Graphite-phase carbon nitride (g-C3N4) material and preparation method and application thereof
A graphitic carbon nitride and carbon nitride technology, applied in chemical instruments and methods, nitrogen compounds, separation methods, etc., can solve the problems of affecting the degradation effect, complex control conditions, difficult operation, etc., to promote the reaction mass transfer, The effect of large active specific surface area and strong redox ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A method for preparing graphite phase carbon nitride material, comprising the steps of:
[0053] (1) Weigh 10g of thiourea and ammonium chloride respectively, pour into a 100mL beaker, add 30mL of pure water to dissolve;
[0054] (2) Move the beaker to a magnetic stirring water bath, continue stirring at 70°C for 60 minutes, and evaporate most of the water to obtain a paste;
[0055] (3) Put the obtained paste-like object into a vacuum drying oven, and vacuum-dry it at 60° C. for 16 hours to completely remove water to obtain a solid;
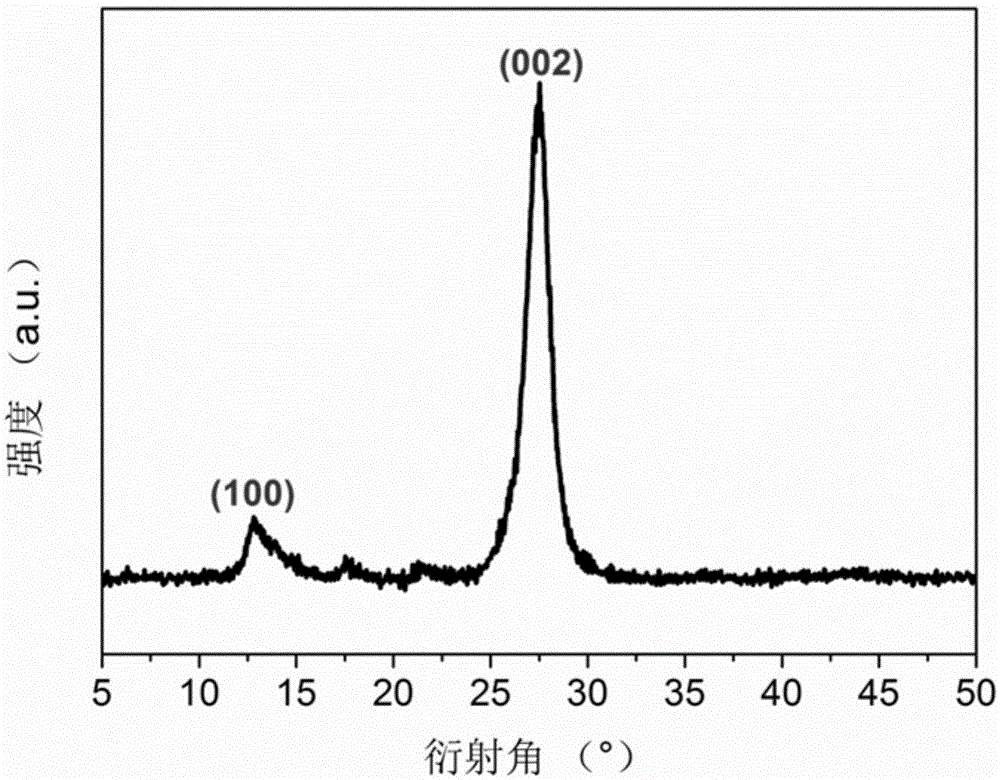
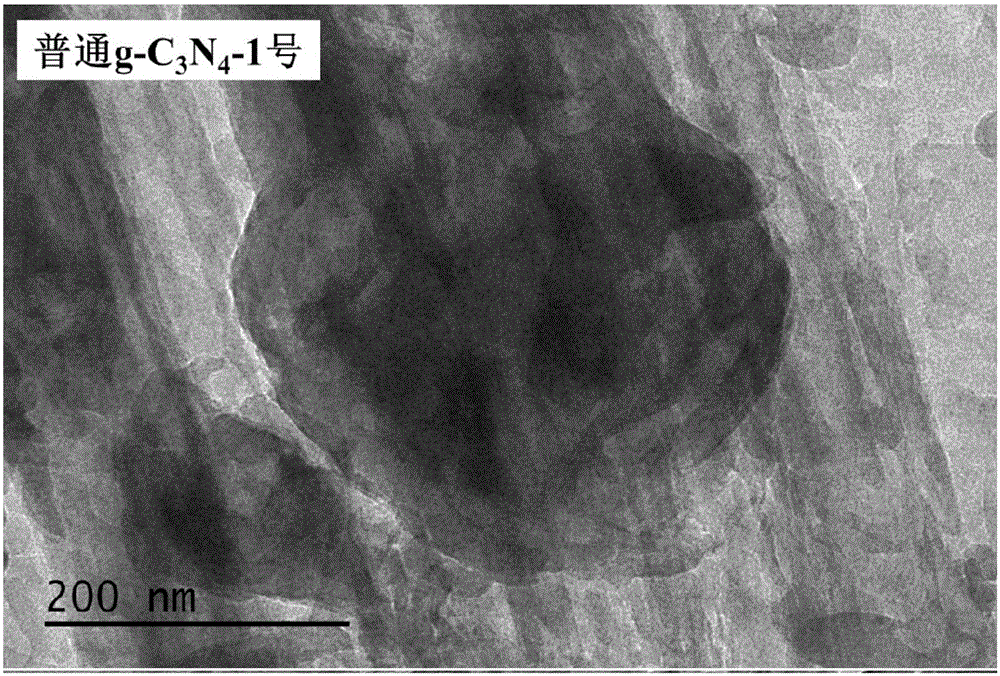
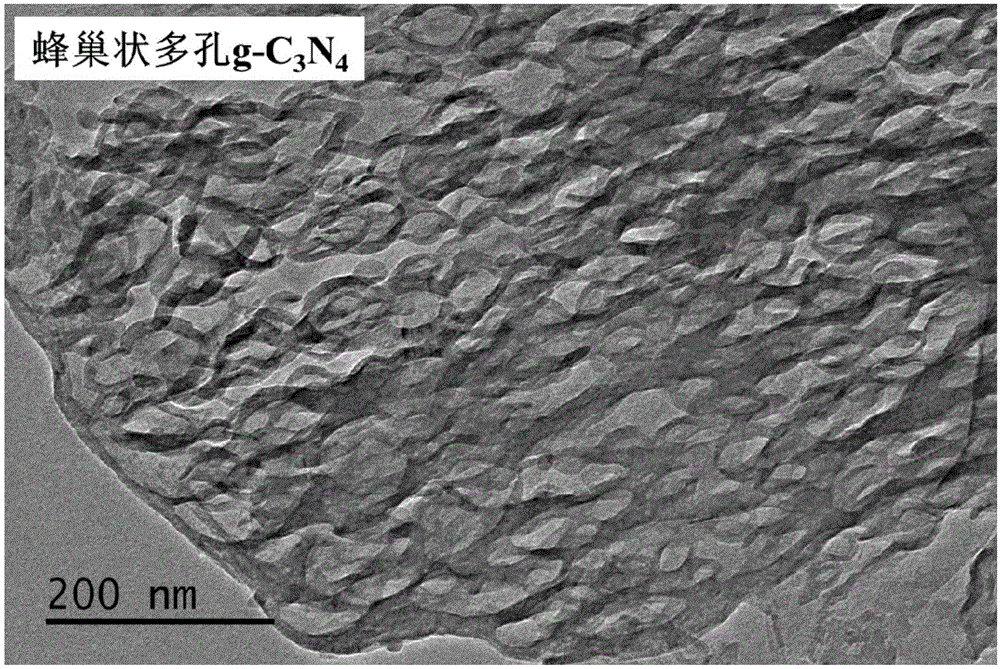
[0056] (4) Put the obtained solid into a corundum crucible, place it in a muffle furnace, raise the temperature from room temperature to 550°C at a rate of 15°C / min, keep it for 2 hours and then cool it naturally, the obtained powder is honeycomb porous graphite phase nitrogen carbonized material (g-C 3 N 4 ).
Embodiment 2
[0060] A method for preparing graphite phase carbon nitride material, comprising the steps of:
[0061] (1) Take by weighing the mixture of 10g dicyandiamide and 15g ammonium carbonate and ammonium bicarbonate (mass ratio 1:1) respectively, dissolve in 60mL ethanol;
[0062] (2) The mixture of step (1) was continuously heated and stirred at 30° C. for 6 h, and most of the ethanol was removed by evaporation;
[0063] (3) Freeze and vacuum-dry the product of step (2) for 48 hours at -50° C., completely remove ethanol to obtain a solid;
[0064] (4) Put the dried solid in a corundum crucible, put it into a tube furnace and blow in air, raise the temperature to 550°C at a rate of 1°C / min, keep it for 4 hours and then cool it naturally, the obtained powder is honeycomb-shaped porous Graphite phase carbon nitride material (g-C 3 N 4 ).
Embodiment 3
[0068] A method for preparing graphite phase carbon nitride material, comprising the steps of:
[0069] (1) Weigh 20g of urea and 8g of ammonium oxalate respectively, pour them into a mixed solution of 40mL of ethanol and water, and the volume ratio of ethanol and water is 1:1;
[0070] (2) After the mixture in step (1) was continuously stirred at 90° C. for 0.5 h, most of the water and ethanol were evaporated to remove;
[0071] (3) The product of step (2) was vacuum-dried at 80° C. for 24 hours, and the ethanol was completely removed to obtain a solid;
[0072] (4) Put the dried solid in a corundum crucible, put it into a muffle furnace, raise the temperature from room temperature to 700°C at a heating rate of 8°C / min, and keep it for 1.5h, then cool naturally, and the obtained powder is honeycomb shape Porous graphitic carbon nitride material (g-C 3 N 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com