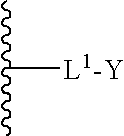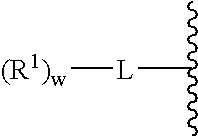Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
201 results about "Sugar moiety" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Moiety is a term that means a part or a portion. So sugar moiety means the sugar molecule in a compound (sugar portion of a complex molecule like nucleic acid). For example when we say that in DNA molecules, the sugar moiety is β-D-2-deoxyribose, it means that the sugar molecule is β-D-2-deoxyribose and describes the position...
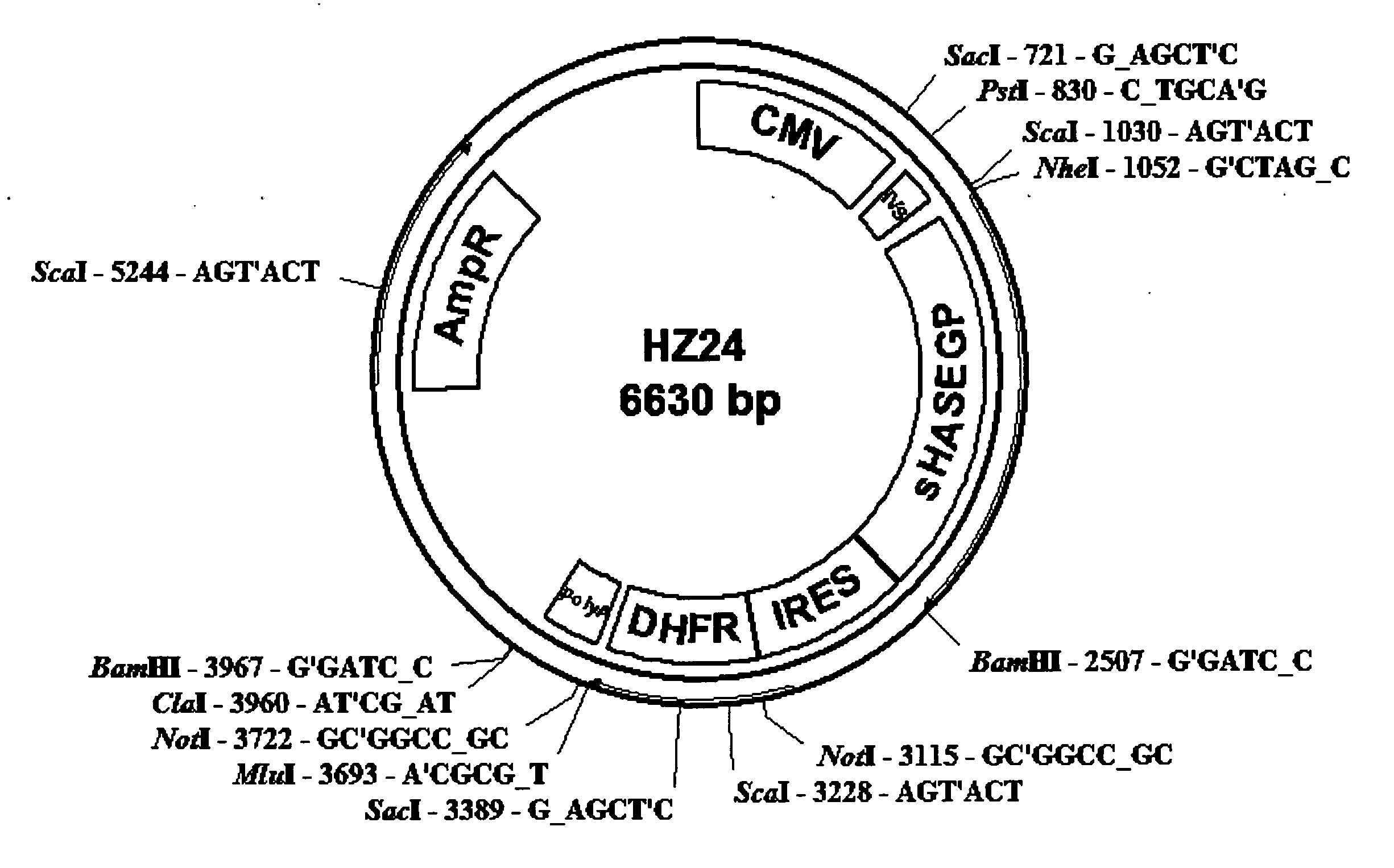
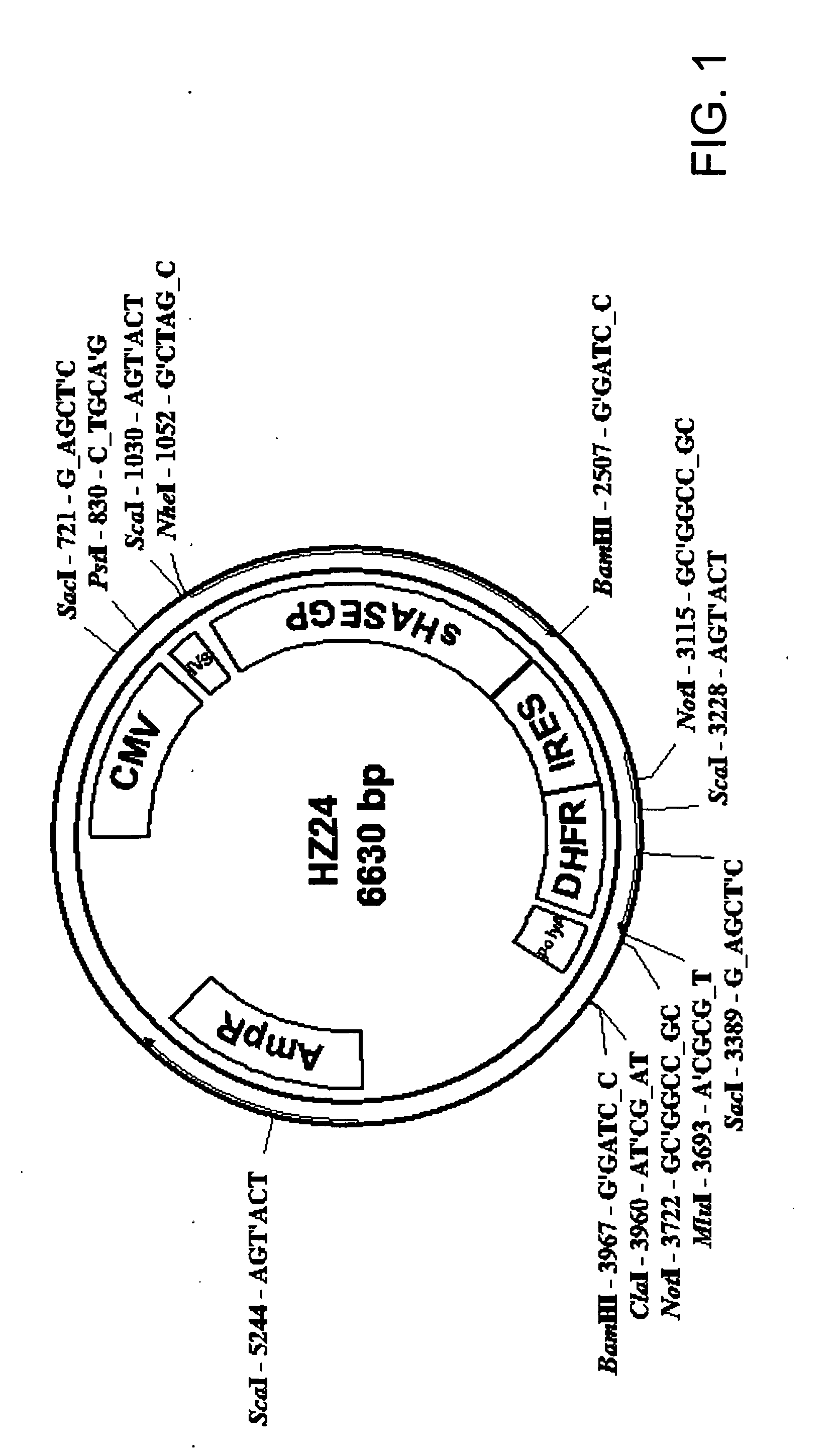
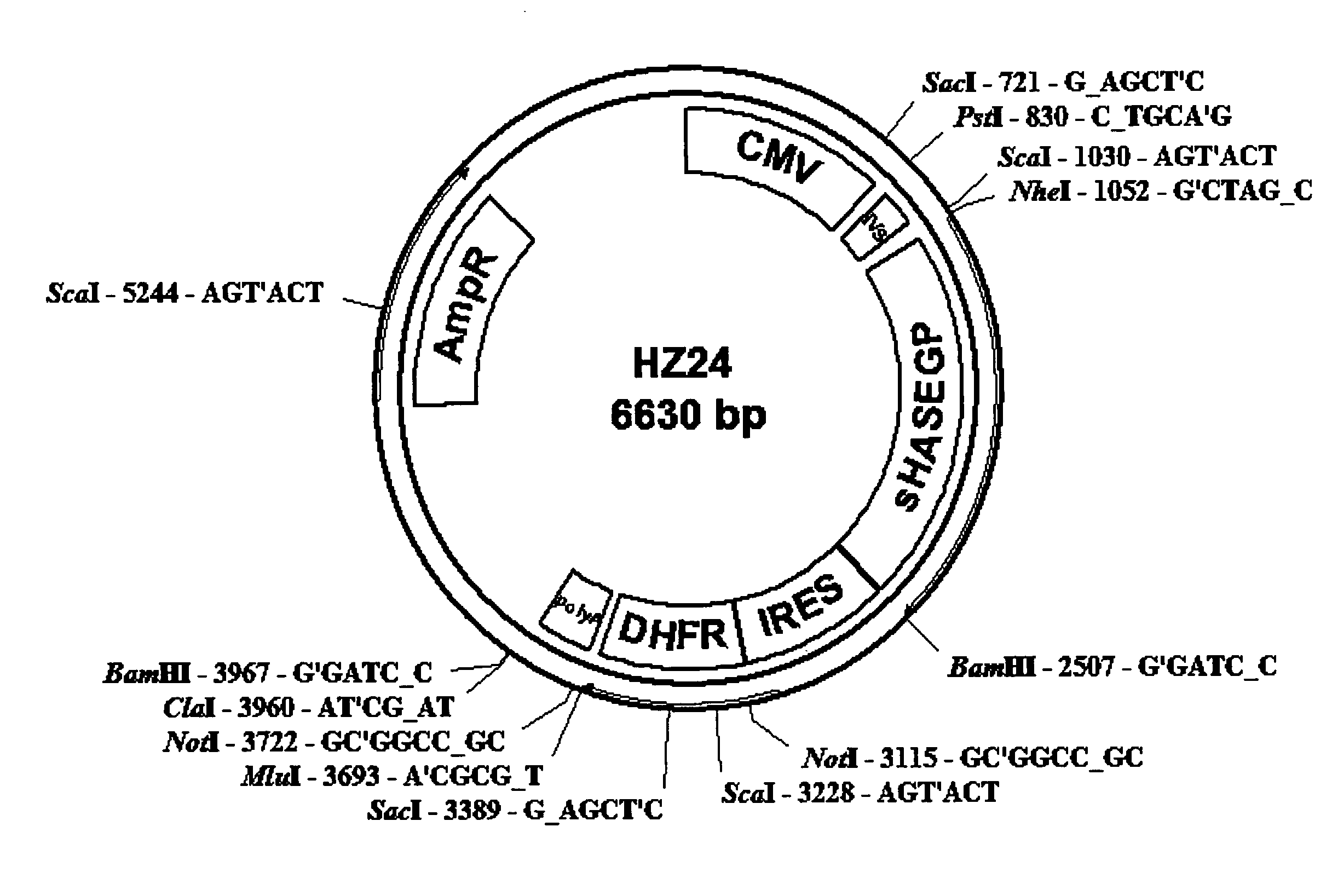
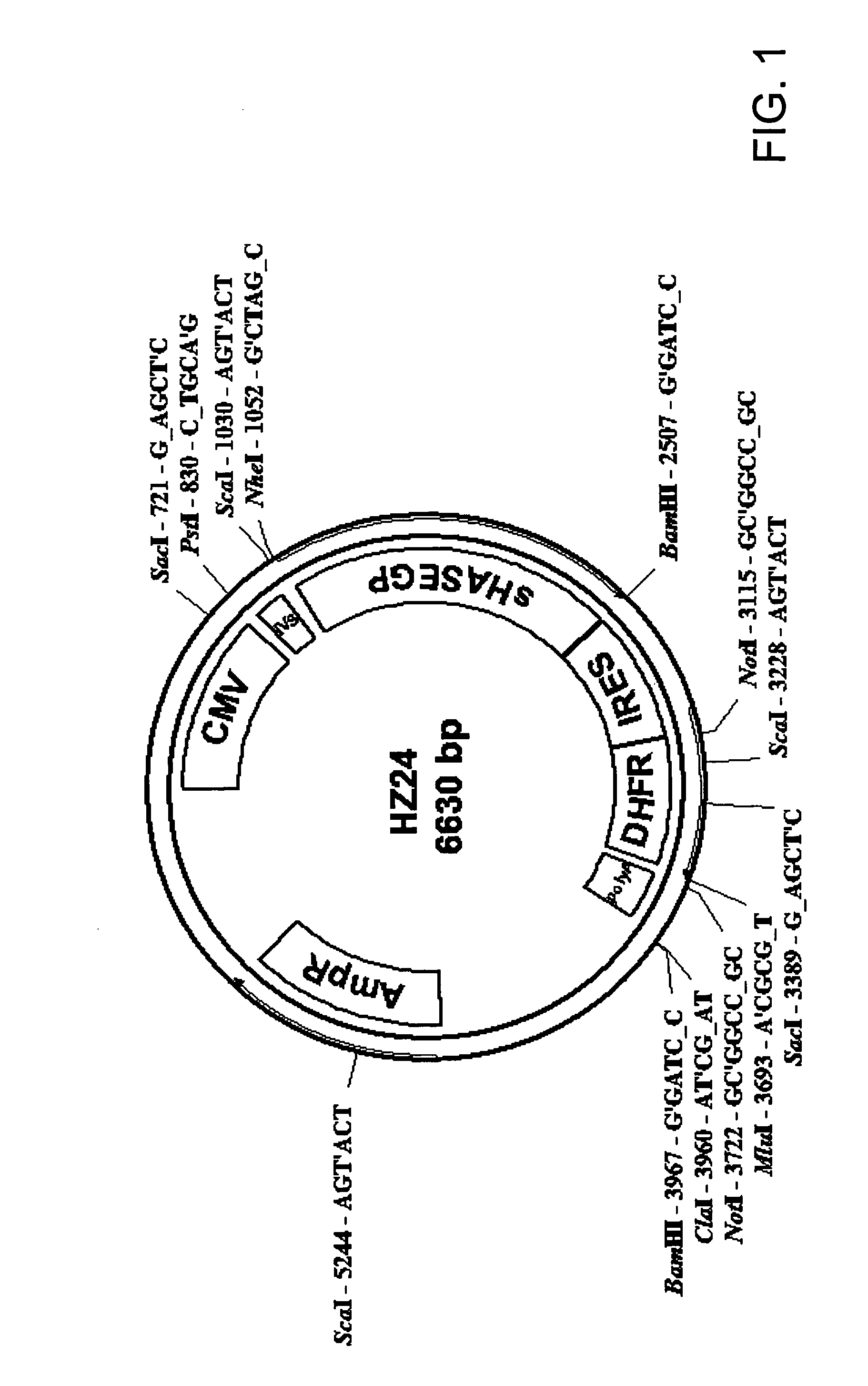
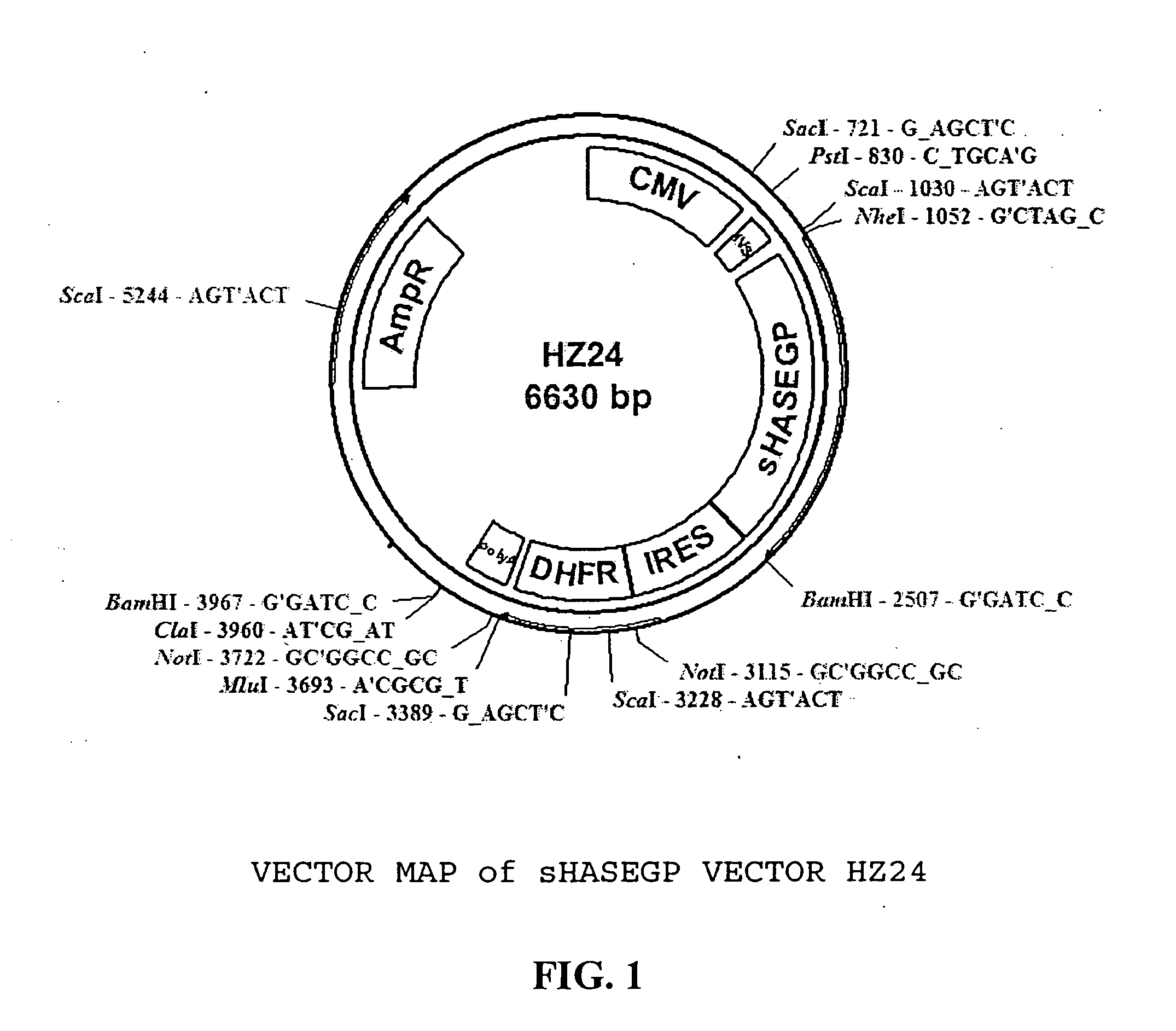
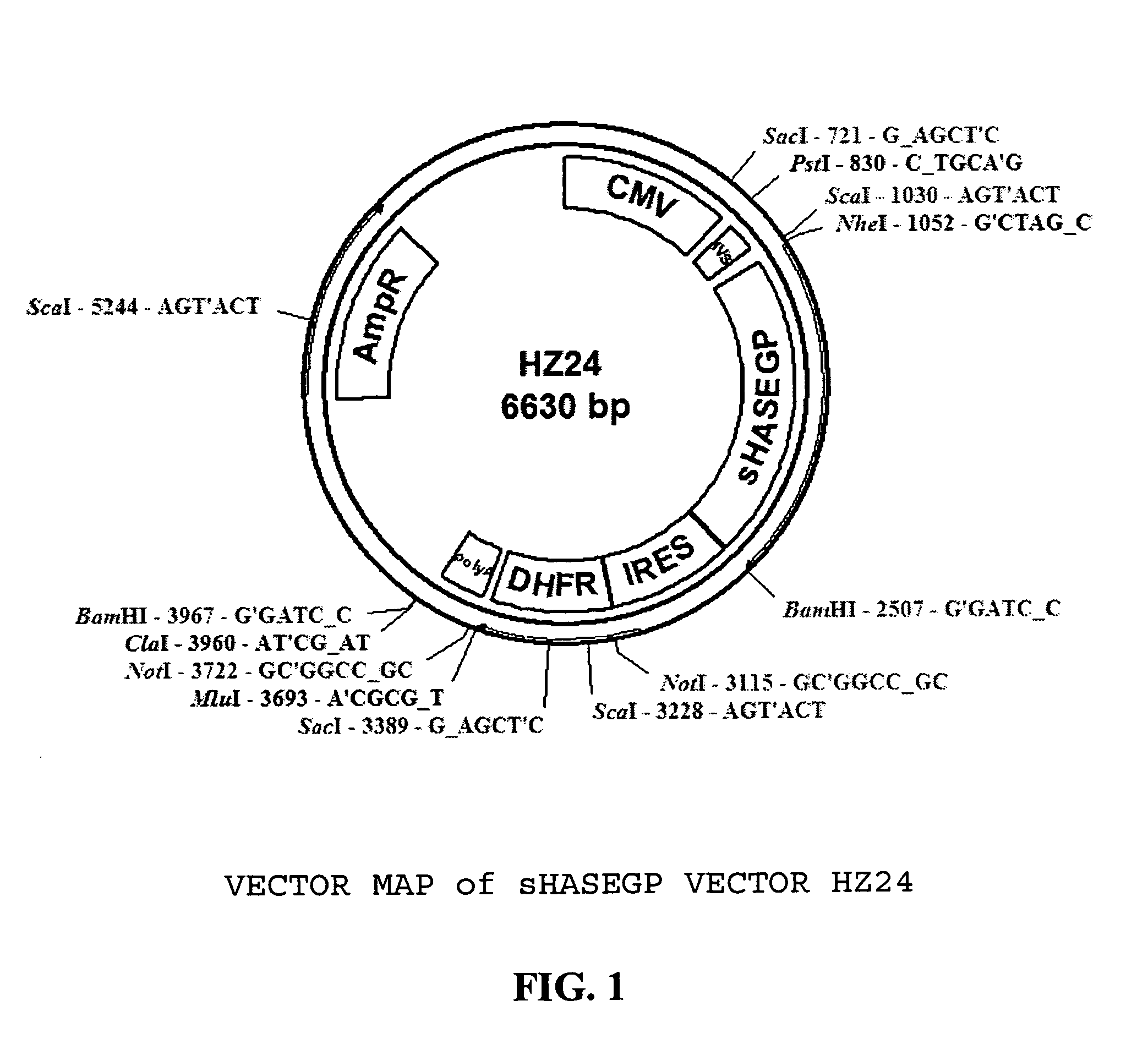
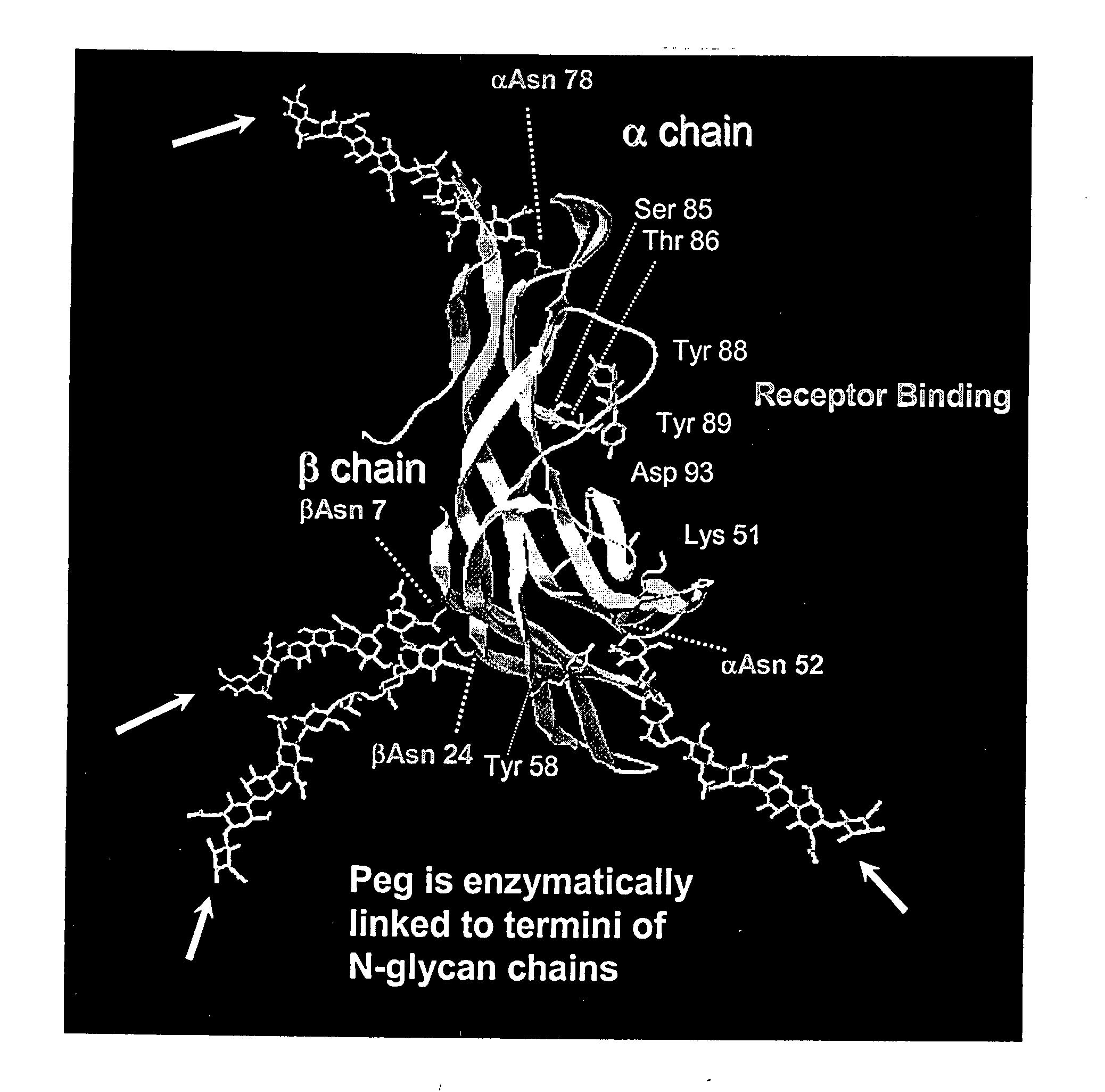
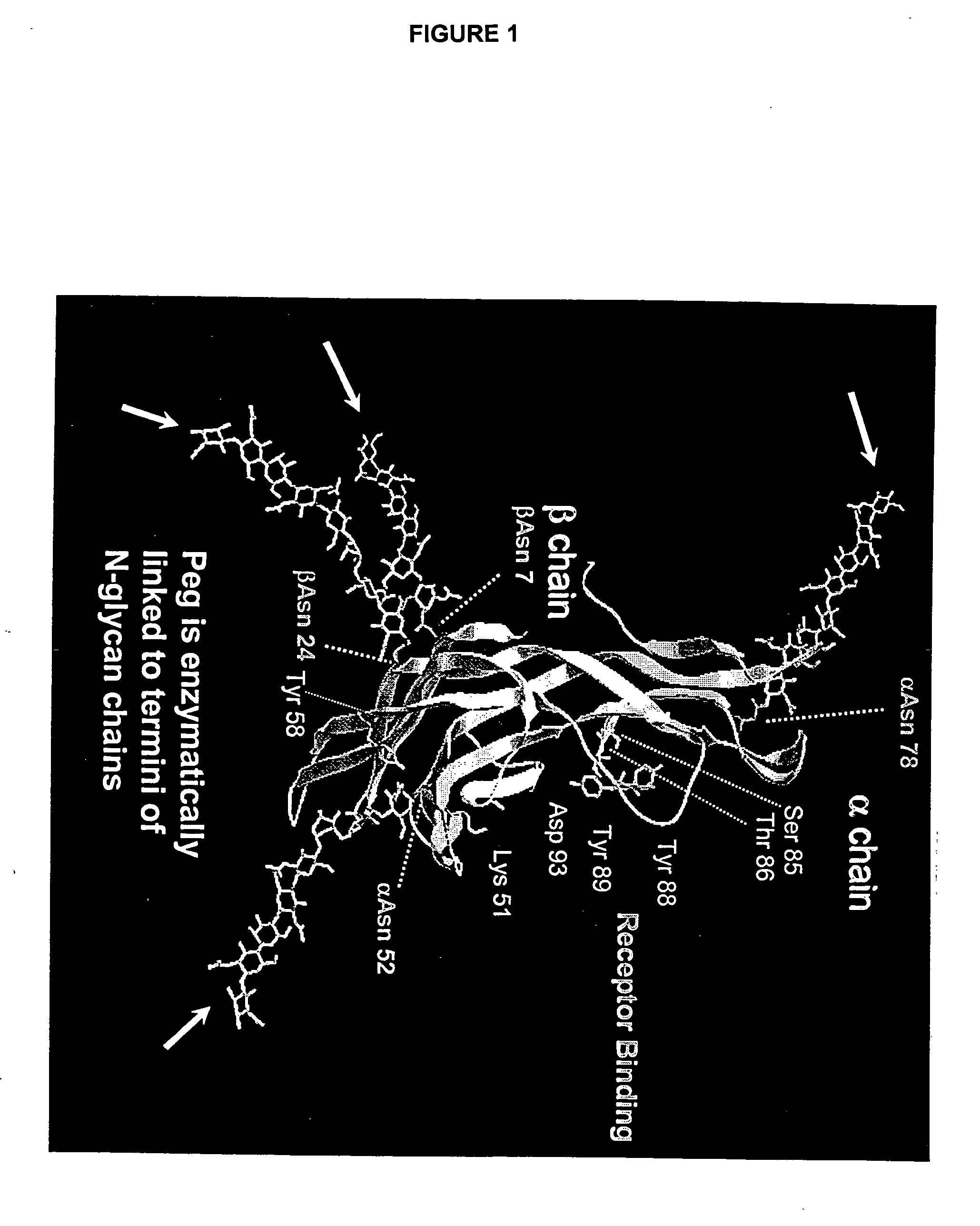
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
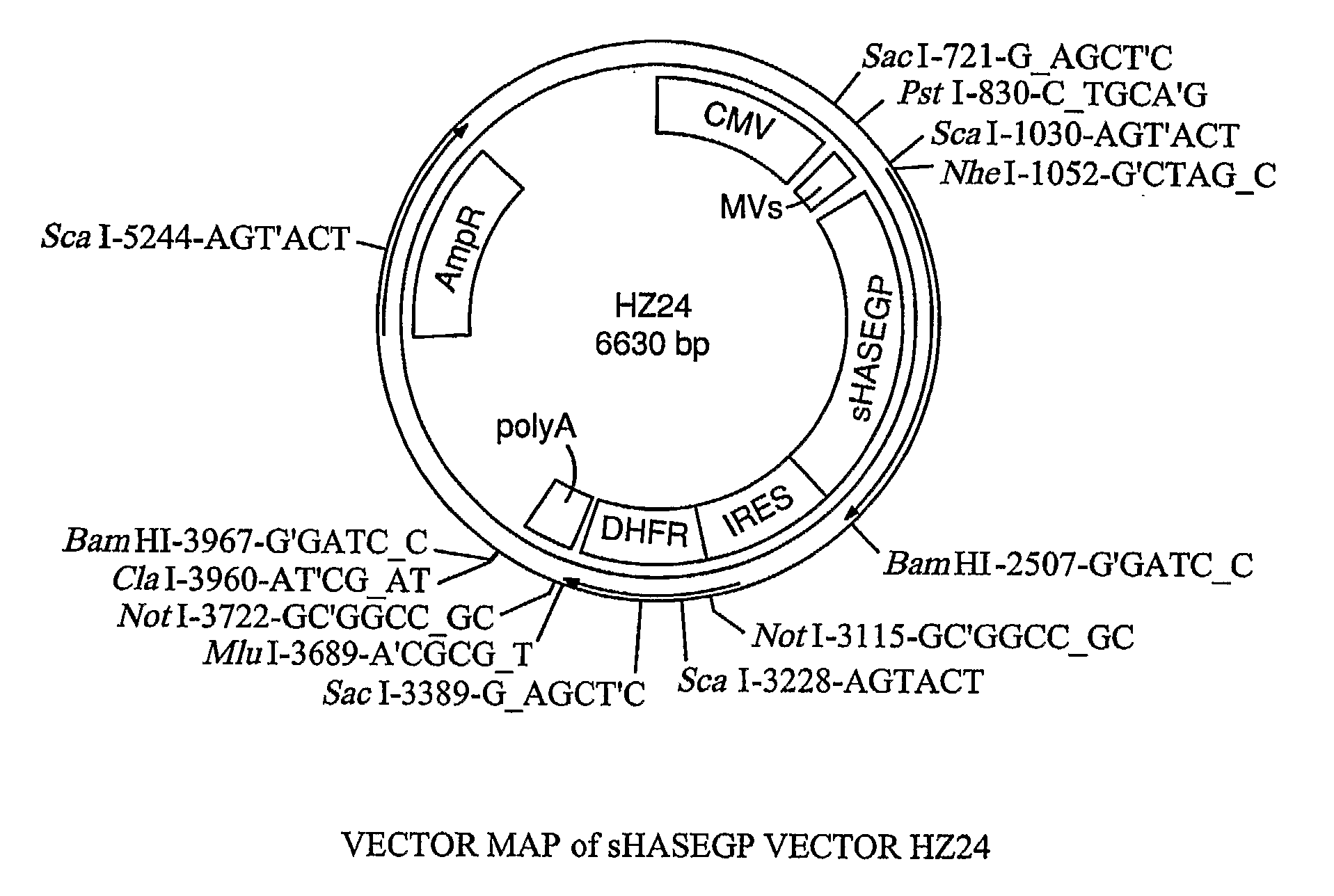
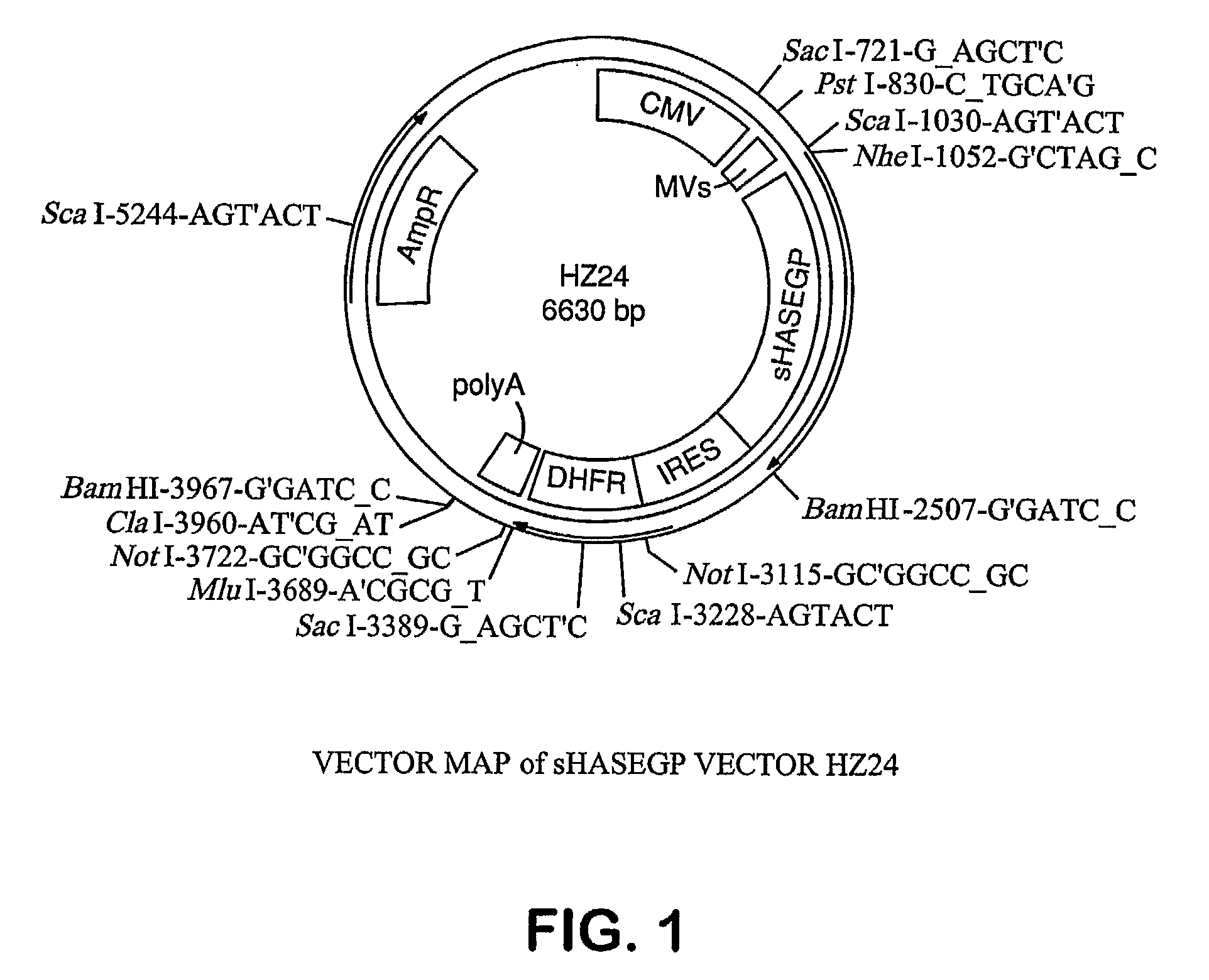
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Oligonucleotides having chiral phosphorus linkages
InactiveUS6239265B1Improved pharmacokinetic propertiesImprove propertiesPeptide/protein ingredientsGenetic material ingredientsSugar moietyPhosphoramidate
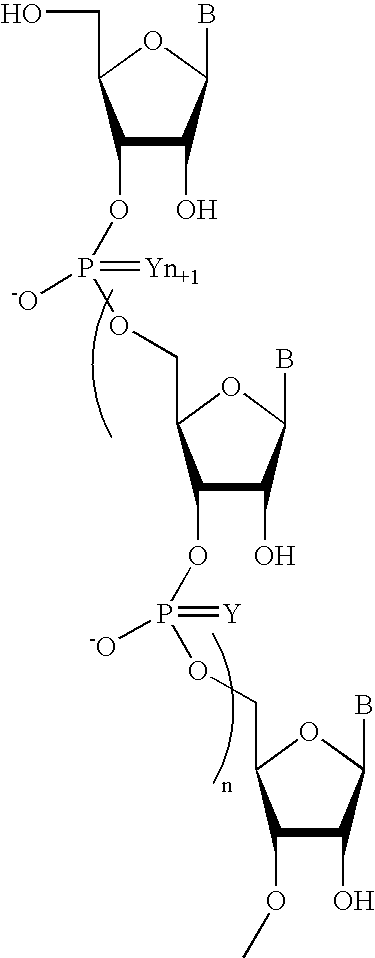
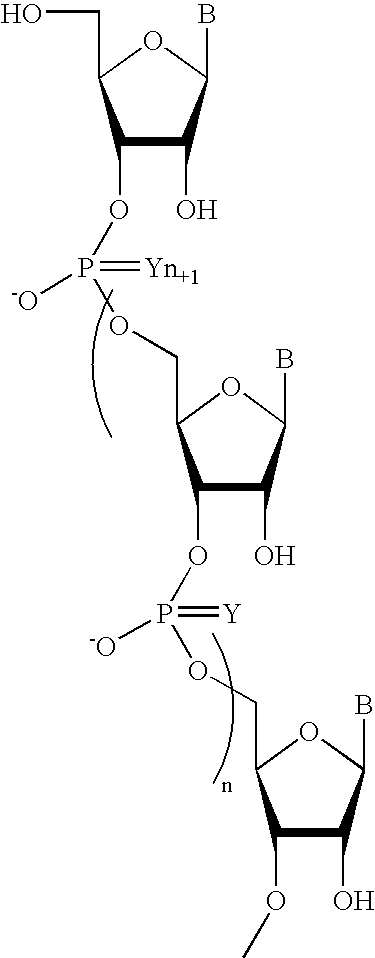
Sequence-specific oligonucleotides are provided having substantially pure chiral Sp phosphorothioate, chiral Rp phosphorothioate, chiral Sp alkylphosphonate, chiral Rp alkylphosphonate, chiral Sp phosphoamidate, chiral Rp phosphoamidate, chiral Sp phosphotriester, and chiral Rp phosphotriester linkages. The novel oligonucleotides are prepared via a stereospecific SN2 nucleophilic attack of a phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate anion on the 3' position of a xylonucleotide. The reaction proceeds via inversion at the 3' position of the xylo reactant species, resulting in the incorporation of phosphodiester, phosphorothioate, phosphoramidate, phosphotriester or alkylphosphonate linked ribofuranosyl sugar moieties into the oligonucleotide.
Owner:IONIS PHARMA INC
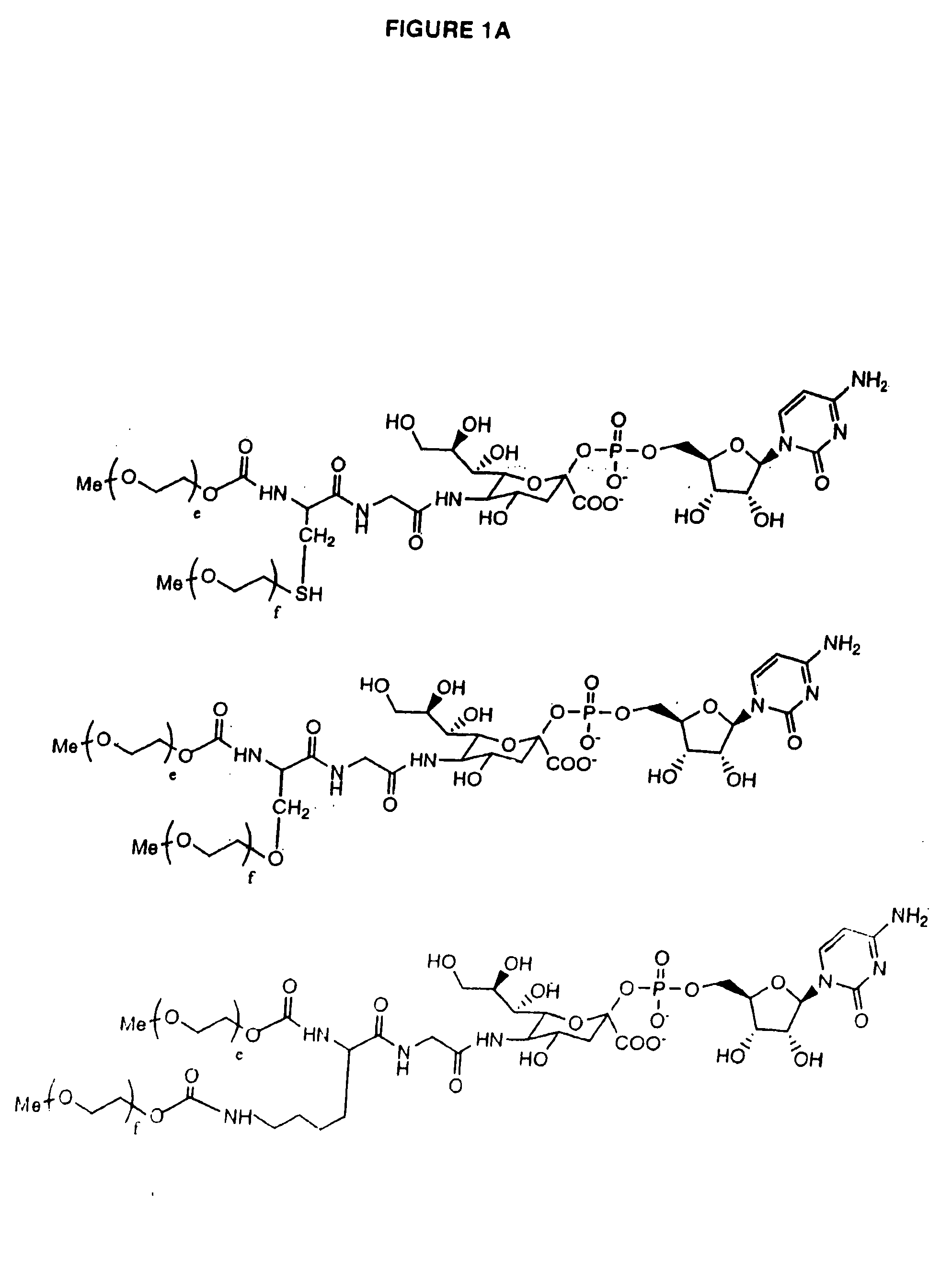
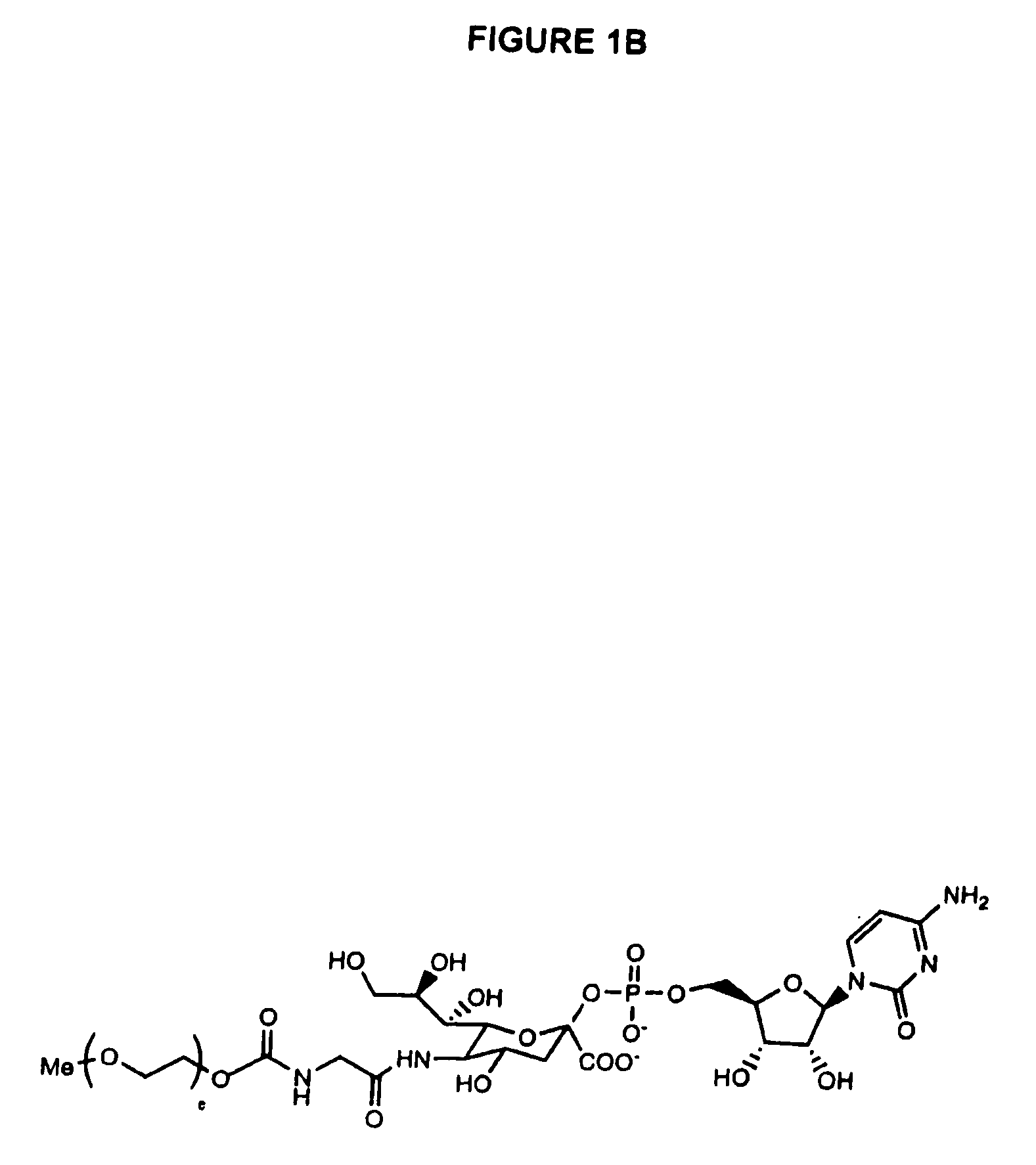
Glycopegylated erythropoietin
InactiveUS20060111279A1Improved pharmacokinetic propertiesCost effectiveSaccharide peptide ingredientsDepsipeptidesDiseaseSugar moiety
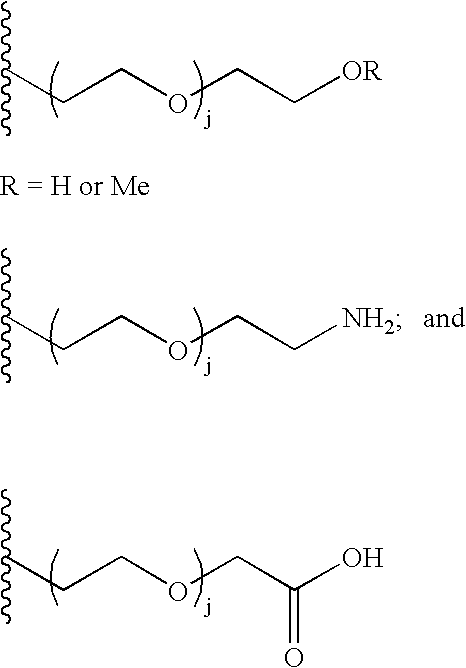
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Glycopegylated erythropoietin
InactiveUS20050143292A1Improved pharmacokinetic propertiesCost-effectiveSugar derivativesPeptide/protein ingredientsDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Single-stranded and double-stranded oligonucleotides comprising a 2-arylpropyl moiety
ActiveUS20060008822A1Improved pharmacokinetic propertiesAntibacterial agentsSenses disorderNucleotidePhosphate
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one aralkyl ligand. In certain embodiments, an aralkyl ligand is bound to only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, an aralkyl ligand is bound to both of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage. In a preferred embodiment, the aralkyl ligand is naproxen or ibuprofen. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one aralkyl ligand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage. In a preferred embodiment, the aralkyl ligand is naproxen or ibuprofen. The aralkyl ligand improves the pharmacokinetic properties of the oligonucleotide.
Owner:ALNYLAM PHARM INC
Oligonucleotides comprising a non-phosphate backbone linkage
One aspect of the present invention relates to a ribonucleoside substituted with a phosphonamidite group at the 3′-position. In certain embodiments, the phosphonamidite is an alkyl phosphonamidite. Another aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one non-phosphate linkage. Representative non-phosphate linkages include phosphonate, hydroxylamine, hydroxylhydrazinyl, amide, and carbamate linkages. In certain embodiments, the non-phosphate linkage is a phosphonate linkage. In certain embodiments, a non-phosphate linkage occurs in only one strand. In certain embodiments, a non-phosphate linkage occurs in both strands. In certain embodiments, a ligand is bound to one of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, a ligand is bound to both of the oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one non-phosphate linkage. Representative non-phosphate linkages include phosphonate, hydroxylamine, hydroxylhydrazinyl, amide, and carbamate linkages. In certain embodiments, the non-phosphate linkage is a phosphonate linkage. In certain embodiments, a ligand is bound to the oligonucleotide strand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety.
Owner:ALNYLAM PHARM INC
Oligonucleotides comprising a modified or non-natural nucleobase
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide contains a non-natural nucleobase. In certain embodiments, both of the oligonucleotide strands comprising the double-stranded oligonucleotide independently contain a non-natural nucleobase. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one non-natural nucleobase. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, the ribose sugar moiety that occurs naturally in nucleosides is replaced with a hexose sugar, polycyclic heteroalkyl ring, or cyclohexenyl group. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage.
Owner:ALNYLAM PHARM INC
Glycopegylated factor IX
InactiveUS20060040856A1Improved pharmacokinetic propertiesRetain pharmacological activitySaccharide peptide ingredientsMammal material medical ingredientsGlycoPEGylated factor VIIaPharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NEOSE TECH
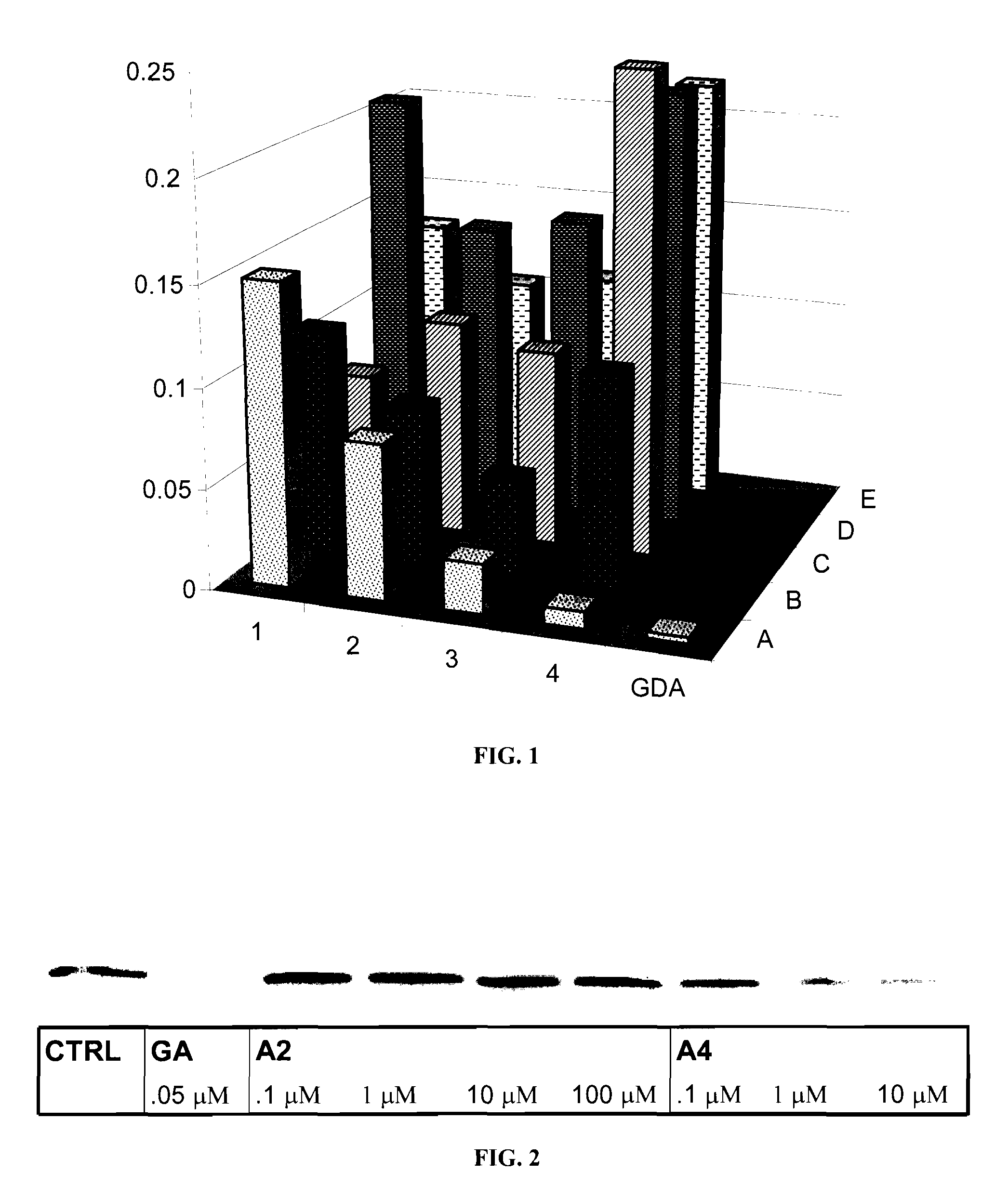
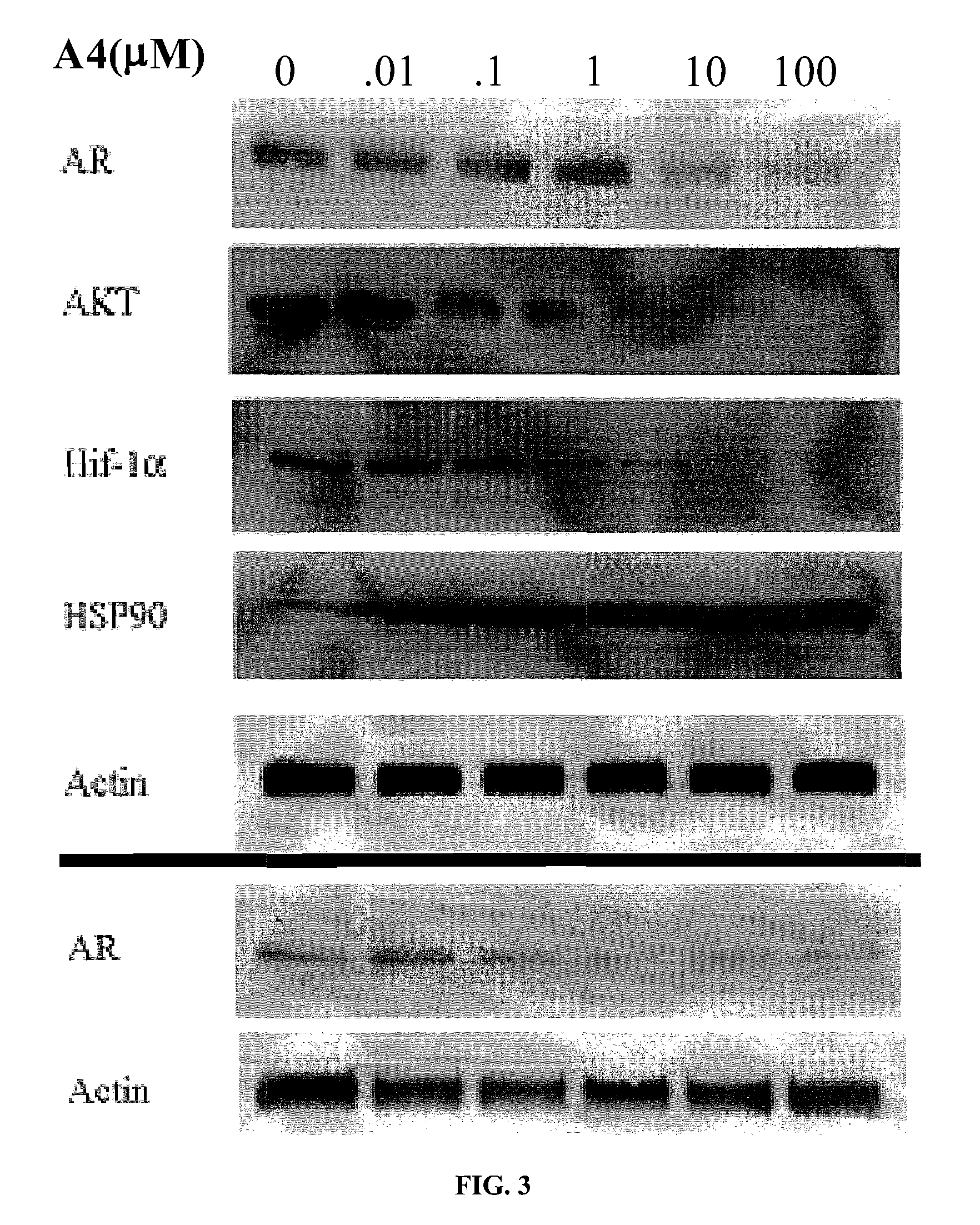
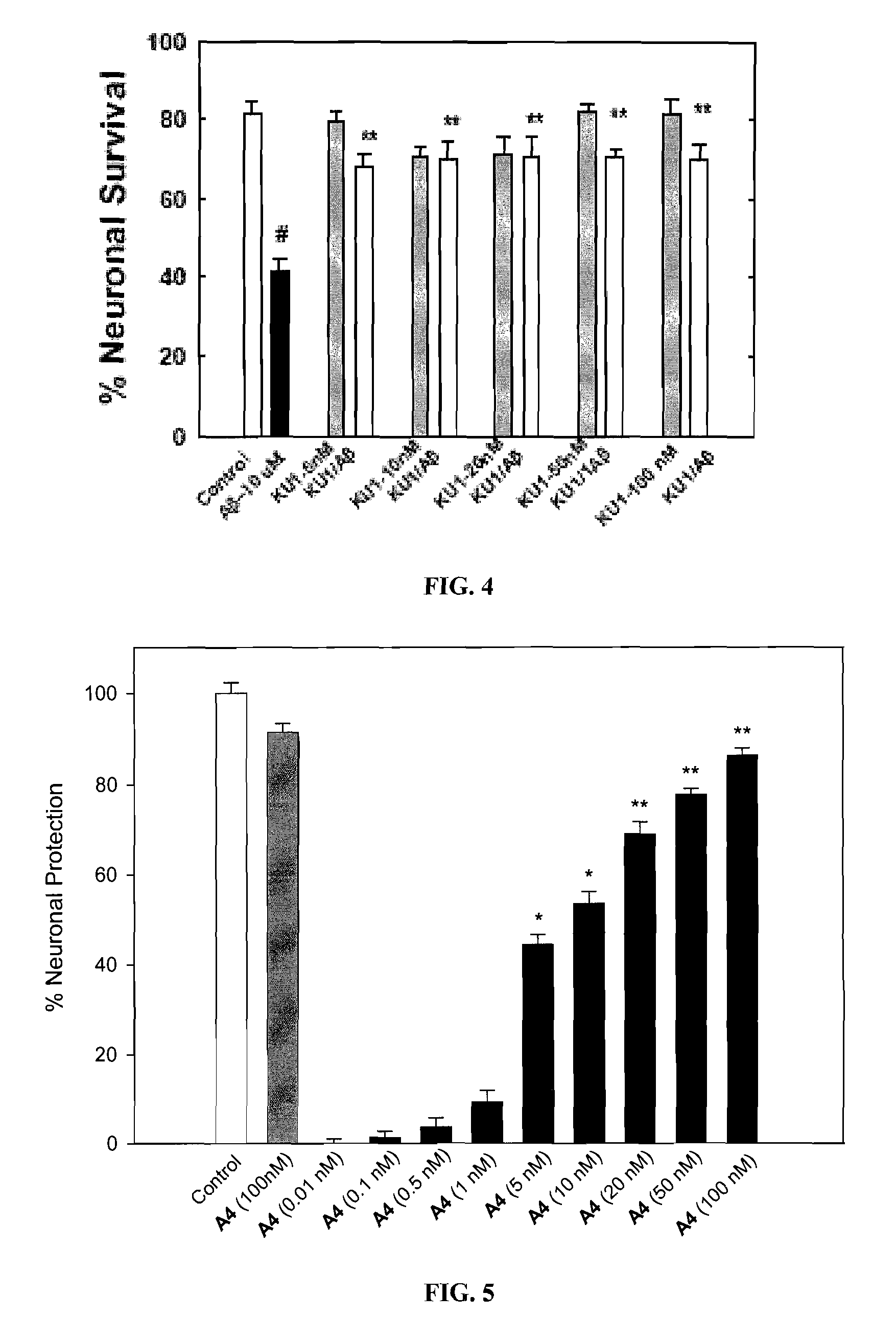
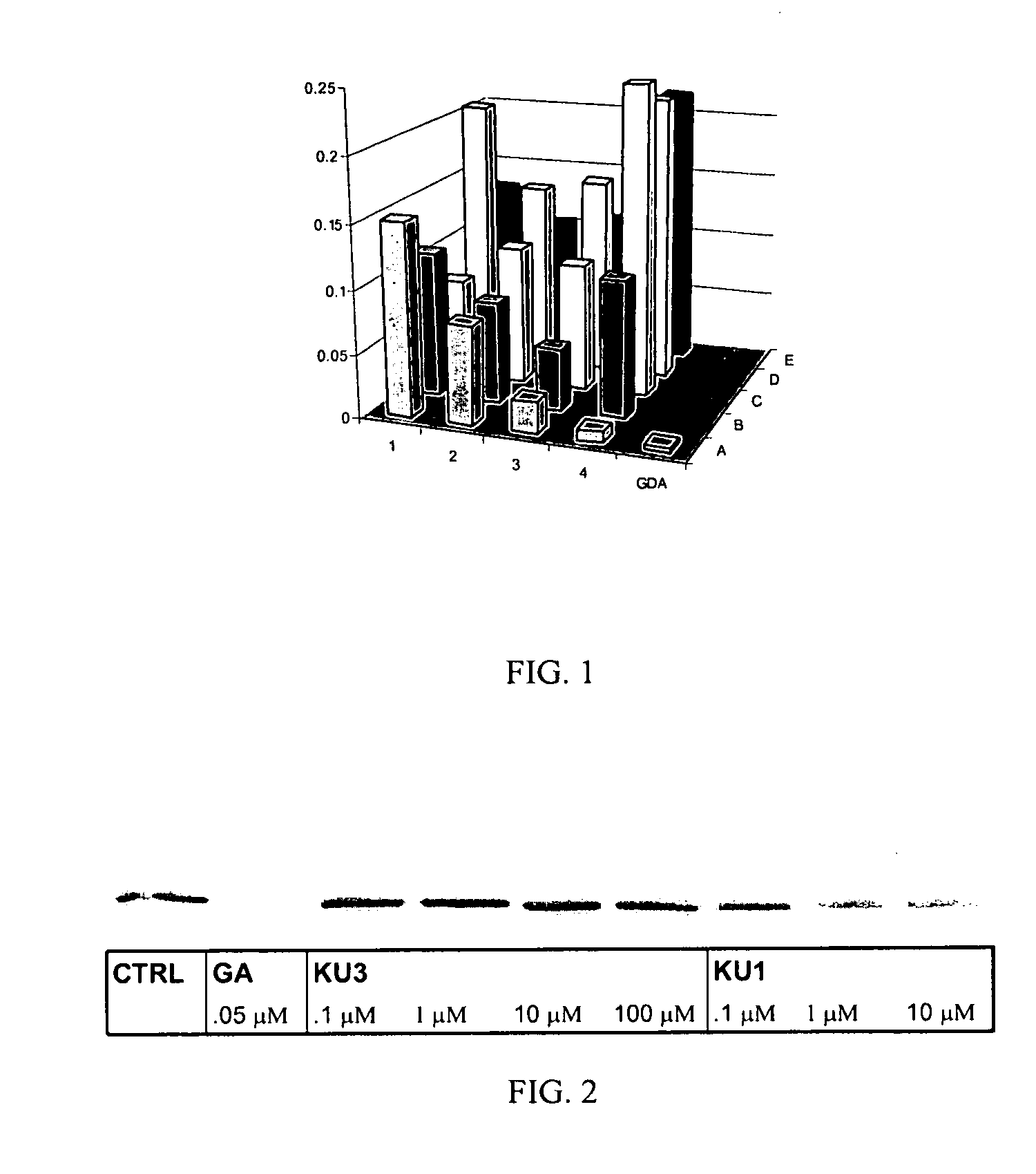
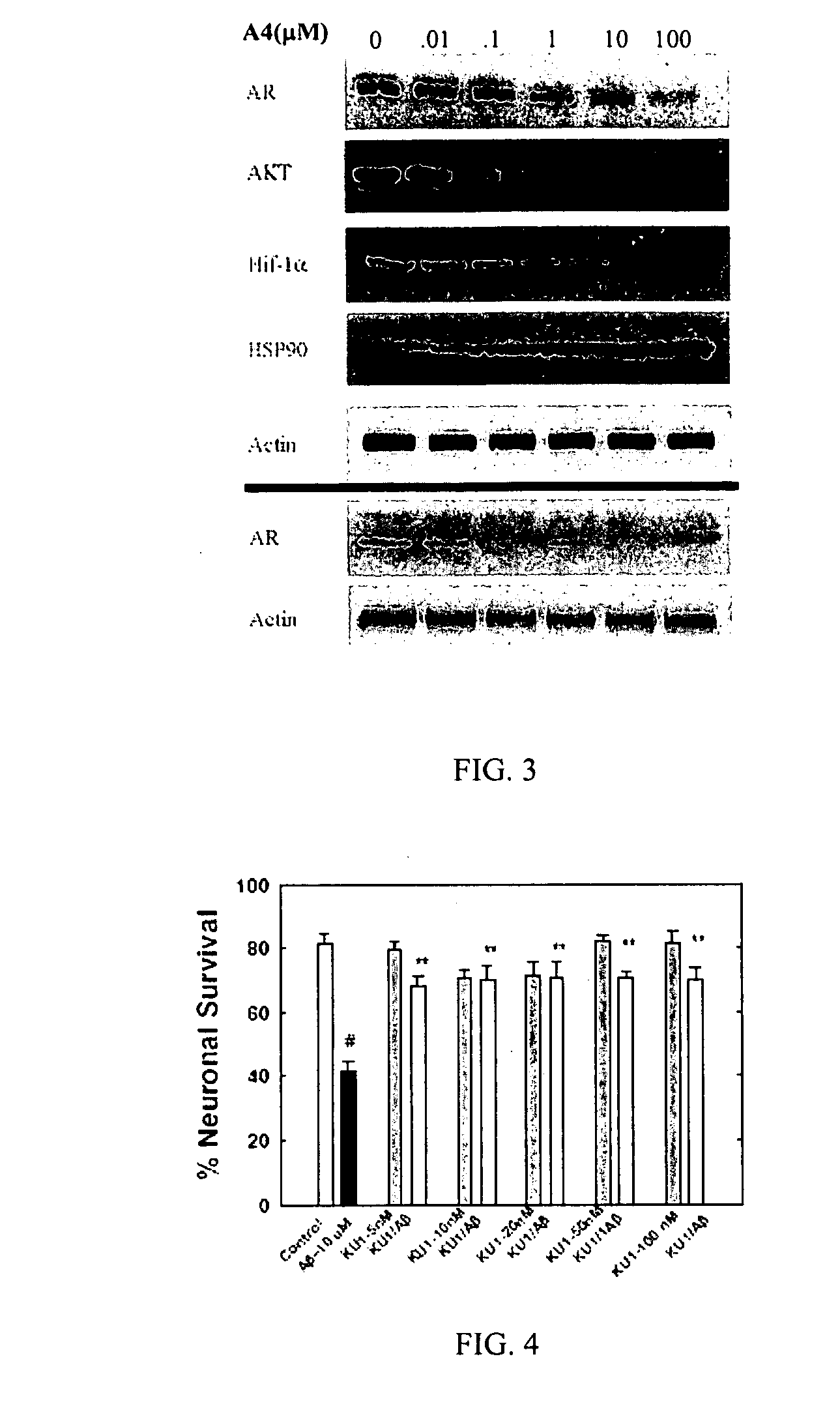
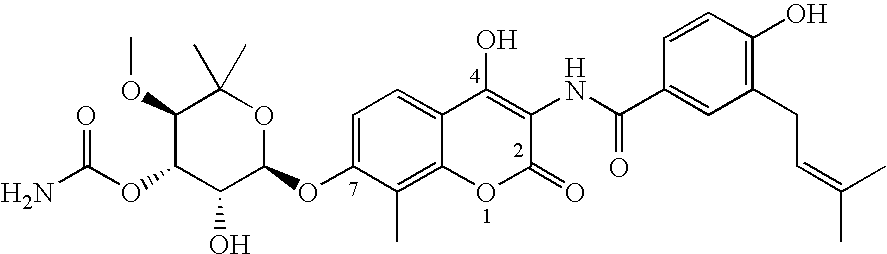
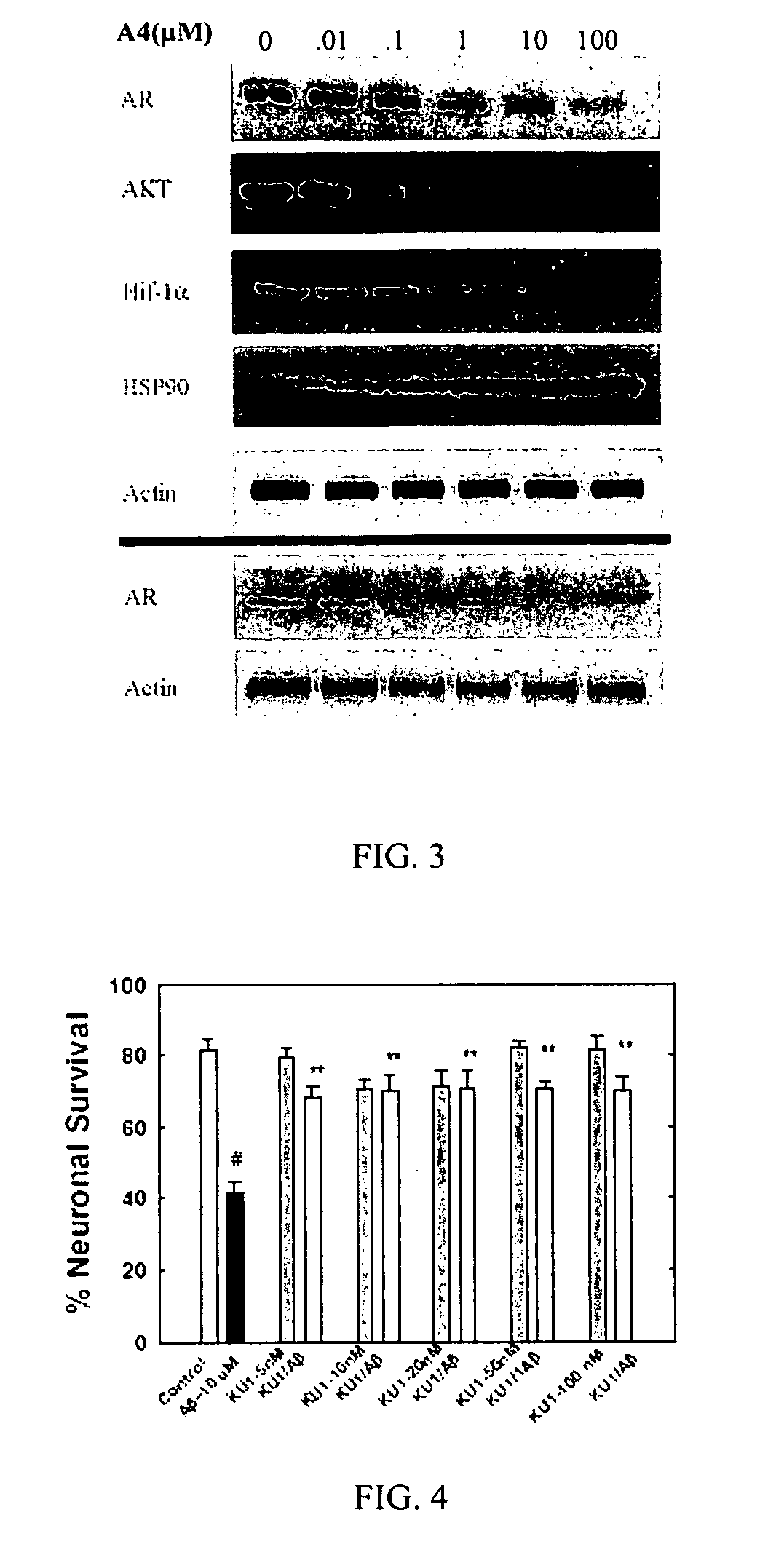
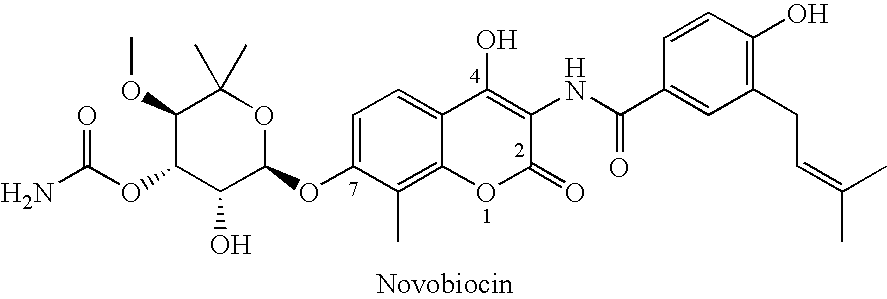
Novobiocin Analogues Having Modified Sugar Moieties
Novobiocin analogues useful as Hsp90 inhibitors in the treatment of cancer, neuroprotection, and autoimmune disorders.
Owner:UNIVERSITY OF KANSAS
Novobiocin analogues as anticancer agents
Novel analogues and derivatives of novobiocin are provided, including compounds having modifications to the amide side chain, coumarin ring, and sugar moieties. The compounds of the present invention are useful as heat shock protein 90 inhibitors, and may be used as anticancer and neuroprotective agents.
Owner:UNIVERSITY OF KANSAS +1
Oligonucleotides comprising a C5-modified pyrimidine
ActiveUS20050288244A1Improved pharmacokinetic propertiesAntibacterial agentsNervous disorderBenzoic acidPhosphate
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one ligand. In certain embodiments, a ligand is bound to only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide. In certain embodiments, both of the oligonucleotide strands of the double-stranded oligonucleotide independently comprise a bound ligand. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. In certain embodiments, a phosphate linkage in one or both of the strands of the oligonucleotide has been replaced with a phosphorothioate or phosphorodithioate linkage. In a preferred embodiment, the ligand is cholesterol or 5β-cholanic acid. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one ligand. In certain embodiments, the oligonucleotide comprises at least one modified sugar moiety. In certain embodiments, a phosphate linkage of the oligonucleotide has been replaced with a phosphorothioate or phosphorodithioate linkage. In a preferred embodiment, the ligand is cholesterol or 5β-cholanic acid. The ligand improves the pharmacokinetic properties of the oligonucleotide.
Owner:ALNYLAM PHARM INC
One pot desialylation and glycopegylation of therapeutic peptides
InactiveUS20070105755A1Improved pharmacokinetic propertiesCost effectiveSaccharide peptide ingredientsDepsipeptidesSugar moietyGlycosyltransferase
Owner:NOVO NORDISK AS
Novobiocin analogues as anticancer agents
Novel analogues and derivatives of novobiocin are provided, including compounds having modifications to the amide side chain, coumarin ring, and sugar moieties. The compounds of the present invention are useful as heat shock protein 90 inhibitors, and may be used as anticancer and neuroprotective agents.
Owner:UNIVERSITY OF KANSAS +1
Glycopegylated Follicle Stimulating Hormone
InactiveUS20080015142A1BioavailabilityImproved pharmacokinetic propertiesBiocideOrganic active ingredientsFollicle-stimulating hormoneSugar moiety
The present invention provides conjugates between follicle stimulating hormone and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Soluble Glycosaminoglycanases and Methods of Preparing and Using Soluble Glycosaminoglycanases
InactiveUS20090123367A1Facilitated DiffusionEnhance convective transportBacterial antigen ingredientsPeptide/protein ingredientsHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME +6
Redundant pacing system with leaded and leadless pacing
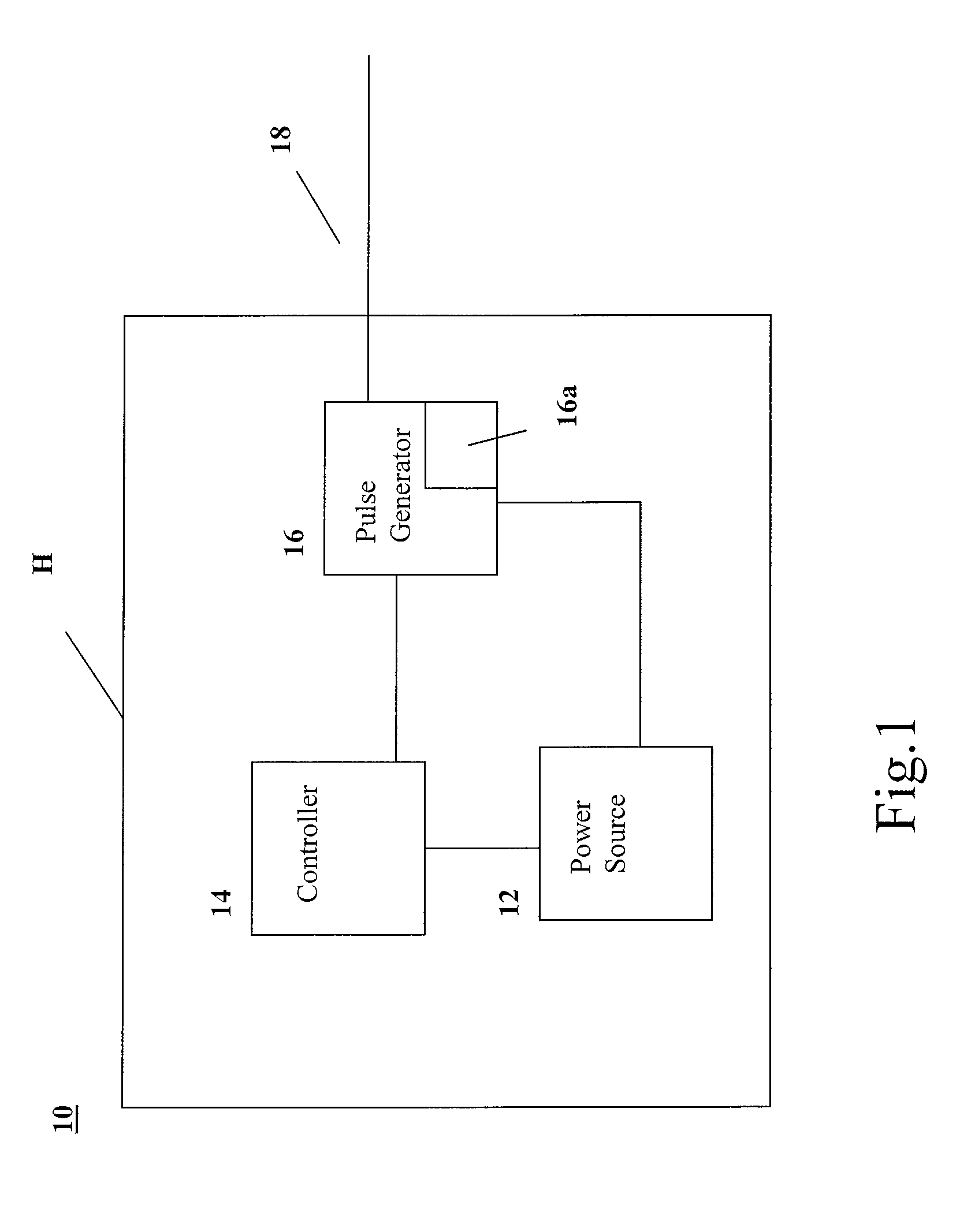
InactiveUS20110276102A1Transvascular endocardial electrodesHeart defibrillatorsTransceiverControl signal
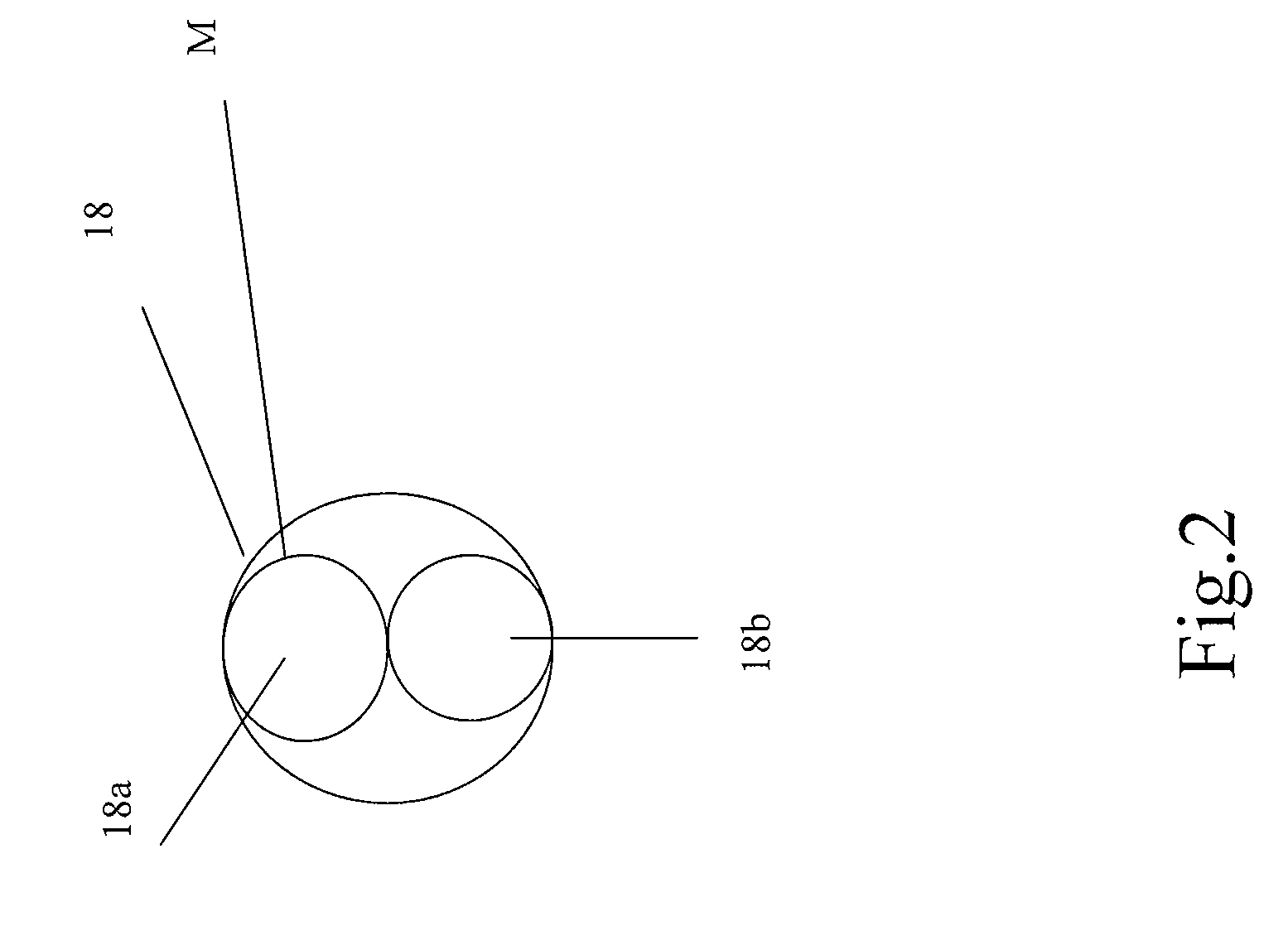
A pacing system includes a controller operable to provide control signals indicating desired pacing signals, a pulse generator connected to the controller and operable to receive the control signals and to generate the desired pacing signals based on the control signals, at least one lead electrically connected to the pulse generator and extending into a user's heart and operable to provide the pacing signals to the heart, at least one electrode positioned in the user's heart and electrically connected to the at least one lead, the at least one electrode in contact with the user's heart and operable to stimulate the heart based on the pacing signals; and a transceiver, in communication with the pulse generator and operable to selectively transmit the pacing signals to the electrode wirelessly. The transceiver is controlled by the controller to transmit the pacing signals when pacing signals are not received by the electrode from the at least one lead. The lead may include multiple leads held together in a sugar moiety as a unitary body for insertion into the heart. Once in the heart, the sugar moiety dissolves to allow the leads to separate for implantation at different points in the heart.
Owner:WINTHROP UNIV HOSPITAL
Glycopegylated factor ix
InactiveUS20090081188A1Improved pharmacokinetic propertiesRetain pharmacological activityPeptide/protein ingredientsEnzyme stabilisationDiseasePharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
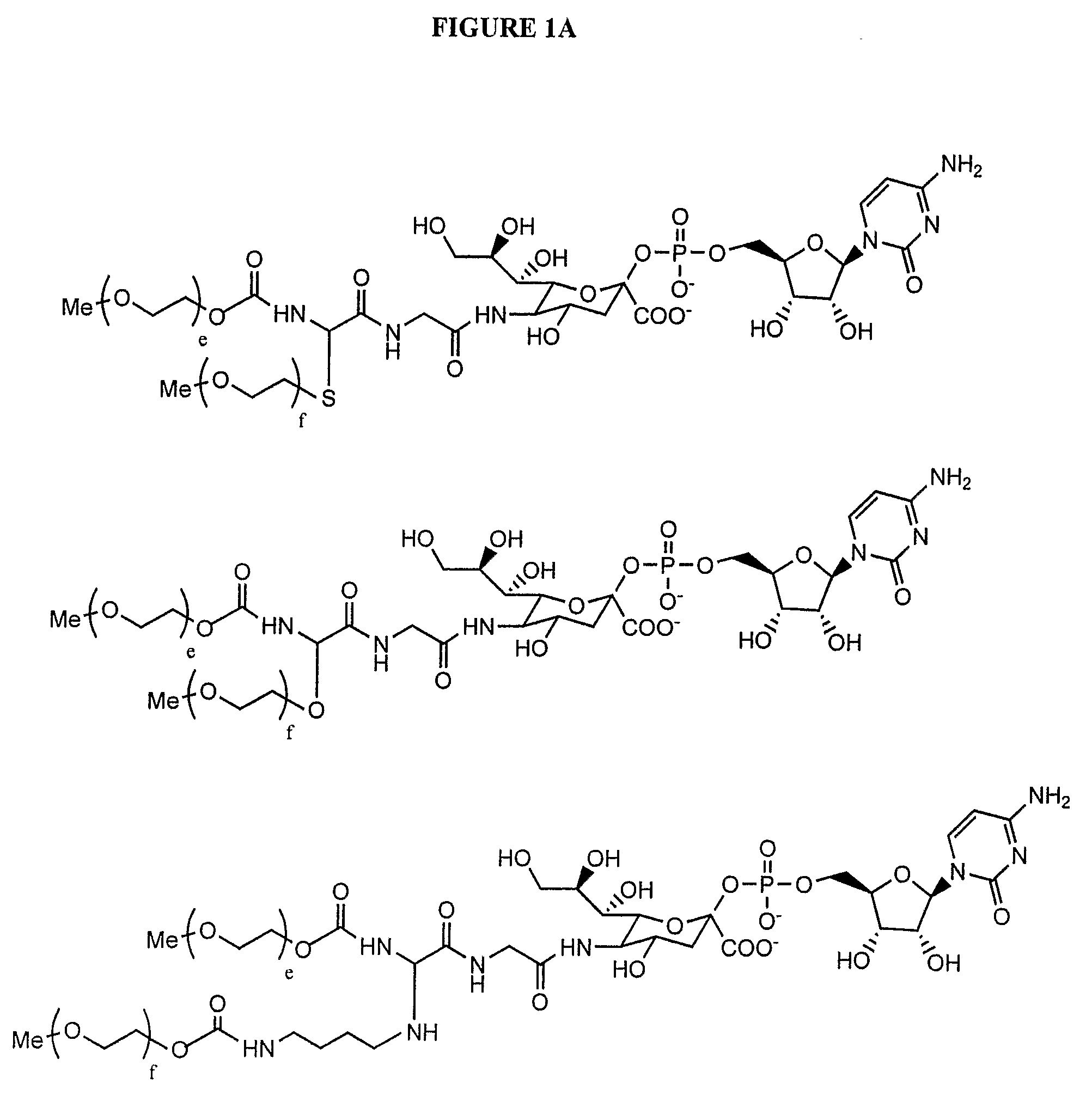
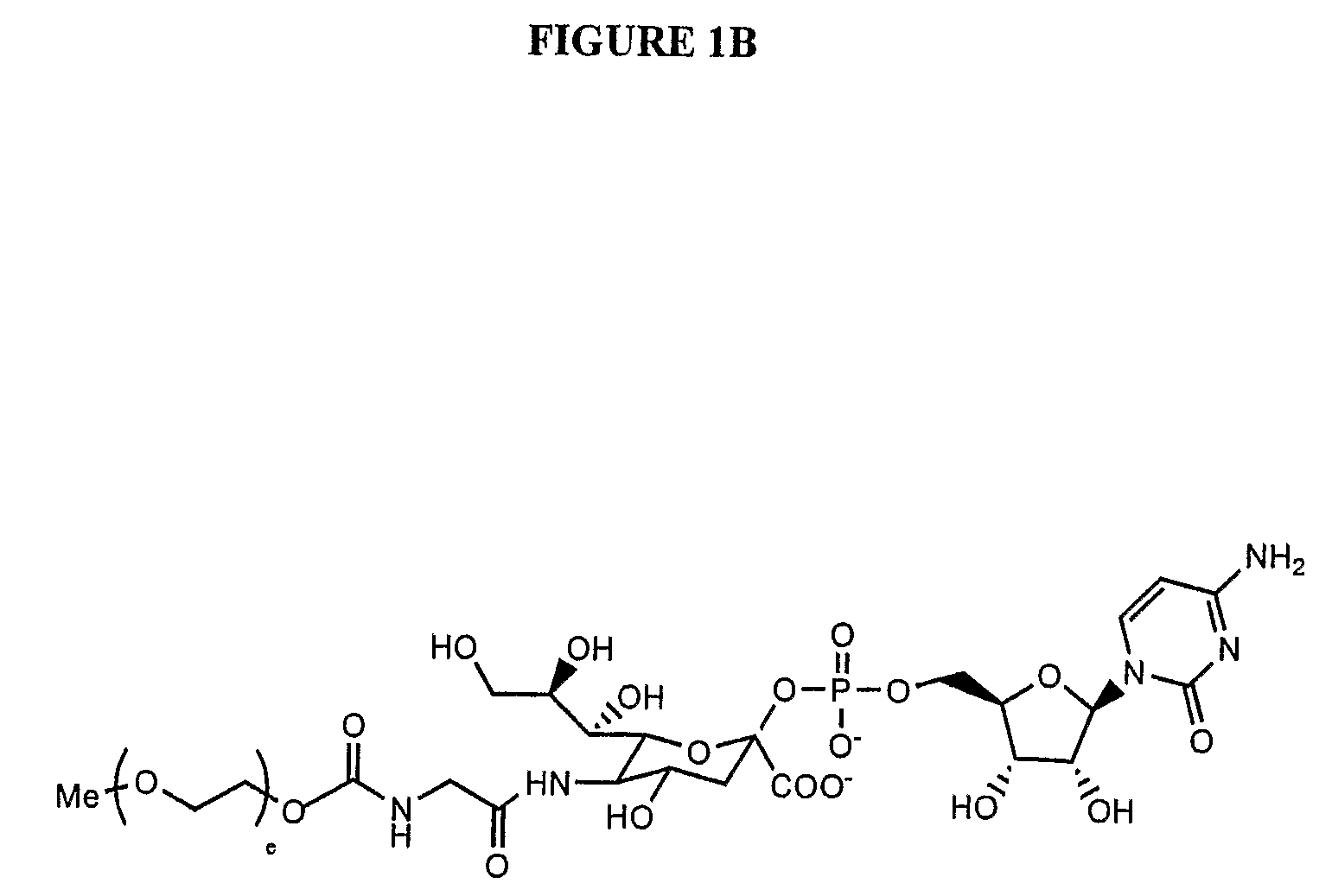
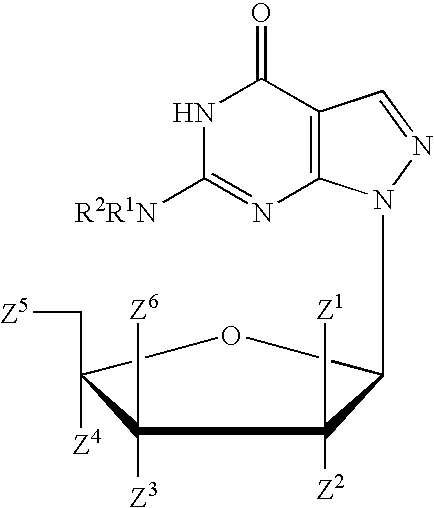
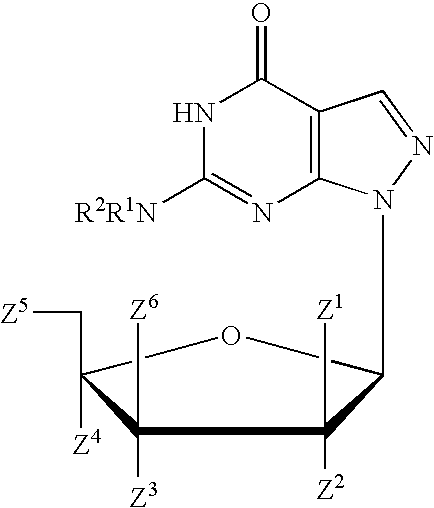
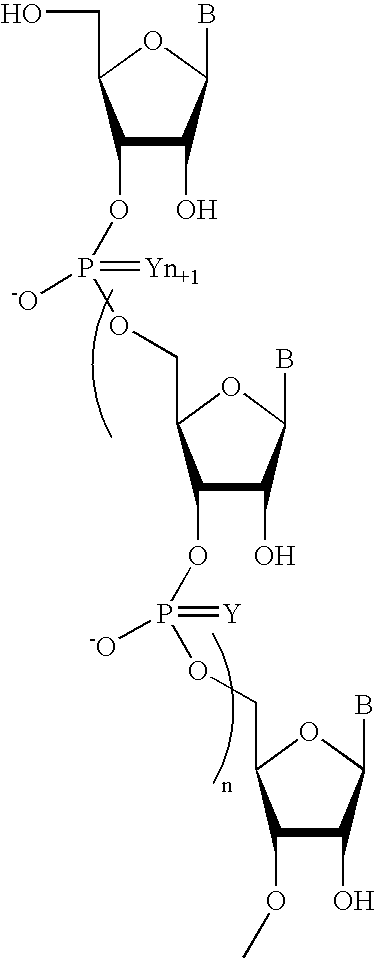
Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides
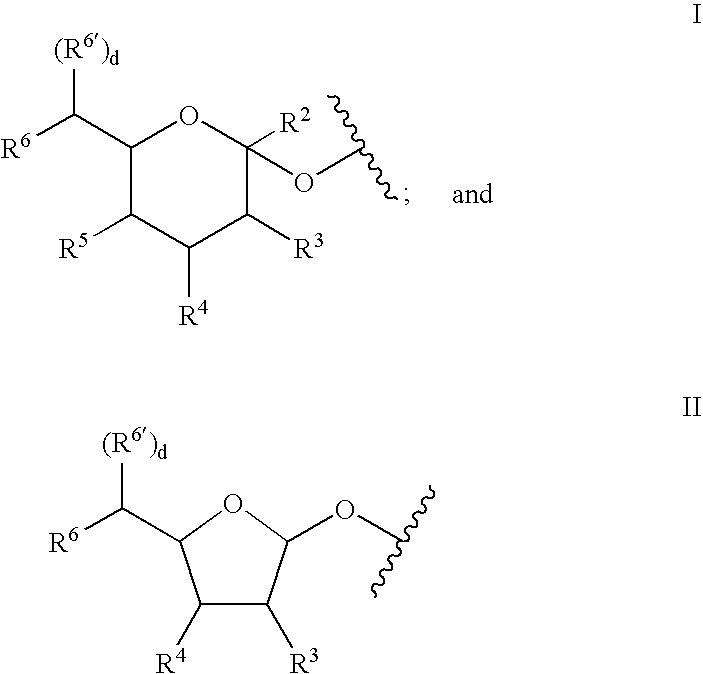
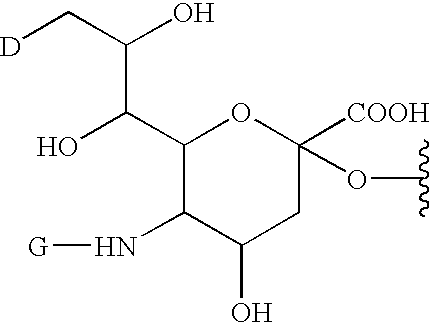
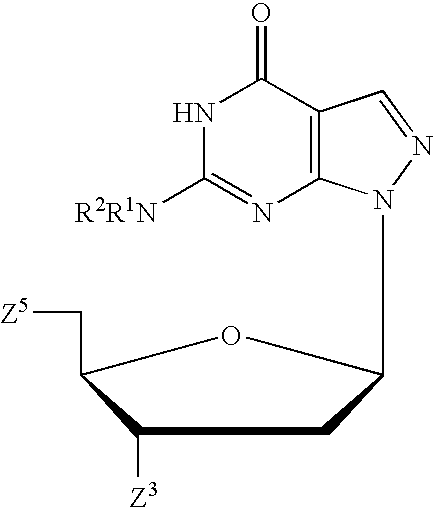
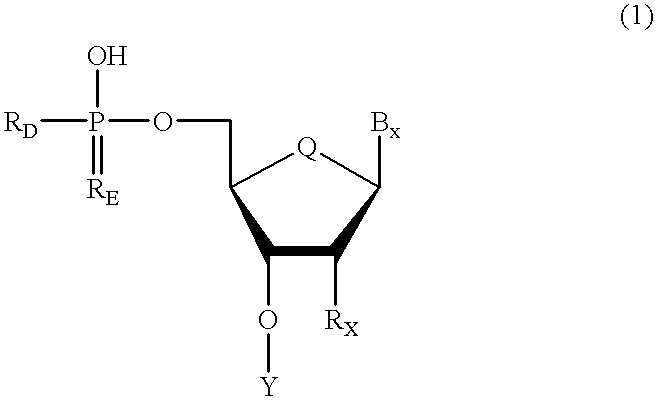
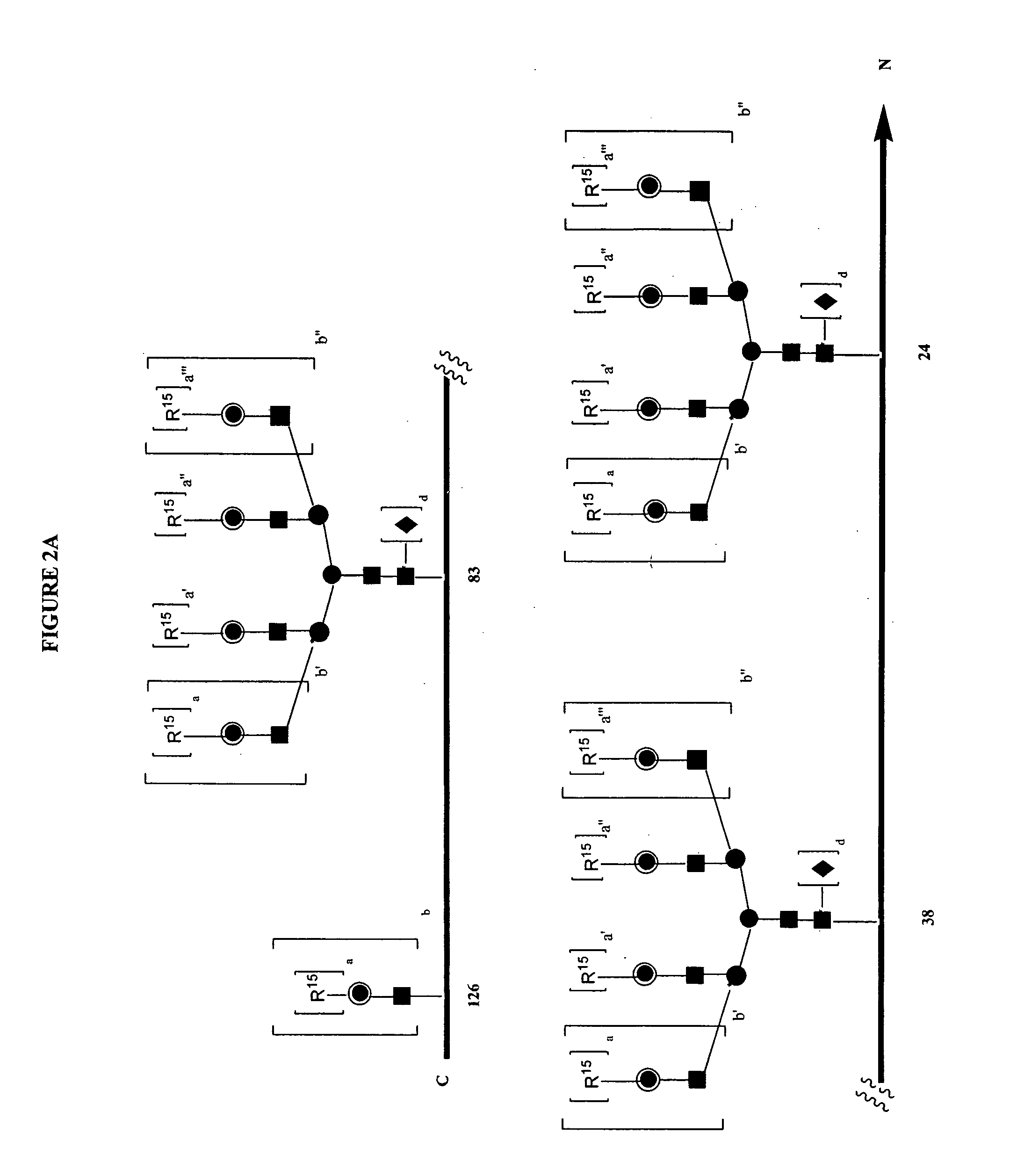
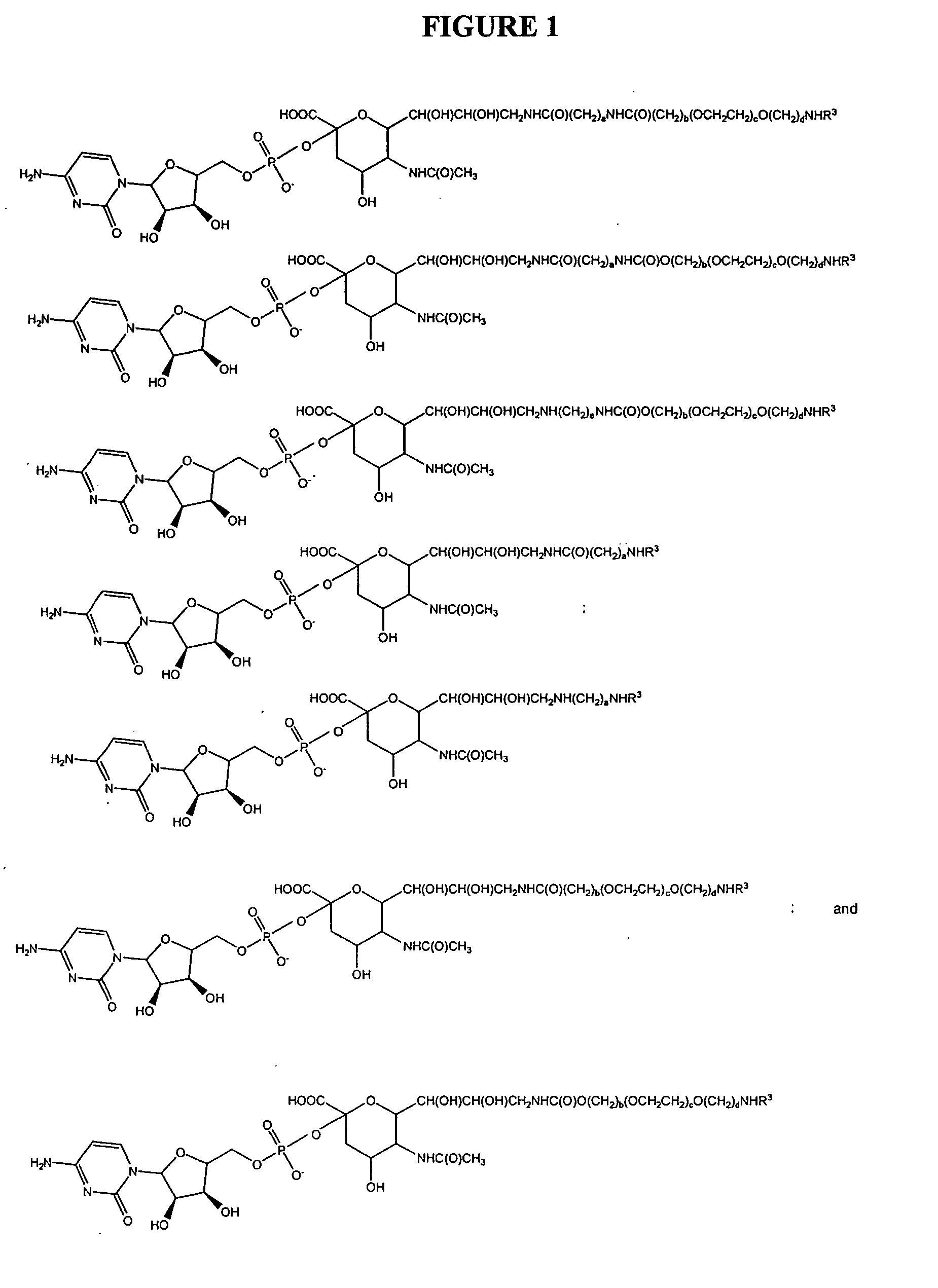
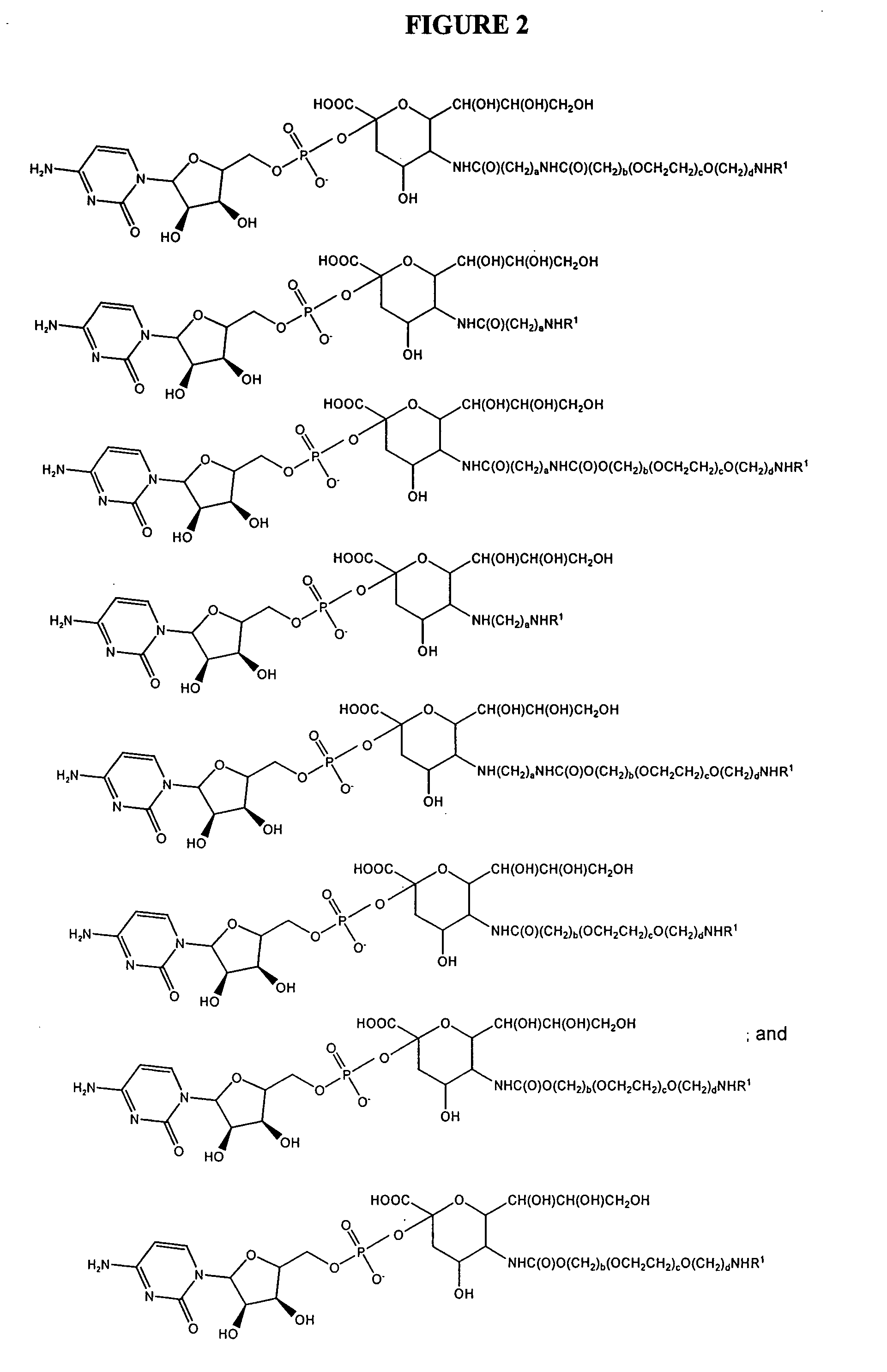
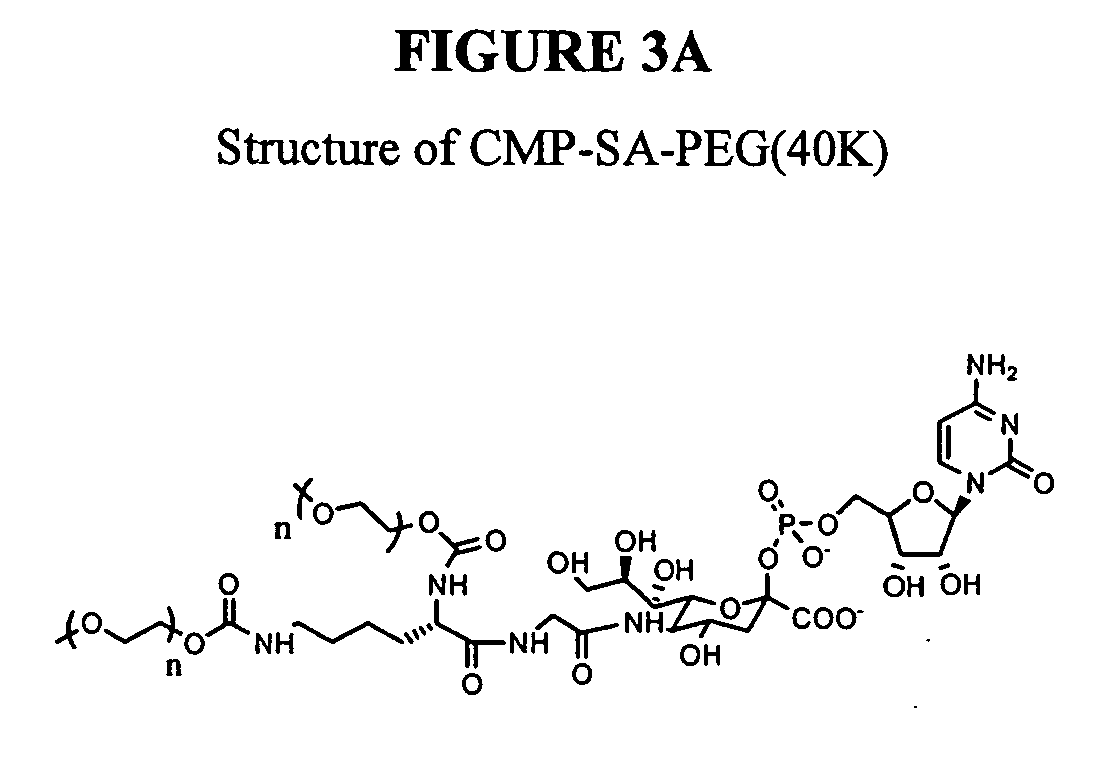
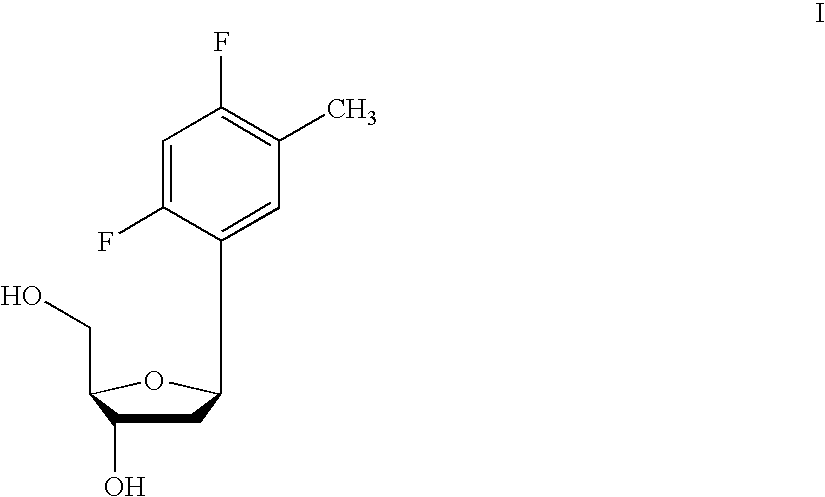
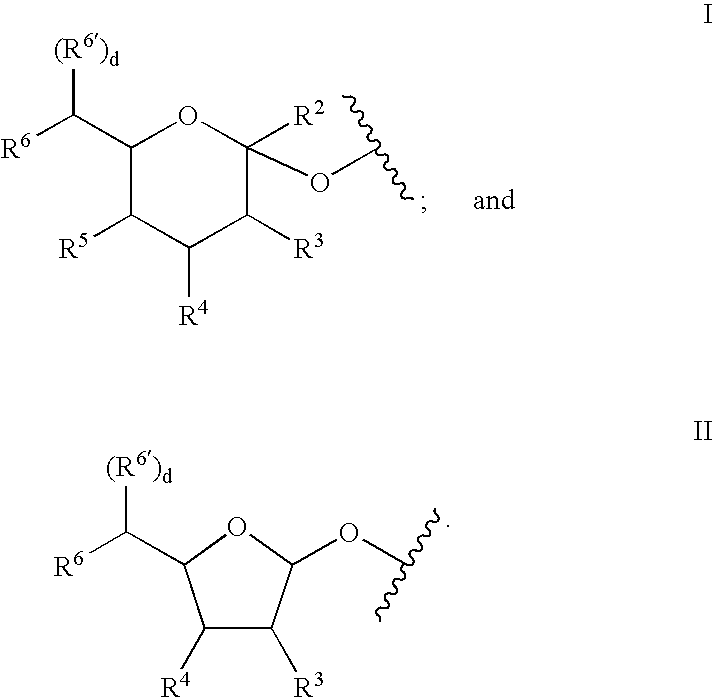
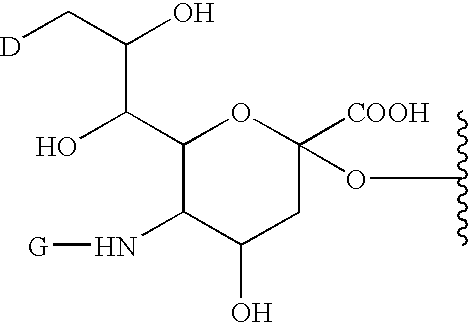
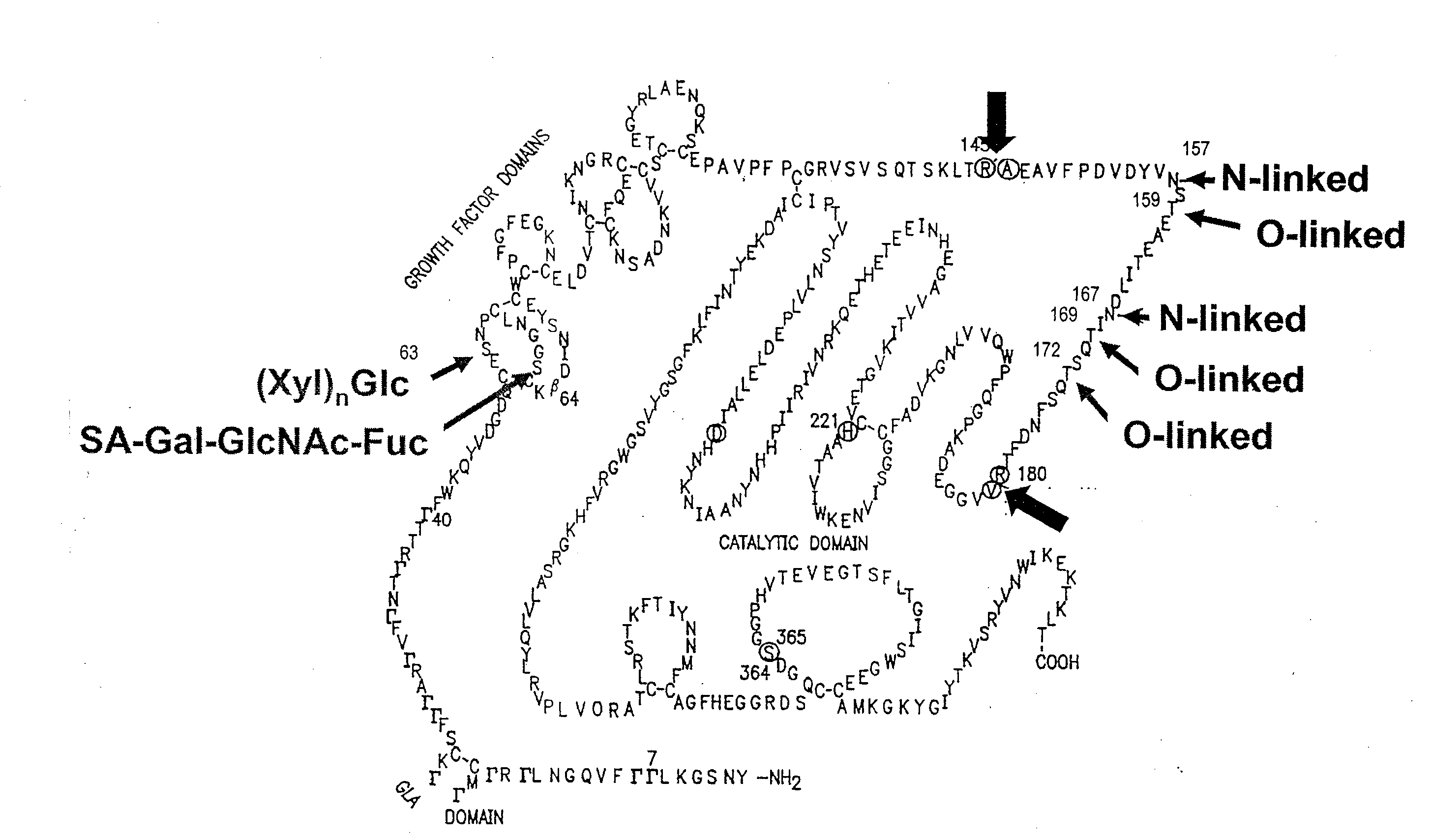
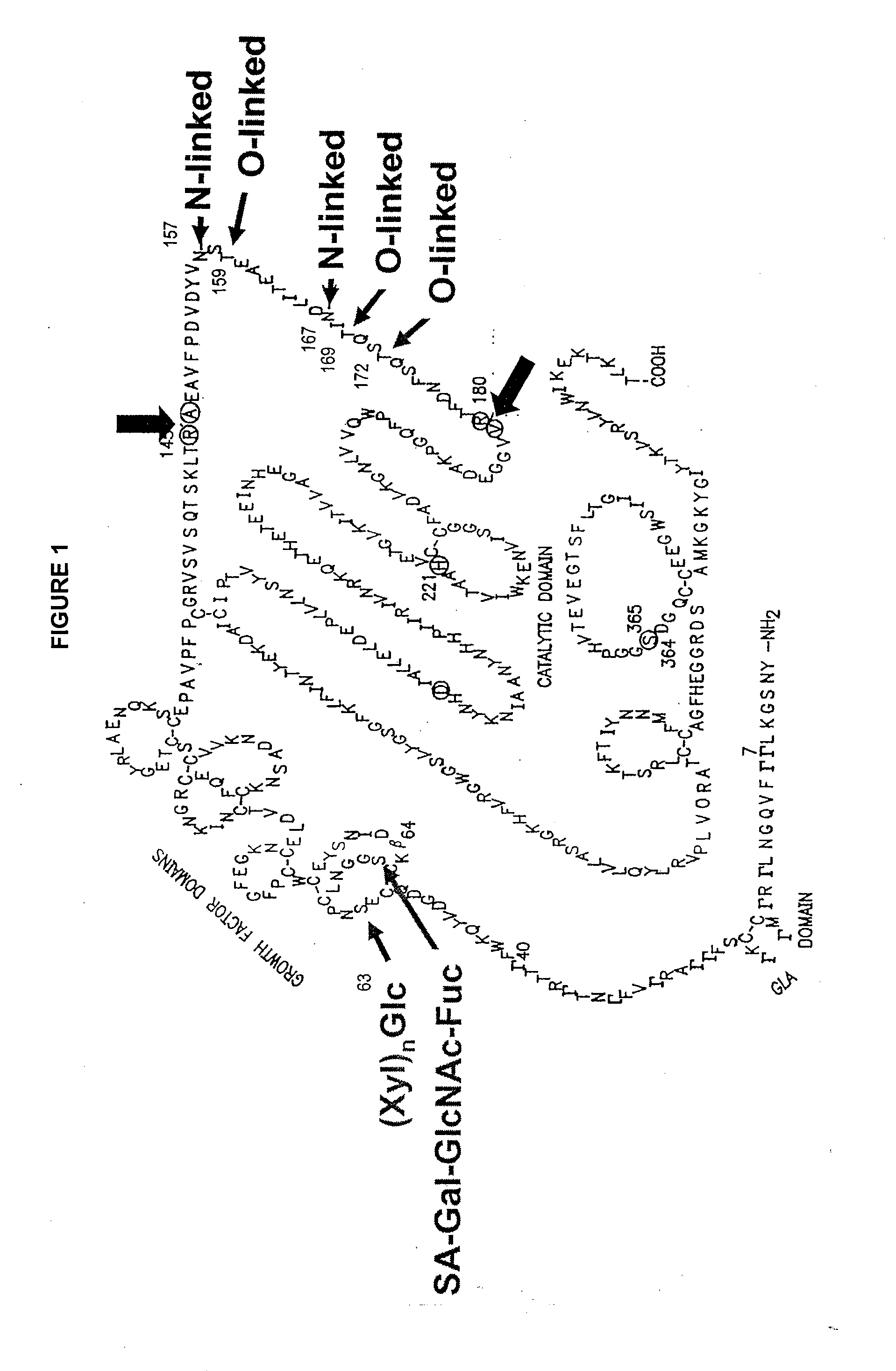
A purine nucleoside analog includes a pyrido[2,3-d]pyrimidine or a pyrimido[4,5-d]pyrimidine and further has a sugar moiety that is optionally modified at the C2′, C3′, C4′ and / or C5′ position. Particularly contemplated compounds also include prodrug forms of the purine nucleoside analogs, and both purine nucleoside analogs and the corresponding prodrugs are employed in the reduction of growth of neoplastic cells.
Owner:VALEANT RES & DEV +1
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090214505A1Extended half-lifeOptimize allocationAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
Provided are soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. Sialated and pegylated forms of the sHASEGPs also are provided. Methods of treatment by administering sHASEGPs and modified forms thereof also are provided.
Owner:HALOZYME
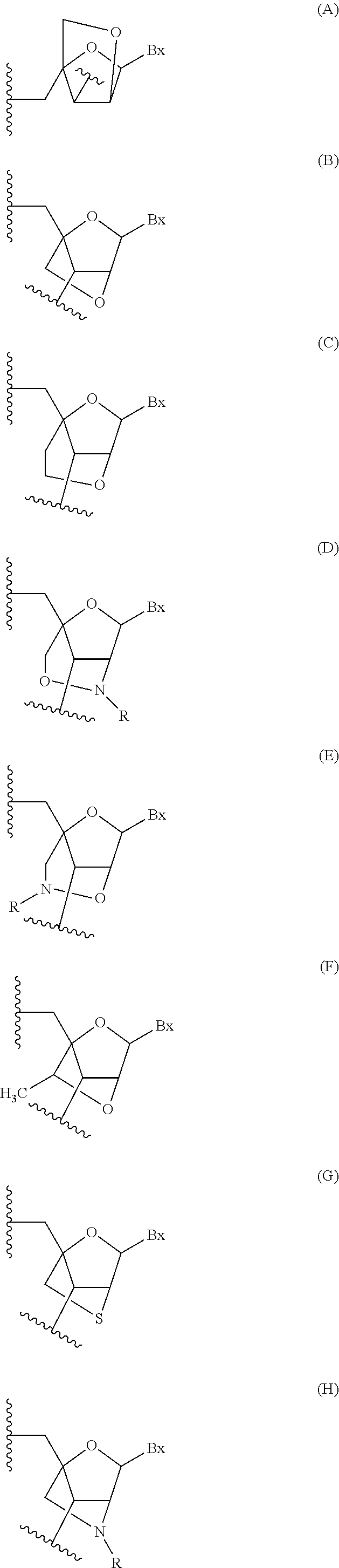
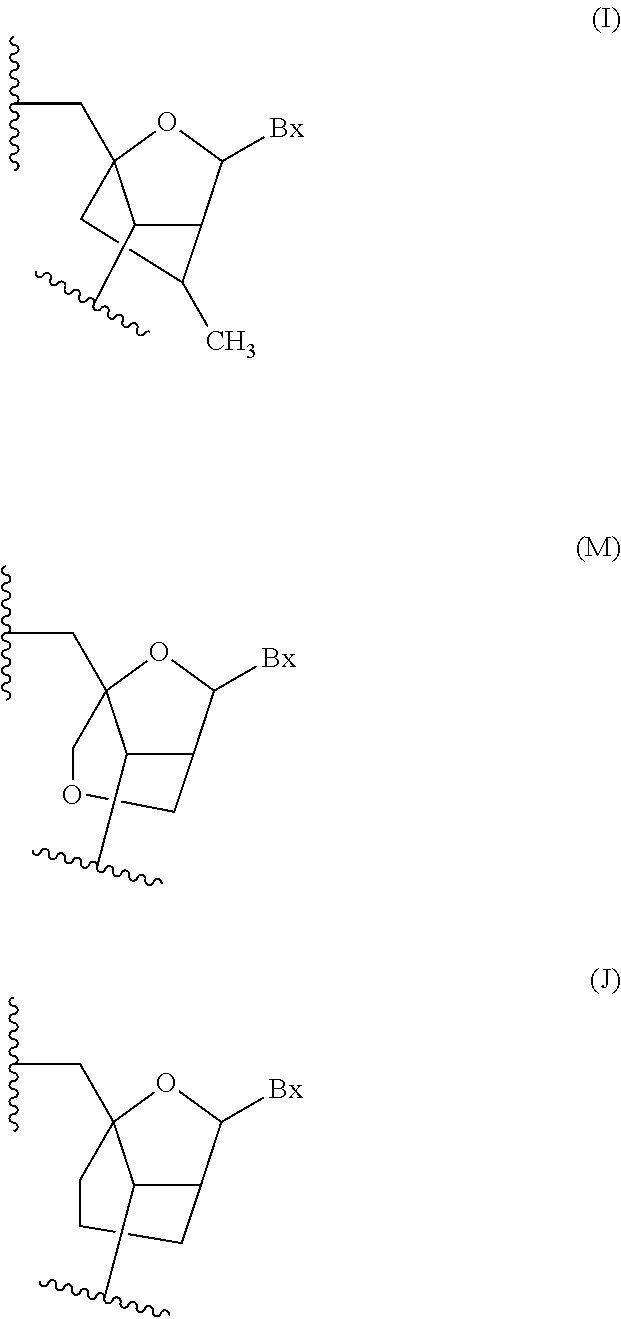
Oligomeric compounds comprising bicyclic nucleosides and uses thereof
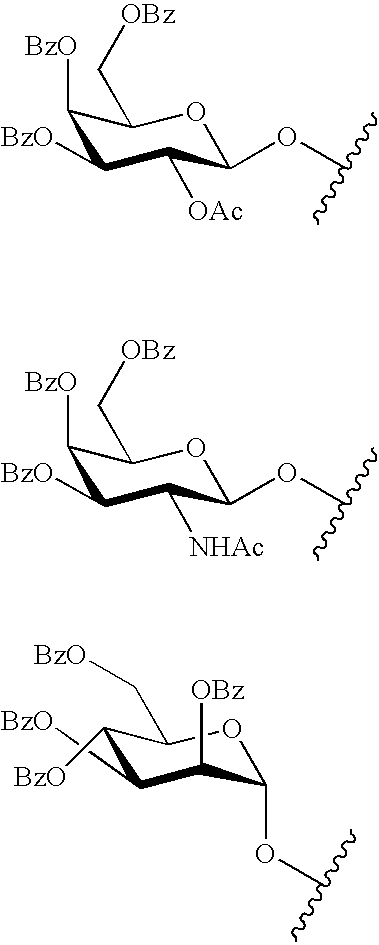
The present invention provides oligomeric compounds. Certain such oligomeric compounds are useful for hybridizing to a complementary nucleic acid, including but not limited, to nucleic acids in a cell. In certain embodiments, hybridization results in modulation of the amount activity or expression of the target nucleic acid in a cell. In certain embodiments, the present invention provides compounds comprising oligonucleotides. In certain embodiments, such oligonucleotides comprise a region having a gapmer sugar motif. In certain embodiments, oligonucleotides comprise one or more type of modified sugar moieties and / or naturally occurring sugar moieties arranged along an oligonucleotide or region thereof in a defined pattern or sugar modification motif.
Owner:IONIS PHARMA INC
Glycopegylated factor ix
ActiveUS20100081791A1Promote recoveryPeptide/protein ingredientsMammal material medical ingredientsSugar moietyPharmaceutical formulation
The present invention provides conjugates between Factor IX and PEG moieties. The conjugates are linked via a glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. Conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
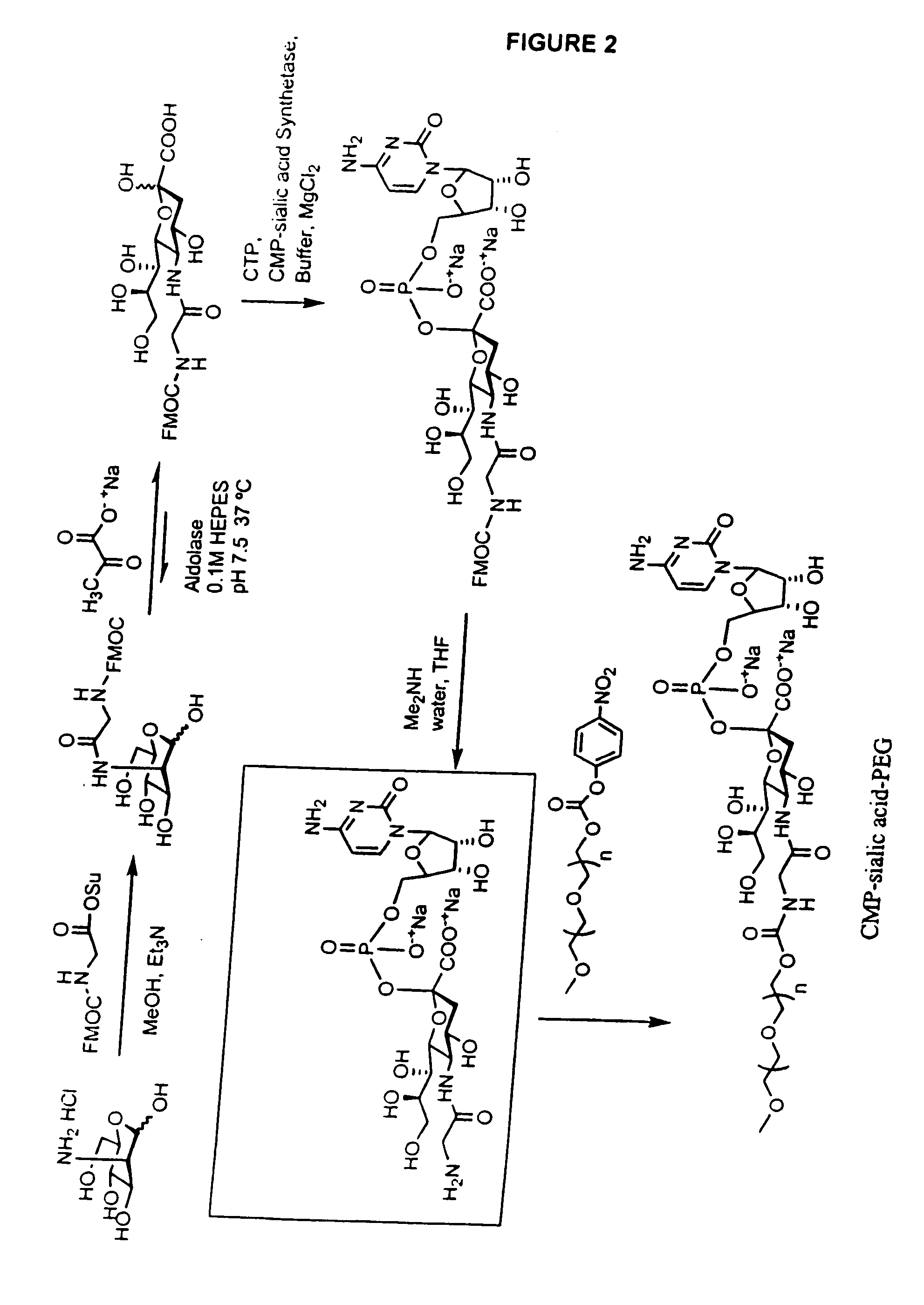
Production of Oligosaccharides By Microorganisms
InactiveUS20080145899A1Highly efficient and rapid and relatively low cost synthesisFermentationEnzymatic synthesisOligosaccharide
The present invention relates to the enzymatic synthesis of oligosaccharides, including sialylated product saccharides. In particular, it relates to the use of recombinant cells to take up low cost precursors such as glucose, pyruvate and N-actyl-glucosamine, and to synthesize activated sugar moieties that are used in oligosaccharide synthesis. The methods make possible the synthesis of many oligosaccharides using microorganisms and readily available, relatively inexpensive starting materials.
Owner:SENEB BIOSCI
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181032A1Extended half-lifeImprove distributionAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Glycopegylated erythropoietin formulations
InactiveUS20060287224A1Improved pharmacokinetic propertiesCost effectiveOrganic active ingredientsBiocideDiseasePharmaceutical formulation
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
Glycopegylated Erythropoietin Formulations
InactiveUS20110003744A1Improved pharmacokinetic propertiesCost effectivePeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseSugar moiety
The present invention provides conjugates between erythropoietin and PEG moieties. The conjugates are linked via an intact glycosyl linking group interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from glycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto a glycosyl residue on the peptide. Also provided are methods for preparing the conjugates, methods for treating various disease conditions with the conjugates, and pharmaceutical formulations including the conjugates.
Owner:NOVO NORDISK AS
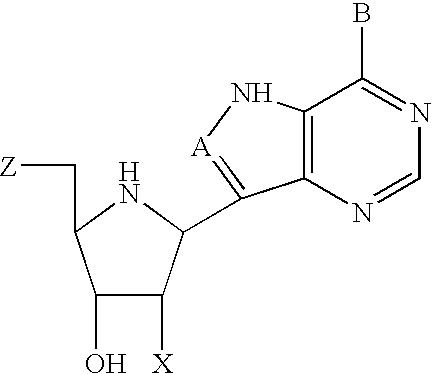
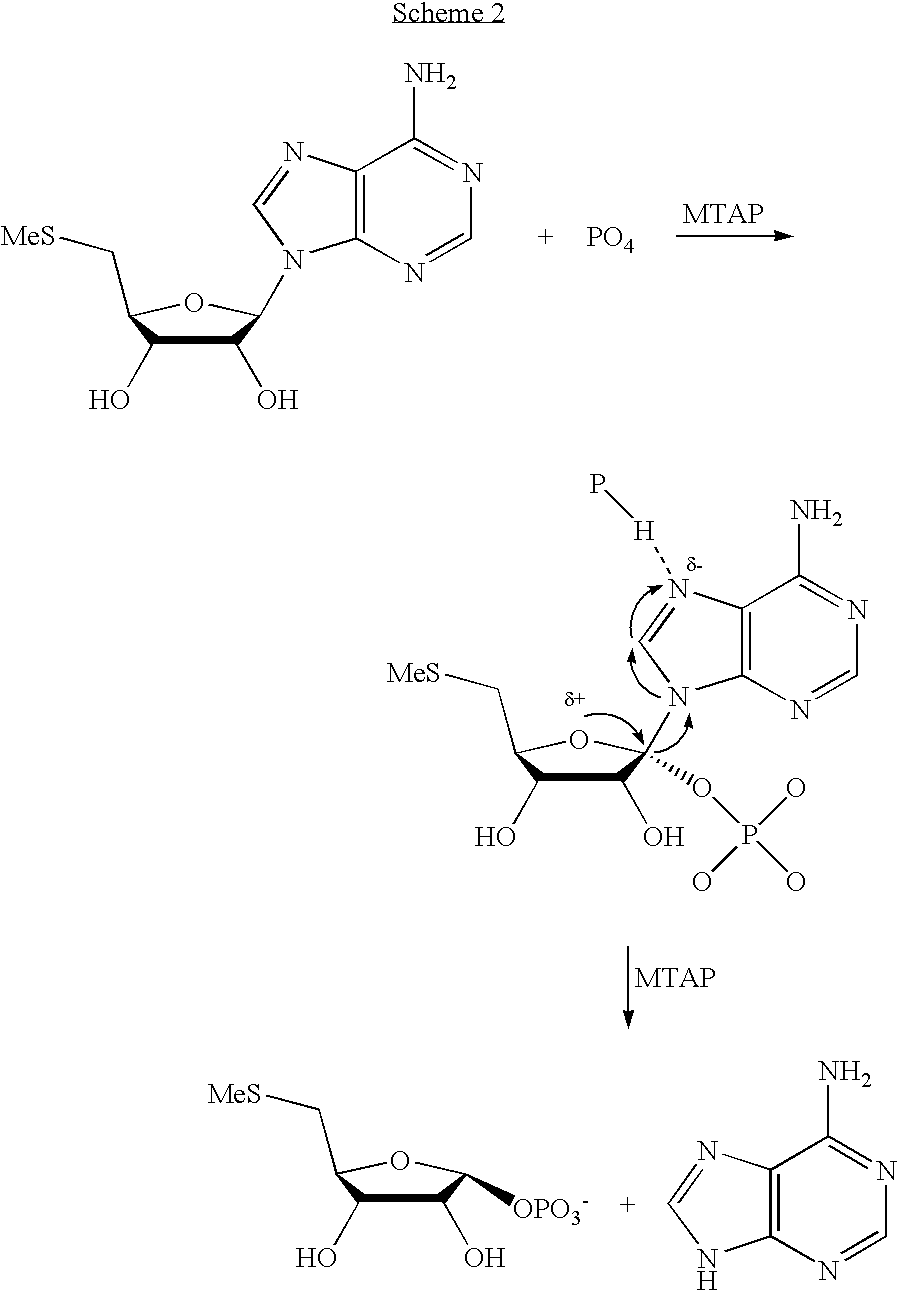
Inhibitors of nucleoside phosphorylases and nucleosidases
The invention provides a compound of the formula: wherein A is selected from N, CH and CR; R is selected from halogen, optionally substituted alkyl, aralkyl and aryl, OH, NH2, NHR<1>, NR<1>R<2 >and SR<3>; R<1>, R<2 >and R<3 >are each optionally substituted alkyl, aralkyl or aryl groups; B is selected from NH2 and NHR<4>; R<4 >is an optionally substituted alkyl, aralkyl or aryl group; X is selected from H, OH and halogen; Z is selected from H, Q, SQ and OQ; Q is an optionally substituted alkyl, aralkyl or aryl group; or a tautomer, a pharmaceutically acceptable salt, an ester, or a prodrug thereof; with the proviso that the stereochemistry of the aza-sugar moiety is D-ribo or 2'-deoxy-D-erythro-; pharmaceutical compositions comprising said compound; and methods of treatment using said compound.
Owner:IND RES LTD +1
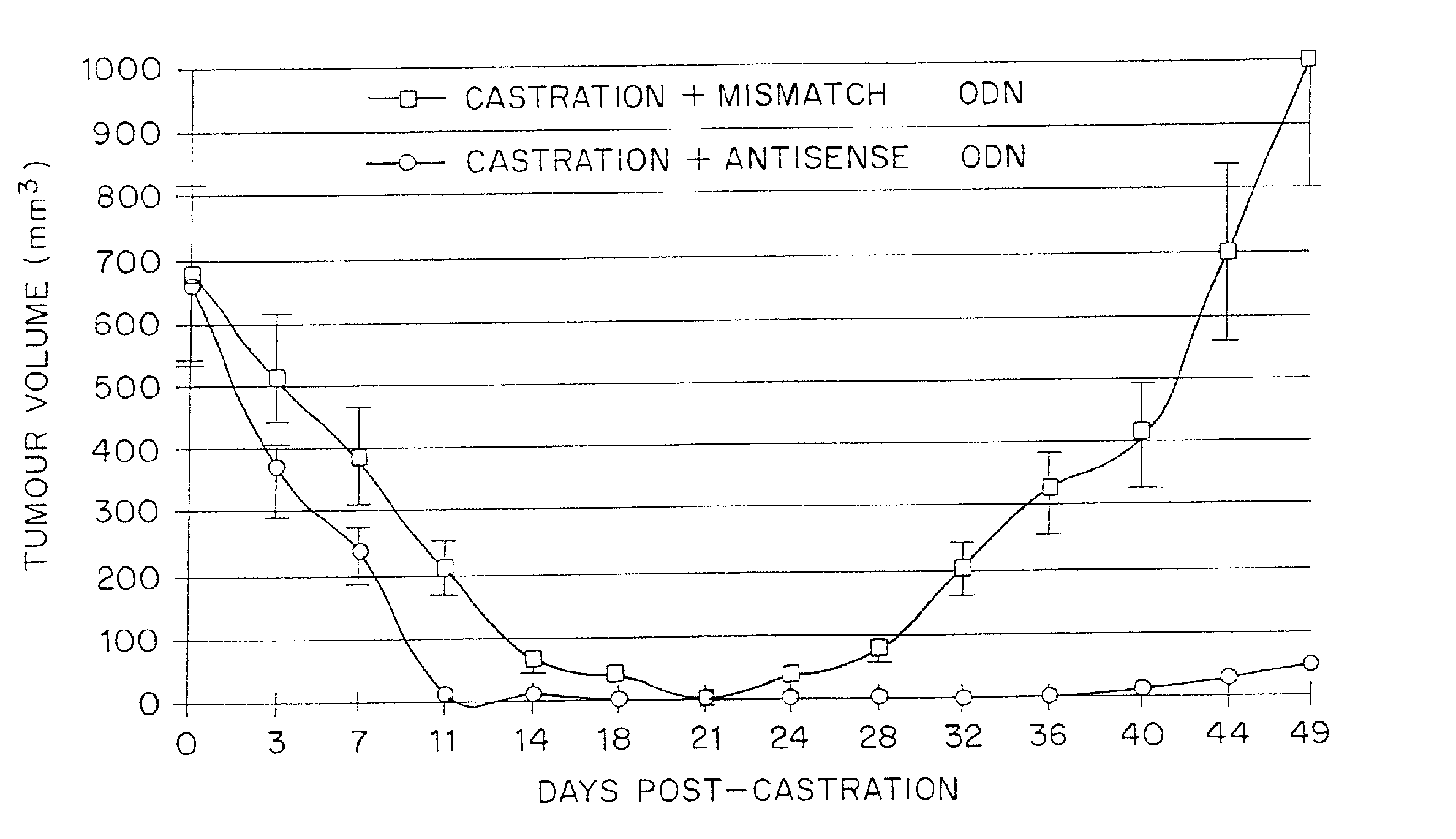
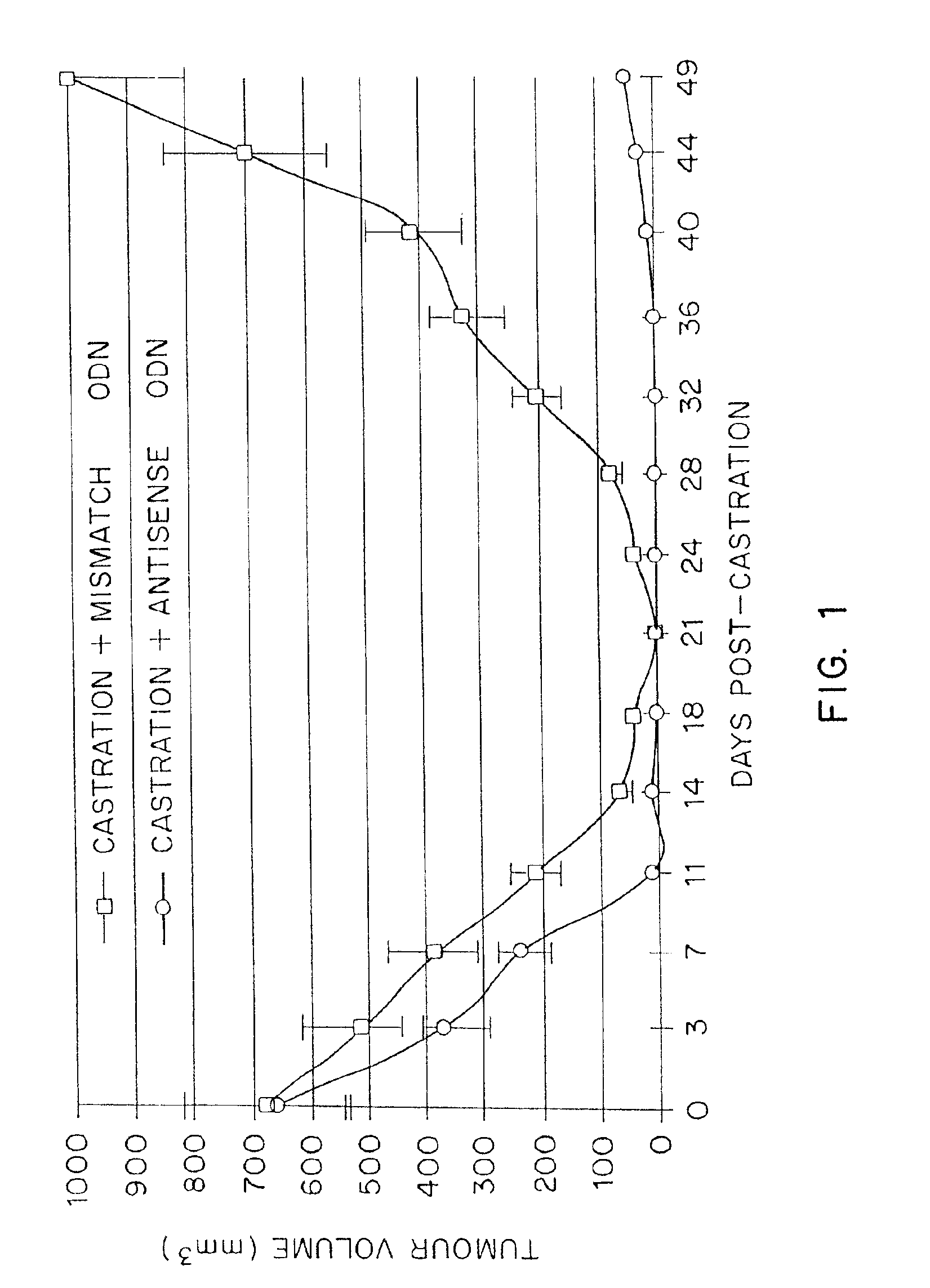
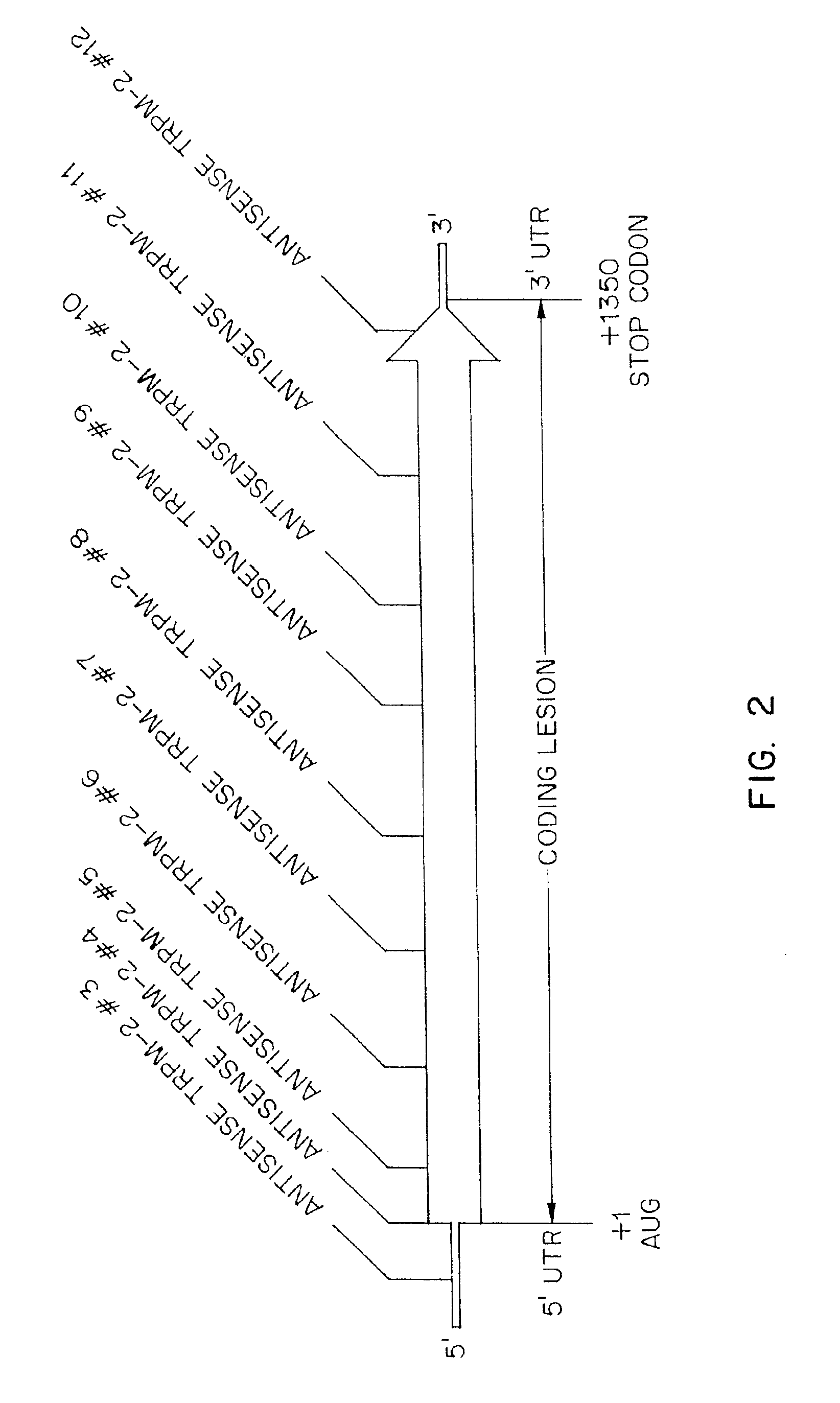
TRPM-2 antisense therapy using an oligonucleotide having 2′-O-(2-methoxy)ethyl modifications
InactiveUS6900187B2Improve in vivo stabilityImproved in vitroPeptide/protein ingredientsGenetic material ingredientsIn vivoOligonucleotide
A compound consisting of an oligonucleotide of sequence CAGCAGCAGAGTCTTCATCAT, where the oligonucleotide has a phosphorothioate backbone throughout, the sugar moieties of nucleotides 1-4 and 18-21 bear 2′-O-methoxyethyl modifications, and the remaining nucleotides (nucleotides 5-17) are 2′-deoxynucleotides, and where the cytosines of nucleotides 1, 4 and 19 are 5-methylcytosines. The compound has increased stability in vivo and improved in vitro and in vivo antitumor activity.
Owner:THE UNIV OF BRITISH COLUMBIA
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181013A1Reduce deliveryImprove usabilityAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Process for the synthesis of pyrazolopyrimidines
The present invention provides a nucleoside comprising a pyrazolopyrimidine base and a process for producing the same. In particular, the processes of the present invention comprises using a halogenated pyrazolopyrimidine base and removing the halogen after the base is coupled to a sugar moiety. The presence of the halogen on the nucleoside base allows facile and economical production of a large quantity of nucleosides.
Owner:EPOCH BIOSCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
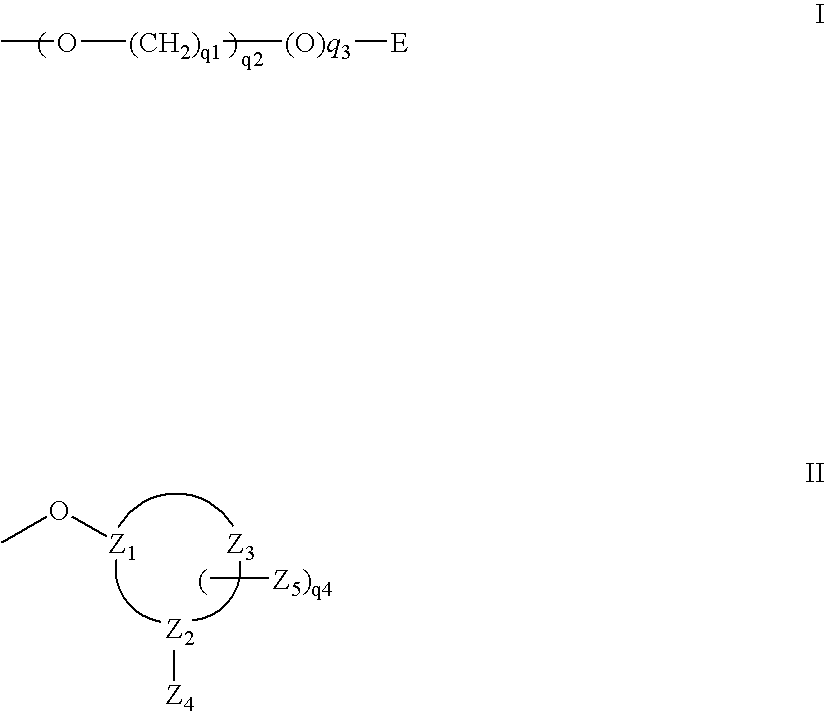
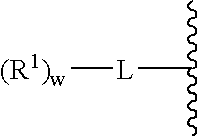

![Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8c82ec8-4d70-46a2-b118-a3a1eb303404/US07081449-20060725-D00001.png)
![Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8c82ec8-4d70-46a2-b118-a3a1eb303404/US07081449-20060725-D00002.png)
![Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides Pyrido[2,3-d]pyrimidine and pyrimido[4,5-d]pyrimidine nucleosides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b8c82ec8-4d70-46a2-b118-a3a1eb303404/US07081449-20060725-D00003.png)