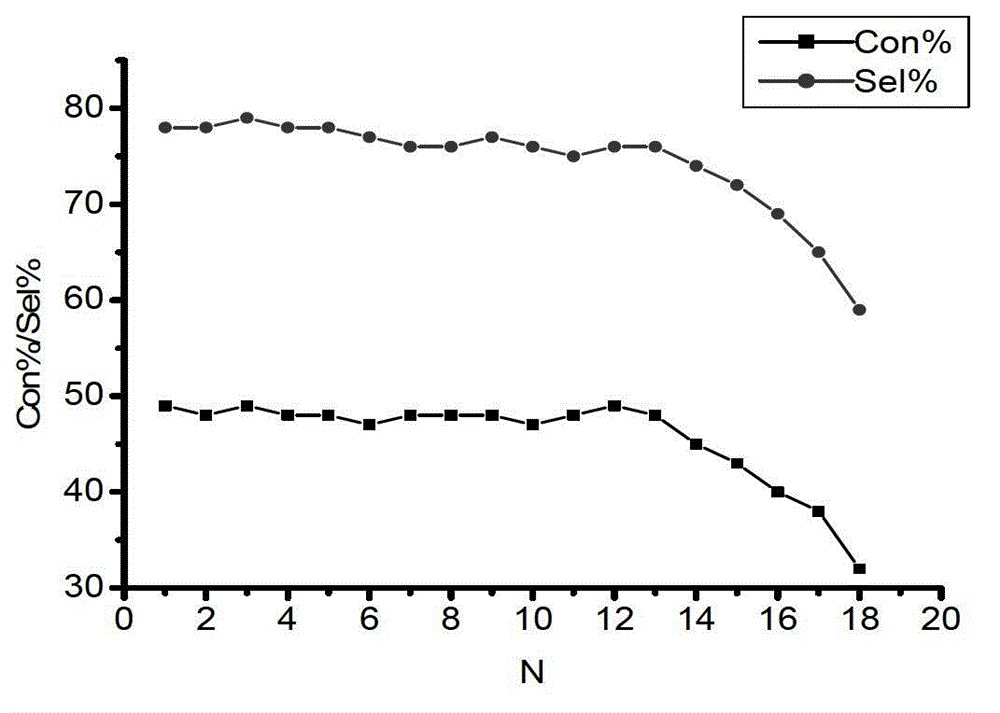
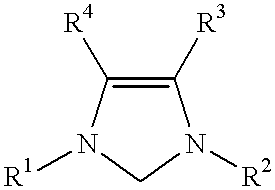
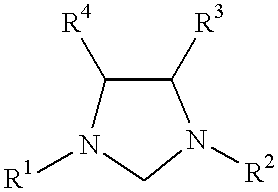
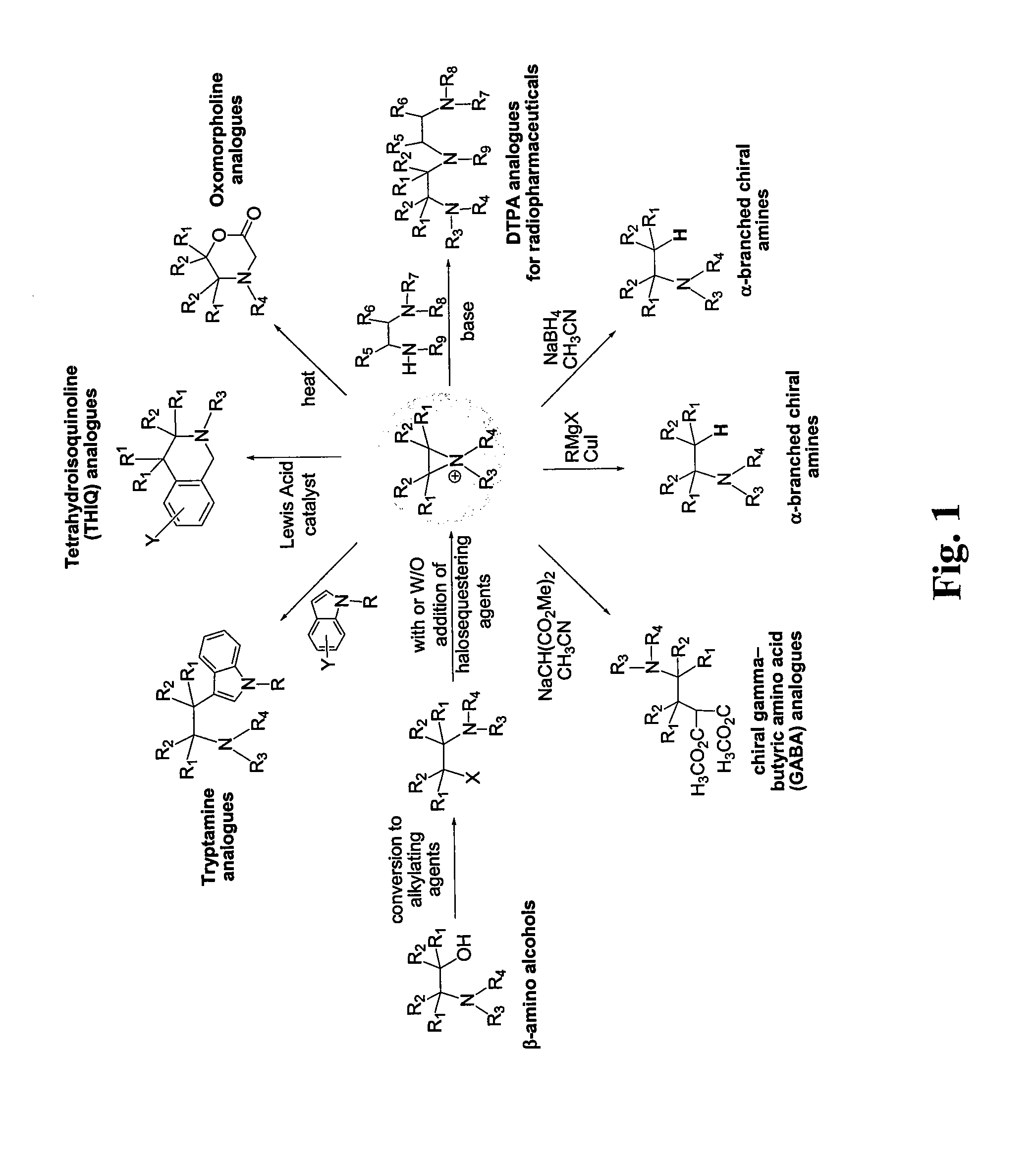
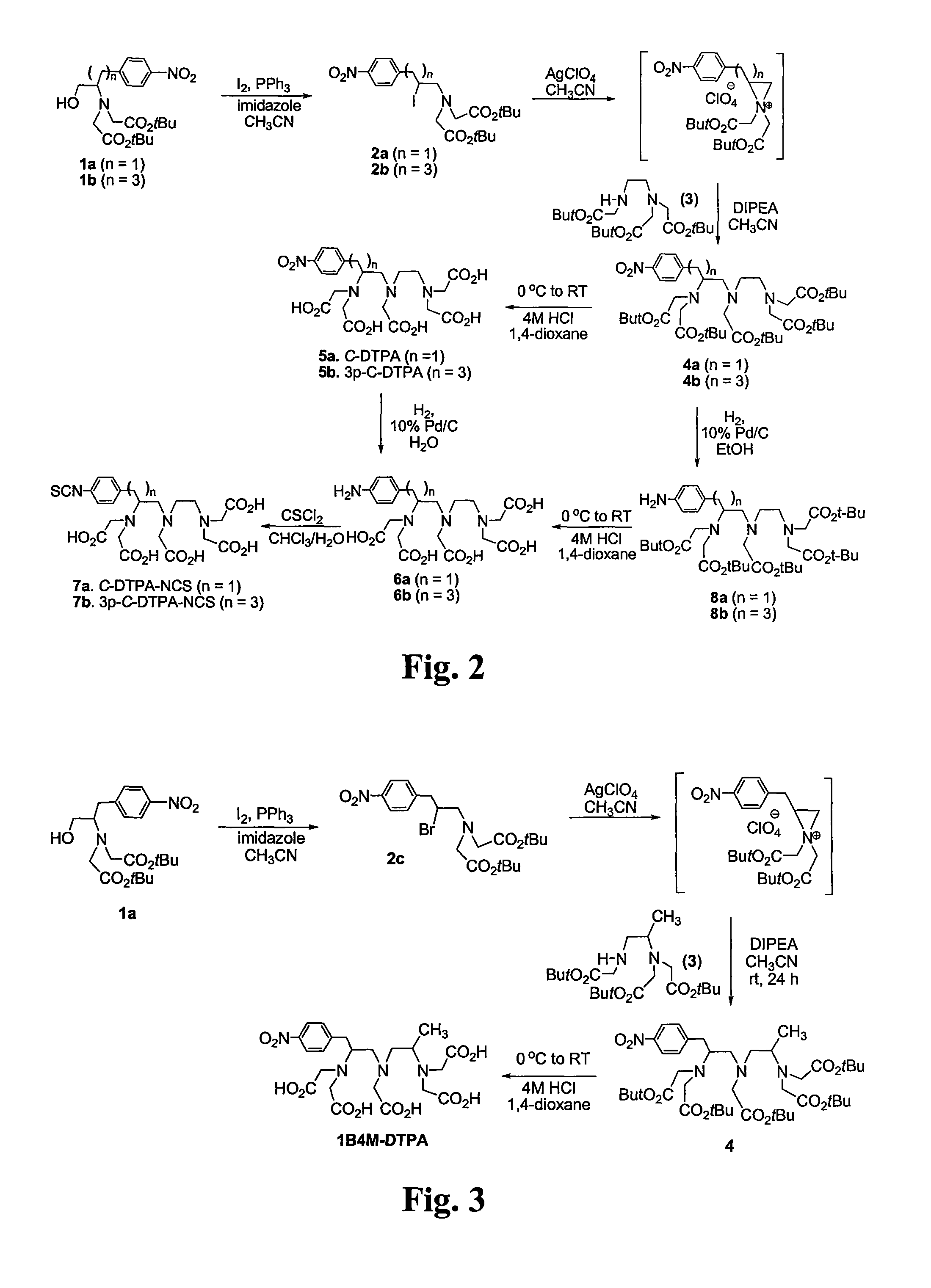
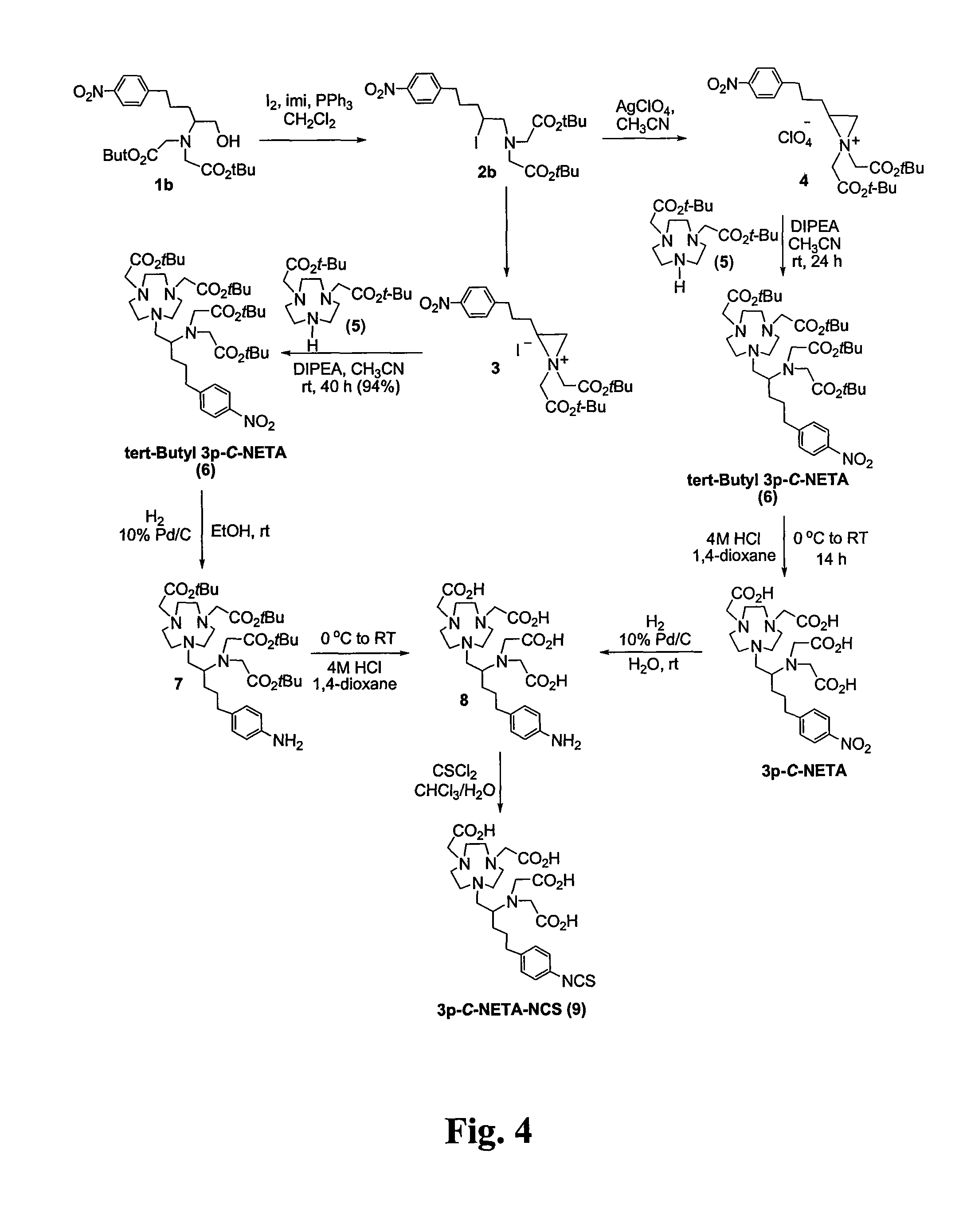
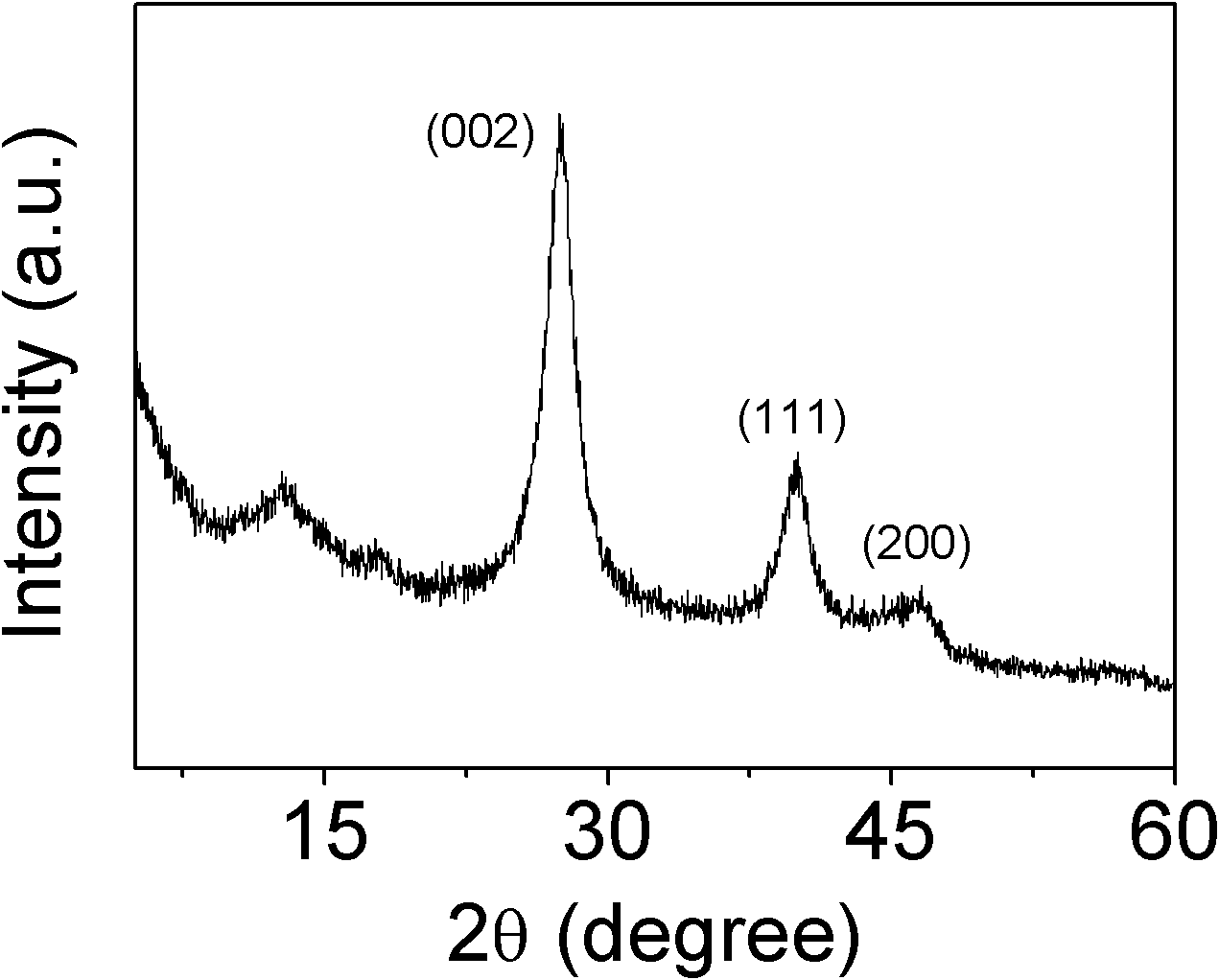
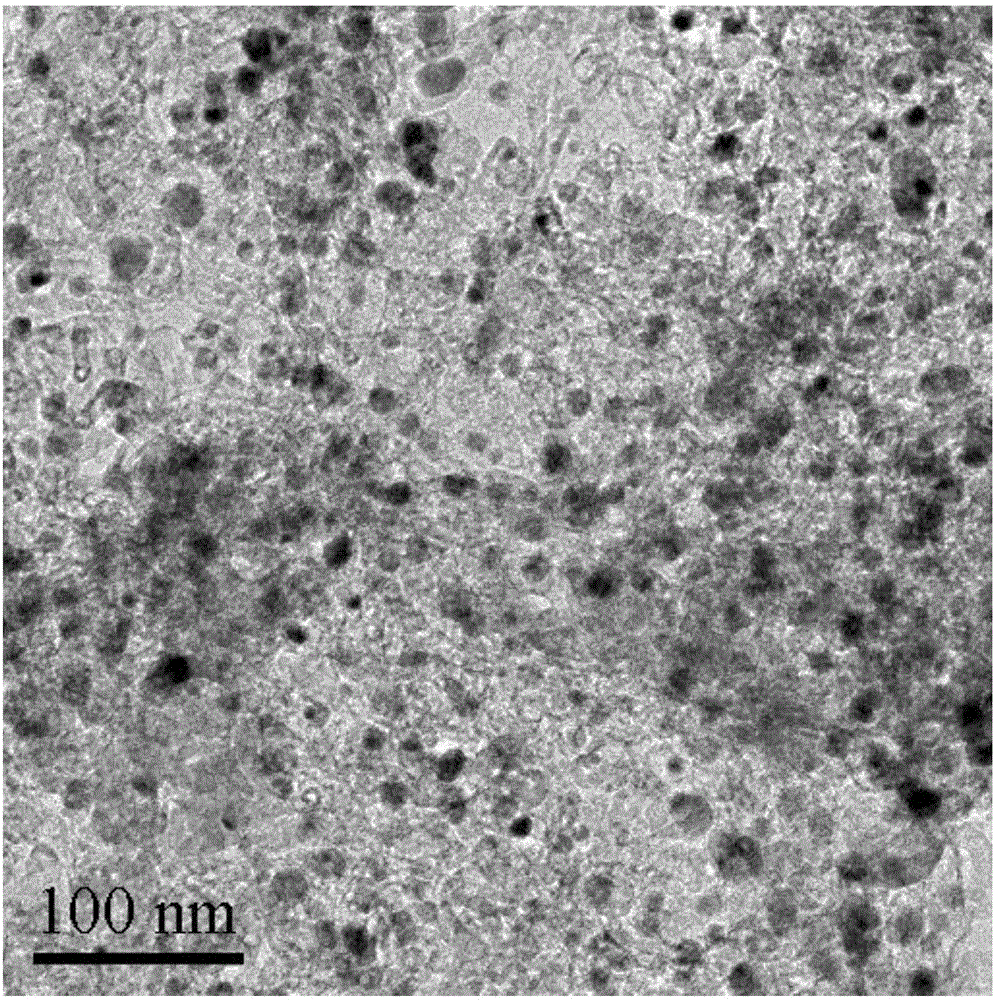
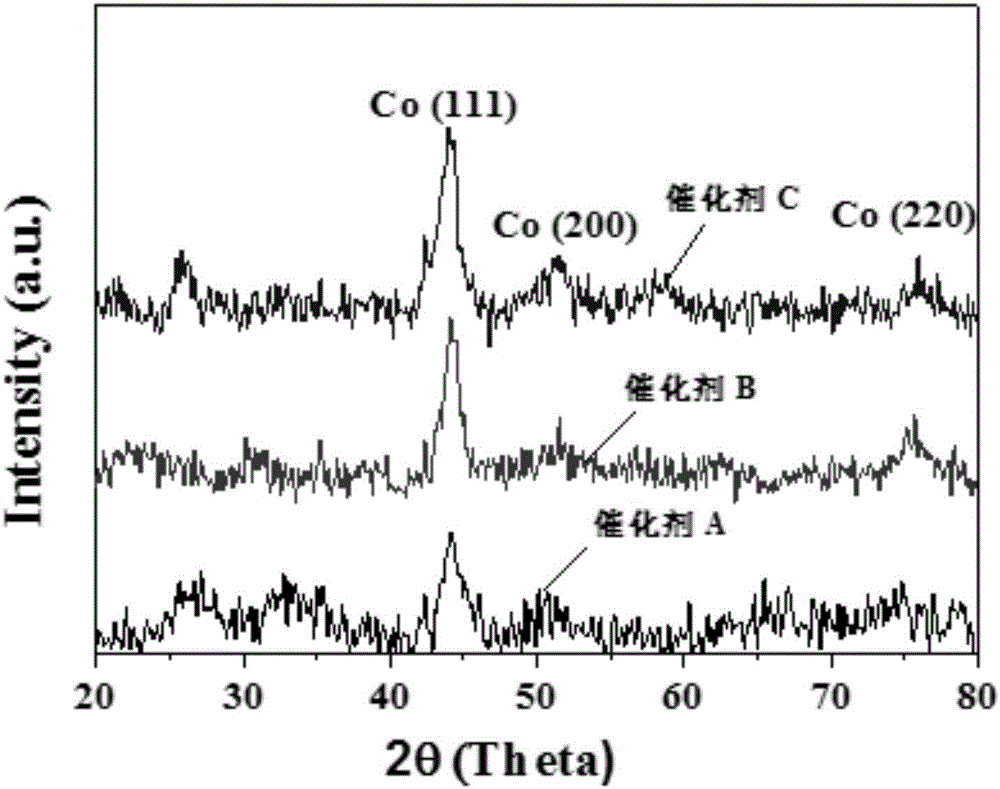
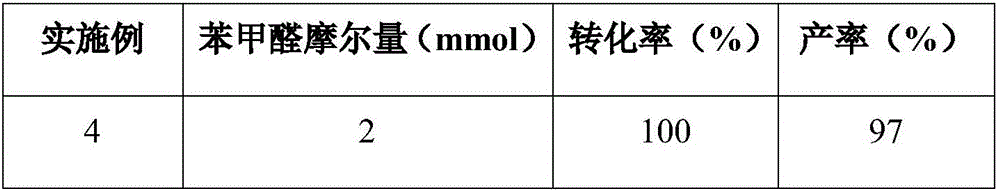
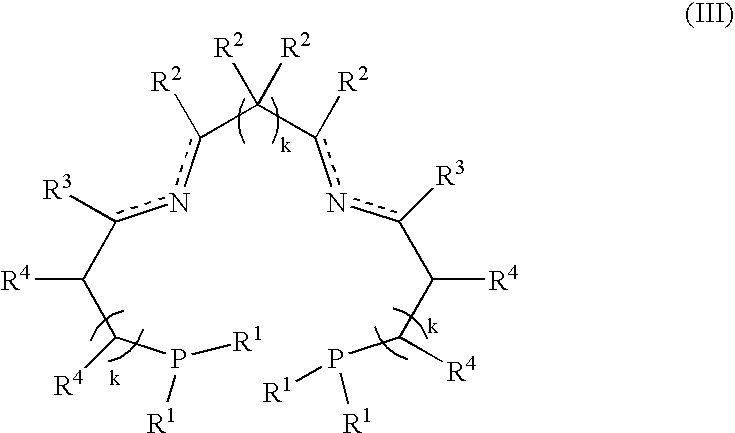
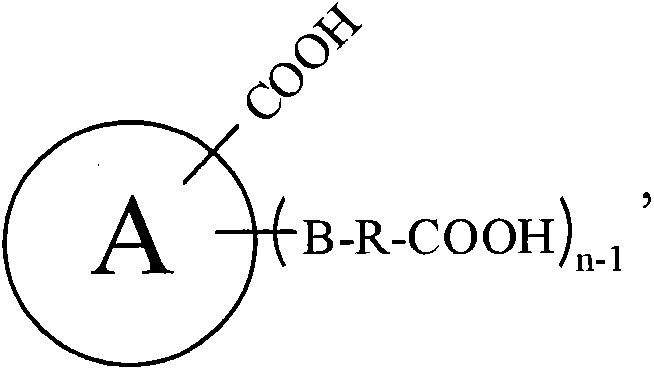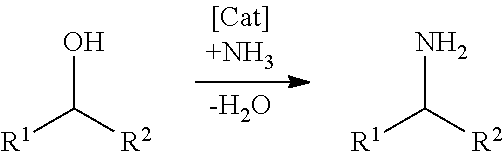Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
406results about "Amino group formation/introduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Catalyst for selective hydrogenation reaction of aromatic nitrocompound and preparation method of catalyst
InactiveCN105032424AEasy to storeLow costCarboxylic acid nitrile preparationOrganic compound preparationNitro compoundAlcohol
The invention relates to a catalyst for selective hydrogenation reaction of an aromatic nitrocompound and a preparation method of the catalyst. The catalyst consists of a catalyst carrier and active metal coated with carbon, wherein the catalyst carrier includes a carbon-base carrier, SiO2, TiO2 or Al2O3; the active metal is selected from Co, Fe, Ni or Cu and other poor and noble metals. The catalyst is prepared by adopting a Pechini type sol-gel process which comprises the steps of dispersing an active metal precursor to water containing a coordination compound, adding a polyhydric alcohol solution and a macromolecule auxiliary, then adding the carrier, stirring for dispersion, carrying out hydrothermal reaction, separating out solid on the lower layer, and calcining in the inert atmosphere to obtain the catalyst in which carbon coats the active metal. Compared with the prior art, the catalyst can realize the hydrogenation reaction of a substituted aromatic nitrocompound in the mild condition; substrate conversion rate and production selectivity are high; the catalyst has recyclable economy and good application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
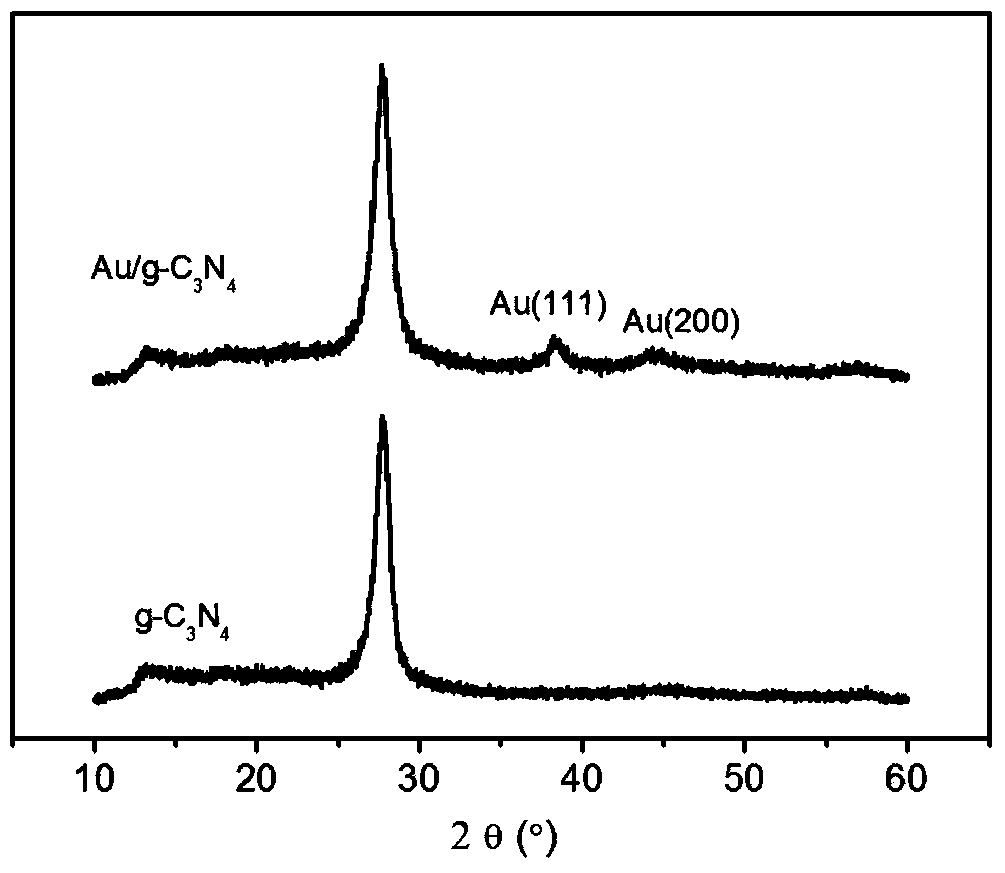
Metal/graphite-like carbon nitride compound catalyst and preparing method thereof
InactiveCN103586064APhysical/chemical process catalystsOrganic compound preparationNitrobenzeneCarbon nitride
The invention relates to a metal / graphite-like carbon nitride (g-C3N4) compound catalyst and a preparing method thereof in the field of catalysis. The chemical constitution of a metal / graphite-like carbon nitride provided by the invention is M / g-C3N4, wherein the metal M is Au, Ag, Pt, Pd, Bi, Cu, Ru or Rh, wherein the content of metal is 0-50% (mass fraction). The metal / graphite-like carbon nitride compound is a catalytic reducing agent at normal pressure and temperature, and the material is suitable for reducing derivants of nitrobenzene.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
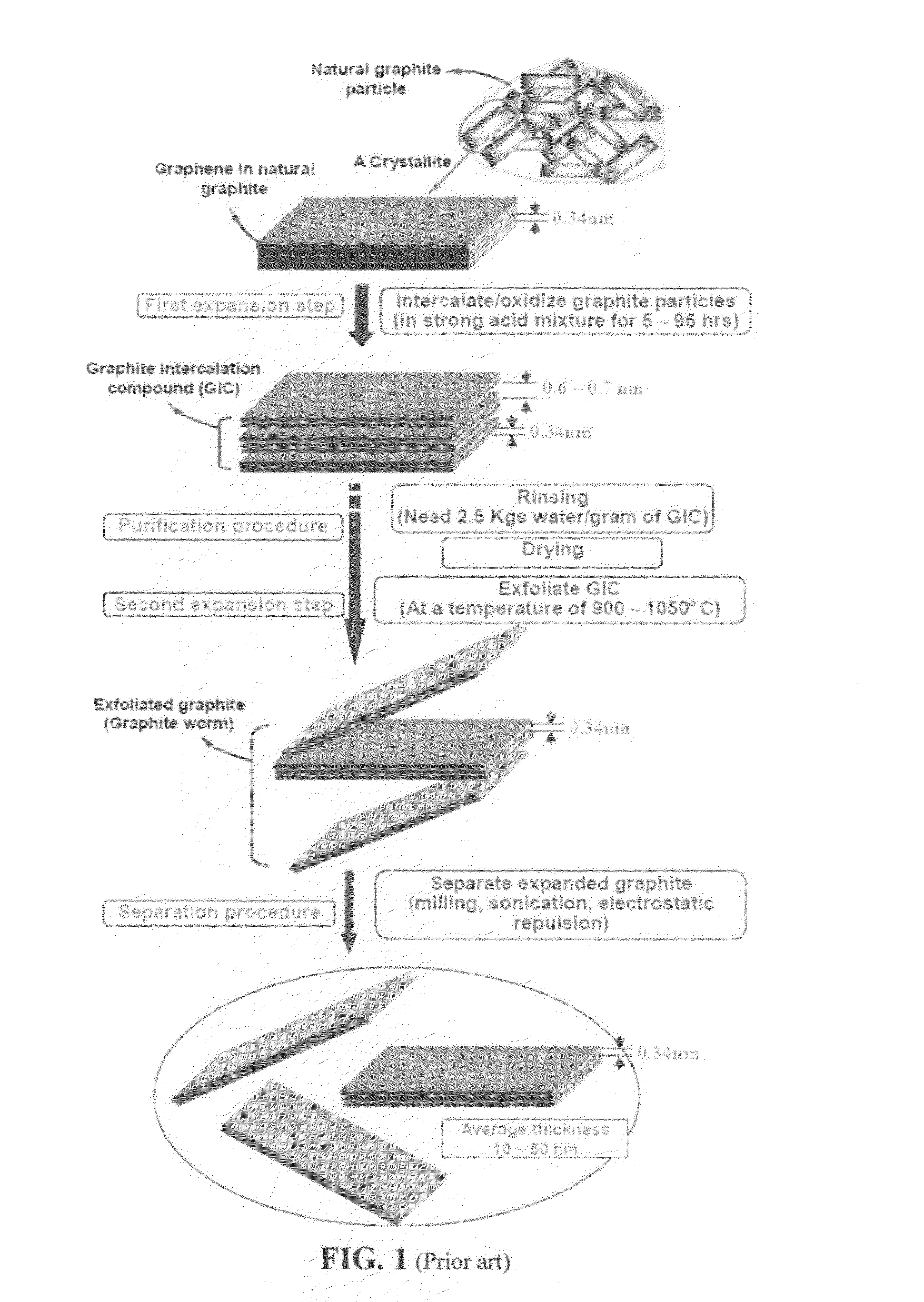
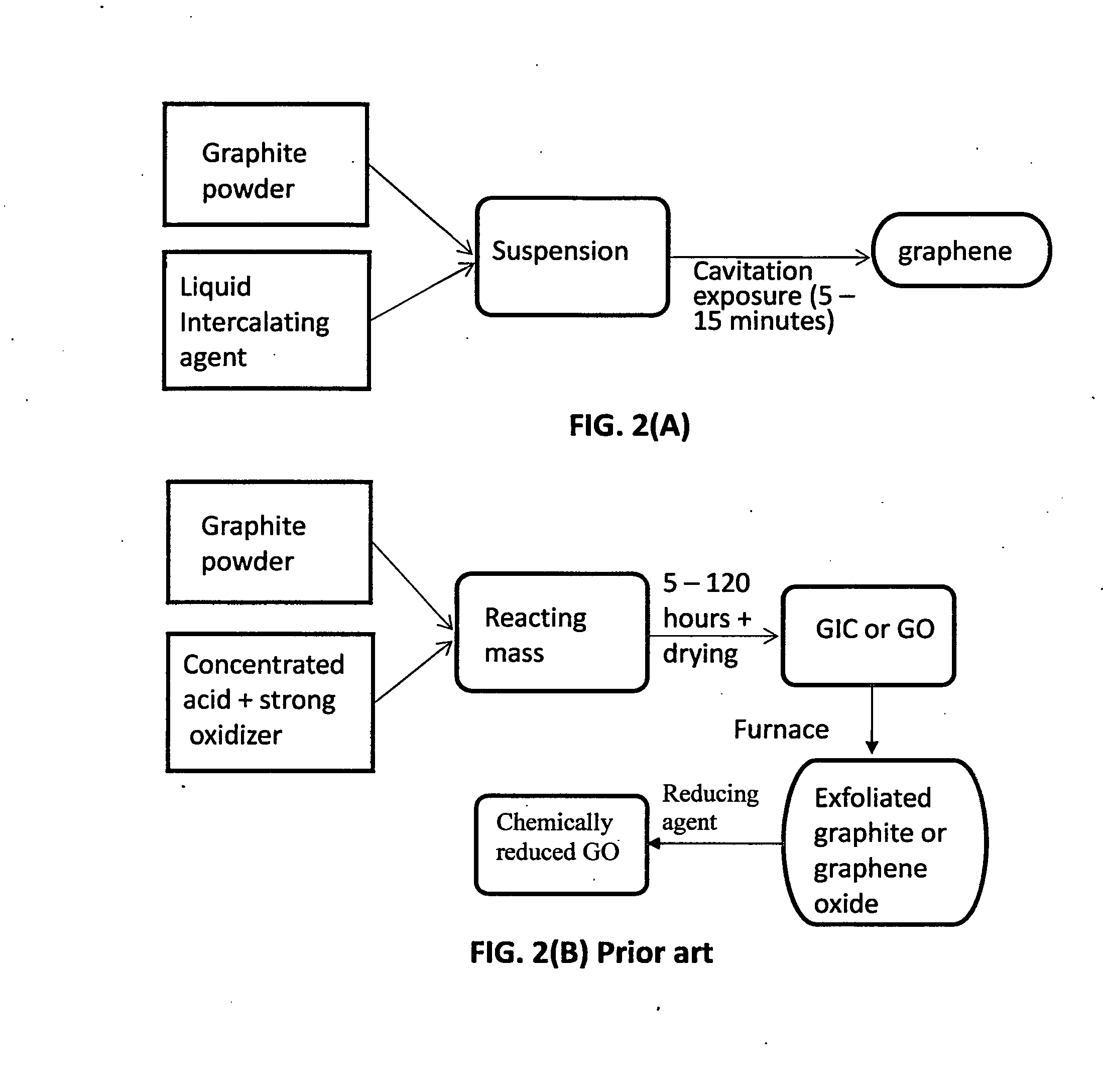
Production of graphene materials in a cavitating fluid
ActiveUS20150239741A1Shorten the timeReduce lossesCarboxylic acid nitrile preparationGraphiteCavitationLiquid medium
The invention provides a method of producing a graphene material from a starting graphitic material. In an embodiment, the method comprises: (a) dispersing the starting graphitic material in a liquid medium to form a graphite suspension; and (b) introducing the graphite suspension into a hydrodynamic cavitation reactor that generates and collapses cavitation or bubbles in the liquid medium to exfoliate and separate graphene planes from the starting graphitic material for producing the graphene material. The process is fast (minutes as opposed to hours or days of conventional processes), environmentally benign, and highly scalable. The reactor can concurrently perform the functions of graphene production, chemical functionalization, dispersion, and mixing with a polymer to make a composite.
Owner:GLOBAL GRAPHENE GRP INC
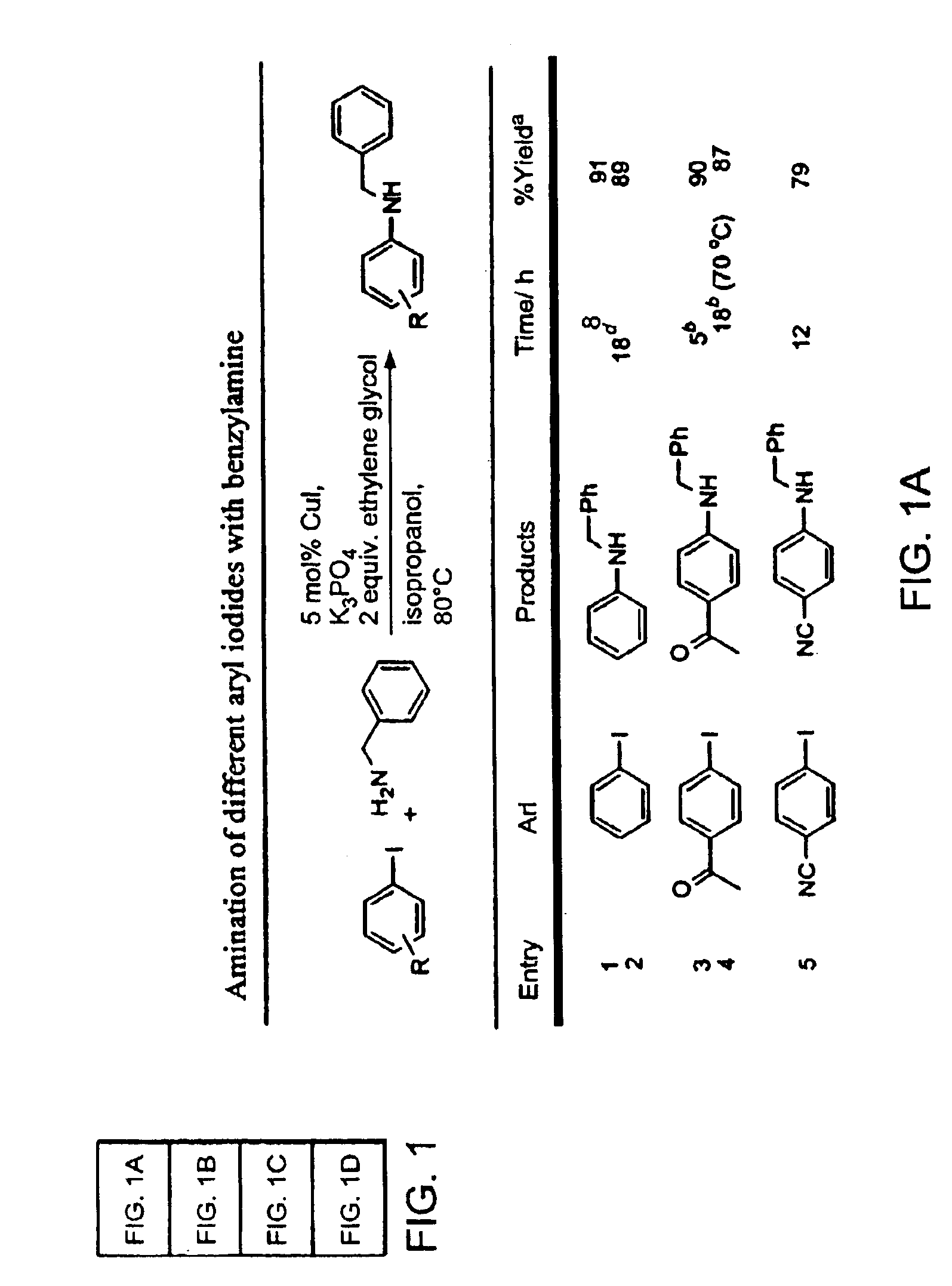
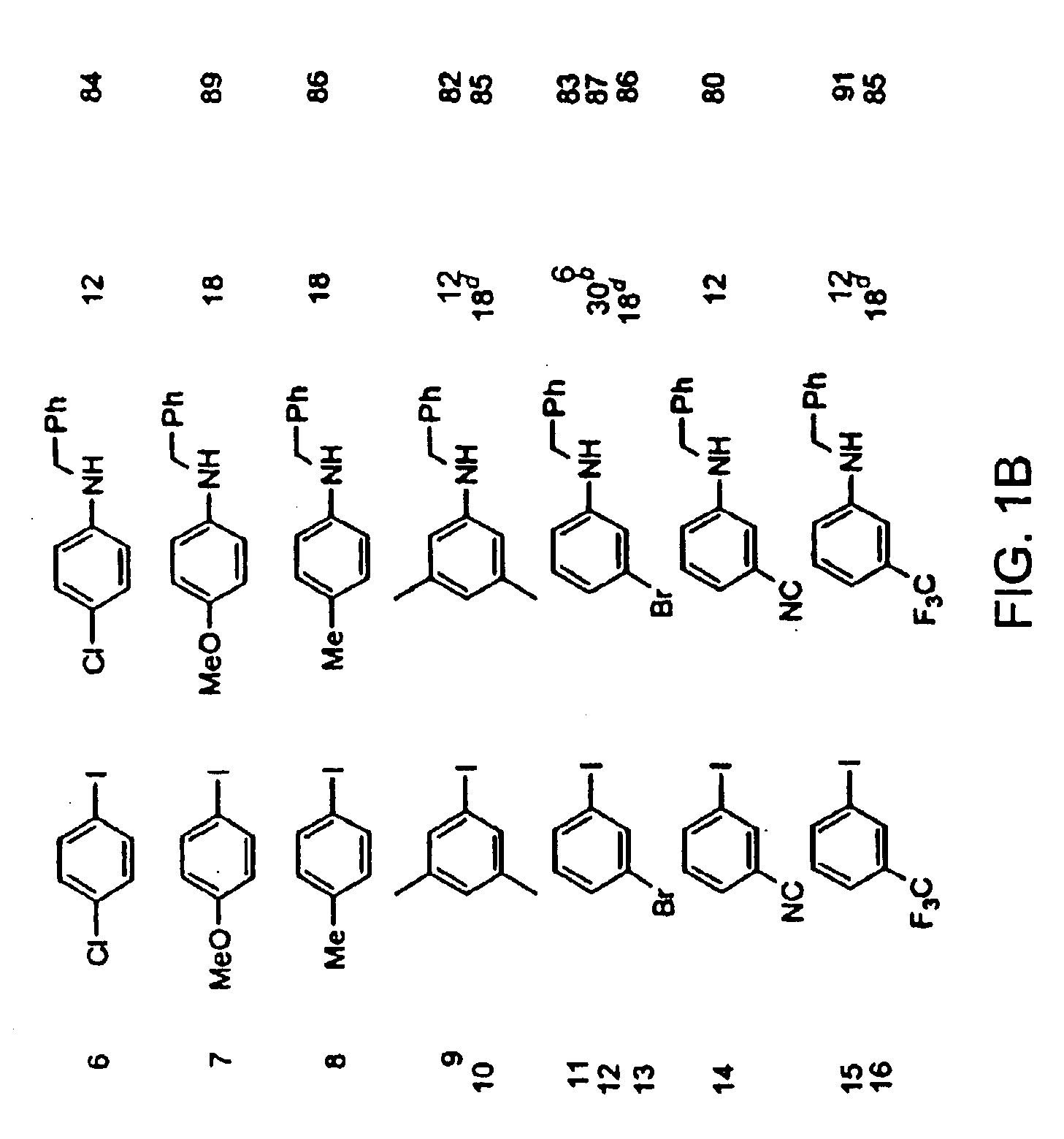
Copper-catalyzed formation of carbon-heteroatom and carbon-carbon bonds
InactiveUS6867298B2Cheap and practicalLow costUrea derivatives preparationCarbamic acid derivatives preparationCarbon–oxygen bondHydrazine compound
The present invention relates to copper-catalyzed carbon-heteroatom and carbon-carbon bond-forming methods. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of an amide or amine moiety and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In additional embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between a nitrogen atom of an acyl hydrazine and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In other embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-nitrogen bond between the nitrogen atom of a nitrogen-containing heteroaromatic, e.g., indole, pyrazole, and indazole, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. In certain embodiments, the present invention relates to copper-catalyzed methods of forming a carbon-oxygen bond between the oxygen atom of an alcohol and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. The present invention also relates to copper-catalyzed methods of forming a carbon-carbon bond between a reactant comprising a nucleophilic carbon atom, e.g., an enolate or malonate anion, and the activated carbon of an aryl, heteroaryl, or vinyl halide or sulfonate. Importantly, all the methods of the present invention are relatively inexpensive to practice due to the low cost of the copper comprised by the catalysts.
Owner:MASSACHUSETTS INST OF TECH
Activated carbon monolith catalyst, methods for making same, and uses thereof
InactiveUS20060229476A1High activityHigh selectivityOxygen-containing compound preparationOrganic compound preparationActivated carbonSupport matrix
An activated carbon monolith catalyst comprising a finished self-supporting activated carbon monolith having at least one passage therethrough, and comprising a supporting matrix and substantially discontinuous activated carbon particles dispersed throughout the supporting matrix and at least one catalyst precursor on the finished self-supporting activated carbon monolith. A method for making, and a method for use, of such an activated carbon monolith catalyst in catalytic chemical reactions are also disclosed.
Owner:APPL TECH LLP
Nitrogen-doped carbon material supporting cobalt catalyst and method therewith of catalytic hydrogenation reductive amination to produce amine compounds
InactiveCN106552661AEasy to prepareReduce manufacturing costOrganic compound preparationCatalyst activation/preparationReaction temperatureNitrogen gas
The invention discloses a nitrogen-doped carbon material supporting cobalt catalyst and a method therewith of catalytic hydrogenation reductive amination to produce primary amine compounds. The catalyst is prepared through the steps of: 1) preparing a MOF material ZIF-67; and 2) under protection of nitrogen gas, calcining the MOF material ZIF-67 at 200-1000 DEG C for 1-10 h to produce the nitrogen-doped carbon material supporting cobalt catalyst Co / N-C. The method of catalytic hydrogenation reductive amination to produce the primary amine compounds with the catalyst includes the steps of: in an organic solvent, adding a carbonyl compound, ammonia water and the nitrogen-doped carbon material supporting cobalt catalyst, feeding hydrogen gas, and performing a reaction at 50-150 DEG C under 0.1-5 MPa for 0.5-20.0 h to produce the amine compounds. The preparation method of the catalyst is simple in operations. The catalyst can be used for producing the primary amine compounds in a manner of catalytic hydrogenation reductive amination. In the method, the primary amine compounds are produced with the carbonyl compound as a raw material through catalytic hydrogenation by means of the catalyst, so that the method has mild reaction conditions and is high in yield.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Catalyst for synthesizing ethylene amine and method for preparing ethylene amine
ActiveCN102658162AModulation compositionHigh activityOrganic compound preparationAmino group formation/introductionHydrogenActive component
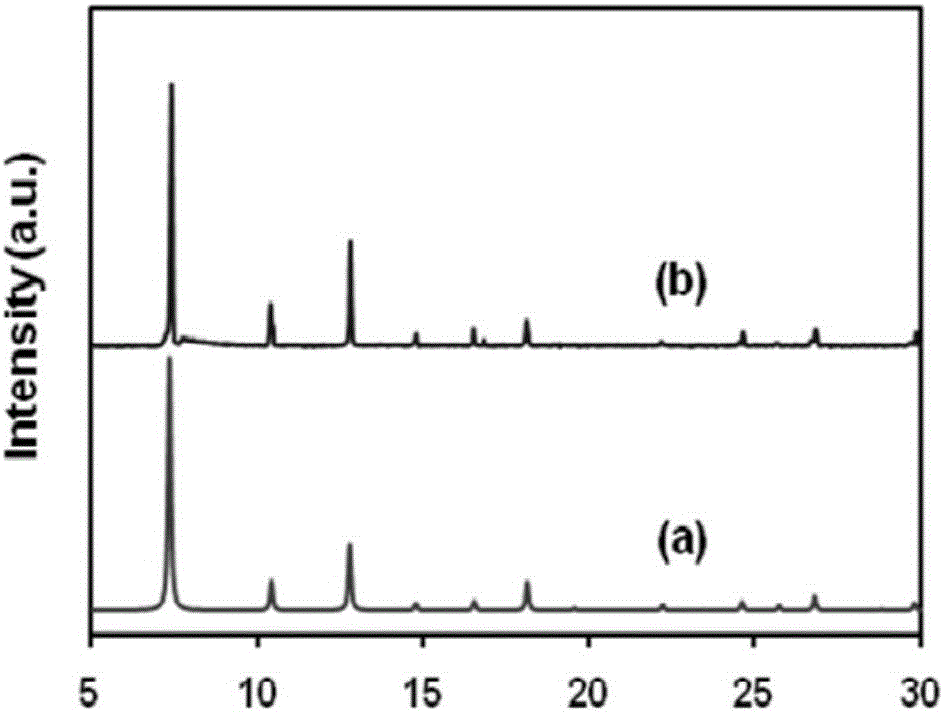
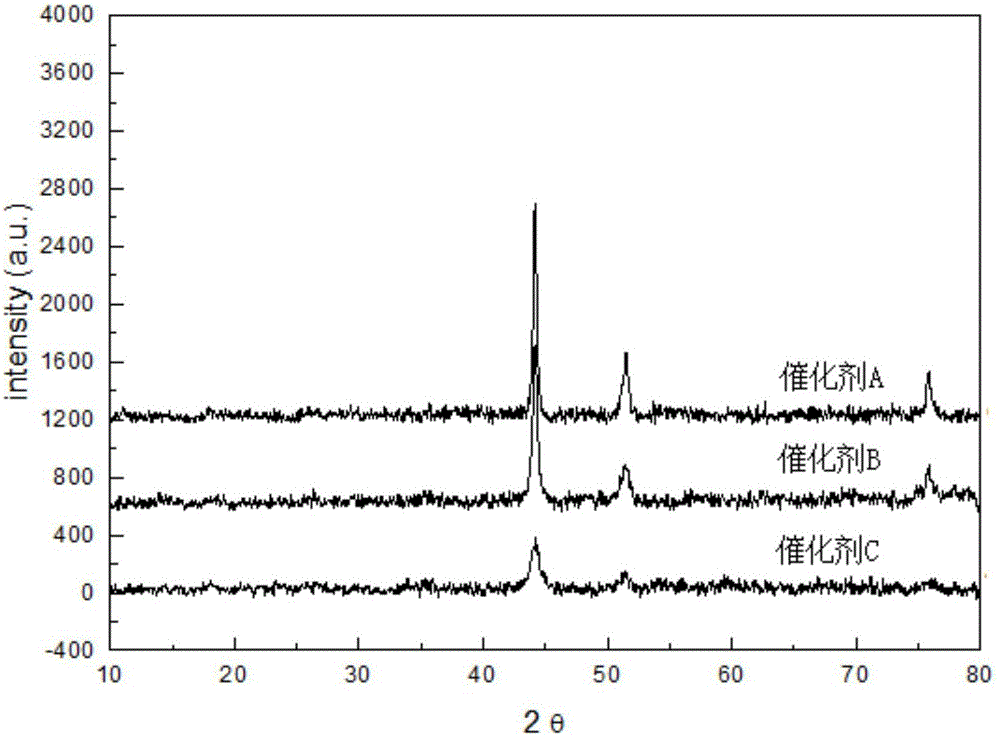
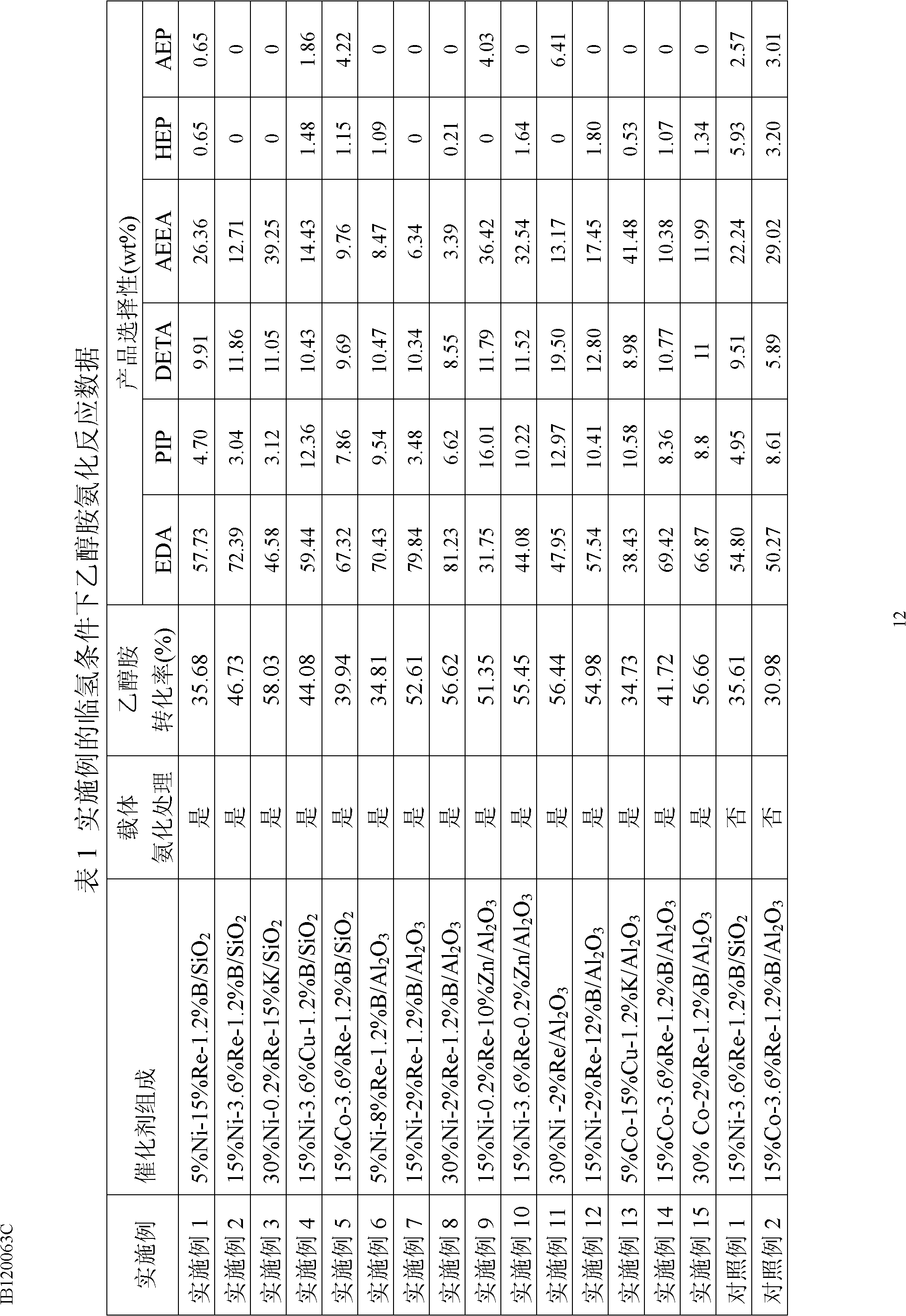
The invention relates to a catalyst for synthesizing ethylene amine and a method for preparing ethylene amine. The catalyst comprises 1-40 wt% of a main active component, 0.1-20 wt% of an auxiliary agent, and a carrier processed by ammonification, wherein the main active component is Ni or Co, the auxiliary agent comprises one ore more of Fe, Cu, Ru, Re, K, Zn, B and other metal or oxides, and the ammonified carrier is ammonified SiO2 or Al2O3. The catalyst is characterized in that the used carrier needs to be ammonified. The ethylene amine product synthesized in hydrogen environment by using the catalyst of the invention to carry out ethanolamine ammonification reaction has high activity, high selectivity and stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS20130310555A1Short reaction stepsHigh yieldOrganic compound preparationThiol preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Preparation methods of nitrile and corresponding amine
ActiveCN105016944AReduce dosageIncrease profitOrganic compound preparationPreparation by carboxylic acid amide dehydrationAmmoniaNitrile
The invention relates to a preparation method of nitrile. Compared with the prior art, the preparation method has the characteristics of obvious reduction of the usage amount of ammonia sources, low environmental pressure, low energy consumption, low production cost, high purity and yields of nitrile products, and the like, and can be used for obtaining nitrile with a more complex structure. The invention also relates to a method for preparing corresponding amine with nitrile.
Owner:SINOPEC YANGZI PETROCHEM +1
Shaped, activated metal, fixed-bed catalyst
InactiveUS6262307B1Small volumeOrganic reductionOxygen-containing compound preparationFixed bedVolumetric Mass Density
A shaped, activated metal, fixed-bed catalyst with a pore volume of 0.05 to 1 ml / g and an outer activated layer consisting of a sintered, finely-divided, catalyst alloy and optionally promoters. The catalyst alloy has metallurgical phase domains, resulting from the method of preparation of the alloy, in which the largest phase by volume has a specific interface density of more than 0.5 mum-1.
Owner:EVONIK DEGUSSA GMBH
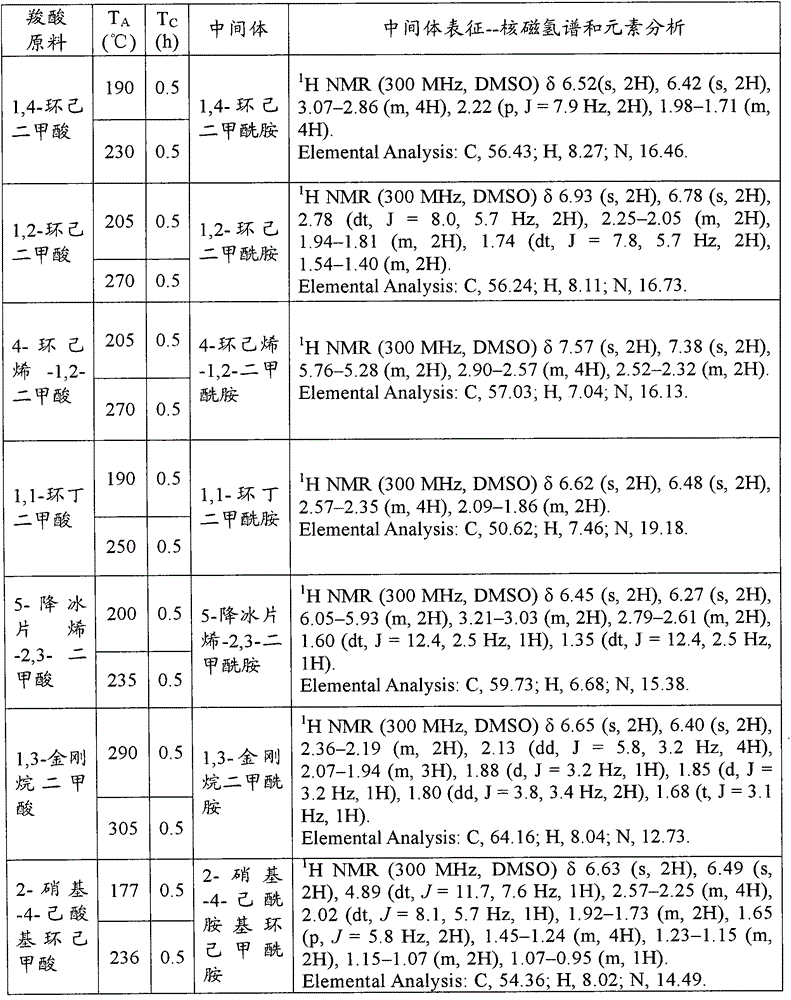
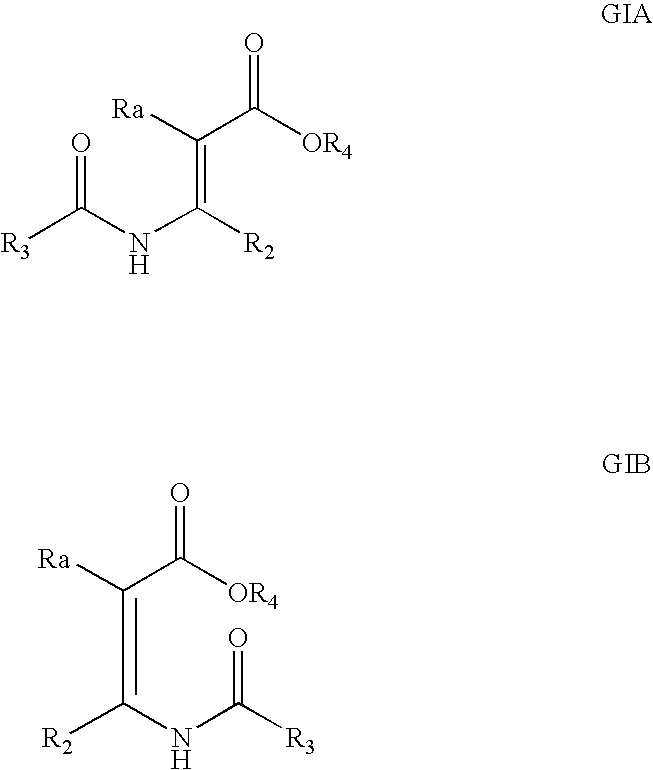
Processes and intermediates
The invention relates to processes and compounds useful for producing modified aspartic acid derivatives, such as aspartic acid aldehyde moieties. Aspartic acid derivatives are useful for preparing caspase inhibitors and / or prodrugs thereof.
Owner:VERTEX PHARMA INC
Method for preparing amino acid schiff base metal complexes without solvent
InactiveCN101811919AReduce usageCompliant with Green Chemistry RequirementsAmino group formation/introductionImino compound preparationBenzaldehydePotassium hydroxide
The invention discloses a method for preparing amino acid schiff base metal complexes without solvent, and relates to a method for preparing the amino acid-Schiff base metal complexes. The invention solves the problems of high solvent consumption and long reaction time in the conventional method for preparing the amino acid schiff base metal complexes. The method comprises the following steps of: 1, under the anhydrous condition, mixing L-amino acid, ortho-hydroxy-benzaldehyde and potassium hydroxide, and grinding for 2 to 5 minutes to obtain amino acid schiff base; and 2, adding bivalent copper salt or bivalent zinc salt into the amino acid schiff base obtained in the step 1, and grinding for 3 to 6 minutes to obtain the amino acid schiff base metal complexes, wherein the step 1 and the step 2 are all completed at room temperature. The whole preparation process needs only 5 to 11 minutes, the reaction time is short, the solvent is not needed, the process is simple, the work efficiency is high, the posttreatment is simple and the yield reaches 80 to 95 percent.
Owner:HARBIN UNIV OF SCI & TECH
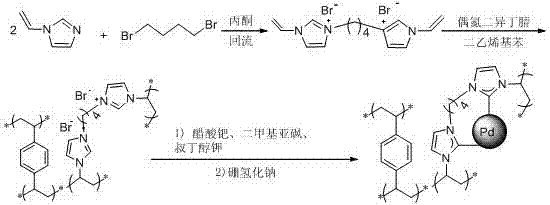
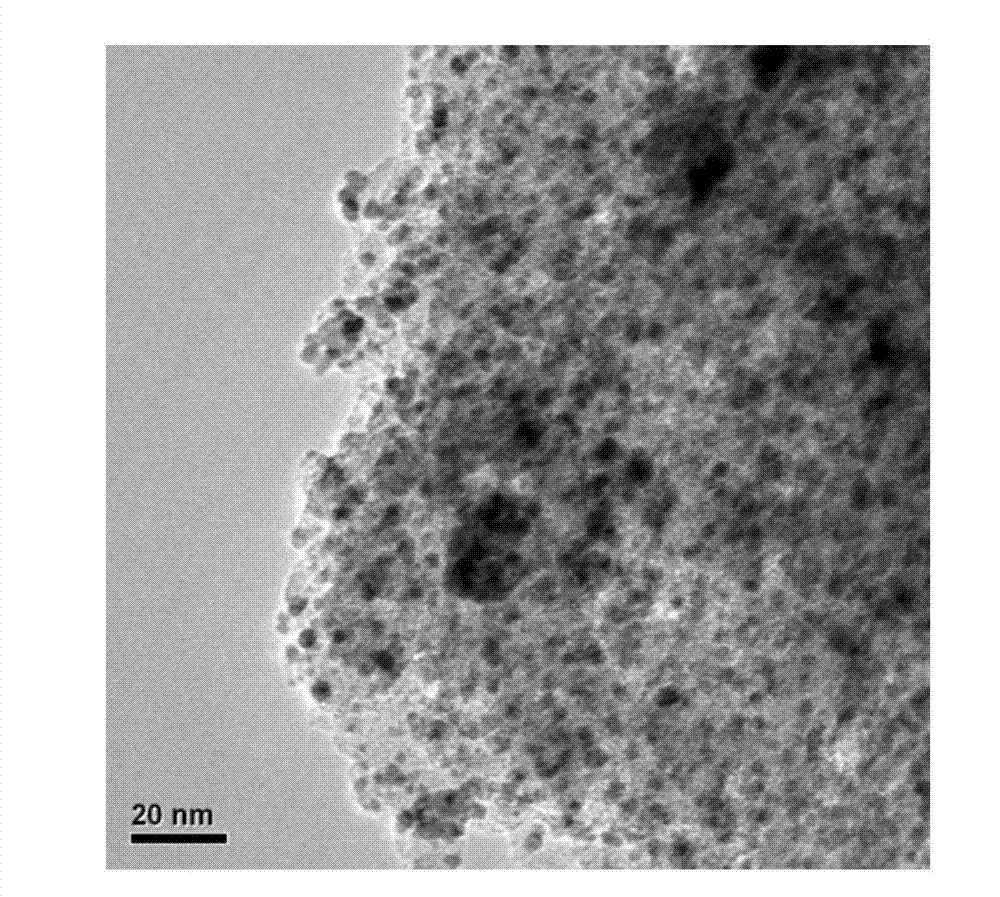
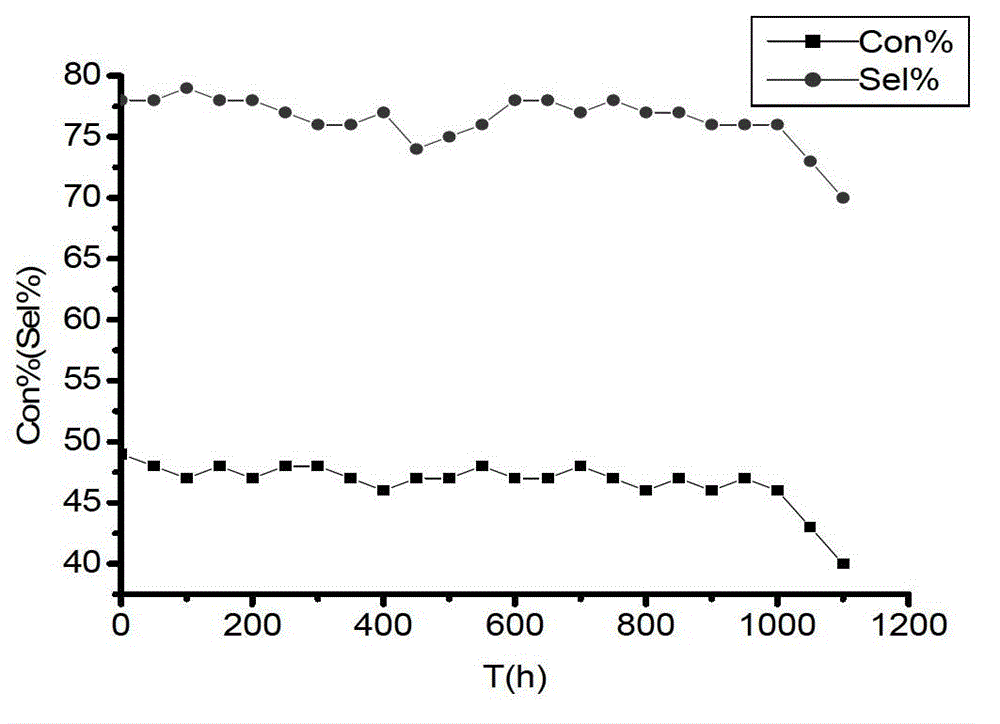
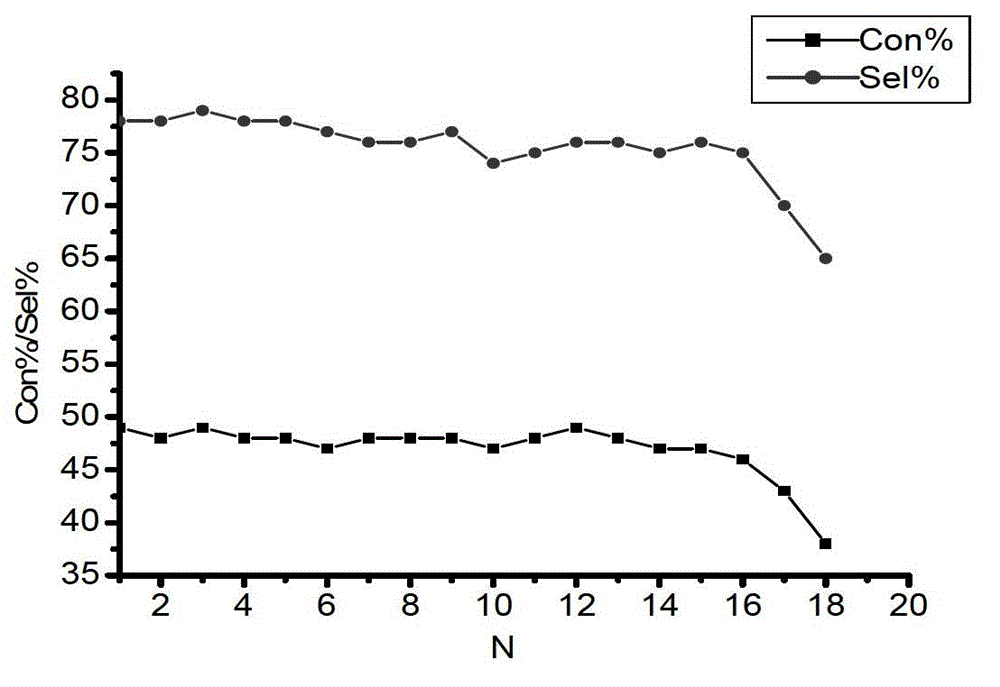
Ion liquid cross-linked polymer supported nanometer palladium metal catalytic material, preparation method and applications thereof
ActiveCN107486240AEasy to prepareStable in natureCarboxylic acid nitrile preparationOrganic compound preparationCross-linkIonic liquid
The present invention discloses an ion liquid cross-linked polymer supported nanometer palladium metal catalytic material, which is short for P(DVB-DIIL)-Pd, and has the structure defined in the specification. The invention further discloses a preparation method of the catalytic material, and applications of the catalytic material in preparation of aromatic amine compounds through liquid phase catalytic hydrogenation of nitro aromatic hydrocarbons. According to the present invention, the ion liquid cross-linked polymer supported nanometer palladium metal catalytic material can provide high catalytic selectivity and high conversion rate for the liquid phase hydrogenation reactions of a variety of nitro aromatic hydrocarbon compounds, and can be recycled.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for catalyzing transfer of hydrogen controllable reduction nitro-compound in formic acid or formate
InactiveCN103539596ALow costImprove securityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitro compoundFormate
The invention relates to a method for catalyzing the transfer of a hydrogen controllable reduction nitro-compound in formic acid or formate. Carbon nitride supported nano palladium or polymer semiconductor supported nano metal is used as a catalyst, the formic acid or formate is used as a hydrogen source, the reaction temperature is 0-15 DEG C, and the transfer of hydrogen in the formic acid or formate to a substrate nitro is efficiently catalyzed so as to selectively generate corresponding amine. Compared with the prior art, the method disclosed by the invention has the advantages that the reaction conditions are mild, the substrate conversion rate and product selectivity are high, the catalyst is easy to recover with good circularity, the system is environment-friendly and low in energy consumption, and the method has a vitally important value in industrial application.
Owner:SHANGHAI JIAO TONG UNIV
Regeneration method for modified zeolite molecular sieve amination catalyst
ActiveCN102974393AUnobstructed channel structureSmooth structureMolecular sieve catalystsCatalyst regeneration/reactivationMolecular sieveOrganic acid
The invention discloses a regeneration treatment method for a modified zeolite molecular sieve amination catalyst. The regeneration method comprises the following steps of: regenerating soluble coke by inorganic acid or organic acid; smelting most coking materials and non-framework aluminum formed by hydrothermal dealumination; and performing charking regeneration on the smelted zeolite molecular sieve amination catalyst by N2 and O2 mixed gas to completely remove non-smelted coke, so that the performance of the modified zeolite molecular sieve amination catalyst is recovered. By the regeneration treatment method, the problem of activity loss caused by the hydrothermal dealumination is solved; and the service life of the modified zeolite molecular sieve amination catalyst is prolonged. The regeneration treatment method is mainly applied to a regeneration treatment process of the modified zeolite molecular sieve amination catalyst under the ethanol amine and the ammonia condensation and amination reaction.
Owner:XIAN MODERN CHEM RES INST
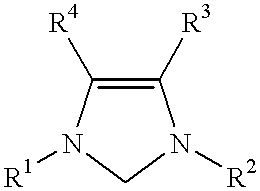
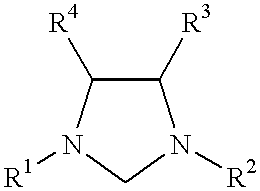
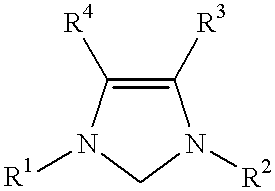
Use of a catalyst system comprising nickel palladium or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in stille coupling reactions
InactiveUS6362357B1High yieldEliminate needHydrocarbon by isomerisationOrganic compound preparationLiquid mediumOxidation state
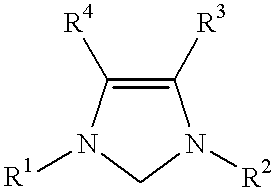
This invention provides a process for conducting Stille coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Stille couplings of aryl halides. A Stille coupling can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than stannyl groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one organotin compound wherein the tin atom is quaternary, wherein one group bound to the tin atom is unsaturated at the alpha or beta position, and wherein each of the remaining groups bound to the tin atom is a saturated group; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:RES & TECH FOUND UNIV OF NEW ORLEANS
Process for homogeneously catalyzed, highly selective direct amination of primary alcohols with ammonia to primary amines with a high volume ratio of liquid phase to gas phase and/or high pressures
InactiveUS20130245276A1Organic compound preparationCarboxylic acid amides preparationGas phaseHigh pressure
The present invention relates to a process for preparing primary amines comprising the process stepsA) provision of a solution of a primary alcohol in a fluid, nongaseous phase,B) contacting of the phase with free ammonia and / or at least one ammonia-releasing compound and a homogeneous catalyst and optionallyC) isolation of the primary amine formed in process step B),characterized in thatthe volume ratio of the volume of the liquid phase to the volume of the gas phase in process step B is greater than 0.05 and / or in that process step B is carried out at pressures greater than 10 bar.
Owner:EVONIK DEGUSSA GMBH
Synthesis of therapeutic and diagnostic drugs centered on regioselective and stereoselective ring opening of aziridinium ions
ActiveUS9446995B2Short reaction stepsHigh yieldThiol preparationOrganic compound preparationRegioselectivityStereoselectivity
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
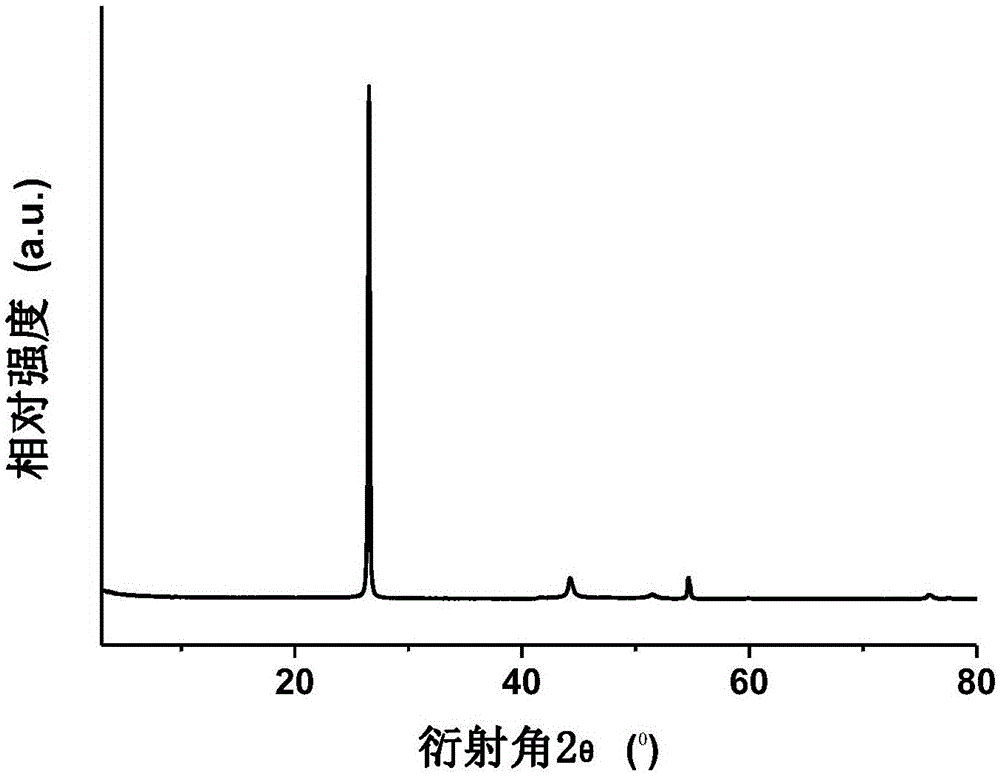
Selective hydrogenation reduction method for aromatic nitro compound
ActiveCN102491863AAchieve restorationPracticalPhysical/chemical process catalystsCarboxylic acid nitrile preparationNitro compoundHydrogen atmosphere
The invention discloses a selective hydrogenation reduction method for an aromatic nitro compound. The selective hydrogenation reduction method for the aromatic nitro compound comprises the following steps of: adding nanopalladium catalyst loaded by mesoporous carbon nitride, water and aromatic nitro compound into a reaction container; reacting completely in a hydrogen atmosphere; and processing to obtain a corresponding aromatic amino compound. In the method, the nanopalladium catalyst loaded by the mesoporous carbon nitride is used for selectively reducing the aromatic nitro compound, and the selectivity and the conversion rate are above 99 percent; reducing conditions are mild; and the selective hydrogenation reduction method for the aromatic nitro compound is high in operability, so that the selective hydrogenation reduction method for the aromatic nitro compound has a bright marketing prospect.
Owner:ZHEJIANG UNIV
Nitrogen-doped carbon material encapsulated cobalt catalyst and method of using the catalyst to prepare secondary amine compounds
InactiveCN106622244AEasy to prepareLow costOrganic compound preparationPreparation by reductive alkylationNitro compoundHydrofluoric acid
The present invention discloses a nitrogen-doped carbon material encapsulated cobalt catalyst and a method of using the catalyst to prepare secondary amine compounds. The preparation method of the catalyst comprises the following steps: 1) silica sol is added into a 2-methylimidazole solution and dispersed uniformly to obtain liquid sol, a stirring is conducted while adding cobalt nitrate liquid into the liquid sol, a stirring reaction is conducted for 3-10 h, and after the reaction is completed, centrifuging, filtering, washing and drying are conducted to obtain ZIF-67@SiO<2> materials; 2) under nitrogen protection, the ZIF-67@SiO<2> materials are calcined at 400-1,200 DEG C for 6-10 h, the calcined materials are soaked with hydrofluoric acid solution, the soaked calcined materials are water washed to neutral, the washed calcined materials are dried to obtain CN@Co materials. The method of using the catalyst to prepare the secondary amine compounds is as follows: in an organic solvent, nitro-compounds, formic acids, carbonyl compounds and the CN@Co materials are added, nitrogen is ventilated to replace oxygen in the reaction system, and the reaction is conducted at 90-220 DEG C for 15-18 hours in an air isolation condition to obtain the secondary amine compounds. The catalyst can be used in the preparation of the secondary amine compounds, reduces the requirements and costs of the reaction to devices and is relatively high in reaction selectivity and yield.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Preparation method for palbociclib
ActiveCN104447743AEase of industrial productionRaw materials are easy to getOrganic active ingredientsAmino group formation/introductionDehydrogenationKetone
The invention discloses a preparation method for palbociclib (I). The preparation method comprises the following steps: performing cyclization reaction on 2-acetyl-2-butenoic acid methyl ester and malononitrile under an alkaline condition to generate 1,4,5,6-4H-2-methoxyl-4-methyl-5-acetyl-6-oxo-3-pyridine carbonitrile (II); performing substitution reaction on the intermediate (II) and cyclopentane halide (III) under the action of an acid-binding agent to generate N-cyclopentyl-1,4,5,6-4H-2-methoxyl-4-methyl-5-acetyl-6-oxo-3-pyridine carbonitrile (IV); performing condensation reaction on the intermediate (IV) and N-[5-(1-piperazinyl)-2-piperidyl]carbamidine (V)to generate 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-5,6-dihydropyrido[2,3-d]pyrimidine-7(8H)-ketone (VI); performing dehydrogenation reaction on the intermediate (VI) and sodium selenate to generate the palbociclib (I). The preparation method for the palbociclib has the advantages that the raw material is easy to obtain, the process is simple, high efficiency and environmental protection are achieved, and the preparation method is suitable for industrial production.
Owner:铜陵王燕堂生物科技有限公司
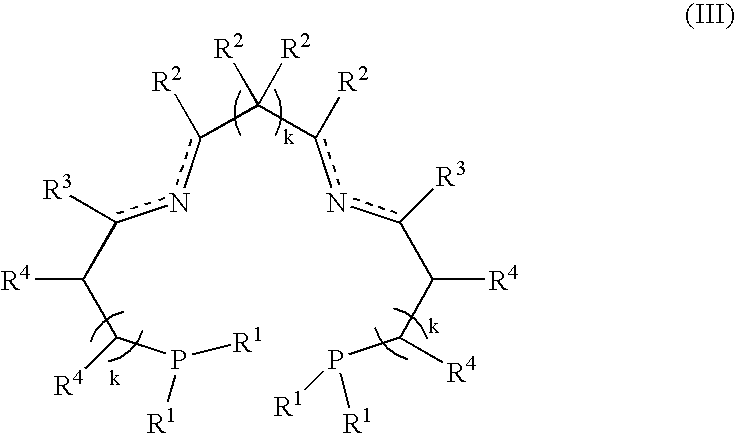
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
A process for the hydrogenation, using molecular hydrogen (H2), of a catalytic system, wherein the catalytic system comprises a base and a complex of formula; (II) wherein Y represents simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and N2P2 is a tetradentate diimine-diphosphino ligand.
Owner:FIRMENICH SA
Branched chain-containing aromatic compound
ActiveUS20120059149A1Easy to operatePeptide/protein ingredientsCarboxylic acid amides preparationOperabilityCrystallization
Owner:AJINOMOTO CO INC
Method for processing asymmetric hydroxylamination and dihydroxylation reaction by use of supported bi-cinchoni alkaloid ligand
InactiveCN1524835AOrganic compound preparationHydroxy group formation/introductionHydroxylamineSide chain
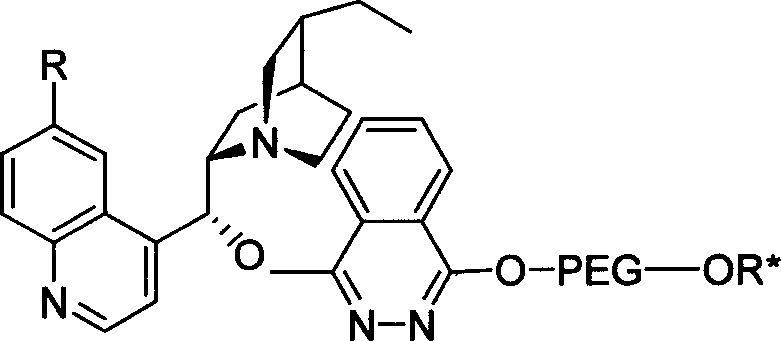
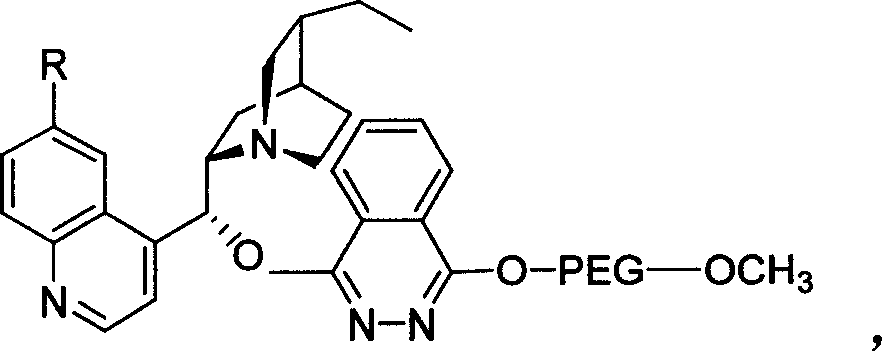
The invention relates to a process for carrying out asymmetrical hydroxylamine amination reaction and dual hydroxide reaction, wherein synthesized high polymer tethered Cinchonideine alkaloid ligand is utilized for raising yield and selectivity for the reaction product, the catalyst can be used repeatedly. The process is especially suitable for the mass production of Taxol synthesis and its C13 side chains, the used synthesized high polymer tethered Cinchonideine alkaloid ligand has the structural formula as disclosed in the specification, wherein PEG is polyoxyethylene glycols, R=H or OCH3.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Application of supported gold cluster catalyst
ActiveCN105985208AHigh selectivityLow priceCarboxylic acid nitrile preparationOrganic compound preparationNitro compoundDeposition precipitation
The invention relates to application of a supported gold cluster catalyst to selective hydrogenation of an aromatic nitrocompound. A preparation method for the catalyst comprises the following steps: with a biological reagent containing mercaptan as a protective agent, preparing a precursor containing a gold cluster in advance; then impregnating a carrier with the precursor; and carrying out drying and roasting at a certain temperature so as to obtain the catalyst which can be directly applied to selective hydrogenation of the aromatic nitrocompound. The catalyst shows excellent activity and selectivity under mild reaction conditions; and compared with supported gold catalysts prepared by using a coprecipitation or deposition-precipitation method, the catalyst prepared in the invention is substantially advantaged in activity and selectivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for hydrogenation of carbonyl and iminocarbonyl compounds using ruthenium catalysts comprising tetradentate diimino-diphosphine ligands
A process for the hydrogenation, using molecular hydrogen (H2) of a catalytic system, wherein the catalytic system includes a base and a complex of formula (II):Ru(P2N2)Y2 (II)wherein Y represent simultaneously or independently a hydrogen or halogen atom, a hydroxy group, or an alkoxy, carboxyl or other anionic radical, and P2N2 is a tetradentate diimino-diphosphine ligand.
Owner:FIRMENICH SA
Asymmetric Diamine Compounds Containing Two Functional Groups and Polymers Therefrom
InactiveUS20150045481A1Improve solubilityLow refractive indexPlastic/resin/waxes insulatorsOrganic compound preparationSolubilityOptical property
The present invention relates to a novel diamine compound, wherein two substituents R and R′ are introduced asymmetrically, and a polymer thereof. The polymer may have excellent solubility in the organic solvent and allows for easy processibility after imidization, thus giving proper film maintaining superior properties, such as thermal, mechanical, and optical properties for applications in electrical, electronic, or optical materials.
Owner:KOREA ADVANCED INST OF SCI & TECH
Use of a catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in kumada coupling reactions
InactiveUS6369265B1Good yieldEliminate needOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsLiquid mediumGrignard reagent
This invention provides a process for conducting Kumada coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Kumada couplings of aryl halides. A Kumada coupling can be carried out by mixing, in a liquid medium, at least one aryl halide, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one Grignard reagent; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group. Homocoupling of aryl pseudohalides is also feasible using the processes of this invention.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Use of catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in amination reactions
InactiveUS6403802B1High yieldEliminate needGroup 5/15 element organic compoundsCarboxylic acid esters preparationLiquid mediumOxidation state
This invention provides a process for conducting amination reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in aminations of aryl halides and aryl pseudohalides. An amination can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than amino groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one primary amine and / or at least one secondary amine; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Method for preparing aromatic primary amine by taking ammonia water as ammonia source in water phase system
ActiveCN102050687AImprove friendlinessEasy to operateOrganic compound preparationAmino group formation/introductionAlkaline earth metalPhosphate
The invention provides a method for preparing aromatic primary amine by taking ammonia water as an ammonia source in a water phase system. In the method, aromatic halogenate and the ammonia water are used as raw materials, water is used as a solvent, at room temperature or under the heating condition of common oil bath, carbonates, fluorides, phosphates and hydroxides of alkali metals or alkaline-earth metals are taken as alkalis, and a catalytic reaction is preformed through adding a surfactant and using a temary catalysis system composed of copper catalyst, hydrazide and ketone. The method has the characteristics that the operation is simple, wide substrate application range is wide, the product is simple and easy to separate, the yield is high, the process is economic and is environment-friendly and the like; and reaction conditions are flexible, thus corresponding room temperature or heating mode can be selected according to practical requirements. In addition, the environment friendliness of the reaction is effectively improved by using water as a reaction solvent, thereby better conforming to the development requirements of green chemistry. Especially, the substrate is wide in application range and has wide application prospects in the aspects of preparing natural products, medicines and pesticides.
Owner:SUN YAT SEN UNIV
Popular searches
Amino-carboxyl compound preparation Chemical recycling Amino compound preparation Metal/metal-oxides/metal-hydroxide catalysts Amino-hyroxy compound preparation Hydroxy compound preparation Cyano group formation/introduction Ketenes preparation Oxygen compounds preparation by hydrocarbon oxidation Graphene
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com