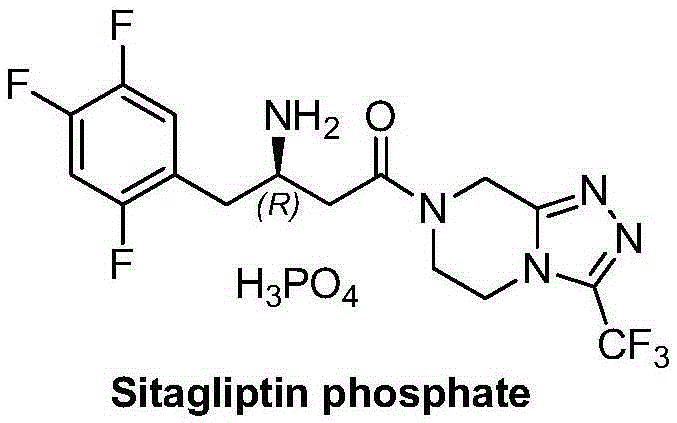
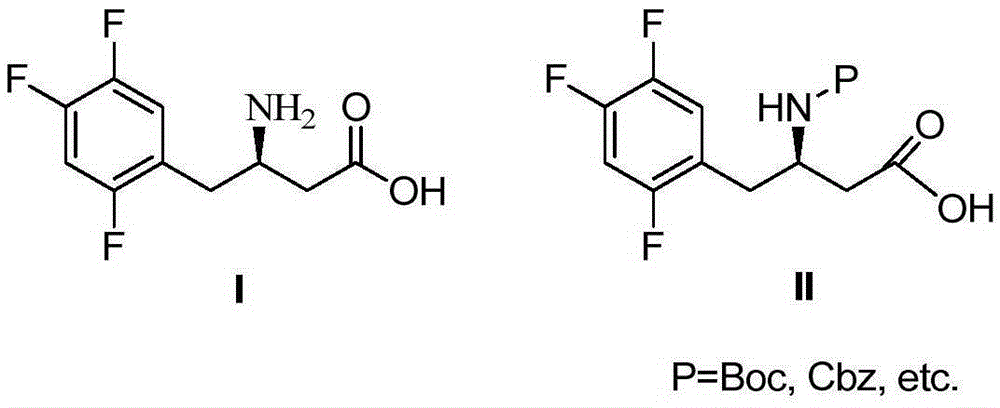
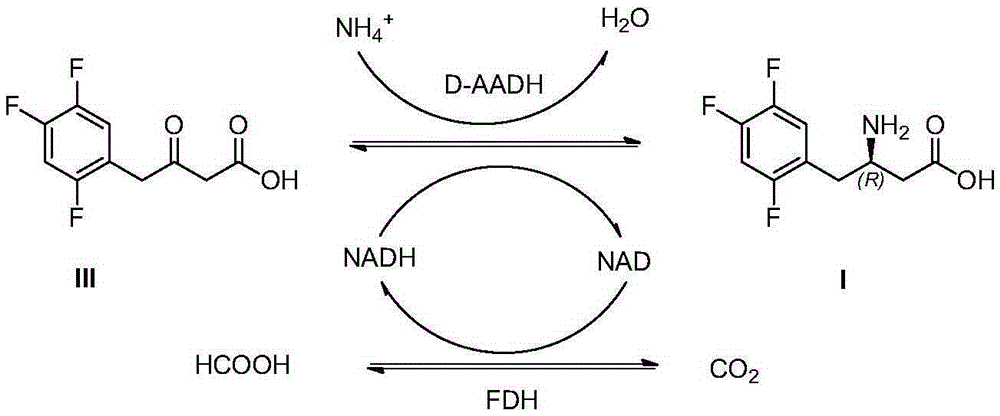
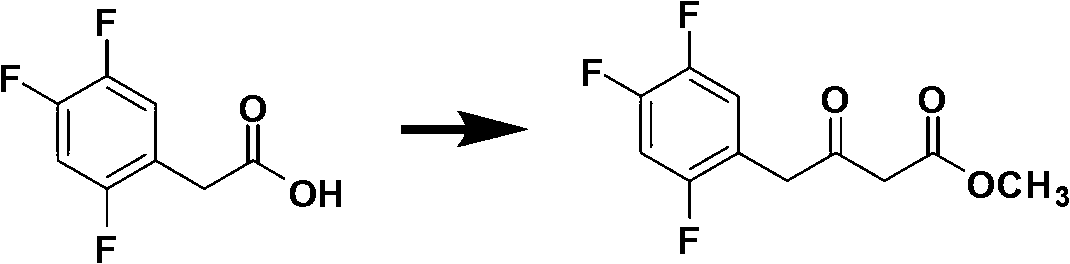
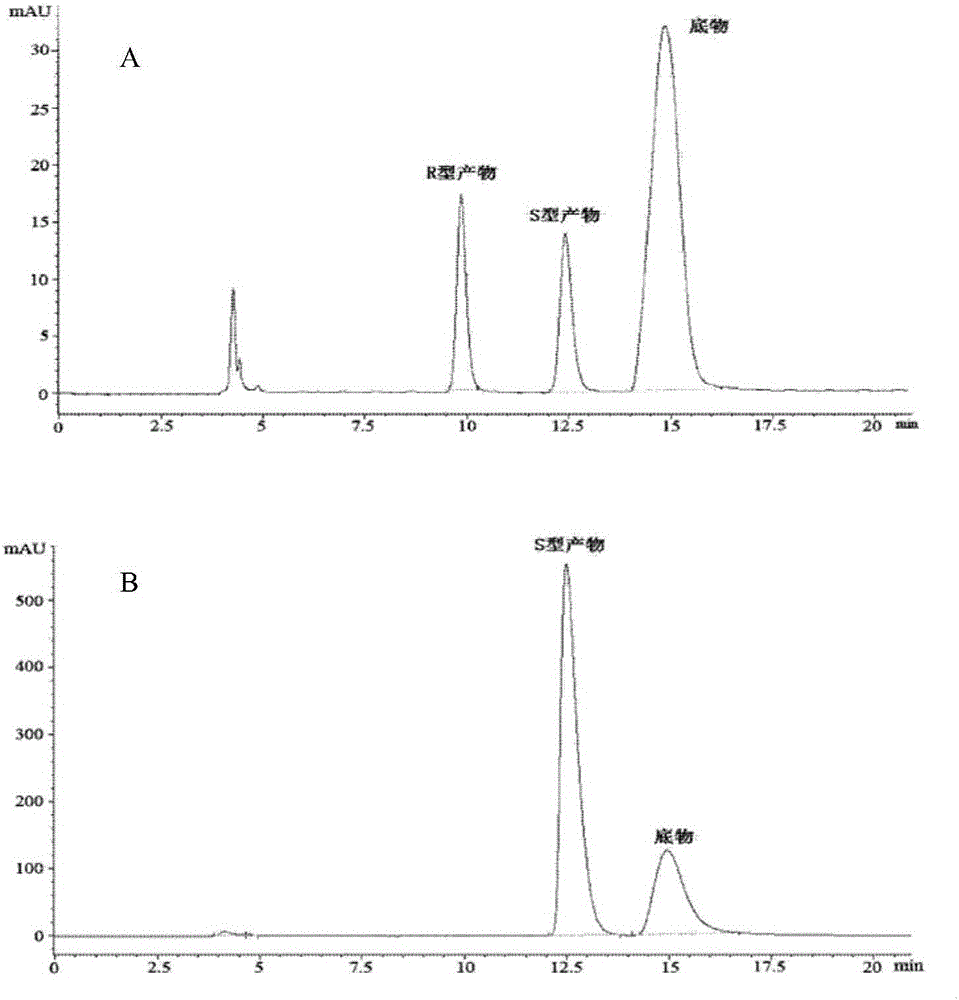
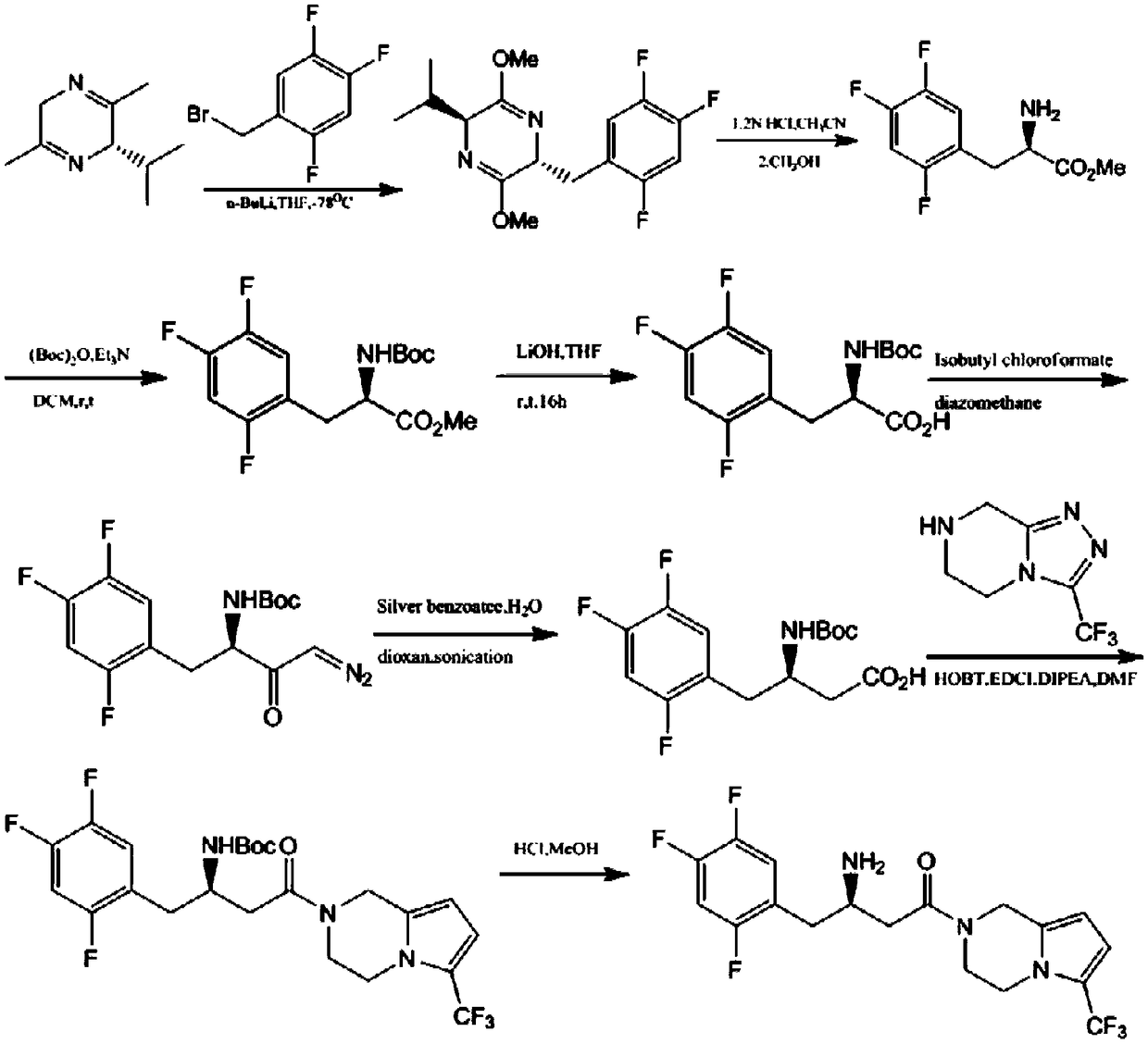
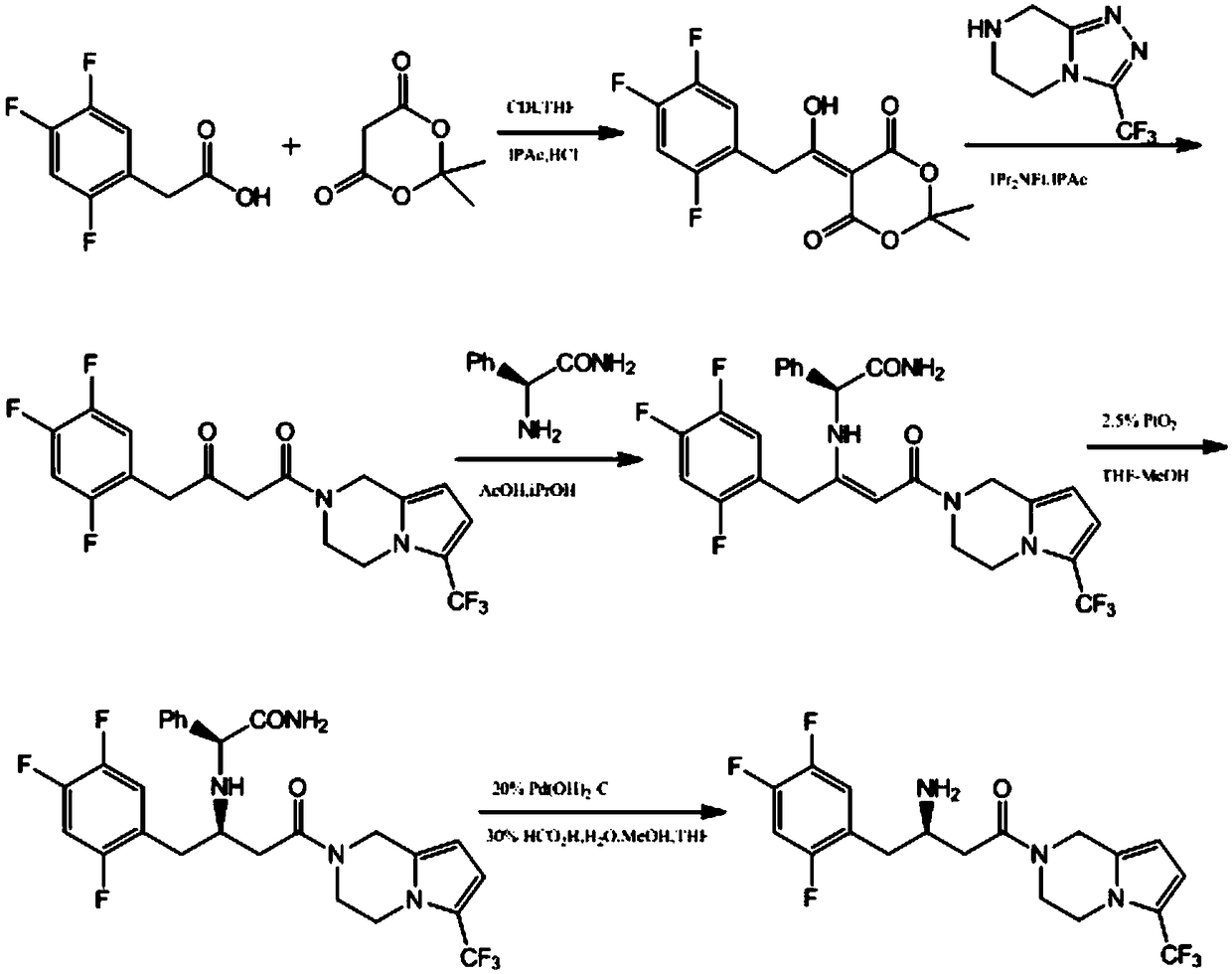
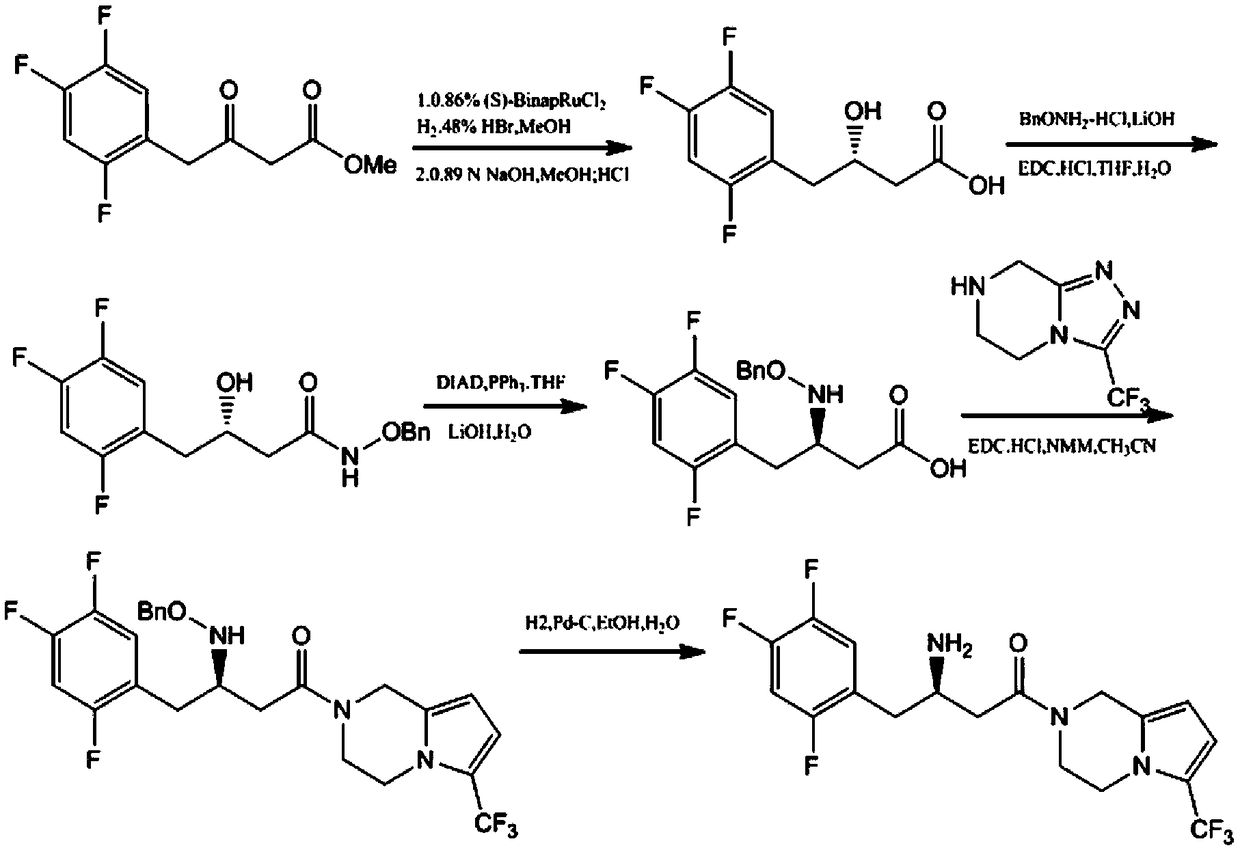
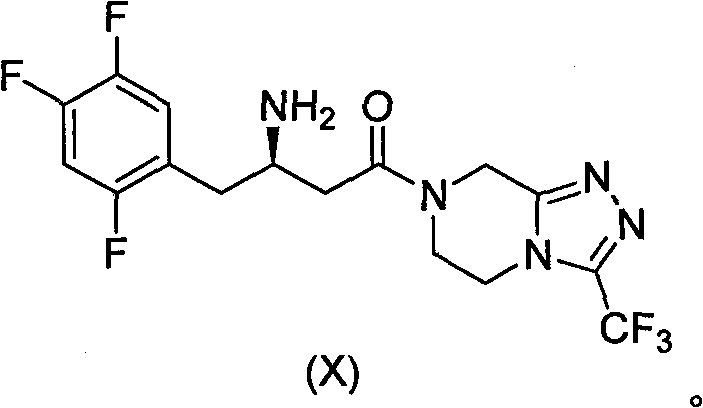
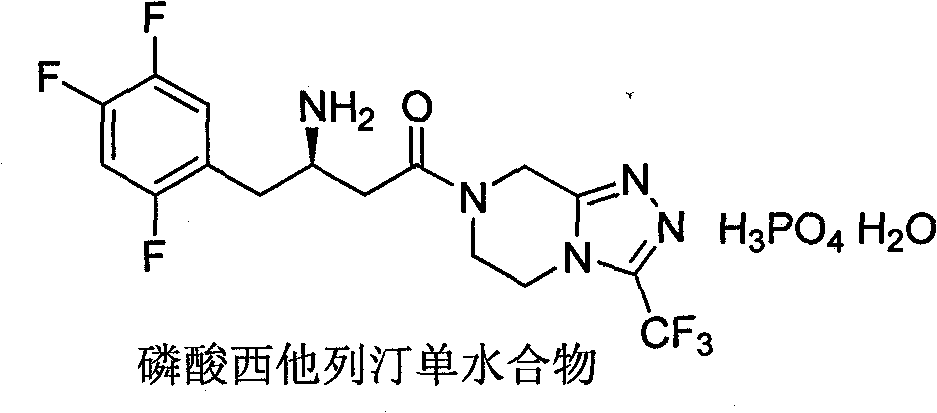
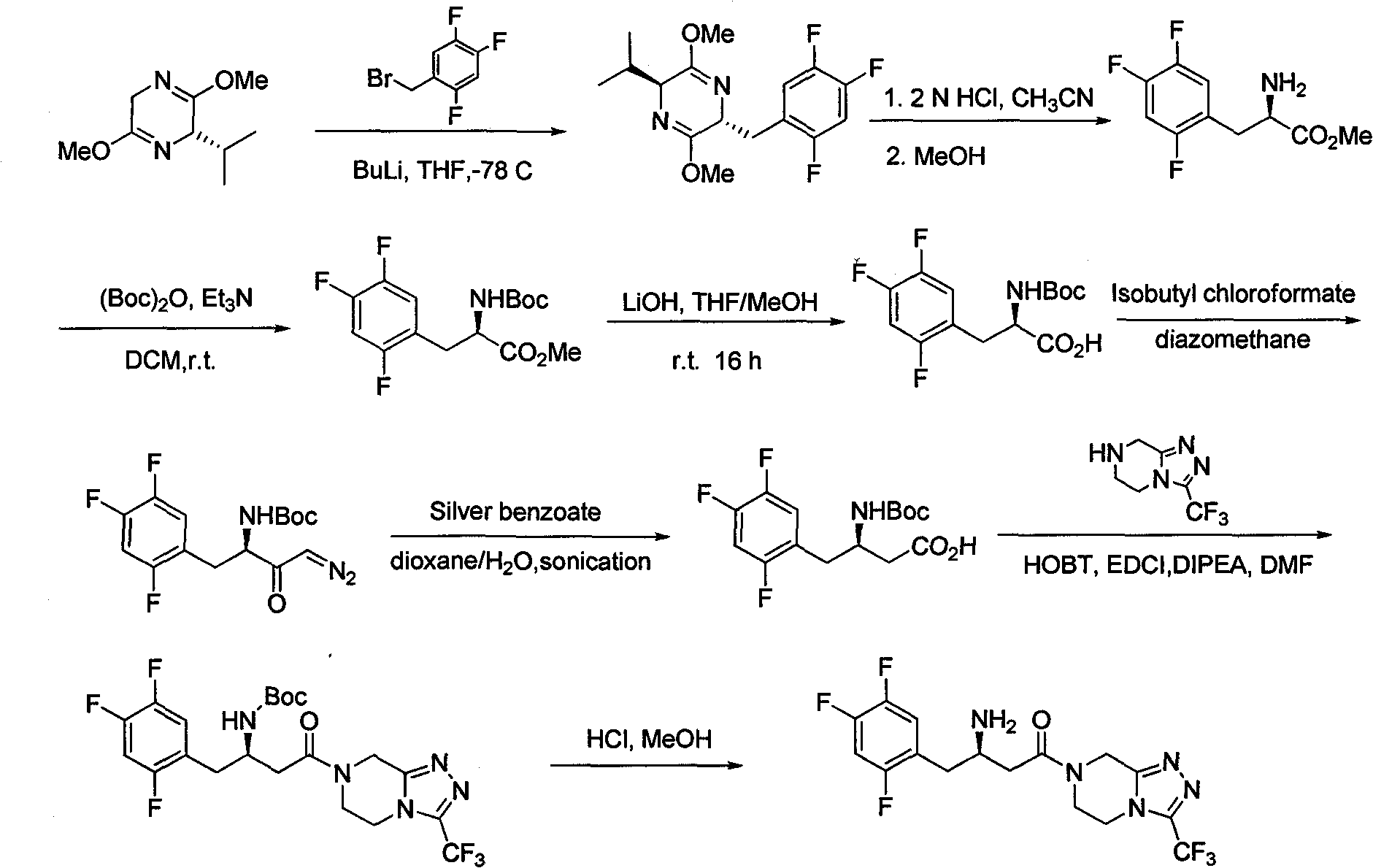
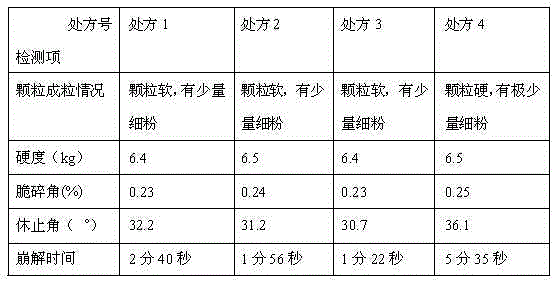
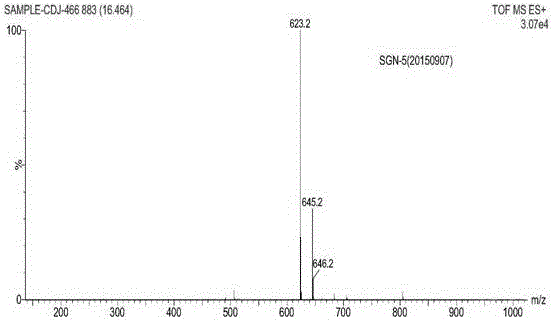
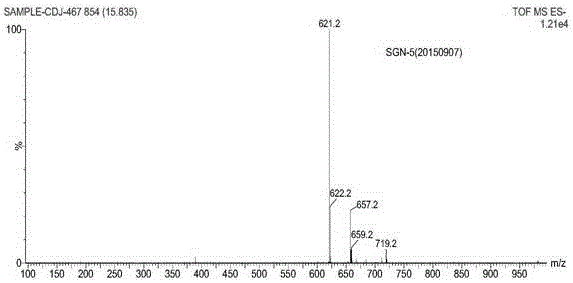
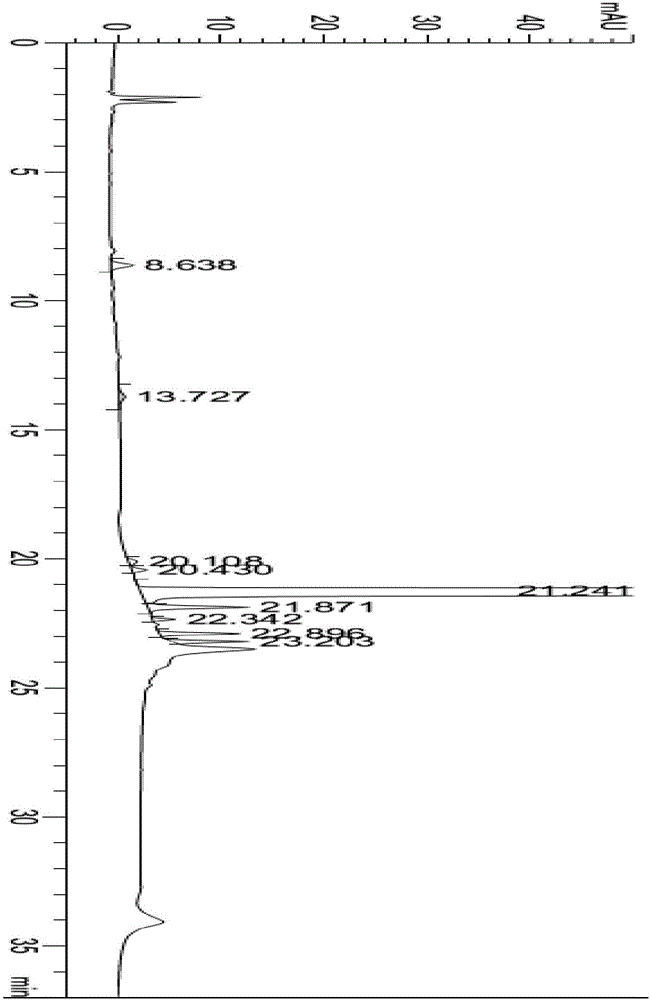
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
249 results about "Sitagliptin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
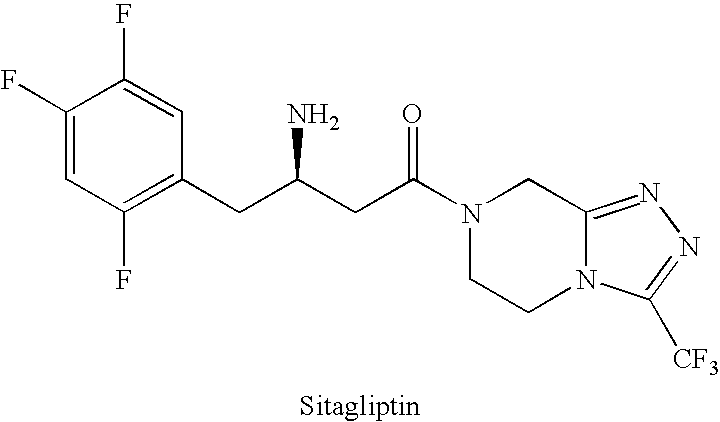
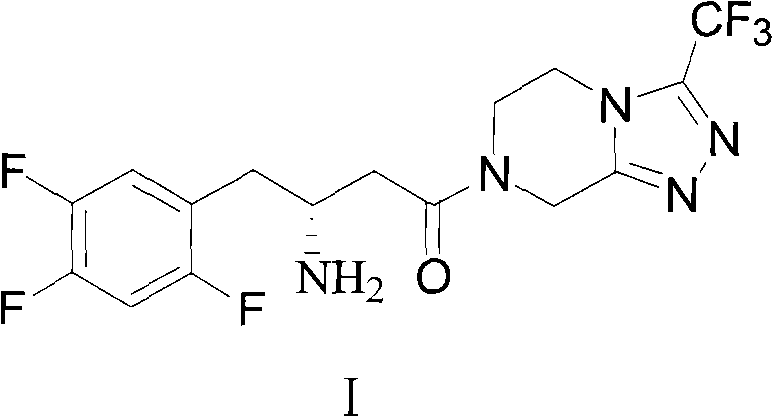
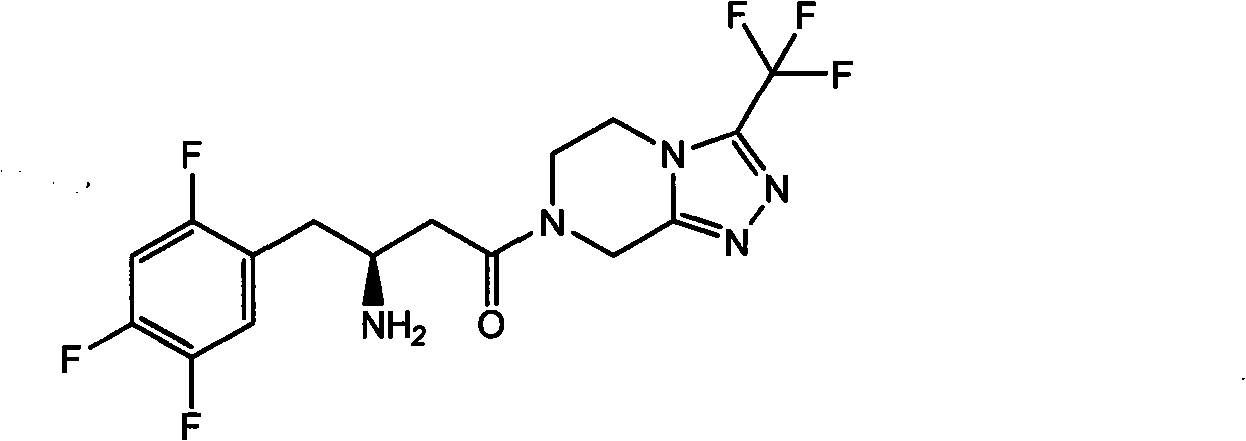
Sitagliptin, sold under the brand name Januvia among others, is a medication used to treat diabetes mellitus type 2. It is generally less preferred than metformin or a sulfonylurea. It is taken by mouth. It is also available within a single pill as metformin/sitagliptin.
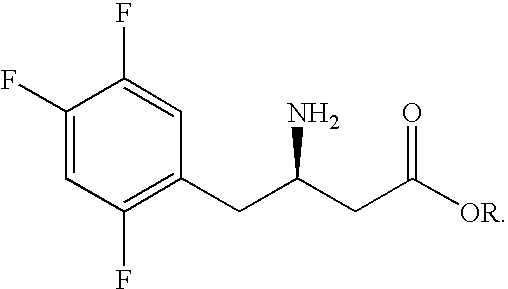
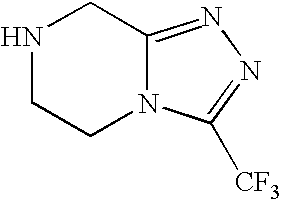
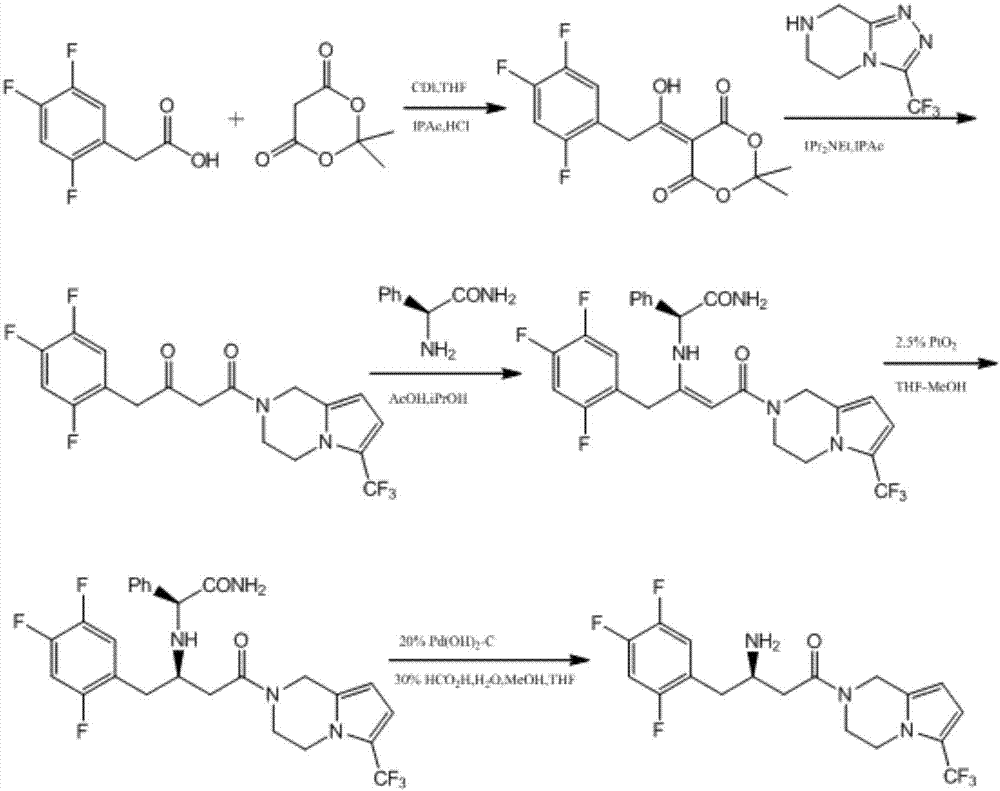
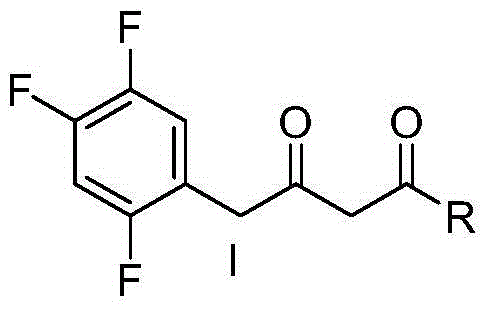
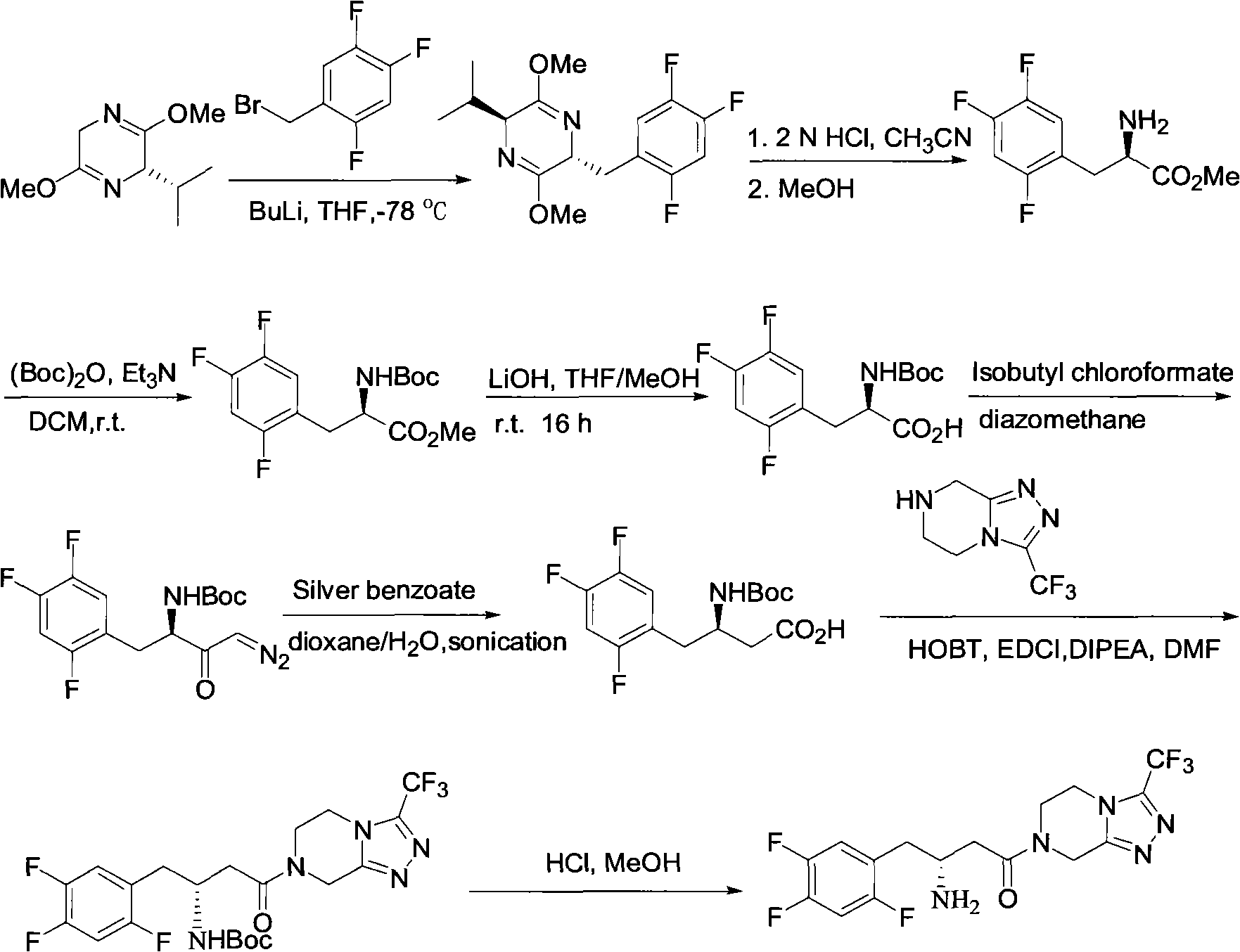
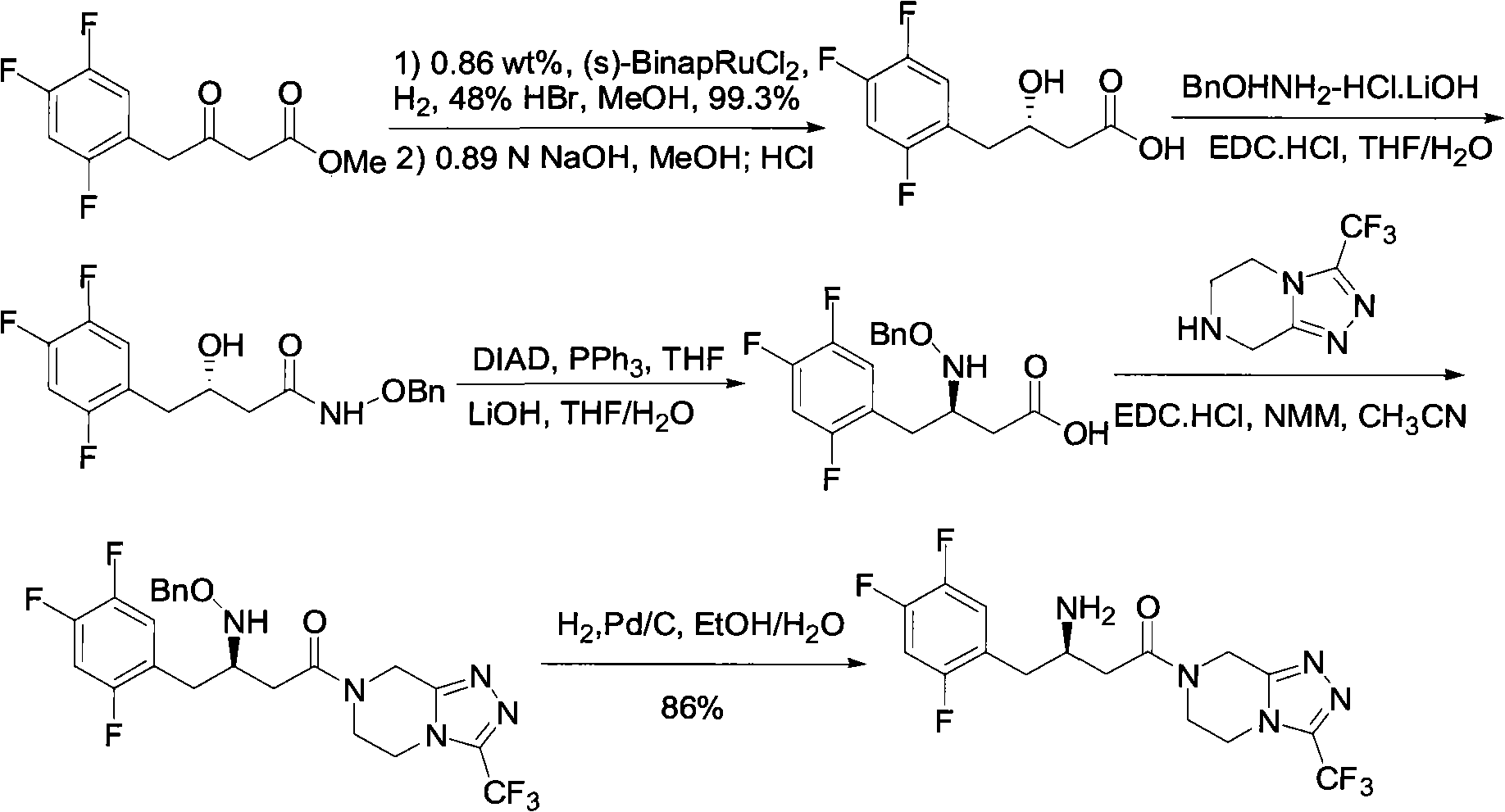
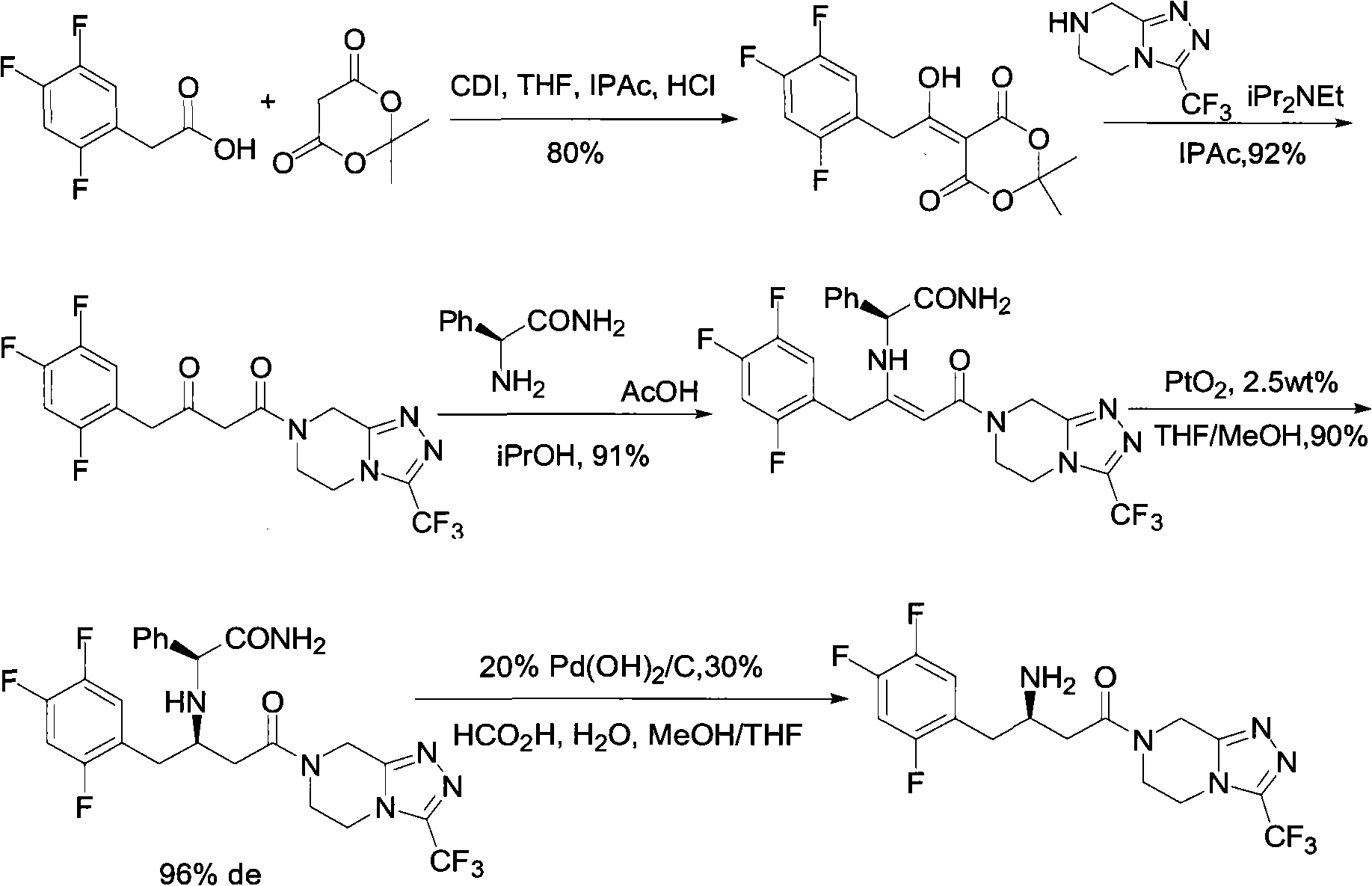
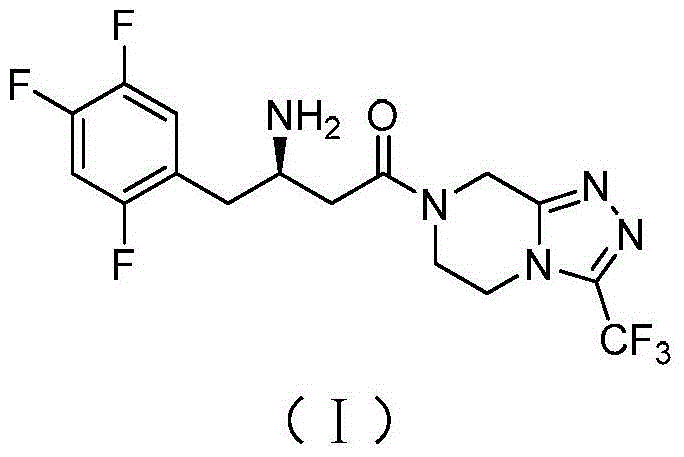
Preparation of sitagliptin intermediate
InactiveUS20090192326A1Organic compound preparationAmino-carboxyl compound preparationSitagliptinMedicinal chemistry
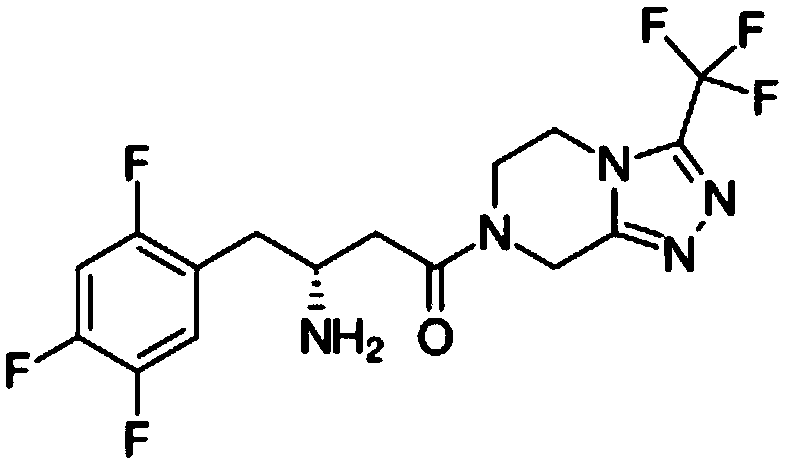
Intermediate compounds in the synthesis of Sitagliptin, 3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid alkyl ester, and amino protected-3-amino-4-(2,4,5-trifluorophenyl)but-2-enoic acid alkyl ester, and the stereoselective reduction of these compound to give Synthon I, or the amino-protected Synthon I, are provided.
Owner:TEVA PHARM USA INC +1
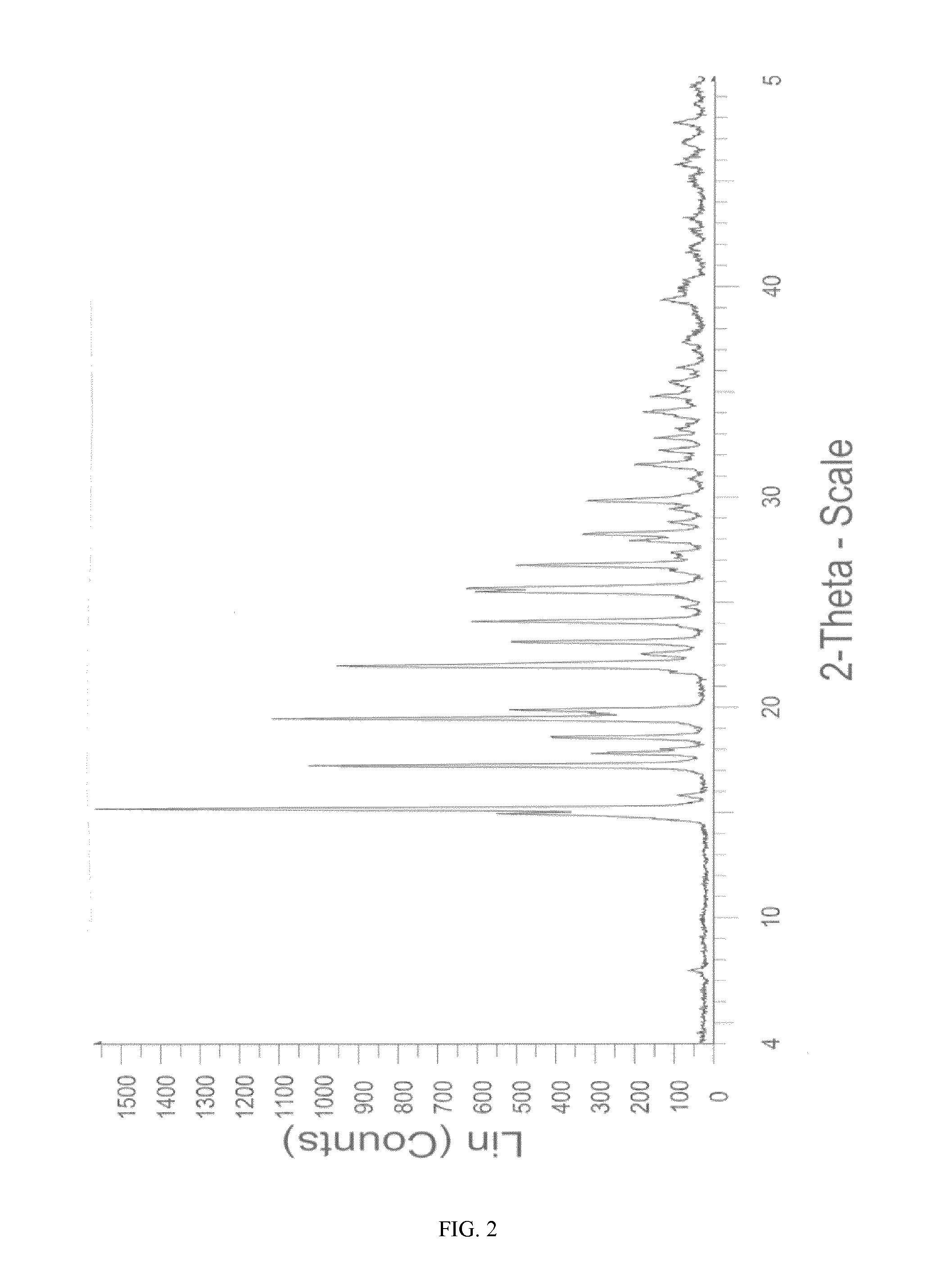
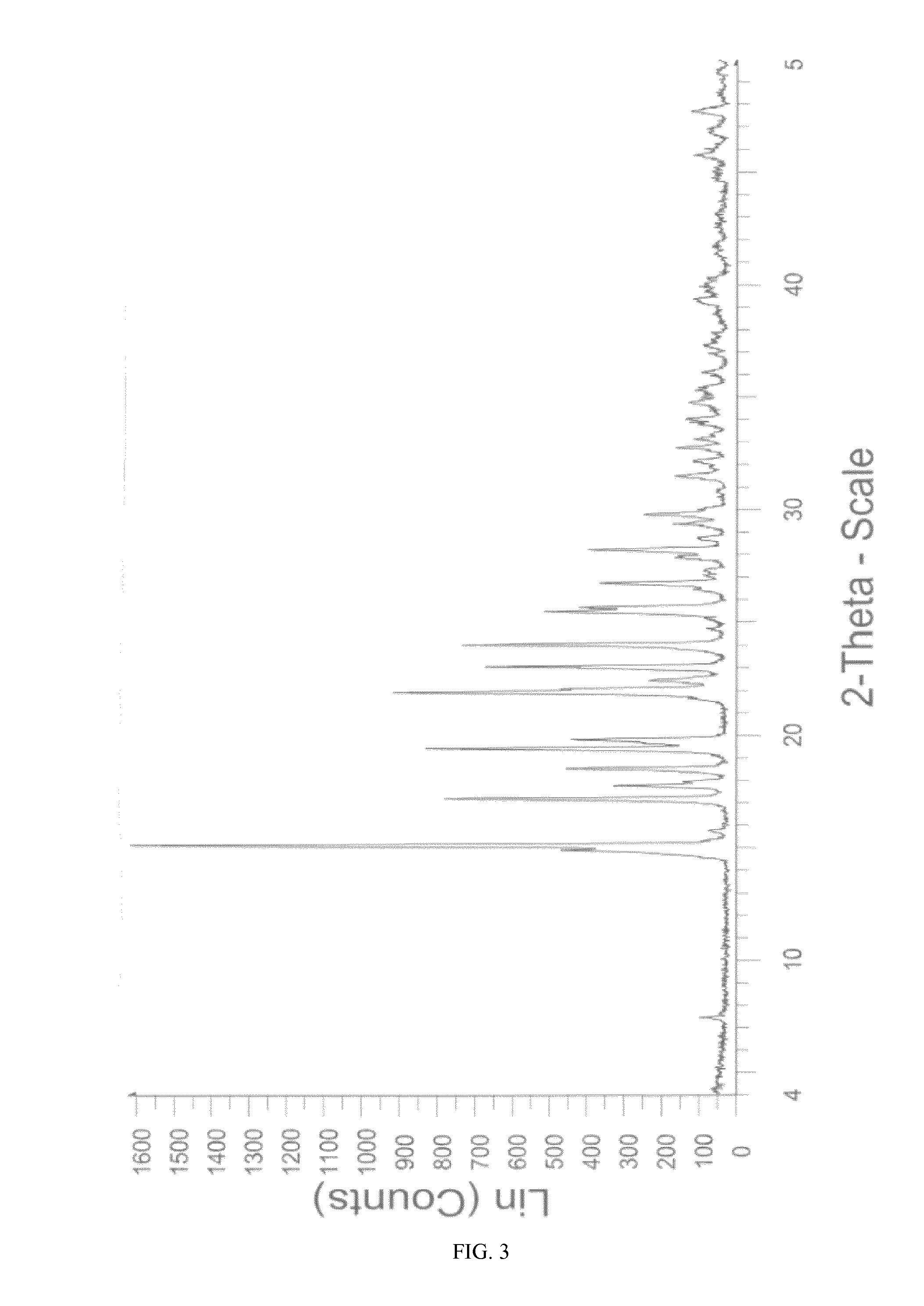
Novel crystalline form of sitagliptin sulfate
InactiveUS20150037406A1Physical improvementImprove pharmaceutical propertiesBiocideOrganic chemistrySitagliptinSulfate
A novel crystalline form of sitagliptin sulfate is provided. In addition, a method for obtaining the crystalline form, pharmaceutical compositions comprising the novel crystalline form and the crystalline form for use as a medicament are provided.
Owner:MOEHS IBERICA
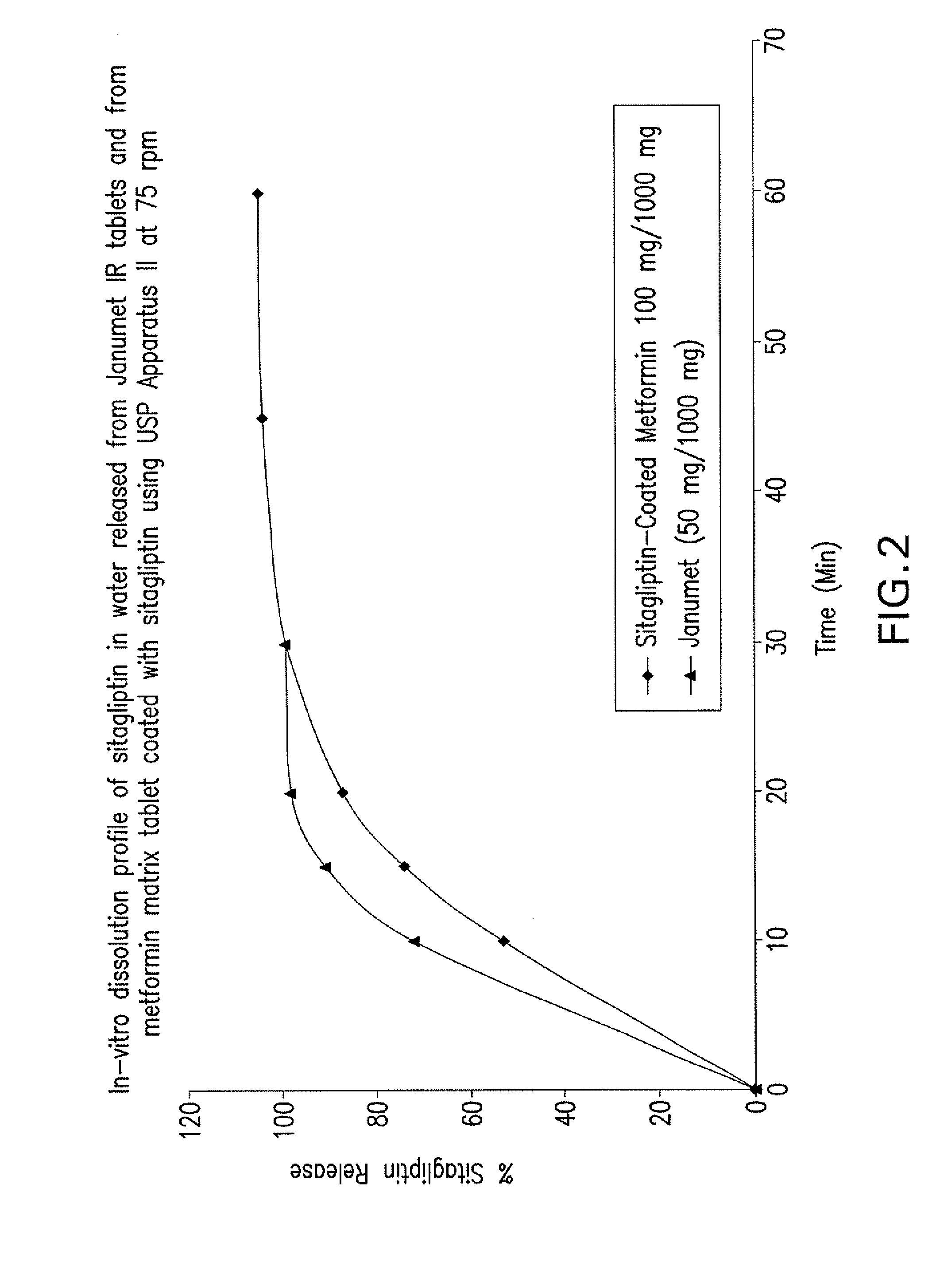
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Process for producing trifluoro benzene acetic acid and sitagliptin
ActiveCN101429115AHigh process yieldHigh purityOrganic compound preparationCarboxylic compound preparationSitagliptinBenzene
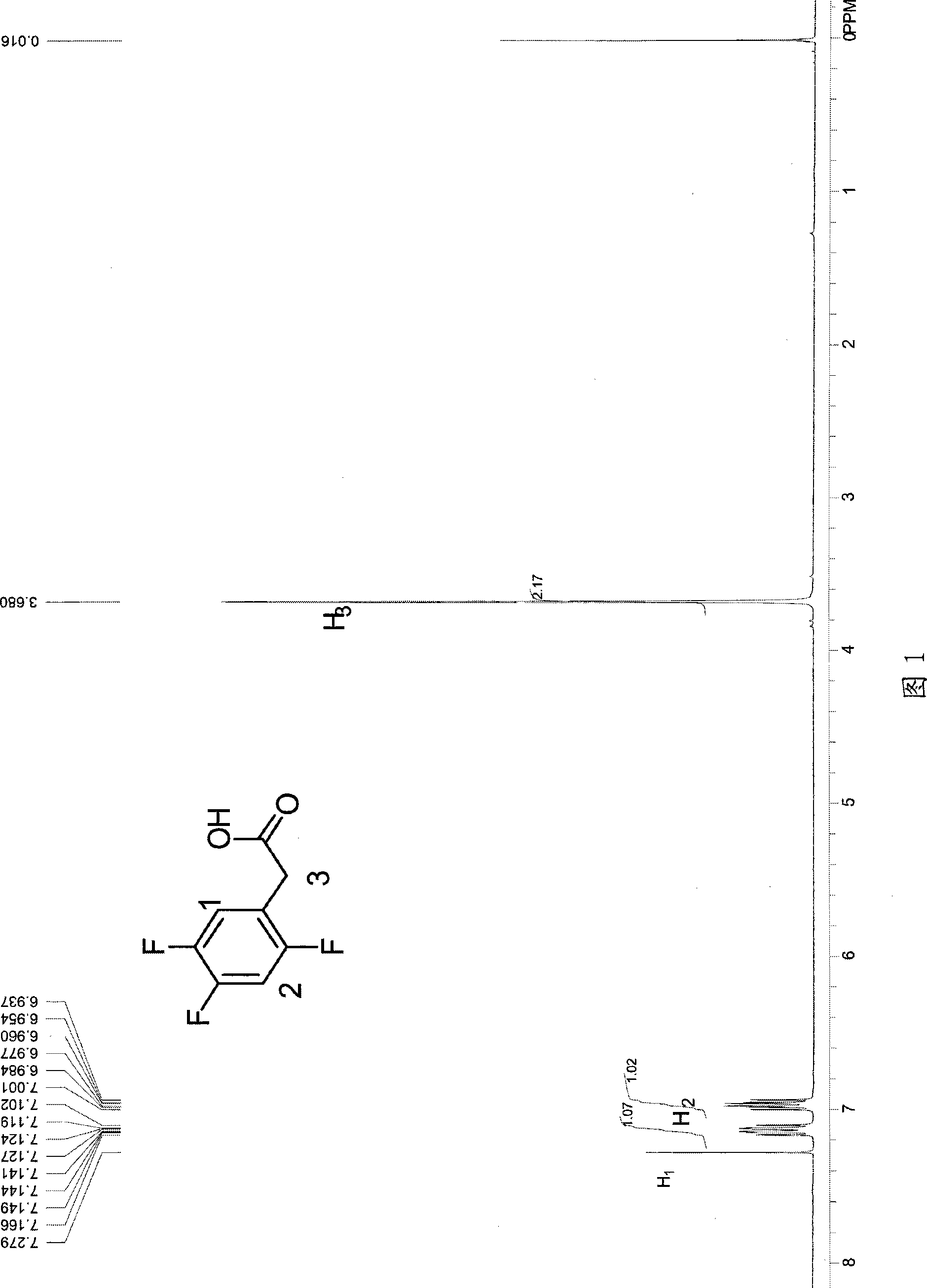
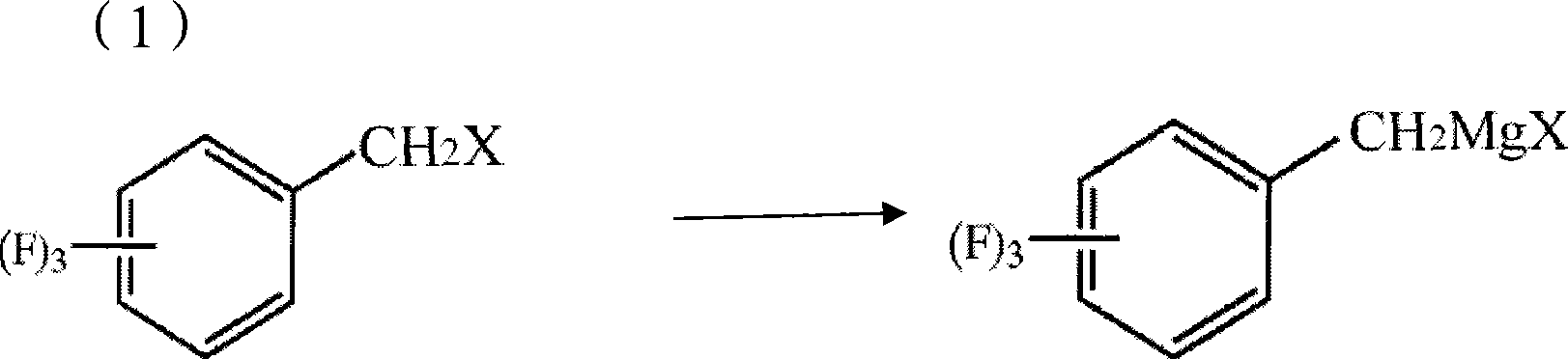
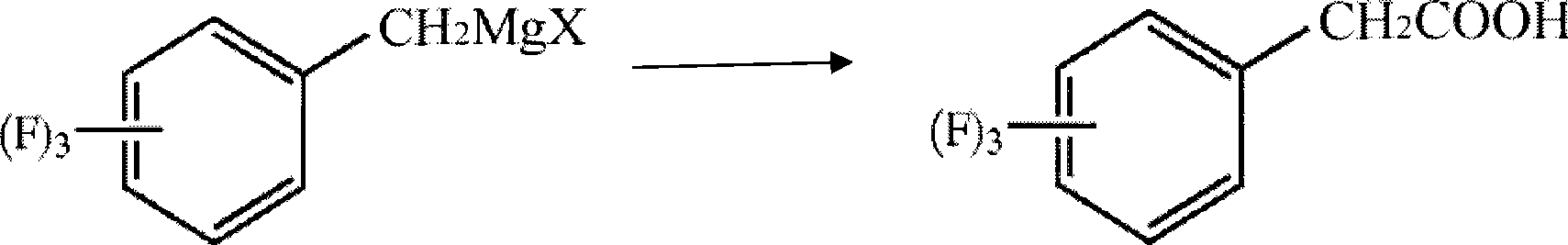
The invention discloses a method for preparing trifluoro-phenylacetic acid. The method comprises the following steps: (1) in the presence of an evocating agent, trifluoro-benzyl halides and magnesium in an organic solvent react to obtain a Grignard reagent; (2) carbon dioxide gas is introduced into the Grignard reagent for reaction; and (3) a product obtained in the step (2) is hydrolyzed to obtain the trifluoro-phenylacetic acid. The invention also discloses a method for preparing sitagliptin. The method has the characteristics of high yield, good purity, low cost, simple process, mild condition, few three wastes and good safety, and is suitable for industrialized production.
Owner:ZHEJIANG HISOAR PHARMA
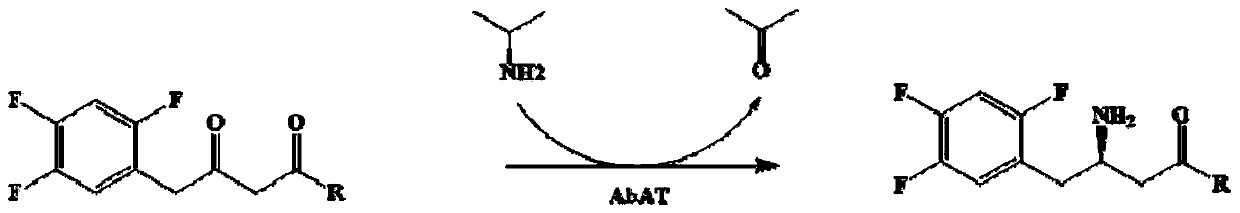
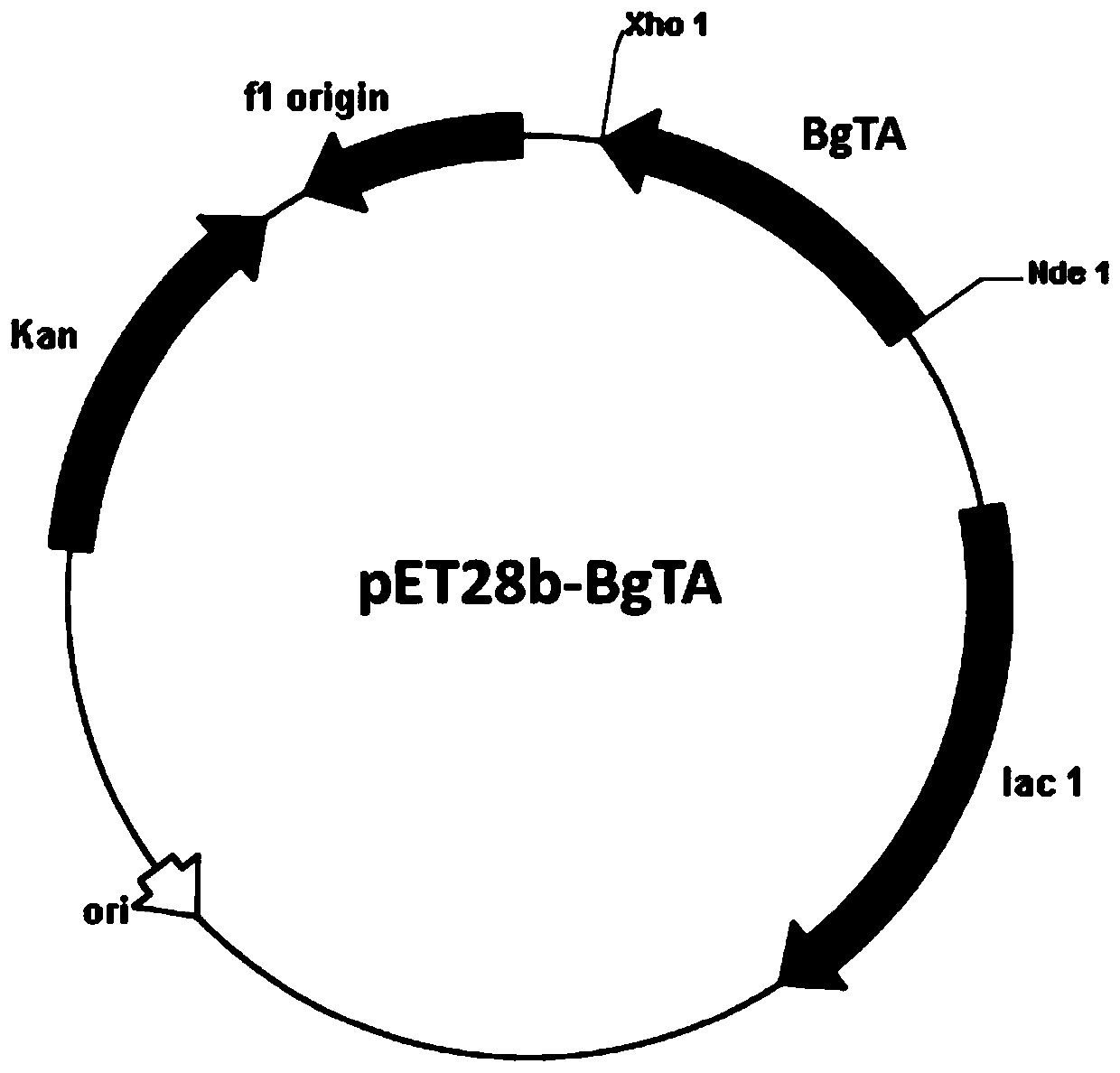
Aminotransferase, mutant and application to Sitagliptin preparation
The invention discloses aminotransferase, a mutant and application to Sitagliptin preparation. According to the application, wet thalli obtained by performing fermentation culture on recombinant escherichia coli containing aminotransferase encoding genes are used as biocatalysts; Sitagliptin precursor ketone is used as a substrate; dimethyl sulfoxide is used as a latent solvent; phosphopyridoxal is used as a coenzyme; isopropylamine is used as an auxiliary substrate; a trolamine buffer solution with the pH being 8 to 9 is used as a reaction medium; a reaction system is formed; the biocatalytic reaction is performed under the conditions of the temperature being 30 to 45 DEG C and the stirring speed being 100 to 250 r / min; after the reaction is completed, the reaction liquid is separated and purified; the Sitagliptin is obtained. The aminotransferase and the mutant are used as biocatalysts; the latent carbonyl compound of Sitagliptin precursor ketone is directly used as the substrate; meanwhile, biocatalytic reaction is performed by using isopropylamine as the auxiliary substrate and using the pyridoxal phosphate as the coenzyme; the separation and purification is performed; Sitagliptin with high optical purity is prepared. The method has the advantages that the total yield is 76 percent; the product e.e. value reaches 99 percent.
Owner:ZHEJIANG UNIV OF TECH +2
Pharmaceutical compositions of a combination of metformin and a dipeptidyl peptidase-iv inhibitor
Disclosed are pharmaceutical compositions comprising fixed-dose combinations of an extended-release form of metformin, or a pharmaceutically acceptable salt thereof, coated with an immediate-release form of the DPP-4 inhibitor sitagliptin, or a pharmaceutically acceptable salt thereof.
Owner:MERCK SHARP & DOHME CORP
Sitagliptin and enzyme-chemical preparation method of intermediate of sitagliptin
The invention discloses sitagliptin and an enzyme-chemical preparation method of an intermediate of the sitagliptin. The method comprises the steps of mixing 3-carbonyl-4-(2, 4, 5-trifluoro-phenyl)butyrate, D-amino acid dehydrogenase, a cofactor, cofactor-catalyzing cyclophorase, an amino doner, a cosolvent and a buffer solution according to a certain ratio to obtain a product (R)-3-amino-4-(2, 4, 5 trifluoro-phenyl)butyrate; enabling the (R)-3-amino-4-(2, 4, 5 trifluoro-phenyl)butyrate to be subjected to the steps of amino protection, condensation and deprotection to obtain sitagliptin free alkali. Compared with the prior art, the method adopted by the invention is less in process route, shorter in reaction time, improved in product purity and yield and lower in production cost, and is suitable for industrial production.
Owner:ZHEJIANG SUPOR PHARM CO LTD
Preparation method of sitagliptin
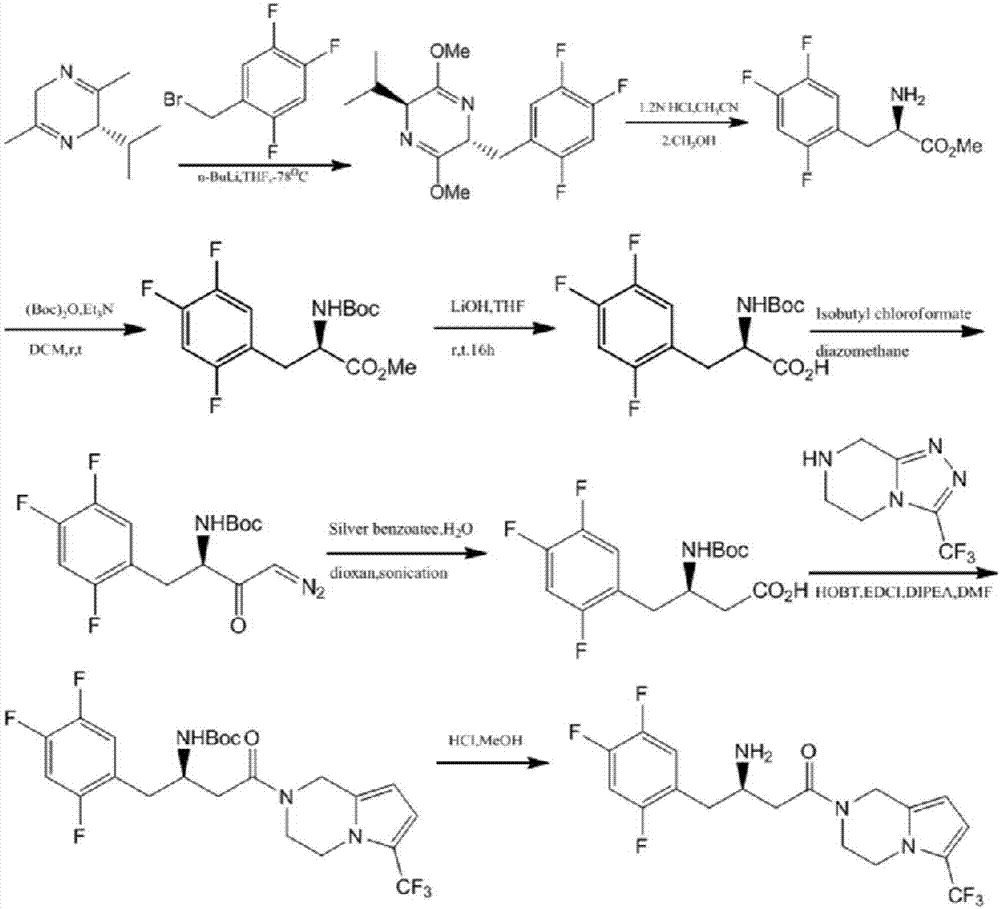
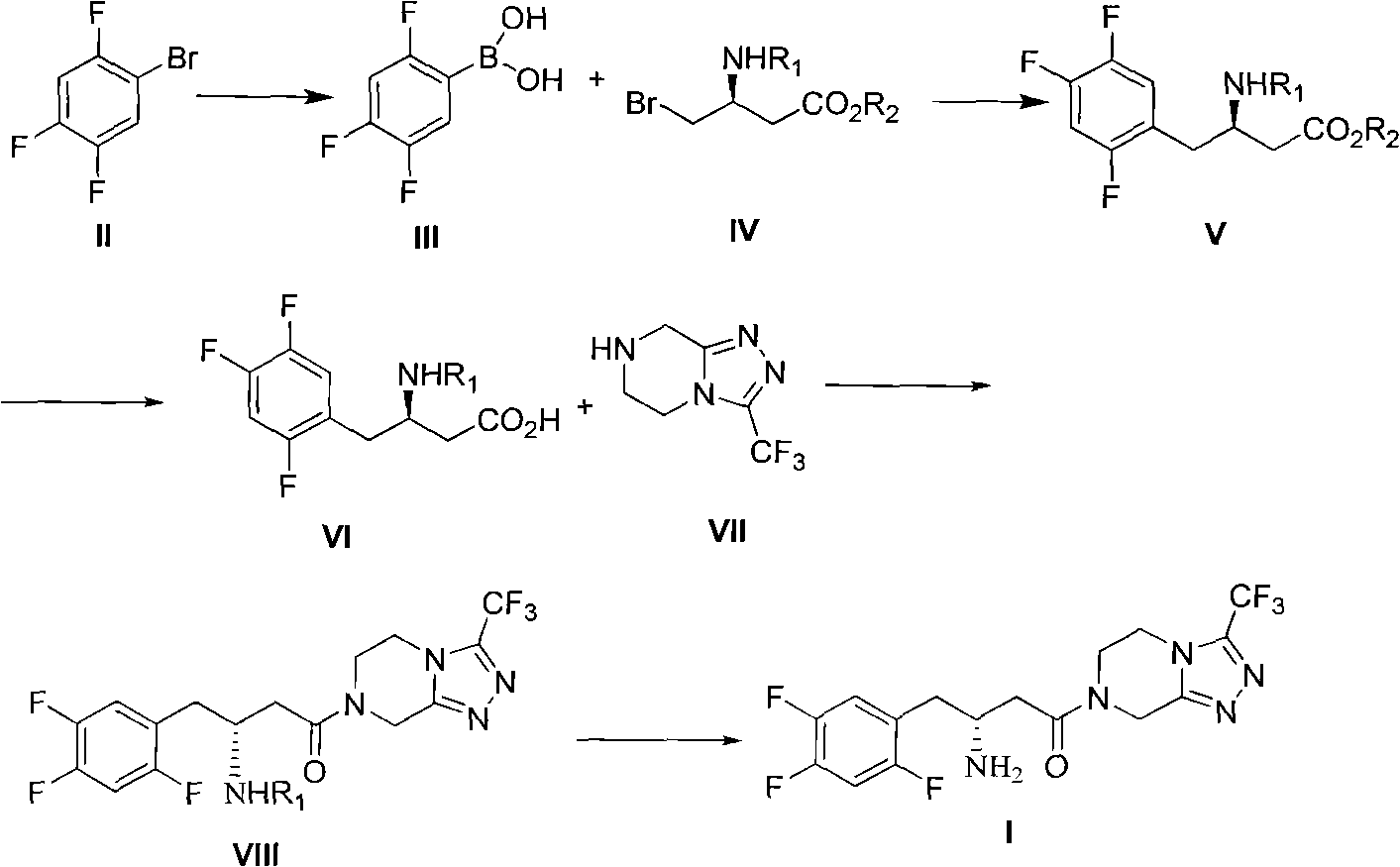
The invention discloses a preparation method of sitagliptin, comprising the following steps: reacting 2, 4, 5-trifluoro-phenylacetic acid with malonic cyclo (sub) isopropyl ester; reacting 3-oxo-4-(2, 4, 5-trifluorophenyl) methyl butyrate with ammonium acetate; reacting 3-amino-4-(2, 4, 5-trifluorophenyl) methyl crotonate with hydrogen; performing hydrolysis reaction to (R)-3-amino-4-(2, 4, 5-trifluorophenyl) methyl butyrate; and reacting (R)-3-amino-4-(2, 4, 5-trifluorophenyl) butyric acid with 3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-[1, 2, 4] triazol [4, 3-a] pyrazine hydrochloride to obtain sitagliptin. According to the invention, EE value greater than 90% is obtained through high-efficiency catalytic hydrogenation; sitagliptin is prepared by only five steps; yield is higher; technical conditions are mild; operations are simple; cost is low; and yield and purity of products are high.
Owner:SUZHOU XINKAI BIOLOGICAL MEDICINE TECH
Preparation method of sitagliptin intermediate, sitagliptin or salts thereof
ActiveCN102093245AAvoid low synthesis efficiency and high cost technical defectsOrganic compound preparationCarboxylic acid amides preparationSitagliptinOrganic solvent
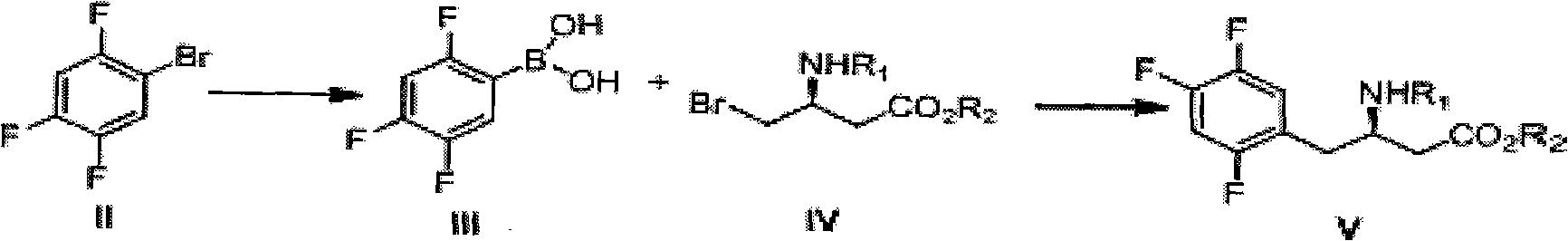
The invention discloses a preparation method of sitagliptin intermediate, sitagliptin or salts thereof. The method comprises the following steps of: (1) preparing a compound, namely 2,4,5-trifluorobromobenzene shown as a formula (II) into a Grignard reagent; (2) reacting the Grignard reagent with boric acid trimester in an organic solvent under the action of a catalyst to obtain a compound, namely 2,4,5-trifluorobenzolc acid shown as a formula (III); and (3) preparing a compound, namely (3R)-3-substituted amido-4-bromine-butyric ester shown as a formula (IV) and the 2,4,5-trifluorobenzolc acid shown as the formula (II) into a compound, namely (3R)-3-substituted amido-4-(2,4,5-trifluorophenyl)-butyric ester shown as a formula (V) under the action of a transition metal catalyst and alkali. By using the method, the synthesis efficiency is increased, and the cost is lowered.
Owner:ZHEJIANG HISOAR PHARMA
Pharmaceutical composition for the prevention or the treatment of non-alcoholic fatty liver disease and the method for prevention or treatment of non-alcoholic fatty liver disease using the same
InactiveCN102883721AInhibit progressInhibitory activityOrganic active ingredientsMetabolism disorderSitagliptinBULK ACTIVE INGREDIENT
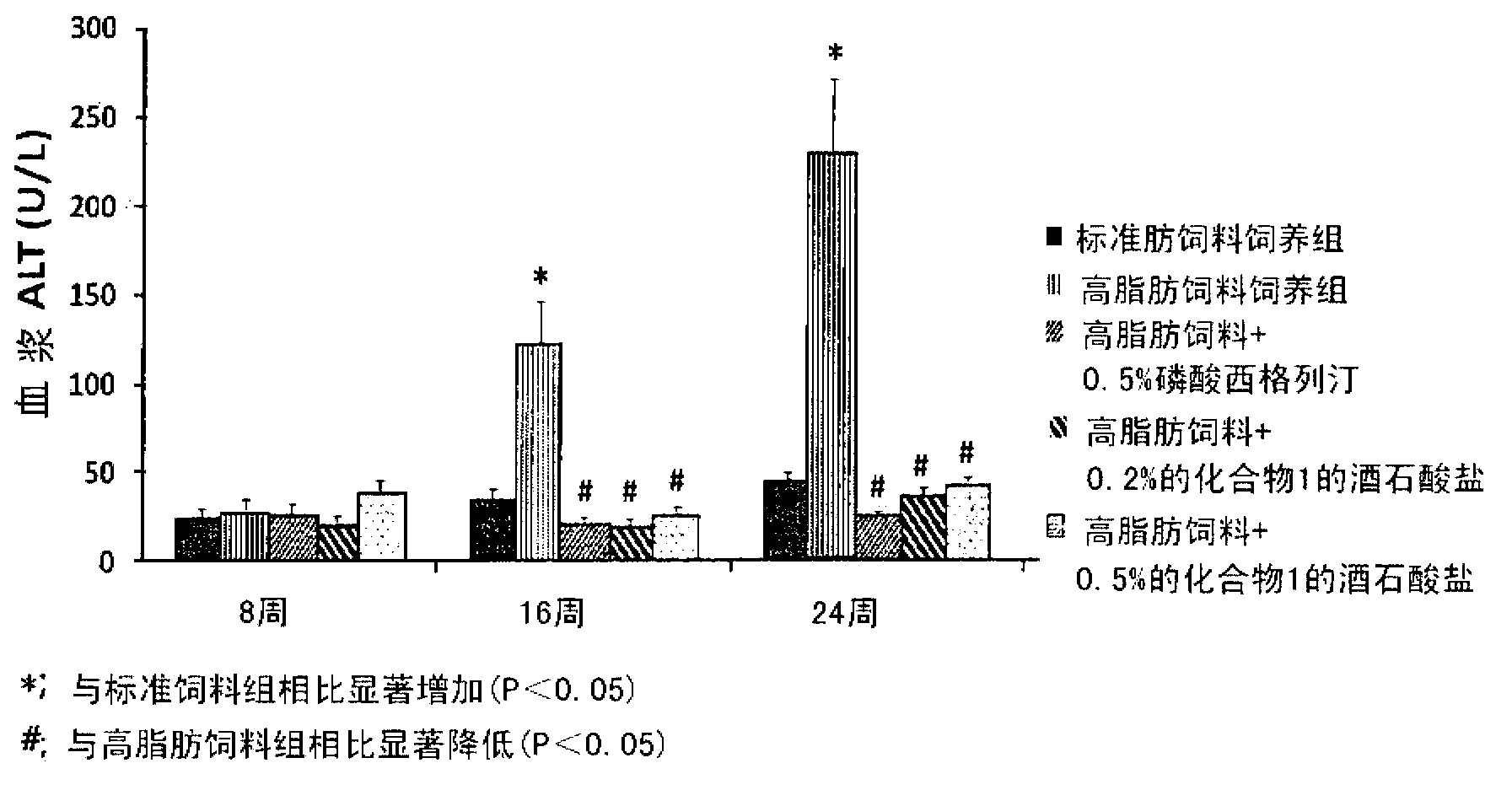
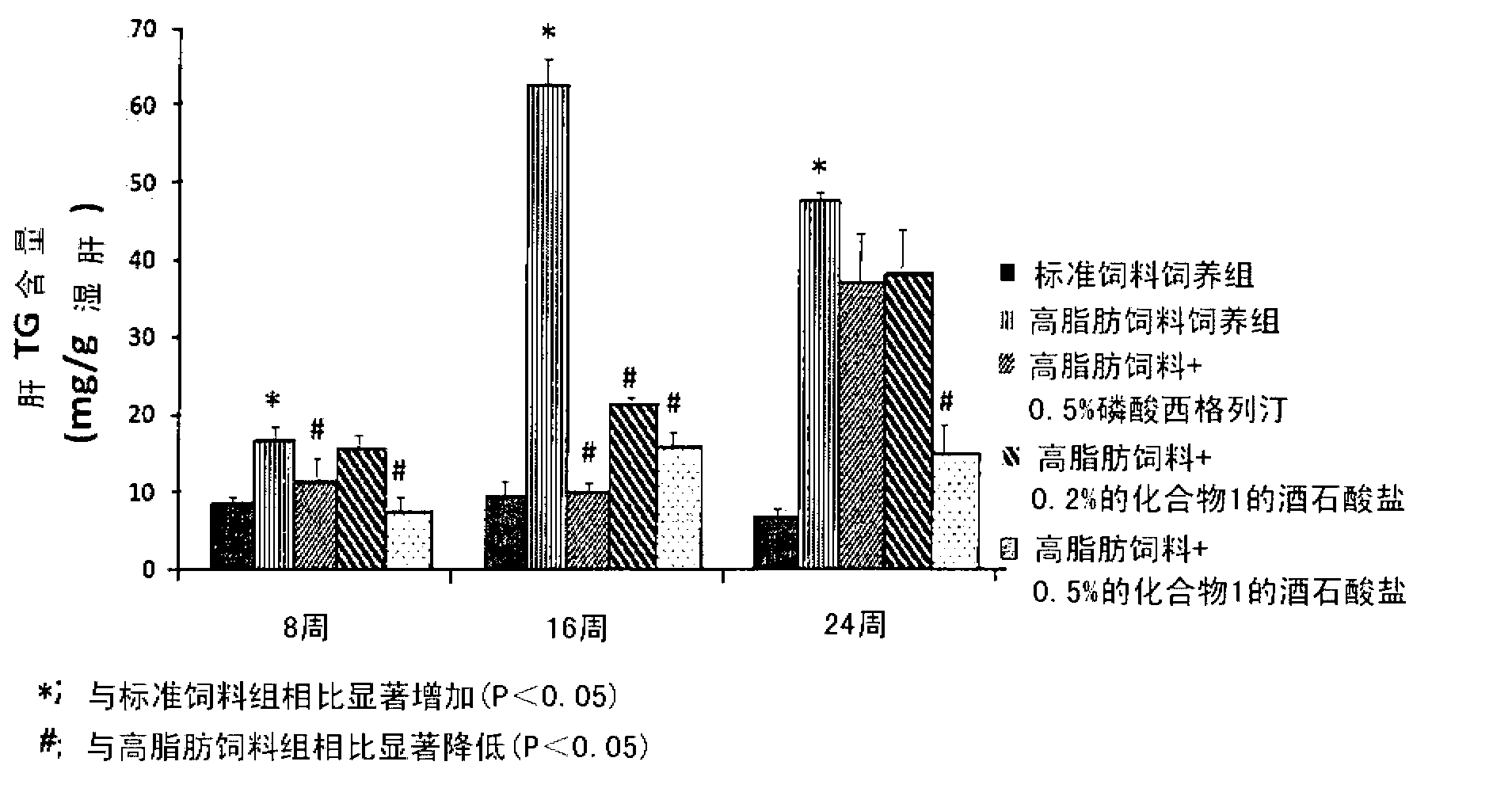
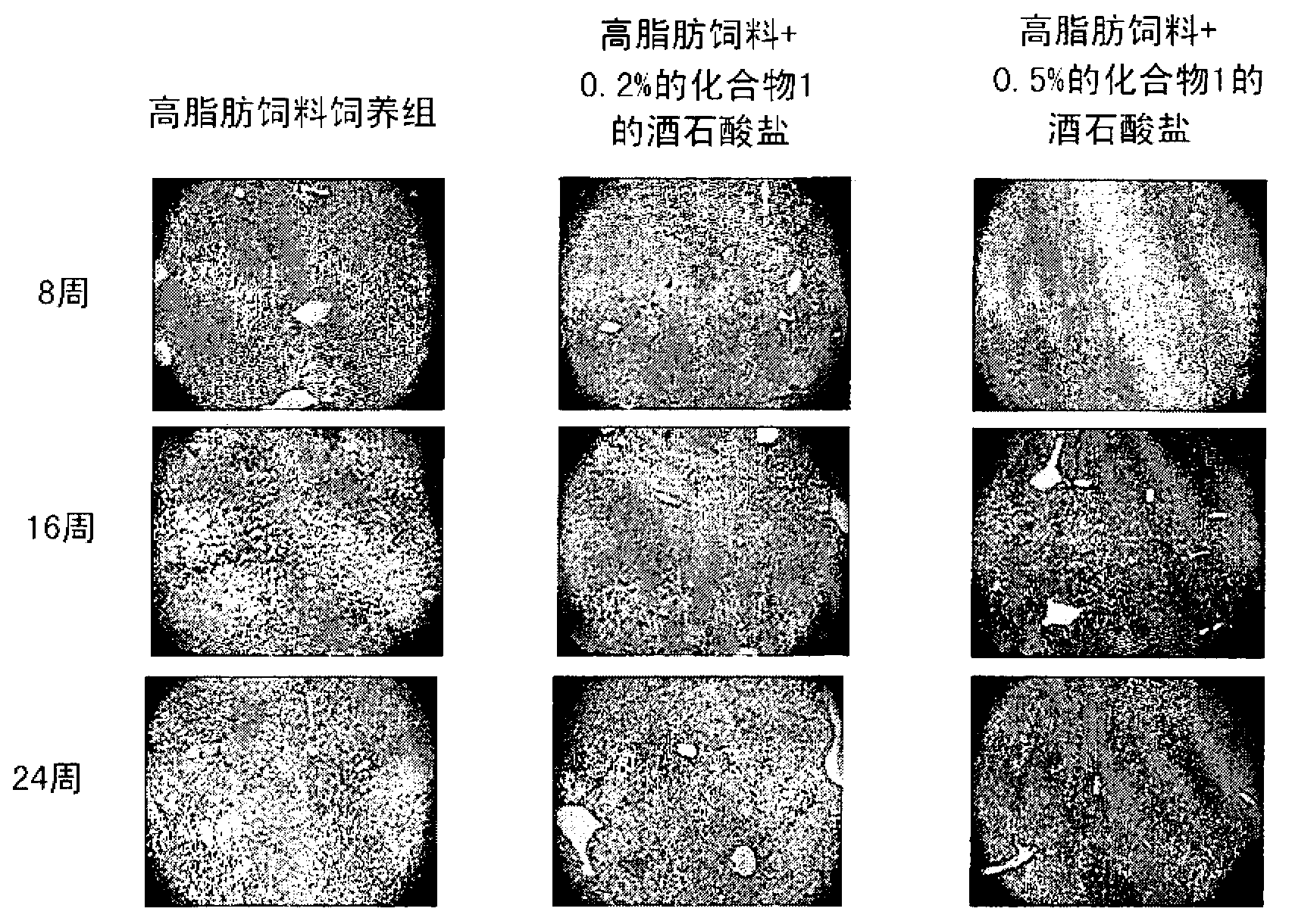
The present invention provides a pharmaceutical composition for the prevention and treatment of a non-alcoholic fatty liver disease (NAFLD), containing an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof. Further, the present invention provides a method for the prevention or treatment of a non-alcoholic fatty liver disease, including administering an effective amount of an active ingredient selected from the group consisting of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof to a mammal including a human in need thereof.; Further, the present invention provides use of Compound 1 represented by formula 1, sitagliptin, vildagliptin, linagliptin or a pharmaceutically acceptable salt thereof, for manufacturing a pharmaceutical composition for the prevention or treatment of a non-alcoholic fatty liver disease.
Owner:DONG A PHARMA
Immobilized transaminase and application of immobilized transaminase in synthesis of sitagliptin intermediate
The invention provides immobilized transaminase. The immobilized transaminase is obtained by fixing transaminase, with histidine tag, derived from Mycobacterium vanbaalenii PYR-1 onto an enzyme immobilization carrier; the enzyme immobilization carrier is obtained by derivatization of epoxy resin through IDA (imino diethyl acetic acid) and cobalt chloride or derivatization of ionic chelate resin through cobalt chloride. The immobilized transaminase has the advantages of firmness in combination, low enzyme activity loss, simplicity in separation and high reusability; low cost of an asymmetric conversion process, mild reaction conditions, environment friendliness, simplicity and convenience in operation, easiness in industrial expanding and promising industrial application prospect are realized.
Owner:ABIOCHEM BIOTECH CO LTD
Sitagliptin intermediates as well as preparation method and application of intermediate
InactiveCN102838511AAvoiding asymmetric catalytic hydrogenation reactionsImprove economyPreparation by cyanide reactionPreparation from nitrilesSitagliptinEpoxy
The invention relates to a Sitagliptin intermediates and a preparation method thereof as well as application of the intermediates in a method for preparing the Sitagliptin. According to the invention, epoxy chloropropane which is low in price and easy to obtain is used as a raw material to synthesize intermediates (S)-(2, 4, 5-trifluorophenyl) epoxy propane and (S)-3-hydroxy-4-(2, 4, 5-trifluorophenyl) butyronitrile, thus a chiral center is introduced, the use of various complex chiral reagents are avoided, and the chiral asymmetric catalytic hydrogenation reaction can be avoided; and the preparation method has the advantages of simple synthetic route, environment conservation, low raw material cost, etc.
Owner:ZHEJIANG HISOAR PHARMA
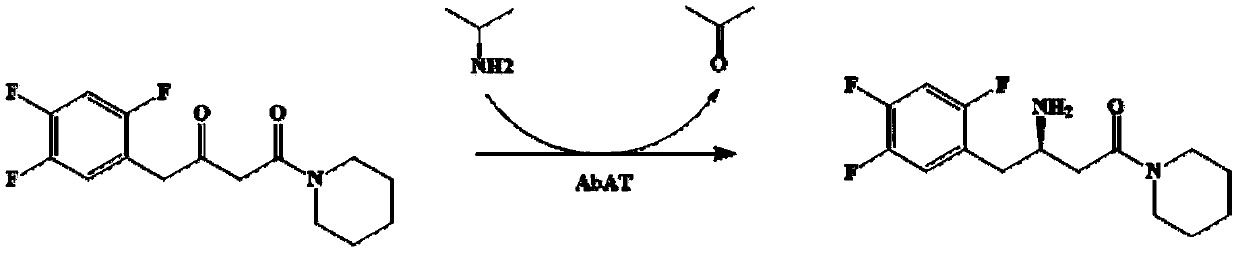
Omega-transaminase mutant and application thereof in preparation of sitagliptin intermediate
The invention discloses an omega-transaminase mutant and application thereof in preparation of sitagliptin intermediate. The omega-transaminase mutant is obtained by performing single mutation on the56 position or the 134 position of an amino acid sequence as shown in a SEQ ID No.2; the 56 phenylalanine is substituted by valine, histidine or tyrosine; and the 134 threonine is substituted by glycine. The omega-transaminase mutant has relatively high enzyme activity which is higher than 300U / g and is at least 2.7 times that of a wild type since the omega-transaminase mutant is subjected to site-specific mutagenesis by acquiring certain sites possibly influencing the catalytic activity of the omega-transaminase mutant through molecular docking, homologous modeling and other modes, can be used for efficiently catalyzing precursor ketone 1-piperidine-4-(2,4,5-trifluorophenyl)-1,3-dibutanone of the sitagliptin intermediate to synthesize the sitagliptin intermediate (R)-3-amino-1-piperidine-4-(2,4,5-trifluorophenyl)-1-butanone, and has a conversion rate of 85 percent.
Owner:ZHEJIANG UNIV OF TECH +2
Pseudomonas aeruginosa ZJPH1504 and application thereof in preparation of sitagliptin chiral intermediate
InactiveCN105925506AHigh optical purityHigh catalytic efficiencyBacteriaMicroorganism based processesSitagliptinDi n butyl phthalate
The invention discloses a pseudomonas aeruginosa ZJPH1504 and an application of pseudomonas aeruginosa ZJPH1504 in preparation of a sitagliptin chiral intermediate. The pseudomonas aeruginosa ZJPH1504 can be used for asymmetric reduction of a prochiral ketone compound (II) with high stereoselectivity to prepare a sitagliptin chiral intermediate (I-a) compound, the product prepared by using the pseudomonas aeruginosa ZJPH1504 is high in optical purity, and the e.e. (Errors Excepted) value is greater than 99.9%, 9g / L of a substrate is added in a phosphate buffer solution system of which the pH is 7.5, and reacted for 30 hours, and the yield of an S-reduction product is 60.2%. When an organic solvent is added in a reaction system, the catalytic efficiency can be effectively improved, the reaction yield can be increased, and the reaction time can be shortened, especially when di-n-butyl phthalate is added in the reaction system, the reaction yield can be increased to 75.6%, the reaction time can be shortened to 24 hours and the e.e. value is greater than 99.9%.
Owner:ZHEJIANG UNIV OF TECH
Transaminase mutant as well as application thereof to preparation of sitagliptin midbody
The invention discloses a transaminase mutant as well as application thereof to preparation of sitagliptin midbody. The application adopts a wet thallus obtained by fermenting and culturing recombinant escherichia coli comprising aminotransferase coded gene as a biological catalyst, adopts sitagliptin midbody precursor ketone as a substrate, adopts dimethyl sulfoxide as a co-solvent and , adopts phosphopyridoxal as co-enzyme, adopts isopropylamine as an auxiliary substrate, adopts a pH 8-9 triethanolamine buffering solution as a reaction medium to form a reaction system to perform the biological catalytic reaction under the conditions that the temperature is 30 to 45 DEG C, and the stirring rate is 100 to 250 r / min, after the reaction is ended, the reaction solution is separated and purified to obtain sitagliptin; and the total yield of the method is about 81 percent, and a e.e. value of the product reaches 99 percent.
Owner:ZHEJIANG UNIV OF TECH +2
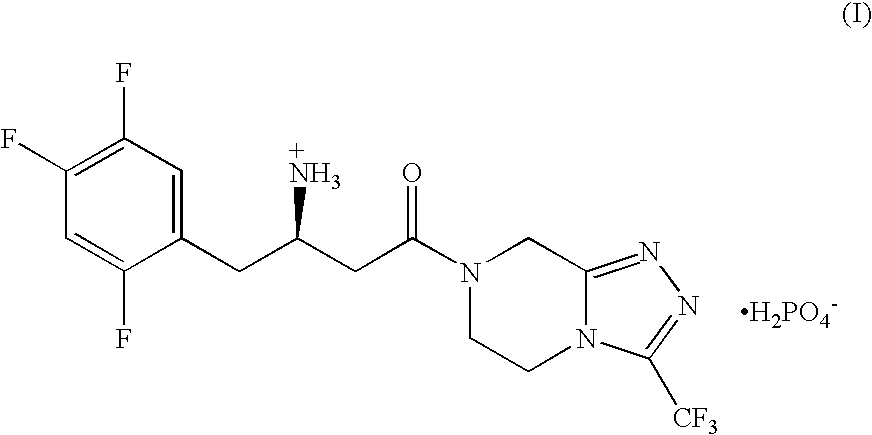
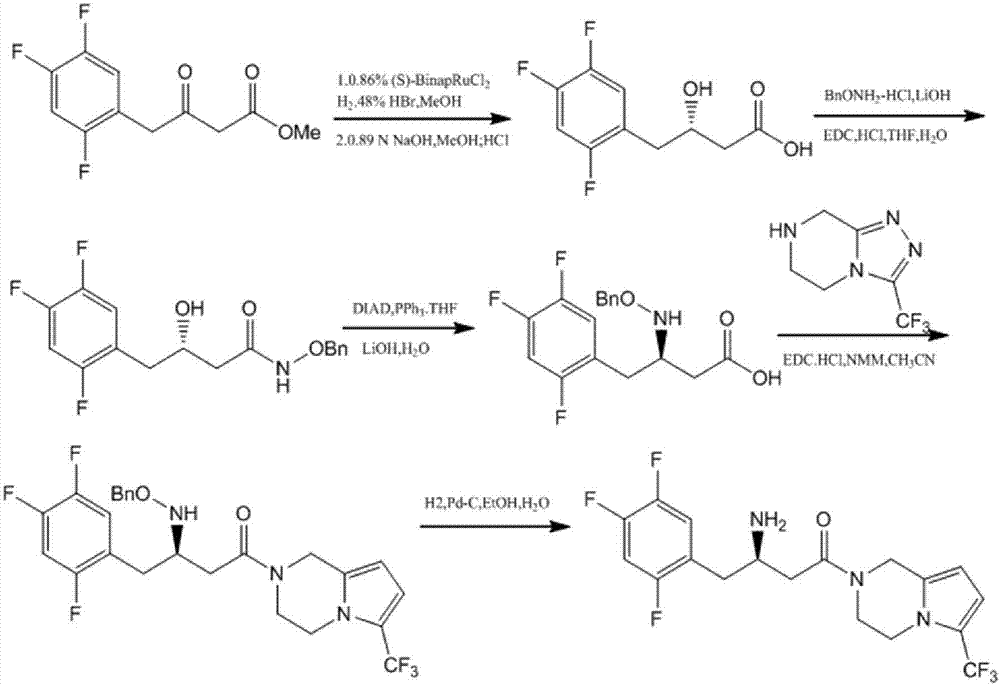
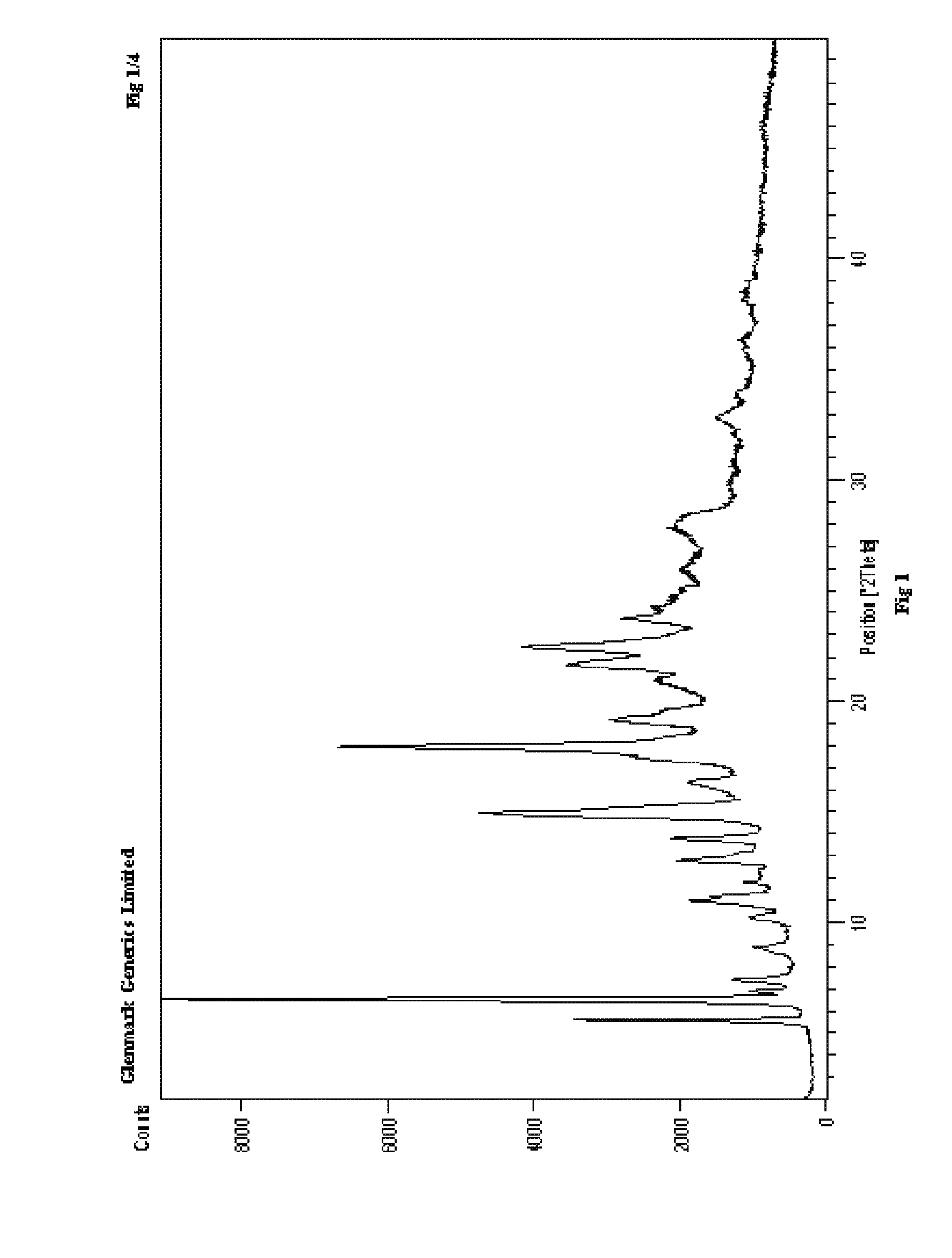
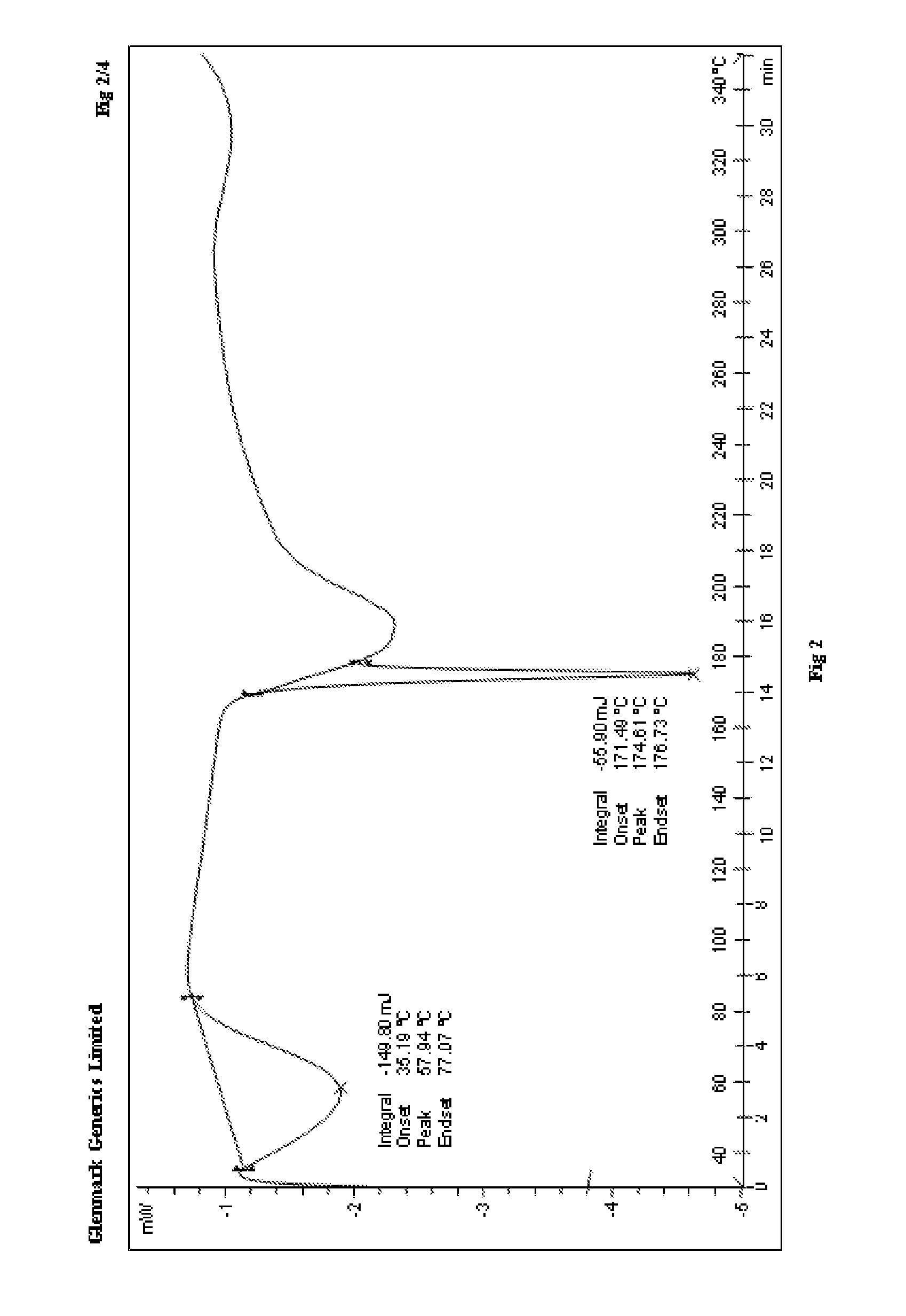
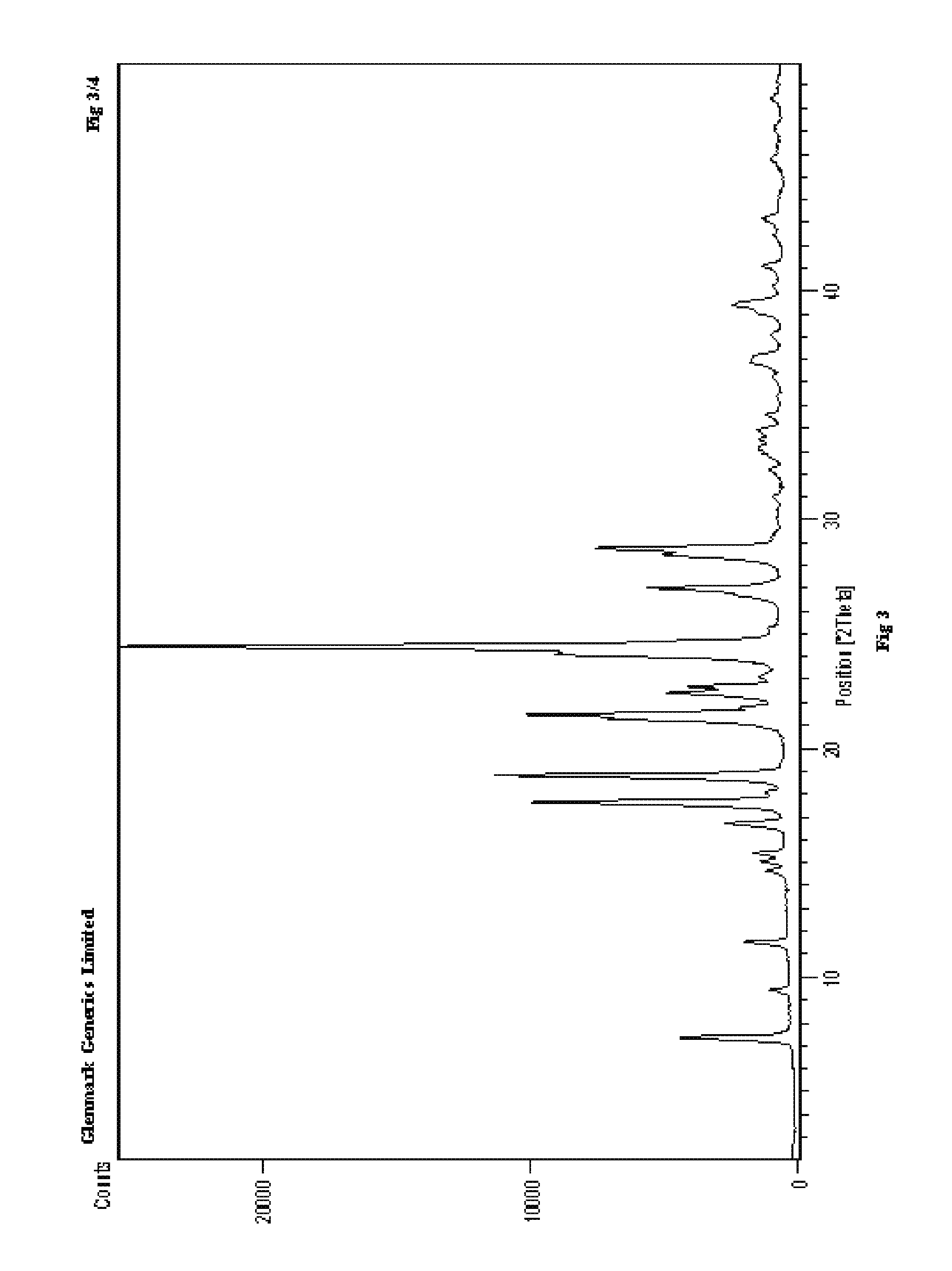
Process for the preparation of r-sitagliptin and its pharmaceutically acceptable salts thereof
The present invention provides processes for the preparation of R-sitagliptin and its pharmaceutically acceptable salts thereof.
Owner:GLENMARK LIFE SCI LTD
Immobilized transaminase and applications thereof in synthesizing of Sitagliptin intermediate
ActiveCN104805069AImprove bindingLittle loss of enzyme activityChemical industryOn/in organic carrierSitagliptinEnzyme
The invention provides an immobilized transaminase obtained via immobilizing recombined transaminase on sodium alginate. Firm bonding of the immobilized transaminase is realized; enzyme activity loss is less; separation is simple; recycling can be realized for a plurality of times; asymmetric conversion process cost is low; reaction conditions are mild; the immobilized transaminase is friendly to the environment; operation is simple; industrial enlarged production is convenient to realize; and industrial application prospect is promising.
Owner:ABIOCHEM BIOTECH CO LTD
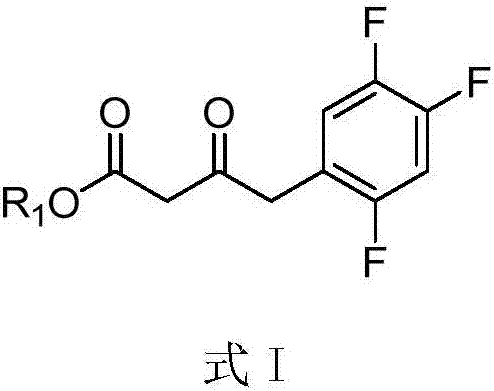
Purpose of compound for preparing Sitagliptin and Sitagliptin preparation method
ActiveCN107286164AHigh yieldImprove conversion efficiencyOrganic chemistryFermentationSitagliptinSolubility
The invention provides a compound shown by a formula I, a purpose of a stereoisomer, a geometrical isomer, a tautomer, an oxynitride, a hydrate, a solvate, a metabolite, a pharmacologically acceptable salt or prodrug of the compound in Sitagliptin preparation, and a Sitagliptin preparation method. The formula I is shown in the description, wherein R1 is a substitutional group containing the hydroxyl group. The compound shown by the formula I or the stereoisomer, the geometrical isomer, the tautomer, the oxynitride, the hydrate, the solvate, the metabolite, the pharmacologically acceptable salt or the prodrug of the compound shown by the formula I has high water solubility, and is used for obtaining a chiral amino intermediate to further obtain the Sitagliptin. Compared with the prior art, the compound has the advantages that the amino group conversion rate is greatly improved; the yield of the Sitagliptin is also greatly improved.
Owner:WUHAN HESHENG TECH CO LTD
Novel method for synthesizing sitagliptin
ActiveCN102757431AReduce pollutionHigh purityOrganic compound preparationAmino-carboxyl compound preparationSitagliptinChemistry
The invention discloses a novel method for synthesizing sitagliptin. The method has the advantages of low cost, simplicity in operation, low environmental pollution, high yield and purity of product and the like, and is particularly applicable to industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +1
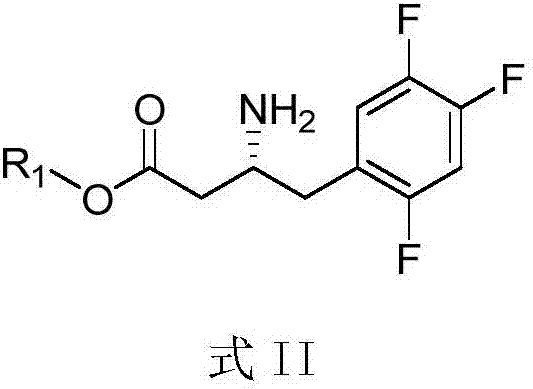
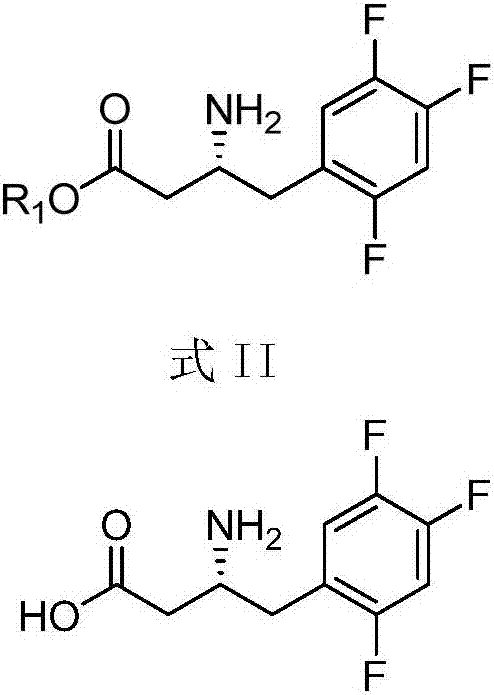
Intermediates of Sitagliptin and preparation method thereof
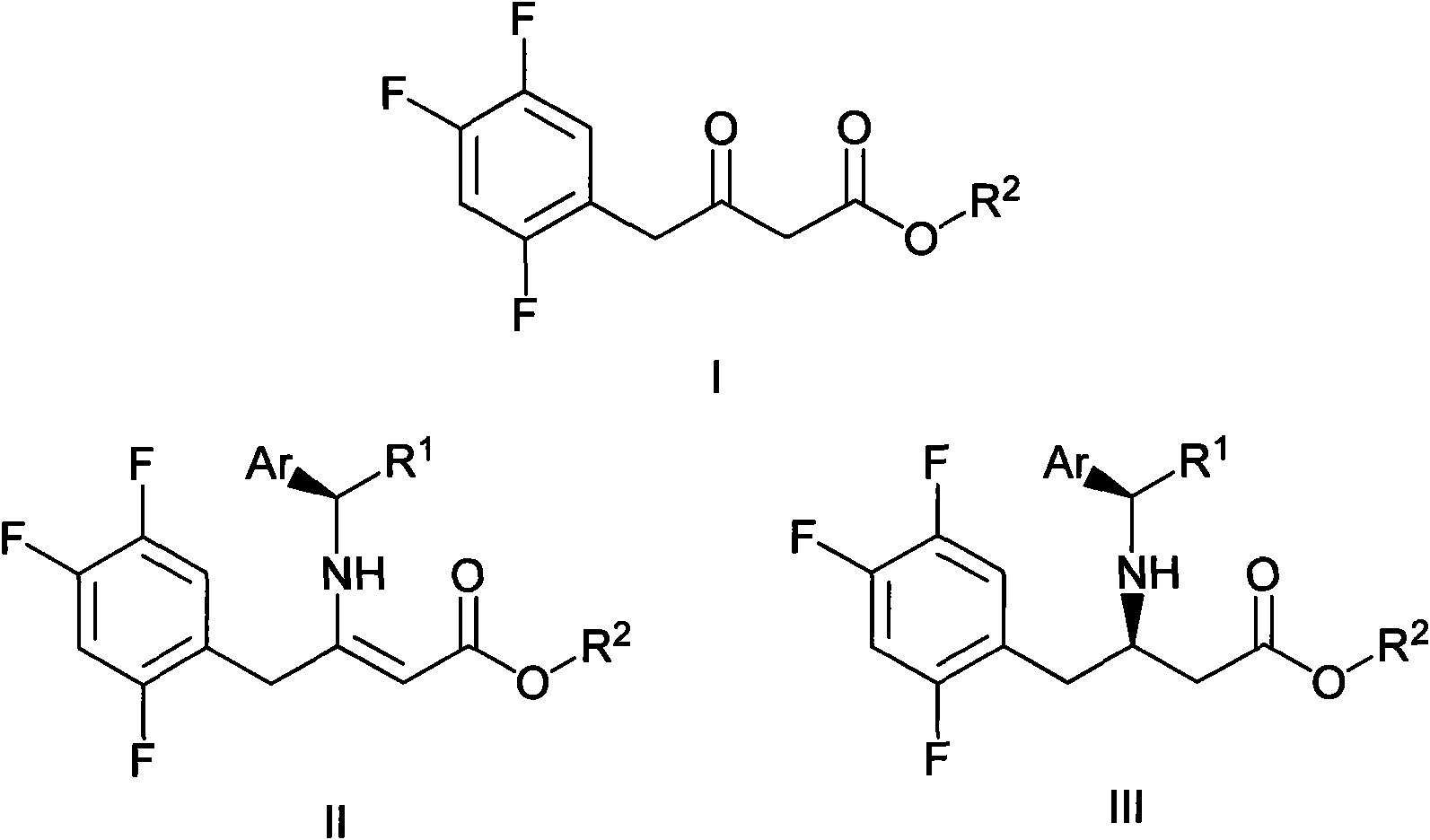
ActiveCN102126976ASimple processReduce dosageOrganic compound preparationAmino-carboxyl compound preparationSitagliptinChemical structure
The invention mainly relates to intermediates of Sitagliptin and a preparation method thereof. The intermediates of the Sitagliptin have chemical structures shown as II and III, wherein definitions of Ar, R1 and R2 are shown in the specifications. The compounds shown as the II and the III are prepared by reacting a compound shown as a formula I with a substituent of phenylethylamine, which serve as raw materials, under hydrogen pressure. The intermediates are used for synthetizing the Sitagliptin, so synthetic difficulty is reduced, purity of a product is improved, the using amount of a high-price catalyst is reduced, production cost is effectively reduced, and the intermediates and the method are suitable for industrialized production.
Owner:JIANGSU SENRAN CHEM +1
Synthetic method of sitagliptin and salt thereof
The invention discloses a synthetic method of sitagliptin and a salt thereof. The synthetic method comprises the steps: carrying out an esterification reaction, a reduction reaction, an oxidizing reaction and a witting reaction on 2,4,5-trifluorophenylacetic acid as a starting raw material to obtain 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate; then carrying out a hydroamination reaction on 4-(2,4,5-trifluorophenyl)-2-ethyl crotonate and chiral amine in the presence of butyl lithium or hexamethyldisilazane sodium to form a chiral hydroamination product; carrying out an esterolysis reaction, a condensation reaction and a hydrogenation reaction to obtain sitagliptin. The raw materials used in the synthetic method of sitagliptin are low in price and easy to obtain; the synthetic method of sitagliptin is less in step, easy to operate and capable of effectively reducing cost. By the use of the method, high-purity sitagliptin can be obtained, a sitgliptin phosphate obtained through salifying has an HPLC (High Performance Liquid Chromatography) and an ee (enantiomeric excess) value of more than 99% and can be applied to the field of medicine.
Owner:ZHEJIANG NHU CO LTD +1
Preparation method of sitagliptin
ActiveCN102627648ALow costHigh yieldOrganic chemistryBulk chemical productionPhenylacetic acidMethyl palmoxirate
The invention provides a preparation method of sitagliptin. The preparation method comprises the following steps of: performing condensation reaction on hydrochloride of 3-trifluoromethyl-[1,2,4] triazol [4,3-a] piperazine serving as a starting raw material and methyl malonyl chloride under a normal temperature condition; reacting an obtained product with 2,4,5-trifluorophenylacetic acid under an alkaline condition and then performing condensation reaction with (S)-phenylglycinamide under normal temperature condition to obtain a product; reducing the obtained product through a reducing agent; removing an ester group through heating reflux; and reacting with a hydrogenation reducing reagent to obtain the sitagliptin. The preparation method has the advantages of low cost, high yield, easiness in operation, all used reagents of conventional reagents, simple post-treatment and convenience for industrial production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Medicinal composition containing sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof and application
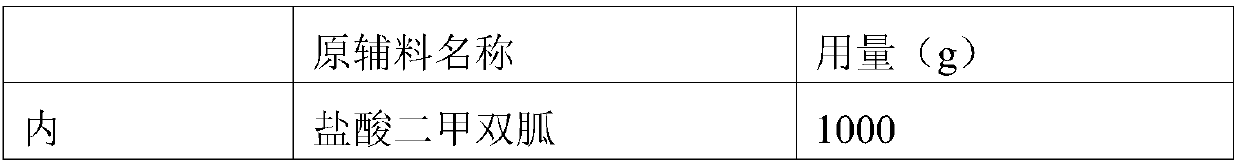
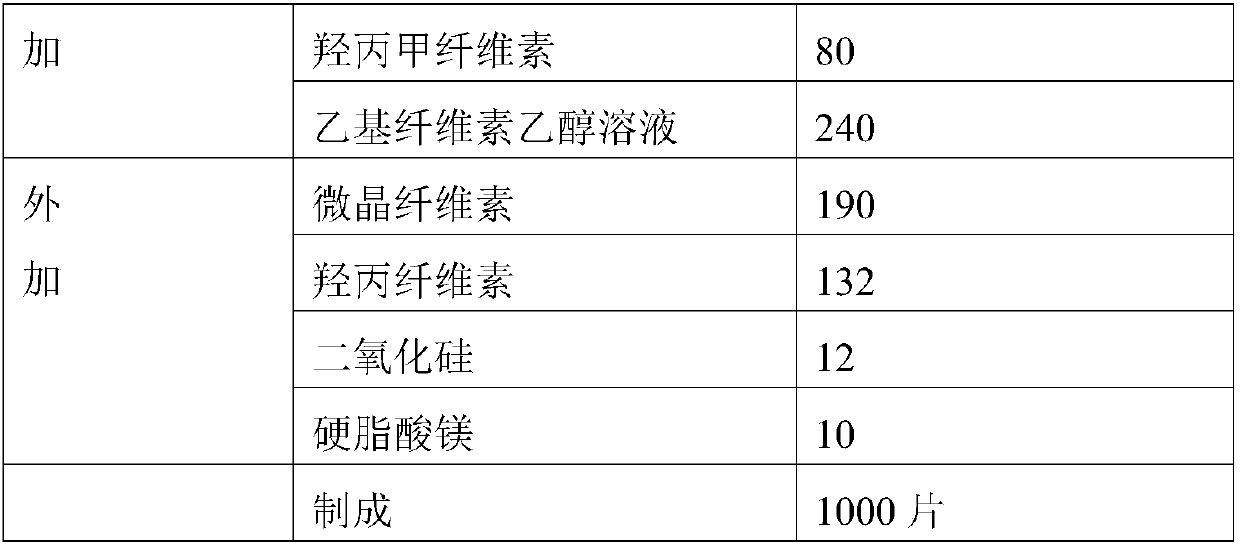
ActiveCN109157522AQuality improvementIncrease contentOrganic active ingredientsMetabolism disorderSitagliptinHydrogen phosphate
The invention relates to a medicinal composition. The medicinal composition comprises sitagliptin, pharmaceutically acceptable salt thereof and / or hydrate of the salt, and anhydrous calcium hydrogen phosphate. The medicinal composition is further used for preparing a solid preparation, and is especially prepared into a tablet by a direct tabletting method. Through selection of the medicinal composition, especially selection of the specific anhydrous calcium hydrogen phosphate as a raw material, the quality control way of the medicinal composition using the sitagliptin, the pharmaceutically acceptable salt thereof and / or the hydrate of the salt as an active component can be significantly improved, and even production of related impurities in the medicinal composition can be significantly improved so as to improve the stability of the medicinal composition and relevant dosage forms.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Glucokinase Activator Compositions for the Treatment of Diabetes
ActiveUS20140066372A1Improve glucose toleranceIncrease secretionBiocidePeptide/protein ingredientsSitagliptinAcetic acid
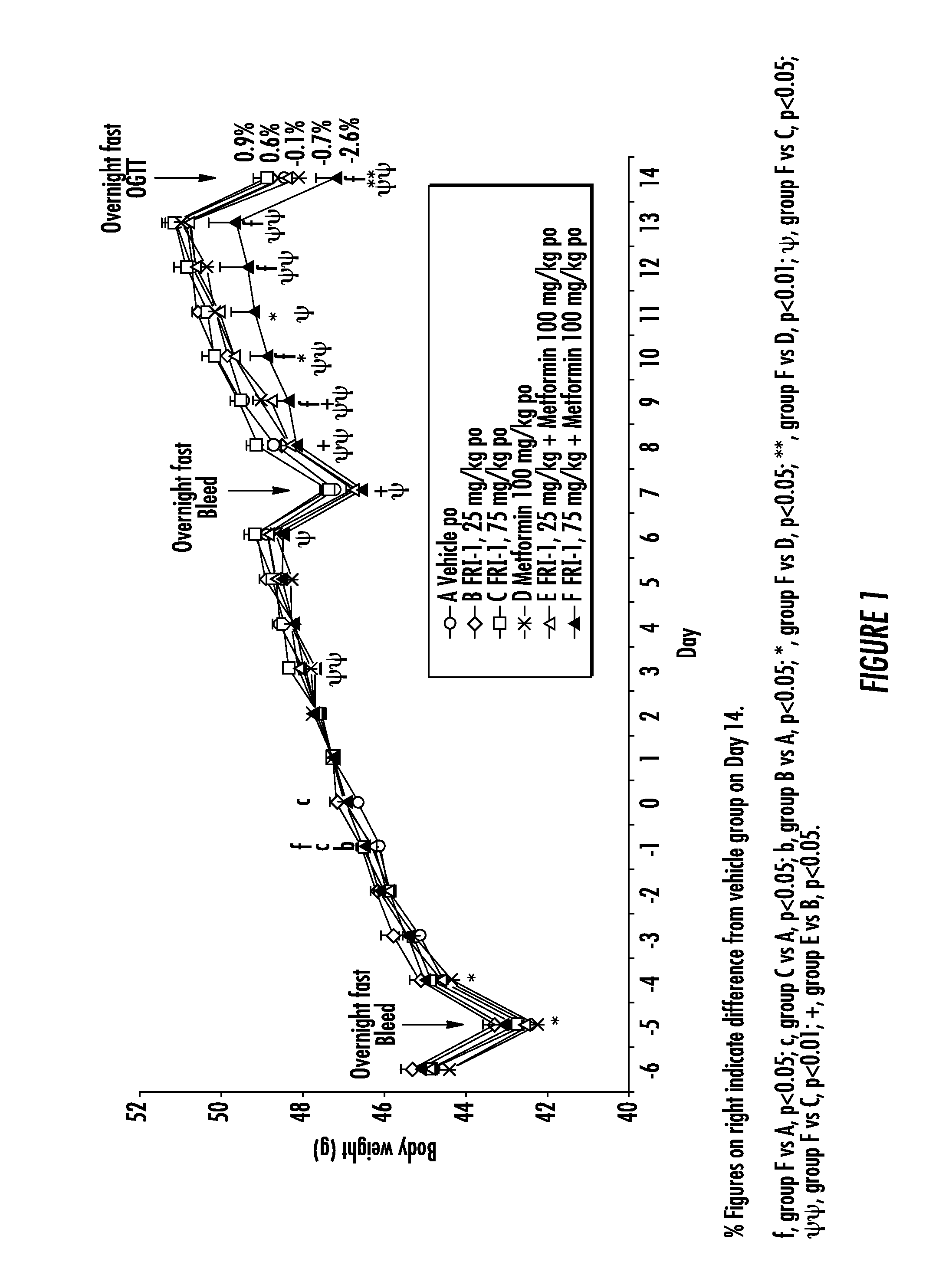
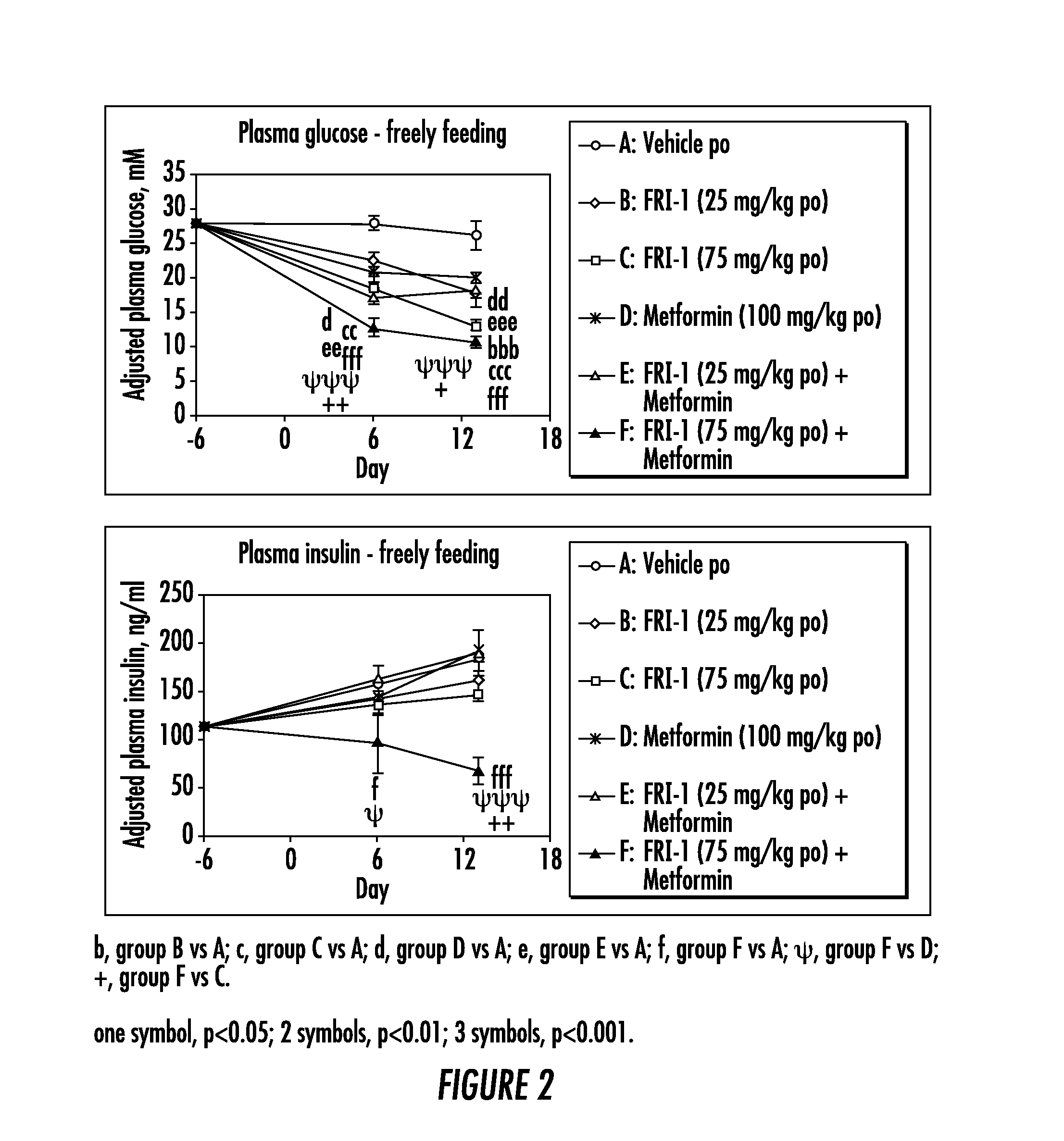
The present invention relates to pharmaceutical compositions comprising {2-[3-cyclohexyl-3-(trans-4-propoxy-cyclohexyl)-ureido]-thiazol-5-ylsulfanyl}-acetic acid (FRI-1) in combination with an anti-diabetic drug selected from the group consisting of metformin, sitagliptin or exenatide. The present invention also relates to the use of the pharmaceutical compositions in restoring insulin sensitivity and treating type II diabetes, including reducing body weight in subjects undergoing type II diabetes treatment.
Owner:VTV THERAPEUTICS LLC
Pharmaceutical composition of sitagliptin
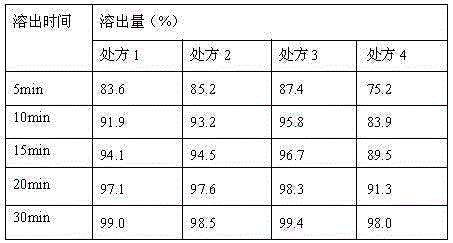
InactiveCN106176653AImprove stabilityHigh dissolution rateOrganic active ingredientsMetabolism disorderSitagliptinDissolution
Belonging to the technical field of pharmaceutical preparations, the invention in particular relates to a pharmaceutical composition of sitagliptin and a preparation method thereof. The pharmaceutical composition of sitagliptin provided by the invention employs the process of mixing sitagliptin and specific auxiliary materials in certain proportion, and obviously overcomes the defects of poor stability, complex production process and incapability of mass production in the prior art. At the same time, the invention accidentally finds that the tablet prepared by the method involved in the invention has better dissolution rate.
Owner:TIANJIN HANRUI PHARMA
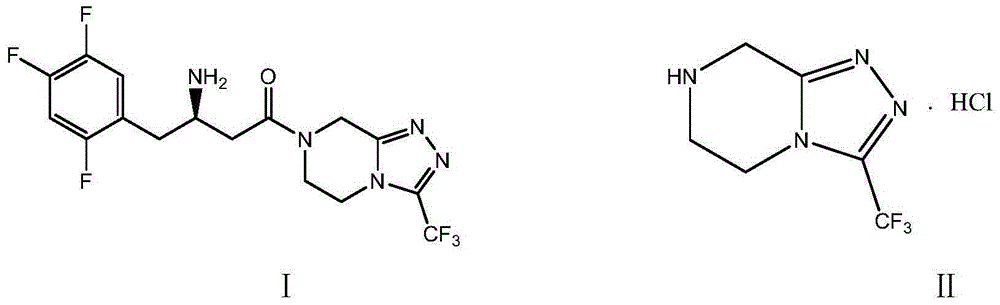
Sitagliptin impurity and preparation and detection method thereof
ActiveCN106478637AEnsuring Safety and ReliabilityHigh yieldOrganic chemistryComponent separationSitagliptinPyrazine
The present invention provides sitagliptin impurity compound I and a preparation method and application thereof in the quality control study of sitagliptin. The sitagliptin impurity compound I is as follows: (R)-3-amino-N-[(R)-4-oxy-4-(3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazole[ 4, 3-a] pyrazine-7(8H)-yl)-1-(2, 4, 5-trifluorophenyl) butyl-2-yl)-4-(2, 4, 5-trifluorophenyl) butyramide.
Owner:NANJING CHIA TAI TIANQING PHARMA
Drug composition containing sitagliptin and melbine
ActiveCN107669683AQuick effectLong-term maintenance of blood sugar balanceOrganic active ingredientsMetabolism disorderSustained Release TabletSitagliptin
The invention discloses a drug composition containing sitagliptin and melbine. A melbine sustained release tablet core is coated with sitagliptin in a mixed suspension mode. The invention also discloses composition of a sitagliptin suspension. By carefully selecting preparation prescriptions, antioxidants are not used in the prescriptions, a production process is simple, and meanwhile and the technical advantages of uniform preparation content, consistent dissolution, good stability and the like are also achieved.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Transaminase-PLP co-immobilized enzyme and preparation and application thereof
The invention relates to a transaminase-PLP co-immobilized enzyme and a preparation and application thereof, and application of the co-immobilized transaminase for preparing a Sitagliptin medicine chiral midbody. Epoxy resin is adopted as a carrier, and the transaminase-PLP co-immobilized enzyme is obtained by covalence immobilizing of transaminase and coenzyme pyridoxal phosphate (PLP for short).The transaminase-PLP co-immobilized enzyme is adopted as a catalyst, the stability is good, the service life is long, the organic solvent tolerance is good, the transaminase-PLP co-immobilized enzymecan be repeatedly used, no expensive exogenous coenzymes are added in the reaction process, and the production cost is greatly lowered. The method is simple in technology, low in cost, and high in product yield and purity, and the enzyme has the great application value in industrial production of the Sitagliptin medicine chiral midbody.
Owner:ZHEJIANG UNIV OF TECH
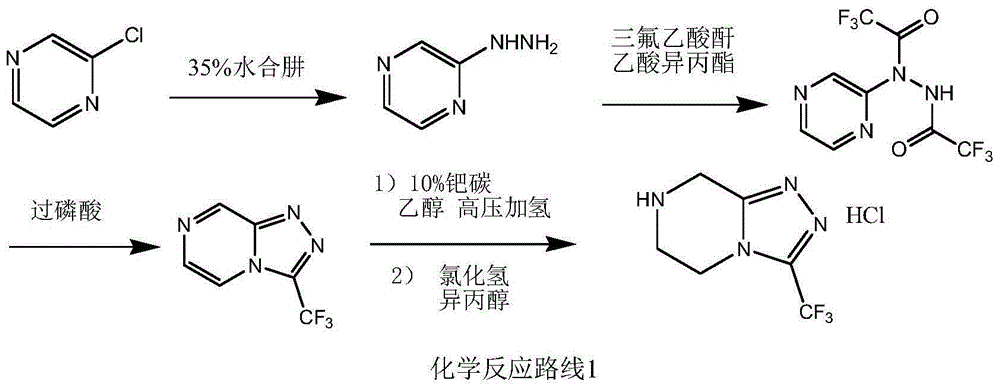
Preparation method of sitagliptin intermediate triazolopyrazine derivative
The invention relates to a preparation method of a sitagliptin intermediate triazolopyrazine derivative. The method comprises the following steps: by using 2-chlorethamin hydrochloride as an initial raw material, reacting the 2-chlorethamin hydrochloride with trifluoroacetate and aminoacetate hydrochloride to generate 1-trifluoroacetyl-2-piperazino ketone (III), and carrying out condensation ring formation on the compound (III), hydrazine hydrate and hydrochloric acid to obtain the sitagliptin intermediate 3-trifluoromethyl-5,6,7,8-tetrahydro-1,2,4-triazolyl[4,3-a]pyrazine hydrochloride (II). The high-activity triluoroacetyl carbonyl functional group and hydrazine hydrate are dehydrated into hydrazone, and the hydrochloric acid is directly added without separation, thereby performing intramolecular dehydration to generate the triazole ring. The method can avoid using thioketone, has the advantages of cheap and accessible raw materials, high reaction selectivity, short process, simple technical operation, high safety and environment friendliness.
Owner:XINFA PHARMA
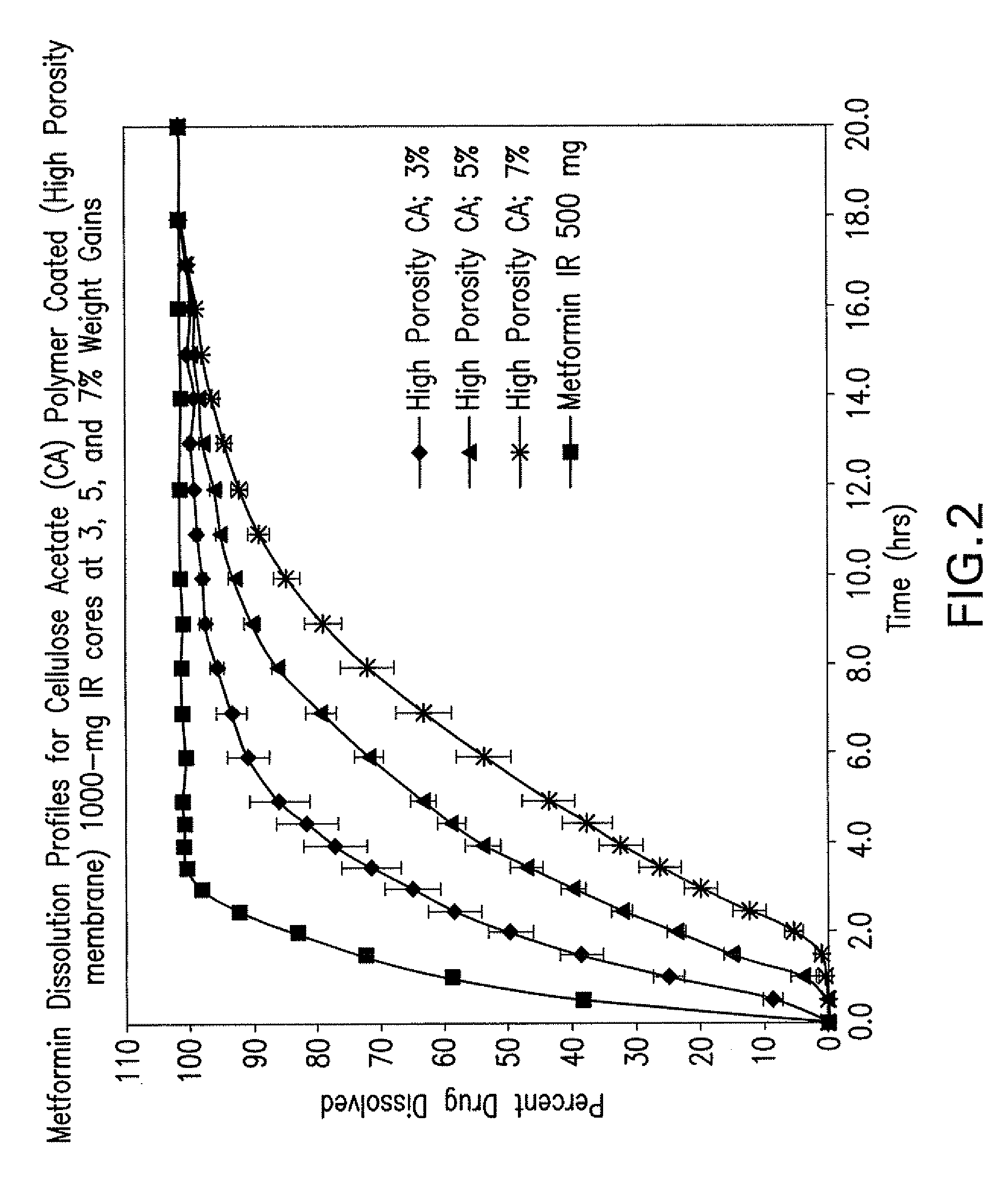
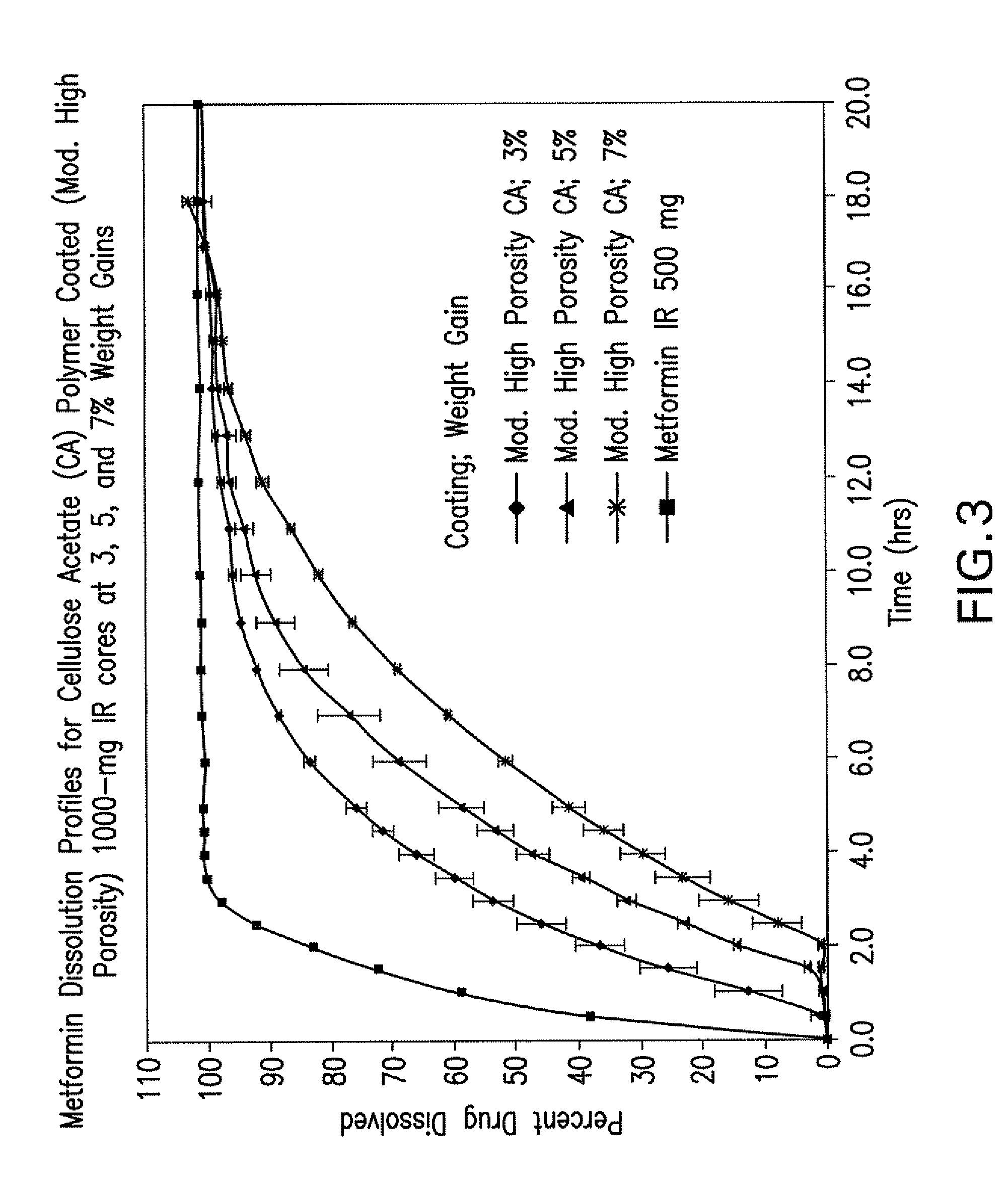
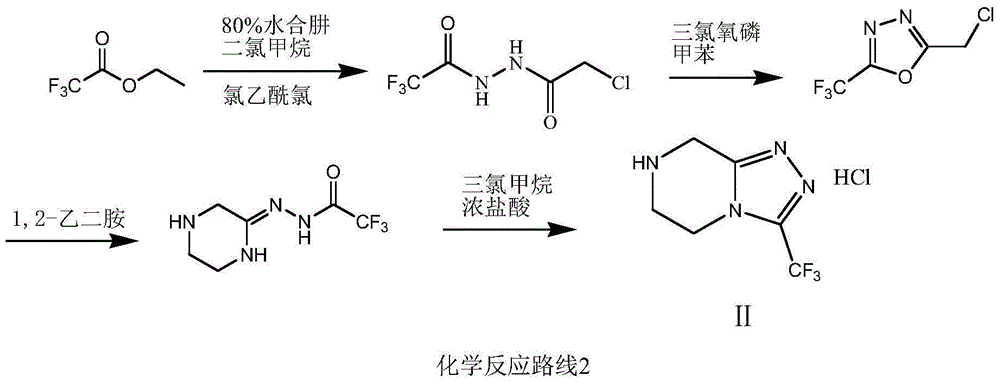
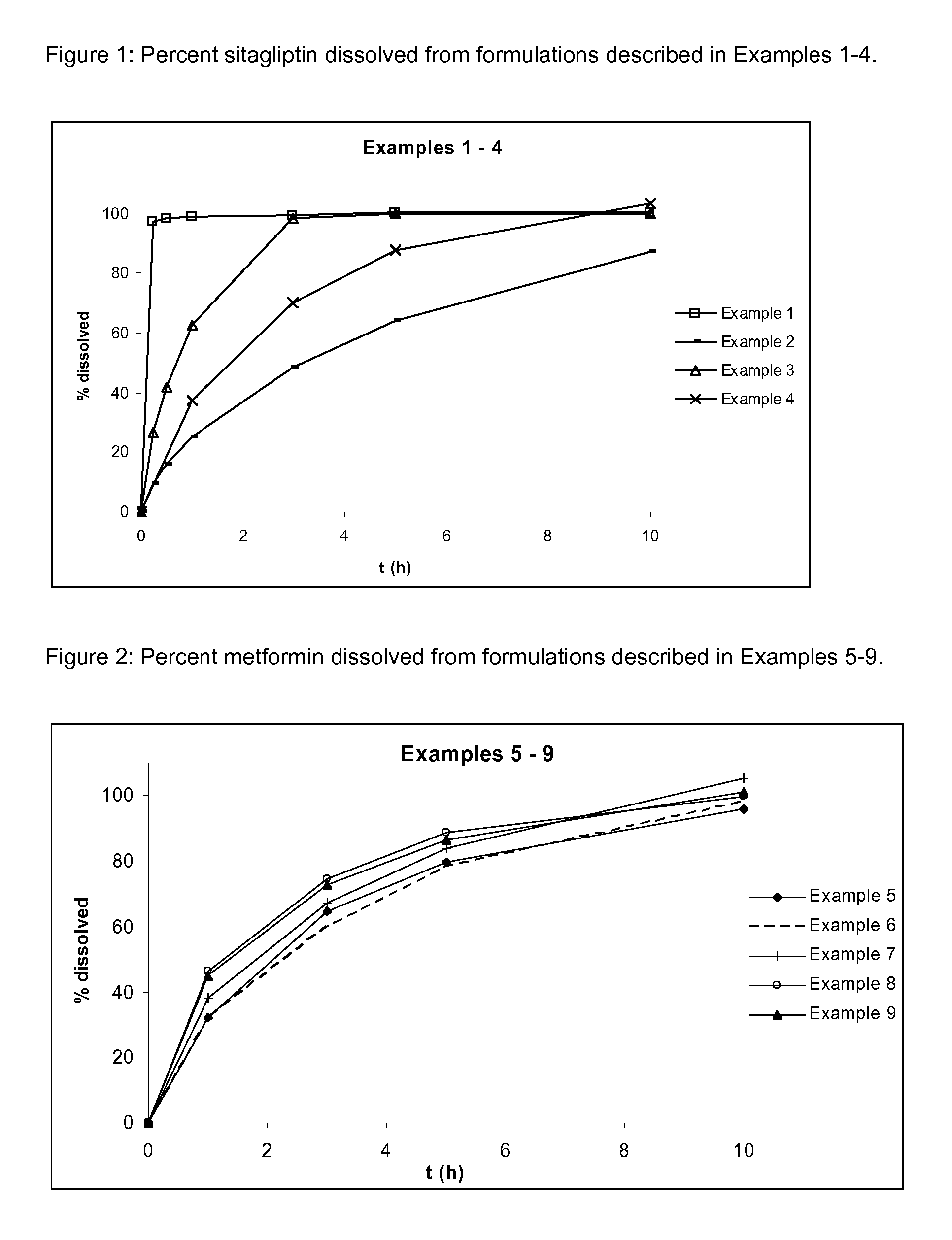
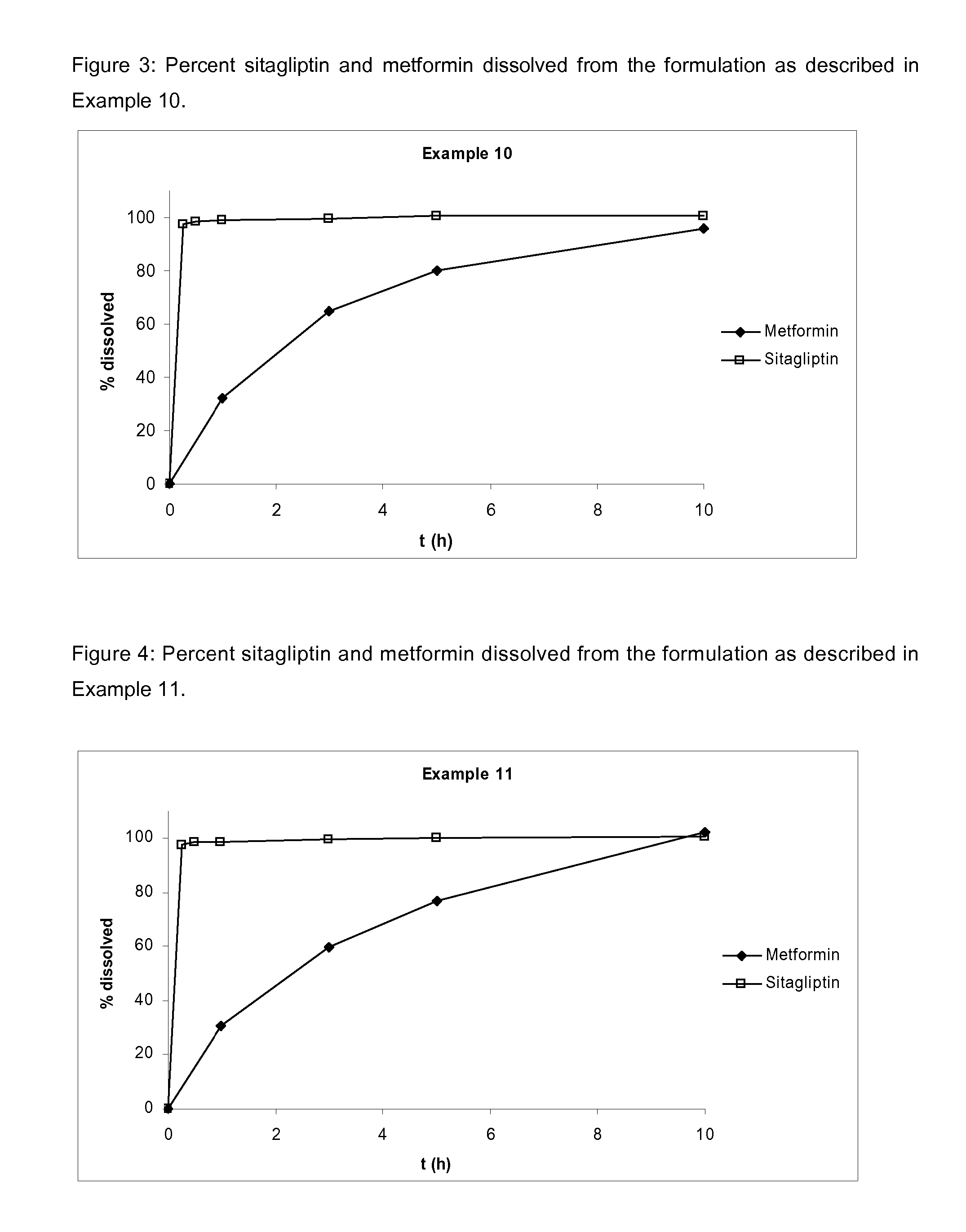
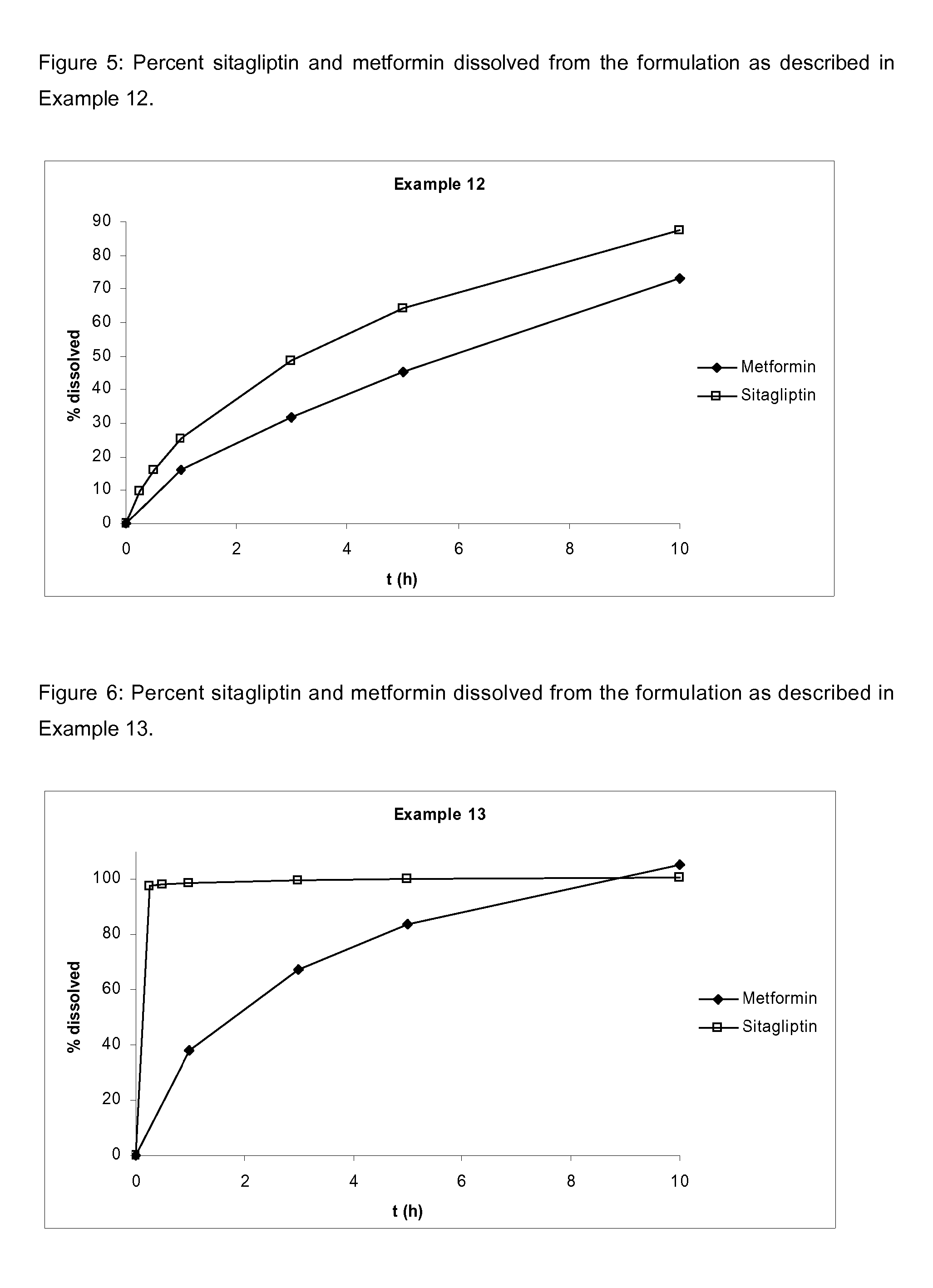
Pharmaceutical compositions comprising a combination of metformin and sitagliptin
ActiveUS20130059002A1Potential for interactionReduce the amount of solutionBiocideMetabolism disorderSitagliptinDosage form
The present invention relates to a pharmaceutical composition, preferably a pharmaceutical dosage form, comprising at least two separate compartments, wherein one compartment contains a composition comprising metformin or a pharmaceutically acceptable salt thereof and wherein another compartment contains a composition comprising sitagliptin.The present invention also relates to a process for preparing dosage forms comprising metformin or a pharmaceutically acceptable salt thereof and sitagliptin or a pharmaceutically acceptable salt thereof, the process comprising the steps of:a) providing one composition containing metformin or a pharmaceutically acceptable salt thereof and optionally also sitagliptin,b) providing a further composition containing sitagliptin or a pharmaceutically acceptable salt thereof and optionally also metformin, andc) combining the compositions to form compartments.The present invention also refers to a process for preparing dosage forms comprising at least one compartment comprising metformin or a pharmaceutically acceptable salt thereof and sitagliptin or a pharmaceutically acceptable salt thereof, the process comprises providing a composition containing metformin or a pharmaceutically acceptable salt thereof, and sitagliptin or a pharmaceutically acceptable salt thereof, and a matrix agent.Moreover, the present invention related to a dosage form obtained by said process, and to the use of said dosage form for the treatment of diabetes.
Owner:LEK PHARMA D D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com