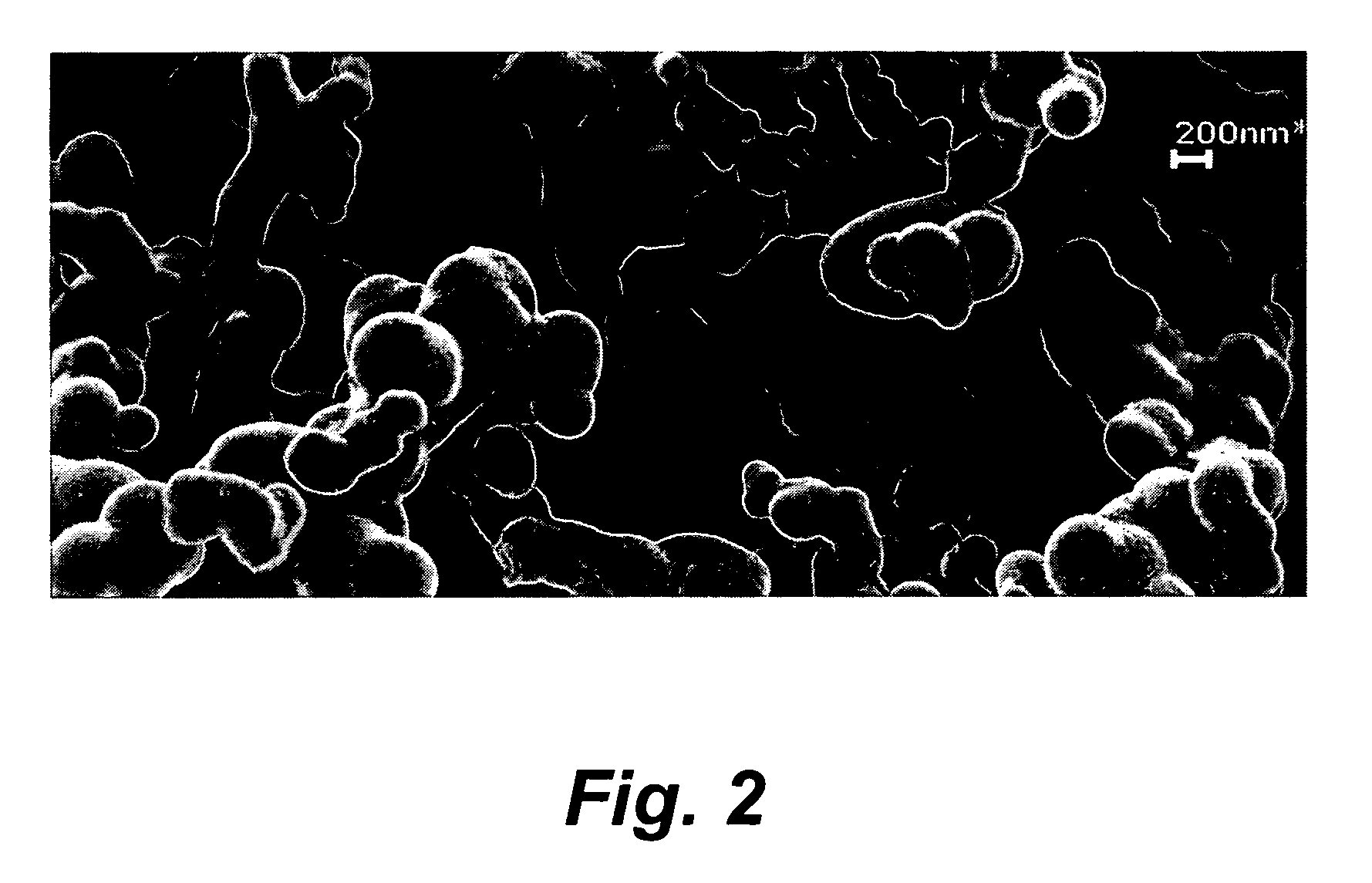
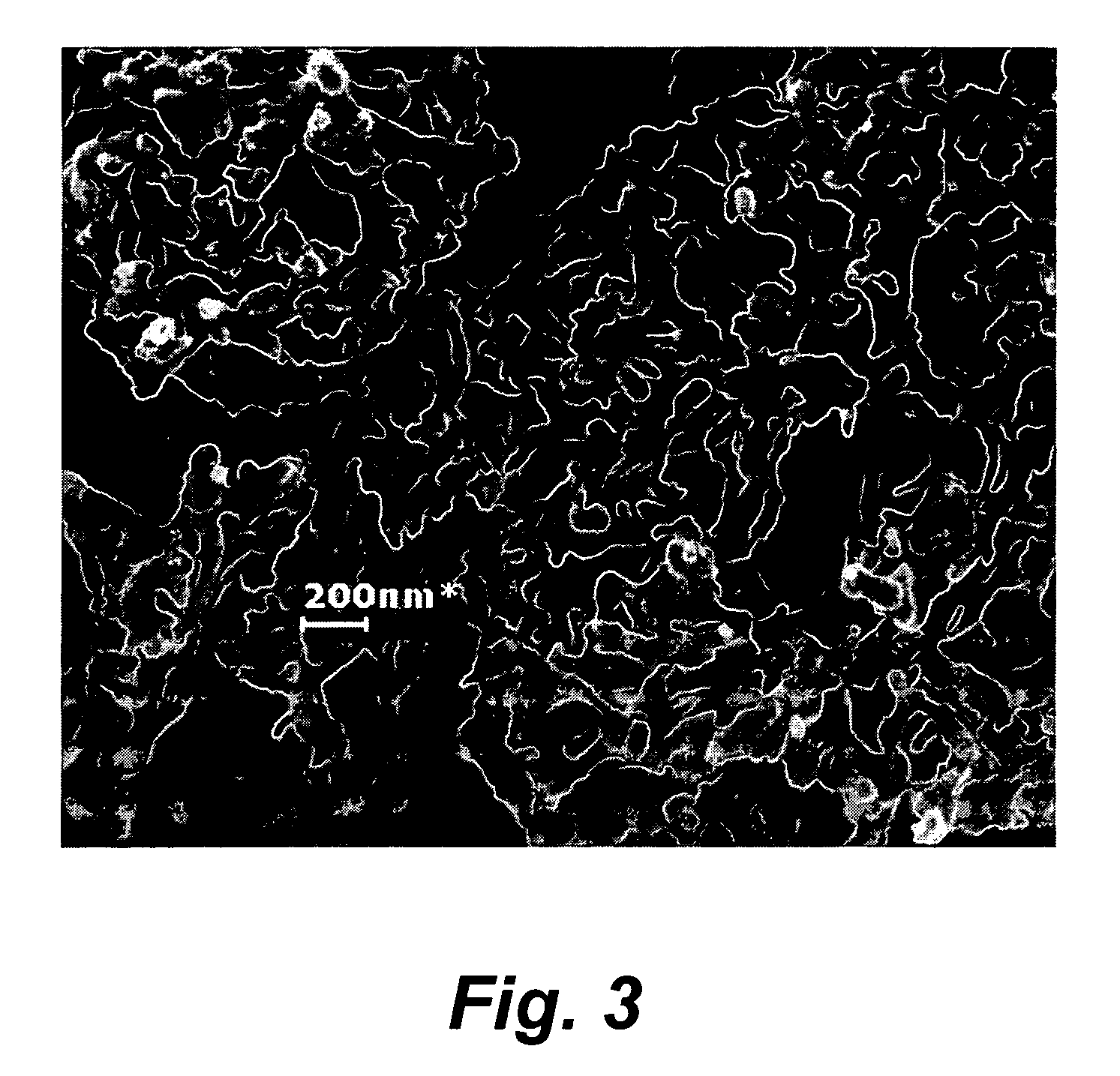
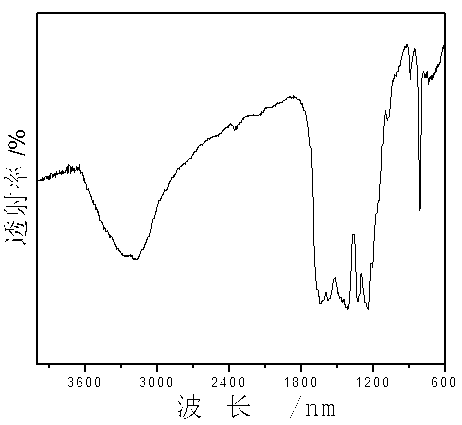
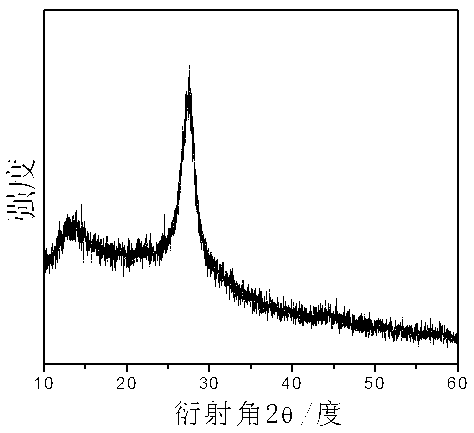
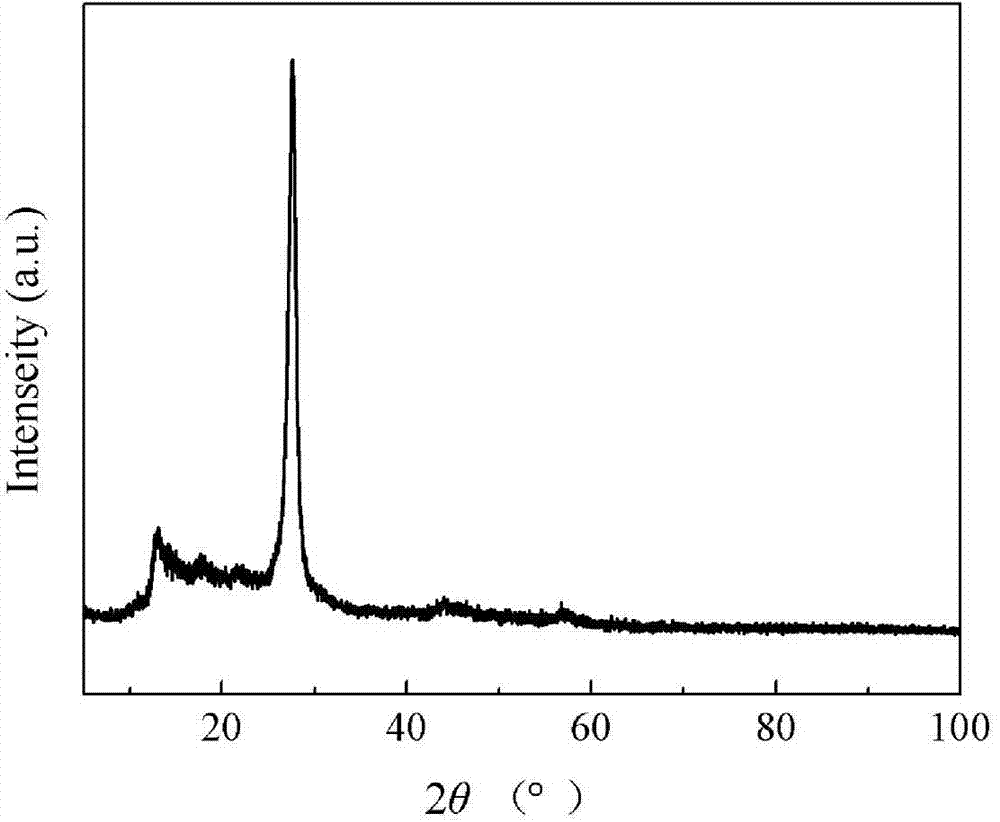
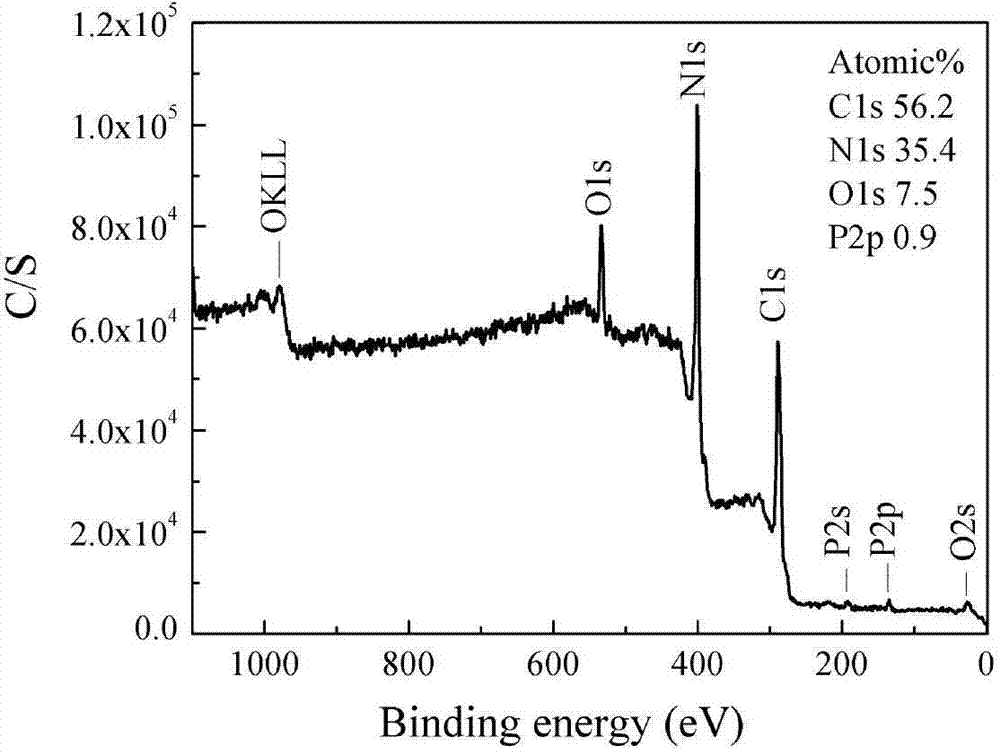
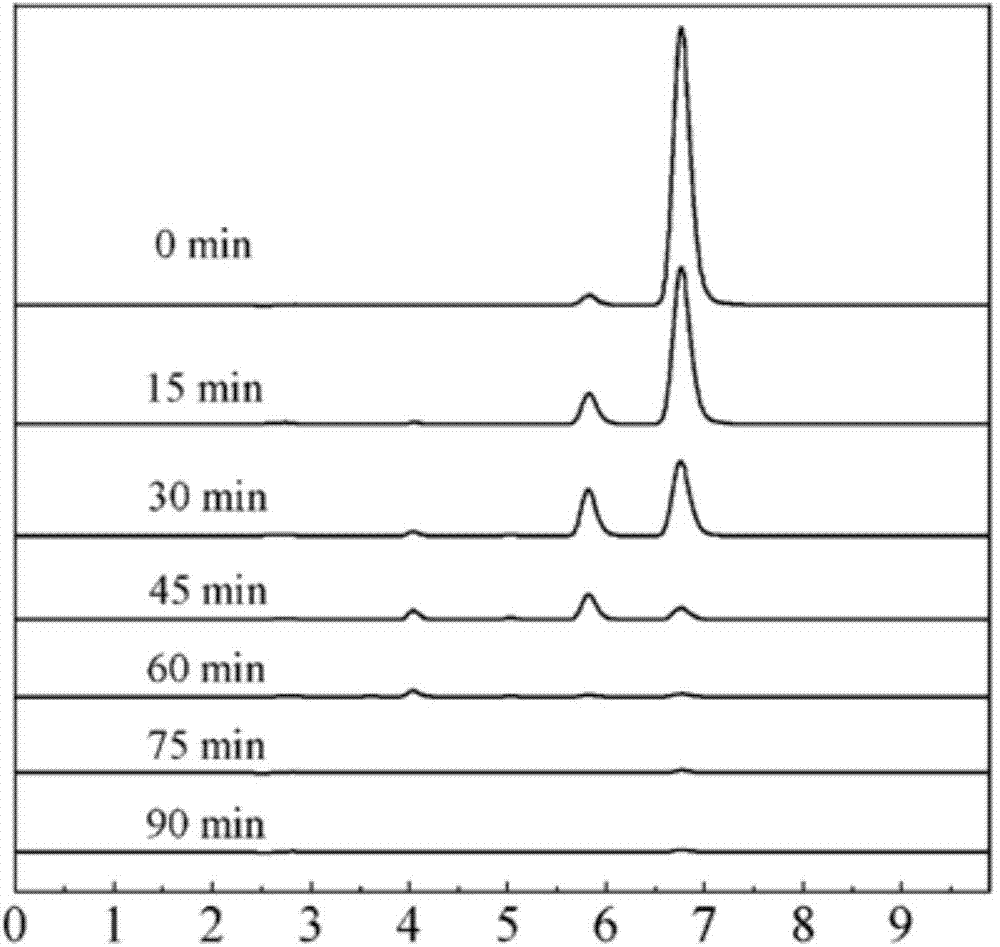
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3099 results about "Carbon nitride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbon nitrides are compounds of carbon and nitrogen.
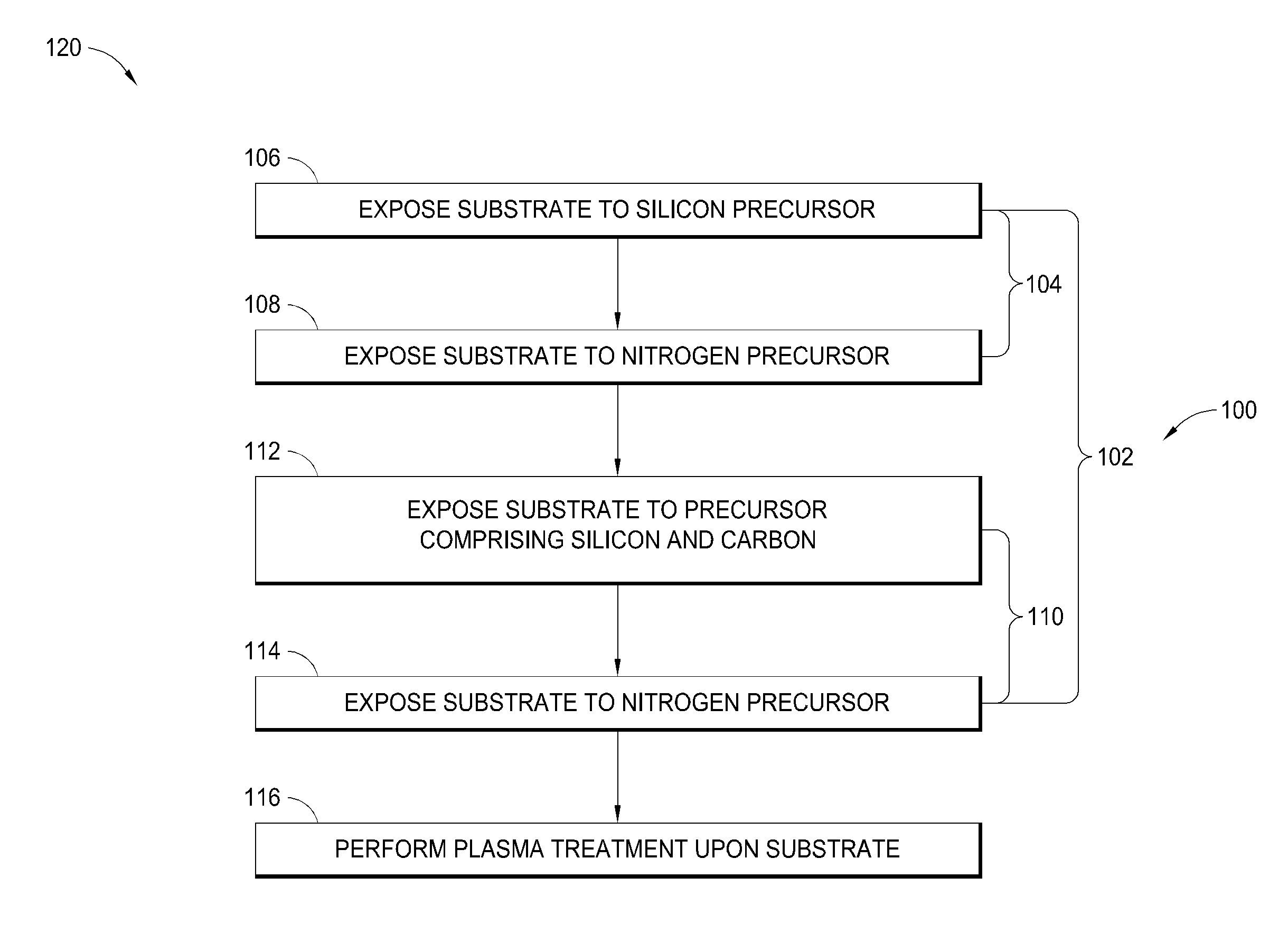
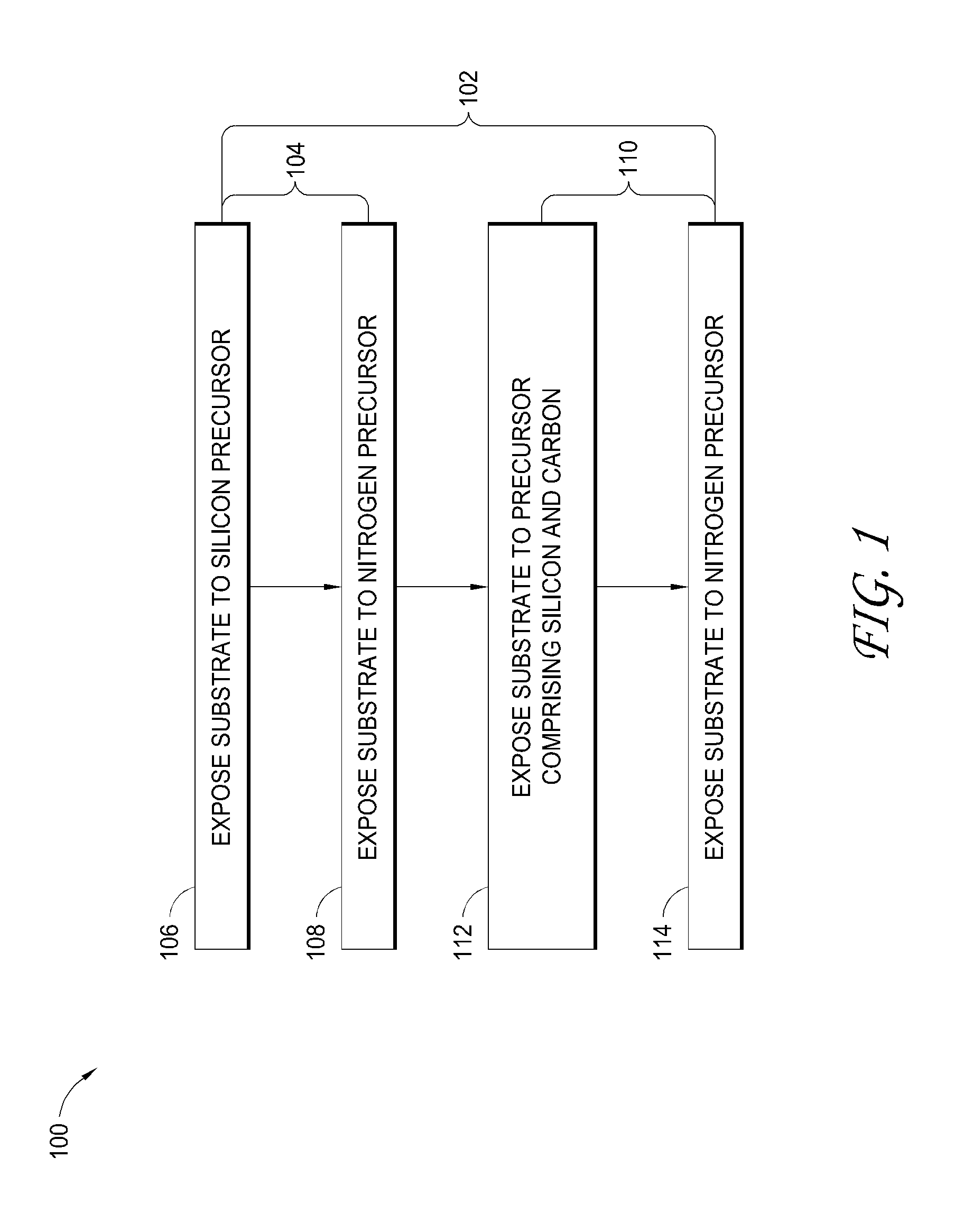
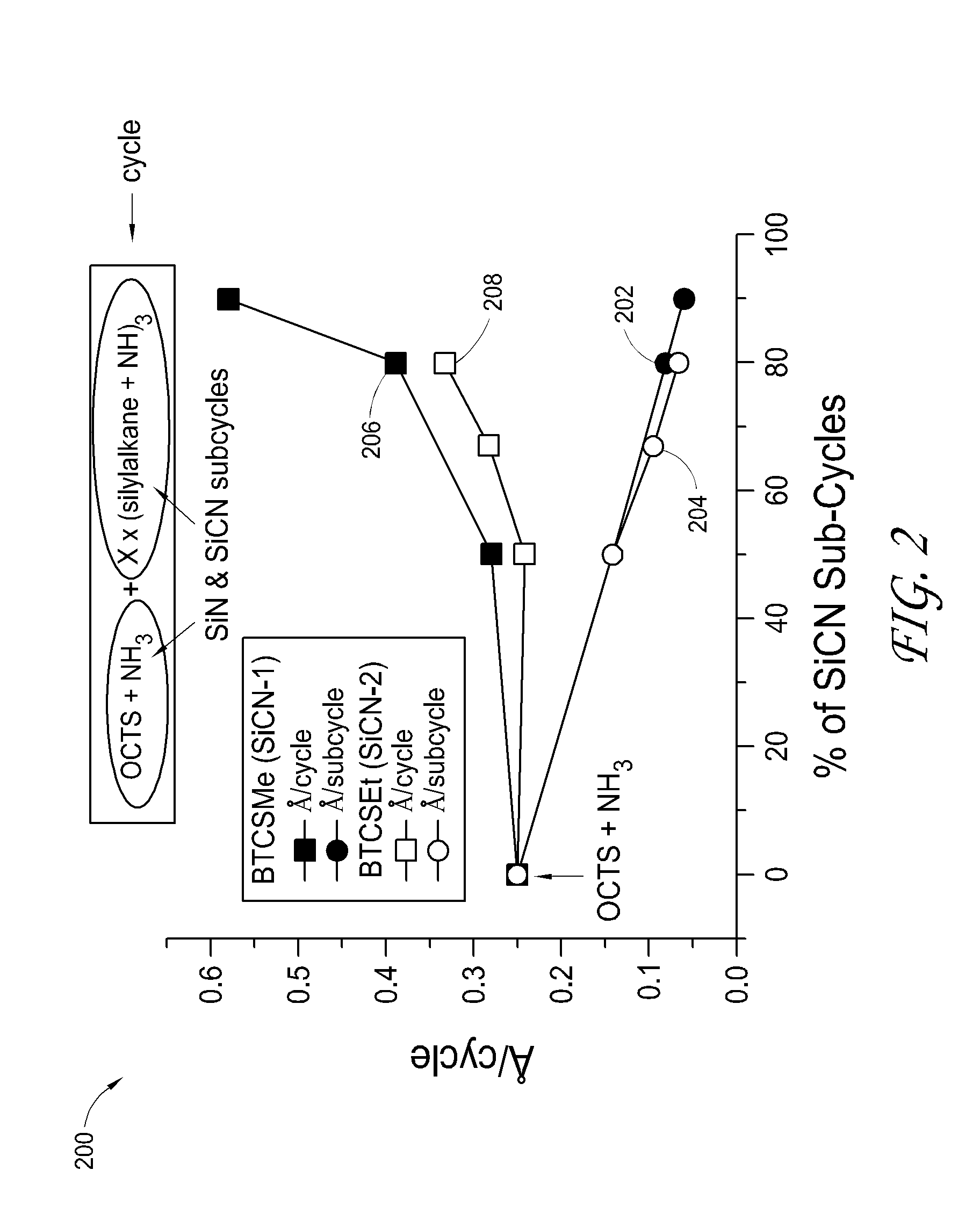
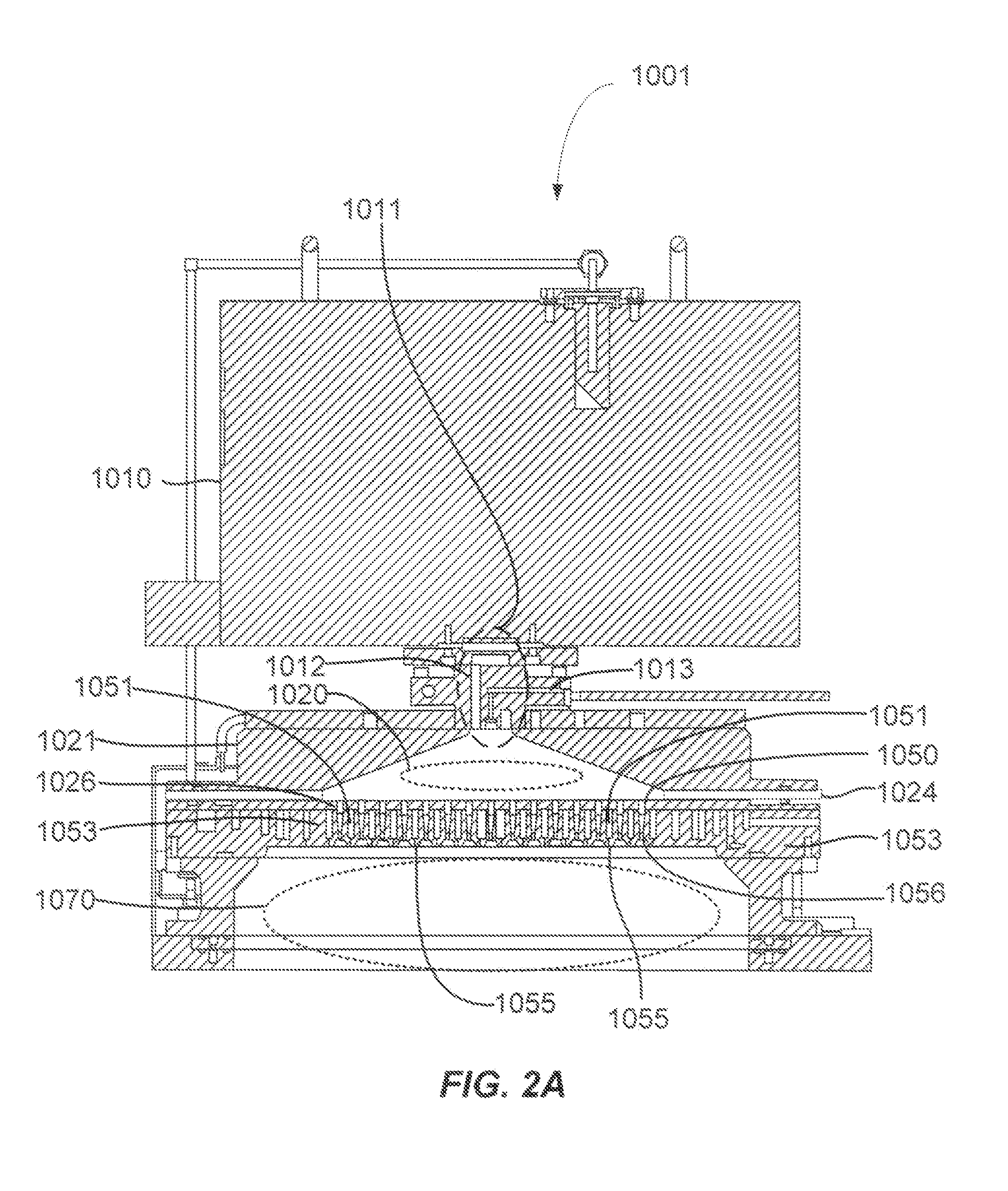
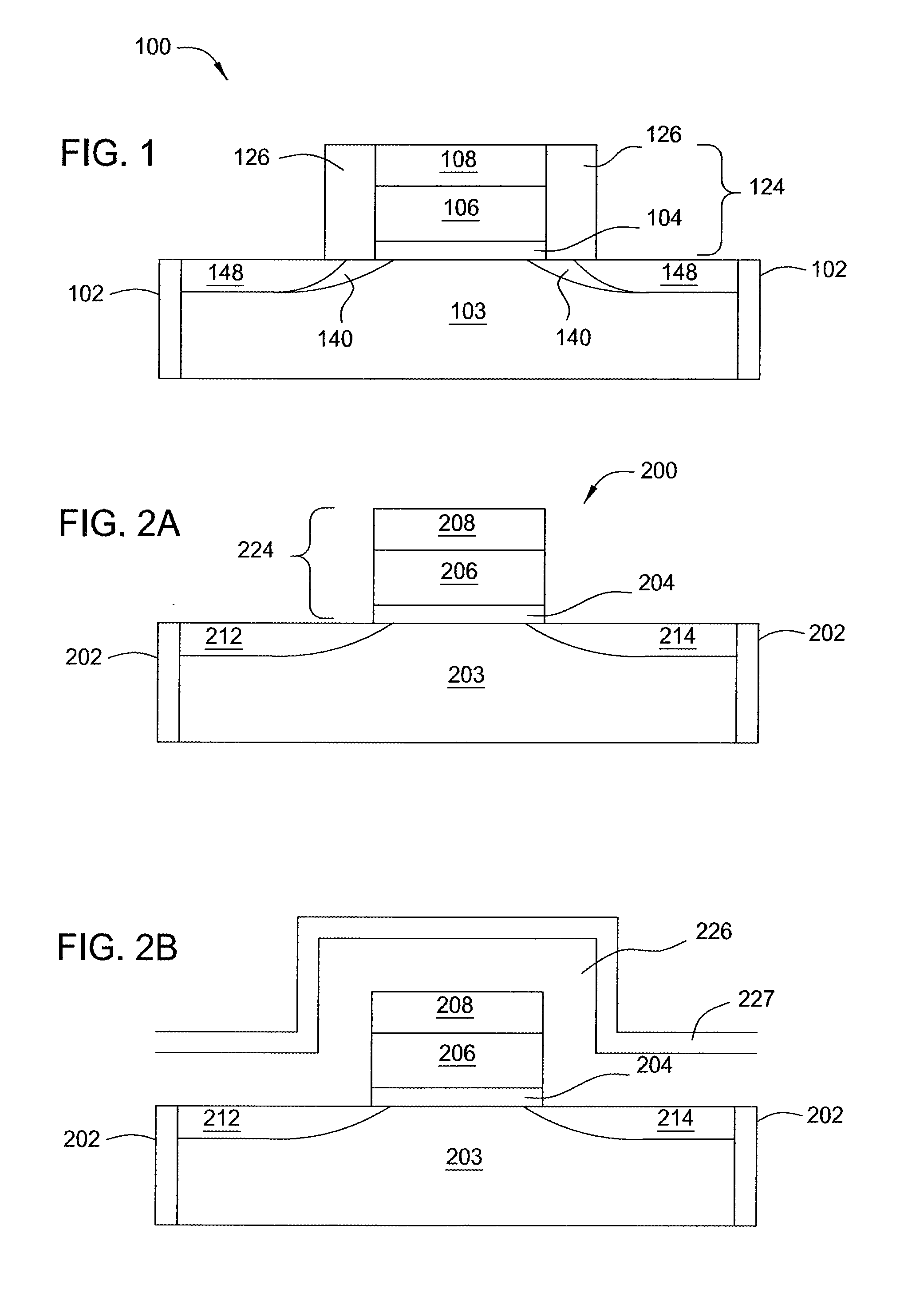
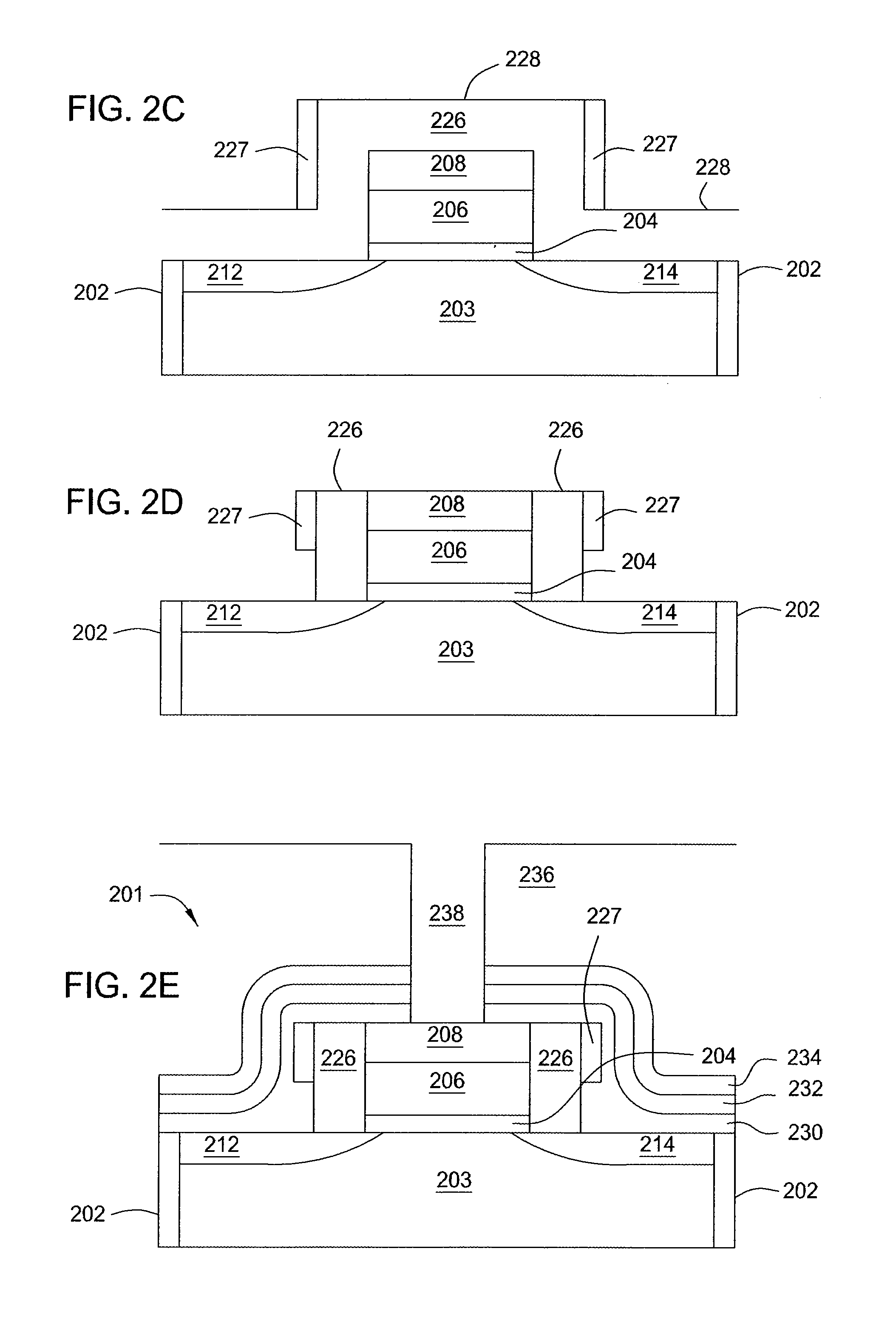
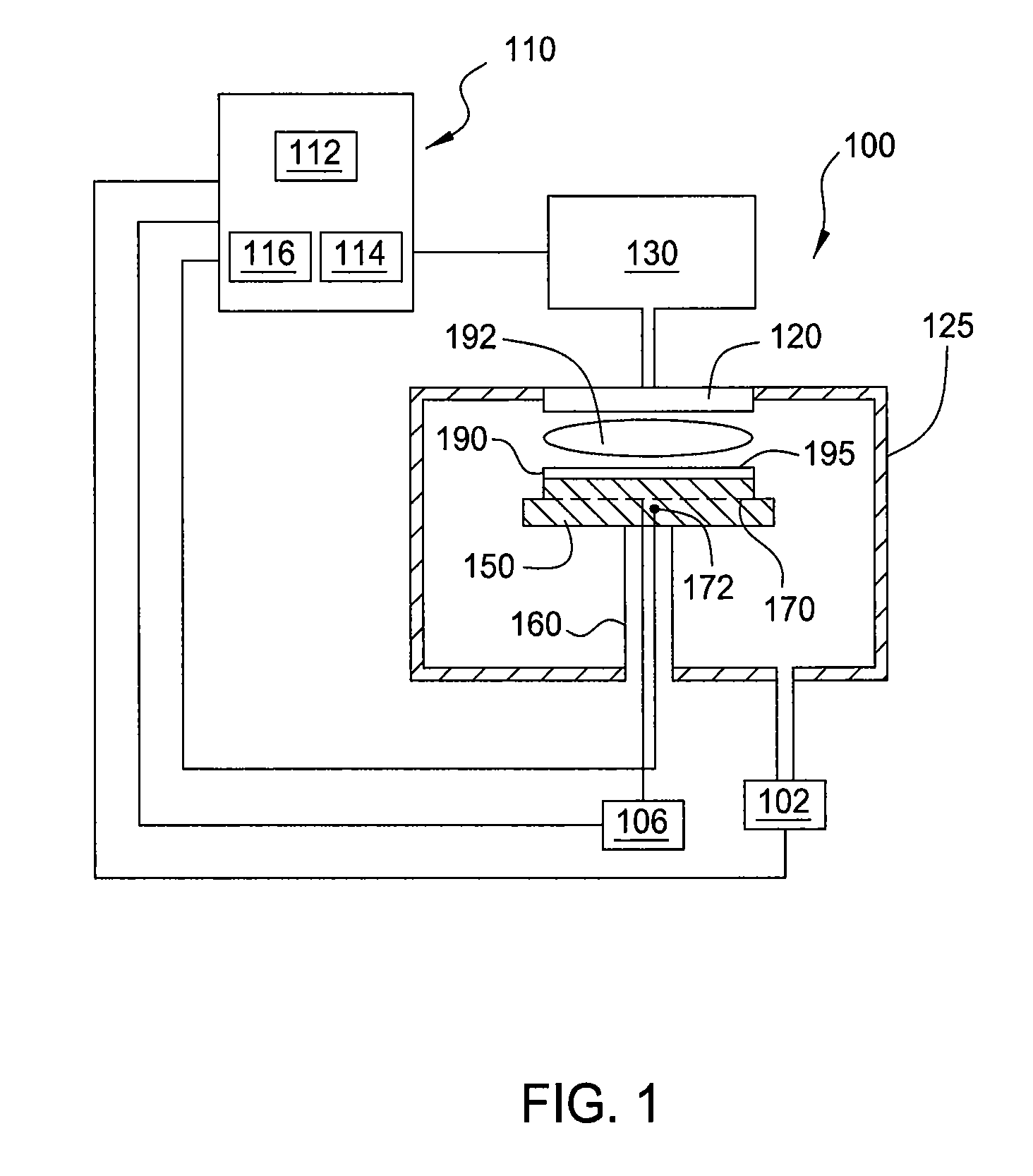
Atomic layer deposition of silicon carbon nitride based materials
ActiveUS20150162185A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingCarbon nitrideNitrogen
A process for depositing a silicon carbon nitride film on a substrate can include a plurality of complete deposition cycles, each complete deposition cycle having a SiN sub-cycle and a SiCN sub-cycle. The SiN sub-cycle can include alternately and sequentially contacting the substrate with a silicon precursor and a SiN sub-cycle nitrogen precursor. The SiCN sub-cycle can include alternately and sequentially contacting the substrate with carbon-containing precursor and a SiCN sub-cycle nitrogen precursor. The SiN sub-cycle and the SiCN sub-cycle can include atomic layer deposition (ALD). The process for depositing the silicon carbon nitride film can include a plasma treatment. The plasma treatment can follow a completed plurality of complete deposition cycles.
Owner:ASM IP HLDG BV
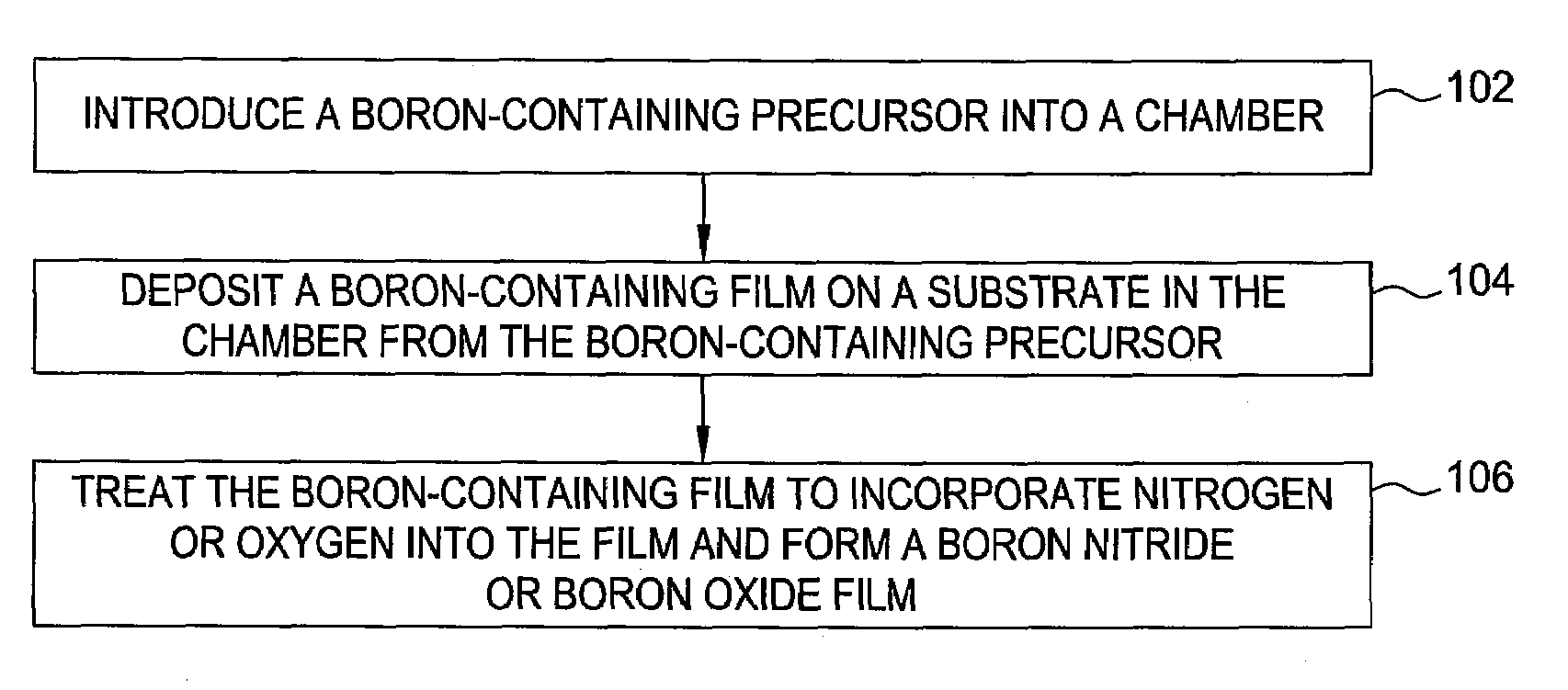
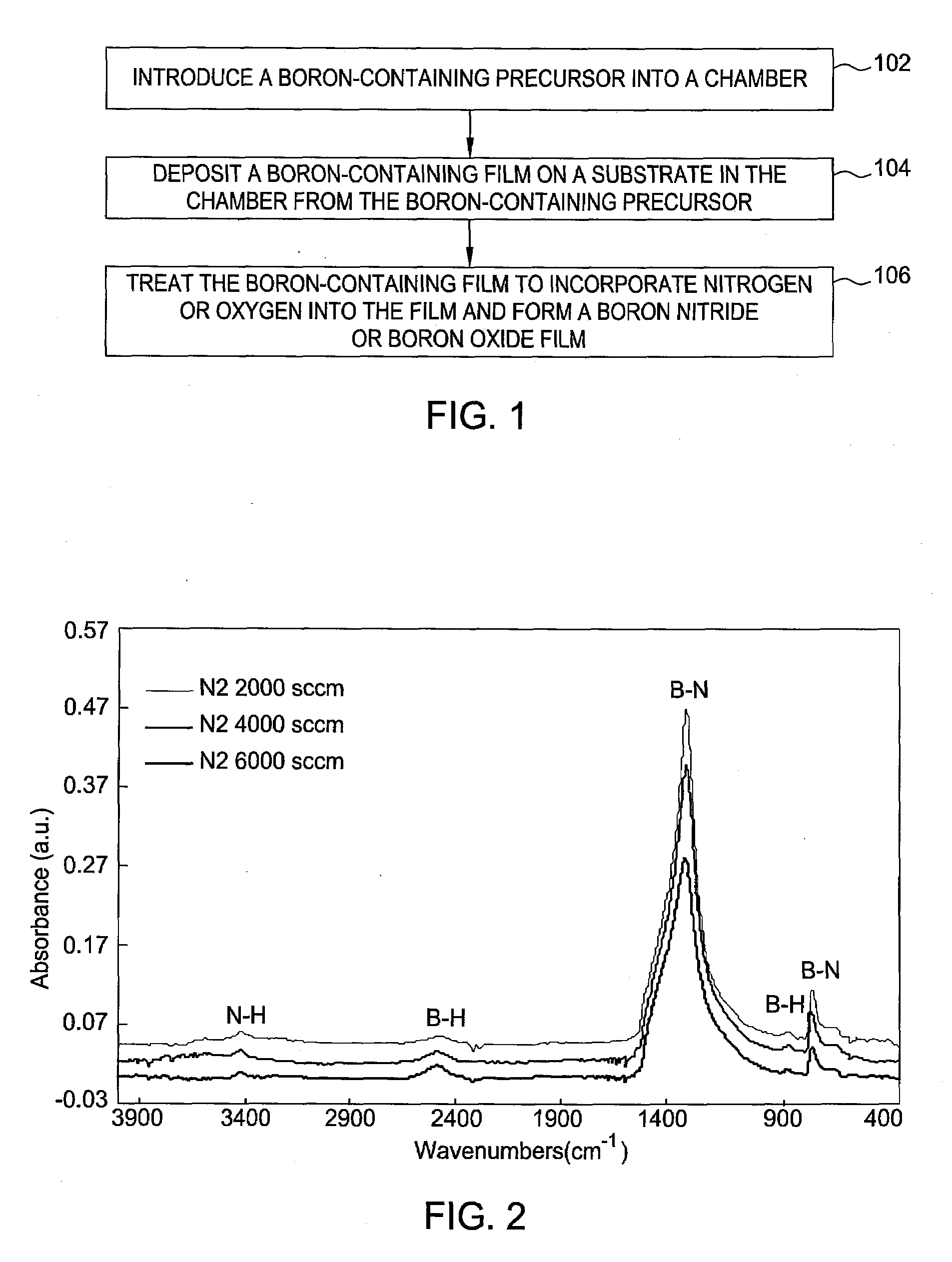
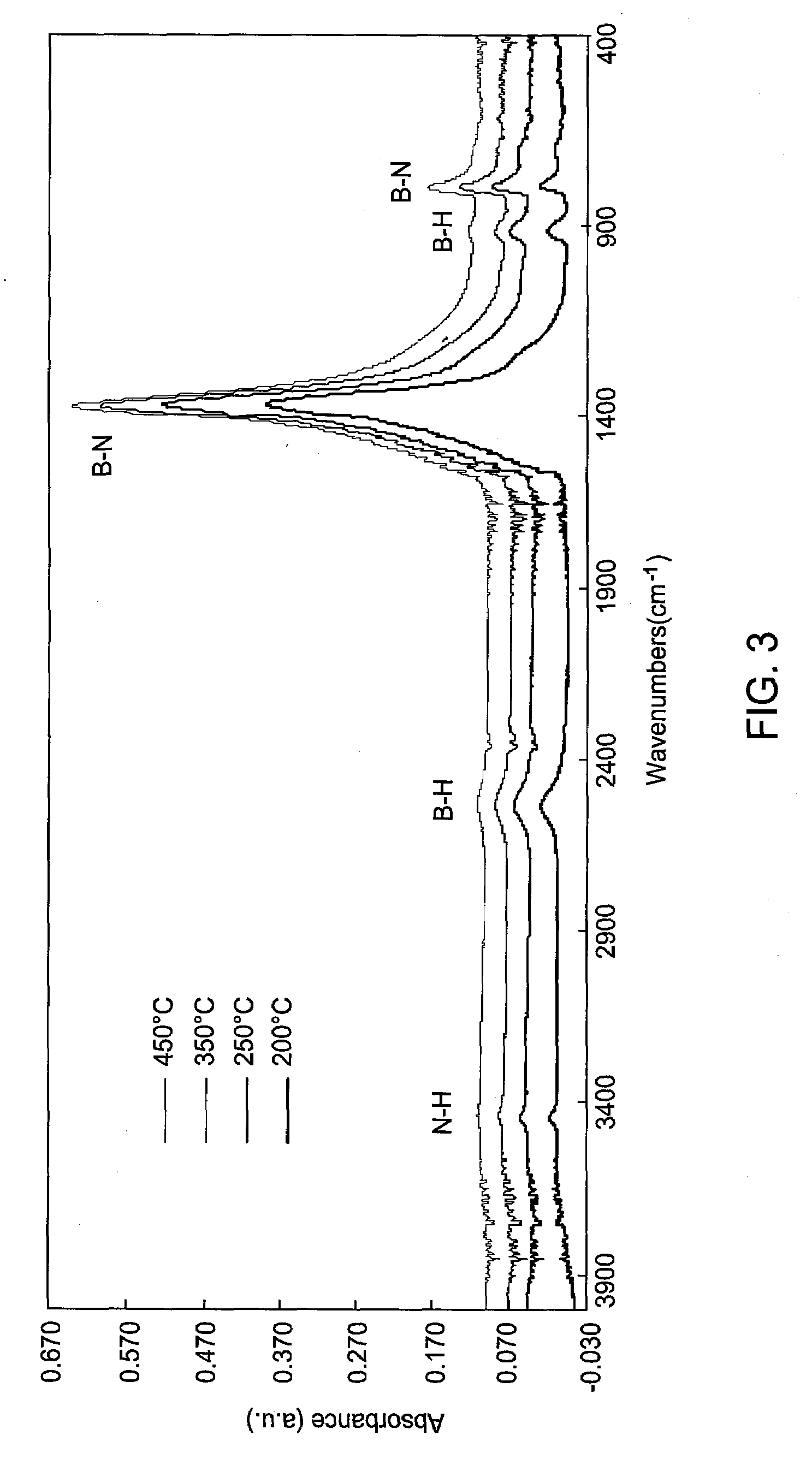
Boron nitride and boron nitride-derived materials deposition method
ActiveUS20080292798A1High nitrogen contentIncrease oxygen contentPretreated surfacesSemiconductor/solid-state device manufacturingMetallurgyBoron containing
Methods for forming boron-containing films are provided. The methods include introducing a boron-containing precursor and a nitrogen or oxygen-containing precursor into a chamber and forming a boron nitride or boron oxide film on a substrate in the chamber. In one aspect, the method includes depositing a boron-containing film and then exposing the boron-containing film to the nitrogen-containing or oxygen-containing precursor to incorporate nitrogen or oxygen into the film. The deposition of the boron-containing film and exposure of the film to the precursor may be performed for multiple cycles to obtain a desired thickness of the film. In another aspect, the method includes reacting the boron-containing precursor and the nitrogen-containing or oxygen-containing precursor to chemically vapor deposit the boron nitride or boron oxide film.
Owner:APPLIED MATERIALS INC
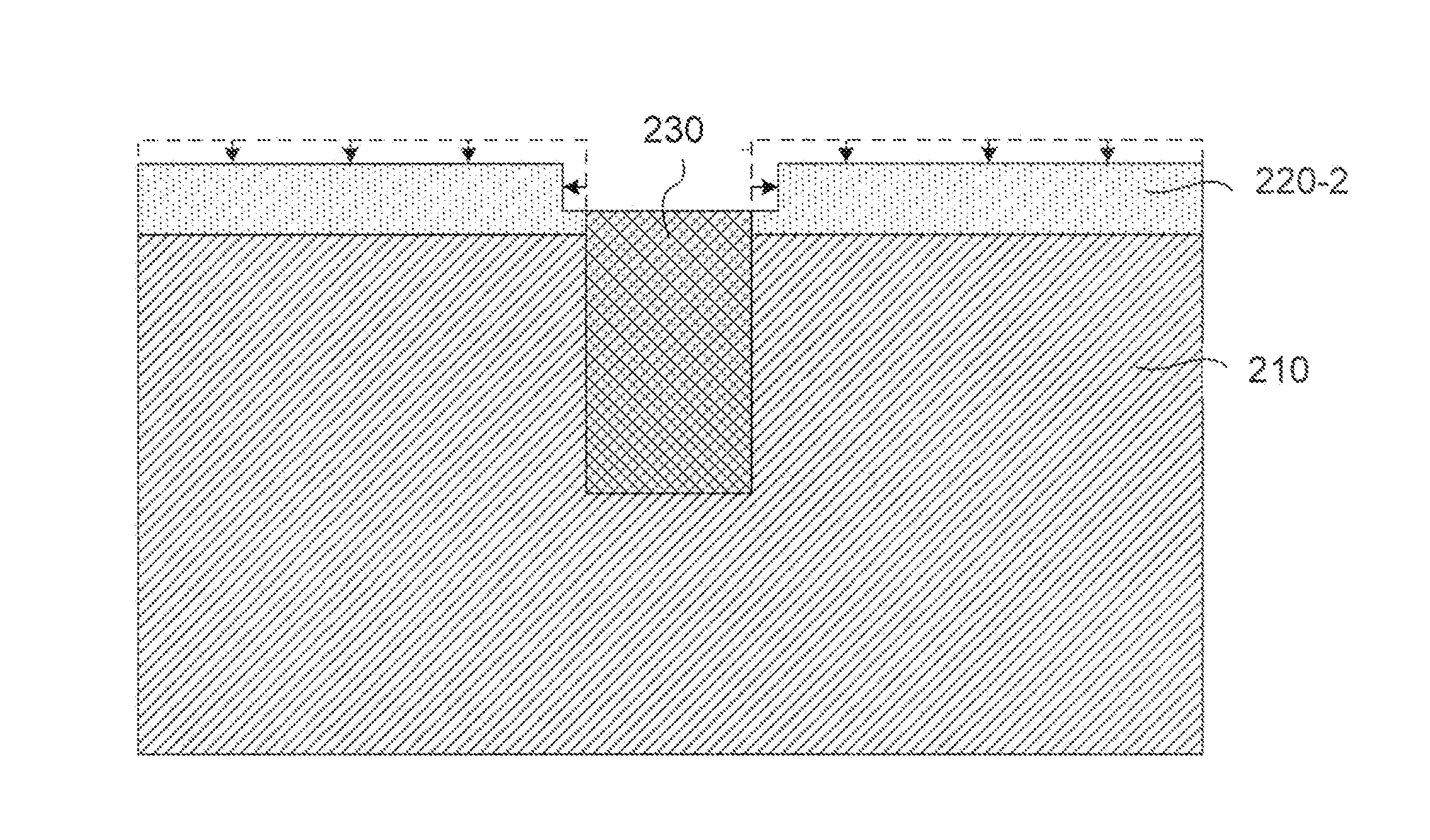
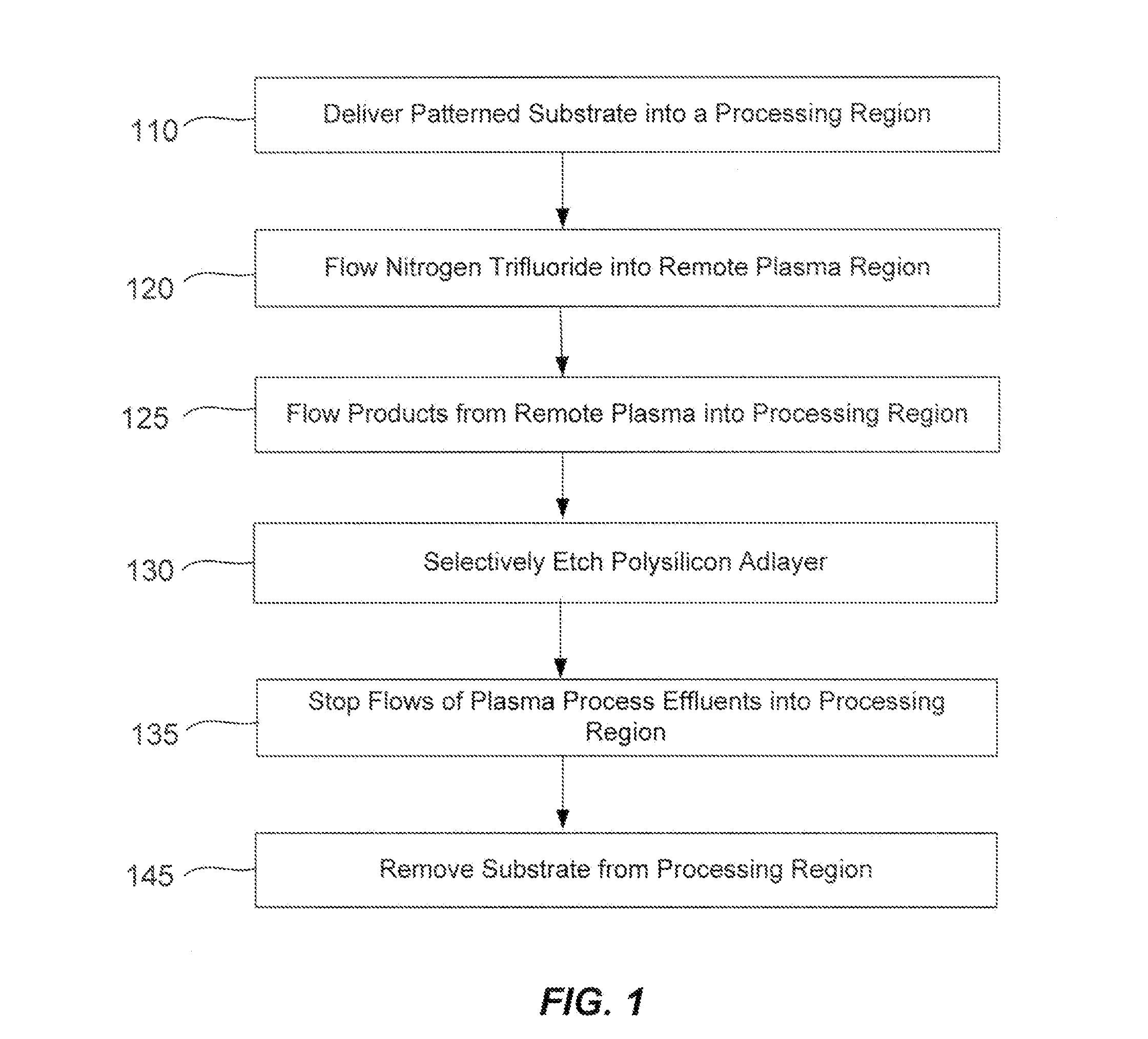
Selective etch for silicon films
ActiveUS20110294300A1Less oxygenEasy to removeSemiconductor/solid-state device manufacturingPlasma techniqueRemote plasmaNitrogen
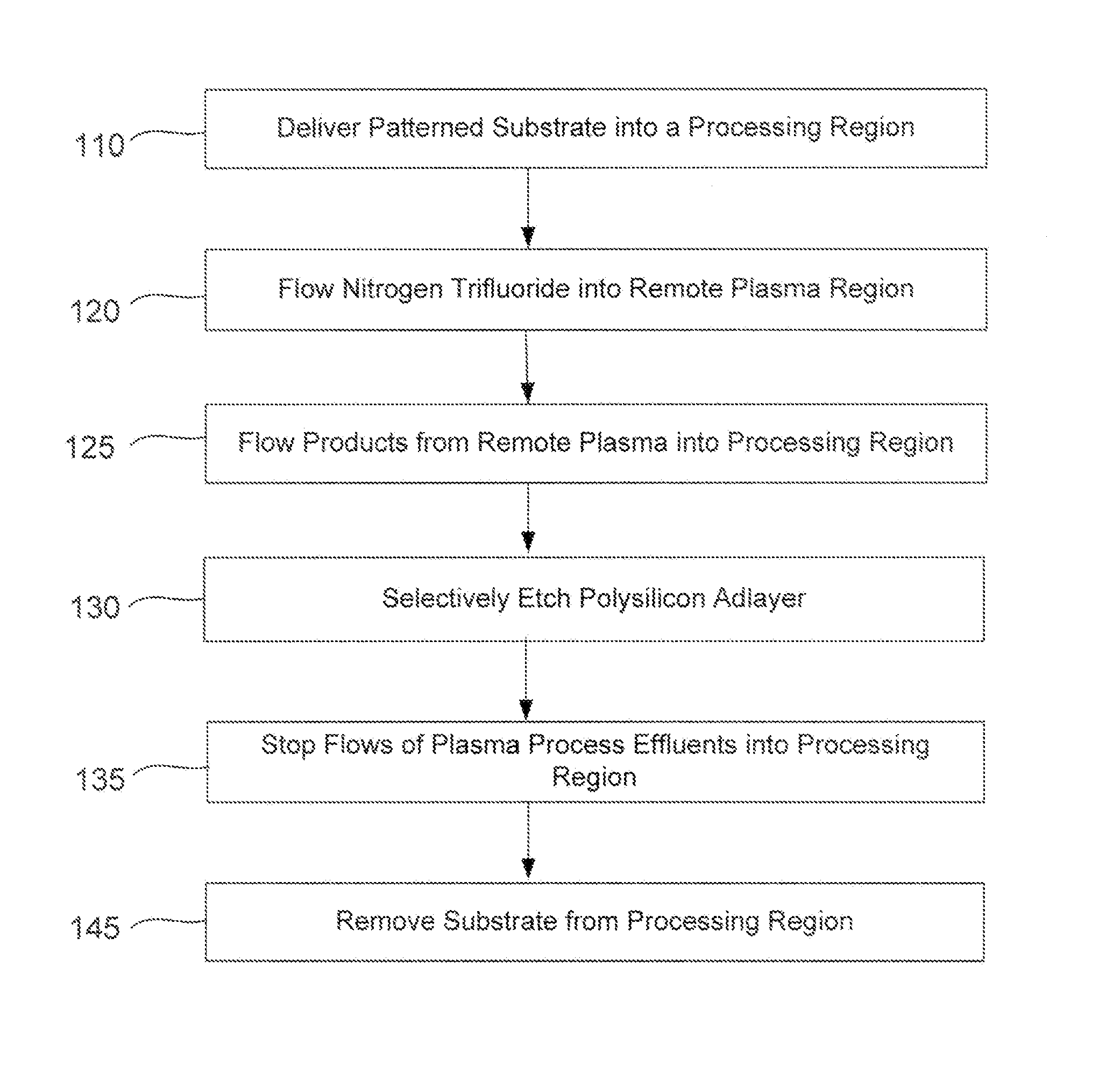
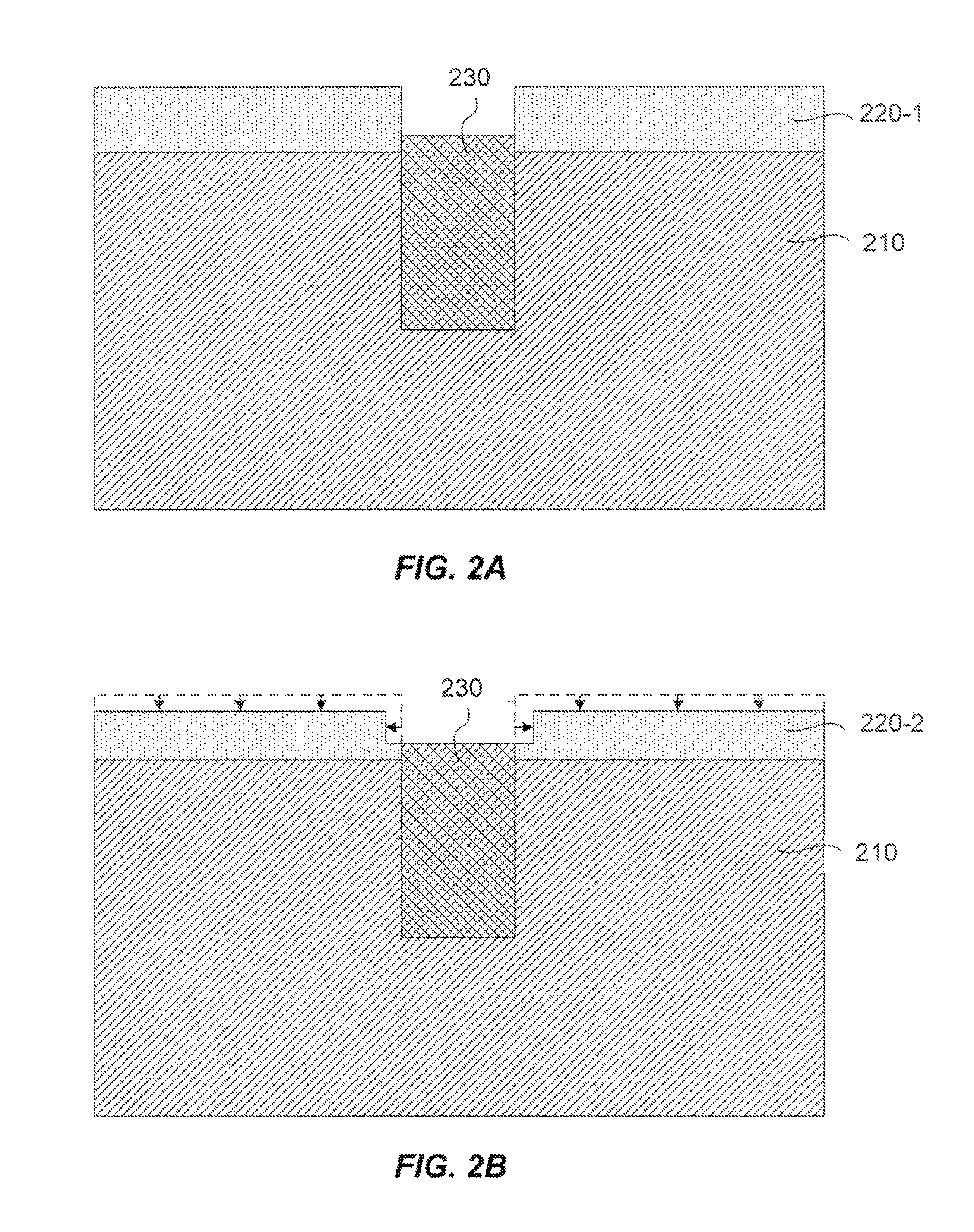
A method of etching patterned heterogeneous silicon-containing structures is described and includes a remote plasma etch with inverted selectivity compared to existing remote plasma etches. The methods may be used to conformally trim polysilicon while removing little or no silicon oxide. More generally, silicon-containing films containing less oxygen are removed more rapidly than silicon-containing films which contain more oxygen. Other exemplary applications include trimming silicon carbon nitride films while essentially retaining silicon oxycarbide. Applications such as these are enabled by the methods presented herein and enable new process flows. These process flows are expected to become desirable for a variety of finer linewidth structures. Methods contained herein may also be used to etch silicon-containing films faster than nitrogen-and-silicon containing films having a greater concentration of nitrogen.
Owner:APPLIED MATERIALS INC
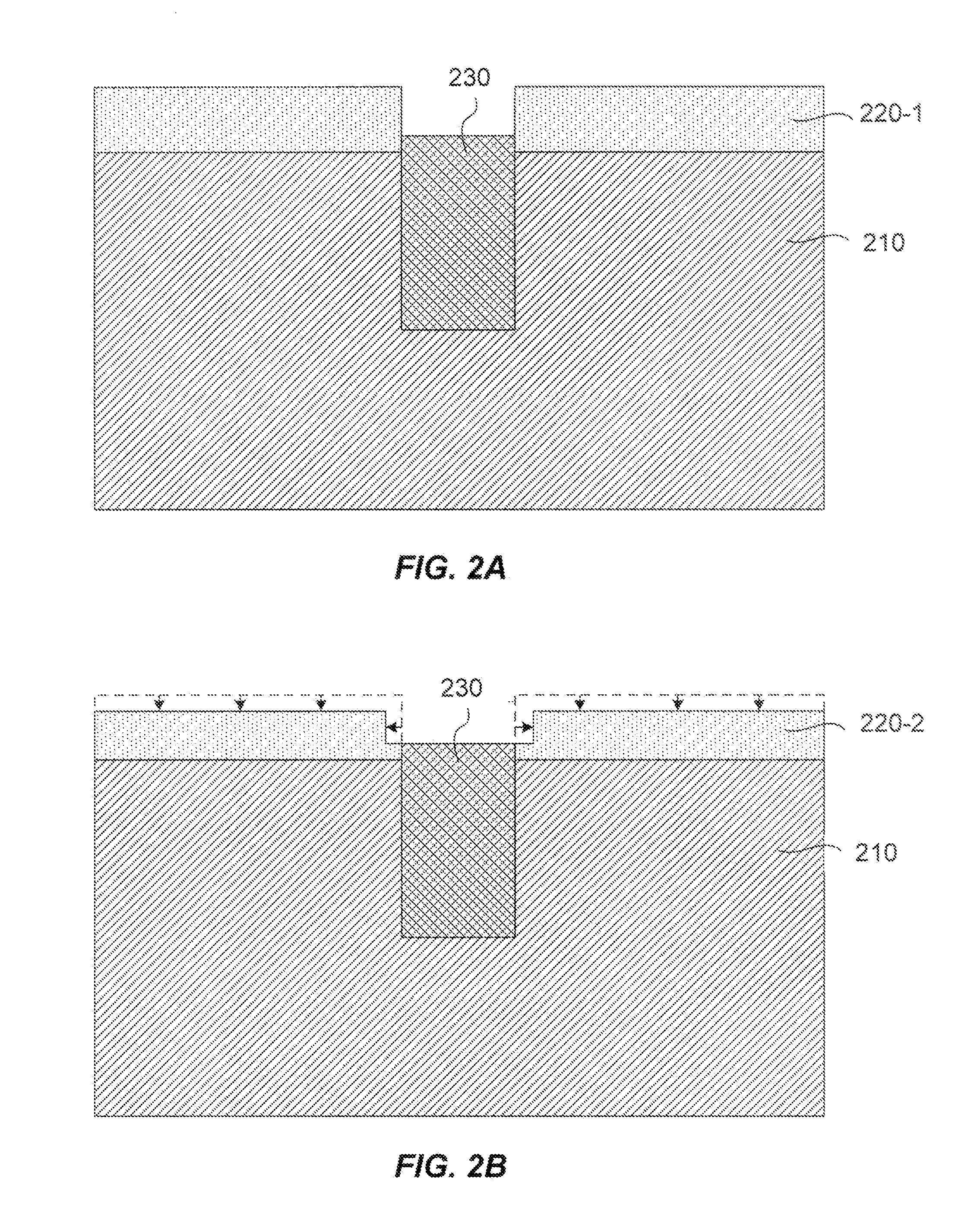
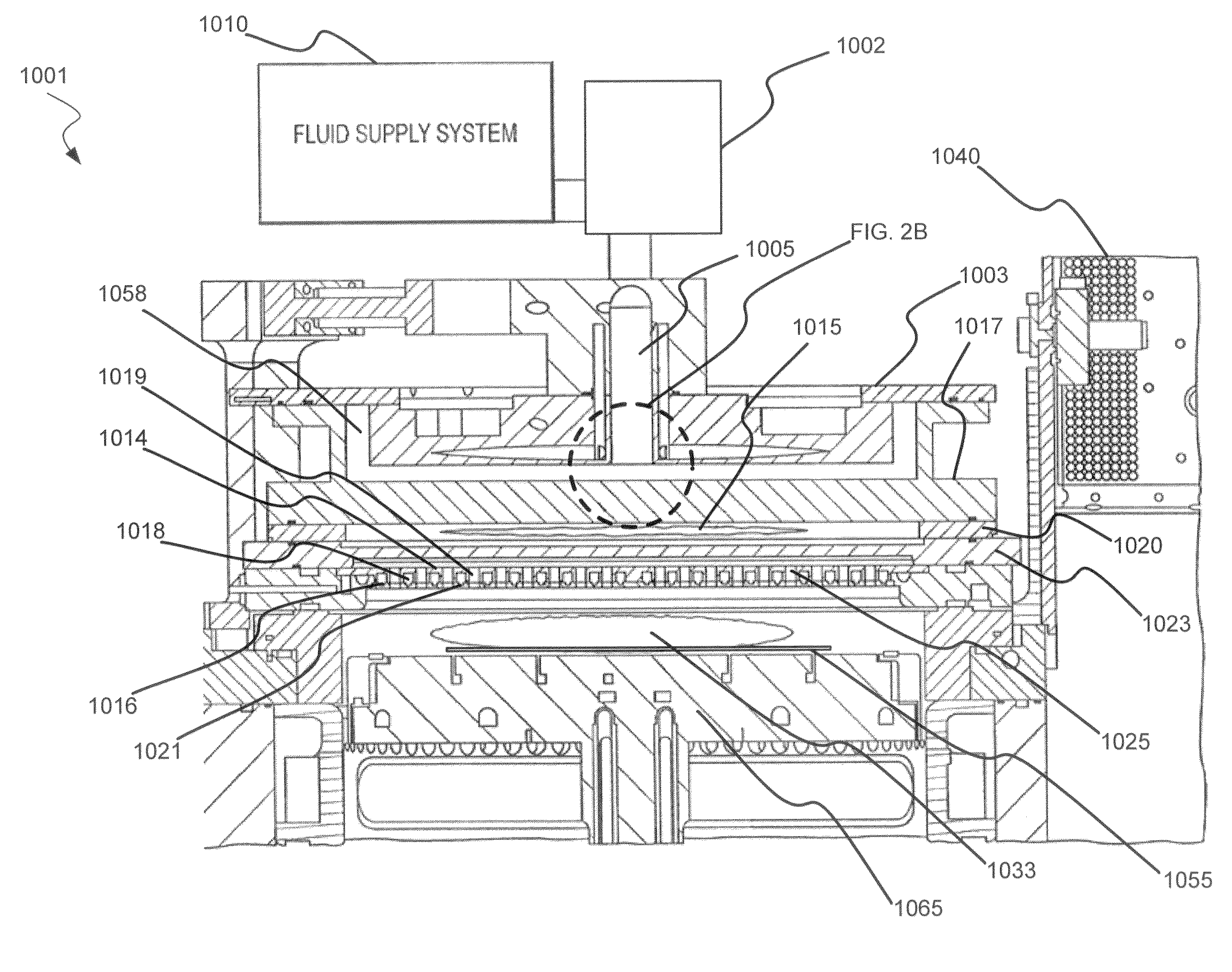
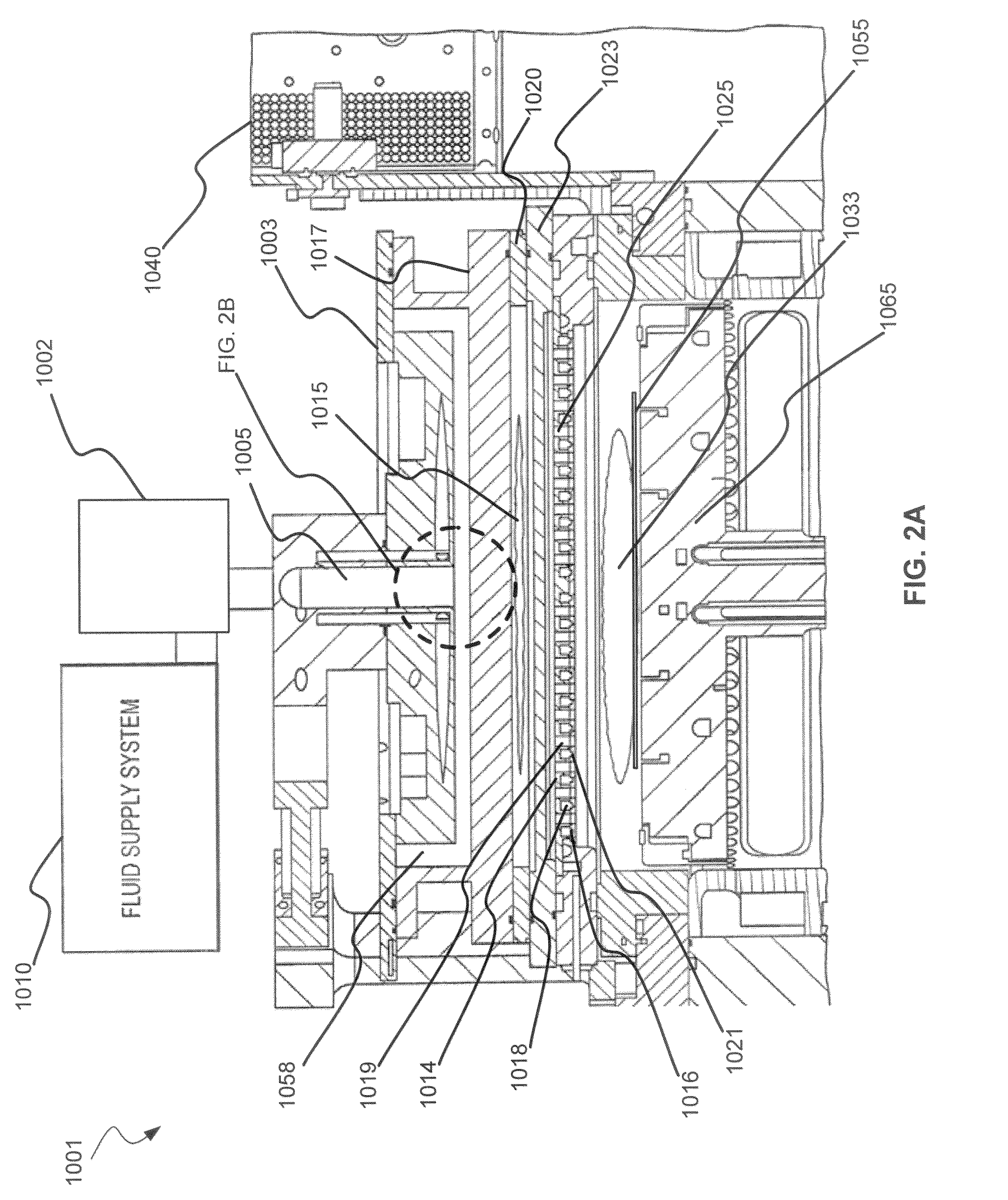
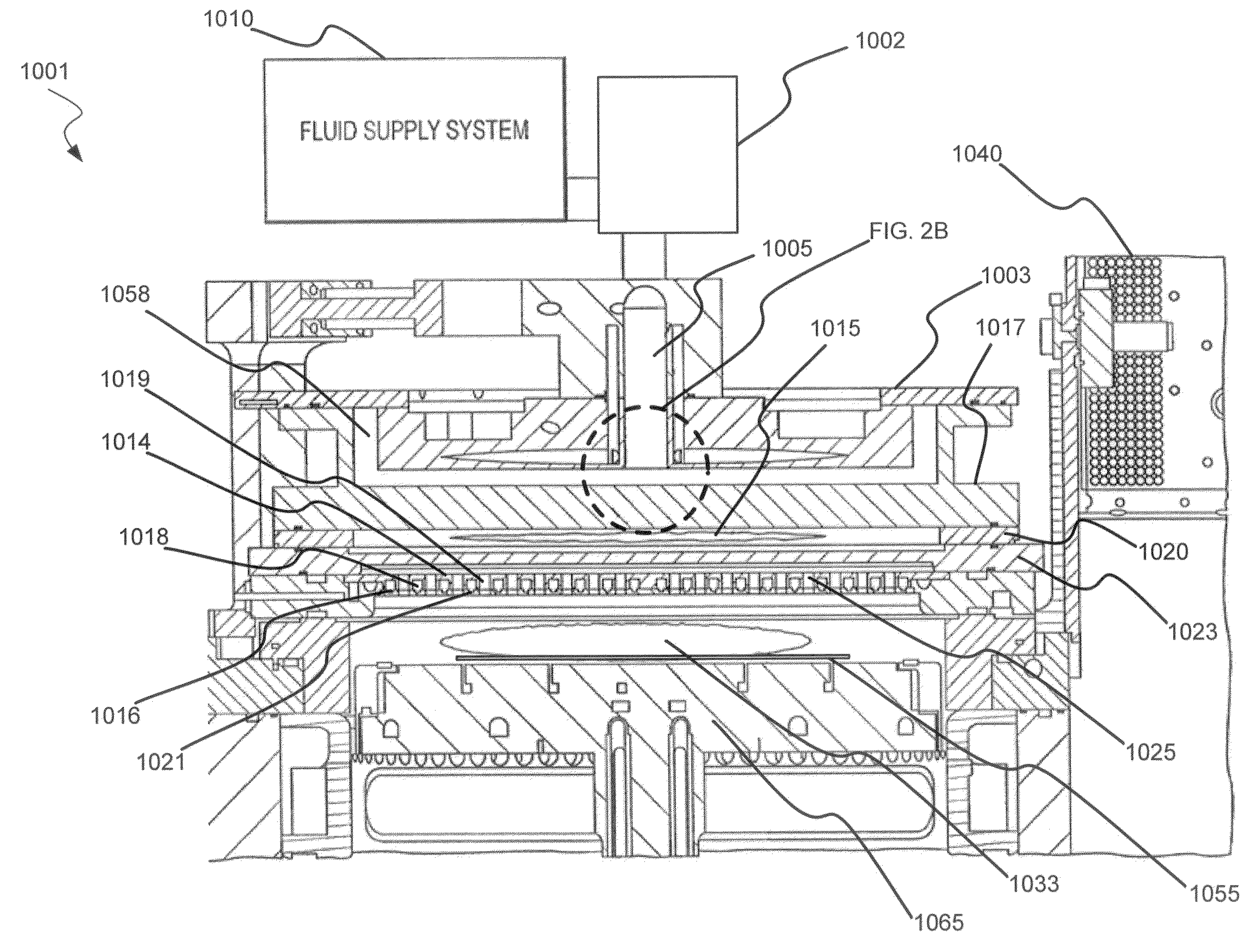
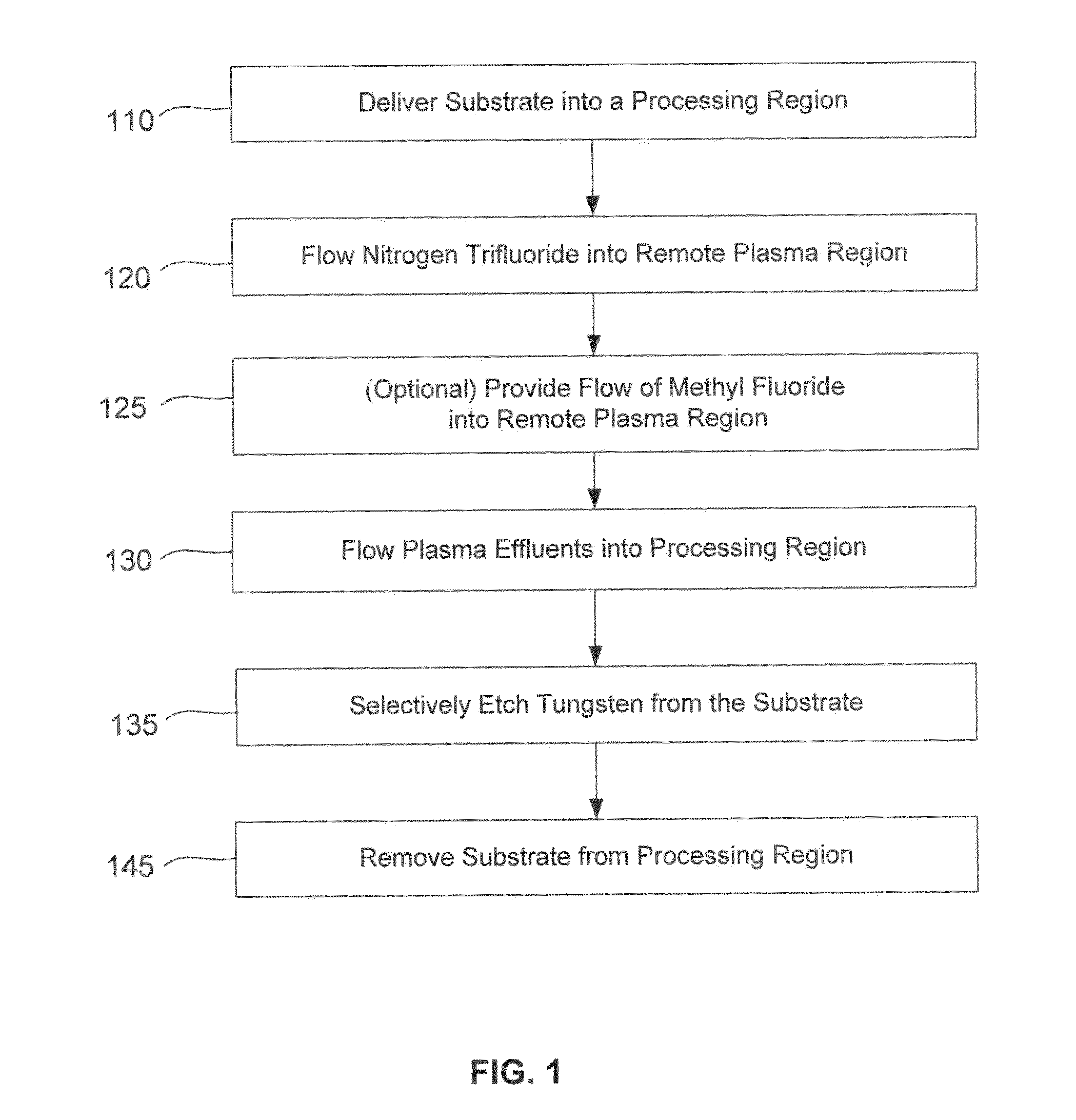
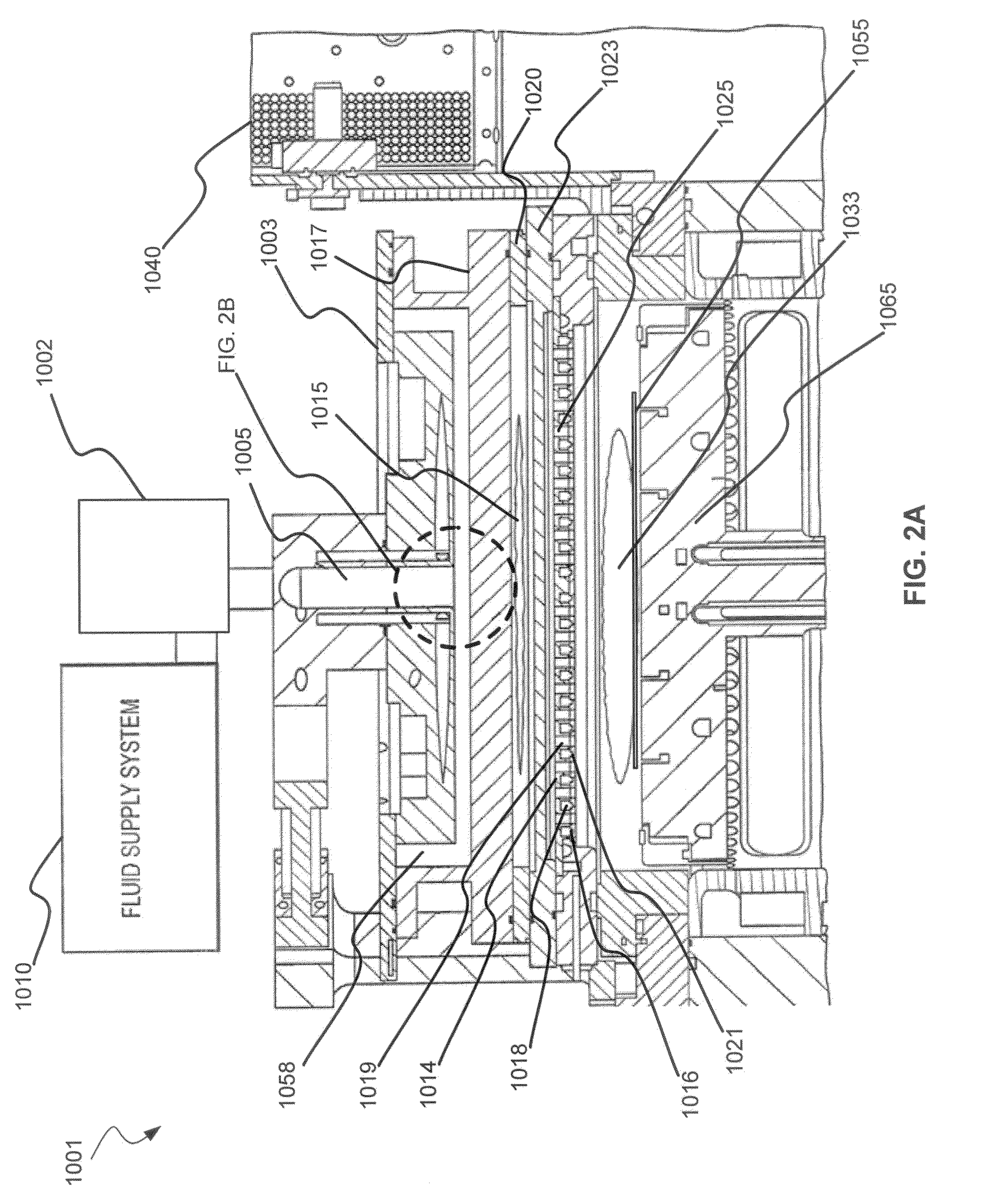
Dry-etch for selective tungsten removal
Methods of selectively etching tungsten relative to silicon-containing films (e.g. silicon oxide, silicon carbon nitride and (poly)silicon) as well as tungsten oxide are described. The methods include a remote plasma etch formed from a fluorine-containing precursor and / or hydrogen (H2). Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the tungsten. The plasma effluents react with exposed surfaces and selectively remove tungsten while very slowly removing other exposed materials. Sequential and simultaneous methods are included to remove thin tungsten oxide which may, for example, result from exposure to the atmosphere.
Owner:APPLIED MATERIALS INC
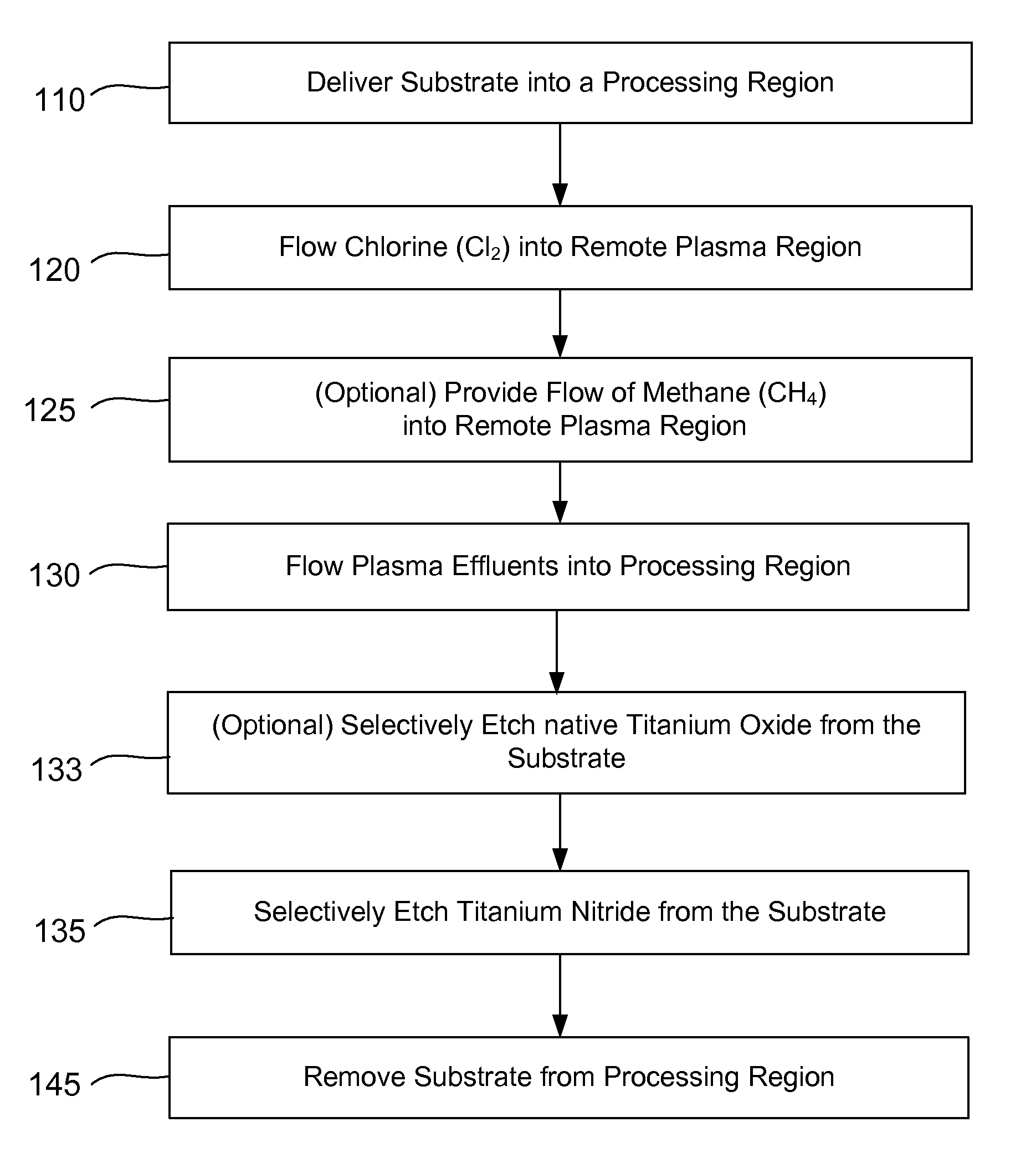
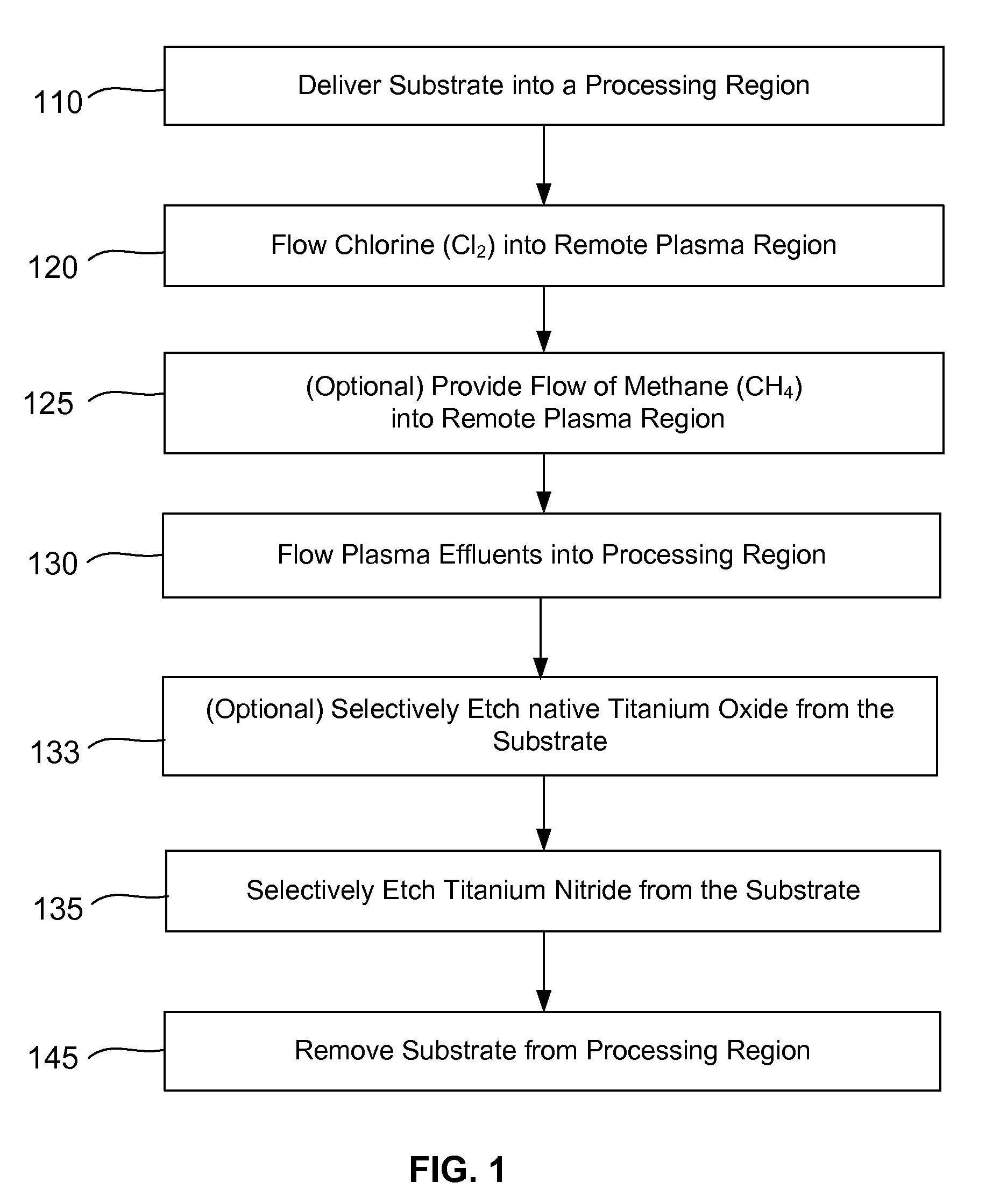
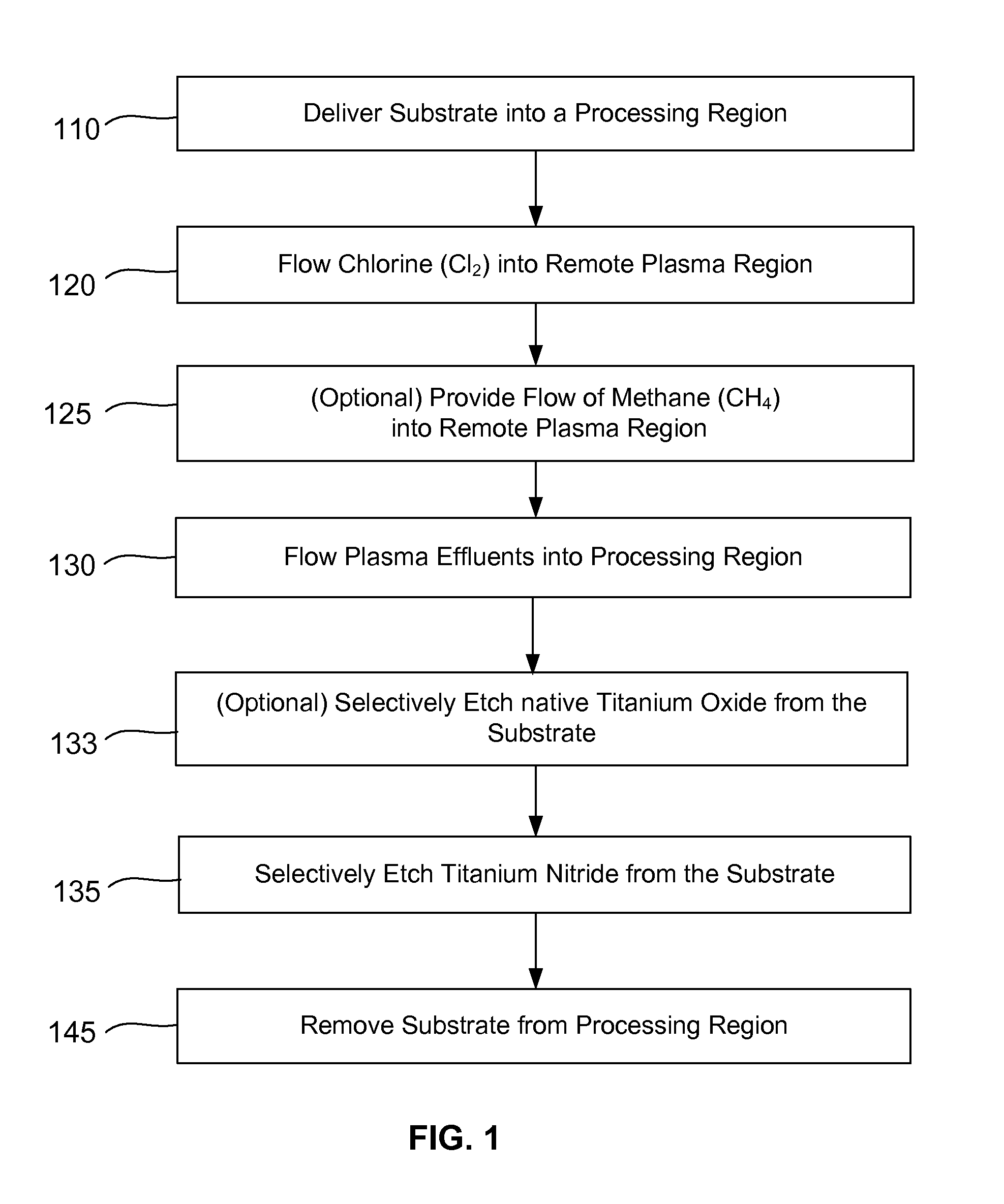
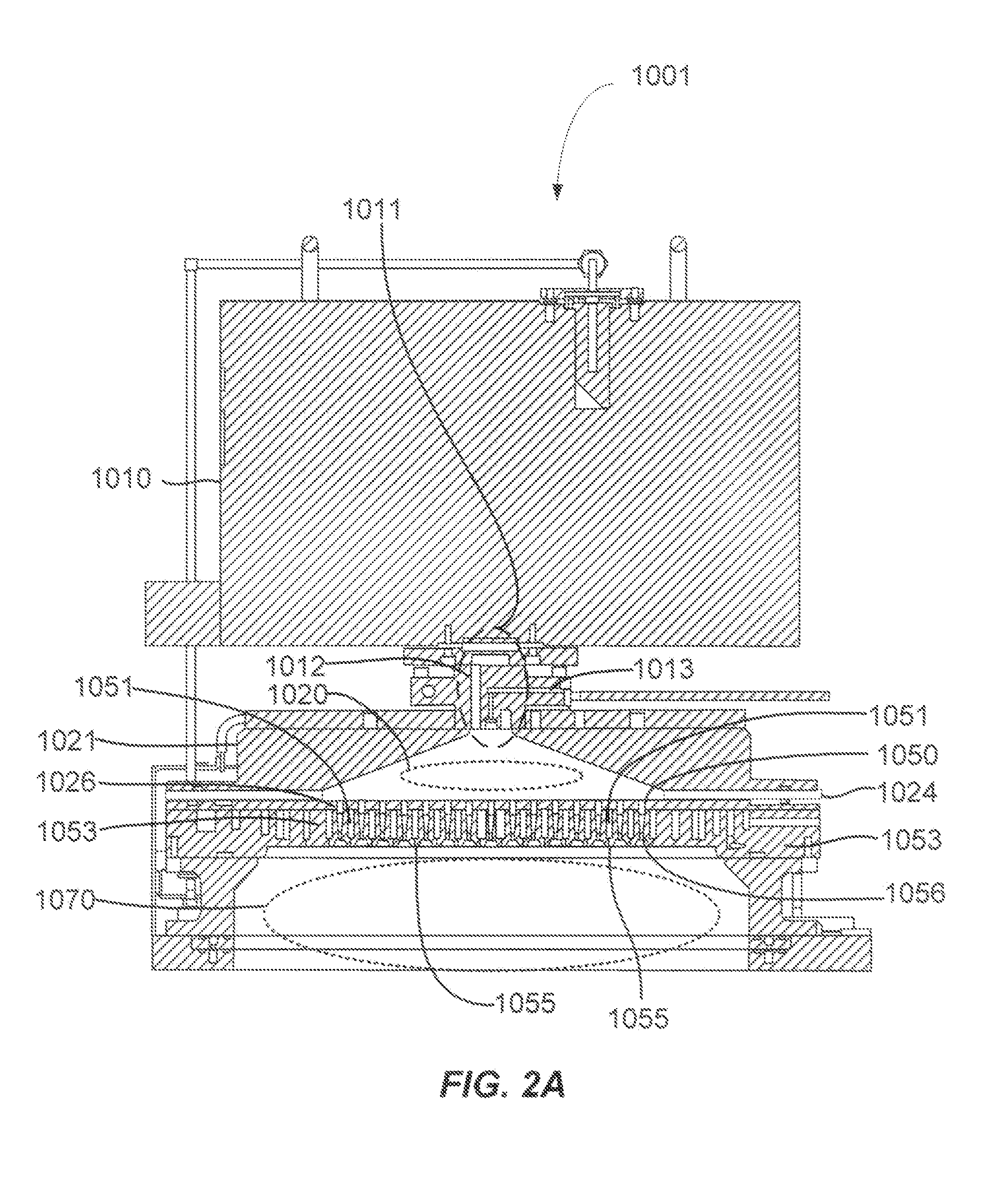
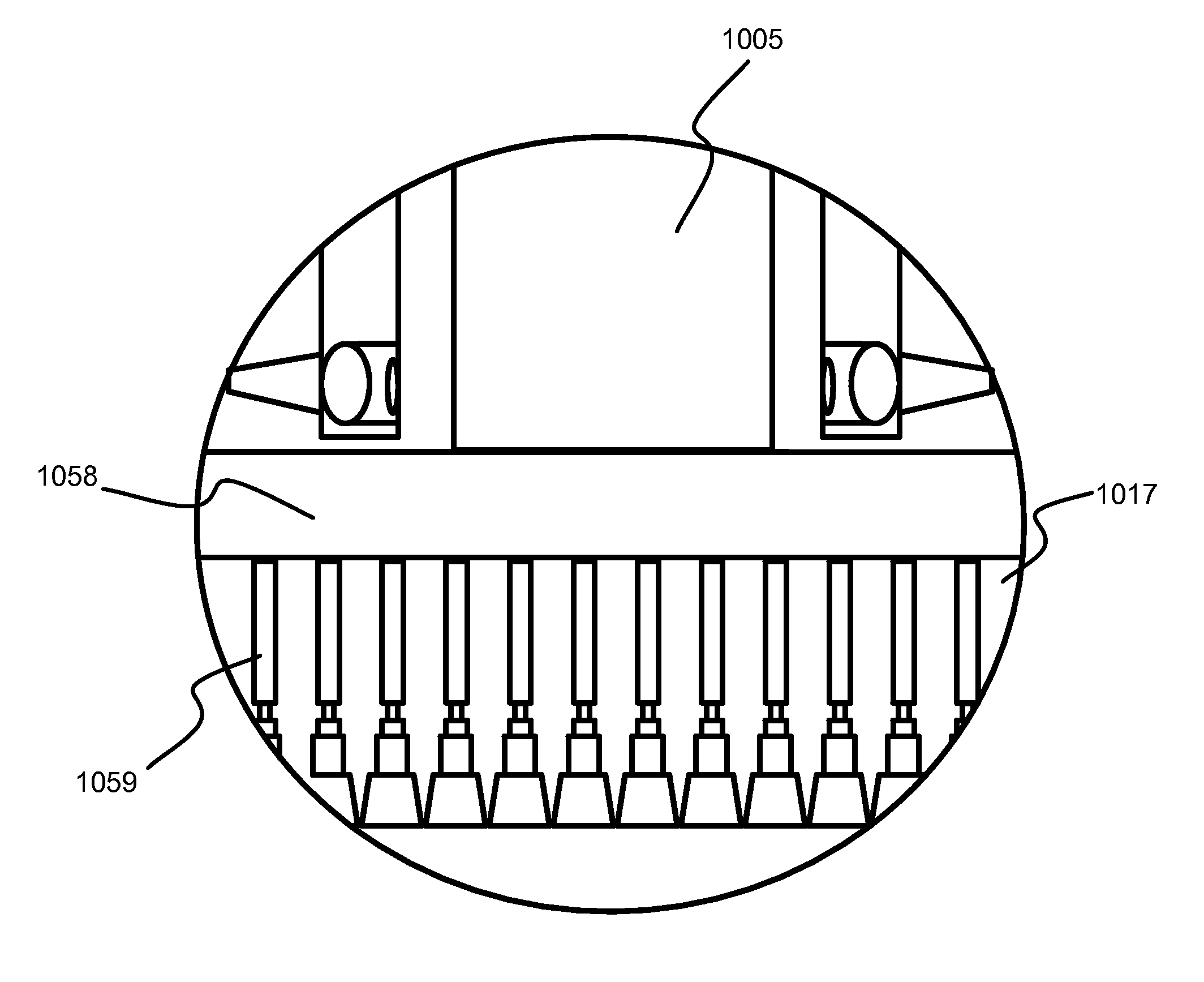
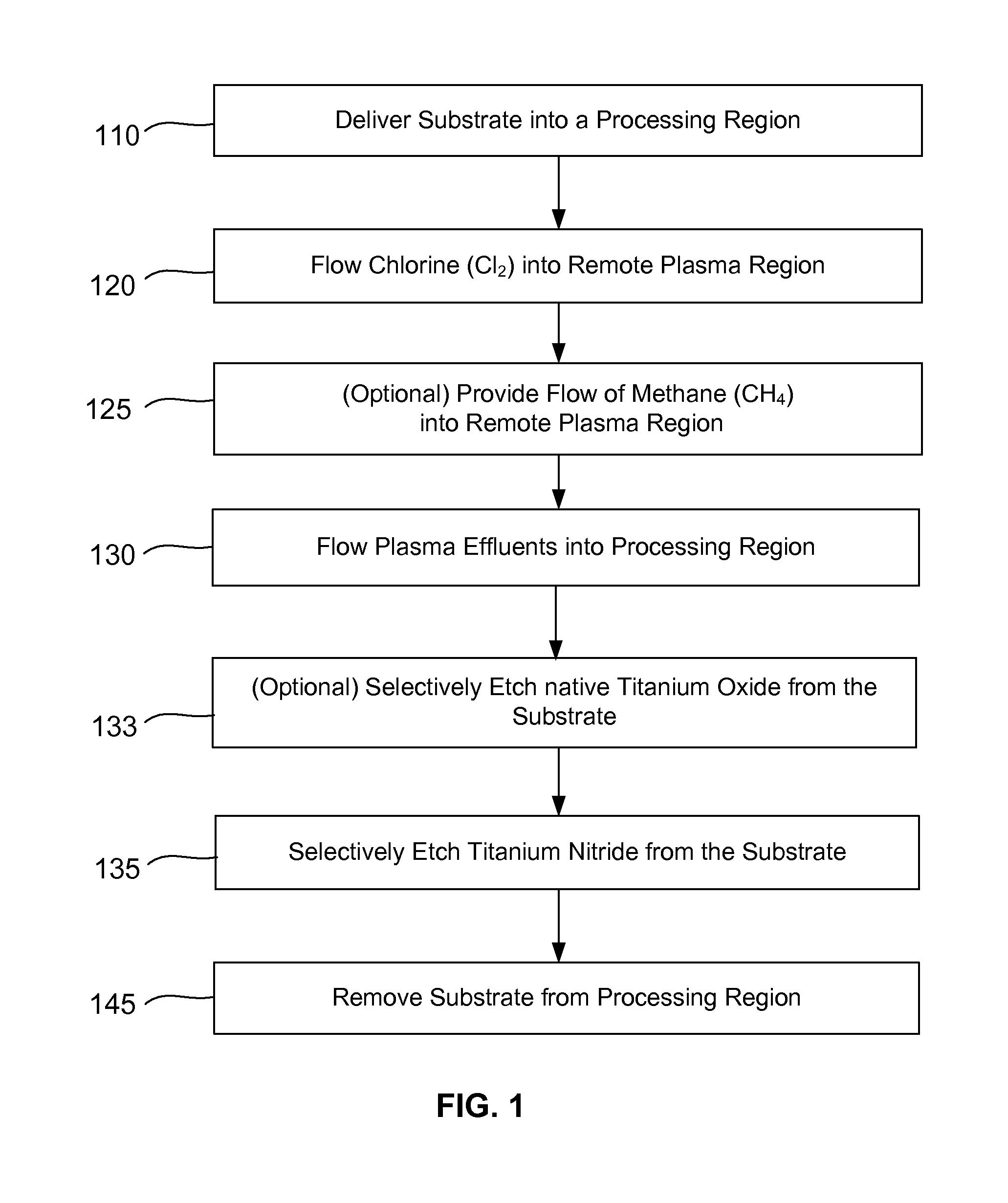
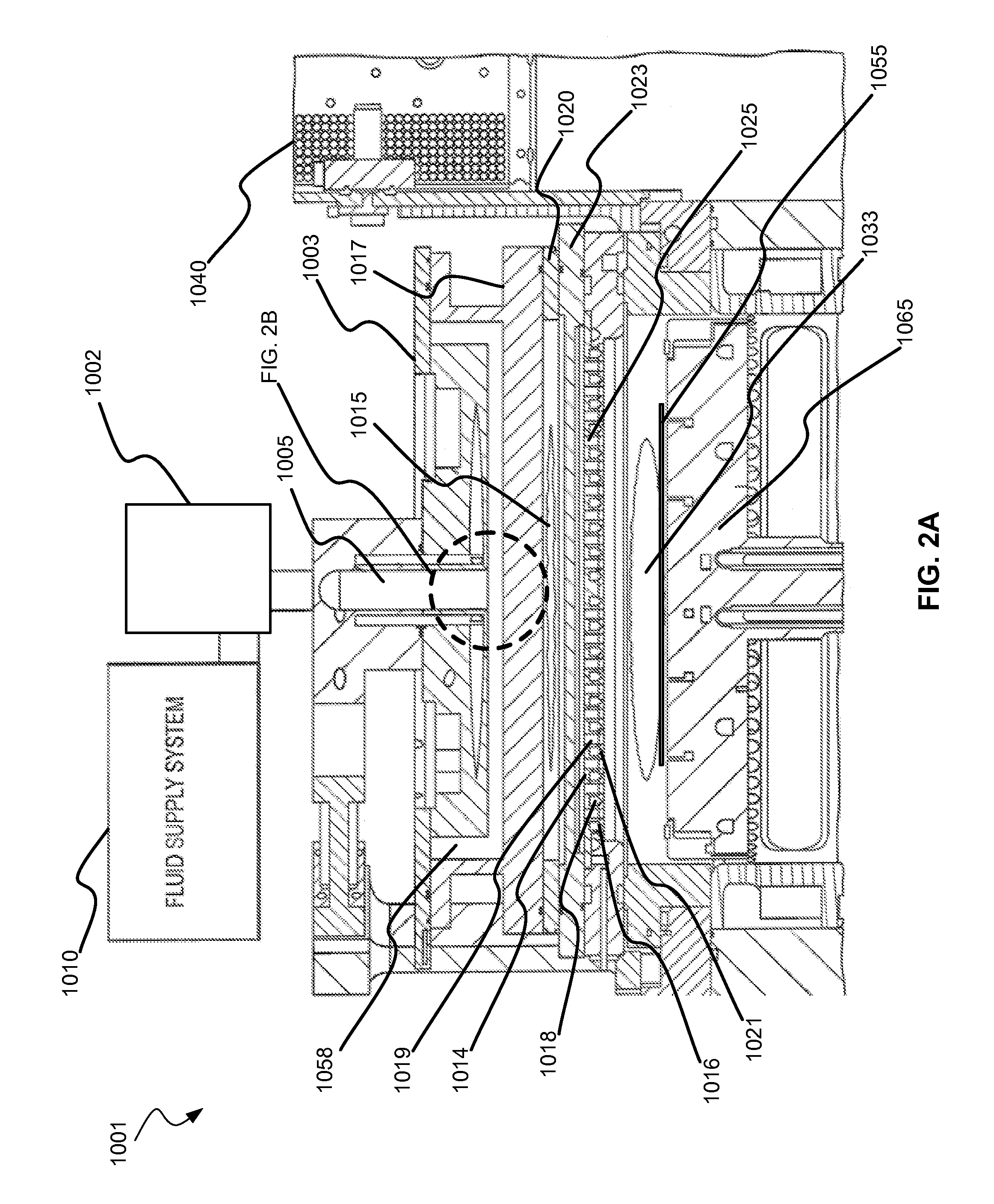
Selective titanium nitride removal
ActiveUS9040422B2Convenient restHigh removal rateElectric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaTitanium nitride
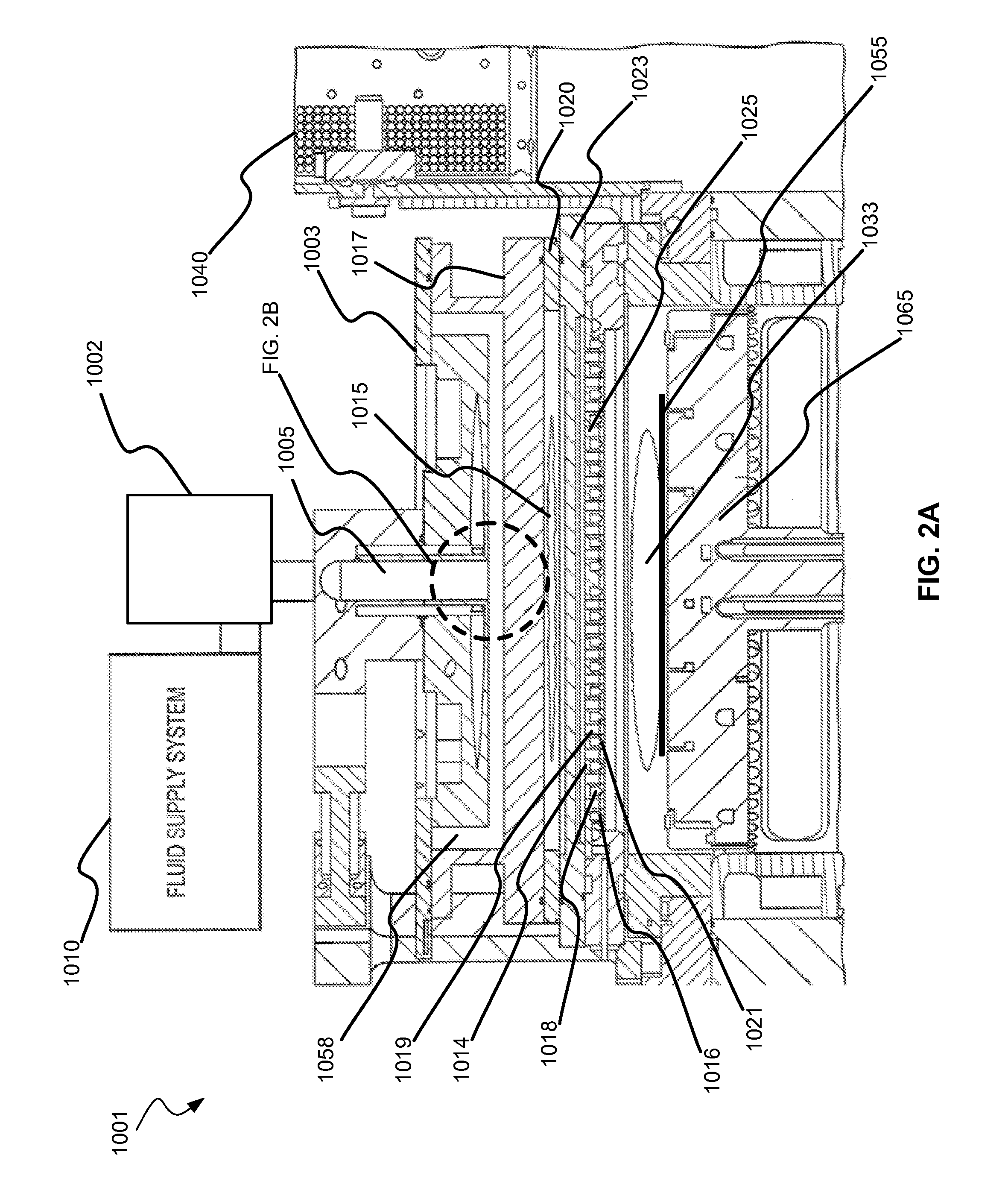
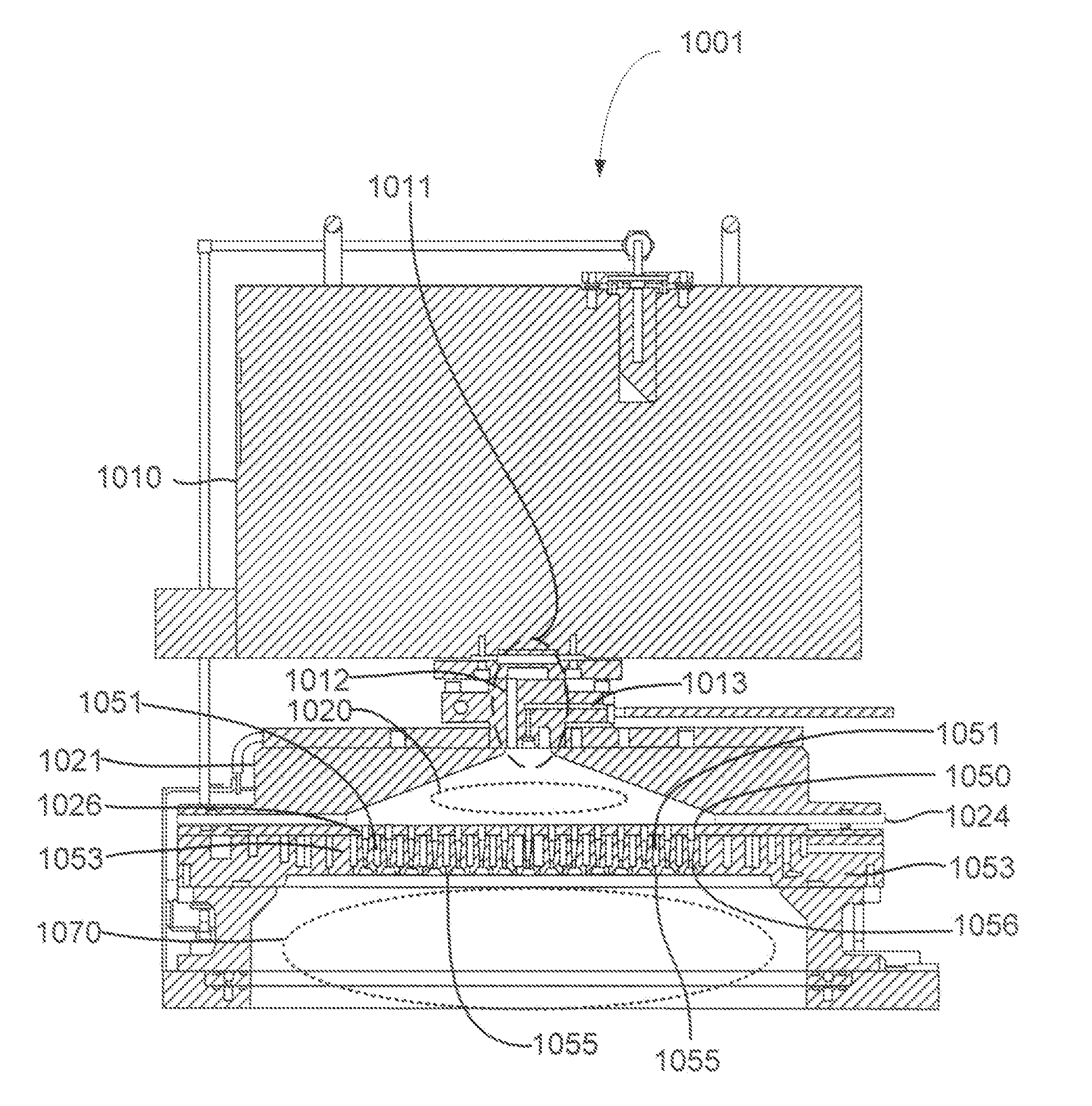
Methods are described herein for selectively etching titanium nitride relative to dielectric films, which may include, for example, alternative metals and metal oxides lacking in titanium and / or silicon-containing films (e.g. silicon oxide, silicon carbon nitride and low-K dielectric films). The methods include a remote plasma etch formed from a chlorine-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the titanium nitride. The plasma effluents react with exposed surfaces and selectively remove titanium nitride while very slowly removing the other exposed materials. The substrate processing region may also contain a plasma to facilitate breaking through any titanium oxide layer present on the titanium nitride. The plasma in the substrate processing region may be gently biased relative to the substrate to enhance removal rate of the titanium oxide layer.
Owner:APPLIED MATERIALS INC
Selective titanium nitride removal
ActiveUS20140256131A1Convenient restHigh removal rateElectric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaTitanium nitride
Methods are described herein for selectively etching titanium nitride relative to dielectric films, which may include, for example, alternative metals and metal oxides lacking in titanium and / or silicon-containing films (e.g. silicon oxide, silicon carbon nitride and low-K dielectric films). The methods include a remote plasma etch formed from a chlorine-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the titanium nitride. The plasma effluents react with exposed surfaces and selectively remove titanium nitride while very slowly removing the other exposed materials. The substrate processing region may also contain a plasma to facilitate breaking through any titanium oxide layer present on the titanium nitride. The plasma in the substrate processing region may be gently biased relative to the substrate to enhance removal rate of the titanium oxide layer.
Owner:APPLIED MATERIALS INC
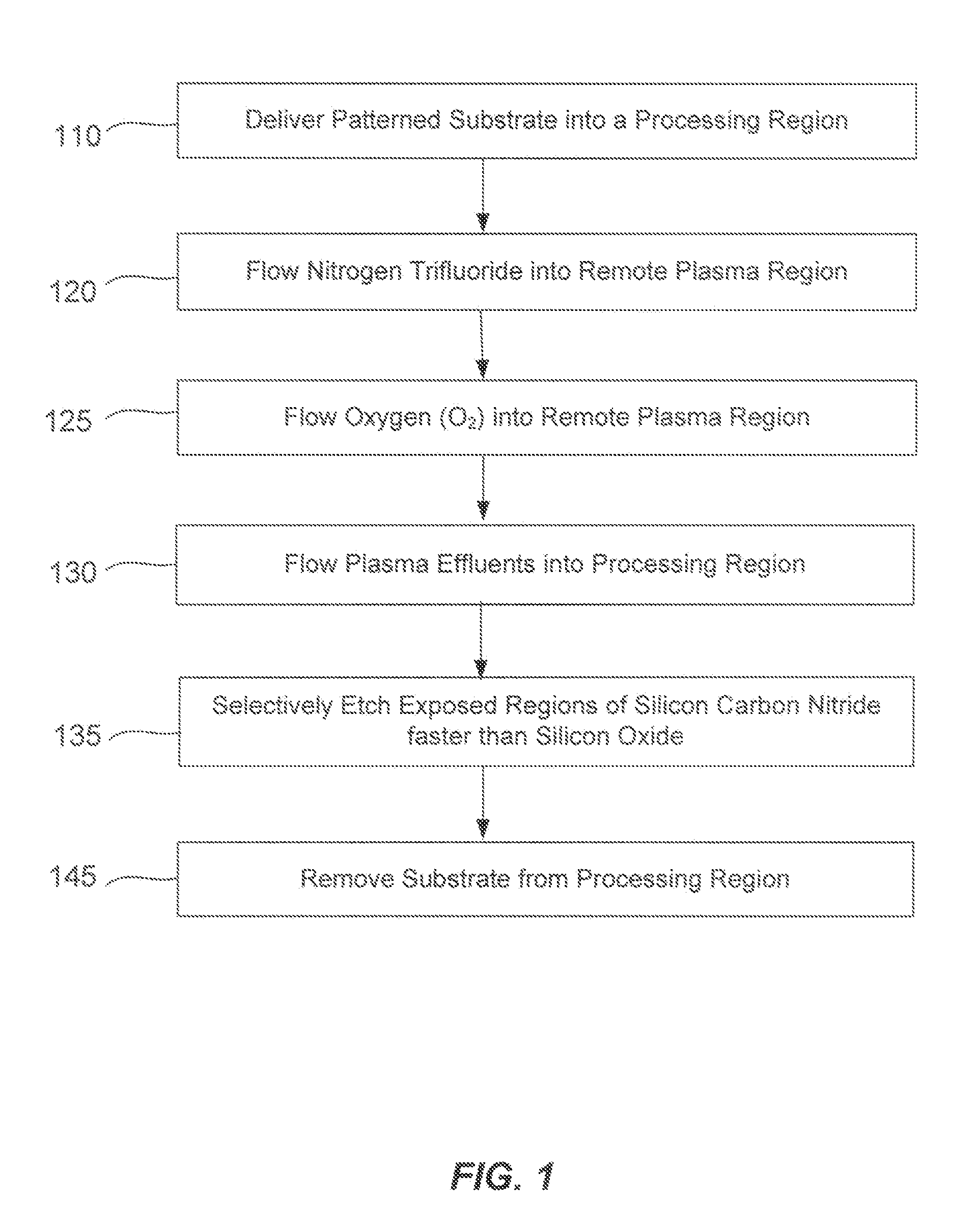
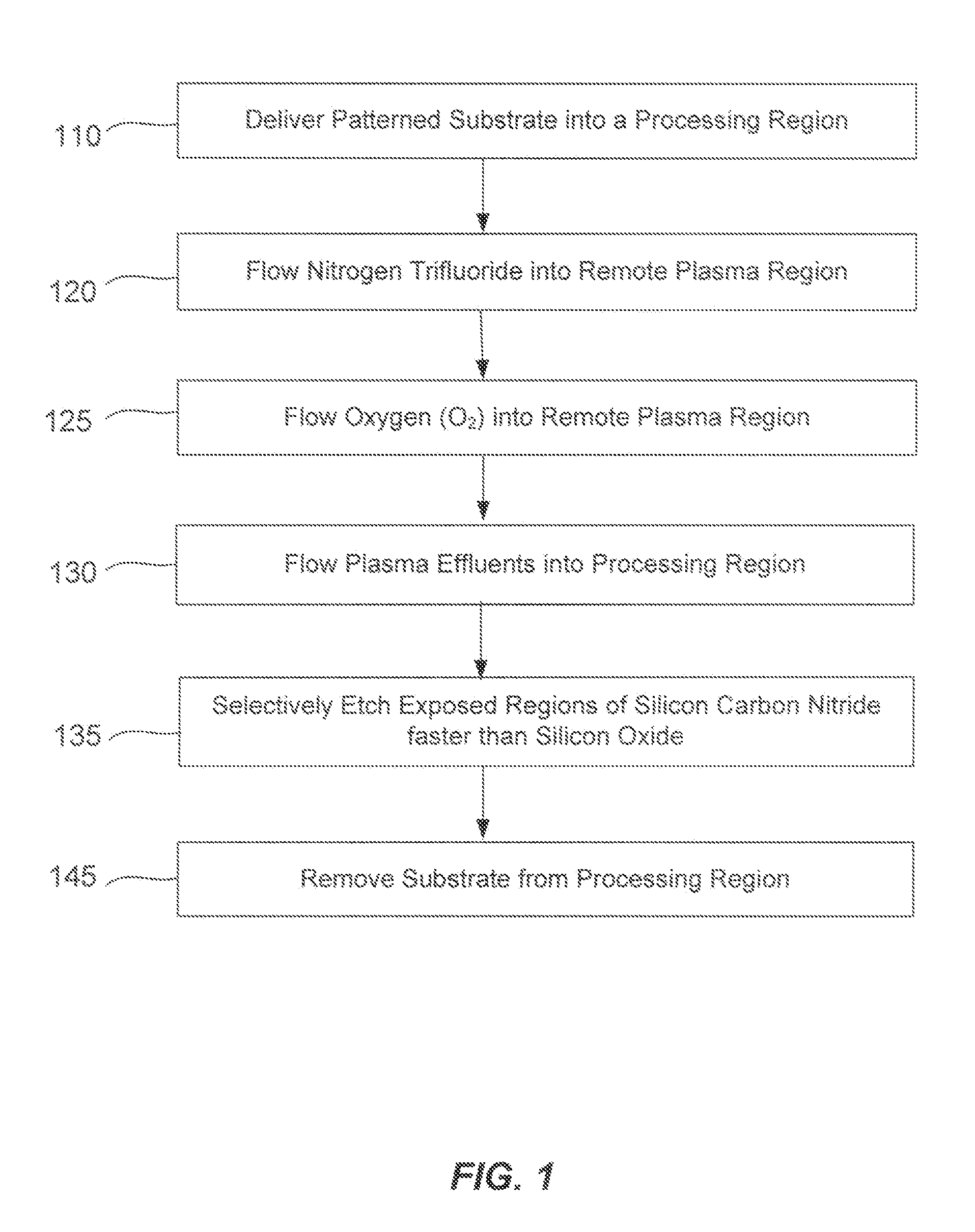
Silicon-carbon-nitride selective etch
ActiveUS20140080310A1Faster rateElectric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaRegioselectivity
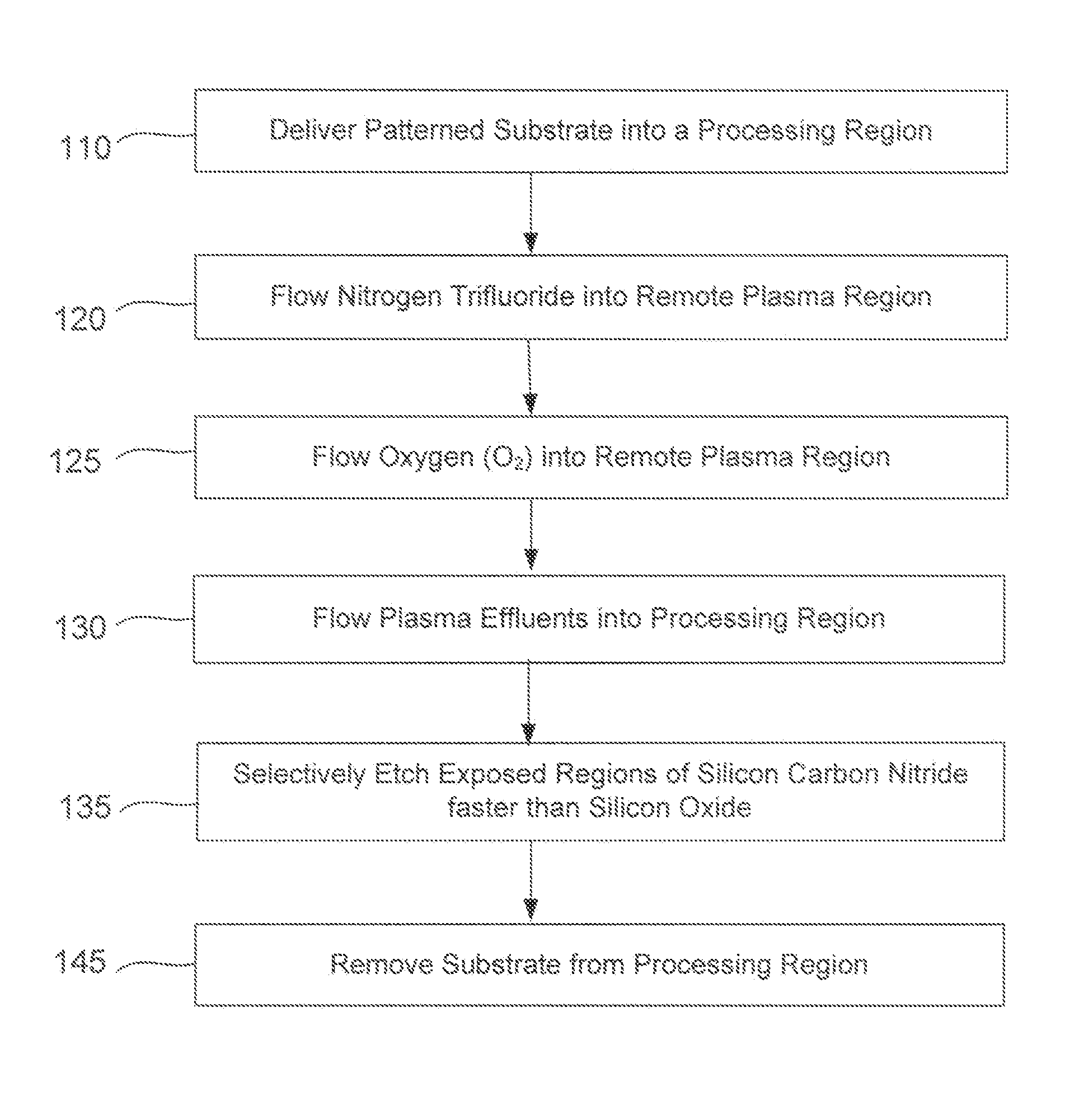
A method of etching exposed silicon-nitrogen-and-carbon-containing material on patterned heterogeneous structures is described and includes a remote plasma etch formed from a fluorine-containing precursor and an oxygen-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the exposed regions of silicon-nitrogen-and-carbon-containing material. The plasma effluents react with the patterned heterogeneous structures to selectively remove silicon-nitrogen-and-carbon-containing material from the exposed silicon-nitrogen-and-carbon-containing material regions while very slowly removing selected other exposed materials. The silicon-nitrogen-and-carbon-containing material selectivity results partly from the presence of an ion suppression element positioned between the remote plasma and the substrate processing region. The ion suppression element controls the number of ionically-charged species that reach the substrate. The methods may be used to selectively remove silicon-nitrogen-and-carbon-containing material at a faster rate than exposed silicon oxide or exposed silicon nitride.
Owner:APPLIED MATERIALS INC
Selective titanium nitride removal
ActiveUS20150357205A1Convenient restHigh removal rateElectric discharge tubesSemiconductor/solid-state device manufacturingTitanium nitrideSilicon oxide
Methods are described herein for selectively etching titanium nitride relative to dielectric films, which may include, for example, alternative metals and metal oxides lacking in titanium and / or silicon-containing films (e.g. silicon oxide, silicon carbon nitride and low-K dielectric films). The methods include a remote plasma etch formed from a chlorine-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the titanium nitride. The plasma effluents react with exposed surfaces and selectively remove titanium nitride while very slowly removing the other exposed materials. The substrate processing region may also contain a plasma to facilitate breaking through any titanium oxide layer present on the titanium nitride. The plasma in the substrate processing region may be gently biased relative to the substrate to enhance removal rate of the titanium oxide layer.
Owner:APPLIED MATERIALS INC
Selective etch for silicon films
ActiveUS9324576B2Decorative surface effectsSemiconductor/solid-state device manufacturingRemote plasmaSilicon membrane
A method of etching patterned heterogeneous silicon-containing structures is described and includes a remote plasma etch with inverted selectivity compared to existing remote plasma etches. The methods may be used to conformally trim polysilicon while removing little or no silicon oxide. More generally, silicon-containing films containing less oxygen are removed more rapidly than silicon-containing films which contain more oxygen. Other exemplary applications include trimming silicon carbon nitride films while essentially retaining silicon oxycarbide. Applications such as these are enabled by the methods presented herein and enable new process flows. These process flows are expected to become desirable for a variety of finer linewidth structures. Methods contained herein may also be used to etch silicon-containing films faster than nitrogen-and-silicon containing films having a greater concentration of nitrogen.
Owner:APPLIED MATERIALS INC
Silicon-carbon-nitride selective etch
ActiveUS9390937B2Faster rateElectric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaCarbon nitride
A method of etching exposed silicon-nitrogen-and-carbon-containing material on patterned heterogeneous structures is described and includes a remote plasma etch formed from a fluorine-containing precursor and an oxygen-containing precursor. Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the exposed regions of silicon-nitrogen-and-carbon-containing material. The plasma effluents react with the patterned heterogeneous structures to selectively remove silicon-nitrogen-and-carbon-containing material from the exposed silicon-nitrogen-and-carbon-containing material regions while very slowly removing selected other exposed materials. The silicon-nitrogen-and-carbon-containing material selectivity results partly from the presence of an ion suppression element positioned between the remote plasma and the substrate processing region. The ion suppression element controls the number of ionically-charged species that reach the substrate. The methods may be used to selectively remove silicon-nitrogen-and-carbon-containing material at a faster rate than exposed silicon oxide or exposed silicon nitride.
Owner:APPLIED MATERIALS INC
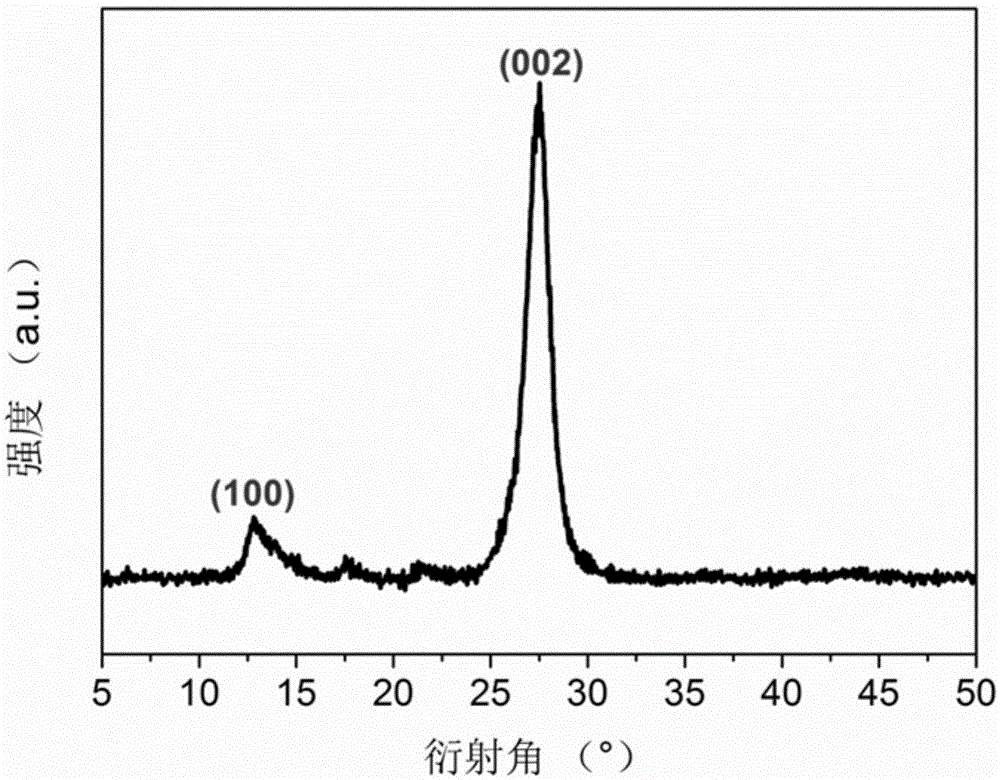
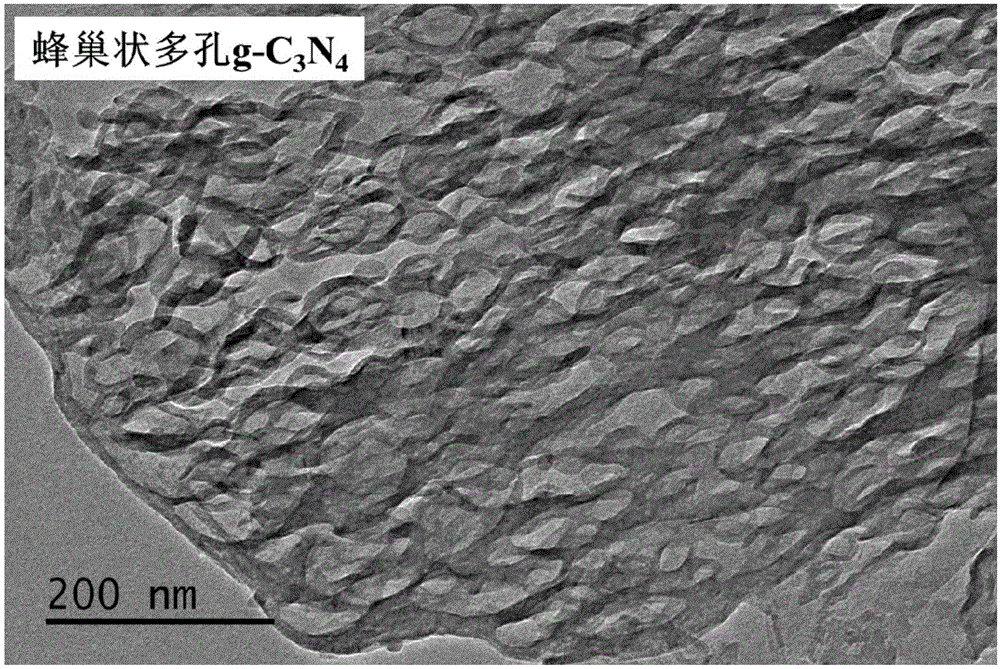
Graphite-phase carbon nitride (g-C3N4) material and preparation method and application thereof
ActiveCN105126893APlay a pore-forming roleAppropriate speedPhysical/chemical process catalystsWater/sewage treatment by irradiationCalcinationMaterials science
The invention relates to a method for preparing a graphite-phase carbon nitride (g-C3N4) material. The method includes the steps that a carbon nitride precursor and ammonium salt are evenly mixed, and then calcination is conducted so that the porous g-C3N4 material can be obtained. The ammonium salt is any one of ammonium base salts capable of generating ammonia gas through thermal decomposition or is the combination of at least two of the ammonium base salts. In the preparation process of the g-C3N4 material, the ammonium salt is added into the carbon nitride precursor to be mixed. In the high-temperature calcining process, the ammonium salt is subjected to pyrogenic decomposition to generate gas, a pore-forming effect on the g-C3N4 material is achieved, and the cellular porous g-C3N4 material is obtained. In the preparation process of the g-C3N4 material, template agents are not used, and thus the method is simple, efficient and environmentally friendly; the prepared g-C3N4 material is high in photocatalytic activity and can be used in the pollution control processes such as exhaust gas and wastewater treatment.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Dry-etch for selective tungsten removal
ActiveUS20140154889A1Electric discharge tubesSemiconductor/solid-state device manufacturingSilicon oxideMaterials science
Methods of selectively etching tungsten relative to silicon-containing films (e.g. silicon oxide, silicon carbon nitride and (poly)silicon) as well as tungsten oxide are described. The methods include a remote plasma etch formed from a fluorine-containing precursor and / or hydrogen (H2). Plasma effluents from the remote plasma are flowed into a substrate processing region where the plasma effluents react with the tungsten. The plasma effluents react with exposed surfaces and selectively remove tungsten while very slowly removing other exposed materials. Sequential and simultaneous methods are included to remove thin tungsten oxide which may, for example, result from exposure to the atmosphere.
Owner:APPLIED MATERIALS INC
Boron nitride and boron-nitride derived materials deposition method
ActiveUS20090263972A1Semiconductor/solid-state device manufacturingSemiconductor devicesCarbon nitrideBoron nitride
A method and apparatus are provided to form spacer materials adjacent substrate structures. In one embodiment, a method is provided for processing a substrate including placing a substrate having a substrate structure adjacent a substrate surface in a deposition chamber, depositing a spacer layer on the substrate structure and substrate surface, and etching the spacer layer to expose the substrate structure and a portion of the substrate surface, wherein the spacer layer is disposed adjacent the substrate structure. The spacer layer may comprise a boron nitride material. The spacer layer may comprise a base spacer layer and a liner layer, and the spacer layer may be etched in a two-step etching process.
Owner:APPLIED MATERIALS INC
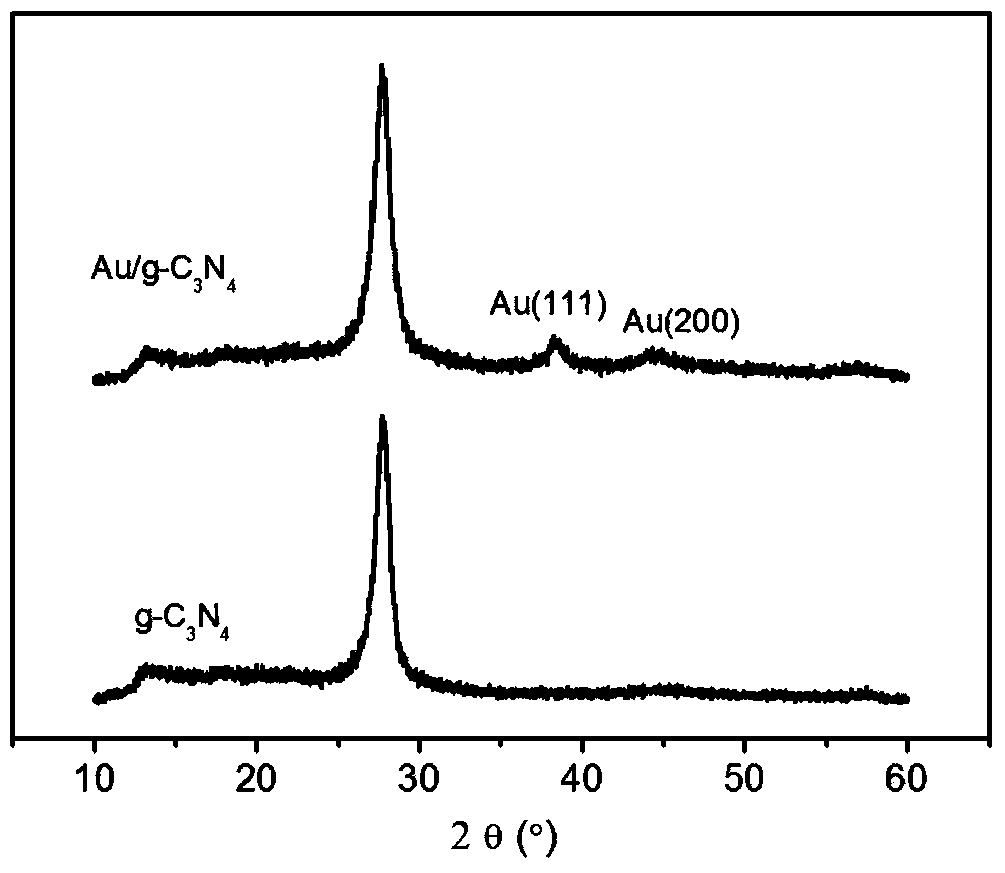
Metal/graphite-like carbon nitride compound catalyst and preparing method thereof
InactiveCN103586064APhysical/chemical process catalystsOrganic compound preparationNitrobenzeneCarbon nitride
The invention relates to a metal / graphite-like carbon nitride (g-C3N4) compound catalyst and a preparing method thereof in the field of catalysis. The chemical constitution of a metal / graphite-like carbon nitride provided by the invention is M / g-C3N4, wherein the metal M is Au, Ag, Pt, Pd, Bi, Cu, Ru or Rh, wherein the content of metal is 0-50% (mass fraction). The metal / graphite-like carbon nitride compound is a catalytic reducing agent at normal pressure and temperature, and the material is suitable for reducing derivants of nitrobenzene.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Composite and process for the production thereof
InactiveUS6124040AHigh hardnessHigh strengthVacuum evaporation coatingSputtering coatingHard metalCarbon nitride
PCT No. PCT / DE95 / 00548 Sec. 371 Date Nov. 20, 1997 Sec. 102(e) Date Nov. 20, 1997 PCT Filed Apr. 26, 1995 PCT Pub. No. WO96 / 33830 PCT Pub. Date Oct. 31, 1996The invention concerns composites substantially consisting of: a cermet material having a binder metal phase of between 5 and 30 mass % and the remainder comprising at least one carbon nitride phase; or a hard metal with a hard material phase of between 70 and 100 %, the remainder being a binder metal phase, with the exception of a WC-Co hard metal, with up to 25 mass % cobalt as binder metal; or a powder-metallurgically produced steel. The invention further concerns a process for producing this composite. In order to improve bending strength and hardness, sintering is carried out in a microwave field.
Owner:WIDIA
Nitride and Carbide Anode Materials
ActiveUS20100233546A1High chemicalHigh environmental stabilityFinal product manufactureConductive materialNanoparticleVanadium nitride
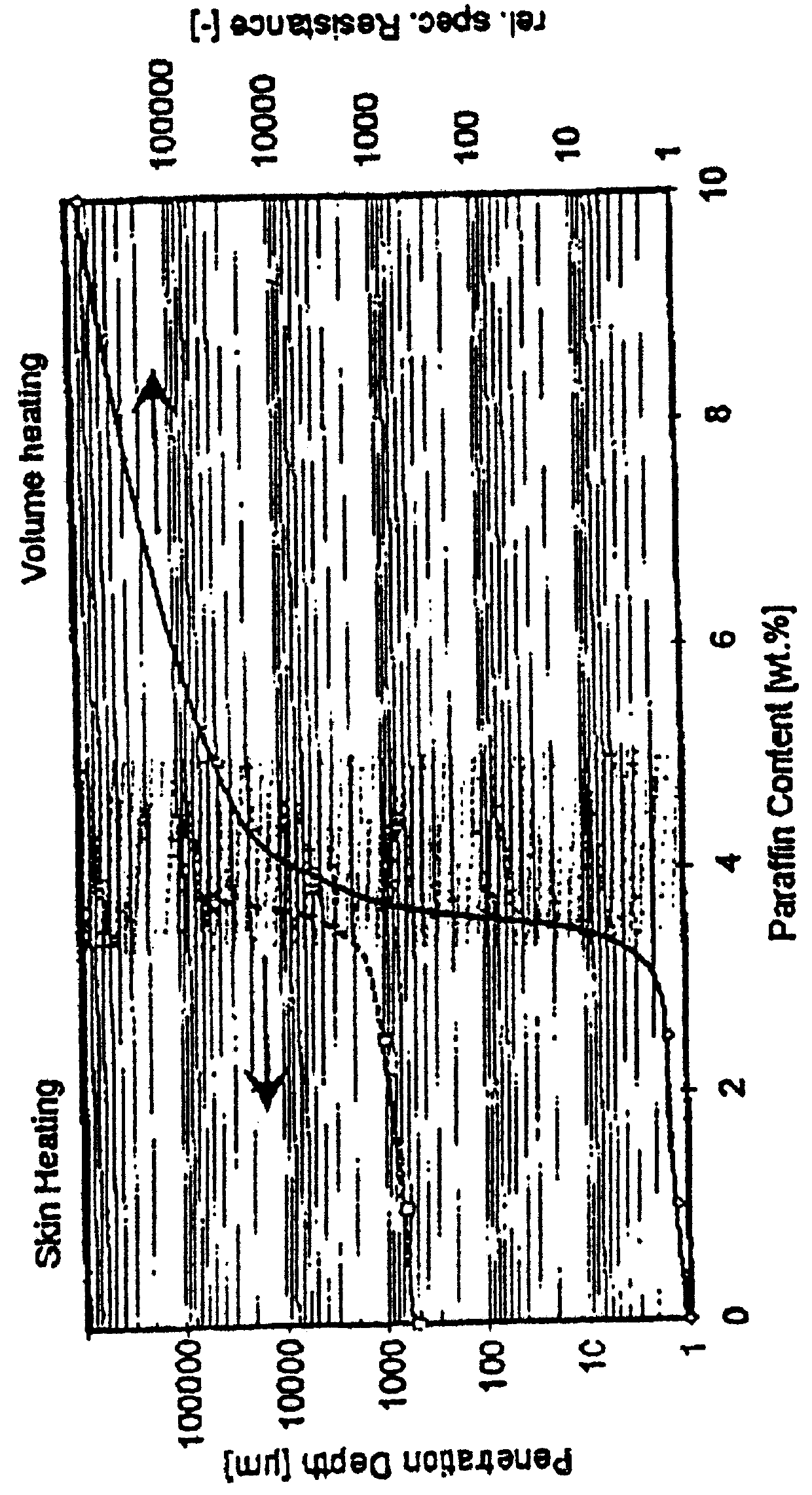
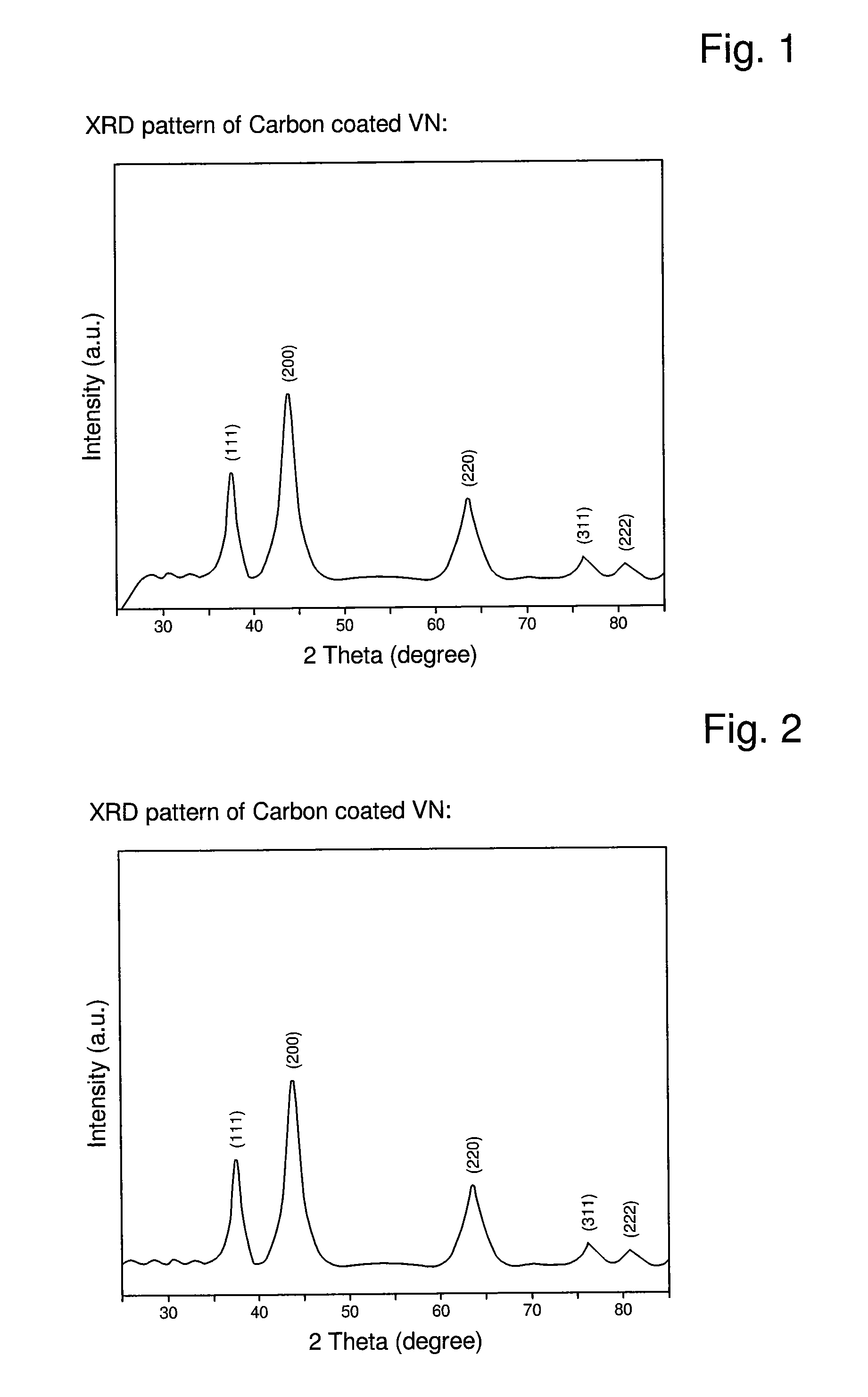
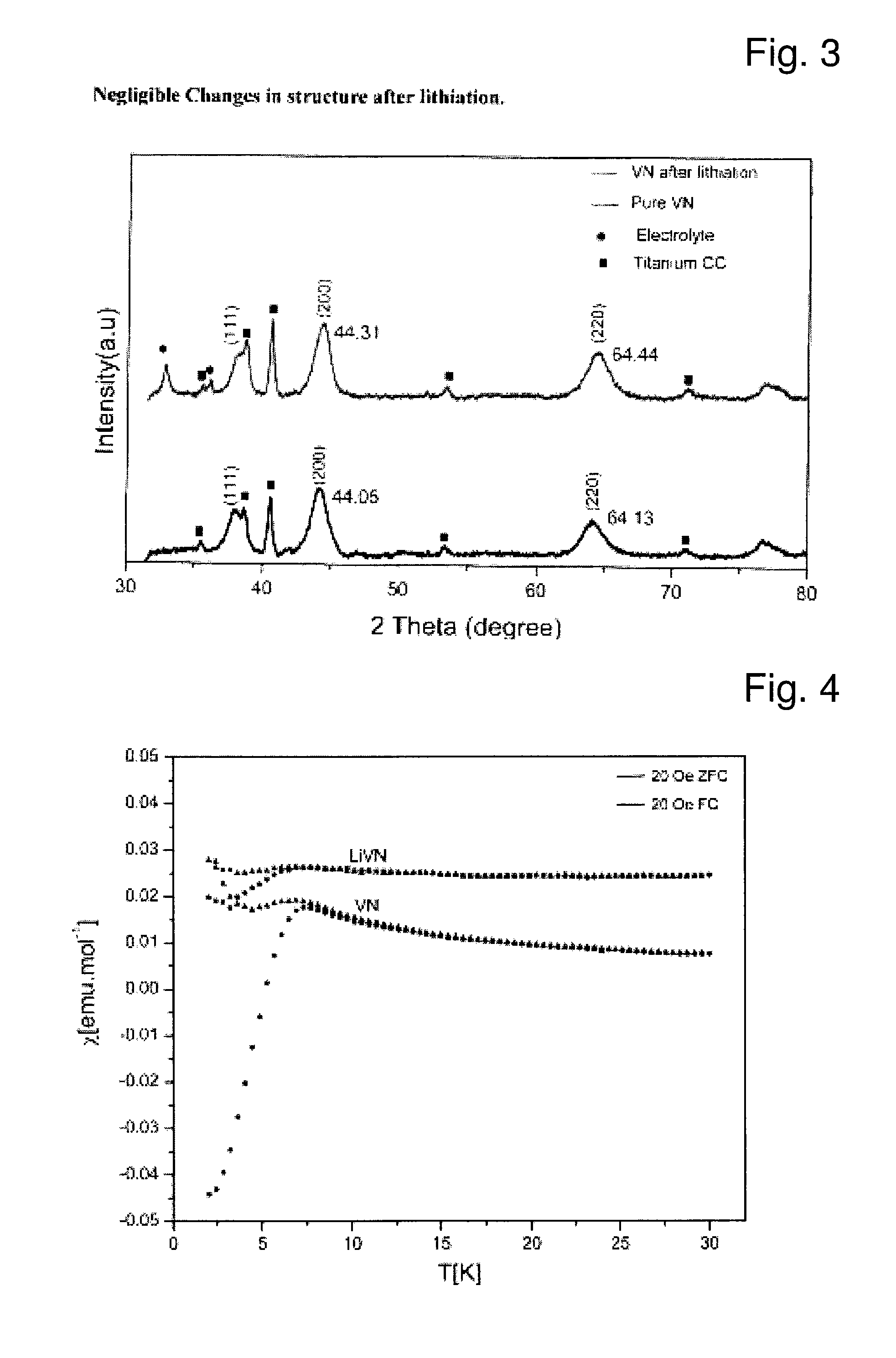
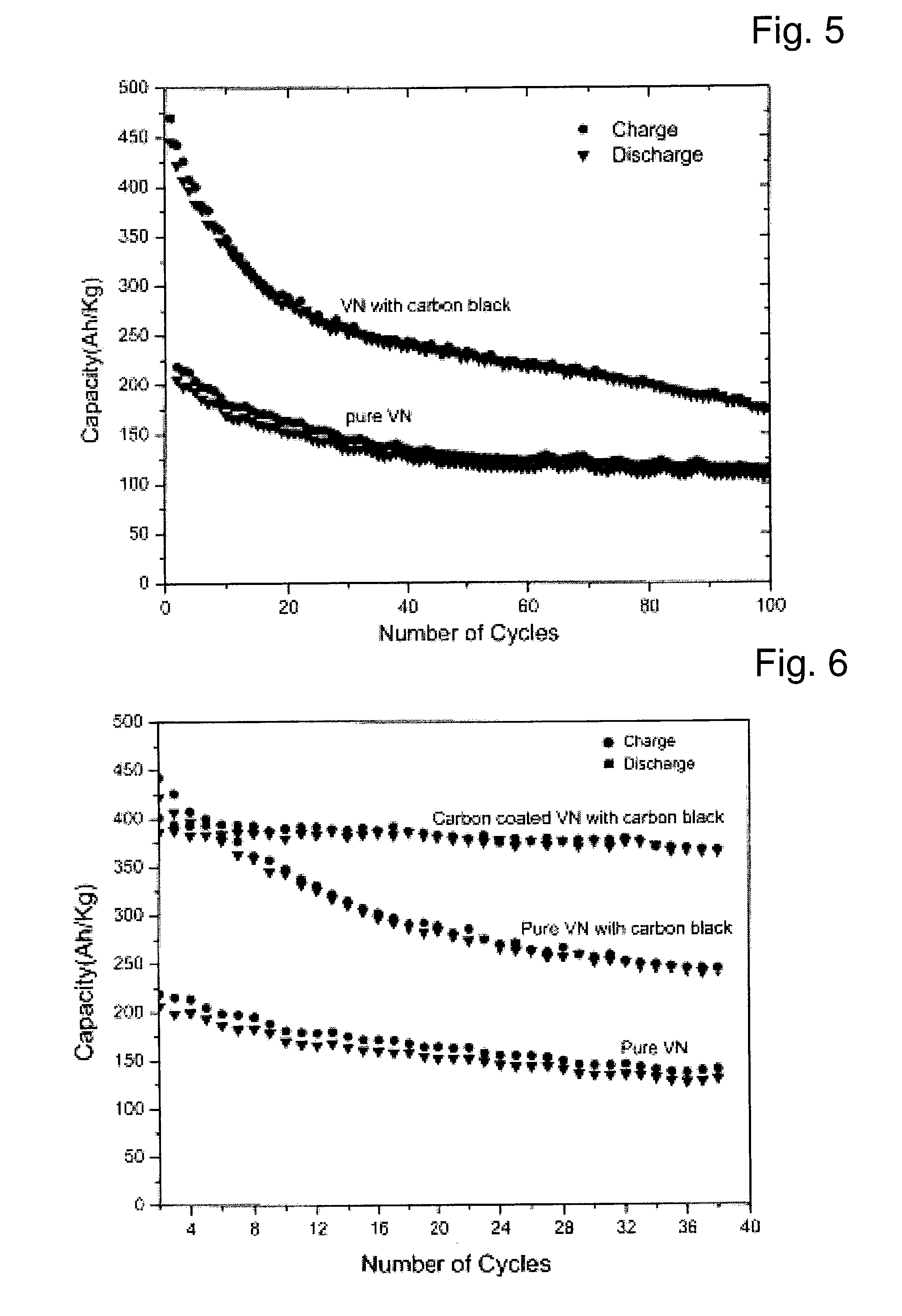
Described is an anode material which is a transition metal nitride or carbide in form of nanoparticles, preferably a nitride or carbide with one nitrogen or carbon per metal, and especially a nitride or carbide having rock salt structure. A preferred anode material is vanadium nitride, in particular carbon coated vanadium nitride having a mean particle size of <500 nm. Embedded in an electrically conducting environment, such nanoparticulate material, in particular the vanadium nitride shows exceptional good charging-discharging cycle stability.
Owner:BELENOS CLEAN POWER HLDG
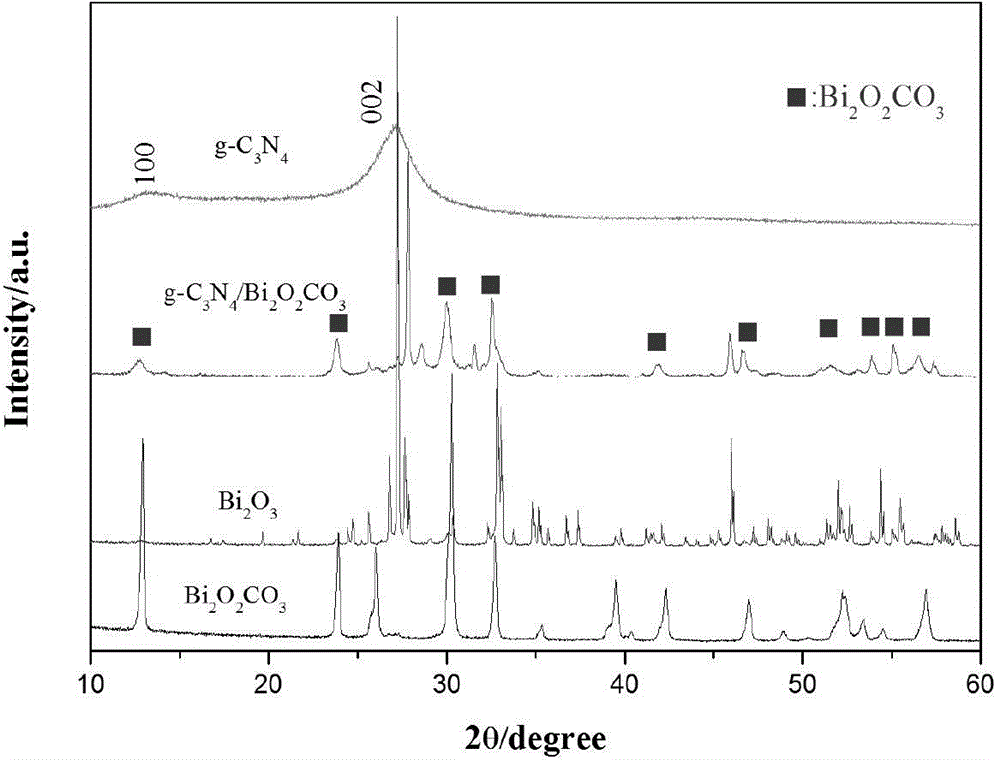
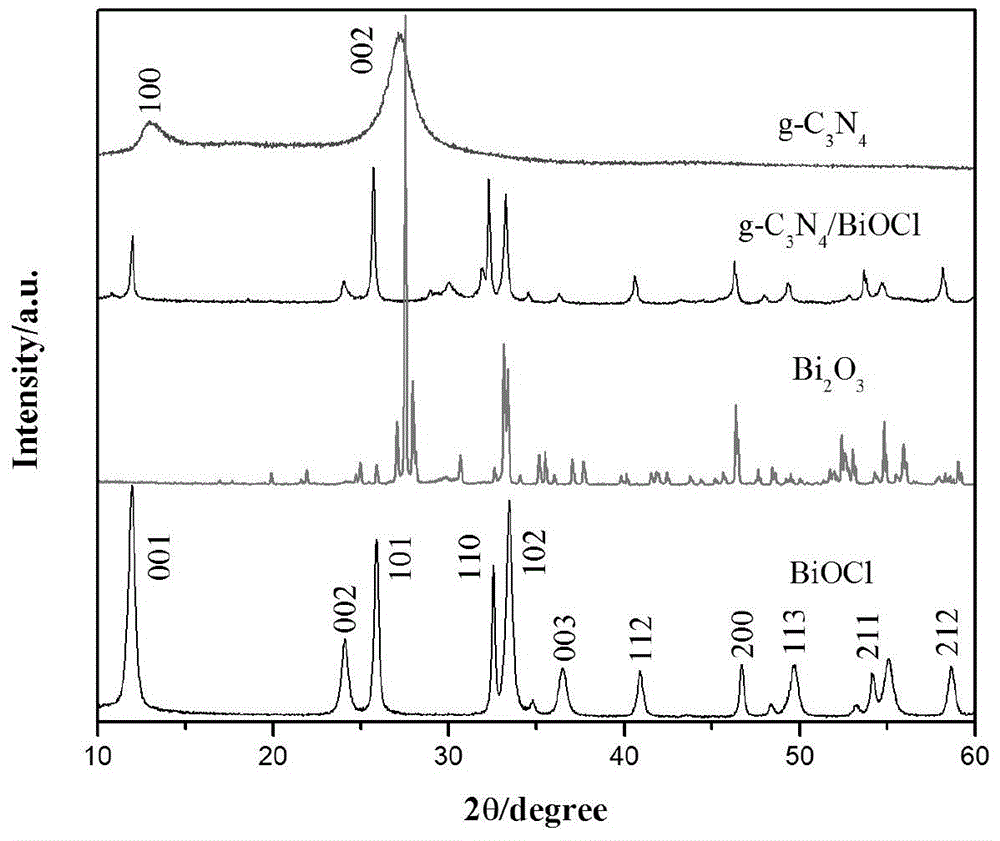
Composite visible light catalyst of g-C3N4/bismuth-based oxide and preparation method and application of composite visible light catalyst
ActiveCN104549406AImprove photocatalytic activitySimple processPhysical/chemical process catalystsWater/sewage treatment by irradiationPtru catalystCarbon nitride
The invention discloses a composite visible light catalyst of a g-C3N4 / bismuth-based oxide and a preparation method and an application of the composite visible light catalyst. Carbon nitride in the composite light catalyst is graphite-phase carbon nitride; and the bismuth-based oxide is more than one of Bi2O2CO3, BiOBr, BiOCl, Bi2WO6, BiVO4 and BiOI. The components of the obtained composite catalyst are tight in contact, so that separation of photo electron-hole pairs is facilitated, and the photocatalytic efficiency is also improved; the composite catalyst has good degradation efficiency on organic pollutants represented by methyl orange under sunlight illumination; the preparation method disclosed by the invention has the characteristics of being simple in process, simple and convenient to operate, and cheap in raw materials; the prepared composite catalyst can be widely applied to waste water treatment, atmospheric purification and the like; and a new concept is provided for design of the novel composite visible light catalyst by the preparation method.
Owner:JIHE SMART URBAN TECH (GUANGZHOU) CO LTD
Preparation method for multi-hole carbon nitride photocatalytic material doped with sulphur
InactiveCN103861632AReduce pollutionGood repeatabilityPhysical/chemical process catalystsHydrogen productionSurface-active agentsSolvent
The invention discloses a preparation method for a multi-hole carbon nitride photocatalytic material doped with sulphur, belonging to the technical field of synthesis of photocatalytic materials. The preparation method comprises the following steps of preparing a super-molecule polymer through simple hydro-thermal treatment by taking melamine and trithiocyanuric acid as a raw material and taking water as a solvent, and burning in an inert atmosphere so as to obtain a three-dimensional network sulphur-doped multi-hole carbon nitride photocatalytic material. The preparation method provided by the invention has the advantages that simplicity and easiness in preparation are achieved, a method for burning a super-molecule polymer is adopted, any template agent and a surface active agent do not need to be added, a reaction system is simplified, the cost is low, the pollution of a reagent is slight, the reaction repeatability is good, a preparation condition is mild, the time consumption of a synthetic process is short, and the requirement on equipment is high; the multi-hole carbon nitride photocatalytic material doped with sulphur has excellent catalytic activity in a catalyzing hydrogen production reaction, and the hydrogen production rate of the material is 8.3 times and 5.2times that of a product obtained by burning melamine and trithiocyanuric acid under the same condition.
Owner:JILIN UNIV
Copolymerization modified graphite-phase carbon nitride nanosheet visible-light-driven photocatalyst
ActiveCN103272639AEasy to separateImprove migration efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionCarbon nitrideGraphite
The invention discloses a copolymerization modified graphite-phase carbon nitride nanosheet visible-light-driven photocatalyst as well as a preparation method and an application thereof, and belongs to the technical field of material preparation and photocatalysis. The graphite-phase carbon nitride nanosheet visible-light-driven photocatalyst which adopts a nanosheet structure and synthesized with a copolymerization method is formed by taking urea and different small organic molecule monomers as precursors through the high-temperature copolymerization action. The prepared graphite-phase carbon nitride has a lower-dimension nanosheet microstructure and a proper band gap; compared with conventional bulk-phase carbon nitride, the graphite-phase carbon nitride effectively increases the specific surface area, enhances the utilization rate of sunlight, and has efficient photocatalysis hydrogen production performance in visible light. According to the copolymerization modified graphite-phase carbon nitride nanosheet visible-light-driven photocatalyst, the synthetic process is simple, the cost is low, the catalytic efficiency is high, the actual production requirements are met, and the photocatalyst has broad application prospects in the field of photocatalysis.
Owner:FUZHOU UNIV
Boron nitride nanotubes
ActiveUS8206674B2Promote resultsMaterial nanotechnologyPolycrystalline material growthMetallurgyCarbon nitride
Owner:NASA +2
High-entropy alloy binder phase-based nitrogen-containing hard alloy and preparation method thereof
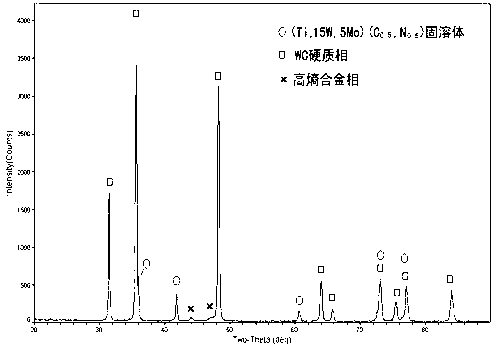
The invention discloses a high-entropy alloy binder phase-based nitrogen-containing hard alloy, wherein the binder phase of the nitrogen-containing hard alloy is high-entropy alloy, and the hard phase of the nitrogen-containing hard alloy is uniformly distributed WC (Wolfram Carbide) and carbon nitride solid solution; the high-entropy alloy binder phase is at least four of iron, cobalt, nickel, chromium, aluminum, vanadium, titanium, copper, zirconium, molybdenum and manganese; the molar ratio of content of each element is 5-35 percent; the carbon nitride solid solution is at least one of Ti(Cx, N1-x) and (Ti, M1, ......)(Cx, N1-x); and M1 component in the (Ti, M1, ......)(Cx, N1-x) is at least one of W, Mo, Ta, Nb, V, Cr, Zr, Hf, Y and lanthanides. According to a preparation method of the high-entropy alloy binder phase-based nitrogen-containing hard alloy, the alloy comprises the raw material components in percentage by weight: 3-25 percent of high-entropy alloy binder phase, 45-96.9 percent of WC powder and 0.1-30 percent of carbon nitride solid solution powder, wherein the nitrogen element in the nitrogen-containing hard alloy is introduced through the carbon nitride solid solution. The preparation method of the multi-element composite carbon nitride solid solution-based nitrogen-containing hard alloy comprises the following steps of: (1) mixing through ball milling; (2) shaping; and (3) performing low-pressure sintering.
Owner:SICHUAN UNIV
Method for preparing carbon nitride quantum dots
InactiveCN104140084AIncrease useHigh yieldNitrogen and non-metal compoundsFluorescent radiationCarbon nitride
A method for preparing carbon nitride quantum dots comprises the following steps of (1) material preparation, (2) drying and tabletting, (3) heating, (4) separation and (5) drying. Sodium chloride crystals are used as a template, the preparing process is simple and low in cost, and the sodium chloride crystals can be easily removed through water dissolution; melamine is used as the raw material, and annular carbon nitride basic structural units exist in melamine molecules, so that the yield of preparing the quantum dots is high, and product size uniformity is good; the prepared quantum dots are high in specific area, good in water solubility and good in dispersity, have strong fluorescent radiation visible under naked eyes, and have wide application prospects in the fields of fluorescence detection, light emitting devices, biomarker and the like.
Owner:NAT UNIV OF DEFENSE TECH
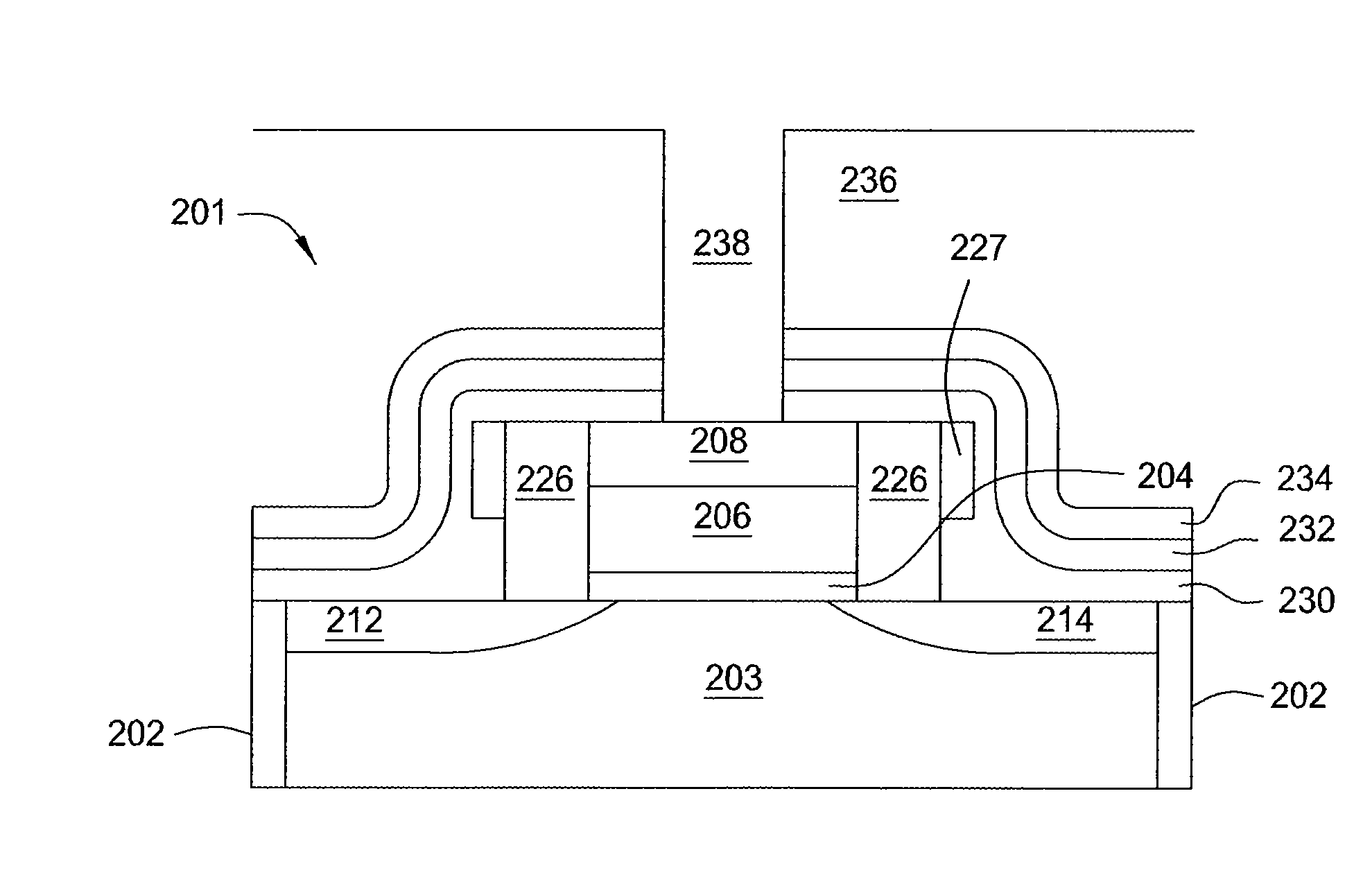
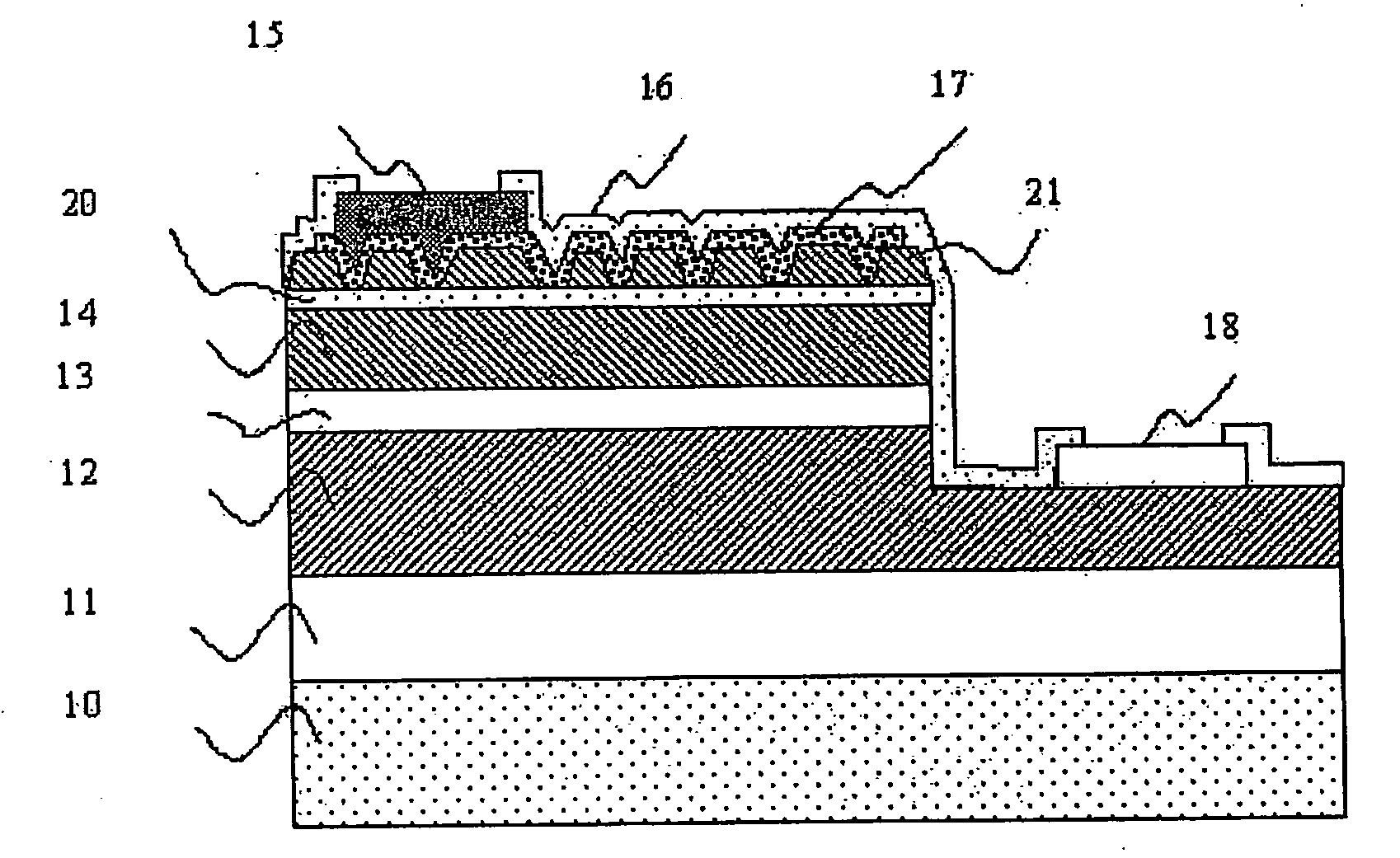
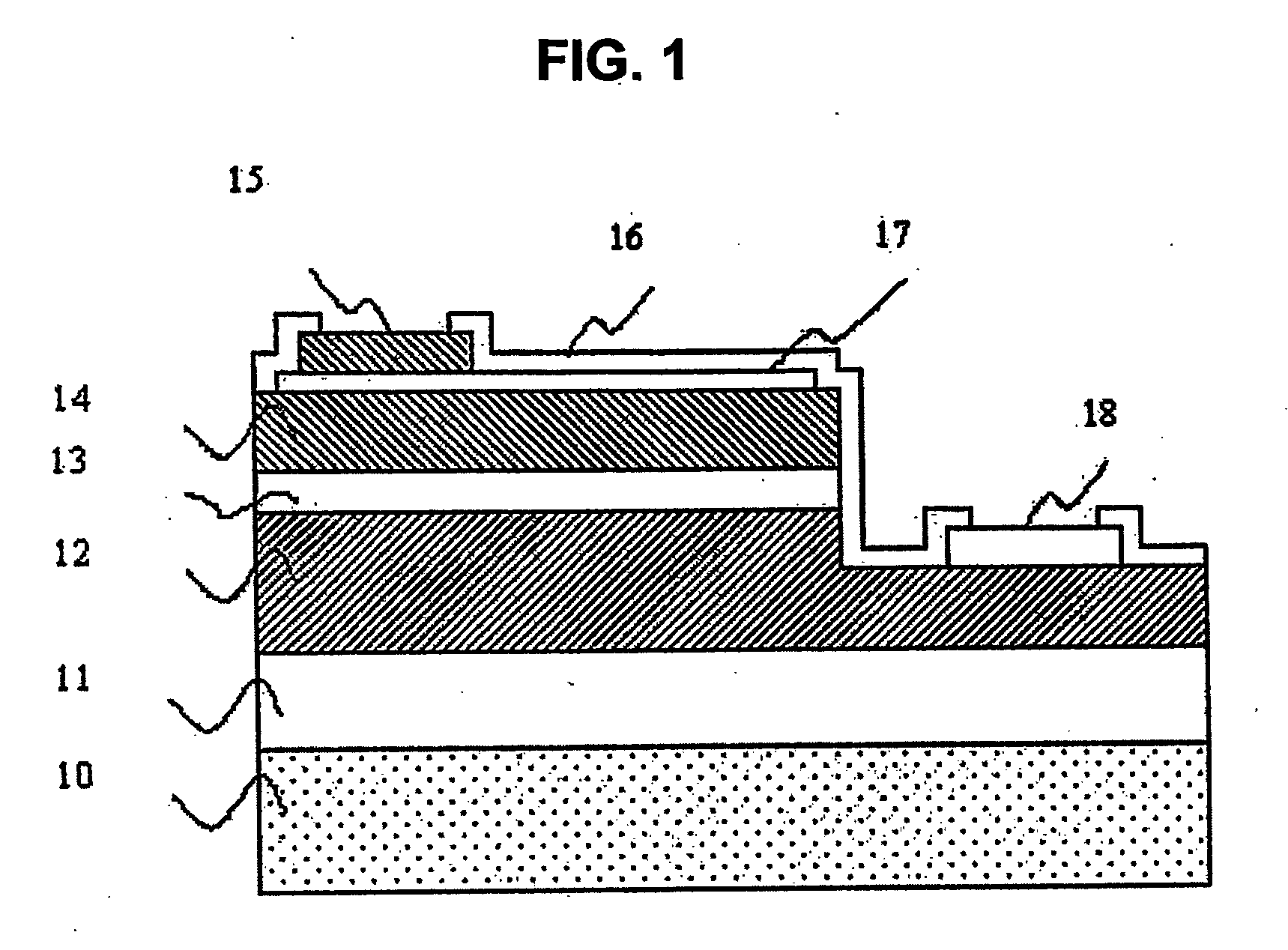
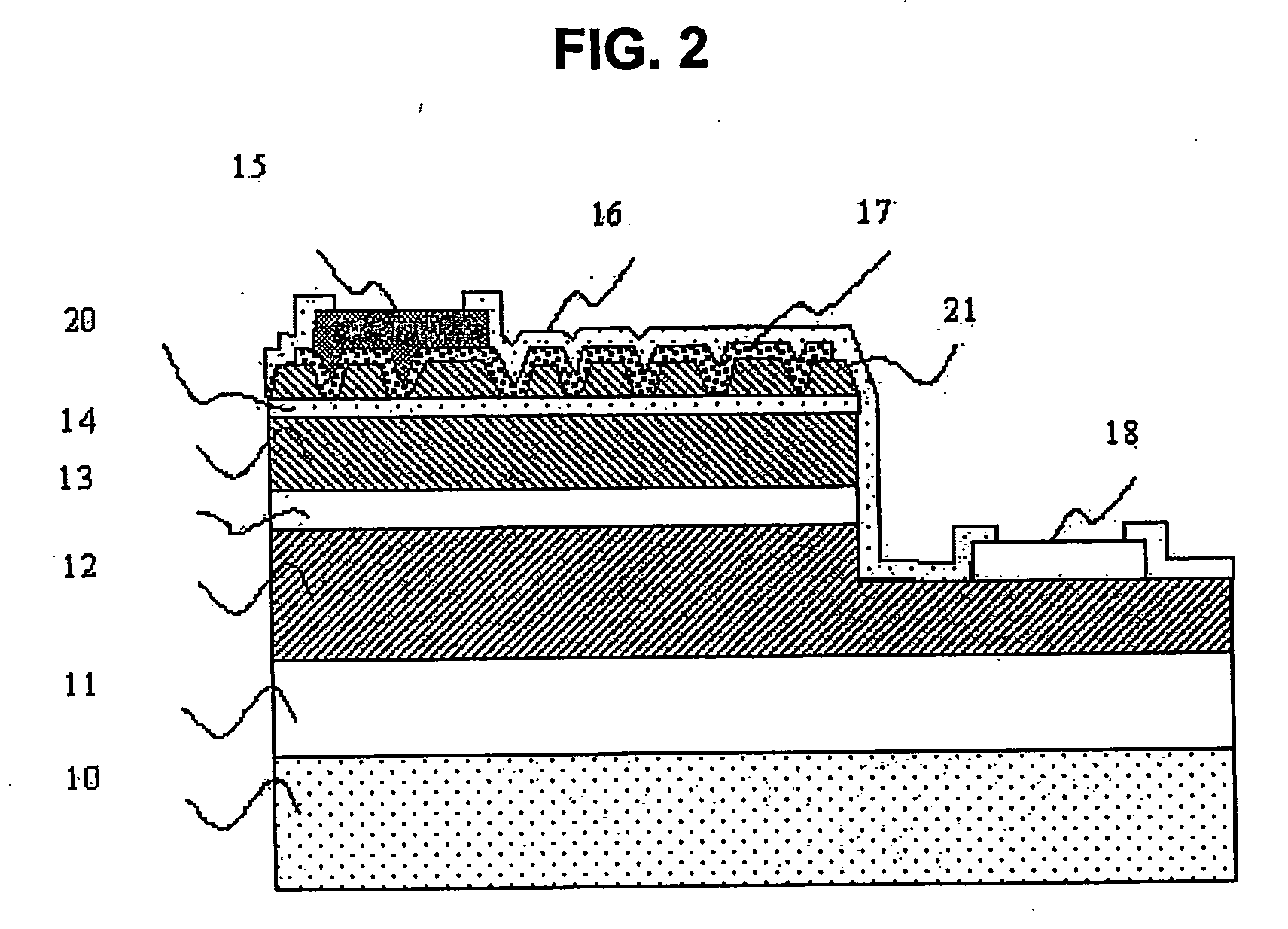
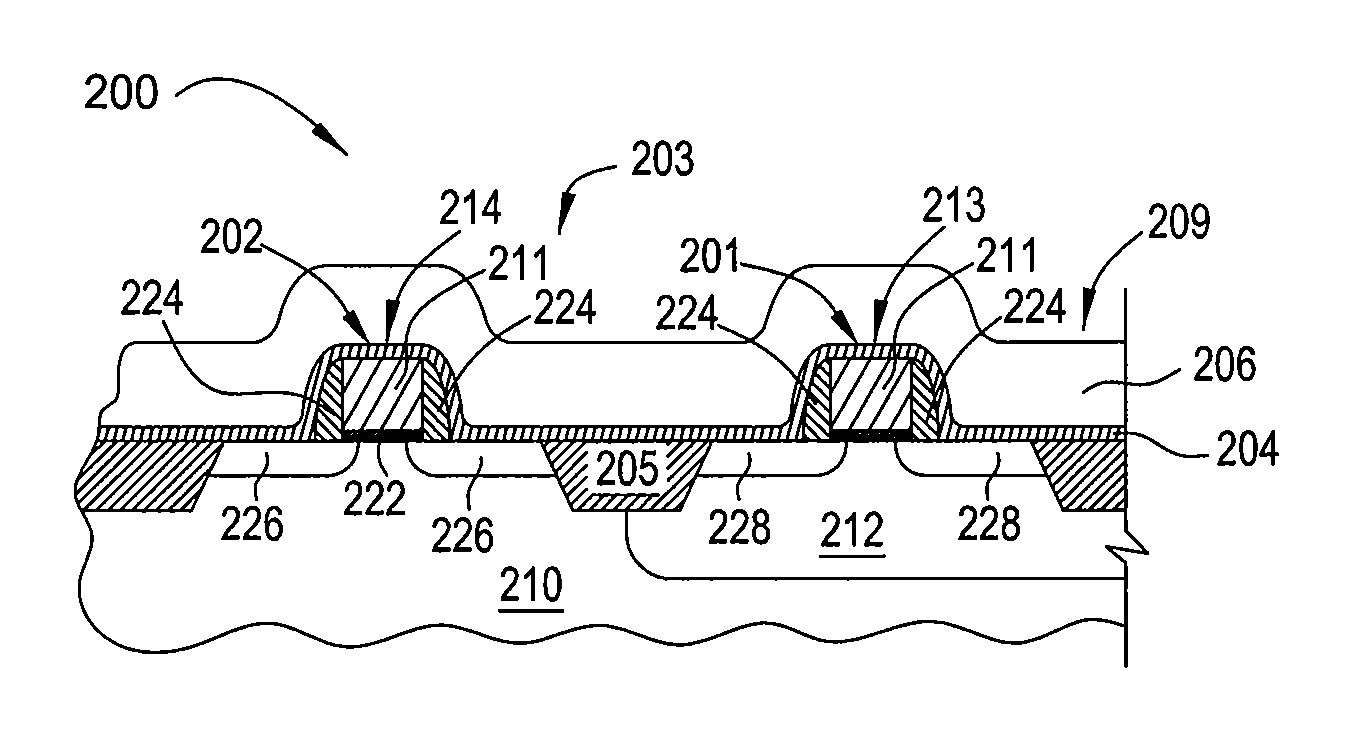
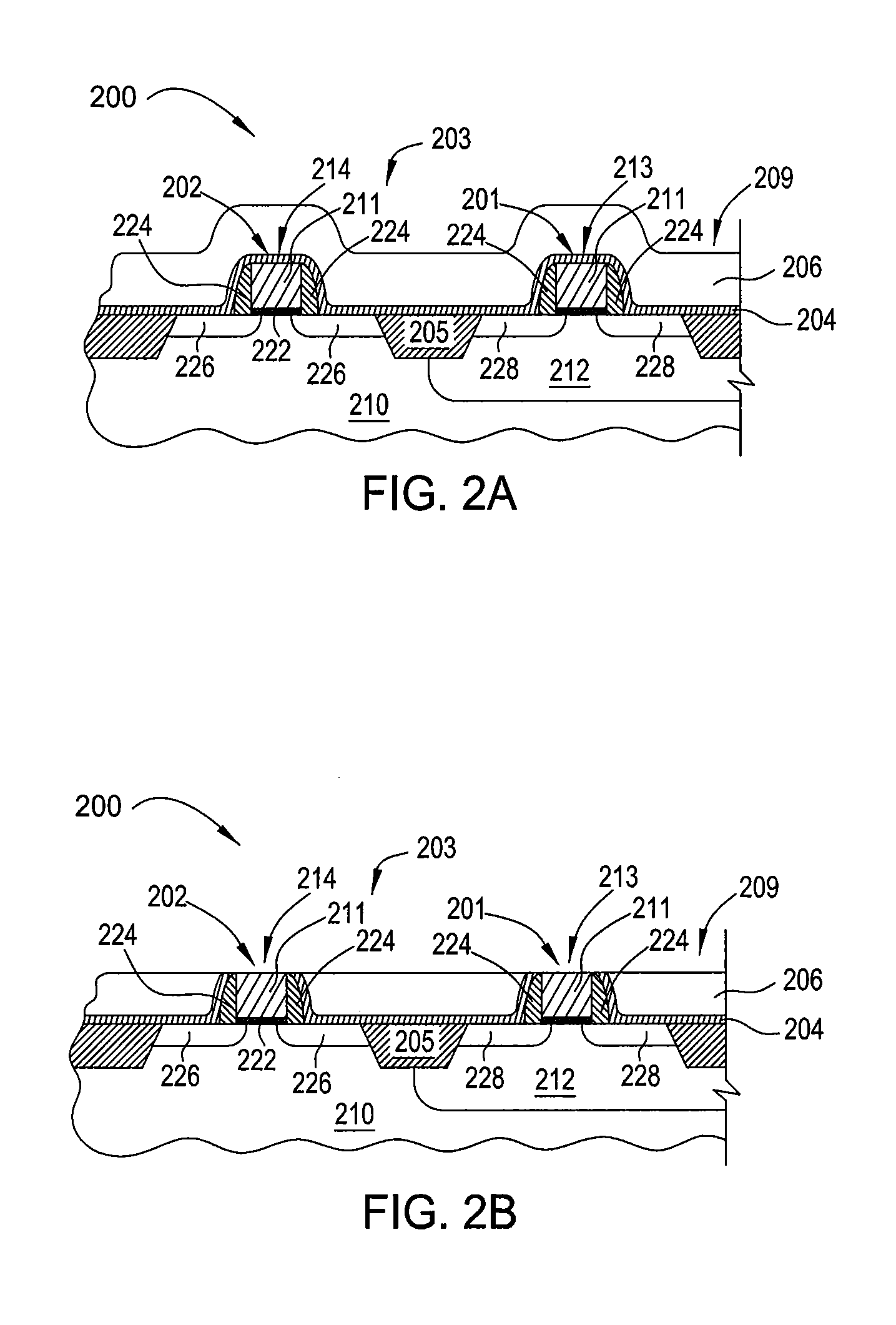
Semiconductor devices and method for manufacturing the same
InactiveUS6844227B2Improve featuresSufficient reliabilityTransistorSolid-state devicesDevice materialCarbon nitride
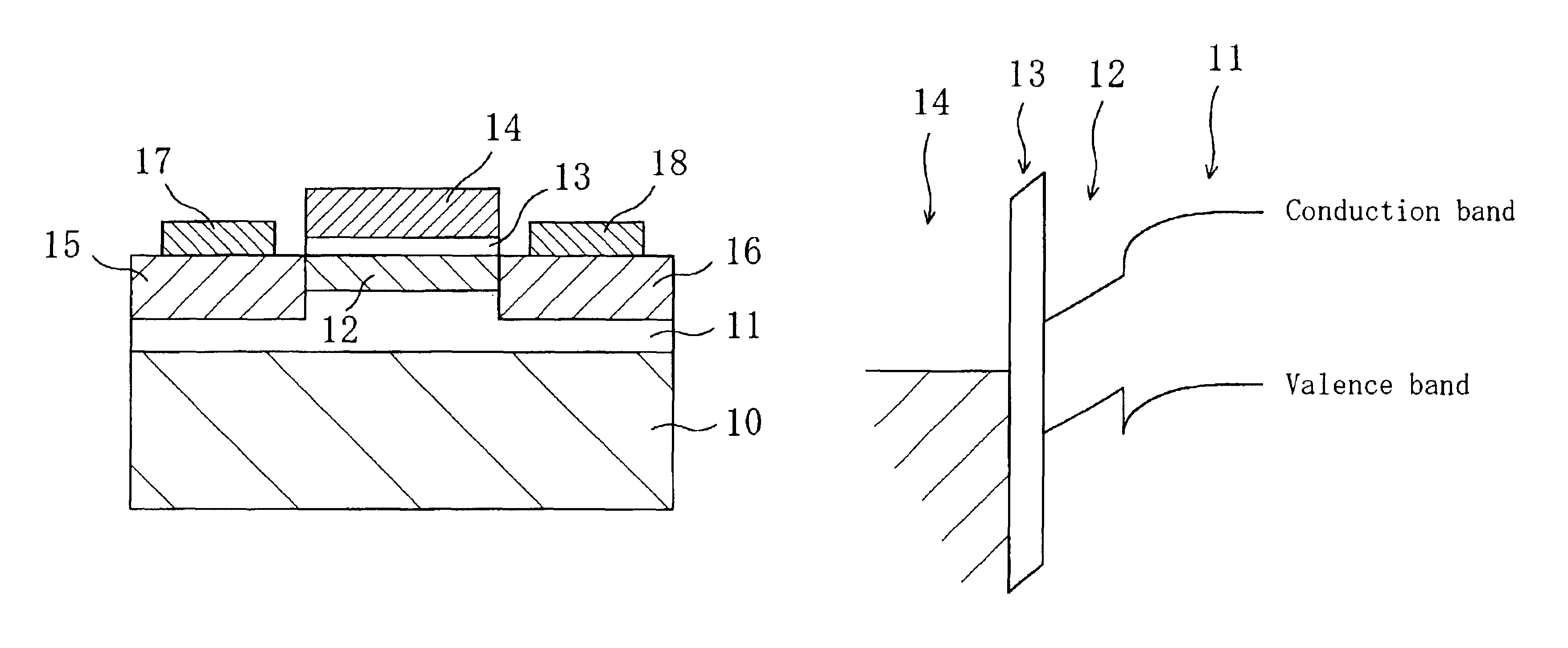
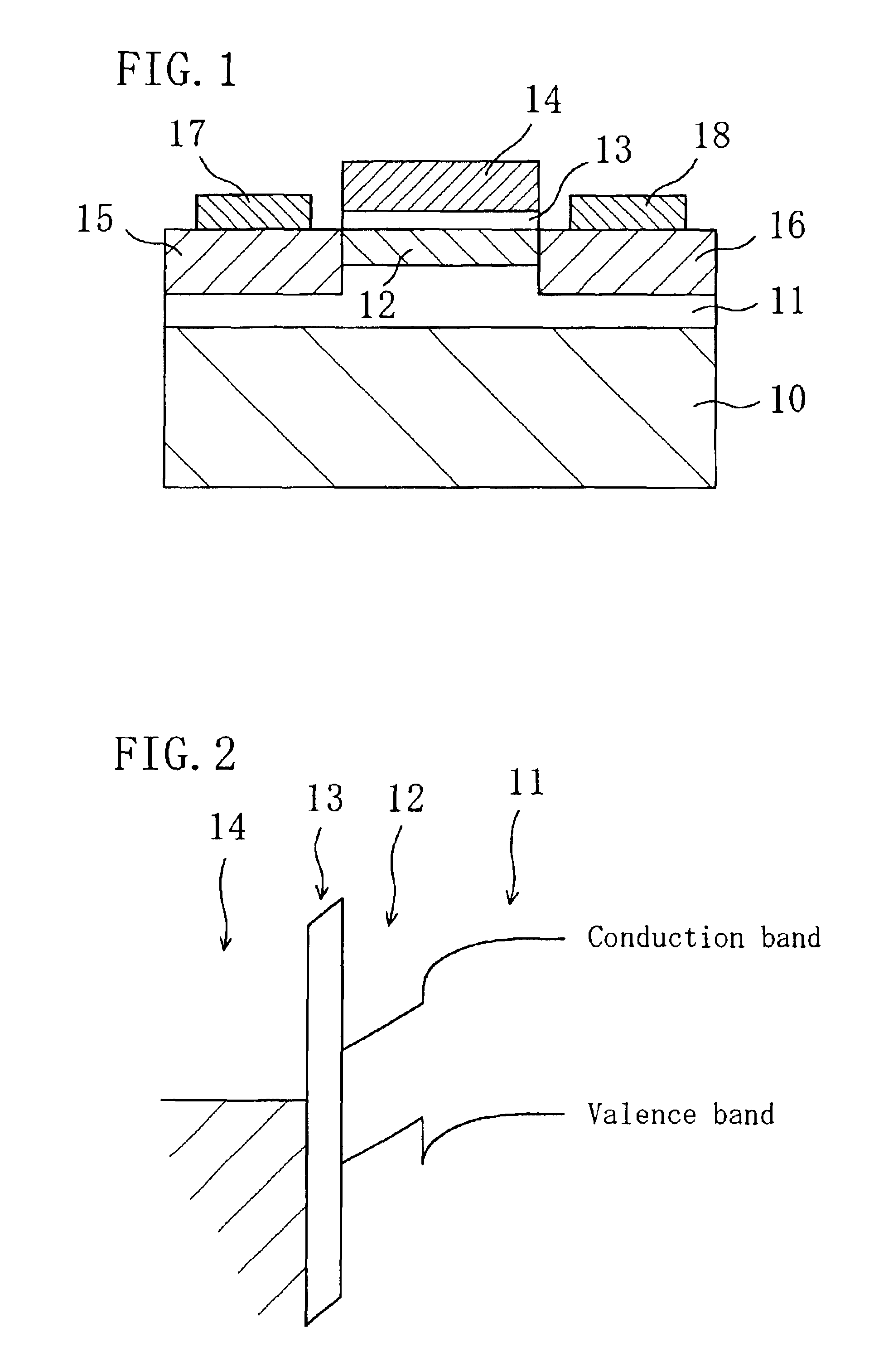
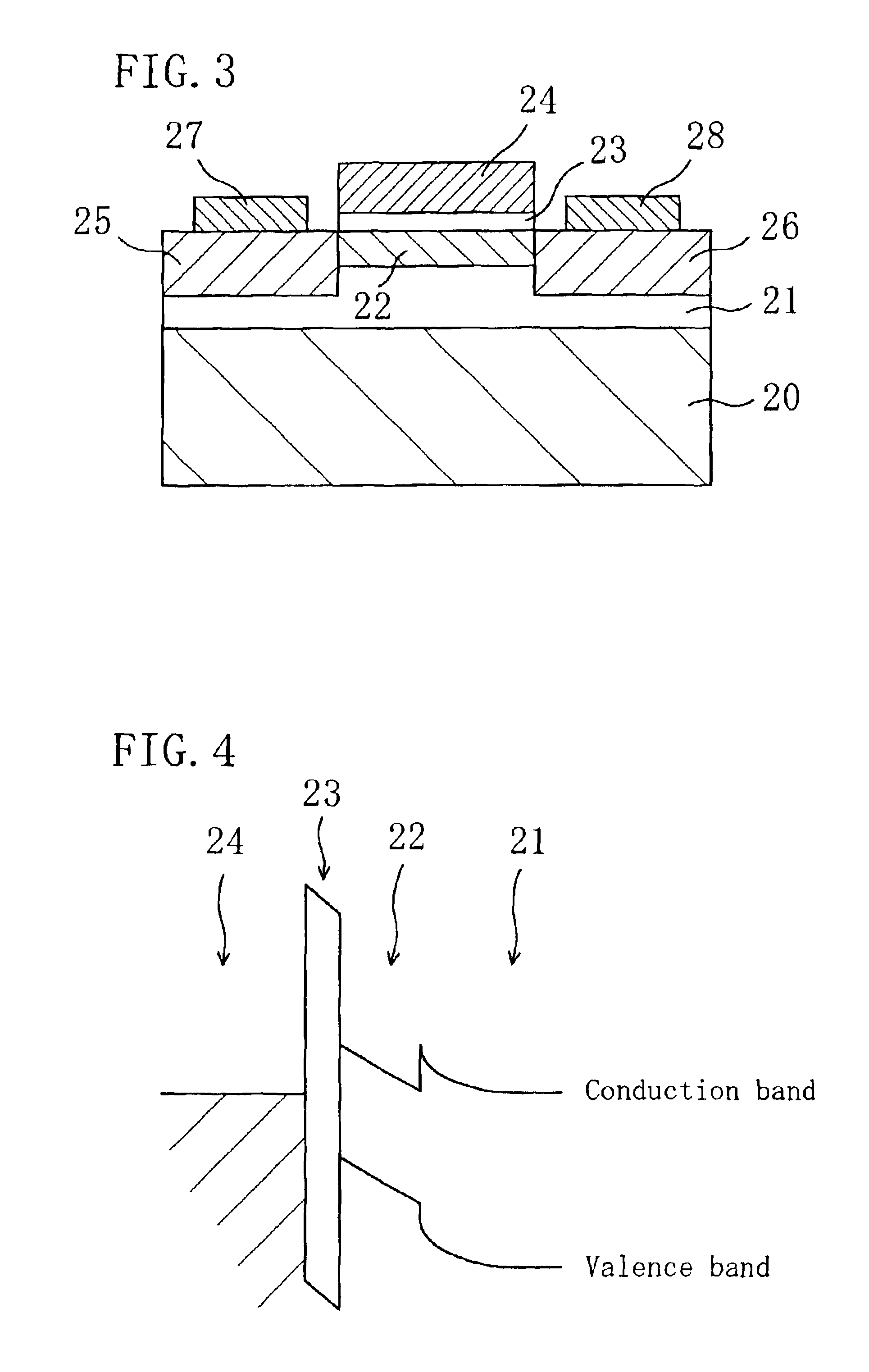
In a field effect transistor, an Si layer, an SiC (Si1-yCy) channel layer, a CN gate insulating film made of a carbon nitride layer (CN) and a gate electrode are deposited in this order on an Si substrate. The thickness of the SiC channel layer is set to a value that is less than or equal to the critical thickness so that a dislocation due to a strain does not occur according to the carbon content. A source region and a drain region are formed on opposite sides of the SiC channel layer, and a source electrode and a drain electrode are provided on the source region and the drain region, respectively.
Owner:PANASONIC CORP
Chip assembly system
ActiveUS20130207268A1Semiconductor/solid-state device detailsSolid-state devicesCarbon nitrideCopper
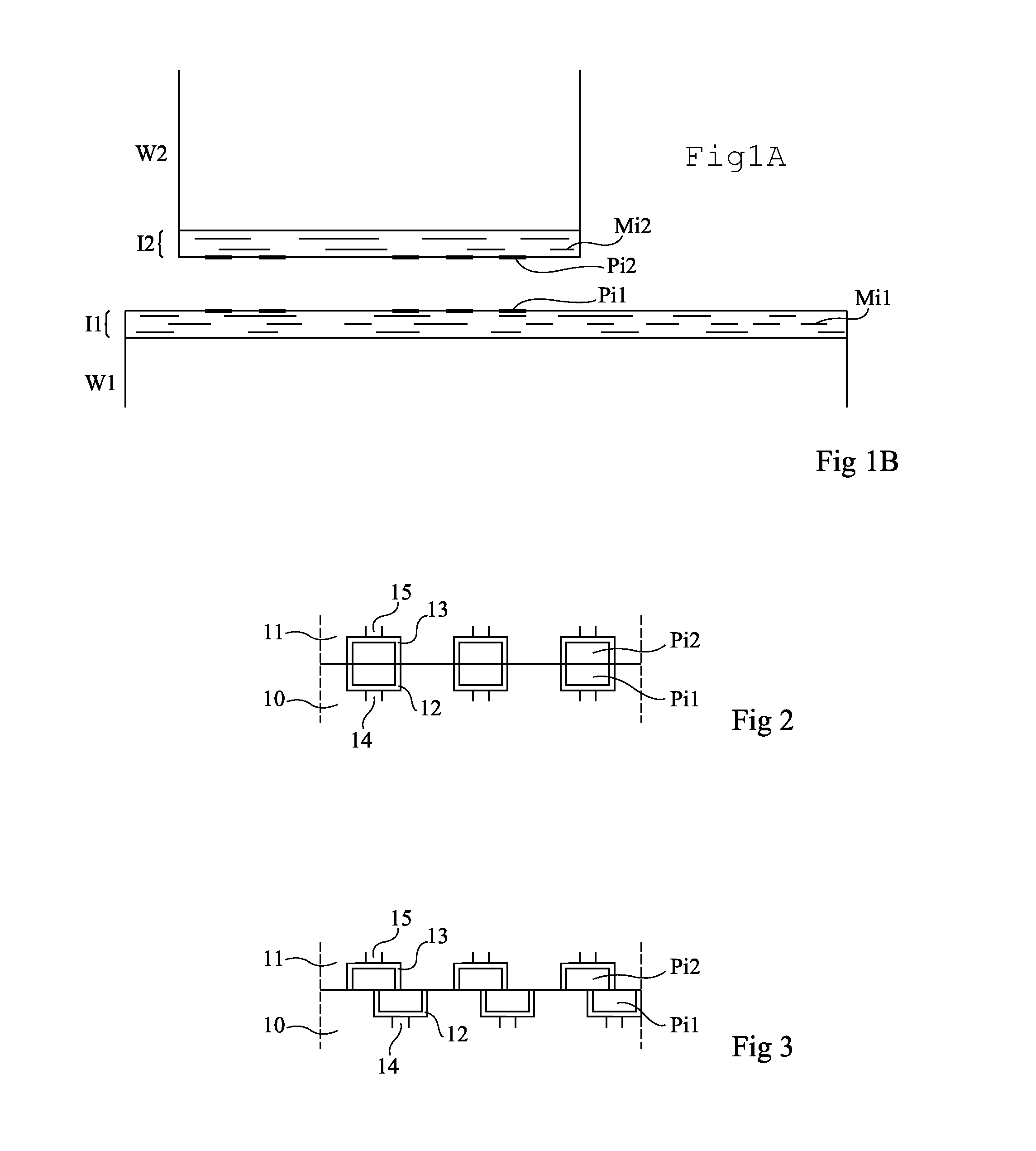
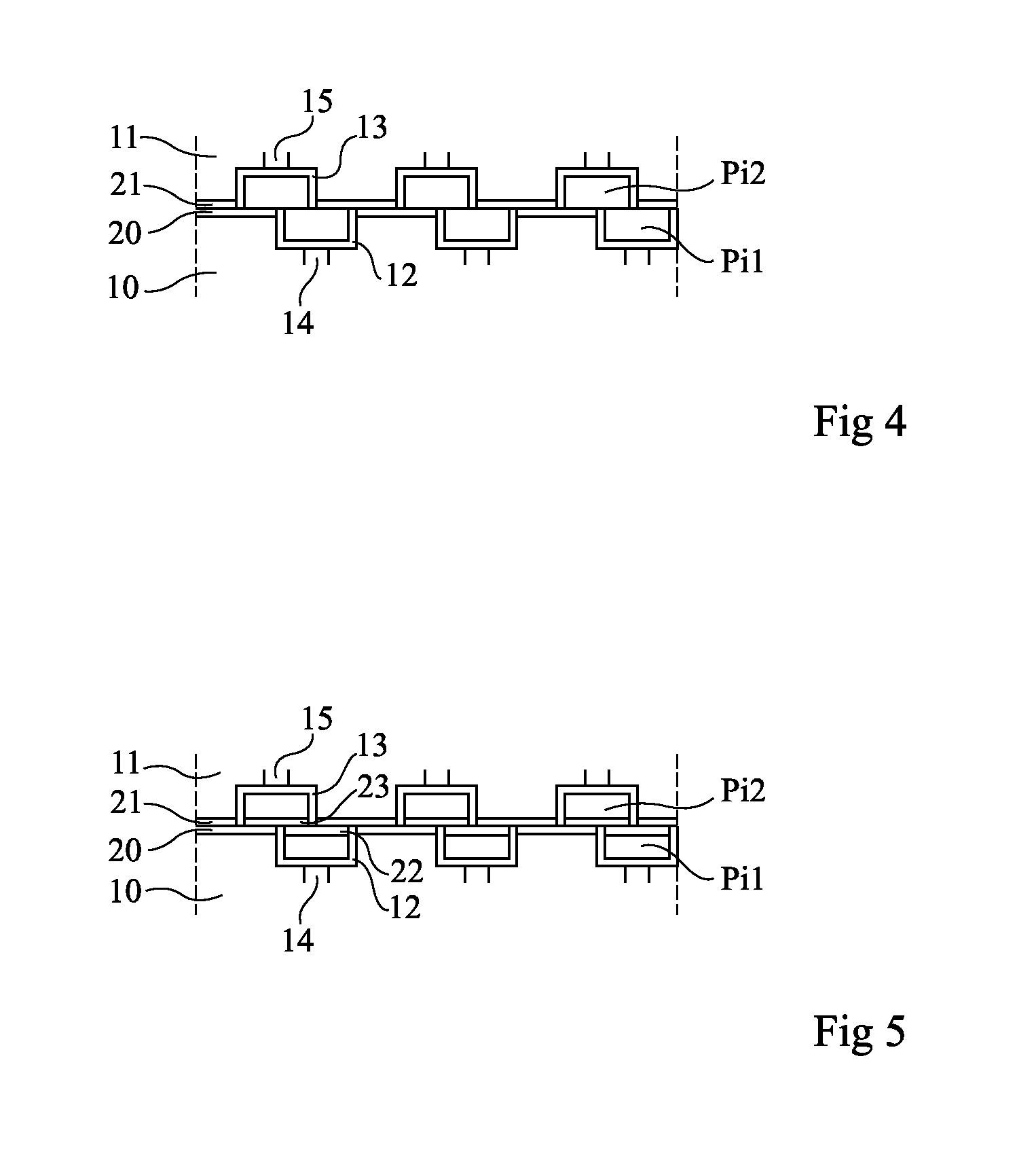
An assembly of semiconductor wafers / chips wherein the adjacent surfaces of the two wafers / chips comprise an insulating layer having opposite copper pads inserted therein. The insulating layer is made of a material selected from the group including silicon nitride and silicon carbon nitride.
Owner:STMICROELECTRONICS (CROLLES 2) SAS
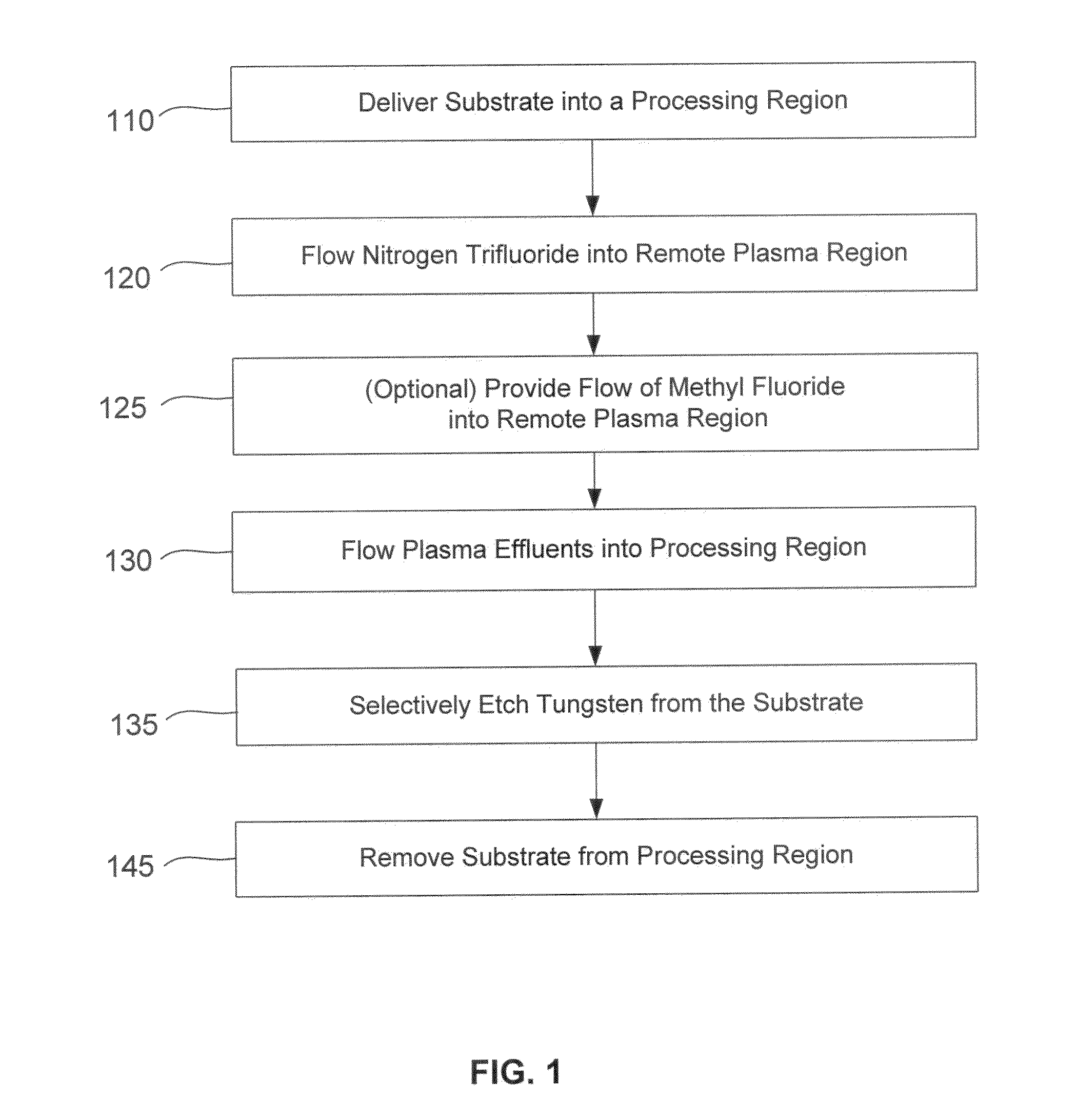
Protective coating comprising boron nitride for refractory material members of an ingot mold for continuous casting of metals
InactiveUS6051058AImprove protectionLow wettabilityMelt-holding vesselsAlkali metal silicate coatingsIngot castingCarbon nitride
The invention relates to a protective coating for a refractory material member of an ingot mold for continuous casting of metals, especially of steel, which consists of a dispersion of particles in a solvent, said particles including essentially boron nitride and at least one of the following metal oxides: zircon, zirconia, alumina and silica, the boron nitride representing between 20 and 50% by weight of said particles.
Owner:USINOR SA +1
III-Nitride Compound Semiconductor Light Emitting Device
InactiveUS20080283865A1Effectively getting outMaximize light efficiencySpectales/gogglesAuxillary optical partsCarbon nitrideEngineering
The present invention relates a III-nitride compound semiconductor light emitting device in which a first layer composed of a carbon-containing compound layer, such as an n-type or p-type silicon carbide (SiC), silicon carbon nitride (SiCN) or carbon nitride layer (CN) layer, is formed on the p-type III-nitride semiconductor layer of the existing III-nitride semiconductor light emitting device, and a second layer composed of a III-nitride semiconductor layer with a given thickness is formed on the first layer.
Owner:EPIVALLEY
Preparation method for phosphor-doped graphite-phase carbon nitride visible-light catalyst
InactiveCN103769213AWide variety of sourcesEasy to prepareOrganic-compounds/hydrides/coordination-complexes catalystsPhosphorCarbon nitride
The invention belongs to the technical field of environment purification photocatalytic material and discloses a preparation method for a phosphor-doped graphite-phase carbon nitride visible-light catalyst. The method comprises the following steps: dispersing melamine into a dilute phosphoric acid solution to enable phosphoric acid molecules to be uniformly adsorbed and dispersed to the surface of melamine particles; then heating to remove solvent water, and after the melamine particles are fully dried, performing programmed heating on the dried melamine particles to 520 DEG C to enable the melamine to generate thermal polycondensation; and cooling and grinding a product to obtain the phosphor-doped graphite-phase carbon nitride visible-light catalyst. The raw materials of the phosphor-doped graphite-phase carbon nitride visible-light catalyst are readily available; the preparation method is simple; complicated equipment is not needed; the preparation method is suitable for large-scale production; the prepared phosphor-doped graphite-phase carbon nitride composite visible-light catalyst is high in visible-light catalysis performance and high in catalysis stability.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Preparation method of carbon nitride supported monoatomic metal catalysis material
ActiveCN106944119AGood dispersionImprove stabilityPhysical/chemical process catalystsHydrogenNitrogen
The invention mainly relates to a preparation method of a carbon nitride supported monoatomic metal catalysis material, concretely relates to a preparation method of a layered graphite phase carbon nitride supported monatomic metal M catalysis material (M is one or two or more of Ag, Pd, Rh and Pt), and belongs to the field of catalysis materials. The carbon nitride supported monoatomic metal catalysis material is prepared through one-step pyrolysis of a carbon and hydrogen precursor and a metal precursor in a carbon and hydrogen precursor and metal precursor complexing manner through using the inhibition of the aggregation of metal atoms by the interaction of a metal center and a ligand, so the stability and the dispersibility of the monatomic metal catalysis material are improved. The metal in the material prepared through the method is supported on the surface of layered graphite phase carbon nitride in a zero-valence monoatomic dispersion form, and the kind and composition of supported metal atoms can be controlled as needed.
Owner:BEIJING NORMAL UNIVERSITY
Low temperature plasma enhanced chemical vapor deposition of conformal silicon carbon nitride and silicon nitride films
ActiveUS20130183835A1Semiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseCarbon nitride
Methods and apparatus for forming conformal silicon nitride films at low temperatures on a substrate are provided. The methods of forming a silicon nitride layer include performing a deposition cycle including flowing a processing gas mixture into a processing chamber having a substrate therein, wherein the processing gas mixture comprises precursor gas molecules having labile silicon to nitrogen, silicon to carbon, or nitrogen to carbon bonds, activating the precursor gas at a temperature between about 20° C. to about 480° C. by preferentially breaking labile bonds to provide one or more reaction sites along a precursor gas molecule, forming a precursor material layer on the substrate, wherein the activated precursor gas molecules bond with a surface on the substrate at the one or more reaction sites, and performing a plasma treatment process on the precursor material layer to form a conformal silicon nitride layer.
Owner:APPLIED MATERIALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com