Tumor vaccines
a vaccine and tumor technology, applied in the field of tumor vaccines, can solve the problems of inability to concentrate extracts, inability to administer extracts in a large amount, and very limited clinical cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
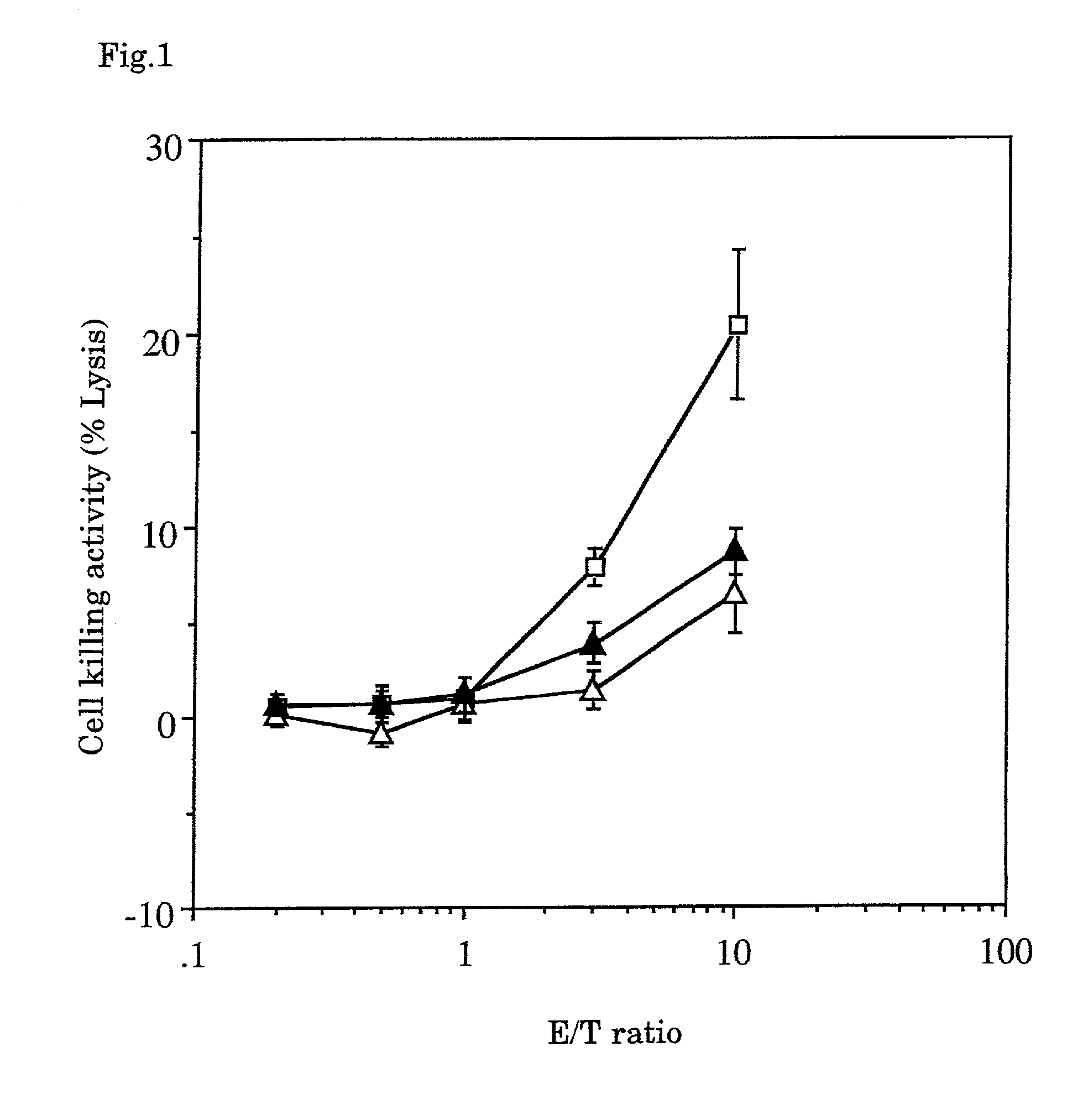
The Action of Tumor Vaccine of the Present Invention
[0035]Using syngeneic transplanted mouse hepatoma with well-known low antigenicity (Guo, Y. J., et al., Nat. Med. 3:451-5, 1997) as the target, the tumor vaccine of the combination of fixed tumor cells as tumor antigens, GM-CSF, IL-2 and an adjuvant was investigated to see whether or not the vaccine inhibited the hepatoma formation.
[Method]
1. Fixed Tumor Cells
[0036]Hepatoma cells Hepa 1-6 developed in C57BL / 6 (obtained from The Cell Bank of The Institute of Physical and Chemical Research) were cultured and fixed with 3% paraformaldehyde in Dalbecco's phosphate buffered saline (hereinafter abbreviated as “PBS”) for 2 hours. The fixed cells were washed once with 70% alcohol for sterilization and aseptically washed four times with PBS, then added with the Dulbecco's minimum essential medium (hereinafter abbreviated as “DMEM”) containing 10% fetal bovine serum, and incubated in a carbon dioxide gas incubator at 37° C. for 2 days. After...
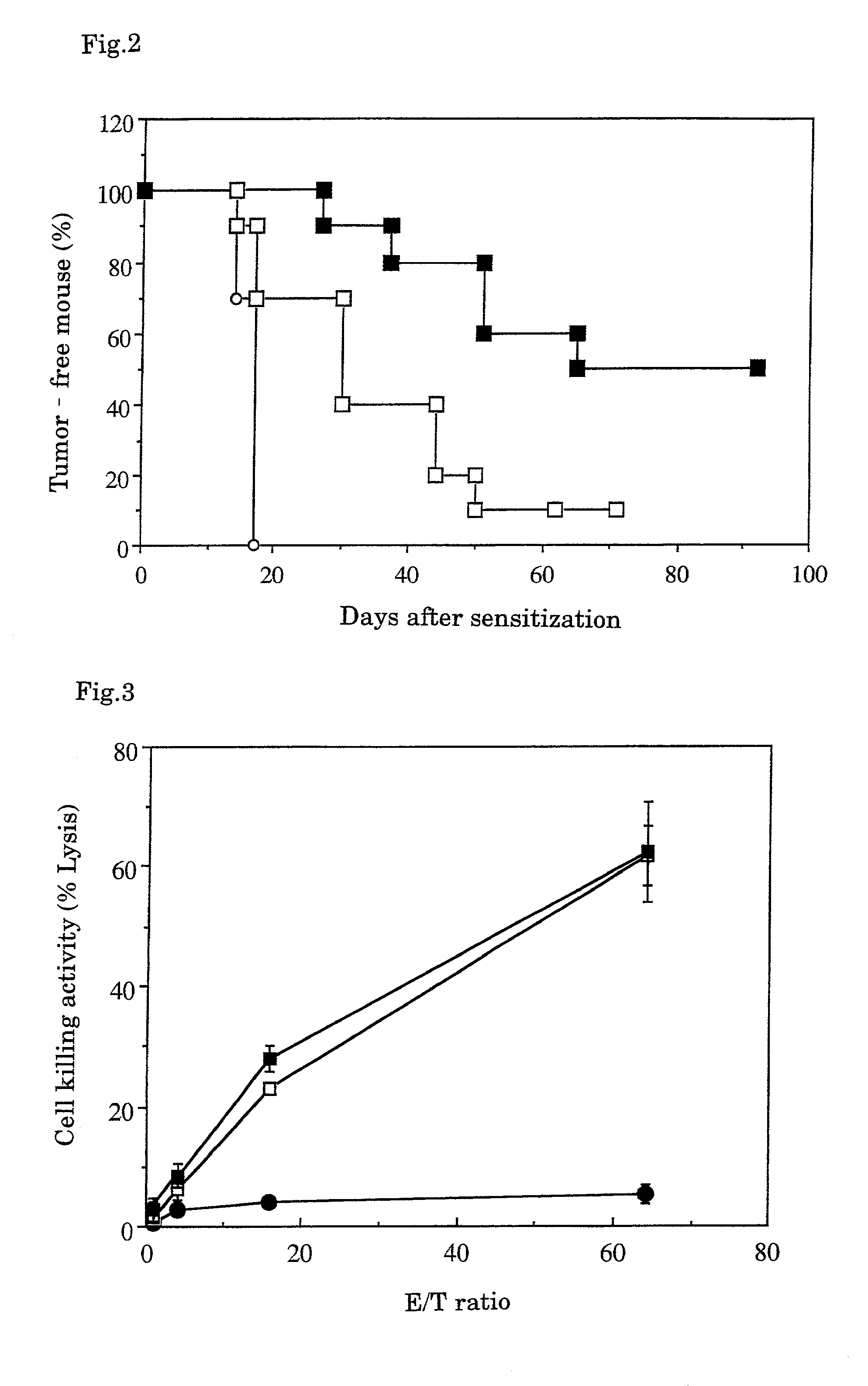
example 2
Method for Preparation of Microparticulate Tumor Antigens from Fixed Tumor Tissues
[0050]Fixed tumor tissues containing fixed tumor cells were ground to prepare fine solidified tumor antigens.
[Method]
[0051]The Hepa 1-6 cells used for the mice in the control group (A) in Example 1 were subcutaneously transplanted in the same amount to the mouse thigh, and the developed hepatoma tissue was isolated after 3 weeks and fixed by soaking in a commercially available neutral formalin solution at room temperature for 3 days. The tissue was taken out, cut with ophthalmic scissors into a fine mince having the diameter of about 1 mm, added with PBS in 10 times amount of the original hepatoma wet weight, and homogenized with ice cooling by a homogenizer (Heidorf Co., DIAX-600, 6G Generator shaft) for 30 seconds. The homogenization was repeated 5 times with intervals of 3 minutes or more for ice cooling. 1.2 ml of the homogenate was placed in a 1.5 m Eppendorf centrifugation tube, and centrifuged b...
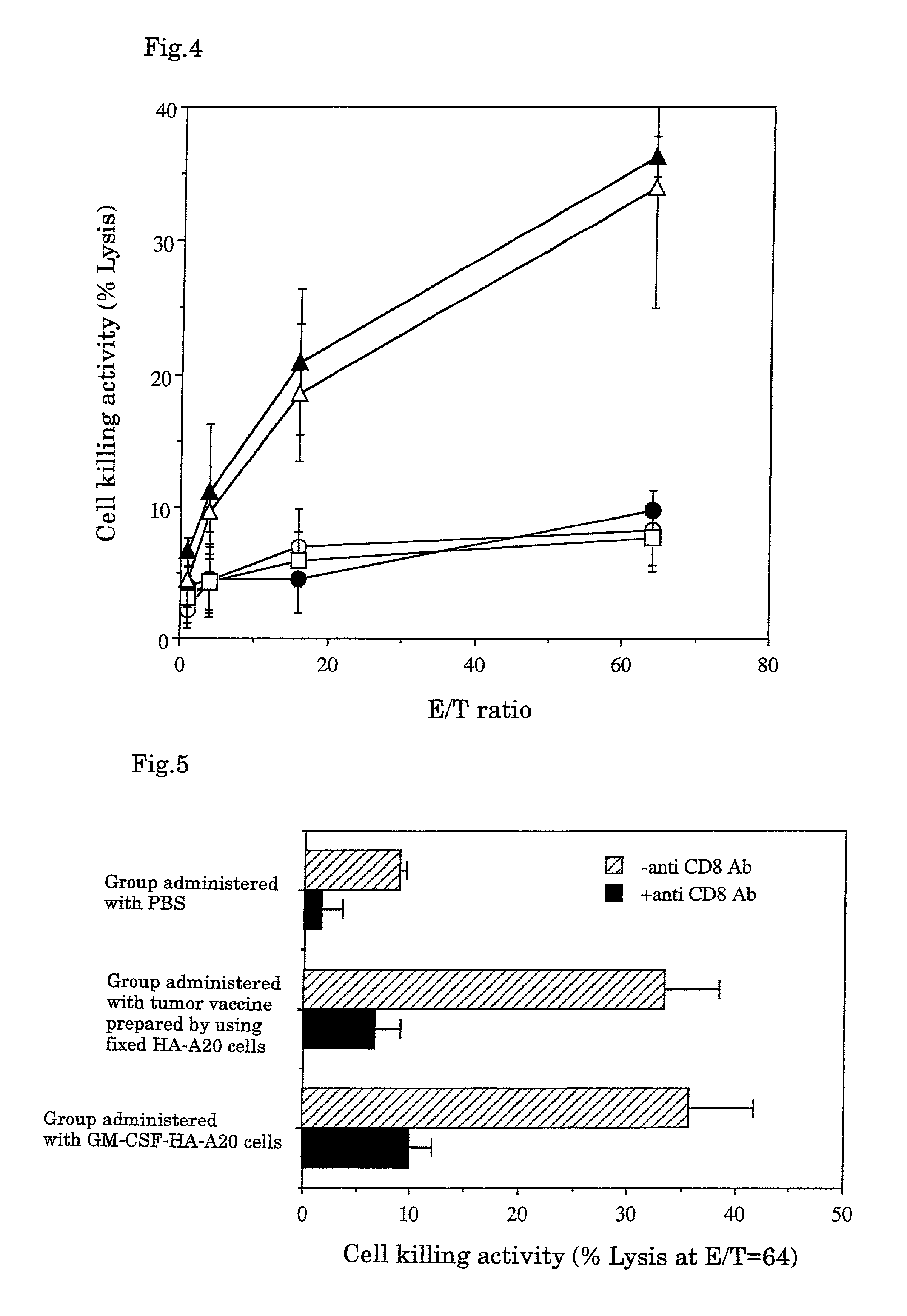
example 3
Antitumor Effect of CTL Induced In Vitro
[0054]The tumor cell killing activity and the specificity were investigated when CTL was induced using fixed tumor cells as a target.
[Method]
1. Fixed Tumor Cells
[0055]108 to 109 cells of substrains B16-F10 of melanoma cell B16 developed by C57BL / 6 mice (obtained from American Type Culture Collection, Bethesda, Mass., USA) were soaked in 10% formalin solution and fixed at 4° C. for 2 to 4 weeks. The resultant was suspended in 30 ml of 70% ethanol, washed by centrifugation, and further suspended in PBS and washed by centrifugation 3 times. The resultant was suspended in an appropriate amount of the MEM medium for cell culture containing 10% fetal bovine serum, and warmed to 37° C. for 2 to 3 days or to 60° C. for 4 hours. Then the resulting cells were recovered by centrifugation (hereinafter the cells subjected to this treatment are referred to as “fixed B16-F10 cells”) and suspended to adjust to 5×108 cells / ml.
2. Determination of Antitumor Effe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com