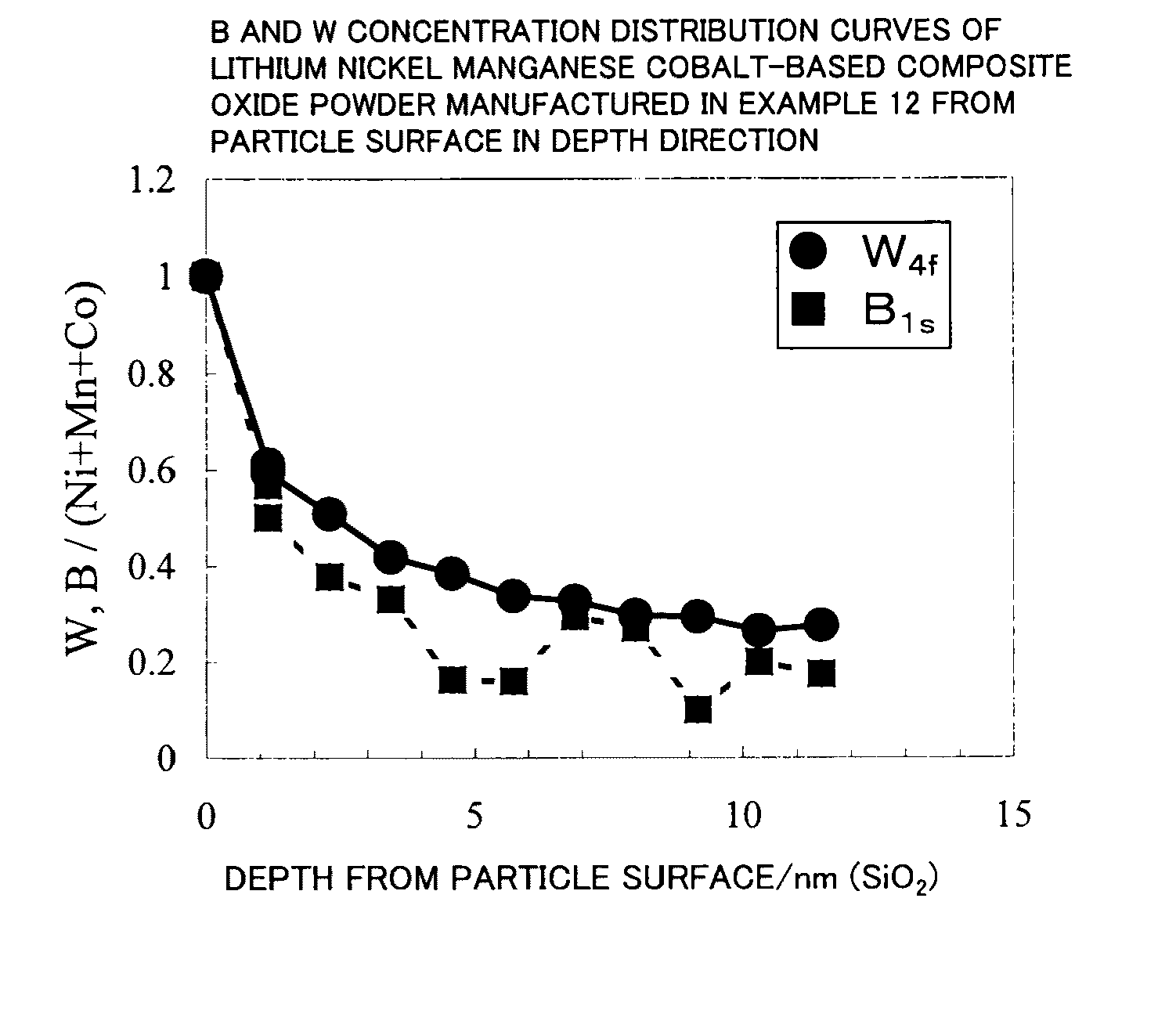
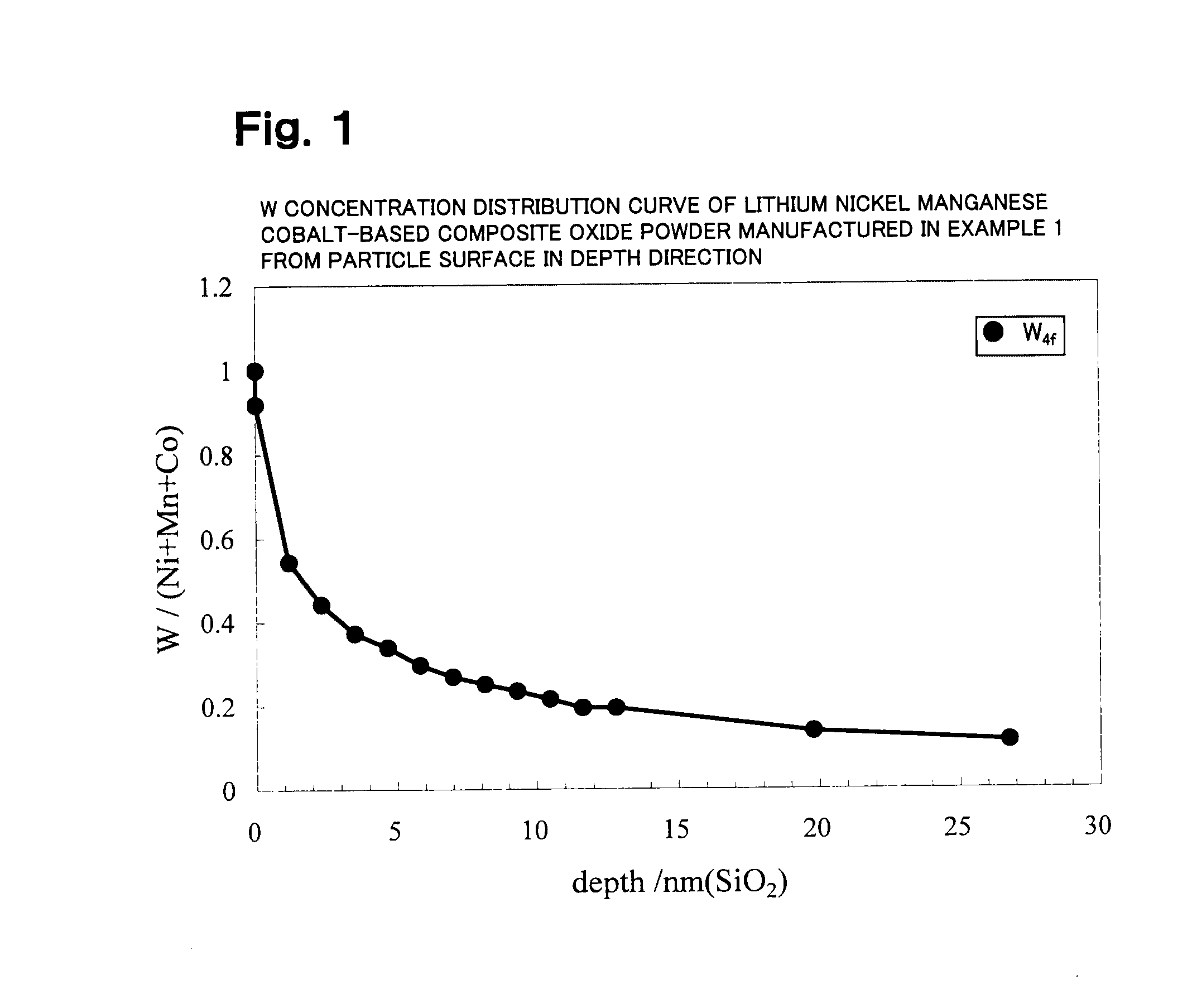
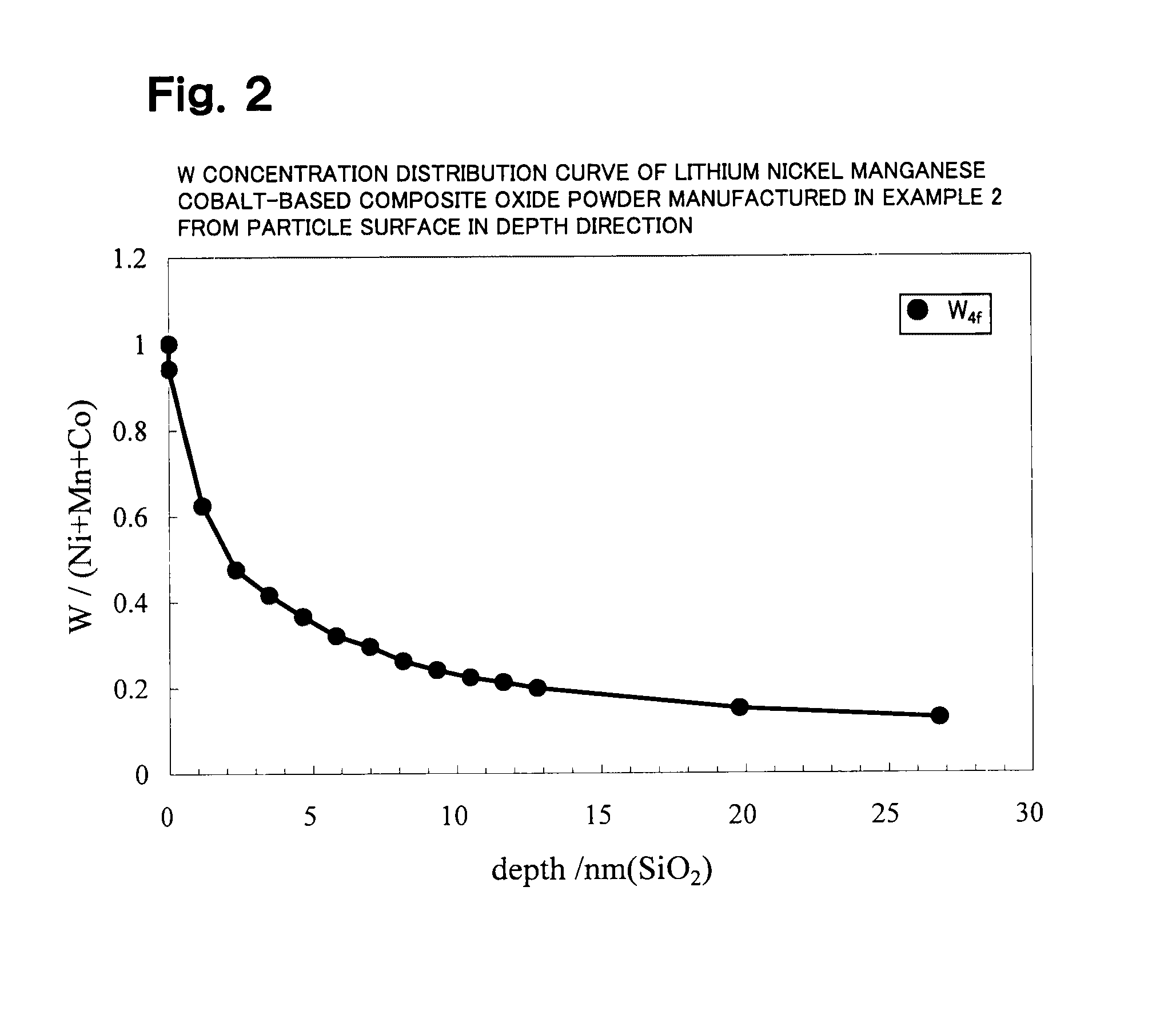
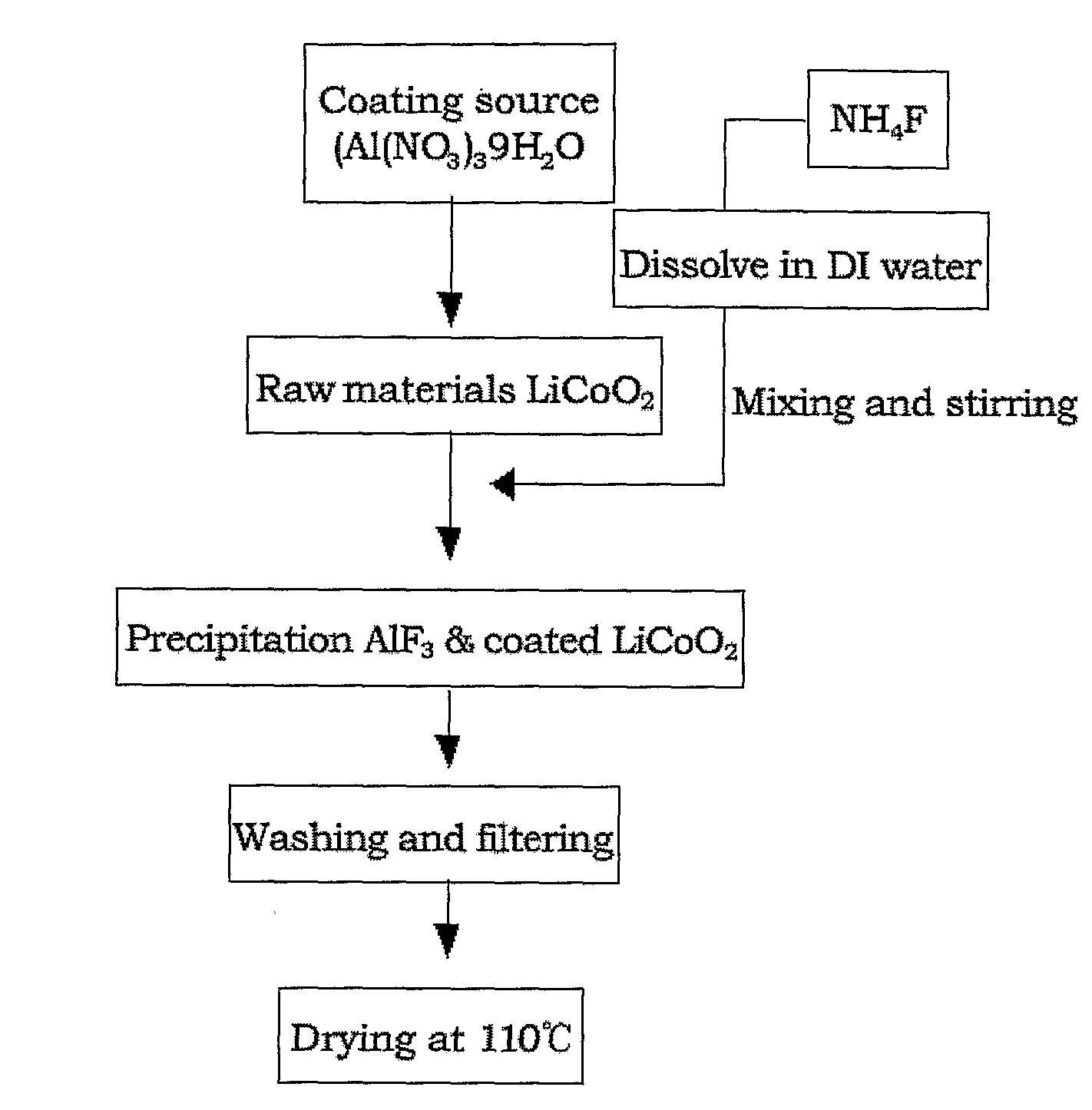
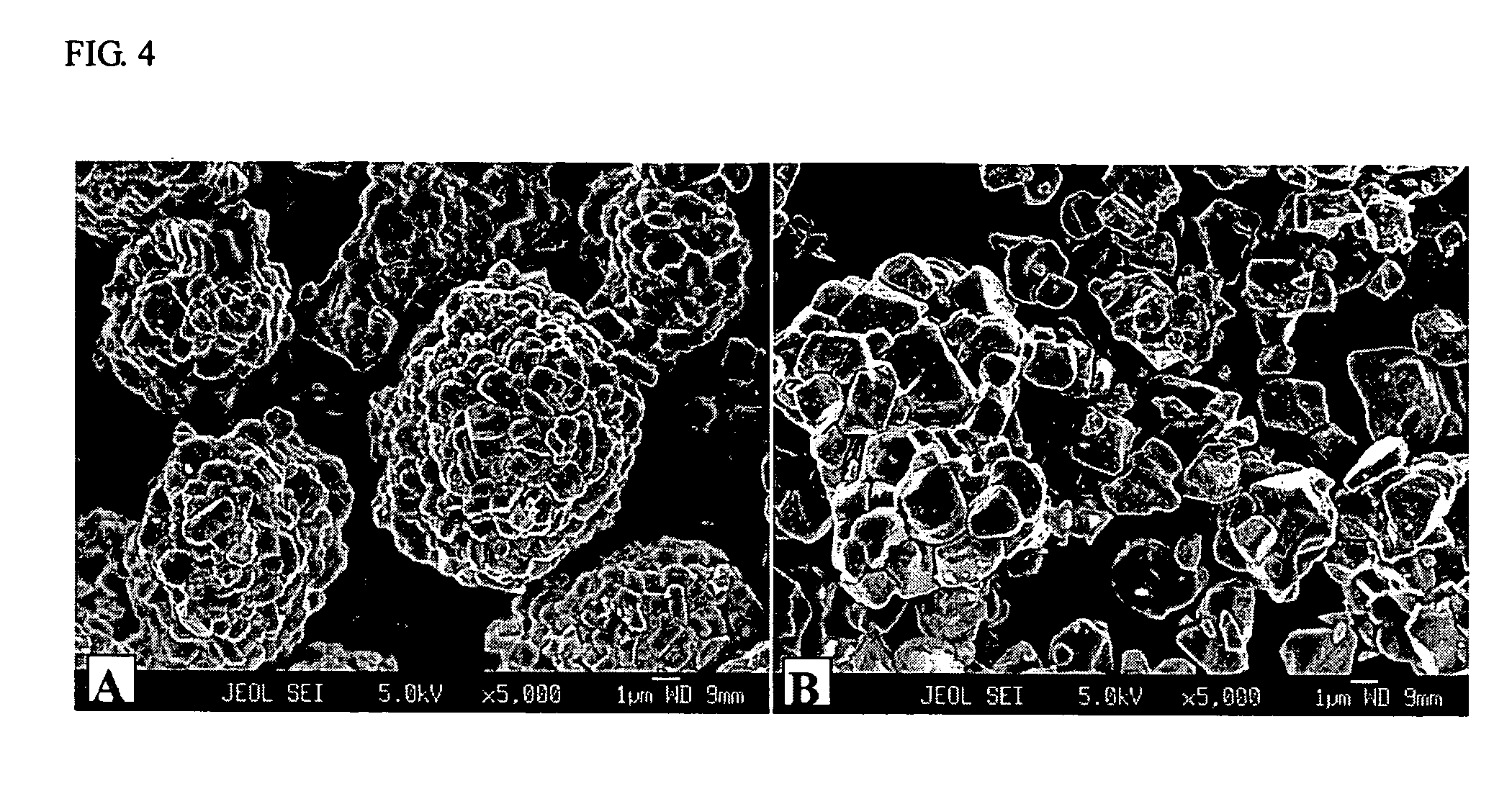
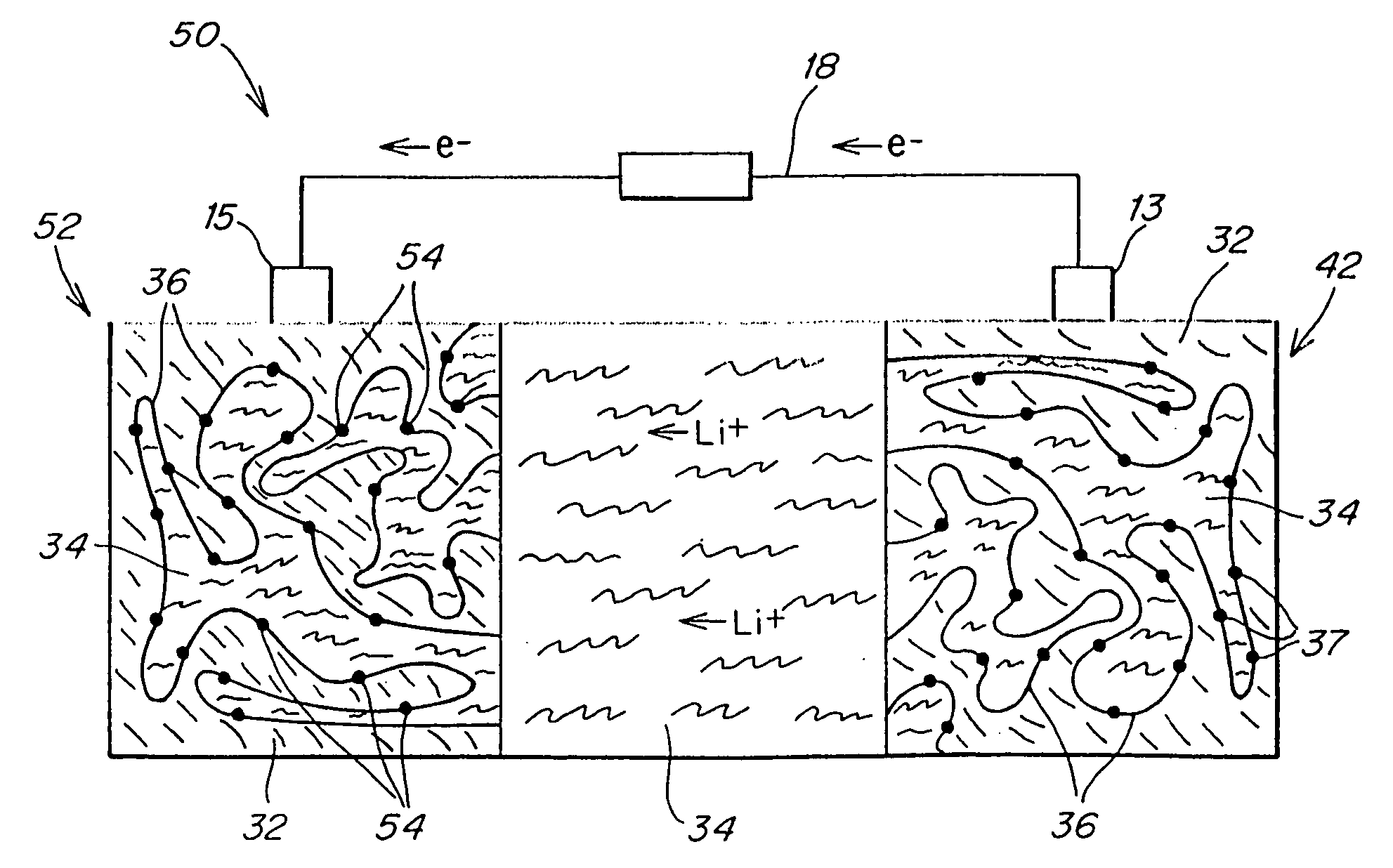
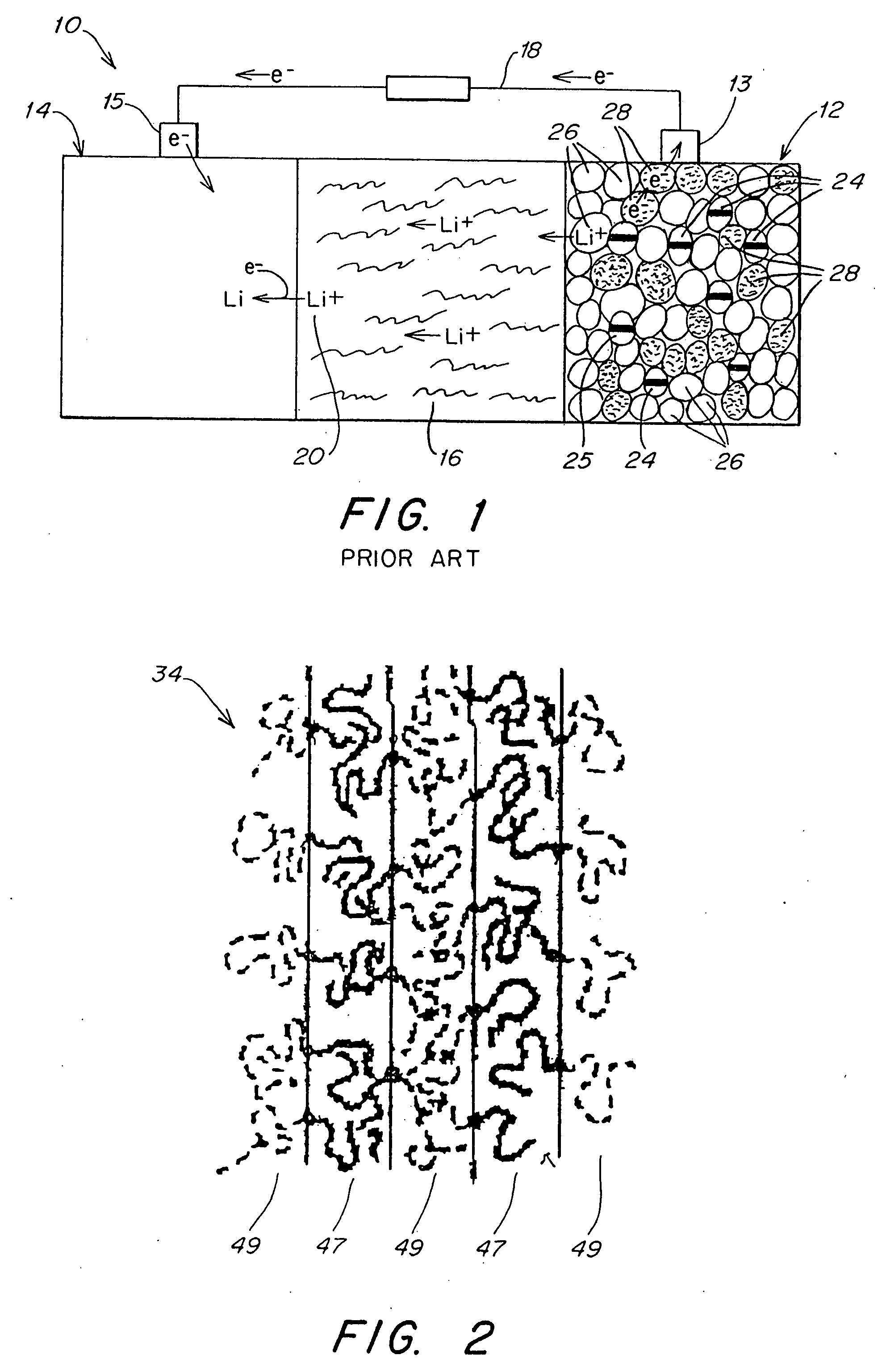
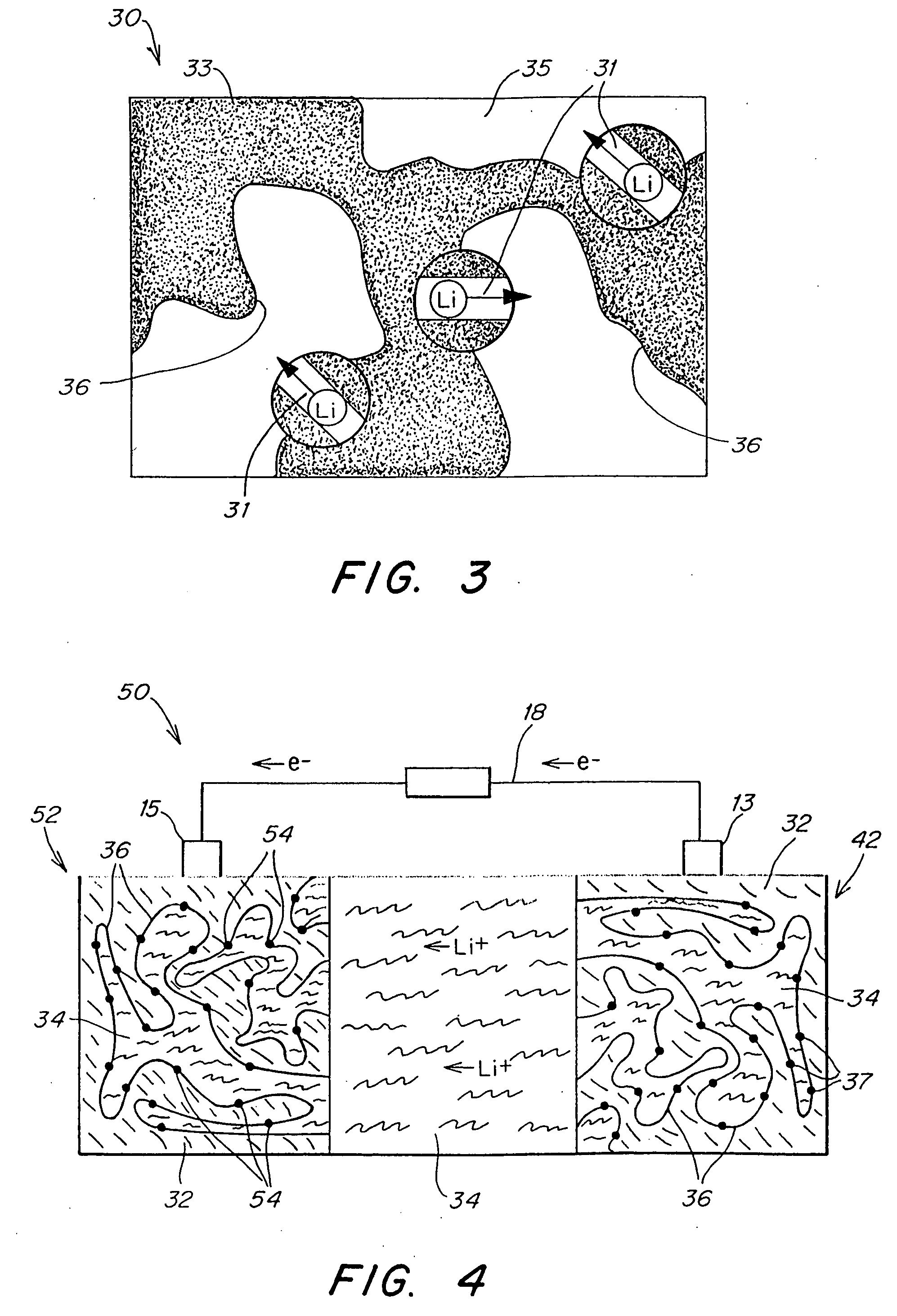
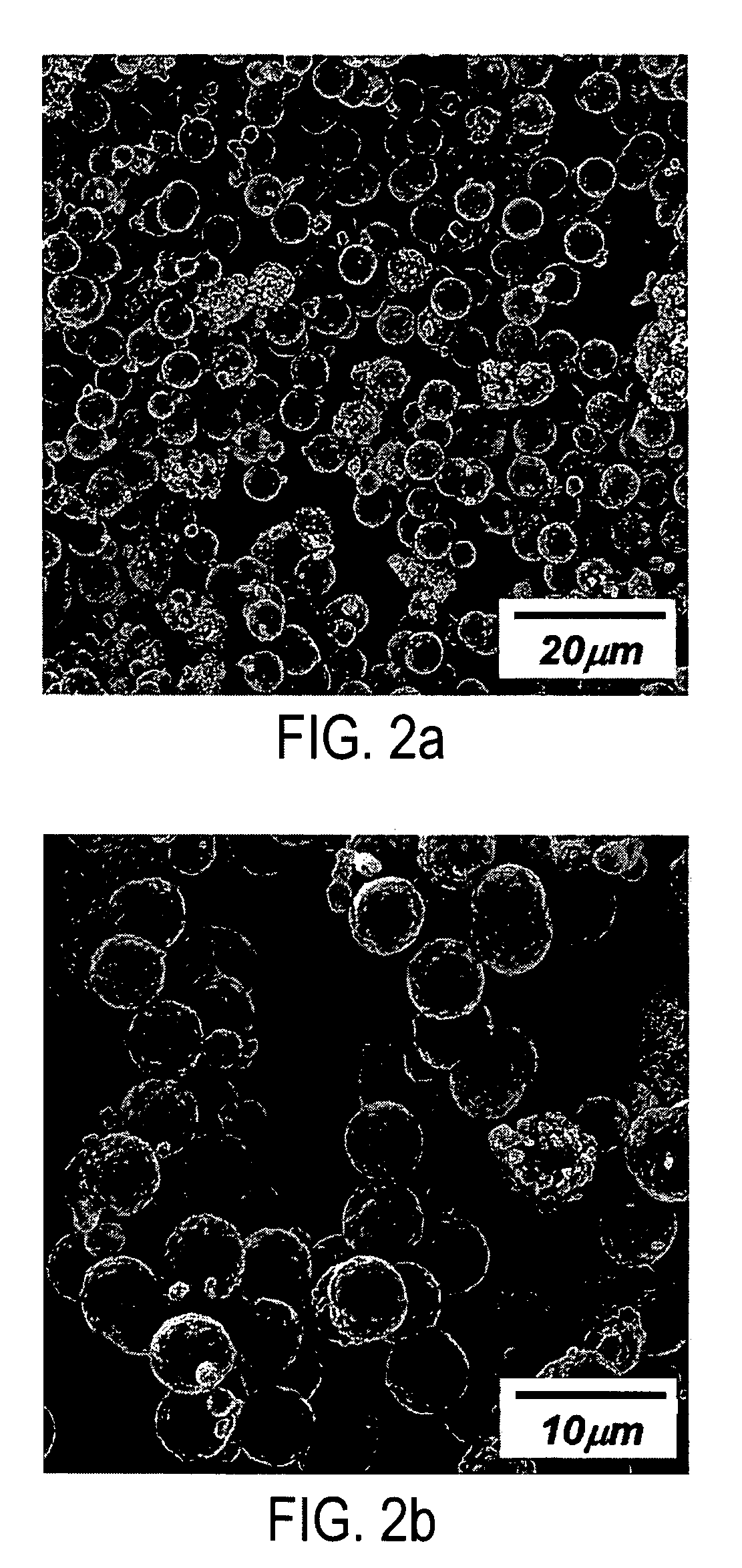
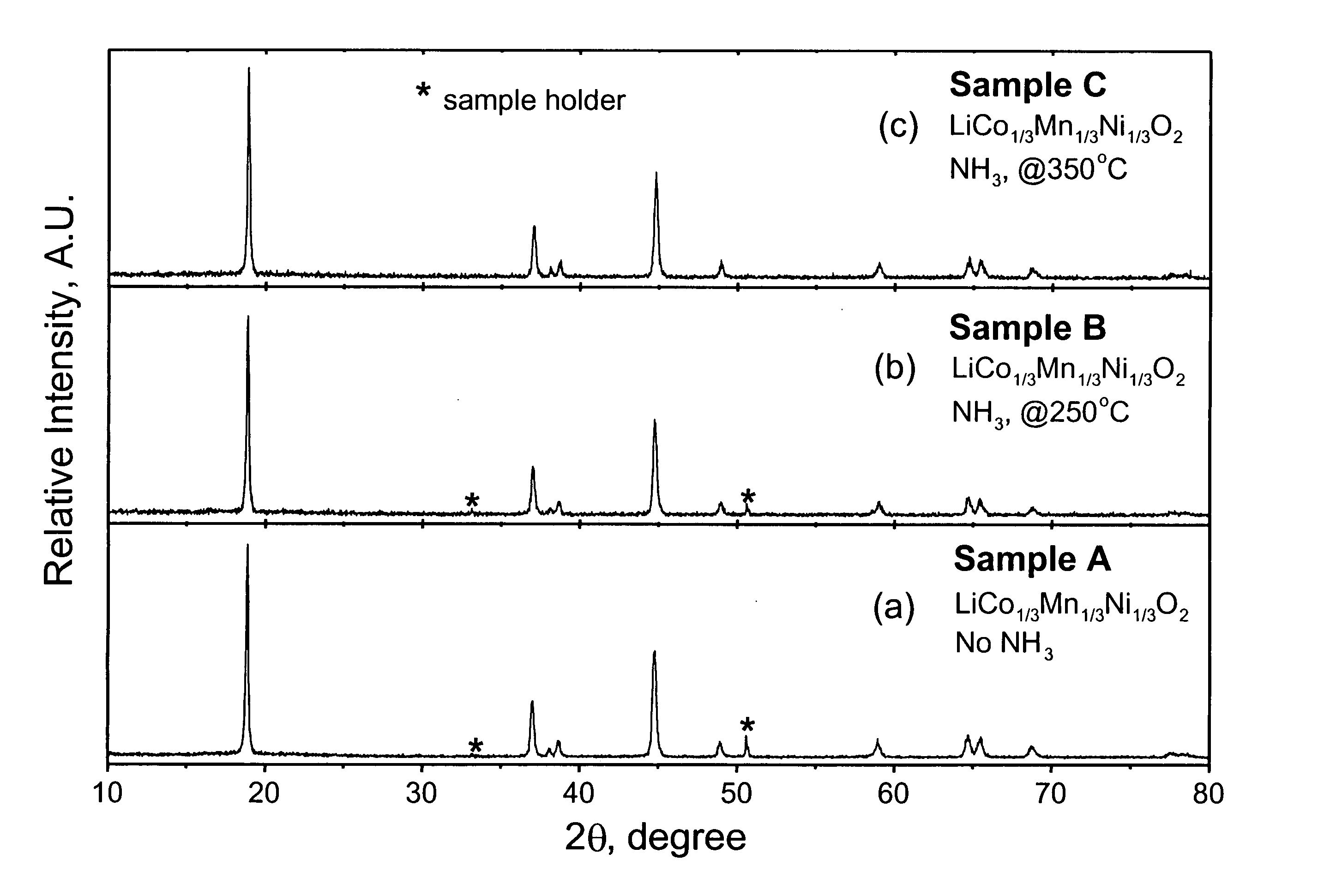
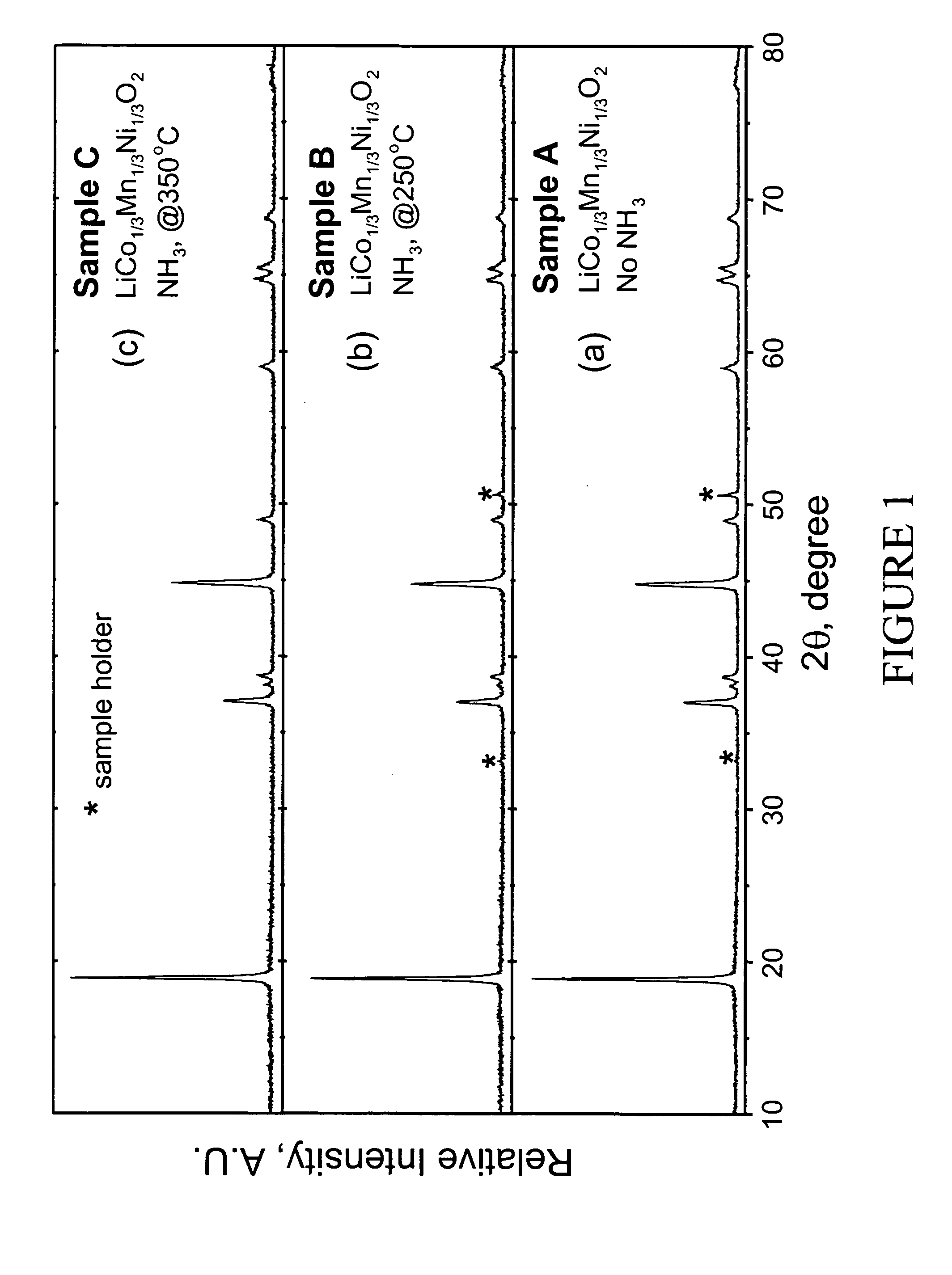
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5548results about "Nickel compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
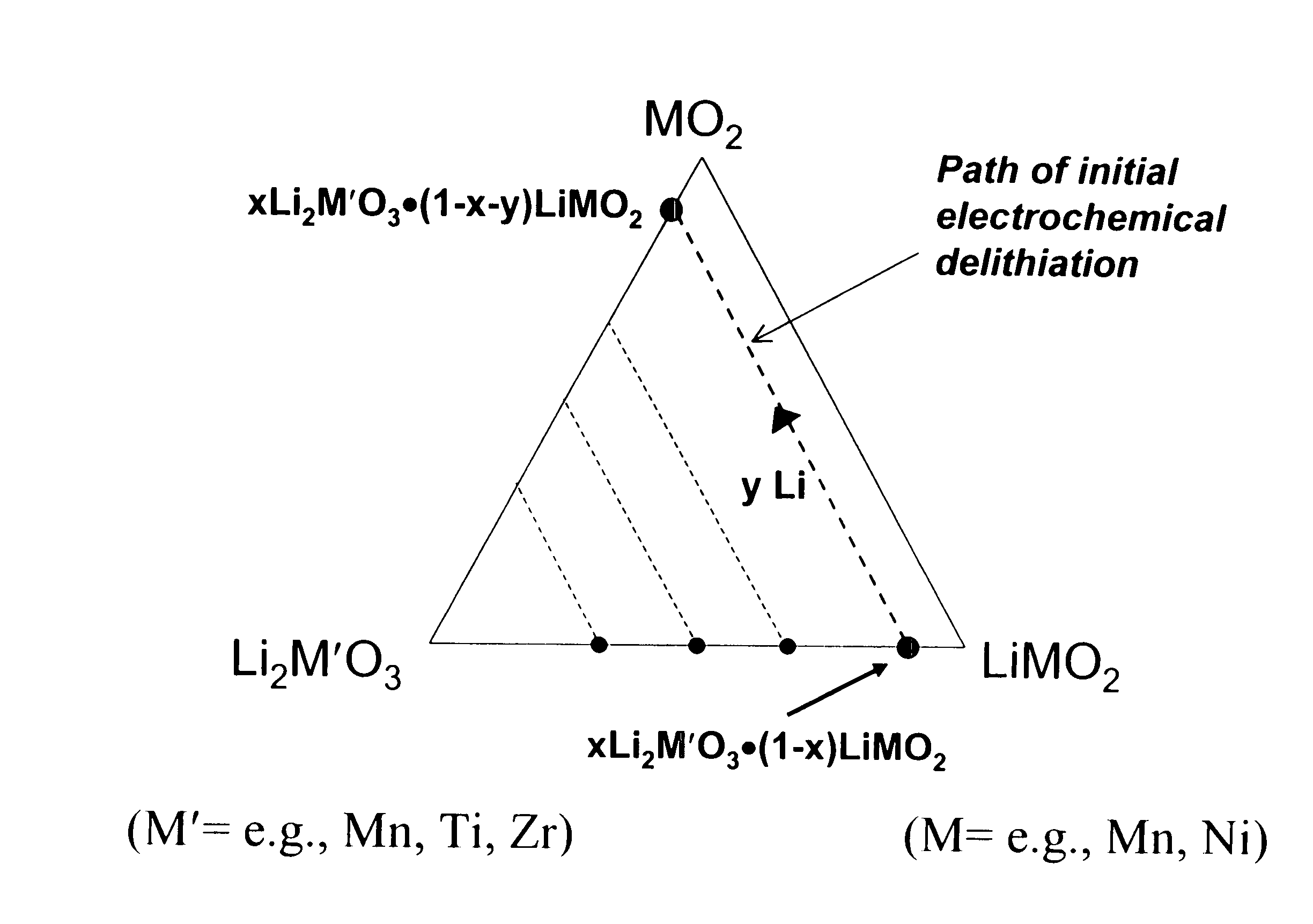
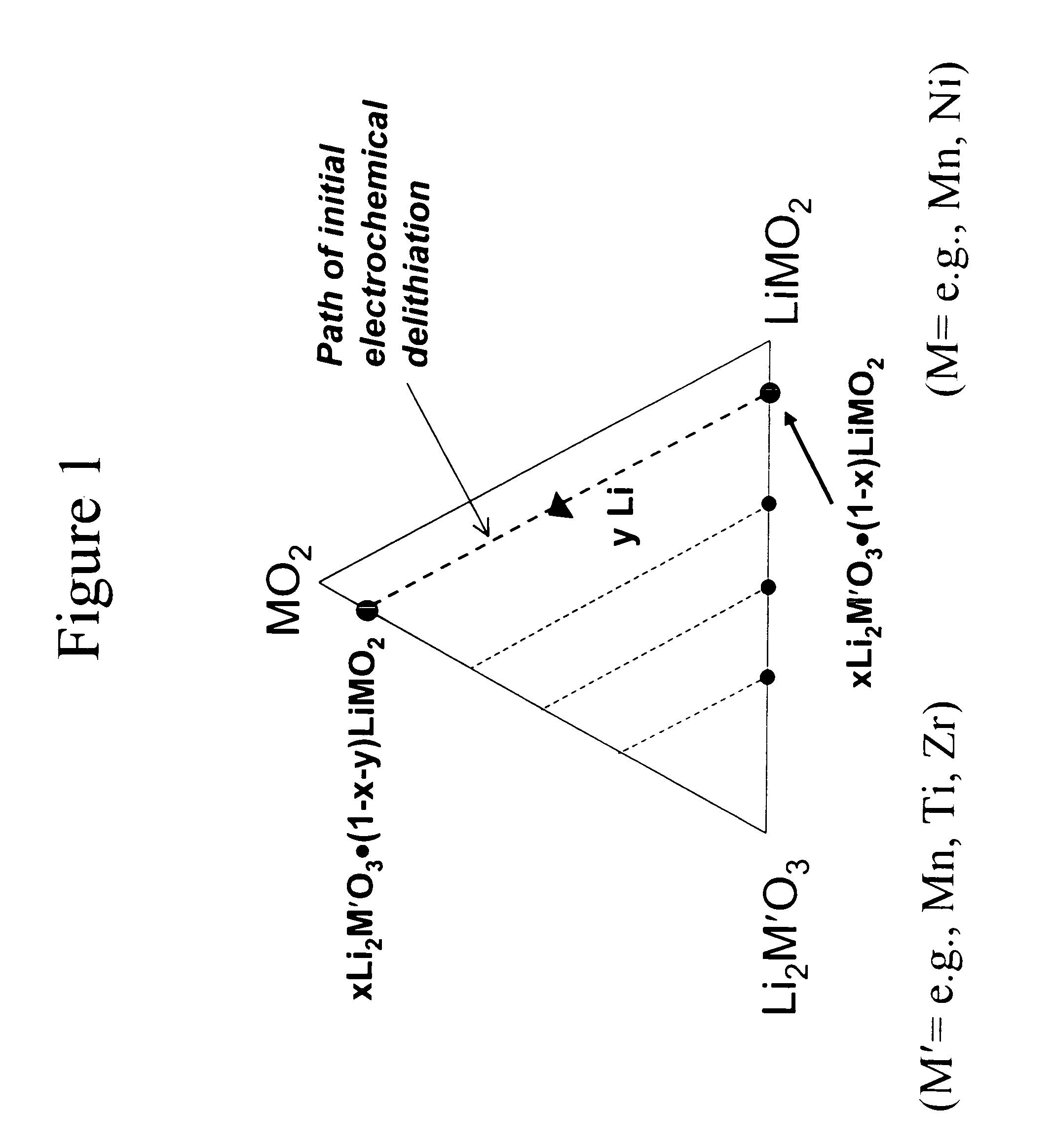
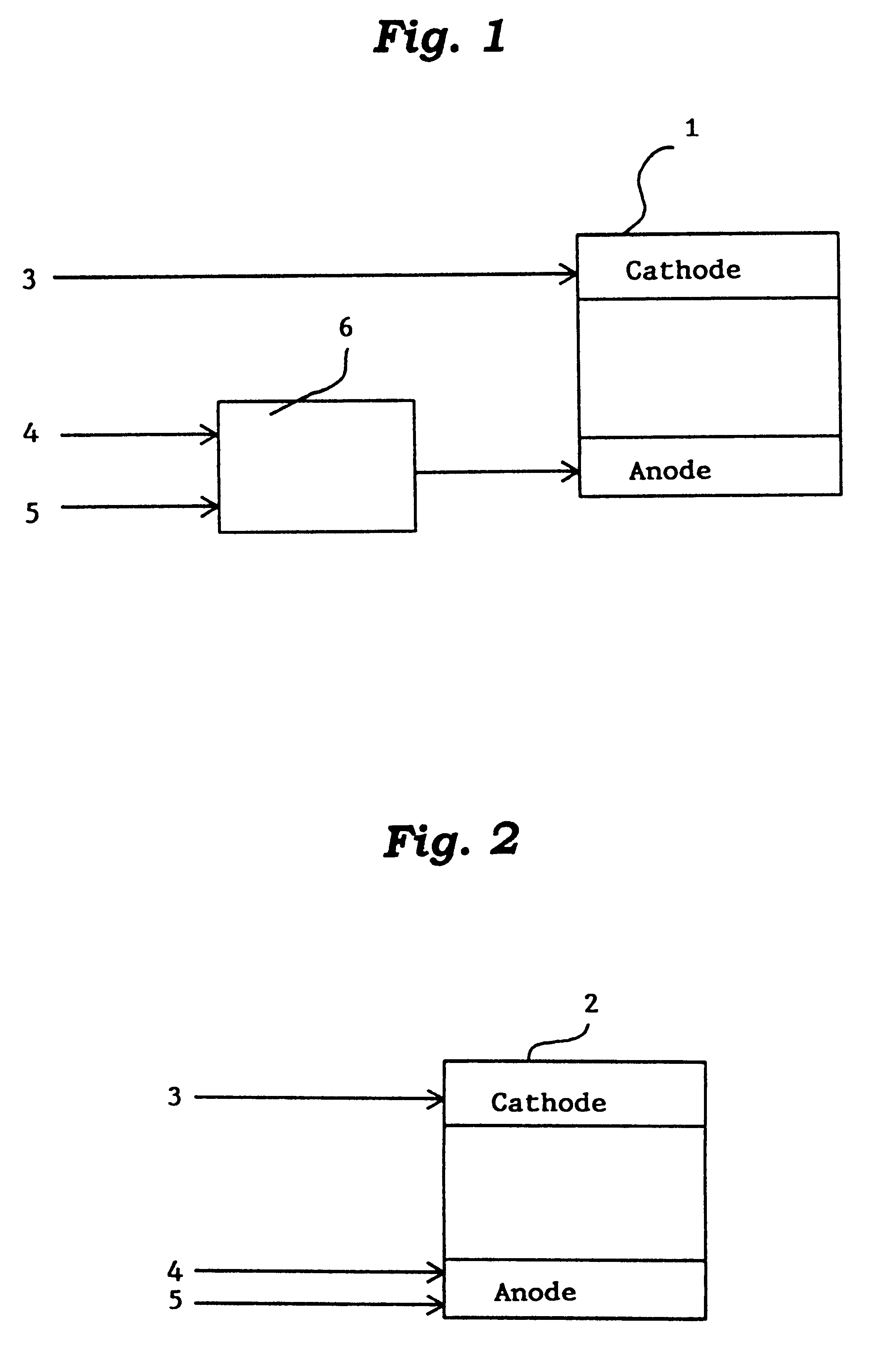
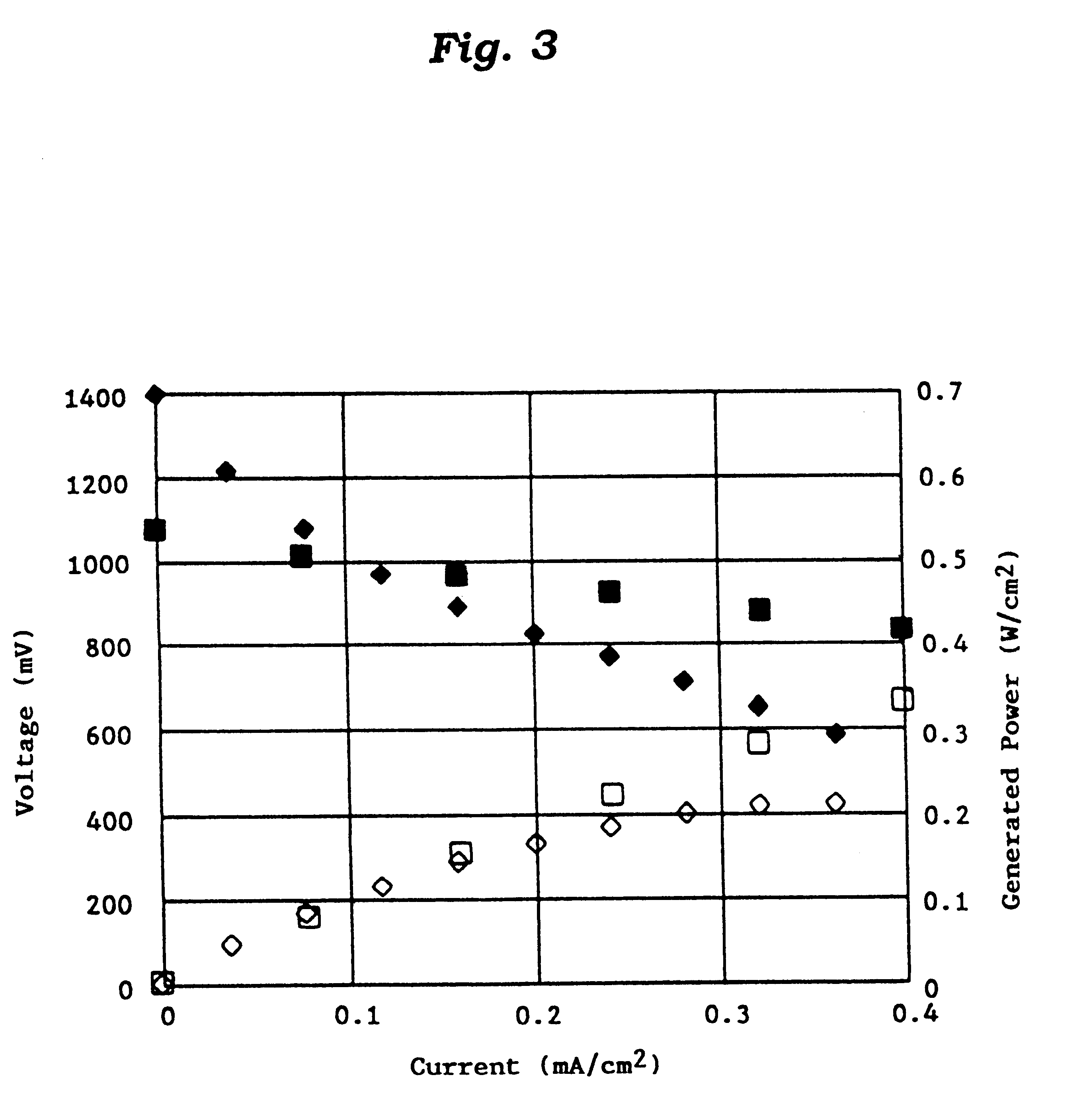
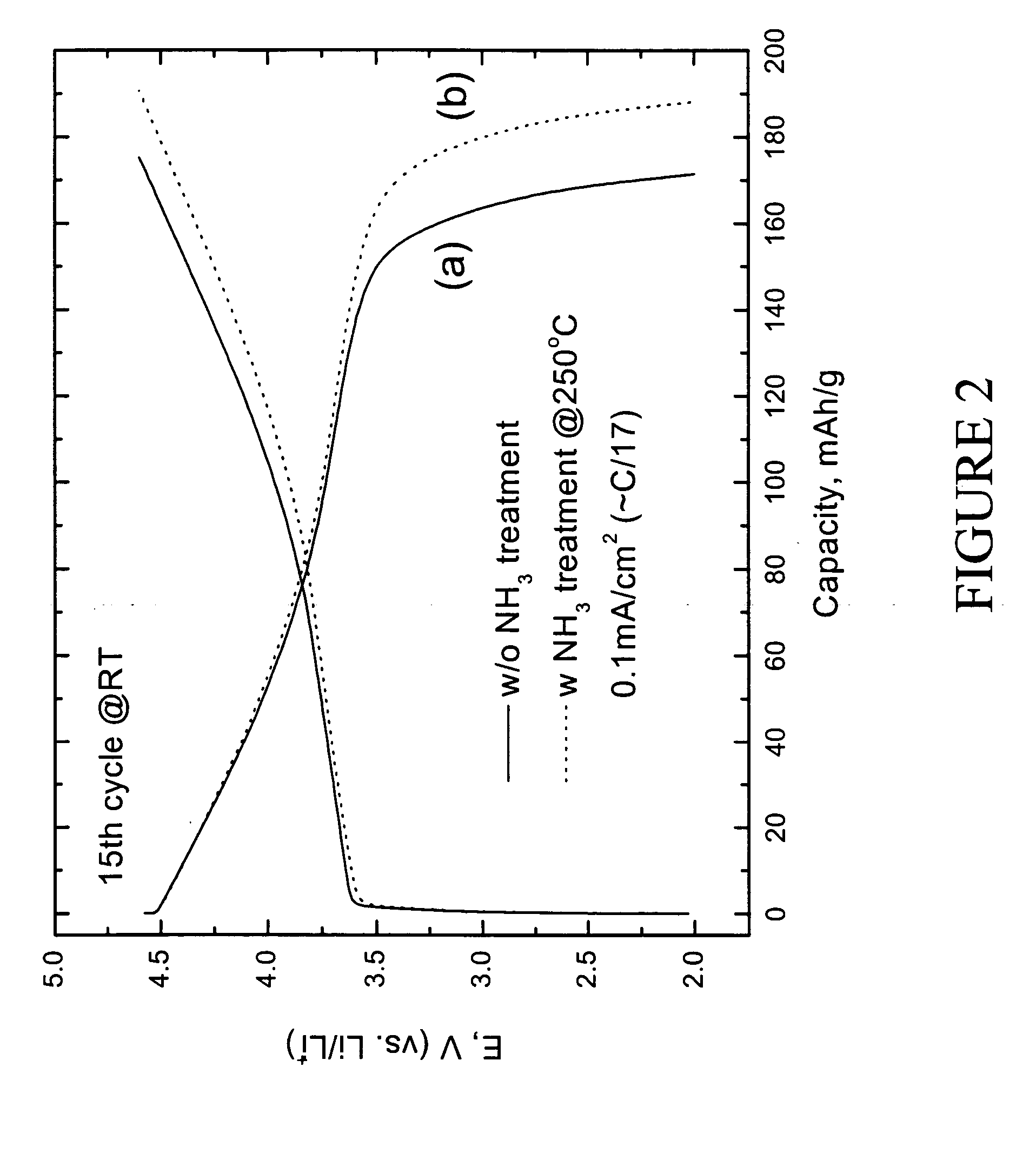
Lithium metal oxide electrodes for lithium cells and batteries
InactiveUS7135252B2Improve structural stabilityImprove stabilityZirconium compoundsSecondary cellsLithium metalOxidation state
A lithium metal oxide positive electrode for a non-aqueous lithium cell is disclosed. The cell is prepared in its initial discharged state and has a general formula xLiMO2.(1-x)Li2M′O3 in which 0<x<1, and where M is more than one ion with an average trivalent oxidation state and with at least one ion being Ni, and where M′ is one or more ions with an average tetravalent oxidation state. Complete cells or batteries are disclosed with anode, cathode and electrolyte as are batteries of several cells connected in parallel or series or both.
Owner:CHICAGO UNIV OF THE +1
Metal oxide coated positive electrode materials for lithium-based batteries
Positive electrode active materials are formed with various metal oxide coatings. Excellent results have been obtained with the coatings on lithium rich metal oxide active materials. Surprisingly improved results are obtained with metal oxide coatings with lower amounts of coating material. High specific capacity results are obtained even at higher discharge rates.
Owner:IONBLOX INC
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS20100086853A1Electrode manufacturing processesAlkali metal oxidesDischarge rateLithium-ion battery
Owner:IONBLOX INC
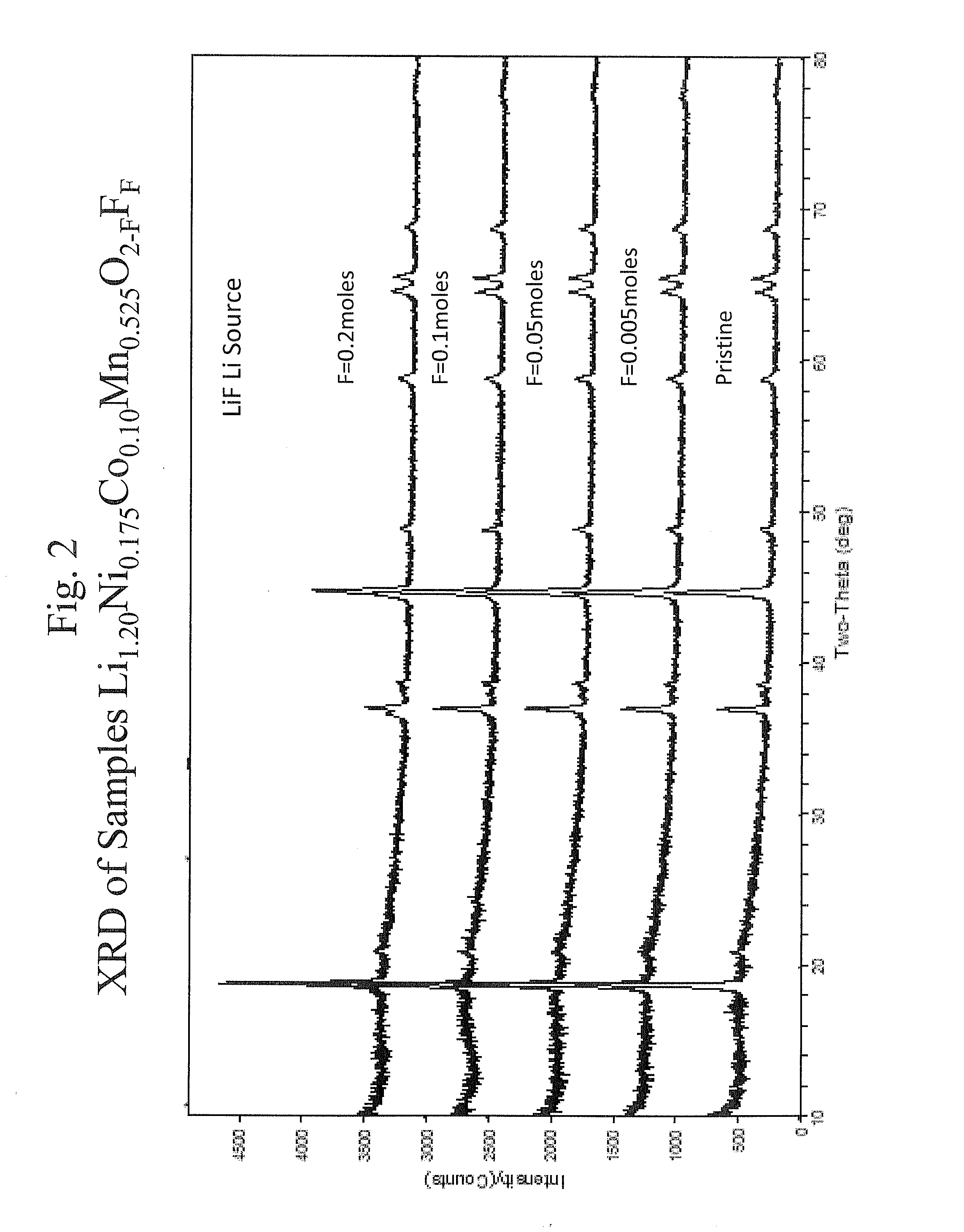
Layered cathode materials for lithium ion rechargeable batteries
ActiveUS7205072B2Improve impedance characteristicsImprove stabilityAlkali metal oxidesNon-aqueous electrolyte accumulator electrodesDopantRechargeable cell
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling.
Owner:UCHICAGO ARGONNE LLC +2
Fluorine doped lithium rich metal oxide positive electrode battery materials with high specific capacity and corresponding batteries
ActiveUS20100086854A1Desirable battery performanceElectrode manufacturing processesFinal product manufactureLithiumDopant
Lithium rich metal oxyfluorides are described with high specific capacity and, good cycling properties. The materials have particularly good high rate capabilities. The fluorine dopant can be introduced in a low temperature process to yield the materials with desirable cycling properties. In some embodiments, the positive electrode active materials have a composition represented approximately by the formula Li1+xNiαMnβCoγAδO2−zFz where:x is from about 0.02 to about 0.19,α is from about 0.1 to about 0.4,β is from about 0.35 to about 0.869,γ is from about 0.01 to about 0.2,δ is from 0.0 to about 0.1 andz is from about 0.01 to about 0.2,where A is Mg, Zn, Al, Ga, B, Zr, Ti, Ca, Ce, Y, Nb or combinations thereof.
Owner:IONBLOX INC
Coated positive electrode materials for lithium ion batteries
ActiveUS20110111298A1Secondary cellsNon-aqueous electrolyte accumulator electrodesLithium metalInorganic composition
High specific capacity lithium rich lithium metal oxide materials are coated with inorganic compositions, such as metal fluorides, to improve the performance of the materials as a positive electrode active material. The resulting coated material can exhibit an increased specific capacity, and the material can also exhibit improved cycling. The materials can be formed while maintaining a desired relatively high average voltage such that the materials are suitable for the formation of commercial batteries. Suitable processes are described for the synthesis of the desired coated compositions that can be adapted for commercial production.
Owner:IONBLOX INC
Two stage process for hydrodesulfurizing distillates using bulk multimetallic catalyst
InactiveUS6929738B1Preparation by oxo-reaction and reductionOrganic compound preparationLiquid productHydrogen
A two stage hydrodesulfurizing process for producing low sulfur distillates. A distillate boiling range feedstock containing in excess of about 3,000 wppm sulfur is hydrodesulfurized in a first hydrodesulfurizing stage containing one or more reaction zones in the presence of hydrogen and a hydrodesulfurizing catalyst. The liquid product stream thereof is passed to a first separation stage wherein a vapor phase product stream and a liquid product stream are produced. The liquid product stream, which has a substantially lower sulfur and nitrogen content then the original feedstream is passed to a second hydrodesulfurizing stage also containing one or more reaction zones where it is reacted in the presence of hydrogen and a second hydrodesulfurizing catalyst at hydrodesulfurizing conditions. The catalyst in any one or more reaction zones is a bulk multimetallic catalyst comprised of at least one Group VIII non-noble metal and at least two Group VIB metals.
Owner:EXXON RES & ENG CO
Ternary oxide nanostructures and methods of making same
InactiveUS20070138459A1Suitable for preparationFrom gel stateAlkaline earth titanatesNanostructureDislocation
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Ti, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Lithium transition metal-based compound powder for positive electrode material in lithium rechargeable battery, method for manufacturing the powder, spray dried product of the powder, firing precursor of the powder, and positive electrode for lithium rechargeable battery and lithium rechargeable battery using the powder
ActiveUS20090104530A1Low costImprove security levelElectrode manufacturing processesNon-aqueous electrolyte accumulator electrodesMetalHigh voltage
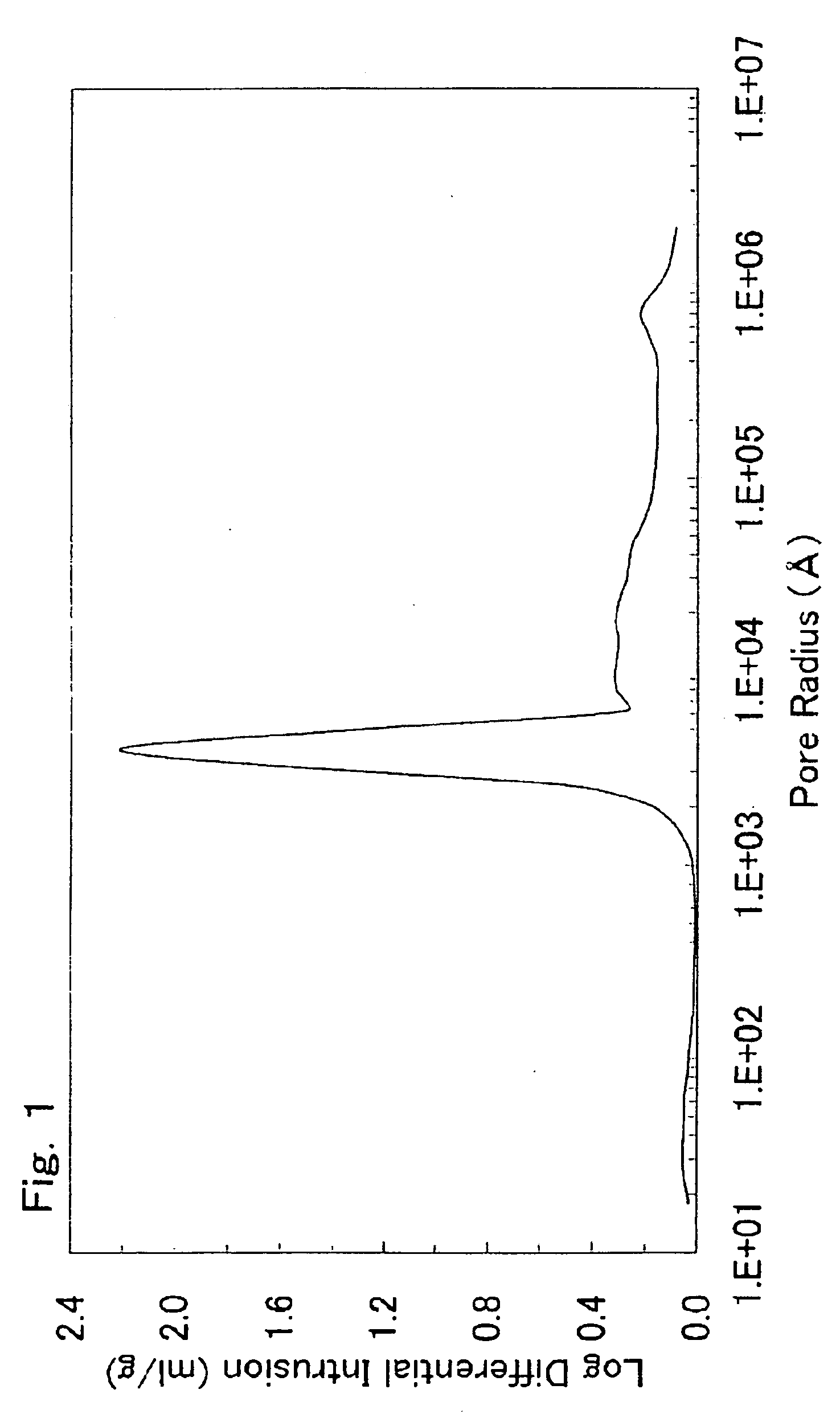
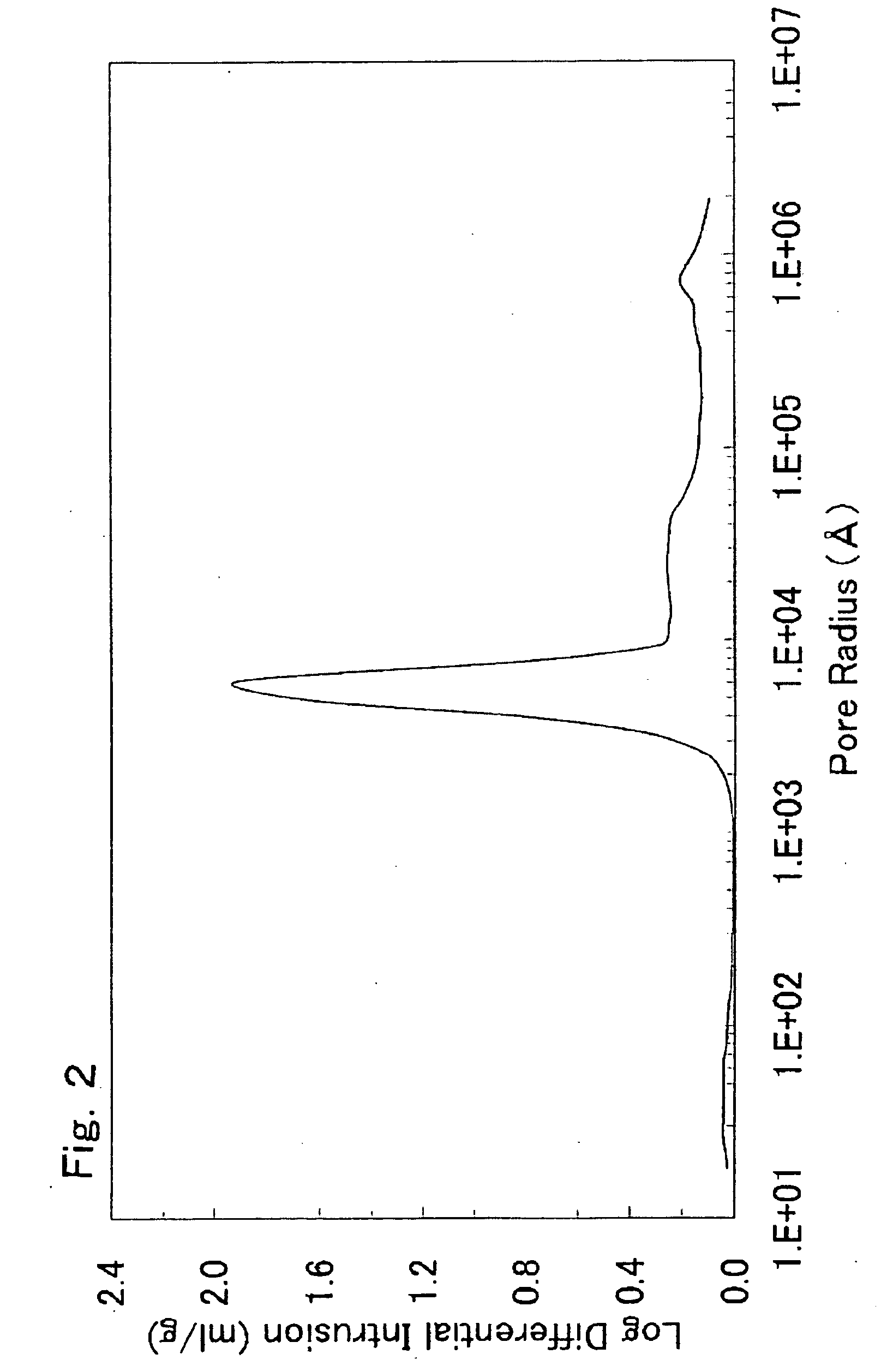
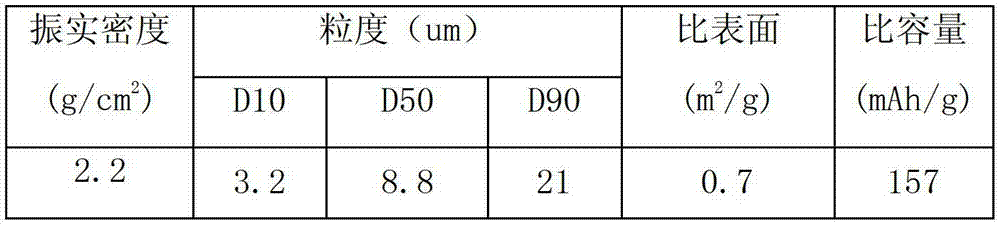
There is provided a powder of a lithium transition-metal compound for a positive-electrode material in a lithium secondary battery, in which the use of the powder as that of a positive-electrode material in a lithium secondary battery achieves a good balance among improvement in battery performance, cost reduction, resistance to a higher voltage, and a higher level of safety. The powder of the lithium transition-metal compound for a positive-electrode material in a lithium secondary battery is characterized in that in a mercury intrusion curve obtained by mercury intrusion porosimetry, the amount of mercury intruded is in the range of 0.8 cm3 / g to 3 cm3 / g when the pressure is increased from 3.86 kPa to 413 MPa.
Owner:MITSUBISHI CHEM CORP
Layer-layer lithium rich complex metal oxides with high specific capacity and excellent cycling
Lithium rich and manganese rich lithium metal oxides are described that provide for excellent performance in lithium-based batteries. The specific compositions can be engineered within a specified range of compositions to provide desired performance characteristics. Selected compositions can provide high values of specific capacity with a reasonably high average voltage. Compositions of particular interest can be represented by the formula, xLi2MnO3.(1−x)LiNiu+ΔMnu−ΔCowAyO2. The compositions undergo significant first cycle irreversible changes, but the compositions cycle stably after the first cycle.
Owner:IONBLOX INC
Positive active material for lithium battery, method of preparing the same, and lithium battery including the same
ActiveUS20090068561A1Conductive materialActive material electrodesConcentration gradientThermal stability
A positive active material according to one embodiment of the present invention includes an internal bulk part and an external bulk part surrounding the internal bulk part and has a continuous concentration gradient of the metal composition from an interface between the internal bulk part and the external bulk part to the surface of the active material. The provided positive active material in which the metal composition is distributed in a continuous concentration gradient has excellent electrochemical characteristics such as a cycle life, capacity, and thermal stability.
Owner:IUCF HYU (IND UNIV COOP FOUNDATION HANYANG UNIV)
Lithium transition metal-based compound powder, method for manufacturing the same, spray-dried substance serving as firing precursor thereof, and lithium secondary battery positive electrode and lithium secondary battery using the same
ActiveUS20100209771A1Improve load characteristicsHigh densityMaterial nanotechnologyAlkaline accumulatorsLithiumHigh density
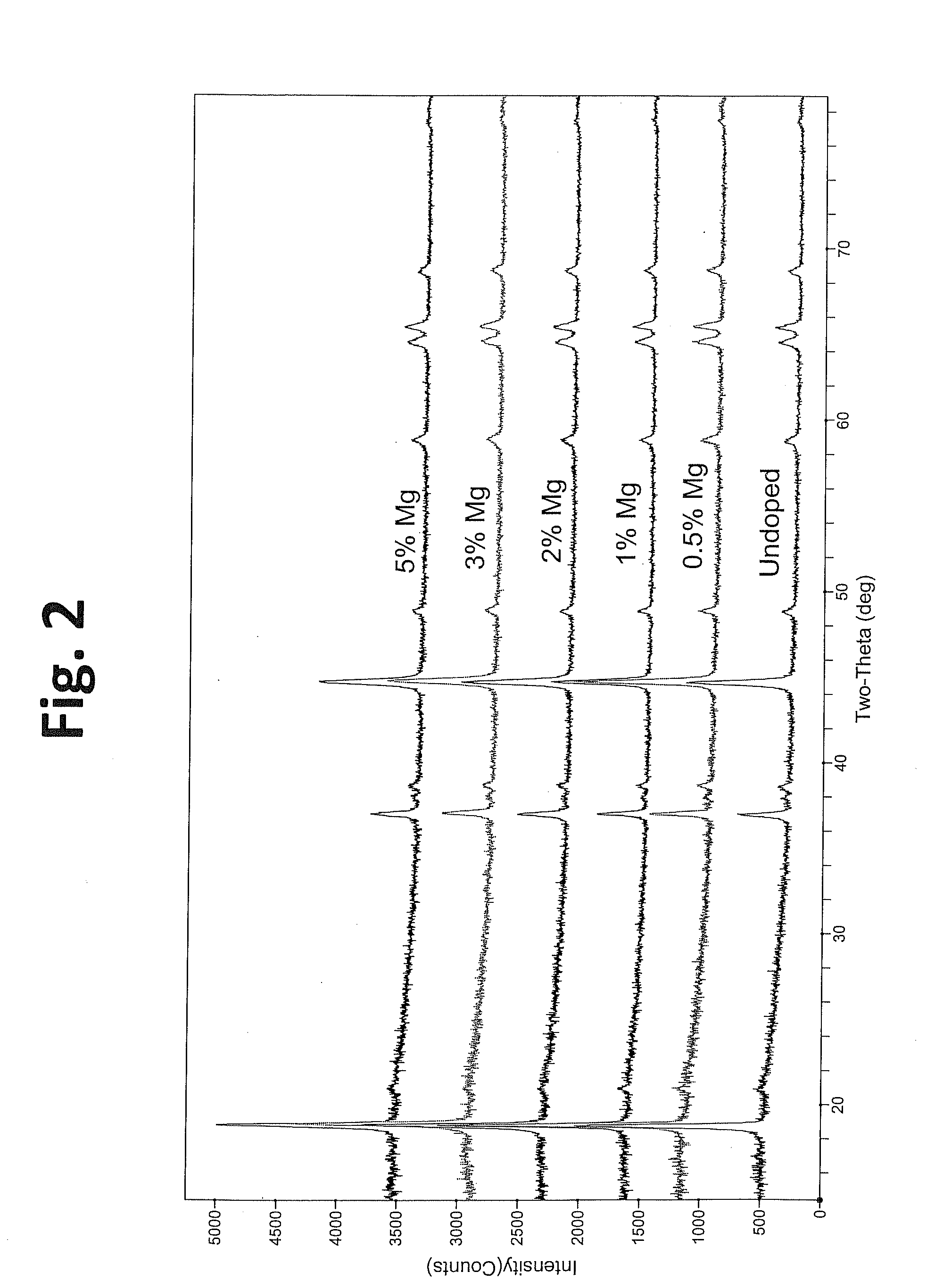
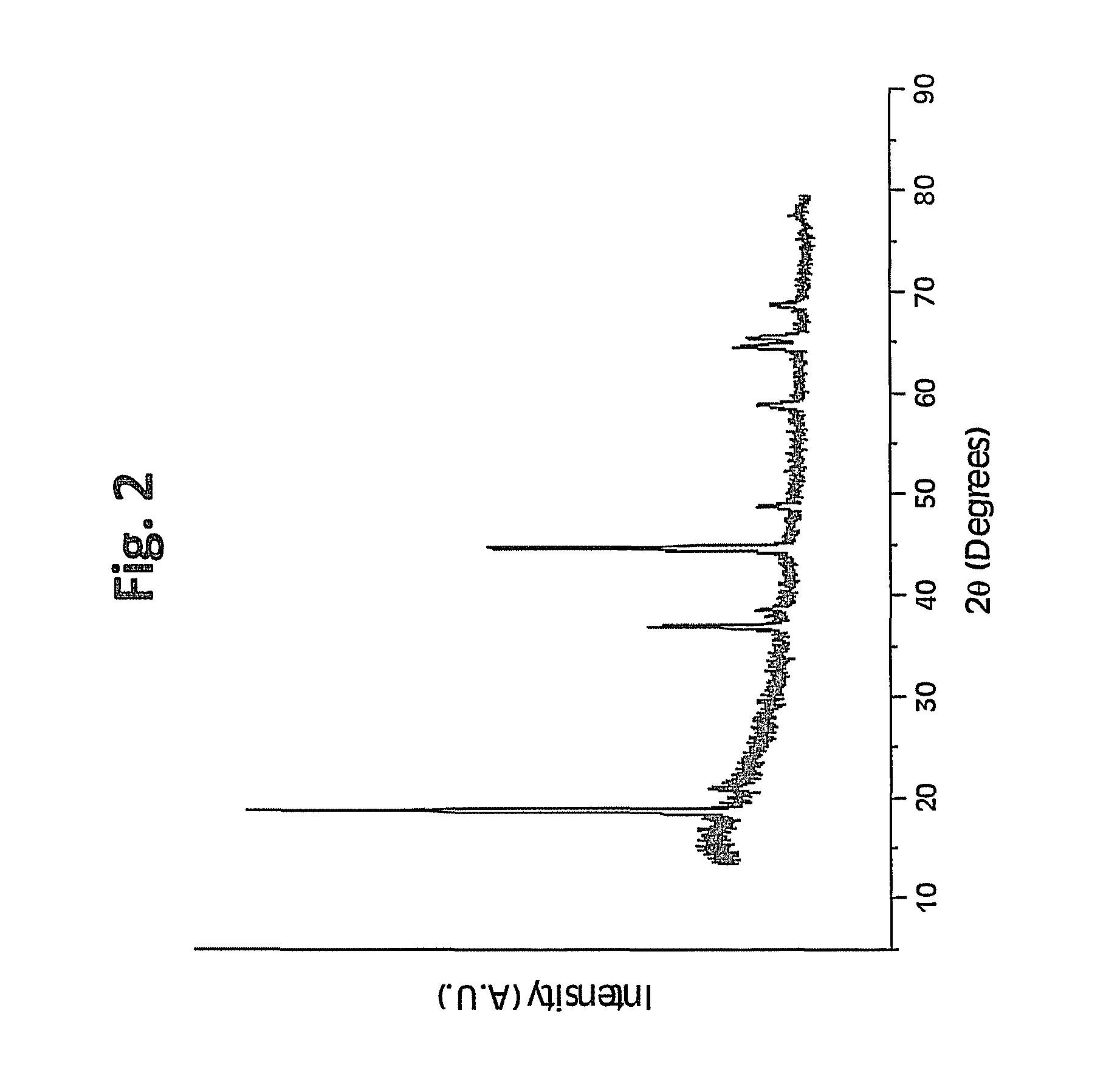
A lithium transition metal-based compound powder for a lithium secondary battery positive electrode material that can achieve both improvements of load characteristics such as rate and output characteristics and a higher density is a lithium transition metal-based compound powder containing, as a main component, a lithium transition metal-based compound that has a function of allowing elimination and insertion of lithium ions, and including a crystal structure belonging to a layer structure, wherein primary particles are aggregated to form secondary particles, the ratio A / B of a median diameter A of the secondary particles to an average diameter (average primary particle diameter B) is in the range of 8 to 100, and 0.01≦FWHM(110)≦0.5 where FWHM(110) is the half width of a (110) diffraction peak present near a diffraction angle 2θ of 64.5° in a powder X-ray diffraction analysis using a CuKα line.
Owner:MITSUBISHI CHEM CORP
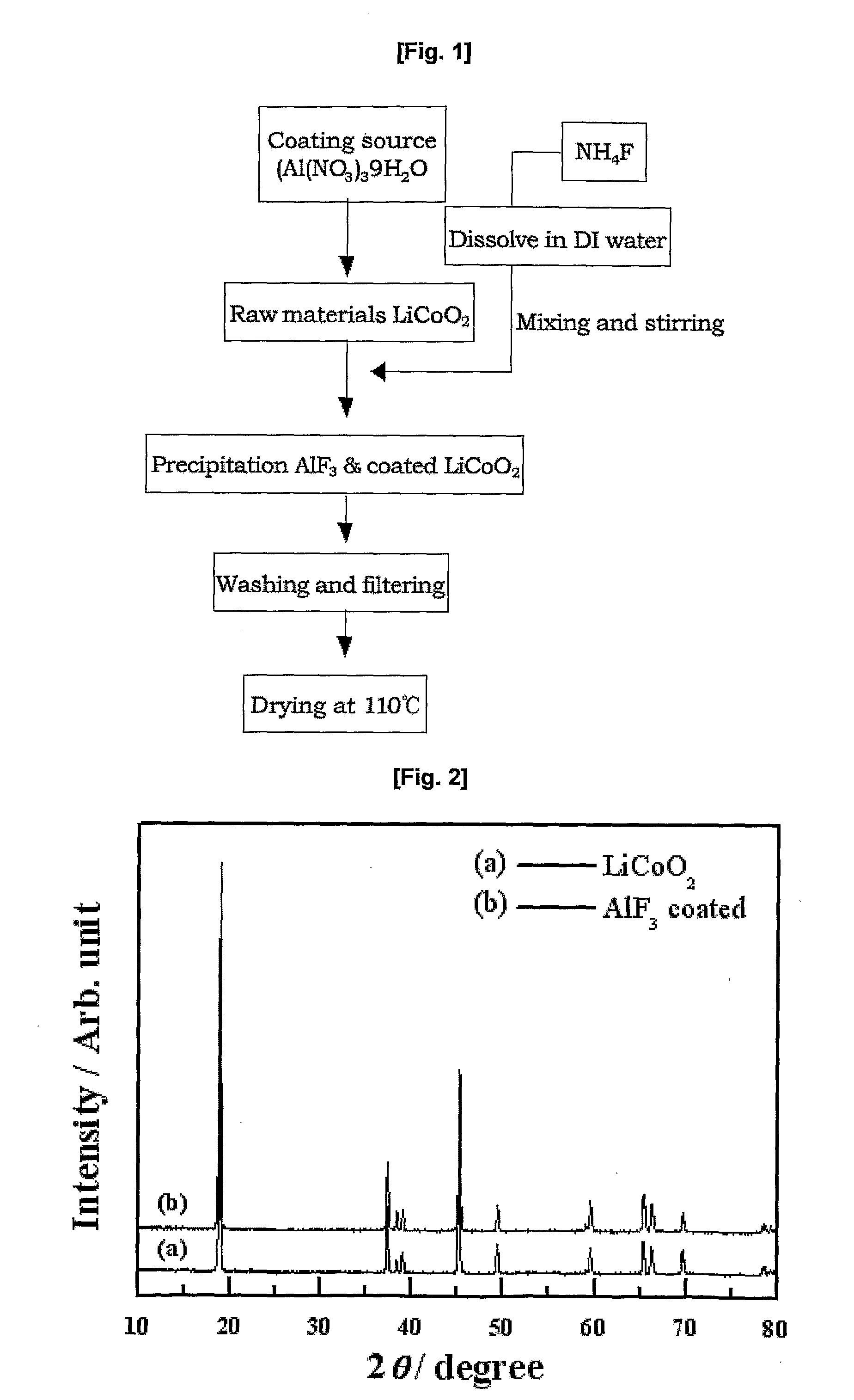
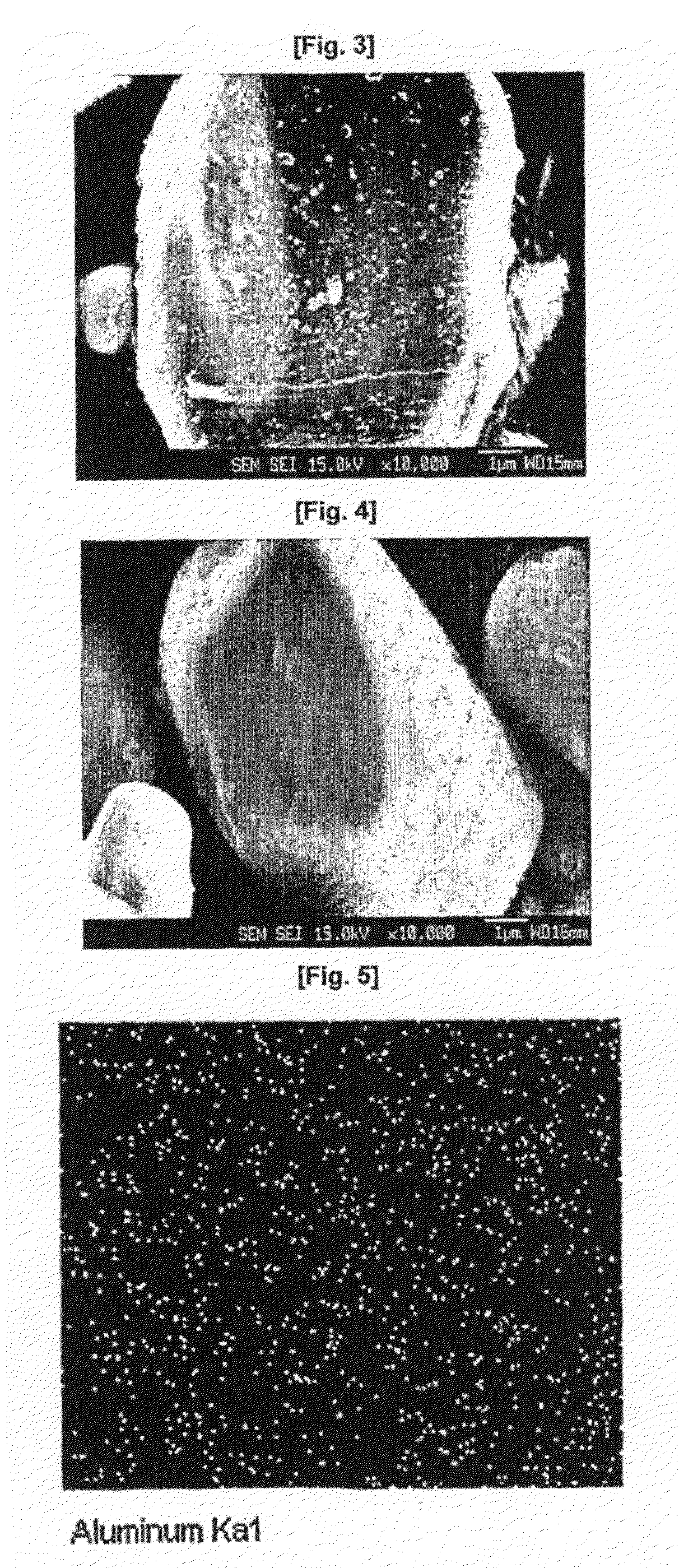
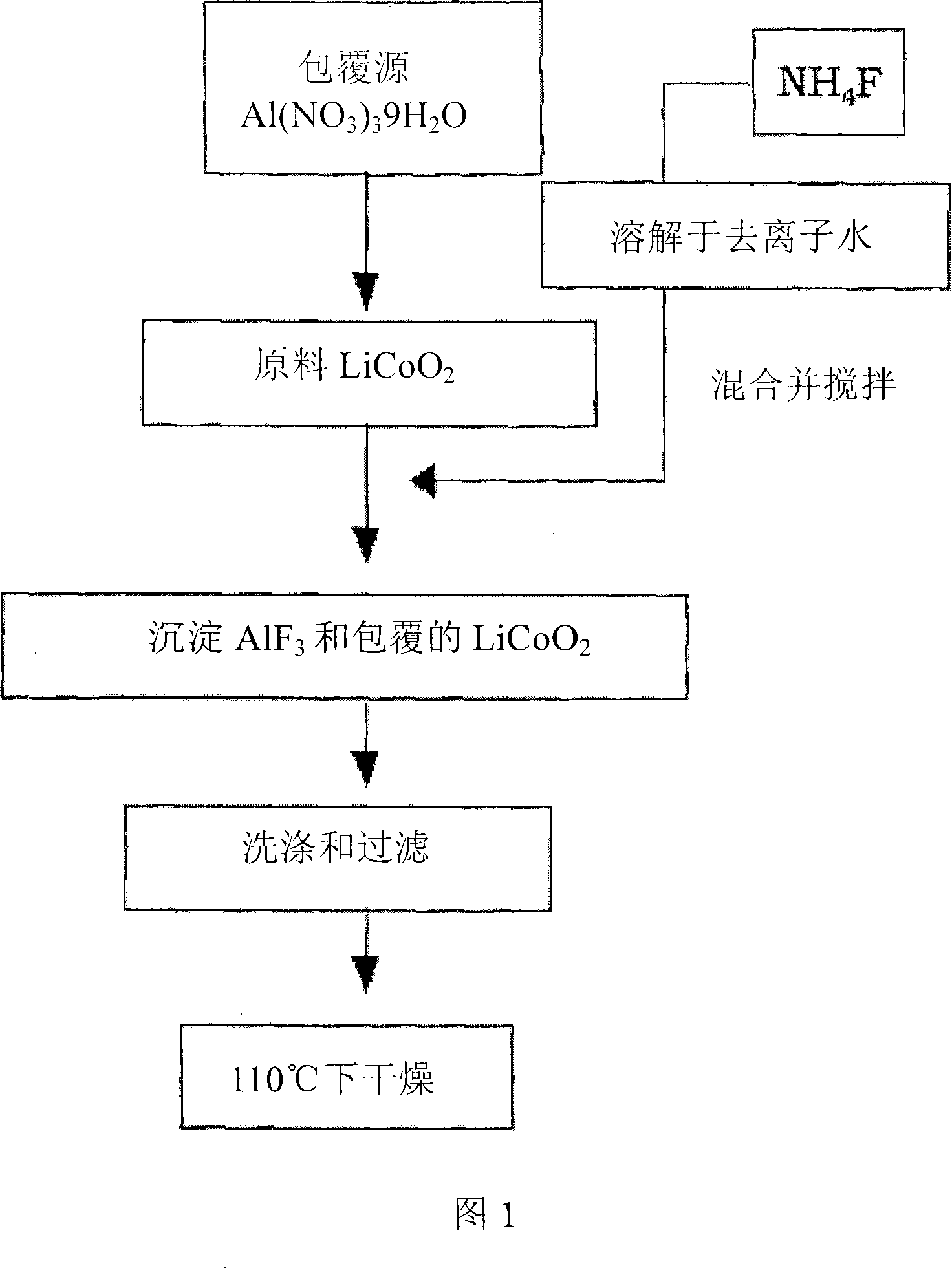
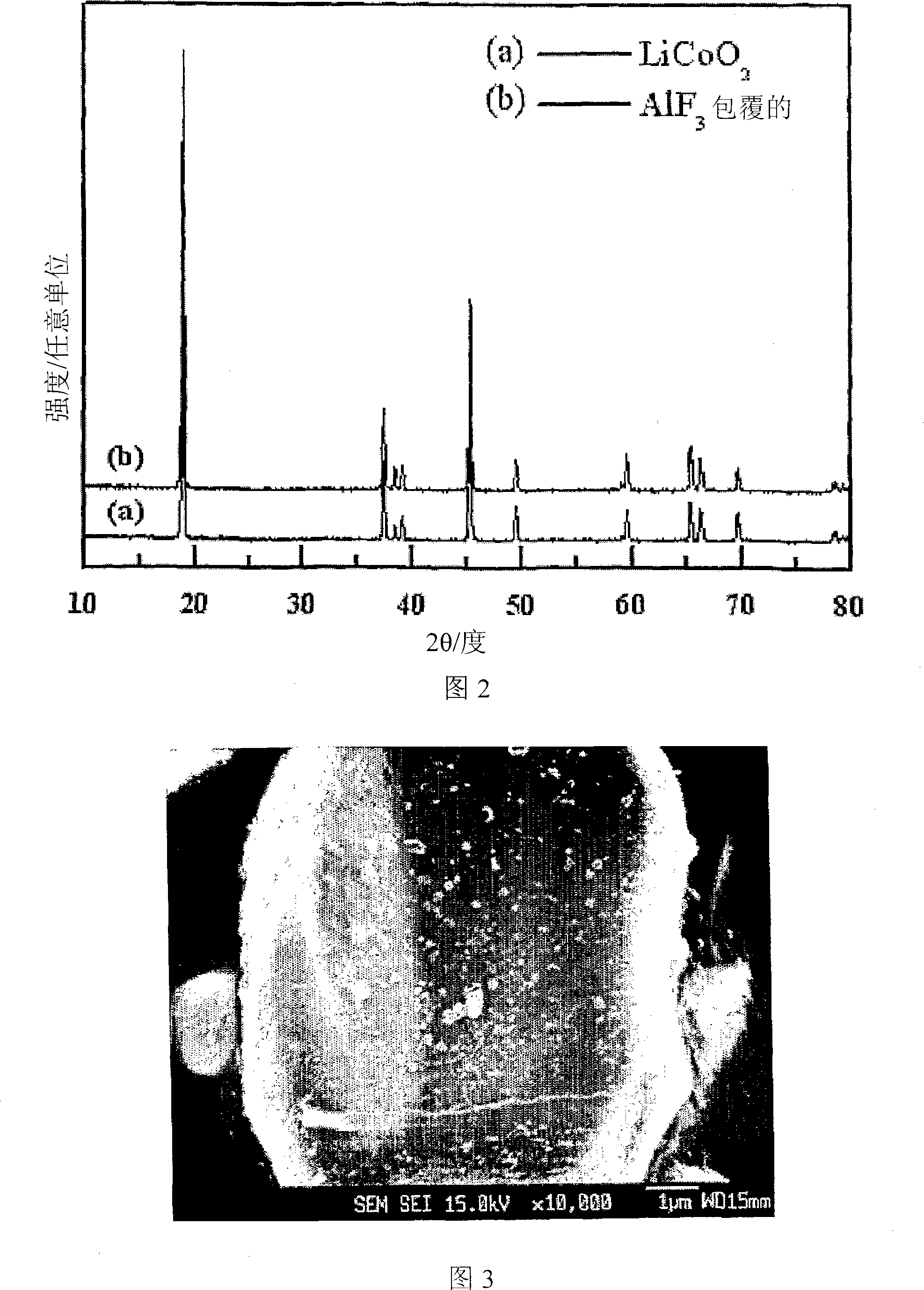
Cathode Active Material Coated With Fluorine Compound for Lithium Secondary Batteries and Method for Preparing the Same
InactiveUS20090087362A1Inhibition of performance deteriorationHigh voltageElectrode manufacturing processesLithium compoundsLithiumHigh rate
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Cathode active material coated with fluorine compound for lithium secondary batteries and method for preparing the same
Disclosed herein is a cathode active material coated with a fluorine compound for lithium secondary batteries. The cathode active material is structurally stable, and improves the charge-discharge characteristics, cycle characteristics, high-voltage characteristics, high-rate characteristics and thermal stability of batteries.
Owner:ENERCERAMIC
Positive electrode active material precursor for lithium secondary battery, positive electrode active material manufactured by using thereof, and lithium secondary battery including same
ActiveUS20140158932A1Large capacityEasy to insertFinal product manufactureAlkali metal oxidesLithiumEngineering
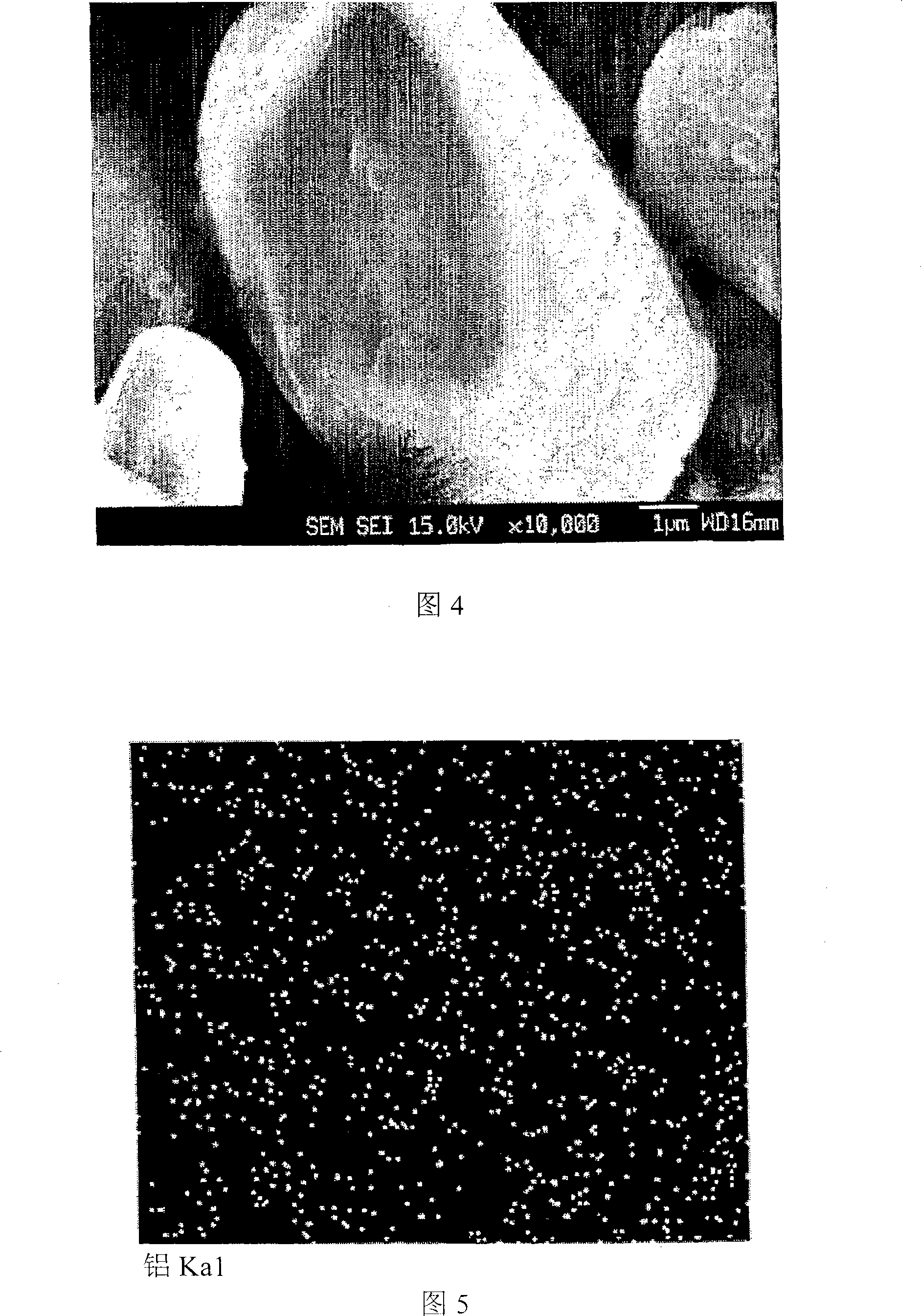
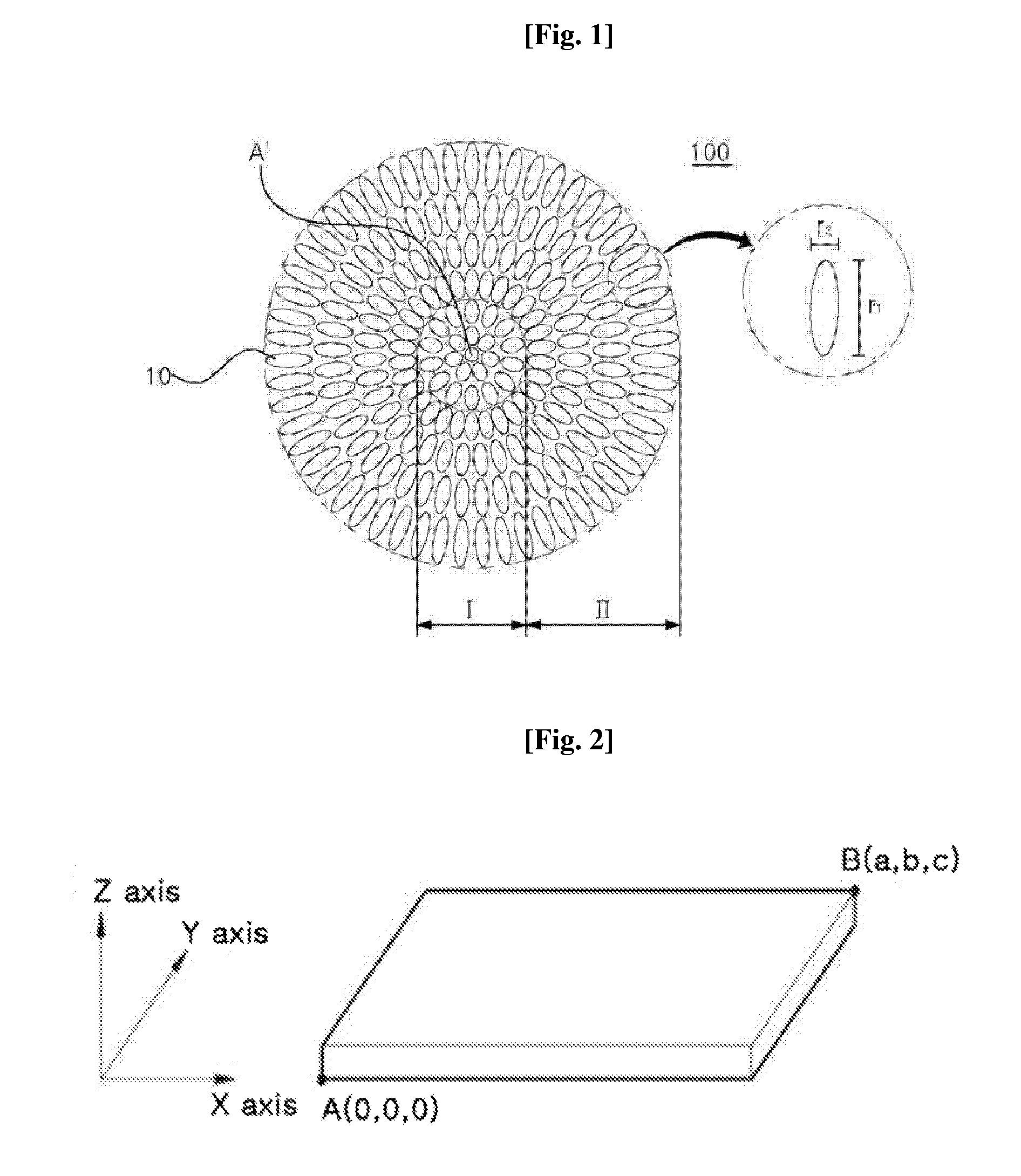
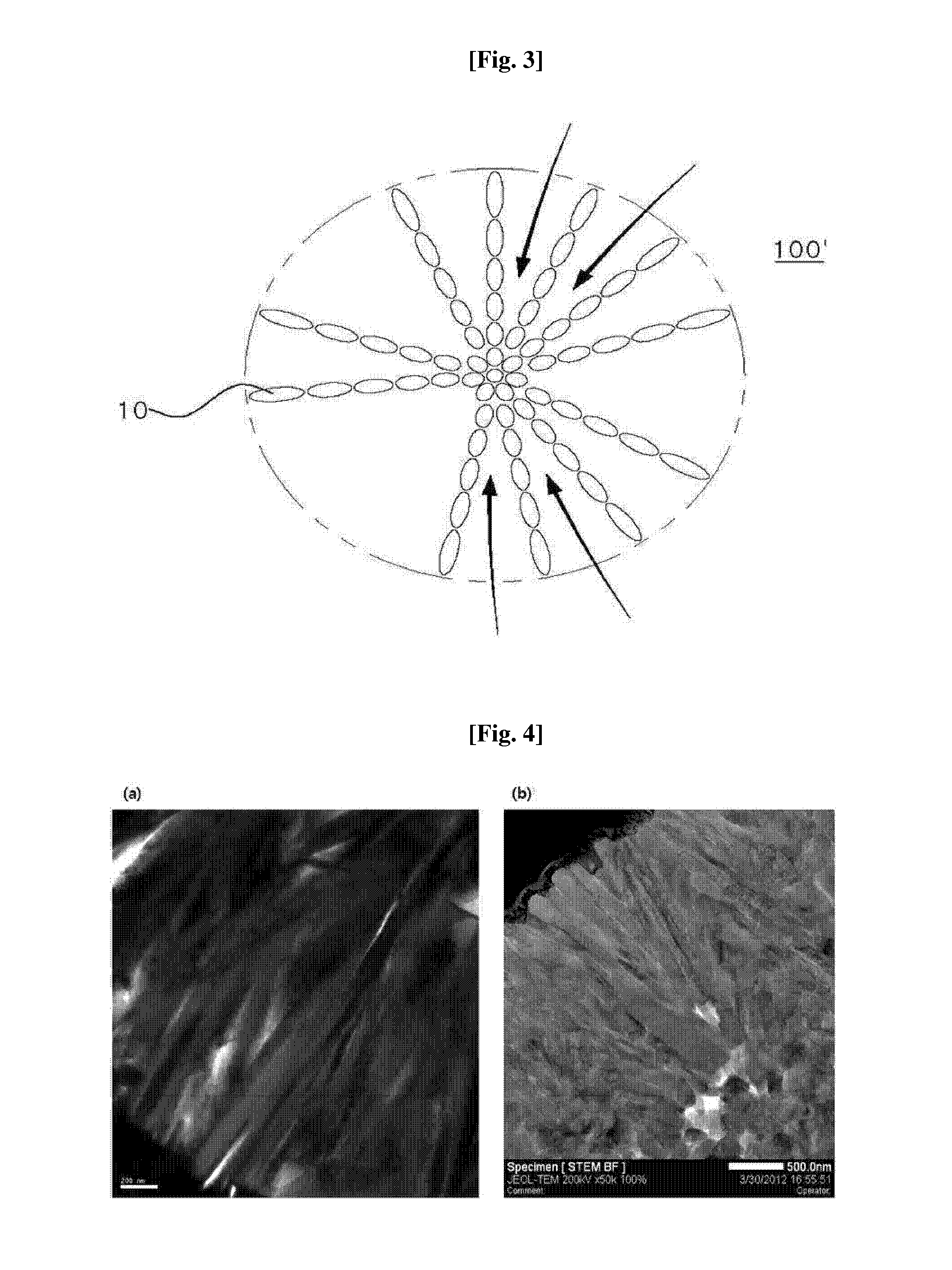
The present disclosure relates to a positive electrode active material precursor for a lithium secondary battery, a positive electrode active material manufactured by using thereof, and a lithium secondary battery comprising the same. More specifically, it relates to a positive electrode active material precursor for a lithium secondary battery as a secondary particle comprising transition metals, and formed by gathering of a plurality of primary particles having different a-axis direction length to c-axis direction length ratio, wherein the a-axis direction length to c-axis direction length ratio of the primary particle making up the secondary particle is increased from the center to the surface of the secondary particle; a positive electrode active material; and a lithium secondary battery comprising the same.
Owner:LG CHEM LTD
Active material for positive electrode used in lithium secondary battery and method of manufacturing same
InactiveUS6372385B1Easy to implementEvenly dispersedElectrode thermal treatmentActive material electrodesLithium-ion batteryMetal
Disclosed is active material for a positive electrode used in lithium secondary batteries of Formula 1 below and a method manufacturing the same, a surface of the active material being coated with metal oxide. The method includes the steps of producing a crystalline powder or a semi-crystalline powder of Formula 1; coating the crystalline powder or the semi-crystalline powder with metal alkoxide sol; and heat-treating the powder coated with the metal alkoxide sol.where 0<x<=0.3, 0<=y<=0.01, andA is an element selected from the group consisting of Ni, Co and Mn; B is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al; and C is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al.
Owner:SAMSUNG ELECTRONICS DEVICES CO LTD
Monodisperse noble metal nanocrystals
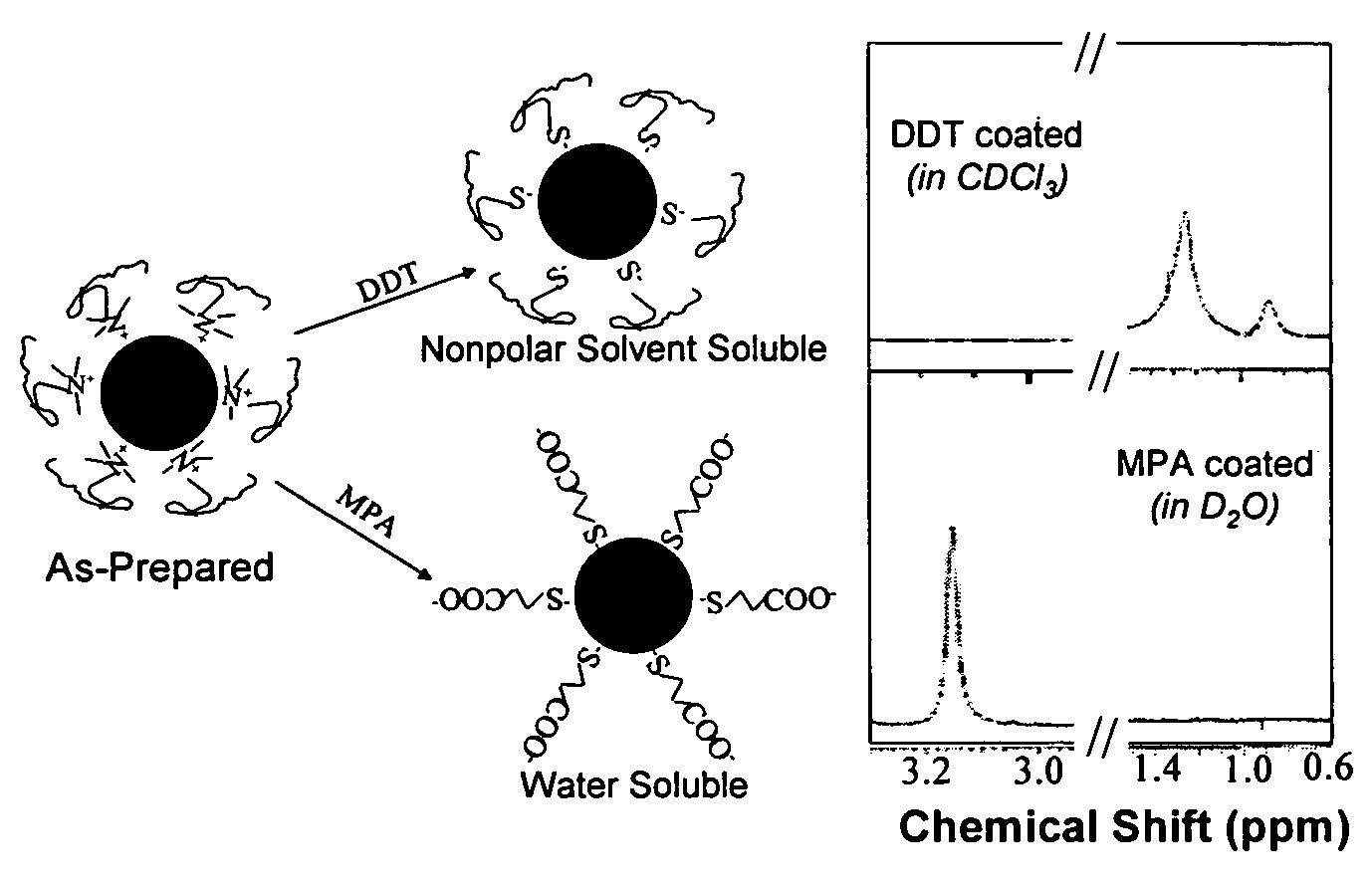
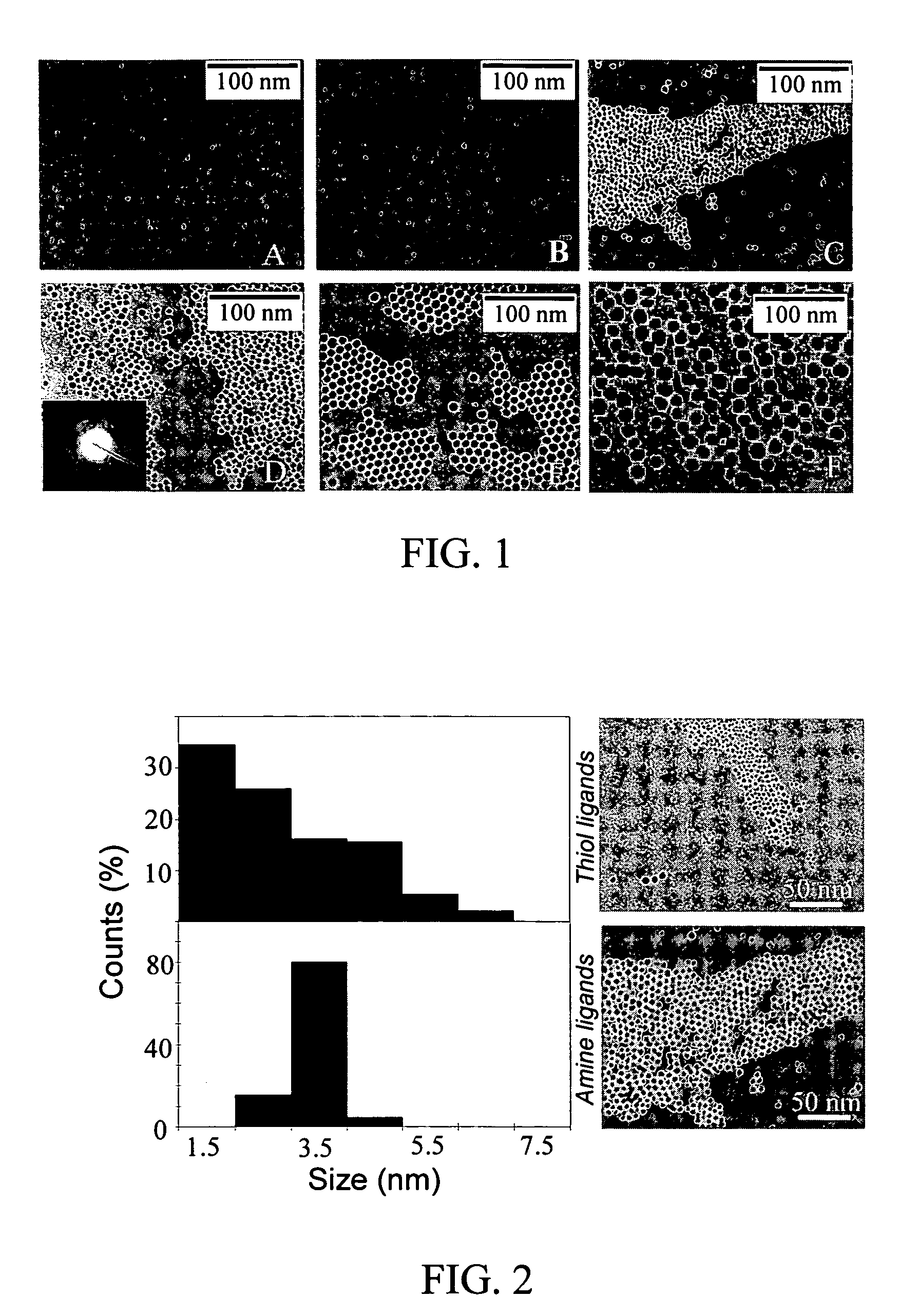
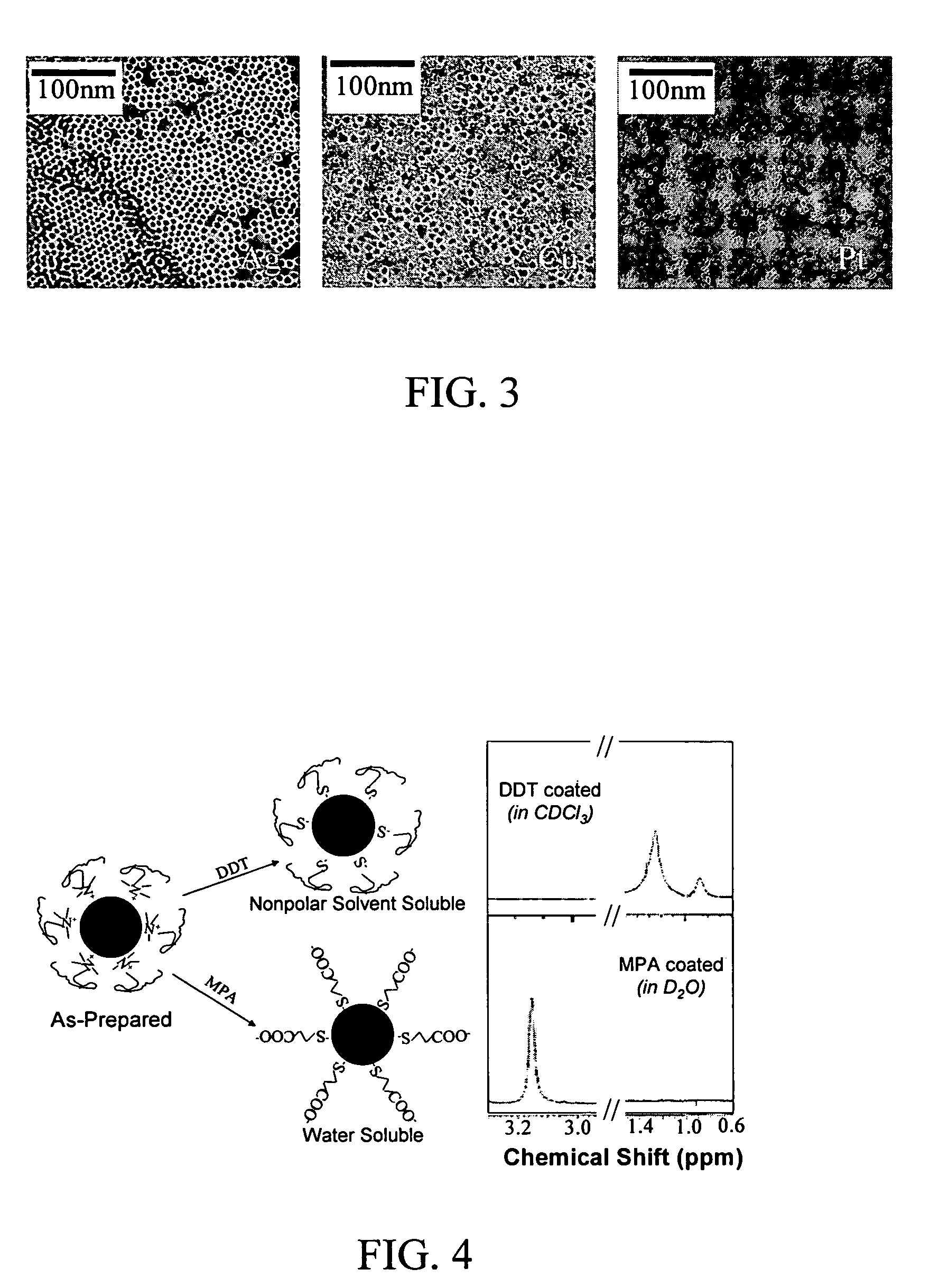
Nanoparticle compositions of noble metals, and methods of making them, are described. The nanoparticle compositions are made by reacting a salt or complex of a noble metal, such as Au, Ag, Cu or Pt, with a weak ligand, and a reducing agent, in a single liquid phase. The noble metal is typically provided as a halide or carboxylate. The ligand is preferably a fatty acid or aliphatic amine. The reducing agent is preferably a borohydride reagent, hydrazine, or a mixture thereof. Nanocrystals in the size range of 1 nm to 20 nm are produced, and can be made in substantially monodisperse form.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
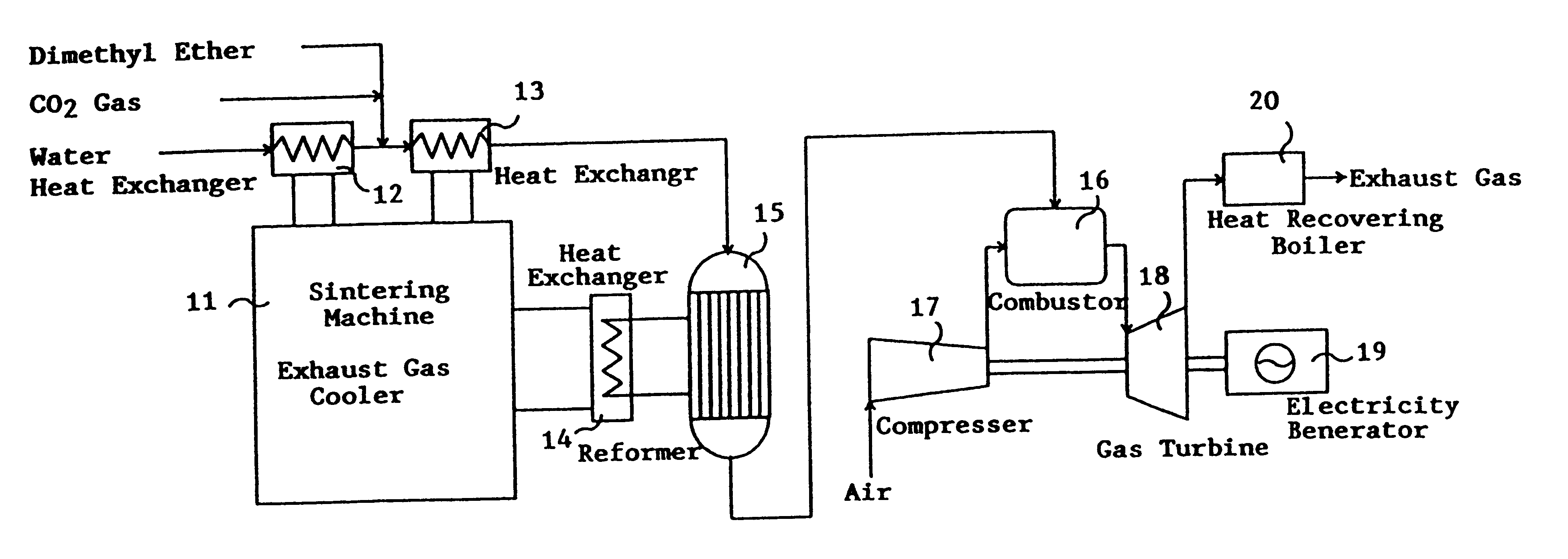
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Ni-based lithium transition metal oxide
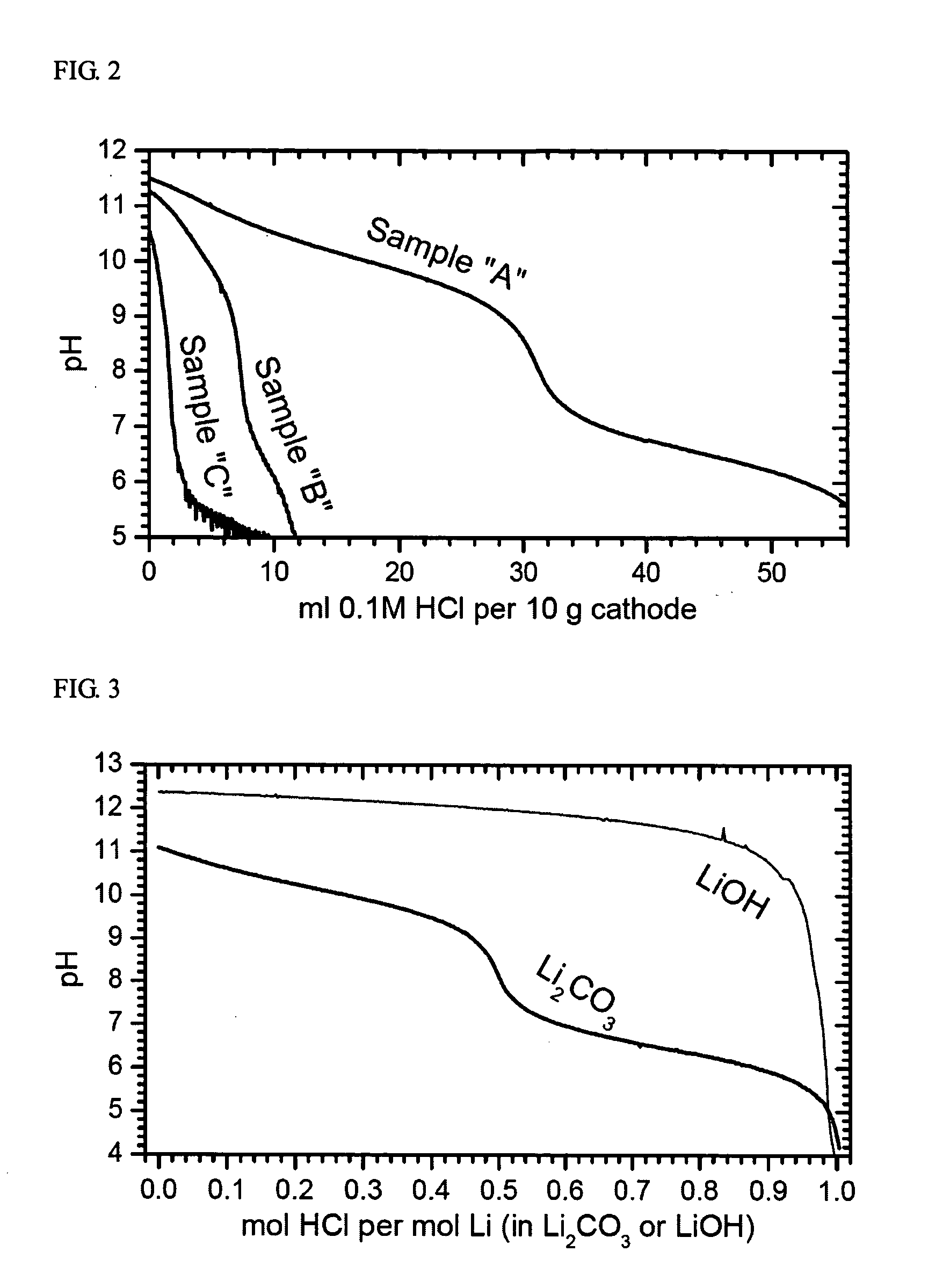
The present invention provides a powderous lithium transition metal oxide with the composition as represented by the below Formula and prepared by solid state reaction in air from a mixed transition metal precursor and Li2CO3, with being practically free of Li2CO3 impurity: LixMyO2 wherein M=M′l-kAk, where M′=Nil-a-b(Ni1 / 2Mn1 / 2)aCob on condition of 0.65≦a+b≦0.85 and 0.1≦b≦0.4; A is a dopant; and 0≦k≦0.05; and x+y=2 on condition of 0.95≦x≦1.05. The Ni-based lithium transition metal oxide according to the present invention has a well-layered structure, and also improved safety, cycling stability and stability against aging and low gas evolution during storage, when used as an active material for cathode of lithium secondary batteries, because it has a high sintering stability and is substantially free of soluble bases. Moreover, the lithium transition metal oxide of the present invention can be prepared by a low-cost process using a mixed transition metal precursor and Li2CO3 as raw stocks and under relatively unrestricted conditions.
Owner:LG ENERGY SOLUTION LTD
Polymer electrolyte, intercalation compounds and electrodes for batteries
Solid battery components are provided. A block copolymeric electrolyte is non-crosslinked and non-glassy through the entire range of typical battery service temperatures, that is, through the entire range of at least from about 0° C. to about 70° C. The chains of which the copolymer is made each include at least one ionically-conductive block and at least one second block immiscible with the ionically-conductive block. The chains form an amorphous association and are arranged in an ordered nanostructure including a continuous matrix of amorphous ionically-conductive domains and amorphous second domains that are immiscible with the ionically-conductive domains. A compound is provided that has a formula of LixMyNzO2. M and N are each metal atoms or a main group elements, and x, y and z are each numbers from about 0 to about 1. y and z are chosen such that a formal charge on the MyNz portion of the compound is (4-x). In certain embodiments, these compounds are used in the cathodes of rechargeable batteries. The present invention also includes methods of predicting the potential utility of metal dichalgogenide compounds for use in lithium intercalation compounds. It also provides methods for processing lithium intercalation oxides with the structure and compositional homogeneity necessary to realize the increased formation energies of said compounds. An article is made of a dimensionally-stable, interpenetrating microstructure of a first phase including a first component and a second phase, immiscible with the first phase, including a second component. The first and second phases define interphase boundaries between them, and at least one particle is positioned between a first phase and a second phase at an interphase boundary. When the first and second phases are electronically-conductive and ionically-conductive polymers, respectively, and the particles are ion host particles, the arrangement is an electrode of a battery.
Owner:MASSACHUSETTS INST OF TECH
Anode active material, manufacturing method thereof, and non-aqueous electrolyte secondary battery
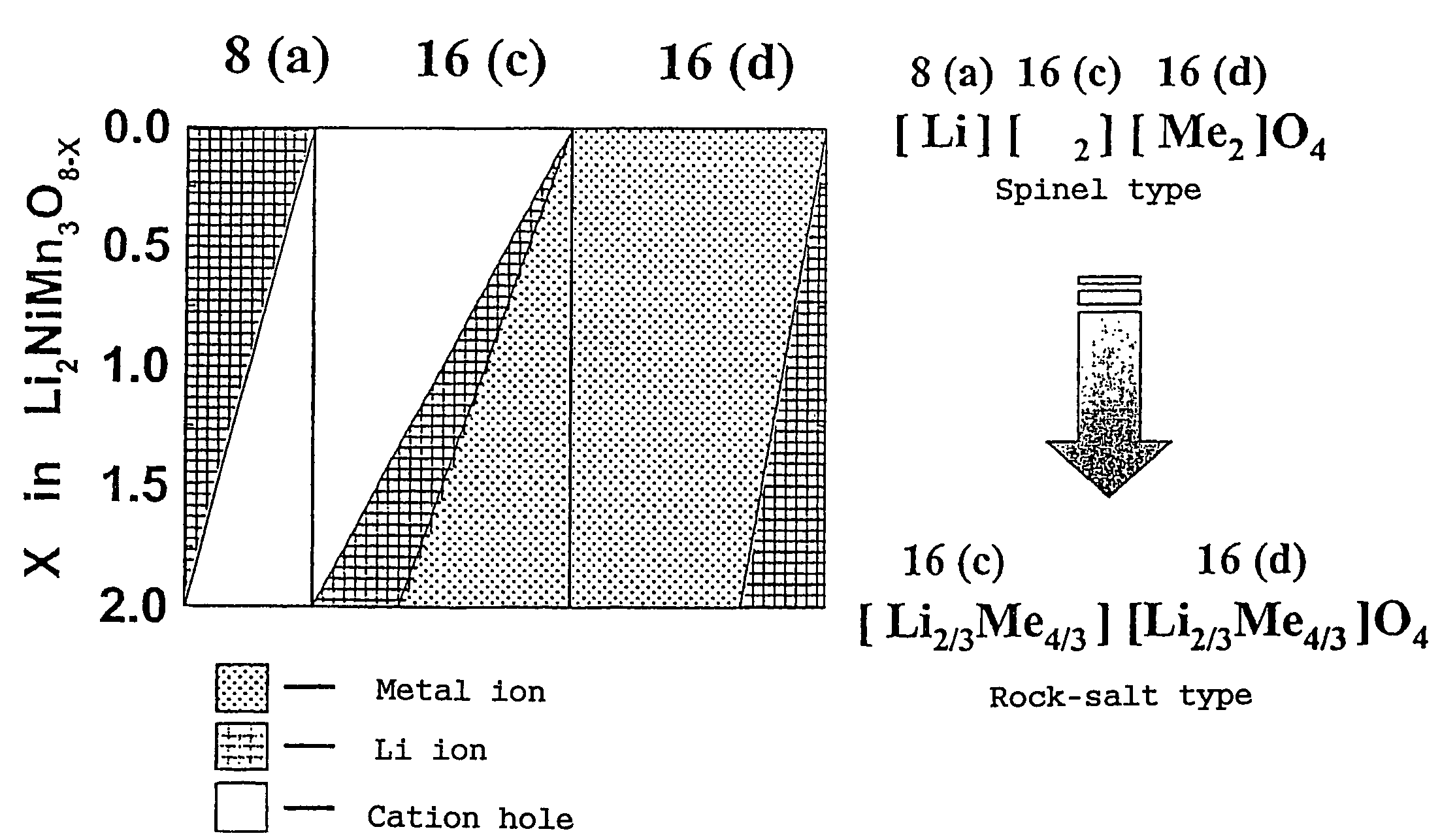
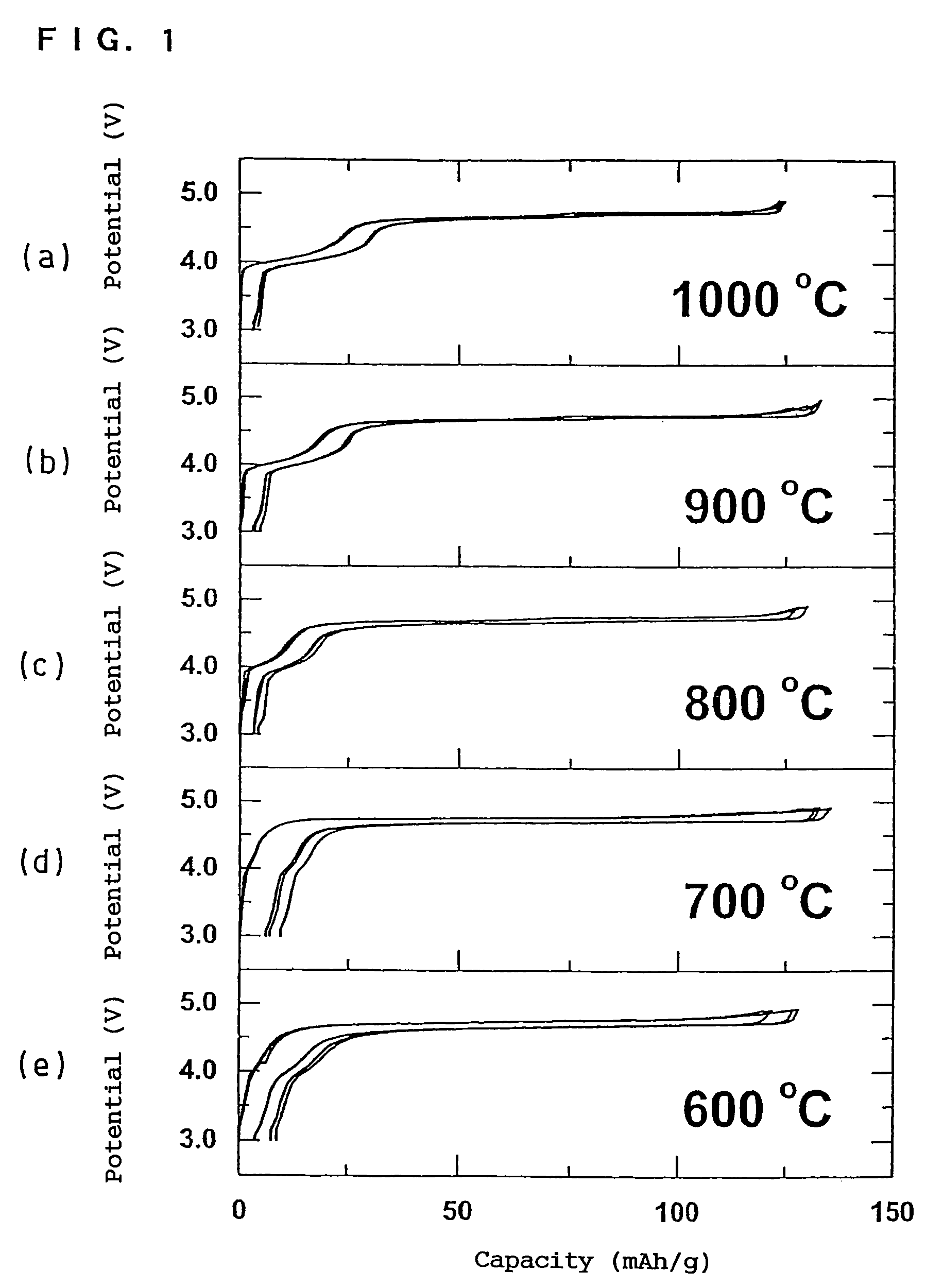
In order to provide a 3V level non-aqueous electrolyte secondary battery with a flat voltage and excellent cycle life at a high rate with low cost, the present invention provides a positive electrode represented by the formula: Li2±α[Me]4O8−x, wherein 0≦α<0.4, 0≦x<2, and Me is a transition metal containing Mn and at least one selected from the group consisting of Ni, Cr, Fe, Co and Cu, said active material exhibiting topotactic two-phase reactions during charge and discharge.
Owner:OSAKA CITY UNIV +1
Lithium ion battery positive pole material cobalt nickel oxide manganses lithium and method for making same
ActiveCN101202343AHigh specific capacityExcellent cycle characteristicsElectrode manufacturing processesLithium compoundsLithium oxideAntioxidant
The invention relates to a nickel cobalt manganese lithium oxide material used for an anode of a li-ion battery and a preparation method. The invention belongs to the li-ion battery technical field. The nickel cobalt manganese lithium oxide material used for the anode of the li-ion battery is a li-rich laminated structure with the chemical component of Li1+zM1-x-yNixCoyO2; wherein, z is less than or equal to 0.2 and more than or equal to 0.05, x is less than or equal to 0.8 and more than 0.1, and y is less than or equal to 0.5 and more than 0.1. The preparation method of the invention is that dissoluble salt of the nickel, cobalt and manganese is taken as the raw material; ammonia or ammonium salt is taken as complexing agent; sodium hydroxide is taken as precipitator; water-dissoluble dispersant and water-dissoluble antioxidant or inert gas are added for control and protection; in a cocurrent flow type the solution is added to a reaction vessel for reaction; after alkalescence disposal, aging procedure, solid-liquid separation and washing and drying, the nickel cobalt manganese oxide is uniformly mixed with the lithium raw material; the nickel cobalt manganese lithium oxide powder is obtained by sintering the mixed powder which is divided into three temperature areas. The invention has the advantages of high specific capacity, good circulation performance, ideal crystal texture, short production period, low power loss, and being suitable for industrial production, etc.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST +1
Method and apparatus for preparation of spherical metal carbonates and lithium metal oxides for lithium rechargeable batteries
ActiveUS7435402B2Improve impedance characteristicsImproved stability of the layered oxide structureConductive materialOxide conductorsDopantLithium metal
A number of materials with the composition Li1+xNiαMnβCoγM′δO2−zFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti) for use with rechargeable batteries, wherein x is between about 0 and 0.3, α is between about 0.2 and 0.6, β is between about 0.2 and 0.6, γ is between about 0 and 0.3, δ is between about 0 and 0.15, and z is between about 0 and 0.2. Adding the above metal and fluorine dopants affects capacity, impedance, and stability of the layered oxide structure during electrochemical cycling. Another aspect of the invention includes materials with the composition Li1+xNiαCoβMnγM′δOyFz (M′=Mg,Zn,Al,Ga,B,Zr,Ti), where the x is between 0 and 0.2, the α between 0 and 1, the β between 0 and 1, the γ between 0 and 2, the δ between about 0 and about 0.2, the y is between 2 and 4, and the z is between 0 and 0.5.
Owner:UCHICAGO ARGONNE LLC
Multicomponent material with multilayer coating structure for lithium ion battery and preparation method thereof
InactiveCN101997113AIncrease capacityReduce bloatElectrode manufacturing processesSecondary cellsSodium-ion batteryLithium-ion battery
The invention discloses a multicomponent material for a lithium ion battery and a preparation method thereof, and particularly relates to a multicomponent material with a multilayer coating structure for a lithium ion battery and a preparation method thereof. The multicomponent material with the multilayer coating structure comprises an inner core and coating layers, wherein both the inner core and the coating layers comprise lithium-containing metallic oxides; and the number n of the coating layers is 1 to 5. The multicomponent material with the multilayer coating structure has a simple preparation process, relatively low cost and high processability; and the lithium ion battery made of the material has a small amount of swelling, high capacity, high high-temperature cyclical stability and high safety.
Owner:BEIJING EASPRING MATERIAL TECH CO LTD
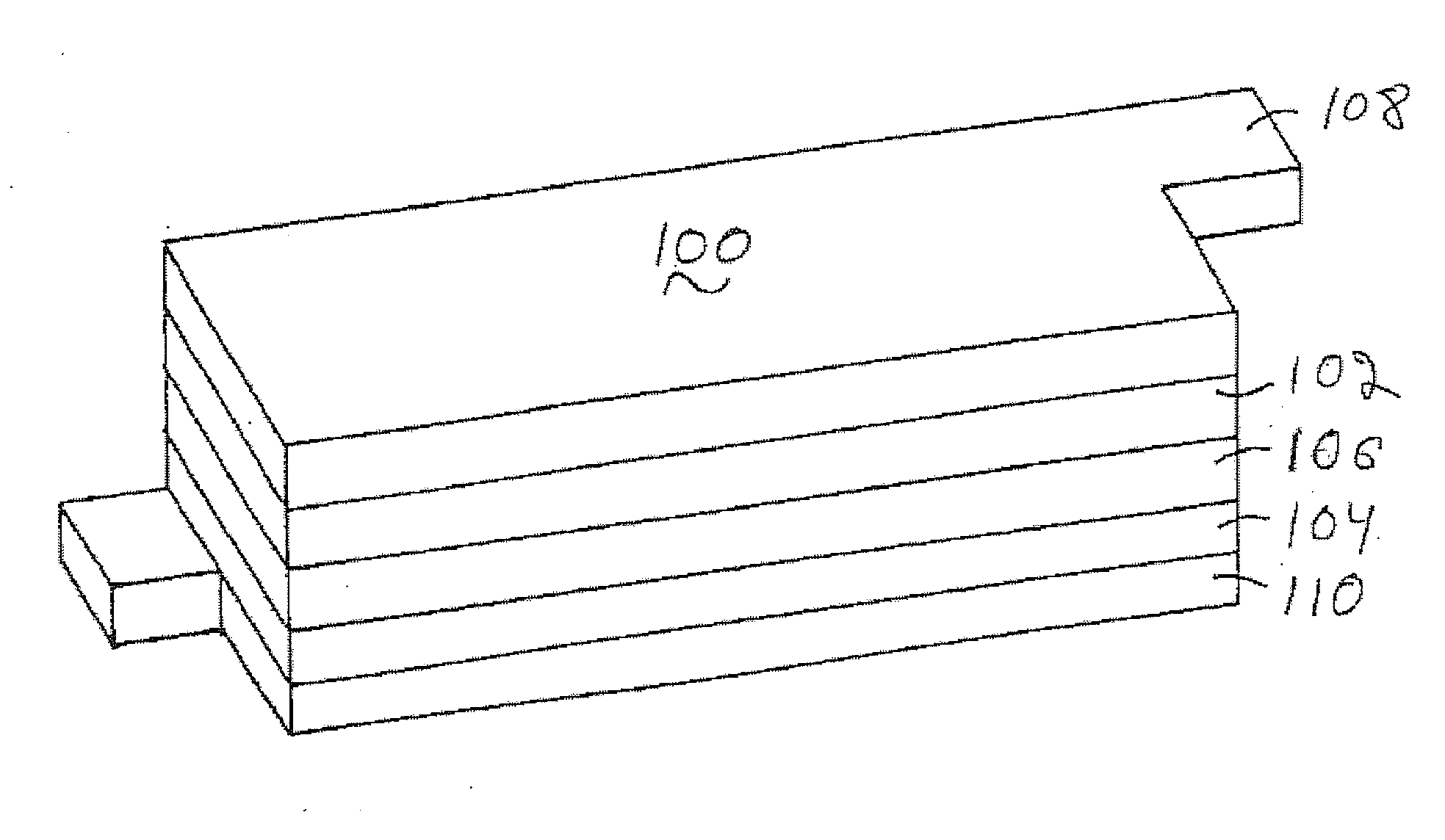
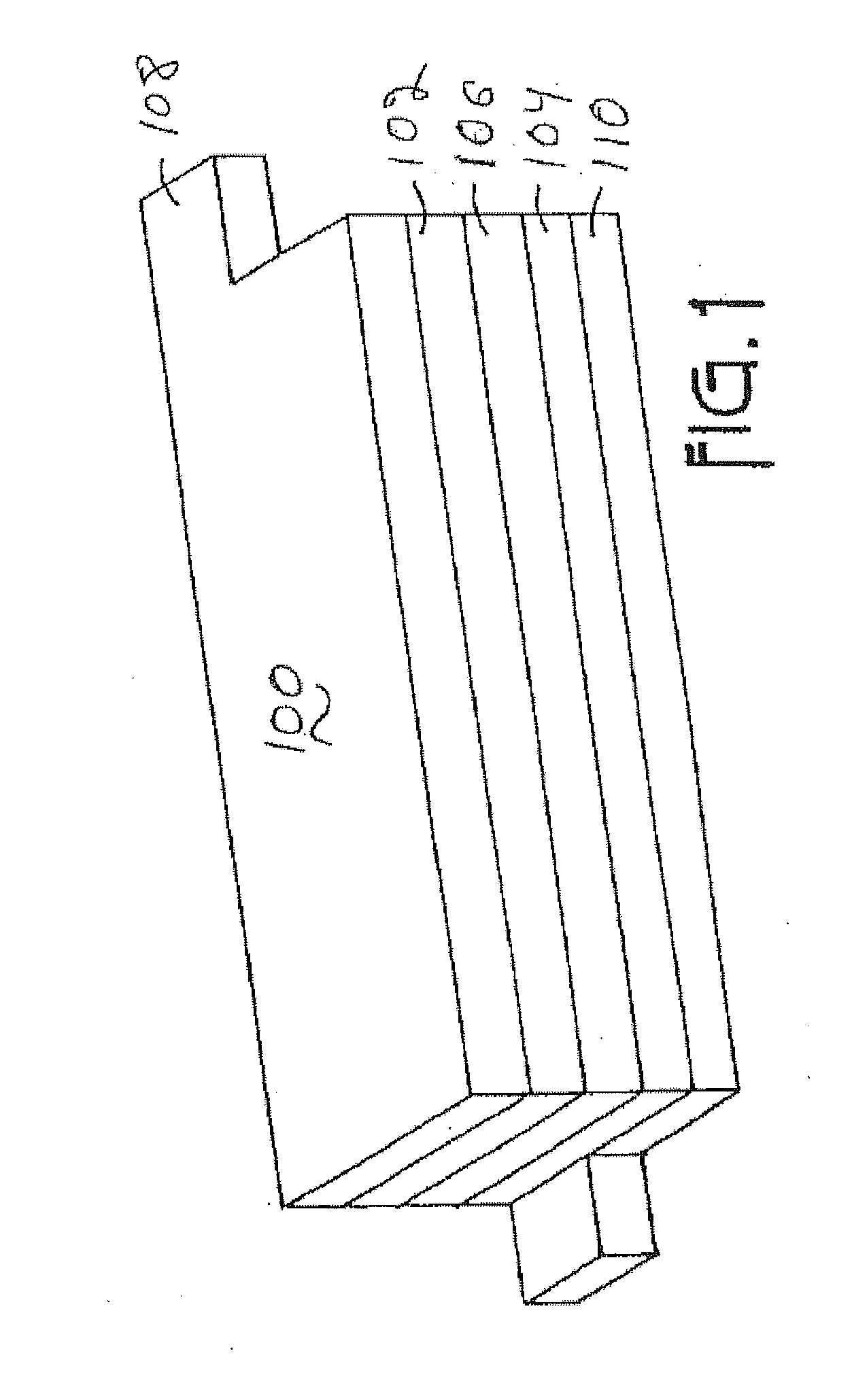
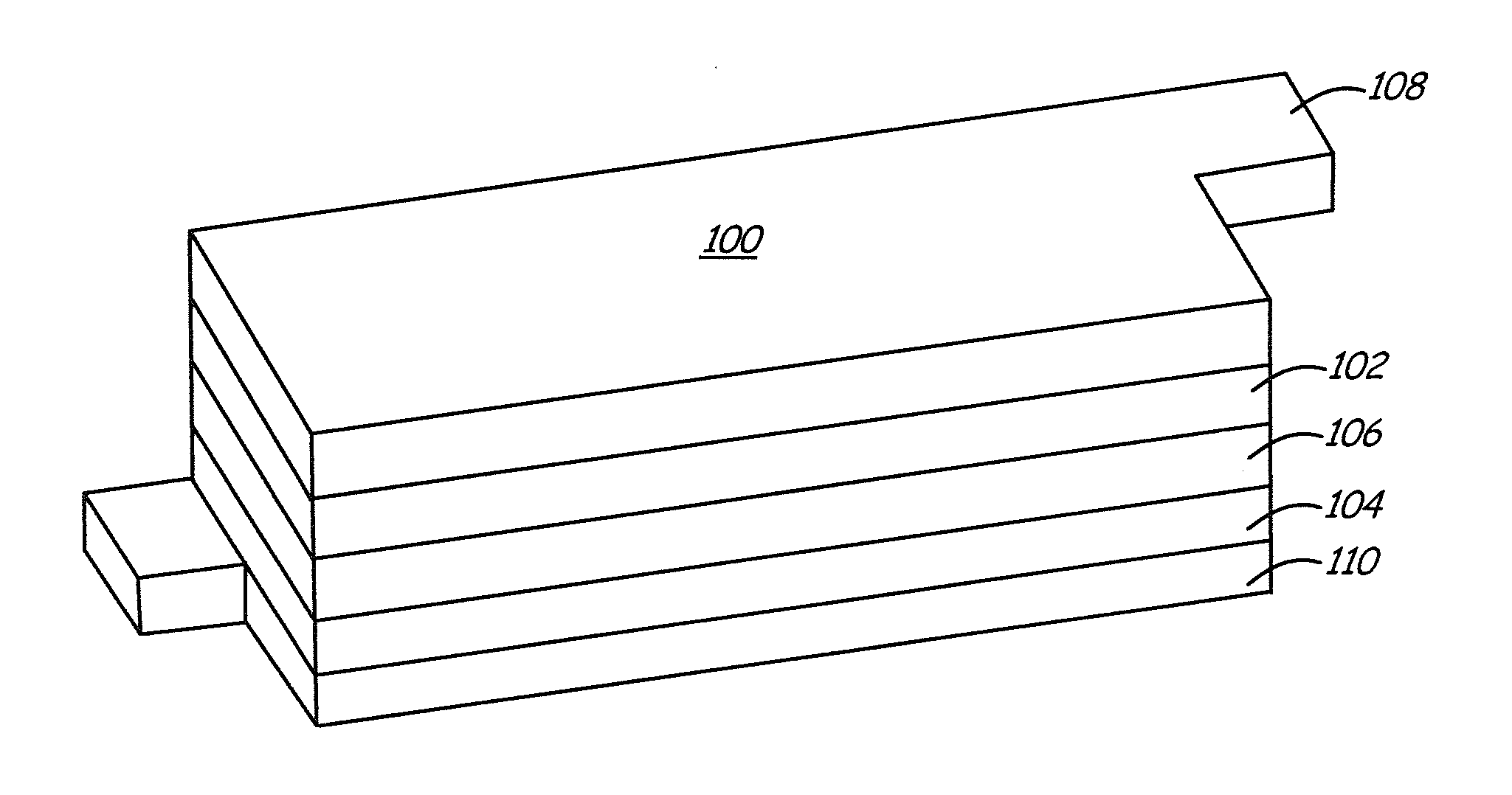
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Lithium ion batteries with long cycling performance
ActiveUS20110017528A1Maintain discharge capacitySmall-sized cells cases/jacketsElectric propulsion mountingHigh energyEngineering
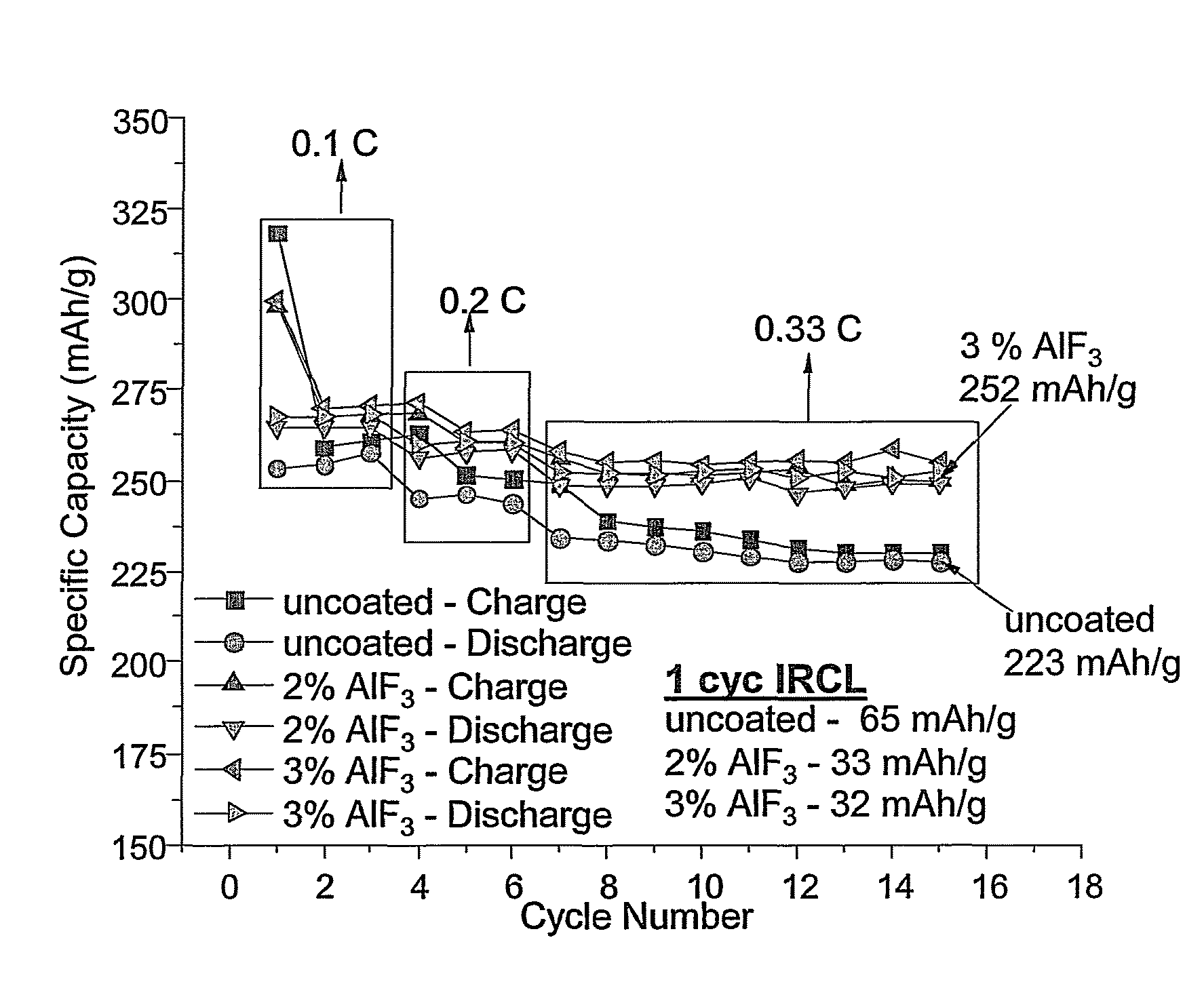
Batteries with high energy and high capacity are described that have a long cycle life upon cycling at a moderate discharge rate. Specifically, the batteries may have a room temperature fifth cycle discharge specific energy of at least about 175 Wh / kg discharged at a C / 3 discharge rate from 4.2V to 2.5V. Additionally, the batteries can maintain at least about 70% discharge capacity at 1000 cycles relative to the fifth cycle, with the battery being discharged from 4.2V to 2.5V at a C / 2 rate from the fifth cycle through the 1000th cycle. In some embodiment, the positive electrode of the battery comprises a lithium intercalation composition with optional metal fluoride coating. Stabilizing additive maybe added to the electrolyte of the battery to further improve the battery performance. The batteries are particularly suitable for use in electric vehicles.
Owner:IONBLOX INC
Doped positive electrode active materials and lithium ion secondary battery constructed therefrom
Positive electrode active materials comprising a dopant in an amount of 0.1 to 10 mole percent of Mg, Ca, Sr, Ba, Zn, Cd or a combination thereof are described that have high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβ-δCoγAδXμO2−zFz, where x ranges from about 0.01 to about 0.3, δ ranges from about 0.001 to about 0.15, and the sum x+α+β+γ+δ+μ can approximately equal 1.0. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. The materials generally can have a tap density of at least 1.8 g / mL. Also, the materials can have an average discharge voltage of around 3.6 V.
Owner:IONBLOX INC
Cathode active material, method of preparing the same, and cathode and lithium battery containing the material
ActiveUS20060257745A1Improve high voltage stabilityImprove thermal stabilityWalking sticksElectrode rolling/calenderingComposite cathodeHigh rate
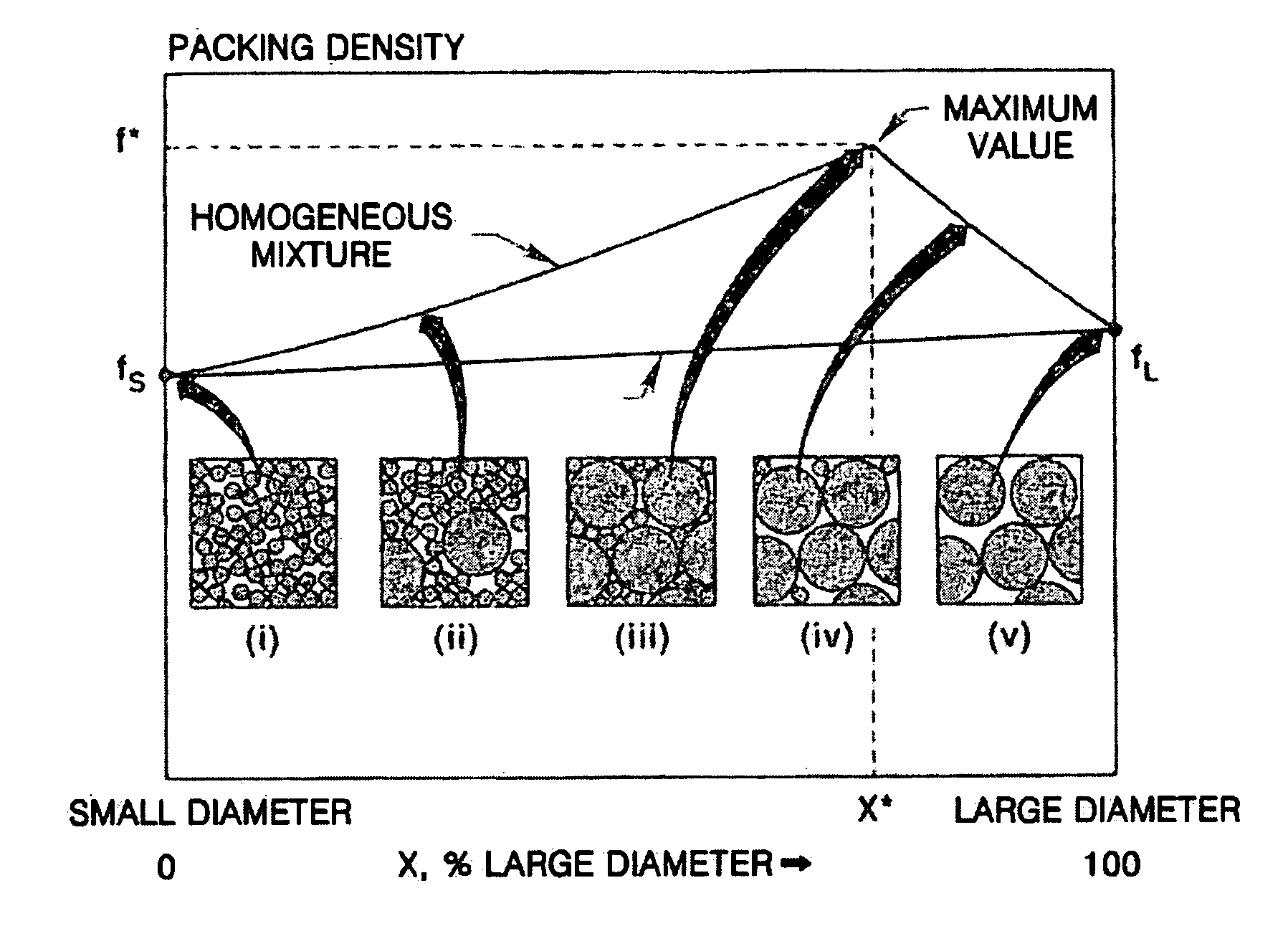
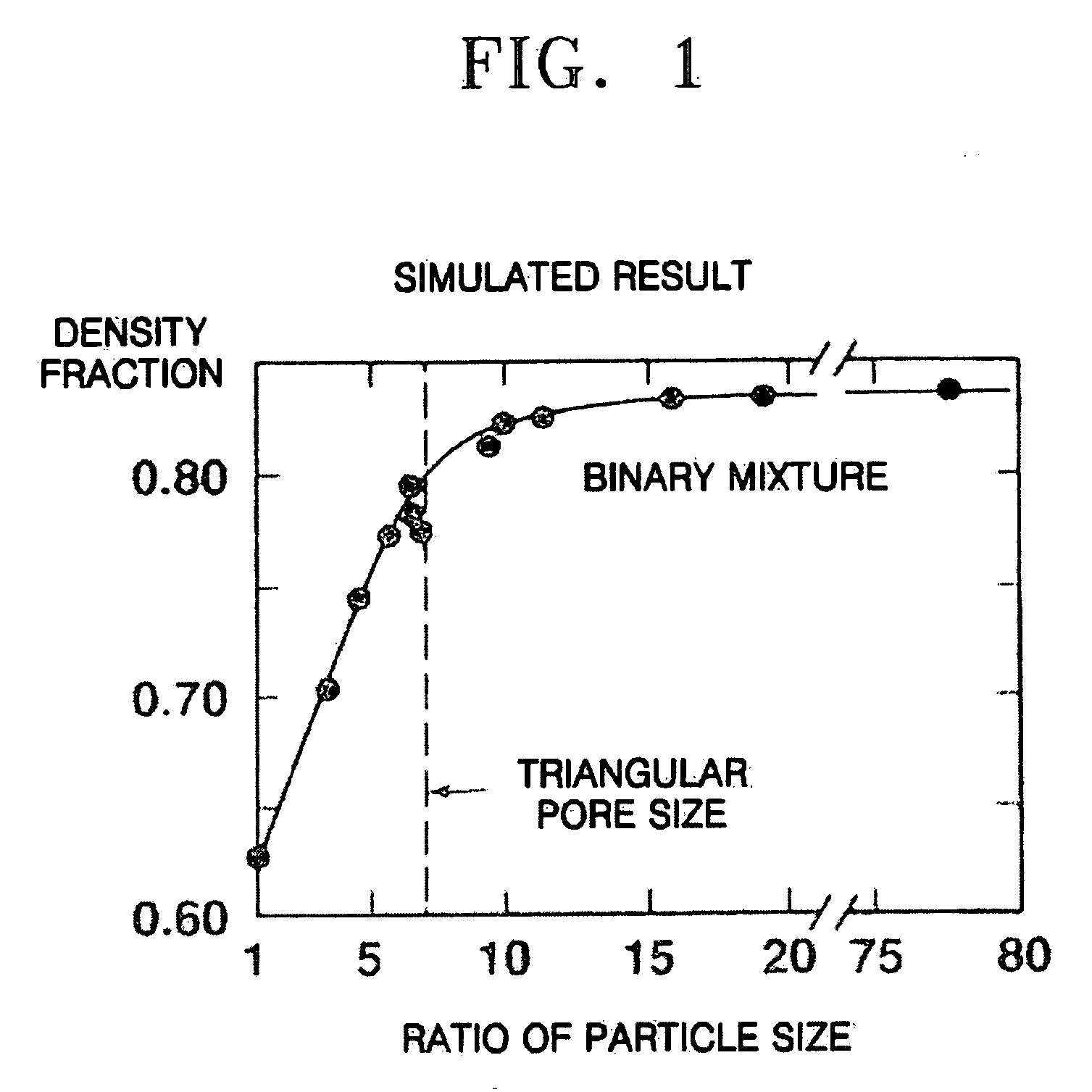
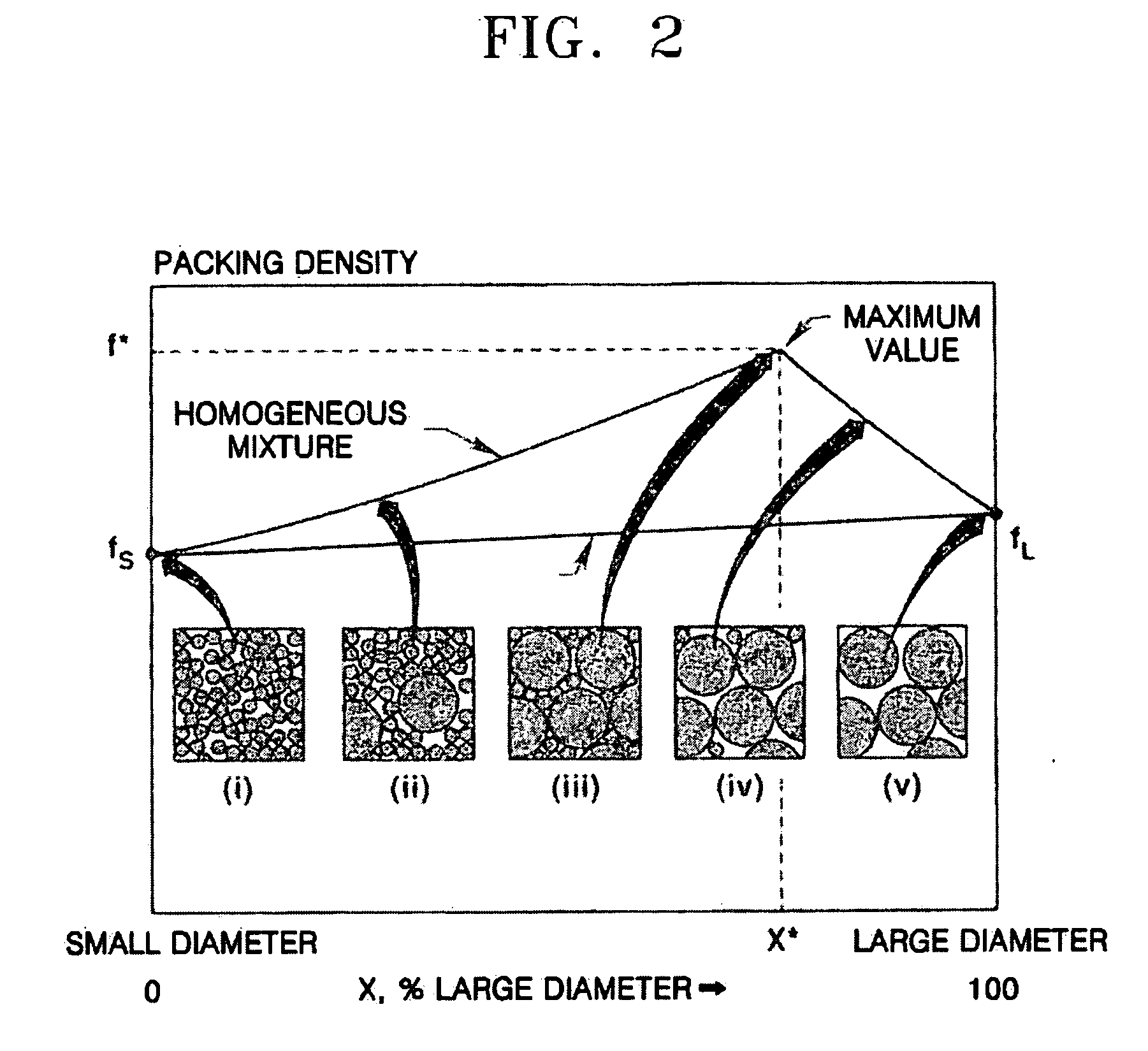
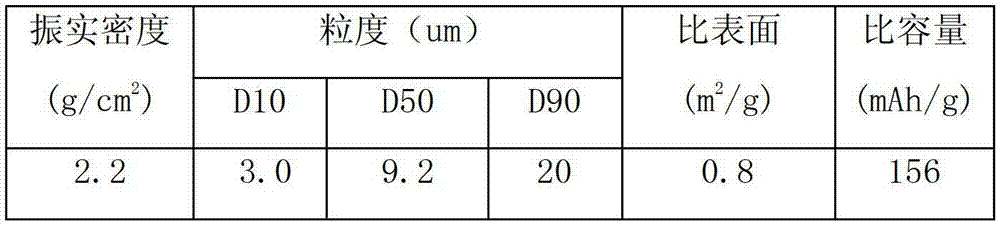
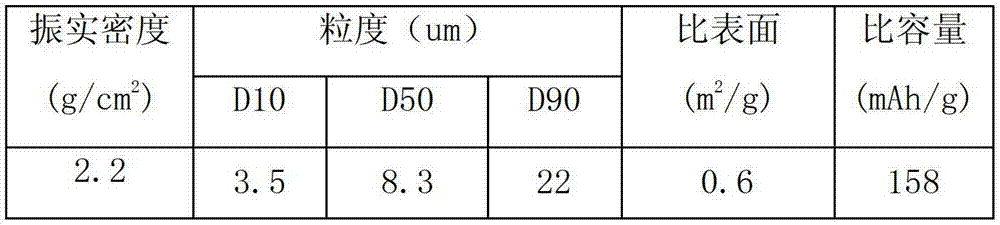
Composite cathode active materials having a large diameter active material and a small diameter active material are provided. The ratio of the average particle diameter of the large diameter active material to the average particle diameter of the small diameter active material ranges from about 6:1 to about 100:1. Mixing the large and small diameter active materials in a proper weight ratio improves packing density Additionally, including highly stable materials and highly conductive materials in the composite cathode active materials improves volume density, discharge capacity and high rate discharge capacity.
Owner:SAMSUNG SDI CO LTD
Method for recycling nickel-cobalt-manganese ternary anode material
ActiveCN103199320AAchieve recyclingPromote sustainable developmentWaste accumulators reclaimingNickel compoundsManganeseCobalt
The invention relates to a method for recycling a nickel-cobalt-manganese ternary anode material, belongs to the technical field of recovery of waste cells, and solves the technical problem that a method for recycling the nickel-cobalt-manganese ternary anode material is provided. The method for recycling the nickel-cobalt-manganese ternary anode material comprises a step of removing a binder from the positiveplate of the nickel-cobalt-manganese ternary material by thermal treatment, and the method for removing the binder in the positive plate of the nickel-cobalt-manganese ternary material is thermal treatment of the positive plate of the nickel-cobalt-manganese ternary anode material at 400-1000 DEG C for 0.5-5 hours.
Owner:天齐锂业(江苏)有限公司 +2
Positive electrode materials for lithium ion batteries having a high specific discharge capacity and processes for the synthesis of these materials
ActiveUS8389160B2Electrode manufacturing processesAlkali metal oxidesElectrical batteryDischarge rate
Positive electrode active materials are described that have a very high specific discharge capacity upon cycling at room temperature and at a moderate discharge rate. Some materials of interest have the formula Li1+xNiαMnβCOγO2, where x ranges from about 0.05 to about 0.25, α ranges from about 0.1 to about 0.4, β ranges from about 0.4 to about 0.65, and γ ranges from about 0.05 to about 0.3. The materials can be coated with a metal fluoride to improve the performance of the materials especially upon cycling. Also, the coated materials can exhibit a very significant decrease in the irreversible capacity lose upon the first charge and discharge of the cell. Methods for producing these materials include, for example, a co-precipitation approach involving metal hydroxides and sol-gel approaches.
Owner:IONBLOX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com