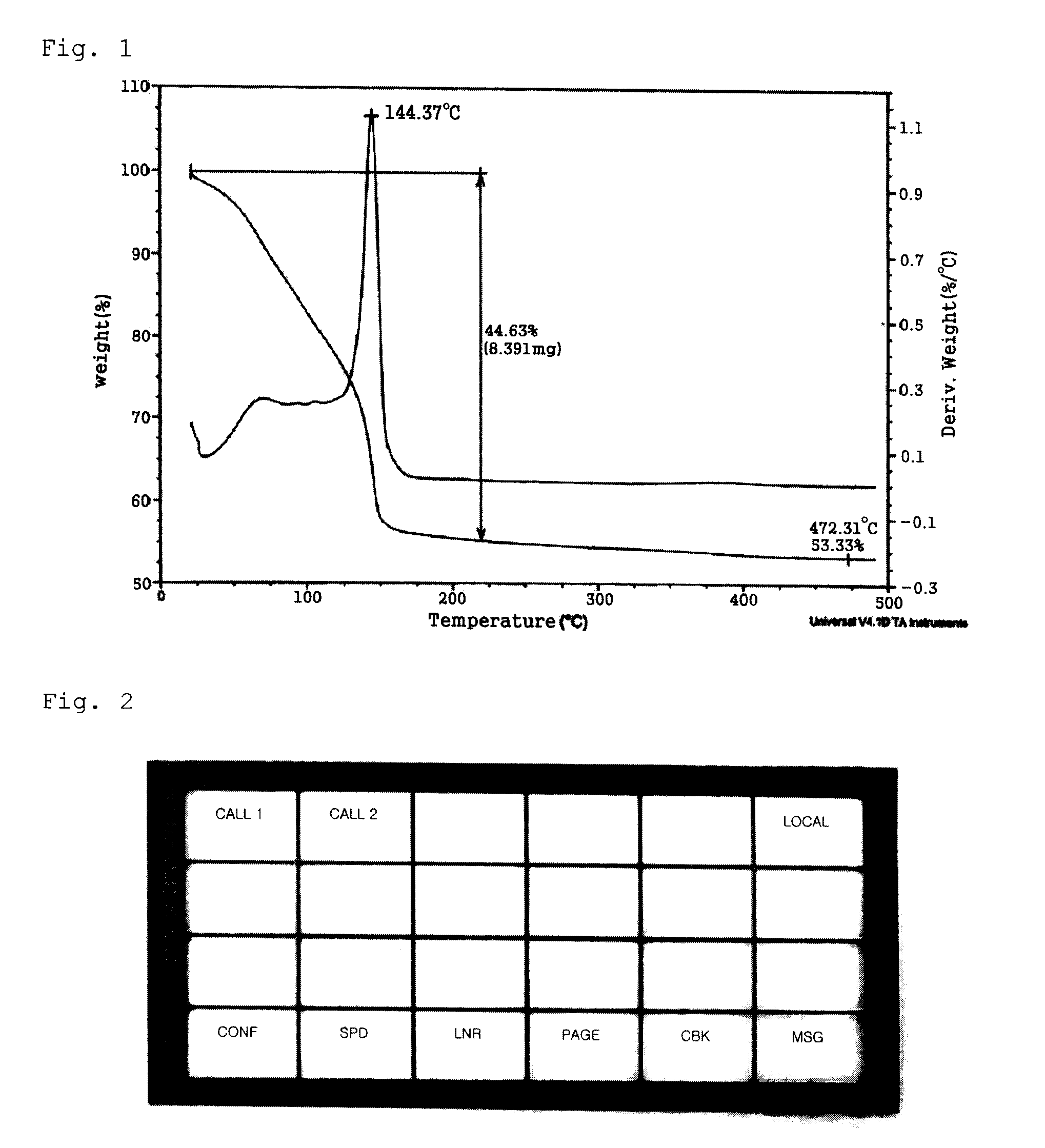
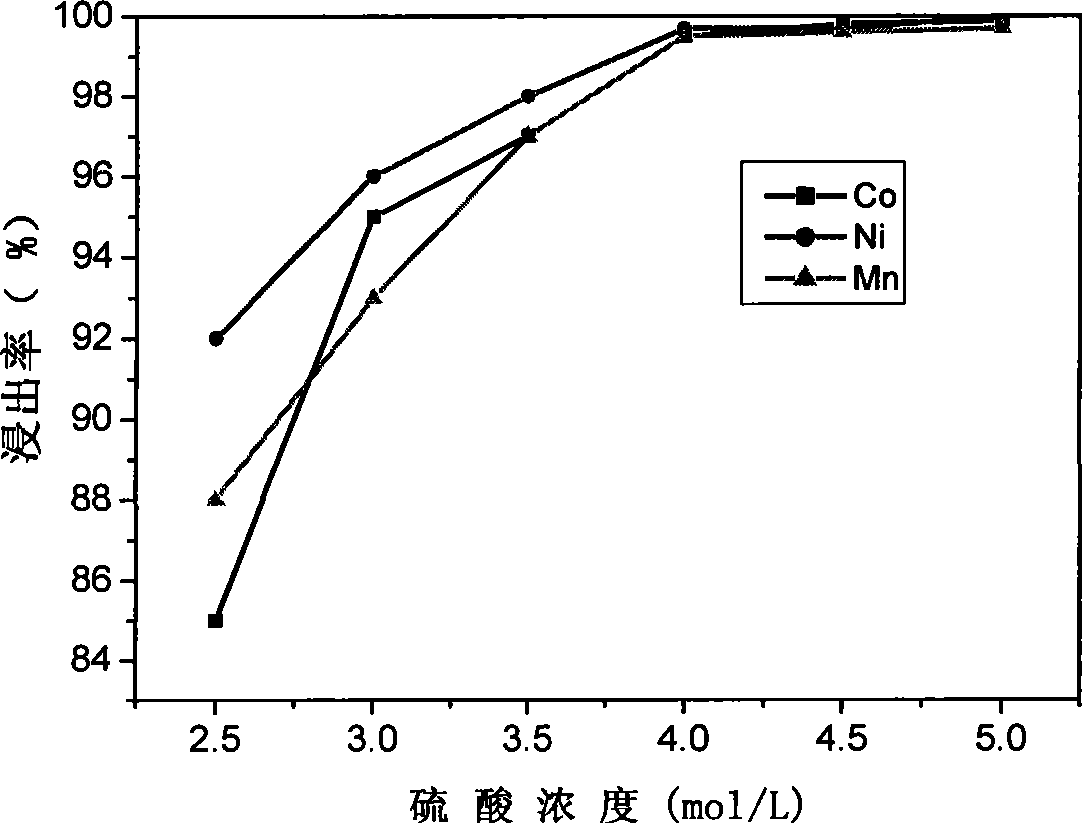
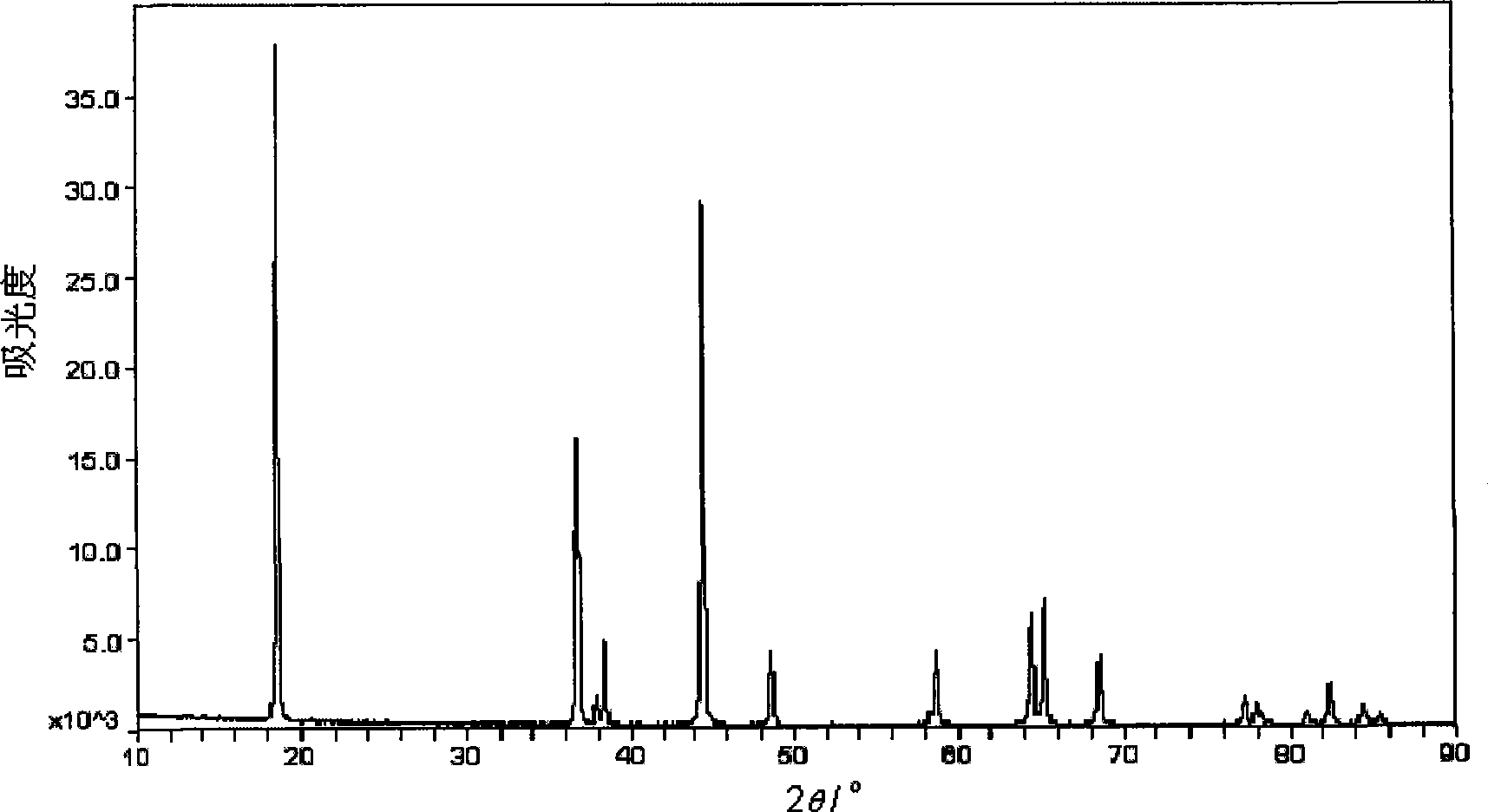
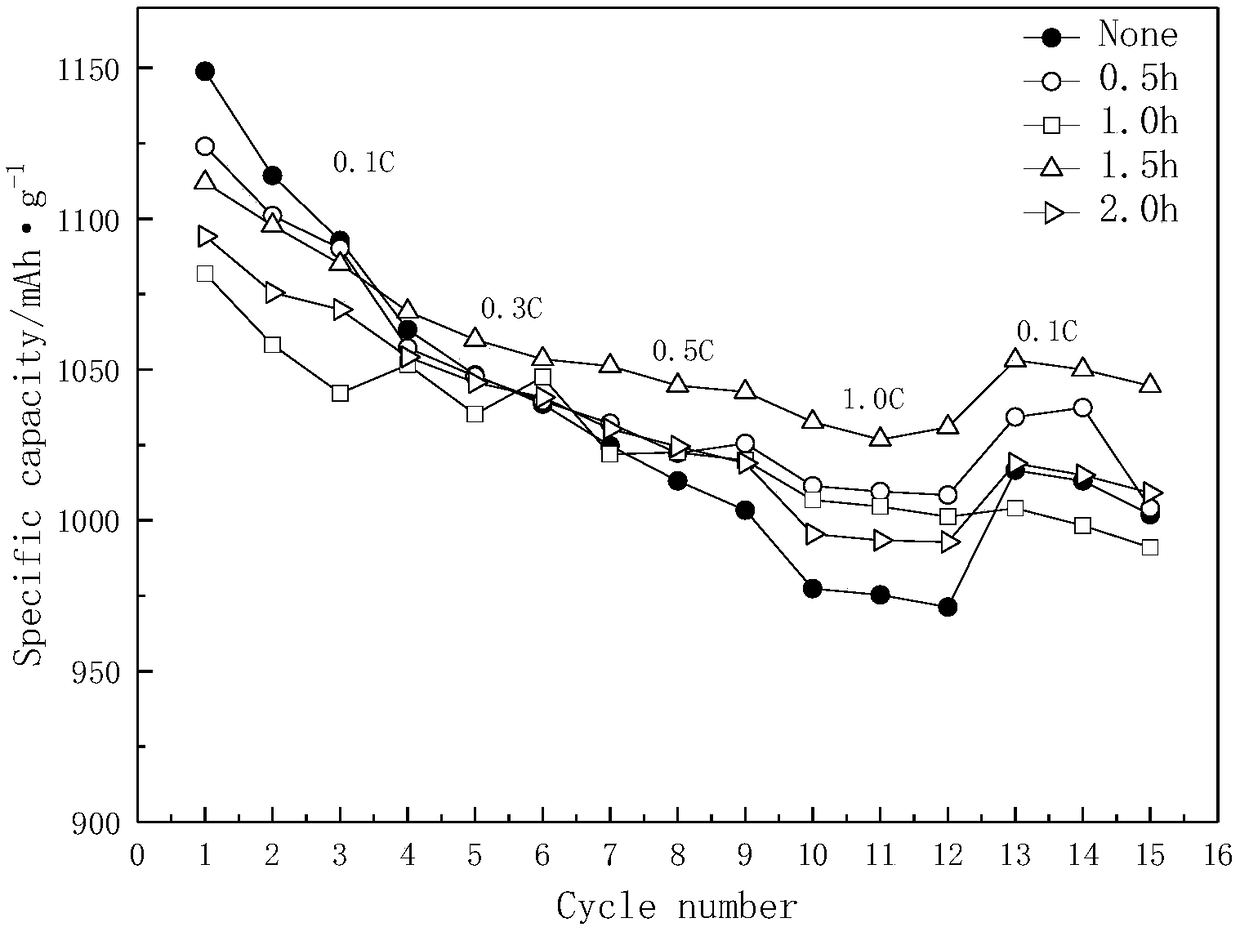
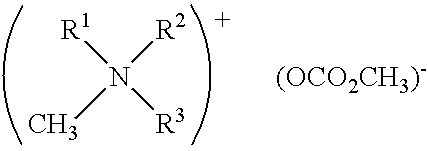Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1357 results about "Ammonium carbonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ammonium carbonate is a salt with the chemical formula (NH₄)₂CO₃. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn.
Method for preparing lithium cobaltate by directly using invalid lithium ion battery
InactiveCN102030375AReduce dispersionHigh purityCell electrodesCobalt compoundsElectrical batteryPotassium hydroxide
The invention provides a method for preparing lithium cobaltate by directly using an invalid lithium ion battery. The method comprises the following steps: crushing the invalid lithium ion battery or scraps generated when a lithium cobaltate battery is produced by a mechanical crusher at normal temperature; adding water and one or more of acetic acid, sulfuric acid, hydrochloric acid or nitric acid to produce mixed aqueous solution of the battery scraps and acid; filling the mixed aqueous solution into a hermetic pressure reactor, and controlling the temperature in the reactor to be between 50 and 150 DEG C; introducing or adding one leaching additive of sulfur dioxide or hydrogen, or adding hydrazine hydrate; stirring and leaching, cooling, and filtering; adding one precipitator of sodium carbonate, potassium carbonate and ammonium carbonate, or adding composite precipitator consisting of one of the sodium carbonate, the potassium carbonate and the ammonium carbonate and one of sodium hydroxide and potassium hydroxide to obtain mixture of lithium carbonate, cobalt carbonate and cobalt hydroxide; drying and calcining at high temperature to produce a lithium cobaltate product. The method is particularly suitable for the treatment scale of medium-sized and small enterprises, and is an effective method for directly materializing cobalt secondary resources.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Conductive Inks and Manufacturing Method Thereof
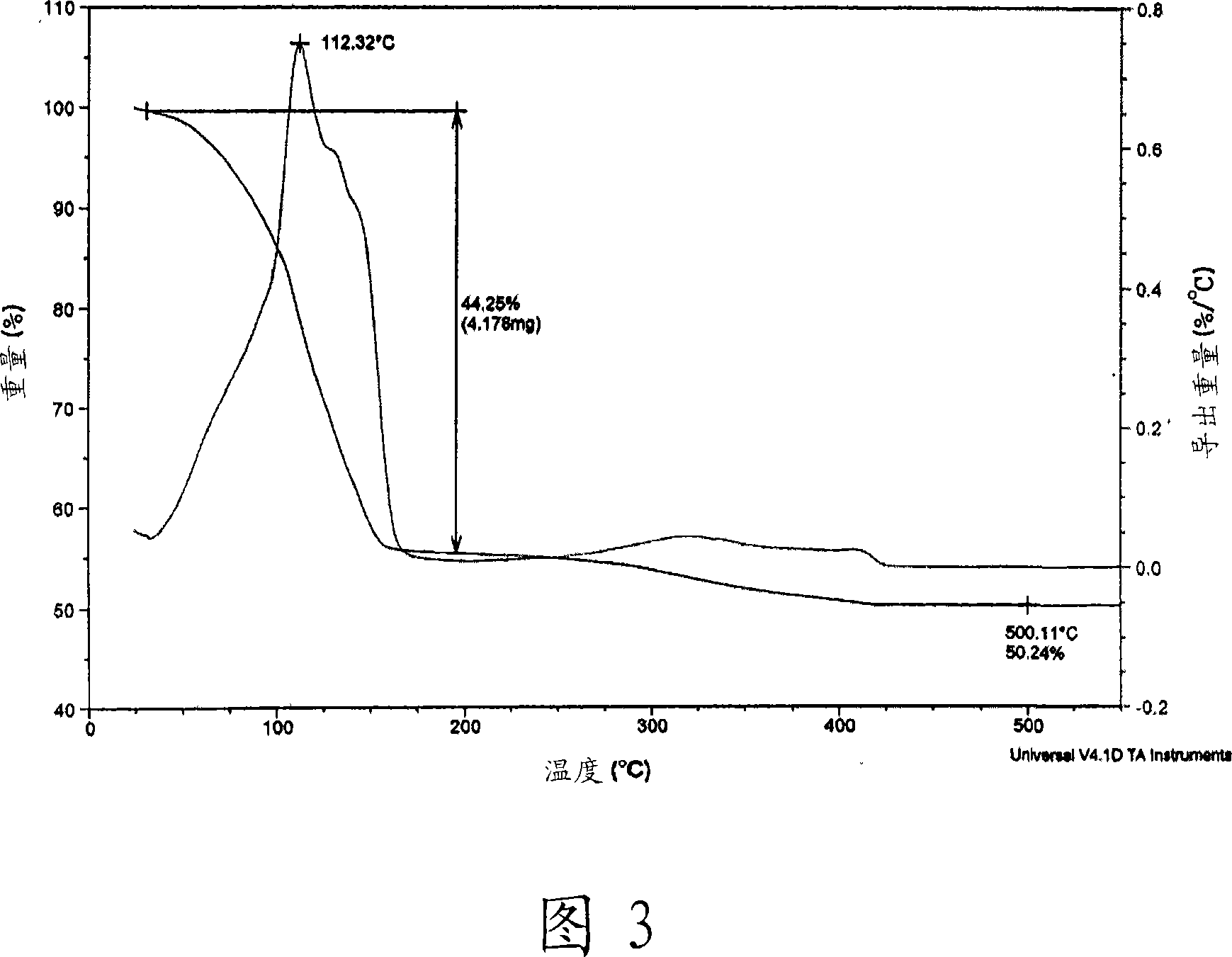
ActiveUS20080206488A1Improve stabilityImprove solubilityElectric discharge heatingConductive materialAmmonium carbonateAmmonium carbamate
The present invention relates to a variety of conductive ink compositions comprising a metal complex compound having a special structure and an additive and a method for preparing the same, more particularly to conductive ink compositions comprising a metal complex compound obtained by reacting a metal or metal compound with an ammonium carbamate- or ammonium carbonate-based compound and an additive and a method for preparing the same.
Owner:INKTEC CO LTD
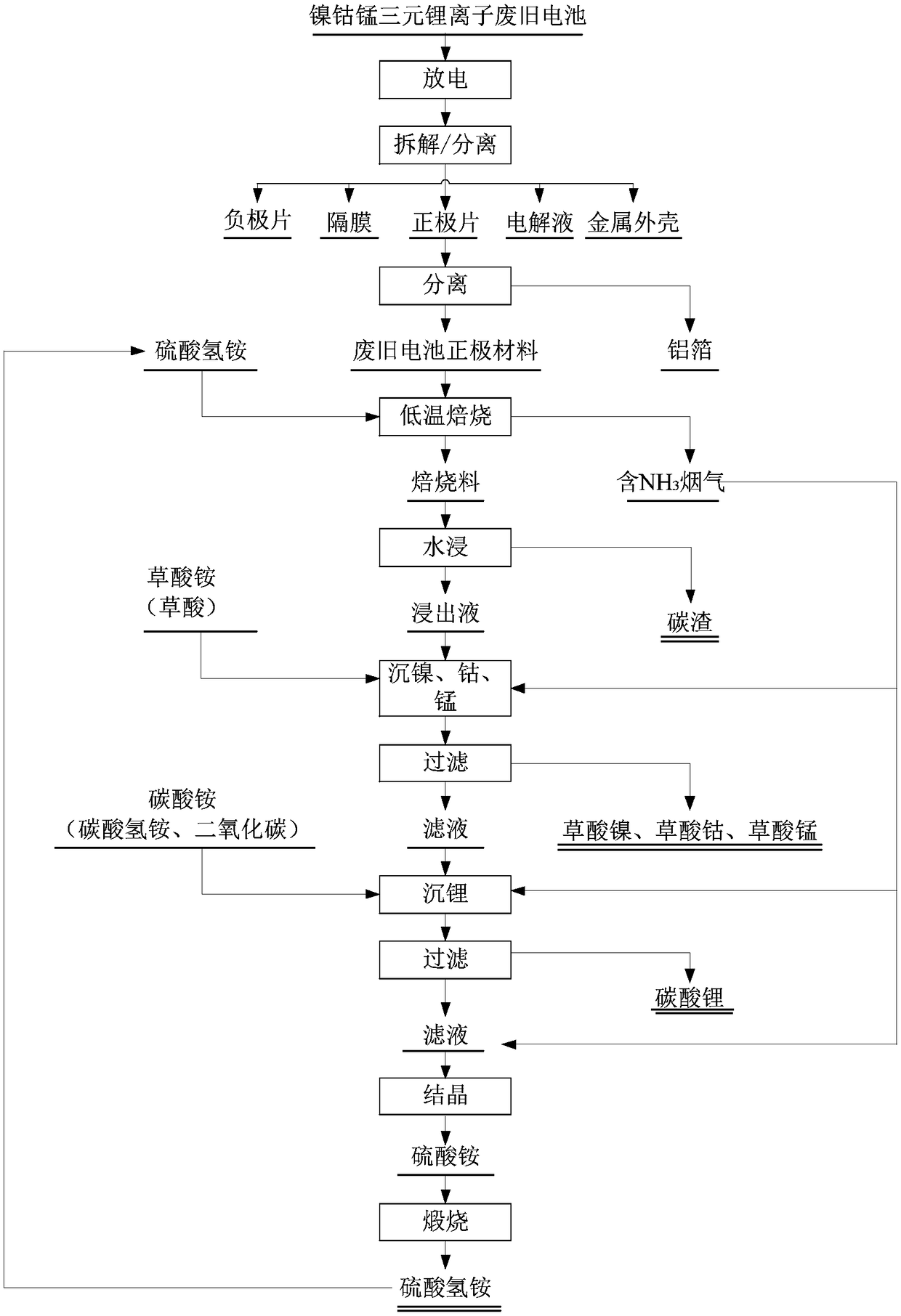
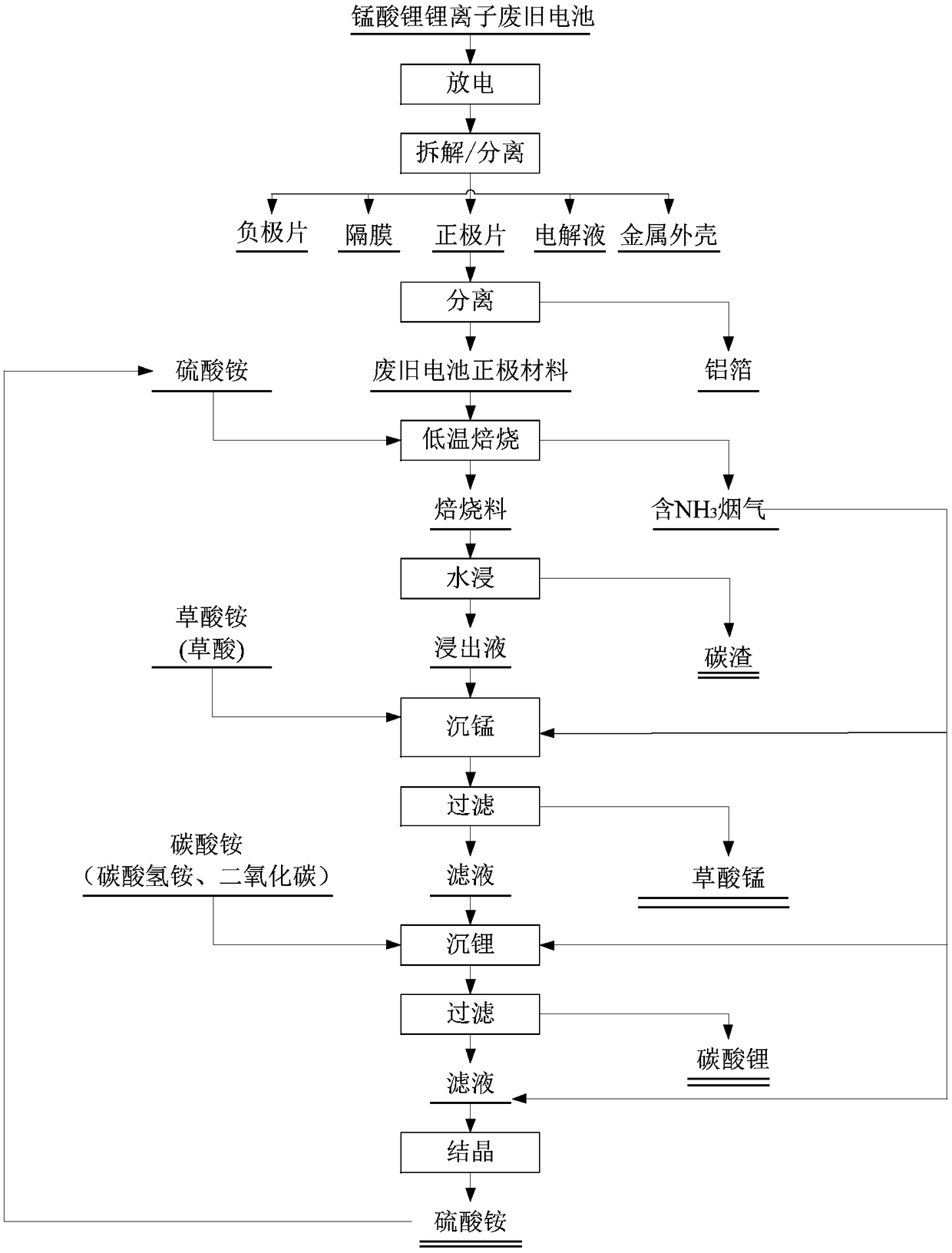
Method for preparing nickel and cobalt doped lithium manganate by using waste and old lithium ionic cell as raw material
InactiveCN101450815ASimultaneous recyclingShort processManganates/permanganatesManganateManganese oxide
The invention discloses a method for preparing lithium nickel cobalt manganese oxide by taking a waste lithium ion battery as a raw material. The method is mainly characterized in that a waste lithium ion battery taking the lithium nickel cobalt manganese oxide, lithium nickel cobalt oxide and so on as a battery positive material is selected as the raw material and is pretreated through disassembly, separation, crushing, screening and so on, and then processes such as adhesive removal at high temperature and aluminum removal by sodium hydroxide are adopted to obtain an inactivated positive material containing nickel, cobalt and manganese; then a sulfuric acid and hydrogen peroxide system is adopted to leach, and P204 is adopted to remove impurities by extraction to obtain pure nickel, cobalt and manganese solution, and proper manganese sulfate, nickel sulfate or cobalt sulfate is blended to ensure that the mol ratio of nickel, cobalt and manganese elements in the solution is 1: 1: 1; and then ammonium carbonate is adopted to adjust the pH value to form a nickel cobalt manganese carbonate precursor, and then a proper amount of lithium carbonate is blended for high temperature sintering to synthesize a lithium nickel cobalt manganese oxide battery material. The first discharge capacity of the material is 150 mAh / g, the discharge capacity is still kept more than 130mAh / g after the circulation for 30 times, and the material has good electrochemical performance.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
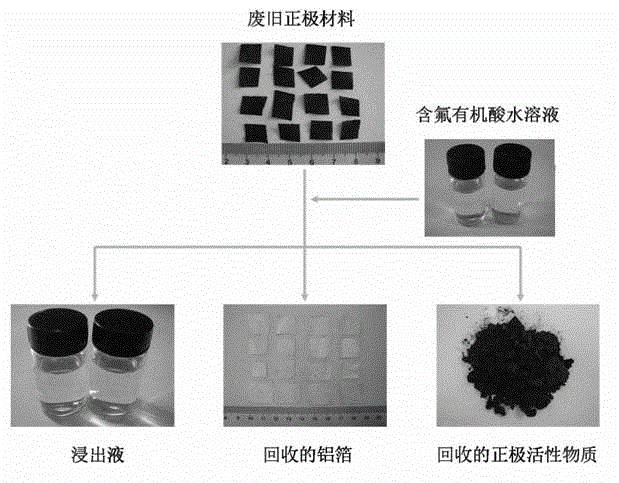
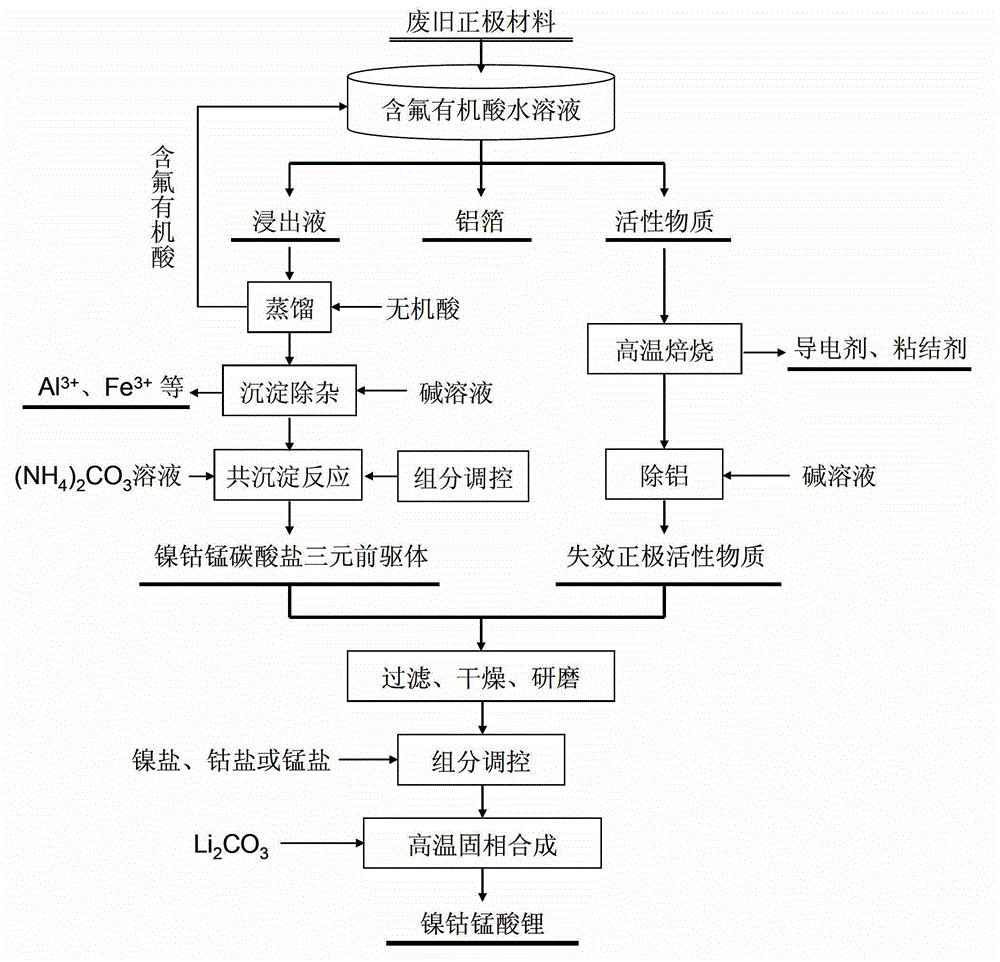
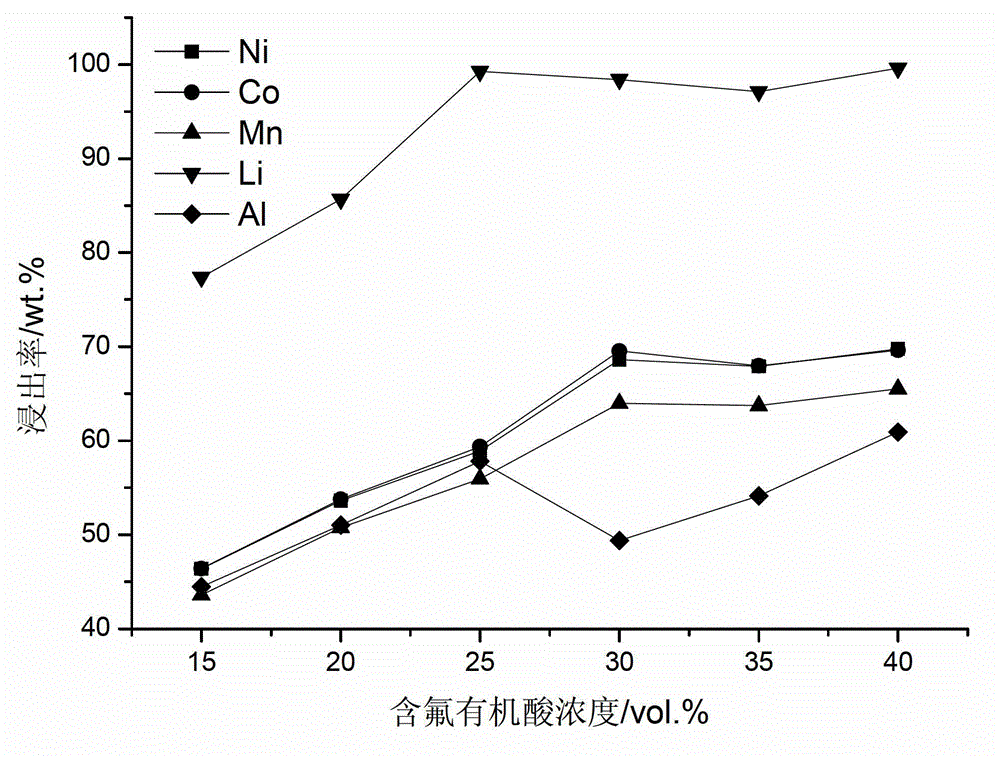
Full-component resource reclamation method for waste positive electrode materials of lithium ion batteries
ActiveCN102751549AAvoid secondary pollutionImprove separation efficiencyWaste accumulators reclaimingBattery recyclingRecovery methodDistillation
The invention provides a full-component resource reclamation method for waste positive electrode materials of lithium ion batteries. The method comprises the following steps: 1) separating active substances and aluminum foils in waste positive electrode materials of lithium ion batteries by using an aqueous solution of fluorine-containing organic acid and carrying out liquid-solid-solid separation so as to obtain leachate, the lithium-containing active substances and the aluminum foils; 2) respectively carrying out high temperature roasting and impurity removal with alkali liquor on the lithium-containing active substances; 3) respectively carrying out recovery of the fluorine-containing organic acid through addition of acid and distillation, deposition of impurity ions through addition of alkali and ammonium carbonate coprecipitation on the leachate so as to prepare nickel-cobalt-manganese carbonate ternary precursor; and 4) carrying out component regulation on a mixture of the treated active substances and the nickel-cobalt-manganese carbonate ternary precursor, adding lithium carbonate in a certain proportion and carrying out high temperature solid phase sintering so as to prepare a lithium nickel cobalt manganese oxide ternary positive electrode material. The method provided in the invention has the following advantages: the application scope of the method is wide; separation efficiency of the lithium-containing active substances and the aluminum foils is high; short-flow direct re-preparation of positive electrode materials in waste lithium ion batteries is realized; and the method is applicable to large-scale resource reclamation of waste lithium ion batteries.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
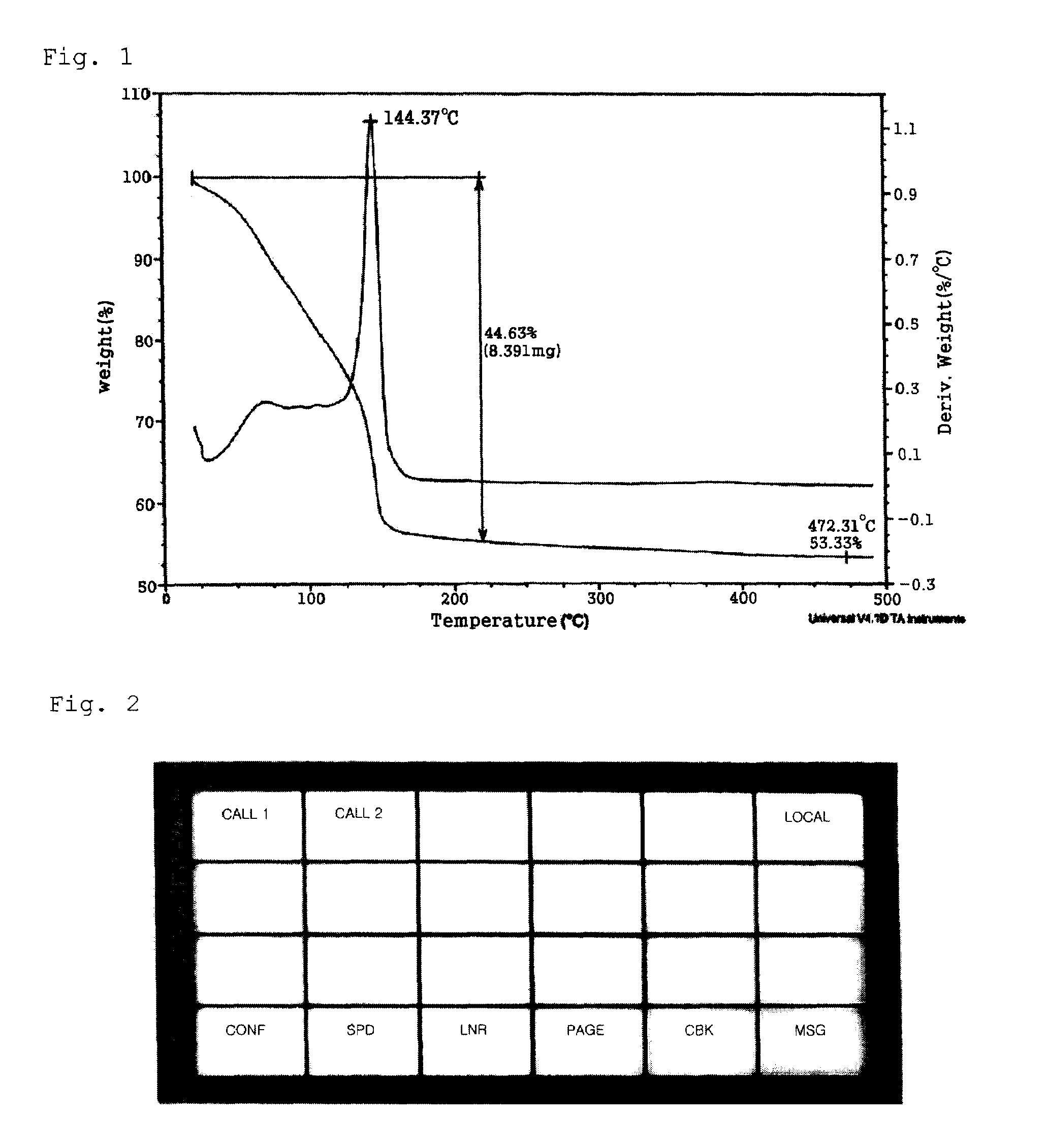
Conductive inks and manufacturing method thereof
The present invention relates to a variety of conductive ink compositions comprising a metal complex compound having a special structure and an additive and a method for preparing the same, more particularly to conductive ink compositions comprising a metal complex compound obtained by reacting a metal or metal compound with an ammonium carbamate- or ammonium carbonate-based compound and an additive and a method for preparing the same.
Owner:INKTEC CO LTD
Conductive inks and manufacturing method thereof
ActiveUS7691294B2Easy to controlWell formedElectric discharge heatingFilament handlingAmmonium carbonateAmmonium carbamate
The present invention relates to a variety of conductive ink compositions comprising a metal complex compound having a special structure and an additive and a method for preparing the same, more particularly to conductive ink compositions comprising a metal complex compound obtained by reacting a metal or metal compound with an ammonium carbamate- or ammonium carbonate-based compound and an additive and a method for preparing the same.
Owner:INKTEC CO LTD
Method for preparing carbon nanofibers
InactiveCN101718011AIncreased interlaminar shear strengthImprove efficiencyCarbon fibresFibre chemical featuresIonElectrospinning
The invention relates to a method for preparing carbon nanofibers. The method comprises the following steps: taking a polyacrylonitrile solution or an acrylonitrile copolymer solution or melt as materials, preparing nanofibers by the electrostatic spinning or the airflow spray method, and then preparing the nanofibers into carbon nanofibers. The method is characterized in that the carbon nanofibers are acidified or electrochemically treated into carbon nanofibers containing oxygen or nitrogen functional groups. The acidifying process comprises the following steps of: placing the carbon nanofibers in a nitric acid solution with the mass concentration of 30-68% and treating for 1-10 hours at 10-50 DEG C; rinsing with deionized water and drying; thus, obtaining the carbon nanofibers containing carboxyl functional groups. The electrochemical treating process comprises the steps of: placing the carbon nanofibers in a nitric acid or phosphoric acid solution with the mass percentage concentration of 1-20% at 10-50 DEG C, or in ammonium carbonate or ammonium hydrogen carbonate, and treating with the apparent current density of 10-100A / m2 for 1-120 minutes to obtain the carbon nanofibers containing carboxyl or amino functional groups.
Owner:TIANJIN POLYTECHNIC UNIV
Method for extracting vanadium from vanadium slag clinker leached by ammonium carbonate
ActiveCN102560086ALow costImprove leaching rateProcess efficiency improvementPregnant leach solutionMetallurgy
The invention discloses a method for extracting vanadium from vanadium slag clinker leached by ammonium carbonate. The method comprises the following steps of: calcifying and roasting vanadium slag of which the molar ratio of CaO to V2O5 is (2-3):1 at the temperature of between 700 and 900 DEG C, grinding the vanadium slag clinker, sieving, and leaching by an ammonium carbonate solution at the leaching temperature of between 60 and 98 DEG C for 30 to 120 minutes, filtering to obtain vanadium-containing leachate, performing vanadium deposition on the vanadium-containing leachate to obtain a vanadium finished product, wherein when the ammonium carbonate solution is leached, the concentration of the ammonium carbonate solution is between 200 and 800 g / L, and a liquid / solid ratio of the ammonium carbonate solution to the vanadium slag clinker is (5-30):1. According to the method, a leaching process is easy to operate and low in cost, and has low requirement on the equipment; and a leaching agent is low in cost and can be recycled, so that the production cost is reduced. By the method, the leaching rate of the vanadium is high, namely is over 90 percent, impurity elements and particularly a phosphorus element in the leachate are reduced, and the leaching rate of phosphorus is less than 10 percent.
Owner:CHONGQING UNIV
Calcium carbonate precipitation method
InactiveUS6036933ACalcium/strontium/barium carbonatesMagnesium carbonatesNitratePrecipitated calcium carbonate
PCT No. PCT / GB96 / 00488 Sec. 371 Date Oct. 22, 1997 Sec. 102(e) Date Oct. 22, 1997 PCT Filed Mar. 1, 1996 PCT Pub. No. WO96 / 26902 PCT Pub. Date Sep. 6, 1996A method for producing precipitated calcium carbonate by reacting an aqueous solution of calcium nitrate [Ca(NO3)2] with an aqueous solution of ammonium carbonate [(NH4)2CO3] and allowing calcium carbonate to precipitate from the resultant mixture containing nitrate [NH4NO3] in the mother liquor, the process being characterized in that: (i) the calcium nitrate [Ca(NO3)2)] solution utilized in the processes is prepared by slaking lime [CaO] in water in the presence of ammonium nitrate [NH4NO3] to form calcium nitrate [Ca(NO3)2] and ammonium hydroxide [NH4OH] in solution, filtering the solution to render it solids free, and heating the filtrate to dissociate the ammonium hydroxide [NH4OH] and to drive ammonia gas [NH3] from the solution; (ii) the ammonium carbonate (NH4)2CO3 solution utilized is prepared by absorbing ammonia gas [NH3] and carbon dioxide gas [CO2] in water, the ammonia gas preferably being derived from the step in (i) above in which the Ca(NO3)2 solution is heated; and (iii) the ammonium nitrate used is derived from the precipitation phase during which calcium carbonate is precipitated from the mother liquor containing ammonium nitrate.
Owner:PRETORIA PORTLAND CEMENT COMPANY +1
Method and equipment for processing organic material
InactiveUS7160456B2Organic material longerEasy to storeBio-organic fraction processingAnimal corpse fertilisersBuffer solutionAmmonia
A method for processing organic material, in which method bioconversion is performed on the organic material in at least one first reactor, the biogas formed in the bioconversion is treated with ammonia in at least one second reactor and buffer solution produced in the second reactor is recycled to the bioconversion in the first reactor. Thus, the carbon dioxide of the mixed methane / carbon dioxide gas reacts with the ammonia and forms a buffer compound, such as ammonium bicarbonate and / or ammonium carbonate.
Owner:PRESECO
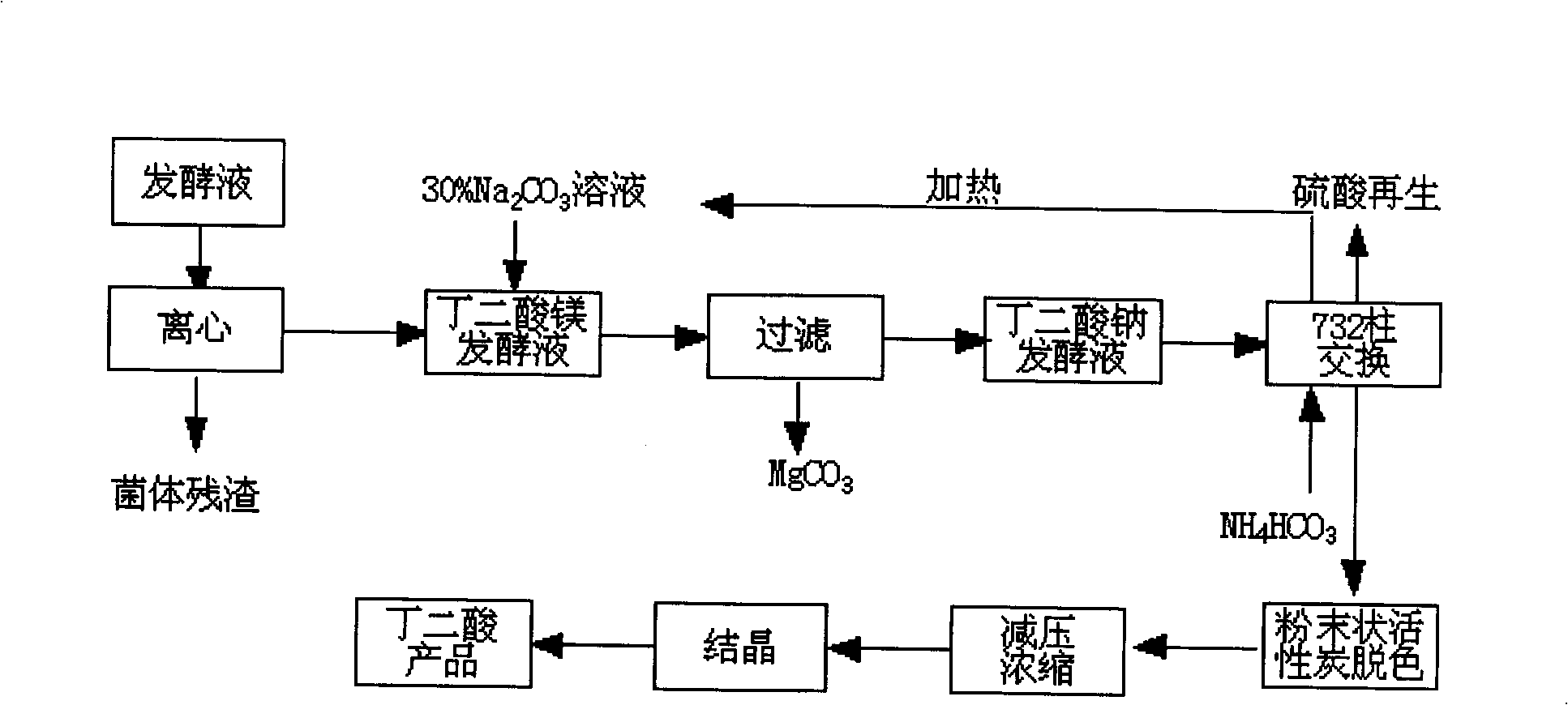
Method for extracting amber acid in fermentation liquor by cationic resin exchange
InactiveCN101348429AAchieving a circular economyShort process routeAmmonium sulfatesFermentationSodium bicarbonateSuccinic acid
A method for extracting succinic acid from fermentation broth through cation resin exchange belongs to the biochemical technical field. The method comprises the following steps that fermentation broth undergoes heated centrifugal filtration or membrane filtration so as to eliminate thalli; sodium carbonate is added in the clear solution to generate magnesium carbonate precipitation; a filter cake is reclaimed after filtration; then, the filtrate flows through a cation resin column, and the effluent is succinic acid solution; a succinic acid product is obtained after decoloring, concentration and crystallization; the cation resin column is eluted by ammonium carbonate after exchange so as to obtain sodium bicarbonate effluent; sodium salt is reclaimed and reused in fermentation broth treatment after ammonia is eliminated by heating; furthermore, resin regeneration can be realized by sulphuric acid through a conventional method, and the generated (NH4)2 SO4 can be used as a fertilizer. The method does not need to carry out acidification for the filtrate, and has the advantages of short technological line and high yield, thereby realizing element cycle economy. The invention provides a method for extracting succinic acid from microorganism fermentation broth with simple operation and economical efficiency.
Owner:JIANGNAN UNIV
Superfine and spheroidizing rare-earth polish and preparing process thereof
ActiveCN101284983AIncrease ionic strengthIncrease surface chargeOther chemical processesIonDiammonium carbonate
The invention provides ultrafine spheroidized rare earth polishing powder and a process for making the same. The process comprises the following steps that: one of or the mixture of ammonia water, ammonium bicarbonate and ammonium carbonate is used as precipitant; ammonium salt and fluoride ions are added into precipitated slurry to adjust the ionic strength in mother liquid, so as to increase the surface electrical property of solid particles in the slurry; after high temperature aging, the ultrafine rare earth polishing powder with good dispersity and high spheroidization degree can be obtained by filtering the slurry, drying, burning, ball-milling and sieving filter cakes. The average particle size of the obtained polishing powder is between 0.02 mu m and 2.0 mu m, wherein the specific surface area BET is more than 0 and less than 10 m<2> / g, and the powder is in a well-dispersed spherical shape. The powder is used for polishing optical glass, crystal, display screens, etc., strong in cutting force, few in scratch and long in service time.
Owner:GRIREM ADVANCED MATERIALS CO LTD
Organic silver complexes, their preparation methods and their methods for forming thin layers
ActiveUS8226755B2Superior stability and solubilityEasy to prepareGroup 1/11 organic compounds without C-metal linkagesRadiation applicationsThin layerAmmonium carbonate
Owner:INKTEC CO LTD
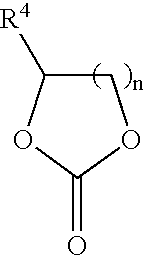
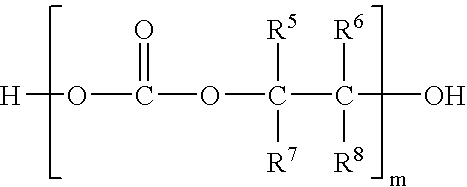
In situ process for preparing quaternary ammonium bicarbonates and quaternary ammonium carbonates
Applicant has discovered an in situ method of preparing quaternary ammonium methocarbonate salts and quaternary ammonium alkylcarbonate salts in high yield from tertiary amines, methanol, and at least one of a cyclic carbonate, an aliphatic polyester (such as a polycarbonate), or an ester (such as a carbonate ester), and their subsequent conversion to quaternary ammonium bicarbonates, quaternary ammonium carbonates or both in a one-pot reaction. According to one embodiment of the invention, the method includes reacting an amine and methanol with at least one of a cyclic carbonate and an aliphatic polyester to yield a quaternary ammonium methocarbonate. This method does not produce or require the handling of corrosive quaternary ammonium hydroxides. Another embodiment is a method of preparing quaternary ammonium alkylcarbonate salts by reacting tertiary amines, methanol, and an ester. The quaternary ammonium methocarbonate or alkylcarbonate can be converted to the corresponding bicarbonate, carbonate, or mixture thereof by methods known in the art.
Owner:ARXADA LLC
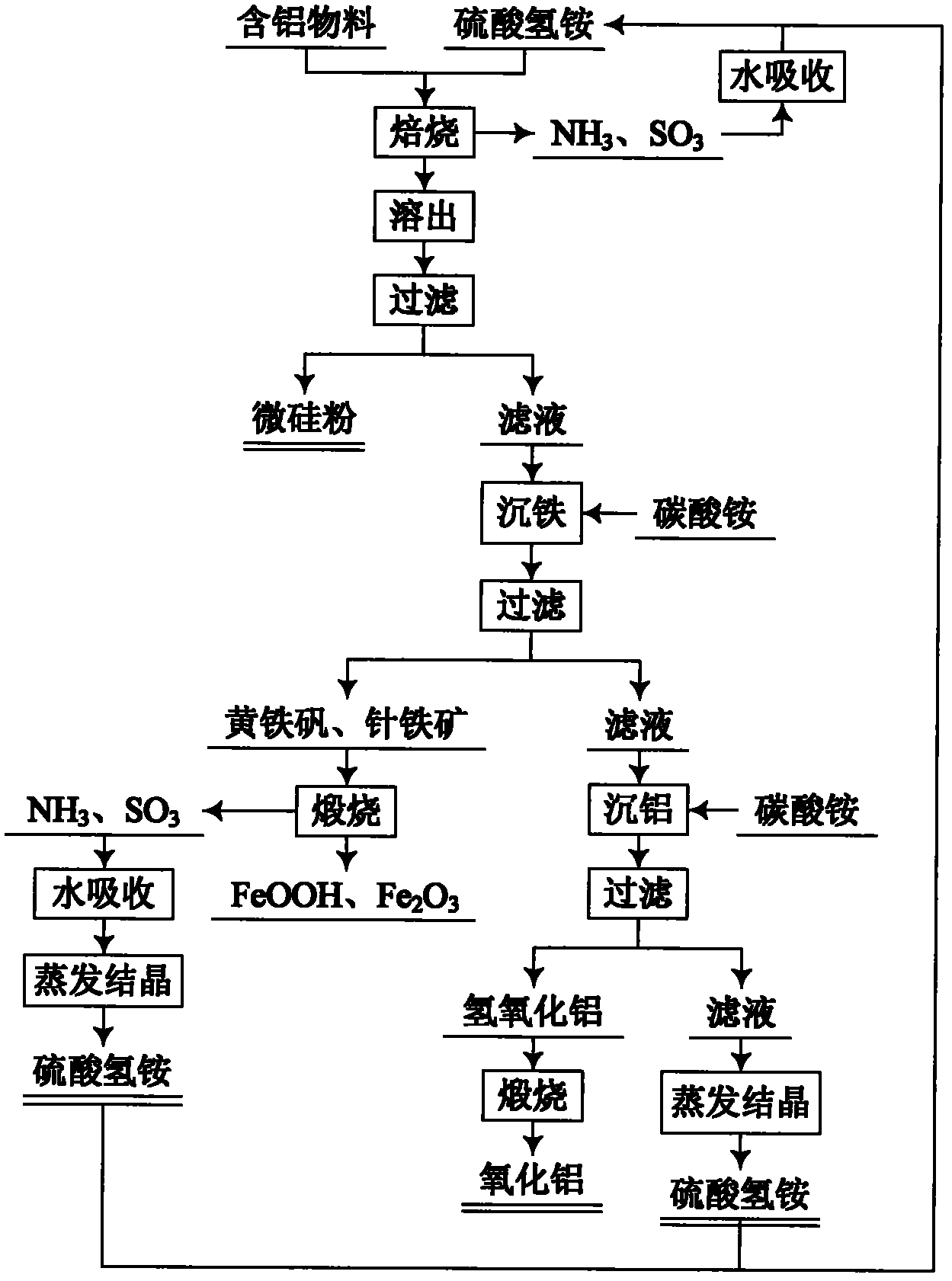
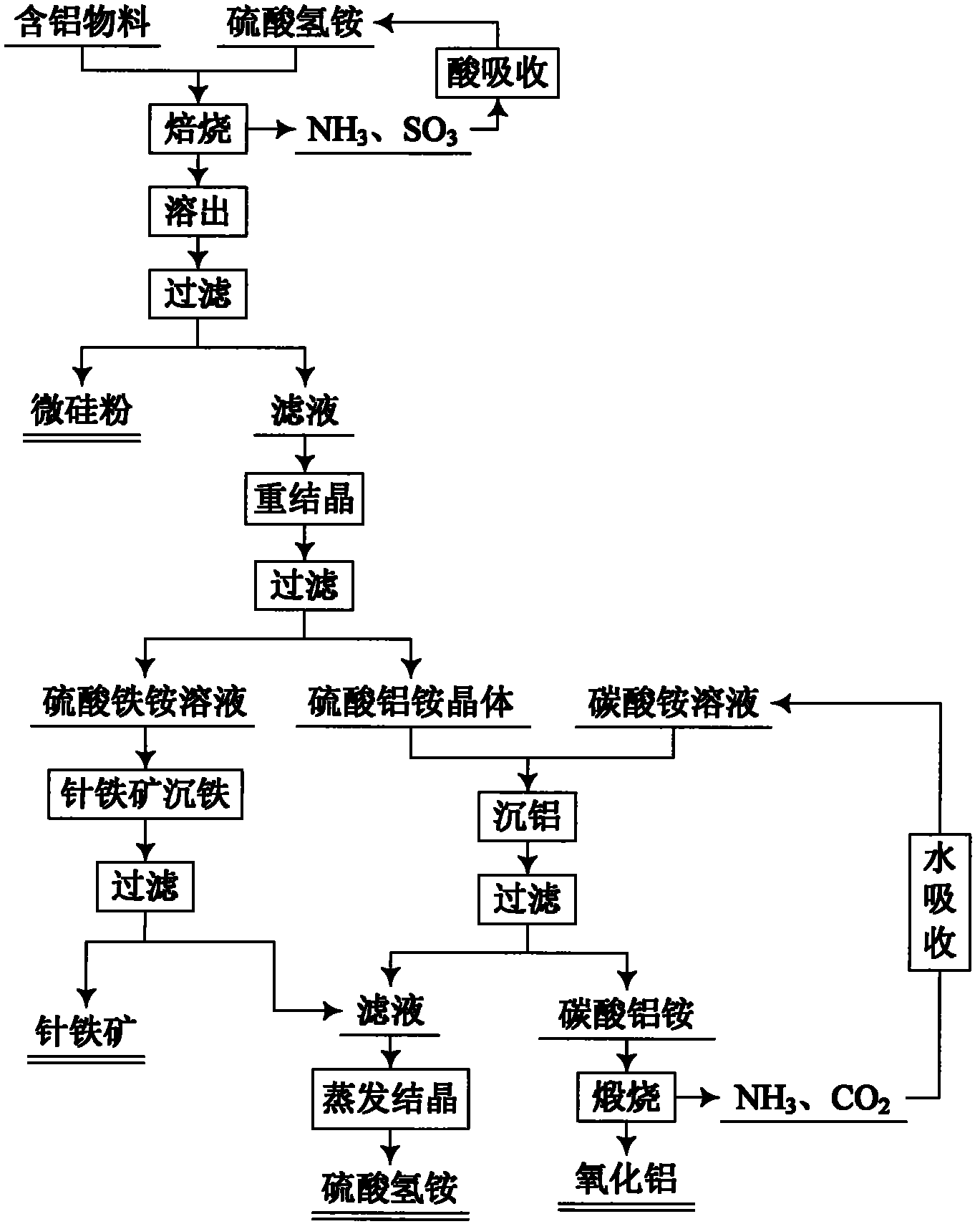
Method for comprehensive utilization of aluminum-containing material
The invention relates to a method for preparing aluminum oxide and other products by aluminum-containing materials of bauxite, alunite, nepheline, fly ash, kaolin, coal gangue and clay. The method comprises the following steps: (1) crushing and grinding an aluminum-containing material, carrying out mixing and baking for the treated aluminum-containing material and ammonium bisulfate; (2) carryingout dissolution and filtering for the baked clinker to obtain a crude ammonium aluminum sulfate solution and aluminum extracting residue; (3) carrying out an iron precipitating treatment for the ammonium aluminum sulfate solution with the concentration more than 1 g / L by adopting a jarosite method, then carrying out an iron precipitating treatment by a goethite method, carrying out an aluminum precipitating treatment for the resulting solution, carrying out calcination for the resulting aluminium hydroxide to prepare aluminum oxide; (4) carrying out an iron precipitating treatment for the ammonium aluminum sulfate solution with the concentration less than 1 g / L by adopting the goethite method, and carrying out an aluminum precipitating treatment to prepare aluminum oxide, or adopting a recrystallization method to carry out purification, adopting a reaction of the ammonium aluminum sulfate crystal and a ammonium carbonate solution to precipitate the aluminum to obtain ammonium aluminumcarbonate, carrying out calcination for the ammonium aluminum carbonate, and adopting a Bayer method to treat the calcined ammonium aluminum carbonate to prepare sandy aluminum oxide; (5) washing anddrying the aluminum extracting residue, wherein the dried aluminum extracting residue is adopted as the silicon dioxide product.
Owner:NORTHEASTERN UNIV
System for Highlighting Hair
The present invention relates to a system to highlight the hair. The system comprises a device (10) and a composition (50). Device (10) comprises a first portion movably joined to a second portion. Composition (50) comprises a percentage of hydrogen peroxide and a percentage of an alkalizer by weight of said composition (50). The weight percentages of hydrogen peroxide and of an alkalizer are defined by equation (I): X+1.5Y≧15 (I) wherein X is the percentage by weight of hydrogen peroxide by weight of the composition (50) and wherein Y is the percentage by weight of an alkalizer by weight of the composition (50). Said composition (50) comprises at least 2% of hydrogen peroxide by weight of said composition (50) and said alkalizer is an inorganic salt selected from the group consisting of sodium silicate, sodium metasilicate, potassium hydrogen carbonate, ammonium carbonate, ammonium hydrogen carbonate and sodium hydrogen carbonate and mixtures thereof. Said composition (50) allows to perform highlighting when applied with the device (10) as described herein.
Owner:WELLA OPERATIONS US LLC
Method for recovering valuable metals from positive electrode material of waste lithium ion battery
ActiveCN108767354AHigh recovery rateAvoid it happening againWaste accumulators reclaimingProcess efficiency improvementLithium-ion batteryMaterials science
The invention provides a simple, efficient and environment-friendly method for recovering valuable metals from a positive electrode material of a waste lithium ion battery; the method comprises the following steps of performing discharging of a salt solution; performing disassembling to separate a positive plate; performing smashing on the positive plate to separate a positive electrode material and an aluminum foil; performing mixed low-temperature roasting on the positive electrode material and a roasting agent ammonium sulfate and / or ammonium bisulfate; performing leaching on the roasted material and separating to obtain carbon and a lixivium; adding a precipitant into the lixivium, and regulating the pH value by using NH<3>-containing flue gas, performing deposition on other metals except Li, and carrying out solid-liquid separation; regulating the pH value of a filtrate by using the NH3-containing flue gas, adding ammonium carbonate or ammonium bicarbonate or pumping CO<2> gas, and carrying out lithium precipitation to obtain a lithium carbonate product. The preparation process is simple, the process conditions are mild, the time required by the process is short, a large amount of acid and alkali do not need to be consumed, and the cost is low; and in addition, recovery of the valuable metals and carbon from the positive electrode material can be effectively realized, themethod is green and environment-friendly, and a large amount of solid waste and wastewater cannot be generated.
Owner:CENT SOUTH UNIV
Light and color glazed tile for building
The invention discloses a light and color glazed tile for a building, which is obtained by the following steps: adding 0.4 to 0.6 kg of magnesium oxide to each kilo of a magnesium chloride solution; agitating the mixture for 6 to 8 minutes; successively adding 0.05 to 0.1 kg of magnesium silicate, 0.02 to 0.03 kg of urea-formaldehyde resin, 0.06 to 0.1 g of ammonium carbonate, 2 to 3 g of trisodium phosphate and 2 to 3 g of pigment; agitating the obtained mixture for 20 to 30 minutes; pouring the agitated mixture to a glass fiber cloth; scraping and compacting the poured mixture; and performing natural cure and drying to obtain the light and color glazed tile for the building. In the way above, the light and color glazed tile for the building has the advantages of smooth and clean surface, bright color, color retention, fracture resistance, compression resistance, thermal and cooling shock resistance, acid and alkali resistance, freezing resistance, light weight, low water absorption, good thermal insulation, good sound insulation and low cost.
Owner:CHINA CHANGSHU CONSTR GRP
Silicon-based electrode with adjustable pore structure and preparation method of silicon-based electrode
ActiveCN108767195AIntegrity guaranteedImprove distributionElectrode rolling/calenderingSecondary cellsPorosityCyclic process
The invention provides a silicon-based electrode with an adjustable pore structure; the porosity of the silicon-based electrode is 30%-60%; the pore structure of the silicon-based electrode is adjusted by controlling the compaction density of the electrode and adding a pore-forming additive, wherein the pore-forming additive is one or more of ammonium carbonate, ammonium hydrogen carbonate, ammonium acetate, ammonium nitrate and ammonium chloride. The invention further provides a preparation method of the silicon-based electrode. The porosity of the electrode is controlled by changing the compaction density, and the appropriate porosity can be consistent with the volume expansion rate of a high specific capacity silicon carbon negative electrode material in a lithium intercalation state, so that the integrity of the structure is kept in the circulation process of the electrode; and the volume change of silicon can be effectively buffered through the high-capacity silicon-based negativeelectrode with the variable pore structure, so that the diffusion speed of lithium ions and electrons is increased, the cycling stability of the electrode is obviously improved, and the large-currentdischarge performance of the electrode is improved.
Owner:CHINA AUTOMOTIVE BATTERY RES INST CO LTD
Method for producing o-chloroaniline
InactiveCN101333169AHigh yieldLow costOrganic compound preparationAmino compound preparationEthylenediamineO-nitrochlorobenzene
An o-chloroaniline production method takes o-nitrochlorobenzene as raw material and is characterized in that the o-nitrochlorobenzene is dissolved in alcohol solvent in the presence of catalyst and additive and reacted with hydrogen at 10-120 DEG C and under 0.3-4.0 MPa; the reaction process is continuous reaction; after the completion of the reaction, the o-chloroaniline is obtained through treatment, wherein, the catalyst can be selected from one of the following: Ni / Al2O3, Raney Ni, Pt / C and Pd / C; while the additive can be selected from one, or two, or three of the following compounds: cyclohexylamine, ethylenediamine, ethanolamine, diethanolamine, triethanolamine, pyridine, liquid ammonia, ammonium bicarbonate, ammonium carbonate, sodium carbonate, sodium bicarbonate, potassium bicarbonate, potassium carbonate, potassium hydrogen phosphate, potassium dihydrogen phosphate, sodium hydrogen phosphate and sodium dihydrogen phosphate; the dosage of the catalyst takes up 0.05% to 20% of the mass of the o-nitrochlorobenzene; the dosage of the additive takes up 0. 5% to 20% of the mass of the o-nitrochlorobenzene; the alcohol can be methanol or ethanol; the dosage of alcohol takes up 30% to 150% of the dosage of the o-nitrochlorobenzene; the continuous reaction is realized through 1 to 6 tank reactors which are connected in series.
Owner:淮安嘉诚高新化工股份有限公司
Alumina supporter and preparation method thereof
ActiveCN101462074AImprove hydrogenation reactivityLower Diffusion LimitsCatalyst carriersPore distributionSlurry
The invention relates to an alumina carrier and a preparation method thereof. The pore distribution of the alumina carrier is as follows: the pore volume of holes with the pore diameter of less than 10 nanometers accounts for less than 10 percent of the total pore volume; the pore volume of holes with the pore diameter between 10 and 50 nanometers accounts for 40 to 80 percent of the total pore volume; the pore volume of holes with the pore diameter between 100 and 1,000 nanometers accounts for 1 to 40 percent of the total pore volume; and the pore volume of holes with the pore diameter between 5,000 and 10,000 nanometers accounts for 1 to 20 percent of the total pore volume. The preparation method for the alumina carrier comprises: adopting a small quantity of starch and / or carbon black as crystal seeds, adopting a method of co-current flow first and swing then to prepare a serum containing aluminum hydroxide, using ammonium carbonate and / or ammonium bicarbonate to adjust the pH value, and finally preparing the alumina carrier. Alumina obtained by the method still maintains high strength under the condition of enlarged pore volume and pore diameter. The alumina carrier is particularly suitable to be a carrier of a residual oil hydrotreating catalyst.
Owner:FUSHUN RES INST OF PETROLEUM & PETROCHEMICALS SINOPEC CORP
Method for preparing high-sphericity-degree and large-particle cobaltosic oxide
InactiveCN105800699AGuarantee quality stabilityPromote productionCell electrodesCobalt oxides/hydroxidesSodium bicarbonateReaction temperature
The invention relates to a method for preparing high-sphericity-degree and large-particle cobaltosic oxide. The method includes the steps that at least one of cobalt chloride, cobaltous sulfate and cobalt nitrate serves as a raw cobalt salt material, and is purified and prepared to obtain a cobalt salt solution; at least one of ammonium carbonate, ammonium bicarbonate, sodium carbonate and sodium bicarbonate serves as a precipitant; a precursor is prepared with the solution direct precipitation method, and added into a reaction vessel with the concurrent adding method, the parameters of the injecting speed, the reaction temperature, the stirring speed, the PH value and the like of the solution are controlled, the batch-type crystallization technology and the kettle dividing technology are adopted, the chemical impurities of the precursor and the crystallinity degree, the granularity, the density and the like of crystals are effectively controlled, a precipitate precursor is synthesized, washing and centrifuging are carried out, then sintering is carried out twice, and the high-sphericity-degree and large-particle cobaltosic oxide is finally obtained. The method is simple in technology and low in cost, and the prepared cobaltosic oxide is high in cut-off voltage, tap density and stability.
Owner:HUNAN HINA NEW MATERIALS
Melt-blown polypropylene as well as preparation method and application thereof
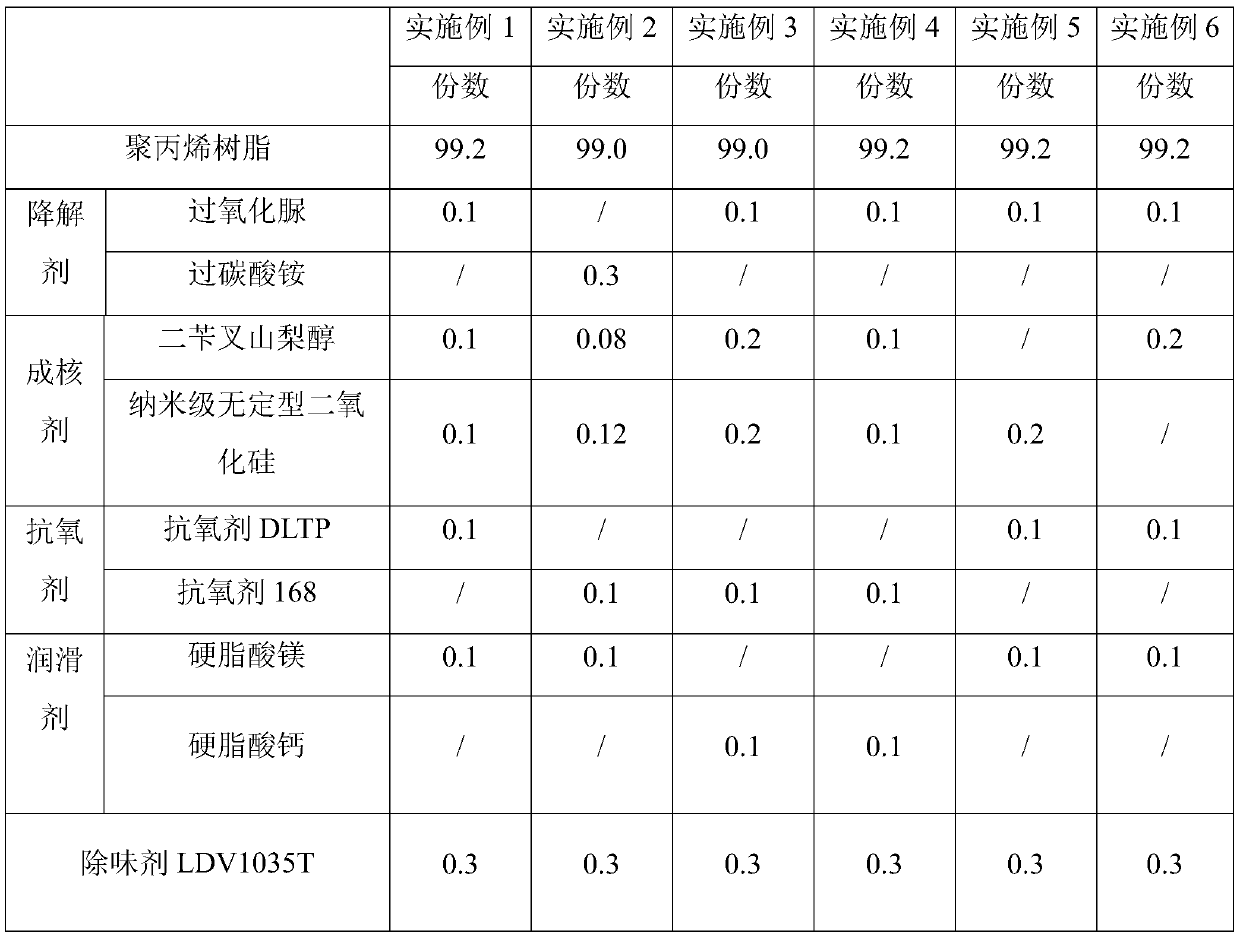
The invention discloses melt-blown polypropylene as well as a preparation method and application thereof. The melt-blown polypropylene comprises following raw polypropylene resin, a degradation agent,an antioxidant and a lubricant, wherein the degradation agent is selected from one or more of hydrogen peroxide, sodium percarbonate, ammonium percarbonate and urea peroxide. The melt-blown polypropylene also comprises a nucleating agent selected from one or more of dibenzylidene sorbitol, aryl phosphate and nano scale amorphous silicon dioxide, wherein the degradation agent and the nucleating agent respectively account for 0.05-0.5% of the mass percentage of the raw materials. The preparation method comprises the following steps: premixing raw materials, stirring, then selectively adding a deodorant; and melting and granulating by an extruder to obtain the product. The invention also discloses an application of the melt-blown polypropylene in manufacturing melt-blown non-woven fabrics, melt-blown filter elements and sound-absorbing cotton. The melt-blown polypropylene has the advantages of low odor, high electret charge stability and the like.
Owner:JIANGSU DEWEI ADVANCED MATERIALS
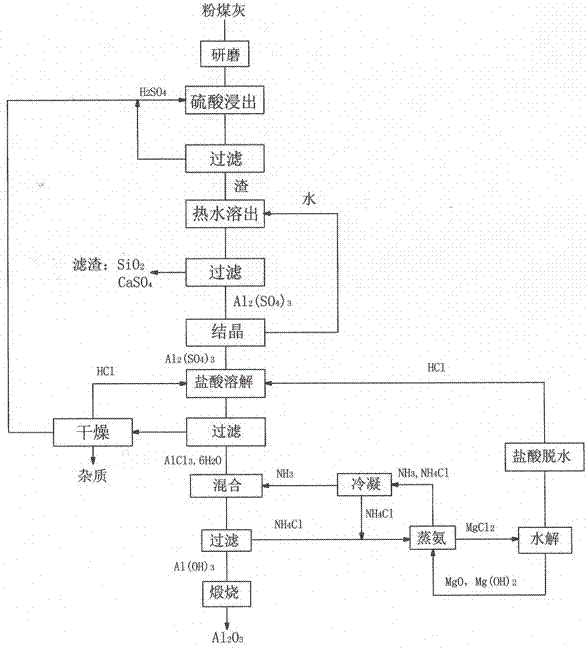
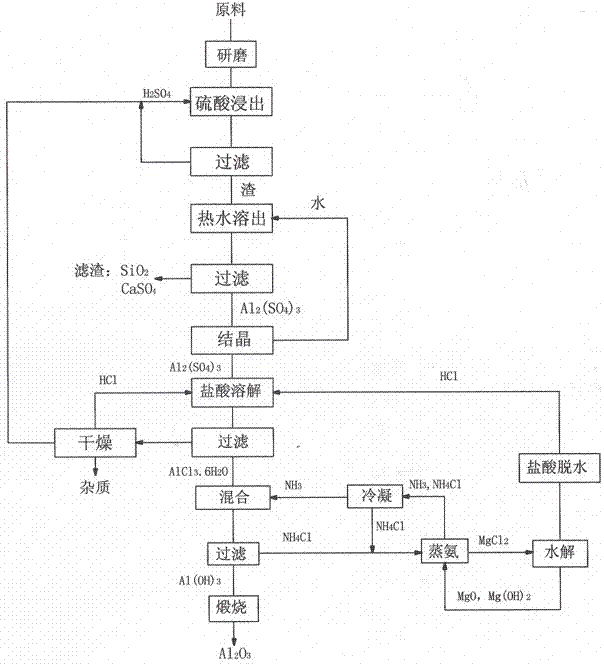
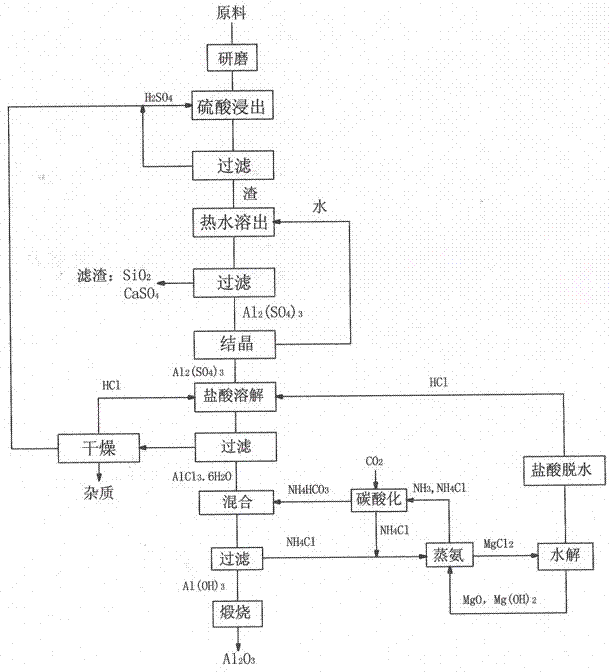
Method for preparing alumina by using power plant fly ash
InactiveCN102849767ASaturated precipitation with high purityThe process steps are simpleAluminium oxide/hydroxide preparationAluminium hydroxideAmmonium chloride mixture
The invention discloses a method for preparing alumina by using power plant fly ash, characterized by: grinding the fly ash, leaching with concentrated sulfuric acid to obtain an aluminum sulfate solution, precipitating aluminum sulfate crystals by concentrating and cooling, dissolving the aluminum sulfate crystals with hydrochloric acid, then letting an HCl gas in the solution to saturate the solution to precipitate AlCl3-6H2O crystals, reacting the AlCl3-6H2O crystals with an ammonium hydroxide solution or liquid ammonia or an ammonium bicarbonate solution or an ammonium carbonate solution to obtain an aluminium hydroxide and ammonium chloride solution, calcining aluminium hydroxide to obtain alumina, displacing ammonium chloride by using magnesium oxide to obtain ammonia gas and magnesium chloride, and hydrolyzing magnesium chloride to obtain magnesium oxide and hydrochloric acid for recycling.
Owner:李景江
Latent epoxy resin curing-foaming agent and method for preparing the same
ActiveCN104945599ASingle-packageIncrease profitCarbamic acid derivatives preparationOrganic compound preparationEpoxyFoaming agent
The invention discloses a latent epoxy resin curing-foaming agent and a method for preparing the same and belongs to the technical field of plastic additives. A commercialized amine curing agent for epoxy resin is utilized to absorb and fix carbon dioxide, so as to synthesize a series of novel alkyl ammonium carbamate compounds or alkyl ammonium carbonate compounds. The alkyl ammonium carbamate compounds or the alkyl ammonium carbonate compounds are stable at normal temperature and can release carbon dioxide gas and the amine curing agent again when heated, so as to effectively foam and cure the epoxy resin, and the latent epoxy resin curing-foaming agent is environmentally friendly.
Owner:CHANGZHOU UNIV
Anti-reburning environment-friendly power transmission line forest fire extinguishing agent
The invention discloses an anti-reburning environment-friendly power transmission line forest fire extinguishing agent. The anti-reburning environment-friendly power transmission line forest fire extinguishing agent comprises the following components in parts by weight: 5-20 parts of ammonium carbonate, 10-45 parts of ammonium polyphosphate, 10-30 parts of sodium silicate, 1-10 parts of a foaming agent, 0.5-5 parts of polyethylene glycol, 1-5 parts of a thickening agent, 4-10 parts of a composite anti-freezing agent and 55-80 parts of water, wherein the foaming agent is selected from one of alkylethoxylate carboxylate, an imidazoline amphoteric surfactant and a biological surfactant; the composite anti-freezing agent is a compound which is prepared by compounding inorganic salt and polyhydric alcohol according to a mole ratio of (1 to 3)-(1 to 9). All the raw materials of the anti-reburning environment-friendly power transmission line forest fire extinguishing agent are environment-friendly materials, so that the fire extinguishing agent is free of fluorine compound, toxicity, smell and pollution; a fire retardant is added into the fire extinguishing agent, so that the fire extinguishing agent is capable of effectively preventing the fire from spreading and is high in fire extinguishing efficiency; the environment-friendly surfactant serves as the foaming agent, so that the fire extinguishing agent is high in foaming performance and is capable of effectively isolating contact between the combustion material and the air and accelerating the fire extinguishing speed; the anti-freezing agent is compounded by the inorganic salt and polyhydric alcohol, so that the freezing point of the fire extinguishing agent is reduced to be -30 DEG C.
Owner:STATE GRID CORP OF CHINA +2
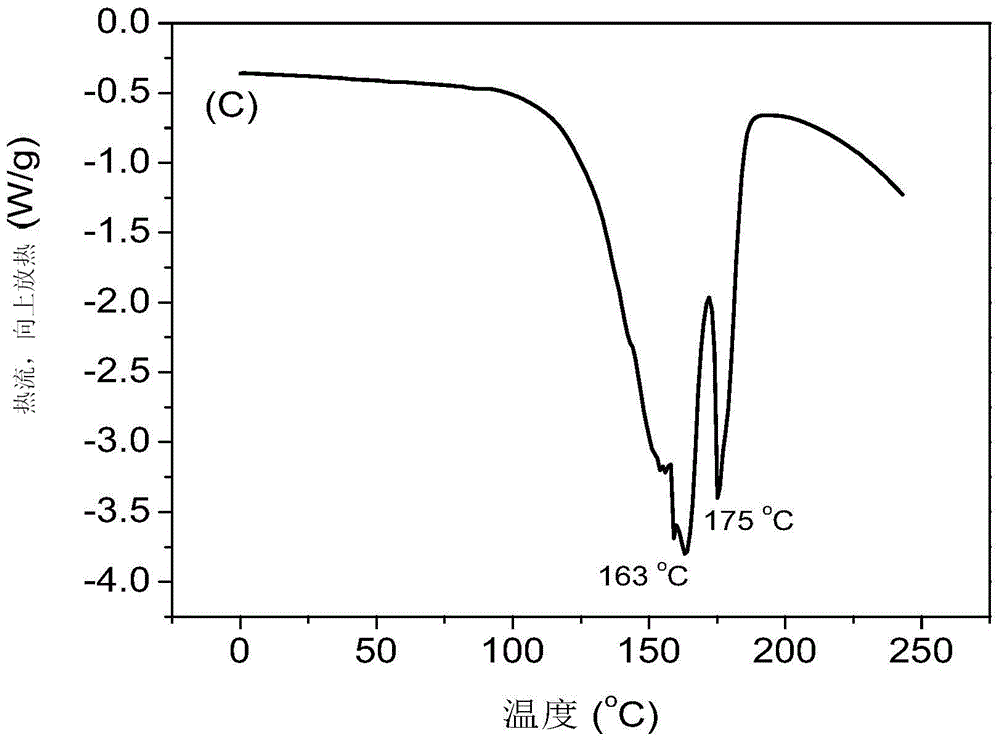
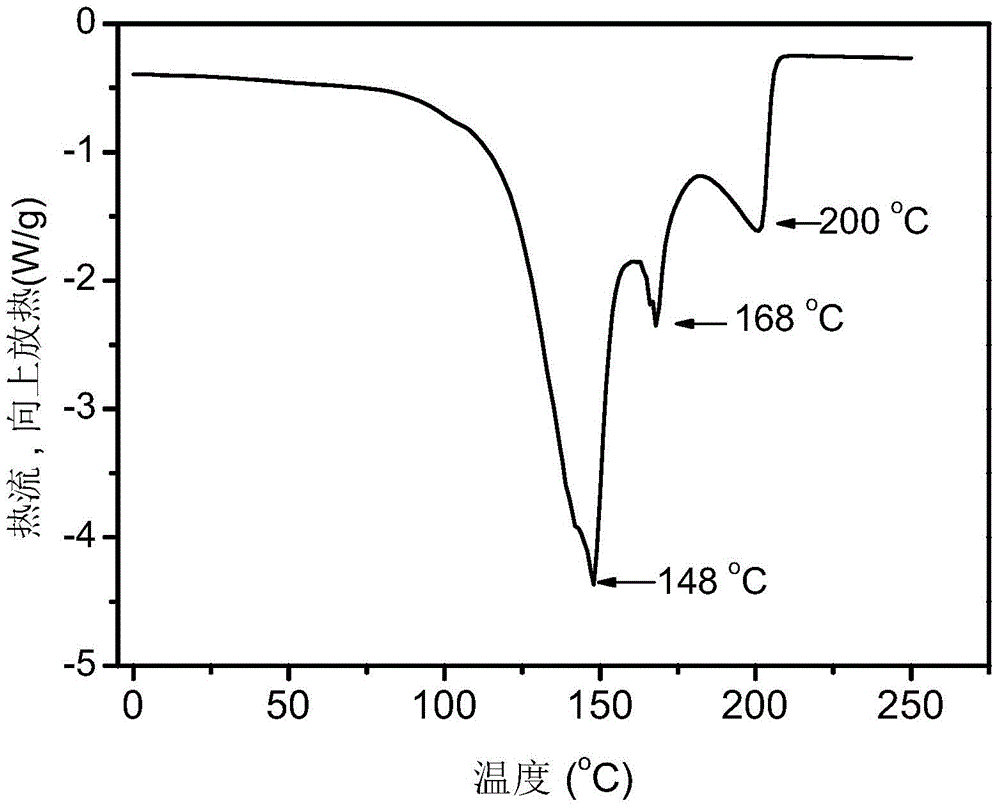
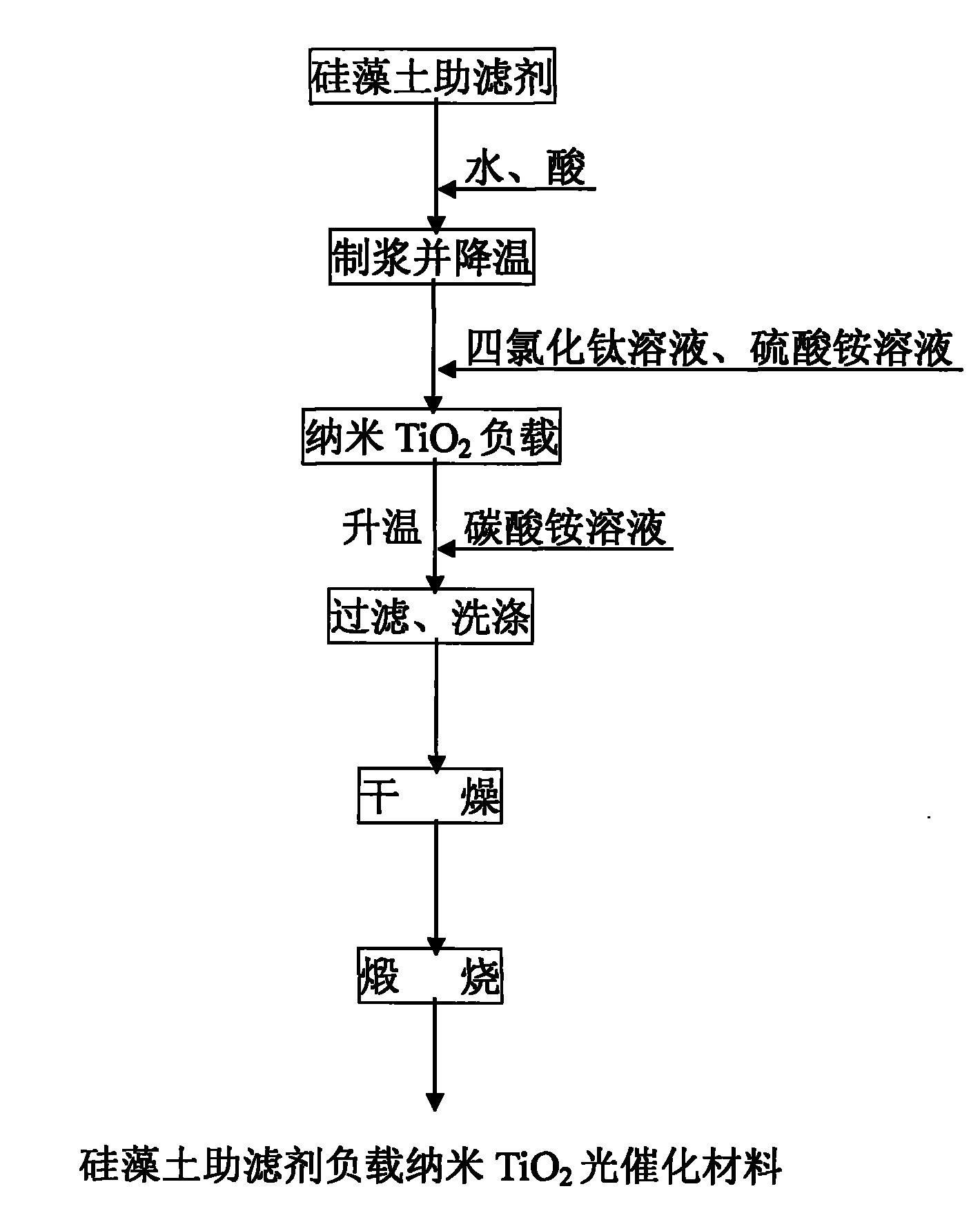
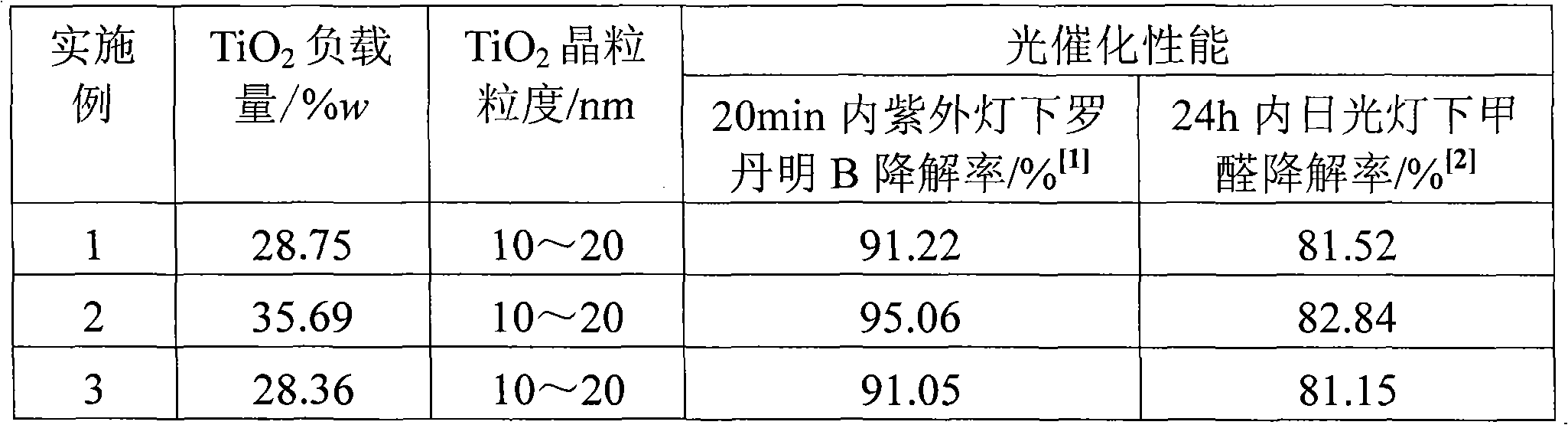
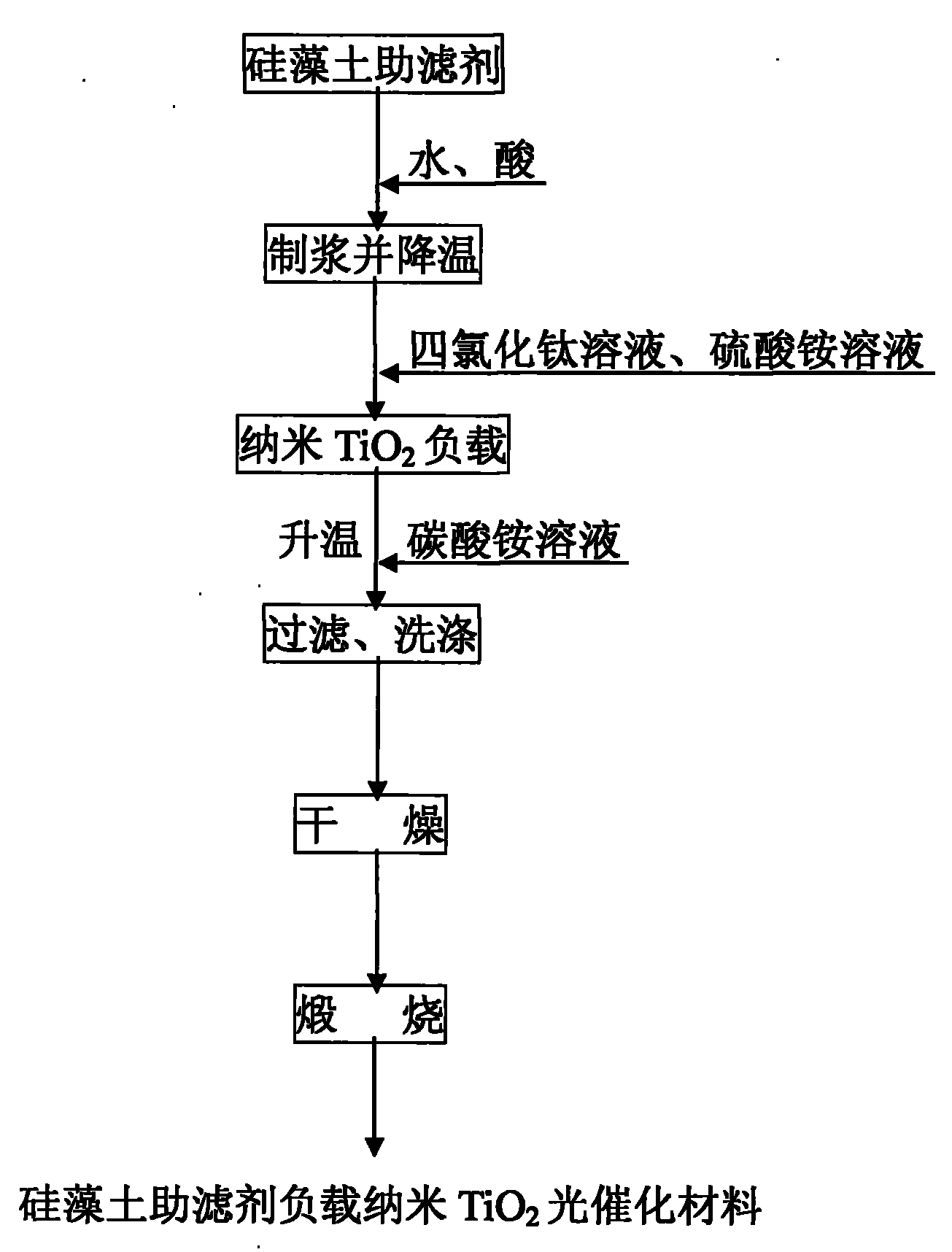
Preparation method of supported nano TiO2 photocatalytic material with diatomite filter aid as carrier
The invention relates to a preparation method of a supported nano TiO2 photocatalysis material with a diatomite filter aid as a carrier. The preparation method comprises the steps of adding water into the diatomite filter aid, stirring the mixture to prepare slurry and adding acid to regulate pH value; then cooling the slurry, then sequentially adding TiCl4 solution and ammonium sulphate for reaction, heating reaction liquid to a certain temperature, then adding ammonium carbonate solution to regulate the pH value of solution and ageing the reaction liquid for a period of time; and finally filtering, washing, drying and calcining the reaction product, thus obtaining the diatomite filter aid supported nano TiO2 photocatalytic material. The supported nano TiO2 photocatalytic material has excellent photocatalytic performance under ultraviolet light and visible light and has more than 80% of degradation removal rate on formaldehyde in 24 hours under a fluorescent lamp.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Technology for combined production of sodium carbonate and ammonium chloride through sodium sulfate type brine thermal cycle method
InactiveCN105000579AAdaptableThe main product is of high qualityAmmonium halidesCarbonate preparationSodium bicarbonateSodium sulfate
The invention discloses a technology for combined production of sodium carbonate and ammonium chloride through a sodium sulfate type brine thermal cycle method. The technology comprises the following steps: (1) carrying out a metathesis reaction on sodium sulfate type brine, ammonia and carbon dioxide as raw materials, separating to obtain sodium bicarbonate and an alkali production mother liquor containing ammonium chloride, sodium sulfate, sodium chloride, ammonium bicarbonate and ammonium carbonate, and calcining the obtained sodium bicarbonate to obtain a sodium carbonate product; (2) preheating the alkali production mother liquor to carry out high temperature removal of ammonium bicarbonate and ammonium carbonate in order to obtain a deaminized mother liquor containing ammonium chloride, sodium sulfate and sodium chloride; (3) adding lime with the amount equal to that of sodium sulfate to the deaminized mother liquor, and reacting to remove sodium sulfate in order to obtain calcium sulfate, ammonia and a denitrified mother liquor containing ammonium chloride and sodium chloride; and (4) evaporating the denitrified mother liquor, and separating to obtain ammonium chloride and an ammonium production mother liquor. The technology has the characteristics of high quality of main products, strong adaptability of the raw materials, low cost, low energy consumption, closed loop, and no discharge of three wastes.
Owner:CHINA LIGHT IND INT ENG CO LTD
Method for treating wastewater of dilute thiamine
ActiveCN101092265AEfficient removalReduce dosageCalcium/strontium/barium sulfatesAmmonium carbonates/bicarbonatesIon exchangeIon-exchange resin
This invention relates to a method for treating diluted ammonium sulfate wastewater. The method solves the problems of high solid waste and organic nitrile impurity contents, low ammonium sulfate concentration, and difficult ammonium sulfate recovery. The method comprises: (1) removing solid impurities from diluted ammonium sulfate wastewater, adjusting the pH value to 6.5-7.5, performing flash evaporation, stripping or rectification, removing lightweight organic fractions at the overhead, collecting heavy fractions at the bottom, and extracting the rest ammonium sulfate wastewater at 1st-5th theoretical plates from the overhead; (2) sending the rest ammonium sulfate wastewater to ion exchange reaction, and removing NH4+; (3) treating regenerated liquid of the ion exchange resin with Ca(OH)2 aqueous solution to produce CaSO4 and diluted ammonia solution, performing solid-liquid separation, reacting CaSO4 and diluted ammonia solution with CO2 to obtain ammonium carbonate or ammonium hydrogen carbonate.
Owner:CHINA PETROLEUM & CHEM CORP +1
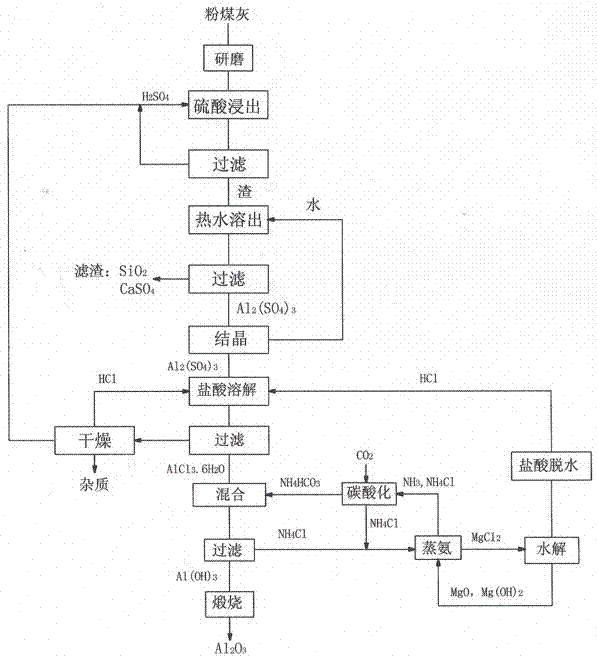
Method for preparing alumina from low-grade bauxite by acid leaching
InactiveCN102849765ASaturated precipitation with high purityThe process steps are simpleAluminium compoundsAluminium hydroxideAmmonium chloride mixture
The invention discloses a method for preparing alumina from low-grade bauxite by acid leaching, characterized by: grinding the low-grade bauxite, leaching with concentrated sulfuric acid to obtain an aluminum sulfate solution, precipitating aluminum sulfate crystals by concentrating and cooling, dissolving the aluminum sulfate crystals with hydrochloric acid, then introducing an HCl gas in the solution to saturate the solution to precipitate AlCl3.6H2O crystals, reacting the AlCl3.6H2O crystals with an ammonium hydroxide solution or liquid ammonia or an ammonium bicarbonate solution or an ammonium carbonate solution to obtain aluminium hydroxide and an ammonium chloride solution, calcining aluminium hydroxide to obtain alumina, carrying out displacement reaction on ammonium chloride and magnesium oxide to obtain ammonia gas and magnesium chloride, and hydrolyzing magnesium chloride to obtain magnesium oxide and hydrochloric acid for recycling.
Owner:李景江
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com