Method for preparing alumina from low-grade bauxite by acid leaching
A low-grade bauxite and alumina technology, applied in chemical instruments and methods, aluminum compounds, inorganic chemistry, etc., can solve the problem of high alumina process cost, and achieve high energy utilization, easy equipment and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
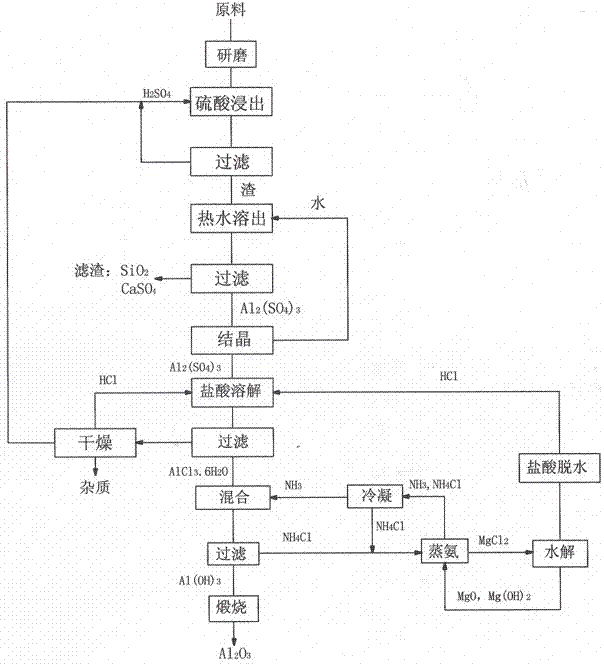
[0022] Take 700 grams of ore sample 1 bauxite, grind it to 100 μm, mix the bauxite with 2600 ml of sulfuric acid with a mass percentage of 98%, and leach at 100 ° C for 1 hour; filter the bauxite leached by sulfuric acid, and make the remaining acid Separate from the filter residue. Add 3000ml of water to the filter residue, boil and dissolve at 50°C for 30 minutes to dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution.
[0023] The aluminum sulfate solution was heated and concentrated to 1000ml, 3000ml of 38% hydrochloric acid was added, and then HCl gas was introduced to precipitate aluminum chloride crystals, which were filtered to obtain 2057 grams of aluminum chloride crystals.
[0024] Take NH 3 2000ml of 25%wt ammonium hydroxide solution is reacted with aluminum chloride solution to obtain aluminum hydroxide precipitate and ammonium chloride solution. After filtration, aluminum hydroxide crystals are calcined at 1100°C to obtai...
Embodiment 2
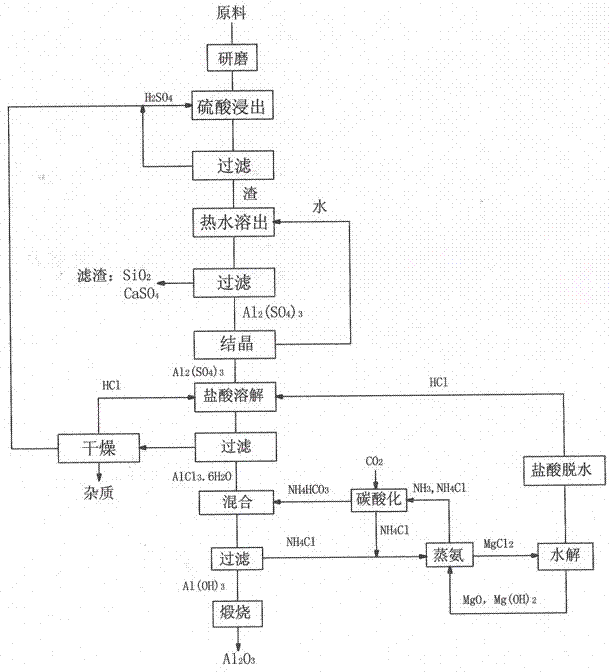
[0027] Take 800 grams of ore sample 2 bauxite, grind it to 150 μm, mix the bauxite with 3500 ml of sulfuric acid with a mass percentage of 75%, and leaching at 250 ° C for 1.5 hours; filter the bauxite leached by sulfuric acid, and make the remaining acid Separate from the filter residue. Add 3000ml of water to the filter residue, boil and dissolve at 50°C for 30 minutes to dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution.
[0028] The aluminum sulfate solution was evaporated and crystallized to obtain 2718 grams of Al 2 (SO4) 3 18H 2 O crystals, aluminum sulfate crystals are mixed with 32% hydrochloric acid 3000ml, then feed HCl gas, separate out aluminum chloride crystals, filter to obtain 2006 grams of aluminum chloride crystals.
[0029] Take NH 3 Content 21%wt ammonium carbonate solution 2000m, aluminum chloride was added into water 2500ml to dissolve, ammonium carbonate solution and aluminum chloride solution were mixed to ...
Embodiment 3
[0032] Take 1,400 grams of kaolin, grind it to 150 μm, mix kaolin with 4,000 ml of sulfuric acid with a mass percentage of 90%, and leach at 200°C for 2 hours; filter the kaolin leached by sulfuric acid to separate the residual acid from the filter residue. Add 4000ml of water to the filter residue, boil and dissolve at 70°C for 45 minutes to dissolve the reactant, filter to remove the residue, and obtain an aluminum sulfate solution.
[0033] The aluminum sulfate solution was heated and concentrated to 1000ml, 3500ml of 25% hydrochloric acid was added, and then HCl gas was introduced to precipitate aluminum chloride crystals, which were filtered to obtain 1929 grams of aluminum chloride crystals.
[0034] Take 400g of liquid ammonia, add aluminum chloride to 2500ml of water to dissolve, and pass liquid ammonia into the aluminum chloride solution to obtain aluminum hydroxide precipitate and ammonium chloride solution. After filtration, the aluminum hydroxide crystals are calci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com