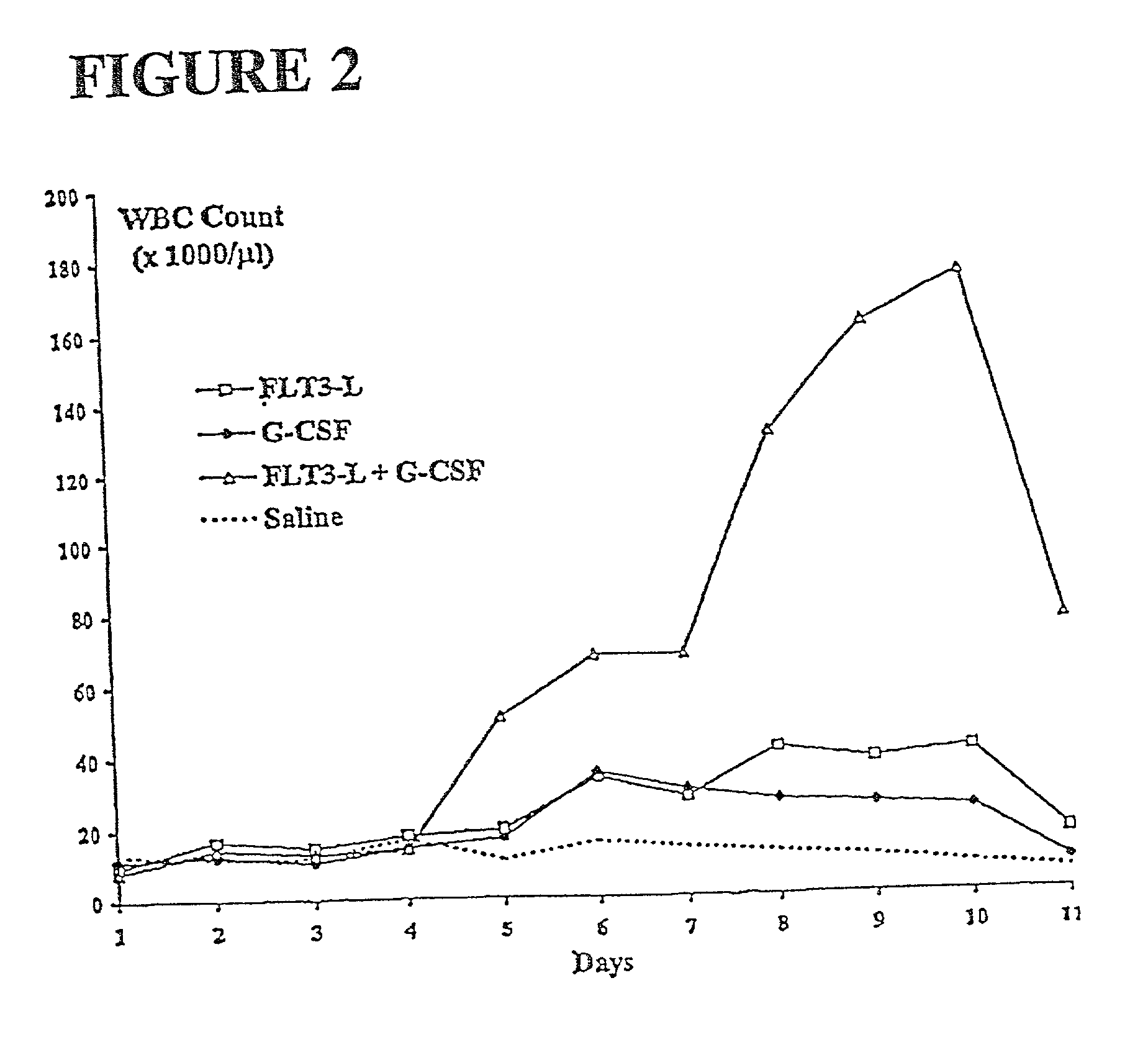
Methods for mobilizing hematopoietic facilitating cells and hematopoietic stem cells into the peripheral blood
The present invention relates to methods for mobilizing hematopoietic facilitating cells (FC) and hematopoietic stem cells (HSC) into a subject's peripheral blood (PB). In particular, the invention relates to the activation of both FLT3 and granulocyte-colony stimulating factor (G-CSF) receptor to increase the numbers of FC and HSC in the PB of a donor. The donor's blood contains both mobilized FC and HSC, and can be processed and used to repopulate the destroyed lymphohematopoietic system of a recipient. Therefore, PB containing FC and HSC mobilized by the method of the invention is useful as a source of donor cells in bone marrow transplantation for the treatment of a variety of disorders, including cancer, anemia, autoimmunity and immunodeficiency. Alternatively, the donor's hematopoietic tissue, such as bone marrow, can be treated ex vivo to enrich selectively for FC and HSC populations by activating appropriate cell surface receptors.
Owner:ILDSTAD SUZANNE T +1
Treatment of osteolytic disorders and cancer using CSF1R extracellular domain fusion molecules
ActiveUS8183207B2Improve propertiesGrowth inhibitionPeptide/protein ingredientsAntibody mimetics/scaffoldsColony-stimulating factorColony Stimulating Factor Receptor
Methods of using colony stimulating factor receptor (CSF1R) extracellular domain (ECD) fusion molecules for treating osteolytic bone loss, cancer metastasis, cancer metastasis-induced osteolytic bone loss, and tumor growth are provided. CSF1R ECD fusion molecules, polynucleotides encoding CSF1R ECD fusion molecules, and methods of making CSF1R ECD fusion molecules are also provided.
Owner:FIVE PRIME THERAPEUTICS
Recombinant II type herpes simplex virus vector, preparation method of recombinant II type herpes simplex virus vector, recombinant virus, medicinal composition and application
ActiveCN102146418AGenetic material ingredientsViral/bacteriophage medical ingredientsCurative effectRecombinant virus vaccine
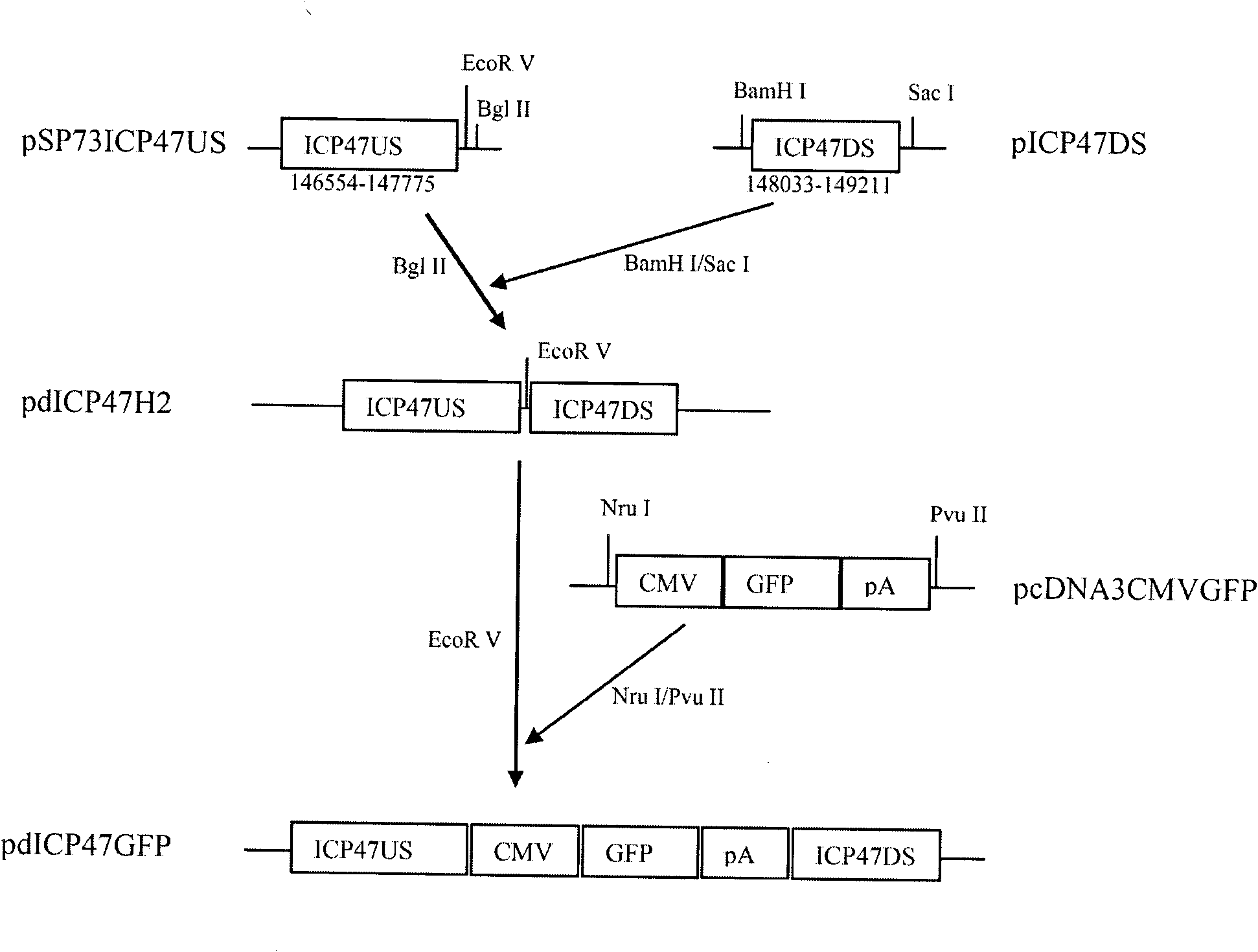
The invention provides a recombinant II type herpes simplex virus vector. An ICP34.5 gene and an ICP47 gene of a wild II type herpes simplex virus HG52 strain are removed in the virus vector, and preferably a human granulocyte macrophage-colony stimulating factor (hGM-CSF) expression box is inserted into the position where the ICP34.5 gene is removed. The invention also provides a preparation method of the recombinant II type herpes simplex virus vector, a recombinant virus using the recombinant II type herpes simplex virus as a vector, a medicinal composition consisting of the recombinant II type herpes simplex virus vector and a pharmaceutically acceptable vector or excipient, and application of the recombinant II type herpes simplex virus vector in preparation of a gene medicament for treating tumors. As the ICP34.5 gene is removed in the recombinant II type herpes simplex virus vector provided by the invention, the oncolysis virus is safe and can selectively grow and propagate in tumor cells; the ICP47 gene is removed to promote immune response and enhance oncolysis activity; and the curative effect of the recombinant II type herpes simplex virus vector is superior to that of the conventional recombinant I type herpes simplex virus vector, and the recombinant II type herpes simplex virus vector has high safety.
Owner:WUHAN BINHUI BIOTECH CO LTD
Tumor vaccines
InactiveUS7247310B1Easy to handleImprove anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsAbnormal tissue growthGranulocyte colony-stimulating factor
A tumor vaccine which comprises a microparticle or a lysate prepared from a solidified tumor material selected from the group consisting of a tumor tissue, a tumor cell, and a component thereof, and at least one cytokine and / or cytokine-inducing agent (e.g., a granulocyte-macrophage-colony stimulating factor and / or interleukin-2 and the like), and optionally an adjuvant. The vaccine can be easily prepared and widely applied for prevention of recurrence, inhibition of metastasis and therapeutic treatment regardless of a type of a tumor, and has excellent antitumor effect.
Owner:RIKEN +1
Methods of perispinal extrathecal administration of large molecules for diagnostic use in mammals
InactiveUS20090130019A1Improving Imaging EfficiencyImprove efficiencyIn-vivo radioactive preparationsDrug compositionsMammalImaging agent
This application concerns novel methods which enable or improve the ability of molecules, particularly large molecules, to cross the blood-brain barrier, the blood-eye barrier, and / or the blood-nerve barrier and therefore be of improved diagnostic and / or therapeutic use in humans and other mammals. These methods involve perispinal administration of imaging agents without direct intrathecal injection. Perispinal administration is defined as administration of the molecule into the anatomic area within 10 cm of the spine. Perispinal administration results in absorption of the imaging agent into the vertebral venous system. The vertebral venous system is capable of transporting molecules into the brain, the eye, the retina, the auditory apparatus, the cranial nerves, the head, the spine, the spinal cord, the vertebral bodies, the dorsal root ganglia, and the nerve roots via retrograde venous flow, thereby bypassing the blood-brain barrier and similar barriers and delivering the molecules to the brain, the eye, the retina, the auditory apparatus, the cranial nerves, the head, the spine (including the vertebral bodies), the spinal cord, the dorsal root ganglia, or the nerve roots. This method may be utilized for a wide variety of diagnostic agents, including, but not limited to biologics, monoclonal antibodies, fusion proteins, monoclonal antibody fragments, antibodies to tumor antigens, hormones, cytokines, anti-cytokines, interleukins, anti-interleukins, interferons, colony-stimulating factors, cancer chemotherapeutic agents, growth factors, anti-virals and antibiotics, including those which are radiolabeled, iodinated, or otherwise altered to facilitate diagnostic imaging. Included in these novel methods are perispinal delivery of amyloid imaging agents, and other ligands radiolabeled with [11C] or [18F] to faciliate PET imaging of the brain.
Owner:TACT IP
Preparation method of recombination human granular cell colony stimulating factor
A process for preparing the recombinant human granulocyte colony stimulating factor includes such steps as fermenting culture of DH5 alpha-PBV220-hGCSF strain, separating cytoryctes, washing, modifying renaturation, ultrafilter, the second renaturation, and chromatography. Its advantages are high purity and high activity.
Owner:深圳未名新鹏生物医药有限公司
Treatment of colony-stimulating factor 3 (CSF3) related diseases by inhibition of natural antisense transcript to csf3
ActiveUS20130035372A1Modulate its functionOrganic active ingredientsNervous disorderDiseaseColony-stimulating factor
The present invention relates to antisense oligonucleotides that modulate the expression of and / or function of Colony-stimulating factor 3 (CSF3), in particular, by targeting natural antisense polynucleotides of Colony-stimulating factor 3 (CSF3). The invention also relates to the identification of these antisense oligonucleotides and their use in treating diseases and disorders associated with the expression of CSF3.
Owner:CURNA INC
Methods for the modulation of neovascularization and/or the growth of collateral arteries and/or other arteries from preexisting arteriolar connections
InactiveUS7507705B2Promote growthEnhancing neovascularizationBiocideSenses disorderColony-stimulating factorNeovascularization
Described is the modulation of the neovascularization and / or growth of collateral arteries and / or other arteries from preexisting arteriolar connections. Methods are provided for enhancing neovascularization and / or the growth of collateral arteries and / or other arteries from preexisting arteriolar connections comprising contacting organs, tissue or cells with a colony stimulating factor (CSF) or a nucleic acid molecule encoding a CSF. Furthermore, the use of a CSF or a nucleic acid molecule encoding a CSF for the preparation of pharmaceutical compositions for enhancing neovascularization and / or collateral growth of collateral arteries and / or other arteries from preexisting arteriolar connections is described. Also provided are methods for the treatment of tumors comprising contacting an organ, tissue or cells with an agent which suppresses neovascularization and / or the growth of collateral arteries and / or other arteries from preexisting arteriolar connections through the inhibition of the biological activity of CSFs. Described is further the use of an agent which suppresses neovascularization and / or the growth of collateral arteries and / or other arteries from preexisting arteriolar connections through inhibition of the biological activity of CSFs for the preparation of pharmaceutical compositions for the treatment of tumors.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Synthetic aperture radar (SAR) image quality evaluation method based on contrast sensitivity characteristics
ActiveCN103106660AIncrease ratingsThe evaluation lacks the improvement of subjective characteristicsImage analysisSynthetic aperture radarImaging quality
The invention discloses a synthetic aperture radar (SAR) image quality evaluation method based on contrast sensitivity characteristics. The method can effectively eliminate noise interference of a high frequency part in an SAR image by utilizing similarity of wavelet decomposition and contrast sensitivity colony stimulating factor (CSF) function space frequency band pass. Simultaneously, the method is simple, convenient and accurate to calculate, free from referring the SAR image and capable of evaluating the image quality comprehensively and effectively only according to an HVSR parameter index. The method can be applied to the SAR image obtained by airborne or satellite borne synthetic aperture radar. A subjective no-reference image type SAR image quality evaluation model is established through the multichannel characteristics of contrast sensitivity in human eye vision and by combining a method of wavelet transform in image processing, and evaluation of the SAR image by real subjectivity is achieved. According to the SAR image quality evaluation method based on the contrast sensitivity characteristics, the defect that existing SAR image quality evaluation lacks subjective characteristics is remedied, and solid technical support for evaluating the SAR image and improving the SAR image quality in future is established.
Owner:BEIHANG UNIV
Methods and compositions for delivery of pharmaceutical agents
InactiveUS7320963B2Good effectEnhanced transfectionBiocideOrganic active ingredientsCholesterolWhite blood cell
Methods and compositions for delivering pharmaceutical agents into cells, in particular urothelial cells of the bladder, are provided. In the methods and compositions of the invention, a solubilized cholesterol composition is used to facilitate delivery of pharmaceutical agents. Preferably, the cholesterol is solubilized by a cyclodextrin (e.g., methyl-β-cyclodextrin) and the pharmaceutical agent comprises a polynucleotide and either a cationic lipid, a cationic polymer or a dendrimer. Improved methods for transfecting polynucleotides into cells thus are also provided, using cationic lipids, cationic polymers or dendrimers and solubilized cholesterol, wherein the transfection efficiency is enhanced compared to use of cationic lipids, cationic polymers or dendrimers alone. Preferred methods of the invention involve transfecting polynucleotides into urothelial cells, preferably for therapeutic treatment of bladder cancer using, for example, a polynucleotide(s) encoding an interleukin(s), an interferon(s), a colony stimulating factor(s) and / or a tumor suppressor(s).
Owner:GENECURE PTE
Compositions and Methods of Use for Mgd-Csf in Disease Treatment
InactiveUS20070264277A1Promotes differentiationPromotes proliferationAntibacterial agentsAntimycoticsDendritic cellPregnancy
Disclosed is a newly identified secreted molecule, identified herein as “monocyte, granulocyte, and dendritic cell colony stimulating factor” (MGD-CSF), the polypeptide sequence, and polynucleotides encoding the polypeptide sequence. Also provided is a procedure for producing the polypeptide by recombinant techniques employing, for example, vectors and host cells. Additionally, procedures are described to modify the disclosed novel molecules of the invention to prepare fusion molecules. Also disclosed are methods for using the polypeptides and active fragments thereof for treatment of a variety of diseases, including, for example, cancer, autoimmune and inflammatory diseases, infectious diseases, and recurrent pregnancy loss.
Owner:FIVE PRIME THERAPEUTICS
Method for producing recombinant human granulocyte colony stimulating factor
ActiveCN1814779ASimple production methodProduction method is stablePeptide preparation methodsCytokines/lymphokines/interferonsInclusion bodiesIon exchange
This invention relates to a method for producing recombined human granular leukocyte colony stimulating factors including: fermenting, breaking bactrriums, extracting occlusion bodies, chromatographing with molecular sieves, renaturating, exchanging anions, exchanging cations to get the raw fluid, in which, BL21 is selected as the host bacterium and the engineering bacterium type is got in high expression volume, quick reproduction and stable passage, several purification steps greatly reduce the residural toxin in the bacterium and the specific activity is increased greatly, a dialysis method is applied for the renaturation to reduce the concentration of the denaturalization agent steadily so the renaturation rate is at high level.
Owner:山东泉港药业有限公司
Dipurification process of recombinant humangranulocyte colony stimulating factor
ActiveCN101045742AHigh annealing quality yieldPeptide preparation methodsRecombinant DNA-technologyInclusion bodiesGlycerol
This invention relates to a preparation method of recombinant human granulocyte colony stimulating factor (rhG - CSF). It includes cracking liquid of inclusion bodies, composition of renaturation liquid, cracking and renaturation method.The renaturation liquid composed by guanidine hydrochloride ( Guanidine - HCl),urea, trihydroxymethyl aminomethane hydrochloride ( Tris - HCl), cysteine, cystine and glycerol.
Owner:NCPC NEW DRUG RES & DEV +1
Construction method and application of non-human animal modified by humanized cell factor IL3 (interleukin3) gene
ActiveCN111118019AAntibody mimetics/scaffoldsGenetically modified cellsBiotechnologyColony-stimulating factor
The invention relates to the modification of a non-human animal by a humanized gene, in particular to a construction method of a non-human animal modified by a humanized cell factor IL3 (interleukin3)gene, and application of the construction method in the biological medicine field, and in particular to an animal model which expresses a humanized IL3 protein. In certain examples, the genetic modification non-human animal which expresses the human IL3 protein also contains IL2rg deletion and / or contains the humanization of more cell factor, including CSF2 (colony stimulating factor 2) and thelike. The invention also provides a construction method for the above animal model and the application of the construction method in the biological medicine field.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
Use of recombinant human granulocyte macrophagocyte colony stimulating factor in preparation of drug for preventing and treating hepatitis B
ActiveCN1990043APeptide/protein ingredientsDigestive systemColony-stimulating factorGenetic engineering
The invention discloses a use of recombinant human granulocyte macrophage colony stimulating factor in the preparation of treatment or prevention hepatitis B, while includes simultaneous or successive given recombinant human granulocyte macrophage colony stimulating factor and genetic engineering hepatitis B vaccine for the prevention or treatment of hepatitis B.
Owner:华北制药金坦生物技术股份有限公司
Method for treatment of demyelinating central nervous system disease
ActiveUS7732407B2Treating and diminishing symptomPrevent demyelinatingNervous disorderPeptide/protein ingredientsMedicineColony-stimulating factor
A method for treating demyelinating central nervous system diseases in a subject that comprises administering to the subject a composition comprising a therapeutically active amount of colony stimulating factor or CSF-like ligand. In a preferred embodiment of the present invention, the CSF is a GM-CSF. In a most preferred embodiment of the present invention, CSF is sargramostim.
Owner:HUNTER SAMUEL F
Protein cross linking agent and method therefor
InactiveCN1524880AStrong process controllabilityHaemoglobins/myoglobinsAlbumin peptidesCross-linkColony-stimulating factor
The invention provides a method for using homotype double functional cross linking agent with epoxy radicals for protein and polypeptide cross bonding, the cross linking agent used by the present invention can realize an ideal reaction velocity for the cross bonding between proteins and polypeptides with good controllability. When applied to the hemoglobin cross bonding under controlled conditions, the method can raise the yield for inter-molecule cross bonding to above 90%.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for treatment of demyelinating central nervous system disease
ActiveUS20070048255A1Treating and diminishing symptomPrevent demyelinatingNervous disorderPeptide/protein ingredientsDiseaseCerebrospinal fluid
A method for treating demyelinating central nervous system diseases in a subject that comprises administering to the subject a composition comprising a therapeutically active amount of colony stimulating factor or CSF-like ligand. In a preferred embodiment of the present invention, the CSF is a GM-CSF. In a most preferred embodiment of the present invention, CSF is sargramostim.
Owner:HUNTER SAMUEL F
Synthetic gene coding for human granulocyte-colony stimulating factor for the expression in E. coli
InactiveUS7655437B2Improved expression level (accumulation)Antibacterial agentsBacteriaEscherichia coliColony-stimulating factor
Owner:LEK PHARMA D D
Methods and compositions for delivery of pharmaceutical agents
InactiveUS7709457B2Good effectEnhanced transfectionBiocideOrganic active ingredientsCholesterolCyclodextrin
Methods and compositions for delivering pharmaceutical agents into cells, in particular urothelial cells of the bladder, are provided. In the methods and compositions of the invention, a solubilized cholesterol composition is used to facilitate delivery of pharmaceutical agents. Preferably, the cholesterol is solubilized by a cyclodextrin (e.g., methyl-β-cyclodextrin) and the pharmaceutical agent comprises a polynucleotide and either a cationic lipid, a cationic polymer or a dendrimer. Improved methods for transfecting polynucleotides into cells thus are also provided, using cationic lipids, cationic polymers or dendrimers and solubilized cholesterol, wherein the transfection efficiency is enhanced compared to use of cationic lipids, cationic polymers or dendrimers alone. Preferred methods of the invention involve transfecting polynucleotides into urothelial cells, preferably for therapeutic treatment of bladder cancer using, for example, a polynucleotide(s) encoding an interleukin(s), an interferon(s), a colony stimulating factor(s) and / or a tumor suppressor(s).
Owner:GENECURE PTE
Purification method of recombinant yeast strain and rhGM-CSF to express human granulocyte-macrophage colony stimulating factor
InactiveCN1487086AHigh specific activityReduce separation and purification stepsFungiPeptide preparation methodsEscherichia coliBiotechnology
The present invention discloses one kind of secretion vector of recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF) and its simple purification method, host cell transformed with the secretion vector, as well as the method of fermentation with the recombinant pichia yeast engineering strain of the said expression vector to express rhGM-CSF. The present inention replaces colibacillus occlusion body expression system with yeast secreting expression sytem and has no need of renaturation of target protein rhGM-CSF, biological specific activity up to 3.4E7 unit / mg protein and less toxic side effect. Therefore, the rhGM-CSF of the present invention has high yield, simple purification process, low cost and less toxic side effect.
Owner:海南国栋药物研究所有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com