Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1949 results about "Boron oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Boron oxide may refer to: Boron trioxide - the most common form Boron monoxide Boron suboxide
Boron nitride and boron nitride-derived materials deposition method
ActiveUS20080292798A1High nitrogen contentIncrease oxygen contentPretreated surfacesSemiconductor/solid-state device manufacturingMetallurgyBoron containing
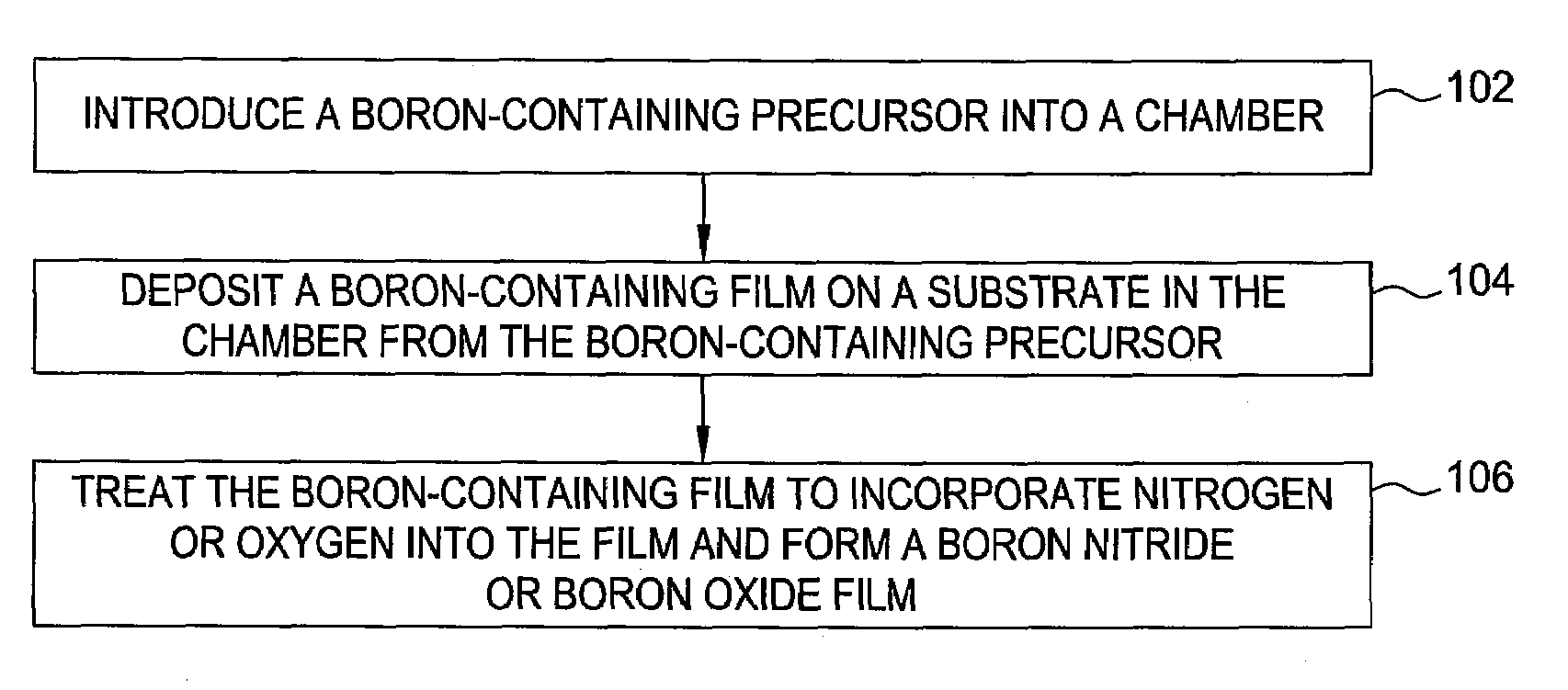
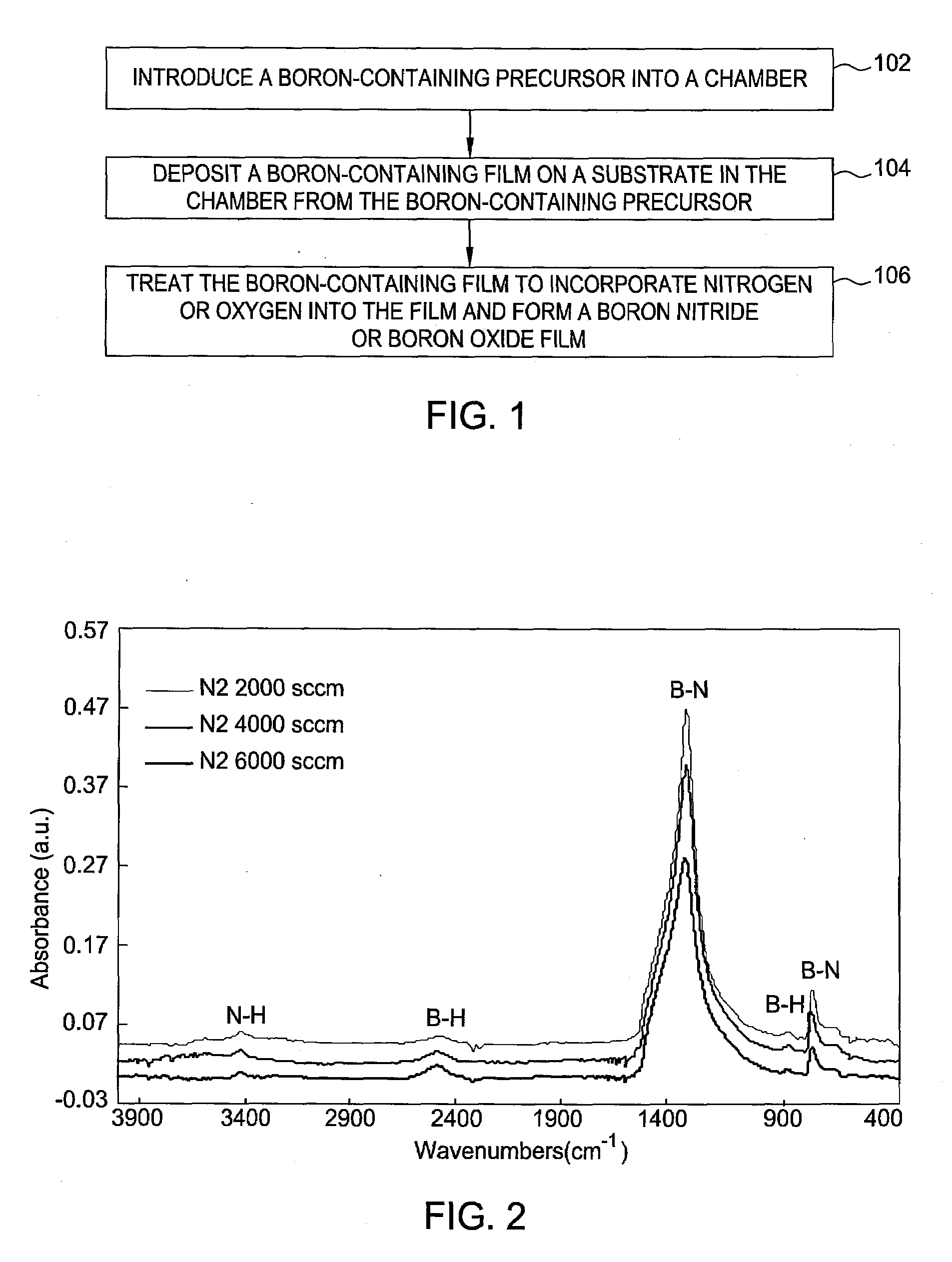
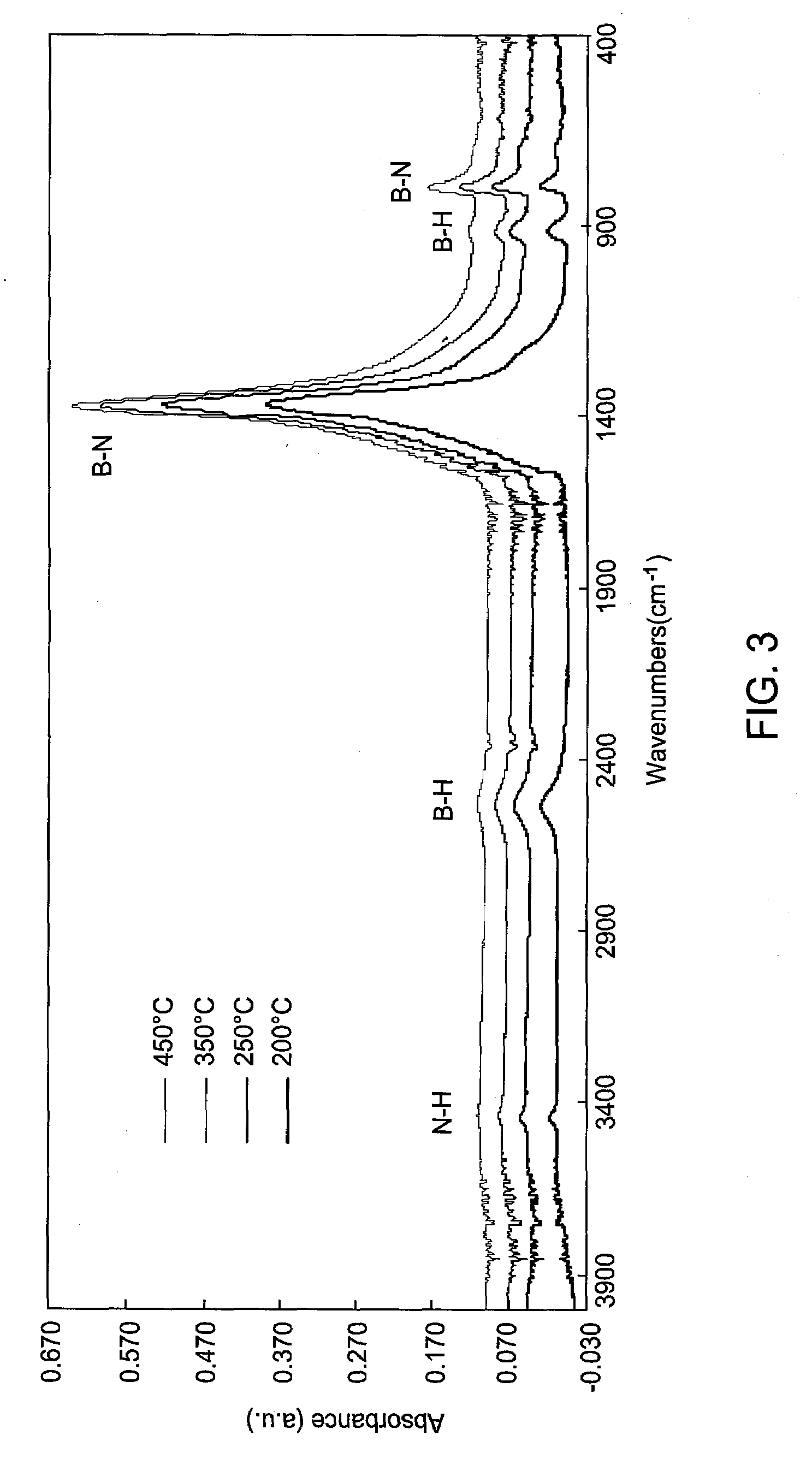
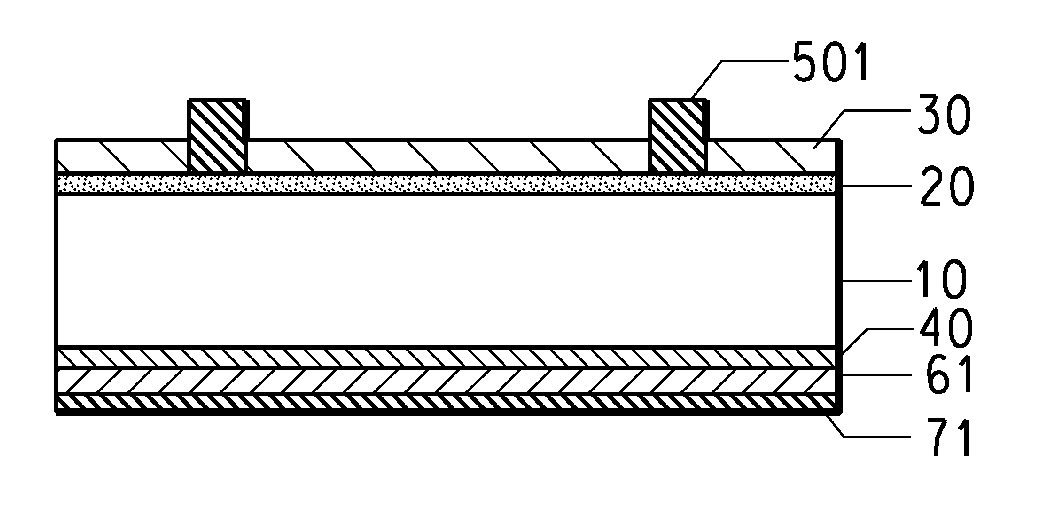
Methods for forming boron-containing films are provided. The methods include introducing a boron-containing precursor and a nitrogen or oxygen-containing precursor into a chamber and forming a boron nitride or boron oxide film on a substrate in the chamber. In one aspect, the method includes depositing a boron-containing film and then exposing the boron-containing film to the nitrogen-containing or oxygen-containing precursor to incorporate nitrogen or oxygen into the film. The deposition of the boron-containing film and exposure of the film to the precursor may be performed for multiple cycles to obtain a desired thickness of the film. In another aspect, the method includes reacting the boron-containing precursor and the nitrogen-containing or oxygen-containing precursor to chemically vapor deposit the boron nitride or boron oxide film.
Owner:APPLIED MATERIALS INC
Thick-film pastes containing lead-tellurium-boron-oxides, and their use in the manufacture of semiconductor devices
The present invention provides a thick-film paste for printing the front side of a solar cell device having one or more insulating layers. The thick-film paste comprises an electrically conductive metal and a lead-tellurium-boron-oxide dispersed in an organic medium.
Owner:SOLAR PASTE LLC
Protected polymeric film
InactiveUS20060063015A1Reduce inherentReduce but potentially undesirableSynthetic resin layered productsSolid-state devicesOptoelectronicsPolymer thin films
Owner:3M INNOVATIVE PROPERTIES CO
Low iron high transmission glass with boron oxide for improved optics, durability and refining, and corresponding method
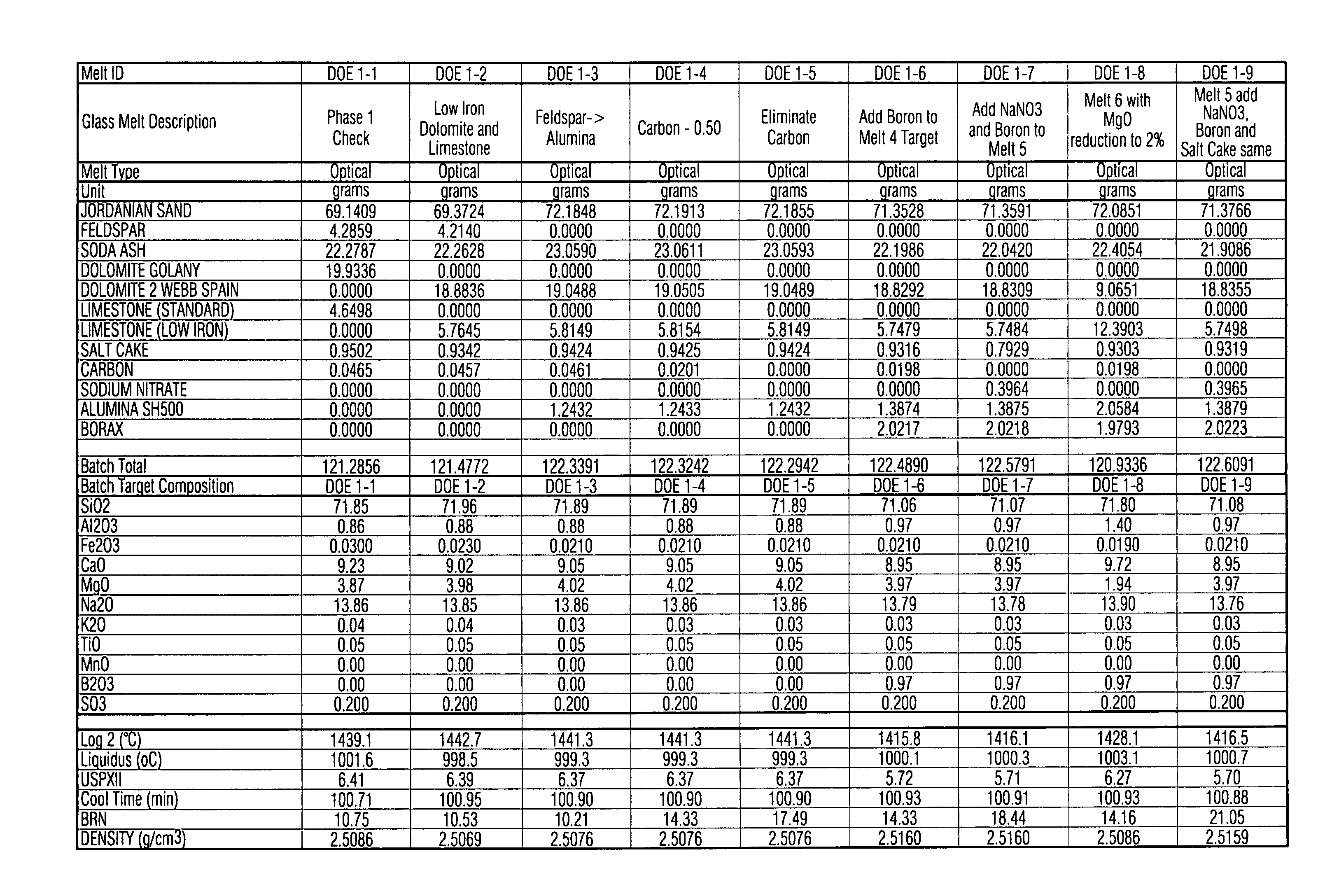
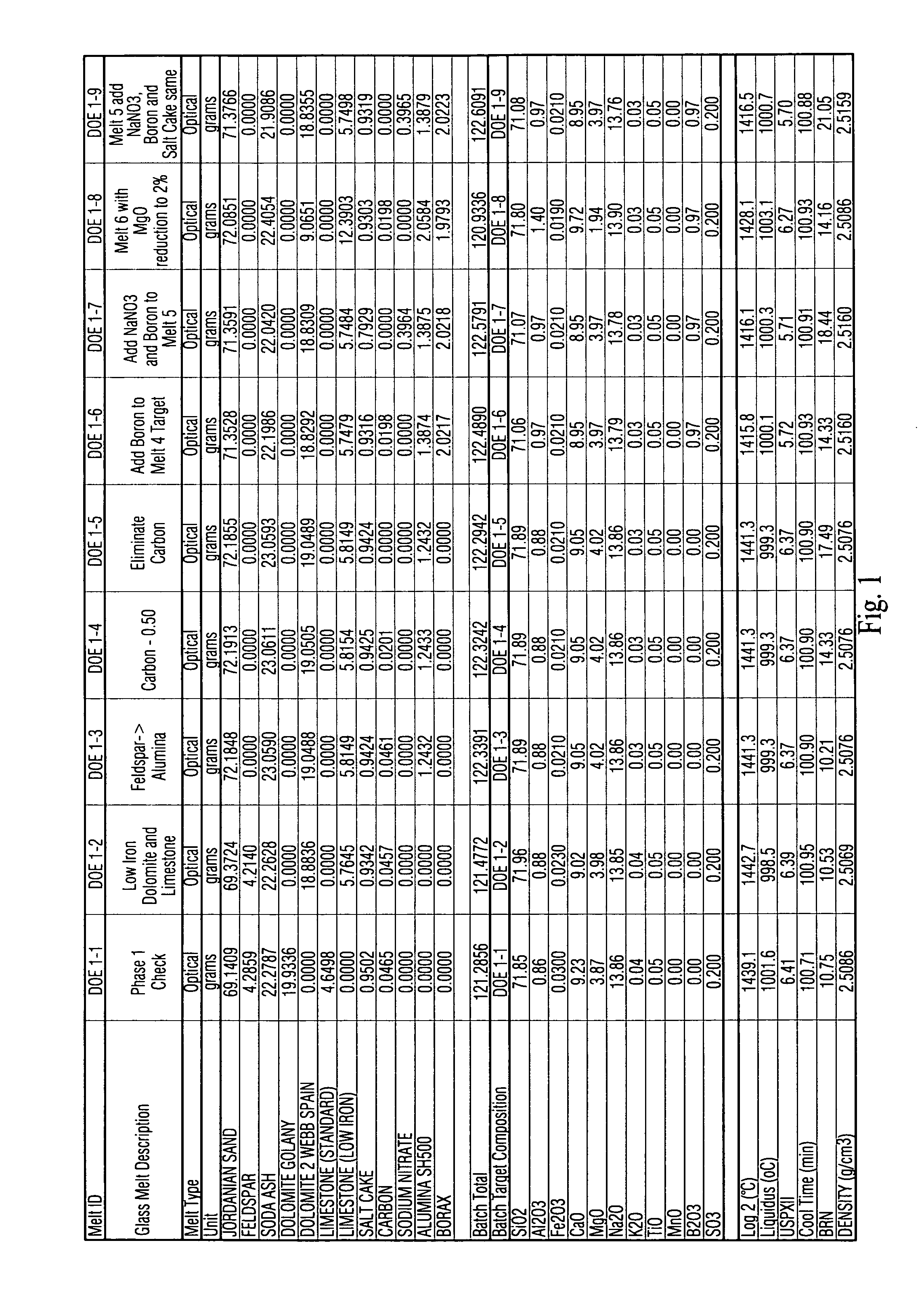
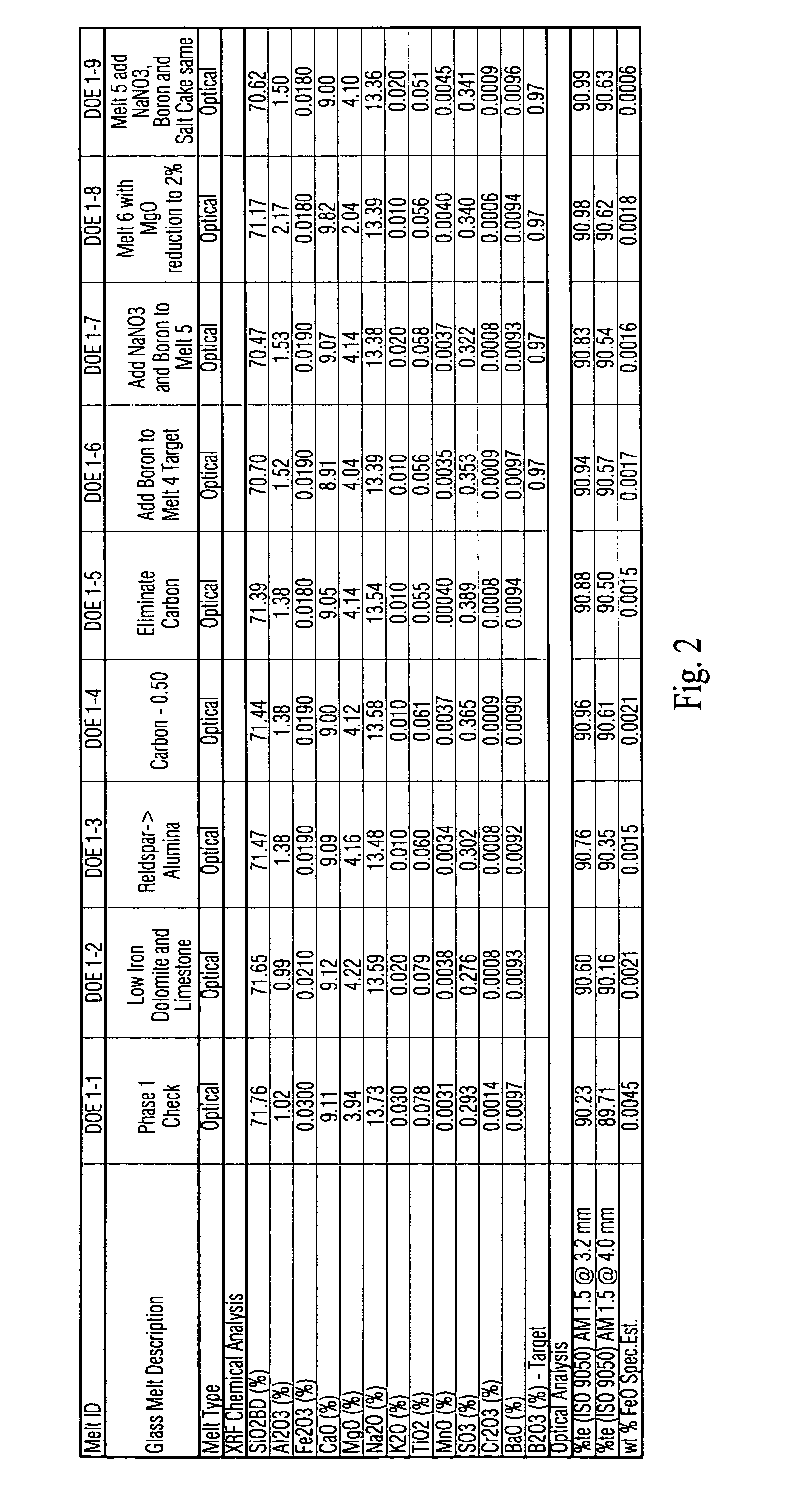
This invention relates to a high transmission low iron glass that includes boron oxide. The boron oxide, added to this low iron glass, has the effect of improving glass refining, homogeneity and quality (lower seed count) through its flux action and improves glass optical parameters of green and clear glass through the change in refractive index and surface tension. Boron oxide lends to broader and weaker absorption band of such transition element(s) as iron which additionally improves the transmittance of low iron clear glass in certain example embodiments of this invention. In certain example embodiments, the addition of boron oxide in certain quantities in advantageous in that it improves the chemical durability of the glass by decreasing the USPX (or USPXIII) value of the glass via suppression of the silica, sodium ions in the glass structure.
Owner:PHOENICIA AMERICA ISRAEL FLAT GLASS +1
Thermal protective coating for ceramic surfaces
ActiveUS6921431B2Extended shelf lifeConvenience to workFireproof paintsOther chemical processesColloidal silicaSodium Bentonite
A coating admixture, method of coating and substrates coated thereby, wherein the coating contains colloidal silica, colloidal alumina, or combinations thereof; a filler such as silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides, copper chromium oxides, cerium oxides, terbium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide is also added.
Owner:WESSEX
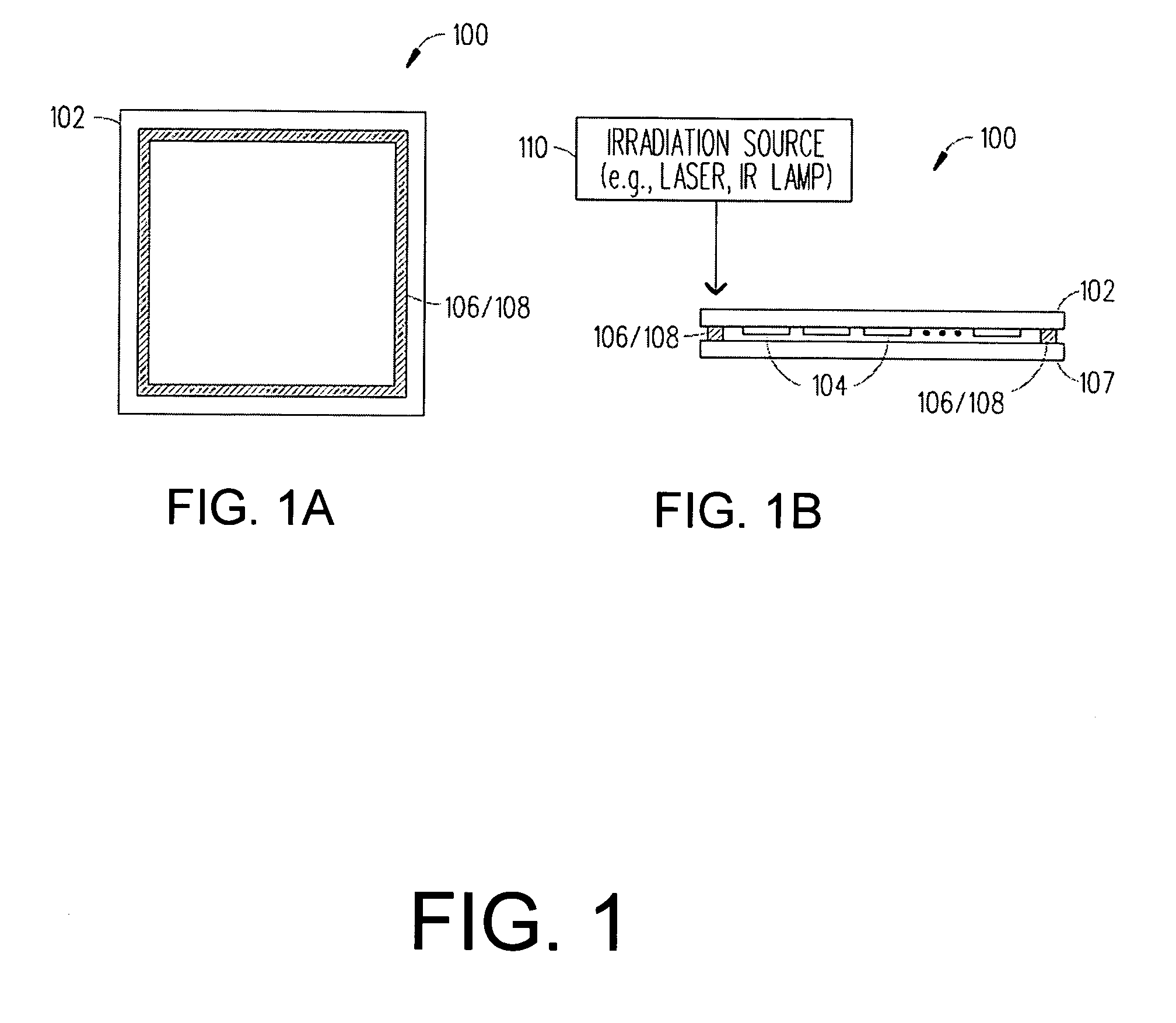
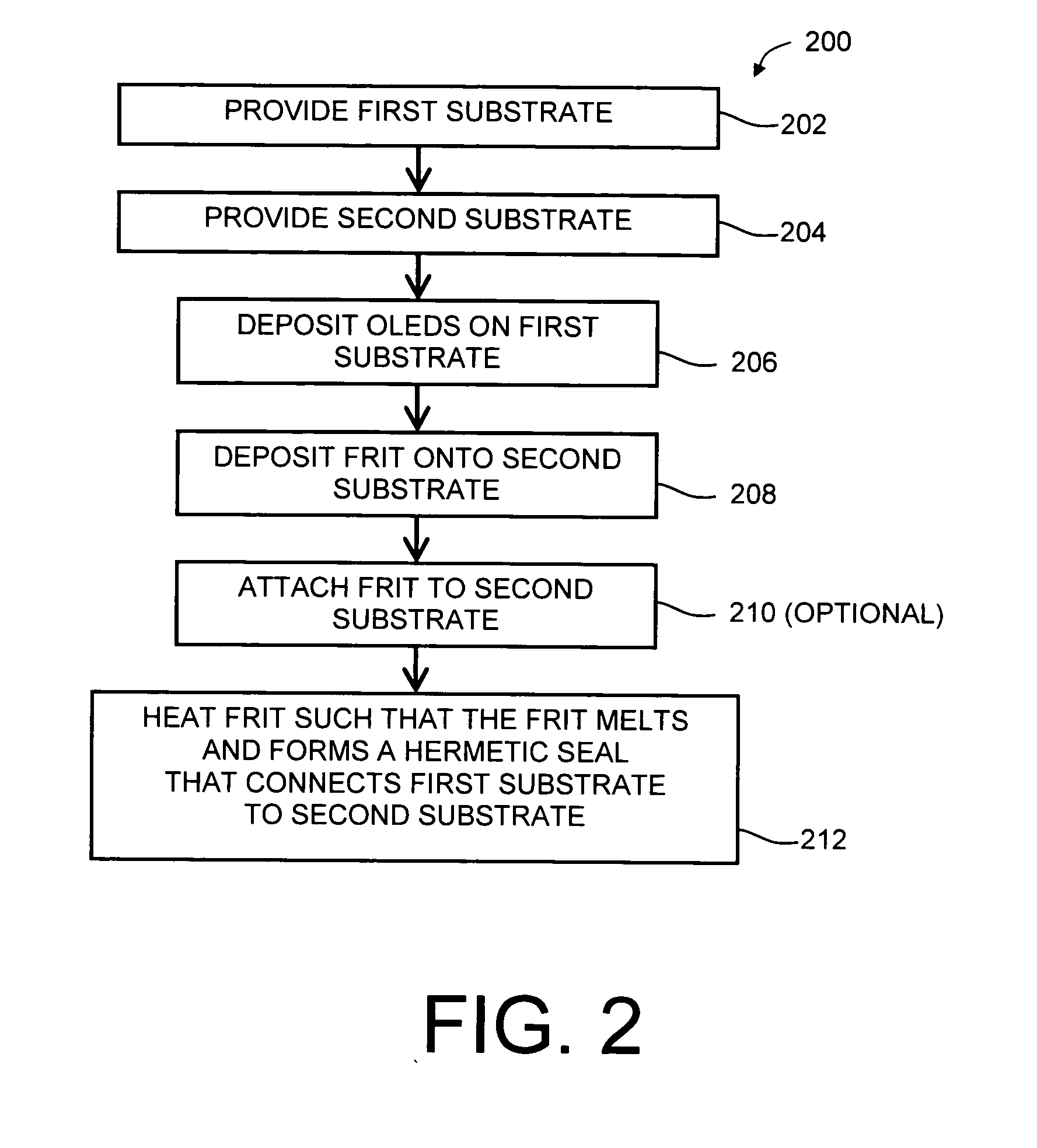
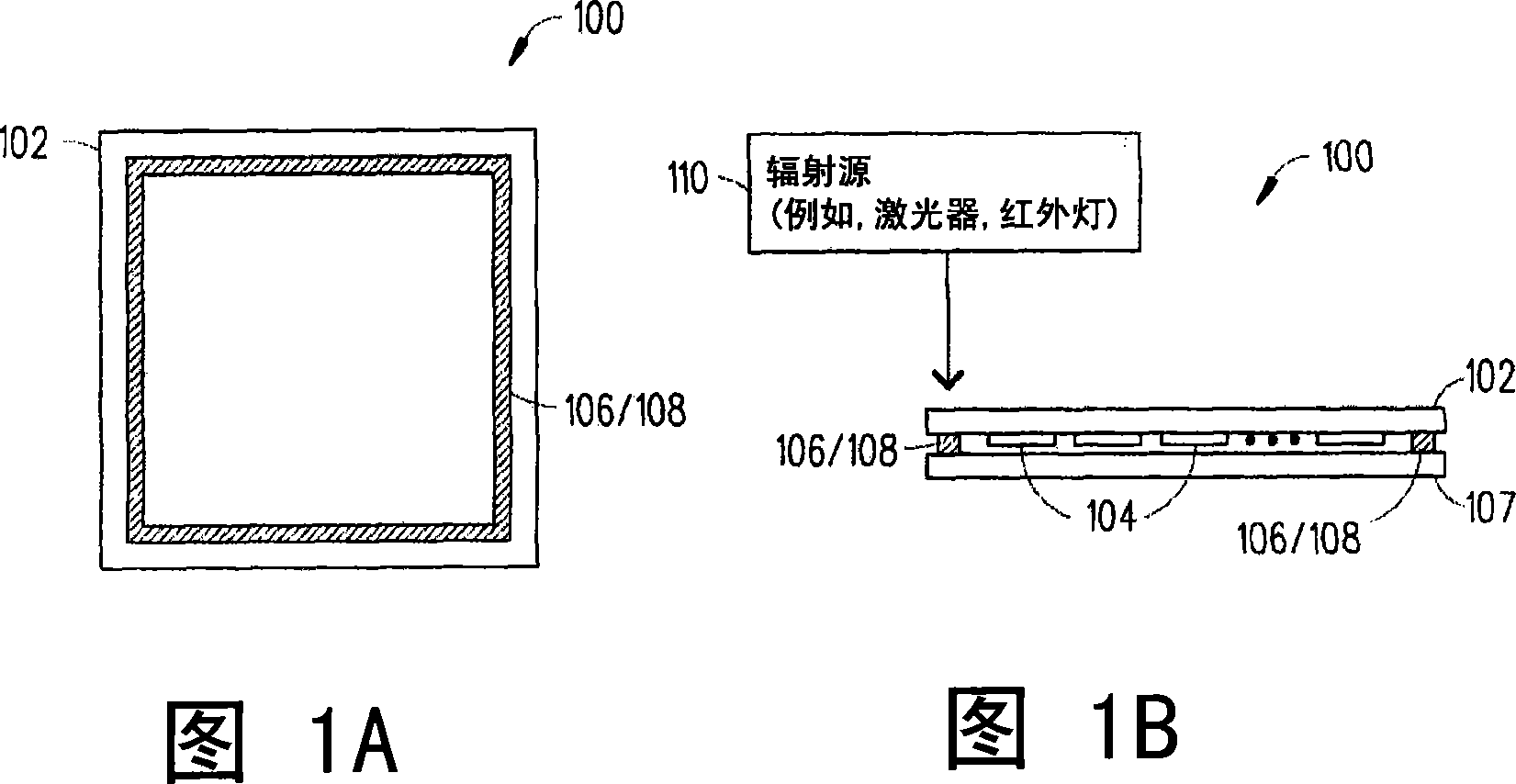
Boro-silicate glass frits for hermetic sealing of light emitting device displays
InactiveUS20080124558A1Problem be addressLamination ancillary operationsSolid-state devicesFritSilicate glass
A frit composition useful for sealing a light emitting device is disclosed. The frit composition comprises a glass portion comprising a base component and at least one absorbing component. The glass portion of the frit comprises silica, boron oxide, optionally alumina, and (a) cupric oxide and / or a (b) combination of ferric oxide, vanadium pentoxide, and optionally titanium dioxide. Also disclosed is an article comprising a substrate and a frit, and a glass package comprising two substrates and a frit positioned between the substrates. A method for manufacturing a hermetically sealed glass package comprising the deposition of a glass frit and heating of the glass frit to form a hermetic seal is also disclosed.
Owner:CORNING INC
High temperature glass fiber insulation
Owner:GLASS
Thermal protective coating for ceramic surfaces
ActiveUS20050051057A1Extend working lifeReduce surface temperatureFireproof paintsOther chemical processesColloidal silicaSodium Bentonite
A coating admixture, method of coating and substrates coated thereby, wherein the coating contains colloidal silica, colloidal alumina, or combinations thereof; a filler such as silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides, copper chromium oxides, cerium oxides, terbium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide is also added.
Owner:WESSEX
Thermal protective coating
ActiveUS7105047B2Extended shelf lifeReduce weightAlkali metal silicate coatingsPretreated surfacesCalcium silicateSodium Bentonite
A coating, method of coating and substrates coated thereby, wherein the coating contains an inorganic adhesive such as an alkali / alkaline earth metal silicate such as sodium silicate, potassium silicate, calcium silicate, and magnesium silicate; a filler such as a metal oxide for example silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides and copper chromium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide may be added.
Owner:WESSEX
Solid-state neutron and alpha particles detector and methods for manufacturing and use thereof
InactiveUS20090302226A1Optimize detection resultsMeasurement with semiconductor devicesSolid-state devicesParticulatesSemiconductor materials
A solid-state detector for detection of neutron and alpha particles detector and methods for manufacturing and use thereof are described. The detector has an active region formed of a polycrystalline semiconductor compound comprising a particulate semiconductor material sensitive to neutron and alpha particles radiation imbedded in a binder. The particulate semiconductor material contains at least one element sensitive to neutron and alpha particles radiation, selected from a group including 10Boron, 6Lithium, 113Cadmium, 157Gadolinium and 199Mercury. The semiconductor compound is sandwiched between an electrode assembly configured to detect the neutron and alpha particles interacting with the bulk of the active region. The binder can be either an organic polymer binder or inorganic binder. The organic polymer binder comprises at least one polymer that can be selected from the group comprising polystyrene, polypropylene, Humiseal™ and Nylon-6. The inorganic binder can be selected from B2O3, PbO / B2O3 / , Bi2O3 / PbO, Borax glass, Bismuth Borate glass and Boron Oxide based glass.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Fire resistant flexible ceramic resin blend and composite products formed therefrom
InactiveUS20100304152A1Improve fire performanceConducive to lightweightSynthetic resin layered productsCellulosic plastic layered productsAdhesiveComposite laminates
High heat resistant elastic composite laminates, sealants, adhesives, and coatings developed from a resin blend. The resin blend is made up of methyl and optionally phenyl silsequioxane resins selected to produce silanol-silanol condensation silicone polymers formed in a slowly evolving reaction mass containing submicron boron nitride, silica and boron oxide fillers. The required ratio of submicron boron nitride to silica has been discovered for assuring the formation of a high temperature resistant elastic composite blend that will form intermediate flexible ceramic products up to 600 deg C., then continue to form preceramic then dense ceramic products from 600 to 1000 deg C. The thermal yield of the composite is generally greater than 90 wt. % at 1000 deg C. Composite products with different levels of heat transformation can be fabricated within the same product depending upon the thickness of the layers of reinforcement.
Owner:FLEXIBLE CERAMICS
Optical glass and optical product
When a glass melt of an optical glass having a refractive index (nd) of at least 1.7 and an Abbe number (νd) of 28 to 41 is flowed down from a flow pipe made of Pt or a Pt alloy to form glass gobs continuously, there is caused a problem that the glass gobs have striae or that the weight variability among the glass gobs is large, and the problem can be overcome by the use of an optical glass comprising silicon oxide and boron oxide, the ratio of a content of the silicon oxide to a content of the boron oxide being greater than 0.78, the optical glass having a contact angle of at least 40° to Pt or a Pt alloy at a predetermined temperature equivalent to, or higher than, its liquidus temperature or in a predetermined temperature range whose lower limit is equivalent to, or higher than, the liquidus temperature and having a sag temperature Ts of 580° C. or lower.
Owner:HOYA CORP
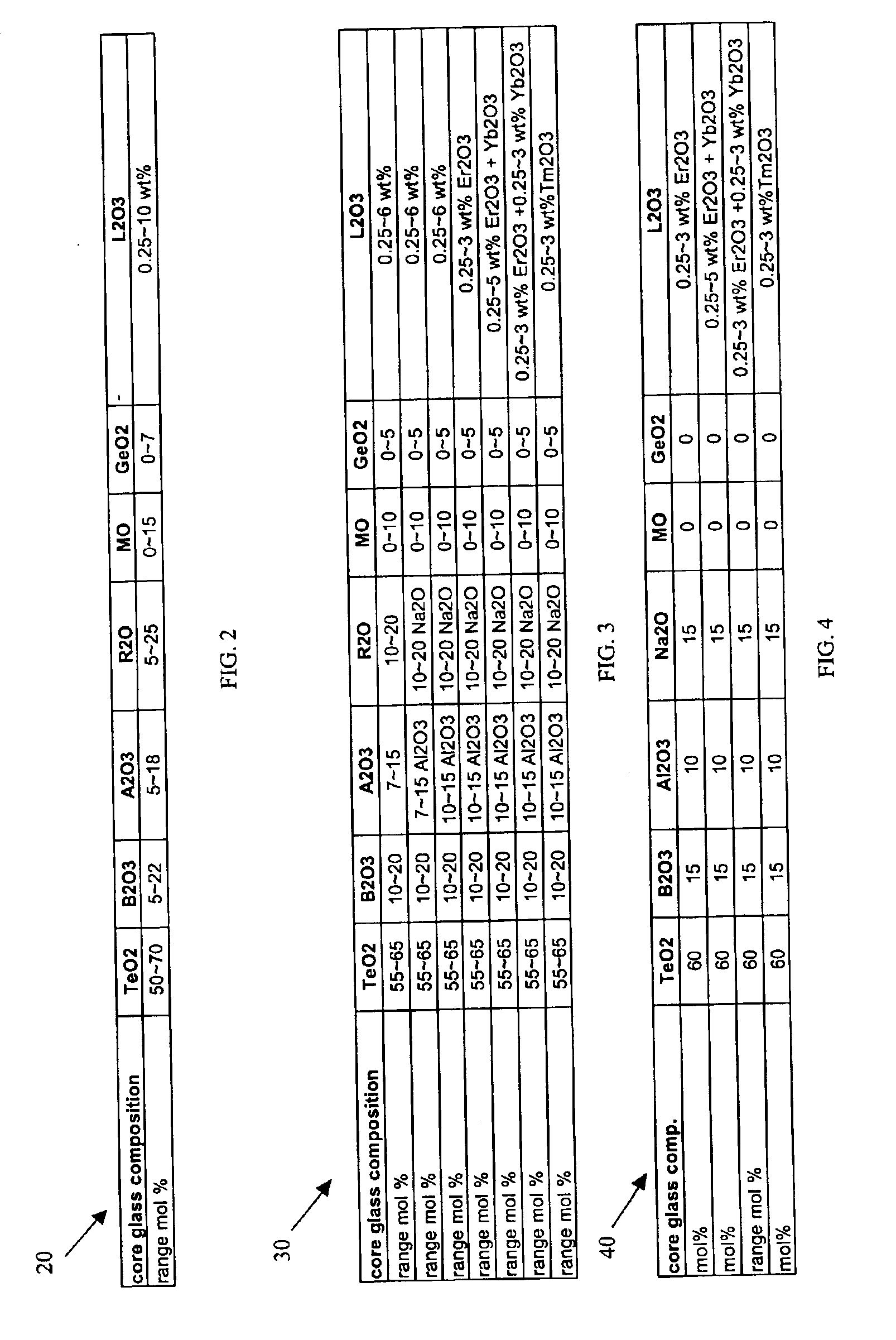
Glass compositions, glass fibers, and methods of inhibiting boron volatization from glass compositions
Embodiments of the present invention relate generally to fiberizable glass compositions comprising boron oxide at least one rare earth oxide in a molar ratio of rare earth oxide to boron oxide ranging from 0.01 to 0.33. Other embodiments of the present invention relate to methods of inhibiting boron volatization from glass compositions comprising boron oxide by adding a rare earth oxide to the glass composition before melting the glass compositions. Still other embodiments of the present invention relate to glass fibers formed from the fiberizable glass compositions of the present invention, and polymeric composites and printed circuit boards made therefrom.
Owner:PPG IND OHIO INC
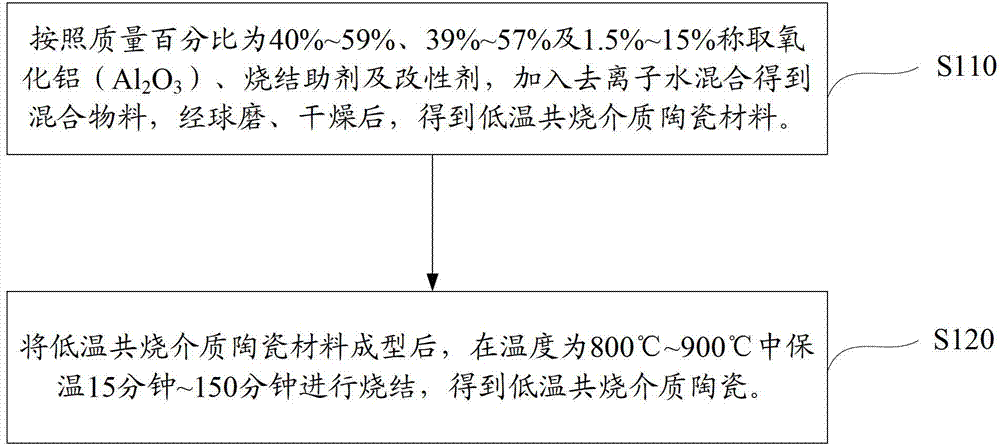
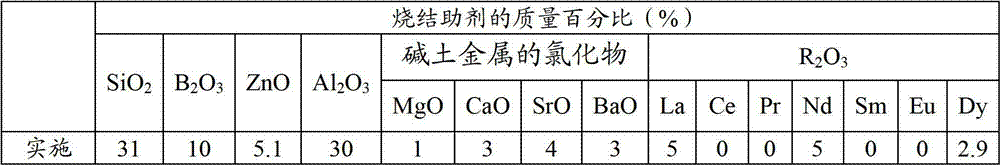
Method, sintering aid and materials for preparation of low-temperature cofired medium ceramic and application
A sintering aid for a low-temperature cofired medium ceramic material is composed of, by weight, 31%-45% of silicon dioxide, 1%-10% of boron oxide, 5.1%-10% of zinc oxide, 18%-30% of aluminum oxide, 11%-24% of alkaline earth metallic oxide and 5%-15% of oxide with the general formula of R2O3, wherein R refers to at least one of lanthanum, cerium, praseodymium, neodymium, samarium, europium and dysprosium, and the alkaline earth metallic oxide refers to one of magnesium oxide, calcium oxide, barium oxide and strontium oxide. Adding the sintering aid into the low-temperature cofired medium ceramic material enables the prepared low-temperature cofired medium ceramic to have excellent thermal mechanical performance and dielectric performance. In addition, the invention provides the low-temperature cofired medium ceramic material and application thereof and a method for preparing the low-temperature cofired medium ceramic.
Owner:GUANGDONG FENGHUA ADVANCED TECH HLDG
Positive electrode active material for lithium secondary battery, method of manufacturing the same, and lithium secondary battery using the same
InactiveUS20110200880A1Improve discharge capacityImprove discharge performanceNon-aqueous electrolyte accumulator electrodesSpecial surfacesLithiumBoron oxide
A positive electrode active material for lithium secondary batteries having a lithium-containing transition metal oxide having a layered structure and represented by the general formula Li1+xMn1-x-yMyO2, where 0<x<0.33, 0<y<0.66, and M is at least one transition metal other than Mn, the lithium-containing transition metal oxide having a boron oxide layer formed on the surface thereof.
Owner:SANYO ELECTRIC CO LTD
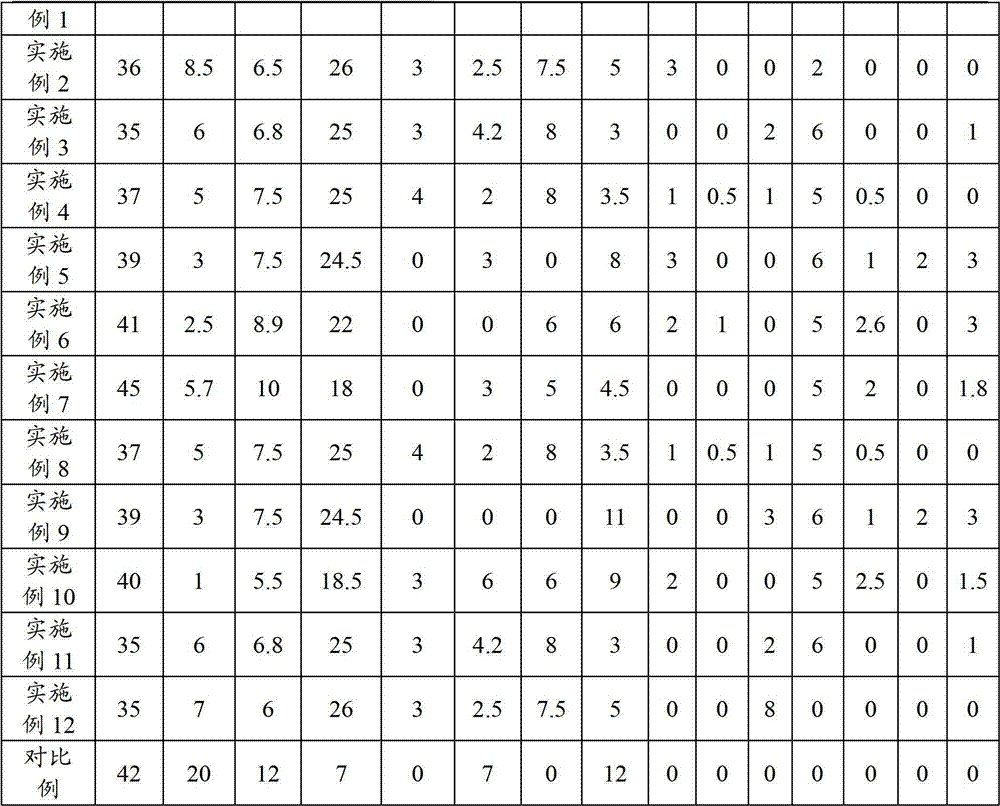
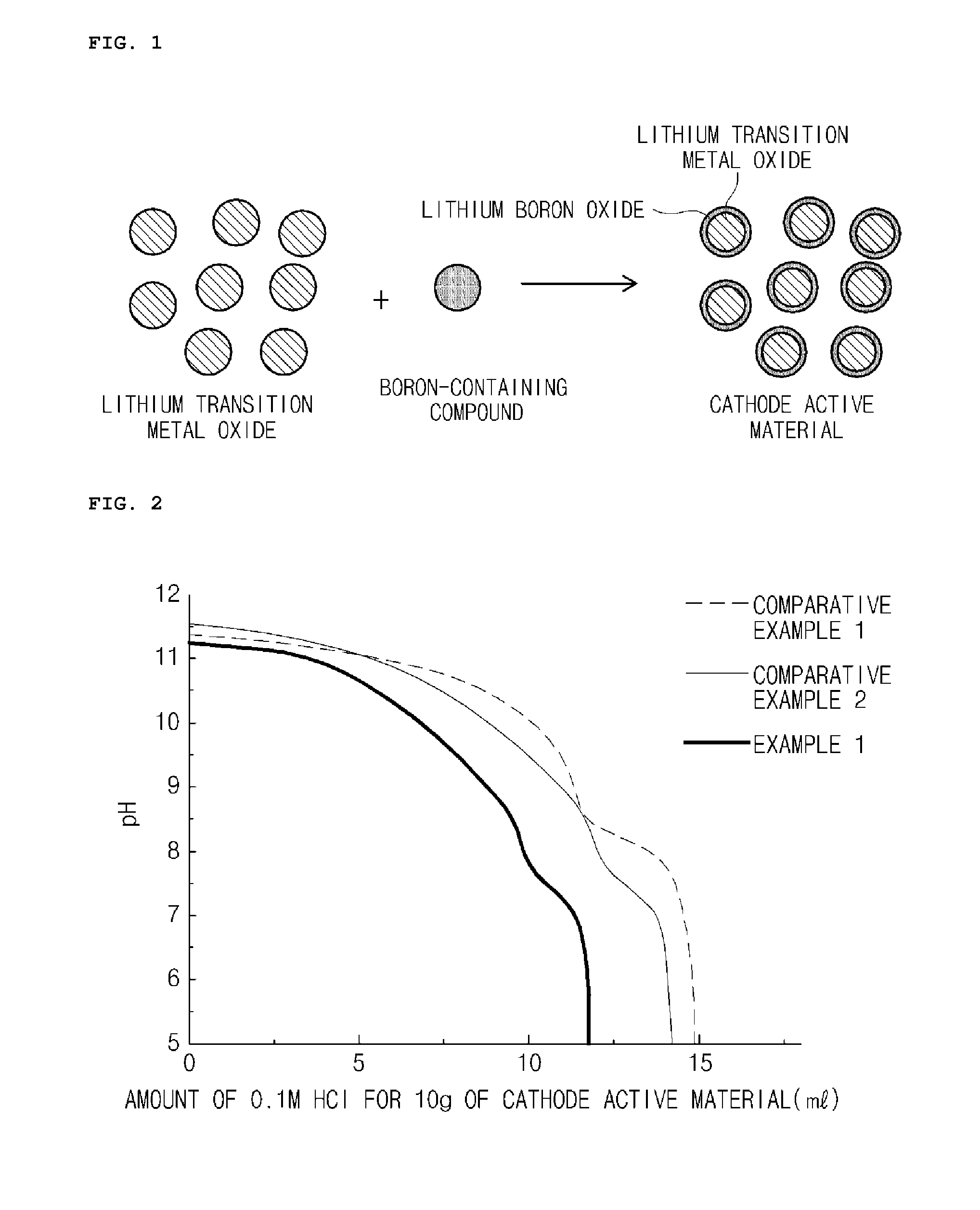
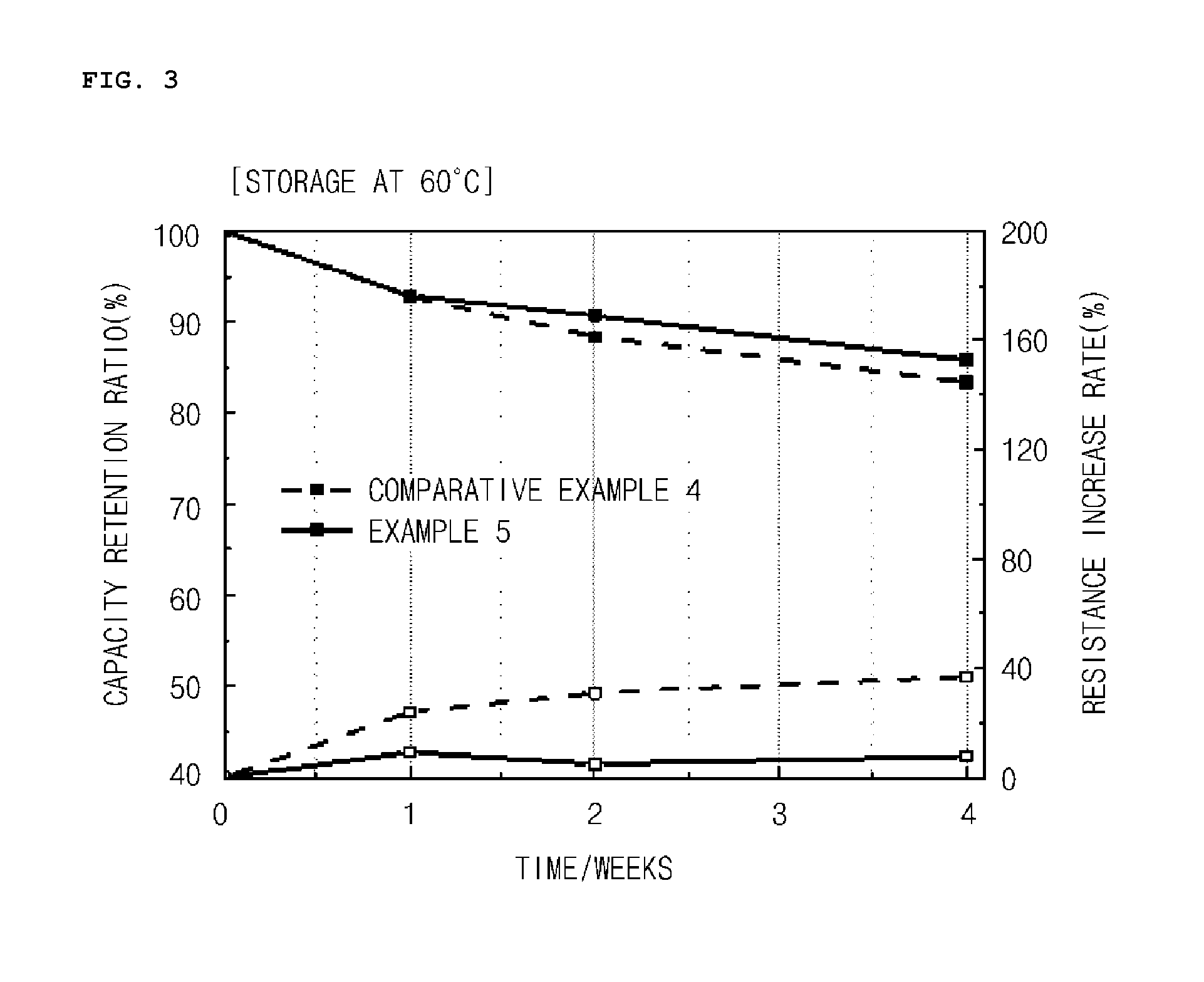
Manufacturing method of cathode active material, and cathode active material for lithium secondary battery manufactured thereby
ActiveUS20160013476A1Easy to transformElectrode thermal treatmentPositive electrodesLithiumBoron containing
Provided are a method of preparing a cathode active material including coating a surface of a lithium transition metal oxide with a lithium boron oxide by dry mixing the lithium transition metal oxide and a boron-containing compound and performing a heat treatment, and a cathode active material prepared thereby.A method of preparing a cathode active material according to an embodiment of the present invention may easily transform lithium impurities present in a lithium transition metal oxide into a structurally stable lithium boron oxide by performing a heat treatment near the melting point of a boron-containing compound.Also, a coating layer may be formed in which the lithium boron oxide is uniformly coated in an amount proportional to the used amount of the boron-containing compound even at a low heat treatment temperature.
Owner:LG ENERGY SOLUTION LTD
Hollow glass microballoons and production method thereof
InactiveCN101638295AGood chemical stabilityIncrease Young's modulusGlass shaping apparatusSilicon dioxideQuenching
The invention relates to hollow glass microballoons and a production method thereof. The hollow glass microballoons are characterized by comprising the following raw materials in portion by weight: 75to 80 portions of silicon dioxide, 7 to 15 portions of boron oxide, 1 to 4 portions of aluminium oxide, 0.5 to 1.5 portions of calcium oxide, 0.8 to 1.2 portions of magnesium oxide, 5 to 6 portions of sodium oxide, 0.0 to 0.3 portion of sulfur trioxide and 0 to 0.05 portion of ferric oxide. The production method of the hollow glass microballoons comprises the following steps: compounding, melting, water quenching, drying, crushing, grading, hollow balling, collection and air separation, and is characterized in that the hollow balling comprises the following steps: ejecting a glass raw material, gas and combustion-supporting gas upwards from the bottom of a balling furnace for combustion, and cooling the glass microballoons upwards by a cooling device after melting under the action of a draft fan. The hollow glass microballoons have the advantages of good surface tension, high compression strength and chemical stability. The production method can better ensure material balling, and hassimple process, less equipment and good effect as much as possible.
Owner:CHINA TRIUMPH INT ENG +1
Protection Against the Oxidation of Composite Materials Containing Carbon
ActiveUS20080311301A1Efficient oxidationEasy to implementPretreated surfacesCoatingsCarbon compositesAlkaline earth metal
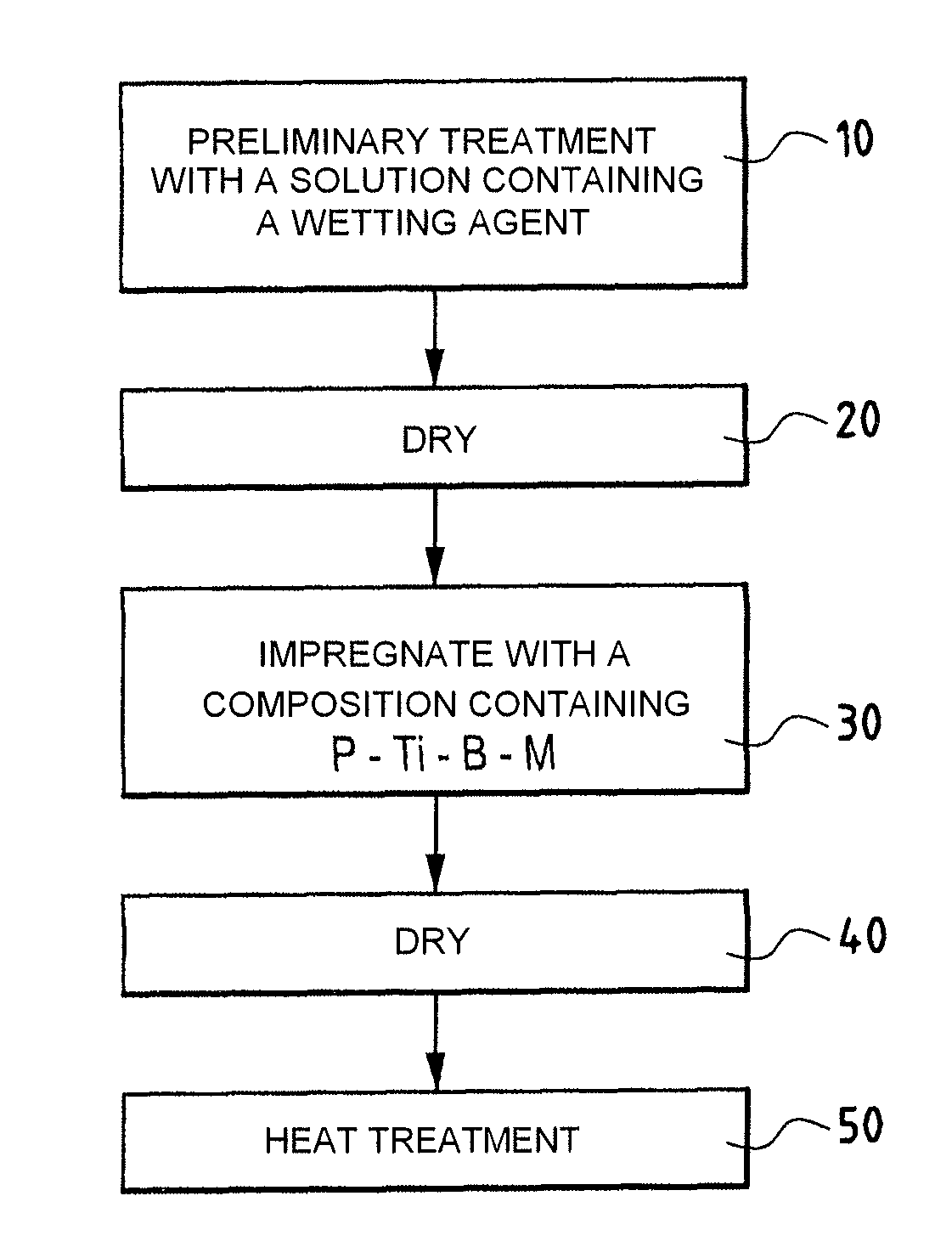
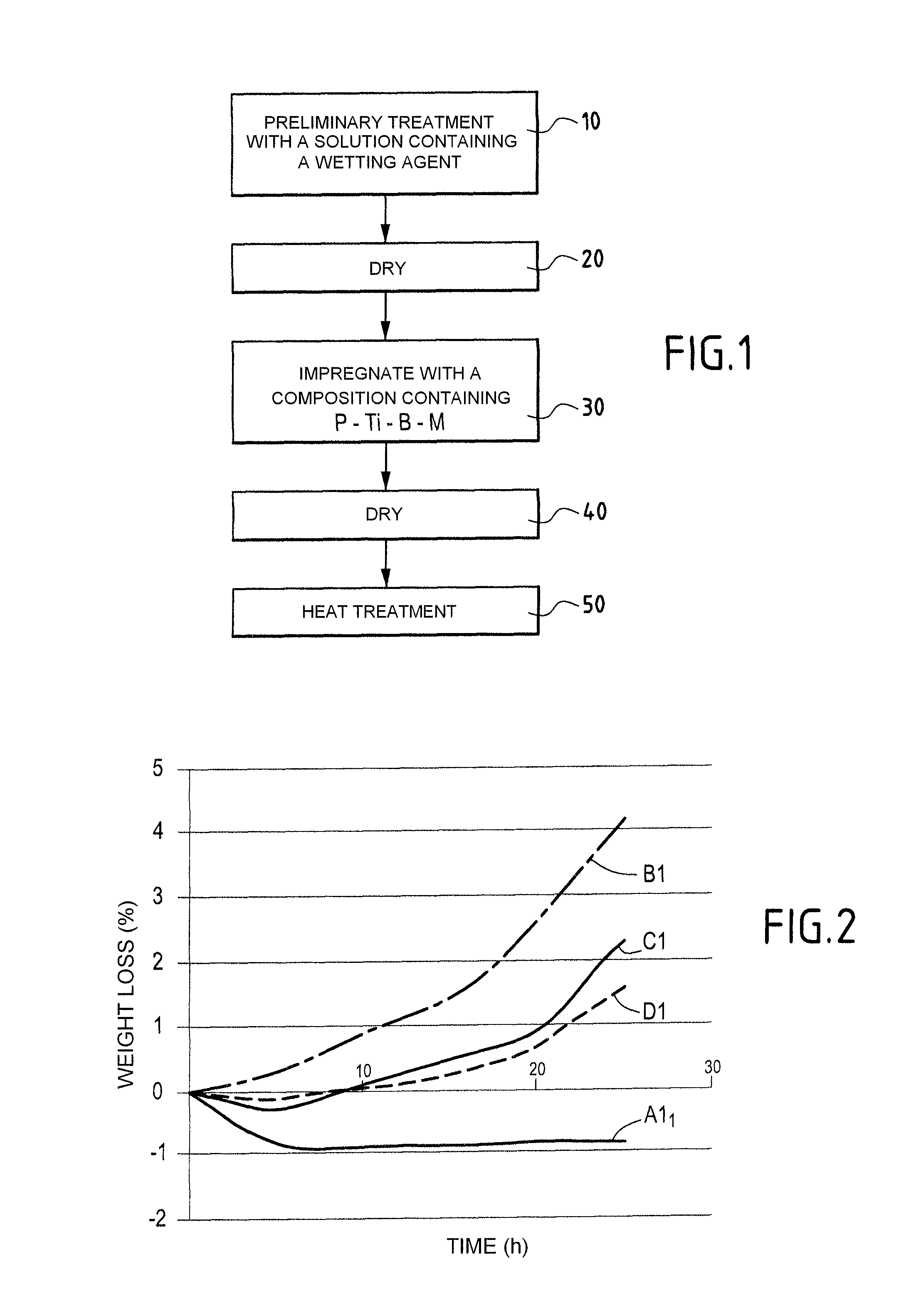
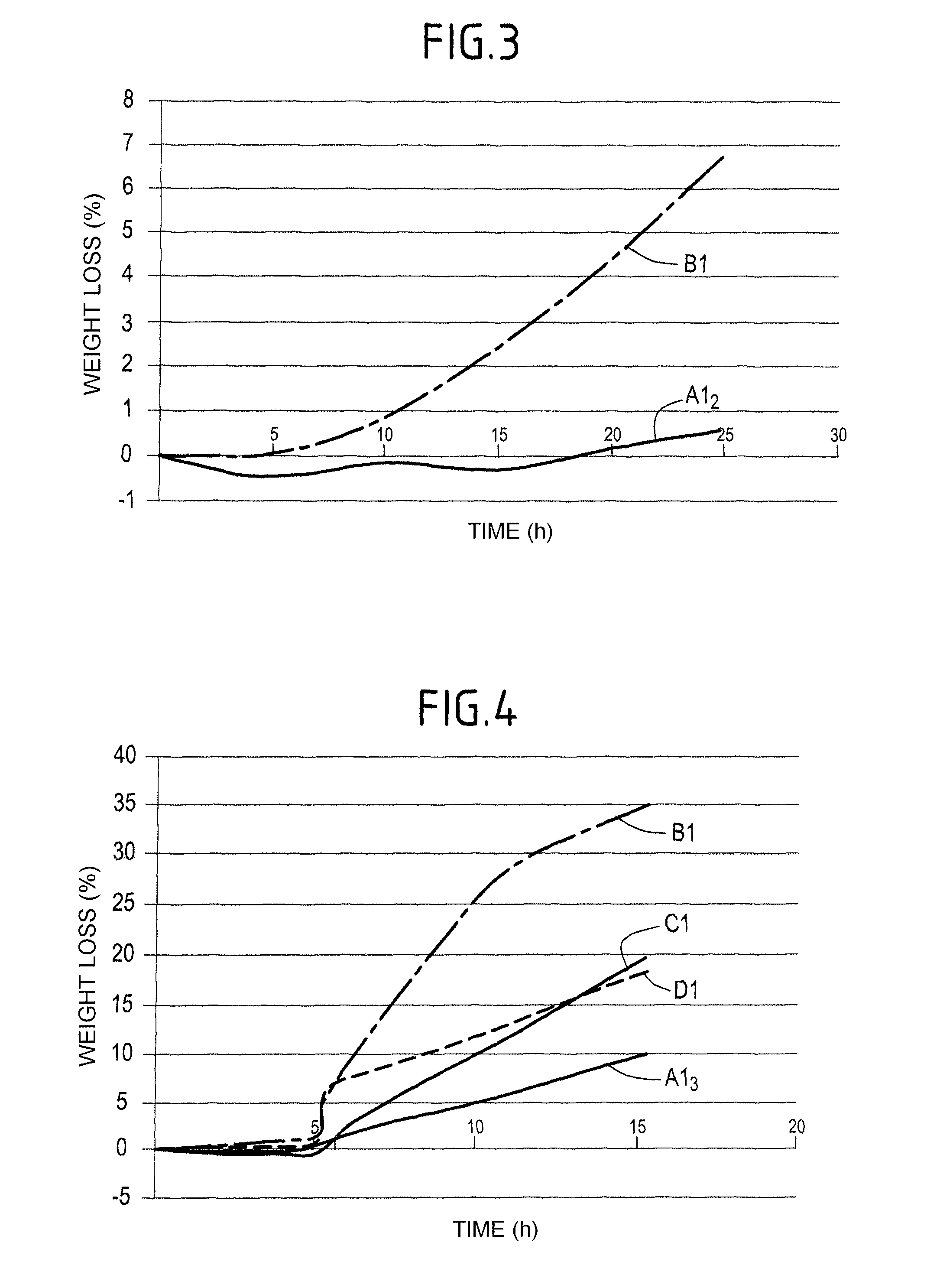
A part made of a porous material containing carbon, in particular a C / C composite material, is protected against oxidation by being impregnated with a composition in an aqueous medium containing at least a phosphorous compound, elemental titanium, and boron or a boron compound other than titanium diboride, to form in the presence of oxygen and at least one alkali or alkaline-earth element M that catalyses oxidation of carbon, at least one P—O—Ti-M type association bonded by boron oxide B2O3 and trapping the element M.
Owner:SN DETUDE & DE CONSTR DE MOTEURS DAVIATION S N E C M A
Glass compositions
Improved glass fibers compositions, typically useful for fire resistant blankets or containers to provide high burn-through resistance at high temperatures of 2,400° F. and higher, and typically comprising silica, sodium oxide, potassium oxide, calcium oxide, magnesium oxide, ferrous+ferric oxide, and titanium oxide; the improved glass compositions may further include alumina, lithium oxide, and boron oxide.
Owner:GLASS
Rare earth doped luminescent glass
InactiveUS20050253113A1Reduce maximum phonon energyLow on OH−Other chemical processesGas discharge lamp usageAlkaline earth metalAlkali metal oxide
A white cold light source uses an LED or a gas discharge lamp and a luminescent rare earth doped glass comprising multiple rare earth cations and a particularly high total rare earth content to generate white light emission. Preferably, the luminescent glass has a 2700K to 7000K black body temperature and color rendering index value exceeding 80. A first embodiment of the glass is composed primarily of P2O5, Al2O3, and alkaline earth and alkali metal oxides, and possesses other properties such as physical and thermal properties that are compatible with conventional melting, forming and other manufacturing steps. Other embodiments of the luminescent glass have a maximum water content of 0.1 wt-% and do not contain any boron. Also the luminescent glass is preferably free of water, boron oxides and nitrides. The luminescent glass can be used as a wavelength converter to produce bright white light emission when pumped by conventional commercially available blue and UV light emitting diode sources.
Owner:SCHOTT AG
Organic luminescence device and its production method
InactiveUS20050122039A1Excellent gas barrier propertiesDischarge tube luminescnet screensLayered productsWater vaporOxygen
An organic luminescence device uses a substrate with a gas-barrier film in which a gas-barrier film containing an amorphous oxide and at least two kinds of oxides selected from the group consisting of boron oxide, phosphorus oxide, sodium oxide, potassium oxide, lead oxide, titanium oxide, magnesium oxide, and barium oxide is formed on a substrate. The selected two kinds of oxides are a combination of an oxide of an element having a large atomic radius and an oxide of an element having a small atomic radius. The substrate is made of glass or plastic. As a result, the organic luminescence device using a substrate excellent in gas-barrier capability to prevent the infiltration of oxygen, water vapor, etc. from outside is provided.
Owner:PANASONIC CORP
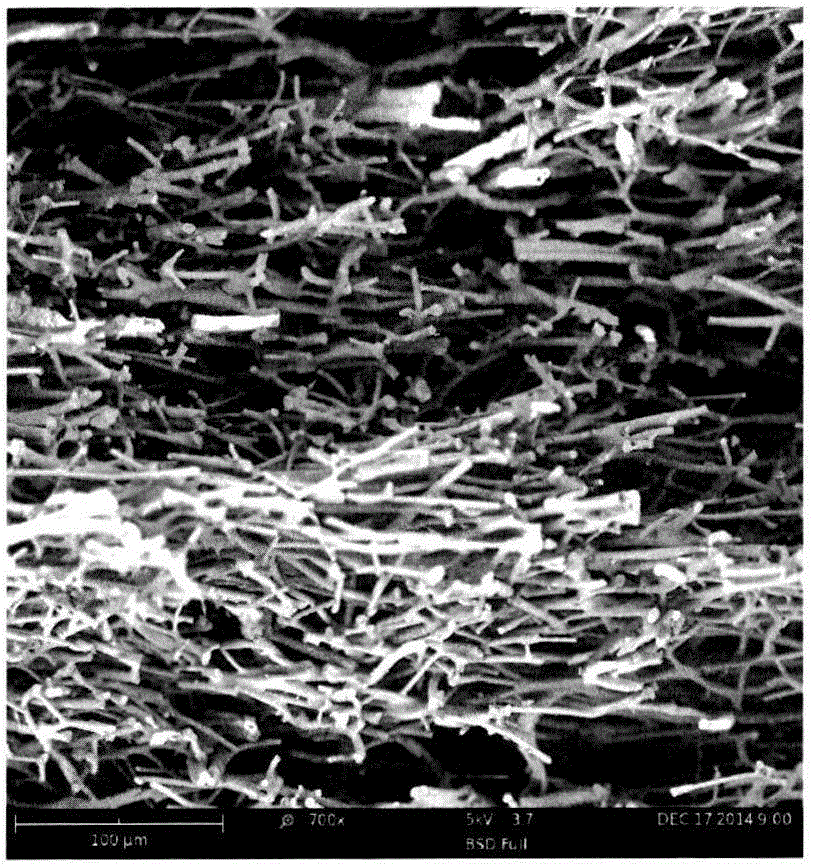
Light-weight, high temperature-resistance and heat-insulation ceramic fiber tile and making method thereof
ActiveCN105272322AOvercome the problem of uneven distributionImprove mechanical propertiesRoom temperatureSlurry
The invention relates to a light-weight, high temperature-resistance and heat-insulation ceramic fiber tile and a making method thereof. The heat insulation tile comprises ceramic fibers and boron oxide, wherein the ceramic fibers comprise quartz fibers, alumina fibers and yttrium oxide stabilized zirconia fibers. The making method of the light-weight, high temperature-resistance and heat-insulation ceramic fiber tile comprises the following steps: preparing a sintering aid suspension, preparing a ceramic fiber slurry, carrying out wet green body molding, drying the obtained wet green body, and carrying out pressurization sintering. The heat insulation tile has good heat insulation effect and mechanical performances, has light weight and resists high temperature; the density is controllable between 0.10g / cm<3> and 0.90g / cm<3>; the lowest apparent heat conduction coefficient at room temperature reaches 0.033W / (m.K); the compressive strength at room temperature is greater than 3.0Mpa; and the long-time use temperature can reach 1350DEG C.
Owner:AEROSPACE INST OF ADVANCED MATERIALS & PROCESSING TECH
ER3+ doped boro-tellurite glasses for 1.5 mum broadband amplification
InactiveUS6859606B2Lower average energyHigher propertiesActive medium materialActive medium shape and constructionBroadbandBoron oxide
A tellurite-based glass composition for use in EDFAs exhibits higher phonon energy without sacrificing optical, thermal or chemical durability properties. The introduction of boron oxide (B2O3) into the Er3+-doped tellurite glasses increases the phonon energy from typically 785 cm−1 up to 1335 cm−1. The inclusion of additional glass components such as Al2O3 has been shown to enhance the thermal stability and particularly the chemical durability of the boro-tellurite glasses. Er:Yb codoping of the glass does further enhance its gain characteristics.
Owner:NP PHOTONICS A CORP OF DELAWARE
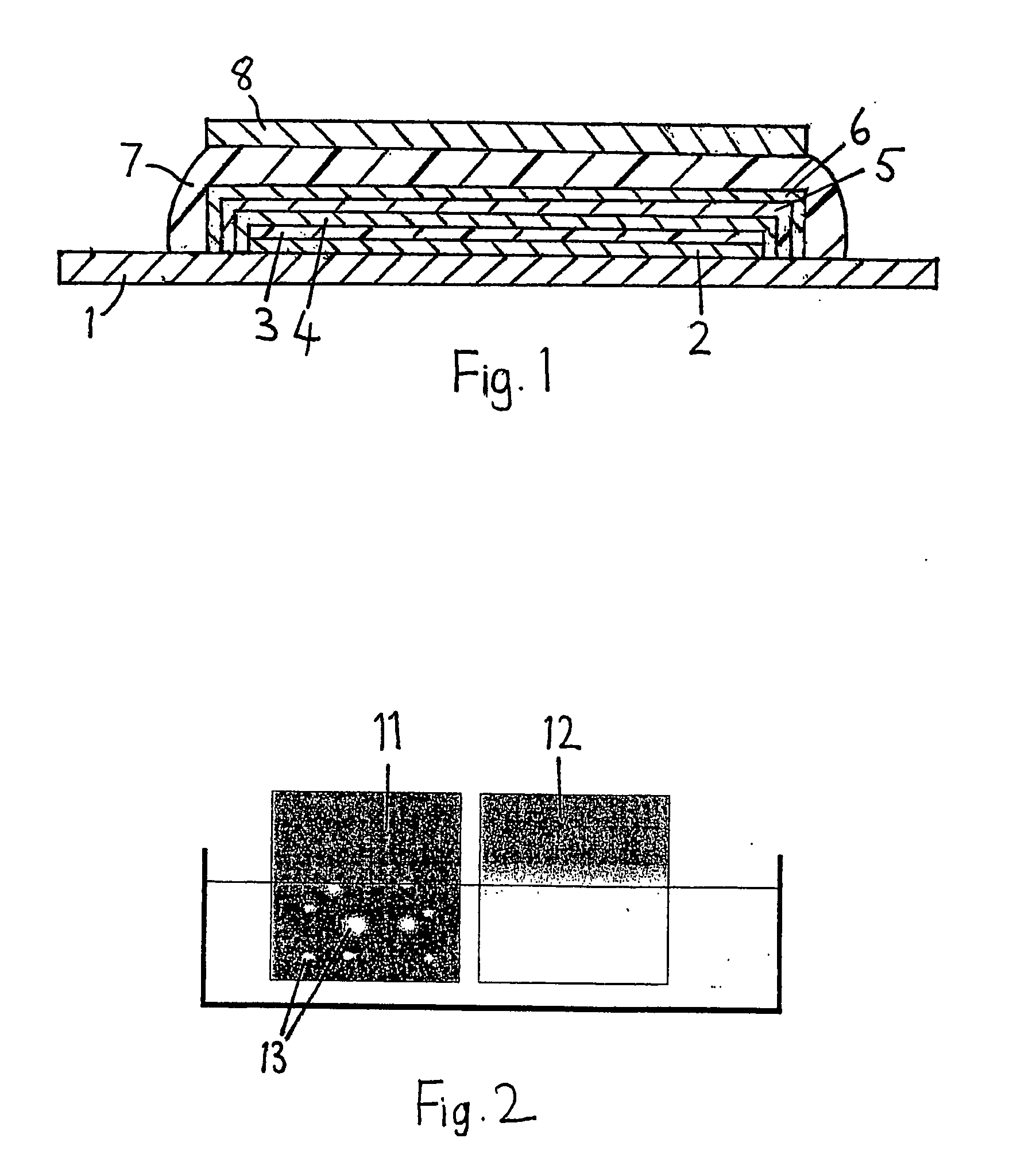
Passivation layer
InactiveUS20060006798A1Few pinholeEffective protectionDischarge tube luminescnet screensElectroluminescent light sourcesWork functionBoron oxide
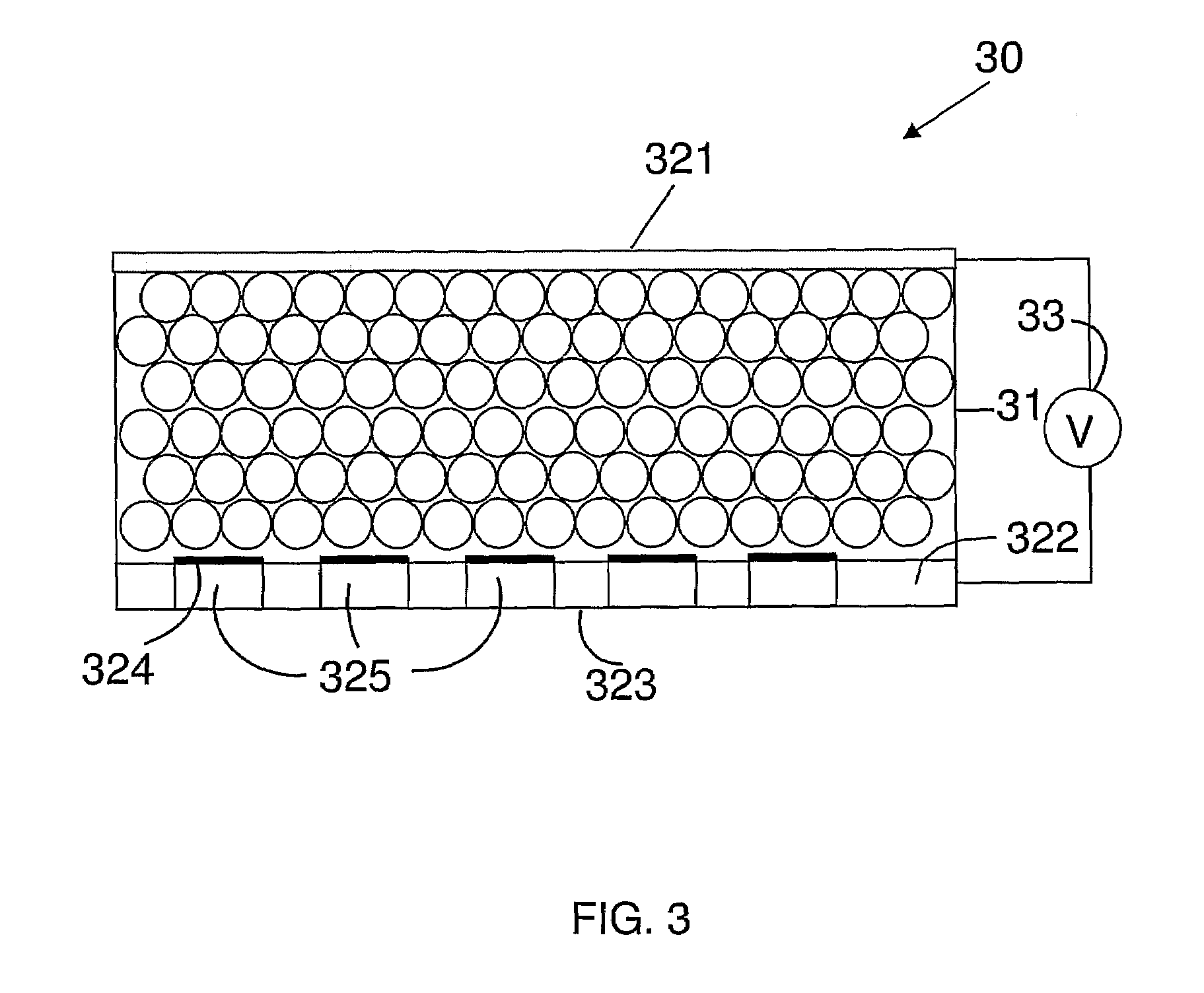
An organic light emitting diode device comprises a substrate (1), a layer (3) of organic, preferably polymeric, light emitting material, and a transparent cathode (4) comprising a layer of material with a work function less than 4 eV. The device has a passivation layer (5) comprising boron oxide.
Owner:MICROEMISSIVE DISPLAY
Thermal protective coating
ActiveUS20060156958A1Extended shelf lifeExcellent optical propertiesAlkali metal silicate coatingsPretreated surfacesCalcium silicateSodium Bentonite
A coating, method of coating and substrates coated thereby, wherein the coating contains an inorganic adhesive such as an alkali / alkaline earth metal silicate such as sodium silicate, potassium silicate, calcium silicate, and magnesium silicate; a filler such as a metal oxide for example silicon dioxide, aluminum oxide, titanium dioxide, magnesium oxide, calcium oxide and boron oxide; and one or more emissivity agents such as silicon hexaboride, carbon tetraboride, silicon tetraboride, silicon carbide, molybdenum disilicide, tungsten disilicide, zirconium diboride, cupric chromite, or metallic oxides such as iron oxides, magnesium oxides, manganese oxides, chromium oxides and copper chromium oxides, and derivatives thereof. In a coating solution, an admixture of the coating contains water. A stabilizer such as bentonite, kaolin, magnesium alumina silicon clay, tabular alumina and stabilized zirconium oxide may be added.
Owner:WESSEX
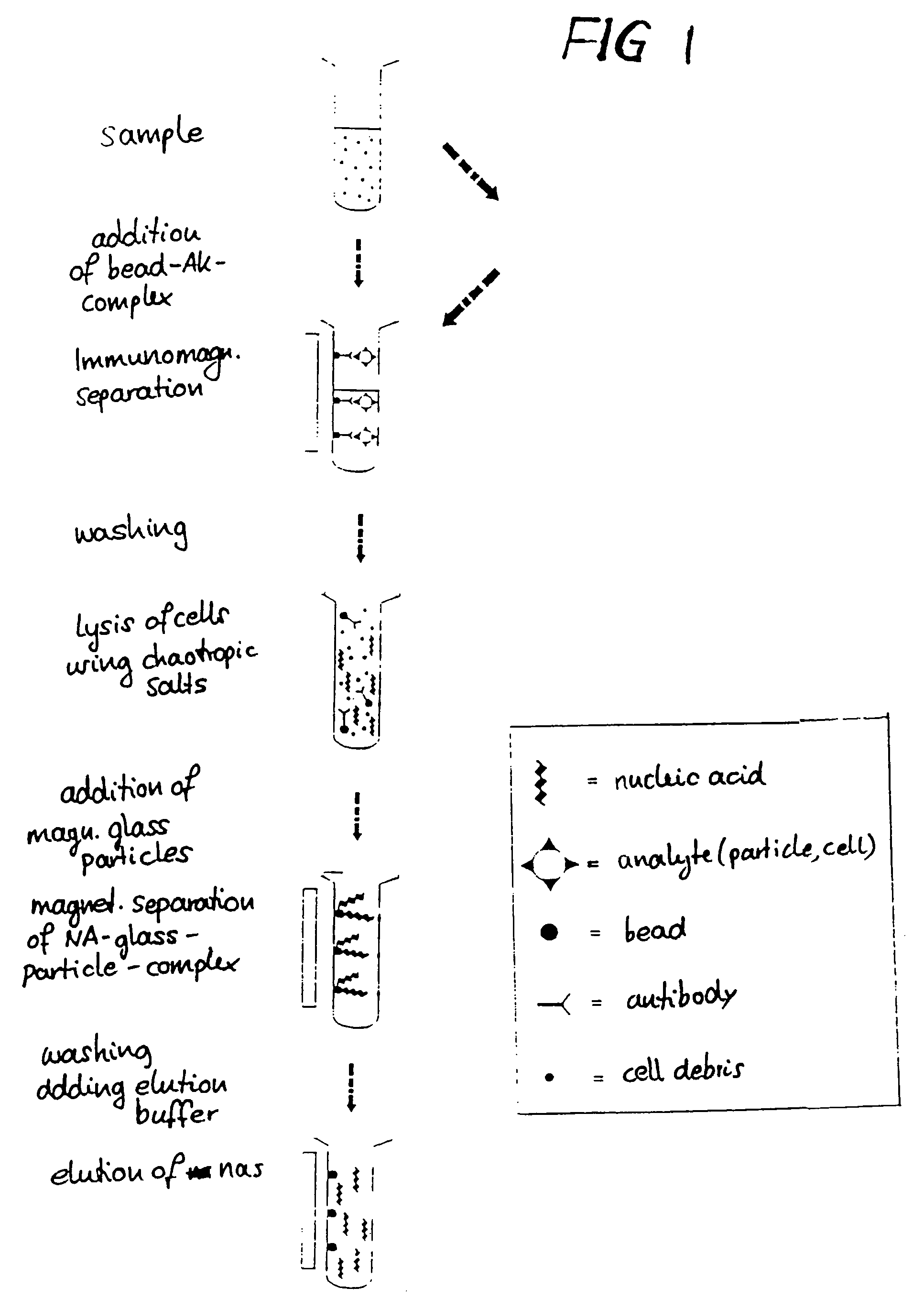
Method for separating biological material from a fluid using magnetic particles
InactiveUS7371830B2Simple procedureSimple materialImmobilised enzymesMicroorganism separationAlcoholMagnetite
Magnetic particles are prepared containing a magnetic core coated with a glass layer having a substantially pore-free glass surface or having pores with a diameter of less than 10 nm. The particles are used for separating biological material such as nucleic acids. A preferred process of preparing the particles is by forming a mixture of magnetic cores with a sol formed from an alcohol and a metal alkoxide, spray-drying the mixture to coat the cores with a layer of gelled sol, and heating the coated cores to obtain the magnetic glass particles. Preferably, the particles have an average particle size of less than 100 μm. The magnetic core may be a composite material containing a mica core and magnetite particles immobilized on the mica core, and the glass layer may contain boron oxide. Magnetic core materials include magnetite (Fe3O4) and Fe2O3.
Owner:ROCHE DIAGNOSTICS GMBH
Boro-silicate glass frits for hermetic sealing of light emitting device displays
A frit composition useful for sealing a light emitting device is disclosed. The frit composition comprises a glass portion comprising a base component and at least one absorbing component. The glass portion of the frit comprises silica, boron oxide, optionally alumina, and (a) cupric oxide and / or a (b) combination of ferric oxide, vanadium pentoxide, and optionally titanium dioxide. Also disclosed is an article comprising a substrate and a frit, and a glass package comprising two substrates and a frit positioned between the substrates. A method for manufacturing a hermetically sealed glass package comprising the deposition of a glass frit and heating of the glass frit to form a hermetic seal is also disclosed.
Owner:CORNING INC
Method for coating high-nickel ternary material with aluminum oxide and boron oxide
InactiveCN108091830AEfficient removalUniform coverageCell electrodesBoron containingElectrical performance
The invention discloses a method for coating a high-nickel ternary material with aluminum oxide and boron oxide. The method includes the steps: 1) mixing an aluminum oxide precursor and water to prepare aluminum oxide coating liquid; 2) mixing the high-nickel ternary material with the aluminum oxide coating liquid to obtain aluminum oxide coating turbid liquid; 3) performing solid-liquid separation on the aluminum oxide coating turbid liquid, and drying obtained solid materials to obtain dry materials; 4) mixing the dry materials and a boron-containing compound and then sintering the dry materials and the boron-containing compound to obtain the finished high-nickel ternary material coated with the aluminum oxide and the boron oxide. According to the treatment method, washing and coating are combined, surface impurities of the high-nickel ternary material can be more effectively removed, coating is performed in a water phase, a coating can more uniformly cover the surface of the material, and further a positive electrode material with a good electrical performance is obtained. According to the coating method, process steps can be simplified, tempering temperature is reduced, tempering time is shortened, production cycle can be shortened, and production cost is saved.
Owner:GUANGDONG BRUNP RECYCLING TECH +1
Amorphous polyamide resin composition and molded product
Provided are an amorphous polyamide resin composition having high transparency, and is excellent in heat resistance and stiffness, and a molded product thereof. The glass filler contains, expressed in terms of oxides by mass %, 68 to 74% of silicon dioxide (SiO2), to 5% of aluminum oxide (Al2O3), 2 to 5% of boron oxide (B2O3), 2 to 10% of calcium oxide (CaO), 0 to 5% of zinc oxide (ZnO), 0 to 5% of strontium oxide (SrO), 0 to 1% of barium oxide (BaO), 1 to 5% of magnesium oxide (MgO), 0 to 5% of lithium oxide (Li2O), 5 to 12% of sodium oxide (Na2O), and 0 to 10% of potassium oxide (K2O), where a total amount of lithium oxide (Li2O), sodium oxide (Na2O), and potassium oxide (K2O) is 8 to 12%.
Owner:ASAHI FIBER GLASS CO LTD
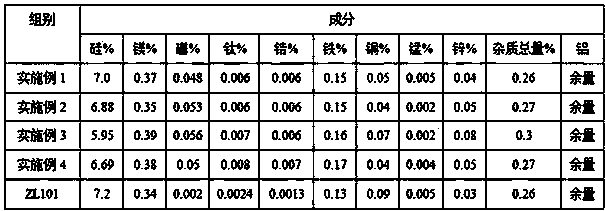
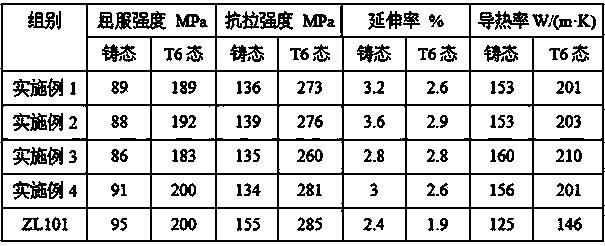
High thermal conductivity cast aluminium alloy and preparation method thereof
InactiveCN103526082AExpand the range of industrial applicationsImprove thermal conductivityTitanium zirconiumManganese
The invention discloses a high thermal conductivity cast aluminium alloy and a preparation method thereof. The high thermal conductivity cast aluminium alloy comprises the following components by weight percent: 5.0-7.5% of silicone, 0.25-0.5% of magnesium, 0.01-0.06% of boron, 0.005-0.02% of titanium, 0.005-0.02% of zirconium, less than 0.2% of iron, less than 0.2% of copper, less than 0.1% of manganese, less than 0.1% of zinc, no more than 0.3% of all the other impurities and the balance of aluminium. The preparation method comprises that boron oxide is taken as a modificator, and then titanium and zirconium elements are added, thus a cast aluminium alloy with thermal conductivity as high as 210W / (m.K) can be prepared. The high thermal conductivity cast aluminium alloy has the advantages that thermal conductivity of a Al7SiMg cast aluminium alloy is obviously improved on the basis of the existing good casting property, mechanical property and thermal treatment property, and an industrial application range of the Al7SiMg cast aluminium alloy is expanded.
Owner:GUANGZHOU KINBON NON FERROUS ALLOY METALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com