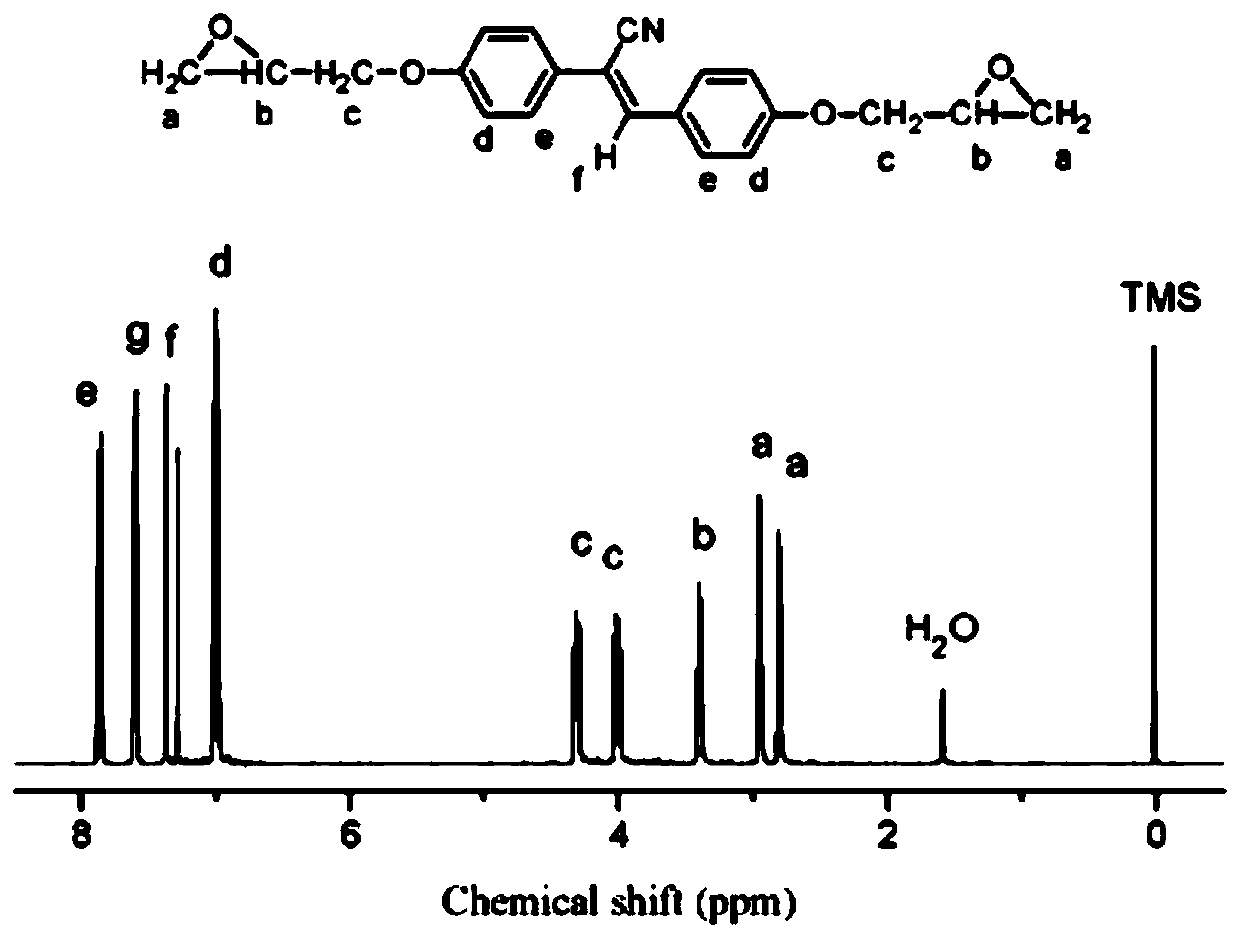
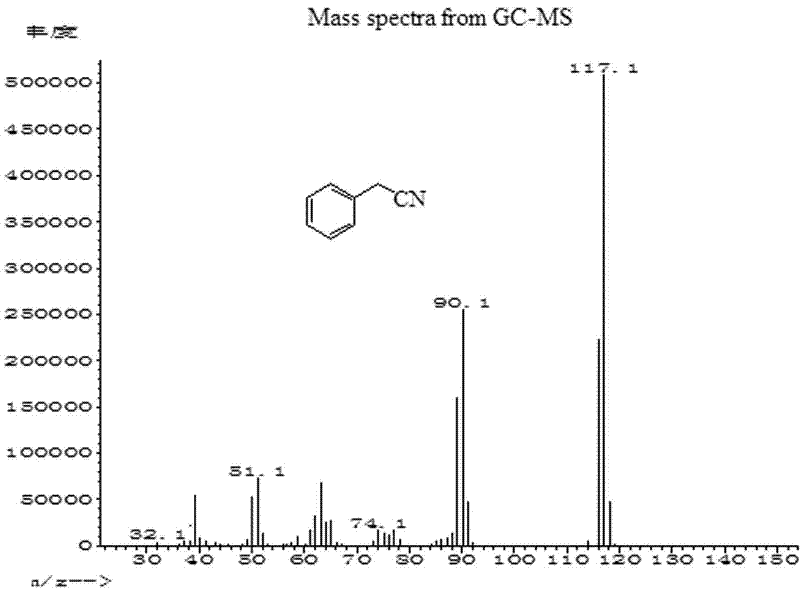
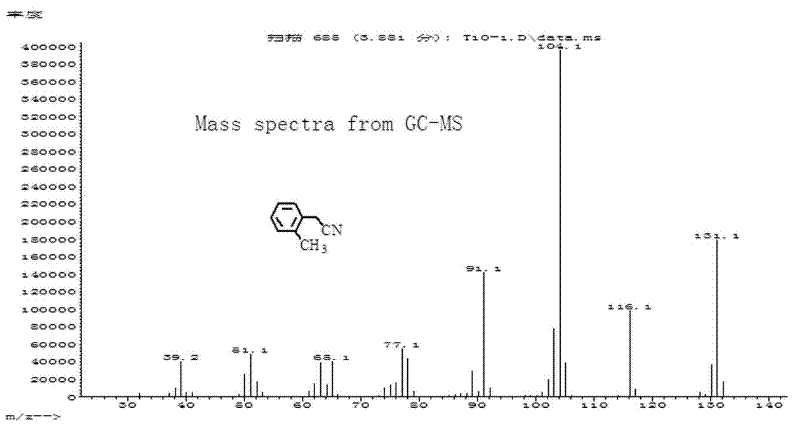
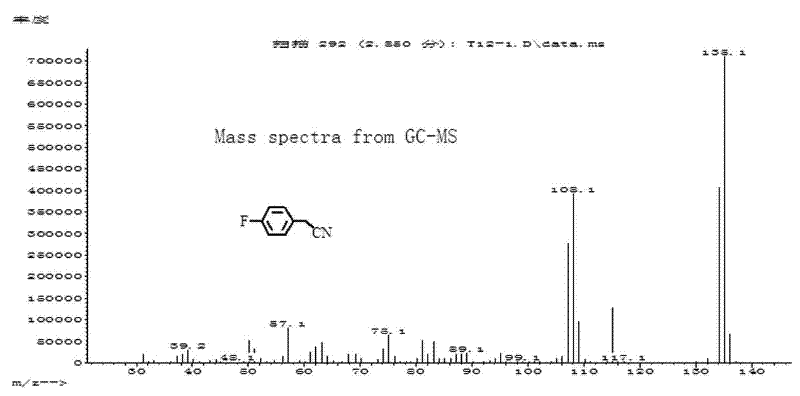
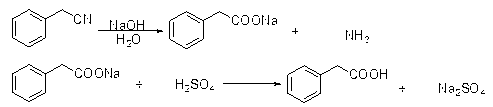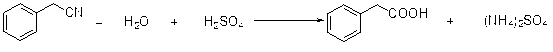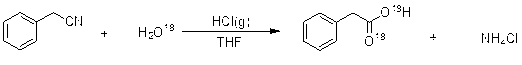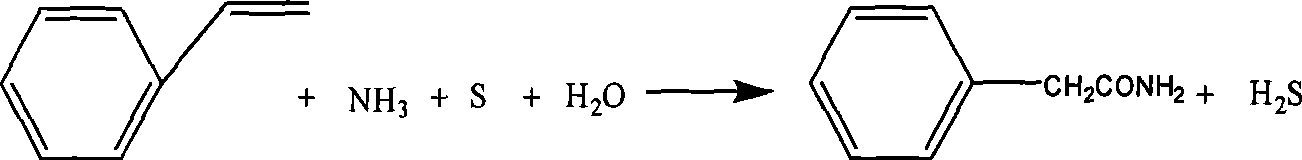Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
194 results about "Benzyl cyanide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C₆H₅CH₂CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.
Vinyl cyanide compounds, preparation and application thereof
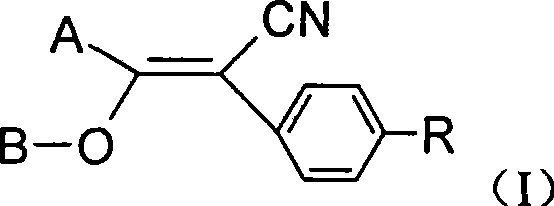
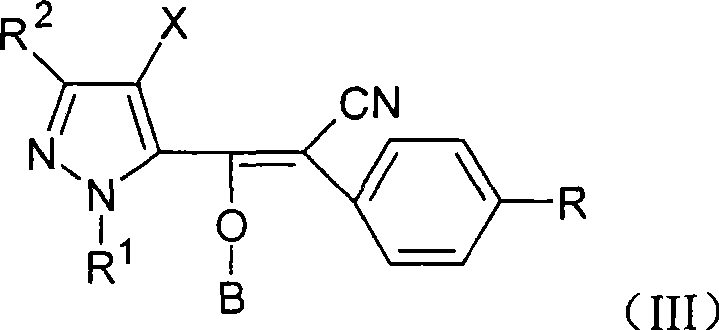
The present invention discloses an acrylonitrile compound, a preparation method thereof, and a pest prevention and curing agent with the compound as an active constituent. The compound has structural characteristics as shown in Formula (I). In the preparation method, under the condition with alkaline, an organic solvent at the temperature between minus 10 DEG C and 150 DEG C is used for the reaction between benzofuran acyl chloride or pyrazole acyl chloride and substituted benzyl cyanide, so as to produce an intermediate, the produced intermediate reacts with acyl chloride, and the (I) can be separated. The compound can be used as an active constituent in a pest prevention and curing agent. The present invention has the advantages that the compound has excellent prevention and curing effects on harmful insects and acarus category, the activity of the compound is high, the consumption is low and the security is excellent.
Owner:JIANGSU PESTICIDE RES INST
Method for machining flower-fragrance black tea in low-temperature compounding mode
ActiveCN103749747AAvoid damageControllable weight loss ratePre-extraction tea treatmentNerolidolBlack tea
The invention relates to a method for machining tea, and in particular relates to a method for machining flower-fragrance black tea in a low-temperature compounding mode. The method comprises the following steps: spreading to cool high-fragrance type oolong tea with two to three fresh leaves on one bud as a raw material, finely manipulating the green tea leaves, kneading, thinly spreading, fermenting at high temperature and low humidity, and drying so as to obtain coarse tea; appropriately selecting to remove peduncles and pieces, highlighting the fragrance and thus accomplishing the machining. According to the flower-fragrance black tea machined by using the method, the weight loss of the green leaves in the machining process can be controlled, and the machining time is shortened, particularly under poor weather conditions; fragrant substances such as flower and fruit fragrance of alcohols, alkenes, esters and ketones are added, and the content of benzyl cyanide with pungent odor, indole and main fragrant substances, namely, nerolidol, in oolong tea, are increased, so that the fragrance and the taste quality of the tea are improved, and then the quality of the black tea is improved. Sensory evaluation on the finished tea shows that the finished tea is fat, strong, compact, black and tender in appearance, tea tips are visible, the fragrance is strong and heavy, the fragrance of flower and fruit appears, the taste is fresh and mellow, the bottom of a cup has the flower fragrance, and the quality is superior to that of black tea manufactured by using the conventional method.
Owner:TEA RES INST OF FUJIAN ACADEMY OF AGRI SCI +1
Method for preparing loxoprofen intermediate
InactiveCN105753685AAvoid bringing inHigh selectivityCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidPropionitrile
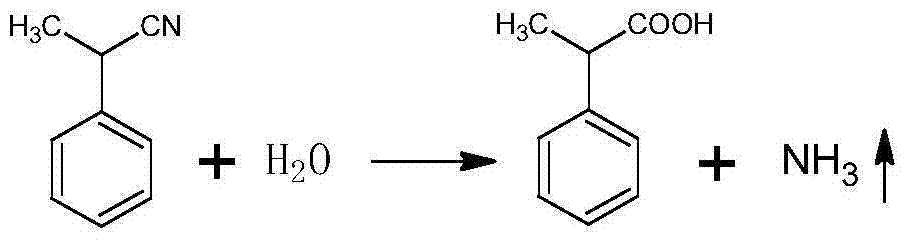
A method for preparing a loxoprofen intermediate comprises the following steps that 1, on the presence of sodium alkoxide, benzyl cyanide and dimethyl carbonate are subjected to methylation in an organic solvent, and 2-(phenyl cyano) sodium propionate is obtained; 2, 2-(phenyl cyano) sodium propionate and dimethyl sulfate react in an organic solvent to obtain 2-(phenyl cyano) methyl propionate; 4, 2-(phenyl cyano) methyl propionate reacts under the alkaline condition to obtain 2-phenyl propionitrile; 4, 2-phenyl propionitrile is hydrolyzed under the alkaline condition, acid is added for acidizing after the reaction to obtain 2-phenylpropionic acid; 5, 2-phenylpropionic acid, hydrobromic acid and paraformaldehyde are mixed and subjected to a bromine methylation reaction under the acidic condition, and 2-(4-tribromomethyl phenyl) propionic acid is obtained.According to the method, a new synthesis route is designed, product selectivity is good, the purity is high, the conversion rate is high, and few by-products are generated; the raw materials are simple and easy to obtain, the production conditions are mild, the process is simple, production cost is low, and pollution is small.
Owner:UPCHEM CHINA
Preparation method of 2-(4-bromomethylphenyl) propionic acid
ActiveCN104744237AHigh purityHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidMethyl carbonate
The invention discloses a preparation method of 2-(4-bromomethylphenyl) propionic acid. The preparation method is characterized by comprising a preparation stage of 2-phenyl propionitrile, a preparation stage of 2-phenyl propionic acid and a preparation stage of 2-(4-bromomethylphenyl) propionic acid. The preparation stage of 2-phenyl propionitrile comprises the following steps: mixing benzyl cyanide, dimethyl carbonate and potassium carbonate; heating to 100-300 DEG C and carrying out thermal reaction for 5-50h under the pressure of 0.5-6MPa; and by the end of the reaction, carrying out filter pressing and rinsing to be neutral; distilling to removal excessive dimethyl carbonate; and carrying out high-vacuum rectification to obtain 2-phenyl propionitrile. Compared with the prior art, the preparation method disclosed by the invention has the following advantages and effects that the main raw material is benzyl cyanide which is subjected to methylation, hydrolysis reaction and bromomethylation to finally obtain the finished product; the purity of the finished product is high and the yield is up to 90% or above; and in addition, the preparation method has the advantages of simple production process, low production cost and low environment pollution.
Owner:ZHEJIANG BOJU NEW MATERIALS CO LTD
4-aroyl-1,8-naphthalimide compound and preparation method and use thereof
InactiveCN104003935AReduce manufacturing costNovel synthetic routeOrganic chemistryMaterial analysis by observing effect on chemical indicatorAcyl groupOrganosolv
The invention discloses a 4-aroyl-1,8-naphthalimide compound and a preparation method and use thereof, wherein the 4-aroyl-1,8-naphthalimide compound has a structural formula as shown in the specification, R1 is C1-C10 straight chain or branched chain alkyl; and R2 is phenyl, naphthyl, biphenylyl, substituted phenyl, quinary or senary heteroaryl or benzo quinary or senary heteroaryl. The preparation method is as follows: a 4 bromo-1,8-naphthalimide compound is used as a raw material to react with substituted phenylacetonitrile or aromatic ring acetonitrile in an organic solvent in the presence of an alkali catalyst to obtain a 4-aryl acetonitrile-1,8-naphthalimide compound, and then the 4-aroyl-1,8-naphthalimide compound is obtained in the effects of fluoride ions or cyanide ions. The 4-aryl acetonitrile-1, 8-naphthalimide compound is used as a color or fluorescence sensor for detection of the fluoride ions or cyanide ions, and has high sensitivity and high selectivity during identifying of the cyanide ions in a mixed solvent.
Owner:SHANGHAI INST OF TECH
Method for preparing 2, 4, 5-trifluoro-phenylacetic-acid
ActiveCN101659611AEasy to separateMeet the requirementsOrganic-compounds/hydrides/coordination-complexes catalystsPreparation from nitrilesAcetic acidPhenylacetic acid
The invention provides a method for preparing 2, 4, 5-trifluoro-phenylacetic-acid. The method adopts 1, 2, 4-trifluoro-benzene to react with polyformaldehyde and chlorinating agent to obtain 2, 4, 5-trifluoro-benzyl chloride, and adopts the 2, 4, 5-trifluoro-benzyl chloride to react with cyaniding agent in ionic liquid to obtain 2, 4, 5-trifluoro-benzyl cyanide, and then the 2, 4, 5-trifluoro-benzyl cyanide is hydrolyzed in acid or alkalic conditions to obtain the 2, 4, 5-trifluoro-phenylacetic-acid. The preparation method for preparing 2, 4, 5-trifluoro-phenylacetic-acid requires cheap and available materials, and has moderate reaction conditions, less three-wastes and better safety; industrialized production can be easily realized, the product purity is high, and quality is stable, thuscompletely meeting the using requirement of 2, 4, 5-trifluoro-phenylacetic-acid as a pharmaceutical intermediate.
Owner:ZHEJIANG YONGTAI TECH CO LTD
Preparation method of baloxavir marboxil intermediate
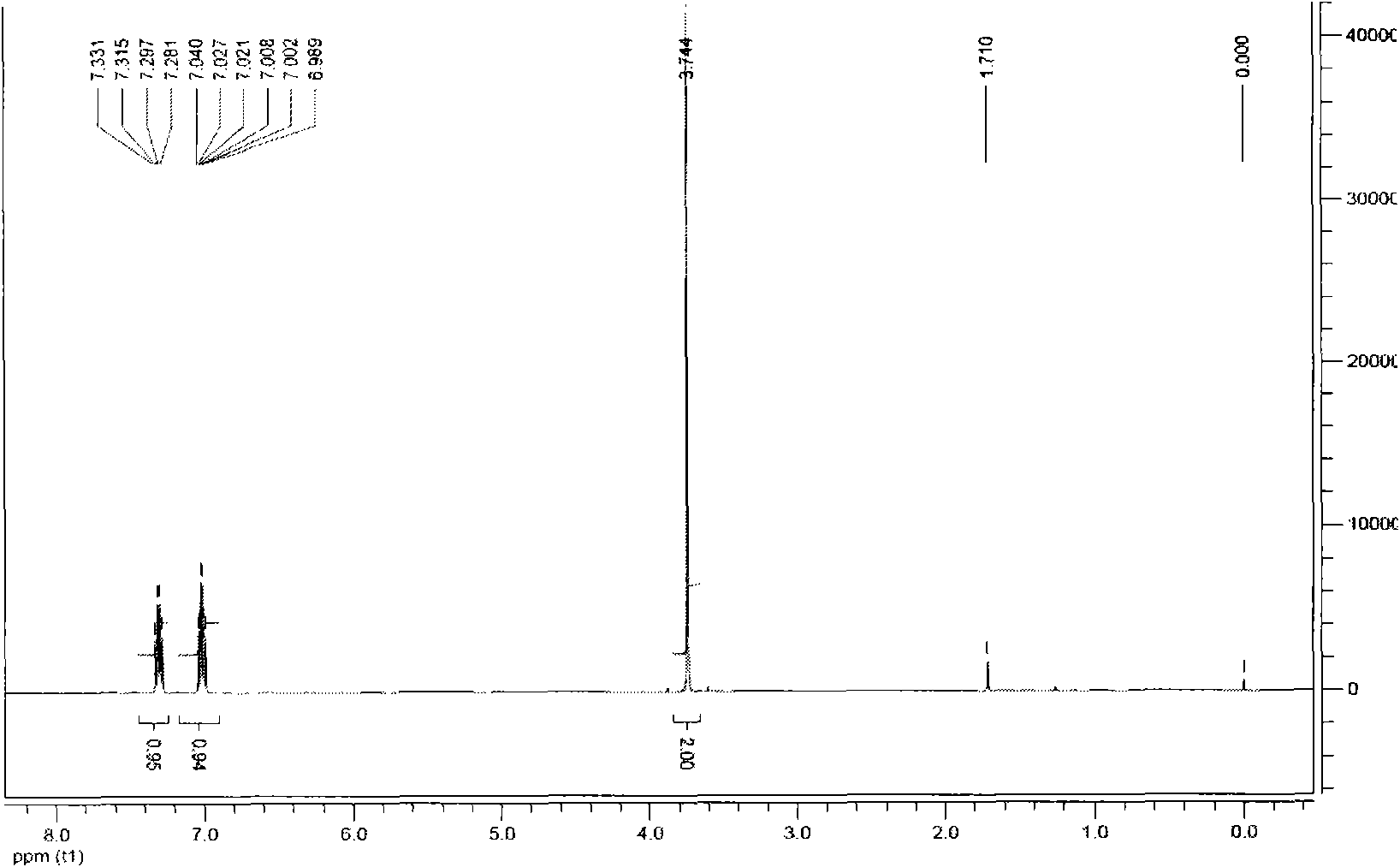
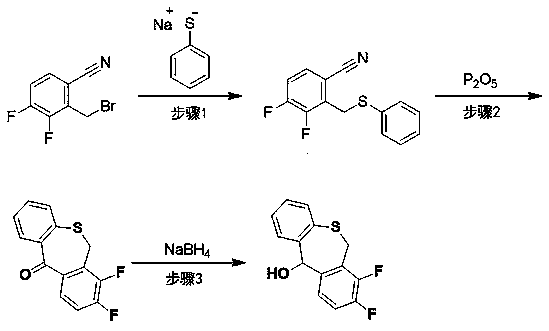
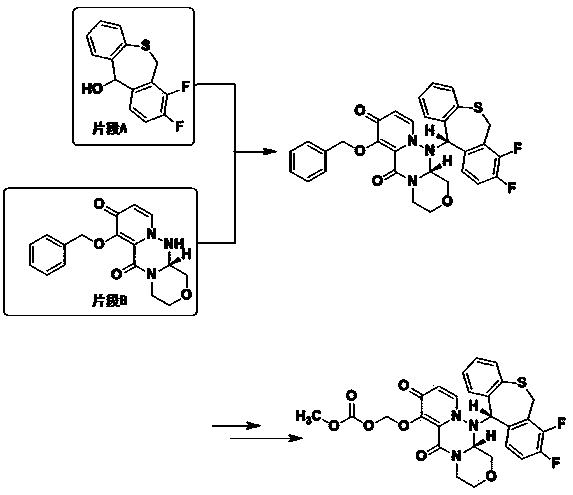
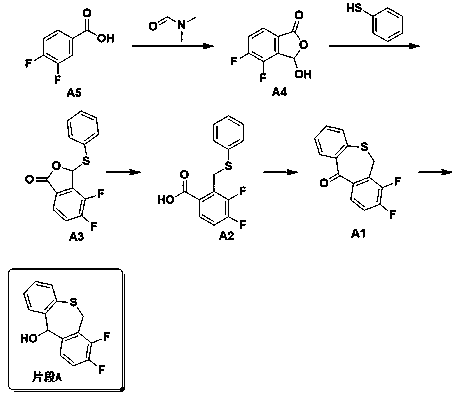
The invention discloses a preparation method of a baloxavir marboxil intermediate. The preparation method is characterized by comprising the following steps: (1) substituting 2-bromomethyl-3,4-difluorophenylacetonitrile through sodium thiophenolate in a solvent A to obtain 3,4-difluoro-2-[(thiophenyl) methyl] benzyl cyanide; (2) reacting the 3,4-difluoro-2-[(thiophenyl) methyl] benzyl cyanide obtained in the step (1) with a cyclizing agent in a solvent B, and carrying out cyclization to obtain 7,8-difluoro dibenzo [b,e] thia heptacyclic-11(6H)-ketone; and (3) reducing the 7,8-difluoro dibenzo[b,e] thia heptacyclic-11(6H)-ketone obtained in the step (2) to obtain 7,8-difluoro dibenzo [b,e] thia heptacyclic-11(6H)-alcohol. The reaction steps in the preparation method are reduced to a six-step reaction from a five-step reaction in the prior art, so that the generation of a side reaction is reduced, and the reaction efficiency is improved. The sodium thiophenolate is used for replacing thiophenol with foul smell and high toxicity, and harm to experimenters during the production is reduced.
Owner:NANJING POLYTECHNIC INSITUTE
Method for separating m-ethyltoluene and p-ethyltoluene by extractive distillation
ActiveCN102795957AImprove separation efficiencyIncrease relative volatilityDistillation purification/separationBenzeneExtractive distillation
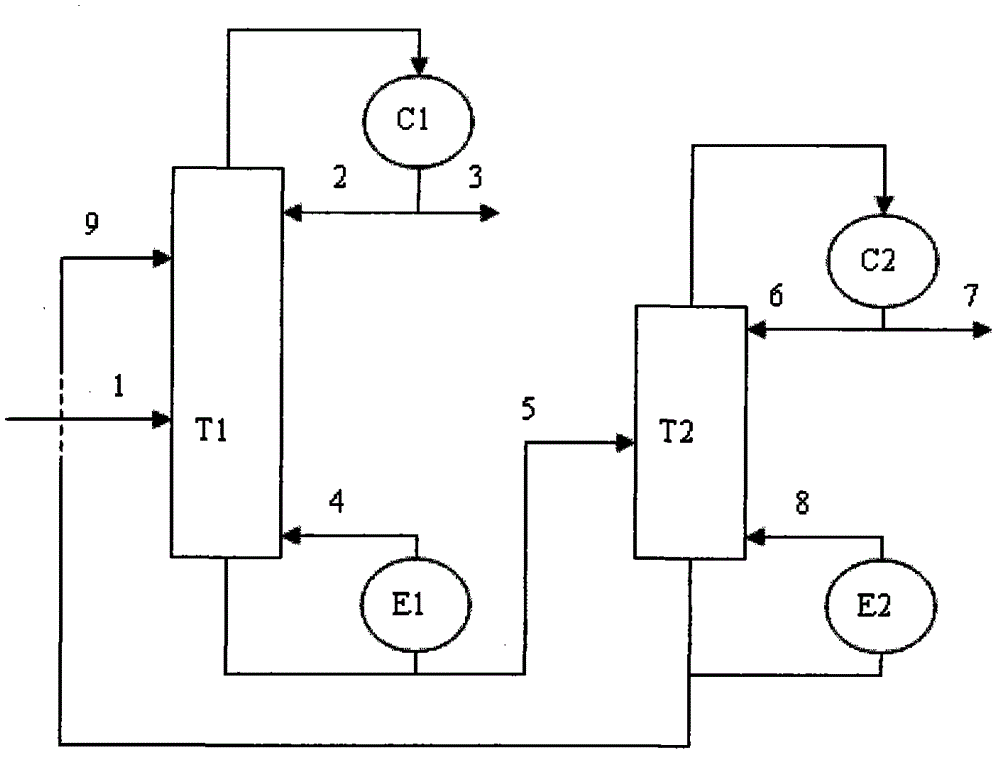
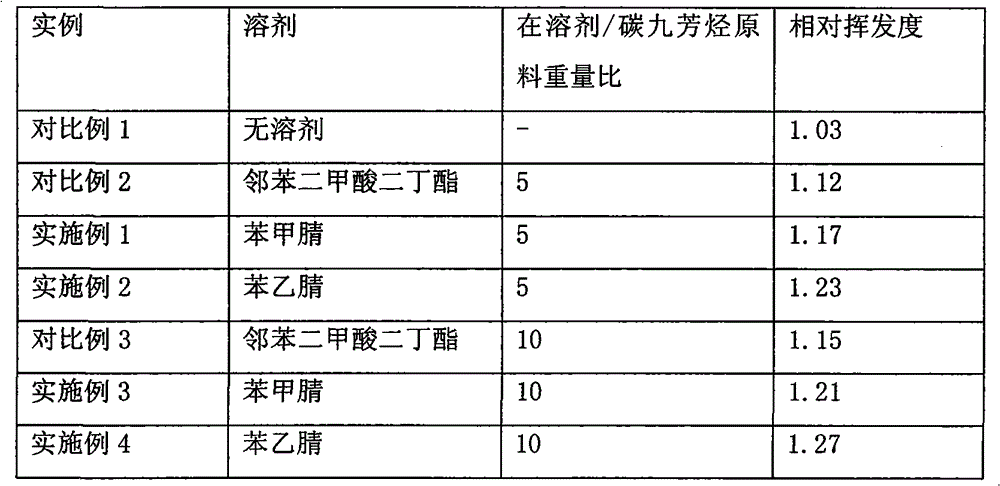
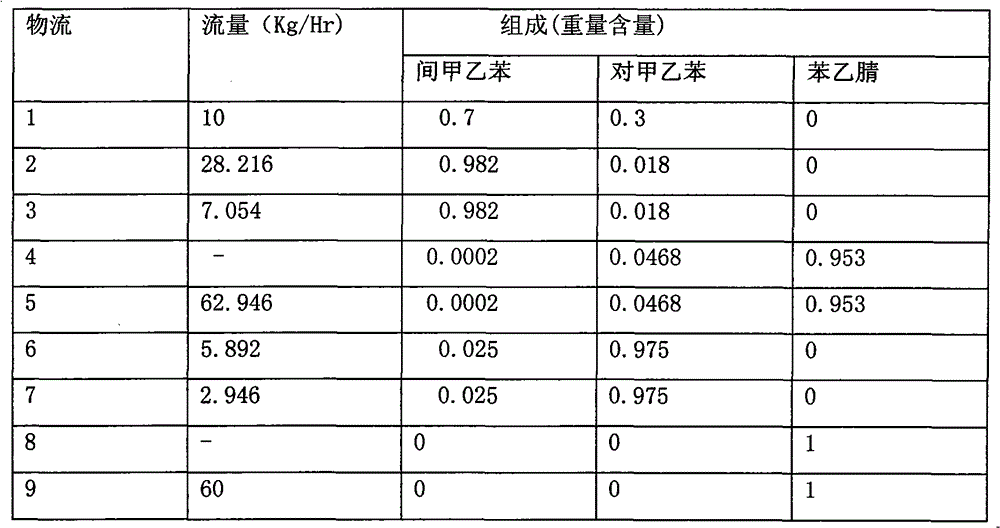
The invention discloses a method for separating m-ethyltoluene and p-ethyltoluene by extractive distillation. The extractive distillation solvent adopted by the method is a nitrogenous compound, preferably cyanobenzene or benzyl cyanide, and more preferably benzyl cyanide. The method comprises the following steps: introducing an m-ethyltoluene / p-ethyltoluene mixture from the middle of the extractive distillation tower, and introducing an extractive distillation solvent from the tower top; and after carrying out extractive distillation, discharging the m-ethyltoluene from the top of the extractive distillation tower, discharging the rich solvent containing rich p-ethyltoluene from the tower bottom, sending the rich solvent into a solvent recovery tower, discharging the p-ethyltoluene from the top of the recovery tower, and recycling the extractive distillation solvent discharged from the bottom of the recovery tower. The invention has the advantages of low operation energy consumption and high product purity; and the solvent adopted by the method can obviously improve the relative volatility between m-ethyltoluene and p-ethyltoluene, is easy to recycle, and has high chemical thermal stability.
Owner:CHINA PETROLEUM & CHEM CORP +1
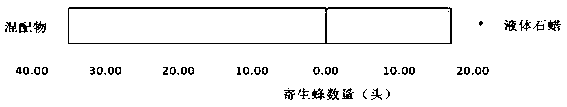
Cotesia plutellae kurdjumov attractant and preparation method thereof
ActiveCN103271034AThere is no problem of long residual period of chemical pesticidesHigh selectivityBiocidePest attractantsBenzaldehydeBenzyl cyanide
The present invention discloses a cotesia plutellae kurdjumov attractant and a preparation method thereof, wherein liquid paraffin is adopted as a solvent, and 100 mul / ml benzaldehyde, farnesene, phenylacetonitrile, cis-3-hexenol, trans-2-hexenal, eucalyptol and nonanal are subjected to the same ratio mixing to obtain the attractant. The attractant is specific component screened from plant volatile substances, and has characteristics of high selectivity and strong targeting property. With the attractant, cotesia plutellae kurdjumov early enters a target prevention and control field, enters a search state, and do not flee so as to enhance host search and attack efficiency, increase parasitism rate, and provide important importance for cotesia plutellae kurdjumov pest controlling effect increase, vegetable field pest management improvement and the like.
Owner:FUJIAN AGRI & FORESTRY UNIV
Preparation method for D, L-phenylglycine and analogue thereof
ActiveCN106380415ASave inorganic saltReduce pollutionCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeBenzyl cyanide
The invention provides a preparation method for D, L-phenylglycine and an analogue thereof. According to the method, benzaldehyde, an analogue thereof and hydrocyanic acid are adopted as raw materials and subjected to cyanidation reaction, and then 2-hydroxy-benzyl cyanide or 2-hydroxy-benzyl cyanide analogue (cyanohydrin for short) is generated. Cyanohydrin reacts with carbon dioxide and the aqueous solution of ammonia, and then 5-phenyl-hydantoin and an analogue thereof (hydantoin for short) are generated. hydantoin is successively subjected to steam stripping, alkaline hydrolysis, steam stripping, decolorization, neutralization, crystallization, washing, centrifuging, drying and the like to obtain D, L-phenylglycine and the analogue thereof. Compared with the prior art, the preparation method for D, L-phenylglycine and the analogue thereof can significantly and effectively reduce the pollution, and fewer inorganic salt by-products are generated. Meanwhile, the prepared D, L-phenylglycine and the analogue thereof are high in product yield and high in purity. Counted in benzaldehyde and the analogue thereof, the yield of D, L-phenylglycine and the analogue thereof is larger than or equal to 96%, and the product purity is larger than or equal to 99%. Meanwhile, the process flow is simple and feasible, so that the method is worthy of market popularization and application.
Owner:NINGXIA UNISPLENDOUR TIANHUA METHIONINE CO LTD
Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof
InactiveCN104926811AImprove universalitySimple and fast operationOrganic chemistryIodideEconomic benefits
The invention discloses a synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and an application thereof. Materials such as 2-aminopyridine compounds, methyl ketone compounds and benzyl cyanide are used to be reacted with N-heteroaryl nitrile through cheap and efficient cuprous iodide in catalyzing, oxidizing and cyclizing modes, and a series of the 3-cyano group imidazo [1, 2-a] pyridine compounds are constructed. The synthetic method has the advantages that the materials are easy to obtain, the operation is simple and convenient, the condition is gentle, the substrate is good in universality, the economic benefit is obtained, and the efficiency is achieved, and the method can be applied to efficient and simple and convenient composition of medicine molecules such as saripidem and necopidem.
Owner:ZHEJIANG UNIV
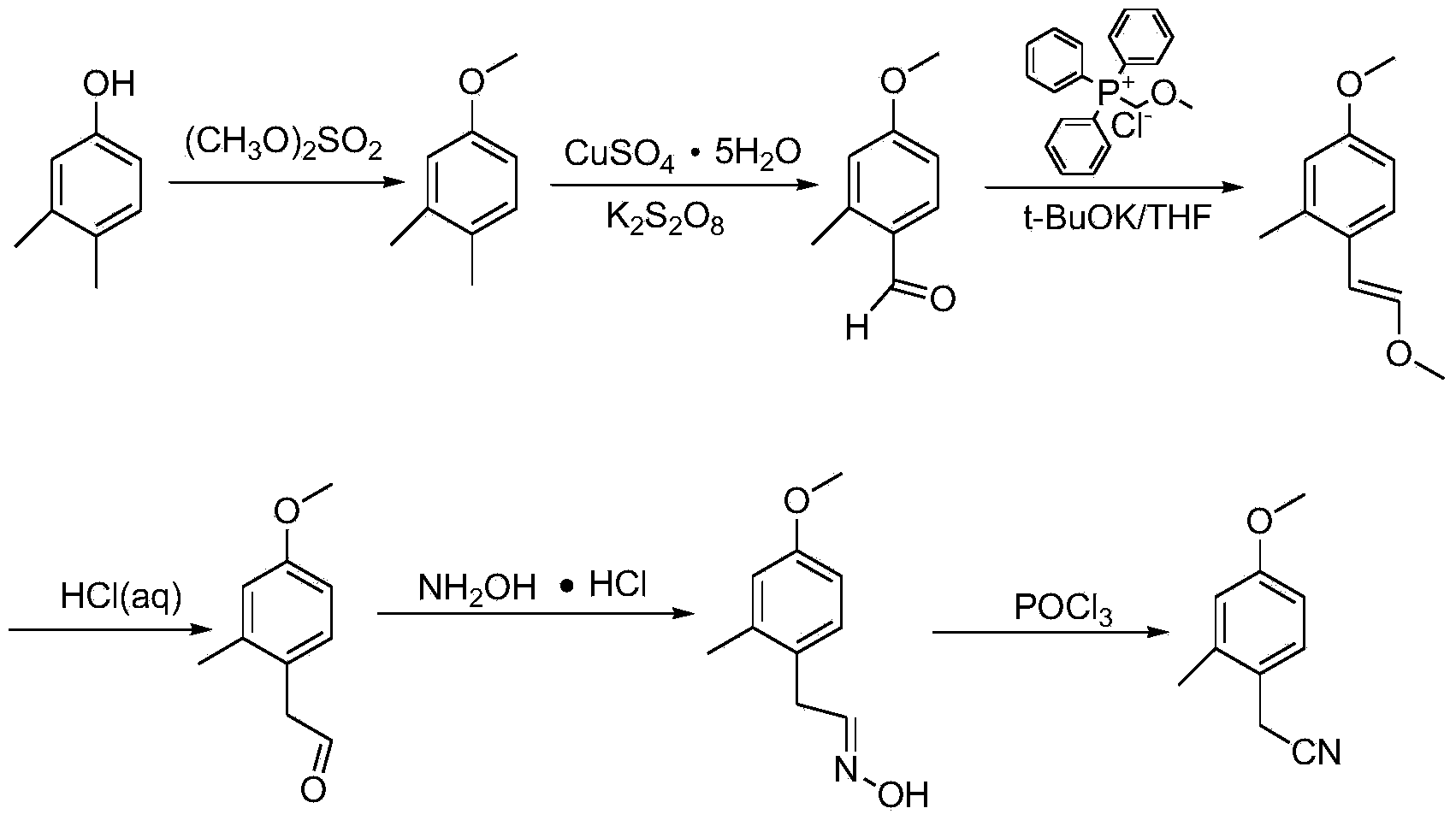
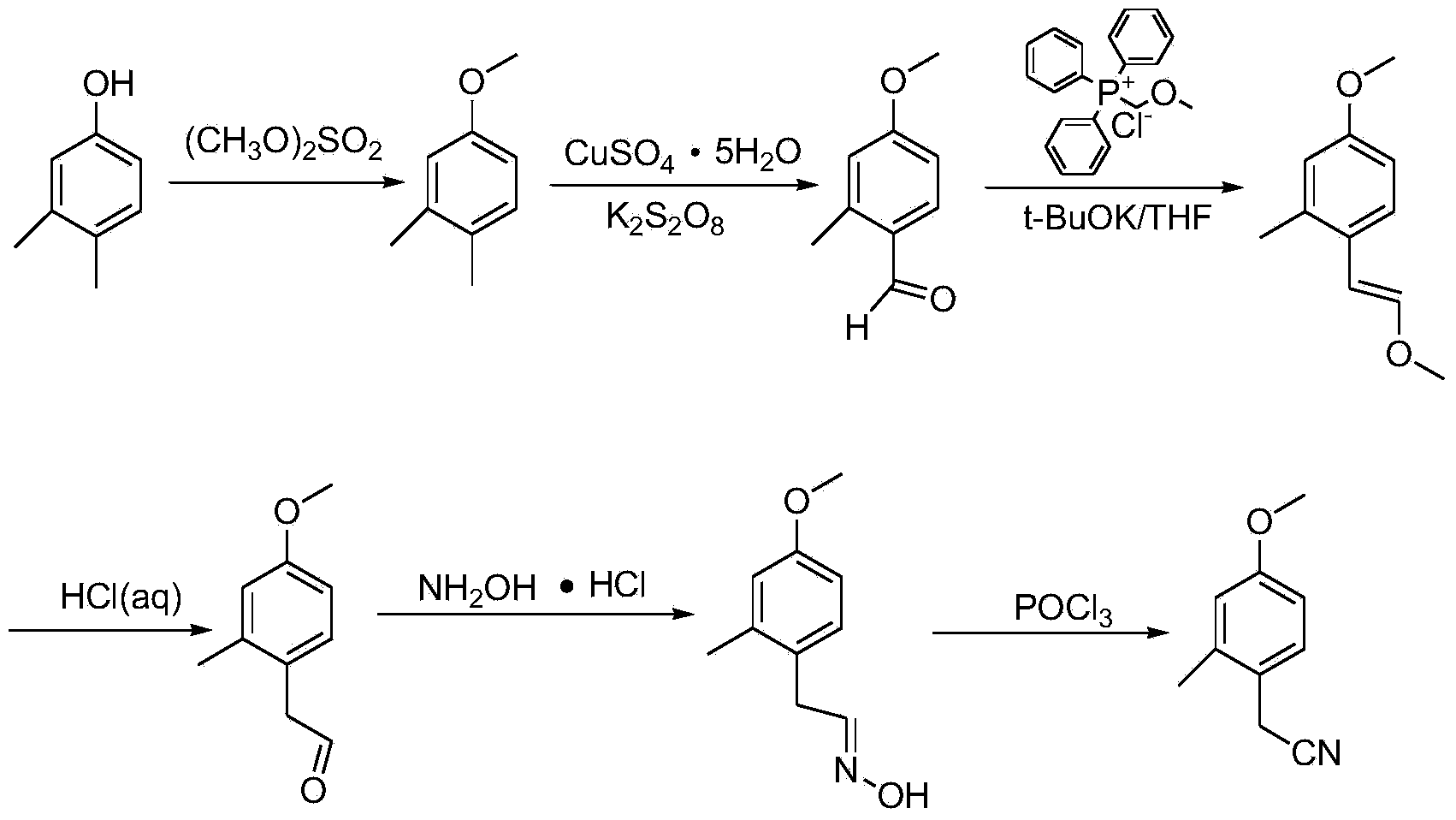
Synthesis method for 4-methoxy-2-methyl benzyl cyanide
ActiveCN103922965AReduce usageAvoid disadvantagesCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsEther
The invention relates to a synthesis method for 4-methoxy-2-methyl benzyl cyanide. The synthesis method takes 3, 4-dimethylphenol which is cheap and simple and easy to purchase as the raw material, and comprises six steps: methylation, oxidation, Witting reaction, alkene ether hydrolysis, oximation and dehydration. The synthesis method uses the simple and cheap raw material, is simple to operate, is easy for industrial production, has short production cycle, only needs purification in the last step of the six so as to obtain high-quality 4-methoxy-2-methyl benzyl cyanide, and avoids using extremely toxic substances like NaCN and KCN, thus filling the blank home and abroad.
Owner:渭南瑞联制药有限责任公司
Continuous synthetic method for benzyl cyanide
The invention provides a continuous synthetic method for benzyl cyanide. Multi-temperature serially connected micro-reactors are adopted; a low temperature micro-reactor is arranged after the benzyl cyanide synthetic reaction; sodium cyanide, phase transfer catalyst component and benzyl chloride component are respectively introduced into two continuous micro-channel reactors; the temperature of asecond micro-channel reactor is slightly lower than the temperature of a first micro-channel reactor, so that the 'overheat' phenomenon under a microcosmic condition can be solved; side reaction during reaction process is reduced; purity and yield of products are increased.
Owner:营创三征(营口)精细化工有限公司 +2
Preparation process of polyhydroxy isoflavone
ActiveCN103087027AHarm reductionAchieve room temperature reactionOrganic chemistryCyanideBenzyl cyanide
The invention relates to a preparation method of polyhydroxy isoflavone. The polyhydroxy isoflavone comprises 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone. According to the invention, the large-scale preparation of 4',6,7-trihydroxy isoflavone and 3',4',6,7-tetrahydroxy isoflavone can be realized by treating cheap and easily-acquired chemical raw materials including 3,4-dimethoxy phenol, 3,4-dimethoxy benzyl cyanide and hydroxybenzyl cyanide as starting materials through optimal research on Hoesch reaction, carburization n-cyclohexylmaleimide reaction and demethylation protection. The preparation method of polyhydroxy isoflavone disclosed by the invention is economic, efficient, environment-friendly, safe and easy to industrialize. The 4',6,7-trihydroxy isoflavone and the 3',4',6,7-tetrahydroxy isoflavone can be applied to the research and the development of new medicines in the aspects of medicines, food hygiene and the like.
Owner:中国人民解放军第三军医大学军事预防医学院
Prepn process of 2-fluoro-5-trifluoromethyl benzyl cyanide
InactiveCN1740146AHigh purityHigh yieldPreparation by cyanide reactionCarboxylic acid nitrile purification/separationBenzyl cyanideAcetonitrile
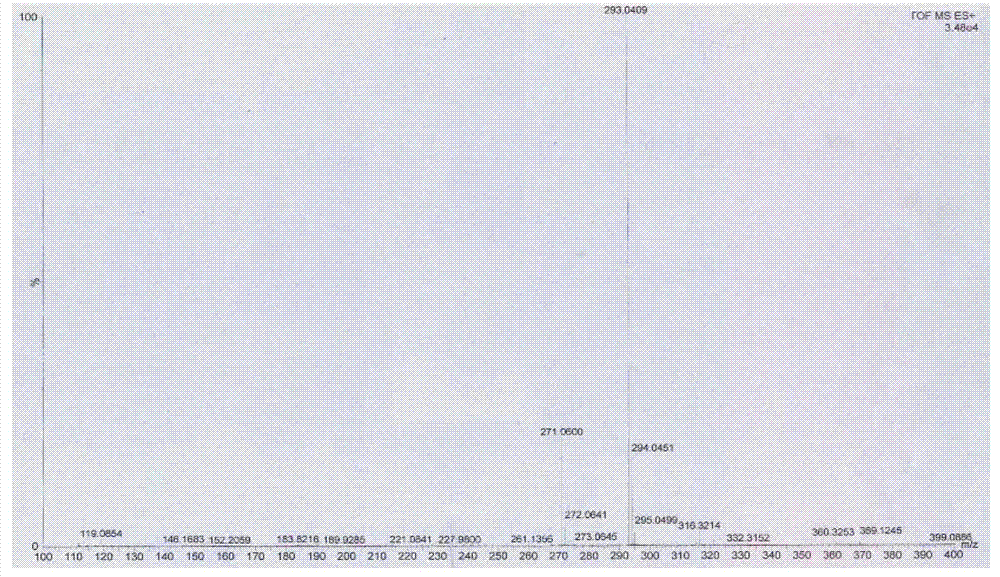
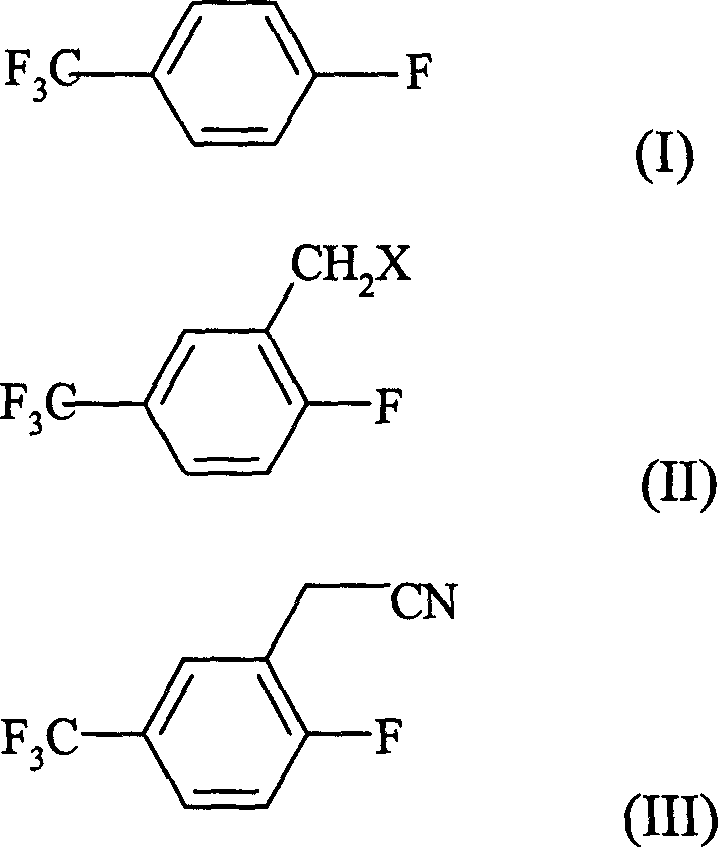
The present invention discloses the preparation process of 2-fluoro-5-trifluoromethyl benzyl cyanide. The preparation process includes the halogenomethylation of p-fluoro trifluoro toluene to produce 2-fluoro-5-trifluoromethyl benzyl halide, and the nitrilation of 2-fluoro-5-trifluoromethyl benzyl halide to produce 2-fluoro-5-trifluoromethyl benzyl cyanide. The present invention has high yield, low cost and high product purity.
Owner:LYNCHEM
Composition for odour improvement
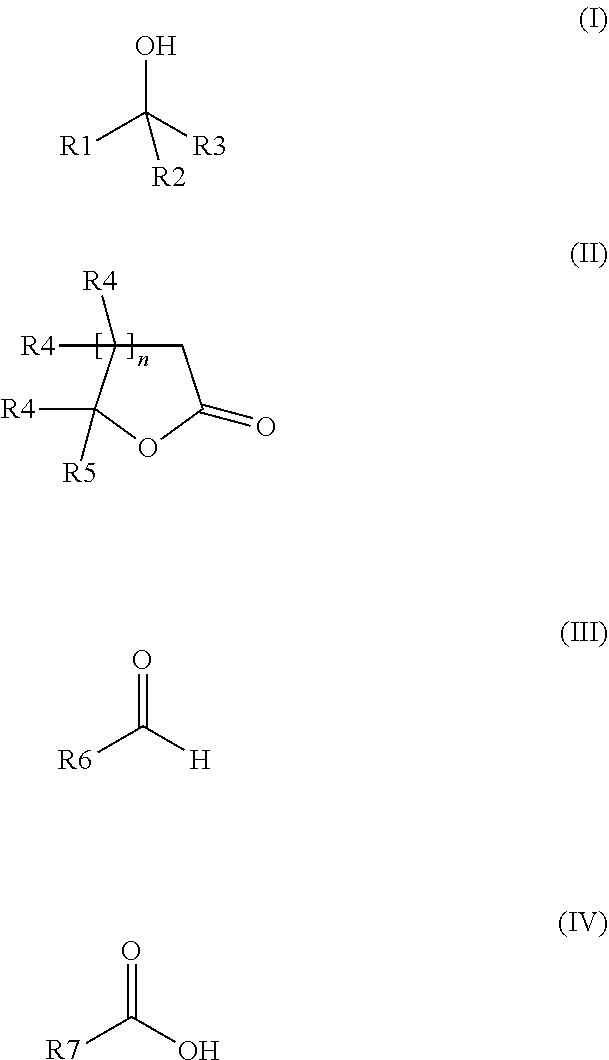
The invention relates to a preparation containing: (i) a composition containing (a) one, two or a plurality of compounds selected from the group consisting of (a1) alcohol monoterpenes of formula (I) in which R1 is H or methyl, R2 is H or C2-alkenyl, and R3 is a linear or branched, saturated or unsaturated hydrocarbon radical with 4 to 10 carbon atoms, and the enantiomers, diastereomers, racemates, solvates and physiologically compatible salts thereof, and / or (a2) bicyclic epoxy-monoterpenes, (b) at least two lactones of formula (II) in which R4 is H or methyl, R5 is a linear or branched, saturated or unsaturated hydrocarbon radical with 2 to 10 hydrocarbon atoms and n is the number 1 or 2, and the enantiomers, diastereomers and racemates thereof, (c) one, two or a plurality of solvents selected from the group consisting of ethanol, water, dipropylene glycol (DPG), diethyl phtalate (DEP), propylene glycol (PG), isopropyl myristate (IPM), isopropyl palmitate (IPP), triethyl citrate (TEC), triacetin (TRI), 1,2-Propanediol, 1,3-Propanediol, Propanethiol, Pentanediol, Hexanediol, Octanediol, Decanediol (SymClariol®), Dodecanol, 4-hydroxy-acetophenone (SymSave® H), glycerine, butylene glycol, pentylene glycol, hexylene glycol, decylene glycol, propylene carbonate, butylene carbonate, glycerine carbonate, 2-5 benzyl heptanol, lauryl alcohol, trimethyl-hydroxypentyl-isobutyrate, glyceryl-caprylate, ethylhexyl glycerine, benzyl benzoate (BB), and optionally (d) other flavouring agents or aromatic substances selected from the group consisting of 3-phenylbutanal (Trifernal), acetyl methyl carbinol, anethole, anisyl acetate, dihydroeugenol, linalyl formate, 2-methyldecanal, 2-benzyl-2-methylbut-3-ene nitrile (Ci-trowanil® B), 3-hexenyl acetate, styrallyl acetate, belanis, citronellal, cinnamyl acetate, rhubafuran, beta-ions, anther, prenyl acetate, 2-phenyl propanal, 4-(4-hydroxyphenyl)butan-2-one (Frambinon®), ethyl phenoxyacetate, isoralderine, gamma-terpinene, limonene, neocyclocitral, methyl lavender ketone, styrallyl propionate, phenyl ethyl propionate, limonenal, 4-isopentylcyclohexanol (Symrose®), 4-methyl-2-phenyl-3,6-dihydro-2H-pyran / 4-methylene-2-phenyl-tetrahydropyrane (Rosyrane super), hydrocitronitril, phenoxanol, isoamyl phenylacetate, damascone, silvial, nectaryl, ambroxide, acetyl pyrazine, trimethyl pyrazine, isoamyl acetate, para-cresyl methyl ether, filbertone, cyclohexyl acetate, heliotropin, acetophenone, anisaldehyde, para-methyl acetophenone, veratraldehyde, methyl anisate and vertoprenal; (ii) aldehydes of formula (III) in which R6 is a saturated or non-saturated, linear hydrocarbon radical; and / or (iii) free fatty acids of formula (IV), in which R7 is a linear or branched, saturated hydrocarbon radical.
Owner:SYMRISE GMBH & CO KG
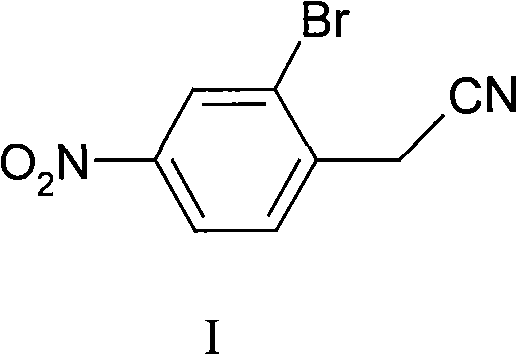
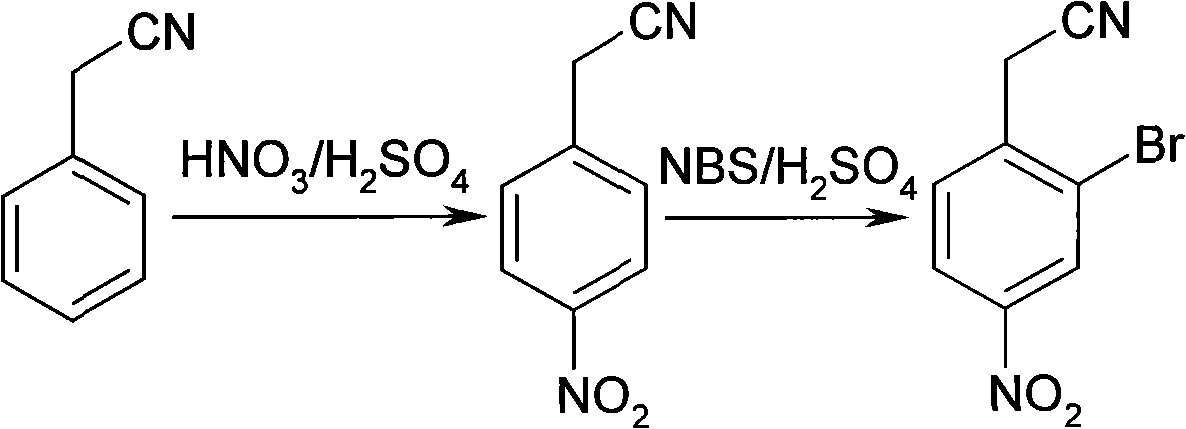
Synthesis of 2-bromine-4-nitrobenzene ethane nitrile
InactiveCN101402589AHigh yieldLow costCarboxylic acid nitrile preparationOrganic compound preparationN-BromosuccinimideIce water
The invention discloses a synthesis method for 2-bromine-4-nitryl phenylacetonitrile. The synthesis method uses phenylacetonitrile as a main starting material and comprises the following: 1) a step of nitrifying: stirring the phenylacetonitrile in mixed acid consisting of concentrated sulfuric acid and concentrated nitric acid to react, putting the obtained reaction product in ice water, separating out solid for filtering to obtain precipitate, and then washing the precipitate by water and drying the precipitation in turn to obtain n-nitro-phenylacetonitrile; and 2) a step of bromizing: dissolving the n-nitro-phenylacetonitrile in the concentrated sulfuric acid, adding N-bromosuccinimide to bromize, putting the obtained reaction mixture in the ice water, separating out solid for filtering to obtain precipitate, and then washing the precipitate by water and drying the precipitate in turn to obtain the 2-bromine-4-nitryl phenylacetonitrile. The 2-bromine-4-nitryl phenylacetonitrile synthesized by the method has the characteristics of simple technique, low cost and high yield.
Owner:ZHEJIANG UNIV
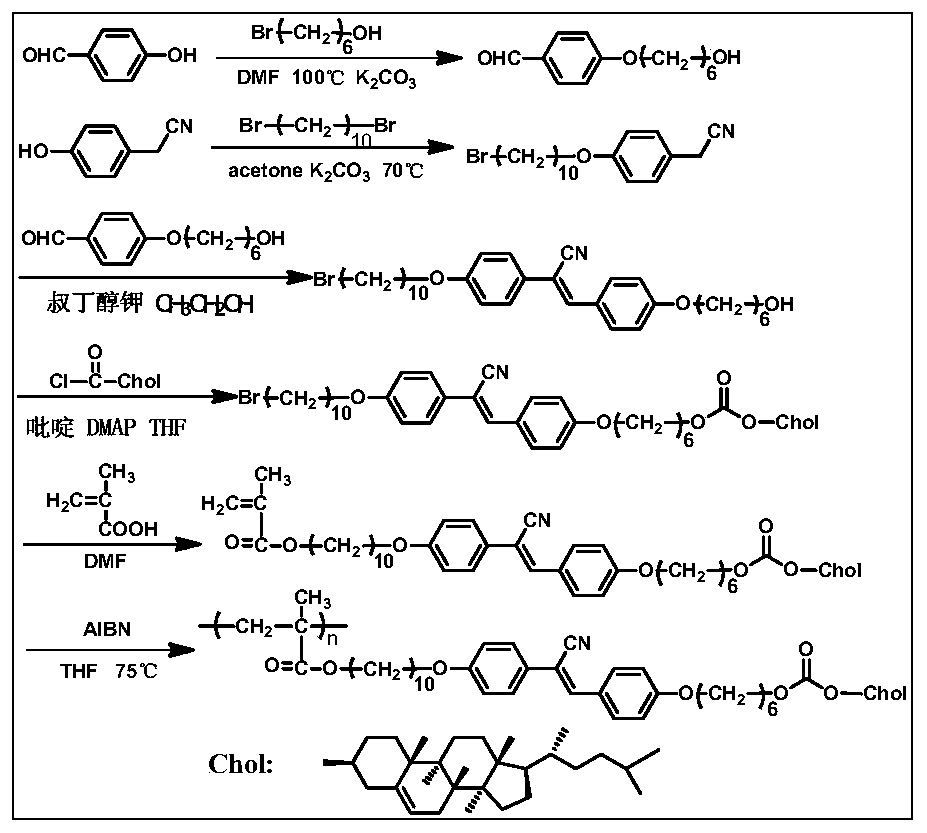
Chiral light-emitting liquid crystal polymer with circular polarization light-emitting property and preparation method thereof
PendingCN111154499ASimple structureEasy to synthesizeLiquid crystal compositionsLuminescent compositionsMeth-Fluorescence
The invention discloses a chiral light-emitting liquid crystal polymer with a circular polarization light-emitting property and a preparation method thereof. The preparation method comprises the following steps: firstly, reacting 4-hydroxybenzaldehyde or 4-hydroxybiphenyl formaldehyde with bromine alcohol to generate benzene or a biphenyl formaldehyde derivative; preparing benzene or a derivativeof biphenyl acetonitrile from 4-hydroxyphenylacetonitrile or 4-hydroxybiphenyl acetonitrile and dihaloalkane; carrying out a Knoevenagel reaction on two derivatives to generate a cyano-stilbene derivative, carrying out a reaction on the obtained derivative and cholesteryl formyl chloride to generate a cyano-stilbene derivative containing a cholesteric chiral structure, and carrying out a reactionon the derivative and (meth)acrylic acid to generate a polymerizable monomer. The monomer can be subjected to free radical polymerization to obtain a polymer taking poly(meth)acrylic acid as a main chain, and can also be subjected to hydrosilylation reaction with polysiloxane to obtain a polymer taking polysiloxane as a main chain. The obtained polymer has liquid crystallinity, an aggregation-induced fluorescence enhanced property and a circular polarization luminescence property, and has a good application prospect.
Owner:XIANGTAN UNIV
Diazosulfide benzyl cyanide derivative and preparation method and application thereof
ActiveCN109651293AObvious color changeHigh fluorescence intensityOrganic chemistryTenebresent compositionsQuantum efficiencyCoupling
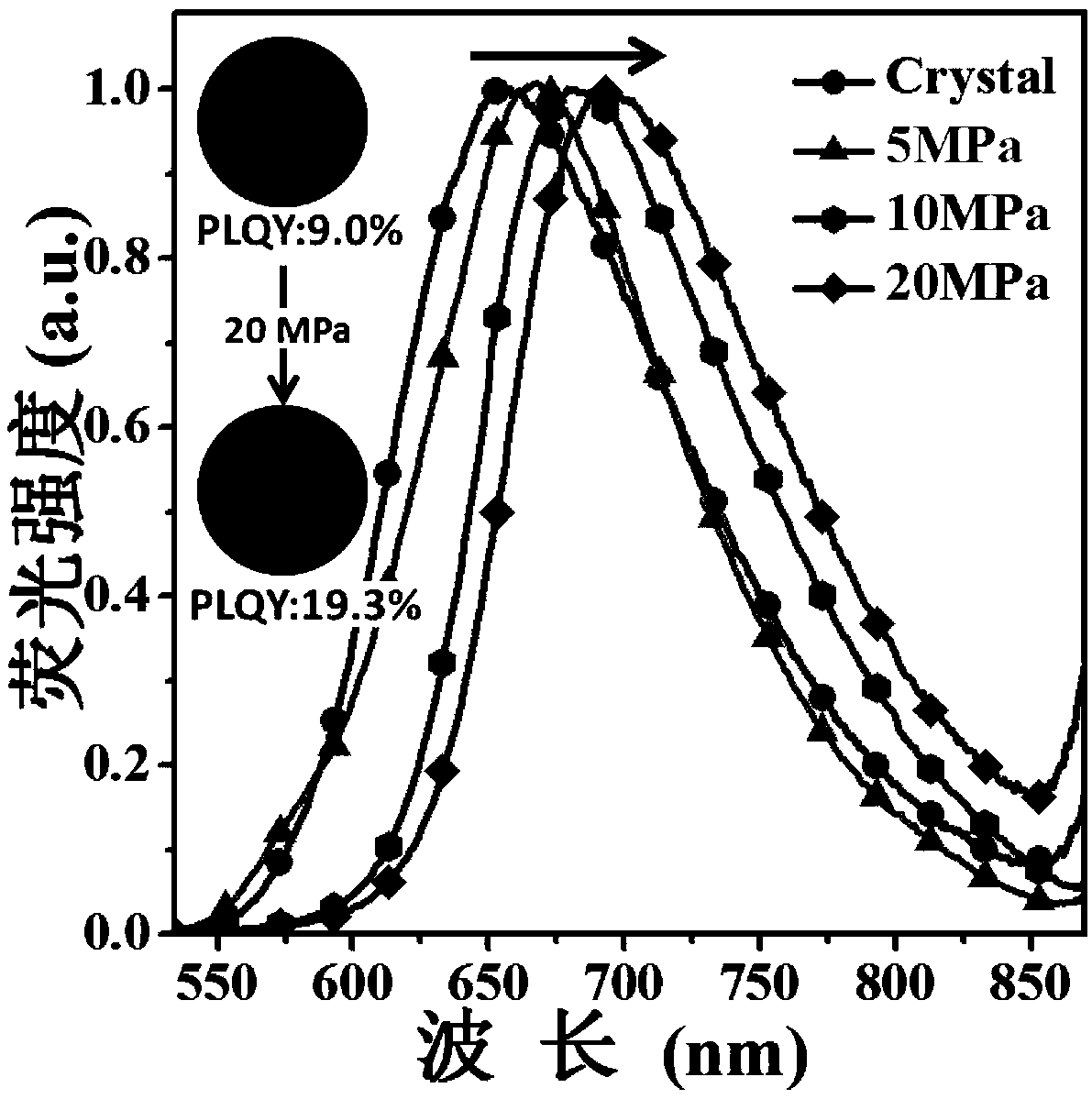
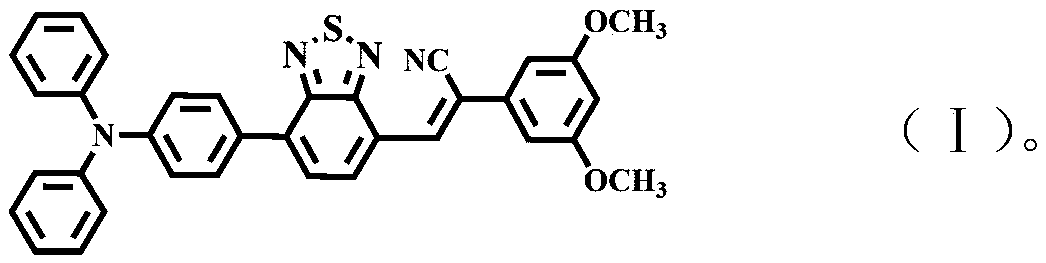
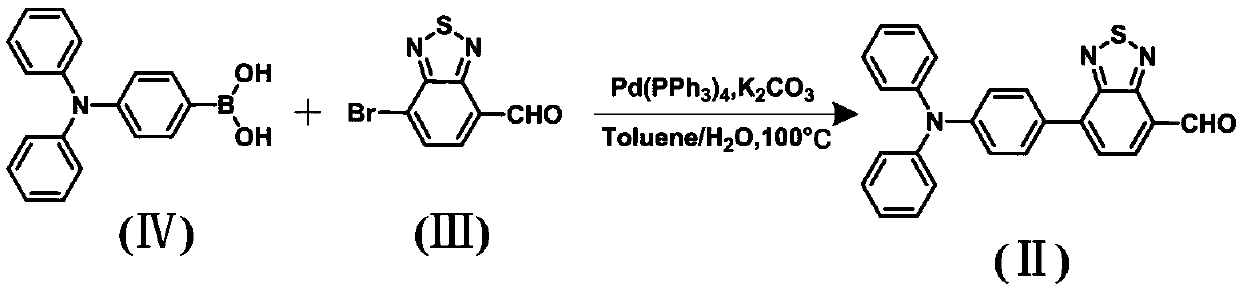
The invention discloses a diazosulfide benzyl cyanide derivative and a preparation method and application thereof. The preparation method for the derivative comprises the following steps that triphenylamine boric acid, diazosulfide and a benzyl cyanide derivative are taken as raw materials and are subjected to Suzuki coupling and Knoevenagel condensation reactions successively to synthesize the target product diazosulfide benzyl cyanide derivative, namely a force-induced fluorescence enhancement material. The color of the material changes under stimulation of lower pressure (the Mpa level), and the material has the advantages that the contrast ratio is high, and fluorescence intensity is improved significantly (the quantum efficiency is improved to 19.3% from 9%), and can be applied to a pressure sensing system.
Owner:HUZHOU TEACHERS COLLEGE
Method for preparing phenylacetic acid by non-catalyzed hydrolysis of benzene acetonitrile in near-critical water medium
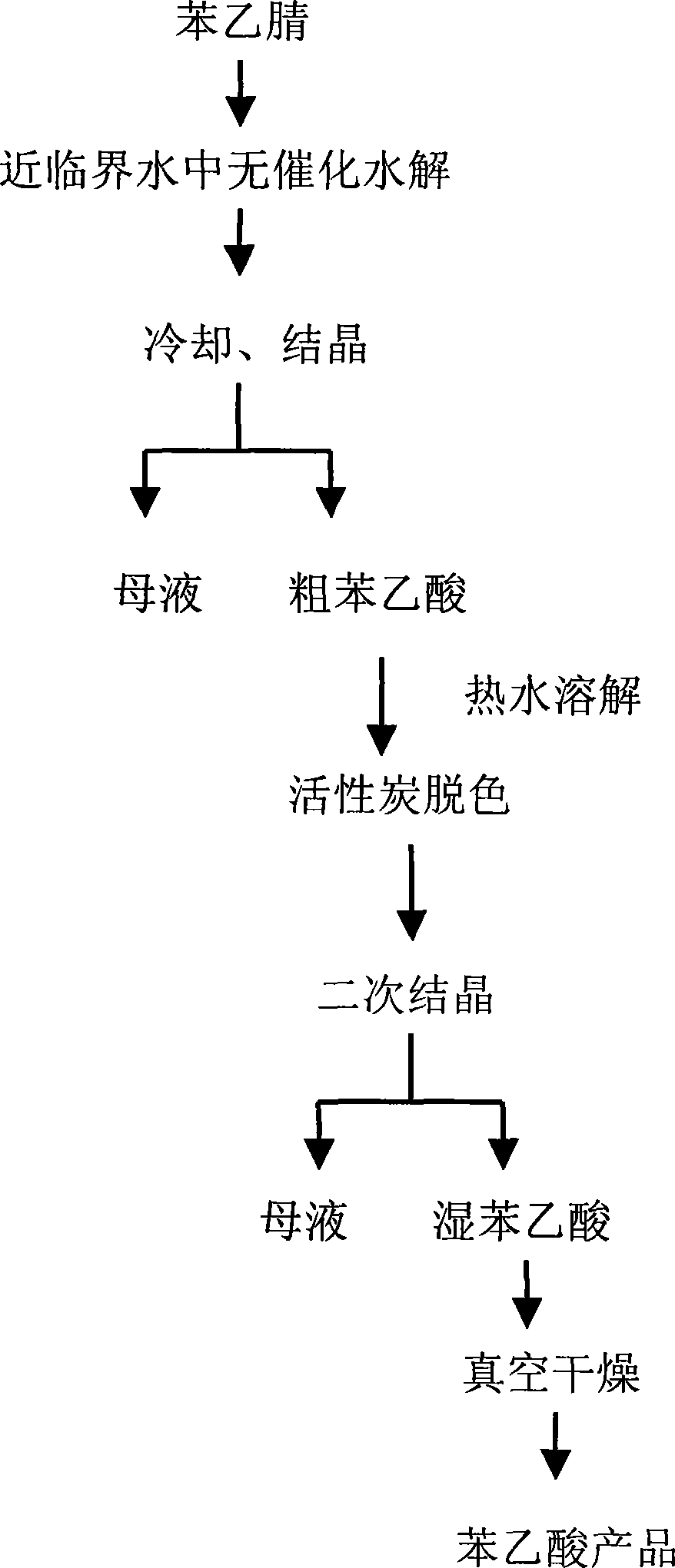
The present invention discloses a no-catalysis hydrolysis preparation method of benzeneacetic acid with benzyl cyanide in critical water mediator. The method includes the following procedures that: firstly deionized water and benzyl cyanide are added into a high-pressure reactor with the mass ratio of 2:1-8:1, the mixture is agitated openly and the temperature is raised up to boiling point under the normal pressure, then the exhaust valve is kept open for 2-5 minutes; secondly the exhaust valve is kept closed, and the temperature is continuously raised up to 240 DEG C to 310 DEG C for hydrolysis for 1-8 hours; thirdly the hydrolysate is cooled down and crystallized, and crude benzeneacetic acid is obtained; fourthly the crude benzeneacetic acid is decolored with active carbon, secondarily crystallized and dried in vacuum to obtain the product of benzeneacetic acid. The present invention has no need to add any catalyst during the reaction process, thereby solving the puzzle of pollution of catalyzed hydrolyzation of acid and alkali, and having the advantages of simple process, green operation, high purity and high yield.
Owner:ZHEJIANG UNIV
Cyanostilbene fluorescence epoxy compound, and preparation method and application thereof
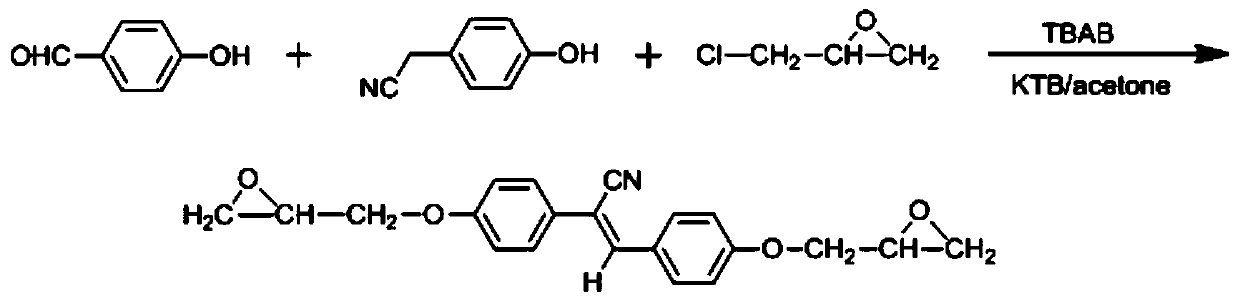
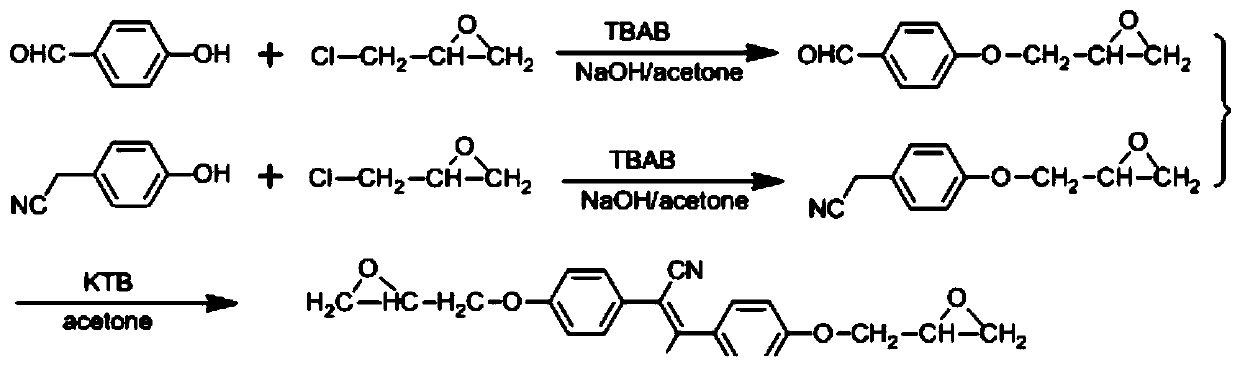
ActiveCN110407772AThe synthetic route is simpleEasy to purifyOrganic chemistryLuminescent paintsEpoxyBenzene
The invention discloses a cyanostilbene fluorescence epoxy compound having AIEE properties, and a preparation method and an application thereof. The compound is prepared by a step-by-step process, that is, a benzaldehyde or phenylacetonitrile compound containing an epoxy group is prepared by a substitution reaction and a ring closure reaction, and then a Knoevenagel condensation reaction is carried out to prepare the cyanostilbene fluorescence epoxy compound; or the compound is prepared by a one-pot process, that is, all the raw materials are mixed, and then the obtained mixture undergoes thesubstitution reaction, the ring closure reaction and the Knoevenagel condensation reaction to prepare the compound. The cyanostilbene fluorescence epoxy compound of the invention has the advantages ofsimple synthesis route, easiness in purification, high yield and low preparation cost, also has the AIEE properties, can emit strong fluorescence when in the aggregate state, and has wide applicationprospects in the fields of fluorescent coatings, fluorescent casting materials, fluorescent adhesives and fluorescent laminates.
Owner:XIANGTAN UNIV
Method for synthesizing benzyl cyanide compound by using benzyl chloride compound
InactiveCN102381918AReduce manufacturing costPreparation by cyanide reactionCyano group formation/introductionCyanideOrganosolv
The invention belongs to the technical field of synthesis of benzyl cyanide compounds and in particular relates to a method for synthesizing a benzyl cyanide compound by using a benzyl chloride compound. The method comprises the following step of: reacting the benzyl chloride compound with potassium ferrocyanide in an organic solvent by using a copper salt as a catalyst to obtain the benzyl cyanide compound. As the less toxic potassium ferrocyanide is used as a cyanide reagent and the inexpensive copper salt is used as the catalyst, the method for synthesizing the benzyl cyanide compound provided by the invention has lower production cost and no highly toxic raw material.
Owner:HENAN UNIV OF SCI & TECH
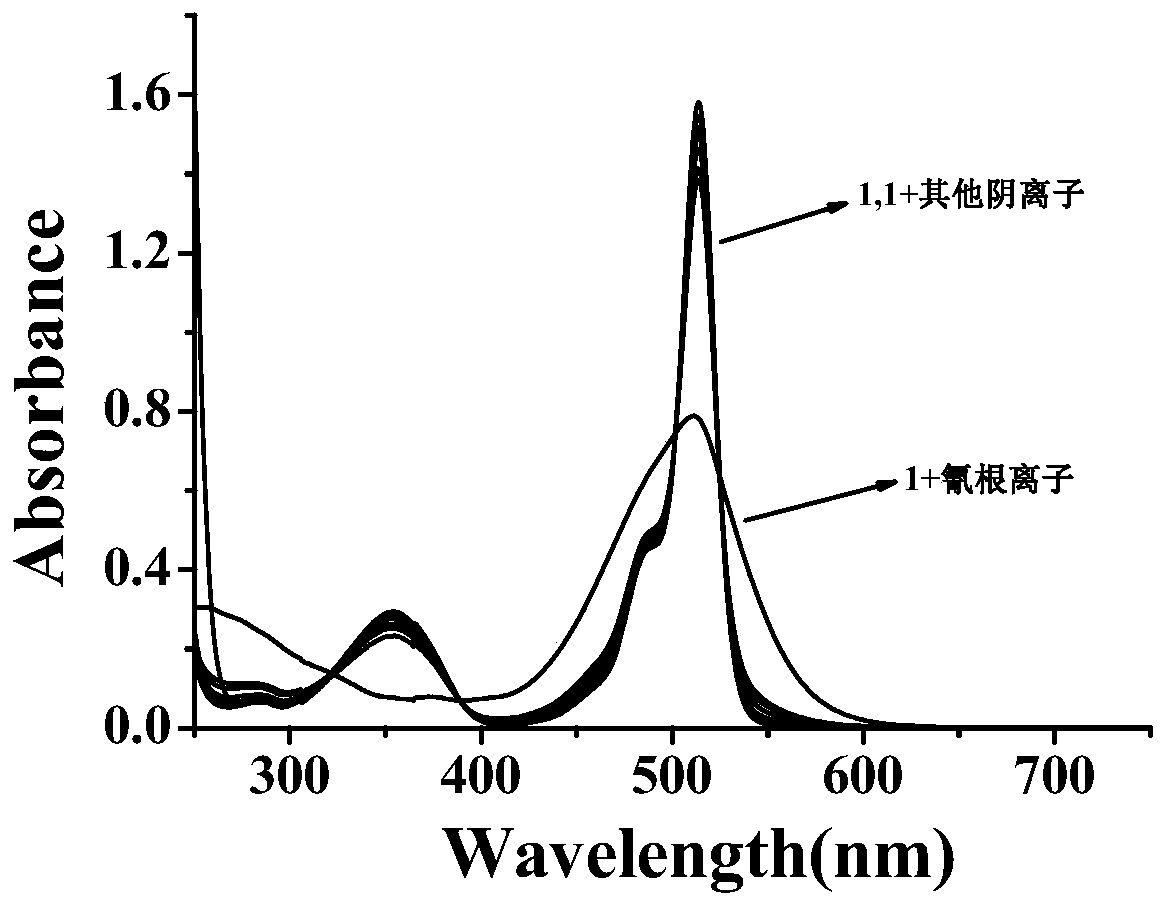
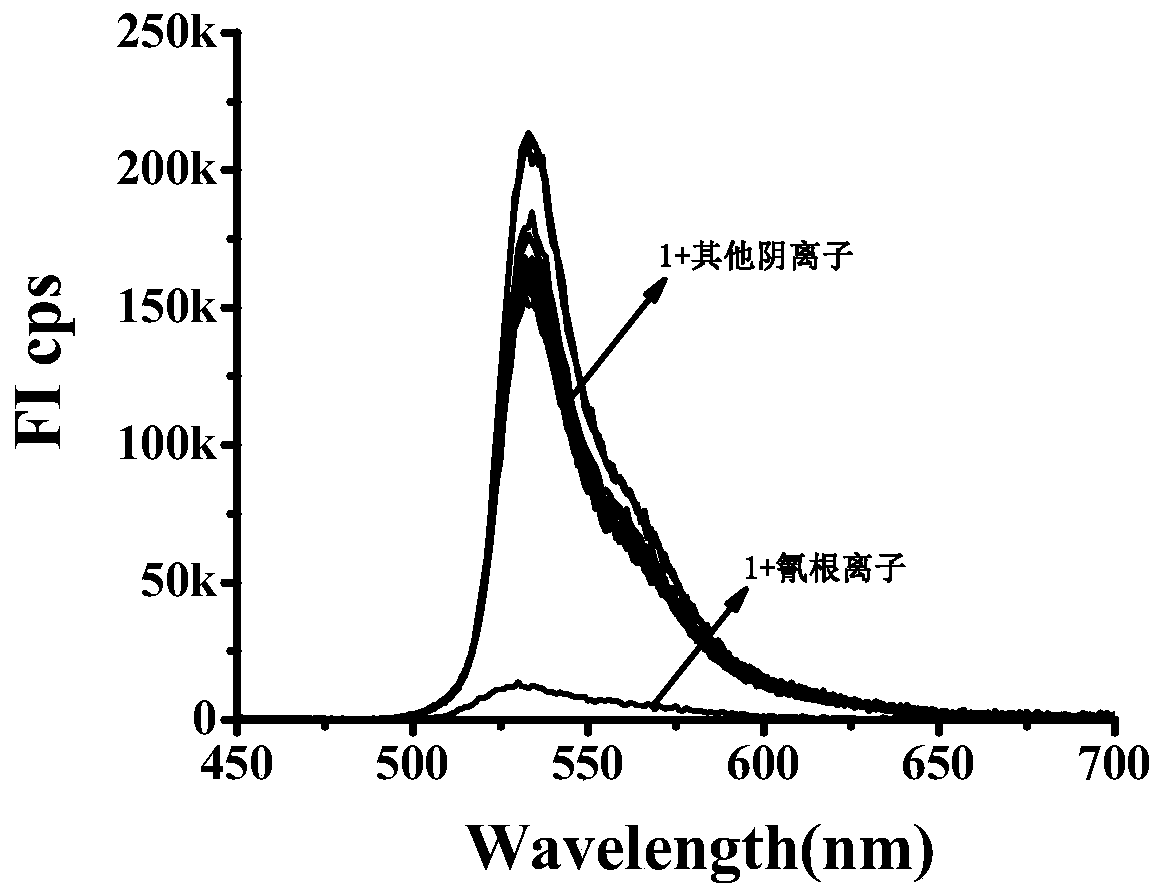
Fluorescence probe for detecting cyanide ions and preparation method and application of fluorescence probe
ActiveCN110204564ACyclicalReversibleGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluorescenceSynthesis methods
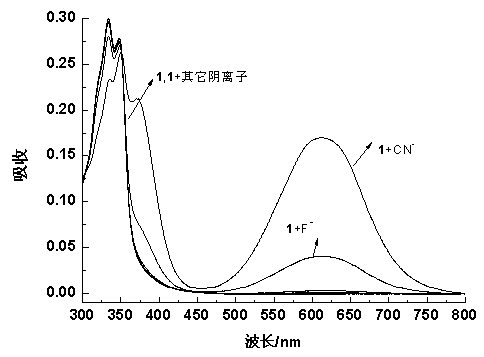
The invention relates to a fluorescence probe for detecting cyanide ions and a preparation method and application of the fluorescence probe. The fluorescence probe is a cyanide ion fluorescence probetaking boron dipyrromethene (BODIPY) as a fluorescence signal group and taking an activated CH group in a benzyl cyanide group as a recognition site, and the probe is obtained through a nucleophilic substitution reaction of 3,5-dibromo8-phenyl boron fluoride dipyrrole and benzyl cyanide. Through the research of an ultraviolet absorption and a fluorescence emission spectroscopy method, it is foundthat the probe can identify the cyanide ions singly, the lowest detectable limit in a 70% aqueous solution can reach 148 nM, and the recognition process is not disturbed by other anions. Compared withthe prior art, the fluorescence probe has the advantages that the synthesis method is simple, the raw materials are easy to obtain, the cost is lower, the selectivity to the cyanide ions is good, thesensitivity is high, reversibility is provided, the detection can be performed in the aqueous solution, and therefore the fluorescence probe has a good application prospect in the detection of the cyanide ions.
Owner:SHANGHAI INST OF TECH
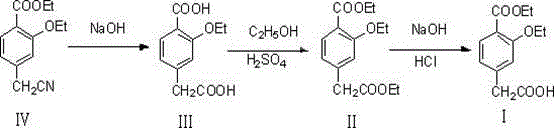
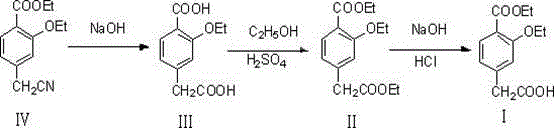
Method for synthesizing repaglinide key intermediate
InactiveCN104628518AHigh yieldAvoid highOxygen-containing compound preparationOrganic compound preparationPhenylacetic acidFirst pass yield
The invention relates to a method for synthesizing a repaglinide key intermediate, namely 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid. The method comprises the following steps: by taking 3-ethyoxy-4-ethoxycarbonyl-benzyl cyanide as an initial raw material, performing hydrolysis, esterification, selective hydrolysis and the like, thereby obtaining the important intermediate 4-ethoxycarbonyl-3-ethyoxy phenylacetic acid of repaglinide. According to the key intermediate, the impurities of the product is less, the purity of the product is high and can reach over 99.7%, so that the quality of the subsequently synthesized repaglinide product is improved, the 100 percent first-pass yield of the subsequently synthesized repaglinide product can be basically reached, a refining step is avoided, and the synthetic yield is effectively improved. The process is easy and convenient to operate, the yield is high, the molar yield is 69.1 percent, the production cost is low, and the method is suitable for industrial production.
Owner:HUBEI YITAI PHARMA
Method for synchronizing m-benzenyl trifluoride di-cyan acetonphenone
ActiveCN102766073AEasy to recycleSmooth responseCarboxylic acid nitrile preparationOrganic compound preparationBenzoic acidMetaclazepam
The invention discloses a method for synchronizing m-benzenyl trifluoride di-cyan acetonephenone which is an important intermediate for metaflumizone, a kind of efficient insecticide. The method includes utilizing 4-cyanobenzylchloride as raw materials to generate 4-cyano phenylacetonitrile to be reacted with methyl 3-benzoate in organic solvent for 5-8 hours. The yield of the intermediate of the metaflumizone produced by the method is higher than 90%, and content is higher than 89%. The method for synchronizing m-benzenyl trifluoride di-cyan acetonphenone is high in yield, low in cost and can be used for industrial production.
Owner:JINGBO AGROCHEM TECH CO LTD
Preparation method for fosamprenir intermediate
InactiveCN104803954ASignificant technological progressReasonable workmanshipOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupGrignard reagent
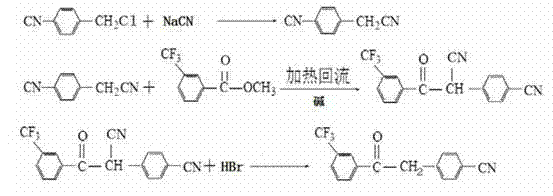
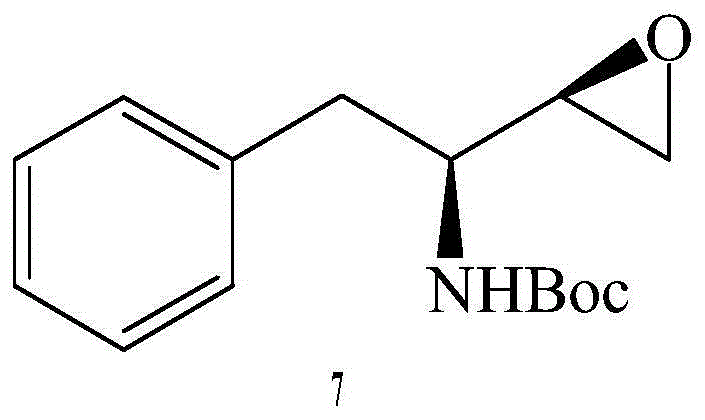
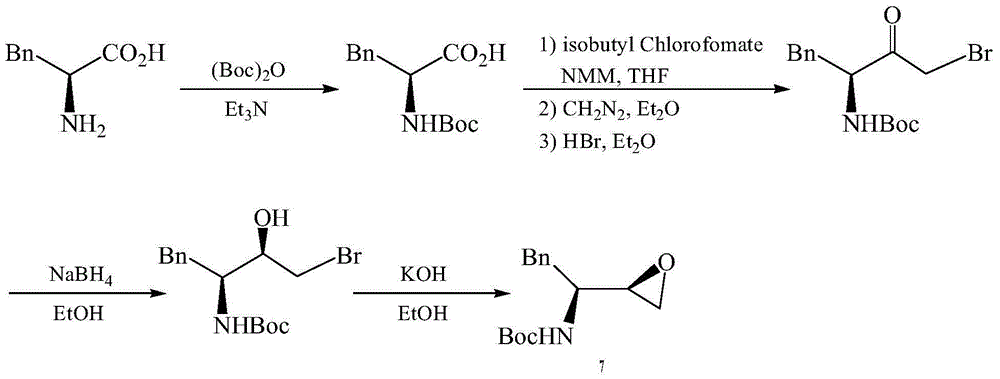
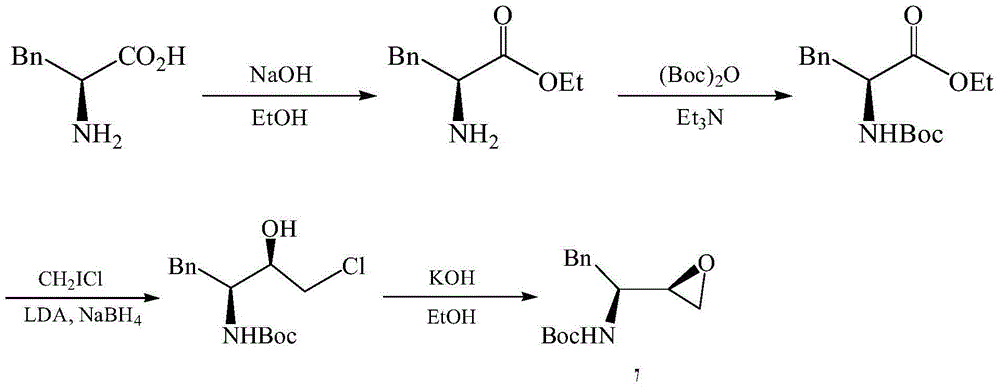
The invention provides a preparation method for a fosamprenir intermediate. The preparation method comprises the following steps: taking benzyl cyanide as a raw material, and performing steps of nitrile hydrolysis, acylation, reaction with a Grignard reagent, ammonization, cyclizing and the like for synthesizing (2R,3S)-1,2-epoxy-3-t-butyloxycarborylamino-4-phenyl butane. The method is reasonable in process, simple to operate, low in cost and high in yield; with the method, industrialization can be well realized and the production efficiency is improved.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Industrial preparation method for 3-amino phenylacetic acid
InactiveCN1634871AHigh selectivityMild reaction conditionsOrganic compound preparationAmino-carboxyl compound preparationPhenylacetic acidAcetylation
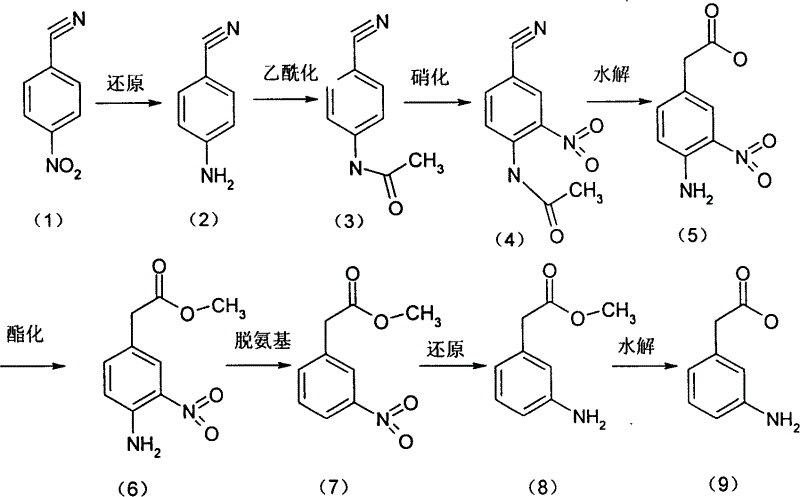
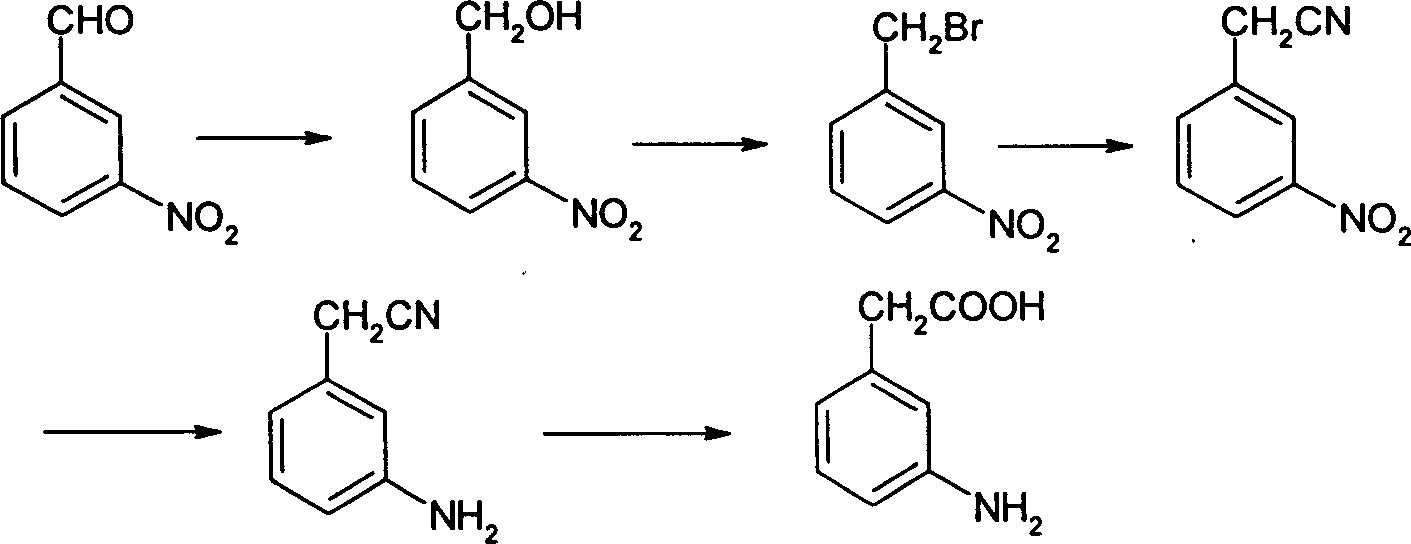
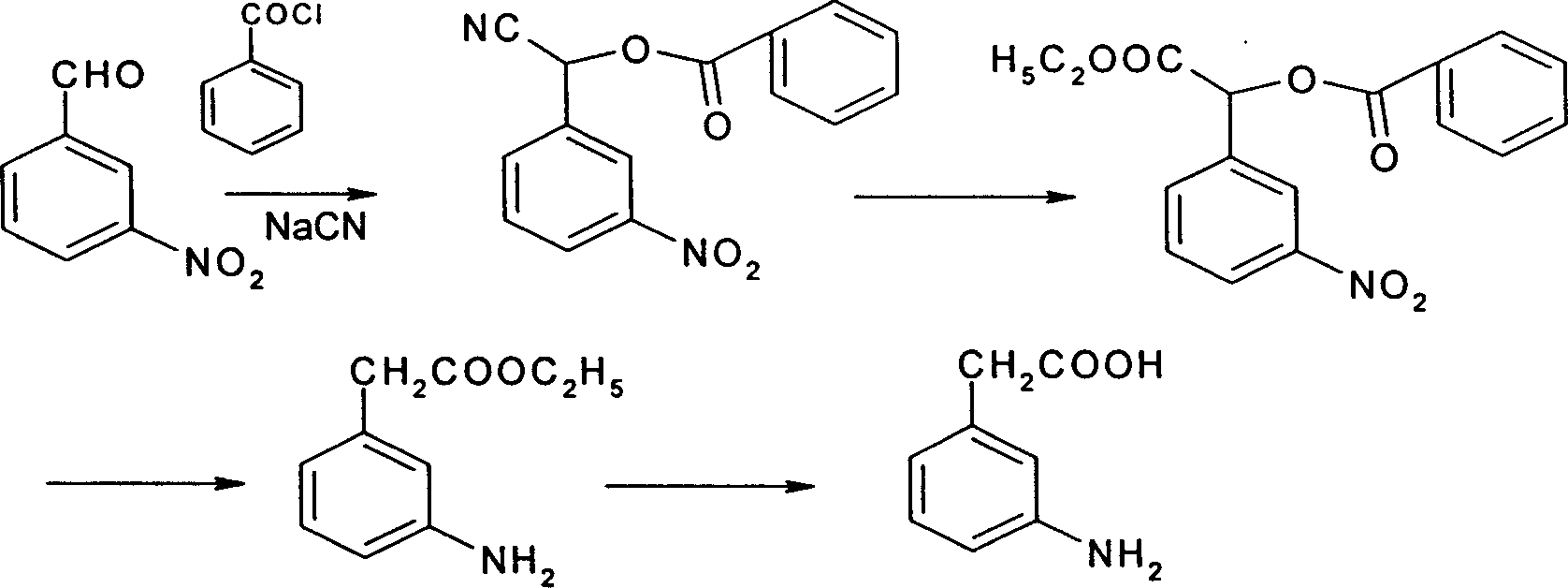
The invention relates to a process for preparing 3-amino phenylacetic acid with p-nitro benzyl cyanide as raw materials, by reduction, acetylation, nitrification, hydrolysis, esterification, deamination, reduction and hydrolysis. The invention has high selectivity and the raw materials are cheap and easily available.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of phenylacetic acid
InactiveCN103232338AReduce typesReduce consumptionPreparation from nitrilesDistillationPhenylacetic acid
The invention discloses a preparation method of phenylacetic acid, which comprises the following steps of: 1) heating benzyl cyanide to 50-100 DEG C, dropwise adding hydrochloric acid to the benzyl cyanide or dropwise adding the molten benzyl cyanide to hydrochloric acid, wherein the molar ratio of the hydrochloric acid to the benzyl cyanide is (1.2-5):1; after dropwise adding, performing a heat preservation reaction for 1-5 hours; and ending the reaction when the mass content of the benzyl cyanide in the reaction system is less than 5%; and 2) performing reduced-pressure distillation for recovering the unreacted benzyl cyanide in the reaction system of the step 1) until the mass content of the benzyl cyanide in the reaction system is less than 0.2%; adding water to the reaction system, and stirring and mixing; cooling for crystallization and performing suction filtration; and washing the obtained crystal with water, and drying to obtain a phenylacetic acid product. According to the preparation method disclosed by the invention, a technology of directly hydrolyzing benzyl cyanide with hydrochloric acid to prepare phenylacetic acid is adopted, few kinds of reactants are used, other organic matters except the raw material benzyl cyanide are not required, the consumption is low, the after-treatment is simple, and both the appearance and quality of the product meet requirements.
Owner:CHONGQING UNISPLENDOUR CHEM
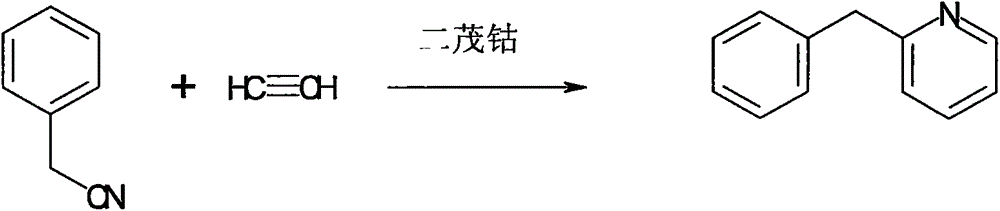
Synthesis method of pheniramine maleate
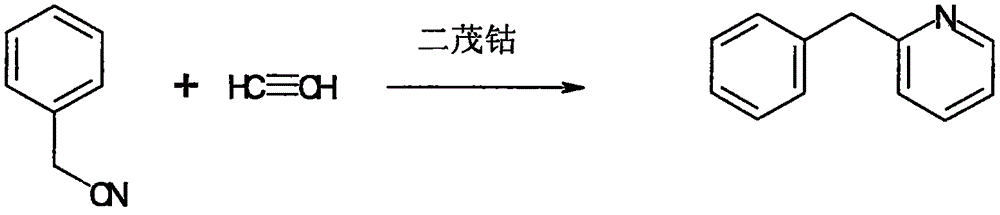
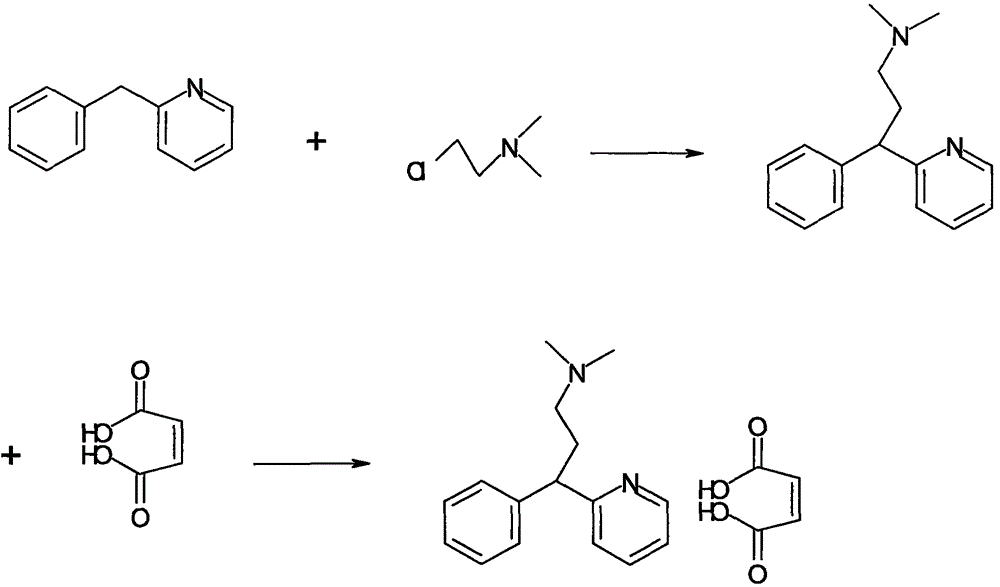
The invention belongs to the field of medical chemistry, and in particular relates to a synthesis method of pheniramine maleate. The method includes the following steps: (1) reacting benzyl cyanide with acetylene gas in the presence of a cobaltocene catalyst to generate benzyl pyridine; (2) reacting benzyl pyridine with N,N-dimethyl chloroethane under the catalysis of sodium amide to generate pheniramine, and conducting neutralization reaction on pheniramine with maleate to generate pheniramine maleate.
Owner:张燕梅
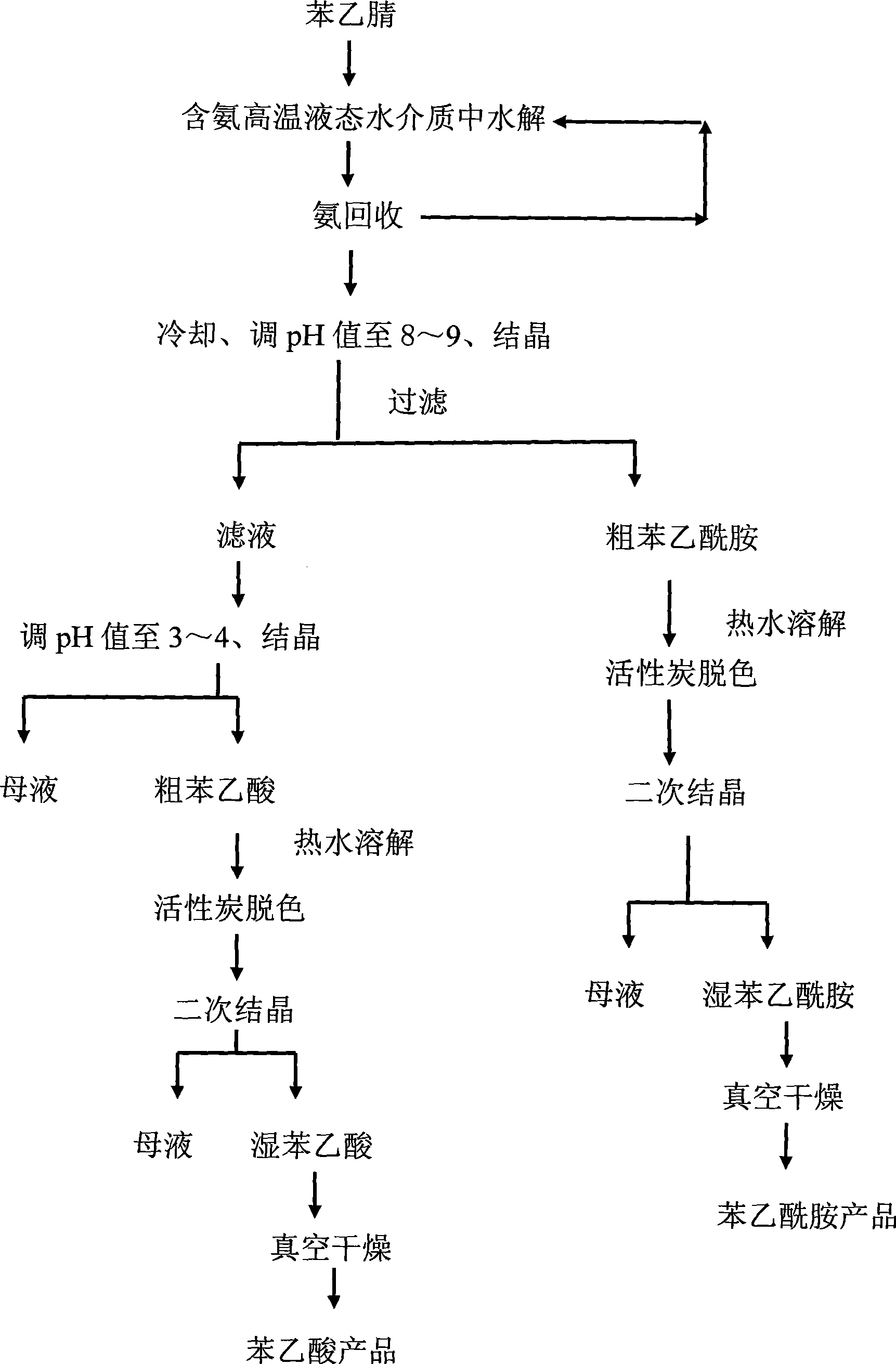
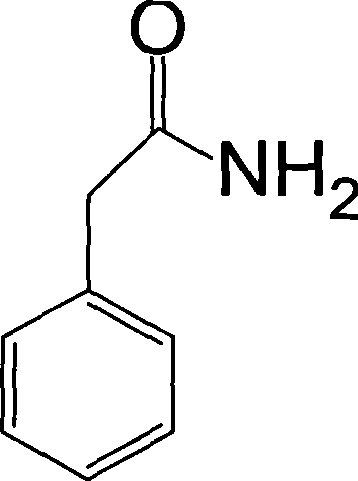
Method for simultaneously preparing phenylacetamide and phenylacetic acid by benzyl cyanide hydrolysis in ammonia-containing high temperature aqueous water medium
InactiveCN101381325AQuick responseLow reaction temperatureOrganic compound preparationCarboxylic acid amides preparationExhaust valvePhenylacetic acid
The invention discloses a method for hydrolyzing phenylacetonitrile in an ammonia-containing high-temperature liquid water medium and preparing phenyl acetamide and phenylacetic acid simultaneously. The method comprises the following steps: 1) deionized water and the phenylacetonitrile with the mass ratio of between 2 to 1 and 8 to 1 are added into a high-pressure reaction kettle, are stirred, and are heated to boil, and an exhaust valve is opened for 2 to 5 minutes; 2) 25 percent ammonia water is pumped through a metering pump to ensure that the ammonia concentration in reactants in the kettle is between 0.05 and 4g / L, and the temperature is increased to be between 180 and 250 DEG C to hydrolyze for 5 to 120 minutes; 3) the temperature is reduced to recover ammonia gas in the kettle; 4) a hydrolysate is cooled, the pH value of the hydrolysate is adjusted to between 8 and 9 , and the hydrolysate is crystallized, and filtered to obtain crude phenyl acetamide; 5) the crude phenyl acetamide is subjected to decolorization by activated carbon, secondary crystallization and vacuum drying to obtain a phenyl acetamide product; and 6) the pH value of filtrate in step 4) is adjusted to between 3 and 4, and the filtrate is crystallized to obtain crude phenylacetic acid, and then the crude phenylacetic acid is subjected to decolorization by the activated carbon, secondary crystallization and vacuum drying to obtain a phenylacetic acid product. The method has a simple and environment-friendly technical process.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


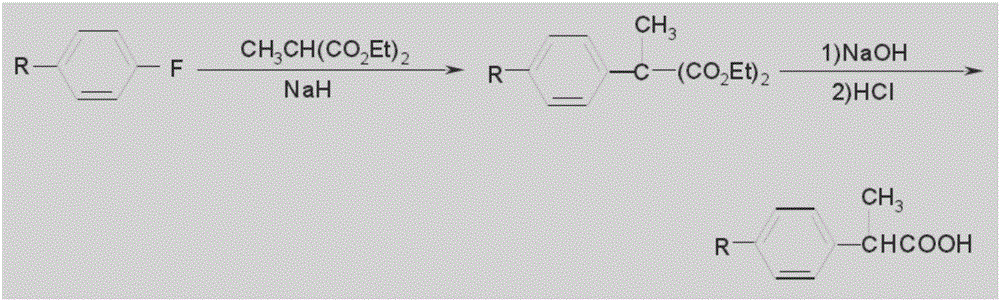
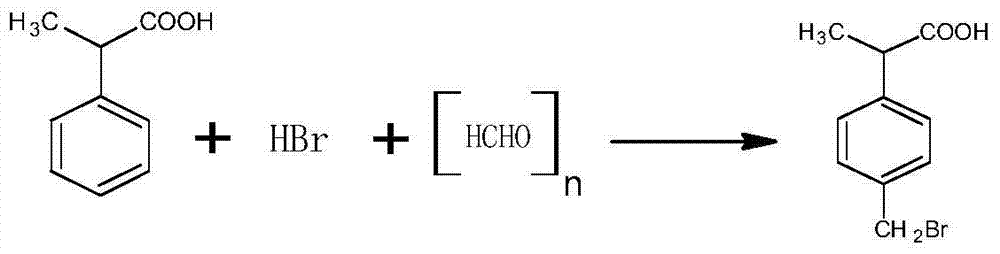


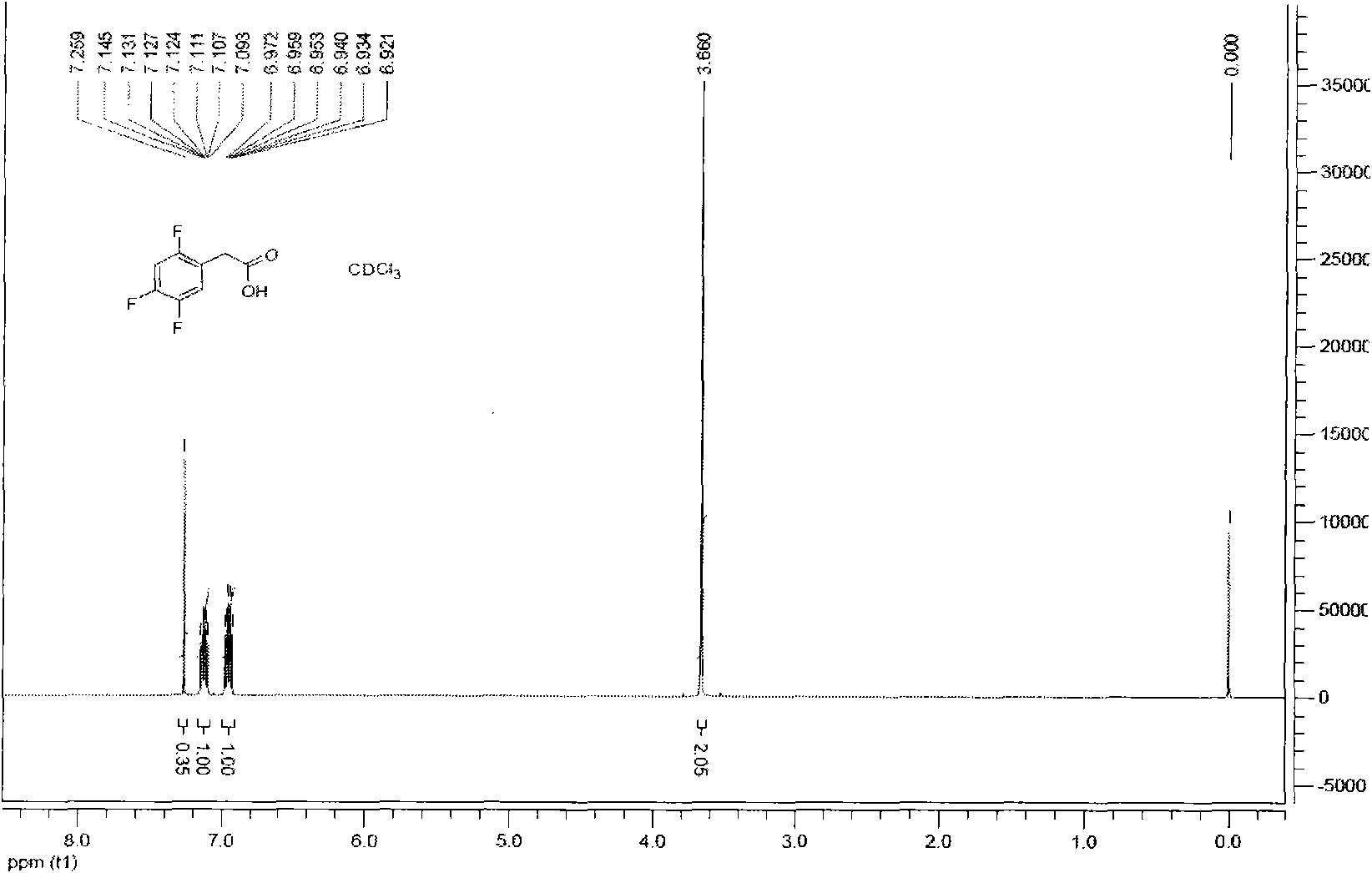


![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000011.PNG)
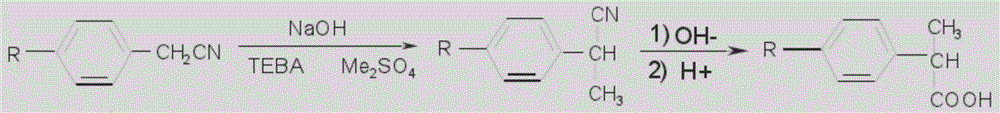
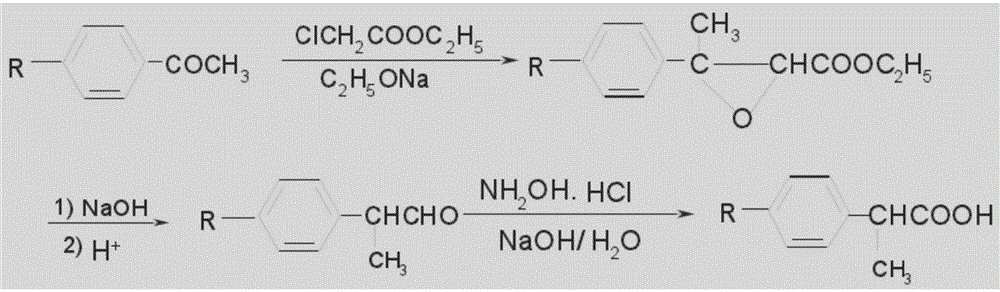
![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000021.PNG)
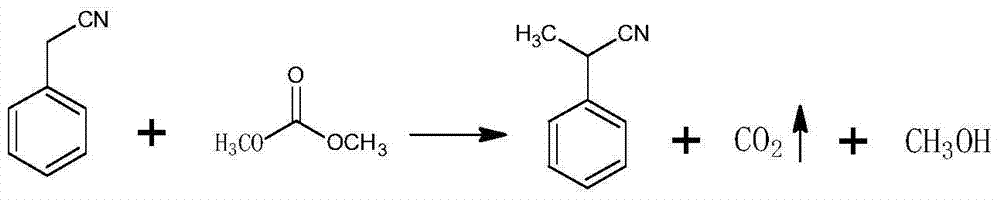
![Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof Synthetic method for 3-cyano group imidazo [1, 2-a] pyridine compounds and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/45f3da4a-19ff-4e24-b23a-0e6c4c4b5110/BDA0000739945350000022.PNG)