Preparation method for 2-(4-brooethylphenyl) propionic acid
A technology of bromomethylphenyl and tolyl propionic acid is applied in the field of preparation of 2-(4-bromomethylphenyl) propionic acid, and can solve the problems of complex production process, high production cost, low yield of finished products and the like , to achieve the effect of simple production process, low production cost and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
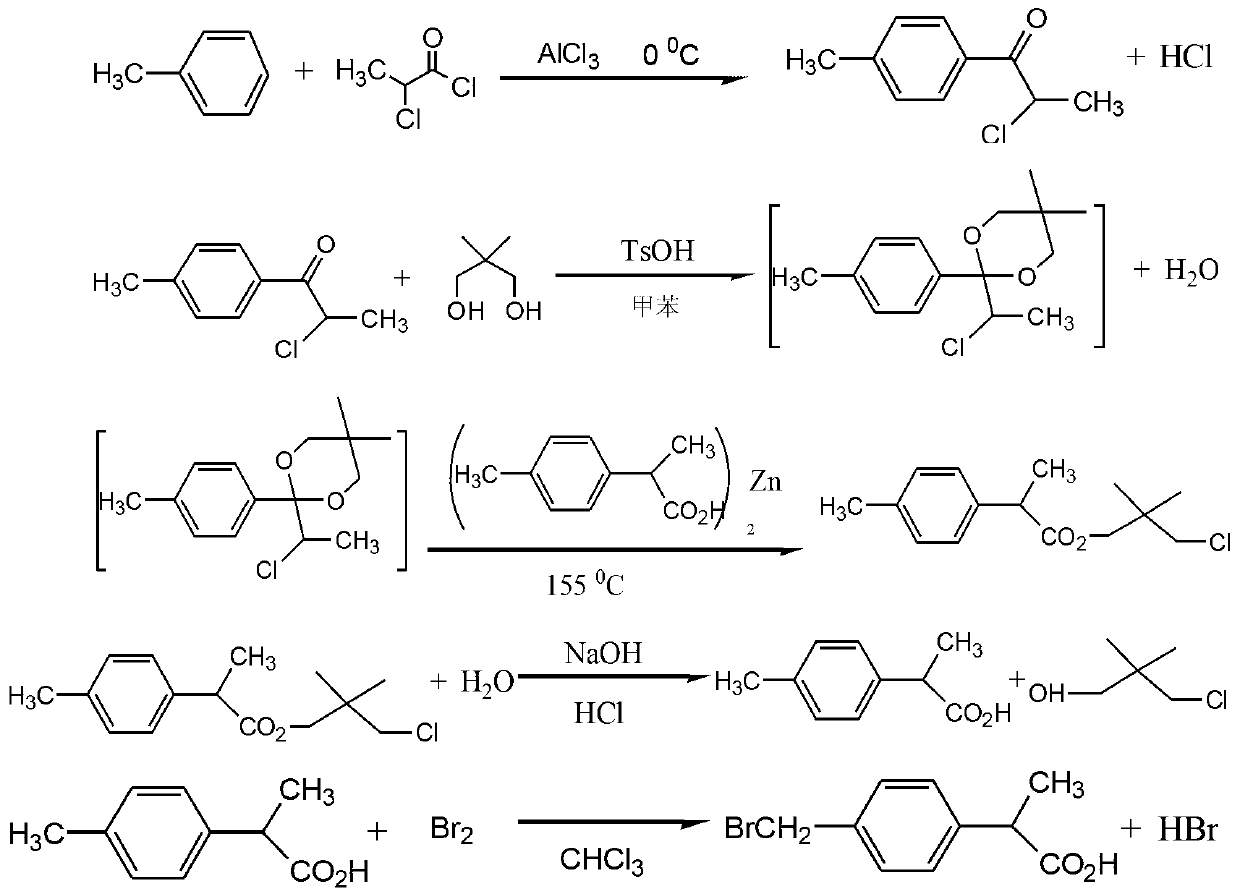
[0016] The preparation method of 2-(4-bromomethylphenyl)propionic acid, including p-tolyl-2-chloro-1-one preparation stage, 2-p-tolylpropionic acid preparation stage and 2-(4-bromomethyl Phenyl)propionic acid stage, the specific steps are as follows:
[0017] (1), p-tolyl-2-chloro-1-one preparation stage
[0018] Add anhydrous aluminum trichloride:anhydrous toluene at a molar ratio of 1:1 to a dry acylation reaction kettle, cool to 0°C, and slowly add 2-chloropropionyl chloride in a molar ratio of 1 dropwise under stirring, dropwise The completion time is 2 hours, and the reaction is completed at a temperature of 0°C for 8 hours; put the hydrolysis kettle into ice water and stir slowly, put the reactants into the hydrolysis kettle, control the hydrolysis temperature at 1°C when discharging, and continue stirring for 30 minutes after discharging , and then static layering, the lower aluminum chloride aqueous solution is separated, the organic layer is washed with sodium chlori...
Embodiment 2
[0025] The preparation method of 2-(4-bromomethylphenyl)propionic acid, including p-tolyl-2-chloro-1-one preparation stage, 2-p-tolylpropionic acid preparation stage and 2-(4-bromomethyl Phenyl)propionic acid stage, the specific steps are as follows:
[0026] (1), p-tolyl-2-chloro-1-one preparation stage
[0027] Add anhydrous aluminum trichloride:anhydrous toluene at a molar ratio of 10:20 to the dry acylation reaction kettle, cool to 3°C, and slowly add 2-chloropropionyl chloride in a molar ratio of 5 under stirring, dropwise The completion time is 2.5 hours, and the reaction is completed at a temperature of 3°C for 8 hours; put the hydrolysis kettle into ice water and stir slowly, put the reactants into the hydrolysis kettle, control the hydrolysis temperature at 3°C when discharging, and continue stirring for 30 minutes after discharging , and then static layering, the lower aluminum chloride aqueous solution is separated, the organic layer is washed with sodium chlorid...
Embodiment 3
[0033] The preparation method of 2-(4-bromomethylphenyl)propionic acid is characterized in that it comprises p-tolyl-2-chloro-1-one preparation stage, 2-p-tolylpropionic acid preparation stage and 2-(4 -bromomethylphenyl) propionic acid stage, the concrete steps are as follows:
[0034] (1), p-tolyl-2-chloro-1-one preparation stage
[0035] Add anhydrous aluminum trichloride:anhydrous toluene at a molar ratio of 20:30 to a dry acylation reaction kettle, cool to 5°C, and slowly add 2-chloropropionyl chloride at a molar ratio of 10 under stirring, dropwise The completion time is 3 hours, and the reaction is completed at a temperature of 5°C for 8 hours; put the hydrolysis kettle into ice water and stir slowly, put the reactants into the hydrolysis kettle, control the hydrolysis temperature at 5°C when discharging, and continue stirring for 30 minutes after discharging , and then static layering, the lower aluminum chloride aqueous solution is separated, the organic layer is was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com