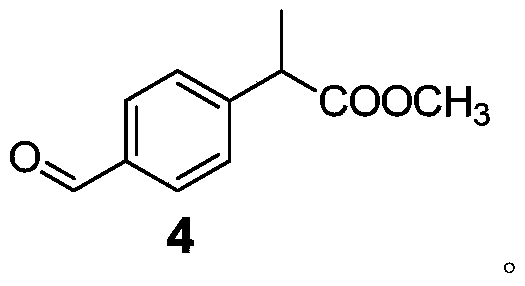
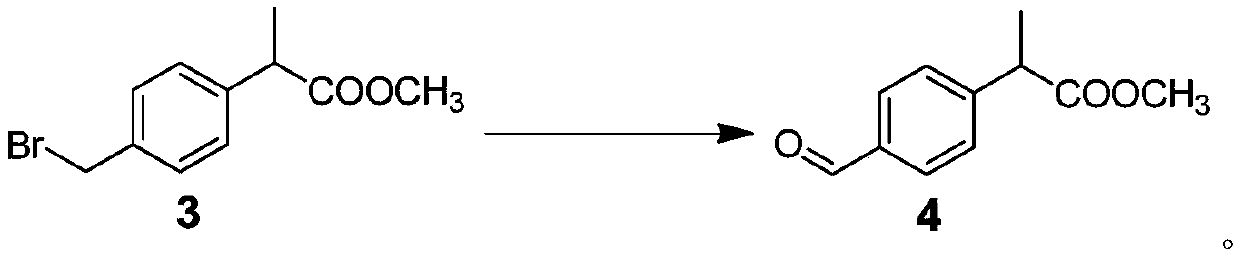
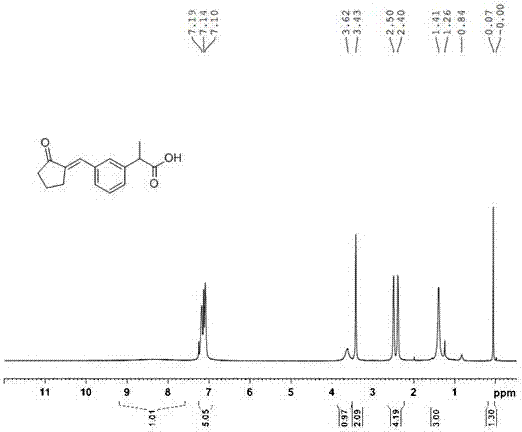
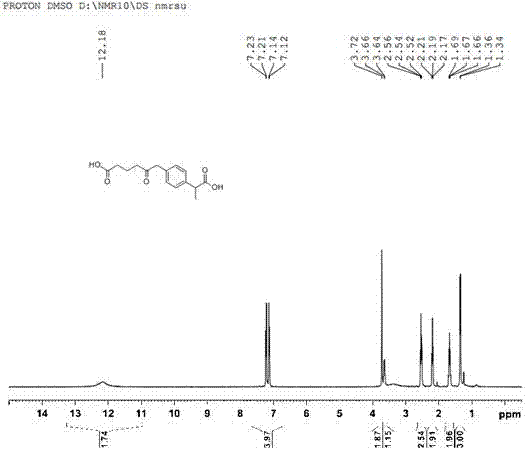
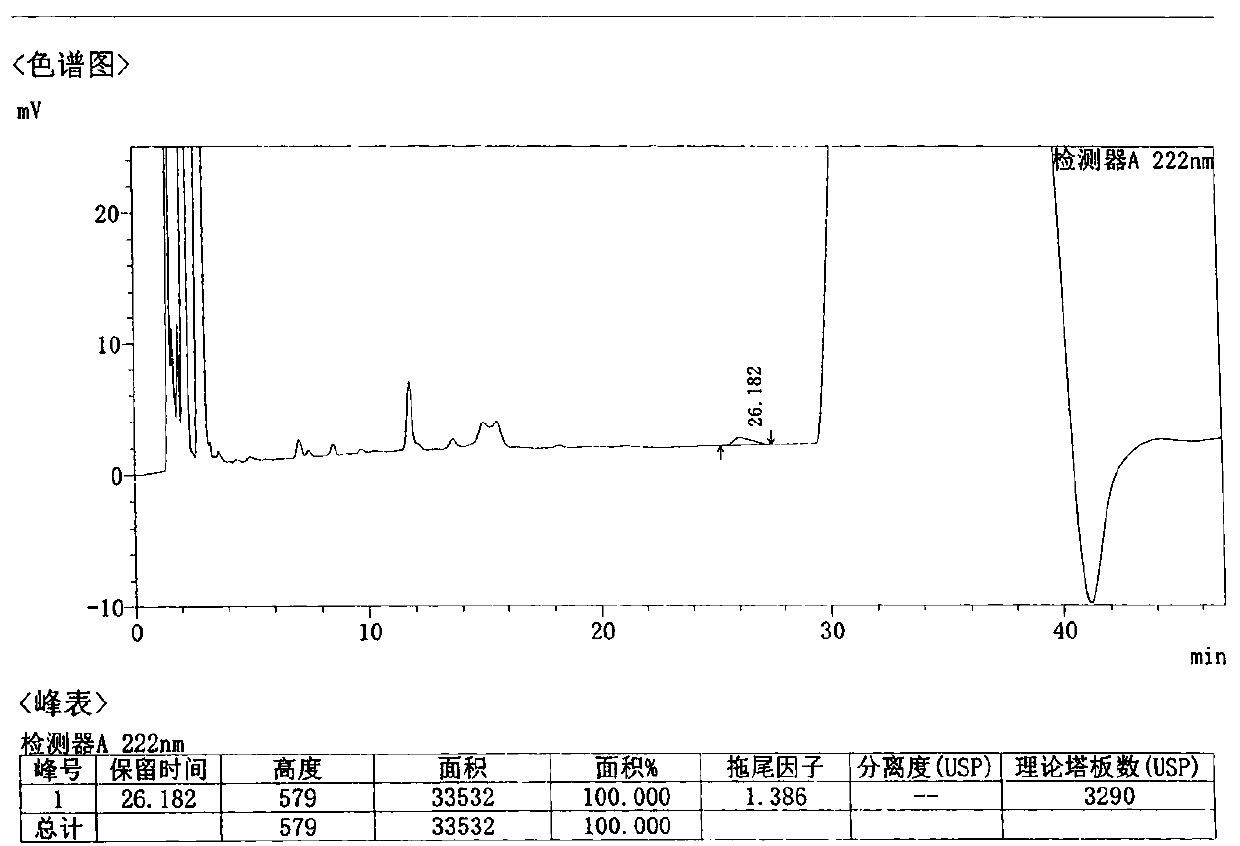
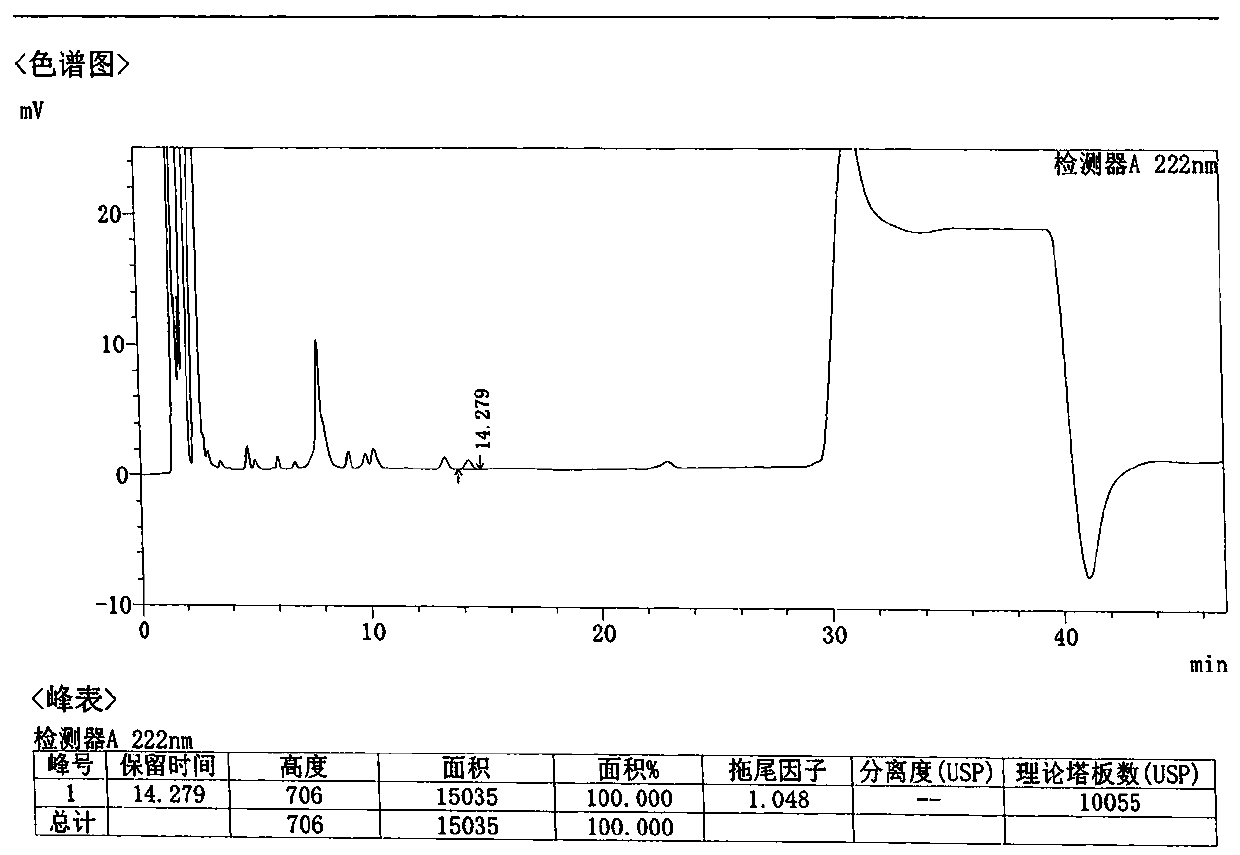
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Loxoprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
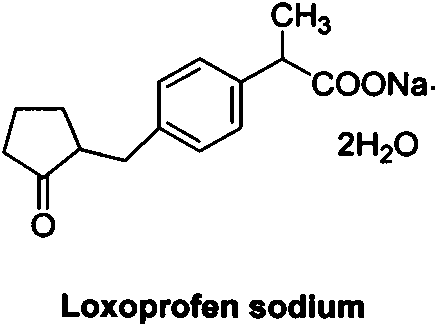
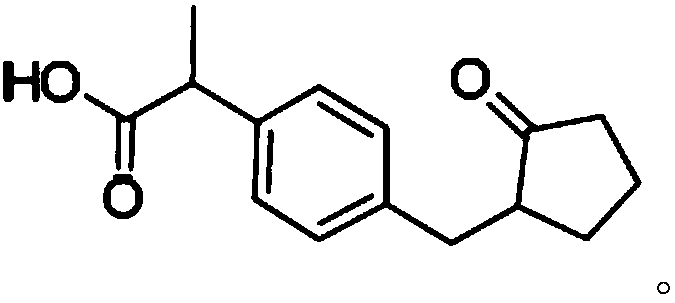
Loxoprofen is a nonsteroidal anti-inflammatory drug (NSAID) in the propionic acid derivatives group, which also includes ibuprofen and naproxen among others. It is available in some countries for oral administration. A transdermal preparation was approved for sale in Japan on January 2006.
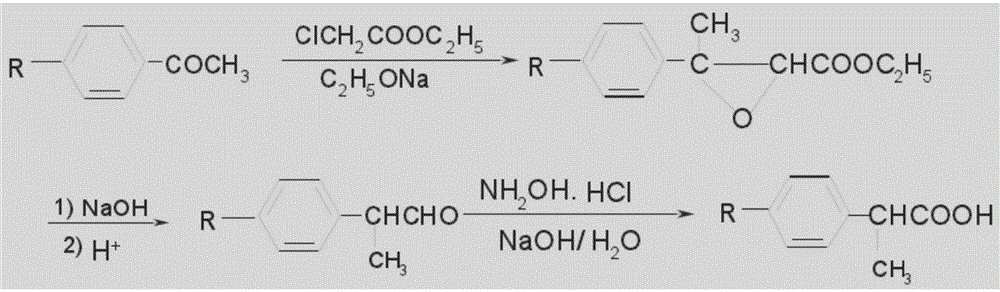
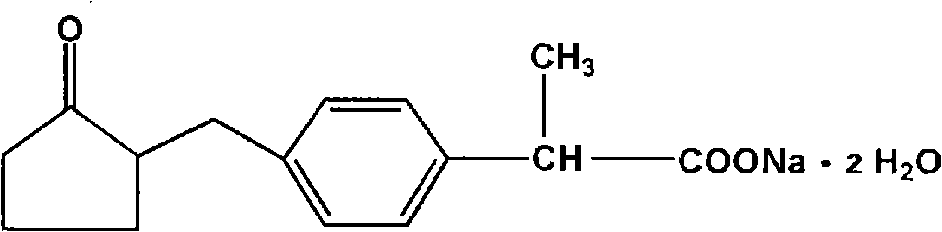
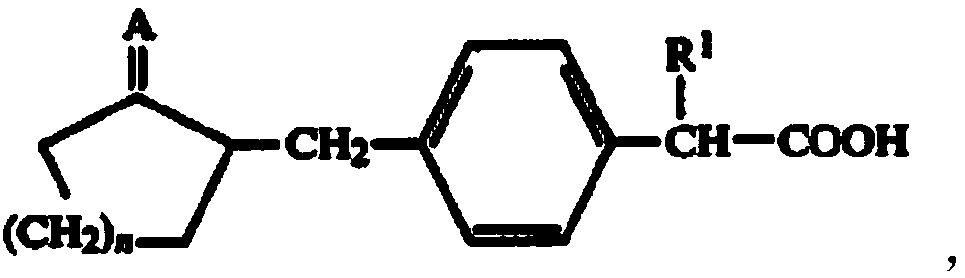
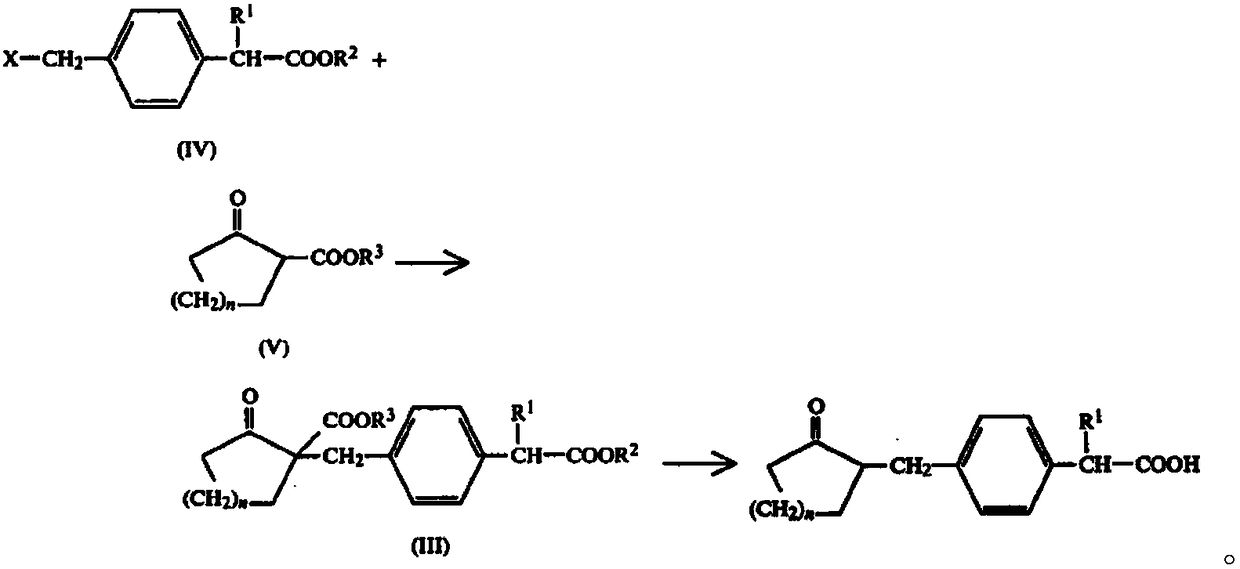
Method for synthesizing loxoprofen sodium
ActiveCN101412670ARaw materials are easy to getUnique craftOrganic compound preparationCarboxylic compound preparationSolventHydrolysis
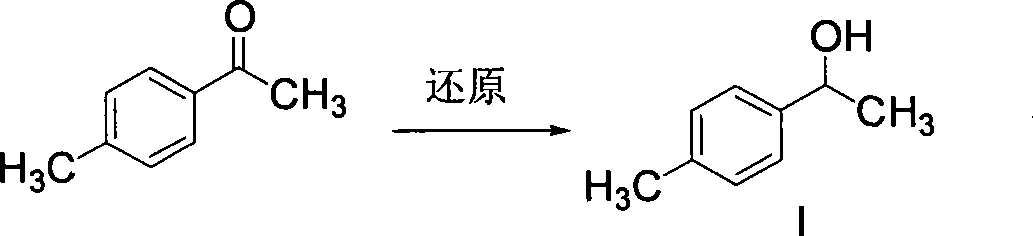
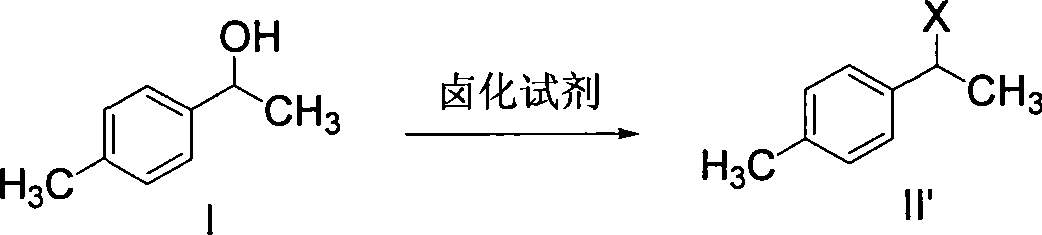
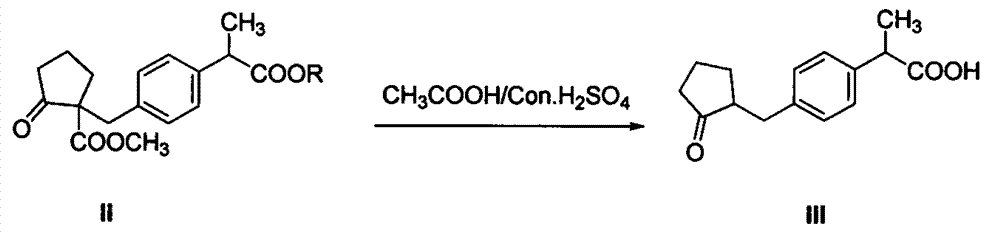
The invention discloses a synthetic method for loxoprofen sodium, which is prepared by taking methyl acetophenone as an initial raw material through reduction, acylation or halogen substituent, cyanation, hydrolysis, bromination, condensation, decarboxylation and salifying. The method has the advantages of easily obtained raw material, unique technology, simple and stable operation, and high productive rate in each step of reaction; and all solvents used in the synthesis process can be recycled, so the production cost is reduced greatly. Tests show that the obtained product has reliable quality and stable performance, and can be further used for making preparation of non-steroidal anti-inflammatory drugs such as the loxoprofen sodium.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Medicament for treatment of nocturia
InactiveUS20040054008A1BiocideAntipyreticNonsteroidal Antiinflammatory Drugs/NSAIDsTherapeutic treatment
A method for preventive and / or therapeutic treatment of nocturia, which comprises the step of administering to a mammal including a human in need of such treatment a preventively and / or therapeutically effective amount of a nonsteroidal anti-inflammatory drug such as loxoprofen.
Owner:SANKYO CO LTD
Synthetic methods of loxoprofen sodium and intermediate thereof
InactiveCN104710309ASimple and fast operationEasy to operateOrganic compound preparationCarboxylic acid esters preparationPropanoic acidLoxoprofen
The invention discloses synthetic methods of loxoprofen sodium and an intermediate thereof; with benzene as a starting material, the key intermediate of loxoprofen sodium is obtained through acylation, halogenation, ketalation, rearrangement and Blanc chloromethylation, then the key intermediate is subjected to condensation, decarboxylation and salt forming reaction to obtain loxoprofen sodium. The methods have the advantages of easily obtained raw materials, simple operation, stable process and high yield; 2-(4-chloromethyl phenyl)propionic acid or propionate is prepared by adopting a method of direct carrying out chloromethylation on a substituted benzene ring, the operation is simple, the yield is high, the use of a chloromethyl ether reagent or stannic chloride and other Lewis acids is avoided, the environmental protection property is high, and relatively good industrial application value is achieved.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Method for preparing loxoprofen intermediate
InactiveCN105753685AAvoid bringing inHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidPropionitrile
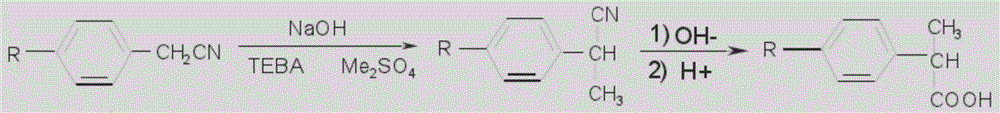
A method for preparing a loxoprofen intermediate comprises the following steps that 1, on the presence of sodium alkoxide, benzyl cyanide and dimethyl carbonate are subjected to methylation in an organic solvent, and 2-(phenyl cyano) sodium propionate is obtained; 2, 2-(phenyl cyano) sodium propionate and dimethyl sulfate react in an organic solvent to obtain 2-(phenyl cyano) methyl propionate; 4, 2-(phenyl cyano) methyl propionate reacts under the alkaline condition to obtain 2-phenyl propionitrile; 4, 2-phenyl propionitrile is hydrolyzed under the alkaline condition, acid is added for acidizing after the reaction to obtain 2-phenylpropionic acid; 5, 2-phenylpropionic acid, hydrobromic acid and paraformaldehyde are mixed and subjected to a bromine methylation reaction under the acidic condition, and 2-(4-tribromomethyl phenyl) propionic acid is obtained.According to the method, a new synthesis route is designed, product selectivity is good, the purity is high, the conversion rate is high, and few by-products are generated; the raw materials are simple and easy to obtain, the production conditions are mild, the process is simple, production cost is low, and pollution is small.
Owner:UPCHEM CHINA
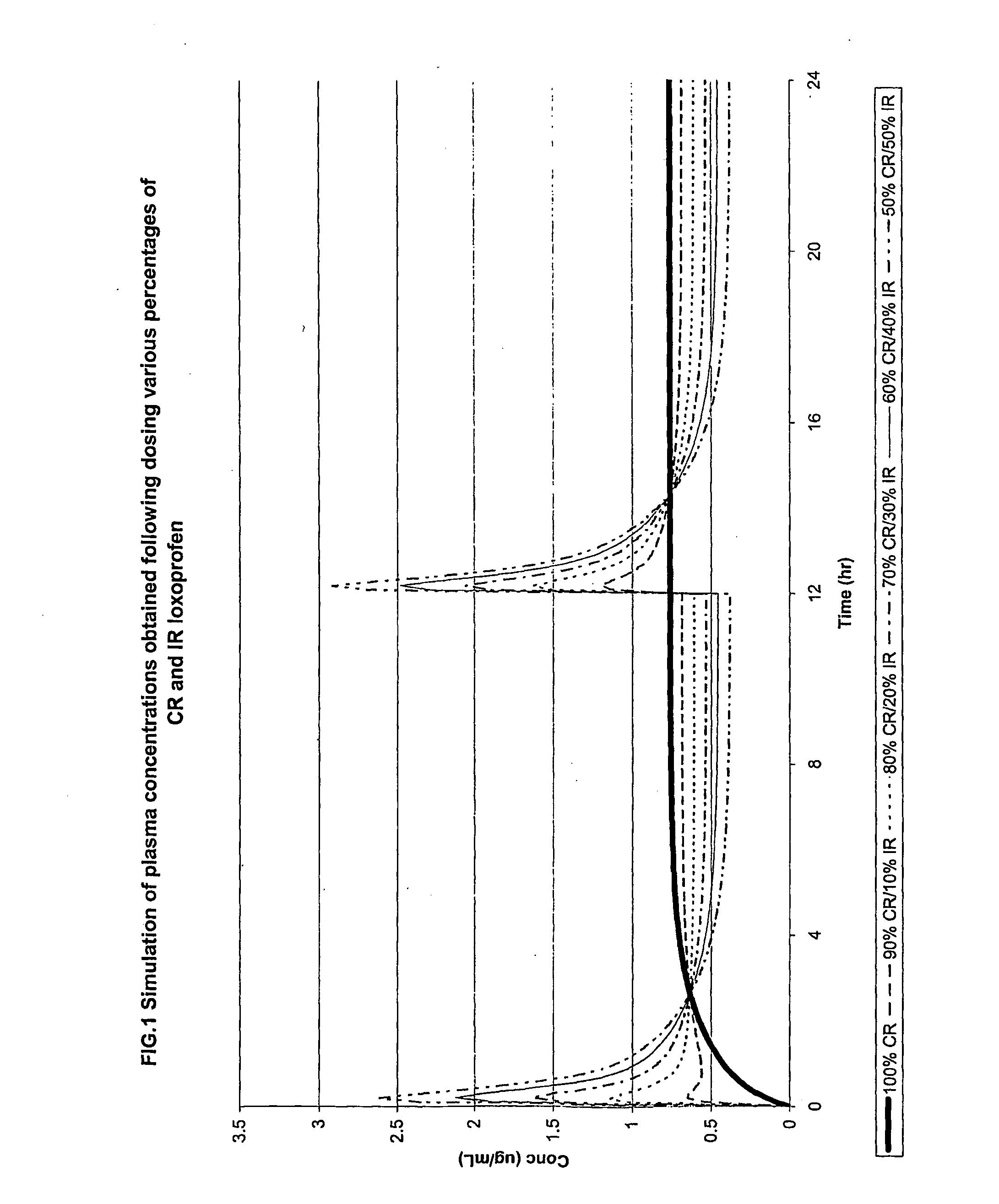
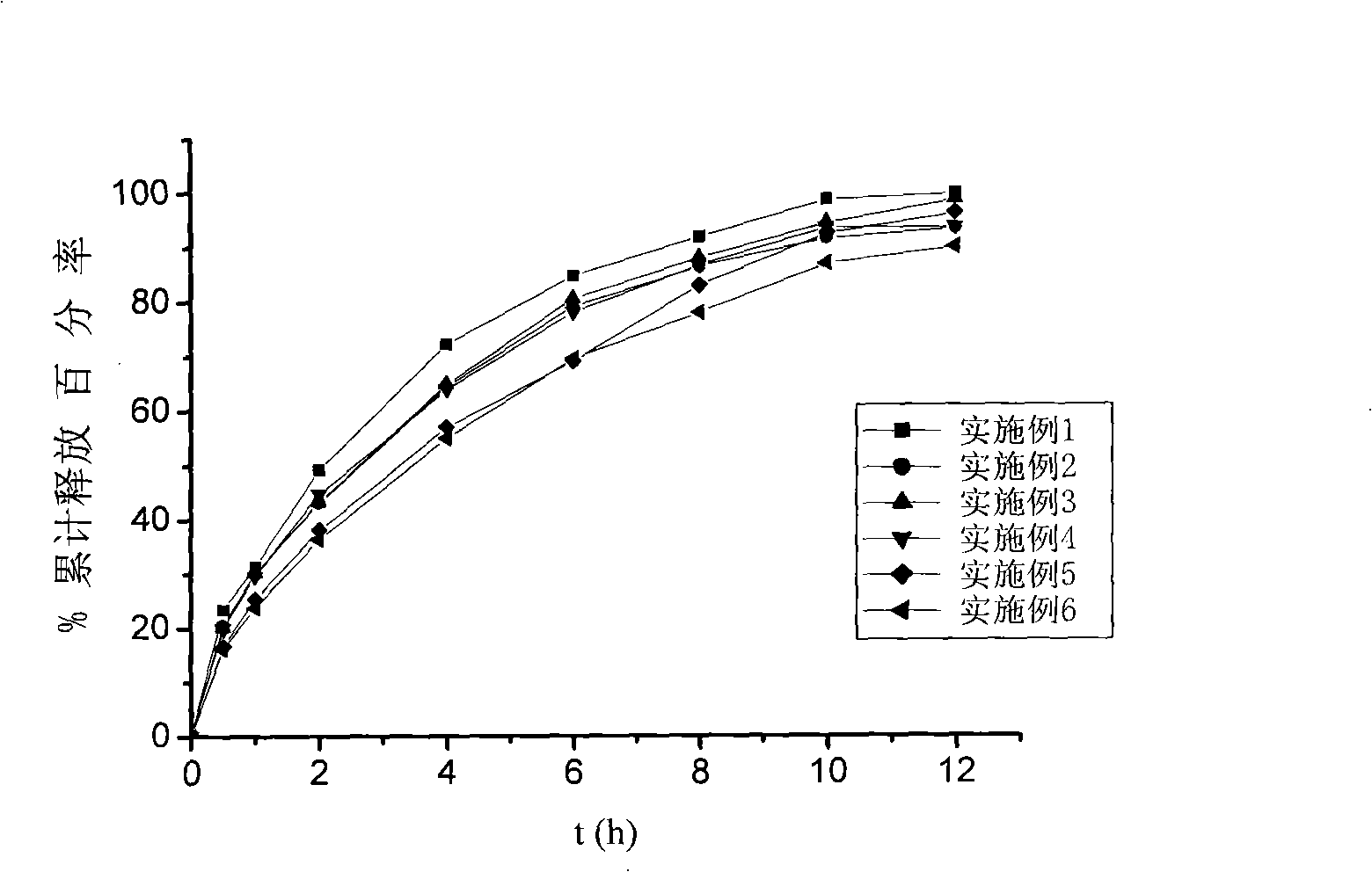
Modified Release Loxoprofen Compositions
The invention relates to a modified release composition comprising loxoprofen or a salt or derivative thereof that in operation delivers the drug in a pulsatile or continuous manner for the treatment of pain and / or inflammation. The composition may comprise a first loxoprofen component and one subsequent loxoprofen component, wherein the first loxoprofen component comprises an immediate release component and the subsequent loxoprofen component comprises a modified release component.
Owner:ALKERMES PHARMA IRELAND LTD
Loxoprofen-containing pharmaceutical composition
InactiveCN102740854AGood storage stabilityReduce or inhibit damageOrganic active ingredientsPowder deliveryBromineLoxoprofen
Disclosed is a pharmaceutical composition containing loxoprofen or a salt thereof and codeine or the like, which has excellent storage stability. Specifically disclosed is a pharmaceutical composition which contains at least one component selected from the group consisting of codeine, carbinoxamine or a salt thereof, clemastine or a salt thereof, chlorpheniramine or a salt thereof, diphenylpyrraline or a salt thereof, bromhexine or a salt thereof, ambroxol or a salt thereof, lysozyme or a salt thereof and dextromethorphan or a salt thereof and loxoprofen or a salt thereof in such a manner that the at least one component and loxoprofen or a salt thereof are substantially not in contact with each other.
Owner:KOWA CO LTD
Aryl propionic acid derivative composition and pharmaceutical purpose
ActiveCN106661061ALess irritatingImprove medication complianceOrganic active ingredientsAntipyreticMetabolitePhosphate
The invention discloses an aryl propionic acid derivative composition and a pharmaceutical purpose. Alcohol metabolites of Loxoprofen having highest activity in metabolism is used as a nuclear parent to produce a series of derivatives of phosphate, sulphonate, carbonic ester, and amino-acid ester. The derivatives are used to enhance pesticide effect of anti-inflammatory and analgesic effects, and reduce toxic reaction, and have good pharmacokinetic property. And at the same time, the stimulation of the medicine on the gastrointestinal tract, and medication compliance of patients is improved, and therefore the aryl propionic acid derivative composition is the non-steroidal anti-inflammatory drugs having potentials.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
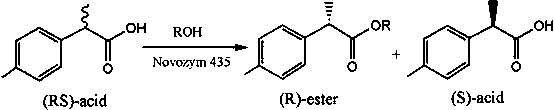
Method for catalytic esterification resolution of 2-(4-methylphenyl) propionic acid enantiomer via stereoselective enzyme
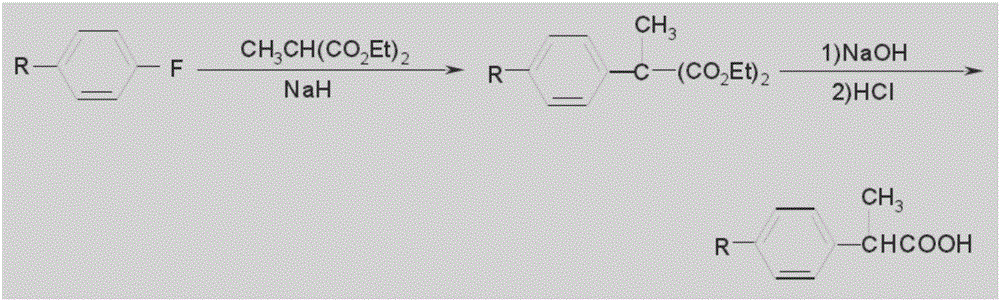
The invention discloses a method for catalytic esterification resolution of a 2-(4-methylphenyl) propionic acid enantiomer via stereoselective enzyme. On the basis of high catalysis efficiency and high stereoselectivity of lipase, catalytic esterification resolution of racemic 2-(4-methylphenyl) propionic acid is conducted in an organic solvent medium, so that the (S)-2-(4-methylphenyl) propionicacid is prepared. The reaction system, with the application of an organic solvent system, can improve thermal stability and catalysis efficiency of the lipase to a great extent, so that a substrate conversion rate and optical activity are greatly improved, and the optical activity of the substrate is greater than or identical to 97.84%. In comparison with other resolution technologies, the methodis gentle in reaction conditions, simple to operate and low in environmental pollution, and the obtained (S)-2-(4-methylphenyl) propionic acid is relatively high in optical purity; and with the (S)-2-(4-methylphenyl) propionic acid serving as a key intermediate product, a feasible method is provided for the preparation of loxoprofen sodium.
Owner:HUNAN INSTITUTE OF SCIENCE AND TECHNOLOGY
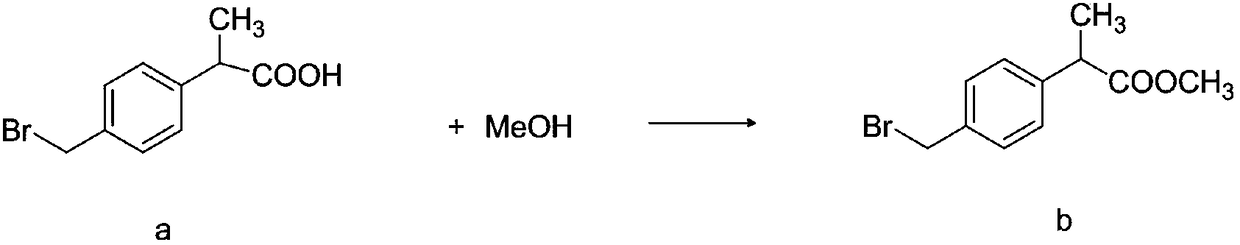
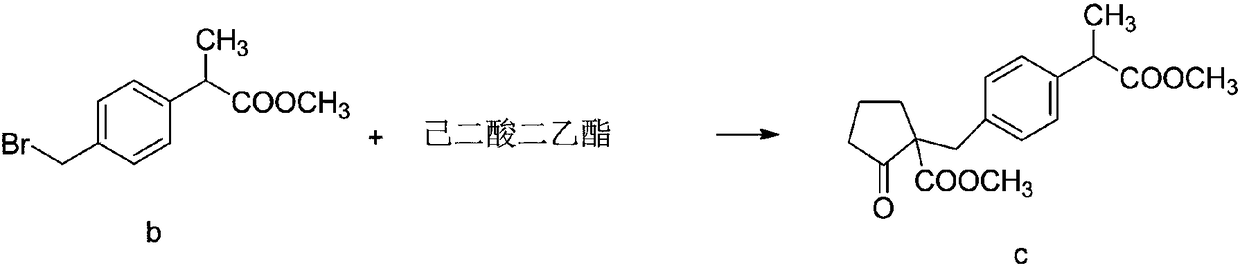
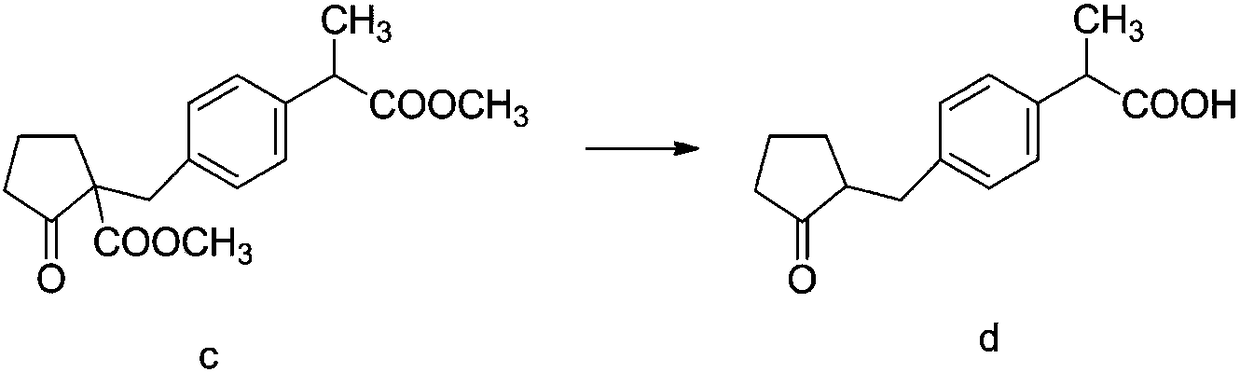
Method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium
InactiveCN108440274AHigh HPLC contentThe reaction mechanism is simpleOrganic compound preparationCarboxylic acid esters preparationCompound cMethyl propionate
The invention discloses a method for synthesizing high-purity non-steroidal anti-inflammatory drug loxoprofen sodium, 2-(4-bromomethylphenyl) methyl propionate b is synthesized by esterification of raw material a and methanol, intermediate compound c and loxoprofen acid are synthesize sequentially, and the loxoprofen sodium is finally synthesized. The reaction mechanism is simple, by-products arefew, synthesis steps are easy to control, the raw material is easily available, and the impurity content of each step is strictly controlled. The purifying method is easy to operate and suitable for industrial production. The white flake crystal loxoprofen sodium is prepared. Finally, HPLC analysis shows that the loxoprofen sodium content detected by HPLC is as high as 99.95%.
Owner:大桐制药(中国)有限责任公司
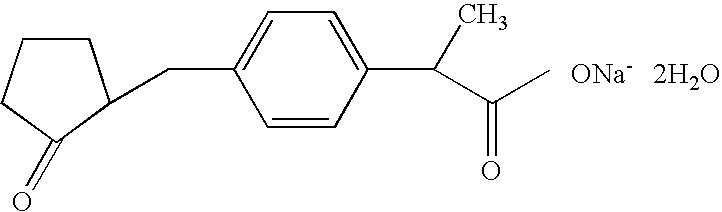
Industrial production method of high purity loxoprofen sodium dehydrate
InactiveCN104326903AHigh yieldImprove qualityOrganic compound preparationCarboxylic acid esters preparationDistillationAdipic acid
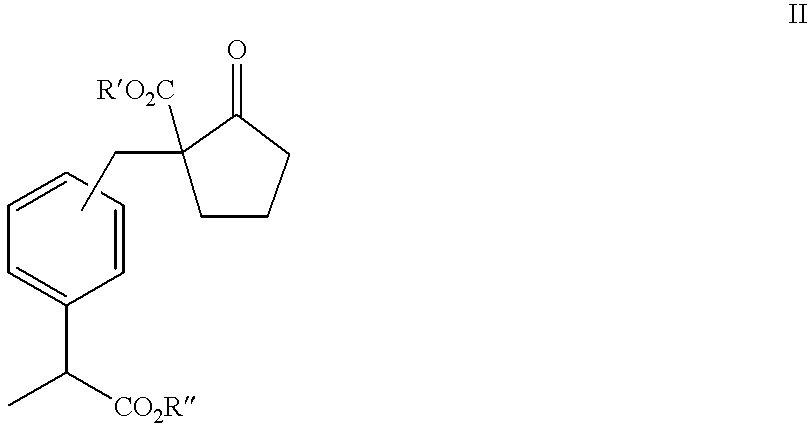
The invention discloses an industrial production method of high purity loxoprofen sodium dehydrate; according to the method, adipic acid diester is taken as a starting material for Dieckmann condensation reaction with 2-(4-bromomethyl phenyl) propionate under strong alkaline conditions, the yield and quality of compound II are effectively improved, because the adipic acid diester on the market is lower in cost than 2-ethoxy carbonyl cyclopentanone, the production cost can be greatly reduced; the compound obtained by reaction is processed sequentially by hydrolysis and decarboxylation under acidic conditions, in the hydrolysis and decarboxylation process, generated low boiling point alcohol solvents and carbon dioxide gas can be removed by atmospheric distillation, the reaction can be effectively promoted, the reaction time is shortened, the conversion rate is improved; and finally the final product loxoprofen sodium dehydrate is generated under the condition of aqueous sodium hydroxide solution. The preparation method can greatly reduce the manufacturing cost of loxoprofen sodium, and is more suitable for the industrialized production than the existing technology.
Owner:合肥远志医药科技开发有限公司
Novel synthetic method of key intermediate2-anisacetone of loxoprofen
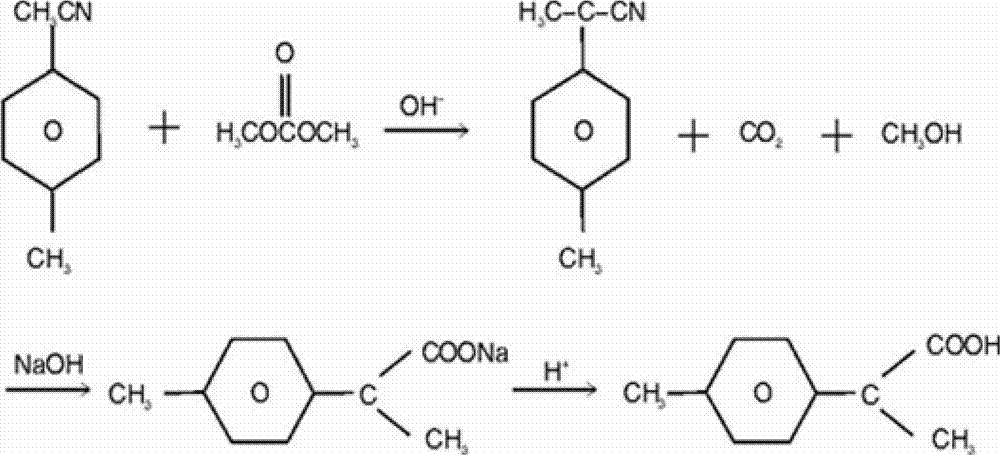
The invention relates to a synthetic method for loxoprofen, in particular to a novel synthetic method of key intermediate2-anisacetone of loxoprofen. The method comprises the following steps: taking methylbenzyl cyanide as an initial material to generate alpha-methylation with dimethyl carbonate in an alkaline environment so as to generate alpha-methyl-methyl phenylacetonitrile, and hydrolyzing the alpha-methyl-methyl phenylacetonitrile in alkaline water to obtain the 2-anisacetone. The method provided by the invention has the advantages of simplicity, easiness in control and small waste water amount. Moreover, the synthesized 2-anisacetone is high in purity and stable in quality, which lays a good foundation for synthesizing the high-purity loxoprofen.
Owner:ANHUI HERYI CHEM
Preparation method for 2-(4-brooethylphenyl) propionic acid
InactiveCN103342636AHigh purityGood anti-rheumatic effectOrganic compound preparationCarboxylic compound preparationPropanoic acidRheumatism
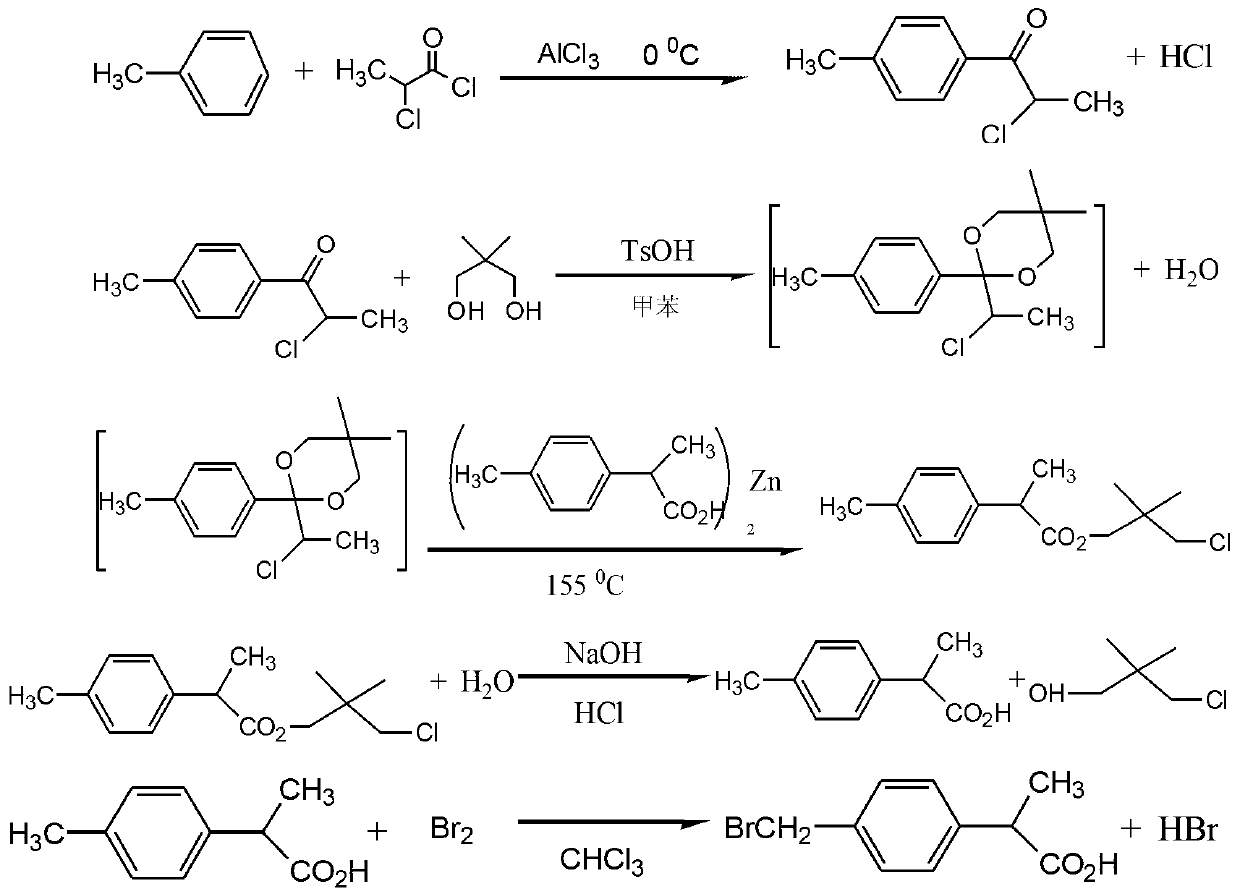
The invention relates to the technical field of 2-(4-brooethylphenyl) propionic acid, and in particular relates to a preparation method for 2-(4-brooethylphenyl) propionic acid. The preparation method comprises the following steps of: taking p-methylphenyl-2-chloro-1-ketone and 2-p-methylphenyl propionic acid as main raw materials, and finally preparing a finished product via a dry acylation reaction, a condensation reaction and a bromination reaction, wherein the finished product is high in purity and high in yield which is up to more than 98%, is one of key intermediates for synthesising loxoprofen sodium, and is clinically indicated to be the greatest in the effects of easing pain, resisting inflammation and resisting rheumatism within the presently known aryl propionic acid medicines, thus being greatly preferred by national and international experts and scholars, and promoted by the medical circle and patients. Additionally, the preparation method disclosed by the invention is simple in production process, low in production cost, and less in pollution.
Owner:NANTONG TAITONG CHEM TECH
Loxoprofen sodium framework tablet
InactiveCN101342147AReduce releaseImprove securityOrganic active ingredientsAntipyreticSide effectActive component
The invention belongs to the technical field of medicines, particularly relates to a composition containing loxoprofen sodium, and more particularly, the invention relates to a loxoprofen sodium matrix tablet. The loxoprofen sodium matrix tablet is composed of loxoprofen sodium of an active component, hydroxypropyl methylcellulose (HPMC), ethyl cellulose (EC) and acrylics of polymer materials and filling agents. The loxoprofen sodium matrix tablet of the invention has longer action time than a common tablet and reduces the side effects. The invention has simple operation process, low cost and easy control and is suitable for industry large scale production.
Owner:FUDAN UNIV
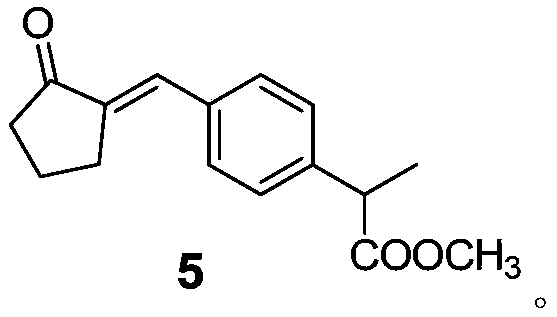
Method for preparing loxoprofen sodium
ActiveCN106699559AHigh purityStrong industrial operationOrganic compound preparationCarboxylic acid esters preparationOrganic synthesisCombinatorial chemistry
The invention relates to the technical field of organic synthesis, in particular to a method for preparing loxoprofen sodium. The invention provides a compound with the structure shown in formula 5 and a preparation method and application of the compound, (the formula is defined in the description). The loxoprofen sodium obtained according to the scheme is high in purity, high in industrialized operation, and good in application prospect.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Drug composition containing loxoprofen sodium and preparation method of drug composition
InactiveCN105380929AEvenly dispersedImprove solubilityOrganic active ingredientsAntipyreticAdhesive cementCurative effect
The invention relates to a drug composition containing loxoprofen sodium and a preparation method of the drug composition. A loxoprofen sodium patch is successively formed by an antisticking film, a drug layer smeared on the antisticking film and a substrate layer covering the drug layer; and the drug layer is prepared from the following components: loxoprofen sodium, a skeleton material, an adhesive, a transdermal enhancers a skeleton material solvent, and the weight ratio of the loxoprofen sodium to the skeleton material is 1 to (1 to 2). When the drug layer is prepared, adhesive cement is homogenized by adopting a homogenizing machine, so that various components are more uniformly dispersed, the uniformity of the drug release speed is guaranteed, and the curative effect is relatively good.
Owner:蚌埠丰原涂山制药有限公司
Loxoprofen sodium coating agent and preparation method thereof
InactiveCN105106180AGood biocompatibilityGood film formingOrganic active ingredientsAntipyreticDiseaseIrritation
The invention discloses a loxoprofen sodium coating agent smeared on the surface of skin, relieve and cure rheumatoid arthritis, osteoarthritis, lumbago, scapulohumeral periarthritis, and diseases of shoulders, necks and wrists, and a preparation method of the loxoprofen sodium coating agent. The loxoprofen sodium coating agent comprises chitosan and glycerol, wherein chitosan is a deacetylation product of crustaceans, has favorable biocompatibility, film-forming property, bacterium resistance, inflammation resistance and hemostasis, and serves as a favorable auxiliary material for preparation of gelata; glycerol is taken as a plasticizer and humectant, and used for enabling a film to be high in elasticity and low in fracture possibility, and enhancing the percutaneous absorption of medicine; the coating agent made of glycerol and loxoprofen sodium can be used for overcoming the first pass effect of a liver and gastrointestinal tract effect, improving bioavailability and enhancing curative effect through local skin application. The coating agent has the advantages that the effect taking period is short; the effect is durable; the spreadability and the adhesive force are high; skin irritation is avoided; the drug delivery is convenient. The preparation method is relatively reasonable; the specificity of a content determination method is high; the result is accurate; the standards can be used for controlling the quality of the coating agent.
Owner:CHENGDU AIBIKE BIOTECH
Medicine composition soft capsule and its preparing process
InactiveCN1857233AEvenly dispersedDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineActive component
The present invention relates to medicine composition soft capsule with loxoprofen sodium as active component and its preparation process. The soft capsule is prepared through preparing the inclusion with the mixture of loxoprofen sodium and several of diluent, co-solvent, solubilizer, suspending agent and surfactant; and the subsequent preparing soft capsule. The soft capsule has high stability of loxoprofen sodium, raised medicine leaching quantity, high bioavailability, high safety and high effectiveness.
Owner:宛六一 +1
Loxoprofen sodium sustained-release pellet
ActiveCN105769773AInhibition of burst releaseGood sustained release propertiesOrganic active ingredientsAntipyreticSustained release pelletsBlood concentration
The invention provides a loxoprofen sodium sustained-release pellet. The pellet comprises a drug-loaded pellet core, an isolating layer coating the drug-loaded pellet core and a sustained-release layer coating the isolating layer, wherein the weight ratio of the drug-loaded pellet core to the isolating layer to the sustained-release layer is 80:(1-15):(2-20). The loxoprofen sodium sustained-release pellet shows an excellent release characteristic in vitro, the frequency of administration can be reduced, the medication compliance of patients can be improved, the blood concentration can be stabilized, and toxic and side effects of drugs on the stomach and intestines can be reduced. The invention further discloses a method for preparing the loxoprofen sodium sustained-release pellet. The method is simple in process, and is easy to industrially produce.
Owner:CHINA PHARM UNIV
Method for preparing loxoprofen active metabolite
ActiveCN106045842ABeneficial technical effectBeneficial progressCarbamic acid derivatives preparationOrganic compound preparationCompound aPropanoic acid
The invention discloses a method for compounding a trans-hydroxyl active metabolite of loxoprofen. The method comprises the following steps: taking 2-[p(bromomethyl)phenyl]propionic acid as a raw material and carrying out resolution and methyl esterification, thus obtaining an intermediate, namely, a compound as shown in a formula 3; preparing a chiral assistant, namely, a compound as shown in a formula 7, by starting from L-phenylalaninol; firstly forming Schiff base as shown in a formula 9 by cyclopentanone and the chiral assistant, namely, the compound as shown in the formula 7, and then condensing the Schiff base as shown in the formula 9 and the intermediate, namely, the compound as shown in the formula 3, into an intermediate, namely, a compound as shown in a formula 11; carrying out acidic hydrolysis on the intermediate, namely, the compound as shown in the formula 11, and perfroming stereoselective reduction on cyclopentanone carbonyl groups, thus obtaining the trans-hydroxyl active metabolite, namely, a compound as shown in a formula TM. In a compounding path, raw materials can be easily obtained, the operation is convenient, environmental friendliness is realized, a separation means of column chromatography is prevented from being used, and the technical requirements of industrial large-scale production can be completely met.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD
Preparation method of loxoprofen sodium ring opened impurity
ActiveCN107353195ASimple processSimple and fast operationOrganic compound preparationCarboxylic preparation by oxidationOrganic solventTetramine
The invention relates to a preparation method of a loxoprofen sodium ring opened impurity, and belongs to the technical field of bulk drug preparation. The preparation method comprises the following steps: step one, carrying out substitution reactions between a compound represented by a formula I and hexamethylene tetramine in an organic solvent A, hydrolyzing the reaction product by inorganic acids to obtain a compound represented by a formula II; carrying out condensation reactions between the compound represented by the formula II and a compound represented by a formula III in an organic solvent B to obtain a compound represented by a formula IV; carrying out oxidation and ring opening reactions of the compound represented by the formula IV under the effect of an oxidant and inorganic alkalis to obtain the loxoprofen sodium ring opened impurity represented by a formula V. The invention provides a preparation method of the loxoprofen sodium ring opened impurity.
Owner:迪嘉药业集团股份有限公司
Supported solid catalyst and preparation method and application thereof
ActiveCN105214692AThe preparation process is simple and reliableReduce manufacturing costOrganic compound preparationCarboxylic acid esters preparationPropionateIndustrial waste water
The invention relates to a supported solid catalyst and a preparation method and application thereof. The chemical formula of the catalyst is MxOy-SO42- / TiO2-Al2O3-SiO2, wherein M is metal ions, x and y are valence state balance numbers, and carriers are TiO2-Al2O3-SiO2. The catalyst is prepared in a step-by-step impregnation method, and nitrate or hydrochloride or ammonium persulfate is sequentially loaded to the carriers TiO2-Al2O3-SiO2 so that the catalyst can be prepared. The catalyst is particularly suitable for being used as a catalyst in the technology of synthesizing loxoprofen intermediate 2-(4'-bro-methyl phenyl) propionate and has the advantages that selectivity is high, the conversation rate is high, reaction conditions are moderate, separation of the catalyst is easy, industrial waste water is little, and industrial production is facilitated.
Owner:HUBEI XUNDA PHARMA
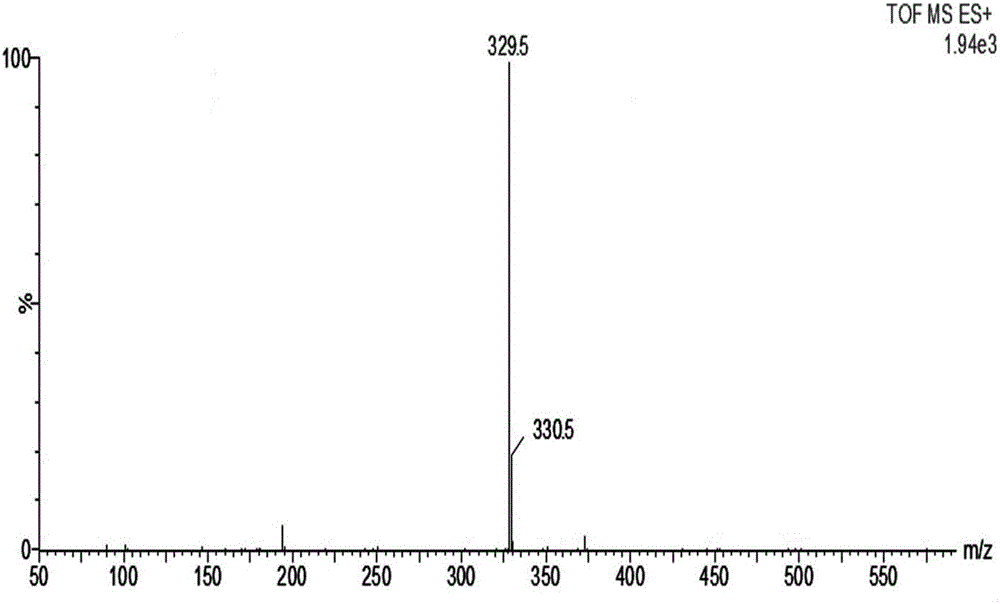
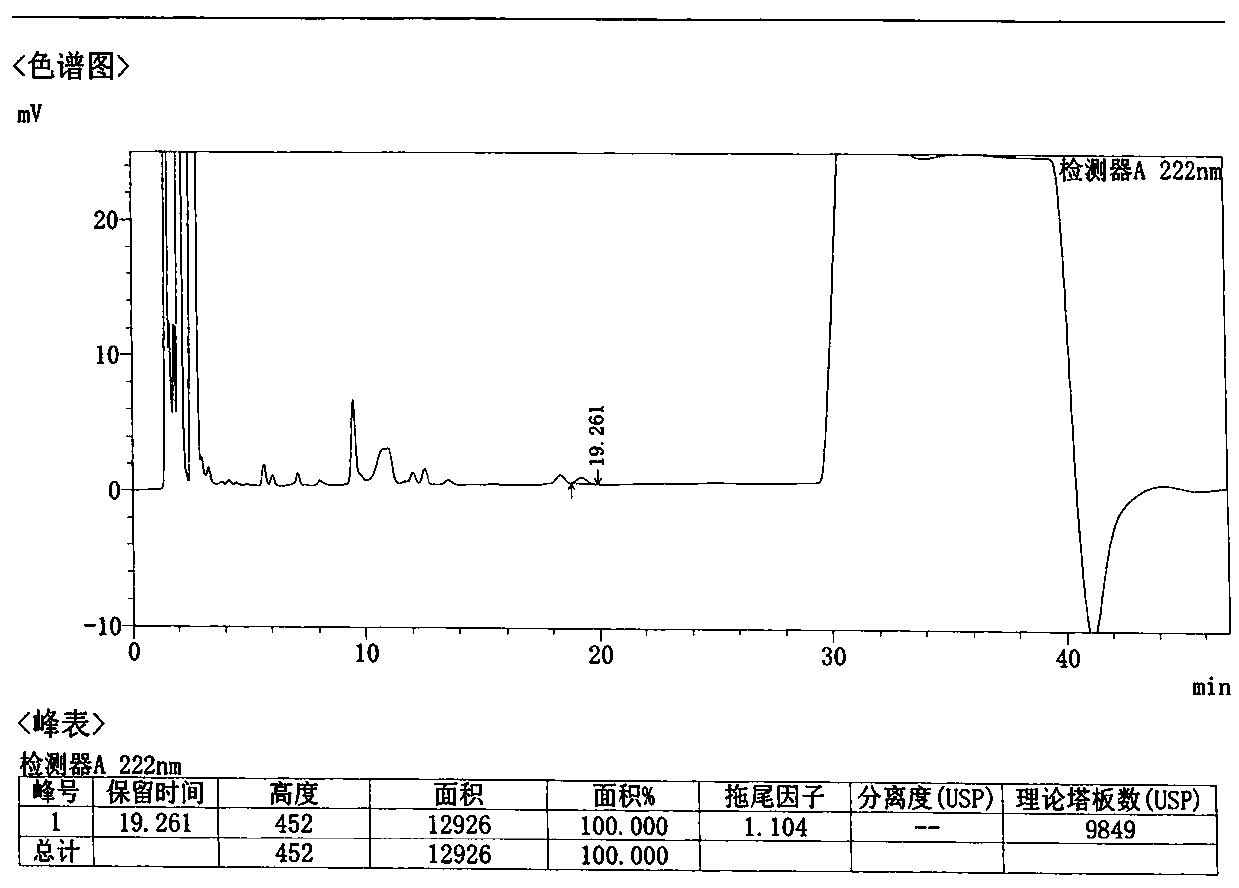
Method for detecting related substances in Loxoprofen or sodium salt thereof
The invention discloses a method for detecting related substances in Loxoprofen or sodium salt thereof. The method uses a high performance liquid chromatography, gives unique chromatographic conditions and successfully detects a process impurity in the Loxoprofen sodium salt. According to the method, the impurities in the process are detected by using a reverse chromatography, the peak shape is better, the separation degree between the peak shape and an adjacent chromatographic peak is high, the research and development cost is saved, the experiment difficulty is reduced, and the reproducibility is good.
Owner:HUNAN JIUDIAN PHARMA +1
Method for protecting bamboo chips against mold and moth in bamboo-wood composite floor manufacture process
InactiveCN102490226AConsistent colorImprove aestheticsWood charring/burningWood impregnation detailsPulp and paper industryThermal water
The invention relates to a bamboo-wood composite floor manufacture process, in particular to a method for protecting bamboo chips against mold and moth in the bamboo-wood composite floor manufacture process. The invention is realized through the technical scheme that the method for protecting bamboo chips against mold and moth in the bamboo-wood composite floor manufacture process comprises the following steps: (1) bamboo chips are placed in a hot water storage tank at a temperature between 60 DEG C and 70 DEG C, and stand for 5-8 min; (2) hydrogen peroxide with 30% concentration and loxoprofen sodium are added to the hot water storage tank, the weight ratio of hydrogen peroxide with 30% concentration to the bamboo chips is 1:12.5-20, the weight ratio of loxoprofen sodium to the bamboo chips is 1:100-120, and the bamboo chips, hydrogen peroxide and loxoprofen sodium are stirred for 2-3min; and (3) steam is used to heat water in the tank to increase the temperature of the water to 100 DEG C in 5-10min, and the water is kept in boiling state for 6-8h. The method achieves excellent mold and moth prevention.
Owner:朱江福
Preparation method of substituted phenylacetic acid derivative
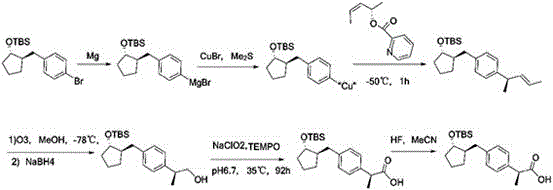
ActiveCN109020808AEasy accessPreparation from carboxylic acid halidesOrganic compound preparationState of artPhenylacetic acid
The invention belongs to the field of drug synthesis and relates to a preparation method of a substituted phenylacetic acid derivative, especially to a preparation method for preparing 2-[4-(2-oxyamylmethyl)phenylpropionic acid]. The preparation method includes a Friedel-Crafts reaction, ring-closure reaction and a coupled reaction which are sequentially exchangeable, and a reduction reaction. Thepreparation method hasn't been enlightened by the prior art and also cannot obtain technical enlightenment from the prior art. The preparation method is suitable for production on a commercial scaleand provides another technical scheme for the industrial production of loxoprofen sodium.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Process for producing 2-substituted propionic acid
Adipic acid diester is caused to react with alkoxide M(OR)n, wherein R is an alkyl group and M is an alkali metal or alkaline earth metal, the reaction product is successively subjected either to coupling with halomethylstyrene followed by carbonylation, or to coupling with 2-(halomethylphenyl)propionic acid or its ester followed by decarboxylation and hydrolysis. With this process, it is possible to produce more efficiently a specific 2-substituted propionic acid, loxoprofen.
Owner:NIPPON PETROCHEMICAL CO LTD
Positively charged water-soluble prodrugs of aryl- and heteroarylpropionic acids with very fast skin penetration rate
ActiveCN101506161AGood absorption rateImprove solubilitySenses disorderNervous disorderSolubilityPhosphate
Owner:TECHFIELDS BIOCHEM CO LTD
Loxoprofen sodium matrix sustained-release tablet
ActiveCN102525989AIncreased diffusion resistanceReduced Diffusion ResistanceOrganic active ingredientsAntipyreticSustained Release TabletLoxoprofen
The invention relates to a novel matrix sustained-release tablet containing loxoprofen sodium, in particular to a locoprofen sodium matrix sustained-release tablet with a regulation layer, which is capable of stably releasing for 8 hours, good in releasing effect, simple in process and low in cost.
Owner:北京天衡药物研究院有限公司
Loxoprofen sodium composition
ActiveCN102670531AWell mixedReduce viscosityOrganic active ingredientsAntipyreticDissolutionMoisture absorption
The invention relates to a loxoprofen sodium composition. Due to the high viscosity of loxoprofen sodium, on one hand, the dispersible tablet of the loxoprofen sodium is easy to clot after being disintegrated, so that the dispersible tablet cannot completely pass through a 2# sieve easily; and on the other hand, the dispersible tablet is easy to absorb moisture, and after the moisture absorption, the tablet is fluffy, the content of the main medicament is decreased, and particularly, the dissolution rate is decreased obviously. According to the loxoprofen sodium composition, a preparation process is changed, and silicon dioxide in the formula is added by an interior addition, so that the composition with high dispersible uniformity and stability is obtained.
Owner:DISHA PHARMA GRP
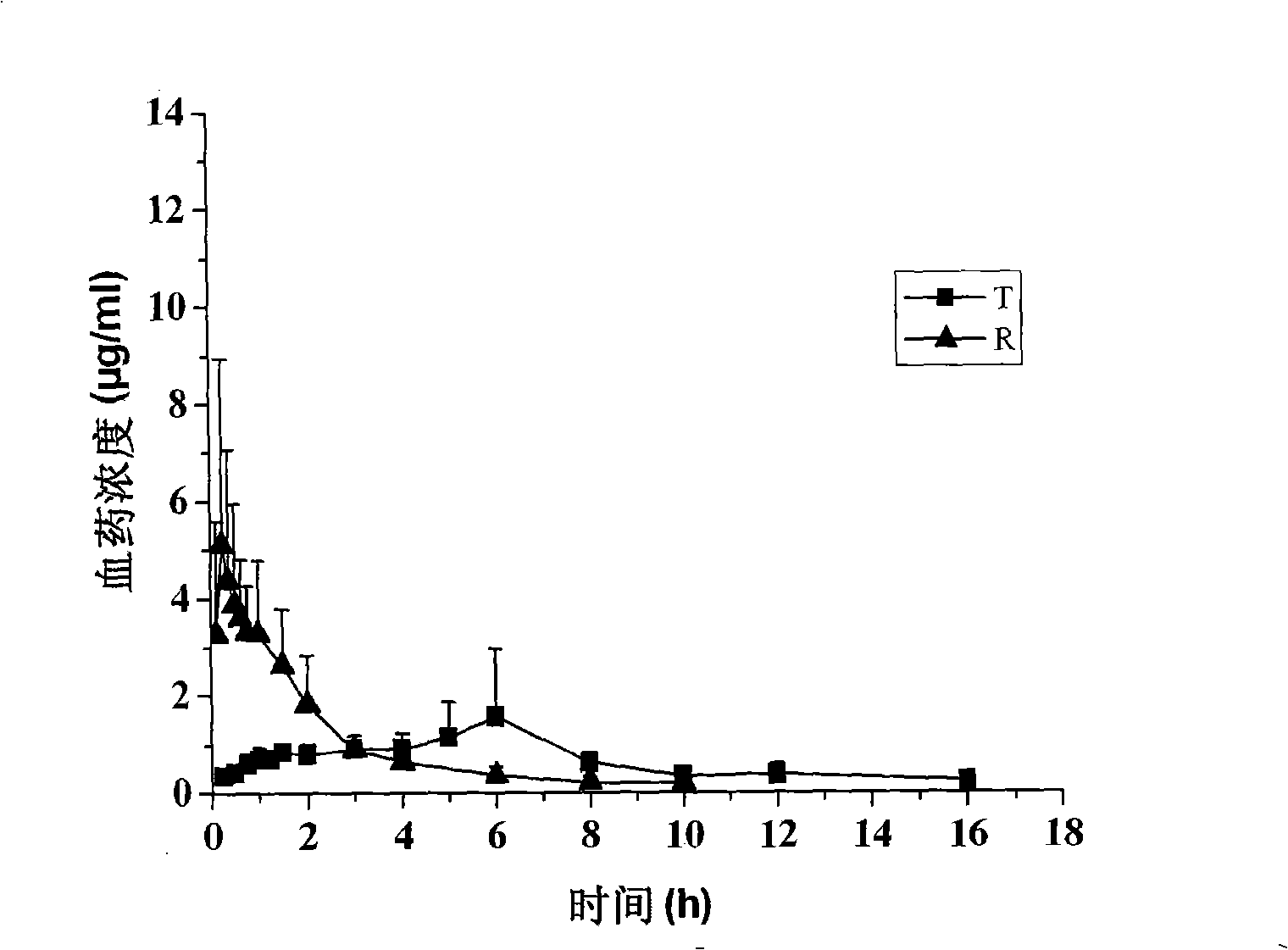
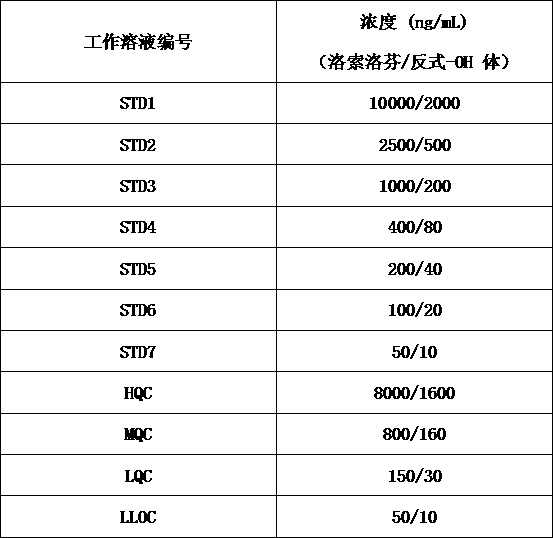
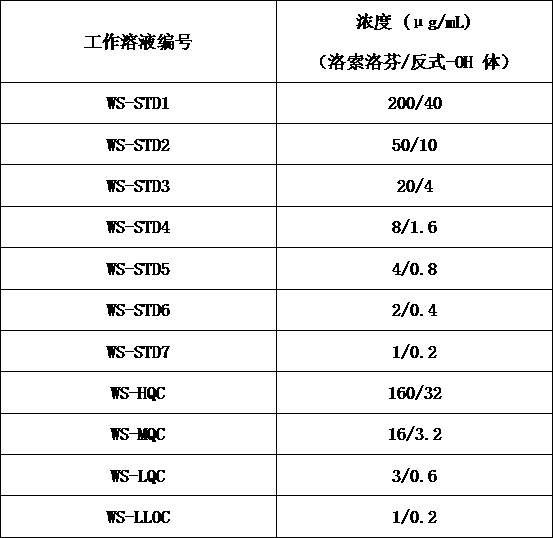
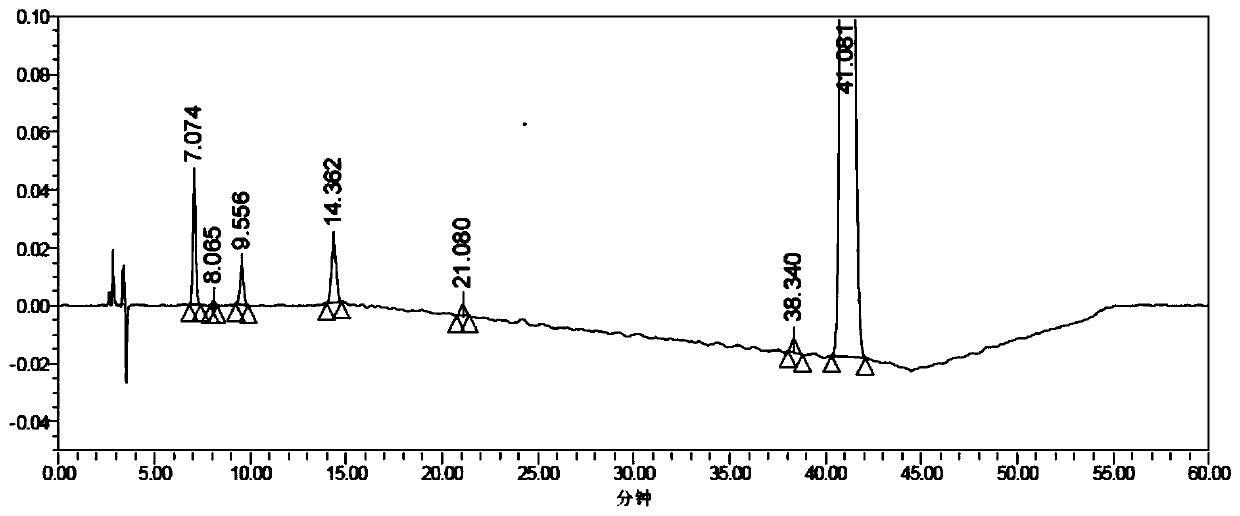
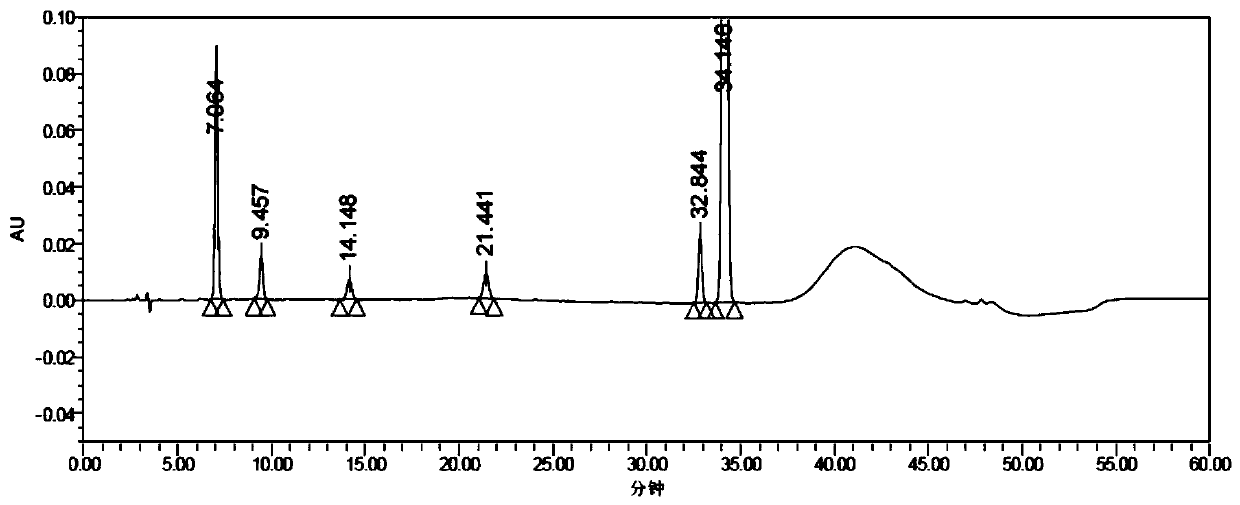
Method for determining concentration of loxoprofen and trans-hydroxyl matrix thereof in plasma
The invention discloses a method for determining concentration of loxoprofen and trans-hydroxyl matrix thereof in plasma, which includes the steps pf preparation of reserve solution, preparation of working solution, preparation of standard curve and quality control sample, sample pretreatment method and LC-MS / MS analysis.The method establishes a simple, rapid, durable, high-sensitivity and good-selectivity LC-MS / MS method for simultaneously determining concentration of loxoprofen and the active metabolite trans-OH thereof in EDTA-K2 anticoagulated human plasma, which can be used for simultaneous quantitative analysis of loxoprofen and trans-OH loxoprofen in EDTA-K2 anticoagulated human plasma. Compared with fluorescence method and ultraviolet detection method, the method provided by the invention is more sensitive, whereby the quantitative lower limit of loxoprofen is reduced from 0.31 mg / mL and 0.20 mg / mL to 0.050 mg / mL, and the quantitative lower limit of the trans-OH loxoprofen is reduced to from 0.31 mg / mL and 0.20 mg / mL 0.010 mg / mL, and meanwhile, the analysis time is greatly shortened from 25 min and 30 min to 3.5 min.
Owner:武汉伯瑞恒医药科技有限公司
Method for separating and measuring related substances contained in loxoprofen Acid and salt thereof
ActiveCN109725074AQuality improvementRealize quality controlComponent separationChromatography columnLoxoprofen
The invention provides a method for separating and measuring related substances contained in loxoprofen acid and salt thereof. The method comprises the steps of: injecting loxoprofen Acid and salt thereof samples into a liquid chromatograph for separation and detection, wherein the chromatographic column of the liquid chromatograph is a C8-C18 column, and the mobile phase is an aqueous phase-organic phase mixed in a certain ratio. The method can realize quantitative and qualitative measure of the related substances contained in loxoprofen Acid and salts thereof, thereby the quality of loxoprofen Acid and salt thereof is effectively controlled, and more effective quality monitoring of loxoprofen Acid and salt product thereof is realized. According to the method of the invention, there is practical guiding significance on improving the quality of related products. In addition, the method is strong in specificity, high in accuracy, convenient to operate, and worthy of being widely popularized and applied.
Owner:康美(北京)药物研究院有限公司 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com