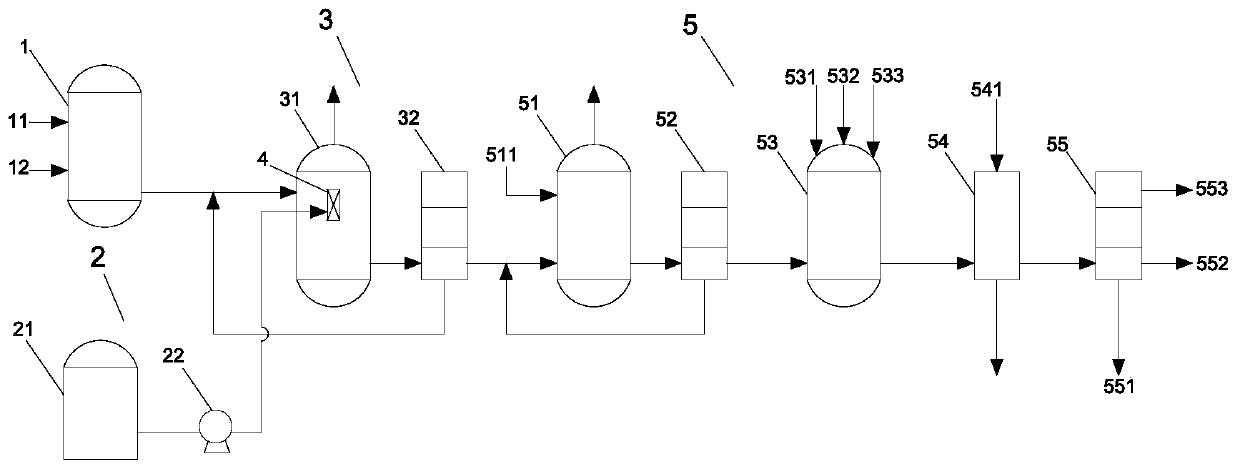
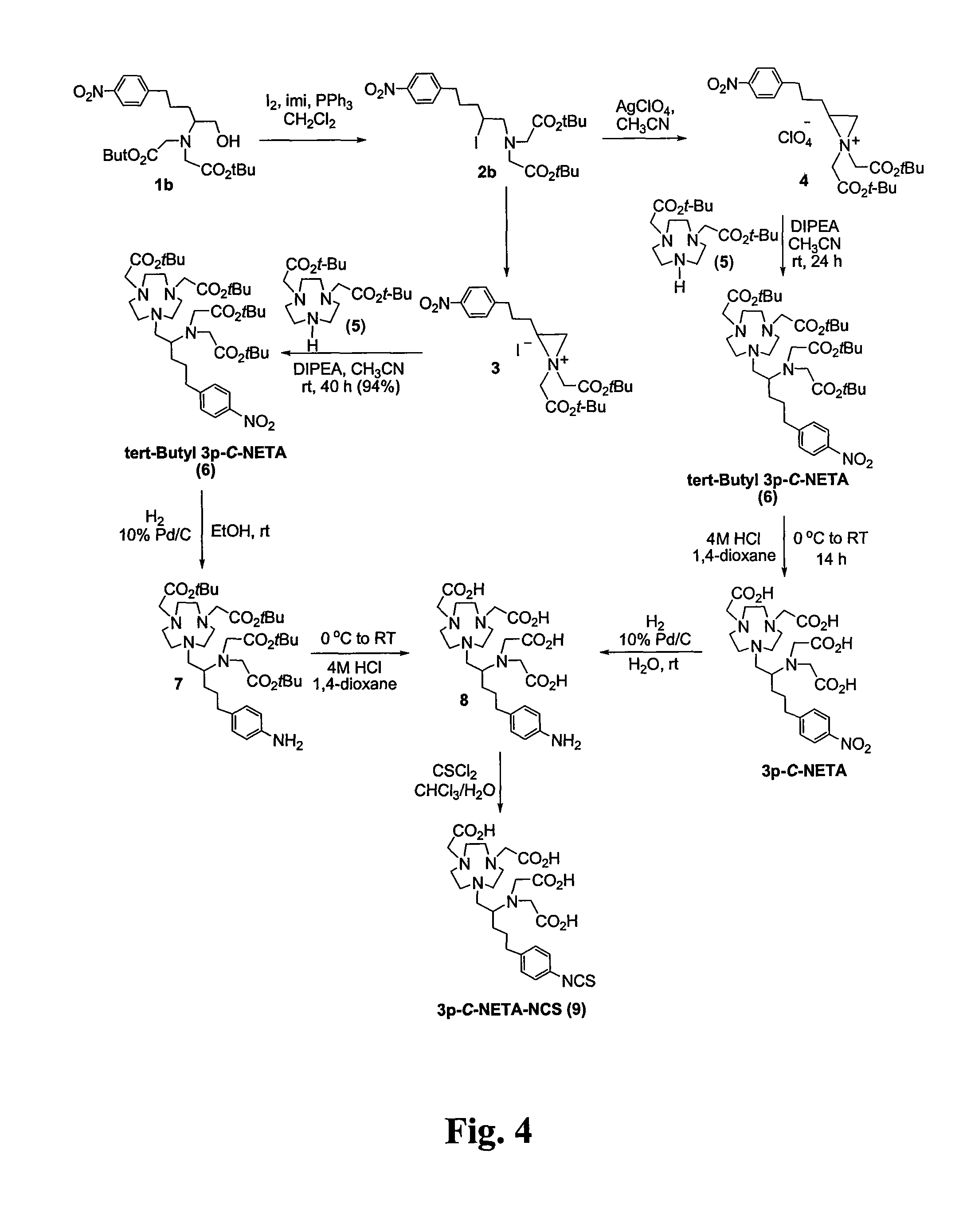
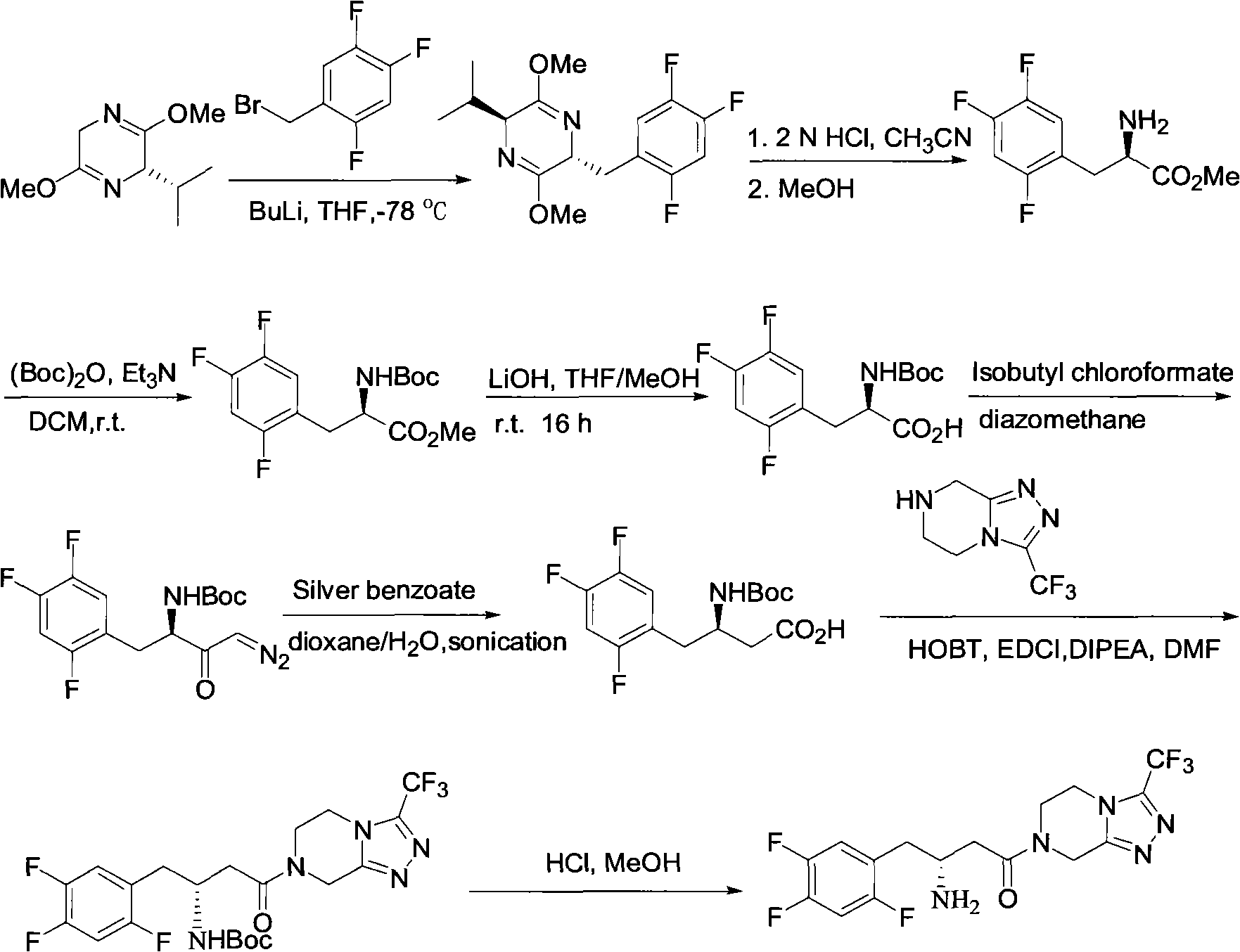
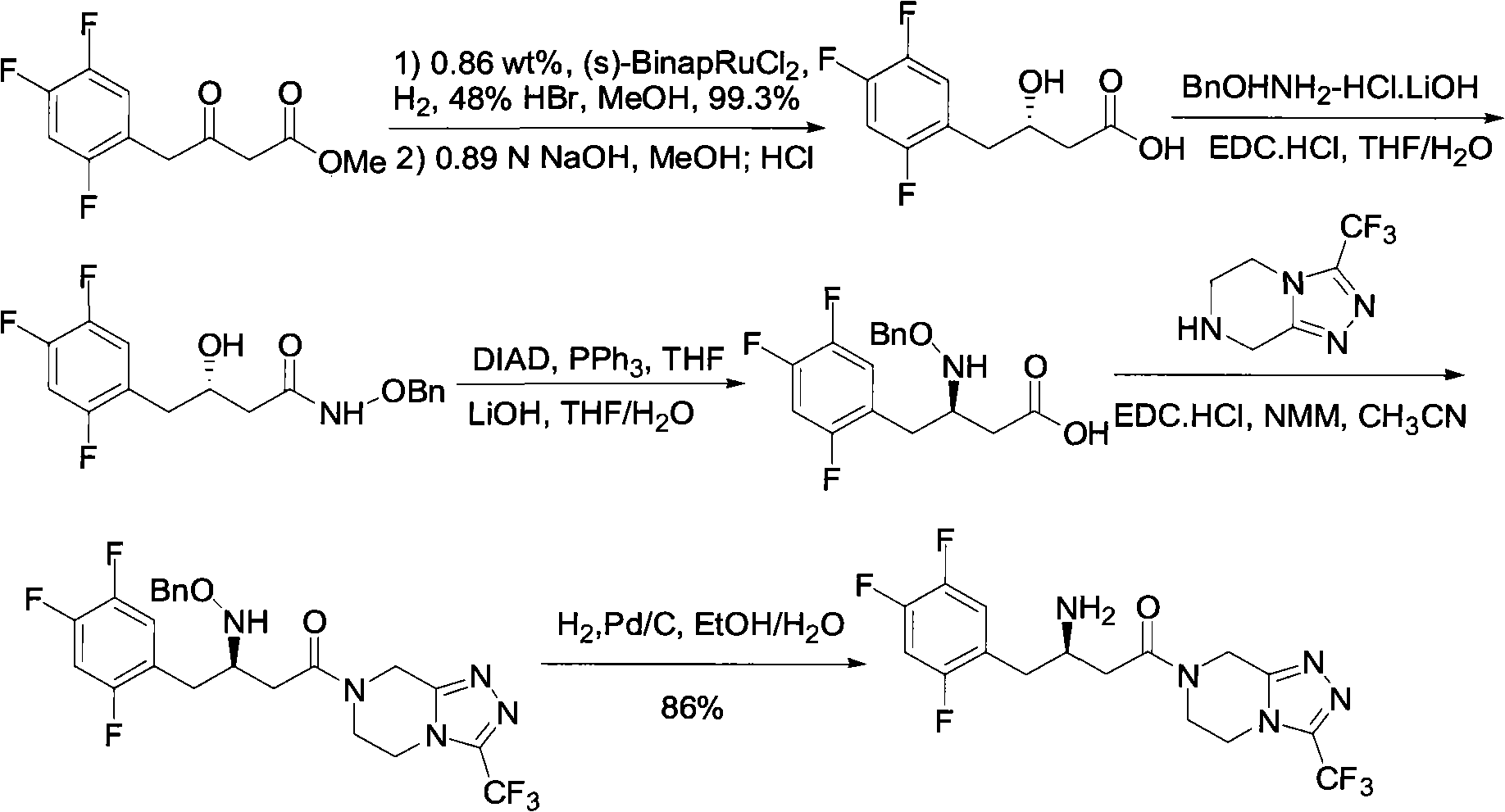
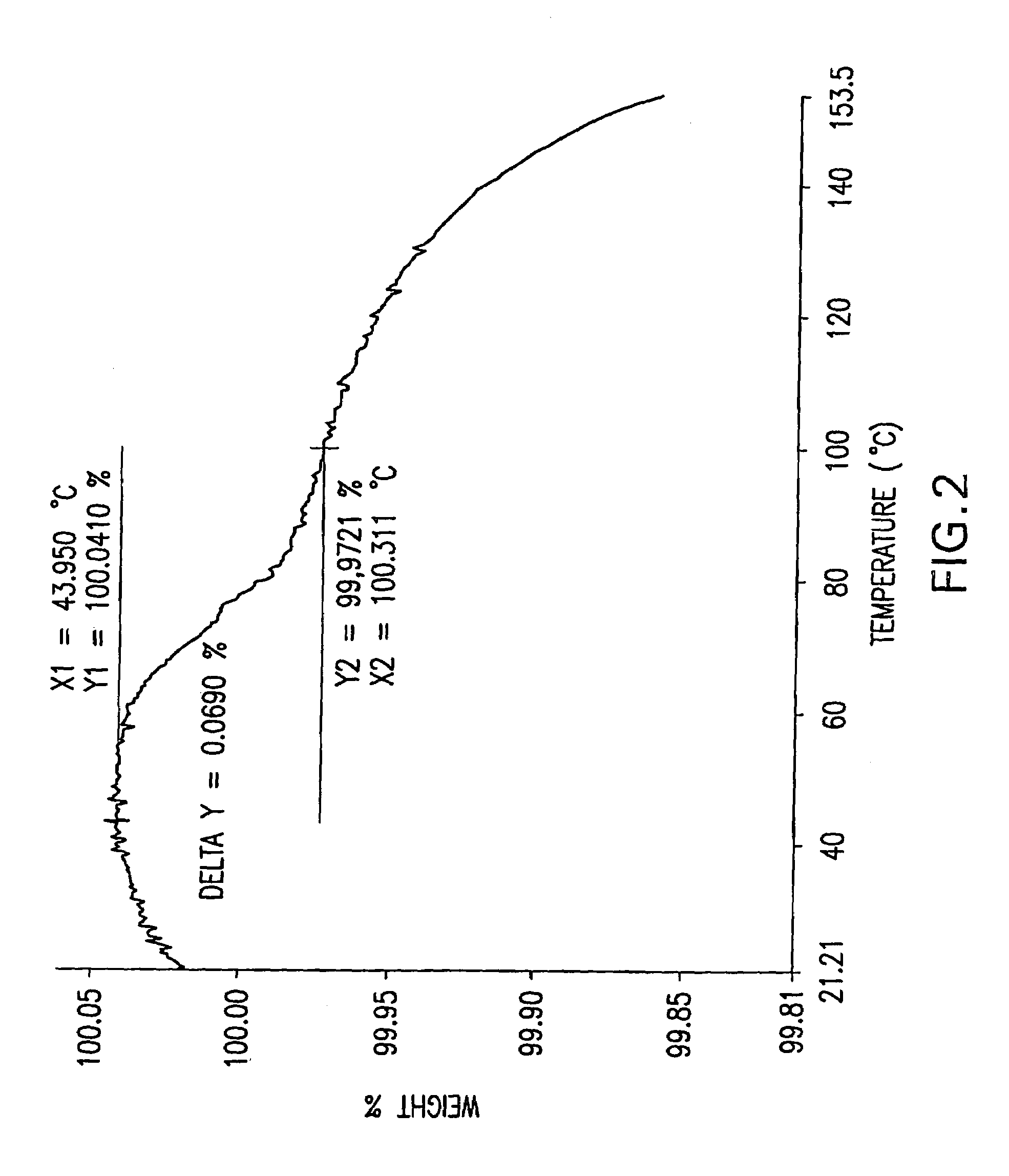
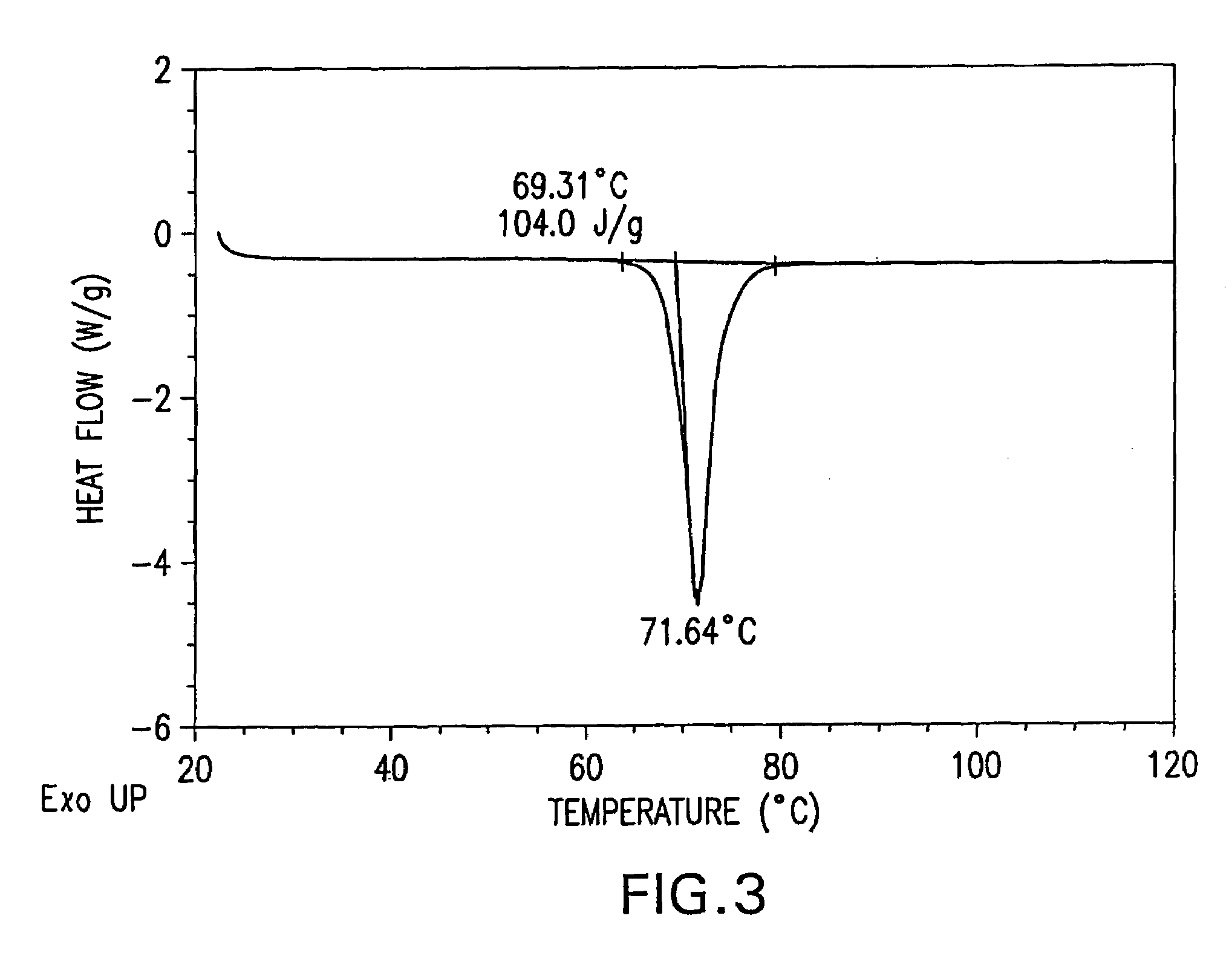
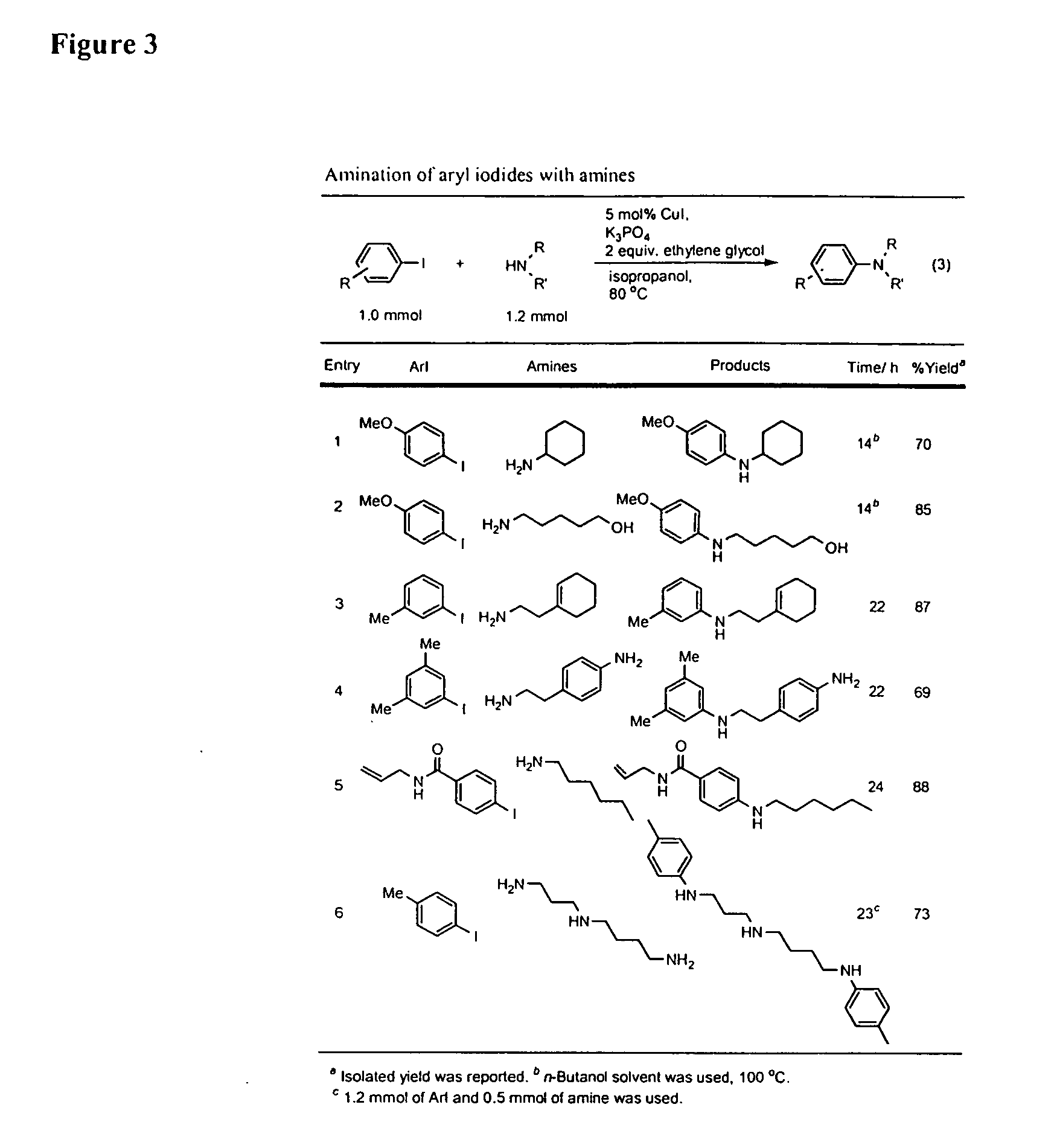
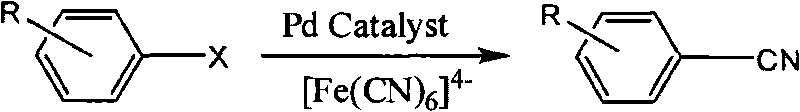Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
426results about "Preparation by cyanide reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
InactiveUS6867298B2Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
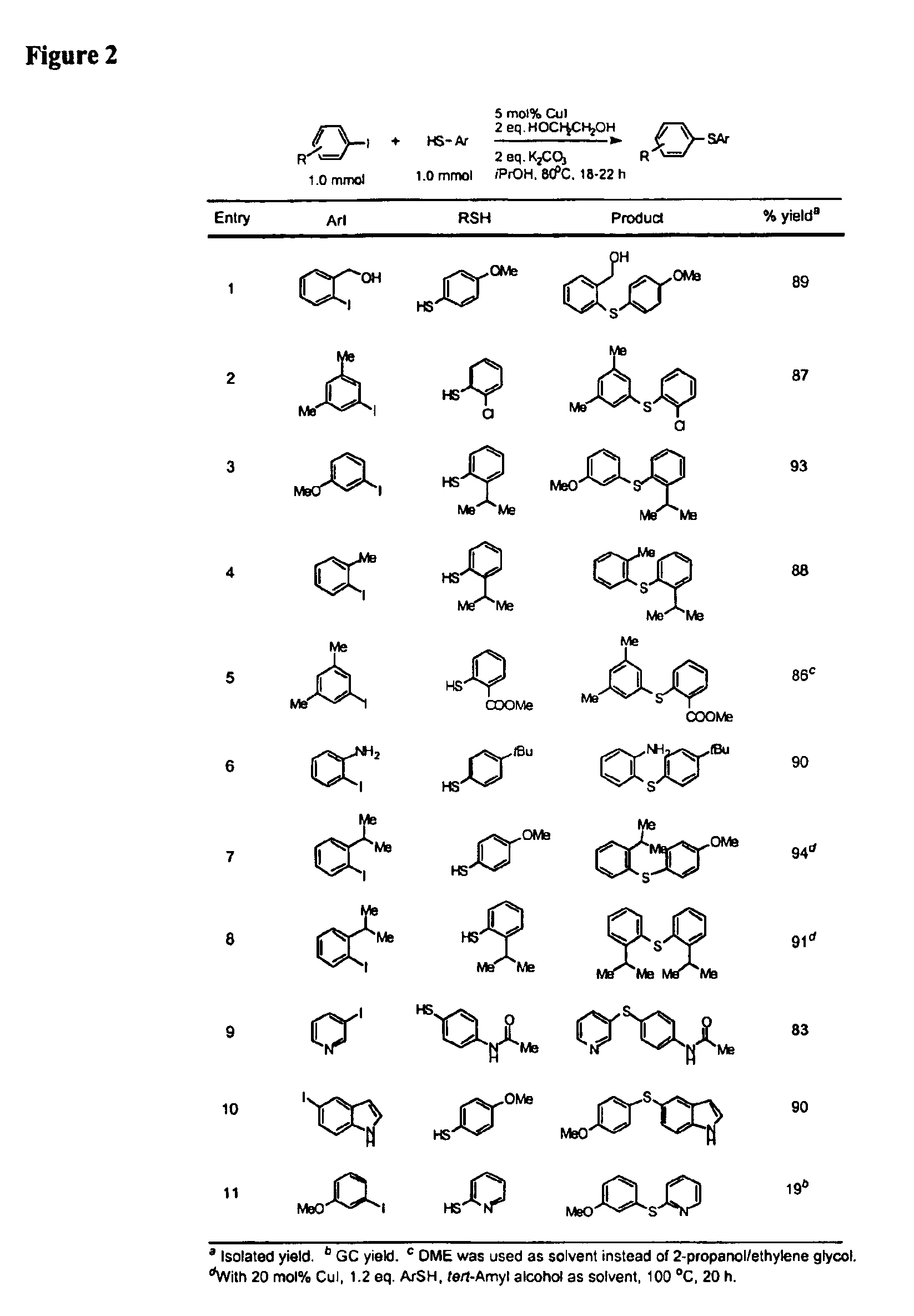
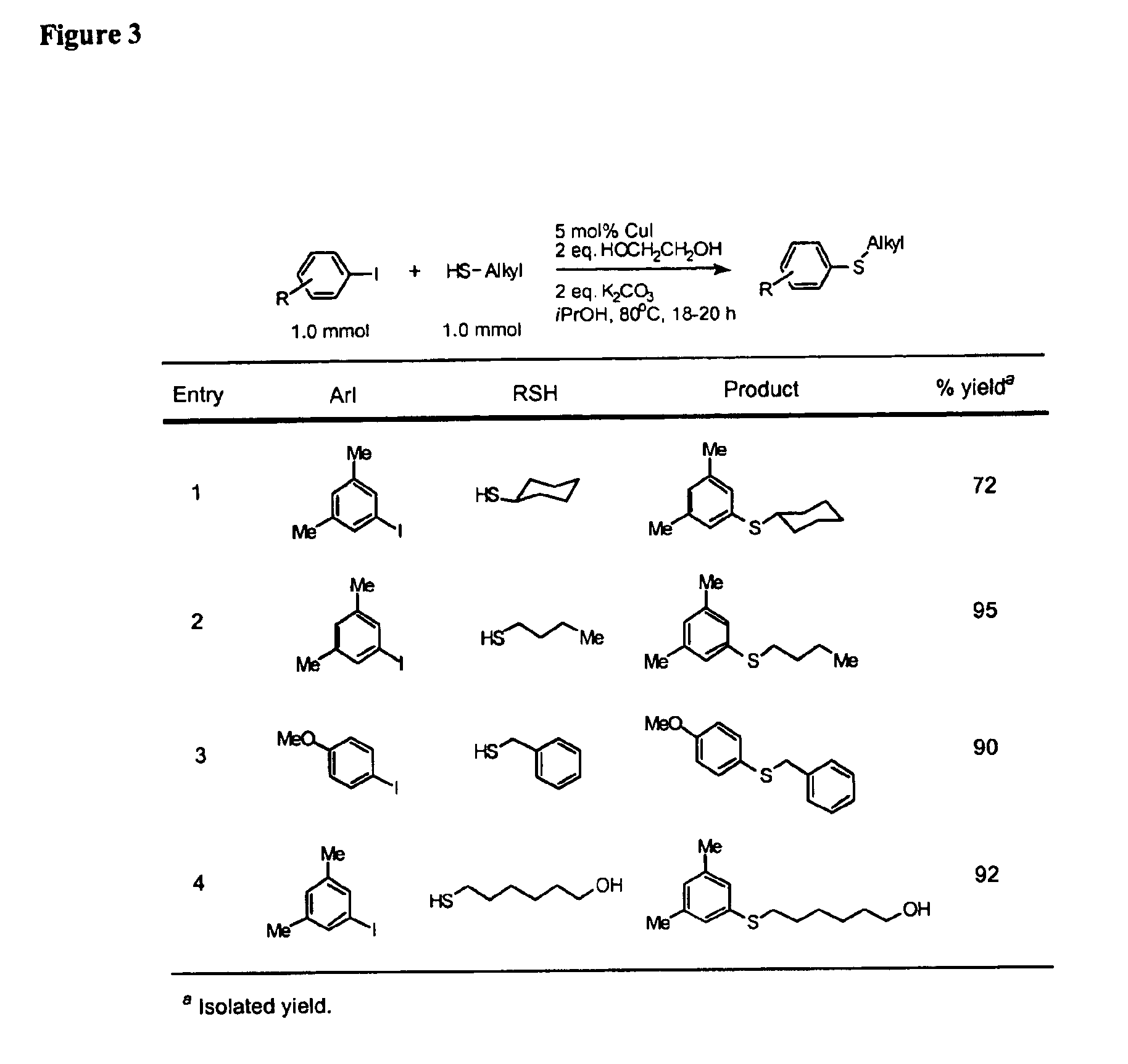
InactiveUS6888032B2Cheap and practicalLow costOrganic compound preparationThiol preparationCarbon–carbon bondSulfide
One aspect of the present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-sulfur bond between the sulfur atom of a thiol moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper(II)-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-carbon bond between the carbon atom of cyanide ion and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In another embodiment, the present invention relates to a copper-catalyzed method of transforming an aryl, heteroaryl, or vinyl chloride or bromide into the corresponding aryl, heteroaryl, or vinyl iodide. Yet another embodient of the present invention relates to a tandem method, which may be practiced in a single reaction vessel, wherein the first step of the method involves the copper-catalyzed formation of an aryl, heteroaryl, or vinyl iodide from the corresponding aryl, heteroaryl, or vinyl chloride or bromide; and the second step of the method involves the copper-catalyzed formation of an aryl, heteroaryl, or vinyl nitrile, amide or sulfide from the aryl, heteroaryl, or vinyl iodide formed in the first step.
Owner:MASSACHUSETTS INST OF TECH
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS20130310555A1Short reaction stepsHigh yieldOrganic compound preparationThiol preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
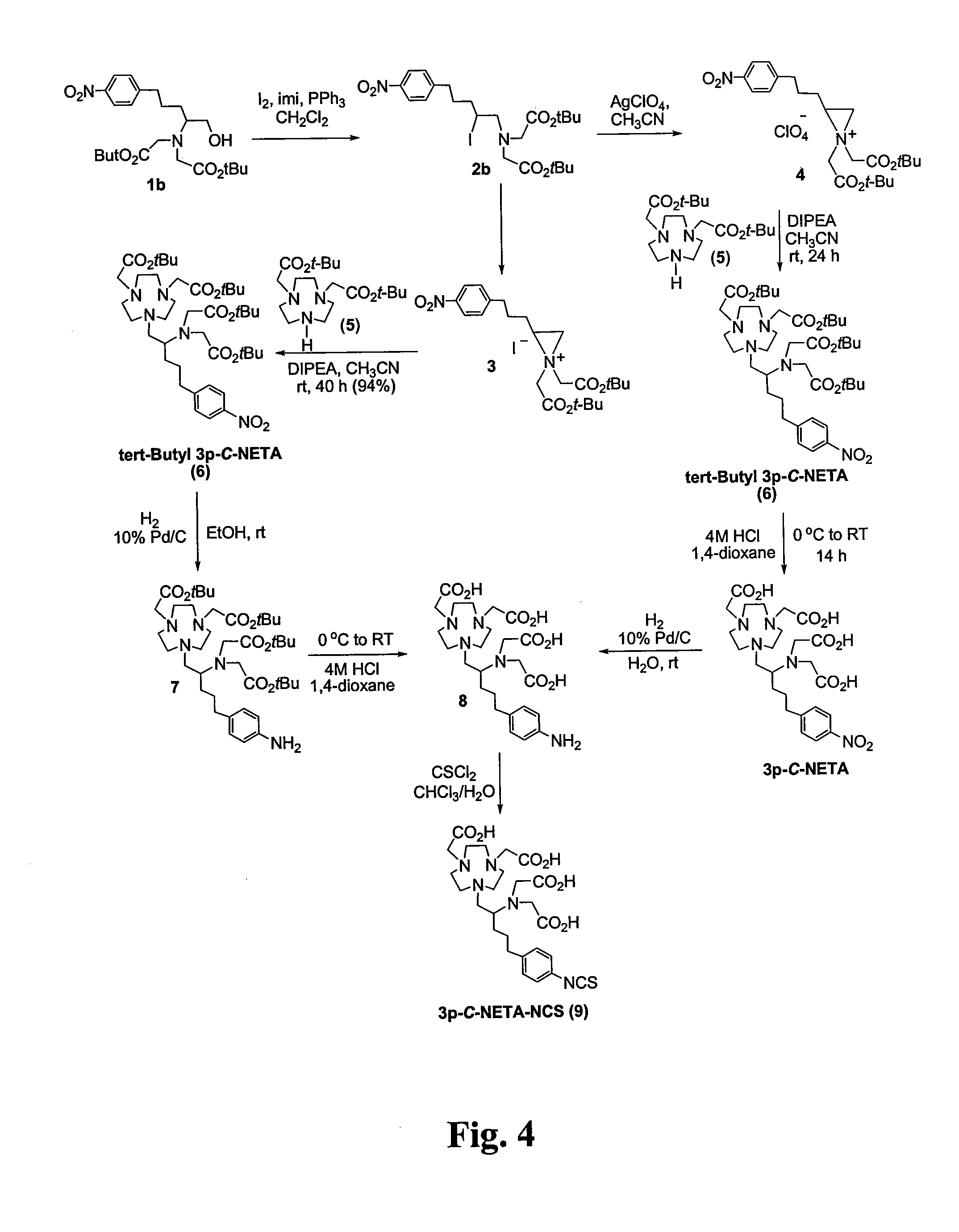
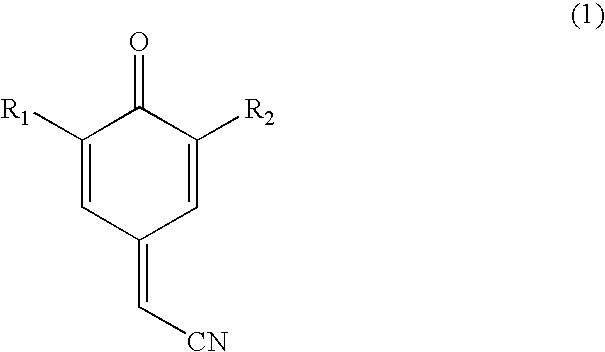
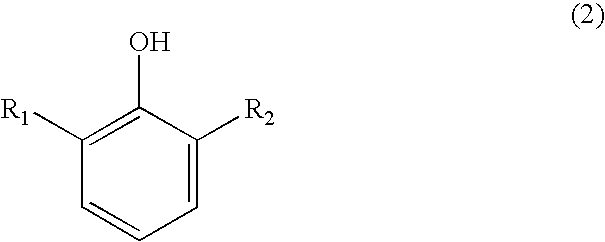
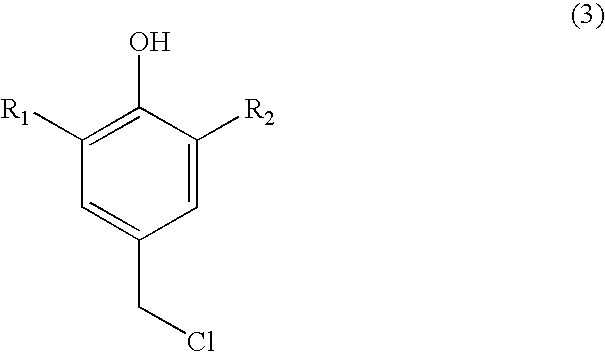
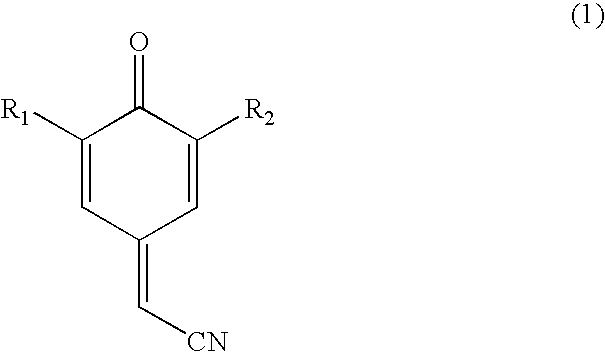
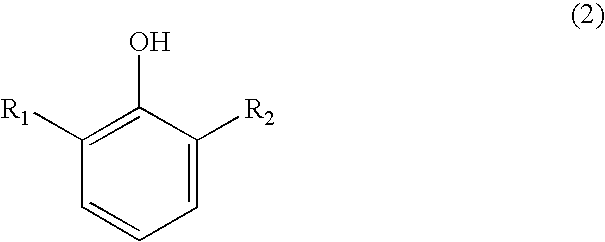
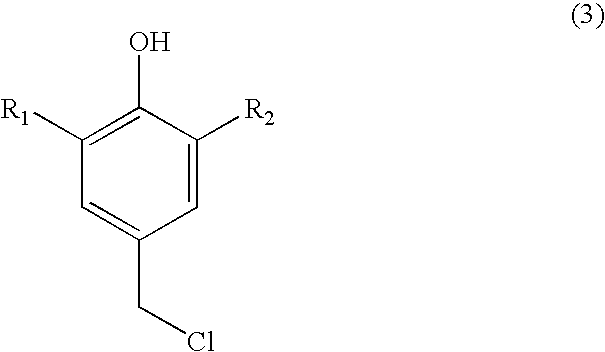
Process for preparing substituted 7-cyano quinone methides
ActiveUS20090287013A1Organic compound preparationPreparation by cyanide reactionOrganic solventQuinone methide
A one-pot process of preparing a substituted 7-cyano quinone methide in which i) a substituted phenol is chloromethylated to form a substituted 4-chloromethylphenol; ii) converting the substituted 4-chloromethylphenol to a substituted 4-cyanomethylphenol; and iii) oxidizing the substituted 4-cyanomethylphenol to the substituted 7-cyano quinone methide, where steps i)-iii) are carried out in a single reaction vessel in a solvent system comprising water and one or more organic solvents and where after steps i) and ii) the aqueous portion of the reaction mixture is removed and the reagents for the subsequent step are added in aqueous solution. The 7-cyano quinone methides are effective inhibitors of the polymerization of reactive monomers.
Owner:ECOLAB USA INC
Manufacturing method of atorvastatin intermediate (R)-(-)-4-nitrile-3-hydroxybutyrate
InactiveCN101838221AIncrease profitEmission reductionPreparation by cyanide reactionState of artEnantiomer
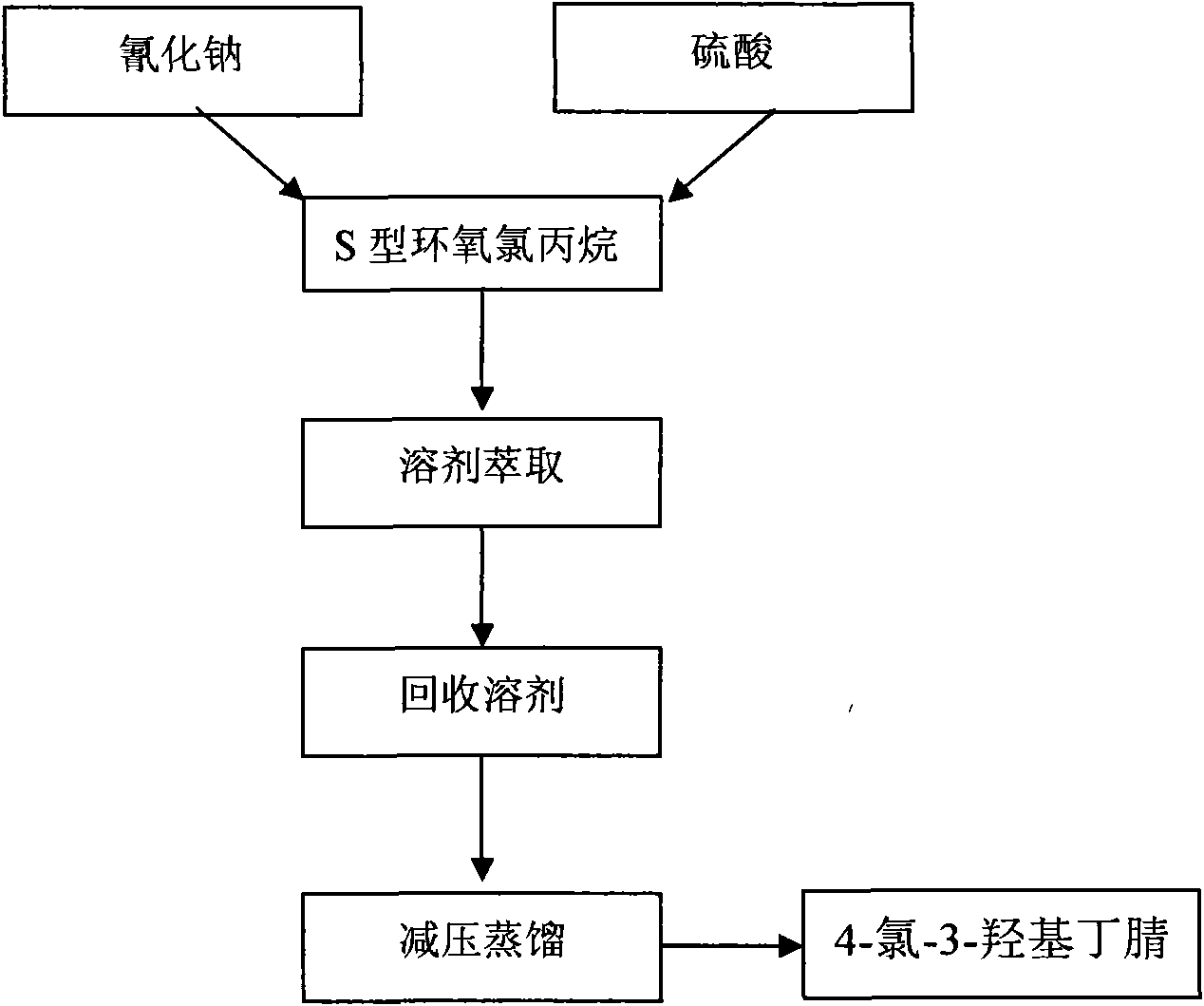
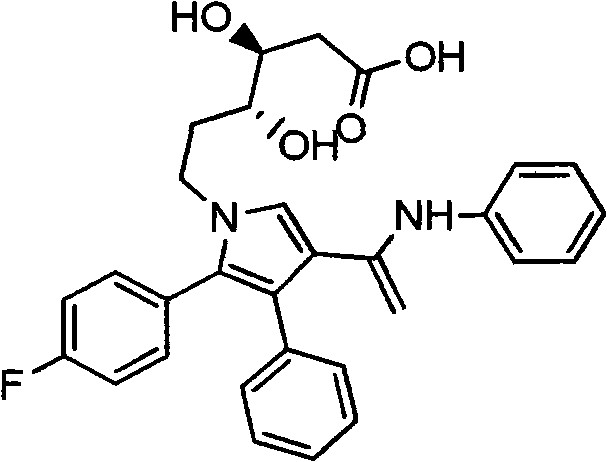
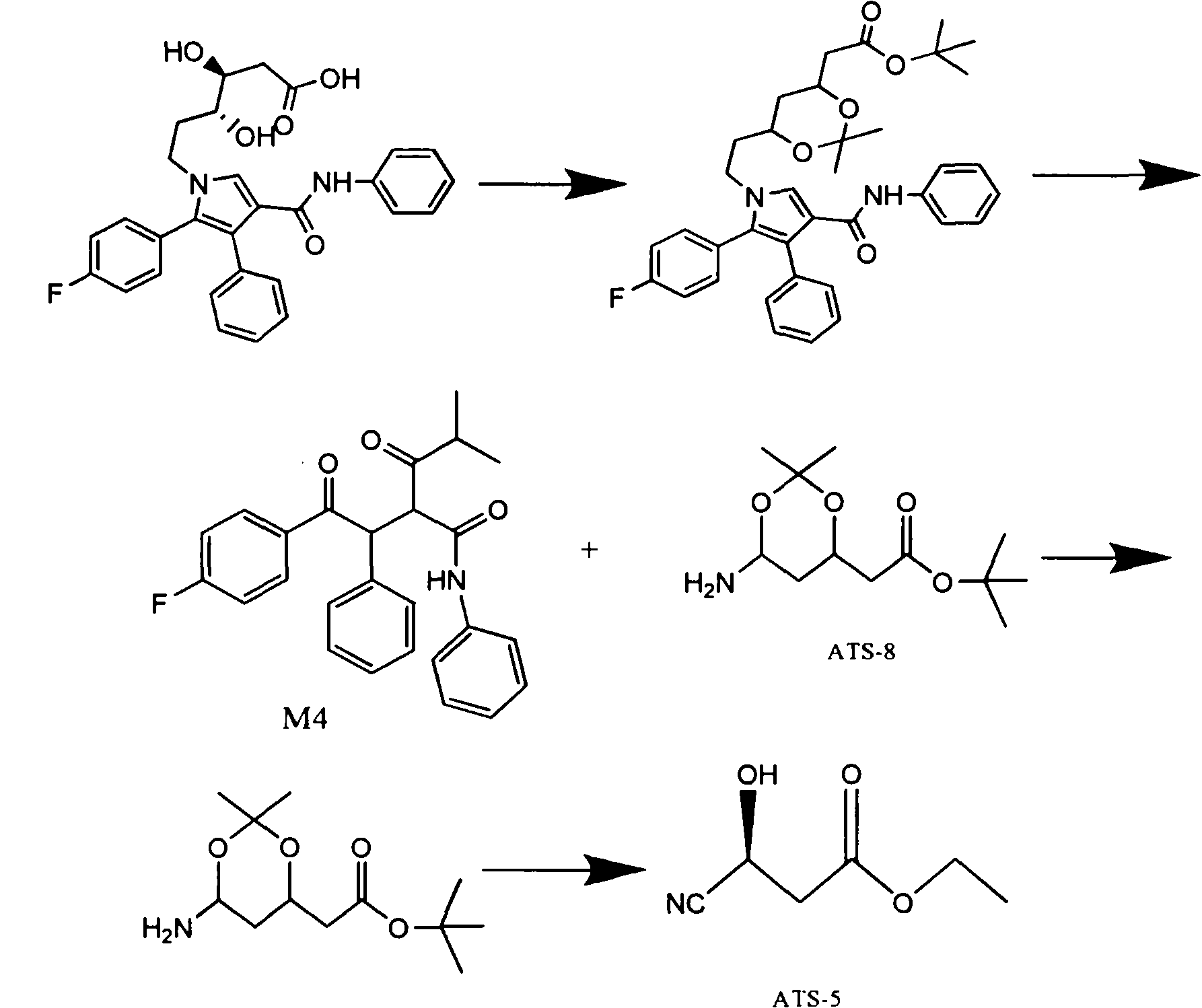
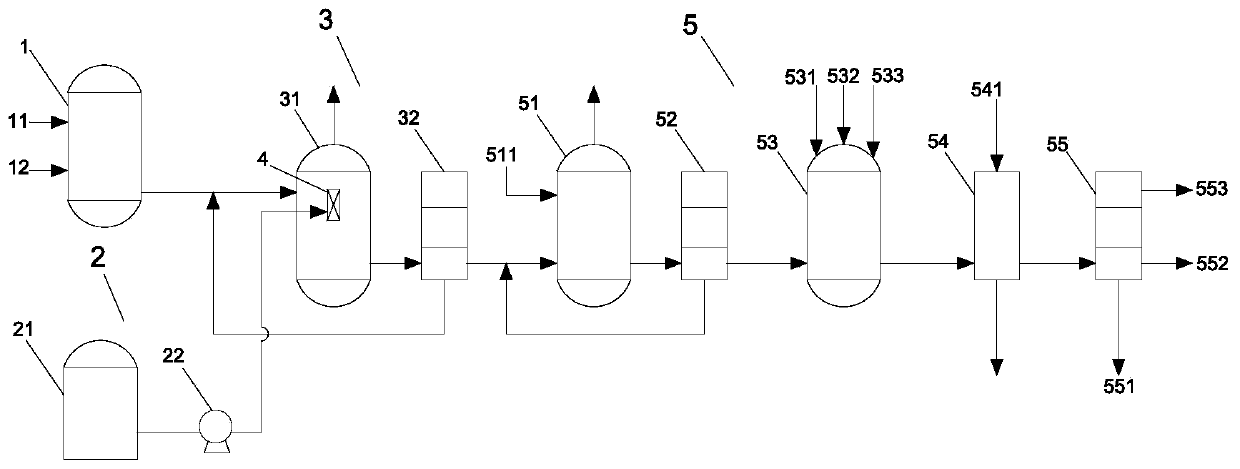
The invention relates to a manufacturing method of atorvastatin intermediate (R)-(-)-4-nitrile-3-hydroxybutyrate (ATS-5), which solves the problems of high cost, expensive raw material price and the like of the prior art. The manufacturing method is characterized in that the atorvastatin intermediate is prepared from epichlorohydrin as a raw material through ring-opening addition, glycolysis esterification, cyaniding substitution synthesis and refining. The invention has simple synthesis route, abundant raw material resources, low equipment requirements and no high-temperature high-pressure reaction; the content of produced ATS-5 is more than 98 percent, the content of water is lower than 0.2 percent, and the content of enantiomer is less than 1.0 percent. The manufacturing method is a mature production process for manufacturing the intermediate of medicine atorvastatin and is applicable to medium-sized and small enterprises.
Owner:HUANGGANG HUAYANG PHARMA
Synthetic system of 3-cyanomethylbenzoic acid methyl ester and method thereof
InactiveCN110002993AIncrease the mass transfer area of the phase boundaryImprove mass transfer efficiencyOrganic compound preparationPreparation by cyanide reactionChlorideMethyl benzoate
The invention provides a synthetic system of 3-cyanomethylbenzoic acid methyl ester and a method thereof. The system comprises an acylation reaction vessel used for preparation of m-Toluoyl chloride;a liquid chlorine feeding unit used for storing and conveying liquid chlorine; a chlorination reaction unit which is connected with the acylation reaction vessel and used as a chlorination reaction chamber of m-Toluoyl chloride; a micro-interface generator which is respectively connected with the liquid chlorine feeding unit and the chlorination reaction unit and used for receiving liquid chlorineconveyed by the liquid chlorine feeding unit, crushing the liquid chlorine into micro-droplets with the diameter being micron level and conveying the micro-droplets to the chlorination reaction unitafter the crushing; and an esterification and cyanation reaction unit which is connected with the chlorination reaction unit. In the prior art, m-Toluoyl chloride and liquid chlorine cannot be fully mixed when m-Toluoyl chloride and liquid chlorine react such that yield of 3-cyanomethylbenzoic acid methyl ester is reduced. The above problem is solved by the synthetic system and the method of the invention.
Owner:NANJING UNIV
Process for the preparation of protected L-alanine derivatives
ActiveUS8710256B2Carbamic acid derivatives preparationOrganic compound preparationMedicinal chemistryL-alanosine
Owner:JANSSEN PHARMA NV
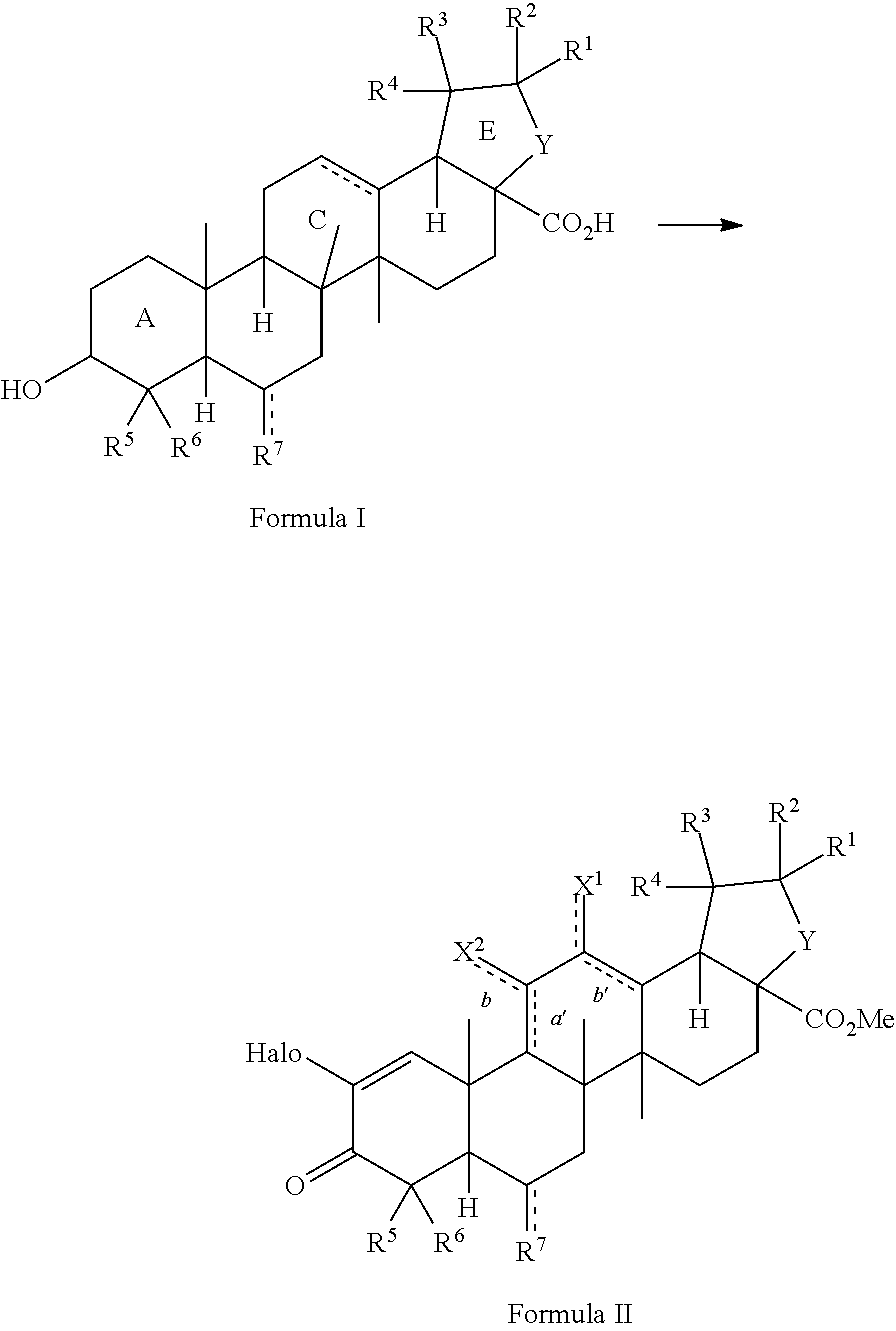
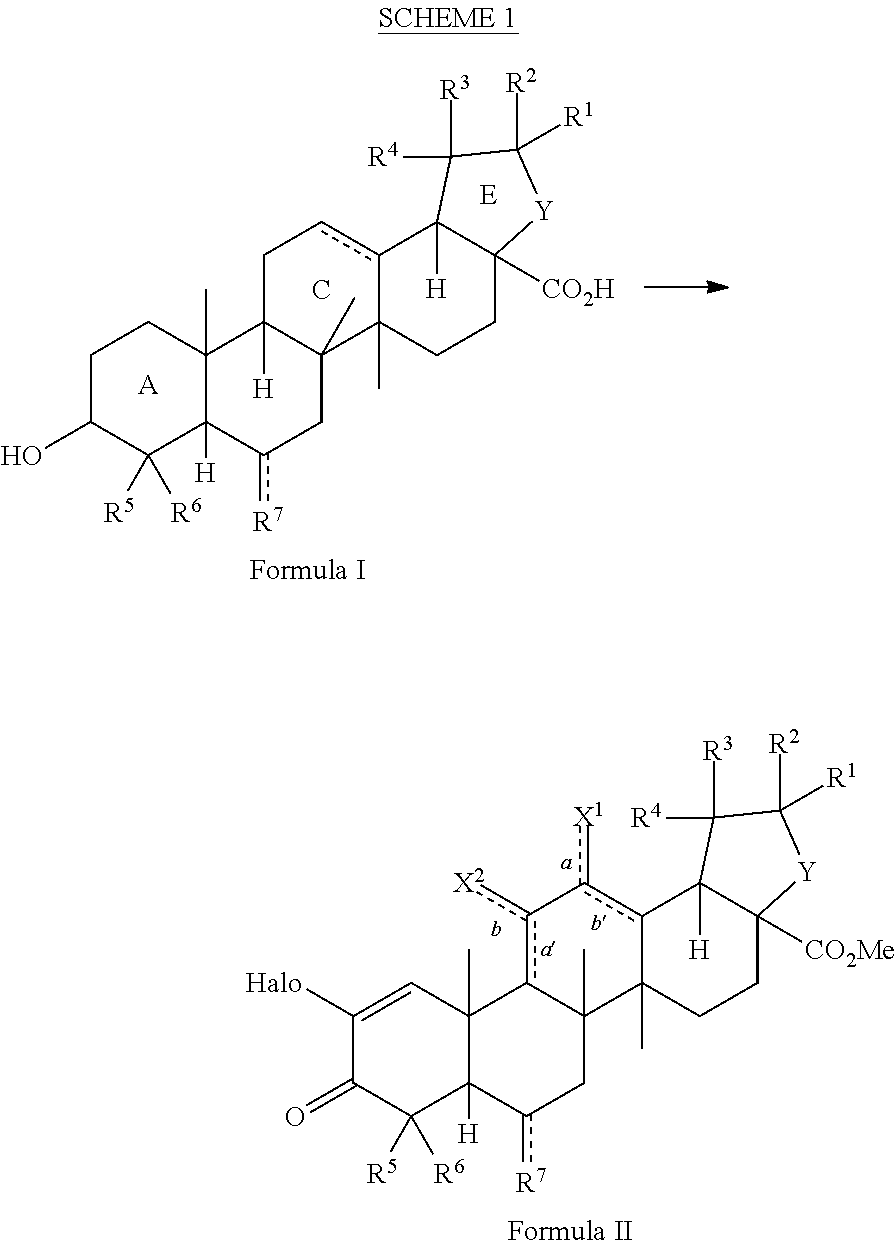
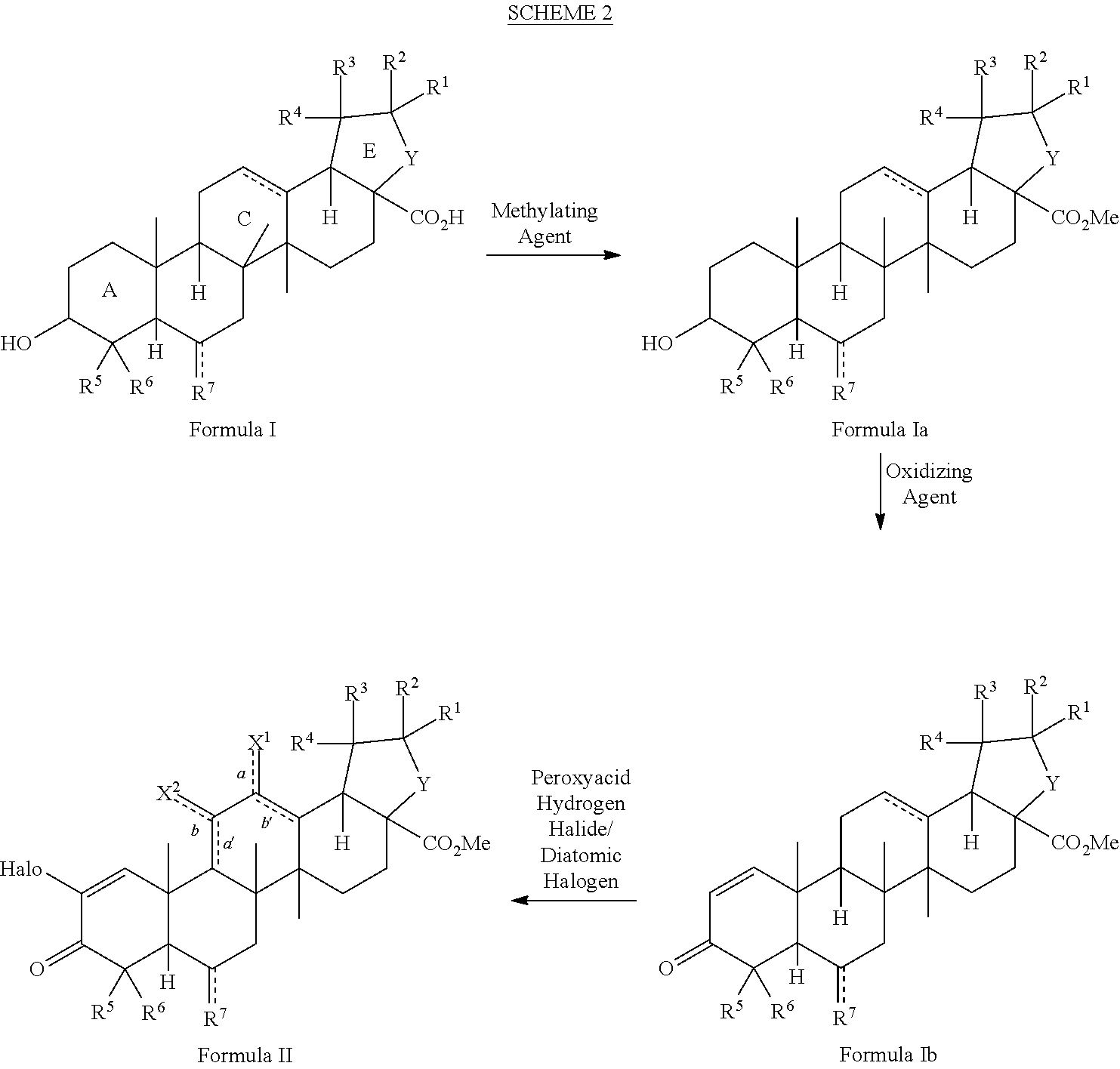
Method for synthesizing 2-cyano-3,12-dioxoolean-1, 9(11)-dien-28-oic acid methyl ester and derivatives thereof
ActiveUS20130303797A1Organic compound preparationCarboxylic acid esters preparationSumaresinolic acidUrsolic acid
The present invention is a method for preparing triterpenoids such as 2-cyano-3,12-dioxoolean-1,9-dien-28-methyl ester and derivatives thereof from oleanic acid, ursolic acid, betulinic acid, sumaresinolic acid or hederagenin.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
2-(4-methylphenyl)propionic acid syntehsis method
ActiveCN104402698AShort process routeReduce the discharge of three wastesPreparation from carboxylic acid saltsOrganic compound preparationPropanoic acidPropionitrile
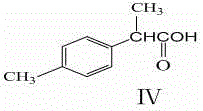
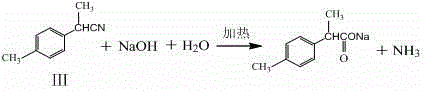
The invention relates to a synthesis method of a fine chemical product 2-(4-methylphenyl)propionic acid. According to the method, para-xylene is adopted as a raw material, and a chlorination reaction is carried out, such that a compound I which is p-methylbenzyl chloride is obtained; p-methylbenzyl chloride is subjected to a nitrilation reaction, such that a compound II which is p-methylbenzyl cyanide is adopted; p-methylbenzyl cyanide is subjected to methylation, such that a compound III which is 2-(4-methylphenyl)propionitrile is obtained; and 2-(4-methylphenyl)propionitrile is subjected to hydrolysis acidification, such that a compound IV which is 2-(4-methylphenyl)propionic acid is obtained. The method provided by the invention has the advantages of short process route, low three-waste discharge, low environment pollution, no solid waste production during reaction processes, relatively low production cost, less chemical substances used during reaction processes, less raw material types, easy-to-obtain raw materials, simple operation, high yield, relatively mild process conditions, and the like.
Owner:柳州丰康泰科技有限公司 +2
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS9446995B2Short reaction stepsHigh yieldThiol preparationOrganic compound preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Monoazo compound as well as preparation method and application thereof
ActiveCN102603572AImprove washing fastnessGood fastness to sublimationAzo dye preparationPreparation by cyanide reactionColour fastnessDisperse dye
The invention relates to the technical field of disperse dyes and provides a monoazo compound as well as a preparation method and application thereof for the purposes of solving the problems that dyeing is carried out on fabrics such as terylene and superfine fiber so as to obtain very deep color and excellent washing resistance fastness and sublimation resistance fastness. The monoazo compound has a general formula (I). When the monoazo compound is dyed on fabrics such as terylene and superfine fiber, the obtained fabrics have very deep color, excellent washing resistance fastness and sublimation resistance fastness, friction resistance color fastness and light fastness and good color fixation rate in dip dyeing. The monoazo compound provided by the invention is also suitable for compounding of mixed tones with other disperse dyes.
Owner:HANGZHOU JIHUA JIANGDONG CHEMICAL CO LTD
Method for preparing high purity solid cyanoacetic acid
InactiveCN101270063AHigh purityImprove efficiencyPreparation by cyanide reactionAcetic acidSodium chloroacetate
The present invention discloses a preparation method of high-purity solid cyano acetic acid. The preparation method comprises the following steps: (1) chloroactic acid and sodium hydroxide have the reaction of neutralization and cyanidation to prepare sodium chloroacetate aqueous solution; hydrochloric acid is added for neutralization; then the mixed solution of cyano acetic acid and sodium chloride can be prepared; (2) at the temperature of 20 to 90 DEG C, ester solvents are used for extracting the mixed solution prepared in the first step; the volume ratio of the mixed solution and the ester solvents is 1 : 1 to 5; the time of extraction is 4 to 10; the time of each extraction step is 5 to 15 minutes; (3) the extract is condensed through vacuum evaporation to prepare the high-purity solid cyano acetic acid. The preparation method has the advantages of simple process and low investment. The extractant is stable, cheap and easily available; and the extractant can be recycled and reused. More importantly, the preparation method can be used for preparing the high-purity solid cyano acetic acid and can satisfy the requirements for refining the high-purity solid cyano acetic acid in the industry.
Owner:TIANJIN UNIV
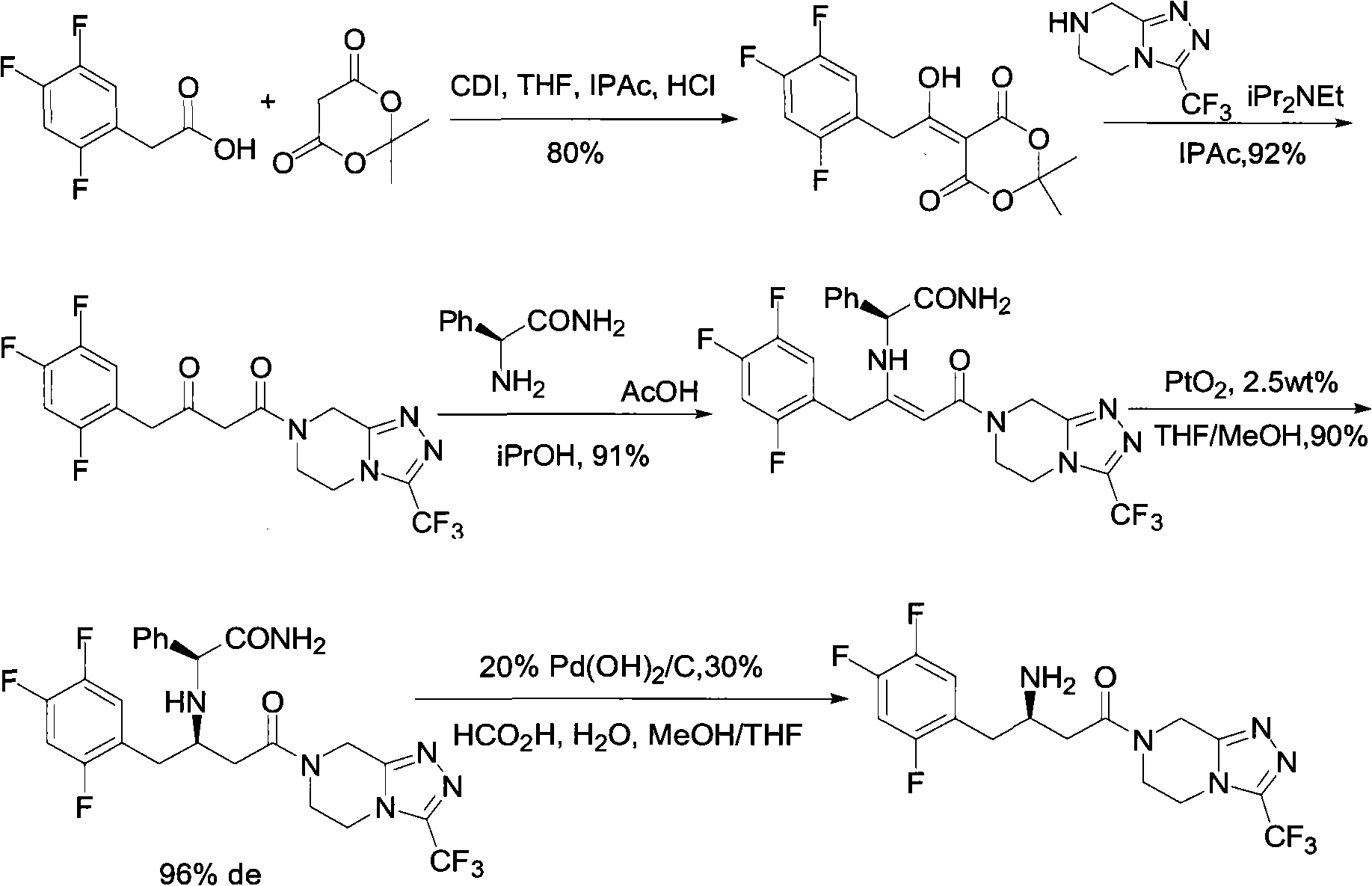
Sitagliptin intermediates as well as preparation method and application of intermediate
InactiveCN102838511AAvoiding asymmetric catalytic hydrogenation reactionsImprove economyPreparation by cyanide reactionPreparation from nitrilesSitagliptinEpoxy
The invention relates to a Sitagliptin intermediates and a preparation method thereof as well as application of the intermediates in a method for preparing the Sitagliptin. According to the invention, epoxy chloropropane which is low in price and easy to obtain is used as a raw material to synthesize intermediates (S)-(2, 4, 5-trifluorophenyl) epoxy propane and (S)-3-hydroxy-4-(2, 4, 5-trifluorophenyl) butyronitrile, thus a chiral center is introduced, the use of various complex chiral reagents are avoided, and the chiral asymmetric catalytic hydrogenation reaction can be avoided; and the preparation method has the advantages of simple synthetic route, environment conservation, low raw material cost, etc.
Owner:ZHEJIANG HISOAR PHARMA
Process for preparing substituted 7-cyano quinone methides
ActiveUS8247593B2Organic compound preparationPreparation by cyanide reactionOrganic solventQuinone methide
A one-pot process of preparing a substituted 7-cyano quinone methide in which i) a substituted phenol is chloromethylated to form a substituted 4-chloromethylphenol; ii) converting the substituted 4-chloromethylphenol to a substituted 4-cyanomethylphenol; and iii) oxidizing the substituted 4-cyanomethylphenol to the substituted 7-cyano quinone methide, where steps i)-iii) are carried out in a single reaction vessel in a solvent system comprising water and one or more organic solvents and where after steps i) and ii) the aqueous portion of the reaction mixture is removed and the reagents for the subsequent step are added in aqueous solution. The 7-cyano quinone methides are effective inhibitors of the polymerization of reactive monomers.
Owner:ECOLAB USA INC
Synthetic method of aryl cyanide in water solution
InactiveCN101717350AEasy to separateReduce pollutionOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by cyanide reactionSolubilityPalladium catalyst
The invention relates to a synthetic method of an aryl cyanide in a water solution, which comprises the following steps: adding an aryl compound, a ferrocyanide and a phase transfer agent into an alkaline water solution, or adding the aryl compound and the ferrocyanide into a mixed solution of alkaline water and an organic solution; controlling the reaction temperature at 30 DEG C-140 DEG C; and synthesizing the aryl cyanide by catalytic coupling of a metal palladium catalyst. In the invention, the non-toxic environment-friendly ferrocyanide is used as a cyanide source for synthesizing the aryl cyanide, and water is used as an environment-friendly solvent to substitute the virulent cyanide and the organic solvent in the prior art, thereby solving the problem of environment pollution in the process of synthesizing the aryl cyanide. In the invention, good solubility of the ferrocyanide in the water and the strong polarity of the ferrocyanide are used, thereby greatly promoting the generation of the aryl cyaniding reaction, the cyaniding reaction can be finished at lower temperature (30 DEG C-140 DEG C), the reaction yield is high, and the application range of the aryl cyaniding reaction is improved.
Owner:NANJING UNIV OF TECH
Process for making organic products and improving the quality of non-product streams using phase transfer catalysis
InactiveUS6846946B2Low costLow cost of treatmentCarbamic acid derivatives preparationOrganic compound preparationChemical speciesOrganic solvent
A method for preparing organic products from aqueous solutions, such as waste or byproduct liquid streams and waste or byproduct gas or vapor streams, uses phase transfer catalysis to transfer a chemical species in low concentration from the aqueous solution to the organic phase or the aqueous-organic interface. The system has little or no organic solvent, and the organic phase contains an electrophile which participates in the reaction. In one embodiment, the aqueous solution is contacted with the electrophile and a phase transfer catalyst and, optionally, a pH adjusting agent in the event that the chemical species in the aqueous solution is not sufficiently ionized to react with the electrophile, and optionally an organic solvent. A method for continuously converting a chemical species involves this contacting step, separating the phases, then dividing the organic phase into the product, the phase transfer catalyst, and the optional organic solvent.
Owner:VALUE RECOVERY
Functionalized column aromatic hydrocarbon derivative and preparation method thereof
ActiveCN109400501AThe means of expanding functionalityAddresses less functional flawsOrganic compound preparationSulfonic acid esters preparationAromatic hydrocarbonOxime
The invention discloses a functionalized column aromatic hydrocarbon derivative and a preparation method thereof. The functionalized column aromatic hydrocarbon derivative comprises an X unit, a Y unit and a Z unit, wherein the X unit is shown in a formula (I), the Y unit is shown in a formula (II), the Z unit is shown in a formula (III), and the six-membered rings of the X unit, the Y unit and the Z unit all have two para-position substituents and four unsubstituted positions; the formulas are as shown in the specification, each unit is connected through CH2 groups to form a ring-shaped structure, and each unit is connected with the other two adjacent units in a para-position manner at an unsubstituted position; and the formed ring-shaped structure is the functionalized column aromatic hydrocarbon derivative. Cyano groups, carboxyl groups and oxime are directly connected to the benzene ring monomer of the column aromatic hydrocarbon, so that the method for functionalizing the column aromatic hydrocarbon is expanded; and the connected groups can effectively change the electronic environment of the column aromatic hydrocarbon rings, so that the functionalized column aromatic hydrocarbon derivative has wide application in the fields of supramolecular host-guest identification and the like.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
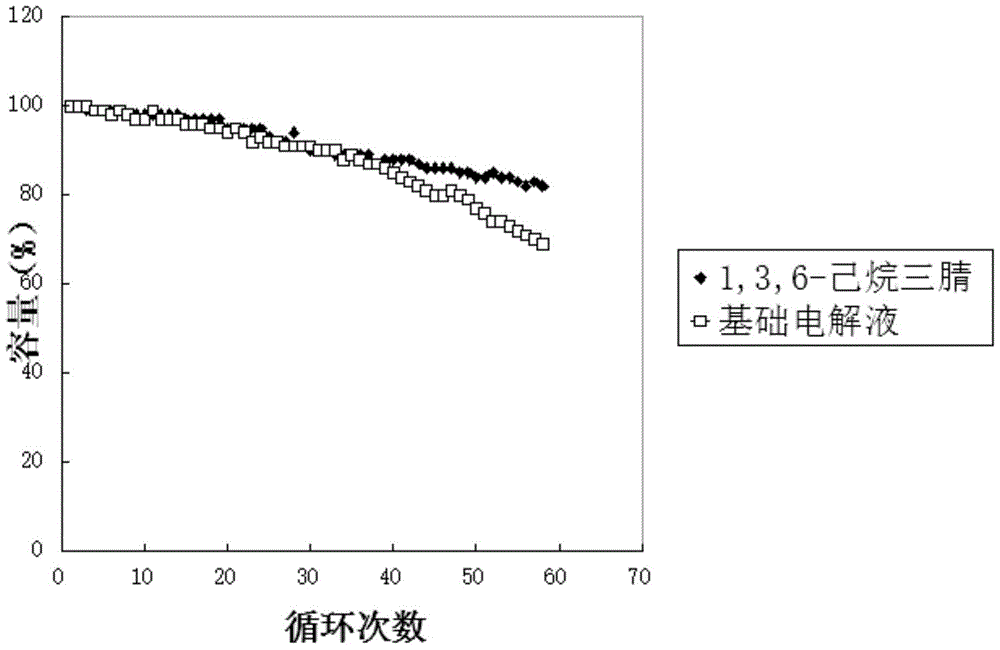
Preparation method of 1,3,6-hexanetricarbonitrile
ActiveCN105037203AImprove performanceAvoid security issuesSecondary cellsPreparation by cyanide reactionRaw materialSodium
The invention relates to a preparation method of 1,3,6-hexanetricarbonitrile, which comprises the following steps: A. preparation of 1,3-dicyano-2-butylene; and B. preparation of the 1,3,6-hexanetricarbonitrile. The invention provides a new preparation method of 1,3,6-hexanetricarbonitrile, which is completely different from the traditional technique and solves the safety problem in the 1,3,6-hexanetricarbonitrile preparation process in the presence of sodium. Thus, the technique is safer, and satisfies the demands for large-scale industrial production. Besides, the preparation method has the advantages of high yield, accessible raw materials, simple reaction control, high product purity and the like.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Technology of synthesizing 2,3-dicyano ethyl propionate
InactiveCN1785966AHigh yieldImprove product qualityPreparation by cyanide reactionParaformaldehydeEthyl fumarate
The present invention relates to a process for synthesizing ethyl-2,3-dicyano-propionate which is used for produce pesticide intermediate. The basic method of said process includes the following steps: using the raw materials of ethyl cyanoacetate, paraformaldehyde and sodium cyanide to synthesize ethyl-2,3-dicyano-propionate in the medium dimethyl sulfoxide; after the ethyl-2,3-dicyano-propionate is synthesized, using solvent dichloromethane to extract the synthesized ethyl-2,3-dicyano-propionate from medium dimethyl sulfoxide, reduced pressure desolventizing to obtain crude product, then rectifying said crude product so as to obtain the refined product ethyl-2,3-cyano-propionate. Said invention also provides the concrete requirements of the above-mentioned every step. The purity of the obtained product is greater than 98%.
Owner:栾忠岳
Process for preparation of substituted aromatic compound
InactiveUS6469224B1High yieldEfficient methodOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCompound aHydrogen atom
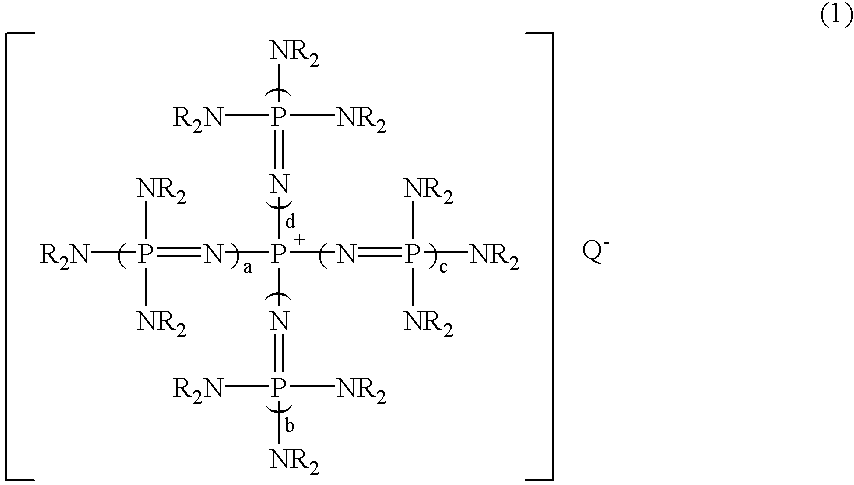
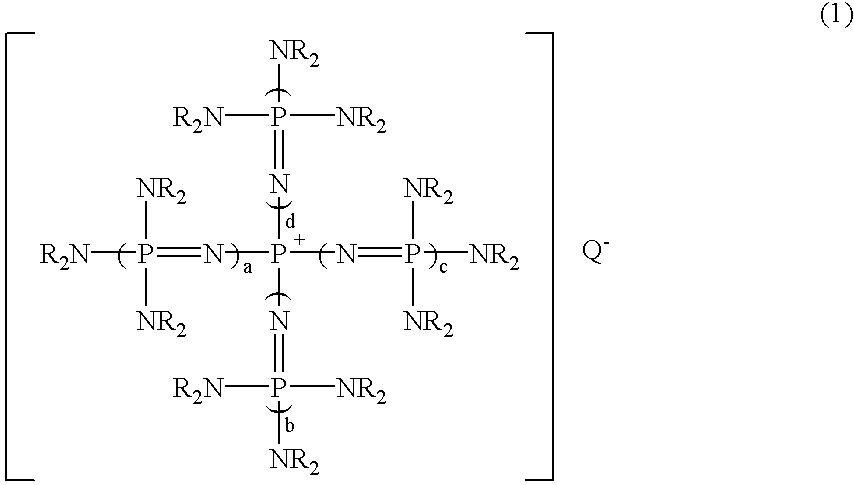
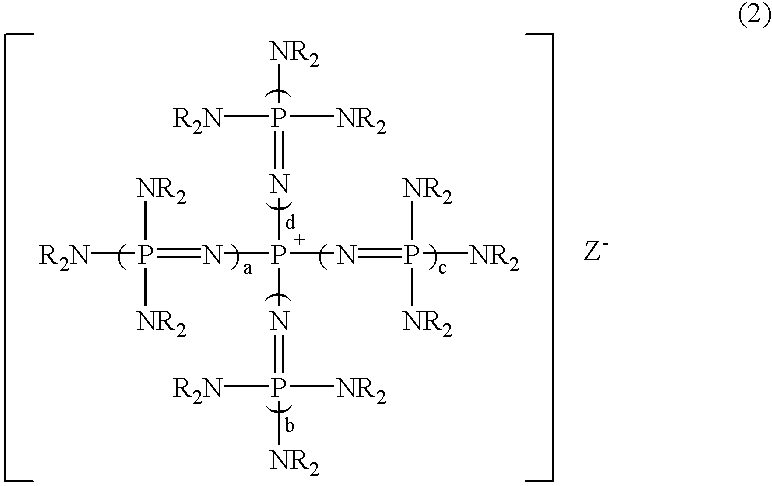
A substituted aromatic compound substituted with Q is obtained by reacting a phosphazenium compound represented by formula (1)(in the formula, Q- represents an anion in a form derived by elimination of a proton from an inorganic acid, or an active hydrogen compound having an active hydrogen on an oxygen atom, a nitrogen atom or a sulfur atom; a, b, c and d, each independently, is 0 or 1, but all of them are not 0 simultaneously; and R groups represent the same or different hydrocarbon groups having 1 to 10 carbon atoms, or two Rs on each common nitrogen atom may be bonded together to form a ring structure) with a halogenated aromatic compound having halogen atoms; whereby, at least one halogen atom in the halogenated aromatic compound is substituted with Q (where, Q represents an inorganic group or an organic group in a form derived by elimination of one electron from Q- in formula (1)).
Owner:MITSUI CHEM INC
Formation of tetra-substituted enamides and stereoselective reduction thereof
InactiveUS7629470B2Carboxylic acid amides optical isomer preparationPreparation by cyanide reactionCouplingTetra
The present invention is directed to a practical process for the preparation of an enamide (II) by palladium catalyzed coupling of a primary amide (IV) with a compound of structural formula (III), as shown below: As well as to crystalline forms of a compound produced by this process, in particular, an anhydrous crystal form, Form B, and crystalline solvates falling into three patterns, Type 1, Type 2, and Type 3, and crystalline intermediate compounds produced in the process. Still further, the present invention relates to the stereoselective reduction of the tetrasubstituted enamide (II) to the corresponding amide (I).
Owner:MERCK SHARP & DOHME CORP
Method for synthesizing phenylacetonitrile by performing continuous reaction
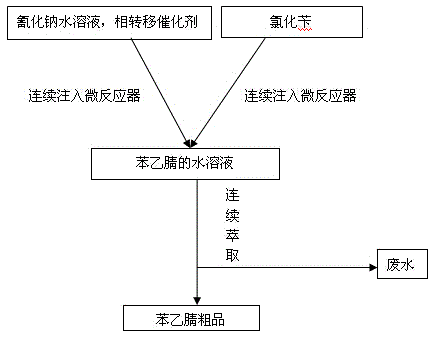
ActiveCN105218401ARealize continuous productionIncrease productivityPreparation by cyanide reactionAutomatic controlBenzyl chloride
The invention discloses a method for synthesizing phenylacetonitrile by performing continuous reaction, and relates to the field of preparing a compound containing six-membered aromatic ring connected with cyano group by saturated carbon chain. The method comprises the following steps: adjusting the temperature of a micro-reactor to 80-400 DEG C; adding a mixture of a sodium cyanide aqueous solution and phase-transfer catalyst, and benzyl chloride in the micro-reactor continuously; reacting in the micro-reactor for 20-300 s to get a phenylacetonitrile aqueous solution. The method has rapid reaction speed, high production efficiency, low cost, good product quality, is easy to realize automatic control, is beneficial to subsequent post-treatment, and can raise the phenylacetonitrile yield, reduce the side reaction, and save labour.
Owner:HEBEI CHENGXIN
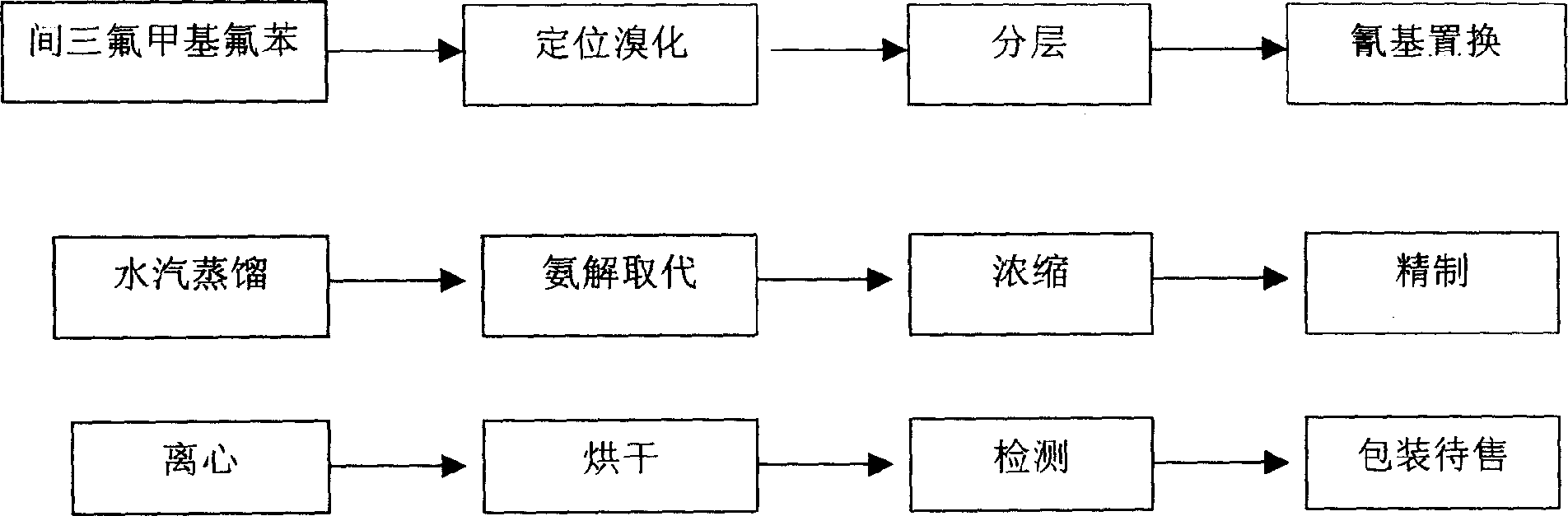
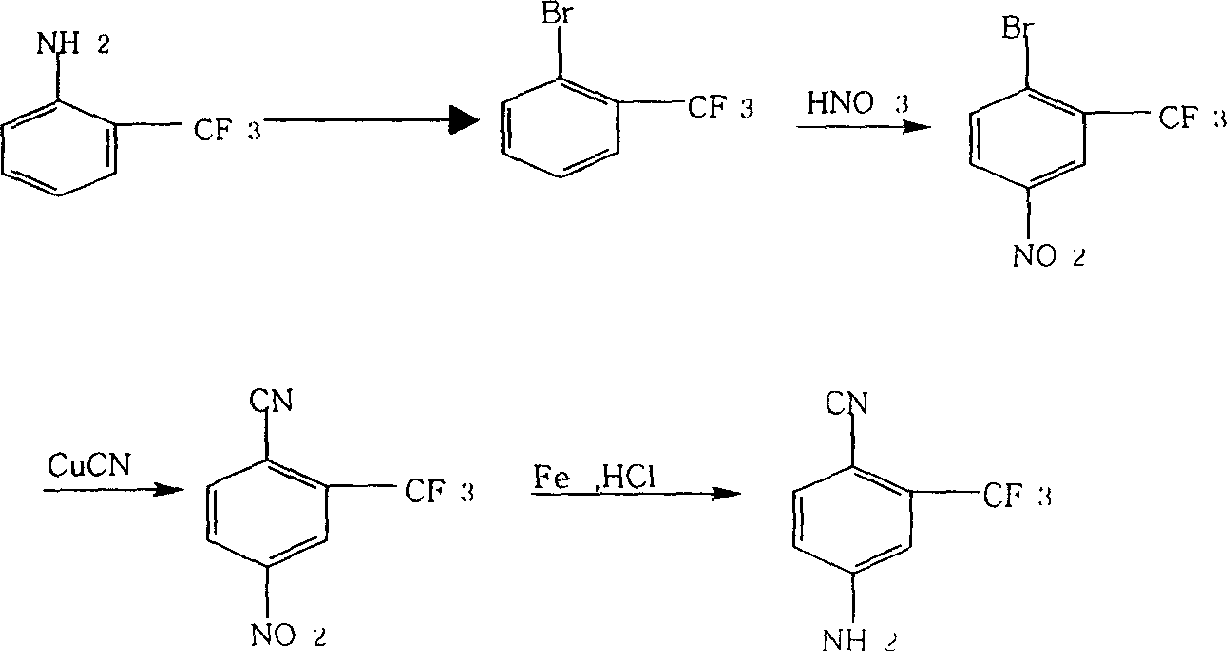
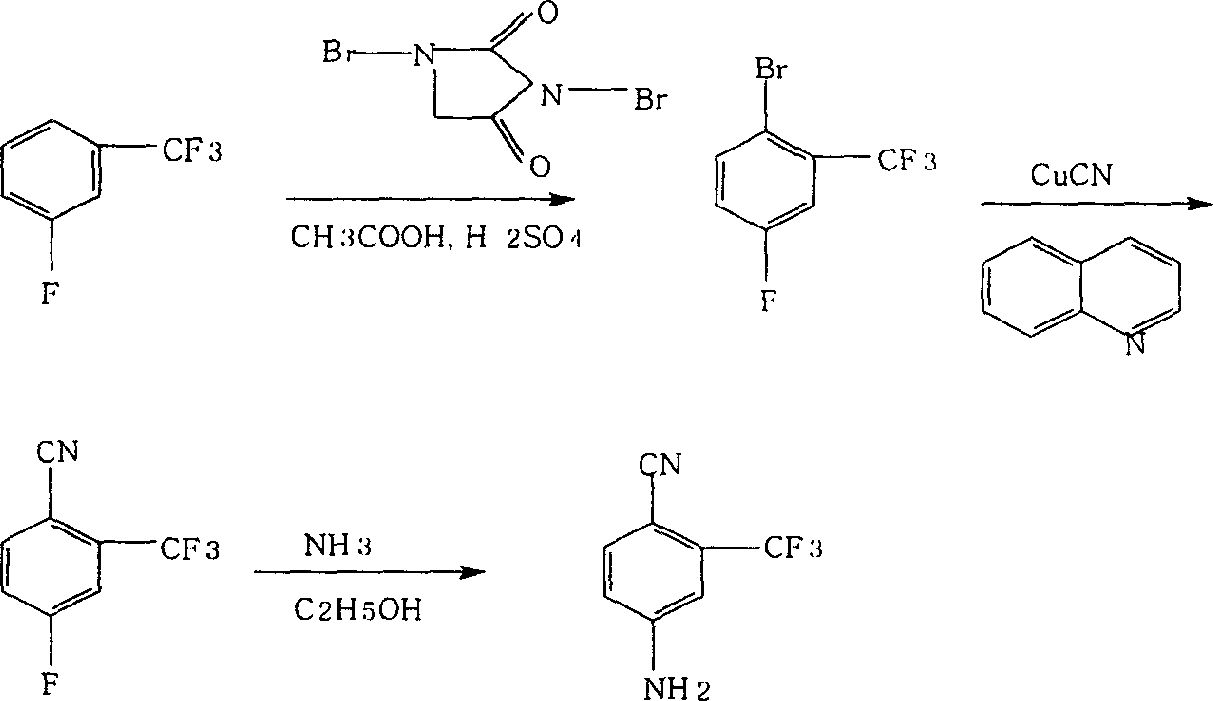
Prepn process of 4-amino-2-trifluoromethyl benzonitrile
The present invention discloses improved process of preparing 4-amino-2-trifluoromethyl benzoitrile. The present invention prepares 4-amino-2-trifluoromethyl benzoitrile with trifluoro methyl fluorobenzene as main material and through three steps of positioning bromination, cyano group replacement and aminolysis substitution. The preparation process uses marketable glacial acetic acid, concentrated sulfuric acid, dibromo hydantoin, cuprous cyanide, liquid ammonia and alcohol, and has less consumption of strong acid and cuprous cyanide. The preparation process has facile materials, simple operation, short path, product purity over 99 % and total product yield up to 73-75 %, less exhaust of harmful matter, and low production cost.
Owner:唐保清 +1
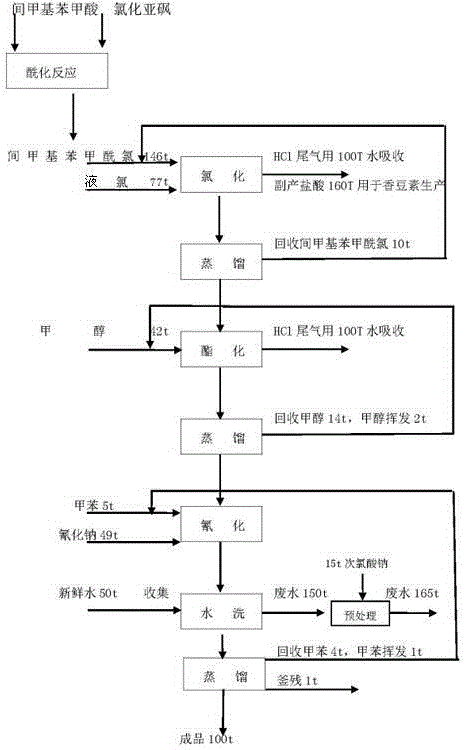
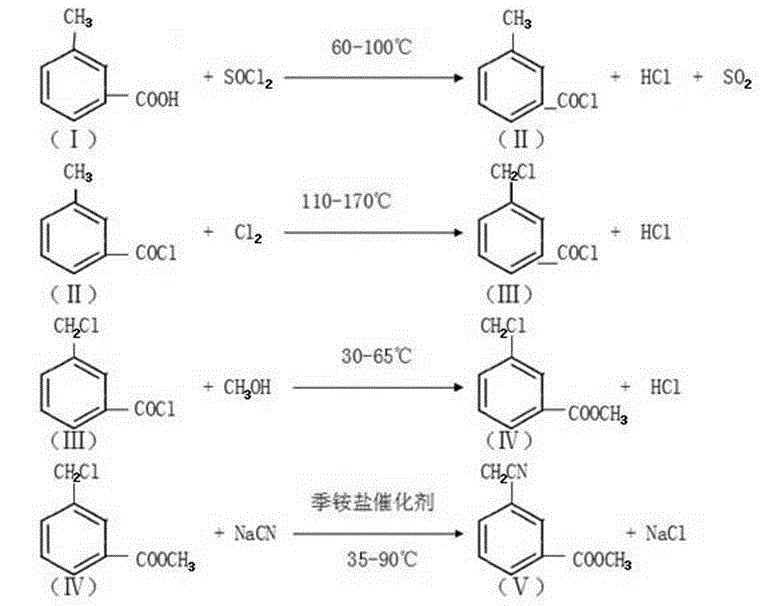
Methyl 3-(cyanomethyl)benzoate synthetic method
InactiveCN105130846AMild reaction conditionsEasy to operatePreparation by cyanide reactionMethyl benzoateSodium cyanide
The invention discloses a methyl 3-(cyanomethyl)benzoate synthetic method. The method comprises the following steps: taking m-toluic acid as a starting material, adding sulphone chloride for an acylation reaction, and flowing into liquid chlorine for a chlorination reaction, adding non-aqueous methanol dropwise for an esterification reaction to prepare ester, adding toluene and sodium cyanide into a cyanation reaction still, raising the temperature for backflow, adding the ester dropwise, carrying out backflow for 2 hours to prepare the methyl 3-(cyanomethyl)benzoate, and after the reaction, adding water to extract the methyl 3-(cyanomethyl)benzoate. The methyl 3-(cyanomethyl)benzoate synthetic method is high in yield, avoids serious three-waste pollution, and is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY +1
Microporous polymer-nano-metal particle catalyst and its preparation method and use
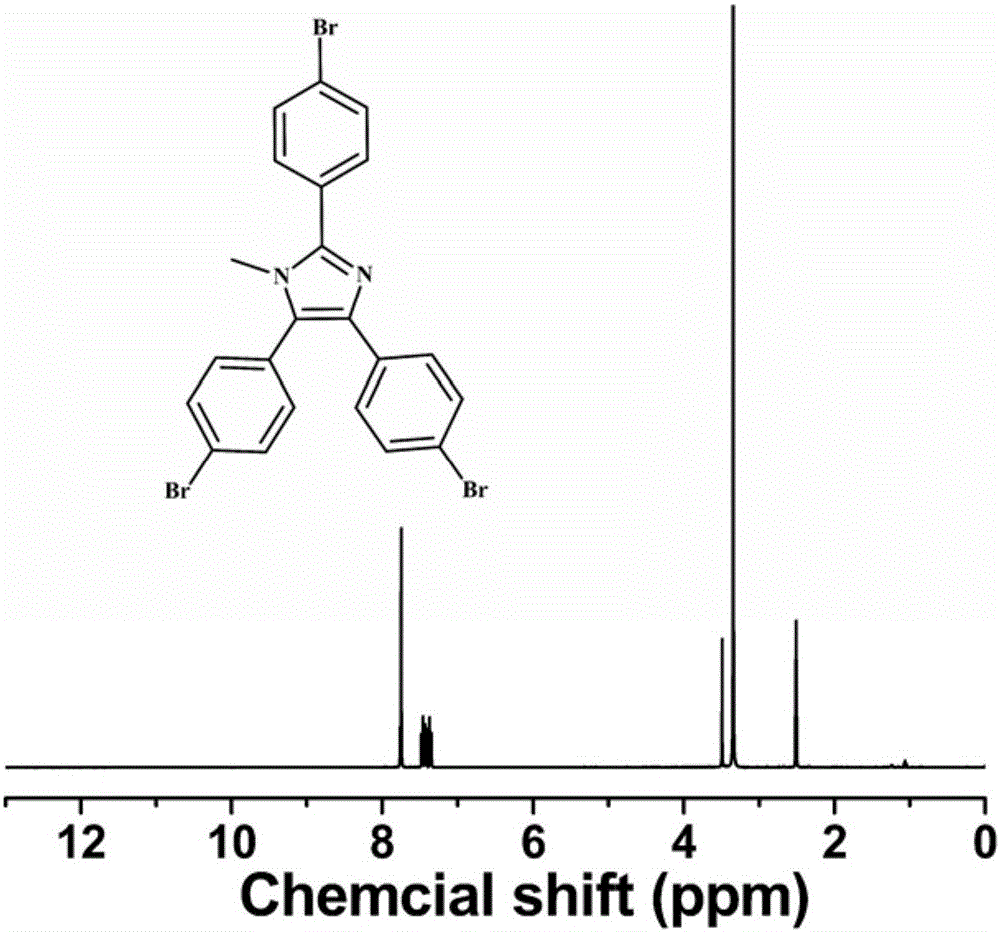
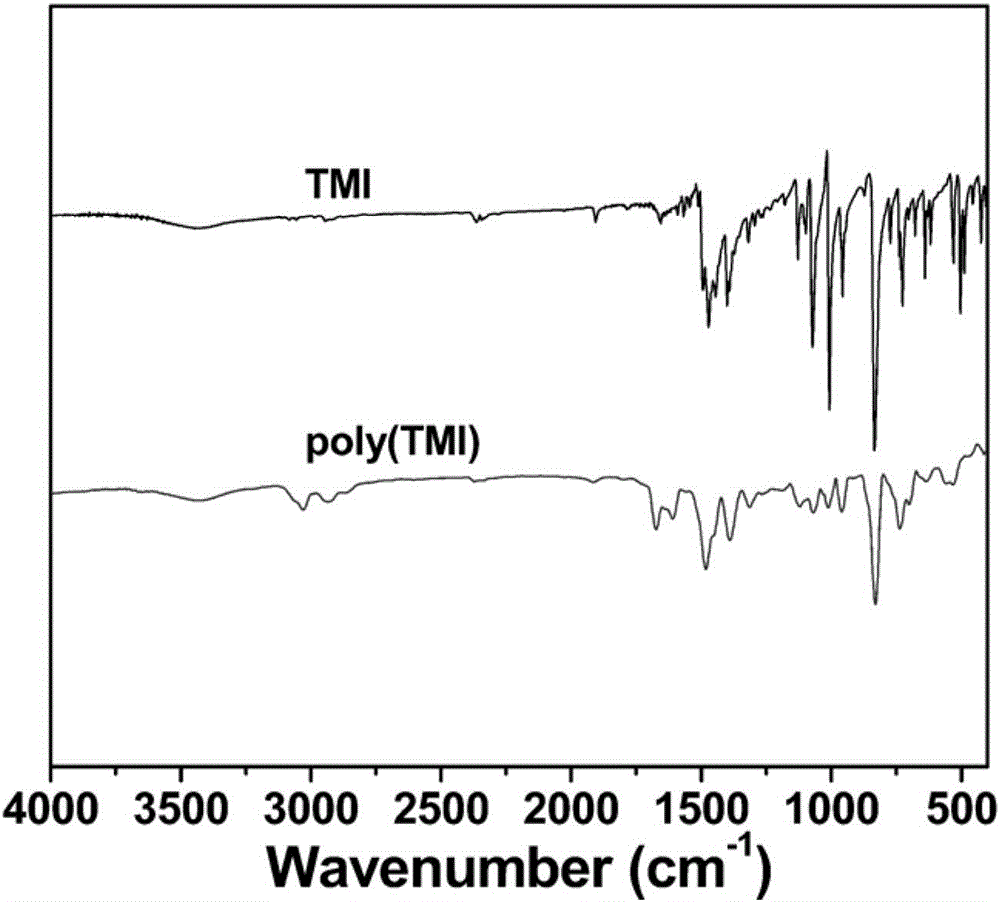
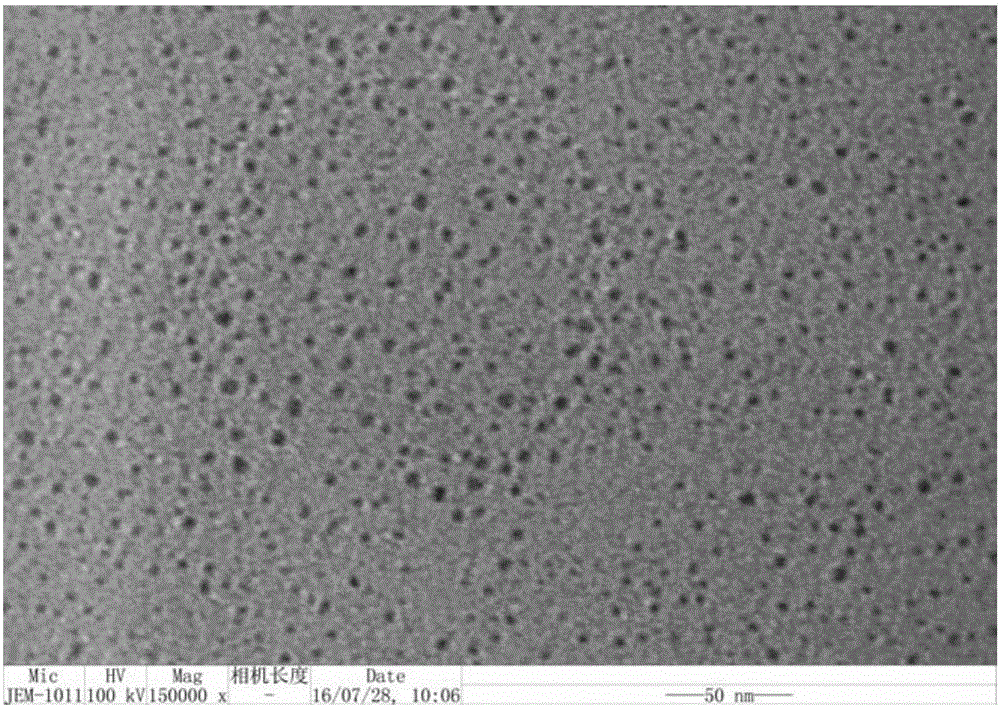
InactiveCN106607091AReduce dosageQuick responseOrganic-compounds/hydrides/coordination-complexes catalystsCatalystsPolymer scienceBromine
The invention discloses a microporous polymer-nano-metal particle catalyst and especially relates to a microporous polymer-nano-palladium catalyst and a preparation method thereof. The preparation method comprises that prepared bromine-substituted triarylimidazole group-containing monomer 2, 4, 5-tris(4-bromophenyl)-1-alkylimidazole (TAI) undergoes a reaction to produce a microporous polymer, the microporous polymer is dissolved in DMF, and an appropriate amount of a H2PdCl4 aqueous solution is added into the DMF solution and undergoes a reaction, and excess NaBH4 is added into the reaction product so that the microporous polymer-nano-metal particle catalyst is obtained. The microporous polymer has high nitrogen content, has a multi-pore structure and helps to increase the loading amount of the transition metal. The catalyst has strong catalytic activity, good selectivity, mild reaction conditions, good reusability and a good market application value.
Owner:XIANGTAN UNIV
Phosphorus-doped boron nitride acid-base catalyst and preparation method and application thereof
ActiveCN108295887AHas dual functions of acid-base catalysisHas the effect of acid-base catalysisCatalyst activation/preparationPreparation by cyanide reactionSolid acidBoron nitride
The invention discloses a phosphorus-doped boron nitride acid-base catalyst and a preparation method and application thereof. The preparation method includes the steps that a solution containing a phosphorus source, a boron source and a nitrogen source is recrystallized to obtain a recrystallization product, the recrystallization product is dried and then roasted at 700-850 DEG C in an inert atmosphere, and after roasting is completed, the phosphorus-doped boron nitride acid-base catalyst is obtained. The prepared phosphorus-doped boron nitride acid-base catalyst is a bifunctional catalyst which has a nanosheet structure with the thickness of 0.8-2 nm and also has acid-base active sites. The catalyst is a non-metal solid acid-base catalyst, has the advantages of being high in acid-base property, good in stability and easy to prepare, and shows excellent activity in one-pot cascade reaction under acid-base catalysis.
Owner:CENT SOUTH UNIV
Copper-catalyzed formation of carbon heteroatom and carbon-carbon bonds
InactiveUS20050215794A1Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Process for catalytically preparing aromatic or heteroaromatic nitriles
InactiveUS20090062541A1Speed up the processEffectively usableCandle holdersLighting support devicesPotassiumCopper
The present invention relates to a process for preparing optionally substituted aromatic or heteroaromatic nitriles starting from haloaromatics. These are reacted in a copper-catalysed reaction with potassium hexacyanoferrate(II) or potassium hexacyanoferrate(III) in the presence of heteroaromatic amines.
Owner:SALTIGO GMBH
Catalytic preparation process for aromatic nitrile or heteroaromatic nitrile
InactiveCN102898264AMild reaction conditionsThe reaction process is shortPreparation by cyanide reactionCyano group formation/introductionIodideTechnical grade
The invention discloses a catalytic preparation process for aromatic nitrile or heteroaromatic nitrile. According to the process, water is used as a reaction solvent, nanometer cuprous iodide, potassium iodide and N,N'-dimethylethylenediamine are used as a combined catalyst, substituted aryl halide or heteroaryl halide reacts with potassium ferrocyanide at a temperature of 150 to 170 DEG C for 24 to 48 h, and purification is carried out to obtain corresponding aromatic nitrile or heteroaromatic nitrile. Compared with synthetic methods in the prior art, the catalytic preparation process provided by the invention has the following advantages: (1) reaction conditions are mild; (2) reaction process flow is short; (3) a cheap nontoxic cyanated reagent is used, and a wide range of substrates can be used; and (4) industrial-grade aromatic nitrile or heteroaromatic nitrile is easy to use and is more economic and environment-friendly.
Owner:ZHEJIANG UNIV
Preparation method for 2-nitro-4-(trifluoromethyl)benzonitrile
InactiveCN105175282AHigh selectivityImprove conversion rateOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by cyanide reactionPotassium cyanideReaction temperature
The invention provides a preparation method for 2-nitro-4-(trifluoromethyl)benzonitrile. The method comprises the following steps: under the protection of nitrogen, successively adding 3-nitro-4-chlorobenzotrifluoride, cyanide, a catalyst II, a catalyst II and a solvent N-methyl-2-pyrrolidone into a reactor; carrying out heating under mechanical stirring; carrying out a reaction at temperature of 185 to 195 DEG C for 5 to 9 h; and then carrying out rectification so as to obtain 2-nitro-4-(trifluoromethyl)benzonitrile. The cyanide is one or more selected from the group consisting of sodium cyanide, potassium cyanide and cuprous cyanide; the catalyst I is one or two selected from the group consisting of cuprous bromide and nickel bromide; and the catalyst II is methylimidazole ionic liquid. According to the preparation method in the invention, the two catalysts are used and reaction temperature is adjusted in stages, so selectivity and conversion rate of the reaction are improved, cost is substantially reduced, and the preparation method is simple.
Owner:青岛和兴精细化学有限公司 +1
Popular searches
Carboxylic acid amides preparation Sulfonic acid amide preparation Group 3/13 element organic compounds Amino-carboxyl compound preparation Amino group formation/introduction Phosphorus organic compounds Organic substitution Amino-hyroxy compound preparation Hydrazide preparation Ether/acetal/ketal group formation/introduction
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com