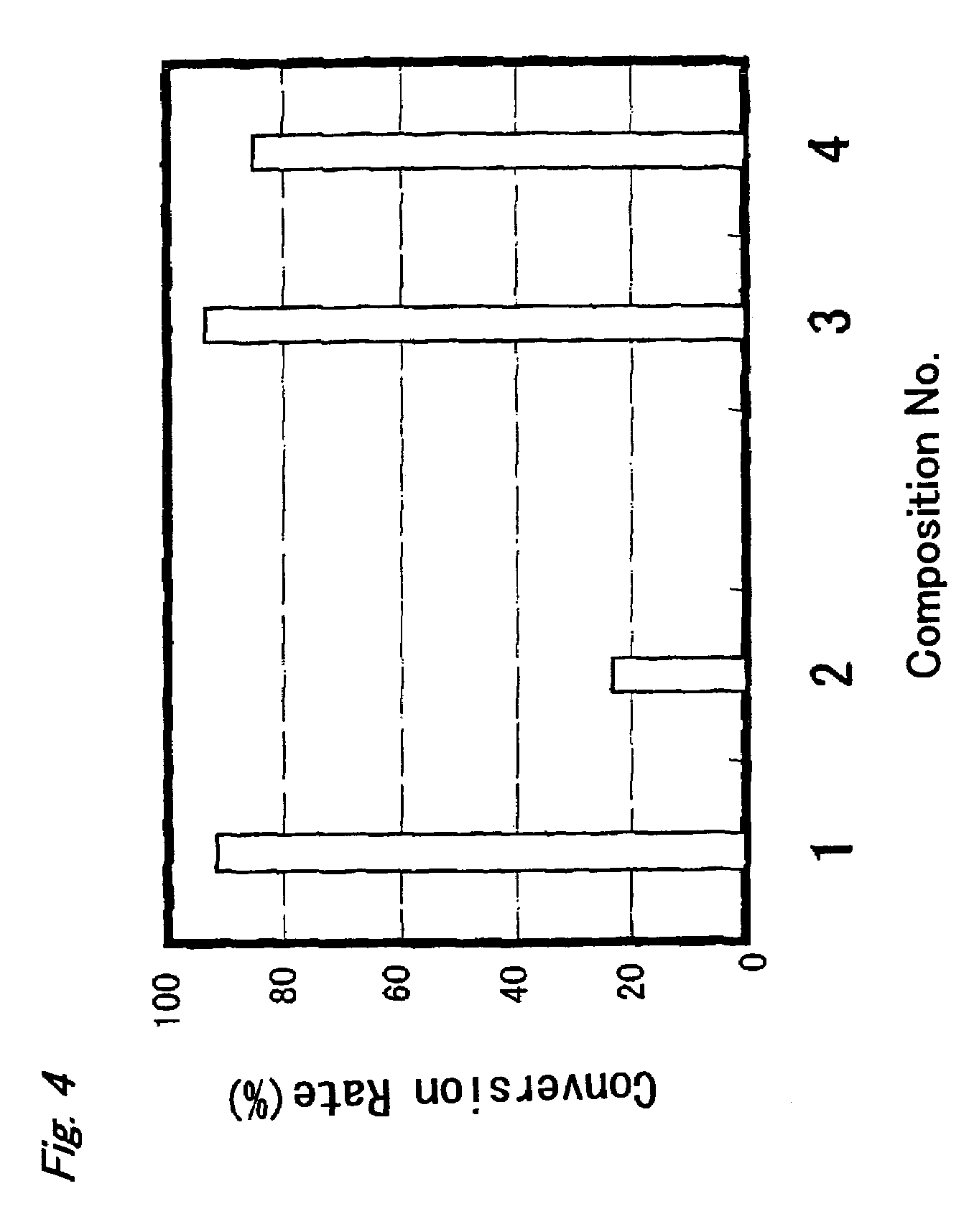
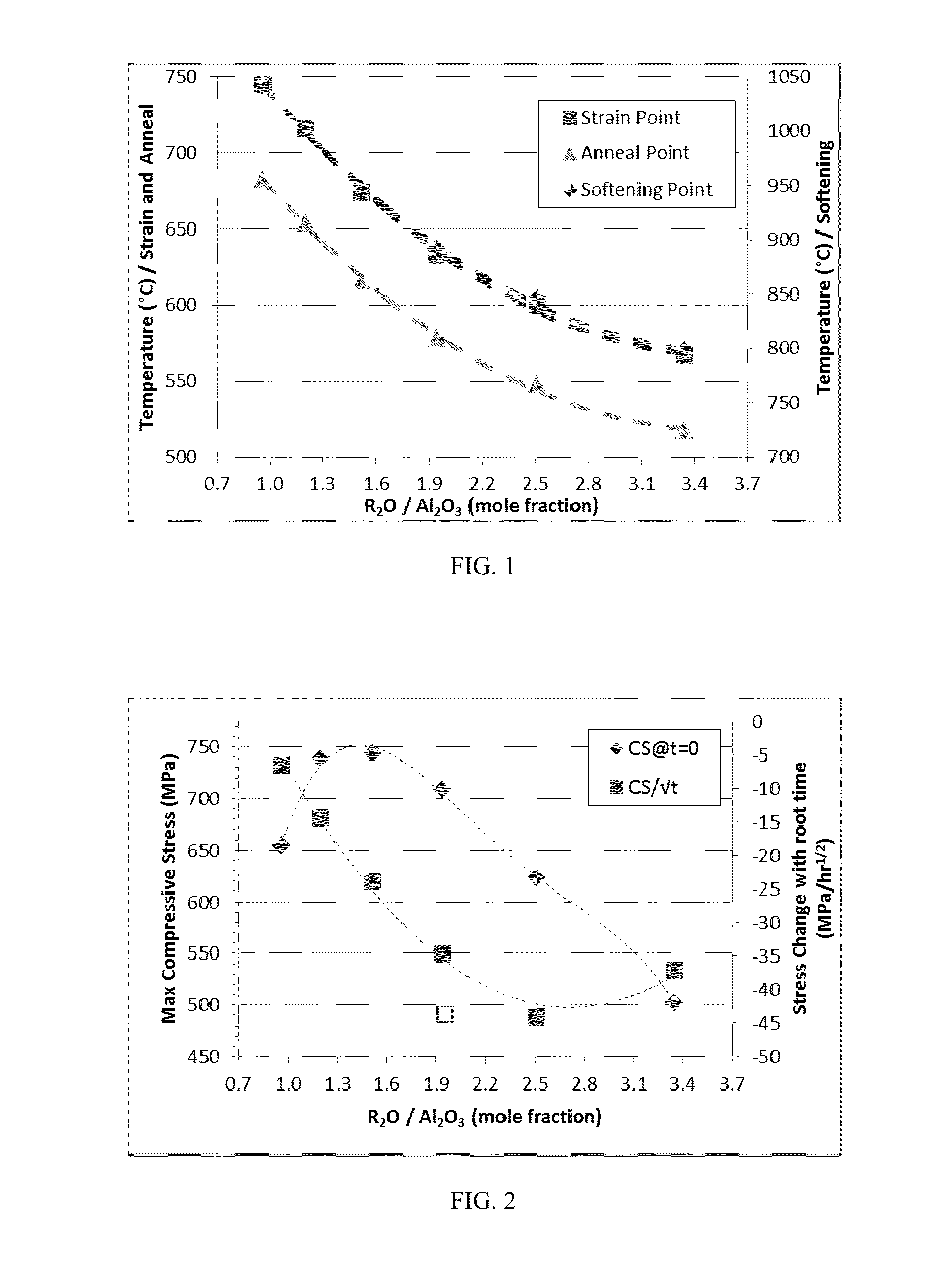
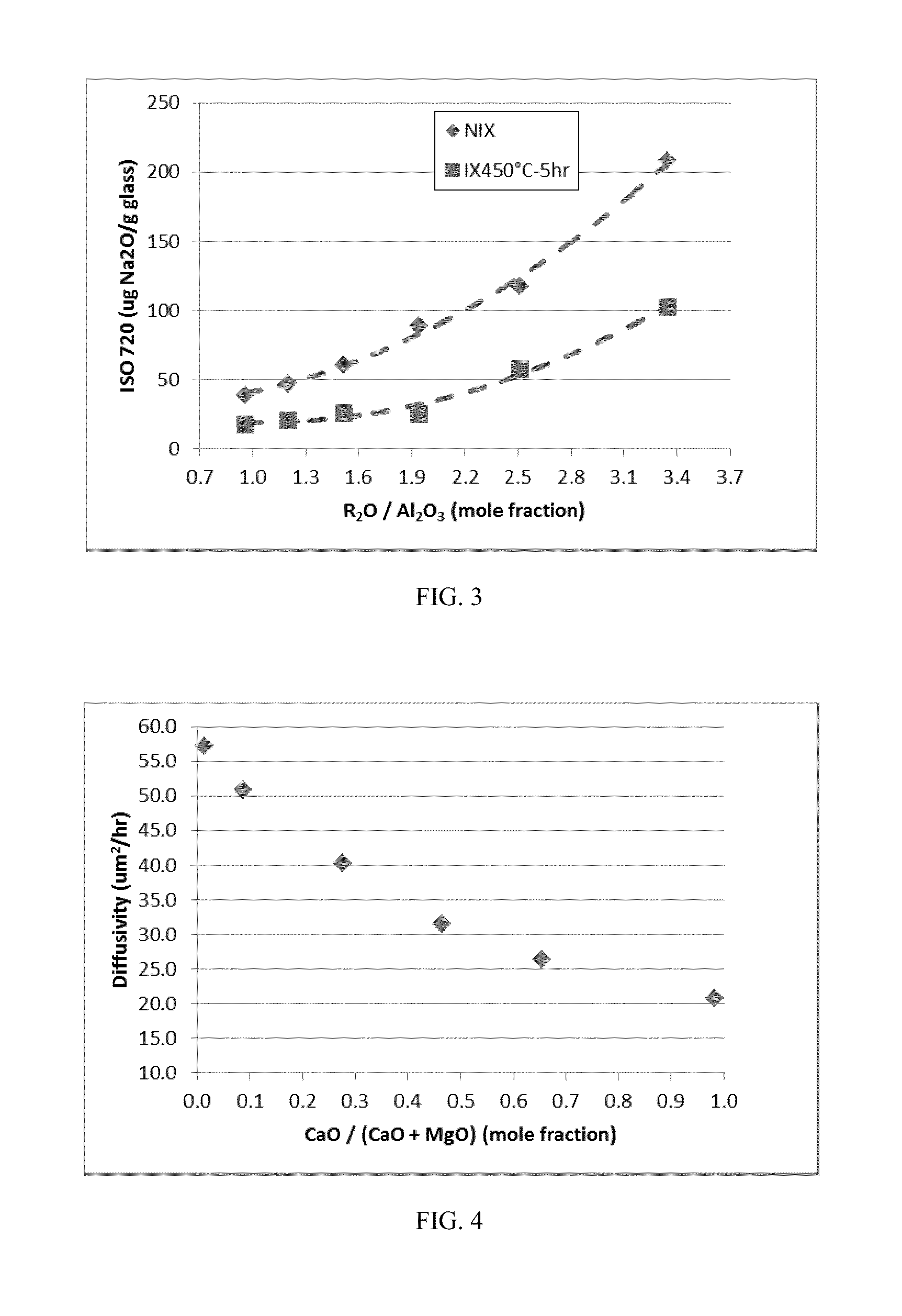
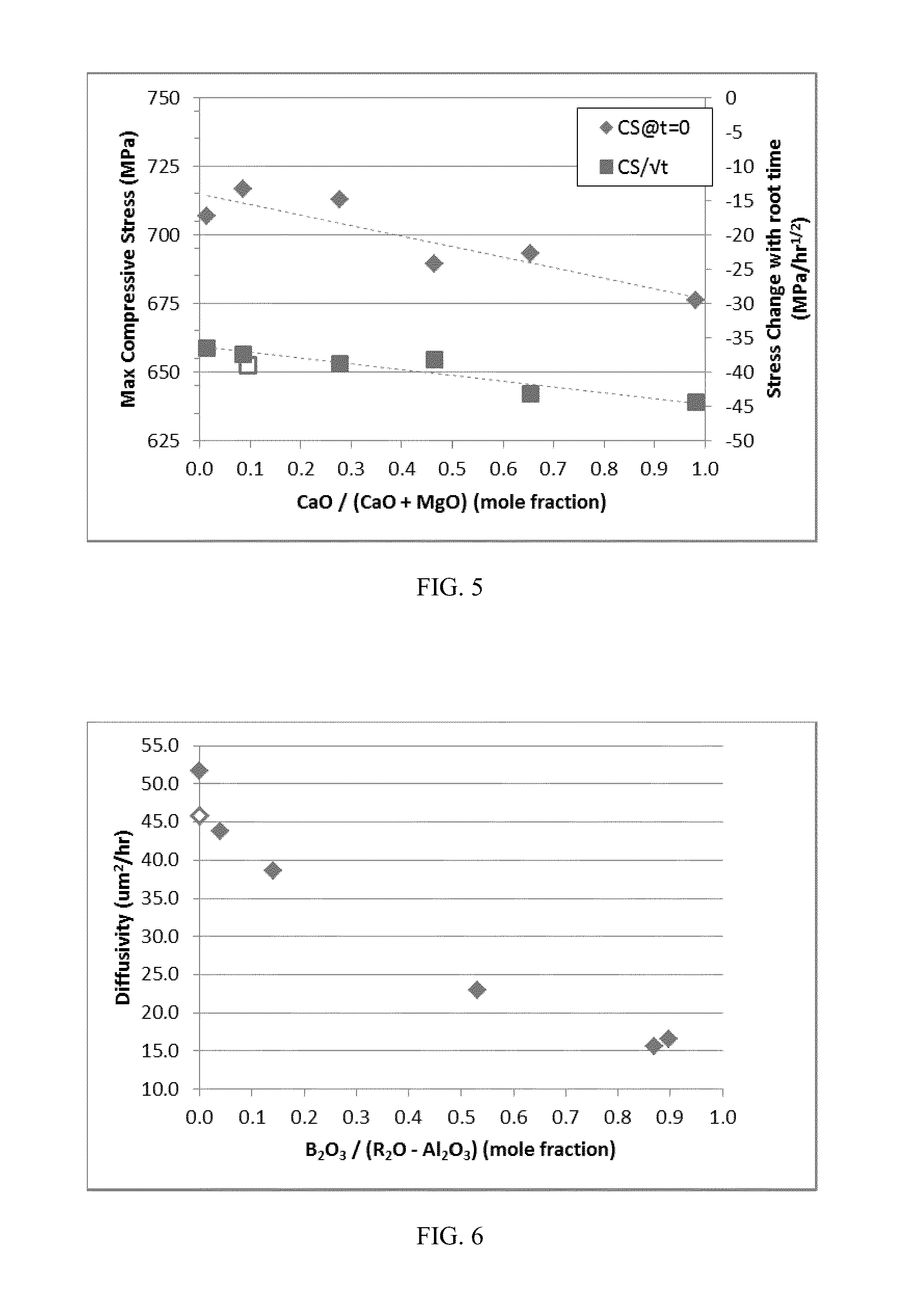
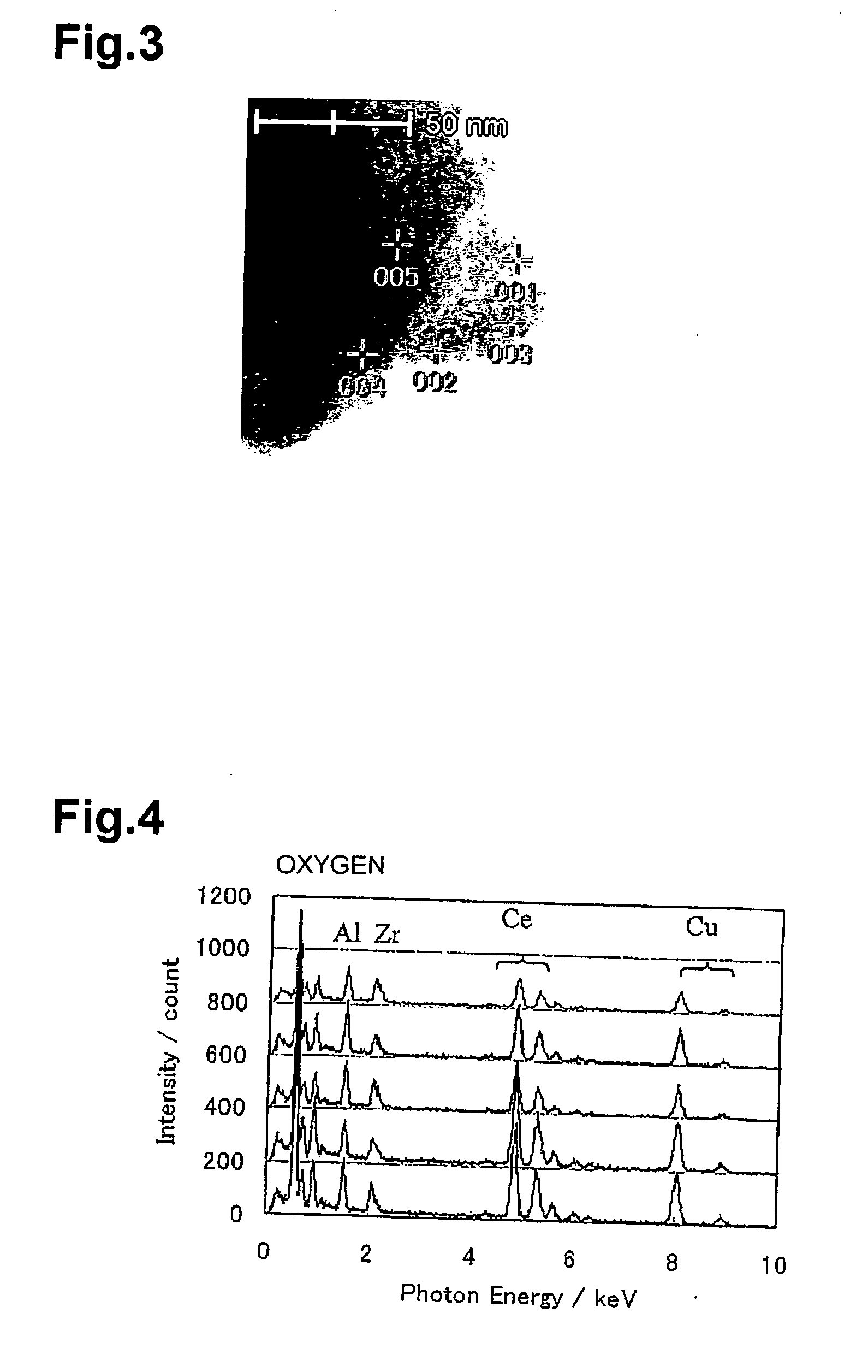
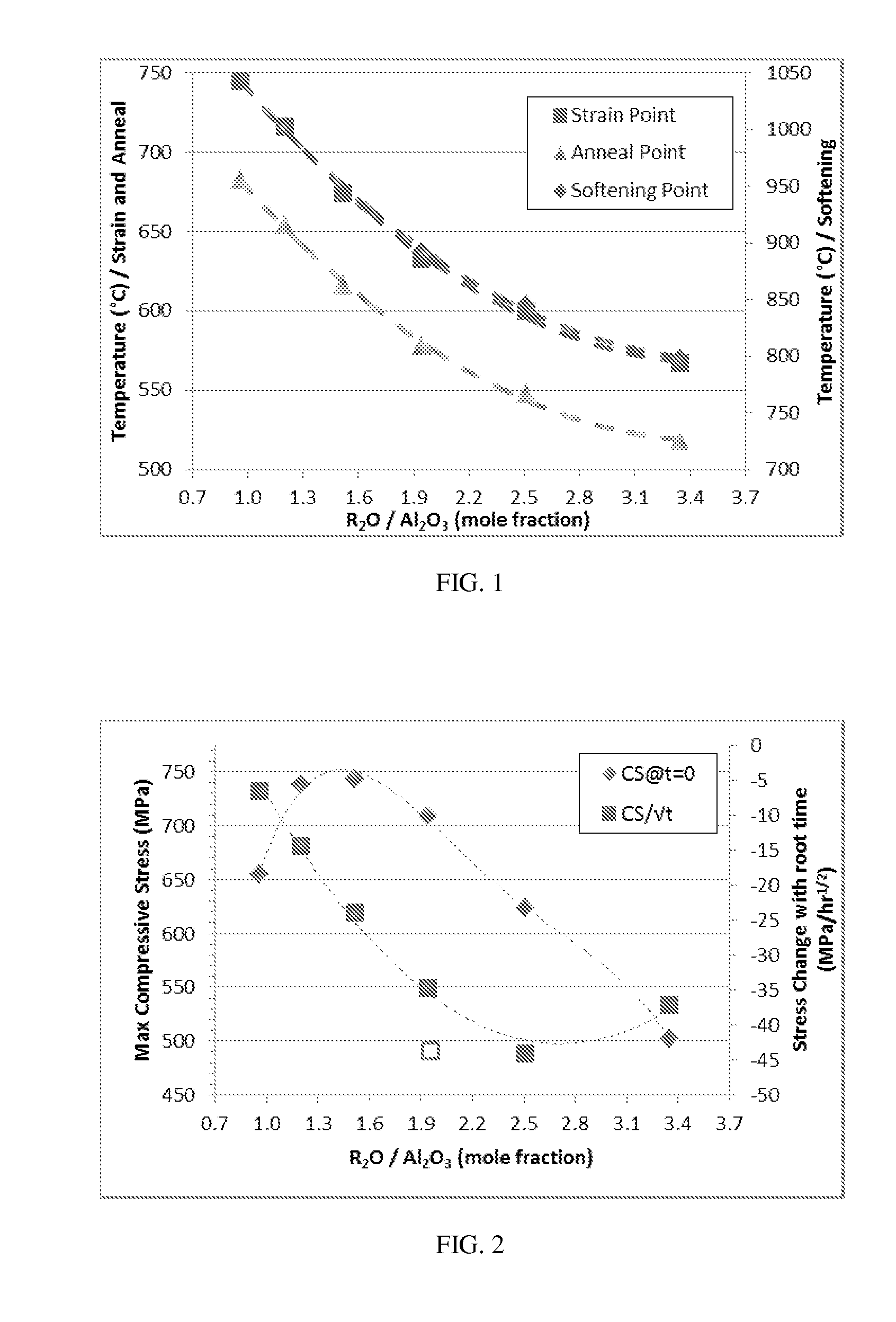
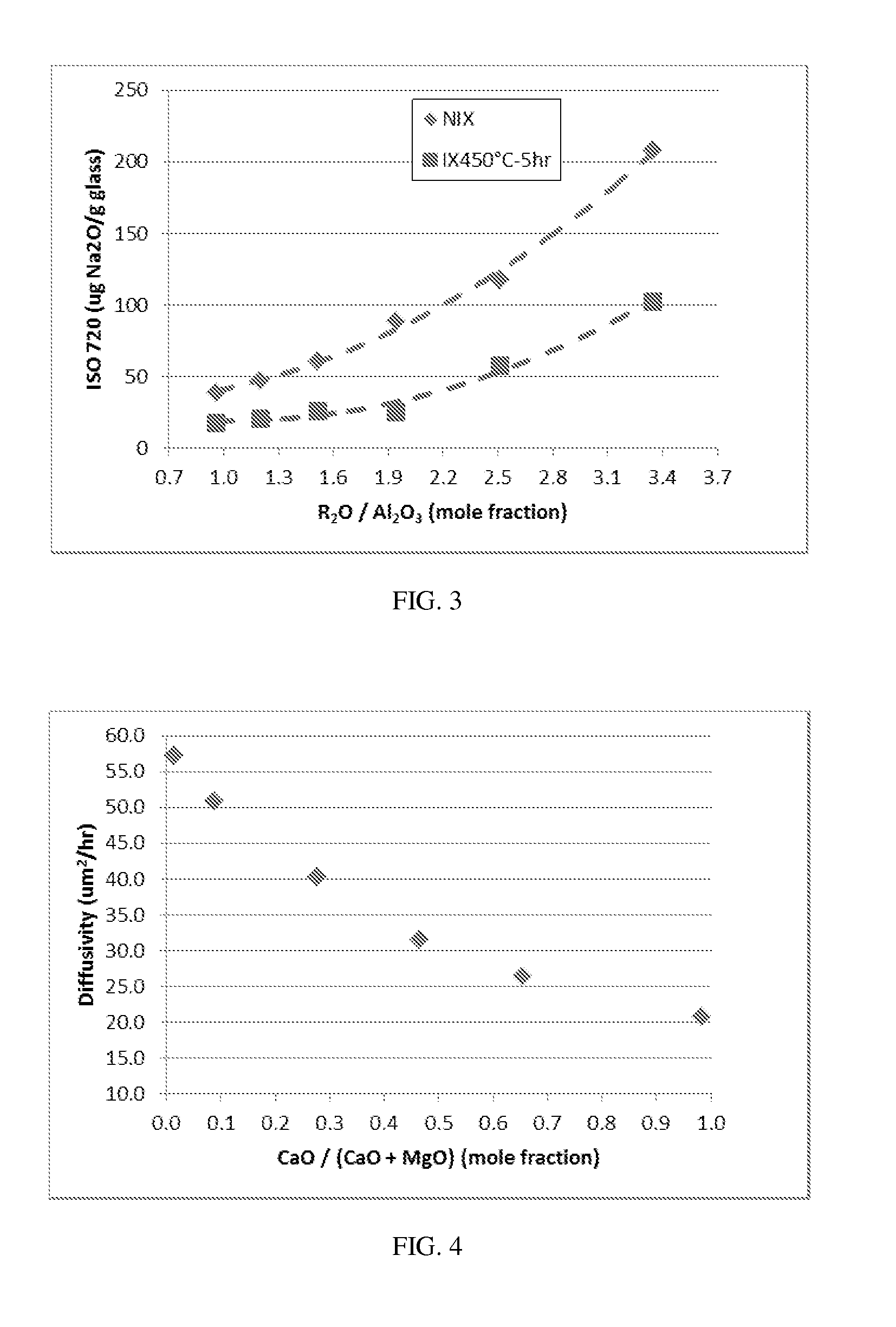
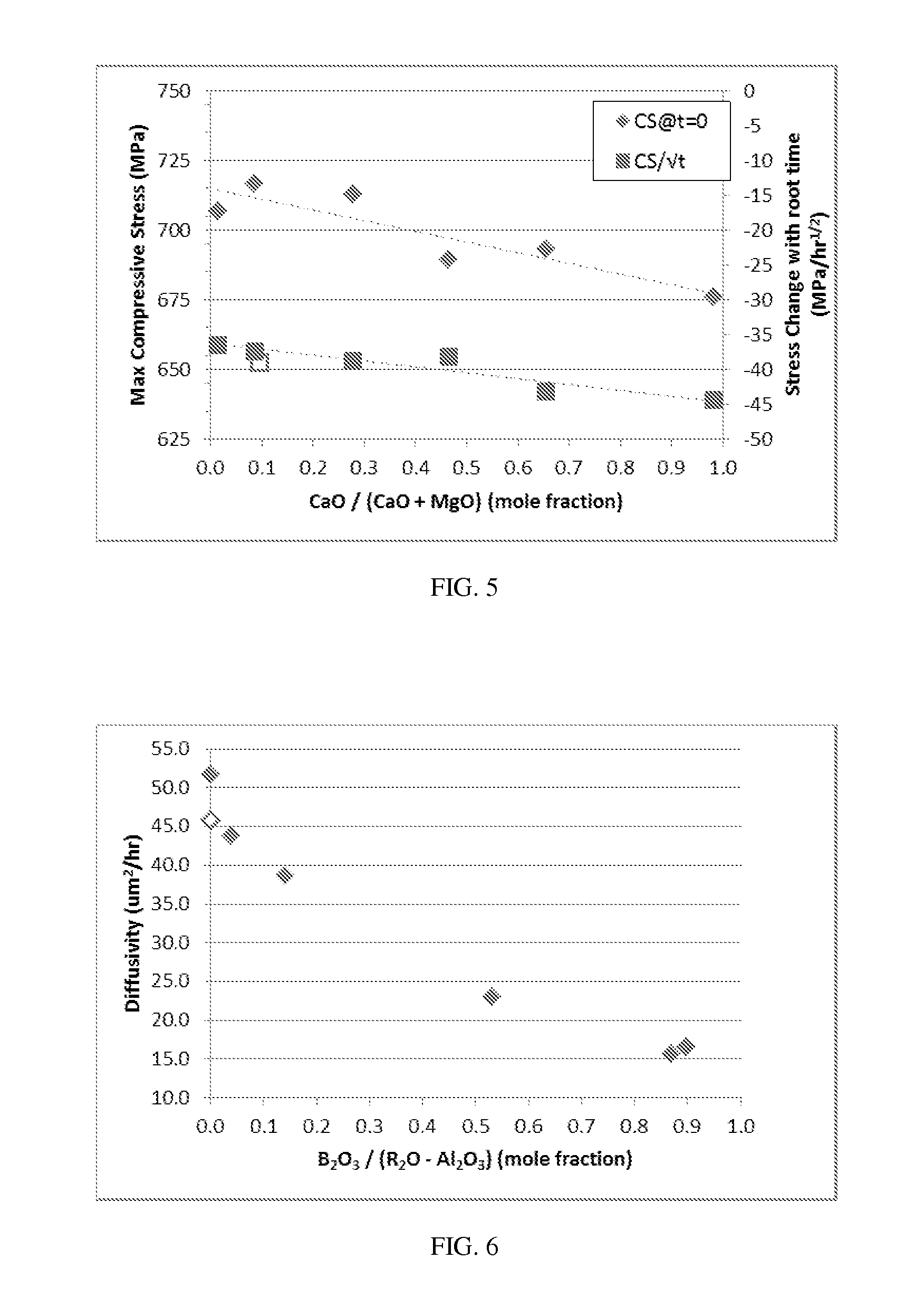
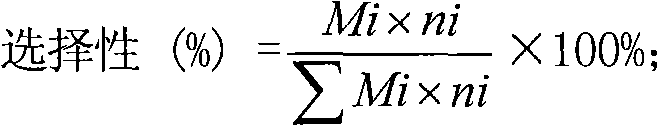Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1123 results about "Alkali metal oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The alkali metals react with oxygen to form several different compounds: suboxides, oxides, peroxides, superoxides, and ozonides. They all react violently with water.
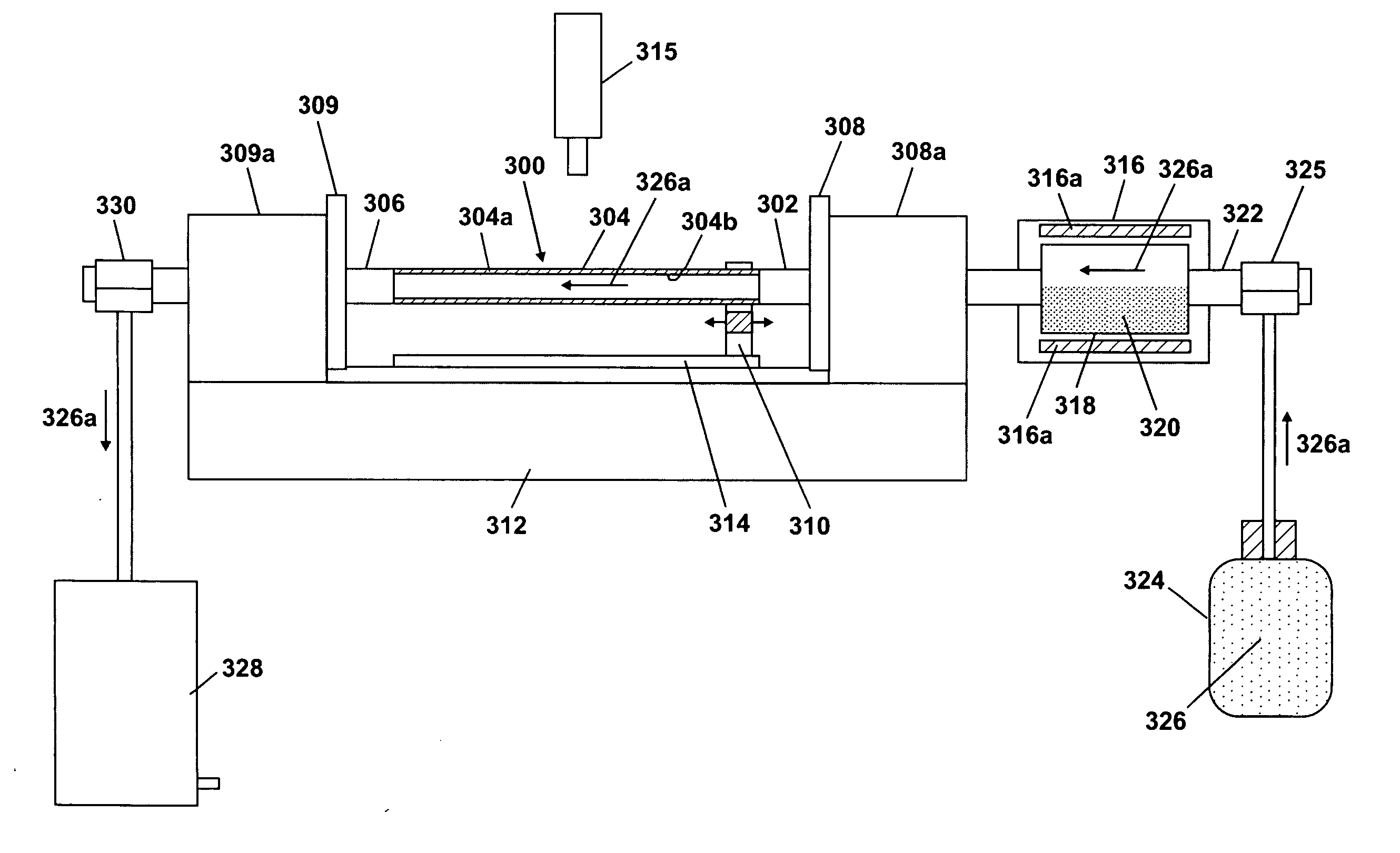
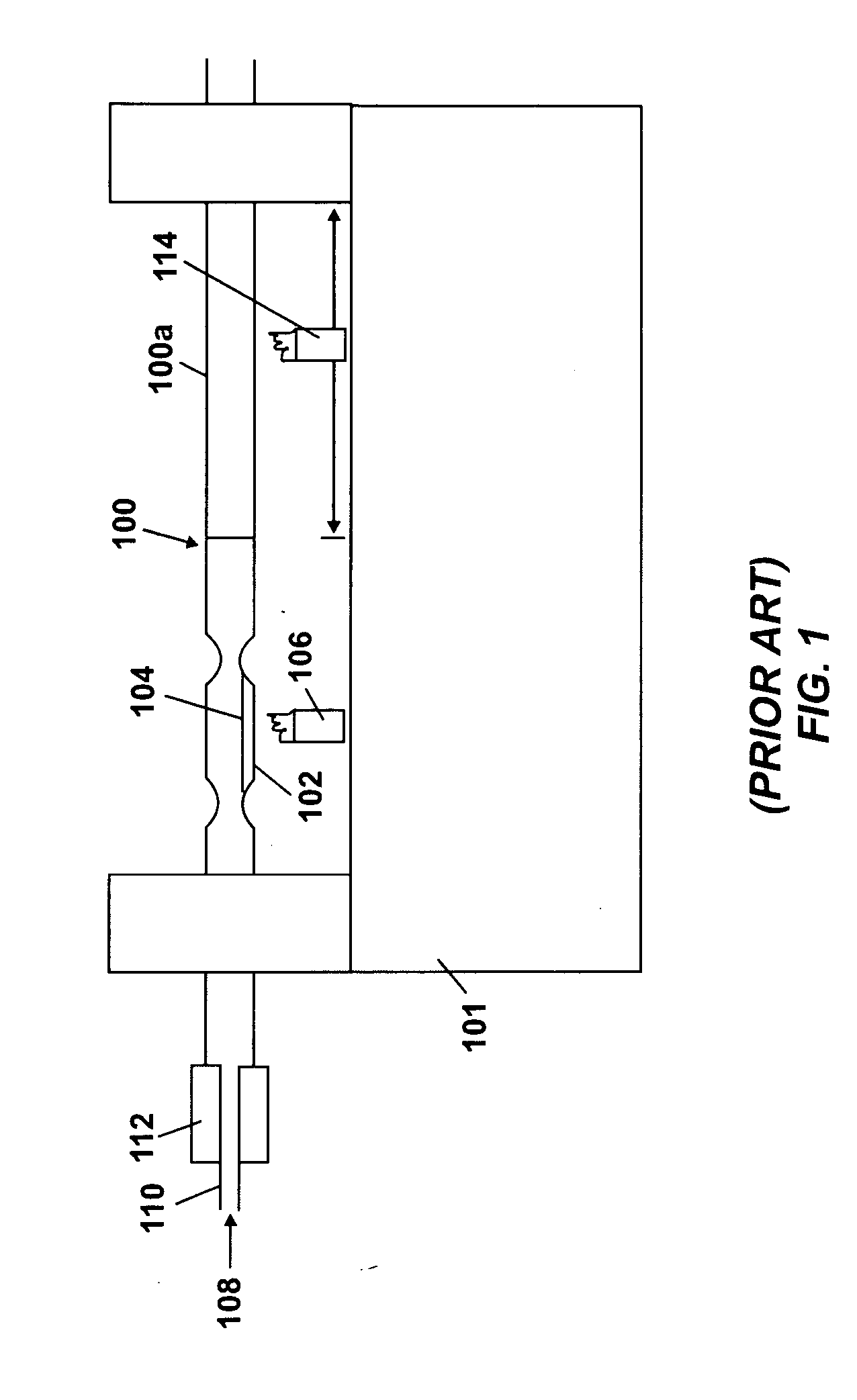
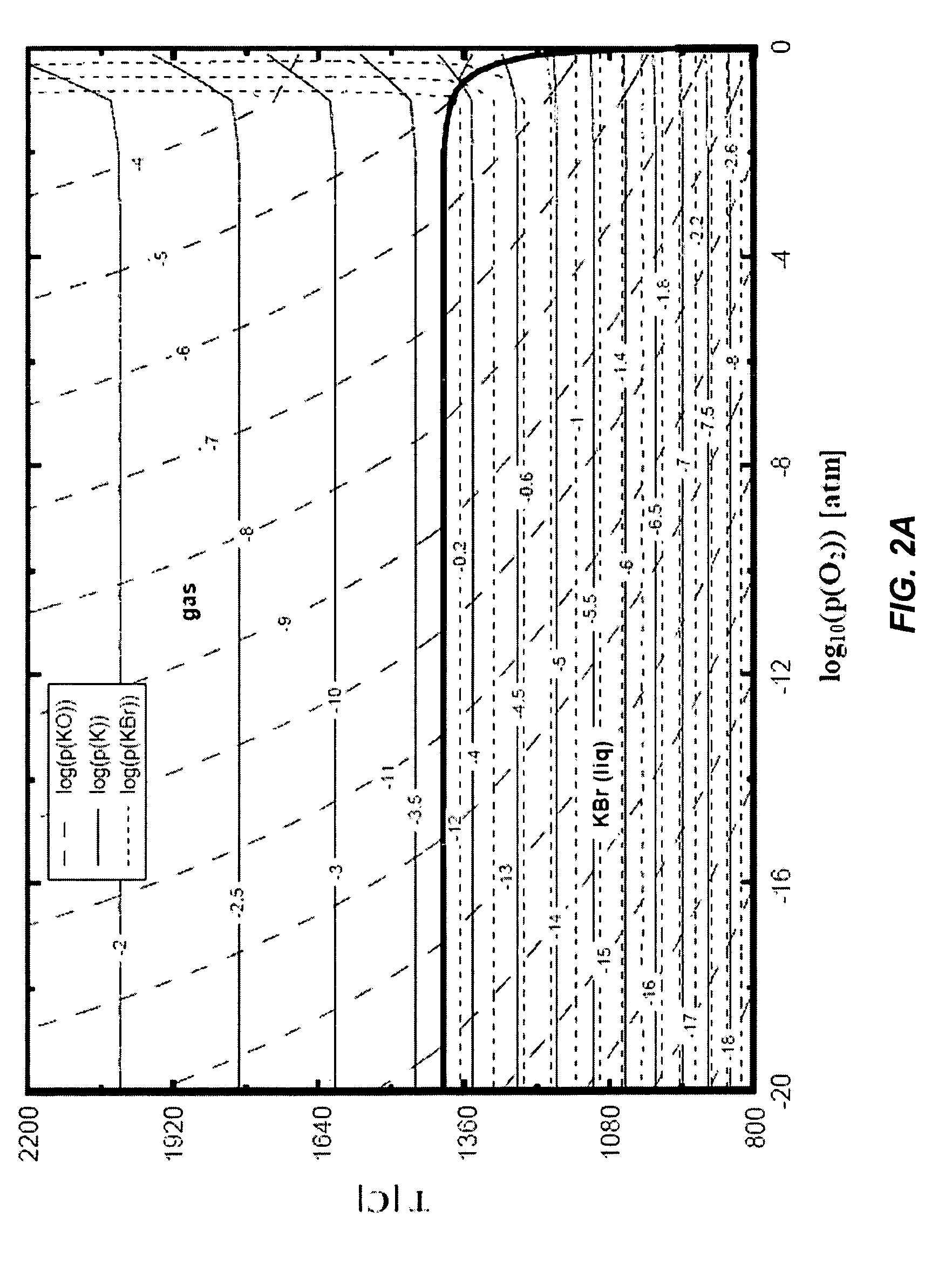
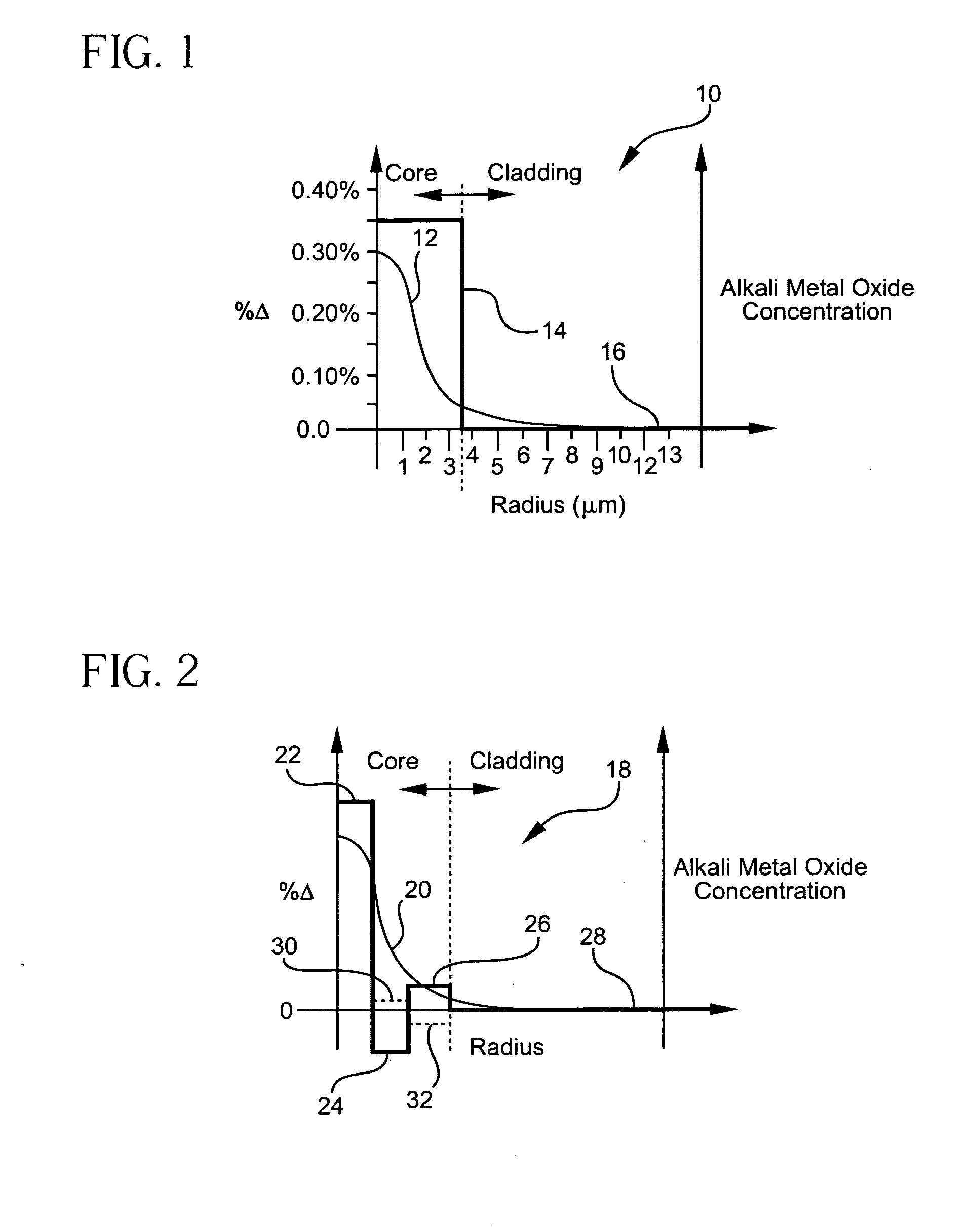
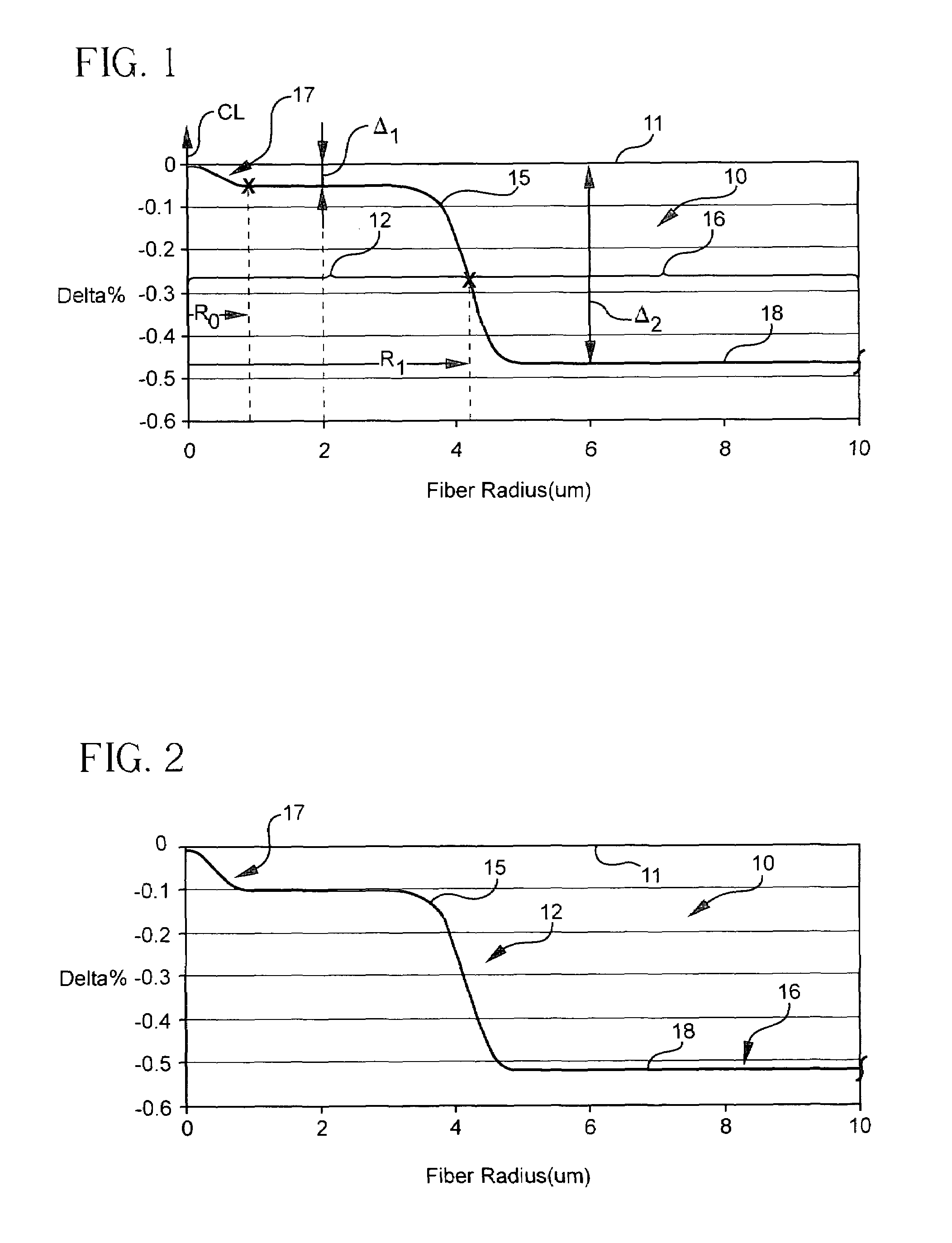
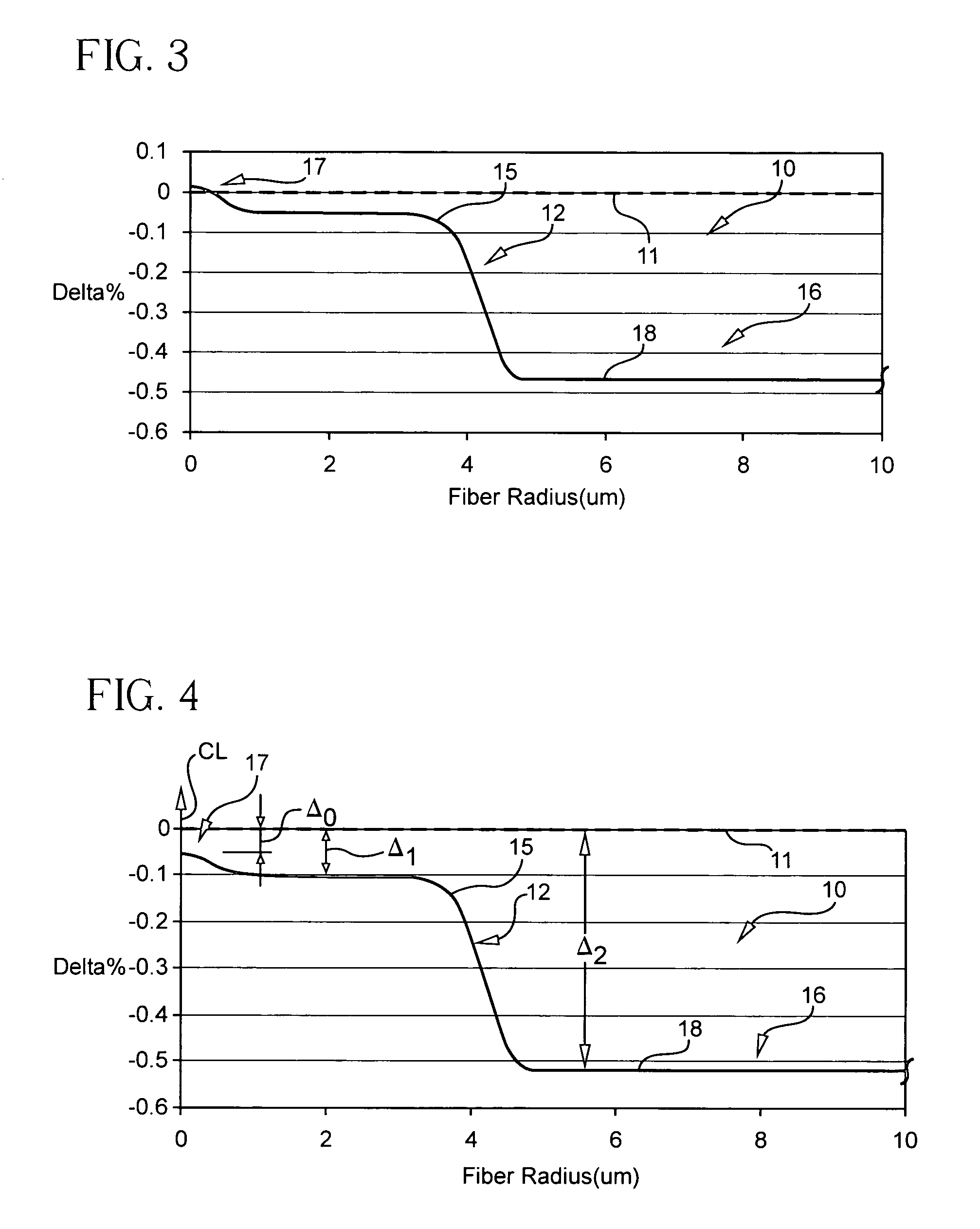
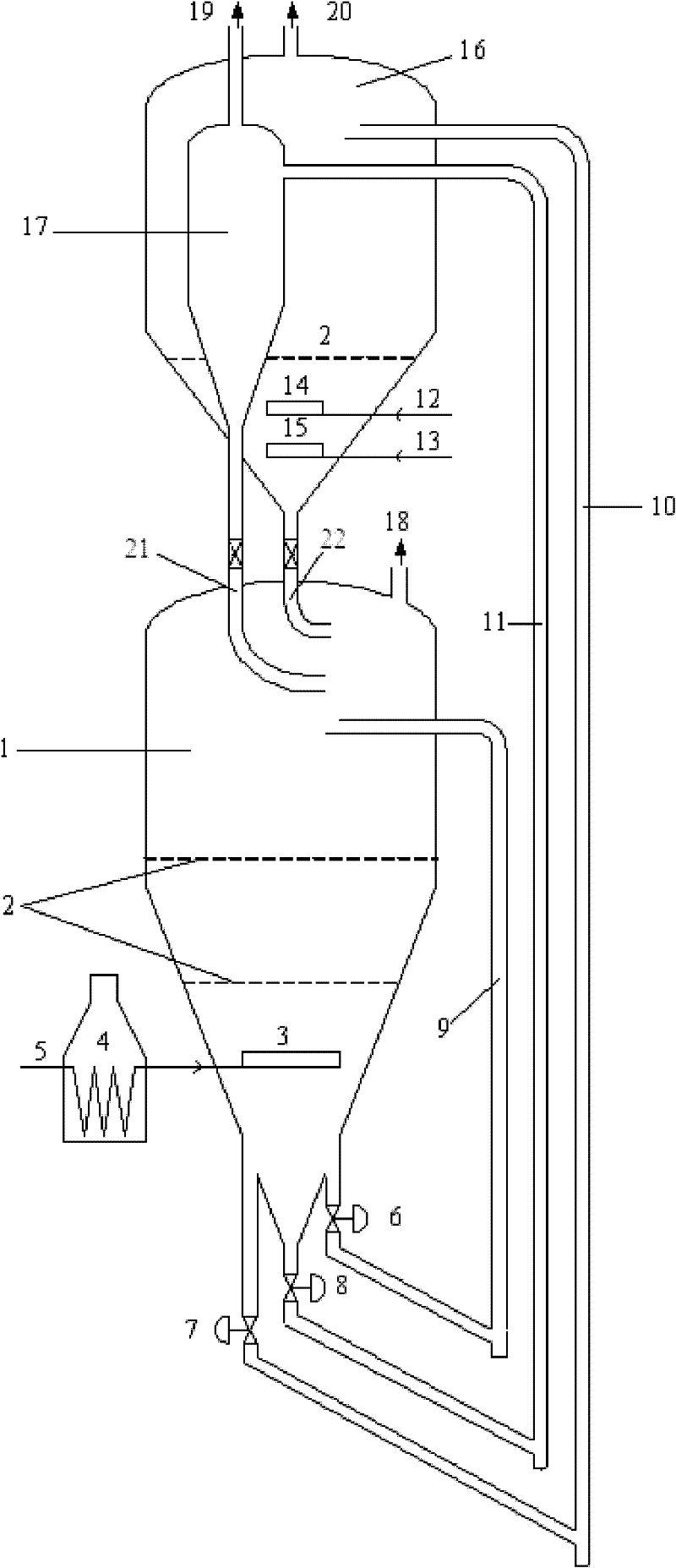
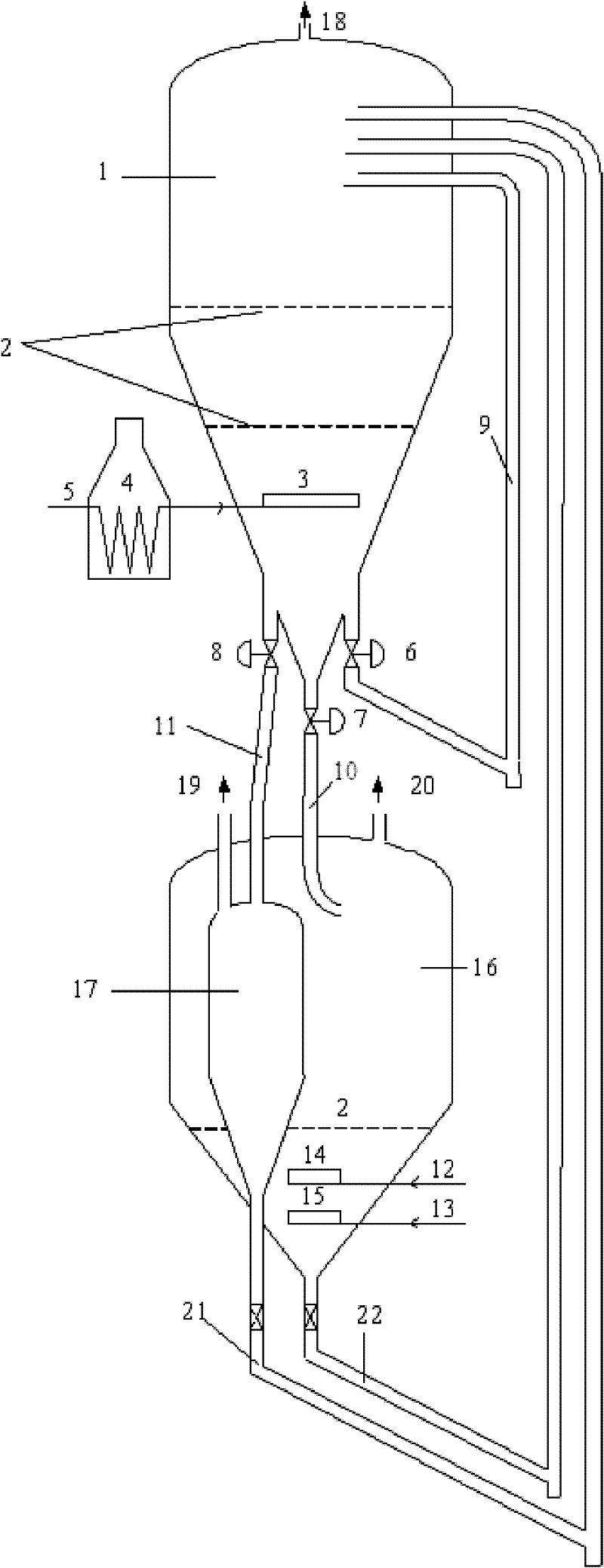
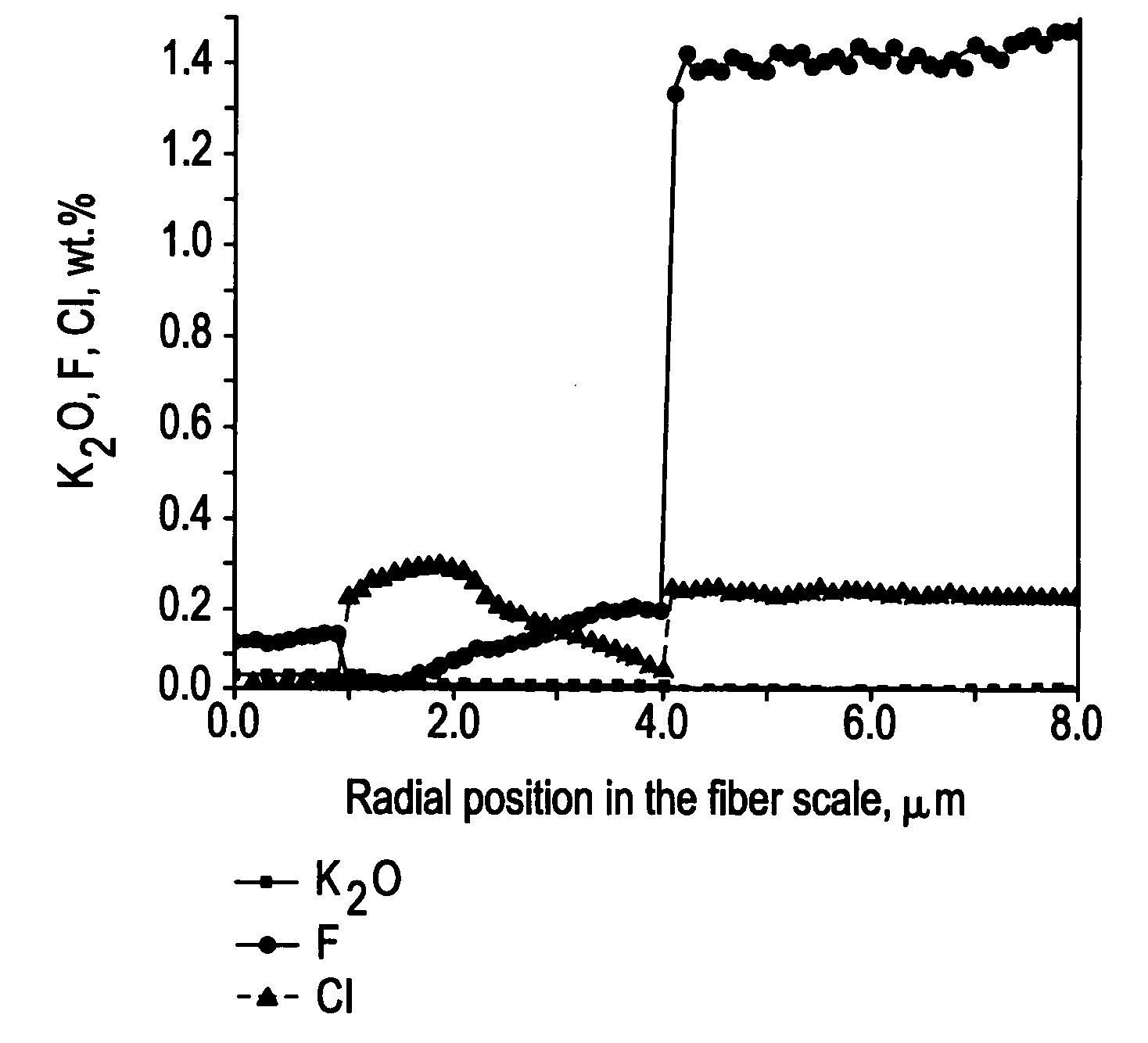
Method of doping silica glass with an alkali metal, and optical fiber precursor formed therefrom
A method of making an optical fiber precursor includes generating vapors from an alkali metal source comprising compound containing oxygen and one or more alkali metals and applying the vapors to a surface of a glass article comprising silica at a temperature that promotes diffusion of the alkali metal into the surface of the glass article. An optical fiber has a core comprising silica and an alkali metal oxide of the form X2O, where X is selected from the group consisting of K, Na, Li, Cs, and Rb, wherein a concentration of the alkali metal oxide along a length of the core is uniform.
Owner:CORNING INC
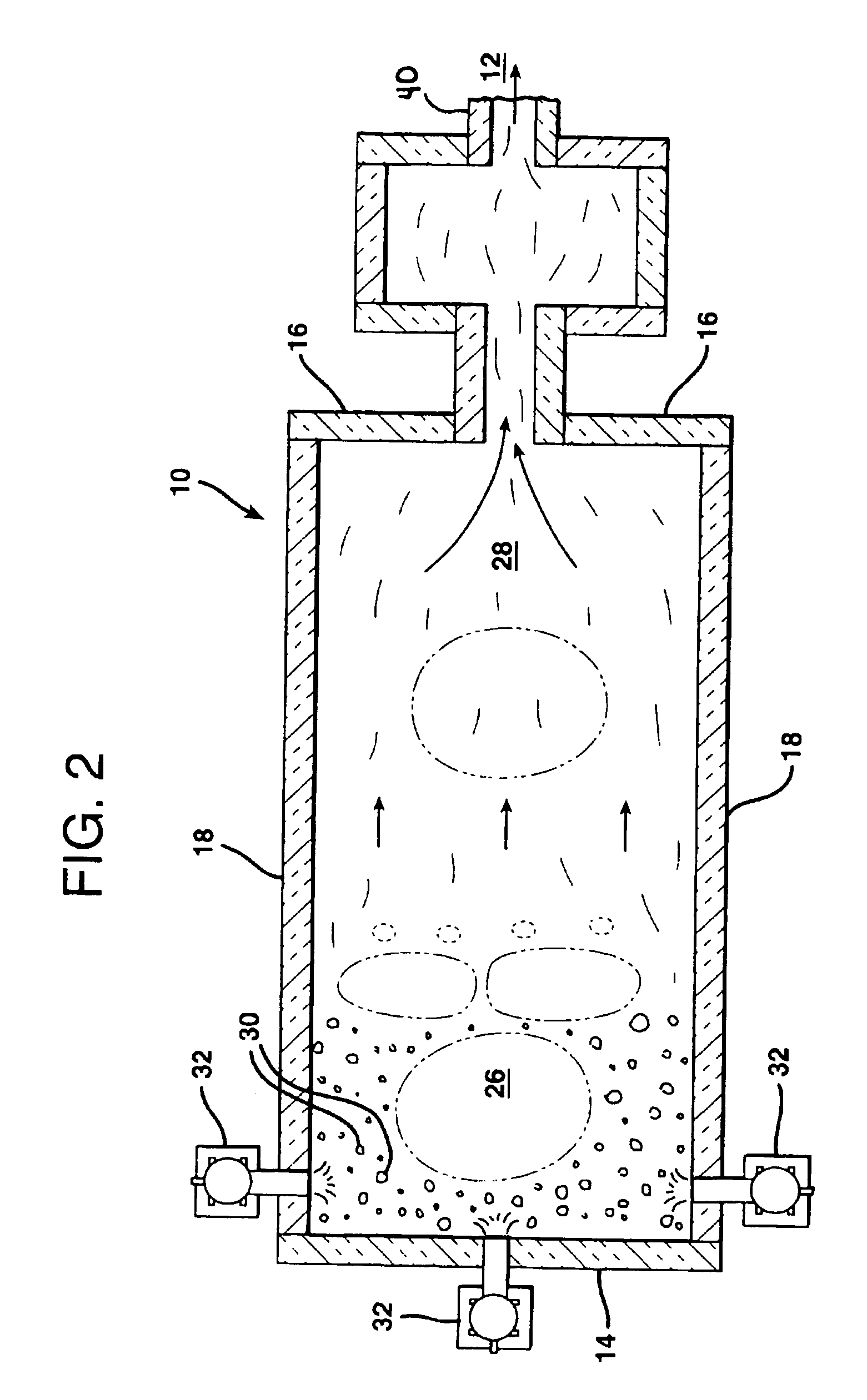
Optical fiber containing an alkali metal oxide and methods and apparatus for manufacturing same
ActiveUS20050063663A1Increase radiusReduce doping concentrationOptical fibre with graded refractive index core/claddingOptical fibre with multilayer core/claddingDopantAlkali metal oxide
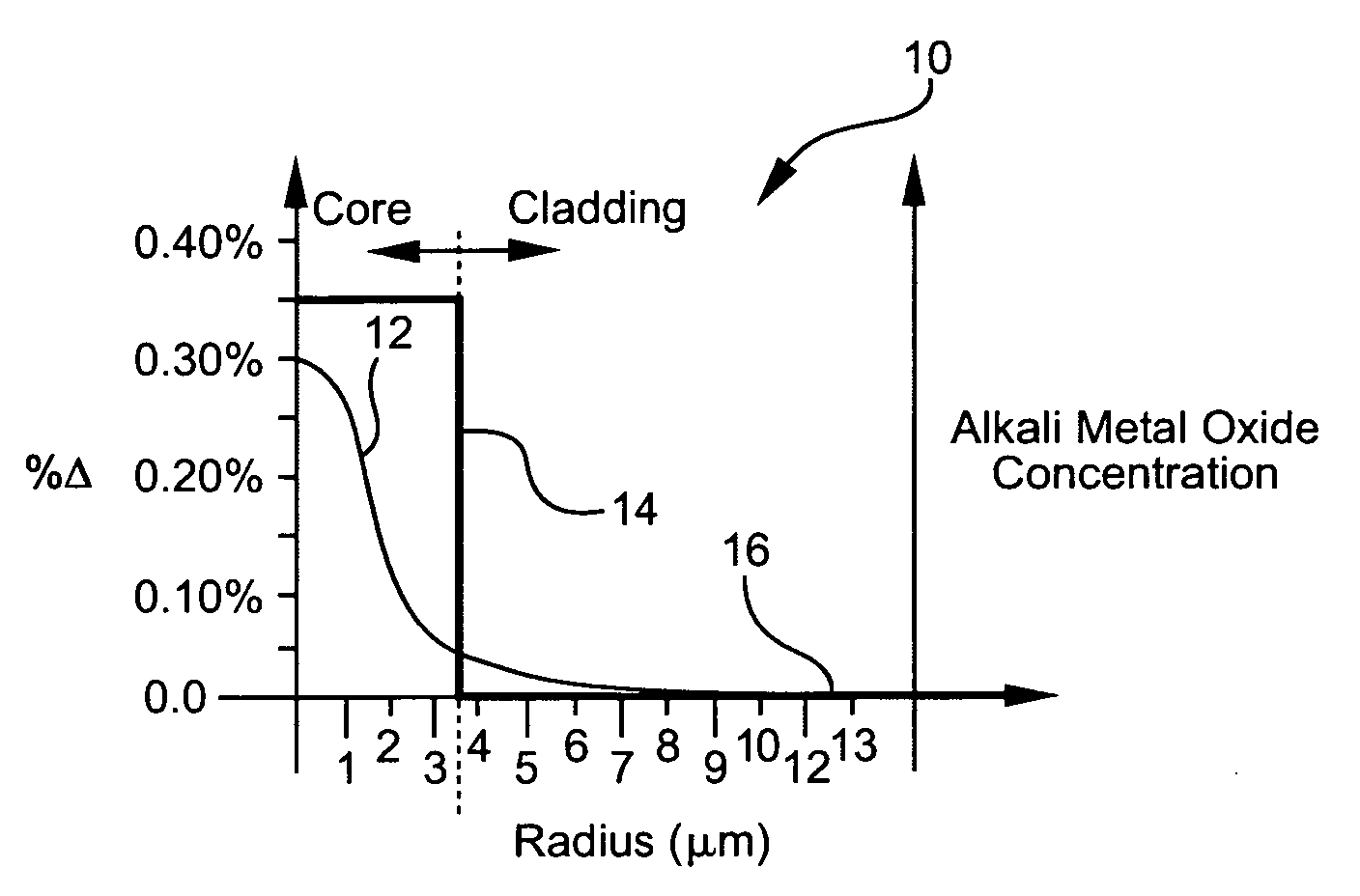
Disclosed is an optical fiber having a core with an alkali metal oxide dopant in an peak amount greater than about 0.002 wt. % and less than about 0.1 wt. %. The alkali metal oxide concentration varies with a radius of the optical fiber. By appropriately selecting the concentration of alkali metal oxide dopant in the core and the cladding, a low loss optical fiber may be obtained. Also disclosed are several methods of making the optical fiber including the steps of forming an alkali metal oxide-doped rod, and adding additional glass to form a draw perform. Preferably, the draw preform has a final outer dimension (d2), wherein an outer dimension (d1) of the rod is less than or equal to 0.06 times the final outer dimension (d2). In a preferred embodiment, the alkali metal oxide-doped rod is inserted into the centerline hole of a preform to form an assembly.
Owner:CORNING INC
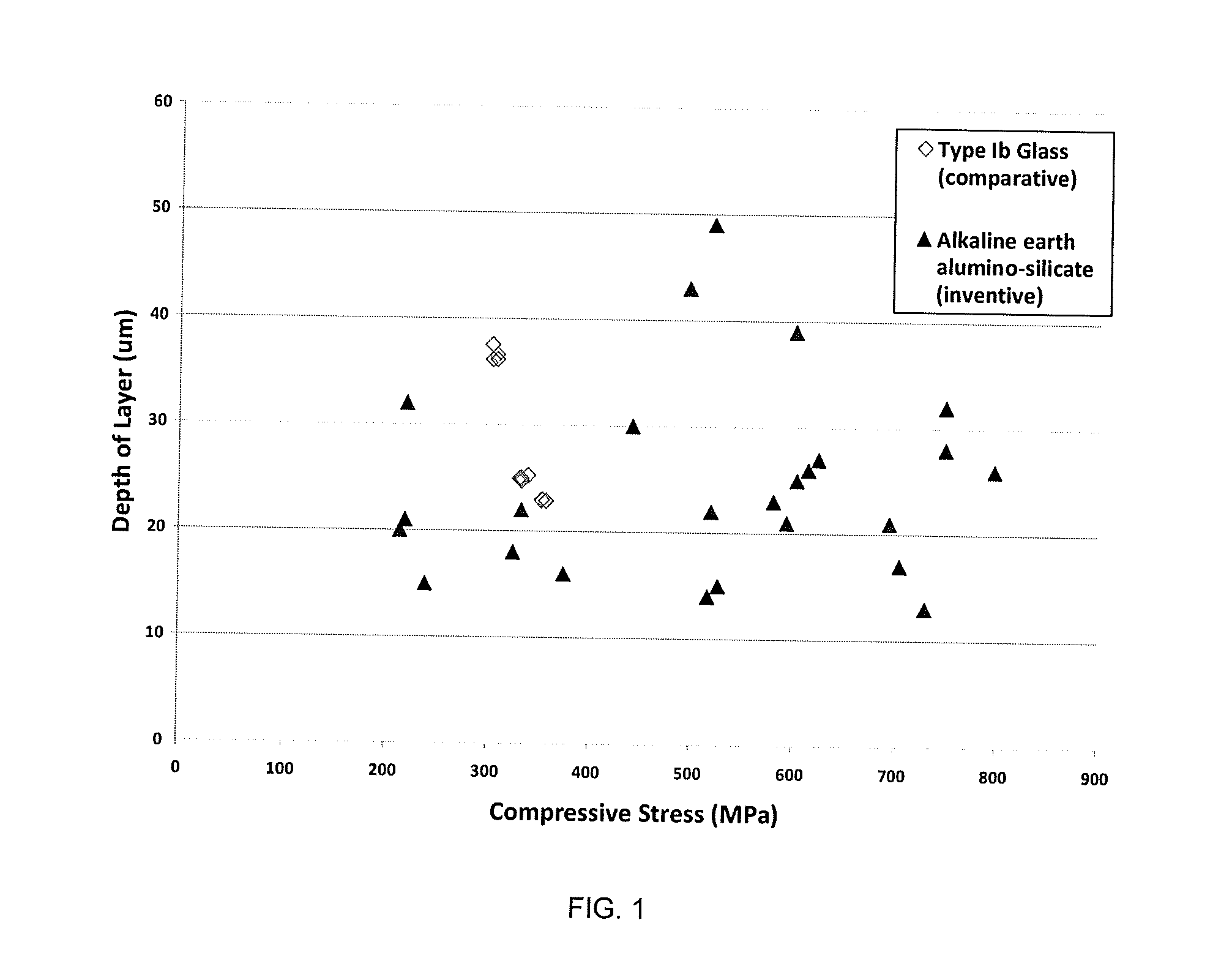
Alkaline earth alumino-silicate glass compositions with improved chemical and mechanical durability
Owner:CORNING INC
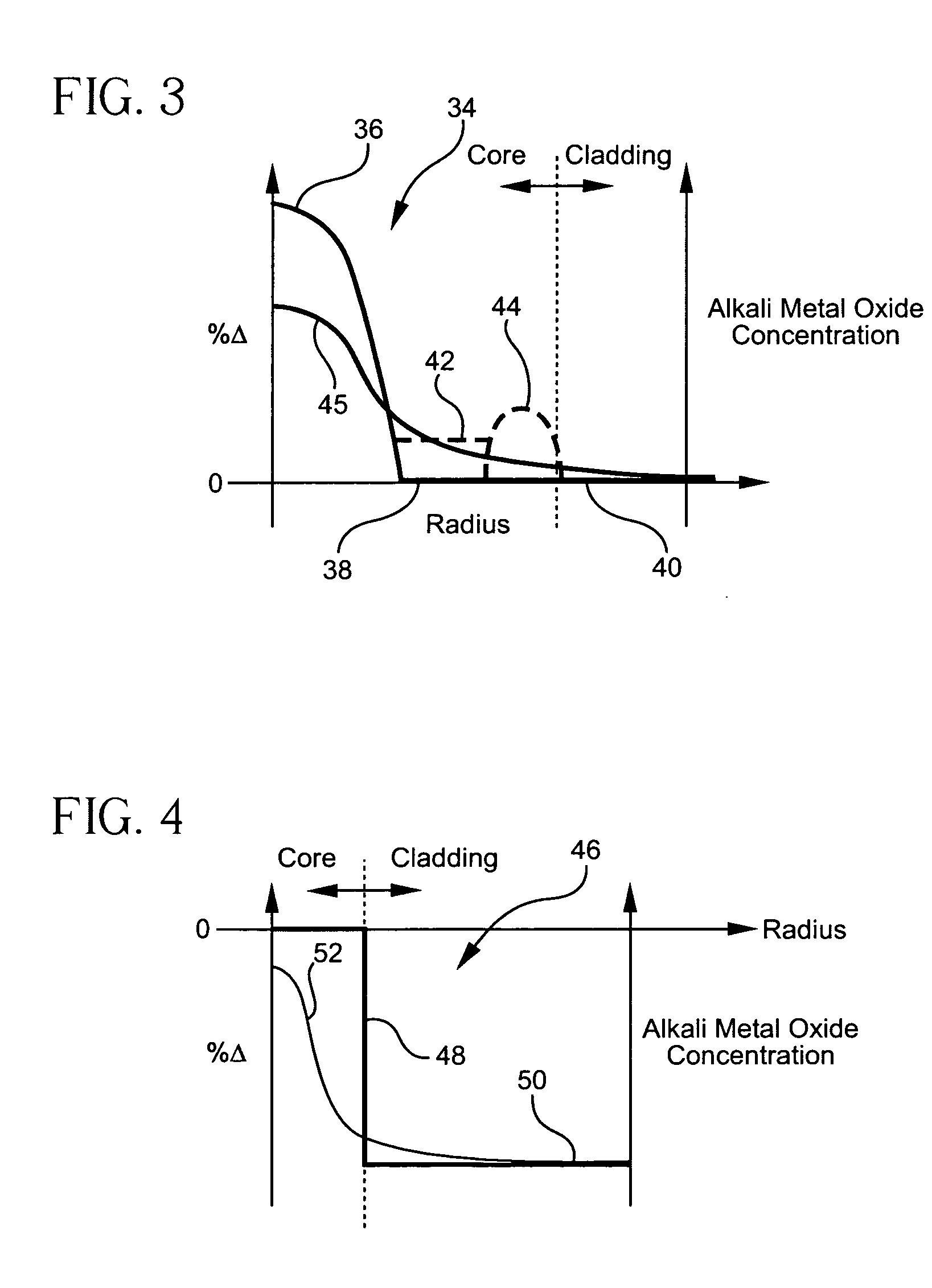
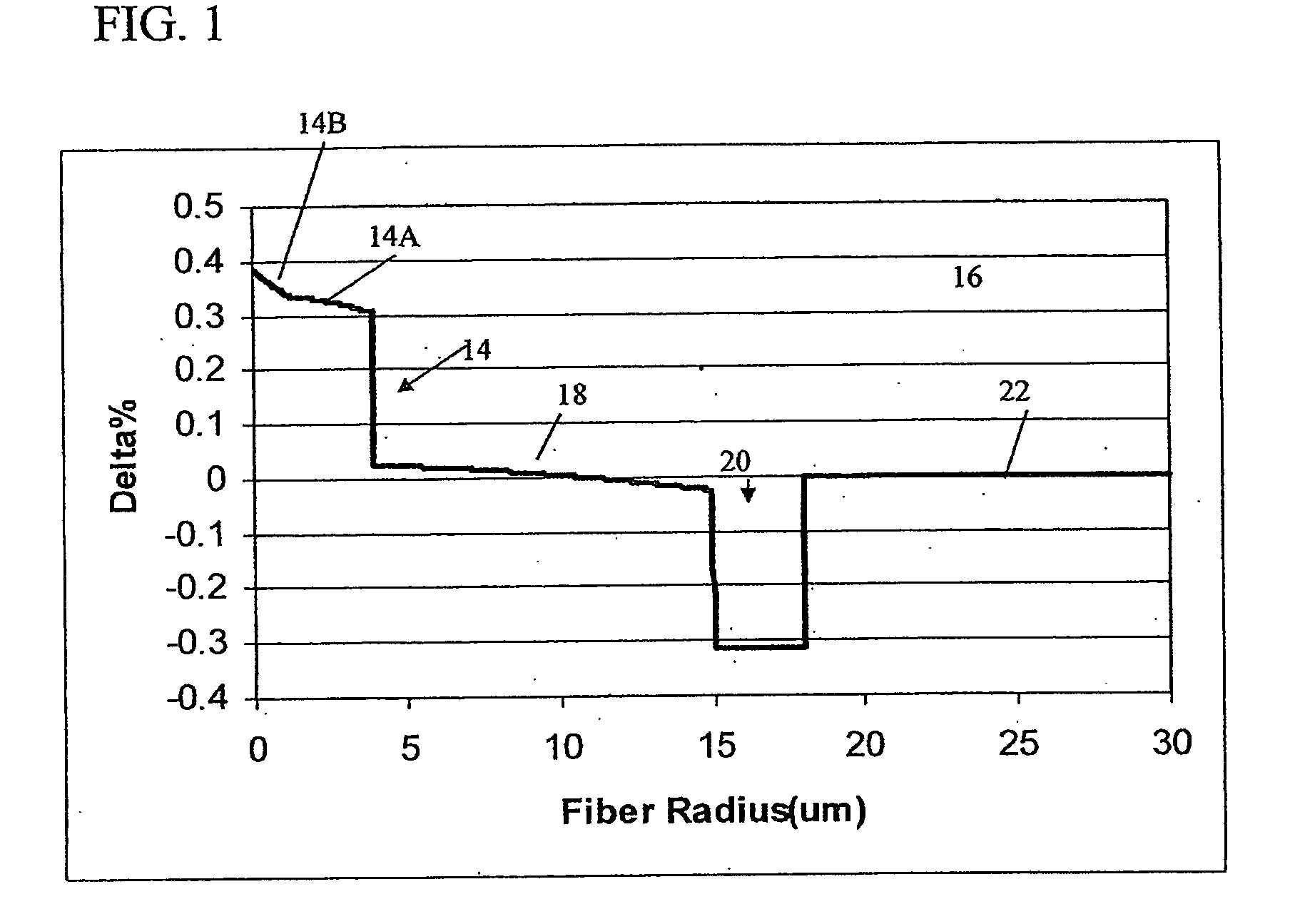
Alkali and fluorine doped optical fiber
ActiveUS7088900B1Reduce decreaseOptical fibre with graded refractive index core/claddingOptical fibre with multilayer core/claddingDopantSilica measurement
Disclosed is an optical fiber having a core of SiO2 doped with fluorine and an alkali metal oxide dopant. The alkali metal oxide is selected from the group consisting of K, Na, Li, Cs and Rb and is provided in amount of at least 20 ppm wt. %. The fiber has an inner cladding surrounding the core, which also includes fluorine. A relative refractive index of the inner cladding (Δ2%), measured relative to pure silica, is preferably between −0.39% and −0.7%. The fiber preferably exhibits attenuation at 1550 nm of less than or equal to 0.178 dB / km.
Owner:CORNING INC
Catalyst support and method of producing the same
ActiveUS7618919B2Improve adhesionImprove reforming performanceHydrogenCatalyst activation/preparationRare-earth elementAlkali metal oxide
A method of producing a catalyst support comprising a substrate, and coating formed on the surface of the substrate and including powder of a first metal oxide of at least one member selected from the group consisting of alumina, zirconia, titania, iron oxides, oxides of rare earth elements, alkali metal oxides and alkali earth metal oxides, wherein the coating is obtained by heat treating the substrate after applied with a coating composition obtained by mixing the first metal oxide powder together with a fluid raw material composition containing raw material of a second metal oxide of at least one member selected from the group consisting of alumina, zirconia, titania, iron oxides, oxides of rare earth elements, alkali metal oxides and alkali earth metal oxides, at a shear rate of 1000 sec−1 or higher.
Owner:TOYOTA CENT RES & DEV LAB INC
Glass compositions with improved chemical and mechanical durability
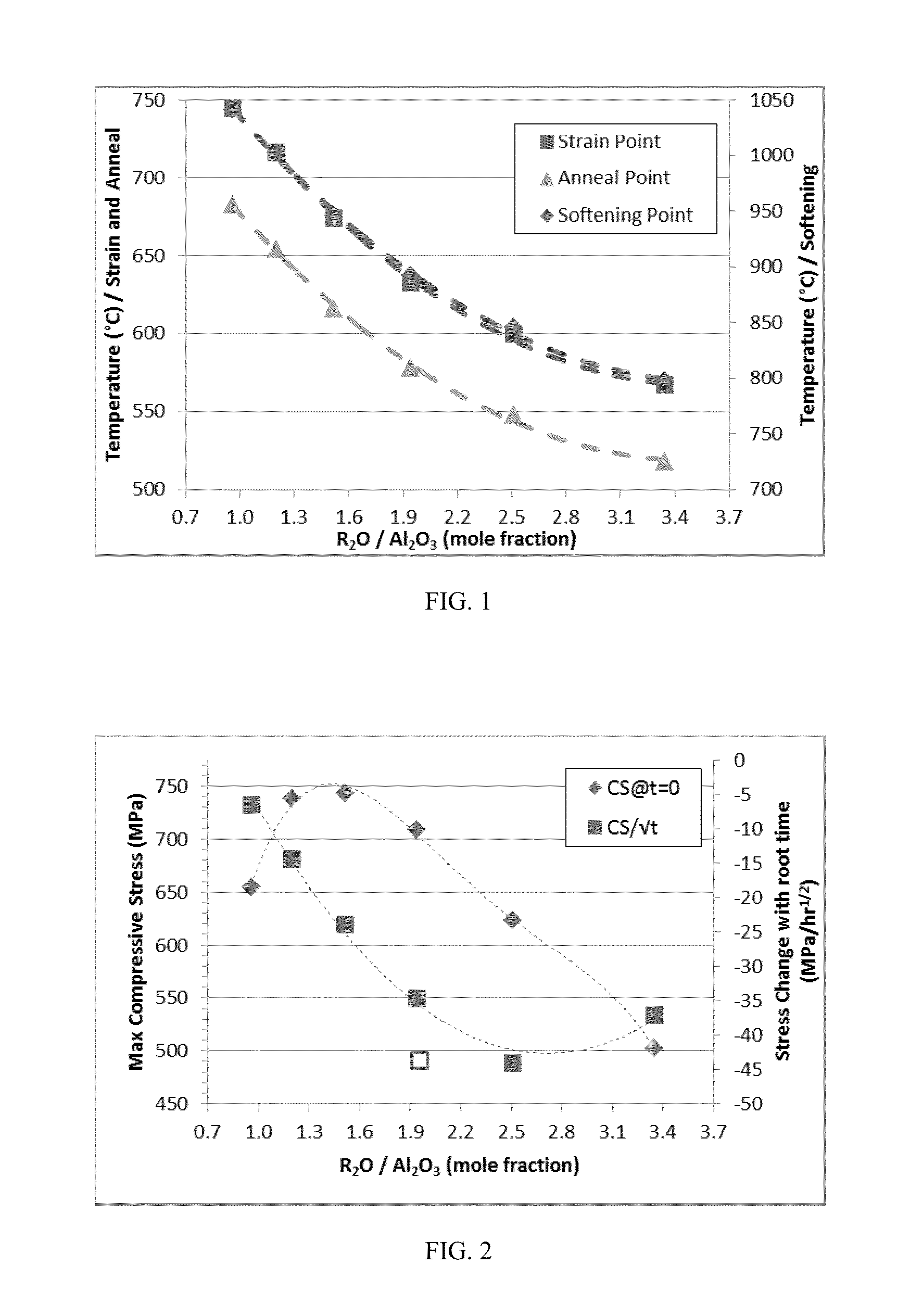
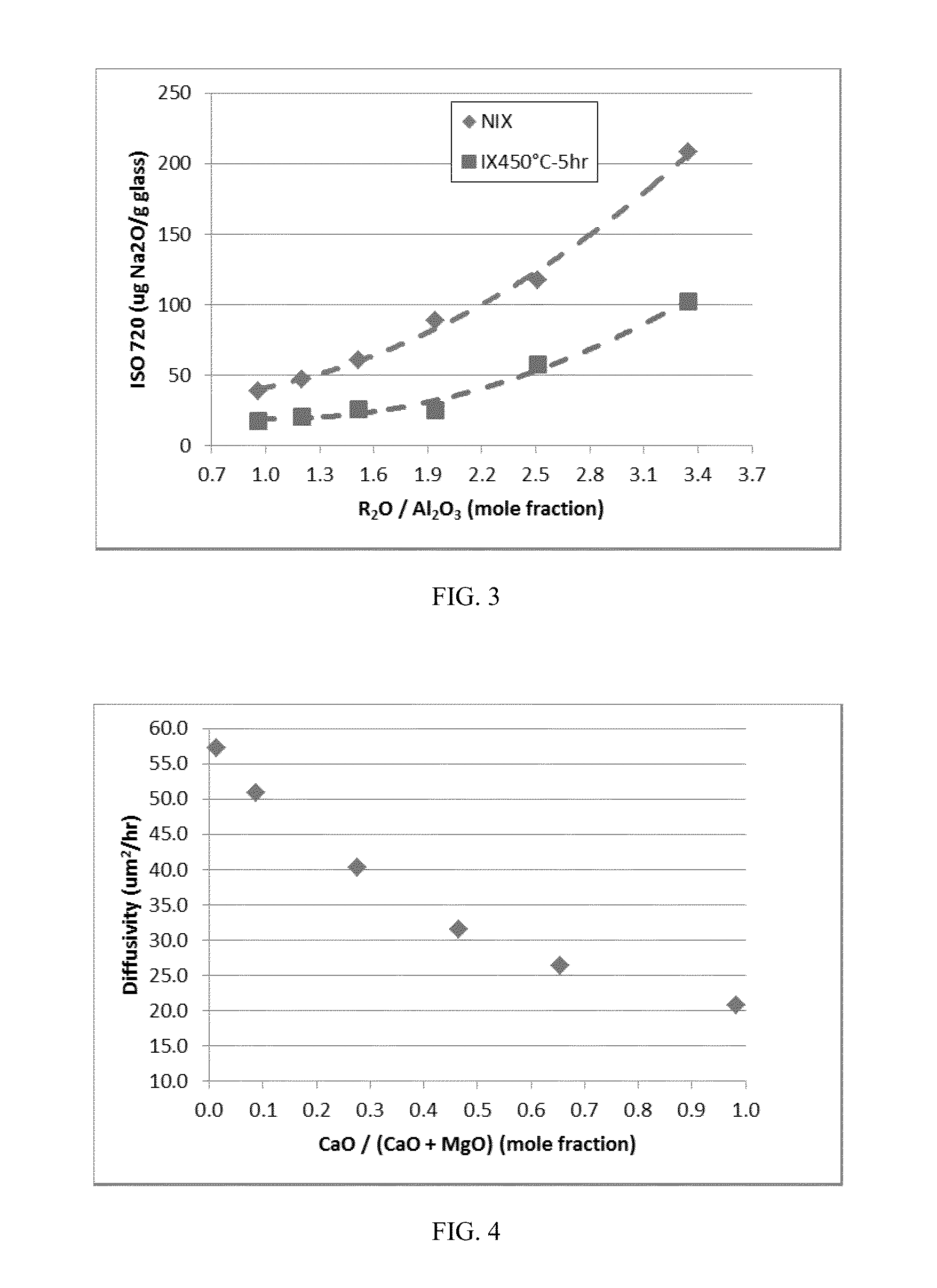
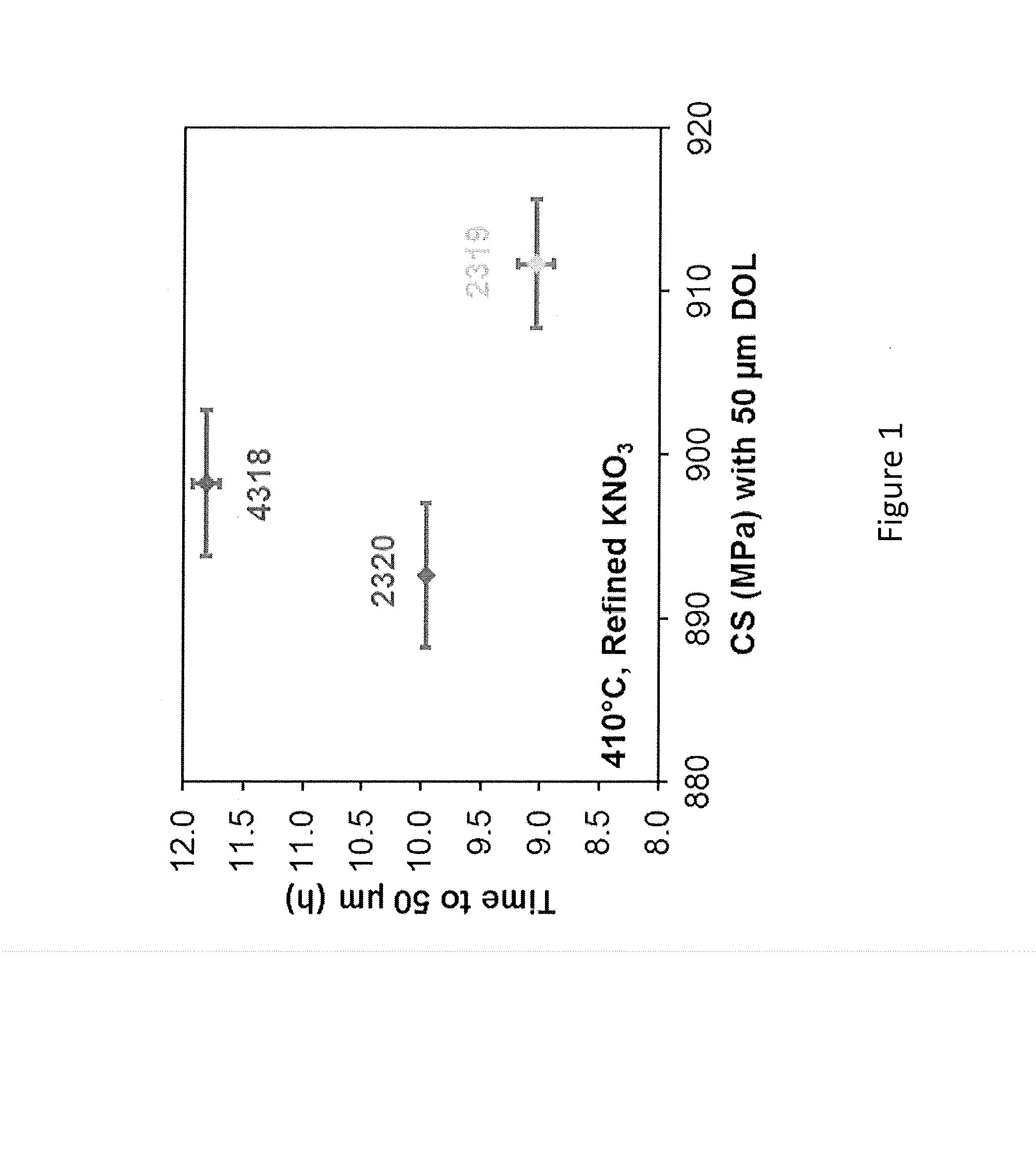
The embodiments described herein relate to chemically and mechanically durable glass compositions and glass articles formed from the same. In another embodiment, a glass composition may include from about 70 mol. % to about 80 mol. % SiO2; from about 3 mol. % to about 13 mol. % alkaline earth oxide; X mol. % Al2O3; and Y mol. % alkali oxide. The alkali oxide may include Na2O in an amount greater than about 8 mol. %. A ratio of Y:X may be greater than 1 and the glass composition may be free of boron and compounds of boron. In some embodiments, the glass composition may also be free of phosphorous and compounds of phosphorous. Glass articles formed from the glass composition may have at least a class S3 acid resistance according to DIN 12116, at least a class A2 base resistance according to ISO 695, and a type HGA1 hydrolytic resistance according to ISO 720.
Owner:CORNING INC
Composition for high performance glass, high performance glass fibers and articles therefrom
ActiveUS20080009403A1Inexpensively formed into glass fiberReduce the temperatureReaction enginesGlass fiberFiber
Glass batch compositions for the formation of high-modulus, and high-strength glass fibers as well as fibers suitable for use as textile and reinforcements are disclosed. Fibers formed of the composition are especially suitable for use in high-strength, low-weight applications such as windmill blades and high strength and modulus applications where strength and stiffness are required in the composite. The glass composition is up to about 70.5 weight % SiO2, about 24.5 weight % Al2O3, about 22 weight % alkaline earth oxides and may include small amounts of alkali metal oxides and ZrO2. Fiberglass-reinforced composite articles such as windmill blades are also disclosed.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
Glass Articles with Improved Chemical and Mechanical Durability
InactiveUS20130101764A1Pharmaceutical containersMedical packagingAlkali metal oxideAlkaline earth oxides
A glass article may formed from a glass composition that may include from about 70 mol. % to about 78 mol. % SiO2, from about 3 mol. % to about 13 mol. % alkaline earth oxide, X mol. % Al2O3, and Y mol. % alkali oxide. The alkali oxide may include Na2O in an amount greater than or equal to about 9 mol. % and less than or equal to about 15 mol. %. The ratio of Y:X may be greater than 1. The glass article may be free of boron and compounds of boron. The glass article may have a compressive stress layer with a compressive stress greater than or equal to about 250 MPa and depth of layer greater than or equal to about 25 μm. The glass article may have at least a type HGA2 hydrolytic resistance according to the ISO 720 standard.
Owner:CORNING INC
Zircon compatible, ion exchangeable glass with high damage resistance
An ion exchangeable glass having a high degree of resistance to damage caused by abrasion, scratching, indentation, and the like. The glass comprises alumina, B2O3, and alkali metal oxides, and contains boron cations having three-fold coordination. The glass, when ion exchanged, has a Vickers crack initiation threshold of at least 10 kilogram force (kgf).
Owner:CORNING INC
Method of manufacturing high performance glass fibers in a refractory lined melter and fiber formed thereby
ActiveUS20070105701A1Inexpensively formed into glass fiberUse in some applicationCharging furnaceGlass furnace apparatusFiberAlkali metal oxide
A method of forming high strength glass fibers in a refractory lined glass melter is disclosed. The refractory fined melter is suited to the batch compositions disclosed for the formation high modulus, and high-strength glass fibers. The glass composition for use in the method of the present invention is up to about 70.5 Weight % SiO2, 24.5 weight % Al2O3, 22 weight % alkaline earth oxides and may include small amounts of alkali metal oxides and ZrO2. Oxide based refractories included alumina, chromic oxide, silica, alumina-silica, zircon, zirconia-alumina-silica and combinations thereof. By using oxide based refractory lined furnaces the cost of production of glass fibers is substantially reduced in comparison with the cost of fibers using a platinum lined melting furnace. Fibers formed by the present invention are also disclosed.
Owner:OWENS CORNING INTELLECTUAL CAPITAL LLC
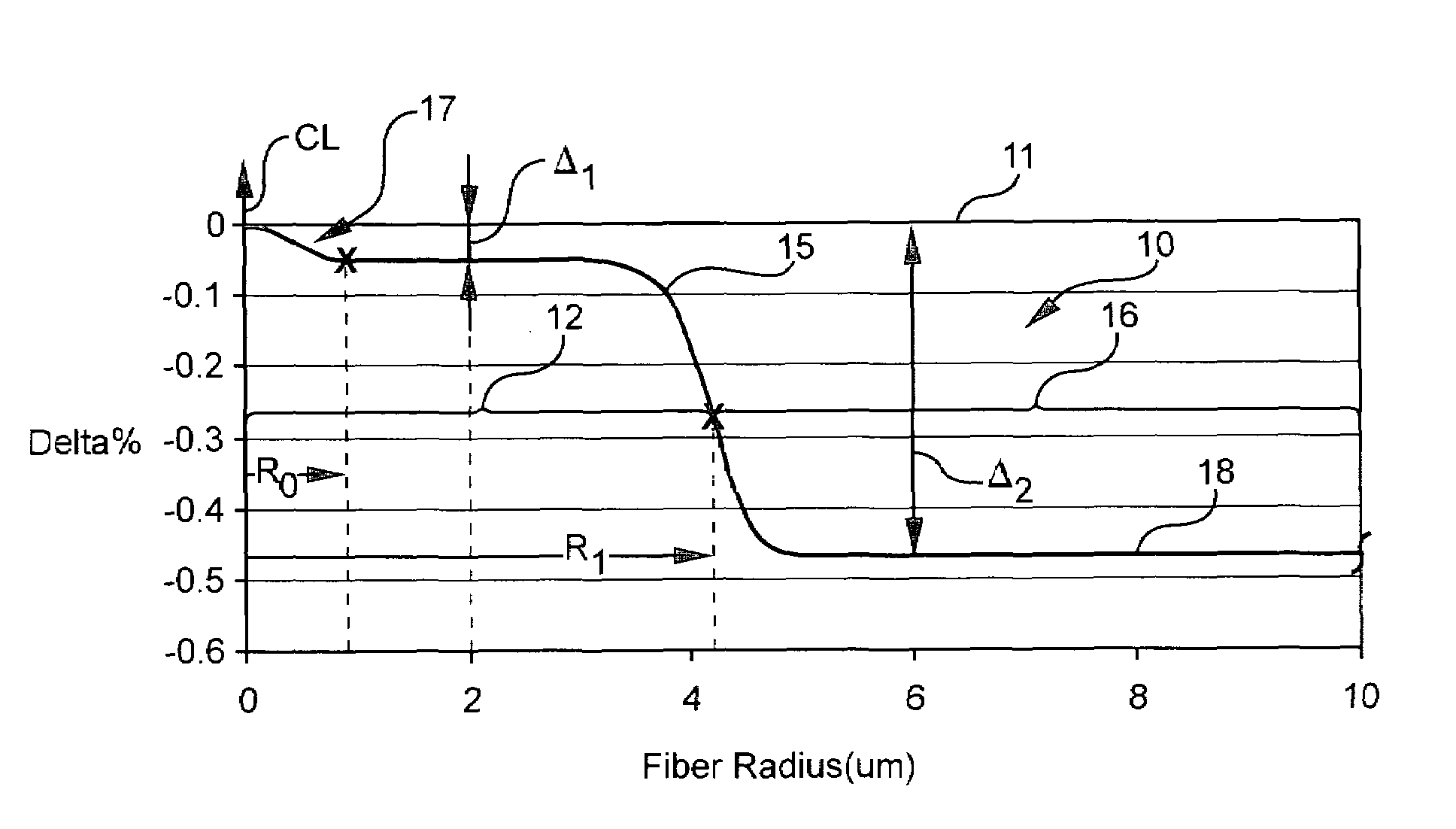
Optical fiber containing alkali metal oxide
InactiveUS20080279515A1Bending loss can be improvedGlass making apparatusOptical fibre with multilayer core/claddingDopantZero-dispersion wavelength
Disclosed is an optical fiber having a silica-based core comprising an alkali metal oxide a silica-based core, said core comprising an alkali metal oxide selected from the group consisting of K2O, Na2O, LiO2, Rb2O, Cs2O and mixtures thereof in an average concentration in said core between about 10 and 10000 ppm by weight, and a silica-based cladding surrounding and directly adjacent the core, the cladding including a region having a lower index of refraction than the remainder of such cladding. By appropriately selecting the concentration of alkali metal oxide dopant in the core and the cladding, a low loss optical fiber may be obtained which exhibits a cable cutoff less than 1400 nm chromatic dispersion at 1550 nm between about 13 and 19 ps / nm / km, and a zero dispersion wavelength less than about 1324 nm.
Owner:CORNING INC
Using nanoparticles for water flow control in subterranean formations
ActiveUS8053397B2Inhibiting and preventing flow of waterAvoid flowFlushingDrilling compositionHigh concentrationAlkali metal oxide
Non-aqueous carrier fluids containing nano-sized particles in high concentration are effective for zone isolation and flow control in water shutoff applications for subterranean formations. The nanoparticles interact with water and solidify it to inhibit its flow, but do not have the same effect on hydrocarbons and thus selectively assist the production of hydrocarbons while suppressing water. Suitable nanoparticles include alkaline earth metal oxides, alkaline earth metal hydroxides, alkali metal oxides, alkali metal hydroxides, transition metal oxides, transition metal hydroxides, post-transition metal oxides, post-transition metal hydroxides, piezoelectric crystals, and / or pyroelectric crystals.
Owner:BAKER HUGHES INC
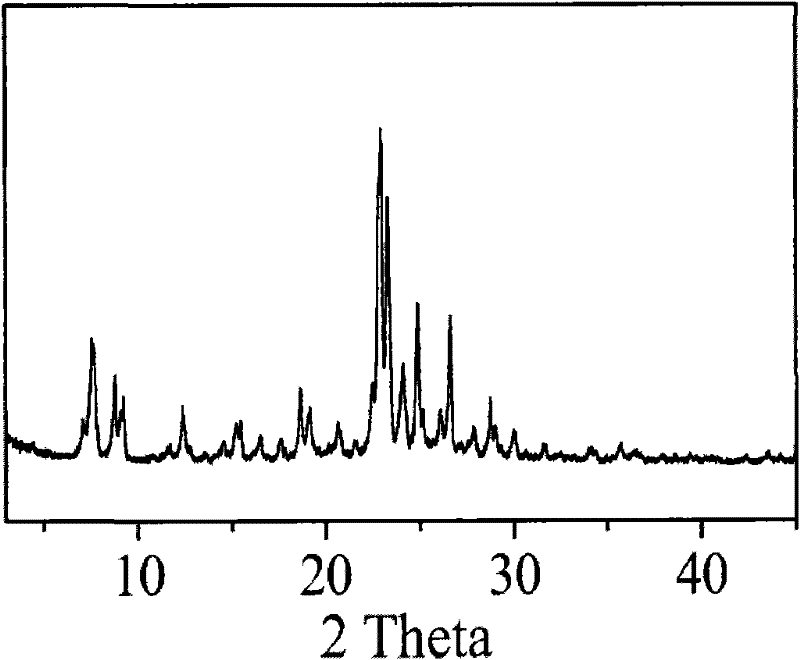
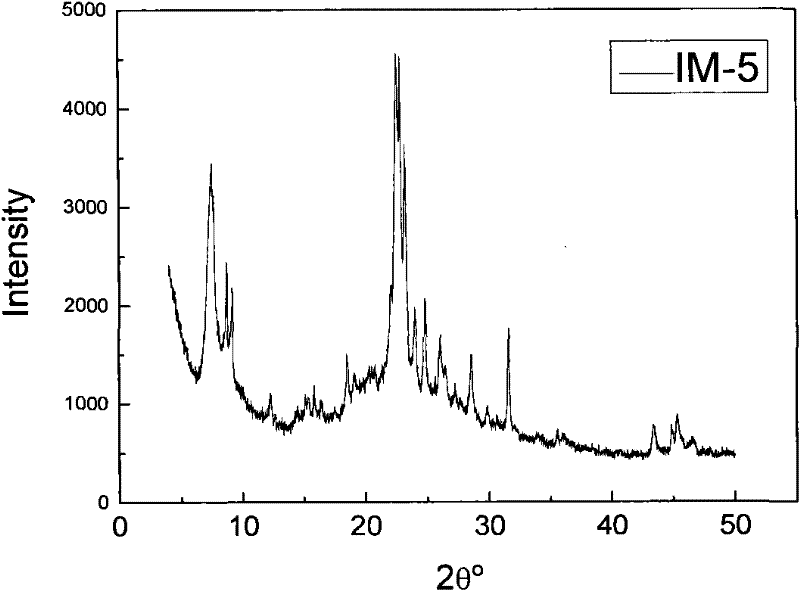
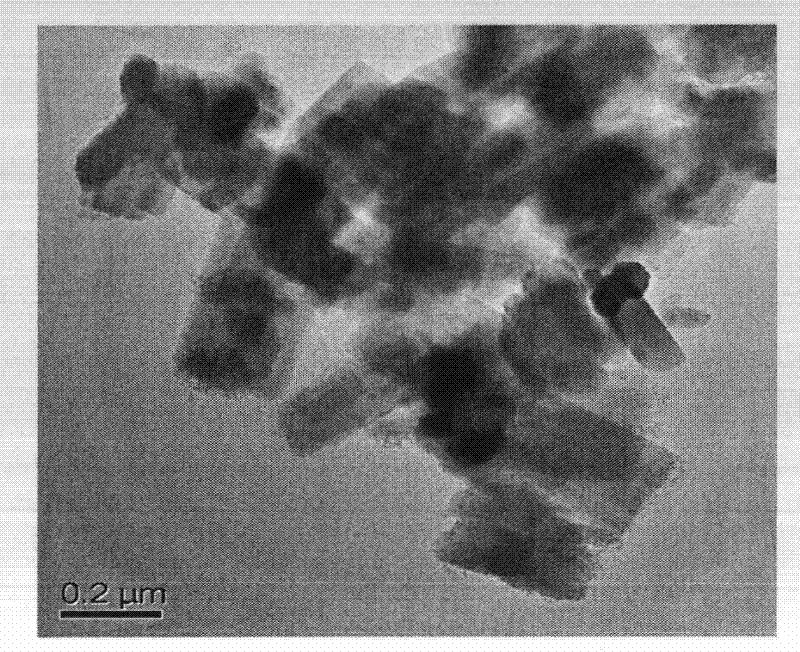
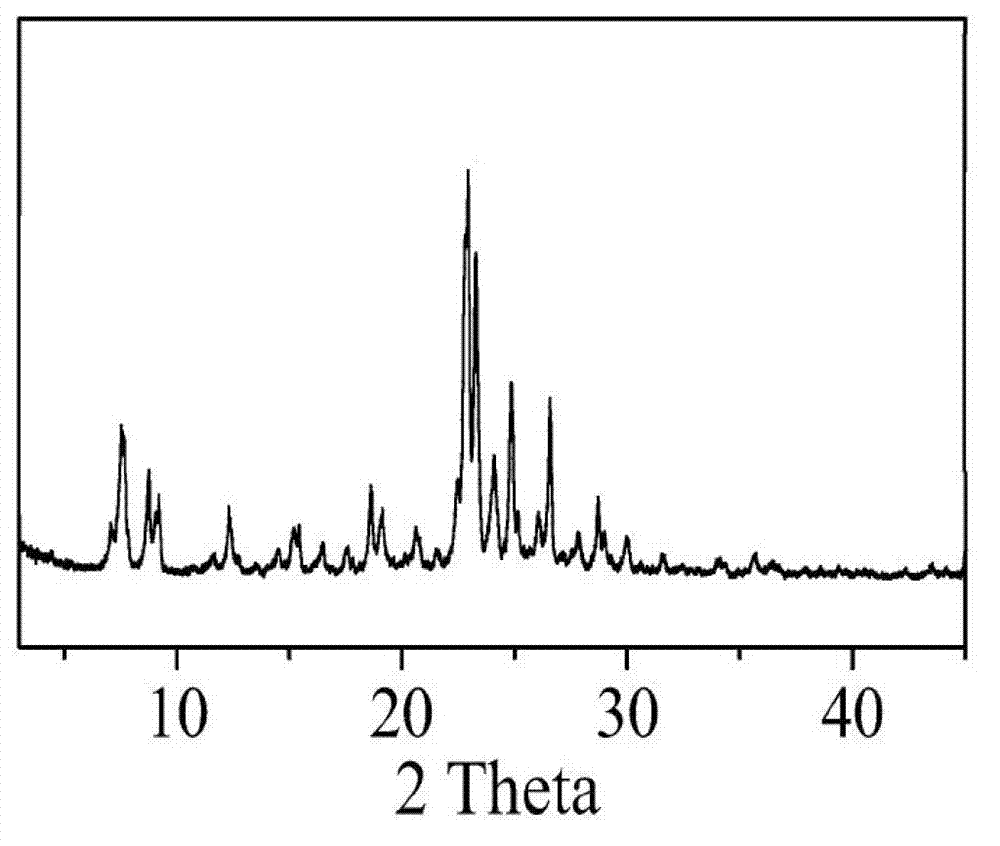
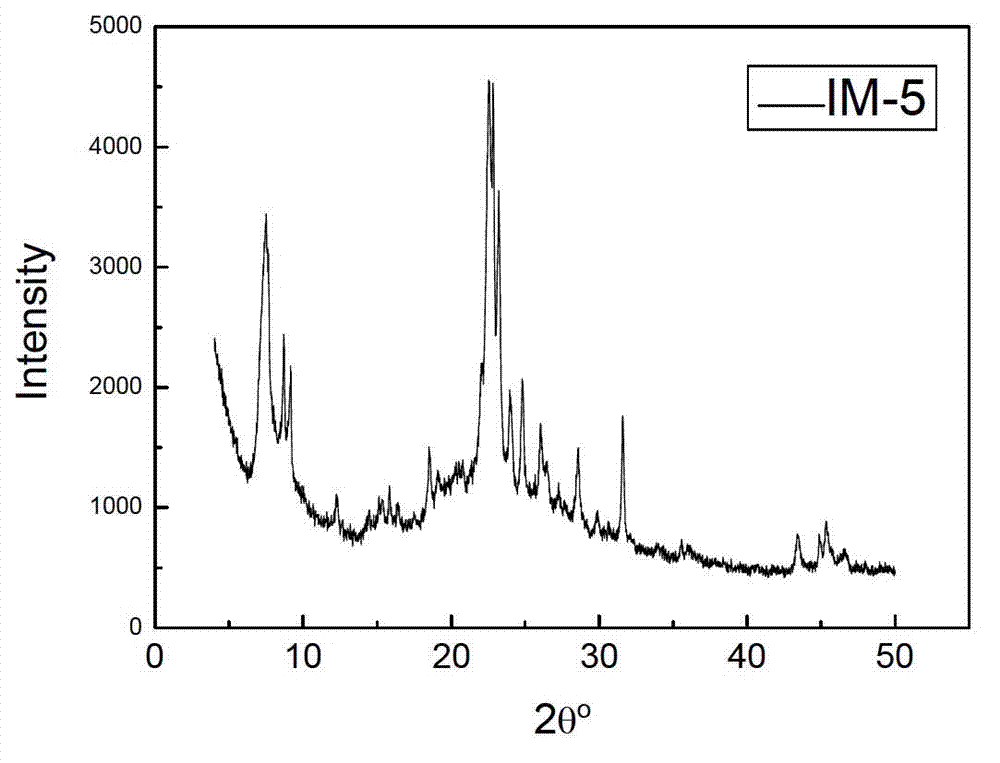
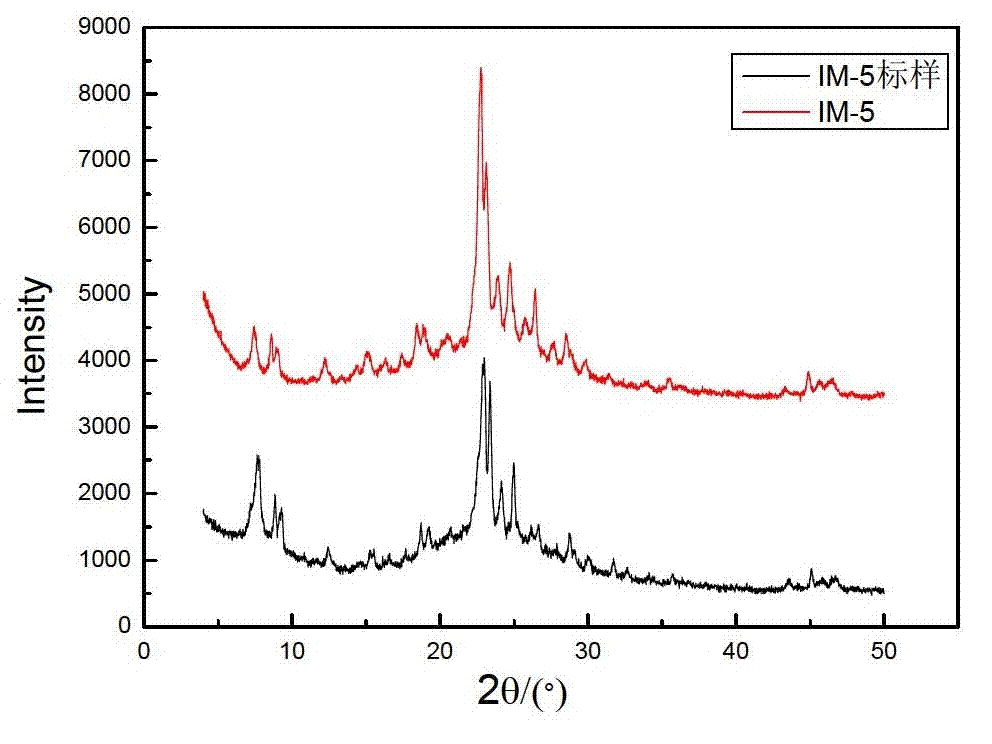
Method for synthesizing IM-5 molecular sieve
ActiveCN102452666AShorten crystallization timeWide ratio rangeCrystalline aluminosilicate zeolitesMolecular sieveAlkali metal oxide
The invention relates to a method for synthesizing IM-5 molecular sieve, which comprises the following steps: 1) dissolving inorganic base, aluminum source and a template in deionized water, uniformly mixing; 2) adding silicon source in a mixture obtained in the step 1), adding an additive or not to prepare colloid or a solid-liquid mixture, uniformly mixing; 3) moving the colloid or the solid-liquid mixture obtained in the step 2) to a crystallization kettle, carrying out hydrothermal dynamic crystallization at the temperature of 120-200 DEG C for 1-10 days, cooling after finishing the crystallization, washing the obtained mixed liquor, filtering and drying to obtain the IM-5 molecular sieve raw powder; wherein the mole ratio of the reactant is SiO2: Al2O3:M2O: R: H2O with 60: (0.3-6): (6-20): (0.6-18): (300-1800), M2O is alkali metal oxide and R is the template. The method of the invention has the advantages that IM-5 molecular sieve crystallization time is shortened, the raw material ratio range is wide, the single kettle yield is enhanced and the industrial amplification can be achieved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Jade-type glass-ceramic and preparation method thereof
The invention relates to a jade-type glass-ceramic and a preparation method thereof. The jade-type glass-ceramic contains a main crystal phase and can also contain a secondary crystal phase, wherein the maximum crystallite dimension of the secondary crystal phase is equal to or smaller than visible wavelength, the jade-type glass-ceramic contains the following components in percentage by weight: 45.0-75.0% of SiO2, 1.0-25.0% of Al2O3, 0-20.0% of alkaline earth metal oxide, 3.0-15.0% of alkali metal oxide, 0-4.0% of TiO2, 0-2.5% of ZrO2, 0-8.0% of F and the balance of flux and clarifier. The preparation method of the jade-type glass-ceramic comprises the steps that the main raw materials and the auxiliary materials are mixed uniformly and then are melted at high temperature to form molten glass, then the molten glass is thermally treated after being cooled and molded so as to form an organization structure beneficial to the penetration of the visible light part in molten glass, and after thermal treatment, the glass-ceramic is in a semitransparent neftdegil shape and becomes a jade-type glass-ceramic. The jade-type glass-ceramic has delicate texture, is translucent, can be widely used as a decorative plate, and can also be carved into handicrafts and prepared into tableware and sanitary ware.
Owner:香港福山实业有限公司
Sealing glass composition, sealing glass frit, and sealing glass sheet
A sealing glass composition which has an alkali metal oxide content kept down as much as possible, and can stably join metal or ceramic members together in a temperature range of 600 to 900° C. The sealing glass composition is used to join together members each selected from the group consisting of metal members and ceramic members, and comprises, as essential components, 20 to 50 mol % of SiO2, 1 to 9 mol % of Al2O3, 5 to 25 mol % of B2O3, 10 to 40 mol % of BaO, and 5 to 20 mol % of SrO. ZnO content is 0 to 10 mol %. An alkali metal oxide content is not more than 5 mol %. PbO is substantially not contained. The total content of MgO, CaO, SrO, BaO and ZnO is 30 to 50 mol %.
Owner:NIPPON SHEET GLASS CO LTD
Propane dehydrogenation to propylene catalyst and preparation and applications thereof
InactiveCN102019178ALow costSimple methodCatalyst activation/preparationHydrocarbonsAir atmosphereAlkaline earth metal
The invention relates to a propane dehydrogenation to propylene catalyst and preparation and applications thereof. The propane dehydrogenation to propylene catalyst is characterized by being a composition prepared from Gamma-Al2O3, a kind or many kinds of chromium oxides, a kind or many kinds of rare earth oxides and a kind or many kinds of alkali metal oxides, wherein the Gamma-Al2O3 accounts for 30-95%, the chromium oxides account for 1-50%, the rare earth oxides account for 0-30%, and the alkali metal oxides account for 0-10%. The preparation process comprises the following steps of: adding active components, such as chromium, rare earth, alkali metal and the like into aluminum oxide grout, regulating the pH value of 1-5 to form gelatum with concentrated nitric acid, and spraying and drying to form microballoons; then roasting for 0-5 hours at 250-750 DEG C in a nitrogen atmosphere; and roasting for 0.1-20 hours at 250-1000 DEG C in an air atmosphere. The obtained catalyst is in a fluid bed reactor, and reaction conditions comprise 400-700 DEG C of temperature, 0.01-3MPa of absolute pressure and 10-10000 h-1 of volume hourly space velocity.
Owner:卓润生
Metal Oxide Nanoporous Material, Coating Composition to Obtain the Same, and Methods of Manufacturing Them
ActiveUS20070215009A1Improve adhesionImprove heat resistanceInternal combustion piston enginesAlkali metal silicate coatingsAlkali metal oxideRare earth
A metal oxide nanoporous material comprises two or more kinds of first metal oxides selected from the group consisting of alumina, zirconia, titania, iron oxide, rare-earth oxides, alkali metal oxides and alkaline-earth metal oxides. The metal oxide nanoporous material has nanopores, each with a diameter of 10 nm or smaller, in which the metal oxides are dispersed homogeneously in the wall forming the nanopores.
Owner:TOYOTA CENT RES & DEV LAB INC
Low-density glass for flat panel display substrates
Disclosed is a glass material essentially free of BaO and alkali oxide particularly suitable for the glass substrate of LCDs. The glass material consists essentially, expressed in mole percent on an oxide basis, of 70–80%, preferably 72–77% of SiO2, 3–9%, preferably 4–7% of Al2O3, 8–18%, preferably 10–16% B2O3, 3–10%, preferably 3–8% of CaO, 0–4%, preferably 0–3% RO, 0–0.2%, preferably 0–0.1% SnO, 0–1%, preferably 0 to 0.5% of XO, where RO represents, collectively, MgO, SrO and ZnO, XO represents, collectively, TiO2, ZrO2, Y2O3 and La2O3. The glass has a strain point in the range of over about 600° C., a coefficient of thermal expansion (CTE) in the range of about 23–35×10−7 / ° C., a density lower than about 2.35 g / cm3, a liquidus temperature lower than or equal to about 1200° C. and a durability in BHF less than or equal to 0.5 mg / cm2 weight loss.
Owner:CORNING INC
Glass compositions with improved chemical and mechanical durability
ActiveUS20130101766A1Record information storageLight beam reproducingAlkali metal oxideAlkaline earth oxides
The embodiments described herein relate to chemically and mechanically durable glass compositions and glass articles formed from the same. In another embodiment, a glass composition may include from about 70 mol. % to about 80 mol. % SiO2; from about 3 mol. % to about 13 mol. % alkaline earth oxide; X mol. % Al2O3; and Y mol. % alkali oxide. The alkali oxide may include Na2O in an amount greater than about 8 mol. %. A ratio of Y:X may be greater than 1 and the glass composition may be free of boron and compounds of boron. In some embodiments, the glass composition may also be free of phosphorous and compounds of phosphorous. Glass articles formed from the glass composition may have at least a class S3 acid resistance according to DIN 12116, at least a class A2 base resistance according to ISO 695, and a type HGA1 hydrolytic resistance according to ISO 720.
Owner:CORNING INC
Catalyst for alkane dehydrogenation and device
ActiveCN102451677AHigh catalytic activityHigh selectivityHydrocarbonsChemical recyclingAlkaneAlkali metal oxide
The invention provides a catalyst for alkane dehydrogenation as well as a continuous reaction regeneration device and method using the catalyst for catalytic dehydrogenation. The catalyst comprises four ingredients, the ingredient A is oxides of one kind of element or several kinds of elements from Ti, Nb, Ta, Mo, W, Re, In or Ga, the ingredient B is one kind or several kinds of materials in MgO, P2O5, ZrO2, Al2O3 or SiO2, the ingredient C is oxides of one kind of material or several kinds of materials from Zn, Cd and Sn, and the ingredient D is one kind of material or a mixture of several kinds of materials from alkali oxides or alkaline earth metal oxides. The catalyst does not contain noble metal such as Pt and the like, does not contain toxic ingredients such as Cr and the like and cannot pollute the environment. The activity of the catalyst is high, the selectivity of olefin generated by alkane dehydrogenation is high, the stability of the catalyst is good, the mechanical intensity is high, the continuous reaction regeneration device is used for alkane dehydrogenation, the reaction and the catalyst regeneration are continuously carried out, the efficiency is high, and the safety is high.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Methods of vitrifying waste with low melting high lithia glass compositions
InactiveUS6258994B1Reduce pointsReduce the temperatureSludge treatmentSolid waste disposalAlkali metal oxideRadioactive agent
The invention relates to methods of vitrifying waste and for lowering the melting point of glass forming systems by including lithia formers in the glass forming composition in significant amounts, typically from about 0.16 wt % to about 11 wt %, based on the total glass forming oxides. The lithia is typically included as a replacement for alkali oxide glass formers that would normally be present in a particular glass forming system. Replacement can occur on a mole percent or weight percent basis, and typically results in a composition wherein lithia forms about 10 wt % to about 100 wt % of the alkali oxide glass formers present in the composition. The present invention also relates to the high lithia glass compositions formed by these methods. The invention is useful for stabilization of numerous types of waste materials, including aqueous waste streams, sludge solids, mixtures of aqueous supernate and sludge solids, combinations of spent filter aids from waste water treatment and waste sludges, supernate alone, incinerator ash, incinerator offgas blowdown, or combinations thereof, geological mine tailings and sludges, asbestos, inorganic filter media, cement waste forms in need of remediation, spent or partially spent ion exchange resins or zeolites, contaminated soils, lead paint, etc. The decrease in melting point achieved by the present invention desirably prevents volatilization of hazardous or radioactive species during vitrification.
Owner:SAVANNAH RIVER NUCLEAR SOLUTIONS
Optical fiber containing alkali metal oxide
ActiveUS20070297735A1Reduce lossReduce concentrationGlass making apparatusOptical fibre with multilayer core/claddingDopantAlkali metal oxide
Disclosed is an optical fiber having a silica-based core comprising an alkali metal oxide selected from the group consisting of K2O, Na2O, LiO2, Rb2O, Cs2O and mixtures thereof in an average concentration in said core between about 50 and 500 ppm by weight, said core further comprising chlorine and fluorine, wherein the average concentration of fluorine in said core is greater than the average concentration of alkali metal oxide in said core and the average concentration of chlorine in said core is greater than the average concentration of alkali metal oxide in said core; and a silica-based cladding surrounding and directly adjacent the core. By appropriately selecting the concentration of alkali metal oxide dopant in the core and the cladding, a low loss optical fiber may be obtained.
Owner:CORNING INC
Alkali-free glass and alkali-free glass substrate, and method of producing the same
ActiveUS20090226671A1Improve productivityExcellent in devitrificationGlass drawing apparatusGlass forming apparatusDevitrificationAlkali free
A technical object of the present invention is to satisfy various properties required in glass for liquid crystal displays and the like, in particular, fusibility, devitrification resistance, and like properties, and then to design a glass composition in which components harmful to the environment are reduced or substantially not contained, thereby obtaining a glass substrate that takes the environment into consideration. An alkali-free glass of the present invention contains the following glass composition in percent by weight based on oxide: 50 to 70% of SiO2, 10 to 20% of Al2O3, 8 to 12% of B2O3, 0 to 3% of MgO, 4 to 15% of CaO, 0 to 10% of SrO, 0 to 1% of BaO, and 0 to 5% of ZnO, and substantially free of alkali metal oxide and As2O3.
Owner:NIPPON ELECTRIC GLASS CO LTD
Method of synthesizing IM-5 molecular sieve by using composite template
ActiveCN102452667AReduced nucleation timeShorten crystallization timeCrystalline aluminosilicate zeolitesMolecular sieveAlkali metal oxide
The invention provides a method of synthesizing IM-5 molecular sieve by using a composite template. The method comprises the following steps: (1) dissolving an inorganic base, an aluminum source and two templates in deionized water and carrying out uniform mixing so as to prepare a mixed solution; (2) adding a silicon source into the mixed solution obtained in step (1), optionally adding an additive and carrying out uniform mixing so as to prepare colloid or a solid-liquid mixture; (3) transferring the colloid or the solid-liquid mixture obtained in step (2) to a crystallization kettle, carrying out hydrothermal crystallization at a temperature of 120 to 200 DEG C for 1 to 10 d, carrying out cooling after crystallization and rinsing, filtering and drying the obtained mixed liquor so as to obtain raw powder of the IM-5 molecular sieve. A mole ratio of reactants satisfies the equation of SiO2: Al2O3: M2O: R1: R2: H2O = 60: 0.4-6: 6-21: 0.6-18: 0.6-12: 300-1800, wherein M2O is an alkali metal oxide, R1 is a first template, and R2 is a second template. The method provided in the invention widens the range of mixing ratios of raw materials, improves yield of a single kettle and enables time for crystallization to be further shortened and enlarged production to be easier.
Owner:CHINA PETROLEUM & CHEM CORP +1
IM-5 molecular sieve synthesis method
ActiveCN103708491APromote depolymerizationIncrease concentrationCrystalline aluminosilicate zeolitesMolecular sieveSynthesis methods
An IM-5 molecular sieve synthesis method comprises the following steps: 1, dissolving an inorganic alkali, an aluminum source and a template in deionized water, and uniformly mixing to prepare a mixed solution; 2, adding a solid silica gel to the mixed solution obtained in step 1, adding a template precursor, and uniformly mixing to prepare a colloid or a solid-liquid mixture; and 3, transferring the colloid or solid-liquid mixture obtained in step 2 to a crystallization kettle, carrying out hydrothermal crystallization for 1-10 days, cooling after the crystallization to obtain a mixed liquid, and washing, filtering and drying the mixed liquid to obtain raw IM-5 molecular sieve powder, wherein reactants comprise SiO2, Al2O3, M2O, R, A and H2O, a molar ratio of SiO2:Al2O3:M2O:R:A:H2O is 60:(0.3-6):(6-20):(4-15):(1.2-6):(300-1200), M2O is an alkali metal oxide, R is the template, and A is the template precursor. The IM-5 molecular sieve synthesis method has the advantages of good product repeatability, effective reduction of the water content, increase of the concentration of the template, crystallization time shortening and single kettle yield increase.
Owner:CHINA PETROLEUM & CHEM CORP +1
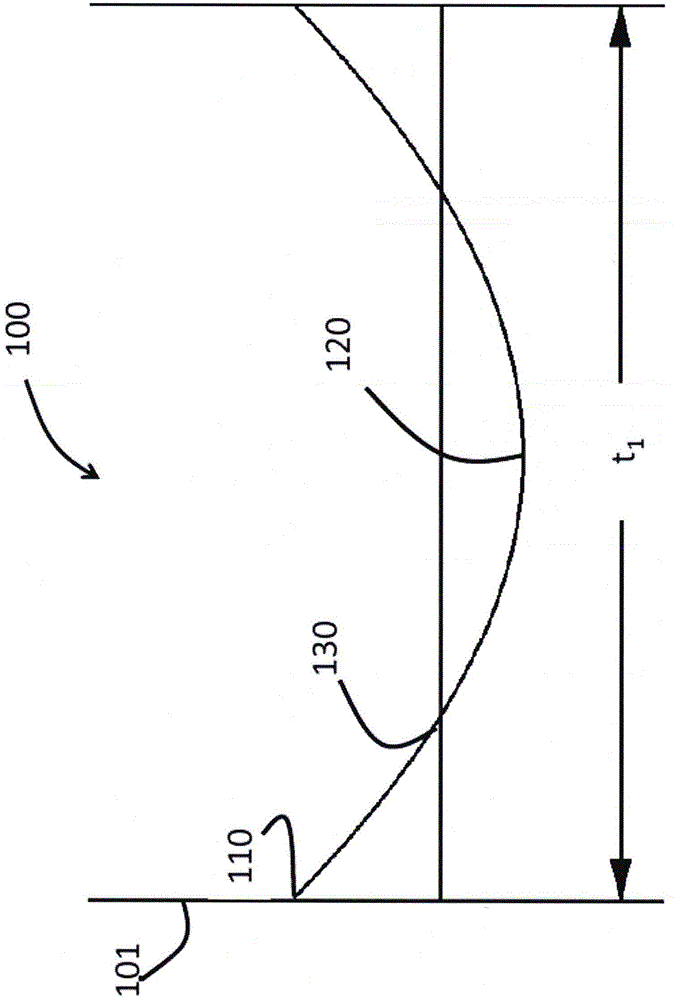
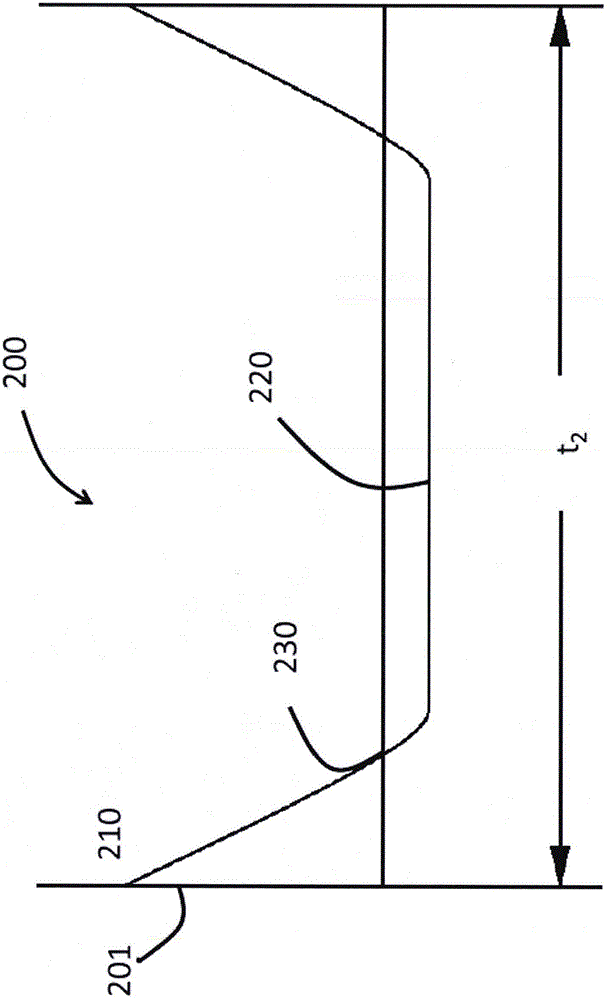
Glasses and glass ceramics including a metal oxide concentration gradient
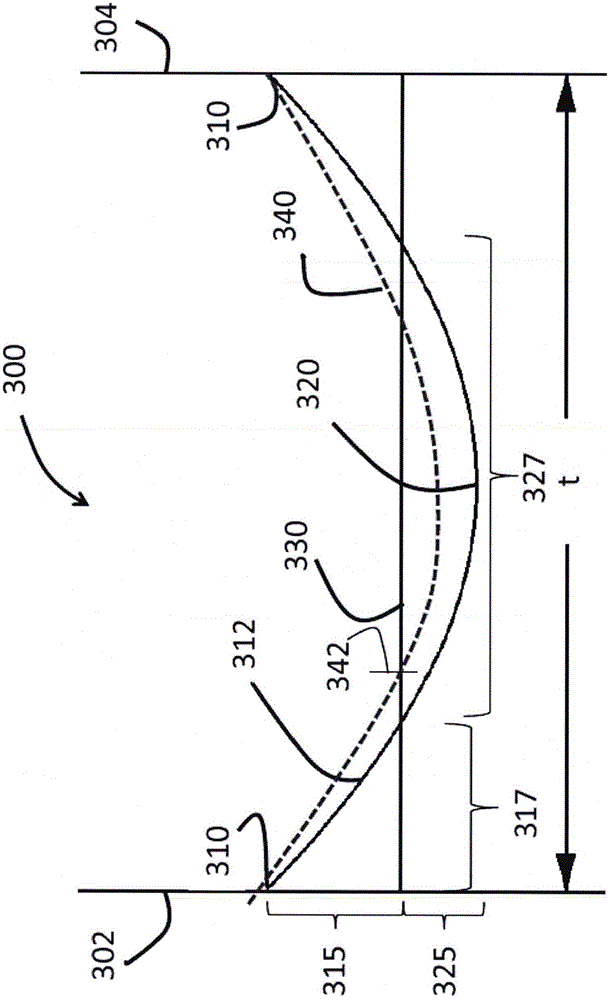
The invention relates to glasses and glass ceramics including a metal oxide concentration gradient. Embodiments of a glass-based article including a first surface and a second surface opposing the first surface defining a thickness (t) of about 3 millimeters or less (e.g., about 1 millimeter or less), and a stress profile, wherein all points of the stress profile between a thickness range from about 0t up to 0.3t and from greater than 0.7t, comprise a tangent that is less than about -0.1 MPa / micrometers or greater than about 0.1 MPa / micrometers, are disclosed. In some embodiments, the glass-based article includes a non-zero metal oxide concentration that varies along at least a portion of the thickness (e.g., 0t to about 0.3t). In some embodiments, the concentration of metal oxide or alkali metal oxide decreases from the first surface to a point between the first surface and the second surface and increases from the point to the second surface. The concentration of the metal oxide may be about 0.05 mol % or greater or about 0.5 mol % or greater throughout the thickness. Methods for forming such glass-based articles are also disclosed.
Owner:CORNING INC
Alkali-free glass and glass plate for a display
An alkali-free glass consists essentially of, in mass percent, 58-70% SiO2, 10-19% Al2O3, 6.5-15% B2O3, 0-2% MgO, 3-12% CaO, 0.1-5% BaO, 0-4% SrO, 0.1-6% BaO+SrO, 0-5% ZnO, 5-15% MgO+CaO+BaO+SrO+ZnO, 0-5% ZrO2, 0-5% TiO2, and 0-5% P2O5. The alkali-free glass contains substantially no alkali metal oxide and has a density of 2.45g / cm3 or less, an average coefficient of thermal expansion of 25×10−7 / ° C.-36×10−7 / ° C. within a temperature range between 30 and 380° C., and a strain point not lower than 640° C.
Owner:NIPPON ELECTRIC GLASS CO LTD
Catalytic combustion catalyst and preparing method thereof
InactiveCN1488435AImprove high temperature resistanceNot easy to fall offDispersed particle separationCatalyst activation/preparationActive componentAlkali metal oxide
The invention refers to a kind of catalyst in organic waster gas purification. It is made up of honeycomb pottery carrier and the paint layer on the carrier, and the precious metal active components. The layer has characters such as stable paint layer, excellent high temperature resistant performance, the efficiency is high. The paint layer is made up of Al2O3, SiO2 and a one kind of or more alkali metal oxide. The paint layer combines with the honeycomb pottery carrier stably.
Owner:CHINA PETROLEUM & CHEM CORP +1
Technology device and method for preparing propylene by dehydrogenating propane or propane-enriched low carbon hydrocarbon
ActiveCN102040445AReduce energy consumptionLow unit priceHydrogen separationDistillation purification/separationGas phaseAlkali metal oxide
The invention provides a technology device and method for preparing propylene by dehydrogenating propane or propane-enriching low carbon hydrocarbon. The device comprises a reacting / regenerating part and a product fractionating part. A reacting / regenerating system is divided into a parallel type arrangement scheme and a coaxial type arrangement scheme by a fluidized bed reacting / regenerating technology, wherein a raising pipe of the parallel type reacting / regenerating system is an internal or external raising pipe, and a raising pipe of the coaxial type reacting / regenerating system is an external raising pipe. The method comprises the steps: leading raw materials and reaction products to enter into a raising pipe reactor after heat exchange; performing a dehydrogenating conversion reaction under the conditions that the reaction temperature is 500-700 DEG C, the pressure is 0.1-0.4MPa, and the ratio of a microspherical catalyst to oil is 5-60, wherein the microspherical catalyst consists of chromium, rare earth oxides, alkali metal oxides and a gamma-Al2O3 carrier, preferably, the temperature is 550-670 DEG C, the pressure is 0.1-0.15MPa, and the ratio of the microspherical catalyst to oil is 6-14; leading the reaction products and the raw materials to enter into a gas-oil separator to separate a gas phase, a liquid phase from a water phase after heat exchange and cooling; leading the separated gas to enter into a gas compressor to be compressed and be transported to an absorbing / stabilizing part; pumping the liquid to an absorbing tower; transporting the liquefied gas from a return tank at the top of a stabilizing tower to a propylene / propane separating tower by a charging pump of a propylene tower; discharging the fine propylene separated from the top of the tower out of the device as a product; discharging the separated byproduct hydrogen out of the device; and returning products at bottom of the tower to the reacting / regenerating part to be recycled.
Owner:卓润生 +1
Low melting glass, sealing composition and sealing paste
ActiveUS20060105898A1Wide sealable temperature rangeErosion to the refractories when melting can be suppressed effectivelyPlatinumAlkali metal oxide
A low melting glass, which contains substantially no lead and contains 70-90% of Bi2O3, 1-20% of ZnO, 2-12% of B2O3, 0.1-5% of Al2O3, 0.1-5% of CeO2, 0-5% of CuO, 0-0.2% of Fe2O3and 0.05-5% of CuO+Fe2O3 in mass %, wherein a content of alkali metal oxides in the glass composition is less than 0.1%, and the glass does not crystallize by pre-baking at a sealing temperature or more, can be used for sealing later, and can suppress deterioration of a material such as platinum or platinum-rhodium to provide a stable melting operation for a long period.
Owner:ASAHI TECHNO GLASS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com