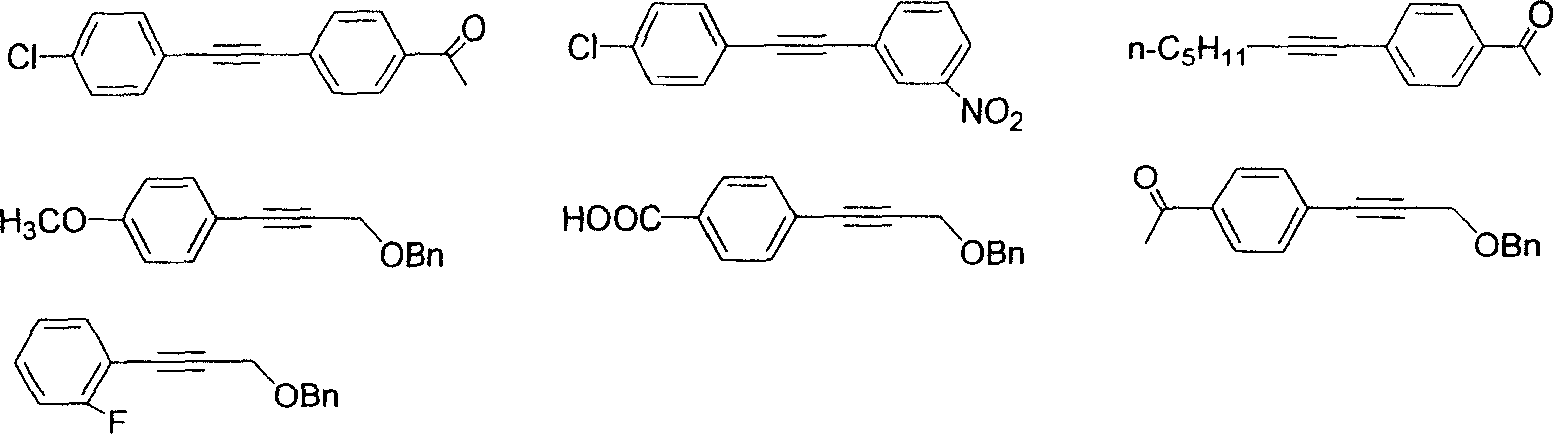
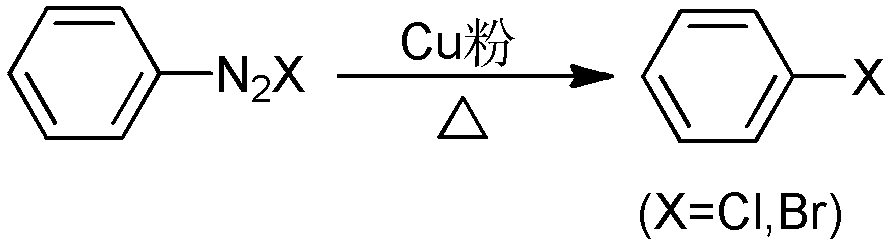
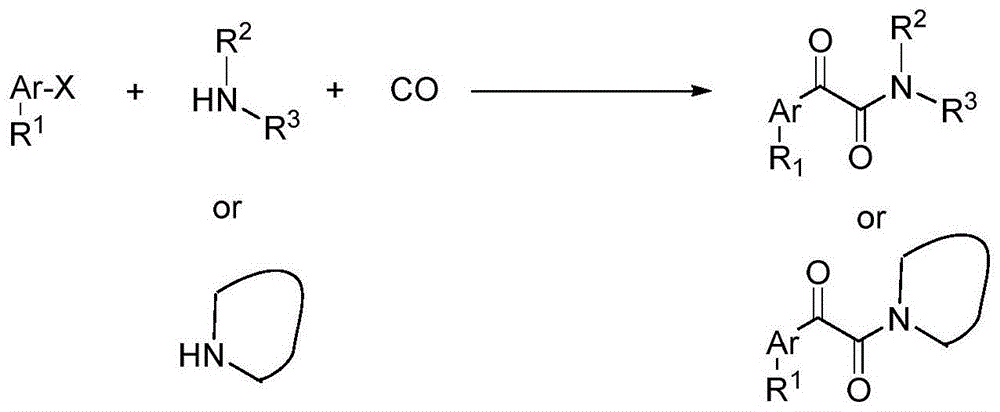
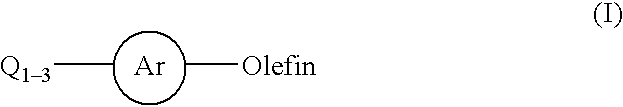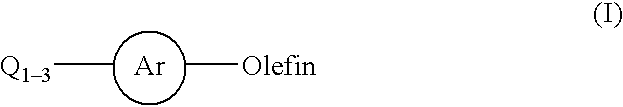Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
391 results about "Aryl halide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
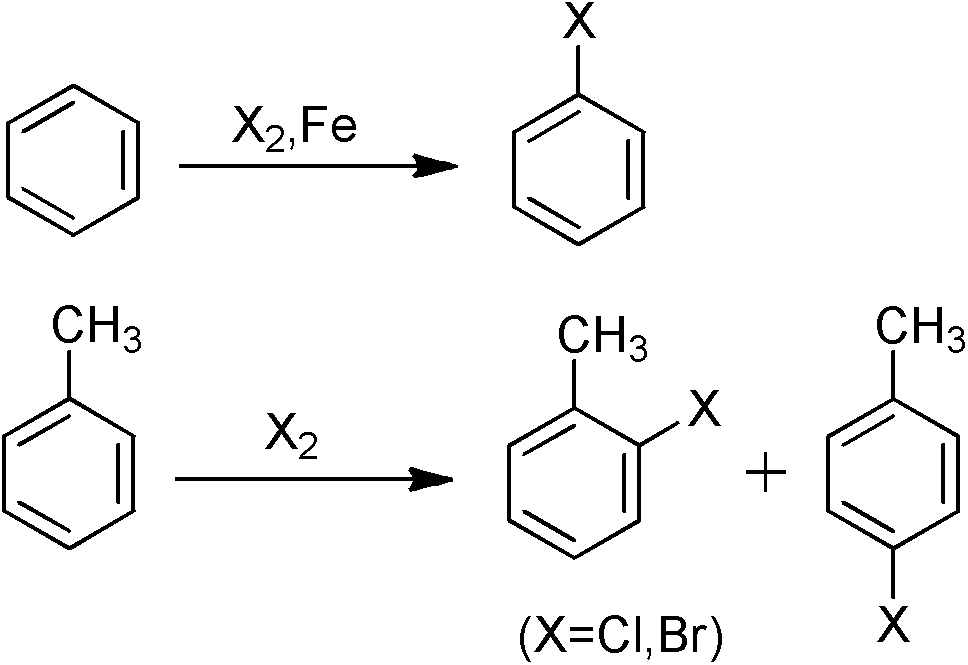
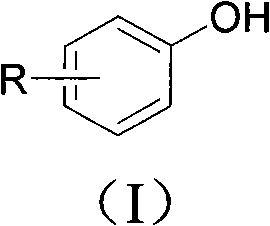
In organic chemistry, an aryl halide (also known as haloarene or halogenoarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide. The haloarene are distinguished from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that many derivatives enjoy niche applications.
2-2′-disubstituted 9,9′-spirobifluorene-base triaryldiamines and their application
ActiveUS7714145B2Simple wayEasy to practiceSilicon organic compoundsDischarge tube luminescnet screensOrganic light emitting deviceSubstitution reaction
The present invention discloses synthesis of 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamine. First, 2,2′-diamino-9,9′-spirobifluorene, a Pd-catalyst as auxiliary and aryl halide BX are provided, wherein X is selected from the group consisting of: Cl, Br and I, B comprises one of the following group: aryl moiety, hetero cycle, multiple fused ring, multiple fused ring with hetero atom(s). Next, a substitution reaction is performed to react the 2,2′-diamino-9,9′-spirobifluorene with the aryl halide BX to produce the 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamines. In addition, the present invention discloses organic light emitting devices comprising hole transporting material comprising 2,2′-bis(N,N-disubstituted amino)-9,9′-spirobifluorenes.
Owner:E RAY OPTOELECTRONICS TECH
Method for producing highly purified, granular silicium
InactiveUS7001579B2Minimum formation of dustMinimizing reactionSiliconChemical/physical processesHydrogen halideHydrogen
The invention relates to a method for producing granular silicon by thermal decomposition of a gas containing silicon in a fluidized bed, said decomposition occurring in the presence of free-flowing mobile elements. Preferably, said free-flowing mobile elements become devoid of silicon in a separate procedural step, said silicon being deposited during decomposition of gas containing silicon, by reacting with hydrogen halides, halogens, alkyl halogenides, aryl halogenides or combinations of halogen and / or hydrogen halide and / or oxidized mineral acids and / or by thermal treatment of said elements.
Owner:SOLARWORLD
2,2'-Disubstituted 9,9'-Spirobifluorene-based Triaryldiamines and Their Application
ActiveUS20070262703A1Simple wayEasy to practiceSilicon organic compoundsDischarge tube luminescnet screensOrganic light emitting deviceSubstitution reaction
The present invention discloses synthesis of 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamine. First, 2,2′-diamino-9,9′-spirobifluorene, a Pd-catalyst as auxiliary and aryl halide BX are provided, wherein X is selected from the group consisting of: Cl, Br and I, B comprises one of the following group: aryl moiety, hetero cycle, multiple fused ring, multiple fused ring with hetero atom(s). Next, a substitution reaction is performed to react the 2,2′-diamino-9,9′-spirobifluorene with the aryl halide BX to produce the 2,2′-disubstituted 9,9′-spirobifluorene-based triaryldiamines. In addition, the present invention discloses organic light emitting devices comprising hole transporting material comprising 2,2′-bis(N,N-disubstituted amino)-9,9′-spirobifluorenes.
Owner:E RAY OPTOELECTRONICS TECH
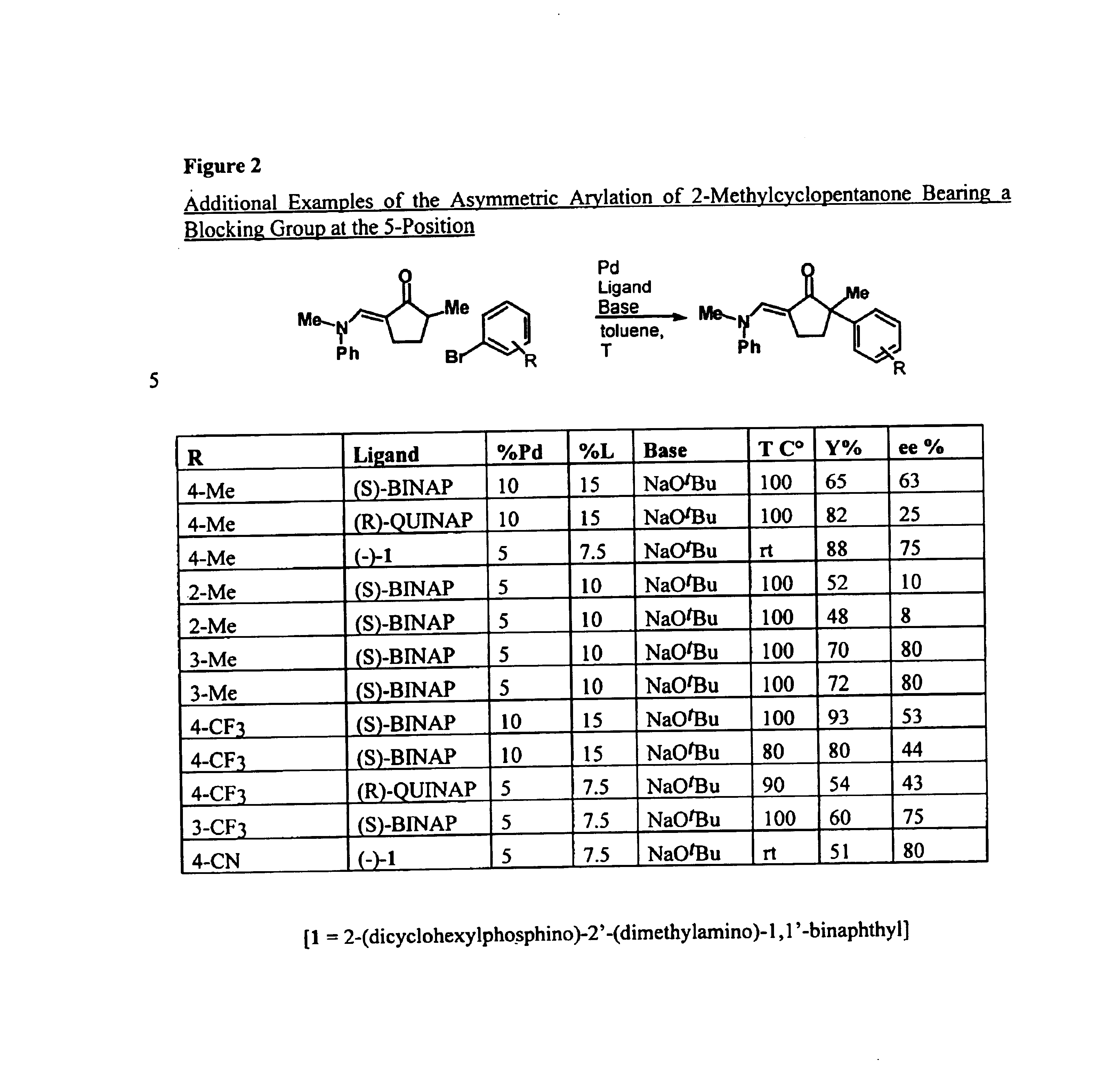
Arylation and vinylation of activated carbons
InactiveUS6867310B1Carboxylic acid nitrile preparationGroup 5/15 element organic compoundsActivated carbonHydrazone
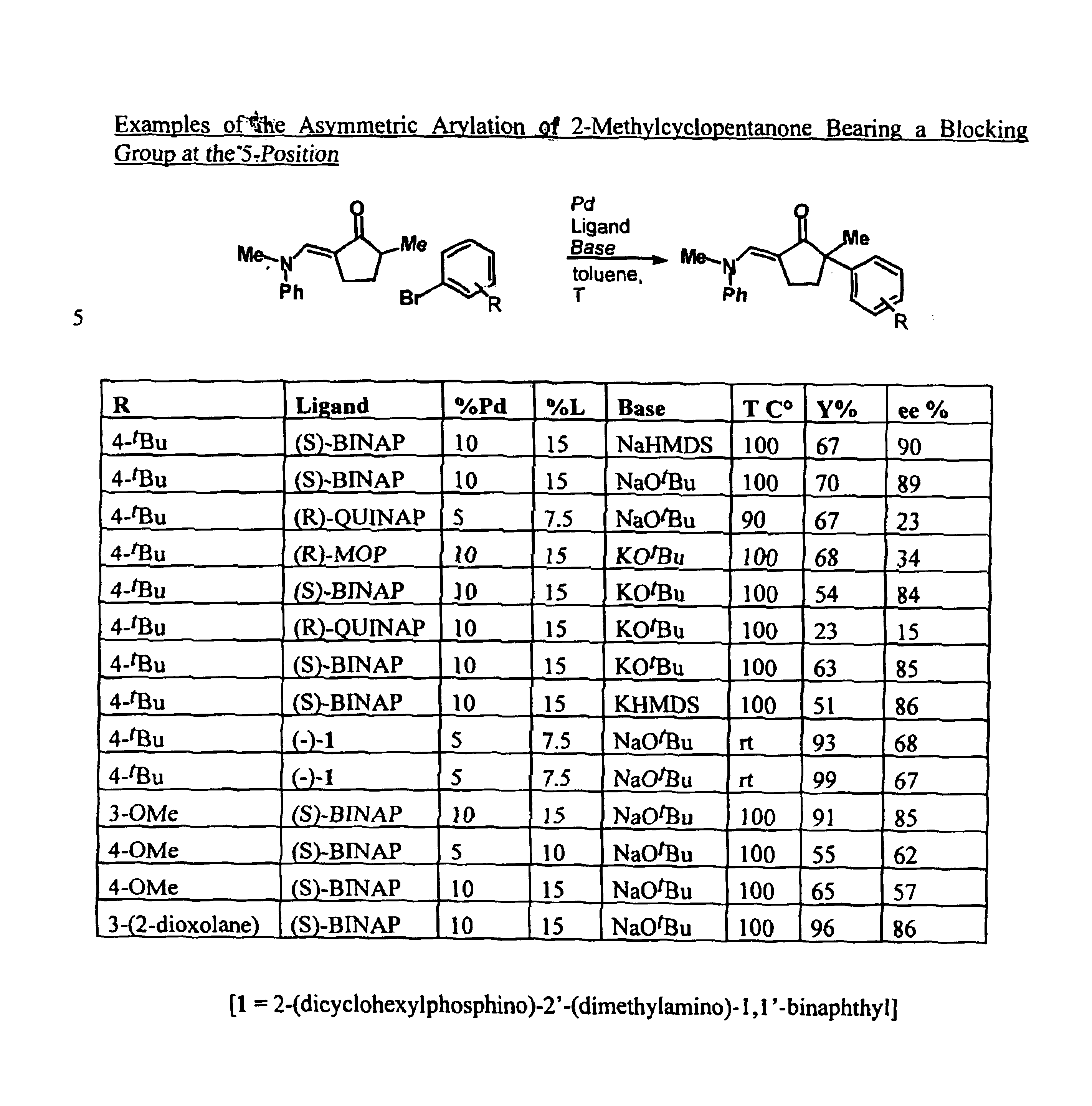
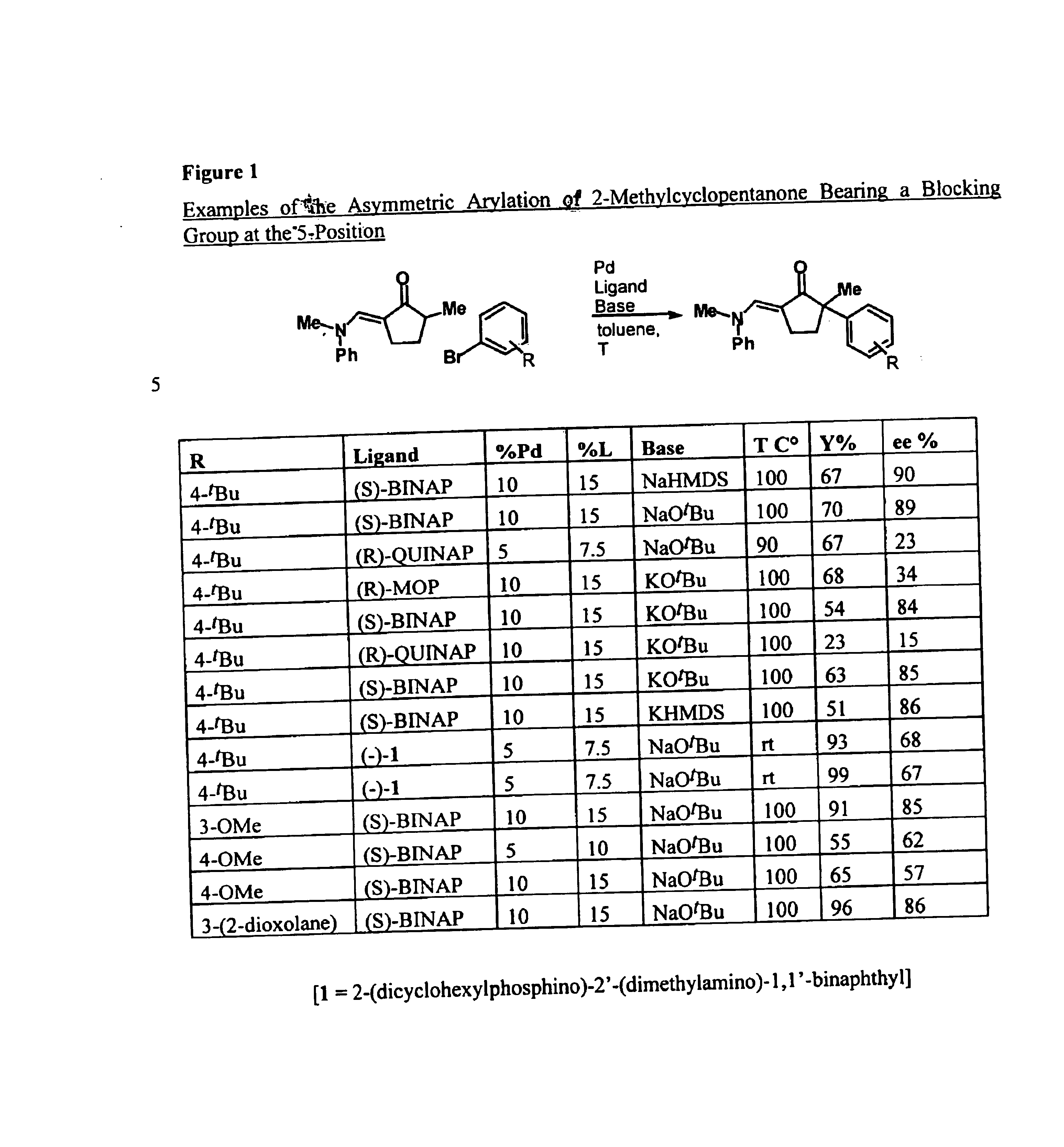
The present invention provides transition-metal-catalyst-based methods for the arylation and vinylation of activated methyl, methylene, and methine carbons with aryl halides, vinyl halides, and the like. The methods of the invention provide several improvements over existing methods, including the ability to synthesize efficiently and under mild conditions α-aryl and α-vinyl products from a wide range of starting materials, including ketones, esters, hydrazones, and imines. Furthermore, the methods of the invention may be used in an asymmetric sense, i.e. to produce enantiomerically-enriched chiral α-aryl and α-vinyl products.
Owner:MASSACHUSETTS INST OF TECH
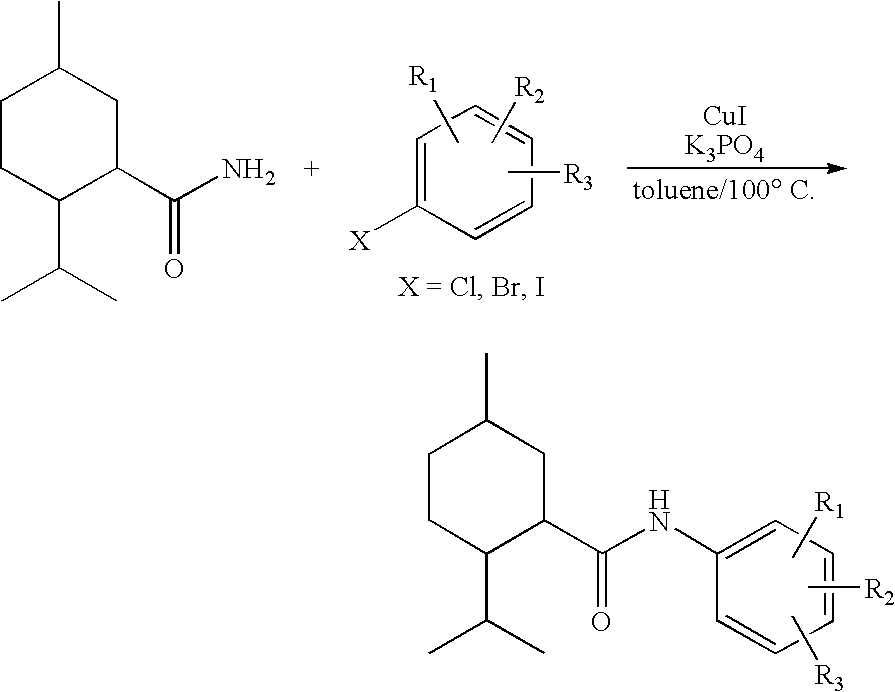
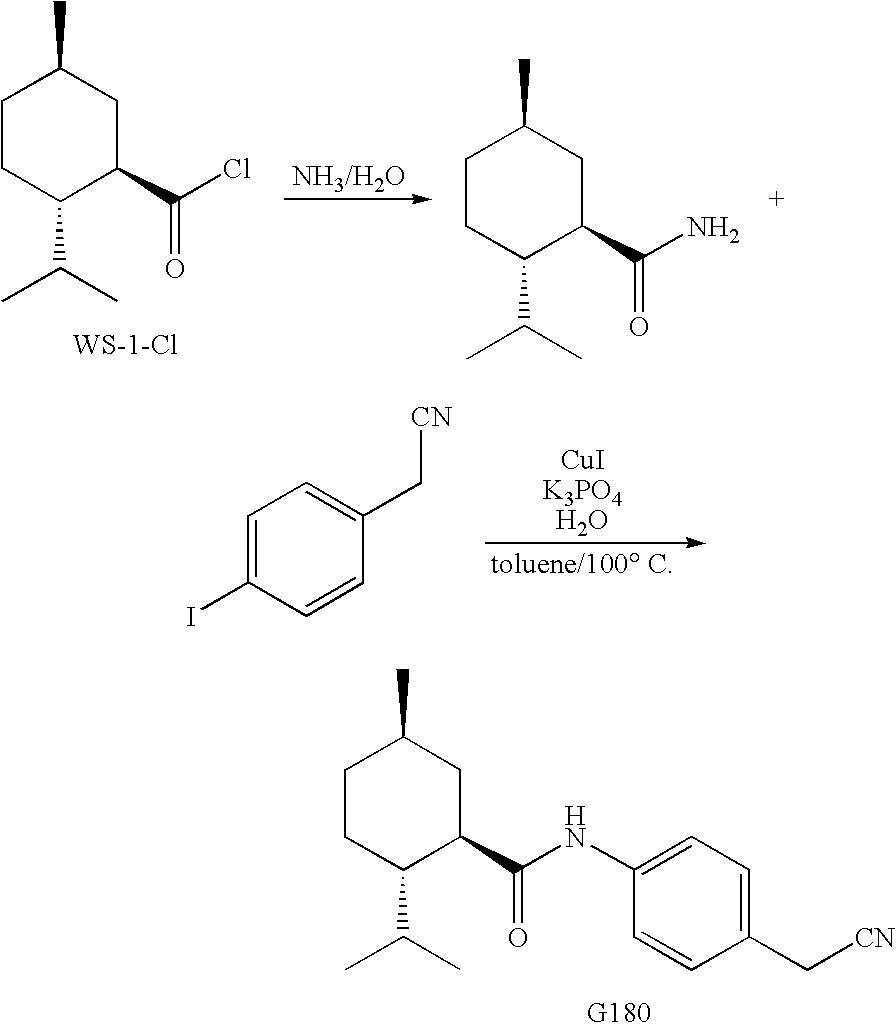
Synthesis of Cyclohexane Derivatives Useful as Sensates in Consumer Products
ActiveUS20100076080A1Improve cooling effectPotent and long lasting cooling effectBiocideCosmetic preparationsCooling effectL menthol
The present invention provides synthetic routes for preparing various isomers of cyclohexane-based coolants, such as menthyl esters and menthanecarboxamide derivatives, in particular those substituted at the amide nitrogen, for example with an aromatic ring or aryl moiety. Such structures have high cooling potency and long lasting sensory effect, which make them useful in a wide variety of consumer products. One synthetic route involves a copper catalyzed coupling of a primary menthanecarboxamide with an aryl halide, such reaction working best in the presence of potassium phosphate and water. Using this synthetic route, specific isomers can be prepared including the menthanecarboxamide isomer having the same configuration as l-menthol and new isomers such as a neoisomer having opposite stereochemistry at the carboxamide (C-1) position. The neoisomer unexpectedly has potent and long lasting cooling effect. Preparation schemes for neoisomers of other menthyl derivatives which are useful as coolants, including esters, ethers, carboxy esters and other N-substituted carboxamides are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
Copper-catalyzed c-h bond arylation
ActiveUS20090076266A1Carboxylic acid nitrile preparationOrganic compound preparationHydrogenOrganic synthesis
The present invention is a one-step method for efficiently converting carbon-hydrogen bonds into carbon-carbon bonds using a combination of aryl halides, a substrate, and a copper salt as catalyst. This method allows faster introduction of complex molecular entities, a process that would otherwise require many more steps. This invention is particularly relevant for the organic synthesis of complex molecules such as, but not limited to, pharmacophores and explosives.
Owner:UNIV HOUSTON SYST
Method for synthesizing flame retardant hexaphenoxy cyclotriphosphazene
ActiveCN101985455ASimple and fast operationShort reaction timeGroup 5/15 element organic compoundsDistillationFire retardant
The invention discloses a method for synthesizing a flame retardant hexaphenoxy cyclotriphosphazene. The method is characterized by comprising the following steps of: performing a one-pot reaction on hexachlorocyclotriphosphazene, phenol, alkali metal hydroxides and other raw materials in a solvent system consisting of aromatic hydrocarbon or aryl halide and water by adopting noncyclic polyether as a catalyst at the temperature of between 20 and 30 DEG C for 3 to 5 hours; heating to the temperature of between 80 and 100 DEG C, reacting for 3 to 5 hours; reclaiming hydrated aromatic hydrocarbon or aryl halide through cooling, separation, washing and depressed pressure distillation after the reaction is completed; and cooling and coagulating the residues to obtain a white crystal solid powder product, wherein the yield is over 95 percent. The method has the characteristics of short reaction time, low cost, good quality and the like, is simple and safe in operation, and is environmentally-friendly and suitable for industrial production and application.
Owner:SICHUAN DONGFANG INSULATING MATERIAL
Use of a catalyst system comprising nickel palladium or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in stille coupling reactions
InactiveUS6362357B1High yieldEliminate needHydrocarbon by isomerisationOrganic compound preparationLiquid mediumOxidation state
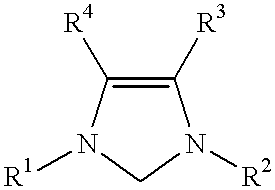
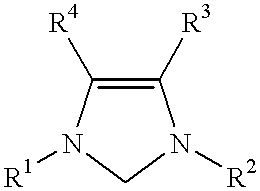
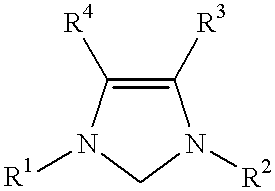
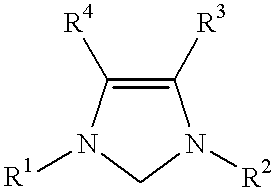
This invention provides a process for conducting Stille coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Stille couplings of aryl halides. A Stille coupling can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than stannyl groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one organotin compound wherein the tin atom is quaternary, wherein one group bound to the tin atom is unsaturated at the alpha or beta position, and wherein each of the remaining groups bound to the tin atom is a saturated group; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:RES & TECH FOUND UNIV OF NEW ORLEANS
9,9'-spiral acridine-containing compound and preparation method and application thereof
InactiveCN106831773ALow singlet-triplet energy level differenceImprove exciton utilizationOrganic chemistrySolid-state devicesAcridineHalogen
The invention discloses a 9,9'-spiral acridine-containing compound. 9,9'-spiral acridine is taken as a core and the 9,9'-spiral acridine is combined with peripheral electrophilic Ar and Ar' units. The invention further discloses a preparation method of the 9,9'-spiral acridine-containing compound. The 9,9'-spiral acridine-containing compound is obtained through selecting different halogen-substituted Ar unit and Ar' unit, carrying out Buchwald-Hartwig coupled reaction on 10H, 10'H-9,9'-spiral acridine, carrying out copper-catalyzed aryl halide amination reaction or carrying out nucleophilic substitution reaction under a strong alkaline condition. The invention further discloses an application of the 9,9'-spiral acridine-containing compound. The 9,9'-spiral acridine-containing compound has high fluorescence and certain conductivity, and can be used for fabricating a light-emitting layer of an organic light-emitting diode as a luminous body.
Owner:SOUTH CHINA UNIV OF TECH +1
Coupling reaction of end group alkine and aryl halide
InactiveCN1634810ALow priceReduce the temperatureOrganic compound preparationHydrocarbonsEnd-groupReaction temperature
The present invention relates to a method for the coupling reaction of terminal alkynes and aryl halides. The invention provides a coupling reaction method, that is, amino acid is used as an additive and CuI is used as a catalyst, so that the coupling reaction of terminal alkynes and aryl halides can be carried out under relatively mild conditions. The invention also provides some new coupling reaction products. The reactions involved in the present invention can be represented by the following general reaction formula. The catalyst and ligand used in the present invention are cheap, easy to get, and stable in the air. Compared with the same type of reaction reported in the literature, the average temperature of the reaction is reduced by about 20 ° C, and the reaction conditions are very mild. Good application prospects.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Use of a catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in kumada coupling reactions
InactiveUS6369265B1Good yieldEliminate needOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsLiquid mediumGrignard reagent
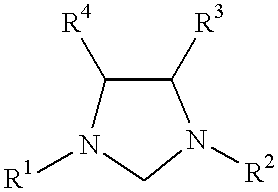
This invention provides a process for conducting Kumada coupling reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in Kumada couplings of aryl halides. A Kumada coupling can be carried out by mixing, in a liquid medium, at least one aryl halide, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one Grignard reagent; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group. Homocoupling of aryl pseudohalides is also feasible using the processes of this invention.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Use of catalyst system comprising nickel, palladium, or platinum and imidazoline-2-ylidene or imidazolidine-2-ylidene in amination reactions
InactiveUS6403802B1High yieldEliminate needGroup 5/15 element organic compoundsCarboxylic acid esters preparationLiquid mediumOxidation state
This invention provides a process for conducting amination reactions. The processes of the present invention make use of N-heterocyclic carbenes as ancillary ligands in aminations of aryl halides and aryl pseudohalides. An amination can be carried out by mixing, in a liquid medium, at least one strong base; at least one aryl halide or aryl pseudohalide in which all substituents are other than amino groups, wherein the aryl halide has, directly bonded to the aromatic ring(s), at least one halogen atom selected from the group consisting of a chlorine atom, a bromine atom, and an iodine atom; at least one primary amine and / or at least one secondary amine; at least one metal compound comprising at least one metal atom selected from nickel, palladium, and platinum, wherein the formal oxidation state of the metal is zero or two; and at least one N-heterocyclic carbene. One preferred type of N-heterocyclic carbene is an imidazoline-2-ylidene of the formulawherein R1 and R2 are each, independently, alkyl or aryl groups having at least 3 carbon atoms, R3 and R4 are each, independently, a hydrogen atom, a halogen atom, or a hydrocarbyl group.
Owner:UNIV OF NEW ORLEANS RES TECH FOUND
Process for manufacturing diphenylamines
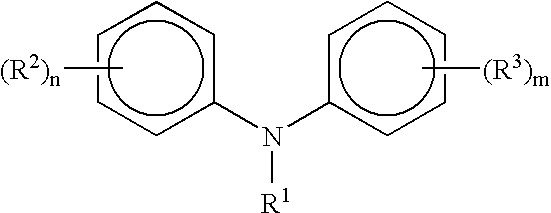
InactiveUS20070073086A1Organic compound preparationAmino preparation by hydrogen substitutionHeat sensitiveAniline
The invention teaches novel process steps for the rapid high yield manufacture of diphenylamines of the formula wherein R1 is selected from hydrogen, alkyl, and aryl; wherein each R2 and R3 is the same or different and each is independently selected from hydrogen, alkyl, alkoxy, aralkyl, dialkylamino, alkylarylamino and substituted or unsubstituted aryl, the substituents on aryl being each independently selected from alkyl (C1-C8), alkoxy (C1-C8), aroxy, aralkoxy and halogen; wherein n and m are each independently an integer from 1 to 5. Diphenylamines are key intermediates for the production of leuco dyes used in pressure-sensitive and heat-sensitive imaging systems. The process in at least one embodiment comprises reacting at elevated temperature an aryl halide with an aromatic amine in an organic solvent and aqueous alkaline solution and optionally in some embodiments, phase-transfer agent, followed by addition of catalytic amounts of bis[tri(t-butylphosphine)]palladium at a suitable temperature to rapidly form diphenylamine.
Owner:APPLETON PAPERS INC
Method and apparatus for continuously producing aryl boric acid
InactiveCN102190676AAvoid it happening againEasy to removeGroup 3/13 element organic compoundsLithiumBoric acid
The invention relates to a method and an apparatus for continuously producing aryl boric acid. The method comprises the following steps: a first mixture is obtained by mixing aryl halide or an aryl heterocyclic compound with lithium alkyl in a first micro mixer, and a stay period of the first mixture is 0.5 to 3 s; a second mixture is obtained by mixing the first mixture with boric acid ester in a second micro mixer; and aryl boric acid is prepared by carrying out a reaction on the second mixture in a micro reactor. According to the method and the apparatus provided by the invention, aryl boric acid can be prepared continuously, rapidly and safely to achieve industrialization of aryl boric acid.
Owner:BAYER TECH & ENG SHANGHAI
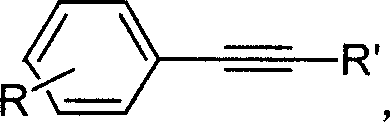
Aryl halide derivatives and synthesis method thereof
ActiveCN104016901AHigh yieldConvenient modification reactionOrganic compound preparationCarboxylic acid esters preparationSynthesis methodsOrganic synthesis
The invention relates to aryl halide derivatives and a synthesis method thereof. The structural formula of the aryl halide derivatives is disclosed in the specification. The synthesis method comprises the following steps: precursor synthesis, cyclic addition and purification. The condensed ring aryl halide derivatives can be used as a drug intermediate or drug, or an important intermediate in organic synthesis, and has wide application prospects in the field of organic synthesis.
Owner:ANHUI NORMAL UNIV
Catalytic carbonylation method for synthesis of alpha-keto amide
InactiveCN104892547AGood compatibilityEnvironmentally friendlySugar derivativesOrganic compound preparationNano catalystSynthesis methods
The invention discloses a catalytic carbonylation method for synthesis of alpha-keto amide. A palladium source in an aqueous solution of polyethylene glycol or polyethylene glycol is used for in-situ formation of a highly active palladium nano-catalyst; and the palladium nano-catalyst can efficiently catalyze bis-carbonylation reaction of aryl halide and aliphatic amine for one-step synthesis of an alpha-keto amide compound by using carbon monoxide as a dicarbonyl source in the presence of alkali or without alkali, and does not require ligand. The synthesis method of alpha-keto amide uses the palladium nano-catalyst formed in situ, the reaction does not require ligand promotion and can achieve conversion with high efficiency and high selectivity; the substrate is stable, cheap and readily available, has good universality of functional groups; and the operation is safe and simple.
Owner:NANJING NORMAL UNIVERSITY
Ionic liquid type monophosphine monoimidazolium salt nickel (II) complex and preparation and application thereof
InactiveCN101591360ARich choiceVarious structural changesOrganic-compounds/hydrides/coordination-complexes catalystsNickel organic compoundsGrignard reagentPhenyl group
The invention discloses an ionic liquid type monophosphine monoimidazolium salt nickel (II) complex and preparation and application thereof. The chemical molecular formula of the monophosphine monoimidazolium salt nickel (II) complex is (Ph3P)Ni{[(RNCHCHNR)CH]X}X2, wherein R is selected from one of C1-C4 saturated alkyl, phenmethyl, 2,6-diisopropylphenyl and mesitylene; and X is halogen and selected from one of chlorine, bromine or iodine. The monophosphine monoimidazolium salt nickel (II) complex has good catalytic activity on Kumada cross-coupling reaction between aryl Grignard reagent and aryl halide or halogenated pyridine.
Owner:SUZHOU UNIV
Method for degrading aryl halide organic pollutants
InactiveCN101954166ACatalytic degradationLow costCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsHydrotalciteSpinel
The invention relates to treatment of aryl halide organic pollutants, in particular to a method for degrading the aryl halide organic pollutants. An active ingredient spinel of a catalyst has the chemical general formula of AB2O4, wherein A is one or two of Mg2+, Ni2+, Ba2+, Zn2+, Cu2+, Ca2+ and Fe2+; and B is Al3+ or Fe3+. A method for preparing the catalyst comprises the following steps of: synthesizing hydrotalcite-like compounds by using a coprecipitation method; and filtering and drying, and roasting at the temperature of between 400 and 900 DEG C. The prepared catalyst is future ground and mixed with aryl halides-containing pollutants, or further processed and formed and put in an aryl halides-containing environment to realize catalytic degradation at the temperature of between 200 and 450 DEG C. The method has the advantages of low cost, simple equipment, low energy consumption, high efficiency and the like, and the production and using processes of the catalyst have the advantage of good environmental protection.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for a carbon-carbon coupling reaction of aryl halides with olefins by heterogeneous catalysts
InactiveUS20020128478A1Organic compound preparationCarboxylic acid esters preparationPalladium catalystChloride
This invention relates to a process for the Heck coupling reaction where heterogeneous palladium catalysts are used to activate aryl halides for a carbon-carbon coupling with olefins in the presence a base and an aprotic solvent to produce aryl-olefin compounds. The process, in particular, provides for the use of aryl chlorides substituted with electron-withdrawing or electron-donating group for the cross coupling with olefins.
Owner:KRSKA SHANE W +2
Preparation method of metal carrier Tenax coating stirring and extracting rod
InactiveCN101992069AAchieve enrichmentAvoid competitive adsorptionIon-exchange process apparatusOther chemical processesEpoxyTenax
The invention relates to a preparation method of a metal carrier Tenax coating stirring and extracting rod, which comprises the following steps of: a, selecting and preprocessing a metal rod; b, synthesizing heat-resistant epoxy resin; c, manufacturing a stirring rod coating; d, curing the coating; and e, removing impurities of the extracting rod body, wherein the extracting rod is used in a stirring manner, and the metal is iron, nickel, cobalt, stainless steel or alloy; and when the extracting rod is used in a headspace or ultrasonic manner, the metal is stainless steel, copper, aluminum or alloy. Tenax is fixed on the surface of the metal rod by using the synthesized heat-resistant epoxy resin or organic silicon resin to form a coating material with heat stability and solvent washing resistance, thus the extracting rod realizes the enrichment of trace organic matters. The invention is especially suitable for processing samples of soil, air, essence and spice, indoor air, alcohol, phenol, amine-aldehyde, ketone, aryl halides and the like.
Owner:TIANJIN CHUNFA BIO TECH GRP
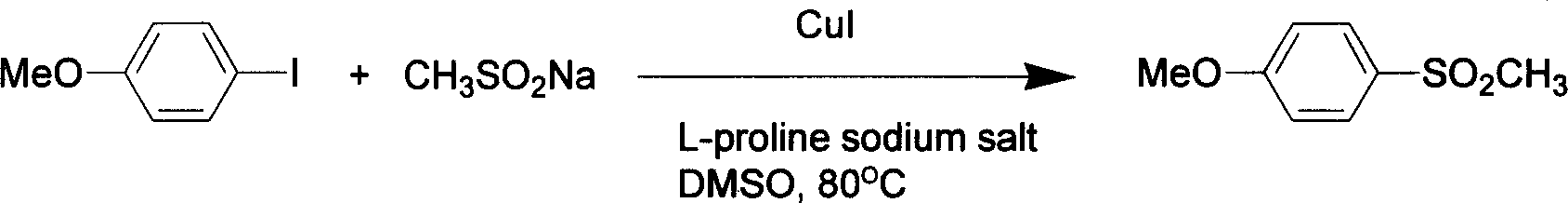
Amino acid accelerated CuI catalyzed aryl halide and coupling reaction of alkyl sulfonate
InactiveCN1651408ALow priceReduce the temperatureOrganic chemistryOrganic compound preparationCoupling reactionOrganic chemistry
A process for preparing arylsulfone by the coupling reaction between arylhalide and hydroxysulfonite features that the amin acid is used as the reaction promoter and the CuI is used as catalyst for creating gentle reaction conditions.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
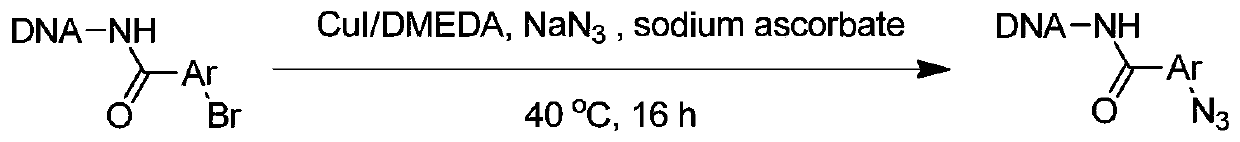
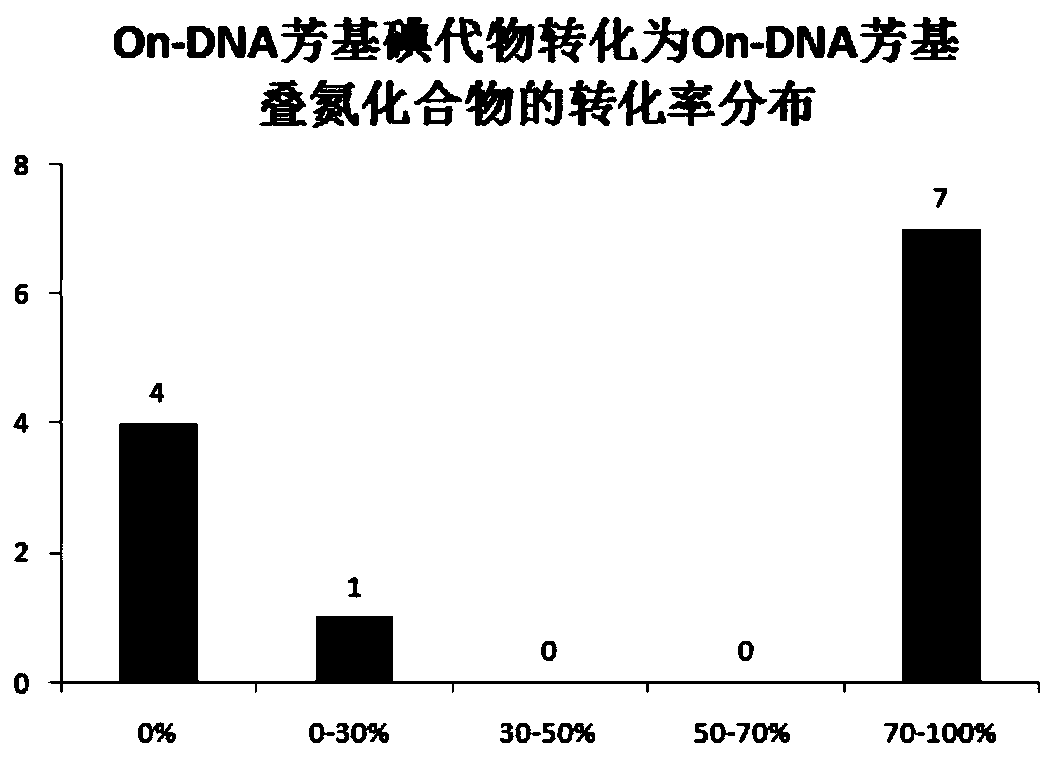
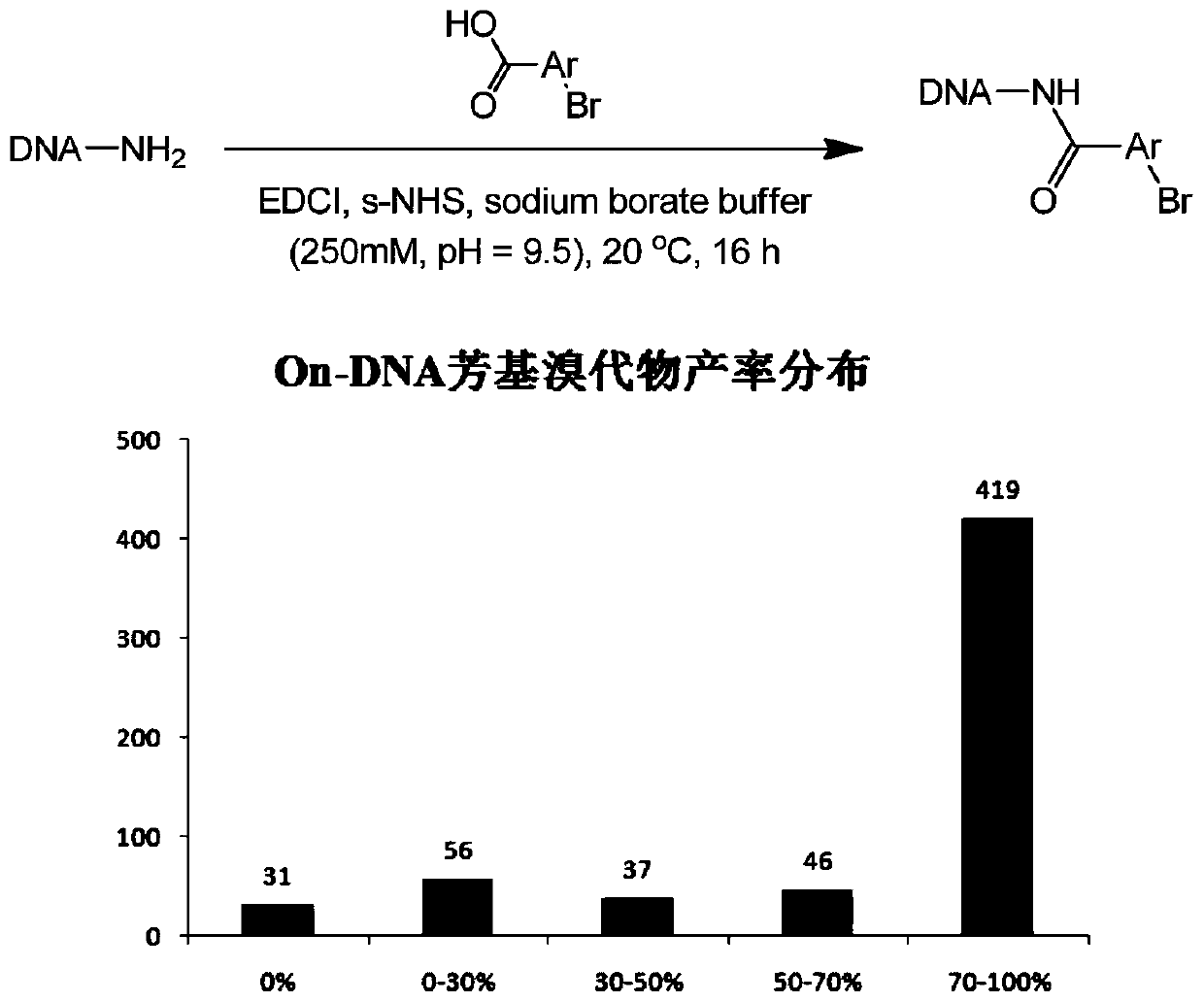
Synthesis method of On-DNA aryl azide compound in construction of DNA coding compound library
ActiveCN110105408AMild reaction conditionsEasy to operateSugar derivativesSugar derivatives preparationSynthesis methodsNitrogen
The invention discloses a preparation method of an On-DNA aryl azide compound used for constructing a DNA coding compound library. According to the method, an On-DNA aryl halide is taken as a substrate to react with an azide reagent to prepare the On0DNA aryl azide compound in the presence of a copper catalyst, a ligand and a base. The preparation method of the On-DNA aryl azide compound has the advantages that the catalyst is cheap and easy to obtain, the reaction condition is mild, the operation is convenient, and the method is suitable for the synthesis of the DNA coding compound library performed by a porous plate.
Owner:上海药明康德新药开发有限公司
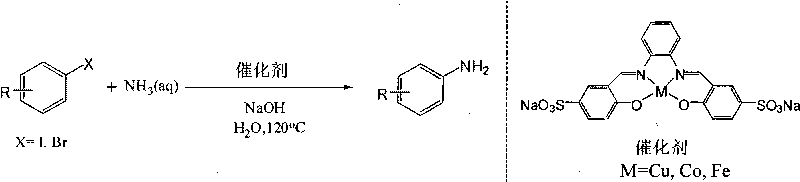
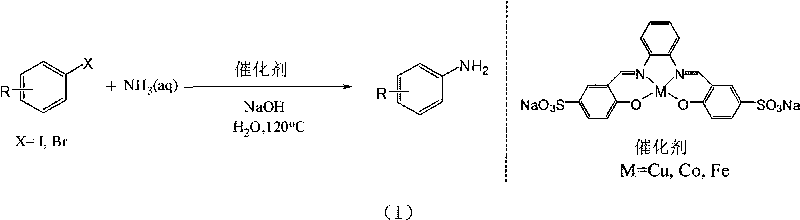
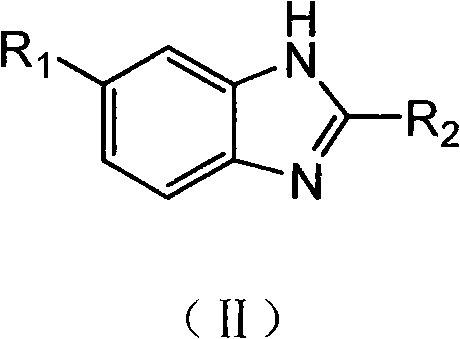
Method for preparing arylamine by catalysis in aqueous phase
InactiveCN101717369AHigh yieldEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsState of artWater soluble
The invention provides a novel method for preparing arylamine by catalysis in aqueous phase, disclosing a primary arylamine preparation method which is safe, cheap, and environment-friendly and has atom economy by efficiently catalyzing ammonia water in aqueous phase to generate a coupling reaction with aryl halide through utilizing a water soluble coordination compound. The invention can be applied to water phase synthesis of important pharmaceutical intermediates, such as substituted benzimidazole, and the like, and chemical materials. Compared with the prior art, the method not only is suitable for wider functional groups, and has high yield, less byproducts, has simple operation, safety, low cost and less pollution.
Owner:SICHUAN UNIV
Aryl halide to olefin arylation carrying bimetallic catalyst, and its preparing method
InactiveCN1709572AHigh catalytic activityImprove stabilityMetal/metal-oxides/metal-hydroxide catalystsAlkeneAqueous solution
The invention relates to a kind of supported bimetallic complex catalyst of arylation to alkene by aryl halogenide and its preparing method. The catalyst contains precious metal Pd and / or base metal Cu, or Mo, or Ni, or Co, or Mn and carrier; the weight ratio of content of precious metal Pd counted as Pd and added carrier is 0.1% - 2.0%; the ratio of content of base metal counted as metal and added carrier is 0.5% - 10%. On the basis of precious metal Pd, add the other base metal Cu, or Mo, or Ni, or Co, or Mn; using chemical reduction method, prepare supported bimetallic complex catalyst used in Heck reaction; the watery solution concentration of reducing agent is 0.5% - 10%. The invention has following advantages: it has high catalyzing activeness, and short reacting time; it reduces the Pd dosage, and is easy to realize separation of output and catalyst.
Owner:SICHUAN UNIV
Phenolic compound synthesizing method taking water as solvent
InactiveCN101774873AImprove toleranceReduce pollutionOrganic compound preparationHydroxy group formation/introductionIodideSynthesis methods
The invention relates to a phenolic compound synthesizing method taking water as a solvent. Under a mild reaction condition, the method takes aryl halides as the raw material, takes water as the solvent, takes alkali metal hydroxides (including sodium, potassium, cesium and the like) as alkalis, takes cuprous oxide, cuprous iodide, cuprous bromide or cuprous chloride as the catalyst, takes 2-pyridine formaldoxime as a ligand and takes 4-n-butyl ammonium bromide as the phase transfer catalyst, the reaction temperature is about 100-110 DEG C, and then phenolic compound is efficiently synthesized. Compared with the conventional phenolic synthesis method, the method takes the cheap and easily obtained aryl halides as the raw materials, takes the cheapest water as the solvent and takes the cheap cuprous oxide as the catalyst, the reaction condition is mild, the environmental pollution is small, the yield of generated phenol is high, the tolerance on various functional groups on an aromatic ring is high, separation and purification are convenient and the like, and the method can be widely applied in synthesis in the fields of medicines, polymers, natural products and the like of industrial community and academia.
Owner:TSINGHUA UNIV
N-arylation process with hydrazone as ligand in aqueous phase system
InactiveCN1803760AEasy to operateSimple reaction timeOrganic compound preparationAmino compound preparationAlkaline earth metalPhosphate
The provided N-arylation method in water phase system for C-N coupling reaction comprises: using aryl halide and amine or nitrogen-contained heteroaromatic compound as material and water as solvent, heating traditionally or with microwave; using the carbonate, fluoride, phosphate and hydrate of alkali metal or alkali earth metal as base; with transient metal catalysis, adding surfactant to promote the C-N coupling reaction with acylated hydrazone compound as ligand. This invention just needs mild reaction condition, and has wide application.
Owner:SUN YAT SEN UNIV
Method for synthesizing 2,4-dichloro-5-fluorobenzoyl
ActiveCN104725221AReduce the ratioAvoid pollutionOrganic compound preparationHalogenated hydrocarbon preparationBenzeneBiopolymer
The invention discloses a method for synthesizing 2,4-dichloro-5-fluorobenzoyl. The method comprises the following steps: reacting 2,4-dichlor fluorbenzene and CCl4 serving as initial raw materials under actions of solid acid catalyst S2O82- / Sm2O3-ZrO2-Al2O3 and AlCl3 to generate 2,4-dichloro-5-fluoro-(trichloromethyl) benzene, and catalyzing and hydrolyzing by FeCl3. The method has the advantages that: the solid acid catalyst S2O82- / Sm2O3-ZrO2-Al2O3 can be conveniently recycled, the yield is high, so that the proportion of biopolymer byproducts can be reduced; use of high-pollution high-risk raw materials can be avoided, the operation safety is high, and pollutant emission is little; and the method can be used for breaking through the technical bottleneck of the reaction of aryl halide and CCl4, and massively consuming CCl4 which destroys the ozone layer by introducing acyl chloride functional group on an aromatic ring by adopting the CCl4 as a raw material.
Owner:ZHEJIANG BENLI TECH CO LTD
Method for obtaining On-DNA aromatic hydrocarbon compound by Suzuki coupling reaction in construction of DNA encoded compound library
ActiveCN110359097AIncrease diversitySuitable for productionNucleotide librariesLibrary creationDna encodingA-DNA
The invention discloses a method for obtaining an On-DNA aromatic hydrocarbon compound by a Suzuki coupling reaction of an On-DNA aryl halide and an organic trifluoroborate reagent in the constructionof a DNA encoded compound library. The On-DNA aryl halide used as a substrate reacts with the n organic potassium trifluoroborate reagent in the presence of a Pd catalyst, a ligand and an alkali to prepare the On-DNA aromatic compound. The reaction method of the invention provides a new idea for the functional group transformation and capping method of the On-DNA aryl halide, can significantly increase the diversity of the DNA encoded compound library of On-DNA aryl halides, has the advantages of high yield, wide universality of the substrate, mild conditions, and convenience in operation, and is suitable for synthesizing the DNA encoded compound library on a perforated plate.
Owner:上海药明康德新药开发有限公司
N-heterocyclic carbene-palladium complex, and preparation method and application thereof
InactiveCN106892945AHigh catalytic efficiencyReduce dosageOrganic compound preparationGroup 8/9/10/18 element organic compoundsIsoquinolineNitrogen
The invention discloses an N-heterocyclic carbene-palladium complex, and a preparation method and application thereof. The N-heterocyclic carbene-palladium complex disclosed by the invention is shown in Formula (I), wherein L1 is a quinoline ligand or isoquinoline ligand, and N in the L1 is connected with Pd; L2 is an N-heterocyclic carbene ligand, and carbene carbon in the L2 is connected with the Pd; and X1 and X2 are respectively independently an anionic ligand. The N-heterocyclic carbene-palladium complex disclosed by the invention can efficiently catalyze carbon-carbon or carbon-heteroatom coupling reaction using aryl halide as a substrate. The formula is shown in the specification.
Owner:WENZHOU UNIVERSITY
High stability polymer-based nano catalyst and preparation method and use thereof
InactiveCN101362088AGood reproducibilityThe synthetic route is simpleOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsNano catalystO-aminothiophenol
The invention provides a nanometer gold catalyst based on high-stability polymer, a preparation method thereof and the application thereof. The nanometer gold catalyst based on high-stability polymer, the grain size of the nanometer gold of which is 1.0nm to 5.0nm and the macromolecular protective agent of which is poly-o-amino-thiophenol, is prepared by the method as follows: o-amino-thiophenol is dissolved in a 1.0mol / L hydrochloric acid water solution, the concentration of the o-amino-thiophenol is controlled to be 0.02-0.08mol / L; after being fully stirred, the obtained solution is added with a chloroauric acid water solution at one time, and the mole ratio of the o-amino-thiophenol and the chloroauric acid is controlled to be 1:1-8:1; and then the obtained solution is fully stirred for half a minute and reacts for 12h under the temperature between 0 DEG C to 30 DEG C to obtain the stable nanometer gold catalyst of poly-o-amino-thiophenol. The nanometer gold catalyst based on high-stability polymer has the advantages that the preparation of the nanometer gold catalyst is simple and convenient to be carried out, and the obtained nanometer gold catalyst is high in stability and good in reproducibility and has good catalytic activity to the Suzuki coupling reaction between aryl halide and aryl boronic acid.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com