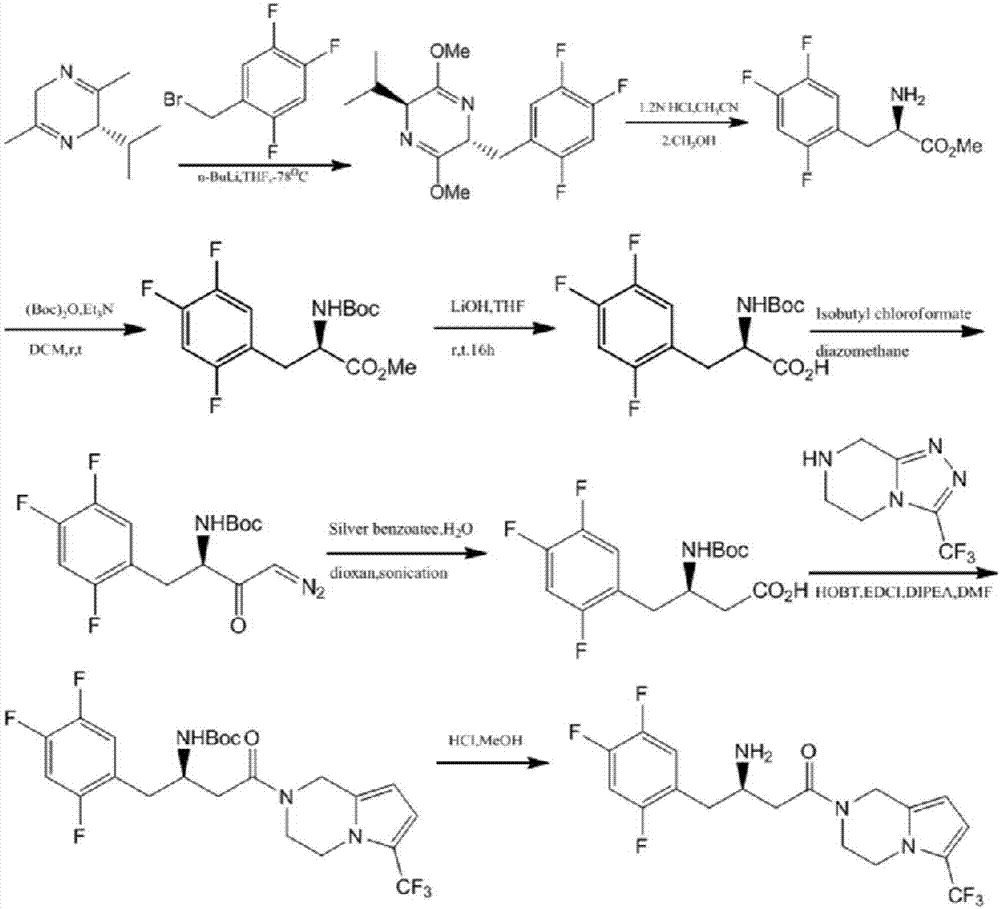
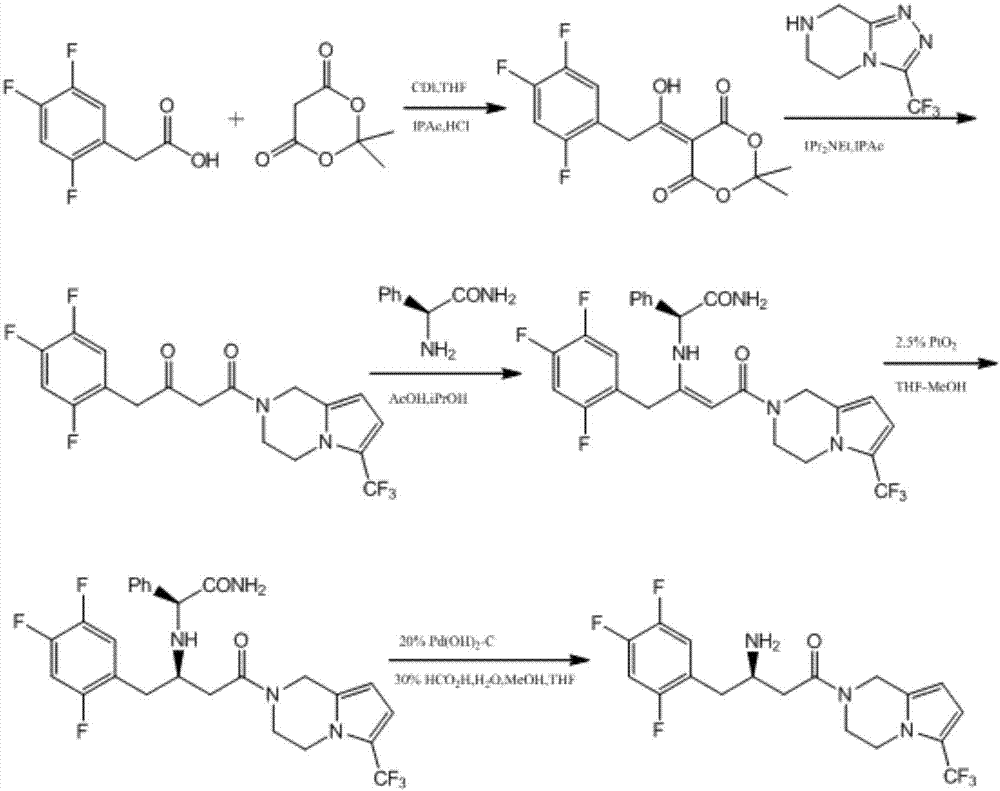
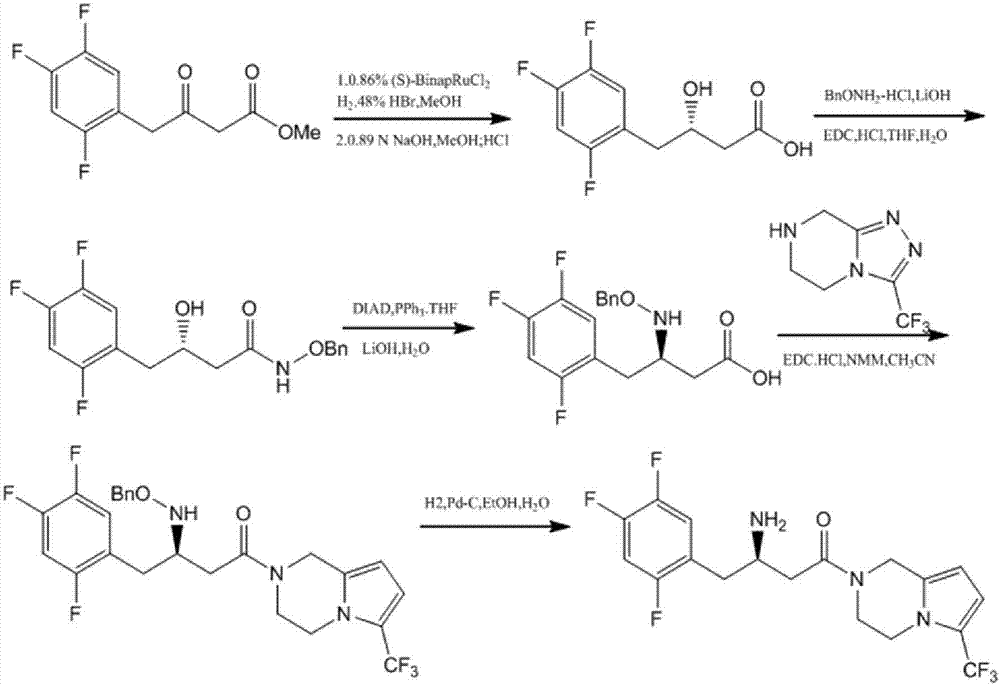
Aminotransferase, mutant and application to Sitagliptin preparation
A technology of aminotransferase and sitagliptin, which is applied in the preparation of chiral drug sitagliptin, can solve the problems of difficult recovery of solvents, poor stereoselectivity, and expensive catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Amplification of Aminotransferase Gene BgTA
[0053] Gladiolus Burkholderia (Burkholderia gladioli) ZJB-1216 is isolated from the soil, preserved in the China Center for Type Culture Collection (preservation number CCTCC NO: 2012379, has been disclosed in the patent application, application number CN201510026596 .7).
[0054] According to the aminotransferase gene sequencing information from Burkholderia sp. collected in Genbank, the Burkholderia gladioli ZJB-1216 cell was extracted with a rapid nucleic acid extraction instrument Using the genomic DNA as a template, PCR amplification was performed under the action of primer 1 (ATGGCTATCATCCAGGTTCAGCAGATC) and primer 2 (AGCCGGAACAGAAGAGAAGTATTC). PCR reaction system (total volume 50 μL): 5 μL of 10×Pfu DNA Polymerase Buffer, 1 μL of 10 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), 1 μL of cloning primer 1 and primer 2 each at a concentration of 50 μM, 1 μL of genomic DNA , Pfu DNA Polymerase 1...
Embodiment 2
[0057] Embodiment 2: Construction of recombinant Escherichia coli BL21 / pET28b-BgTA
[0058] According to embodiment 1BgTA gene sequence design primer 3 (CCG CATATG GCTATCATCCAG
[0059] GTTCAGC), Primer 4 (TTG CTCGAG TCAAGCCGGAACAGAAGAG), and Nde I and Xho I restriction enzyme sites (underlined) were introduced into primer 3 and primer 4, respectively. Under the initiation of primer 3 and primer 4, the high-fidelity Pfu DNA polymerase was used to amplify, and the recombinant plasmid pMD18-T-BgTA was used as a template (obtained in Example 1) to obtain the BgTA gene sequence, which was sequenced using Nde I and The amplified fragment was treated with Xho I restriction endonuclease (TaKaRa), and the fragment was ligated with the commercial vector pET28b (Invitrogen) treated with the same restriction endonuclease using T4 DNA ligase (TaKaRa) to construct Expression vector pET28b-BgTA ( Figure 8 ). The constructed expression vector pET28b-BgTA was transformed into Escheric...
Embodiment 3
[0060] Example 3: Induced expression of aminotransferase (ω-BgTA)
[0061] The recombinant Escherichia coli BL21(DE3) / pET28b-BgTA obtained in Example 2 was inoculated into LB liquid medium containing 50 μg / ml kanamycin resistance, cultivated at 37° C. for 12 hours at 200 rpm, and then treated with 1% (v / v) The inoculum is inoculated into fresh LB liquid medium containing 50 μg / ml kanamycin resistance, cultivated at 37°C and 150 rpm until the OD of the bacteria 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 5000rpm at 4°C for 20min, discard the supernatant, collect the precipitate, and obtain recombinant Escherichia coli BL21 / pET28b-BgTA wet cells. The bacterium can be used directly as a biocatalyst or for protein purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com