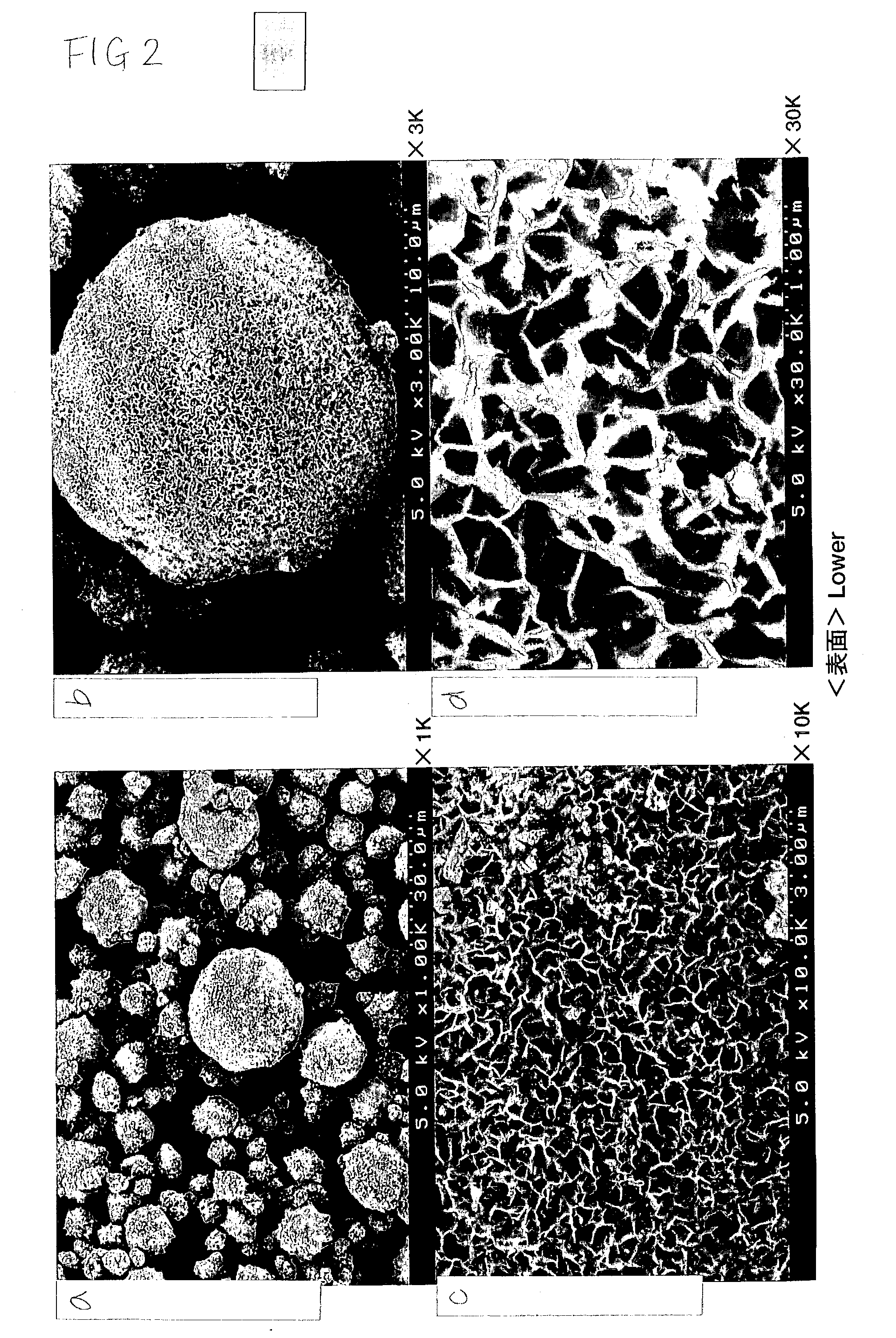
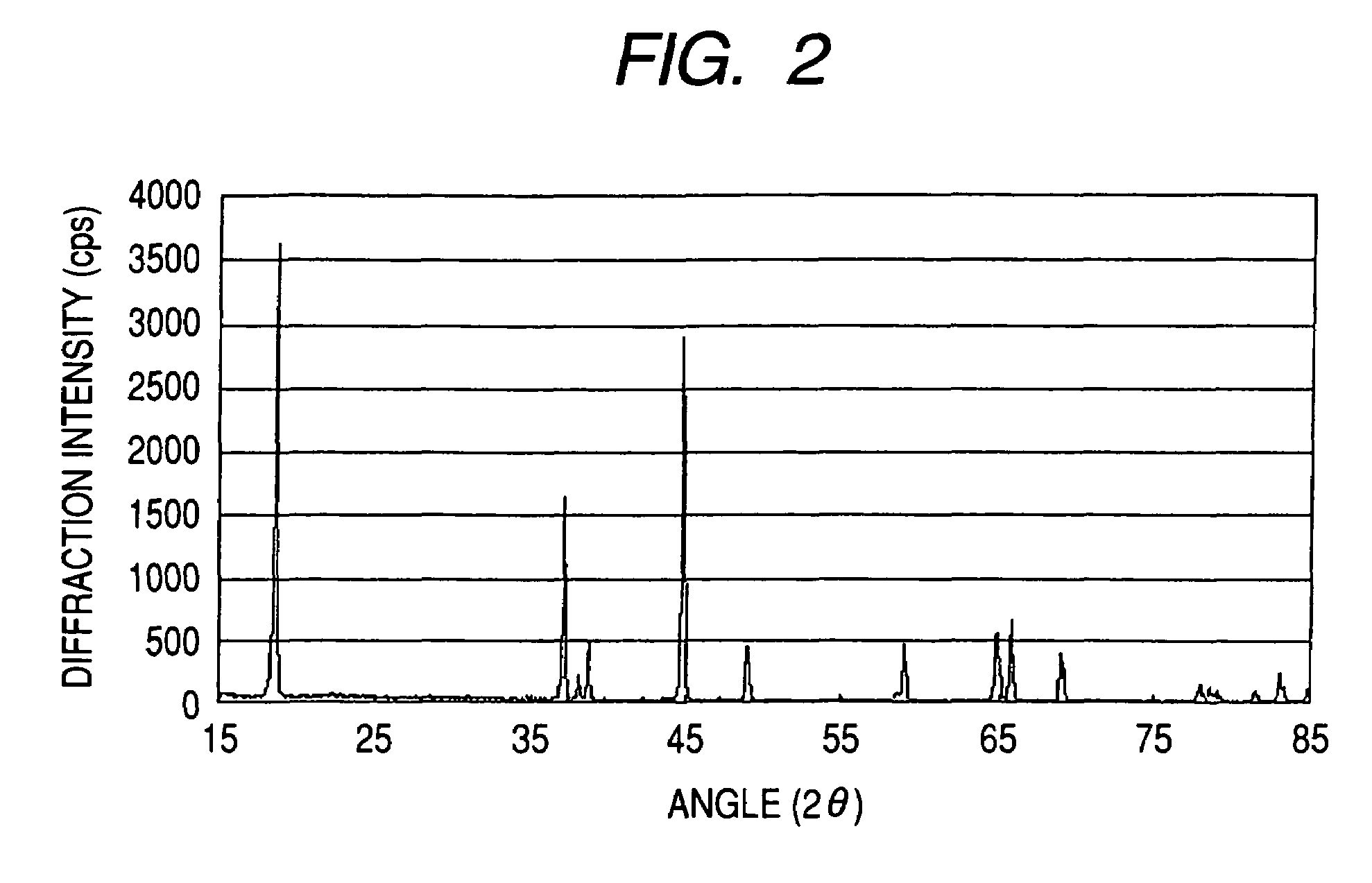
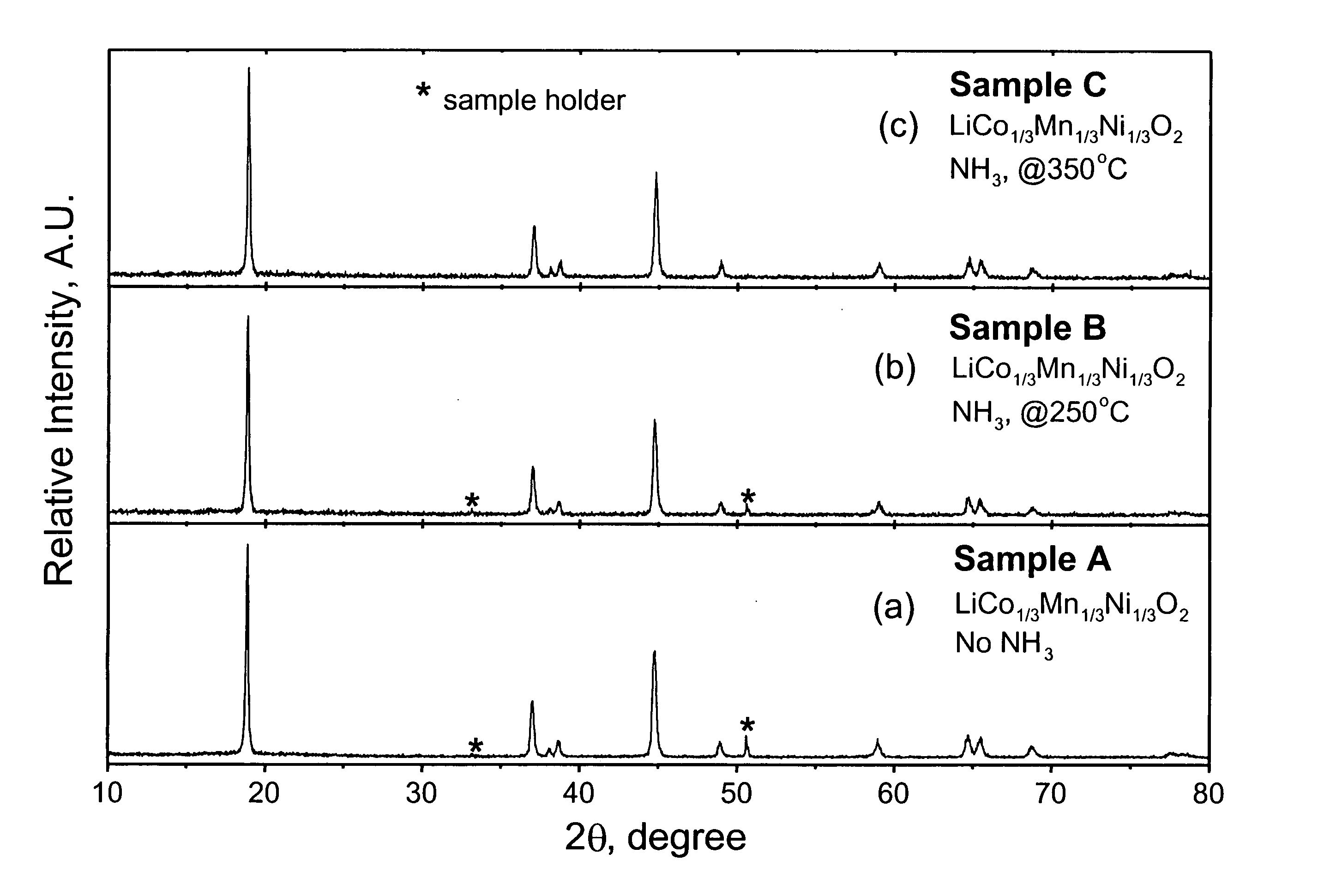
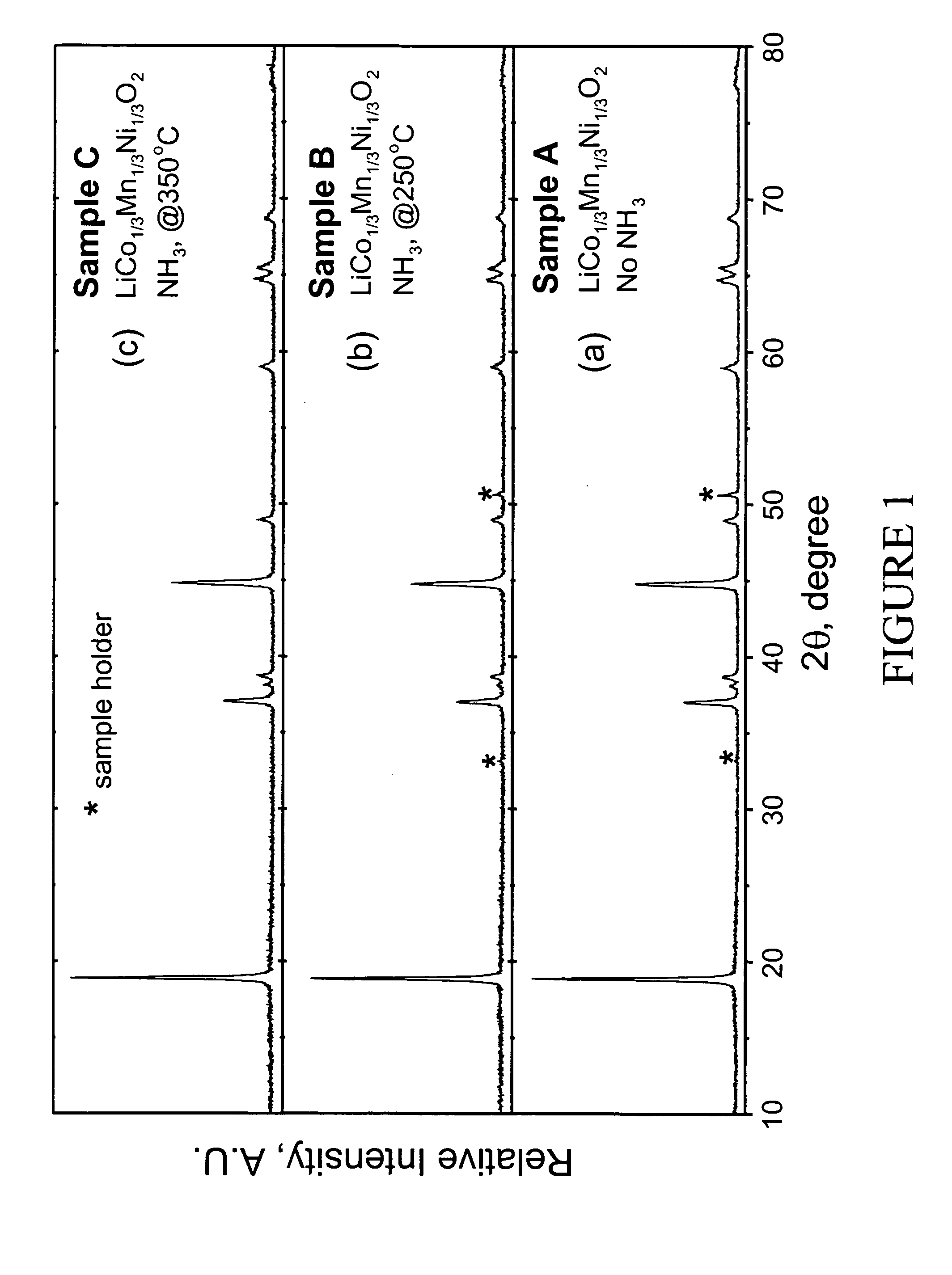
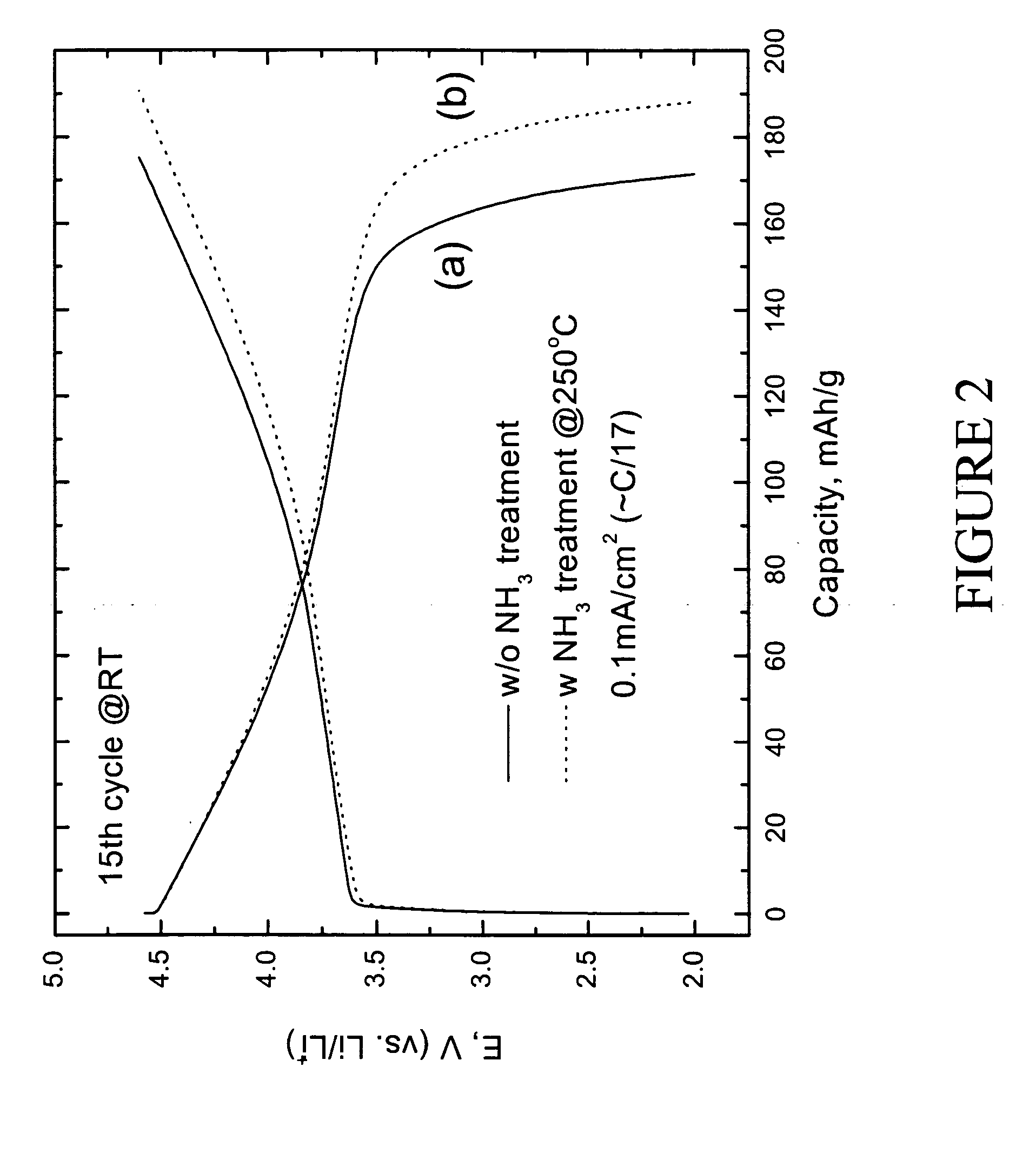
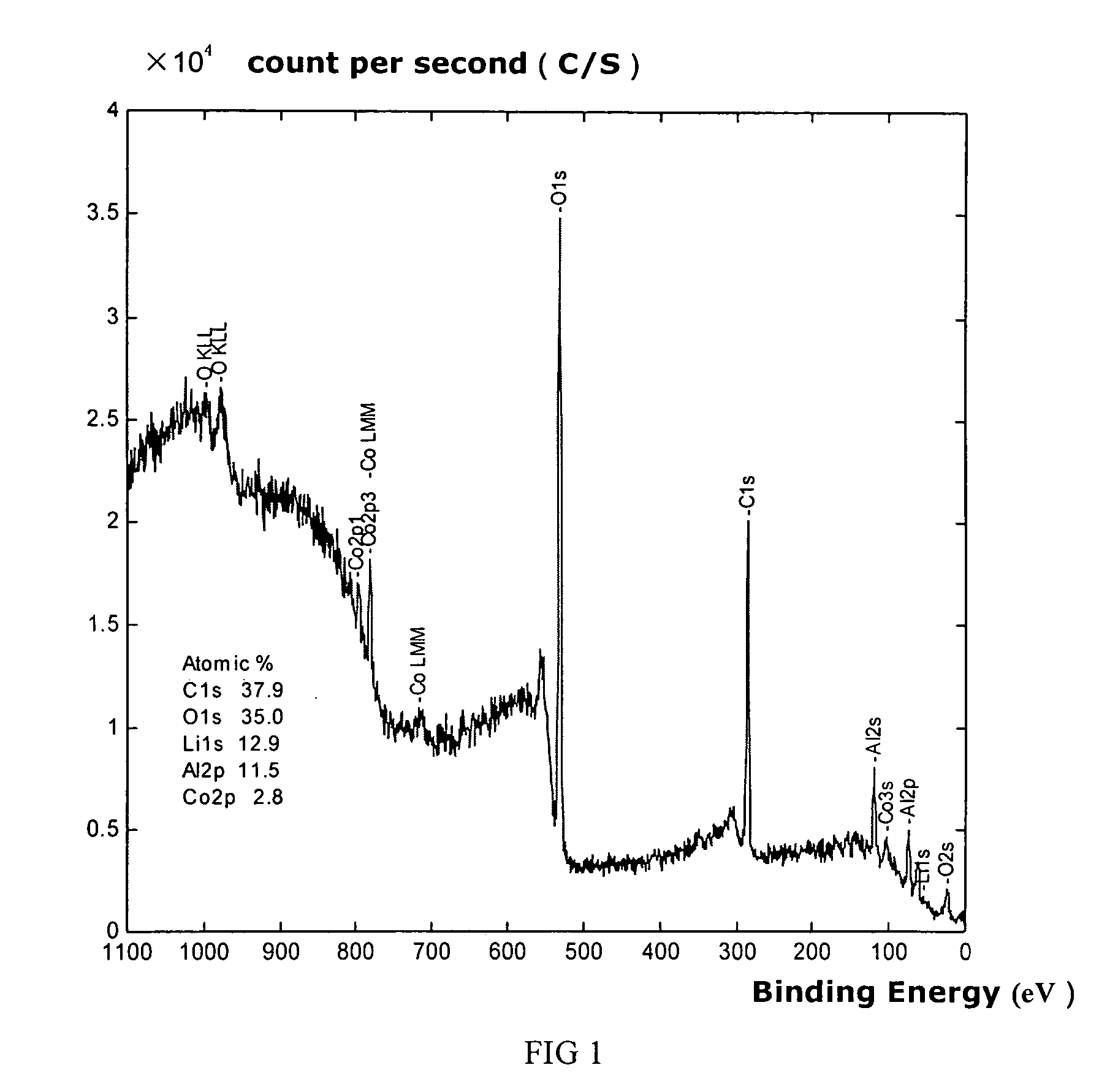
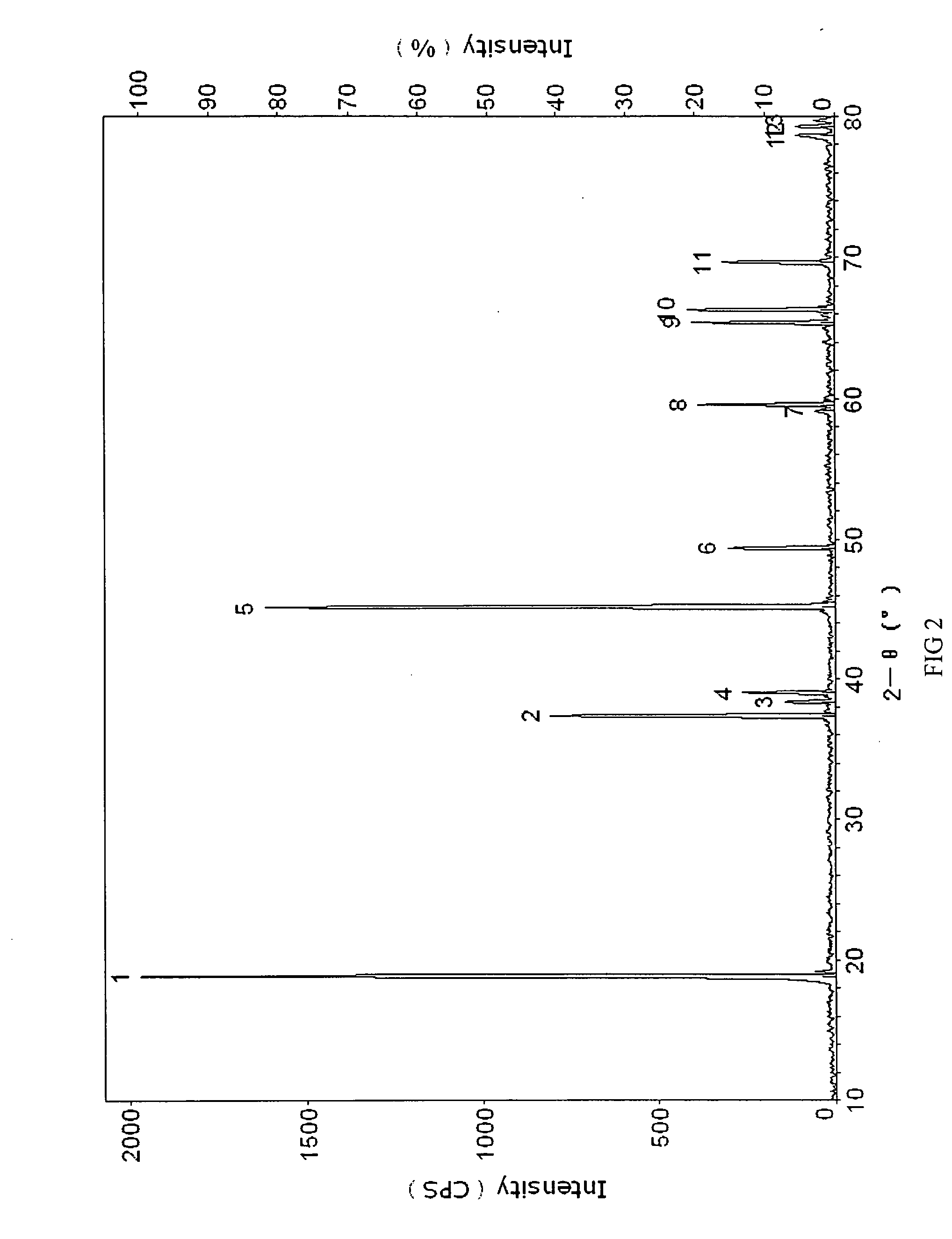
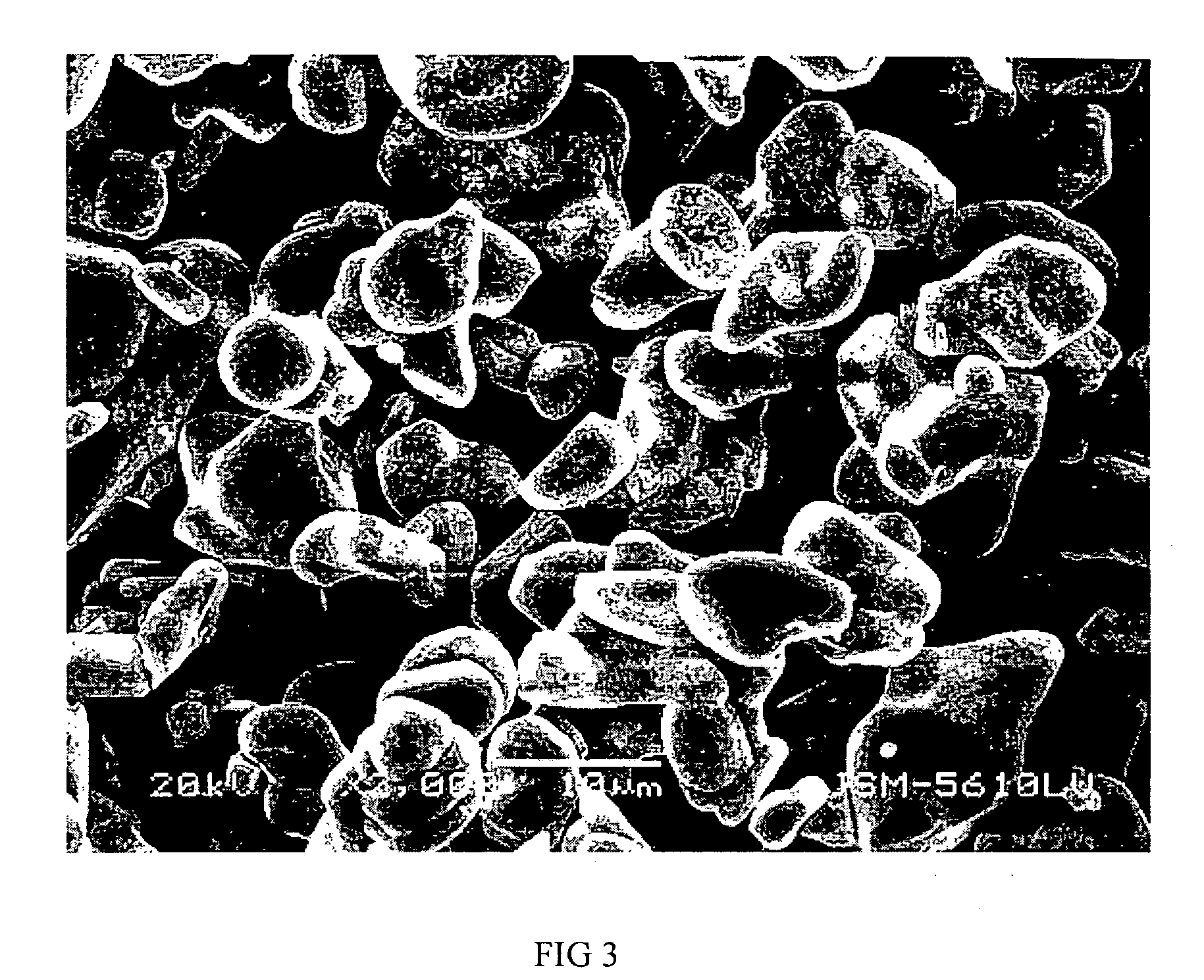
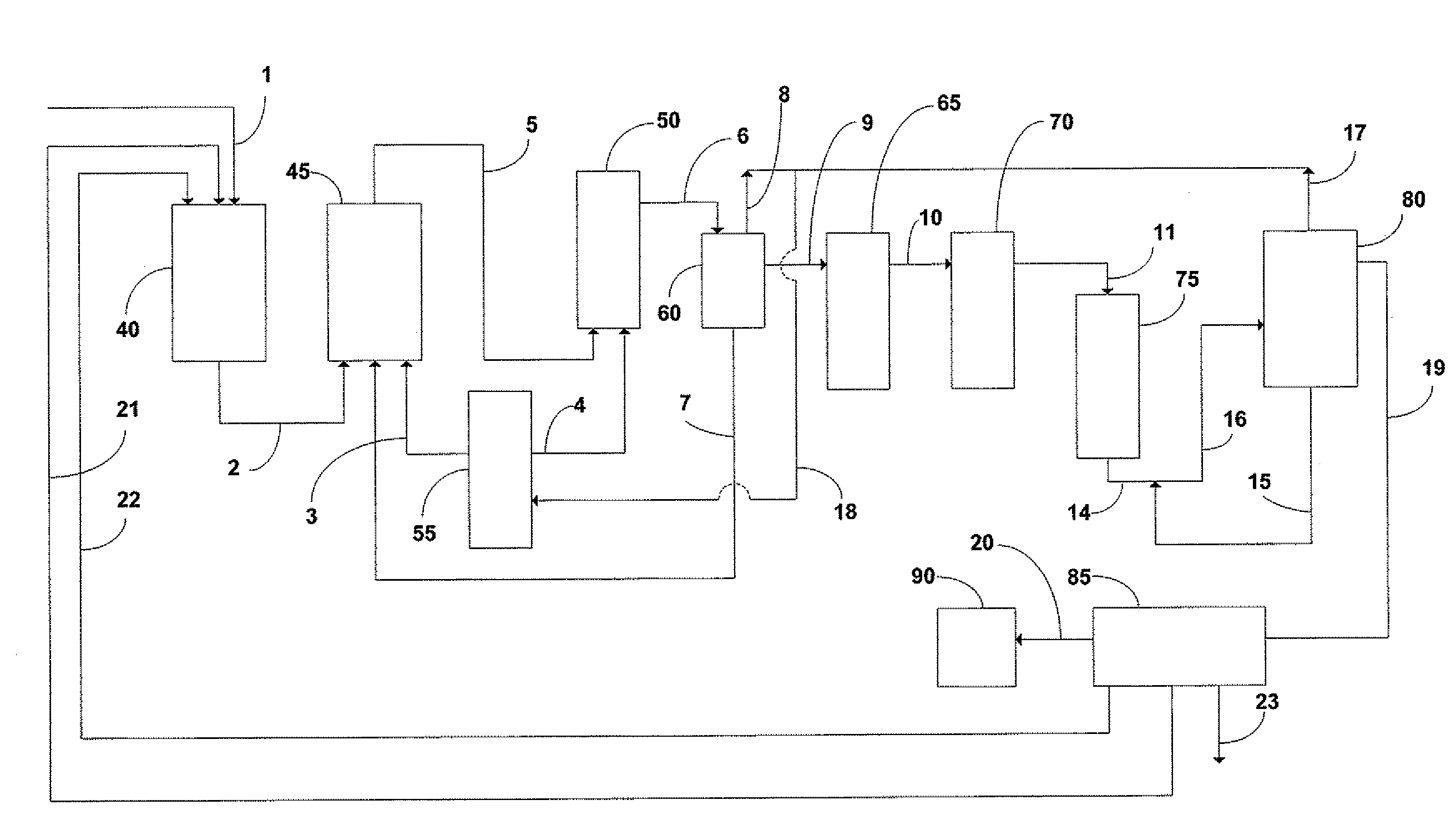
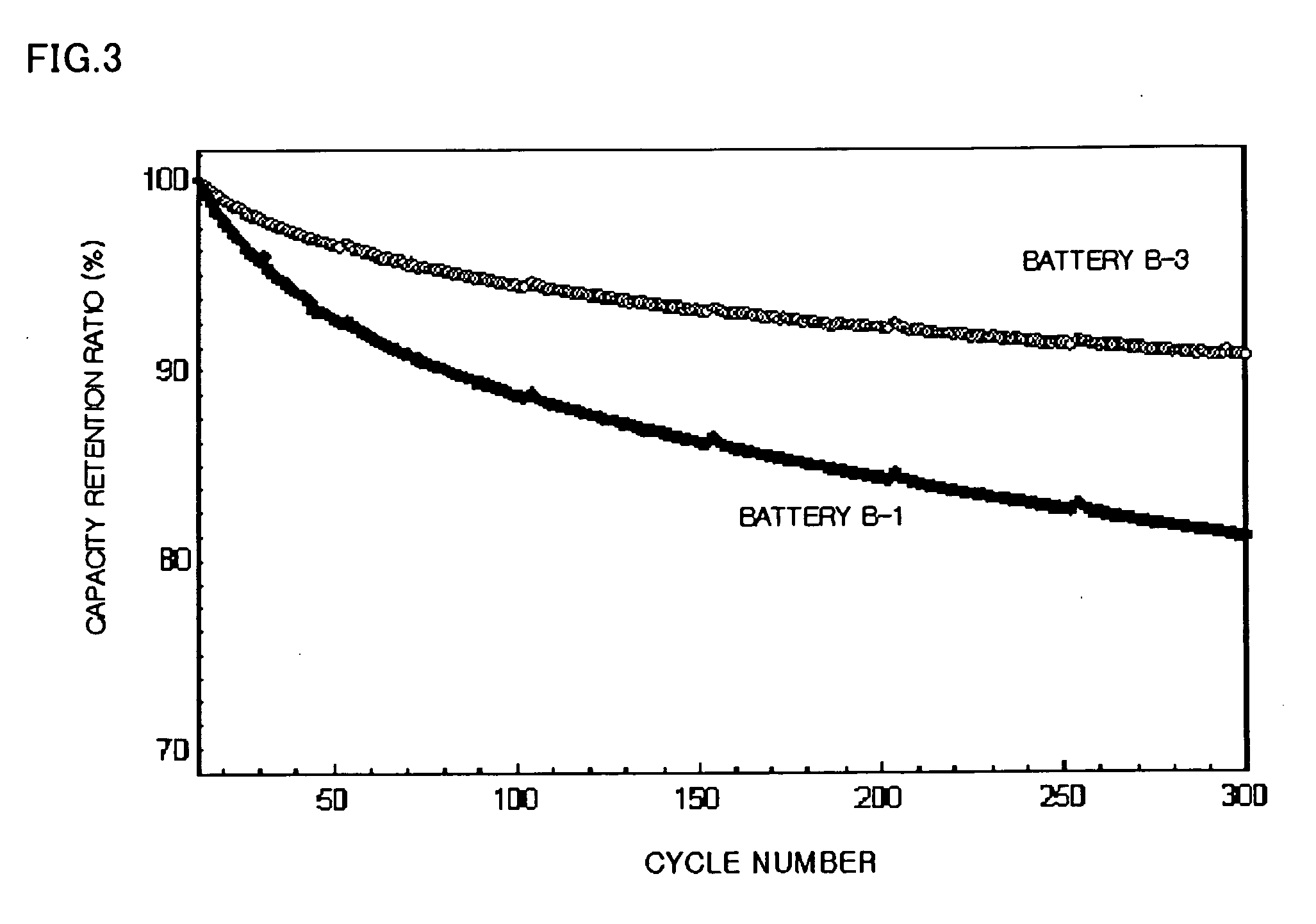
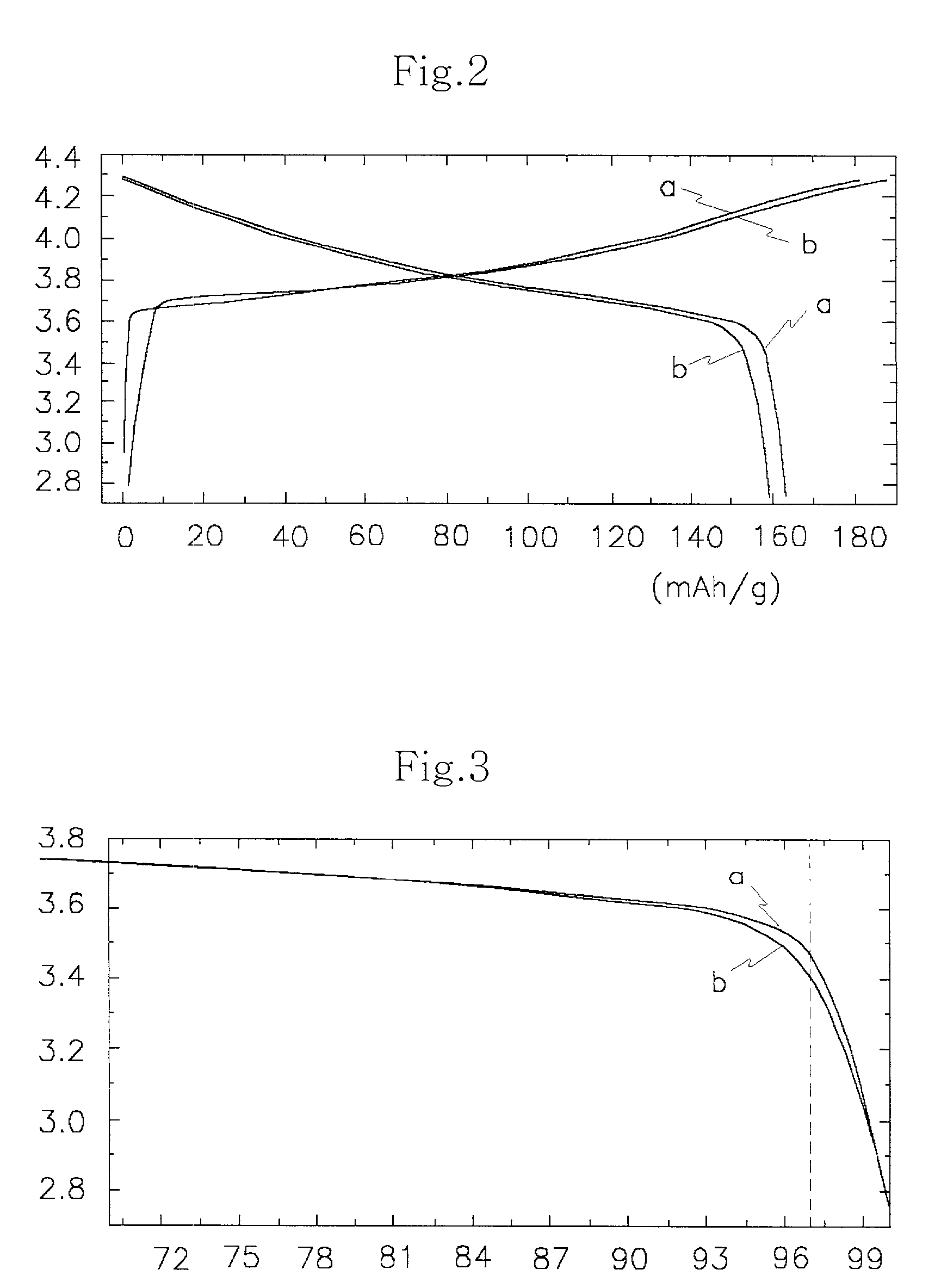
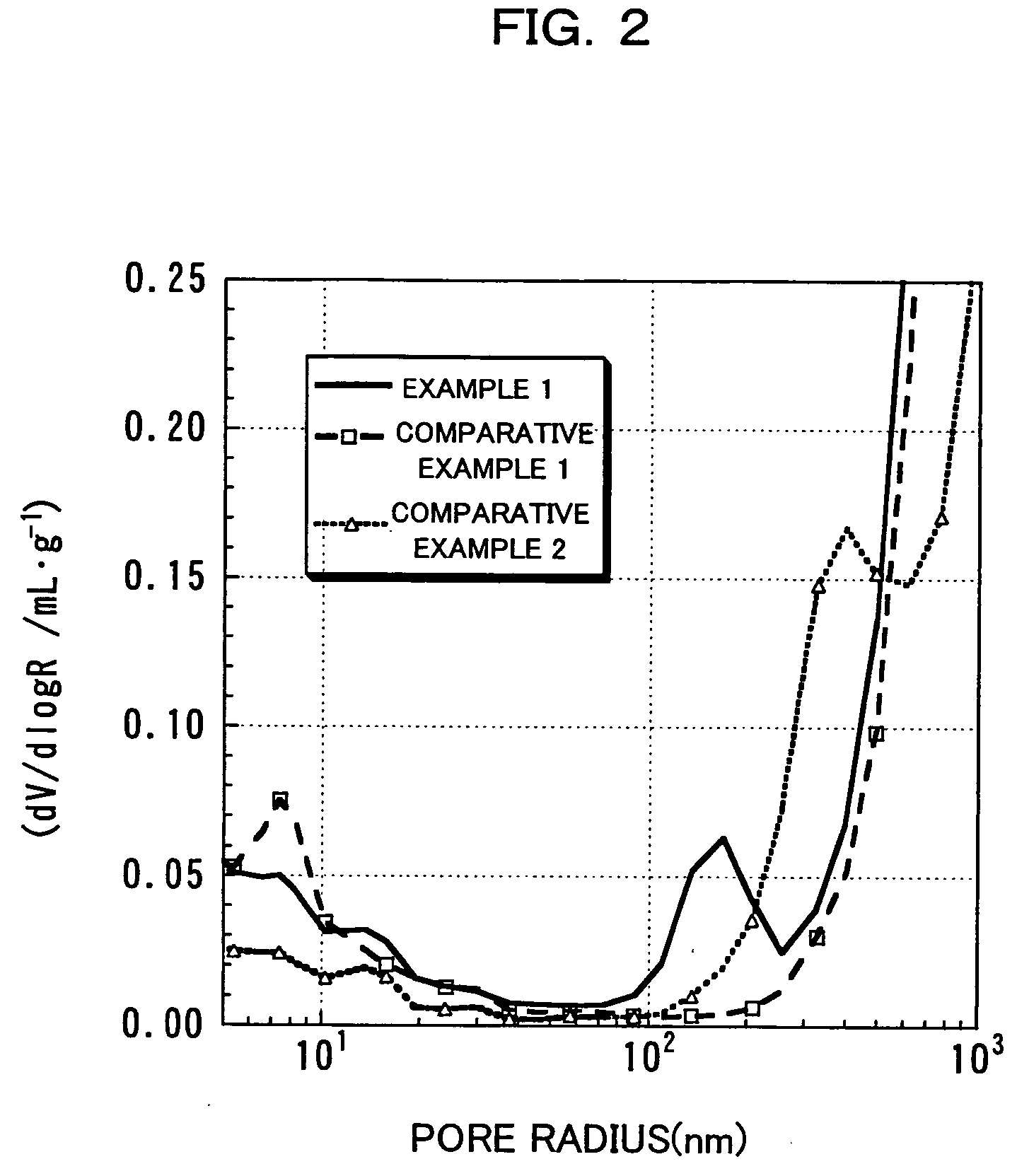
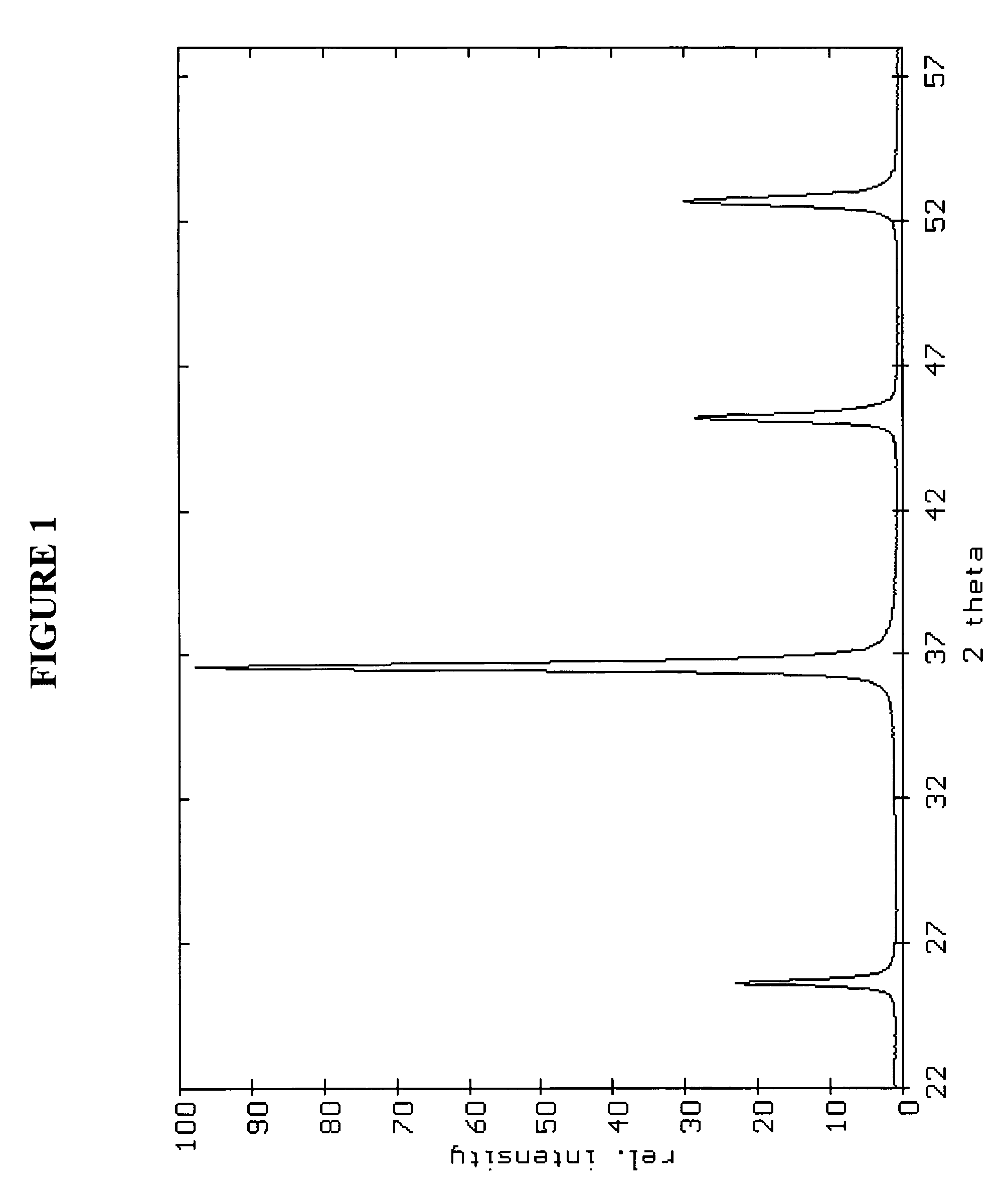
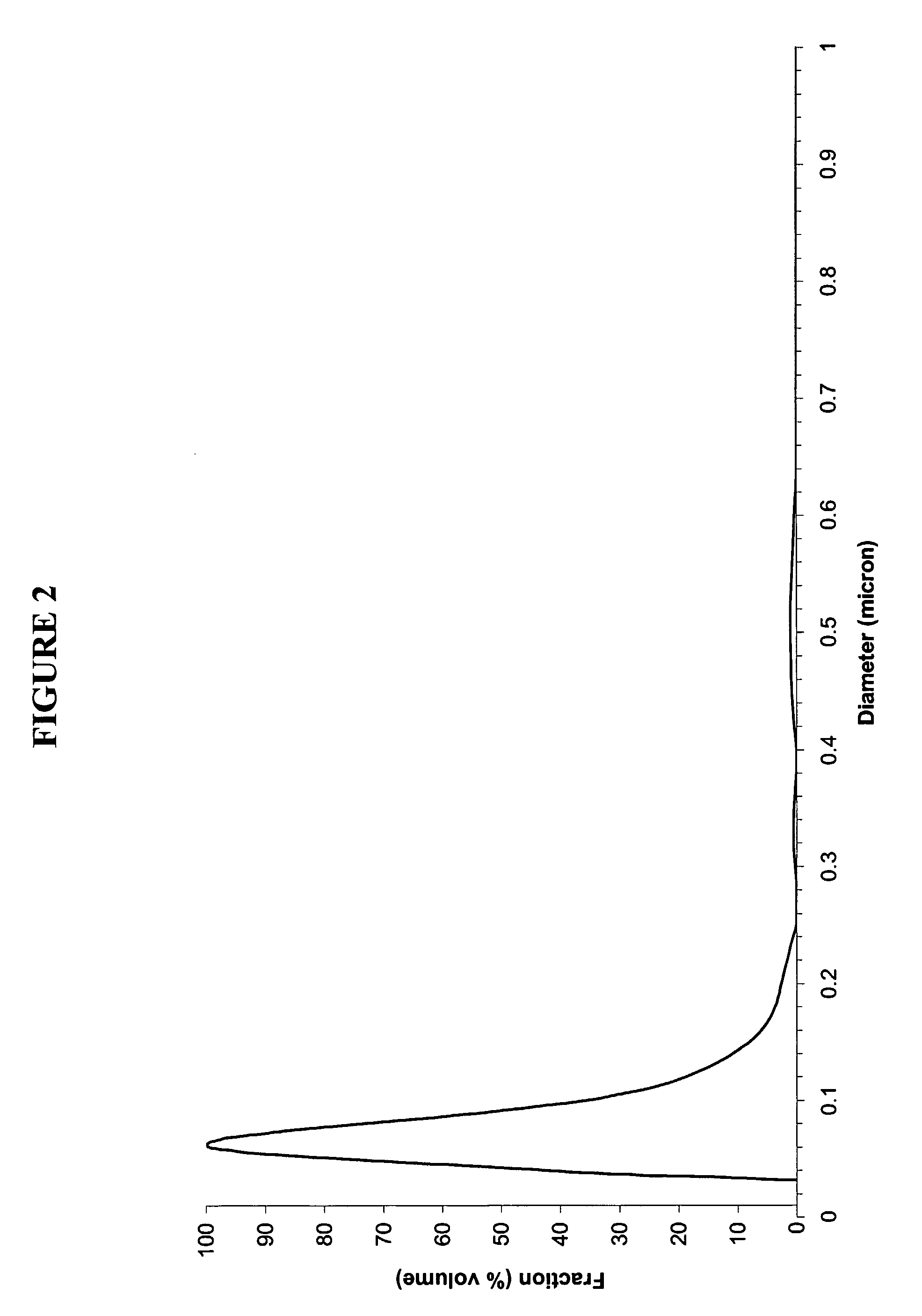
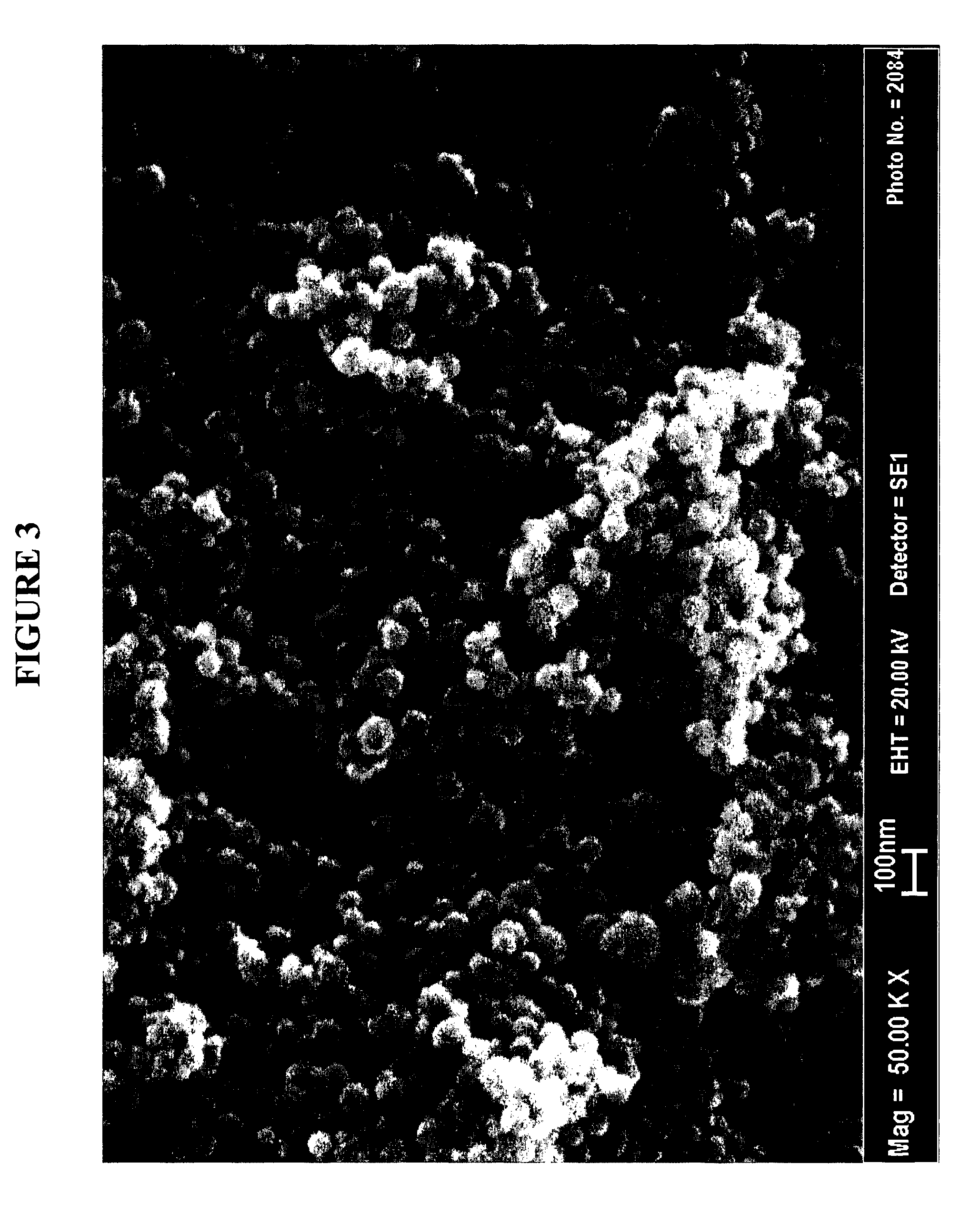
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1375results about "Manganates/permanganates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lithium metal oxide electrodes for lithium cells and batteries
InactiveUS7135252B2Improve structural stabilityImprove stabilityZirconium compoundsSecondary cellsLithium metalOxidation state
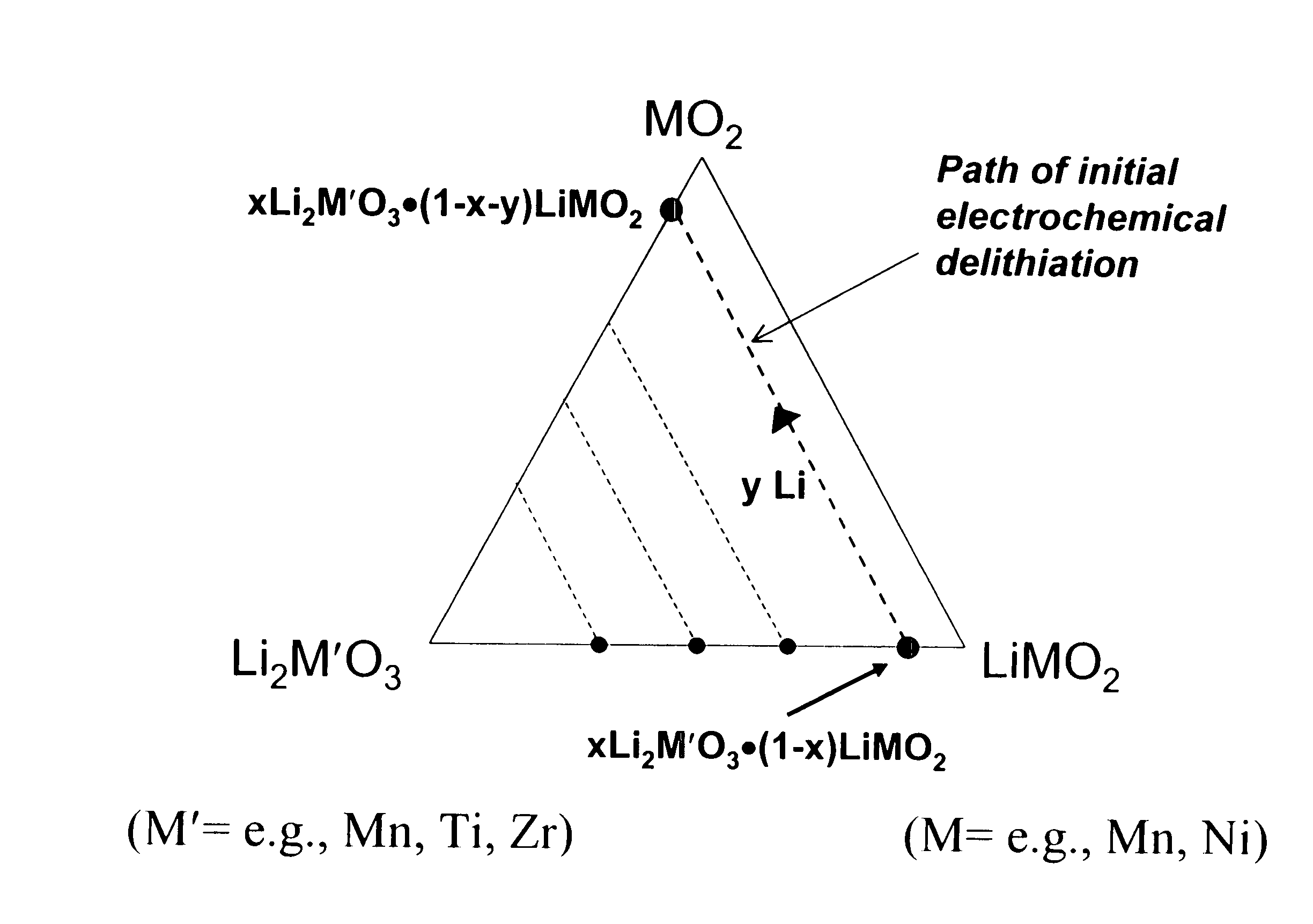
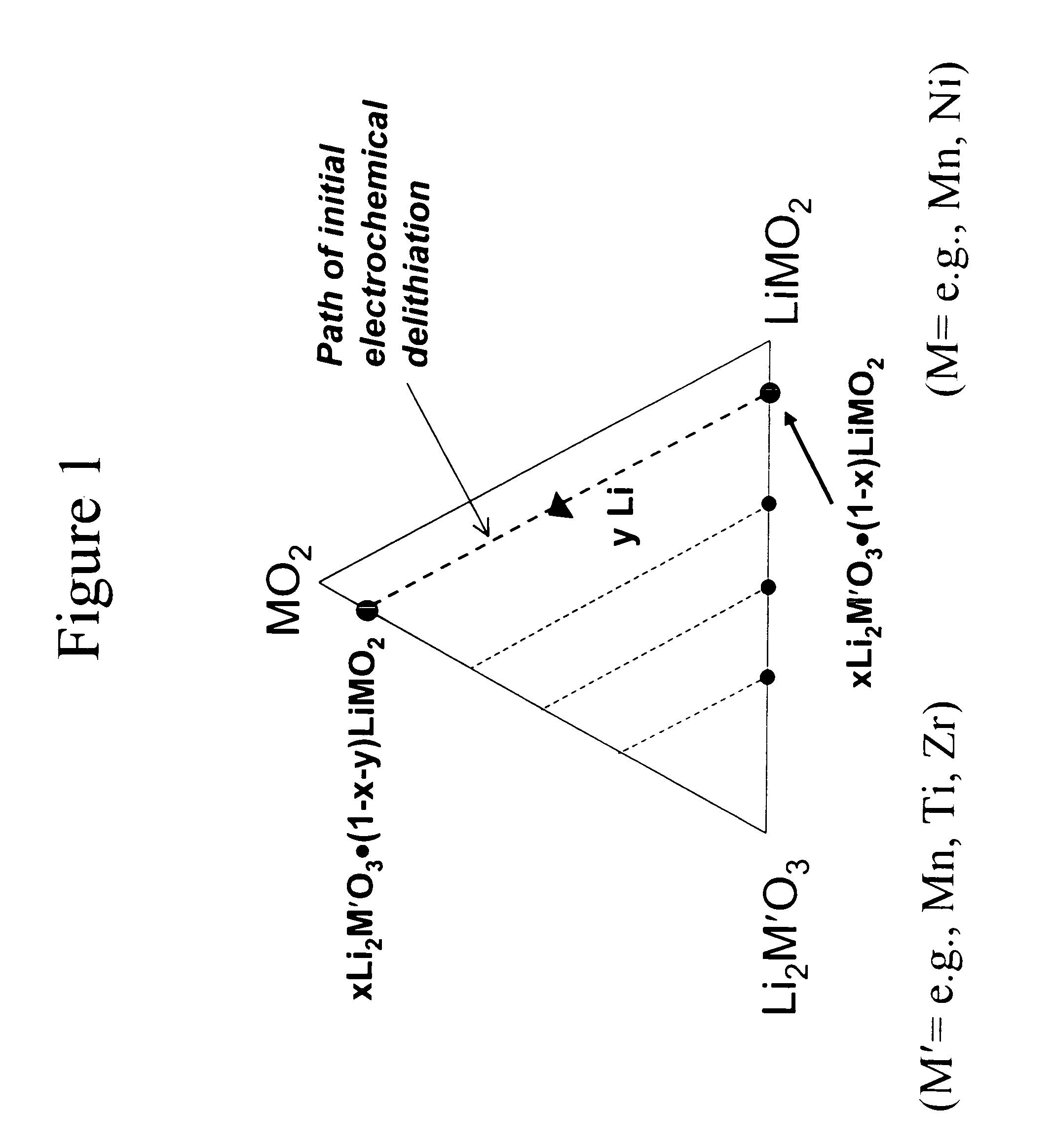
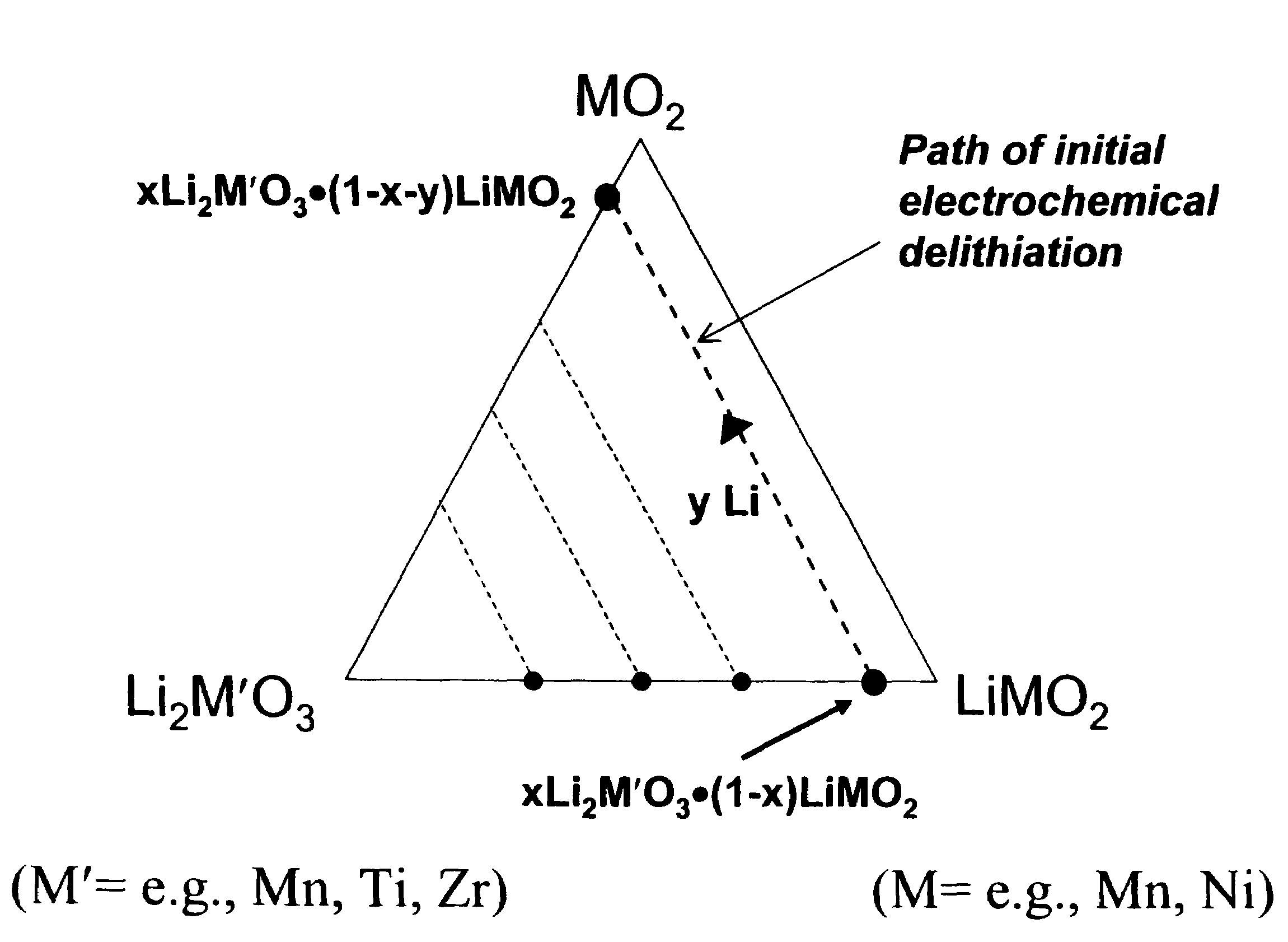
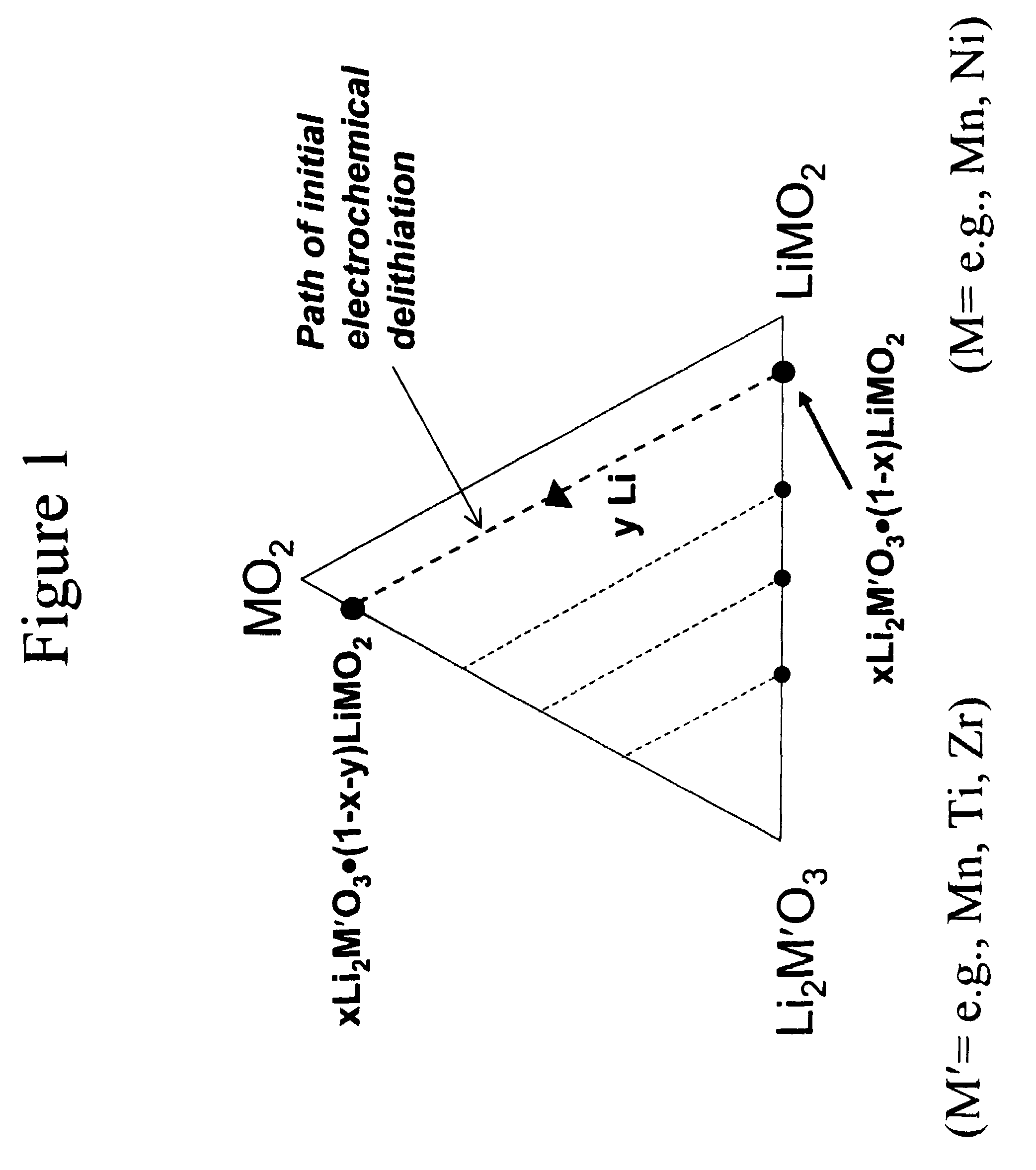
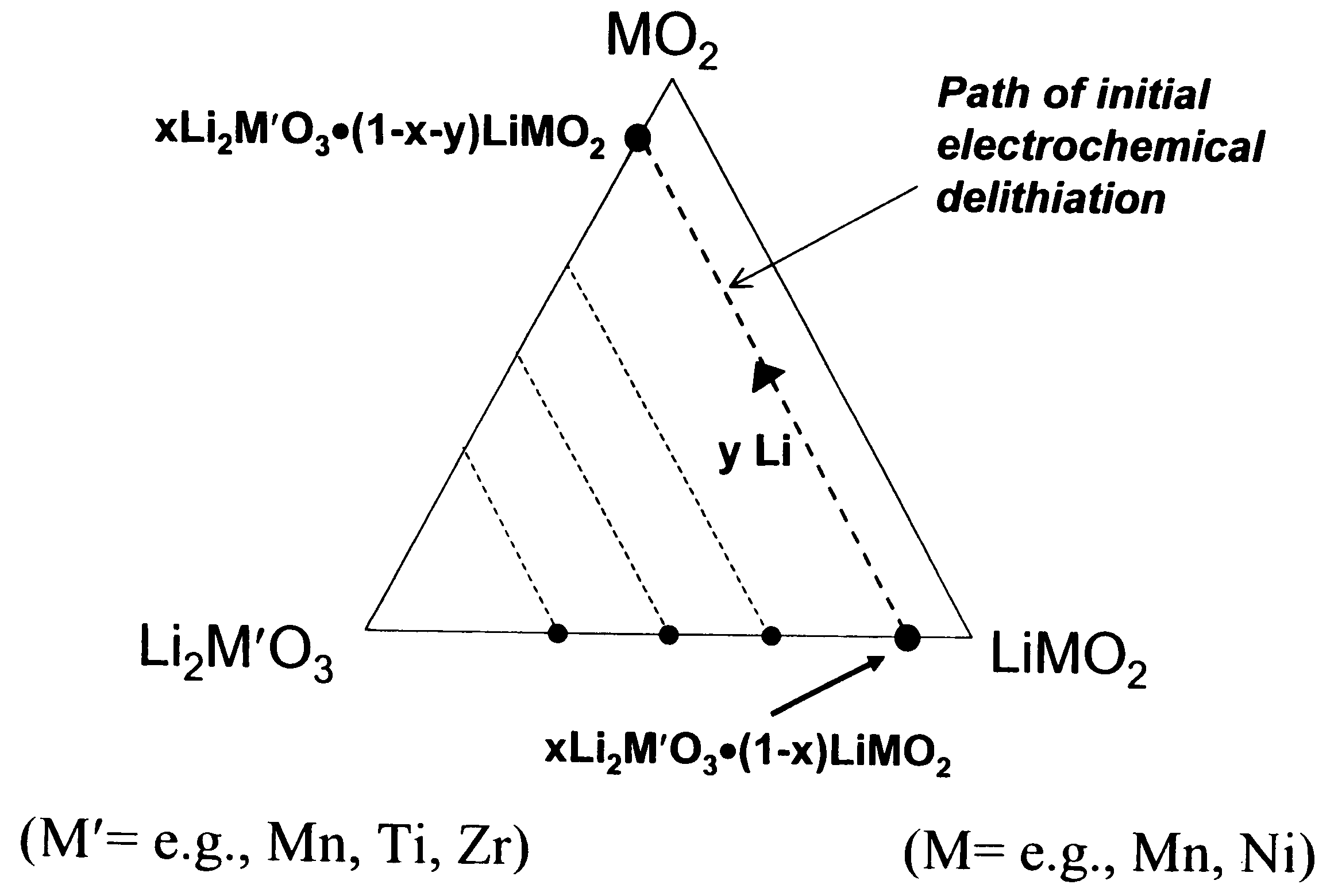
A lithium metal oxide positive electrode for a non-aqueous lithium cell is disclosed. The cell is prepared in its initial discharged state and has a general formula xLiMO2.(1-x)Li2M′O3 in which 0<x<1, and where M is more than one ion with an average trivalent oxidation state and with at least one ion being Ni, and where M′ is one or more ions with an average tetravalent oxidation state. Complete cells or batteries are disclosed with anode, cathode and electrolyte as are batteries of several cells connected in parallel or series or both.
Owner:CHICAGO UNIV OF THE +1
Layered cathode materials for lithium ion rechargeable batteries
ActiveUS7205072B2Improve impedance characteristicsImprove stabilityAlkali metal oxidesNon-aqueous electrolyte accumulator electrodesDopantRechargeable cell
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling.
Owner:UCHICAGO ARGONNE LLC +2
Lithium metal oxide electrodes for lithium cells and batteries
InactiveUS7468223B2Improve electronic conductivityLess costlyLi-accumulatorsNon-aqueous electrolyte accumulator electrodesLithium metalOxidation state
Owner:UCHICAGO ARGONNE LLC +1
Catalysts for petrochemical catalysis
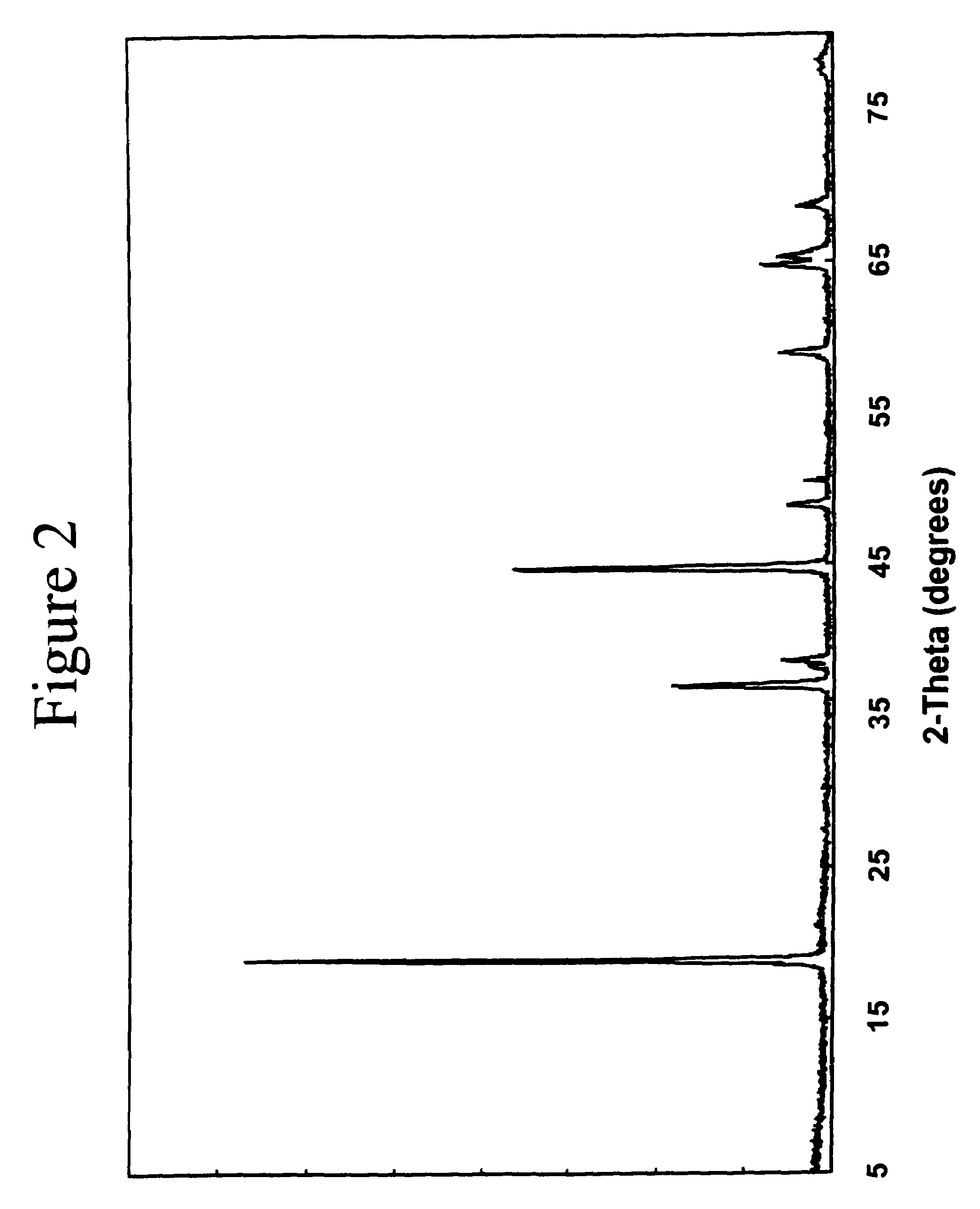
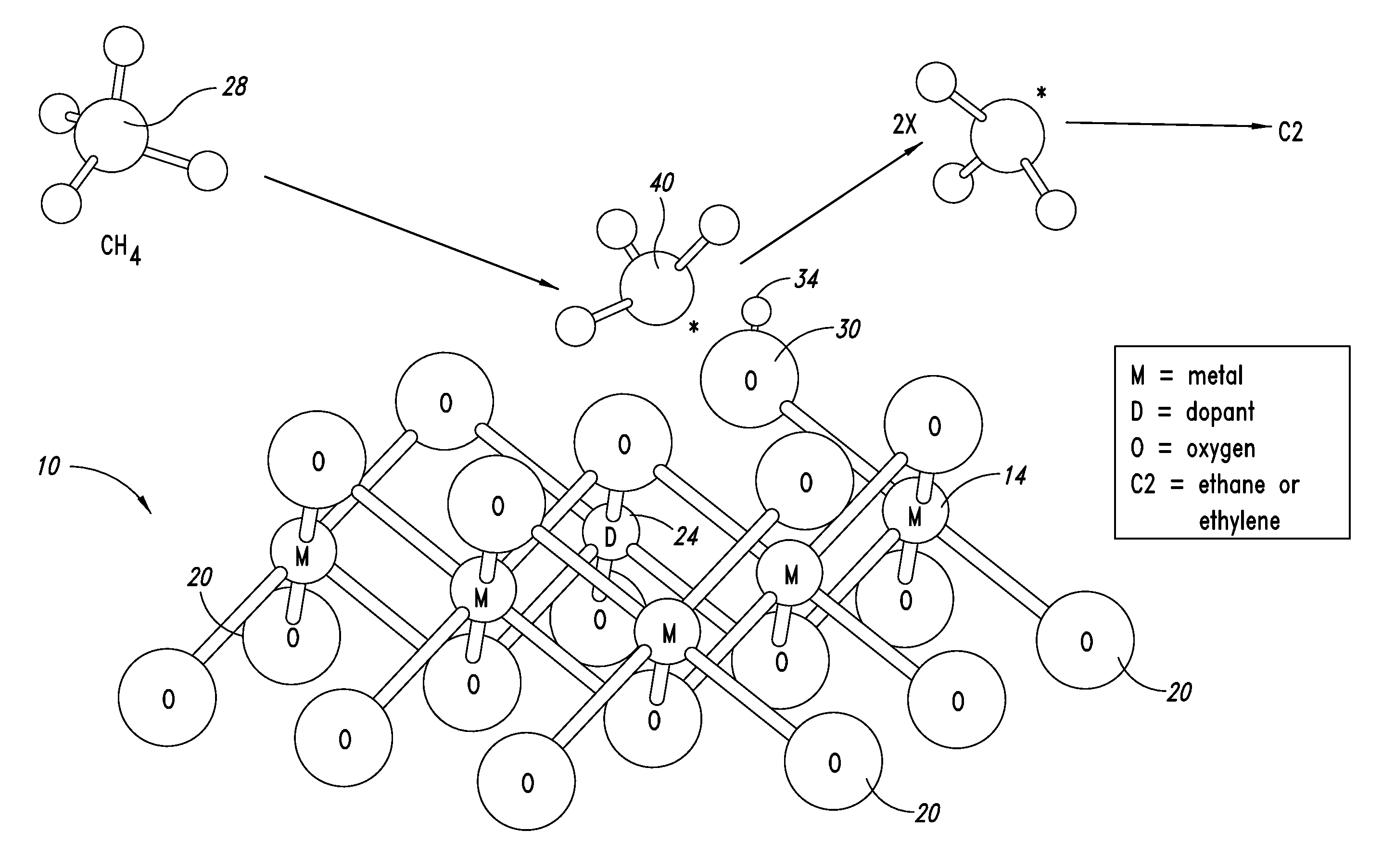
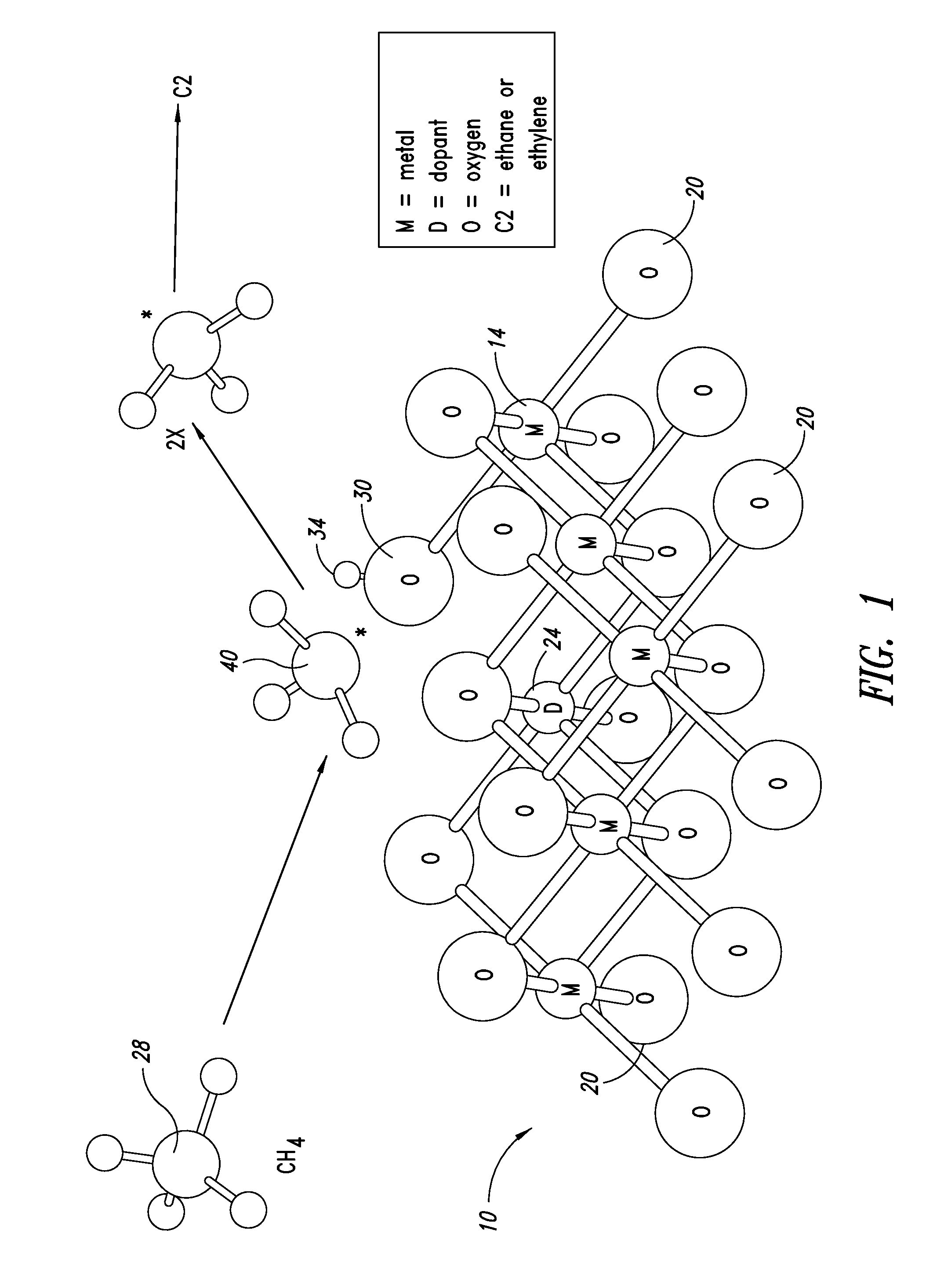
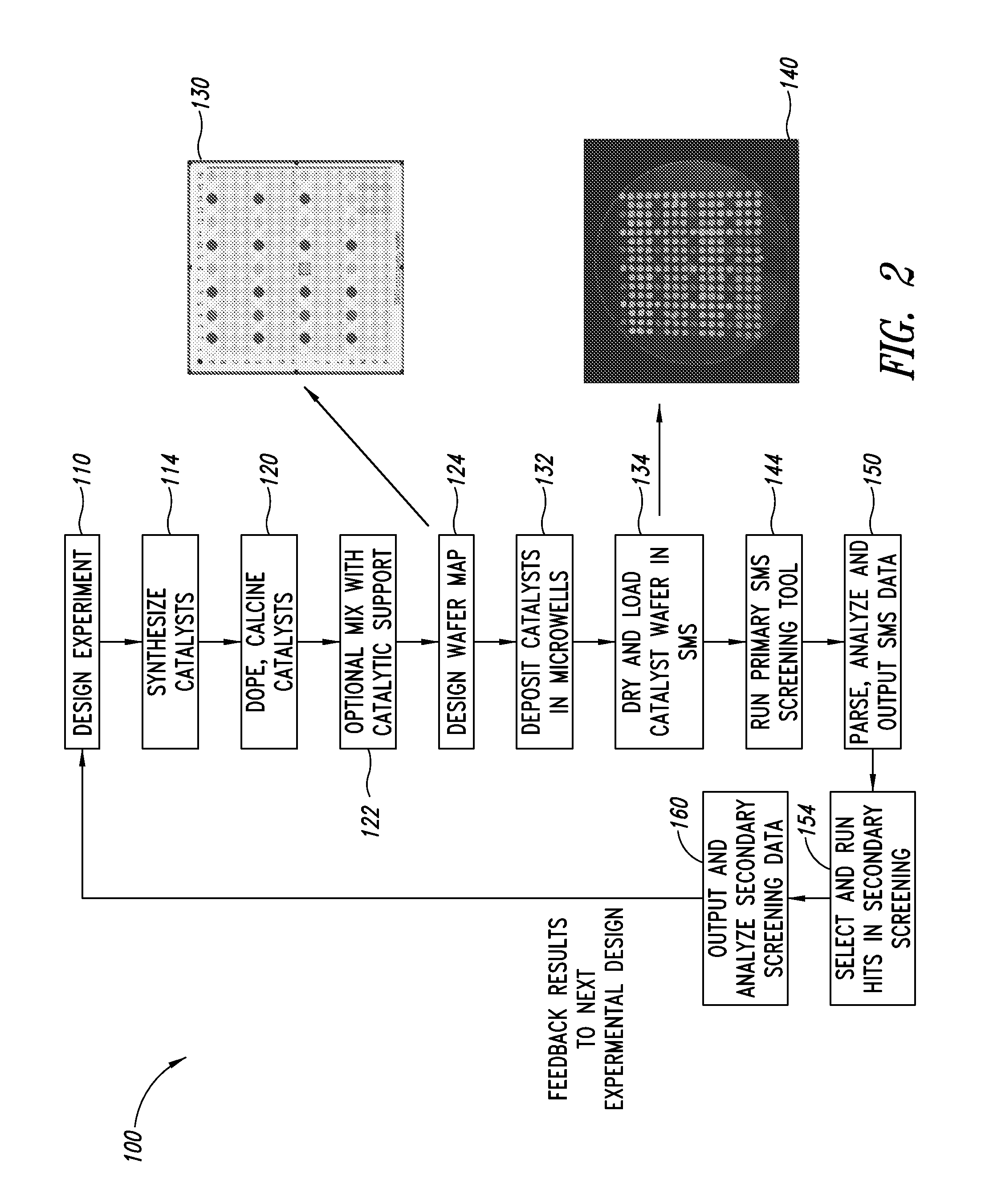
ActiveUS20130023709A1Sequential/parallel process reactionsManganese oxides/hydroxidesDopantPetrochemical
Metal oxide catalysts comprising various dopants are provided. The catalysts are useful as heterogenous catalysts in a variety of catalytic reactions, for example, the oxidative coupling of methane to C2 hydrocarbons such as ethane and ethylene. Related methods for use and manufacture of the same are also disclosed.
Owner:SILURIA TECH INC
Ni-based lithium transition metal oxide
The present invention provides a powderous lithium transition metal oxide with the composition as represented by the below Formula and prepared by solid state reaction in air from a mixed transition metal precursor and Li2CO3, with being practically free of Li2CO3 impurity: LixMyO2 wherein M=M′l-kAk, where M′=Nil-a-b(Ni1 / 2Mn1 / 2)aCob on condition of 0.65≦a+b≦0.85 and 0.1≦b≦0.4; A is a dopant; and 0≦k≦0.05; and x+y=2 on condition of 0.95≦x≦1.05. The Ni-based lithium transition metal oxide according to the present invention has a well-layered structure, and also improved safety, cycling stability and stability against aging and low gas evolution during storage, when used as an active material for cathode of lithium secondary batteries, because it has a high sintering stability and is substantially free of soluble bases. Moreover, the lithium transition metal oxide of the present invention can be prepared by a low-cost process using a mixed transition metal precursor and Li2CO3 as raw stocks and under relatively unrestricted conditions.
Owner:LG ENERGY SOLUTION LTD
Lithium-ion secondary battery
InactiveUS20070026315A1Safer chemistry characteristicLow cathode costPrimary cell to battery groupingFinal product manufactureManganateSpinel
A lithium-ion battery includes a cathode that includes an active cathode material. The active cathode material includes a cathode mixture that includes a lithium cobaltate and a manganate spinel a manganate spinel represented by an empirical formula of Li(1+x1)(Mn1−y1A′y2)2−x2Oz1. The lithium cobaltate and the manganate spinel are in a weight ratio of lithium cobaltate: manganate spinel between about 0.95:0.05 to about 0.55:0.45. A lithium-ion battery pack employs a cathode that includes an active cathode material as described above. A method of forming a lithium-ion battery includes the steps of forming an active cathode material as described above; forming a cathode electrode with the active cathode material; and forming an anode electrode in electrical contact with the cathode via an electrolyte.
Owner:BOSTON POWER INC
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
Lithium metal oxide electrodes for lithium cells and batteries
InactiveUS20060099508A1Improve structural stabilityImprove stabilityLi-accumulatorsNon-aqueous electrolyte accumulator electrodesLithium metalOxidation state
A lithium metal oxide positive electrode for a non-aqueous lithium cell is disclosed. The cell is prepared in its initial discharged state and has a general formula xLiMO2.(1−x)Li2M′O3 in which 0<x<1, and where M is one or more ion with an average trivalent oxidation state and with at least one ion being Mn or Ni, and where M′ is one or more ion with an average tetravalent oxidation state. Complete cells or batteries are disclosed with anode, cathode and electrolyte as are batteries of several cells connected in parallel or series or both.
Owner:UCHICAGO ARGONNE LLC +1
Layered Lithium Nickel Manganese Cobalt Composite Oxide Powder For Material Of Positive Electrode Of Lithium Secondary Battery, Process For Producing The Same, Positive Electrode Of Lithium Secondary Battery Therefrom, And Lithium Secondary Battery
ActiveUS20070202405A1Improve securityImprove battery performanceNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsManganeseCobalt
A powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is provided which, when used as a positive-electrode material for lithium secondary battery, enables a cost reduction and higher safety to be reconciled with improved battery performances. The powder of a layered lithium-nickel-manganese-cobalt composite oxide for use as a positive-electrode material for lithium secondary battery is composed of secondary particles formed by the aggregation of primary particles. It has a composition represented by the following formula (I), has a volume resistivity of 5×105 Ω·cm or lower in the state of being compacted at a pressure of 40 MPa, and has a value of C / S, wherein C is the concentration of carbon contained therein (% by weight) and S is the BET specific surface area thereof (m2 / g), of 0.025 or smaller. The powder has been regulated so as to have a volume resistivity not higher than the specified value and a considerably reduced carbon content while having a composition in a limited range, whereby a cost reduction and higher safety can be reconciled with improved battery performances. Li1+zNixMnyCo1−x−yOδ (I) (0<z≦0.91, 0.1≦x≦0.55, 0.20≦y≦0.90, 0.50≦x+y≦1, 1.9≦δ≦3)
Owner:MITSUBISHI CHEM CORP
Anode active material, manufacturing method thereof, and non-aqueous electrolyte secondary battery
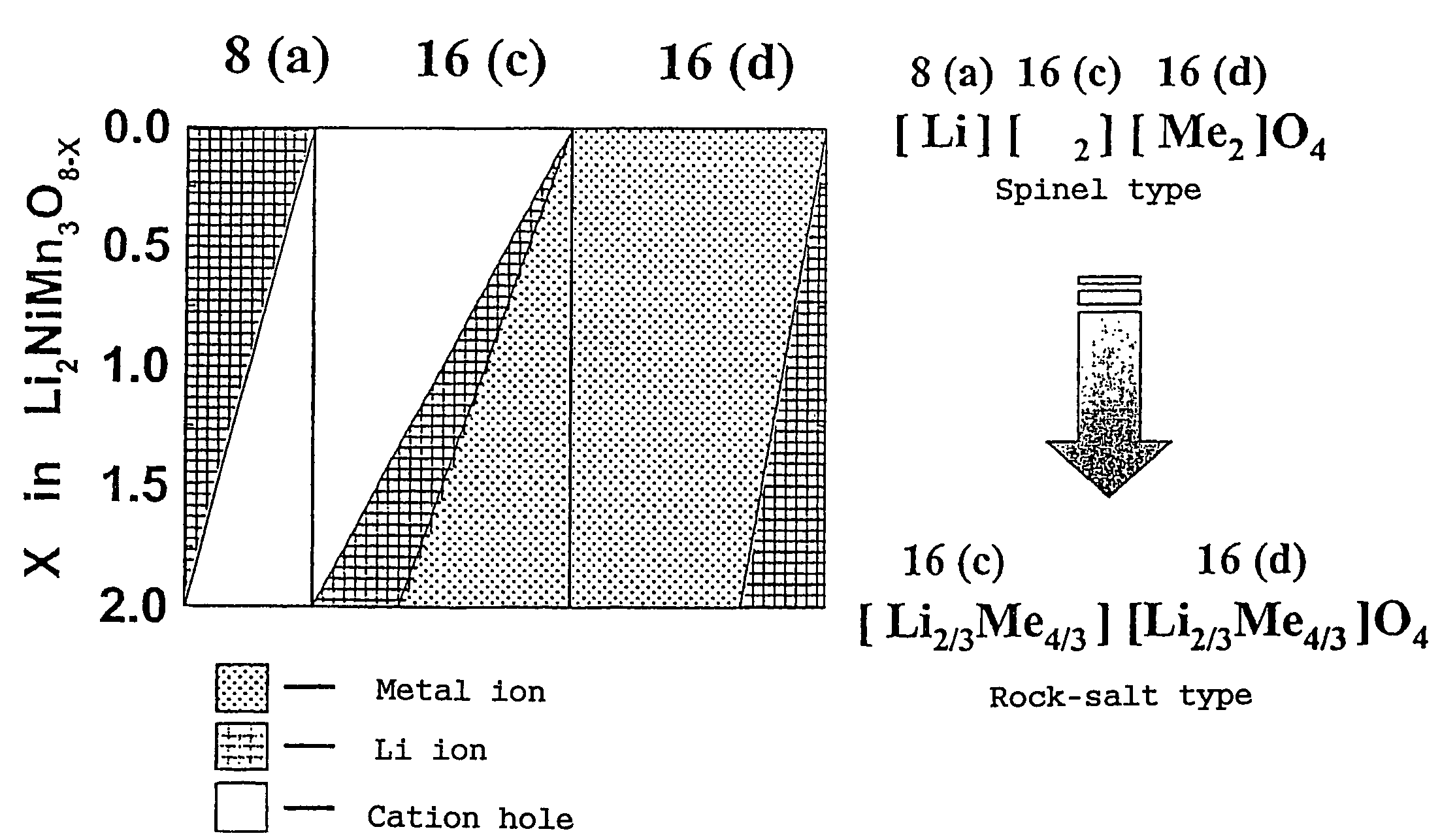
In order to provide a 3V level non-aqueous electrolyte secondary battery with a flat voltage and excellent cycle life at a high rate with low cost, the present invention provides a positive electrode represented by the formula: Li2±α[Me]4O8−x, wherein 0≦α<0.4, 0≦x<2, and Me is a transition metal containing Mn and at least one selected from the group consisting of Ni, Cr, Fe, Co and Cu, said active material exhibiting topotactic two-phase reactions during charge and discharge.
Owner:OSAKA CITY UNIV +1
Positive electrode active material for lithium secondary cell and lithium secondary cell
ActiveUS7393476B2Improve cycle performanceIncrease energy densityMaterial nanotechnologyCell temperature controlCrystallographyComposite oxide
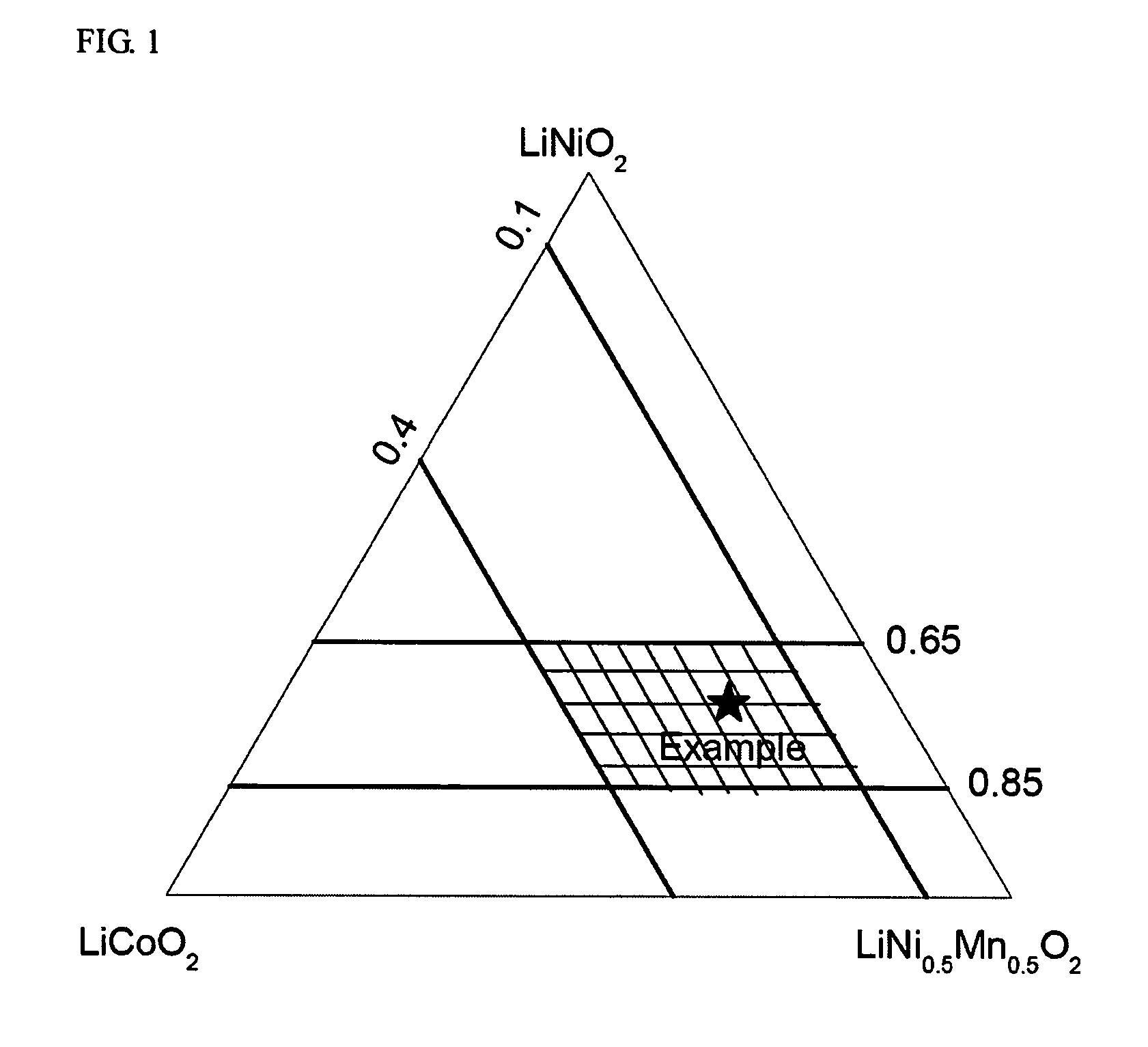
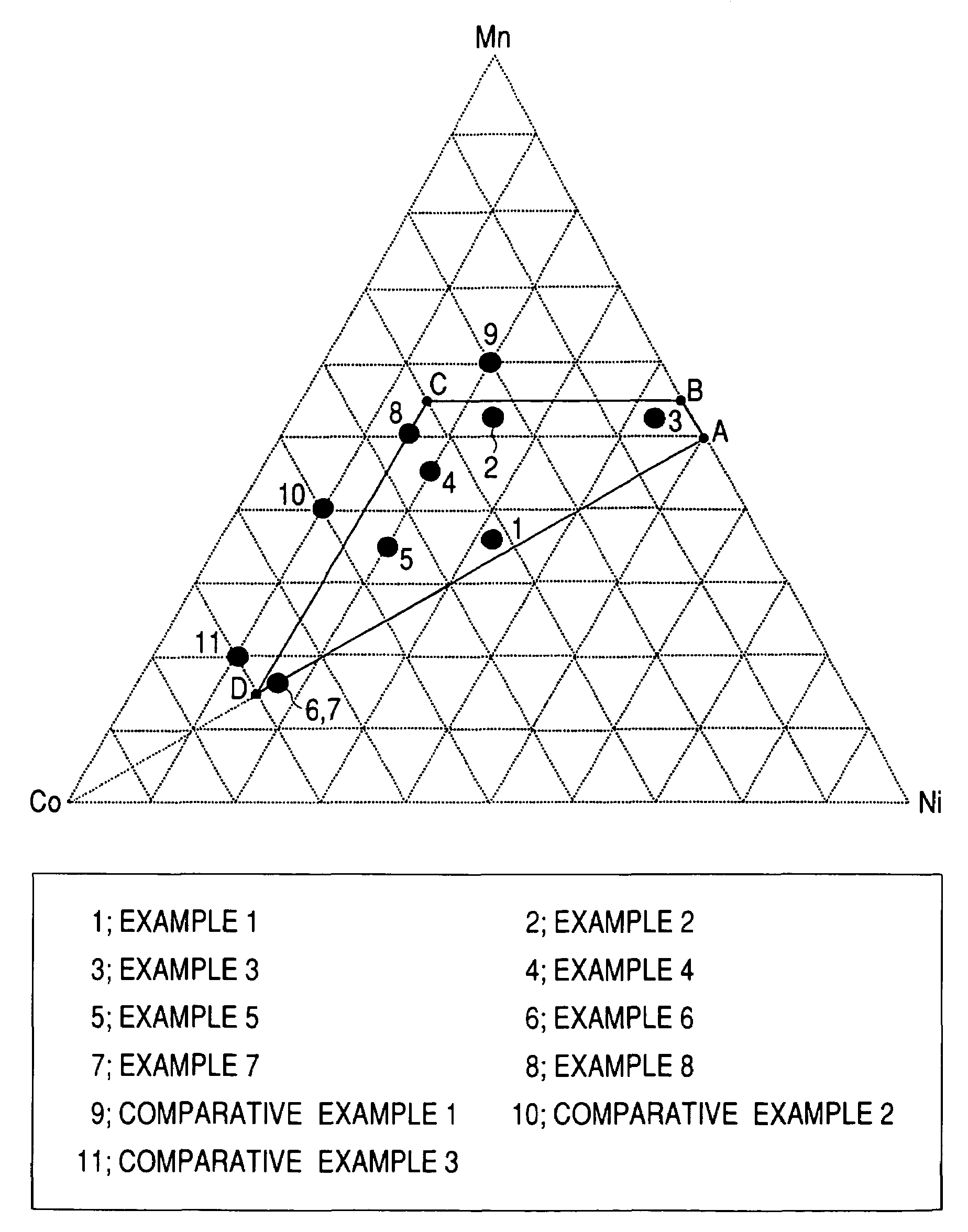
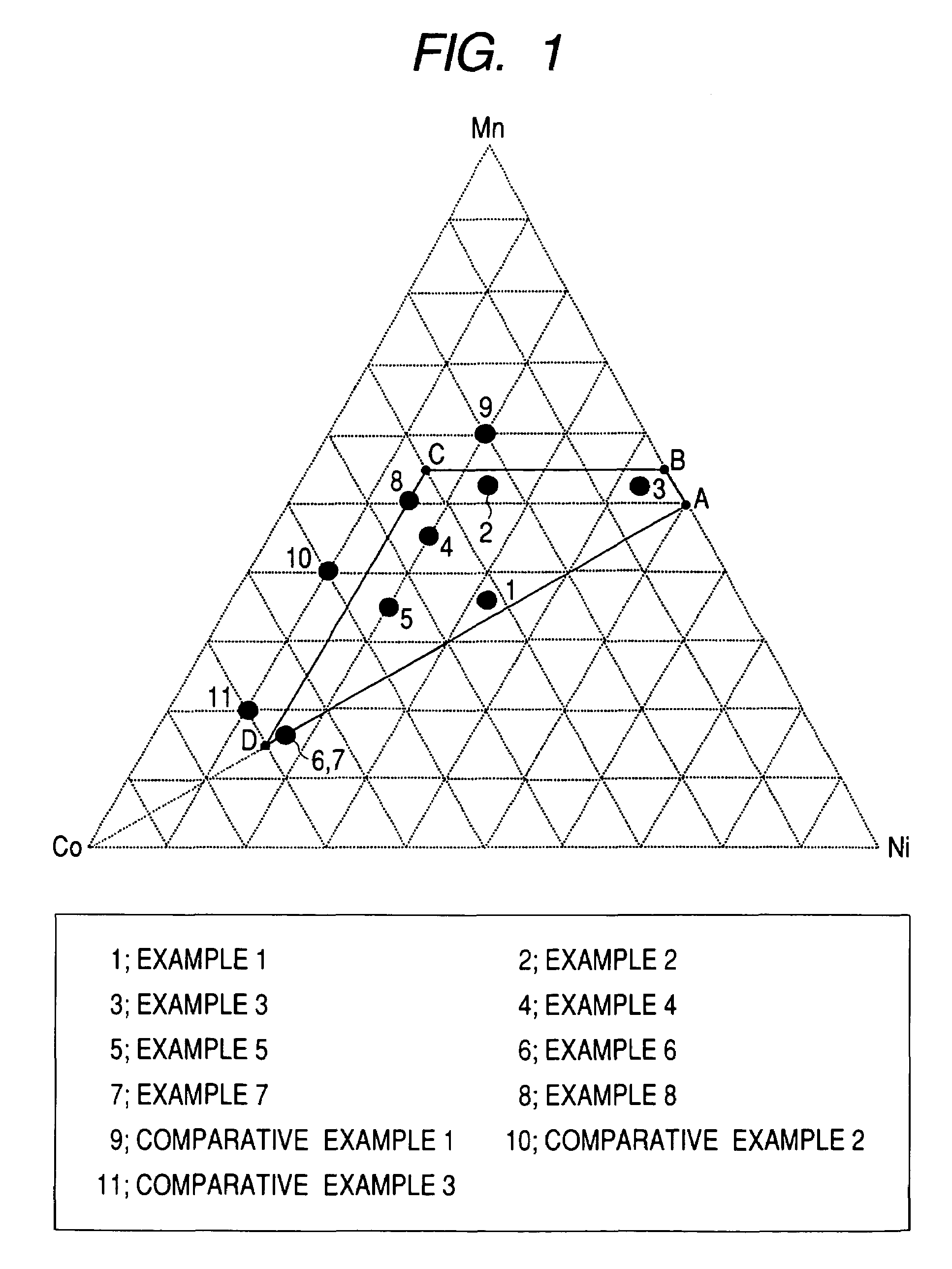
A positive active material for lithium secondary batteries, includes a composite oxide including an oxide which is represented by the composite formula LixMnaNibCocO2 and has an α-NaFeO2 structure, and an impurity phase including Li2MnO3. The values a, b, and c are within such a range that in a ternary phase diagram showing the relationship among these, (a, b, c) is present on the perimeter of or inside the quadrilateral ABCD defined by point A (0.5, 0.5, 0), point B (0.55, 0.45, 0), point C (0.55, 0.15, 0.30), and point D (0.15, 0.15, 0.7) as vertexes, and 0.95<x / (a+b+c)<1.35.
Owner:GS YUASA INT LTD
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Processes for preparing highly pure lithium carbonate and other highly pure lithium containing compounds
ActiveUS20110200508A1Electrolysis componentsLithium organic compoundsLithium carbonateLithium hydroxide
Owner:TERRALITHIUM LLC
Lithium metal oxide electrodes for lithium batteries
InactiveUS20060188781A1Increase capacityImprove cycle stabilityActive material electrodesAlkali metal oxidesLithium metalOxidation state
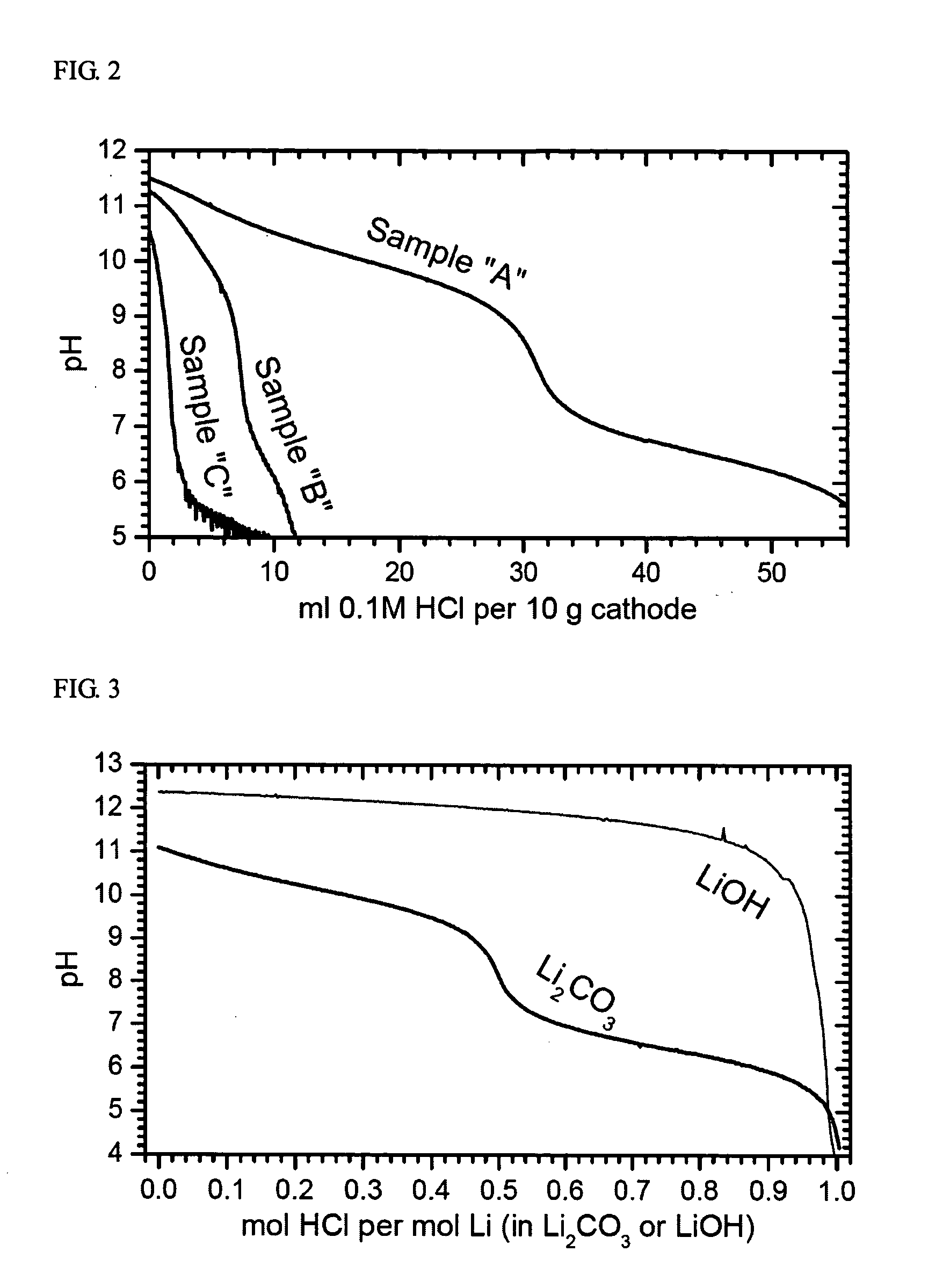
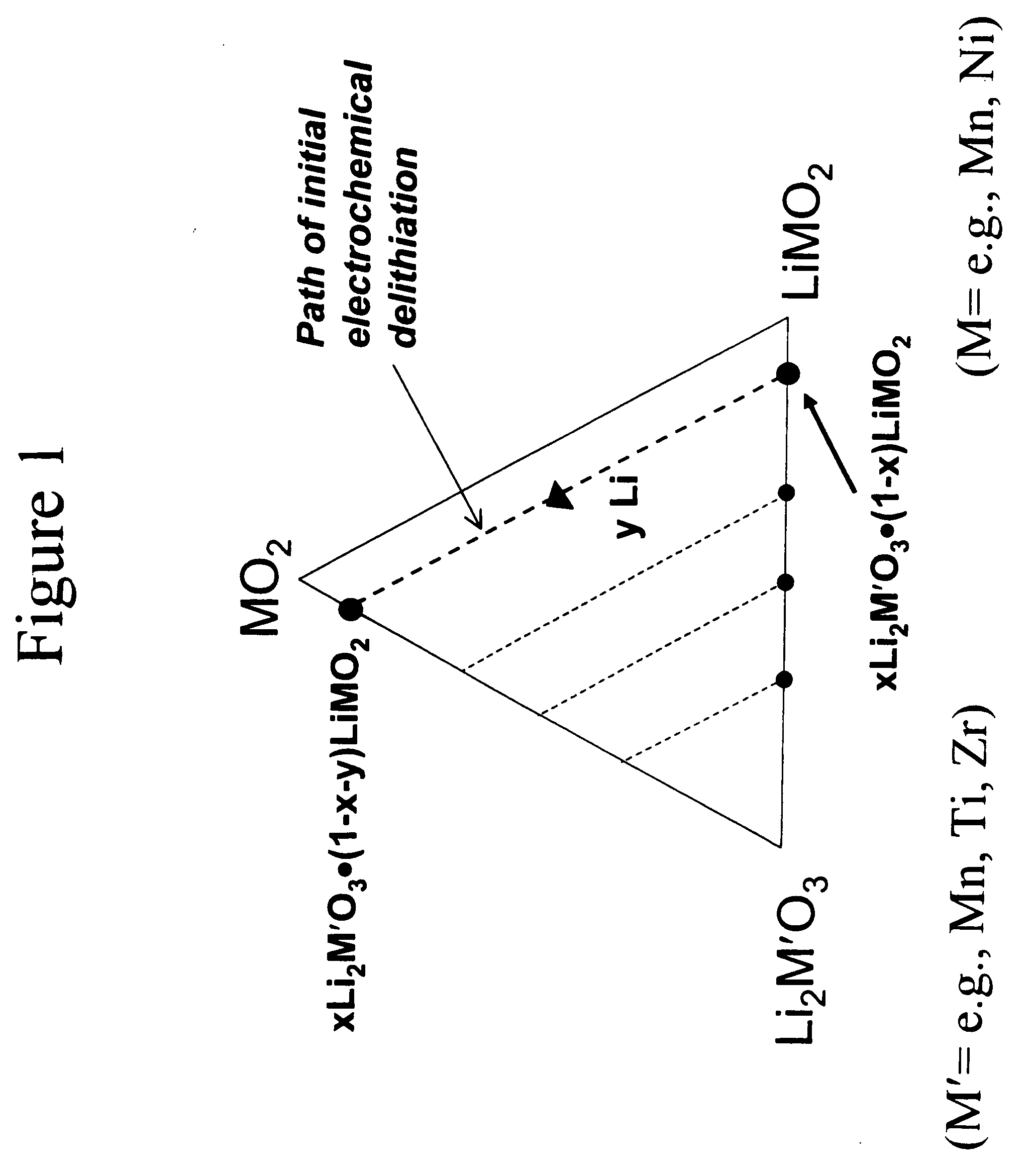
An uncycled preconditioned electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn, and an uncycled electrode of the formula xLi2M′O3.(1−x)LiMO2, in which 0≦x<1, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more first-row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first- and second-row transition metal elements and Sn, the electrode being preconditioned in a proton-containing medium with a pH<7.0. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC +1
Lithium metal oxide electrodes for lithium batteries
InactiveUS7314682B2Electrode thermal treatmentActive material electrodesLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2−2x) / (2+x)O2−δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
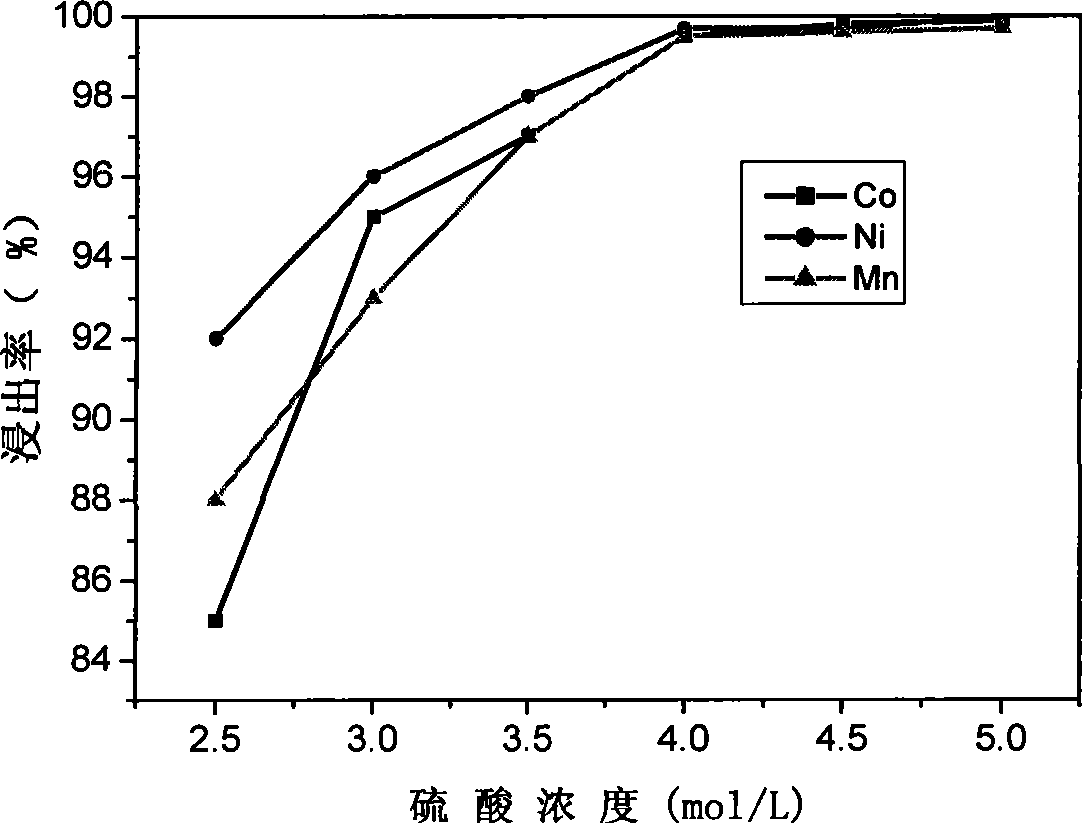
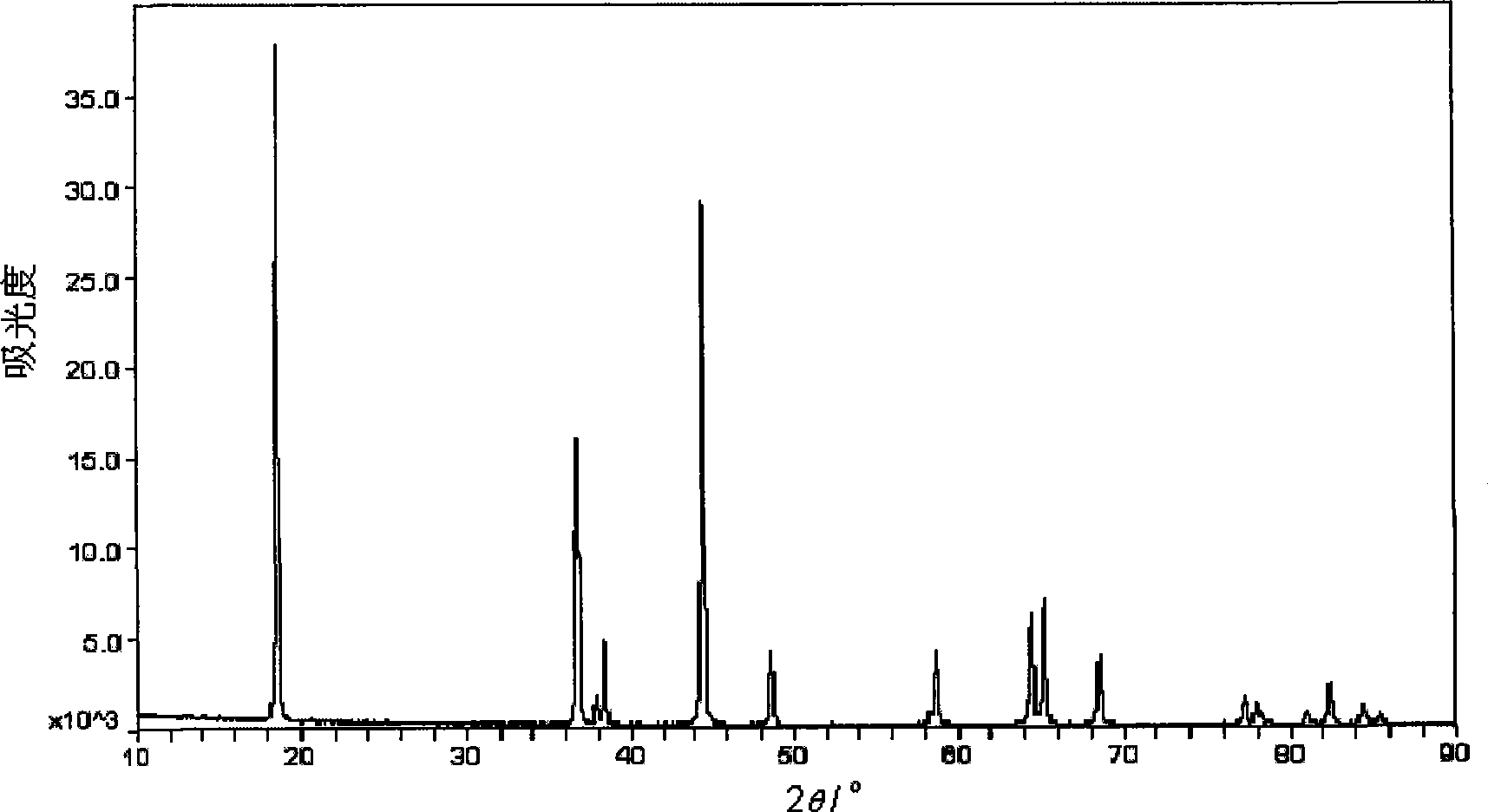
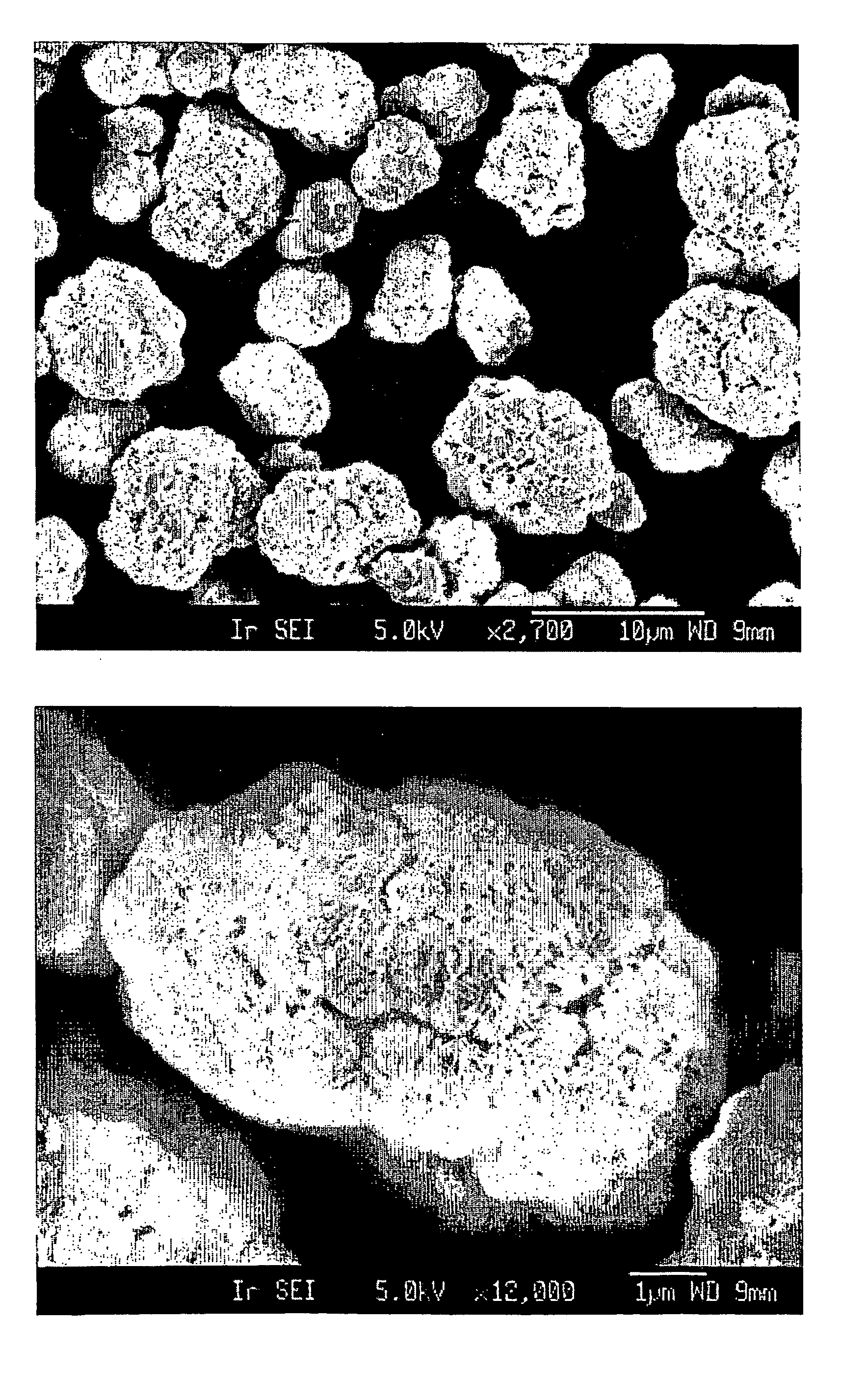
Method for preparing nickel and cobalt doped lithium manganate by using waste and old lithium ionic cell as raw material
InactiveCN101450815ASimultaneous recyclingShort processManganates/permanganatesManganateManganese oxide
The invention discloses a method for preparing lithium nickel cobalt manganese oxide by taking a waste lithium ion battery as a raw material. The method is mainly characterized in that a waste lithium ion battery taking the lithium nickel cobalt manganese oxide, lithium nickel cobalt oxide and so on as a battery positive material is selected as the raw material and is pretreated through disassembly, separation, crushing, screening and so on, and then processes such as adhesive removal at high temperature and aluminum removal by sodium hydroxide are adopted to obtain an inactivated positive material containing nickel, cobalt and manganese; then a sulfuric acid and hydrogen peroxide system is adopted to leach, and P204 is adopted to remove impurities by extraction to obtain pure nickel, cobalt and manganese solution, and proper manganese sulfate, nickel sulfate or cobalt sulfate is blended to ensure that the mol ratio of nickel, cobalt and manganese elements in the solution is 1: 1: 1; and then ammonium carbonate is adopted to adjust the pH value to form a nickel cobalt manganese carbonate precursor, and then a proper amount of lithium carbonate is blended for high temperature sintering to synthesize a lithium nickel cobalt manganese oxide battery material. The first discharge capacity of the material is 150 mAh / g, the discharge capacity is still kept more than 130mAh / g after the circulation for 30 times, and the material has good electrochemical performance.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
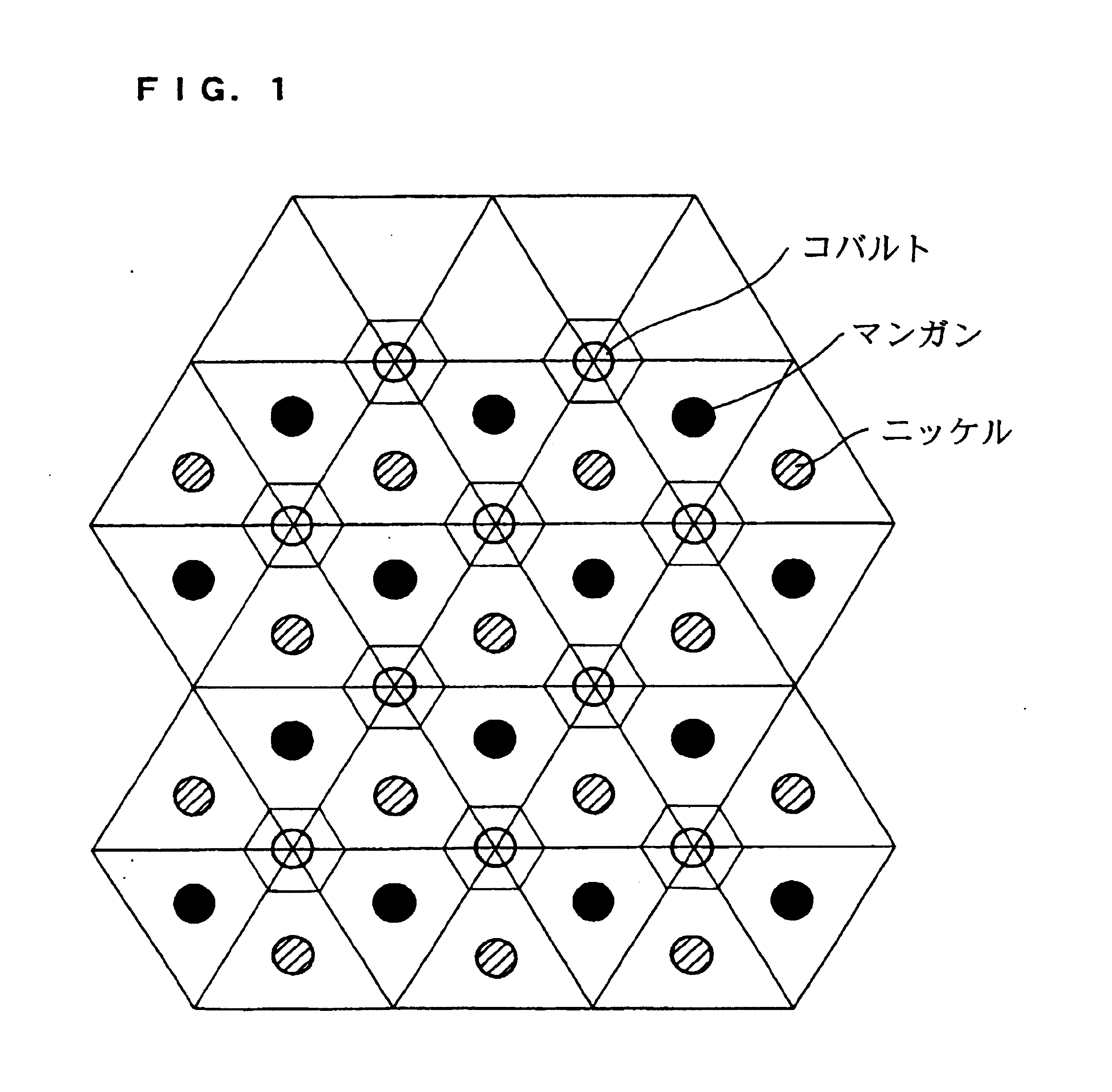
Lithium transition metal oxide with gradient of metal composition
ActiveUS20060105239A1Improve electrochemical performanceIncrease energy densityCell electrodesManganese oxides/hydroxidesManganeseMaterials science
Disclosed are primary materials, precursor materials and final materials as well as methods to prepare these materials. The final materials are mixed lithium transition metal oxides, useful as performance optimized cathode materials for rechargeable lithium batteries. The transition metal is a solid solution mixture of manganese, nickel and cobalt, M=(Mn1-uNiu)1-u-yCoy, with 0.2
Owner:LG ENERGY SOLUTION LTD
Positive electrode active material and nonaqueous electrolyte secondary cell including the same
InactiveUS20050079416A1Large capacityImprove charge and discharge efficiencyOxygen/ozone/oxide/hydroxideIron oxides/hydroxidesMetallic lithiumManganese
A nonaqueous electrolytic secondary cell produced at low cost and having a large capacity comprises a negative electrode having an active material mainly composed of a material that at least absorbs and releases lithium ions or metallic lithium, a positive electrode, and an electrolyte. The active material of the positive electrode is an oxide containing nickel, manganese, and cobalt, and the contents of the elements are substantial the same.
Owner:PANASONIC CORP +1
Active substance of positive electrode and nonaqueous electrolyte battery containing the same
ActiveUS20050019659A1Increase energy densityImprove high rate discharge performanceSecondary cellsAlkali metal oxidesHigh rateHigh energy
A positive active material is provided which can give a battery having a high energy density and excellent high-rate discharge performance and inhibited from decreasing in battery, performance even in the case of high-temperature charge. Also provided is a non-aqueous electrolyte battery employing the positive active material. The positive active material contains a composite oxide which is constituted of at least lithium (Li), manganese (Mn), nickel (Ni), cobalt (Co), and oxygen (O) and is represented by the following chemical composition formula: LiaMnbNicCodOe (wherein 0<a≦1.3, |b−c|≦0.05, 0.6≦d<1, 1.7≦e≦2.3, and b+c+d=1). The non-aqueous electrolyte battery has a positive electrode containing the positive active material, a negative electrode, and a non-aqueous electrolyte.
Owner:GS YUASA INT LTD
Cathode active material for lithium secondary battery, process for preparing the same and reactor for use in the same process
ActiveUS20070111098A1Large capacityHigh tap densityElectrode manufacturing processesPhosphatesLithiumHigh rate
The present invention relates to a cathode active material for a lithium secondary battery and a process for preparing the same. In accordance with the present invention, the cathode active material having a high packing density was designed and synthesized and thus provided is a cathode active material for a lithium secondary battery exhibiting structural stability such as improved characteristics for charge / discharge, service life and high-rate and thermal stability, by modifying surface of the electrode active material with amphoteric or basic compounds capable of neutralizing acid produced around the cathode active material.
Owner:LG CHEM LTD
Lithium-containing composite oxide and nonaqueous secondary cell using the same, and method for manufacturing the same
InactiveUS20030082452A1Stable structureHigh energy density per volumeElectrode thermal treatmentOrganic electrolyte cellsLithiumHigh density
Because of the composition represented by General Formula: Li1+x+alphaNi(1-x-y+delta) / 2Mn(1-x-y-delta) / 2MyO2 (where 0<=x<=0.05, -0.05<=x+alpha<=0.05, 0<=y<=0.4; -0.1<=delta<=0.1 (when 0<=y<=0.2) or -0.24<=delta<=0.24 (when 0.2<=y<=0.4); and M is at least one element selected from the group consisting of Ti, Cr, Fe, Co, Cu, Zn, Al, Ge and Sn), a high-density lithium-containing complex oxide with high stability of a layered crystal structure and excellent reversibility of charging / discharging can be provided, and a high-capacity non-aqueous secondary battery excellent in durability is realized by using such an oxide for a positive electrode.
Owner:MAXELL HLDG LTD
Manganese oxide based materials as ion intercalation hosts in lithium batteries
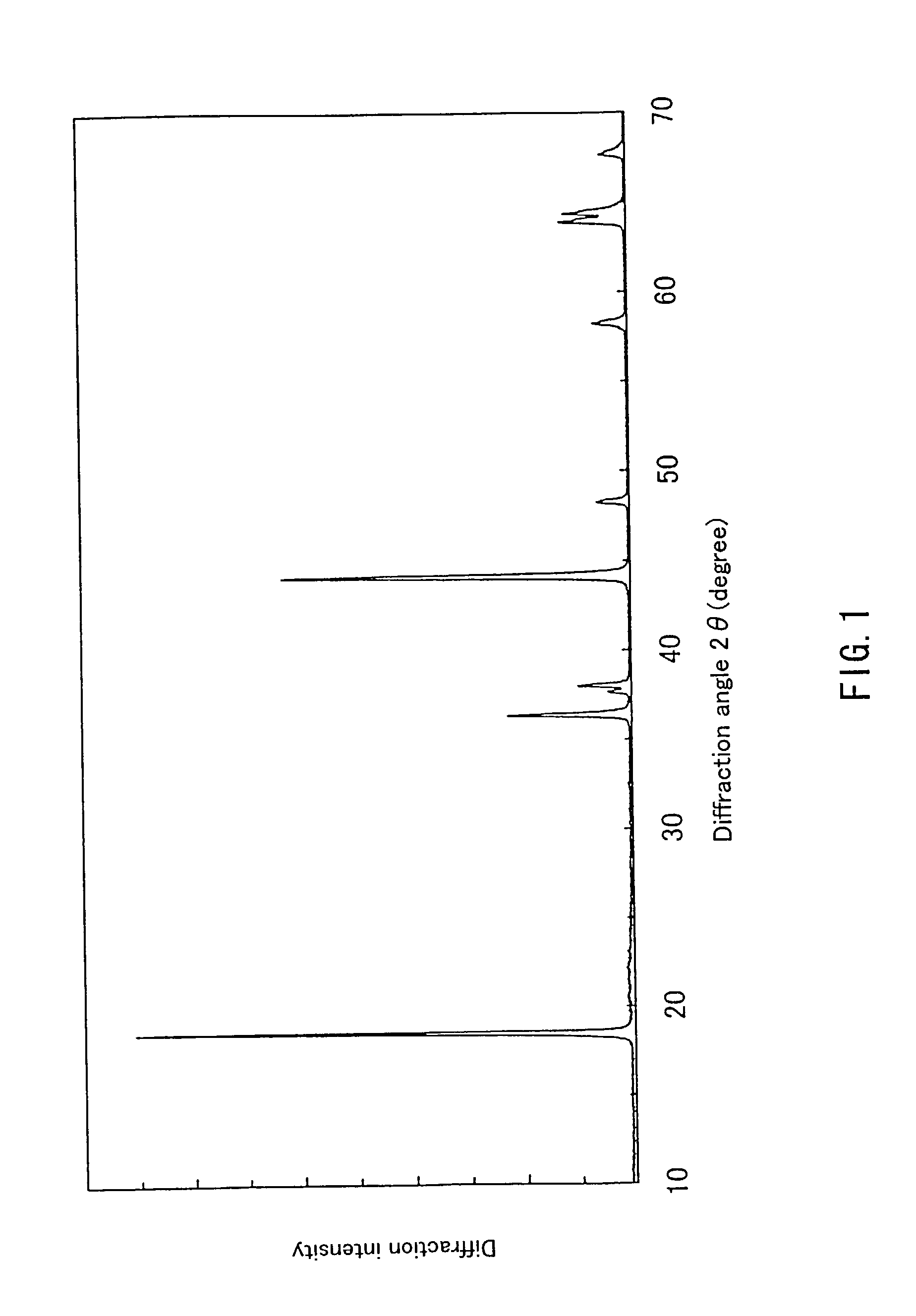
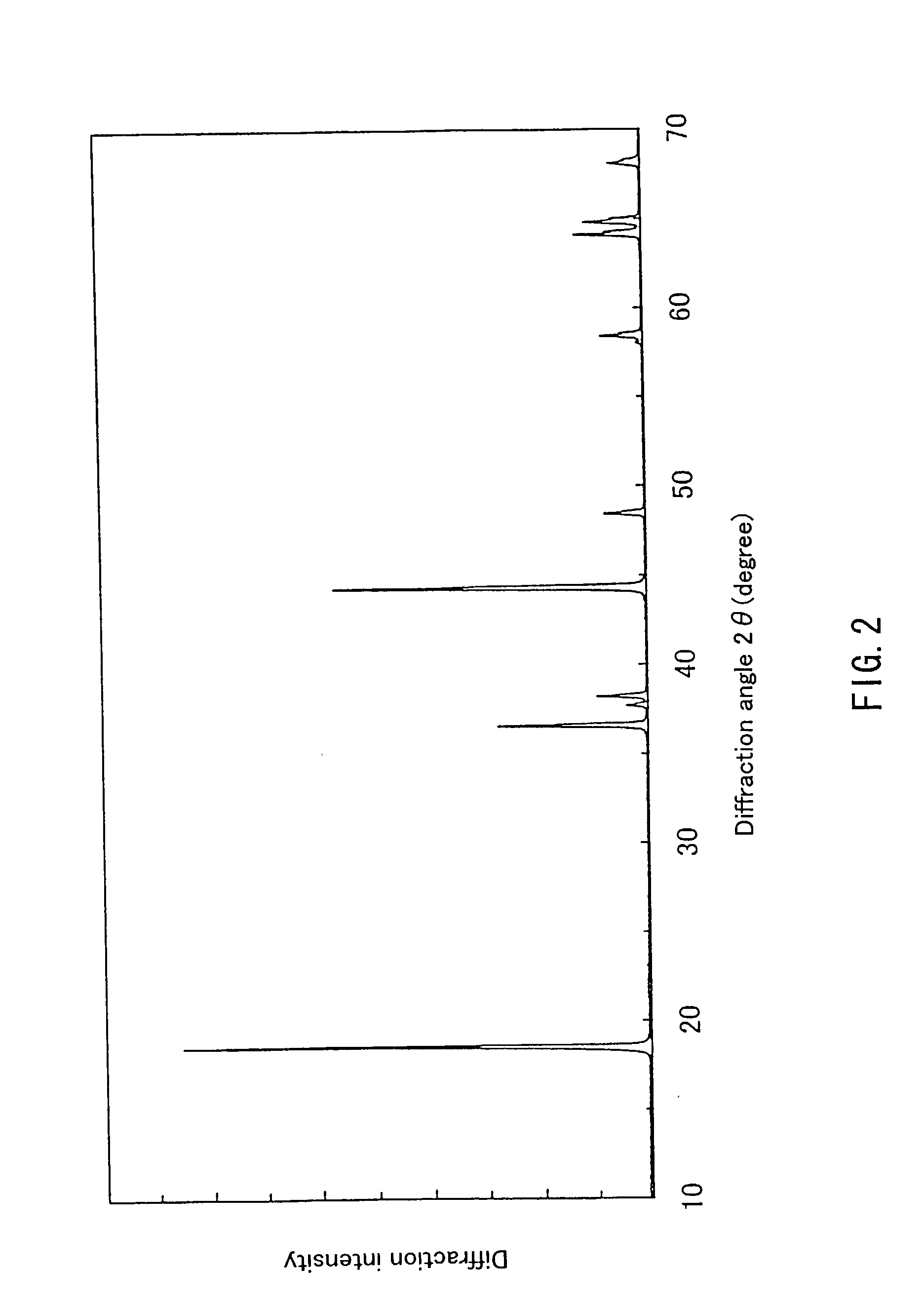
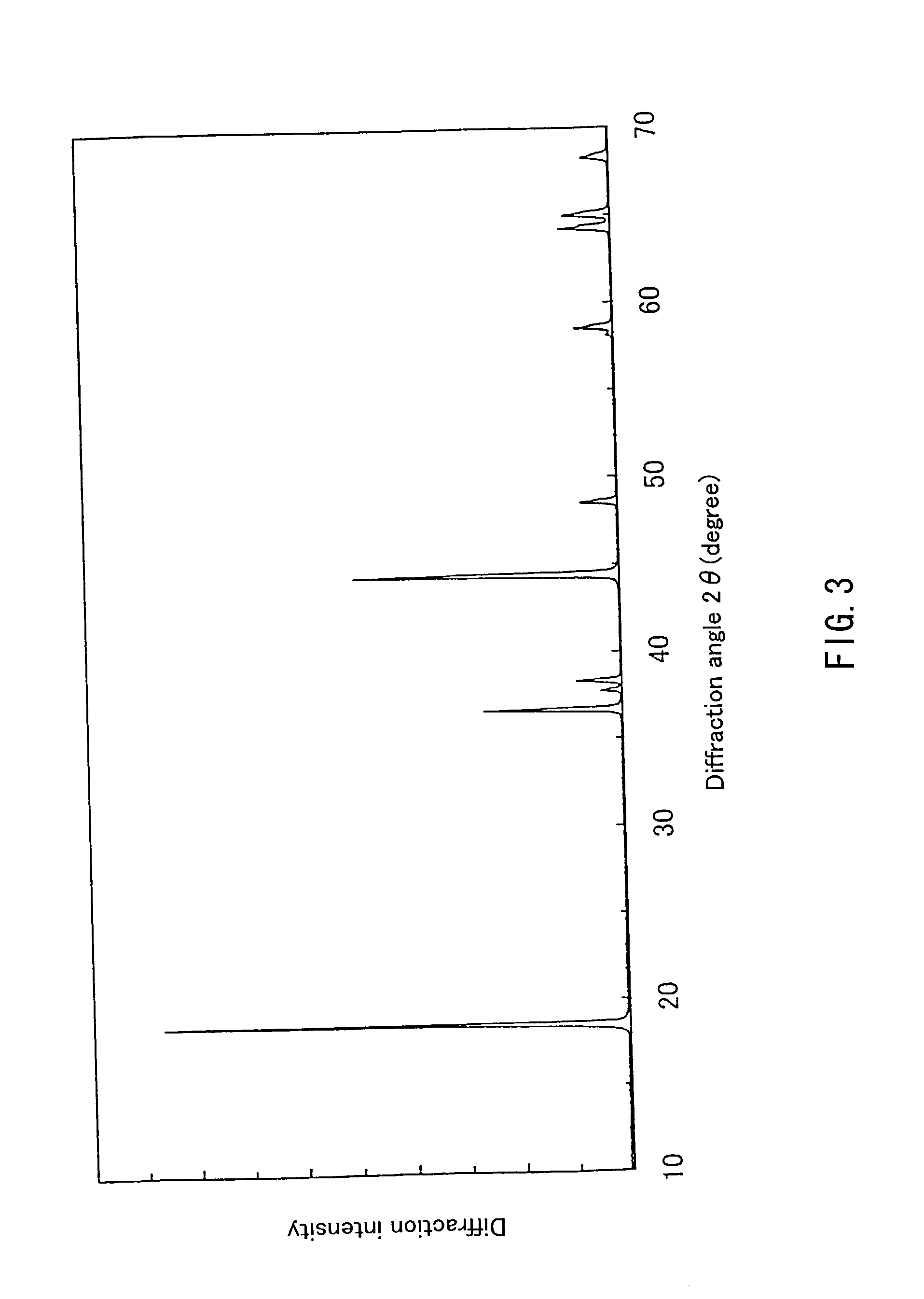
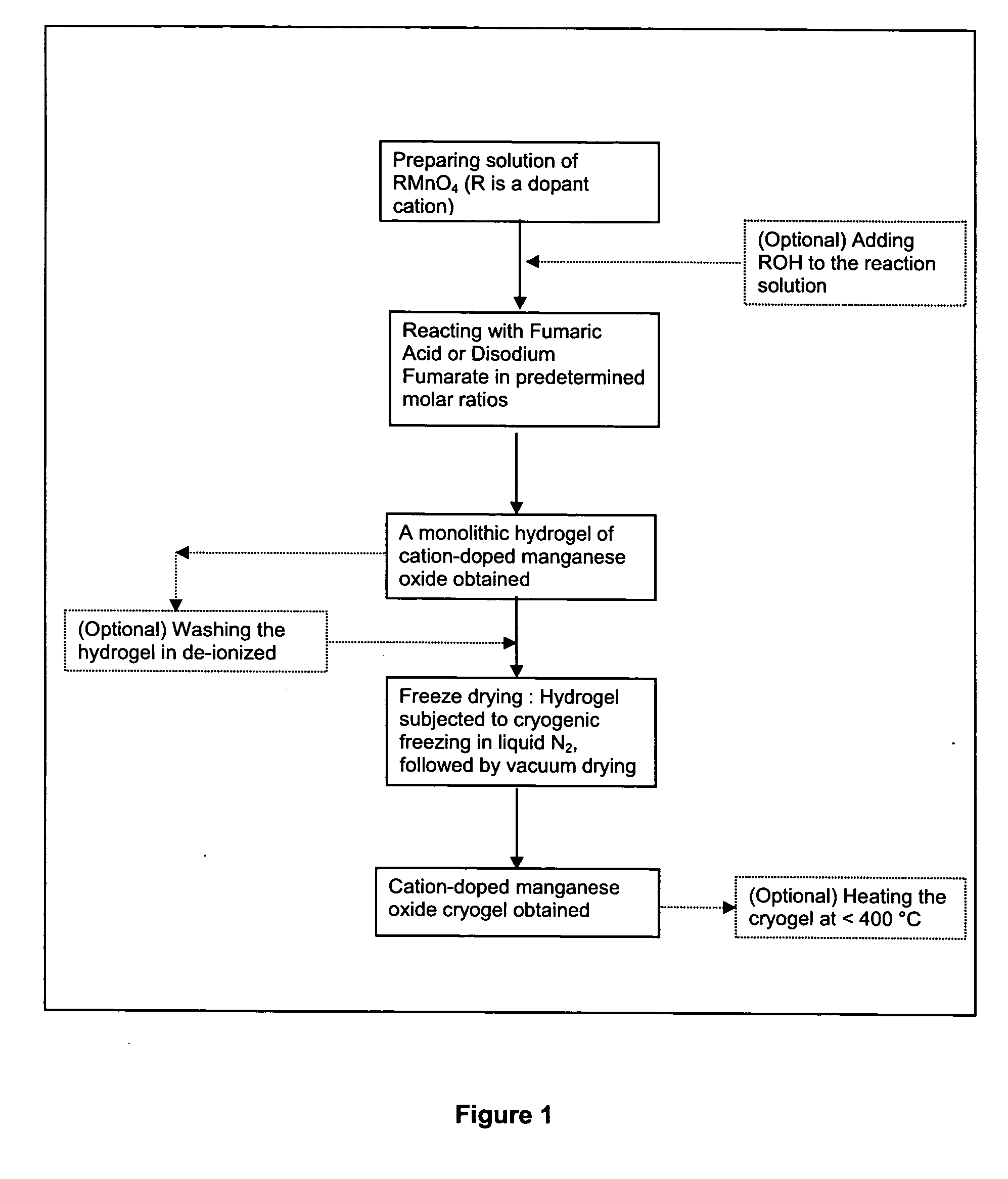
InactiveUS20050135993A1Enhances electrochemical property and performance of materialImprove stabilityActive material electrodesManganese oxides/hydroxidesRechargeable cellPERMANGANATE ION
The present invention is directed to a process for making an amorphous nanostructured cation-doped manganese oxide material useful as an ion intercalation host for rechargeable batteries, including the steps of preparing a solution containing cation permanganate combined optionally with a cation donor compound, mixing the solution with a reducing agent to yield a hydrogel comprising a manganese oxide compound, cryogenically freezing the hydrogel, drying the frozen gel to yield a cryogel amorphous nanostructured cation-doped manganese oxide, and heat treating the dried cryogel.
Owner:RUTGERS THE STATE UNIV
Positive electrode active material for secondary battery, positive electrode for secondary battery, secondary battery and method for producing positive electrode active material for secondary battery
ActiveUS20060035151A1Minimization of increase in resistanceMinimization of in reduction in capacityNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsManganateManganese
A life of a secondary battery is extended, increase in a resistance when storing a secondary battery at an elevated temperature is prevented, and increase in a resistance during a charge-discharge cycle is prevented. A positive electrode active material comprising a lithium manganate and a lithium nickelate are used. The lithium manganate is a compound represented by the following formula (1) or the compound in which some of Mn or O sites are replaced with another element: Li1+xMn2−xO4 (1) (in the above-shown formula (1), 0.15≦x≦0.24).
Owner:NEC CORP
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS20020055042A1Improve thermal stabilityElectrode manufacturing processesZirconium compoundsPhysical chemistryLithium battery
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 andl a metal oxide or composite metal oxide layer formed on the compound. <table-cwu id="TABLE-US-00001"> <number>1< / number> <tgroup align="left" colsep="0" rowsep="0" cols="3"> <colspec colname="OFFSET" colwidth="42PT" align="left" / > <colspec colname="1" colwidth="77PT" align="left" / > <colspec colname="2" colwidth="98PT" align="center" / > <row> <entry>< / entry> <entry>< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyF2< / entry> <entry>(1)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyS2< / entry> <entry>(2)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aFa< / entry> <entry>(3)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aSa< / entry> <entry>(4)< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> < / tgroup> < / table-cwu> (where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, IPu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<=y<=0.99, 0<=,z<=0.5, and 0<=a<=0.5)
Owner:SAMSUNG SDI CO LTD
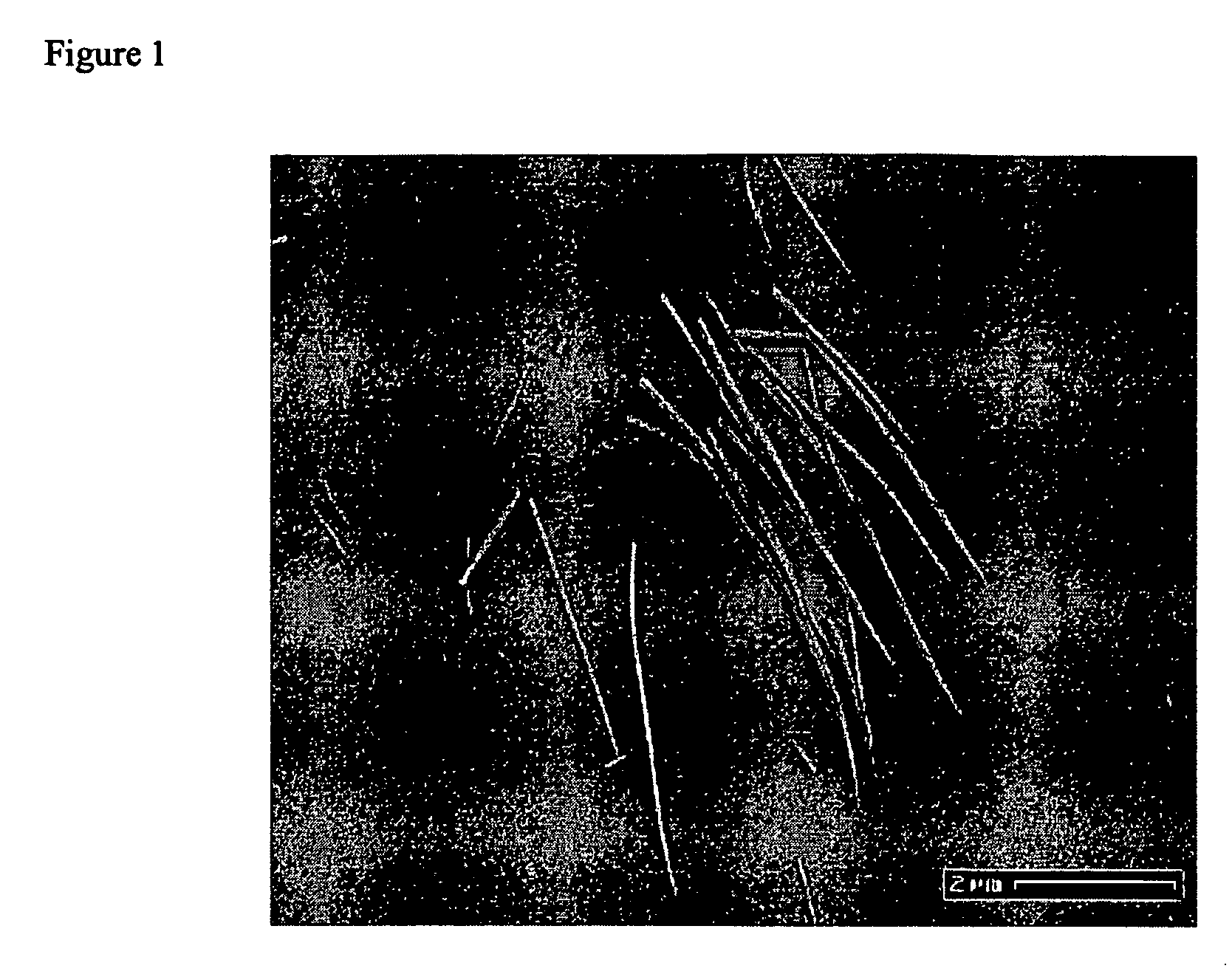
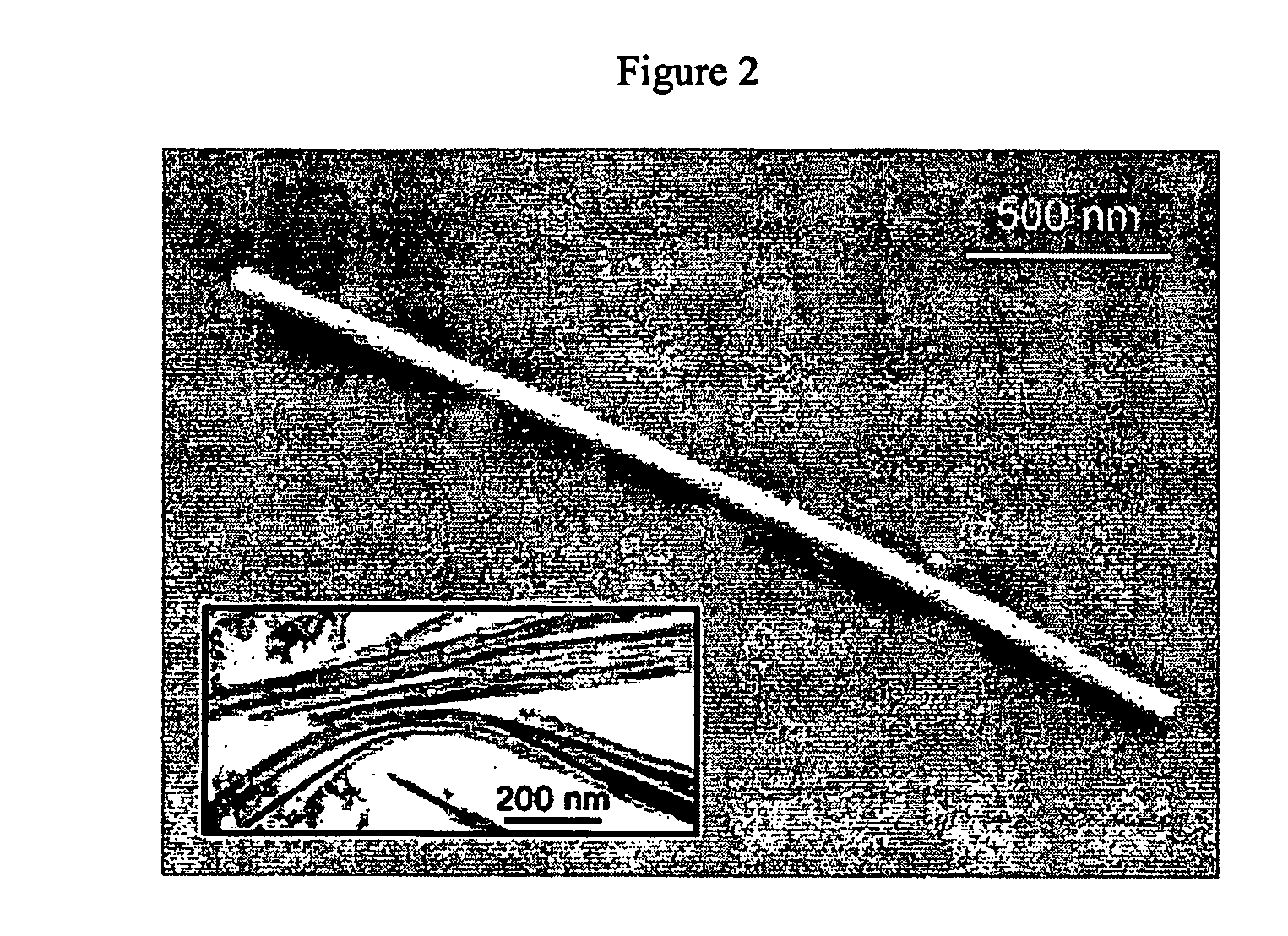
Transition metal oxide nanowires
Nanowires are disclosed which comprise transition metal oxides. The transition metal oxides may include oxides of group II, group III, group IV and lanthanide metals. Also disclosed are methods for making nanowires which comprise injecting decomposition agents into a solution comprising solvents and metallic alkoxide or metallic salt precursors.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Anode active material, manufacturing method thereof, and non-aqueous electrolyte secondary battery
In order to provide a 3V level non-aqueous electrolyte secondary battery with a flat voltage and excellent cycle life at a high rate with low cost, the present invention provides a positive electrode represented by the formula: Li2±α[Me]4O8−x, wherein 0≦α<0.4, 0≦x<2, and Me is a transition metal containing Mn and at least one selected from the group consisting of Ni, Cr, Fe, Co and Cu, said active material exhibiting topotactic two-phase reactions during charge and discharge.
Owner:OSAKA CITY UNIVERSITY +1
Lithium composite oxide particle for positive electrode material of lithium secondary battery, and lithium secondary battery positive electrode and lithium secondary battery using the same
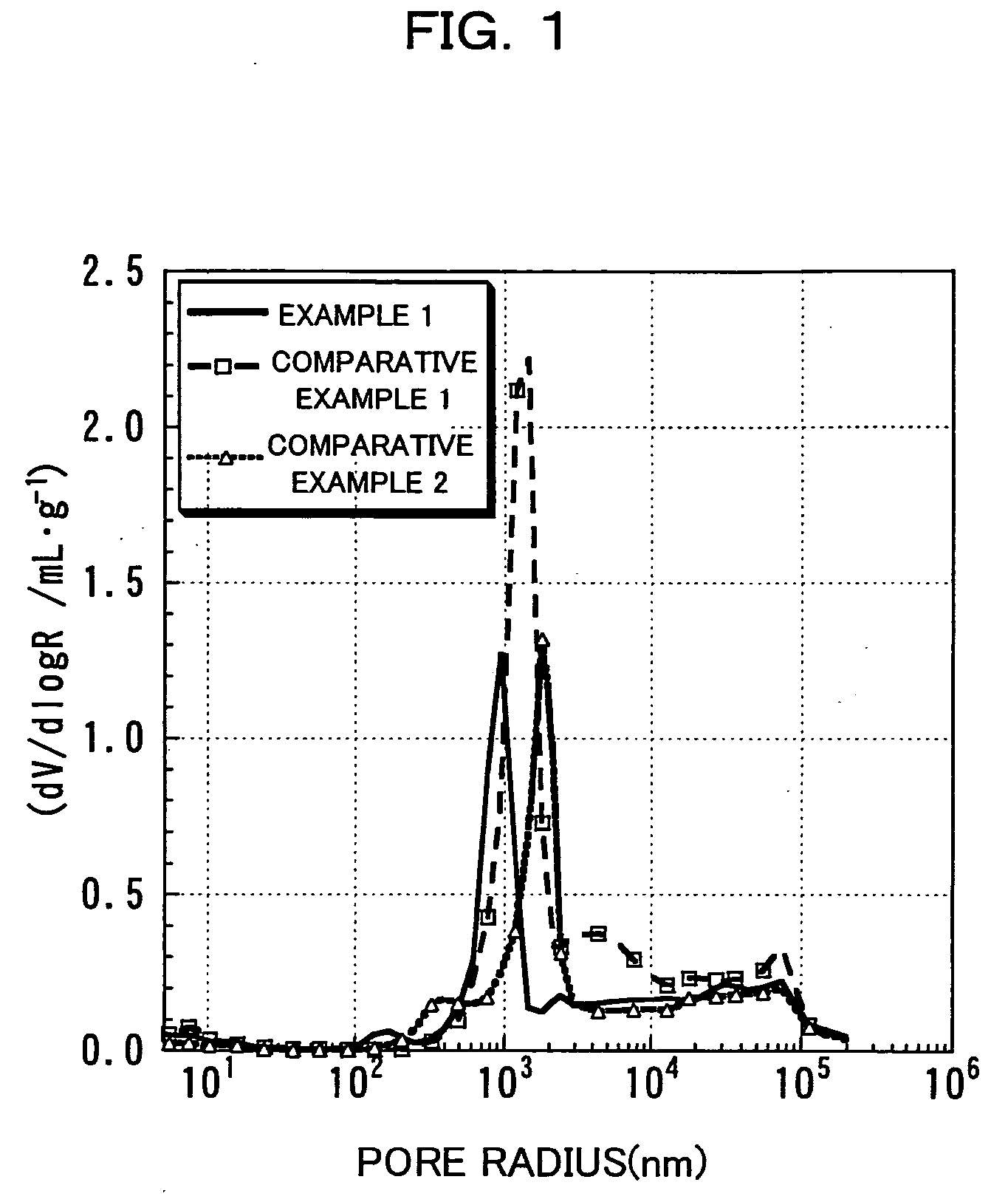
InactiveUS20060134521A1Improving low-temperature load characteristicGood paintabilityRuthenium/rhodium/palladium/osmium/iridium/platinum compoundsAlkali metal oxidesElectrical batteryComposite oxide
An excellent positive electrode material for a lithium secondary battery is provided that can increase low-temperature load characteristics of the battery as well as improving coatability. When measured by mercury intrusion porosimetry, the material meets Condition (A) and at least either Condition (B) or Condition (C). Condition (A) : on a mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.02 cm3 / g or smaller. Condition (B): on the mercury intrusion curve, the mercury intrusion volume from 50 MPa to 150 MPa is 0.01 cm3 / g or larger. Condition (C): the average pore radius is within 10-100 nm, and the pore-size distribution curve has a main peak (with peak top at a pore radius of within 0.5-50 μm) and a sub peak (with peak top at a pore radius of within 80-300 nm).
Owner:MITSUBISHI CHEM CORP
Production Of Barium Titanate Compounds
InactiveUS20070202036A1High purityLow costMaterial nanotechnologyAlkaline earth titanatesBarium titanateSpherical shaped
An ultrafine powder of barium titanate including solid solutions and doped compounds that meets up to specific characteristics is produced by method comprising two main steps. The first step is a reaction, typically in a Segmented Flow Tubular Reactor, between reactants to produce cubic-structure barium titanate composed of non-agglomerated ultrafine particles having a shape of given aspect ratio, usually a generally spherical shape, of low density corresponding at most to 90% of the intrinsic density, all particles being smaller than 1 micron and having a narrow particle size distribution and wherein the ratio of Ba:Ti including substitutents and dopants is very close to the ideal stoichiometry. This is followed by subjecting the powder produced in the first step to a second stage solvothermal post treatment typically in an autoclave at temperature less than 400° C. to convert the cubic-structure particles of low density to ultrafine tetragonal particles of increased density corresponding to at least 90% of the intrinsic density while maintaining the same aspect ratio, and maintaining the size of all particles below 1 micron, the narrow particle size distribution span, and the given ideal stoichiometry. The produced particles can have a non-spherical facetted shape such as cube-like.
Owner:JONGEN NATHALIE +1
Electrode active material powder with size dependent composition and method to prepare the same
ActiveUS20070122705A1Increased gravimetric energy densityLow costElectrode thermal treatmentActive material electrodesLithiumPhysical chemistry
The present invention relates to a powderous electrode active material of lithium transition metal oxide LiaMbO2, wherein 0.9 < a < 1.1, 0.9 < b < 1.1 and M is dominantly transition metal chosen from Mn, Co and Nickel, having particles with a distribution of sizes, where the composition M varies with the size of the particles, and a preparation method thereof. The present invention also relates to an electrochemical cell, particularly rechargeable lithium battery, using the powderous electrode active material.
Owner:LG ENERGY SOLUTION LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com