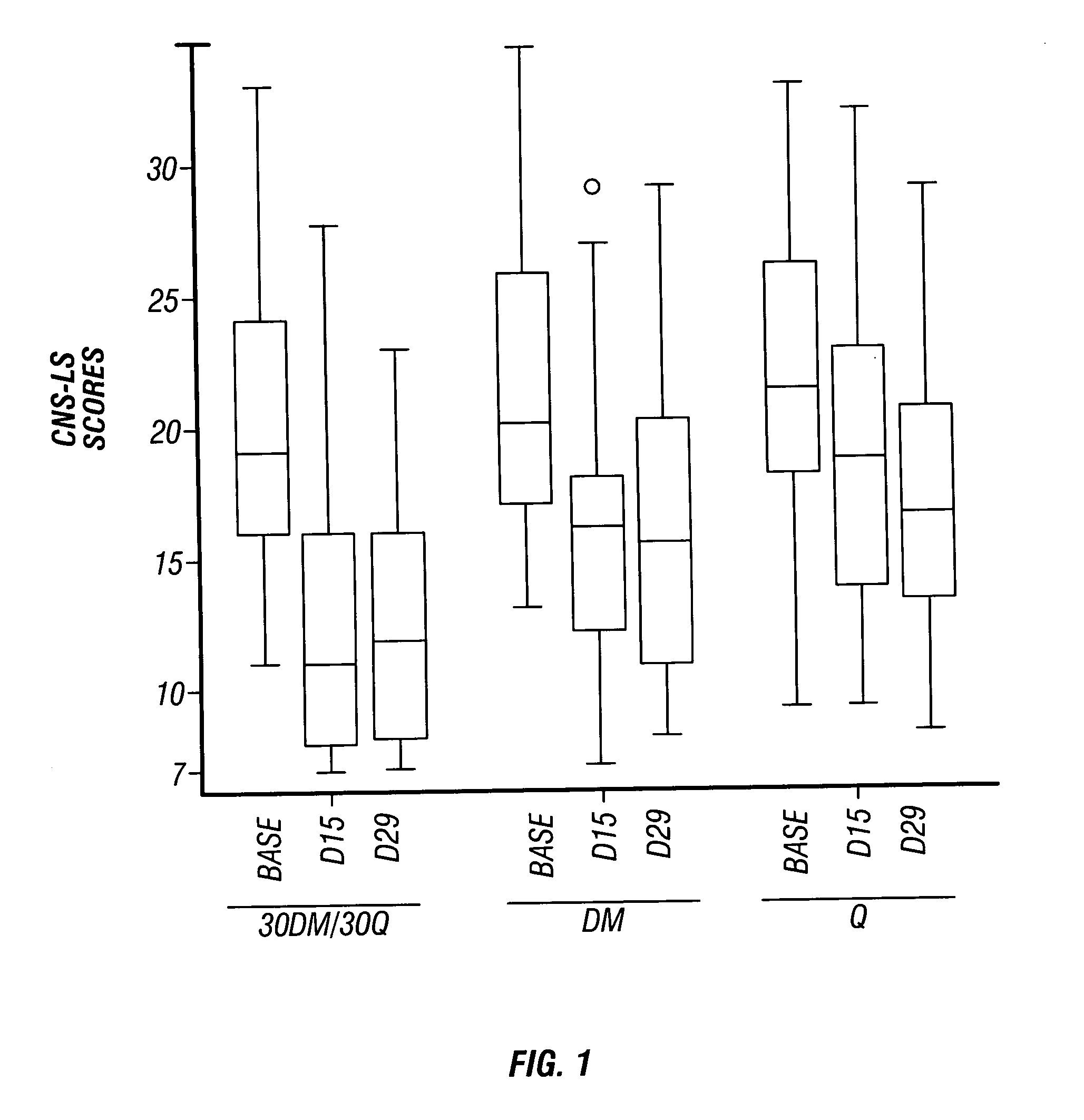
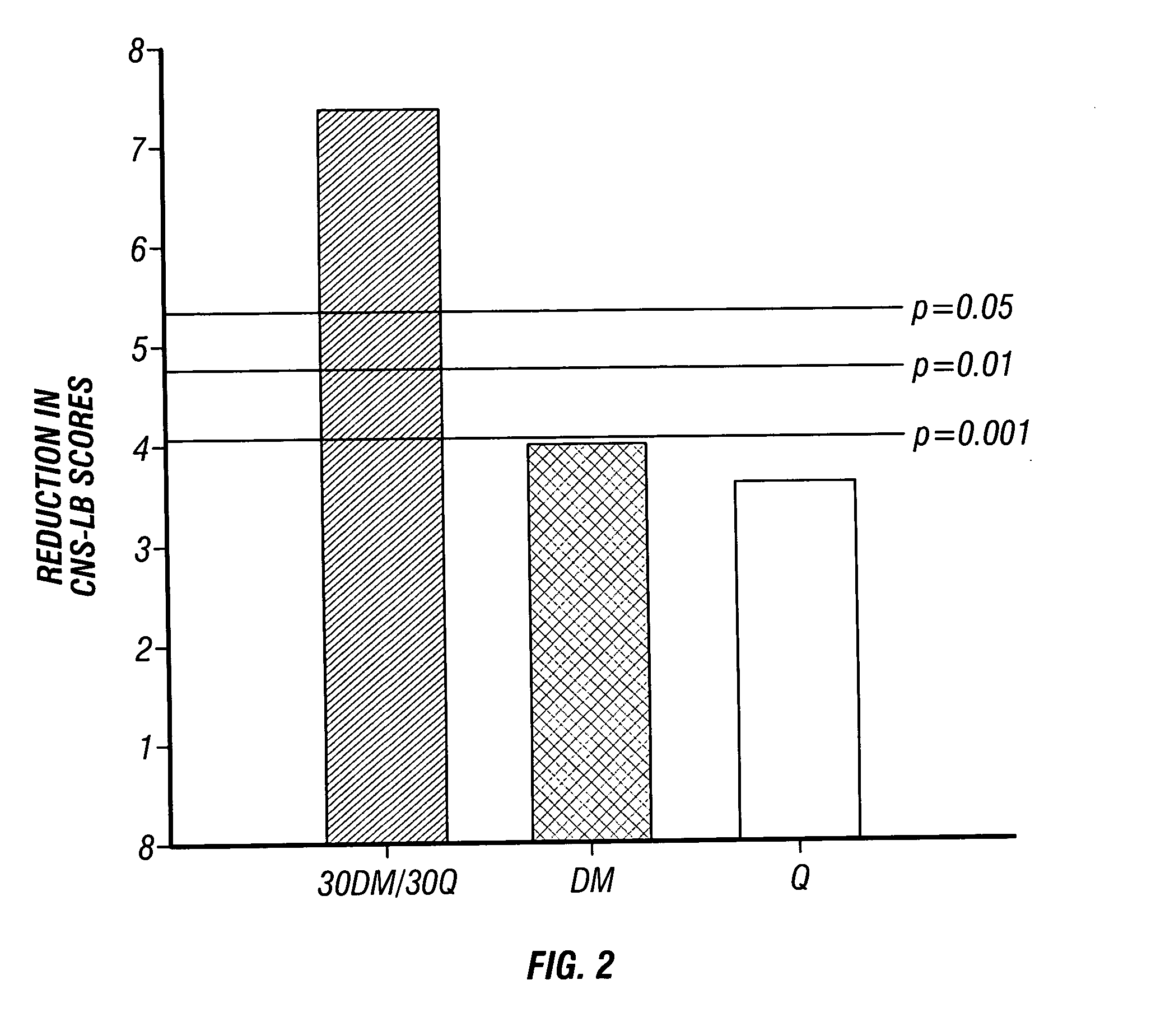
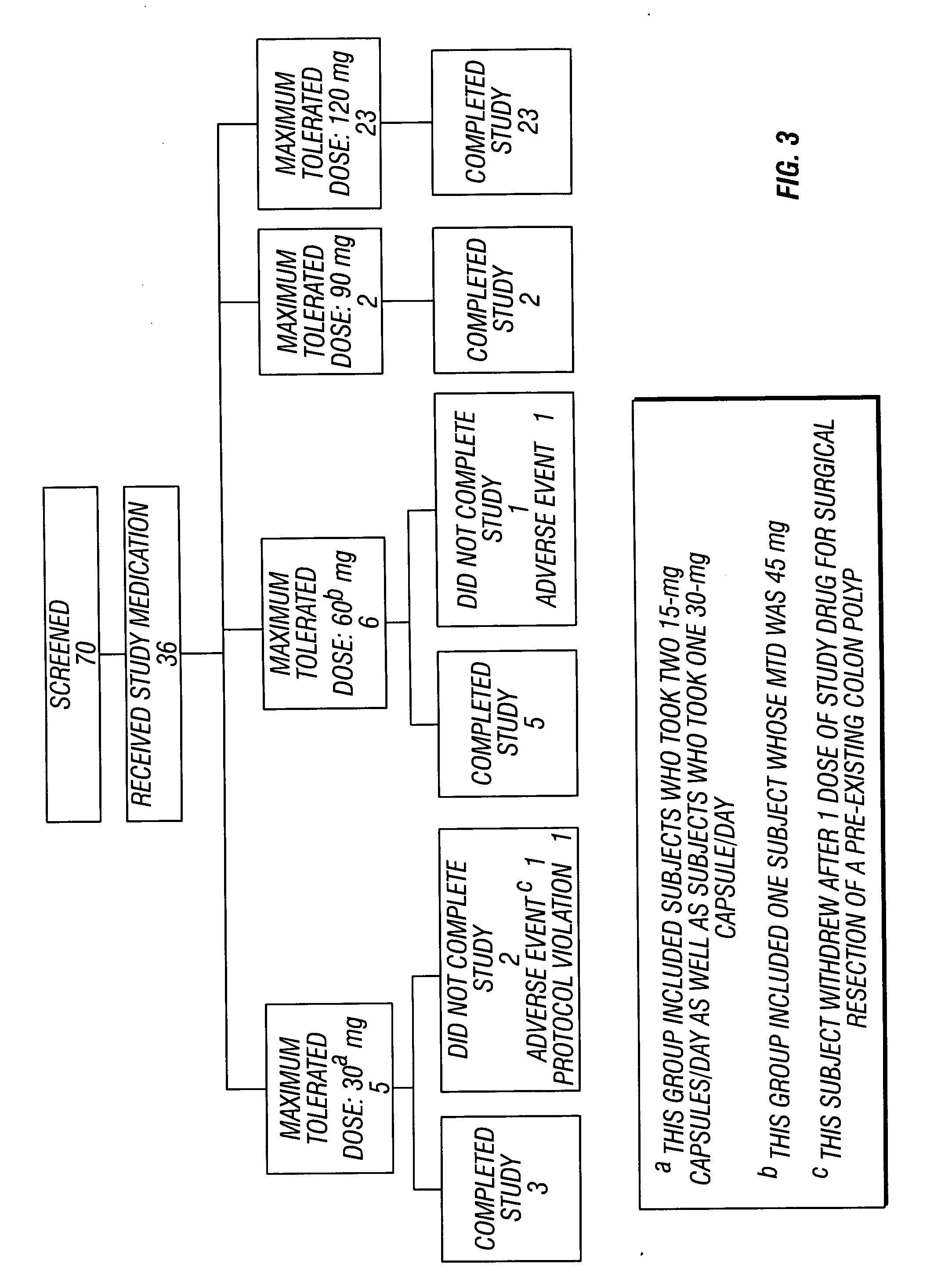
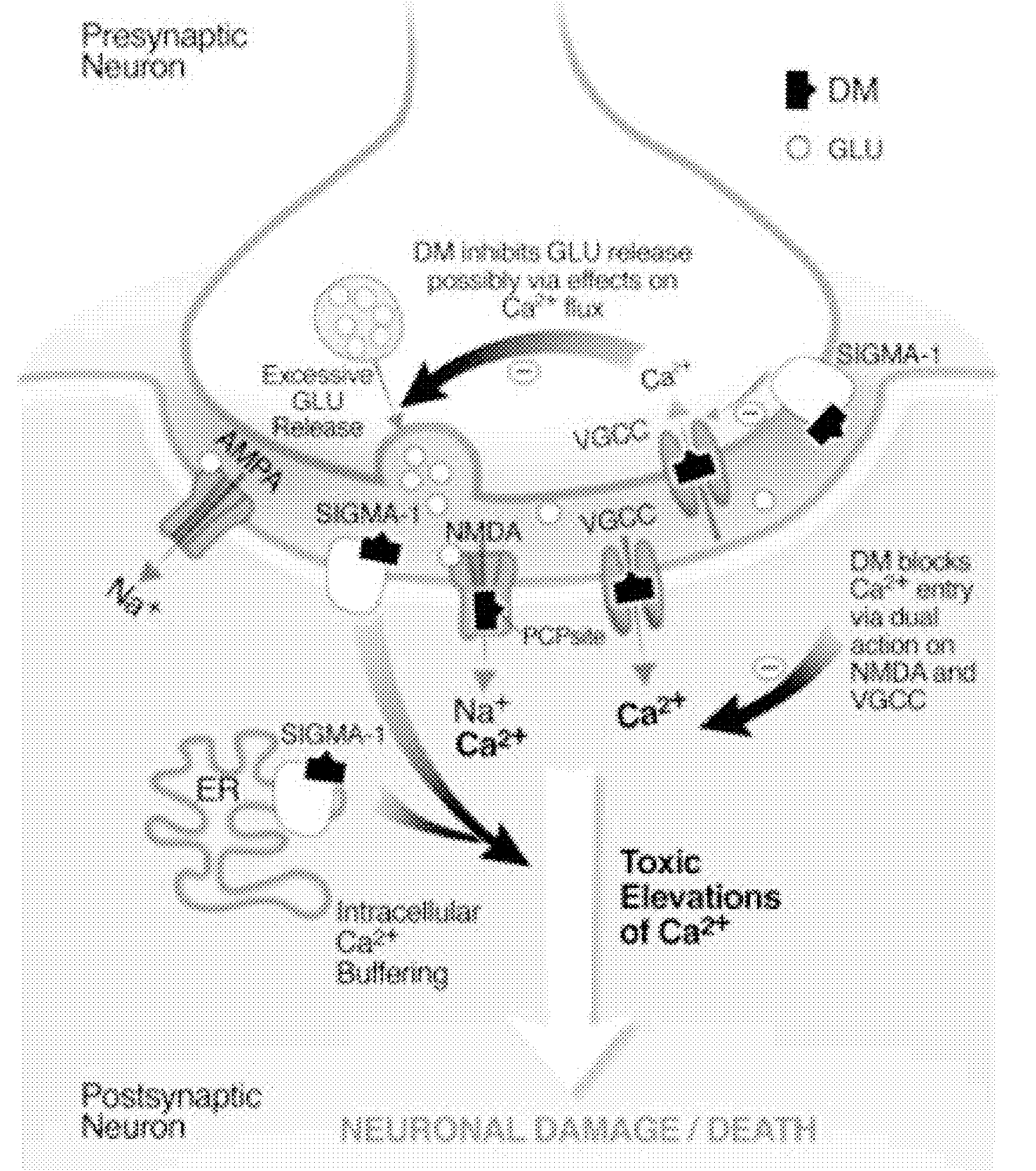
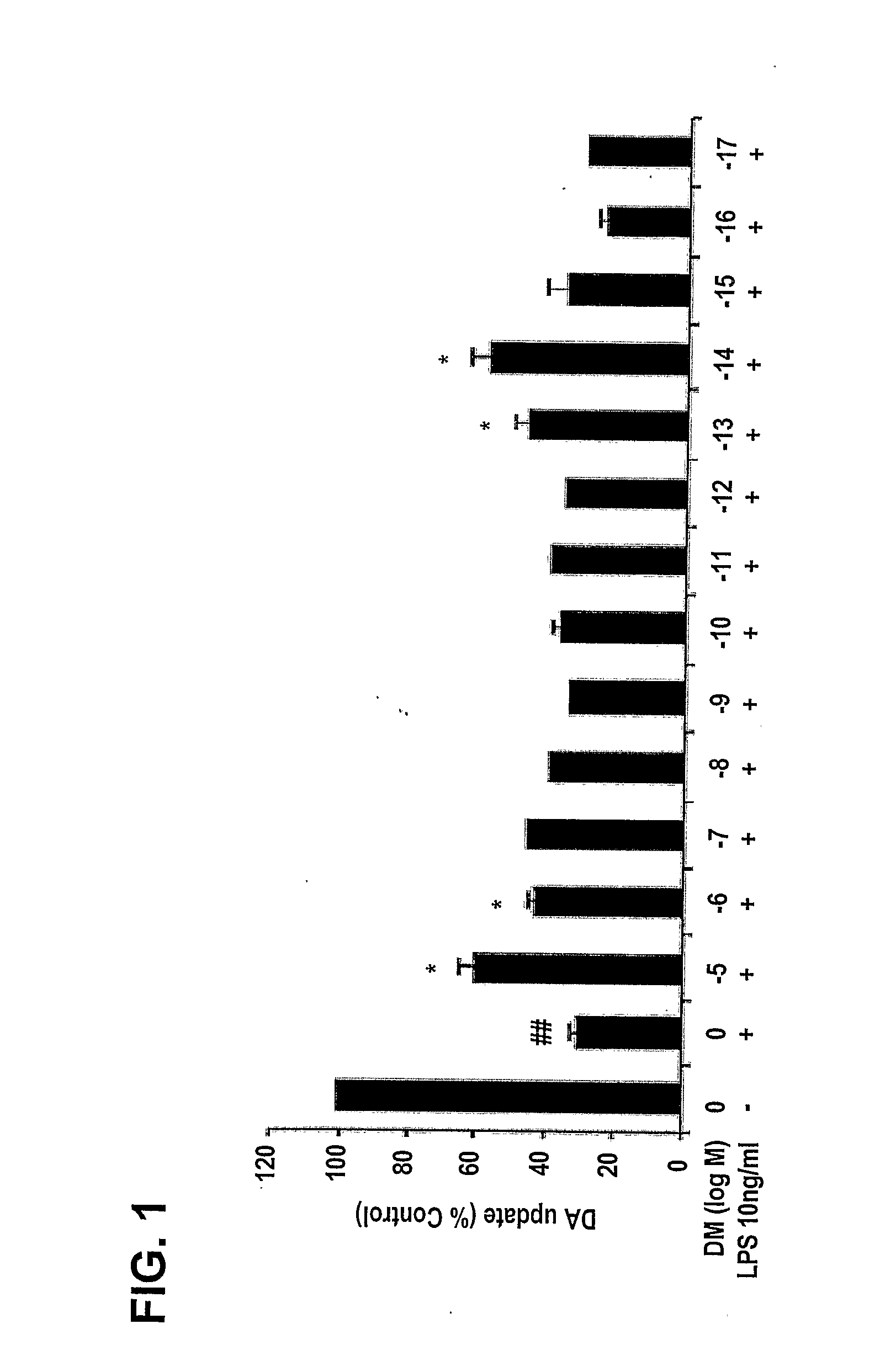
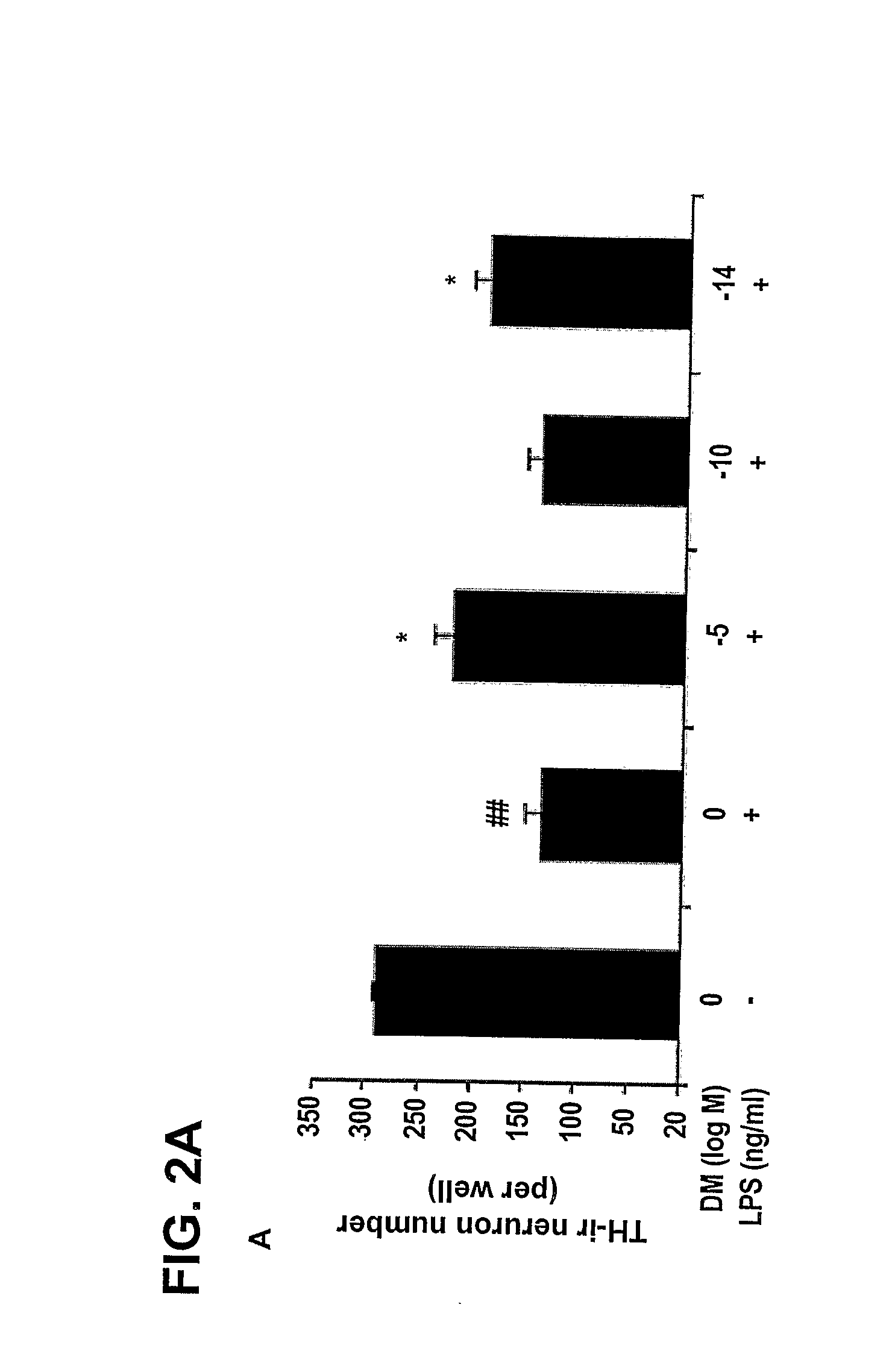
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
124 results about "Dextromethorphan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
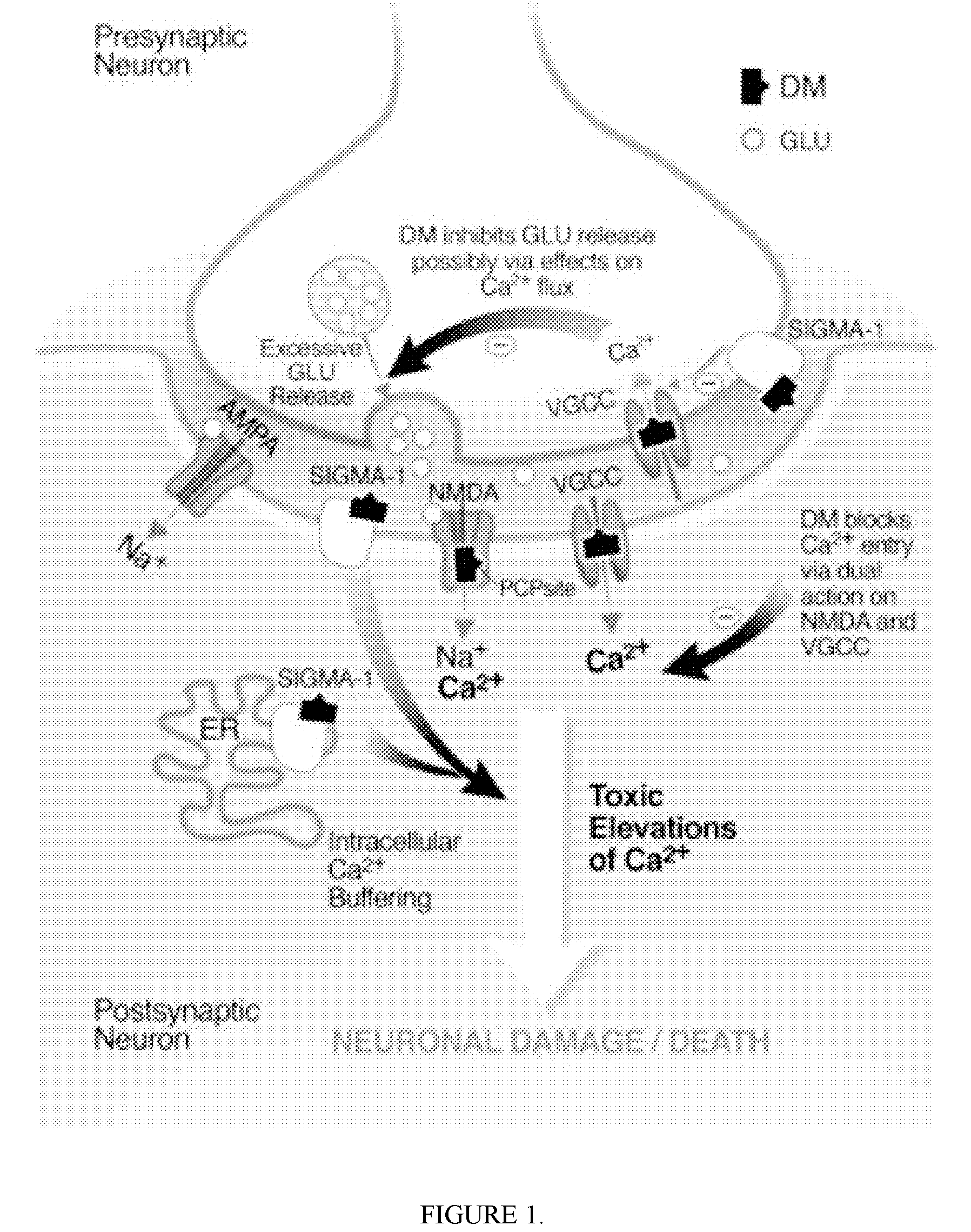
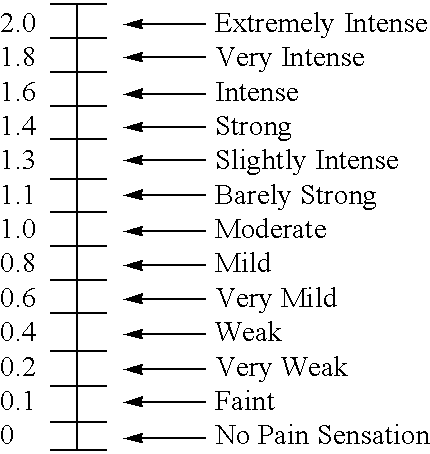
This medication is used for temporary relief of coughs without phlegm that are caused by certain infections of the air passages (e.g., sinusitis, common cold). This product should not usually be used for an ongoing cough from smoking or long-term breathing problems (e.g., chronic bronchitis, emphysema) unless directed by your doctor.
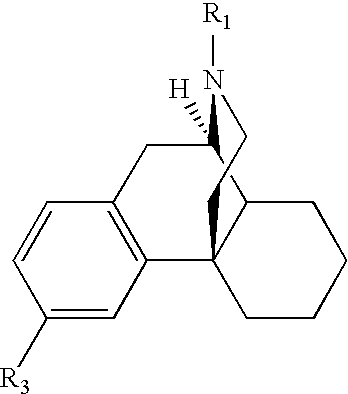
Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of neurological disorders
Pharmaceutical compositions and methods for treating neurological disorders by administering same are provided. The compositions comprise dextromethorphan in combination with quinidine.
Owner:AVANIR PHARMA
Pharmaceutical compositions comprising dextromethorphan and quinidine for the treatment of depression, anxiety, and neurodegenerative disorders
Pharmaceutical compositions and methods for treating depression, anxiety, and neurodegenerative diseases and cognitive disorders, such as dementia and Alzheimer's disease, by administering same are provided. The compositions comprise dextromethorphan in combination with quinidine.
Owner:AVANIR PHARMA
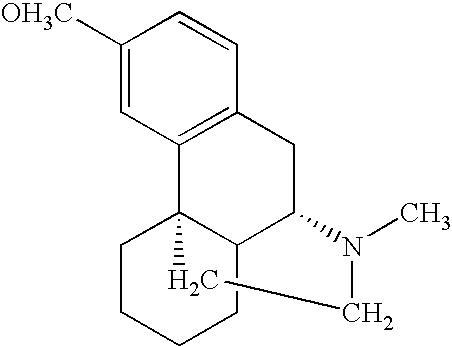
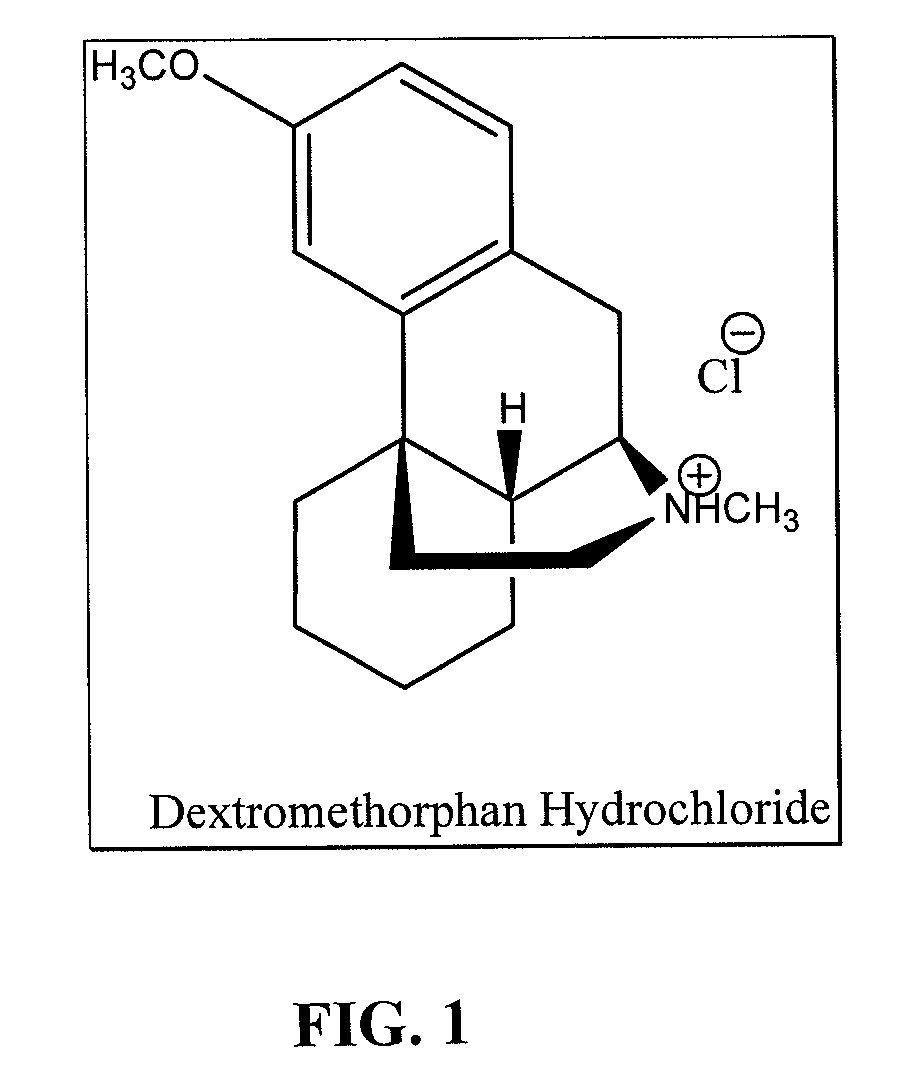
Dextromethorphan hydrochloride
ActiveUS8017623B2Eliminate riskBiocideNervous disorderDextromethorphanDextromethorphan Hydrochloride
The present invention provides pharmaceutical compositions comprising dextromethorphan hydrochloride.
Owner:LUMINUS BIOSCIENCES INC
Preparation of topical regional compositions for the relief of pain
Owner:FROME BRUCE M
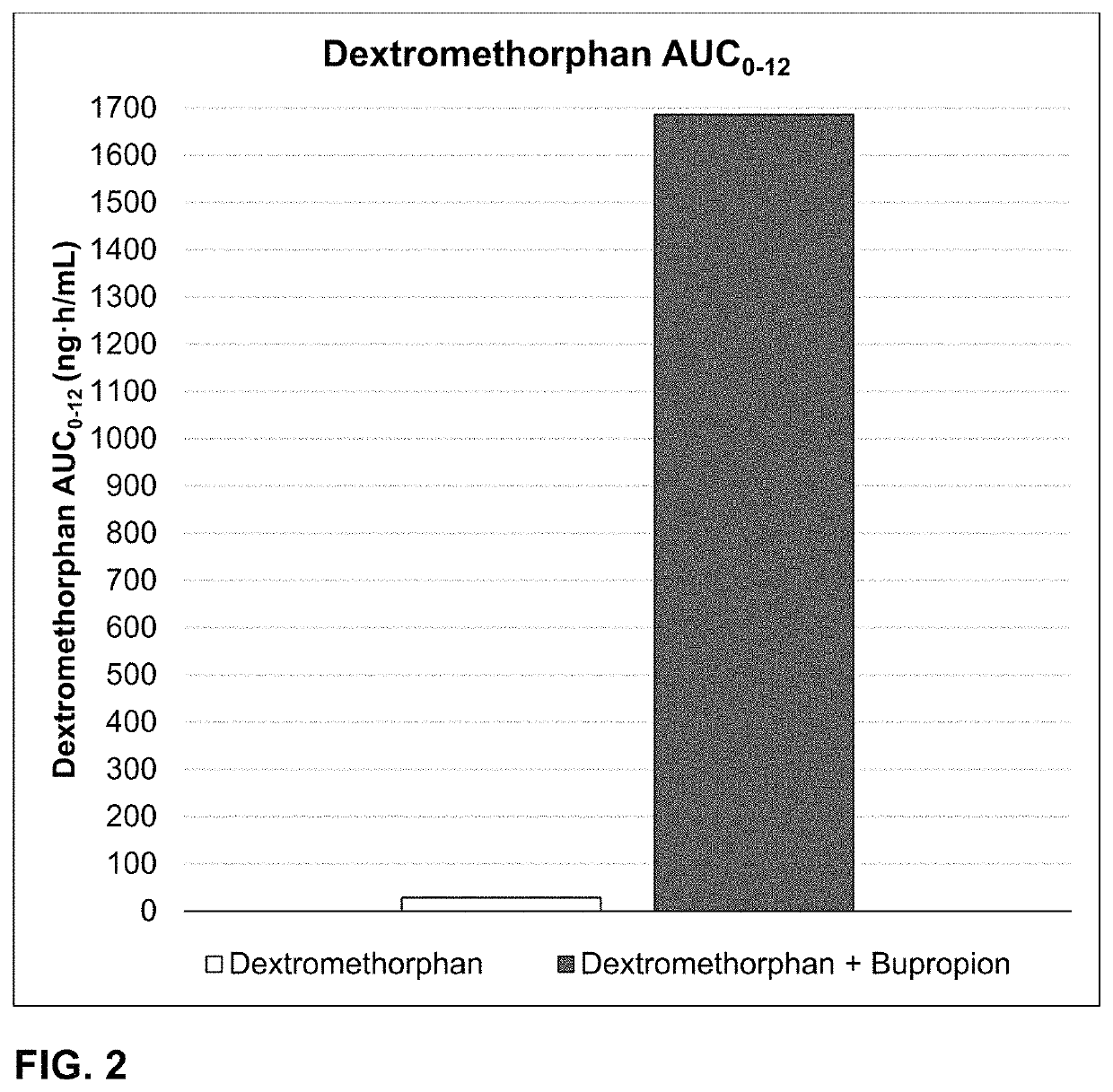
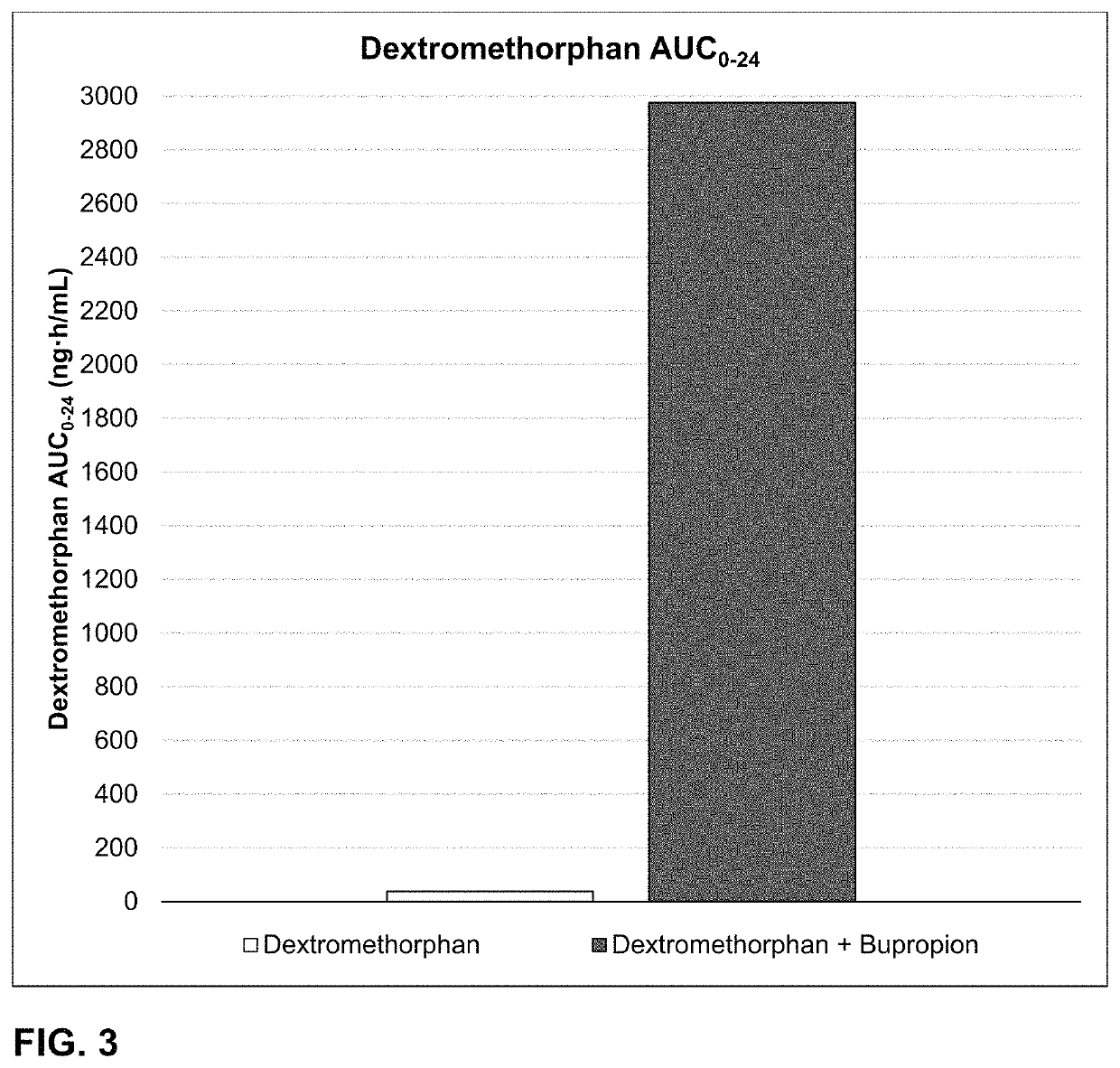
Bupropion and dextromethorphan for treating nicotine addiction
ActiveUS10688066B2Reduce in quantityOrganic active ingredientsNervous disorderPharmaceutical drugTherapeutic effect
Dosage forms, drug delivery systems, and methods related to sustained release of dextromethorphan or improved therapeutic effects are disclosed. Typically, bupropion or a related compound is orally administered to a human being to be treated with, or being treated with, dextromethorphan.
Owner:ANTECIP BIOVENTURES II
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Dextromethorphan and an oxidase inhibitor for treating intractable conditions
Methods are disclosed for increasing the effectiveness of dextromethorphan in treating chronic or intractable pain, for treating tinnitus and for treating sexual dysfunction comprising administering dextromethorphan in combination with a therapeutically effective dosage of a debrisoquin hydroxylase inhibitor. A preferred combination is dextromethorphan and the oxidative inhibitor quinidine.
Owner:AVANIR PHARMA
Enhancement of impaired motor and mental functions, using dextromethorphan and oxidase enzyme inhibitor
InactiveUS20070191411A1Improve abilitiesImprove motor controlBiocideNervous disorderDiseaseClinical trial
During clinical trials on patients suffering from neurological disorders, it has been observed that some patients obtain dramatic improvements in motor control and / or higher mental functioning, when they receive a combination of dextromethorphan and quinidine, at suitable dosages. Improved motor control has been exemplified to date by improved ability to swallow and / or speak, among victims of stroke, head injury, or ALS. Improved higher mental functioning has been exemplified better job performance, increased ability to analyze and solve problems, and increased ability to have successful and satisfying interactions with other people. These types of effects can be seen in a relatively brief time period, such as within several days to a week.
Owner:CENT FOR NEUROLOGIC STUDY
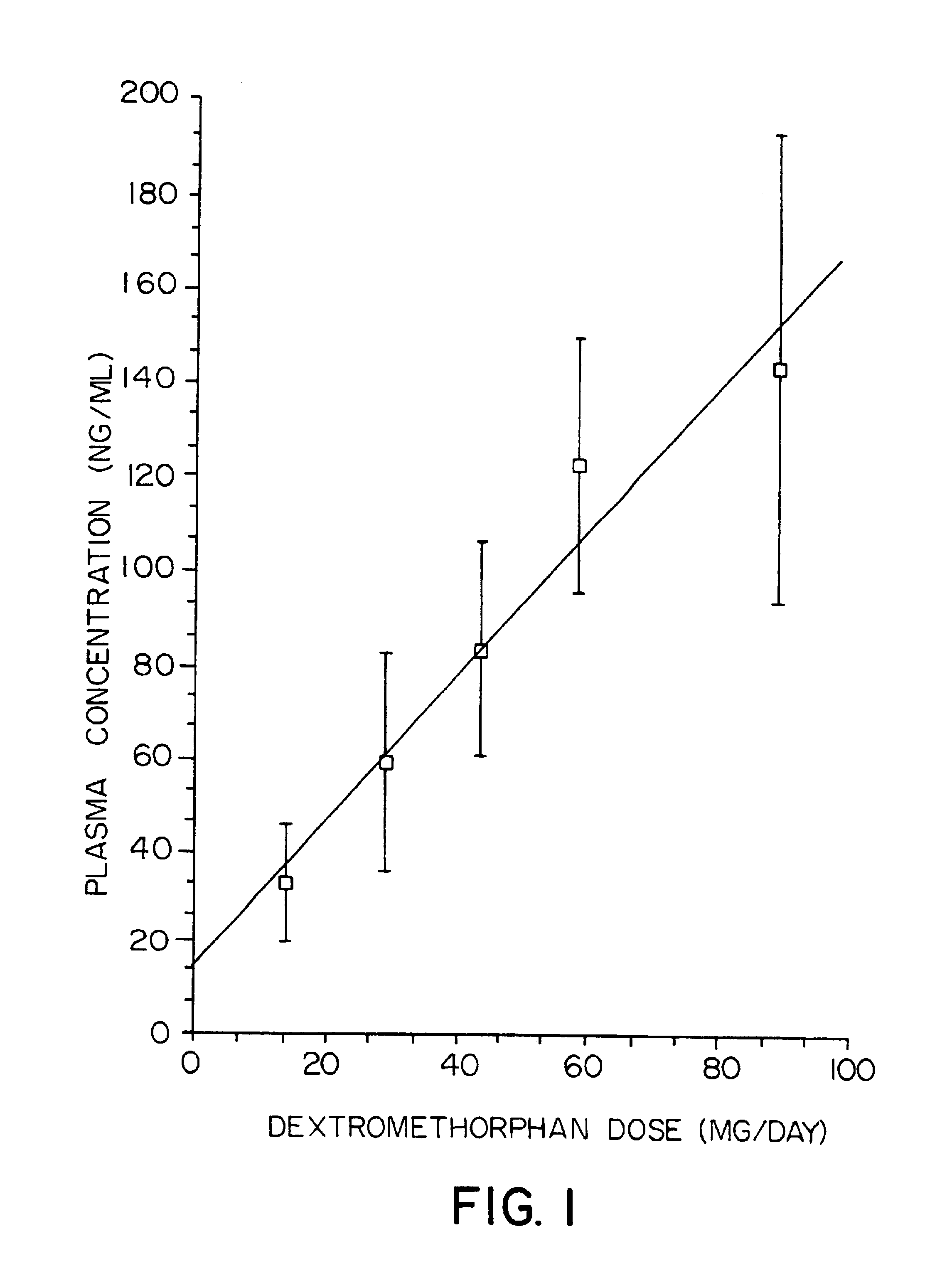
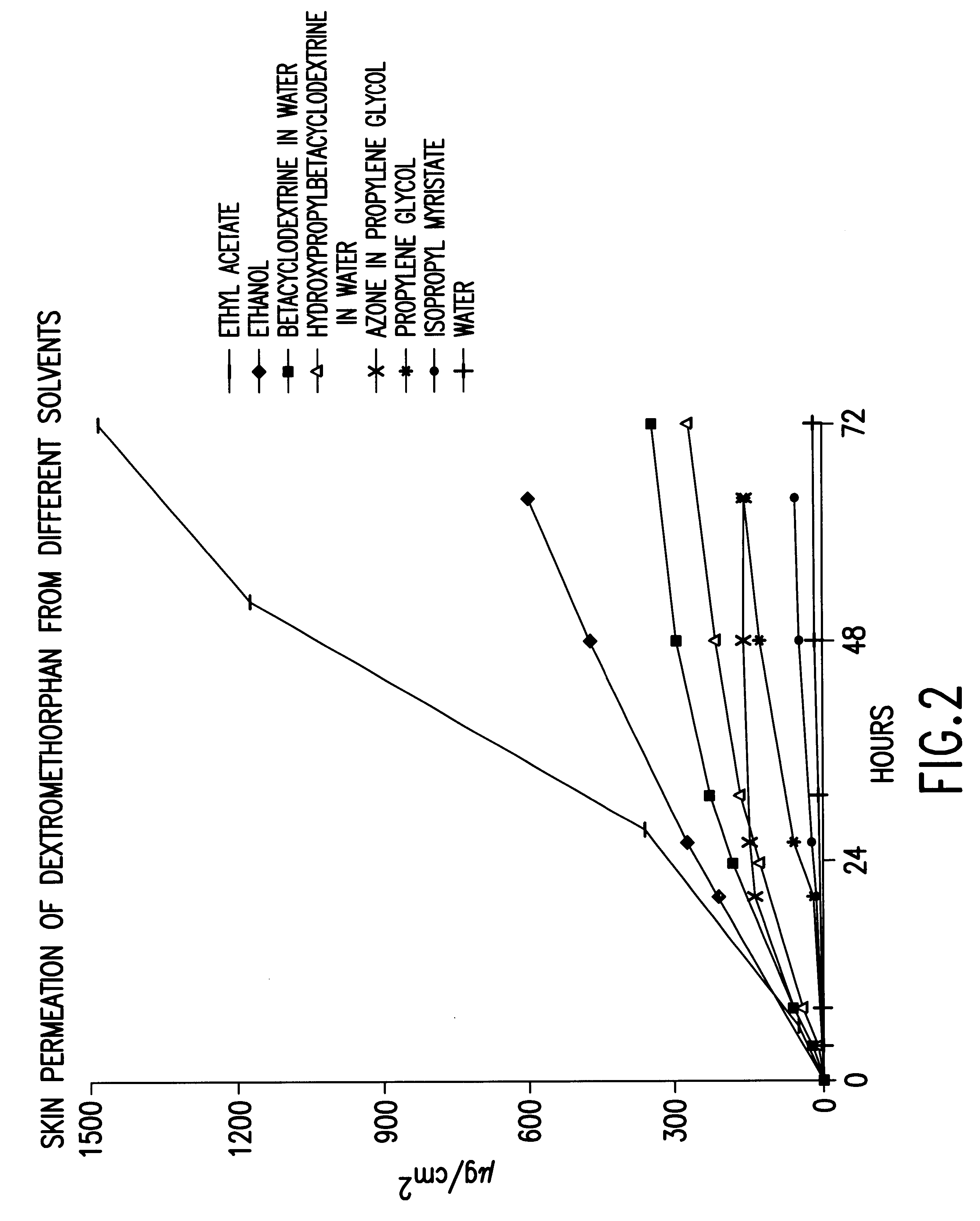
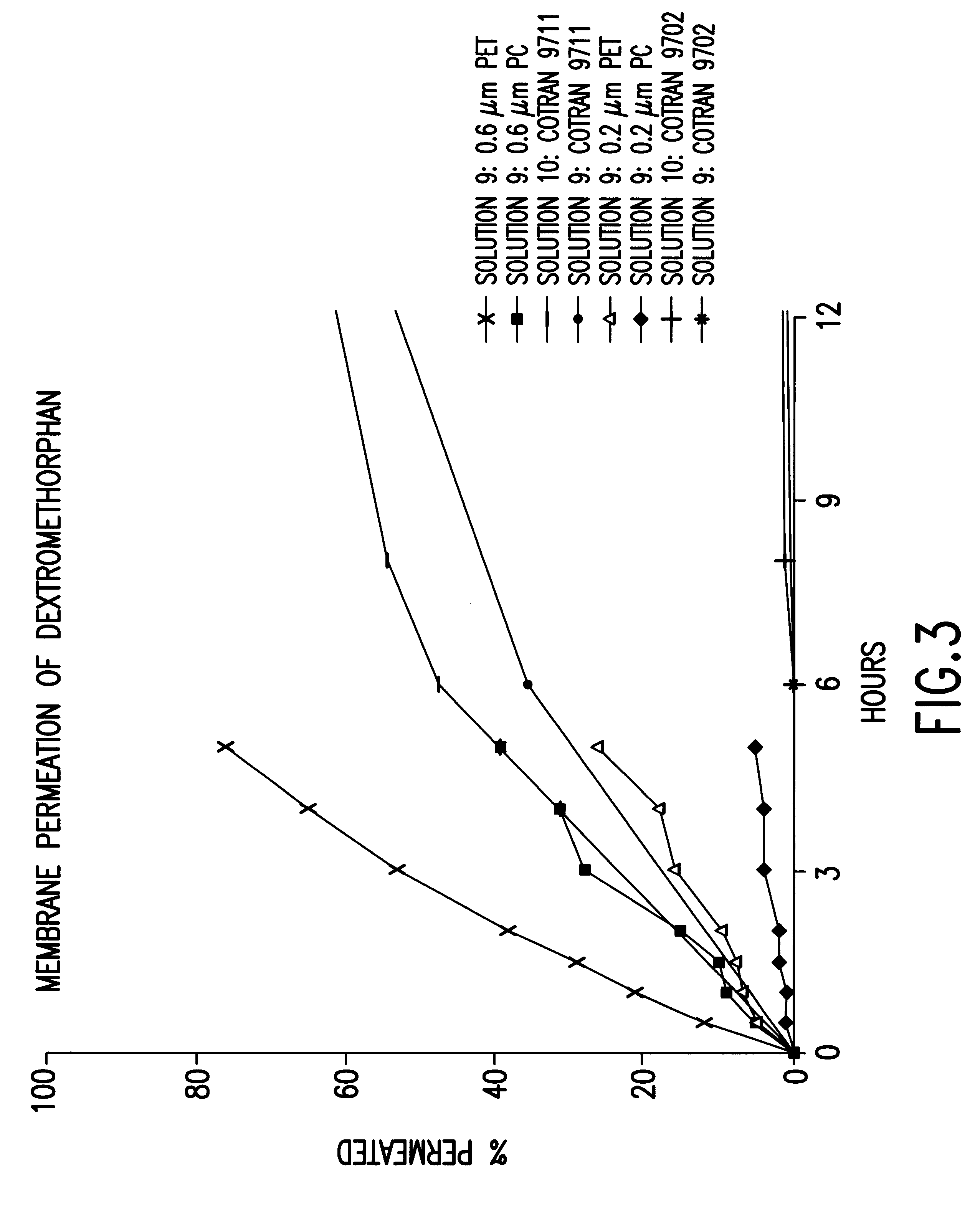
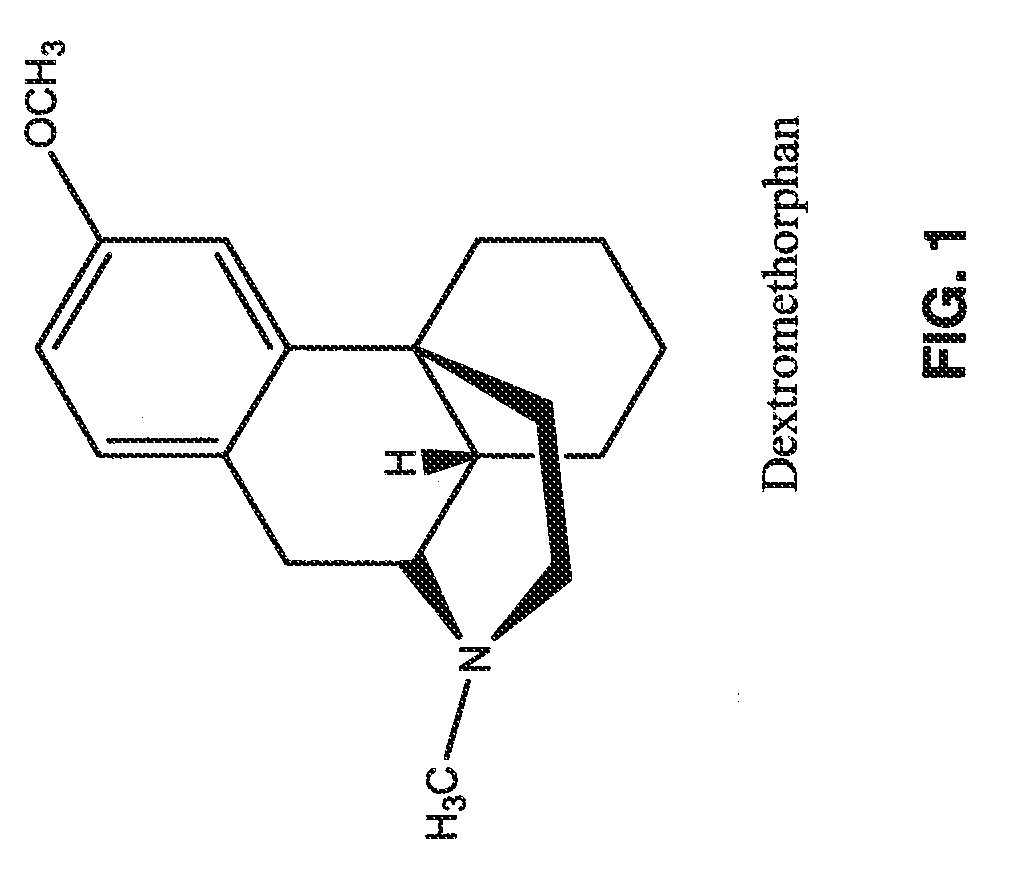
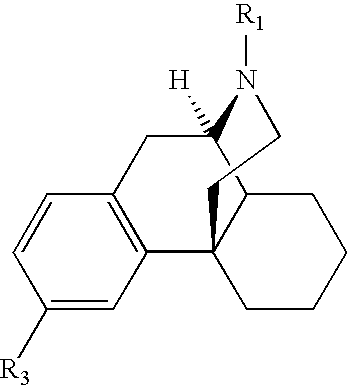
Transdermally administered dextromethorphan as antitussive agent
The present invention is drawn to a device for the transdermal administration of dextromethorphan, (+)-3-methoxy-17-methyl-9a,13a,14a-morphanin, and salts, prodrugs and metabolites thereof, together with a pharmaceutically acceptable carrier, to a human being or animal in need thereof, to achieve an antitussive effect. The present invention is further drawn to a method of achieving an antitussive effect in a human being or animal which comprises transdermally administering dextromethorphan, (+)-3-methoxy-17-methyl-9a,13a,14a-morphanin, and salts, prodrugs and metabolites thereof, together with a pharmaceutically acceptable carrier.
Owner:MCNEIL AB +1
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise an expectorant, an extended release antitussive, and an extended release decongestant. Specifically, the compositions comprise guaifenesin, phenylephrine tannate, and dextromethorphan tannate. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Pharmaceutical and nutraceutical compositions for treating respiratory disease and associated phlegm
InactiveUS20120121730A1Strong vascular dilating effectControl vascular toneAntibacterial agentsBiocideDiseaseDietary supplement
The present invention relates to pharmaceutical compositions comprising dextromethorphan or a physiologically acceptable salt thereof, quercetin, resveratrol, and hesperidin. The invention further relates to nutraceutical or dietary supplement composition comprising a physiologically acceptable salt of magnesium, quercetin, resveratrol, and hesperidin. Further provided are methods for treating respiratory disease in a human subject by substantially eliminating phlegm from the lung through administering to the subject effective amounts of compositions of the invention.
Owner:TRINITY LAB INC
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
Owner:EVERETT LAB
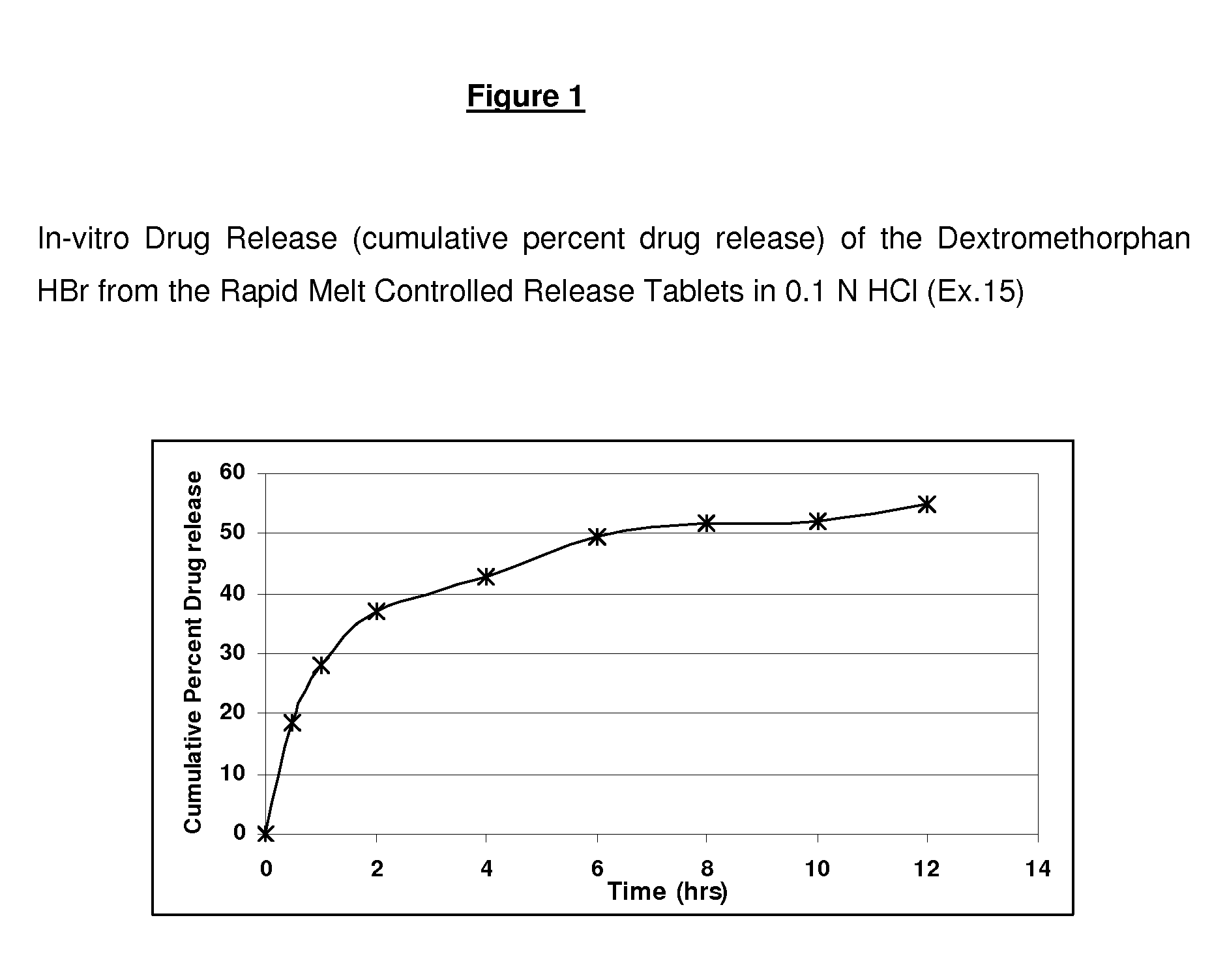
Rapid Melt Controlled Release Taste-Masked Compositions
InactiveUS20130071476A1Unpleasant tasteUnpleasant odorPowder deliveryBiocideWater insolubleAdditive ingredient
Rapid melt tablets that dissolve and release an active component in the oral cavity are comprised of a pharmaceutical active ingredient such as dextromethorphan complexed with a resin that is effective in taste-masking the otherwise bitter taste of the active making it convenient for oral administration. The drug / resin-complexed particles can be coated with water swellable or water insoluble polymers to impart controlled release properties to the active ingredient. A rapid melt tablet also comprises diluents, sweeteners, flavors, disintegrants and other excipients to form granules that can be compressed into tablets at low pressure without the need for a binding agent.
Owner:CHERUKURI SUBRAMAN RAO
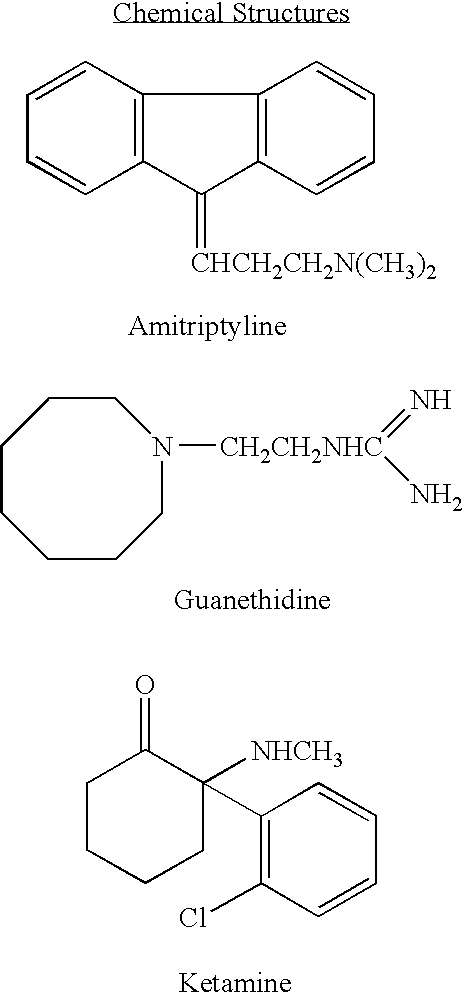
Ketamine or dextromethorphan formulations and methods of use
ActiveUS20150342947A1Act quicklyEliminating the digestive tractBiocideOrganic chemistryDiseaseRapid-acting antidepressant
The present invention provides a method of treating depression disease in a patient comprising administering to a mucosal membrane of a patient an effective amount of a pharmaceutically acceptable composition comprising an effective amount of ketamine or dextromethorphan, or both ketamine and dextromethorphan, wherein the mucosal administration of the ketamine or dextromethorphan containing composition allows for the mucosal absorption of the composition eliminating the digestive tract of the patient for effecting a rapid acting antidepressant treatment of the patient. Preferably, this method includes administering the composition to the oral cavity, and more preferably to the buccal cavity, of the patient. A pharmaceutically acceptable composition comprising ketamine or dextromethorphan and a vehicle is disclosed. A biomarker for identifying a depressive disease is set forth. A method of treating depressive illness in a patient using dextromethorphan via the oral route is provided.
Owner:WEST VIRGINIA UNIVERSITY
Combination cough treatment compounds and method of treating common coughs
A novel composition of three recognized antitussive agents, when used in combination, work in an additive fashion to suppress cough. Each drug has a desirable effect of suppressing cough in a unique fashion. However, undesirable side effects can occur in humans at concentrations at which the drug has its maximal antitussive effect. Pharmaceutical compositions of theobromine, dextromethorphan, and an antihistamine with central nervous system effect, such as dexbrompheniramine, maximize cough suppression while decreasing the likelihood of side effects when used in combination.
Owner:LEVINE BRIAN M +1
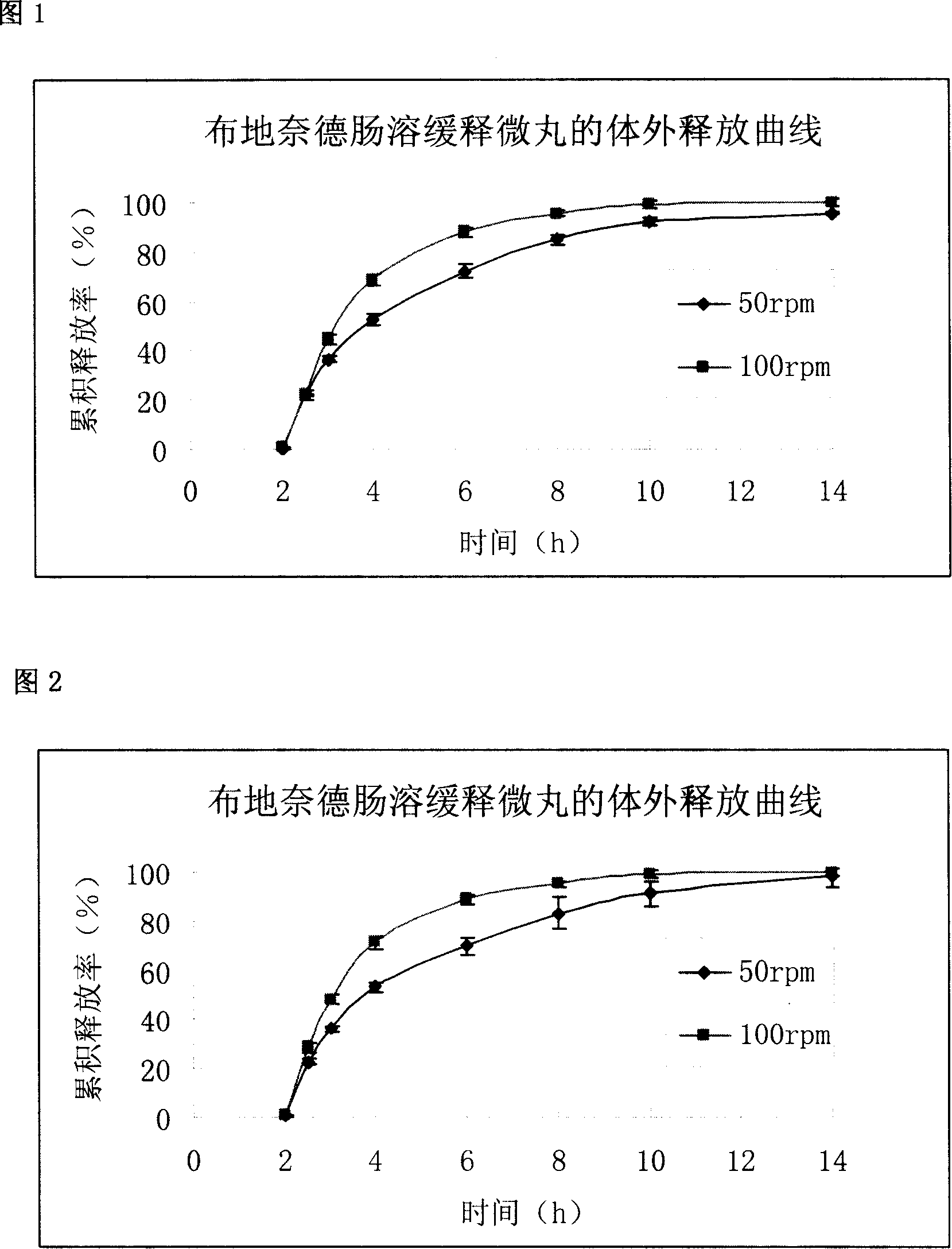
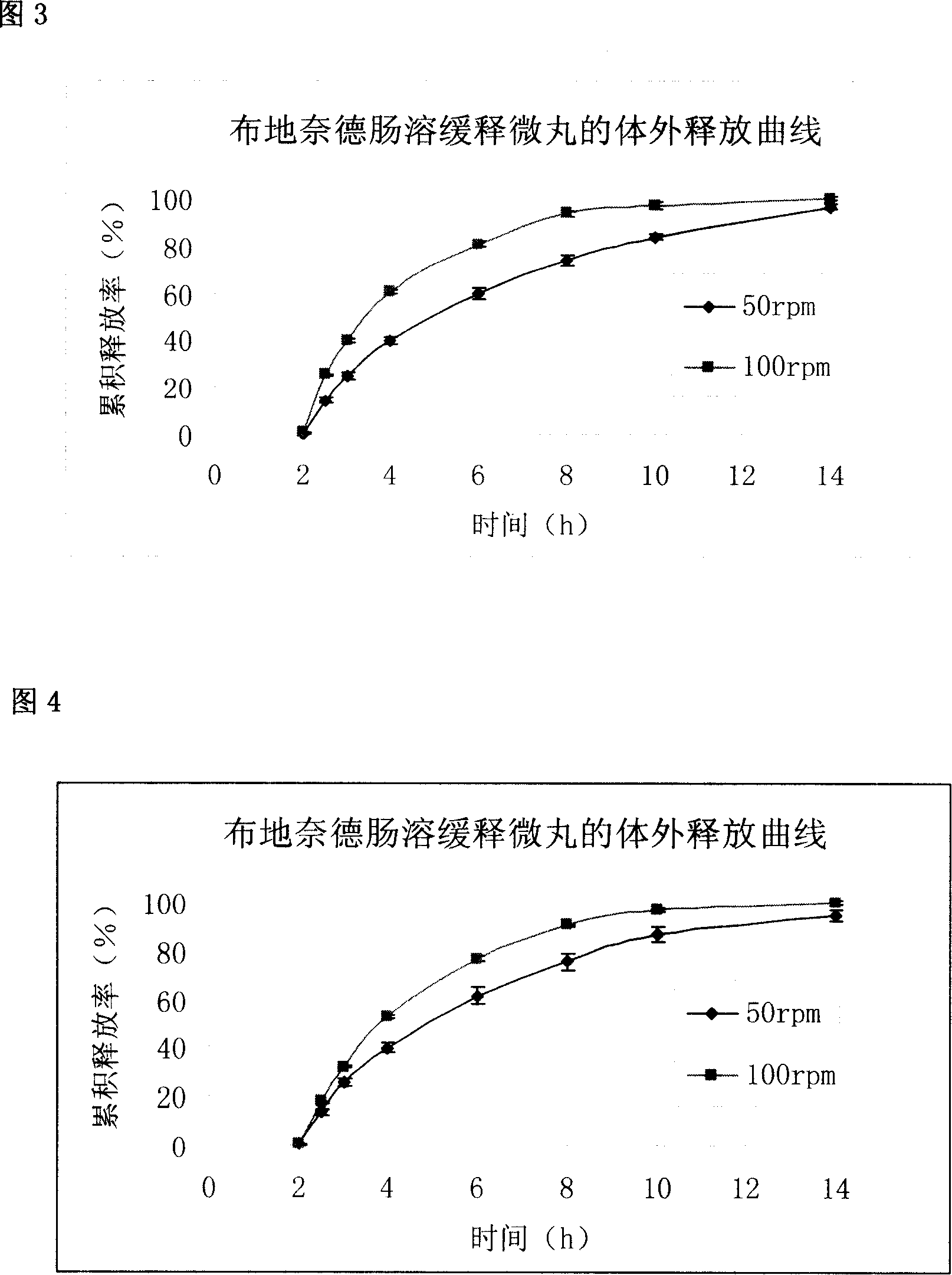
Budesonide intestines sustained release dextromethorphan pellets and method of manufacturing the same
InactiveCN101108171ARegulated release rateGood film formingOrganic active ingredientsAntipyreticSustained release pelletsMedicine
The invention belongs to the technical field of medicinal preparation and relates to an enteric preparation and sustained or controlled release preparation, in particular to an enteric controlled release micro-granule with budesonide and its preparation method. The invention, which comprises a quick release celphere, a controlled release layer and an enteric layer, takes budesonide as active ingredient of the drug to coordinate with medicinal carrier supplementary materials. After the release level testing, it is proved that the enteric controlled release micro-granule, which adopts different enteric materials, controlled release material and different coating conditions, does not release in stomach but began to release slowly after entering the small intestine with even release level. Therefore, the enteric controlled release micro-granule in the invention can effectively prevent the release in stomach, ensure the slow release in small intestine and the release of drug in the affected section of small incestine, ileocecus and colon.
Owner:FUDAN UNIV
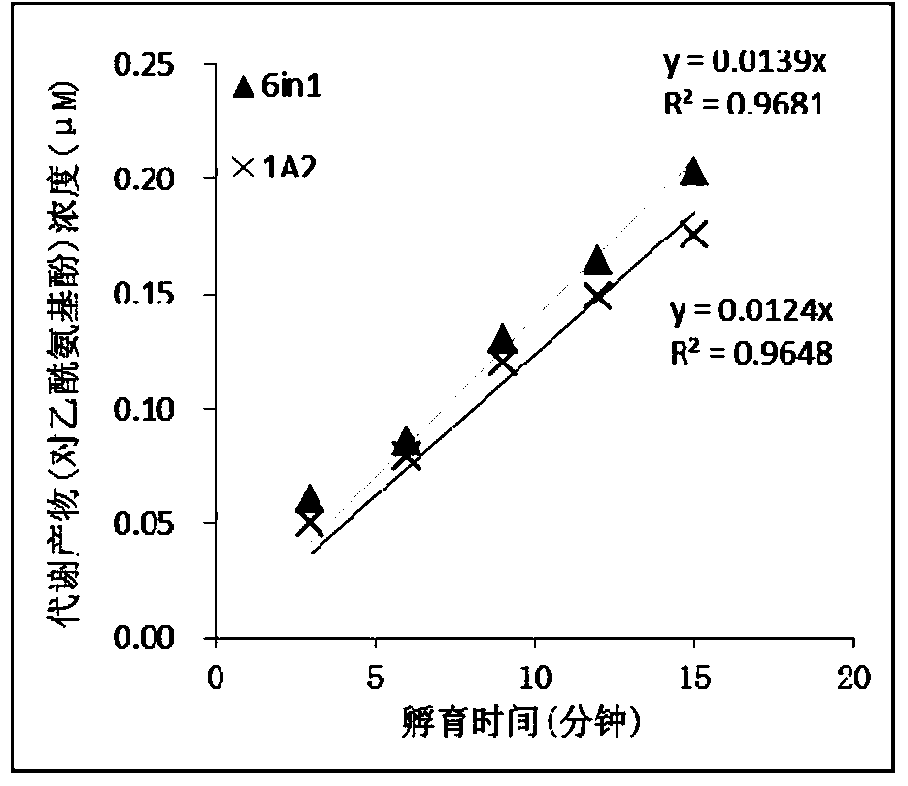
Specific probe substrate composition of cytochrome P450 enzyme and application of specific probe substrate composition
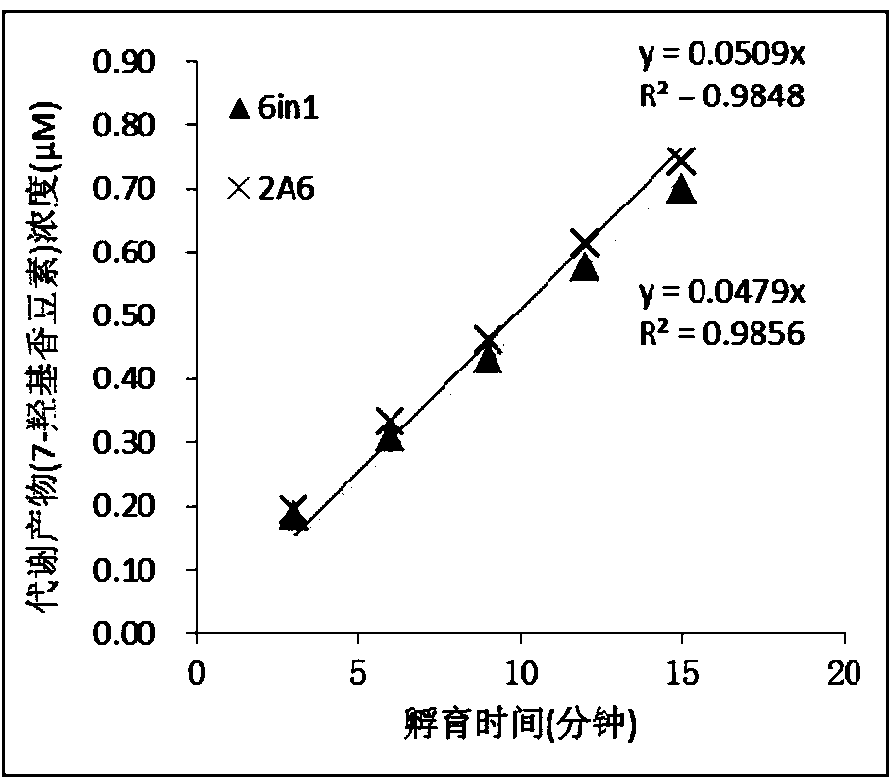
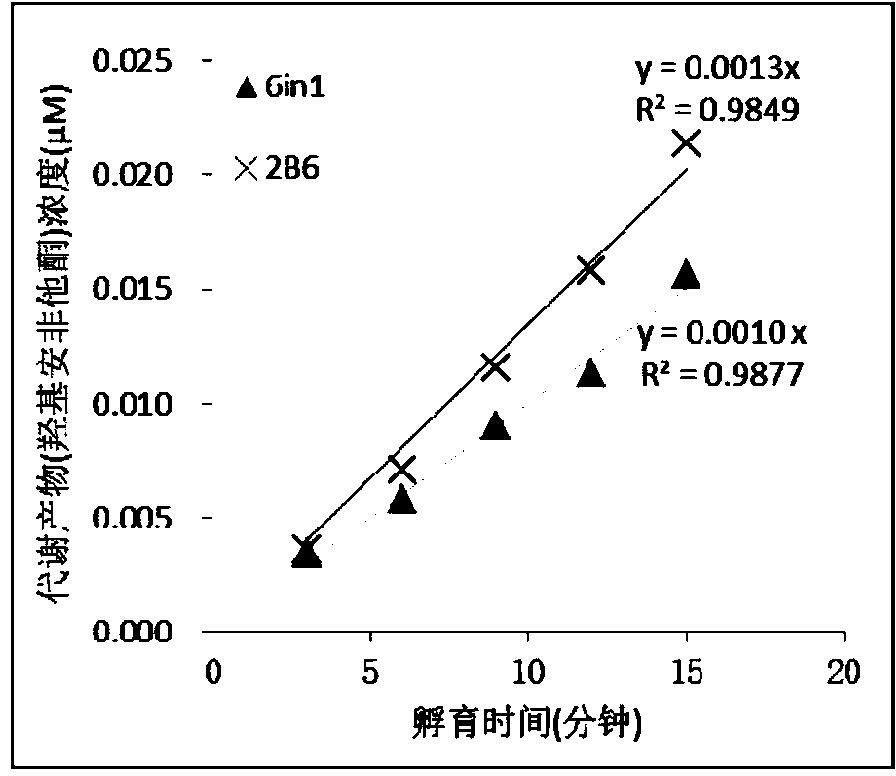
InactiveCN104195218AStrong specificityReduce distractionsComponent separationMicrobiological testing/measurementLiquid chromatography mass spectroscopyIonic Channels
The invention relates to a specific probe substrate composition of cytochrome P450 (CYP450) enzyme and an application of the specific probe substrate composition in subtype enzyme activity detection of the cytochrome P450 enzyme. The composition comprises at least two of phenacetin, coumarin, amfebutamone, dextromethorphan, diclofenac and testosterone. The characteristics of multi-ion channels can be simultaneously detected by the specific probe substrate composition and a liquid chromatography-mass spectrometry technology, and the inhibition conditions of six CYP450 enzymes are determined simultaneously, so that the experiment flux efficiency is greatly improved, and the experiment cost is reduced.
Owner:广东中西达一新药开发有限公司
Non-narcotic biphasic release compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions that comprise immediate release and extended release guaifenesin, extended release phenylephrine and immediate and extended release dextromethorphan. The present invention also includes methods for using these compositions for treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
Resolution of 1-(4-methoxybenzyl)-octahydro-isoquinoline
The invention provides a resolution method of racemic 1-(4-methoxybenzyl)-octahydro-isoquinoline by (R)-2-(6-methoxy-2- naphthyl) propionic acid with good yield, wherein the racemic 1-(4-methoxybenzyl)-octahydro-isoquinoline is a key intermediate for synthetizing a cough-relieving medicine dextromethorphan. A resolving agent and unneeded isomers of octahydro-isoquinoline can well be recovered.
Owner:DIVI S LAB LTD
Loxoprofen-containing pharmaceutical composition
InactiveCN102740854AGood storage stabilityReduce or inhibit damageOrganic active ingredientsPowder deliveryBromineLoxoprofen
Disclosed is a pharmaceutical composition containing loxoprofen or a salt thereof and codeine or the like, which has excellent storage stability. Specifically disclosed is a pharmaceutical composition which contains at least one component selected from the group consisting of codeine, carbinoxamine or a salt thereof, clemastine or a salt thereof, chlorpheniramine or a salt thereof, diphenylpyrraline or a salt thereof, bromhexine or a salt thereof, ambroxol or a salt thereof, lysozyme or a salt thereof and dextromethorphan or a salt thereof and loxoprofen or a salt thereof in such a manner that the at least one component and loxoprofen or a salt thereof are substantially not in contact with each other.
Owner:KOWA CO LTD
Composition and method for reducing the risk or progression of cardiovascular, glaucoma and tardive dyskinesia diseases
InactiveUS20030220225A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, beta-carotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such as grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
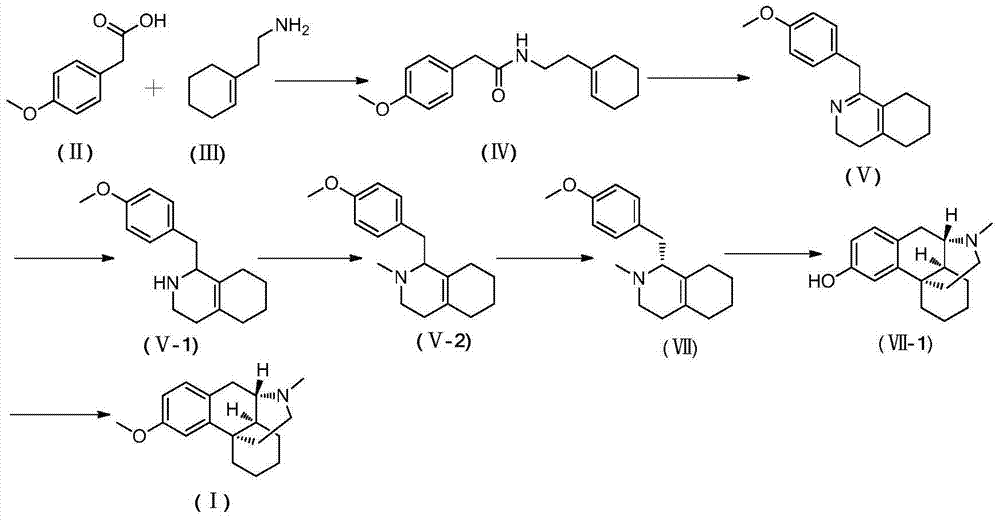
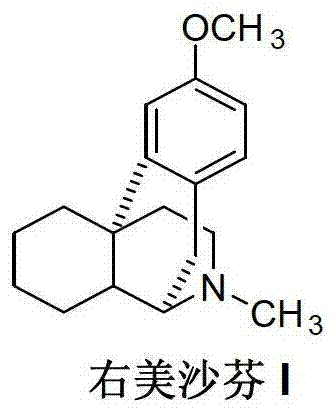
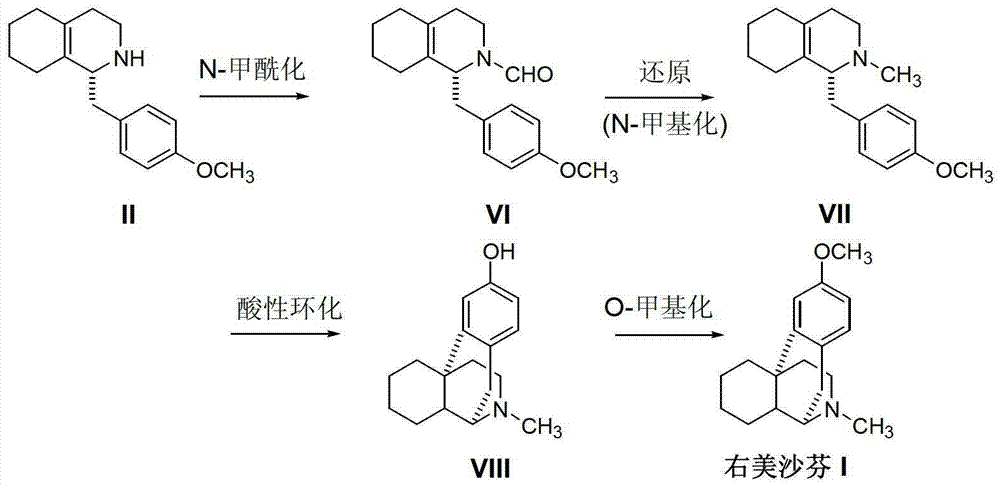
Novel method for preparing dextromethorphan
ActiveCN104119273AOrganic compound preparationCarboxylic acid amides preparationIsoquinolineQuinoline
The invention relates to a novel method for preparing dextromethorphan. When the method is used for preparing an intermediate (+)-1-(4-methoxy) benzyl-1,2,3,4,5,6,7,8-hexahydroisoquinoline (VI), a catalytic reducing method is adopted to carry out chiral reduction on 1-(4-methoxy) benzyl-3,4,5,6,7,8-hexahydroisoquinoline (VI), so that the intermediate is prepared with high selectivity. The novel method disclosed by the invention can cancel complex operations such as chiral resolution, is simple to operate, gentle in reaction condition, short in total time, wide in material source, and very suitable for industrially producing dextromethorphan.
Owner:SHANGHAI TIANCI INT PHARMA
Method for treating autism
A method for treating autism comprising the step of administering an effective amount of Memantine and dextromethorphan or pharmaceutically acceptable derivatives and / or salts thereof.
Owner:FOREST LAB HLDG LTD
Novel method for resolving 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline through enzyme catalysis
The invention relates to a method for resolving 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline through enzyme catalysis dynamic kinetics. Specifically, the invention provides a method for resolving 1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline racemate into a single configuration product (S)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline through dynamic kinetics and by utilizing cyclohexane oxidase and a mutant thereof, wherein (S)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline is a key intermediate for synthesizing the central antitussive drug dextromethorphan. The method has the outstanding characteristics of high yield, good stereoselectivity, mild reaction conditions and the like.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Methods and compositions for bactericide, bacteriostatic and anti-inflammation
ActiveCN101023947AAntibacterial agentsOrganic active ingredientsDiseaseBACTERIAL INFECTIOUS DISEASES
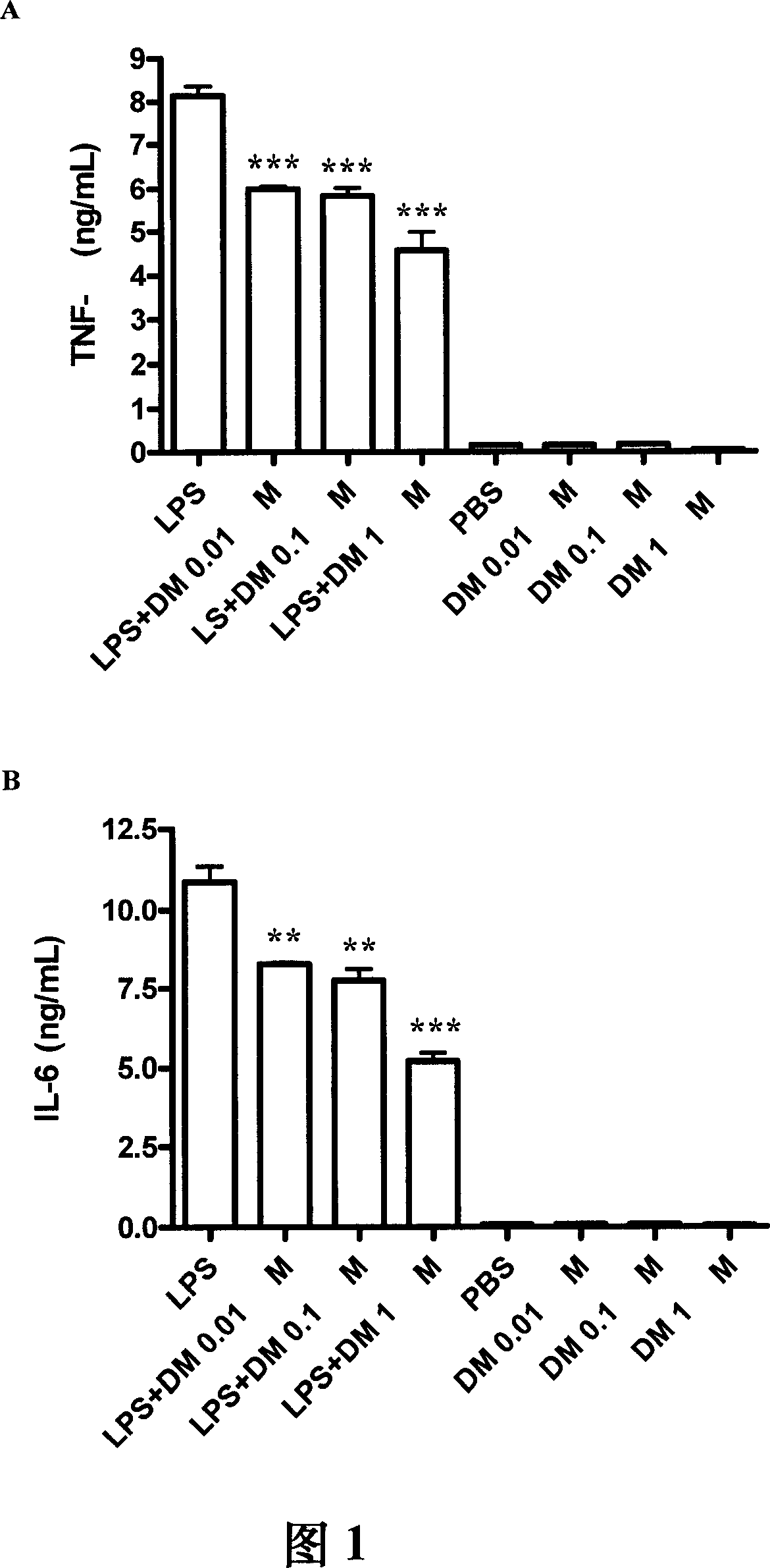
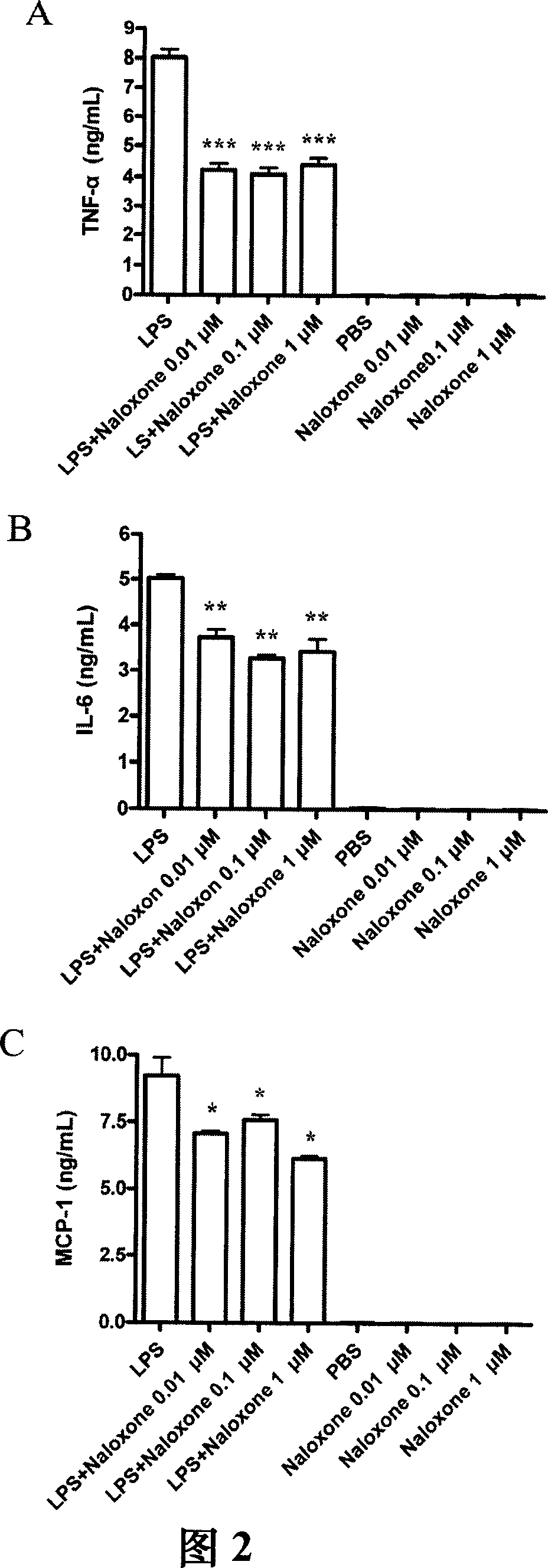
The present invention relates to a method for providing bactericide or bacteriostatic, especially for treating disease due to bacterial infection. The method comprising administering a patient in need of such treatment a therapeutically effective amount of a compound of dextromethorphan or naloxone or a pharmaceutically acceptable salt or an analog thereof. The compound is applied to skin or mucosal surface of the patient. The invention also relates to a method of treating inflammation caused by suppressing secretion of TNF-alpha, IL-6, or MCP-1 from macrophage comprising administering a patient in need of such treatment a therapeutically effective amount of NADPH oxidase inhibitor.
Owner:BLUE BLOOD BIOTECH CORP
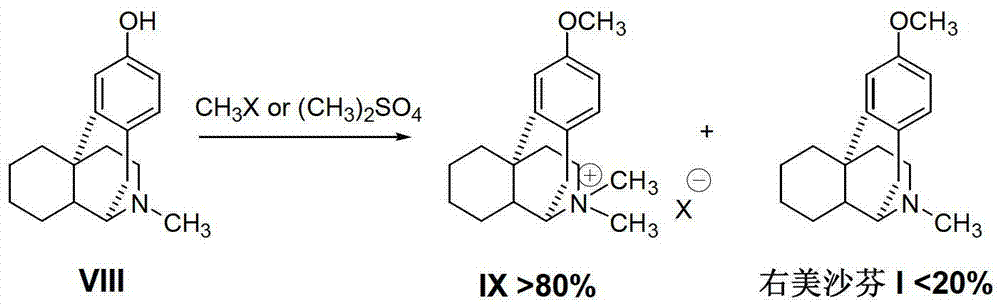
Preparation method of dextromethorphan
ActiveCN103044327AReduce manufacturing costPromote the development of economy and technologyOrganic chemistryIsoquinolineN methylation
The invention discloses a preparation method of dextromethorphan ((+)-3-methoxy-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane, I). The method comprises the following steps that a dextromethorphan intermediate (+)-1-(4-methoxy) benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (II) conducts N-benzylation reaction with a benzylation reagent under alkaline conditions to form (+)-1-(4-methoxy) benzyl-N-benzyl-1,2,3,4,5,6,7,8-octahydro-isoquinoline (III); the intermediate (III) is subjected to acid cyclization reaction to form (+)-3-hydroxy-17-benzyl-(9 alpha,13 alpha,14 alpha)-levorphane, (IV); the intermediate (IV) reacts with dimethyl sulfate or methine halide, and is subjected to O-methylation and N-methylation reaction to form (+)-3-methoxy group-17-benzyl-17-methyl-(9 alpha,13 alpha,14 alpha)-levorphane quaternary ammonium salt (V); (V) is subjected to catalytic hydrogenation reaction; benzyl is removed; and dextromethorphan (I) is obtained. Therefore, the preparation method can use a common and cheap methylation reagent to substitute an unusual methylation reagent such as phenyltrimethylammonium hydroxide, and the yield of reaction can be increased.
Owner:SUZHOU LIXIN PHARMA
Methods Related to the Treatment of Neurodegenerative and Inflammatory Conditions
The invention includes methods of neuroprotection, inducing release of neurotrophic factors, inhibiting the over-activation of innate immune cells, attenuating the toxin-induced death and / or damage of tissues, reducing inflammation, treating an inflammation-related condition, and inhibiting NADPH oxidase, that includes contacting or administering an effective amount of at least one compound of the invention that include: valproic acid, sodium butyrate, and salts thereof; opioid peptides; a peptide comprising the tripeptide GGF; and morphinans, such as naloxone, naltrexone, 3-hydroxy-morphinan and dextromethorphan.
Owner:UNITED STATES OF AMERICA
Lozenge for delivery of dextromethorphan
InactiveUS20050238695A1Powder deliveryOrganic active ingredientsDextromethorphan+diphenhydramineEthylmorphine
The present invention provides an organoleptically pleasing lozenge containing an antitussive selected from the group consisting of dextromethorphan, diphenhydramine, caramiphen, carbapentane, ethylmorphine, noscapine, codeine, and mixtures thereof, complexed with an ion exchange resin wherein the particle size of the resin is 38 μm or less in diameter. Also provided is a process for producing the lozenge and methods of administering the lozenge.
Owner:MCNEIL PPC INC +1
Pharmaceutical composition
InactiveUS20100015248A1Reduce severityShorten the durationBiocideHydroxy compound active ingredientsVitamin CMedicine
A pharmaceutical composition for the treatment of colds and influenza. The pharmaceutical composition is a mixture of: acetaminophen, diphenhydramine, dextromethorphan, arabinogalactan, vitamin C, zinc, olive leaf extract, resveratrol and elderberry extract.
Owner:VANTERPOOL ELAINE A
Compound formula dextro methaphen oral disintegration tablet and its preparation method
InactiveCN1830442AShort disintegration timeGreat tasteOrganic active ingredientsPill deliveryMANNITOL/SORBITOLPseudoephedrine
An oral disintegrating tablet of dextromethorphan for treating the cold caused cough, nasal congestion and rhinorrhea is proportionally prepared from dextromethorphan, chlorphenamine, pseudoephedrine, beta-cyclodextrin or ion exchange resin, mannitol or lactose starch, and disintegrant through sieving, flavouring, mixing and die pressing.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com